Abstract
Background
Despite several decades of research, managing body weight remains an unsolved clinical problem. Health problems associated with dysregulated body weight, such as obesity and cachexia, exhibit several gut microbiota alterations. There is an increased interest in utilising the gut microbiota for body weight control, as it responds to intervention and plays an important role in energy extraction from food, as well as biotransformation of nutrients.
Scope of the review
This review provides an overview of the role of the gut microbiota in the physiological and metabolic alterations observed in two body weight dysregulation-related disorders, namely obesity and cachexia. Second, we assess the available evidence for different strategies, including caloric restriction, intermittent fasting, ketogenic diet, bariatric surgery, probiotics, prebiotics, synbiotics, high-fibre diet, and fermented foods – effects on body weight and gut microbiota composition. This approach was used to give insights into the possible link between body weight control and gut microbiota configuration.
Major conclusions
Despite extensive associations between body weight and gut microbiota composition, limited success could be achieved in the translation of microbiota-related interventions for body weight control in humans. Manipulation of the gut microbiota alone is insufficient to alter body weight and future research is needed with a combination of strategies to enhance the effects of lifestyle interventions.
Keywords: Body weight, Obesity, Gut microbiota, Cachexia, Dietary intervention, Metabolism
Highlights
-
•
The gut microbiota is involved in the control of nutrient availability, appetite, and body weight.
-
•
Both obesity and cachexia are associated with altered gut microbiota.
-
•
Specific dietary and surgical approaches positively impact body weight and gut microbiota.
-
•
Manipulation of the gut microbiota alone is insufficient to alter body weight in humans.
List of Abbreviations
- 16S
Subunit 16
- ADF
Alternate-day fasting
- AIDS
Acquired immunodeficiency syndrome
- BMI
Body-mass index
- CCK
Cholecystokinin
- ClpB
Caseinolytic Mitochondrial Matrix Peptidase Chaperone Subunit B
- CR
Calorie restriction
- CRP
C-reactive protein
- db/db
Leptin receptor diabetic gene deficiency
- DIO
Diet-induced obesity
- FA
Fatty acid
- FMT
Faecal microbiota transplantation
- GDF15
Growth differentiation factor 15
- GF
Germ-free
- GIT
Gastrointestinal tract
- GLP-1
Glucagon-like peptide-1
- GOS
Galacto-oligosaccharide
- GPR
G-protein receptor
- HDL
High-density lipoprotein
- HF
High-fermentable
- HFD
High-fat diet
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- ICR
Intermittent caloric restriction
- IF
Intermittent fasting
- IL
Interleukin
- ITS
Internal transcribed spacer
- KD
Ketogenic diet
- LDL
Low-density lipoprotein
- LF
Low-fermentable
- LPS
Lipopolysaccharide
- ob/ob
Loss-of-function leptin gene mutation
- PF
Prolonged fasting
- PYY
Peptide tyrosine tyrosine
- RNA
Ribonucleic acid
- rRNA
Ribosomal ribonucleic acid
- SCFA
Short-chain fatty acid
- T2D
Type-2 diabetes
- TRF
Time-restricted feeding
1. Introduction
The gut microbiota, defined as the trillions of microorganisms living in the gastrointestinal tract (GIT), is intricately linked with the health of the host. Several pathological conditions, including metabolic, mental, immune diseases, and cancer, have been associated with an alteration in the configuration and function of the gut microbiota [[1], [2], [3], [4], [5]]. Therefore, there is growing interest in identifying microbiota-targeted therapeutic strategies [6].
Bodyweight is the result of cumulative signals that regulate energy balance. The main factors that influence energy balance are feeding behaviour and energy intake, intestinal energy absorption, energy expenditure, and energy storage [[7], [8], [9]]. Long-term disruption of energy homeostasis leads to the development of several chronic disorders such as obesity (positive energy balance), cachexia and malnutrition (negative energy balance) [10,11]. Obesity is a multifactorial condition whereby the energy extracted from the food consumed is greater than what is used by the body. This leads to an expansion of adipose tissue mass and, consequently, weight gain as well as other comorbidities [12,13]. Conversely, cachexia is a multifactorial condition characterized by excessive weight loss following a severe illness such as cancer [14,15]. Alterations in the composition and functionality of gut microbiota have been observed during the pathogenesis of both obesity and cachexia [16,17]. This occurs because the gut microbiota is wholly dependent on the host organism for water and nutrients, and one of the main processes by which the gut microbiota can affect host physiology is the production of bioactive metabolites from the gastrointestinal contents. Moreover, accumulating evidence demonstrates additional mechanisms and functions of the gut microbiota that go beyond peripheral effects and span both the gut and brain [18]. For example, microbiota-derived metabolites, such as short-chain fatty acids (SCFAs), have been shown to be involved in the control of glucose homeostasis, adipose tissue biology, secretion of gut hormones and neuroactive compounds, and the modulation of vagal nerve activity, all of which can influence metabolism, central appetite regulation, and drive eating behavior [19,20].
The current review provides an update on the effect of the microbiota on host metabolism, appetite, and eating behaviour as well as the role of the gut microbiota in the pathogenesis of obesity and cachexia. This is followed by a critical discussion on the effect of different dietary, surgical, and supplementary approaches, such as calorie restriction (CR), intermittent fasting (IF), ketogenic diet (KD), bariatric surgery, probiotics, prebiotics, synbiotics, high-fibre diet, and fermented foods, in shaping the gut microbiota while having an impact on the bodyweight in the context of obesity and cachexia.
2. The gut microbiota
2.1. Introduction to the gut microbiota
Research over the past two decades has demonstrated the central role of mammalian-associated microbiota in host physiology [21] with the ‘holobiont’ concept proposed to encompass both the eukaryotic host and its microbiota as basic biological units [22]. The gut microbiota incorporates the bacteria, viruses, fungi, and archaea that reside within the GIT, with revised estimates of over 10 trillion total microorganisms, with a huge majority of them residing in the large intestine [23].
Metagenomic analysis of microbial composition is typically performed on faecal samples by sequencing marker genes (such as the 16S rRNA gene in bacteria and the internal transcribed spacer (ITS) in fungi) or whole metagenome sequencing approaches, allowing for both high-resolution taxonomic and functional characterization [24]. Furthermore, functional approaches such as metatranscriptomics, metaproteomics, and metabolomics are also increasingly employed to describe the behavior of these communities [25]. The developments in culture-based approaches have also significantly increased the proportion of cultivable micro-organisms available for in vitro analysis [26]. At the forefront of population-level microbiota research, several large studies are available, notably the National Institutes of Health (NIH)-funded Human Microbiome Project [27] in the United States and the European Commission-funded Metagenomics of the Human Intestinal Tract (MetaHIT) consortium [28], as well as national population cohorts [[29], [30], [31]]. These studies have documented unprecedented diversity in the human microbiota, with recently updated global estimates of about 2,500 species, extending observations beyond cohorts of largely European ancestry [32]. They have also shown large degrees of inter-individual variability in terms of composition with a more conserved functional make-up. Attempts to group individuals into categories based on their microbiota (termed ‘enterotypes’) have identified 3 clusters, based on enrichments in Prevotella, Bacteroides, and Ruminococcus [33], the generalizability, the biological and clinical relevance of which are active areas of research [34,35]. Beyond bacteria, comparatively neglected though important fungal [36] and viral [37] components are present. The gut microbiota has been implicated in a wide range of diseases, notably inflammatory and autoimmune disorders [25,38], a number of cancers [[39], [40], [41]], cardiometabolic [42], and neurological and psychiatric conditions [43,44]. However, many human observational microbiota studies remain underpowered, hampered by the large degree of inter-personal variability in microbiota communities and numerous confounding variables, including diet, medication use, body mass index (BMI), alcohol consumption, age, physical activity, and stool consistency [29,45].
2.2. Application of animal models and tools in the study of the microbiota
There are many ways to examine the contribution of the microbiota to host physiology (Figure 1), including experiments with germ-free (GF) or antibiotic-treated animal models. Moreover, faecal microbiota transplant (FMT) studies can be conducted to provide further proof-of-concept evidence of the association of the gut microbiota with altered disease phenotypes. The studies conducted to examine the role of the gut microbiota in body weight control began with the identification of reduced body weight in GF mice despite an increased caloric intake [46] followed by the demonstration of resistance to diet-induced obesity [47] and the transferability of this phenotype by FMT [48].
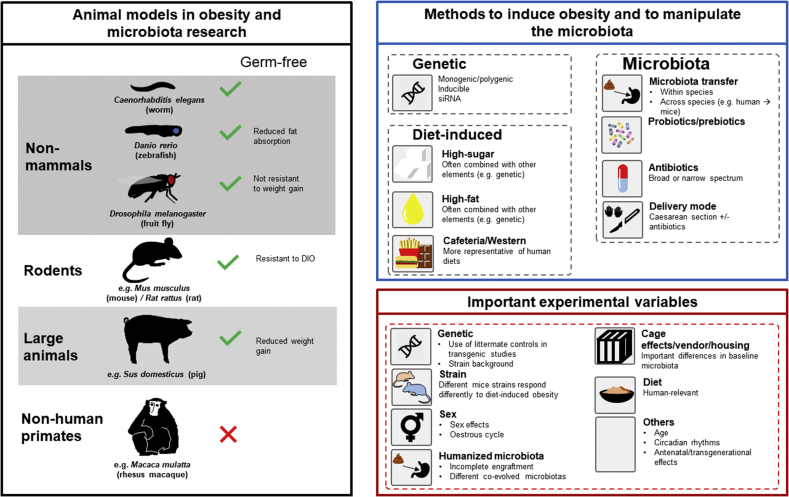
Figure 1.
Animal models used as tools in obesity and microbiome research. Animal models used in obesity and microbiome research (left panel). Methods employed to induce obesity and to manipulate the gut microbiota in animal models. Also indicated are the co-factors (variables) to take into account during the preclinical experiment on obesity and microbiota analysis (right panel). Abbreviation: DIO; diet-induced obesity, siRNA; small interfering ribonucleic acid.
Animal models, including non-mammalian model organisms (such as Drosophila melanogaster), rodents, large animals (such as pigs) and non-human primates are essential tools in obesity and microbiome research (Figure 1). Several approaches are commonly employed by in vivo preclinical studies to investigate the role of the gut microbiota in different circumstances, for example during diet intervention, drug administration, genetic effects or disease models. The following methods aim to disturb or manipulate the gut microbiota in order to assess the consequent amelioration or worsening of the original phenotype in the animal model. These methods are used alone or in combination with the above approaches. Some common strategies employed in rodents, the model organism most commonly used in the studies reviewed in the present paper, are described below (Figure 1).
-
-
Germ-free rodents- GF animals are generated from founder animals that are delivered via aseptic caesarean section (C-section) and are not exposed to microorganisms since their birth and throughout the lifespan. GF rodents differ with respect to the development and physiology, relative to animals with commensal bacteria. For example, they are characterized by lower body weight, as well as impaired intestinal function, immune system, metabolism, neurodevelopment and hormone signalling regulation [[49], [50], [51], [52], [53]]. In addition to comparative studies with conventionally raised animals, GF rodents also allow for colonisation with known communities (gnotobiotic), providing exquisite control over otherwise complex communities. Relevant examples include the production of the tryptophan metabolite indole propionic acid [54,55] in Clostridium sporogenes and bacterial bile salt hydrolase expressed in Escherichia coli [56] influencing host immunity, gut permeability, and metabolism.
-
-
Antibiotic treatment. Different antibiotics target and inhibit/interfere with a specific function essential for the bacterial cell, such as DNA replication, RNA synthesis, protein synthesis, and cell wall biosynthesis [57]. Antibiotics can be broad-spectrum, acting on different bacterial taxa, and narrow-spectrum, targeting specific taxa. Broad-spectrum antibiotic usage can have the undesirable effect of also impacting non-pathogenic bacteria [58]. A single antibiotic or an antibiotic cocktail, usually broad-spectrum, are given to the rodents dissolved in drinking water or placed directly into the GIT using oral gavage [59]. However, compared to GF rodents, antibiotic treatment offers a rapid, affordable, and achievable method to study the effects of gut microbiota depletion. The main advantages in the usage of antibiotics versus GF rodents are [59] that antibiotics can rapidly deplete gut microbiota in any rodent genotype or condition and allow for microbial depletion in rodents that were normally colonized since birth. This allows the researchers to evaluate the effects of microbial depletion independent of the numerous developmental impairments observed in GF rodents. However, this gut microbiota depletion method has some disadvantages. First, all bacteria are not killed by antibiotic treatment and antibiotic-resistant pathogens and non-bacteria, e.g., fungi, can flourish. Second, the antibiotic treatment is not always localized within the intestine, as many antibiotics exert systemic effects, influencing other organs and eukaryotic cells. This leads to antibiotic-induced side effects, such as problems with immunomodulation, metabolism, and digestion [60,61]. The use of gut-specific antibiotics such as oral vancomycin has been reported in humans though few benefits have initially been suggested for metabolic syndrome [62]. Further, a recent randomized controlled study of antibiotics in obese pre-diabetic men demonstrated no significant effect of microbiota manipulation on host metabolism, despite having significant and sustained alterations to key members of Firmicutes which have been implicated in energy harvest [63]. This study not only suggests that host metabolism and dietary energy harvest are largely resilient to gut microbiota manipulation in adult humans but also provides invaluable evidence that is not readily available from rodent studies.
-
-
Probiotics, prebiotics, and synbiotics interventions. Probiotics are defined as a live microorganism that confers health benefits to the host when consumed in adequate amounts [64] while prebiotics are selectively fermented non-digestible food ingredients that bring about specific changes in the composition and/or activity of the gastrointestinal microbiota, thereby conferring benefits upon the host health [65,66]. The mixtures comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confer a health benefit on the host are called synbiotics [67].
-
-
Faecal microbiota transplant. Transfer of the gut microbiota can be achieved through co-housing of rodents or directly by FMT. FMT involves the administration of faecal material from a donor to a recipient directly into the GIT [[48], [68], [69], [70]]. FMT is being investigated as a treatment across a huge range of conditions and has demonstrated some benefit in the induction of remission in ulcerative colitis [[71], [72], [73], [74]], improving response to immune checkpoint inhibitors [75,76], and as a potential treatment in metabolic syndrome [77,78]. Through FMT, it is also possible to create the so-called humanized rodents by administering stool from a human donor into a rodent recipient [79,80]. Humanized gnotobiotic rodents, animals into which a human microbiota has been transplanted, have been used to demonstrate causality; the transfer of human disease traits has been reported across a broad spectrum of conditions, including obesity [81,82]. However, the near-universal demonstration of such ‘phenotype-transfer’ has raised concerns about the methodological and statistical approaches employed [83,84]. Particularly, Walter et al. noted that human microbiota-associated animal studies frequently fail to demonstrate recapitulation of disease-associated microbiota changes (or ‘dysbiosis’) in recipient rodents (<30%), while >80% of studies use pseudoreplication and are thus statistically underpowered to reach their conclusions [83], leading to guideline aimed at standardizing approaches to FMT in rodent models [84,85]. In addition to improving the rigor of these studies, Walter et al. suggest several approaches, including using large animal models, experimental designs aimed at addressing causality, and intervention studies in humans where the microbiota is directly manipulated.
-
-
Mode of delivery at birth. Delivery mode is one of the most important factors that shape the gut microbiota in early life. Infants born by caesarean section have significantly different early gut microbial features compared to vaginally delivered infants [86]. Rodent models of caesarean section are used to study the effects of early life manipulations of the gut microbiota on different aspects of health and disease [87,88].
2.3. The gut microbiota depends fully on the host for its energy requirements
Microbial metabolism of undigested material in the GIT is a key mechanism by which the gut microbiota and host interact; the substances ingested or secreted into the gut lumen by the host organism provide essential nourishment for the microbes and shapes gut microbiota composition, and the products of microbial enzymes can then interact with host tissues, either locally at the gut epithelium or following absorption [89,90]. The gut microbiota depends on the host for meeting all its nutritional and water requirements. While some ingested nutrients are utilised directly by gut bacteria, most energy requirements are met by bacteria exploiting food remnants, that the host has already digested and acquired nutrients from, bacterial proteins and polysaccharides, and non-digestible fibre [91,92]. To take full advantage of the vast range of substances that make their way into the GIT, a healthy gut microbiota exhibits a substantial enzymatic repertoire [93], thus producing a wide range of metabolites and compounds capable of impacting host physiology.
In order to maintain a stable community and gut ecosystem, the gut microbiota must regulate its growth rate and population size in response to nutrient availability and population density, communicated through quorum signalling of the gut microbiota. This large contribution to bacterial growth in an organism is further modified by several host factors [94,95], such as digestive enzymes, peristalsis, and excretion. This results in a cascade of cyclical changes in gut microbiota density and diversity over the day, aligning with feeding and fasting patterns, in both rodents [96] and humans [97]. Bacterial duplication is an energy-expensive process, as ∼ 4 kJ is required for duplication by 1 g of gut bacteria [98]. We know that approximately 15 g of bacteria are lost daily in faeces [99], the rate of bacterial turnover in the gut remains unknown and understanding this rate is very important for understanding the daily energy requirements of the gut microbiota. GF mice exhibit 27% reduced oxygen consumption [46], which may represent the gut microbiota's energy requirements. The gut microbiota's reliance upon the host for energy implies that the gut bacteria should be able to manipulate host mechanisms of energy intake, and bacterial growth should align with feeding behaviours and interoceptive hunger and satiety [98].
2.4. The gut microbiota modifies the host metabolome and nutrient availability
Gut microbiota composition is shaped by habitual diet across mammalian species [100,101] including humans [94,[102], [103], [104], [105]]. In adults, rapid, reversible shifts in the composition and function of the microbiota on an animal-based diet have been described, including switching from carbohydrate to protein fermentation, increases in the secondary bile acid deoxycholic acid, and increased abundance of bile tolerant organisms such as Alistipes and Bilophila [94]. However, microbiota resilience to dietary intervention has also been reported [106], while the beneficial effects of dietary fibre in type 2 diabetes (T2D) appear to be associated with a limited number of species (termed a ‘guild’) that can utilise fibre and produce SCFAs [107] and response to resistant starch is dependent on the presence of a specific species [108].
The gut microbiota itself exerts a profound effect on the mammalian host's global metabolome [55], with SCFAs [109], l-carnitine and choline derivatives [110], tryptophan metabolites [111], branched-chain amino acids [112], and transformed bile acids [56] being key classes in metabolic health that are influenced by diet (specific examples are discussed below).
The gut microbiota also contributes directly to the energy harvest of non-digestible carbohydrates from dietary fibre and this has been proposed as a mechanism by which differences in the composition of the gut microbiota can influence energy balance [[113], [114], [115]]. The vast majority of micro-organisms in the GIT (>97%) are located in the large intestine [23], where between 10 g and 60 g of carbohydrates are estimated to become available to the gut microbiota per day [116,117]. Dietary fibre is fermented in the large intestine to produce SCFAs, predominantly butyrate, propionate, and acetate, which act as fuel as well as diverse signalling molecules; SCFAs have been reported to increase in overweight individuals [118]. In addition to having anti-proliferative effects and influencing mucosal immunity [119,120], butyrate is an important energy source for the colonic epithelium. Propionate has an important role in the control and acts as a substrate for intestinal gluconeogenesis, as well as the release of the gut hormones peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) [[121], [122]]. Acetate circulates systemically and is important in peripheral lipid metabolism, as well as in central appetite regulation [122]. SCFA also exerts an influence on gut permeability, which has important repercussions for host inflammation and metabolism [122].
Two things remain unclear: the extent of the direct contribution of gut microbiota energy harvest to net energy balance and whether alterations in the gut microbiota meaningfully influence this balance. A figure of up to 10% contribution to total energy gain from non-digestible carbohydrates is often cited based on a UK diet [123]. However, in revising this estimate, it was recently suggested that based on a daily fibre intake of 25 g, the contribution of bacterial-derived SCFAs to total energy absorption would be approximately 2.5% [124]. Therefore, even if gut microbiota with a high capacity for energy harvest exist [113], their maximum theoretical contribution to total energy yield would be more modest than previous estimates suggest. Furthermore, by increasing the proportion of carbohydrates from dietary fibre (and thus the influence of the gut microbiota on energy harvest), total energy gain for a fixed calorie intake is decreased [124], implying a comparatively minor role of the gut microbiota in obesogenic diets, which are associated with low levels of fibre.
These calculations may be supported by observations in patients who have had a pan-proctocolectomy (removal of the colon and rectum), an established surgical treatment for ulcerative colitis, and a prophylactic measure for patients with hereditary colorectal cancer syndromes. While most long-term follow-up focuses on quality of life (QOL), functional outcomes and specific nutritional deficiencies [125], removal of the large intestine (and thus the fermentative energy-harvesting capacity of the gut microbiota) do not appear to be associated with significant long-term weight loss [126,127]. Additionally, manipulation of the gut microbiota with antibiotics in a randomised controlled trial did not affect energy harvest (or other clinically relevant outcomes) in obese prediabetic men, following 7 days of treatment or 8 weeks after, despite showing dramatic global change in composition and diversity of the gut microbiota [63]. This finding challenges the role of variations in the energy-harvesting capacity of the gut microbiota as a key influence on host energy balance.
