Abstract
Injuries to articular cartilage of the knee are increasingly common. The operative management of these focal chondral lesions continues to be problematic for the treating orthopedic surgeon secondary to the limited regenerative capacity of articular cartilage. The pericellular matrix (PCM) is a specialized, thin layer of the extracellular matrix that immediately surrounds chondrocytes forming a unit together called the chondron. The advancements in our knowledge base with regard to the PCM/chondrons as well as interterritorial matrix has permeated and led to advancements in product development in conjunction with minced cartilage, marrow stimulation, osteochondral allograft, and autologous chondrocyte implantation (ACI). This review intends to summarize recent progress in chondrocytes with matrix research, with an emphasis on the role the PCM/extracellular matrix (ECM) plays for favorable chondrogenic gene expression, as a barrier/filtration unit, and in osteoarthritis. The bulk of the review describes cutting-edge and evolving clinical developments and discuss these developments in light of underlying basic science applications. Clinical applications of chondrocytes with matrix science include Reveille Cartilage Processor, Cartiform, and ACI with Spherox (which was recently recommended for the treatment of grade III or IV articular cartilage defects over 2 cm2 by the National Institute of Health and Care Excellence [NICE] in the United Kingdom). The current article presents a comprehensive overview of both the basic science and clinical results of these next-generation cartilage repair techniques by focusing specifically on the scientific evolution in each category as it pertains with underlying chondrocytes with matrix theory.
Keywords: chondrons, pericellular matrix, cartilage repair, autologous chondrocyte implantation (ACI)
Basic Science of Chondrons, Pericellular Matrix, and Interterritorial Matrix
In normal articular cartilage, chondrocytes are embedded within an abundant extracellular matrix (ECM). The solid matrix of the ECM is composed of a crosslinked network of type II collagen, proteoglycans, and several important other collagens (e.g., VI, IX, X, XI) and noncollagenous proteins.1,2 The ECM consists of discrete regions based on proximity to the chondrocytes, composition, and collagen fibril diameter and organization. The ECM can be divided into pericellular matrix (PCM), territorial, and interterritorial regions. 3 The PCM is a specialized, thin layer of the ECM that immediately surrounds chondrocytes 4 forming a unit together called the chondron. 5 This PCM (mainly type VI collagen) has an important role in the metabolic activity of the chondrocyte and the mechanical signaling from and to the ECM.6-8 The PCM has a patent structure, defined molecular composition, and unique physical properties that support the chondrocyte ( Fig. 1 ). Given this spatial position, the PCM is pivotal in mediating communication between chondrocytes and the ECM and, thus, plays a critical role in cartilage homeostasis. The biological function and mechanical properties of the PCM have been comprehensively studied, mostly in the form of chondrons.
Figure 1.
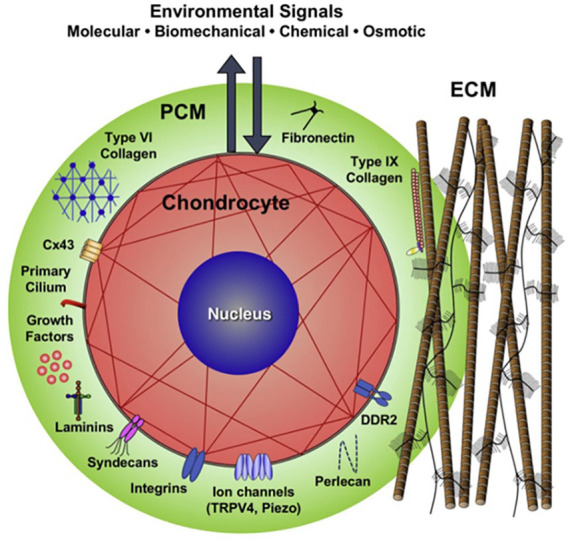
Schematic of a chondron (formed by the chondrocyte together with its surrounding pericellular matrix (PCM). The PCM is a specialized, thin layer of the extracellular matrix. The PCM has a patent structure, defined molecular composition, and unique physical properties that support the chondrocyte. Image used with permission from Guilak et al. 9
Interterritorial ECM
The PCM integrates with the surrounding tissue via the territorial matrix connecting the PCM to the interterritorial matrix. 9 The interterritorial region of the ECM is the largest matrix region, and its hallmark is randomly oriented bundles of large collagen fibrils. These fibers are arranged based on location in articular cartilage: parallel to the surface of the superficial zone, obliquely in the middle zone, and perpendicular to the joint surface in the deep zone. 3
The interterritorial matrix contains abundant proteoglycans and contributes to the biomechanical properties of articular cartilage. It has elongated parallel ridges with significantly greater surface roughness versus than the PCM. The average Young’s modulus of the interterritorial matrix is significantly greater than the PCM. 10 Thus, the interterritorial matrix appears to possess not only distinct microtopographic contours in comparison with the PCM, but also significantly greater mechanical stiffness. “These distinctive nanostructural and nanomechanical properties may have implications in nutrient diffusion and fluid dynamics, both of which are of vital importance for cartilage health and function.” 10
Biomechanical Importance of the PCM
The PCM has been found to have biomechanical importance to support the chondrocyte. First, within the chondron, the PCM allows the construct to be stiffer versus chondrocytes alone. One study found the PCM allowed for retaining the width and volume of the enclosed chondrocytes via collagen fibrils during compression. 11 Second, through the molecular complex, the PCM has a role in transduction of a mechano-biological signal. This molecular complex, consisting of type VI collagen, biglycan, decorin, and matrilins, interact with other components of the PCM. Specifically, matrilin-1 and -3 attach to the cell membrane, via integrin, and other ECM molecules in order to receive extracellular signals. 12 Another example localized in the chondrocyte PCM, is fibroblast growth factor 2, which is a growth factor that participates in mechanical signal transduction. 13 Finally, the role of transduction translates to gene expression. Mechanical forces regulate chondrocyte differentiation and proliferation via gene expression, that is, Indian hedgehog gene. The upregulation for the expression of Indian hedgehog gene (a vital signal in chondrocyte fate determination and proliferation) 14 was found to be dependent on domain A of matrilin-3. 15 These studies help illustrate a key role of PCM to translate mechanical signals to biological events.
