Summary
Individuals often respond differently to identical treatments, and characterizing such variability in treatment response is an important aim in the practice of personalized medicine. In this article, we describe a nonparametric accelerated failure time model that can be used to analyze heterogeneous treatment effects (HTE) when patient outcomes are time-to-event. By utilizing Bayesian additive regression trees and a mean-constrained Dirichlet process mixture model, our approach offers a flexible model for the regression function while placing few restrictions on the baseline hazard. Our nonparametric method leads to natural estimates of individual treatment effect and has the flexibility to address many major goals of HTE assessment. Moreover, our method requires little user input in terms of model specification for treatment covariate interactions or for tuning parameter selection. Our procedure shows strong predictive performance while also exhibiting good frequentist properties in terms of parameter coverage and mitigation of spurious findings of HTE. We illustrate the merits of our proposed approach with a detailed analysis of two large clinical trials (N = 6769) for the prevention and treatment of congestive heart failure using an angiotensin-converting enzyme inhibitor. The analysis revealed considerable evidence for the presence of HTE in both trials as demonstrated by substantial estimated variation in treatment effect and by high proportions of patients exhibiting strong evidence of having treatment effects which differ from the overall treatment effect.
Keywords: Dirichlet process mixture, Ensemble methods, Heterogeneity of treatment effect, Interaction, Personalized medicine, Subgroup analysis
1. Introduction
While the main focus of clinical trials is on evaluating the average effect of a particular treatment, assessing heterogeneity in treatment effect (HTE) across key patient sub-populations remains an important task in evaluating the results of clinical studies. Accurate evaluations of HTE that is attributable to variation in baseline patient characteristics offers many potential benefits in terms of informing patient decision-making and in appropriately targeting existing therapies. HTE assessment can encompass a wide range of goals: quantification of overall heterogeneity in treatment response, identification of important patient characteristics related to HTE, estimation of proportion who benefits from the treatment, identification of patient sub-populations deriving most benefit from treatment, detection of cross-over (qualitative) interactions, identifying patients who are harmed by treatment, estimation of individualized treatment effects, optimal treatment allocation for individuals, and predicting treatment effect for a future patient.
Recently, there has been increasing methodology development in the arena of HTE assessment. However, each developed method has usually been targeted to address one specific goal of HTE analysis. For example, Xu and others (2015) and Foster and others (2011) proposed methods to identify patient subgroups whose response to treatment differs substantially from the average treatment effect. Weisberg and Pontes (2015) and Lamont and others (2018) discuss estimation of individualized treatment effects. Zhao and others (2012) discuss construction of optimal individualized treatment rules through minimization of a weighted classification error. Shen and Cai (2016) focus on detection of biomarkers which are predictive of treatment effect heterogeneity. Thus, most existing methods are not sufficiently flexible to address multiple goals of HTE analysis.
The aim of this article is to construct a unified methodology for analyzing and exploring HTE with a particular focus on cases where the responses are time-to-event. The methodology is readily extended to continuous and binary response data. The motivation for investigating such a framework is the recognition that most, if not all, of the above-stated goals of personalized medicine could be directly addressed if a sufficiently rich approximation to the true data generating model for patient outcomes were available. Bayesian nonparametric methods are well-suited to provide this more unified framework for HTE analysis because they place few a priori restrictions on the form of the data-generating model and provide great adaptivity. Bayesian nonparametrics allow construction of flexible models for patient outcomes coupled with probability modeling of all unknown quantities which generates a full posterior distribution over the desired response surface. This allows researchers to directly address a wide range of inferential targets without the need to fit a series of separate models or to employ a series of different procedures. Our methodology has the flexibility to address all of the HTE goals previously highlighted. For example, researchers could quantify overall HTE; identify most important patient characteristics pertaining to HTE; estimate the proportion benefiting from, or harmed by, the treatment; and predict treatment effect for a future patient.
Bayesian additive regression trees (BART) (Chipman and others, 2010) provide a flexible means of modeling patient outcomes without the need for making specific parametric assumptions, specifying a functional form for a regression model, or for using pre-specified patient subgroups. Because it relies on an ensemble of regression trees, BART has the capability to automatically detect non-linearities and covariate interactions. As reported by Hill (2011) in the context of using BART for causal inference, BART has the advantage of exhibiting strong predictive performance in a variety of settings while requiring little user input in terms of selecting tuning parameters. Crucially, using BART for HTE analysis also allows the user to avoid the need to pre-specify patient subgroups or to specify a potentially large number of treatment-covariate interaction terms. While tree-based methods have been employed in the context of personalized medicine and subgroup identification by a variety of investigators including, for example, Su and others (2009), Loh and others (2015), and Foster and others (2011), BART offers several advantages for the analysis of HTE. In contrast to many other tree-based procedures that use a more algorithmic approach, BART is model-based and utilizes a full likelihood function and corresponding prior over the tree-related parameters. Because of this, BART automatically generates measures of posterior uncertainty; on the other hand, reporting uncertainty intervals is often quite challenging for other frequentist tree-based procedures though there has been interesting recent work on constructing confidence intervals for random forests (Wager and others, 2014; Wager and Athey, 2015). In addition, because inference with BART relies on posterior sampling, analysis of HTE on alternative treatment scales can be done directly by simply transforming the desired parameters in posterior sampling. Moreover, any quantity of interest for individualized decisions or HTE evaluation can be readily accommodated by the Bayesian framework. In this article, we aim to utilize and incorporate these advantages of BART into our approach for analyzing HTE with censored data.
Accelerated failure time (AFT) models (Wei, 1992) represent an alternative to Cox-proportional hazards models in the analysis of time-to-event data. AFT models have a number of features which make them appealing in the context of personalized medicine and investigating the comparative effectiveness of different treatments. Because they involve a regression with log-failure times as the response variable, AFT models provide a direct interpretation of the relationship between patient covariates and failure times. Moreover, treatment effects may be defined directly in terms of the underlying failure times for the two different treatments.
Bayesian semi-parametric approaches to the accelerated failure time model have been investigated by a number of authors including Komárek and Lesaffre (2007), Johnson and Christensen (1988), Kuo and Mallick (1997), Hanson and Johnson (2002), and Hanson (2006). Kuo and Mallick (1997) assume a parametric model for the regression function and suggest either modeling the distribution of the residual term or of the exponential of the residual term via a Dirichlet process (DP) mixture model, while Hanson (2006) proposed modeling the residual distribution with a DP mixture of Gamma densities. Our approach for modeling the residual distribution resembles that of Kuo and Mallick (1997). Similar to these approaches, we model the residual distribution as a location-mixture of Gaussian densities, and by utilizing constrained DPs, we constrain the mean of the residual distribution to be zero, thereby clarifying the interpretation of the regression function.
Extensions of the original BART procedure to handle time-to-event outcomes have been proposed and investigated by Bonato and others (2011) and Sparapani and others (2016). In Bonato and others (2011), the authors introduce several sum-of-trees models and examine their use in utilizing gene expression measurements for survival prediction. Among the survival models proposed by Bonato and others (2011) is an AFT model with a sum-of-trees regression function and a normally distributed residual term. Sparapani and others (2016) introduce a nonparametric approach that employs BART to directly model the individual-specific probabilities of an event occurring at the observed event and censoring times. To harness the advantages of both BART and AFT models for HTE analysis, we propose a nonparametric version of the AFT model which combines a sum-of-trees model for the regression function with a DP mixture model for the residual distribution. Such an approach has the advantage of providing great flexibility while generating interpretable measures of covariate-specific treatment effects thus facilitating the analysis of HTE.
This article is organized as follows. In Section 2, we describe the general structure of our nonparametric, tree-based AFT model, discuss its use in estimating individualized treatment effects, detail new choices for the BART hyperparameters, and describe our approach for posterior computation. Section 3 examines key inferential targets in the analysis of HTE and describes how the nonparametric AFT model may be utilized to estimate these targets. Moreover, in this section, we demonstrate the use of our nonparametric AFT method to investigate HTE in two large clinical trials involving the use of an ACE inhibitor. In Section 4, we detail the results of two simulation studies that evaluate our procedure in terms of individualized treatment effect estimation, coverage, and treatment assignment. We conclude in Section 5 with a short discussion.
2. Methods
2.1. Notation and nonparametric AFT model
We assume that study participants have been randomized to one of two treatments, which we
denote by either 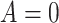 or
or 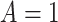 . We let
. We let
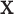 denote a
denote a
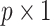 vector of baseline covariates
and let
vector of baseline covariates
and let 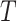 denote patient failure time. Given
censoring time
denote patient failure time. Given
censoring time 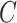 , we observe
, we observe 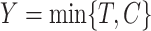 and a failure indicator
and a failure indicator 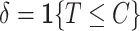 We assume
also that censoring is noninformative, that is,
We assume
also that censoring is noninformative, that is, 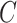 and
and
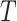 are independent given
are independent given
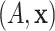 . The data consist of
. The data consist of
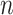 independent measurements
independent measurements
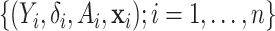 .
Although, we assume randomized treatment assignment here, our approach may certainly be
applied in observational settings. In such settings, however, one should ensure that
appropriate unconfoundedness assumptions (e.g. Hill,
2011) are reasonable, so that the individualized treatment effects defined in
(2.2) correspond to an expected
difference in potential outcomes under the two treatments.
.
Although, we assume randomized treatment assignment here, our approach may certainly be
applied in observational settings. In such settings, however, one should ensure that
appropriate unconfoundedness assumptions (e.g. Hill,
2011) are reasonable, so that the individualized treatment effects defined in
(2.2) correspond to an expected
difference in potential outcomes under the two treatments.
The conventional AFT model assumes that log-failure times are linearly related to patient
covariates. We consider here a nonparametric analogue of the AFT model in which the
failure time 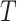 is related to the covariates and
treatment assignment through
is related to the covariates and
treatment assignment through
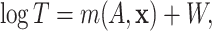 |
(2.1) |
and where the distribution of the residual term 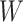 is assumed to satisfy
is assumed to satisfy
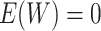 . With the mean-zero constraint on
the residual distribution, the regression function
. With the mean-zero constraint on
the residual distribution, the regression function 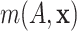 has a
direct interpretation as the expected log-failure time given treatment assignment and
baseline covariates.
has a
direct interpretation as the expected log-failure time given treatment assignment and
baseline covariates.
