Abstract
Ancylostoma caninum is the most prevalent nematode parasite of dogs. We confirmed multiple-drug resistance (MDR) in several A. caninum isolates to all anthelmintic drug classes approved for the treatment of hookworms in dogs in the USA. Cases of MDR hookworms appear to be highly overrepresented in greyhounds. The aims of this study were to evaluate the drug-resistant phenotypes and genotypes of the A. caninum infecting greyhounds. Fecal samples from greyhounds of the USA were acquired from two greyhound adoption kennels, one active greyhound racing kennel, and three veterinary practices. Fecal egg counts (FECs) were performed on fecal samples from 219 greyhounds, and despite treatment with anthelmintics, the mean FEC was 822.4 eggs per gram (EPG). Resistance to benzimidazoles and macrocyclic lactones were measured using the egg hatch assay (EHA) and the larval development assay (LDA), respectively. We performed 23 EHA and 22 LDA on either individual or pooled feces, representing 54 animals. Mean and median IC50 and IC95 values for the EHA were 5.3 μM, 3.6 μM, and 24.5 μM, 23.4 μM, respectively. For the LDA, the median IC50 value was >1000 nM. These values ranged 62–81 times higher than our susceptible laboratory isolate. Only post-treatment samples were available. For samples collected <10 days post-treatment with albendazole, moxidectin, or a combination of febantel-pyrantel-moxidectin, the mean FEC were 349, 333, and 835 EPG, respectively. We obtained DNA from hookworm eggs isolated from 70 fecal samples, comprised of 60 individual dogs and 10 pools. Deep sequencing of the isotype 1 β-tubulin gene only revealed the presence of the F167Y (TTC>TAC) resistance polymorphism in 99% of these samples. These clinical, in vitro, and genetic data provide strong evidence that greyhound dogs in the USA are infected with MDR A. caninum at very high levels in prevalence and infection intensity.
Keywords: Ancylostoma caninum, hookworms; Multiple-drug resistance (MDR); Deep-amplicon; Greyhounds
Graphical abstract
Highlights
-
•
Conclusive evidence that most racing greyhounds in the USA are infected with MDR A. caninum.
-
•
79% of the samples from racing or retired greyhounds were positive.
-
•
IC50 values for BZs and MLs were 62–81 times higher in greyhounds.
-
•
The F167Y SNP was detected in 99% of samples, and in more than 2/3 the frequency was ≥ 75%.
1. Introduction
The canine hookworm, Ancylostoma caninum, is the most prevalent and important intestinal nematode parasite of dogs in the USA, with the prevalence depending on age, level of care and geographic location of the dog (Little et al., 2009). A recent study evaluating over 39 million fecal samples from 2012 to 2018, found that the prevalence of hookworms remained very stable from 2012 to 2014 at around 2%, but then from 2015 onwards, there was a steady yearly increase, with an overall increase of 47% by 2018 (Drake and Carey, 2019). Moreover, in a study assessing intestinal parasites from 3006 dog fecal samples collected in 288 off-leash dog parks across the USA in 2019, the prevalence of A. caninum was 7.1% (Stafford et al., 2020). Interestingly, this prevalence is more than twice as high as that reported for 2018 by (Drake and Carey, 2019), and is more than 70% higher than the mean prevalence for 2017–2019 reported by (Sweet et al., 2021). Taken together these data suggest that hookworm prevalence is rapidly increasing, and that dogs that visit dog parks are at a higher risk of infection.
Anthelmintic drugs currently approved for the treatment of A. caninum in the United States include, febantel and fenbendazole, moxidectin and milbemycin oxime, and pyrantel, of the benzimidazole, avermectin/milbemycin, and tetrahydropyrimidine classes, respectively. In registration studies, febantel, moxidectin and milbemycin oxime all demonstrated efficacies of >99% (F.D.A, 1994, 1998, 2006), fenbendazole demonstrated efficacy of >98% (F.D.A, 1983) and pyrantel demonstrated a slightly variable efficacy, with a mean across studies of approximately 94%, where more than half of those studies yielded >99% (F.D.A, 1993).
Hookworms are blood-feeding nematodes that use a cutting apparatus to attach to the intestinal mucosa and submucosa, and contract their muscular esophagus to create negative pressure, which sucks a plug of tissue into their buccal capsules (Hotez et al., 2004). Bleeding is facilitated by both mechanical damage and chemical action by hydrolytic enzymes that cause rupture of capillaries and arterioles (Stassens et al., 1996). Pathological consequences of infection in dogs include iron-deficiency anaemia, hypoalbuminemia, and an enteritis characterized by diarrhoea, that may contain fresh (haematochezia) or digested blood (melena) (Kalkofen, 1987; Epe, 2009; Taylor et al., 2016).
In the past few years, there is empirical evidence that veterinarians are diagnosing increasing numbers of cases of persistent hookworm infections that appear refractory to typical anthelmintic therapy. Recent work in our laboratory confirmed that many, if not most, of these persistent hookworm cases are likely due to multiple-drug resistance (MDR) in A. caninum, with retired racing greyhounds highly over-represented among the cases reported to our laboratory (Jimenez Castro et al., 2019). Our laboratory established one of these A. caninum isolates (Worthy), which we obtained from a recently adopted retired racing greyhound dog in late 2017. In a controlled efficacy study using this isolate, we confirmed high levels of resistance to all classes of drugs approved for treatment of hookworm in dogs; fenbendazole, pyrantel pamoate and milbemycin oxime yielded efficacies of 26%, 23% and 9%, respectively (Jimenez Castro et al., 2020).
At its peak in 1991, greyhound racing was rated the sixth most popular sporting activity in the USA, was legal in 19 states, and generated around 100,000 jobs with wager revenues of $3.5 billion USD (Theil, 2021). At that time there were 38,000 individual pups and 5,700 registered racers, but by 2020 those numbers had dramatically decreased to 4,300 and 850, respectively (Gartland, 2021). Kansas (KS), the state with the most greyhound breeding farms, had 274 greyhound breeding farms in the 1990's, but by 2015 this number had decreased by more than half to 130 (Hall, 2016), and continues to fall. These farms tend to have large dog populations; more than 60% and 80% of all racing greyhounds come from farms with >250 dogs and >100 dogs, respectively (Hall, 2016).
In November 2018, voters in Florida (FL) passed Amendment 13, a constitutional amendment which banned wagering on live dog races, including greyhound racing in the state as of January 1st, 2021 (State, 2018). In 2018, 65% of the greyhound tracks in the USA were in FL. However, with the closure of these tracks and others in several other states in the past few years, to our knowledge there are currently only 7 tracks in 5 states remaining. This change likely represents the beginning of the end for greyhound racing in the USA. In parallel, a large network of greyhound adoption groups has been active for many years, having over 160 organizations across the USA and Canada. Though we could not find more recent data, Lord and colleagues reported that 120,000 Greyhounds lived in homes as pets as compared to 55,000 Greyhounds in racetracks, with numbers of adoptions in the early-mid 2000's ranging from 15,000–18,000 per year (Lord et al., 2007). With the recent demise of the greyhound racing industry, the number of annual adoptions has most likely increased again. Thus, it is important for the health of both racing greyhounds and pet dogs to determine the extent of the MDR hookworm problem in racing greyhounds.
The aims of this study were to investigate the prevalence of infection, the range of in vitro and in vivo drug susceptible/resistant phenotypes, and the frequency of benzimidazole-resistant β-tubulin genotypes in greyhound dogs infected with A. caninum.
2. Materials and methods
2.1. Sample collection
From February 2019 to February 2020, fecal samples were acquired from two greyhound adoption kennels located in Birmingham, Alabama (AL), and Dallas, Texas (TX), one active greyhound racing kennel in Sanford, FL, and three veterinary practices located in Acworth, Georgia (GA), Columbia, South Carolina (SC), and St. Petersburg, FL, USA that work with greyhound adoption organizations. Most samples were collected from individual dogs, but from the Sanford, FL site, only anonymous samples from the ground were available. The dogs residing in these kennels originated from 16 different locations in 8 different states. These included five breeding farms located in Kansas (KS), Colorado (CO), Arkansas (AR), Texas (TX), or Oklahoma (OK), and 11 racing tracks located in AL, FL, AR, or West Virginia (WV).
