Abstract
Conventional anti-tuberculosis (TB) therapies comprise lengthy antibiotic treatment regimens, exacerbated by multi-drug resistant and extensively drug resistant mycobacterial strains. We assessed the ability of all-trans retinoic acid (ATRA), as repurposed compound serving as host-directed therapy (HDT), to counteract the suppressive effects of myeloid-derived suppressor cells (MDSCs) obtained from active TB cases (untreated or during week one of treatment) on T-cell responsiveness. We show for the first time that MDSCs suppress non-specific T-cell activation and production of interleukin (IL)-2, IL-4, IL-13 and GM-CSF via contact-dependent mechanisms. ATRA treatment decreases MDSC frequency, but fails to mature MDSCs to non-suppressive, terminally differentiated myeloid cells and does not restore T-cell function or cytokine production in the presence of MDSCs. The impact of ATRA treatment on improved immunity, using the concentration tested here, is likely to be minimal, but further identification and development of MDSC-targeting TB host-directed therapies are warranted.
Keywords: Tuberculosis, Mycobacterium tuberculosis, Host-directed therapies, Myeloid-derived suppressor cells, All-trans retinoic acid
1. Introduction
It is estimated that a quarter of the global population is infected by Mycobacterium tuberculosis (M.tb), and is thus vulnerable to developing tuberculosis (TB) [1]. Those with active TB are prescribed a 6-month minimum treatment regimen consisting of isoniazid, rifampicin, pyrazinamide and ethambutol (HRZE) for two months, followed by 4-months of isoniazid and rifampicin [2]. This lengthy regimen, achieves an 82% cure rate in treatment adherent, drug-sensitive TB patients [1]. Multi-drug resistant (MDR) TB, diabetes mellitus type II (DM2) and human immunodeficiency virus (HIV) co-infection add to an already demanding treatment regimen, hampering adherence to therapy. Non-adherence results in treatment failure, relapse of disease, disease spread and further increases the emergence of resistant M.tb strains [3]. Taken together, these challenges support the need for development of more effective anti-TB drugs and/or adjunct therapies to improve TB treatment outcomes.
Host-directed therapies (HDT) encompass compounds that improve beneficial host immunity and/or decrease inflammation and associated tissue pathology and thus facilitate cure [4]. Repurposed drugs have already passed FDA/EMA safety requirements for other conditions and may only need proven efficacy against M.tb to allow introduction to clinical care. In the context of TB, there are 24 HDT agents which have shown efficacy in vitro [5,6]. Of these, 6 are currently in clinical trials to be tested as adjunct TB therapies [7]. These HDTs can modulate host factors associated with pathogenic responses, interfere with host mechanisms exploited by the pathogen to persist or replicate, target pathways that reduce hyper-inflammation or boost host immune defences against the pathogen [6].
Immune mechanisms exist that suppress effector responses in the presence of persistent antigens to limit immune-mediated tissue pathology. Regulatory cells fulfil such a role but also hinder effective M.tb clearance by effector T-cells, allowing for increased M.tb survival [8,9]. MDSCs contribute to suppressive protective immunity in TB. MDSCs are a heterogeneous population of myeloid cells, at various stages of differentiation, consisting of immature myeloid cells and also further differentiated cells, with the capacity to suppress T-cell functions [10]. Accelerated myelopoiesis occurs in response to chronic inflammation, caused by persistent pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs) and chronic inflammatory mediator stimulation [11]. This state licenses and activates myeloid cells with potent ability to suppress immune responses [12,13].
Human MDSCs share several phenotypic markers with their non-suppressive counterparts developing under steady state conditions, however a combination of markers accompanied by demonstration of suppressive functions, are used to reliably characterize MDSCs in humans [10]. Total MDSCs (T-MDSC) can be identified as HLA-DR−/loCD11b + CD33+, and further classified into 3 subsets, monocytic-MDSCs (M–MDSC) CD11b + HLA-DR−/loCD14 + CD15-, polymorphonuclear-MDSCs (PMN-MDSC) CD66 + CD15 + CD14-CD33dimHLA-DR−/lo [10,14] and recently described eosinophilic MDSCs (eo-MDSC) CD11b + Siglec-F + CCR3 + IL-5R+ [15]. Another population of MDSCs, thought to be precursors of T-MDSC, have been described and are classified as early-stage MDSCs (E-MDSC), are absent of lineage markers (CD3, CD4, CD8, CD19, CD20), and express CD33 and HLA-DR−/lo [10].
We have previously observed that total MDSC frequencies are increased and suppress non-specifically activated T-cell function in TB patients and recently exposed contacts of TB patients [16]. Others have also found an association in mice between TB progression and cells expressing Gr-1 (Ly-6C and Ly-6G) [17] as well as higher expression of MDSCs in the blood and lung components of TB patients [9]. MDSC suppressive mechanisms in TB have been linked to arginase-1 (ARG1) and nitric oxide (NO) production [9,17,18]. Immunosuppressive MDSCs accumulated during experimental TB and contained M.tb [19]. This work suggests that MDSCs have a dual role in TB disease, firstly, by suppressing T-cell function, and secondly harbouring M.tb.
Attempts to reverse MDSC-induced immunosuppression in cancer include blocking MDSC activating factors [20,21], inhibition of MDSC generating signalling pathways [22,23], prevention of recruitment to the site of disease [24,25], blockade of T-cell-immunosuppression [26] and differentiation of MDSC to mature non-suppressive myeloid cells [27].
All-trans retinoic acid, ATRA, a vitamin A metabolite, has been used successfully in leukaemia to mature immature myeloid cells [28–30]. ATRA is a FDA/EMA approved drug with low toxicity and high efficacy in various cancers and its pharmacokinetics (PK) are well characterized. In humans, it has a half-life (HL) of 45 min, prescribed as 1 tablet per day for 90 days when dosed at 45 mg/m2/day to achieve a peak plasma concentration of 1 uM [31,32]. In the context of MDSCs, glutathione synthase (GSS) is a target gene under retinoic acid (RA) control [28]. ATRA specifically upregulates GSS expression at protein level within MDSCs. The resultant upregulation of GSS in MDSCs leads to an increase in glutathione (GSH), a potent scavenger of ROS, involved in MDSC immunosuppressive functions. ROS neutralization within MDSCs due to ATRA also results in differentiation of MDSCs [28].
In a murine model, ATRA administration in combination with HRZ has demonstrated its potential to shorten TB treatment and decrease relapse events [33], as well as decreasing the frequency of MDSCs in the lungs of mice with TB, significantly reducing bacterial loads, decreasing granuloma size and ameliorating pathology [19]. ATRA treatment also enhanced mucosal immune responses following parenteral vaccination with a TB subunit vaccine, by programming T and B cells to home to mucosal compartments [34].
This improved treatment efficacy and shortened duration holds promise for HDT inclusion into standard treatment regimens. Such a strategy could also promote decreased transmission. For these reasons, experimental proof of principle would allow rapid progression towards clinical testing. Currently, no information on ATRA in TB patients, or TB-derived MDSCs, exist.
We hypothesize that ATRA treatment of TB patient-derived MDSCs, will (1) induce their maturation and resultantly (2) decrease their immunosuppressive effects, thereby (3) improving innate and adaptive immunity in TB patients. Here we measured the effect of in vitro ATRA treatment of TB patient-derived MDSCs, on MDSC maturation and subsequent anti-TB innate and adaptive immune responses.
