Abstract
Cannabis sativa, widely known as ‘Marijuana’ poses a dilemma for being a blend of both good and bad medicinal effects. The historical use of Cannabis for both medicinal and recreational purposes suggests it to be a friendly plant. However, whether the misuse of Cannabis and the cannabinoids derived from it can hamper normal body physiology is a focus of ongoing research. On the one hand, there is enough evidence to suggest that misuse of marijuana can cause deleterious effects on various organs like the lungs, immune system, cardiovascular system, etc. and also influence fertility and cause teratogenic effects. However, on the other hand, marijuana has been found to offer a magical cure for anorexia, chronic pain, muscle spasticity, nausea, and disturbed sleep. Indeed, most recently, the United Nations has given its verdict in favour of Cannabis declaring it as a non-dangerous narcotic. This review provides insights into the various health effects of Cannabis and its specialized metabolites and indicates how wise steps can be taken to promote good use and prevent misuse of the metabolites derived from this plant.
Keyword: Cannabis sativa, Tetrahydrocannabinol, Cannabinoids, Psychoactive, Medical marijuana, Phytochemicals, Chemical components, Therapeutic roles
1. Introduction
The Mexican slang ‘marijuana’ connotes the medicinal part of Cannabis, which was used for smoking by Mexican soldiers (Johnson, 2017). Dioecious, rarely monoecious, and dimorphic in nature (Rupasinghe et al., 2020). Marijuana plant (Cannabis sativa) is an annual wind-pollinated plant. This plant is assumed to have evolved from the steppes of Central Asia, explicitly in the regions, which are now located in Mongolia and south Siberia (McPartland et al., 2019). The fossil records of Cannabis go back about 12,000 years, making it the oldest cultivated crop plant (Peltzer and Pengpid, 2017). According to Vedic philosophy, Cannabis is one of five sacred plants with the guardian angel residing in the leaves of this plant. Furthermore, Vedas associated the Cannabis plant with happiness and as a saviour, which was purposely provided to humans to achieve satisfaction and overcome their phobias (Clarke and Merlin, 2016). Historically, the usage of marijuana could be found during 6000 BC when the seeds of Cannabis were used as food material in China, while in 4000 BC, Chinese designed textile material from hemp (Monthony et al., 2021). The first record found for the association of Cannabis with the field of medicine was in 2727 BC in China (Hand et al., 2016). In Traditional Chinese Medicine (TCM) it was usually used to treat constipation, malaria, rheumatic pains as well as pain during childbirth (Anand et al., 2019). In 1500 BC, Cannabis was commonly cultivated as a source of food and fiber (Deguchi et al., 2020). This timeline continues to the date. Marijuana has numerous uses such as fiber, pulp and paper, seeds, medicine, cement (concrete), plaster, oilseed use, geotextiles, animal bedding, personal care products, etc. (Stasiłowicz et al., 2021). The use of marijuana is not limited to the production of cigarettes, which is formed by drying the flowers and leaves of female hemp, but it also has many other important uses indicated below (Small, 2015).
Several studies have shown that the phytochemicals of Cannabis play an important role in the prevention and treatment of various diseases, indicating its multifaceted role as a valuable therapeutic agent. Therefore, the present study will be significant in the medical field to understand the therapeutic benefits of marijuana. Due to the frequent changes in the medical marijuana landscape, healthcare workers must stay up to date on existing information regarding both the health hazards and health benefits of its use. Although it is imperative to evaluate possible benefits exhibited in precise states of ailment, however, there is insufficient evidence in maximum qualifying indications, in which most randomized controlled trials (RCTs) are lacking (Breijyeh et al., 2021). Therefore, possible health hazards and health benefits must be carefully assessed to make suitable clinical choices due to the limited high-quality evidence and lack of regulation.
Over the years, researchers have studied the effect of various bioactive compounds to study the medicinal properties of Cannabis, but little effort has been made to put the entire literature review of these bioactive compounds their therapeutic characteristics in one refereed paper. Therefore, the present review paper is an attempt to compile and better understand the existing information on various aspects of the Cannabis plant and provide appropriate information to emphasize the need for new guidelines and strategies to regulate Cannabis use. The present review article aims to provide an overall update on various phytochemicals of Cannabis and their biological properties by highlighting their therapeutic uses. The action mechanism of tetrahydrocannabinol is also emphasized. An overview of the adverse effects of marijuana and possible therapeutic promise are also discussed. This review also summarizes the marijuana withdrawal by studying its treatment, observation, and bottlenecks.
2. Chemical components of Cannabis
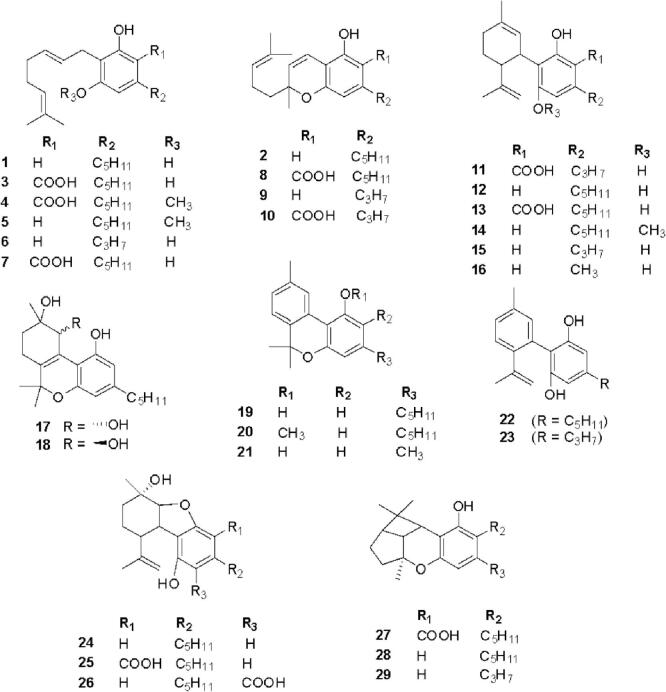
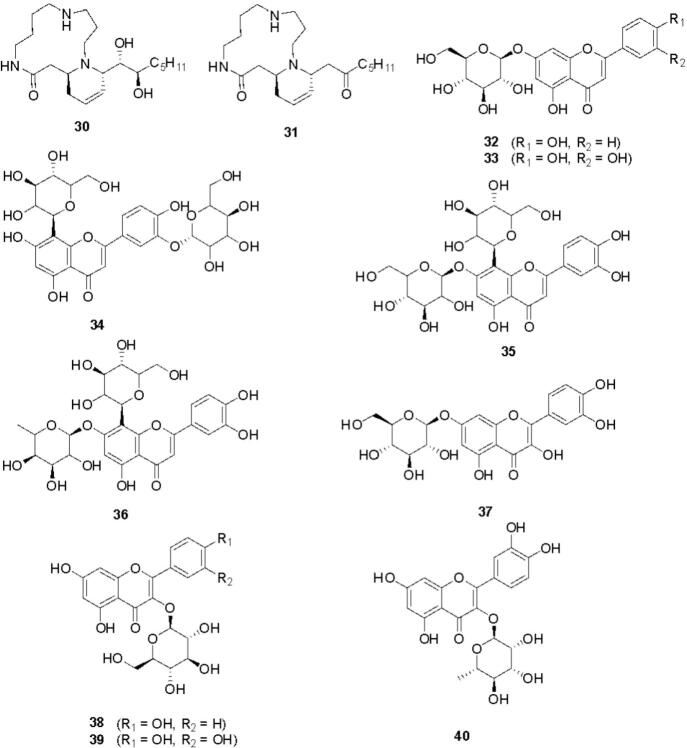
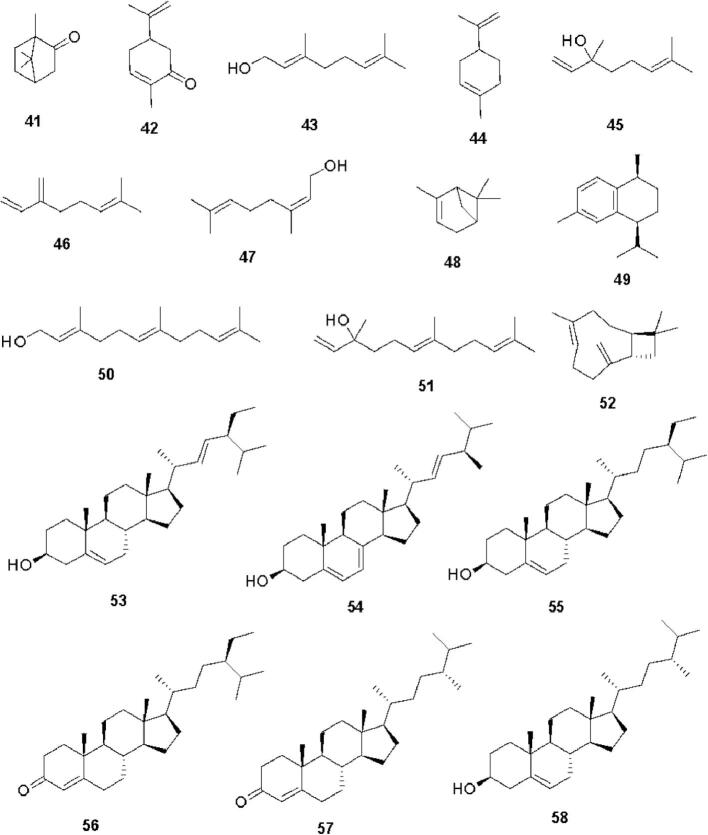
Cannabis constitutes a rich diversity of compounds that belong to different chemical classes such as cannabinoids, alkaloids, flavonoids, terpenoids, steroids, fatty acids, amino acids, etc. (Pollastro et al., 2018). Cannabinoids are a group of C21 compounds present mainly in Cannabis sativa (Stasiłowicz et al., 2021). In 1964, Gaoni and Mechoulam isolated cannabigerol (1) and cannabichromene (2) from the hexane extract of hashish followed by cannabigerolic acid (3) and methyl ester of cannabigerolic acid (4) from the acid fraction of hashish sole (Gaoni and Mechoulam, 1964). Shoyama et al. isolated cannabigerolic acid monomethyl ether (5), cannabigerovarin (6), cannabigerovarin acid (7), and cannabichromenic acid (8) from the benzene fraction of Cannabis sativa (Shoyama et al., 1972). Shoyama et al. isolated cannabichromevarin (9) from chloroform extract of hashish (Shoyama et al., 1975). Cannabichromevarinic acid (10) and cannabidivarinic acid (11) were isolated from the benzene extract of Cannabis (Turner et al., 1980). Adams et al. isolated cannabidiol (12) from an ethanolic extract of wild hemp (Adams et al., 1940). Cannabidiolic acid (13) was isolated from the benzene extract of leaves and the fresh top of Cannabis sativa (Kabelik et al., 1960). Cannabidiolic acid monomethyl ether (14) was isolated from the ethanolic extract of domestic hemp (Shoyama et al., 1970a). Cannabidivarin (15) was isolated from ligroin extract of hashish (Irie et al., 1975). Cannabidiorcol (16) was isolated from the hexane extract of Lebanese hashish (Vree et al., 1972). (+) Cannabitriol (17) and (−) cannabitriol (18) were isolated from the ethanolic extract of Cannabis (ElSohly and Slade, 2005). Cannabinol (19) was isolated from the ether extract of Indian hump (charas) (Wood et al., 1899). Cannabinol methyl ether (20) was isolated from the ethanolic extract of Lebanese hashish (Bercht et al., 1974). Cannabiorcol (21) was isolated from the hexane extract of Brazilian marihuana (Vree et al., 1972). Cannabinodiol (22) and cannabinodivarin (23) were isolated from the hexane ether extract of Lebanese hashish and Nepalese hashish, respectively. Cannabinol and its derivatives have been reported to have anticonvulsant, anti-inflammatory, and immunological properties. Cannabielsoin (24) was isolated from the ethanolic extract of Lebanese hashish, while cannabielsoinic acid A (25) and cannabielsoinic acid B (26) were isolated from a boiling benzene extract of Lebanese hashish (Bercht et al., 1974). Cannabicyclol (27) was isolated from Cannabis using thin layer chromatography of silica gel (Gaoni and Mechoulam, 1964). Cannabicyclolic acid (28) was isolated from dried leaves of Cannabis (Shoyama et al., 1970b). Cannabicyclovarin (29) was isolated from the ether extract of Congo marijuana (Turner et al., 1980). Fig. 1 represents the structures of cannabinoids present in Cannabis. Cannabisativine (30) and anhydrocannabisativine (31) are alkaloids isolated from alcoholic extracts of dry leaves and small stems of Mexican Cannabis (Atakan, 2012). A group of flavonoid glycosides such as apigenin-7-O-glucoside (32), luteolin-O-glycoside (33), orientin-3′-O-glucoside (34), orientin-7-O-glucoside (35), orientin-7-O-rhamnoside (36), quercetin-7-O-glucoside (37), kaempferol-3-O-glycoside (38), quercetin-3-O-glucopyranoside (39), quercetin-3-O-rhamnoside (40) etc. were isolated from various species of Cannabis (Andre et al., 2016, Do, 2018). Fig. 2 presents the structures of alkaloids and flavonoid glycosides present in Cannabis. A group of monoterpenes [camphor (41), carvone (42), geraniol (43), limonene (44), linalool (45), myrcene (46), nerol (47), α-pinene (48) etc.] and sesquiterpene [calamenene (49), farnesol (50), nerolidol (51), caryophyllene (52) etc.] were reported from different species of Cannabis. Several steroids such as stigmasterol (53), ergosterol (54), β-sitosterol (55), stigmast-4-en-3-one (56), campest-4-en-3-one (57), campesterol (58), etc. were also isolated from different species of Cannabis (Aizpurua-Olaizola et al., 2016, Booth and Bohlmann, 2019). Fig. 3 presents the structures of terpenoids and steroids found in Cannabis. Several fatty acids (arachidic acid, behenic acid, eicosadienic acid, eicosenoic acid, linoleic acid, linolenic acid, oleic acid, palmitic acid, palmitoleic acid, sativic acid, stearic acid etc.) and amino acids (alanine, aspartic acid, cystine, glutamic acid, glycine, serine, arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, threonine, tryptophan, tyrosine, valine etc.) have also been isolated from different species of Cannabis (Asadi et al., 2019, Watson et al., 2019). In summary, Cannabis is a polypharmacy with a rich diversity of nutritionally and pharmaceutically important metabolites.
Fig. 1.
Structures of cannabinoids present in Cannabis.
Fig. 2.
Structures of alkaloids and flavonoid glycosides present in Cannabis.
Fig. 3.
Structures of terpenoids and steroids present in Cannabis.
3. Therapeutic roles of phytochemical constituents of Cannabis sp.
The therapeutic use of Cannabis has been noted in ancient texts from various traditional medicinal systems (Brand and Zhao, 2017). The multipurpose usage of medicinal Cannabis has mostly addressed the pharmacological attributes of cannabinoids and their derivatives. To date, 113 phytocannabinoids and 120 terpenes have been characterized (Calvi et al., 2018, Pollastro et al., 2018), but the large contribution and synergistic interaction of non-cannabinoids or polyphenolic secondary metabolites have not yet been studied. It was noted that along with the seed, the other parts of the plant of Cannabis are also enriched with a variety of biologically active metabolites and many researchers have worked extensively on the complex biosynthetic pathways of these phytochemicals, their synergistic effects, isolation, as well as characterization and medicinal applications (Andre et al., 2016). Lignanammides, prenylated flavonoids, and stilbenoid derivatives, which have shown different biological activities, play an important protective role against various types of pathogenic attacks (Pollastro et al., 2018). Flavonoids such as kaempferol, apigenin, vitexin, isovitexin, cannflavin A, B, luteolin, and quercetin have been investigated for their inhibitory activities of lipoxygenase and cyclooxygenase and their role in chemoprevention (Birt et al., 2001, Moon et al., 2006). Another crucial lacuna present in research in the field of Cannabis phytochemistry is the incomplete elucidation of the chemical profile of Cannabis cultivars of medicinal or ‘approved drug category’. Cannabis chemotypes differ significantly in both cannabinoid and non-cannabinoid content. To determine the medicinal, narcotic or sedative values of any cultivar, it is important to examine the qualitative and quantitative details of phytoconstituents using a rigorous analytical process. Several analytical methods, such as optical rotatory dispersion (ORD) and electronic circular dichroism (CD) based studies, liquid chromatography coupled to high resolution tandem mass spectrometry (LC - MS / MS), headspace solid phase microextraction (HS-SPME), overpressured layer chromatography (OPLC), high-performance thin-layer chromatography (HPTLC), high-performance liquid chromatography with diode array detector (HPLC-DAD), liquid chromatography with high resolution mass spectrometry (LC-HRMS), gas chromatography with flame ionization detector (GC-FID) and gas chromatography – mass spectrometry (GC–MS)-based qualitative and quantitative determination, nuclear magnetic resonance (NMR) and mass spectrometry (MS) based structural determination have played a critical role in phytochemical profiling of different medicinal and nontherapeutic cultivars (Calvi et al., 2018). The content of tetrahydrocannabinol and cannabidiol in any cultivar is essentially important for its main prescribed and ‘legally safe’ recommendation (Raderman, 2016, Lowe et al., 2021). Therefore, commercially marketed cultivars must be studied for all possible types of synergistic interactions and chemical components present to determine the safe use of Cannabis as a useful medicinal herb (Jin et al., 2017). Table 1 summarizes the different cannabinoid and non-cannabinoid phytochemical constituents of Cannabis sp. and their origin, pharmacological activities, therapeutic role, and experimental details. Table 2 represents the systematic application of Cannabis-derived phytochemicals to clinically focus psychiatric disorders.
Table 1.
Phytochemical constituents of Cannabis sativa with pharmacological activities.
