Abstract
Chlamydomonas reinhardtii can grow photosynthetically using CO2 or in the dark using acetate as the carbon source. In the light in air, the CO2 concentrating mechanism (CCM) of C. reinhardtii accumulates CO2, enhancing photosynthesis. A combination of carbonic anhydrases (CAs) and bicarbonate transporters in the CCM of C. reinhardtii increases the CO2 concentration at Ribulose 1,5-bisphosphate carboxylase oxygenase (Rubisco) in the chloroplast pyrenoid. Previously, CAs important to the CCM have been found in the periplasmic space, surrounding the pyrenoid and inside the thylakoid lumen. Two almost identical mitochondrial CAs, CAH4 and CAH5, are also highly expressed when the CCM is made, but their role in the CCM is not understood. Here, we adopted an RNAi approach to reduce the expression of CAH4 and CAH5 to study their possible physiological functions. RNAi mutants with low expression of CAH4 and CAH5 had impaired rates of photosynthesis under ambient levels of CO2 (0.04% CO2 [v/v] in air). These strains were not able to grow at very low CO2 (<0.02% CO2 [v/v] in air), and their ability to accumulate inorganic carbon (Ci = CO2 + ) was reduced. At low CO2 concentrations, the CCM is needed to both deliver Ci to Rubisco and to minimize the leak of CO2 generated by respiration and photorespiration. We hypothesize that CAH4 and CAH5 in the mitochondria convert the CO2 released from respiration and photorespiration as well as the CO2 leaked from the chloroplast to thus “recapturing” this potentially lost CO2.
Mitochondrial carbonic anhydrases CAH4 and CAH5 in Chlamydomonas reinhardtii are involved in maintaining optimal photosynthesis.
Introduction
Aquatic photosynthetic organisms face several challenges obtaining CO2 from the environment including slow diffusion of gases in water, pH fluctuations, and the slow interconversion of inorganic carbon (Ci) forms. Aquatic photosynthetic organisms have adapted to these changing conditions by developing a carbon dioxide concentrating mechanism (CCM). In Chlamydomonas reinhardtii (referred to as Chlamydomonas hereafter), the CCM occurs only when it is grown in a low CO2 environment (Moroney and Somanchi, 1999; Spalding, 2008). The CCM increases the CO2 around Ribulose 1,5-bisphosphate carboxylase oxygenase (Rubisco) enhancing its carboxylase activity (Moroney and Ynalvez, 2007). Oxygen competes with CO2 for the active site of Rubisco, hence the CCM favors the carboxylase activity of Rubisco at the same time as reducing the oxygenation process. In low CO2 conditions, Rubisco is packaged inside a structure called the pyrenoid, and is surrounded by a starch sheath (Ramazanov et al., 1994; Freeman Rosenzweig et al., 2017; Itakura et al., 2019). Chlamydomonas acclimates to low CO2 by maintaining a CCM, which includes proteins that aid in the delivery of Ci (Ci = CO2 + HCO3−) to Rubisco in the pyrenoid.
Two major components of the CCM are Ci transporters and carbonic anhydrases (CAs). The known Ci transporters are low CO2-induced 1 (LCI1), high-light activated 3 located on the plasma membrane, LCI A (LCIA) on the chloroplast envelope, and three bestrophin-like proteins (BST1, BST2, and BST3) on the thylakoid membrane (Ohnishi et al., 2010; Yamano et al., 2015; Mukherjee et al., 2019; Kono et al., 2020). These transporters increase the bicarbonate concentration inside the chloroplast relative to the external HCO3− concentration. Chlamydomonas has several CAs and some of these are important in the functioning of the CCM (Moroney et al., 2011). One of them is CA 3 (CAH3), an α-type CA located inside the thylakoid lumen (Karlsson et al., 1998). CAH3 is essential for maintaining high CO2 inside the chloroplast by converting HCO3− to CO2 in the acidic thylakoid lumen (Moroney and Ynalvez, 2007; Spalding, 2008). CAs 1 and 2 (CAH1 and CAH2) are two other α-type CAs that are present in the periplasmic space (Fujiwara et al., 1990; Rawat and Moroney, 1991). CAH1 is highly upregulated when Chlamydomonas is grown in low CO2, as are all the Ci transporters. In addition, the expression of CAH1 and the Ci transporters require the transcription activator Ci accumulation deficient 5 (CIA5), as cells with mutations in CIA5 fail to express these proteins. The CCM components are under regulation by CIA5 and they show high expression in low CO2 conditions (Moroney et al., 1989; Fang et al., 2012).
CAs 4 and 5 (CAH4 and CAH5) were the first β-type CAs to be discovered in Chlamydomonas and they are present in mitochondria (Eriksson et al., 1995). CAH4 and CAH5 are almost identical, and the genes encoding these proteins are present as an inverted repeat on chromosome 5 (Eriksson et al., 1996). Since CAH4 and CAH5 are nearly identical, they will be referred to a CAH4/5 for the remainder of this manuscript. CAH4/5 are among the most highly upregulated genes when Chlamydomonas is grown in low CO2 conditions (Fang et al., 2012). An immunogold labeling experiment confirmed that CAH4/5 are present in mitochondria in cells grown under low CO2 conditions and are undetectable in high CO2 conditions (Moroney et al., 2011). The expression pattern of CAH4/5 strongly resembles that of a CCM component, which is surprising since they are in the mitochondria.
The physiological role of CAH4/5 is still not clear although a number of hypotheses have been proposed about their function in Chlamydomonas. One suggested role of CAH4/5 is to maintain the pH of the mitochondrial matrix by generating H+ ions during hydration of CO2. These H+ ions would balance the pH change caused by the production of NH3 by glycine decarboxylation (Eriksson et al., 1998). Another idea by Raven (2001) proposes CAH4/5 is needed to retain CO2 generated by the mitochondria. In another study, CAH4/5 was hypothesized to be required for anaplerotic reactions (Giordano et al., 2003). Giordano et al. (2003) showed that the levels of CAH4/5 decrease with increasing ammonium concentration in growth media as HCO3− produced by CAH4/5 is used by PEP carboxylase for assimilation. However, until now, no mutant for CAH4/5 protein has been reported, so there is little experimental evidence testing these hypotheses.
In this report, we sought to elucidate the function of CAH4/5 in Chlamydomonas by using an RNAi strategy where we screened for mutants exhibiting low mRNA and protein expression levels of CAH4/5. Here, we describe the growth and physiological properties of cah4/5 RNAi mutants under a variety of autotrophic conditions. We found that wild-type (WT) levels of expression of CAH4/5 are required to maintain optimal rates of photoautotrophic growth on ambient levels of CO2.
Results
CAH4/5 expression is strongly affected by CO2 levels and is under the control of CIA5
CAH4/5 expression was measured in photoautotrophically grown cells using a CAH4/5 specific antibody (Figure 1). In the light, CAH4/5 was nearly absent in cells maintained on high CO2 (5% CO2 [v/v] in air) conditions but was strongly expressed when cells were transferred to ambient CO2 (0.04% CO2 [v/v] in air; Figure 1). The results agree with early reports by Eriksson et al. (1998) and Giordano et al. (2003). In addition, CAH4/5 is under the control of CIA5 (Figure 2). CAH4/5 expression was not visible in cia5 cells in light conditions at ambient CO2 or high CO2 concentrations (Figure 2). Thus, the expression of CAH4/5 closely matches the expression of proteins involved in the CCM.
