Abstract
This Graphical Review provides a concise overview of the manifold and mechanistically diverse methods that enable the functionalization of sp3 C–H bonds in amines and their derivatives.
Keywords: C–H bond functionalization, amines, heterocycles, catalysis, synthesis
Graphical Abstract

1. Introduction
The development of methods for the C–H bond functionalization of amines continues to be a topic of significant interest. Given the potential to lead to real-world applications, coupled with the intellectually stimulating nature of the field, this sustained high level of interest is hardly surprising. A plethora of approaches have emerged over the years, exhibiting significant mechanistic diversity. In addition, an almost overwhelming number of contributions continue to be published at an ever-accelerating pace, making it challenging to keep up with what has already been accomplished, and to put new discoveries into perspective. The rapid speed of development can also obscure what has already been done well versus which transformations need further improvement (regarding scope, ease of use, cost, scalability, etc.), and which worth-while unsolved challenges remain to be addressed. The goal of this Graphical Review is to provide a concise overview of the manifold methods that achieve the functionalization of sp3 C–H bonds in amines and their protected derivatives (e.g., amides, carbamates, N-aryl amines, etc.). We aim to cover the most important methods while highlighting the underlying mechanisms. Throughout, we have attempted to trace the origin of each approach back to a seminal report or important literature precedent. A focus is placed on historical contributions, key innovations, and the most recent cutting-edge advances. While reactions are grouped by mechanism, clear categorization of a given process is not always possible. Clearly, certain transformations would fit well into different categories. Due to the format of this review and the vast number of contributions published to date, this overview could not possibly be comprehensive, nor does it aim to be. Coverage extends to the end of 2020, with selected contributions from early 2021. We hope that this review will offer something of value to novices and experts alike. Feedback from the community is welcomed, so that a future, updated version of this review can be improved upon.
Regarding the structure of this Graphical Review, abbreviated references including prior reviews are provided within the Figures at the appropriate places. Full references are shown in the reference section and are grouped by Figure number. A note on the use of color: Amine substrates are shown in black, while groups that are being added are colored in light or dark blue. Catalysts are shown in purple or green. Other colors are used on occasion to highlight certain aspects (e.g., green for directing groups, red for hydrogens that are being functionalized, and orange for curly arrows).
Figure 1.
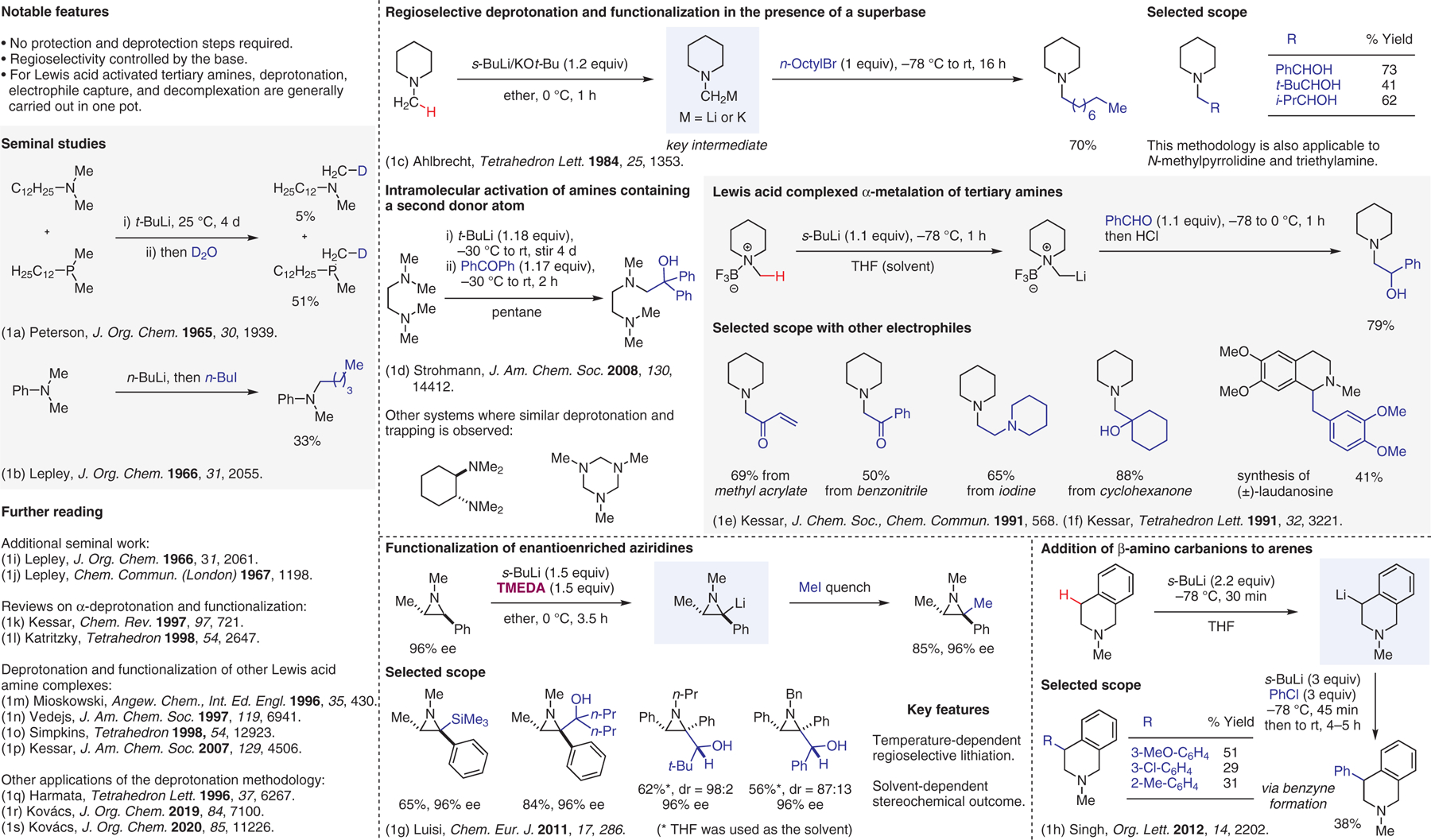
Deprotonation of tertiary amines.1
Figure 2.
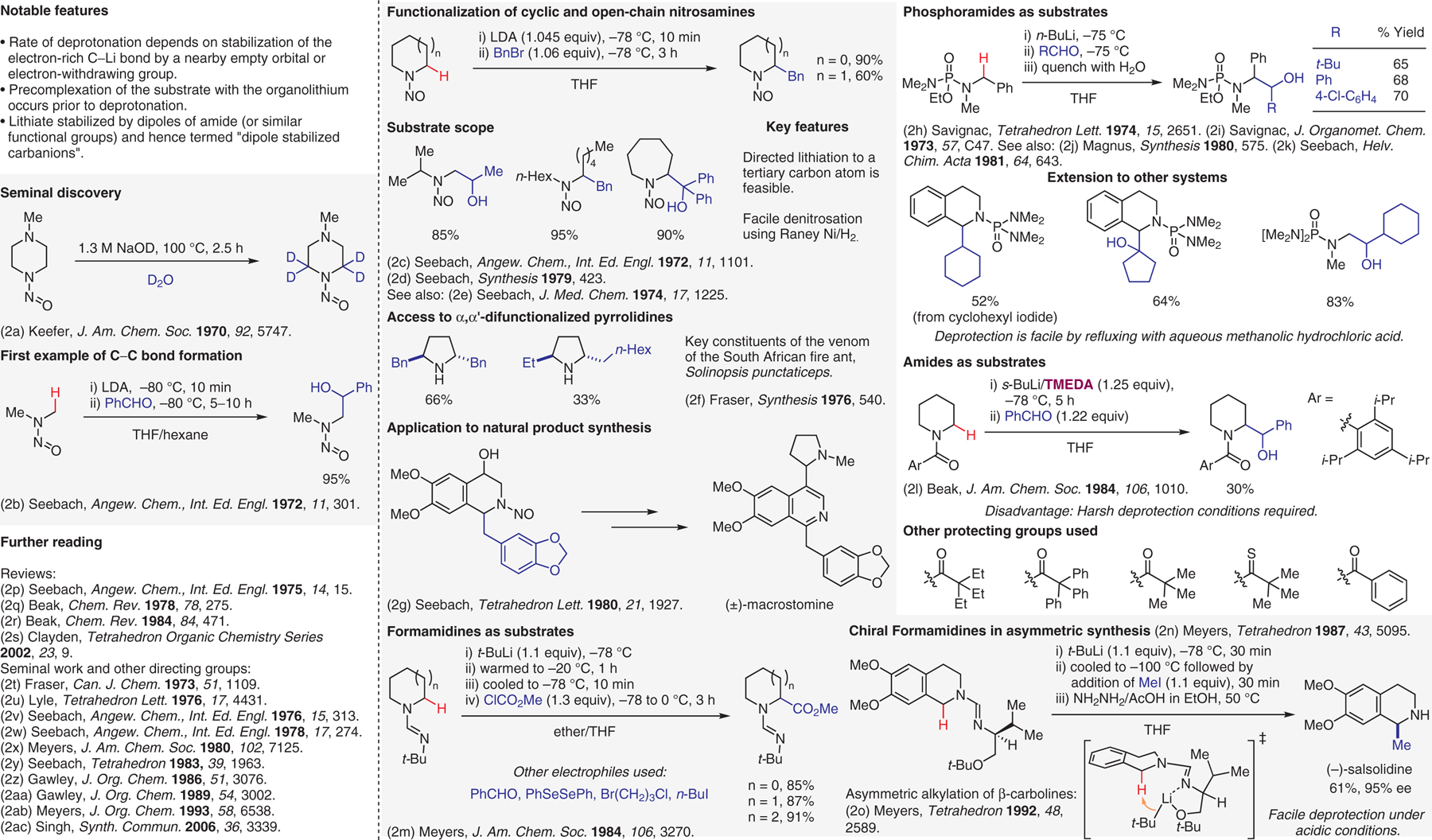
Deprotonation of protected amines, part I.2
Figure 3.
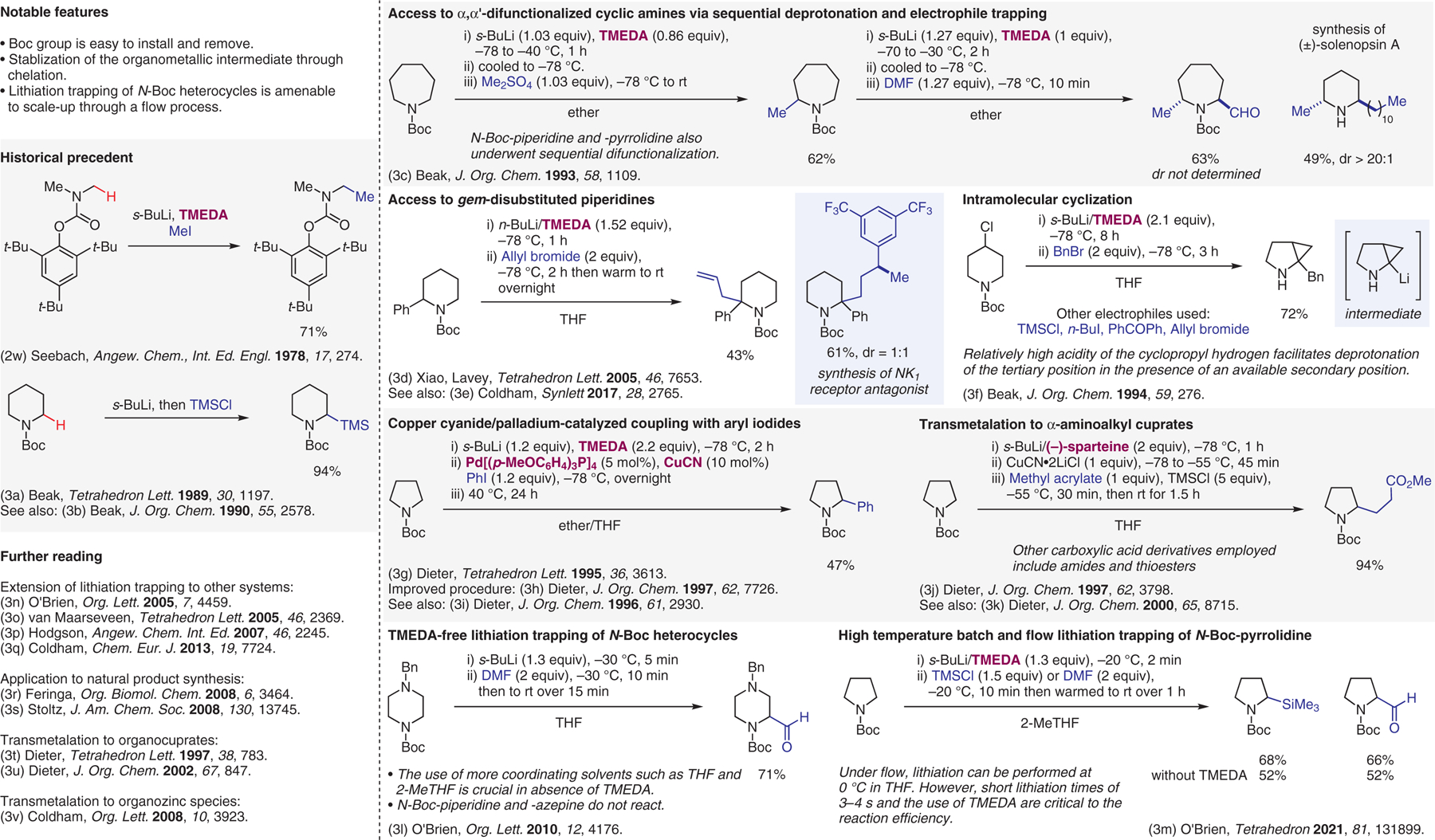
Deprotonation of protected amines, part II.3
Figure 4.
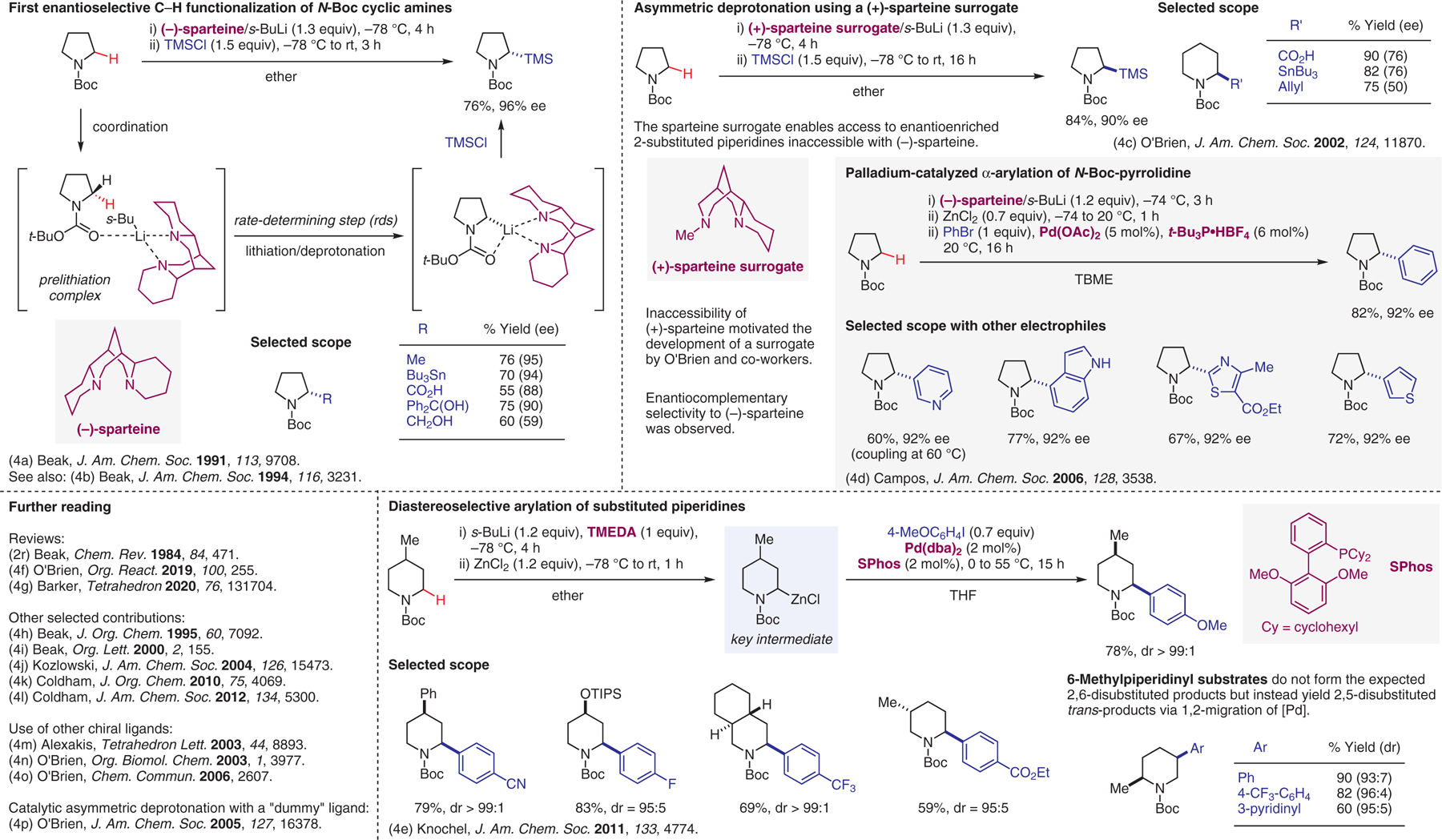
Deprotonation of protected amines, part III.4
Figure 5.
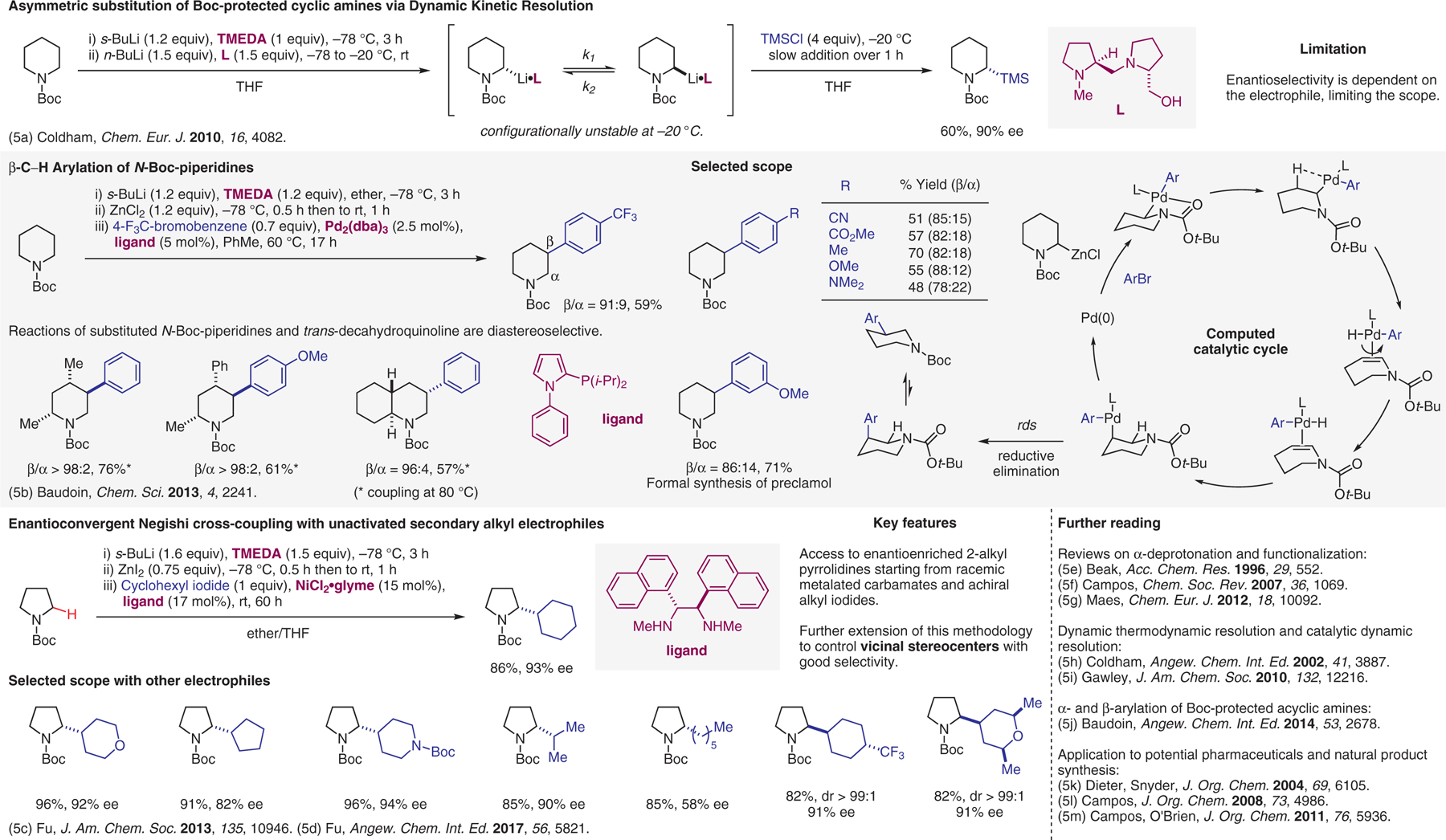
Deprotonation of protected amines, part IV.5
Figure 6.
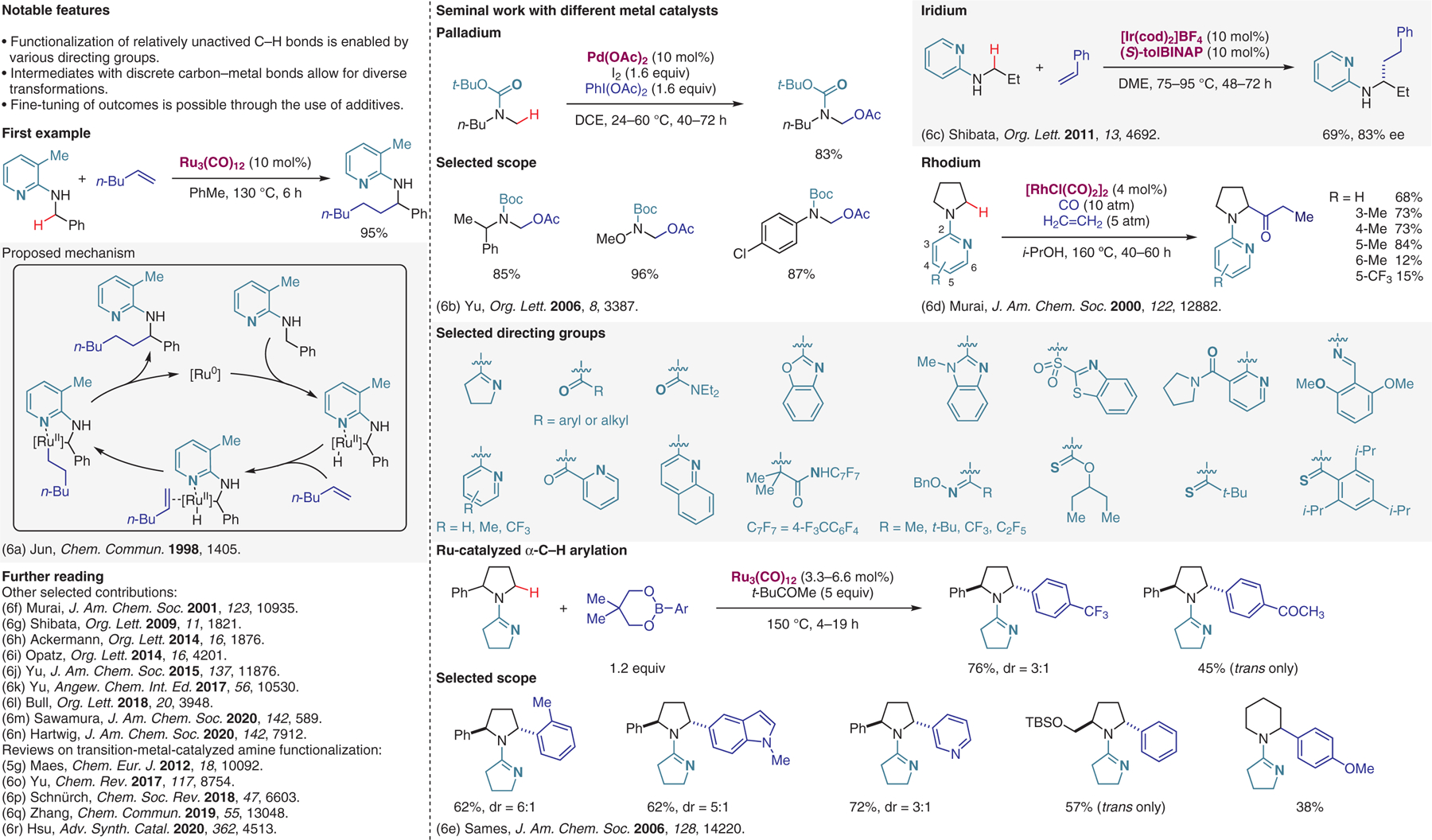
Transition-metal-catalyzed reactions with substrates containing directing groups, part I.6
Figure 7.
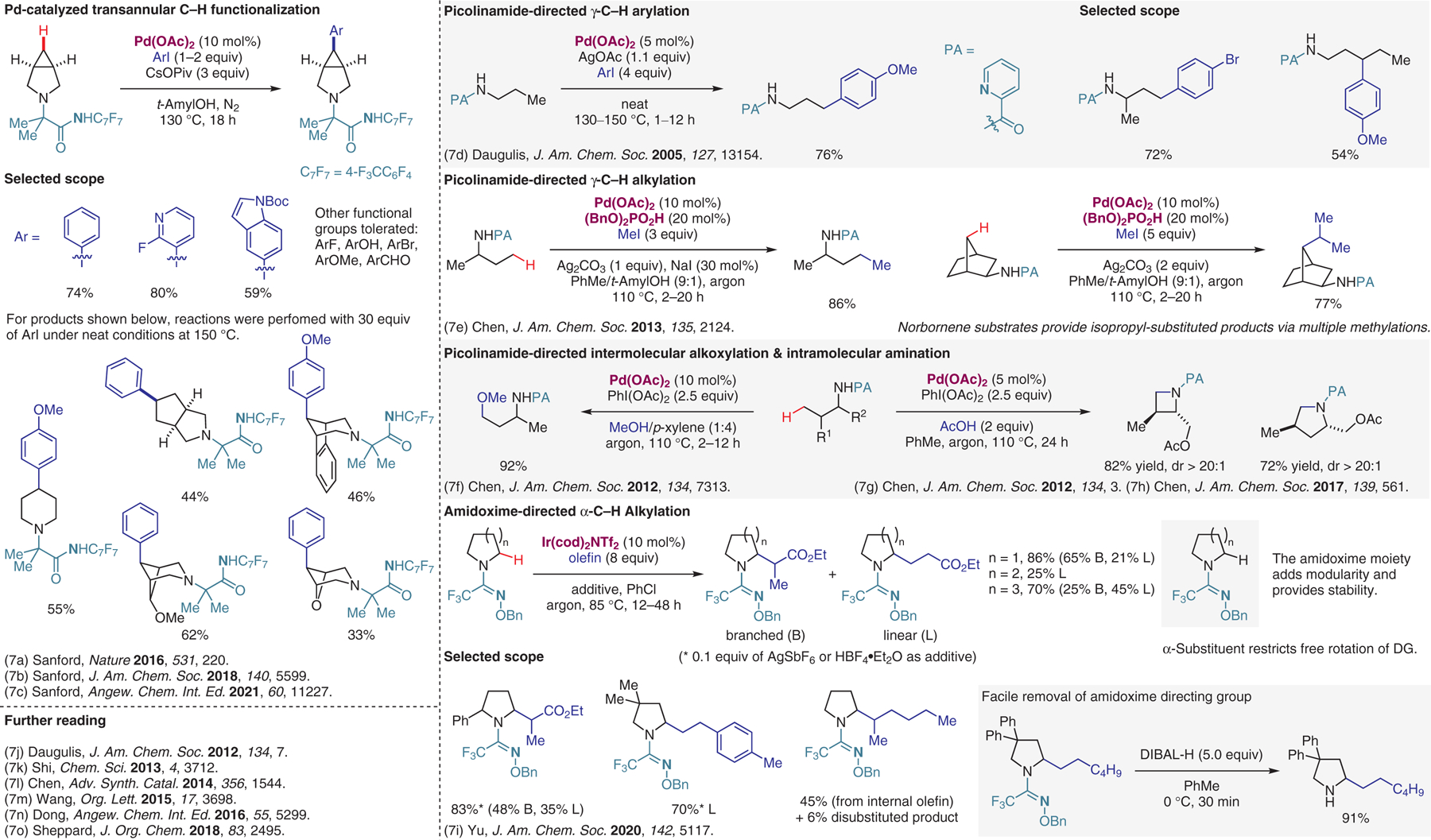
Transition-metal-catalyzed reactions with substrates containing directing groups, part II.7
Figure 8.
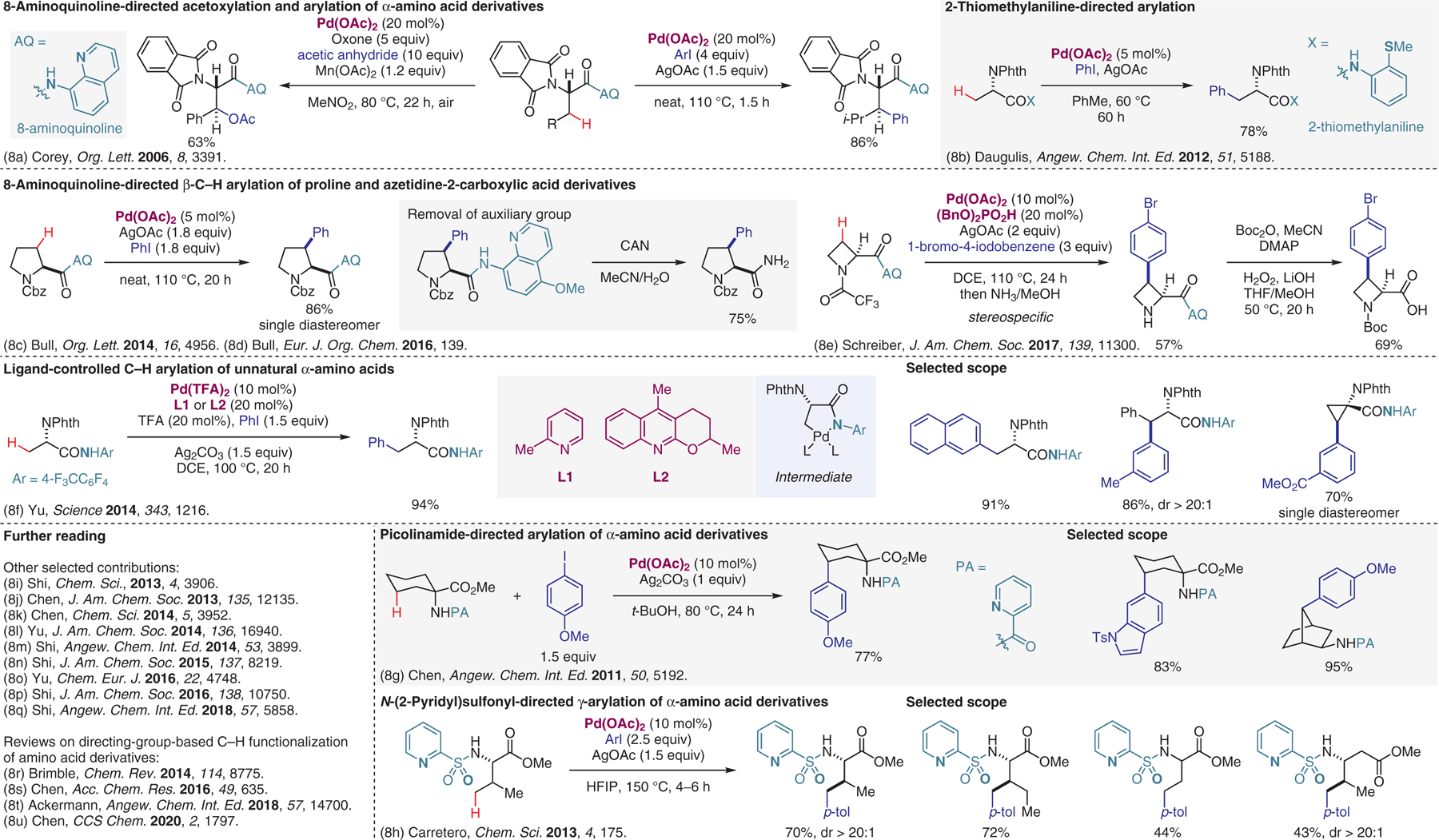
Transition-metal-catalyzed reactions with substrates containing directing groups, functionalization of amino acid derivatives.8
Figure 9.
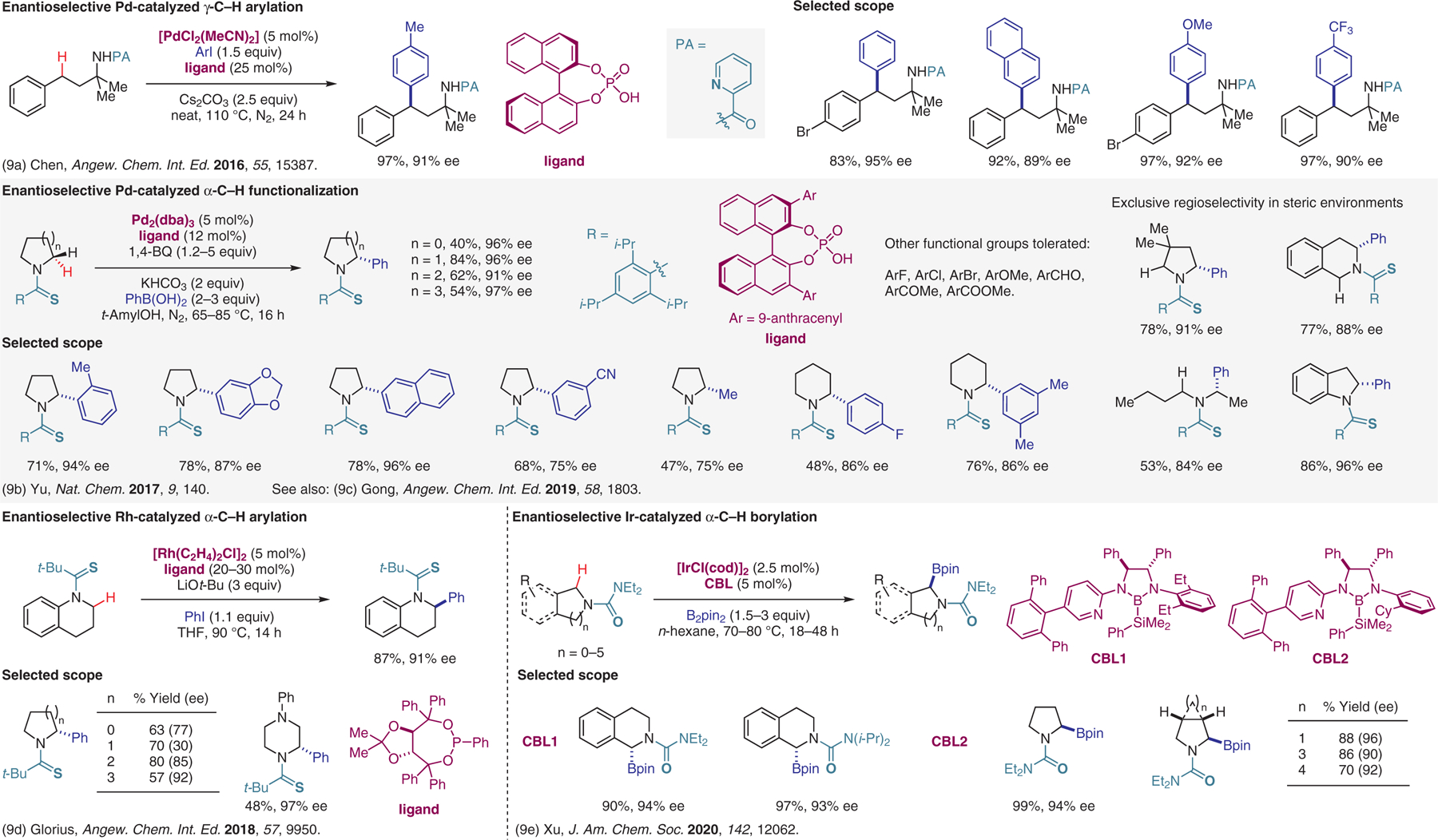
Transition-metal-catalyzed reactions with substrates containing directing groups, catalytic enantioselective approaches.9
Figure 10.