The AFT model (2.1) leads to a
natural, directly interpretable definition of the individualized treatment effect (ITE),
namely, the difference in expected log-failure in treatment 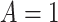 versus
versus
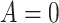 . Specifically, we define the ITE
. Specifically, we define the ITE
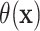 for a patient with
covariate vector
for a patient with
covariate vector 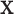 as
as
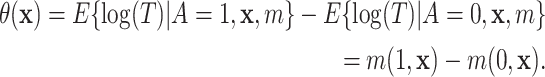 |
(2.2) |
The distribution of 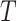 in the accelerated failure time model
(2.1) is characterized by both the
regression function
in the accelerated failure time model
(2.1) is characterized by both the
regression function 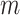 and the distribution
and the distribution
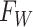 of the residual term. In the following,
we outline a model for the regression function that utilizes additive regression trees,
and we describe a flexible nonparametric mixture model for the residual distribution
of the residual term. In the following,
we outline a model for the regression function that utilizes additive regression trees,
and we describe a flexible nonparametric mixture model for the residual distribution
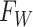 .
.
2.2. Overview of BART
BART is an ensemble method in which the regression function is represented as the sum of
individual regression trees. The BART model for the regression function relies on a
collection of 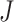 binary trees
binary trees 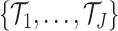 and an associated set of terminal node values
and an associated set of terminal node values 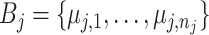 for each binary tree
for each binary tree 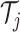 . Each tree
. Each tree
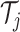 consists of a sequence of
decision rules through which any covariate vector can be assigned to one terminal node of
consists of a sequence of
decision rules through which any covariate vector can be assigned to one terminal node of
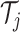 by following the decision
rules prescribed at each of the interior nodes. In other words, each binary tree generates
a partition of the predictor space where each element
by following the decision
rules prescribed at each of the interior nodes. In other words, each binary tree generates
a partition of the predictor space where each element 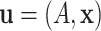 of the
predictor space belongs to exactly one of the
of the
predictor space belongs to exactly one of the  terminal nodes of
terminal nodes of
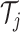 . The decision rules at the
interior nodes of
. The decision rules at the
interior nodes of 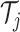 are of the form
are of the form
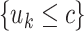 vs.
vs.
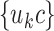 , where
, where
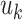 denotes the
denotes the 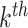 element
of
element
of 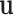 . A covariate
. A covariate
 that corresponds to the
that corresponds to the
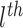 terminal node of
terminal node of
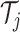 is assigned the value
is assigned the value
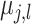 and
and 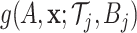 is
used to denote the function returning
is
used to denote the function returning 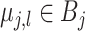 whenever
whenever  is assigned to the
is assigned to the
 terminal node of
terminal node of
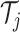 .
.
The regression function 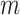 is represented in BART as a sum of the
individual tree contributions
is represented in BART as a sum of the
individual tree contributions
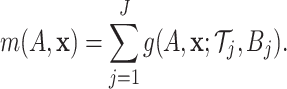 |
(2.3) |
Trees 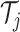 and node values
and node values
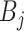 can be thought of as model parameters.
The prior distribution on these parameters induces a prior on
can be thought of as model parameters.
The prior distribution on these parameters induces a prior on  and hence induces a prior on the regression function
and hence induces a prior on the regression function  via (2.3). To complete the description of the
prior on
via (2.3). To complete the description of the
prior on  ,
one needs to specify the following: (i) the distribution on the choice of splitting
variable at each internal node; (ii) the distribution of the splitting value
,
one needs to specify the following: (i) the distribution on the choice of splitting
variable at each internal node; (ii) the distribution of the splitting value
 used at each internal node; (iii) the
probability that a node at a given node-depth
used at each internal node; (iii) the
probability that a node at a given node-depth 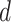 splits, which is assumed
to be equal to
splits, which is assumed
to be equal to 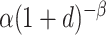 ; and (iv) the
distribution of the terminal node values
; and (iv) the
distribution of the terminal node values 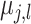 which is assumed
to be
which is assumed
to be 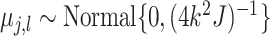 .
In order to ensure that the prior variance
.
In order to ensure that the prior variance 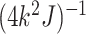 for
for
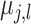 induces a prior on the regression
function that assigns high probability to the observed range of the data, Chipman and others (2010) center and
scale the response so that the minimum and maximum values of the transformed response are
induces a prior on the regression
function that assigns high probability to the observed range of the data, Chipman and others (2010) center and
scale the response so that the minimum and maximum values of the transformed response are
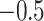 and
and 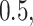 respectively.
Regarding (i), at each interior node, the splitting variable is chosen uniformly from the
set of available splitting variables. Regarding (ii), Chipman and others (2010) suggest a uniform prior on the
discrete set of available splitting values though alternative prior distributions for the
splitting value are implemented in the R package
BayesTree. We discuss our choice for the prior distribution on
the splitting values in more detail in Section
2.4.
respectively.
Regarding (i), at each interior node, the splitting variable is chosen uniformly from the
set of available splitting variables. Regarding (ii), Chipman and others (2010) suggest a uniform prior on the
discrete set of available splitting values though alternative prior distributions for the
splitting value are implemented in the R package
BayesTree. We discuss our choice for the prior distribution on
the splitting values in more detail in Section
2.4.
To denote the distribution on the regression function 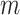 induced by the
prior distribution on
induced by the
prior distribution on 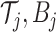 with parameter
values
with parameter
values 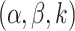 and
and
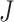 total trees, we use the notation
total trees, we use the notation
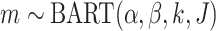 .
Choices for the hyperparameters
.
Choices for the hyperparameters 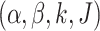 are described in more
detail in Section 2.4.
are described in more
detail in Section 2.4.
2.3. Centered DP mixture prior
We model the density 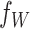 of
of 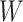 as a
location-mixture of Gaussian densities with common scale parameter
as a
location-mixture of Gaussian densities with common scale parameter
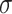 . Letting
. Letting 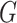 denote the
distribution of the locations, we assume the density of
denote the
distribution of the locations, we assume the density of 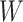 (conditional
on
(conditional
on 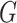 and
and 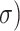 can be expressed as
can be expressed as
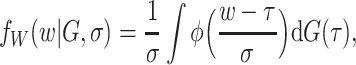 |
(2.4) |
where 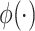 is the standard normal density
function. The DP is a widely used choice for a nonparametric prior on an unknown
probability distribution, and when placing a DP prior on G, the resulting DP mixture model
for the distribution of
is the standard normal density
function. The DP is a widely used choice for a nonparametric prior on an unknown
probability distribution, and when placing a DP prior on G, the resulting DP mixture model
for the distribution of 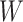 provides a flexible prior for the
residual density. Indeed, a DP mixture model similar to (2.4) was used by Kuo and Mallick
(1997) as a prior for a smooth residual distribution in a semi-parametric
accelerated failure time model. The Gaussian location-mixture model in (2.4) is also similar to the flexible
approach described in Komárek and others
(2005) for modeling the residual distribution in an AFT setting.
provides a flexible prior for the
residual density. Indeed, a DP mixture model similar to (2.4) was used by Kuo and Mallick
(1997) as a prior for a smooth residual distribution in a semi-parametric
accelerated failure time model. The Gaussian location-mixture model in (2.4) is also similar to the flexible
approach described in Komárek and others
(2005) for modeling the residual distribution in an AFT setting.
Because of the zero-mean constraint on the residual distribution, the DP is not an
appropriate choice for a prior on 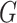 . A direct approach proposed by Yang and others (2010) addresses the
problem of placing mean and variance constraints on an unknown probability measure by
utilizing a parameter-expanded version of the DP which the authors refer to as the
centered DP (CDP). As formulated by Yang and
others (2010), the CDP with mass parameter
. A direct approach proposed by Yang and others (2010) addresses the
problem of placing mean and variance constraints on an unknown probability measure by
utilizing a parameter-expanded version of the DP which the authors refer to as the
centered DP (CDP). As formulated by Yang and
others (2010), the CDP with mass parameter 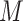 and base
measure
and base
measure 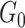 has the following stick-breaking
representation
has the following stick-breaking
representation
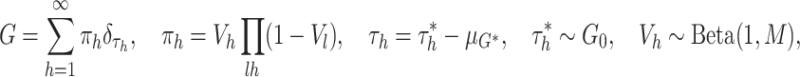 |
where 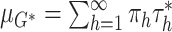 and where
and where 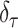 denotes a distribution
consisting only of a point mass at
denotes a distribution
consisting only of a point mass at 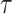 . We denote that a random measure
. We denote that a random measure
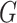 follows a CDP with the notation
follows a CDP with the notation
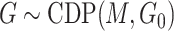 . From the
above representation of the CDP, it is clear that the mixture model (2.4) for
. From the
above representation of the CDP, it is clear that the mixture model (2.4) for  and the
assumption that
and the
assumption that 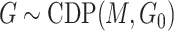 together
imply the mean-zero constraint, since the expectation of
together
imply the mean-zero constraint, since the expectation of  may be
expressed as
may be
expressed as
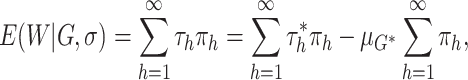 |
which equals zero almost surely.
For the scale parameter 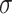 of
of 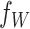 , we assume
that
, we assume
that 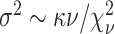 ,
with the default degrees of freedom
,
with the default degrees of freedom 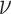 set to
set to 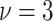 . Instead
of specifying a particular value for the mass parameter, we allow for learning about this
parameter by assuming
. Instead
of specifying a particular value for the mass parameter, we allow for learning about this
parameter by assuming 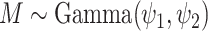 where
where 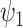 and
and 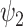 refer
to the shape and rate parameters of the Gamma distribution, respectively.
refer
to the shape and rate parameters of the Gamma distribution, respectively.
Our nonparametric model that combines the BART model for the regression function and DP mixture model for the residual density can now be expressed hierarchically as
| (2.5) |
In our implementation, the base measure 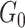 is assumed to be
Gaussian with mean zero and variance
is assumed to be
Gaussian with mean zero and variance 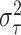 . Choosing
. Choosing
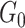 to be conjugate to the Normal
distribution simplifies posterior computation considerably, but other choices of
to be conjugate to the Normal
distribution simplifies posterior computation considerably, but other choices of
 could be considered. For example, a
t-distributed base measure could be implemented by introducing an additional latent scale
parameter.
could be considered. For example, a
t-distributed base measure could be implemented by introducing an additional latent scale
parameter.