3. The role of the microbiota-gut-brain axis in homeostatic and hedonic regulation of appetite
Bodyweight control and energy homeostasis are essential for survival and health [128]. Signals from multiple organ systems are integrated into the hypothalamus and other brain regions, which then regulate food-seeking behaviour and consumption. Macronutrients and hormones released from the gut, liver, and pancreas are generated during discrete meals and act as satiation signals to halt or reduce discrete feeding bouts, while leptin and other adipokines are released proportional to total body fat and are thought to regulate long-term energy intake and expenditure [129] (For recent comprehensive reviews on the central and peripheral appetite and satiety systems please see [[130], [131], [132]]). In parallel and in combination with homeostatic appetite regulation, brain regions involved in reward regulate energy intake by modifying palatable food-seeking [133] and may also be modulated by the gut microbiota. A recent study found that FMT from obese mice transferred reduced preference for a high-fat high-sugar diet to recipient mice while microbial depletion with antibiotics altered intake of high-fat high-sugar diet in lean mice [134]. A number of these peripheral appetites and satiety-related molecules send a signal to the brain via the gut-brain axis, defined as a bidirectional communication between the central nervous system and the gut. In addition, the gut microbiota is an important modulator of immune system development and homeostasis [135] and can regulate cytokine production [136]; therefore, it may modify feeding behaviour indirectly through interactions with the immune system. Indeed, some pathogenic microbes have evolved mechanisms to inhibit sickness-associated anorexia to improve host health and increase transmission [137].
Accumulating evidence demonstrates a strong influence of the gut microbiota on gut-brain axis structure and function with important consequences for overall host physiology [18]. Several mechanisms of microbiota-gut-brain communication have been described. They include microbiota-mediated effects on the afferent and efferent fibres of the vagus nerve, nutrient sensing in the gut, intestinal barrier function, modulation of the immune system, microbiota-mediated changes in gut hormone secretion, fermentation of nutrients, microbial metabolite signalling, and production of neuroactive signalling molecules. Taken together, these multifaceted pathways have profound effects on host metabolism, energy balance, and different aspects of centrally regulated appetite and eating behaviour, including food reward (Figure 2, Figure 3) [98,[138], [139], [140]]. Here we focus on the effects of bacterial products on components of the gut-brain axis involved in appetite regulation.
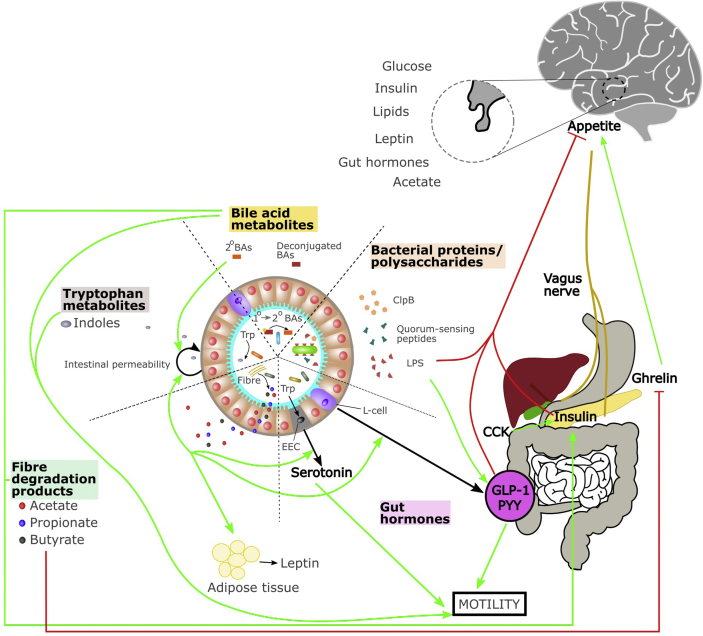
Figure 2.
The gut microbiota-gut-brain axis and the control of appetite and energy metabolism. Schematic representation of the microbiota-gut-brain axis communication pathways involved in appetite control and peripheral energy metabolism. Green arrows indicate an enhancement and red arrows indicate inhibition. Abbreviation: LPS; lipopolysaccharide, PYY; peptide YY, GLP-1; glucagon-like peptide 1, CCK; cholecystokinin, 2°BAs; secondary bile acids, BAs; bile acids, ClpB; Caseinolytic Mitochondrial Matrix Peptidase Chaperone Subunit B, EEC; enteroendocrine cell.
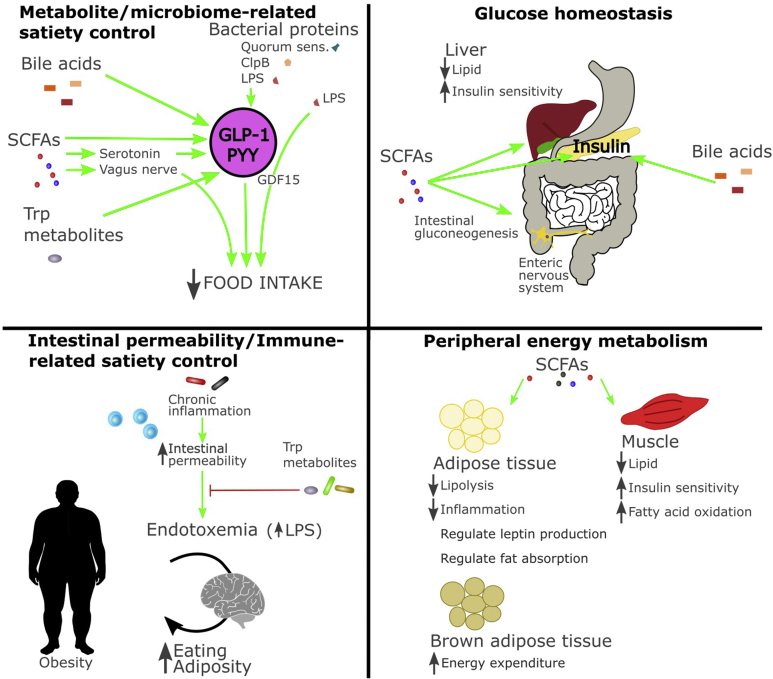
Figure 3.
Influence of the gut microbiota on metabolism in regulating appetite and energy balance. Role of the gut microbiota and its metabolites in metabolite-satiety control, glucose homeostasis, immune-related satiety control, and peripheral energy metabolism. Abbreviation: SCFAs; short-chain fatty acids, Trp; tryptophan, LPS; lipopolysaccharide, C1pB; Caseinolytic Mitochondrial Matrix Peptidase Chaperone Subunit B, GDF15; growth differentiation factor 15, PYY; peptide YY, GLP-1; glucagon-like peptide 1.
3.1. Bacterial proteins and polysaccharides
If the strong association between bacterial growth in the gut and satiation following a meal is causally established, altered bacterial growth may be communicated to the host by a simple concentration gradient of one or many microbially-derived molecules [98]. While a limited number of experiments have been conducted to test this hypothesis, so far, the data support a role for bacterial proteins and components in satiety (Figure 2, Figure 3). Proteins collected from commensal E. coli during both exponential and stationary growth phases increased plasma GLP-1 and PYY in rats. Stationary growth phase proteins activated c-Fos expression in anorexigenic neurons in the brain and both acutely and chronically reduced food intake [141]. Numerous proteins could underlie this response, including quorum-sensing peptides, caseinolytic mitochondrial matrix peptidase chaperone subunit B (ClpB) and bacterial cell components among others. While quorum-sensing peptides have not yet been shown to interact with the peripheral and central appetite regulation, they may act on the brain to modify feeding behaviour and satiety. Numerous quorum-sensing peptides are measurable in the circulation, and some of these can cross the blood-brain barrier [142] where they might exert effects on central appetite circuitry and other brain regions. In vitro screening of quorum sensing peptides revealed that some peptides can alter neurite outgrowth, can induce microgliosis or astrogliosis, or exhibit neuronal and microglial toxicity [143].
Protein ClpB from commensal E. coli is a conformation antigen mimetic of the alpha-melanocyte stimulating hormone, a key anorexigenic neuropeptide, that has been associated with eating disorders and obesity in human populations [144,145] and is increased during the stationary growth phase of E. coli that express it [141]. In vitro experiments indicate the ClpB mRNA and protein expression is increased specifically in response to protein supplementation [146]. Mice immunized to ClpB exhibited altered food intake and body weight, and chronic oral E. coli K12 positive for ClpB decreased food intake in healthy mice while oral administration ClpB-deficient E. coli did not affect food intake [145]. ClpB is thought to act on melanocortin-4 receptors expressed on enteroendocrine L cells [147], increasing PYY production in primary rat intestinal mucosal cells in vitro [146,148]. This protein has recently been shown to reduce cumulative food intake when administered systemically to both healthy and ob/ob mice [149], indicating its therapeutic value in obesity.
Bacterial cell wall components, particularly lipopolysaccharide (LPS), have a long-established role in modifying food intake through activation of the host immune system via pattern recognition receptors in the context of a bacterial infection or sepsis [150]. In healthy animals, signalling from these innate immune receptors is necessary for intestinal epithelial homeostasis and contributes to intestinal epithelial circadian rhythmicity [151]. Energy intake is positively associated with circulating LPS in humans and mice [152] and a single meal rich in fat increased post-prandial plasma endotoxin in people with T2D [153]. Circulating LPS rapidly increases plasma GLP-1 in both rodents and humans prior to measurable pro-inflammatory response [154], indicating that LPS may act on host appetite independently of cytokine-induced sickness behaviour. LPS also exerts effects on the host's sweet taste receptors: seven days following oral LPS administration, mice exhibited reduced sucrose sensitivity with corresponding reductions in lingual sweet taste receptor expression which normalized after an additional seven days [155]. Both acute and chronic LPS gavage decreased licking responses to sucrose, saccharin, and sodium chloride in mice, with concomitant reductions in neural response to these compounds measured by recording from the chorda tympani [156], indicating the role of LPS in the hedonic and/or motivational aspects of palatable solutions. Muramyl dipeptide (C19H32N4O11), a cell wall component of mycobacteria, reduces feeding behaviour and inhibits gastric emptying similar to LPS and independent of vagal afferent signalling [157]. LPS transiently increases circulating growth differentiation factor 15 (GDF15) [158], a newly-identified anorexigenic protein that reduces food intake in response to metabolic and toxin-related stressors [159] including bacterial and viral inflammation [160]. However, a recent study indicates that LPS does not reduce food intake through activation of this protein as reducing GDF15 activity by both targeted monoclonal antibody administration and by genetic knockout did not impact the effect of LPS on food intake and body weight in mice [158]. However, this does not preclude other microbial proteins and components acting via this pathway. Notably, chronic inflammation caused by a high-fat diet (HFD) is associated with increased LPS (metabolic endotoxemia) which may trigger body weight gain [[114], [161]].
Overall, several bacterial proteins and cell components can induce satiety signals and reduce food intake. Further research examining the function of other bacterial proteins produced during gut microbiota exponential growth and stationary growth phases may lead to additional pathways by which the gut microbiota can induce satiety. Furthermore, an investigation into proteins released during bacterial population decline may be of interest in determining whether the gut microbiota can elicit hunger in host organisms.
3.2. Products of fibre degradation: short-chain fatty acids, succinate, and lactate
Fibre intake is associated with reduced subjective appetite and small reductions in acute energy intake [162]. This may be through the effects of SCFAs, and other fibre-derived microbial metabolites, which reduce food intake and improve glucose homeostasis and insulin sensitivity in preclinical models [163] (Figure 2, Figure 3). So far acute and chronic SCFA supplementation has produced mixed effects on appetite and metabolic health in humans [122,[164], [165], [166], [167]].
Butyrate, propionate [168], and succinate [169] promote intestinal gluconeogenesis, a process known to improve glucose handling [170] and prevent hepatic steatosis, in mice fed a hypercaloric diet [171]. Of note, the metabolic benefits of administration of butyrate, propionate, or succinate on body weight and glucose handling were absent in mice that were genetically modified to prevent intestinal gluconeogenesis [168,169]. Acetate and l-lactate produced by bacteria are also able to modify intestinal fat absorption: both compounds inhibit chylomicron secretion from enterocytes while acetate promotes lipid oxidation and l-lactate promotes lipid storage [172]. Additionally, SCFAs enhance colonic secretion of serotonin in mice [173] and gut hormone, including the anorexigenic hormones (i.e., GLP-1 and PYY), from L cells in the GIT (Figure 2) [174,175]. The gut microbiota also regulates the release of ghrelin [174], an orexigenic hormone released predominantly in the stomach. Besides, SCFAs and specific microbiota strains can attenuate ghrelin receptor signalling in vitro [176]. Furthermore, adipocytes express specific receptors for SCFAs, which can promote the expression and release of leptin [177], potentially affecting long-term appetite regulation.
Recent preclinical studies indicate that SCFAs are also able to regulate sympathetic and parasympathetic nervous system activation. Propionate [178] and butyrate [179] increase sympathetic tone, elevating energy expenditure in brown adipose tissue. Conversely, increased gut acetate has been shown to activate the parasympathetic nervous system, which promotes increased glucose-stimulated insulin secretion, increased ghrelin secretion, hyperphagia, and obesity [180]. The effects of SCFAs on the autonomic nervous system appear to be via vagal afferents projecting from the distal gut [181].
Optogenetic manipulation of right vagal afferent signalling from the upper GIT increases striatal dopamine and appetitive behaviour in mice [182]. This effect may be mediated by propionate which can suppress food intake through vagal afferent neurons [20] and appears to be involved in hedonic feeding. In a small study, acute colon-specific propionate delivery reduced blood oxygen level-dependent signal during food picture evaluation in brain regions involved in reward and reduced energy intake during an ad libitum meal in healthy men [164].
Overall, microbial products of dietary fibre appear to modulate several processes associated with body weight control of metabolism. Through changes to intestinal gluconeogenesis and lipid absorption, as well as altered autonomic and reward signalling, these metabolites shift body weight in preclinical models of obesity with some effects in the human experiments conducted.
3.3. Tryptophan metabolites
Tryptophan metabolites another key group of microbially-derived metabolites, exert effects on host physiology, acting on gut health and function, and the immune system [[183], [184], [185]] with reported effects on food intake and gut satiety hormone signalling (Figure 2, Figure 3). Several bacterial taxa metabolize tryptophan, altering host tryptophan availability and metabolism, through the expression of tryptophanase and other bacterial enzymes [186]. Infusions of l-tryptophan into the gut reduce food intake in both mice [187] and humans [188], where it increases plasma cholecystokinin (CCK), glucagon, GLP-1, and PYY. The effects of tryptophan do not appear to be through conditioned taste aversion or the induction of sickness behaviour [187]. It is unclear whether these effects are via direct actions of tryptophan on the host or through the production of microbial tryptophan metabolites, which also exert effects on food intake.
Bacteria produce indole using tryptophan in response to environmental stress, improving survival of less-resistant communities members [189]. Acute administration of indole in colonic cells in vitro and in mice in vivo increases GLP-1 through activating the aryl hydrocarbon receptor and can reverse dietary- and genetic-induced metabolic impairments in mice [190]. Bacterial tryptamine activated intestinal epithelial G-protein-coupled receptor 5-HT4 to increase ionic flux and fluid secretion in vitro, thereby increasing GI transit in mice in vivo [191]. Furthermore, multiple tryptophan metabolites have been implicated in gut barrier function [192], which may impact nutrient absorption and immune signalling, indirectly affecting food intake and appetite.
3.4. Bile acids
Bile acids are enterohepatic hormones that are critical for the digestion and absorption of lipids. They are involved in glucose and lipid metabolism, energy expenditure, inflammation, cholesterol homeostasis, and enterohepatic protection. Bile acids have been implicated in cancer, cardiovascular disease (CVD), and T2D. Primary bile acids, such as cholate and chenodeoxycholate, are synthesized by the host organism in the liver and then secreted into the gut lumen, where gut microbial taxa interact with bile acids to form secondary bile acids. Bile acids exert antimicrobial actions, shape the composition of gut microbiota, and undergo extensive modification by microbes expressing bile salt hydrolase enzymes, which modifies mucosal colonization resistance, immune activity, and gut tissue physiology [193]. Bile acids increase satiety signalling in the gut, by increasing secretion of gut satiety hormones including GLP-1, PYY, glucagon, and insulin in rats [[194], [195], [196]] (Figure 2, Figure 3) and in the human intestinal mucosa [197] through activating TGR5 receptor and altering intestinal lipid sensing by modifying G protein-coupled receptor 119 (GPR119) activity in the distal intestine [198,199]. Additionally, commensal gut bacteria modulate host gut serotonin expression in part through modifying deoxycholate levels in the mouse gut, altering gastrointestinal motility in mice [200]. Specific bile acids appear to have different effects on glucose tolerance [196,[201], [202], [203]] and further work is required to confirm these differences and determine how different bile acids exert their effects on glucose handling. The metabolic improvements both through bariatric surgery [204] and Parabacteroides distasonis [201] can be simulated by targeted bile acid replacement. Finally, bile acids that reach the brain reduce food intake through activation of TGR5 receptors on agouti-related-peptide (AgRP) expressing inhibiting neurons [205].
4. Obesity and cachexia: pathogenesis and related microbiota alterations
4.1. Obesity
Obesity is a chronic, relapsing, progressive condition, that is characterized by an abnormal or excessive accumulation of white adipose tissue [206]. It is associated with dysfunctions across several organ systems, including adipose tissue [13], gut [207], pancreas [208], and the brain [209]. Obesity contributes to premature disability and death by increasing the risk of several diseases [12], including T2D, CVD, some cancers, and neuropsychiatric disorders. Due to the rapid increase in obesity prevalence and limited evolutionary pressure in recent decades, there is a growing recognition that the current obesity epidemic is driven primarily by environmental factors, namely diet and lifestyle [12]. This simplistic view is further complicated by the discovery that diet-induced changes in gut microbiota composition may also impact body weight control and energy balance [17].
Alterations in gut microbiota composition, with a concurrent reduction in overall microbial species diversity, have repeatedly been observed in the context of obesity [210,211]. In studies conducted both in obese mice [113,212] and in humans [213], the proportion of the phylum Firmicutes was observed to increase with decreases in the Bacteroidetes in the obese condition compared to their normal-weight counterparts. These studies suggested that an increased Firmicutes/Bacteroidetes ratio could be used as an obesity biomarker. However, other studies have subsequently contradicted this rather simplistic reductionist view [118,[214], [215], [216]] and the precise taxonomic alterations that characterise obesity remain the subject of debate. However, a recent meta-analysis examining the effects of obesity and high-fat diet on microbiota composition in both mice and humans concluded that the Firmicute/Bacteroidetes ratio is reproducibly increased across 25 studies [217]. Large-scale analyses that compared lean vs obese humans showed no relationship between BMI and gut microbiota phylum-level composition, suggesting that a simple taxonomic signature of obesity was not detected in the gut microbiota [216]. Interpersonal variables such as dietary habits and lifestyle as well as effect size and technical differences in the analyses of the microbiota might be the causes of conflicting results between different studies [215,216]. However, a significant, but small, relationship between obesity status and microbiota diversity and richness has been observed [211,215,218]. Despite these inconsistencies in the literature, obesity has been associated with an alteration of gut microbiota. Most of the observational studies that established associations and much of the evidence of a role of the gut microbiota in body weight control comes from rodent studies [94,[101], [102], [103], [104], [105],213].
Early work by Turnbaugh and colleagues indicated that the obesity-associated microbiota characterized by an increased capacity for energy absorption from the diet, and also that the obese phenotype can be induced in rodents through FMT of samples from obese humans, independent of diet [113]. Though this study has subsequently been criticized for overstating the effect of FMT on fat mass (for example, see analysis by Dalby [219]), it was an important catalyst for subsequent research into the role of the gut microbiota in obesity. Moreover, experiments in GF mice have illustrated the role of the gut microbiota in obesity pathogenesis and susceptibility. Bäckhed and colleagues showed that GF mice have 42% less total body fat than conventional mice, although they ingest 29% more calories than their conventionally raised littermates [46]. GF mice also gain less weight than conventional mice when exposed to an HFD and appear protected against diet-induced glucose intolerance and the development of Insulin resistance (IR) [47,220,221]. In addition, FMT from conventionally raised mice to GF mice triggered a 57% increase in the amount of body fat and a dramatic increase in hepatic triglyceride levels and IR without modifying the amount of food consumed [46]. Regarding the link between the gut microbiota and body fat, studies showed that the gut microbiota could influence the deposition of fatty acids (FAs) in the adipocytes by suppressing the intestinal fasting-induced adipocyte factor, a circulating lipoprotein lipase inhibitor [46]. Lipoprotein lipase is a key enzyme involved in the hydrolysation of triglycerides in free FAs before their transport into the adipocytes [222].
Furthermore, rodent studies have indicated that altering microbiota composition may be sufficient to induce or reverse increased weight gain. For example, wild-derived microbiota transplanted in laboratory mice during the neonatal period protected against excessive weight gain and metabolic syndrome during a 10-week course of HFD [223]. In another study, FMT from obese and lean twin donors was performed in mice. Mice colonized with obese microbiota showed a significant increase in fat mass compared to the lean counterpart. Cohousing of the mice with donated obese and lean microbiota prevented the development of increased body mass and obesity-associated metabolic phenotypes in mice that received FMT from obese donors [81]. In addition, diet-induced obesity (DIO) promotes a microbiota composition that exacerbates weight regain in previously obese mice that can also exacerbate weight gain in naïve mice receiving FMT [224]. However, a pilot trial in people with obesity reported that FMT from lean donors did not reduce body weight while effectively shifting microbiota composition and metabolite production [225]. Another recent clinical study showed that FMT capsules from lean donors administered to adults with obesity did not cause significant changes in body weight, IR, and body composition [226]. These data indicate the insufficient role played by the gut microbiota composition in humans in improving to induce healthy weight loss.