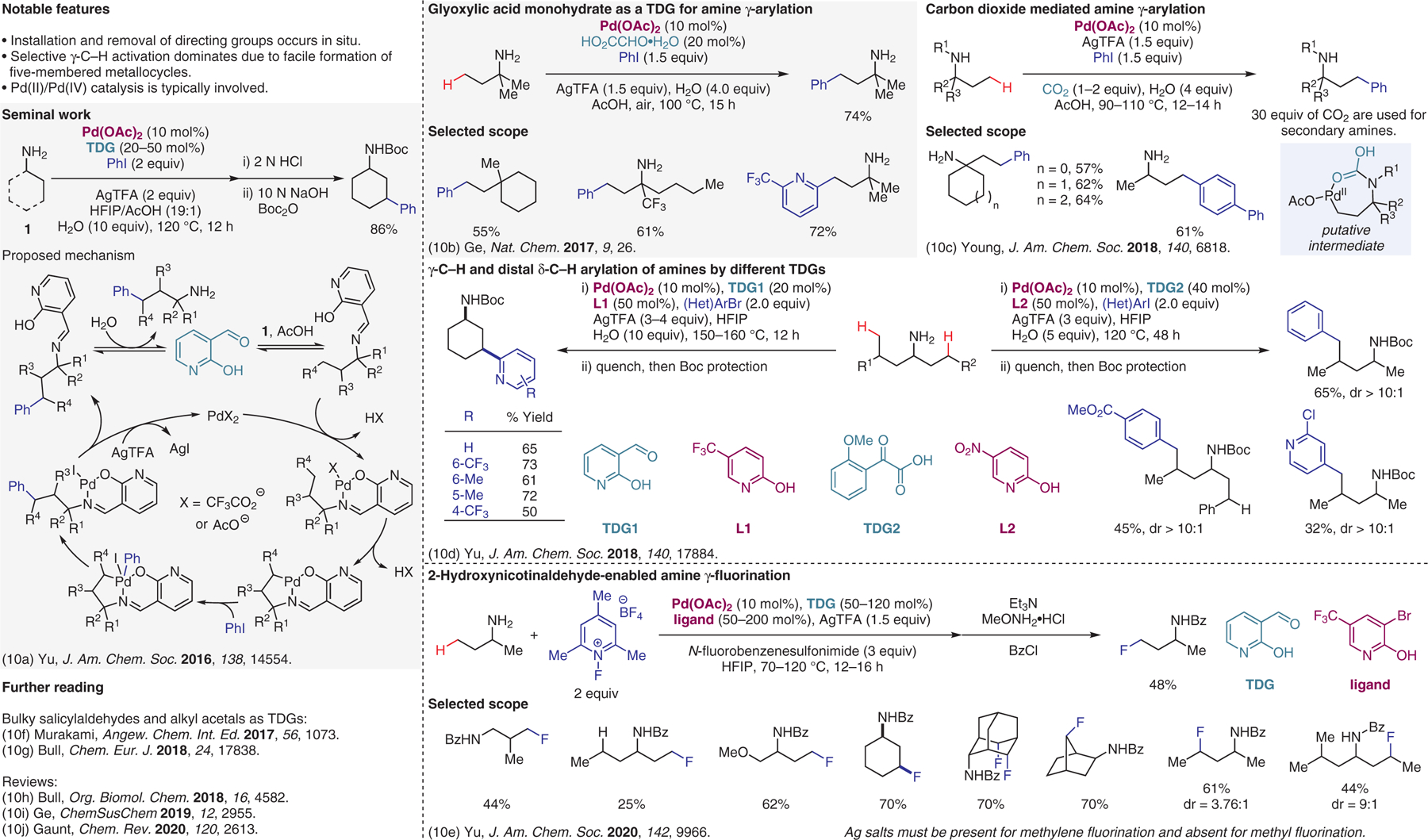
Transition-metal-catalyzed reactions involving transient directing groups (TDGs).10
Figure 11.
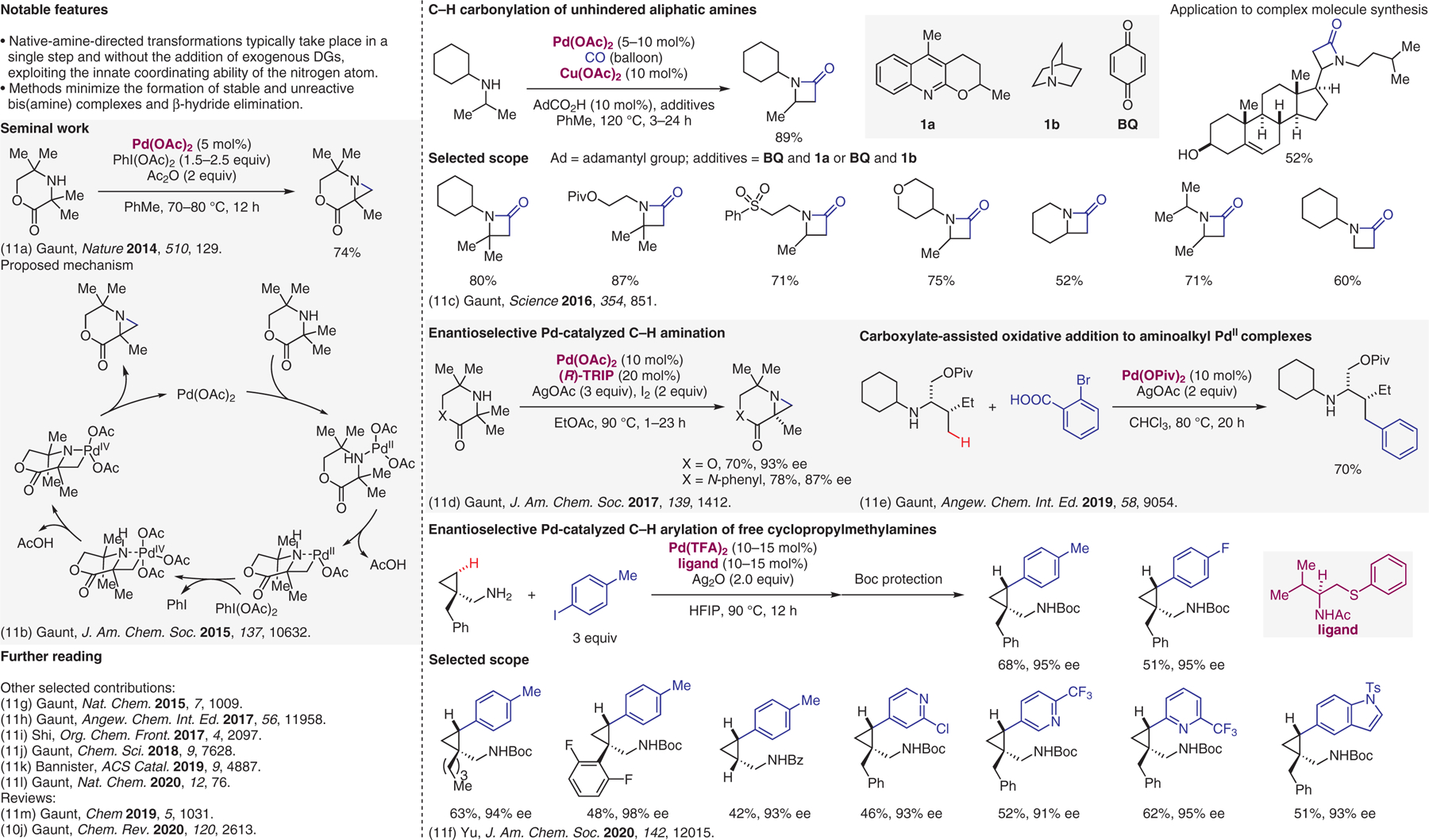
Native-amine-directed transition-metal-catalyzed reactions.11
Figure 12.
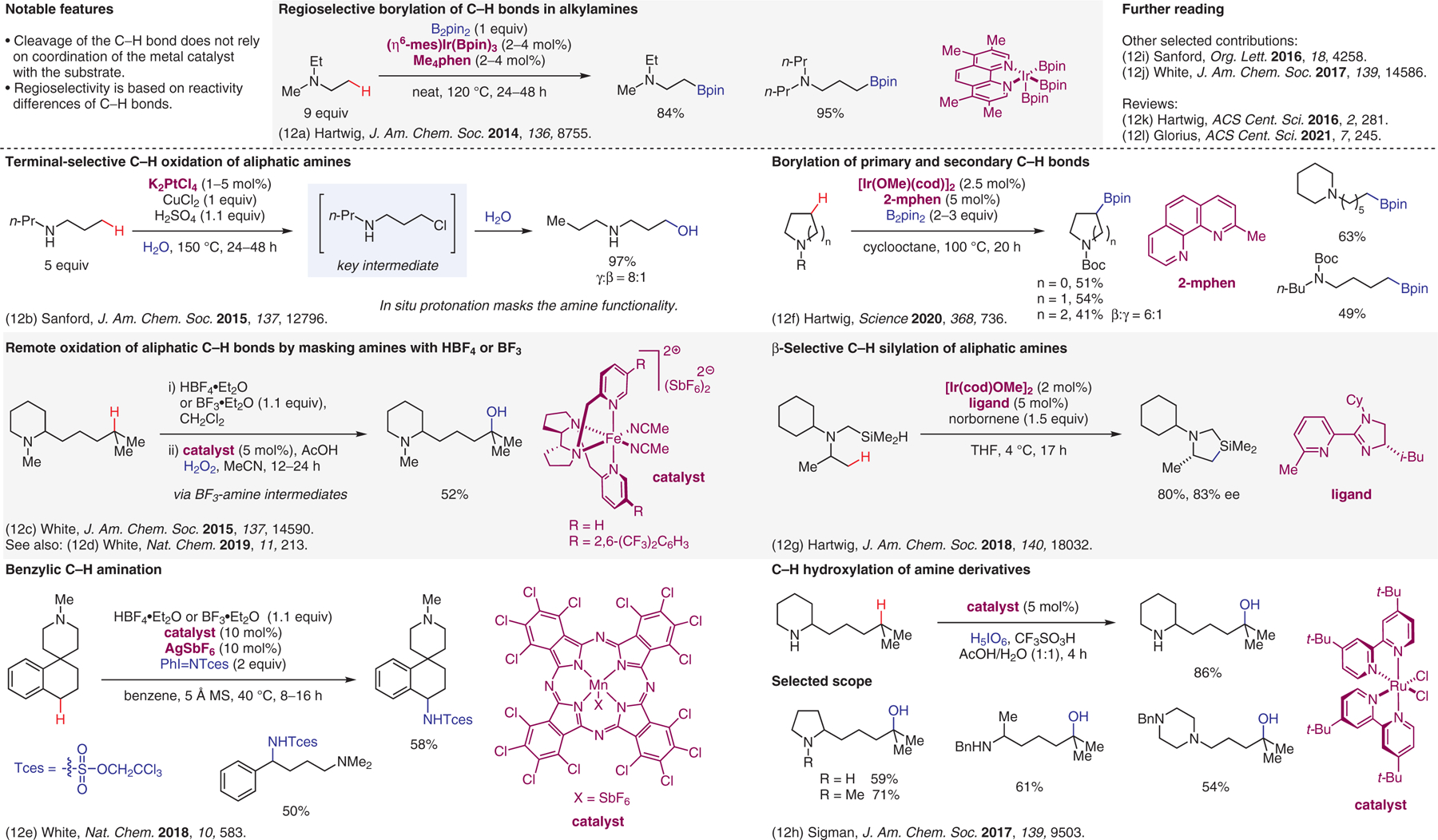
Undirected transition-metal-catalyzed reactions.12
Figure 13.
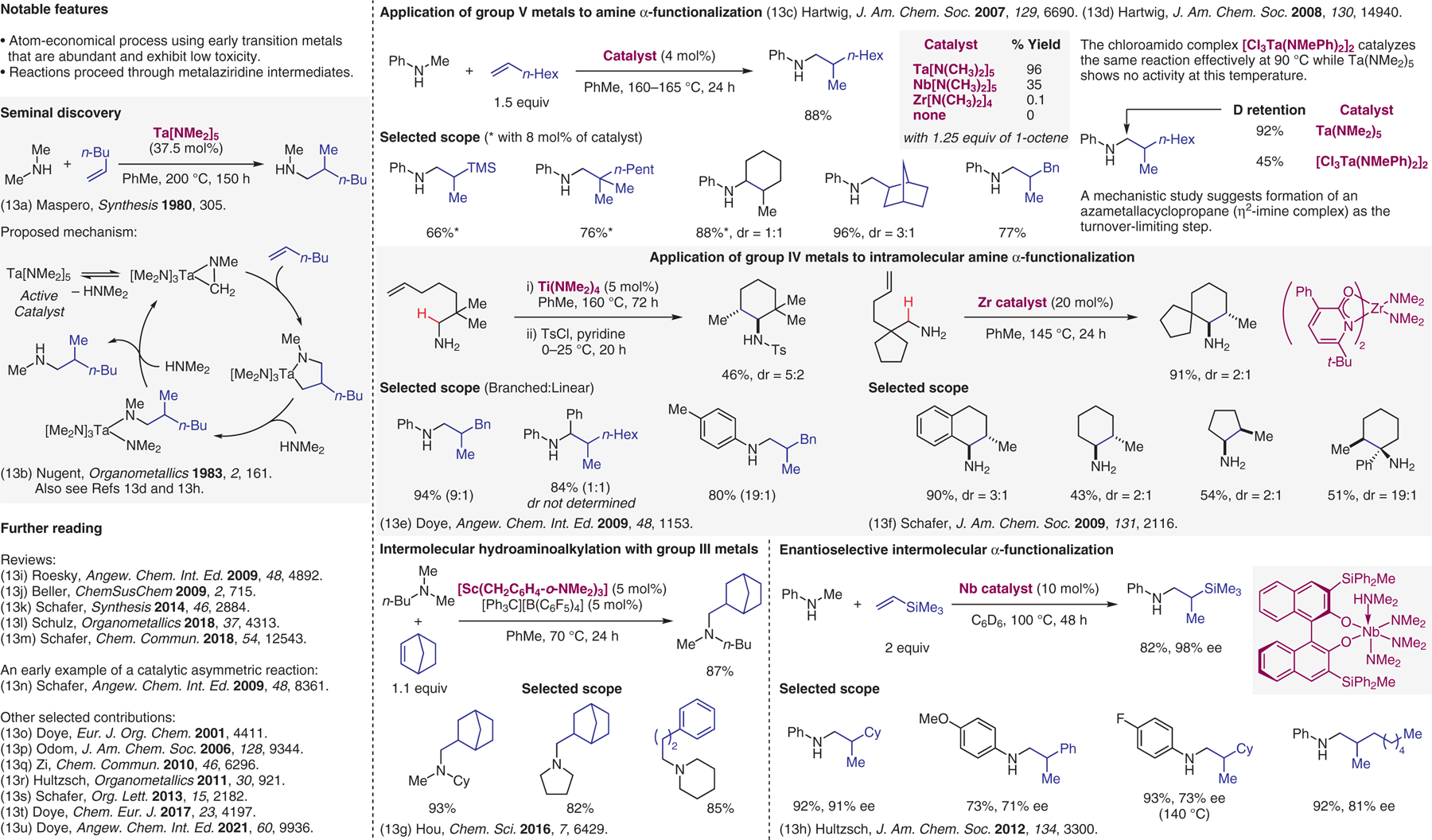
Hydroaminoalkylation.13
Figure 14.
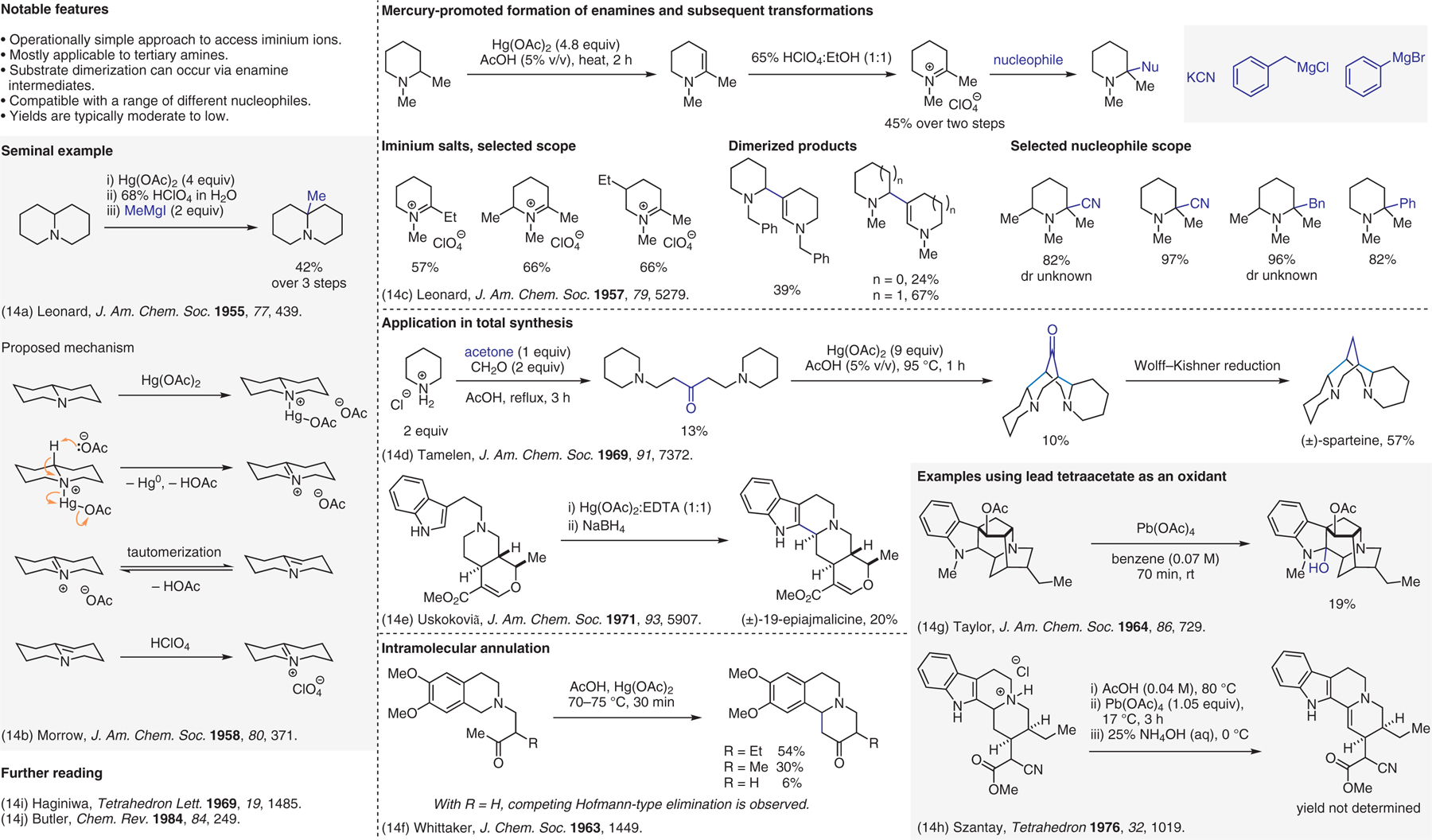
Oxidative methods, stoichiometric metal-based oxidants.14
Figure 15.
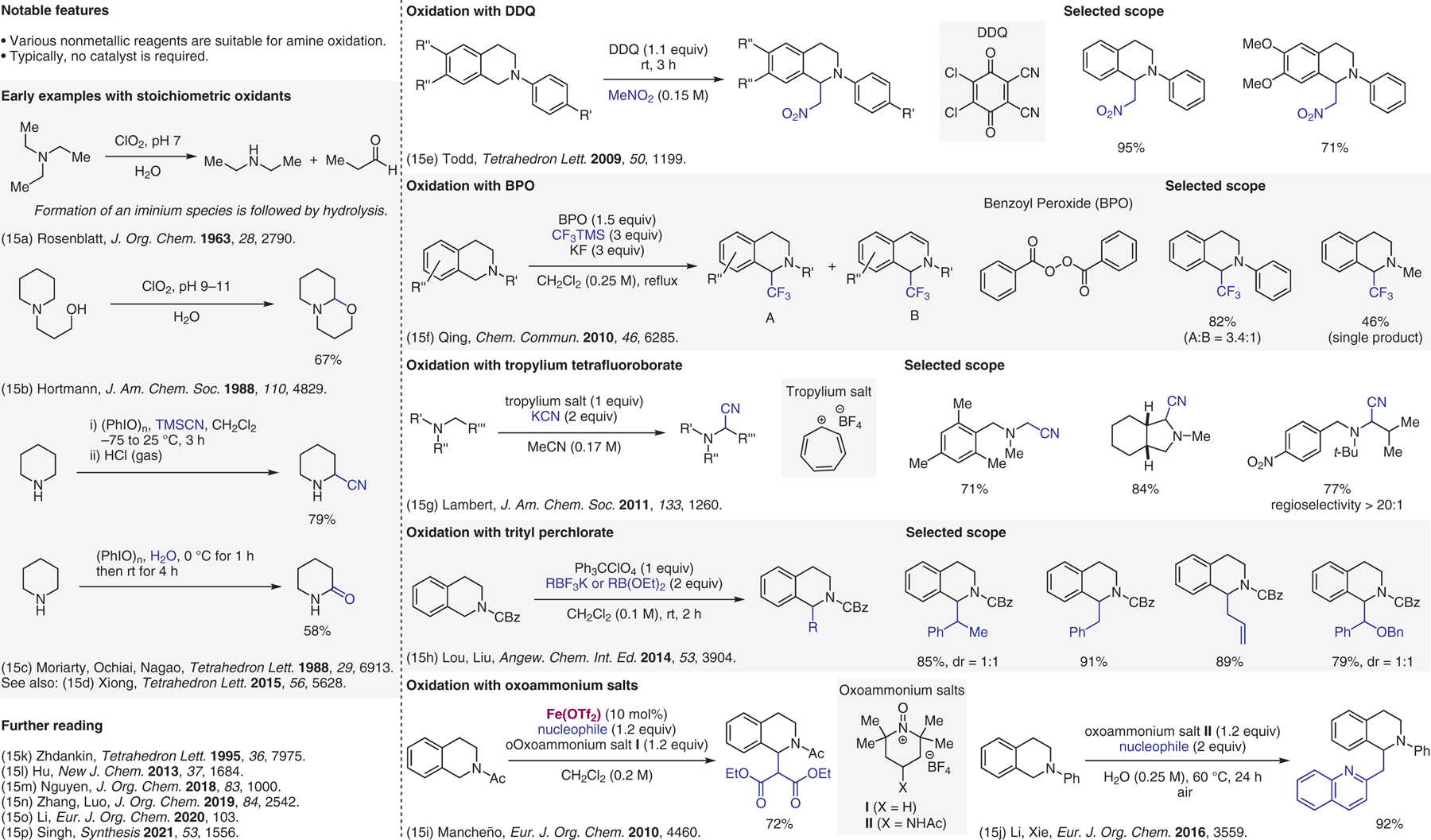
Oxidative methods, stoichiometric nonmetallic oxidants.15
Figure 16.
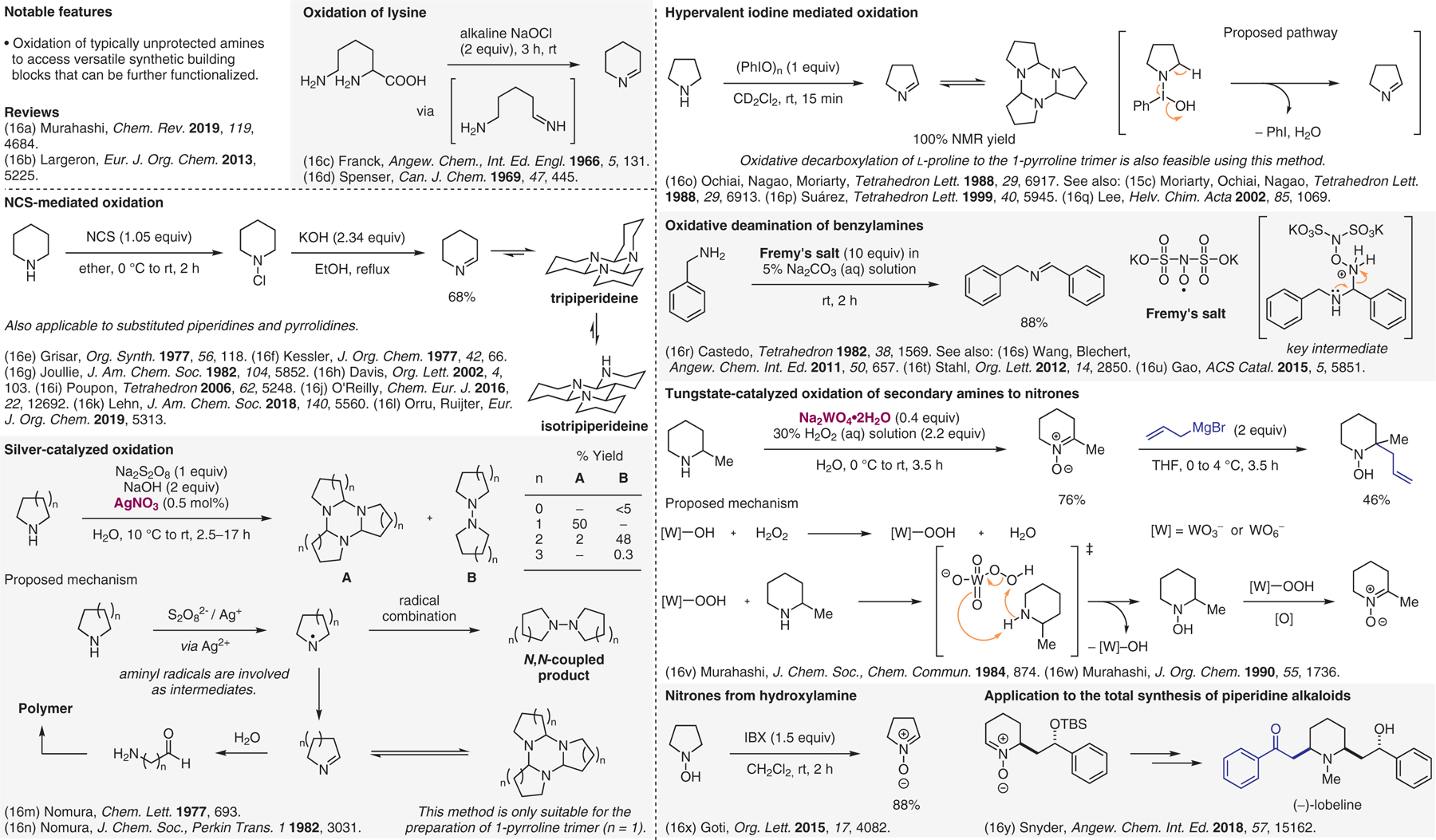
Oxidative preparation of building blocks.16
Figure 17.
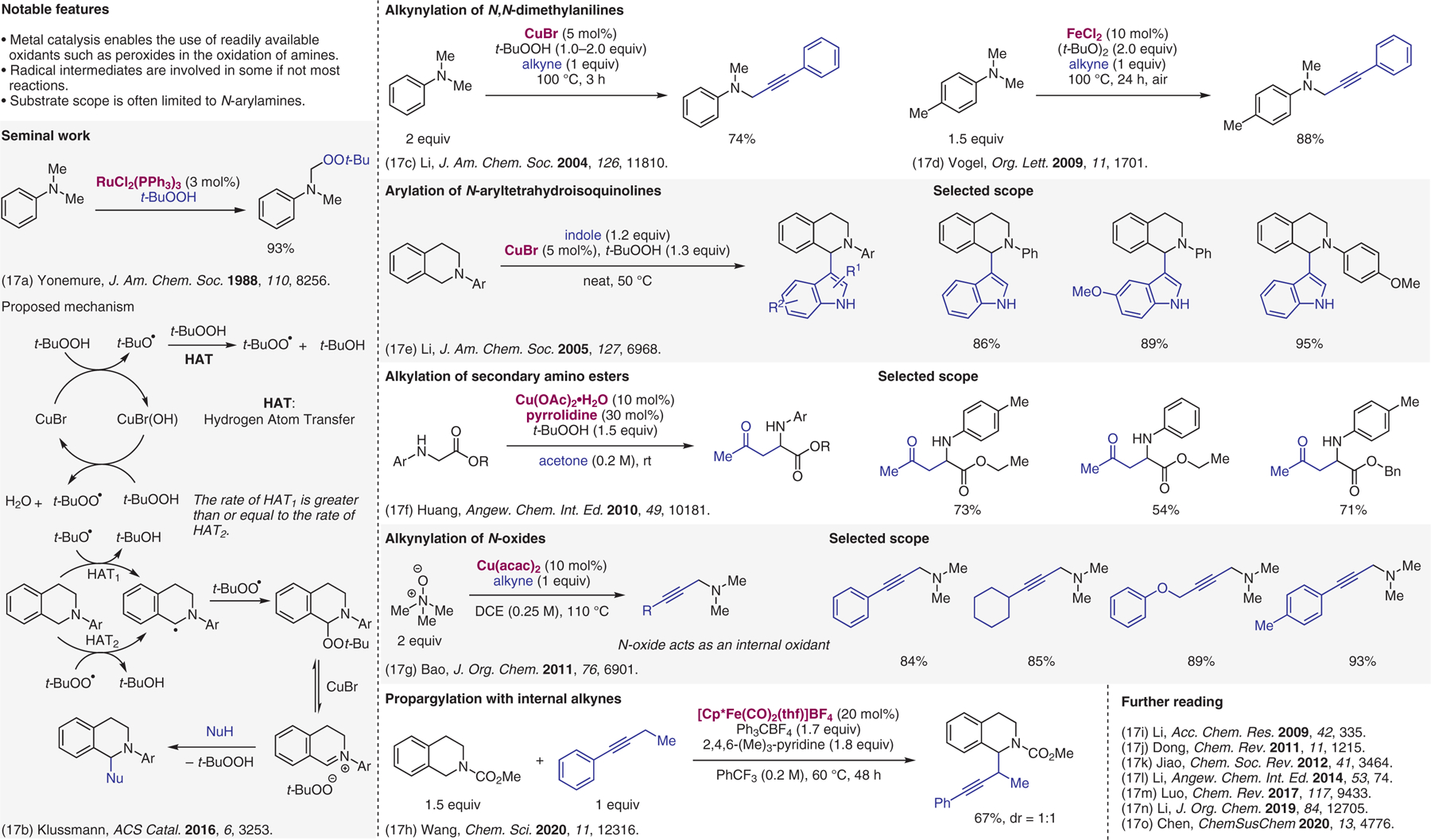
Metal-catalyzed cross-dehydrogenative-coupling (CDC) reactions.17
Figure 18.
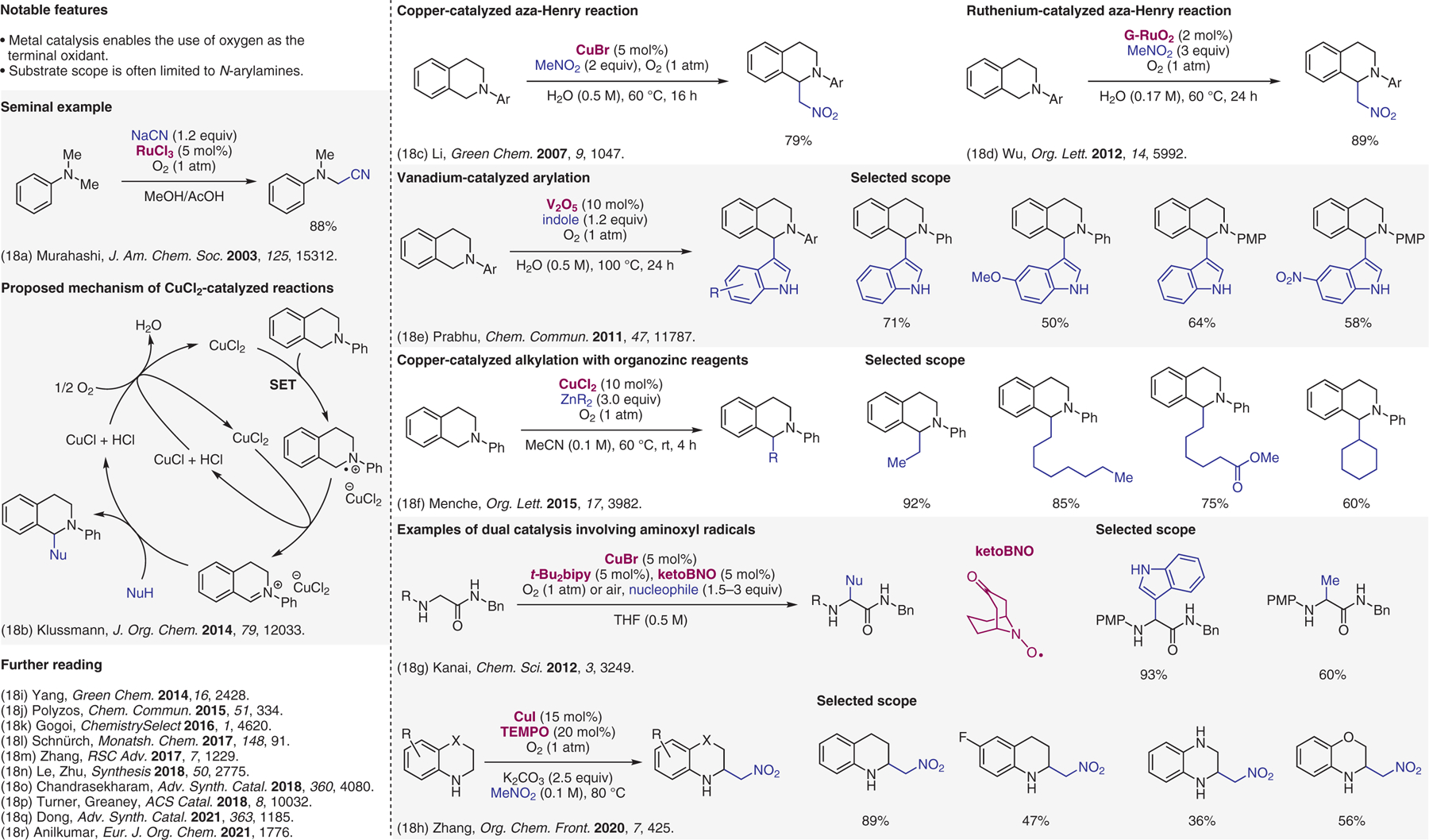
Metal-catalyzed cross-dehydrogenative-coupling (CDC) reactions with oxygen as the terminal oxidant.18
Figure 19.
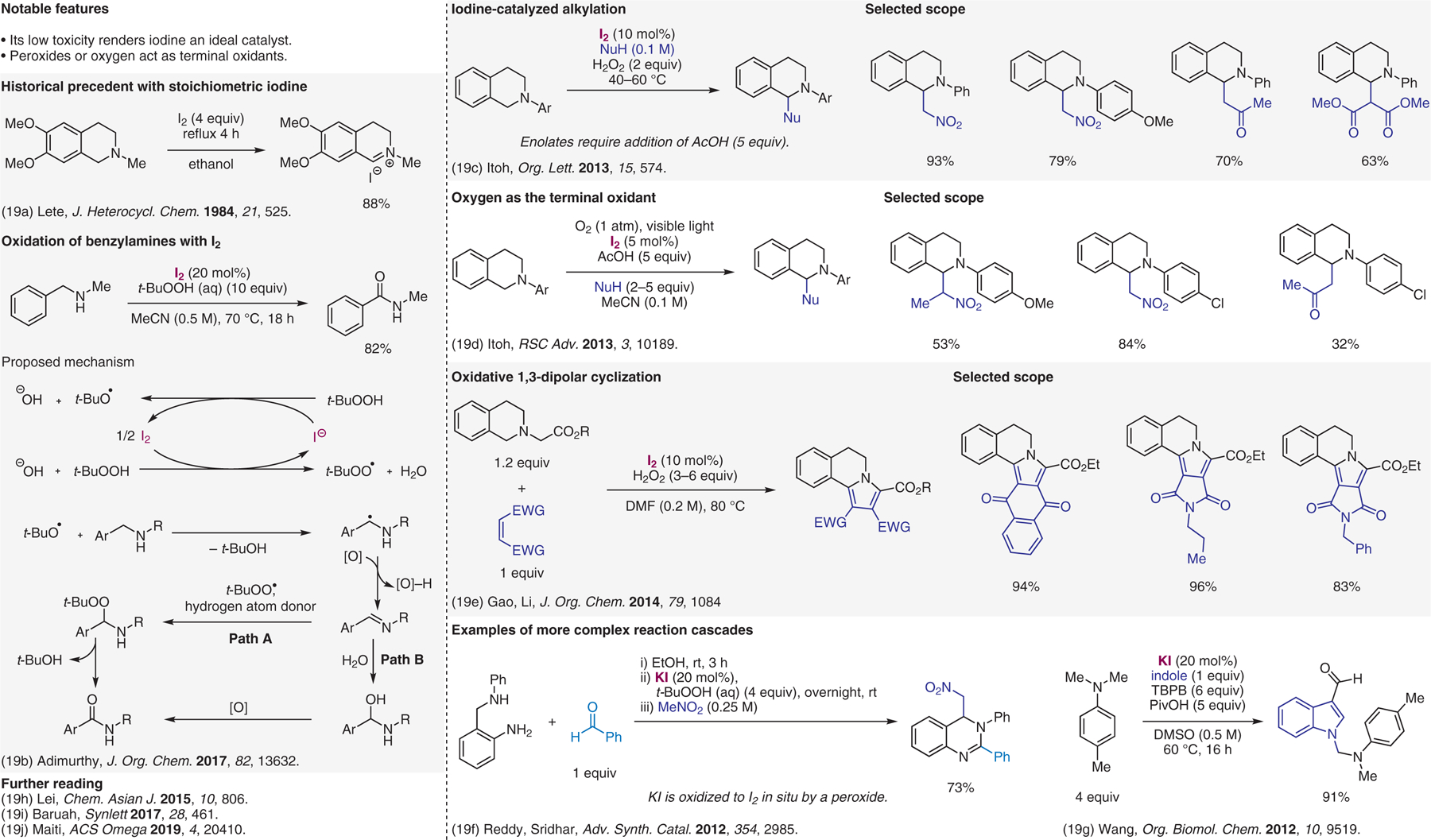
Iodine-catalyzed cross-dehydrogenative-coupling (CDC) reactions.19
Figure 20.
