Abstract
Learning objective
After participating in this activity, learners should be better able to:
• Discuss and outline the general and overlapping effects of the menstrual cycle on women’s mental health
Abstract
A growing body of research demonstrates menstrual cycle–dependent fluctuations in psychiatric symptoms; these fluctuations can therefore be considered as prevalent phenomena. Possible mechanisms underlying these fluctuations posit behavioral, psychological, and neuroendocrine influences. Recent reviews document cyclic exacerbation of symptoms and explore these mechanisms in the context of specific and often single disorders. The question remains, however, as to whether there are general and overlapping effects of the menstrual cycle on women’s mental health. To address this gap, we synthesized the literature examining the exacerbation of a variety of psychiatric symptoms across the menstrual cycle in adult women. Results show that the premenstrual and menstrual phases are most consistently implicated in transdiagnostic symptom exacerbation. Specifically, strong evidence indicates increases in psychosis, mania, depression, suicide/suicide attempts, and alcohol use during these phases. Anxiety, stress, and binge eating appear to be elevated more generally throughout the luteal phase. The subjective effects of smoking and cocaine use are reduced during the luteal phase, but fewer data are available for other substances. Less consistent patterns are demonstrated for panic disorder, symptoms of posttraumatic stress disorder, and borderline personality disorder, and it is difficult to draw conclusions for symptoms of generalized anxiety disorder, social anxiety disorder, obsessive-compulsive disorder, and trichotillomania because of the limited data. Future research should focus on developing standardized approaches to identifying menstrual cycle phases and adapting pharmacological and behavioral interventions for managing fluctuations in psychiatric symptoms across the menstrual cycle.
Keywords: estrogen, progesterone, menstrual cycle, mental health, women’s health
The menstrual cycle is characterized by predictable and recurrent fluctuations in hormones—namely, the ovarian hormones estrogen and progesterone. The cycle is separated into two distinct phases: the follicular phase, which consists of the first part of the cycle lasting from menstruation to ovulation and which varies in length but typically lasts 14 days; and the luteal phase, which is the second half of the cycle following ovulation and leading up to menstruation, and consistently lasts 14 days (see Mihm et al.1 for an overview). The days immediately prior to menstruation are often termed the premenstrual phase.
During menstruation, estrogen and progesterone levels are relatively low (see Figure 1).2 As the cycle advances through the follicular phase, estrogen levels spike, causing the pituitary gland to release a surge of follicle-stimulating hormone and luteinizing hormone—which facilitates the maturing of eggs within the ovaries.3 When the most mature egg is released, the follicle transforms into a corpus luteum, which produces gradually increasing amounts of progesterone; a moderate amount of estrogen is also produced.3 If the egg is not fertilized, progesterone and estrogen levels fall, the uterine lining breaks down, and the menstrual cycle resumes with menstruation, which typically lasts between 1 and 7 days.4
Figure 1.
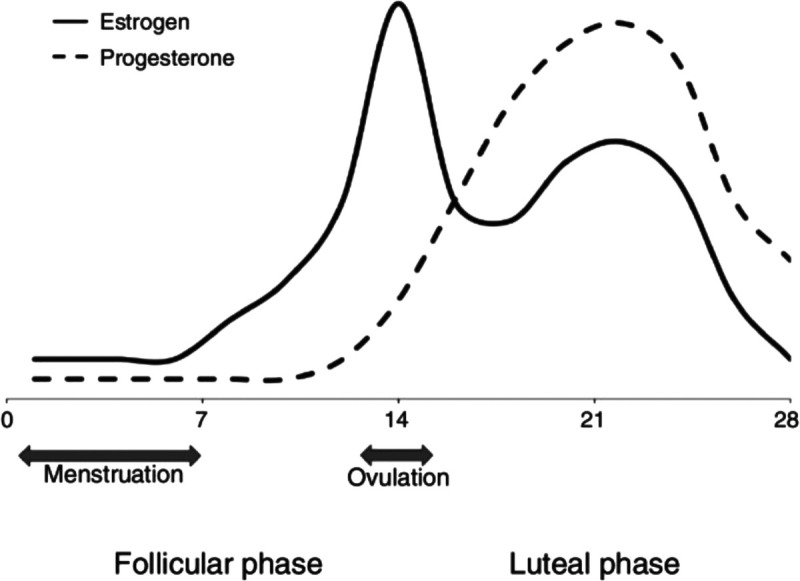
Estrogen and progesterone levels across a typical 28-day menstrual cycle (adapted from Glover et al. (2013).2
This cyclic experience may influence women’s mental health through a variety of mechanisms. For example, many women experience physical discomfort (e.g., dysmenorrhea, breast tenderness, joint pain5) around menstruation. This physical discomfort can be associated with increases in psychological distress and irritability, and decreased self-esteem.6 Many women additionally report increased interpersonal conflicts and reduced social engagement premenstrually and during menstruation7,8—which may contribute to depression and isolation.8 Negative affect is linked with increased impulsivity,9 substance use,10 and nonsuicidal self-injury.11 As such, it is unsurprising that systematic and meta-analytic reviews find exacerbations of psychiatric symptoms across the menstrual cycle (e.g., Carroll et al.12).
In addition to affective and behavioral impacts of the menstrual cycle, there are also several direct biological effects on mental health. For example, estrogen downregulates dopamine transmission, which mimics the antidopaminergic action of many antipsychotic medications.13 Higher estrogen levels are hypothesized to protect against psychiatric symptoms, such as psychosis, thereby increasing vulnerability to psychosis when estrogen is low (e.g., menstruation, postpartum14,15). Estrogen also assists in memory consolidation through increased hippocampal activation,16,17 which, in the context of treatment for posttraumatic stress disorder (PTSD), has been shown to facilitate fear extinction recall.18 Progesterone can have anxiolytic effects19 through increases in allopregnanolone and, subsequently, increased GABA potentiation.20–22 Other progesterone metabolites, however, are not anxiolytic. In the presence of stress, progesterone is converted into cortisol, increasing stress responses and impairing emotional processing.23 To this end, it has been suggested that progesterone may underlie menstrual-related mood symptoms.23
Recently, several elegant reviews documented menstrual exacerbations of numerous psychiatric symptoms,24 including addictive behaviors,25 psychosis,15 suicidality,26 anxiety, and posttraumatic stress disorder.27 These reviews increased the scientific understanding of the effects of the menstrual cycle on women’s mental health, including the more pointed effects of estrogen and progesterone. Yet, no review to date comprehensively evaluated the impact of the menstrual cycle on psychiatric symptoms. To further this growing body of research, we conducted a comprehensive review synthesizing the literature on fluctuations in a broad spectrum of psychiatric disorders and symptoms across the menstrual cycle. Summarizing these data in such a way will allow us to potentially identify patterns and draw conclusions beyond what previous reviews examined.
METHODS
Search Strategy
We conducted a comprehensive search using the PubMed database for articles focusing on psychiatric symptoms across the menstrual cycle. We used combinations of the following search terms to identify potentially relevant articles: menstrual cycle, psychosis, bipolar, mania, depression, suicide, anxiety, obsessive-compulsive disorder (OCD), body dysmorphic disorder, trichotillomania, excoriation, hoarding, impulse control, kleptomania, PTSD, eating disorder, anorexia, bulimia, binge eating, borderline personality disorder (BPD), intermittent explosive disorder, conduct disorder, pyromania, substance use, alcohol, and smoking. We compiled the results, removed duplicates, and reviewed the titles and abstracts of the remaining articles. We then read and assessed for eligibility the full texts of the remaining articles using the following criteria: studies (1) were published in English, (2) presented original findings, (3) included premenopausal women at least 18 years old, and (4) assessed relevant psychiatric symptoms during at least two menstrual cycle phases. We did not include articles focusing on symptoms in women with premenstrual syndrome (PMS) or premenstrual dysphoric disorder, as those conditions inherently vary across the menstrual cycle. See inclusion diagram (Supplemental Figure 1, http://links.lww.com/HRP/A187), and Supplemental Tables 1–10, http://links.lww.com/HRP/A188, for full descriptions of the articles included.
Definition of Menstrual Cycle Phases
Most menstrual cycle research uses a “count” method to estimate cycle phases based on the first day of menstruation (Day 1). Estimates are often based on a 28-day cycle, although a substantial body of evidence suggests significant variation in cycle length in healthy women.196 Indeed, the follicular phase has been found to vary by as much as 12 days.197 The luteal phase, by contrast, lasts a relatively consistent amount of time—approximately 14 days. Some exceptions to this pattern include women with luteal phase disorders (e.g., short luteal phase).198,199
Many articles included in this review have varying terminology and definitions of menstrual cycle phases. For example, occasionally the “premenstrual” phase is referred to as the mid- or late-luteal phase. “Perimenstrual” can include both premenstrual and menstrual phases, whereas “early follicular” can include menstruation and the initial days following menstruation. We report the findings using the language described in the articles reviewed; however, our synthesis of the data focuses on overall patterns during specific phases, regardless of terminology.
Quality Rating
To strengthen our synthesis of study results, we developed a quality rating based on study design. We considered studies to be of high quality if they included at least one biological indicator of the menstrual cycle (e.g., basal body temperature, hormone levels), sample size was ≥30 women per group, and, when applicable, clinical diagnoses were made.
RESULTS
Psychosis
Most reports on the exacerbation of psychotic symptoms across the menstrual cycle are single case studies. These studies retrospectively rely on a patient’s self-reported or clinician-observed experiences over several years or decades. In all but one37 of the identified case studies, the authors reported recurrent psychosis during the premenstrual phase.28,29,32–34,36,38 Often, symptom onset occurred at menarche.34 Though many authors reported that psychotic or manic symptoms occurred during the week or days before menstruation, several noted that psychotic symptoms remitted upon menstruation.28,38
Findings become less clear when examining larger populations. Ray and colleagues39 followed 40 women with schizophrenia in an inpatient unit in India. Clinicians rated patients’ symptoms weekly for two consecutive menstrual cycles and found that positive symptoms of schizophrenia—namely, excitability and hostility—were highest during the premenstrual phase. Negative symptoms, such as withdrawal and difficulty with abstract thinking, were highest during menstruation. These findings complement earlier work by Harris,30 who found that, in a sample of 39 inpatient women with schizophrenia, most women experienced affective changes, rather than overt psychosis, during the premenstrual phase. Conversely, in a large, community-based study of 278 healthy women from the United Kingdom, persecutorial thoughts increased during the paramenstrual phase (day 1 of menstruation ± 3 days) compared to midcycle (11–17 days prior to menstruation).6 It is possible that certain menstrual fluctuations in paranoia exist in nonclinical samples and that affective fluctuations may be more common in clinical samples, where paranoia is more consistently pronounced. For women who also experience menstrual exacerbation of overt psychosis, the exacerbation appears to occur predominantly during the premenstrual phase.31
Results from high-quality studies
The one high-quality study found that 32.4% of a sample of women with schizophrenia had cyclical worsening of psychotic symptoms.35
Bipolar Disorders
Several case studies document menstrual fluctuations in symptoms of bipolar disorder. All but one study43 identified in the present review reported instances of hypomania,47 mania,41 or psychosis50 during the premenstrual and menstrual phases. Kukopoulos and colleagues40 reported that a 28-year-old Sardinian woman regularly experienced hypomania two weeks and depression two days prior to menstruation, with gradual improvement throughout menstruation. Subsequent reports similarly note the onset of mania and hypomania days prior to menstruation, with some symptoms ceasing upon menstruation41 or days after menstruation began.47 The sole case study to report incongruent findings described one woman’s experience with menstrual onset of depression and luteal-phase onset of hypomania.43
Results from larger clinical and community samples speak to these individual differences, as many studies report no overwhelming effect of the menstrual cycle on bipolar symptoms.42,45,46,48 Cyclic effects were found, however, in numerous subgroups of study samples. In one study of 41 women, increases in depression and mania were observed during the luteal phase for some women (n’s of 8 and 5, respectively).42,46 Similarly, Leibenluft and colleagues42 found that, in a sample of 25 women with rapid-cycling bipolar disorder, 6 showed an increase in depression and 5 an increase in hypomania in the days following menstruation. Sit and colleagues48 posit that the use of mood stabilizers and antipsychotics in treating bipolar disorders could mask patterns in symptom expression across the menstrual cycle. Indeed, one study examining menstrual cycle effects on mood in 34 Turkish women taking lithium or valproate for bipolar disorder and 35 healthy controls found greater mood variability across the cycle in healthy controls.44 Properly treated bipolar disorder can stabilize naturally occurring menstrual effects on mood,49 which may explain why large studies often find little or no effects of the menstrual cycle on symptom expression. Alternatively, it is possible that, in the study by Leibenluft and colleagues,42 mood changes among women with rapid-cycling bipolar disorder occurred too quickly to demonstrate any effect of the menstrual cycle.