2. Results
2.1. MDSCs from TB patients suppress polyclonal T-cell responses in a contact-dependent manner.
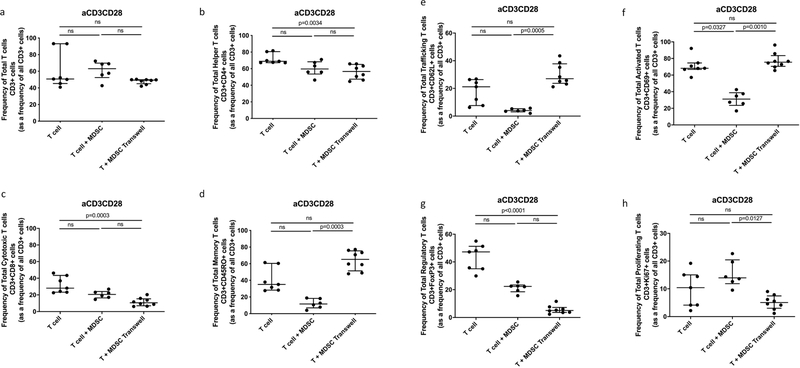
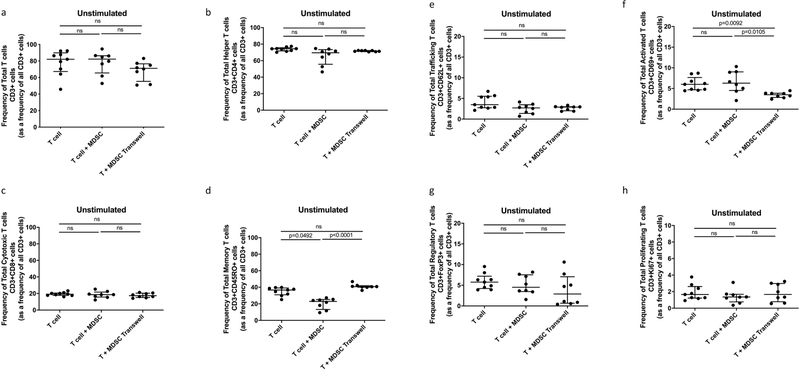
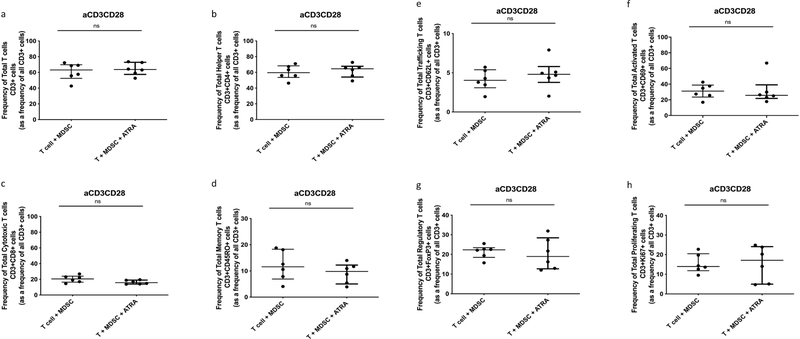
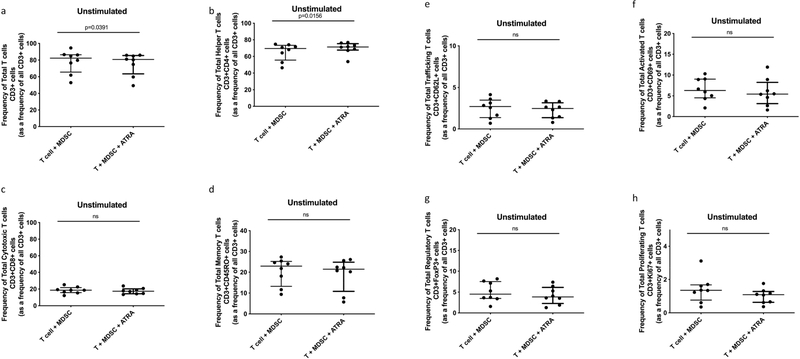
Participant demographics are shown in Table 1. MDSCs are increased in TB patients and suppress non-specific T-cell activation, proliferation and cytokine production [16]. We set out to verify the suppressive potential of TB-derived MDSCs on polyclonally, non-specifically, activated (anti-CD2-CD3-CD28-loaded beads) T-cells in active TB patients. Human T-cell phenotype, function and cytokine production were measured following cellular co-culture and contact-independent trans-well coculture in the presence or absence of MDSCs. Coculture of polyclonally activated T cells in the presence of MDSCs did not alter the frequency of Total T cells, helper T cells (CD4) or cytotoxic T cells (CD8) (Fig. 1 a–c). MDSCs did not suppress Total Memory T cells (CD45RO), Total Trafficking T cells (CD62L), Total Regulatory T cells (FoxP3) or Total T cell proliferation (Ki67) (Fig. 1 d, e, g and h). However, we confirmed data from previous reports, MDSCs suppressed T-cell activation (CD69) (p = 0.0327, Fig. 1 f) in the polyclonally activated condition. MDSCs also reduced the frequency of memory T-cells (p = 0.0492, Fig. 2 d) in the unstimulated condition.
Table 1.
Patient Demographics. 25 Adult, HIV negative patients, recently diagnosed with active pulmonary TB disease.
| Patient demographics | ||
|---|---|---|
| Male | Female | |
|
| ||
| Count (Percentage) | 21 (84%) | 4 (16%) |
| Median Age (Range) | 44 (25–61) | 36 (25–44) |
| TB Treatment: | ||
| 0 days | 18 | 4 |
| 1–2 days | 2 | 0 |
| 3–7 days | 1 | 0 |
Fig. 1.
Effect of MDSC on non-specifically activated (anti-CD3CD28 beads) Total T cell and T cell subset responses. Frequency of Total T cells (CD3+) and T cell subsets from in vitro T cell co-culture as well as trans-well co-culture of allogeneic T cells (CD3+ cells) isolated from PBMC of a Quantiferon positive individual (8 replicates), and MDSC (HLA-CD33+ cells) isolated from PBMC of individuals with active TB disease (n = 8). Plots show QFT+ individual T cell replicates. Panels a-g show T cell frequency of various subsets and panel h shows cell proliferation as measure of T cell response. Kruskal-Wallis test with Dunns post-hoc test. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
Fig. 2.
Effect of MDSC on Unstimulated Total T cell and T cell subset responses. Frequency of Total T cells (CD3+) and T cell subsets from in vitro T cell co-culture as well as trans-well co-culture of allogeneic T cells (CD3+ cells) isolated from PBMC of a Quantiferon positive individual (9 replicates), and MDSC (HLA-CD33+ cells) isolated from PBMC of individuals with active TB disease (n = 9). Plots show QFT+ individual T cell replicates. Panels a-g show T cell frequency of various subsets and panel h shows cell proliferation as measure of T cell response. Kruskal-Wallis test with Dunns post-hoc test. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
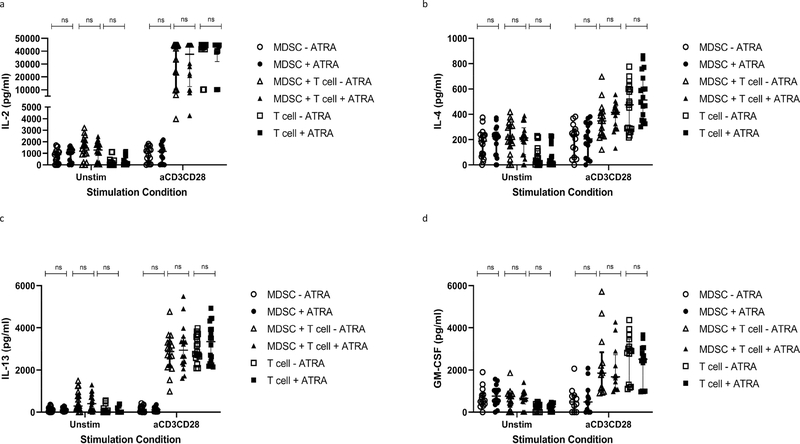
The MDSC-mediated T-cell suppression measured by flow cytometry, was reflected in the cell supernatant, measured by multiplex experiment. MDSCs significantly suppressed T-cell production of IL-2 (p = 0.000074), IL-4 (p = 0.000086), IL-13 (p = 0.041371) and GM-CSF (p = 0.011171) during co-culture in the polyclonally activated condition (Fig. 3 a–d and Table 2).
