Abstract
The real epidemiology and the possible consequences of anabolic-androgenic steroids (AAS) use still represent a very tricky task due to the difficulties in the quantification and detection of these drugs. Chronic use of AAS, frequently combined with other illicit substances, can induce tremendous negative effects on the reproductive system, but it is also associated with an increased overall and cardiovascular mortality risk. In the present review we summarize and discuss the available evidence regarding the negative impact of AAS on the male reproductive system, providing practical suggestions to manage these problems. For this purpose a meta-analysis evaluating the effects of AAS abusers vs. controls on several hormonal, reproductive and metabolic parameters was performed. In addition, in order to overcome possible limitations related to the combined use of different AAS preparations, we also retrospectively re-analyzed data on animal models treated with supraphysiological dosage of testosterone (T), performed in our laboratory. Available data clearly indicated that AAS negatively affect endogenous T production. In addition, increased T and estradiol circulating levels were also observed according to the type of preparations used. The latter leads to an impairment of sperm production and to the development of side effects such as acne, hair loss and gynecomastia. Furthermore, a worse metabolic profile, characterized by reduced high density lipoprotein and increased low density lipoprotein cholesterol levels along with an increased risk of hypertension has been also detected. Finally sexual dysfunctions, often observed upon doping, represent one the most probable unfavorable effects of AAS abuse.
Keywords: Cardiovascular risk; Doping in sports; Hypogonadism; Infertility, gynecomastia; Sperm; Testosterone congeners
INTRODUCTION
Anabolic-androgenic steroids (AAS) represent a group of heterogeneous compounds, which include testosterone (T) and its derivate substances, largely used to enhance physical performance, sense of well-being and cosmetic appearance among athletes [1,2,3,4]. Some years after T isolation and synthesis in 1935 [5], Bøje [6] had already discussed the possible use of sex steroids in athletes. Alleged information suggests that the Germans gave AAS to solders during World War II and to athletes in preparation for the 1936 Berlin Olympic Games [4]. The first documented use of AAS in an athletic competition dates back to a Russian team at the weightlifting championship during the 1954 Vienna Olympic Games [4]. From strength-intensive sports, the use of AAS gradually spread to other disciplines and to non-professional competitions. In 1990, the possibility of having open access to classified documents, after the fall of Communist government in the German Democratic Republic, allowed for the discovery of a secret State program to improve national athletes' performance using AAS during the 1970s and 1980s [4]. Similarly, more recently, the Russian track and field team was banned from the 2016 Olympic Games due to suspicions about a State program supporting the use of illicit drugs among their athletes [3]. The epidemiology of AAS use, either in professional athletes or in the general population, is a difficult task due to reluctance to declare openly the consumption and the introduction of chemical modifications in order to reduce the detection of AAS [3,4]. For the first time in 1975, the International Olympic Committee created a list of banned illicit substances including stimulants and narcotics, which, since 2004, is nowadays under the responsibility of the World Anti-Doping Agency (WADA) [3,4].
Official data resulting from Olympic-level athletes, tested between 1987 and 2013, indicated a prevalence of positive test, ranging from 0.96% to 2.45% [7]. However, WADA data showed that, of elite professional athletes, from 44% up to 70% have declared past use of illicit drugs, although only less than 1% of those tested were caught [7]. The figure is even more impressive considering that more than 75% of AAS users are noncompetitive athletes [1,2,3,4]. A meta-analysis, including 187 studies and providing data from 271 lifetime prevalence rates, indicated a global rate of AAS use of 3.3%, being four times higher in males than in females (6.4% vs. 1.6%) [1]. In addition, data derived from ten studies, investigating AAS dependence, concluded that 32.5% of AAS users developed, at some time, a drug-dependence [4]. Applying these data to the US population, the prevalence of AAS users is similar to that observed for other diseases such as Human Immunodeficiency Virus infection or type 1 diabetes [4].
Due to their influence on hypothalamus-pituitary testis axis, the chronic use of AAS could have a tremendous impact on endogenous T production, fertility and general well-being. In addition, the combination with the abuse of other substances such as opiates, other anabolic drugs (human chorionic gonadotropin hormone [hCG], growth hormone [GH], insulin) can have even more deleterious effects not only on the reproductive system, but also increasing overall and cardiovascular (CV) mortality risk [1,2,3,4]. A previous meta-analysis, including 11 studies and less than 100 abusers, has documented that the use of AAS resulted in a rapid reduction in gonadotropins and T levels, which gradually return to the baseline within 13–24 weeks [8]. No data on comparisons between AAS-abusers and non-abuser athletes were reported.
The aim of the present study is to summarize and critically discuss the available evidence regarding the impact of AAS on the male reproductive system providing practical suggestions to manage these problems. A meta-analysis including those studies comparing the effects of AAS vs. controls on reproductive systems will be also provided.
METHODS
A comprehensive narrative review and meta-analysis was performed using Medline, Embase and Cochrane search and including the following words: (“steroidal”[All Fields] OR “steroidals”[All Fields] OR “steroidic”[All Fields] OR “steroids”[MeSH Terms] OR “steroids”[All Fields] OR “steroid”[All Fields]) AND (“abusable”[All Fields] OR “abuse s”[All Fields] OR “abused”[All Fields] OR “abuser”[All Fields] OR “abuser s”[All Fields] OR “abusers”[All Fields] OR “abuses”[All Fields] OR “abusing”[All Fields] OR “abusive”[All Fields] OR “abusively”[All Fields] OR “abusiveness”[All Fields] OR “substance related disorders”[MeSH Terms] OR (“substance related”[All Fields] AND “disorders”[All Fields]) OR “substance related disorders”[All Fields] OR “abuse”[All Fields]) AND (“testosterone”[MeSH Terms] OR “testosterone”[All Fields] OR “testosterone”[All Fields] OR “testosterones”[Al l Fields] OR “testosterones”[All Fields]) AND (“sperms”[All Fields] OR “spermatozoa”[MeSH Terms] OR “spermatozoa”[All Fields] OR “sperm”[All Fields] OR “sperms”[All Fields]). Publications from January 1, 1969 up to January 15th, 2021 were included. The search was restricted to humans and males. In addition, only English papers were considered.
Preclinical data were derived from a previously published series of eugonadal rabbits or rats treated with or without supra-physiological doses of T (see below).
ANABOLIC-ANDROGENIC STEROID COMPOUNDS
A complete biochemical analysis of the most used AAS is beyond the aim of the present review. Hence, only a short overview will be presented here, while more complete information is available elsewhere [3,4]. Endogenous T levels or actions can be increased by the administration of T or its derivates or by the use of drugs that can increase endogenous T production such as hCG, selective estrogen modulators (SERM) or T precursors (Table 1). Overall, the final effects of AAS and their adverse consequences depend upon their chemical structure and on their possibility to cover all T biological actions through aromatization or 5-alpha reduction. The most common used AAS such as T, boldenone, metadione and nortestosterone can be aromatized and 5-alpha-reduced, whereas fluoxymesterone and formebolone cannot be aromatized but can be reduced and other molecules neither reduced or aromatized (Table 1). In addition, it is important to recognize that the widespread, unregulated, web-market has increased the number of anabolic steroids available, frequently contained in dietary supplements (see also https://www.usada.org/athletes/substances/supplement-411/) [3,4]. The interpretation of the final effects of AAS is further complicated by the combination of different steroids in cycles of increasing and decreasing concentrations along with several other drugs to support the anabolic action (GH, insulin), to counteract possible adverse effects (SERM or aromatase inhibitors, hCG) or reduce the risk of detection (diuretics and probenecid) [3,4].
