Abstract
The human vaginal microbiota is a critical determinant of vaginal health. These communities live in close association with the vaginal epithelium and rely on host tissues for resources. Although often dominated by lactobacilli, the vaginal microbiota is frequently comprised of a collection of facultative and obligate anaerobes. The prevalence of these communities with a paucity of Lactobacillus varies among women and epidemiological studies have associated them with an increased risk of adverse health outcomes. The mechanisms that drive these associations have yet to be described in detail with few studies establishing causative relationships. Here, we review our current understanding of the vaginal microbiota and its connection with host health. We center our discussion around the biology of the vaginal microbiota when Lactobacillus species are dominant versus when they are not, including host factors that are implicated in shaping these microbial communities and the resulting adverse health outcomes. We discuss current approaches to modulate the vaginal microbiota, including probiotics and vaginal microbiome transplants, and argue that novel model systems of the cervicovaginal environment that incorporate the vaginal microbiota are needed to progress from association to mechanism and this will prove invaluable for future research.
Introduction
The microbial communities that inhabit the human vagina are unique. Unlike the relatively diverse and even communities found at other body sites1, the vaginal microbiota of reproductive-age cisgender women is often dominated by single species of Lactobacillus2–4. This Lactobacillus-dominant configuration was first reported in 1892 by Donderlein5 and has long been considered to be a hallmark of vaginal health6–9. The production of lactic acid as a fermentation end-product by Lactobacillus spp. lowers vaginal pH (~4.0) and is thought to constrain the growth of many pathogenic microbes10,11 and has a beneficial effect on the host epithelium, such as immune modulation12,13. However, around 25% of North American women have communities that are not dominated by Lactobacillus spp. and are instead comprised of a more proportionally even collection of obligate and facultative anaerobes (e.g. species in the genera Gardnerella, Prevotella, Atopobium, Sneathia, Megasphaera, Peptoniphilus)2,4,14–17. These women are often diagnosed with bacterial vaginosis (BV), a common vaginal condition poorly characterized as a dysbiosis of the vaginal microbiota18,19. Many of these women do not report experiencing adverse vaginal symptoms, for example odor and discharge, and appear to be otherwise “healthy” upon gynecological examination2,14,20,21. Epidemiological studies have linked the presence of these non-Lactobacillus-dominant communities with increased risk for adverse health outcomes including sexually transmitted infection (STI) acquisition22–25 and spontaneous preterm birth26–36, indicating that they may be less protective, hence non-optimal37. The mechanistic underpinnings of these epidemiological associations have yet to be described in detail. Here, we discuss our current understanding of the vaginal microbiota, how these communities interact with host tissues, and propose the next steps on the path towards a deeper understanding of their relationship to health.
This review is focused on the vaginal microbiota of cisgender female individuals, primarily of reproductive age. A brief discussion on the vaginal microbiota of premenarchal girls and postmenopausal women is included and highlights gaps in our knowledge of these age groups. We know comparably little about the vaginal microbiota of other individuals with a vagina, including transgender individuals. This topic was reviewed recently in Krakowsky, et al.38. More study is needed to comprehensively characterize these microbial communities and their relationships with health.
Composition of the vaginal microbiota
Advances in molecular biology and DNA sequencing have enabled the high throughput characterization of the taxonomic composition of the vaginal microbiota2,39. Composition is often established via sequencing of 16S rRNA gene amplicons, although others have utilized sequencing of cpn60 gene amplicons40, or a battery of taxon-specific qPCR assays41. Although the bulk of these data describe the vaginal microbiota of reproductive age North American women, a growing number of studies have examined women from other regions15,25,42–48. Most reproductive age women have a vaginal microbiota whose taxonomic composition resembles one of a limited number of configurations termed community state types (CSTs; also referred to as vaginotypes or cervicotypes, see McKinnon, et al.37). These configurations can be represented by five CSTs, four of which are dominated by single species of Lactobacillus (CST I-L. crispatus, CST II-L. gasseri, CST III-L. iners, CST V-L. jensenii). A fifth configuration, CST IV, represents the more proportionally even collection of facultative and obligate anaerobes. The phylotypes common to CST IV include, among others: Gardnerella, Atopobium, Prevotella, Candidatus Lachnocurva vaginae (formerly BVAB149), Sneathia, Peptoniphilus, Finegoldia, and Megasphaera39,50. These are largely fastidious bacteria that are either difficult to cultivate, or so far uncultivatable (e.g. Ca. L. vaginae49). CSTs I, III, and IV are the most prevalent and account for around 90% of reproductive-age women2. Larger studies have employed finer resolution classification schemes that split the five CSTs into subtypes39, most of which distinguish between variations of CST IV and describe uncommon communities (e.g. Bifidobacterium or Streptococcus dominated communities). Although the CST approach does simplify community composition, it continues to be an important framework for the study of the vaginal microbiota.
The term “Community State Type” was originally meant to convey its representation of the taxonomic composition at a single timepoint2. This distinction is important because the vaginal microbiota of some women has been documented to vary, including shifts in CST51,52. Changes in composition are sometimes explicable, occurring at the onset of menstruation or following unprotected vaginal intercourse. Menstruation is accompanied by biophysical and hormonal fluctuations which impacts host physiology and thus the microbial communities present. Unprotected vaginal intercourse introduces semen into the vagina, an alkaline substance that temporarily raises vaginal pH53, and has the potential to bring new microbial species and strains into the community from the penile microbiota54. Other changes in the vaginal microbiota cannot be obviously attributed to a specific factor and may be the result of fluctuations in host physiology, competitive interactions between members of the community, bacteriophage activity, ecological drift, or some other mechanisms55. The vaginal microbiota of some individuals, however, have been shown to not demonstrate temporal variation and instead maintain their community composition over several menstrual cycles51. It is not clear if this stability is a property of the microbiota, host physiology, or a combination of the two. Understanding the factors that drive temporal variation in the vaginal microbiota will be critical in the development of strategies to modulate these communities.
The vaginal microenvironment
The estrogenized vaginal epithelium consists of several squamous layers, with a superficial outermost layer overlying an intermediate, parabasal, and basal layer beneath56 (Fig. 1). The upper layer is composed of flattened, dead cells that have undergone cornification, offering a physical protective barrier57. This barrier also serves as an immune junction, separate from that of the cervix. While immune cells are present at the transformation zone of the cervix58, vaginal mucosal tissue harbors few T cells and antigen presenting cells (APCs) under normal conditions but displays increased numbers in response to inflammatory triggers. Additionally, the vaginal mucosal immune profile fluctuates with hormonal cycles, such that the highest levels of IgA and IgG are present just prior to ovulation, with lower levels at the time of menses59.
Fig. 1 |. Effect of the menstrual cycle on the vaginal microenvironment.
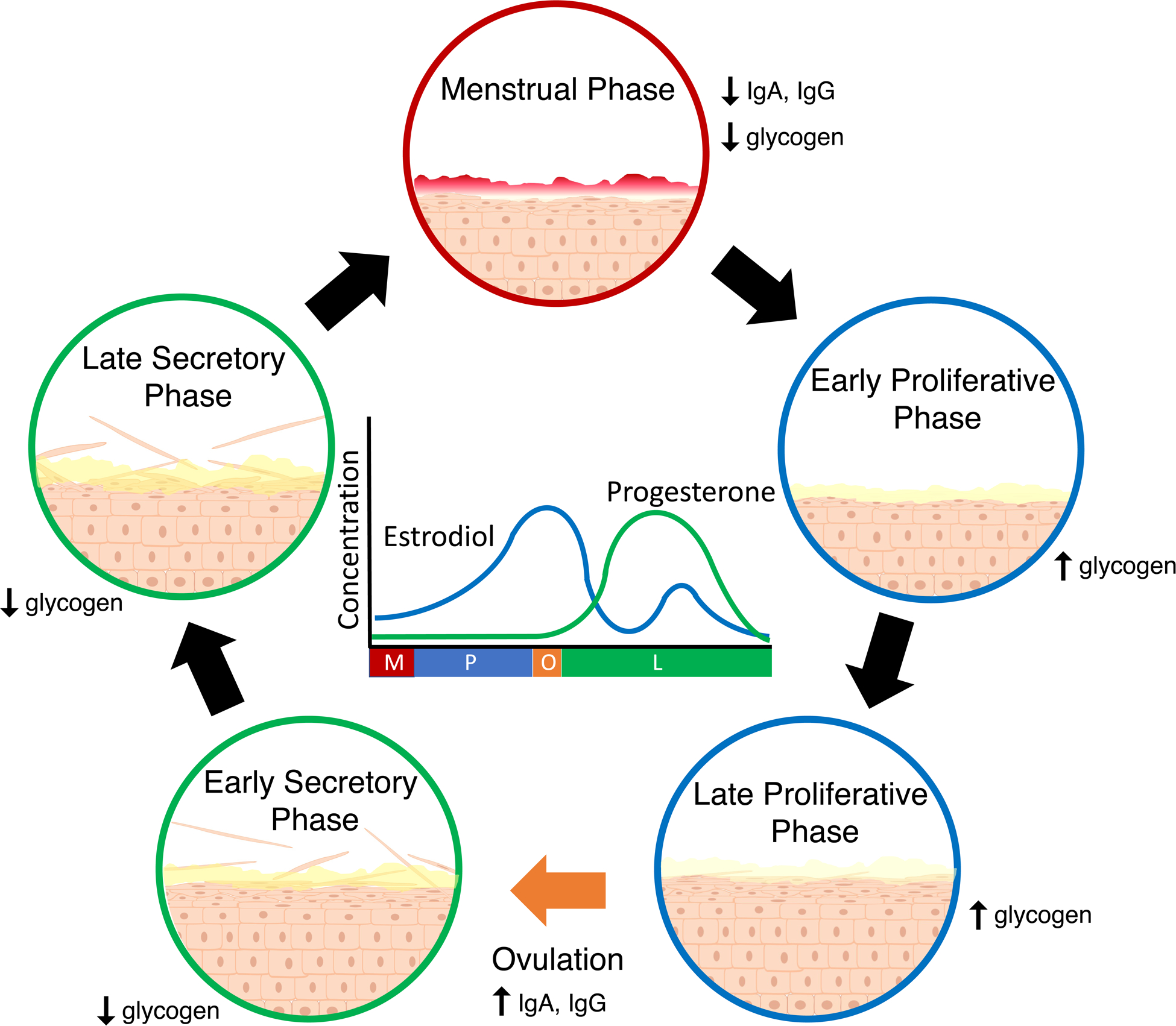
During the menstrual phase (M; red), blood and the shed functional layer of the uterine endometrium flow through the vagina. During the subsequent proliferative phase (P; blue), higher estrodiol levels promote the growth and maturation of the vaginal epithelium. The mucus is thinner during this stage, which is thought to facilitate sperm penetration. Following ovulation (O; orange), progestrerone levels rise during the secretory phase (L; green), halting growth and maturation of the epithelium. Superficial cells of the epithelium are shed and the protective mucus layer is thicker.