2.2. In vitro assays
To evaluate drug response phenotypes, the egg hatch assay (EHA) and larval development assay (LDA) were used for benzimidazoles (BZs), and macrocyclic lactones (MLs), respectively as previously described (Jimenez Castro et al., 2019). The concentration ranges for ivermectin aglycone (1.9–1000 nM) in the LDA, and thiabendazole (0.075–40 μM) in the EHA were selected based on our previous work to allow the discrimination of susceptible versus resistant isolates of A. caninum (Jimenez Castro et al., 2019). EHA plates were sealed with parafilm and stored in the refrigerator at 4 °C for a maximum of one week. LDA plates (Microbial Screening Technologies, Armidale, New South Wales, Australia) were vacuum sealed upon arrival and stored in the refrigerator at 4 °C for a maximum of 10 months. Previous experience in our laboratory has demonstrated that LDA plates containing ML stored in this manner provided consistent results for well over a year. Prior to performing, the assays plates were removed from the refrigerator and permitted to reach room temperature. Eggs were isolated using 50 mL tubes containing activated charcoal granules (0.5–1 cm) and specialized lids, which essentially were a filter with a mesh size of 1 mm that could attach firmly to 15 mL centrifuge tubes (Supplementary Fig. 1). Five to 10 g of feces and approximately 15 mL of water were added to the 50 mL tube containing approximately 5 g of activated charcoal and vigorously shaken by hand to break up the feces. The lid of the 50 mL tube was removed and replaced with the specialized lid, and a 15 mL tube was attached to the other end. The apparatus was then shaken again which allowed the filtered fecal suspension to fill the 15 mL tube, which was then centrifuged at 240×g for 10 min. The supernatant was discarded, 10 mL of sodium nitrate (Feca-Med®, Vedco, Inc. St. Joseph; MO, USA specific gravity = 1.2) were added and the tube was vortexed to disperse the 1–2 mls of fecal material left as sediment. The tube was then centrifuged again at 240×g for 10 min. Following centrifugation, the supernatant containing the eggs was passed through a 20 μm stainless steel sieve, rinsed with distilled water, transferred to a new tube, and then the volume was adjusted to yield 50–60 eggs per 20 μl using distilled water. If insufficient eggs were recovered to perform both the EHA and the LDA, then only the EHA was performed.
2.3. In vivo measurements
Every dog sampled in this study was treated with anthelmintics either every two weeks or once a month; therefore, all the samples were collected relatively recently post-treatment. Samples were refrigerated immediately after collection and shipped to the Kaplan lab at the University of Georgia in a container with ice packs. In order to account for the differences in timeframe since the previous anthelmintic treatment, dogs were assigned to one of three categories based on the following biological factors: (A) = <10 days, as this can be too soon to measure an accurate fecal egg count reduction (FECR) and can lead to false positives due to temporary inhibition of egg production (Jimenez Castro et al., 2019, 2020), (B) = 10–21 days, as this would be an optimal timeframe for measuring the FECR, and (C) =>21 days, as there is the possibility that eggs could be shed from reactivated encysted/arrested larvae that migrated to the small intestine and completed development to sexually mature adults following the anthelmintic treatment (due to “larval leak”) (Jimenez Castro and Kaplan, 2020). Fecal egg counts (FEC) were performed using the Mini-FLOTAC (University of Naples Federico II, Naples, Italy) procedure with a detection threshold of 5 EPG (Maurelli et al., 2014; Lima et al., 2015), adding 2 g of feces to 18 mL of sodium nitrate (Feca-Med®, Vedco, Inc. St. Joseph; MO, USA specific gravity = 1.25 to 1.30). Positive samples were defined as having a FEC of ≥ 5 EPG. All anthelmintic treatments were administered by either kennel or veterinary practice personnel. Where products approved for use in dogs were used, treatments were administered according to label instructions; these included febantel-pyrantel pamoate, Drontal® Plus (Elanco, Greenfield, IN), moxidectin, Advantage® Multi (Elanco, Greenfield, IN) and pyrantel pamoate, Nemex-2® (Zoetis, Kalamazoo, MI). In some instances, products labelled for large animals were used; these included moxidectin, Quest® Plus (Zoetis, Kalamazoo, MI) and albendazole, Valbazen® (Zoetis, Kalamazoo, MI). These products were administered orally at approximately 3.3 mg/kg and 19 mg/kg, respectively. In some cases, compounded drugs were used, such as pyrantel pamoate, praziquantel, and mebendazole which are included in the PPM Triwormer (Roadrunner pharmacy, Phoenix, AZ).
2.4. Ancylostoma caninum isotype-1 β-tubulin deep amplicon sequencing
2.4.1. DNA preparation
After setting up the in vitro assays, the remaining eggs were transferred to 2 mL cryotubes (Sigma-Aldrich, St. Louis, MO), suspended in a final concentration of 70% ETOH and stored at −80 °C until further use. DNA lysates were prepared from individual or pooled egg samples. Briefly, 3 freeze-thaw cycles were carried out at −80 °C and at 55 °C respectively, followed by adding 180 μL of DirectPCR (Cell) Lysis Buffer (Catalog No. 301-C, Viagen Biotech, St. Louis, MO) and 20 μL of Proteinase K (Catalog No. 19133, QIAGEN, Hilden, Germany). Samples were then incubated for at least 12h at 65 °C, then 1h at 95 °C, and were then cooled to 4 °C. DNA was purified from the crude DNA lysates using the QIAGEN QIAmp DNA mini kit (Cat# 51306), following the manufacturer's recommended protocol, and stored at −80 °C.
2.4.2. Deep-amplicon sequencing assay analysis
Deep amplicon sequencing assays developed to evaluate the frequency of non-synonymous single nucleotide polymorphisms (SNP) at codons 167, 198 and 200 of the A. caninum isotype-1 β-tubulin gene were applied to 70 samples ranging from 200 to 20,000 eggs (mean of 1670, standard error of mean 296.9) from the two adoption kennels, one active racing kennel, and from one of the veterinary practices. Using adapted primers suitable for Illumina deep-sequencing, two separate regions of the A. caninum isotype-1 β-tubulin gene, comprising 293 bp and 340 bp which encompass codon 167, and codons 198 and 200, respectively, were PCR amplified (Jimenez Castro et al., 2019). The following PCR conditions were used: 5 μL KAPA HiFi Hotstart fidelity buffer (5X) (KAPA Biosystems, USA), 1.25 μL forward primer (10 μM), 1.25 μL reverse primer (10 μM), 0.75 μL dNTPs (10 μM), 0.5 μL KAPA HiFi polymerase (0.5U), 0.1 μL bovine serum albumin (Thermo Fisher Scientific), 14.15 μL H2O, and 2 μL of DNA lysate. The thermocycling parameters were 95 °C for 3 min, followed by 45 cycles of 98 °C for 20 s, 65 °C for 15 s, and 72 °C for 30 s, followed by 72 °C for 2 min. Sample purification and addition of barcoded primers followed the protocols defined in (Avramenko et al., 2019). Library preparation was as previously described and library sequencing performed using the Illumina MiSeq platform with the 2 × 300v3 Reagent Kit (Illumina Inc., San Diego, CA, USA) (Avramenko et al., 2015). For the fragment encompassing codon 167, two independent PCR reactions were performed on 66 samples and the libraries were sequenced in two independent sequencing runs using the Illumina MiSeq platform with the 2 × 300 v3 Reagent Kit.
2.4.3. Sequence analysis
Cutadapt v3.2 (Martin, 2011) was used to remove the A. caninum forward and reverse primer sequences. Following adapter trimming, all the forward and reverse reads were processed using the DADA2 bioinformatic pipeline to obtain Amplicon Sequence Variants (ASVs) (Callahan et al., 2016). During the quality filtering step of the DADA2 pipeline, the default setting with the following additional settings were used: (i) forward and reverse reads were trimmed to a length of 280 bp and 190 bp, respectively and (ii) reads shorter than 50 bp or with an expected error of>1 or>2 in the forward and reverse reads respectively were removed. The DADA2 algorithm was then applied to the filtered and trimmed reads to identify the ASVs. Following this, the overlapping forward and reverse reads were merged, allowing a maximum mismatch of 4 bp in the overlap region. The ASVs generated using the DADA2 pipeline were then aligned to the A. caninum isotype-1 β-tubulin reference sequence (Genbank Accession: DQ459314.1) using a global (Needleman-Wunsch) pairwise alignment algorithm without end gap penalties. Following alignment, the ASVs were discarded if they were <180 bp or >350 bp long, or if they had a percentage identity <70% to the reference sequence, or if the ASVs had fewer than 200 reads in a sample, or if they were not present in two or more samples. This additional filtering ensures the removal of spurious sequences. In summary, of 2,081,485 total paired-end reads inputted into the DADA 2 analysis pipeline, 822,023 merged paired-end reads were outputted for ASV generation and analysis. Following mapping of ASVs to the A. caninum isotype-1 β-tubulin reference sequence, and removal of ASVs that had less than a total of 200 mapped reads or were present in only one sample, 783,793 reads remained for variant calling. The codons 167, 198 and 200 were then analyzed for the presence of any variants resulting in non-synonymous changes. The MUSCLE alignment tool was used to align the filtered ASVs from both the fragments with isotype-1 and 2 β-tubulins from other nematodes present in Clade V of the nematode phylogeny. Using the Geneious tree builder, a neighbour-joining tree, utilizing the Jukes Cantor tree building method, was constructed from the trimmed alignment, and having H. contortus isotype-3 β-tubulin as the outgroup (Genbank Accession: HE604101) and 2000 bootstrap replicates.