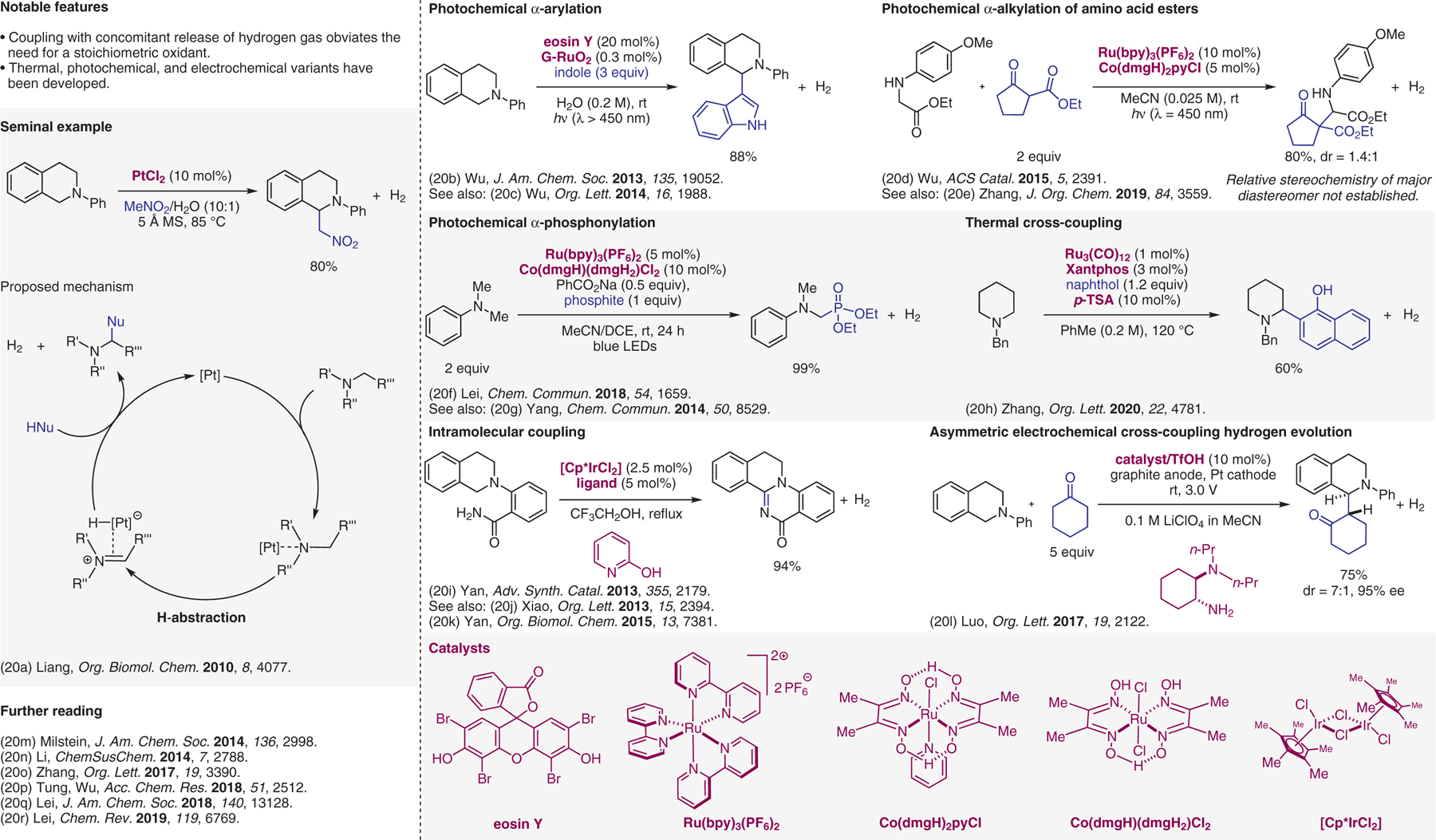
Acceptorless cross-dehydrogenative-coupling (CDC) reactions with hydrogen evolution.20
Figure 21.
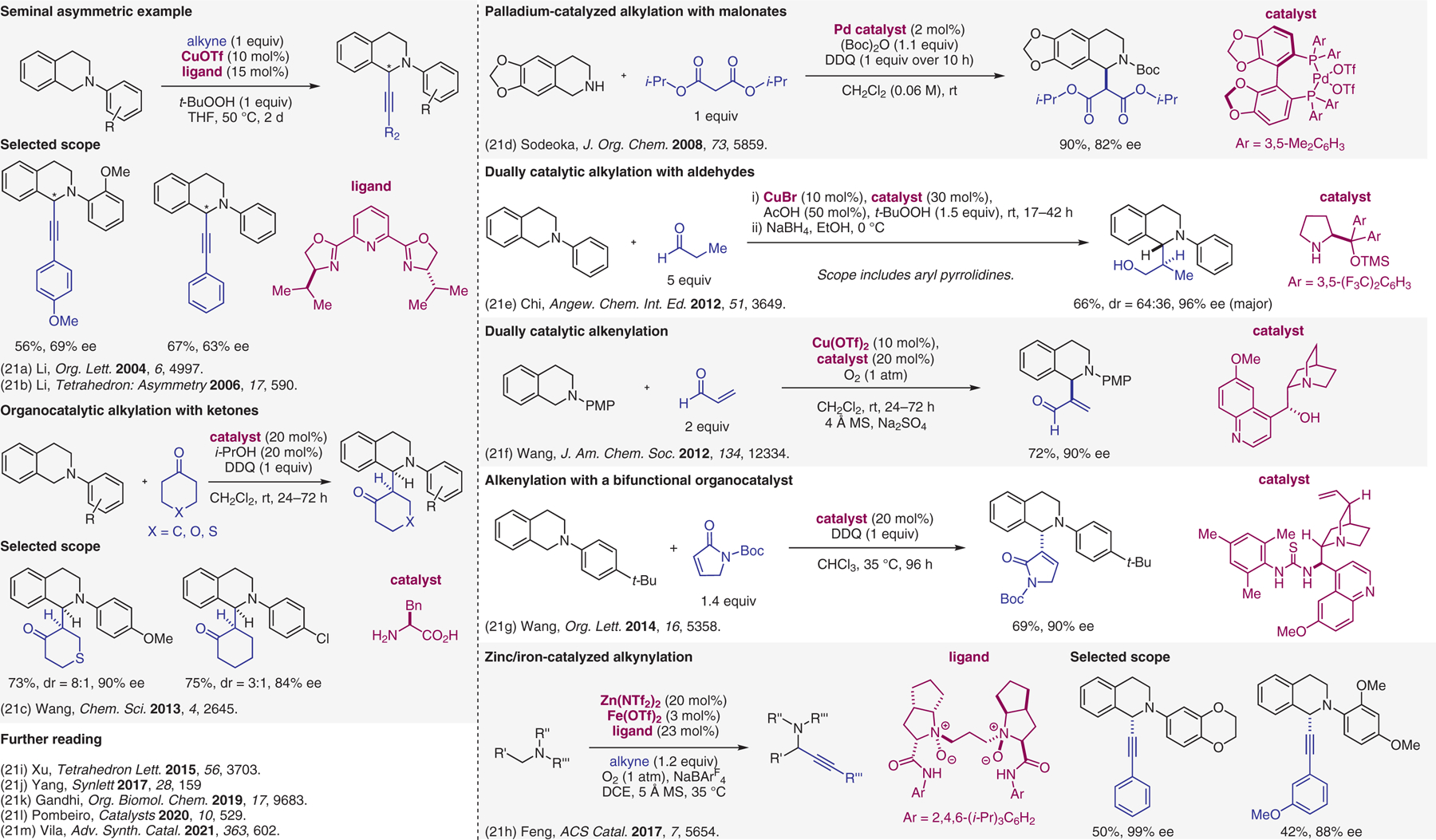
Catalytic enantioselective cross-dehydrogenative-coupling (CDC) reactions.21
Figure 22.
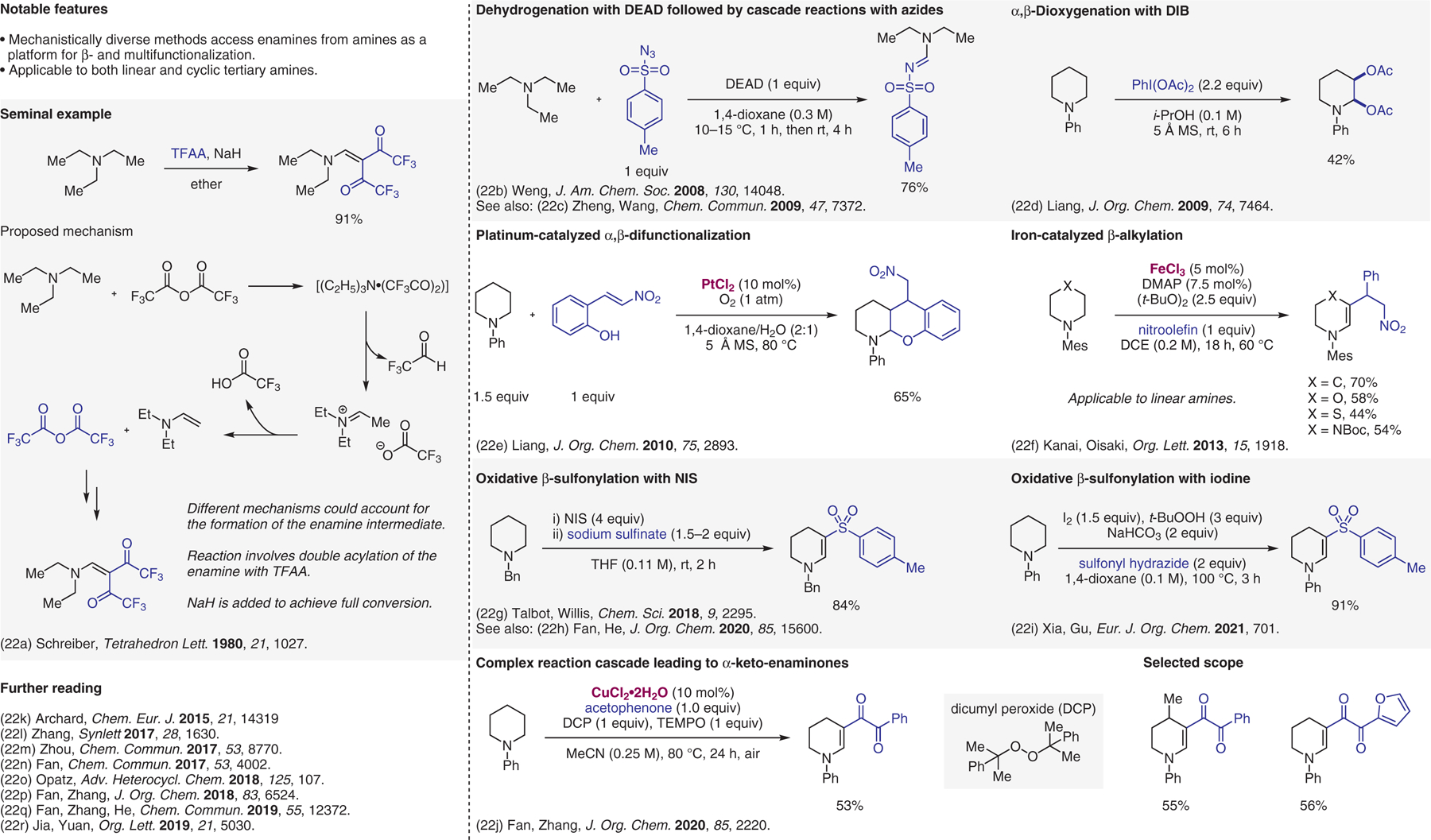
Oxidative β-functionalization.22
Figure 23.
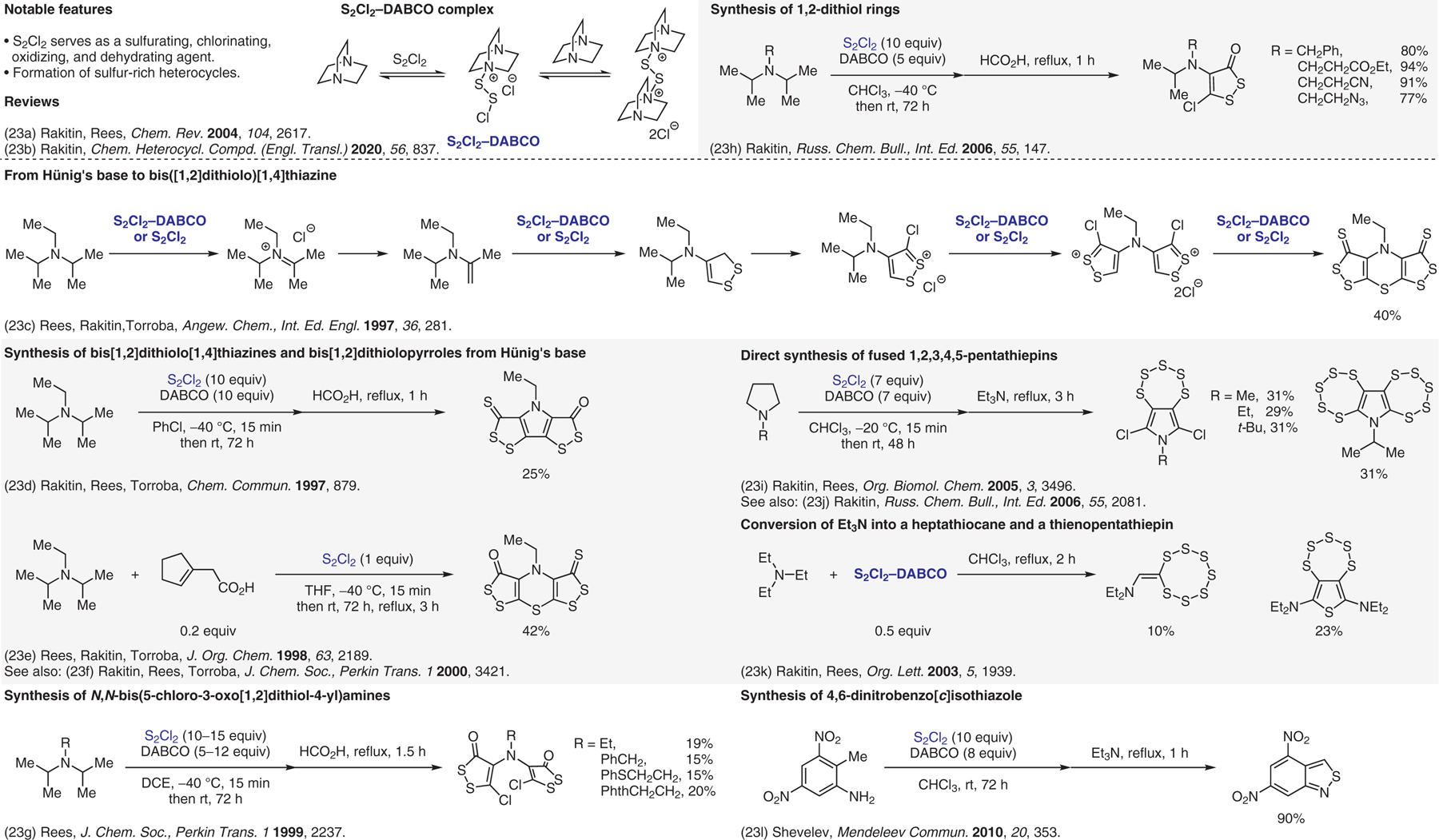
Oxidative formation of sulfur-rich heterocycles.23
Figure 24.
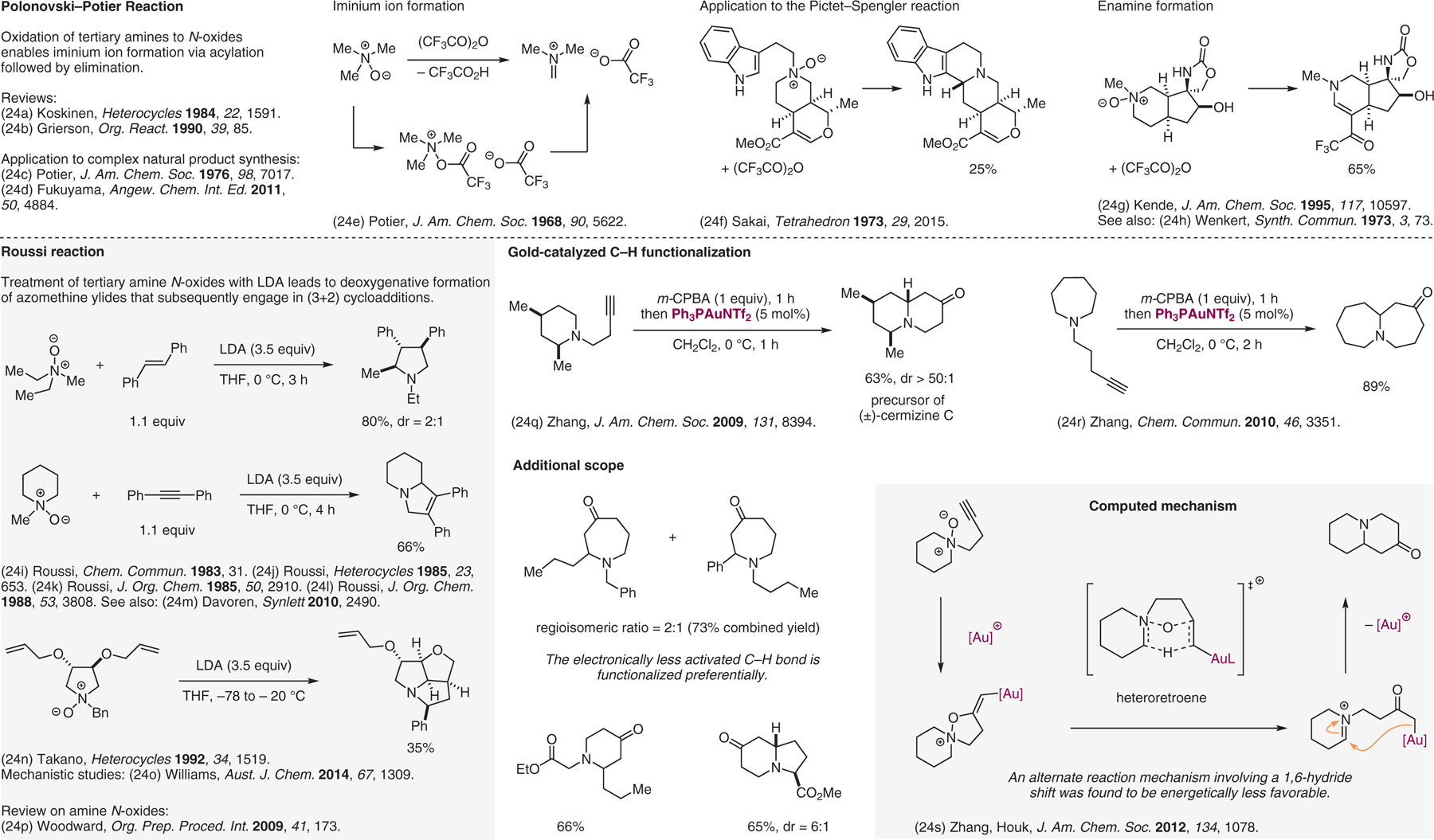
Reactions involving amine N-oxides.24
Figure 25.
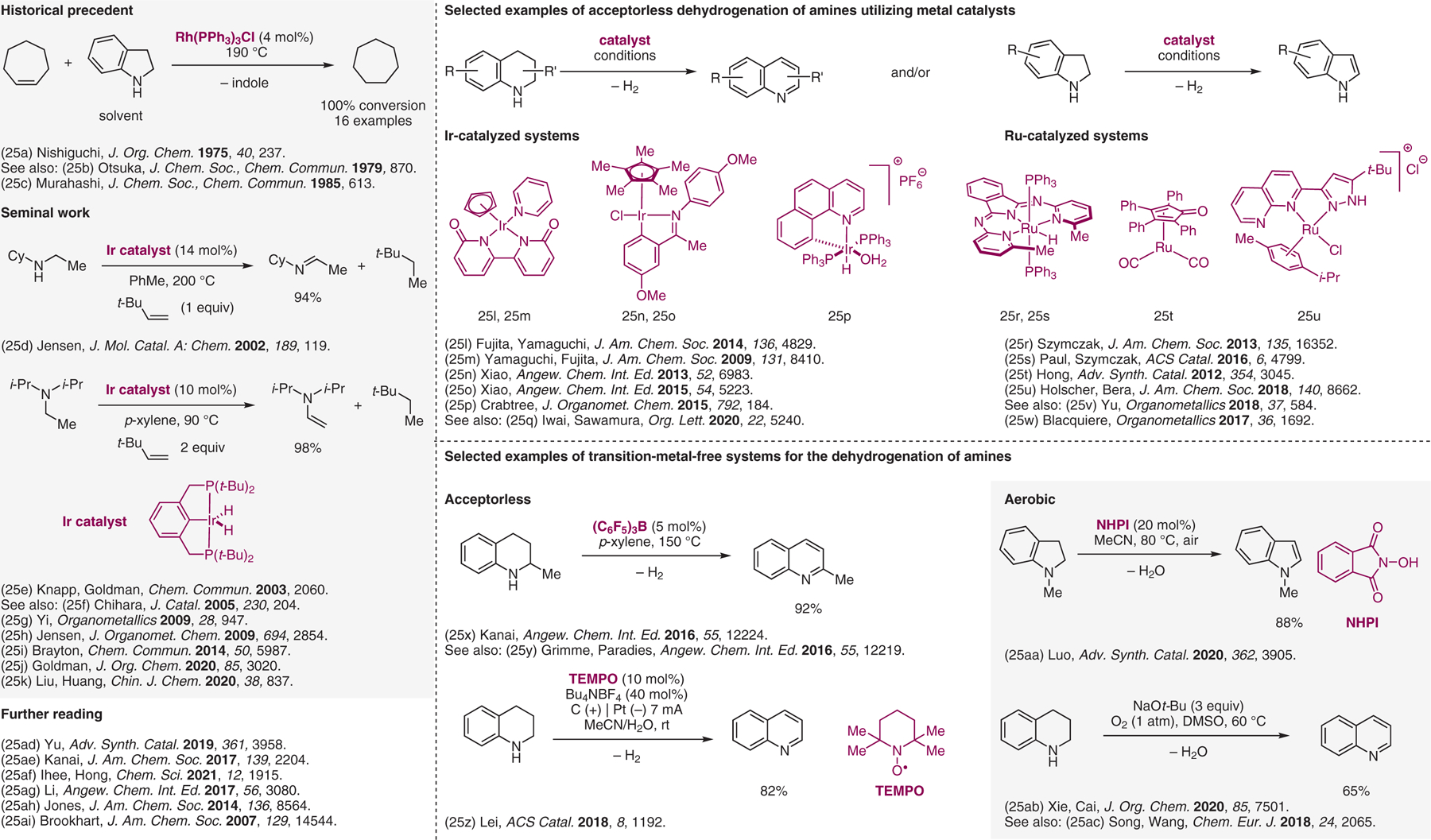
Dehydrogenation/aromatization.25
Figure 26.
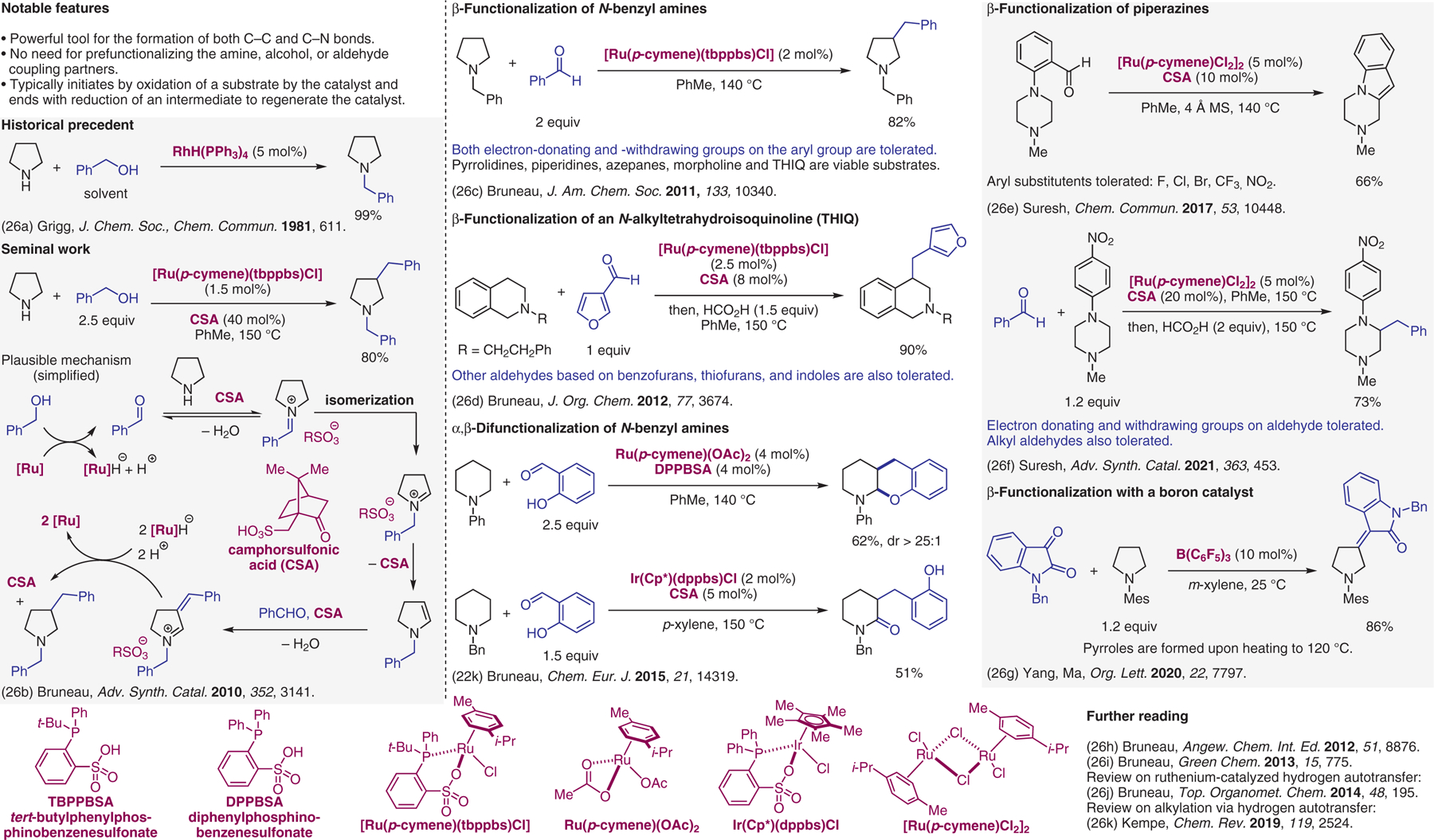
Hydrogen borrowing.26
Figure 27.
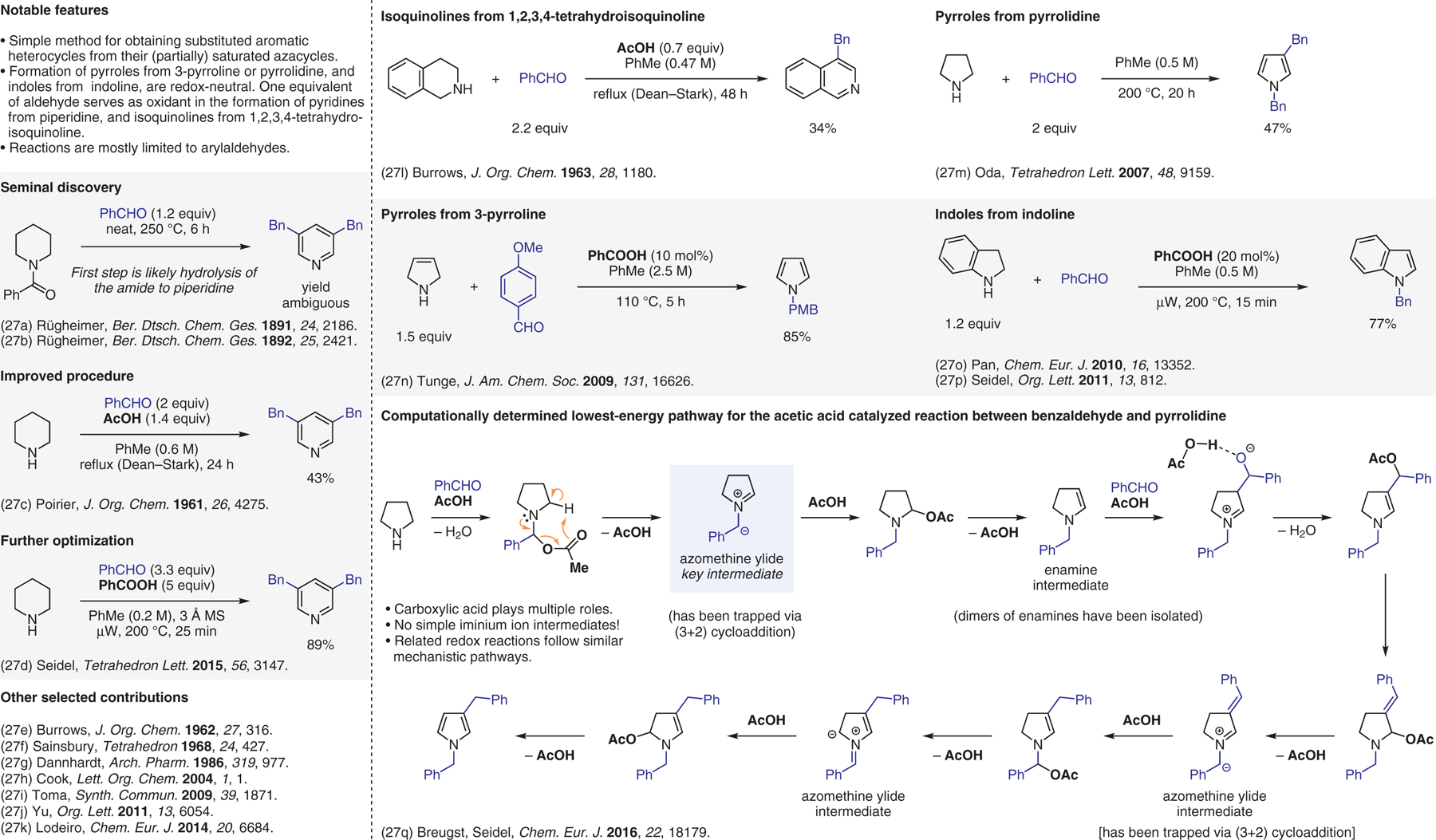
Condensation-based methods involving azomethine ylide intermediates, aromatization.27
Figure 28.
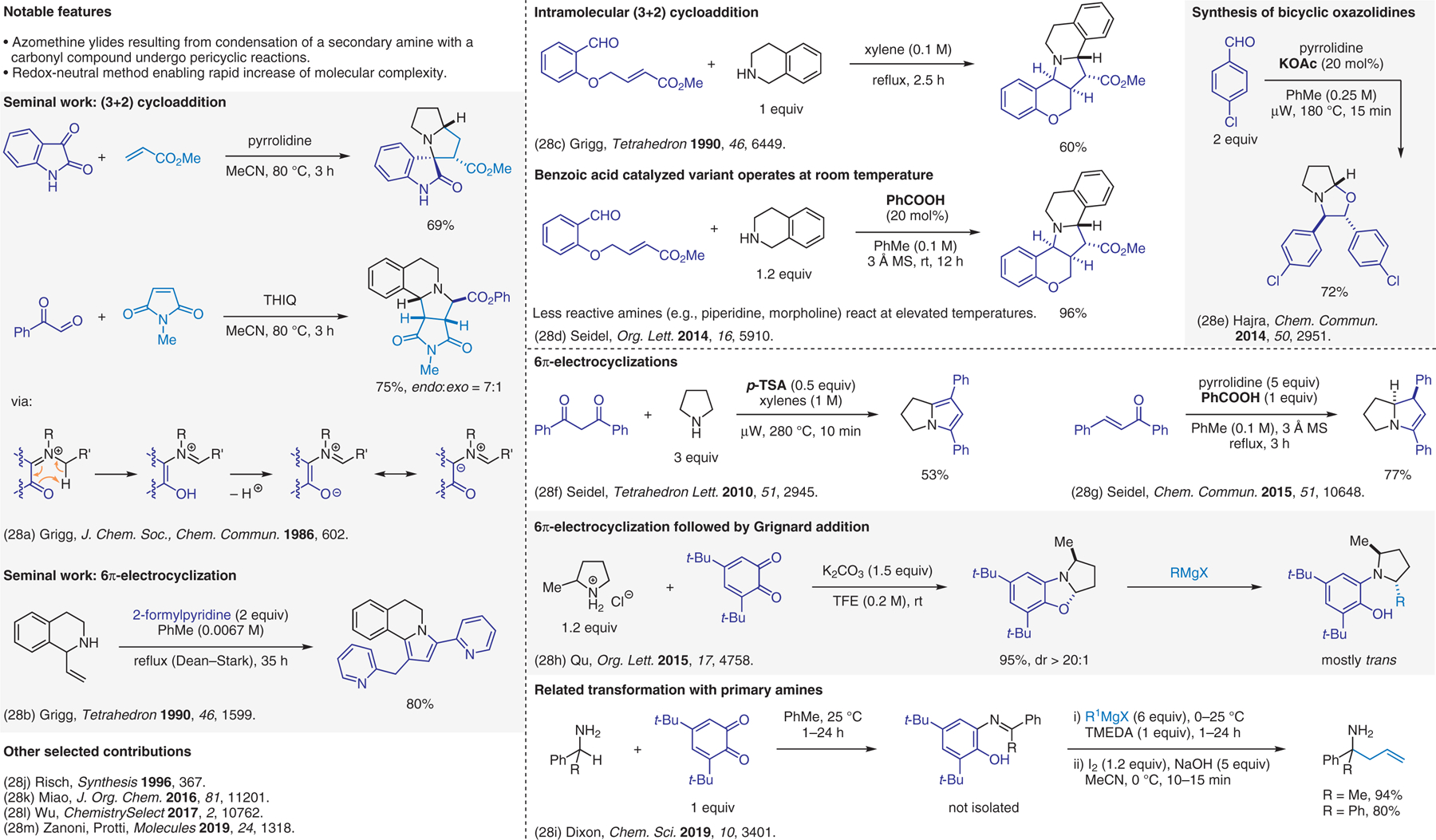
Condensation-based methods involving azomethine ylide intermediates, pericyclic reactions.28
Figure 29.
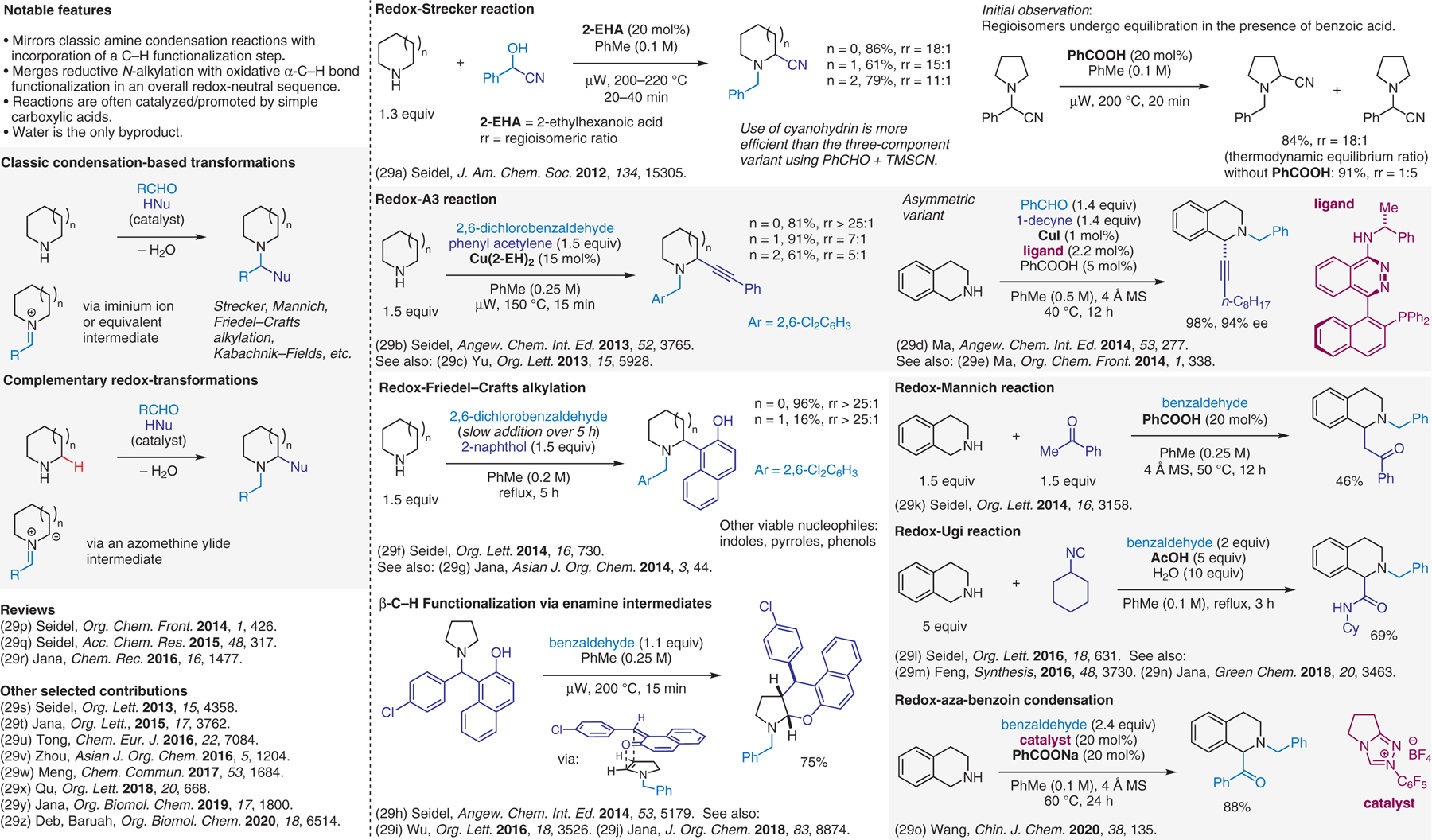
Condensation-based methods involving azomethine ylide intermediates, redox-neutral 3-component coupling reactions.29
Figure 30.