Figure 1.
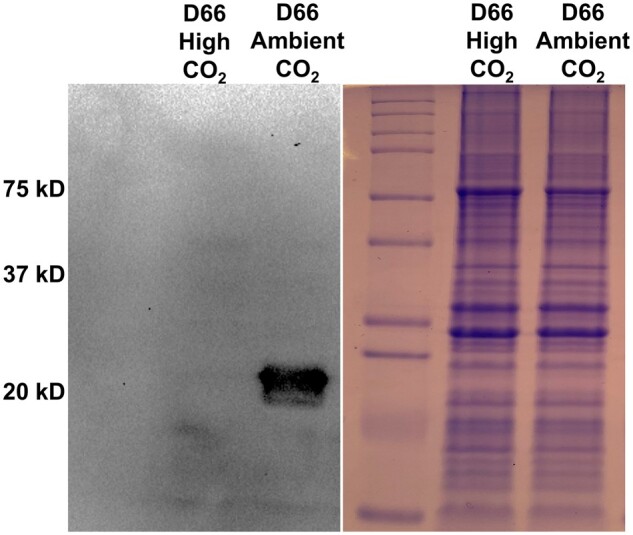
CAH4/5 protein levels are strongly affected by CO2 levels. The left panel is an immunoblot probed with anti-CAH4 antibodies showing the CAH4/5 levels in D66 grown on minimal media in light at high CO2 or ambient CO2. Cells were grown in MIN media for 72 h in high CO2 conditions before incubating them under the respective CO2 conditions. The right panel is an SDS–PAGE gel of the same samples stained with Coomassie Blue.
Figure 2.
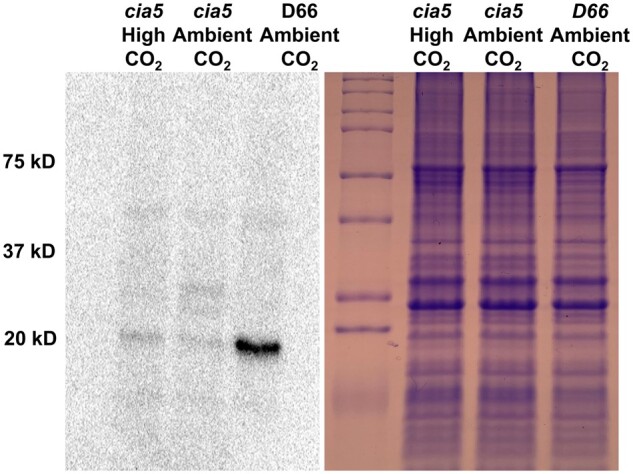
CAH4/5 protein expression is under the control of CIA5. The left panel is an immunoblot probed with anti-CAH4 antibodies showing CAH4/5 levels in cia5 grown on MIN media in light at high CO2 or ambient CO2 compared to D66 grown at ambient CO2. Cells were grown in MIN media for 72 h in high CO2 conditions before incubating them under the respective CO2 conditions. The right panel is an SDS–PAGE gel of the same samples stained with Coomassie Blue.
Identification of cah4/5 RNAi lines with reduced levels of CAH4/5
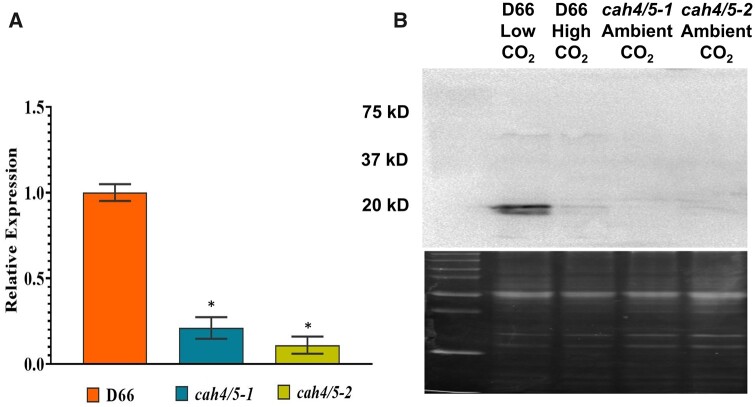
CAH4 and CAH5 proteins differ by only one amino acid (Supplemental Figure S1; Eriksson et al. 1995), so they likely have similar functions in vivo. Therefore, the expression of both genes was reduced to help elucidate their physiological functions. To this end, an RNAi construct that targets a common region of the CAH4 and CAH5 genes was designed (Supplemental Table S1). This construct was used to transform strain D66 (the WT or WT strain), and transformants were selected on paromomycin and kept at high CO2 levels. The resultant transformants were then screened by growing them on both high and very low CO2 (<0.02% CO2 [v/v] in air). Colonies that grew well on high CO2 but poorly on very low CO2 were selected, and their expression of CAH4/5 was examined. Two independent colonies were selected that showed reduced expression of CAH4/5 transcript and protein. These strains were designated as cah4/5-1 and cah4/5-2. Figure 3A shows the CAH4/5 mRNA levels in the two cah4/5 RNAi strains as well as WT cells after cells were switched from 5% CO2 in air to ambient CO2 levels for 12 h. Both RNAi strains had less CAH4/5 mRNA than WT cells but cah4/5-2 consistently had less mRNA expression than cah4/5-1 (Figure 3A). Immunoblot analysis using polyclonal antibodies raised against the CAH4/5 protein from Chlamydomonas showed a corresponding decrease in CAH4/5 proteins as compared to the WT D66 (Figure 3B) with both strains having less than 20% of the WT amount of CAH4/5. In WT cells, the CAH4/5 protein is highly expressed when cells are grown in ambient CO2 conditions but this expression is reduced drastically in high CO2 conditions (Figure 3B, lanes 1 and 2). Neither RNAi strain had elevated levels of CAH4/5 even when grown in ambient CO2. In agreement with the mRNA results, the amount of CAH4/5 protein was reduced in both RNAi lines (Figure 3B).
Figure 3.
Relative mRNA and protein expression of CAH4/5 in D66 and the knockdown mutants. A, RT-qPCR shows expression of CAH4/5 genes in knockdown lines cah4/5-1 and cah4/5-2 and in D66. Cells were grown in MIN media for 48 h in high CO2 conditions before transferring them to ambient CO2 for 12 h before harvesting for RNA. Error bars represent standard deviation from three biological replicates. Transcript levels were calculated using 2−ΔΔCT relative to the reference gene CBLP and reported relative to the corresponding WT D66 cells. The asterisk indicates the value is significantly different from the control (*P < 0.05 by Student’s t test). B, Western blot showing protein levels of CAH4/5 in knockdown lines cah4/5-1 and cah4/5-2 and D66 grown in ambient CO2 (0.04% CO2 (v/v) in air. Cells were initially grown in MIN media in the light for 48 h in high CO2 conditions before incubating them for 12 h at ambient CO2. The top panel is an immunoblot using an antibody raised against CAH4; the bottom panel is SDS–PAGE of the samples stained with Coomassie Blue.