| Name of phytochemicals isolated from Cannabis sativa | Source organ or plant part | Bioactivity as reported by the researchers or experimentally analysed | Key points of the experimental approach | References |
|---|---|---|---|---|
| Delta-9-tetrahydrocannabinol and cannabidiol, cannabinol, cannabigerol, cannabigerolic acid and cannabichromene | Flower | Psychoactive properties* | Phytochemical screening of marketed medical marihuana samples was carried out. | Mudge et al., 2016 |
| tetrahydrocannabinol, cannabidiol, cannabigerol, cannabichromene, tetrahydrocannabivarin | Flowers and inflorescence leaves | Psychoactive properties* | Chemical profiling of cannabinoids demonstrated organ- and location-specific regulation of accumulation | Bernstein et al., 2019 |
| Essential oil component rich in terpenes and cannabinoid | Dried flower | Antioxidant properties and anti acetylcholinesterase activities** | GC–FID and GC–MS analyses revealed the chemical composition and indicated a possible synergistic attribution of terpenes and phytocannabinoids | Smeriglio et al., 2020 |
| (±)-6,7-trans-epoxycannabigerolic acid, (±)-6,7-cis-epoxycannabigerolic acid, (±)-6,7-cis-epoxycannabigerol, (±)-6,7-trans-epoxycannabigerol, 5′-methyl-4-pentylbiphenyl-2,2′,6-triol, and 7-methoxycannabispirone, cannabigerolic acid, 5′-methoxycannabigerolic acid, cannabispirone, β-cannabispiranol, dehydrocannabifuran, cannflavin B and cannabigerol | Whole buds of mature female plants | Antimicrobial and antileishmanial activities** | Identification, isolation and characterization of novel Cannabis constituents and their pharmacological activities were determined. | Radwan et al., 2008a |
| Cannabichromanones A, B, C, D | Whole buds of mature female plants | Analgesic, antidepressant, anticancer, and psychoactive properties* | ORD and CD were used to determine the configuration of cannabichromanone derivatives. | Ahmed et al., 2008a |
| Δ9‐tetrahydrocannabiorcol, cannabidivarin, cannabicitran, Δ9‐tetrahydrocannabivarin, cannabicyclol, cannabidiol, cannabichromene, Δ9‐tetrahydrocannabinol, cannabigerol, cannabinol, dihydrocannabinol, cannabielsoin, 6a, 7, 10a‐trihydroxytetrahydro cannabinol, 9, 10‐epoxycannabitriol, 10‐O‐ethylcannabitriol, and 7, 8‐dehydro‐10‐O‐ethylcannabitriol, kaempferol 3‐O‐sophoroside and quercetin 3‐O‐sophoroside |
Pollen grains | Antioxidant and anti-inflammatory activities* | High‐field 2D-NMR experiments, application of GC‐FID and GC‐MS analyses revealed the presence of cannabinoids and glycosides | Ross et al., 2005 |
| β-ocimene, α-terpinolene, α- and ß-pinene, ß-myrcene, sesquiterpenes (trans-caryophyllene and α-humulene), tetrahydrocannabinol, cannabidiol, cannabidiolic acid, cannabigerolic acid, cannabidivarin | Inflorescences | Dietary supplement* | HPLC-high-resolution mass spectrometry, LC-MS/MS, HS-SPME GC–MS, and GC-FID were performed to illustrate the complete chemical profile. | Pavlovic et al., 2019 |
| Orientin, vitexin, isovitexin, quercetin, luteolin, kaempferol, apigenin | Flowers, seedlings and fruits. | Carcinogens detoxification, enzyme activation** | Inhibited prostaglandin E2 production in rheumatoid synovial cells | Schewe et al., 2002, Moon et al., 2006 |
| Cannflavins A, B, and C | Sprouts, flower bud | Antimicrobial and antileishmanial activity** | Therapeutic potential revealed along with antioxidant efficacy | Radwan et al., 2008a, Radwan et al., 2008b |
| Cannabis in-B, C, D, G, grossamide | Fruit, root | Chemical protection against pathogenic attack* | Isolation and characterization of polyphenols | Calvi et al., 2018 |
| Cannabispirone, iso-cannabispirone, iso-cannabispiradienone, cannabispirenone-B, β-cannabispiranol, α -cannabispiranol, 5,7-dihydroxyindan-1-spiro-cyclohexane, 7-hydroxy-5-methoxyindan-1-spirociclohexane | Resin, flower heads, stem, and leaves | Antioxidant activities* | Isolation, biosynthesis and bioactivity of stilbenoids | Calvi et al., 2018 |
| Tetrahydrocannabinol, tetrahydrocannabinolic acid synthase, cannabinol | Leaves | Psychoactive agent* | The oldest documentation of pharmacological application revealed | Russo et al., 2008 |
| α-zone and β-zone | Leaves | Psychoactive properties* | The OPLC method was applied to separate neutral and acidic cannabinoids | Oroszlán et al., 1987 |
| Cannabidiolic acid, cannabidiol, cannabidiol monomethyl ether, cannabidiol-C4, cannabidivarinic acid, cannabidivarin, cannabidiorcol | Leaves/plant | Psychotropic, analgesic, anti-inflammatory activities* | The therapeutic potential and occurrence of different cannabinoids were discussed. | Asati et al., 2017 |
| (E)‐caryophyllene, cannabidiol, cannabidiolic acid, kaempferol, and apigenin | Leaves, male and female inflorescences | CB2 agonists with nutraceutical and pharmaceutical properties* | GC/MS, NMR, and LC‐DAD‐MS techniques revealed essential oil composition | Nagy et al., 2019 |
| Δ9-tetrahydrocannabinol and cannabidiol (resorcinol and 2-p-mentha-1,8-dien-4-yl-5-pentyl) | Leaves, stems, and seeds | Antimicrobial activities (detected by agar well diffusion method against four pathogenic bacteria strains) ** | Mineral analysis, GC–MS, and antimicrobial assay were conducted to determine the bioactivity of cannabinoids. | Isahq et al., 2015 |
| Δ9-trans-tetrahydrocannabivarin, Δ9-trans-tetrahydrocannabinol, sterols (campesterol, stigmasterol, p-sitosterol), amino acid (L-proline) | Young stem | Psychotomimetic activity* | Phytochemical screening was performed via GLC-MS. | Mole and Turner, 1974 |
| rac-6′,7′-dihydro,6′,7′-dihydroxycannabigerol | Aerial parts | Antibacterial activity** | Polar cannabinoid isolated and characterized by spectrometric analysis | Appendino et al., 2008 |
|
Denbinobin |
Whole plant |
Antiproliferative activity** |
Causes mitochondrial dysfunction, protein kinase B (Akt) and NF-kB pathway inactivation, Bcl-2- associated death promoter and caspase 3 activation and releases apoptosis-inducing factor (AIF) in human lung adenocarcinoma, Jurkat and other human leukemia cell lines |
Kuo et al., 2008, Sánchez-Duffhues et al., 2009 |
| Tetrahydrocannabinol, cannabidiol, cannabinol, cannabichromene, cannabigerol, α- pinene, 1,8-cineole, pulegone, d- limonene, β – caryophyllene, apigenin, β- sitosterol, quercetin |
Plant |
Antidepressant, sedative, anti-inflammatory, antioxidant analgesic activities* | Dopamine antagonists and their role against Cannabis dependency were noted. |
Oladimeji and Valan, 2020 |
|
Cannabidiol |
Plant |
Anticancer activity** |
The antiproliferative activity of cannabidiol on different cervical cancer cell lines was tested | Lukhele and Motadi, 2016 |
| β-fenchyl Δ9-tetrahydrocannabinolate, epi-bornyl Δ9-tetrahydrocannabinolate, α-terpenyl Δ9-tetrahydrocannabinolate, 4-terpenyl Δ9-tetrahydrocannabinolate, α-cadinyl Δ9-tetrahydrocannabinolate, γ-eudesmyl Δ9-tetrahydrocannabinolate, γ-eudesmyl cannabigerolate, 4-terpenyl cannabinolate, bornyl Δ9-tetrahydrocannabinolate (9), α-fenchyl Δ9-tetrahydrocannabinolate, α-cadinyl cannabigerolate, Δ9-tetrahydrocannabinol, Δ9-tetrahydrocannabinolic acid A, cannabinolic acid A, and cannabigerolic acid |
Plant |
Moderate antimicrobial activity** |
Extensive spectroscopic analyses revealed their structural identity |
Ahmed et al., 2008b |
| Δ9-tetrahydrocannabinol, Δ9-tetrahydrocannabinolic acid |
Plant (water extracts) |
Psychoactive herbal remedies* |
Diffusion-edited 1H NMR (1D DOSY) and 1H NMR provided semiquantitative data on phyto-cannabinoids |
Politi et al., 2008 |
| Caffeoyltyramine, cannabisin A, B, C, ω-6 linoleic acid |
Seed, sprouts |
Cellular antioxidant activity, anti-mutagenic** | Spectrophotometric analysis of phytochemicals and antioxidant assays revealed its potency as a functional food | Frassinetti et al., 2018 |
| Cannabisin I (1), together with seven known lignanamides, cannabisins A, B, C, F, M, 3,3′-demethylgrossamide, grossamide, N-trans-caffeoyltyramine and N-trans-caffeoyloctopamine |
Hempseed cakes |
Arginase inhibitory property and antioxidant capacity** |
NMR spectroscopy and mass spectrometry data determined the structure of lignanamides and phenylpropanoid amides |
Bourjot et al., 2017 |
| Linoleic, α-linolenic and oleic acid, β-sitosterol, campesterol, phytol, cycloartenol, γ-tocopherol |
Seed oil |
Antioxidant activity* |
A qualitative and quantitative characterization of the unsaponifiable fraction was performed. |
Paz et al., 2014 |
|
Hemp protein isolate (HPI) containing edestin (hexameric legumin) |
Seed |
Nutraceutical value** |
Emulsifying activity index, emulsion stability index, water holding capacity, and fat adsorption capacity indicate the role of HPI as a functional food |
Tang et al., 2006 |
|
Δ9‐tetrahydrocannabinol and other neutral cannabinoids |
Seed |
Psychoactive properties and other pharmacological activities* | Simple, reproducible, and accurate, the HPTLC method was standardized for the quantification of Δ9‐THC | Fischedick et al., 2009 |
| Linolenic acid, oleic acid, sitosterol, campesterol, phytol, cycloartenol and tocopherol |
Seed |
Nutraceutical value** |
Phytochemical characterization of the unsaponifiable fraction determined via analytical studies |
Paz et al., 2014 |
| 3,3′-demethyl-grossamide, cannabisin-M 111, cannabisin- N, (2,3-trans)-3-(3-hydroxy-5-methoxyphenyl)- N-(4-hydroxyphenethyl)-7-{(E)-3-[(4-hydroxyphenethyl) amino]-3-oxoprop-1-enyl}-2,3-dihydrobenzo [b] [1,4] dioxine-2-carboxamide, cannabisin-O, and 3,3′-demethyl-heliotropamide |
Seed |
Antioxidant and acetylcholinesterase inhibitory activities** |
Phytochemical characterization and pharmacological studies revealed the presence of phenolic amides and lignanamides. |
Yan et al., 2015 |
| Quercetin, gallic acid, p Coumaric acid, m-coumaric acid, caffeic acid, cinnamic acid, ferulic acid, benzoic acid, and kaempferol |
Seed |
Antioxidant and chemopreventive activities* | High-throughput phytochemical characterization of noncannabinoid compounds via HPLC screening |
Ahmad et al., 2018 |
|
Phenylpropionamides |
Seed |
Ameliorated LPS stimulated memory dysfunction and neuroinflammation in mice** | Reversal of hippocampal neuronal damage supports the nutraceutical potential and the neuroprotective perspective of hemp seed. |
Zhou et al., 2018a |
| Cannabisin A, B, F, G, M, 3,3′-demethyl-grossamide,N-trans-coumaroyloctopamine, N-trans-coumaroyltyramine, N-trans-feryroyltyramine, N-trans-caffeoyltyramine, (S)-N-(2-(4-hydroxyphenyl)-2-methoxyethyl)cinnamamide,4-[(E)-p-coumaroylamino]butan-1-ol, trans-ferulic acid-4-O-β-D-glucopyranoside, adenosine, p-hydroxybenzaldehyde, and 4-hydroxy-3-acid |
Seed |
Anti-neuroinflammatory activity** |
Many of these compounds inhibited TNF-α release from LPS-induced BV2 microglia cells, which is an important therapeutic approach for neurodegenerative diseases. |
Zhou et al., 2018b |
| Polyunsaturated fatty acids, protein (ß-conglycinin and vicilin) | Seed | Health-promoting property* | Cannabinoid profiling performed using an untargeted metabolomics approach | Pavlovic et al., 2019 |
| Fatty acids (palmitic, stearic, oleic, linoleic, γ-linolenic and α-linolenic acid), tocopherols, carotenoids (lutein, β-carotene, zeaxanthin), protocatechuic acid, p-hydroxybenzoic acid, cinnamic acid, trans-caffeoyltyramine, cannabisin A |
Seed |
Antioxidant activity (detected by ABTS and FRAP assays) ** |
The nutritional value, phytochemical composition, and antioxidant properties of seven hemp cultivars were detected. |
Irakli et al., 2019 |
| Monoterpene (limonene, β-myrcene, and α-pinene), sesquiterpene (caryophyllene and humulene) |
Seed |
Various pharmacological activities* | The terpene metabolomics study (based on GC–MS) helped in phytochemical screening | Mudge et al., 2019 |
| Hemp protein hydrolysates (HPH20A and HPH60A + 15AF) |
Seed |
Anti-neuroinflammatory activities** |
Down-regulated transcriptional levels of TNF-α, IL-1β, and IL-6 mRNA in LPS-stimulated BV-2 microglial cells; up-regulated expression of the IL-10 cytokine gene |
Rodriguez-Martin et al., 2019 |
| (−)-trans-Δ9-tetrahydrocannabiphorol (Δ9-THCP) | Seed | In vivo cannabimimetic activity** | As a potent CB1 agonist, it induced catalepsy, analgesia, and hypomotility; decreased rectal temperature |
Citti et al., 2019 |
| Δ9-tetrahydrocannabinolic acid (A) and its neutral derivative trans-Δ9-tetrahydrocannabinol-C5, Cis and trans-Δ9-tetrahydrocannabinol-C7 isomers |
Seed |
Psychoactive properties* |
A phytochemical investigation by mass spectrometry revealed the presence of homologues of trimethylsilyl (TMS) derivatives | Basas-Jaumandreu and De Las Heras, 2020 |
| Cannabidiol, cannabidiolic acid, cannabinol, tetrahydrocannabinol, tetrahydrocannabinolic acid | Cannabis medicinal extracts (oil and alcohol-based CMEs) |
Psychoactive properties* |
LC–MS/MS using an untargeted metabolomics approach revealed the effect of decarboxylated cannabinoids on pharmacological activity |
Citti et al., 2018 |
*Activities are reported based on previous research
**Activities are experimentally determined by the corresponding researchers
Table 2.
Systematic application of Cannabis-derived phytochemicals against clinically focused psychiatric disorders.
| Applied drug and/or phytochemical | Neurological ailment or psychiatric disorder | Experiment type and duration | Experimental design | Result | Remark | Reference |
|---|---|---|---|---|---|---|
| Nabiximols [THC (2.7 mg), CBD (2.5 mg)] and placebo | Attention deficit hyperactivity disorder (ADHD) | Randomized trial; 6 weeks | 30 patients were randomly treated with drug or placebo; cognitive performance, the appearance of ADHD symptoms and emotional stability were evaluated |
Quantitative behavioral test results were better for the drug-treated group | ADHD-related symptoms improved significantly | Cooper et al., 2017 |
| CBD (400 mg) | Social anxiety disorder (SAD) | Double-blind crossover study | Ten patients received CBD or placebo and their regional cerebral blood flow activity was analyzed. | CBD treatment modulated blood flow in the hippocampus, inferior temporal gyrus, left parahippocampal gyrus, and right posterior cingulate gyrus | CBD showed anxiolytic effects | Crippa et al., 2011 |
| CBD (600 mg) | Social phobia | A biopsychosocial model of social anxiety was applied; 1.5 h before public speaking | 24 patients randomly received CBD or placebo; Twelve healthy controls and other physiological measures were observed 6 times during the study |
Prior treatment with CBD reduced anxiety, hyperalertness, and cognitive speech impairment and performance discomfort | The visual analog mood scale and negative self-statement scale were used to study the effect of CBD | Bergamaschi et al., 2011 |
| CBD (600–800 mg) or amisulpride | Schizophrenia | Randomized trial; 4 weeks | 42 patients were randomly treated with drug or CBD; positive and negative syndromes were checked after every 2 weeks, psychiatric behavior was analysed | Both the drug and phytochemical treated groups showed recovery | The CBD treated group showed some side effects | Leweke et al., 2012 |
| CBD (1000 mg) and placebo [existing antipsychotic drug] | Schizophrenia | Randomized trial; 6 weeks | 88 patients were treated with phytochemical or continuing antipsychotic drug; behavioral pattern, positive and negative set of symptoms, and cognition level were observed | The CBD-treated group showed faster recovery, fewer symptoms, and illness | CBD-treated group showed improved cognitive function and motor activity compared to placebo | McGuire et al., 2018 |
| CBD (600 mg) and placebo (antipsychotic Medication) | Schizophrenia | Randomized trial; 6 weeks | 36 patients with schizophrenics received CBD or placebo; positive and negative set of symptoms were checked | Both groups showed signs of recovery and less symptoms | The CBD treated group showed some side effects | Boggs et al., 2018 |
| CBD(600–1200 mg) | Bipolar disorder | 30 day trial | 2 patients were treated with CBD, received placebo for 5 days; mania and psychiatric symptoms were checked every 7 days | Improvements were greater in the case of CBD + olanzapine treatment; CBD monotherapy did not show significant development with symptoms |
No side effects with CBD were reported | Zuardi et al., 1995 |
| Nabiximols: THC (2.7 mg) + CBD (2.5 mg) | Chronic depression | Randomized, placebo-controlled, graded-dose trial; 5 weeks | 263 patients with advanced cancer stage received placebo and drug in low, medium, and high dosage; drug tolerability, opioid-refractory pain reversal, sleep quality, and quality of life were evaluated |
All groups reported significant pain relief | The high dose (11–16 sprays) group reported a mood swing | Portenoy et al., 2012 |
| CBD capsules (25 mg) + liquid (6–25 mg) |
Insomnia | 5-month trial with the single patient (age 10 years; childhood trauma history) | The patient received fish oil (750 mg/day) and CBD oil capsule/day; for 1 month, the patient also received CBD liquid (12–24 mg) and later used it. 6–12 mg of CBD when needed; Sleep Disturbance Scale for children applied to monitor sleep patterns |
Sleep quality and quantity improved significantly | No cognitive characteristics or other effects on mental health were measured. | Shannon and Opila-Lehman, 2016 |
| CBD capsules (mainly 25 mg/day) |
Insomnia | Large retrospective case series-based study; 12 weeks | 72 patients received CBD in addition to their current medication | Hamilton anxiety rating scale showed improvement in sleep quality, decreased anxiety pattern | Data were not statistically significant for an experimental group | Shannon et al., 2019 |
| Nabiximols: [THC (2.7 mg) + CBD (2.5 mg) or only THC (2.7 mg)] | Insomnia | Large retrospective case series-based study; 5 weeks | 43 patients with advanced cancer stage received both types of drug combination; drug tolerability, opioid-refractory pain reversal, and quality of life were evaluated. | Pain, insomnia, and fatigue decreased over time | Johnson et al., 2013 | |
| Cannabis sample | Post-traumatic stress disorder (PTSD) | Clinician administered a posttraumatic scale or CAPS data analysis | Retrospectively, collected data of 80 patients suffering from PTSD and analyzed | More than 75% decrease in posttraumatic syndrome was reported in patients reported; less anxiety and better sleep were also marked by patients | The form of administration dosage and application procedure are not defined | Greer et al., 2014 |
| CBD (capsule or spray; 8–49 mg/week) | PTSD | Open-label retrospective case study; 8 weeks | Data from 11 patients with PTSD were collected and analyzed | Significant reduction in symptoms is noted | Data were not statistically significant | Elms et al., 2019 |
| Nabiximols [THC (2.7 mg), CBD (2.5 mg)] and placebo |
Attention deficit hyperactivity disorder (ADHD) | Randomized trial; 6 weeks | 30 patients were randomly treated with drugs or placebo; cognitive performance, the occurrence of symptoms of ADHD, and emotional stability were assessed | Quantitative behavioral test results were better for the rug-treated group | ADHD-related symptoms were improved significantly | Cooper et al., 2017 |
| CBD (400 mg) | Social anxiety disorder (SAD) | Double-blind crossover study | Ten patients received CBD or placebo and their regional cerebral blood flow activity was analyzed |
CBD treatment modulated blood flow in the hippocampus, inferior temporal gyrus, left parahippocampal gyrus, and right posterior cingulate gyrus | CBD showed anxiolytic effects | Crippa et al., 2011 |
| CBD (600 mg) | Social phobia | A biopsychosocial model of social anxiety was applied; 1.5 h before public speaking | 24 patients randomly received CBD or placebo; Twelve healthy controls and other physiological measures were observed 6 times during the study |
Prior treatment with CBD reduced anxiety, hyperalertness, cognitive speech impairment and performance discomfort | The visual analog mood scale and negative self-statement scale were used to study the effect of CBD | Bergamaschi et al., 2011 |
| CBD (600–800 mg) or amisulpride | Schizophrenia | Randomized trial; 4 weeks | 42 patients were randomly treated with drugs or CBD; positive and negative syndromes were checked after every 2 weeks, psychiatric behavior was analyzed | Both the drug and phytochemical treated groups showed recovery | CBD treated group showed some side effects | Leweke et al., 2012 |
| CBD (1000 mg) and placebo [existing antipsychotic drug] | Schizophrenia | Randomized trial; 6 weeks | 88 patients were treated with phytochemical or continuing antipsychotic drug; behavioral pattern, positive and negative set of symptoms, and cognition level were observed. | CBD treated group showed faster recovery, fewer symptoms and illness | CBD-treated group showed improved cognitive function and motor activity compared to placebo | McGuire et al., 2018 |
| CBD (600 mg) and placebo (antipsychotic Medication) | Schizophrenia | Randomized trial; 6 weeks | 36 patients with schizophrenics received CBD or placebo; positive and negative set of symptoms were checked | Both groups showed signs of recovery and less symptoms | CBD treated group showed some side effects | Boggs et al., 2018 |
| CBD (600–1200 mg) | Bipolar disorder | 30 day trial | 2 patients were treated with CBD, received placebo for 5 days; mania and psychiatric symptoms were checked every 7 days |
Improvements were higher in the case of CBD + olanzapine treatment; CBD monotherapy did not show significant development with symptoms | No side effects with CBD were reported | Zuardi et al., 1995 |
| Nabiximols: THC (2.7 mg) + CBD (2.5 mg) |
Chronic depression | Randomized, placebo-controlled, graded-dose trial; 5 weeks | 263 patients with advanced cancer stage received placebo and drug in low, medium, and high dosage; drug tolerability, opioid-refractory pain reversal, sleep quality, and quality of life were assessed | All groups reported significant pain relief | The high dose (11–16 sprays) group reported a mood swing | Portenoy et al., 2012 |
| CBD capsules (25 mg) + liquid (6–25 mg) |
Insomnia | 5-month trial with a single patient (age 10 years; childhood trauma history) | The patient received fish oil (750 mg/day) and a capsule of CBD oil capsule/day; for 1 month, the patient also received CBD liquid (12–24 mg) and then used 6–12 mg of CBD when needed; Sleep disturbance scale for children applied to monitor the sleep pattern | Sleep quality and quantity improved significantly | No cognitive characteristics or other effects on mental health were measured | Shannon and Opila-Lehman, 2016 |
| CBD capsules (mainly 25 mg/day) |
Insomnia | Large retrospective case series-based study; 12 weeks | 72 patients received CBD in addition to their current medication | Hamilton anxiety rating scale showed improvement in sleep quality, decreased anxiety pattern | Data were not statistically significant for one experimental group | Shannon et al., 2019 |
| Nabiximols: [THC (2.7 mg) + CBD (2.5 mg) or only THC (2.7 mg)] | Insomnia | Large retrospective case series-based study; 5 weeks | 43 patients with advanced cancer stage received both types of drug combination; drug tolerability, opioid-refractory pain reversal, and quality of life were evaluated. | Pain, insomnia, and fatigue decreased over time | Johnson et al., 2013 | |
| Cannabis sample | Post-traumatic stress disorder (PTSD) | Clinician administered a posttraumatic scale or CAPS data analysis | Retrospectively, collected data of 80 patients suffering from PTSD and analyzed | More than 75% decrease in posttraumatic syndrome was reported in patients reported; less anxiety and better sleep were also marked by patients | The form of administration dosage and application procedure are not defined | Greer et al., 2014 |
| CBD (capsule or spray; 8–49 mg/week) | PTSD | Open-label retrospective case study; 8 weeks | Data from 11 patients with PTSD were collected and analyzed | Significant reduction in symptoms is noted | Data were not statistically significant | Elms et al., 2019 |
4. Tobacco vs. marijuana and tetrahydrocannabinol (THC)
Various experiments have been conducted to date to assess the constituents in the smoke of marijuana and tobacco. On evaluation, it was found that the same constituents were present in both samples with chemicals, which were toxic to the respiratory system (Melamede, 2005). Tar, which is the particulate phase, comprises quite similar components in tobacco and marijuana with the exception of the presence of tetrahydrocannabinol (THC) and a trace amount of THC-like constituents (cannabinoids) in marijuana, while tobacco does not contain these compounds (Sharma et al., 2012). Tobacco tar was found to contain nicotine that is absent in marijuana. Due to the similarity between tobacco smoke and marijuana, it makes marijuana carcinogenic, as it consists of compounds named polycyclic aromatic hydrocarbons such as benzo[a]pyrene, which provides expression as a key factor that progresses cancer in the lungs of humans (Do, 2018). A key point that should be acknowledged is that tobacco cigarettes at present contain a filter tip, which lowers the tar content. However, in contrast, marijuana cigarettes lack filter tips, which assists in the generation of tar about two-fold more than that of tobacco per unit weight in addition to the same profile of smoke (Aldington et al., 2008). Furthermore, the breathing and puff volume in the case of marijuana is around two-thirds bigger, the depth of smoke after inhalation is 40% greater, and the holding time of breath is four times stretched in contrast to the characteristics of the smoke of tobacco. Thus, the illustration of variations between filtration along with smoking results in the delivery and retention of a four-fold excessive amount of tar in the lung by marijuana smoke compared to tobacco smoke (Tashkin and Roth, 2019).
