Abstract
Background: In spite of increasing evidence highlighting the role of dynamic functional connectivity (FC) in characterizing mental disorders, there is a lack of (a) reliable statistical methods to compute dynamic connectivity and (b) rigorous dynamic FC-based approaches for predicting mental health outcomes in heterogeneous disorders such as post-traumatic stress disorder (PTSD).
Methods: In one of the first such efforts, we develop a reliable and accurate approach for estimating dynamic FC guided by brain structural connectivity (SC) computed using diffusion tensor imaging data and investigate the potential of the proposed multimodal dynamic FC to predict continuous mental health outcomes. We develop concrete measures of temporal network variability that are predictive of PTSD resilience, and identify regions whose temporal connectivity fluctuations are significantly related to resilience.
Results: Our results illustrate that the multimodal approach is more sensitive to connectivity change points, it can clearly detect localized brain regions with the dynamic network features such as small-worldedness, clustering coefficients, and efficiency associated with resilience, and that it has superior predictive performance compared with existing static and dynamic network models when modeling PTSD resilience.
Discussion: While the majority of resting-state network modeling in psychiatry has focused on static FC, our novel multimodal dynamic network analyses that are sensitive to network fluctuations allowed us to provide a model of neural correlates of resilience with high accuracy compared with existing static connectivity approaches or those that do not use brain SC information, and provided us with an expanded understanding of the neurobiological causes for PTSD.
Impact statement
The methods developed in this article provide reliable and accurate dynamic functional connectivity (FC) approaches by fusing multimodal imaging data that are highly predictive of continuous clinical phenotypes in heterogeneous mental disorders. Currently, there is very little theoretical work to explain how network dynamics might contribute to individual differences in behavior or psychiatric symptoms. Our analysis conclusively discovers localized brain resting-state networks, regions, and connections where variations in dynamic FC (that is estimated after incorporating brain structural connectivity information) are associated with post-traumatic stress disorder resilience, which could potentially provide valuable tools for the development of neural circuit modeling in psychiatry in the future.
Keywords: dynamic functional connectivity, Gaussian graphical models, multimodal imaging, post-traumatic stress disorder, scalar-on-function regression, trauma resilience
Introduction
Over the last decade, numerous advances have been made in developing neuroimaging biomarkers for mental illnesses that offer tremendous versatility in terms of understanding and targeting pathophysiological mechanisms. Of these, static functional connectivity (FC) has emerged as one of the most promising biomarkers capable of classifying and predicting mental disorders (see Du et al., 2018 for a recent review).
However, it is increasingly recognized that the brain connectivity may not remain constant over time, and is likely to exhibit dynamic variations that may be linked to changes in vigilance (Thompson et al., 2013), arousal (Chang et al., 2013), personality traits (Kabbara et al., 2020), behavioral performance (Jia et al., 2014), disease status (Jin et al., 2017), and so on. Most existing methods use the sliding window approach (Sagoklu et al., 2010) to compute dynamic connectivity, although other approaches such as hidden Markov models (Vidaurre et al., 2016) and change point estimation (Kundu et al., 2018) are gaining in prominence.
There has been limited literature on disease phenotypic classification using dynamic FC (Du et al., 2018), with the overwhelming majority of methods investigating differences in dynamic FC between prespecified clinical phenotypes (Fu et al., 2019; Jin et al., 2017). However, predefined clinical labels may be erroneous due to gaps in diagnosis, which is especially true in heterogeneous disorders such as post-traumatic stress disorder (PTSD) where a gold standard for classification may not be present. Hence, more recently, there has been a push toward continuous measures of assessment, for example, the research domain criterion (Insel and Cuthbert, 2015). Compared with existing classification approaches, there are even lesser number of approaches that use dynamic FC to model continuous clinical measures (Haslam, 2003; Widiger, 2005), although the need for such predictive approaches for heterogeneous mental disorders such as PTSD is clear. Some challenges include the following: (a) developing reliable measures of dynamic FC based on single subject data and identifying quantifiable dynamic network summaries that provide the highest differentiating power with respect to the continuous clinical outcomes of interest; (b) discovering localized brain regions whose dynamic FC signatures are directly related to behavior; and (c) developing predictive models for continuous clinical outcomes based on dynamic networks, which goes beyond disease phenotype classification. Challenges (b)–(c) can only be tackled adequately, provided one can find accurate and reliable estimates for dynamic FC [issue (a)]. However, current approaches for dynamic FC based on single subject data may be compromised due to noise in the functional magnetic resonance imaging (fMRI) data and other factors (Kundu et al., 2018), which demands more innovative and novel statistical methods.
The goal of this work is twofold. First, to develop novel, accurate, and reliable methods for dynamic FC estimation using resting-state fMRI (Rs-fMRI) data that are guided by brain structural connectivity (SC) information obtained through diffusion tensor imaging (DTI) data; and second, to develop a scalar-on-function regression framework for predictive modeling of resilience in PTSD using dynamic FC. To address issue (a), we identify time-varying network summaries such as network efficiency, clustering coefficient, and small-worldedness that encode patterns of information transmission and are highly predictive of PTSD resilience. Our analysis helps identify localized brain regions and network edges, as well as resting-state networks, that are most strongly associated with resilience [question (b)]. The dynamic networks obtained through the multimodal approach provide greater predictive accuracy when modeling trauma resilience [issue (c)]. Using extensive validation studies, we conclusively illustrate that the multimodal dynamic FC approach is able to recover the true dynamic network with high accuracy. A graph theoretic approach is used for network modeling, where brain regions are perceived as nodes and edges encode network connectivity, and strength of edges is measured through partial correlations.
To our knowledge, we are one of the first to develop and investigate multimodal dynamic FC (mDFC) as a neuroimaging biomarker for predictive analysis of continuous mental health outcomes. Of course, the motivation for structurally guided dynamic FC stems from a well-established literature illustrating the relationship between static FC and SC (Sporns, 2013). Such evidence has given rise to some limited development of static FC approaches guided by SC knowledge (Higgins et al., 2018). Since static FC can be interpreted as an average of dynamic FC values over time, it is natural to conjecture that dynamic FC is also regulated by brain SC to some degree. However, it is nontrivial to generalize the existing approaches to our settings of interest involving the estimation of dynamic networks guided by SC, and this requires major methodological and scientific innovations undertaken in the article, with the ultimate goal of developing neuroimaging biomarkers based on dynamic FC.