Results from high-quality studies
Only one study met criteria for being considered high quality. This study found greater mood variability across the menstrual cycle in healthy controls compared to those taking lithium or valproate.
Depression
Depression is a profound and often debilitating disorder that disproportionately affects women.200 Research suggests that interactions among emotional and behavioral sensitivities to fluctuations in ovarian hormones across the menstrual cycle may be, for some women (e.g., see Schmidt et al.201), primary factors leading to depressive symptoms. The concept of premenstrual mood worsening has been a focus of investigation for decades, with many,52–54,57,58,68 but not all,7,55,56,156 studies finding some evidence of symptom exacerbation (i.e., mood worsening) in this phase. Indeed, women have been treated with hormonal therapy for improving premenstrual symptoms,51,202 with some success. Ongoing work seeks to better characterize the nature, timing, and mechanisms of changes in depression across the menstrual cycle to help develop and evaluate additional therapies.
Epidemiological and self-report studies assessing depressive symptoms in healthy women produced inconsistent findings. In a large study of 248 adult, premenopausal women (60% White), depressive symptoms and hormone levels were measured across at least two menstrual cycles.71 The authors found no relationship between depressive symptoms and absolute levels of hormonal changes across the menstrual cycle, although women with more depressive symptoms also had worse premenstrual mood changes. Premenstrual mood worsening was also found in an earlier study,59 and healthy women have reported higher symptoms of depression in both early and late follicular phases compared to the mid-luteal phase.70 Another study, however, showed no relationship between menstrual cycle phase and negative affect.74 It is possible that premenstrual mood worsening is related to decreases in reward responsivity. In general, reward responsivity (a possible biomarker for depression, with low reward sensitivity correlated with depression), appears to be highest in the follicular phase and lowest in the luteal phase.76,77,191,192,203 Women in the late luteal phase of the menstrual cycle may also have difficulty with emotion perception as evidenced by neural differences in brain activation compared to men,77 which could make them vulnerable to depression at this time. Another possibility is that individual women experience different patterns of mood changes throughout the cycle, with the consequence that analyzing data as a group may obfuscate individual differences.69
An early study found that women with a history of treated depression reported greater symptoms of depression across the menstrual cycle compared to women without a history of depression. There was no phase-specific pattern in this sample; however, these data were collected retrospectively, which may have introduced recall bias.61 In women with major depression, one study compared menstrual cycle phases at the time of psychiatric admissions, but these data were inconsistent54 and did not reveal a clear pattern. In a large, community-based sample of 900 girls and women ages 13–54 years (n = 111 Black; n = 121 Hispanic), participants underwent diagnostic interviews and tracked their moods for two cycles.65 Fifty-two participants had clinical (i.e., major depressive disorder or dysthymia) or subclinical depression. Women with clinical and subclinical depression reported greater symptom exacerbation in the premenstrual phase than did nondepressed women, though premenstrual symptom exacerbation was observed in all participants. Symptom worsening during the follicular phase was highest for clinically depressed, moderate for subclinically depressed, and low for non-depressed women. These data suggest that women with depression are at increased risk of premenstrual symptom worsening,60 possibly due to impaired estrogen-related modulation of stress reactivity;72 however, the pattern of increased mood symptoms premenstrually is also generally true across girls and women with and without depression.78 Similar findings were demonstrated in a sample of Chinese women with depressive disorders.66 Luteal phase decrease in positive affect has also been shown in women with fibromyalgia and rheumatoid arthritis,64 and women with epilepsy show lower moods and increased seizures during menstruation.78
The use of oral contraceptives further complicates the identification of clear patterns of how the menstrual cycle affects mood. In Sweden, a randomized, controlled trial of combined oral contraception (n = 84) compared to placebo (n = 94) examined the effect on mood during three consecutive menstrual cycles.75 Cycle phases were menstrual (days 1 to 4), premenstrual (days −7 to −1), and intermenstrual (all remaining days). The study found that oral contraceptive users reported a small, significant worsening of mood symptoms during the intermenstrual phase; however, additional analyses revealed that this effect was primarily driven by a subgroup of women with previous significant mood symptoms associated with oral contraceptive use. Other studies reported similar findings,62 regardless of the type of oral contraceptive used.63 Interestingly, another randomized, controlled trial in Germany found the opposite pattern: healthy, PMS-free women taking oral contraceptives reported slightly better mood across the cycle compared to naturally cycling women.73
Results from high-quality studies
Data from the high-quality studies do not offer much clarity. Several found that symptoms of depression were not related to absolute hormone levels, although one study focusing on women with clinical symptoms of depression and one study in healthy women both noted a premenstrual worsening of symptoms. Others found no relationship of depression to the menstrual cycle.
Suicide
Most research in this domain employs psychiatric hospitalization admission data or interviews immediately following hospitalization for suicide attempts. Research consistently demonstrates that rates of self-harm, suicide, and suicide attempts are significantly elevated during the premenstrual and menstrual phases.81–87,92,93 Histopathological reports suggest rates of completed suicides during the menstrual phase to range from 25%89 to 54%.88 Via autopsy, Leenaars and colleagues89 and Dogra and colleagues88 compared menstrual cycle phase at time of death in women who died by suicide versus other causes (e.g., motor vehicle accident). Despite the large difference in rates of suicides occurring during menstruation noted above, both studies reported similar rates of death by other causes during menstruation: 4.5% and 6.75%, respectively. Nonetheless, a disproportionate number of suicides occur during the menstrual phase compared to other phases of the menstrual cycle and other causes of death.
These rates are largely consistent with those seen in suicide attempts, with reported rates of attempts occurring during the menstrual phase ranging between 26%84 and 42%.79,84 Research grouping the premenstrual and menstrual phases reported rates of 47%,80 and one study reported luteal-phase attempt rates as high as 67%.91 Baca-García and colleagues83 posit that women with histories of diagnosed psychiatric disorders are five times more likely to attempt suicide during the menstrual phase than those with no such history. Variations in rates of suicide attempts across the menstrual cycle appear to be unique to naturally cycling women. Fourestié and colleagues79 found that, in a sample of 108 French women (35 naturally cycling) who attempted suicide, 42% of naturally cycling women attempted suicide while menstruating and 12% attempted during the premenstrual phase. They did not find associations with cycle phase and suicide among women using hormonal contraceptives.
Suicide is highly heritable, with rates ranging from 17%–55%.204–208 Furthermore, serotonergic function and the serotonin transport gene 5-HTT are highly related to suicidal behavior (as reviewed in Kenna et al.209). Researchers examined the possibility of gene × hormone interactions in rates of suicide and suicide attempts across the menstrual cycle. Baca-García and colleagues84 assessed the role of allele variants in rates of suicide attempts among 104 naturally cycling, White women. Serum assays indicated that, of these women, 17 had two long alleles, 38 had two short alleles, and 49 had one long and one short allele. Among women with two long alleles, a significant proportion of suicide attempts occurred during the menstrual phase (41%). Furthermore, estradiol levels were significantly lower in women with long rather than short alleles. No significant phasic differences emerged for women with two short alleles. As such, it is possible that genetic vulnerabilities may underlie menstrual cycle exacerbations of suicidal behavior.
Results from high-quality studies
Eleven studies met criteria for being considered high quality, with nine studies indicating that rates of completed or attempted suicide were highest during menstruation and two studies indicating these rates were highest premenstrually.
Anxiety and Anxiety Disorders I: Anxiety and Stress
Symptoms of anxiety and stress have been examined in healthy women’s menstrual cycles across a variety of laboratory protocols. Research focusing on daily symptoms of anxiety, as well as anxiety in response to stressors, has found clear premenstrual exacerbations of anxiety,60,67,94–96,103,115 although six studies (three of which had very small sample sizes) found no significant changes in anxiety related to the menstrual cycle.55,56,98,99,102,113 Some research found a divergence between self-reported stress and cortisol responses to stress in healthy women,98,107 while high levels of trait anxiety in women are associated with cortisol only in the follicular phase.105 State and trait anxiety were related to daily reports of anxiety during the luteal phase compared to the follicular phase in a healthy sample of 203 women,104 although another study showed that women high in state and trait anxiety do not show changes in symptoms across their menstrual cycles.100 In the one study that examined the role of ovulation during the menstrual cycle, there were no differences in symptoms, regardless of whether the cycle was ovulatory or anovulatory.68 A separate study assessing acoustic startle responses in women (as a proxy for anxiety) found larger startle magnitudes during ovulation and the late luteal phase, suggesting a potential vulnerability to anxiety during these phases.109 Other research has also shown higher levels of anxiety in response to stressors, including exercise,119 during the luteal phase.108
Other psychological characteristics representative of anxiety have been explored as factors contributing to menstrual cycle symptom severity. Lower perceived levels of control over anxiety have been correlated with higher levels of menstrual severity,111 which may be related to overall difficulty regulating emotions.112 Similarly, higher levels of health anxiety are associated with increased perceived stress, but only during the late luteal phase of the menstrual cycle.118 One recent study found that calmness was highest during the late luteal and menstrual phases; however, anxiety moderated the relationship between irritability and cycle phase, such that highly anxious women were more irritable during the late luteal and menstrual phases, when estrogen and progesterone levels are low.114 Chronic anxiety may therefore be a risk factor for more severe premenstrual and menstrual symptoms.210 In fact, high levels of estrogen may serve as a protective factor against psychosocial stress, as evidenced by changes in brain activation110 and cardiovascular responses to stress.106 Evidence also suggests that anxiety may be directly related to progesterone levels across the menstrual cycle,116 although one recent study found no relationship of anxious jealousy to progesterone levels across the menstrual cycle.117
Despite relatively consistent evidence of premenstrual anxiety exacerbation across the menstrual cycle, data also suggest that some women experience symptom exacerbation at mid-cycle and decreased symptoms premenstrually.52,69 In a sample of 213 young women attending college in Italy, participant responses were separated into four groups using cluster analysis, with two of the groups suggesting a “classic” PMS pattern, one group revealing a non-cyclic pattern, and the last group suggesting the mid-cycle pattern described earlier.69 This study represents an important step to examining individual differences across the menstrual cycle, rather than just assuming women experience similar changes across cycle phases—an approach demonstrated by earlier research comparing community volunteers with women who reported high levels of premenstrual symptoms.101
Although most studies have excluded women using oral contraceptives, several studies have compared anxiety in women who were and were not using exogenous hormones. Generally, naturally cycling women demonstrate the expected pattern of anxiety (higher during menstrual and premenstrual phases), whereas women using oral contraceptives showed no change in anxiety.97,98 One small study showed no cycle-phase or group differences in anxiety in women who were and were not taking oral contraceptives.56
Results from high-quality studies
When examining high-quality studies only, the majority found no effect of menstrual cycle phase on anxiety, although women with high baseline anxiety seemed to experience more symptoms in the luteal phase.
Anxiety and Anxiety Disorders II: Generalized Anxiety Disorder
A recent study compared women with and without generalized anxiety disorder (GAD) on measures of mental and physical fatigue during the early follicular and mid-luteal phases.121 The only difference to emerge was that women with GAD had higher mental fatigue in the early follicular phase. Furthermore, salivary estradiol and progesterone were not associated with measures of fatigue during any cycle phase. This is consistent with earlier research demonstrating increased symptoms of depression, anxiety, and hostility in women with GAD; however, symptom exacerbation was even greater for women with GAD and PMS, particularly in the premenstrual phase.120
Results from high-quality studies
Neither study met our criteria for being considered high quality.
Anxiety and Anxiety Disorders III: Social Anxiety Disorder
In women with social anxiety disorder, higher social anxiety and avoidance were reported in the premenstrual phase of the menstrual cycle (week 4) compared to the three previous weeks.122 In a separate study of Chinese women, salivary progesterone was positively correlated with self-reported social feedback sensitivity, regardless of menstrual cycle phase (late follicular or mid-luteal).124 This finding is supported by a previous study in which progesterone levels were associated with increased attention to social stimuli.123
Results from high-quality studies
Analysis of high-quality studies revealed no cycle effect of interpersonal sensitivity in healthy women, although the luteal phase was associated with greater attention to social stimuli and higher interpersonal anxiety.