2.5. Data analyses
Dogs treated solely with albendazole and moxidectin were represented in each of the post-treatment timeframe categories, therefore for each drug individually, statistical analyses were performed to determine if the FEC of the dogs differed between those categories. For this, a Kruswal-Wallis test was performed for the overall comparison with a Benjamini-Krieger-Yekutielli procedure for individual pairwise comparisons. For the EHA and LDA, dose-response analyses were performed after log transformation of the drug concentrations and constraining the bottom value to zero. The top parameter for samples that did not reach 100% inhibition was constrained to 100 to avoid introducing artificial bias to the model. Data were then fitted to a four-parameter non-linear regression algorithm with variable slope. The IC50 or IC95 values, which represent the concentration of drug required to inhibit hatching (EHA) or development to the third larval stage (LDA) by 50% or 95%, respectively of the maximal response were calculated.
To compare the infection prevalence between states, a Chi-square test was performed for the overall comparison, followed by a Fisher's exact test with a Bonferroni procedure for individual pairwise comparisons between states. All analyses were designed to maintain the overall type I error rate at 5%. To evaluate the level of agreement between the two independent PCR amplifications and separate sequencing runs of the fragment encompassing codon 167, a Bland-Altman analysis was performed. To quantify the relationship between the EHA and the deep-amplicon sequencing assay to measure levels of BZ resistance, a Spearman correlation analysis was performed comparing both IC50 and IC95 values with the F167Y SNP frequencies. All statistical analyses were performed in GraphPad Prism® version 9.0.2, GraphPad Software, San Diego, CA, USA.
3. Results
3.1. Fecal egg count data
171 of the 219 fecal samples from racing or recently retired greyhounds were positive for hookworm eggs, yielding an overall prevalence of 79%, with a mean FEC of 822.4 EPG (Table 1). The prevalence of infection for the Birmingham, AL (p = 0.0063) and Sanford, FL (p < 0.0001) kennels were significantly higher than for the Dallas, TX kennel. Percent reductions in FEC following treatments could not be calculated since pre-treatment FEC were not available. However, in the 197 (89.9%) samples collected 2–21 days post-treatment, the mean FEC was 721 EPG, indicating a major lack of efficacy across the different treatments. For the group of samples collected following moxidectin treatment, samples collected>21 days post-treatment had a statistically significant higher mean FEC when compared to the other two categories (Table 2). For the group of samples collected following albendazole treatment, the 10–21 and>21 days categories had a statistically significant higher mean FEC when compared to the <10 days. Interestingly, a single group of samples from nine dogs recently acquired from a breeding farm in KS had <5 EPG following treatment. For all other sites, mean FEC were 330 EPG or greater.
Table 1.
Mean fecal egg count (FEC) data and percent prevalence of hookworm infections from 219 greyhound dog samples obtained from two greyhound adoption kennels, one greyhound racing kennel, and veterinary practices. Data from the three veterinary practices were combined for reporting purposes. Positive samples were defined as having a FEC of ≥ 5 eggs per gram (EPG).
| Adoption kennel | FEC (EPG) | Prevalence (%) (95%CI)b | Total # of samples |
|---|---|---|---|
| Birmingham, AL | 527.8 | 76.3 (66.2,84.7)a | 80 |
| Sanford, FL | 1288.6 | 87.5 (79.1,93.5)a | 80 |
| Dallas, TX | 683 | 48.1 (30.1,66.5)b | 27 |
| Veterinary practicesa | 529.3 | 90.6 (77.5,97.6) | 32 |
| Overall means | 822.4 | 79 (73.3,84.0) | 219 |
Three veterinary practices located in Acworth, GA, Columbia, SC, and St. Petersburg, FL.
Shared superscripts denote non-significant differences.
Table 2.
Mean fecal egg counts (FEC) in eggs per gram (EPG) with the standard error of the mean (SEM) of fecal samples from greyhound dogs. Samples were obtained at varying intervals following treatments with several different anthelmintics. State of origin of the dogs is provided, and where more than one city was represented and city was known, a subscript letter indicates the number of cities represented. Timeframes since the previous anthelmintic treatment were categorized as: (A): <10 days, (B): 10–21 days, and (C):>21 days. Dogs treated solely with albendazole and moxidectin were represented in each of the post-treatment timeframe categories, therefore for each drug individually, statistical analyses were performed to determine if the FEC of the dogs differed between those categories.
| Treatment | No. ofgreyhounds | Categories post-treatment | FEC (SEM) (EPG)f | State of origine |
|---|---|---|---|---|
| Single drugs | ||||
| Moxidectina | 20 | A | 333.3 (108.9)a | AL, FL1, FL2, FL3, AR, TX, WV, KS |
| Moxidectina | 41 | B | 334.6 (93.5)a | AL, AR, FL1, FL2, FL4, WV, AR, CO, TX |
| Moxidectina | 53 | C | 1117 (189.4)b | AL, AR, FL1, FL3, FL4, FL5, FL6, WV |
| Pyrantel pamoateb | 3 | C | 600 (263.2) | FL, AL |
| Albendazolec | 20 | A | 349.3 (150)c | AR, FL |
| Albendazolec | 21 | B | 1874 (396.4)d | AR, FL |
| Albendazolec | 10 | C | 1819 (524.1)d | FL |
| Combinations | ||||
| Febantel-pyrantel-moxidectind | 20 | A | 834.8 (271.9) | AL, AR, AR1, FL1, FL3, FL4, FL7 |
| Moxidectina + pyrantelb | 9 | C | 0 | KS |
Quest® Plus.
Nemex®-2.
Valbazen®.
Drontal® Plus and Advantage® Multi.
FL1 = Palm Beach, FL2 = Daytona Beach, FL3 = Jacksonville, FL4 = Naples-Ft. Myers, FL5 = Sanford, FL6 = Clermont, FL6 = Pensacola, AR1 = West Memphis.
For moxidectin and albendazole, shared superscripts denote non-significant differences.
3.2. In vitro assays
EHA and LDA were performed on 35 samples, yielding dose-response data on 23 and 22 samples, respectively, which represented samples collected from 54 greyhounds (Fig. 1).
Fig. 1.
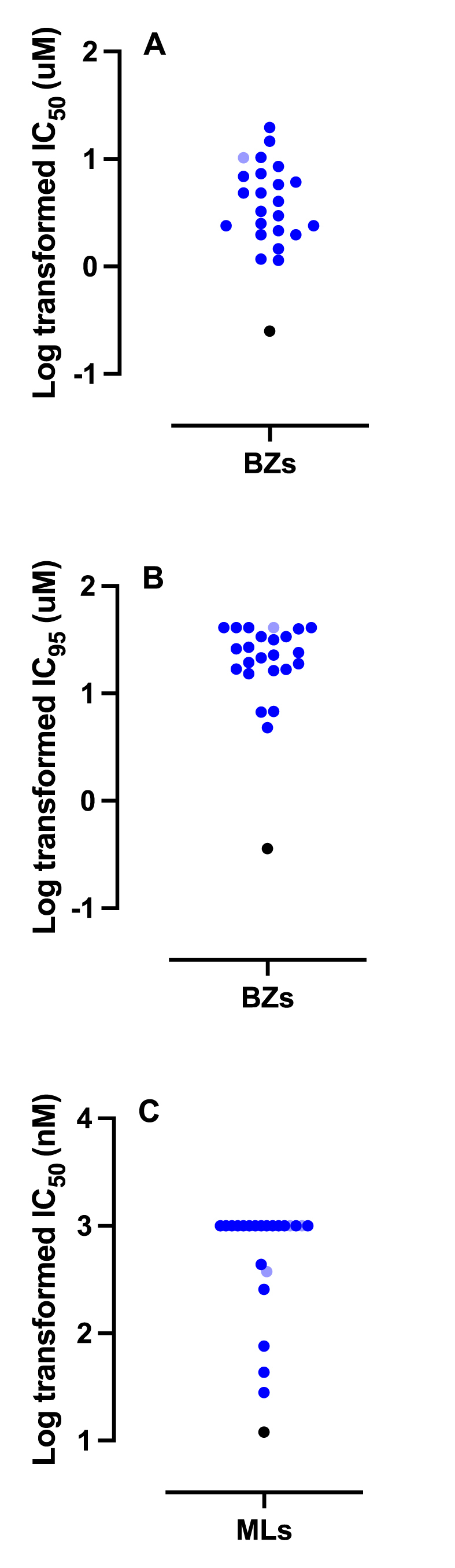
Scatter dot plots of greyhound samples showing the log transformed Egg Hatch Assay (EHA) IC50 (A) and IC95 (B) values, and the Larval Development Assay (LDA) IC50 (C) values for the benzimidazoles (BZs) and macrocyclic lactones (MLs), respectively. Each dark blue and light blue dot represent an assay performed on an individual or a pooled sample, respectively. The black dot represents the value of our susceptible laboratory isolate for reference (Jimenez Castro et al., 2019). Dose-responses were analyzed using the variable slope nonlinear regression model analysis contained in GraphPad 9.0.2. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Mean hatching rates of eggs in the assays ranged from 82 to 98%. For the EHA, mean and median IC50 and IC95 values were 5.3 μM, 3.6 μM, and 24.5 μM, 23.4 μM, respectively (Table 3). For the LDA, IC50 and IC95 values were calculated in a subset of assays, however for the majority of assays, IC50 values could not be calculated since this value was greater than the highest concentration tested (1000 nM). Likewise, IC95 values could not be calculated for the majority of assays performed. IC50 and IC95 values were calculated for 22 samples with a range of 28.1 nM to>1000 nM; 16 samples (73%) had IC50>1000 nM, and hence the median value was also>1000 nM.