Materials and Methods
Description of Grady Trauma Project data
Our study involves female African American participants from the Grady Trauma Project (GTP). These participants were recruited from primary care clinics at Grady Health System, a publicly funded, tertiary care center in Atlanta, Georgia. A majority of these participants have experienced significant psychological trauma of various types (Gillespie et al., 2009). Imaging modalities including Rs-fMRI and DTI data were collected for each individual—the details for the preprocessing steps are provided in the Supplementary Data. The participants were all female and African American (age 25.8 ± 3.1) and did not have any disability or head injury, and were not on psychoactive medications. We confined our analysis to a subset of 31 younger individuals who were aged <30 years to bypass any potential confounding effects due to aging that may interfere with our final findings and interpretations, and to protect against potential white matter integrity issues that are expected to affect the DTI data (Gunning-Dixon et al., 2009) in older adults. Of these participants, 16 subjects were diagnosed with PTSD (resilience score within −1.11 to 28.9), and the other 15 subjects were not (resilience score within −13.4 to 17.5); the procedure for determining the PTSD status is similar to Falsetti et al.'s (1993; Supplementary Data). Our clinical outcome of interest is the resilience score that is a transdiagnostic indicator of mental health in the face of adversity and is measured through the approach of Ioannidis et al. (2020). The resilience score was computed as the deviation in PTSD symptoms beyond what would be expected based on childhood trauma exposure using residuals in a linear regression model (Supplementary Data). Negative residuals indicate lower-than-expected PTSD symptoms, representing psychiatric resilience, while positive residuals indicate higher-than-expected PTSD that represents psychiatric risk.
Power atlas and functional modules
We use a whole-brain parcellation corresponding with 264 Region of Interest (ROI) under the Power system (Power et al., 2011). Further, we group these ROIs into 10 functional modules as identified by Cole et al. (2013), which better characterize resting-state functional networks. These modules included sensory/somatomotor, cingulo-opercular (CON), salience (SAL), auditory (AUD), subcortical (SCOR), default mode network (DMN), visual, frontoparietal (FPL), ventral-attention network (VAN), and dorsal-attention network (DAN). The coordinates for the ROIs and their allocation to these modules are presented in Supplementary Table S1.
Overview of statistical approach
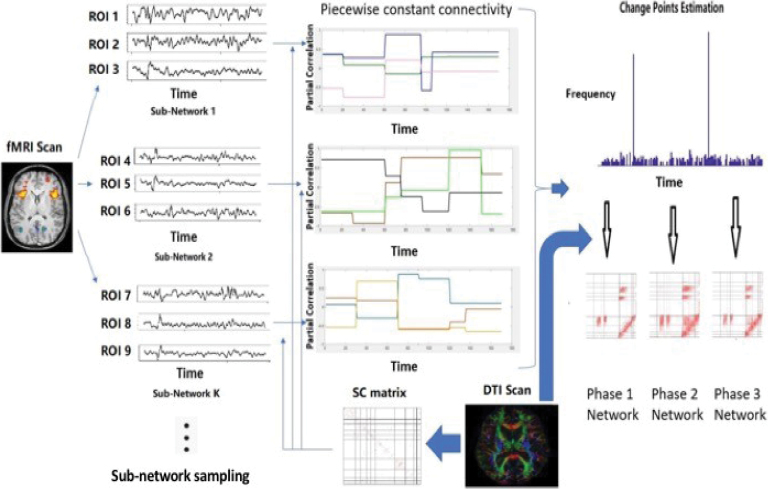
Our goal is to develop an approach for estimating dynamic connectivity based on Gaussian graphical models (GGMs) that are guided by SC knowledge. GGMs assume that the fMRI measurements are normally distributed, and are characterized by a sparse inverse covariance or precision matrix that has zero off-diagonals corresponding to absent edges in the network. Moreover, the nonzero elements of the precision matrix encode the strength of the important edges, which are estimated after incorporating brain SC strengths, which are in turn computed using probabilistic tractography on the DTI data as in Higgins et al. (2018; Supplementary Data). The proposed approach uses a change point estimation approach to identify the number and locations of state phases, which results in a piecewise constant connectivity matrix that is learnt from the data. Moreover, the pattern of zeros in the precision matrix at each time point essentially provides all the necessary information about the time-varying network. We denote our approach as mDFC through the rest of the article. A diagrammatic illustration is provided in Figure 1, and full details regarding the statistical method are provided in the Supplementary Data.
FIG. 1.
A diagrammatic illustration of our novel mDFC approach using Rs-fMRI data, which is guided by brain SC information computed from DTI data. Given a set of nodes in the network, the approach is able to learn change points or jumps in the network in an unsupervised manner, where the number and locations of the change points are unknown and the network is assumed to remain constant within a state phase defined as the time interval between two consecutive change points. The greedy partitioning scheme used to compute change points uses state phase-specific networks that are computed after incorporating brain SC knowledge—in this manner, the change point estimation procedure is influenced by the given brain SC information. To scale up the mDFC approach to high-dimensional networks, we propose a subnetwork sampling scheme where we use the mDFC approach to compute change points using several smaller subsets of nodes or subnetworks. This process is applied repeatedly for a large number of subnetworks, and the set of change points for each subnetwork is recorded. The subnetwork sampling scheme yields a frequency or score for each time point to be identified as a network-level change point, and a systematic data-adaptive thresholding strategy to determine frequency cutoffs that can be used to determine network-level change points that are consistently identified across most subnetworks. Conditional on the estimated network-level change points, the structurally informed precision matrix estimation is applied once again to compute a distinct sparse inverse covariance matrix encoding the network separately for each state phase. The state phase-specific networks are computed by integrating brain SC information that encourages greater weights for FC corresponding to those edges with strong SC under a Gaussian graphical model. DTI, diffusion tensor imaging; FC, functional connectivity; fMRI, functional magnetic resonance imaging; mDFC, multimodal dynamic FC; Rs-fMRI, resting-state fMRI; SC, structural connectivity. Color images are available online.
