ABSTRACT
The amount of time spent in poor health at the end of life is increasing. This narrative review summarizes consistent evidence indicating that healthy dietary patterns and maintenance of a healthy weight in the years leading to old age are associated with broad prevention of all the archetypal diseases and impairments associated with aging including: noncommunicable diseases, sarcopenia, cognitive decline and dementia, osteoporosis, age-related macular degeneration, diabetic retinopathy, hearing loss, obstructive sleep apnea, urinary incontinence, and constipation. In addition, randomized clinical trials show that disease-specific nutrition interventions can attenuate progression—and in some cases effectively treat—many established aging-associated conditions. However, middle-aged and older adults are vulnerable to unhealthy dietary patterns, and typically consume diets with inadequate servings of healthy food groups and essential nutrients, along with an abundance of energy-dense but nutrient-weak foods that contribute to obesity. However, based on menu examples, diets that are nutrient-dense, plant-based, and with a moderately low glycemic load are better equipped to meet the nutritional needs of many older adults than current recommendations in US Dietary Guidelines. These summary findings indicate that healthy nutrition is more important for healthy aging than generally recognized. Improved public health messaging about nutrition and aging, combined with routine screening and medical referrals for age-related conditions that can be treated with a nutrition prescription, should form core components of a national nutrition roadmap to reduce the epidemic of unhealthy aging.
Keywords: aging, nutrition, noncommunicable diseases, sarcopenia, cognition, age-related macular degeneration, diabetic retinopathy, obstructive sleep apnea, urinary incontinence, constipation
Healthy nutrition has a broad, underrecognized role in preventing aging-related diseases and conditions. Updated public health recommendations on nutrition are needed to support healthy aging.
Background: Living Longer Compared with Living Healthier
Leading a long and healthy life is a goal that is embraced worldwide (1), and fear of death has long been proposed to be a defining characteristic of humans (2, 3). From these perspectives, the 30-y increase in life expectancy during the 20th century is a transformational advance. Furthermore, life expectancy continues to increase for adults aged >65 y (4), and adults >85 y are the fastest growing demographic (5). However, a little-recognized corollary of the recent trends is that older adults are now living in an ill and disabled state for longer: the mean duration of disability at the end of life was just 5.3 y in the 1960s (6), whereas more recent calculations indicate that the duration of poor health and functional impairments has increased from 8.9 to 10.2 y between 1990 and 2017 (7). This extension of unhealthy life is unprecedented in human history, and presents major personal and public health burdens. This is particularly evident during the current coronavirus disease-19 (COVID-19) pandemic, because the association of COVID-19 severity and age is substantially weakened when comorbidities are taken into account (8), and highlights the need to identify ways to support healthy aging (9). This review summarizes current knowledge of the underrecognized role of diet in prevention and treatment of diseases and functional losses that become increasingly prevalent during aging, with a focus on data available from research conducted in North America and Europe.
There is no single definition of “healthy aging” or the related term “healthspan” (1, 10, 11), but it is generally taken to mean the absence of the archetypal diseases and functional impairments associated with old age. The specific diseases and functional losses associated with aging have been defined as those conditions where there is a quadratic relation between disease prevalence and chronological age (12). These include: sarcopenia [loss of skeletal muscle (13)], wasting, and osteoporosis (14, 15), which are linked to frailty and falls (16); impaired cognitive function and increased risk of dementia (17, 18); impaired vision via age-related macular degeneration, cataracts, and diabetic retinopathy (19); hearing loss (20); noncommunicable diseases (NCDs) such as type 2 diabetes, cardiovascular disease, and cancer (12); obstructive sleep apnea (21, 22) and poor sleep quality (22, 23); and urinary incontinence (24) and chronic constipation (25, 26). The prevalence of these problems is often >50% in adults aged >85 y, especially in racial and ethnic minorities (26–28). The proposed underlying mechanisms of the aging-associated pathologies include inflammation, oxidative stress, and limited capacity for removal of damaged proteins and DNA repair (29–34). Because these changes affect all organ systems, aging with multiple comorbidities is the norm (35, 36).
Current Status of Knowledge
Unhealthy nutrition throughout life, but especially in old age
American adults of all ages typically eat a broadly unhealthy diet relative to national recommendations (37). Figure 1A illustrates the percentage of adults in different age groups who consume less than the estimated average requirements (EARs) of micronutrients (38). Mean intakes of choline, vitamin B-6, zinc, magnesium, and calcium are increasingly inadequate as adults age. In addition, 21% of women and 13% of men aged >70 y consume less than the RDA for protein (39), which is an important concern because emerging evidence suggests that protein levels higher than the RDA (1.0–1.2 g/kg) can be optimal for older adults to prevent muscle wasting (40) due to factors such as decreased muscle uptake of dietary amino acids and reduced anabolic signaling for protein synthesis (41). These findings of broadly low intakes of essential nutrients throughout adult life and especially in older adults are based on self-reported diet, but are consistent with nationally representative biochemical data showing that 30–36% of older adults have ≥1 micronutrients in the deficient range (42). Low micronutrient intakes have also been documented in older adults living in other countries (46), indicating this is likely a global, rather than a specifically US, phenomenon.
FIGURE 1.

Dietary adequacy in different age-groups. (A) Percentage of adults consuming below the estimated average requirement (EAR), or at or below the adequate intake (AI) when EAR values are not available, based on reported usual intakes in the NHANES 2009–2012. Includes nonconsumers of supplements examined in NHANES 2009–2012. Figure adapted from published information (39, 43). (B) Percentage of adults consuming above, below, or at the recommended intakes for food groups in the 2020–2025 Dietary Guidelines (44) by sex and age group, based on dietary data obtained from the 2007–2010 NHANES. A: Whole grains, B: Dairy, C: Seafood, D: Vegetables, E: Fruit, F: Oils, G: Nuts, seeds, soy, H: Protein, I: Meat, poultry, eggs, J: Refined grains, K: SoFAS. Note: Total vegetables includes beans and peas. Protein excludes beans and peas (45). SoFAS, solid fats and added sugars.
The dietary patterns of older adults are also broadly inadequate compared with food-based recommendations. Figure 1B shows that adults of all ages typically consume less than the 2020–2025 Dietary Guidelines recommended portions of most healthy food groups including whole grains, dairy, seafood, vegetables, fruits, nuts, seeds, and legumes (44). The figure also shows consumption of excess amounts of meats, saturated fats, and added sugars compared with the recommendations. Using currently available nutritional benchmarks, the majority of adults aged >50 y consume diets that fall far short of recommendations (37, 39, 43). Some groups are especially vulnerable, including low-income and minority populations (37), those participating in the national supplemental nutrition assistance program (47), and older adults with obesity (41% of adults >60 y) (48, 49).
In relation to these observations, it should be noted that current dietary recommendations for older adults are largely based on requirements measured in young adults. Thus, further research is needed to refine essential nutrient and food group recommendations for healthy aging (50). Nevertheless, empirical considerations suggest that mean requirements for protein and several micronutrients can increase during aging, with only a few energy-related vitamins (such as thiamin) decreasing (51–53).