2.4. Prior specification
2.4.1. Prior for trees.
For the hyperparameters of the trees 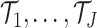 , we
defer to the defaults suggested in Chipman and
others (2010); namely,
, we
defer to the defaults suggested in Chipman and
others (2010); namely, 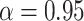 ,
,
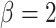 , and
, and 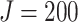 . These
default settings seem to work quite well in practice, and in part F of the supplementary material available
at Biostatistics online, we investigate the impact of varying
. These
default settings seem to work quite well in practice, and in part F of the supplementary material available
at Biostatistics online, we investigate the impact of varying
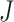 through cross-validation estimates of
prediction performance. Our choice for the prior distribution of the splitting value
through cross-validation estimates of
prediction performance. Our choice for the prior distribution of the splitting value
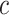 is uniform over the covariate quantiles
which is is based on the implementation in the BayesTree package
(Chipman and McCulloch, 2016). Further details
are provided in part D of the supplementary material available at Biostatistics online.
is uniform over the covariate quantiles
which is is based on the implementation in the BayesTree package
(Chipman and McCulloch, 2016). Further details
are provided in part D of the supplementary material available at Biostatistics online.
2.4.2. Prior for terminal node parameters.
As discussed in Section 2.2, the original
description of BART in Chipman and
others (2010) employs a transformation of the response variable and
sets the hyperparameter 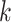 to
to 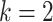 so that the
regression function is assigned substantial prior probability to the observed range of
the response. Because our responses
so that the
regression function is assigned substantial prior probability to the observed range of
the response. Because our responses 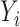 are right-censored,
we propose an alternative approach to transforming the responses and to setting the
prior variance of the terminal node parameters. Our suggested approach is to first fit a
parametric AFT model that only has an intercept in the model and that assumes log-normal
residuals. This produces estimates of the intercept
are right-censored,
we propose an alternative approach to transforming the responses and to setting the
prior variance of the terminal node parameters. Our suggested approach is to first fit a
parametric AFT model that only has an intercept in the model and that assumes log-normal
residuals. This produces estimates of the intercept 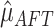 and the
residual scale
and the
residual scale 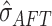 which allows us to
define transformed “centered” responses
which allows us to
define transformed “centered” responses 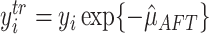 .
Turning to the prior variance of the terminal node parameters
.
Turning to the prior variance of the terminal node parameters 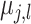 , we
assign the terminal node values the prior distribution
, we
assign the terminal node values the prior distribution 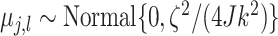 ,
where
,
where 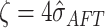 . This prior
on
. This prior
on 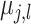 induces a
induces a
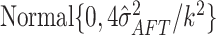 prior on the regression function
prior on the regression function  and hence assigns
approximately
and hence assigns
approximately 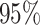 prior probability to the interval
prior probability to the interval
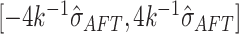 .
Thus, the default setting of
.
Thus, the default setting of 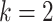 assigns
assigns 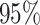 prior
probability to the interval
prior
probability to the interval 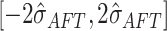 .
Note that assigning most of the prior probability to the interval
.
Note that assigning most of the prior probability to the interval
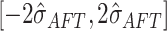 is sensible because this corresponds to the regression function for the “centered”
responses
is sensible because this corresponds to the regression function for the “centered”
responses 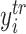 rather than the original
responses.
rather than the original
responses.
2.4.3. Residual distribution prior.
Under the assumed prior for the mass parameter, we have 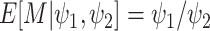 and
and 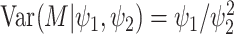 .
We set
.
We set 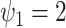 and
and
 so that the resulting
prior on
so that the resulting
prior on 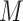 is relatively diffuse with
is relatively diffuse with
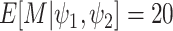 ,
,
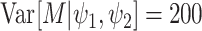 .
.
When setting the defaults for the remaining hyperparameters 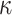 and
and
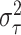 , we adopt a similar
strategy to that used by Chipman and
others (2010) for BART when calibrating the prior for the residual
variance. There, they rely on a preliminary, rough overestimate
, we adopt a similar
strategy to that used by Chipman and
others (2010) for BART when calibrating the prior for the residual
variance. There, they rely on a preliminary, rough overestimate
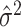 of the residual variance
parameter
of the residual variance
parameter 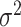 and define the prior for
and define the prior for
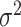 in such a way that there is
in such a way that there is
 prior probability that
prior probability that
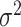 is greater than the rough
estimate
is greater than the rough
estimate 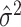 . Here,
. Here,
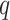 may be regarded as an additional
hyperparameter with the value of
may be regarded as an additional
hyperparameter with the value of 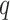 determining how conservative the prior
of
determining how conservative the prior
of 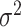 is relative to the initial
estimate of the residual variance. Chipman and
others (2010) suggest using
is relative to the initial
estimate of the residual variance. Chipman and
others (2010) suggest using 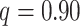 as the default
whenever
as the default
whenever 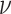 is set to
is set to  .
.
Similar to the approach described above, we begin with a rough over-estimate
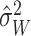 of the variance of
of the variance of
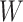 to calibrate our choices of
to calibrate our choices of
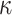 and
and 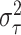 . A direct way of
generating the estimate
. A direct way of
generating the estimate 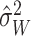 is to fit a
parametric AFT model with log-normal residuals and use the resulting estimate of the
residual variance, but other estimates could potentially be used. To connect the
estimate
is to fit a
parametric AFT model with log-normal residuals and use the resulting estimate of the
residual variance, but other estimates could potentially be used. To connect the
estimate 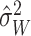 with the
hyperparameters
with the
hyperparameters 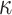 and
and 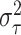 described in (2.5), it is helpful to first note that
the conditional variance of the residual term can be expressed as
described in (2.5), it is helpful to first note that
the conditional variance of the residual term can be expressed as
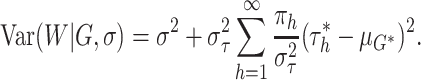 |
(2.6) |
Our aim then is to select 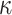 and
and 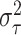 so that the induced prior
on the variance of
so that the induced prior
on the variance of 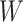 assigns approximately
assigns approximately
 probability to the event
probability to the event
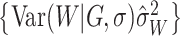 ,
where
,
where 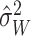 is treated here as a
fixed quantity. As an approximation to the distribution of (2.6), we use the approximation that
is treated here as a
fixed quantity. As an approximation to the distribution of (2.6), we use the approximation that
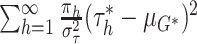 has a
has a 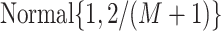 distribution (see part E of the supplementary material available at Biostatistics online for
further details about this approximation). Assuming further that
distribution (see part E of the supplementary material available at Biostatistics online for
further details about this approximation). Assuming further that
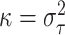 , we have that
the variance of
, we have that
the variance of 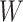 is approximately distributed as
is approximately distributed as
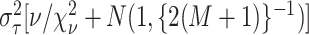 where
where 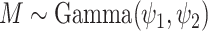 ,
and with this approximation, we can directly find a value of
,
and with this approximation, we can directly find a value of 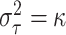 such that
such that
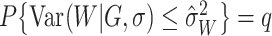 .
In contrast to the
.
In contrast to the 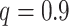 setting suggested in Chipman and others (2010), we set the
default to
setting suggested in Chipman and others (2010), we set the
default to 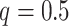 .
.
With the normal approximation to 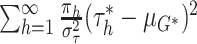 ,
the prior for
,
the prior for 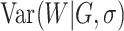 has a mean of
has a mean of
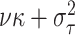 where
where
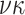 is the variance of
is the variance of
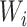 conditional on a known location
conditional on a known location
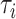 and
and 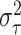 is the variance of the
locations. Thus, when
is the variance of the
locations. Thus, when 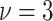 , our default setting of
, our default setting of
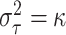 means that
roughly three-fourths of the prior variation in
means that
roughly three-fourths of the prior variation in 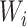 is attributable to
the variance of
is attributable to
the variance of 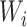 conditional on location. Rather
than fixing
conditional on location. Rather
than fixing 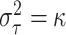 , one could
introduce an additional hyperparameter that represents the proportion of variation in
, one could
introduce an additional hyperparameter that represents the proportion of variation in
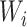 that is due to variation conditional
on location, but we have chosen to fix
that is due to variation conditional
on location, but we have chosen to fix 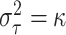 in order to keep
the number of model hyperparameters manageable.
in order to keep
the number of model hyperparameters manageable.
2.5. Posterior computation
The original Metropolis-within-Gibbs sampler proposed in Chipman and others (2010) works by sequentially updating each
tree while holding all other 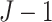 trees fixed. As a result, each
iteration of the Gibbs sampler consists of
trees fixed. As a result, each
iteration of the Gibbs sampler consists of 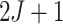 steps where the
first
steps where the
first 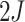 steps involve updating either one of the
trees
steps involve updating either one of the
trees 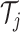 or terminal node parameters
or terminal node parameters
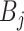 and the last step involves updating the
residual variance parameter. The Metropolis-Hastings algorithm used to update the
individual trees is discussed in Chipman and
others (1998). Our strategy for posterior computation is a direct
extension of the original Gibbs sampler, viz., after updating trees and terminal node
parameters, we update the parameters related to the residual distribution. In addition,
censored values are handled through a data augmentation approach where unobserved survival
times are imputed in each Gibbs iteration.
and the last step involves updating the
residual variance parameter. The Metropolis-Hastings algorithm used to update the
individual trees is discussed in Chipman and
others (1998). Our strategy for posterior computation is a direct
extension of the original Gibbs sampler, viz., after updating trees and terminal node
parameters, we update the parameters related to the residual distribution. In addition,
censored values are handled through a data augmentation approach where unobserved survival
times are imputed in each Gibbs iteration.
To sample from the posterior of the CDP, we adopt the blocked Gibbs sampling approach
described in Ishwaran and James (2001). In this
approach, the mixing distribution 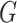 is truncated so that it only has a large,
finite number of components
is truncated so that it only has a large,
finite number of components 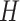 which is done by assuming that,
which is done by assuming that,
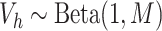 for
for
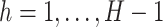 and
and
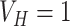 . This modification of the
stick-breaking weights ensures that
. This modification of the
stick-breaking weights ensures that 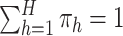 . One advantage
of using the truncation approximation is that it makes posterior inferences regarding
. One advantage
of using the truncation approximation is that it makes posterior inferences regarding
 straightforward. Additionally, when
truncating the stick-breaking distribution, using the CDP prior as opposed to a DP prior
does not present any additional challenges for posterior computation because the
unconstrained parameters
straightforward. Additionally, when
truncating the stick-breaking distribution, using the CDP prior as opposed to a DP prior
does not present any additional challenges for posterior computation because the
unconstrained parameters 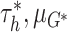 in (2.5) may be updated as described in
Ishwaran and James (2001) with the parameters of
interest
in (2.5) may be updated as described in
Ishwaran and James (2001) with the parameters of
interest 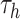 then being updated through the
simple transformation
then being updated through the
simple transformation 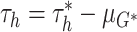 . The
upper bound on the number of components
. The
upper bound on the number of components 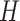 should be chosen to be
relatively large (as a default, we set
should be chosen to be
relatively large (as a default, we set 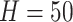 ), and in the Gibbs
sampler, the maximum index of the occupied clusters should be monitored. If a maximum
index equal to
), and in the Gibbs
sampler, the maximum index of the occupied clusters should be monitored. If a maximum
index equal to 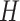 occurs frequently in posterior sampling,
occurs frequently in posterior sampling,
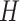 should be increased. A detailed description
of our Metropolis-within-Gibbs sampler used for posterior computation is given in part A
of the supplementary material
available at Biostatistics online. It is worth mentioning that in our
implementation, we assume that there is no missing data. A number of missing-data models
for the covariates could potentially be directly incorporated into our posterior sampling
scheme. In part H of the supplementary
material available at Biostatistics online, we describe two
particular missing-data models for the covariates, and we discuss how they would be into
integrated into our nonparametric AFT model.
should be increased. A detailed description
of our Metropolis-within-Gibbs sampler used for posterior computation is given in part A
of the supplementary material
available at Biostatistics online. It is worth mentioning that in our
implementation, we assume that there is no missing data. A number of missing-data models
for the covariates could potentially be directly incorporated into our posterior sampling
scheme. In part H of the supplementary
material available at Biostatistics online, we describe two
particular missing-data models for the covariates, and we discuss how they would be into
integrated into our nonparametric AFT model.
