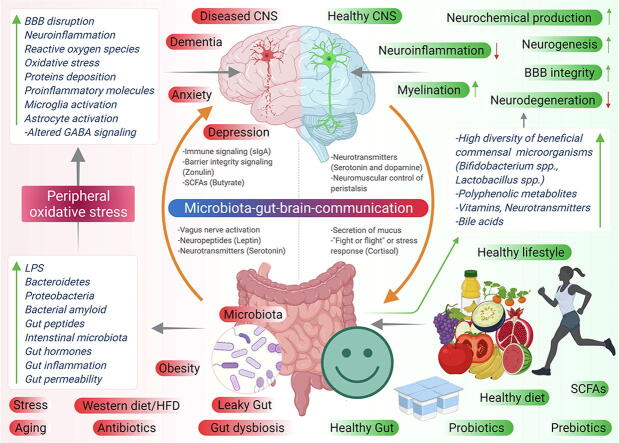
Graphical abstract
Keywords: Gut-microbiota, Oxidative stress, Neurodegeneration, Alzheimer’s disease, Parkinson's disease, Neurotherapeutics
Highlights
-
•
Owing to high O2 consumption, brain is highly vulnerable to oxidative stress.
-
•
Gut-brain axis act as a vital pathway of communication and physiological regulation.
-
•
Altered gut microbiota-mediated oxidative stress is associated with neurodegeneration.
-
•
Healthy gut microbiota has an immense antioxidative and anti-inflammatory role.
-
•
Antioxidative prebiotics and probiotics attenuates neurodegenerative symptoms.
-
•
Current databases and in silico tools will help to develop new therapeutic regimens.
Abstract
Background
Recent research on the implications of gut microbiota on brain functions has helped to gather important information on the relationship between them. Pathogenesis of neurological disorders is found to be associated with dysregulation of gut-brain axis. Some gut bacteria metabolites are found to be directly associated with the increase in reactive oxygen species levels, one of the most important risk factors of neurodegeneration. Besides their morbid association, gut bacteria metabolites are also found to play a significant role in reducing the onset of these life-threatening brain disorders.
Aim of Review
Studies done in the recent past raises two most important link between gut microbiota and the brain: “gut microbiota-oxidative stress-neurodegeneration” and gut microbiota-antioxidant-neuroprotection. This review aims to gives a deep insight to our readers, of the collective studies done, focusing on the gut microbiota mediated oxidative stress involved in neurodegeneration along with a focus on those studies showing the involvement of gut microbiota and their metabolites in neuroprotection.
Key Scientific Concepts of Review
This review is focused on three main key concepts. Firstly, the mounting evidences from clinical and preclinical arenas shows the influence of gut microbiota mediated oxidative stress resulting in dysfunctional neurological processes. Therefore, we describe the potential role of gut microbiota influencing the vulnerability of brain to oxidative stress, and a budding causative in Alzheimer's and Parkinson’s disease. Secondly, contributing roles of gut microbiota has been observed in attenuating oxidative stress and inflammation via its own metabolites or by producing secondary metabolites and, also modulation in gut microbiota population with antioxidative and anti-inflammatory probiotics have shown promising neuro resilience. Thirdly, high throughput in silico tools and databases also gives a correlation of gut microbiome, their metabolites and brain health, thus providing fascinating perspective and promising new avenues for therapeutic options.
Introduction
Enteric nervous system (ENS), also known as the second brain of the body acts as a key link in understanding the bidirectional communication between the gastrointestinal (GI) tract and the central nervous system (CNS) [1]. Enteric neurons communicate with the CNS via neuronal (vagus nerve), endocrine, and immune pathways, which are involved in maintaining gut health [2]. However, the modulating factor which can regulate this communication is gut microbiota. Among the gut microbial population (fungi, archaea, virus, bacteria and parasites), particularly gut bacteria constitute equal number to that of human cells in the body [3] and interestingly possess a metabolic potential equivalent to that of human liver [4]. Continuous research in this area shows the immense importance of gut microbiota not only in the functioning of the immune system [5] and regulatory metabolism [6] but even in the development of various organs [7]. Variable environmental factors such as diet [8], drugs [9] and host factors including age and genetics [10] not only alter the composition of gut microbiota but also can cause the change in signaling activity of them. Also, Immunoglobulin A (IgA), which is the most abundant antibody secreted at mucosal surface, coats the commensal bacteria in the gut and maintains diverse and stable gut microbiota community [11], [12]. Even, gut microbiota is known to influence the serum protein zonulin, which is required to regulate intestinal and vascular endothelium (blood-brain barrier) tight junctions [13]. Changes in gut microbiota directly affect zonulin pathway resulting in leaky gut [13]. Moreover, gut microbiota is one of the contributing factors controlling gut peristalsis [14]. Among the endocrine factors, elevated levels of cortisol has been observed as contributing factors in gut dysbiosis and is considered a cause of stress and depression [15]. Thus, gut microbiota directly influences human health by sensing, modulating and circulating a vast number of chemical signals coming from the environment.
The relationship between intestinal bacteria and neurological diseases was first hypothesized by Elie Metchnikoff and colleagues in 1900s, which has now been recognized by many research groups. Neurodegenerative diseases (NDDs) were first believed to be caused by defects in the nervous system neglecting the facts that microorganisms in the gut possess the ability to produce and modify various immune, metabolic and neurochemical factors which are known to directly affect the nervous system [16]. NDDs are mainly caused by oxidative damage, increased reactive oxygen species (ROS) production, neuroinflammation and disruptive energy metabolism, which on the other hand also affects the gut microbial population [17]. Surprisingly, gut microbial composition alters with the changes in the metabolism of the body from healthy to diseased state [18], [19]. This shows the intersection of gut microbiota between the host and the environment, and its morbid association with various neurological and psychological disorders. Gut dysbiosis and neuroinflammation are consistent factors in the pathophysiology of various neurological disorders. In this review, we emphasized showcasing the role gut microbiota-mediated oxidative stress in neurodegeneration, including the explanation of mechanisms of ROS production, why the brain is more susceptible to oxidative stress and how gut microbial metabolites influence the oxidative stress-induced damage in the brain with a focus on Alzheimer’s disease (AD), Parkinson’s disease (PD) and Traumatic brain injury (TBI).
Despite the role of gut microbiota in the pathology of NDDs, unsurprisingly, gut microbiota also has the potential to protect the brain from damage either by releasing the metabolites generated by them from converting dietary fibers, polyphenols or host molecules like bile acids and steroid hormones or their composition in the gut can be modulated by prebiotics in order to promote neuro resilience [20]. Neuroprotective role of gut microbiota is well documented in recent studies, it was observed that Lactobacillus buchneri KU200793 isolated from Korean fermented food showed higher antioxidant activity and was able to protect the SH-SY5Y cells from 1-methyl-4-phenylpyridinium (MPP+), suggesting its probiotic and neuroprotective effects [21]. Similarly, it was reported that exopolysaccharides isolated from Lactobacillus delbrueckii ssp. bulgaricus B3 and Lactobacillus plantarum GD2 protected SH-SY5Y cells from Aβ(1–42)-induced apoptosis [22], which potentiates their role as a promising natural chemical constituent for the pharmacological therapy of AD. Moreover, treatment of SH-SY5Y and mouse model with heat-killed strain of Rumnicoccus albus showed the neuroprotective effects, it was found to be very effective in reducing ROS levels and increasing superoxide dismutase (SOD) and glutathione (GSH) levels in hydrogen peroxide (H2O2) treated SH-SY5Y cells and in sodium arsenate treated animal models [23]. Likewise, anti-Alzheimer’s actions of Lactobacillus plantarum MTCC1325 have been studied in Albino rats with AD induced by D-Galactose (D-Gal) [24]. It has been shown that the L. plantarum protects against memory defect in D-Gal and scopolamine-induced AD in mice [25]. Taken together, these studies reflect the promising role of gut microbiota, their antioxidative and subsequently, their neuroprotective roles. Based on these foundational discoveries, we describe the role of gut microbiota, their metabolites, along with anti-inflammatory and antioxidative probiotics in neuroprotection. Also, we have gathered information about the databases and in silico strategies utilized to study gut-brain interactions. Altogether, this review will give a deep insight into the dual role of gut microbiota in oxidative stress-induced neurodegeneration and gut microbial metabolites-mediated antioxidative mechanism-based neuroprotection.
ROS and oxidative stress
Oxidative stress corresponds to the disruption of redox signaling pathway in cells due to an increase of ROS level more than that of antioxidant levels. This state of imbalance results in deleterious effects and is a leading cause of many neurological diseases. Every chemical reaction involved in aerobic metabolism results in the formation of reactive intermediate products which are unstable and short-lived, known as ROS [26], [27].
Biomolecular oxygen (O2) possesses two unpaired electrons which cannot be reduced completely, thus its incomplete reduction results in the formation of highly electrophilic and short-lived ROS like H2O2, superoxide anion, nitric oxide, peroxynitrite anion, hydroxyl and peroxyl radicals [28]. Either ROS is generated as an intermediate in normal cellular processes via ROS generating enzymes or in the presence of exogenous factors like drugs, toxins, and radiations [29]. Neuronal tissues due to their high metabolic rate produces a large amount of ROS in comparison to the other organs. Mitochondria is considered as the main cellular site of ROS generation in brain, where during the process of ATP generation (mitochondrial oxidative phosphorylation), anion superoxide is generated as a byproduct, which is then rapidly transformed into H2O2 and O2 by SOD [30]. The greater the amount of O2, the greater is the formation of superoxide, which further results in more ROS like H2O2 and hydroxyl radicals due to the addition of electrons from leaky electron transport chain (ETC) (complex I and III) [31]. Mitochondrial ROS production also indicates neuronal activity, as intense synaptic transmission boosts superoxide production [32]. Though on one hand mitochondrial ROS can be regulated by intracellular calcium (Ca2+) levels [33], moreover, increased mitochondrial ROS production is also associated with the increased mitochondrial membrane potential. Besides inner mitochondrial membrane, mitochondrial matrix enzyme, aconitase also contributes to ROS production by transforming H2O2 into hydroxyl radicals facilitated by iron-sulphur cluster in Fenton reaction. Many other enzymes like external NADH dehydrogenase, proline dehydrogenase, dihydroorotate dehydrogenase, and complex IV, also have been reported as contributors in mitochondrial ROS production [34].