The vaginal epithelium itself also responds to hormonal fluctuations, undergoing cyclic proliferation throughout the menstrual cycle with a peak at ovulation (Fig. 1), though changes are not as drastic as those of the uterus60. The vaginal epithelium is coated in a cervical mucus layer that is subject to regulation by hormonal fluctuation, with progesterone associated thickening seen in the peri-ovulatory period61. Although the vagina doesn’t produce its own mucus, cervical mucus is produced in high enough abundance to flow down and coat the vaginal epithelium62. The mucus is composed primarily of proteins, lipids, water, and glycoproteins referred to as mucins63,64. Every mucin is rich in sequences of repeating serines and threonines, with the repeat regions serving as the location for O-linked glycosylations chains comprised of N-acetylgalactosamine, galactose, and N-actylglucosamine and capped with fucose or sialic acid65,66. These glycosylation chains play a key role in mucin function, and alterations to these patterns are associated with several adverse health conditions including spontaneous preterm birth67. Mucins are hypothesized to play a protective role for the vaginal epithelium68,69 and they may also serve as a source of nutrition for the vaginal microbiota70,71. Mucin levels vary throughout the menstrual cycle; for instance, the amount of MUC5B peaks mid-cycle at ovulation72 and is accompanied by an increase in the glycosylation of several mucins73. Glycogen made by the vaginal epithelium is also thought to be a nutrient source for vaginal bacteria74,75. Vaginal epithelial cells, in particular, contain an overabundance of glycogen relative to other epithelial tissues76. Higher free glycogen concentrations are associated with lower progesterone levels77, while intracellular concentrations are associated with higher estrogen levels78. Both free and intracellular glycogen fluctuate throughout the menstrual cycle.
Many characteristics of vaginal physiology are altered following hormonal changes associated with the onset of menopause. The predominant cell type of the parabasal layer changes from stratum spinosum to predominantly basophilic stratum granulosum with clear cell nuclei79,80. Cycles of epithelial cell proliferation no longer occur due to the reduction in circulating estrogen levels, and vaginal atrophy is common81. Additionally, there are decreases in cervical mucus production82, and changes in mucus composition83, concomitant with the decline in estrogen and testosterone levels observed in this period. Free and intracellular levels of glycogen also decline84. Additionally, an increase in vaginal pH to ≥ 4.7 has been found to be one of the more sensitive markers of menopause85. Altogether, these changes contribute to a vastly different microenvironment for the microorganisms residing in the vagina. These differences are thought to be responsible for menopause-associated changes in vaginal microbiota composition86 and the genitourinary syndrome of menopause (GSM)87. Hormonal replacement therapy is often used to treat GSM and may also impact the vaginal microbiota via its effect on the vaginal microenvironment.
Lactobacillus spp. and the vaginal microbiota in reproductive age women
It is well accepted that a vaginal microbiota dominated by Lactobacillus offers a greater degree of protection to their host compared to a more diverse microbiota. Recent work has highlighted that populations of Lactobacillus are typically not comprised of a single strain and display a substantial amount of intraspecies diversity88. Considering the continual supply of new mutants originating from each genetic background89, these populations might best be thought of as clouds of related genotypes rather than single entities. This intraspecies diversity could be a critical determinant of community stability by buffering the dominant Lactobacillus population against perturbations90. There is consensus that the Lactobacillus species common to the human vagina are likely not equivalent with respect to their positive impacts on the host. Communities dominated by L. crispatus are thought to offer the most protective benefits and those dominated by L. iners, the least. It could be that L. jensenii and L. gasseri are equivalent to L. crispatus as they are more similar to this species in their metabolic capabilities than to L. iners91, but their rarity impedes the investigation of their relationships to host health. Many hypotheses exist to explain the associations between Lactobacillus dominance and vaginal health, and they have varying degrees of evidential support9,92,93. In this section, we review our current understanding of the mechanistic explanations for these associations and discuss the ecology of the vaginal microbiome when Lactobacillus species are abundant. We focus our discussion on the biology of L. crispatus in the vaginal microenvironment (Fig. 2), followed by a brief examination of L. iners and how it differs from the other vaginal lactobacilli.
Fig. 2 |. The biology of Lactobacillus crispatus in the vaginal microbiota.
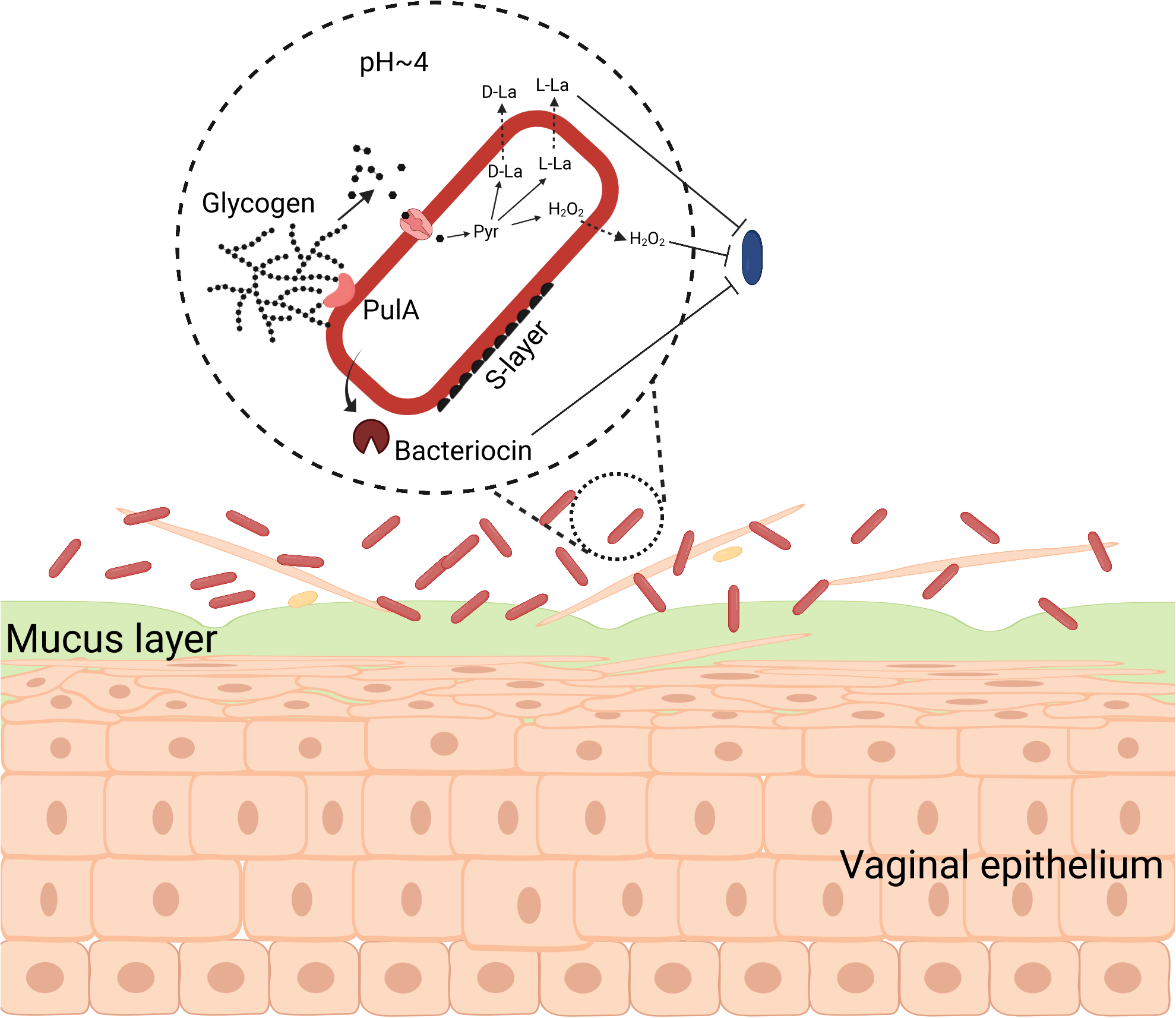
When lactobacilli (red) dominate the vaginal microbiota, less beneficial bacteria (blue) are lower in abundance. Lactobacillus spp. produce PulA, a glycogen-degrading enzyme that generates smaller glucose polymers that are then imported into the cell and fermented via pyruvate (Pyr), producing lactic acid isomers (D-La and L-La). This acidifies the microenvironment to a pH <4. Glycogen breakdown products can also be used to produce hydrogen peroxide (H2O2). Growth of less beneficial bacteria is suppressed by the low vaginal pH and bacterial products, such as lactic acid, bacteriocins and H2O2. D-lactic acid production and S-layer proteins can modulate host immune function in an anti-inflammatory manner.
L. crispatus is a Gram positive, facultative, anaerobic bacterium that produces both the L- and D-lactic acid isomers as its primary fermentation end-products94 (Fig. 2). Although originally thought to lack the intrinsic ability to degrade glycogen without the help of host amylases95,96, studies have now confirmed and described this metabolic capability in L. crispatus, including the identification of PulA homologs97–99. As the human vaginal epithelium is glycogen-rich100, L. crispatus likely derives the majority of its carbon and energy through the fermentation of glycogen, converting it ultimately into lactic acid. Lactic acid production lowers vaginal pH, often to level less than pH 4.211,39, and this acidification of the vaginal microenvironment is one hypothesized means by which L. crispatus benefits the host. In vitro studies have demonstrated that acidic conditions can preclude or inhibit the growth of less beneficial bacterial species including Gardnerella, Prevotella, Mobiluncus, and E. coli101–103. Lactic acid may also have direct effects on host tissues by modulating the immune system and gene expression. For example, D-lactic acid, which is produced by L. crispatus (as well as L. gasseri and L. jensenii) but not L. iners91,104, has been associated with differential expression of immune factors by host tissues105,106. A study by Hearps et al., on the other hand, found that the ionization status of lactic acid, which is a function of pH, had a larger impact on its ability to suppress inflammation than the isomer form13. Lactic acid more readily diffuses through epithelial cell membranes when in the non-ionized form107. It is clear that the relationship between lactic acid and vaginal health is multifaceted and its effects extend beyond lowering vaginal pH.
There are other mechanisms by which L. crispatus is thought to exert beneficial effects on vaginal health. L. crispatus (and L. gasseri and L. jensenii) have long been known to produce hydrogen peroxide in the presence of oxygen108 (Fig. 2). It was thought their production of H2O2 also served to inhibit the growth of anaerobic bacteria in the vaginal microenvironment109,110. Observational studies found associations between the presence of H2O2 producing lactobacilii and vaginal health7,110,111. We now know that only L. iners does not produce hydrogen peroxide3 confounding this observation with other factors that distinguish L. iners from the other lactobacilli104,112. In vitro studies have shown that Lactobacillus-produced H2O2 can inhibit the growth of many of these less beneficial bacteria113, although Gardnerella spp. seem to have the capability to resist H2O2114. These studies do not necessarily have relevance to the in vivo production of H2O2 by Lactobacillus. The reactions require molecular oxygen, which is likely rare in the microaerobic vaginal microenvironment where O2 concentrations are 1/10 to 1/5 that of atmospheric concentrations115. Further, any H2O2 that is produced can be quenched through reactions with various non-microbial components of vaginal fluid116. If H2O2 production does play an inhibitory role in the vaginal microenvironment it is likely limited to localized interactions between the lactobacilli and their competitors. L. crispatus and other vaginal lactobacilli may also have other means of inhibiting the growth of competitors including the production of bacteriocins117,118.