Table 1. Biological proprieties of the commonly used anabolic androgenic steroids (AAS).
| AAS | Substrate for aromatization | Substrate for 5-alpha reductase |
|---|---|---|
| Testosterone | + | + |
| Boldenone | + | + |
| Metadione | + | + |
| Nortestosterone | + | + |
| Metandienone | + | + |
| Fluoxymesterone | + | - |
| Formebolone | + | - |
| Nadrolone | + | - |
| Bolandiola | + | - |
| Oxymetholone | + | Already 5-alpha reduced |
| Stanazol | - | + |
| Oxandrolone | - | + |
| Trenbolone | - | + |
| Danazol | - | + |
| Dostranolone | - | + |
| Metenolone | - | Already 5-alpha reduced |
| Clostebol | - | - |
| Gestrinone | - | - |
| Tetrahydrogestrinone | - | - |
aNadrolone precursors.
CLINICAL DATA
1. General characteristics
Out of 738 studies, 24 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] specifically analyzed the andrologic consequences of AAS in males. These trials included 2,411 patients with a mean age of 29.7 years and a mean follow-up of 148.7 weeks. The trials differ in basal characteristics and type of AAS used (Table 2 and Supplement Table 1). In particular, 13 studies compared the effects of AAS to controls whereas 11 reported only side effects in the active group. Bodybuilders or weightlifters were the most common athletes considered, the list of which included also soccer players, other professional athletes as well as recreational exercisers. In the vast majority of the cases, control groups were made up of non-abuser athletes but also of normal sedentary groups (Table 2).
Table 2. Characteristics of trials included in the meta-analysis.
| Authors | AAS (N°) |
Controls (N°) |
Study area | Population type | Type of controls | Type of AAS | Follow-up (wk) |
Mean age (y) |
|---|---|---|---|---|---|---|---|---|
| Aakvaag and Stromme, 1974 [9]a | 10 | 11 | Norway | Normal subjects | Normal subjects | Mesterolone | 8 | |
| Strauss et al, 1983 [10] | 20 | 7 | USA | Bodybuilders | Bodybuilders | Mixed | 27.7 | |
| Alén et al, 1985 [11] | 5 | 6 | Finland | Not specified athletes | Not specified athletes | Mixed | 26 | 26.3 |
| Yesalis et al, 1988 [12] | 15 | - | USA | Weightlifters | - | Mixed | - | 27 |
| Knuth et al, 1989 [13] | 19 | 10 | Germany | Bodybuilders | Bodybuilders | Mixed | - | 26.7 |
| Moss et al, 1993 [14] | 15 | - | USA | Amateur body building athletes | - | Mixed | - | 25.2 |
| Evans, 1997 [15] | 100 | - | UK | Bodybuilders | - | Mixed | - | From 16 up to >40 |
| Korkia and Stimson, 1997 [16] | 97 | - | UK | Amateur athletes | - | Mixed | - | 27.3 |
| Torres-Calleja et al, 2001 [17] | 15 | 15 | Mexico | Bodybuilders | Bodybuilders | Mixed | 8.7 | 26.3 |
| Fudala et al, 2003 [18] | 88 | - | USA | Not specified athletes | - | Mixed | - | 18–59 |
| Perry et al, 2005 [19] | 207 | - | USA | Bodybuilder and other athletes | - | Mixed | - | 27.2 |
| Taher et al, 2008 [20] | 15 | 15 | Iran | Bodybuilders | Healthy sedentary | Mixed | - | - |
| Ip et al, 2010 [21] | 506 | - | USA | Mixed population: recreational exercisers, competitive bodybuilders, competitive weightlifters, competitive athletes | Mixed population: recreational exercisers, competitive bodybuilders, competitive weightlifters, competitive athletes | Mixed | 260 | 29.3 |
| Al-Janabi et al, 2011 [22] | 15 | 15 | Iraq | Bodybuilders | Bodybuilders | Mixed | - | 15–28 |
| Coward et al, 2013 [23] | 80 | - | USA | Bodybuilders | Bodybuilders | Mixed | - | 40.4 |
| Razavi et al, 2014 [24] | 72 | - | Iran | Not specified abusers | - | Mixed | From <6 months up to >12 years | 25.5 |
| Rasmussen et al, 2016 [25], 2017 [26], 2018 [27] | 37 | 30 | Denmark | Not specified abusers | Healthy volunteers | Mixed | 142.3 | 31.4 |
| Baggish et al, 2017 [28] | 86 | 54 | USA | Weightlifters | Weightlifters | Mixed | 384 | 42 |
| Bordin et al, 2017 [29] | 20 | 20 | Brazil | Soccer and body builder professional athletes | Non abuser professional athletes | Mixed | 21–27 | |
| Barbosa Neto et al, 2018 [30] | 15 | 15 | Brazil | Bodybuilders | Healthy volunteers | Mixed | 197.6 | |
| Horwitz et al, 2019 [31] | 545 | - | Denmark | Tested positive for AAS in Danish Fitness centres |
- | Mixed | - | 26.2 |
| Smit and de Ronde, 2018 [32] | 180 | - | Netherlands | Not specified abusers | - | Mixed | - | 34 |
| Souza et al, 2019 [33] | 20 | 10 | Brazil | Not specified abusers | Healthy sedentary subjects | Mixed | 104 | 29 |
| Börjesson et al, 2020 [34] | 16 | 5 | Sweden | Mixed athletes | Past AAS users | Mixed | 208 | 33 |
UK: United Kingdom, USA: United States of America, AAS: anabolic androgenic steroids.
aRandomized controlled trial.
2. Hormonal parameters
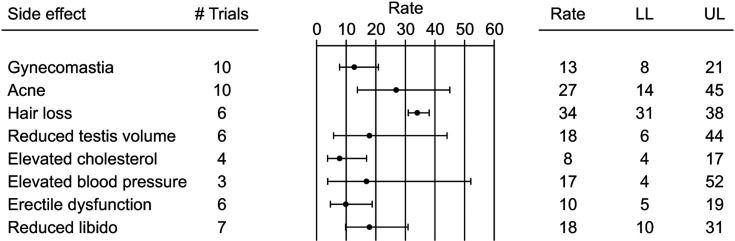
Abusers were characterized by reduced luteinizing hormone (LH) and follicular stimulating hormone (FSH) and increased total T and estradiol levels (Table 3 and Supplement Fig. 1A-D). Similarly, reduced sex hormone binding globuline levels were observed in abusers, when compared to controls (Table 3 and Supplement Fig. 1E). Accordingly, gynecomastia, acne, hair loss and decreased testis volume were quite frequently reported as possible side effects during AAS use (Fig. 1 and Supplement Fig. 2A-D).