Growth analysis of CAH4/5 RNAi mutants
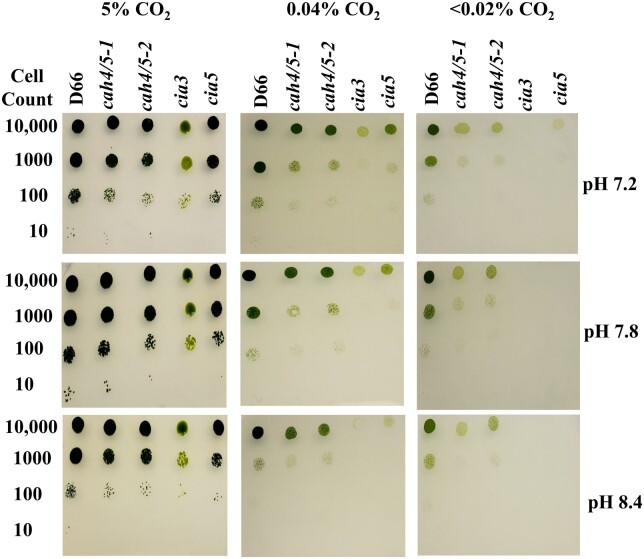
The growth of cah4/5-1 and cah4/5-2 was tested on high, ambient, and very low CO2 at different pH levels. For this experiment, the strains cia3 and cia5 were used as controls. The first control, cia3, is a knockout of CAH3, which is responsible for encoding the thylakoid lumen CA. The second control, cia5, is a knockout of gene CIA5, which is a transcription factor controlling the expression of many CCM genes (Moroney et al., 1989; Fukuzawa et al., 2001; Xiang et al., 2001). CAH4/5 mutants showed reduced growth at all three pH levels tested (7.2, 7.8, 8.4) in ambient and very low CO2 conditions (Figure 4A). The poor growth phenotype was more severe at pH 7.8 and 8.4 and worse under very low CO2 conditions (Figure 4A). However, at high CO2, the growth of the cah4/5 RNAi mutants was similar to the WT at all pH. Therefore, the cah4/5 RNAi lines show a classic CCM phenotype, growing well on high CO2 but poorly under ambient or very low CO2 concentrations. In addition, growth was worse at high pH where most of the Ci is in the form of HCO3−. Therefore, CAH4/5 is required for optimal photoautotrophic growth of Chlamydomonas under ambient or very low CO2 conditions.
Figure 4.
Growth of mutants cah4/5-1 and cah4/5-2 at different CO2 levels. Growth analysis showing D66, cah4/5-1, cah4/5-2, cia3, and cia5. Cells were diluted to 6.6 × 106 cells mL−1, followed by 1:10 serial dilution three times at very low CO2, ambient CO2, and high CO2 at pH 7.2, pH 7.8, and pH 8.4, respectively. Cells were grown for 6 d. The cia3 and cia5 mutants were included as a CCM-deficient control. Cells were initially grown in TAP media at ambient CO2 in the light before spotting them onto plates.
To confirm the reduction of CAH4/5 is the reason for the phenotype, a reverse transcription quantitative polymerase chain reaction (RT-qPCR) experiment was performed to determine whether the expression of other CCM genes was affected by the reduction in the CAH4/5 transcript. All the CCM genes in the RNAi strains were expressed at levels equal to or slightly greater than seen in WT cells (Supplemental Figure S2). As an additional confirmation that the reduction in the CAH4/5 transcripts was the cause of the poor growth phenotype, CAH5 was transformed back into the cah4/5-2 RNAi line. The complementation of cah4/5-2 was achieved by expressing CAH5 CDS (804 bp) without including 5′ UTR and 3′ UTR in the invitrogen pChlamy _4 vector under control of Hsp70A-Rbc S2 hybrid promoter. Results of growth experiments show that the WT phenotype in low CO2 conditions was restored in complemented lines (referred to as com1, com2, and com 3; Supplemental Figure S3).
Reduction in CAH4/5 expression decreases the cells’ affinity for Ci
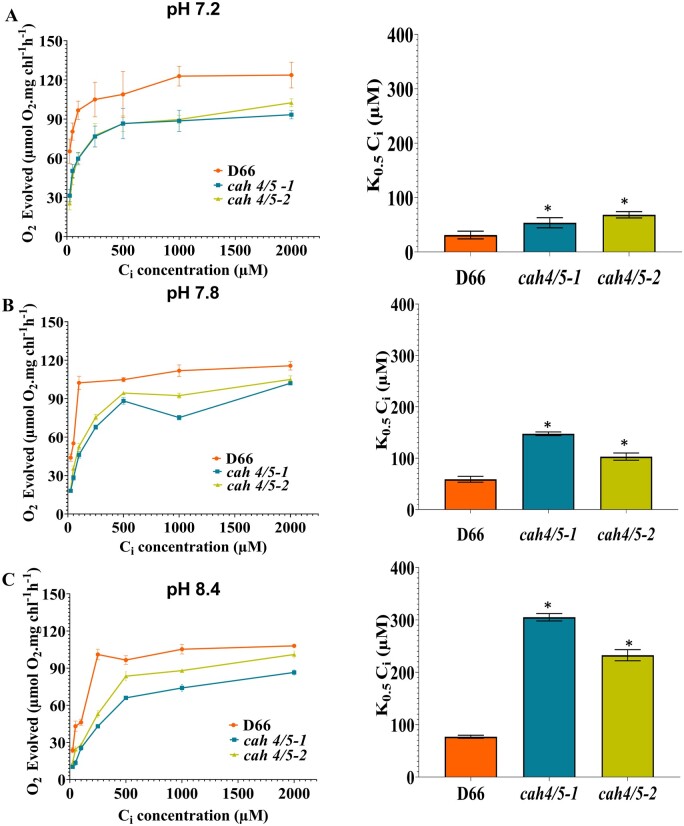
The ability of algal cells to accumulate Ci increases when the CCM is induced and cells with an active CCM have very high affinities for Ci. The CCM allows cells to accumulate Ci to higher levels than that can be attained by diffusion. Both cah4/5-1 and cah4/5-2 show a severe reduction in Ci affinity at pH 7.8 and 8.4 when grown under low CO2 conditions (Figure 5). The higher K0.5(Ci) observed in the mutants indicates they have a lower Ci affinity as compared to the WT, D66. In addition, the reduced affinity for Ci, as indicated by the higher K0.5(Ci), becomes more severe at higher pH as shown in Figure 5, B and C.
Figure 5.
Photosynthetic oxygen evolution of the cah4/5 knockdown RNAi lines and D66. Ci affinity and K0.5(Ci) were estimated for cah4/5-1, cah4/5-2, and D66 acclimated to ambient CO2 for 12 h at (A) pH 7.2, (B) pH 7.8, and (C) pH 8.4. K0.5 (Ci) values (Ci concentration needed for half maximum oxygen evolution) were calculated from the O2 evolution versus Ci curves. Asterisk indicates the value is significantly different from the control (*P < 0.05 by Student’s t test). Cells were grown in MIN media for 48 h in high CO2 conditions before incubating them for 12 h at ambient CO2 at the indicated pH. Each point in O2 evolution versus Ci curves represents the mean and sd of three technical replicates from a representative experiment. Error bars in K0.5 (Ci) values indicate sd.