In addition to its thick cell wall, L. crispatus also produces a proteinaceous outer surface layer, called the S-layer119,120 (Fig. 2). The S-layer, and its associated proteins, is thought to contribute to the species’ ability to adhere to host cells119,120 and its’ immunomodulatory capabilities121,122. The adherence of L. crispatus to vaginal epithelial cells is thought to block adhesion of pathogens123,124, although the role of adhesion to a rapidly shedding vaginal epithelium is unclear. Vaginal microbiota that are dominated by L. crispatus have been associated with lowered vaginal inflammation28,125, although a complete mechanistic explanation of the immunomodulatory capacity of the species has not been described. It is likely that proteins in the S-layer contribute. Efforts to further characterize the biology of L. crispatus and many other vaginal bacteria have been severely hampered by a lack of tools to manipulate the species’ genetics. Methods to generate targeted gene knockout mutants of these species will prove critical in future research.
One final aspect of L. crispatus biology that is often overlooked but may be relevant to vaginal health is the dominance of L. crispatus in the vaginal microbiota and therefore the low proportion of other bacteria. L. crispatus, and the other vaginal Lactobacillus, can dominate the vaginal niche, often accounting for 99% of the sequences in 16S rRNA gene amplicon data2,39. Some women also maintain L. crispatus dominance over several menstrual cycles, indicating the dominance of these populations can be fairly stable51. By dominating the vaginal niche, L. crispatus reduces and precludes the growth of other, potentially harmful bacteria. This concept, termed “pathogen resistance”, is certainly a benefit provided by a L. crispatus-dominant vaginal microbiota126. Ecological theory predicts that a more complex community utilizes more resources in an environment than a simple community, due to the non-overlapping portions of the constituent’s niches127. A community that is mostly comprised of a single species should therefore not exploit the vaginal environment to the same extent as the more proportionally even CST IV community. For example, L. crispatus is not predicted to be a substantial degrader of host protective mucus as it is not known to be capable of removing terminal sialic acid and fucose residues from mucin glycosylation chains128,129. This is in contrast with some of the other, non-Lactobacillus species that are capable of these metabolic feats128–133. L. crispatus can therefore preserve this critical barrier that protects the vaginal epithelium. Additionally, L. crispatus does not produce a cytolysin that would allow it to liberate resources through the lysis of host cells134,135, nor does it appear capable of producing many of the biogenic amines thought to be responsible for vaginal odor136 (e.g. trimethylamine, cadaverine).
L. iners is perhaps the most common vaginal bacteria and is unique among the Lactobacillus2,39. The species was first identified as the vaginal lactobacilli that did not produce hydrogen peroxide109,111. Compared to other vaginal Lactobacillus, L. iners has a smaller genome104,112, produces a cytolysin134, and does not produce the D isomer of lactic acid91,105. Its relevance to vaginal health has been a topic of much discussion137. The dominance of L. iners in the vaginal microbiota is associated with low vaginal pH (<4.5). due to its production of L-lactic acid as a fermentation end-product2,39. Longitudinal studies have also found that L. iners dominated communities are less stable than those dominated by other lactobacilli, and often transition to CST IV, which may contribute to its limited association with vaginal health51,138. In line with this, L. iners is sometimes found in low to moderate relative abundances in CST IV communities2,39,109,111. This species has been shown to vary its gene expression when found within CST IV communities, including higher expression of its cytolysin139,140. These results suggest that the impact of L. iners on vaginal health may be community composition dependent. Although more study is needed to define the relationship between L. iners and vaginal health, all indications are that L. iners offers fewer benefits to its host than L. crispatus, or the other vaginal lactobacilli, although strain-level variations might modulate these benefits. A study by Bloom et al., has indicated that metabolic differences between L. iners and the other vaginal lactobacilli could be leveraged to selectively inhibit L. iners141.
The vaginal microbiota when Lactobacillus does not dominate
Many women have a vaginal microbiota that is comprised of other facultative and obligate anaerobic bacteria2,4,14–17 (Fig. 3). These communities are associated with a higher vaginal pH (>4.5) and with symptoms such as abnormal discharge and/or odor, although many are asymptomatic2,14,20,21. It is estimated that somewhere between 23–29% of reproductive-age women have BV18,19, which is diagnosed on the basis of a high vaginal pH, a paucity of Lactobacillus, an increased abundance of odorific biogenic amines, and the presence of clue cells (shed vaginal epithelial cells coated in bacteria)142. In research settings, BV is typically identified using a Gram-stain procedure that produces a Nugent score143. Standard of care treatment for BV includes the use of metronidazole (topical or systemic) or clindamycin (topical)144 and often fails to produce a lasting resolution of the condition145,146. The connections between BV and CST IV are clear—both are defined by a lack of lactobacilli and a higher vaginal pH. However, CST IV communities are not always associated with vaginal symptoms and this is often described as asymptomatic BV. The question of whether to treat remains controversial as epidemiological studies have linked asymptotic BV with increased risk to adverse health outcomes147. Understanding which, if any, CST IV communities do not cause vaginal symptoms and/or do not increase risk to adverse health outcomes will go a long way toward understanding when treatment is necessary.
Fig. 3 |. The CST IV vaginal microbiota.
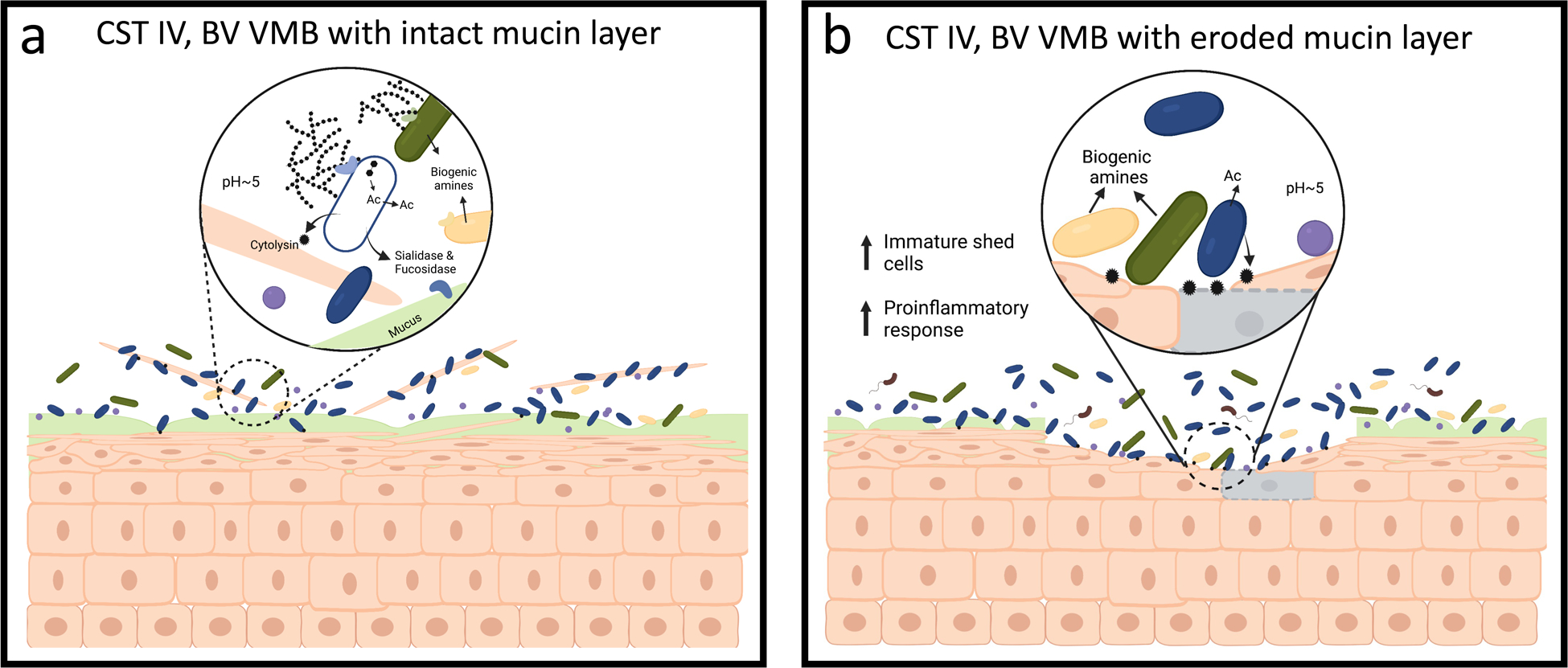
a, The CST IV vaginal microbiota is comprised of a more even collection of Gardnerella (Blue), Prevotella (Green, Yellow), Atopobium (Purple), Ca. L. vaginae (Brown, flagellated) and is associated with a higher pH (>4.5). These bacteria produce biogenic amines that raise vaginal pH, impact host physiology, and inhibit the growth of Lactobacillus. These species can break down glycogen produced by the host to produce acetate (Ac), for example, using sialidase and fucosidase enzymes. Production of cytolysin enzymes by Gardnerella and other species allow the community to liberate more resources through the lysis of host cells. b, A subset of CST IV communities has the potential to degrade host mucin glycochains due to their ability to produce sialidase and fucosidase enzymes. If the mucus layer is degraded faster than it can be replenished, the integrity of the protective mucus layer might become compromised, exposing the vaginal epithelium to further damaged by cytolytic activity.
Similar to lactobacilli, host-produced glycogen is likely to be a major source of carbon and energy for CST IV bacteria. Gardnerella and many of the other species common to CST IV have been shown to have genes associated with glycogen degradation98,140,148. Expression levels of predicted glycogen debranching enzymes are high in these communities and similar to that observed in Lactobacillus dominant communities140. Studies have shown a positive association between free glycogen in vaginal fluid and Lactobacillus75,149; however, we argue that this does not conflict with the observation that CST IV bacteria also utilize glycogen. The CST IV vaginal microbiota, which is often higher in bacterial load and more diverse, might simply consume more of the host-produced glycogen. The species common to CST IV have at least two other metabolic capabilities that likely allow them to access more host-produced resources (Fig. 3). First, various Gardnerella spp. and Prevotella spp. are known to produce sialidase and fucosidase enzymes capable of degrading mucin glycan chains128–133. Second, Gardnerella (and other species) produce a cholesterol-dependent cytolysin that is capable of lysing epithelial cells, thereby liberating their intracellular contents for use by the microbiota135,150,151. Damage to the vaginal epithelium likely activates proinflammatory signaling pathways, drawing leukocytes to the area152. These two metabolic feats, mucin degradation and host cell lysis, might act synergistically to damage the vaginal epithelium: removing the mucin layer would give the cytolysin better access to epithelial cells. While mature vaginal epithelium cells are regularly shed, the CST IV microbiota is likely capable of actively depleting the vaginal epithelium (Fig. 3). Consistent with this hypothesis is the observation that women with symptomatic BV experience higher cell shedding while those with asymptomatic BV shed fewer, but more immature epithelial cells153. We argue that these results indicate that the vaginal epithelium of some women with CST IV microbiota is damaged and might require repair before a Lactobacillus dominant microbiota can re-establish. This hypothesis may explain the frequency of recurrence following treatment of BV.