Besides inner mitochondrial membrane, monoamine oxidases (MAO) present in outer mitochondrial membrane, present in most of the cell types including neurons and is among the primary source of ROS. MAO catalyzes the oxidative deamination of monoamines, requiring cofactor FAD (flavin adenine dinucleotide), by utilizing molecular O2 to remove an amine group from the molecule, where H2O2 is produced as a byproduct of the reaction [35]..The two isoforms of MAO, namely MAO-A and MAO-B have been known to regulate the redox state of glia and neuronal cells. MAO-A is mainly found in the catecholaminergic neurons and is involved in oxidation of noradrenaline and serotonin, whereas MAO-B is particularly expressed in serotonergic neurons and glial cells and oxidizes β-phenylethylamine [36]. Thirdly, an isoform of nitric oxide synthase (NOS) nNOS present in neurons is also one of the sources of ROS in brain, which is regulated by Ca2+ binding protein calmodulin. NOS catalyzes the oxidation of L-arginine to L-citruline, producing nitric oxide (NO), utilizing NADPH, tetrahydrobiopterin and O2 as a cofactor [37]. Though on one hand NO acts as a critical signaling molecule which regulates synaptic transmission, but is also capable of interfering the redox homoeostasis by interacting with superoxides to form highly reactive peroxynitrite compounds, which are directly involved in causing nitrosative stress in cells and is associated with apoptotic and necrotic cell death at low concentration and high concentrations, respectively [37].
Besides other enzymes, one of the major endogenous sources of ROS in brain during physiological conditions includes NADPH oxidases (NOX) which catalyze the oxidation of NADPH producing superoxide as its main product. Though, NOX are found to be primarily located in plasma membrane and phagosomes of polymorphonuclear neutrophils, previous immunohistology assessments of mouse and rat tissues showed its abundance in cortex and hippocampus regions of brain [38], [39]. Ca2+acts as a prime activator of NOX, leading to the post synaptic localization of the enzyme complex in neurons, thus showing the involvement of NOX in neuronal activity [40]. Seven paralogs of NOX have been reported, namely NOX (1-5), dual oxidase DUOX (1 and 2), which differ in their size and domain structure but are mainly involved in ROS generation. The proteins get activated, undergo maturation, stabilization and translocation across the membrane on interaction with the other proteins, like NOX (1–3) gets activated by interacting with p22 (phox) transmembrane protein along with G-protein Rac. Similarly, NOX-4 gets activated by interacting with only p22 (phox) transmembrane protein, whereas NOX-5 and DUOX (1 and 2) gets activated by direct binding to Ca2+. Upon activation NOX (1–3) and NOX-5 mainly catalyzes the production of superoxide and NOX-4, DUOX (1 and 2) are involved in the direct production of H2O2 [41]. NOX paralogs are found to be widely distributed in cortex, hippocampus and cerebellum in brain, most prominently NOX-2, NOX-3 and NOX-4. Previous reports also revealed the synergistic relation between mitochondrial ROS and NOX-ROS, thus supporting each other’s ROS generation [42]. To date, studies reveal a pivotal role of NOX in mediating the progression of chronic CNS diseases like AD, PD, amyotrophic lateral sclerosis (ALS) and huntington’s disease (HD) [41]. Thus, development of isoform selective NOX inhibitor can be a promising therapeutic approach for the treatment of acute and chronic CNS disorders.
Within the cytoplasm, non-heme iron enzymes such as lipoxygenases catalyze the peroxidation of arachidonic acid in the presence of molecular O2 and generate superoxide and hydroxyl radicals [43]. Many other enzymes in the cytoplasm like xanthine oxidase, cytochrome P450 monooxygenase, cyclooxygenase, D-amino oxidase are also important ROS producers [44]. In concert with mitochondria, organelles like peroxisomes are also the site of ROS production, where beta-oxidation of fatty acids catalyzed by glycolate oxidase and xanthine oxidase results in superoxide and H2O2 [27]. Also, increased ROS production occurs in endoplasmic reticulum due to unfolded protein response (UPR) [45].
Oxidative stress results in cellular damage by mediating three main reactions namely lipid peroxidation, oxidation of proteins and nucleic acid damage [46]. In fact, oxidative stress is considered as a part of the normal physiological process during aging, but have been known to be involved in chronic disorders of the brain like AD, PD, HD, ischemic stroke, depression and sclerosis [46]. Moreover, it plays a significant role in lifestyle-related metabolic disorders like Type 2 diabetes (T2D), non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, obesity, cardiovascular diseases and cancer [47].
Role of ROS in brain under physiological condition
Though ROS at higher levels is considered harmful causing biomolecular damage resulting in a wide range of cellular dysfunctions, whereas at a safe steady level ROS play a useful biological role. Under normal physiological conditions, extracellular ROS helps in mitigating infections by eliciting innate immunity responses. On the other hand, free radicals produced intracellularly helps to stimulate signaling pathways, apoptosis and defense system against oxidative stress. ROS also plays an important role in activating nuclear transcription factor NF-κB, which triggers inflammation leading to oxidative stress. Free radicals like hypochlorous acid (HOCl) are produced in lysosomes by the action of myeloperoxidases, which is used as a strong oxidative agent against pathogens. Thus, ROS act as a strong participant in signal transduction pathways regulating intracellular signaling [27], [44].
With respect to CNS, ROS produced as a byproduct in many reactions under the physiological condition not only helps in modulating intracellular signal transduction pathways but also regulates cell proliferation, differentiation, and maturation [48]. Previous reports showed that ROS production and redox balance helps in mediating neuronal differentiation from precursor neuronal progenitor cells and the axon formation [49], also, it helps in neuronal cell expansion in their niches [50]. Moreover, It has been observed that redox signaling (ROS and oxidative states) also regulates the functioning of transcription factors like (NF-κB), nuclear factor of activated T-cells and the activator protein 1 (AP-1) along with the redox state of tyrosine phosphorylated protein PKC, thus plays an important role in influencing signaling cascades involved in neurogenesis [51]. Also, ROS like H2O2 have been observed to modulate the excitability of cortical neurons by enhancing the intracellular Ca2+signaling [52]. In a similar study it was observed that H2O2 increases the phosphorylation of ERK and cAMP-response element-binding protein (CREB) in cortical neurons and PC12 cells [53], [54]. This shows that ROS plays a crucial role in influencing signaling cascades in nervous system and can act as a messenger in the signal transduction pathways.
ROS acts as a secondary messenger in various parts of the brain like hippocampus, cereberal cortex, hypothalamus, amygdala and spinal cord, thus helps in maintaining synaptic plasticity [55]. ROS was also found to be necessary for the long term potentiation (LTP) in the hippocampus, which is associated with learning and memory in mammals, thus, showing the involvement of ROS in synaptic enhancement [56], [57]. ROS also affects the pain related behavior by its involvement in increasing the excitability of the central nucleus of amygdala, a region of brain responsible for emotional aspect of pain modulation [58]. Likewise, in spinal cord, neuroplasticity processes related to neuropathic and inflammatory pain is also controlled by ROS acting as a signaling molecule [59]. Reports from animal studies showed that NOX mediated direct production of ROS act as an important physiological process which helps in maintaining synaptic plasticity mechanisms particularly in hippocampus and visual cortex [60]. Moreover, dose dependent effect of H2O2 was observed to be an obligatory process to bring the redox changes and thus regulating synaptic plasticity [61].
Why brain is vulnerable to oxidative stress?
Approximately 20% of the basal O2 is consumed by brain to support the ATP driven activity of ∼ 86 billion neurons connected by trillions of synapses, supported by 250–300 billion glia [62]. It was reported that neurons (∼1.9 million) and synapses (∼14 million) begin to perish every minute when brain is deprived of O2 [63]. Though O2 is essential for the brain functioning, but ambiguity always lies in the fact that how oxidative stress causes neurodegeneration. O2 derived free radical and non-radical species produced during redox signaling equally sustain brain health depicting its potential positive functionality [64]. It has been reported that O2.- and H2O2 produced by the action of NADPH oxidase (NOX-2) are involved in maintaining the growth of neuronal progenitor cells via PI3K/AKT pathway [49], also deletion of NOX-2 shows its importance in regulating cognitive functions of brain [65]. Recently, it is reported that NOX-2 derived H2O2 acts as an endogenous chemo-attractant supporting axonal path finding as well in its regeneration [66]. Thus, diverse reactive species are used by brain to perform signaling functions which make it more susceptible to oxidative stress. There are many other biochemical events which make the brain more vulnerable to oxidative stress that includes:
-
a)
Ca2+ transients during action potential are required to maintain the bidirectional synaptic plasticity. When high Ca2+ transients across the membrane are disrupted, the free intracellular Ca2+ concentration increases, which then activates neuronal NOS (production of NO.), phospholipase A2 and calpins damaging cytoskeleton. High NO. also binds to cytochrome C oxidase, inhibiting mitochondrial respiration. Further, NO. reacts with O2.- (from dysfunctional mitochondria) to form ONOO–. Ca2+ overloads in mitochondria abolishes the ATP generation by mediating efflux of Ca2+/ H2O2 via mitochondrial permeability transition pore (mPTP), this leads to necroptosis. Thus, disruption of Ca2+ homeostasis makes brain susceptible to oxidative stress [67], [68].
-
b)
Glutamate act as an excitotoxic amino acid for neurons and when taken in large amounts, it damages the cell by necrosis. This damage results in the release of large amount of glutamate in the extracellular environment, which also binds to receptors on adjacent neurons leading to a sustained release of Ca2+and Na+ in the cell. Reactive species like ONOO– inhibits the conversion of glutamate to glutamine by inactivating glutamine synthetase. Also, glutamate inhibits the exchange for intracellular glutamate for intracellular cysteine via Xc- transporters, resulting in depletion of GSH, which in turn leads to ferroptosis mediated death of neurons [69], [70].
-
c)
Brain is enriched with redox transition metal ions like Fe2+and Cu+ which act as cofactors for many enzymes. Stress factors causing brain damage release metal ions capable of catalyzing free radical reactions. Moreover, the iron released have a prolonged existence because cerebrospinal fluid (CSF) has little or no iron binding capacity [71], [72].
-
d)
Neurotransmitters like dopamine, serotonin and norepinephrine undergo auto-oxidation to generate ROS . Briefly, dopamine reacts with O2 to produce semiquinone and O2., which further reacts with O2 to generate quinone. Quinone so produced is re-oxidized by O2 to quinol and H2O2, where Mn2+ reacts with H2O2 to produce OH. Such auto-oxidation results in mitochondrial and lysosomal dysfunction [73], [74].
-
e)
Brain is also sensitive to oxidative stress induced by glucose. To utilize most of the glucose for pentose phosphate pathway, neurons degrade phosphofructokinase, a rate-limiting glycolytic enzyme. The absence of glycolytic rate results in protein glycation and the formation of advanced glycation end products (AGE). AGE impairs proteins and mitochondrial function by inflammation-induced oxidative stress [75], [76], [77].
-
f)
Brain possesses a high content of polyunsaturated fatty acids particularly docosahexaenoic acid (DHA), which makes it more prone to oxidative stress because of lipid peroxidation and using peroxide lipids by brain to signal. Products of lipid peroxidation like 4-Hydroxynonenal inactivates glutamate transporters by increasing Ca2+ levels, thus are neurotoxic. Lipids peroxides also inactivate alpha-ketoglutarate dehydrogenase, act as vasoconstrictive agent, damages proteasome, and is found to be a consistent factor in NDDs like AD [78], [79].