Marijuana, which is a blend of shreddsed dried leaves, stems, flowers, and seeds shredded from the Cannabis sativa plant, appears to be harmless but is quite known for its toxic effects (DeFilippis et al., 2020). Cannabis is said to be toxic due to the presence of a psychoactive chemical, named delta-9-tetrahydrocannabinol (Fig. 4), commonly known as THC (Roychoudhury et al., 2021). The potency or strength of marijuana is directly proportional to the amount of THC present in it. Marijuana is often smoked by rolling into a cigarette called ‘joint’ and smoked through hollowed cigars (Cooper and Haney, 2009). A survey conducted in 2001 in US secondary school students of 12th grade revealed that 19% were cigarette smokers, 5.8% smoke marijuana, and 3.6% consume alcohol daily. This also revealed and gave the estimate that about 49% of 12th grade students have smoked marijuana at some point in their life and about 29.5% are smoking marijuana in the past months (Grucza and Bierut, 2006). Considerable distress associated with the consumption of marijuana by young people in this population acts as a ‘gateway drug’, which drove them toward ingesting more illicit compounds such as cocaine or heroin (Volkow et al., 2014a). The initiation of smoking marijuana typically starts at the beginning of adolescence and the stage of life of young adulthood. It is found that two-thirds of the new marijuana smokers belonged to the age range of 12–17 of youth, whereas the rest of the third belongs to young adults in the age range of 18–25 (Ellickson et al., 2005).
Fig. 4.
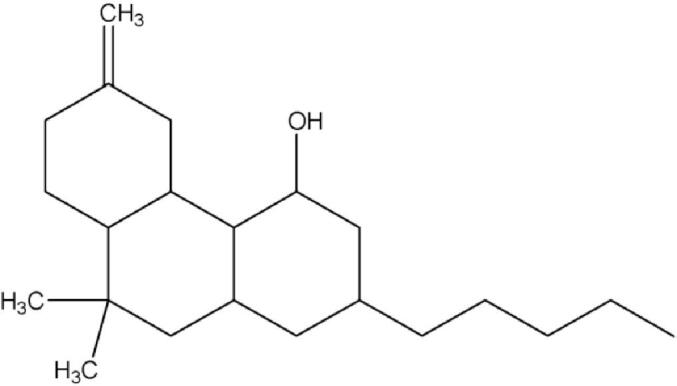
Structure of delta-9-tetrahydrocannabinol (Chemdraw Version 8.0.3).
5. Mode of action (MOA) of THC
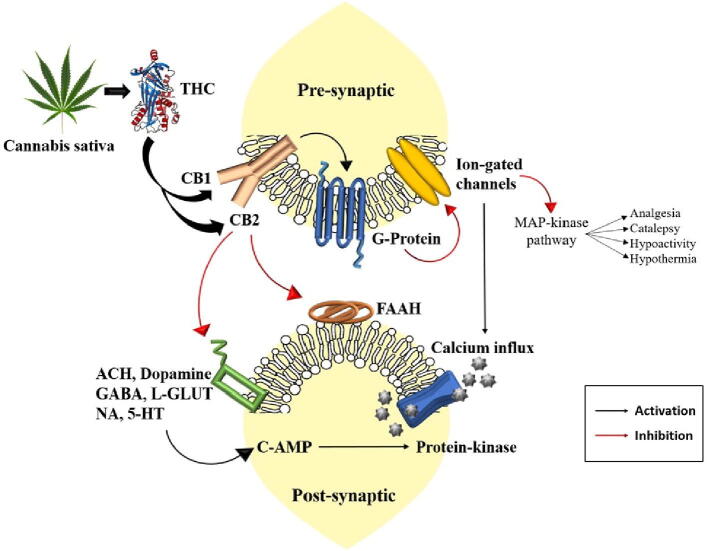
Cannabis shares a brief history due to its consumption either for recreational and medicinal purposes. Ongoing research and recent findings have provided significant insight to understand the chemical reaction process of Cannabis and associated compounds named cannabinoids, which affect the normal function of the body (Finn et al., 2021). The term cannabinoids means a family of 21 carbon alkaloids that are found exclusively in Cannabis plants. Marijuana smoke contains up to 61 different cannabinoids (Bridgeman and Abazia, 2017). Out of all, the principal active compound found by Gaoni and Mechoulam, named delta-9-tetrahydrocannabinol (delta-9 THC) is believed to be associated with almost every addictive and psychotropic characteristic of Cannabis (Gaoni and Mechoulam, 1964, Seely et al., 2011). Behavioral and physiological changes due to the consumption of cannabinoids are considered associated with nonspecific interactions rather than interactions with specific membrane-bound receptors present on cell membranes. The two primary receptors for cannabinoids are CB1 and CB2 (Glogauer and Blay, 2021). In which, the CB1 receptor is associated with a maximum number of actions carried out by cannabinoids. These receptors are commonly found in animals and even in birds, fish, mammals, and reptiles (Silver, 2019). Cannabinoids are associated with a wide range of actions in the brain; they hinder learning and storing of information in the hippocampus, control of locomotion action and reward pathways of the basal ganglia, and modulate appetite in the hypothalamus (Reggio, 2010). Thus, their action is opposite to that of, for example, Bacopa monnieri bacosides, which are well known as a potent ‘tonic for the human brain’, which serves as a memory enhancer (Banerjee et al., 2021). Additionally, the mechanisms and pharmacological action of herbal bioactive compounds to improve memory and use in the near future for the treatment of severe brain disorders were recently discussed (Halder et al., 2021), including anti-Parkinsonian medicinal crops for industrial use (e.g., Tandon et al., 2021).
THC is a cannabinoid compound that interacts with the CB1 receptor in the human brain. THC mimics naturally synthesized endocannabinoids, which are produced by the brain, and has a strong impact. CB receptors are predominantly found in the frontal region of the cerebral cortex and many other parts of the brain such as the basal ganglia, cerebellum, anterior cingulate cortex, hippocampus, and hypothalamus (Zou and Kumar, 2018). Many neurotransmitter enzymes, viz. acetylcholine, dopamine, GABA, 5-HT, L-glutamate, and noradrenaline are inhibited by the action of THC, while most of the time endocannabinoids are ineffective due to the enzyme fatty acid amide hydrolase (FAAH) (Atakan, 2012). In contrast, exogenous cannabinoids like THC endure for a long time, which in the result shows recorded physiological properties (Wyrofsky et al., 2019). The interaction between the THC and CB receptors occur in the presynaptic neurons. The interaction passes the signal to activate G-proteins, which further transfer the signal eighter to activate or inhibit a number of signals associated with transduction pathways. The G-protein is found to be directly involved in the inhibition of voltage-gated channels of Ca and Na, which, when inhibited, lead to activation of the MAP kinase pathway. Due to the integration of these pathways, we precede towards severe effects (Howlett et al., 2010) (Fig. 5).
Fig. 5.
Severe effects of Cannabis sativa and THC on the human nervous system.
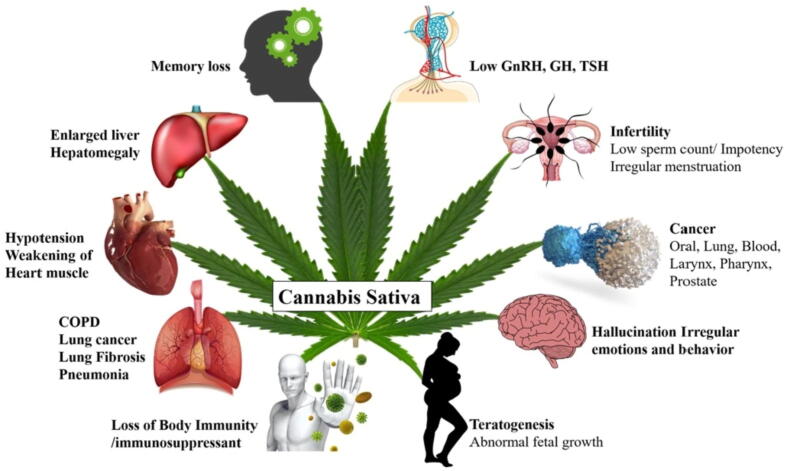
In animals, a combination of characteristic features of four syndromes, such as analgesia, catalepsy, hypoactivity, and hypothermia, occurs due to the synthesis of cannabinoid agonists (Manzanares et al., 2006). There are many other effects associated with the consumption of Cannabis, including antinociception, inconvenience while concentrating, increase in perception of senses, tachycardia, nausea repression, and increased hunger, which collectively lead to impaired thinking, storage of information, and locomotive and cognitive skills (Gonçalves et al., 2020). Dissociative disorder and panic attacks emerge as adverse effects, which are commonly seen in young people, leading further to the development of motivational syndrome in them (Radhakrishnan et al., 2014). The multifaceted medicinal attributes of the health hazards of Cannabis are presented in Fig. 6.
Fig. 6.
Multifaceted medicinal attributes of the health hazards of Cannabis sativa.
6. Gateway theory and its connection with Cannabis sativa
The theory, which is connected with consumption, is known as the gateway drug theory and is recogn ized by different names such as gateway effect and gateway hypothesis. This hypothesis states that the consumption of less-lethal drugs can advance the risk in the future of consuming more lethal and hard drugs and committing criminal activity (Nkansah-Amankra and Minelli, 2016). Even many reports have been documented which reveal that many hard drug consumers have started using Cannabis or alcohol before shifting towards harder substances, whereas another research indicates that few vital drug users have consumed different drugs before consuming alcohol or Cannabis (Budney et al., 2007). Thus, the gateway drug hypothesis proclaims and supports that the consumption of Cannabis can eventually precede the use of harder drugs. In the majority, Cannabis is believed to act as a gateway to different drugs due to its connection with many social aspects (Secades-Villa et al., 2015).
7. Marijuana and health effects: Conflicting but interesting outcomes
Animals are often exposed to marijuana due to accidental ingestion. Scientific reports have revealed that in the case of marijuana inhalation by humans, the concentration of the THC peak is observed in plasma for about 5 to 12 min, while it remains around for 2–3 h when it is ingested. The path, which is most common in the case of the animal, is oral. The result of the intake is visible after 6–12 min in the case of inhalation and 30–60 min in the case of consumption orally (Huestis,2007). On evaluation, the cases studied by APCC (Animal Poison Control Center, US) claimed that clinical traits prevailed within two hours after consumption. In one case reported on the APCC, a 13.5 kg dog ingested 3 g of marijuana exhibited ataxia, head bobbing, and vocalization. Furthermore, in another case, bradycardia and ataxia were observed in a dog weighing about 17 kg who had ingested only 1 tsp of marijuana. In the evaluation of both cases, it was found that the beginning of the effects of Cannabis ingestion started within 30 to 90 m, and the effect lasted for 72 h (Fitzgerald et al., 2013). In the case of humans, cannabinoids were found to be soluble in lipids; whereas 97–99 percentiles were found to be protein bound and segregated to different parts of the body that comprise organs such as the brain, heart, kidneys, liver, and salivary glands. As soon as THC is ingested, it has a substantial first-pass effect. It is metabolized by microsomal hydroxylation and nonmicrosomal oxidation within the liver. The route followed for excretion passes through bile. After the consumption of marijuana within 72 h, approximately 35% of the dose taken orally is excreted in the form of feces as unconjugated metabolites, while 10%-15% is excreted in the form of urine as acidic metabolites and conjugates, and the rest of the minor portion is excreted in the unchanged form via urine. After five days of exposure, almost 80–90% of marijuana has been found to be eliminated (Fitzgerald et al., 2013). An LD50 for marijuana has not been determined in animals such as cats and dogs. In conducting the experimentation, the researchers found that ingestion of delta 9-THC and delta 8-THC through the oral route, ranging between 3,000 and 9,000 mg / kg in dogs, was not found lethal; Furthermore, all dogs were well again within 24 h after consumption. Dosing rats with 3,600 mg/kg of delta 9-THC resulted in arousal of mild to severe lesions; in addition to that they also acquired adrenal congestion, hemorrhage, lung congestion, pneumonitis, splenic hypocellularity, thymic congestion, and but no death has occurred (Taffe, 2012, Thompson et al., 1973).
Studies conducted on animals with prolonged use of the drug resulted in a decreased desire for food as well as sex, an improvement in behavioural tolerance, a decline in stimulated aggressive behavior, and sedative effects upon acquiring information (Colizzi et al., 2018a, Zamberletti et al., 2012). The chronic effect, which is associated with consuming marijuana, is brain lesions and mental deterioration that are as damaging as the consequences of persistent alcohol consumption (Mandelbaum and Suzanne, 2017). The actual study conducted on consistent marijuana smokers is quite rare, as the researchers experienced various substantial deficiencies, which have remained as unquantified documented information as proof, the employment of biased, aberrant consumers as a subject of the study, and no adequate regulation was used in executing the research plan. The current study was conducted to find a solution to improve these defects in the context of an evaluation of the consequences of persistent heavy use on a number of issues of brain functioning with little interest for a better understanding of drug experience compared to expectations (Colizzi et al., 2018b).
The lethal single dose, which was found to be toxic in rats, was about 50% and can be associated with other animals. It means that L1, 0 of Delta-9-THC will be lethal, if given by intravenous injection in the range of 20–40 mg/kg, and if taken orally in the range of 800–1400 mg/kg which also depends on sex in addition to species (Justinova et al., 2005). Animals that consume these compounds in high doses are extremely susceptible to the risk of respiratory arrest as a cause of death. Marijuana consumption is not limited to these medical side problems, but also affects the social life of a person. It retards the performance of the individual in academics and jobs, makes the person lethargic and less interested in outdoor activities, and isolates the person from its social groups, such as friends and family. It also leads to a financial crisis and motivates the other person to commit criminal offenses. Thus, we see that marijuana causes many effects in the body of animals depending on the time and severity of the dose. Effects on various organs are discussed hereafter (Wang et al., 2018)
7.1. Marijuana, endocannabinoid systems and their cumulative effect on cardioprotection
Marijuana and the cannabinoid compound delta9-THC are associated with an elevation in heart rate and a minute increase in blood pressure during the supine position and, in rare cases, generate marked postural hypotension (Hiley and Ford, 2004, Pacher et al., 2018). It also contributes to redness in the eyes by causing swelling in the conjunctival vessels. Marijuana also leads to temporary weakening of heart muscle contractions. People suffering from some health problems encounter chest pain due to an improper oxygen level reaching the heart, which is frequently found in people who abuse marijuana in contrast to tobacco smokers (Montecucco and Di Marzo, 2012). Bradycardia and hypotension are also two of the most typical responses to cardiovascular effects. The overall output of the heart increases, the peripheral vascular system sustains resistance, and this decreases the overall efficiency of the body. Due to recurrent encounters of supine blood pressure, it slowly decreases, orthostatic hypotension is perished, the volume of blood intensifies, the rate of heart decreases, which causes a malfunction of the circulatory system, such as decreased sympathetic activity and increased parasympathetic activity (Jouanjus et al., 2014). After smoking marijuana, it has been found that the heart starts to beat faster along with a decrease in blood pressure, causing the risk of cardiac infarction to increase four times during the first hour (Goyal et al., 2017). Marijuana smoking causes elevated heart functioning, which also elevates catecholamine level and carboxyhaemoglobin, resulting in postural hypotension (Goyal et al., 2017, Jouanjus et al., 2014). Cannabidiol is a nonpsychoactive ingredient of marijuana and has shown multiple defensive functions to recover cardiac injuries preliminarily by decreasing oxidative stress through the elimination of free radicals, apoptosis, and inflammation, therefore ultimately showing its antioxidant and antiapoptotic effects based on nonclinical studies (Shayesteh et al., 2019). A relationship was demonstrated between cannabinoids and metabolic processes due to the lower prevalence of obesity and diabetes mellitus among marijuana users compared to those who never used marijuana, as shown by a number of epidemiologic studies. The lower level of fasting insulin and HOMA-IR and a smaller waist circumference were found through the use of marijuana in a study that has been conducted in 4657 adult Americans from the National Health and Nutrition Examination Survey (Goyal et al., 2017).
7.2. Hepatotoxicity and the role of marijuana
A much less documented context related to the effect of persistent use of marijuana on the liver is found. As its consumption can damage the liver (Ewing et al., 2019). Inflammation of the liver is associated with the disease hepatitis. People with hepatitis C are prohibited from consuming marijuana because it plays the role of an inflammatory agent on the liver, increasing the risk of liver fibrosis and even causing wounds in liver tissue. Problems such as enlarged liver and hepatomegaly are also caused by marijuana use (Wijarnpreecha et al., 2018). Furthermore, it has been found that people who consume alcohol at moderate to high levels with Cannabis are at high risk of liver fibrosis. Those patients who consume Cannabis every day, are found to have a considerably low Quetelet index compared to occasional users (25.2% versus 26.4%), were more prospective to those who consume Cannabis on the basis of medical prescription (57.1% versus 8.79%), and are highly prone to getting affected by HIV co-infection (39.3% versus 18.2%) (Nada et al., 2017, Santos et al., 2020). Liver safety concerns were raised in randomized controlled trials from healthy adults who received therapeutic daily doses of cannabidiol for ~ 3.5 weeks and it has been shown that consumption of cannabidiol by healthy adults caused an increase in serum alanine aminotransferase, consistent with drug-induced liver injury (Watkins et al., 2021).
7.3. Marijuana consumption and possible inflammatory effects on lung function
Marijuana is generally smoked, whereas it is also taken orally. It can lead to pulmonary edema with hemorrhage (Yayan and Rasche, 2016). The general presumption of people is that smoking Cannabis generates similar consequences as smoking tobacco in its persistent use. With regular evaluations, a connection of Cannabis smoking with inflammation in the large airway has been established; with that, it is also associated with bronchitis symptoms and resistance to increased airway in the lungs. Even the particulate, as well as the gaseous composition of cigarettes of both marijuana and tobacco, are indistinguishable, but they differ due to active constituents, named THC and nicotine (Biehl and Burnham, 2015). Marijuana fumes are inhaled to a maximum limit and held for a long time in the lung to increase the absorption of THC. As a result, the major variation in tar and carbon monoxide largely depends on the entry route, the depth to which it is inhaled, and how long the breath was held (Hancox et al., 2010).