Fig. 3.
Effect of MDSC on Total T cell cytokine production. Analyte concentration (pg/ml) was measured for various culture combinations (MDSC only, T cell only, MDSC-T cell coculture), under differing stimulation combinations (Unstimulated and anti-CD3CD28 beads) by multiplex analysis (n = 18). Plots show analyte concentration in coculture supernatant. Mixed model analysis, LS means and Type II decomposition analysis followed. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
Table 2.
Effect of MDSC on T cell production of 27 Innate Cytokines, under differing stimulation combinations (Unstimulated and anti-CD3CD28 beads), measured by Luminex. Data analysed by Mixed Model, LS means and Type III decomposition using Statistica (Quest Software, Elliot Management Group). A p-value less than 0.05 was considered significant.
| Analytes | T cell vs T cell co-cultured with MDSC |
|||
|---|---|---|---|---|
| Unstim (p-value) | Significant Increase/Decrease/ns | aCD3CD28 (p-value) | Significant Increase/Decrease/ns | |
|
| ||||
| IL1b | 0.136537 | ns | 0.020006 | n/a |
| IL1ra | 0.000006 | n/a | 0 | n/a |
| IL2 | 0.467837 | ns | 0.000074 | decrease |
| IL4 | 0 | n/a | 0.000086 | decrease |
| IL6 | 0 | n/a | 0.000001 | n/a |
| IL7 | 0 | n/a | 0.017214 | n/a |
| IL9 | 0.000009 | n/a | 0.669309 | ns |
| IL10 | 0 | n/a | 0.944703 | ns |
| IL12 | 0.110671 | ns | 0.436979 | ns |
| IL13 | 0.000603 | n/a | 0.041371 | decrease |
| IL15 | 0 | n/a | 0 | n/a |
| IL17A | 0 | n/a | 0.855873 | ns |
| FGF basic | 0.000033 | n/a | 0.19259 | ns |
| IFNg | 0.89317 | ns | 0.878242 | ns |
| MCP1 | 0 | n/a | 0 | n/a |
| MIP1a | 0.000016 | n/a | 0.144661 | ns |
| PDGF-bb | 0 | n/a | 0.00032 | n/a |
| MIP1b | 0 | n/a | 0.552896 | ns |
| TNFa | 0 | n/a | 0.031027 | n/a |
| IL5 | 0 | n/a | 0.69174 | ns |
| IL8 | 0 | n/a | 0 | n/a |
| Eotaxin | 0 | n/a | 0.425538 | ns |
| G-CSF | 0.000003 | n/a | 0.923801 | ns |
| IP10 | 0 | n/a | 0.000001 | n/a |
| RANTES | 0 | n/a | 0.193398 | ns |
| GM-CSF | 0.031863 | n/a | 0.011171 | decrease |
| VEGF | 0 | n/a | 0.086424 | ns |
We confirmed that the suppression observed was indeed specific to the MDSC enriched population by testing the ability of the control monocyte population from the same participants to replicate this effect. The control monocyte population did not suppress T-cell activation, memory or proliferation in either of the culture conditions (Supplementary Figs. 1 and 2 d and f). Taken together, these data confirm our previous study by showing that MDSCs from TB patients supress T-cell responses [16].
To expand on our previous work, we investigated whether MDSC-mediated suppression of T-cell responses occurred via contact dependent mechanisms or by soluble factors (contact-independent mechanisms). We physically separated MDSCs and T-cells using trans-well coculture plates and found that MDSCs were no longer able to suppress T-cell activation in the polyclonally activated condition (p = 0.0010, Fig. 1 f). MDSCs were also unable to reduce the frequency of memory T-cells in the unstimulated condition (p < 0.0001, Fig. 2 d) when physically separated. These results indicate, that in this context, MDSC-mediated suppression of T-cells requires close proximity between these cell populations.
2.2. ATRA treatment reduces M-MDSC, but does not mature MDSC into terminally differentiated myeloid cells
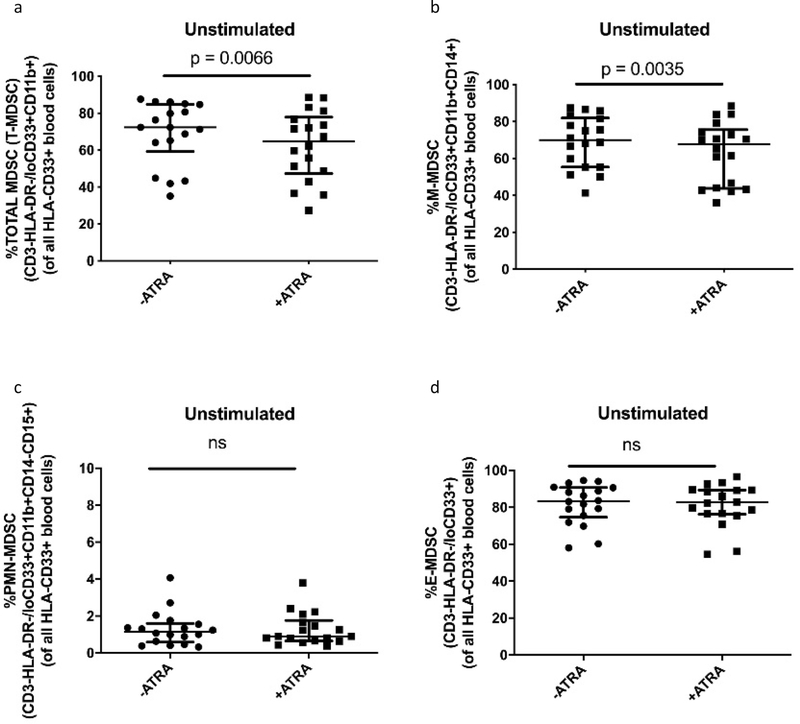
In oncology, ATRA was shown to mature MDSCs into non-suppressive terminally differentiated myeloid cells [20,28,35]. We investigated whether ATRA, at peak plasma concentration as dosed for AML (1uM), would mature TB patient blood-derived MDSCs, into non-suppressive terminally differentiated myeloid cell subsets. We assessed the frequency of MDSCs and mature myeloid populations when cultured in the presence or absence of ATRA. We observed a significant reduction in total MDSC frequency (p = 0.0066, Fig. 4 a), driven by a significant reduction in M-MDSC frequency (p = 0.0035, Fig. 4 b). No reduction in frequency was seen for PMN-MDSC or E-MDSC (Fig. 4 c–d).
Fig. 4.