Analysis outline
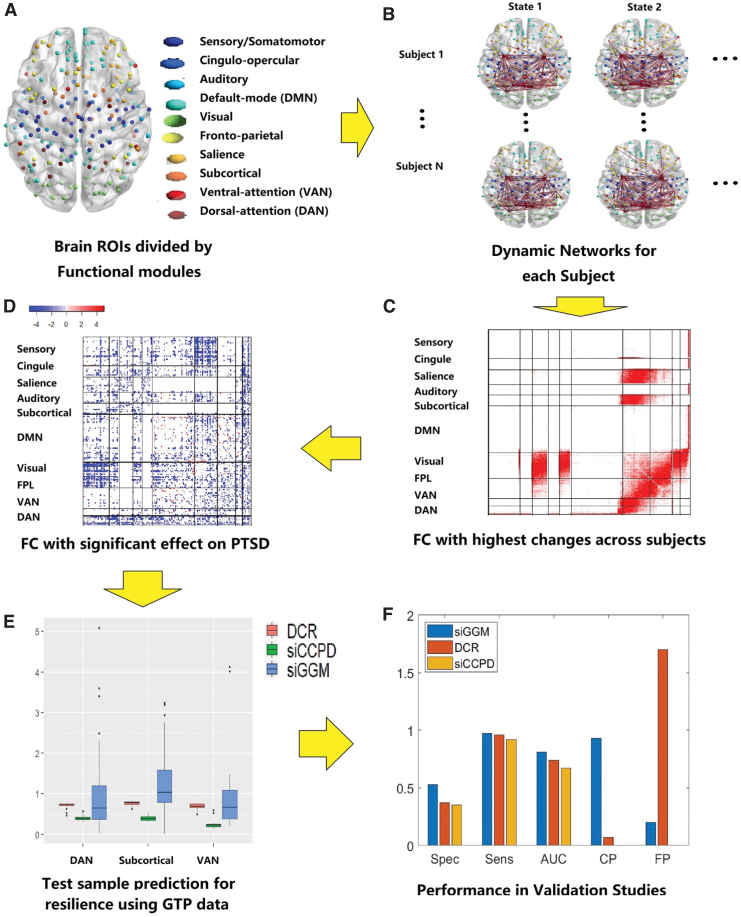
Using the mDFC approach, we first compute the dynamic resting-state network and subsequently investigate whether the temporal connectivity changes can explain variations in disease severity (resilience). Our primary analysis does not involve confounding variables such as depression (measured through Beck depression inventory or BDI) since they are often strongly associated with PTSD severity (Kim et al., 2019), and may mask the brain network effects on resilience. However, we also performed a secondary analysis that adjusts for BDI when modeling resilience using dynamic networks (prediction results in the Supplementary Data). Figure 2 provides an illustration of the different steps in our BDI-unadjusted analysis, which are described in detail below. We compare our method with two alternate approaches: a Bayesian structurally informed GGM by Higgins et al. (2018), which estimates a static network guided by brain SC information, and the SC naive version of the proposed approach that is similar to dynamic connectivity regression proposed by Cribben et al. (2013). The performance metrics used for comparison are described in detail in the Supplementary Data.
FIG. 2.
The proposed analysis pipeline. (A) Illustrates the nodes used in brain functional connectivity that are distinguished based on the known functional modules. (B) Illustrates the computed dynamic functional connectivity separately for each individual, the method for which is detailed in the Methods section and Figure 1. (C) Depicts a heatmap with summary measures that reflect the degree of temporal variation for edges across all the individuals. (D) Illustrates our discovery regarding the edges whose temporal fluctuations are directly related to trauma resilience. (E) Provides boxplots for out-of-sample prediction accuracy using the edge-wise dynamic connections to predict the continuous clinical outcome, through the scalar-on-function statistical methodology. (F) Provides a visual depiction of the performance metrics from our extensive validation studies comparing the proposed approach with alternative methods. AUC, area under the ROC curve; CP, change Point Detection; DAN, dorsal-attention network; DCR, dynamic connectivity regression; DMN, default mode network; FP, false positive of CP; FPL, frontoparietal; GTP, Grady Trauma Project; PTSD, post-traumatic stress disorder; ROI, region of Interest; siCCPD, proposed method; siGGM, Bayesian structurally informed Gaussian graphical models; VAN, ventral-attention network. Color images are available online.
Detecting temporal connectivity fluctuations related to resilience
To assess potential associations between temporal variability in FC and disease severity, we (a) investigated whether temporal edge variability in terms of the number of fluctuations between the present and absent states (measured through change points) is significantly different between individuals with and without PTSD (or equivalently high and low resilience groups); (b) examined if fluctuations in connectivity strengths (partial correlations) are related to the continuous resilience measure; and (c) identified which network nodes are most significantly associated with resilience in terms of node-level dynamic network features. For (b), we performed a univariate regression analysis (one edge at-a-time) to identify those edges whose standard deviation of the partial correlations is significantly related to the resilience score. For (c), we performed a univariate scalar-on-function regression model (Prediction based on dynamic FC through scalar-on-function regression) with the explanatory variables being the node-level dynamic network features (one node at-a-time). Because multiple regression models were fit, significant effects were identified after multiplicity adjustment for p-values using the Benjamini–Hochberg correction (Benjamini and Hochberg, 1995). Edges having a significantly positive (or negative) association will imply connections where greater variability in connectivity strength enhances (or decreases) resilience and vice versa.
Prediction based on dynamic FC through scalar-on-function regression
We use scalar-on-function regression to predict the resilience score using the dynamic functional connections as a function of time. The scalar-on-function regression (Ramsay and Silverman, 2005) can be expressed as zi = ∫0Tρˆi(t)β (t)dt + ɛi, where ɛi denotes the residual that is assigned a Gaussian distribution, zi represents the scalar clinical outcome, and ρi(t) denotes some network summary measure of the estimated time-varying FC for the i-th individual derived under the dynamic network (see the sequel for more details), and β (t) denotes the time-varying coefficient function that weights the dynamic connection over time to model the outcome and can be interpreted as a dynamic analog of regression coefficients in usual linear regression models. We used the R package FDBoost (Brockhaus et al., 2018) for implementing the scalar-on-function regression. The predictive accuracy of the scalar-on-function regression was assessed using out-of-sample mean squared error that calculates the averaged squared difference between the observed and predictive values in the test sample. We performed a leave-one-out prediction strategy that excludes the t-th individual to be kept aside as the test set, and then fit the model using the training data involving the remaining N-1 individuals, where N is the total sample size. Out-of-sample prediction corresponding to the t-th test sample is performed, and the predictive accuracy is computed. This process is repeated by cycling over all individuals as test samples one at a time, and the predictive accuracy is averaged over all test samples. We did not include gender, race, or age in our regression model since the entire sample comprised of African American females between 19 and 30 years.
Dynamic network summaries for prediction of disease severity
Edge-level analyses, although more easily interpretable, may often be subject to greater levels of noise and may be less reproducible across studies. Hence, instead of using edge-level features, we investigated the predictive ability of global dynamic network summaries such as small-worldedness, global efficiency, and global clustering coefficients, as well as local clustering coefficient and local efficiency corresponding to some local functional modules. These include Visual, SAL, SCOR, VAN, and DAN modules that were identified as regions with the highest FC changes for trauma-exposed individuals under our dynamic network analysis (Fig. 4). We also reported the prediction results corresponding to the small-worldedness for all modules. Clustering coefficient and small worldedness were chosen based on recent findings of differences in these metrics in static networks between individuals with and without PTSD (Rowland et al., 2018), whereas the global and local efficiency are additional network metrics that we chose to investigate in the context of predicting resilience. We note that all the network metrics in our analysis are time dependent. The network metrics were computed using the Matlab toolbox Brain Connectivity Toolbox (Rubinov and Sporns, 2010) and are described in Supplementary Data. Our analysis results correspond to networks with ∼15% density for all participants, which seem to reflect an acceptable sparsity level in connectome studies, although the predictive performance for 10% and 5% network densities is also presented in the Supplementary Data.