Table 3.
Geographic origin of the dogs, fecal egg counts (FEC) in eggs per gram (EPG), egg hatch assay (EHA) IC50 and IC95 values (uM), larval development assay (LDA) IC50 values and IC95 values (nM), last anthelmintic treatment administered, and days from treatment to sample collection. All dose-response analyses were performed after log transformation of the drug concentrations and constraining the bottom value to zero. Data were then fitted to a four-parameter non-linear regression algorithm with variable slope. Data for the susceptible isolate, Barrow 1.0, was taken from Jimenez Castro et al. (2019).
| Sample ID codea | FEC (EPG) | EHA IC50 (BZ) | EHA IC95 (BZ) | LDA IC50 (ML) | LDA IC95 (ML) | Last treatment administered | Days since last treatment | City and State of sample origing |
|---|---|---|---|---|---|---|---|---|
| Barrow 1.0 | N/A | 0.17 | 0.36 | 12.3 | 241.8 | N/A | N/A | N/A |
| Bh 2 | 4725 | 1.2 | 16.8 | 255.5 | >1000 | Febantel-pyrantel-moxidectinb | 4 | Birmingham, AL |
| Bh 7 | 1895 | 2.4 | 21.5 | 43.3 | >1000 | Febantel-pyrantel-moxidectinb | 4 | Palmbeach, FL |
| Bh 19 | 1825 | 2 | 24 | 28.1 | >1000 | Febantel-pyrantel-moxidectinb | 4 | Palmbeach, FL |
| Bh 20 | 2635 | 1.14 | >40 | NA | NA | Febantel-pyrantel-moxidectinb | 4 | Birmingham, AL |
| Bh 25 | 1440 | 3.3 | 15.3 | 75.8 | 315.6 | Moxidectinc | 16 | Birmingham, AL |
| Bh 28 | 1410 | 2.9 | 22.7 | 438.5 | >1000 | Moxidectinc | 16 | Birmingham, AL |
| Bh 32 | 520 | 5.8 | 26.1 | >1000 | >1000 | Moxidectinc | 16 | Birmingham, AL |
| Bh 40 | 690 | 4 | >40 | >1000 | >1000 | Moxidectinc | 16 | Daytona beach, FL |
| Bh 41 | 615 | 8.5 | 16.3 | >1000 | >1000 | Moxidectinc | 2 | Birmingham, AL |
| Bh 43 | 1675 | 10.3 | >40 | >1000 | >1000 | Moxidectinc | 2 | Birmingham, AL |
| Bh 51 | 205 | 14.6 | 26.9 | >1000 | >1000 | Moxidectinc | 18 | Birmingham, AL |
| Bh 53 | 2560 | NA | NA | >1000 | >1000 | Moxidectinc | 11 | Birmingham, AL |
| Sf 10–20h | 472.7 | NA | NA | 375.4 | >1000 | Moxidectinc | 31 | Sanford, FL |
| Sf 61–70h | 1353 | 10.2 | >40 | >1000 | >1000 | Moxidectinc | 30 | Sanford, FL |
| Sf 71–80h | 1819 | NA | NA | >1000 | >1000 | Albendazoled | 30 | Sanford, FL |
| Dl 12 | 4450 | 4.8 | 33.8 | >1000 | >1000 | Unknown | 25 | KS |
| Dl 13 | 600 | 2.1 | 6.7 | NA | NA | Unknown | 25 | KS |
| Dl 14 | 620 | 1.5 | 4.8 | NA | NA | Unknown | 25 | KS |
| Cl 9 | 1110 | 4.8 | 33.8 | >1000 | >1000 | Pyrantel-mebendazole-moxidectine | 4 | Sanford, FL |
| Cl 12 | 490 | NA | NA | >1000 | >1000 | Pyrantel-mebendazole-moxidectine | 9 | Sanford, FL |
| Cl 14 | 1180 | 2.4 | 18.9 | >1000 | >1000 | Moxidectinc | 5 | Birmingham, AL |
| Cl 16 | 1665 | 1.9 | 6.8 | NA | NA | Moxidectinc | 24 | Fort Myers, FL |
| Sp 1 | 905 | 19.6 | >40 | >1000 | >1000 | Moxidectinc | 23 | FL |
| Sp 4 | 1125 | 6.1 | 16.9 | NT | NT | Pyrantelf | 23 | FL |
| Sp 5 | 2870 | 6.9 | 31.5 | >1000 | >1000 | Unknown | 23 | FL |
| Sp 6 | 560 | 7.3 | >40 | >1000 | >1000 | Moxidectinc | 23 | FL |
| Ac 1 | 390 | 2.5 | 19.4 | >1000 | >1000 | Unknown | Unknown | FL |
NA: Assay data not available.
NT: Assay data not available due to insufficient amount of eggs.
N/A: Not applicable.
Samples were received from these locations, greyhound adoption kennels: Bh = (Birmingham, AL), and Dl = (Dallas, TX); greyhound racing kennel: Sf = (Sanford, FL), and veterinary practices: Cl = (Columbia, SC), Sp= (St. Petersburg, FL), and Ac = (Acworth, GA).
Drontal® Plus and Advantage® Multi.
Quest® Plus.
Valbazen® (albendazole).
PPM Triwormer and Advantage® Multi.
Nemex®-2.
For some samples the city of origin was not available. City/state refers only to the origin of the sample tested, but does not necessarily reflect the origin of the dogs prior to arriving at the respective kennels or veterinary practices.
Pooled samples comprising ten dogs.
3.3. Relative frequencies of the isotype-1 β-tubulin benzimidazole resistance associated polymorphisms
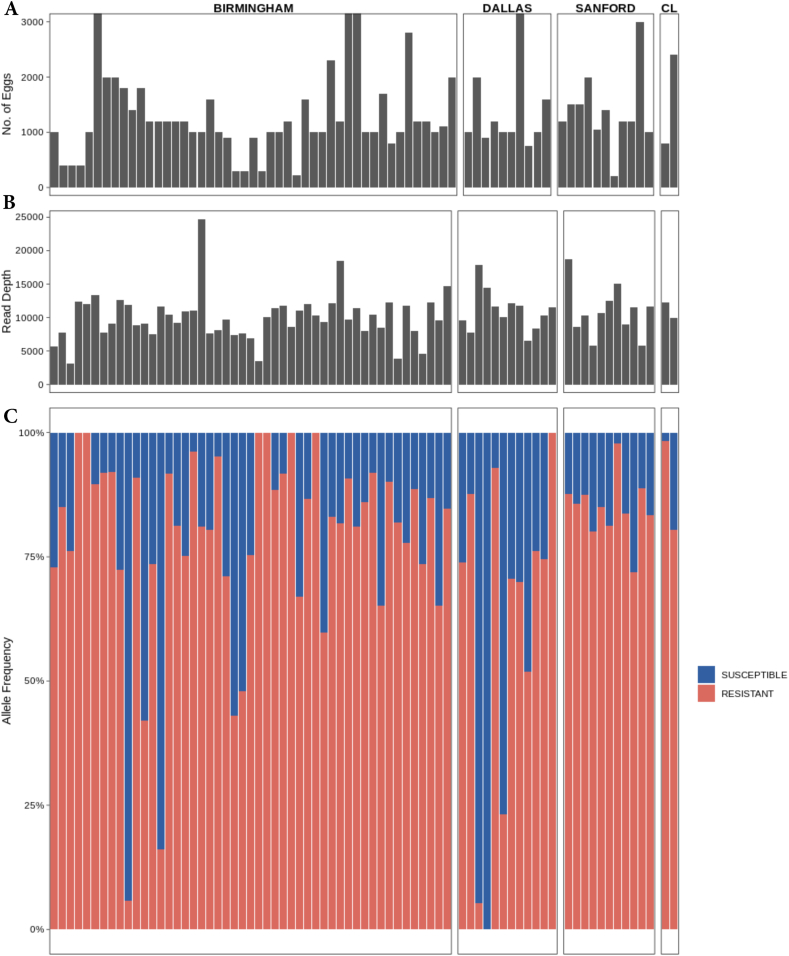
The three codons in the isotype-1 β-tubulin gene known to have BZ resistance-associated polymorphisms (167, 198 and 200) in Strongylid nematodes were examined using deep-amplicon sequencing. A minimum of 200 eggs was contained within each DNA lysate; range 200–20,000 eggs/sample, mean 1670, standard error mean of 296.9 (Fig. 2A). The average read depth for the fragment containing codon 167 was ∼10,400, ranging between 3,180 and 24,732 reads across the samples (Fig. 2B). For the fragment containing codons 198 and 200, the average read depth was ∼22,700, ranging between 7,890 and 37,060 reads. Only the F167Y (TTC>TAC) resistance polymorphism was detected and this was present in 99% (69/70) of the samples that were sequenced, and this polymorphism was found at high frequencies in most positive samples (Fig. 2C). In 48 out of the 70 samples (69%), the frequency of the resistant allele was ≥75%. In 29% of the samples, and in at least one sample from each greyhound kennel, the allele frequency was ≥90%. All greyhound kennels had samples with at least a 60% frequency of the F167Y SNP. Only 7% of the samples had <25% of the resistant allele, and only one sample had 100% frequency of the susceptible allele. These frequencies had a high level of agreement (bias = −0.02 and 95% limits of agreement of −0.14 to 0.10) between the two independent sequencing runs for the fragment containing codon 167 (Supplementary Fig. 2). No SNPs were detected at codons 198 nor 200 (Supplementary Fig. 3). The ASVs for the amplicons spanning codons 167 and codons 198 and 200 were aligned to other nematode β-tubulins using the MUSCLE alignment tool. This alignment was trimmed, and a neighbour-joining tree was constructed using Geneious tree builder with H. contortus isotype-3 β-tubulin (Genbank accession: HE604101) as the outgroup. All the ASVs formed a monophyletic cluster with Ancylostoma isotype-1 β-tubulin (Supplementary Figs. 4 and 5).