Low energy requirements contribute to unhealthy nutrition in older adults
An important yet underrecognized factor in unhealthy dietary patterns in old age is that there is a large decrease in typical energy requirements as individuals age (54). Figure 2 shows the Institute of Medicine's estimated energy requirements of men and women of different ages and heights for the healthy weight range (BMI = 18.5–25.0 kg/m2), which were based on measurements of energy expenditure using the gold-standard doubly labeled water method (38). The equations used to generate the figure are given in Supplemental Table 1. As shown, the decrease in energy requirements to maintain healthy weight during adult life is substantial, with a typical reduction of ≥500–700 kcal/d between early adulthood and late life in healthy women and men. This creates the challenge that to meet the same or increased absolute intakes of protein and micronutrients in a diet containing a diminishing level of energy, the proportion of nutrient-dense foods in the diet has to keep increasing over time, with a parallel decrease of greater magnitude in the quantity of low-nutrient foods. In other words, a healthier diet is needed in older age to counterbalance decreasing energy requirements. Supplemental Table 2 shows EARs for protein and micronutrients as a percentage of 1000 kcal of typical energy requirements, illustrating that the density of most micronutrients needs to increase in older adults by 50%, and by nearly 100% for nutrients that are required in greater absolute amounts.
FIGURE 2.
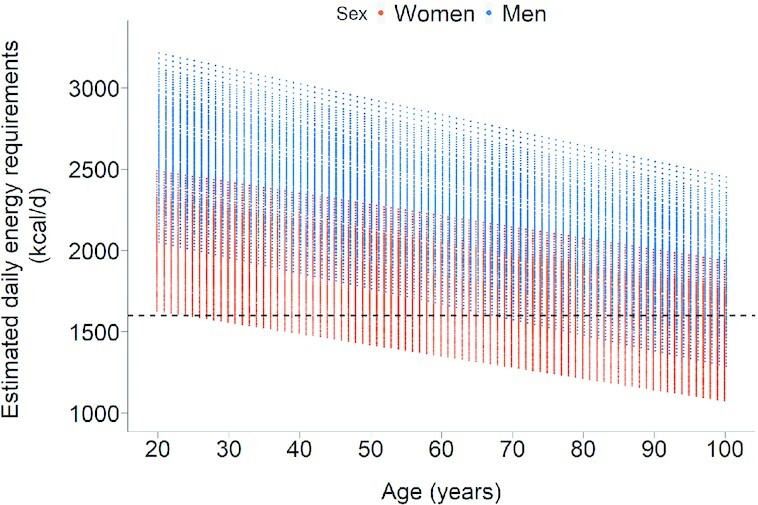
Energy requirements for individuals in the healthy weight range at different ages. Data are based on the Institute of Medicine's equations for predicting energy requirements of individuals with typical heights (for men: 1.58–1.9 m; for women: 1.45–1.78 m), a BMI in the healthy range of 18.5–25 kg/m2, and sedentary or light activity levels (38). The dotted line represents the lowest energy menu examples in the US Dietary Guidelines.
US Dietary Guidelines for 2020–2025 (44) give examples of healthy dietary patterns designed for all Americans, and include portion guidelines for adults with requirements of ≥1600 kcal/d (lower-calorie menus are illustrated for children, who have different nutritional needs). However, as illustrated in Figure 2, many older individuals, particularly women, require <1600 kcal/d to maintain a healthy weight, and some frail older adults will need as little as 1000 kcal/d to maintain a healthy weight depending on their age, weight, and height and health status. Thus, current Dietary Guidelines do not provide adequate guidance on healthful dietary patterns for the increasing population of older adults.
Functional losses are contributors to unhealthy nutrition in older adults
There is a negative cycle between functional losses and inadequate nutrition in older adults that accelerates unhealthy aging. Sarcopenia, the age-associated loss in skeletal muscle mass and function, is a key underlying cause of decreases in movement, physiological capacity, and functional performance, and increased disability and mortality observed with advancing age (55, 56). The causes of sarcopenia are multifactorial but include inadequate nutrition, low physical activity, inflammation, and multiple NCDs and other comorbidities (57, 58). Sarcopenia also has a profoundly negative impact on nutritional status in older adults, because decreased muscle mass contributes to reduced energy requirements, and can also limit the ability to shop for food and prepare meals (58).
As summarized below, poor vision in old age also limits the capability to purchase, prepare, and consume healthy food. For example, many older adults cannot see clearly the food on their plate. Similarly, reduced dental health, taste, smell, and hunger are associated with aging and also reduce the drive to eat (54, 59–62). Older adults are also more likely to take medications that impact food intake (63) and have digestive problems including gastric atrophy, chronic constipation, and/or malabsorption (64, 65) that negatively impact appetite and nutrient absorption. Older adults additionally have changes in homeostatic mechanisms regulating thirst sensation and renal water absorption, resulting in a higher risk of dehydration, (66, 67), which can be exacerbated by the use of common diuretics and fear of incontinence due to limited mobility (68).
Socioeconomic factors are contributors to unhealthy nutrition in older adults
In addition to physiological and genetic factors influencing nutritional status during aging, there are widely recognized demographic and social factors that increase the risk of consuming an unhealthy diet as adults grow older. These include poverty and food insecurity, which make it harder to purchase the nutrient-rich foods that are both more necessary and more expensive (69, 70). Older adults are also more likely to live alone and be socially isolated, factors that limit food preparation and predict unhealthy dietary intake (61, 62).
Dietary patterns, nutrients, and weight management for prevention and treatment of aging-associated diseases and conditions
Dietary patterns and nutrients
Table 1 summarizes the evidence from recent consensus reports, umbrella and systematic reviews, and meta-analyses for the associations of specific dietary patterns, nutrients, and BMI with prevention of age-related diseases and functional impairments. Table 1 also summarizes data from randomized controlled trials of nutritional treatments for specific conditions.
TABLE 1.