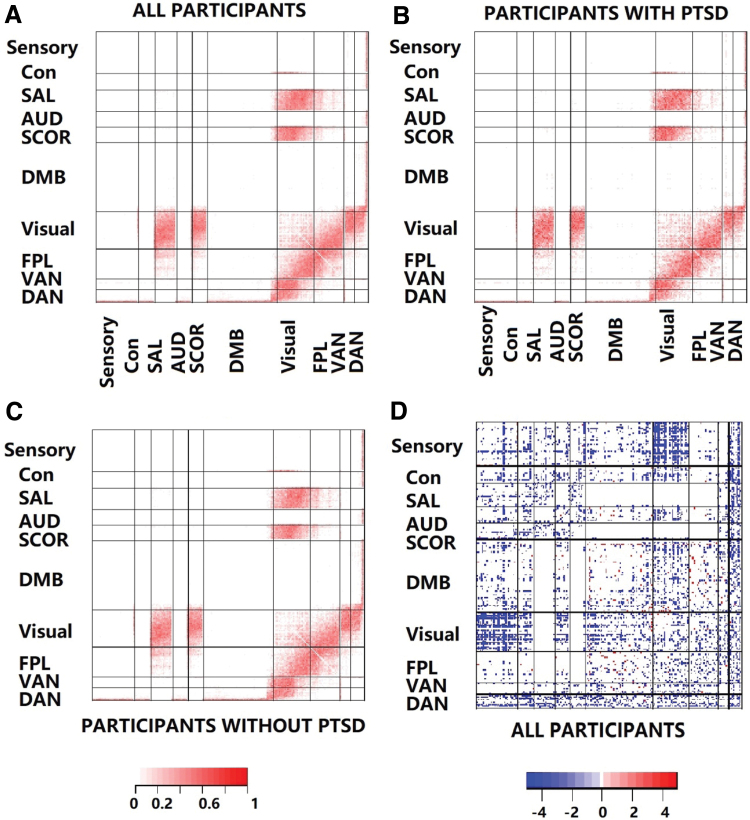
FIG. 4.
(A–C) Illustrates the edge-wise temporal variation averaged over all individuals, individuals with PTSD, and those without PTSD, respectively. Here, the temporal variation for an edge was calculated as the ratio of the number of state changes for that edge divided by the number of state changes in the network. Edges in several modules including Visual, SAL, SCOR, VAN, and DAN show strong temporal fluctuations resulting from frequent state changes. On the contrary, the Sensory, Cingulomotor, and DMN register the fewest temporal fluctuations over time. Only 213 edges illustrated significant differences in terms of the proportion of edge-specific state changes between the PTSD and non-PTSD groups, which suggests the inadequacy of this measure to distinguish disease severity. Of these 213 edges, almost all had a higher frequency corresponding to the PTSD group, illustrating higher temporal fluctuations in this cohort. (D) Illustrates edges whose fluctuations in terms of the edge strength (measured through edge-specific standard deviations for partial correlations over time) are significantly related to PTSD resilience (multiplicity adjusted). Most of these edges lie between functional modules and are contained between the Visual and other modules, as well as between the DAN and other modules. Blue and red colors imply a negative and positive association with PTSD resilience, respectively. It is clear that an increase in temporal edge strength fluctuations in most edges leads to decrease in resilience and vice versa. However, a small number of connections within DMN and between DMN and other modules lead to increased resilience corresponding to higher fluctuations in edge strength and vice versa. AUD, auditory; CON, cingulo-opercular; DMB, default mode network; SAL, salience; SCOR, subcortical. Color images are available online.
Results
Findings in PTSD data analysis
Multimodal approach is more sensitive to dynamic network changes
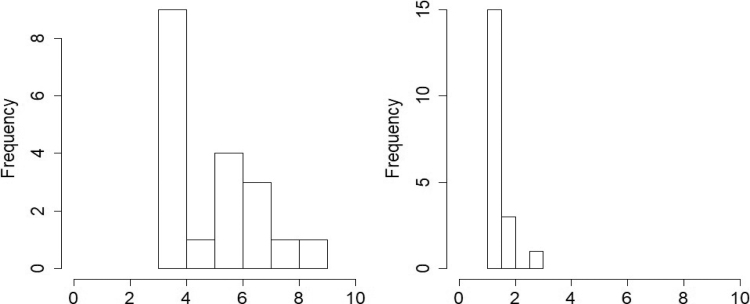
Figure 3 provides the histogram for the number of change points for the proposed approach and the SC naive version of the method. Our method detected 5 change points on average across all participants, with the number of change points ranging from 3 to 7. On the contrary, the SC naive version of the method registers only 1 change point for a large majority of participants, and only one subject has 3 change points. Given 146 brain volumes in the fMRI time series and recent findings that some brain networks may change within as little as 30–60 sec (Sagoklu et al., 2010; Shirer et al., 2012), the number of change points under the SC naive version seems to be unrealistic, whereas the number of change points under the proposed method appears more practical and supported by previous evidence. Our findings reveal that the brain network computed through the proposed multimodal FC method is much more sensitive to temporal fluctuations in the network than the SC naive version. Hence, incorporating brain SC information provides greater power to detect dynamic FC changes.
FIG. 3.
Histogram for the number of FC change points detected in the PTSD data analysis. The left and right panels depict the results under the proposed approach and under the SC naive version of the method. The multimodal dynamic FC approach seems to be more sensitive to network changes.
The edges with the largest temporal fluctuations, identified as those that consistently switch over the different state phases, are illustrated in Figures 4A–C. This figure depicts the proportion of times each edge flips (changes from present to absent state from one time point to the next, and vice versa) over the scanning session averaged over all individuals (Fig. 4A), and separately for the PTSD (Fig. 4B) and non-PTSD (Fig. 4C) cohorts. From Figure 4A, we observe that connectivity within and between the Visual, FPL, DAN, and VAN resting-state networks exhibits the highest number of temporal fluctuations. We also report those edges that exhibit the largest fluctuations in terms of edge strengths in Table 1, which is another way of assessing temporal fluctuations. This table indicates that these edges are concentrated in the superior parietal lobule, middle frontal gyrus, and the fusiform gyrus regions located in the DAN and VAN modules. Interestingly, the highest temporal fluctuations in connectivity occur between the different functional networks, whereas the temporal fluctuations within each functional module are largely limited. The least temporal variability was observed within and between Sensory, CON, AUD, and DMN resting-state networks, which implies stable connections. The Visual network had the largest share of edges with large temporal fluctuations that were concentrated in left occipital inf, left lingual, right inferior temporal gyrus, right cuneus, and left middle occipital lobes. These regions have been shown to be involved in upregulated perception of environmental stimuli that could be arousal mediated (Mueller-Pfeiffer, et al., 2013). The SAL and SCOR modules have previously demonstrated altered resting-state connectivity (Brown, et al., 2014; Rabinak, et al., 2011), and gray matter alterations were discovered in subcortical areas for PTSD individuals (O'Doherty, et al., 2017).
Table 1.