Recent studies have further defined potential bacterial candidates underlying the effects of gut microbiota composition on body weight control and energy balance. Unexpectedly, the opportunistic pathogen, Enterobacter cloacae B29, isolated from the gut of an obese human, caused obesity and insulin resistance in GF mice [227]. In another study using gnotobiotic mice fed a HFD or low-fat diet, Clostridium ramosum was associated with the upregulation of glucose and fat transporters in the intestine, as well as increased body fat deposition [228]. Contrastively, other bacterial taxa in the gut appear to have a protective role against obesity and adiposity both in rodents and in humans. Among them, Akkermansia muciniphila, a mucin-degrading bacteria, is present in significantly lower levels in genetically obese ob/ob (leptin-deficient) and db/db (leptin receptor-deficient) mice [229,230] and oral administration of A. muciniphila reverses HFD associated metabolic disorders including fat mass gain and IR in these mice [230]. A recent pilot study in people with overweight and obesity showed that A. muciniphila supplementation over 3 months could not alter body weight or adiposity, but reduced plasma insulin and cholesterol levels [146]. In addition, the abundance of A. muciniphila within the gut microbiota is associated with the decreased risk of metabolic syndrome in a dose-dependent manner in humans [231]. Moreover, Christensenellaceae, a bacterial family present in lean individuals, has been shown to promote a lean host phenotype and has an impact on the diversity of the bacterial community when transplanted to mice [232].
In summary, specific gut microbiota components may underlie pathological weight gain observed in obesity, but additional work in humans is required to confirm the translatability of promising results observed in rodent studies.
4.2. Cachexia
Similar to excessive weight gain, abnormal weight loss is also associated with increased mortality [233] and reduced quality of life [234] and can result in permanent damage to several organ systems. Abnormal weight loss is primarily differentiated into four syndromes: starvation or malabsorption (caloric deficit), dehydration (fluid loss or deficit), sarcopenia (muscle atrophy in the context of aging), and cachexia. According to the most recent evidence on cachexia diagnosis, cachexia is defined as a multifactorial syndrome with involuntary progressive weight loss as a result of reduction of skeletal muscle mass with or without depletion of adipose tissue. Additionally, cachexia is characterized by loss of muscle strength, anorexia, lean tissue depletion, distinct fatigue, or abnormal metabolism (i.e., decreased albumin, increased C-reactive protein (CRP) levels [14,15]. Cachexia is observed in a subset of patients with cancer, AIDS, chronic obstructive pulmonary disease, kidney or heart failure, and during long-term care in nursing homes [235]. Overproduction of cytokines may play a role in the pathogenesis of cachexia, as well as the alterations in hormones including testosterone, insulin-like growth factor I, myostatin, and glucocorticoids [236]. However, therapeutic approaches that included the treatment with anti-cytokines and anti-inflammatory components have mainly failed to ameliorate the symptoms of cachexia in cancer patients [[237], [238], [239]]. While a wide range of nutritional, lifestyle and pharmacological interventions have been proposed and trialled in the treatment of cachexia, these have generally resulted in limited clinical benefit, although preliminary trials combining therapies have shown promising results [240]. The gut microbiota is known to modulate several of the systems thought to underlie cachexia, namely energy availability and absorption, and cytokine production [136], and it can interact with a number of hormone signalling pathways [241]. Subsequently, interest has been increasingly shown in the potential of the gut microbiota as a target in the treatment of cachexia.
While intentional weight loss and malnutrition are known to be associated with shifts in the gut microbiota, less is known about the effects of cachexia on gut microbiota composition and function. A recent small study in patients with lung cancer showed that patients with cachexia exhibited a significantly altered gut microbiota composition, along with differences in functional metagenomic pathways and relevant plasma metabolites. Specifically, branched-chain amino acids, vitamins, and methylhistamine were relatively reduced in the plasma of patients with cachexia, while the gut microbiota's capacity for synthesis of LPS was enriched [16]. This finding is in line with that in a separate study on colorectal and lung cancer patients where serum LPS content was shown to be an independent predictor of cachexia and survival [242]. A recent faecal transfer study showed that cachectic patients with advanced gastroesophageal cancer exhibited improved treatment response and survival following FMT from healthy obese donors relative to autologous FMT (from faeces collected prior to cancer therapy) with no improvement in any cachexia outcomes [243], indicating that the microbiota changes associated with cachexia may underlie the related differences in patient prognosis but not necessarily relate to the syndrome itself.
While there is limited human data available, rodent models of cachexia demonstrate that the condition is associated with altered gut microbiota composition and function. Preclinical models of cancer cachexia are characterized by a bloom of Enterobacteriaceae, particularly Klebsiella oxytoca [244,245], and increased gut barrier permeability [242,244,246]. In line with these microbiota compositional differences, cachectic mice exhibit altered caecal metabolomic relative to control mice, including reduced caecal acetate and butyrate levels and increased bile acid and amino acid content [247]. Furthermore, cachectic mice also exhibit an altered mycobiota with compositional changes similar to those observed in several gastrointestinal inflammatory diseases [248], which may also contribute to the local gut barrier changes as well as altered immune profile. Overall, there is strong preliminary evidence supporting further research into the role of the microbiota in cachexia in clinical populations, as well as further investigation into the therapeutic potential of bacterial strains and their products for cachexia management.
5. Dietary interventions that act on body weight and that shape the microbiota
5.1. Calorie restriction (CR)
CR, a nutritional intervention, includes a reduced daily energy intake maintaining healthy dietary habits without malnutrition. Several studies on obesity in humans and rodents have demonstrated the multiple beneficial effects of CR. Among these, body weight loss, fat loss, reduced risk of hyperglycaemia and hyperinsulinemia, and reduced inflammation have been reported [[249], [250], [251]].
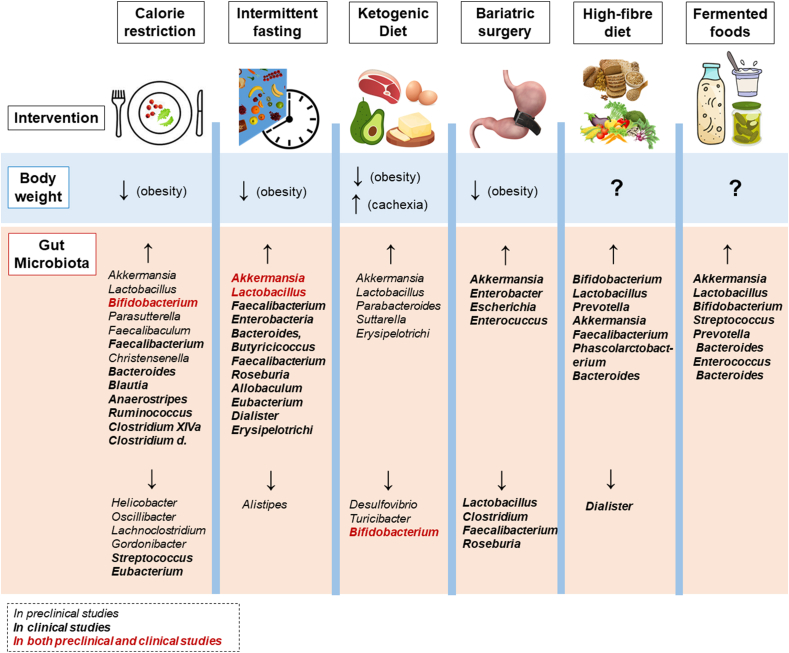
The involvement of the gut microbiota in the body weight loss exerted by CR has also been explored (Figure 4). Mice treated with antibiotics were resistant to the body weight loss induced by CR. In addition, mice that underwent CR showed a higher proportion of Lactobacillus, Bifidobacterium, Parasutterella, Clostridiales vadinBB60 as well as a lower proportion of Helicobacter, Lachnospiraceae NK4A136, Lachnoclostridium, Oscillibacter, Roseburia, and Gordonibacter within the gut. FMT from CR-fed mice in HFD-fed mice prevented HFD-induced weight gain and other obesity-related metabolic features, demonstrating that CR potentially induces weight loss via gut microbiota modulation [252]. Mice fed with HFD that received FMT from CR-fed donors exhibited an increased abundance of Firmicutes and Actinobacteria, together with a decrease in the abundance of Bacteroidetes. At the genus level, these mice exhibited significant enrichment of Faecalibaculum, Coriobacteriaceae UCG-002, and Coprococcus [252].
Figure 4.
Body weight and gut microbiota changes following dietary and surgical interventions. The upper part of the figure summarizes the effect of calorie restriction, intermittent fasting, ketogenic diet, bariatric surgery, high-fibre diet, and fermented foods on body weight during obesity or cachexia. The question mark (?) indicates the lack of scientific evidence or contrasting endpoints. The lower part of the figure summarizes the general changes in the gut microbiota (at the genus level) upon the interventions. Terms in italics indicate the genera found in preclinical models, in bold are indicated the genera found in clinical models and terms in bold-red indicate the genera found in both preclinical and clinical models.
Other studies performed on rodents showed that mice receiving a CR diet had a significantly higher proportion of Lactobacillus, frequently represented by Lactobacillus murinus, which is linked with an improved metabolic profile [[253], [254], [255], [256]] and a lower proportion of Lachnospiraceae [255,257] Additionally, CR and a very-low calorie ketogenic diet enriched both Akkermansia muciniphila and Christensenella minuta [258,259]. In most of these studies, the bodyweight loss exerted by CR is linked to decrease inflammation and metabolic dysfunctions. During CR, together with the modification of the gut microbiota composition, fecal metaproteomic and the functional capacity of the microbiota changed, including the expression of propiogenic enzymes [260].
In non-obese adults, short-term CR intervention (40% reduction in daily calorie intake) was associated with more marked body weight loss in subjects with the Prevotella gut microbiota enterotype relative to Bacteroides enterotype subjects, indicating that CR can have different effects based on the baseline gut microbiota composition [261]. Long-term CR in obese adolescents promoted weight and adiposity loss and higher metabolic performance linked with enrichment of Bacteroides, Roseburia, Faecalibacterium, and Clostridium XIVa and with a lower abundance of Coriobacteriaceae, Streptococcus, Clostridiales, Eubacterium, and Coprococcus [262,263]. Short-term CR in obese women resulted in a significant decrease in body weight, inflammation, and gut permeability, as well as an improvement in leptin production and glucose tolerance levels. Within the gut, these obese women had a higher abundance of selected operational taxonomic units from Lachnospiraceae (including Blautia and Anaerostripes hadrus), as well as from Ruminococcus and Bifidobacterium [264].
A recent human study in overweight and obese women showed that a 16-week CR intervention (800 Kcal per day) reduced body weight, adiposity, hyperglycaemia, gut microbiota diversity, and SCFAs production effectively reduced body weight. Bodyweight loss and the improvement in the metabolic parameters were transmitted to the recipient via the donor microbiota in an FMT study in GF mice. Moreover, it was shown that the gut microbiota post-CR was characterized by a lower capacity in extracting energy from the diet. In addition, GF mice receiving FMT from post-CR women showed a higher abundance of the pathogenic bacteria Clostridium difficile in their gut, which, in part, contributed to the weight loss in a toxin-dependent manner. The authors also showed that the changes within the gut microbiota upon CR are reversible [265].
Low-calorie diets are not always a long-term solution; however, since in 80% of the cases recurrent weight gain and relapsing metabolic complications are observed after an individual resumes normal calorie intake [224,266]. This phenomenon is commonly called “weight cycling” or the “yo-yo effect”. It was shown that one of the causes of this effect lies within the gut microbiota configuration. After dieting, some modifications of the microbiota do not normalize even if weight normalization after obesity is achieved. This contributes to the susceptibility to develop aggravated metabolic complications upon re-exposure to obesity-inducing conditions. The “post-obesity microbiota” seems linked with reduced production of certain flavonoids, which are important postbiotics, and energy expenditure capacity. This demonstrated that, to efficiently act to control body weight, more interventions that take into account the baseline gut microbiota composition are necessary [224,267].
5.2. Intermittent fasting (IF)
IF, a dietary pattern, include periods where energy intake is zero or extremely low. There are different ways to implement IF [298,299] including the following:
-
•
Prolonged fasting (PF) or intermittent caloric restriction (ICR, fasting for up to 24 h once or twice a week with ad libitum food intake in the remaining days of the week),
-
•
Time-restricted feeding (TRF, 8-hour window of eating and 16 h of fasting for most days of the week),
-
•
Alternate-day fasting (ADF, consists of a “feed day” i.e., ad libitum food intake for 24 h, alternated with a “fast day” i.e., complete fast for 24 h).
All of these IF methods have shown efficacy in reducing body weight gain by the 2.5–9.9%, as well as reducing hyperglycaemia, energy intake, and cardiovascular disturbances (Figure 4). Conversely, a recent study in overweight/obese individuals showed no difference in body weight loss among the groups upon time-restricted feeding (TRF) [300] intervention although there were marked inter-individual differences in response. However, IF protocol, duration, and baseline characteristics of the sample population remain highly variable [299,[301], [302], [303]].
Recent literature reviews have summarized the effects of IF on the gut microbiota [304,305] (Figure 4). In healthy mice, one month of ADF intervention led to an increase Lactobacillaceae, Bacteroidaceae, and Prevotellaceae family bacteria, whereas one month of 16-hour TRF was linked with an increase in A. muciniphila and a decrease in Alistipes genus bacteria [306,307]. In addition, ketone body metabolism and glutathione metabolism-related pathways were upregulated [308]. Both short-term (4 weeks) and long-term (7 months) ADF increased the abundance of Lactobacillus and improved gut function and physiology in db/db mice [309,310]. In addition, ADF increased the browning of the white adipose tissue and counteracted the metabolic derangements induced by HFD in a gut microbiota-dependent manner [311]. Moreover, studies showed that HFD-induced obese mice exhibited reduced body weight and improved lipid metabolism linked with a higher proportion of A. muciniphila and Lactobacillus following 4 days of complete food withdrawal [312].
In non-obese adults, both TRF and ADF lead to an increase in the abundance of Bacteroides [307,313,314]. In healthy adults that underwent 29 days of TRF (Ramadan fasting regimen), an increase in Bacteroides fragilis, A. muciniphila, Butyricicoccus, Faecalibacterium, Roseburia, Allobaculum, Eubacterium, Dialister, and Erysipelotrichi was observed [313,314]. In overweight or obese adults, an increase in Lactobacillus, A. muciniphila, Faecalibacterium prausnizii, and Enterobacteria abundances was reported after a TRF intervention [315]. In contrast, no differences within the microbiota were found in a more recent study in which adults with obesity had lost weight upon TRF [316]. Further studies are needed to define the effect of long-term IF in humans and well-defined IF protocols are needed to better establish the relationship between IF benefits and the gut microbiota. Moreover, it is highly likely that the gut microbiota may play a role in the inter-individual responses to time-restricted feeding in controlled trials [300].
5.3. Ketogenic diet
The KD is a dietary strategy that includes a very low quantity of carbohydrates and a high quantity of fats. This diet accounts for approximately 10% carbohydrates, 70% fats, and 20% proteins. The objective is to minimize the dietary carbohydrates as much as possible and thus glucose and to promote the mobilization of fats directed at the liver. This facilitates the production of ketone bodies that are utilized as the main fuel by the host, rather than glucose [317]. Weight loss, optimization of metabolism, prevention/amelioration of IR syndrome and neuropsychiatric disorders (i.e., epilepsy and depression) as well as reduction of neuro-inflammation are among the advantages of a KD [[318], [319], [320]].
In recent years, numerous studies have demonstrated the efficacy of a KD in targeting obesity (Figure 4); however, the mechanisms underlying this effect remain unresolved. The possible causes of weight loss upon KD consumption include appetite suppression mediated by ketone bodies, as well as an improvement in fat metabolism efficiency that is secondary to consuming fats [321]. In addition, KD leads to a strong modulation of the gut microbiota (Figure 4). Healthy mice fed KD for 16 weeks showed a significant increase in the abundance of A. muciniphila and Lactobacillus and a decrease in Desulfovibrio and Turicibacter [322]. These changes were coupled with a improvement in neurovascular function. Accordingly, another study performed in mice with refractory epilepsy showed an increase in A. muciniphila, Parabacteroides, Suttarella, and Erysipelotrichaceae upon KD consumption. By using gnotobiotic mice colonized with A. muciniphila and Parabacteroides, they demonstrated that the anti-seizure effect on mice was exerted by these two taxa [323].
In a recent study, 17 overweight or obese nondiabetic men were recruited and they spent two months as inpatients during which they were carefully monitored. The participants consumed a standard diet (50% carbs, 15% protein, and 35% fat) or a KD (5% carbs, 15% protein, and 80% fat) for 4 weeks and they switched the diets at the end of the 4 weeks. The abundance of Actinobacteria, Bacteroidetes, and Firmicutes phyla and in 19 genera was different between groups after KD [324]. FMT from human donors on a KD into mice could recapitulate the gut microbiota composition and the ‘humanised’ mice showed a decrease in pro-inflammatory marker Th17 cells both in the gut and in the AT. Thus, a decrease in Bifidobacterium was linked with a reduction of inflammation. In vitro, they demonstrated that Bifidobacterium adolescentis is the species that is most likely inhibited by the ketone bodies. They also observed that mice fed with classical HFD had an opposite gut microbiota configuration (i.e., higher Actinobacteria and Firmicutes and lower Bacteroidetes) compared to mice fed with KD, with the latter group exhibiting reduced body weight [324]. Decreased Bifidobacterium abundance upon KD consumption has also been observed in children with severe epilepsy [325].
KD could be a beneficial strategy to adopt in particular cases such as metabolic diseases and epilepsy. However, the same diet for a long-term period could reveal different side effects. KD, being very low in carbohydrates, is also very low in fibres which are very important for the maintenance of a healthy GIT as well as healthy gut microbiota. Accordingly, KD causes a decrease in the diversity and richness of the gut microbiota [326]. Thus, the main therapeutic approach seems to suggest increasing water and fibre intake (i.e., dietary fibre supplementation) [327].
Moreover, the kind of fats that are predominant in KD must be taken into account since the proportion of saturated FAs vs monounsaturated FAs content in the diet differentially affects the gut microbiota [326,328]. Thus, the use of KD should be carefully regulated and monitored based on the specific medical condition and patient response. For example, concerns in the use of KD have been raised particularly for people with GI disorders or at risk of nutritional deficiency [329,330]. In addition, it was recently shown that KD aggravates cognitive impairment in mice that underwent intermittent hypoxia, by influencing the gut microbiota and by increasing intestinal IFNg-producing Th1 cells [331].
KD was shown to exert a positive effect on cancer-associated cachexia, preventing muscular degradation and general weight loss as well as improving motor function strength (Figure 4). These beneficial outcomes were accompanied by a reduction of the tumour mass [[332], [333], [334]]. Whether the modulation of the gut microbiota by KD consumption could be involved in the amelioration of cachexia-related symptoms is still unexplored.
5.4. Bariatric surgery
Bariatric surgery encompasses surgical procedures that alter the GIT resulting in malabsorption, diminished food intake, and substantial weight loss [335,336] (Figure 4). Among all the weight loss strategies, bariatric surgery is the most effective and long-lasting. It causes a profound change in the production of satiety hormones, altering carbohydrate and bile acid metabolism [337]. Several recent studies and literature reviews have focused in depth on the dramatic effect of bariatric surgery on the gut microbiota in humans with obesity [[338], [339], [340]] (Figure 4). In summary, bariatric surgery leads to a decrease in Firmicutes (including Lactobacillus, Clostridium, Faecalibacterium, Roseburia) and an increase in A. muciniphila and Proteobacteria (including Enterobacter, Escherichia, Enterococcus) [338,[341], [342], [343]]. Additionally, a significant reduction in SCFAs, and energy and amino acid metabolism were detected post-bariatric surgery [340,344]. A longitudinal study revealed that the changes in the gut microbiota and in the faecal metabolomics were present even 4 years post surgery [344]. Furthermore, bariatric surgery response can vary among individuals and the gut microbiota configuration pre-surgery was discovered to be an important predictor of surgery outcomes [343].
Besides the beneficial effect shown against obesity, bariatric surgery can lead to some collateral effects at the GIT level such as nausea, vomiting, obstruction of the GIT, infections, neuropathies due to nutritional deficiencies and emotional disorders (e.g., depression, anxiety, drugs-alcohol abuse) [345,346]. It was shown that the post-surgery mood-related disorders are linked with changes in the production of appetite-related gut peptides as well as with the alteration of the gut microbiota composition and function (i.e., bile acids profile). However, further investigations in the context of changes in gut-brain signalling after bariatric surgery are needed to better elucidate the related mechanisms [347].
5.5. Probiotics, prebiotics, and synbiotics
5.5.1. Probiotics
Several probiotics effectively reduce body weight gain and adiposity in individuals with obesity. Specific strains or combinations of strains such as Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium lactis, B. animalislactis, L. gasseri, L. curvatus, Lactobacillus rhamnosus, Lactobacillus plantarum, L. fermentum, B. adolescentis, L. sakei, B. bifidum, B. infantis, B. breve, Enterococcus faecalis, L. parakefir, L. kefir, B. brevis, B. casei, Bacteroides vulgatus, Bacteroides dorei and Dysosmobacter welbionis have been detailed elsewhere to be effective for weight loss during obesity in humans and/or rodents [139,279,[348], [349], [350], [351], [352], [353], [354], [355], [356]]. However, other probiotic strains such as B. longum, L. reuteri and L. salivarius cause a decrease in body weight and metabolic syndrome-related parameters in obese rodents but are mainly ineffective or partially effective in humans [349,357]. It was proposed that probiotics can be beneficial for body weight control and metabolic syndrome through the modulation of the gut microbiota, inflammation and intestinal barrier protection [139,348,357]. Table 1 details the studies on the efficacy of specific probiotics in ameliorating obesity and their effect on the gut microbiota in humans. The effect of each probiotic on the body weight, glucose tolerance, and gut microbiota is very variable. Thus, it is hard to associate the lower body weight caused by probiotics with changes in specific microbial taxa per se, also because several studies showed the beneficial effect of single strains of probiotics without changes in the gut microbiota [358]. Also, the positive effects of probiotics on glucose tolerance do not always translate into changes in body weight [279].
Table 1.