Consistent evidence for nutrition parameters and prevention and treatment of common aging-associated diseases and functional losses1
| Prevention(derived from expert consensus reports, or umbrella/systematic reviews, or meta analyses) | Reduced disease progression and or remission(derived from expert consensus reports, or powered randomized trials) | |
|---|---|---|
| Musculoskeletal | ||
| Frailty/sarcopenia, risk falls | • Healthy BMI (18.5–25 kg/m2) (71)• Dietary patterns: “Prudent” (72), Mediterranean (73) | • Specific nutrients: High protein: 1.3–1.5 g/kg protein alone or combined with exercise (74–76) |
| • Specific nutrients: Recommended protein (77), high total antioxidants (40, 78) | • For sarcopenic obesity: high-protein and weight loss with or without exercise (79) | |
| Osteoarthritis | • Healthy BMI (80) | • Weight loss (81) |
| Osteoporosis | • Specific nutrients: Adequate intakes of calcium (82), protein (83), vitamin D (84) | • Specific nutrients: 1200 mg Ca + 800 IU vitamin D + weight-bearing exercise (85, 86) |
| Cognition | ||
| Cognitive decline | • Healthy BMI (87) | • Weight loss (88) |
| • Dietary patterns: Mediterranean diet (89, 90), HEI, WHO's Healthy Diet Indicator (91) | ||
| Dementia/Alzheimer disease | • Healthy BMI (87) | |
| • Dietary patterns: Mediterranean diet (90, 92) | ||
| • Specific nutrients: Low saturated fat (92, 93) | ||
| Sense-organ diseases | ||
| Age-related macular degeneration | • Healthy BMI (7, 94)• Dietary patterns: Mediterranean diet, oriental diet, low-glycemic-index diet (95) | • Nutrients: High vitamins C + E, lutein, zeaxanthin, zinc, copper (96) |
| Cataracts | • Healthy BMI (97), healthy glycemic control in type 2 diabetes (98) | |
| • Specific nutrients: Multivitamin-mineral supplement (99, 100) | ||
| Hearing loss | • Healthy BMI (101)• Dietary patterns: HEI, low-glycemic-index carbohydrates (102, 103) | • Nutrients: Folic acid in individuals with high homocysteine (104) |
| Noncommunicable diseases | ||
| Type 2 diabetes | • Healthy BMI (105, 106)• Dietary patterns: Mediterranean (107, 108), DASH, and HEI (109), plant-based (110), low glycemic index, and low glycemic load (111) | • Lifestyle intervention with weight loss, healthy diet, and exercise (105, 112, 113)• Dietary patterns: Mediterranean, plant based (114, 115), low-carbohydrate (116, 117) |
| Cardiovascular disease | • Healthy BMI, weight loss if obesity (105, 106) | • Weight loss with healthy diet, exercise (105, 118) |
| • Dietary patterns: Mediterranean (107, 119, 120), DASH, and HEI (121, 119, 109), plant-based (119) [not low-carbohydrate (122)] | • Dietary patterns: Mediterranean (123), DASH (124) | |
| Cancers | • Healthy BMI (125, 126) | |
| • Dietary patterns: Mediterranean (107, 127–129, 120), DASH/HEI (109), plant-based diet (130, 131) | ||
| Sleep | ||
| Obstructive sleep apnea | • Healthy BMI (21) | • Weight loss (132) |
| Gastrointestinal | ||
| Chronic constipation | • Specific nutrients: Recommended fiber intake, including coarse wheat bran fiber (133–135) | • Specific nutrients: Coarse wheat bran fiber, adequate fluid (133) |
| Urinary incontinence | • Healthy BMI (136) | • Weight loss (24) |
DASH, Dietary Approaches to Stop Hypertension Trial; HEI, Healthy Eating Index.
A variety of dietary patterns and indices have been evaluated for their association with age-associated diseases and conditions, including Mediterranean-style diets (137), US Dietary Guidelines and the related Healthy Eating Index (138, 139), the WHO's Healthy Diet Indicator (140), Dietary Inflammatory Index (29), the MIND diet (141), and low glycemic index carbohydrate and high-fiber diets (142). These dietary profiles are associated with prevention of a broad range of age-associated diseases and conditions including: frailty and risk of falls, osteoporosis, cognitive decline, dementia, age-related macular degeneration, cataracts, hearing loss, NCDs (including type 2 diabetes, cardiovascular disease, and many cancers), and chronic constipation (72, 73, 89–92, 95, 102, 103, 107, 108, 121, 119, 109, 127–129, 120, 130, 131). In some cases, these associations are confirmed with randomized trials (143, 123). In addition Table 1 highlights specific nutrients associated with preventing unhealthy aging including: dietary protein at least equal to current RDAs for preventing sarcopenia, frailty, and falls in combination with exercise (77); adequate calcium and vitamin D intake with recommended protein for preventing osteoporosis (82–84); and whole grains and dietary fiber (in particular, coarse wheat bran fiber) for preventing type 2 diabetes and chronic constipation (133, 134).
There is also evidence that healthy aging is fostered by the cumulative effects of healthy nutrition earlier in life. For example, for prevention of osteoporosis late in life, attaining a high peak bone mineral density by age 30 is required (after which bone mineral density falls) and this requires consuming recommended levels of calcium throughout childhood and young adulthood (144). Similarly, high dietary flavanol intakes over 2 decades are associated with a reduced risk of Alzheimer disease and related dementias (145), and greater adherence to a Mediterranean diet for >5 y is associated with a 1–3-fold reduction in risk of frailty (146, 147), a 30% reduction in risk of a major cardiovascular event (123), and a 41% reduced risk of incident advanced age-related macular degeneration (148).
In addition there are a number of age-related diseases and conditions that randomized trials indicate can be treated to attenuate progression (and in some cases support remission) with a nutrition regimen (Table 1). These include sarcopenia, osteoporosis and fractures, age-related macular degeneration, type 2 diabetes, and chronic constipation (133, 74–76, 85, 86, 96). However, not all age-related diseases and conditions that are apparently prevented by healthy nutrition can also be treated after their diagnosis. For example, randomized trials have indicated no significant effect of omega-3 fatty acids, B vitamins, vitamin D, or soy protein on recurrence of various cancers (149, 150).
Although food-based nutrition is the focus of this report, a strong case can be made for targeted supplementation with specific nutrients that are hard to achieve in old age through a healthy diet. In particular, the mean intake of vitamin D in US women aged 51–70 is only about one-fourth of the RDA, and lower intakes are reported for ages ≥71 y (151). Similarly, mean calcium intake is less than one-third of the RDA in older adults (151). Some older adults can also benefit from supplemental vitamin B-12 because they are at increased risk of deficiency due to chronic atrophic gastritis [present in 30–50% of older adults (152)] and the widespread use of gastric acid–blocking drugs that inhibit digestion of food-based vitamin B-12 to an absorbable form (153).
Weight management
BMI values above the healthy range (>25.0) are strongly associated with increased risk of a wide range of age-associated diseases (Table 1). Older adults with obesity [41% of adults >60 y (154)] are at higher risk of frailty and osteoarthritis, and consequently have more functional limitations than those who are not obese (155). Obesity also increases the risk of all the major NCDs, cognitive decline and dementia, obstructive sleep apnea, sensory impairments (age-related macular degeneration, cataracts, diabetic retinopathy, and hearing loss), and urinary incontinence (7, 21, 156, 105, 106, 136, 71, 80, 87, 94). It should also be noted that unhealthy dietary patterns with high intakes of sugar-sweetened beverages, processed snack foods, and red meat, and low intakes of vegetables, whole grains, fruits, and nuts are associated with weight gain (157), which emphasizes the key link between diet, BMI, and health. As observed with dietary patterns, the risks of obesity for unhealthy aging increase over time, and there is a progressive increase in the risks of type 2 diabetes (158), cardiovascular disease (159), and cancer (160) with every year that obesity is maintained. Conversely, reduced energy intake promotes healthy aging, with data from studies of nonhuman primates (161) and a 2-y trial of calorie restriction in nonobese humans (162, 163) indicating that low energy intake promotes favorable changes in a broad range of age-related biomarkers of healthspan (162, 164).