3. Posterior Inferences for the Analysis of Heterogeneous Treatment Effects with an Application to Two Large Clinical Trials
The nonparametric AFT model (2.5)
generates a full posterior over the entire regression function 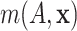 and the residual distribution. As such, this model has the flexibility to address a variety
of questions related to heterogeneity of treatment effect. In particular, we focus in this
section on the use of the nonparametric AFT model to answer the following key HTE questions:
overall variation in response to treatment, individual-specific treatment effects, evidence
for the presence of HTE, and the proportion of patients likely to benefit from treatment. We
use two large clinical trials (the SOLVD trials) to illustrate the use of the BART-based
nonparametric AFT model in addressing these HTE inferential targets. Applications of the
nonparametric AFT model to answer other HTE questions of interest from the SOLVD trial are
described in part B of the supplementary
material available at Biostatistics online.
and the residual distribution. As such, this model has the flexibility to address a variety
of questions related to heterogeneity of treatment effect. In particular, we focus in this
section on the use of the nonparametric AFT model to answer the following key HTE questions:
overall variation in response to treatment, individual-specific treatment effects, evidence
for the presence of HTE, and the proportion of patients likely to benefit from treatment. We
use two large clinical trials (the SOLVD trials) to illustrate the use of the BART-based
nonparametric AFT model in addressing these HTE inferential targets. Applications of the
nonparametric AFT model to answer other HTE questions of interest from the SOLVD trial are
described in part B of the supplementary
material available at Biostatistics online.
The Studies of Left Ventricular Dysfunction (SOLVD) were devised to investigate the
efficacy of the angiotensin-converting enzyme (ACE) inhibitor enalapril in a target
population with low left-ventricular ejection fractions. The SOLVD treatment trial (SOLVD-T)
enrolled patients determined to have a history of overt congestive heart failure, and the
SOLVD prevention trial (SOLVD-P) enrolled patients without overt congestive heart failure.
In total, 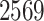 patients were enrolled in the treatment
trial while
patients were enrolled in the treatment
trial while 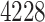 patients were enrolled in the prevention
trial. The survival endpoint that we examine in our analysis is time until death or
hospitalization where time is reported in days from enrollment.
patients were enrolled in the prevention
trial. The survival endpoint that we examine in our analysis is time until death or
hospitalization where time is reported in days from enrollment.
In our analysis of the SOLVD-T and SOLVD-P trials, we included 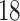 patient
covariates common to both trials, in addition to using treatment and study indicators as
covariates. These
patient
covariates common to both trials, in addition to using treatment and study indicators as
covariates. These 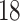 patient covariates contained information
from key patient characteristics recorded at baseline (e.g. age, sex, weight, ejection
fraction, blood pressure, sodium level, and diabetic status) along with information about
patient history (e.g. history of myocardial infarction, history of stroke, smoking history).
In our analysis, we dropped those patients who had one or more missing covariates, which
resulted in
patient covariates contained information
from key patient characteristics recorded at baseline (e.g. age, sex, weight, ejection
fraction, blood pressure, sodium level, and diabetic status) along with information about
patient history (e.g. history of myocardial infarction, history of stroke, smoking history).
In our analysis, we dropped those patients who had one or more missing covariates, which
resulted in 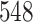 patients being dropped from the total of
patients being dropped from the total of
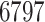 enrolled in either trial. Currently, our
software does not support an analysis where the design matrix contains missing values.
However, a number of missing-data models could be directly incorporated into our Gibbs
sampling scheme though the computational efficiency of any such scheme will of course depend
on specific model details and the size of the dataset to be analyzed. In the supplementary material available at
Biostatistics online, we discuss several potential missing-data models
and how they could be incorporated into our posterior computation scheme.
enrolled in either trial. Currently, our
software does not support an analysis where the design matrix contains missing values.
However, a number of missing-data models could be directly incorporated into our Gibbs
sampling scheme though the computational efficiency of any such scheme will of course depend
on specific model details and the size of the dataset to be analyzed. In the supplementary material available at
Biostatistics online, we discuss several potential missing-data models
and how they could be incorporated into our posterior computation scheme.
3.1. Individualized Treatment Effects
As discussed in Section 2.1, a natural
definition of the individual treatment effects in the context of an AFT model is the
difference in expected log-survival 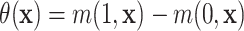 .
Draws from the posterior distribution of
.
Draws from the posterior distribution of 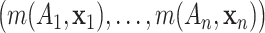 allow one to compute fully nonparametric estimates
allow one to compute fully nonparametric estimates 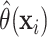 of the
treatment effects along with corresponding
of the
treatment effects along with corresponding 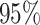 credible intervals.
As is natural with an AFT model, the treatment difference
credible intervals.
As is natural with an AFT model, the treatment difference 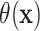 in (2.2) is examined on the scale of
log-survival time, but other, more interpretable scales on which to report treatment
effects could be easily computed. For example, ratios in expected survival times
in (2.2) is examined on the scale of
log-survival time, but other, more interpretable scales on which to report treatment
effects could be easily computed. For example, ratios in expected survival times
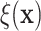 defined by
defined by
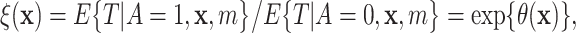 |
(3.1) |
could be estimated via posterior output. Likewise, one could estimate differences in
expected failure time by using both posterior draws of 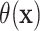 and of the residual
distribution. Posterior information regarding treatment effects may be used to stratify
patients into different groups based on anticipated treatment benefit. Stratification
could be done using the posterior mean, the posterior probability of treatment benefit, or
some other relevant measure.
and of the residual
distribution. Posterior information regarding treatment effects may be used to stratify
patients into different groups based on anticipated treatment benefit. Stratification
could be done using the posterior mean, the posterior probability of treatment benefit, or
some other relevant measure.
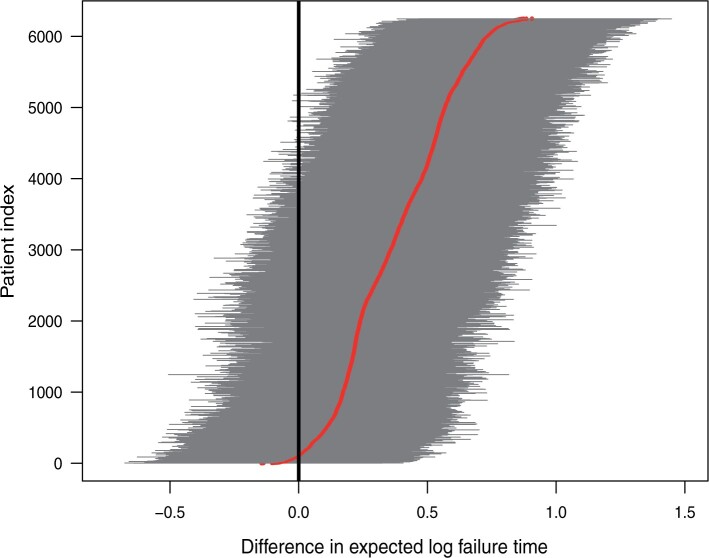
Figure 1 shows point estimates of the ITEs
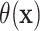 for patients in both the
SOLVD-T and SOLVD-P trials. While the plot in Figure
1 indicates a clear, overall benefit from the treatment, the variation in the
ITEs suggests substantial heterogeneity in response to treatment. In the following
subsection, we further investigate the evidence for the presence of HTE in the SOLVD
trials.
for patients in both the
SOLVD-T and SOLVD-P trials. While the plot in Figure
1 indicates a clear, overall benefit from the treatment, the variation in the
ITEs suggests substantial heterogeneity in response to treatment. In the following
subsection, we further investigate the evidence for the presence of HTE in the SOLVD
trials.
Fig. 1.
Posterior means of 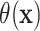 with corresponding
with corresponding
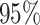 credible intervals for patients in
the SOLVD-T and SOLVD-P trials.
credible intervals for patients in
the SOLVD-T and SOLVD-P trials.
3.2. Assessing Evidence for Heterogeneity of Treatment Effect
As a way of detecting the presence of HTE, we utilize the posterior probabilities of differential treatment effect
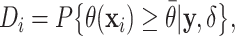 |
(3.2) |
along with the closely related quantity
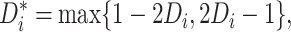 |
(3.3) |
where, in (3.2),
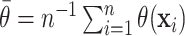 is the conditional average treatment effect. Note that
is the conditional average treatment effect. Note that 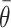 is
a model parameter that represents the average value of the individual
is
a model parameter that represents the average value of the individual
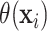 and does not
represent a posterior mean. The posterior probability
and does not
represent a posterior mean. The posterior probability 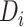 is a measure of the
evidence that the ITE
is a measure of the
evidence that the ITE 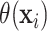 is greater or equal
to
is greater or equal
to 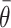 , and thus we should expect
both high and low values of
, and thus we should expect
both high and low values of 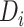 in settings where substantial HTE is
present. Note that
in settings where substantial HTE is
present. Note that 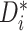 approaches
approaches
 as the value of
as the value of 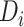 approaches
either
approaches
either 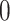 or
or 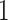 , and
, and
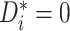 whenever
whenever
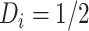 . For a given individual
. For a given individual
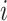 , we consider there to be strong evidence of
a differential treatment effect if
, we consider there to be strong evidence of
a differential treatment effect if 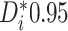 (equivalently, if
(equivalently, if
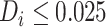 or
or
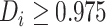 ), and we define an
individual as having mild evidence of a differential treatment effect provided that
), and we define an
individual as having mild evidence of a differential treatment effect provided that
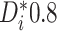 (equivalently, if
(equivalently, if
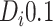 or
or 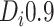 ).