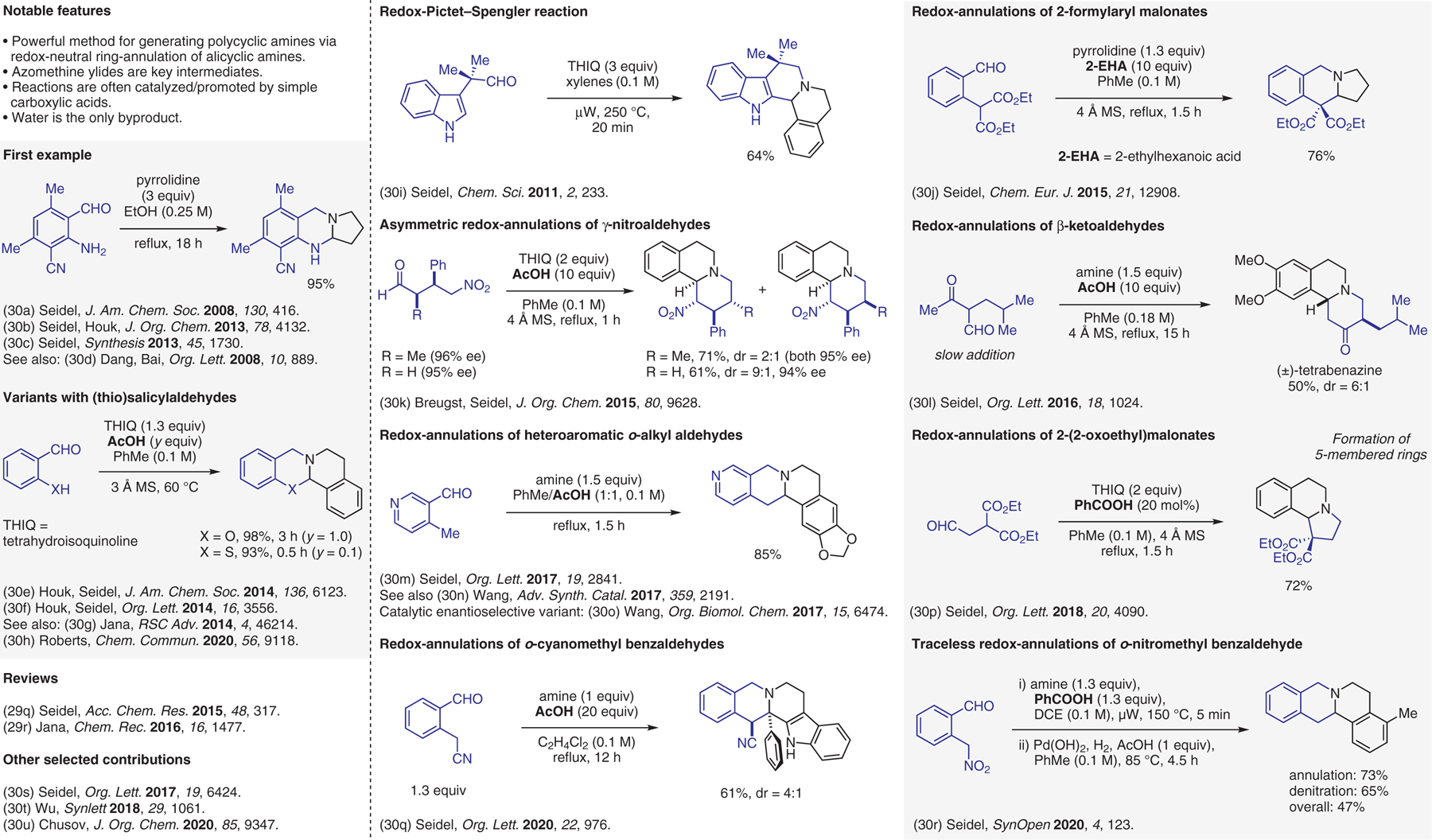
Condensation-based methods involving azomethine ylide intermediates, redox-annulations.30
Figure 31.
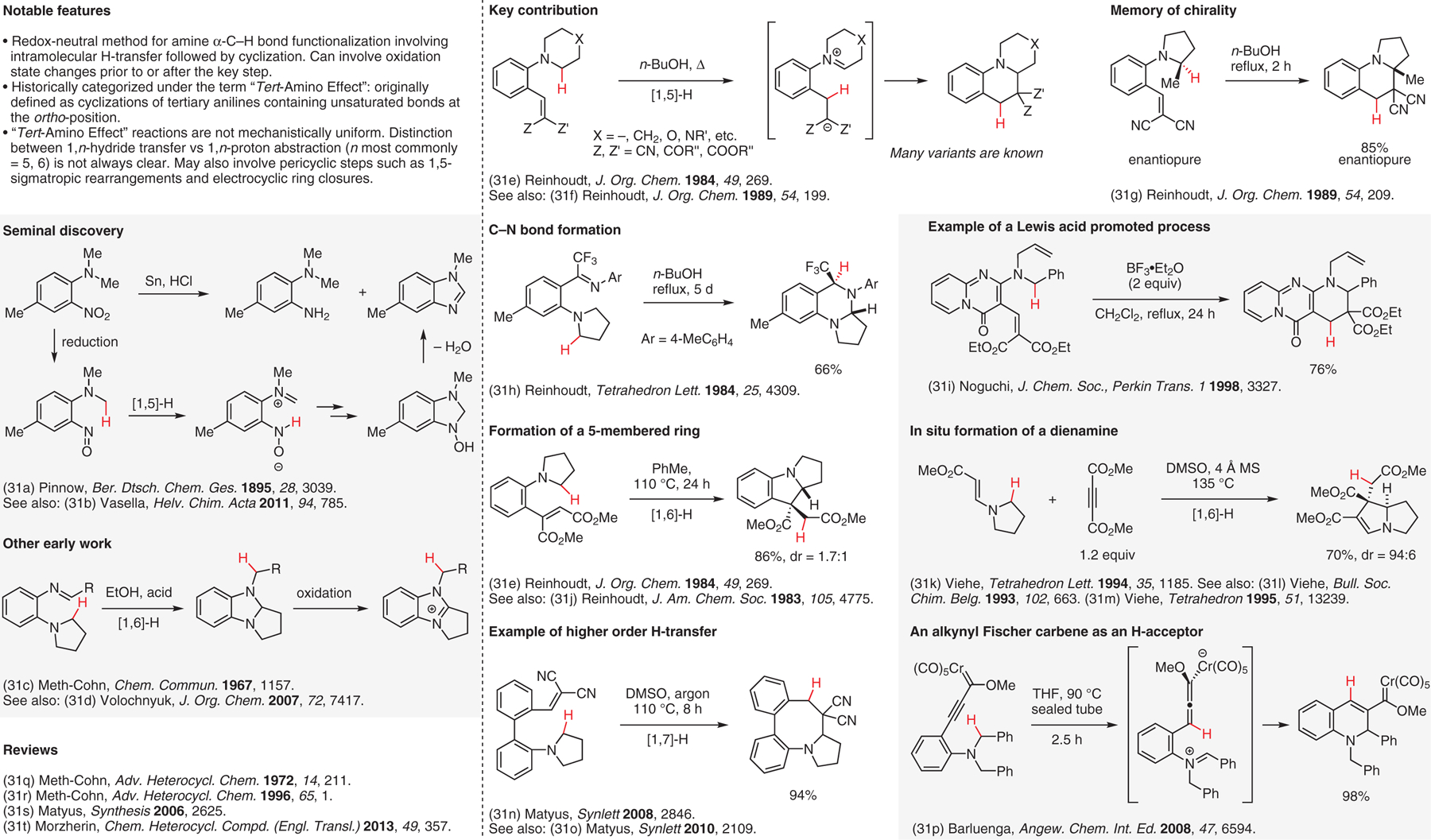
Internal redox transformations involving [1,n]-H transfers, the ‘tert-amino effect’.31
Figure 32.
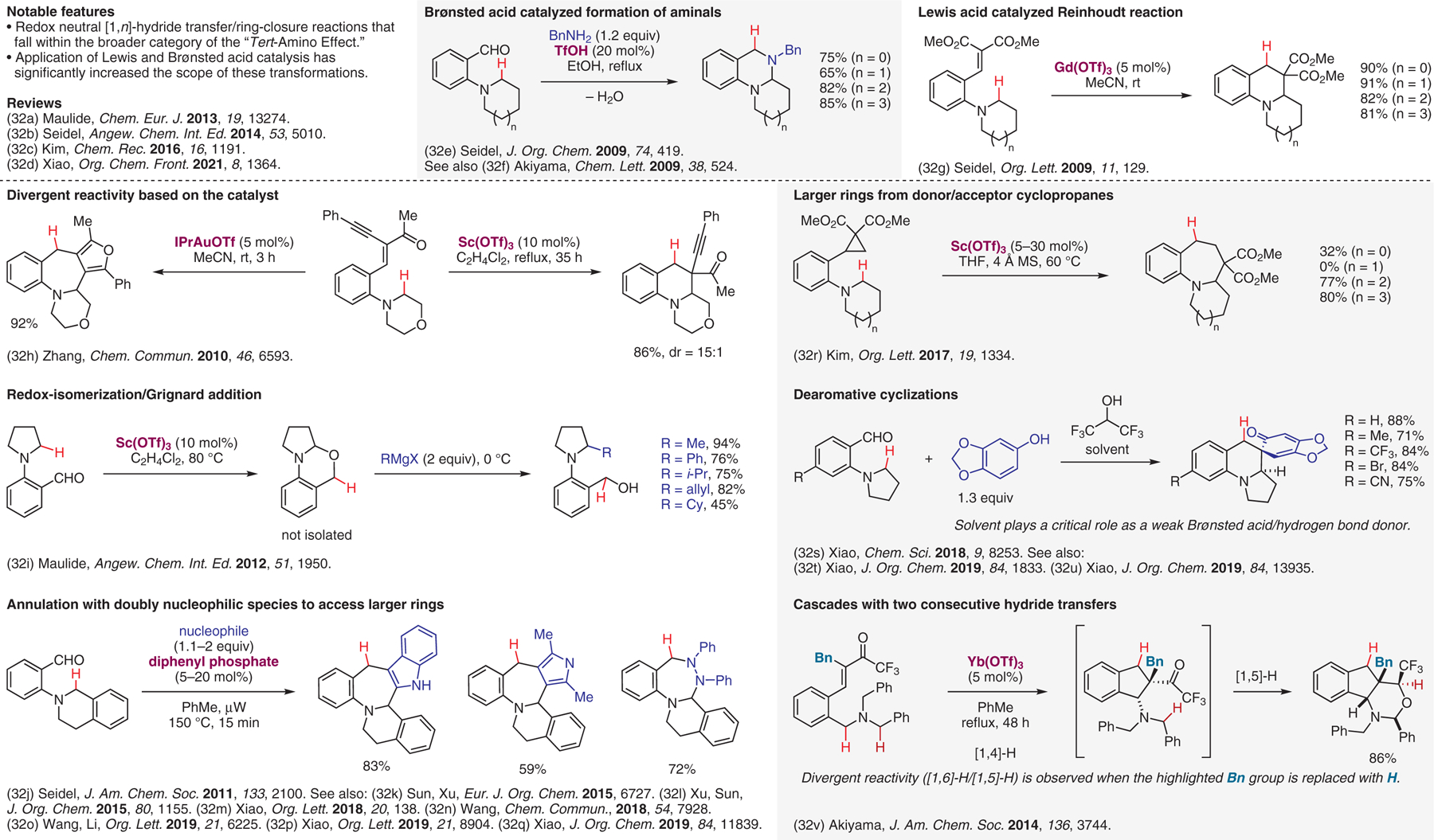
Lewis and Brønsted acid catalyzed internal redox transformations involving [1,n]-H transfers.32
Figure 33.
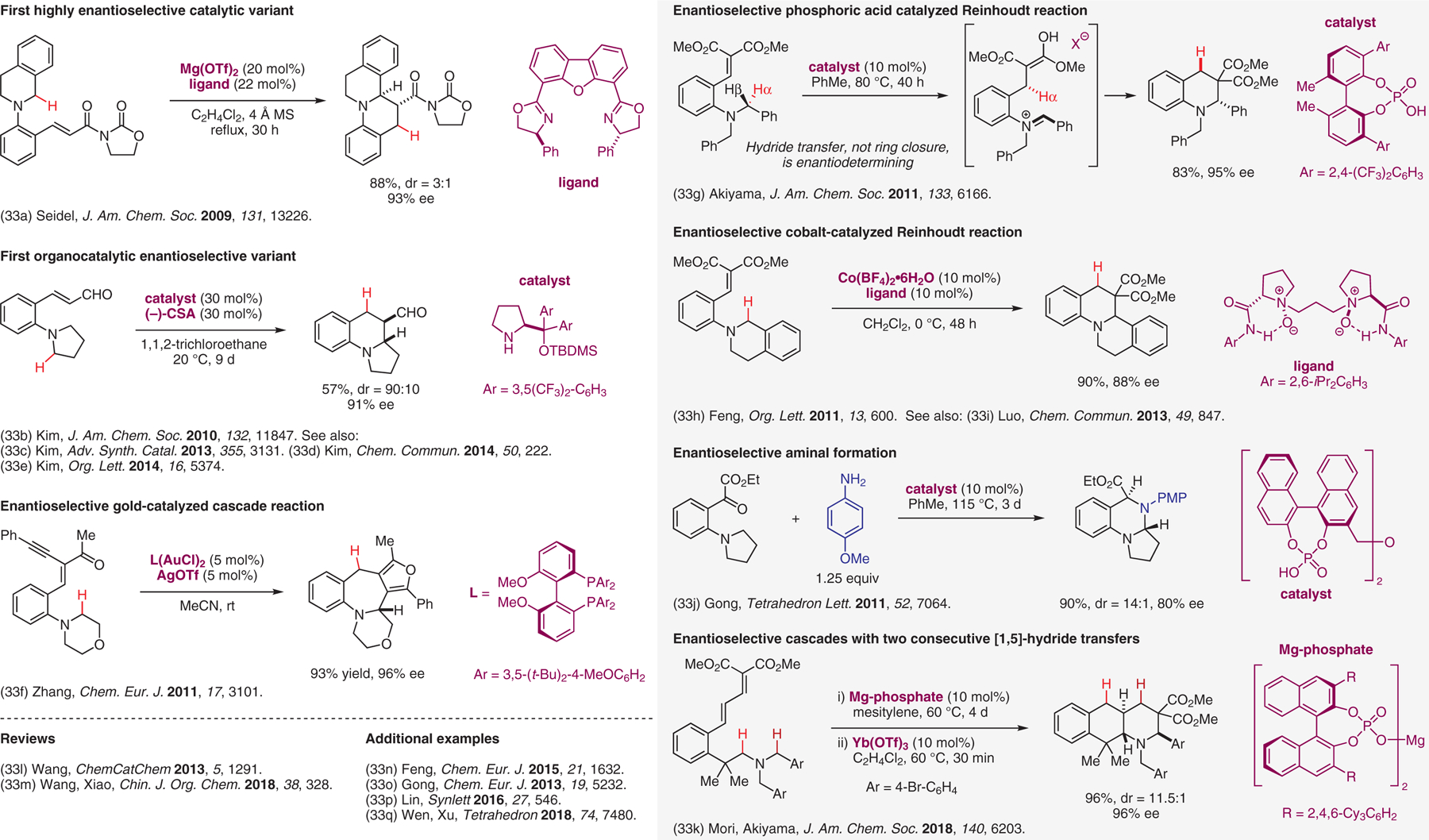
Catalytic enantioselective internal redox transformations involving [1,n]-H transfers.33
Figure 34.
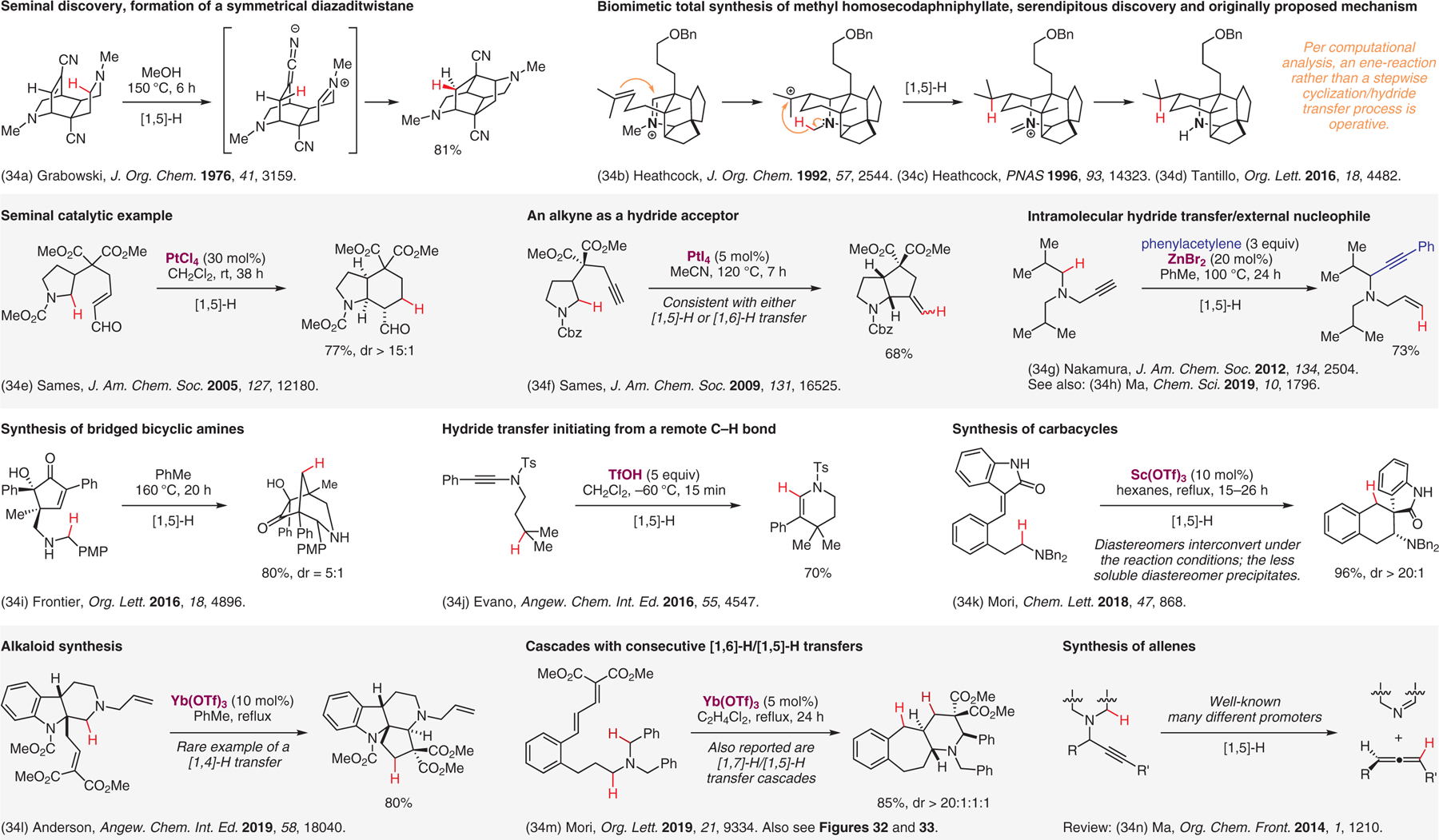
Internal redox transformations involving [1,n]-H transfers in non-conjugated systems.34
Figure 35.
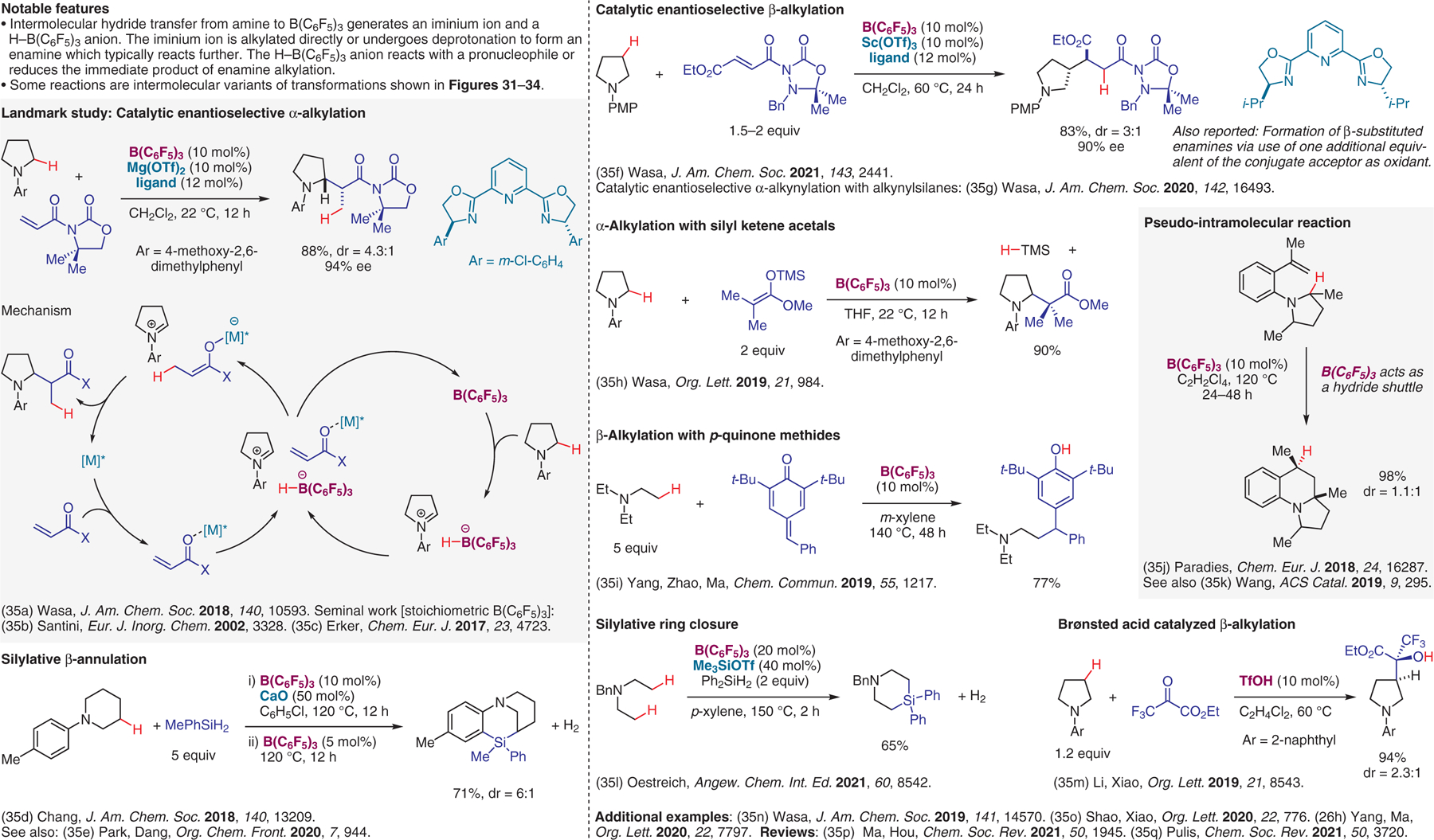
(Redox-neutral) methods involving intermolecular hydride transfer.35
Figure 36.
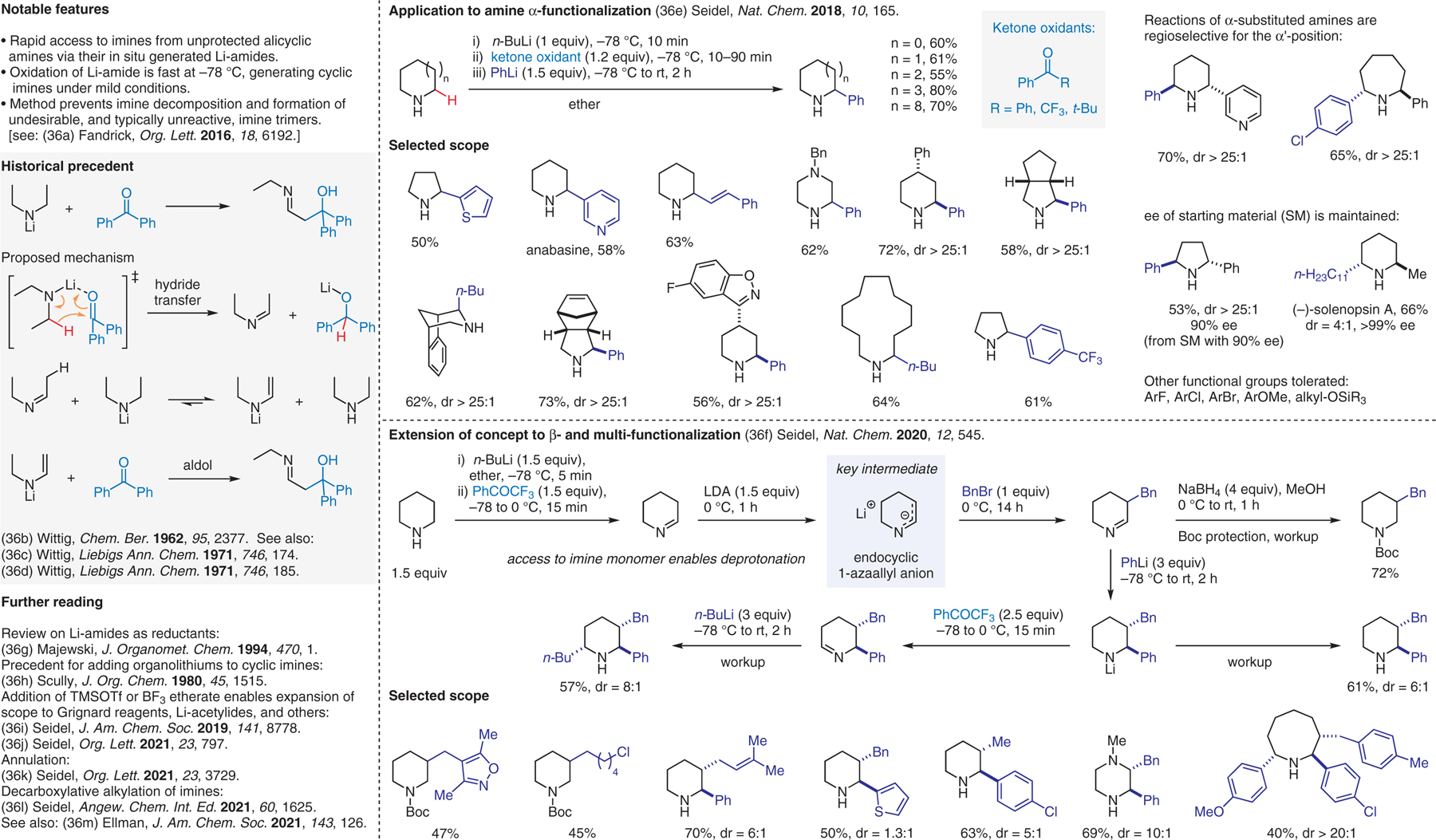
Li-amide-based imine and 1-azaallyl anion generation from unprotected azacycles.36
Figure 37.
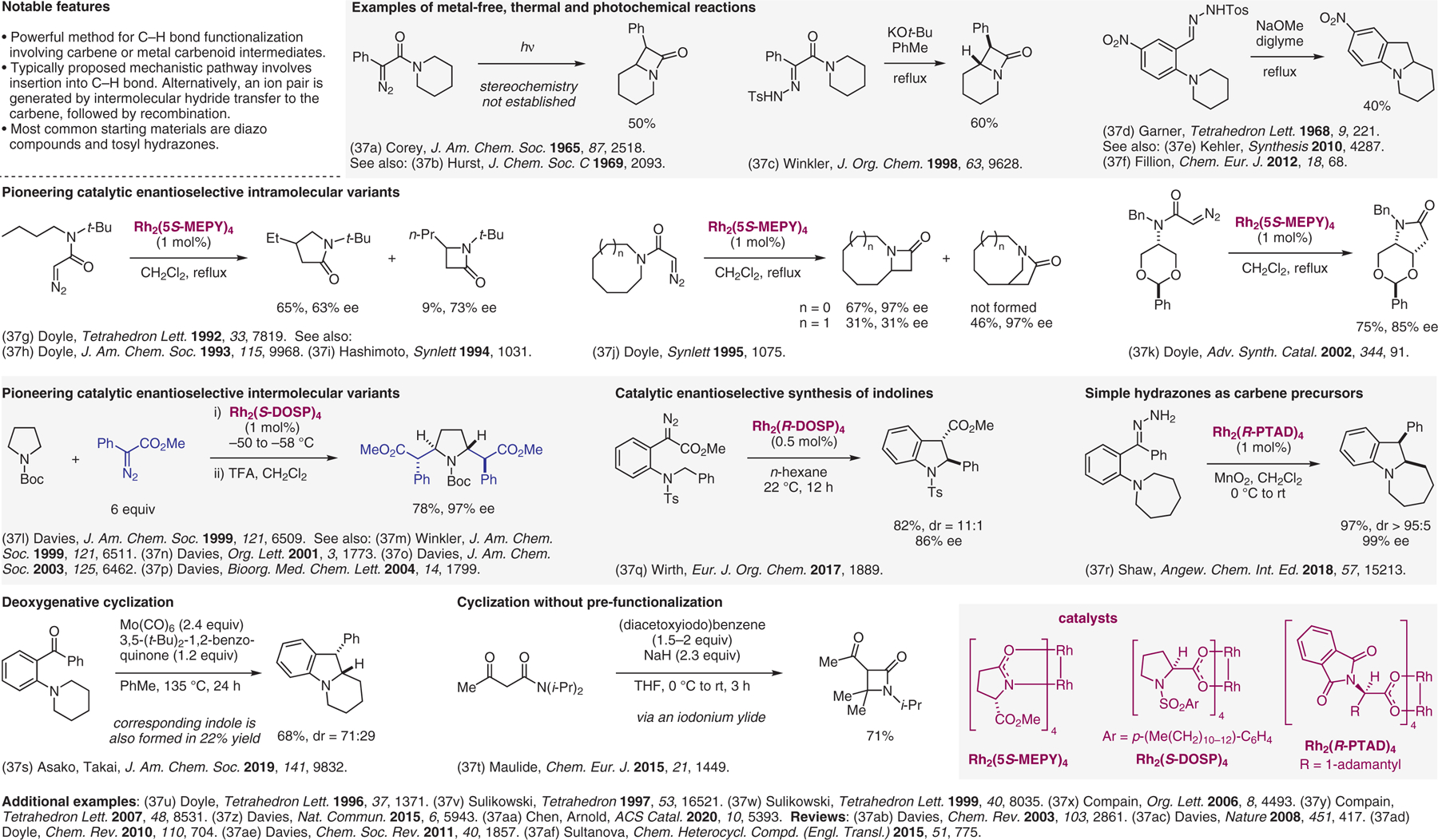
Reactions involving carbenes or metal carbenoids.37
Figure 38.
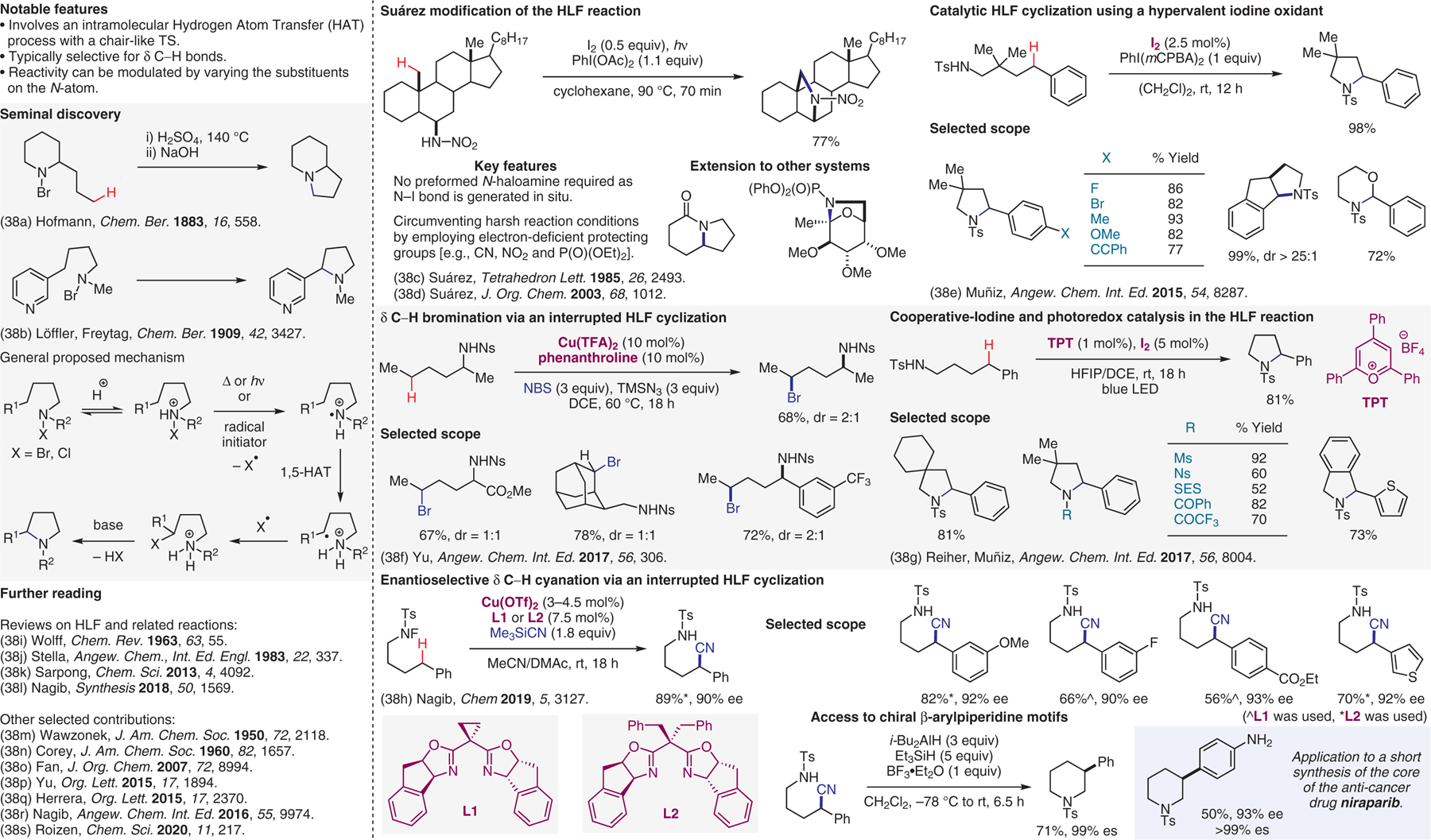
Hofmann–Löffler–Freytag (HLF) reaction.38
Figure 39.
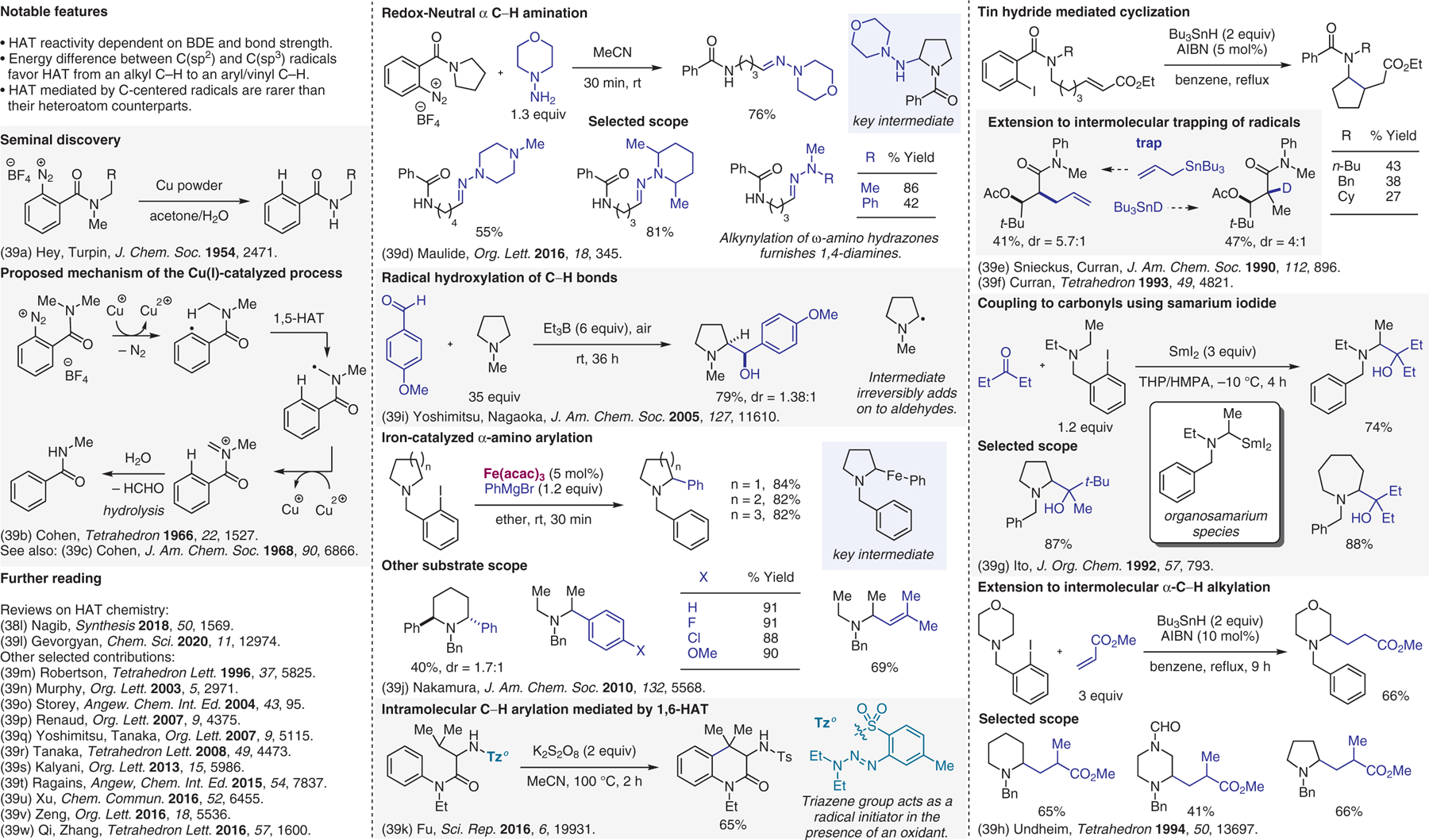
Miscellaneous radical-based methods.39
Figure 40.