Weight loss is also an effective first-line therapy for treatment of several age-related diseases and conditions in individuals with obesity, including urinary incontinence and sleep apnea (24, 132). Furthermore, a mean weight loss of 10% has been reported to achieve remission of type 2 diabetes in 50% of cases when implemented within 7 y of onset, providing a remarkable example of the potential for nutrition to impact age-related disease more effectively than current medication regimens (112).
It is also important to note that recommendations for maintaining physical activity into old age can play a valuable role in supporting nutritional health in old age, not only by preserving musculoskeletal health (165, 166) but also by attenuating the decline in energy requirements with aging. These findings are consistent with a recent federal report noting the lack of specificity in nutrition assistance programs to support healthy aging in current government programs (50).
Are All Generally Healthy Dietary Patterns Equivalent for Achieving Nutritional Health in Old Age?
There is currently insufficient information to categorically differentiate the effects of consuming the different broadly healthy dietary patterns discussed above. This is because they share multiple common features including an emphasis on regular consumption of vegetables and fruits, whole grains, legumes, nuts and seeds, seafood, and liquid oils such as olive and canola, and with low intakes of saturated fat and nutrient-weak foods such as sugar-sweetened beverages. However, different dietary patterns can differ in the extent to which adequate portion sizes of healthy foods can be achieved despite the decreased energy requirements associated with aging. We therefore created typical example menus for 3 healthy dietary patterns (Dietary Guidelines MyPlate, a Mediterranean-style diet, and a Vegetarian diet) and analyzed them for their ability to support nutritional sufficiency for older adults at different levels of energy requirements.
A summary of the results is provided in Figure 3, with descriptions of the menu items given in the Supplemental Information on Menu Calculations. As shown, for dietary energy ≥1600 kcal/d, all of the healthy dietary patterns could provide recommended portions of different recommended food groups, and also provide a calorie allowance for other “discretionary” foods of 32 (Mediterranean-style) to 246 (Vegetarian) kcal/d. However, for the lower dietary energy requirements observed in many older adults, the menus increasingly did not provide recommended portions of all healthy foods without exceeding total energy requirements, even when discretionary calories were reduced to zero (which is unrealistic). The Vegetarian menu was the one that best met portion recommendations for all food groups and protein at lower energy levels (including possibly higher protein needs than current US RDAs), and is consistent with the United Nations’ calls for greater reliance on plant-based foods (167). Among MyPlate and Mediterranean menus, reducing dairy and grain servings (selectively removing refined grains to preserve whole grain intake) resulted in moderately low carbohydrate options that did allow proteins, fruits, and vegetable servings to be as recommended at the lower calorie levels. These calculations suggest that lower-carbohydrate Dietary Guidelines menus provide another practical approach to meeting healthy nutrition guidelines for older adults at lower levels of energy intake.
FIGURE 3.

Illustration of the adequacy of healthy food group servings that can be achieved with different dietary patterns at lower levels of energy requirements in older adults. Typical menu examples were used to calculate the number of servings per day of foods in key healthy food groups (fruit, vegetables, proteins, dairy, grains, and oils) for 3 healthy dietary patterns (US Dietary Guidelines, Mediterranean, and Vegetarian) implemented at 4 energy levels (1600, 1400, 1200, and 1000 kcal/d). Suggested servings reflect a mean of 3 different menus that adhere to the respective dietary pattern and are shown relative to the serving size recommendations outlined in the Dietary Guidelines for Americans for each dietary pattern at 1600 kcal/d intakes. The vertical black line represents 100% of the recommended servings for the specified dietary patterns, and for discretionary calories * (all calories not included in healthy food group servings) represents 200 kcal/d. Note: Legumes are included in the protein category not vegetables, and oils do not reflect oils included in food items (e.g., avocado, nuts). Discretionary calories reflect calories that remain for other uses after meeting recommended servings of fruit, vegetables, protein, dairy, and grains.
Opportunities for a National Nutrition Strategy to Reduce Unhealthy Aging
The United States currently ranks only #55 in a global assessment of years of age-related disease burden at the end of life (#1 being best-ranked) (12) despite health care expenditures that are approximately twice those of other affluent nations (168). This striking public health failure has occurred despite acknowledgement of the general importance of nutrition across the lifespan (11, 169–171). The breadth of healthy aging benefits achievable with healthy nutrition described herein clarifies the broad and important role that nutrition can play to keep older adults healthy, and supports the development of a national nutrition strategy with clinical involvement for healthy aging.
One important element of a successful nutrition strategy for healthy aging would be increasing investment in federal nutrition research directed to this goal (172), with coordination among stakeholders to maximize research efficiency. This would recognize a strong role for nutrition in supporting healthy aging (as summarized here), the relative shortage of data from conventional randomized trials of specific interventions, and the need for fresh approaches to conduct rapid, rigorous testing of different dietary interventions in diverse populations. Stakeholders in a national nutrition strategy for healthy aging would include consumers, government agencies, food producers, the food industry, health professionals, and community organizations. Health professionals would play a pivotal role by leading the development of consensus recommendations (e.g., within societies for nutrition, geriatric medicine, primary care, nursing, physician assistant, occupational therapy) that would aid diagnosis and evidence-based treatments based on existing knowledge and identify priorities for next-generation research. This work could also be a springboard for developing training modules and continuing education for health care professionals.
Another important key to healthy aging would be the development of routine nutrition screening, implemented years before age-related diseases become prevalent, combined with research initiatives to develop and refine lifestyle interventions supporting aging-focused healthy behavior changes in different population groups. Direct nutrition screening is currently not performed in primary care and current indirect measures, such as BMI and lipid panels, do not provide adequate information to understand the specific nutritional vulnerabilities of individuals. Ideally, the development and use of broad nutrition screening panels to support healthy aging would allow for identification of at-risk individuals within primary care and either treatment within primary care or referral to specialized services (11, 70, 169, 173). In addition, artificial intelligence could be used to add nutrition screening data in real time, for rapid identification of time-sensitive nutritional risks, and such information could also be used as the basis for artificial intelligence–enabled personalized interventions. As well as evaluating dietary intake, screening assessments could include BMI and weight change. This inclusion would recognize both that obesity is a major risk factor for unhealthy aging (105), and that weight loss and protein-energy malnutrition with a low BMI are increasingly prevalent as adults age (174) and are similarly linked to reduced independence and greater risk of poor health. The apparent paradox that both obesity and weight loss with low BMI are risk factors emphasizes the importance of screening to allow for personalized nutrition support for healthy aging. There is currently no validated screening tool for the range of dietary intakes, BMI, and weight change seen in community-dwelling adults beginning in midlife on, but scales used in hospitalized patients (175) have potential for adaptation to standardized instruments for primary care.