Importance of the PCM as Barrier/Filtration Unit
For the molecules that enter or exit the chondrocytes, the PCM acts as a barrier. An intact PCM helps balance the anabolic and catabolic processes of chondrocytes. Studies have shown cultured Chondrons expressed less matrix metalloproteinase proteins (MMP) in comparison with isolated chondrocytes.16,17 When chondrons were challenged with varied osmolarities, the chondrocyte volumes in the chondrons varied less compared with that of the isolated chondrocytes displaying the most efficient volume regulation. 18 Additionally, acting as a barrier to harmful pathways, chondrons have shown resilience to apoptosis induction versus isolated chondrocytes. Peters et al. 19 demonstrated monoiodoacetate treatment induced 1.6% of chondrons to undergo apoptosis, compared with 9% of chondrocytes. Disruption of integrin-ligand interactions from detachment of cells from the matrix may activate cell death pathways. 20 Furthermore, individual PCM molecules, namely type VI collagen, has been shown to protect cells from apoptosis. Cheng et al. 21 suggested a potential protective mechanism by which neurons upregulate collagen VI production under stress conditions to activate an anti-apoptotic signaling pathway. In summary, the PCM (1) balances the anabolic and catabolic processes of chondrocytes, (2) acts as a barrier to harmful pathways, and (3) protects cells from apoptosis.
Favorable Chondrogenic Gene Expression and Growth Factors within the PCM
The PCM has a meaningful impact on the functionality of chondrocytes. 22 Zhang et al. 23 reported over 200 genes involved in chondrocyte proliferation, phenotype, and metabolism differentially expressed in chondrons versus chondrocytes using cDNA microarray. Likewise, chondrons have been shown to upregulate the expression of type II collagen and aggrecan to a greater extent than chondrocytes in response to dynamic compression. 6 Not only does the PCM (type VI collagen) stabilize the chondrocyte phenotype, 22 it also protects chondrocytes from MMP13 expression, an enzyme that can degrade collagen from the cartilage ECM.16,24 Furthermore, PCM molecules have been shown to have an association with chondrogenic differentiation of mesenchymal cells and chondrocytes. French et al. 25 demonstrated domain I of perlecan (the binding site of numerous growth factors localized to the PCM) promoted the chondrogenic differentiation of an embryonic fibroblast cell line in vitro. Perlecan regulates chondrogenic differentiation through bone morphogenetic protein (BMP)-2 signaling26,27 and directs vascular invasion into cartilage during endochondral ossification through vascular endothelial growth factor receptor (VEGFR). 28 Furthermore, it has been proposed that an intact PCM improves matrix production and cell-induced cartilage formation. Larson et al. 8 showed chondron pellets had a 10-fold increase in proteoglycan content versus a 6-fold increase for chondrocyte only pellets over 8 weeks (P < 0.0001) secondary to increased matrix deposition rather than cell division (unclear whether due to differences in rates of synthesis or greater retention and assembly). Vonk et al. 17 presented data suggesting that preserving the PCM has a positive effect on cell-induced cartilage production secondary to increased quantities of proteoglycans and decreased gene expression of MMP13 in chondrons versus chondrocytes taken from articular cartilage. Also, the PCM also acts as storage and maintenance of growth factors, where they are activated, degraded, or transported. 29
The Role of the PCM in Osteoarthritis
Growing evidence suggests that many of the features of osteoarthritis (OA) are possibly initiated in the PCM. 9 In healthy articular cartilage, the ECM is maintained in homeostasis (a balance of matrix synthesis and degradation). The PCM acts as a transitional zone between the interterritorial matrix and chondrocytes to modulate environmental signals before they reach the chondrocytes. Changes to PCM properties, such as in a pathologic state like OA, may not only represent the disease state but may also influence the regulatory function of PCM and therefore chondrocyte activity. 9 Multiple studies have suggested that associated change in PCM composition, structure, and mechanical function is present in OA.