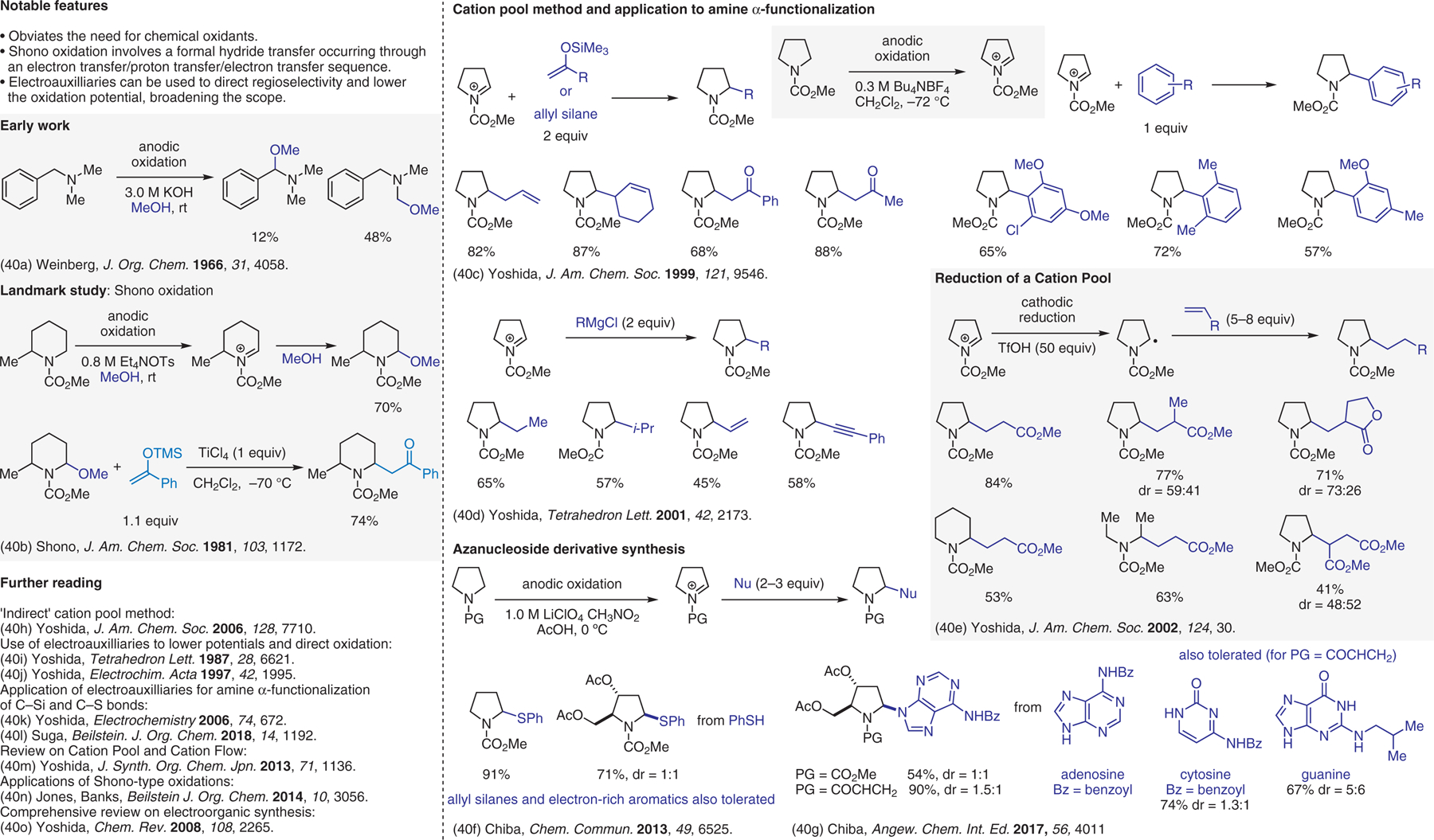
Electrochemical approaches, cation pool method.40
Figure 41.
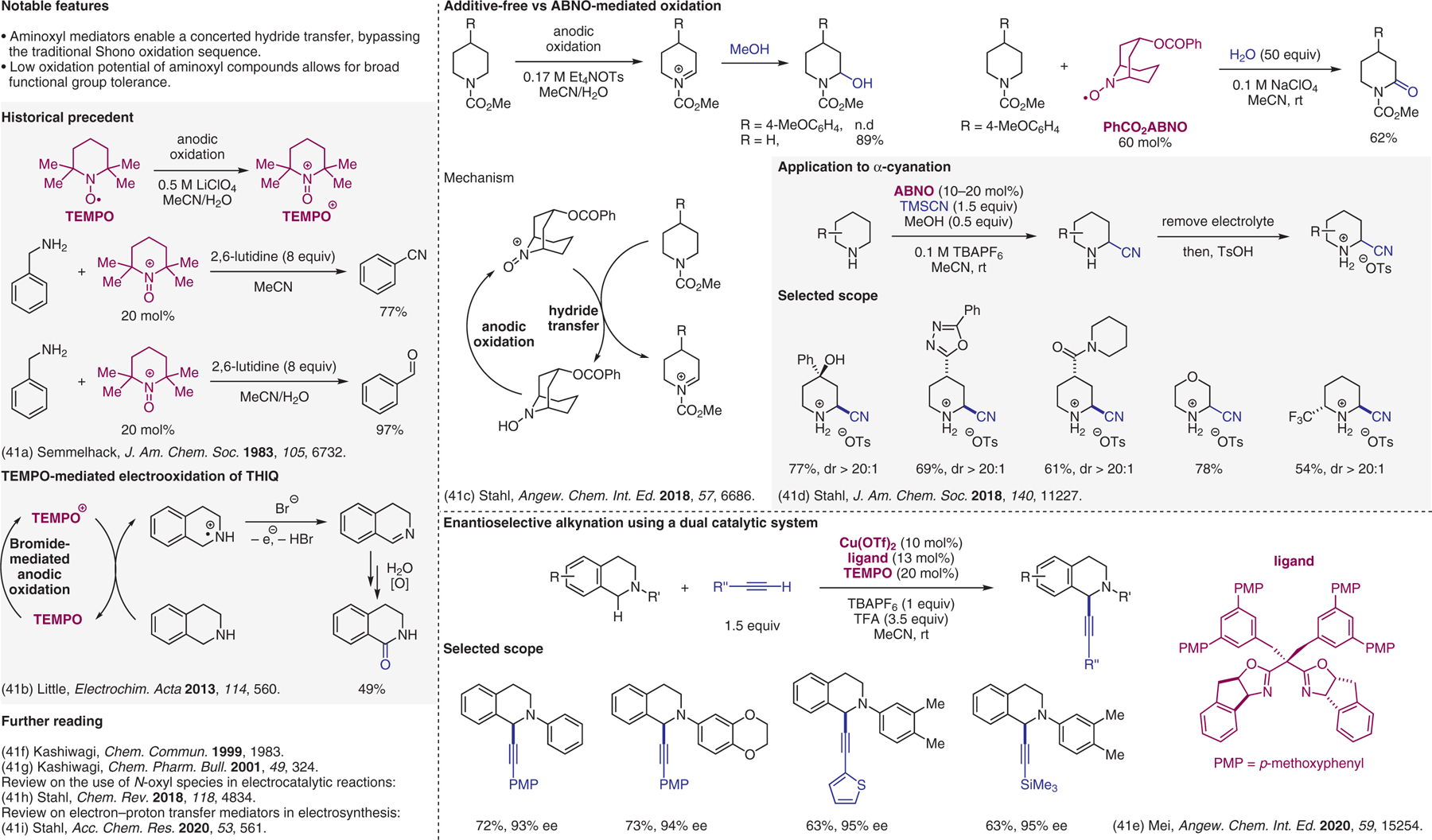
Electrochemical approaches, 9-azabicyclo[3.3.1]nonane N-oxyl (ABNO) catalysis.41
Figure 42.
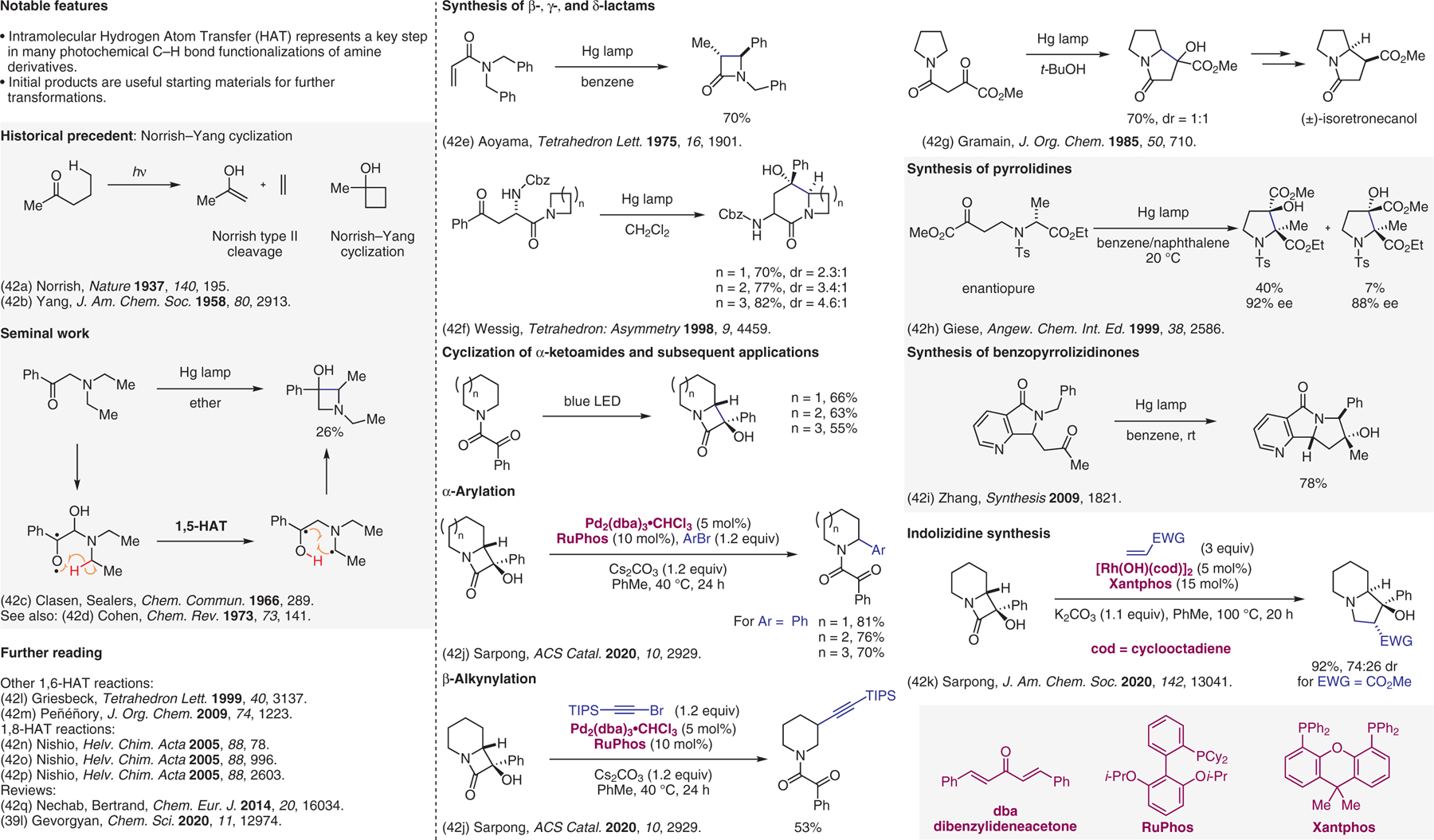
Intramolecular hydrogen atom transfer (HAT).42
Figure 43.
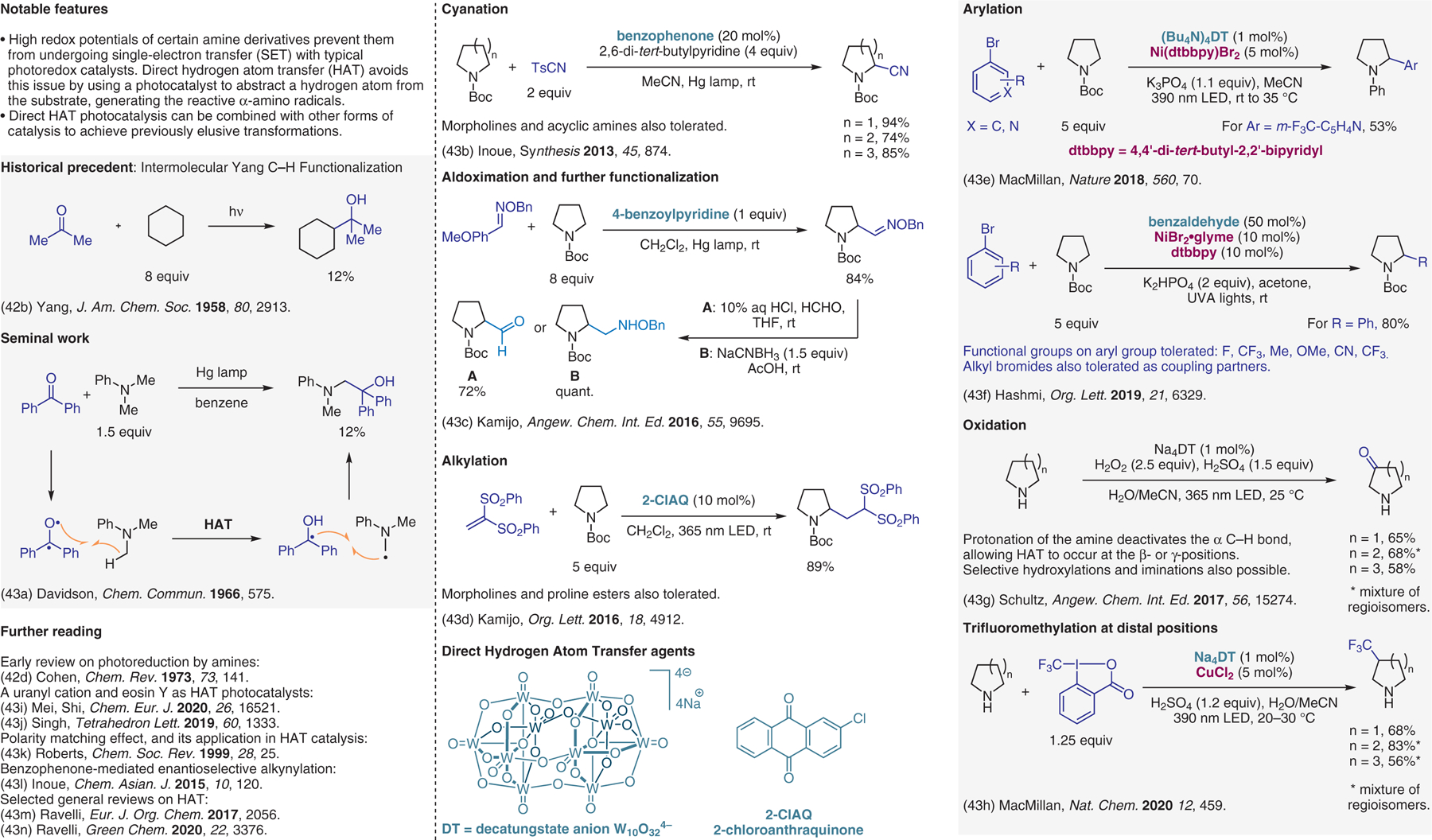
Direct hydrogen atom transfer (HAT).43
Figure 44.
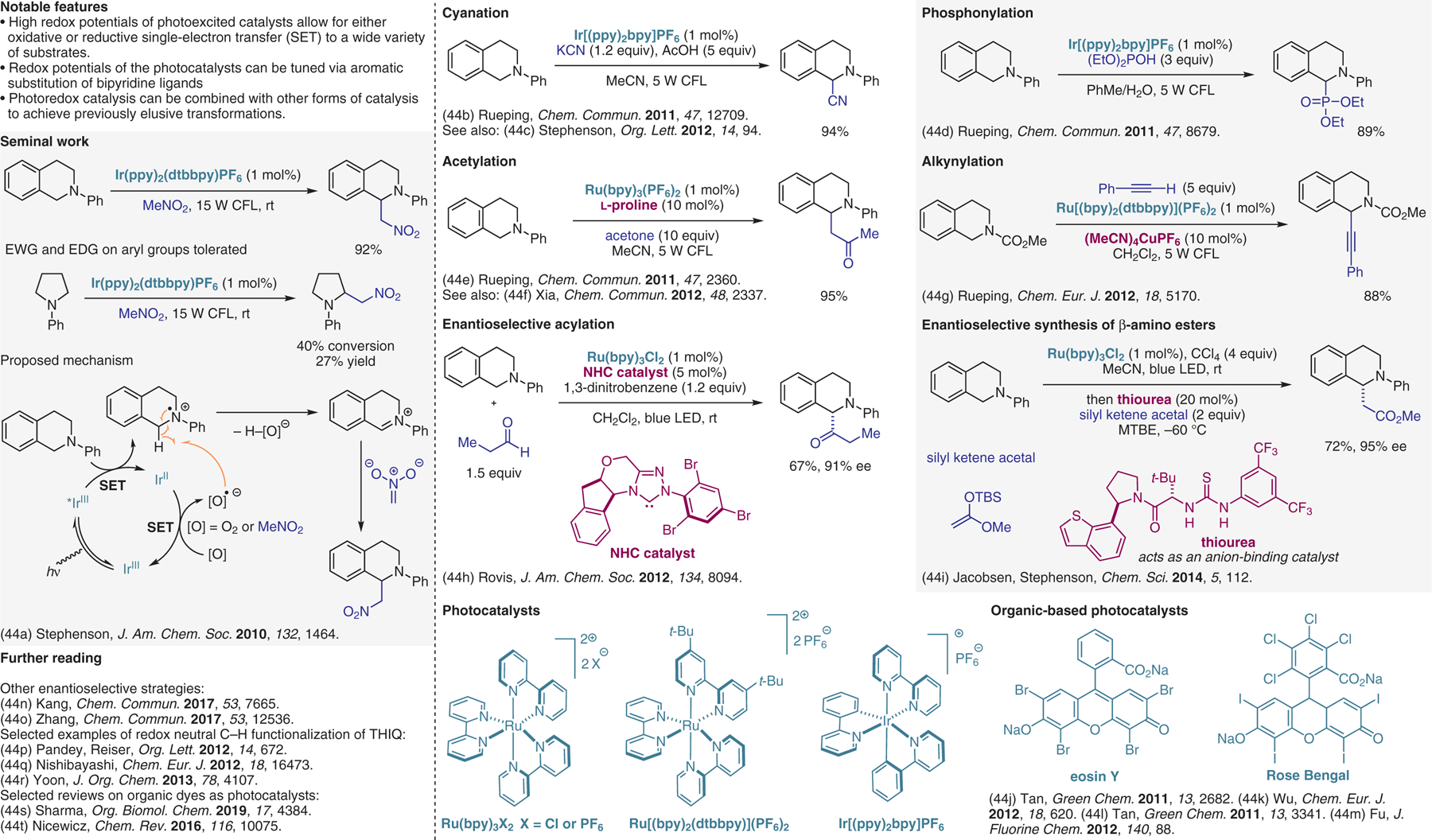
Photoredox approaches, part I.44
Figure 45.
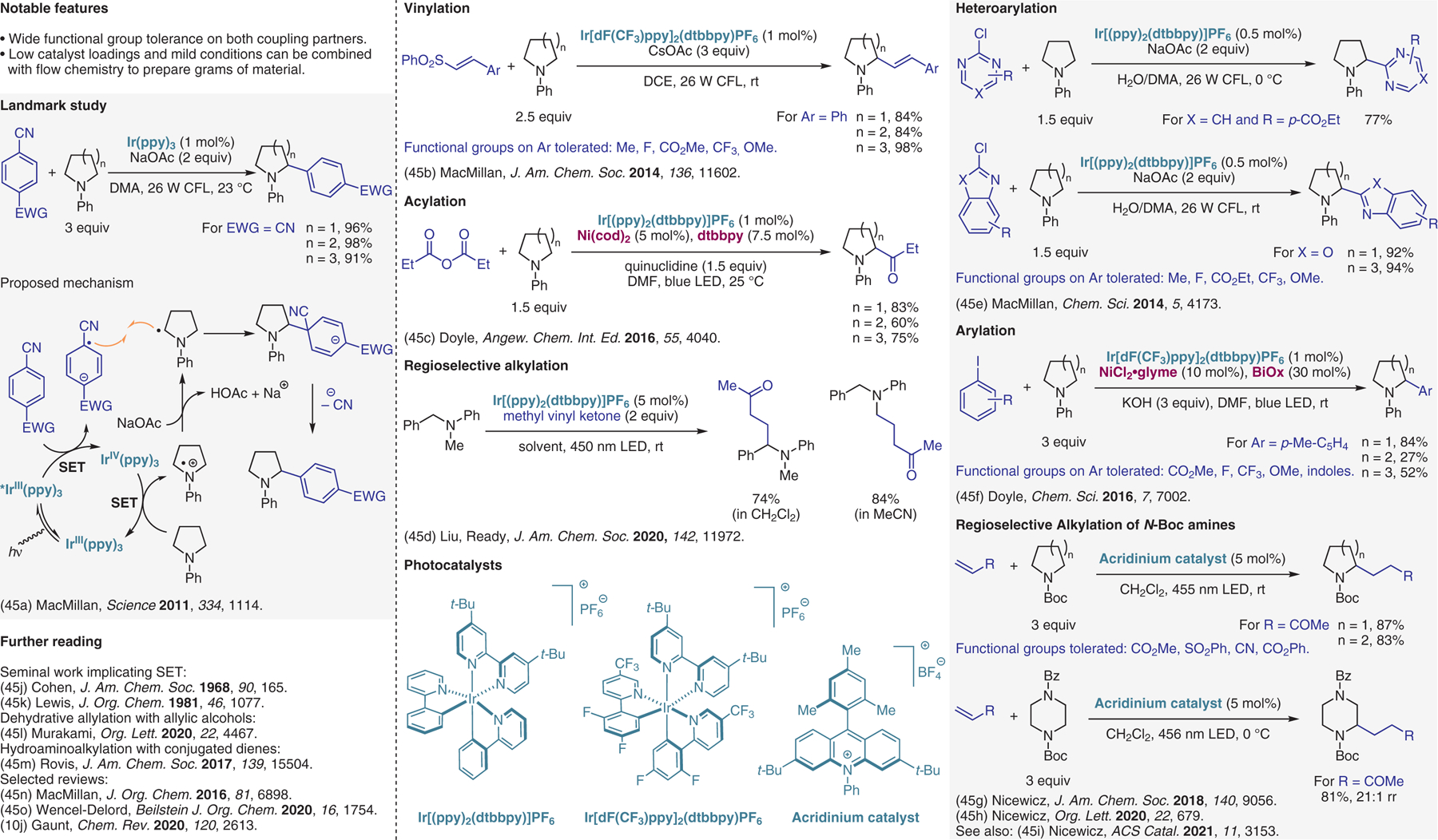
Photoredox approaches, part II.45
Figure 46.
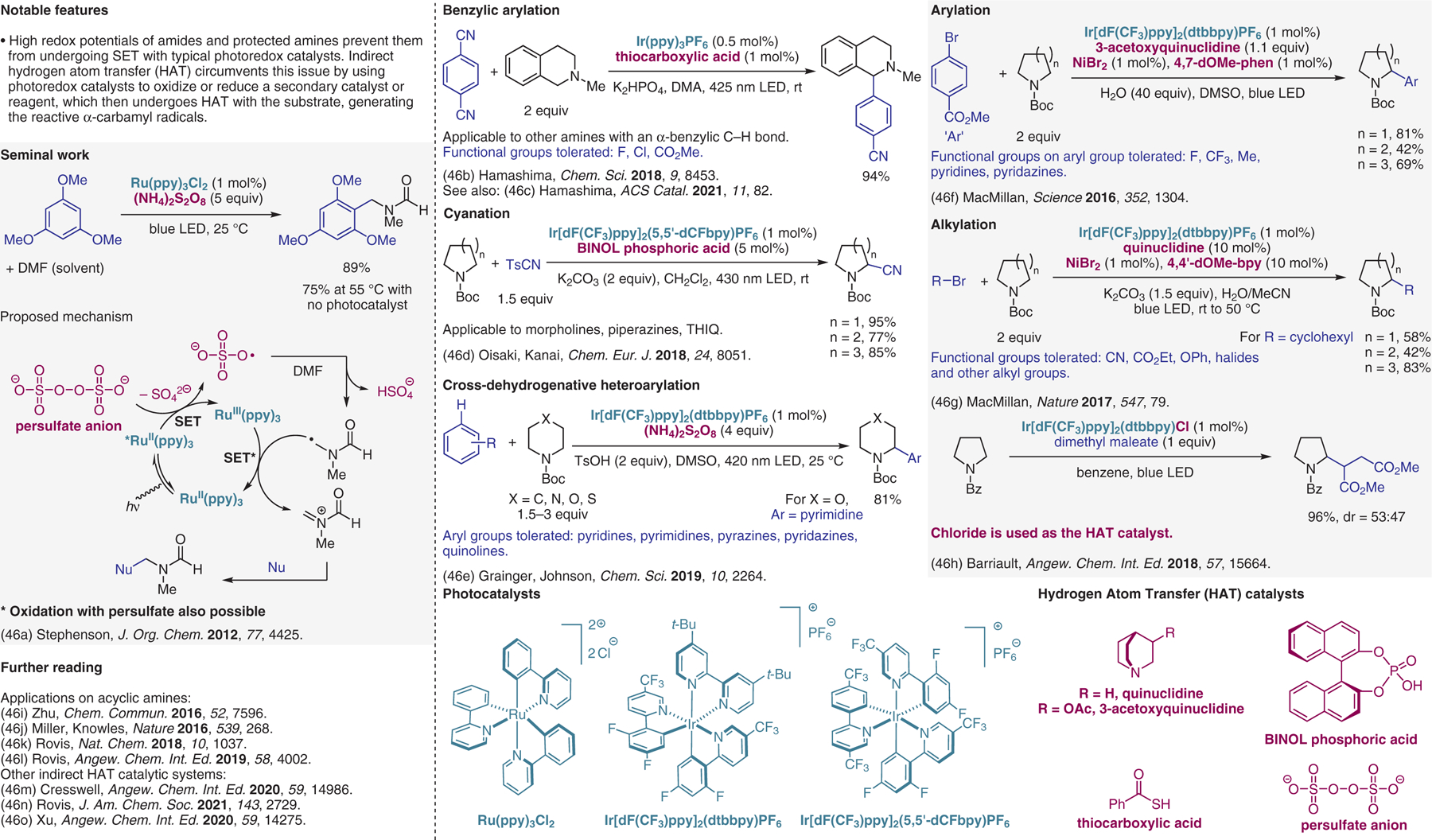
Indirect hydrogen atom transfer (HAT).46
Figure 47.
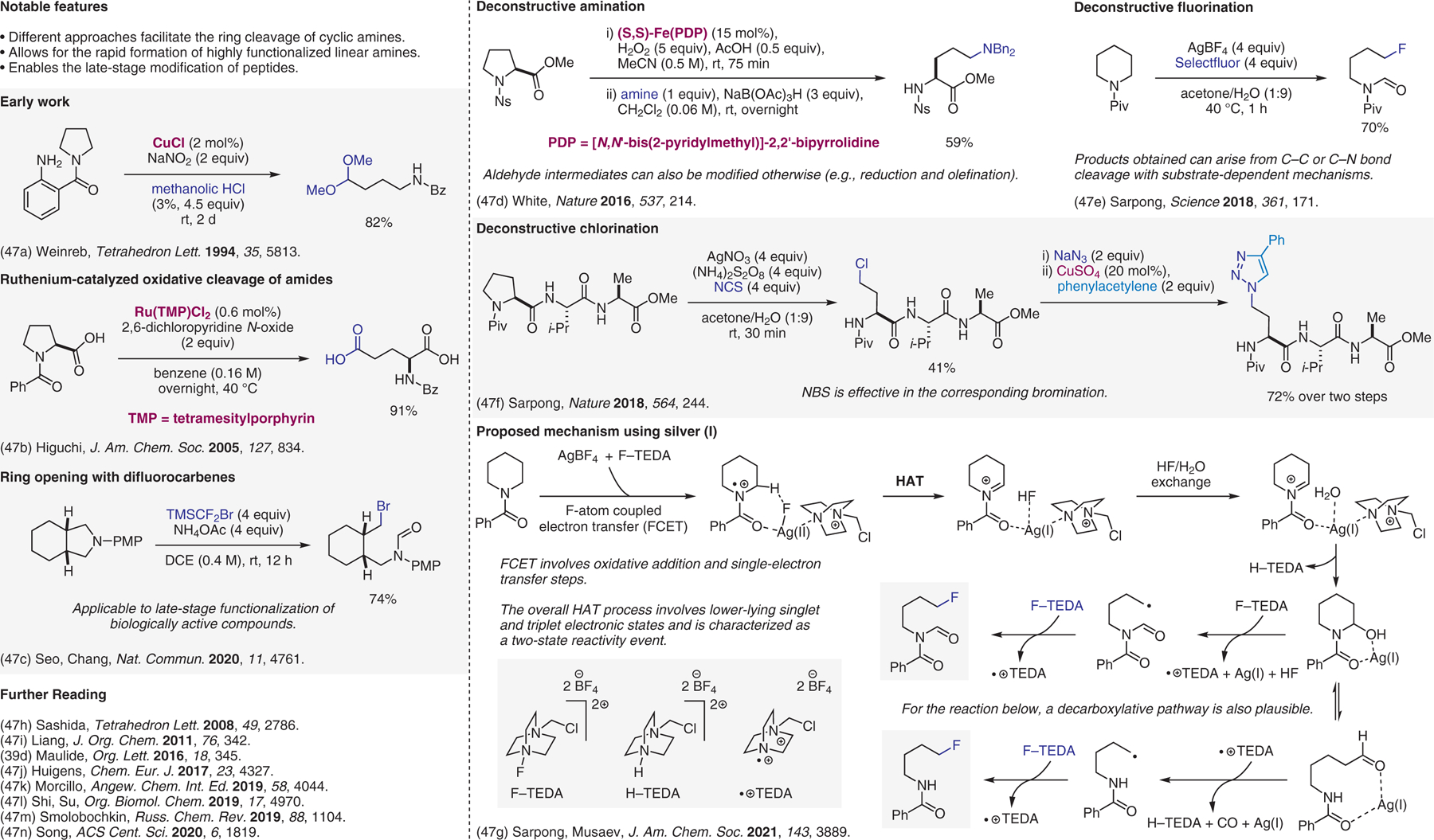
Deconstructive functionalization.47
Acknowledgment
We are grateful to the current and former members of the Seidel research group who have contributed to the development of this field.
Funding Information
Financial support from the NIH-NIGMS (grant no. R01GM101389) is gratefully acknowledged.
Biographies

Subhradeep Dutta was born and raised in West Bengal, India. He earned a B.Sc. degree in chemistry from Calcutta University (India) in 2016 and an M.Sc. degree in chemistry from the Indian Institute of Technology Kanpur (IITK) in 2018 under the guidance of Prof. Basker Sundararaju. In August 2018, he moved to the University of Florida (USA) for his graduate studies, joining the group of Prof. Daniel Seidel. His research focuses on developing methods towards the C–H bond functionalization of cyclic amines.

Bowen Li was born and raised in Shandong, P. R. of China. He earned a B.Sc. degree in the School of Chemistry and Chemical Engineering at Shanghai Jiao Tong University (P. R. of China) working with Prof. Wanbin Zhang. In 2019, he moved to the University of Florida (USA) for his graduate studies, joining the group of Prof. Daniel Seidel. His research focuses on asymmetric catalysis and C–H bond functionalization.

Dillon Rickertsen was born in Denver, Colorado, USA. He earned a B.Sc. degree in the Department of Chemistry at the University of Colorado, Denver (USA), working with Prof. Scott Reed. In 2019, he moved to the University of Florida for his graduate studies, joining the group of Prof. Daniel Seidel. His research is focused on developing methodologies for the C–H bond functionalization of amines.

Daniel Valles was born in Caracas, Venezuela and raised in Weston, Florida, USA. He attended the California Institute of Technology (Caltech) (USA) working with Prof. Peter Dervan, Prof. Sarah Reisman, and Dr. Scott Virgil. In 2018, he started his Ph.D. research at the University of Florida under the direction of Prof. Daniel Seidel. His research focuses on the functionalization of C–H bonds on cyclic amines.