For cases with no HTE present, the proportion of patients exhibiting strong evidence of
differential treatment effect should, ideally, be zero or quite close to zero. For this
reason, the proportion of patients with
).
For cases with no HTE present, the proportion of patients exhibiting strong evidence of
differential treatment effect should, ideally, be zero or quite close to zero. For this
reason, the proportion of patients with 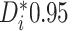 can
potentially be a useful summary measure for detecting the presence of HTE. In this
article, we do not explore explicit choices of a threshold for this proportion, but we
examine, through a simulation study in Section
4.2, the value of this proportion for scenarios where no HTE is present. It is
worth mentioning that the quantity
can
potentially be a useful summary measure for detecting the presence of HTE. In this
article, we do not explore explicit choices of a threshold for this proportion, but we
examine, through a simulation study in Section
4.2, the value of this proportion for scenarios where no HTE is present. It is
worth mentioning that the quantity 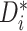 represents evidence that the
treatment effect for patient
represents evidence that the
treatment effect for patient 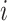 differs from the overall treatment
effect, and by itself, is not a robust indicator of HTE across patients in the trial.
Rather, the proportion of patients with high values of
differs from the overall treatment
effect, and by itself, is not a robust indicator of HTE across patients in the trial.
Rather, the proportion of patients with high values of
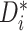 is what we use to assess evidence
for HTE.
is what we use to assess evidence
for HTE.
It is worth noting that the presence or absence of HTE depends on the treatment effect
scale, and 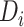 is designed for cases, such as the
AFT model, where HTE is difference in expected log-failure time. For example, it is
possible to have heterogeneity on the log-hazard ratio scale while having no heterogeneity
in the ITEs
is designed for cases, such as the
AFT model, where HTE is difference in expected log-failure time. For example, it is
possible to have heterogeneity on the log-hazard ratio scale while having no heterogeneity
in the ITEs 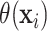 across patients.
across patients.
Examining the posterior probabilities of differential treatment effect offers further
evidence for the presence of meaningful HTE in the SOLVD trials. Table 1 shows that, in the SOLVD-T trial, approximately
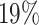 of patients had strong evidence of a
differential treatment effect (i.e.
of patients had strong evidence of a
differential treatment effect (i.e. 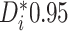 ), and approximately
), and approximately
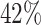 of patients had mild evidence (i.e.
of patients had mild evidence (i.e.
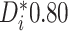 ). In the SOLVD-P trial,
approximately
). In the SOLVD-P trial,
approximately 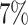 of patients had strong evidence of a
differential treatment effect while approximately
of patients had strong evidence of a
differential treatment effect while approximately  had mild evidence.
Comparison of these percentages with the results from the simulations of Section 4.2 suggests the presence of HTE. In the null
simulation scenarios of Section 4.2, the
proportion of cases with strong evidence of differential treatment was very close to zero.
Thus, the large proportion of patients with strong evidence for differential treatment
effect is an indication that there is HTE in the SOLVD trials that deserves further
exploration.
had mild evidence.
Comparison of these percentages with the results from the simulations of Section 4.2 suggests the presence of HTE. In the null
simulation scenarios of Section 4.2, the
proportion of cases with strong evidence of differential treatment was very close to zero.
Thus, the large proportion of patients with strong evidence for differential treatment
effect is an indication that there is HTE in the SOLVD trials that deserves further
exploration.
Table 1.
Tabulation of posterior probabilities of treatment benefit and posterior
probabilities of differential treatment effect 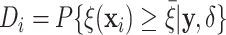 for patients in the SOLVD trials
for patients in the SOLVD trials
| SOLVD Treatment Trial | SOLVD Prevention Trial | |
|---|---|---|
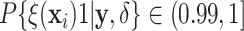
|
51.38 | 20.47 |
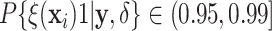
|
24.69 | 23.71 |
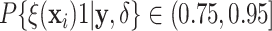
|
20.08 | 41.98 |
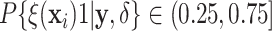
|
3.85 | 13.84 |
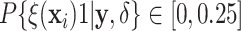
|
0.00 | 0.00 |
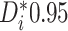
|
19.36 | 7.30 |
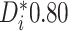
|
41.93 | 31.58 |
For each trial, the empirical percentage of patients whose estimated posterior
probability of treatment benefit lies within each of the intervals
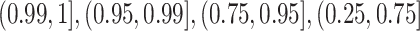 ,
and
,
and 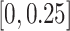 is reported. In addition,
the percentages of patients in each trial that exhibit “strong” (i.e.
is reported. In addition,
the percentages of patients in each trial that exhibit “strong” (i.e.
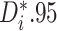 ) and “mild” (i.e.
) and “mild” (i.e.
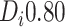 ) evidence of
differential treatment effect are shown.
) evidence of
differential treatment effect are shown.
3.3. Characterizing heterogeneity of treatment effect
Variability in treatment effect across patients in the study is a prime target of
interest when evaluating the extent of HTE from the results of a clinical trial.
Assessments of HTE can be used to evaluate consistency of response to treatment across
patient sub-populations or to assess whether or not there are patient subgroups that
appear to respond especially strongly to treatment. In more conventional subgroup analyses
(e.g. Jones and others, 2011), HTE
is frequently reported in terms of the posterior variation in treatment effect across
patient subgroups. While the variance of treatment effect is a useful measure, especially
in the context of subgroup analysis, we can provide a more detailed view of HTE by
examining the full distribution of the individualized treatment effects defined by (2.2) where the distribution may be
captured by the latent empirical distribution function 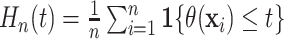 .
Such an approach to examining the “distribution” of a large collection of parameters has
been explored in Louis and Shen (1999). The
distribution function
.
Such an approach to examining the “distribution” of a large collection of parameters has
been explored in Louis and Shen (1999). The
distribution function 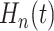 may be regarded as a model
parameter that may be directly estimated by
may be regarded as a model
parameter that may be directly estimated by
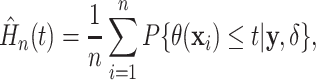 |
(3.4) |
and credible bands for 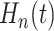 may be obtained from posterior
samples. For improved visualization of the spread of treatment effects, it is often better
to display a density function
may be obtained from posterior
samples. For improved visualization of the spread of treatment effects, it is often better
to display a density function 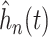 associated with (3.4) which could be obtained through
direct differentiation of (3.4).
Alternatively, a smooth estimate can be found by computing the posterior mean of a kernel
function
associated with (3.4) which could be obtained through
direct differentiation of (3.4).
Alternatively, a smooth estimate can be found by computing the posterior mean of a kernel
function 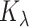 with bandwidth
with bandwidth
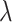
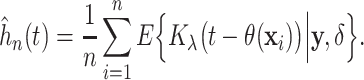 |
(3.5) |
The posterior of 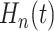 provides a direct assessment of
the variation in the underlying treatment effects, and as such, serves as a useful overall
evaluation of HTE.
provides a direct assessment of
the variation in the underlying treatment effects, and as such, serves as a useful overall
evaluation of HTE.
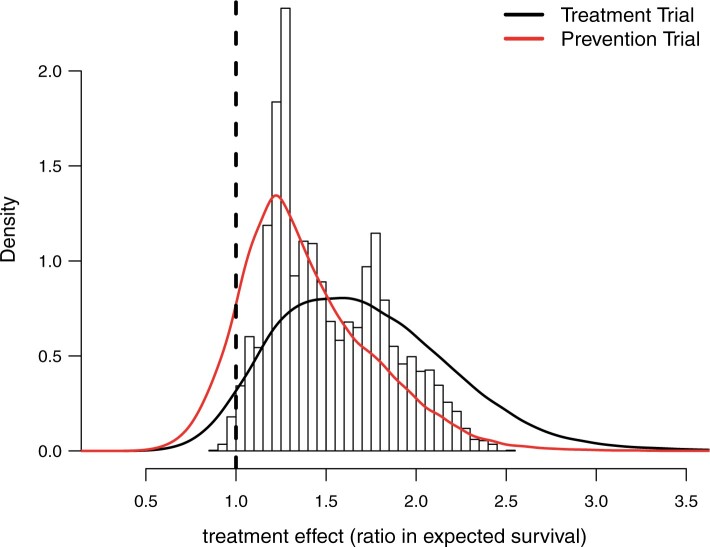
Figure 2 displays a histogram of the posterior means
of the treatment ratios 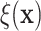 (see eq. (3.1)), for each patient in the SOLVD-T
and SOLVD-P trials. In contrast to the ITE scale used in Figure 1, defining the ITEs in terms of the ratios of expected failure times may
provide a more interpretable scale by which to describe HTE. As may be inferred from the
histogram in Figure 2, nearly all patients have a
positive estimated treatment effect with
(see eq. (3.1)), for each patient in the SOLVD-T
and SOLVD-P trials. In contrast to the ITE scale used in Figure 1, defining the ITEs in terms of the ratios of expected failure times may
provide a more interpretable scale by which to describe HTE. As may be inferred from the
histogram in Figure 2, nearly all patients have a
positive estimated treatment effect with 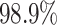 having an estimated
value of
having an estimated
value of 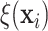 greater than one. Of
those in the SOLVD-T trial, all the patients had
greater than one. Of
those in the SOLVD-T trial, all the patients had 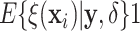 ,
and
,
and 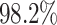 of patients in the SOLVD-P trial had
of patients in the SOLVD-P trial had
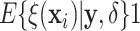 .
.
Fig. 2.
Histogram of point estimates (i.e. posterior means) of the treatment effects
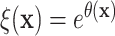 and smooth posterior estimates
and smooth posterior estimates 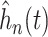 of the treatment effect
distribution. The histogram is constructed using all point estimates from both the
SOLVD treatment and prevention trials. Smooth estimates,
of the treatment effect
distribution. The histogram is constructed using all point estimates from both the
SOLVD treatment and prevention trials. Smooth estimates, 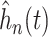 , of the distribution of
treatment effects were computed as described in equation (3.5) for the two trials
separately. The kernel bandwidth
, of the distribution of
treatment effects were computed as described in equation (3.5) for the two trials
separately. The kernel bandwidth 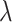 for each trial was chosen using
the rule
for each trial was chosen using
the rule 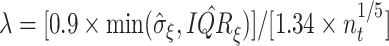 ,
where
,
where 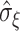 and
and
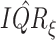 are posterior means of
the standard deviation and inter-quartile range of
are posterior means of
the standard deviation and inter-quartile range of 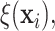 respectively and
where
respectively and
where 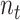 is the trial-specific sample
size.
is the trial-specific sample
size.