Table 3. Overall weighted differences (with 95% CI) in hormonal, sperm, body composition and metabolic parameters as derived from meta-analysis in men using or not using anabolic androgenic steroids.
| Parameter | Study no. | Mean (95% CI) | p-value | |
|---|---|---|---|---|
| Hormonal parameters | ||||
| Lutenizing hormone (mU/mL) | 8 | -2.60 (-3.18 to -2.01) | <0.0001 | |
| Follicular stimulating hormone (mU/mL) | 8 | -2.61 (-3.32 to -1.91) | <0.0001 | |
| Total testosterone (nmol/L) | 9 | 8.75 (0.79 to 16.72) | <0.03 | |
| Total estradiol (pmol/L) | 5 | 170.23 (81.10 to 259.36) | <0.0001 | |
| Sex hormone binding globulin (nmol/L) | 2 | -17.06 (-33.58 to -0.54) | 0.04 | |
| Sperm parameters | ||||
| Sperm count (×106) | 2 | -47.28 (-79.93 to -14.62) | <0.0001 | |
| Sperm concentration (×106/mL) | 3 | -39.59 (-82.82 to 3.63) | 0.07 | |
| Normal morphology | 3 | -2.00 (-2.95 to -1.04)a | <0.0001 | |
| Body composition and metabolic parameters | ||||
| Lean mass | 5 | 1.24 (0.62 to 1.87)a | <0.0001 | |
| Fat mass | 4 | -1.07 (-1.53 to -0.61)a | <0.0001 | |
| Low density lipoprotein-cholesterol (mmol/L) | 4 | 0.63 (0.38 to 0.88) | <0.0001 | |
| High density lipoprotein-cholesterol (mmol/L) | 5 | -0.43 (-0.56 to -0.31) | <0.0001 | |
| Total cholesterol (mmol/L) | 4 | 0.13 (-0.24 to 0.51) | 0.48 | |
| Triglycerides (mmol/L) | 3 | 0.16 (-0.23 to 0.55) | 0.43 | |
| Fasting glucose (mmol/L) | 2 | 0.11 (-0.36 to 0.59) | 0.63 | |
CI: confidence interval.
aStandardized mean (95% CI).
Fig. 1. Rate (95% confidence interval) of the main adverse events related to the abuse of anabolic androgen steroids. LL: lower levels, UP: upper levels.
3. Sperm parameters
When sperm parameters were evaluated, reduced total sperm count and sperm morphology were observed in abusers, when compared to controls (Table 3 and Supplement Fig. 3A, 3C). In addition, a trend towards a statistically significant reduction in sperm concentration in abusers was also detected (Supplement Fig. 3B). Insufficient data were available to analyze other sperm parameters (not shown).
4. Body composition and metabolic parameters
AAS was characterized by an increased free-fat and reduced fat mass (Table 3 and Supplement Fig. 4A, 4B). In addition, higher low-density lipoprotein (LDL) and lower high-density lipoprotein (HDL)-cholesterol levels were observed in AAS when compared to controls (Table 3 and Supplement Fig. 4C, 4D). In line with the latter results, dyslipidemia was reportedinup to 17% of abusers (Fig. 1 and Supplement Fig. 2E). Conversely, no difference in total cholesterol, triglycerides and glucose levels were observed between groups (Table 3 and Supplement Fig. 4E–G). Finally, elevated blood pressure (BP) was reported in up to 50% of cases (Fig. 1 and Supplement Fig. 2F). However, insufficient data were available to compare mean BP in cases and controls (not shown).
5. Sexual function
Limited information regarding sexual function was available. No comparisons between cases and controls were reported (not shown). Data regarding erectile dysfunction (ED) and low libido were recorded in six and seven studies, respectively. Combining the results of those studied, ED and reduced sexual desire were reportedin up to 19% and 31% of cases (Fig. 1 and Supplement Fig. 2G, 2H).
6. Discussion
The effects of AAS on hypothalamus-pituitary-testis (HPT) axis are not surprising. A previous meta-analysis showed that, during AAS use, a significant reduction of LH and FSH was observed when compared to baseline levels [8]. We here confirmed similar findings when AAS-abusers were compared to controls. Our data also indicated that, when compared to a control group, AAS-abusers have higher T and estradiol levels. The latter results are in line with the observed higher risk of side effects such as acne, hair loss and gynecomastia as well as reduced testis volume. Data derived from the Christou et al. [8] meta-analysis previously documented reduced and not increased T levels after AAS use. The different patterns of AAS can be used to explain the latter differences. In fact, although it is clear that the use of AAS causes a reduction of endogenous T production, the type of drug used can modify T circulating levels differently. Accordingly, AAS can be converted or not to T and/or to estrogens (Table 1). Unfortunately, information related to the specific type of AAS used in the different studies was limited and no specific subanalyses were therefore possible. The role played by T and dihydrotestosterone in regulating sebum production is well recognized, so that the increased risk of acne associated with AAS is expected [3,4]. Similar considerations can be drawn for hair loss [35]. In addition, the alteration in estrogen-to-androgen balance induced by AAS is probably the cause of the reported high risk of gynecomastia [36,37]. The previously described modifications of the HPT axis, induced by the use of AAS, eventually cause a sperm production impairment and a testis volume reduction, since tubules account for more than 90% of the testis volume and their deterioration induces shrinkages of the testes. Considering the different preparations and combinations used, general conclusions related to the HPT axis recovery after AAS discontinuation appear difficult to draw. A previous meta-analysis documented that gonadotropin levels recover from 13 to 24 weeks after AAS withdrawal, whereas serum endogenous T levels are still reduced at 16 weeks [8]. The time of AAS exposure as well as the dosages used and the type of preparations assumed can specifically affect HPT axis recovery. It is reasonable to suppose that a shorter duration, lower dosages and younger age of the athletes can guarantee a faster recovery after AAS cessation. In particular, the type of AAS used seems to play a crucial role. In fact, nandrolone metabolites can be detected in the urine of the abusers after more than a year in some men [38]. Limited information related to sperm recovery after AAS interruption is available. However, data derived from male hormonal contraceptive trials showed that sperm concentration normalization is achieved in 100% of subjects within 24 months [39].
Androgens play a crucial role in regulating male sexual function, acting either at a central or peripheral level [40,41,42]. Data derived from available meta-analyses showed that the use of testosterone replacement therapy (TRT) is useless in eugonadal men for improving erectile function [43,44,45,46]. Conversely, limited effects have been reported for libido [43,46]. Present data indicate a prevalence of ED and low libido similar to that reported in the general population [47]. It has been suggested that the type of AAS could represent a crucial factor, with sexual dysfunction being more prevalent especially among those men abusing aromatizable AAS [2]. Accordingly, high levels of circulating estrogens and/or anestrogen-to-androgen imbalance have been described as a possible factor negatively influencing male sexual function [48].
In AAS abusers, the observed increase in lean mass and decrease in fat mass were expected findings; in fact, they are in line with what has been reportedin hypogonadal men during TRT [49]. Interestingly, the effect size observed in the present meta-analysis is two times higher than that reported by our group in a previous meta-analysis including all randomized placebo controlled trials (RCTs) evaluating the effects of TRT in patients with late onset hypogonadism [50]. The higher dosages applied and the use of T preparations with higher anabolic effects can explain, at least partially, the observed differences. Accordingly, supraphysiological doses of T combined with strength trainingis able to increase fat-free mass and muscle size and strength in normal men [51].
The role of TRT in the regulation of lipid and glycometabolic profiles in hypogonadal men is more conflicting [52]. In particular, whereas some positive outcomes for fasting and post-loading glucose levels have been observed [52,53,54,55,56], the role of TRT on glycated hemoglobin, in diabetic subjects, and on lipid profile are more conflicting [50,51,52]. The present data documented that AAS resulted in more atherogenic lipid profile (increased HDL, LDL, and increased LDL cholesterol, HDL, levels) whereas no modification of fasting glucose levels have been observed. The specific mechanisms through which T can regulate endogenous metabolism are beyond the aim of the present study and revised elsewhere [52]. However, the worse lipid profile along with other effects related to AAS abuse such as cardiac mass modulation, higher risk of hypertension and thromboembolic events can all contribute to the observed increased CV risk related to the use of AAS [4].