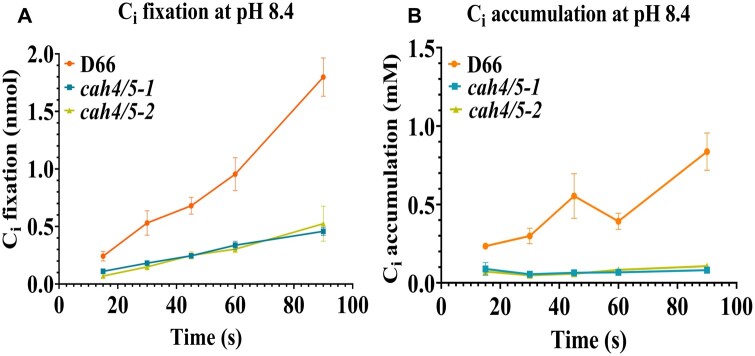
At pH 8.4, the K0.5(Ci) for cah4/5-1 is 300 μM, and cah4/5-2 is 225 μM, as compared to a K0.5(Ci) of 75 μM in D66. Similarly, the K0.5(Ci) of cah4/5-1, cah4/5-2 and D66 at pH 7.8 are 150, 98, and 55 μM, respectively. These data, like the growth data, show that the cah4/5 RNAi strains have a reduced ability to use Ci especially at higher pH.
RNAi silenced strains exhibit reduced Ci accumulation
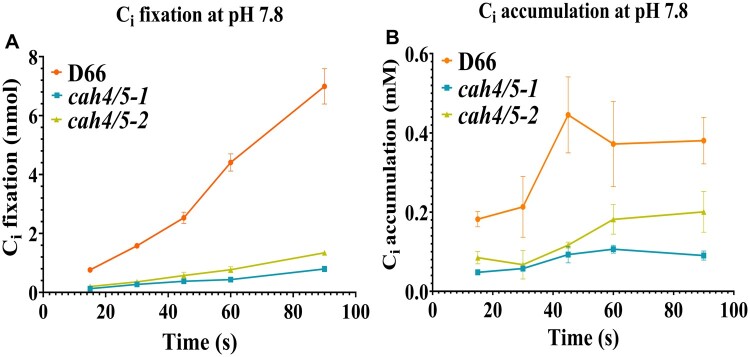
Ci accumulation was measured in WT (D66) and the cah4/5 RNAi strains at low Ci conditions to assess the importance of CAH4 and CAH5 in accumulation and fixation of Ci. At pH 7.8, there is a substanstial decrease in Ci fixation and Ci accumulation for the cah4/5 mutants as compared to D66 (Figure 6, A and B). Ci accumulation and fixation is further reduced in both the RNAi lines when pH is increased from 7.8 to 8.4 (Figure 7, A and B). The difference in accumulation was visible from earliest time point, that is, 15 s up to and including 90 s. In both mutants, Ci accumulation is only 30%–40% of the levels observed in WT D66 cells. This clearly indicates that CAH4 and CAH5 in the mitochondria play an important role in Ci accumulation and retention in ambient CO2 conditions in Chlamydomonas.
Figure 6.
Ci uptake of D66 and cah4/5 RNAi knockdown lines at pH 7.8. The silicone oil method was used to estimate Ci fixation and Ci accumulation (see “Materials and methods”). Cells were grown in elevated CO2 and then acclimated to ambient CO2 for 12 h prior to the assays. Cells were depleted of endogenous Ci in a 6-mL chamber with a Clark electrode before performing the assays. Time courses of CO2 fixation (A) and Ci accumulation (B) are shown at pH 7.8. Each point represents the mean and sd of three technical replicates from a representative experiment.
Figure 7.
Ci uptake of D66 and cah4/5 RNAi knockdown lines at pH 8.4. The silicone oil method was used to estimate Ci fixation and Ci accumulation (see “Materials and methods” section). Cells were grown in elevated CO2 and then acclimated to ambient CO2 for 12 h prior to the assays. Cells were depleted of endogenous Ci in a 6-mL chamber with a Clark electrode before running the assays. Time courses of CO2 fixation (A) and Ci accumulation (B) are shown at pH 8.4. Each point represents the mean and SD of three technical replicates from a representative experiment.
Mitochondria and CAH4/5 are located to the cell periphery in cells growing under low CO2 conditions
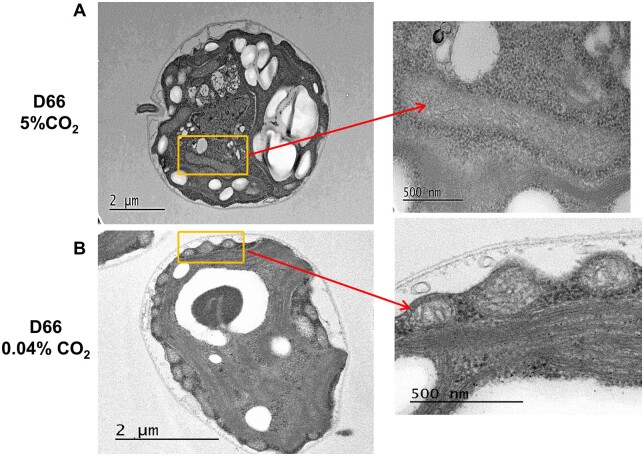
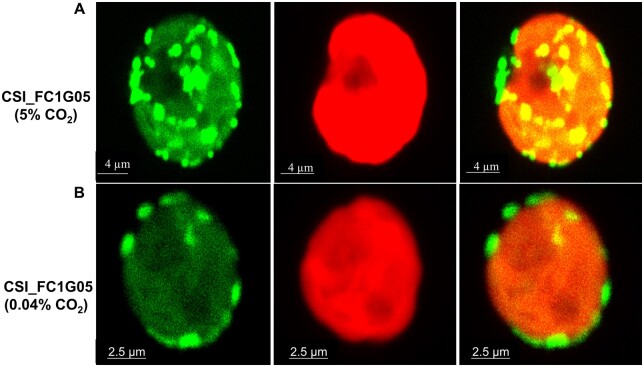
When observed using transmission electron microscopy (TEM) the number and localization of Chlamydomonas mitochondria varies with the cells growth conditions (Geraghty and Spalding, 1996). When grown in high CO2, D66 cells had fewer mitochondria as compared to D66 cells grown in ambient CO2 (Figure 8, A and B). However, the cross sections of the mitochondria seen in cells growing at high CO2 were larger than those observed in cells grown under ambient CO2 (Figure 8B). In addition, the distribution of the mitochondria was different. In cells grown in high CO2, the mitochondria were distributed throughout the cells (Figure 8A). However, in cells grown under ambient CO2, the mitochondria can be clearly seen distributed near plasma membrane (Figure 8B). These observations are similar to those reported by Geraghty and Spalding (1996). This observation can also be seen in Supplemental Figure S4, where TEM images of multiple cells are shown. The mitochondrial location was confirmed using cells containing CAH5 tagged with Venus. In those cells expressing the CAH5-venus protein, in high CO2 grown cells CAH5 is localized mostly in the center of the cell, whereas in all cells acclimated to ambient CO2, CAH5 was localized to the periphery of the cell (Figure 9; Supplemental Figure S5).