The metabolic activities of the microorganisms that comprise the CST IV vaginal microbiota also impact the vaginal metabolome. One prominent example is that these communities are associated with an increased abundance of biogenic amines including: putrescine, cadaverine, and tyramine136,154 (Fig. 3). Biogenic amines are hypothesized to explain the connection between BV and vaginal odor. However, their role in the vaginal microenvironment likely extends beyond this symptom. Production of biogenic amines is a mechanism of acid tolerance which could be necessary for these bacteria to survive in the vagina155. Several biogenic amines have also been shown to either increase the lag time or decrease the growth rate of the vaginal Lactobacillus, suggesting that they may drive the establishment and maintenance of the CST IV microbiota156. Gardnerella is not thought to be a primary producer of these metabolites; species within the Prevotella, Mobiluncus, Dialister, Parvimonas, Megasphaera, and Peptostreptococcus genera are instead suspected to be responsible136. The metabolic pathways that microorganisms use to produce biogenic amines are generally not well characterized so other bacteria could also be involved in their generation. For example, it is not known how trimethylamine (TMA), the compound thought to be responsible for the fishy odor symptom of BV, is produced in the vagina. Mobiluncus spp. are capable of producing TMA157 but it seems unlikely that this is the only source as these bacteria are not common in the vaginal microbiota.
It is critical to also recognize that the CST IV microbiota is not monolithic. A unifying characteristic of these communities is that they are not dominated by lactobacilli but their composition can take a number of forms. While the presence of Gardnerella, Atopobium, and various Prevotella spp. is a common motif, some women have CST IV communities that also include high proportions of Ca. L. vaginae, Sneathia, Mobiluncus, and even L. iners39,41,158. It could be that a subset of species common to CST IV are responsible for the majority of its association with adverse health outcomes, or that these associations could be strengthened by looking at subtypes of these communities. Compositional characterizations of the vaginal microbiota have largely been derived from 16S rRNA gene amplicon survey data, which has likely underestimated diversity within CST IV communities. G. vaginalis, for example, has long been known to be a diverse species159 and has recently been split into multiple genomospecies160. Most women who are colonized by Gardnerella have several of these species in their vaginal microbiota88,159. Over the years many genomic and in vitro phenotypic comparisons of Gardnerella strains have been conducted, some of which suggest that there is variation in pathogenic potential within Gardnerella (e.g. not all Gardnerella genomes encode a known sialidase)159,161–163. Shotgun metagenomic studies are necessary to disentangle diversity within Gardnerella and many of the other species common to CST IV communities. Disentangling the diversity of CST IV will prove critical for resolving the connection between these communities and vaginal health and will lead to improved targeted treatments.
Host factors affecting vaginal microbiota composition
Early epidemiology studies observed that vaginal microbiota composition exhibits variation that is dependent on a woman’s ethnicity or race. Some studies found Black women in North America and Europe were less likely to have a vaginal microbiota dominated by Lactobacillus than white women in these populations2,16. For example, in a study of 396 North American women, 10.3% of those who identified as white or Caucasian had a CST IV vaginal microbiota, compared to 40.4% of those who identified as Black or African American2. Another study identified a subtype of CST IV, defined by the presence of Ca. Lachnocurva vaginae, that was not prevalent in North American women who identified as Asian39. Given that race is a social construct, the factors that drive these differences are multifaceted and it has been hypothesized that socioeconomic, cultural, genetic, and/or behavioral factors, as well as inequalities in healthcare, are responsible164. However, it is important to note that these differences have largely not been found to extend within CSTs. The taxonomic composition of a vaginal microbiota assigned to CST IV, or any other for that matter, does not appear to depend on race or ethnicity. One exception is that Prevotella spp. may be more abundant in CST IV communities from women in African populations25,43. An in-depth comparison of African women and women with African ancestry living on other continents is necessary to confirm this observation.
Moreover, it is important to recognize and discuss the concordance in the composition of the human vaginal microbiota among reproductive-age women from around the world. L. iners, L. crispatus, and G. vaginalis are three of the most prevalent bacterial species in the vaginal microbiota of women from every population examined thus far, including: North American39, South American165, European166, African25,46, and Asian15,42 populations. A study of Amerindian women living a pre-agricultural lifestyle found that their vaginal microbiota was commonly comprised of L. iners or G. vaginalis but L. crispatus was less prevalent than in other populations48. All indications are that the taxonomic composition of the vaginal microbiota is a shared distinguishing trait of humanity. Lactobacillus do not dominate the vaginal microbiota of any other known mammal167, and many species common to our vaginal microbiota have not been identified in the vaginal microbiota of other mammals, including non-human primates. Gardnerella have been identified in rhesus macaques, but less frequently than in humans and at lower relative abundances168. It remains to be seen if these Gardnerella species are distinct from those found in humans. The driving factors behind the development of our unique vaginal microbiota are not known.
Age is also known to impact the vaginal microbiota. Less is known about the communities that reside in the vagina during and prior to puberty or during/following menopause. This lack of knowledge should not be interpreted as a reflection of the vaginal microbiota’s relative importance to health in these populations. For example, the vaginal microbiota is thought to play a role in urinary tract infections during childhood, which afflict 3–7% of premenarchal girls169,170. For post-menopausal women, the vaginal microbiota is thought to contribute to atrophic vaginitis and associated sexual dysfunction171,172. Both premenarchal girls and postmenopausal women are less likely to have communities dominated by Lactobacillus, although their composition is also somewhat distinct from the CST IV communities commonly found in reproductive age women173,174. One commonality between these two age groups is their propensity to have lower levels of circulating estrogen than reproductive-age women100. Low estrogen levels are thought to result in a thinner vaginal epithelium that is not as glycogen rich175. It could be that without this glycogen, the environment is less conducive for the growth of lactobacilli and other species common to the reproductive age vaginal microbiota. The number of bacteria in these communities is typically several logs lower than that found in reproductive-age women, which could be driven by lower nutrient levels176. Additional studies are needed to define the relationship between the vaginal microbiota and health in these age groups (see Laniewski and Herbst-Kralovetz for more in depth discussion on the menopause and vaginal microbiota177).
Although often overlooked, the vaginal microbiota of premenarchal girls is of particular interest as it may influence the future composition of these communities. At birth, neonatal estrogen levels are high due to their mother’s circulating hormones. However, the estrogen levels decline during the first weeks of life, and normally remain low until the initiation of puberty178. A recent study examined the vaginal microbiota of 4–6-year-old Chinese girls and found their communities were comprised of a diverse collection of Peptoniphilus, Porphyromonas, Prevotella, Pseudomonas, and Escherichia coli179. The timing of the transition towards a vaginal microbiota that resembles that at reproductive-age is not well characterized. A study by Hickey et al. found that the adolescent vaginal microbiota (aged 10–13) resembled that of a reproductive-age woman prior to their first menses, indicating the transition must happen earlier in life180. If we generalize the results of these two studies, we can posit that the transition must occur sometime between the ages of 6 and 12. Estrogen levels begin to rise during this time period indicating that it may be a driving force behind the transition. The source of the species that gain dominance in the vaginal microbiota during reproductive ages (e.g. L. crispatus, L. iners, G. vaginalis) is also not clear. It could be that these species are vertically transmitted from mother to offspring during the birthing process or early in life. Under this scenario the species would need to persist in the vagina throughout early childhood and then increase in abundance during adolescence. However, the vaginal microbiota might experience more frequent influxes of new strains and species through another mechanism and transmission happens later in life.
Epidemiologic associations between the vaginal microbiota and adverse health outcomes
The results from epidemiological studies have described associations between the composition of the vaginal microbiota and adverse health outcomes (Table 1). In this section, we will refer to a community with lower proportion or abundance of Lactobacillus and a higher proportion or abundance of facultative and obligate anaerobes (e.g. Gardnerella, Prevotella, Atopobium, Sneathia etc.) as a “non-optimal vaginal microbiota”. Note that this definition includes women with asymptotic and symptomatic BV. There is strong and consistent evidence from longitudinal studies linking this non-optimal microbiota to an increased risk of acquiring and transmitting human immunodeficiency virus (HIV)181–185. Similar associations have been identified between these communities and an increased risk for acquiring other STIs, including gonorrhea, chlamydia, trichomonas, herpes simplex virus 2 (HSV-2), and syphilis22,186–189. The non-optimal microbiota has also been linked to both incidence and prevalence of human papillomavirus (HPV), as well as the associated development and progression of cervical intraepithelial neoplasia and increased risk for cervical cancer190–195. Again though, there are contrary reports196–199. The composition of the vaginal microbiota has also been associated with increased risk for non-sexually transmitted infections, including urinary tract infections200,201, vulvovaginal candidiasis202–204, and pelvic inflammatory disease205–207. There is evidence supporting an association between the composition of the vaginal microbiota and reproductive health including risk for spontaneous preterm birth33,208–210. Studies that utilized sequence-based methodologies have found associations between specific vaginal bacteria, and bacterial community structures and preterm birth, spontaneous preterm birth, and preterm premature rupture of fetal membranes; however, results were heterogeneous across studies, with some finding no association27–29,211–214.
Table 1:
Epidemiological associations between the composition of the vaginal microbiota and vaginal health
| Outcomes | Summary of findings | Citations |
|---|---|---|
| STI acquisition (including HIV, gonorrhea, chlamydia, trichomonas, HSV, HPV, syphilis) | Results of studies vary, especially depending on the STI; presence or increased relative abundances of Lactobacillus spp. are generally associated with decreased risk; BV, a CST IV vaginal microbiota, and particular BV-associated phylotypes have been found to be associated with increased risk. | 22,181–185,187–189,195–199 |
| Vulvovaginal candidiasis | Results of studies vary with one finding no evidence for differences in vaginal microbiota of women with and without recurrent vulvovaginal candidiasis but another suggested risk of symptomatic candidiasis may be higher for a Lactobacillus dominant community. | 202,203 |
| Urinary tract infection (UTI) | UTIs were more common among women with vaginal E. coli colonization, and without H202-producing Lactobacillus. | 200 |
| Pelvic inflammatory disease (PID) | Higher growth of several BV-associated bacteria was associated with increased risk for PID, while there was no association between carriage of non-BV-associated bacteria and PID risk. | 205–207 |
| Preterm delivery | Results of studies vary; increased relative abundances of Lactobacillus spp. have generally been shown to be associated with decreased risk; BV, a CST IV vaginal microbiota, and particular BV-associated phylotypes have been found to be associated with increased risk. | 27–29,33,208,210–213,233 |
Despite the volume of work establishing associations between the vaginal microbiota and health, we are still lacking descriptions of the causal mechanisms and pathways. It is particularly difficult to determine whether the associations are driven by the microbiota influencing host physiology or by changes in host physiology impacting the composition of the microbiota. Parsing these tripartite associations will require the development of animal and cell culture model systems that incorporate the vaginal microbiota (Box 1).