MANAGEMENT
The lack of conclusive and well-performed RCTs prevents the possibility of giving clear recommendations on how to manage an eventual AAS abuse and its consequences, in particular in male fertility. In fact, even in our experience, couple infertility represents one of the most common reasons for consultation in men using AAS. As reported above, AAS withdrawal allows normalization of the HPT axis within 24 weeks, especially when ASS abuse dates to less than one year. In this case, couple reassurance should be sufficient. However, a longer recovery time, up to 2 years, has been described for those men who declared a longer period and larger dosages of AAS [2,3,4]. Considering that nowadays most couples decide to conceive at an older age than in the previous decades, such a long time for spermatogenesis recovery is often unacceptable. In addition, a withdrawal syndrome has been described after long-term AAS cessation. Various therapeutic approaches have been reported to overcome the situation allowing a faster recovery. In particular, the use of hCG, along with SERM such as tamoxifen and clomiphene, are the most commonly suggested therapies. However, when fertility represents an issue, hCG alone cannot be sufficient and the combination with FSH is often required [2,3,4]. Finally, when AAS is associated with other illicit drugs, including opioids, the management of AAS withdrawal syndrome can be more difficult to manage, requiring interaction with psychologists and psychiatrics [2,3,4].
ANIMAL MODELS OF DOPING
As stated before, the most important motivation prompting AAS abuse in men is to increase muscle mass and physical performance. The lack of well-designed, placebo-controlled RCT studies on the effect of AAS on male sexuality and fertility, along with the often-used combination of several abused drugs in the available trials, severely hampered the possibility to dissect the specific effects of AAS on the male genital tract. To overcome these problems, we retrospectively re-analyzed previously published data on animal models performed in our laboratory [57,58,59,60,61] adding few unpublished observations. We selected data involving eugonadal experimental animals (rabbits and rats) fed a regular diet (RD) treated (RD+T) or not with supraphysiological doses of T (rats: T proprionate, 100 mg/kg for 2 weeks and rabbits: T propionate 30 mg/kg weekly for 12 weeks) focusing on sexual and fertility data. We also re-analyzed data on the effect of such supraphysiological doses of T on muscle biology and on physical performance, using a rabbit model in which eugonadal rabbits fed a RD were trained to perform an endurance exercise (treadmill) (RD+PhyEx) treated (RD+T+PhyEx) or not with the aforementioned supraphysiological dose of T enanthate [57,58]. Finally, we re-analyzed data on eugonadal rats treated with or not with T, monitoring intracavernous pressure (ICP) following electro-stimulation of the cavernous nerve [61].
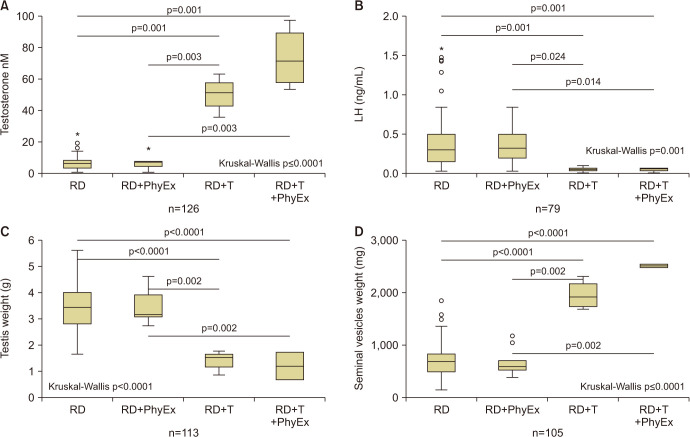
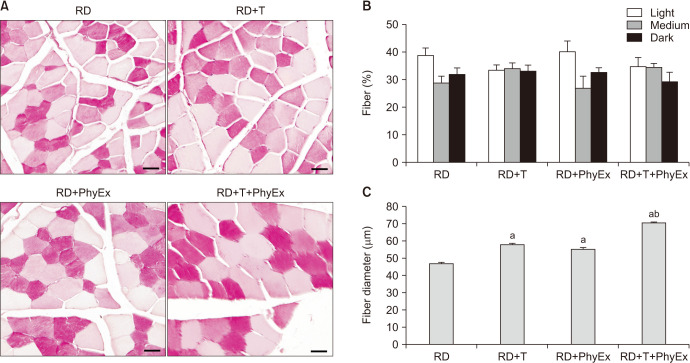
Fig. 2 shows the effect of T administration on otherwise eugonadal rabbits, which performed or not physical exercise. In T-treated arms, we observed a sharp increase in circulating T with a parallel two-fold increase in seminal vesicle weight (Fig. 2A, 2C). As expected, the supraphysiological level of circulating T downregulated LH levels and significantly decreased testis volume (Fig. 2B, 2D). Overall, no significant differences were found between rabbits performing or not physical exercise. Interestingly, we did not notice any difference in the ability to perform physical exercise, consisting in running on a treadmill. In fact running distance, running time and lactate production was similar among groups (not shown) [57,58]. Accordingly, histomorphological analysis by PAS staining of vastus medialis cross-sections did not reveala difference in glycogen content, which classifies fibers as light-, medium- and dark staining, ranging from oxidative to glycolytic (Fig. 3A, 3B). However, fiber diameters were increased by both physical exercise and T administration with an additive effect (Fig. 3C). At a molecular level, the most striking effect was a T-induced increase in MYH7 gene expression, which is a marker of slow/oxidative fibers, independently from performing or not a physical exercise (Supplement Fig. 5) [57]. In addition, T administration induced an increase in myogenic and differentiation markers, such as MY5, PAX7, and ITGAT7 mRNA, respectively, independently from the physical activity performed (Supplement Fig. 6A-C) [57]. Finally, physical activity and T administration increased significantly the lipolytic marker lipoprotein lipase mRNA (Supplement Fig. 6D) [57]. All of this evidence indicates that T administration to eugonadal animals leads to an increase in muscle fiber mass by stimulating myogenesis and differentiation of the fibers, without evident results in performance, as often observed in humans. Hence, this animal model seems to recapitulate the human phenotype of eugonadal subjects assuming supraphysiological doses of androgen to increase muscle mass. Therefore, analyzing the effect on sexual and reproductive parameters could be interesting.
Fig. 2. Circulating testosterone (T; A) and luteinizing hormone (LH; B) levels in eugonadal rabbits fed a regular diet (RD), which performed (RD+PhyEx) or not physical exercise (PhyEx). The effect of supraphysiological dosing with T is also showed in rabbits which performed physical exercise (RD+T+PhyEx) or not (RD+T). Weight of testis (C) and seminal vesicles (D) in the four aforementioned rabbit arms. Level of significance upon Kruskal–Wallis and post-hoc Mann–Whitney analyses is also indicated, along with number of observations. *Outlier.