Recent studies on the effect of probiotics/prebiotics/synbiotics on body weight and gut microbiota in obese humans.
| Obesity | Dose | Model | Treatment length | Changes in the gut microbiota | Bodyweight | Other physiological and metabolic changes | Reference |
|---|---|---|---|---|---|---|---|
| Probiotics | |||||||
| (a) Lactobacillus amylovorus or (b) Lactobacillus fermentum in yogurt | (a) 1.39 × 109 CFU, (b) 1.08 × 109 CFU daily | Overweight adults | 3 × 43-day phases, each separated by a 6-week washout | (a) ↓Clostridial cluster IV, ↑ Lactobacillus (b) ↑ Lactobacillus |
= | (a) ↓↓ body fat mass (b) ↓ body fat mass |
[268] |
| VSL#3 (Bifidobacterium longum, B. Infantis, B. breve) | 112.5 × 109 CFU/capsule each strain | Overweight adults | 6 weeks | ↑ Lactobacillus, ↑ Bifidobacterium, ↑ Bacteroides, ↑ Streptococcus | Not reported | ↓ total cholesterol, ↓ LDL, ↓ TAG, ↓ VLDL, ↓ CRP, ↑ insulin sensitivity, ↑ HDL | [269] |
| VSL#3 (Streptococcus thermophilus DSM24731, L. acidophilus DSM24735, L. delbrueckii ssp. Bulgaricus DSM24734, L. paracasei DSM24733, L. plantarum DSM24730, B. longum DSM24736, B. infantis DSM24737, B. breve DSM24732) | 450 billion CFU/sachet | Healthy young adults that consumed HFD (55% fat) | 4 weeks | ↑ L. acidophilus, ↑ S. thermophilus | ↓ body mass (2.47%) | ↓ fat mass gain, = insulin sensitivity and fat oxidation | [270] |
| L. paracasei F19 | 9.4 × 109 CFU/sachet daily | Obese postmenopausal women | 6 weeks | ↑ Eubacterium rectale, ↑ Ruminococcus torques | = | = CRP,= insulin sensitivity and glucose tolerance | [271] |
| L. reuteri DSM 17938 | 108 or 1010 CFU daily | Overweight or obese adults with T2D | 12 weeks | = Increased microbiota diversity at baseline predicted probiotic response |
= | ↑ insulin sensitivity, ↑ serum deoxycholic acid | [272] |
| (a) B. adolescentis IVS-1 and (b) B. animalis subsp. Lactis BB-12 | 1 × 109 CFU daily each | Obese adults | 3 weeks | (a) ↑ Bifidobacterium, ↑ B. adolescentis (b)= |
= | (a) ↓ intestinal permeability during aspirin intake (b)= |
[273] |
| B animalis subsp. Lactis 420™ (B420) | 1010 CFU daily | Overweight/obese human adults | 6 months | ↑ Lactobacillus, ↑ Akkermansia |
Trend to ↓ (∼1.5%) [274] | =SCFAs or other faecal metabolites. ↓ inflammatory markers, ↓ zonulin (intestinal permeability), ↓ CRP, ↓ body fat mass, ↓ waist circumference [274] | [275] |
| B. animalis subsp. Lactis CECT 8145 | 1010 CFU daily | Obese adults | 3 months | ↑ Akkermansia | ↓ BMI (- 0.349 kg/m2) | ↓ waist circumference, ↓ visceral fat, ↓ conicity index, ↓ diastolic blood pressure, ↓ HOMA-IR index | [276] |
| B. pseudocatenulatum CECT 7765 | 109−10 CFU daily | Obese children | 13 weeks | ↓ Rikenellaceae, ↑ Alistipes | ↓ BMI (−0.38 kg/m2) | ↓ CRP, ↓ MPC-1 (inflammation), ↑ HDL, ↑ omentin-1 | [277] |
| Akkermansia muciniphila alive (a) or pasteurized (p) | 1010 CFU daily | Overweight/obese insulin-resistant adults | 3 months | = | (p) ↓ (1.88%) (a)= |
(p) ↑ insulin sensitivity, ↓ hyperinsulinemia, ↓ plasma total cholesterol, ↓ fat mass, ↓ hip circumference (a) ↓ blood markers of liver dysfunctions and inflammation |
[146] |
| L. acidophilus, B. lactis, B. bifidum, B. longum | 1.5 × 1010 CFU each | Overweight or obese people with prediabetes | 6 months | ↑ Bacillus fragilis/E. coli ratio ↓ F/B ration |
Not reported | =food intake | [278] |
| B. longum APC1472 | 1 × 1010 CFU capsule daily | Overweight/obese human adults | 12 weeks | ↑ Bifidobacterium | = | ↑ fasting glucose levels, ↓ ghrelin, ↓ cortisol awakening response levels | [279] |
| L. plantarum Dad-13 | 2 × 109 CFU/g/sachet | Overweight human adults | 90 days | ↓ Coprococcus, ↑ Bacteroides L. plantarum Dad-13 Compared to baseline |
↓ (1.64%) BMI ↓ (- 0.53 kg/m2) |
=SCFAs and lipid profile | [280] |
| Prebiotics | |||||||
| Inulin + oligofructose | 8 g/day + 8 g/day | Obese adult women | 3 months | ↑Bifidobacterium, ↑Faecalibacterium prausnitzii, ↓Bacteroides intestinalis, ↓Bacteroides vulgatus, ↓Propionibacterium | = | A slight reduction in % fat mass | [281] |
| Bi2muno (mixture of trans- galactooligosaccharides) | 5.5 g/day | Overweight adults | 3 months | ↑Bifidobacterium, ↓Bacteroides, ↓C. hystolicum ↓Desulfovibrio |
= | ↓ faecal calprotectin and secretory IgA, ↓ total cholesterol, ↓ insulin, ↓ CRP, ↓ TAG | [282] |
| Inulin + β-glucan + blueberry anthocyanins + blueberry polyphenols | 3.79 g/day + 2.03 g/day + 162.53 mg/day + 723.99 mg/day | Overweight or obese adults | 1 month | = | ↓ fasting glucose and PYY levels, ↓ reported desire to eat and prospective intake, ↑ fasting plasma ghrelin levels, ↑ faecal SCFA content | [283] | |
| Inulin, sodium butyrate, Inulin + sodium butyrate | 10 g/day, 600 mg/day, 10 g/day + 600 mg/day | Overweight or obese adults with T2D | 45 days | ↑Akkermansia muciniphila in groups supplemented with inulin or sodium butyrate alone | = | ↓ CRP and malondialdehyde serum levels, ↓ diastolic blood pressure in all supplemented groups | [284] |
| Litesse Ultra polydextrose | 12 g/day | Overweight or obese adults | 6 months | ↑ Christensenellaceae | Not reported | = | [275] |
| Arabinoxylan | Female: 25 g/day Male: 35 g/day |
Overweight or obese adults | 6 weeks | ↓ alpha-diversity ↑ Bifidobacterium, ↑ Blautia. ↓ Parasutterella, ↓ Ruminococcus, ↓ Clostridium XVIII, ↓ Lachnospiraceae incertae sedis |
Not reported | Not reported | [285] |
| Synbiotics | |||||||
| B. lactis Bb12 + oligofructose | 2010 CFU + 16 g/day | Obese adults | 6 weeks | ↑ Bifidobacterium | = | = | [286] |
| B. adolescentis IVS-1 + galactooligosaccharide | 109 CFU + 5 g/day | Obese adults | 3 weeks | ↑ Bifidobacterium, ↑ B. adolescentis | = | = | [273] |
| B. animalis subsp. Lactis BB-12 + galactooligosaccharide | 109 CFU + 5 g/day | Overweight or obese adults | 6 months | ↑ Bifidobacterium, UP B. animalis subsp. Lactis BB-12 | = | = | [273] |
| Bifidobacterium animalis subsp. Lactis 420 + Litesse Ultra polydextrose | 1010 CFU + 12 g/day | Overweight or obese adults | 3 weeks | ↑Akkermansia, ↑ Christensenellaceae, ↑ Methanobrevibacter, ↓ Paraprevotella |
Not reported | ↓ bile acids glycholic acid, glycoursodeoxycholic acid, taurohyodeoxycholic acid, tauroursodeoxycholic acid | [275] |
| Bacillus coagulans BC30, 6086 + β-glucans | 5 × 108 CFU + 13 g/day | Overweight and obese adults | 3 months | ↑Faecalibacterium prausnitzii, ↓ Bifidobacterium spp. | = | ↑ plasma gamma-glutamyltransferase, ↓ plasma CRP, resistin and LDL/HDL cholesterol ratio | [287] |
| Omnibiotic Hetox: B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W37, L. casei W56, L. brevis W63, L. salivarius W24, Lc. Lactis W58 and Lc. Lactis W19 + Omnilogic Plus: GOS P11, FOS P6, konjac glucomannan P13 (E425), calcium carbonate (E170), zinc citrate 3-hydrate, vitamin D3 (cholecalciferol) and vitamin B2 (riboflavin) (E101) and a matrix containing maltodextrin, natural elderflower flavouring and Gum Arabic (E414) |
1.5 × 1010 CFU total + 8 g/day | Obese adults with T2D | 6 months | = | = | ↓ hip circumference, ↓ zonulin, ↑ QoL (physical functioning), = glucose metabolism | [288] |
| L. acidophilus, B. lactis, B. longum, B. bifidum + GOS | 15 × 109 CFU + 5.5 g/day |
Overweight/obese humans that underwent weight loss program | 3 months | ↑richness, ↑Bifidobacterium, ↑Lactobacillus, ↓Prevotella, ↓Gardnerella | = | = | [289] |
| L. acidophilus, B. lactis, B. bifidum, B. longum + inulin | 1.5 × 1010 CFU each +6 g/day | Overweight or obese people with prediabetes | 6 months | = | Not reported | =food intake | [278] |
| 2 Bifidobaterium +5 Lactobacillus strains + FOS + inulin | 1 × 109/g (total) + 9.6 g + 110.4 g | Overweight/obese humans | 3 months | ↑Lactobacillus, ↑E. coli, ↑Enterococcus | = | Not measured | [290] |
| L. casei, L. paracasei, B. breve + GOS | 3 × 108 CFU each + 7.5 g daily | Obese humans with T2D | 24 weeks | ↑Bifidobacterium, ↑Lactobacillus, ↑B. adolescentis, ↑B. pseudocatenulatum | = | ↑ acetic and butyric acids in faeces | [291] |
This table summarises recent studies, examining the unique effect of microbiota modifications on body weight and gut microbiota composition in people with overweight or obesity. Studies that involved concomitant dietary interventions or do not report gut microbiota changes are excluded. Abbreviations: CFU; colony-forming unit, LDL; low-density lipoprotein, TAG; triacylglycerol, VLDL; very-low-density lipoprotein, CRP; C-reactive protein, HDL; high-density lipoprotein, HFD; high-fat diet, T2D; type-2 diabetes, SCFAs; short-chain fatty acids, HOMA-IR; homeostatic model assessment of Insulin Resistance, MCP-1; macrophages chemoattractant protein 1, BMI; body mass index, IgA antibody; immunoglobulin A, PYY; peptide YY, QoL; quality of life.
Probiotics such as L. gasseri and L. reuteri have been shown to have a positive effect in mice with cachexia, by decreasing muscle atrophy, inflammation and by increasing intestinal health as well as survival [[359], [360], [361]]. Upon administration of these probiotics, the level of Lactobacillus spp. was restored within the gut. However, bodyweight was not significantly altered or was not reported (Table 2); thus, it is still not possible to establish whether a link between probiotic consumption, cancer cachexia-induced body weight loss, and gut microbiota configuration exists. The safety of probiotic administration in cancer patients is under examination [362].
Table 2.
Studies on the effect of probiotics/prebiotics/synbiotics on bodyweight and gut microbiota in pre-clinical and clinical models of cachexia.
| Cachexia | Treatment | Dose | Model | Treatment length | Changes in the gut microbiota | Bodyweight | Other physiological and metabolic changes | References |
|---|---|---|---|---|---|---|---|---|
| Probiotics | ||||||||
| Preclinical studies | Lactobacillus reuteri 100-23 + Lactobacillus gasseri 311476 | 2 × 108 CFU/ml of each strain | Leukemic BALB/c mice with cachexia | 13 days | ↑ L. reuteri, ↑ L. gasseri/L. johnsonii | = | ↓ pro-inflammatory markers,↓ muscle atrophy markers | [292] |
| Lactobacillus reuteri ATCC-PTA-6475 | 3.5 × 105 CFU daily | C57BL/6 ApcMIN mice | ∼ 3 months | Not measured | Not reported | ↓ muscle atrophy, ↑ muscle weight, ↓ inflammation | [293] | |
| Prebiotics | ||||||||
| Preclinical studies | POS | 5% (w/w) | Leukemic BALB/c mice with cachexia | 15 days | ↑ Bacteroides dorei, ↑ Bifidobacterium, ↑ Roseburia | = | ↓ body fat loss and β-oxidation, ↑ SCFAs acetate and propionate, ↓ isovalerate and branched SCFAs | [294] |
| POS | 200 mg/day | BALB/c mice with neuroblastoma | Not reported | ↑ Clostridial Family XIII, ↓Muribaculum | = | No changes | [295] | |
| Clinical studies | inulin + FOS | Elderly people with frailty syndrome | 13 weeks | Not measured | Not reported | ↓exhaustion, ↑ handgrip strength | [296] | |
| Synbiotics | ||||||||
| Preclinical studies | Lactobacillus reuteri 100-23 + oligofructose Orafti | 6.34 × 108 CFU + 0.2 g daily | Leukemic BALB/c mice with cachexia | 13 days | ↑ Lactobacillus spp (reuteri), ↓ Enterobacteriaceae, ↑ Clostridium cluster XVIII | Not reported | ↓ muscle waste, ↑ survival, improved gut function and immunity | [245] |
| Kimchi enriched with Leuconostoc mesenteroides + L. plantarum | 5.1 g/kg/day | Cachectic BALB/c mice with adenocarcinoma | 3 weeks | Not reported | ↑ (∼ 11%) | ↑ survival, ↑ muscle preservation, ↓ inflammation, ↓ lipolysis | [297] | |
Abbreviation; CFU; colony-forming unit, POS; pectic oligosaccharides. FOS; fructo-oligosaccharides.
5.5.2. Prebiotics
Prebiotics can be found naturally in fruits, vegetables, milk, and unrefined cereals and grains [363]. The consumption of specific prebiotics, such as resistant starch, oligo-fructose, xylooligosaccharides, fructooligosaccharides, arabinoxylan, chitin-β-glucan, soluble corn fiber, fungus-derived (1,3)/(1,6)-β-glucan, 2′-fucosyllactose and inulin showed several beneficial effects against obesity and related comorbidities [[363], [364], [365], [366], [367], [368], [369], [370], [371]]. They resulted in a decrease of fat mass, attenuation of systemic inflammation, reduced serum LPS concentration, improved glucose tolerance and lipid metabolism in obese rodents and/or humans. These beneficial metabolic outcomes were accompanied by an increased abundance of Lactobacillus, Bifidobacterium, Faecalibacterium, Bacteroides, Prevotella. and Roseburia [363,365,372]. It was recently demonstrated that the efficacy in ameliorating metabolic syndrome of a specific prebiotic depends on the pre-intervention microbiota configuration of the individual [371]. The studies on the effect of prebiotics on obesity and microbiota in humans are reported in Table 1.
In contrast, galacto-oligosaccharide (GOS) is not linked with modifications of the anthropometric parameters of individuals with metabolic syndrome and overweight. However, this prebiotic can modify the gut microbiota configuration, plasma lipid/biochemical, and immune profile [365,372]. Another prebiotic mixture, called GSK457, produced a strong weight loss effect in DIO and db/db mice but this effect was not recapitulated in overweight adults with T2D [373]. One of the possible mechanisms by which prebiotics ameliorate metabolic syndrome is through SCFAs production and signalling to the host [363,365].
Very few studies assessing the effects of prebiotics on cachexia (cancer and age-related) and gut microbiota are available (Table 2), leaving this topic mainly unexplored.
5.5.3. Synbiotics
Some synbiotics have been tested in obese rodents and humans giving contrasting outcomes. In HFD-induced obese mice, the symbiotic B. animalis subsp. Lactis, Lactobacillus paracasei subsp. Paracasei + oat β-glucan supplementation had a stronger effect in ameliorating the metabolic syndrome and in shaping the gut microbiota compared to the probiotics mix and β-glucan given separately [374].
There is evidence that synbiotics can have a beneficial impact on anthropometric measures and body composition in obese humans coupled with a weight loss intervention [375], but recent human studies showed limited improvement in metabolic syndrome dysfunctions upon synbiotic supplementation only, even if the gut microbiota was affected (Table 1). One study showed that Bifidobacterium strains and GOS improved gut health in obese adults without showing synergism when used together as synbiotics [273]. Therefore, further efforts are necessary to design and study novel synbiotics for the prevention/treatment of obesity.
Few studies have investigated the potential benefits of synbiotics on excessive weight loss, muscle waste, inflammation, and intestinal health in individuals with cachexia [376] (Table 2). Since just one study in rodents includes the analysis of the gut microbiota, further studies that test the involvement of the gut microbiota in the amelioration of cachexia upon synbiotics supplementation are required.
5.6. High-fibre diet
Dietary fibres are classified as soluble (i.e. able to dissolve in water and to be digested) and insoluble (i.e., indigestible fibres that reach the colon and are fermented through several gut bacteria) [377]. Different proportions of fibres are contained in fruits, vegetables, legumes, cereals, nuts, and seeds [377]. The Mediterranean, vegetarian and vegan diets include a high amount of these foods [378,379]. High-fibre intake has been associated with intestinal health, improved bowel motility, low risk of intestinal inflammation and colorectal cancer, low cholesterol absorption and improvement in cognitive functions [[379], [380], [381]].
Numerous clinical trials have tested the effect of a high-fibre diet in obese individuals, which revealed positive effects in lowering body weight gain and adiposity, improving satiety and glucose tolerance [380]. In addition, high-fibre diets are associated with a modulation in the gut microbiota, in particular an increase in Bifidobacterium, Lactobacillus, Prevotella, A. muciniphila, F. prausnitzii, and Ruminococcus [378,382] (Figure 4). In most of the studies, the changes in the gut microbiota are linked with an increase in SCFAs [383]. Indeed, it was shown that dietary fibres intake promoted a greater gut microbiota diversity and the growth of a select group of SCFA-producing strains within the gut in diabetic humans. These changes were accompanied by improvements in GLP-1 production and glucose tolerance [107]. However, in some studies, fibre consumption significantly changed the gut microbiota configuration without affecting glucose tolerance, IR, and other metabolic parameters [382,384]. This is due to the high variability of the diets tested as well as the different proportions of fibre within the diets and the baseline fibre intake of the participants. In a recent study, people with severe obesity were selected and underwent FMT from healthy donors. Following FMT, the participants consumed high-fermentable (HF) or low-fermentable (LF) fibre supplements for 6 weeks. Unlike HF fibre, LF fibre significantly improved insulin sensitivity and satiety hormones production. However, the cessation of LF fibre consumption reversed the beneficial effects on insulin sensitivity. There were no differences in body weight gain among the groups. Moreover, the FMT-LF group showed an increase in bacterial richness, an increase in the abundance of Phascolarcobacterium, Christensenellaceae, Bacteroides, and A. muciniphila, and a decrease in Dialister and Ruminococcus torques. In addition, the abundance of Phascolarctobacterium, Bacteroides stercoris, and Bacteroides caccae was significantly associated with HOMA-IR and insulin sensitivity [368].
Deeper and long-term investigations on the consumption of different kinds of fibre as well as more knowledge on the effect of soluble vs non-soluble fibres on metabolism and gut microbiota are needed. This would help to design specific high-fibre diets for defined pathological conditions.
5.7. Fermented foods
Fermented foods are foods enriched in live microorganisms, bacterial-derived molecules and compounds, and micronutrients that are potentially good for human health. Fruit, vegetables, and legumes, can be spontaneously fermented by promoting the growth of bacteria that naturally colonize the food (i.e., sauerkrauts and kimchi). Other foods, such as milk, tea, and soybeans, can be fermented with starter cultures (i.e., yogurt, kefir, kombucha, and natto) [385].
Few studies have investigated the effect of certain fermented foods on health, even if most of them are largely undocumented (Figure 4). It was shown that some fermented foods such as kefir, natto, miso, and kimchi are linked with the increase of Lactobacillus and Bifidobacterium spp. within the gut [386]. In another study, the gut microbiota of a large cohort of fermented foods consumers was assessed. Higher proportions of Streptococcus, Prevotella, Bacteroides, Enterococcus spp. were also observed as well as a different metabolomics profile compared to non-consumers [387]. Recently, it was shown that 17 weeks of high-fermented food consumption (i.e., 6 servings per day) increased gut microbiota diversity and decreased inflammatory markers in a small cohort of healthy volunteers [388].
Products derived from the fermentation of milk have shown positive effects in people with T2D and CVDs, by improving glucose metabolism [389]. Kefir showed a beneficial effect on GIT health both in healthy individuals and in people with inflammatory bowel disease [386]. In addition, fermented foods consumption exerts anti-inflammatory properties and could prevent cancer development [[390], [391], [392], [393]]. Recently, it was observed that kefir consumption can regulate the microbiota-gut-brain axis by influencing mood and behaviour [394,395].
Limited studies aiming to look at the effects of fermented foods on body weight control and gut microbiota have been carried out. In rodents with HFD-induced obesity, some fermented foods such as Chinese fermented dark tea and fermented ginger showed positive effects by reducing body weight gain and improving obesity-related metabolic markers [396,397]. In humans, Chungkookjang (Korean fermented soybeans, similar to natto) and kimchi significantly improved body fat mass and metabolic parameters [398,399]. However, the composition of the gut microbiota was not assessed in these studies. In another study, HFD-fed mice were supplemented with fermented rice enriched in Bifidobacterium sp. MKK4. After 8 weeks of intervention, a reduction in body weight was observed, as well as an improvement of metabolic parameters, glucose tolerance, and lipolysis. These changes were associated with increased proportions of Lactobacillus, Bifidobacterium and Bacteroides within the gut [400]. In a recent clinical study, the improvements in LDL/HDL ratio and CRP levels, a higher abundance of Akkermansia, Bacteroides and acetate within the gut were reported upon yogurt consumption. Here, the changes in body weight were not significant [401].