Daniel Seidel studied chemistry at the Friedrich-Schiller-Universität Jena (Germany) and at the University of Texas at Austin (USA) (Diplom 1998). He performed his graduate studies in the lab of Prof. Jonathan L. Sessler, obtaining his Ph.D. in 2002. From 2002–2005, he was an Ernst Schering Postdoctoral Fellow in the group of Prof. David A. Evans at Harvard University (USA). He started his independent career at Rutgers University (USA) in 2005 and was promoted to Associate Professor in 2011 and Full Professor in 2014. In the summer of 2017, his research group moved to the University of Florida (USA).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- (1).(a) Peterson DJ; Hays HR J. Org. Chem 1965, 30, 1939. [Google Scholar]; (b) Lepley AR; Giumanini AG J. Org. Chem 1966, 31, 2055. [Google Scholar]; (c) Ahlbrecht H; Dollinger H Tetrahedron Lett. 1984, 25, 1353. [Google Scholar]; (d) Gessner VH; Strohmann C J. Am. Chem. Soc 2008, 130, 14412. [DOI] [PubMed] [Google Scholar]; (e) Kessar SV; Singh P; Vohra R; Kaur NP; Singh KN J. Chem. Soc., Chem. Commun 1991, 568. [Google Scholar]; (f) Kessar SV; Vohra R; Kaur NP Tetrahedron Lett. 1991, 32, 3221. [Google Scholar]; (g) De Ceglie MC; Musio B; Affortunato F; Moliterni A; Altomare A; Florio S; Luisi R Chem. Eur. J 2011, 17, 286. [DOI] [PubMed] [Google Scholar]; (h) Singh KN; Singh P; Singh P; Deol YS Org. Lett 2012, 14, 2202. [DOI] [PubMed] [Google Scholar]; (i) Lepley AR; Khan WA J. Org. Chem 1966, 31, 2061. [Google Scholar]; (j) Lepley AR; Khan WA Chem. Commun 1967, 1198. [Google Scholar]; (k) Kessar SV; Singh P Chem. Rev 1997, 97, 721. [DOI] [PubMed] [Google Scholar]; (l) Katritzky AR; Qi M Tetrahedron 1998, 54, 2647. [Google Scholar]; (m) Ferey V; Toupet L; Le Gall T; Mioskowski C Angew. Chem., Int. Ed. Engl 1996, 35, 430. [Google Scholar]; (n) Vedejs E; Kendall JT J. Am. Chem. Soc 1997, 119, 6941. [Google Scholar]; (o) Ebden MR; Simpkins NS; Fox DNA Tetrahedron 1998, 54, 12923. [Google Scholar]; (p) Kessar SV; Singh P; Singh KN; Venugopalan P; Kaur A; Bharatam PV; Sharma AK J. Am. Chem. Soc 2007, 129, 4506. [DOI] [PubMed] [Google Scholar]; (q) Harmata M; Carter KW; Jones DE; Kahraman M Tetrahedron Lett. 1996, 37, 6267. [Google Scholar]; (r) Kovács E; Huszka B; Gáti T; Nyerges M; Faigl F; Mucsi Z J. Org. Chem 2019, 84, 7100. [DOI] [PubMed] [Google Scholar]; (s) Kovács E; Faigl F; Mucsi Z J. Org. Chem 2020, 85, 11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Keefer LK; Fodor CH J. Am. Chem. Soc 1970, 92, 5747. [Google Scholar]; (b) Seebach D; Enders D Angew. Chem., Int. Ed. Engl 1972, 11, 301. [Google Scholar]; (c) Seebach D; Enders D Angew. Chem., Int. Ed. Engl 1972, 11, 1101. [Google Scholar]; (d) Seebach D; Wykypiel W Synthesis 1979, 423. [Google Scholar]; (e) Seebach D; Enders D J. Med. Chem 1974, 17, 1225. [DOI] [PubMed] [Google Scholar]; (f) Fraser RR; Passannanti S Synthesis 1976, 540. [Google Scholar]; (g) Wykypiel W; Seebach D Tetrahedron Lett. 1980, 21, 1927. [Google Scholar]; (h) Savignac P; Dreux M; Leroux Y Tetrahedron Lett. 1974, 15, 2651. [Google Scholar]; (i) Savignac P; Leroux Y J. Organomet. Chem 1973, 57, C47. [Google Scholar]; (j) Magnus P; Roy G Synthesis 1980, 575. [Google Scholar]; (k) Seebach D; Yoshifuji M Helv. Chim. Acta 1981, 64, 643. [Google Scholar]; (l) Beak P; Zajdel WJ J. Am. Chem. Soc 1984, 106, 1010. [Google Scholar]; (m) Meyers AI; Edwards PD; Rieker WF; Bailey TR J. Am. Chem. Soc 1984, 106, 3270. [Google Scholar]; (n) Meyers AL; Dickman DA; Boes M Tetrahedron 1987, 43, 5095. [Google Scholar]; (o) Meyers AI Tetrahedron 1992, 48, 2589. [Google Scholar]; (p) Seebach D; Enders D Angew. Chem., Int. Ed. Engl 1975, 14, 15. [Google Scholar]; (q) Beak P; Reitz DB Chem. Rev 1978, 78, 275. [Google Scholar]; (r) Beak P; Zajdel WJ; Reitz DB Chem. Rev 1984, 84, 471. [Google Scholar]; (s) Clayden J Organolithiums: Selectivity for Synthesis, In Tetrahedron Organic Chemistry Series, Vol. 23; Clayden J, Ed.; Pergamon: Amsterdam, 2002, 9. [Google Scholar]; (t) Fraser RR; Boussard G; Postescu ID; Whiting JJ; Wigfield YY Can. J. Chem 1973, 51, 1109. [Google Scholar]; (u) Lyle RE; Saavedra JE; Lyle GG; Fribush HM; Marshall JL; Lijinsky W; Singer GM Tetrahedron Lett. 1976, 17, 4431. [Google Scholar]; (v) Seebach D; Lubosch W Angew. Chem., Int. Ed. Engl 1976, 15, 313. [Google Scholar]; (w) Seebach D; Hassel T Angew. Chem., Int. Ed. Engl 1978, 17, 274. [Google Scholar]; (x) Meyers AI; Ten Hoeve W J. Am. Chem. Soc 1980, 102, 7125. [Google Scholar]; (y) Seebach D; Lohmann J-J; Syfrig MA; Yoshifuji M Tetrahedron 1983, 39, 1963. [Google Scholar]; (z) Gawley RE; Hart G; Goicoechea-Pappas M; Smith AL J. Org. Chem 1986, 51, 3076. [Google Scholar]; (aa) Gawley RE; Rein K; Chemburkar S J. Org. Chem 1989, 54, 3002. [Google Scholar]; (ab) Meyers AI; Milot G J. Org. Chem 1993, 58, 6538. [Google Scholar]; (ac) Nain Singh K; Singh P; Kaur A Synth. Commun 2006, 36, 3339. [Google Scholar]
- (3).(a) Beak P; Lee W-K Tetrahedron Lett. 1989, 30, 1197. [Google Scholar]; (b) Beak P; Lee WK J. Org. Chem 1990, 55, 2578. [Google Scholar]; (c) Beak P; Lee WK J. Org. Chem 1993, 58, 1109. [Google Scholar]; (d) Xiao D; Lavey BJ; Palani A; Wang C; Aslanian RG; Kozlowski JA; Shih N-Y; McPhail AT; Randolph GP; Lachowicz JE; Duffy RA Tetrahedron Lett. 2005, 46, 7653. [Google Scholar]; (e) Aeyad T; Williams JD; Meijer AJHM; Coldham I Synlett 2017, 28, 2765. [Google Scholar]; (f) Beak P; Wu S; Yum EK; Jun YM J. Org. Chem 1994, 59, 276. [Google Scholar]; (g) Dieter RK; Li S Tetrahedron Lett. 1995, 36, 3613. [Google Scholar]; (h) Dieter RK; Li S J. Org. Chem 1997, 62, 7726. [Google Scholar]; (i) Dieter RK; Dieter JW; Alexander CW; Bhinderwala NS J. Org. Chem 1996, 61, 2930. [DOI] [PubMed] [Google Scholar]; (j) Dieter RK; Velu SE J. Org. Chem 1997, 62, 3798. [DOI] [PubMed] [Google Scholar]; (k) Dieter RK; Lu K; Velu SE J. Org. Chem 2000, 65, 8715. [DOI] [PubMed] [Google Scholar]; (l) Barker G; O’Brien P; Campos KR Org. Lett 2010, 12, 4176. [DOI] [PubMed] [Google Scholar]; (m) Kwong A; Firth JD; Farmer TJ; O’Brien P Tetrahedron 2021, 81, 131899. [Google Scholar]; (n) Stead D; O’Brien P; Sanderson AJ Org. Lett 2005, 7, 4459. [DOI] [PubMed] [Google Scholar]; (o) Berkheij M; van der Sluis L; Sewing C; den Boer DJ; Terpstra JW; Hiemstra H; Iwema Bakker WI; van den Hoogenband A; van Maarseveen JH Tetrahedron Lett. 2005, 46, 2369. [Google Scholar]; (p) Hodgson DM; Humphreys PG; Xu Z; Ward JG Angew. Chem. Int. Ed 2007, 46, 2245. [DOI] [PubMed] [Google Scholar]; (q) Li X; Leonori D; Sheikh NS; Coldham I Chem. Eur. J 2013, 19, 7724. [DOI] [PubMed] [Google Scholar]; (r) Pizzuti MG; Minnaard AJ; Feringa BL Org. Biomol. Chem 2008, 6, 3464. [DOI] [PubMed] [Google Scholar]; (s) Krishnan S; Bagdanoff JT; Ebner DC; Ramtohul YK; Tambar UK; Stoltz BM J. Am. Chem. Soc 2008, 130, 13745. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Dieter RK; Sharma RR; Ryan W Tetrahedron Lett. 1997, 38, 783. [Google Scholar]; (u) Dieter RK; Lu K J. Org. Chem 2002, 67, 847. [DOI] [PubMed] [Google Scholar]; (v) Coldham I; Leonori D Org. Lett 2008, 10, 3923. [DOI] [PubMed] [Google Scholar]
- (4).(a) Kerrick ST; Beak P J. Am. Chem. Soc 1991, 113, 9708. [Google Scholar]; (b) Beak P; Kerrick ST; Wu S; Chu J J. Am. Chem. Soc 1994, 116, 3231. [Google Scholar]; (c) Dearden MJ; Firkin CR; Hermet J-PR; O’Brien P J. Am. Chem. Soc 2002, 124, 11870. [DOI] [PubMed] [Google Scholar]; (d) Campos KR; Klapars A; Waldman JH; Dormer PG; Chen C-Y J. Am. Chem. Soc 2006, 128, 3538. [DOI] [PubMed] [Google Scholar]; (e) Seel S; Thaler T; Takatsu K; Zhang C; Zipse H; Straub BF; Mayer P; Knochel P J. Am. Chem. Soc 2011, 133, 4774. [DOI] [PubMed] [Google Scholar]; (f) Kasten K; Seling N; O’Brien P Org. React 2019, 100, 255. [Google Scholar]; (g) Wong JYF; Barker G Tetrahedron 2020, 76, 131704. [Google Scholar]; (h) Gallagher DJ; Beak P J. Org. Chem 1995, 60, 7092. [Google Scholar]; (i) Wilkinson TJ; Stehle NW; Beak P Org. Lett 2000, 2, 155. [DOI] [PubMed] [Google Scholar]; (j) Phuan P-W; Ianni JC; Kozlowski MC J. Am. Chem. Soc 2004, 126, 15473. [DOI] [PubMed] [Google Scholar]; (k) Coldham I; Leonori D J. Org. Chem 2010, 75, 4069. [DOI] [PubMed] [Google Scholar]; (l) Sheikh NS; Leonori D; Barker G; Firth JD; Campos KR; Meijer AJHM; O’Brien P; Coldham I J. Am. Chem. Soc 2012, 134, 5300. [DOI] [PubMed] [Google Scholar]; (m) Kizirian J-C; Caille J-C; Alexakis A Tetrahedron Lett. 2003, 44, 8893. [Google Scholar]; (n) Hermet J-PR; Porter DW; Dearden MJ; Harrison JR; Koplin T; O’Brien P; Parmene J; Tyurin V; Whitwood AC; Gilday J; Smith NM Org. Biomol. Chem 2003, 1, 3977. [DOI] [PubMed] [Google Scholar]; (o) McGrath MJ; Bilke JL; O’Brien P Chem. Commun 2006, 2607. [DOI] [PubMed] [Google Scholar]; (p) McGrath MJ; O’Brien P J. Am. Chem. Soc 2005, 127, 16378. [DOI] [PubMed] [Google Scholar]
- (5).(a) Coldham I; Raimbault S; Whittaker DTE; Chovatia PT; Leonori D; Patel JJ; Sheikh NS Chem. Eur. J 2010, 16, 4082. [DOI] [PubMed] [Google Scholar]; (b) Millet A; Larini P; Clot E; Baudoin O Chem. Sci 2013, 4, 2241. [Google Scholar]; (c) Cordier CJ; Lundgren RJ; Fu GC J. Am. Chem. Soc 2013, 135, 10946. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mu X; Shibata Y; Makida Y; Fu GC Angew. Chem. Int. Ed 2017, 56, 5821. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Beak P; Basu A; Gallagher DJ; Park YS; Thayumanavan S Acc. Chem. Res 1996, 29, 552. [Google Scholar]; (f) Campos KR Chem. Soc. Rev 2007, 36, 1069. [DOI] [PubMed] [Google Scholar]; (g) Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW Chem. Eur. J 2012, 18, 10092. [DOI] [PubMed] [Google Scholar]; (h) Coldham I; Dufour S; Haxell TFN; Howard S; Vennall GP Angew. Chem. Int. Ed 2002, 41, 3887. [DOI] [PubMed] [Google Scholar]; (i) Beng TK; Gawley RE J. Am. Chem. Soc 2010, 132, 12216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Millet A; Dailler D; Larini P; Baudoin O Angew. Chem. Int. Ed 2014, 53, 2678. [DOI] [PubMed] [Google Scholar]; (k) Watson RT; Gore VK; Chandupatla KR; Dieter RK; Snyder JP J. Org. Chem 2004, 69, 6105. [DOI] [PubMed] [Google Scholar]; (l) Klapars A; Campos KR; Waldman JH; Zewge D; Dormer PG; Chen C-Y J. Org. Chem 2008, 73, 4986. [DOI] [PubMed] [Google Scholar]; (m) Barker G; McGrath JL; Klapars A; Stead D; Zhou G; Campos KR; O’Brien P J. Org. Chem 2011, 76, 5936. [DOI] [PubMed] [Google Scholar]
- (6).(a) Jun C-H Chem. Commun 1998, 1405. [Google Scholar]; (b) Wang D-H; Hao X-S; Wu D-F; Yu J-Q Org. Lett 2006, 8, 3387. [DOI] [PubMed] [Google Scholar]; (c) Pan S; Endo K; Shibata T Org. Lett 2011, 13, 4692. [DOI] [PubMed] [Google Scholar]; (d) Chatani N; Asaumi T; Ikeda T; Yorimitsu S; Ishii Y; Kakiuchi F; Murai S J. Am. Chem. Soc 2000, 122, 12882. [DOI] [PubMed] [Google Scholar]; (e) Pastine SJ; Gribkov DV; Sames D J. Am. Chem. Soc 2006, 128, 14220. [DOI] [PubMed] [Google Scholar]; (f) Chatani N; Asaumi T; Yorimitsu S; Ikeda T; Kakiuchi F; Murai S J. Am. Chem. Soc 2001, 123, 10935. [DOI] [PubMed] [Google Scholar]; (g) Tsuchikama K; Kasagawa M; Endo K; Shibata T Org. Lett 2009, 11, 1821. [DOI] [PubMed] [Google Scholar]; (h) Schinkel M; Wang L; Bielefeld K; Ackermann L Org. Lett 2014, 16, 1876. [DOI] [PubMed] [Google Scholar]; (i) Lahm G; Opatz T Org. Lett 2014, 16, 4201. [DOI] [PubMed] [Google Scholar]; (j) Spangler JE; Kobayashi Y; Verma P; Wang D-H; Yu J-Q J. Am. Chem. Soc 2015, 137, 11876. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Tran AT; Yu J-Q Angew. Chem. Int. Ed 2017, 56, 10530. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Antermite D; Affron DP; Bull JA Org. Lett 2018, 20, 3948. [DOI] [PubMed] [Google Scholar]; (m) Reyes RL; Sato M; Iwai T; Sawamura M J. Am. Chem. Soc 2020, 142, 589. [DOI] [PubMed] [Google Scholar]; (n) Su B; Bunescu A; Qiu Y; Zuend SJ; Ernst M; Hartwig JF J. Am. Chem. Soc 2020, 142, 7912. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) He J; Wasa M; Chan KSL; Shao Q; Yu J-Q Chem. Rev 2017, 117, 8754. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Sambiagio C; Schönbauer D; Blieck R; Dao-Huy T; Pototschnig G; Schaaf P; Wiesinger T; Zia MF; Wencel-Delord J; Besset T; Maes BUW; Schnürch M Chem. Soc. Rev 2018, 47, 6603. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Zhang M; Wang Q; Peng Y; Chen Z; Wan C; Chen J; Zhao Y; Zhang R; Zhang AQ Chem. Commun 2019, 55, 13048. [DOI] [PubMed] [Google Scholar]; (r) Kapoor M; Singh A; Sharma K; Hsu MH Adv. Synth. Catal 2020, 362, 4513. [Google Scholar]
- (7).(a) Topczewski JJ; Cabrera PJ; Saper NI; Sanford MS Nature 2016, 531, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cabrera PJ; Lee M; Sanford MS J. Am. Chem. Soc 2018, 140, 5599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Aguilera EY; Sanford MS Angew. Chem. Int. Ed 2021, 60, 11227. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zaitsev VG; Shabashov D; Daugulis O J. Am. Chem. Soc 2005, 127, 13154. [DOI] [PubMed] [Google Scholar]; (e) Zhang S-Y; He G; Nack WA; Zhao Y; Li Q; Chen G J. Am. Chem. Soc 2013, 135, 2124. [DOI] [PubMed] [Google Scholar]; (f) Zhang S-Y; He G; Zhao Y; Wright K; Nack WA; Chen G J. Am. Chem. Soc 2012, 134, 7313. [DOI] [PubMed] [Google Scholar]; (g) He G; Zhao Y; Zhang S; Lu C; Chen G J. Am. Chem. Soc 2012, 134, 3. [DOI] [PubMed] [Google Scholar]; (h) He G; Zhao Y; Zhang S; Lu C; Chen G J. Am. Chem. Soc 2017, 139, 561. [DOI] [PubMed] [Google Scholar]; (i) Verma P; Richter JM; Chekshin N; Qiao JX; Yu J-Q J. Am. Chem. Soc 2020, 142, 5117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Nadres ET; Daugulis O J. Am. Chem. Soc 2012, 134, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ye X; He Z; Ahmed T; Weise K; Akhmedov NG; Petersen JL; Shi X Chem. Sci 2013, 4, 3712. [Google Scholar]; (l) Li Q; Zhang S-Y; He G; Nack WA; Chen G Adv. Synth. Catal 2014, 356, 1544. [Google Scholar]; (m) Wang P-L; Li Y; Wu Y; Li C; Lan Q; Wang X-S Org. Lett 2015, 17, 3698. [DOI] [PubMed] [Google Scholar]; (n) Huang Z; Wang C; Dong G Angew. Chem. Int. Ed 2016, 55, 5299. [DOI] [PubMed] [Google Scholar]; (o) Coomber CE; Benhamou L; Bučar D-K; Smith PD; Porter MJ; Sheppard TD J. Org. Chem 2018, 83, 2495. [DOI] [PubMed] [Google Scholar]
- (8).(a) Reddy BVS; Reddy LR; Corey EJ Org. Lett 2006, 8, 3391. [DOI] [PubMed] [Google Scholar]; (b) Tran LD; Daugulis O Angew. Chem. Int. Ed 2012, 51, 5188. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Affron DP; Davis OA; Bull JA Org. Lett 2014, 16, 4956. [DOI] [PubMed] [Google Scholar]; (d) Affron DP; Bull JA Eur. J. Org. Chem 2016, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Maetani M; Zoller J; Melillo B; Verho O; Kato N; Pu J; Comer E; Schreiber SL J. Am. Chem. Soc 2017, 139, 11300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) He J; Li S; Deng Y; Fu H; Laforteza BN; Spangler JE; Homs A; Yu J-Q Science 2014, 343, 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) He G; Chen G Angew. Chem. Int. Ed 2011, 50, 5192. [DOI] [PubMed] [Google Scholar]; (h) Rodríguez N; Romero-Revilla JA; Fernández-Ibáñez MÁ; Carretero JC Chem. Sci 2013, 4, 175. [Google Scholar]; (i) Chen K; Hu F; Zhang S-Q; Shi B-F Chem. Sci 2013, 4, 3906. [Google Scholar]; (j) Zhang S-Y; Li Q; He G; Nack WA; Chen G J. Am. Chem. Soc 2013, 135, 12135. [DOI] [PubMed] [Google Scholar]; (k) Wang B; Nack WA; He G; Zhang S-Y; Chen G Chem. Sci 2014, 5, 3952. [Google Scholar]; (l) Gong W; Zhang G; Liu T; Giri R; Yu J-Q J. Am. Chem. Soc 2014, 136, 16940. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Zhang L-S; Chen G; Wang X; Guo Q-Y; Zhang X-S; Pan F; Chen K; Shi Z-J Angew. Chem. Int. Ed 2014, 53, 3899. [DOI] [PubMed] [Google Scholar]; (n) Zhang Q; Yin X-S; Chen K; Zhang S-Q; Shi B-F J. Am. Chem. Soc 2015, 137, 8219. [DOI] [PubMed] [Google Scholar]; (o) Ye S; Yang W; Coon T; Fanning D; Neubert T; Stamos D; Yu J-Q Chem. Eur. J 2016, 22, 4748. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Xu J-W; Zhang Z-Z; Rao W-H; Shi B-F J. Am. Chem. Soc 2016, 138, 10750. [DOI] [PubMed] [Google Scholar]; (q) Zhan B-B; Li Y; Xu J-W; Nie X-L; Fan J; Jin L; Shi B-F Angew. Chem. Int. Ed 2018, 57, 5858. [DOI] [PubMed] [Google Scholar]; (r) Noisier AFM; Brimble MA Chem. Rev 2014, 114, 8775. [DOI] [PubMed] [Google Scholar]; (s) He G; Wang B; Nack WA; Chen G Acc. Chem. Res 2016, 49, 635. [DOI] [PubMed] [Google Scholar]; (t) Wang W; Lorion MM; Shah J; Kapdi AR; Ackermann L Angew. Chem. Int. Ed 2018, 57, 14700. [DOI] [PubMed] [Google Scholar]; (u) Tong H-R; Li B; Li G; He G; Chen G CCS Chem. 2020, 3, 1797. [Google Scholar]
- (9).(a) Wang H; Tong H-R; He G; Chen G Angew. Chem. Int. Ed 2016, 55, 15387. [DOI] [PubMed] [Google Scholar]; (b) Jain P; Verma P; Xia G; Yu J-Q Nat. Chem 2017, 9, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jiang H-J; Zhong X-M; Yu J; Zhang Y; Zhang X; Wu Y-D; Gong L-Z Angew. Chem. Int. Ed 2019, 58, 1803. [DOI] [PubMed] [Google Scholar]; (d) Greßies S; Klauck FJR; Kim JH; Daniliuc CG; Glorius F Angew. Chem. Int. Ed 2018, 57, 9950. [DOI] [PubMed] [Google Scholar]; (e) Chen L; Yang Y; Liu L; Gao Q; Xu S J. Am. Chem. Soc 2020, 142, 12062. [DOI] [PubMed] [Google Scholar]
- (10).(a) Wu Y; Chen Y-Q; Liu T; Eastgate MD; Yu J-Q J. Am. Chem. Soc 2016, 138, 14554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu Y; Ge H Nat. Chem 2017, 9, 26. [Google Scholar]; (c) Kapoor M; Liu D; Young MC J. Am. Chem. Soc 2018, 140, 6818. [DOI] [PubMed] [Google Scholar]; (d) Chen Y-Q; Wang Z; Wu Y; Wisniewski SR; Qiao JX; Ewing WR; Eastgate MD; Yu J-Q J. Am. Chem. Soc 2018, 140, 17884. [DOI] [PubMed] [Google Scholar]; (e) Chen Y-Q; Singh S; Wu Y; Wang Z; Hao W; Verma P; Qiao JX; Sunoj RB; Yu J-Q J. Am. Chem. Soc 2020, 142, 9966. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Yada A; Liao W; Sato Y; Murakami M Angew. Chem. Int. Ed 2017, 56, 1073. [DOI] [PubMed] [Google Scholar]; (g) St John-Campbell S; Ou AK; Bull JA Chem. Eur. J 2018, 24, 17838. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) St John-Campbell S; Bull JA Org. Biomol. Chem 2018, 16, 4582. [DOI] [PubMed] [Google Scholar]; (i) Niu B; Yang K; Lawrence B; Ge H ChemSusChem 2019, 12, 2955. [DOI] [PubMed] [Google Scholar]; (j) Trowbridge A; Walton SM; Gaunt MJ Chem. Rev 2020, 120, 2613. [DOI] [PubMed] [Google Scholar]
- (11).(a) McNally A; Haffemayer B; Collins BSL; Gaunt MJ Nature 2014, 510, 129. [DOI] [PubMed] [Google Scholar]; (b) Smalley AP; Gaunt MJ J. Am. Chem. Soc 2015, 137, 10632. [DOI] [PubMed] [Google Scholar]; (c) Willcox D; Chappell BGN; Hogg KF; Calleja J; Smalley AP; Gaunt MJ Science 2016, 354, 851. [DOI] [PubMed] [Google Scholar]; (d) Smalley AP; Cuthbertson JD; Gaunt MJ J. Am. Chem. Soc 2017, 139, 1412. [DOI] [PubMed] [Google Scholar]; (e) Whitehurst WG; Blackwell JH; Hermann GN; Gaunt MJ Angew. Chem. Int. Ed 2019, 58, 9054. [DOI] [PubMed] [Google Scholar]; (f) Zhuang Z; Yu J-Q J. Am. Chem. Soc 2020, 142, 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Calleja J; Pla D; Gorman TW; Domingo V; Haffemayer B; Gaunt MJ Nat. Chem 2015, 7, 1009. [DOI] [PubMed] [Google Scholar]; (h) Cabrera-Pardo JR; Trowbridge A; Nappi M; Ozaki K; Gaunt MJ Angew. Chem. Int. Ed 2017, 56, 11958. [DOI] [PubMed] [Google Scholar]; (i) Chen K; Wang D; Li Z-W; Liu Z; Pan F; Zhang Y-F; Shi Z-J Org. Chem. Front 2017, 4, 2097. [Google Scholar]; (j) Png ZM; Cabrera-Pardo JR; Peiró Cadahía J; Gaunt MJ Chem. Sci 2018, 9, 7628. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Lin H; Pan X; Barsamian AL; Kamenecka TM; Bannister TD ACS Catal. 2019, 9, 4887. [Google Scholar]; (l) Rodrigalvarez J; Nappi M; Azuma H; Flodén NJ; Burns ME; Gaunt MJ Nat. Chem 2020, 12, 76. [DOI] [PubMed] [Google Scholar]; (m) He C; Whitehurst WG; Gaunt MJ Chem 2019, 5, 1031. [Google Scholar]
- (12).(a) Li Q; Liskey CW; Hartwig JF J. Am. Chem. Soc 2014, 136, 8755. [DOI] [PubMed] [Google Scholar]; (b) Lee M; Sanford MS J. Am. Chem. Soc 2015, 137, 12796. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Howell JM; Feng K; Clark JR; Trzepkowski LJ; White MC J. Am. Chem. Soc 2015, 137, 14590. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao J; Nanjo T; de Lucca EC; White MC Nat. Chem 2019, 11, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Clark JR; Feng K; Sookezian A; White MC Nat. Chem 2018, 10, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Oeschger R; Su B; Yu I; Ehinger C; Romero E; He S; Hartwig J Science 2020, 368, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Su B; Lee T; Hartwig JF J. Am. Chem. Soc 2018, 140, 18032. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Mack JBC; Gipson JD; Du Bois J; Sigman MS J. Am. Chem. Soc 2017, 139, 9503. [DOI] [PubMed] [Google Scholar]; (i) Mbofana CT; Chong E; Lawniczak J; Sanford MS Org. Lett 2016, 18, 4258. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Nanjo T; de Lucca EC; White MC J. Am. Chem. Soc 2017, 139, 14586. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Hartwig JF; Larsen MA ACS Cent. Sci 2016, 2, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Dalton T; Faber T; Glorius F ACS Cent. Sci 2021, 7, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Clerici MG; Maspero F Synthesis 1980, 305. [Google Scholar]; (b) Nugent WA; Ovenall DW; Holmes SJ Organometallics 1983, 2, 161. [Google Scholar]; (c) Herzon SB; Hartwig JF J. Am. Chem. Soc 2007, 129, 6690. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Herzon SB; Hartwig JF J. Am. Chem. Soc 2008, 130, 14940. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kubiak R; Prochnow I; Doye S Angew. Chem. Int. Ed 2009, 48, 1153. [DOI] [PubMed] [Google Scholar]; (f) Bexrud JA; Eisenberger P; Leitch DC; Payne PR; Schafer LL J. Am. Chem. Soc 2009, 131, 2116. [DOI] [PubMed] [Google Scholar]; (g) Nako AE; Oyamada J; Nishiura M; Hou Z Chem. Sci 2016, 7, 6429. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Reznichenko AL; Hultzsch KC J. Am. Chem. Soc 2012, 134, 3300. [DOI] [PubMed] [Google Scholar]; (i) Roesky PW Angew. Chem. Int. Ed 2009, 48, 4892. [DOI] [PubMed] [Google Scholar]; (j) Kruger K; Tillack A; Beller M ChemSusChem 2009, 2, 715. [DOI] [PubMed] [Google Scholar]; (k) Chong E; Garcia P; Schafer L Synthesis 2014, 46, 2884. [Google Scholar]; (l) Hannedouche J; Schulz E Organometallics 2018, 37, 4313. [Google Scholar]; (m) Edwards PM; Schafer LL Chem. Commun 2018, 54, 12543. [DOI] [PubMed] [Google Scholar]; (n) Eisenberger P; Ayinla RO; Lauzon JM; Schafer LL Angew. Chem. Int. Ed 2009, 48, 8361. [DOI] [PubMed] [Google Scholar]; (o) Bytschkov I; Doye S Eur. J. Org. Chem 2001, 4411. [Google Scholar]; (p) Ramanathan B; Odom AL J. Am. Chem. Soc 2006, 128, 9344. [DOI] [PubMed] [Google Scholar]; (q) Zi G; Zhang F; Song H Chem. Commun 2010, 46, 6296. [DOI] [PubMed] [Google Scholar]; (r) Reznichenko AL; Emge TJ; Audörsch S; Klauber EG; Hultzsch KC; Schmidt B Organometallics 2011, 30, 921. [Google Scholar]; (s) Payne PR; Garcia P; Eisenberger P; Yim JC-H; Schafer LL Org. Lett 2013, 15, 2182. [DOI] [PubMed] [Google Scholar]; (t) Luhning LH; Strehl J; Schmidtmann M; Doye S Chem. Eur. J 2017, 23, 4197. [DOI] [PubMed] [Google Scholar]; (u) Geik D; Rosien M; Bielefeld J; Schmidtmann M; Doye S Angew. Chem. Int. Ed 2021, 60, 9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Leonard NJ; Hay AS; Fulmer RW; Gash VW J. Am. Chem. Soc 1955, 77, 439. [Google Scholar]; (b) Leonard NJ; Morrow DF J. Am. Chem. Soc 1958, 80, 371. [Google Scholar]; (c) Leonard NJ; Hauck FP J. Am. Chem. Soc 1957, 79, 5279. [Google Scholar]; (d) Van Tamelen EE; Foltz RL J. Am. Chem. Soc 1969, 91, 7372. [Google Scholar]; (e) Gutzwiller J; Pizzolato G; Uskoković M J. Am. Chem. Soc 1971, 93, 5907. [Google Scholar]; (f) Openshaw HT; Whittaker N J. Chem. Soc 1963, 1449. [Google Scholar]; (g) Bartlett MF; Lambert BF; Taylor WI J. Am. Chem. Soc 1964, 86, 729. [Google Scholar]; (h) Barczaibeke M; Dornyei G; Kajtar M; Szantay C Tetrahedron 1976, 32, 1019. [Google Scholar]; (i) Sakai S; Kubo A; Haginiwa J Tetrahedron Lett. 1969, 10, 1485. [DOI] [PubMed] [Google Scholar]; (j) Butler RN Chem. Rev 1984, 84, 249. [Google Scholar]
- (15).(a) Rosenblatt DH; Moore KA; Streaty RA; Hayes AJ; Harrison BL J. Org. Chem 1963, 28, 2790. [Google Scholar]; (b) Chen CK; Hortmann AG; Marzabadi MR J. Am. Chem. Soc 1988, 110, 4829. [Google Scholar]; (c) Moriarty RM; Vaid RK; Duncan MP; Ochiai M; Inenaga M; Nagao Y Tetrahedron Lett. 1988, 29, 6913. [Google Scholar]; (d) Shen H; Zhang XH; Liu Q; Pan J; Hu W; Xiong Y; Zhu XM Tetrahedron Lett. 2015, 56, 5628. [Google Scholar]; (e) Tsang ASK; Todd MH Tetrahedron Lett. 2009, 50, 1199. [Google Scholar]; (f) Chu LL; Qing FL Chem. Commun 2010, 46, 6285. [DOI] [PubMed] [Google Scholar]; (g) Allen JM; Lambert TH J. Am. Chem. Soc 2011, 133, 1260. [DOI] [PubMed] [Google Scholar]; (h) Xie ZY; Liu L; Chen WF; Zheng HB; Xu QQ; Yuan HQ; Lou HX Angew. Chem. Int. Ed 2014, 53, 3904. [DOI] [PubMed] [Google Scholar]; (i) Richter H; Mancheño OG Eur. J. Org. Chem 2010, 4460. [Google Scholar]; (j) Fang L; Li ZH; Jiang ZJ; Tan ZY; Xie YY Eur. J. Org. Chem 2016, 3559. [Google Scholar]; (k) Zhdankin VV; Kuehl CJ; Krasutsky AP; Bolz JT; Mismash B; Woodward JK; Simonsen AJ Tetrahedron Lett. 1995, 36, 7975. [Google Scholar]; (l) Huang WZ; Ni CF; Zhao YC; Hu JB New J. Chem 2013, 37, 1684. [Google Scholar]; (m) Oss G; de Vos SD; Luc KNH; Harper JB; Nguyen TV J. Org. Chem 2018, 83, 1000. [DOI] [PubMed] [Google Scholar]; (n) Zhang RP; Qin Y; Zhang L; Luo SZ J. Org. Chem 2019, 84, 2542. [DOI] [PubMed] [Google Scholar]; (o) Chen WL; Wang LY; Li YJ Eur. J. Org. Chem 2020, 103. [Google Scholar]; (p) Singh P; Batra A; Singh KN; Mritunjay M Synthesis 2021, 53, 1556. [Google Scholar]
- (16).(a) Murahashi S-I; Imada Y Chem. Rev 2019, 119, 4684. [DOI] [PubMed] [Google Scholar]; (b) Largeron M Eur. J. Org. Chem 2013, 5225. [Google Scholar]; (c) Franck B; Randau D Angew. Chem., Int. Ed. Engl 1966, 5, 131. [DOI] [PubMed] [Google Scholar]; (d) Gupta RN; Spenser ID Can. J. Chem 1969, 47, 445. [Google Scholar]; (e) Claxton GP; Allen L; Grisar JM Org. Synth 1977, 56, 118. [Google Scholar]; (f) Kessler H; Moehrle H; Zimmermann G J. Org. Chem 1977, 42, 66. [Google Scholar]; (g) Nutt RF; Joullie MM J. Am. Chem. Soc 1982, 104, 5852. [Google Scholar]; (h) Davis BG; Maughan MAT; Chapman TM; Villard R; Courtney S Org. Lett 2002, 4, 103. [DOI] [PubMed] [Google Scholar]; (i) Gravel E; Poupon E; Hocquemiller R Tetrahedron 2006, 62, 5248. [Google Scholar]; (j) Gomm A; Lewis W; Green AP; O’Reilly E Chem. Eur. J 2016, 22, 12692. [DOI] [PubMed] [Google Scholar]; (k) Gu R; Flidrova K; Lehn J-M J. Am. Chem. Soc 2018, 140, 5560. [DOI] [PubMed] [Google Scholar]; (l) van der Heijden G; van Schaik TB; Mouarrawis V; de Wit MJM; Velde CMLV; Ruijter E; Orru RVA Eur. J. Org. Chem 2019, 5313. [Google Scholar]; (m) Nomura Y; Ogawa K; Takeuchi Y; Tomoda S Chem. Lett 1977, 693. [Google Scholar]; (n) Ogawa K; Nomura Y; Takeuchi Y; Tomoda S J. Chem. Soc., Perkin Trans 1 1982, 3031. [Google Scholar]; (o) Ochiai M; Inenaga M; Nagao Y; Moriarty RM; Vaid RK; Duncan MP Tetrahedron Lett. 1988, 29, 6917. [Google Scholar]; (p) Boto A; Hernández R; Suárez E Tetrahedron Lett. 1999, 40, 5945. [Google Scholar]; (q) Huang W-J; Singh OV; Chen C-H; Chiou S-Y; Lee S-S Helv. Chim. Acta 2002, 85, 1069. [Google Scholar]; (r) Castedo L; Riguera R; Rodriguez MJ Tetrahedron 1982, 38, 1569. [Google Scholar]; (s) Su F; Mathew SC; Möhlmann L; Antonietti M; Wang X; Blechert S Angew. Chem. Int. Ed 2011, 50, 657. [DOI] [PubMed] [Google Scholar]; (t) Wendlandt AE; Stahl SS Org. Lett 2012, 14, 2850. [DOI] [PubMed] [Google Scholar]; (u) Chen B; Wang L; Gao S ACS Catal. 2015, 5, 5851. [Google Scholar]; (v) Mitsui H; Zenki S-I; Shiota T; Murahashi S-I J. Chem. Soc., Chem. Commun 1984, 874. [Google Scholar]; (w) Murahashi S; Mitsui H; Shiota T; Tsuda T; Watanabe S J. Org. Chem 1990, 55, 1736. [Google Scholar]; (x) Matassini C; Parmeggiani C; Cardona F; Goti A Org. Lett 2015, 17, 4082. [DOI] [PubMed] [Google Scholar]; (y) Lisnyak VG; Lynch-Colameta T; Snyder SA Angew. Chem. Int. Ed 2018, 57, 15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Murahashi S; Naota T; Yonemura K J. Am. Chem. Soc 1988, 110, 8256. [Google Scholar]; (b) Boess E; Wolf LM; Malakar S; Salamone M; Bietti M; Thiel W; Klussmann M ACS Catal. 2016, 6, 3253. [Google Scholar]; (c) Li ZP; Li CJ J. Am. Chem. Soc 2004, 126, 11810. [DOI] [PubMed] [Google Scholar]; (d) Volla CMR; Vogel P Org. Lett 2009, 11, 1701. [DOI] [PubMed] [Google Scholar]; (e) Li ZP; Li CJ J. Am. Chem. Soc 2005, 127, 6968. [DOI] [PubMed] [Google Scholar]; (f) Xie J; Huang ZZ Angew. Chem. Int. Ed 2010, 49, 10181. [DOI] [PubMed] [Google Scholar]; (g) Xu ZW; Yu XQ; Feng XJ; Bao M J. Org. Chem 2011, 76, 6901. [DOI] [PubMed] [Google Scholar]; (h) Wang YD; Zhu J; Guo R; Lindberg H; Wang YM Chem. Sci 2020, 11, 12316. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Li CJ Acc. Chem. Res 2009, 42, 335. [DOI] [PubMed] [Google Scholar]; (j) Yeung CS; Dong VM Chem. Rev 2011, 111, 1215. [DOI] [PubMed] [Google Scholar]; (k) Zhang C; Tang CH; Jiao N Chem. Soc. Rev 2012, 41, 3464. [DOI] [PubMed] [Google Scholar]; (l) Girard SA; Knauber T; Li CJ Angew. Chem. Int. Ed 2014, 53, 74. [DOI] [PubMed] [Google Scholar]; (m) Qin Y; Zhu LH; Luo SZ Chem. Rev 2017, 117, 9433. [DOI] [PubMed] [Google Scholar]; (n) Huang CY; Kang H; Li JB; Li CJ J. Org. Chem 2019, 84, 12705. [DOI] [PubMed] [Google Scholar]; (o) Zhang JS; Liu L; Chen TQ; Han LB ChemSusChem 2020, 13, 4776. [DOI] [PubMed] [Google Scholar]