Studies examining advanced stages of OA show composition changes of collagen expression including significant upregulated expression of collagen type VI 30 and overlapping localization of collagens I, II and III in the PCM. 31 Via the chondrocyte’s primary cilium (organelle that projects from the cell surface into the PCM) these changes in composition can significantly affect mechanotransduction in chondrocytes. 9 Distorted cilia length and incidence has been reported with OA severity, implying that there is altered cilia-mediated signaling in degenerative cartilage. 32 The cilium has mechanosensors, a variety of ion channels such as the transient receptor potential vanilloid 4 (TRPV4), and an ability to interact with matrix proteins such as collagens type II and VI. One study showed TRPV4 provokes intracellular Ca2+ signaling cascades in response to mechanical stimuli. 33
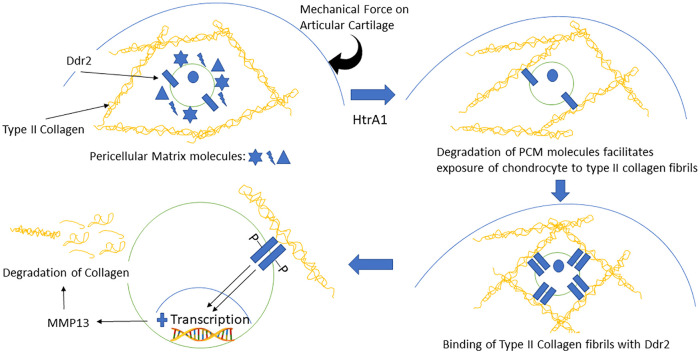
Degradation of PCM structure may be one of the earliest events during OA onset 9 secondary to elevated serine proteases 34 that digest several major PCM components 35 leading to the chondrocyte’s increased contact with type II collagen fibrils. Binding of cell receptors, such as DDR2, to type II collagen has been shown to upregulates production of MMP-13 in chondrocytes leading to degradation of type II collagen in cartilage matrix. 36 This highlights the feedback loop of serine proteases (HtrA1) to cell receptors (DDR2) and the MMP13 degradative pathway in OA progression. 37 In summary, a mechanical force on the articular cartilage activates HtrA1 which in turn degrades PCM proteins, thus leading to exposure of the chondrocyte’s surface receptors to type II collagen fibrils in the ECM. Then, binding of type II collagen fibrils to DDR2 upregulates MMP13, thus leading to degradation of collagen ( Fig. 2 ). Moreover, a disruption of PCM structure may trigger the release of modulatory growth factors, normally sequestered and localized to the PCM, such as syndecan, which plays a vital role in modulating the phenotypic changes of chondrocyte through Wnt signaling 38 or perlecan which modulates cell binding of fibroblast growth factor (FGF) 13 to alter the proliferation and metabolism of chondrocytes in response to injury. 9
Figure 2.
Feedback loop of serine proteases (HtrA1) to cell receptors (DDR2) and the matrix metalloproteinas-13 (MMP13) degradative pathway in osteoarthritis progression. A mechanical force on the articular cartilage activates HtrA1, which in turn degrades pericellular matrix (PCM) molecules, thus leading to exposure of the chondrocyte’s surface receptors to type II collagen fibrils in the extracellular matrix (ECM). Then, binding of type II collagen fibrils to DDR2 upregulates MMP13, thus leading to degradation of collagen.
Clinical Applications Evolving from Chondron and Chondrocytes with Matrix Basic Science
The PCM plays a role in biomechanical transduction, chondrogenic phenotype expression, induction of OA disease processes, and as a filtration unit. Underlying principles regarding the importance of the matrix, including both PCM and interterritorial matrix, has brought about change in multiple cartilage repair procedures including minced cartilage, marrow stimulation, osteochondral allograft, and autologous chondrocyte implantation (ACI).
It has been proposed that the outgrowth of embedded chondrocytes (limited chondrocyte migration is contributing factor to poor cartilage self-healing) can be achieved through increased tissue surface area by mincing the cartilage tissue mechanically into small tissue fragments that covers a large area for new tissue formation. 39 Lu et al. 39 suggested a correlation between chondrocyte mincing and mitogenic activation to produce a more robust neocartilage by demonstrating direct treatment of full thickness chondral defects in goats using cartilage fragments on a resorbable scaffold produced hyaline-like repair tissue at 6 months. This mitogenic activation from the minced particles induces migration of chondrocytes and resulting cartilaginous PCM/ECM deposition more analogous to the chondron. As noted above, the native articular cartilage PCM and ECM components provide signals to drive undifferentiated cells toward chondrogenesis. In a porcine model, Cheng et al. 40 investigated the chondrogenic effects on adipose-derived stem cells (ADSC) with ECM-derived scaffolds compared to ADSCs with granules. The results showed two chondrogenic markers (AGC1 and COL2A1) and aggregate modulus of ECM material were significantly higher in ECM-derived scaffold group versus control (both P < 0.05).
Multipotent mesenchymal stromal cells (MSCs) describe the nonhematopoietic adult cell population present in various tissues such as bone marrow and adipose tissue that have chondrogenic differentiation capabilities. 41 Marrow stimulation (MS) techniques induce an influx of marrow substrates (MSCs, growth factors, and cytokines) to repopulate a cartilage defect. 42 Stimulation of cartilage matrix production and upregulation of cartilage-specific matrix genes were observed when chondrocytes were combined with MSCs,43,44 thus suggesting that successful combination with freshly isolated articular chondrocytes could promote chondrocytic differentiation of MSC in a 1-step procedure. In a goat model, Bekkers et al. 5 showed that chondrons generated from debrided cartilage combined with MSCs resulted in better histological quality of regenerated tissue (determined by O’Driscoll score), mean ICRS macroscopic score for cartilage repair, and mean absolute glycosaminoglycan production (all statistically significant) compared with microfracture (MFx) alone. Even when obtained from damaged articular cartilage, chondrons also have a higher regenerative capacity when compared to chondrocytes without their PCM. Vonk et al. 45 showed in a goat model that chondrons isolated from a damaged joint outperformed chondrocytes on cell-by-cell basis in vitro with a statistically significant higher content of proteoglycan and collagen. de Windt et al. 46 showed in a 1-year follow-up pilot study of ten patients a statistically significant improvement in mean Knee Injury and Osteoarthritis. Outcome Score (KOOS) and significant decrease in mean visual analogue scale (VAS) score with complete defect filling on magnetic resonance imaging (MRI) with chondrons + MSCs. This was followed up with a second-look biopsy study of 35 patients showing newly formed cartilage tissue with hyaline-like features containing a high concentration of proteoglycans and type II collagen. 47 Of note, no comparisons to chondrocytes instead of chondrons were made in either de Windt study.