Conclusions
Maintenance of functional independence and quality of life are of primary importance to older adults. Although aging is clearly programmed and progressive, a cohesive body of research finds that a healthy diet and weight management are able to not only reliably delay the onset of most typical diseases and functional losses in aging, but also arrest progression and severity, and even support remission for some conditions. Public health measures to facilitate healthy aging are currently lacking, but can be developed based on existing research to reduce the growing burden of poor health in old age.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Peggy Connolly for expert support during preparation of this review.
The authors’ responsibilities were as follows—All authors contributed material within their area of expertise and read and approved the final manuscript. JMK and RES also performed literature reviews and summaries, and prepared figures.
Notes
Supported by the AARP Foundation, and the USDA, Agricultural Research Service under Cooperative Agreement No. 58-8050-7-005. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA.
Author disclosures: SBR receives grant support from Danone, serves on the advisory board of Integrative Phenomics, and founded www.theidiet.com. RAF serves on advisory boards for Amazentis, Segterra, Acella Health, Juvicell, and Biophytis, has consulted for Pfizer, GSK, Nestlé, and Rejuvenate Biomed, and has equity in Axcella Health, Juvicell, and Segterra. PFJ receives grant support from Danone and serves on the Danone North America Essential Dairy and Plant-Based Advisory Board. JBM serves on the advisory board of Care/of; NMM receives grant support from Danone, Proctor & Gamble, ILSI North America, and the General Mills Bell Institute of Health & Nutrition, serves on the advisory board of the Whole Grains Council, and has consulted for General Mills, Canned Manufacturers Association, Nestlé Nutrition Fund, and Alliance for Potato Research & Education. All other authors report no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Information on Menu Calculations are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: COVID-19, coronavirus disease-19; DASH, Dietary Approaches to Stop Hypertension Trial; EAR, Estimated Average Requirement; NCD, noncommunicable disease.
Contributor Information
Susan B Roberts, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Rachel E Silver, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Sai Krupa Das, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Roger A Fielding, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Cheryl H Gilhooly, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Paul F Jacques, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Jennifer M Kelly, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Joel B Mason, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Nicola M McKeown, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Meaghan A Reardon, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Sheldon Rowan, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Edward Saltzman, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Barbara Shukitt-Hale, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Caren E Smith, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Allen A Taylor, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Dayong Wu, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Fang Fang Zhang, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Karen Panetta, School of Engineering, Tufts University, Medford, MA, USA.
Sarah Booth, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
References
- 1. World Health Organization . Ageing: Healthy ageing and functional ability. 2020; [Internet]. [cited 15 Jan 2021]. Available from: https://www.who.int/ageing/healthy-ageing/en/ [Google Scholar]
- 2. Joachim HH, Rees DA. Aristotle: the Nicomachean ethics. Oxford: Clarendon Press; 1952. [Google Scholar]
- 3. Becker E. The denial of death. New York (NY): Simon and Schuster; 2007. [Google Scholar]
- 4. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 2019;322(20):1996–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. United States Census Bureau, Economics and Statistics Administration; 2014. [Google Scholar]
- 6. Sullivan DF. A single index of mortality and morbidity. HSMHA Health Rep. 1971;86(4):347–54. [PMC free article] [PubMed] [Google Scholar]
- 7. US Burden of Disease Collaborators, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi Aet al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romero Starke K, Petereit-Haack G, Schubert M, Kämpf D, Schliebner A, Hegewald J, Seidler A. The age-related risk of severe outcomes due to COVID-19 infection: a rapid review, meta-analysis, and meta-regression. Int J Environ Res Public Health. 2020;17(16):5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dzau VJ, Inouye SK, Rowe JW, Finkelman E, Yamada T. Enabling healthful aging for all – the National Academy of Medicine grand challenge in healthy longevity. N Engl J Med. 2019;381(18):1699–701. [DOI] [PubMed] [Google Scholar]
- 10. Kaeberlein M. How healthy is the healthspan concept?. Geroscience. 2018;40(4):361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J-P, Lloyd-Sherlock P, Epping-Jordan JE, Peeters G, Mahanani WRet al. The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang AY Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health. 2019;4:e159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, van Kan GA, Andrieu S, Bauer J, Breuille D. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–24. [DOI] [PubMed] [Google Scholar]
- 15. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, Weir DR. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quillen DA. Common causes of vision loss in elderly patients. Am Fam Physician. 1999;60(1):99–108. [PubMed] [Google Scholar]
- 20. National Institute on Deafness and Other Communication Disorders (NIDCD) . Hearing loss and older adults. [Internet]. 2019; [cited 15 Jan 2021]. from: https://www.nidcd.nih.gov/health/hearing-loss-older-adults [Google Scholar]
- 21. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174(1):190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–9. [DOI] [PubMed] [Google Scholar]
- 27. Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, Xue Q-L, Walston JD, Kasper JD. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70(11):1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention . Arthritis-related statistics. [Internet]. 2018; [cited 15 Jan 2021]. Available from: https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm#prevspecific [Google Scholar]
- 29. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us?. Oxid Med Cell Longev. 2016;2016:7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, Wickramasinghe SN, Everson RB, Ames BN. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci USA. 1997;94(7):3290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc Natl Acad Sci USA. 2006;103(47):17589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin K. Modern biological theories of aging. Aging Dis. 2010;1(2):72. [PMC free article] [PubMed] [Google Scholar]
- 34. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention. Trends in aging–United States and worldwide. MMWR. 2003;52(6):101. [PubMed] [Google Scholar]
- 36. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE. Aging: a common driver of chronic diseases and a target for novel interventions. Cell. 2014;159(4):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Otten JJ, Hellwig JP, Meyers LD. Dietary reference intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press;2006. [Google Scholar]