Fig. 2.
The relative proportions of isotype-1 β-tubulin alleles encoding resistance conferring polymorphisms at F167Y vs wild type susceptible as measured by deep-amplicon sequencing in 70 samples from greyhounds that originated from 16 different locations in 8 different states (C). Only four samples had more than 3000 eggs, so we set the max at 3000 eggs because those outliers with large numbers would distort the overall chart (A). The average read depth is also reported (B).
3.4. Comparison of F167Y (TTC>TAC) frequency and egg hatch assay phenotype
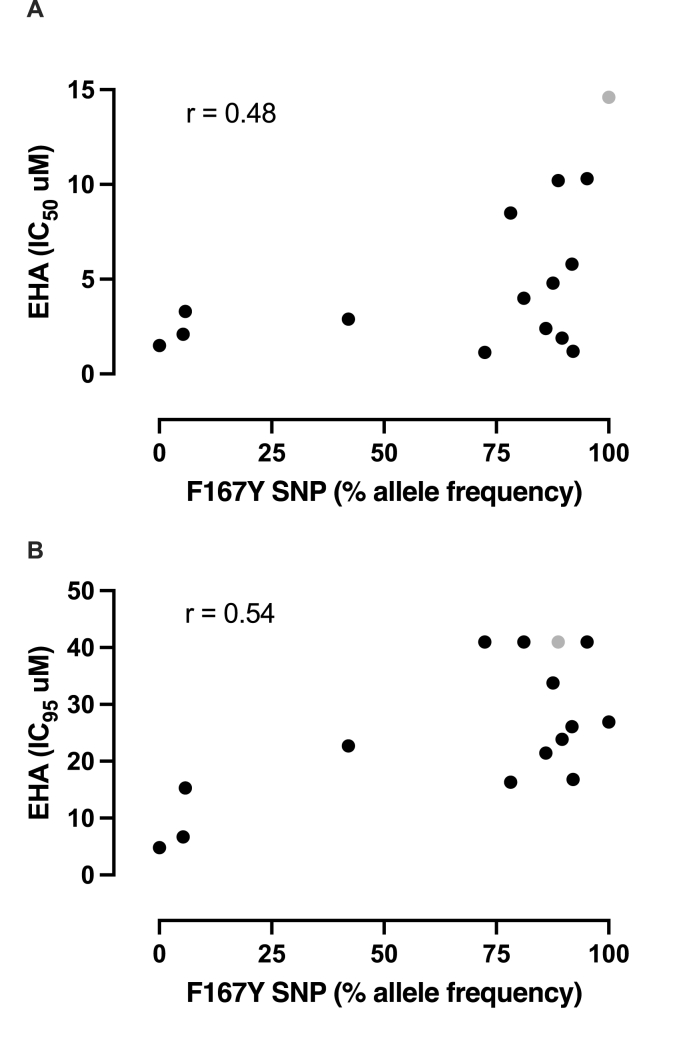
Both EHA (phenotypic) and β-tubulin allele (genotypic) data were only available for 15 samples. There was not a statistically significant correlation between the IC50 and resistant SNP F167Y allele frequency (p = 0.08), (r = 0.48), however, there was a significant correlation with the IC95 (p = 0.04), (r = 0.54) (Fig. 3).
Fig. 3.
Scatterplots of EHA (A) IC50 values (r = 0.48) or (B) IC95 values (r = 0.54) vs. F167Y SNP frequency based on deep-amplicon sequencing. Only 15 samples had results from both assays. The gray dot represents a pooled sample. The highest concentration tested in the EHA was 40 μM, therefore the four values with IC95 of 40 μM likely would have been greater if higher concentrations were tested.
4. Discussion and conclusions
The present study provides strong and conclusive evidence that racing greyhounds in the USA are infected with MDR A. caninum at a very high prevalence, and with wide geographic distribution. Very high IC50 and IC95 values were measured for both the BZs and MLs when compared to the susceptible isolate from our previous work (Jimenez Castro et al., 2019), indicating that almost every sample was resistant to both drugs. The F167Y (TTC>TAC) BZ resistance polymorphism was detected in 99% of the samples, and at high frequencies in more than 2/3 of the samples. All three greyhound kennels had samples with at least a 60% frequency of the F167Y SNP, and every sample from the Sanford, FL site had at least a 70% frequency of the resistant SNP. These data are consistent with levels we reported for our MDR (Worthy) lab isolate, which yielded F167Y SNP frequencies of 87.6–94.5% over several different passages (Jimenez Castro et al., 2019). Furthermore, in a controlled efficacy study using Worthy 4.1F3P, we measured an efficacy of 26% for fenbendazole, confirming that this high F167Y SNP frequency was associated with a very low in vivo efficacy (Jimenez Castro et al., 2020).
It has been noted that there are important epidemiological and biological differences between helminths of dogs and livestock with respect to the development of anthelmintic resistance, particularly in the levels of refugia and in husbandry practices (Von Samson-Himmelstjerna et al., 2021). However, these acknowledged differences focus on the comparison between small numbers of pet dogs raised in many individual homes and livestock raised in relatively large herds on farms. This comparison does not include the situation where large numbers of dogs are raised on farms, as is done in the greyhound racing industry (Hall, 2016). Although additional genetic analyses are required for confirmation, the available clinical and genetic evidence strongly suggests that these MDR A. caninum evolved on greyhound breeding farms and kennels. Thus, it is germane to this issue to examine the clinical and epidemiological factors that may be responsible for the development of these MDR worms, and to hypothesize why/how this problem became so prevalent and widespread before it was recognized. Ancylostoma caninum is the most prevalent parasitic nematode in racing greyhounds (Jacobs and Prole, 1976; Ash et al., 2019), and this is attributed to the near constant exposure of these dogs to infective third stage larvae in the sand/dirt exercise run/pens, which are ideal for hookworm transmission (Ridley et al., 1994; M.W. Dryden personal communication). The combination of large numbers of animals and high transmission rates produces large effective populations of worms and increases the probabilities that resistance mutations will occur (Gilleard, 2006; Redman et al., 2015). Racing greyhounds are also treated extremely frequently with multiple different anthelmintics (e.g., fenbendazole, ivermectin, pyrantel) throughout their lives (Ridley et al., 1994; M.W. Dryden personal communication). The intervals between these treatments often are less than the pre-patent period for hookworms, which will minimize the amount of refugia. Thus, genetically-resistant worms surviving treatment will have a large reproductive advantage, and the lack of refugia will lead to a rapid increase in their frequency (Martin et al., 1981; van Wyk, 2001). This combination of factors is known to place heavy selection pressure for drug resistance in nematodes (Wolstenholme et al., 2004), and is very similar to the epidemiological factors that have led to high levels of MDR in gastrointestinal nematodes of sheep and goats, worldwide (Kaplan and Vidyashankar, 2012).
Another factor that may have played a contributory role in the development of hookworm drug resistance in greyhounds are physiological differences, as compared to other dog breeds. There is strong evidence that breed-related differences in both metabolic and physiologic responses can influence drug pharmacokinetics and pharmacodynamics (Fleischer et al., 2008). With regard to greyhounds, there are several known breed-related differences. Greyhounds are deficient in CYP2B11, which impairs drug metabolism of some types of drugs (Zoran et al., 1993; Martinez et al., 2020). Additionally, the low percent body fat in greyhounds can lead to a lower-than-expected volume of distribution for lipophilic compounds (Zoran et al., 1993). These differences could potentially influence the drug selection dynamics that lead to the development of anthelmintic resistance. Thus, differences in pharmacokinetics and pharmacodynamics could be a potential contributing factor. Moreover, there is evidence that greyhounds have lower leukocyte (Porter and Canaday, 1971) and neutrophil counts (Steiss et al., 2000) as compared to the reference canine values. Additionally, greyhounds are hypoproteinemic relative to other dog breeds due to low serum α and β-globulin concentrations (Fayos et al., 2005). This could possibly impact the nematode susceptibility of greyhounds as compared to other breeds, though whether this occurs is unknown.