Anxiety and Anxiety Disorders IV: Panic Disorder
One of the earliest studies compared retrospective versus prospective reports of anxiety and panic in a small sample of adult women with panic disorder.127 Interestingly, most women (79%; n = 15) retrospectively reported worsening anxiety symptoms premenstrually, but prospective self-reported anxiety and daily frequency of panic attacks were similar pre- to postmenstrually. These data mirror two earlier published reports,125,126 although another study found that prospective report of panic and anxiety clearly demonstrated premenstrual exacerbation of symptoms.128
Research on mechanisms of panic disorder, such as anxiety sensitivity, has produced some important findings.211 In a 1996 study, 337 college women were screened for anxiety sensitivity, and the lower and upper quartiles of respondents participated during either the intermenstrual (days 8 to 22) or premenstrual (days 24 to 28) phase of their cycles.130 Women in the high anxiety-sensitivity group demonstrated elevated skin conductance reactivity to anxiety-provoking scenes in the premenstrual phase compared to those with low anxiety sensitivity or those in other phases of the menstrual cycle. The authors propose that these data were the first to link both state (menstrual cycle phase) and trait (high anxiety sensitivity) factors that may contribute to vulnerability to panic in women. Similar patterns were found in more recent studies in healthy women with high/low anxiety sensitivity.134 Additionally, women with high anxiety sensitivity report more menstrual-related symptoms,131 and women with asthma with or without panic disorder report more state anxiety,133 regardless of cycle phase.135
Laboratory-based studies often use what is known as a “CO2 challenge” to evaluate reactivity to the sensation of difficulty breathing.212,213 In this approach, participants inhale a full lung capacity of a gas mixture (typically 35% CO2/65% O2) and rate their levels of anxiety. One of the first studies to explore menstrual cycle effects in this paradigm found that women with panic disorder experience significantly more reactivity during the early follicular phase (day 4 of the menstrual cycle) than during the mid-luteal phase (8 days prior to menstruation), whereas healthy women show no differences across the menstrual cycle.129 A separate laboratory study demonstrated elevated skin conductance responses to anxiety-provoking stimuli during the premenstrual phase in women with panic disorder compared to women without.132
Results from high-quality studies
Two studies of healthy women were considered to be of high quality. They both found no menstrual cycle effects for anxiety generally, although high anxiety sensitivity was associated with higher cognitive panic symptoms in the premenstrual phase.
Obsessive-Compulsive and Related Disorders I: Obsessive-Compulsive Disorder
Early retrospective studies suggested a link between exacerbation of OCD symptoms and the premenstrual phase, specifically.136,137 The first study to evaluate this relationship prospectively included 101 women who met diagnostic criteria for OCD.138 Approximately half of the women reported premenstrual worsening of OCD symptoms, as demonstrated by significantly higher scores on a self-report measure of OCD symptoms. In a laboratory-based study designed to measure OCD-related checking symptoms, no menstrual cycle phase differences (comparing mid-luteal and mid-follicular) were identified.139
Results from high-quality studies
Only one study meeting criteria for high quality found no differences in checking behaviors.
Obsessive-Compulsive and Related Disorders II: Trichotillomania (Hairpulling)
A single published study explored the relationships among menstrual cycle phases and trichotillomania in 59 adult women.140 Participants were retrospectively asked whether they believed whether their menstrual cycles and hairpulling were related, and 53.3% indicated that they were. Participants also reported a clear effect of menstrual phase when asked to indicate symptoms premenstrually, during menstruation, and postmenstrually, such that greater frequency and intensity of urges, greater frequency of hairpulling, and decreased ability to control hairpulling were all significantly higher in the premenstrual phase than in the other phases.
Results from high-quality studies
The one identified study did not meet our criteria for being considered high quality.
Posttraumatic Stress Disorder
Assessment of trauma and PTSD symptoms and their relationship to the menstrual cycle is complicated by differences in when the trauma occurred and when it was assessed. One study looked at this specific issue in a large sample of women (n = 147) with various types of trauma, including motor vehicle accidents, falls, and nonsexual assaults.141 Based on retrospective self-reports of their last menstrual periods, women who were in the mid-luteal phase at the time of the trauma (20% of the sample) or at the time of assessment (16% of the sample) reported experiencing significantly more frequent and severe flashbacks than women who experienced trauma in other cycle phases. This finding remained even after controlling for number of days in the hospital, injury severity, age, trauma type, and mild traumatic brain injury. In healthy populations,142 women exposed to a distressing film during the early luteal phase were more likely to experience intrusive memories of the film in the days following than women who watched the film in the mid-follicular or late luteal phases (when progesterone levels are low).143 Furthermore, the frequency of intrusions was negatively correlated with the estrogen-to-progesterone ratio, suggesting that estrogen may have protective effects and that both hormones may be important for encoding distressing memories. Similar results have been demonstrated in other intrusive-memory paradigms.144 In the luteal phase, when the estrogen-to-progesterone ratio is lower than in the follicular phase, women may experience more intrusive memories and impaired fear inhibition.2
Laboratory assessment of fear also sheds light on biomarkers of PTSD. Prepulse inhibition is a neurobiological process typically assessed through a startle-response paradigm. A weaker version of the stimulus (prepulse) is administered prior to a startle stimulus (pulse), which results in a decreased startle response compared to when no prepulse is administered. This paradigm aims to measure the brain’s ability to effectively filter interruptions (i.e., the startle stimulus) from ongoing processing of the prepulse stimulus.214 Pineles and colleagues146 examined prepulse inhibition in women with PTSD and trauma-exposed women without PTSD in the early follicular and mid-luteal phases of the menstrual cycle. Although group differences were found, there were no main effects or interactions with menstrual cycle phase, estradiol, or progesterone levels, suggesting that menstrual cycle phase was not associated with prepulse inhibition. The authors suggest that these null results may indicate that prepulse inhibition evokes early stages of information processing that may not be influenced by the menstrual cycle. Other studies comparing women with PTSD to trauma-exposed women without PTSD found that deficits in extinction learning (i.e., learning such that a stimulus previously associated with a shock is no longer associated with the shock) were present in the mid-luteal phase but only for women with PTSD.147,150 A possible explanation for this deficit is that estrogen, which is lower in the luteal than late follicular phase, may be important for higher-order processes such as extinction learning. Relatedly, it is possible that women with PTSD have deficits in the conversion of progesterone, which is typically higher in the luteal phase, to the GABAergic neurosteroid allopregnanolone, which affects differential fear conditioning and extinction.148 Although GABA plasma levels appear to be positively correlated with PTSD symptoms in women with PTSD compared to trauma-exposed healthy controls, menstrual cycle phase was not related to GABA levels in either group, according to a recent study.151
The course of other symptoms of PTSD across the menstrual cycle is not entirely clear. Anxiety sensitivity (i.e., fear of the physical symptoms of anxiety), for example, appears to be stable across the menstrual cycle in women with and without PTSD.149 However, interpersonal sensitivity, depression, anxiety, hostility, and phobic anxiety are significantly higher in women with PTSD than in those without.145 Women with PTSD report more phobic anxiety in the early follicular phase compared to the mid-luteal phase, and women without PTSD report no changes across the menstrual cycle.145
Results from high-quality studies
Three studies met our established criteria for high-quality evidence, and all of these explored intrusive memories in healthy women. The data suggest that intrusive memories are more frequent in the luteal phase and when estradiol levels are low.
Eating Disorders
Much research examining eating disorders across the menstrual cycle focuses on binge eating rather than caloric restriction or compensatory behaviors (e.g., purging). As such, results presented in this review surround emotional and binge eating. Research examining emotional or binge eating appears to indicate consistent cyclic effects (see Fowler et al.164 and Leon et al.152). In both clinical and community-based studies of women diagnosed with bulimia nervosa, significant increases in binge eating were reported during the mid-luteal and premenstrual phases.153–155,159 Similar results were noted in community samples of women without diagnosed eating disorders.157,158,160–162 In a convenience sample of 148 women (84% White), naturally cycling women (n = 67) reported increased hunger during the menstrual phase and increased food cravings and amount of food eaten during both the premenstrual and menstrual phases.158 Women using hormonal contraceptives (n = 81) demonstrated the same pattern with the addition of increased hunger during the premenstrual phase.
To better understand the biological underpinnings of these fluctuations, researchers investigated associations among progesterone, estradiol, and eating behaviors. In a sample of nine women with bulimia nervosa and eight healthy controls (82.4% White), Edler and colleagues155 found significant negative associations between binge eating and estradiol, and significant positive associations between binge eating and progesterone. Similarly, Baker and colleagues163 reported that, when women had low progesterone levels, an inverse relationship between estradiol and body dissatisfaction emerged. When progesterone levels were high, however, positive relationships among estradiol, body dissatisfaction, and binge eating emerged.
Results from high-quality studies
Data from the three high-quality studies do not reflect a consistent pattern, with two studies reporting no direct hormonal associations, and one study reporting positive associations, among emotional eating, progesterone, and estradiol.
Borderline Personality Disorder
BPD is characterized by intense and frequent emotional dysregulation, often resulting in anger and aggressive behavior toward others. Individuals with BPD are highly sensitive to criticism and may experience intense mood fluctuations throughout a day. However, few studies examined the role of the menstrual cycle or ovarian hormones in BPD symptoms. In a convenience sample of 226 undergraduate women, researchers found that women using oral contraceptives endorsed significantly more BPD symptoms on a self-report questionnaire.165 Moreover, the phase of the menstrual cycle when estrogen was rising (days 5 to 10; mid- to late-follicular phase) was associated with more symptoms than those in a low-estrogen phase (days 0 to 3 and 26 to 29). This association was confirmed in a second study reported in the same article that measured salivary estradiol and found a significant positive relationship between rising, but not absolute, estrogen levels and BPD symptoms.
Subsequent studies sought to better understand the relationship between estrogen-to-progesterone ratios and key symptoms. One study found that within-person higher-than-average progesterone levels and lower-than-average estrogen levels predicted increased symptoms for women with high baseline BPD symptoms.166 In another study of women with BPD, symptoms were generally worse in the perimenstrual phase than mid-luteal, ovulatory, and follicular phases. High-arousal symptoms (e.g., anger) returned to baseline, however, in the early follicular phase (i.e., when estrogen levels are low), whereas low-arousal symptoms (e.g., depression) persisted until ovulation (i.e., when estrogen levels are high).167 In this same sample, anger/irritability was highest in the perimenstrual phase, with reactive aggression highest in the mid-luteal phase and proactive aggression highest during ovulation and lowest perimenstrually.168
Results from high-quality studies
Two high-quality studies of BPD symptoms in healthy women suggest that BPD symptoms may change as a function of variability in estradiol and progesterone, as opposed to being associated with absolute ovarian hormone levels.
Substance Use Disorders I: Alcohol Use
The literature assessing the relationship between alcohol use and the menstrual cycle is mixed.169–171 In a 2015 meta-analysis, Carroll and colleagues12 found that 7 of the 13 identified articles reported increased drinking during the premenstrual phase, one reported decreased drinking during the premenstrual phase, and five reported no significant menstrual cycle effects. More recent research suggests that drinking may indeed fluctuate across the menstrual cycle, and this may be linked with progesterone-to-estradiol ratios. In a study by Joyce and colleagues,173 94 naturally cycling women (76.6% White) documented the quantity of alcohol consumed and reasons for drinking across a full menstrual cycle. Women reported slight increases in drinking during the premenstrual and menstrual phases, and motivations related to coping were significantly associated with these increases (see also Hayaki et al.174). Similarly, social motivations (e.g., “because [drinking] makes social gatherings more fun”) were associated with alcohol consumption around ovulation.173 These findings align with Martel and colleagues’ work172 demonstrating increases in drinking and binge drinking during the premenstrual phase and ovulation. High levels of estradiol predicted alcohol consumption, and these effects increased when progesterone was low and decreased when progesterone was high. Mood may also moderate these effects. Research shows that, when progesterone is low, women are more likely to drink when their mood is negative and that, when progesterone is high, women are more likely to drink when their mood is positive.175 Taken together, recent research has elucidated that menstrual cycle effects may be moderated by positive or negative affect.
Results from high-quality studies
The two high-quality studies reported relatively consistent findings. When progesterone is low in the premenstrual and menstrual phases, alcohol consumption appears to be associated with negative mood. Around ovulation and when progesterone rises, alcohol consumption appears to be associated with positive mood.