- (18).(a) Murahashi SI; Komiya N; Terai H; Nakae T J. Am. Chem. Soc 2003, 125, 15312. [DOI] [PubMed] [Google Scholar]; (b) Scott M; Sud A; Boess E; Klussmann M J. Org. Chem 2014, 79, 12033. [DOI] [PubMed] [Google Scholar]; (c) Basle O; Li CJ Green Chem. 2007, 9, 1047. [Google Scholar]; (d) Meng QY; Liu Q; Zhong JJ; Zhang HH; Li ZJ; Chen B; Tung CH; Wu LZ Org. Lett 2012, 14, 5992. [DOI] [PubMed] [Google Scholar]; (e) Alagiri K; Kumara RGS; Prabhu KR Chem. Commun 2011, 47, 11787. [DOI] [PubMed] [Google Scholar]; (f) Wang TT; Schrempp M; Berndhauser A; Schiemann O; Menche D Org. Lett 2015, 17, 3982. [DOI] [PubMed] [Google Scholar]; (g) Sonobe T; Oisaki K; Kanai M Chem. Sci 2012, 3, 3249. [Google Scholar]; (h) Li X; Zhao H; Chen XW; Jiang HF; Zhang M Org. Chem. Front 2020, 7, 425. [Google Scholar]; (i) Wang FF; Luo CP; Deng GJ; Yang L Green Chem. 2014, 16, 2428. [Google Scholar]; (j) Brzozowski M; Forni JA; Savage GP; Polyzos A Chem. Commun 2015, 51, 334. [DOI] [PubMed] [Google Scholar]; (k) Sharma K; Borah A; Neog K; Gogoi P ChemistrySelect 2016, 1, 4620. [Google Scholar]; (l) Groll B; Schaaf P; Schnürch M Monatsh. Chem 2017, 148, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Zhang Y; Wei BW; Wang WX; Deng LL; Nie LJ; Luo HQ; Fan XL RSC Adv. 2017, 7, 1229. [Google Scholar]; (n) Zhu ZQ; Xiao LJ; Chen Y; Xie ZB; Zhu HB; Le ZG Synthesis 2018, 50, 2775. [Google Scholar]; (o) Ramana DV; Chandrasekharam M Adv. Synth. Catal 2018, 360, 4080. [Google Scholar]; (p) Odachowski M; Greaney MF; Turner NJ ACS Catal. 2018, 8, 10032. [Google Scholar]; (q) Peng K; Dong ZB Adv. Synth. Catal 2021, 363, 1185. [Google Scholar]; (r) Afsina CMA; Aneeja T; Neetha M; Anilkumar G Eur. J. Org. Chem 2021, 1776. [Google Scholar]
- (19).(a) Dominguez E; Lete E J. Heterocycl. Chem 1984, 21, 525. [Google Scholar]; (b) Rao SN; Reddy NNK; Samanta S; Adimurthy S J. Org. Chem 2017, 82, 13632. [DOI] [PubMed] [Google Scholar]; (c) Nobuta T; Tada N; Fujiya A; Kariya A; Miura T; Itoh A Org. Lett 2013, 15, 574. [DOI] [PubMed] [Google Scholar]; (d) Nobuta T; Fujiya A; Yamaguchi T; Tada N; Miura T; Itoh A RSC Adv. 2013, 3, 10189. [Google Scholar]; (e) Huang HM; Li YJ; Ye Q; Yu WB; Han L; Jia JH; Gao JR J. Org. Chem 2014, 79, 1084. [DOI] [PubMed] [Google Scholar]; (f) Kumar RA; Saidulu G; Prasad KR; Kumar GS; Sridhar B; Reddy KR Adv. Synth. Catal 2012, 354, 2985. [Google Scholar]; (g) Li LT; Li HY; Xing LJ; Wen LJ; Wang P; Wang B Org. Biomol. Chem 2012, 10, 9519. [DOI] [PubMed] [Google Scholar]; (h) Liu D; Lei AW Chem. Asian J 2015, 10, 806. [DOI] [PubMed] [Google Scholar]; (i) Deb ML; Borpatra PJ; Saikia PJ; Baruah PK Synlett 2017, 28, 461. [DOI] [PubMed] [Google Scholar]; (j) Debnath S; Das T; Gayen S; Ghosh T; Maiti DK ACS Omega 2019, 4, 20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Shu XZ; Yang YF; Xia XF; Ji KG; Liu XY; Liang YM Org. Biomol. Chem 2010, 8, 4077. [DOI] [PubMed] [Google Scholar]; (b) Meng QY; Zhong JJ; Liu Q; Gao XW; Zhang HH; Lei T; Li ZJ; Feng K; Chen B; Tung CH; Wu LZ J. Am. Chem. Soc 2013, 135, 19052. [DOI] [PubMed] [Google Scholar]; (c) Zhong JJ; Meng QY; Liu B; Li XB; Gao XW; Lei T; Wu CJ; Li ZJ; Tung CH; Wu LZ Org. Lett 2014, 16, 1988. [DOI] [PubMed] [Google Scholar]; (d) Gao XW; Meng QY; Li JX; Zhong JJ; Lei T; Li XB; Tung CH; Wu LZ ACS Catal. 2015, 5, 2391. [Google Scholar]; (e) Chen XW; Li YB; Chen L; Zhu ZZ; Li B; Huang YB; Zhang M J. Org. Chem 2019, 84, 3559. [DOI] [PubMed] [Google Scholar]; (f) Niu LB; Wang SC; Liu JM; Yi H; Liang XA; Liu TY; Lei AW Chem. Commun 2018, 54, 1659. [DOI] [PubMed] [Google Scholar]; (g) Zhou AX; Mao LL; Wang GW; Yang SD Chem. Commun 2014, 50, 8529. [DOI] [PubMed] [Google Scholar]; (h) Cao L; Zhao H; Tan ZD; Guan RQ; Jiang HF; Zhang M Org. Lett 2020, 22, 4781. [DOI] [PubMed] [Google Scholar]; (i) Sun X; Hu Y; Nie SZ; Yan YY; Zhang XJ; Yan M Adv. Synth. Catal 2013, 355, 2179. [Google Scholar]; (j) Nie SZ; Sun X; Wei WT; Zhang XJ; Yan M; Xiao JL Org. Lett 2013, 15, 2394. [DOI] [PubMed] [Google Scholar]; (k) Sun X; Lv XH; Ye LM; Hu Y; Chen YY; Zhang XJ; Yan M Org. Biomol. Chem 2015, 13, 7381. [DOI] [PubMed] [Google Scholar]; (l) Fu NK; Li LJ; Yang Q; Luo SZ Org. Lett 2017, 19, 2122. [DOI] [PubMed] [Google Scholar]; (m) Khusnutdinova JR; Ben-David Y; Milstein D J. Am. Chem. Soc 2014, 136, 2998. [DOI] [PubMed] [Google Scholar]; (n) He KH; Li Y ChemSusChem 2014, 7, 2788. [DOI] [PubMed] [Google Scholar]; (o) Chen C; Chen X; Zhao H; Jiang H; Zhang M Org. Lett 2017, 19, 3390. [DOI] [PubMed] [Google Scholar]; (p) Chen B; Wu LZ; Tung CH Acc. Chem. Res 2018, 51, 2512. [DOI] [PubMed] [Google Scholar]; (q) Tang S; Zeng L; Lei AW J. Am. Chem. Soc 2018, 140, 13128. [DOI] [PubMed] [Google Scholar]; (r) Wang HM; Gao XL; Lv ZC; Abdelilah T; Lei AW Chem. Rev 2019, 119, 6769. [DOI] [PubMed] [Google Scholar]
- (21).(a) Li ZP; Li CJ Org. Lett 2004, 6, 4997. [DOI] [PubMed] [Google Scholar]; (b) Li ZP; MacLeod PD; Li CJ Tetrahedron: Asymmetry 2006, 17, 590. [Google Scholar]; (c) Zhang G; Ma YX; Wang SL; Kong WD; Wang R Chem. Sci 2013, 4, 2645. [Google Scholar]; (d) Dubs C; Hamashima Y; Sasamoto N; Seidel TM; Suzuki S; Hashizume D; Sodeoka M J. Org. Chem 2008, 73, 5859. [DOI] [PubMed] [Google Scholar]; (e) Zhang JM; Tiwari B; Xing C; Chen XK; Chi YGR Angew. Chem. Int. Ed 2012, 51, 3649. [DOI] [PubMed] [Google Scholar]; (f) Zhang G; Ma YX; Wang SL; Zhang YH; Wang R J. Am. Chem. Soc 2012, 134, 12334. [DOI] [PubMed] [Google Scholar]; (g) Ma YX; Zhang G; Zhang JL; Yang DX; Wang R Org. Lett 2014, 16, 5358. [DOI] [PubMed] [Google Scholar]; (h) Huang TY; Liu XH; Lang JW; Xu J; Lin LL; Feng XM ACS Catal. 2017, 7, 5654. [Google Scholar]; (i) Zhao YL; Wang Y; Luo YC; Fu XZ; Xu PF Tetrahedron Lett. 2015, 56, 3703. [Google Scholar]; (j) Cheng MX; Yang SD Synlett 2017, 28, 159. [Google Scholar]; (k) Gandhi S Org. Biomol. Chem 2019, 17, 9683. [DOI] [PubMed] [Google Scholar]; (l) Phillips AMF; da Silva M; Pombeiro AJL Catalysts 2020, 10, 529. [Google Scholar]; (m) Rostoll-Berenguer J; Blay G; Pedro JR; Vila C Adv. Synth. Catal 2021, 363, 602. [Google Scholar]
- (22).(a) Schreiber SL Tetrahedron Lett. 1980, 21, 1027. [Google Scholar]; (b) Xu XL; Li XN; Ma L; Ye N; Weng BJ J. Am. Chem. Soc 2008, 130, 14048. [DOI] [PubMed] [Google Scholar]; (c) Wang SJ; Wang ZY; Zheng XQ Chem. Commun 2009, 47, 7372. [DOI] [PubMed] [Google Scholar]; (d) Shu XZ; Xia XF; Yang YF; Ji KG; Liu XY; Liang YM J. Org. Chem 2009, 74, 7464. [DOI] [PubMed] [Google Scholar]; (e) Xia XF; Shu XZ; Ji KG; Yang YF; Shaukat A; Liu XY; Liang YM J. Org. Chem 2010, 75, 2893. [DOI] [PubMed] [Google Scholar]; (f) Takasu N; Oisaki K; Kanai M Org. Lett 2013, 15, 1918. [DOI] [PubMed] [Google Scholar]; (g) Griffiths RJ; Kong WC; Richards SA; Burley GA; Willis MC; Talbot EPA Chem. Sci 2018, 9, 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) He Y; Yang JT; Liu QM; Zhang XY; Fan XS J. Org. Chem 2020, 85, 15600. [DOI] [PubMed] [Google Scholar]; (i) Rong XN; Guo JW; Hu ZQ; Huang LH; Gu YG; Cai YP; Liang G; Xia QQ Eur. J. Org. Chem 2021, 701. [Google Scholar]; (j) Wang F; Zhang XY; He Y; Fan XS J. Org. Chem 2020, 85, 2220. [DOI] [PubMed] [Google Scholar]; (k) Jiang F; Achard M; Bruneau C Chem. Eur. J 2015, 21, 14319. [DOI] [PubMed] [Google Scholar]; (l) Cai YG; Zhang RH; Sun DL; Xu S; Zhou QG Synlett 2017, 28, 1630. [Google Scholar]; (m) Zhou MJ; Zhu SF; Zhou QL Chem. Commun 2017, 53, 8770. [DOI] [PubMed] [Google Scholar]; (n) He Y; Wang F; Zhang XY; Fan XS Chem. Commun 2017, 53, 4002. [DOI] [PubMed] [Google Scholar]; (o) Liu GQ; Opatz T Adv. Heterocycl. Chem 2018, 125, 107. [Google Scholar]; (p) Shi XN; Chen X; Wang MH; Zhang XY; Fan XS J. Org. Chem 2018, 83, 6524. [DOI] [PubMed] [Google Scholar]; (q) He Y; Zheng Z; Liu YJ; Qiao JJ; Zhang XY; Fan XS Chem. Commun 2019, 55, 12372. [DOI] [PubMed] [Google Scholar]; (r) He KX; Zhang T; Zhang SW; Sun Z; Zhang YX; Yuan Y; Jia XD Org. Lett 2019, 21, 5030. [DOI] [PubMed] [Google Scholar]
- (23).(a) Konstantinova LS; Rakitin OA; Rees CW Chem. Rev 2004, 104, 2617. [DOI] [PubMed] [Google Scholar]; (b) Rakitin OА Chem. Heterocycl. Compd. (Engl. Transl.) 2020, 56, 837. [Google Scholar]; (c) Rees CW; Marcos CF; Polo C; Torroba T; Rakitin OA Angew. Chem., Int. Ed. Engl 1997, 36, 281. [Google Scholar]; (d) Marcos CF; Polo C; Rakitin OA; Rees CW; Torroba T Chem. Commun 1997, 879. [Google Scholar]; (e) Rees CW; White AJP; Williams DJ; Rakitin OA; Marcos CF; Polo C; Torroba T J. Org. Chem 1998, 63, 2189. [Google Scholar]; (f) Konstantinova LS; Obruchnikova NV; Rakitin OA; Rees CW; Torroba T J. Chem. Soc., Perkin Trans 1 2000, 3421. [Google Scholar]; (g) Barriga S; Konstantinova SL; Marcos FC; Rakitin AO; Rees WC; Torroba T; White JPA; Williams JD J. Chem. Soc., Perkin Trans 1 1999, 2237. [Google Scholar]; (h) Konstantinova LS; Berezin AA; Lysov KA; Rakitin OA Russ. Chem. Bull., Int. Ed 2006, 55, 147. [Google Scholar]; (i) Amelichev SA; Konstantinova LS; Lyssenko KA; Rakitin OA; Rees CW Org. Biomol. Chem 2005, 3, 3496. [DOI] [PubMed] [Google Scholar]; (j) Konstantinova LS; Amelichev SA; Rakitin OA Russ. Chem. Bull., Int. Ed 2006, 55, 2081. [Google Scholar]; (k) Konstantinova LS; Rakitin OA; Rees CW; Souvorova LI; Golovanov DG; Lyssenko KA Org. Lett 2003, 5, 1939. [DOI] [PubMed] [Google Scholar]; (l) Konstantinova LS; Bastrakov MA; Starosotnikov AM; Glukhov IV; Lysov KA; Rakitin OA; Shevelev SA Mendeleev Commun. 2010, 20, 353. [Google Scholar]
- (24).(a) Lounasmaa M; Koskinen A Heterocycles 1984, 22, 1591. [Google Scholar]; (b) Grierson D Org. React 1990, 39, 85. [Google Scholar]; (c) Langlois N; Gueritte F; Langlois Y; Potier P J. Am. Chem. Soc 1976, 98, 7017. [DOI] [PubMed] [Google Scholar]; (d) Han-ya Y; Tokuyama H; Fukuyama T Angew. Chem. Int. Ed 2011, 50, 4884. [DOI] [PubMed] [Google Scholar]; (e) Ahond A; Cave A; Kan-Fan C; Husson HP; De Rostolan J; Potier P J. Am. Chem. Soc 1968, 90, 5622. [Google Scholar]; (f) Aimi N; Yamanaka E; Endo J; Sakai S; Haginiwa J Tetrahedron 1973, 29, 2015. [Google Scholar]; (g) Kende AS; Liu K; Jos Brands KM J. Am. Chem. Soc 1995, 117, 10597. [Google Scholar]; (h) Wenkert E; Chauncy B; Wentland SH Synth. Commun 1973, 3, 73. [Google Scholar]; (i) Beugelmans R; Negron G; Roussi G J. Chem. Soc., Chem. Commun 1983, 31. [Google Scholar]; (j) Chastanet J; Roussi G Heterocycles 1985, 23, 653. [Google Scholar]; (k) Chastanet J; Roussi G J. Org. Chem 1985, 50, 2910. [Google Scholar]; (l) Chastanet J; Roussi G J. Org. Chem 1988, 53, 3808. [Google Scholar]; (m) Davoren JE; Gray DL; Harris AR; Nason DM; Xu W Synlett 2010, 2490. [Google Scholar]; (n) Takano S; Sugihara Y; Ogasawara K Heterocycles 1992, 34, 1519. [Google Scholar]; (o) Mirzayans PM; Krenske EH; Williams CM Aust. J. Chem 2014, 67, 1309. [Google Scholar]; (p) Bernier D; Wefelscheid UK; Woodward S Org. Prep. Proced. Int 2009, 41, 173. [Google Scholar]; (q) Cui L; Peng Y; Zhang L J. Am. Chem. Soc 2009, 131, 8394. [DOI] [PubMed] [Google Scholar]; (r) Cui L; Ye L; Zhang L Chem. Commun 2010, 46, 3351. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Noey EL; Luo YD; Zhang LM; Houk KN J. Am. Chem. Soc 2012, 134, 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).(a) Nishiguchi T; Tachi K; Fukuzumi K J. Org. Chem 1975, 40, 237. [Google Scholar]; (b) Yoshida T; Okano T; Otsuka S J. Chem. Soc., Chem. Commun 1979, 870. [Google Scholar]; (c) Murahashi SI; Naota T; Taki H J. Chem. Soc., Chem. Commun 1985, 613. [Google Scholar]; (d) Gu XQ; Chen W; Morales-Morales D; Jensen CM J. Mol. Catal. A: Chem 2002, 189, 119. [Google Scholar]; (e) Zhang XW; Fried A; Knapp S; Goldman AS Chem. Commun 2003, 2060. [PubMed] [Google Scholar]; (f) Kamiguchi S; Nakamura A; Suzuki A; Kodomari M; Nomura M; Iwasawa Y; Chihara T J. Catal 2005, 230, 204. [Google Scholar]; (g) Yi CS; Lee DW Organometallics 2009, 28, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Wang ZH; Tonks I; Belli J; Jensen CM J. Organomet. Chem 2009, 694, 2854. [Google Scholar]; (i) Brayton DF; Jensen CM Chem. Commun 2014, 50, 5987. [DOI] [PubMed] [Google Scholar]; (j) Lu YSJ; Zhang XW; Malakar S; Krogh-Jespersen K; Hasanayn F; Goldman AS J. Org. Chem 2020, 85, 3020. [DOI] [PubMed] [Google Scholar]; (k) Wang YL; Qian L; Huang ZD; Liu GX; Huang Z Chin. J. Chem 2020, 38, 837. [Google Scholar]; (l) Fujita K; Tanaka Y; Kobayashi M; Yamaguchi R J. Am. Chem. Soc 2014, 136, 4829. [DOI] [PubMed] [Google Scholar]; (m) Yamaguchi R; Ikeda C; Takahashi Y; Fujita K J. Am. Chem. Soc 2009, 131, 8410. [DOI] [PubMed] [Google Scholar]; (n) Wu JJ; Talwar D; Johnston S; Yan M; Xiao JL Angew. Chem. Int. Ed 2013, 52, 6983. [DOI] [PubMed] [Google Scholar]; (o) Talwar D; Gonzalez-de-Castro A; Li HY; Xiao JL Angew. Chem. Int. Ed 2015, 54, 5223. [DOI] [PubMed] [Google Scholar]; (p) Manas MG; Sharninghausen LS; Lin E; Crabtree RH J. Organomet. Chem 2015, 792, 184. [Google Scholar]; (q) Zhang DL; Iwai T; Sawamura M Org. Lett 2020, 22, 5240. [DOI] [PubMed] [Google Scholar]; (r) Tseng KNT; Rizzi AM; Szymczak NK J. Am. Chem. Soc 2013, 135, 16352. [DOI] [PubMed] [Google Scholar]; (s) Hale LVA; Malakar T; Tseng KNT; Zimmerman PM; Paul A; Szymczak NK ACS Catal. 2016, 6, 4799. [Google Scholar]; (t) Muthaiah S; Hong SH Adv. Synth. Catal 2012, 354, 3045. [Google Scholar]; (u) Dutta I; Yadav S; Sarbajna A; De S; Holscher M; Leitner W; Bera JK J. Am. Chem. Soc 2018, 140, 8662. [DOI] [PubMed] [Google Scholar]; (v) Wang QF; Chai HN; Yu ZK Organometallics 2018, 37, 584. [Google Scholar]; (w) Stubbs JM; Hazlehurst RJ; Boyle PD; Blacquiere JM Organometallics 2017, 36, 1692. [Google Scholar]; (x) Kojima M; Kanai M Angew. Chem. Int. Ed 2016, 55, 12224. [DOI] [PubMed] [Google Scholar]; (y) Maier AFG; Tussing S; Schneider T; Florke U; Qu ZW; Grimme S; Paradies J Angew. Chem. Int. Ed 2016, 55, 12219. [DOI] [PubMed] [Google Scholar]; (z) Wu Y; Yi H; Lei AW ACS Catal. 2018, 8, 1192. [Google Scholar]; (aa) Chen WD; Tang H; Wang WL; Fu Q; Luo JF Adv. Synth. Catal 2020, 362, 3905. [Google Scholar]; (ab) Yang RC; Yue SS; Tan W; Xie YF; Cai H J. Org. Chem 2020, 85, 7501. [DOI] [PubMed] [Google Scholar]; (ac) Huang YQ; Song HJ; Liu YX; Wang QM Chem. Eur. J 2018, 24, 2065. [DOI] [PubMed] [Google Scholar]; (ad) Liu TT; Wu KK; Wang LD; Yu ZK Adv. Synth. Catal 2019, 361, 3958. [Google Scholar]; (ae) Kato S; Saga Y; Kojima M; Fuse H; Matsunaga S; Fukatsu A; Kondo M; Masaoka S; Kanai M J. Am. Chem. Soc 2017, 139, 2204. [DOI] [PubMed] [Google Scholar]; (af) Kim J; Kim S; Choi G; Lee GS; Kim D; Choi J; Ihee H; Hong SH Chem. Sci 2021, 12, 1915. [DOI] [PMC free article] [PubMed] [Google Scholar]; (ag) He KH; Tan FF; Zhou CZ; Zhou GJ; Yang XL; Li Y Angew. Chem. Int. Ed 2017, 56, 3080. [DOI] [PubMed] [Google Scholar]; (ah) Chakraborty S; Brennessel WW; Jones WD J. Am. Chem. Soc 2014, 136, 8564. [DOI] [PubMed] [Google Scholar]; (ai) Bolig AD; Brookhart M J. Am. Chem. Soc 2007, 129, 14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).(a) Grigg R; Mitchell TRB; Sutthivaiyakit S; Tongpenyai N J. Chem. Soc., Chem. Commun 1981, 611. [Google Scholar]; (b) Sundararaju B; Tang Z; Achard M; Sharma GVM; Toupet L; Bruneau C Adv. Synth. Catal 2010, 352, 3141. [Google Scholar]; (c) Sundararaju B; Achard M; Sharma GVM; Bruneau C J. Am. Chem. Soc 2011, 133, 10340. [DOI] [PubMed] [Google Scholar]; (d) Boudiar T; Sahli Z; Sundararaju B; Achard M; Kabouche Z; Doucet H; Bruneau C J. Org. Chem 2012, 77, 3674. [DOI] [PubMed] [Google Scholar]; (e) Murugesh V; Bruneau C; Achard M; Sahoo AR; Sharma GVM; Suresh S Chem. Commun 2017, 53, 10448. [DOI] [PubMed] [Google Scholar]; (f) Murugesh V; Sahoo AR; Achard M; Sharma GVM; Bruneau C; Suresh S Adv. Synth. Catal 2021, 363, 453. [Google Scholar]; (g) Chen Y; Wan HL; Huang Y; Liu S; Wang FY; Lu CF; Nie JQ; Chen ZX; Yang GC; Ma C Org. Lett 2020, 22, 7797. [DOI] [PubMed] [Google Scholar]; (h) Yuan KD; Jiang F; Sahli Z; Achard M; Roisnel T; Bruneau C Angew. Chem. Int. Ed 2012, 51, 8876. [DOI] [PubMed] [Google Scholar]; (i) Sahli Z; Sundararaju B; Achard M; Bruneau C Green Chem. 2013, 15, 775. [Google Scholar]; (j) Bruneau C. Top. Organomet. Chem 2014, 48, 195. [Google Scholar]; (k) Irrgang T; Kempe R Chem. Rev 2019, 119, 2524. [DOI] [PubMed] [Google Scholar]
- (27).(a) Rügheimer L Ber. Dtsch. Chem. Ges 1891, 24, 2186. [Google Scholar]; (b) Rügheimer L Ber. Dtsch. Chem. Ges 1892, 25, 2421. [Google Scholar]; (c) Poirier RH; Morin RD; McKim AM; Bearse AE J. Org. Chem 1961, 26, 4275. [Google Scholar]; (d) Platonova AY; Seidel D Tetrahedron Lett. 2015, 56, 3147. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Burrows EP; Hutton RF; Burrows WD J. Org. Chem 1962, 27, 316. [Google Scholar]; (f) Sainsbury M; Dyke SF; Brown DW; Lugton WGD Tetrahedron 1968, 24, 427. [Google Scholar]; (g) Dannhardt G; Mayer KK; Obergrusberger I; Roelcke J Arch. Pharm.(Weinheim Ger.) 1986, 319, 977. [Google Scholar]; (h) Cook AG; Switek KA; Cutler KA; Witt AN Lett. Org. Chem 2004, 1, 1. [Google Scholar]; (i) Polackova V; Veverkova E; Toma S; Bogdal D Synth. Commun 2009, 39, 1871. [Google Scholar]; (j) Xue X; Yu A; Cai Y; Cheng J-P Org. Lett 2011, 13, 6054. [DOI] [PubMed] [Google Scholar]; (k) Moura NMM; Nunez C; Santos SM; Faustino MAF; Cavaleiro JAS; Paz FAA; Neves M; Capelo JL; Lodeiro C Chem. Eur. J 2014, 20, 6684. [DOI] [PubMed] [Google Scholar]; (l) Burrows WD; Burrows EP J. Org. Chem 1963, 28, 1180. [Google Scholar]; (m) Oda M; Fukuchi Y; Ito S; Thanh NC; Kuroda S Tetrahedron Lett. 2007, 48, 9159. [Google Scholar]; (n) Pahadi NK; Paley M; Jana R; Waetzig SR; Tunge JA J. Am. Chem. Soc 2009, 131, 16626. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Mao H; Xu R; Wan J; Jiang Z; Sun C; Pan Y Chem. Eur. J 2010, 16, 13352. [DOI] [PubMed] [Google Scholar]; (p) Deb I; Das D; Seidel D Org. Lett 2011, 13, 812. [DOI] [PubMed] [Google Scholar]; (q) Ma L; Paul A; Breugst M; Seidel D Chem. Eur. J 2016, 22, 18179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).(a) Ardill H; Grigg R; Sridharan V; Surendrakumar S; Thianpatanagul S; Kanajun S J. Chem. Soc., Chem. Commun 1986, 602. [Google Scholar]; (b) Grigg R; Gunaratne HQN; Henderson D; Sridharan V Tetrahedron 1990, 46, 1599. [Google Scholar]; (c) Ardill H; Fontaine XLR; Grigg R; Henderson D; Montgomery J; Sridharan V; Surendrakumar S Tetrahedron 1990, 46, 6449. [Google Scholar]; (d) Mantelingu K; Lin Y; Seidel D Org. Lett 2014, 16, 5910. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Rahman M; Bagdi AK; Mishra S; Hajra A Chem. Commun 2014, 50, 2951. [DOI] [PubMed] [Google Scholar]; (f) Deb I; Seidel D Tetrahedron Lett. 2010, 51, 2945. [Google Scholar]; (g) Kang Y; Richers MT; Sawicki CH; Seidel D Chem. Commun 2015, 51, 10648. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Cheng Y-F; Rong H-J; Yi C-B; Yao J-J; Qu J Org. Lett 2015, 17, 4758. [DOI] [PubMed] [Google Scholar]; (i) Vasu D; Fuentes de Arriba AL; Leitch JA; de Gombert A; Dixon DJ Chem. Sci 2019, 10, 3401. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Wittland C; Arend M; Risch N Synthesis 1996, 367. [Google Scholar]; (k) Yang H-T; Tan Y-C; Ge J; Wu H; Li J-X; Yang Y; Sun XQ; Miao C-B J. Org. Chem 2016, 81, 11201. [DOI] [PubMed] [Google Scholar]; (l) Zheng K; Zhuang S; You M; Shu W; Wu A; Wu Y ChemistrySelect 2017, 2, 10762. [Google Scholar]; (m) Strada A; Fredditori M; Zanoni G; Protti S Molecules 2019, 24, 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).(a) Ma L; Chen W; Seidel D J. Am. Chem. Soc 2012, 134, 15305. [DOI] [PubMed] [Google Scholar]; (b) Das D; Sun AX; Seidel D Angew. Chem. Int. Ed 2013, 52, 3765. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zheng Q-H; Meng W; Jiang G-J; Yu Z-X Org. Lett 2013, 15, 5928. [DOI] [PubMed] [Google Scholar]; (d) Lin W; Cao T; Fan W; Han Y; Kuang J; Luo H; Miao B; Tang X; Yu Q; Yuan W; Zhang J; Zhu C; Ma S Angew. Chem. Int. Ed 2014, 53, 277. [DOI] [PubMed] [Google Scholar]; (e) Lin W; Ma S Org. Chem. Front 2014, 1, 338. [Google Scholar]; (f) Chen W; Wilde RG; Seidel D Org. Lett 2014, 16, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Haldar S; Mahato S; Jana CK Asian J. Org. Chem 2014, 3, 44. [Google Scholar]; (h) Chen W; Kang Y; Wilde RG; Seidel D Angew. Chem. Int. Ed 2014, 53, 5179. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zheng K-L; Shu W-M; Ma J-R; Wu Y-D; Wu A-X Org. Lett 2016, 18, 3526. [DOI] [PubMed] [Google Scholar]; (j) Mandal S; Dwari S; Jana CK J. Org. Chem 2018, 83, 8874. [DOI] [PubMed] [Google Scholar]; (k) Chen W; Seidel D Org. Lett 2014, 16, 3158. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Zhu Z; Seidel D Org. Lett 2016, 18, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Yan J-M; Bai Q-F; Xu C; Feng G Synthesis 2016, 48, 3730. [Google Scholar]; (n) Haldar S; Saha S; Mandal S; Jana CK Green Chem. 2018, 20, 3463. [Google Scholar]; (o) Jiang D; Wu Z; Wang J Chin. J. Chem 2020, 38, 135. [Google Scholar]; (p) Seidel D Org. Chem. Front 2014, 1, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Seidel D Acc. Chem. Res 2015, 48, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Mahato S; Jana CK Chem. Rec 2016, 16, 1477. [DOI] [PubMed] [Google Scholar]; (s) Das D; Seidel D Org. Lett 2013, 15, 4358. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Mandal S; Mahato S; Jana CK Org. Lett 2015, 17, 3762. [DOI] [PubMed] [Google Scholar]; (u) Zhou S; Tong R Chem. Eur. J 2016, 22, 7084. [DOI] [PubMed] [Google Scholar]; (v) Huang J; Li L; Xiao T; Mao Z.-w.; Zhou L Asian J. Org. Chem 2016, 5, 1204. [Google Scholar]; (w) Du Y; Yu A; Jia J; Zhang Y; Meng X Chem. Commun 2017, 53, 1684. [DOI] [PubMed] [Google Scholar]; (x) Yi C-B; She Z-Y; Cheng Y-F; Qu J Org. Lett 2018, 20, 668. [DOI] [PubMed] [Google Scholar]; (y) Haldar S; Jana CK Org. Biomol. Chem 2019, 17, 1800. [DOI] [PubMed] [Google Scholar]; (z) Rahman I; Deka B; Thakuria R; Deb ML; Baruah PK Org. Biomol. Chem 2020, 18, 6514. [DOI] [PubMed] [Google Scholar]
- (30).(a) Zhang C; De C K; Mal R; Seidel D J. Am. Chem. Soc 2008, 130, 416. [DOI] [PubMed] [Google Scholar]; (b) Dieckmann A; Richers MT; Platonova AY; Zhang C; Seidel D; Houk KN J. Org. Chem 2013, 78, 4132. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Richers MT; Deb I; Platonova AY; Zhang C; Seidel D Synthesis 2013, 45, 1730. [PMC free article] [PubMed] [Google Scholar]; (d) Zheng L; Yang F; Dang Q; Bai X Org. Lett 2008, 10, 889. [DOI] [PubMed] [Google Scholar]; (e) Richers MT; Breugst M; Platonova AY; Ullrich A; Dieckmann A; Houk KN; Seidel D J. Am. Chem. Soc 2014, 136, 6123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Jarvis CL; Richers MT; Breugst M; Houk KN; Seidel D Org. Lett 2014, 16, 3556. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Mahato S; Haque MA; Dwari S; Jana CK RSC Adv. 2014, 4, 46214. [Google Scholar]; (h) Kirkeby EK; Roberts AG Chem. Commun 2020, 56, 9118. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zhang C; Das D; Seidel D Chem. Sci 2011, 2, 233. [Google Scholar]; (j) Ma L; Seidel D Chem. Eur. J 2015, 21, 12908. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Kang Y; Chen W; Breugst M; Seidel D J. Org. Chem 2015, 80, 9628. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Chen W; Seidel D Org. Lett 2016, 18, 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Zhu Z; Seidel D Org. Lett 2017, 19, 2841. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Li J; Qin C; Yu Y; Fan H; Fu Y; Li H; Wang W Adv. Synth. Catal 2017, 359, 2191. [Google Scholar]; (o) Li J; Fu Y; Qin C; Yu Y; Li H; Wang W Org. Biomol. Chem 2017, 15, 6474. [DOI] [PubMed] [Google Scholar]; (p) Zhu Z; Chandak HS; Seidel D Org. Lett 2018, 20, 4090. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Paul A; Chandak HS; Ma L; Seidel D Org. Lett 2020, 22, 976. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Rickertsen DRL; Ma L; Paul A; Abboud KA; Seidel D SynOpen 2020, 4, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Zhu Z; Lv X; Anesini JE; Seidel D Org. Lett 2017, 19, 6424. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Liu Y; Wu J; Jin Z; Jiang H Synlett 2018, 29, 1061. [Google Scholar]; (u) Afanasyev OI; Podyacheva E; Rudenko A; Tsygankov AA; Makarova M; Chusov D J. Org. Chem 2020, 85, 9347. [DOI] [PubMed] [Google Scholar]
- (31).(a) Pinnow J Ber. Dtsch. Chem. Ges 1895, 28, 3039. [Google Scholar]; (b) Ruiz MDR; Vasella A Helv. Chim. Acta 2011, 94, 785. [Google Scholar]; (c) Meth-Cohn O; Naqui MA Chem. Commun 1967, 1157. [Google Scholar]; (d) Ryabukhin SV; Plaskon AS; Volochnyuk DM; Shivanyuk AN; Tolmachev AA J. Org. Chem 2007, 72, 7417. [DOI] [PubMed] [Google Scholar]; (e) Verboom W; Reinhoudt DN; Visser R; Harkema S J. Org. Chem 1984, 49, 269. [Google Scholar]; (f) Nijhuis WHN; Verboom W; Abu El-Fadl A; Harkema S; Reinhoudt DN J. Org. Chem 1989, 54, 199. [Google Scholar]; (g) Nijhuis WHN; Verboom W; Abu El-Fadl A; Van Hummel GJ; Reinhoudt DN J. Org. Chem 1989, 54, 209. [Google Scholar]; (h) Verboom W; Hamzink MRJ; Reinhoudt DN; Visser R Tetrahedron Lett. 1984, 25, 4309. [Google Scholar]; (i) Noguchi M; Yamada H; Sunagawa T J. Chem. Soc., Perkin Trans 1 1998, 3327. [Google Scholar]; (j) Reinhoudt DN; Visser GW; Verboom W; Benders PH; Pennings MLM. J. Am. Chem. Soc 1983, 105, 4775. [Google Scholar]; (k) Jiang S; Janousek Z; Viehe HG Tetrahedron Lett. 1994, 35, 1185. [Google Scholar]; (l) Jiang S; Janousek Z; Viehe HG Bull. Soc. Chim. Belg 1993, 102, 663. [Google Scholar]; (m) De Boeck B; Janousek Z; Viehe HG Tetrahedron 1995, 51, 13239. [Google Scholar]; (n) Polonka-Balint A; Saraceno C; Ludányi K; Bényei A; Matyus P Synlett 2008, 2846. [Google Scholar]; (o) Foldi AA; Ludanyi K; Benyei AC; Matyus P Synlett 2010, 2109. [Google Scholar]; (p) Barluenga J; Fananas-Mastral M; Aznar F; Valdes C Angew. Chem. Int. Ed 2008, 47, 6594. [DOI] [PubMed] [Google Scholar]; (q) Meth-Cohn O; Suschitzky H Adv. Heterocycl. Chem 1972, 14, 211. [Google Scholar]; (r) Meth-Cohn O Adv. Heterocycl. Chem 1996, 65, 1. [Google Scholar]; (s) Matyus P; Elias O; Tapolcsanyi P; Polonka-Balint A; Halasz-Dajka B Synthesis 2006, 2625. [Google Scholar]; (t) Platonova AY; Glukhareva TV; Zimovets OA; Morzherin YY Chem. Heterocycl. Compd. (Engl. Transl.) 2013, 49, 357. [Google Scholar]