Nevertheless, even if these factors are given due consideration, no evidence exists to support these factors as being primarily responsible for the development of the MDR situation we have identified. To attribute the development of anthelmintic resistance to these physiological peculiarities of greyhounds, there would need to be examples of other breed(s) of dogs raised in similar epidemiological circumstances, but where resistance did not develop. However, to our knowledge, the epidemiological circumstances of greyhound breeding farms and racing kennels do not exist for any other breeds of dogs. Consequently, we feel there is insufficient evidence to attribute the development of MDR hookworms in greyhounds to those factors. Our hypothesis is that MDR A. caninum evolved as a consequence of epidemiological circumstances where high levels of parasitism and frequent anthelmintic treatments led to high numbers of hookworm genotypes under selection, and thus a high probability that drug-resistance mutations would emerge and then increase in frequency. Once resistance became established on any single farm/kennel, the high level of movement and mixing of dogs and the high frequency of anthelmintic treatments that are typical of the racing greyhound industry would allow the resistant worms to spread throughout the greater population of racing greyhounds. This hypothesis relies on well-established biological and genetic principles regarding the emergence and spread of drug resistance in nematode parasites (Prichard et al., 1980; Martin et al., 1982; Barnes et al., 1995; Le Jambre et al., 1999; Wolstenholme et al., 2004; Redman et al., 2015; Chaudhry et al., 2020).
P-glycoproteins (Kerboeuf et al., 2003), and more specifically PunPgp-2, PunPgp-9, and PunPgp-11 (Gerhard et al., 2020), have been implicated as being potentially involved in MDR in nematode parasites. However, no definitive mechanism that can explain levels of resistance observed in field isolates has been identified (Kotze and Prichard, 2016). Thus, the evidence points to these genes only being involved as contributory mechanisms, and not primary causative mechanisms of resistance. The body of evidence on this issue suggests that anthelmintic resistance develops independently to each drug class by a different mechanism (Kotze and Prichard, 2016). Given these facts, and the evidence for the near ubiquitous nature of MDR A. caninum to all three major anthelmintic classes in racing greyhounds in the USA, one must ask the question; “why has there not been any published reports of resistance to any single drug class previously in the USA greyhound population?” To address this question, it is necessary to examine how veterinarians typically manage hookworm infections in dogs. Typically, when a dog presents to a veterinarian with a fecal positive for hookworms, the dog is treated with one or more drugs from the BZ, ML or tetrahydropyrimidine classes. If the dog then tests positive again in a future exam, the infection is attributed to reinfection or reactivation of encysted/arrested larvae (larval leak). Consequently, the same treatment regimen is often repeated, or the veterinarian may choose to use a drug from a different drug class. Small animal veterinarians never previously considered measuring the efficacy of the treatment using a fecal egg count reduction test (FECRT) (Jimenez Castro and Kaplan, 2020). In contrast, FECRT, where both pre- and post-treatment FEC are performed and percent reduction in FEC is calculated, is commonly performed by large animal veterinarians (Kaplan, 2002, 2020). Without diagnostic surveillance, anthelmintic resistance is not diagnosed, and most often is not even considered as a likely cause of the recurrent hookworm infections. Therefore, as resistance evolves and leads to more recurrent hookworm infections, small animal veterinarians typically treat more often, and rotate and/or combine drugs. But they do not perform FECRT to measure the efficacy of the various drugs administered. Thus, as long as infections remain at subclinical levels, one drug remains effective, and/or several drugs each retain some modest level of efficacy, the problem will appear to be managed by the drug(s), and recurrent infections will continue to be attributed to reinfection or reactivation of encysted/arrested larvae. However, once high levels of MDR to all drugs evolves, and infections reach levels that cause clinically apparent hookworm disease, it becomes difficult to manage the infections, and the likelihood that anthelmintic resistance will be recognized increases. However, even then resistance may not be diagnosed without diagnostic surveillance. Early in our investigations, and prior to demonstrating that MDR indeed existed in A. caninum (Jimenez Castro et al., 2019, 2020), we spoke with many veterinarians and parasitologists who were aware of a serious hookworm problem in greyhounds, but continued to attribute the issue to increasing rates of larval leak. Within this context, it is noteworthy to compare the prevalence of infection we measured here in 219 greyhounds as compared to the prevalence reported in a previous 1991 study in 218 greyhounds. Ridley et al. (1994) reported that the prevalence of A. caninum in racing greyhounds examined at the University of Kansas during the summer of 1991 was 16%. Interestingly, the authors felt this level was surprisingly high given the intense levels of anthelmintic treatments administered, but this pales in comparison to the prevalence of 79% we report here.
Our in vitro, in vivo, and molecular data demonstrate strong evidence of very high levels of resistance to both BZs and the MLs. With regards to pyrantel, no in vitro or molecular assays currently exist for measuring resistance. However, in every suspected MDR case we have treated with pyrantel pamoate there is virtually no efficacy based on FEC reduction, (Jimenez Castro et al., 2019), and in a controlled efficacy study using Worthy 4.1F3P, pyrantel pamoate yielded an efficacy of only 23% (Jimenez Castro et al., 2020).
Regarding BZ resistance, the Sanford, FL kennel applied the greatest BZ selection pressure of any of the kennels, treating all dogs twice a month with albendazole, and all dogs tested had F167Y (TTC>TAC) frequencies of at least 70%. When comparing the phenotypic and genotypic data for BZs, the IC95 yielded a significant correlation (p = 0.04) but the IC50 did not (p = 0.08). The lack of significance for the IC50 may be due to low power as a consequence of only having 15 samples with both types of data. However, this finding is consistent with our previous work, where we found that the IC95 was more appropriate for discriminating susceptible vs resistant isolates using the EHA (Jimenez Castro et al., 2019). Interestingly, there were two samples, Dl 13 and Dl 14 that had IC95 values 13 and 18 times higher than the susceptible isolate from our previous work (Jimenez Castro et al., 2019), but which had a resistant F167Y SNP allele frequency of only 5%, and 0%, respectively (Supplementary Table 1). This lack of correlation between phenotype and genotype could have three possible explanations: (1) the EHA has a high interassay variability, (2) there are mutations in β-tubulin other than at codons 167, 198, and 200 that are involved with resistance to BZ drugs, or (3) there are loci other than β-tubulin that are involved with resistance to BZ drugs. Interassay variability seems highly unlikely to explain this, as in on our previous work we tested multiple isolates and biological replicates and had rather low interassay variability (Jimenez Castro et al., 2019). Additionally, we previously measured a >100-fold increase in the EHA IC50 in the Worthy isolate following treatment with fenbendazole, but the SNP allele frequency remained relatively unchanged (Jimenez Castro et al., 2019). Together, these findings lend support to the hypothesis that there are non-β-tubulin mutations that are involved in resistance to BZ drugs in A. caninum. Evidence of this has already been reported in Caenorhabditis spp. where a quantitative trait loci that did not overlap with β-tubulin genes was identified in two genetically divergent isolates (Zamanian et al., 2018). Also, using genome wide association mappings in C. elegans, novel genomic regions independent of ben-1 and other β-tubulin loci were correlated with resistance to albendazole (Hahnel et al., 2018). Additionally, disparity in responses in C. elegans to fenbendazole and albendazole provided evidence that the former could have additional targets beyond β-tubulin, such as genes that encode β-tubulin interacting proteins (Dilks et al., 2020). Thus, our observations demand further study.
Our previous work demonstrated that the LDA provided excellent discrimination between susceptible and resistant A. caninum isolates for the MLs (Jimenez Castro et al., 2019). In the current study the median IC50 value was >83 times higher than the susceptible isolate value from our previous work. This is both extremely high and an underestimation, since the IC50 of many samples could not be accurately measured given that they exceeded the highest concentration tested. Furthermore, of all the samples that had LDA assays with IC50 values>1000 nM, all but one had moxidectin as the last treatment administered, and that one group was administered moxidectin in the previous treatment. Macrocyclic lactones, particularly, ivermectin, have been used intensively by the greyhound industry for parasite control for the past several decades (Ridley et al., 1994; M.W. Dryden personal communication). However, to our knowledge, moxidectin, which is a substantially more potent member of this drug class (Prichard et al., 2012), has only recently started to be used on greyhound farms and kennels. It is well documented in the literature in small ruminants that resistance in MLs often has a stepwise evolution, where worms first become resistant to avermectin drugs, and then with further re-infection and selection become resistant to moxidectin (Leathwick et al., 2000; Kaplan et al., 2007). In H. contortus, ivermectin resistant worms that are naïve to moxidectin are typically killed at very high efficacy following administration of moxidectin (Craig et al., 1992; Oosthuizen and Erasmus, 1993); however, once moxidectin is used regularly in an ivermectin-resistant population, resistance to moxidectin can develop rapidly (Kaplan et al., 2007). Evidence suggests this same pattern is occurring in canine hookworms as well.