Fig. 3. Effect of physical exercise (PhyEx) and testosterone (T) on skeletal muscle fiber composition. (A) Representative images of periodic acid–Schiff (PAS) staining of vastus medialis cross-sections from untreated rabbits (RD) and rabbits fed a regular diet (RD) and treated with testosterone (RD+T) and/or physical exercise (RD+PhyEx, RD+T+PhyEx). Scale bar 50 µm. Based on PAS staining intensity, indicative of glycogen content, fibers are classified as light-, medium- or dark-stained, ranging from oxidative to glycolytic. T and PhyEx treatments do not affect the overall fiber composition, maintaining the ratio between oxidative (light) and glycolytic (medium/dark) fibers, as quantified by counting ten fields from muscle sections of at least three animals for each experimental group (B). (C) An increased fiber diameter was detected in PAS-stained muscle cross-sections from PhyEx and T-treated groups, as analyzed using Image J 1.51f software (National Institutes of Health). ap<0.001 vs. RD; bp<0.001 vs. RD+PhyEx.
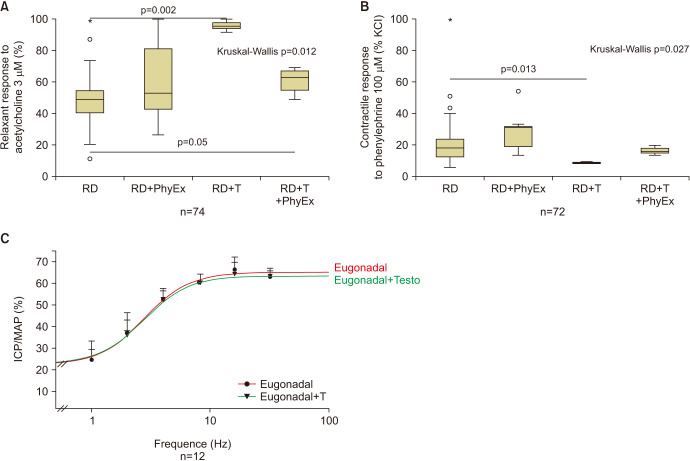
Concerning the molecular machinery leading to penile erection, we did not find any significant effect on the genes involved in the main pathways stimulating or inhibiting erection, including NO-cGMP-PDE5 and RhoA-ROCK expression, respectively (not shown). However, functional studies on penile strips indicated that T administration, independently from performing or not physical activity, increased responsiveness to acetylcholine, the main neurotransmitter initiating the NO cascade and exploring the endothelial-dependent relaxation (Fig. 4A). In addition, T administration decreased the contractile effect of the adrenergic agonist phenylephrine (Fig. 4B). Hence, because T facilitated Ach-induced relaxation and decreased contractile response to an adrenergic stimulation of penile strips in vitro, T should have an overall positive effect on erection. However, this is not the case when considering in vivo experiments. Fig. 4B shows results of increasing frequency of stimulation of the exposed cavernous nerves by recording the maximal ICP, normalized to mean arterial pressure (MAP). In control rats, the mean T level was 3.8±0.68 nM and the maximal ICP/MAP obtained was=65.1±1.7, with an EC50=2.8±0.28 Hz. T administration induced a 10-fold increase in circulating T levels (T=34.98±9 nM) but was completely ineffective, at all the frequencies tested, in increasing penile erection (Fig. 4C) [60]. Accordingly, even in this model, we did not observe any variation at a molecular level in genes related to penile erection (not shown) [60].
Fig. 4. Relaxant (A) and contractile (B) response to acetylcholine (3 µM) or phenylephrine (100 µM), respectively, on isolated rabbit corpora cavernosa strips from eugonadal rabbit fed a regular diet (RD) and treated with (RD+T) or not with supraphysiological concentration of testosterone (T). Results in rabbit treated as before but performing physical exercise (PhyEx) are also showed (RD+PhyEx and RD+T+PhyEx). Level of significance upon Kruskal–Wallis and post-hoc Mann–Whitney analyses is also indicated, along with number of observations. Panel (C) shows frequency-dependent erectile response of eugonadal rat treated or not with a supraphysiological dose of T. Erection was elicited by electrical stimulation (2.5 V, 5 msec, 30 s) of the cavernous nerve at varying stimulation frequency (1–32 Hz). Erectile function was quantified by intracavernous pressure/mean arterial pressure (ICP/MAP)×100 in the indicated numer of rats. *Outlier.
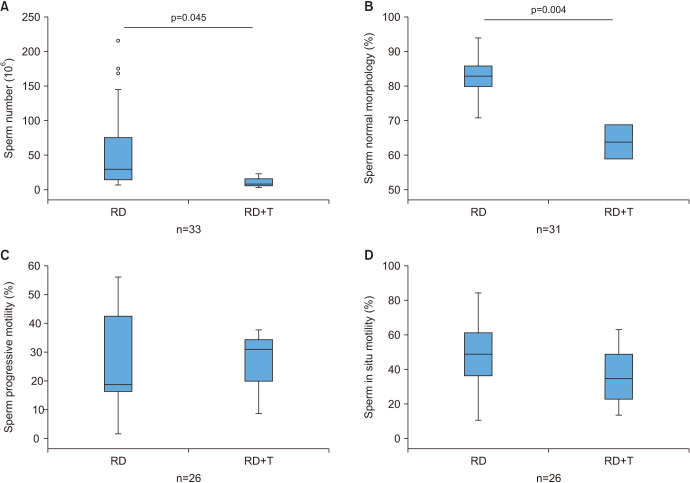
Concerning sperm parameters, in the aforementioned rabbit model we found that supraphysiological dosing of T (Fig. 5) was able to significantly decrease sperm number and to deteriorate sperm morphology (Fig. 5A, 5B) [61], without apparent effects on sperm motility (Fig. 5C, 5D) [61].
Fig. 5. Sperm parameters in eugonadal rabbit fed a regular (RD) treated (RD+T) or not with supraphysiological dosing of testosterone (T). T significantly decreased sperm number (A) and % normal morphology (B), while it did affect sperm motility (C, D). Level of significance at Mann–Whitney analysis is indicated, along with number of observations.
Finally, concerning glucolipid metabolism and mean blood pressure, Kruskal–Wallis analysis did not reveal significant variations among groups treated or not with supraphysiological doses of T and performing or not physical exercise (not shown) [57,58].
CONCLUSIONS
T, often considered the hormone for youthful vigor and sexual potency, could be indeed venomfor fertility and sexuality, if abused. The animal models here described demonstrated that the decrease in gonadotrophins and testis volume, as well as in alterations in sperm parameters, i.e., sperm concentration and normal morphology, are genuine effects of supraphysiological dosing of androgens [57,58,59,60,61]. In contrast, a supraphysiological dose of Tin animal studies resulted in a modest or null effect on erection, in contrast to that observed in some, but not all clinical studies (Fig. 1 and Supplement Fig. 2G). It should be recognized that changes in sexuality are not a primary endpoint in such clinical studies and that, quite often, androgens are not the only substance abused. This might justify the negative effect of androgen abuse recorded in the present meta-analysis. In fact, in clinical trials, where a standardized dose of T was administered to otherwise eugonadal subjects or in mixed populations, libido increased and did not decrease [43,44,45,46] with a null effect on erections. In our animal models, modifications in sexual desire were not investigated and, therefore, final conclusions cannot be drawn. Clinical studies, here scrutinized and summarized in a meta-analysis, indicated that AAS was associated with an unfavorable lipid profile and with an increase in blood pressure. All these conditions might be associated with a decreased blood flow within cavernous arteries, finally leading to erectile dysfunction. We did not observesuch changes in the rabbit model, where all the animals were maintained in a standardized condition and fed a regular diet. Hence, it is possible that the adverse modifications in lipid metabolism and in the blood pressure observed in clinical trials were most probably due to abuse of other doping substances or to an unhealthy lifestyle.