-
g)
Microglia, the resident immune cells of the brain are important for brain development and function, but during the normal activity of phagocytosis, it produces O2-. and other reactive species. Active microglia produce O2-. via NOX-2. Thus, microglia activity depends on the total O2 bioavailability and depicting damage to synapse by consuming more O2 to produce O2-.. Reactive species like H2O2 and NO. attracts microglia at the site to enforce local inflammation driving neurodegeneration [80].
-
h)
Brain is prone to disrupted redox homeostasis due to modest antioxidant defense system in comparison to other tissues. Catalase content in neurons is much lower (50 times) than that of hepatocytes. Moreover, their presence in peroxisomes restricts its activity to act on H2O2 produced in other subcellular compartments. Similarly, neurons possess very low levels of GSH, which makes them sensitive to ferroptosis and resist them to metabolize electrophiles [81], [82], [83].
-
i)
Hemoglobin is considered as neurotoxic for the brain when its reaction with excess of H2O2 results in the release of prooxidant iron ions and heme. Free heme is the strongest promoter of lipid peroxidation. Moreover, its binding with NO. leads to vasoconstriction [84], [85].
-
j)
Though brain contains low levels of cytochromes P450 enzyme, CYP2E1 that makes brain prone to oxidative stress due to leakage of electrons while catalyzing reactions. Study showed that its level might increases due to the consumption of ethanol and smoking [86].
-
k)
DNA repair enzymes like poly-ADP-ribose polymerase (PARP-1), repairs DNA damage by cleaving NAD+ and binding ADP ribose to nuclear proteins. But, overactivation of this enzyme leads to depletion of neuroprotective NAD+, restricting energy production and opening of transient receptor potential melastatin (TRPM2) Ca2+ channels that results in neuronal cell death [87].
-
l)
Being single-stranded and non-protected by histone proteins, RNA is more vulnerable to oxidation. Oxidized RNA halts the protein synthesis by ribosomes and might result in unfolded, truncated proteins if left unrepaired. It was reported that oxidized RNA along with redox-active transition metals catalyzes Fenton’s reaction. It was also reported that oxidized CuZn-SOD mRNA is a preclinical sign of ALS. However, there is a need to investigate its potent role in neurodegeneration [88].
Gut microbiota, oxidative stress and neurodegeneration
Gut-brain axis under physiological condition
Gastrointestinal (GI) tract encompasses trillions of commensal microorganisms and ∼1000 of its species which plays an important role in preserving membrane barrier functions [2]. These microorganisms are permanent residents of small intestine and colon, involving the constant flux of molecules within the host organisms, thus regulating a variety of metabolic functions [58]. The microbial community gets stabilized in the host GI tract within two years after birth, but their composition varies among individuals and can change depending upon external factors like age, health, genetics and lifestyle [89]. Luminal side of the GI tract is exposed to dietary components and gut microbiota, moreover, gut tissue is housed by 70% of immune cells and innervated by neurons which connects the gut and brain, involving constant communication between the gut and the brain [2]. Communication between the gut microbiota and brain involves the four main routes; the first important mode includes the activation of vagus nerve which connects the muscular and mucosal layer of GI tract to the brain stem. Recent reports show that enteric pathogens and probiotics regulates the host behaviors like anxiety, feeding, and depression by altering γ-Aminobutyric acid (GABA), oxytocin and brain derived neurotrophic factor (BDNF) signaling in brain via activation of vagal neurons [90], [91]. Though, one report showed that indole, a bacterial metabolite obtained from tryptophan, increases anxiety-like behavior in rats via vagus nerve activation, but specific metabolites mediating these effects still need to be identified [92]. The second route of communication influencing brain activity directly or indirectly involves the signaling through serotonin released by enterochromaffin cells (EC) present in gut lining. A study showed the increase in serotonin and serotonin precursor levels in mouse models of depression, when treated with probiotic Bifidobacterium spp., improving their depressive state [93]. Similarly, it was reported that metabolites from spore-forming bacteria (Clostridium spp.) were able to stimulate serotonin production from EC [94]. Thirdly, gut microbiota plays an essential role in development, maturation and activation of microglia. In a study, it was reported that germ free (GF) mice carries larger number of immature microglia than conventional mice, moreover when treated with Bifidobacterium spp. activates microglial cells through transcriptional activation [95]. Changes in microglia functioning have been observed in behavioral and NDDs, showing the influence of gut microbiota on NDDs mediated via microglia. Gut microbiota also affects the nervous system via systemic immune system i.e., cytokines and chemokines. Study showed that GF mice possess greater blood–brain barrier (BBB) permeability in comparison to conventional mice, thus making brain accessible to microbial products subsequently leads to neuropathological conditions [96]. Last but not the least, gut microbiota communicates by a direct transfer of chemical signals to the brain. For example, fermentation of dietary fiber by intestinal bacteria produces short chain fatty acids (SCFAs) which have been shown to regulate neuroplasticity in CNS, and was also reported to improve the depressive behavior in mice [97]. Furthermore, gut microbiota like Bacteroides, Bifidobacterium, Parabacteroides and Escherichia spp. are capable of producing neurotransmitter GABA which implicates that gut microbiota modulates the concentration of neurotransmitters in host organism [98].
Gut microbiota-mediated oxidative stress and neurodegeneration
Four main phyla of commensal bacteria colonize in human gut which includes Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. Among them, Firmicutes including Lactobacillus, Streptococcus, Mycoplasma and Clostridium along with Bacteroidetes encompass 90% of the total. Both commensal and pathogenic bacteria in gut are able to alter the cellular ROS by modulating mitochondrial activity [99]. Commensal bacteria produce formylated peptides which bind to G protein-coupled receptors (GPCRs) on macrophages and neutrophils, which triggers inflammation in epithelial cells. This process results in superoxide production by NOX-1, increasing cellular ROS [100]. Gut Lactobacilli and Bifidobacterium possess the ability to convert nitrate and nitrites into NO, making the gut epithelia a rich source of NO. similarly, Streptococcus and bacilli produce NO from L-arginine using NOS [101]. NO in nanomolar concentration is considered to be neuroprotective and is a neurotransmitter for noradrenergic, noncholinergic enteric neurons. While at a higher concentration, it results in the detrimental effect caused by the production of reactive oxygen and nitrogen species (RONS) like superoxide and H2O2, which further forms highly reactive hydroxyl radicals associating it to neuroinflammation, axonal degeneration and NDDs [18]. Beneficial metabolites like SCFAs produced by gut bacteria help to reduce ROS by influencing mitochondrial activity. This will be discussed more in detail in another section.
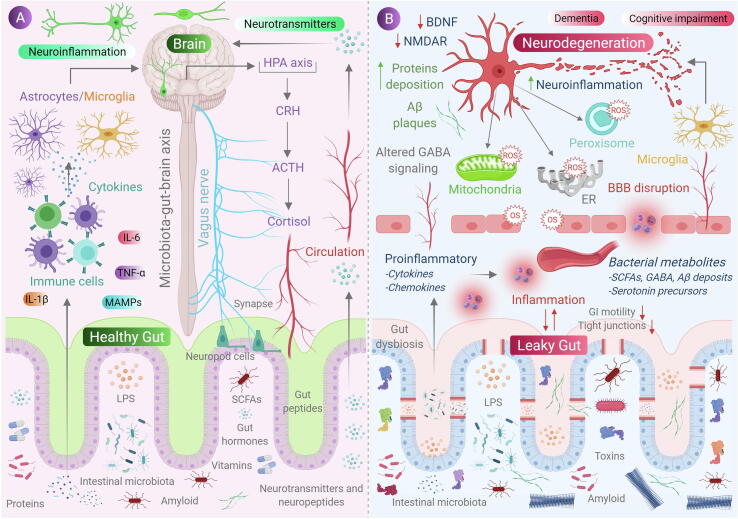
Membrane associated molecular pattern (MAMP) maintains structural integrity and basic functions of all classes of microorganisms and are detected even by brain. These are diverse chemical groups including peptides, nucleotides, carbohydrates and lipids [102]. When such molecular patterns are undetected by the host, it may escort acute to chronic inflammation and is found to alter brain development and function. These highly conserved structural motifs bind to pattern recognition receptors (PRR) present on cells of innate immune system, thus inducing mitochondrial ROS production and activation of NF-κB pathway leading to inflammatory responses, causing neuronal stress and cell death [103]. In a recent study, it was reported that bacterial cell-wall component peptidoglycan translocate to the developing brain affecting gene expression and causes a change in social behavior [104]. Similarly, lipopolysaccharide present in Gram-negative bacterial cell-wall was found to impair fetal brain development, acute depression and cognitive impairment in mice [105]. Moreover, acute and chronic exposure of MAMP was observed to be a responsible factor in developing disease symptoms in PD, autism spectrum disorder (ASD) and synucleinopathy models [106]. Apart from MAMPs, bacterial toxins produced by opportunistic pathogens also exert a negative impact on host nervous system. Lethal toxins like toxin B, enterotoxin and epsilon toxin produced by Clostridium spp. were discovered to decrease neuronal viability and also inhibit the release of neurotransmitters by reaching the brain via disrupted BBB [107]. Enterotoxins and cereulide produced by Staphylococcus spp. and Bacillus spp. were found to induce vomiting and sickness behavior by stimulating vagus nerve [108], [109]. Pathogenic bacteria like Salmonella and E. coli are able to degrade sulphur containing amino acids, thus producing hydrogen sulphide (H2S) in the gut. An increase in H2S levels imposes change in various metabolic activities like increased lactate and decreased ATP production, inhibition of cyclooxygenase 2 (COX-2) activity [110], decreased consumption of O2 by mitochondria and an increased expression of pro-inflammatory cytokines [111] and is known to stimulate hypertension and neuroinflammation [112]. Fig. 1 shows the role of gut microbiota-mediated oxidative stress in neurodegeneration.
Fig. 1.
Role of gut microbiota in neurodegeneration. (A) Communication between gut and the brain involves neural, metabolic, endocrine and immunological pathways. Gut microbial molecules like neurotransmitters, amino acids, short-chain fatty acids (SCFAs), amyloid, lipopolysaccharide (LPS) and microbe-associated molecular patterns (MAMPs) interacts with host immune system via circulation affecting metabolism and the nervous system of the host and, also it affects the brain by direct activation of the vagus nerve via enteric nervous system to the brain. Conditions like stress causes the hypothalamic neurons to secrete corticotrophic receptor harmone (CRH), triggering the release of adrenocorticotrophic releasing hormone (ACTH), subsequently activating the release of cortisol, which affects intestinal barrier integrity affecting gut health. (B) when, there occurs a condition of gut dysbiosis, there is a decrease of anti-inflammtory molecules (like SCFAs, H2) to that of pro-inflammatory molecules (LPS, amyloids) also with an alteration of beneficial bacteria to that of pathogenic in the gut. This results in increase of intestinal and blood brain barrier permeability, subsequently causing increase in peripheral immune responses, thus increasing oxidative stress in central nervous system (CNS). An increased production of reactive oxygen species (ROS) can be observed in cell organelles like mitochondria, endoplasmic reticulum (ER) and peroxisomes in neurons, along with neurotoxin aggregation, resulting in neurodegeneration.