Substance Use Disorders II: Smoking
Studies have examined menstrual cycle effects on ad lib smoking (i.e., smoking at will), subjective effects of nicotine, cravings, withdrawal symptoms, and smoking cessation. We found no consistent pattern for ad lib smoking, with studies documenting no cyclic effect,187 increased smoking during the luteal phase,179,184 or increased smoking during menstruation.176 In a study by Schiller and colleagues,183 98 female smokers (79% White) attended two laboratory sessions spaced two weeks apart, during which they smoked ad lib for one hour. Researchers found that women’s progesterone-to-estradiol ratios were negatively associated with smoking behavior; women with lower levels of progesterone compared to estradiol smoked more. The authors proposed that these relative levels may partly explain inconsistencies in the extant literature, as relative amounts of these hormones may be an important factor in menstrual cycle–related smoking behavior.
Progesterone may also diminish subjective effects of nicotine.185 In a study by Goletiani and colleagues,186 23 naturally cycling female smokers rated the subjective effects of cigarettes throughout two-hour ad lib smoking sessions twice during their menstrual cycles. No phasic effects were found on subjective effects of cigarettes. However, when data collected during the luteal phase were grouped based on progesterone levels, researchers found that women with high levels of progesterone reported significantly lower subjective effects. Similar findings show the effects of progesterone on reducing cravings.181,188,189,215
Conversely, symptoms of nicotine withdrawal appear to be highest during the luteal phase.177,178 This increase may be related to premenstrual symptoms, which are greater during the luteal phase and include symptoms like those of nicotine withdrawal (e.g., fatigue, headache, anxiety; see Weinberger et al.216). It is unclear whether this is related to the effectiveness of smoking cessation (e.g., quit attempts); however, while the research is limited, there are studies that indicate superior outcomes for smoking cessation initiated during both the luteal182 and follicular180 phases. These studies are also limited as they vary regarding whether cessation is assisted by pharmacotherapies such as nicotine replacement, bupropion, or varenicline. Given the negative correlation between progesterone-to-estradiol ratios and smoking behavior,183 quit attempts made during the follicular phase may be more successful.
Results from high-quality studies
Seven studies assessing smoking behavior across the menstrual cycle were considered high quality. Overall, these studies suggest that cravings and affective responses to nicotine are lower in the luteal phase, when progesterone levels are relatively high, compared to the follicular phase, when progesterone levels are relatively low.
Substance Use Disorders III: Cocaine Use
Like menstrual effects on nicotine use, research indicates an attenuation of subjective effects of smoked cocaine when progesterone levels are high (see Collins et al.193 and Reed et al.194). Sofuoglu and colleagues190 reported that women (n = 21) had lower ratings of feeling “high” and “stimulated” during the luteal phase than the follicular phase. Evans and colleagues191 similarly reported that, although women (n = 11; 91% African American) reported greater desire for cocaine during the luteal phase, their ratings of drug effects such as feeling “high,” “stimulated,” “alert,” and “self-confident” were significantly reduced compared to ratings in the follicular phase. Evans and colleagues192 later examined subjective responses to smoked cocaine in 11 naturally cycling women (91% African American) during the follicular phase, luteal phase, and follicular phase with exogenous progesterone administration. Ten men served as a control group. Subjective effects of cocaine were significantly lower during the luteal phase and when the follicular phase was supplemented with exogenous progesterone compared to the follicular phase and men’s responses. No differences were found between follicular phase responses and men’s responses, which continues to suggest that progesterone modulates subjective responses to smoked cocaine.
To assess the potential role of allopregnanolone, a progesterone metabolite, on cocaine cravings, Milivojevic and associates195 randomized 46 cocaine-dependent men and women (n’s of 29 and 17, respectively; 73.9% African American) to receive either a progesterone supplement or placebo, and measured blood concentrations of allopregnanolone and self-reported cravings. They found that, through increases in allopregnanolone, those who received progesterone supplementation reported significantly lower cravings than those who received placebo.
Results from high-quality studies
No study in this section met our criteria for being of high quality.
Miscellaneous Disorders
We found only one study relating to symptom fluctuation in a woman with kleptomania.217 The authors did not formally test her symptoms during different cycle phases, but the patient reported experiencing intensified urges to steal during the luteal phase. We felt that the data from this single case study were not sufficient to justify inclusion in the review. There were no studies available for body dysmorphic disorder, excoriation, hoarding, intermittent explosive disorder, conduct disorder, or pyromania.
DISCUSSION
The aim of this comprehensive review is to describe the findings of previous research examining psychiatric symptom variability across the menstrual cycle. Each study included (1) a comparison of at least two menstrual cycle phases, (2) data not derived from evaluation of an intervention, and (3) premenopausal women, age 18 years or older. Across psychiatric diagnoses, we saw evidence of symptom exacerbation primarily in the luteal, premenstrual, and menstrual phases.
Evidence of Potential Mechanisms Involved in Symptom Fluctuation
Several studies included in this review examined possible mechanisms that may underlie menstrual-related changes in symptoms. Regarding depression, for example, the literature indicates that both healthy women and women with a depressive disorder experience perimenstrual increase of depressive symptoms. However, data exploring potential mechanisms are not as clear. fMRI studies examining functional brain changes show inconsistent results.218,219 Similarly, estradiol may help regulate stress for healthy women but not for women who have experienced clinical depression,72 suggesting that even a history of depression could make women vulnerable to increased perimenstrual mood changes.61 These data highlight the complexities of determining how depression changes over the course of the menstrual cycle and whether these processes may be different between healthy and clinical populations, perhaps suggesting the need for different treatment approaches.220,221
Ovarian Hormone Mechanisms
Several studies hypothesized a specific link between ovarian hormones and symptom fluctuation. Regarding substance use, the decrease in cravings in the luteal phase may be due, in part, to increases in allopregnanolone, a progesterone-derived neuroactive steroid. Allopregnanolone produces anxiolytic and hypnotic effects via increased GABA potentiation (see Lambert et al.21 for a review), which could lessen the subjective effects (e.g., reportedly feeling “high,” “stimulated”) of substances such as cocaine. High levels of progesterone in the mid-luteal phase are also associated with release of glucocorticoids, which help consolidate memories, potentially increasing susceptibility to developing PTSD.18,141 Once symptoms of PTSD have developed, reductions in the conversion of progesterone to allopregnanolone and pregnanolone can further impair learning of new, non-fearful associations.150 These and other studies have led to the hypothesis that allopregnanolone-to-progesterone ratios may be better biomarkers for psychiatric symptoms, as decreases in this ratio from the follicular to luteal phases are evident despite increases in absolute levels across the menstrual cycle.222 Impairments in allopregnanolone synthesis may further impair GABAergic function and leave some women at risk for psychiatric disorders,20,22 although this causal link has not yet been clearly demonstrated.
Additionally, according to the estrogen hypothesis, estrogen is protective against psychosis (see Reilly et al.15 for a review). Reductions in estrogen can facilitate or exacerbate psychosis—which is exemplified by the increased risk of psychosis in postmenopausal and postpartum periods. It is therefore consistent with these data that increases in psychotic experiences tend to occur as estrogen levels decline throughout the premenstrual phase.
Regarding cyclic effects on emotional and binge eating, there are likely both hormonal and genetic underpinnings of these behaviors. The pattern of decreased food intake during the first half of the menstrual cycle and increased food intake during the second half of the menstrual cycle is observed in many mammalian species (see Schneider et al.223 for a review). From an evolutionary standpoint, it is theorized that this pattern allows for a shift in motivational priorities from reproduction to eating. During the first half of the menstrual cycle, motivational priorities surround increasing sexual desire as ovulation approaches. As the likelihood of conception decreases the further from ovulation a woman is in her cycle, motivational priorities shift toward eating.223 One hypothesis is that a gene × hormone effect could exaggerate this process in women who have binge-eating behavior.161 For example, an individual who is genetically vulnerable to binge eating may experience increased activation by certain concentrations of estradiol and progesterone during the luteal phase of the menstrual cycle compared to someone without this genetic predisposition.
Finally, the ratio between progesterone and estradiol appears to play an important role in symptom expression, though not in a consistent direction. Lower levels of progesterone coupled with higher levels of estradiol, for example, have been associated with increased smoking,183 alcohol consumption,172 and body dissatisfaction.163 Conversely, higher levels of progesterone compared to estradiol has been associated with increases in intrusive memories in the context of PTSD.143
Although we did not examine reproductive mood disorders (e.g., premenstrual dysphoric disorder, postpartum depression, perimenopausal depression) specifically, the literature in these areas suggests that there is a large amount of individual variability in mood sensitivity to ovarian hormones.201,224–226 Indeed, it is likely that a minority of women exhibit psychological sensitivity to ovarian hormones across the menstrual cycle and that collapsing participant data into groups may mask this variability. As such, researchers are encouraged to assess for subgroups when analyzing menstrual cycle data.
It is also possible that psychiatric symptoms may be a delayed response to hormonal changes and may therefore not reflect the hormonal phase when the symptoms arise. For example, symptoms that have onset in the luteal phase may be in response to increases in estradiol or progesterone, and symptoms in the early follicular phase may be a response to hormone withdrawal. Indeed, research suggests that some symptoms may peak several weeks following exogenous hormonal manipulation.201 Schmidt and colleagues226 argue that menstrual cycle studies may not be able to accurately tease apart the effect of hormonal changes versus absolute hormone levels on psychiatric symptoms, and they encourages the use of hormonal manipulation to address this possible limitation.
Results from High-Quality Studies
Overall, a paucity of studies met our criteria to be considered of high quality. Of the 16 areas examined, three (GAD, trichotillomania, and cocaine use) had no high-quality studies, and three (psychosis, bipolar disorders, and OCD) each had one high-quality study. As such, no strong conclusions can be made for these psychiatric disorders/symptoms. It is recommended that future research continue to explore the presentation of these psychiatric disorders/symptoms across the menstrual cycle. Furthermore, researchers are encouraged to use the flexible design recommendations made by Schmalenberger and colleagues227 (e.g., using within-subjects designs, incorporating ovulation predictor testing) to enhance study quality and validity of results.
Social anxiety disorder, PTSD, alcohol use, and smoking each had two to three high-quality studies demonstrating relatively consistent results. For social anxiety disorder and PTSD, it appears as though symptoms may worsen in the luteal phase (e.g., greater interpersonal anxiety, more frequent intrusive memories). Alcohol use across the menstrual cycle appears to be influenced by mood such that alcohol use is associated with negative mood in the premenstrual and menstrual phases and is associated with positive mood around ovulation. Regarding smoking, cravings appear to be lower in the luteal phase than in the follicular phase. This may help to explain why quit attempts appear to be more successful when made during the follicular phase: if women experience a reduction in cravings in the luteal phase following quit attempts made during the follicular phase, women may be able to sustain these attempts for a longer period.178 Cumulatively, these results provide a preliminary understanding of the effect of the menstrual cycle on these symptoms, and more high-quality research within each area is needed.
Results from the two to three high-quality studies for each of the following diagnoses—panic disorder, eating disorders, and BPD—were unclear or inconsistent, and more research is needed to determine the effect of the menstrual cycle on these disorders. We identified 7 high-quality studies examining depression and 14 examining anxiety/stress. Similarly, results from these areas were unclear or inconsistent. Given that the studies in these two areas yielded inconsistent results, it may be that the menstrual cycle has no consistent effects on symptom expression in these areas, although a history of depression or higher levels of baseline depression or anxiety may be a risk factor for menstrual cycle–related exacerbation of symptoms. Alternatively, it may be that the ways in which any menstrual cycle effect is expressed are nuanced and possibly masked by the varying populations and study designs in these studies. Future research aimed at replicating study designs used in these high-quality studies would help elucidate any true menstrual cycle effects.
Research on suicide/suicide attempts was the only area sufficiently studied, with 11 studies meeting criteria to be considered high-quality and yielding consistent results. Overall, results from these studies indicate that rates of suicide/suicide attempts are highest during menstruation.
Limitations
Overall, the data on psychiatric symptoms across the menstrual cycle are limited because of the lack of prospective studies of women with a range of psychiatric disorders in which standardized assessment of the menstrual cycle is collected. This limits our understanding and knowledge of these phenomena, including the implications for specific disorders and the investigation of underlying mechanisms. Varied definitions and assessment of menstrual cycle phases, as well as a lack of standardized assessments of the menstrual cycle—including biological assays of estrogen, estradiol, allopregnanolone, and progesterone, along with their relative ratios, on and off oral contraceptives—all limit existing information. Inconsistencies in the literature may also result from individual variation (see Kiesner69 for a review). In addition, studies vary in standardization of symptoms for specific psychiatric disorders, further limiting assessment of the existing data.