Effect of ATRA on the frequency of (a) Total MDSC, (b) M-MDSC, (c) PMN-MDSC, (d) E-MDSC from in vitro cultures of MDSC (HLA-CD33+ cells) isolated from PBMC of individuals with active TB disease (n = 18). Plots show individual TB patient data. Wilcoxon matched pairs signed rank test. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
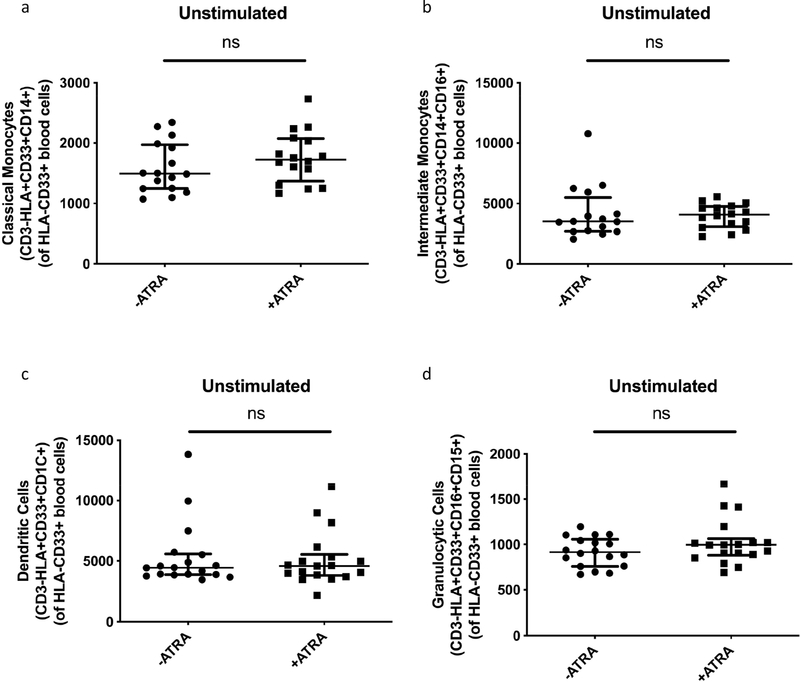
We found no significant increases in terminally differentiated myeloid subsets, including classical monocytes, intermediate monocytes, dendritic cells (DC) or granulocytes, following ATRA treatment of MDSCs (Fig. 5 a–d). We also treated the control monocyte population with ATRA, and investigated mature monocyte populations, following treatment. We observed no significant increases in classical monocytes, intermediate monocytes or granulocytes, but ATRA treatment significantly increased DC (p = 0.0244, Supplementary Fig. 3 c). Taken together, these data show that ATRA marginally decreases the magnitude of markers used to classify M-MSDC frequencies with statistical significance. ATRA treatment also does not mature these PBMC-enriched MDSCs into non-suppressive terminally differentiated myeloid cells.
Fig. 5.
Effect of ATRA on MDSC maturation to non-suppressive mature myeloid cell subsets. Median Fluorescence Intensity of HLA-DR on isolated MDSC (HLA-CD33+) from ATRA-treated in vitro culture (n = 16 a&b and n = 18c&d). Plots show individual TB patient data. Wilcoxon matched pairs signed rank test. Median with interquartile range. P < 0.05 significant.
2.3. ATRA supplementation does not restore T-cell function in the presence of MDSC.
Next we investigated ATRA’s ability to reverse MDSC-mediated T-cell suppression. The addition of ATRA to the cocultures did not affect Total T cell frequency nor helper T cell and cytotoxic T cell frequencies (Fig. 6 a–c). The addition of ATRA to polyclonally activated T cell and MDSC cocultures resulted in no significant differences in the frequencies of Total memory T cells, Total Trafficking T cells, Total Activation, Total Regulatory T cells and Total Proliferating T cells, at specified dose and treatment period (Fig. 6 d–h). This was consistent for the unstimulated condition (Fig. 7 d–h). The addition of ATRA to these MDSC and T cell cocultures did not demonstrate reversal of T-cell function. Similar to the flow data, ATRA treatment at the indicated dose, was unable to restore T-cell production of IL-2, IL-4, IL-13 and GM-CSF, when co-cultured in the presence of MDSCs (Fig. 8 a–d and Tables 3–5). We could also not detect any effects of ATRA treatment on MDSC or T-cell single culture cytokine responses (Fig. 8). Taken together, these data show that ATRA does not restore the MDSC-mediated suppression of selected T-cell responses measured here.
Fig. 6.
Effect of ATRA on restoration of non-specifically activated (anti-CD3CD28 beads) T cell responses in the presence of MDSC. ATRA treated in vitro co-culture of allogeneic T cells (CD3+ cells) isolated from PBMC of a Quantiferon positive individual (6 replicates), and MDSC (HLA-CD33+ cells) isolated from PBMC of individuals with active TB disease (n = 6). Plots show QFT+ individual T cell replicates. Panels a-g show T cell frequency of various subsets and panel h shows cell proliferation as measure of T cell response. Wilcoxon matched pairs signed rank test. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
Fig. 7.
Effect of ATRA on restoration of Unstimulated T cell responses in the presence of MDSC. ATRA treated in vitro co-culture of allogeneic T cells (CD3+ cells) isolated from PBMC of a Quantiferon positive individual (8 replicates), and MDSC (HLA-CD33+ cells) isolated from PBMC of individuals with active TB disease (n = 8). Plots show QFT+ individual T cell replicates. Panels a-g show T cell frequency of various subsets and panel h shows cell proliferation as measure of T cell response. Wilcoxon matched pairs signed rank test. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
Fig. 8.
Effect of ATRA on restoration of T cell responses in the presence of MDSC. Analyte concentration (pg/ml) was measured for various culture combinations (MDSC only, T cell only, MDSC-T cell coculture), under differing stimulation combinations (Unstimulated and anti-CD3CD28 beads) with or without ATRA treatment, by multiplex analysis. Plots show analyte concentration in coculture supernatant. Mixed model analysis, LS means and Type II decomposition analysis. Median with interquartile range indicated on plots. P < 0.05 was considered significant.
Table 3.
Effect of ATRA treatment on MDSC production of 27 Innate Cytokines, under differing stimulation combinations (Unstimulated and anti-CD3CD28 beads), measured by Luminex. Data analysed by Mixed Model, LS means and Type III decomposition using Statistica (Quest Software, Elliot Management Group). A p-value less than 0.05 was considered significant.
| Analytes | MDSC treated with ATRA vs MDSC untreated |
|||
|---|---|---|---|---|
| Unstim (p-value) | Significant Increase/Decrease/ns | aCD3CD28 (p-value) | Significant Increase/Decrease/ns | |
|
| ||||
| IL1b | 0.040585 | increase | 0.058276 | n/a |
| IL1ra | 0.000267 | decrease | 0.00953 | decrease |
| IL2 | 0.9408 | ns | 0.733743 | ns |
| IL4 | 0.198769 | ns | 0.938564 | ns |
| IL6 | 0.575273 | ns | 0.298997 | ns |
| IL7 | 0.456974 | ns | 0.421494 | ns |
| IL9 | 0.427765 | ns | 0.111893 | ns |
| IL10 | 0.794165 | ns | 0.389357 | ns |
| IL12 | 0.586326 | ns | 0.675968 | ns |
| IL13 | 0.913091 | ns | 0.537514 | ns |
| IL15 | 0.484428 | ns | 0.603196 | ns |
| IL17A | 0.114309 | ns | 0.856363 | ns |
| FGF basic | 0.857615 | ns | 0.1313 | ns |
| IFNg | 0.821907 | ns | 0.574484 | ns |
| MCP1 | 0.167234 | ns | 0.666422 | ns |
| MIP1a | 0.545664 | ns | 0.603152 | ns |
| PDGF-bb | 0.372726 | ns | 0.133662 | ns |
| MIP1b | 0.963856 | ns | 0.403096 | ns |
| TNFa | 0.773995 | ns | 0.808988 | ns |
| IL5 | 0.683855 | ns | 0.6876 | ns |
| IL8 | 0.187782 | ns | 0.4381 | ns |
| Eotaxin | 0.718447 | ns | 0.912494 | ns |
| G-CSF | 0.362893 | ns | 0.333957 | ns |
| IP10 | 0.014096 | decrease | 0.00045 | decrease |
| RANTES | 0.660844 | ns | 0.929136 | ns |
| GM-CSF | 0.42946 | ns | 0.413523 | ns |
| VEGF | 0.225454 | ns | 0.611222 | ns |
Table 5.