With all their beneficial properties and excellent nutritional values, fermented foods could also be a helpful dietary solution for people with cachexia. Only one study in mice has been performed. Here, kimchi consumption suppressed IL-6 levels and ameliorated body weight loss, muscle atrophy, and lipolysis in mice with cancer cachexia [297]. More pre-clinical and clinical studies are needed to better explore the potential of fermented foods in cachexia improvement.
6. Conclusions & future perspectives
Substantial work has demonstrated strong associational relationships between gut microbiota composition and function and body weight regulation. Preclinical evidence in both obesity and cachexia supports a role of the gut microbiota in associated body weight and metabolic dysfunction. However, these encouraging results have had limited translatability when assessed in clinical populations. In addition, associations and causality of human diseases and gut microbiota are better explored in rodents than humans but gaps remain for many conditions [82,402]. A key challenge for researchers investigating the role of the microbiota in body weight control is determining whether specific taxa or microbial functions can underlie bodyweight dysregulation in these pathologies.
The strategies investigated to rebalance the body weight lead to the modification in the proportion of different taxa and current research implicates several bacterial taxa in body weight control (detailed in Table 3). It remains unclear whether these taxa exert effects directly on body weight, or if their benefits depend on modifications to other metabolic processes, such as changes in glycaemic control, IR, intestinal health, and immune function. Furthermore, bodyweight control may depend on a specific bacterial function or product that may be redundant across multiple strains.
Table 3.
Several candidate taxa involved in the control of body weight.
| Phylum | Family | Genus (Species) | How might it affect body weight? |
|---|---|---|---|
| Firmicutes | Lactobacillaceae | Lactobacillus (plantarum, rhamnosus, gasseri, curvatus, sakei, kefir, parakefir) | ↑ after CR, IF, KD, FF.
|
| Lachnospiraceae | Roseburia (hominis, intestinalis, inulinivorans, faecis) | ↑ after CR, IF.
|
|
| Ruminococcaceae | Faecalibacterium (prausnitzii) | ↑ after CR, IF. | |
| Christensenellaceae | Christensenella (minuta) | ↑ after CR, HFi.
|
|
| Bacteroidetes | Bacteroidaceae | Bacteroides (uniformis, thetaiotaomicron) | ↑ after CR, IF, HFi, FF. |
| Actinobacteria | Bifidobacteriaceae | Bifidobacterium (lactis, animalis, adolescentis, bifidum, infantis, breve, brevis, casei) | ↑ after CR, FF; ↓ KD
|
| Verrucomicrobia | Akkermansiaceae | Akkermansia (muciniphila) | ↑ after CR, IF, KD, BS, HFi, FF.
|
Abbreviation; CR; calorie restriction, IF; intermittent fasting. KD; ketogenic diet, BS; bariatric surgery, FF; fermented foods, HFi; high-fibre diet, NF-kB; nuclear factor kappa B, GLP-1; glucagon-like peptide, PYY; peptide tyrosine tyrosine.
Currently, the literature is highly variable in terms of intervention length, formulation, and dose, making comparison difficult. Thus, more consistency in the intervention protocols might help to reduce variability in the results. Furthermore, there is a lack of long-term experiments and follow-up. Since in many clinical studies, the response to a certain bodyweight control intervention is not the same for each individual, in the future it is essential to always include baseline gut microbiota assessment and focus on individual response to intervention longitudinally [6]. This could help understand the temporal relationship between gut microbiota and metabolic changes, and is key for determining causality in human studies. Accordingly, a recent human study showed that the baseline microbiota composition can determine the efficacy of responses to weight loss interventions [403]. In addition, we now need to go deeper into the analysis of bacterial strains by using more precise methods such as the shotgun metagenomics [24] and to investigate more about the function, combined with metabolomics, of the candidate strains [404,405]. These deeper analyses, rather than superficial classification at the genus level, are essential for determining the underlying mechanisms by which potential probiotics exert effects in body weight control.
In summary, while there are strong relationships between body weight and gut microbiota composition both in preclinical and clinical studies, so far there is limited evidence for a causal role of the gut microbiota in body weight control in both healthy people, and people suffering from bodyweight dysregulation. Addressing dysregulated body weight remains a key clinical challenge and manipulation of the gut microbiota alone may not be sufficient to exert beneficial effects in this context and future research should focus on combining strategies to potentiate the benefits of lifestyle interventions.
Funding
Supported by a Science Foundation Ireland (SFI) grant to APC Microbiome Ireland through the Irish Government's National Development Plan (grant SFI/12/RC/2273_P2) and Saks-Kavanaugh Foundation.
Conflict of interest
None.
References
- 1.Chen Y., Zhou J., Wang L. Role and mechanism of gut microbiota in human disease. Frontiers in Cellular and Infection Microbiology. 2021;11:625913. doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani P.D. Microbiota and metabolites in metabolic diseases. Nature Reviews Endocrinology. 2019;15:69–70. doi: 10.1038/s41574-018-0143-9. [DOI] [PubMed] [Google Scholar]
- 3.Roy S., Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nature Reviews Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 4.Rea K., Dinan T.G., Cryan J.F. Gut microbiota: a perspective for psychiatrists. Neuropsychobiology. 2020;79:50–62. doi: 10.1159/000504495. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X., Chen B.D., Zhao L.D., Li H. The gut microbiota: emerging evidence in autoimmune diseases. Trends in Molecular Medicine. 2020;26:862–873. doi: 10.1016/j.molmed.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Schupack D.A., Mars R.A.T., Voelker D.H., Abeykoon J.P., Kashyap P.C. The promise of the gut microbiome as part of individualized treatment strategies. Nature Reviews Gastroenterology & Hepatology. 2021 doi: 10.1038/s41575-021-00499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill J.O., Wyatt H.R., Peters J.C. Energy balance and obesity. Circulation. 2012;126:126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill J.O., Wyatt H.R., Peters J.C. The importance of energy balance. European Journal of Endocrinology. 2013;9:111–115. doi: 10.17925/EE.2013.09.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer P.V., Hamr S.C., Duca F.A. Regulation of energy balance by a gut–brain axis and involvement of the gut microbiota. Cellular and Molecular Life Sciences. 2016;73:737–755. doi: 10.1007/s00018-015-2083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurpad A.V., Muthayya S., Vaz M. Consequences of inadequate food energy and negative energy balance in humans. Public Health Nutrition. 2005;8:1053–1076. doi: 10.1079/phn2005796. [DOI] [PubMed] [Google Scholar]
- 11.Uauy R., Díaz E. Consequences of food energy excess and positive energy balance. Public Health Nutrition. 2005;8:1077–1099. doi: 10.1079/phn2005797. [DOI] [PubMed] [Google Scholar]
- 12.Blüher M. Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 13.Sam S., Mazzone T. Adipose tissue changes in obesity and the impact on metabolic function. Translational Research. 2014;164:284–292. doi: 10.1016/j.trsl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Vanhoutte G., van de Wiel M., Wouters K., Sels M., Bartolomeeussen L., De Keersmaecker S., et al. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterology. 2016;3 doi: 10.1136/bmjgast-2016-000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans W.J., Morley J.E., Argilés J., Bales C., Baracos V., Guttridge D., et al. Cachexia: a new definition. Clinical Nutrition. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Ni Y., Lohinai Z., Heshiki Y., Dome B., Moldvay J., Dulka E., et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. The ISME Journal. 2021;15:3207–3220. doi: 10.1038/s41396-021-00998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres-Fuentes C., Schellekens H., Dinan T.G., Cryan J.F. The microbiota-gut-brain axis in obesity. The Lancet Gastroenterology & Hepatology. 2017;2:747–756. doi: 10.1016/S2468-1253(17)30147-4. [DOI] [PubMed] [Google Scholar]
- 18.Cryan J.F., O'Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., et al. The microbiota-gut-brain Axis. Physiological Reviews. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 19.Byrne C.S., Chambers E.S., Morrison D.J., Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. International Journal of Obesity. 2015;39:1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami C., Iwasaki Y., Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. The Journal of Nutritional Biochemistry. 2018;57:130–135. doi: 10.1016/j.jnutbio.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder B.O., Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nature Medicine. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 22.Bordenstein S.R., Theis K.R. Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biology. 2015;13 doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biology. 2016;14 doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie H., Guo R., Zhong H., Feng Q., Lan Z., Qin B., et al. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Systems. 2016;3(6):572–584. doi: 10.1016/j.cels.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagier J.-C., Dubourg G., Million M., Cadoret F., Bilen M., Fenollar F., et al. Culturing the human microbiota and culturomics. Nature Reviews Microbiology. 2018;16:540–550. doi: 10.1038/s41579-018-0041-0. [DOI] [PubMed] [Google Scholar]
- 27.Huttenhower C., Gevers D., Knight R., Abubucker S., Badger J.H., Chinwalla A.T., et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K., et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 30.McDonald D., Hyde E., Debelius J.W., Morton J.T., Gonzalez A., Ackermann G., et al. American gut: an open platform for citizen science microbiome research. mSystems. 2018;3 doi: 10.1128/mSystems.00031-18. e00031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tigchelaar E.F., Zhernakova A., Dekens J.A.M., Hermes G., Baranska A., Mujagic Z., et al. Cohort profile: LifeLines DEEP, a prospective, general population cohort study in the northern Netherlands: study design and baseline characteristics. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida A., Mitchell A.L., Boland M., Forster S.C., Gloor G.B., Tarkowska A., et al. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandeputte D., Kathagen G., D'Hoe K., Vieira-Silva S., Valles-Colomer M., Sabino J., et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 35.Costea P.I., Hildebrand F., Arumugam M., Bäckhed F., Blaser M.J., Bushman F.D., et al. Enterotypes in the landscape of gut microbial community composition. Nature Microbiology. 2018;3:8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richard M.L., Sokol H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nature Reviews Gastroenterology & Hepatology. 2019;16:331–345. doi: 10.1038/s41575-019-0121-2. [DOI] [PubMed] [Google Scholar]
- 37.Shkoporov A.N., Clooney A.G., Sutton T.D.S., Ryan F.J., Daly K.M., Nolan J.A., et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host & Microbe. 2019;26:527–541.e5. doi: 10.1016/j.chom.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Feehley T., Plunkett C.H., Bao R., Choi Hong S.M., Culleen E., Belda-Ferre P., et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nature Medicine. 2019;25:448–453. doi: 10.1038/s41591-018-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kostic A.D., Gevers D., Pedamallu C.S., Michaud M., Duke F., Earl A.M., et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Research. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J.I., et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S., et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 42.Tang W.H., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly J.R., Borre Y., C O.B., Patterson E., El Aidy S., Deane J., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vujkovic-Cvijin I., Sklar J., Jiang L., Natarajan L., Knight R., Belkaid Y. Host variables confound gut microbiota studies of human disease. Nature. 2020;587:448–454. doi: 10.1038/s41586-020-2881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15718. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bäckhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences. 2007;104:979. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turnbaugh P.J., Bäckhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host & Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeppsson B., James J.H., Hummel R.P., Brenner W., West R., Fischer J.E. Increased blood-brain transport of tryptophan after portacaval anastomoses in germ-free rats. Metabolism. 1983;32:4–8. doi: 10.1016/0026-0495(83)90147-6. [DOI] [PubMed] [Google Scholar]
- 51.Savage D.C., Siegel J.E., Snellen J., Whitt D. Transit time of epithelial cells in the small intestines of germfree mice and ex-germfree mice associated with indigenous microorganisms. Applied and Environmental Microbiology. 1981;42:996–1001. doi: 10.1128/aem.42.6.996-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawase T., Nagasawa M., Ikeda H., Yasuo S., Koga Y., Furuse M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. British Journal of Nutrition. 2017;117:775–783. doi: 10.1017/S0007114517000678. [DOI] [PubMed] [Google Scholar]
- 53.Weger B.D., Gobet C., Yeung J., Martin E., Jimenez S., Betrisey B., et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metabolism. 2019;29:362–382.e8. doi: 10.1016/j.cmet.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dodd D., Spitzer M.H., Van Treuren W., Merrill B.D., Hryckowian A.J., Higginbottom S.K., et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joyce S.A., MacSharry J., Casey P.G., Kinsella M., Murphy E.F., Shanahan F., et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proceedings of the National Academy of Sciences. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nature Reviews Microbiology. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melander R.J., Zurawski D.V., Melander C. Narrow-spectrum antibacterial agents. MedChemComm. 2018;9:12–21. doi: 10.1039/c7md00528h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy E.A., King K.Y., Baldridge M.T. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Frontiers in Physiology. 2018;9 doi: 10.3389/fphys.2018.01534. 1534-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaser M.J. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–545. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Medicine. 2016;8 doi: 10.1186/s13073-016-0294-z. 39-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vrieze A., Out C., Fuentes S., Jonker L., Reuling I., Kootte R.S., et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. Journal of Hepatology. 2014;60:824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 63.Reijnders D., Goossens G.H., Hermes G.D., Neis E.P., van der Beek C.M., Most J., et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metabolism. 2016;24:63–74. doi: 10.1016/j.cmet.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 65.Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A., et al. Dietary prebiotics: current status and new definition. Food Science & Technology Bulletin: Functional Foods. 2010;7:1–19. [Google Scholar]
- 66.Bindels L.B., Delzenne N.M., Cani P.D., Walter J. Towards a more comprehensive concept for prebiotics. Nature Reviews Gastroenterology & Hepatology. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 67.Swanson K.S., Gibson G.R., Hutkins R., Reimer R.A., Reid G., Verbeke K., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nature Reviews Gastroenterology & Hepatology. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim K.O., Gluck M. Fecal microbiota transplantation: an update on clinical practice. Clinical Endoscopy. 2019;52:137–143. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai Z.-L., Tseng C.-H., Ho H.J., Cheung C.K.Y., Lin J.-Y., Chen Y.-J., et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Scientific Reports. 2018;8:15625. doi: 10.1038/s41598-018-33893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellekilde M., Selfjord E., Larsen C.S., Jakesevic M., Rune I., Tranberg B., et al. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Scientific Reports. 2014;4 doi: 10.1038/srep05922. 5922-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Costello S.P., Hughes P.A., Waters O., Bryant R.V., Vincent A.D., Blatchford P., et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. Journal of the American Medical Association. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–109.e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Paramsothy S., Kamm M.A., Kaakoush N.O., Walsh A.J., van den Bogaerde J., Samuel D., et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 74.Rossen N.G., Fuentes S., van der Spek M.J., Tijssen J.G., Hartman J.H., Duflou A., et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–118.e4. doi: 10.1053/j.gastro.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 75.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 77.Kootte R.S., Levin E., Salojärvi J., Smits L.P., Hartstra A.V., Udayappan S.D., et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metabolism. 2017;26:611–619.e6. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Vrieze A., Van Nood E., Holleman F., Salojärvi J., Kootte R.S., Bartelsman J.F., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen T.L.A., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Disease Models & Mechanisms. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hugenholtz F., de Vos W.M. Mouse models for human intestinal microbiota research: a critical evaluation. Cellular and Molecular Life Sciences. 2018;75:149–160. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ridaura V.K., Faith J.J., Rey F.E., Cheng J., Duncan A.E., Kau A.L., et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaudhari S.N., McCurry M.D., Devlin A.S. Chains of evidence from correlations to causal molecules in microbiome-linked diseases. Nature Chemical Biology. 2021;17:1046–1056. doi: 10.1038/s41589-021-00861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walter J., Armet A.M., Finlay B.B., Shanahan F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020;180:221–232. doi: 10.1016/j.cell.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 84.Gheorghe C.E., Ritz N.L., Martin J.A., Wardill H.R., Cryan J.F., Clarke G. Investigating causality with fecal microbiota transplantation in rodents: applications, recommendations and pitfalls. Gut Microbes. 2021;13:1941711. doi: 10.1080/19490976.2021.1941711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Secombe K.R., Al-Qadami G.H., Subramaniam C.B., Bowen J.M., Scott J., Van Sebille Y.Z.A., et al. Guidelines for reporting on animal fecal transplantation (GRAFT) studies: recommendations from a systematic review of murine transplantation protocols. Gut Microbes. 2021;13:1979878. doi: 10.1080/19490976.2021.1979878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell C.M., Mazzoni C., Hogstrom L., Bryant A., Bergerat A., Cher A., et al. Delivery mode affects stability of early infant gut microbiota. Cell Reports Medicine. 2020;1 doi: 10.1016/j.xcrm.2020.100156. 100156-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hansen C.H., Andersen L.S., Krych L., Metzdorff S.B., Hasselby J.P., Skov S., et al. Mode of delivery shapes gut colonization pattern and modulates regulatory immunity in mice. The Journal of Immunology. 2014;193:1213–1222. doi: 10.4049/jimmunol.1400085. [DOI] [PubMed] [Google Scholar]
- 88.Morais L.H., Golubeva A.V., Moloney G.M., Moya-Pérez A., Ventura-Silva A.P., Arboleya S., et al. Enduring behavioral effects induced by birth by caesarean section in the mouse. Current Biology. 2020;30:3761–3774.e6. doi: 10.1016/j.cub.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 89.Clarke G., Sandhu K.V., Griffin B.T., Dinan T.G., Cryan J.F., Hyland N.P. Gut reactions: breaking down xenobiotic–microbiome interactions. Pharmacological Reviews. 2019;71:198. doi: 10.1124/pr.118.015768. [DOI] [PubMed] [Google Scholar]
- 90.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., et al. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dethlefsen L., McFall-Ngai M., Relman D.A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glowacki R.W.P., Martens E.C. If you eat it, or secrete it, they will grow: the expanding list of nutrients utilized by human gut bacteria. Journal of Bacteriology. 2020;203 doi: 10.1128/JB.00481-20. e00481-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gill S.R., Pop M., Deboy R.T., Eckburg P.B., Turnbaugh P.J., Samuel B.S., et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Townsend G.E., 2nd, Han W., Schwalm N.D., 3rd, Raghavan V., Barry N.A., Goodman A.L., et al. Dietary sugar silences a colonization factor in a mammalian gut symbiont. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:233–238. doi: 10.1073/pnas.1813780115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zarrinpar A., Chaix A., Yooseph S., Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metabolism. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaczmarek J.L., Musaad S.M., Holscher H.D. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. The American Journal of Clinical Nutrition. 2017;106:1220–1231. doi: 10.3945/ajcn.117.156380. [DOI] [PubMed] [Google Scholar]
- 98.Fetissov S.O. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nature Reviews Endocrinology. 2017;13:11–25. doi: 10.1038/nrendo.2016.150. [DOI] [PubMed] [Google Scholar]
- 99.Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Critical Reviews in Environmental Science and Technology. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ley R.E., Hamady M., Lozupone C., Turnbaugh P.J., Ramey R.R., Bircher J.S., et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muegge B.D., Kuczynski J., Knights D., Clemente J.C., González A., Fontana L., et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–974. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M., Li H., et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Science Translational Medicine. 2016;8:343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 104.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fragiadakis G.K., Wastyk H.C., Robinson J.L., Sonnenburg E.D., Sonnenburg J.L., Gardner C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. The American Journal of Clinical Nutrition. 2020;111:1127–1136. doi: 10.1093/ajcn/nqaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X., et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 108.Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. Journal of the International Society for Microbial Ecology. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein–coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shan Z., Sun T., Huang H., Chen S., Chen L., Luo C., et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. The American Journal of Clinical Nutrition. 2017;106:888–894. doi: 10.3945/ajcn.117.157107. [DOI] [PubMed] [Google Scholar]
- 111.Agus A., Clément K., Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pedersen H.K., Gudmundsdottir V., Nielsen H.B., Hyotylainen T., Nielsen T., Jensen B.A.H., et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 113.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 114.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 115.Jumpertz R., Le D.S., Turnbaugh P.J., Trinidad C., Bogardus C., Gordon J.I., et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. The American Journal of Clinical Nutrition. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cummings J.H., Macfarlane G.T. The control and consequences of bacterial fermentation in the human colon. Journal of Applied Microbiology. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- 117.Silvester K.R., Englyst H.N., Cummings J.H. Ileal recovery of starch from whole diets containing resistant starch measured in vitro and fermentation of ileal effluent. The American Journal of Clinical Nutrition. 1995;62:403–411. doi: 10.1093/ajcn/62.2.403. [DOI] [PubMed] [Google Scholar]