Figure 8.
Mitochondrial localization in WT D66 cells with change in CO2 levels. TEM of sectioned Chlamydomonas WT cells at (A) high CO2 and (B) ambient CO2 levels. WT cells were grown in MIN media for 48 h in high CO2 before incubating them for 12 h at their respective conditions. Areas shown by the rectangles are enlarged (right) to reveal mitochondrial structures. Scale bar, 2 µm (A), 2 µm (B), and 500 nm (enlargements).
Figure 9.
CAH5 protein localization in WT cells with changes depending on the CO2 levels during growth. Confocal images were taken of CSI_FC1G05 cells expressing pLM005-CAH5-Venus-3xFLAG at (A) high CO2 and (B) ambient CO2 levels. WT cells were grown in MIN media for 48 h in high CO2 before incubating them for 12 h at their respective conditions. Scale bar, 4.2 µm (A), 2.5 µm (B).
Discussion
CAH4 and CAH5 were initially discovered in Chlamydomonas in 1996 (Eriksson et al, 1996). The genes encoding CAH4 and CAH5 are arranged as an inverted repeat on chromosome 5 and are controlled by a single promoter (Eriksson et al., 1996). The expression of CAH4/5 increases dramatically when cells are shifted from 5% CO2 conditions to ambient levels of CO2 or lower in the light (Eriksson et al., 1998; Giordano et al., 2003; Fang et al., 2012). Immunogold labeling and Venus tagging experiments both show that CAH4/5 is localized to the mitochondrial matrix (Geraghty and Spalding, 1996; Moroney et al., 2011; Tirumani et al., 2014; Mackinder et al., 2017). This report presents evidence that CAH4 and CAH5 are required for autotrophic growth in the light when the CO2 level is low. Since CAH4 and CAH5 have almost identical open reading frames, we used an RNAi approach to target both genes simultaneously. A similar approach was recently used to reduce the expression of three similar bestrophin genes in Chlamydomonas (Mukherjee et al., 2019). The RNAi construct successfully reduced the amount of CAH4/5 mRNA (Figure 3A). The reduction of CAH4/5 mRNA expression in the RNAi mutants was mirrored by a similar reduction in protein abundance as estimated by immunoblots (Figure 3B). The expression of other CCM genes was also tested and they were expressed in the RNAi strains at levels equal to or slightly higher than in the WT D66 (Supplemental Figure S2). In complementation experiments, the expression of the CAH5 coding region from the WT gene was able to restore the normal growth phenotype (Supplemental Figure S3). These results suggest that the RNAi construct is specifically affecting the transcript abundance of CAH4 and CAH5.
The cah4/5 RNAi silenced strains exhibited reduced rates of photosynthesis at low concentrations of dissolved Ci (DIC), and over a range of pH levels (Figure 5). The RNAi strains also exhibited slower growth under ambient CO2 and very low CO2 when compared to WT cells (Figure 4). On the other hand, the RNAi strains grew at a rate similar to the WT parent in high CO2 conditions (Figure 4). It is likely that at high CO2 levels there is no need for a CCM in C. reinhardtii cells.
The slow growth observed in low CO2 (400 ppm CO2 or lower; Figure 4) is likely due to the reduced ability of the RNAi mutants to accumulate and retain Ci. The RNAi lines had a lower apparent affinity for Ci (Figure 5) and a reduced ability to accumulate added 14Ci (Figures 6 and 7). Algae with a CCM must not only be able to accumulate Ci for photosynthesis, but they also must reduce CO2 leakage. An important aspect of preventing the leakage is the pKa of the interconversion of HCO3− and CO2, which is about 6.4. CO2 is thought to be able to readily cross cell membranes while HCO3− is about 100-fold slower in crossing membranes (Gutknecht et al., 1977; Tolleter et al., 2017). The chloroplast stroma has a pH close to 8 in the light while the thylakoid lumen has a pH close to 5.5. The acidic environment inside the thylakoid lumen favors the conversion of HCO3− to CO2, a reaction catalyzed by the luminal CA, CAH3. This increases the CO2 concentration around Rubisco, but the resulting high CO2 concentration also leads to CO2 leakage problems (Karlsson et al., 1998; Moroney and Ynalvez, 2007; Spalding, 2008). The CO2 gradient from inside the pyrenoid to the outside of the cell could lead to a significant loss of CO2 by diffusion (Raven, 2001). The LCIB/C complex surrounding the pyrenoid is an important component of Chlamydomonas CCM. It has been proposed that this complex may reduce CO2 leakage from the pyrenoid by converting that CO2 to HCO3− (Yamano et al., 2010; Wang et al., 2015; Mackinder et al., 2017). By converting the CO2 to HCO3−, a reaction favored by the stromal pH, the loss of Ci is less severe since HCO3− crosses membranes more slowly.
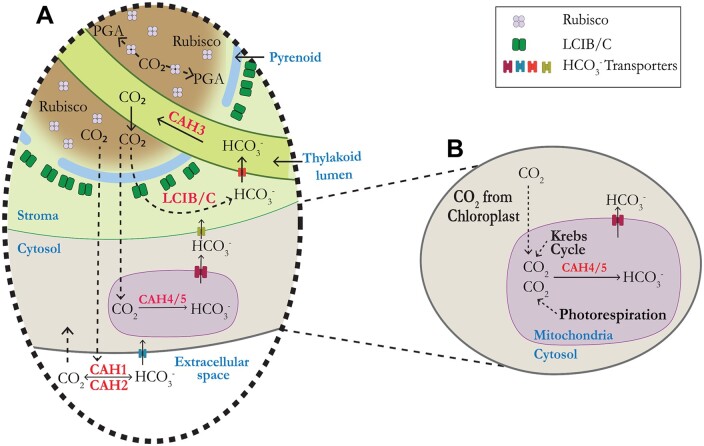
We propose that CAH4 and CAH5 can reduce CO2 leakage from the cell in two ways. First, the mitochondria produce CO2 through respiration and photorespiration. It has been estimated that mitochondrial respiration in the light is about 10% the rate of CO2 fixation (Raven, 1984). In addition, even though the rate of photorespiration is reduced by the CCM, it has been measured to be between 2% and 5% of the rate of CO2 assimilation (Moroney et al., 1986). The CO2 produced by these processes has the potential to be lost from the cell, and cells acclimated to low CO2 cannot afford to lose this carbon. We propose that CAH4/5 is produced at high levels when Chlamydomonas is grown in the light on low CO2 to reduce CO2 leakage. As shown in Figure 10, the CO2 generated in the mitochondria matrix is converted to HCO3− by CAH4/5, and using a possible bicarbonate transporter (Pollock et al., 2004) the HCO3− is brought back to the cytosol. Since no CA has been found in the cytoplasm, the rate of conversion of this HCO3− back to CO2 in the cytosol should be minimal. Thus, the HCO3− can be then transported back to the thylakoid lumen using bicarbonate transporters present on the chloroplast inner membrane and thylakoid membrane (Mukherjee et al., 2019; Figure 10). It is also likely that some of the bicarbonate generated in the mitochondria is used in the anaplerotic reactions (Giordano et al., 2003).