Growth factors in the surrounding ECM may play an important role in cartilage regeneration. Thus, combining the underlying principles of minced cartilage (migration of chondrocytes and resulting cartilaginous matrix [PCM/ECM] deposition beyond that seen in chondrons) along with scaffolding, the Cartilage Autograft Implantation System (CAIS) Harvester and Dispenser (DePuy Mitek, Raynham, MA) was developed. CAIS is a point of care surgical procedure where cartilage is mechanically minced and then affixed using fibrin glue on a synthetic, absorbable scaffold (designed to keep the tissue fragments in place) that is reinforced with a mesh within the defect. 48 Using a low load-bearing surface (i.e., lateral wall of the intercondylar notch) or during debridement of a cartilage defect (often containing viable cartilage cells), 49 cartilage and accompanying ECM can be harvested. Multiple animal models have shown success with a cartilage fragment seeded scaffold demonstrating high cellularity and intense ECM production as well as hyaline-like repair tissue in terms of morphological, mechanical, and histological assessments.50,51 Another study showed CAIS achieved the highest score compared with ACI in a horse model in terms of arthroscopic, histologic, and immunohistochemistry results. 52 A level II randomized controlled trial (RCT) compared CAIS with MFx in 29 patients with 2-years of follow-up. 48 The multicenter study demonstrated CAIS demonstrated significantly improved International Knee Documentation Committee (IKDC) scores and KOOS compared with MFx at 24 months. There was no difference in terms of fill of the graft bed, tissue integration, or presence of subchondral cysts on MRI.
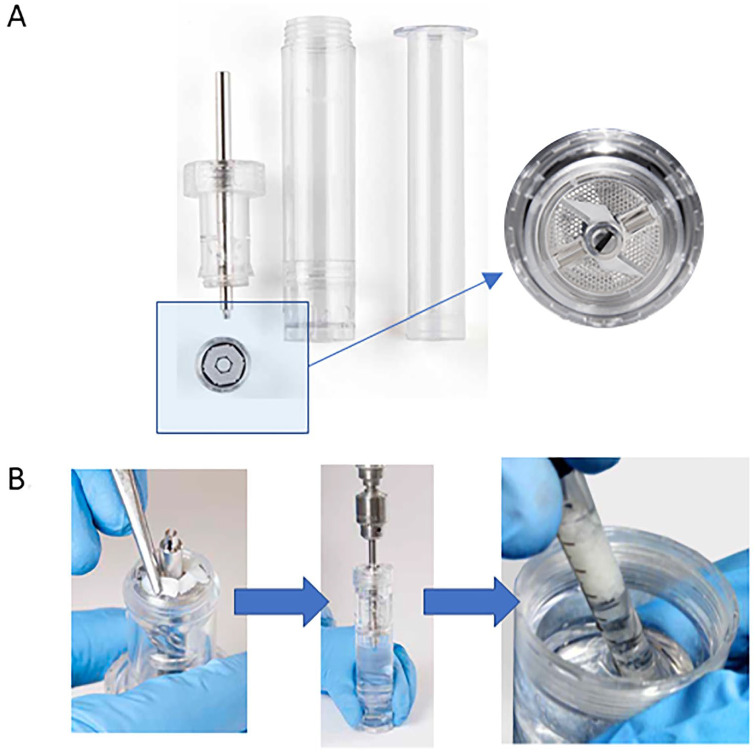
Reveille Cartilage Processor (Exactech, Gainesville, FL) is a single-stage surgical technique that utilizes autologous cartilage combining the underlying principles of minced cartilage (migration of chondrocytes and resulting cartilaginous PCM/ECM deposition analogous to chondrons) along with MS ( Fig. 3 ). It has a 1.3 ± 0.1 minutes intraoperative processing time producing particles sized 0.76 ±0.77 mm2. 53 Particle size has been thought be a vital consideration for the amount of ECM production, in theory secondary to the activation of chondrocytes on the motorized stimulation of the cutting. 54 One recent in vitro study showed chondrocyte outgrowth and matrix deposition from cartilage pieces (using Reveille Cartilage Processor) embedded in the gels harvested from patients undergoing total knee arthroplasty. 53 A preliminary unpublished in vitro study at 4 weeks demonstrated the binding capability of MSCs to porcine cartilage fragments along with outgrown chondrocytes and new tissue formation between graft particles under fluorescent microscopy analysis. There are no published clinical studies currently available.
Figure 3.
Reveille Cartilage Processor technique. (A) Reveille Processor highlighting the particulator and sieve. (B) First, autologous harvested cartilage fragments are placed onto the particulator and spread evenly. Then, tissue is particulated for at least 2 minutes at recommended speed of 1,500 rpm. Finally, after defect preparation followed by marrow stimulation, particulated tissue is ready for transfer. Images used with permission from Exactech, Gainesville, FL. USA.