Etiopathology of NDDs like AD and PD involves the intraneuronal protein misfolding and its aggregation. Moreover, oxidative stress is also considered as one of its pathogenic factors, but the exact underlining mechanism is still unknown. The first evidence showing the relationship of NDD pathology with gut microbiota was shown by Heiko Braak group, where they found the α-synuclein aggregation in submucosal Meissner's and Myenteric Auerbach’s plexuses in the gut of PD patients, suggesting the role of gut microbiota in initiating the α-synuclein aggregation in the gut, which is then ascended trans-synaptically to CNS neurons causing neurodegeneration [113]. Interestingly, microbiota-mediated proteinopathy and neuroinflammation is termed as “mapronosis” showing their interim relationship [114]. So far, numerous studies have shown the altered gut microbiota composition in neurological disease state in comparison to healthy individuals, but further work is needed to identify the mechanisms on how bacteria and bacterial factors influence the disease progression. Here, we describe the recent work depicting the link between gut bacteria, oxidative stress and NDDs, focusing on AD, PD and TBI.
Gut microbiota-mediated oxidative stress in Alzheimer’s disease
Alzheimer’s disease (AD) is the leading cause of dementia, affecting over 50 million population worldwide, where its prevalence is higher in older population, with about 80 per 1000 individuals above 85 years of age. Non-symptomatic pathology of AD is thought to begin approx. 20 years before the symptoms like memory loss and cognitive deficits arise [115]. Pathological changes in brain associated with AD include the extracellular accumulation of protein amyloid-beta (Aβ-amyloid plaques) and the intracellular accumulation of tau proteins (tau tangles) [116]. This abnormal accumulation of proteins leads to activation of microglia for the clearance of Aβ and tau proteins, but with subsequent aging, chronic inflammation occurs causing neuronal cell death, leading to atrophy [117]. Despite of rare genetic mutation causing Aβ accumulation 20 years before onset, followed by decreased glucose consumption by brain 18 years before and atrophy began 13 years before the development of disease [118], many cross-sectional studies have documented modifiable risk factors as an etiopathology of AD [119]. Among the possible risk factors, the role of oxidative stress and gut microbiota has attracted the scientific community and is considered as an immediate plausible consequence of neurodegenerative processes.
In addition to high energy demands of brain, the link between oxidative stress and AD is reflected in many studies which showed the alteration in antioxidant defense system of the brain i.e. changes in activity and levels of SOD and catalase enzyme [120]. Likewise, oxidative stress biomarkers like malondialdehyde, 4-hydroxynonenal, and F2-isoprostane (lipid oxidative damage); protein carbonyls and 3-nitrotyrosine (products of protein oxidation), 8-hydroxydeoxyguanosine (nucleic acid oxidation) were found at high levels in blood and CSF [120], also their concentration was found proportional to that of cognitive impairment and brain weight [121]. ROS production in AD brain is also characterized by dysfunction in cellular organelles like mitochondria (deficiency of cytochrome C oxidase) [122], endoplasmic reticulum due to UPR [123], accumulation of metal ions in neuretic plaques [124] and due to the hyperactivation of microglia followed by the overexpression of NADPH oxidase [125]. There is also an inter-relationship between Aβ deposition and oxidative stress i.e. Aβ aggregation induces oxidative stress (also in organelles like mitochondria, ER and golgi apparatus) and oxidative stress induces Aβ accumulation [126]. Even, aggregation of tau proteins in neurons leads to decreased NADH-ubiquinone reductase activity resulting in increased ROS production and mitochondrial dysfunction [127].
Recent facts and figures show that AD is not only a result of confined brain inflammation but is also a consequence of peripheral inflammation [128]. This is supported by the fact that gut dysbiosis leads to inflammation which increases with age, disruption of BBB, activation of immune system followed by neurodegeneration, on the other hand, healthy and well-balanced gut helps to decrease detrimental effects produced by ROS [19]. Individuals suffering from AD have been identified with decreased population of commensal bacteria such as Bifidobacterium spp. and Firmicutes, and an increased abundance of Escherichia, Shigella spp. and Bacteriodetes, followed by increased inflammation and Aβ accumulation [129]. similarly, decline in Aβ plaque formation was seen in APP/PS1 mice model when treated with the combination of broad-spectrum antibiotics [130]. Likewise, 5xFAD mouse model of AD showed shifts in microbiota population towards proinflammatory species along with the changes in amino acid catabolism, and conversely, treatment with antibiotics reversed the effects, suggesting the possible link between severity of disease and transformed gut population [131]. Microbial amyloid protein formed in the gut activates the Toll-like receptors (TLR) and cluster of differentiation 14 (CD14) facilitated immune response leading to overlooked misfolded Aβ with impaired Aβ clearance, subsequently increasing cytokine production resulting in disrupted intestinal and BBB [114]. Also, it was shown that levels of enteric hormones decrease in AD patients, on the contrary, gut microbiota metabolites like H2S and trimethylamine increase, enhancing its rigor [132].
Age-related decrease of gut microbial biodiversity is also considered as one cause of dementia. It has been shown that with growing age, there is a decrease in Bifidobacterium spp. and an increase in Proteobacteria, where dementia results not because of decrease in SCFA’s but due to interference in lipid metabolism. Bifidobacterium plays an important role in regulating cholesterol levels directly facilitating its fecal elimination and indirectly by increasing the serum leptin levels, thus involved in maintaining hippocampal plasticity and memory functions [133], [134]. Gut bacteria like Lactobacilli and Bifidobacterium metabolize an inhibitory neurotransmitter GABA [135]. In a study, it was observed that synaptic plasticity in hippocampus was altered in APPSwe/PSEN1DeltaE9 bigenic mouse model of AD, where reduced GABA production was found with a concomitant increase in glutamatergic neurotransmission [136], [137]. Although, reports show that phylum cynobacteria produces neurotoxins causing cognitive impairment, but no relation has been observed with AD [138].
Another possible connecting link, cerebral amyloid accumulation and gut microbiota involve the mechanism of cross seeding of microbial amyloids in a manner similar to prion propagation, thus different amyloid conformers formed induces toxicity at distinct levels in their cellular targets, hypothesizing the existence of AD phenotypes [139]. In addition to gut microbiota, a link between commensal bacteria in oral cavity and AD was also studied. Interestingly, poor oral hygiene and teeth loss was found to cause an increased risk of early onset of AD [140].
Gut microbiota-mediated oxidative stress in Parkinson’s disease
Parkinson’s disease (PD) is pathologically characterized by progressive degeneration of dopaminergic neurons, aggregation of phosphorylated protein α-synuclein, excessive ROS production, mitochondrial dysfunction and microglia activation [141]. Symptomatically, it is characterized by inability of the patients to control voluntary movements (tremors, muscle rigidity, difficulty walking and hunched posture) due to damage in substantia nigra and striatum regions of the brain. It is the second most common NDD affecting more than 1% of elderly population worldwide [142]. The first report on relationship between gut and PD was mentioned in an essay on shaking palsy by James Parkinson in 1817 [143]. Braak’s hypothesis supported this view showing the initiation of pathology in the gut, then affecting the brain. Accumulating evidences show that gut inflammation, early accumulation of phosphorylated α-synuclein transcending to the dorsal motor neuron of the vagus nerve, constipation problems and increased intestinal permeability are common in PD patients, suggesting a strong relationship between gut microbiota and PD pathogenesis [144]. Moreover, it was observed that risk of developing PD decreases in those individuals who have gone for vagotomy [145]. In addition, lower levels of GSH, and higher level of iron and H2O2 makes substantia nigra pars compacta (SNc) neurons susceptible to oxidative stress [146]. Also, lipid peroxidation and dopamine oxidation in this region leads to neuronal cell death. Studies also show that mitochondrial respiratory chain dysfunction leads to excessive ROS production. It is supported by the fact that inhibitors of complex 1 induces cytotoxic effects on dopamine neurons [147], also patients with pathology of α-synuclein, phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and Parkin were detected to have mitochondrial dysfunction with increased oxidative stress [148]. Similar to AD, aggregation of abnormal protein α-synuclein is interrelated to increased oxidative stress and vice versa. Coming to the role of gut microbiota, some pathogenic spp. release toxins causing mitochondrial dysfunction in cells of the gut and ENS resulting in neurodegeneration [149]. The pathogenic bacteria increase in gut of PD patients and their microbial products are directly involved in PD pathogenesis. In support of this fact, recently it was reported that pathogenic bacteria Escherichia coli produces amyloid protein known as curli, which promotes α-synuclein aggregation in gut and brain and was observed to cause motor deficits in mice [150], On the other hand, when mice were treated with gut restricted amyloid inhibitor, it has shown the improvements in motor functions along with amelioration of constipation [151], showing the involvement of gut in etiology of PD symptoms, supporting Braak’s hypothesis.
Gut inflammation caused by gut bacteria can be directly correlated with progressive neurodegeneration in PD. Though serum metabolic profile and gut composition are found altered in PD patients, it was observed that in severe PD condition, Enterobacteriaceae level enhances in gut with very low level of anti-inflammatory bacteria, also showing its parallel association with gut inflammation in Crohn’s disease [152]. This shows that patients with Crohn’s disease possess very high risk of developing PD. Similarly, it was observed that Citrobacter rodentium infection in mouse model (PTEN induced Kinase 1 (PINK1) knockout mice), aggravated PD symptoms by inducing gut inflammation [153]. In addition to induce inflammation, gut microbiota also exerts metabolic effects, like its metabolites β-glucoronate, tryptophan and SCFA’s were found to be altered in case of PD patients [154]. Recently, one striking attribute of gut microbiota was also reported showing that it acquires the ability to reduce the efficacy of anti-PD drugs either by decreasing its bioavailability or by increasing drug inactivation, as observed in the case of standard levodopa treatment [155], [156]. Inclusive of these studies, decreased production of H2 by gut bacteria is also found to be one of the contributing factors in PD [157]. A recent report showed that when 50% H2 saturated water was given to rat and MPTP mouse model of PD, it was found to be successful in reducing neuronal loss in substantia nigra and also the oxidative stress markers and when double blind randomized trial was performed in humans, an improvement in motor ratings of PD patients was observed [157], [158]. Further, towards this notion, recent work on GF mouse model of PD with overexpressed human α-synuclein gene showed the reduced level of SCFAs along with the decreased microglia activation and improved motor functions which suggests the direct involvement of gut microbiota in enhancing PD. In addition to it, when gut microbiota from PD patients was transplanted into GF α-synuclein overexpressed mouse model, worsened the motor symptoms, suggesting the role of dysfunctional gut microbiota in PD patients [151]. similarly, mouse model treated with neurotoxin showed altered gut microbiota composition with increased levels of pathogenic Enterobacteriaceae [159]. Furthermore, increased Fermicutes to Bacteroides ratio (condition also in gut inflammation) was observed in mice treated with pesticide rotenone [151]. Strikingly, some specific bacterial species like Proteus mirabilis were found to bolster neurodegeneration in mice [160]. Taken together, these studies suggest that gut microbiota exacerbates neuronal dysfunction and neuroinflammation in both human and animal models of PD.