Effect of ATRA treatment on MDSC and T coculture production of 27 Innate Cytokines, under differing stimulation combinations (Unstimulated and anti-CD3CD28 beads), measured by Luminex. Data analysed by Mixed Model, LS means and Type III decomposition using Statistica (Quest Software, Elliot Management Group). A p-value less than 0.05 was considered significant.
| Analytes | MDSC-T coculture treated with ATRA vs MDSC-T coculture untreated |
|||
|---|---|---|---|---|
| Unstim (p-value) | Significant Increase/Decrease/ns | aCD3CD28 (p-value) | Significant Increase/Decrease/ns | |
|
|
|
|||
| IL1b | 0.048671 | increase | 0.000088 | increase |
| IL1ra | 0.015877 | decrease | 0.574493 | ns |
| IL2 | 0.806956 | ns | 0.782279 | ns |
| IL4 | 0.972355 | ns | 0.18547 | ns |
| IL6 | 0.529845 | ns | 0.097235 | ns |
| IL7 | 0.180003 | ns | 0.502946 | ns |
| IL9 | 0.330493 | ns | 0.000001 | decrease |
| IL10 | 0.004687 | decrease | 0.000073 | decrease |
| IL12 | 0.250421 | ns | 0.439182 | ns |
| IL13 | 0.644473 | ns | 0.142873 | ns |
| IL15 | 0.003601 | decrease | 0.314941 | ns |
| IL17A | 0.134339 | ns | 0.181012 | ns |
| FGF basic | 0.858729 | ns | 0.699784 | ns |
| IFNg | 0.931487 | ns | 0.013263 | decrease |
| MCP1 | 0.223835 | ns | 0.637885 | ns |
| MIP1a | 0.784159 | ns | 0.327488 | ns |
| PDGF-bb | 0.576593 | ns | 0.582702 | ns |
| MIP1b | 0.018211 | decrease | 0.084148 | ns |
| TNFa | 0.918553 | ns | 0.118593 | ns |
| IL5 | 0.850073 | ns | 0.135631 | ns |
| IL8 | 0.333222 | ns | 0.146483 | ns |
| Eotaxin | 0.881097 | ns | 0.79493 | ns |
| G-CSF | 0.727989 | ns | 0.854466 | ns |
| IP10 | 0.005848 | decrease | 0.371339 | ns |
| RANTES | 0.001105 | decrease | 0.191487 | ns |
| GM-CSF | 0.889786 | ns | 0.109635 | ns |
| VEGF | 0.068603 | ns | 0.012675 | decrease |
3. Discussion
This study investigated pharmacological modulation of MDSCs and the subsequent effect on T-cell immune reactivity, specifically T-cell activation, proliferation, trafficking, memory phenotype and regulatory subset induction. Strong evidence on the potential of ATRA to reverse MDSCs immunosuppressive function and reduce MDSC numbers in cancer and TB mouse models supported ATRA treatment as candidate agent for restoring anti-TB host immunity via MDSC regulation. We assessed the effect of ATRA treatment on MDSCs, control monocytes as well as T-cell phenotype and function.
We confirmed our previous study by showing that MDSCs from TB patients supress non-specifically activated T-cell responses [16]. Furthermore we show that MDSCs of TB cases suppress T-cell production of IL-2, IL-4, IL13 and GM-CSF. In TB disease, T-cell secretion of IL-2 is associated with effective clearance and treatment [36,37]. MDSC suppression of T-cell IL-2 production alters this protective proinflammatory environment, promoting TB disease. GM-CSF has been shown to be protective to the host during TB disease, reducing M.tb bacterial burden and activating production of monocytes, granulocytes and dendritic cells [38]. MDSCs reduce GM-CSF mediated immune protection, further modulating the immune response to an anti-inflammatory environment, favourable for disease progression. In contrast to this, MDSCs also reduced IL-4 and IL-13. These are key Th2, anti-inflammatory cytokines, linked through the IL-13/IL-4Ra axis, associated with tissue pathology and undermining protective immunity [39,40]. The magnitude by which MDSCs modulate proinflammatory cytokines is larger than their effect on anti-inflammatory cytokines.
We also showed for the first time that MDSC-mediated suppression of T-cells in TB is conducted via contact-dependant mechanisms. Although not directly tested in this investigation, these findings refine the scope of potential mechanisms of MDSC-mediated suppression, as inferred from mechanisms reported in oncology studies. One such oncology study investigated ATRA treatment on human MDSC expansion and function, showing that ATRA decreases the expression of ARG1, NOX1, INOS and PD-L1 and improved T cell proliferation [41]. MDSCs have also been shown to reduce T-cell activation by mechanisms such as nitrosylation of the TCR resulting in alteration of TCR integrity and disruption of T cell signalling, rendering them unresponsive to antigen specific stimulation [42]. Our future work will thus include investigations into MDSC upregulation of programmed death-ligand 1 (PD-L1) and FASL [43,44], local depletion of L-arginine (L-Arg) or cysteine through arginase 1 (ARG1) and IDO enzymes [45], T-cell receptor functionality affected by MDSC-induced reactive oxygen species (ROS), H202, and reactive nitrogen species (RNS) [46–48].
The effect of ATRA on MDSCs has been thoroughly investigated as a potential immunotherapy for a wide variety of cancers. Previous studies have shown that ATRA decreases the frequency of MDSCs in various tumour models [27,29,49,49]. Consistent with reports on ATRA treatment of tumour-derived MDSCs, we demonstrate that ATRA treatment of TB patient blood-derived MDSCs leads to a statistically significant reduction in the frequency of T-MDSCs, mainly driven by the reduction in M–MDSC in vitro. The effect of ATRA on the PMN-MDSC subset remains to be investigated in ex vivo samples as these cells are sensitive to cryopreservation [50]. The reduced frequency of the PMN-MDSC subset due to cryopreservation in our study could explain the lack of maturation or reversal of immunosuppression as seen in oncology studies. Other studies have reported a corresponding increase in the frequency of mature myeloid cells, DCs and macrophages, upon ATRA treatment of MDSCs [29,49,51]. Our flow cytometry data show that ATRA treatment of TB patient-derived MDSCs, at the dose employed, did not differentiate these cells into classical monocytes, intermediate monocytes, DCs and granulocytes.
No positive control was included in the study to show that ATRA is indeed capable of inducing maturation of primary circulating immature myeloid cells. Nonetheless, the reduction in MDSC frequency without consistent increase in HLADR expression, suggests that ATRA does not mature M-MDSCs. It stands to reason that differentiation could have occurred without upregulation of MDSCs, or alternatively, that these MDSCs were mainly not immature in phenotype. We also considered the possibility that apoptosis might explain the finding. ATRA has been reported to have a role in apoptosis regulation in other cell types [52,53]. The mechanism of action involves ATRA upregulating TRAIL and Caspase-8 signalling pathways, leading to activation of apoptosis [54]. Although apoptosis was not directly measured here, the concentration of ATRA used in our experiments are similar to peak plasma concentrations used for treatment of Acute Promyelocytic Leukaemia (APL). As shown in Supplementary Fig. 8 and in literature [35], this concentration of ATRA does not affect T-cell viability. Another explanation for ATRA’s failure to mature MDSCs in TB might be the difference in disease pathogenesis and the cell types targeted by ATRA in TB and in malignancies. Whereas TB is a bacterial infection, APL is caused by a genetic mutation, t(15;17)(q24;121) [55], whereas melanoma and renal cell carcinomas are caused by a variety of environmental and genetic factors. ATRA-mediated differentiation of promyelocytes by induction of PML-RAR degradation is different to ATRA-mediated maturation of MDSCs in cancer, as these are different myeloid cell subtypes,. Likewise, targeting MDSCs with ATRA in the context of TB may not have the desired effect and other HDTs should be considered. The specific mechanism of ATRA-mediated reduction of M-MDSCs during TB remains an important consideration for future investigations.