Figure 10.
Model showing the proposed physiological role of mitochondrial CAH4/5 in the CCM of Chlamydomonas. In (A) known CAs (CAH1, CAH2, CAH3, and LCIB) are indicated in the periplasmic space, the chloroplast stroma, and the thylakoid lumen, respectively. Dotted lines indicate the leakage of CO2 from the pyrenoid and how it is recaptured by different layers of CAs. PGA, phosphoglyceric acid. B, The proposed recapturing of CO2 in mitochondria. The CO2 leakage arises from the chloroplast, mitochondrial respiration, and photorespiration.
A second way CAH4/5 can help in the accumulation of Ci is by helping LCIB/C in recapturing CO2 leaking from the pyrenoid. The positioning of the mitochondria to the periphery of the cell (Figures 8 and 9;Tirumani et al., 2014), allows CAH4/5 to become a second layer of CA CO2 must cross before exiting the cell.
As suggested by Geraghty and Spalding (1996), the positioning of the mitochondria may also be important in intercepting glycolate coming from the chloroplast. Chlamydomonas cells grown under 5% CO2 in air will excrete large amounts of glycolate into the medium (Hess and Tolbert, 1967) if placed in a low CO2 environment in the light. They will continue to excrete glycolate until the CCM is induced in about 4 h. In addition to upregulating the expression of photorespiratory genes (Chen et al., 1996; Tural and Moroney, 2005) the repositioning of the mitochondria would favor the recapture of glycolate with CAH4/5 converting the CO2 released by glycine decarboxylase to HCO3−. Thus, the upregulation of CAH4/5 in conjunction with repositioning of mitochondria helps in retaining the CO2 generated by the mitochondria as well as helps LCIB/C reduce CO2 leakage from the chloroplast.
Materials and methods
Cell culture and growth
Chlamydomonas reinhardtii culture conditions were similar to those described previously (Ma et al., 2011). The D66 strain (nit2−, cw15, mt+) was obtained from the Chlamydomonas Resource Center (https://www.chlamycollection.org;Zhang et al., 2014). Tris-Acetate-Phosphate (TAP) and Minimal (MIN) media (acetate free) were prepared according to Sueoka (1960). TAP and MIN plates for growth included 1.5% (w/v) agar. The colonies from TAP plate are used to start a mixotrophic culture using 100-mL TAP liquid media. The cells were cultured in TAP media for 48 h at ambient CO2 (0.04% CO2 [v/v] in air) under continuous light illumination of 100 µE m−2 s−1. Cells in early log phase were washed and harvested in MIN media and bubbled with high CO2 (5% CO2 [v/v] in air) for 48 h. The CCM was induced by transferring the cells to low CO2 0.04% CO2 (v/v) in air) bubbling for 12 h. High CO2 was generated by mixing CO2 with air such that final CO2 concentration was (5% CO2 [v/v] in air). For ambient CO2 levels building air measured at (0.04% CO2 [v/v] in air) was used. For very low CO2 ambient air was mixed with the CO2-free air to bring the CO2 level to less than 0.02% v/v CO2.
Generation of RNAi construct and algal transformations
Artificial microRNA constructs for the knockdown of the CAH4 and CAH5 proteins were made using the RNAi protocol of (Molnar et al., 2009). The target sequences aligning to the “common region” of CAH4 and CAH5 were designed using Web MicroRNA Designer website (http://wmd3.weigelworld.org/cgi-bin/webapp.cgi). The Web Micro RNA designer suggests suitable amiRNA based on optimal hybridization properties and it avoids the off-targets to other genes in Chlamydomonas genome. No obvious off targets were identified by the program and BlastN search of the Chlamydomonas genome indicated no other matches to the region of the RNAi target besides these two CA genes. The pChlamyRNA3int plasmid was obtained from the Chlamydomonas Resource Center and the oligos were annealed to it using the SpeI restriction site. Supplemental Table S1 details the oligos used for the miRNA construct. For creation of the RNAi strains, D66 cells were first grown in TAP in the light. When the cells reached an OD730 between 0.2 and 0.3 (∼2–3 × 106 cells mL−1) they were washed and resuspended in GeneArt MAX Efficiency Transformation Reagent for Algae at a density of ∼2–3 × 106 cells mL−1. For electroporation, 2 µg of pChlamyRNA3int plasmid was added to 250 µL of the D66 resuspended cells in an electroporation cuvette (0.4-cm gap width, BioRad). The protocol from Invitrogen was followed (https://www.thermofisher.com/order/catalog/product/A24229#/A24229).
Screening of RNAi mutants
TAP plates containing antibiotic paromomycin (4 µg mL−1, Invitrogen) were used for selecting transformed colonies and TAP paromomycin plates were maintained in high CO2. Around 600 paromomycin resistant transformed colonies were selected by replica plating on MIN media plates and screening them in a high CO2 chamber and a very low CO2 chamber with continuous illumination (100 µmol photons m−2·s−1) for 4 d. The colonies growing poorly on very low CO2 were selected for spot tests. D66 and cia3 (Moroney et al., 1985) were the control strains used for the initial screening. Selected colonies for spot test were grown to log phase in TAP media and then resuspended in MIN media to a final concentration of 6.6 × 105 cells mL−1. Spot tests were done by spotting 15 µL of sample (10,000 cells) followed by three 1:10 serial dilutions. These MIN media plates were screened by keeping them in high, ambient and very low CO2 chambers under continuous illumination at 100 µmol photons m−2 s−1 for 7 d. The level of CO2 was monitored using an Environmental Gas Monitor (EGM-4, PP systems, Massachusetts, USA).
Gene expression and immunoblot analysis
Trizol reagent was used to extract RNA using a protocol from Invitrogen. ProtoScript First Strand cDNA Synthesis Kit (NEB) was used to make cDNA using 1 µg RNA per sample. As per manufacturer’s instructions (Luna Universal One-Step RT-qPCR Kit) 100 ng RNA per sample was used to perform RT-qPCR using QuantStudio 6. G protein beta subunit-like polypeptide (CBLP) was used as a reference gene for all RT-qPCR. A list of primers is provided in Supplemental Table S1. For the immunoblot analysis, cell cultures were grown for 72 h in high CO2 conditions in MIN media and then incubated at ambient CO2 (∼0.04% CO2) for 12 h. Cells were collected by centrifugation and washed with 25-mM Hepes (pH 7.2). Samples were then normalized to 10-µg protein·µL-1 for each sample. Five micrograms of protein was boiled with 2X Laemmli sample buffer and β-mercaptoethanol for 10 min at 80°C prior to analysis. Samples were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on 10% (w/v) polyacrylamide gels (Mini-PROTEAN TGX, Bio-Rad Laboratories). Proteins were transferred to a PVDF-FL membrane using a Bio-Rad semidry blotting system. The membrane was blocked in 1% (w/v) bovine serum albumin and TTBS (TBS containing 0.1% Tween) for 1 h at 4°C. CAH4/5 primary antibody (Agrisera) was used with a dilution of 1:10,000 for 1 h at room temperature. Anti-rabbit secondary antibody diluted in 1% Bovine serum albumin and TTBS was used with a dilution of 1:4,000 at room temperature. A BioRad chemiluminescence instrument was used to observe the CAH4/5 protein bands for the cells.