Varied definitions and assessment of menstrual cycle phases
Menstrual cycle phases are termed and calculated differently across research groups, which may mask or inflate true symptom variability. For example, some researchers define ovulation as a distinct phase or window of days,174 and others include ovulation as a part of the luteal phase.164 Assessment of cycle phase is further complicated when comparing studies using hormonal measures and those employing self-reports. Assessing menstrual phases through self-reported days since menstruation is complicated by the known variability in the length of menstrual cycle phases.196 As such, 16 days since menstruation could, for example, fall during the luteal phase for one woman, coincide with ovulation for another, and, in the case of short luteal phase disorder, fall during the follicular phase for yet another woman.
Lack of standardized assessment of symptoms
Across multiple disorders, symptom assessment is inconsistent. In some studies, researchers employ self-reports, others use behavioral tasks, and still others use observations. These inconsistencies could complicate findings as research indicates there are discrepancies across some forms of symptom assessment.228 Additionally, psychiatric disorders are comprised of constellations of symptoms, all of which may change independently throughout the menstrual cycle. Laboratory paradigms use tasks as proxies for stressors or psychophysiological measures. Taken together, these factors create a highly complex and nuanced picture that can be hard to interpret. Just as one example, in BPD, high- and low-arousal symptoms are each affected separately by the menstrual cycle; teasing apart arousal symptoms may shed additional light on cycle-related and hormonal relationships.229 Across disorders, better understanding these relationships may help inform treatment options or guidance.230,231
Sampling biases
With few exceptions,191,192,195 most studies reviewed either included samples of primarily White women or did not provide information on participants’ racial and ethnic backgrounds. Given the failure to include this information and the general lack of representation of racial and ethnic minority groups, the generalizability of these findings is an open question. Women from underrepresented groups may experience symptom changes differently, either because of the experience of different acute or chronic stressors (e.g., racial trauma) or because of varying cultural interpretations of symptoms or menstruation.
CONCLUSIONS AND FUTURE DIRECTIONS
The existing literature demonstrates that menstrual-related exacerbation of psychiatric symptoms occurs most commonly during the premenstrual and menstrual phases, and that, for some symptoms, progesterone-to-estradiol ratios play important roles in this relationship. Effective treatment for women with psychiatric disorders will require an understanding of the role of ovarian hormones and other neuroactive steroids such as allopregnanolone but perhaps others as well.13 To further elucidate the role of ovarian hormones in psychiatric symptom expression, researchers are encouraged to employ prospective designs and incorporate hormone assays in their relevant research, as research has found that retrospective140 and self-reported232 assessments of the menstrual cycle are less accurate, which may obfuscate potential findings. Given the mixed findings on the influence of oral contraceptives on mood symptoms,73,75 as well as research indicating the likely importance of progesterone-to-estrogen ratios, researchers are also encouraged to further assess the effects of various types of oral contraceptives (e.g., androgenic vs. antiandrogenic; high vs. low doses of ethinylestradiol) on psychiatric symptoms. These findings would better highlight the roles of progesterone and estrogen in women’s mental health, and also possibly identify oral contraceptives that may assist in symptom stabilization. Furthermore, given the lack of diversity in the included samples, future research should focus on women from racially and ethnically diverse backgrounds to assess the generalizability of these results. Clinicians should also be routinely assessing symptom variability across the menstrual cycle in their patients. The ability to predict worsening of symptoms allows clients to better prepare and utilize effective coping strategies to help manage emotional changes. Clinicians are further encouraged to assess other factors that influence ovarian hormone expression, such as pregnancy status and hormonal contraceptive use.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Footnotes
Original manuscript received 9 August 2021; revised manuscript received 19 November 2021, accepted for publication 12 December 2021.
Harvard Review of Psychiatry offers CME for readers who complete questions about featured articles. Questions can be accessed from the Harvard Review of Psychiatry website (www.harvardreviewofpsychiatry.org) by clicking the CME tab. Please read the featured article and then log into the website for this educational offering. If you are already online, click here to go directly to the CME page for further information.
Contributor Information
Ariel B. Handy, Email: arielbhandy@gmail.com.
Shelly F. Greenfield, Email: sgreenfield@mclean.harvard.edu.
Kimberly A. Yonkers, Email: Kimberly.Yonkers@umassmemorial.org.
REFERENCES
- 1.Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci 2011;124:229–36. [DOI] [PubMed] [Google Scholar]
- 2.Glover EM Mercer KB Norrholm SD, et al. Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci 2013;38:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins SM, Matzuk MM. The menstrual cycle: basic biology. Ann N Y Acad Sci 2008;1135:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobeika E, Armouti M, Kala HS, Stocco C. Ovarian hormones. In: Litwack G, ed. Hormonal signaling in biology and medicine: comprehensive modern endocrinology. London: Academic, 2020:565–83. [Google Scholar]
- 5.Zaka M, Mahmood KT. Pre-menstrual syndrome—a review. J Pharm Sci Res 2012;4:1684–91. [Google Scholar]
- 6.Brock R, Rowse G, Slade P. Relationships between paranoid thinking, self-esteem and the menstrual cycle. Arch Womens Ment Health 2016;19:271–9. [DOI] [PubMed] [Google Scholar]
- 7.Laessle RG, Tuschl RJ, Schweiger U, Pirke KM. Mood changes and physical complaints during the normal menstrual cycle in healthy young women. Psychoneuroendocrinology 1990;15:131–8. [DOI] [PubMed] [Google Scholar]
- 8.Owens SA, Eisenlohr-Moul TA, Prinstein MJ. Understanding when and why some adolescent girls attempt suicide: an emerging framework integrating menstrual cycle fluctuations in risk. Child Dev Perspect 2020;14:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman AM, Critchley HD, Duka T. Risk-taking and impulsivity: the role of mood states and interoception. Front Psychol 2018;9:1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Iersel KC, Kiesner J, Pastore M, Scholte RH. The impact of menstrual cycle-related physical symptoms on daily activities and psychological wellness among adolescent girls. J Adolesc 2016;49:81–90. [DOI] [PubMed] [Google Scholar]
- 11.Lockwood J, Daley D, Townsend E, Sayal K. Impulsivity and self-harm in adolescence: a systematic review. Eur Child Adolesc Psychiatry 2017;26:387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll HA, Lustyk MK, Larimer ME. The relationship between alcohol consumption and menstrual cycle: a review of the literature. Arch Womens Ment Health 2015;18:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yum SK, Yum SY, Kim T. The problem of medicating women like the men: conceptual discussion of menstrual cycle-dependent psychopharmacology. Transl Clin Pharmacol 2019;27:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogos A, Sbisa AM, Sun J, Gibbons A, Udawela M, Dean B. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol 2015;2015:615356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly TJ, Sagnay de la Bastida VC, Joyce DW, Cullen AE, McGuire P. Exacerbation of psychosis during the perimenstrual phase of the menstrual cycle: systematic review and meta-analysis. Schizophr Bull 2020;46:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus 2009;19:1142–50. [DOI] [PubMed] [Google Scholar]
- 17.Frick KM, Tuscher JJ, Koss WA, Kim J, Taxier LR. Estrogenic regulation of memory consolidation: a look beyond the hippocampus, ovaries, and females. Physiol Behav 2018;187:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia NM, Walker RS, Zoellner LA. Estrogen, progesterone, and the menstrual cycle: a systematic review of fear learning, intrusive memories, and PTSD. Clin Psychol Rev 2018;66:80–96. [DOI] [PubMed] [Google Scholar]
- 19.Piette PCM. The pharmacodynamics and safety of progesterone. Best Pract Res Clin Obstet Gynaecol 2020;69:13–29. [DOI] [PubMed] [Google Scholar]
- 20.Amin Z, Mason GF, Cavus I, Krystal JH, Rothman DL, Epperson CN. The interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacol Biochem Behav 2006;84:635–43. [DOI] [PubMed] [Google Scholar]
- 21.Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev 2001;37:68–80. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy K, Osborne LM. Allopregnanolone and reproductive psychiatry: an overview. Int Rev Psychiatry 2019;31:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundstrom-Poromaa I, Comasco E, Sumner R, Luders E. Progesterone—friend or foe? Front Neuroendocrinol 2020;59:100856. [DOI] [PubMed] [Google Scholar]
- 24.Barron ML, Flick LH, Cook CA, Homan SM, Campbell C. Associations between psychiatric disorders and menstrual cycle characteristics. Arch Psychiatr Nurs 2008;22:254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce KM, Good KP, Tibbo P, Brown J, Stewart SH. Addictive behaviors across the menstrual cycle: a systematic review. Arch Womens Ment Health 2021;24:529–54. [DOI] [PubMed] [Google Scholar]
- 26.Jang D, Elfenbein HA. Menstrual cycle effects on mental health outcomes: a meta-analysis. Arch Suicide Res 2019;23:312–32. [DOI] [PubMed] [Google Scholar]
- 27.Nillni YI, Rasmusson AM, Paul EL, Pineles SL. The impact of the menstrual cycle and underlying hormones in anxiety and PTSD: what do we know and where do we go from here? Curr Psychiatry Rep 2021;23:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerada C, Reveley A. Schizophreniform psychosis associated with the menstrual cycle. Br J Psychiatry 1988;152:700–2. [DOI] [PubMed] [Google Scholar]
- 29.Lovestone S. Periodic psychosis associated with the menstrual cycle and increased blink rate. Br J Psychiatry 1992;161:402–4. [DOI] [PubMed] [Google Scholar]
- 30.Harris AH. Menstrually related symptom changes in women with schizophrenia. Schizophr Res 1997;27:93–9. [DOI] [PubMed] [Google Scholar]
- 31.Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: evidence supporting the oestrogen hypothesis. Acta Psychiatr Scand 2004;109:269–74. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao MC, Liu CY. Unusual manifestations of premenstrual syndrome. Psychiatry Clin Neurosci 2007;61:120–3. [DOI] [PubMed] [Google Scholar]
- 33.Andreou C, Syngelakis M, Karavatos A. Metformine for psychosis associated with the menstrual cycle in a patient with polycystic ovary syndrome. Arch Womens Ment Health 2008;11:387–8. [DOI] [PubMed] [Google Scholar]
- 34.Lee YT, Chou YH. Lack of efficacy of antipsychotics on premenstrual psychosis: a case report. Psychopharmacol Bull 2012;45:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleeson PC Worsley R Gavrilidis E, et al. Menstrual cycle characteristics in women with persistent schizophrenia. Aust N Z J Psychiatry 2016;50:481–7. [DOI] [PubMed] [Google Scholar]
- 36.Jalnapurkar I, Findley JC. A case of repeated mixed mood episodes with psychotic symptoms associated with the premenstrual period in a patient with polycystic ovarian syndrome. Gynecol Endocrinol 2018;34:467–9. [DOI] [PubMed] [Google Scholar]
- 37.Vengadavaradan A, Sathyanarayanan G, Kuppili PP, Bharadwaj B. Is menstrual psychosis a forgotten entity? Indian J Psychol Med 2018;40:574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahern E, Cohen D, Prior C, Raji E. Menstrual psychosis. Ir J Psychol Med 2019:1–3. [DOI] [PubMed] [Google Scholar]
- 39.Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health 2020;23:113–22. [DOI] [PubMed] [Google Scholar]