Figure 2 also reports the smoothed estimate
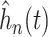 of the distribution of the
treatment effects separately for the two trials. These smoothed posterior estimates of the
treatment effect distribution were computed as described in equation (3.5) where posterior samples of
of the distribution of the
treatment effects separately for the two trials. These smoothed posterior estimates of the
treatment effect distribution were computed as described in equation (3.5) where posterior samples of
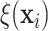 were used in place of
were used in place of
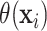 . Note that the
. Note that the
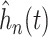 shown in Figure 2 are estimates of the distribution of the
underlying treatment effects and do not represent the posterior distribution of the
overall treatment effects within each trial. As expected, the variation in treatment
effect suggested by the plots of
shown in Figure 2 are estimates of the distribution of the
underlying treatment effects and do not represent the posterior distribution of the
overall treatment effects within each trial. As expected, the variation in treatment
effect suggested by the plots of 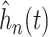 in Figure 2 is greater than the variation exhibited by the posterior means
of
in Figure 2 is greater than the variation exhibited by the posterior means
of 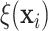 . The estimates
. The estimates
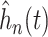 provide informative
characterizations of the distribution of treatment effects in each trial especially for
visualizing the variability in treatment effects in each trial.
provide informative
characterizations of the distribution of treatment effects in each trial especially for
visualizing the variability in treatment effects in each trial.
3.4. Proportion who benefits
Another quantity of interest related to HTE is the proportion of patients who benefit from treatment. Such a measure has a direct interpretation and is also a useful quantity for assessing the presence of cross-over or qualitative interactions, namely, cases where the effect of treatment has the opposite sign as the overall average treatment effect. That is, for situations where an overall treatment benefit has been determined, a low- estimated proportion of patients benefiting may be an indication of the existence of cross-over interactions.
Using the treatment differences 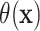 , the proportion who
benefit may be defined as
, the proportion who
benefit may be defined as
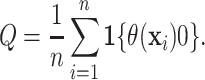 |
(3.6) |
Alternatively, one could define the proportion benefiting relative to a clinically
relevant threshold 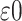 , i.e.
, i.e.
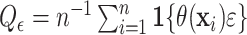 .
The posterior mean of
.
The posterior mean of 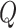 is an average of the posterior
probabilities of treatment benefit
is an average of the posterior
probabilities of treatment benefit 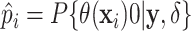 .
Posterior probabilities of treatment benefit can be used for treatment assignment
(
.
Posterior probabilities of treatment benefit can be used for treatment assignment
(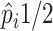 vs.
vs.
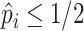 ), or as an additional
summary measure of HTE where one, for example, could tabulate the proportion very likely
to benefit from treatment
), or as an additional
summary measure of HTE where one, for example, could tabulate the proportion very likely
to benefit from treatment 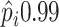 or the proportion
likely to benefit from treatment
or the proportion
likely to benefit from treatment 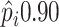 .
.
When using (3.6) to estimate the
proportion of patients benefiting in each of the SOLVD trials, the estimated proportions
of patients (i.e. the posterior mean of 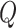 in (3.6)) benefiting were
in (3.6)) benefiting were
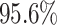 and
and 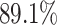 in the
SOLVD-T and SOLVD-P trials, respectively. These proportions are approximately equal to the
area under the curve of
in the
SOLVD-T and SOLVD-P trials, respectively. These proportions are approximately equal to the
area under the curve of 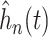 for
for
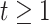 in Figure 2.
in Figure 2.
Table 1 shows a tabulation of patients according to
evidence of treatment benefit. In both trials, all patients have at least a
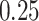 posterior probability of treatment
benefit (i.e.
posterior probability of treatment
benefit (i.e. 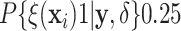 ).
In the treatment trial,
).
In the treatment trial, 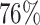 percent of patients exhibit a
posterior probability of benefit greater than
percent of patients exhibit a
posterior probability of benefit greater than 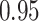 , and the corresponding
percentage for the prevention trial is
, and the corresponding
percentage for the prevention trial is 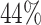 .
.
3.5. Partial dependence and exploring important variables for HTE
To explore patient attributes important in driving differences in treatment effect, we
use a direct approach similar to the “Virtual Twins” method used by Foster and others (2011) in the context of subgroup
identification. In Foster and others
(2011), the authors suggest a two-stage procedure where one first estimates
treatment difference for each individual and then, using these estimated differences as a
new response variable, one estimates a regression model in order to identify a region of
the covariate space where there is an enhanced treatment effect. Similar to this, to
examine important HTE variables in the SOLVD trials, we first fit the full nonparametric
AFT model to generate posterior means 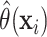 of the
individualized treatment effect for each patient. Then, we fit a (weighted) linear
regression using the previously estimated
of the
individualized treatment effect for each patient. Then, we fit a (weighted) linear
regression using the previously estimated 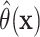 as
the response variable and the patient covariates (except for treatment assignment) as the
predictors. Because the treatment difference
as
the response variable and the patient covariates (except for treatment assignment) as the
predictors. Because the treatment difference 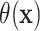 should
only depend on covariates that are predictive of HTE, using estimates of the unobserved
should
only depend on covariates that are predictive of HTE, using estimates of the unobserved
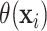 as the responses in
a regression with the patient covariates as predictors represents a direct and efficient
approach to exploring variables involved in driving treatment effect heterogeneity. In
this weighted regression, the residual variances were assumed proportional to the
posterior variances of
as the responses in
a regression with the patient covariates as predictors represents a direct and efficient
approach to exploring variables involved in driving treatment effect heterogeneity. In
this weighted regression, the residual variances were assumed proportional to the
posterior variances of 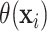 . Additionally, to
make the covariates comparable, all covariates were normalized to have zero mean and unit
variance. The patient covariates with the five largest estimated coefficients in absolute
value were as follows: ejection fraction, history of myocardial infarction, creatinine
levels, gender, and diabetic status. We can further explore the role these key variables
play in driving HTE through the use of partial dependence plots.
. Additionally, to
make the covariates comparable, all covariates were normalized to have zero mean and unit
variance. The patient covariates with the five largest estimated coefficients in absolute
value were as follows: ejection fraction, history of myocardial infarction, creatinine
levels, gender, and diabetic status. We can further explore the role these key variables
play in driving HTE through the use of partial dependence plots.
Partial dependence plots are a useful tool for visually assessing the dependence of an
estimated function on a particular covariate or set of covariates. As described in Friedman (2001), such plots demonstrate the way an
estimated function changes as a particular covariate varies while averaging over the
remaining covariates. For the purposes of examining the impact of a covariate on the
treatment effects, we define the partial dependence function for the
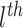 covariate as
covariate as
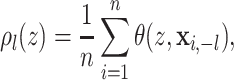 |
where 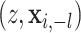 denotes a vector
where the
denotes a vector
where the 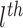 component of
component of
 has been removed and
replaced with the value
has been removed and
replaced with the value 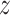 . Estimated partial dependence functions
. Estimated partial dependence functions
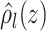 with associated credible
bands may be obtained directly from MCMC output. The supplementary material available at
Biostatistics online contains a figure showing partial dependence plots
for ejection fraction and creatinine, and this figure also displays the posterior
distribution of the overall treatment effect in both the male/female subgroups and the
subgroups defined by history of myocardial infarction.
with associated credible
bands may be obtained directly from MCMC output. The supplementary material available at
Biostatistics online contains a figure showing partial dependence plots
for ejection fraction and creatinine, and this figure also displays the posterior
distribution of the overall treatment effect in both the male/female subgroups and the
subgroups defined by history of myocardial infarction.
4. Simulations studies
To evaluate the performance of the nonparametric, tree-based AFT method, we performed two
main simulation studies. An additional simulation study involving randomly generated
nonlinear regression functions is described in part C of the supplementary material available at
Biostatistics online. For performance related to quantifying HTE, we
recorded the following measures: root mean-squared error (RMSE) of the estimated
individualized treatment effects, the misclassification proportion (MCprop), i.e. the
proportion of patients allocated to the wrong treatment, and the average coverage of the
credible intervals. Average coverage proportions are measured as the average coverage over
individuals, namely, 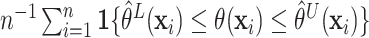 ,
for interval estimates
,
for interval estimates 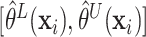 .
.
For the performance measures of RMSE and coverage proportions, we compared our tree-based
nonparametric AFT model (NP-AFTree) with the semi-parametric AFT model (SP-AFTree) where the
BART model is used for the regression function and the residual distribution is assumed to
be Gaussian. In addition, we compared the NP-AFTree procedure with a parametric AFT model
(Param-AFT) which assumes a linear regression with treatment-covariate interactions and
log-normal residuals. For both the NP-AFTree and SP-AFTree methods,
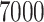 MCMC iterations were used with the first
MCMC iterations were used with the first
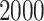 treated as burn-in steps. For both of
these, the default parameters (i.e.
treated as burn-in steps. For both of
these, the default parameters (i.e. 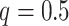 ,
, 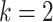 ,
,
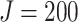 ) were used for each simulation
scenario.
) were used for each simulation
scenario.
4.1. AFT simulations based on the SOLVD trials
In our first set of simulations, we use data from the SOLVD trials (The SOLVD Investigators, 1991) as a guide. To generate our simulated
data, we first took two random subsets of sizes 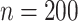 and
and
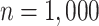 from the SOLVD data. For each
subset, we computed estimates
from the SOLVD data. For each
subset, we computed estimates 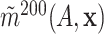 and
and
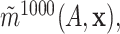 respectively of the regression function for
respectively of the regression function for 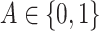 using the
nonparametric AFT Tree model. Simulated responses
using the
nonparametric AFT Tree model. Simulated responses 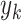 were then generated
as
were then generated
as
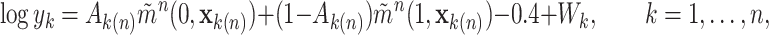 |
(4.1) |
where the regression function was fixed across simulation replications and
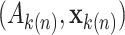 corresponds to
the
corresponds to
the 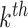 patient’s treatment assignment and
covariate vector in the random subset with
patient’s treatment assignment and
covariate vector in the random subset with 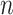 patients. The constant
patients. The constant
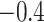 in (4.1) was added so that there was a substantial fraction of
simulated patients that would have an underlying ITE less than zero. For the distribution
of
in (4.1) was added so that there was a substantial fraction of
simulated patients that would have an underlying ITE less than zero. For the distribution
of 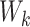 , we considered four different choices:
a Gaussian distribution, a Gumbel distribution with mean zero, a “standardized” Gamma
distribution with mean zero, and a mixture of three t-distributions with
, we considered four different choices:
a Gaussian distribution, a Gumbel distribution with mean zero, a “standardized” Gamma
distribution with mean zero, and a mixture of three t-distributions with
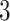 degrees of freedom for each mixture
component. The parameters of these four distributions were chosen so that the variances
were approximately equal, and the levels of censoring was varied across three levels:
none, light censoring (
degrees of freedom for each mixture
component. The parameters of these four distributions were chosen so that the variances
were approximately equal, and the levels of censoring was varied across three levels:
none, light censoring (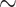
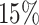 of cases censored),
and heavy censoring (
of cases censored),
and heavy censoring (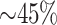 of cases censored).
of cases censored).