Nonetheless, as universally observed in all meta-analyses involving TRT in hypogonadal individuals [49,50], we here report a parallel decrease in fat mass and an increase in lean mass induced by AAS. Accordingly, histomorphological analysis of rabbit vastus medialis sections indicate a T-induced increase in muscle fiber diameter with an exercise-associated additive effect.
Results on sperm parameters were clearly concordant between preclinicaland clinical observations. Chronic exposure to androgens not only resulted in a reduced testis size but also in a reduction of sperm number and normal morphology, supporting fertility problems in androgen abusers.
Finally, due to the activation of androgenic and/or estrogenic signaling, AAS was significantly associated with in one way acne and baldness and, in the other one, with gynecomastia, respectively. Some, but not all, these conditions can be reversed by AAS withdrawal. To restore testicular function after doping, direct (hCG) or indirect (antiestrogen) stimulators of endogenous T synthesis have been suggested, but clear evidence of their efficacy over a natural course are scanty.
In conclusion, illicit use of supraphysiological doses of androgens for non-medical purposes, including increasing muscular performances, is associated with several androgen-dependent consequences, such as acne, gynecomastia and baldness. In addition, due to the well-known negative feedback on the HPT axis it is associated with a fall in gonadotrophin levels and to a reduction in testis size and sperm production. In fact, T is the cornerstone for all the male contraceptive preparation. Sexual dysfunctions, often observed upon doping with androgens, is most probably related to the unfavorable effect of AAS on blood pressure and lipid profile recorded in clinical studies and most probably related to the concomitant abuse of other substances or to an unhealthy lifestyle.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: GC, MM.
- Data curation: GR, LV, SF, SM, AM, ES.
- Formalanalysis: GC, SF, GR, MM.
- Investigation: GC, MM.
- Methodology: GC, MM.
- Project administration: GC, MM.
- Supervision: GC, MM.
- Validation: GC, MM.
- Visualization: GC, MM.
- Writing — original draft: GC, MM.
- Writing — review & editing: GC, SF, GR, MM, AS.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.210021.
Weighted differences (with 95% confidence interval [CI]) in Lutenizing hormone (A; mU/mL), follicular stimulating hormone (B; mU/mL), total testosterone (C; nmol/L), total estradiol (D; pmol/L), and sex hormone binding globulin (E; nmol/L) as derived from meta-analysis in men using or not using anabolic androgenic steroids (AAS). Data are reported as mean (95% CI) and as standardized mean (95% CI).
Rate (95% confidence interval [CI]) of the main adverse events related to the abuse of anabolic androgen steroids (AAS): gynecomastia (A), acne (B), hair loss (C), reduced testis volume (D), elevated cholesterol (E), elevated blood pressure (F), erectile dysfunction (G), reduce libido (H).
Weighted differences (with 95% confidence interval [CI]) of total sperm count (A; ×106), sperm concentration (B; ×106/mL) and sperm normal morphology (C; %) in men using or not using anabolic androgen steroids (AAS). Data are reported as mean (95% CI). Std diff: standardized difference. aStandardized mean (95% CI).
Weighted differences (with 95% confidence interval [CI]) of lean mass (A), fat mass (B), low density lipoprotein-cholesterol (C; mmol/L), high density lipoprotein cholesterol (D; mmol/L), total cholesterol (E; mmol/L), triglycerides (F; mmol/L) and fasting glycaemia (G; mmol/L) in men using or not using anabolic androgen steroids (AAS). Data are reported as mean (95% CI). Std diff: standardized difference. aStandardized mean (95% CI).
Gene expression of the indicated markers of different muscular fibers (A) very fast/glycolytic fibers; (B) fast/glycolytic fibers; (C) fast/oxidative fibers; (D) slow/oxidative fibers) in eugonadal rabbits fed a regular diet (RD), which performed (RD+PhyEx) or not physical exercise. The effect of supraphysiological dosing with T is also showed in rabbits which performed physical exercise (RD+T+PhyEx) or not (RD+T). Level of significance upon Kruskal–Wallis and post-hoc Mann–Whitney analyses is also indicated, along with number of observations. *Outlier.
Gene expression of the indicated myogenic (A, B) or differentiation muscle (C) markers in eugonadal rabbits fed a regular diet (RD), which performed (RD+PhyEx) or not physical exercise (PhyEx). The effect of supraphysiological dosing with testosterone (T) is also showed in rabbits which performed physical exercise (RD+T+PhyEx) or not (RD+T). Panel (D) shows results on lipoprotein lipase (LPL) mRNA, a lipolytic marker. Level of significance upon Kruskal–Wallis and post-hoc Mann–Whitney analyses is also indicated, along with number of observations. *Outlier.
Parameters reported per single trial included in the meta-analysis
References
- 1.Sagoe D, Molde H, Andreassen CS, Torsheim T, Pallesen S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann Epidemiol. 2014;24:383–398. doi: 10.1016/j.annepidem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Nieschlag E, Vorona E. Mechanisms in endocrinology: medical consequences of doping with anabolic androgenic steroids: effects on reproductive functions. Eur J Endocrinol. 2015;173:R47–R58. doi: 10.1530/EJE-15-0080. [DOI] [PubMed] [Google Scholar]
- 3.Anawalt BD. Diagnosis and management of anabolic androgenic steroid use. J Clin Endocrinol Metab. 2019;104:2490–2500. doi: 10.1210/jc.2018-01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope HG, Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014;35:341–375. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15:1903–1926. doi: 10.1517/14656566.2014.944896. [DOI] [PubMed] [Google Scholar]
- 6.Bøje O. Doping: a study of the means employed to raise the level of performance in sport. Bull Health Organ. 1939;8:439–469. [Google Scholar]
- 7.de Hon O, Kuipers H, van Bottenburg M. Prevalence of doping use in elite sports: a review of numbers and methods. Sports Med. 2015;45:57–69. doi: 10.1007/s40279-014-0247-x. [DOI] [PubMed] [Google Scholar]
- 8.Christou MA, Christou PA, Markozannes G, Tsatsoulis A, Mastorakos G, Tigas S. Effects of anabolic androgenic steroids on the reproductive system of athletes and recreational users: a systematic review and meta-analysis. Sports Med. 2017;47:1869–1883. doi: 10.1007/s40279-017-0709-z. [DOI] [PubMed] [Google Scholar]
- 9.Aakvaag A, Stromme SB. The effect of mesterolone administration to normal men on the pituitary-testicular function. Acta Endocrinol (Copenh) 1974;77:380–386. doi: 10.1530/acta.0.0770380. [DOI] [PubMed] [Google Scholar]
- 10.Strauss RH, Wright JE, Finerman GA, Catlin DH. Side effects of anabolic steroids in weight-trained men. Phys Sportsmed. 1983;11:86–98. doi: 10.1080/00913847.1983.11708706. [DOI] [PubMed] [Google Scholar]