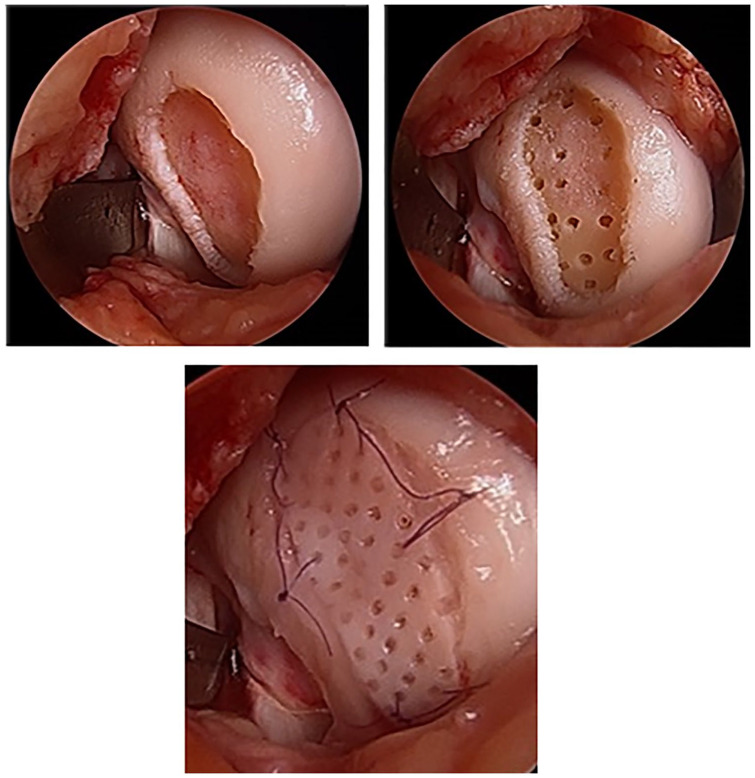
Multiple cartilage repair products have been developed building on the advances made in the fields of chondrons combined with osteochondral allografts and MS, including a cryopreserved, viable osteochondral allograft with pores spanning the thickness of the graft called Cartiform (Arthrex Inc., Naples, FL, USA). Harvested from human cadaveric specimens, the allograft consists of full-thickness articular cartilage and a thin layer of subchondral bone with perforations in the articular cartilage that allows for flexible conformity and improved integration to the underlying subchondral bone. 55 Of note, the implant has a long shelf life (frozen storage for up to 2 years) secondary to pores which enable the cryopreservation solution to penetrate the tissue to maintain cell viability. 56 The chondrocytes along with extracellular matrix proteins, chondrogenic factors (PCM plus surrounding ECM [beyond that seen in chondrons]), and a thin osseous portion are cryopreserved at −80°C. 55 Following MS, Cartiform serves as a scaffold for the MSCs while the viable chondrocytes simultaneously release PCM/ECM inducing the MSCs to undergo chondrogenesis and produce hyaline cartilage. Nonmetallic and nonbone reactive suture anchors may be used to fix Cartiform in place 57 or secured to the surrounding healthy cartilage using absorbable sutures. 58 Fibrin glue is applied between the graft periphery and the native cartilage walls, avoiding the holes in the graft, as a final step to secure the graft in place ( Fig. 4 ). 57 In a goat model, Geraghty et al. 56 showed lesions treated with MFx plus Cartiform had more overall lesion fill at 12 months, but no difference in histological grade versus MFx alone. Another study demonstrated a statistically significant higher percentage of type II collagen staining in the Cartiform plus MSCs pellet group versus MSCs alone. 56 Vangsness et al. 57 showed good results in their pilot 3 patient case series with a return to sport/exercise at 6 months with pain free/satisfactory outcomes at 2 years. In a case study, Hoffman et al. 58 reported complete resolution of pain and improvement in function at 9 months. The patient had returned to sports at 9 months after implantation of a Cartiform implant into the femoral trochlea. Biopsy of the graft site showed repair tissue consisting of 85% hyaline cartilage and excellent basal integration (score 90) based on ICRS (International Cartilage Repair Society) II scores for patient repair tissue. For comparison, a biopsy obtained from a patient 8.2 months after treatment with MS alone contained only 5% hyaline cartilage. 58
Figure 4.
Cartiform (Arthrex Inc., Naples, FL, USA) is a cryopreserved, viable osteochondral allograft (frozen storage for up to 2 years) harvested from human cadaveric specimens characterized by a minimal amount of bone and pores spanning the thickness of the graft. Following marrow stimulation, Cartiform serves as a scaffold for the mesenchymal stem cells (MSCs) while the viable chondrocytes simultaneously release growth factors into the adjacent microenvironment theoretically inducing the MSCs to undergo chondrogenesis. Surgical images courtesy of senior author (KM).