We also assessed the effect of ATRA treatment on the immunosuppressive function of MDSCs when co-cultured with T-cells. ATRA treatment did not reverse MDSC-induced immunosuppressive effects on Total T-cell and T-cell subsets. Decreases in MDSC and Treg frequency by optimised ATRA timing and dosage could favour the pro-inflammatory immune response and increase clearance of M.tb. An important consideration for future research is the effect of pre-treatment of MDSCs with ATRA and subsequent removal of ATRA from culture, to evaluate the direct effect of ATRA-modulated MDSCs on T-cell responses. Correct timing of MDSC treatment by ATRA may restore M.tb antigen specific T-cell responses as evident in murine tumour models where ATRA was protective only when administered 3 days after vaccination [56]. ATRAs direct effect on T cell function would be a useful control for future studies. The specific contact-dependent mechanisms by which MDSC suppress T cells in TB also remains to be tested.
This study was also limited by the origin of the main cell types investigated here. The T cells come from an allogeneic donor to the source of TB patient MDSCs. Due to this, in the contact-dependent cultures, an allogeneic response is possible against the disparate MHC molecules recognized as foreign by the T cells, although this is less likely to occur in the trans-well cultures (assuming MHC molecules are not transported via vesicles through the membrane). The potential mixed-lymphocyte reaction (MLR) would result in high proliferative and cytokine responses which could potentially impede specific responses from being seen. Previous data has shown that MDSC suppress T cell responses in a MLR [9,16], thus the mere presence of MDSC and T cells is not likely to yield a major MLR response as seen for example, for DC + Tcell MLR [57–59]. Our control data also show no statistically significant increase in T cell responses (Supplementary Figs. 3–5 d–h). If the cells were cultured for a longer period of time (>48 h) we may have seen a stronger MLR, but our control data allows us to establish that an MLR was not stimulated. However, the possibility that ATRA’s abrogation of MDSC’s suppressive activity should be considered. It could allow an MLR to be misinterpreted as a new response to antigen revealed by reversal of suppression. To improve this study design future investigations will include autologous T cells and MDSCs.
Here we have shown that MDSCs induced in the context of TB are immunosuppressive and that ATRA-treatment results in a statistically significant decrease in the frequency of these suppressive cells. The biological significance of ATRA treatment is however questioned given the small degree of MDSC frequency reduction, as well as the consideration that ATRA treatment did not restore T-cell responses. Although higher levels of ATRA might yield improved results, the current concentration was selected due to the extensive safety and tolerability data available. ATRA inclusion into the anti-TB treatment regimen is questionable, but synergistic effects with other HDTs could be investigated, especially considering the heterogeneous immunosuppressive mechanisms employed by this cell population. Further investigations into repurposed drug compounds targeting MDSC frequency or function are warranted.
4. Materials and methods
4.1. Study subjects
The study cohort consisted of 25 adult, HIV uninfected patients who were newly diagnosed with active pulmonary TB disease and were preferentially treatment naïve or initiated treatment for less than a week, demographics shown in Table 1. These patients were recruited by the Stellenbosch University Molecular Biology Clinical Research Unit from the Ravensmead, Uitsig, Elsiesriver, Adriaanse and Fisantekraal suburbs of Cape Town, South Africa, following the National TB Program treatment practices. The diagnosis of these individuals was confirmed by a positive sputum MGIT culture or GeneXpert, in addition to radiological evidence of active disease. Written informed consent was obtained from all participants involved in the study, by dedicated research personnel, and study approval was given by the Stellenbosch University Ethics Review Committee (N16/05/070). Sodium heparinised peripheral blood (18 mL) was collected from all individuals. All samples were processed within 2 h of collection. All experiments were performed in accordance with relevant guidelines and regulations.
4.2. Cell isolations
Peripheral blood mononuclear cells (PBMC) were collected by Ficoll centrifugation (400×g for 25 min) according to manufacturer’s instructions. Cell isolations were performed by MACS isolation using LS columns (Miltenyi). Briefly, (HLA-DR−CD33+) MDSCs were enriched from PBMCs by depleting HLA-DR+ cells and positively selecting CD33+ cells from the HLA-DR− population (Miltenyi) (Supplementary Fig. 4). MACS isolated MDSCs, from the cohort of active TB participants, were cryopreserved in cryomedia consisting of 90% Fetal bovine serum (FBS) and 10% Dimethyl sulfoxide (DMSO). Adherent monocytes, to be used as a control population, were obtained from HLA-DR+ cells following overnight plating in Roswell Park Memorial Institute (RPMI) medium at 37 °C with 5% CO2 and cryopreserved as above (Supplementary Fig. 4).
Allogeneic T-cells were isolated from a known Quantiferon (QFN) positive donor using a Pan T-cell isolation kit (Miltenyi) and cryopreserved in aliquots of 5×106 cells (Supplementary Fig. 4). This QFN+ status is known by measuring IFN-γ production from whole blood following TB antigen stimulation, by making use of the QuantiFERON-TB Gold (QFT) commercial test. This donor was also selected due to having a previous case (>5 years prior) case of active TB disease. T cells from this donor would thus be responsive to PPD stimulation.
Purity of all cell fractions exceeded 85% by flow cytometry, representative plots shown in Supplementary Fig. 5.
4.3. Co-culture assays and trans-well cultures
Cryopreserved MDSCs from an individual TB participant, with their corresponding adherent monocytes, as well as an aliquot of allogeneic T-cells isolated from isolated from a QFN positive donor, was removed from liquid nitrogen (LN) storage, thawed, washed and counted. These cells were cultured according the plate layout in Supplementary Fig. 6. Briefly, combinations of 2×105 cells per well, were cultured in RPMI + 10% autologous human serum (AHS) + 1% L-glutamine (L-glut) with or without 1uM ATRA, under 2 T cell conditions including Unstimulated and non-specifically activated (anti-CD3CD28 beads, Miltenyi T-cell activation kit) T cells for 48 h at 37 °C with 5% CO2.
For trans-well co-cultures, 96 well 0.4 um Polycarbonate Membrane Corning Trans-well culture plate inserts (Becton Dickinson, New Jersey, USA) were used to separate MDSCs (top) and T-cells (bottom), following the plate layout in Supplementary Fig. 6. Brefeldin-A was added to all wells, co-culture and trans-well cocultures, at 43 h to facilitate accumulation of proteins in the endoplasmic reticulum (ER) and prevent export of cytokine proteins from the cell. Culture supernatants were harvested at 48 h and stored at −80 °C for multiplex analysis of soluble cytokine production, and the cellular fraction cryopreserved in LN.
4.4. Flow cytometry
MDSC and T-cell fractions were analysed separately by flow cytometry (BD FACS Canto II) using 2 antibody panels, consisting of 8 antibodies each. These panels were designed to (1) evaluate MDSC phenotype/maturation status (CD1c-PE-Cy7, CD3-FITC, CD11b-PerCP, CD14-PacBlue, CD15-BV-510, CD16-APC-Cy7, CD33-PE and HLA-DR-APC) and (2) T-cell function (CD3-FITC, CD4-PerCP-Cy5.5, CD8-APC-Cy7, CD45RO-PacBlue, CD62L-APC, CD69-BV510, FoxP3-PE and Ki67-PE-Cy7). Post-culture, all cells were cryopreserved. In batches, cells were thawed, stained and acquired. Titrations, Compensation and Fluorescence-minus-one (FMO) was performed prior to analysis, on the FACS Canto II, at the BD Flow Cytometry Unit at Stellenbosch University, Tygerberg Campus. Data was analysed using the third party FlowJo Software v10.0.8 using the gating strategies attached in Supplementary Fig. 7.