Gut microbiome and traumatic brain injury (TBI)
Traumatic Brain Injury (TBI) is one of the most prevalent injury types sustained in the world population with an annual incidence of ∼1.4 million cases in the United States, and is among the major cause of death and disability [161]. The disabilities which occur in TBI not only includes a primary mechanical damage of brain but involves post injury secondary damages, which takes place at the cellular and molecular level, and may lead to metabolic anomalies like mitochondrial dysfunction, oxidative stress, inflammation, microglia activation, excitotoxicity, resulting in temporary or lifelong cognitive impairments [162]. Moreover, severity of TBI is not only focused on brain but can be a multiorgan damage and is considered as a heterogenous pathobiological condition. Due to the heterogenous nature of brain injury, therapies for TBI-induced neuropathologies are still lacking, pertaining to the consideration of novel therapeutic regimens. Towards this approach gut eubiotic therapeutics have gained much attention, because of its capability to restore the bifacial relationship between gut dysbiosis and TBI [163], [164].
Growing line of evidences reveal that there exists a bi-directional relationship between a gut microbiome and TBI injury. One of the systemic manifestations of TBI has been observed as a disruption of intestinal motility and permeability, mucosal damage, histopathological alterations of intestinal villi, thus indicating the perturbance in the composition of gut microbiome [165], [166], [167]. Conversely, it has been observed that gut dysbiosis also influences the pathophysiology of traumatic CNS injury, alterations in BBB permeability and activation of microglia, leading to severe repercussions [168], [169], [170]. Recent report showed that a mice exposed to mild and repetitive brain injury for 20 days show a progressive emergence of white matter damage, cognitive deterioration and a mild, transient gut dysbiosis [171]. Similarly, it was observed that depletion of gut microbiota in murine model of TBI prior to and following brain injury resulted in an increased CA1 hippocampal neuronal density, attenuated associative learning deficit and had reduced lesion volume [172]. Recent study also claims that gut dysbiosis takes place after traumatic spinal cord injury which resulted in intraspinal inflammation and lesion pathology [173]. Also, changes in two major order of bacteria in gut Bacteroidales and Clostridiales was observed i.e ∼30% decrease in phylum Bacteroidetes and ∼25% increase in phylum Firmicutes. This was accompanied by the consistent changes in minor taxa including Anaeroplasmatales, Turicibacterales and Lactobacillales. Such changes were found to be persistent and lasted for about 4 weeks post injury [174]. A similar modulation in gut microbiome population, but an inverse relation of decrease in Firmicutes and an increase in Bacteroidetes was found to occur 2 h following injury and being persistent for about 7 days in rodent model of moderate TBI [175]. Likewise, recent report showed that their occurs a rapid significant decrease in three species Lactobacillus gasseri, Ruminococcus flavefaciens, and Eubacterium ventriosum and an significant increase in Eubacterium sulci, and Marvinbryantia formatexigens in human gut microbiome just in 24 h after TBI in mice [176]. Furthermore, a decrease in Bacteroidales, Fusobacteriales, and Verrucomicrobiales, along with an increase in Clostridiales and Enterococcus within 72 h were observed in severely injured patients with polytrauma [177]. Gut microbiota also plays a very important role in recovery from TBI, as observed in a recent report where gut dysbiosis induced by a broad spectrum antibiotic before, during and after TBI resulted in an increased neuronal loss, suppressed neurogenesis as well as altered the microglia and peripheral immune response along with the modulation in fear memory response [178]. Thus, the influence of gut microbiota on TBI patients is of paramount clinical significance because TBI patients become susceptible to alterations in gut microbiota due to regular antibiotic administration and prolonged hospitalization. Furthermore, detection of gut microbiota modulation might provide a diagnostic tool for the identification of TBI severity, thus providing targeted therapeutic approaches.
CNS antioxidants and neuroprotection
CNS is highly vulnerable to oxidative stress and leads the neurological disorders. High level of ROS is generated during the functioning of CNS due to high O2 demand and rush of peroxidation-susceptible lipid cells. This oxidative metabolism generates reactive species for transmitting redox signals to regulate critical functions such as synaptic plasticity [29], [179]. Antioxidants whether enzymatic or non-enzymatic, endogenous, or exogenous, protects the brain against the oxidative stress by preventing the generation of ROS or by scavenging the free radicals or inactivate the free radical product. The first line of antioxidative defence mechanism involves the use of endogenous enzymes like SOD, glutathione peroxidase (GPx), glutathione reductase, and catalase [180]. Whereas, second line of antioxidative defence involves the use of endogenous non-enzymatic molecules like thioredoxin, ferritin, transferrin, ceruloplasmin, albumin, and metallothionein. Also, enzyme co-factors, i.e., coenzyme Q and alpha-lipoic acid, and metabolites, i.e., bilirubin, melatonin, and uric acid plays an important role in antioxidative defence mechanism [181], [182]. Similarly, natural dietary compounds like vitamins A, E, and C, flavonoids, phenolic acids, and carotenoids are also known to possess a strong possible antioxidative defence against oxidative stress-induced neurodegeneration [182].
The antioxidant enzymes such as SOD decreases the concentration of superoxide anion by catalyzing the dismutation of superoxide to O2 and H2O2; GPx reduce H2O2 and lipid peroxides; thiol-specific peroxidases such as peroxiredoxins reduce the amount of hydroxyperoxides, and catalase transforms H2O2 to H2O and ordinary molecular O2 [29], [183]. The free radicals activate the transcription of genes involved in antioxidant pathways and protect the cells from adverse effects. Glutathione is present in low amount in the CNS which reacts with free radicals in its reduced form to convert the H2O2 to H2O by self-catalyzing to GS–SG and regenerates again to glutathione by a reductase [183]. Insufficiency of glutathione may limit the activity of peroxidases that could make the neurons more susceptible to oxidative stress. In-vivo and in-vitro studies performed till date showed that the SOD and catalase improves the neuronal survival following the Aβ-induced neuronal toxicity [184], [185], [186], [187]. Likewise, both thioredoxin and thioredoxin reductase are widely expressed in brain and are known to exert neuroprotective effect against oxidative stress models of HD and AD [188], [189].
Another very important pathway is Kelch-like ECH-Associating protein 1 nuclear factor erythroid 2 related factor 2-antioxidant response element (Keap1-Nrf2-ARE). Keap1-Nrf2-ARE is highly expressed in neurons and linked with the defense mechanisms against oxidative stress associated with NDDs. It modulates the activity of SOD, thioredoxin, peroxiredoxins, and GPx via transcriptional regulation [190]. The importance of NF-κB is also reported as a redox-sensor in CNS that is activated by ROS. Moderate level of ROS phosphorylates NF-κB inhibitor that results in NF-κB activation. Activated NF-κB regulates the expression of anti-apoptotic and inhibits caspase-dependent cell death pathways. However, high levels of ROS inhibit the binding of NF-κB via inactivating it. This mechanism promotes the apoptosis of cells and halts the pro-survival pathways [29]. The regulation of antioxidant metabolism of the CNS is tightly controlled where the role of gut microbes is highly dynamic.
Gut microbiota in neuroprotection
Gut microbe-microbe and microbe-host interactions regulate the level of exogenous and endogenous ROS via various metabolites production such as absorbable vitamins, polyphenols, SCFAs, BDNF, diffusible antioxidant and oxidant gases, etc. Gut microbes also control the permeability of metabolites to BBB, tight junction integrity and intestinal barrier, modulate the immune system and impede the intestinal colonization of pathogens [191]. The vagus nerve of the parasympathetic nervous system senses the gut metabolites and communicates to CNS about the gut information to generate specific responses. Under the stress condition, the vagal tone is repressed and shows detrimental effects such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) due to dysbiosis [192]. Aβ proteins involved in the pathogenesis of AD are expressed by gut bacteria such as Escherichia coli and Salmonella enterica in ENS [183]. Beneficial gut microbes also produce dopamine, serotonin, and GABA. These are the CNS neurotransmitters which modulate the ENS activity and may correlate [179]. In some studies, it was revealed that gut microbes manage the activation and maturation of microglia, and activated microglia releases significant amounts of inducible nitric oxide synthase (iNOS) to regulate NO production. Dysbiosis provokes the inflammatory iNOS and causes neuroinflammation [183].
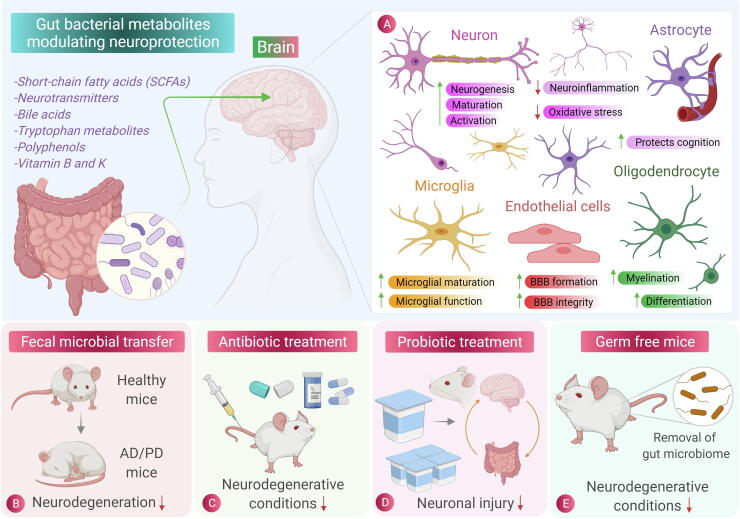
As we have seen the potential role of gut microbiota as well as essential role of oxidative stress in mediating neuronal disorders, in recent years, a growing need and a major scientific focus is on developing antioxidant-based therapies for treating oxidative stress-induced NDDs. Antioxidants are chemical or natural substances capable of counteracting oxidative stress induced by ROS/RNS [193]. Though a potent therapeutic effect of antioxidants has been observed on diseases like diabetes, arthritis, cataract and osteoporosis, antioxidant therapies used for CNS disorders are limited and still requires deep mechanistic understanding [194]. Contrary to the antioxidant therapies, dichotomous role of gut microbiome is observed, which one hand is responsible for the basic underlying mechanism of neurodegeneration (gut dysbiosis and neuroinflammation) and on the other hand, gut microbiome and its metabolites regulate many associated pathways suggesting their neuroprotective therapeutic role. Microbial molecules like protein, vitamins are produced via multistep biosynthetic pathway, which may exert beneficial or detrimental effects on the host system [16]. Therefore, a healthy gut microbiome maintained with a proper diet including prebiotics and probiotics is a prerequisite for maintaining neuronal health [195]. Fig. 2 shows the role of gut microbiota metabolites along with the modulation procedures on gut microbiota and their effect on NDDs. Here, we review how bacterial metabolites (their natural, transformed, and dietary metabolites) help to combat oxidative stress-induced neuronal damage. Moreover, we will further discuss about the role of antioxidative and anti-inflammatory probiotics/prebiotics in neuroprotection. Table 1 shows the recent studies on gut microbiota showcasing its neuroprotective role.