- 11.Alén M, Reinilä M, Vihko R. Response of serum hormones to androgen administration in power athletes. Med Sci Sports Exerc. 1985;17:354–359. [PubMed] [Google Scholar]
- 12.Yesalis CE, 3rd, Herrick RT, Buckley WE, Friedl KE, Brannon D, Wright JE. Self-reported use of anabolic-androgenic steroids by elite power lifters. Phys Sportsmed. 1988;16:91–100. doi: 10.1080/00913847.1988.11709666. [DOI] [PubMed] [Google Scholar]
- 13.Knuth UA, Maniera H, Nieschlag E. Anabolic steroids and semen parameters in bodybuilders. Fertil Steril. 1989;52:1041–1047. doi: 10.1016/s0015-0282(16)53172-0. [DOI] [PubMed] [Google Scholar]
- 14.Moss HB, Panzak GL, Tarter RE. Sexual functioning of male anabolic steroid abusers. Arch Sex Behav. 1993;22:1–12. doi: 10.1007/BF01552908. [DOI] [PubMed] [Google Scholar]
- 15.Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54–58. doi: 10.1136/bjsm.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korkia P, Stimson GV. Indications of prevalence, practice and effects of anabolic steroid use in Great Britain. Int J Sports Med. 1997;18:557–562. doi: 10.1055/s-2007-972681. [DOI] [PubMed] [Google Scholar]
- 17.Torres-Calleja J, González-Unzaga M, DeCelis-Carrillo R, Calzada-Sánchez L, Pedrón N. Effect of androgenic anabolic steroids on sperm quality and serum hormone levels in adult male bodybuilders. Life Sci. 2001;68:1769–1774. doi: 10.1016/s0024-3205(01)00972-9. [DOI] [PubMed] [Google Scholar]
- 18.Fudala PJ, Weinrieb RM, Calarco JS, Kampman KM, Boardman C. An evaluation of anabolic-androgenic steroid abusers over a period of 1 year: seven case studies. Ann Clin Psychiatry. 2003;15:121–130. doi: 10.1023/a:1024640410093. [DOI] [PubMed] [Google Scholar]
- 19.Perry PJ, Lund BC, Deninger MJ, Kutscher EC, Schneider J. Anabolic steroid use in weightlifters and bodybuilders: an internet survey of drug utilization. Clin J Sport Med. 2005;15:326–330. doi: 10.1097/01.jsm.0000180872.22426.bb. [DOI] [PubMed] [Google Scholar]
- 20.Taher AMM, Al-Sabbagh MS, Al-Khashali DK. Effects of abuse of anabolic androgenic steroids on Iraqi athletes. Iraqi J Pharm Sci. 2008;17:9–17. [Google Scholar]
- 21.Ip EJ, Barnett MJ, Tenerowicz MJ, Kim JA, Wei H, Perry PJ. Women and anabolic steroids: an analysis of a dozen users. Clin J Sport Med. 2010;20:475–481. doi: 10.1097/JSM.0b013e3181fb5370. [DOI] [PubMed] [Google Scholar]
- 22.Al-Janabi AS, Kanaan ZA, Al Salih AM. Effect of anabolic-androgenic steroids on semen parameters and serum hormonal levels in Iraqi male bodybuilders. Jordan Med J. 2011;45:159–166. [Google Scholar]
- 23.Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW, Lipshultz LI. Anabolic steroid induced hypogonadism in young men. J Urol. 2013;190:2200–2205. doi: 10.1016/j.juro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Razavi Z, Moeini B, Shafiei Y, Bazmamoun H. Prevalence of anabolic steroid use and associated factors among body-builders in Hamadan, West province of Iran. J Res Health Sci. 2014;14:163–166. [PubMed] [Google Scholar]
- 25.Rasmussen JJ, Selmer C, Østergren PB, Pedersen KB, Schou M, Gustafsson F, et al. Former abusers of anabolic androgenic steroids exhibit decreased testosterone levels and hypogonadal symptoms years after cessation: a case-control study. PLoS One. 2016;11:e0161208. doi: 10.1371/journal.pone.0161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen JJ, Schou M, Selmer C, Johansen ML, Gustafsson F, Frystyk J, et al. Insulin sensitivity in relation to fat distribution and plasma adipocytokines among abusers of anabolic androgenic steroids. Clin Endocrinol (Oxf) 2017;87:249–256. doi: 10.1111/cen.13372. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen JJ, Schou M, Madsen PL, Selmer C, Johansen ML, Hovind P, et al. Increased blood pressure and aortic stiffness among abusers of anabolic androgenic steroids: potential effect of suppressed natriuretic peptides in plasma? J Hypertens. 2018;36:277–285. doi: 10.1097/HJH.0000000000001546. [DOI] [PubMed] [Google Scholar]
- 28.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, et al. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation. 2017;135:1991–2002. doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordin DM, Bettim BB, Perdona GC, de Campos EG, De Martinis BS. Understanding alterations on blood and biochemical parameters in athletes that use dietary supplements, steroids and illicit drugs. Toxicology. 2017;376:75–82. doi: 10.1016/j.tox.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Barbosa Neto O, da Mota GR, De Sordi CC, Resende EAMR, Resende LAPR, Vieira da Silva MA, et al. Long-term anabolic steroids in male bodybuilders induce cardiovascular structural and autonomic abnormalities. Clin Auton Res. 2018;28:231–244. doi: 10.1007/s10286-017-0470-2. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz H, Andersen JT, Dalhoff KP. Health consequences of androgenic anabolic steroid use. J Intern Med. 2019;285:333–340. doi: 10.1111/joim.12850. [DOI] [PubMed] [Google Scholar]
- 32.Smit DL, de Ronde W. Outpatient clinic for users of anabolic androgenic steroids: an overview. Neth J Med. 2018;76:167. [PubMed] [Google Scholar]
- 33.Souza FR, Dos Santos MR, Porello RA, Fonseca GWPD, Sayegh ALC, Lima TP, et al. Diminished cholesterol efflux mediated by HDL and coronary artery disease in young male anabolic androgenic steroid users. Atherosclerosis. 2019;283:100–105. doi: 10.1016/j.atherosclerosis.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Börjesson A, Lehtihet M, Andersson A, Dahl ML, Vicente V, Ericsson M, et al. Studies of athlete biological passport biomarkers and clinical parameters in male and female users of anabolic androgenic steroids and other doping agents. Drug Test Anal. 2020;12:514–523. doi: 10.1002/dta.2763. [DOI] [PubMed] [Google Scholar]
- 35.Heilmann-Heimbach S, Hochfeld LM, Henne SK, Nöthen MM. Hormonal regulation in male androgenetic alopecia-sex hormones and beyond: evidence from recent genetic studies. Exp Dermatol. 2020;29:814–827. doi: 10.1111/exd.14130. [DOI] [PubMed] [Google Scholar]
- 36.Kanakis GA, Nordkap L, Bang AK, Calogero AE, Bártfai G, Corona G, et al. EAA clinical practice guidelines-gynecomastia evaluation and management. Andrology. 2019;7:778–793. doi: 10.1111/andr.12636. [DOI] [PubMed] [Google Scholar]
- 37.Maseroli E, Rastrelli G, Corona G, Boddi V, Amato AM, Mannucci E, et al. Gynecomastia in subjects with sexual dysfunction. J Endocrinol Invest. 2014;37:525–532. doi: 10.1007/s40618-014-0055-z. [DOI] [PubMed] [Google Scholar]
- 38.Gårevik N, Strahm E, Garle M, Lundmark J, Ståhle L, Ekström L, et al. Long term perturbation of endocrine parameters and cholesterol metabolism after discontinued abuse of anabolic androgenic steroids. J Steroid Biochem Mol Biol. 2011;127:295–300. doi: 10.1016/j.jsbmb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Long JE, Lee MS, Blithe DL. Update on novel hormonal and non-hormonal male contraceptive development. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab034. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European Society of Endocrinology. Andrology. 2020;8:970–987. doi: 10.1111/andr.12770. [DOI] [PubMed] [Google Scholar]