Edges with the Highest Variability in Their Edge Strengths (in Terms of Partial Correlations) Over the Duration of the Functional Magnetic Resonance Imaging Experiment
| Node 1 |
Node 2 |
|
||
|---|---|---|---|---|
| Index | Name | Index | Name | SD for edge strength |
| 258 | Superior parietal lobule | 263 | Superior parietal lobule | 0.03 |
| 258 | Superior parietal lobule | 264 | Middle frontal gyrus | 0.03 |
| 260 | Precuneus | 263 | Superior parietal lobule | 0.03 |
| 261 | Middle frontal gyrus | 263 | Superior parietal lobule | 0.03 |
| 261 | Middle frontal gyrus | 264 | Middle frontal gyrus | 0.03 |
| 262 | Fusiform gyrus | 263 | Superior parietal lobule | 0.03 |
| 262 | Fusiform gyrus | 264 | Middle frontal gyrus | 0.04 |
| 263 | Superior parietal lobule | 264 | Middle frontal gyrus | 0.05 |
We observe that most of these edges are concentrated in the superior parietal lobule, middle frontal gyrus, and the fusiform gyrus regions.
SD, standard deviation.
Do temporal network fluctuations drive disease severity?
To investigate this question, we first compared the temporal fluctuations for individuals with and without PTSD in terms of the frequency of state changes in the network in Figures 4A–C. Figure 4B–C clearly illustrate very limited differences in temporal variability patterns in individuals with and without PTSD, although individuals with PTSD tend to have slightly higher number of fluctuations in brain network state phases. In particular, only 213 edges (0.61% of all possible edges in the network) have significantly different frequency of state phase changes between the PTSD and non-PTSD groups at 5% level of significance after adjusting for family-wise error rate over all edges using Bonferroni corrections. Almost all of these edges correspond to a higher frequency of changes under the PTSD group. Overall, this part of our analysis points to the limited ability of the frequency of state phase changes to distinguish between the PTSD and non-PTSD groups. Hence, it is imperative to develop alternate measures of dynamic connectivity that provides a greater distinction with respect to disease severity.
To develop a connectivity measure that captures important associations between the continuous metric of disease severity and the dynamic network, we investigated the ability of temporal variability of edge-wise connectivity strengths (measured as the standard deviation of partial correlations) to predict resilience scores. Figure 4D illustrates those dynamic FC that have significant associations between temporal variability in connectivity strength and resilience. The significant edges were detected using univariate analysis at 5% level of significance and using multiplicity corrections for controlling family-wise error rate over all edges through Bonferroni corrections. The overwhelming number of edges between the Visual and Sensory functional modules had fluctuations in connectivity strength, which were significantly related to resilience. The temporal fluctuations of connectivity strengths between and within the Visual, FPL, VAN, and DAN modules are also often significantly associated with resilience. The functional modules with the smallest proportion of edges whose temporal variability was related to resilience include the DMN, SAL, and subcortical modules. We also listed a subset of edges with the strongest associations with resilience in Table 2. These edges appear to be concentrated in the middle gyral, lingual gyral, angular gyral, occipital gyral, and frontal gyral regions, along with the precuneus, anterior cingulate, and parietal lobe regions.
Table 2.
List of Edges with the Highest Association (Measured through R-Squared Values) between the Edge Strength Variability and Resilience
| Node 1 |
Node 2 |
|
||
|---|---|---|---|---|
| Index | Location | Index | Location | R-squared |
| 5 | Subgyral | 162 | Cuneus | 0.5938 |
| 37 | Sensory/somatomotor RSN | 154 | Middle occipital gyrus | 0.4999 |
| 64 | Insula | 231 | Subcortical RSN | 0.5664 |
| 69 | Postcentral gyrus | 229 | Subcortical RSN | 0.4983 |
| 85 | Inferior frontal gyrus | 212 | Anterior cingulate | 0.4847 |
| 96 | Angular gyrus | 158 | Lingual gyrus | 0.4641 |
| 96 | Angular gyrus | 173 | Middle occipital gyrus | 0.5189 |
| 122 | Anterior cingulate | 144 | Middle temporal gyrus | 0.4643 |
| 136 | Precuneus | 234 | Thalamus | 0.5429 |
| 143 | Parahippocampal gyrus | 213 | Cingulate gyrus | 0.5082 |
| 148 | Lingual gyrus | 206 | Salience RSN | 0.5968 |
| 158 | Lingual gyrus | 243 | Declive | 0.5209 |
| 161 | Middle occipital gyrus | 213 | Cingulate gyrus | 0.4944 |
| 163 | Precuneus | 235 | Inferior parietal lobule | 0.5436 |
| 172 | Middle occipital gyrus | 234 | Thalamus | 0.4968 |
| 174 | Middle frontal gyrus | 187 | Inferior frontal gyrus | 0.5645 |
| 177 | Inferior parietal lobule | 258 | Superior parietal lobule | 0.5821 |
| 199 | Inferior parietal lobule | 213 | Cingulate gyrus | 0.5802 |
| 208 | Salience RSN | 261 | Middle frontal gyrus | 0.5322 |
| 208 | Salience RSN | 264 | Middle frontal gyrus | 0.5058 |
| 217 | Anterior cingulate | 256 | Precuneus | 0.4515 |
| 217 | Anterior cingulate | 261 | Middle frontal gyrus | 0.4652 |
These edges seem to be concentrated in the middle gyral, lingual gyral, angular gyral, occipital gyral, and frontal gyral regions, along with the precuneus, anterior cingulate, and parietal lobe regions.
RSN, resting-state networks.
The overwhelming majority of the significant associations (96.1%) are negative, which means that increased temporal fluctuations for these connections lead to lower PTSD symptoms than predicted, relative to experiences of early adversity (reflecting greater resilience). A small number of positive associations between the temporal edge variability and resilience can be found within the DMN, and between DMN and the Visual module and FPL modules. Since the connections in DMN were shown to be relatively stable (Fig. 4A–C), significant positive associations imply lower resilience resulting from stable connections within DMN. On the contrary, the largely negative associations corresponding to edges with negligible temporal fluctuations in other brain regions (Fig. 4A–C) point to an increase in resilience due to more stable associations. Further, the largely negative associations corresponding to edges with increased temporal connectivity fluctuations within and between the Visual, FPL, VAN, and DAN modules, between the Visual–SAL modules, and the Visual–Subcortical modules, imply greater resilience due to increased dynamic connectivity.
Finally, we also report those network nodes where changes in the dynamic network in terms of nodal efficiency and nodal degree are most strongly associated with changes in resilience (Tables 3 and 4). The nodes whose degree is most strongly associated with resilience were largely situated in the ventral visual stream (bilateral inferior temporal gyri) and other nodes of the visual network, bilateral posterior parietal regions involved in attention regulation, and the right middle frontal gyrus. Further, those nodes whose local efficiency was most strongly associated with resilience were located in the temporal and parietal lobes confined to the VAN and DAN resting-state networks. Two nodes in the DAN resting-state module, one located in the superior parietal gyrus in the left cerebrum ([−32,−46,47]) and the other in the superior parietal lobule in the right cerebrum ([25,−58,60]), are shown to be strongly associated with resilience in terms of both the local degree and local efficiency in the dynamic network. These nodes imply promising findings in terms of gaining deeper understanding of the neurobiological basis of PTSD.