Recent experimentation with Cannabis smoking has provided affirmative results for the development of COPD (Chronic Obstructive Pulmonary Disease) in which obstruction of airflow occurs while breathing and emphysema occurs at the same time (Martinasek et al., 2016). In the recent literature, 11 from a group of 12 people who smoke Cannabis were found to have a severe bronchodilator. However, there are a large number of documented reports of Cannabis smokers who were diagnosed with bullous emphysema. The long-term effect of extensive consumption of Cannabis has created concern about its effect on mental health. The effect on the respiratory system is confirmed by symptoms, and various studies have been conducted in Cannabis smokers. The surveys conducted revealed that cough, sputum production, and wheezing are present in a 3:5 ratio among those who smoke Cannabis (Abdallah et al., 2018, Gracie and Hancox, 2020). Smoking Cannabis and tobacco has been found to cause bronchial impairment and increases the chances of basal cell hyperplasia, disorientation among cells, alteration of nuclei, and even increases the value of the nuclear/cytoplasmic ratio (Tashkin, 2013). Cannabis constituents are found to be more cytogenic, highly mutagenic and have a high tendency to cause damage to chromosomes in much more unpredictable ways in comparison to tobacco. Even infrequent smoking of marijuana will cause a burning sensation in addition to stinging of the mouth and throat. It also results in excessive coughing. Daily coughing and phlegm synthesis result in severe chest pain, which causes obstruction during breathing and makes users prone to lung infection (Tashkin, 2018). Further studies conducted on marijuana smokers revealed symptoms of the respiratory system such as severe bronchitis, pneumonia, recurrent synthesis of phlegm, breathlessness, frequent gasping, and production of chest sound even without having a cold. The results of medical examination of the general chest revealed inadequate functioning of the pulmonary system (Joshi et al., 2002, Owen et al., 2014).
Phyto-cannabinoids exhibited intestinal anti-inflammatory effects. Intraperitoneally and oral gavage application of CBD BDS (CBD botanical drug substance), which was extracted from Cannabis, markedly decreased the damage rate and intestinal hypermotility in intestinal inflammation (Pagano et al., 2016). Iannotti et al. (2019) studied the effect of phytocannabinoids on some pathological characteristics of Duchenne muscular dystrophy (DMD) and observed the action of cannabinoids on cannabinoid CB1 receptors, which is a common GPCR in the mammalian brain and is responsible for numerous euphoric impacts of marijuana. The pathological features of DMD, i.e., damaged myoblast and satellite cell differentiation, unresolved inflammation, and defective autophagy, are significantly counteracted by phyto-cannabinoids. Marijuana smoke has been found to contain a high number of carcinogenic hydrocarbons compared to tobacco smoke (Huang et al., 2015). It also increases the chances of cancer development as it is inhaled deeper and held longer in the lungs than tobacco smoke, thus increasing the contact time of these carcinogenic hydrocarbons and the risk of cancer (Jett et al., 2018). Even the evidence retrieved from studies conducted in animals and humans showed that frequent exposure to marijuana constituents is harmful to the lungs. Studies in which animals were exposed to various doses of marijuana for approximately 12–30 months revealed immense damage to the smaller airways among dogs and monkeys. Interestingly, these are the main sites that are affected by tobacco-associated COPD as well as acute pneumonia among rats and monkeys (Tashkin, 2017). The experiment conducted to assess the structural or functional abnormalities of emphysema did not show an effect in rats that were exposed for 1 year to increasing doses of marijuana compared to rats that were exposed to increasing doses of tobacco. Earlier human studies have provided mixed results in which some preferred consistent marijuana consumption, while some associated it with acute bronchitis and pulmonary emphysema, even some that did not find any relationship (Alqahtani et al., 2019). A survey was conducted in Los Angeles on a large group of people in which 144 were habitual marijuana smokers, 135 were the ones who smoked marijuana as well as tobacco, 70 were tobacco smokers, and 97 were nonsmokers. When the result of nonsmokers was compared with marijuana smokers, it was found that 15–20% of marijuana smokers suffered from acute bronchitis, as symptoms such as persistent cough and phlegm synthesis were predominant. In addition, 20–25% of tobacco smokers were also reported to have acute bronchitis, although the symptoms of tobacco smokers were quite similar to those of marijuana smokers regardless of the graded disproportion in the quantity of each constituent smoked per day, which means that 3 marijuana joints had the same effect as 20 tobacco cigarettes. However, no additional consequences of marijuana and tobacco were observed (Lankenau et al., 2019). A similar type of survey conducted in the residential area of Tucson over a randomly arranged group of individuals in the range of 15–40 years of age reported additive effects of both marijuana and tobacco, which was not noted in the survey conducted in Los Angles (Chase et al., 2016).
Scientists consider marijuana injurious to the lungs as its smoke is inhaled deeply without filtering and is held for a longer time in the lungs. Due to this contact time of smoke with lung tissues, irritation prevails and causes impairment in the normal functioning of the lungs. Even a few of the constituents of marijuana smoke are found to be similar to tobacco smoke, which is also found to be responsible for emphysema and cancer (Lozano et al., 2017). Many marijuana smokers also use cigarettes; thus, the combined effect induced by smoking of these two increases the possibility of severe health problems. On histological analysis of the lungs of marijuana smokers, it was revealed that the association with marijuana causes damage to the epithelium and ciliated epithelial cells present in the respiratory system and decreased their numbers; these similar types of change have been observed among tobacco smokers (Ribeiro and Ind, 2016). Biopsy carried out on the endobronchial tissues revealed metaplasia of the goblet cells, hyperplasia of the reserve cell, and metaplasia of the squamous cells. Further consequences that can arise are the formation of oedema with inflammation in the lung along with hypersecretion of mucus. Therefore, Cannabis consumption is associated with various pernicious consequences on respiratory organs that can lead to abnormal lung functioning, which can cause infection in the respiratory tract and can further lead to the development of cancer (Huang et al., 2015).
7.4. Immunomodulatory attributions of marijuana: Risk versus benefit
Smoking marijuana is associated with inflammatory reactions. Alveolar macrophages have been acquired from the lungs of marijuana and tobacco smokers, two out of three in comparison to nonsmokers. In addition, the effect induced by both was quite similar (Cabral and Staab, 2005). Executing signifies that frequent marijuana smokers stimulate the inflammatory reaction, which leads to the accumulation of alveolar macrophages in a massive amount in the lungs. Alveolar macrophages under the electron microscope from marijuana or tobacco smokers showed a significant expansion in size and due to the intake of particulate from smoke cells showing complexity among the inclusion bodies present in the cytoplasm. In contrast, the alveolar macrophages of shared marijuana and tobacco smokers were found to be completely crowded by inclusion bodies (Tanasescu and Constantinescu, 2010). Recent research focuses on identifying the THC receptors (or cannabinoid receptors) in the WBC (white blood cell) as THC has persuasive effects on the immune system. In vitro analysis conducted on different immune cells, such as macrophages, natural killer cells, and T cells of animals, indicated that THC has an immunosuppressive effect on them (Randall, 2007). Another analysis was carried out on mice, exposing them to Δ9-THC resulted in an incompetent immune response against an opportunistic pathogen, Legionella pneumophilia, which causes infection in the lungs. It has also been observed that alveolar macrophages in the lungs of healthy people were active in killing bacterial and fungal infections along with the ability to suppress tumor cells compared to daily marijuana smokers (Greineisen and Turner, 2010, Rao et al., 2015). Additionally, alveolar macrophages of daily marijuana smokers were incompetent in releasing cytokines to induce a pro-inflammatory response. Thus, these verdicts imply marijuana as an immune suppressant, which impedes the defense system of the host and increases the health risks among those patients who suffer cancer, HIV-AIDS, and organ transplants, as they have a weak immune system. Therefore, marijuana is not found safe due to the health risks associated with it, such as HIV-AIDS (Tashkin et al., 2007), high chances of opportunistic infection in addition to Kaposi’s sarcoma (Tashkin and Roth, 2019).
Downregulation of the neuroinflammatory process in animal models of multiple sclerosis (MS) was promoted by the use of CBD associated with Δ9-THC and also decreased muscle pain and spasticity in patients with MS. Furthermore, the psychotropic effects of THC were attenuated by CBD when practiced in a combined form (Gonçalves et al., 2020). Cannabidivarin (CBDV) exhibited potential intestinal anti-inflammatory impacts in children with active ulcerative colitis (UC) by reducing cytokine expression in colon biopsies of pediatric patients (Pagano et al., 2019). The injection of lipopolysaccharide (LPS) triggered depressive behavior was inhibited by terpineol by modulating the dopamine receptor type 2 (D2R), the cannabinoid receptor type 1 (CB1R), and the cannabinoid receptor type 2 (CB2R) and suggested that the most promising targets of the action of terpineol are the CB1 and CB2 receptors (Vieira et al., 2020).
7.5. Possible carcinogenic effects of marijuana: A compilation of case studies
Marijuana smoke comprises constituents, which are similar to carcinogens and cocarcinogens. Tar, which is found in marijuana smokers, has been claimed to comprise various types of carcinogenic mixture, which is also found in tobacco smoke, such as reactive aldehydes, nitrosamines and approximately 50% of carcinogenic polycyclic hydrocarbons plus benzo[a]pyrene. Benz[a]pyrene is known to be associated with promoting p53 mutation (Hecht, 2008, Omare et al., 2021, Wei et al., 2018). Marijuana consumption is involved in developing the risk of colorectal or lung cancer, but certain studies showed its involvement in cervical and prostate cancer. Based on a survey conducted in hospital patients in the United States, it was found that the use of marijuana increases the susceptibility to developing cancer in the head or neck, with its relation to the response to dosage on the basic rate and time of exposure (Mousa et al., 2019). In Tunisia, cases studied on lung cancer showed an increase in the occurrence of cancer eight times among marijuana users (de Groot et al., 2018). Female smoking marijuana during pregnancy makes the child prone to astrocytoma, blood cancer, and rhabdomyosarcoma (Benevenuto et al., 2017). One marijuana cigarette has been found to accumulate four times more tar inside the lung compared to the filtered tobacco cigarette of the same weight. A high concentration of carcinogenic polycyclic hydrocarbons is found in marijuana tar, due to which the accumulation of tar in the lungs intensifies the exposure of these carcinogens among marijuana smokers. Marijuana smoke contains more carcinogens compared to tobacco smoke. The precancerous stage, named metaplasia, has been observed in lung tissue of long-term marijuana consumers when observed in vitro (Mehra et al., 2006). The tar of marijuana smoke when spread to the skin of an animal led to the development of the tumor. The smoke from marijuana in the fumes when painted on the mice caused the lesions, which were associated with the malignant stage. On biopsy, the internal bronchial lining tissue of chronic marijuana smokers showed massive cellular amendment, some of which can be considered premalignant. Marijuana and tobacco smoking act as supplements, leading to the consequences of cellular aberrations (Popova et al., 2017). Many case studies have revealed that a large portion of marijuana smokers have lung or aerodigestive tract cancers, including oral cavity, larynx, and pharynx cancer. Thus, these case studies indicate the role of marijuana in the development of lung cancer among humans. It also provides the inference that if marijuana is persistently smoked for a long period, it will lead to the development of cancer (Haverkos et al., 2017, Newman et al., 2019).
Tumor growth was found to increase with treatment with low levels of THC-cannabinoid receptor expression, and cytotoxicity was not induced in these cells. It was also concluded that modification of JunD, a transcription factor, can mediate the anticancer effects of THC in breast cancer where JunD was activated by THC through its translocation to the nucleus and increased its expression. In addition to this, overall anticancer effects can be enhanced by the combination of cannabinoids with other therapeutic agents (Tomko et al., 2020). In a study conducted between 1999 and 2003, marijuana usage has decreased the risk of head and neck squamous cell cancer in a population-based case-control study of 434 cases and 547 controls based on age, gender, and the town of residence in Boston (Liang et al., 2009).
7.6. Marijuana and fertility: Myth versus reality
A study conducted on female monkeys illustrated the same result of cannabinoids on their reproductive system as that of humans. The introduction of Δ9-THC led to the declination of LH by 50–80% in monkeys. The implication of GnRH secretion has been observed in direct and indirect ways. Inhibition of gonadotropin secretion was observed due to interference in the mensuration cycle (John et al., 2018). A study conducted in the reproductive psychology department at the University at Buffalo stated that men who smoke marijuana daily have considerably lower amounts of seminal fluid, low sperm count and their sperm showing unusual behaviour, the sum of which adversely affects fertility (Hallak et al., 2019). The sperm of marijuana smokers showed abnormal behavior rather than swimming slowly to sustain energy to reach the egg. Instead of that, they are hyperactive at first and get retarded before reaching the egg. In the general case, the sperm becomes hyperactive when it is present in the cervix region quite close to the egg. While THC-influenced sperm become hyperactive in their early stage and then exhaust themselves (Rajanahally et al., 2019). Even casual marijuana smokers have experienced a reduction in fertility. Impotency has now been associated with marijuana and has also been found to hinder ejaculation. Even the administration of marijuana extract has initiated a reduction in testicular size in rodents and dogs. The deterioration of the seminiferous tubules of the testes may explain the root cause of this problem. Direct inhibition of the normal functioning of Leydig cells by Cannabis has been visualized (Barak and Baker, 2016, Kasman et al., 2020). The negative effect of marijuana has caused a low sperm count due to less synthesis. A report on chronic marijuana smokers showed a 58% declination in sperm amount without significant alteration in LH and testosterone (Sharma, 2017). Women who smoke marijuana have a high risk of infertility due to irregular menstruation. The active component, THC, was evaluated in the reproductive organs along with the vaginal fluid of women smoking marijuana. A similar influence of THC was observed when sperm were exposed to it inside the testes or outside (Lammert et al., 2018). In the female reproductive fluid, the constituents of marijuana, nicotine, and other drugs can be assessed. Marijuana smoking allows THC to reach the woman’s oviduct, as well as her cervix. If the male is a nonsmoker but the female is a smoker, then the sperm encounter THC in the vagina, oviduct, or uterus region, which causes alternation to the male sperm and reduces the productivity of gonadotropic hormones (Kasman et al., 2018a, Kasman et al., 2018b). Certain pieces of evidence are available that claim that chronic marijuana can lead to abnormal ovulation (Dubovis and Muneyyirci-Delale, 2020).
7.7. Effect of marijuana as a teratogen
In a study conducted in the Netherlands, it was found that 2.9% of women have experienced Cannabis in their adolescent and pregnancy stages. Various studies have reported neurological disorders during the development of a child whose mother is a chronic marijuana smoker (Grant et al., 2018). Various studies revealed that exposure to marijuana in the prenatal stage affects the normal reaction to any visual stimulus, increases quavering problems in concentrating, and poor memory power with that poor problem deciphering ability. Marijuana is also found to be associated with slow growth of the child in the prenatal period. Babies that have been born before their time suffer from various health issues compared to babies that have been born on time. Babies that were frequently subjected to marijuana during the prenatal stage displayed withdrawal-type indications, such as weeping for a very long shiver and, in addition to that, they are not able to sleep. Certain studies suggested that the child who has been exposed to Cannabis during pregnancy experiences a problem during concentration, but this does not mean that they have poor IQ (Gunn et al., 2016, Roncero et al., 2020). Marijuana was found to be toxic during foetal development among birds, dogs, fish, hamsters, monkeys, rabbits, and rodents (Beaulieu, 2005). Even children of affected persons are seen to have impeded growth and abnormalities in their behavior. However, no behavioural consequences or conversations related to behavioural abnormalities were restricted to subjective reports of stimulating results of marijuana consumption among humans. In addition to that, the copulation behavior of male mice was also observed (El Marroun et al., 2018). It is associated with defects such as low body weight and smaller head circumference. Marijuana consumption extensively affects and retards foetal development irrespective of the consumption of other factors such as drinking, smoking tobacco, and consuming alternative psychoactive drugs (Fergusson et al., 2002). A study revealed that marijuana has probable chances of brain mutilation during fetal development and causes obstacles during the process of neurophysiological integrity in a child (Wu et al., 2011). Substantial teratogenic effects have been seen in mice, rats, rabbits, and hamsters on dispensing marijuana, including ‘resorption, growth impediments, and deformity’, among which cases of resorption and impediment are more persistently reported than deformity. The basis on which marijuana has emerged as a major concern depends on the THC compound, which has access to pass through the placenta and affect the process of development of the fetus (Gilbert et al., 2016, Janer et al., 2008). Few blood vessels of the fetus are present in the villi region of the placenta, which extends up to the wall of the uterus of the mother. The blood of the mother travels through the intervillous spaces, which facilitates the separation of villi with the help of a thin membrane layer of the placenta. The THC constituents present in marijuana are capable of transferring from the blood of the mother through the membrane of the placenta to the villi region, which comprises the blood vessels of the fetus. Therefore, when entering THC, it travels through the umbilical cord to the foetus (Bauer and Lagasse, 2004, Veit et al., 2017).
At the University of Pittsburgh, a study was conducted to assess the consequences of exposure to marijuana in the prenatal stage on the consequent child’s intellect (Sundram,2006). In another study, it was discovered that exposure to marijuana in the prenatal stage, among students in the first and third trimesters, showed substantially elevated levels of depression symptoms at the age of 10 in 2006. It was also recognized that the consumption of marijuana during pregnancy was correlated with many negative effects, such as poor concentration, short temper, defalcation during learning and memorizing things, and insufficiency while performing administrative functions (Ryan et al., 2018). Furthermore, another study on marijuana smoking revealed that it does not affect the IQ level of the person, but is found to have a negative effect if used in the prenatal stage, such as deficient in performing administrative functions, persuading the integration of skills to solve the problem in different situations, which requires visual integration, psychoanalysis and persistent concentration (Grant et al., 2018). A study conducted on rabbits revealed that marijuana had embryotoxic effects, not teratogenic effect on plasma compared to the results found in human females. Certain studies have also affirmed that exposure to marijuana affects not only the mother, but also her breast milk, providing high concentrations of Cannabis compounds to the infant (Orsolini et al., 2017).
7.8. Modulation of the neuroprotective mechanism after exposure to marijuana
Scientists have acquired knowledge and learned about the mechanism of action of THC and how it affects the normal functioning of the brain and induces other effects. When marijuana is smoked, THC is synthesized that moves to the lungs, from where it enters the bloodstream and is transferred throughout the body, including the brain. THC generally binds to a certain specific region of the brain, named cannabinoid receptors that trigger the cascade reaction that finally induces the ‘high’ sensation, which is usually experienced by marijuana smokers. The number of cannabinoid receptors varies according to the region of the brain. Cannabinoid receptors are found in a large number in that region of the brain, which controls thinking, memorizing, focusing, sensing, and coordination during movement and induces pleasure (Alger, 2013). It is not uncommon that marijuana intoxication leads to indistinct opinions, poor coordination, and inconvenience while thinking and solving a problem, and difficulty while acquiring and memorizing things. Researchers have claimed that the effect of marijuana in chronic smokers can last for a few days to a week, after which the adverse effect of it fades (Dierker et al., 2018, Pillersdorf, 2018). Thus, chronic smokers work at an inadequate intelligence level most of the time. Research has also stated that if marijuana is smoked for a longer period, it will cause alterations in the brain and show similar effects as seen with chronic use of different major drugs. For example, when cannabinoid was not injected into animals, which were previously exposed to a chronic dose, it caused an increase in the level of activation of the acute stress response system and caused a certain variation among the activities of nerve cells, which comprise dopamine (Oberbarnscheidt et al., 2017). Dopamine neurons are generally regulated in response to a motivated and rewarded signal, which is also affected by drug abuse directly or indirectly. Marijuana intrudes the central nervous system by adhering to neurons receptors present in the brain and intervening in normal interactions between neurons. The changed initial behaviour is observed as a response of these nerves (Wise and Robble, 2020). For example, if a nerve is assigned to retrieve information from short-term memory, cannabinoid interactions with its receptors impose them to perform the opposite functions. Therefore, it is a big problem for the person who is ‘high’ on smoking marijuana to recall what he has done in the past 5 min (Zehra et al., 2018).
Marijuana has a negative effect on emotions, as in one instance the person is happy and in another, the person starts acting paranoid. These immediate changes in emotions are associated with the interaction of THC with the limbic system of the brain. The limbic system is the part of the brain that regulates the emotion and behaviour of a person (Phan et al., 2008). Cannabis is traditionally inhaled as nicotine, thus having rapid entry into the circulatory system. Generally, drugs and their metabolites are fat-soluble (mean lipophilic), which facilitates their movement through the blood–brain barrier that regulates the movement of different constituents of the brain. When Cannabis is metabolized in the lungs and liver into THC, it is transferred to lipid-rich regions of the body, including the brain (Cox et al., 2019). Cannabidiol-induced neuroprotection properties were also found on β-amyloid peptide-triggered damage triggered by -amyloid peptide in cultured rat PC12 cells and also regulates microglial cell function in vitro. Learning a spatial navigation task and gene expression of TNF-α and IL-6 was also prevented in mice injected with β-amyloid (Abate et al., 2021). Chronic use of marijuana causes impairment in normal brain functioning. Various studies have revealed that chronic use will lead to obvious mutilation, such as declination in active memory, abnormalities in locomotor action, distorted sense of time, obsessive nature, broken thoughts, and feeling fatigued.