Photosynthetic assay
Chlamydomonas cultures were grown in TAP medium (100 µmol photons m−2 s −1) to logarithmic phase. Cells were washed in MIN media and then bubbled with high CO2 for 24 h in MIN media. The cells were then bubbled with ambient CO2 in the light for 12 h to induce the CCM. Cells normalized to 100-µg chlorophyll were suspended in 25-mM HEPES-NaOH buffer (pH 7.84) or 25-mM EPPS-NaOH buffer (pH 8.4) that had been bubbled with nitrogen gas. A Clark O2 electrode chamber (Rank Brothers, Cambridge, UK) illuminated at 300 µmol photons m−2·s −1 was used to deplete Ci. Then different concentrations of NaHCO−3 were injected into the depleted cells and the slope was calculated for each point. DIC concentration needed for half maximal rate of oxygen evolution was calculated as K1/2 (Ci).
Ci uptake
Silicone oil centrifugation was used to estimate intercellular concentration of DIC as in Moroney et al. (1985). Cells were initially grown in TAP media and then transferred to high CO2 for 24 h in MIN media. The cells were centrifuged and normalized to a concentration of 25-µg chlorophyll mL−1. Cells (4 mL) were illuminated in a Clark electrode chamber in 25-mM EPPS-NaOH buffer (pH 7.8 or 8.4) until the Ci was depleted to a net O2 evolution rate of zero. Cells were maintained in the light until used. 300 µL of Ci depleted cells were layered into centrifuge tubes containing 25 µL of 1 M glycine (pH 10) with 0.75% (w/v) SDS overlaid with 75 µL of Dow Corning AR200/AR 20 (3:1 [v/v]) silicone oil. Assays were performed at 25°C in 200 µmol photons m−2·s −1 light in a Beckman Microfuge B. Ci uptake was initiated by adding 3 µL of 2.5-mM NaH14CO3 to a final concentration of 25 µM, followed by the indicated time of illumination (between 15 and 90 s at 150 µmol photons m−2 s −1). The reaction was terminated by a 15-s centrifugation in a Microfuge B (Beckman). Internal Ci was calculated using the difference between total and acid stable 14C in the pellet and corrected for cell volume (Machingura et al., 2017).
Transmission electron microscopy
For TEM, Chlamydomonas cells were grown in TAP medium until the OD730 of the cells reached between 0.2 and 0.3. Then, the cells were washed and bubbled with high CO2 for 24 h. After that, cells were incubated at high CO2 or ambient CO2 for 12 h. The cells were processed for TEM as described previously (Mitra et al., 2004). The cells were washed in MIN media and fixed in growth medium containing equal volume of 1% OsO4, 2% formaldehyde, 0.5% glutaraldehyde, and 0.1-mM sodium cacodylate buffer (pH 7.2) for 30 min. Cells were then washed and negatively stained with 0.5% uranyl acetate for 30 min in dark, dehydrated in ethanol and embedded in London resin white. Ultrathin sections (∼70 nm) were mounted onto collodion-coated nickel grids, and micrographs were obtained using a JEOL JEM-1400 TEM.
Visualization of CAH5 protein
Venus fluorescence was visualized using a Chlamydomonas strain cMJ030 expressing pLM005-CAH5-Venus-3xFLAG (obtained from the Chlamydomonas Resource Center). Chlamydomonas cells overexpressing CAH5 were grown in TAP medium until the OD730 of the cells reached between 0.2 and 0.3. Then, the cells were washed in MIN media and bubbled with high CO2 for 24 h in MIN media. Cells were then incubated at high CO2 and ambient CO2 for 12 h. The Venus fluorescence was observed from the cells with a Leica SP8 confocal microscope using a 63× water-emersion lens. The white light laser was used with 5% laser power and smart gain was adjusted to 100%. Two fluorescence detection channels were used, the green channel for Venus signals (excited at 514 nm) and red channel for chlorophyll autofluorescence (excited at 488 nm). The emission wavelength of the fluorescence filter for Venus and chlorophyll was 520–560 and 640–721 nm, respectively. The images received from the Leica LAS X software was processed through standard deconvolution using Huygens Essential software.
Complementation of cah4/5 RNAi mutant
Complementation of the cah4/5-2 RNAi mutant was achieved by transformation of cah4/5-2 RNAi mutant cells with invitrogen pChlamy _4 vector containing a CDS of CAH5 driven by the Hsp70A-Rbc S2 hybrid promoter. The construct consists of no untranslated regions of the original CAH5 gene as the area targeted by the RNAi was in the 3′ UTR of the CAH4 and CAH5 genes. The electroporation method was used for cell transformation, and strains were selected for zeocin resistance. The presence of complemented DNA in selected Chlamydomonas strains was confirmed by PCR. The growth of the complemented line was then compared to the growth of the RNAi lines and D66 as described in the screening RNAi lines section.
Quantification and statistical analysis
Statistical analyses were performed using Student’s t test in GraphPad Prism 8. In all cases, at least at least three separate experiments were performed that showed similar trends. The data in the figures are the means and standard deviations for three technical replicates from one of the experiments. Statistical significance is defined as P < 0.05. Details are in the figure legends.
Accession numbers
CAH4 and CAH5 can be found in the Phytozome under the accession numbers Cre05.g248400.t1.2 and Cre05.g248450.t1.1
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . Multiple sequence alignment of the CAH4 and CAH5 protein sequences.
Supplemental Figure S2 . RT-qPCR of other CCM genes.
Supplemental Figure S3 . Growth of strains complementing the cah4/5 RNAi knockdown line at different CO2 levels.
Supplemental Figure S4 . Additional TEM images of whole cells of the D66 strain.
Supplemental Figure S5 . Additional confocal images of whole cells of the CSI_FC1G05 expressing pLM005-CAH5-Venus-3xFLAG.
Supplemental Table S1 . List of primers used in this study.
Supplementary Material
Acknowledgments
We thank Ms Ying Xiao from the Shared Instrumentation Facility at LSU for transmission electron microscopy support.
Funding
This work was supported by the Realizing Improved Photosynthetic Efficiency (RIPE) initiative awarded to JVM by the University of Illinois, USA. RIPE is made possible through support from the Bill & Melinda Gates Foundation, Foundation for Food and Agricultural Research and the Foreign, Commonwealth & Development Office (grant no. OPP1172157).
Conflict of interest statement. None declared.
A.K.R. and J.V.M. designed the research; A.K.R., T.C., and J.V.M. performed the research; A.K.R., T.C., and J.V.M. analyzed the data; and A.K.R. and J.V.M. wrote the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is James Moroney (btmoro@lsu.edu).