Table 3.
Nodes Whose Degree in the Dynamic Network Were Associated with Resilience Score as Indicated by R-Squared Values >0.25
| Node index | R-square | Resting-state network | MNI coordinate | Region |
|---|---|---|---|---|
| 250 | 0.49844 | Uncertain | (−50,−7,−39) | Inferior temporal gyrus |
| 259 | 0.351967 | DAN | (−32,−46,47) | Superior parietal gyrus |
| 232 | 0.349966 | Subcortical | (−31,−11,0.3) | Putamen |
| 236 | 0.346671 | VAN | (−56,−50,9) | Superior temporal gyrus |
| 243 | 0.321025 | Cerebellar | (−16,−65,−19) | Cerebellum—declive |
| 246 | 0.305447 | Cerebellar | (0.5,−61,−18) | Cerebellum—declive |
| 247 | 0.302259 | Uncertain | (32,−12,−34) | Parahippocampal gyrus |
| 258 | 0.29569 | DMN | (25,−58,60) | Superior parietal lobule |
| 249 | 0.285476 | Uncertain | (48,−2,−38) | Inferior temporal gyrus |
| 257 | 0.260607 | DAN | (46,−58,3) | Middle temporal gyrus |
| 264 | 0.255546 | DAN | (28,−4,53) | Middle frontal gyrus |
| 262 | 0.253069 | DAN | (−42,−60,−8) | Fusiform gyrus |
DAN, dorsal-attention network; MNI, Montreal Neurological Institute; VAN, ventral-attention network.
Table 4.
Nodes Whose Local Efficiency in the Dynamic Network is Strongly Associated with Resilience as Indicated by R-Squared Values >0.25
| Node index | R-square | Resting-state network | MNI coordinate | Region |
|---|---|---|---|---|
| 259 | 0.406118 | DAN | (−32,−46,47) | Superior parietal gyrus |
| 252 | 0.390547 | DAN | (−52,−63,5) | Middle temporal gyrus |
| 235 | 0.351281 | VAN | (53,−42,21) | Inferior parietal lobule |
| 258 | 0.282145 | DAN | (25,−58,60) | Superior parietal lobule |
| 234 | 0.255562 | Subcortical | (8,−3,5) | Thalamus |
All of these nodes are located in the right or left cerebrum, and concentrated in the temporal and parietal lobes. These nodes are primarily located in the VAN and DAN resting-state networks.
Dynamic networks provide higher predictive accuracy for trauma resilience
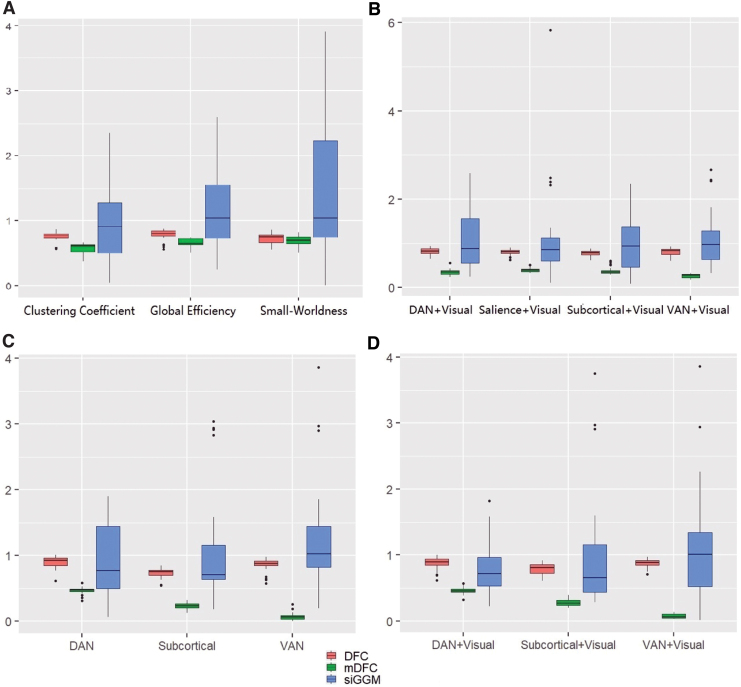
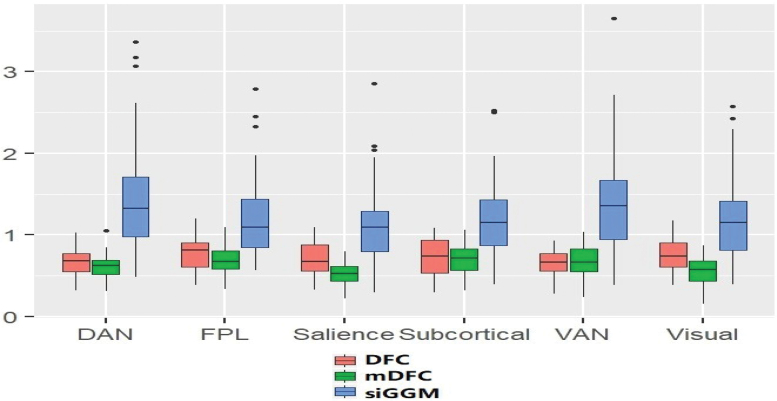
The results of our predictive analysis are reported through boxplots for out-of-sample MSE corresponding to the different network metrics are presented in Figure 5. An additional Figure 6 also demonstrates the predictive accuracy using small-worldedness derived from different localized functional modules.
FIG. 5.
Prediction performance in terms of MSE when using dynamic network metrics for predicting resilience score under mDFC, SC naive version of mDFC (denoted as DFC), and siGGM. The subplots indicate MSE values when using the following time-varying explanatory variables in scalar-on-function regression. (A) Global clustering coefficient and global efficiency. (B) Local clustering coefficient for DAN+VIS, SAL+VIS, SCOR+VIS, VAN+VIS functional modules. (C) Local efficiency in DAN, SCOR, and VAN functional modules. (D) Local efficiency in DAN+VIS, SCOR+VIS, VAN+VIS functional modules. MSE, mean squared error; VIS, visual. Color images are available online.
FIG. 6.
Prediction performance in terms of MSE when using small-worldedness derived from localized functional modules for predicting resilience scores. Results are reported for mDFC and the SC-naive version of the method (denoted as DFC), along with the siGGM approach that computes static networks. The results indicate that MSE values are lower or comparable under the mDFC method across all local functional modules, with significant improvements corresponding to the salience network. Color images are available online.
A multiplicity-adjusted permutation test revealed that the improvements in predictive accuracy (compared with the alternative network modeling methods) were significantly better under mDFC when using dynamic global efficiency and clustering coefficient using all the network nodes as well as localized resting-state networks with a subset of nodes (Fig. 5; Supplementary Table S1). The predictive performance under the global small-worldedness as well as corresponding to most localized modules was also improved compared with the existing approaches (Fig. 1). The highest predictive accuracy was obtained under the local efficiency corresponding to the VAN and VAN and Visual resting-state networks, which showcases the importance of these regions in characterizing PTSD.