7.9. Impact of marijuana on memory and cognition
Marijuana adversely affects short-term memory by affecting the normal functionality of the hippocampus (Gar et al., 2020). A report has been surveyed, documented, and published in India and discussed short-term memory mutilation among chronic marijuana smokers. Mutilation is evaluated by the concentration ability of the subject. Marijuana has been found to cause inebriation that leads to a shortfall in spatial learning assignment, induces impediments in task matching and non-matching, and the exiguous follow-up of rodents was assessed in the radial arm maze test (Biswas et al., 2017, Dager et al., 2018). Furthermore, an extremely supervised analysis was carried out on middle-aged adolescents in America. Those who consumed marijuana resulted in short-term memory mutilation. These types of memory deficiency are illustrated when there is an injury in the hippocampus (Gar et al., 2020). Another study revealed that THC gets attached to the receptors of the hippocampus and other areas of the brain that are assumed to be associated with storing memory (Blest-Hopley et al., 2020, Pezdek et al., 2020). Willford et al. (2021) conducted a study to evaluate the effect of early and/or current marijuana use on young adult memory to check control for prenatal exposure to marijuana use. It was concluded that young adults before age 15 were at increased risk of memory deficits after starting marijuana use. Young adult memory function via childhood memory deficits and early initiation of marijuana were indirectly predicted by first- trimester marijuana exposure.
7.10. Endocannabinoid versus endocrine system: Possible contribution of marijuana
It is well established that sex hormones in both males and females are administered by the pituitary, whereas the hypothalamus plays an influential role. Hypothalamic cells secrete a gonadotropin-releasing hormone (GnRH), due to the impact of various constituents such as catecholamines, corticotropin-releasing hormone (CRH), neuropeptide, and prolactin (Emons and Gründker, 2021). GnRH triggers the synthesis of follicle stimulating hormone (FSH) and luteinizing hormone (LH) within the anterior pituitary gland; FSH and LH synthesized in both male and female influence over the gonads, causing the release of testosterone in males and oestrogen and progesterone in females (Bosch et al., 2021). Cannabis constituents such as Δ9-THC, and other cannabinoids have been found to markedly alter hypothalamic-pituitary–gonadal (HPG) stability and hinder reproductive functioning, which is controlled by the hypothalamus under direct influence of GnRH or indirect influence of different modulators (Ketcherside et al., 2016, Vuong et al., 2010). Studies conducted using the acute dose of Δ9-THC in male rodents revealed a significant reduction in the levels of testosterone and gonadotropin hormones. In another study, it was found that THC leads to a 65% reduction in testosterone levels, which lasted for an hour. Therefore, it was concluded that the consequences of chronic exposure are less severe compared to acute administration and can be related to the development of resistance against other drugs (Bovolin et al., 2017, Sharma and Balhara, 2018). In experiments conducted on different animals for the study, the effect of acute dose of cannabinoids showed an increase in ACTH and GC levels with respect to the dose of cannabinoids and showed a similar effect as perceived by the increase in the CRH level (Franks et al., 2020). Cannabinoids have been discovered to stimulate the secretion of somatostatin, which further causes inhibition of GH secretion. In a clinical trial, THC injection in rats showed a severe declination in GH level (Al-Massadi et al., 2010). The impact of cannabinoids on the endocrine system was observed when the extract of Cannabis exhibited a decrease in the level of iodine aggregation in the thyroid of rats. In a study conducted in rodents, administration of an acute dose of THC led to a decrease in thyroxine and TSH levels by 90% and was persistent for 6 h (Haney, 2007). Plasma cortisol levels are increased by intravenous treatment with delta-9-THC in a dose-dependent manner in both controls and Cannabis-dependent individuals. However, a blunted response was observed in frequent Cannabis users compared to healthy controls (Sharma and Balhara, 2018). Ovulation is allowed through the presence of high intrafollicular levels of endocannabinoids, i.e. N-arachidonoylethanolamine (AEA), while the level of plasma and intrauterine must be reduced to allow the establishment of a fertilized oocyte, demonstrating that AEA was involved in the maturation of the follicles and the oocyte. Processes such as ovulation can be altered, and activity also changes not only at the hypothalamic level but also at the level of ovarian granulosa cells, due to the presence of excessive levels of cannabinoids (O’Llenecia et al., 2019).
8. Critical assessment of the study: Overview of the adverse effects of marijuana and the possible therapeutic promise
Two bioactive compounds of the Cannabis genus have been extensively studied in connection with the human system, cannabidiol (CBD) and tetrahydrocannabinol (THC). However, in the case of marijuana, the possible medical attributions and the profound hazards and implications of THC are immense. THC is one of the highly effective psychoactive compounds that affect neurotransmitter release from the brain; thus, it hampers neuronal signaling, promotes a higher sense of euphoria and long-term exposure or ‘drug relapse syndrome’ is connected with numerous psychiatric adversities, including structural alteration of the brain and a decrease in intelligence quotient (IQ) (Moore et al., 2007, Bhattacharyya et al., 2010, Meier et al., 2012, Leweke et al., 2012). However, regulated clinical trials have shown the beneficial effects of THC where it acts primarily as a pain reliever in patients with chemotherapy or neuropathic diseases. Profound positive effects were documented in the reduction of nausea, insomnia, sleep apnea, and appetite recovery. Many reports indicated its possible role against multiple sclerosis, glaucoma, posttraumatic stress disorder, Alzheimer’s disease, etc. However, there is a conflict in medicalization (behavioral or therapeutic studies) of the entire marijuana plant and its main active compound Δ9-tetrahydrocannabinol or THC (Wilkinson et al., 2016). The conversion of status from an investigational drug to a legalized drug has inferred a wrong message about its medicinal use and substantially accelerated its prevalence. According to a report, an almost 65% increase in marijuana intake was recorded among adolescents since its legalization, which is truly worrying (Rocky Mountain HIDTA, 2015). However, the role of therapeutic compounds including the chemical class of cannabinoids, cannabinols, cannabidiol, cannabitriol, cannabicyclol, cannabichromene, cannabielsoin or other miscellaneous phytoconstituents could only be authorized after conducting ‘randomized, double-blind, placebo and active-controlled clinical trials’ after detection, isolation and analytical quantification (Elsohly and Slade, 2005, Wilkinson et al., 2016). Various modulatory roles within the gastrointestinal tract have been exhibited by the therapeutic use of nonpsychotropic phytocannabinoids in inflammatory bowel diseases (IBD) by altering the performance of the local immune system, that is, affecting cytokine, immunoglobulin production, and immune cell migration (Martínez et al., 2020).
Interestingly, placebo-controlled, within-subject studies have revealed that the application of a cannabinoid agonist, oral THC, can alleviate marijuana dependence in humans when investigated with a mood stabilizer, divalproex, as the latter subjectively worsened cognitive performance and elicited negative emotions, namely irritation, anxiety, depression, fatigue, etc. (Haney et al., 2004). Furthermore, combinatorial drug treatment composed of lofexidine and THC warranted a promising result in marijuana withdrawal (Haney et al., 2008). Marijuana’s health hazards have been investigated for a long time and its legalization has triggered strong conservative attitudes against plant and plant-based products among psychiatrists and professionals related to the field of public health and medicine. Although there are some studies that noted that the controlled application of marijuana might be positively attributed in the case of humans, there are many conflicts over the issue. Marijuana is mainly ingested as smoke, food additives based on brew-tea oil or in the form of hashish (Volkow et al., 2014a). Interestingly, the latter itself is the origin of the word ‘assassin’, which is self-explanatory to assume the role marijuana could play for human subjects; especially among young people. Dependence or addiction is the first fatal effect of marijuana where withdrawal, as well as recovery, takes longer. The active ingredient, THC, as mentioned earlier, could modulate the dynamics of the cytoskeleton, possibly alter awareness, reduce the volume of the hippocampus, and cause neurotransmitter malfunction, as well as mental illness, which could have devastating consequences during the formative days of young adults in terms of education and career advancement (Batalla et al., 2013). In addition to sudden death due to accidents (lack of coordination of motor function coordination), suicide (cognitive impairment), or chronic depression, it can cause the onset of chronic bronchitis and other forms of marijuana intoxication leading to cancer (Thomas et al., 2014). The study of cell surface receptors and the THC binding pattern with CB1 and CB2 in and out of the nervous system has revealed the importance of preventing atherosclerosis. In some cases, THC in some instances could prevent the onset of heart attack, but in vivo animal models and in vitro human cell studies have clearly indicated that it could suppress protective cytokines to increase the production of immunosuppressive cytokines, which destroy natural immunity against microbial infection and cancer development (Klein et al., 2003). However, during smoking, THC increases pulse rate, causes a rapid rise and fall in blood pressure, increases the level of carboxyhaemoglobin that delimits oxygen supply through blood vessels, and could effectively stage the perfect state for a deadly heart attack (Roth, 2005). However, reports have suggested that the use of marijuana is not associated with the progression of advanced liver fibrosis in immunosuppressed patients (HIV-hepatitis C co-infected) (Kelly et al., 2016) and may show an inverse effect in patients with nonalcoholic fatty liver disease (Kim et al., 2017). In the case of lung function, quite naturally, the results are disturbing as long-term marijuana smoking causes serious lung injury and triggers chronic obstructive lung disease (Tashkin, 2005, Tan et al., 2009, Tashkin, 2013). Many epidemiological period-wise cohort studies on populations have established a clear association between marijuana smoking and lung cancer (Mehra et al., 2006, Callaghan et al., 2013); lung and upper aerodigestive tract cancers (Hashibe et al., 2006); head and neck cancer (Hashibe et al., 2002); cancer and formation of testicular germ cell tumors (Daling et al., 2009, Lacson et al., 2012) etc. According to reports, parental marijuana use may increase the risk of childhood nonlymphoblastic acute myeloid leukemia (Robison et al., 1989, Trivers et al., 2006). The progression of cancer and respiratory tract diseases is the result of cannabinoid-induced immune suppression that causes antigen modulation. Cannabinoid ligands have represented an agonist-antagonist framework and their immunomodulatory effects are still a subject of future research, but the present data have provided some insight that the ‘context-dependent targeted therapeutic approach’ of marijuana may prove beneficial for the treatment of inflammatory diseases (Tanasescu and Constantinescu, 2010). Zumbrun et al. suggested that epigenetic regulation could affect immunological function, as well as prenatal deaths in offspring of parents under long-term marijuana abuse (Zumbrun et al., 2015). Furthermore, the disrupted endocannabinoid system, which is the result of long-term smoking of marijuana, negatively affects the reproductive potential of male candidates, as it alters the hypothalamus-pituitary-gonadal axis, which is coordinated with the process of spermatogenesis. Furthermore, it disrupts sperm capacitation, acrosome reaction, and motility (du Plessis et al., 2015). Again, two contradictory reports have been published very recently on the effect of marijuana on male fertility. In one experiment, participants with substantial exposure to marijuana were detected with loss of sperm motility and sperm morphology leading to infertility (Carroll et al., 2020), while Nassan et al. reported higher sperm count and lower serum FSH concentration in men with present and past exposure to marijuana (Nassan et al., 2019). Such outcomes may again trigger some deleterious choices among youth if the true nature of marijuana exposure is not validated with urgency.
The accelerated popularity of marijuana among young people is highly detrimental to the structure of society, as young people recognize the health outcome of adulthood and the healthy progeny afterward. Therefore, the onset of gross neurocognitive deficits at a younger age would affect not only the individual but also his or her future social relationships and population health. The process of neuromaturation is highly linked with skill, coordination, cognition, alertness, self-acceptance, decision-making, and overall social behaviour, which occur during adolescence and adulthood. Thus, early exposure to marijuana followed by incapacity to cause withdrawal, behavioral problems, impulsive choice making, aggressiveness, and frequent mood swings (Wijaya, 2021), poor attention and school performance, cognitive inhibition, structural changes in brain configuration, altered brain tissue composition, altered brain glucose metabolism, changes in regional cerebral blood flow (rCBF), lower IQ, lower self-esteem, acute energy depletion, loss of appetite, visual disturbances, the onset of mental retardation, and problems with learning and memory (Matochik et al., 2005, O'Leary et al., 2007, Lisdahl et al., 2013, Filbey et al., 2014). Volkow et al. suggested that decreased dopamine sensation reactivity in the brains of marijuana smokers is completely related to their pattern of negative emotion and the severity of drug addiction (Volkow et al., 2014a). In fact, numerous studies have been conducted to understand the role of marijuana in brain development, function, and cognitive stimulation. Filbey et al., 2014, reported the combined and independent effects of the CNR1 and FAAH genes on neuronal function after exposure to marijuana. Furthermore, marijuana use was reported with comorbidities along with attention deficit hyperactivity disorder (ADHD) or schizophrenia where gene polymorphisms of cannabinoid receptor 1 and/or interaction of MAPK14 and CNR1 gene variants affected cognitive deficits (Ho et al., 2011, Onwuameze et al., 2013). Despite the odds, the application of medical marijuana to selected neurological disorders has attracted the attention of researchers around the world (Koppel et al., 2014, Wilkinson et al., 2016, Kim et al., 2019). Therefore, the takeaway message regarding the use of marijuana manifests the use of medical marijuana as a psychotherapeutic drug, under controlled setup, and lower dosage may induce the occurrence of numerous neurodegenerative disorders as well as other physiological anomalies if ingested in an uncontrolled, regular pattern from a very early age.
It is necessary to understand that specific phytoconstituents of marijuana may have some therapeutic potential to alleviate certain neurodegenerative disorders or other physiological ailments, but all should be subjected to rigorous clinical investigation based on evidence, as mainly the whole marijuana plant is ingested as hallucinating drugs to feel ‘high’. Such regulatory trials are important for its primary compound THC, which may alleviate some debilitating medical complications (Volkow et al., 2014b). In no case should medical practitioners, drug dealers, marijuana-exposed youth and adults or regulatory authorities undermine the fact that both short- and long-term exposure to marijuana has its own share of adverse effects, which are scientifically validated both in animal and human subjects. Another cautionary note is that smoking marijuana causes persistent functional and structural changes in the brain, which could jeopardize the social, educational and professional achievements of an individual in a fatal way (Memedovich et al., 2018). Legalization of marijuana for medical purposes should be strictly controlled and its widespread use in young people and adults must be checked in a sensitive manner to maintain social harmony and negative impact on the population and individual’s progeny.
9. Marijuana withdrawal: Treatment, observation, and bottlenecks
The first problem that arises with statistical analysis of clinical trials focused on marijuana withdrawal syndrome and treatment is the experimental design. In both randomized controlled and non-controlled trials, the preparation of the data set is always complicated due to multifactorial influences (McRae et al., 2003). Nature, age, sex, duration of exposure to marijuana, symptomatic frequency, and severity of dependence, along with epidemiological data, must be assessed. Often, participants have declared exposure to other types of drugs and nicotine and the variability of symptoms is quite high among heavy and light users. However, the marijuana treatment project has highlighted intermingled parameters and their cumulative effect on participants (McRae et al., 2003). In the majority cases, antidepressants and anxiolytic medications are applied, but customized treatment is actually recommended. Marijuana dependence is often kept secret by most adults and adolescents, and the practice of self-medication or other drug abuse may play a significant role in that process (Haney,2005). Regular follow-up, exercise, and intake of nutritious foods often provide combinatorial treatment along with psychological counseling. However, most clinical studies have not taken into account factors such as genetic predisposition, ethnic diversity, mode of treatment, and socioeconomical background of participants, which can create data discrepancy. However, public awareness and thoughtful acceptance of marijuana legalization cannot be regulated only by data interpretation. The problems of marijuana addiction, the emergence of mental health problems, lower rate of participation in long-term treatment, and frequent relapse of the signature syndrome (irritability, sleeping problems, tension, loss of appetite, fatigue, and cravings) could only be alleviated with proper collateral participation of common people, medical practitioners, and government agencies (Budney et al., 2007).
10. Prospects and preventive measures
The internal cardiac cannabinoid system was found to be associated with various incidents in which it exhibited cardioprotective effects. Cannabinoids have also been found to have the ability to reduce heart size, which is confirmed in vivo in anesthetized mice. Certain reports have suggested and even shown the effective result of marijuana in reducing different types of pain, controlling nausea, puking, and in soothing the symptoms caused by diseases such as cancer, HIV, and disseminated sclerosis in contrast to the harsh drugs used for treating these diseases. The usage of marijuana is found to be quite safe and less lethal than other drugs prescribed daily by doctors. Oral THC is recommended to AIDS victims to increase their appetite to maintain body mass. Scientists are exploring THC and other cannabinoids present in marijuana, which has medicinal applications. In many cases, it has proven to be a very good pain reliever with not much negative effect if the dose is taken appropriately. In 1997, 6059 articles related to Cannabis published in the medical literature exhibited 194 titles related to the properties of promethazine, 56 related to acute glaucoma, 10 related to disseminated sclerosis, 23 related to appetite, and 11 related to relief or terminal aid. Smoking marijuana has been associated with various consequences, which are certainly not effective in alleviating the symptoms of any particular disease among the large number of patients. THC, the active constituent of Cannabis, has been found to be beneficial for sufferers, those who have severe pain, exclusively in fractious cases of disseminated sclerosis and sufferers of AIDS-related wasting syndrome. Among these diseases, few patients take advantage and infer the benefit of being free from undesirable consequences.
11. Conclusions
With a thorough evaluation of the literature in context of the effects of Cannabis on animals and its association with many toxic chemicals, which have an adverse effect on both humans and animals, a large number of studies denied support for the term ‘medical marijuana’. Smoking of marijuana is associated with severe side effects for which it could not be used for the treatment of particular diseases among outnumbered patients. Even with the role of the active compound, THC from marijuana cannot be neglected in patients who experience severe pain, particularly in patients suffering from multiple sclerosis. However, it does not deny the destructive effect of extensive marijuana consumption on brain signaling that is retarded in the area influenced by THC. However, for proper functioning, other regions of the brain amend abnormalities and loss, which are triggered by long-term use of Cannabis regardless of the age of the user. The defect attributed to marijuana consumption will be noticed in short- and long-term memory loss or in association with other mental illnesses. In addition to the effects on the brain, marijuana also increases the chances of respiratory cancer or may exhibit adverse consequences in certain consumers. In addition to drug abuse and dependence, the main concern is the side effects induced on the heart, hormonal system, immune system, and cerebral functions. It has been found that the consumption of marijuana in pregnancy leads to a lower intellectual ability of the child, in addition to increasing the chances of mental illness. In the analysis of the overall scenario, marijuana is not considered safe for consumption even if a physician prescribes it. In younger generations, there is an increasing craze to smoke marijuana and many take it vigorously under peer pressure. This affects their overall lives and puts their future at stake. Various scientific reports have denied that marijuana is safe and that it could be smoked for entertainment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abhijit Dey, Email: abhijit.dbs@presiuniv.ac.in.
Jarosław Proćków, Email: jaroslaw.prockow@upwr.edu.pl.
Joginder Singh, Email: joginder.15005@lpu.co.in.