- (32).(a) Peng B; Maulide N Chem. Eur. J 2013, 19, 13274. [DOI] [PubMed] [Google Scholar]; (b) Haibach MC; Seidel D Angew. Chem. Int. Ed 2014, 53, 5010. [DOI] [PubMed] [Google Scholar]; (c) Kwon SJ; Kim DY Chem. Rec 2016, 16, 1191. [DOI] [PubMed] [Google Scholar]; (d) An X-D; Xiao J Org. Chem. Front 2021, 8, 1364. [Google Scholar]; (e) Zhang C; Murarka S; Seidel DJ Org. Chem 2009, 74, 419. [DOI] [PubMed] [Google Scholar]; (f) Mori K; Ohshima Y; Ehara K; Akiyama T Chem. Lett 2009, 38, 524. [Google Scholar]; (g) Murarka S; Zhang C; Konieczynska MD; Seidel D Org. Lett 2009, 11, 129. [DOI] [PubMed] [Google Scholar]; (h) Zhou G; Zhang J Chem. Commun 2010, 46, 6593. [DOI] [PubMed] [Google Scholar]; (i) Jurberg ID; Peng B; Woestefeld E; Wasserloos M; Maulide N Angew. Chem. Int. Ed 2012, 51, 1950. [DOI] [PubMed] [Google Scholar]; (j) Haibach MC; Deb I; De C K; Seidel D J. Am. Chem. Soc 2011, 133, 2100. [DOI] [PubMed] [Google Scholar]; (k) Wang P-F; Huang Y-P; Wen X; Sun H; Xu Q-L Eur. J. Org. Chem 2015, 6727. [Google Scholar]; (l) Wang P-F; Jiang C-H; Wen X; Xu Q-L; Sun H J. Org. Chem 2015, 80, 1155. [DOI] [PubMed] [Google Scholar]; (m) Li S-S; Zhou L; Wang L; Zhao H; Yu L; Xiao J Org. Lett 2018, 20, 138. [DOI] [PubMed] [Google Scholar]; (n) Liu S; Qu J; Wang B Chem. Commun 2018, 54, 7928. [DOI] [PubMed] [Google Scholar]; (o) Bai G; Dong F; Xu L; Liu Y; Wang L; Li S-S Org. Lett 2019, 21, 6225. [DOI] [PubMed] [Google Scholar]; (p) Wang S; Shen Y-B; Li L-F; Qiu B; Yu L; Liu Q; Xiao J Org. Lett 2019, 21, 8904. [DOI] [PubMed] [Google Scholar]; (q) An X-D; Duan K; Li X-J; Yang J-M; Lu Y-N; Liu Q; Xiao J J. Org. Chem 2019, 84, 11839. [DOI] [PubMed] [Google Scholar]; (r) Suh CW; Kwon SJ; Kim DY Org. Lett 2017, 19, 1334. [DOI] [PubMed] [Google Scholar]; (s) Li S-S; Lv X; Ren D; Shao C-L; Liu Q; Xiao J Chem. Sci 2018, 9, 8253. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Lv X; Hu F; Duan K; Li S-S; Liu Q; Xiao J J. Org. Chem 2019, 84, 1833. [DOI] [PubMed] [Google Scholar]; (u) Shen Y-B; Li L-F; Xiao M-Y; Yang J-M; Liu Q; Xiao J J. Org. Chem 2019, 84, 13935. [DOI] [PubMed] [Google Scholar]; (v) Mori K; Kurihara K; Yabe S; Yamanaka M; Akiyama T J. Am. Chem. Soc 2014, 136, 3744. [DOI] [PubMed] [Google Scholar]
- (33).(a) Murarka S; Deb I; Zhang C; Seidel D J. Am. Chem. Soc 2009, 131, 13226. [DOI] [PubMed] [Google Scholar]; (b) Kang YK; Kim SM; Kim DY J. Am. Chem. Soc 2010, 132, 11847. [DOI] [PubMed] [Google Scholar]; (c) Kang YK; Kim DY Adv. Synth. Catal 2013, 355, 3131. [Google Scholar]; (d) Kang YK; Kim DY Chem. Commun 2014, 50, 222. [DOI] [PubMed] [Google Scholar]; (e) Suh CW; Kim DY Org. Lett 2014, 16, 5374. [DOI] [PubMed] [Google Scholar]; (f) Zhou G; Liu F; Zhang J Chem. Eur. J 2011, 17, 3101. [DOI] [PubMed] [Google Scholar]; (g) Mori K; Ehara K; Kurihara K; Akiyama T J. Am. Chem. Soc 2011, 133, 6166. [DOI] [PubMed] [Google Scholar]; (h) Cao W; Liu X; Wang W; Lin L; Feng X Org. Lett 2011, 13, 600. [DOI] [PubMed] [Google Scholar]; (i) Lv J; Luo S Chem. Commun 2013, 49, 847. [DOI] [PubMed] [Google Scholar]; (j) He Y-P; Du Y-L; Luo S-W; Gong L-Z Tetrahedron Lett. 2011, 52, 7064. [Google Scholar]; (k) Mori K; Isogai R; Kamei Y; Yamanaka M; Akiyama T J. Am. Chem. Soc 2018, 140, 6203. [DOI] [PubMed] [Google Scholar]; (l) Wang M ChemCatChem 2013, 5, 1291. [Google Scholar]; (m) Xiao M; Zhu S; Shen Y; Wang L; Xiao J Chin. J. Org. Chem 2018, 38, 328. [Google Scholar]; (n) Cao W; Liu X; Guo J; Lin L; Feng X Chem. Eur. J 2015, 21, 1632. [DOI] [PubMed] [Google Scholar]; (o) He Y-P; Wu H; Chen D-F; Yu J; Gong L-Z Chem. Eur. J 2013, 19, 5232. [DOI] [PubMed] [Google Scholar]; (p) Mao Z; Mo F; Lin X Synlett 2016, 27, 546. [Google Scholar]; (q) Du H-J; Lin C; Wen X; Xu Q-L Tetrahedron 2018, 74, 7480. [Google Scholar]
- (34).(a) Ten Broeke J; Douglas AW; Grabowski EJJ J. Org. Chem 1976, 41, 3159. [Google Scholar]; (b) Heathcock CH; Hansen MM; Ruggeri RB; Kath JC J. Org. Chem 1992, 57, 2544. [Google Scholar]; (c) Heathcock CH Proc. Natl. Acad. Sci. U.S.A 1996, 93, 14323. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tantillo D. J. Org. Lett 2016, 18, 4482. [DOI] [PubMed] [Google Scholar]; (e) Pastine SJ; McQuaid KM; Sames D J. Am. Chem. Soc 2005, 127, 12180. [DOI] [PubMed] [Google Scholar]; (f) Vadola PA; Sames D J. Am. Chem. Soc 2009, 131, 16525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Sugiishi T; Nakamura H J. Am. Chem. Soc 2012, 134, 2504. [DOI] [PubMed] [Google Scholar]; (h) Cui Y; Lin W; Ma S Chem. Sci 2019, 10, 1796. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Huang Y-W; Frontier AJ Org. Lett 2016, 18, 4896. [DOI] [PubMed] [Google Scholar]; (j) Lecomte M; Evano G Angew. Chem. Int. Ed 2016, 55, 4547. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Machida M; Mori K Chem. Lett 2018, 47, 868. [Google Scholar]; (l) Zhang X; Anderson JC Angew. Chem. Int. Ed 2019, 58, 18040. [DOI] [PubMed] [Google Scholar]; (m) Kataoka M; Otawa Y; Ido N; Mori K Org. Lett 2019, 21, 9334. [DOI] [PubMed] [Google Scholar]; (n) Ye J; Ma S Org. Chem. Front 2014, 1, 1210. [Google Scholar]
- (35).(a) Shang M; Chan JZ; Cao M; Chang Y; Wang Q; Cook B; Torker S; Wasa M J. Am. Chem. Soc 2018, 140, 10593. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Millot N; Santini CC; Fenet B; Basset JM Eur. J. Inorg. Chem 2002, 3328. [Google Scholar]; (c) Chen G-Q; Kehr G; Daniliuc CG; Bursch M; Grimme S; Erker G Chem. Eur. J 2017, 23, 4723. [DOI] [PubMed] [Google Scholar]; (d) Zhang J; Park S; Chang S J. Am. Chem. Soc 2018, 140, 13209. [DOI] [PubMed] [Google Scholar]; (e) Zhou M; Park S; Dang L Org. Chem. Front 2020, 7, 944. [Google Scholar]; (f) Chang Y; Cao M; Chan JZ; Zhao C; Wang Y; Yang R; Wasa M J. Am. Chem. Soc 2021, 143, 2441. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chan JZ; Yesilcimen A; Cao M; Zhang Y; Zhang B; Wasa M J. Am. Chem. Soc 2020, 142, 16493. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Chan JZ; Chang Y; Wasa M Org. Lett 2019, 21, 984. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Li R; Chen Y; Jiang K; Wang F; Lu C; Nie J; Chen Z; Yang G; Chen Y-C; Zhao Y; Ma C Chem. Commun 2019, 55, 1217. [DOI] [PubMed] [Google Scholar]; (j) Maier AFG; Tussing S; Zhu H; Wicker G; Tzvetkova P; Flörke U; Daniliuc CG; Grimme S; Paradies J Chem. Eur. J 2018, 24, 16287. [DOI] [PubMed] [Google Scholar]; (k) Tian J-J; Zeng N-N; Liu N; Tu X-S; Wang X-C ACS Catal. 2019, 9, 295. [Google Scholar]; (l) Fang H; Xie K; Kemper S; Oestreich M Angew. Chem. Int. Ed 2021, 60, 8542. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Zhou L; Shen Y-B; An X-D; Li X-J; Li S-S; Liu Q; Xiao J Org. Lett 2019, 21, 8543. [DOI] [PubMed] [Google Scholar]; (n) Chang Y; Yesilcimen A; Cao M; Zhang Y; Zhang B; Chan JZ; Wasa M J. Am. Chem. Soc 2019, 141, 14570. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Zhou L; An X-D; Yang S; Li X-J; Shao C-L; Liu Q; Xiao J Org. Lett 2020, 22, 776. [DOI] [PubMed] [Google Scholar]; (p) Ma Y; Lou S-J; Hou Z Chem. Soc. Rev 2021, 50, 1945. [DOI] [PubMed] [Google Scholar]; (q) Basak S; Winfrey L; Kustiana BA; Melen RL; Morrill LC; Pulis AP Chem. Soc. Rev 2021, 50, 3720. [DOI] [PubMed] [Google Scholar]
- (36).(a) Fandrick DR; Hart CA; Okafor IS; Mercadante MA; Sanyal S; Masters JT; Sarvestani M; Fandrick KR; Stockdill JL; Grinberg N; Gonnella N; Lee H; Senanayake CH Org. Lett 2016, 18, 6192. [DOI] [PubMed] [Google Scholar]; (b) Wittig G; Schmidt HJ; Renner H Chem. Ber 1962, 95, 2377. [Google Scholar]; (c) Wittig G; Hesse A Liebigs Ann. Chem 1971, 746, 174. [Google Scholar]; (d) Wittig G; Häusler G Liebigs Ann. Chem 1971, 746, 185. [Google Scholar]; (e) Chen W; Ma L; Paul A; Seidel D Nat. Chem 2018, 10, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen W; Paul A; Abboud KA; Seidel D Nat. Chem 2020, 12, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Majewski M; Gleave DM J. Organomet. Chem 1994, 470, 1. [Google Scholar]; (h) Scully FE J. Org. Chem 1980, 45, 1515. [Google Scholar]; (i) Paul A; Seidel D J. Am. Chem. Soc 2019, 141, 8778. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Kim JH; Paul A; Ghiviriga I; Seidel D Org. Lett 2021, 23, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Chen W; Seidel D Org. Lett 2021, 23, 3729. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Paul A; Kim JH; Daniel SD; Seidel D Angew. Chem. Int. Ed 2021, 60, 1625. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Shen Z; Walker MM; Chen S; Parada GA; Chu DM; Dongbang S; Mayer JM; Houk KN; Ellman JA J. Am. Chem. Soc 2021, 143, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).(a) Corey EJ; Felix AM J. Am. Chem. Soc 1965, 87, 2518. [DOI] [PubMed] [Google Scholar]; (b) Earle RH; Hurst DT; Viney M J. Chem. Soc. C 1969, 2093. [Google Scholar]; (c) Axten JM; Krim L; Kung HF; Winkler JD J. Org. Chem 1998, 63, 9628. [Google Scholar]; (d) Garner R Tetrahedron Lett. 1968, 9, 221. [Google Scholar]; (e) Krogsgaard-Larsen N; Begtrup M; Herth MM; Kehler J Synthesis 2010, 4287. [Google Scholar]; (f) Mahoney SJ; Fillion E Chem. Eur. J 2012, 18, 68. [DOI] [PubMed] [Google Scholar]; (g) Doyle MP; Protopopova MN; Winchester WR; Daniel KL Tetrahedron Lett. 1992, 33, 7819. [Google Scholar]; (h) Doyle MP; Winchester WR; Hoorn JAA; Lynch V; Simonsen SH; Ghosh R J. Am. Chem. Soc 1993, 115, 9968. [Google Scholar]; (i) Watanabe N; Anada M; Hashimoto S.-i.; Ikegami S Synlett 1994, 1031. [Google Scholar]; (j) Doyle MP; Kalinin AV Synlett 1995, 1075. [Google Scholar]; (k) Doyle MP; Yan M; Phillips IM; Timmons DJ Adv. Synth. Catal 2002, 344, 91. [Google Scholar]; (l) Davies HML; Hansen T; Hopper DW; Panaro SA J. Am. Chem. Soc 1999, 121, 6509. [Google Scholar]; (m) Axten JM; Ivy R; Krim L; Winkler JD J. Am. Chem. Soc 1999, 121, 6511. [Google Scholar]; (n) Davies HML; Venkataramani C Org. Lett 2001, 3, 1773. [DOI] [PubMed] [Google Scholar]; (o) Davies HML; Venkataramani C; Hansen T; Hopper DW J. Am. Chem. Soc 2003, 125, 6462. [DOI] [PubMed] [Google Scholar]; (p) Davies HML; Hopper DW; Hansen T; Liu Q; Childers SR Bioorg. Med. Chem. Lett 2004, 14, 1799. [DOI] [PubMed] [Google Scholar]; (q) Santi M; Müller STR; Folgueiras-Amador AA; Uttry A; Hellier P; Wirth T Eur. J. Org. Chem 2017, 1889. [Google Scholar]; (r) Souza LW; Squitieri RA; Dimirjian CA; Hodur BM; Nickerson LA; Penrod CN; Cordova J; Fettinger JC; Shaw JT Angew. Chem. Int. Ed 2018, 57, 15213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Asako S; Ishihara S; Hirata K; Takai K J. Am. Chem. Soc 2019, 141, 9832. [DOI] [PubMed] [Google Scholar]; (t) Gomes LFR; Veiros LF; Maulide N; Afonso CAM Chem. Eur. J 2015, 21, 1449. [DOI] [PubMed] [Google Scholar]; (u) Doyle MP; Kalinin AV Tetrahedron Lett. 1996, 37, 1371. [Google Scholar]; (v) Lee S; Lim H-J; Cha KL; Sulikowski GA Tetrahedron 1997, 53, 16521. [Google Scholar]; (w) Sulikowski GA; Lee S Tetrahedron Lett. 1999, 40, 8035. [Google Scholar]; (x) Toumieux S; Compain P; Martin OR; Selkti M Org. Lett 2006, 8, 4493. [DOI] [PubMed] [Google Scholar]; (y) Morin MST; Toumieux S; Compain P; Peyrat S; Kalinowska-Tluscik J Tetrahedron Lett. 2007, 48, 8531. [Google Scholar]; (z) He J; Hamann LG; Davies HML; Beckwith RE J. Nat. Commun 2015, 6, 5943. [DOI] [PubMed] [Google Scholar]; (aa) Zhou AZ; Chen K; Arnold FH ACS Catal. 2020, 10, 5393. [Google Scholar]; (ab) Davies HML; Beckwith RE J. Chem. Rev 2003, 103, 2861. [DOI] [PubMed] [Google Scholar]; (ac) Davies HML; Manning JR Nature 2008, 451, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]; (ad) Doyle MP; Duffy R; Ratnikov M; Zhou L Chem. Rev 2010, 110, 704. [DOI] [PubMed] [Google Scholar]; (ae) Davies HML; Morton D Chem. Soc. Rev 2011, 40, 1857. [DOI] [PubMed] [Google Scholar]; (af) Sultanova RM; Khanova MD; Zlotskii SS Chem. Heterocycl. Compd. (Engl. Transl.) 2015, 51, 775. [Google Scholar]
- (38).(a) Hofmann AW Chem. Ber 1883, 16, 558. [Google Scholar]; (b) Löffler K; Freytag C Chem. Ber 1909, 42, 3427. [Google Scholar]; (c) De Armas P; Carrau R; Concepción JI; Francisco CG; Hernández R; Suárez E Tetrahedron Lett. 1985, 26, 2493. [Google Scholar]; (d) Francisco CG; Herrera AJ; Suárez E J. Org. Chem 2003, 68, 1012. [DOI] [PubMed] [Google Scholar]; (e) Martínez C; Muñiz K Angew. Chem. Int. Ed 2015, 54, 8287. [DOI] [PubMed] [Google Scholar]; (f) Liu T; Myers MC; Yu J-Q Angew. Chem. Int. Ed 2017, 56, 306. [DOI] [PubMed] [Google Scholar]; (g) Becker P; Duhamel T; Stein CJ; Reiher M; Muñiz K Angew. Chem. Int. Ed 2017, 56, 8004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhang Z; Zhang X; Nagib DA Chem 2019, 5, 3127. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wolff ME Chem. Rev 1963, 63, 55. [Google Scholar]; (j) Stella L Angew. Chem., Int. Ed. Engl 1983, 22, 337. [Google Scholar]; (k) Jeffrey JL; Sarpong R Chem. Sci 2013, 4, 4092. [Google Scholar]; (l) Stateman L; Nakafuku K; Nagib DA Synthesis 2018, 50, 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Wawzonek S; Thelen PJ J. Am. Chem. Soc 1950, 72, 2118. [Google Scholar]; (n) Corey EJ; Hertler WR J. Am. Chem. Soc 1960, 82, 1657. [Google Scholar]; (o) Fan R; Pu D; Wen F; Wu J J. Org. Chem 2007, 72, 8994. [DOI] [PubMed] [Google Scholar]; (p) Qin Q; Yu S Org. Lett 2015, 17, 1894. [DOI] [PubMed] [Google Scholar]; (q) Paz NR; Rodríguez-Sosa D; Valdés H; Marticorena R; Melián D; Copano MB; González CC; Herrera AJ Org. Lett 2015, 17, 2370. [DOI] [PubMed] [Google Scholar]; (r) Wappes EA; Fosu SC; Chopko TC; Nagib DA Angew. Chem. Int. Ed 2016, 55, 9974. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Short MA; Shehata MF; Sanders MA; Roizen JL Chem. Sci 2020, 11, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).(a) Hey DH; Turpin DG J. Chem. Soc 1954, 2471. [Google Scholar]; (b) Lewin AH; Dinwoodie AH; Cohen T Tetrahedron 1966, 22, 1527. [Google Scholar]; (c) Cohen T; McMullen CH; Smith K J. Am. Chem. Soc 1968, 90, 6866. [Google Scholar]; (d) Shaaban S; Oh J; Maulide N Org. Lett 2016, 18, 345. [DOI] [PubMed] [Google Scholar]; (e) Snieckus V; Cuevas JC; Sloan CP; Liu H; Curran DP J. Am. Chem. Soc 1990, 112, 896. [Google Scholar]; (f) Curran DP; Abraham AC Tetrahedron 1993, 49, 4821. [Google Scholar]; (g) Murakami M; Hayashi M; Ito Y J. Org. Chem 1992, 57, 793. [Google Scholar]; (h) Williams L; Booth SE; Undheim K Tetrahedron 1994, 50, 13697. [Google Scholar]; (i) Yoshimitsu T; Arano Y; Nagaoka H J. Am. Chem. Soc 2005, 127, 11610. [DOI] [PubMed] [Google Scholar]; (j) Yoshikai N; Mieczkowski A; Matsumoto A; Ilies L; Nakamura E J. Am. Chem. Soc 2010, 132, 5568. [DOI] [PubMed] [Google Scholar]; (k) Tian H; Yang H; Zhu C; Fu H Sci. Rep 2016, 6, 19931. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Sarkar S; Cheung KPS; Gevorgyan V Chem. Sci 2020, 11, 12974. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Robertson J; Peplow MA; Pillai J Tetrahedron Lett. 1996, 37, 5825. [Google Scholar]; (n) Khan TA; Tripoli R; Crawford JJ; Martin CG; Murphy JA Org. Lett 2003, 5, 2971. [DOI] [PubMed] [Google Scholar]; (o) Beckwith ALJ; Bowry VW; Bowman WR; Mann E; Parr J; Storey JMD Angew. Chem. Int. Ed 2004, 43, 95. [DOI] [PubMed] [Google Scholar]; (p) Dénès F; Beaufils F; Renaud P Org. Lett 2007, 9, 4375. [DOI] [PubMed] [Google Scholar]; (q) Yoshimitsu T; Matsuda K; Nagaoka H; Tsukamoto K; Tanaka T Org. Lett 2007, 9, 5115. [DOI] [PubMed] [Google Scholar]; (r) Yoshimitsu T; Atsumi C; Iimori E; Nagaoka H; Tanaka T Tetrahedron Lett. 2008, 49, 4473. [Google Scholar]; (s) Wertjes WC; Wolfe LC; Waller PJ; Kalyani D Org. Lett 2013, 15, 5986. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Hollister KA; Conner ES; Spell ML; Deveaux K; Maneval L; Beal MW; Ragains JR Angew. Chem. Int. Ed 2015, 54, 7837. [DOI] [PubMed] [Google Scholar]; (u) Chen J-Q; Wei Y-L; Xu G-Q; Liang Y-M; Xu P-F Chem. Commun 2016, 52, 6455. [DOI] [PubMed] [Google Scholar]; (v) Liu P; Tang J; Zeng X Org. Lett 2016, 18, 5536. [DOI] [PubMed] [Google Scholar]; (w) Huang F-Q; Dong X; Qi L-W; Zhang B Tetrahedron Lett. 2016, 57, 1600. [Google Scholar]
- (40).(a) Weinberg NL; Brown EA J. Org. Chem 1966, 31, 4058. [Google Scholar]; (b) Shono T; Matsumura Y; Tsubata K J. Am. Chem. Soc 1981, 103, 1172. [Google Scholar]; (c) Yoshida J; Suga S; Suzuki S; Kinomura N; Yamamoto A; Fujiwara K J. Am. Chem. Soc 1999, 121, 9546. [Google Scholar]; (d) Suga S; Okajima M; Yoshida J Tetrahedron Lett. 2001, 42, 2173. [Google Scholar]; (e) Suga S; Suzuki S; Yoshida J J. Am. Chem. Soc 2002, 124, 30. [DOI] [PubMed] [Google Scholar]; (f) Kim S; Shoji T; Kitano Y; Chiba K Chem. Commun 2013, 49, 6525. [DOI] [PubMed] [Google Scholar]; (g) Shoji T; Kim S; Chiba K Angew. Chem. Int. Ed 2017, 56, 4011. [DOI] [PubMed] [Google Scholar]; (h) Suga S; Matsumoto K; Ueoka K; Yoshida JI J. Am. Chem. Soc 2006, 128, 7710. [DOI] [PubMed] [Google Scholar]; (i) Yoshida J; Isoe S Tetrahedron Lett. 1987, 28, 6621. [Google Scholar]; (j) Sugawara M; Mori K; Yoshida JI Electrochim. Acta 1997, 42, 1995. [Google Scholar]; (k) Suga S; Watanabe M; Song CH; Yoshida JI Electrochemistry 2006, 74, 672. [Google Scholar]; (l) Mitsudo K; Yamamoto J; Akagi T; Yamashita A; Haisa M; Yoshioka K; Mandai H; Ueoka K; Hempel C; Yoshida J; Suga S Beilstein J. Org. Chem 2018, 14, 1192. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Yoshida J; Ashikari Y; Matsumoto K; Nokami T J. Synth. Org. Chem. Jpn 2013, 71, 1136. [Google Scholar]; (n) Jones AM; Banks CE Beilstein J. Org. Chem 2014, 10, 3056. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Yoshida J; Kataoka K; Horcajada R; Nagaki A Chem. Rev 2008, 108, 2265. [DOI] [PubMed] [Google Scholar]
- (41).(a) Semmelhack MF; Schmid CR J. Am. Chem. Soc 1983, 105, 6732. [Google Scholar]; (b) Li C; Zeng CC; Hu LM; Yang FL; Yoo SJ; Little RD Electrochim. Acta 2013, 114, 560. [Google Scholar]; (c) Wang F; Rafiee M; Stahl SS Angew. Chem. Int. Ed 2018, 57, 6686. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lennox AJJ; Goes SL; Webster MP; Koolman HF; Djuric SW; Stahl SS J. Am. Chem. Soc 2018, 140, 11227. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gao PS; Weng XJ; Wang ZH; Zheng C; Sun B; Chen ZH; You SL; Mei TS Angew. Chem. Int. Ed 2020, 59, 15254. [DOI] [PubMed] [Google Scholar]; (f) Kashiwagi Y; Kurashima F; Kikuchi C; Anzai J; Osa T; Bobbitt JM Chem. Commun 1999, 1983. [DOI] [PubMed] [Google Scholar]; (g) Kashiwagi Y; Anzai J Chem. Pharm. Bull 2001, 49, 324. [DOI] [PubMed] [Google Scholar]; (h) Nutting JE; Rafiee M; Stahl SS Chem. Rev 2018, 118, 4834. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wang F; Stahl SS Acc. Chem. Res 2020, 53, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).(a) Norrish RGW; Bamford CH Nature 1937, 140, 195. [Google Scholar]; (b) Yang NC; Yang DDH J. Am. Chem. Soc 1958, 80, 2913. [Google Scholar]; (c) Clasen RA; Searles S Chem. Commun 1966, 289. [Google Scholar]; (d) Cohen SG; Parola A; Parsons GH Chem. Rev 1973, 73, 141. [Google Scholar]; (e) Hasegawa T; Aoyama H; Omote Y Tetrahedron Lett. 1975, 16, 1901. [Google Scholar]; (f) Lindemann U; Wulff-Molder D; Wessig P Tetrahedron: Asymmetry 1998, 9, 4459. [Google Scholar]; (g) Gramain JC; Remuson R; Vallee D J. Org. Chem 1985, 50, 710. [Google Scholar]; (h) Giese B; Wettstein P; Stahelin C; Barbosa F; Neuburger M; Zehnder M; Wessig P Angew. Chem. Int. Ed 1999, 38, 2586. [PubMed] [Google Scholar]; (i) Wu JF; Zhang W; Wang CL Synthesis 2009, 1821. [Google Scholar]; (j) Roque JB; Kuroda Y; Jurczyk J; Xu LP; Ham JS; Gottemann LT; Roberts CA; Adpressa D; Sauri J; Joyce LA; Musaev DG; Yeung CS; Sarpong R ACS Catal. 2020, 10, 2929. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ham JS; Park B; Son M; Roque JB; Jurczyk J; Yeung CS; Baik MH; Sarpong R J. Am. Chem. Soc 2020, 142, 13041. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Griesbeck AG; Heckroth H; Schmickler H Tetrahedron Lett. 1999, 40, 3137. [Google Scholar]; (m) Rey V; Pierini AB; Peñéñory AB J. Org. Chem 2009, 74, 1223. [DOI] [PubMed] [Google Scholar]; (n) Nishio T; Tabata M; Koyama H; Sakamoto M Helv. Chim. Acta 2005, 88, 78. [Google Scholar]; (o) Nishio T; Koyama H; Sasaki D; Sakamoto M Helv. Chim. Acta 2005, 88, 996. [Google Scholar]; (p) Nishio T; Sakurai N; Iba K; Hamano Y; Sakamoto M Helv. Chim. Acta 2005, 88, 2603. [Google Scholar]; (q) Nechab M; Mondal S; Bertrand MP Chem. Eur. J 2014, 20, 16034. [DOI] [PubMed] [Google Scholar]
- (43).(a) Davidson RS Chem. Commun 1966, 575. [Google Scholar]; (b) Hoshikawa T; Yoshioka S; Kamijo S; Inoue M Synthesis 2013, 45, 874. [Google Scholar]; (c) Kamijo S; Takao G; Kamijo K; Hirota M; Tao K; Murafuji T Angew. Chem. Int. Ed 2016, 55, 9695. [DOI] [PubMed] [Google Scholar]; (d) Kamijo S; Takao G; Kamijo K; Tsuno T; Ishiguro K; Murafuji T Org. Lett 2016, 18, 4912. [DOI] [PubMed] [Google Scholar]; (e) Perry IB; Brewer TF; Sarver PJ; Schultz DM; DiRocco DA; MacMillan DWC Nature 2018, 560, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Si XJ; Zhang LM; Hashmi ASK Org. Lett 2019, 21, 6329. [DOI] [PubMed] [Google Scholar]; (g) Schultz DM; Levesque F; DiRocco DA; Reibarkh M; Ji YN; Joyce LA; Dropinski JF; Sheng HM; Sherry BD; Davies IW Angew. Chem. Int. Ed 2017, 56, 15274. [DOI] [PubMed] [Google Scholar]; (h) Sarver PJ; Bacauanu V; Schultz DM; DiRocco DA; Lam YH; Sherer EC; MacMillan DWC Nat. Chem 2020, 12, 459. [DOI] [PubMed] [Google Scholar]; (i) Yu JP; Zhao CY; Zhou R; Gao WC; Wang S; Liu K; Chen SY; Hu KQ; Mei L; Yuan LY; Chai ZF; Hu HS; Shi WQ Chem. Eur. J 2020, 26, 16521. [DOI] [PubMed] [Google Scholar]; (j) Srivastava V; Singh PK; Singh PP Tetrahedron Lett. 2019, 60, 1333. [Google Scholar]; (k) Roberts BP Chem. Soc. Rev 1999, 28, 25. [Google Scholar]; (l) Nagatomo M; Yoshioka S; Inoue M Chem. Asian J 2015, 10, 120. [DOI] [PubMed] [Google Scholar]; (m) Capaldo L; Ravelli D Eur. J. Org. Chem 2017, 2056. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Capaldo L; Quadri LL; Ravelli D Green Chem. 2020, 22, 3376. [Google Scholar]
- (44).(a) Condie AG; Gonzalez-Gomez JC; Stephenson CRJ J. Am. Chem. Soc 2010, 132, 1464. [DOI] [PubMed] [Google Scholar]; (b) Rueping M; Zhu SQ; Koenigs RM Chem. Commun 2011, 47, 12709. [DOI] [PubMed] [Google Scholar]; (c) Freeman DB; Furst L; Condie AG; Stephenson CR J. Org. Lett 2012, 14, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Rueping M; Zhu SQ; Koenigs RM Chem. Commun 2011, 47, 8679. [DOI] [PubMed] [Google Scholar]; (e) Rueping M; Vila C; Koenigs RM; Poscharny K; Fabry DC Chem. Commun 2011, 47, 2360. [DOI] [PubMed] [Google Scholar]; (f) Zhao GL; Yang C; Guo L; Sun HN; Chen C; Xia WJ Chem. Commun 2012, 48, 2337. [DOI] [PubMed] [Google Scholar]; (g) Rueping M; Koenigs RM; Poscharny K; Fabry DC; Leonori D; Vila C Chem. Eur. J 2012, 18, 5170. [DOI] [PubMed] [Google Scholar]; (h) DiRocco DA; Rovis T J. Am. Chem. Soc 2012, 134, 8094. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Bergonzini G; Schindler CS; Wallentin CJ; Jacobsen EN; Stephenson CR J. Chem. Sci 2014, 5, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Pan YH; Kee CW; Chen L; Tan CH Green Chem. 2011, 13, 2682. [Google Scholar]; (k) Liu Q; Li YN; Zhang HH; Chen B; Tung CH; Wu LZ Chem. Eur. J 2012, 18, 620. [DOI] [PubMed] [Google Scholar]; (l) Pan YH; Wang S; Kee CW; Dubuisson E; Yang YY; Loh KP; Tan CH Green Chem. 2011, 13, 3341. [Google Scholar]; (m) Fu WJ; Guo WB; Zou GL; Xu C J. Fluorine Chem 2012, 140, 88. [Google Scholar]; (n) Lin SX; Sun GJ; Kang Q Chem. Commun 2017, 53, 7665. [DOI] [PubMed] [Google Scholar]; (o) Zhang T; Liang WW; Huang YX; Li XR; Liu YZ; Yang B; He CX; Zhou XC; Zhang JM Chem. Commun 2017, 53, 12536. [DOI] [PubMed] [Google Scholar]; (p) Kohls P; Jadhav D; Pandey G; Reiser O Org. Lett 2012, 14, 672. [DOI] [PubMed] [Google Scholar]; (q) Miyake Y; Nakajima K; Nishibayashi Y Chem. Eur. J 2012, 18, 16473. [DOI] [PubMed] [Google Scholar]; (r) Espelt LR; Wiensch EM; Yoon TP J. Org. Chem 2013, 78, 4107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Sharma S; Sharma A Org. Biomol. Chem 2019, 17, 4384. [DOI] [PubMed] [Google Scholar]; (t) Romero NA; Nicewicz DA Chem. Rev 2016, 116, 10075. [DOI] [PubMed] [Google Scholar]
- (45).(a) McNally A; Prier CK; MacMillan DWC Science 2011, 334, 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Noble A; MacMillan DWC J. Am. Chem. Soc 2014, 136, 11602. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Joe CL; Doyle AG Angew. Chem. Int. Ed 2016, 55, 4040. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Leng LY; Fu Y; Liu P; Ready JM J. Am. Chem. Soc 2020, 142, 11972. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Prier CK; MacMillan DWC Chem. Sci 2014, 5, 4173. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ahneman DT; Doyle AG Chem. Sci 2016, 7, 7002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) McManus JB; Onuska NPR; Nicewicz DA J. Am. Chem. Soc 2018, 140, 9056. [DOI] [PubMed] [Google Scholar]; (h) McManus JB; Onuska NPR; Jeffreys MS; Goodwin NC; Nicewicz DA Org. Lett 2020, 22, 679. [DOI] [PubMed] [Google Scholar]; (i) Holmberg-Douglas N; Choi Y; Aquila B; Huynh H; Nicewicz DA ACS Catal. 2021, 11, 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Cohen SG; Chao HM J. Am. Chem. Soc 1968, 90, 165. [DOI] [PubMed] [Google Scholar]; (k) Lewis FD; Ho TI; Simpson JT J. Org. Chem 1981, 46, 1077. [Google Scholar]; (l) Masuda Y; Ito M; Murakami M Org. Lett 2020, 22, 4467. [DOI] [PubMed] [Google Scholar]; (m) Thullen SM; Rovis T J. Am. Chem. Soc 2017, 139, 15504. [DOI] [PubMed] [Google Scholar]; (n) Shaw MH; Twilton J; MacMillan DWC J. Org. Chem 2016, 81, 6898. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Guillemard L; Wencel-Delord J Beilstein J. Org. Chem 2020, 16, 1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).(a) Dai C; Meschini F; Narayanam JMR; Stephenson CRJ J. Org. Chem 2012, 77, 4425. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ide T; Barham JP; Fujita M; Kawato Y; Egami H; Hamashima Y Chem. Sci 2018, 9, 8453. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kobayashi F; Fujita M; Ide T; Ito Y; Yamashita K; Egami H; Hamashima Y ACS Catal. 2021, 11, 82. [Google Scholar]; (d) Wakaki T; Sakai K; Enomoto T; Kondo M; Masaoka S; Oisaki K; Kanai M Chem. Eur. J 2018, 24, 8051. [DOI] [PubMed] [Google Scholar]; (e) Grainger R; Heightman TD; Ley SV; Lima F; Johnson CN Chem. Sci 2019, 10, 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shaw MH; Shurtleff VW; Terrett JA; Cuthbertson JD; MacMillan DWC Science 2016, 352, 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Le C; Liang YF; Evans RW; Li XM; MacMillan DWC Nature 2017, 547, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Rohe S; Morris AO; McCallum T; Barriault L Angew. Chem. Int. Ed 2018, 57, 15664. [DOI] [PubMed] [Google Scholar]; (i) Li WP; Duan YQ; Zhang ML; Cheng J; Zhu CJ Chem. Commun 2016, 52, 7596. [DOI] [PubMed] [Google Scholar]; (j) Choi GJ; Zhu QL; Miller DC; Gu CJ; Knowles RR Nature 2016, 539, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Ye JT; Kalvet I; Schoenebeck F; Rovis T Nat. Chem 2018, 10, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Ashley MA; Yamauchi C; Chu JCK; Otsuka S; Yorimitsu H; Rovis T Angew. Chem. Int. Ed 2019, 58, 4002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Ryder ASH; Cunningham WB; Ballantyne G; Mules T; Kinsella AG; Turner-Dore J; Alder CM; Edwards LJ; McKay BSJ; Grayson MN; Cresswell AJ Angew. Chem. Int. Ed 2020, 59, 14986. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Treacy SM; Rovis T J. Am. Chem. Soc 2021, 143, 2729. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Xu P; Chen PY; Xu HC Angew. Chem. Int. Ed 2020, 59, 14275. [DOI] [PubMed] [Google Scholar]
- (47).(a) Han GH; McIntosh MC; Weinreb SM Tetrahedron Lett. 1994, 35, 5813. [Google Scholar]; (b) Ito R; Umezawa N; Higuchi T J. Am. Chem. Soc 2005, 127, 834. [DOI] [PubMed] [Google Scholar]; (c) Kim Y; Heo J; Kim D; Chang S; Seo S Nat. Commun 2020, 11, 4761. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Osberger TJ; Rogness DC; Kohrt JT; Stepan AF; White MC Nature 2016, 537, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Roque JB; Kuroda Y; Gottemann LT; Sarpong R Science 2018, 361, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Roque JB; Kuroda Y; Gottemann LT; Sarpong R Nature 2018, 564, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Roque JB; Sarpong R; Musaev DG J. Am. Chem. Soc 2021, 143, 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kaname M; Yoshifuji S; Sashida H Tetrahedron Lett. 2008, 49, 2786. [Google Scholar]; (i) Xia XF; Shu XZ; Ji KG; Shaukat A; Liu XY; Liang YM J. Org. Chem 2011, 76, 342. [DOI] [PubMed] [Google Scholar]; (j) Paciaroni NG; Ratnayake R; Matthews JH; Norwood VM; Arnold AC; Dang LH; Luesch H; Huigens RW Chem. Eur. J 2017, 23, 4327. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Morcillo SP Angew. Chem. Int. Ed 2019, 58, 14044. [DOI] [PubMed] [Google Scholar]; (l) Zhang YJ; Sun S; Su YJ; Zhao J; Li YH; Han B; Shi F Org. Biomol. Chem 2019, 17, 4970. [DOI] [PubMed] [Google Scholar]; (m) Smolobochkin AV; Gazizov AS; Burilov AR; Pudovik MA; Sinyashin OG Russ. Chem. Rev 2019, 88, 1104. [Google Scholar]; (n) Su JK; Ma XX; Ou ZL; Song QL ACS Cent. Sci 2020, 6, 1819. [DOI] [PMC free article] [PubMed] [Google Scholar]