- 118.Schwiertz A., Taras D., Schäfer K., Beijer S., Bos N.A., Donus C., et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 119.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 120.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 122.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Current Nutrition Reports. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McNeil N.I. The contribution of the large intestine to energy supplies in man. American Journal of Clinical Nutrition. 1984;39:338–342. doi: 10.1093/ajcn/39.2.338. [DOI] [PubMed] [Google Scholar]
- 124.Flint H.J. Why gut microbes matter: understanding our microbiome. Springer International Publishing; Cham: 2020. Do my microbes make me fat? Potential for the gut microbiota to influence energy balance, obesity and metabolic health in humans; pp. 97–108. [Google Scholar]
- 125.Khanna R., Shen B. Adverse metabolic sequelae following restorative proctocolectomy with an ileal pouch. Gastroenterology and Hepatology. 2012;8:322–326. [PMC free article] [PubMed] [Google Scholar]
- 126.Hettiarachchi P., Wickremasinghe A.R., Frost G.S., Deen K.I., Pathirana A.A., Murphy K.G., et al. Resection of the large bowel suppresses hunger and food intake and modulates gastrointestinal fermentation. Obesity. 2016;24:1723–1730. doi: 10.1002/oby.21550. [DOI] [PubMed] [Google Scholar]
- 127.Kuisma J., Nuutinen H., Luukkonen P., Järvinen H., Kahri A., Färkkilä M. Long term metabolic consequences of ileal pouch-anal anastomosis for ulcerative colitis. American Journal of Gastroenterology. 2001;96:3110–3116. doi: 10.1111/j.1572-0241.2001.05256.x. [DOI] [PubMed] [Google Scholar]
- 128.Müller M.J., Geisler C., Heymsfield S.B., Bosy-Westphal A. Recent advances in understanding body weight homeostasis in humans. F1000Research. 2018;7:F1000. doi: 10.12688/f1000research.14151.1. Faculty Rev-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woods S.C., D'Alessio D.A. Central control of body weight and appetite. Journal of Clinical Endocrinology & Metabolism. 2008;93:S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.MacLean P.S., Blundell J.E., Mennella J.A., Batterham R.L. Biological control of appetite: a daunting complexity. Obesity. 2017;25:S8–S16. doi: 10.1002/oby.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zimmerman C.A., Knight Z.A. Layers of signals that regulate appetite. Current Opinion in Neurobiology. 2020;64:79–88. doi: 10.1016/j.conb.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim K.-S., Seeley R.J., Sandoval D.A. Signalling from the periphery to the brain that regulates energy homeostasis. Nature Reviews Neuroscience. 2018;19:185–196. doi: 10.1038/nrn.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leigh S.J., Morris M.J. The role of reward circuitry and food addiction in the obesity epidemic: an update. Biological Psychology. 2018;131:31–42. doi: 10.1016/j.biopsycho.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 134.de Wouters d'Oplinter A., Rastelli M., Van Hul M., Delzenne N.M., Cani P.D., Everard A. Gut microbes participate in food preference alterations during obesity. Gut Microbes. 2021;13:1959242. doi: 10.1080/19490976.2021.1959242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mezouar S., Chantran Y., Michel J., Fabre A., Dubus J.-C., Leone M., et al. Microbiome and the immune system: from a healthy steady-state to allergy associated disruption. Human Microbiome Journal. 2018;10:11–20. [Google Scholar]
- 136.Schirmer M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1125–1136.e8. doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rao S., Schieber A.M.P., O'Connor C.P., Leblanc M., Michel D., Ayres J.S. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell. 2017;168:503–516.e12. doi: 10.1016/j.cell.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gupta A., Osadchiy V., Mayer E.A. Brain–gut–microbiome interactions in obesity and food addiction. Nature Reviews Gastroenterology & Hepatology. 2020;17:655–672. doi: 10.1038/s41575-020-0341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van de Wouw M., Schellekens H., Dinan T.G., Cryan J.F. Microbiota-gut-brain Axis: modulator of host metabolism and appetite. Journal of Nutrition. 2017;147:727–745. doi: 10.3945/jn.116.240481. [DOI] [PubMed] [Google Scholar]
- 140.García-Cabrerizo R., Carbia C., KJ O.R., Schellekens H., Cryan J.F. Microbiota-gut-brain axis as a regulator of reward processes. Journal of Neurochemistry. 2021;157:1495–1524. doi: 10.1111/jnc.15284. [DOI] [PubMed] [Google Scholar]
- 141.Breton J., Tennoune N., Lucas N., Francois M., Legrand R., Jacquemot J., et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metabolism. 2016;23:324–334. doi: 10.1016/j.cmet.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 142.Wynendaele E., Verbeke F., Stalmans S., Gevaert B., Janssens Y., Van De Wiele C., et al. Quorum sensing peptides selectively penetrate the blood-brain barrier. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142071. e0142071-e0142071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janssens Y., Wynendaele E., Verbeke F., Debunne N., Gevaert B., Audenaert K., et al. Screening of quorum sensing peptides for biological effects in neuronal cells. Peptides. 2018;101:150–156. doi: 10.1016/j.peptides.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 144.Breton J., Legrand R., Akkermann K., Järv A., Harro J., Déchelotte P., et al. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. International Journal of Eating Disorders. 2016;49:805–808. doi: 10.1002/eat.22531. [DOI] [PubMed] [Google Scholar]
- 145.Tennoune N., Chan P., Breton J., Legrand R., Chabane Y.N., Akkermann K., et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide α-MSH, at the origin of eating disorders. Translational Psychiatry. 2014;4:e458. doi: 10.1038/tp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Depommier C., Everard A., Druart C., Plovier H., Van Hul M., Vieira-Silva S., et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nature Medicine. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Panaro B.L., Tough I.R., Engelstoft M.S., Matthews R.T., Digby G.J., Møller C.L., et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metabolism. 2014;20:1018–1029. doi: 10.1016/j.cmet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dominique M., Breton J., Guérin C., Bole-Feysot C., Lambert G., Déchelotte P., et al. Effects of macronutrients on the in vitro production of ClpB, a bacterial mimetic protein of α-MSH and its possible role in satiety signaling. Nutrients. 2019;11:2115. doi: 10.3390/nu11092115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dominique M., Lucas N., Legrand R., Bouleté I.M., Bôle-Feysot C., Deroissart C., et al. Effects of bacterial CLPB protein fragments on food intake and PYY secretion. Nutrients. 2021;13:2223. doi: 10.3390/nu13072223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Konsman J.P., Parnet P., Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in Neurosciences. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 151.Mukherji A., Kobiita A., Ye T., Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 152.Amar J., Burcelin R., Ruidavets J.B., Cani P.D., Fauvel J., Alessi M.C., et al. Energy intake is associated with endotoxemia in apparently healthy men. American Journal of Clinical Nutrition. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 153.Harte A.L., Varma M.C., Tripathi G., McGee K.C., Al-Daghri N.M., Al-Attas O.S., et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lebrun L.J., Lenaerts K., Kiers D., Pais de Barros J.P., Le Guern N., Plesnik J., et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Reports. 2017;21:1160–1168. doi: 10.1016/j.celrep.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 155.Zhu X., He L., McCluskey L.P. Ingestion of bacterial lipopolysaccharide inhibits peripheral taste responses to sucrose in mice. Neuroscience. 2014;258:47–61. doi: 10.1016/j.neuroscience.2013.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pittman D.W., Dong G., Brantly A.M., He L., Nelson T.S., Kogan S., et al. Behavioral and neurophysiological taste responses to sweet and salt are diminished in a model of subclinical intestinal inflammation. Scientific Reports. 2020;10:17611. doi: 10.1038/s41598-020-74632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Langhans W., Harlacher R., Balkowski G., Scharrer E. Comparison of the effects of bacterial lipopolysaccharide and muramyl dipeptide on food intake. Physiology & Behavior. 1990;47:805–813. doi: 10.1016/0031-9384(90)90001-k. [DOI] [PubMed] [Google Scholar]
- 158.Breen D.M., Jagarlapudi S., Patel A., Zou C., Joaquim S., Li X., et al. Growth differentiation factor 15 neutralization does not impact anorexia or survival in lipopolysaccharide-induced inflammation. iScience. 2021;24:102554. doi: 10.1016/j.isci.2021.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hsu J.-Y., Crawley S., Chen M., Ayupova D.A., Lindhout D.A., Higbee J., et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550:255–259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 160.Luan H.H., Wang A., Hilliard B.K., Carvalho F., Rosen C.E., Ahasic A.M., et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178:1231–1244.e11. doi: 10.1016/j.cell.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 162.Wanders A.J., van den Borne J.J., de Graaf C., Hulshof T., Jonathan M.C., Kristensen M., et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obesity Reviews. 2011;12:724–739. doi: 10.1111/j.1467-789X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 163.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nature Reviews Endocrinology. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 164.Byrne C.S., Chambers E.S., Alhabeeb H., Chhina N., Morrison D.J., Preston T., et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. American Journal of Clinical Nutrition. 2016;104:5–14. doi: 10.3945/ajcn.115.126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chambers E.S., Viardot A., Psichas A., Morrison D.J., Murphy K.G., Zac-Varghese S.E., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Canfora E.E., van der Beek C.M., Jocken J.W.E., Goossens G.H., Holst J.J., Olde Damink S.W.M., et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Scientific Reports. 2017;7:2360. doi: 10.1038/s41598-017-02546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bouter K., Bakker G.J., Levin E., Hartstra A.V., Kootte R.S., Udayappan S.D., et al. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clinical and Translational Gastroenterology. 2018;9 doi: 10.1038/s41424-018-0025-4. 155-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 169.De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metabolism. 2016;24 doi: 10.1016/j.cmet.2016.06.013. 151-157. [DOI] [PubMed] [Google Scholar]
- 170.Mithieux G., Andreelli F., Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12:419–423. doi: 10.1097/MCO.0b013e32832c4d6a. [DOI] [PubMed] [Google Scholar]
- 171.Vily-Petit J., Soty-Roca M., Silva M., Raffin M., Gautier-Stein A., Rajas F., et al. Intestinal gluconeogenesis prevents obesity-linked liver steatosis and non-alcoholic fatty liver disease. Gut. 2020;69:2193–2202. doi: 10.1136/gutjnl-2019-319745. [DOI] [PubMed] [Google Scholar]
- 172.Araújo J.R., Tazi A., Burlen-Defranoux O., Vichier-Guerre S., Nigro G., Licandro H., et al. Fermentation products of commensal bacteria alter enterocyte lipid metabolism. Cell Host & Microbe. 2020;27:358–375.e7. doi: 10.1016/j.chom.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 173.Reigstad C.S., Salmonson C.E., Rainey J.F., 3rd, Szurszewski J.H., Linden D.R., Sonnenburg J.L., et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leeuwendaal N.K., Cryan J.F., Schellekens H. Gut peptides and the microbiome: focus on ghrelin. Current Opinion in Endocrinology Diabetes and Obesity. 2021;28:243–252. doi: 10.1097/MED.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dumoulin V., Moro F., Barcelo A., Dakka T., Cuber J.C. Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780–3786. doi: 10.1210/endo.139.9.6202. [DOI] [PubMed] [Google Scholar]
- 176.Torres-Fuentes C., Golubeva A.V., Zhdanov A.V., Wallace S., Arboleya S., Papkovsky D.B., et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. The FASEB Journal. 2019;33:13546–13559. doi: 10.1096/fj.201901433R. [DOI] [PubMed] [Google Scholar]
- 177.Gabriel F.C., Fantuzzi G. The association of short-chain fatty acids and leptin metabolism: a systematic review. Nutrition Research. 2019;72:18–35. doi: 10.1016/j.nutres.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 178.Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proceedings of the National Academy of Sciences. 2011;108:8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li Z., Yi C.X., Katiraei S., Kooijman S., Zhou E., Chung C.K., et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 180.Perry R.J., Peng L., Barry N.A., Cline G.W., Zhang D., Cardone R.L., et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Muller P.A., Schneeberger M., Matheis F., Wang P., Kerner Z., Ilanges A., et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Han W., Tellez L.A., Perkins M.H., Perez I.O., Qu T., Ferreira J., et al. A neural circuit for gut-induced reward. Cell. 2018;175:665–678.e23. doi: 10.1016/j.cell.2018.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nature Communications. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.O'Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Research. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 185.Clarke G., Villalobos-Manriquez F., Campos Marin D. In: The oxford handbook of the microbiome-gut-brain Axis. Burnet P.W.J., editor. Oxford Handbooks Online; 2021. Tryptophan metabolism and the microbiome-gut-brain Axis. [Google Scholar]
- 186.Gheorghe C.E., Martin J.A., Manriquez F.V., Dinan T.G., Cryan J.F., Clarke G. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Current Opinion in Pharmacology. 2019;48:137–145. doi: 10.1016/j.coph.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 187.Gartner S.N., Aidney F., Klockars A., Prosser C., Carpenter E.A., Isgrove K., et al. Intragastric preloads of l-tryptophan reduce ingestive behavior via oxytocinergic neural mechanisms in male mice. Appetite. 2018;125:278–286. doi: 10.1016/j.appet.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 188.Steinert R.E., Luscombe-Marsh N.D., Little T.J., Standfield S., Otto B., Horowitz M., et al. Effects of intraduodenal infusion of L-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. Journal of Clinical Endocrinology & Metabolism. 2014;99:3275–3284. doi: 10.1210/jc.2014-1943. [DOI] [PubMed] [Google Scholar]
- 189.Lee H.H., Molla M.N., Cantor C.R., Collins J.J. Bacterial charity work leads to population-wide resistance. Nature. 2010;467:82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Natividad J.M., Agus A., Planchais J., Lamas B., Jarry A.C., Martin R., et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metabolism. 2018;28:737–749.e4. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 191.Bhattarai Y., Williams B.B., Battaglioli E.J., Whitaker W.R., Till L., Grover M., et al. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host & Microbe. 2018;23:775–785.e5. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Scott S.A., Fu J., Chang P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proceedings of the National Academy of Sciences. 2020;117:19376–19387. doi: 10.1073/pnas.2000047117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grüner N., Mattner J. Bile acids and microbiota: multifaceted and versatile regulators of the liver-gut Axis. International Journal of Molecular Sciences. 2021;22:1397. doi: 10.3390/ijms22031397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kuhre R.E., Wewer Albrechtsen N.J., Larsen O., Jepsen S.L., Balk-Møller E., Andersen D.B., et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Molecular Metabolism. 2018;11:84–95. doi: 10.1016/j.molmet.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Christiansen C.B., Trammell S.A.J., Wewer Albrechtsen N.J., Schoonjans K., Albrechtsen R., Gillum M.P., et al. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. American Journal of Physiology-Gastrointestinal and Liver. 2019;316:g574–g584. doi: 10.1152/ajpgi.00010.2019. [DOI] [PubMed] [Google Scholar]
- 196.Cheng Z., Liu G., Zhang X., Bi D., Hu S. Improvement of glucose metabolism following long-term taurocholic acid gavage in a diabetic rat model. Medical Science Monitor. 2018;24:7206–7212. doi: 10.12659/MSM.912429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Calderon G., McRae A., Rievaj J., Davis J., Zandvakili I., Linker-Nord S., et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine. 2020;55:102759. doi: 10.1016/j.ebiom.2020.102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Higuchi S., Ahmad T.R., Argueta D.A., Perez P.A., Zhao C., Schwartz G.J., et al. Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice. Gut. 2020;69:1620–1628. doi: 10.1136/gutjnl-2019-319693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cryan J.F., Clarke G., Dinan T.G., Schellekens H. A microbial drugstore for motility. Cell Host & Microbe. 2018;23:691–692. doi: 10.1016/j.chom.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 200.Yano Jessica M., Yu K., Donaldson Gregory P., Shastri Gauri G., Ann P., Ma L., et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang K., Liao M., Zhou N., Bao L., Ma K., Zheng Z., et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Reports. 2019;26:222–235.e5. doi: 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 202.Zietak M., Kozak L.P. Bile acids induce uncoupling protein 1-dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. American Journal of Physiology. Endocrinology and Metabolism. 2016;310:E346–E354. doi: 10.1152/ajpendo.00485.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zaborska K.E., Lee S.A., Garribay D., Cha E., Cummings B.P. Deoxycholic acid supplementation impairs glucose homeostasis in mice. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Quante M., Iske J., Heinbokel T., Desai B.N., Cetina Biefer H.R., Nian Y., et al. Restored TDCA and valine levels imitate the effects of bariatric surgery. Elife. 2021;10 doi: 10.7554/eLife.62928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Perino A., Velázquez-Villegas L.A., Bresciani N., Sun Y., Huang Q., Fénelon V.S., et al. Central anorexigenic actions of bile acids are mediated by TGR5. Nature Metabolism. 2021;3:595–603. doi: 10.1038/s42255-021-00398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.WHO global status report on noncommunicable diseases 2014. 2014. [DOI] [PubMed] [Google Scholar]
- 207.Halsted C.H. Obesity: effects on the liver and gastrointestinal system. Current Opinion in Clinical Nutrition and Metabolic Care. 1999;2:425–429. doi: 10.1097/00075197-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 208.Wiese M.L., Aghdassi A.A., Lerch M.M., Steveling A. Excess body weight and pancreatic disease. Visceral Medicine. 2021;37:281–286. doi: 10.1159/000517147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Williams L.M. Hypothalamic dysfunction in obesity. Proceedings of the Nutrition Society. 2012;71:521–533. doi: 10.1017/S002966511200078X. [DOI] [PubMed] [Google Scholar]
- 210.Turnbaugh P.J., Gordon J.I. The core gut microbiome, energy balance and obesity. The Journal of Physiology. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 212.Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 214.Duncan S.H., Lobley G.E., Holtrop G., Ince J., Johnstone A.M., Louis P., et al. Human colonic microbiota associated with diet, obesity and weight loss. International Journal of Obesity. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 215.Sze M.A., Schloss P.D., Fraser C.M. Looking for a signal in the noise: revisiting obesity and the microbiome. mBio. 2016;7 doi: 10.1128/mBio.01018-16. e01018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Finucane M.M., Sharpton T.J., Laurent T.J., Pollard K.S. A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bisanz J.E., Upadhyay V., Turnbaugh J.A., Ly K., Turnbaugh P.J. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host & Microbe. 2019;26:265–272.e4. doi: 10.1016/j.chom.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cotillard A., Kennedy S.P., Kong L.C., Prifti E., Pons N., Le Chatelier E., et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 219.Dalby M. The call of the Honeyguide. 2018. Graphic faecal transplants and obesity.honey-guide.com [Google Scholar]
- 220.Caesar R., Reigstad C.S., Bäckhed H.K., Reinhardt C., Ketonen M., Lundén G., et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61:1701–1707. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rabot S., Membrez M., Bruneau A., Gérard P., Harach T., Moser M., et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. The FASEB Journal. 2010;24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 222.Kersten S. Physiological regulation of lipoprotein lipase. Biochimica et Biophysica Acta. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 223.Hild B., Dreier M.S., Oh J.H., McCulloch J.A., Badger J.H., Guo J., et al. Neonatal exposure to a wild-derived microbiome protects mice against diet-induced obesity. Nature Metabolism. 2021;3:1042–1057. doi: 10.1038/s42255-021-00439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thaiss C.A., Itav S., Rothschild D., Meijer M.T., Levy M., Moresi C., et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 225.Allegretti J.R., Kassam Z., Mullish B.H., Chiang A., Carrellas M., Hurtado J., et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clinical Gastroenterology and Hepatology. 2019;18:855–863. doi: 10.1016/j.cgh.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 226.Yu E.W., Gao L., Stastka P., Cheney M.C., Mahabamunuge J., Torres Soto M., et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Medicine. 2020;17 doi: 10.1371/journal.pmed.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fei N., Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. Journal of the International Society for Microbial Ecology. 2013;7:880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Woting A., Pfeiffer N., Loh G., Klaus S., Blaut M. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. mBio. 2014;5:e01530–e01614. doi: 10.1128/mBio.01530-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Suriano F., Vieira-Silva S., Falony G., Roumain M., Paquot A., Pelicaen R., et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: two sides of the same coin. Microbiome. 2021;9:147. doi: 10.1186/s40168-021-01097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences. 2013;110:9066. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhou Q., Pang G., Zhang Z., Yuan H., Chen C., Zhang N., et al. Association between gut Akkermansia and metabolic syndrome is dose-dependent and affected by microbial interactions: a cross-sectional study. Diabetes, Metabolic Syndrome and Obesity. 2021;14:2177–2188. doi: 10.2147/DMSO.S311388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Goodrich Julia K., Waters Jillian L., Poole Angela C., Sutter Jessica L., Koren O., Blekhman R., et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.De Stefani F.d.C., Pietraroia P.S., Fernandes-Silva M.M., Faria-Neto J., Baena C.P. Observational evidence for unintentional weight loss in all-cause mortality and major cardiovascular events: a systematic review and meta-analysis. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-33563-z. 15447-15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fine J.T., Colditz G.A., Coakley E.H., Moseley G., Manson J.E., Willett W.C., et al. A prospective study of weight change and health-related quality of life in women. Journal of the American Medical Association. 1999;282:2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 235.Sharma R., Anker S. First cachexia symposium, berlin, Germany, 1st–2nd december, 2000. European Journal of Heart Failure. 2001;3:751–754. doi: 10.1016/s1388-9842(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 236.Fearon Kenneth C.H., Glass David J., Guttridge Denis C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabolism. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 237.Prado B.L., Qian Y. Anti-cytokines in the treatment of cancer cachexia. Annual Palitychny Medicine. 2018;8:67–79. doi: 10.21037/apm.2018.07.06. [DOI] [PubMed] [Google Scholar]