References
- Abate G., Uberti D., Tambaro S. Potential and Limits of Cannabinoids in Alzheimer’s Disease Therapy. Biology. 2021;10(6):542. doi: 10.3390/biology10060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah S.J., Smith B.M., Ware M.A., Moore M., Zhi Li P., Bourbeau J., Jensen D., Edwards A. Effect of Vaporized Cannabis on Exertional Breathlessness and Exercise Endurance in Advanced Chronic Obstructive Pulmonary Disease A Randomized Controlled Trial. Ann. Am. Thoracic Soc. 2018;15(10):1146–1158. doi: 10.1513/AnnalsATS.201803-198OC. [DOI] [PubMed] [Google Scholar]
- Adams R., Pease D.C., Clark J.H. Isolation of Cannabinol, Cannabidiol and Quebrachitol from Red Oil of Minnesota Wild Hemp. J. Am. Chem. Soc. 1940;62(8):2194–2196. doi: 10.1021/ja01865a080. [DOI] [Google Scholar]
- Ahmad F., Abbas T., Farman K., Akrem A., Asif M., Saleem M.U.I., Baloch F.S., Mahmood S. High-throughput phytochemical characterization of non-cannabinoid compounds of Cannabis plant and seed, from Pakistan. Pak. J. Bot. 2018;50(2):639–643. [Google Scholar]
- Ahmed S.A., Ross S.A., Slade D., Radwan M.M., Khan I.A., ElSohly M.A. Structure determination and absolute configuration of cannabichromanone derivatives from high potency Cannabis sativa. Tetrahedron Lett. 2008;49(42):6050–6053. doi: 10.1016/j.tetlet.2008.07.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.A., Ross S.A., Slade D., Radwan M.M., Zulfiqar F., ElSohly M.A. Cannabinoid ester constituents from high-potency Cannabis sativa. J. Nat. Prod. 2008;71(4):536–542. doi: 10.1021/np070454a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizpurua-Olaizola O., Soydaner U., Öztürk E., Schibano D., Simsir Y., Navarro P., Etxebarria N., Usobiaga A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016;79(2):324–331. doi: 10.1021/acs.jnatprod.5b0094910.1021/acs.jnatprod.5b00949.s001. [DOI] [PubMed] [Google Scholar]
- Aldington S., Harwood M., Cox B., Weatherall M., Beckert L., Hansell A., Pritchard A., Robinson G., Beasley R. Cannabis use and risk of lung cancer: A case-control study. Eur. Respir. J. 2008;31(2):280–286. doi: 10.1183/09031936.00065707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger, B.E., 2013. Getting high on the endocannabinoid system. Cerebrum: The Dana Forum on Brain Science. [PMC free article] [PubMed]
- Al-Massadi O., Gabellieri E., Trujillo M.L., Señaris R., Pagotto U., Pasquali R., Casanueva F.F., Seoane L.M. Peripheral endocannabinoid system-mediated actions of rimonabant on growth hormone secretion are ghrelin-dependent. J. Neuroendocrinol. 2010;22(11):1127–1136. doi: 10.1111/j.1365-2826.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- Alqahtani A., Ammari Z., Ramahi A., Said Ahmed T.S., Klada E. Cannabis Smoking-induced Diffuse Alveolar Hemorrhage. Cureus. 2019;11(7) doi: 10.7759/cureus.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G., Giana A., Gibbons S., Maffei M., Gnavi G., Grassi G., Sterner O. A polar cannabinoid from Cannabis sativa var. carma. Nat. Prod. Commun. 2008;3(12):1977–1980. doi: 10.1177/1934578x0800301207. [DOI] [Google Scholar]
- Asadi S., Moghadam H., Naghdi Badi H., Naghdi Badi H., Naghavi M.R., Salami S.A.R. A review on agronomic, phytochemical and pharmacological aspects of cannabis (Cannabis sativa L.) J. Med. Plants. 2019;2(70):1–20. [Google Scholar]
- Asati A., Sahoo H., Sahu S., Dwivedi A. Phytochemical and pharmacological profile of Cannabis sativa L. Drugs. 2017;2(2):37–45. [Google Scholar]
- Atakan Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Therap. Adv. Psychopharmacol. 2012;2(6):241–254. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Anand U., Ghosh S., Ray D., Ray P., Nandy S., Dewaji Deshmukh G., Tripathi V., Dey A. Bacosides from Bacopa monnieri extract: An overview of the effects on neurological disorders. Phytother. Res. 2021;1–12 doi: 10.1002/ptr.7203. [DOI] [PubMed] [Google Scholar]
- Barak, S., Baker, H., 2016. Clinical Management of Male Infertility. Endotext.
- Basas-Jaumandreu J., De Las Heras F.X.C. GC-MS Metabolite Profile and Identification of Unusual Homologous Cannabinoids in High Potency Cannabis sativa. Planta Med. 2020;86(5):338–347. doi: 10.1055/a-1110-1045. [DOI] [PubMed] [Google Scholar]
- Batalla A., Bhattacharyya S., Yücel M., Fusar-Poli P., Crippa J.A., Nogué S., Torrens M., Pujol J., Farré M., Martin-Santos R., Lu L. Structural and Functional Imaging Studies in Chronic Cannabis Users: A Systematic Review of Adolescent and Adult Findings. European Psychiatry. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, C.R., Lagasse, L.L., 2004. The Maternal Lifestyle Study: Cognitive, Motor, and Behavioral Outcomes of Cocaine-Exposed and Opiate-Exposed Infants Through Three Years of Age. Am Acad Pediatrics, 113(6), 1677–1685. https://doi.org/10.1542/peds.113.6.1677 [DOI] [PubMed]
- Beaulieu P. Toxic effects of cannabis and cannabinoids: Animal data. Pain Res. Manage. 2005;10(suppl a):23A–26A. doi: 10.1155/2005/763623. [DOI] [PubMed] [Google Scholar]
- Benevenuto S.G., Domenico M.D., Martins M.A.G., Costa N.S., de Souza A.R.L., Costa J.L., Tavares M.F.M., Dolhnikoff M., Veras M.M. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: An experimental study in mice. Toxicology. 2017;376:94–101. doi: 10.1016/j.tox.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Bercht C.A.L., Lousberg R.J.J.C., Küppers F.J.E.M., Salemink C.A. Cannabicitran: A new naturally occurring tetracyclic diether from lebanese Cannabis sativa. Phytochemistry. 1974;13(3):619–621. doi: 10.1016/S0031-9422(00)91362-1. [DOI] [Google Scholar]
- Bergamaschi M.M., Queiroz R.H.C., Chagas M.H.N., De Oliveira D.C.G., De Martinis B.S., Kapczinski F., Crippa J.A.S. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N., Gorelick J., Koch S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.) Ind. Crops Prod. 2019;129:185–194. doi: 10.1016/j.indcrop.2018.11.039. [DOI] [Google Scholar]
- Bhattacharyya S., Morrison P.D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., Nosarti C., O' Carroll C.M., Seal M., Allen P., Mehta M.A., Stone J.M., Tunstall N., Giampietro V., Kapur S., Murray R.M., Zuardi A.W., Crippa J.A., Atakan Z., McGuire P.K. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl J.R., Burnham E.L. Cannabis smoking in 2015: A concern for lung health? Chest. 2015;148(3):596–606. doi: 10.1378/chest.15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D.F., Hendrich S., Wang W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001;90(2-3):157–177. doi: 10.1016/S0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Biswas P., Mishra P., Bose D., Durgbanshi A. Cannabis: A Neurological Remedy or a Drug of Abuse in India. CNS & Neurological Disorders - Drug. Targets. 2017;16(5) doi: 10.2174/1871527316666170424115008. [DOI] [PubMed] [Google Scholar]
- Blest-Hopley G., Giampietro V., Bhattacharyya S. A Systematic Review of Human Neuroimaging Evidence of Memory-Related Functional Alterations Associated with Cannabis Use Complemented with Preclinical and Human Evidence of Memory Performance Alterations. Brain Sciences. 2020;10(2):102. doi: 10.3390/brainsci10020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs D.L., Surti T., Gupta A., Gupta S., Niciu M., Pittman B., Schnakenberg Martin A.M., Thurnauer H., Davies A., D’Souza D.C., Ranganathan M. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology. 2018;235(7):1923–1932. doi: 10.1007/s00213-018-4885-9. [DOI] [PubMed] [Google Scholar]
- Booth J.K., Bohlmann J. Terpenes in Cannabis sativa – From plant genome to humans. Plant Sci. 2019;284:67–72. doi: 10.1016/j.plantsci.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Bosch, E., Alviggi, C., Lispi, M., Conforti, A., Hanyaloglu, A.C., Chuderland, D., Simoni, M., Raine-Fenning, N., Crépieux, P., Kol, S., Rochira, V., 2021. Reduced FSH and LH action: implications for medically assisted reproduction. Human Reproduct. 36(6), 1469-1480. https://doi.org/10.1093/humrep/deab065 [DOI] [PMC free article] [PubMed]
- Bourjot M., Zedet A., Demange B., Pudlo M., Girard-Thernier C. In Vitro Mammalian Arginase Inhibitory and Antioxidant Effects of Amide Derivatives Isolated from the Hempseed Cakes (Cannabis sativa) Planta Medica Int. Open. 2017;3(3):64–67. doi: 10.1055/s-0042-119400. [DOI] [Google Scholar]
- Bovolin P., D’Atri I., Cottone E., Pomatto V., Zuccarini G., Gothilf Y., Merlo G.R. Exposure to cannabinoid receptor 1 ligands induces miswiring of GnRH axons in the brain of zebrafish embryos. Reprod. Toxicol. 2017;72:33. doi: 10.1016/j.reprotox.2017.06.144. [DOI] [Google Scholar]
- Brand E.J., Zhao Z. Cannabis in Chinese medicine: Are some traditional indications referenced in ancient literature related to cannabinoids? Front. Pharmacol. 2017;8:108. doi: 10.3389/fphar.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breijyeh Z., Jubeh B., Bufo S.A., Karaman R., Scrano L. Cannabis: A Toxin-Producing Plant with Potential Therapeutic Uses. Toxins. 2021;13(2):117. doi: 10.3390/toxins13020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman M.B., Abazia D.T. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. Pharm. Therap. 2017;42(3):180. [PMC free article] [PubMed] [Google Scholar]
- Budney A., Roffman R., Stephens R., Walker D. Marijuana dependence and its treatment. Add. Sci. Clin. Pract. 2007;4(1):4–16. doi: 10.1151/ascp10.1151/ascp074110.1151/ASCP07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral, G.A., Staab, A., 2005. Effects on the immune system. Cannabinoids, 385–423. https://doi.org/10.1007/3-540-26573-2_13. [DOI] [PubMed]
- Callaghan R.C., Allebeck P., Sidorchuk A. Marijuana use and risk of lung cancer: A 40-year cohort study. Cancer Causes Control. 2013;24(10):1811–1820. doi: 10.1007/s10552-013-0259-0. [DOI] [PubMed] [Google Scholar]
- Calvi L., Pentimalli D., Panseri S., Giupponi L., Gelmini F., Beretta G., Vitali D., Bruno M., Zilio E., Pavlovic R., Giorgi A. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018;150:208–219. doi: 10.1016/j.jpba.2017.11.073. [DOI] [PubMed] [Google Scholar]
- Carroll K., Pottinger A.M., Wynter S., DaCosta V. Marijuana use and its influence on sperm morphology and motility: identified risk for fertility among Jamaican men. Andrology. 2020;8(1):136–142. doi: 10.1111/andr.v8.110.1111/andr.12670. [DOI] [PubMed] [Google Scholar]
- Chase P.B., Hawkins J., Mosier J., Jimenez E., Boesen K., Logan B.K., Walter F.G. Differential physiological and behavioral cues observed in individuals smoking botanical marijuana versus synthetic cannabinoid drugs. Clin. Toxicol. 2016;54(1):14–19. doi: 10.3109/15563650.2015.1101769. [DOI] [PubMed] [Google Scholar]
- Citti C., Battisti U.M., Braghiroli D., Ciccarella G., Schmid M., Vandelli M.A., Cannazza G. A Metabolomic Approach Applied to a Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry Method (HPLC-ESI-HRMS/MS): Towards the Comprehensive Evaluation of the Chemical Composition of Cannabis Medicinal Extracts. Phytochem. Anal. 2018;29(2):144–155. doi: 10.1002/pca.v29.210.1002/pca.2722. [DOI] [PubMed] [Google Scholar]
- Citti C., Linciano P., Russo F., Luongo L., Iannotta M., Maione S., Laganà A., Capriotti A.L., Forni F., Vandelli M.A., Gigli G., Cannazza G. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-56785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R.C., Merlin M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. Crit. Rev. Plant Sci. 2016;35(5–6):293–327. doi: 10.1080/07352689.2016.1267498. [DOI] [Google Scholar]
- Colizzi M., McGuire P., Giampietro V., Williams S., Brammer M., Bhattacharyya S. Modulation of acute effects of delta-9-tetrahydrocannabinol on psychotomimetic effects, cognition and brain function by previous cannabis exposure. Eur. Neuropsychopharmacol. 2018;28(7):850–862. doi: 10.1016/j.euroneuro.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Colizzi M., McGuire P., Giampietro V., Williams S., Brammer M., Bhattacharyya S. Previous cannabis exposure modulates the acute effects of delta-9-tetrahydrocannabinol on attentional salience and fear processing. Exp. Clin. Psychopharmacol. 2018;26(6):582–598. doi: 10.1037/pha0000221. [DOI] [PubMed] [Google Scholar]
- Cooper R.E., Williams E., Seegobin S., Tye C., Kuntsi J., Asherson P. Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial. Eur. Neuropsychopharmacol. 2017;27(8):795–808. doi: 10.1016/j.euroneuro.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Cooper Z.D., Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103(3):107–113. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E.J., Maharao N., Patilea-Vrana G., Unadkat J.D., Rettie A.E., McCune J.S., Paine M.F. A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 2019;201:25–38. doi: 10.1016/j.pharmthera.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa J.A.S., Derenusson G.N., Ferrari T.B., Wichert-Ana L., Duran F.LS., Martin-Santos R., Simões M.V., Bhattacharyya S., Fusar-Poli P., Atakan Z., Filho A.S., Freitas-Ferrari M.C., McGuire P.K., Zuardi A.W., Busatto G.F., Hallak J.E.C. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011;25(1):121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- Dager A.D., Tice M.R., Book G.A., Tennen H., Raskin S.A., Austad C.S., Wood R.M., Fallahi C.R., Hawkins K.A., Pearlson G.D. Relationship between fMRI response during a nonverbal memory task and marijuana use in college students. Drug Alcohol Depend. 2018;188:71–78. doi: 10.1016/j.drugalcdep.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daling J.R., Doody D.R., Sun X., Trabert B.L., Weiss N.S., Chen C., Biggs M.L., Starr J.R., Dey S.K., Schwartz S.M. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115(6):1215–1223. doi: 10.1002/cncr.v115:610.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot P.M., Wu C.C., Carter B.W., Munden R.F. The epidemiology of lung cancer. Trans. Lung Cancer Res. 2018;7(3):220–233. doi: 10.21037/tlcr.2018.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis E.M., Bajaj N.S., Singh A., Malloy R., Givertz M.M., Blankstein R., Bhatt D.L., Vaduganathan M. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2020;75(3):320–332. doi: 10.1016/j.jacc.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi, M., Kane, S., Potlakayala, S., George, H., Proano, R., Sheri, V., Curtis, W.R., Rudrabhatla, S., 2020. Metabolic Engineering Strategies of Industrial Hemp (Cannabis sativa L.): A Brief Review of the Advances and Challenges. Front. Plant Sci. 11.10.3389/fpls.2020.580621 [DOI] [PMC free article] [PubMed]
- Dierker L., Selya A., Lanza S., Li R., Rose J. Depression and marijuana use disorder symptoms among current marijuana users. Addict. Behav. 2018;76:161–168. doi: 10.1016/j.addbeh.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, E.P.B., 2018. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Wiley Online Library, 58(7). https://doi.org/10.1111/head.13345. [DOI] [PubMed]
- du Plessis S.S., Agarwal A., Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015;32(11):1575–1588. doi: 10.1007/s10815-015-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovis M., Muneyyirci-Delale O. Effects of marijuana on human reproduction. Reprod. Toxicol. 2020;94:22–30. doi: 10.1016/j.reprotox.2020.04.071. [DOI] [PubMed] [Google Scholar]
- El Marroun H., Brown Q.L., Lund I.O., Coleman-Cowger V.H., Loree A.M., Chawla D., Washio Y. An epidemiological, developmental and clinical overview of cannabis use during pregnancy. Prev. Med. 2018;116:1–5. doi: 10.1016/j.ypmed.2018.08.036. [DOI] [PubMed] [Google Scholar]
- Ellickson P.L., D’Amico E.J., Collins R.L., Klein D.J. Marijuana use and later problems: When frequency of recent use explains age of initiation effects (and when it does not) Subst. Use Misuse. 2005;40(3):343–359. doi: 10.1081/JA-200049356. [DOI] [PubMed] [Google Scholar]
- Elms L., Shannon S., Hughes S., Lewis N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complement. Med. 2019;25(4):392–397. doi: 10.1089/acm.2018.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohly M.A., Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Elsevier; 2005. pp. 539–548. [DOI] [PubMed] [Google Scholar]
- Emons G., Gründker C. The Role of Gonadotropin-Releasing Hormone (GnRH) in Endometrial Cancer. Cells. 2021;10(2):292.. doi: 10.3390/cells10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing L.E., Skinner C.M., Quick C.M., Kennon-McGill S., McGill M.R., Walker L.A., ElSohly M.A., Gurley B.J., Koturbash I. Hepatotoxicity of a cannabidiol-rich cannabis extract in the mouse model. Molecules. 2019;24(9):1694.. doi: 10.3390/molecules24091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood L.J., Northstone K. Maternal use of cannabis and pregnancy outcome. BJOG: Int. J. Obstetrics Gynaecol. 2002;109(1):21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., Aslan S., Calhoun V.D., Spence J.S., Damaraju E., Caprihan A., Segall J. Long-term effects of marijuana use on the brain. Nat. Acad. Sci. 2014;111(47):16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn D.P., Haroutounian S., Hohmann A.G., Krane E., Soliman N., Rice A.S. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain. 2021;162:S5–S25. doi: 10.1097/j.pain.0000000000002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischedick J.T., Glas R., Hazekamp A., Verpoorte R. A qualitative and quantitative HPTLC densitometry method for the analysis of cannabinoids in Cannabis sativa L. Phytochem. Anal. 2009;20(5):421–426. doi: 10.1002/pca.v20:510.1002/pca.1143. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, K.T., Bronstein, A.C., Newquist, K.L., 2013. Topical Review Marijuana Poisoning. Elsevier, 28(1), 8–12. https://doi.org/10.1053/j.tcam.2013.03.004. [DOI] [PubMed]
- Franks A.L., Berry K.J., DeFranco D.B. Prenatal drug exposure and neurodevelopmental programming of glucocorticoid signalling. J. Neuroendocrinol. 2020;32(1) doi: 10.1111/jne.v32.110.1111/jne.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassinetti S., Moccia E., Caltavuturo L., Gabriele M., Longo V., Bellani L., Giorgi G., Giorgetti L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018;262:56–66. doi: 10.1016/j.foodchem.2018.04.078. [DOI] [PubMed] [Google Scholar]
- Gaoni Y., Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964;86(8):1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- Gar A., Alluri V., Garimella A., Rajguru S., Singla U. Marijuana and the hippocampus: A longitudinal study on the effects of marijuana on hippocampal subfields. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;101 doi: 10.1016/j.pnpbp.2020.109897. [DOI] [PubMed] [Google Scholar]
- Gilbert M.T., Sulik K.K., Fish E.W., Baker L.K., Dehart D.B., Parnell S.E. Dose-dependent teratogenicity of the synthetic cannabinoid CP-55,940 in mice. Neurotoxicol. Teratol. 2016;58:15–22. doi: 10.1016/j.ntt.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogauer, J., Blay, J., 2021. Cannabinoids, their cellular receptors, and effects on the invasive phenotype of carcinoma and metastasis. Cancer Reports, e1475. https://doi.org/10.1002/cnr2.1475. [DOI] [PMC free article] [PubMed]
- Gonçalves E.C., Baldasso G.M., Bicca M.A., Paes R.S., Capasso R., Dutra R.C. Terpenoids, cannabimimetic ligands, beyond the cannabis plant. Molecules. 2020;25(7):1567. doi: 10.3390/molecules25071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal H., Awad H.H., Ghali J.K. Role of cannabis in cardiovascular disorders. J. Thoracic Disease. 2017;9(7):2079–2092. doi: 10.21037/jtd.2017.06.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracie K., Hancox R.J. Cannabis use disorder and the lungs. Addiction. 2020;116(1):182–190. doi: 10.1111/add.v116.110.1111/add.15075. [DOI] [PubMed] [Google Scholar]
- Grant K.S., Petroff R., Isoherranen N., Stella N., Burbacher T.M. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol. Ther. 2018;182:133–151. doi: 10.1016/j.pharmthera.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer G.R., Grob C.S., Halberstadt A.L. PTSD Symptom Reports of Patients Evaluated for the New Mexico Medical Cannabis Program. J. Psychoact. Drugs. 2014;46(1):73–77. doi: 10.1080/02791072.2013.873843. [DOI] [PubMed] [Google Scholar]
- Greineisen W.E., Turner H. Immunoactive effects of cannabinoids: Considerations for the therapeutic use of cannabinoid receptor agonists and antagonists. Int. Immunopharmacol. 2010;10(5):547–555. doi: 10.1016/j.intimp.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza R.A., Bierut L.J. Cigarette smoking and the risk for alcohol use disorders among adolescent drinkers. Alcohol. Clin. Exp. Res. 2006;30(12):2046–2054. doi: 10.1111/j.1530-0277.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J.K.L., Rosales C.B., Center K.E., Nuñez A., Gibson S.J., Christ C., Ehiri J.E. Prenatal exposure to cannabis and maternal and child health outcomes: A systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S., Anand U., Nandy S., Oleksak P., Qusti S., Alshammari E.M., El-Saber Batiha G., Koshy E.P., Dey A. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitives and brain boosters perspectives. Saudi Pharm. J. 2021;29(8):879–907. doi: 10.1016/j.jsps.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak J., Teixeira T.A., Pariz J.R., Iori I., Costa E., Saldiva P.H. MP75-09 Marijuana consumption has a direct deleterious effect on spermatozoa by increasing intracellular reactive oxygen species levels 20 times more than tobacco smoking: reasons for concern on widespread use. J. Urol. 2019;201(4) doi: 10.1097/01.ju.0000557221.22634.47. [DOI] [Google Scholar]