Box 1: Model systems for studying the vaginal microbiota.
A major obstacle in vaginal microbiome research is a lack of suitable animal and cell culture model systems. These model systems are needed to investigate and test mechanistic hypotheses generated through observational studies of the vaginal microbiota. Unfortunately, the uniqueness of the human vaginal microenvironment and the human vaginal microbiota means that routinely used animal model systems lack relevance. Mouse models, that have proven so useful for investigations of the intestinal tract microbiota234,235, have also been used in studies of the vaginal microbiota236–238. However, because these animals do not naturally have a vaginal microbiota that resembles that of humans, it is difficult to interpret whether results are generalizable to humans. Animal models are more frequently used in STI research239–242, but these studies have historically, and unfortunately, not considered the role of the microbiota in the host-pathogen interaction. Two- and three-dimensional cell culture models have been developed and used in vaginal microbiome research including: cellular hydrogels243,244, self-assembled organoids245,246, and microfluidic organ-on-a-chip models247. Notably, microfluidic organ-on-a-chip models offer the ability to place cells within defined geometries, can reproduce key microenvironment conditions, and can be maintained for longer durations. They also allow the integration of immune cells, the use of hormonal control, and the application of relevant mechanical forces248. An ideal organ-on-a-chip model would include cervical and vaginal tissues with a transition zone between them. The vaginal epithelium should be stratified in multiple layers and should shed superficial cells that contain glycogen stores and vaginal mucus, either produced by the cervical tissue or supplied from an external source, should coat the vaginal tissue. The application of spatial transcriptomics to such a model system would allow the researcher to characterize the local host response to the microbiota and would be critical for the multi-layered vaginal epithelium249,250. The development and use of such a model would be a major breakthrough for vaginal microbiota research and will enable mechanistic hypothesis testing.
Efforts to modulate the vaginal microbiota
Efforts to impart lasting change in the composition of the vaginal microbiota have largely proven unsuccessful. Standard of care antibiotic treatment for BV often yields only temporary resolution of the condition215–217. Other methods to repress the growth of BV-associated anaerobes and/or support the growth of lactobacilli include estrogen therapy172 and treatment with lactic218 or boric acid219 (Fig. 4). Many have also suggested probiotics for the modulation of the vaginal microbiota, either following antibiotic treatment or primary treatment. Several vaginal probiotics containing Lactobacillus species have been designed and tested, largely yielding mixed results220–227. There are a number of reasons why the efficacies of these probiotics fell short of expectations. In some cases, the probiotic formulations did not utilize species that are common to the human vagina, opting instead to use those that were already in gut probiotics222,227. Other probiotics were given to women in the form of oral tablets with the expectation that such a probiotic might influence host physiology, creating a vaginal environment favorable for Lactobacillus221,227. A recent randomized, double-blind, placebo controlled clinical trial, was conducted to test the efficacy of a vaginally delivered L. crispatus probiotic called Lactin-V. The probiotic was provided to women with BV, following metronidazole treatment, and resulted in a difference of 15% in the rate of BV recurrence between the treatment and placebo groups (30% versus 45% recurrence)228. This result is encouraging but still over a quarter of treated women experienced BV recurrence within 12 weeks. Identifying the factors that drive treatment failure will prove critical for the development of more effective vaginal probiotics.
Fig. 4 |. Vaginal microbiota interventions to treat bacterial vaginosis.
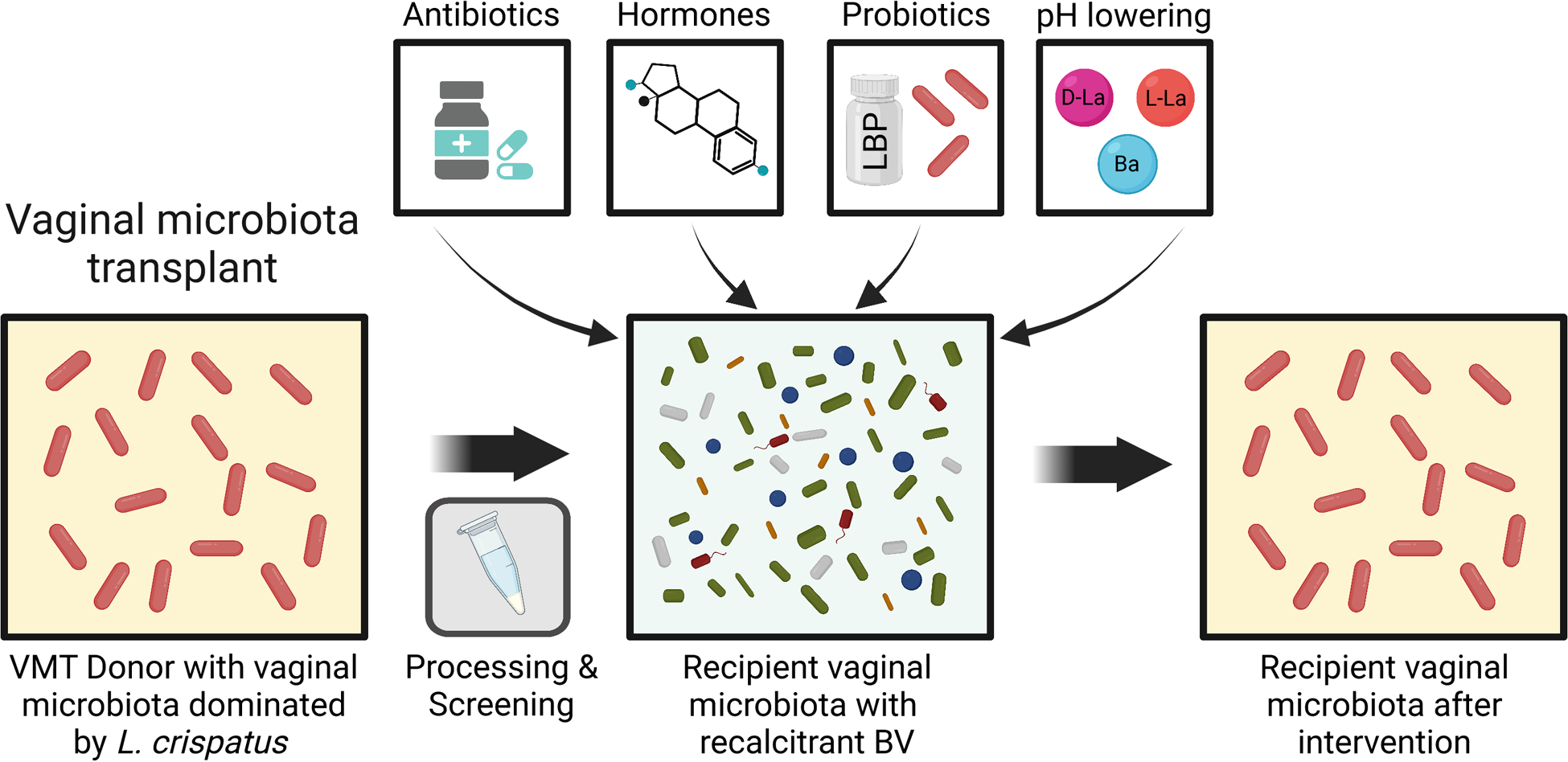
Existing treatments include antibiotics such as metronidazole, estrogen therapy, lactic and boric acid, and vaginal lactobacilli probiotics. However, these interventions vary in their success and do not effectively prevent recurrent/recalcitrant BV. Vaginal microbiota transplants (VMTs) are a promising intervention for BV. A suitable donor with a Lactobacillus dominant vaginal microbiota is identified. Vaginal secretions are collected from the donor, screened for various STIs, and processed. The processed vaginal secretions are then introduced into the vagina of a recipient who is typically experiencing recurrent/recalcitrant BV. The recipient may or may not be treated with antibiotics prior to the transplant. Success is defined as a long-lasting resolution of the recipient’s BV and a shift of their vaginal microbiota to the Lactobacillus dominant configuration.
Promising results from studies reporting the efficacy of fecal microbiota transplants to treat recurrent Clostridioides difficile infections229 have motivated the investigation of vaginal microbiota transplants (VMT) as a potential approach to treat recurrent BV (Fig. 4). The concept involves sampling vaginal secretions from an individual with a Lactobacillus-dominant vaginal microbiota and introduce it into the vagina with recurrent and/or recalcitrant BV230. An exploratory study on women with recurrent BV indicated the potential efficacy for this approach as long-term remission was achieved for four of the five recipients of the VMT231. It is not clear how VMT could be implemented at scale safely, as each donation requires extensive testing for vaginal pathogens and viruses (e.g. HSV or HPV) and contains a relatively small bacterial load232. However, studies on the mechanisms of VMT are likely to yield novel insights into the factors that influence the successful modulation of the vaginal microbiota. These insights could then be translated to traditional Lactobacillus probiotic formulations with increased safety and can be produced at scale.
Outlook
Over the past decade we have learned a great deal about the vaginal microbiome and how it relates to host health. Unfortunately, our reliance on observational studies and amplicon-based compositional survey data has stymied the progress towards a mechanistic understanding of these communities and their impact on host physiology. These observational studies have generated innumerable hypotheses that must be tested in the laboratory. Recent in vitro work has characterized aspects of the biology of individual bacteria (e.g. on glycogen debranching enzymes of the vaginal bacteria97,99) but these studies often do not include the microbiota and/or the host. A major barrier towards the development of a mechanistic understanding is the dearth of suitable model systems for in vitro experimentation. While it is true that no model is perfect, some models are certainly better than others and a cervicovaginal model that incorporates the vaginal microbiota is sorely needed. Progress must be made in the field of multi-omics as integration of metagenomic, metatranscriptomic, metabolomic, and immunology datasets could afford a detailed look into the biology of the microbiota-host relationship as it exists in vivo. Results from such in vitro and in vivo studies, along with interventional clinical trials will likely drive the development of advanced and innovative treatment options and preventative measures for the myriad of adverse health outcomes that impact individuals with a vagina and remain unaddressed.
Acknowledgements
This review was supported by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Nursing Research (NINR) of the National Institute of Health (NIH) under awards number U19AI158930, R21AI162006 and R01NR01549. MA acknowledges support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of NIH under award number T32DK067872.