- 238.Garcia J.M., Shamliyan T.A. Nonsteroidal anti-inflammatory drugs in patients with anorexia-cachexia syndrome associated with malignancy and its treatments. The American Journal of Medicine. 2017;130:1033–1036. doi: 10.1016/j.amjmed.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 239.Seelaender M., Batista M., Jr., Lira F., Silverio R., Rossi-Fanelli F. Inflammation in cancer cachexia: to resolve or not to resolve (is that the question?) Clinical Nutrition. 2012;31:562–566. doi: 10.1016/j.clnu.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 240.Del Fabbro E. Combination therapy in cachexia. Annals of Palliative Medicine. 2018;8:59–66. doi: 10.21037/apm.2018.08.05. [DOI] [PubMed] [Google Scholar]
- 241.Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: gut microbiota: the neglected endocrine organ. Molecular Endocrinology. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bindels L.B., Neyrinck A.M., Loumaye A., Catry E., Walgrave H., Cherbuy C., et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9:18224–18238. doi: 10.18632/oncotarget.24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.de Clercq N.C., van den Ende T., Prodan A., Hemke R., Davids M., Pedersen H.K., et al. Fecal microbiota transplantation from overweight or obese donors in cachectic patients with advanced gastroesophageal cancer: a randomized, double-blind, placebo-controlled, phase II study. Clinical Cancer Research. 2021;27:3784–3792. doi: 10.1158/1078-0432.CCR-20-4918. [DOI] [PubMed] [Google Scholar]
- 244.Pötgens S.A., Brossel H., Sboarina M., Catry E., Cani P.D., Neyrinck A.M., et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-30569-5. 12321-12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bindels L.B., Neyrinck A.M., Claus S.P., Le Roy C.I., Grangette C., Pot B., et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. The ISME Journal. 2016;10:1456–1470. doi: 10.1038/ismej.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Puppa M.J., White J.P., Sato S., Cairns M., Baynes J.W., Carson J.A. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochimica et Biophysica Acta. 2011;1812:1601–1606. doi: 10.1016/j.bbadis.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pötgens S.A., Thibaut M.M., Joudiou N., Sboarina M., Neyrinck A.M., Cani P.D., et al. Multi-compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. Journal of Cachexia, Sarcopenia and Muscle. 2021;12:456–475. doi: 10.1002/jcsm.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jabes D.L., de Maria Y., Aciole Barbosa D., Santos K., Carvalho L.M., Humberto A.C., et al. Fungal dysbiosis correlates with the development of tumor-induced cachexia in mice. Journal of Fungi. 2020;6:364. doi: 10.3390/jof6040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Most J., Tosti V., Redman L.M., Fontana L. Calorie restriction in humans: an update. Ageing Research Reviews. 2017;39:36–45. doi: 10.1016/j.arr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang J., Vanegas S.M., Du X., Noble T., Zingg J.M., Meydani M., et al. Caloric restriction favorably impacts metabolic and immune/inflammatory profiles in obese mice but curcumin/piperine consumption adds no further benefit. Nutrition and Metabolism. 2013;10:29. doi: 10.1186/1743-7075-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.López-Lluch G., Navas P. Calorie restriction as an intervention in ageing. The Journal of Physiology. 2016;594:2043–2060. doi: 10.1113/JP270543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang S., Huang M., You X., Zhao J., Chen L., Wang L., et al. Gut microbiota mediates the anti-obesity effect of calorie restriction in mice. Scientific Reports. 2018;8:13037. doi: 10.1038/s41598-018-31353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fraumene C., Manghina V., Cadoni E., Marongiu F., Abbondio M., Serra M., et al. Caloric restriction promotes rapid expansion and long-lasting increase of Lactobacillus in the rat fecal microbiota. Gut Microbes. 2018;9:104–114. doi: 10.1080/19490976.2017.1371894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pan F., Zhang L., Li M., Hu Y., Zeng B., Yuan H., et al. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome. 2018;6:54. doi: 10.1186/s40168-018-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang C., Li S., Yang L., Huang P., Li W., Wang S., et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nature Communications. 2013;4 doi: 10.1038/ncomms3163. 2163-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang Z., Chen X., Loh Y.J., Yang X., Zhang C. The effect of calorie intake, fasting, and dietary composition on metabolic health and gut microbiota in mice. BMC Biology. 2021;19:51. doi: 10.1186/s12915-021-00987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zeng T., Cui H., Tang D., Garside G.B., Wang Y., Wu J., et al. Short-term dietary restriction in old mice rejuvenates the aging-induced structural imbalance of gut microbiota. Biogerontology. 2019;20:837–848. doi: 10.1007/s10522-019-09830-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fabbiano S., Suárez-Zamorano N., Chevalier C., Lazarević V., Kieser S., Rigo D., et al. Functional gut microbiota remodeling contributes to the caloric restriction-induced metabolic improvements. Cell Metabolism. 2018;28:907–921.e7. doi: 10.1016/j.cmet.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rondanelli M., Gasparri C., Peroni G., Faliva M.A., Naso M., Perna S., et al. The potential roles of very low calorie, very low calorie ketogenic diets and very low carbohydrate diets on the gut microbiota composition. Frontiers in Endocrinology. 2021;12:662591. doi: 10.3389/fendo.2021.662591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tanca A., Abbondio M., Palomba A., Fraumene C., Marongiu F., Serra M., et al. Caloric restriction promotes functional changes involving short-chain fatty acid biosynthesis in the rat gut microbiota. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-33100-y. 14778-14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zou H., Wang D., Ren H., Cai K., Chen P., Fang C., et al. Effect of caloric restriction on BMI, gut microbiota, and blood amino acid levels in non-obese adults. Nutrients. 2020;12:631. doi: 10.3390/nu12030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Simões C.D., Maukonen J., Scott K.P., Virtanen K.A., Pietiläinen K.H., Saarela M. Impact of a very low-energy diet on the fecal microbiota of obese individuals. European Journal of Nutrition. 2014;53:1421–1429. doi: 10.1007/s00394-013-0645-0. [DOI] [PubMed] [Google Scholar]
- 263.Ruiz A., Cerdó T., Jáuregui R., Pieper D.H., Marcos A., Clemente A., et al. One-year calorie restriction impacts gut microbial composition but not its metabolic performance in obese adolescents. Environmental Microbiology. 2017;19:1536–1551. doi: 10.1111/1462-2920.13713. [DOI] [PubMed] [Google Scholar]
- 264.Ott B., Skurk T., Hastreiter L., Lagkouvardos I., Fischer S., Büttner J., et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Scientific Reports. 2017;7:11955. doi: 10.1038/s41598-017-12109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.von Schwartzenberg R.J., Bisanz J.E., Lyalina S., Spanogiannopoulos P., Ang Q.Y., Cai J., et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature. 2021;595:272–277. doi: 10.1038/s41586-021-03663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Anastasiou C.A., Karfopoulou E., Yannakoulia M. Weight regaining: from statistics and behaviors to physiology and metabolism. Metabolism. 2015;64:1395–1407. doi: 10.1016/j.metabol.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 267.Thaiss C.A., Shapiro H., Elinav E. Post-dieting weight gain: the role of persistent microbiome changes. Future Microbiology. 2017;12:555–559. doi: 10.2217/fmb-2017-0045. [DOI] [PubMed] [Google Scholar]
- 268.Omar J.M., Chan Y.-M., Jones M.L., Prakash S., Jones P. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. Journal of Functional Foods. 2013;5:116–123. [Google Scholar]
- 269.Rajkumar H., Mahmood N., Kumar M., Varikuti S.R., Challa H.R., Myakala S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators of Inflammation. 2014;2014:348959. doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Osterberg K.L., Boutagy N.E., McMillan R.P., Stevens J.R., Frisard M.I., Kavanaugh J.W., et al. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity. 2015;23:2364–2370. doi: 10.1002/oby.21230. [DOI] [PubMed] [Google Scholar]
- 271.Brahe L.K., Le Chatelier E., Prifti E., Pons N., Kennedy S., Blædel T., et al. Dietary modulation of the gut microbiota-a randomised controlled trial in obese postmenopausal women. British Journal of Nutrition. 2015;114:406–417. doi: 10.1017/S0007114515001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mobini R., Tremaroli V., Ståhlman M., Karlsson F., Levin M., Ljungberg M., et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes, Obesity and Metabolism. 2017;19:579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 273.Krumbeck J.A., Rasmussen H.E., Hutkins R.W., Clarke J., Shawron K., Keshavarzian A., et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6:121. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Stenman L.K., Lehtinen M.J., Meland N., Christensen J.E., Yeung N., Saarinen M.T., et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults—randomized controlled trial. EBioMedicine. 2016;13:190–200. doi: 10.1016/j.ebiom.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hibberd A.A., Yde C.C., Ziegler M.L., Honoré A.H., Saarinen M.T., Lahtinen S., et al. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Beneficial Microbes. 2019;10:121–135. doi: 10.3920/BM2018.0028. [DOI] [PubMed] [Google Scholar]
- 276.Pedret A., Valls R.M., Calderón-Pérez L., Llauradó E., Companys J., Pla-Pagà L., et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. International Journal of Obesity. 2019;43:1863–1868. doi: 10.1038/s41366-018-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sanchis-Chordà J., Del Pulgar E.M.G., Carrasco-Luna J., Benítez-Páez A., Sanz Y., Codoñer-Franch P. Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. European Journal of Nutrition. 2019;58:2789–2800. doi: 10.1007/s00394-018-1828-5. [DOI] [PubMed] [Google Scholar]
- 278.Kassaian N., Feizi A., Rostami S., Aminorroaya A., Yaran M., Amini M. The effects of 6 mo of supplementation with probiotics and synbiotics on gut microbiota in the adults with prediabetes: a double blind randomized clinical trial. Nutrition. 2020;79–80:110854. doi: 10.1016/j.nut.2020.110854. [DOI] [PubMed] [Google Scholar]
- 279.Schellekens H., Torres-Fuentes C., van de Wouw M., Long-Smith C.M., Mitchell A., Strain C., et al. Bifidobacterium longum counters the effects of obesity: partial successful translation from rodent to human. EBioMedicine. 2021;63:103176. doi: 10.1016/j.ebiom.2020.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rahayu E.S., Mariyatun M., Putri Manurung N.E., Hasan P.N., Therdtatha P., Mishima R., et al. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World Journal of Gastroenterology. 2021;27:107–128. doi: 10.3748/wjg.v27.i1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Dewulf E.M., Cani P.D., Claus S.P., Fuentes S., Puylaert P.G., Neyrinck A.M., et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62:1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Vulevic J., Juric A., Tzortzis G., Gibson G.R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight Adults. Journal of Nutrition. 2013;143:324–331. doi: 10.3945/jn.112.166132. [DOI] [PubMed] [Google Scholar]
- 283.Rebello C.J., Burton J., Heiman M., Greenway F.L. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: a randomized controlled pilot trial. Journal of Diabetes and Its Complications. 2015;29:1272–1276. doi: 10.1016/j.jdiacomp.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Roshanravan N., Mahdavi R., Alizadeh E., Ghavami A., Rahbar Saadat Y., Mesri Alamdari N., et al. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. Journal of Cardiovascular and Thoracic Research. 2017;9:183–190. doi: 10.15171/jcvtr.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nguyen N.K., Deehan E.C., Zhang Z., Jin M., Baskota N., Perez-Muñoz M.E., et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome. 2020;8:118. doi: 10.1186/s40168-020-00887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Peña F., Luisa Mizgier M., Morales P., Rios I., Carrasco-Pozo C., Diaz E., et al. Effect of the synbiotic (B. Animalis spp. lactis Bb12 + oligofructose) in obese subjects. A randomized, double-blind, controlled clinical trial. Journal of Food and Nutrition Research. 2014;2:491–498. [Google Scholar]
- 287.Angelino D., Martina A., Rosi A., Veronesi L., Antonini M., Mennella I., et al. Glucose- and lipid-related biomarkers are affected in healthy obese or hyperglycemic adults consuming a whole-grain pasta enriched in prebiotics and probiotics: a 12-week randomized controlled trial. Journal of Nutrition. 2019;149:1714–1723. doi: 10.1093/jn/nxz071. [DOI] [PubMed] [Google Scholar]
- 288.Horvath A., Leber B., Feldbacher N., Tripolt N., Rainer F., Blesl A., et al. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: a randomized, double-blind, placebo-controlled pilot study. European Journal of Nutrition. 2020;59:2969–2983. doi: 10.1007/s00394-019-02135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sergeev I.N., Aljutaily T., Walton G., Huarte E. Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients. 2020;12:222. doi: 10.3390/nu12010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Janczy A., Aleksandrowicz-Wrona E., Kochan Z., Małgorzewicz S. Impact of diet and synbiotics on selected gut bacteria and intestinal permeability in individuals with excess body weight - a Prospective, Randomized Study. Acta Biochimica Polonica. 2020;67:571–578. doi: 10.18388/abp.2020_5443. [DOI] [PubMed] [Google Scholar]
- 291.Kanazawa A., Aida M., Yoshida Y., Kaga H., Katahira T., Suzuki L., et al. Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: a randomized controlled study. Nutrients. 2021;13:558. doi: 10.3390/nu13020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bindels L.B., Beck R., Schakman O., Martin J.C., De Backer F., Sohet F.M., et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Varian B.J., Gourishetti S., Poutahidis T., Lakritz J.R., Levkovich T., Kwok C., et al. Beneficial bacteria inhibit cachexia. Oncotarget. 2016;7:11803–11816. doi: 10.18632/oncotarget.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bindels L.B., Neyrinck A.M., Salazar N., Taminiau B., Druart C., Muccioli G.G., et al. Non digestible oligosaccharides modulate the gut microbiota to control the development of leukemia and associated cachexia in mice. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Obermüller B., Singer G., Kienesberger B., Klymiuk I., Sperl D., Stadlbauer V., et al. The effects of prebiotic supplementation with OMNi-LOGiC® FIBRE on fecal microbiome, fecal volatile organic compounds, and gut permeability in murine neuroblastoma-induced tumor-associated cachexia. Nutrients. 2020;12:2029. doi: 10.3390/nu12072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Buigues C., Fernández-Garrido J., Pruimboom L., Hoogland A.J., Navarro-Martínez R., Martínez-Martínez M., et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. International Journal of Molecular Sciences. 2016;17:932. doi: 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.An J.M., Kang E.A., Han Y.-M., Oh J.Y., Lee D.Y., Choi S.H., et al. Dietary intake of probiotic kimchi ameliorated IL-6-driven cancer cachexia. Journal of Clinical Biochemistry & Nutrition. 2019;65:109–117. doi: 10.3164/jcbn.19-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Varady K.A., Bhutani S., Church E.C., Klempel M.C. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. The American Journal of Clinical Nutrition. 2009;90:1138–1143. doi: 10.3945/ajcn.2009.28380. [DOI] [PubMed] [Google Scholar]
- 299.Stockman M.-C., Thomas D., Burke J., Apovian C.M. Intermittent fasting: is the wait worth the weight? Current Obesity Reports. 2018;7:172–185. doi: 10.1007/s13679-018-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lowe D.A., Wu N., Rohdin-Bibby L., Moore A.H., Kelly N., Liu Y.E., et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. Journal of American Medical Association Internal Medicine. 2020;180:1491–1499. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Templeman I., Gonzalez J.T., Thompson D., Betts J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proceedings of the Nutrition Society. 2020;79:76–87. doi: 10.1017/S0029665119000636. [DOI] [PubMed] [Google Scholar]
- 302.Antoni R., Johnston K.L., Collins A.L., Robertson M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proceedings of the Nutrition Society. 2017;76:361–368. doi: 10.1017/S0029665116002986. [DOI] [PubMed] [Google Scholar]
- 303.Frank J., Gupta A., Osadchiy V., Mayer E.A. Brain–gut–microbiome interactions and intermittent fasting in obesity. Nutrients. 2021;13:584. doi: 10.3390/nu13020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mohr A.E., Gumpricht E., Sears D.D., Sweazea K.L. Recent advances and health implications of dietary fasting regimens on the gut microbiome. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2021;320:G847–G863. doi: 10.1152/ajpgi.00475.2020. [DOI] [PubMed] [Google Scholar]
- 305.Llewellyn-Waters K., Abdullah M.M. Intermittent fasting - a potential approach to modulate the gut microbiota in humans? A systematic review. Nutrition and Healthy Aging. 2021;6:87–94. [Google Scholar]
- 306.Li L., Su Y., Li F., Wang Y., Ma Z., Li Z., et al. The effects of daily fasting hours on shaping gut microbiota in mice. BMC Microbiology. 2020;20:65. doi: 10.1186/s12866-020-01754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cignarella F., Cantoni C., Ghezzi L., Salter A., Dorsett Y., Chen L., et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metabolism. 2018;27:1222–1235.e6. doi: 10.1016/j.cmet.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mallick H., Franzosa E.A., McLver L.J., Banerjee S., Sirota-Madi A., Kostic A.D., et al. Predictive metabolomic profiling of microbial communities using amplicon or metagenomic sequences. Nature Communications. 2019;10 doi: 10.1038/s41467-019-10927-1. 3136-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Beli E., Yan Y., Moldovan L., Vieira C.P., Gao R., Duan Y., et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. 2018;67:1867–1879. doi: 10.2337/db18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liu Z., Dai X., Zhang H., Shi R., Hui Y., Jin X., et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nature Communications. 2020;11:855. doi: 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L., et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metabolism. 2017;26:672–685.e4. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zheng X., Zhou K., Zhang Y., Han X., Zhao A., Liu J., et al. Food withdrawal alters the gut microbiota and metabolome in mice. Federation of American Societies for Experimental Biology Journal. 2018;32:4878–4888. doi: 10.1096/fj.201700614R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ozkul C., Yalinay M., Karakan T. Structural changes in gut microbiome after Ramadan fasting: a pilot study. Beneficial Microbes. 2020;11:227–233. doi: 10.3920/BM2019.0039. [DOI] [PubMed] [Google Scholar]
- 314.Özkul C., Yalınay M., Karakan T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: a preliminary study on intermittent fasting. Turkish Journal of Gastroenterology. 2019;30:1030–1035. doi: 10.5152/tjg.2019.19185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Remely M., Hippe B., Geretschlaeger I., Stegmayer S., Hoefinger I., Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wiener Klinische Wochenschrift. 2015;127:394–398. doi: 10.1007/s00508-015-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gabel K., Marcell J., Cares K., Kalam F., Cienfuegos S., Ezpeleta M., et al. Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutrition and Health. 2020;26:79–85. doi: 10.1177/0260106020910907. [DOI] [PubMed] [Google Scholar]
- 317.Masood W., Annamaraju P., Uppaluri K.R. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; Treasure Island (FL): 2021. Ketogenic diet. [Google Scholar]
- 318.Koh S., Dupuis N., Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Research. 2020;167:106454. doi: 10.1016/j.eplepsyres.2020.106454. [DOI] [PubMed] [Google Scholar]
- 319.Yuan X., Wang J., Yang S., Gao M., Cao L., Li X., et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutrition & Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kumar S., Behl T., Sachdeva M., Sehgal A., Kumari S., Kumar A., et al. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sciences. 2021;264:118661. doi: 10.1016/j.lfs.2020.118661. [DOI] [PubMed] [Google Scholar]
- 321.Paoli A. Ketogenic diet for obesity: friend or foe? International Journal of Environmental Research and Public Health. 2014;11:2092–2107. doi: 10.3390/ijerph110202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ma D., Wang A.C., Parikh I., Green S.J., Hoffman J.D., Chlipala G., et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-25190-5. 6670-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;174:497. doi: 10.1016/j.cell.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ang Q.Y., Alexander M., Newman J.C., Tian Y., Cai J., Upadhyay V., et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181:1263–1275.e16. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lindefeldt M., Eng A., Darban H., Bjerkner A., Zetterström C.K., Allander T., et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes. 2019;5:1–13. doi: 10.1038/s41522-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Paoli A., Mancin L., Bianco A., Thomas E., Mota J.F., Piccini F. Ketogenic diet and microbiota: friends or enemies? Genes. 2019;10:534. doi: 10.3390/genes10070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Muscogiuri G., Barrea L., Laudisio D., Pugliese G., Salzano C., Savastano S., et al. The management of very low-calorie ketogenic diet in obesity outpatient clinic: a practical guide. Journal of Translational Medicine. 2019;17:356. doi: 10.1186/s12967-019-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wolters M., Ahrens J., Romaní-Pérez M., Watkins C., Sanz Y., Benítez-Páez A., et al. Dietary fat, the gut microbiota, and metabolic health - a systematic review conducted within the MyNewGut project. Clinical Nutrition. 2019;38:2504–2520. doi: 10.1016/j.clnu.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 329.Tuck C.J., Staudacher H.M. The keto diet and the gut: cause for concern? The Lancet Gastroenterology & Hepatology. 2019;4:908–909. doi: 10.1016/S2468-1253(19)30353-X. [DOI] [PubMed] [Google Scholar]
- 330.Reddel S., Putignani L., Del Chierico F. The impact of low-FODMAPs, gluten-free, and ketogenic diets on gut microbiota modulation in pathological conditions. Nutrients. 2019;11:373. doi: 10.3390/nu11020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Olson C.A., Iñiguez A.J., Yang G.E., Fang P., Pronovost G.N., Jameson K.G., et al. Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host & Microbe. 2021;29:1378–1392.e6. doi: 10.1016/j.chom.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cortez N., Hong B., Villareal E., Mackenzie G. A ketogenic diet mitigates pancreatic cancer-associated cachexia in mice. Current Developments in Nutrition. 2020;4 315-315. [Google Scholar]
- 333.Shukla S.K., Gebregiworgis T., Purohit V., Chaika N.V., Gunda V., Radhakrishnan P., et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer & Metabolism. 2014;2:18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Fearon K.C., Borland W., Preston T., Tisdale M.J., Shenkin A., Calman K.C. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. The American Journal of Clinical Nutrition. 1988;47:42–48. doi: 10.1093/ajcn/47.1.42. [DOI] [PubMed] [Google Scholar]
- 335.Mason E.E., Ito C. Gastric bypass in obesity. Surgical Clinics of North America. 1967;47:1345–1351. doi: 10.1016/s0039-6109(16)38384-0. [DOI] [PubMed] [Google Scholar]