- Hancox R.J., Poulton R., Ely M., Welch D., Taylor D.R., McLachlan C.R., Greene J.M., Moffitt T.E., Caspi A., Sears M.R. Effects of cannabis on lung function: A population-based cohort study. Eur. Respir. J. 2010;35(1):42–47. doi: 10.1183/09031936.00065009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand A., Blake A., Kerrigan P., Samuel P., Friedberg J. History of medical cannabis. J. Pain Manage. 2016;9(4):387–394. [Google Scholar]
- Haney M., Hart C.L., Vosburg S.K., Nasser J., Bennett A., Zubaran C., Foltin R.W. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M., Hart C.L., Vosburg S.K., Comer S.D., Reed S.C., Foltin R.W. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: Diagnosis and treatment. Current Psychiatry Reports. 2005;7(5):360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: Differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32(6):1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Hashibe M., Ford D.E., Zhang Z.-F. Marijuana Smoking and Head and Neck Cancer. J. Clin. Pharmacol. 2002;42(1):103–107. doi: 10.1002/j.1552-4604.2002.tb06010.x. [DOI] [PubMed] [Google Scholar]
- Hashibe M., Morgenstern H., Cui Y., Tashkin D.P., Zhang Z.-F., Cozen W., Mack T.M., Greenland S. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol. Prevent. Biomarkers. 2006;15(10):1829–1834. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- Haverkos H.W., Haverkos G.P., O’Mara M. Co-carcinogenesis: Human papillomaviruses, coal tar derivatives, and squamous cell cervical cancer. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S.S. Progress and challenges in selected areas of tobacco carcinogenesis. Chem. Res. Toxicol. 2008;21(1):160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley C.R., Ford W.R. Cannabinoid pharmacology in the cardiovascular system: Potential protective mechanisms through lipid signalling. Biol. Rev. Camb. Philos. Soc. 2004;79(1):187–205. doi: 10.1017/S1464793103006201. [DOI] [PubMed] [Google Scholar]
- Ho B.C., Wassink T.H., Ziebell S., Andreasen N.C. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr. Res. 2011;128(1–3):66–75. doi: 10.1016/j.schres.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A., Blume L., Dalton G. CB1 Cannabinoid Receptors and their Associated Proteins. Curr. Med. Chem. 2010;17(14):1382–1393. doi: 10.2174/092986710790980023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H.J., Zhang Z.F., Tashkin D.P., Feng B., Straif K., Hashibe M. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol. Prevent. Biomarkers. 2015;24(1):15–31. doi: 10.1158/1055-9965.EPI-14-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis M. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007;4(8):1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti F.A., Pagano E., Moriello A.S., Alvino F.G., Sorrentino N.C., D’Orsi L., Gazzerro E., Capasso R., De Leonibus E., De Petrocellis L., Di Marzo V. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Br. J. Pharmacol. 2019;176(10):1568–1584. doi: 10.1111/bph.14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irakli M., Tsaliki E., Kalivas A., Kleisiaris F., Sarrou E., Cook C.M. Effect of genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants. 2019;8(10):491. doi: 10.3390/antiox8100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie H., Ohno K., Ito Y., Uyeo S. Isolation and Characterization of 10,11-Dihydroatlantone and Related Compounds from Ginkgo biloba L. Chem. Pharm. Bull. 1975;23(8):1892–1894. doi: 10.1248/cpb.23.1892. [DOI] [Google Scholar]
- Isahq M.S., Afridi M.S., Ali J., Hussain M.M., Ahmad S., Kanwal F. Proximate composition, phytochemical screening, GC-MS studies of biologically active cannabinoids and antimicrobial activities of Cannabis indica. Asian Pacific J. Tropical Disease. 2015;5(11):897–902. doi: 10.1016/S2222-1808(15)60953-7. [DOI] [Google Scholar]
- Janer G., Slob W., Hakkert B.C., Vermeire T., Piersma A.H. A retrospective analysis of developmental toxicity studies in rat and rabbit: What is the added value of the rabbit as an additional test species? Regul. Toxicol. Pharm. 2008;50(2):206–217. doi: 10.1016/j.yrtph.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Jett J., Stone E., Warren G., Cummings K.M. Cannabis Use, Lung Cancer, and Related Issues. J. Thoracic Oncol. 2018;13(4):480–487. doi: 10.1016/j.jtho.2017.12.013. [DOI] [PubMed] [Google Scholar]
- Jin D., Jin S., Yu Y., Lee C., Chen J. Classification of Cannabis Cultivars Marketed in Canada for Medical Purposes by Quantification of Cannabinoids and Terpenes Using HPLC-DAD and GC-MS Analytical & Bioanalytical Techniques. J. Analy. Bioanaly. Tech. 2017;8:1. doi: 10.4172/2155-9872.1000349. [DOI] [Google Scholar]
- John W.S., Martin T.J., Solingapuram Sai K.K., Nader S.H., Donald Gage H., Mintz A., Nader M.A. Chronic d9-THC in rhesus monkeys: Effects on cognitive performance and dopamine D2/D3 receptor availability. J. Pharmacol. Exp. Ther. 2018;364(2):300–310. doi: 10.1124/jpet.117.244194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.R., Lossignol D., Burnell-Nugent M., Fallon M.T. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J. Pain Symptom Manage. 2013;46(2):207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Johnson N. Workers’ weed: Cannabis, sugar beets, and landscapes of labor in the American West, 1900–1946. Agric. Hist. 2017;91(3):320–341. doi: 10.3098/ah.2017.091.3.320. [DOI] [Google Scholar]
- Joshi M., Joshi A., Bartter T. Marijuana and lung diseases. CE: Namrta; MCP. 2002;20(2):173–179. doi: 10.1097/MCP.0000000000000026. [DOI] [PubMed] [Google Scholar]
- Jouanjus E., Lapeyre‐Mestre M., Micallef J. Cannabis use: signal of increasing risk of serious cardiovascular disorders. J. Am. Heart Assoc. 2014;3(2) doi: 10.1161/JAHA.113.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z., Goldberg S.R., Heishman S.J., Tanda G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol. Biochem. Behav. 2005;81(2):285–299. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik J., Krejci Z., Santavy F. Cannabis as a medicament. Bull Narc. 1960;12(3):5–23. [Google Scholar]
- Kasman A., Thoma M., McLain A., Eisenberg M. PD09-09 Association between use of marijuana and infertility in men and women: findings from the national survey of family growth. J. Urol. 2018;199(4) doi: 10.1016/j.juro.2018.02.606. [DOI] [PubMed] [Google Scholar]
- Kasman A.M., Giudice F.D., Eisenberg M.L. New insights to guide patient care: the bidirectional relationship between male infertility and male health. Elsevier. 2020;113(3):469–477. doi: 10.1016/j.fertnstert.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Kasman A.M., Thoma M.E., McLain A.C., Eisenberg M.L. Association between use of marijuana and time to pregnancy in men and women: findings from the National Survey of Family Growth. Fertil. Steril. 2018;109(5):866–871. doi: 10.1016/j.fertnstert.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Kelly E.M., Dodge J.L., Sarkar M., French A.L., Tien P.C., Glesby M.J., Golub E.T., Augenbraun M., Plankey M., Peters M.G. Marijuana use is not associated with progression to advanced liver fibrosis in HIV/hepatitis C virus–coinfected women. Rev. Infect. Dis. 2016;63(4):512–518. doi: 10.1093/cid/ciw350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcherside A., Baine J., Filbey F. Sex Effects of Marijuana on Brain Structure and Function. Current Addiction Reports. 2016;3(3):323–331. doi: 10.1007/s40429-016-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Kim W., Kwak M.-S., Chung G.E., Yim J.Y., Ahmed A., Strnad P. Inverse association of marijuana use with nonalcoholic fatty liver disease among adults in the United States. PLoS ONE. 2017;12(10):e0186702. doi: 10.1371/journal.pone.0186702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Yang J.W., Kim K.H., Kim J.U., Yook T.H. A Review on Studies of Marijuana for Alzheimer’s Disease-Focusing on CBD, THC. J. Pharm. 2019;22(4):225. doi: 10.3831/KPI.2019.22.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T.W., Newton C., Larsen K., Lu L., Perkins I., Nong L., Friedman H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003;74(4):486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Koppel B.S., Brust J.C., Fife T., Bronstein J., Youssof S., Gronseth G., Gloss D. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.T., Hsu M.J., Chen B.C., Chen C.C., Teng C.M., Pan S.L., Lin C.H. Denbinobin induces apoptosis in human lung adenocarcinoma cells via Akt inactivation, Bad activation, and mitochondrial dysfunction. Toxicol. Lett. 2008;177(1):48–58. doi: 10.1016/j.toxlet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Lacson J.C.A., Carroll J.D., Tuazon E., Castelao E.J., Bernstein L., Cortessis V.K. Population-based case-control study of recreational drug use and testis cancer risk confirms an association between marijuana use and nonseminoma risk. Cancer. 2012;118(21):5374–5383. doi: 10.1002/cncr.27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert S., Harrison K., Tosun N., Allen S. Menstrual Cycle in Women Who Co-use Marijuana and Tobacco. J. Addict. Med. 2018;12(3):207–211. doi: 10.1097/ADM.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau S.E., Tabb L.P., Kioumarsi A., Ataiants J., Iverson E., Wong C.F. Density of Medical Marijuana Dispensaries and Current Marijuana Use among Young Adult Marijuana Users in Los Angeles. Subst. Use Misuse. 2019;54(11):1862–1874. doi: 10.1080/10826084.2019.1618332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke F.M., Piomelli D., Pahlisch F., Muhl D., Gerth C.W., Hoyer C., Klosterkötter J., Hellmich M., Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry. 2012;2(3):e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., McClean M.D., Marsit C., Christensen B., Peters E., Nelson H.H., Kelsey K.T. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prevention Res. 2009;2(8):759–768. doi: 10.1158/1940-6207.CAPR-09-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M., Gilbart E.R., Wright N.E., Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe H., Steele B., Bryant J., Toyang N., Ngwa W. Non-cannabinoid metabolites of Cannabis sativa l. With therapeutic potential. Plants. 2021;10(2):400.. doi: 10.3390/plants10020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano P., Barrientos-Gutierrez I., Arillo-Santillan E., Morello P., Mejia R., Sargent J.D., Thrasher J.F. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427–430. doi: 10.1016/j.drugalcdep.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukhele S.T., Motadi L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Alternative Med. 2016;16(1) doi: 10.1186/s12906-016-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum D.E., Suzanne M. Adverse structural and functional effects of marijuana on the brain: evidence reviewed. Pediatr. Neurol. 2017;66:12–20. doi: 10.1016/j.pediatrneurol.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J., Julian M., Carrascosa A. Role of the Cannabinoid System in Pain Control and Therapeutic Implications for the Management of Acute and Chronic Pain Episodes. Curr. Neuropharmacol. 2006;4(3):239–257. doi: 10.2174/157015906778019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinasek M.P., Rrt M., Mcgrogan J.B., Maysonet A. A Systematic Review of the Respiratory Effects of Inhalational Marijuana. Respiratory care. 2016;61(11):1543–1551. doi: 10.4187/respcare.04846. [DOI] [PubMed] [Google Scholar]
- Martínez V., Iriondo De-Hond A., Borrelli F., Capasso R., Del Castillo M.D., Abalo R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020;21(9):3067. doi: 10.3390/ijms21093067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik J.A., Eldreth D.A., Cadet J.L., Bolla K.I. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McGuire P., Robson P., Cubala W.J., Vasile D., Morrison P.D., Barron R., Taylor A., Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry. 2018;175(3):225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- McPartland J.M., Hegman W., Long T. Cannabis in Asia: its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Vegetation History Archaeobotany. 2019;28(6):691–702. [Google Scholar]
- McRae A.L., Budney A.J., Brady K.T. Treatment of marijuana dependence: a review of the literature. J. Subst. Abuse Treat. 2003;24(4):369–376. doi: 10.1016/S0740-5472(03)00041-2. [DOI] [PubMed] [Google Scholar]
- Mehra R., Moore B.A., Crothers K., Tetrault J., Fiellin D.A. The association between marijuana smoking and lung cancer: a systematic review. Arch. Intern. Med. 2006;166(13):1359–1367. doi: 10.1001/archinte.166.13.1359. [DOI] [PubMed] [Google Scholar]
- Meier M.H., Caspi A., Ambler A., Harrington H., Houts R., Keefe R.S.E., Mcdonald K., Ward A., Poulton R., Moffitt T.E., Designed T.E.M., Performed T.E.M. Persistent cannabis users show neuropsychological decline from childhood to midlife. Nat. Acad Sci. 2012;109:15980. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamede R.J. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduction J. 2005;2 doi: 10.1186/1477-7517-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memedovich K.A., Dowsett L.E., Spackman E., Noseworthy T., Clement F. The adverse health effects and harms related to marijuana use: an overview review. CMAJ Open. 2018;6(3):E339–E346. doi: 10.9778/cmajo.20180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole M.L., Turner C.E. Phytochemical screening of Cannabis sativa L. I: Constituents of an indian variant. J. Pharm. Sci. 1974;63(1):154–156. doi: 10.1002/jps.2600630144. [DOI] [PubMed] [Google Scholar]
- Montecucco F., Di Marzo V. At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol. Sci. 2012.At;33(6):331–340. doi: 10.1016/j.tips.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Monthony A.S., Page S.R., Hesami M., Jones A.M.P. The past, present and future of Cannabis sativa tissue culture. Plants. 2021;10(1):185. doi: 10.3390/plants10010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat-De La Paz S., Marín-Aguilar F., García-Giménez M.D., Fernández-Arche M.A. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food. Chem. 2014;62(5):1105–1110. doi: 10.1021/jf404278q. [DOI] [PubMed] [Google Scholar]
- Moon Y.J., Wang X., Morris M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Moore T.H., Zammit S., Lingford-Hughes A., Barnes T.R., Jones P.B., Burke M., Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Mousa A., Petrovic M., Fleshner N.E. Prevalence and predictors of cannabis use among men receiving androgen-deprivation therapy for advanced prostate cancer. Canadian Urol. Assoc. J. 2019;14(1) doi: 10.5489/cuaj.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge E., Murch S., Brown P. Evaluating phytochemical variation in medical marihuana. Planta Med. 2016;81(1):1–381. doi: 10.1055/s-0036-1596181. [DOI] [Google Scholar]
- Mudge E.M., Brown P.N., Murch S.J. The Terroir of Cannabis: Terpene Metabolomics as a Tool to Understand Cannabis sativa Selections. Planta Med. 2019;85(9–10):781–796. doi: 10.1055/a-0915-2550. [DOI] [PubMed] [Google Scholar]
- Nada, S.A., Abdel-Salam, O.M.E., Sleem, A.A., 2017. Cannabis and Hepatic Injury. In Handbook of Cannabis and Related Pathologies, 505–516. https://doi.org/10.1016/B978-0-12-800756-3.00062-4.
- Nagy D.U., Cianfaglione K., Maggi F., Sut S., Dall'Acqua S. Chemical Characterization of Leaves, Male and Female Flowers from Spontaneous Cannabis (Cannabis sativa L.) Growing in Hungary. Chem. Biodivers. 2019;16(3):e1800562. doi: 10.1002/cbdv.v16.310.1002/cbdv.201800562. [DOI] [PubMed] [Google Scholar]
- Nassan F.L., Arvizu M., Mínguez-Alarcón L., Williams P.L., Attaman J., Petrozza J., Hauser R., Chavarro J., Ford J.B., Keller M.G. Marijuana smoking and markers of testicular function among men from a fertility centre. Hum. Reprod. 2019;34(4):715–723. doi: 10.1093/humrep/dez002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T.M., Krishnan L.P., Lee J., Adami G.R. Microbiomic differences at cancer-prone oral mucosa sites with marijuana usage. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-48768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkansah-Amankra S., Minelli M. “Gateway hypothesis” and early drug use: Additional findings from tracking a population-based sample of adolescents to adulthood. Prevent. Med. Reports. 2016;4:134–141. doi: 10.1016/j.pmedr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary D.S., Block R.I., Koeppel J.A., Schultz S.K., Magnotta V.A., Ponto L.B., Watkins G.L., Hichwa R.D. Effects of smoking marijuana on focal attention and brain blood flow. Hum. Psychopharmacol. 2007;22(3):135–148. doi: 10.1002/hup.v22:310.1002/hup.832. [DOI] [PubMed] [Google Scholar]
- O’Llenecia S.W., Holloway A.C., Raha S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019;12(1):1–10. doi: 10.1186/s13048-018-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbarnscheidt T., Gold M., Miller N.S., Gold M.S. Marijuana Addictive Disorders and DSM-5 Substance-Related Disorders Marijuana Addictive Disorders: DSM-5 Substance-Related Disorders. J. Addict. Res. Ther. 2017;11:2–8. doi: 10.4172/2155-6105.S11-013. [DOI] [Google Scholar]
- Oladimeji A.V., Valan M.F. Phytochemical profile of cannabis plant: A review. J. Pharm. Phytochem. 2020;9(3):680–687. doi: 10.22271/phyto.2020.v9.i3k.11350. [DOI] [Google Scholar]
- Omare M.O., Kibet J.K., Cherutoi J.K., Kengara F.O. Current Trends in the Use of Cannabis sativa: Beyond Recreational and Medicinal Applications. Open Access Library J. 2021;8(6):1–15. doi: 10.4236/oalib.1107132. [DOI] [Google Scholar]
- Onwuameze O.E., Nam K.W., Epping E.A., Wassink T.H., Ziebell S., Andreasen N.C., Ho B.-C. MAPK14 and CNR1 gene variant interactions: effects on brain volume deficits in schizophrenia patients with marijuana misuse. Psychol. Med. 2013;43(3):619–631. doi: 10.1017/S0033291712001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlán P., Verzár-petri G., Mincsovics E., Székely T. Separation, quantitation and isolation of cannabinoids from Cannabis sativa L. by overpressured layer chromatography. J. Chromatogr. A. 1987;388:217–224. doi: 10.1016/S0021-9673(01)94481-4. [DOI] [PubMed] [Google Scholar]
- Orsolini L., Papanti D., Corkery J., De Luca M.A., Cadoni C., Di Chiara G., Schifano F. Is there a teratogenicity risk associated with cannabis and synthetic cannabimimetics’(‘spice’) intake? CNS Neurol. Disorders-Drug Targets. 2017;16(5):585–591. doi: 10.2174/1871527316666170413101257. [DOI] [PubMed] [Google Scholar]
- Owen, K.P., Sutter, M.E., Albertson, T.E., 2014. Marijuana: Respiratory Tract Effects. Springer, 46(1), 65–81. https://doi.org/10.1007/s12016-013-8374-y. [DOI] [PubMed]
- Pacher P., Steffens S., Haskó G., Schindler T.H., Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat. Rev. Cardiol. 2018;15(3):151–166. doi: 10.1038/nrcardio.2017.130. [DOI] [PubMed] [Google Scholar]
- Pagano E., Capasso R., Piscitelli F., Romano B., Parisi O.A., Finizio S., Lauritano A., Marzo V.D., Izzo A.A., Borrelli F. An orally active cannabis extract with high content in cannabidiol attenuates chemically-induced intestinal inflammation and hypermotility in the mouse. Front. Pharmacol. 2016;7:341. doi: 10.3389/fphar.2016.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano E., Romano B., Iannotti F.A., Parisi O.A., D’Armiento M., Pignatiello S., Coretti L., Lucafò M., Venneri T., Stocco G., Lembo F., Orlando P., Capasso R., Di Marzo V., Izzo A.A., Borrelli F. The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol. Res. 2019;149:104464. doi: 10.1016/j.phrs.2019.104464. [DOI] [PubMed] [Google Scholar]
- Pavlovic R., Panseri S., Giupponi L., Leoni V., Citti C., Cattaneo C., Giorgi A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Frontiers. Plant Sci. 2019;10:1265. doi: 10.3389/fpls.2019.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K., Pengpid S. Cannabis and Amphetamine Use Among Adolescents in Five Asian Countries. Central Asian Journal of. Global Health. 2017;6(1) doi: 10.5195/cajgh.2017.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezdek K., Abed E., Reisberg D. Marijuana Impairs the Accuracy of Eyewitness Memory and the Confidence-Accuracy Relationship Too. J. Appl. Res. Memory Cognit. 2020;9(1):60–67. doi: 10.1016/j.jarmac.2019.11.005. [DOI] [Google Scholar]
- Phan K.L., Angstadt M., Golden J., Onyewuenyi I., Popovska A., De Wit H. Cannabinoid Modulation of Amygdala Reactivity to Social Signals of Threat in Humans. Soc Neuroscience. 2008;28(10):2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillersdorf, D., 2018. Cannabis-associated Impairments in Autobiographical Memory Specificity and the Fading Affect Bias. https://scholar.uwindsor.ca/etd/7559. [DOI] [PubMed]
- Politi M., Peschel W., Wilson N., Zloh M., Prieto J.M., Heinrich M. Direct NMR analysis of cannabis water extracts and tinctures and semi-quantitative data on Δ9-THC and Δ9-THC-acid. Phytochemistry. 2008;69(2):562–570. doi: 10.1016/j.phytochem.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Pollastro F., Minassi A., Fresu L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018;25(10):1160–1185. doi: 10.2174/0929867324666170810164636. [DOI] [PubMed] [Google Scholar]
- Popova L., McDonald E.A., Sidhu S., Barry R., Richers Maruyama T.A., Sheon N.M., Ling P.M. Perceived harms and benefits of tobacco, marijuana, and electronic vaporizers among young adults in Colorado: implications for health education and research. Addiction. 2017;112(10):1821–1829. doi: 10.1111/add.13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy R.K., Ganae-Motan E.D., Allende S., Yanagihara R., Shaiova L., Weinstein S., McQuade R., Wright S., Fallon M.T. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J. Pain. 2012;13(5):438–449. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Raderman, J.M., 2016. Method for modifying THC content in a lipid-based extract of Cannabis.