- 39. Berryman CE, Lieberman HR, Fulgoni VL III, SM Pasiakos. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am J Clin Nutr. 2018;108(2):405–13. [DOI] [PubMed] [Google Scholar]
- 40. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta D. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 41. Damanti S, Azzolino D, Roncaglione C, Arosio B, Rossi P, Cesari M. Efficacy of nutritional interventions as stand-alone or synergistic treatments with exercise for the management of sarcopenia. Nutrients. 2019;11(9):1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bird JK, Murphy RA, Ciappio ED, McBurney MI. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9(7):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blumberg JB, Frei B, Fulgoni VL, Weaver CM, Zeisel SH. Contribution of dietary supplements to nutritional adequacy in various adult age groups. Nutrients. 2017;9(12):1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. U.S. Department of Agriculture and U.S. Department of Health and Human Services . Dietary Guidelines for Americans, 2020–2025. 9th ed [Internet]. 2020. Available from: https://www.dietaryguidelines.gov/ [Google Scholar]
- 45. National Cancer Institute . Usual dietary intakes: food intakes, U.S. population, 2007–10. [Internet]. Epidemiology and Genomics Research Program; Updated October 31, 2019; [cited 15 Jan 2021]. Available from: https://epi.grants.cancer.gov/diet/usualintakes/national-data-usual-dietary-intakes-2007-to-2010.pdf#search=usual%20dietary%20intakes [Google Scholar]
- 46. Ruxton CH, Derbyshire E, Toribio-Mateas M. Role of fatty acids and micronutrients in healthy ageing: a systematic review of randomised controlled trials set in the context of European dietary surveys of older adults. J Hum Nutr Diet. 2016;29(3):308–24. [DOI] [PubMed] [Google Scholar]
- 47. Zhang FF, Liu J, Rehm CD, Wilde P, Mande JR, Mozaffarian D. Trends and disparities in diet quality among US adults by Supplemental Nutrition Assistance Program participation status. JAMA Netw Open. 2018;1(2):e180237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;(288):1–8. [PubMed] [Google Scholar]
- 49. Asghari G, Mirmiran P, Yuzbashian E, Azizi F. A systematic review of diet quality indices in relation to obesity. Br J Nutr. 2017;117(8):1055–65. [DOI] [PubMed] [Google Scholar]
- 50. United States Government Accountability Office, Nutrition Assistance Programs . Agencies could do more to help address the nutritional needs of older adults. Washington (DC): USGA Office; 2019. p. 1–64. [Google Scholar]
- 51. National Institutes of Health, Office of Dietary Supplements . Nutrient recommendations: dietary reference intakes (DRI). 2011; [cited August 20, 2018] [Internet]. Available from: https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx [Google Scholar]
- 52. Baum JI, Kim I-Y, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake?. Nutrients. 2016;8(6):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance?J Gerontol A Biol Sci Med Sci 2013;68(6):677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86(2):651–67. [DOI] [PubMed] [Google Scholar]
- 55. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, Magaziner JM, Newman AB, Kiel DP, Cooper C. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2020;68(7):1410–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, Santanasto AJ, Ensrud KE, Xue QL, Shardell M. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68(7):1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aversa Z, Zhang X, Fielding RA, Lanza I, LeBrasseur NK. The clinical impact and biological mechanisms of skeletal muscle aging. Bone. 2019;127:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rolland Y, Czerwinski S, Van Kan GA, Morley J, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lichtenstein AH. Optimal nutrition for older adults. In: Rippe JM, editor. Nutrition in Lifestyle Medicine. Cham: Humana Press; 2017. p. 355–66.. https://doi.org/10.1007/978-3-319-43027-0_19. [Google Scholar]
- 60. Morley JE. Nutrition and aging well. J Am Med Dir Assoc. 2017;18(2):91–4. [DOI] [PubMed] [Google Scholar]
- 61. Kaushik S, Wang JJ, Flood V, Tan JSL, Barclay AW, Wong TY, Brand-Miller J, Mitchell P. Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr. 2008;88(4):1104–10. [DOI] [PubMed] [Google Scholar]
- 62. Payette H, Shatenstein B. Determinants of healthy eating in community-dwelling elderly people. Can J Public Health. 2005;96(Suppl 3):S27–31. [PubMed] [Google Scholar]
- 63. Little MO. Updates in nutrition and polypharmacy. Curr Opin Clin Nutr Metab Care. 2018;21(1):4–9. [DOI] [PubMed] [Google Scholar]
- 64. Whitcomb EA, Chiu C-J, Taylor A. Dietary glycemia as a determinant of health and longevity. Mol Aspects Med. 2015;46:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ellis AC. Nutrition and healthy aging. In: Coll P. Healthy Aging. Cham: Springer; 2019. p. 263–74.. https://doi.org/10.1007/978-3-030-06200-2_22. [Google Scholar]
- 66. Miller M. Hormonal aspects of fluid and sodium balance in the elderly. Endocrinol Metab Clin North Am. 1995;24(2):233–53. [PubMed] [Google Scholar]
- 67. Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311(12):753–9. [DOI] [PubMed] [Google Scholar]
- 68. Lavizzo-Mourey RJ. Dehydration in the elderly: a short review. J Natl Med Assoc. 1987;79(10):1033–8. [PMC free article] [PubMed] [Google Scholar]
- 69. Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, Freund-Levi Y, Fielding RA, Cheng FW, Jensen GL, Wu Det al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr. 2017;8(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuczmarski MF, Weddle DO, American Dietetic Association . Position paper of the American Dietetic Association: nutrition across the spectrum of aging. J Am Diet Assoc. 2005;105(4):616–33. [DOI] [PubMed] [Google Scholar]
- 71. Amiri S, Behnezhad S, Hasani J. Body mass index and risk of frailty in older adults: a systematic review and meta-analysis. Obes Med. 2020;18:100196. [Google Scholar]
- 72. Rashidi Pour Fard N, Amirabdollahian F, Haghighatdoost F. Dietary patterns and frailty: a systematic review and meta-analysis. Nutr Rev. 2019;77(7):498–513. [DOI] [PubMed] [Google Scholar]
- 73. Silva R, Pizato N, Da Mata F, Figueiredo A, Ito M, Pereira M. Mediterranean diet and musculoskeletal-functional outcomes in community-dwelling older people: a systematic review and meta-analysis. J Nutr Health Aging. 2018;22(6):655–63. [DOI] [PubMed] [Google Scholar]
- 74. Park Y, Choi J-E, Hwang H-S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2018;108(5):1026–33. [DOI] [PubMed] [Google Scholar]
- 75. Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, Van De Rest O, de Groot LC, Van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–19. [DOI] [PubMed] [Google Scholar]
- 76. ten Haaf DS, Eijsvogels TM, Bongers CC, Horstman AM, Timmers S, de Groot LC, Hopman MT. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2019;10(2):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Beasley JM, LaCroix AZ, Neuhouser ML, Huang Y, Tinker L, Woods N, Michael Y, Curb JD, Prentice RL. Protein intake and incident frailty in the Women's Health Initiative observational study. J Am Geriatr Soc. 2010;58(6):1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Coelho-Júnior HJ, Rodrigues B, Uchida M, Marzetti E. Low protein intake is associated with frailty in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10(9):1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Starr KNP, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J Am Med Dir Assoc. 2014;15(4):240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan J, Protheroe J, Jordan K. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(4):507–15. [DOI] [PubMed] [Google Scholar]
- 81. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the US bone and joint initiative. Semin Arthritis Rheum. 2014;43:701–12. [DOI] [PubMed] [Google Scholar]