We note that our findings go further than the results of Rowland et al. (2018) who illustrated differences between individuals with and without PTSD based on small-worldedness and global clustering coefficient derived from static networks. Our investigation not only reveals the importance of these metrics along with network efficiency under dynamic networks for modeling disease severity but also identifies more localized functional modules whose clustering coefficient and efficiency based on the dynamic network are related to resilience. Moreover, identical analyses as above but using 10% and 5% network densities revealed consistent gains in predictive accuracy, although some variations in relative performance were noticed when using global dynamic network features (results in Supplementary Data). Our secondary BDI-adjusted analysis also suggested significantly improved predictive performance for resilience under the proposed mDFC approach, compared with BDI-adjusted models that employ alternative dynamic network modeling methods. However, the gains in prediction accuracy decreased under the BDI-adjusted analysis compared with the BDI-unadjusted analysis, due to the fact that BDI is often significantly associated with PTSD symptoms, so that the predictive ability of BDI is likely to overshadow the contributions by the dynamic brain network. Hence, we chose to not include BDI in our primary analysis focused on discovering localized dynamic network features associated with PTSD.
Results from additional validation studies
In addition to the analysis of PTSD data, we performed extensive numerical studies using simulated data involving different network types and network dimensions, which are presented in full detail in the Supplementary Data. These studies clearly illustrated the prowess of the proposed multimodal approach in terms of near-perfect change point estimation, as well as accurate recovery of the true underlying network compared with existing methods.
Discussion
Our findings suggest that (1) estimating dynamic network connectivity models can be improved with the addition of DTI-based structural constraints; and (2) including metrics of dynamic change in resting networks will improve models for predicting psychiatric risk and resilience to trauma and stress. One possible explanation for the association between SC and network changes is that the dynamic FC can be considered as distinct manifestations of an underlying intrinsic network that is associated with the brain SC, in a manner that is similar to the association between static FC and SC. Additional work is needed to investigate the above conjecture; but if true, then this could potentially be a novel finding with considerable implications. Our PTSD data analysis discovers resting-state network alterations among participants exposed to varying degrees of trauma based on dynamic connectivity, and illustrates the direct link between temporal fluctuations of connections with PTSD resilience. The ability of small-worldedness, clustering coefficients, and efficiency computed from the dynamic network to accurately predict trauma resilience points to the potential of these dynamic network metrics as neuroimaging biomarkers in trauma resilience studies. Identifying biomarkers of risk and resilience for post-trauma psychopathology is critical for the development of targeted treatment approaches, particularly in the case of early interventions (Watkins et al., 2018). Localization of these effects to brain networks such as visual attention network, VAN, and DAN may have strong clinical interpretations in terms of PTSD diagnosis and treatment in a civilian, highly traumatized sample of African American women.
In addition, the two nodes that are strongly associated with resilience in terms of both nodal degree and nodal efficiency are located in the bilateral superior parietal nodes of the DAN, and are known to be involved in the regulation of attention. Theories of attention posit that DAN and VAN may compete or collaborate (Majerus et al., 2012; Vossel et al., 2014) in the allocation and direction of attentional resources, particularly in the case of visual attention. Our findings fit these theories, in that it is likely that DAN and VAN regulation of visual attention requires temporal shifts in the engagement of each network with visual cortex. Patients with PTSD show difficulties in disengaging spatial attention, with less connectivity between SAL network and DAN nodes during static rsfMRI (Block et al., 2017).
These findings build upon traditional neural circuit models of trauma-related psychopathology and resilience, which have focused primarily on individual differences in fear learning and extinction, supported by plasticity within an amygdala–mPFC–hippocampal circuit (Johnson, et al., 2012). Recent findings suggest that stress resilience also depends on the instantiation of fear memories throughout a broader network of regions, including the primary sensory cortex through which initial information about the trauma or threat was gathered (Ressler, 2020). Network-based resting-state FC findings have supported this idea in PTSD. For example, visual and sensorimotor networks were implicated in identifying subtypes of PTSD among war-exposed male military veterans (Maron-Katz, et al., 2019). Furthermore, Harnett et al. (2020) have shown that in the early weeks after trauma exposure, both the structure and static FC of the visual network predicts the emergence of later PTSD. We extend such findings by showing that, in our structure-guided dynamic connectivity analyses, greater temporal fluctuation in the visual network and its connections with the SAL, subcortical, and attention networks were associated with greater resilience in the context of a sample of female civilian trauma survivors with histories of multiple trauma spread over the life span, supporting the importance of sensory resting-state nodes to trauma-related pathology across multiple populations. The current findings may be consistent with an “overconsolidation” hypothesis (Yehuda and LeDoux, 2007), such that individuals at risk of PTSD or other forms of psychopathology after trauma may have very efficient communication between various aspects of the network supporting fear memory, and thus encode and stabilize a very strong fear memory when exposed to trauma. Given that currently, there is very little theoretical work to explain how network dynamics contribute to individual differences in behavior or psychiatric symptoms, our contributions in this work are important for future efforts in neural circuit modeling in psychiatry.
One limitation of the study (that is consistent with most brain network studies in the literature) is that the results are potentially sensitive to the brain network density used in the analysis. One way of choosing the network density involves a data-adaptive approach through some goodness-of-fit criteria such as Bayesian information criteria, which does not assume a predetermined network density level. While appealing, one disadvantage of this approach is that the results of our analysis are likely to be suboptimal when the data-adaptive network density varies widely across samples. Moreover, the predictive results of the scalar-on-function regression approach may not be comparable between different network estimation approaches if they report networks with very different network densities. Although our analysis does not require the network density to be similar across individuals, to ensure meaningful comparisons between competing methods, we prefixed the average density across all samples. The prefixed network density was set to ∼15% for all samples in our analysis, which is within the range of network densities encountered in practical neuroimaging applications (Higgins et al., 2018); however, we also investigated network densities of 10% and 5% as mentioned previously, with results included in the Supplementary Data. Another potential limitation is the smaller sample size of the study that was carefully chosen to include only younger participants so as to bypass any white matter integrity issues associated with aging. In future work, we plan to investigate how our findings generalize to a larger cohort of individuals with trauma exposure and/or PTSD diagnosis.
Conclusion
Notably, our study was novel within the field of neural circuit-based models of trauma resilience, because we study a high-risk but understudied population. Previous models of the neural correlates of resilience have often focused on populations of treatment-seeking patients with either depression or PTSD, whereas in this study we recruited broadly among a sample at high risk for trauma but without respect to any mental health diagnosis or complaint. We note that while the majority of resting-state network modeling in psychiatry has focused on static FC, our novel analyses that are highly sensitive to network dynamics allowed us to provide a model of the neural correlates of resilience with much lower error than a static connectivity-based comparison model (refer to Figs. 5 and 6).