Fig. 2.
Role of gut microbiota in neuroprotection. (A) Scheme of brain cell specific effects in response to beneficial metabolites released by gut microbiota in reducing inflammation and oxidative stress. (B) Fecal microbial transfer (FMT) which involves the transfer of fecal bacteria from healthy individual to one having pathological condition and was found to be an effective procedure in reducing the pathophysiology of neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and conditions like Multiple sclerosis (MS). (C) and (D) Antibiotic treatment and Probiotic treatment, respectively, which have shown the considerable effectiveness in decreasing the pathogenesis of neurodegenerative diseases. (E) The germ-free mice (mice free from gut microbiota), showed the decrease in neurodegenerative conditions and also used to study the effect of gut microbiota on brain physiology, thus showing the involvement of gut microbiota in neurodegeneration.
Table 1.
Summary of various reported studies on the role of gut microbiota in neuroprotection.
| Microbiota | Neurodegenerative disease | Model | Study outcome | Reference(s) |
|---|---|---|---|---|
| Lactobacillus buchneri KU200793 | PD/AD | SH-SY5Y cells | Treatment with heat killed strain reduced Bax/Bcl-2 ratio and increased BDNF expression | [288] |
| Lactobacillus delbrueckii ssp. bulgaricus B3 and Lactobacillus plantarum | AD | SH-SY5Y cells | Protected cells from Aβ-induced cytotoxicity | [22] |
| Lactococcus lactis p62(SQSTM1)-engineered | AD | 3xTg-AD mice | Diminished oxidative stress and inflammation, reduced levels of amyloid peptides and improved memory | [289] |
| Lacticaseibacillus rhamnosus HA-114 | ALS/HD | Caenorhabditis elegans and mouse model of ALS | Restores lipid homeostasis and energy balance through mitochondrial β oxidation | [290] |
| Clostridium butyricum | AD | APP/PS1 mouse model of AD | Prevents Aβ deposition, microglia activation, production of TNF-α and IL-1β | [291] |
|
Lactobacillus fermentum NCIMB 5221 |
AD | APPswe and PS11E9 mutant transgenic mice |
Ferulic acid produced by bacretia reduces oxidative stress, Aβ fibrillation and improves memory | [292] |
|
Lactobacillus plantarum WCFS1, E.coli Nissle and Bifidobacterium infantis spp. |
AD/PD | NA | Proficient in producing butyrate, propionate and acetate | [293] |
|
Lactobacillus plantarum MTCC1325 |
AD | D-galactose- induced AD mice model | ATPase enzyme levels and Na+ and K+ ATPase activity was restored required for potential neural activity | [294], [295] |
|
SLAB51 (Streptococcus thermophilus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus delbrueckii subsp. bulgaricus, L. brevis) |
AD | 3xTg-AD mice | Inhibits Aβ deposition, decreased acylation of p53 protein along with increase in SIRT1 deacetylase activity and ADAM10-α secretase activity | [274] |
| Rumnicoccus albus | NA | Oxidative stress induced Sprague Dawley rats and SH-SY5Y cells | Reduced ROS levels and increased SOD and GSH levels in oxidative stress condition | [23] |
| Lactobacillus acidophilus and Bifidobacterium infantis | PD | Human PD patients | Reduced symptoms of abdominal pain and bloating | [296] |
| Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum | PD | Human PD patients | Decreased movement disorder society-unified Parkinson’s disease rating scale scores | [297] |
| Lactobacillus casei Shirota | PD | Human PD patients | Improved abdominal symptoms | [298] |
|
Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum |
AD | Human AD patients | Decreased C- reactive protein (CRP) levels and insulin resistance and neuronal cell death | [299] |
| Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, and Lactobacillus fermentum | PD | 6-OHDA treated male Wister rats | Improved rotational behavior, cognitive function, lipid peroxidation, and neuronal damage | [300] |
| Lactobacillus acidophilus, Bifidobacterium bifidum and Bifidobacterium longum | AD | Animal model of AD | Improved cognitive performance and restored synaptic plasticity | [301] |
| L. casei LC122 and B. longum BL986 | Age related neurodegeneration | C57BL/6 mice | Attenuated oxidative stress, improved gut barrier function and inhibited hepatic lipid accumulation | [302] |
| Lactobacillus johnsonii | AD | Germ free mice | Decreased kynurenine and increased serotonin levels | [303] |
Gut microbiota metabolites in neuroprotection with their cell-specific responses
Gut microbiota interaction with host molecules
Bile acids
Bile acids are produced in liver and are released in the intestinal lumen, primarily involved in the solubilization of lipids and fat-soluble vitamins, signaling in energy metabolism and is also known to play an important role in physiology and pathophysiology of the brain [196]. Bile acid influences neuronal activity in different brain regions and also vagal neuronal activity either by directly binding the receptors in brain crossing BBB, or by indirectly inducing the release of fibroblast growth factor (FGF) and glucagon-like peptide 1 by binding to gut receptors [197]. Bile acids like ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) possess neuroprotective properties and lack of cytotoxicity as proved in their phase III clinical trials [198] and in an animal study [199], respectively. Recent data shows that TUDCA helps to attenuate autophagy, α- synuclein aggregation and protein oxidation in chronic mouse model of PD [200]. Moreover, it helps to prevent neuronal apoptosis in rats affected by subarachnoid hemorrhage via Takeda G protein-coupled receptor 5/sirtuin-3 (TGR5/SIRT-3) pathway [201]. Likewise, UDCA showed a neuroprotective effect in charged multivesicular body protein 2B (CHMP2B) Intron 5 models of frontotemporal dementia [202]. Gut microbiota in the intestinal lumen converts primary bile acids (cholic acid and chenodeoxycholic acid) into secondary bile acids by the action of dehydratases, involves deconjugation of amino acids with bile salt hydrolases and other enzymatic processes, which alters their nuclear receptor binding, solubility and circulation [203]. Changes in secondary bile acid levels have been found in human and mouse model of AD [204], PD [205], ASD [206] and multiple sclerosis [207]. Also, bacteria-modified bile acids were found to be neuroprotective in case of ALS and stroke [208]. Modulation of gut microbiota community can cause changes in the level and properties of bile acids, which might be neurodegenerative or neuroprotective. It has also been reported that gut microbiota-mediated increase in deoxycholic acids induces the release of neurotransmitter serotonin in EC in mice [94]. Bile acid metabolites were found to improve demyelination [209] and reducing oxidative stress [210], potentiating its neuroprotective role via acting on oligodendrocytes and microglia, respectively. Still, the potential role and effects of microbiota manipulated bile acid is unknown and remains to be defined clearly.
Steroid hormones
Signaling by steroid hormones is crucial for brain development and functions (memory, decision making and sexual behavior) [211]. While circulating throughout the body, steroid hormone encounters microbiota in intestinal lumen [212]. Gut bacteria modify steroid hormone in a deconjugation reaction mediated by the action of β-glucuronidases and β-glucosidases, which reactivates the hormone and prevent it from excretion. Thus, gut microbiome influences the level of active and inactive steroid hormones via degradation and activation pathways [213]. Androgens and oestrogens are found to be influenced by gut microbiome. A large number of enteric bacteria were found to metabolize oestrogens [214] and oestrogens also undergo oxidation–reduction reactions in faecal samples [215], suggesting the role of gut microbiota. It has been observed that gut microbiota also possesses the ability to convert testosterone [216] and cholesterol to androgens [217]. Microbial influenced oestrogens were found to be neuroprotective, showing anti-inflammatory effects on microglia [218]. Also, altered gut microbial community was observed to result in low levels of oestrogen, which resulted in chronic inflammation[219] and cognitive impairment [220]. Oestrogenic molecules also affects differentiation and myelination in oligodendrocytes [221]. Recent report also showed that even progesterone treatment in MPTP parkinsonian mouse model showed neuroprotective, anti-inflammatory and immune-modulatory effects, but whether the neuroprotective role begins in gut or brain is still unknown [222].
Gut microbiota interaction with dietary molecules
Amino acids
Dietary amino acids can also be metabolized by gut microorganisms and their effect on the brain varies depending upon the type and frequency of diet intake [223]. Although, gut microbiota encoded amino acids circulate in the host, but those affecting CNS are dietary amino acids metabolized by gut bacteria [224]. Aromatic amino acids like tyrosine, tryptophan and phenylalanine are metabolized by gut bacteria into SCFAs, indole derivatives, neurotransmitters, organic acids, amines and ammonia [225]. The end product of tyrosine metabolism is the formation of two catecholamines, dopamine and noradrenaline from tyramine intermediate. In vitro studies showed that large number of gut bacteria can produce noradrenaline in millimolar range [226]. Recent report showed that non-adrenaline increases the supply of glutathione from astrocytes by stimulating B-3 adrenoceptor, thus protecting neurons from H2O2- induced neuronal death [227]. It is found that tyrosine is also metabolized by gut microbiota in phenols like 4-ethyl phenol, which subsequently get sulphated in host to 4-ethyl phenol sulphate, which is found elevated in a mouse model of ASD, and also a urinary biomarker for children with ASD [228]. Indole derivatives like tryptamine and kynurenine are the products of tryptophan metabolism by gut bacteria, which are neuroactive molecules [92]. Indole propanoic acid, an indole derivative act as an antioxidant reducing neuroinflammation, and is observed to have a potential role in decreasing AD pathology [229]. Kynurenine metabolites were found to affect anxiety, memory and stress-like behavior [230]. It has also been observed that disturbances in kynurenine metabolic pathway promote inflammation, excitotoxic glutamate production, and free radical attack, suggesting the neuroprotective role of balanced kynurenine and its anti-inflammatory role in AD, PD and HD [231]. Tryptophan metabolites help to decrease the inflammatory responses of astrocytes by modulating its aryl hydrocarbon receptor and also affects their interaction with microglia. Likewise, it has also been observed that indoxyl-3-sulphate controls activation of microglia and subsequently its interaction with astrocytes [232]. Amino acid glutamate is also converted by gut bacterial glutamate decarboxylases to form GABA, an inhibitory neurotransmitter, which is observed to reduce depression and anxiety symptoms in mouse model [90]. In case of amino acid arginine, it is metabolized into four polyamines agmatine, putrescine, spermidine and spermine, which act via glutamate receptors and are involved in maintaining synaptic plasticity and memory formation [233]. Agmatine shows the therapeutic effects in case of CNS disorders while acting as a ligand for α-2 adregenic and imidazole receptors in brain [234]. Spermidine also showed its neuroprotective effect in 3-nitropropionic acid 3-NP model of HD [235]. Moreover, agmatine was found to stimulate Nrf-2 signaling pathway, ameliorating ROS production induced by lipopolysaccharide (LPS) [236]. In vitro and in vivo studies also revealed that agmatine protects astrocytes and microglia from damage induced by oxidative stress [237]. These studies suggest the potential role of gut microbial endocrinology in neuroscience.