- 82. Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr. 2000;19(Suppl 2):83S–99S. [DOI] [PubMed] [Google Scholar]
- 83. Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC, LeBoff MS, Shapses SA, Sackey J, Wallace TC. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. 2017;105(6):1528–43. [DOI] [PubMed] [Google Scholar]
- 84. Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD, Cole DE, Atkinson SA, Josse RG, Feldman S, Kline GA. Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. Can Med Assoc J. 2010;182(12):E610–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kanis J, Burlet N, Cooper C, Delmas P, Reginster J-Y, Borgstrom F, Rizzoli R. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19(4):399–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weaver C, Alexander D, Boushey C, Dawson-Hughes B, Lappe JM, LeBoff M, Liu S, Looker A, Wallace T, Wang D. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Beydoun MA, Beydoun H, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9(3):204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Veronese N, Facchini S, Stubbs B, Luchini C, Solmi M, Manzato E, Sergi G, Maggi S, Cosco T, Fontana L. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;72:87–94. [DOI] [PubMed] [Google Scholar]
- 89. Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cao L, Tan L, Wang H-F, Jiang T, Zhu X-C, Lu H, Tan M-S, Yu J-T. Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53(9):6144–54. [DOI] [PubMed] [Google Scholar]
- 93. Ruan Y, Tang J, Guo X, Li K, Li D. Dietary fat intake and risk of Alzheimer's disease and dementia: a meta-analysis of cohort studies. Curr Alzheimer Res. 2018;15(9):869–76. [DOI] [PubMed] [Google Scholar]
- 94. Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chapman NA, Jacobs RJ, Braakhuis AJ. Role of diet and food intake in age-related macular degeneration: a systematic review. Clin Exp Ophthalmol. 2019;47(1):106–27. [DOI] [PubMed] [Google Scholar]
- 96. Age-Related Eye Disease Study 2 Research Group . Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–15. [DOI] [PubMed] [Google Scholar]
- 97. Ye J, Lou L-X, He J-J, Xu Y-F. Body mass index and risk of age-related cataract: a meta-analysis of prospective cohort studies. PLoS One. 2014;9(2):e89923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Drinkwater JJ, Davis WA, Davis TM. A systematic review of risk factors for cataract in type 2 diabetes. Diabetes Metab Res Rev. 2019;35(1):e3073. [DOI] [PubMed] [Google Scholar]
- 99. Zhao L-Q, Li L-M, Zhu H. The effect of multivitamin/mineral supplements on age-related cataracts: a systematic review and meta-analysis. Nutrients. 2014;6(3):931–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jiang H, Yin Y, Wu C-R, Liu Y, Guo F, Li M, Ma L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am J Clin Nutr. 2019;109(1):43–54. [DOI] [PubMed] [Google Scholar]
- 101. Dhanda N, Taheri S. A narrative review of obesity and hearing loss. Int J Obes. 2017;41(7):1066–73. [DOI] [PubMed] [Google Scholar]
- 102. Puga A, Pajares M, Varela-Moreiras G, Partearroyo T. Interplay between nutrition and hearing loss: state of art. Nutrients. 2018;11(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jung SY, Kim SH, Yeo SG. Association of nutritional factors with hearing loss. Nutrients. 2019;11(2):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ. Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med. 2007;146(1):1–9. [DOI] [PubMed] [Google Scholar]
- 105. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RFet al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yamaoka K, Nemoto A, Tango T. Comparison of the effectiveness of lifestyle modification with other treatments on the incidence of type 2 diabetes in people at high risk: a network meta-analysis. Nutrients. 2019;11(6):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72(1):30–43. [DOI] [PubMed] [Google Scholar]
- 108. Schwingshackl L, Missbach B, König J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18(7):1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74. [DOI] [PubMed] [Google Scholar]
- 110. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Livesey G, Taylor R, Livesey HF, Buyken AE, Jenkins DJ, Augustin LS, Sievenpiper JL, Barclay AW, Liu S, Wolever T. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 2019;11(6):1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018(391):541–51. [DOI] [PubMed] [Google Scholar]
- 113. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Papamichou D, Panagiotakos D, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis. 2019;29:531–43. [DOI] [PubMed] [Google Scholar]
- 115. Huo R, Du T, Xu Y, Xu W, Chen X, Sun K, Yu X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200. [DOI] [PubMed] [Google Scholar]
- 116. McArdle P, Greenfield S, Rilstone S, Narendran P, Haque M, Gill P. Carbohydrate restriction for glycaemic control in type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2019;36(3):335–48. [DOI] [PubMed] [Google Scholar]
- 117. Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diab Res Care. 2017;5(1):e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Trial Maruthur N, Wang N, Appel L. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER. Circulation. 2009;119(15):2026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kahleova H, Salas-Salvadó J, Rahelić D, Kendall CW, Rembert E, Sievenpiper JL. Dietary patterns and cardiometabolic outcomes in diabetes: a summary of systematic reviews and meta-analyses. Nutrients. 2019;11(9):2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17(12):2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113(1):1–15. [DOI] [PubMed] [Google Scholar]
- 122. Mazidi M, Katsiki N, Mikhailidis DP, Sattar N, Banach M. Lower carbohydrate diets and all-cause and cause-specific mortality: a population-based cohort study and pooling of prospective studies. Eur Heart J. 2019;40(34):2870–9. [DOI] [PubMed] [Google Scholar]
- 123. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 124. Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, Salas-Salvadó J, Kendall CW, Sievenpiper JL. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Suárez AL. Burden of cancer attributable to obesity, type 2 diabetes and associated risk factors. Metabolism. 2019;92:136–46. [DOI] [PubMed] [Google Scholar]
- 126. World Cancer Research Fund International . Diet, nutrition, physical activity and cancer: a global perspective. The Third Expert Report [Internet]. 2019; [cited 15 Jan 2021]. Available from: https://www.wcrf.org/dietandcancer [Google Scholar]
- 127. Foscolou A, Koloverou E, Matalas A-L, Tyrovolas S, Chrysohoou C, Sidossis L, Rallidis L, Panagiotakos DB. Decomposition of Mediterranean dietary pattern on successful aging, among older adults: a combined analysis of two epidemiological studies. J Aging Health. 2019;31(9):1549–67. [DOI] [PubMed] [Google Scholar]
- 128. Fransen HP, Beulens JW, May AM, Struijk EA, Boer JM, de Wit GA, Onland-Moret NC, van der Schouw YT, Bueno-de-Mesquita HB, Hoekstra Jet al. Dietary patterns in relation to quality-adjusted life years in the EPIC-NL cohort. Prev Med. 2015;77:119–24. [DOI] [PubMed] [Google Scholar]
- 129. Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4(12):1933–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. [DOI] [PubMed] [Google Scholar]
- 131. Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. 2012;60(4):233–40. [DOI] [PubMed] [Google Scholar]
- 132. Mitchell LJ, Davidson ZE, Bonham M, O'Driscoll DM, Hamilton GS, Truby H. Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med. 2014;15(10):1173–83. [DOI] [PubMed] [Google Scholar]