Curiously, there was a single group of nine dogs for which we detected no eggs in the feces (<5 EPG) following treatment with moxidectin. The only information available on this group was that these dogs were acquired from a breeding farm in KS. We could not test the parasites from these 9 dogs with the EHA or LDA, nor for the presence of β-tubulin mutations because there were no eggs in the samples we received. Thus, it was not possible to determine whether these dogs were infected with MDR parasites that retained susceptibility to moxidectin, or if they were not MDR. However, based on the body of our data that encompasses dogs originating from a broad geographical area, it seems most likely that those 9 dogs had ML-resistant worms that had not yet evolved to being moxidectin-resistant. Supporting this assumption are data from our previous studies, where we reported isolates of MDR hookworms in dogs that had levels of resistance to MLs consistent with ivermectin resistance and moxidectin susceptibility (Jimenez Castro et al., 2019).
When examining the FEC data for moxidectin and albendazole by timeframe post-treatment, we found significant differences for both drugs, but in a different pattern. For the group of samples collected following albendazole treatment, the 10–21 (p = 0.0003) and >21 days (p = 0.0004) categories had a statistically significant higher mean FEC when compared to the <10 days category. This finding is consistent with our previous observations in the Worthy isolate, where we documented a temporary suppression on worm fecundity following treatment with fenbendazole (Jimenez Castro et al., 2019, 2020). In contrast, for the dogs treated with moxidectin, there was no difference between the <10 and 10–21 day timeframes, but the >21day period had significantly higher FEC (p = 0.0002) and (p < 0.0001), respectively. This is also consistent with our previous observations where no reduction in fecundity was seen in moxidectin-resistant A. caninum following treatment with ML drugs. The higher EPG in the >21day period is likely due to reinfection and/or reactivation of arrested larvae.
The almost ubiquitous presence of MDR worms in actively racing and recently retired greyhounds combined with the progressive demise of the greyhound racing industry, which most likely will lead to increasing numbers of greyhound adoptions, poses a serious risk to the health of pet dogs. Moreover, from 2009 to 2019, the number of dog parks in the USA increased by 74% (TPL, 2019), and a recent survey showed that the prevalence of A. caninum in dogs visiting these parks was more than 70% higher as compared to the prevalence in all pet dogs recorded over the same general timeframe (Stafford et al., 2020; Sweet et al., 2021). In this context, it is germane to appreciate that a fecal pile deposited by a 30 kg dog with an A. caninum FEC of ∼1000 EPG will contain approximately 500,000 eggs. If not picked up and disposed of properly, tens to hundreds of thousands of infective larvae are likely to contaminate the surrounding soil from this one defecation. Consequently, there is a high probability of transmission to other dogs visiting the dog park, and once infected, anthelmintic treatment of these dogs with the three most commonly used drug classes will have little efficacy. The end result will be a continual cycle of infection and transmission that is not interrupted by monthly treatments with heartworm preventive products, or with anthelmintics administered specifically to treat the hookworm infections. When this is considered in light of the fact that resistance in A. caninum was not reported in greyhounds until the worms were already MDR, we believe it is likely that anthelmintic-resistant A. caninum are already quite common in pet dogs. In terms of public health, A. caninum is also zoonotic, and MDR A. caninum will not be susceptible to usual anthelmintic treatments administered by physicians.
Finally, given these alarming new data, it is urgent that studies be performed to determine the prevalence and geographic distribution of drug-resistant A. caninum in the general pet dog population. Additionally, genetic studies investigating the haplotype diversity of the susceptible and resistant β-tubulin alleles from both greyhounds and the general pet dog population from different geographic regions are likely to provide deeper insights into the molecular epidemiology and the origin(s) of these MDR worms.
Declaration of competing interest
The authors do not report any conflict of interests.
Acknowledgements
We thank the staff at the participating greyhound kennels, adoption groups and veterinarians for their efforts and assistance, which made this project possible. AM was funded through the Georgia Veterinary Scholars Program in the summer of 2019. PDJC was funded in part by a grant from Boehringer Ingelheim Animal Health, and in part by other project funds from the Kaplan laboratory. AV and RC were supported by Alberta Graduate Excellence Scholarships (AGES) and RA by Bill and Melinda Gates Foundation grant number OPP1172974. Work in JG laboratory was funded by NSERC Discovery Grant number RGPIN/371529–2209.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2021.08.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ash A., Lymbery A., Godfrey S., Shiel R., Paul A. Substrate type and age are risk factors for gastrointestinal parasitism in greyhound kennels. Vet. Parasitol. 2019;265:7–14. doi: 10.1016/j.vetpar.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Avramenko R.W., Redman E.M., Lewis R., Yazwinski T.A., Wasmuth J.D., Gilleard J.S. Exploring the gastrointestinal "nemabiome": deep amplicon sequencing to quantify the species composition of parasitic nematode communities. PloS One. 2015;10:1–18. doi: 10.1371/journal.pone.0143559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramenko R.W., Redman E.M., Melville L., Bartley Y., Wit J., Queiroz C., Bartley D.J., Gilleard J.S. Deep amplicon sequencing as a powerful new tool to screen for sequence polymorphisms associated with anthelmintic resistance in parasitic nematode populations. Int. J. Parasitol. 2019;49:13–26. doi: 10.1016/j.ijpara.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Barnes E.H., Dobson R.J., Barger I.A. Worm control and anthelmintic resistance: adventures with a model. Parasitol. Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., Mcmurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry U., Redman E.M., Kaplan R., Yazwinski T., Sargison N., Gilleard J.S. Contrasting patterns of isotype-1 β-tubulin allelic diversity in Haemonchus contortus and Haemonchus placei in the southern USA are consistent with a model of localised emergence of benzimidazole resistance. Vet. Parasitol. 2020;286:109240. doi: 10.1016/j.vetpar.2020.109240. [DOI] [PubMed] [Google Scholar]
- Craig T.M., Hatfield T.A., Pankavich J.A., Wang G.T. Efficacy of moxidectin against an ivermectin-resistant strain of Haemonchus contortus in sheep. Vet. Parasitol. 1992;41:329–333. doi: 10.1016/0304-4017(92)90090-v. [DOI] [PubMed] [Google Scholar]
- Dilks C.M., Hahnel S.R., Sheng Q., Long L., McGrath P.T., Andersen E.C. Quantitative benzimidazole resistance and fitness effects of parasitic nematode beta-tubulin alleles. Int. J. Parasitol.: Drugs and Drug Resistance. 2020;14:28–36. doi: 10.1016/j.ijpddr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J., Carey T. Seasonality and changing prevalence of common canine gastrointestinal nematodes in the USA. Parasites Vectors. 2019;12:430. doi: 10.1186/s13071-019-3701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epe C. Intestinal nematodes: biology and control. Vet. Clin. North Am. Small Anim. Pract. 2009;39:1091–1107. doi: 10.1016/j.cvsm.2009.07.002. vi-vii. [DOI] [PubMed] [Google Scholar]
- F.D.A . Food and Drug Administration; 1983. NADA 121-473 Panacur. [Google Scholar]
- F.D.A . Food and Drug Administration; 1993. NADA 141-008 Drontal. [Google Scholar]
- F.D.A . Food and Drug Administration; 1994. NADA 141-007 Drontal Plus. [Google Scholar]
- F.D.A . Food and Drug Administration; 1998. NADA 140-915 Interceptor. [Google Scholar]
- F.D.A . Food and Drug Administration; 2006. NADA 141-251 Advantage Multi. [Google Scholar]
- Fayos M., Couto C.G., Iazbik M.C., Wellman M.L. Serum protein electrophoresis in retired racing Greyhounds. Vet. Clin. Pathol. 2005;34:397–400. doi: 10.1111/j.1939-165x.2005.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Sharkey M., Mealey K., Ostrander E.A., Martinez M. Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 2008;10:110–119. doi: 10.1208/s12248-008-9011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland J. National Greyhound Association; 2021. [Google Scholar]