Dietary fibers
Undigested dietary fibers like complex carbohydrate polysaccharides are acted upon by intestinal microbial enzymes glycoside hydrolases and polysaccharide lyases and are converted to SCFAs via anaerobic fermentation [238]. Butyrate, acetate, and propionate comprise SCFAs, which act as a source of energy for colonic epithelial cells. Besides this, it also enters systemic circulation, subsequently affecting physiological functions of many organs including neural development and functions directly or indirectly [238]. Reports reveal that when gut microbiota of AD mouse model was modulated by treating with a mixture of probiotics or by using anti-inflammatory SCFAs, it helps to counteract the progression of the disease [239]. Similarly, SCFAs were found effective to exacerbate motor symptoms in GF mouse model of PD [240]. SCFA, acetate crosses the BBB and activates the neurons and modulates the level of neurotransmitters and neurotrophic factors [241]. In a study, it was shown that propionate and butyrate influence the intracellular potassium level in neurons [242]. Butyrate acts as a potent inhibitor of enzyme which regulates epigenetic gene activation (histone deacetylase) and is found to act as a potent anti-inflammatory agent in mouse model of AD, PD, HD, stroke and memory impairment [238]. Interestingly, SCFAs interfere in the interaction between Aβ peptides to form neurotoxic oligomers, thus preventing AD pathology [243]. It was also reported that fecal microbiota transplantation from wild type mice to the animal model of PD along with the butyrate administration remarkably improved the motor symptoms and dopamine deficiency [244]. When we look at the cell-specific responses, butyrate has been shown to reduce neuroinflammation and oxidation in astrocytes in vitro, and acetate is used as a source of energy by these cells [245]. In a study, it was observed that SCFAs increases the expression of tight junction proteins by binding to the SCFA receptors on endothelial cells, thus helps in decreasing the permeability of BBB and preventing the LPS-induced seizures and stroke [246]. SCFAs reduce oxidative stress in the brain by decreasing microglial activation [247], thus combating neuroinflammation in AD and PD. Taken together, SCFAs obtained from dietary fibers helps to improve brain health, depending upon the gut health of the individual.
Polyphenols
Polyphenols are bioactive molecules present in plants that plays a fundamental role in growth, protection and reproduction in plants [248]. Polyphenols are classified as flavonoids, phenolic acids and tannins. The molecular structure of polyphenols i.e., positions of hydroxyl group and nature of the substitution of aromatic rings affords them the ability to scavenge free radicals [248] and are widely studied as an antioxidative therapy for the treatment of NDDs [249]. Unabsorbed polyphenol is converted into bioavailable and bioactive metabolites [250] in the host by the action of gut microbiota via hydrolysis and esterification, which is further followed by modifications like methylation, sulfation, hydroxylation before they reach the peripheral tissues [251]. Studies on different polyphenols like resveratrol obtained from grapes and wines, curcumin and epigallocatechin-3-gallate from green tea exerts neuroprotective effects by activating protein kinase pathways like Keap1/Nrf-2/ARE, which are major pathways in alleviating both endogenous and exogenous ROS. It has also been reported that bacterial polyphenolic metabolites such as 3-hydroxybenzoic acid and 3-(3′-hydroxyphenyl)propionic acid inhibits amyloid aggregation, thus facilitates to inhibit the progression of AD [252]. Similarly, it has been observed that a flavonoid quercetin act as BACE-1 inhibitor [253]. Moreover, natural flavonoid proanthocyanidins mitigate rotenone-induced oxidative stress in dopaminergic neurons in vitro [254]. Also, polyphenols like ferulic acid produced by gut microbiota promote neurogenesis and have shown the neuroprotective role in mouse models of AD [255] and cerebral ischemia [256]. Equol and Enterolactone are also derivatives produced by gut bacteria by metabolizing phytoestrogen, one of the polyphenols [257], which might have an effect on classical neuroprotective pathway mediated by oestrogen receptors. Interestingly, polyphenols can also modulate the composition of gut microbiota [258], which further convert them into anti-inflammatory and neuroprotective metabolites.
Vitamin B and vitamin K
Gut microbiota also acts as an important source of vitamins particularly vitamin B and K, which are not only essential for the gut microbial metabolism but also show it effects on physiological pathways in the host [259]. Gut bacteria like Escherichia coli, Klebsiella pneumoniae, Propionibacterium, and Eubacterium produces vitamin K, B2 (riboflavin) is produced by Bacillus subtilis and E. coli; B9 (folic acid) by Bifidobacterium, Lactococcus lactis and Streptococcus thermophilus; and B12 (cobalamin) is produced by Lactobacillus reuteri and Propionibacterium freudenreichi [260]. Though dietary vitamins are absorbed through small intestine, uptake of microbiota-derived vitamins occurs in the colon. To prevent hemorrhagic disease in neonates, prior to the establishment of gut microbiota, vitamin K is administered as an essential agent for the process of thrombosis [261]. Moreover, vitamin B and K are found to be important for brain development and function [262]. In the case of NDDs, many studies have shown the effective role of vitamin B and K in improving neuronal health. Vitamin K deficiency was found to be correlated with the pathogenesis of AD, similarly, increased uptake of dietary vitamin K has helped to improve the memory functions in elderly patients. Recent report shows that vitamin K2 (menaquione-4) possesses potent antioxidative properties, and was found to significantly inhibit rotenone-induced p38 activation, ROS production and caspase-1 activity and subsequently, restored mitochondrial membrane potential, showing its potential in the treatment of neuroinflammation-induced PD [263]. Similarly, vitamin K2 was found effective to modulate bax and caspase-3 activation in PC12 mouse neuroblastoma cells, protecting it from 6-OHDA induced apoptosis [264]. Notably, one recent report has shown that low levels of vitamin K2 in PD patients are associated with dysregulated inflammatory responses and cascade coagulation signals [265]. Deficiency of vitamin B was also found to be associated with neurological disorders like beriberi, and polyneuropathy [266]. Similarly, folate deficiency was found to be associated with cognitive impairment and progressive dementia in older women [267]. Also, high intake of B6, B9 and B12 decreases the rate of atrophy in the brain region linked with the cognitive decline in AD [268]. Further, more constructive studies will be required to prove the potential link of gut microbiota produced vitamins in neuroprotection.
Antioxidative and anti-inflammatory effects of probiotics
Free radicals in the form of ROS are byproducts of normal cellular metabolic processes. Free radicals are potentially able to damage the genetic material, enzymes inactivation, depolymerization of complex carbohydrates and lipid peroxidation. This vandalization of intracellular molecules leads to the cell death. To balance the free radicals, the body have specific antioxidants such as glutathione [269]. Some products also started to flow in pharmaceutical markets to tackle oxidative stress such as ferulic acid (FA) due to its antioxidative and anti-inflammatory properties. It helps to proliferate neuronal stem cells by enhancing the production of BDNF and nerve growth factor (NGF) and some neuropeptides having anti-inflammatory properties [270], [271]. In 2017, Westfall et al. reported that FA is also produced by probiotic bacteria such as Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum NCIMB 5221 and Bifidum animalis in large quantity by bacterial FA esterase enzymes [272]. Due to its therapeutic effect, it is gaining attention for its use in AD treatment, pre-treatment with FA has been shown to reduce the Aβ fibrils and to treat the neuroinflammation in AD mice [273]. FA-producing probiotic bacteria inhibits the formation and aggregation of beta-amyloid fibrils through scavenging ROS.
Other probiotic bacterial protein, sirtuin-1 (SIRT1) protein deacetylase has been shown to have antioxidant properties. This protein regulates the genes of antioxidant pathways of host and has been documented to have neuroprotective effects. Recent research probed the capability of a formulation of probiotic bacteria named SLAB51 (Streptococcus thermophilus, Lactobacillus acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, L. brevis, Bifidobacterium longum, B. breve, B. infantis) to relieve oxidative stress and discovered the molecular mechanism of its effects [274]. In the treated transgenic 3xTg-AD-mice, the expression and activity of SIRT1 protein in the brain were observed to be retrieved and the formation of Aβ peptide was shown decreased. However, in untreated AD mice, the expression of SIRT1 was found to be substantially decreased. Moreover, the enhanced activity of SIRT1 reduces the p53 protein acetylation and improves the survival of stressed cells by repressing apoptotic pathways. Other studies also showed that probiotic supplements activate the SIRT1 pathway and provoke antioxidative effects. SLAB51 also enhances the activity of GPx and catalase antioxidant enzymes to alleviate oxidative stress-causing impairments. Similar findings were also observed in humans. The concentration of SIRT1 in the brains of AD patients was found significantly reduced which is closely associated with the accumulation of amyloid-β and tau in the cerebral cortex of persons with AD [275]. An improved level of SIRT1 has been reported in a human study supplemented with Lactobacillus casei 01, a probiotic strain [276].
There is a very fragile relationship between gut microbiota and host immune cells. Immune cells are specialized to peculiarly differentiate the host friendly bacteria from pathogenic bacteria. If this relationship hurts, it can lead to unnecessary immune responses, prompting chronic inflammation [277]. This differentiation is started by intestinal epithelial cells who are responsible for producing the educated macrophage phenotype based on bacterial cell surface antigens such as LPS, peptidoglycan and flagellin. When epithelial cells become vulnerable to pathogenic attack, the antigens translocate into vasculature and in response, proinflammatory cytokines such as IL (interleukin)-1, IL- 6, and tumor necrosis factor-alpha (TNF-α) are produced, which leads to septic shock and inflammation in the gut and brain. Some bacterial toxins can also cross the BBB [269], [278]. To gaze at the role of microbiota in amyloidosis, an investigation was conducted through assessing the pro- (CXCL2, CXCL10, IL-1β, IL-6, IL-18, IL-8, inflammasome complex NLRP3, TNF-α) and anti-inflammatory (IL-4, IL-10, IL-13) cytokine activity of several gut microbiota taxa in cognitively impaired patients and it was observed that the number of Escherichia/Shigella increases and Eubacterium rectale decreases significantly correlated with changes in concentration of pro- and anti-inflammatory cytokines in cognitively impaired and amyloid positive patients. In concert, an increased level of IL-6, CXCL2, NLRP3, and IL-1β and a decreased level of IL-10 was observed in AD patients. This study suggests that gut microbiota could initiate, worsen or alleviate peripheral inflammation in neurological diseases [279].