Footnotes
Competing interests
J.R. is co-founder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutics drugs for women’s health. All other authors declare that they have no competing interests.
References
- 1.Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravel J, et al. Vaginal microbiome of reproductive-age women. in Proceedings of the National Academy of Sciences, Vol. 108 4680–4687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio M.a., Hawes SE & Hillier SL The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. in The Journal of infectious diseases, Vol. 180 1950–1956 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, et al. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology (Reading) 150, 2565–2573 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Döderlein A Das Scheidensekret und seine Bedeutung für das Puerperalfieber, (Verlag Von Eduard Besold, Leipzig, Germany, 1892). [Google Scholar]
- 6.Galask RP, Larsen B & Ohm MJ Vaginal flora and its role in disease entities. Clin Obset Gynceol 19, 61–81 (1976). [DOI] [PubMed] [Google Scholar]
- 7.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ & Eschenbach DA The normal vaginal flora, H202-producing Lactobacilli, and bacterial vaginosis in pregnant women. Clinical Infectious Diseases 16 (Suppl 4), S273–S281 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Sobel JD Is there a protective role for vaginal flora? in Current Infectious Disease Reports, Vol. 1 379–383 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Ma B, Forney LJ & Ravel J Vaginal Microbiome: Rethinking Health and Disease. in Annual Review of Microbiology, Vol. 66 371–389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boskey ER, Teslch KM, Whaley KJ, Moench TR & Cone R a. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infection and Immunity 67, 5170–5175 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Hanlon DE, Moench TR & Cone RA Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One 8, e80074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldunate M, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol 6, 164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado-Diaz DJ, et al. Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota. Front Cell Infect Microbiol 9, 446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, et al. Disparity in the vaginal microbial community composition of healthy Caucasian and Black women. ISME J. 1, 121–133 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, et al. The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. in FEMS Immunology and Medical Microbiology, Vol. 58 169–181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 1, 121–133 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Verstraelen H, Verhelst R, Claeys G, Temmerman M & Vaneechoutte M Culture-independent analysis of vaginal microflora: the unrecognized association of Atopobium vaginae with bacterial vaginosis. Am J Obstet Gynecol 191, 1130–1132 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Peebles K, Velloza J, Balkus JE, McClelland RS & Barnabas RV High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex Transm Dis 46, 304–311 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Allsworth JE & Peipert JF Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey. Obstetrics and Gynecology 109, 114–120 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Ravel J & Brotman RM Translating the vaginal microbiome: gaps and challenges. Genome Med 8, 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid G Is bacterial vaginosis a disease? Appl Microbiol Biotechnol 102, 553–558 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Brotman RM, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis 202, 1907–1915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brotman RM, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 39, 807–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin HL, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. in The Journal of infectious diseases, Vol. 180 1863–1868 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Gosmann C, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. in Immunity, Vol. 46 29–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feehily C, et al. Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. NPJ Biofilms Microbiomes 6, 50 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown RG, et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl Res 207, 30–43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elovitz MA, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun 10, 1305 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown RG, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med 16, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freitas AC, Bocking A, Hill JE & Money DM Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. in Microbiome, Vol. 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stafford GP, et al. Spontaneous Preterm Birth Is Associated with Differential Expression of Vaginal Metabolites by Lactobacilli-Dominated Microflora. Front Physiol 8, 615 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett PR, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. in Microbiome, Vol. 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan BJ, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Vol. 114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kindinger LM, et al. Relationship between vaginal microbial dysbiosis, inflammation, and pregnancy outcomes in cervical cerclage. in Science Translational Medicine, Vol. 8 350ra102 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Romero R, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson DB, et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol 28, 88–96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinnon LR, et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res Hum Retroviruses 35, 219–228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krakowsky Y, et al. The effect of gender-affirming medical care on the vaginal and neovaginal microbiomes of transgender and gender diverse people. Front Cell Infect Microbiol 11, 769950 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.France MT, et al. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8, 166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vancuren SJ & Hill JE Update on cpnDB: a reference database of chaperonin sequences. Database (Oxford) 2019(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB & Marrazzo JM Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 45, 3270–3276 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y, Chen L, Tong J & Xu C Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. The Journal of Obstetrics and Gynaecology Research 35, 525–532 (2009). [DOI] [PubMed] [Google Scholar]
- 43.McClelland RS, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. The Lancet Infectious Diseases 18, 554–564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balle C, et al. Hormonal contraception alters vaginal microbiota and cytokines in South African adolescents in a randomized trial. Nat Commun 11, 5578 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaspan HB, et al. Association between vaginal washing and vaginal bacterial concentrations. Plos One 14(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lennard K, et al. Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females. Infection and Immunity 86(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradshaw CS, et al. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. The Journal of Infectious Disease 194, 826–836 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Vargas-Robles D, et al. Changes in the vaginal microbiota across a gradient of urbanization. Sci Rep 10, 12487 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holm JB, et al. Comparative Metagenome-Assembled Genome Analysis of “Candidatus Lachnocurva vaginae”, Formerly Known as Bacterial Vaginosis-Associated Bacterium-1 (BVAB1). Front Cell Infect Microbiol 10, 117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srinivasan S & Fredricks DN The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008, 750479 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajer P, et al. Temporal dynamics of the human vaginal microbiota. in Science Translational Medicine, Vol. 4 132ra152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan S, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5, e10197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tevi-Benissan C, et al. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clinical and Diagnostic Laboratory Immunology 4, 367–374 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta SD, et al. The Microbiome Composition of a Man’s Penis Predicts Incident Bacterial Vaginosis in His Female Sex Partner With High Accuracy. Front Cell Infect Microbiol 10, 433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert JA & Lynch SV Community ecology as a framework for human microbiome research. Nat Med 25, 884–889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheater PR, Burkitt HG & Daniels VG Functional histology. A text and colour atlas, (Churchill Livingstone, 23 Ravelston Terrace, Edinburgh, EH4 3TL., 1979). [Google Scholar]
- 57.Anderson DJ, Marathe J & Pudney J The structure of the human vaginal stratum corneum and its role in immune defense. American journal of reproductive immunology 71, 618–623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pudney J, Quayle AJ & Anderson DJ Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biology of reproduction 73, 1253–1263 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Franklin R & Kutteh W Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. Journal of reproductive immunology 42, 93–106 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Dierks K Der normale mensuelle Zyklus der menschlichen Vaginalschleimhaut. Archiv für Gynäkologie 130, 46–69 (1927). [Google Scholar]
- 61.Han L, Taub R & Jensen JT Cervical mucus and contraception: what we know and what we don’t. Contraception 96, 310–321 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Odeblad E The discovery of different types of cervical mucus and the Billings Ovulation Method. Bulletin of the Natural Family Planning Council of Victoria 21(1994). [Google Scholar]
- 63.Lacroix G, Gouyer V, Gottrand F & Desseyn J-L The cervicovaginal mucus barrier. International Journal of Molecular Sciences 21, 8266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voynow JA & Rubin BK Mucins, mucus, and sputum. Chest 135, 505–512 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Voynow JA & Fischer BM MUCINS. in Encyclopedia of Respiratory Medicine (eds. Laurent GJ & Shapiro SD) 56–62 (Academic Press, Oxford, 2006). [Google Scholar]
- 66.Reily C, Stewart TJ, Renfrow MB & Novak J Glycosylation in health and disease. Nature Reviews Nephrology 15, 346–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Critchfield AS, et al. Cervical mucus properties stratify risk for preterm birth. PLoS One 8, e69528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Domino SE, et al. Cervical mucins carry α (1, 2) fucosylated glycans that partly protect from experimental vaginal candidiasis. Glycoconjugate journal 26, 1125–1134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cone RA Barrier properties of mucus. Advanced drug delivery reviews 61, 75–85 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Agarwal K & Lewis AL Vaginal sialoglycan foraging by Gardnerella vaginalis: mucus barriers as a meal for unwelcome guests? Glycobiology (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vagios S & Mitchell CM Mutual Preservation: A Review of Interactions Between Cervicovaginal Mucus and Microbiota. Frontiers in Cellular and Infection Microbiology, 645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gipson IK, et al. The amount of MUC5B mucin in cervical mucus peaks at midcycle. The Journal of Clinical Endocrinology & Metabolism 86, 594–600 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Andersch-Björkman Y, Thomsson KA, Larsson JMH, Ekerhovd E & Hansson GC Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Molecular & Cellular Proteomics 6, 708–716 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Tester R & Al-Ghazzewi FH Intrinsic and extrinsic carbohydrates in the vagina: a short review on vaginal glycogen. International journal of biological macromolecules 112, 203–206 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Mirmonsef P, et al. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PloS one 9, e102467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seidman JD, Cho KR, Ronnett BM & Kurman RJ Surface epithelial tumors of the ovary. in Blaustein’s pathology of the female genital tract (2002). [Google Scholar]
- 77.Mirmonsef P, et al. Glycogen levels in undiluted genital fluid and their relationship to vaginal pH, estrogen, and progesterone. PloS one 11, e0153553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farage M & Maibach H Lifetime changes in the vulva and vagina. Archives of gynecology and obstetrics 273, 195–202 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Konar H DC Dutta’s textbook of gynecology, (JP Medical Ltd, 2016). [Google Scholar]
- 80.Rousseaux CG, Wallig MA & Haschek WM Fundamentals of toxicologic pathology, (Academic Press, 2009). [Google Scholar]
- 81.Kingsberg SA, Wysocki S, Magnus L & Krychman ML Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. The journal of sexual medicine 10, 1790–1799 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Schwenkhagen A Hormonal changes in menopause and implications on sexual health. The journal of sexual medicine 4, 220–226 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Moncla BJ, Chappell CA, Debo BM & Meyn LA The effects of hormones and vaginal microflora on the glycome of the female genital tract: cervical-vaginal fluid. PLoS One 11, e0158687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirmonsef P, et al. An exploratory comparison of vaginal glycogen and Lactobacillus levels in pre-and post-menopausal women. Menopause (New York, NY) 22, 702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panda S, Das A, Santa Singh A & Pala S Vaginal pH: A marker for menopause. Journal of mid-life health 5, 34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muhleisen AL & Herbst-Kralovetz MM Menopause and the vaginal microbiome. Maturitas 91, 42–50 (2016). [DOI] [PubMed] [Google Scholar]