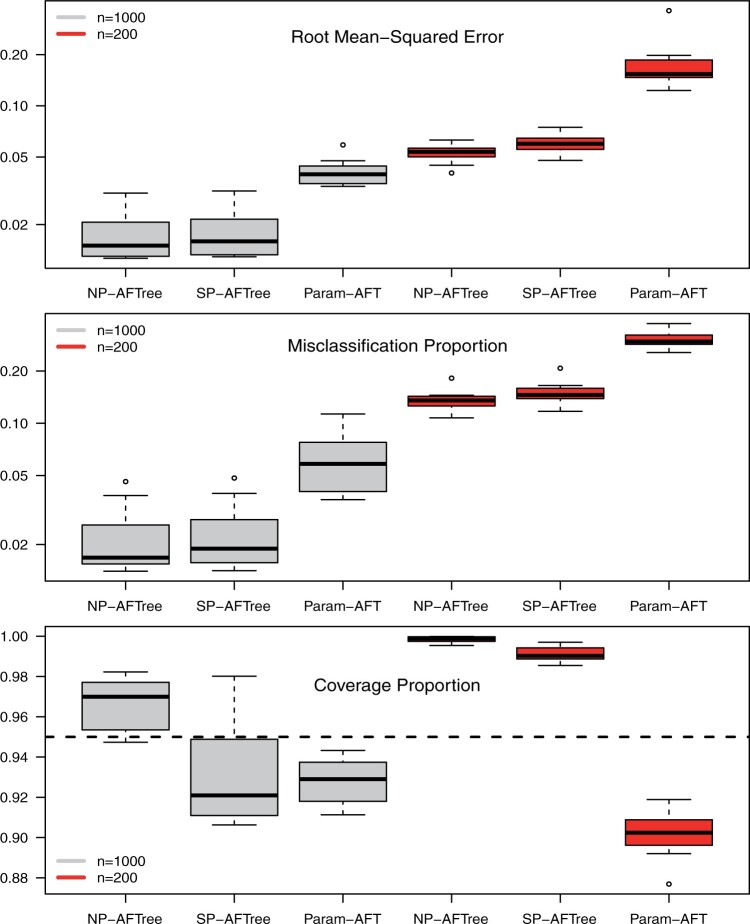
Root mean-squared error, misclassificiation, and coverage results are shown in Figure 3. More detailed results from this simulation
study are detailed in part G of the supplementary material available at Biostatistics online. As
may be inferred from Figure 3, the NP-AFTree method
consistently performs better in terms of RMSE and MCprop than the SP-AFTree procedure.
Moreover, while not apparent from the figure, the NP-AFTree approach performs just as well
as SP-AFTree, even when the true residual distribution is Gaussian (see the supplementary material available at
Biostatistics online). For each residual distribution, the advantage of
NP-AFTree over SP-AFTree is more pronounced for the smaller sample sizes settings
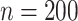 , with closer performance for the
, with closer performance for the
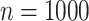 cases. While RMSE and MCprop seem
to be comparable between NP-AFTree and SP-AFTree for the
cases. While RMSE and MCprop seem
to be comparable between NP-AFTree and SP-AFTree for the 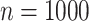 settings,
the coverage for NP-AFTree is consistently closer to the desired
settings,
the coverage for NP-AFTree is consistently closer to the desired 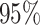 level and
is greater than
level and
is greater than 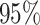 for nearly all settings. When
for nearly all settings. When
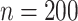 , average coverage often differs
substantially from
, average coverage often differs
substantially from 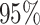 , but in these cases, BART is quite
conservative in the sense that coverage is typically much greater than
, but in these cases, BART is quite
conservative in the sense that coverage is typically much greater than
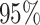 . It is worth mentioning that while we
have observed good frequentist coverage in many settings, BART does not come with strong
frequentist coverage guarantees as the reported uncertainty measures are based on Bayesian
credible intervals. The supplementary
material available at Biostatistics online shows an example
where modest under coverage has been observed. In our experience, such cases of under
coverage can occur when there is both low treatment balance in certain regions of the
covariate space and considerable variation in the ITE function
. It is worth mentioning that while we
have observed good frequentist coverage in many settings, BART does not come with strong
frequentist coverage guarantees as the reported uncertainty measures are based on Bayesian
credible intervals. The supplementary
material available at Biostatistics online shows an example
where modest under coverage has been observed. In our experience, such cases of under
coverage can occur when there is both low treatment balance in certain regions of the
covariate space and considerable variation in the ITE function 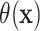 . In these cases, the
regularization prior used by BART imposes a kind of skepticism on very large ITEs meaning
that ITE estimates and credible intervals are shrunken considerably whenever there is not
strong evidence supporting ITEs that differ strongly from the overall treatment effect. In
other words, under coverage is often due to shrinkage towards the overall treatment effect
rather than estimates which overstate the amount of HTE.
. In these cases, the
regularization prior used by BART imposes a kind of skepticism on very large ITEs meaning
that ITE estimates and credible intervals are shrunken considerably whenever there is not
strong evidence supporting ITEs that differ strongly from the overall treatment effect. In
other words, under coverage is often due to shrinkage towards the overall treatment effect
rather than estimates which overstate the amount of HTE.
Fig. 3.
Simulations based on the SOLVD trial data. Results are based on
 simulation replications. Root
mean-squared error, misclassification proportion, and empirical coverage are shown for
each method. Performance measures are shown for the NP-AFTree method, the SP-AFTree,
and the parametric, linear regression-based AFT (Param-AFT) approach. Four different
choices of the residual distribution were chosen: a Gaussian distribution, a Gumbel
distribution with mean zero, a “standardized” Gamma distribution with mean zero, and a
mixture of three t-distributions with
simulation replications. Root
mean-squared error, misclassification proportion, and empirical coverage are shown for
each method. Performance measures are shown for the NP-AFTree method, the SP-AFTree,
and the parametric, linear regression-based AFT (Param-AFT) approach. Four different
choices of the residual distribution were chosen: a Gaussian distribution, a Gumbel
distribution with mean zero, a “standardized” Gamma distribution with mean zero, and a
mixture of three t-distributions with 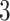 degrees of freedom
for each mixture component.
degrees of freedom
for each mixture component.
4.2. Several “null” simulations
We considered data generated from several “null” cases where the simulation scenarios were designed to have no HTE. For these simulations, we consider four AFT models and one Cox proportional-hazards model. In these “null” simulations, we are primarily interested in the degree to which the NP-AFTree procedure “detects” spurious HTE in settings where no HTE is present in the underlying data generating model. The AFT models used for the simulations assumed a linear regression function with no treatment-covariate interactions
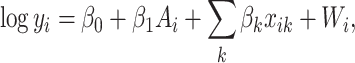 |
(4.2) |
and the hazard functions for the Cox model simulations similarly took the form
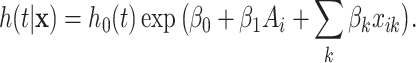 |
(4.3) |
Although there may be a degree of heterogeneity in 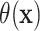 for the
Cox proportional hazards model (4.3), it is still worthwhile to investigate the behavior of
for the
Cox proportional hazards model (4.3), it is still worthwhile to investigate the behavior of
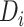 when there is no HTE when treatment
effects are defined in terms of hazard ratios.
when there is no HTE when treatment
effects are defined in terms of hazard ratios.
The parameters in (4.2) were first
estimated from the SOLVD data using a parametric AFT model with log-normal residuals.
Herein, we estimated the parameters in (4.2) separately using the same fixed subsets of size 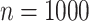 and
and
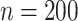 used in Section 4.1. The parameters were fixed across simulation
replications. For the AFT models, we considered the same four choices of the residual term
distribution as in Section 4.1. The parameters
for the hazard functions in (4.3)
were found by fitting a Cox proportional hazards model to the same two subsets of size
used in Section 4.1. The parameters were fixed across simulation
replications. For the AFT models, we considered the same four choices of the residual term
distribution as in Section 4.1. The parameters
for the hazard functions in (4.3)
were found by fitting a Cox proportional hazards model to the same two subsets of size
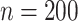 and
and 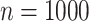 from
the SOVLD trials data, and these parameters were fixed across simulation replications. The
cumulative baseline hazard function used for generating the Cox proportional hazards
simulations was found using Breslow’s estimator.
from
the SOVLD trials data, and these parameters were fixed across simulation replications. The
cumulative baseline hazard function used for generating the Cox proportional hazards
simulations was found using Breslow’s estimator.
For each null simulation scenario, we computed the posterior probabilities of
differential treatment effect 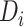 (see equations (3.2) and 3.3)) and tabulated the percentage of patients with either
strong evidence of differential treatment effect (i.e.
(see equations (3.2) and 3.3)) and tabulated the percentage of patients with either
strong evidence of differential treatment effect (i.e. 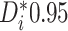 ) or mild evidence (i.e.
) or mild evidence (i.e.
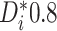 ). Table 2 shows, for each null simulation scenario, the average proportion
of individuals exhibiting strong evidence of a differential treatment effect and the
average proportion of individuals exhibiting mild evidence. As displayed in Table 2, the average percentage of individuals showing
strong evidence of differential treatment effect is less than
). Table 2 shows, for each null simulation scenario, the average proportion
of individuals exhibiting strong evidence of a differential treatment effect and the
average proportion of individuals exhibiting mild evidence. As displayed in Table 2, the average percentage of individuals showing
strong evidence of differential treatment effect is less than 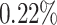 for all
simulation settings. Moreover, the percentages of cases with mild evidence of differential
treatment effect was fairly modest. The average percentage of patients with mild evidence
was less than
for all
simulation settings. Moreover, the percentages of cases with mild evidence of differential
treatment effect was fairly modest. The average percentage of patients with mild evidence
was less than 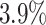 for all except one simulation
scenario, and most of the simulation scenarios had, on average, less than
for all except one simulation
scenario, and most of the simulation scenarios had, on average, less than
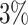 of patients exhibiting mild evidence of
differential treatment effect. Null simulations with
of patients exhibiting mild evidence of
differential treatment effect. Null simulations with 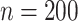 tended to have much
fewer cases of strong or mild evidence than those simulations with
tended to have much
fewer cases of strong or mild evidence than those simulations with
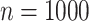 . The results presented in Table 2 suggest that the NP-AFTree procedure rarely
reports any patients as having strong evidence of differential treatment effects in
situations where HTE is absent.