References
- Chen Z-Y, Burow MD, Mason CB, Moroney JV (1996) Characterization of a low CO2-inducible alanine aminotransferase in Chlamydomonas reinhardtii. Plant Physiol 112: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Gardestrom P, Samuelsson G (1995) Isolation, purification, and characterization of mitochondria from Chlamydomonas reinhardtii. Plant Physiol 107: 479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Karlsson J, Ramazanov Z, Gardeström P, Samuelsson G (1996) Discovery of an algal mitochondrial carbonic anhydrase: molecular cloning and characterization of a low-CO2-induced polypeptide in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 93: 12031–12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Villand P, Gardeström P, Samuelsson G (1998) Induction and regulation of expression of a low-CO2-induced mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 116: 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Si Y, Douglass S, Casero D, Merchant SS, Pellegrini M, Ladunga I, Liu P, Spalding MH (2012) Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. Plant Cell 24: 1876–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman Rosenzweig ES, Xu B, Kuhn Cuellar L, Martinez-Sanchez A, Schaffer M, Strauss M, Cartwright HN, Ronceray P, Plitzko JM, Förster F, et al. (2017) The eukaryotic CO2-concentrating organelle is liquid-like and exhibits dynamic reorganization. Cell 171: 148–162.e119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S, Fukuzawa H, Tachiki A, Miyachi S (1990) Structure and differential expression of two genes encoding carbonic anhydrase in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 87: 9779–9783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H, Miura K, Ishizaki K, Kucho KI, Saito T, Kohinata T, Ohyama K (2001) Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci U S A 98: 5347–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty AM, Spalding MH (1996) Molecular and structural changes in Chlamydomonas under limiting CO2 (a possible mitochondrial role in adaptation). Plant Physiol 111: 1339–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J, Bisson MA, Tosteson FC (1977) Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol 69: 779–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Norici A, Forssen M, Eriksson M, Raven JA (2003) An anaplerotic role for mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 132: 2126–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JL, Tolbert NE (1967) Glycolate pathway in algae. Plant Physiol 42: 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura AK, Chan KX, Atkinson N, Pallesen L, Wang L, Reeves G, Patena W, Caspari O, Roth R, Goodenough U, et al. (2019) A Rubisco-binding protein is required for normal pyrenoid number and starch sheath morphology in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 116: 18445–18454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G (1998) A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17: 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono A, Chou T-H, Radhakrishnan A, Bolla JR, Sankar K, Shome S, Su C-C, Jernigan RL, Robinson CV, Yu EW, et al. (2020) Structure and function of LCI1: a plasma membrane CO2 channel in the Chlamydomonas CO2 concentrating mechanism. Plant J 102: 1107–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Pollock SV, Xiao Y, Cunnusamy K, Moroney JV (2011) Identification of a novel gene, CIA6, required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol 156: 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machingura MC, Bajsa-Hirschel J, Laborde SM, Schwartzenburg JB, Mukherjee B, Mukherjee A, Pollock SV, Förster B, Price GD, Moroney JV (2017) Identification and characterization of a solute carrier, CIA8, involved in inorganic carbon acclimation in Chlamydomonas reinhardtii. J Exp Bot 68: 3879–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinder LCM, Chen C, Leib RD, Patena W, Blum SR, Rodman M, Ramundo S, Adams CM, Jonikas MC (2017) A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 171: 133–147.e114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra M, Lato SM, Ynalvez RA, Xiao Y, Moroney JV (2004) Identification of a new chloroplast carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 135: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Bassett A, Thuenemann E, Schwach F, Karkare S, Ossowski S, Weigel D, Baulcombe D (2009) Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant J 58: 165–174 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 79: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE, Kitayama M, Manuel LJ, Togasaki RK (1989) Isolation and characterization of a mutant of Chlamydomonas reinhardtii deficient in the CO2 concentrating mechanism. Plant Physiol 89: 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109: 133–149 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Somanchi A (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119: 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Wilson BJ, Tolbert NE (1986) Glycolate metabolism and excretion by Chlamydomonas reinhardtii Plant Physiol 82: 821–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryotic Cell 6: 1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Lau CS, Walker CE, Rai AK, Prejean CI, Yates G, Emrich-Mills T, Lemoine SG, Vinyard DJ, Mackinder LCM, et al. (2019) Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 116: 16915–16920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi N, Mukherjee B, Tsujikawa T, Yanase M, Nakano H, Moroney JV, Fukuzawa H (2010) Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. Plant Cell 22: 3105–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock SV, Prout DL, Godfrey AC, Lemaire SD, Moroney JV (2004) The Chlamydomonas reinhardtii proteins Ccp1 and Ccp2 are required for long-term growth, but are not necessary for efficient photosynthesis, in a low-CO2 environment. Plant Mol Biol 56: 125–132 [DOI] [PubMed] [Google Scholar]
- Ramazanov Z, Rawat M, Henk MC, Mason CB, Matthews SW, Moroney JV (1994) The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta 195: 210–216 [Google Scholar]
- Raven JA (2001) A role for mitochondrial carbonic anhydrase in limiting CO2 leakage from low CO2-grown cells of Chlamydomonas reinhardtii. Plant Cell Environ 24: 261–265 [Google Scholar]
- Raven JA (1984) Energetics and Transport in Aquatic Plants. A. R. Liss & Co., New York [Google Scholar]
- Rawat M, Moroney JV (1991) Partial characterization of a new isoenzyme of carbonic anhydrase isolated from Chlamydomonas reinhardtii. J Biol Chem 266: 9719–9723 [PubMed] [Google Scholar]
- Spalding MH (2008) Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot 59: 1463–1473 [DOI] [PubMed] [Google Scholar]
- Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 46: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirumani S, Kokkanti M, Chaudhari V, Shukla M, Rao BJ (2014) Regulation of CCM genes in Chlamydomonas reinhardtii during conditions of light–dark cycles in synchronous cultures. Plant Mol Biol 85: 277–286 [DOI] [PubMed] [Google Scholar]
- Tolleter D, Chochois V, Poiré R, Price GD, Badger MR (2017) Measuring CO2 and HCO3-permeabilities of isolated chloroplasts using a MIMS-18O approach. J Exp Bot 68: 3915–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tural B, Moroney JV (2005) Regulation of the expression of photorespiratory genes in Chlamydomonas reinhardtii. Can J Bot 83: 810–819 [Google Scholar]
- Wang Y, Stessman DJ, Spalding MH (2015) The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: how Chlamydomonas works against the gradient. Plant J 82: 429–448 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Zhang J, Weeks DP (2001) The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 98: 5341–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Sato E, Iguchi H, Fukuda Y, Fukuzawa H (2015) Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 112: 7315–7320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Tsujikawa T, Hatano K, Ozawa S-i, Takahashi Y, Fukuzawa H (2010) Light and low-CO2-dependent LCIB–LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 51: 1453–1468 [DOI] [PubMed] [Google Scholar]
- Zhang R, Patena W, Armbruster U, Gang SS, Blum SR, Jonikas MC (2014) High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell 26: 1398–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.