- 87.The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 27, 976–992 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Ma B, et al. A comprehensive non-redundant gene catalog reveals extensive within-community intraspecies diversity in the human vagina. Nat Commun 11, 940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garud NR, Good BH, Hallatschek O & Pollard KS Evolutionary dynamics of bacteria in the gut microbiome within and across hosts. PLoS Biol 17, e3000102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agashe D The Stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. The American Naturalist 174, 255–267 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ & Forneya LJ Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. in Journal of Bacteriology, Vol. 196 1458–1470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta S, Kakkar V & Bhushan I Crosstalk between Vaginal Microbiome and Female Health: A review. Microb Pathog 136, 103696 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Smith SB & Ravel J The vaginal microbiota, host defence and reproductive physiology. in Journal of Physiology, Vol. 595 451–463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vos P, et al. Bergey’s manual of systematic bacteriology: Vol 3: The Firmicutes, (Springer, New York City, 2011). [Google Scholar]
- 95.Stewart-Bull DES Evidence that vaginal lactobacilli do not ferment glycogen. American Journal of Obstetrics and Gynecology 88, 676–679 (1964). [DOI] [PubMed] [Google Scholar]
- 96.Spear GT, et al. Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 210, 1019–1028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van der Veer C, et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: implications for in vivo dominance of the vaginal microbiota. Microbiome 7, 49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nunn KL, et al. Amylases in the human vagina. mSphere 5, e00943–00920 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woolston BM, Jenkins DJ, Hood-Pishchany MI, Nahoum SR & Balskus EP Characterization of vaginal microbial enzymes identifies amylopullulanases that support growth of Lactobacillus crispatus on glycogen. bioRvix (2021). [Google Scholar]
- 100.Cruickshank R & Sharman A The biology of the vagina in the human subject. BJOG 41, 190–207 (1934). [Google Scholar]
- 101.Skarin A & Sylwan J Vaginal lactobacilli inhibiting the growth of Gardnerella vaginalis, Mobiluncus, and other bacterial species cultured from vaginal content of women with bacterial vaginosis. Acta Pathologica Microbiologica Scandinavica Series B: Microbiology 94, 399–403 (1986). [DOI] [PubMed] [Google Scholar]
- 102.Atassi F, Brassart D, Grob P, Graf F & Servin AL Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol 48, 424–432 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Nunn KL, et al. Changes in the Vaginal Microbiome during the Pregnancy to Postpartum Transition. Reprod Sci 28, 1996–2005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.France MT, Mendes-Soares H & Forney LJ Genomic comparisons of Lactobacillus crispatus and Lactobacillus iners reveal potential ecological drivers of community composition in the vagina. in Applied and Environmental Microbiology, Vol. 82 7063–7073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Witkin SS, et al. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: implications for protection against upper genital tract infections. mBio 4(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Manhanzva MT, et al. Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota. Sci Rep 10, 6196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmitt MG Jr., Soergel KH, Wood CM & Steff JJ Absorption of short-chain fatty acids from the human ileum. The American Journal of Digestive Diseases 22, 340–347 (1977). [DOI] [PubMed] [Google Scholar]
- 108.Wylie JG & Henderson A Hydrogen-peroxide formation and catalase activity in the lactic acid bacteria. J Gen Microbiol 35, 13–26 (1964). [DOI] [PubMed] [Google Scholar]
- 109.Eschenbach DA, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. Journal of Clinical Microbiology 27, 251–256 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klebanoff SJ, Hillier SL & Eschenbach DA Control of microbial flora of the vagina by h2o2-generating lactobacilli. The Journal of Infectious Disease 194, 94–100 (1991). [DOI] [PubMed] [Google Scholar]
- 111.Hawes SE, et al. Hydrogen peroxide-producing Lactobacilli and acquisition of vaginal infections. The Journal of Infectious Disease 174, 1058–1063 (1996). [DOI] [PubMed] [Google Scholar]
- 112.Macklaim JM, Gloor GB, Anukam KC, Cribby S & Reid G At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. in Proceedings of the National Academy of Sciences of the United States of America, Vol. 108 4688–4695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strus M, Brzychczy-Wloch M, Gosiewski T, Kochan P & Heczko PB The in vitro effect of hydrogen peroxide on vaginal microbial communities. FEMS Immunol Med Microbiol 48, 56–63 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Patterson JL, Girerd PH, Karjane NW & Jefferson KK Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol 197, 170 e171–177 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hill DR, et al. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J Appl Physiol (1985) 99, 1582–1591 (2005). [DOI] [PubMed] [Google Scholar]
- 116.O’Hanlon DE, Lanier BR, Moench TR & Cone RA Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis 10, 120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abdelmaksoud AA, et al. Comparison of Lactobacillus crispatus isolates from Lactobacillus-dominated vaginal microbiomes with isolates from microbiomes containing bacterial vaginosis-associated bacteria. Microbiology (Reading) 162, 466–475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stoyancheva G, Marzotto M, Dellaglio F & Torriani S Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol 196, 645–653 (2014). [DOI] [PubMed] [Google Scholar]
- 119.Sillanpaa J, et al. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. Journal of Bacteriology 182, 6440–6450 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toba T, et al. A collagen-binding S-layer protein in Lactobacillus crispatus. Applied and Environmental Microbiology 61, 2467–2471 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kobatake E & Kabuki T S-Layer Protein of Lactobacillus helveticus SBT2171 Promotes Human beta-Defensin 2 Expression via TLR2-JNK Signaling. Front Microbiol 10, 2414 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abramov VM, et al. S-layer protein 2 of Lactobacillus crispatus 2029, its structural and immunomodulatory characteristics and roles in protective potential of the whole bacteria against foodborne pathogens. Int J Biol Macromol 150, 400–412 (2020). [DOI] [PubMed] [Google Scholar]
- 123.Kwok L, et al. Adherence of Lactobacillus crispatus to vaginal epithelial cells from women with or without a history of recurrent urinary tract infection. J Urol 176, 2050–2054; discussion 2054 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Osset J, Bartolome RM, Garcia E & Andreu A Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. The Journal of Infectious Disease 183, 485–491 (2001). [DOI] [PubMed] [Google Scholar]
- 125.Anahtar MN, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42, 965–976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McLaren MR & Callahan BJ Pathogen resistance may be the principal evolutionary advantage provided by the microbiome. Philos Trans R Soc Lond B Biol Sci 375, 20190592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grace JB, et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Howe L, et al. Mucinase and sialidase activity of the vaginal microflora: implications for the pathogensis of preterm labour. International Journal of STD and AIDS 10, 442–447 (1999). [DOI] [PubMed] [Google Scholar]
- 129.Briselden AM, Moncla BJ, Stevens CE & Hillier SL Sialidases (Neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. Journal of Clinical Microbiology 30, 663–666 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robinson LS, Schwebke J, Lewis WG & Lewis AL Identification and characterization of NanH2 and NanH3, enzymes responsible for sialidase activity in the vaginal bacterium Gardnerella vaginalis. Journal of Biological Chemistry 294, 5230–5245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hardy L, et al. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One 12, e0172522 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis WG, Robinson LS, Gilbert NM, Perry JC & Lewis AL Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J Biol Chem 288, 12067–12079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smayevsky J, Canigia LF, Lanza A & Bianchini H Vaginal microflora associated with bacterial vaginosis in nonpregnant women: reliability of sialidase detection. Infect Dis Obstet Gynecol 9, 17–22 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rampersaud R, et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol 193, 1034–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gelber SE, Aguilar JL, Lewis KL & Ratner AJ Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol 190, 3896–3903 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nelson TM, et al. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front Physiol 6, 253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petrova MI, Reid G, Vaneechoutte M & Lebeer S Lactobacillus iners: Friend or Foe? Trends Microbiol 25, 182–191 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Munoz A, et al. Modeling the temporal dynamics of cervicovaginal microbiota identifies targets that may promote reproductive health. Microbiome 9, 163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Macklaim JM, et al. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 1(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.France MT, et al. Insight into the ecology of vaginal bacteria through integrative analyses of metagenomic and metatranscriptomic data. bioRvix (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bloom SM, et al. Cysteine dependence in Lactobacillus iners constitutes a novel therapeutic target to modify the vaginal microbiota. Nature Microbiology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Amsel R, et al. Nonspecific Vaginitis: Diagnostic criteria and microbial and epidemiological associations. Clinical Studies 74, 14–22 (1983). [DOI] [PubMed] [Google Scholar]
- 143.Nugent RP, Krohn MA & Hillier SL Reliability of diagnosing bacterial vaginosis is improved by standardized method of gram stain Interpretation. in Journal of Clinical Microbiology, Vol. 29 297–301 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Workowski K, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 70, 1–187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verwijs MC, Agaba SK, Darby AC & van de Wijgert J Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am J Obstet Gynecol 222, 157 e151–157 e113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ferris DG, Litaker MS, Woodward L, Mathis D & Hendrich J Treatment of bacterial vaginosis: a comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. Journal of Family Practice 41, 443–450 (1995). [PubMed] [Google Scholar]
- 147.Muzny CA & Schwebke JR Asymptomatic Bacterial Vaginosis: To Treat or Not to Treat? Curr Infect Dis Rep 22(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bhandari P, Tingley JP, Palmer DRJ, Abbott DW & Hill JE Characterization of an alpha-glucosidase enzyme conserved in Gardnerella spp. isolated from the human vaginal microbiome. Journal of Bacteriology 203, e00213–00221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nunn KL, et al. Vaginal Glycogen, Not Estradiol, Is Associated With Vaginal Bacterial Community Composition in Black Adolescent Women. J Adolesc Health 65, 130–138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ragaliauskas T, et al. Inerolysin and vaginolysin, the cytolysins implicated in vaginal dysbiosis, differently impair molecular integrity of phospholipid membranes. Sci Rep 9, 10606 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Randis TM, et al. Vaginolysin drives epithelial ultrastructural responses to Gardnerella vaginalis. Infect Immun 81, 4544–4550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thapa R, Ray S & Keyel PA Interaction of Macrophages and Cholesterol-Dependent Cytolysins: The Impact on Immune Response and Cellular Survival. Toxins (Basel) 12(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Hanlon DE, Gajer P, Brotman RM & Ravel J Asymptomatic Bacterial Vaginosis Is Associated With Depletion of Mature Superficial Cells Shed From the Vaginal Epithelium. Front Cell Infect Microbiol 10, 106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Srinivasan S, et al. Metabolic Signatures of Bacterial Vaginosis. mBio 6(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chattopadhyay MK, Tabor CW & Tabor H Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc Natl Acad Sci U S A 100, 2261–2265 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Borgogna JL, et al. Biogenic amines increase the odds of bacterial vaginosis and affect the growth of and lactic acid production by vaginal Lactobacillus spp. Appl Environ Microbiol 87, e03068–03020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cruden DL & Galask RP Reduction of Trimethylamine Oxide to Trimethylamine by Mobiluncus Strains Isolated from Patients with Bacterial Vaginosis. Microbial Ecology in Health and Disease 1, 95–100 (2009). [Google Scholar]
- 158.Fredricks DN, Fiedler TL & Marrazzo JM Molecular identification of bacteria associated with bacterial vaginosis. in The New England journal of medicine, Vol. 353 1899–1911 (2005). [DOI] [PubMed] [Google Scholar]
- 159.Piot P, et al. Biotypes of Gardnerella vaginalis. Journal of Clinical Microbiology 20, 677–679 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vaneechoutte M, et al. Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int J Syst Evol Microbiol 69, 679–687 (2019). [DOI] [PubMed] [Google Scholar]