Similarly, ProChondrix (AlloSource, Centennial, CO) is a laser-etched, fresh cryopreserved osteochondral allograft that provides functional cells and PCM plus surrounding ECM (beyond that seen in chondrons) to a defect site. Following MS, ProChondrix works as a scaffold for the MSCs while the viable chondrocytes concurrently release PCM/ECM to help promote chondrogenesis. In a unpublished series, Mehta et al. 59 demonstrated a statistically significant increase in KOOS, Short Form–36 health questionnaire (SF-36) domains, subjective IKDC and Tegner activity scores at a mean follow up of 2.33 years in 17 patients for treatment of isolated, symptomatic articular cartilage surface lesions. Currently, there is an ongoing prospective, multicenter study to evaluate the use of ProChondrix for improvement in physical function and pain when used on symptomatic cartilage defects of the femoral condyle or patella for a period of 60 months follow-up with a target enrollment of 80 patients. 60
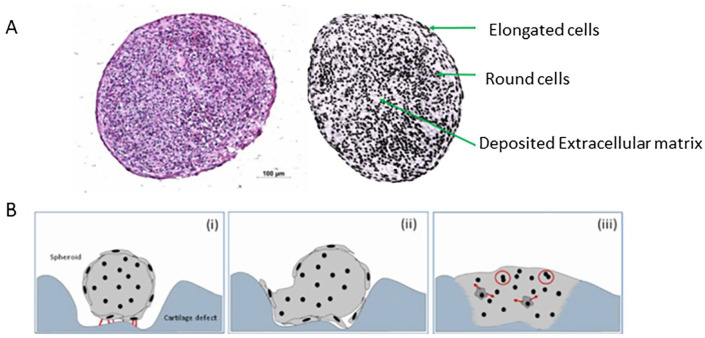
Finally, combining ACI with knowledge learned from studying the PCM has led to advances in technologies. Chondrocyte de-differentiation, 61 during in vitro monolayer expansion, remains a key limitation of ACI. 62 Chondrons have a higher regenerative capacity when compared to chondrocytes without their PCM. 45 Consequently, ACI with Spherox (formerly called chondrosphere) (ACT3D-CS, CO.DON AG, Teltow, Germany) was developed. The chondrocyte spheroids derive from human autologous chondrocytes and associated cartilage-specific matrix, which under defined cell culture conditions can build a 3-dimensional structure ( Fig. 5 ). Unlike chondrocyte cell suspensions that do not begin differentiating and producing matrix proteins until transplantation into the defect, the chondrocytes in the spheroids are in a more developed differentiation state with a pronounced formation of ECM. 63 In mouse model, chondrospheres demonstrated an ability to adhere to full-thickness cartilage defects and produce a cartilaginous extracellular matrix increasing content of collagen type II, glycosaminoglycans and collagenous fibers thus restoring and conserving phenotype. 64 In a 1-year clinical follow-up study of 37 patients treated with Spherox, there was significantly improved scores (Lysholm, IKDC, SF-36, and Tegner) with a sizable degree of defect fill seen at 3 months on MRI. 63 In a RCT, a potential dose relationship was seen in terms of magnetic resonance observation of cartilage repair tissue (MOCART) scores, but no significant correlation for clinical outcomes 65 or any safety criteria. 66 In a 2-year follow-up RCT, matrix-associated ACI with Spherox demonstrated significantly higher KOOS Activities of Daily Living subscore compared to MFx with no other differences noted. 67 However, a histological analysis of biopsies suggested a better quality of repair in the patients treated with Spherox. 67 Ultimately, in the absence of a head-to-head trial, indirect treatment comparisons can be made using network meta-analysis (NMA) methodology. This was used to estimate the relative clinical performance of Spherox versus other ACI. Using this data and a lifetime Markov model, the cost-effectiveness of Spherox was calculated. Estimates for chondrosphere versus MFx and for chondrosphere versus matrix-applied autologous cultured chondrocyte implant (MACI) were £4360 and around £18,000 per quality-adjusted life year gained, respectively. 68 As a result, the National Institute of Health and Care Excellence (NICE) recommended ACI using Chondrosphere for treating adult patients with symptomatic ICRS grades III or IV articular cartilage defects of the femoral condyle and patella if (1) the patient has not had previous cartilage repair surgery, (2) there is minimal osteoarthritic damage, and (3) the defect is over 2 cm2. 69
Figure 5.
Autologous chondrocyte implantation with Spherox (formerly called chondrosphere). (A) Hematoxylin-eosin staining showing the histology and the appearance of chondrocytes in a spheroid (Spherox). They derive from human autologous chondrocytes and associated cartilage-specific matrix in a 3-dimentional structure. (B) Schematic representation of the adhesion (i) of a cartilage cell spheroid to the defect ground through adhesion points (indicated by the red lines) (ii) widening of the spheroids and its integration in to the adhesion area by migration of surface chondrocytes along the irregular surface of the defect ground (iii) spheroid is completely integrated into the cartilage defect. Red arrows indicate secretion of cartilage-specific proteins. Red circles indicate a presumed cell proliferation. Images used with permission from CO.DON AG, Teltow, Germany.
Conclusion
In summary, the use of isolated chondrons as a model for studying the chondrocyte microenvironment including the ECM has enhanced our grasp of cartilage biology. Execution and improvement of cartilage engineering protocols with emphasis on the microenvironment of chondrocytes/MSCs and ECM could lead to the production of more robust and functional cartilage. Lesions in articular cartilage can cause considerable musculoskeletal morbidity including pain and loss of function. With that comes significant economic implications, especially when considering its progression to OA. 70 The ambition of a chondrocytes with matrix therapy aims to bring about a repair without the need for further long-term surgery. Bearing this in mind, we have highlighted the ongoing research encompassing novel tissue sources and cell types that are being developed and employed for cartilage repair utilizing underlying chondrocytes with matrix theory.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Sarav S. Shah  https://orcid.org/0000-0002-7656-8482
https://orcid.org/0000-0002-7656-8482
References
- 1. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1_suppl):30-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinegard D. Fell-Muir lecture: proteoglycans and more—from molecules to biology. Int J Exp Pathol. 2009;90(6):575-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox AJS, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes LC, Archer CW, ap Gwynn I. The ultrastructure of mouse articular cartilage: collagen orientation and implications for tissue functionality. A polarised light and scanning electron microscope study and review. Eur Cell Mater. 2005; 9:68-84. [DOI] [PubMed] [Google Scholar]