- 40.Kukopoulos A, Minnai G, Muller-Oerlinghausen B. The influence of mania and depression on the pharmacokinetics of lithium. A longitudinal single-case study. J Affect Disord 1985;8:159–66. [DOI] [PubMed] [Google Scholar]
- 41.Sothern RB, Slover GP, Morris RW. Circannual and menstrual rhythm characteristics in manic episodes and body temperature. Biol Psychiatry 1993;33:194–203. [DOI] [PubMed] [Google Scholar]
- 42.Leibenluft E, Ashman SB, Feldman-Naim S, Yonkers KA. Lack of relationship between menstrual cycle phase and mood in a sample of women with rapid cycling bipolar disorder. Biol Psychiatry 1999;46:577–80. [DOI] [PubMed] [Google Scholar]
- 43.Becker OV, Rasgon NL, Marsh WK, Glenn T, Ketter TA. Lamotrigine therapy in treatment-resistant menstrually-related rapid cycling bipolar disorder: a case report. Bipolar Disord 2004;6:435–9. [DOI] [PubMed] [Google Scholar]
- 44.Karadag F Akdeniz F Erten E, et al. Menstrually related symptom changes in women with treatment-responsive bipolar disorder. Bipolar Disord 2004;6:253–9. [DOI] [PubMed] [Google Scholar]
- 45.Rasgon N Bauer M Grof P, et al. Sex-specific self-reported mood changes by patients with bipolar disorder. J Psychiatr Res 2005;39:77–83. [DOI] [PubMed] [Google Scholar]
- 46.Shivakumar G Bernstein IH Suppes T, et al. Are bipolar mood symptoms affected by the phase of the menstrual cycle? J Womens Health (Larchmt) 2008;17:473–8. [DOI] [PubMed] [Google Scholar]
- 47.Aalouane R, Rammouz I, Elghazouani F, Aarab C, Blecha L. Hypomanic episodes during menstrual periods: bipolar II disorder? Psychiatry Clin Neurosci 2011;65:112–3. [DOI] [PubMed] [Google Scholar]
- 48.Sit D, Seltman H, Wisner KL. Menstrual effects on mood symptoms in treated women with bipolar disorder. Bipolar Disord 2011;13:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robakis TK, Holtzman J, Stemmle PG, Reynolds-May MF, Kenna HA, Rasgon NL. Lamotrigine and GABAA receptor modulators interact with menstrual cycle phase and oral contraceptives to regulate mood in women with bipolar disorder. J Affect Disord 2015;175:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Susser LC, Hermann AD. Protection against hormone-mediated mood symptoms. Arch Womens Ment Health 2017;20:355–6. [DOI] [PubMed] [Google Scholar]
- 51.Schick A. On premenstrual depression. Am J Psychother 1953;7:664–71. [DOI] [PubMed] [Google Scholar]
- 52.May RR. Mood shifts and the menstrual cycle. J Psychosom Res 1976;20:125–30. [DOI] [PubMed] [Google Scholar]
- 53.Golub S. The magnitude of premenstrual anxiety and depression. Psychosom Med 1976;38:4–12. [DOI] [PubMed] [Google Scholar]
- 54.Abramowitz ES, Baker AH, Fleischer SF. Onset of depressive psychiatric crises and the menstrual cycle. Am J Psychiatry 1982;139:475–8. [DOI] [PubMed] [Google Scholar]
- 55.Lahmeyer HW, Miller M, DeLeon-Jones F. Anxiety and mood fluctuation during the normal menstrual cycle. Psychosom Med 1982;44:183–94. [DOI] [PubMed] [Google Scholar]
- 56.O’Neil MK, Lancee WJ, Freeman SJ. Fluctuations in mood and psychological distress during the menstrual cycle. Can J Psychiatry 1984;29:373–8. [DOI] [PubMed] [Google Scholar]
- 57.Shaver JF, Woods NF. Concordance of perimenstrual symptoms across two cycles. Res Nurs Health 1985;8:313–9. [DOI] [PubMed] [Google Scholar]
- 58.Halbreich U, Endicott J, Goldstein S, Nee J. Premenstrual changes and changes in gonadal hormones. Acta Psychiatr Scand 1986;74:576–86. [DOI] [PubMed] [Google Scholar]
- 59.Chen AW, Filsinger E. Mood across the menstrual cycle and number of menstrual symptoms reported: a cross-sectional study. Can J Psychiatry 1987;32:429–32. [DOI] [PubMed] [Google Scholar]
- 60.Chisholm G, Jung SO, Cumming CE, Fox EE, Cumming DC. Premenstrual anxiety and depression: comparison of objective psychological tests with a retrospective questionnaire. Acta Psychiatr Scand 1990;81:52–7. [DOI] [PubMed] [Google Scholar]
- 61.Bancroft J, Rennie D, Warner P. Vulnerability to perimenstrual mood change: the relevance of a past history of depressive disorder. Psychosom Med 1994;56:225–31. [DOI] [PubMed] [Google Scholar]
- 62.Ross C, Coleman G, Stojanovska C. Relationship between the NEO personality inventory revised neuroticism scale and prospectively reported negative affect across the menstrual cycle. J Psychosom Obstet Gynaecol 2001;22:165–76. [DOI] [PubMed] [Google Scholar]
- 63.Abraham S, Luscombe G, Soo I. Oral contraception and cyclic changes in premenstrual and menstrual experiences. J Psychosom Obstet Gynaecol 2003;24:185–93. [DOI] [PubMed] [Google Scholar]
- 64.Alonso C, Loevinger BL, Muller D, Coe CL. Menstrual cycle influences on pain and emotion in women with fibromyalgia. J Psychosom Res 2004;57:451–8. [DOI] [PubMed] [Google Scholar]
- 65.Hartlage SA, Brandenburg DL, Kravitz HM. Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosom Med 2004;66:698–706. [DOI] [PubMed] [Google Scholar]
- 66.Hsiao MC, Hsiao CC, Liu CY. Premenstrual symptoms and premenstrual exacerbation in patients with psychiatric disorders. Psychiatry Clin Neurosci 2004;58:186–90. [DOI] [PubMed] [Google Scholar]
- 67.Gonda X, Telek T, Juhász G, Lazary J, Vargha A, Bagdy G. Patterns of mood changes throughout the reproductive cycle in healthy women without premenstrual dysphoric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1782–8. [DOI] [PubMed] [Google Scholar]
- 68.Harvey AT, Hitchcock CL, Prior JC. Ovulation disturbances and mood across the menstrual cycles of healthy women. J Psychosom Obstet Gynaecol 2009;30:207–14. [DOI] [PubMed] [Google Scholar]
- 69.Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology 2011;36:68–76. [DOI] [PubMed] [Google Scholar]
- 70.Walder DJ, Statucka M, Daly MP, Axen K, Haber M. Biological sex and menstrual cycle phase modulation of cortisol levels and psychiatric symptoms in a non-clinical sample of young adults. Psychiatry Res 2012;197:314–21. [DOI] [PubMed] [Google Scholar]
- 71.Prasad A Schisterman EF Schliep KC, et al. Depressive symptoms and their relationship with endogenous reproductive hormones and sporadic anovulation in premenopausal women. Ann Epidemiol 2014;24:920–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobs EG Holsen LM Lancaster K, et al. 17beta-estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology 2015;40:566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamstra DA, de Kloet ER, de Rover M, Van der Does W. Oral contraceptives positively affect mood in healthy PMS-free women: a longitudinal study. J Psychosom Res 2017;103:119–26. [DOI] [PubMed] [Google Scholar]
- 74.Hengartner MP Kruger THC Geraedts K, et al. Negative affect is unrelated to fluctuations in hormone levels across the menstrual cycle: evidence from a multisite observational study across two successive cycles. J Psychosom Res 2017;99:21–7. [DOI] [PubMed] [Google Scholar]
- 75.Lundin C Danielsson KG Bixo M, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle—a double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology 2017;76:135–43. [DOI] [PubMed] [Google Scholar]
- 76.Mulligan EM, Nelson BD, Infantolino ZP, Luking KR, Sharma R, Hajcak G. Effects of menstrual cycle phase on electrocortical response to reward and depressive symptoms in women. Psychophysiology 2018;55:e13268. [DOI] [PubMed] [Google Scholar]
- 77.Dan R Canetti L Keadan T, et al. Sex differences during emotion processing are dependent on the menstrual cycle phase. Psychoneuroendocrinology 2019;100:85–95. [DOI] [PubMed] [Google Scholar]
- 78.Huerta-Franco MR, Ulloa-Aguirre A, Geronimo AL, Capaccione K, Marquez-Romero JM. Mood variations and personality traits in patients with epilepsy over the course of their menstrual cycle. Epilepsy Behav 2020;105:106990. [DOI] [PubMed] [Google Scholar]
- 79.Fourestie V de Lignieres B Roudot-Thoraval F, et al. Suicide attempts in hypo-oestrogenic phases of the menstrual cycle. Lancet 1986;2:1357–60. [DOI] [PubMed] [Google Scholar]
- 80.Targum SD, Caputo KP, Ball SK. Menstrual cycle phase and psychiatric admissions. J Affect Disord 1991;22:49–53. [DOI] [PubMed] [Google Scholar]
- 81.Baca-García E, Sánchez-González A, González Diaz-Corralero P, González García I, de Leon J. Menstrual cycle and profiles of suicidal behaviour. Acta Psychiatr Scand 1998;97:32–5. [DOI] [PubMed] [Google Scholar]
- 82.Baca-García E, Díaz-Sastre C, Saiz-Ruiz J, de Leon J. Influence of psychiatric diagnoses on the relationship between suicide attempts and the menstrual cycle. Psychosom Med 2001;63:509–10. [DOI] [PubMed] [Google Scholar]
- 83.Baca-Garcia E, Diaz-Sastre C, Ceverino A, Saiz-Ruiz J, Diaz FJ, de Leon J. Association between the menses and suicide attempts: a replication study. Psychosom Med 2003;65:237–44. [DOI] [PubMed] [Google Scholar]
- 84.Baca-Garcia E Vaquero C Diaz-Sastre C, et al. A pilot study on a gene-hormone interaction in female suicide attempts. Eur Arch Psychiatry Clin Neurosci 2003;253:281–5. [DOI] [PubMed] [Google Scholar]
- 85.Caykoylu A, Capoglu I, Ozturk I. The possible factors affecting suicide attempts in the different phases of the menstrual cycle. Psychiatry Clin Neurosci 2004;58:460–4. [DOI] [PubMed] [Google Scholar]
- 86.Lee DO. Menstrually related self-injurious behavior in adolescents with autism. J Am Acad Child Adolesc Psychiatry 2004;43:1193. [DOI] [PubMed] [Google Scholar]
- 87.Sein Anand J, Chodorowski Z, Ciechanowicz R, Wisniewski M, Pankiewicz P. The relationship between suicidal attempts and menstrual cycle in women. Przegl Lek 2005;62:431–3. [PubMed] [Google Scholar]
- 88.Dogra TD Leenaars AA Raintji R, et al. Menstruation and suicide: an exploratory study. Psychol Rep 2007;101:430–4. [DOI] [PubMed] [Google Scholar]
- 89.Leenaars AA, Dogra TD, Girdhar S, Dattagupta S, Leenaars L. Menstruation and suicide: a histopathological study. Crisis 2009;30:202–7. [DOI] [PubMed] [Google Scholar]
- 90.Baca-Garcia E Diaz-Sastre C Ceverino A, et al. Suicide attempts among women during low estradiol/low progesterone states. J Psychiatr Res 2010;44:209–14. [DOI] [PubMed] [Google Scholar]
- 91.Mousavi SG, Bateni S, Maracy MR, Mardanian F, Mousavi SH. Recurrent suicide attempt and female hormones. Adv Biomed Res 2014;3:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Behera C Sikary AK Mridha AR, et al. Association of menstruation cycle with completed suicide: a hospital-based case-control study. Arch Womens Ment Health 2019;22:771–7. [DOI] [PubMed] [Google Scholar]
- 93.Papadopoulou A Efstathiou V Christodoulou C, et al. Clinical and psychometric features of psychiatric patients after a suicide attempt in relation with menstrual cycle phases. Arch Womens Ment Health 2019;22:605–11. [DOI] [PubMed] [Google Scholar]
- 94.Gottschalk LA, Kaplan SM, Gleser GC, Winget CM. Variations in magnitude of emotion: a method applied to anxiety and hostility during phases of the menstrual cycle. Psychosom Med 1962;24:300–11. [DOI] [PubMed] [Google Scholar]
- 95.Moos RH Kopell BS Melges FT, et al. Fluctuations in symptoms and moods during the menstrual cycle. J Psychosom Res 1969;13:37–44. [DOI] [PubMed] [Google Scholar]
- 96.Ivey ME, Bardwick JM. Patterns of affective fluctuation in the menstrual cycle. Psychosom Med 1968;30:336–45. [DOI] [PubMed] [Google Scholar]
- 97.Paige KE. Effects of oral contraceptives on affective fluctuations associated with the menstrual cycle. Psychosom Med 1971;33:515–37. [DOI] [PubMed] [Google Scholar]
- 98.Marinari KT, Leshner AI, Doyle MP. Menstrual cycle status and adrenocortical reactivity to psychological stress. Psychoneuroendocrinology 1976;1:213–8. [DOI] [PubMed] [Google Scholar]
- 99.Veith JL, Anderson J, Slade SA, Thompson P, Laugel GR, Getzlaf S. Plasma β-endorphin, pain thresholds and anxiety levels across the human menstrual cycle. Physiol Behav 1984;32:31–4. [DOI] [PubMed] [Google Scholar]
- 100.van den Akker O, Steptoe A. The pattern and prevalence of symptoms during the menstrual cycle. Br J Psychiatry 1985;147:164–9. [DOI] [PubMed] [Google Scholar]
- 101.Morse CA, Dennerstein L, Varnavides K, Burrows GD. Menstrual cycle symptoms: comparison of a non-clinical sample with a patient group. J Affect Disord 1988;14:41–50. [DOI] [PubMed] [Google Scholar]
- 102.Gómez-Amor J, Martínez-Selva JM, Román F, Zamora S, Sastre JF. Electrodermal activity, hormonal levels and subjective experience during the menstrual cycle. Biol Psychol 1990;30:125–39. [DOI] [PubMed] [Google Scholar]