- 41.Rastrelli G, Guaraldi F, Reismann Y, Sforza A, Isidori AM, Maggi M, et al. Testosterone replacement therapy for sexual symptoms. Sex Med Rev. 2019;7:464–475. doi: 10.1016/j.sxmr.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Rastrelli G, Corona G, Maggi M. Testosterone and sexual function in men. Maturitas. 2018;112:46–52. doi: 10.1016/j.maturitas.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 44.Boloña ER, Uraga MV, Haddad RM, Tracz MJ, Sideras K, Kennedy CC, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:20–28. doi: 10.4065/82.1.20. [DOI] [PubMed] [Google Scholar]
- 45.Corona G, Isidori AM, Buvat J, Aversa A, Rastrelli G, Hackett G, et al. Testosterone supplementation and sexual function: a meta-analysis study. J Sex Med. 2014;11:1577–1592. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 46.Corona G, Rastrelli G, Morgentaler A, Sforza A, Mannucci E, Maggi M. Meta-analysis of results of testosterone therapy on sexual function based on international index of erectile function scores. Eur Urol. 2017;72:1000–1011. doi: 10.1016/j.eururo.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Corona G, Lee DM, Forti G, O'Connor DB, Maggi M, O'Neill TW, et al. EMAS Study Group. Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS) J Sex Med. 2010;7(4 Pt 1):1362–1380. doi: 10.1111/j.1743-6109.2009.01601.x. [DOI] [PubMed] [Google Scholar]
- 48.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corona G, Torres LO, Maggi M. Testosterone therapy: what we have learned from trials. J Sex Med. 2020;17:447–460. doi: 10.1016/j.jsxm.2019.11.270. [DOI] [PubMed] [Google Scholar]
- 50.Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, et al. Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol. 2016;174:R99–R116. doi: 10.1530/EJE-15-0262. [DOI] [PubMed] [Google Scholar]
- 51.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 52.Grossmann M, Ng Tang Fui M, Cheung AS. Late-onset hypogonadism: metabolic impact. Andrology. 2020;8:1519–1529. doi: 10.1111/andr.12705. [DOI] [PubMed] [Google Scholar]
- 53.Wittert G, Bracken K, Robledo KP, Grossmann M, Yeap BB, Handelsman DJ, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9:32–45. doi: 10.1016/S2213-8587(20)30367-3. [DOI] [PubMed] [Google Scholar]
- 54.Corona G, Mannucci E, Forti G, Maggi M. Following the common association between testosterone deficiency and diabetes mellitus, can testosterone be regarded as a new therapy for diabetes? Int J Androl. 2009;32:431–441. doi: 10.1111/j.1365-2605.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 55.Pizzocaro A, Vena W, Condorelli R, Radicioni A, Rastrelli G, Pasquali D, et al. King, Klinefelter ItaliaN Group. Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest. 2020;43:1675–1687. doi: 10.1007/s40618-020-01299-1. [DOI] [PubMed] [Google Scholar]
- 56.Corona G, Sforza A, Maggi M. Testosterone replacement therapy: long-term safety and efficacy. World J Mens Health. 2017;35:65–76. doi: 10.5534/wjmh.2017.35.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarchielli E, Comeglio P, Filippi S, Cellai I, Guarnieri G, Guasti D, et al. Testosterone improves muscle fiber asset and exercise performance in a metabolic syndrome model. J Endocrinol. 2020;245:259–279. doi: 10.1530/JOE-19-0532. [DOI] [PubMed] [Google Scholar]
- 58.Morelli A, Filippi S, Comeglio P, Sarchielli E, Cellai I, Pallecchi M, et al. Physical activity counteracts metabolic syndrome-induced hypogonadotropic hypogonadism and erectile dysfunction in the rabbit. Am J Physiol Endocrinol Metab. 2019;316:E519–E535. doi: 10.1152/ajpendo.00377.2018. [DOI] [PubMed] [Google Scholar]
- 59.Filippi S, Vignozzi L, Morelli A, Chavalmane AK, Sarchielli E, Fibbi B, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6:3274–3288. doi: 10.1111/j.1743-6109.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 60.Morelli A, Corona G, Filippi S, Ambrosini S, Forti G, Vignozzi L, et al. Which patients with sexual dysfunction are suitable for testosterone replacement therapy? J Endocrinol Invest. 2007;30:880–888. doi: 10.1007/BF03349232. [DOI] [PubMed] [Google Scholar]
- 61.Marchiani S, Vignozzi L, Filippi S, Gurrieri B, Comeglio P, Morelli A, et al. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol Cell Endocrinol. 2015;401:12–24. doi: 10.1016/j.mce.2014.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Weighted differences (with 95% confidence interval [CI]) in Lutenizing hormone (A; mU/mL), follicular stimulating hormone (B; mU/mL), total testosterone (C; nmol/L), total estradiol (D; pmol/L), and sex hormone binding globulin (E; nmol/L) as derived from meta-analysis in men using or not using anabolic androgenic steroids (AAS). Data are reported as mean (95% CI) and as standardized mean (95% CI).
Rate (95% confidence interval [CI]) of the main adverse events related to the abuse of anabolic androgen steroids (AAS): gynecomastia (A), acne (B), hair loss (C), reduced testis volume (D), elevated cholesterol (E), elevated blood pressure (F), erectile dysfunction (G), reduce libido (H).
Weighted differences (with 95% confidence interval [CI]) of total sperm count (A; ×106), sperm concentration (B; ×106/mL) and sperm normal morphology (C; %) in men using or not using anabolic androgen steroids (AAS). Data are reported as mean (95% CI). Std diff: standardized difference. aStandardized mean (95% CI).
Weighted differences (with 95% confidence interval [CI]) of lean mass (A), fat mass (B), low density lipoprotein-cholesterol (C; mmol/L), high density lipoprotein cholesterol (D; mmol/L), total cholesterol (E; mmol/L), triglycerides (F; mmol/L) and fasting glycaemia (G; mmol/L) in men using or not using anabolic androgen steroids (AAS). Data are reported as mean (95% CI). Std diff: standardized difference. aStandardized mean (95% CI).
Gene expression of the indicated markers of different muscular fibers (A) very fast/glycolytic fibers; (B) fast/glycolytic fibers; (C) fast/oxidative fibers; (D) slow/oxidative fibers) in eugonadal rabbits fed a regular diet (RD), which performed (RD+PhyEx) or not physical exercise. The effect of supraphysiological dosing with T is also showed in rabbits which performed physical exercise (RD+T+PhyEx) or not (RD+T). Level of significance upon Kruskal–Wallis and post-hoc Mann–Whitney analyses is also indicated, along with number of observations. *Outlier.
Gene expression of the indicated myogenic (A, B) or differentiation muscle (C) markers in eugonadal rabbits fed a regular diet (RD), which performed (RD+PhyEx) or not physical exercise (PhyEx). The effect of supraphysiological dosing with testosterone (T) is also showed in rabbits which performed physical exercise (RD+T+PhyEx) or not (RD+T). Panel (D) shows results on lipoprotein lipase (LPL) mRNA, a lipolytic marker. Level of significance upon Kruskal–Wallis and post-hoc Mann–Whitney analyses is also indicated, along with number of observations. *Outlier.
Parameters reported per single trial included in the meta-analysis