- 5. Bekkers JE, Tsuchida AI, van Rijen MH, Vonk LA, Dhert WJA, Creemers LB, et al. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am J Sports Med. 2013;41(9):2158-66. [DOI] [PubMed] [Google Scholar]
- 6. Wang QG, Nguyen B, Thomas CR, Zhang Z, El Haj AJ, Kuiper NJ. Molecular profiling of single cells in response to mechanical force: comparison of chondrocytes, chondrons and encapsulated chondrocytes. Biomaterials. 2010;31(7):1619-25. [DOI] [PubMed] [Google Scholar]
- 7. Graff RD, Kelley SS, Lee GM. Role of pericellular matrix in development of a mechanically functional neocartilage. Biotechnol Bioeng. 2003;82(4):457-64. [DOI] [PubMed] [Google Scholar]
- 8. Larson CM, Kelley SS, Blackwood AD, Banes AJ, Lee GM. Retention of the native chondrocyte pericellular matrix results in significantly improved matrix production. Matrix Biol. 2002;21(4):349-59. [DOI] [PubMed] [Google Scholar]
- 9. Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen DM, Mao JJ. Heterogeneous nanostructural and nanoelastic properties of pericellular and interterritorial matrices of chondrocytes by atomic force microscopy. J Struct Biol. 2004;145(3):196-204. [DOI] [PubMed] [Google Scholar]
- 11. Korhonen RK, Herzog W. Depth-dependent analysis of the role of collagen fibrils, fixed charges and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. J Biomech. 2008;41(2):480-5. [DOI] [PubMed] [Google Scholar]
- 12. Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegård D, et al. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278(39):37698-704. [DOI] [PubMed] [Google Scholar]
- 13. Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15(7):752-63. [DOI] [PubMed] [Google Scholar]
- 14. Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58(12):3788-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanbe K, Yang X, Wei L, Sun C, Chen Q. Pericellular matrilins regulate activation of chondrocytes by cyclic load-induced matrix deformation. J Bone Miner Res. 2007;22(2):318-28. [DOI] [PubMed] [Google Scholar]
- 16. Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology (Oxford). 2011;50(3):463-72. [DOI] [PubMed] [Google Scholar]
- 17. Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Preservation of the chondrocyte’s pericellular matrix improves cell-induced cartilage formation. J Cell Biochem. 2010;110(1_suppl):260-71. [DOI] [PubMed] [Google Scholar]
- 18. Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis Cartilage. 2002;10(4):297-307. [DOI] [PubMed] [Google Scholar]
- 19. Peters HC, Otto TJ, Enders JT, Jin W, Moed BR, Zhang Z. The protective role of the pericellular matrix in chondrocyte apoptosis. Tissue Eng Part A. 2011;17(15-16):2017-24. [DOI] [PubMed] [Google Scholar]
- 20. Nagaprashantha LD, Vatsyayan R, Lelsani PC, Awasthi S, Singhal SS. The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int J Cancer. 2011; 128(4):743-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng IH, Lin YC, Hwang E, Huang HT, Chang WH, Liu YL, et al. Collagen VI protects against neuronal apoptosis elicited by ultraviolet irradiation via an Akt/phosphatidylinositol 3-kinase signaling pathway. Neuroscience. 2011;183:178-88. [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z. Chondrons and the pericellular matrix of chondrocytes. Tissue Eng Part B Rev. 2015;21(3):267-77. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Z, Fan J, Becker KG, Graff RD, Lee GM, Francomano CA. Comparison of gene expression profile between human chondrons and chondrocytes: a cDNA microarray study. Osteoarthritis Cartilage. 2006;14(5):449-59. [DOI] [PubMed] [Google Scholar]
- 24. Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, et al. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol. 2011;179(3):1338-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. French MM, Gomes RR, Jr, Timpl R, Höök M, Czymmek K, Farach-Carson MC, et al. Chondrogenic activity of the heparan sulfate proteoglycan perlecan maps to the N-terminal domain I. J Bone Miner Res. 2002;17(1_suppl):48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomes R, Kirn-Safran C, Farach-Carson MC, Carson DD. Perlecan: an important component of the cartilage pericellular matrix. J Musculoskelet Neuronal Interact. 2002;2(6):511-6. [PMC free article] [PubMed] [Google Scholar]
- 27. Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30(36):6964-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishijima M, Suzuki N, Hozumi K, Matsunobu T, Kosaki K, Kaneko H, et al. Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation. Matrix Biol. 2012;31(4):234-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macri L, Silverstein D, Clark RAF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59(13):1366-81. [DOI] [PubMed] [Google Scholar]
- 30. Ramos YF, den Hollander W, Bovee JVMG, Bomer N, van der Breggen R, Lakenberg N, et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS One. 2014;9(7):e103056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mrosek EH, Lahm A, Erggelet C, Uhl M, Kurz H, Eissner B, et al. Subchondral bone trauma causes cartilage matrix degeneration: an immunohistochemical analysis in a canine model. Osteoarthritis Cartilage. 2006;14(2):171-8. [DOI] [PubMed] [Google Scholar]
- 32. McGlashan SR, Cluett EC, Jensen CG, Poole CA. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 2008;237(8):2013-20. [DOI] [PubMed] [Google Scholar]
- 33. Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60(10):3028-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J, Liu W, Bemis A, Wang E, Qiu Y, Morris EA, et al. Comparative proteomic characterization of articular cartilage tissue from normal donors and patients with osteoarthritis. Arthritis Rheum. 2007;56(11):3675-84. [DOI] [PubMed] [Google Scholar]
- 35. Polur I, Lee PL, Servais JM, Xu L, Li Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol. 2010;25(5):599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, et al. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280(1_suppl):548-55. [DOI] [PubMed] [Google Scholar]