- 133. de Vries J, Miller PE, Verbeke K. Effects of cereal fiber on bowel function: a systematic review of intervention trials. World J Gastroenterol. 2015;21(29):8952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Institute of Medicine. Dietary reference intakes: energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): The National Academies Press;2005. [Google Scholar]
- 135. McRorie JW Jr, Fahey GC Jr, Gibb RD, Chey WD. Laxative effects of wheat bran and psyllium: resolving enduring misconceptions about fiber in treatment guidelines for chronic idiopathic constipation. J Am Assoc Nurse Pract. 2020;32(1):15–23. [DOI] [PubMed] [Google Scholar]
- 136. Subak LL, Richter HE, Hunskaar S. Obesity and urinary incontinence: epidemiology and clinical research update. J Urol. 2009;182(6S):S2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–6S. [DOI] [PubMed] [Google Scholar]
- 138. United States Department of Health and Human Services . Dietary guidelines for Americans, 5th ed. Washington (DC): USDA; 2000. [Google Scholar]
- 139. USDA Food and Nutrition Service. Healthy Eating Index (HEI) [Internet]. 2019; [cited 15 Jan 2021]. Available from: https://www.fns.usda.gov/resource/healthy-eating-index-hei [Google Scholar]
- 140. Jankovic N, Geelen A, Streppel MT, de Groot LC, Orfanos P, van den Hooven EH, Pikhart H, Boffetta P, Trichopoulou A, Bobak Met al. Adherence to a healthy diet according to the World Health Organization guidelines and all-cause mortality in elderly adults from Europe and the United States. Am J Epidemiol. 2014;180(10):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement (Amst). 2015;11(9):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jenkins D, Wolever T, Collier GR, Ocana A, Rao AV, Buckley G, Lam Y, Mayer A, Thompson LU. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr. 1987;46(6):968–75. [DOI] [PubMed] [Google Scholar]
- 143. Toledo E, Salas-Salvadó J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, Corella D, Fitó M, Hu FB, Arós F. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med. 2015;175(11):1752–60. [DOI] [PubMed] [Google Scholar]
- 144. Weaver CM. The role of nutrition on optimizing peak bone mass. Asia Pac J Clin Nutr. 2008;17:135–7. [PubMed] [Google Scholar]
- 145. Shishtar E, Rogers GT, Blumberg JB, Au R, Jacques PF. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am J Clin Nutr. 2020;112(112):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Talegawkar SA, Bandinelli S, Bandeen-Roche K, Chen P, Milaneschi Y, Tanaka T, Semba RD, Guralnik JM, Ferrucci L. A higher adherence to a Mediterranean-style diet is inversely associated with the development of frailty in community-dwelling elderly men and women. J Nutr. 2012;142(12):2161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. León-Muñoz LM, Guallar-Castillón P, López-García E, Rodríguez-Artalejo F. Mediterranean diet and risk of frailty in community-dwelling older adults. J Am Med Dir Assoc. 2014;15(12):899–903. [DOI] [PubMed] [Google Scholar]
- 148. Merle BM, Colijn JM, Cougnard-Grégoire A, de Koning-Backus AP, Delyfer M-N, Kiefte-de Jong JC, Meester-Smoor M, Féart C, Verzijden T, Samieri C. Mediterranean diet and incidence of advanced age-related macular degeneration: the EYE-RISK Consortium. Ophthalmology. 2019;126(3):381–90. [DOI] [PubMed] [Google Scholar]
- 149. Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Rueter EE, Melamed J, Kong MX, Macias V, Kajdacsy-Balla A, Lumey L. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. 2013;310(2):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Andreeva VA, Touvier M, Kesse-Guyot E, Julia C, Galan P, Hercberg S. B vitamin and/or ω-3 fatty acid supplementation and cancer: ancillary findings from the supplementation with folate, vitamins B6 and B12, and/or omega-3 fatty acids (SU.FOL.OM3) randomized trial. Arch Intern Med. 2012;172(7):540–7. [DOI] [PubMed] [Google Scholar]
- 151. Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Krasinski SD, Russell RM, Samloff IM, Jacob RA, Dallal GE, McGandy RB, Hartz SC. Fundic atrophic gastritis in an elderly population: effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc. 1986;34(11):800–6. [DOI] [PubMed] [Google Scholar]
- 153. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435–42. [DOI] [PubMed] [Google Scholar]
- 154. Centers for Disease Control and Prevention . Adult obesity facts. [Internet]. 2018; [cited 15 Jan 2021]. Available from: https://www.cdc.gov/obesity/data/adult.html [Google Scholar]
- 155. Crow RS, Lohman MC, Titus AJ, Cook SB, Bruce ML, Mackenzie TA, Bartels SJ, Batsis JA. Association of obesity and frailty in older adults: NHANES 1999–2004. J Nutr Health Aging. 2019;23(2):138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. GBD Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hu Y, Bhupathiraju SN, de Koning L, Hu FB. Duration of obesity and overweight and risk of type 2 diabetes among US women. Obesity. 2014;22(10):2267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–70. [DOI] [PubMed] [Google Scholar]
- 160. Arnold M, Freisling H, Stolzenberg-Solomon R, Kee F, O'Doherty MG, Ordóñez-Mena JM, Wilsgaard T, May AM, Bueno-de-Mesquita HB, Tjønneland A. Overweight duration in older adults and cancer risk: a study of cohorts in Europe and the United States. Eur J Epidemiol. 2016;31(9):893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant Met al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DTet al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Das SK, Balasubramanian P, Weerasekara YK. Nutrition modulation of human aging: the calorie restriction paradigm. Mol Cell Endocrinol. 2017;455:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Das SK, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(9):673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. World Health Organization . Global strategy on diet, physical activity and health. [Internet]. 2004; [cited 15 Jan 2021]. Available from: https://www.who.int/dietphysicalactivity/strategy/eb11344/strategy_english_web.pdf [Google Scholar]
- 166. Dietary Guidelines Advisory Committee . Dietary guidelines for Americans 2015–2020. Office of Disease Prevention and Health Promotion; 2015. [Google Scholar]
- 167. Intergovernmental Panel on Climate Change . Climate change and land. [Internet]. 2019; [cited 15 Jan 2021]. Available from: https://www.ipcc.ch/report/srccl/ [Google Scholar]
- 168. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–39. [DOI] [PubMed] [Google Scholar]
- 169. National Prevention Council . Healthy aging in action. [Internet]. 2016; [cited 15 Jan 2021]. Available from: https://www.cdc.gov/aging/pdf/healthy-aging-in-action508.pdf [PubMed] [Google Scholar]
- 170. Kritchevsky SB. Nutrition and healthy aging. J Gerontol A Biol Sci Med Sci. 2016;71(10):1303–5. [DOI] [PubMed] [Google Scholar]
- 171. Morley JE. Nutrition and aging well. J Am Med Dir Assoc. 2017;18(2):91–4. [DOI] [PubMed] [Google Scholar]
- 172. Fleischhacker SE, Woteki CE, Coates PM, Hubbard VS, Flaherty GE, Glickman DR, Harkin TR, Kessler D, Li WW, Loscalzo J. Strengthening national nutrition research: rationale and options for a new coordinated federal research effort and authority. Am J Clin Nutr. 2020;112(3):721–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Johnson MA, Dwyer JT, Jensen GL, Miller JW, Speakman JR, Starke-Reed P, Volpi E. Challenges and new opportunities for clinical nutrition interventions in the aged. J Nutr. 2011;141(3):535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Defeat Malnutrition Today . Homepage. [Internet]. 2020; [cited 15 Jan 2021]. Available from: https://www.defeatmalnutrition.today/ [Google Scholar]
- 175. Dwyer JT, Gahche JJ, Weiler M, Arensberg MB. Screening community-living older adults for protein energy malnutrition and frailty: update and next steps. J Community Health. 2020;45(3):640–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