- Gerhard A.P., Krücken J., Heitlinger E., Janssen I.J.I., Basiaga M., Kornaś S., Beier C., Nielsen M.K., Davis R.E., Wang J., von Samson-Himmelstjerna G. The P-glycoprotein repertoire of the equine parasitic nematode Parascaris univalens. Sci. Rep. 2020;10:13586. doi: 10.1038/s41598-020-70529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleard J.S. Understanding anthelmintic resistance: the need for genomics and genetics. Int. J. Parasitol. 2006;36 doi: 10.1016/j.ijpara.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hahnel S.R., Zdraljevic S., Rodriguez B.C., Zhao Y., McGrath P.T., Andersen E.C. Extreme allelic heterogeneity at a Caenorhabditis elegans beta-tubulin locus explains natural resistance to benzimidazoles. PLoS Pathog. 2018;14:1–26. doi: 10.1371/journal.ppat.1007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.P. In: The Economics of Restoring Live Horse Racing and Greyhound Racing in Kansas. Alliance T.G.K.R., editor. Kansas University School of Business; 2016. [Google Scholar]
- Hotez P.J., Brooker S., Bethony J., Bottazzi M.E., Loukas A., Xiao S. Hookworm infection. N. Engl. J. Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Jacobs D., Prole J. Helminth infections of British dogs: prevalence in racing greyhounds. Vet. Parasitol. 1976;1:377–387. [Google Scholar]
- Jimenez Castro P.D., Howell S.B., Schaefer J.J., Avramenko R.W., Gilleard J.S., Kaplan R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasites Vectors. 2019;12:576. doi: 10.1186/s13071-019-3828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez Castro P.D., Kaplan R.M. 2020. Persistent or Suspected-Resistant Hookworm Infections; pp. 59–68. Clinician’s Brief. [Google Scholar]
- Jimenez Castro P.D., Mansour A., Charles S., Hostetler J., Settje T., Kulke D., Kaplan R.M. Efficacy evaluation of anthelmintic products against an infection with the canine hookworm (Ancylostoma caninum) isolate Worthy 4.1F3P in dogs. Int J Parasitol Drugs Drug Resist. 2020;13:22–27. doi: 10.1016/j.ijpddr.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkofen U.P. Hookworms of dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 1987;17:1341–1354. doi: 10.1016/s0195-5616(87)50005-5. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Anthelmintic resistance in nematodes of horses. Vet. Res. 2002;33:491–507. doi: 10.1051/vetres:2002035. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Biology, epidemiology, diagnosis, and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Vet. Clin. North Am. Food Anim. Pract. 2020;36:17–30. doi: 10.1016/j.cvfa.2019.12.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N., Howell S.B., Neiss J.M., Williamson L.H., Terrill T.H. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in gastrointestinal nematodes of goats. Int. J. Parasitol. 2007;37:795–804. doi: 10.1016/j.ijpara.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kerboeuf D., Blackhall W., Kaminsky R., von Samson-Himmelstjerna G. P-glycoprotein in helminths: function and perspectives for anthelmintic treatment and reversal of resistance. Int. J. Antimicrob. Agents. 2003;22:332–346. doi: 10.1016/s0924-8579(03)00221-8. [DOI] [PubMed] [Google Scholar]
- Kotze A.C., Prichard R.K. Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv. Parasitol. 2016;93:397–428. doi: 10.1016/bs.apar.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Le Jambre L.F., Dobson R.J., Lenane I.J., Barnes E.H. Selection for anthelmintic resistance by macrocyclic lactones in Haemonchus contortus. Int. J. Parasitol. 1999;29:1101–1111. doi: 10.1016/s0020-7519(99)00074-0. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Moen I.C., Miller C.M., Sutherland I.A. Ivermectin-resistant Ostertagia circumcincta from sheep in the lower North Island and their susceptibility to other macrocyclic lactone anthelmintics. N. Z. Vet. J. 2000;48:151–154. doi: 10.1080/00480169.2000.36183. [DOI] [PubMed] [Google Scholar]
- Lima V.F.S., Cringoli G., Rinaldi L., Monteiro M.F.M., Calado A.M.C., Ramos R.A.N., Meira-Santos P.O., Alves L.C.J.P.R. A comparison of mini-FLOTAC and FLOTAC with classic methods to diagnosing intestinal parasites of dogs from Brazil. Parasitol. Res. 2015;114:3529–3533. doi: 10.1007/s00436-015-4605-x. [DOI] [PubMed] [Google Scholar]
- Little S.E., Johnson E.M., Lewis D., Jaklitsch R.P., Payton M.E., Blagburn B.L., Bowman D.D., Moroff S., Tams T., Rich L., Aucoin D. Prevalence of intestinal parasites in pet dogs in the United States. Vet. Parasitol. 2009;166:144–152. doi: 10.1016/j.vetpar.2009.07.044. [DOI] [PubMed] [Google Scholar]
- Lord L.K., Yaissle J.E., Marin L., Couto C.G. Results of a web‐based health survey of retired racing Greyhounds. J. Vet. Intern. Med. 2007;21:1243–1250. doi: 10.1892/07-063.1. [DOI] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. journal. 2011;17:10–12. [Google Scholar]
- Martin P.J., Anderson N., Jarrett R.G., Brown T.H., Ford G.E. Effects of a preventive and suppressive control scheme on the development of thiabendazole-resistance in Ostertagia spp. Aust. Vet. J. 1982;58:185–190. doi: 10.1111/j.1751-0813.1982.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Martin P.J., Le Jambre L.F., Claxton J.H. The impact of refugia on the development of thiabendazole resistance in Haemonchus contortus. Int. J. Parasitol. 1981;11:35–41. doi: 10.1016/0020-7519(81)90023-0. [DOI] [PubMed] [Google Scholar]
- Martinez S.E., Andresen M.C., Zhu Z., Papageorgiou I., Court M.H. Pharmacogenomics of poor drug metabolism in Greyhounds: cytochrome P450 (CYP) 2B11 genetic variation, breed distribution, and functional characterization. Sci. Rep. 2020;10 doi: 10.1038/s41598-019-56660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli M.P., Rinaldi L., Alfano S., Pepe P., Coles G.C., Cringoli G. Mini-FLOTAC, a new tool for copromicroscopic diagnosis of common intestinal nematodes in dogs. Parasites Vectors. 2014;7:356. doi: 10.1186/1756-3305-7-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen W.T., Erasmus J.B. Efficacy of moxidectin against a strain of Haemonchus contortus resistant to ivermectin, a benzimidazole and a salicylanilide. J. S. Afr. Vet. Assoc. 1993;64:9–12. [PubMed] [Google Scholar]
- Porter J.A., Canaday W.R. Hematologic values in mongrel and greyhound dogs being screened for research use. J. Am. Vet. Med. Assoc. 1971;159:1603–&. [PubMed] [Google Scholar]
- Prichard R., Ménez C., Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol Drugs Drug Resist. 2012;2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard R.K., Hall C.A., Kelly J.D., Martin I.C., Donald A.D. The problem of anthelmintic resistance in nematodes. Aust. Vet. J. 1980;56:239–251. doi: 10.1111/j.1751-0813.1980.tb15983.x. [DOI] [PubMed] [Google Scholar]
- Redman E., Whitelaw F., Tait A., Burgess C., Bartley Y., Skuce P.J., Jackson F., Gilleard J.S. The emergence of resistance to the benzimidazole anthlemintics in parasitic nematodes of livestock is characterised by multiple independent hard and soft selective sweeps. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley R.K., Dryden M.W., Gabbert N.H., Schoning P. Epidemiology and control of helminth-parasites in greyhound breeding farms. Compend. Continuing Educ. Pract. Vet. 1994;16:585–596. [Google Scholar]
- Stafford K., Kollasch T.M., Duncan K.T., Horr S., Goddu T., Heinz-Loomer C., Rumschlag A.J., Ryan W.G., Sweet S., Little S.E. Detection of gastrointestinal parasitism at recreational canine sites in the USA: the DOGPARCS study. Parasites Vectors. 2020;13:275. doi: 10.1186/s13071-020-04147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassens P., Bergum P.W., Gansemans Y., Jespers L., Laroche Y., Huang S., Maki S., Messens J., Lauwereys M., Cappello M., Hotez P.J., Lasters I., Vlasuk G.P. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc. Natl. Acad. Sci. Unit. States Am. 1996;93:2149–2154. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State F.D.O. 2018. Results of Elections. [Google Scholar]
- Steiss J.E., Brewer W.G., Welles E., Wright J.C. Hematologic and serum biochemical reference values in retired greyhounds. Compend. Continuing Educ. Pract. Vet. 2000;22:243–248. [Google Scholar]
- Sweet S., Hegarty E., Mccrann D.J., Coyne M., Kincaid D., Szlosek D. 2021. A 3-year Retrospective Analysis of Canine Intestinal Parasites: Fecal Testing Positivity by Age, U.S. Geographical Region and Reason for Veterinary Visit. Parasit Vectors 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Coop R.L., Wall R. Wiley Blackwell; 2016. Veterinary Parasitology. [Google Scholar]
- Theil C.M. 2021. GREY2K USA. [Google Scholar]
- TPL . 2019. Dog Park Rankings for the 100 Largest U.S. Cities. [Google Scholar]
- van Wyk J.A. Refugia--overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort J. Vet. Res. 2001;68:55–67. [PubMed] [Google Scholar]
- Von Samson-Himmelstjerna G., Thompson R.A., Krücken J., Grant W., Bowman D.D., Schnyder M., Deplazes P. Spread of anthelmintic resistance in intestinal helminths of dogs and cats is currently less pronounced than in ruminants and horses – yet it is of major concern. Int. J. Parasitol.: Drugs and Drug Resistance. 2021 doi: 10.1016/j.ijpddr.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zamanian M., Cook D.E., Zdraljevic S., Brady S.C., Lee D., Lee J., Andersen E.C. Discovery of genomic intervals that underlie nematode responses to benzimidazoles. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran D., Riedesel D., Dyer D. Pharmacokinetics of propofol in mixed-breed dogs and greyhounds. Am. J. Vet. Res. 1993;54:755–760. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.