- 336.Lupoli R., Lembo E., Saldalamacchia G., Avola C.K., Angrisani L., Capaldo B. Bariatric surgery and long-term nutritional issues. World Journal of Diabetes. 2017;8:464–474. doi: 10.4239/wjd.v8.i11.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Valentí V., Cienfuegos J.A., Becerril Mañas S., Frühbeck G. Mechanism of bariatric and metabolic surgery: beyond surgeons, gastroenterologists and endocrinologists. Revista Española de Enfermedades Digestivas. 2020;112:229–233. doi: 10.17235/reed.2020.6925/2020. [DOI] [PubMed] [Google Scholar]
- 338.Ulker İ., Yildiran H. The effects of bariatric surgery on gut microbiota in patients with obesity: a review of the literature. Bioscience of Microbiota, Food and Health. 2019;38:3–9. doi: 10.12938/bmfh.18-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lau E., Belda E., Picq P., Carvalho D., Ferreira-Magalhães M., Silva M.M., et al. Gut microbiota changes after metabolic surgery in adult diabetic patients with mild obesity: a randomised controlled trial. Diabetology & Metabolic Syndrome. 2021;13:56. doi: 10.1186/s13098-021-00672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Gutiérrez-Repiso C., Moreno-Indias I., Tinahones F.J. Shifts in gut microbiota and their metabolites induced by bariatric surgery. Impact of factors shaping gut microbiota on bariatric surgery outcomes. Reviews in Endocrine & Metabolic Disorders. 2021 doi: 10.1007/s11154-021-09676-8. [DOI] [PubMed] [Google Scholar]
- 341.Yan M., Song M.-M., Bai R.-X., Cheng S., Yan W.-M. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World Journal of Gastrointestinal Surgery. 2016;8:301–307. doi: 10.4240/wjgs.v8.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Dao M.C., Belda E., Prifti E., Everard A., Kayser B.D., Bouillot J.L., et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. American Journal of Physiology-Endocrinology and Metabolism. 2019;317:e446–e459. doi: 10.1152/ajpendo.00140.2019. [DOI] [PubMed] [Google Scholar]
- 343.Ben Izhak M., Eshel A., Cohen R., Madar-Shapiro L., Meiri H., Wachtel C., et al. Projection of gut microbiome pre- and post-bariatric surgery to predict surgery outcome. mSystems. 2021;6 doi: 10.1128/mSystems.01367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Juárez-Fernández M., Román-Sagüillo S., Porras D., García-Mediavilla M.V., Linares P., Ballesteros-Pomar M.D., et al. Long-term effects of bariatric surgery on gut microbiota composition and faecal metabolome related to obesity remission. Nutrients. 2021;13:2519. doi: 10.3390/nu13082519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ma I.T., Madura J.A., 2nd Gastrointestinal complications after bariatric surgery. Gastroenterology and Hepatology. 2015;11:526–535. [PMC free article] [PubMed] [Google Scholar]
- 346.Pories W.J. Bariatric surgery: risks and rewards. Journal of Clinical Endocrinology & Metabolism. 2008;93:S89–S96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Brown R.M., Guerrero-Hreins E., Brown W.A., le Roux C.W., Sumithran P. Potential gut–brain mechanisms behind adverse mental health outcomes of bariatric surgery. Nature Reviews Endocrinology. 2021;17:549–559. doi: 10.1038/s41574-021-00520-2. [DOI] [PubMed] [Google Scholar]
- 348.Silva Pontes K.S.d., Guedes M.R., Rabello da Cunha M., de Souza Mattos S., Barreto Silva M.I., Neves M.F., et al. Effects of probiotics on body adiposity and cardiovascular risk markers in individuals with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition. 2021;40:4915–4931. doi: 10.1016/j.clnu.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 349.Mazloom K., Siddiqi I., Covasa M. Probiotics: how effective are they in the fight against obesity? Nutrients. 2019;11:258. doi: 10.3390/nu11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Alipour H., Gazerani P., Heidari M., Dardmeh F. Modulatory effect of probiotic Lactobacillus rhamnosus PB01 on mechanical sensitivity in a female diet-induced obesity model. Pain Research and Management. 2021;2021:5563959. doi: 10.1155/2021/5563959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Chen Y.-T., Hsu A.-H., Chiou S.-Y., Lin Y.-C., Lin J.-S. AB-kefir reduced body weight and ameliorated inflammation in adipose tissue of obese mice fed a high-fat diet, but not a high-sucrose diet. Nutrients. 2021;13:2182. doi: 10.3390/nu13072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Gulnaz A., Nadeem J., Han J.H., Lew L.C., Son J.D., Park Y.H., et al. Lactobacillus sps in reducing the risk of diabetes in high-fat diet-induced diabetic mice by modulating the gut microbiome and inhibiting key digestive enzymes associated with diabetes. Biology. 2021;10:348. doi: 10.3390/biology10040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Joung H., Chu J., Kim B.-K., Choi I.-S., Kim W., Park T.-S. Probiotics ameliorate chronic low-grade inflammation and fat accumulation with gut microbiota composition change in diet-induced obese mice models. Applied Microbiology and Biotechnology. 2021;105:1203–1213. doi: 10.1007/s00253-020-11060-6. [DOI] [PubMed] [Google Scholar]
- 354.Le Roy T., Moens de Hase E., Van Hul M., Paquot A., Pelicaen R., Régnier M., et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut. 2021 doi: 10.1136/gutjnl-2020-323778. gutjnl-2020-323778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Molina-Tijeras J.A., Diez-Echave P., Vezza T., Hidalgo-García L., Ruiz-Malagón A.J., Rodríguez-Sojo M.J., et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacological Research. 2021;167:105471. doi: 10.1016/j.phrs.2021.105471. [DOI] [PubMed] [Google Scholar]
- 356.Yoshida N., Yamashita T., Osone T., Hosooka T., Shinohara M., Kitahama S., et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience. 2021;24:103342. doi: 10.1016/j.isci.2021.103342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Abenavoli L., Scarpellini E., Colica C., Boccuto L., Salehi B., Sharifi-Rad J., et al. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019;11:2690. doi: 10.3390/nu11112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sanders M.E. Probiotics and microbiota composition. BMC Medicine. 2016;14:82. doi: 10.1186/s12916-016-0629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bindels L.B., Thissen J.-P. Nutrition in cancer patients with cachexia: a role for the gut microbiota? Clinical Nutrition Experimental. 2016;6:74–82. [Google Scholar]
- 360.Herremans K.M., Riner A.N., Cameron M.E., Trevino J.G. The microbiota and cancer cachexia. International Journal of Molecular Sciences. 2019;20:6267. doi: 10.3390/ijms20246267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.van Krimpen S.J., Jansen F.A.C., Ottenheim V.L., Belzer C., van der Ende M., van Norren K. The effects of pro-, pre-, and synbiotics on muscle wasting, a systematic review—gut permeability as potential treatment target. Nutrients. 2021;13:1115. doi: 10.3390/nu13041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Redman M.G., Ward E.J., Phillips R.S. The efficacy and safety of probiotics in people with cancer: a systematic review. Annals of Oncology. 2014;25:1919–1929. doi: 10.1093/annonc/mdu106. [DOI] [PubMed] [Google Scholar]
- 363.Green M., Arora K., Prakash S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. International Journal of Molecular Sciences. 2020;21:2890. doi: 10.3390/ijms21082890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hiel S., Neyrinck A.M., Rodriguez J., Pachikian B.D., Bouzin C., Thissen J.-P., et al. Inulin improves postprandial hypertriglyceridemia by modulating gene expression in the small intestine. Nutrients. 2018;10:532. doi: 10.3390/nu10050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Rivera-Piza A., Lee S.-J. Effects of dietary fibers and prebiotics in adiposity regulation via modulation of gut microbiota. Applied Biological Chemistry. 2020;63:2. [Google Scholar]
- 366.Ghosh S., Yang X., Wang L., Zhang C., Zhao L. Active phase prebiotic feeding alters gut microbiota, induces weight-independent alleviation of hepatic steatosis and serum cholesterol in high-fat diet-fed mice. Computational and Structural Biotechnology Journal. 2020;19:448–458. doi: 10.1016/j.csbj.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Lee S., Goodson M., Vang W., Kalanetra K., Barile D., Raybould H. 2′-Fucosyllactose supplementation improves gut-brain signaling and diet-induced obese phenotype and changes the gut microbiota in high fat-fed mice. Nutrients. 2020;12:1003. doi: 10.3390/nu12041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Mocanu V., Zhang Z., Deehan E.C., Kao D.H., Hotte N., Karmali S., et al. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nature Medicine. 2021;27:1272–1279. doi: 10.1038/s41591-021-01399-2. [DOI] [PubMed] [Google Scholar]
- 369.Muthuramalingam K., Singh V., Choi C., Choi S.I., Kim Y.M., Unno T., et al. Dietary intervention using (1,3)/(1,6)-β-glucan, a fungus-derived soluble prebiotic ameliorates high-fat diet-induced metabolic distress and alters beneficially the gut microbiota in mice model. European Journal of Nutrition. 2020;59:2617–2629. doi: 10.1007/s00394-019-02110-5. [DOI] [PubMed] [Google Scholar]
- 370.Muthuramalingam K., Singh V., Choi C., Choi S.I., Kim Y.M., Unno T., et al. Correction to: dietary intervention using (1,3)/(1,6)-β-glucan, a fungus-derived soluble prebiotic ameliorates high-fat diet-induced metabolic distress and alters beneficially the gut microbiota in mice model. European Journal of Nutrition. 2021;60 doi: 10.1007/s00394-021-02537-9. 2273-2273. [DOI] [PubMed] [Google Scholar]
- 371.Rodriguez J., Hiel S., Neyrinck A.M., Le Roy T., Pötgens S.A., Leyrolle Q., et al. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut. 2020;69:1975–1987. doi: 10.1136/gutjnl-2019-319726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Aoun A., Darwish F., Hamod N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Preventive Nutrition and Food Science. 2020;25:113–123. doi: 10.3746/pnf.2020.25.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hodge R.J., Paulik M.A., Walker A., Boucheron J.A., McMullen S.L., Gillmor D.S., et al. Weight and glucose reduction observed with a combination of nutritional agents in rodent models does not translate to humans in a randomized clinical trial with healthy volunteers and subjects with type 2 diabetes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ke X., Walker A., Haange S.-B., Lagkouvardos I., Liu Y., Schmitt-Kopplin P., et al. Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Molecular Metabolism. 2019;22:96–109. doi: 10.1016/j.molmet.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Cao S., Ryan P.M., Salehisahlabadi A., Abdulazeem H.M., Karam G., Černevičiūtė R., et al. Effect of probiotic and synbiotic formulations on anthropometrics and adiponectin in overweight and obese participants: a systematic review and meta-analysis of randomized controlled trials. Journal of King Saud University Science. 2020;32:1738–1748. [Google Scholar]
- 376.Seifi N., Safarian M., Nematy M., Rezvani R., Khadem-Rezaian M., Sedaghat A. Effects of synbiotic supplementation on energy and macronutrients homeostasis and muscle wasting of critical care patients: study protocol and a review of previous studies. Trials. 2020;21:221. doi: 10.1186/s13063-020-4136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Williams B.A., Mikkelsen D., Flanagan B.M., Gidley M.J. Dietary fibre": moving beyond the "soluble/insoluble" classification for monogastric nutrition, with an emphasis on humans and pigs. Journal of Animal Science and Biotechnology. 2019;10 doi: 10.1186/s40104-019-0350-9. 45-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kim B., Choi H.-N., Yim J.-E. Effect of diet on the gut microbiota associated with obesity. Journal of Obesity & Metabolic Syndrome. 2019;28:216–224. doi: 10.7570/jomes.2019.28.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Akbar A., Shreenath A.P. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC; Treasure Island (FL): 2021. High fiber diet. [Google Scholar]
- 380.Barber T.M., Kabisch S., Pfeiffer A.F.H., Weickert M.O. The health benefits of dietary fibre. Nutrients. 2020;12:3209. doi: 10.3390/nu12103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Berding K., Carbia C., Cryan J.F. Going with the grain: fiber, cognition, and the microbiota-gut-brain-axis. Experimental Biology and Medicine. 2021;246:796–811. doi: 10.1177/1535370221995785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Myhrstad M.C.W., Tunsjø H., Charnock C., Telle-Hansen V.H. Dietary fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients. 2020;12:859. doi: 10.3390/nu12030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Kumar J., Rani K., Datt C. Molecular link between dietary fibre, gut microbiota and health. Molecular Biology Reports. 2020;47:6229–6237. doi: 10.1007/s11033-020-05611-3. [DOI] [PubMed] [Google Scholar]
- 384.Ojo O., Feng Q.-Q., Ojo O.O., Wang X.-H. The role of dietary fibre in modulating gut microbiota dysbiosis in patients with type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2020;12:3239. doi: 10.3390/nu12113239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Rezac S., Kok C.R., Heermann M., Hutkins R.J.F.i.m. Fermented foods as a dietary source of live organisms. Frontiers in Microbiology. 2018;9:1785. doi: 10.3389/fmicb.2018.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Dimidi E., Cox S.R., Rossi M., Whelan K. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11:1806. doi: 10.3390/nu11081806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Taylor B.C., Lejzerowicz F., Poirel M., Shaffer J.P., Jiang L., Aksenov A., et al. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems. 2020;5:e00901–e00919. doi: 10.1128/mSystems.00901-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Wastyk H.C., Fragiadakis G.K., Perelman D., Dahan D., Merrill B.D., Yu F.B., et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–4153.e14. doi: 10.1016/j.cell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligné B., et al. Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 390.Aragón F., Perdigón G., de Moreno de LeBlanc A. Modification in the diet can induce beneficial effects against breast cancer. World Journal of Clinical Oncology. 2014;5:455–464. doi: 10.5306/wjco.v5.i3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zhang K., Dai H., Liang W., Zhang L., Deng Z. Fermented dairy foods intake and risk of cancer. International Journal of Cancer. 2019;144:2099–2108. doi: 10.1002/ijc.31959. [DOI] [PubMed] [Google Scholar]
- 392.Shahbazi R., Sharifzad F., Bagheri R., Alsadi N., Yasavoli-Sharahi H., Matar C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients. 2021;13:1516. doi: 10.3390/nu13051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.SaeidiFard N., Djafarian K., Shab-Bidar S. Fermented foods and inflammation: a systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition ESPEN. 2020;35:30–39. doi: 10.1016/j.clnesp.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 394.van de Wouw M., Walsh A.M., Crispie F., van Leuven L., Lyte J.M., Boehme M., et al. Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome. 2020;8:67. doi: 10.1186/s40168-020-00846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.van de Wouw M., Walsh C.J., Vigano G.M.D., Lyte J.M., Boehme M., Gual-Grau A., et al. Kefir ameliorates specific microbiota-gut-brain axis impairments in a mouse model relevant to autism spectrum disorder. Brain, Behavior, and Immunity. 2021;97:119–134. doi: 10.1016/j.bbi.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 396.Sun Y., Wang Y., Song P., Wang H., Xu N., Wang Y., et al. Anti-obesity effects of instant fermented teas in vitro and in mice with high-fat-diet-induced obesity. Food & Function. 2019;10:3502–3513. doi: 10.1039/c9fo00162j. [DOI] [PubMed] [Google Scholar]
- 397.Kang D., Li Z., Ji G.E. Anti-obesity effects of a mixture of fermented ginseng, Bifidobacterium longum BORI, and Lactobacillus paracasei CH88 in high-fat diet-fed mice. Journal of Microbiology and Biotechnology. 2018;28:688–696. doi: 10.4014/jmb.1801.01016. [DOI] [PubMed] [Google Scholar]
- 398.Byun M.S., Yu O.K., Cha Y.S., Park T.S. Korean traditional Chungkookjang improves body composition, lipid profiles and atherogenic indices in overweight/obese subjects: a double-blind, randomized, crossover, placebo-controlled clinical trial. European Journal of Clinical Nutrition. 2016;70:1116–1122. doi: 10.1038/ejcn.2016.77. [DOI] [PubMed] [Google Scholar]
- 399.Kim E.K., An S.Y., Lee M.S., Kim T.H., Lee H.K., Hwang W.S., et al. Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutrition Research. 2011;31:436–443. doi: 10.1016/j.nutres.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 400.Ray M., Hor P.K., Ojha D., Soren J.P., Singh S.N., Mondal K.C. Bifidobacteria and its rice fermented products on diet induced obese mice: analysis of physical status, serum profile and gene expressions. Beneficial Microbes. 2018;9:441–452. doi: 10.3920/BM2017.0056. [DOI] [PubMed] [Google Scholar]
- 401.González S., Fernández-Navarro T., Arboleya S., de Los Reyes-Gavilán C.G., Salazar N., Gueimonde M. Fermented dairy foods: impact on intestinal microbiota and health-linked biomarkers. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.01046. 1046-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Koh A., Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Molecular Cell. 2020;78:584–596. doi: 10.1016/j.molcel.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 403.Diener C., Qin S., Zhou Y., Patwardhan S., Tang L., Lovejoy J.C., et al. Baseline gut metagenomic functional gene signature associated with variable weight loss responses following a healthy lifestyle intervention in humans. mSystems. 2021;6 doi: 10.1128/mSystems.00964-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Zierer J., Jackson M.A., Kastenmüller G., Mangino M., Long T., Telenti A., et al. The fecal metabolome as a functional readout of the gut microbiome. Nature Genetics. 2018;50:790–795. doi: 10.1038/s41588-018-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Ghosh T.S., Rampelli S., Jeffery I.B., Santoro A., Neto M., Capri M., et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Randomized Controlled Trial. 2020;69:1218–1228. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Blackwood B.P., Yuan C.Y., Wood D.R., Nicolas J.D., Grothaus J.S., Hunter C.J. Probiotic Lactobacillus species strengthen intestinal barrier function and tight junction integrity in experimental necrotizing enterocolitis. Journal of Probiotics & Health. 2017;5:159. doi: 10.4172/2329-8901.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Karczewski J., Troost F.J., Konings I., Dekker J., Kleerebezem M., Brummer R.J., et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 408.Wang J., Ji H., Wang S., Liu H., Zhang W., Zhang D., et al. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Frontiers in Microbiology. 2018;9:1953. doi: 10.3389/fmicb.2018.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hosseinifard E.-S., Bavafa-Valenlia K., Saghafi-Asl M., Morshedi M. Antioxidative and metabolic effects of Lactobacillus plantarum, inulin, and their synbiotic on the hypothalamus and serum of healthy rats. Nutrition and Metabolic Insights. 2020;13 doi: 10.1177/1178638820925092. 1178638820925092-1178638820925092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Carvalho B.M., Abdalla Saad M. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators of Inflammation. 2013;2013:986734. doi: 10.1155/2013/986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Forssten S.D., Korczyńska M.Z., Zwijsen R.M., Noordman W.H., Madetoja M., Ouwehand A.C. Changes in satiety hormone concentrations and feed intake in rats in response to lactic acid bacteria. Appetite. 2013;71:16–21. doi: 10.1016/j.appet.2013.06.093. [DOI] [PubMed] [Google Scholar]
- 412.Wiciński M., Gębalski J., Gołębiewski J., Malinowski B. Probiotics for the treatment of overweight and obesity in humans-A review of clinical trials. Microorganisms. 2020;8:1148. doi: 10.3390/microorganisms8081148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.O'Shea E.F., Cotter P.D., Stanton C., Ross R.P., Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. International Journal of Food Microbiology. 2012;152:189–205. doi: 10.1016/j.ijfoodmicro.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 414.Lee H.Y., Park J.H., Seok S.H., Baek M.W., Kim D.J., Lee K.E., et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochimica et Biophysica Acta. 2006;1761:736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 415.Tilg H., Danese S. Roseburia hominis: a novel guilty player in ulcerative colitis pathogenesis? Gut. 2014;63:1204–1205. doi: 10.1136/gutjnl-2013-305799. [DOI] [PubMed] [Google Scholar]
- 416.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Zhang L., Liu C., Jiang Q., Yin Y. Butyrate in energy metabolism: there is still more to learn. Trends in Endocrinology and Metabolism. 2021;32:159–169. doi: 10.1016/j.tem.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 418.Martín R., Miquel S., Benevides L., Bridonneau C., Robert V., Hudault S., et al. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. Prausnitzii as a next-generation probiotic. Frontiers in Microbiology. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Miquel S., Leclerc M., Martin R., Chain F., Lenoir M., Raguideau S., et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio. 2015;6:e00300–e00315. doi: 10.1128/mBio.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Ferreira-Halder C.V., Faria A.V.d.S., Andrade S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Practice & Research Clinical Gastroenterology. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 421.Waters J.L., Ley R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biology. 2019;17:83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Tavella T., Rampelli S., Guidarelli G., Bazzocchi A., Gasperini C., Pujos-Guillot E., et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes. 2021;13:1–19. doi: 10.1080/19490976.2021.1880221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Mazier W., Le Corf K., Martinez C., Tudela H., Kissi D., Kropp C., et al. A new strain of Christensenella minuta as a potential biotherapy for obesity and associated metabolic diseases. Cells. 2021;10:823. doi: 10.3390/cells10040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Rios-Covian D., Salazar N., Gueimonde M., de los Reyes-Gavilan C.G. Shaping the metabolism of intestinal Bacteroides population through diet to improve human health. Frontiers in Microbiology. 2017;8:376. doi: 10.3389/fmicb.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y., et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nature Medicine. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 426.Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 427.Ruiz L., Delgado S., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and their molecular communication with the immune system. Frontiers in Microbiology. 2017;8 doi: 10.3389/fmicb.2017.02345. 2345-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Uusitupa H.-M., Rasinkangas P., Lehtinen M.J., Mäkelä S.M., Airaksinen K., Anglenius H., et al. Bifidobacterium animalis subsp. lactis 420 for metabolic health: review of the research. Nutrients. 2020;12:892. doi: 10.3390/nu12040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Huo Y., Lu X., Wang X., Wang X., Chen L., Guo H., et al. Bifidobacterium animalis subsp. lactis A6 alleviates obesity associated with promoting mitochondrial biogenesis and function of adipose tissue in mice. Molecules. 2020;25:1490. doi: 10.3390/molecules25071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Zhang T., Li Q., Cheng L., Buch H., Zhang F. Akkermansia muciniphila is a promising probiotic. Microbial Biotechnology. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Schneeberger M., Everard A., Gómez-Valadés A.G., Matamoros S., Ramírez S., Delzenne N.M., et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Scientific Reports. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Abuqwider J.N., Mauriello G., Altamimi M. Akkermansia muciniphila, a new generation of beneficial microbiota in modulating obesity: a systematic review. Microorganisms. 2021;9:1098. doi: 10.3390/microorganisms9051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Depommier C., Van Hul M., Everard A., Delzenne N.M., De Vos W.M., Cani P.D.J.G.M. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;11:1231–1245. doi: 10.1080/19490976.2020.1737307. [DOI] [PMC free article] [PubMed] [Google Scholar]