. The results presented in Table 2 suggest that the NP-AFTree procedure rarely
reports any patients as having strong evidence of differential treatment effects in
situations where HTE is absent.
Table 2.
Simulation for settings without any HTE present
| Normal | Gumbel | Std-Gamma | T-mixture | Cox-PH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Censoring | SE | ME | SE | ME | SE | ME | SE | ME | SE | ME |
| 200 | none | 0.000 | 0.095 | 0.000 | 0.040 | 0.000 | 0.070 | 0.000 | 0.060 | 0.000 | 0.140 |
| 200 | light | 0.000 | 0.095 | 0.000 | 0.000 | 0.075 | 0.350 | 0.000 | 0.380 | 0.000 | 0.015 |
| 200 | heavy | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.055 | 0.000 | 0.290 |
| 1000 | none | 0.000 | 0.803 | 0.073 | 2.066 | 0.092 | 1.483 | 0.161 | 3.087 | 0.176 | 3.824 |
| 1000 | light | 0.061 | 1.989 | 0.015 | 0.967 | 0.213 | 2.674 | 0.066 | 3.176 | 0.111 | 3.405 |
| 1000 | heavy | 0.002 | 1.253 | 0.000 | 0.484 | 0.030 | 1.176 | 0.123 | 2.953 | 0.135 | 2.688 |
Results are based on 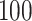 simulation replications. Average
percentage of patients exhibiting strong evidence (SE) of
differential treatment effect (i.e.
simulation replications. Average
percentage of patients exhibiting strong evidence (SE) of
differential treatment effect (i.e. 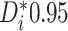 )
and average percentage of patients exhibiting mild evidence (ME) of
differential treatment effect (i.e.
)
and average percentage of patients exhibiting mild evidence (ME) of
differential treatment effect (i.e. 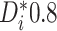 ).
Results are shown for AFT models with the same four residual distributions used in
the simulations from Section 4.1 and for a
Cox-proportional hazards model with no treatment-covariate interactions. Censoring
levels were varied according to: none, light censoring (
).
Results are shown for AFT models with the same four residual distributions used in
the simulations from Section 4.1 and for a
Cox-proportional hazards model with no treatment-covariate interactions. Censoring
levels were varied according to: none, light censoring (
 of
cases censored), and heavy censoring (
of
cases censored), and heavy censoring (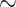
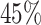 of
cases censored).
of
cases censored).
5. Discussion
In this article, we have described a flexible, tree-based approach to examining heterogeneity of treatment effect with survival endpoints. This method produces estimates of individualized treatment effects and corresponding credible intervals for AFT models with an arbitrary regression function and residual distribution. Moreover, we have demonstrated how this approach provides a useful framework for addressing a variety of other HTE-related questions. When using the default hyperparameter settings, the method only requires the user to input the survival outcomes, treatment assignments, and patient covariates. Due to the tree-based formulation of the regression function, the user does not need to pre-specify any treatment covariate interaction terms or patient subgroups in order to obtain informative characterizations of HTE. As shown in several simulation studies, the default settings exhibit strong predictive performance and good coverage properties. Though quite flexible, our nonparametric AFT model does entail some assumptions regarding the manner in which the patient covariates modify the baseline hazard. Hence, it would be worth further investigating the robustness of the nonparametric AFT method to other forms of model misspecification such as cases where neither an AFT or a Cox proportional hazards assumption holds or cases where the residual distribution depends on the patient covariates.
In addition to describing a novel nonparametric AFT model, we examined a number of measures for reporting HTE including the distribution of individualized treatment effects, the proportion of patients benefiting from treatment, and posterior probabilities of differential treatment effect. Each have potential uses in allowing for more refined interpretations of clinical trial results. The argument has been made by some (e.g. Kent and Hayward, 2007) that the positive results of some clinical trials are driven substantially by the outcomes of high-risk patients. In such cases, the posterior distribution of the ITEs along with the estimated proportion benefiting may help in clarifying the degree to which lower risk patients are expected to benefit from the proposed treatment. In Section 4.2, we explored the use of posterior probabilities of differential treatment effect as a means of detecting the presence of HTE. Such measures show potential for evaluating the consistency of treatment and for assessing whether or not further investigations into HTE are warranted.
6. Software
The methods described in this paper are implemented in the R package AFTrees, which is available for download at https://github.com/nchenderson/AFTrees (accessed July 4, 2018). The AFTrees package and additional supplementary code is also available for download at http://hteguru.com/index.php/software/ (accessed July 4, 2018).
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
Funding
The authors were supported by the National Institute of Health’s (NIH) National Center for the Advancement of Translational Sciences (NCATS) through the grant number UL1TR001079-04S1, and by the NIH grant number P30CA006973. This work was also supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (ME-1303-5896).
References
- Bonato, V., Baladandayuthapani, V., Broom, B. M., Sulman, E. P., Aldape, K. D. and Do, K.-A. (2011). Bayesian ensemble methods for survival prediction in gene expression data. Bioinformatics 27, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman, H. A., George, E. I. and McCulloch, R. E. (1998). Bayesian CART model search (with discussion and a rejoinder by the authors). Journal of the American Statistical Association 93, 935–960. [Google Scholar]
- Chipman, H. A., George, E. I. and McCulloch, R. E. (2010). BART: Bayesian additive regression trees. The Annals of Applied Statistics 4, 266–298. [Google Scholar]
- Chipman, H. and McCulloch, R. (2016). BayesTree: Bayesian Additive Regression Trees. R package version 0.3-1.4. [Google Scholar]
- Foster, J. C., Taylor, J. M. G. and Ruberg, S. J. (2011). Subgroup identification from randomized clinical trial data. Statistics in Medicine 30, 2867–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, J. (2001). Greedy function approximation: a gradient boosting machine. The Annals of Statistics 29, 1189–1232. [Google Scholar]
- Hanson, T. and Johnson, W. O. (2002). Modeling regression error with a mixture of Polya trees. Journal of the American Statistical Association 97, 1020–1033. [Google Scholar]
- Hanson, T. E. (2006). Modeling censored lifetime data using a mixture of gammas baseline. Bayesian Analysis 1, 575–594. [Google Scholar]
- Hill, J. L. (2011). Bayesian nonparametric modeling for causal inference. Journal of Computational and Graphical Statistics 20, 217–240. [Google Scholar]
- Ishwaran, H. and James, L. F. (2001). Gibbs sampling methods for stick-breaking priors. Journal of the American Statistical Association 96, 161–173. [Google Scholar]
- Johnson, W. and Christensen, R. (1988). Modeling accelerated failure time with a Dirichlet process. Biometrika 75, 693–704. [Google Scholar]
- Jones, H. E., Ohlssen, D. I., Neuenschwander, B., Racine, A. and Branson, M. (2011). Bayesian models for subgroup analysis in clinical trials. Clinical Trials 8, 129–143. [DOI] [PubMed] [Google Scholar]
- Kent, D. M. and Hayward, R. A. (2007). Limitations of applying summary results of clinical trials to individual patients - The need for risk stratification. Journal of the American Medical Association 298, 1209–1212. [DOI] [PubMed] [Google Scholar]
- Komárek, A. and Lesaffre, E. (2007). Bayesian accelerated failure time model for correlated interval-censored data with a normal mixture as error distribution. Statistica Sinica 17, 549–569. [Google Scholar]
- Komárek, A., Lesaffre, E. and Hilton, J. F. (2005). Accelerated failure time model for arbitrarily censored data with smoothed error distribution. Journal of Computational and Graphical Statistics 14, 726–745. [Google Scholar]
- Kuo, L. and Mallick, B. (1997). Bayesian semiparametric inference for the accelerated failure-time model. The Canadian Journal of Statistics 25, 457–472. [Google Scholar]
- Lamont, A., Lyons, M. D., Jaki, T., Stuart, E., Feaster, D. J., Tharmaratnam, K., Oberski, D., Ishwaran, H., Wilson, D. K. and Horn, M. L. V. (2018). Identification of predicted individual treatment effects in randomized clinical trials. Statistical Methods in Medical Research 27, 142–157. [DOI] [PubMed] [Google Scholar]
- Loh, W.-Y., He, X. and Man, M. (2015). A regression tree approach to identifying subgroups with differential treatment effects. Statistics in Medicine 34, 1818–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, T. A. and Shen, W. (1999). Innovations in Bayes and empirical Bayes methods: estimating parameters, populations, and ranks. Statistics in Medicine 18, 2493–2505. [DOI] [PubMed] [Google Scholar]
- Shen, Y. and Cai, T. (2016). Identifying predictive markers for personalized treatment selection. Biometrics 72, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparapani, R. A., Logan, B. R., McCulloch, R. E. and Laud, P. W. (2016). Nonparametric survival analysis using Bayesian additive regression trees (BART). Statistics in Medicine 35, 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, X., Tsai, C.-L., Wang, H., Nickerson, D. M. and Li, B. (2009). Subgroup analysis via recursive partitioning. Journal of Machine Learning Research 10, 141–158. [Google Scholar]
- The SOLVD Investigators. (1991). Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. The New England Journal of Medicine 325(5), 293–302. [DOI] [PubMed] [Google Scholar]
- Wager, S. and Athey, S. (2015). Estimation and inference of heterogeneous treatment effects using random forests. ArXiv 1510.04342. [Google Scholar]
- Wager, S., Hastie, T. and Efron, B. (2014). Confidence intervals for random forests: the jackknife and the infinitesimal jackknife. Journal of Machine Learning Research 15, 1625–1651. [PMC free article] [PubMed] [Google Scholar]
- Wei, L. J. (1992). The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Statistics in Medicine 11, 1871–1879. [DOI] [PubMed] [Google Scholar]
- Weisberg, H. I. and Pontes, V. P. (2015). Post hoc subgroups in clinical trials: anathema or analytics? Clinical Trials 12, 357–364. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Yu, M., Zhao, Y.-Q., Li, Q., Wang, S. and Shao, J. (2015). Regularized outcome weighted subgroup identification for differential treatment effects. Biometrics 71, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M., Dunson, D. B. and Baird, D. (2010). Semiparametric Bayes hierarchical models with mean and variance constraints. Computational Statistics & Data Analysis 54, 2172–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y.-Q., Zheng, D., Rush, A. J. and Kosorok, M. R. (2012). Estimating individualized treatment rules using outcome weighted learning. Journal of the American Statistical Association 107, 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.