- 37. Holt DW, Henderson ML, Stockdale CE, Farrell JT, Kooyman DL, Bridgewater LC, et al. Osteoarthritis-like changes in the heterozygous sedc mouse associated with the HtrA1-Ddr2-Mmp-13 degradative pathway: a new model of osteoarthritis. Osteoarthritis Cartilage. 2012;20(5):430-9. [DOI] [PubMed] [Google Scholar]
- 38. Xie Z, Khair M, Shaukat I, Netter P, Mainard D, Barré L, et al. Non-canonical Wnt induces chondrocyte de-differentiation through Frizzled 6 and DVL-2/B-raf/CaMKIIα/syndecan 4 axis. Cell Death Differ. 2018;25(8):1442-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261-70. [DOI] [PubMed] [Google Scholar]
- 40. Cheng NC, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15(2):231-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418(6893):41-9. [DOI] [PubMed] [Google Scholar]
- 42. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:58-69. [DOI] [PubMed] [Google Scholar]
- 43. Tsuchiya K, Chen G, Ushida T, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Materials Sci Eng. 2004;24(3):391-6. [Google Scholar]
- 44. Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, et al. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 2009;60(2):450-9. [DOI] [PubMed] [Google Scholar]
- 45. Vonk LA, de Windt TS, Kragten AH, et al. Enhanced cell-induced articular cartilage regeneration by chondrons; the influence of joint damage and harvest site. Osteoarthritis Cartilage. 2014;22(11):1910-7. [DOI] [PubMed] [Google Scholar]
- 46. de Windt TS, Vonk LA, Slaper-Cortenbach IC, van den Broek MPH, Nizak R, van Rijen MHP, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1_suppl):256-64. [DOI] [PubMed] [Google Scholar]
- 47. de Windt TS, Vonk LA, Slaper-Cortenbach ICM, Nizak R, van Rijen MHP, Saris DBF. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35(8):1984-93. [DOI] [PubMed] [Google Scholar]
- 48. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170-9. [DOI] [PubMed] [Google Scholar]
- 49. Biant LC, Bentley G. Stem cells and debrided waste: two alternative sources of cells for transplantation of cartilage. J Bone Joint Surg Br. 2007;89(8):1110-4. [DOI] [PubMed] [Google Scholar]
- 50. Marmotti A, Bruzzone M, Bonasia DE, Castoldi, Von Degerfeld MM, Bignardi C, et al. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur Cell Mater. 2013;26:15-31. [DOI] [PubMed] [Google Scholar]
- 51. Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2590-601. [DOI] [PubMed] [Google Scholar]
- 52. Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37(Suppl 1):71S-80S. [DOI] [PubMed] [Google Scholar]
- 53. Levinson C, Cavalli E, Sindi DM, Kessel B, Zenobi-Wong M, Preiss S, et al. Chondrocytes from device-minced articular cartilage show potent outgrowth into fibrin and collagen hydrogels. Orthop J Sports Med. 2019;7(9):2325967119867618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonasia DE, Marmotti A, Mattia S, Cosentino A, Spolaore S, Governale G, et al. The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: an in vitro study. Arthroscopy. 2015;31(12): 2335-41. [DOI] [PubMed] [Google Scholar]
- 55. Woodmass JM, Melugin HP, Wu IT, Saris DBF, Stuart MJ, Krych AJ. Viable osteochondral allograft for the treatment of a full-thickness cartilage defect of the patella. Arthroscopy Techniques. 2017;6(5):e1661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Geraghty S, Kuang JQ, Yoo D, LeRoux-Williams M, Vangsness CT, Danilkovitch A. A novel, cryopreserved, viable osteochondral allograft designed to augment marrow stimulation for articular cartilage repair. J Orthop Surg Res. 2015;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vangsness CT, Higgs G, Hoffman JK, et al. Implantation of a novel cryopreserved viable osteochondral allograft for articular cartilage repair in the knee. J Knee Surg. 2018;31(6):528-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow stimulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mehta V, Mandala C, Shriver R. Clinical results of a novel fresh osteochondral allograft for focal articular cartilage defects. Presented at: 5th ICRS Summit Bio-Orthopaedics in Sports Medicine; 2019; San Diego, CA Available from: https://www.allosource.org/wp-content/uploads/2019/10/2019-ICRS-Summit-San-Diego-Poster.pdf [Google Scholar]
- 60. ClinicalTrials.gov. Prospective evaluation of ProChondrix CR for repair of articular cartilage defects on femoral condyle and patella. Available from: https://clinicaltrials.gov/ct2/show/NCT03873545
- 61. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1_suppl):215-24. [DOI] [PubMed] [Google Scholar]
- 62. Davies RL, Kuiper NJ. Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering (Basel). 2019;6(1_suppl):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fickert S, Gerwien P, Helmert B, Schattenberg T, Weckbach S, Kaszkin-Bettag M, et al. One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage. 2012;3(1_suppl):27-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schubert T, Anders S, Neumann E, et al. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int J Mol Med. 2009;23(4):455-60. [DOI] [PubMed] [Google Scholar]
- 65. Niemeyer P, Laute V, John T, Becher C, Diehl P, Kolombe T, et al. The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the knee: a randomized clinical trial. Am J Sports Med. 2016;44(8):2005-14. [DOI] [PubMed] [Google Scholar]
- 66. Becher C, Laute V, Fickert S, Zinser W, Niemeyer P, John T, et al. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res. 2017;12(1_suppl):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Niemeyer P, Laute V, Zinser W, Becher C, Kolombe Y, Fay J, et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7(7):2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Armoiry X, Cummins E, Connock M, Metcalfe A, Royle P, Johnston R, et al. Autologous chondrocyte implantation with chondrosphere for treating articular cartilage defects in the knee: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(7):879-86. [DOI] [PubMed] [Google Scholar]
- 69. National Institute for Health and Care Excellence. Autologous chondrocyte implantation using chondrosphere for treating symptomatic articular cartilage defects of the knee. Available from: https://www.nice.org.uk/guidance/ta508
- 70. Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001(391 Suppl):S14-25. [PubMed] [Google Scholar]