- Radhakrishnan R., Wilkinson S.T., D’Souza D.C. Gone to pot-a review of the association between cannabis and psychosis. Front. Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M.M., ElSohly M.A., Slade D., Ahmed S.A., Wilson L., El-Alfy A.T., Khan I.A., Ross S.A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry. 2008;69(14):2627–2633. doi: 10.1016/j.phytochem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M.M., Ross S.A., Slade D., Ahmed S.A., Zulfiqar F., Elsohly M.A. Isolation and characterization of new cannabis constituents from a high potency variety. Planta Med. 2008;74(3):267–272. doi: 10.1055/s-2008-1034311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanahally S., Raheem O., Rogers M., Brisbane W., Ostrowski K., Lendvay T., Walsh T. The relationship between cannabis and male infertility, sexual health, and neoplasm: a systematic review. Andrology. 2019;7(2):139–147. doi: 10.1111/andr.2019.7.issue-210.1111/andr.12585. [DOI] [PubMed] [Google Scholar]
- Randall M.D. Endocannabinoids and the haematological system. Br. J. Pharmacol. 2007;152(5):671–675. doi: 10.1038/sj.bjp.0707420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R., Nagarkatti P.S., Nagarkatti M. Δ9Tetrahydrocannabinol attenuates Staphylococcal enterotoxin B-induced inflammatory lung injury and prevents mortality in mice by modulation of miR-17-92 cluster and induction of T-regulatory cells. Br. J. Pharmacol. 2015;172(7):1792–1806. doi: 10.1111/bph.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio P. Endocannabinoid Binding to the Cannabinoid Receptors: What Is Known and What Remains Unknown. Curr. Med. Chem. 2010;17(14):1468–1486. doi: 10.2174/092986710790980005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro L.IG., Ind P.W. Effect of cannabis smoking on lung function and respiratory symptoms: A structured literature review. NPJ Primary Care Respir. Med. 2016;26(1) doi: 10.1038/npjpcrm.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison L.L., Buckley J.D., Daigle A.E., Wells R., Benjamin D., Arthur D.C., Hammond G.D. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the childrens cancer study group) Cancer. 1989;63(10):1904–1911. doi: 10.1002/1097-0142(19890515)63:10<1904::AID-CNCR2820631006>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Rocky Mountain High Intensity Drug Trafficking Area, 2015. The legalization ofmarijuana in Colorado: The impact, Vol. 3 http://www.rmhidta.org/html/2015%20PREVIEW%20Legalization%20of%20MJ%20in%20Colorado%20the%20Impact.pdf (Retrieved 30 October 2020).
- Rodriguez-Martin, N.M., Toscano, R., Villanueva, A., Pedroche, J., Millan, F., Montserrat-De La Paz, S., Millan-Linares, M.C., 2019. Neuroprotective protein hydrolysates from hemp (Cannabis sativa L.) seeds. Food Function, 10(10), 6732–6739. https://doi.org/10.1039/c9fo01904a [DOI] [PubMed]
- Roncero C., Valriberas-Herrero I., Mezzatesta-Gava M., Villegas J.L., Aguilar L., Grau-López L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reproductive Health. 2020;17(1) doi: 10.1186/s12978-020-0880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.A., ElSohly M.A., Sultana G.N.N., Mehmedic Z., Hossain C.F., Chandra S. Flavonoid glycosides and cannabinoids from the pollen of Cannabis sativa L. Phytochem. Anal. 2005;16(1):45–48. doi: 10.1002/pca.v16:110.1002/pca.809. [DOI] [PubMed] [Google Scholar]
- Roth M.D. Pharmacology: Marijuana and your heart. Nature. 2005;434(7034):708–709. doi: 10.1038/434708a. [DOI] [PubMed] [Google Scholar]
- Roychoudhury, P., Wang, N.N., Narouze, S.N., 2021. Phytocannabinoids: Tetrahydrocannabinol (THC). In: Narouze S.N. (eds).Cannabinoids and Pain. Springer, Cham, pp. 71-77.https://doi.org/10.1007/978-3-030-69186-8_10
- Rupasinghe H.P., Davis A., Kumar S.K., Murray B., Zheljazkov V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules. 2020;25(18):4078.. doi: 10.3390/molecules25184078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, E.B., Jiang, H.E., Li, X., Sutton, A., Carboni, A., Del Bianco, F., Li, C. Sen., 2008. Phytochemical and genetic analyses of ancient cannabis from Central Asia. Journal of fExperimental Botany, 59(15), 4171–4182. https://doi.org/10.1093/jxb/ern260 [DOI] [PMC free article] [PubMed]
- Ryan S.A., Ammerman S.D., O’Connor M.E., Patrick S.W., Plumb J., Quigley J., Walker-Harding L.R. Marijuana use during pregnancy and breastfeeding: Implications for neonatal and childhood outcomes. Pediatrics. 2018;142(3) doi: 10.1542/peds.2018-1889. [DOI] [PubMed] [Google Scholar]
- Sánchez-Duffhues G., Calzado M.A., de Vinuesa A.G., Appendino G., Fiebich B.L., Loock U., Lefarth-Risse A., Krohn K., Muñoz E. Denbinobin inhibits nuclear factor-κB and induces apoptosis via reactive oxygen species generation in human leukemic cells. Biochem. Pharmacol. 2009;77(8):1401–1409. doi: 10.1016/j.bcp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Santos M.E., Protopopescu C., Zucman D., Marcellin F., Wittkop L., Miailhes P., Sogni P., Carrieri M.P. Benefits of cannabis use for metabolic disorders and survival in people living with HIV with or without hepatitis C co-infection. AIDS. 2020;34(6):953–954. doi: 10.1097/QAD.0000000000002480. [DOI] [PubMed] [Google Scholar]
- Schewe T., Kühn H., Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002;132(7):1825–1829. doi: 10.1093/jn/132.7.1825. [DOI] [PubMed] [Google Scholar]
- Secades-Villa R., Garcia-Rodríguez O., Jin C.J., Wang S., Blanco C. Probability and predictors of the cannabis gateway effect: A national study. Int. J. Drug Policy. 2015;26(2):135–142. doi: 10.1016/j.drugpo.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely K.A., Prather P.L., James L.P., Moran J.H. Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol. Interventions. 2011;11(1):36–51. doi: 10.1124/mi.11.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S., Opila-Lehman J. Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. Permanente J. 2016;20(4):108–111. doi: 10.7812/TPP/16-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S., Lewis N., Lee H., Hughes S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Permanente J. 2019;23:18–41. doi: 10.7812/TPP/18-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. Male infertility; evidences, risk factors, causes, diagnosis and management in human. Ann. Clin. Lab. Res. 2017;5(3):188. doi: 10.21767/2386-5180.1000188. [DOI] [Google Scholar]
- Sharma P., Balhara Y.P.S. Cannabis and Endocrine system: A narrative review. J. Psychiatrists’ Assoc. Nepal. 2018;7(2):5–15. doi: 10.3126/jpan.v7i2.24608. [DOI] [Google Scholar]
- Sharma P., Murthy P., Bharath M.S. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iranian J. Psychiatry. 2012;7(4):149. [PMC free article] [PubMed] [Google Scholar]
- Shayesteh M.R.H., Haghi-Aminjan H., Mousavi M.J., Momtaz S., Abdollahi M. The protective mechanism of cannabidiol in cardiac injury: A systematic review of non-clinical studies. Curr. Pharm. Des. 2019;25(22):2499–2507. doi: 10.2174/2210327909666190710103103. [DOI] [PubMed] [Google Scholar]
- Shoyama Y., Oku R., Yamauchi T., Nishioka I. Cannabis. VI. Cannabicyclolic Acid. Chem. Pharm. Bull. 1972;20(9):1927–1930. doi: 10.1248/cpb.20.1927. [DOI] [Google Scholar]
- Shoyama Y., Yagi M., Nishioka I., Yamauchi T. Biosynthesis of cannabinoid acids. Phytochemistry. 1975;14(10):2189–2192. doi: 10.1016/S0031-9422(00)91096-3. [DOI] [Google Scholar]
- Shoyama, Y., Yamauchi, T., Nishioka, I., 1970a. Cannabis. V1). Cannabigerolic Acid Monomethyl Ether and Cannabinolic Acid. Chem. Pharm. Bull. 18(7), 1327–1332. https://doi.org/10.1248/cpb.18.1327.
- Shoyama Y., Yamauchi T., Nishioka I. Cannabis. V. Cannabigerolic Acid Monomethyl Ether and Cannabinolic Acid. Chem. Pharm. Bull. 1970;18(7):1327–1332. doi: 10.1248/cpb.18.1327. [DOI] [Google Scholar]
- Silver R.J. The endocannabinoid system of animals. Animals. 2019;9(9):686. doi: 10.3390/ani9090686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015;81(3):189–294. doi: 10.1007/s12229-015-9157-3. [DOI] [Google Scholar]
- Smeriglio A., Trombetta D., Alloisio S., Cornara L., Denaro M., Garbati P., Circosta C. Promising in vitro antioxidant, anti-acetylcholinesterase and neuroactive effects of essential oil from two non-psychotropic Cannabis sativa L. biotypes. Phytother. Res. 2020 doi: 10.1002/ptr.6678. [DOI] [PubMed] [Google Scholar]
- Stasiłowicz A., Tomala A., Podolak I., Cielecka-Piontek J. Cannabis sativa L. as a natural drug meeting the criteria of a multitarget approach to treatment. Int. J. Mol. Sci. 2021;22(2):778. doi: 10.3390/ijms22020778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundram S. Cannabis and neurodevelopment: Implications for psychiatric disorders. Hum. Psychopharmacol. 2006;21(4):245–254. doi: 10.1002/hup.v21:410.1002/hup.762. [DOI] [PubMed] [Google Scholar]
- Taffe M.A. δ 9-Tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012;201:125–133. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.C., Lo C., Jong A., Xing L., FitzGerald M.J., Vollmer W.M., Buist S.A., Sin D.D. Marijuana and chronic obstructive lung disease: a population-based study. Can Med Assoc. 2009;180(8):814–820. doi: 10.1503/cmaj.081040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu, R., & Constantinescu, C.S., 2010. Cannabinoids and the immune system: An overview. Elsevier, 215(8), 588–597. https://doi.org/10.1016/j.imbio.2009.12.005. [DOI] [PubMed]
- Tandon B., Anand U., Alex B.K., Kaur P., Nandy S., Shekhawat M.S., Sanyal R., Pandey D.K., Koshy E.P., Dey A. Statistical optimization of in vitro callus induction of wild and cultivated varieties of Mucuna pruriens L. (DC.) using response surface methodology and assessment of L-Dopa biosynthesis. Ind. Crops Prod. 2021;169:113626. doi: 10.1016/j.indcrop.2021.113626. [DOI] [Google Scholar]
- Tang C.H., Ten Z., Wang X.S., Yang X.Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food. Chem. 2006;54(23):8945–8950. doi: 10.1021/jf0619176. [DOI] [PubMed] [Google Scholar]
- Tashkin D.P. Smoked Marijuana as a Cause of Lung Injury. Monaldi Arch Chest Dis. 2005;63(2) doi: 10.4081/monaldi.2005.645. https://monaldi-archives.org/index.php/macd/article/view/645 [DOI] [PubMed] [Google Scholar]
- Tashkin D.P. Effects of marijuana smoking on the lung. Ann. Am. Thoracic Soc. 2013;10(3):239–247. doi: 10.1513/AnnalsATS.201212-127FR. [DOI] [PubMed] [Google Scholar]
- Tashkin, D.P., 2017. Cannabis Smoking and the Lung. Handbook of Cannabis and Related Pathologies, 494–504. https://doi.org/10.1016/B978-0-12-800756-3.00060-0.
- Tashkin D.P. Marijuana and lung disease. Chest. 2018;154(3):653–663. doi: 10.1016/j.chest.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Tashkin, Donald P., Roth, M.D., 2007. Effects of Marijuana on the Lung and Immune Defenses. Marijuana and the Cannabinoids, 253–275. https://doi.org/10.1007/978-1-59259-947-9_11.
- Tashkin D.P., Roth M.D. Pulmonary effects of inhaled cannabis smoke. Am. J. Drug Alcohol Abuse. 2019;45(6):596–609. doi: 10.1080/00952990.2019.1627366. [DOI] [PubMed] [Google Scholar]
- Thomas G., Kloner R.A., Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014;113(1):187–190. doi: 10.1016/j.amjcard.2013.09.042. [DOI] [PubMed] [Google Scholar]
- Thompson G.R., Rosenkrantz H., Schaeppi U.H., Braude M.C. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol. Appl. Pharmacol. 1973;25(3):363–372. doi: 10.1016/0041-008X(73)90310-4. [DOI] [PubMed] [Google Scholar]
- Tomko A.M., Whynot E.G., Ellis L.D., Dupré D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers. 2020;12(7):1985. doi: 10.3390/cancers12071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers K.F., Mertens A.C., Ross J.A., Steinbuch M., Olshan A.F., Robison L.L. Parental marijuana use and risk of childhood acute myeloid leukaemia: A report from the Children’s Cancer Group, United States and Canada) Paediatr. Perinat. Epidemiol. 2006;20(2):110–118. doi: 10.1111/j.1365-3016.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- Turner C.E., Elsohly M.A., Boeren E.G. Constituents of Cannabis sativa l. xvii. a review of the natural constituents. J. Nat. Prod. 1980;43(2):169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- Veit F., Erdmann F., Birngruber C., Dettmeyer R. Detection of drugs in paired maternal and umbilical cord blood samples. Rom J. Leg. Med. 2017;25(2):185–192. doi: 10.4323/rjlm.2017.185. [DOI] [Google Scholar]
- Vieira G., Cavalli J., Gonçalves E.C., Braga S.F., Ferreira R.S., Santos A.R., Cola M., Raposo N.R., Capasso R., Dutra R.C. Antidepressant-like effect of terpineol in an inflammatory model of depression: Involvement of the cannabinoid system and D2 dopamine receptor. Biomolecules. 2020;10(5):792. doi: 10.3390/biom10050792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Baler R.D., Compton W.M., Weiss S.R.B. Adverse health effects of marijuana use. N. Engl. J. Med. 2014;370(23):2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Telang F., Fowler J.S., Alexoff D., Logan J., Jayne M., Wong C., Tomasi D. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. National Acad. Sci. 2014;111(30):E3149–E3156. doi: 10.1073/pnas.1411228111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vree T.B., Breimer D.D., Van Ginneken C.A.M., Van Rossum J.M. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogues with a methyl side-chain. J. Pharm. Pharmacol. 1972;24(1):7–12. doi: 10.1111/j.2042-7158.1972.tb08857.x. [DOI] [PubMed] [Google Scholar]
- Vuong, C., Van Uum, S.H.M., O’dell, L.E., Lutfy, K., Friedman, T.C., 2010. The Effects of Opioids and Opioid Analogs on Animal and Human Endocrine Systems. Endocrine Rev. 31(1), 98–132. https://doi.org/10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed]
- Wang C., Hipp J.R., Butts C.T., Lakon C.M., Lin Z.C. The interdependence of cigarette, alcohol, and marijuana use in the context of school-based social networks. PLoS ONE. 2018;13(7):e0200904. doi: 10.1371/journal.pone.0200904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins P.B., Church R.J., Li J., Knappertz V. Cannabidiol and abnormal liver chemistries in healthy adults: Results of a phase I clinical trial. Clin. Pharmacol. Ther. 2021;109(5):1224–1231. doi: 10.1002/cpt.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J.E., Kim J.S., Das A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019;143 doi: 10.1016/j.prostaglandins.2019.106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Smith D., O’Connor R., Travers M.J., Hyland A. Examining the Association between Body Burdens of Harmful Chemicals and Heaviness of Marijuana Smoking. Chem. Res. Toxicol. 2018;31(8):643–645. doi: 10.1021/acs.chemrestox.8b00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijarnpreecha K., Panjawatanan P., Ungprasert P. Use of cannabis and risk of advanced liver fibrosis in patients with chronic hepatitis C virus infection: A systematic review and meta-analysis. J. Evidence-Based Med. 2018;11(4):272–277. doi: 10.1111/jebm.12317. [DOI] [PubMed] [Google Scholar]
- Wijaya D.S. Cannabis for health. J. Holistic Traditional Med. 2021;5(04):534–537. http://www.jhtm.or.id/index.php/jhtm/article/view/103 [Google Scholar]
- Wilkinson S.T., Radhakrishnan R., D’Souza D.C. A systematic review of the evidence for medical marijuana in psychiatric indications. J. Clin. Psychiatry. 2016;77(08):1050–1064. doi: 10.4088/JCP.15r10036. [DOI] [PubMed] [Google Scholar]
- Willford J.A., Goldschmidt L., De Genna N.M., Day N.L., Richardson G.A. A longitudinal study of the impact of marijuana on adult memory function: Prenatal, adolescent, and young adult exposures. Neurotoxicol. Teratol. 2021;84:106958. doi: 10.1016/j.ntt.2021.106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A., Robble M.A. Dopamine and addiction. Annu. Rev. Psychol. 2020;71(1):79–106. doi: 10.1146/annurev-psych-010418-103337. [DOI] [PubMed] [Google Scholar]
- Wood T.B., Spivey W.T.N., Easterfield T.H. III. - Cannabinol. Part I. J. chem. Soc. Trans. 1899;75(0):20–36. doi: 10.1039/CT8997500020. [DOI] [Google Scholar]
- Wu C.S., Jew C.P., Lu H.C. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011;6(4):459–480. doi: 10.2217/fnl.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrofsky R.R., Reyes B.A.S., Zhang X.-Y., Bhatnagar S., Kirby L.G., Van Bockstaele E.J. Endocannabinoids, stress signaling, and the locus coeruleus-norepinephrine system. Neurobiol. Stress. 2019;11:100176. doi: 10.1016/j.ynstr.2019.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Tang J., dos Santos Passos C., Nurisso A., Simões-Pires C.A., Ji M., Lou H., Fan P. Characterization of Lignanamides from Hemp (Cannabis sativa L.) Seed and Their Antioxidant and Acetylcholinesterase Inhibitory Activities. J. Agric. Food. Chem. 2015;63(49):10611–10619. doi: 10.1021/acs.jafc.5b05282. [DOI] [PubMed] [Google Scholar]
- Yayan J., Rasche K. Damaging effects of cannabis use on the lungs. Adv. Exp. Med. Biol. 2016;952:31–34. doi: 10.1007/5584_2016_71. [DOI] [PubMed] [Google Scholar]
- Zamberletti E., Prini P., Speziali S., Gabaglio M., Solinas M., Parolaro D., Rubino T. Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience. 2012;204:245–257. doi: 10.1016/j.neuroscience.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Zehra A., Burns J., Liu C.K., Manza P., Wiers C.E., Volkow N.D., Wang G.J. Cannabis Addiction and the Brain: a Review. J. Neuroimmune Pharmacol. 2018;13(4):438–452. doi: 10.1007/s11481-018-9782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang S., Ji J., Lou H., Fan P. Hemp (Cannabis sativa L.) Seed Phenylpropionamides Composition and Effects on Memory Dysfunction and Biomarkers of Neuroinflammation Induced by Lipopolysaccharide in Mice. ACS. Omega. 2018;3(11):15988–15995. doi: 10.1021/acsomega.8b02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang S., Lou H., Fan P. Chemical constituents of hemp (Cannabis sativa L.) seed with potential anti-neuroinflammatory activity. Phytochem. Lett. 2018;23:57–61. [Google Scholar]
- Zou S., Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int. J. Mol. Sci. 2018;19(3):833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi A.W., Morais S.L., Guimaraes F.S., Mechoulam R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- Zumbrun E.E., Sido J.M., Nagarkatti P.S., Nagarkatti M. Epigenetic Regulation of Immunological Alterations Following Prenatal Exposure to Marijuana Cannabinoids and its Long Term Consequences in Offspring. J. Neuroimmune Pharmacol. 2015;10(2):245–254. doi: 10.1007/s11481-015-9586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]