4.5. Multiplex assay
Quantification of cytokine levels from culture supernatants was performed using a human 27-plex assay (Biorad Bio-Plex Pro Human Cytokine Standard 27-plex, Group I), on the Bioplex 200 (Biorad, California, USA). The panel comprised of Interleukin (IL)-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL12p70, IL-13, IL-15, IL17A, Eotaxin, Basic Fibroblast growth factor (FGF), Granulocyte-Colony Stimulating Factor (G-CSF), Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF), Interferon gamma (IFNγ), Interferon Gamma-Induced Protein (IP)-10, Monocyte chemoattractant protein (MCP)-1, Macrophage Inflammatory Protein (MIP)-1α, MIP-1β, Platelet-derived growth factor (PDGF), Regulated on Activation, Normal T-cell Expressed and Secreted (RANTES), Tumour Necrosis Factor alpha (TNFα) and Vascular Endothelial Growth Factor (VEGF). Measurements were examined using Bio-Plex Manager v.4.1.1. software against internal quality controls and dilutions of cytokine standard curves were prepared according to manufacturer’s instructions.
4.6. Statistical analysis
Statistical analyses were performed using the Kruskal-Wallis test with Dunns post-hoc test, Wilcoxon matched pairs signed rank test (GraphPadPrism v.8; GraphPad Software, San Diego, CA), and Mixed Model, LS means and Type III decomposition (Luminex data) (Statistica, Quest Software, Elliot Management Group). A p-value less than 0.05 was considered significant.
Supplementary Material
Table 4.
Effect of ATRA treatment on T cell production of 27 Innate Cytokines, under differing stimulation combinations (Unstimulated and anti-CD3CD28 beads), measured by Luminex. Data analysed by Mixed Model, LS means and Type III decomposition using Statistica (Quest Software, Elliot Management Group). A p-value less than 0.05 was considered significant.
| Analytes | T cell treated with ATRA vs T cell untreated |
|||
|---|---|---|---|---|
| Unstim (p-value) | Significant Increase/Decrease/ns | aCD3CD28 (p-value) | Significant Increase/Decrease/ns | |
|
| ||||
| IL1b | 0.93224 | ns | 0.980514 | ns |
| IL1ra | 0.607315 | ns | 0.664356 | ns |
| IL2 | 0.907946 | ns | 0.476152 | ns |
| IL4 | 0.808789 | ns | 0.000012 | increase |
| IL6 | 0.418197 | ns | 0.404916 | ns |
| IL7 | 0.060996 | ns | 0.233229 | ns |
| IL9 | 0.513908 | ns | 0.000004 | decrease |
| IL10 | 0.733901 | ns | 0.029443 | decrease |
| IL12 | 0.000029 | decrease | 0.175689 | ns |
| IL13 | 0.807077 | ns | 0.070138 | ns |
| IL15 | 0.398862 | ns | 0.838695 | ns |
| IL17A | 0.802545 | ns | 0.001726 | decrease |
| FGF basic | 0.579758 | ns | 0.543935 | ns |
| IFNg | 0.953108 | ns | 0.272509 | ns |
| MCP1 | 0.024316 | increase | 0.789536 | ns |
| MIP1a | 0.996559 | ns | 0.000029 | decrease |
| PDGF-bb | 0.006977 | increase | 0.997963 | ns |
| MIP1b | 0.753384 | ns | 0.034838 | decrease |
| TNFa | 0.302195 | ns | 0.010654 | decrease |
| IL5 | 0.718635 | ns | 0.148093 | ns |
| IL8 | 0.056845 | ns | 0.333874 | ns |
| Eotaxin | 0.373247 | ns | 0.626624 | ns |
| G-CSF | 0.907528 | ns | 0.266079 | ns |
| IP10 | 0.023365 | decrease | 0.053419 | ns |
| RANTES | 0.166528 | ns | 0.16034 | ns |
| GM-CSF | 0.957072 | ns | 0.011559 | decrease |
| VEGF | 0.758214 | ns | 0.001114 | decrease |
Acknowledgements
The authors thank the participants who gave consent for their specimens to be used in this study and greatly acknowledge the clinicians, nurses and staff at the Molecular Biology Clinical Research Unit at Stellenbosch University for their contribution to this study. This work was supported by the European & Developing Countries Clinical Trials Partnership (EDCTP; CDF1546), National Institute of Health (NIH) International Collaborations in Infectious Disease Research (ICIDR): Biology and Biosignatures of anti-TB Treatment Response (5U01IA115619/03) and South African National Research Foundation SA Research Chair Initiative (SARCHI; 86535).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellimm.2021.104359.
References
- [1].World Health Organization, Global tuberculosis report 2018. 2018. [Google Scholar]
- [2].“WHO | Guidelines for treatment of drug-susceptible tuberculosis and patient care (2017 update).” http://www.who.int/tb/publications/2017/dstb_guidance_2017/en/ (accessed Feb. 13, 2018).
- [3].Guernsey BG, Alexander MR, Tuberculosis: review of treatment failure, relapse and drug resistance, Am. J. Hosp. Pharm. 35 (6) (Jun. 1978) 690–698. [PubMed] [Google Scholar]
- [4].Hancock REW, Nijnik A, Philpott DJ, Modulating immunity as a therapy for bacterial infections, Nat. Rev. Microbiol. 10 (4) (Apr. 2012) 243–254, 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- [5].Kolloli A, Subbian S, Host-Directed Therapeutic Strategies for Tuberculosis, Front. Med. 4 (October. 2017), 10.3389/fmed.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Palucci I, Delogu G, Host Directed Therapies for Tuberculosis: Futures Strategies for an Ancient Disease, Chemotherapy 63 (3) (2018) 172–180, 10.1159/000490478. [DOI] [PubMed] [Google Scholar]
- [7].“https://clinicaltrials.gov/ct2/results?cond=Tuberculosis&term=Host-directed+therapy&cntry=&state=&city=&dist=&Search=Search.”