- 103.Lane T, Francis A. Premenstrual symptomatology, locus of control, anxiety and depression in women with normal menstrual cycles. Arch Womens Ment Health 2003;6:127–38. [DOI] [PubMed] [Google Scholar]
- 104.Davydov DM, Shapiro D, Goldstein IB, Chicz-DeMet A. Moods in everyday situations: effects of menstrual cycle, work, and stress hormones. J Psychosom Res 2005;58:343–9. [DOI] [PubMed] [Google Scholar]
- 105.Hlavacova N, Wawruch M, Tisonova J, Jezova D. Neuroendocrine activation during combined mental and physical stress in women depends on trait anxiety and the phase of the menstrual cycle. Ann N Y Acad Sci 2008;1148:520–5. [DOI] [PubMed] [Google Scholar]
- 106.Childs E, Dlugos A, De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology 2010;47:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lustyk MK, Olson KC, Gerrish WG, Holder A, Widman L. Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biol Psychol 2010;83:84–92. [DOI] [PubMed] [Google Scholar]
- 108.Lustyk MKB, Douglas HAC, Shilling EA, Woods NF. Hemodynamic and psychological responses to laboratory stressors in women: assessing the roles of menstrual cycle phase, premenstrual symptomatology, and sleep characteristics. Int J Psychophysiol 2012;86:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Armbruster D, Strobel A, Kirschbaum C, Brocke B. The impact of sex and menstrual cycle on the acoustic startle response. Behav Brain Res 2014;274:326–33. [DOI] [PubMed] [Google Scholar]
- 110.Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015;59:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mahon JN, Rohan KJ, Nillni YI, Zvolensky MJ. The role of perceived control over anxiety in prospective symptom reports across the menstrual cycle. Arch Womens Ment Health 2015;18:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manikandan S, Nillni YI, Zvolensky MJ, Rohan KJ, Carkeek KR, Leyro TM. The role of emotion regulation in the experience of menstrual symptoms and perceived control over anxiety-related events across the menstrual cycle. Arch Womens Ment Health 2016;19:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Özer Kaya D, Toprak Çelenay Ş. Fluctuations of state anxiety, spinal structure, and postural stabilityacross the menstrual cycle in active women. Turk J Med Sci 2016:977–84. [DOI] [PubMed] [Google Scholar]
- 114.Welz A, Huffziger S, Reinhard I, Alpers GW, Ebner-Priemer U, Kuehner C. Anxiety and rumination moderate menstrual cycle effects on mood in daily life. Women Health 2016;56:540–60. [DOI] [PubMed] [Google Scholar]
- 115.Villada C, Espin L, Hidalgo V, Rubagotti S, Sgoifo A, Salvador A. The influence of coping strategies and behavior on the physiological response to social stress in women: the role of age and menstrual cycle phase. Physiol Behav 2017;170:37–46. [DOI] [PubMed] [Google Scholar]
- 116.Reynolds TA Makhanova A Marcinkowska UM, et al. Progesterone and women’s anxiety across the menstrual cycle. Horm Behav 2018;102:34–40. [DOI] [PubMed] [Google Scholar]
- 117.Hahn AC, DeBruine LM, Pesce LA, Diaz A, Aberson CL, Jones BC. Does women’s anxious jealousy track changes in steroid hormone levels? Psychoneuroendocrinology 2020;113:104553. [DOI] [PubMed] [Google Scholar]
- 118.Shayani DR, Arditte Hall KA, Isley BC, Rohan KJ, Zvolensky MJ, Nillni YI. The role of health anxiety in the experience of perceived stress across the menstrual cycle. Anxiety Stress Coping 2020;33:706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prado RCR, Silveira R, Kilpatrick MW, Pires FO, Asano RY. The effect of menstrual cycle and exercise intensity on psychological and physiological responses in healthy eumenorrheic women. Physiol Behav 2021;232:113290. [DOI] [PubMed] [Google Scholar]
- 120.McLeod DR, Hoehn-Saric R, Foster GV, Hipsley PA. The influence of premenstrual syndrome on ratings of anxiety in women with generalized anxiety disorder. Acta Psychiatr Scand 1993;88:248–51. [DOI] [PubMed] [Google Scholar]
- 121.Li SH, Lloyd AR, Graham BM. Physical and mental fatigue across the menstrual cycle in women with and without generalised anxiety disorder. Horm Behav 2020;118:104667. [DOI] [PubMed] [Google Scholar]
- 122.van Veen JF, Jonker BW, van Vliet IM, Zitman FG. The effects of female reproductive hormones in generalized social anxiety disorder. Int J Psychiatry Med 2009;39:283–95. [DOI] [PubMed] [Google Scholar]
- 123.Maner JK, Miller SL. Hormones and social monitoring: menstrual cycle shifts in progesterone underlie women’s sensitivity to social information. Evol Hum Behav 2014;35:9–16. [Google Scholar]
- 124.Wang JX, Zhuang JY, Fu L, Lei Q. Social orientation in the luteal phase: increased social feedback sensitivity, inhibitory response, interpersonal anxiety and cooperation preference. Evol Psychol 2021;19:1474704920986866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cameron OG, Kuttesch D, McPhee K, Curtis GC. Menstrual fluctuation in the symptoms of panic anxiety. J Affect Disord 1988;15:169–74. [DOI] [PubMed] [Google Scholar]
- 126.Stein MB, Schmidt PJ, Rubinow DR, Uhde TW. Panic disorder and the menstrual cycle: panic disorder patients, healthy control subjects, and patients with premenstrual syndrome. Am J Psychiatry 1989;146:1299–303. [DOI] [PubMed] [Google Scholar]
- 127.Cook BL, Noyes R, Jr., Garvey MJ, Beach V, Sobotka J, Chaudhry D. Anxiety and the menstrual cycle in panic disorder. J Affect Disord 1990;19:221–6. [DOI] [PubMed] [Google Scholar]
- 128.Kaspi SP, Otto MW, Pollack MH, Eppinger S, Rosenbaum JF. Premenstrual exacerbation of symptoms in women with panic disorder. J Anxiety Disord 1994;8:131–8. [Google Scholar]
- 129.Perna G, Brambilla F, Arancio C, Bellodi L. Menstrual cycle-related sensitivity to 35% CO2 in panic patients. Biol Psychiatry 1995;37:528–32. [DOI] [PubMed] [Google Scholar]
- 130.Sigmon ST, Fink CM, Rohan KJ, Hotovy LA. Anxiety sensitivity and menstrual cycle reactivity: psychophysiological and self-report differences. J Anxiety Disord 1996;10:393–410. [Google Scholar]
- 131.Sigmon ST, Dorhofer DM, Rohan KJ, Boulard NE. The impact of anxiety sensitivity, bodily expectations, and cultural beliefs on menstrual symptom reporting: a test of the menstrual reactivity hypothesis. J Anxiety Disord 2000;14:615–33. [DOI] [PubMed] [Google Scholar]
- 132.Sigmon ST, Dorhofer DM, Rohan KJ, Hotovy LA, Boulard NE, Fink CM. Psychophysiological, somatic, and affective changes across the menstrual cycle in women with panic disorder. J Consult Clin Psychol 2000;68:425–31. [DOI] [PubMed] [Google Scholar]
- 133.Dorhofer DM, Sigmon ST. Physiological and psychological reactivity in women with asthma: the effects of anxiety and menstrual cycle phase. Behav Res Ther 2002;40:3–17. [DOI] [PubMed] [Google Scholar]
- 134.Nillni YI, Rohan KJ, Zvolensky MJ. The role of menstrual cycle phase and anxiety sensitivity in catastrophic misinterpretation of physical symptoms during a CO(2) challenge. Arch Womens Ment Health 2012;15:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nillni YI, Rohan KJ, Mahon JN, Pineles SL, Zvolensky MJ. The role of anxiety sensitivity in the experience of menstrual-related symptoms reported via daily diary. Psychiatry Res 2013;210:564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Williams KE, Koran LM. Obsessive-compulsive disorder in pregnancy, the puerperium, and the premenstruum. J Clin Psychiatry 1997;58:330–4; quiz 5–6. [DOI] [PubMed] [Google Scholar]
- 137.Labad J, Menchon JM, Alonso P, Segalas C, Jimenez S, Vallejo J. Female reproductive cycle and obsessive-compulsive disorder. J Clin Psychiatry 2005;66:428–35; quiz 546. [DOI] [PubMed] [Google Scholar]
- 138.Vulink NC, Denys D, Bus L, Westenberg HG. Female hormones affect symptom severity in obsessive-compulsive disorder. Int Clin Psychopharmacol 2006;21:171–5. [DOI] [PubMed] [Google Scholar]
- 139.Mulligan EM, Hajcak G, Klawohn J, Nelson B, Meyer A. Effects of menstrual cycle phase on associations between the error-related negativity and checking symptoms in women. Psychoneuroendocrinology 2019;103:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keuthen NJ, O’Sullivan RL, Hayday CF, Peets KE, Jenike MA, Baer L. The relationship of menstrual cycle and pregnancy to compulsive hairpulling. Psychother Psychosom 1997;66:33–7. [DOI] [PubMed] [Google Scholar]
- 141.Bryant RA, Felmingham KL, Silove D, Creamer M, O’Donnell M, McFarlane AC. The association between menstrual cycle and traumatic memories. J Affect Disord 2011;131:398–401. [DOI] [PubMed] [Google Scholar]
- 142.Ferree NK, Kamat R, Cahill L. Influences of menstrual cycle position and sex hormone levels on spontaneous intrusive recollections following emotional stimuli. Conscious Cogn 2011;20:1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Soni M, Curran VH, Kamboj SK. Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiol Learn Mem 2013;104:32–8. [DOI] [PubMed] [Google Scholar]
- 144.Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem 2014;116:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nillni YI, Pineles SL, Patton SC, Rouse MH, Sawyer AT, Rasmusson AM. Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress 2015;28:1–7. [DOI] [PubMed] [Google Scholar]
- 146.Pineles SL Blumenthal TD Curreri AJ, et al. Prepulse inhibition deficits in women with PTSD. Psychophysiology 2016;53:1377–85. [DOI] [PubMed] [Google Scholar]
- 147.Pineles SL Nillni YI King MW, et al. Extinction retention and the menstrual cycle: different associations for women with posttraumatic stress disorder. J Abnorm Psychol 2016;125:349–55. [DOI] [PubMed] [Google Scholar]
- 148.Pineles SL Nillni YI Pinna G, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 2018;93:133–41. [DOI] [PubMed] [Google Scholar]
- 149.Nillni YI, Arditte Hall KA, Langdon KJ, Pineles SL. Examination of the stability of the anxiety sensitivity index across the menstrual cycle in trauma-exposed women with and without PTSD. Anxiety Stress Coping 2020;33:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pineles SL Nillni YI Pinna G, et al. Associations between PTSD-related extinction retention deficits in women and plasma steroids that modulate brain GABAA and NMDA receptor activity. Neurobiol Stress 2020;13:100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arditte Hall KA DeLane SE Anderson GM, et al. Plasma gamma-aminobutyric acid (GABA) levels and posttraumatic stress disorder symptoms in trauma-exposed women: a preliminary report. Psychopharmacology (Berl) 2021;238:1541–52. [DOI] [PubMed] [Google Scholar]
- 152.Leon GR, Phelan PW, Kelly JT, Patten SR. The symptoms of bulimia and the menstrual cycle. Psychosom Med 1986;48:415–22. [DOI] [PubMed] [Google Scholar]
- 153.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry 1987;144:1592–5. [DOI] [PubMed] [Google Scholar]
- 154.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med 2003;33:51–60. [DOI] [PubMed] [Google Scholar]
- 155.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med 2007;37:131–41. [DOI] [PubMed] [Google Scholar]
- 156.Gonda X, Lazáry J, Telek T, Pap D, Kátai Z, Bagdy G. Mood parameters and severe physical symptoms of the female reproductive cycle. Neuropsychopharmacol Hung 2008;10:91–6. [PubMed] [Google Scholar]
- 157.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med 2008;38:1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McVay MA, Copeland AL, Geiselman PJ. Eating disorder pathology and menstrual cycle fluctuations in eating variables in oral contraceptive users and non-users. Eat Behav 2011;12:49–55. [DOI] [PubMed] [Google Scholar]
- 159.Schoofs N, Chen F, Braunig P, Stamm T, Kruger S. Binge eating disorder and menstrual cycle in unmedicated women with bipolar disorder. J Affect Disord 2011;129:75–8. [DOI] [PubMed] [Google Scholar]