- 161.Castro J, Jefferson KK & Cerca N Genetic Heterogeneity and Taxonomic Diversity among Gardnerella Species. Trends Microbiol 28, 202–211 (2020). [DOI] [PubMed] [Google Scholar]
- 162.Janulaitiene M, et al. Phenotypic characterization of Gardnerella vaginalis subgroups suggests differences in their virulence potential. PLoS One 13, e0200625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahmed A, et al. Comparative genomic analyses of 17 clinical isolates of Gardnerella vaginalis provide evidence of multiple genetically isolated clades consistent with subspeciation into genovars. J Bacteriol 194, 3922–3937 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Serrano MG, et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat Med 25, 1001–1011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marconi C, et al. Characterization of the Vaginal Microbiome in Women of Reproductive Age From 5 Regions in Brazil. Sex Transm Dis 47, 562–569 (2020). [DOI] [PubMed] [Google Scholar]
- 166.Borgdorff H, et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS One 12, e0181135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yildirim S, et al. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. in ISME Journal, Vol. 8 2431, 2444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rhoades NS, et al. Longitudinal Profiling of the Macaque Vaginal Microbiome Reveals Similarities to Diverse Human Vaginal Communities. mSystems 6(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Curley T & Forster CS Recurrent UTIs in Girls: What Is the Role of the Microbiome? Urology 151, 94–97 (2021). [DOI] [PubMed] [Google Scholar]
- 170.Marild S & Jodal U Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Pædiatr 87, 549–552 (1998). [DOI] [PubMed] [Google Scholar]
- 171.Brotman RM, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 21, 450–458 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shen J, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep 6, 24380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hammerschlag MR, et al. Anaerobic microflora of the vagina in children. American Journal of Obstetrics and Gynecology 131, 853–856 (1978). [DOI] [PubMed] [Google Scholar]
- 174.Mitchell CM, et al. Association between postmenopausal vulvovaginal discomfort, vaginal microbiota, and mucosal inflammation. Am J Obstet Gynecol 225, 159 e151–159 e115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Farage M & Maibach H Lifetime changes in the vulva and vagina. in Archives of Gynecology and Obstetrics, Vol. 273 195–202 (2006). [DOI] [PubMed] [Google Scholar]
- 176.Gliniewicz K, et al. Comparison of the Vaginal Microbiomes of Premenopausal and Postmenopausal Women. Front Microbiol 10, 193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Laniewski P & Herbst-Kralovetz MM Investigating microbiome and menopause for healthy aging in women. Nature Microbiology (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bidlingmaier F, Wagner-Barnack M, Butenandt O & Knorr D Plasma estrogens in childhood and puberty under physiologic and pathologic conditions. Pediat Res 7, 901–907 (1973). [DOI] [PubMed] [Google Scholar]
- 179.Xiaoming W, et al. Characteristics of the vaginal microbiomes in prepubertal girls with and without vulvovaginitis. Eur J Clin Microbiol Infect Dis 40, 1253–1261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hickey RJ, et al. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. in mBio, Vol. 6 e00097–00015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Martin HL, et al. Vaginal Lactobacilli, Microbial Flora, and Risk of Human Immunodeficiency Virus Type 1 and Sexually Transmitted Disease Acquisition. The Journal of Infectious Diseases 180, 1863–1868 (1999). [DOI] [PubMed] [Google Scholar]
- 182.Cohen CR, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med 9, e1001251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Borgdorff H, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J 8, 1781–1793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gosmann C, et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 46, 29–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McClelland RS, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 18, 554–564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brotman RM Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest 121, 4610–4617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cherpes TL, Meyn LA, Krohn MA, Lurie JG & Hillier SL Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 37, 319–325 (2003). [DOI] [PubMed] [Google Scholar]
- 188.Balkus JE, et al. Bacterial vaginosis and the risk of trichomonas vaginalis acquisition among HIV-1-negative women. Sex Transm Dis 41, 123–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van Houdt R, et al. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: a case-control study. Sex Transm Infect 94, 117–123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee JE, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 8, e63514 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Norenhag J, et al. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG 127, 171–180 (2020). [DOI] [PubMed] [Google Scholar]
- 192.Brusselaers N, Shrestha S, van de Wijgert J & Verstraelen H Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol 221, 9–18 e18 (2019). [DOI] [PubMed] [Google Scholar]
- 193.Oh HY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect 21, 674 e671–679 (2015). [DOI] [PubMed] [Google Scholar]
- 194.Mitra A, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep 5, 16865 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shannon B, et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol 10, 1310–1319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.King CC, et al. Bacterial vaginosis and the natural history of human papillomavirus. Infect Dis Obstet Gynecol 2011, 319460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Watts DH, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis 191, 1129–1139 (2005). [DOI] [PubMed] [Google Scholar]
- 198.Brotman RM, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis 210, 1723–1733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Di Paola M, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci Rep 7, 10200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gupta K, et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis 178, 446–450 (1998). [DOI] [PubMed] [Google Scholar]
- 201.Kirjavainen PV, et al. Abnormal immunological profile and vaginal microbiota in women prone to urinary tract infections. Clin Vaccine Immunol 16, 29–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.McClelland RS, et al. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis 199, 1883–1890 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou X, et al. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect Immun 77, 4130–4135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brown SE, et al. The Vaginal Microbiota and Behavioral Factors Associated With Genital Candida albicans Detection in Reproductive-Age Women. Sex Transm Dis 46, 753–758 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ness RB, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 162, 585–590 (2005). [DOI] [PubMed] [Google Scholar]
- 206.Haggerty CL, et al. Presence and Concentrations of Select Bacterial Vaginosis-Associated Bacteria Are Associated With Increased Risk of Pelvic Inflammatory Disease. Sex Transm Dis 47, 344–346 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Haggerty CL, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect 92, 441–446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hillier SL, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 333, 1737–1742 (1995). [DOI] [PubMed] [Google Scholar]
- 209.Leitich H, et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 189, 139–147 (2003). [DOI] [PubMed] [Google Scholar]
- 210.Fettweis JM, et al. The vaginal microbiome and preterm birth. Nat Med 25, 1012–1021 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Petricevic L, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep 4, 5136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Romero R, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2, 18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Freitas AC, Bocking A, Hill JE, Money DM & Group VR Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 6, 117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Peelen MJ, et al. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta 79, 30–39 (2019). [DOI] [PubMed] [Google Scholar]
- 215.Gustin AT, et al. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am J Obstet Gynecol (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Deng Z-L, et al. Metatranscriptome analysis of the vaginal microbiota reveals potential mechanisms for recurrence and protection against metronidazole in bacterial vaginosis. in bioRxiv, Vol. 3 1–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Larsson PG & Forsum U Bacterial vaginosis-a disturbed bacterial flora and treatment enigma. APMIS 113, 305–316 (2005). [DOI] [PubMed] [Google Scholar]
- 218.Plummer EL, et al. Lactic acid-containing products for bacterial vaginosis and their impact on the vaginal microbiota: A systematic review. PLoS One 16, e0246953 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Reichman O, Akins R & Sobel JD Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 36, 732–734 (2009). [DOI] [PubMed] [Google Scholar]
- 220.Macklaim JM, Clemente JC, Knight R, Gloor GB & Reid G Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microbial Ecology in Health & Disease 26(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Husain S, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG 127, 275–284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oerlemans EFM, et al. Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci Rep 10, 7976 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Marcotte H, et al. An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infectious Diseases 19(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bohbot JM, et al. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. J Gynecol Obstet Hum Reprod 47, 81–86 (2018). [DOI] [PubMed] [Google Scholar]
- 225.Mastromarino P, Vitali B & Mosca L Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiologica 36, 229–238 (2013). [PubMed] [Google Scholar]
- 226.Stapleton AE, et al. Randomized, placebo-controlled phase 2 trial of a lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. in Clinical Infectious Diseases, Vol. 52 1212–1217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Reid G, Beuerman D, Heinemann C & Bruce AW Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol 32, 37–41 (2001). [DOI] [PubMed] [Google Scholar]
- 228.Cohen CR, et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N Engl J Med 382, 1906–1915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rohlke F & Stollman N Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol 5, 403–420 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liwinski T & Elinav E Harnessing the microbiota for therapeutic purposes. Am J Transplant 20, 1482–1488 (2020). [DOI] [PubMed] [Google Scholar]
- 231.Lev-Sagie A, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med 25, 1500–1504 (2019). [DOI] [PubMed] [Google Scholar]
- 232.DeLong K, et al. Conceptual Design of a Universal Donor Screening Approach for Vaginal Microbiota Transplant. Front Cell Infect Microbiol 9, 306 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.DiGiulio DB, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 112, 11060–11065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Park JC & Im SH Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med 52, 1383–1396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kennedy EA, King KY & Baldridge MT Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front Physiol 9, 1534 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sierra LJ, et al. Colonization of the cervicovaginal space with Gardnerella vaginalis leads to local inflammation and cervical remodeling in pregnant mice. PLoS One 13, e0191524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gilbert NM, Lewis WG & Lewis AL Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One 8, e59539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gilbert NM, et al. Gardnerella vaginalis and Prevotella bivia Trigger Distinct and Overlapping Phenotypes in a Mouse Model of Bacterial Vaginosis. J Infect Dis 220, 1099–1108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Raterman EL & Jerse AE Female Mouse Model of Neisseria gonorrhoeae Infection. Methods Mol Biol 1997, 413–429 (2019). [DOI] [PubMed] [Google Scholar]
- 240.Kaser T, et al. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine 35, 91–100 (2017). [DOI] [PubMed] [Google Scholar]
- 241.Policicchio BB, Pandrea I & Apetrei C Animal Models for HIV Cure Research. Front Immunol 7, 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.De Clercq E, Kalmar I & Vanrompay D Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun 81, 3060–3067 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ogawa-Tominaga M, Umezu T, Nakajima T & Tomooka Y Stratification of mouse vaginal epithelium. 1. Development of three-dimensional models in vitro with clonal cell lines. Biol Reprod 99, 718–726 (2018). [DOI] [PubMed] [Google Scholar]
- 244.Pyles RB, et al. Cultivated vaginal microbiomes alter HIV-1 infection and antiretroviral efficacy in colonized epithelial multilayer cultures. PLoS One 9, e93419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Barrila J, et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol 8, 791–801 (2010). [DOI] [PubMed] [Google Scholar]
- 246.Hjelm BE, Berta AN, Nickerson CA, Arntzen CJ & Herbst-Kralovetz MM Development and characterization of a three-dimensional organotypic human vaginal epithelial cell model. Biol Reprod 82, 617–627 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xiao S, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8, 14584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rothbauer M, Zirath H & Ertl P Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab Chip 18, 249–270 (2018). [DOI] [PubMed] [Google Scholar]
- 249.Stahl PL, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016). [DOI] [PubMed] [Google Scholar]
- 250.Rodriques SG, et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