- [8].Rubtsov YP, et al. , Regulatory T Cell-Derived Interleukin-10 Limits Inflammation at Environmental Interfaces, Immunity 28 (4) (2008) 546–558, 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- [9].El Daker SK, et al. , Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level, PLOS ONE 10 (4) (Apr. 2015) e0123772, 10.1371/journal.pone.0123772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bronte V, et al. , Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards, Nat. Commun. 7 (1) (2016), 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gabrilovich DI, Myeloid-derived suppressor cells, Cancer Immunol. Res. 5 (1) (2017) 3–8, 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Veglia F, Perego M, Gabrilovich D, Myeloid-derived suppressor cells coming of age, Nat. Immunol. 19 (2) (2018) 108–119, 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ribechini E et al. , “Novel GM-CSF signals via IFN-gR/IRF-1 and AKT/mTOR license monocytes for suppressor function,” vol. 1, no. 14, p. 14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cassetta L, et al. , Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice and non-human primates, Cancer Immunol. Immunother. 68 (4) (2019) 687–697, 10.1007/s00262-019-02302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldmann O, Beineke A, Medina E, Identification of a novel subset of myeloid-derived suppressor cells during chronic staphylococcal infection that resembles immature eosinophils, J. Infect. Dis. 216 (11) (Dec. 2017) 1444–1451, 10.1093/infdis/jix494. [DOI] [PubMed] [Google Scholar]
- [16].du Plessis N, et al. , Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function, Am. J. Respir. Crit. Care Med. 188 (6) (2013) 724–732, 10.1164/rccm.201302-0249OC. [DOI] [PubMed] [Google Scholar]
- [17].Tsiganov EN et al. , “Gr-1dimCD11b+ immature myeloid-derived suppressor cells but not neutrophils are markers of lethal tuberculosis infection in mice,” J. Immunol. Baltim. Md 1950, vol. 192, no. 10, pp. 4718–4727, May 2014, doi: 10.4049/jimmunol.1301365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Obregón-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ, Gr1intCD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection, PloS One 8 (11) (2013) e80669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Knaul JK et al. , Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis, Am. J. Respir. Crit. Care Med, Oct. 2014, doi: 10.1164/rccm.201405-0828OC. [DOI] [PubMed] [Google Scholar]
- [20].Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system, Nat. Rev. Immunol. 9 (3) (Mar. 2009) 162–174, 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pan P-Y et al. , Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function, Blood, vol. 111, no. 1, pp. 219–228, Jan. 2008, doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ko JS et al. , Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients, Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res, vol. 15, no. 6, pp. 2148–2157, Mar. 2009, doi: 10.1158/1078-0432. CCR-08–1332. [DOI] [PubMed] [Google Scholar]
- [23].Ko JS, et al. , Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained, Cancer Res. 70 (9) (2010) 3526–3536, 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rodan GA, Fleisch HA, Bisphosphonates: mechanisms of action, J. Clin. Invest. 97 (12) (1996) 2692–2696, 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Heissig B, et al. , Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand, Cell 109 (5) (2002) 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Serafini P et al. , Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function, J. Exp. Med, vol. 203, no. 12, pp. 2691–2702, Nov. 2006, doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kusmartsev S, et al. , All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination, Cancer Res. 63 (15) (Aug. 2003) 4441–4449. [PubMed] [Google Scholar]
- [28].Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI, Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells, Cancer Res. 67 (22) (Nov. 2007) 11021–11028, 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- [29].Mirza N, et al. , All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients, Cancer Res. 66 (18) (2006) 9299–9307, 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mueller BU, ATRA resolves the differentiation block in t(15;17) acute myeloid leukemia by restoring PU.1 expression, Blood, vol. 107, no. 8, pp. 3330–3338, Apr. 2006, doi: 10.1182/blood-2005-07-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adamson PC, Clinical and pharmacokinetic studies of all-trans-retinoic acid in pediatric patients with cancer, Leukemia 8 (Suppl. 3) (1994) S22–25. [PubMed] [Google Scholar]
- [32].Adamson PC, All-trans-retinoic acid pharmacology and its impact on the treatment of acute promyelocytic leukemia, The Oncologist 1 (5) (1996) 305–314. [PubMed] [Google Scholar]
- [33].Mourik BC et al. , Immunotherapy added to antibiotic treatment reduces relapse of disease in a mouse model of tuberculosis, Am. J. Respir. Cell Mol. Biol, Sep. 2016, doi: 10.1165/rcmb.2016-0185OC. [DOI] [PubMed] [Google Scholar]
- [34].Riccomi A, Piccaro G, Christensen D, Palma C, Andersen P, Vendetti S, Parenteral vaccination with a tuberculosis subunit vaccine in presence of retinoic acid provides early but transient protection to M. tuberculosis infection, Front. Immunol. 10 (2019) 934, 10.3389/fimmu.2019.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM, “Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells,” J. Immunol. Baltim. Md 1950, vol. 166, no. 9, pp. 5398–5406, May 2001. [DOI] [PubMed] [Google Scholar]
- [36].Millington KA et al. , Dynamic relationship between IFN-γ and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load, p. 21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harari A, Vallelian F, Meylan PR, Pantaleo G, Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence, J. Immunol. 174 (2) (2005) 1037–1045, 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- [38].Robinson RT, T cell production of GM-CSF protects the host during experimental tuberculosis” mBio, vol. 8, no. 6, Dec. 2017, doi: 10.1128/mBio.02087-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van Crevel R et al. , Increased Production of Interleukin 4 by CD4 + and CD8 + T Cells from Patients with Tuberculosis Is Related to the Presence of Pulmonary Cavities, J. Infect. Dis, vol. 181, no. 3, pp. 1194–1197, Mar. 2000, doi: 10.1086/315325. [DOI] [PubMed] [Google Scholar]
- [40].Heitmann L, et al. , The IL-13/IL-4R α axis is involved in tuberculosis-associated pathology: IL-13/IL-4R and granuloma necrosis in TB, J. Pathol. 234 (3) (Nov. 2014) 338–350, 10.1002/path.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tobin RP, Jordan KR, Davis D, McCarter M, “Effects of in vitro ATRA treatment on human MDSC expansion and function.,” J. Clin. Oncol, vol. 35, no. 7_suppl, pp. 125–125, Mar. 2017, doi: 10.1200/JCO.2017.35.7_suppl.125. [DOI] [Google Scholar]
- [42].Nagaraj S, Schrum AG, Cho H-I, Celis E, Gabrilovich DI, Mechanism of T cell tolerance induced by myeloid-derived suppressor cells, J. Immunol. 184 (6) (Mar. 2010) 3106–3116, 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Noman MZ et al. , “PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation,” J. Exp. Med, vol. 211, no. 5, pp. 781–790, May 2014, doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu J et al. , “Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes,” Nat. Commun, vol. 8, no. 1, Dec. 2017, doi: 10.1038/s41467-017-00784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raber P, Ochoa AC, Rodríguez PC, Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives, Immunol. Invest. 41 (6–7) (2012) 614–634, 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nagaraj S, et al. , Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer, Nat. Med. 13 (7) (2007) 828–835, 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Raber PL, et al. , Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways, Int. J. Cancer 134 (12) (2014) 2853–2864, 10.1002/ijc.v134.1210.1002/ijc.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rigamonti N et al. , “Modulators of arginine metabolism do not impact on peripheral T-cell tolerance and disease progression in a model of spontaneous prostate cancer,” Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res, vol. 17, no. 5, pp. 1012–1023, Mar. 2011, doi: 10.1158/1078-0432.CCR-10-2547. [DOI] [PubMed] [Google Scholar]
- [49].Almand B et al. , “Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer,” J. Immunol. Baltim. Md 1950, vol. 166, no. 1, pp. 678–689, Jan. 2001. [DOI] [PubMed] [Google Scholar]
- [50].Trellaki S Increased Production of Interleukin 4 by CDset al. , “Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer,” Innate Immun, vol. 19, no. 3, pp. 328–336, Jun. 2013, doi: 10.1177/1753425912463618. [DOI] [PubMed] [Google Scholar]
- [51].Castaigne S, et al. , All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results, Blood 76 (9) (1990) 1704–1709. [PubMed] [Google Scholar]
- [52].Noy N, Between death and survival: retinoic acid in regulation of apoptosis, Annu. Rev. Nutr. 30 (1) (2010) 201–217, 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- [53].De Genaro P, Simón MV, Rotstein NP, Politi LE, Retinoic acid promotes apoptosis and differentiation in photoreceptors by activating the P38 MAP kinase pathway, Investig. Opthalmology Vis. Sci. 54 (5) (2013) 3143, 10.1167/iovs.12-11049. [DOI] [PubMed] [Google Scholar]
- [54].Dhandapani L, Yue P, Ramalingam SS, Khuri FR, Sun S-Y, Retinoic acid enhances TRAIL-induced apoptosis in cancer cells by upregulating TRAIL receptor 1 expression, Cancer Res. 71 (15) (2011) 5245–5254, 10.1158/0008-5472.CAN-10-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Grignani F, et al. , The acute promyelocytic leukemia-specific PML-RAR fusion protein inhibits differentiation and promotes survival of myeloid precursor cells, Cell 74 (1993) 9. [DOI] [PubMed] [Google Scholar]
- [56].Heine A, et al. , Targeting myeloid derived suppressor cells with all-trans retinoic acid is highly time-dependent in therapeutic tumor vaccination, OncoImmunology 6 (8) (2017) e1338995, 10.1080/2162402X.2017.1338995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Inaba K, Romani N, Steinman RM, An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells, J. Exp. Med. 170 (2) (1989) 527–542, 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kondo T et al. , “Dendritic cells signal T cells in the absence of exogenous antigen,” Nat. Immunol, vol. 2, no. 10, Art. no. 10, Oct. 2001, doi: 10.1038/ni711. [DOI] [PubMed] [Google Scholar]
- [59].Revy P, Sospedra M, Barbour B, Trautmann A, Functional antigen-independent synapses formed between T cells and dendritic cells, Nat. Immunol. 2 (10) (2001) 925–931, 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.