- 160.Klump KL Keel PK Racine SE, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol 2013;122:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klump KL Racine SE Hildebrandt B, et al. Ovarian hormone influences on dysregulated eating: a comparison of associations in women with versus without binge episodes. Clin Psychol Sci 2014;2:545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hildebrandt BA Racine SE Keel PK, et al. The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. Int J Eat Disord 2015;48:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Baker JH, Eisenlohr-Moul T, Wu YK, Schiller CE, Bulik CM, Girdler SS. Ovarian hormones influence eating disorder symptom variability during the menopause transition: a pilot study. Eat Behav 2019;35:101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fowler N Keel PK Burt SA, et al. Associations between ovarian hormones and emotional eating across the menstrual cycle: do ovulatory shifts in hormones matter? Int J Eat Disord 2019;52:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.DeSoto MC, Geary DC, Hoard MK, Sheldon MS, Cooper L. Estrogen fluctuations, oral contraceptives and borderline personality. Psychoneuroendocrinology 2003;28:751–66. [DOI] [PubMed] [Google Scholar]
- 166.Eisenlohr-Moul TA, DeWall CN, Girdler SS, Segerstrom SC. Ovarian hormones and borderline personality disorder features: preliminary evidence for interactive effects of estradiol and progesterone. Biol Psychol 2015;109:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eisenlohr-Moul TA, Schmalenberger KM, Owens SA, Peters JR, Dawson DN, Girdler SS. Perimenstrual exacerbation of symptoms in borderline personality disorder: evidence from multilevel models and the Carolina Premenstrual Assessment Scoring System. Psychol Med 2018;48:2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peters JR, Owens SA, Schmalenberger KM, Eisenlohr-Moul TA. Differential effects of the menstrual cycle on reactive and proactive aggression in borderline personality disorder. Aggress Behav 2020;46:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Griffin ML, Mello NK, Mendelson JH, Lex BW. Alcohol use across the menstrual cycle among marihuana users. Alcohol 1987;4:457–62. [DOI] [PubMed] [Google Scholar]
- 170.Charette L, Tate DL, Wilson A. Alcohol consumption and menstrual distress in women at higher and lower risk for alcoholism. Alcohol Clin Exp Res 1990;14:152–7. [DOI] [PubMed] [Google Scholar]
- 171.DiMatteo J, Reed SC, Evans SM. Alcohol consumption as a function of dietary restraint and the menstrual cycle in moderate/heavy (“at-risk”) female drinkers. Eat Behav 2012;13:285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martel MM, Eisenlohr-Moul T, Roberts B. Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. J Abnorm Psychol 2017;126:1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Joyce KM Hudson A O’Connor R, et al. Changes in coping and social motives for drinking and alcohol consumption across the menstrual cycle. Depress Anxiety 2018;35:313–20. [DOI] [PubMed] [Google Scholar]
- 174.Hayaki J Holzhauer CG Epstein EE, et al. Menstrual cycle phase, alcohol consumption, alcohol cravings, and mood among women in outpatient treatment for alcohol use disorder. Psychol Addict Behav 2020;34:680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holzhauer CG, Wemm SE, Wulfert E, Cao ZT. Fluctuations in progesterone moderate the relationship between daily mood and alcohol use in young adult women. Addict Behav 2020;101:106146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marks JL, Hair CS, Klock SC, Ginsburg BE, Pomerleau CS. Effects of menstrual phase on intake of nicotine, caffeine, and alcohol and nonprescribed drugs in women with late luteal phase dysphoric disorder. J Subst Abuse 1994;6:235–43. [DOI] [PubMed] [Google Scholar]
- 177.DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. Addict Behav 1995;20:335–43. [DOI] [PubMed] [Google Scholar]
- 178.Perkins KA Levine M Marcus M, et al. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol 2000;68:176–80. [DOI] [PubMed] [Google Scholar]
- 179.Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior, dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 2000;25:677–91. [DOI] [PubMed] [Google Scholar]
- 180.Carpenter MJ, Saladin ME, Leinbach AS, Larowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: a pilot feasibility study. J Womens Health (Larchmt) 2008;17:293–301. [DOI] [PubMed] [Google Scholar]
- 181.Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe SD, Upadhyaya HP. Menstrual cycle and cue reactivity in women smokers. Nicotine Tob Res 2010;12:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend 2011;114:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ. Association between ovarian hormones and smoking behavior in women. Exp Clin Psychopharmacol 2012;20:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sakai H, Ohashi K. Association of menstrual phase with smoking behavior, mood and menstrual phase-associated symptoms among young Japanese women smokers. BMC Womens Health 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology 2014;39:1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Goletiani NV, Siegel AJ, Lukas SE, Hudson JI. The effects of smoked nicotine on measures of subjective states and hypothalamic-pituitary-adrenal axis hormones in women during the follicular and luteal phases of the menstrual cycle. J Addict Med 2015;9:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saladin ME Wray JM Carpenter MJ, et al. Menstrual cycle phase effects in the gender dimorphic stress cue reactivity of smokers. Nicotine Tob Res 2015;17:607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carlson SC, Allen AM, Allen SS, al’Absi M. Differences in mood and cortisol by menstrual phase during acute smoking abstinence: a within-subject comparison. Exp Clin Psychopharmacol 2017;25:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pang RD, Liautaud MM, Kirkpatrick MG, Huh J, Monterosso J, Leventhal AM. Ovarian hormones and transdermal nicotine administration independently and synergistically suppress tobacco withdrawal symptoms and smoking reinstatement in the human laboratory. Neuropsychopharmacology 2018;43:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 1999;7:274–83. [DOI] [PubMed] [Google Scholar]
- 191.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. [DOI] [PubMed] [Google Scholar]
- 192.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 2006;31:659–74. [DOI] [PubMed] [Google Scholar]
- 193.Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav 2007;86:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Horm Behav 2011;59:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Milivojevic V, Fox HC, Sofuoglu M, Covault J, Sinha R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology 2016;65:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grieger JA, Norman RJ. Menstrual cycle length and patterns in a global cohort of women using a mobile phone app: retrospective cohort study. J Med Internet Res 2020;22:e17109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs 2006;35:376–84. [DOI] [PubMed] [Google Scholar]
- 198.Abdulla SH, Bouchard TP, Leiva RA, Boyle P, Iwaz J, Ecochard R. Hormonal predictors of abnormal luteal phases in normally cycling women. Front Public Health 2018;6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: the sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril 1994;62:54–62. [DOI] [PubMed] [Google Scholar]
- 200.Girgus JS, Yang K, Ferri CV. The gender difference in depression: are elderly women at greater risk for depression than elderly men? Geriatrics (Basel) 2017;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998;338:209–16. [DOI] [PubMed] [Google Scholar]
- 202.Schmidt H. The use of progesterone in the treatment of postpartum psychosis. JAMA 1943;121:190–2. [Google Scholar]
- 203.Sakaki M, Mather M. How reward and emotional stimuli induce different reactions across the menstrual cycle. Soc Personal Psychol Compass 2012;6:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roy A, Segal NL. Suicidal behavior in twins: a replication. J Affect Disord 2001;66:71–4. [DOI] [PubMed] [Google Scholar]
- 205.Ruderfer DM Walsh CG Aguirre MW, et al. Significant shared heritability underlies suicide attempt and clinically predicted probability of attempting suicide. Mol Psychiatry 2020;25:2422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sokolowski M, Wasserman J, Wasserman D. Genome-wide association studies of suicidal behaviors: a review. Eur Neuropsychopharmacol 2014;24:1567–77. [DOI] [PubMed] [Google Scholar]
- 207.Statham DJ Heath AC Madden PA, et al. Suicidal behaviour: an epidemiological and genetic study. Psychol Med 1998;28:839–55. [DOI] [PubMed] [Google Scholar]
- 208.Voracek M, Loibl LM. Genetics of suicide: a systematic review of twin studies. Wien Klin Wochenschr 2007;119:463–75. [DOI] [PubMed] [Google Scholar]
- 209.Kenna GA Roder-Hanna N Leggio L, et al. Association of the 5-HTT gene-linked promoter region (5-HTTLPR) polymorphism with psychiatric disorders: review of psychopathology and pharmacotherapy. Pharmgenomics Pers Med 2012;5:19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Contreras CM, Marván ML, Alcalá-Herrera V, Yeyha A. Relations between anxiety, psychophysiological variables and menstrual cycle in healthy women. Bol Estud Med Biol 1989;37:50–6. [PubMed] [Google Scholar]
- 211.Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clin Psychol Rev 2011;31:1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gorman JM Papp LA Martinez J, et al. High-dose carbon dioxide challenge test in anxiety disorder patients. Biol Psychiatry 1990;28:743–57. [DOI] [PubMed] [Google Scholar]
- 213.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry 1993;50:306–17. [DOI] [PubMed] [Google Scholar]
- 214.Blumenthal TD, Elden A, Flaten MA. A comparison of several methods used to quantify prepulse inhibition of eyeblink responding. Psychophysiology 2004;41:326–32. [DOI] [PubMed] [Google Scholar]
- 215.Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology 2011;36:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weinberger AH Smith PH Allen SS, et al. Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res 2015;17:407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Aboujaoude E, Gamel N, Koran LM. A case of kleptomania correlating with premenstrual dysphoria. J Clin Psychiatry 2004;65:725–6. [DOI] [PubMed] [Google Scholar]
- 218.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A 2007;104:2465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ossewaarde L, van Wingen GA, Kooijman SC, Backstrom T, Fernandez G, Hermans EJ. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Soc Cogn Affect Neurosci 2011;6:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798–811. [DOI] [PubMed] [Google Scholar]
- 221.Lentz MJ, Woods N, Heitkemper M, Mitchell E, Henker R, Shaver J. Ovarian steroids and premenstrual symptoms: a comparison of group differences and intra-individual patterns. Res Nurs Health 2007;30:238–49. [DOI] [PubMed] [Google Scholar]
- 222.Kimball A Dichtel LE Nyer MB, et al. The allopregnanolone to progesterone ratio across the menstrual cycle and in menopause. Psychoneuroendocrinology 2020;112:104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E. When do we eat? Ingestive behavior, survival, and reproductive success. Horm Behav 2013;64:702–28. [DOI] [PubMed] [Google Scholar]
- 224.Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157:924–30. [DOI] [PubMed] [Google Scholar]
- 225.Schmidt PJ Ben Dor R Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry 2015;72:714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schmidt PJ Martinez PE Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry 2017;174:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schmalenberger KM Tauseef HA Barone JC, et al. How to study the menstrual cycle: practical tools and recommendations. Psychoneuroendocrinology 2021;123:104895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dang J, King KM, Inzlicht M. Why are self-report and behavioral measures weakly correlated? Trends Cogn Sci 2020;24:267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dougherty DM, Bjork JM, Moeller FG, Swann AC. The influence of menstrual-cycle phase on the relationship between testosterone and aggression. Physiol Behav 1997;62:431–5. [DOI] [PubMed] [Google Scholar]
- 230.Eriksson E, Sundblad C, Lisjö P, Modigh K, Andersch B. Serum levels of androgens are higher in women with premenstrual irritability and dysphoria than in controls. Psychoneuroendocrinology 1992;17:195–204. [DOI] [PubMed] [Google Scholar]
- 231.Peters JR, Eisenlohr-Moul TA. Ovarian hormones as a source of fluctuating biological vulnerability in borderline personality disorder. Curr Psychiatry Rep 2019;21:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol 2007;17:163–70. [DOI] [PubMed] [Google Scholar]