Supplementary Material
Authors' Contributions
S.K. visualized the main ideas and statistical analysis, and wrote the article. J.M. wrote the code and implemented the numerical analysis. J.S. helped shape the interpretations of the analysis findings, helped write some parts of the article, and was responsible for GTP data collection.
Availability of Materials and Data
The deidentified data used for our analysis will be provided upon request at http://gradytraumaproject.com/ contact/ The code used to produce the results will be made available by the authors on reasonable request.
Ethics Statement
Study procedures were approved by the institutional review board of Emory University, and procedures were consistent with the Declaration of Helsinki.
Use of Experimental Animals and Human Participants
The article does not report experiments on live vertebrates and/or higher invertebrates, and only involves secondary analysis of deidentified data from ADNI.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
All participants provided written informed consent prior to participating. Participants received monetary compensation for their time. The Institutional Review Board of Emory University approved the study procedures, and testing took place at Grady Memorial Hospital and the Biomedical Imaging Technology Center at Emory University Hospital.
This study was funded by award number R01MH120299 from National Institutes of Mental Health.
Supplementary Material
The Supplementary Data contains the coordinates for the ROIs in the power system along with the modules they were classified into. It also contains additional plots for the data analysis and a description of the network metrics used in predictive analysis.
References
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodol) 57:289–300. [Google Scholar]
- Block SR, King AP, Sripada RK, et al. 2017. Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cogn Affect Behav Neurosci 17:422–436. [DOI] [PubMed] [Google Scholar]
- Brockhaus S, Ruegamer D, Stoecker A. 2018. FDboost-package: FDboost: Boosting Functional Regression Models. R Package; pp. 1–79. [Google Scholar]
- Brown VM, Labar KS, Haswell CC, et al. 2014. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes inposttraumatic stress disorder. Neuropsychopharmacology 39:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, et al. 2013. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, et al. 2013. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribben I, Wager T, Lindquist M. 2013. Detecting functional connectivity change points for single-subject fMRI data. Front Comput Neurosci 7:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Fu Z, Calhoun VD. 2018. Classification and prediction of brain disorders using functional connectivity: promising but challenging. Front Neurosci 12:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetti SA, Resnick HS, Resick PA, et al. 1993. The Modified PTSD Symptom Scale: a brief self-report measure of posttraumatic stress disorder. Behav Therap 16:161–162. [Google Scholar]
- Fu S, Ma X, Wu Y, et al. 2019. Altered local and large-scale dynamic functional connectivity variability in posttraumatic stress disorder: a resting-state fMRI study. Front Psychiatry 10:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, et al. 2009. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry 31:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, et al. 2009. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 24:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Stevens JS, Fani N, et al. 2020. Acute posttraumatic symptoms are associated with multimodal neuroimaging structural covariance patterns: a possible role for the neural substrates of visual processing in posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. DOI: 10.1016/j.bpsc.2020.07.019 [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam N. 2003. Categorical versus dimensional models of mental disorder: the taxometric evidence. Austr N Z J Psychiatry 37:696–704. [DOI] [PubMed] [Google Scholar]
- Higgins IA, Kundu S, Guo Y. 2018. Integrative Bayesian analysis of brain functional networks incorporating anatomical knowledge. Neuroimage 181:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. 2015. Medicine. Brain disorders? Precisely Sci 348:499–500. [DOI] [PubMed] [Google Scholar]
- Ioannidis K, Askelund AD, Kievit RA, et al. 2020. The complex neurobiology of resilient functioning after childhood maltreatment. BMC Med 18:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Hu X, Deshpande G. 2014. Behavioral relevance of the dynamics of the functional brain connectome. Brain Connect 4:741–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Jia H, Lanka P, et al. 2017. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp 38:4479–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, McGuire J, Lazarus R, et al. 2012. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology 62:638–646. [DOI] [PubMed] [Google Scholar]
- Kabbara A, Paban V, Weill A, et al. 2020. Brain network dynamics correlate with personality traits. Brain Connect 10:108–120. [DOI] [PubMed] [Google Scholar]
- Kim, YJ, van Rooij, SJH, Ely, TD, et al. 2019. Association between posttraumatic stress disorder severity and amygdala habituation to fearful stimuli. Depress Anxiety 36:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Ming J, Pierce J, et al. 2018. Estimating dynamic brain functional networks using multi-subject fMRI data. Neuroimage 183:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S, Attout L, D'Argembeau A, et al. 2012. Attention supports verbal short-term memory via competition between dorsal and ventral attention networks. Cerebral Cortex 22:1086–1097. [DOI] [PubMed] [Google Scholar]
- Maron-Katz A, Zhang Y, Narayan M, et al. 2020. Individual patterns of abnormality in resting-state functional connectivity reveal two data-driven PTSD subgroups. Am J Psychiatry 177:244–253. [DOI] [PubMed] [Google Scholar]
- Mueller-Pfeiffer C, Schick M, Schulte-Vels T, et al. 2013. Atypical visual processing in posttraumatic stress disorder. Neuroimage Clin 3:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty DC, Tickell A, Ryder W, et al. 2017. Frontal and subcortical grey matter reductions in PTSD. Psychiatry Res Neuroimag 266:1–9. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, et al. 2011. Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M., Welsh RC, et al. 2011. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Inpsychiatry 2:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay J, Silverman B. 2005. Principal components analysis for functional data. In: Functional Data Analysis. New York: Springer-Verlag; pp. 147–172. [Google Scholar]
- Ressler KJ. 2020. Translating across circuits and genetics toward progress in fear-and anxiety-related disorders. Am J Psychiatry 177:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JA, Stapleton-Kotloski JR, Dobbins DL, et al. 2018. Increased small-world network topology following deployment-acquired traumatic brain injury associated with the development of post-traumatic stress disorder. Brain Connect 8:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Sakoğlu Ü, Pearlson GD, Kiehl K, et al. 2010. A method for evaluating dynamic functional network connectivity and task- modulation: application to schizophrenia. Magnetic Resonance Materials in Physics, Biology and Medicine 2:351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, et al. 2012. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. 2013. The human connectome: origins and challenges. Neuroimage 80:53–61. [DOI] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, et al. 2013. Short-time windows of correlation between large-scale functional brain networks predict vigilance intra-individually and inter-individually. Hum Brain Mapp 34:3280–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D, Quinn AJ, Baker AP, et al. 2016. Spectrally resolved fast transient brain states in electrophysiological data. Neuroimage 126:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. 2014. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LE, Sprang KR, Rothbaum BO. 2018. Treating PTSD: a review of evidence-based psychotherapy interventions. Front Behav Neurosci 12:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA, Samuel DB. 2005. Diagnostic categories or dimensions? A question for the Diagnostic and statistical manual of mental disorders. J Abnormal Psychol 114:494. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. 2007. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56:19–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.