In silico strategies to advance the gut-brain research
With the advancement of technology, knowledge about the brain is increasing exponentially. The neuroscientists have generated plenty of data from molecular biology, molecular genetics, brain imaging, and other new technologies, and have a significant interest in sharing the neuroscience data for various analysis. Neuroimaging is also very helpful in predicting and detecting NDDs and mental disorders. In the interest of neuroscience data collection and analysis, various bioinformatics tools are being developed for the further development of devices based on brain functions. To date, researchers have a rudimentary knowledge of molecular pathways involved in neurodegeneration due to various stress conditions. The integrated databases will be helpful to collect the different types of data related to neurodegeneration diseases from publicly available sources (Table 2).
Table 2.
Overview of currently active databases related to neurological data.
| Database | Web address | Data type | Reference(s) |
|---|---|---|---|
| PDGene | http://www.pdgene.org/ | Meta-analysis of genome-wide association studies (GWAS) | [304] |
| MDSGene | http://www.mdsgene.org/ | Genetic mutations, movement disorder genes related to PD | [305] |
| Alzforum | https://www.alzforum.org/mutations | Repository of variants in genes linked to AD | [306] |
| AlzGene | http://www.alzgene.org/ | Systematic meta-analyses of AD | [307] |
| Neuroinformatics Database (NiDB) | https://www.nitrc.org/projects/nidb/ | Imaging | [308] |
| Neuroscience Information Framework (NIF) | https://neuinfo.org/ | Metadata (imaging, genes, omics, function, grants, protocols etc.) | [309] |
| BrainMaps | http://brainmaps.org/ | Brain structure and function | [310] |
| Hippocampome Portal | http://www.hippocampome.org/php/index.php | Morphology, molecular marker, membrane biophysics and synaptic physiology | [311] |
| NeuroElectro | https://neuroelectro.org/ | Electrophysiological properties | [312] |
| NeuroMorpho | http://neuromorpho.org/index.jsp | Neuromorphology and metadata | [313] |
| NeuronDB | https://senselab.med.yale.edu/NeuronDB/ | Voltage gated conductances, neurotransmitter receptors, and neurotransmitter substances | [313] |
| Collaborative Research in Computational Neuroscience (CRCNS) |
http://crcns.org/ | Electrophysiology and behavioral data | [314] |
| NeuroImaging Tools and Resources Collaboratory (NITRC) | https://www.nitrc.org/ | Neuroimaging, imaging genomics software tools, data, and computational resources | [315] |
| BigBrain | https://bigbrainproject.org/ | Ultrahigh-Resolution 3D Human Brain Model | [316] |
| Brain Transcriptome database (BrainTx) | http://www.cdtdb.brain.riken.jp | Gene expression | [317] |
| Marmoset Gene Atlas | https://gene-atlas.brainminds.riken.jp/ | Gene expression | [318] |
| Allen Brain Map | https://portal.brain-map.org/ | Metadata (gene expression, imaging toolkits etc.) |
[319] |
| BrainCloud | https://www.libd.org/brain-cloud/ | Gene expression | [320] |
| M2IA | http://m2ia.met-bioinformatics.cn/ | Microbiome and metabolome integrative analysis | [283] |
| Microbiota-Active Substance Interaction Database (MASI) |
http://www.aiddlab.com/MASI/ | Effect of bioactive compounds on microbiota and vice versa | [284] |
The comprehensive knowledge of microbiome and their importance in human health is increasing at a fast pace with the aggrandizement of high throughput technologies. Microbiota interacts with the host metabolites and converts them to secondary metabolites. Gut microbiota also produces their metabolites which play very important role in human health. Comprehending the interaction of metabolites and gut microbiota, it is important to know the function of microbiota in health and disease status and to develop natural therapies [280]. In the recent past, it has been proved that gut bacteria can alter CNS physiology and dysbiosis could be a potential factor in neuroinflammation [281]. In 2019, Lu and Claud reported that initial colonization and microbiota development affect brain development in preterm infants [282]. To manage and facilitate this exponentially increasing information, microbiota databases are being developed for in-depth understanding of the role of microbiota in human health. To establish the correlation between microbiota and metabolites, new pipelines are being developed such as M2IA, an automated microbiome and metabolome integrative analysis pipeline [283]. Other microbiota databases provide useful information about disease-associated microbes, modulation of drugs by microbiota, the effect of diet on gut microbes. To distribute the knowledge about the alteration of active substances such as therapeutic drugs, dietary components, herbal products, probiotics and environmental chemicals by microbiota and alteration of microbiota by active substances, Microbiota–Active Substance Interactions (MASI) database is developed [284]. These databases will be very helpful to establish the correlation between bacterial metabolites and neuronal health and pathogenesis. It has been reported that in clinical trials, the outcome of L-dopa treatment for PD varies between the recruiting subjects. This variation is because of their microbiota. L-dopa is metabolized by Tyrosine decarboxylase (TDC) and dopamine dehydroxylase (Dadh) of different gut bacterial species namely Enterococcus faecalis and Eggerthella lenta A2 [285]. For the inactivation of bacterial L-dopa decarboxylase, a drug, (S)-α-fluoromethyltyrosine (AFMT) was discovered. The combination of L-dopa and AFMT is being used for Parkinson's treatment. Zhuang et al. showed that gut microbiota is altered in patients with AD compared to healthy individuals at taxonomic levels, such as Bacteroides, Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales [286]. Metagenomic data/16S RNA sequencing data support firm association between dysbiosis and AD. Bacterial products such as LPS and SCFAs are associated with amyloid pathology [287]. The main objective of the development of these databases is to organize data in a set of structured records that can be helpful to retrieve the information for different analysis by analyst to provide the solutions to different unsolved mysteries related to neurodegeneration.
Conclusion
Interconnection of gut microbiota and brain has led to transformative advances in neuroscience research. To date, most of the studies found are related to gut bacterial neuroscience research, but the gut microbiome is vast and requires the attention of scientists to identify and characterize the role of gut micro-organisms and their communities, affecting gut-brain signaling routes. There is a need for additional understanding of pathways employed by gut microbiota in mediating communication between the gut and brain in the process of neurodegeneration and neuroprotection, which may open the way to new hypotheses in pathology and treatment of the disease. New treatment regimens like fecal microbial transfer and probiotics, though have helped in improving brain health to some extent, but lacking empiric pieces of evidence in commercial probiotic strains provokes the need to study particular probiotics for the treatment of particular NDDs. Further, the growing potential of in-silico strategies will help to gain a deep understanding of microbial effector molecules in neurodegeneration and neuroprotection, but unfortunately, these studies are very limited. Bioinformatic tools are the need of the hour, which could potentiate the designing of such drugs that can target the specific bacterial enzymes mediating the production of harmful metabolites. Neuroprotective and neurodegenerative roles of gut microbial metabolites are continued to be uncovered, providing opportunities to develop novel therapeutic modalities.
Funding
Authors received no specific funding for this work. Department of Applied Physics, Aalto University has supported the APC or open access fee.
Consent for publication
All authors have read the final version of the manuscript and have given their consent for publication.
CRediT authorship contribution statement
Shruti Shandilya: Conceptualization, Writing – original draft, Writing – review & editing, Artwork & schemes, Visualization. Sandeep Kumar: Writing – original draft, Writing – review & editing, Software. Niraj Kumar Jha: Formal analysis, Editing, Artwork & schemes. Kavindra Kumar Kesari: Supervision, Writing – review & editing, Validation. Janne Ruokolainen: Supervision, Validation, Data curation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Shruti Shandilya is a Ph.D. research scholar in Aalto University. Her research is focused on the importance of natural compounds to be used as therapeutics to cure neurological disorders. She is gold medalist in M.Sc. Biochemistry and obscured best thesis award for her excellent research during master’s degree. She also patented a method for siRNA encapsulation in exosomes, filed in India. She has published many scientific research articles in the internationally recognized peer-review journals with high impact factors.

Sandeep Kumar is currently working with the clinical research company as a data analyst. He completed his Ph.D. degree from Animal Biochemistry Department, National Dairy Research Institute (NDRI), India. His work was focused on the commensal bacteria and acquired resistance by them. He has expertise in biochemical techniques and bioinformatics data analysis. He has published many research articles in recognized peer review journals.

Dr. Niraj Kumar Jha is currently working as an Assistant Professor in the Department of Biotechnology at Sharda University, Greater Noida, India. He is a former Assistant Professor of the faculty of Biotechnology, Noida Institute of Engineering and Technology (NIET), affiliated with Abdul Kalam Technical University (AKTU), India. He holds a doctorate in Biotechnology from Delhi Technological University (DTU) and a postgraduate degree in Biotechnology from Vellore Institute of Technology, TN, India. During the doctorate program, DBT granted him a fellowship as a financial boost to accomplish research work. He has published over 90 quality scholarly work and 5 book chapters in prestigious international journals and presented over 10 research papers in top national and international conferences. He is an editorial board member of various high repute international journals and assisted significantly in reviewing articles of many international journals such as Scientific reports, Life sciences, European journal of pharmaceutical sciences and Journal of food biochemistry. Currently, he is also serving as a guest editor for various top journals of international repute including Antioxidants, Environmental science and pollution research, Frontiers in Bioscience-Landmark and Immuno. He has executed various research projects and supervised several doctoral scholars, masters, and bachelor students. His vast research expertise covers the areas of Natural products, Cell signaling and Disease.

Kavindra Kesari is currently working as Senior Researcher in the Department of Applied Physics at the Aalto University, Espoo, Finland. He obtained Doctoral, Master, and Bachelor degreés in Biotechnology from India. He served as an Assistant Professor position at Jaipur National University, Jaipur, India. He obtained his first Postdoctoral research from University of Eastern Finland, Kuopio, Finland. Currently, he is working on cell ultrastructure study with micro & macro spectroscopic techniques. He has developed various methods for cell culture and animal exposure to radiations and theurapeutic measures. His study explores the medicinal applications of plant derived bioactive compounds and nanoparticles for disease treatment. He has published over 85 scientific papers in scientific journals, 22 book chapters, 5 books and presented over 25 papers at national and international scientific meetings. His Hirsch index (h-index) as of August 2021 is 29 on Google Scholar, 24 on Scopus. He has received four times young scientist award. His research interest is focus on neurobiology, radiation and cancer biology and therapeutic research.

Janne Ruokolainen is a Professor in the Department of Applied Physics, Aalto University, Espoo, Finland. He gained his PhD (Tech) in Materials Physics from Helsinki University of Technology and Docentship in polymer physics and soft materials microscopy. His current research activities focus the characterization of molecular bioactive compounds extracted from plant species in neurodegenerative disease treatment. He has published over 250 scientific papers in peer reviewed and refereed journals. Since 1995, he has gained high bibliometric factor, where H-index 62 (google scholar). His awards and research honours include invitation to the Alfred Nobel Symposium (2005), Young Scientist Award by Foundation of Technology (2002), Prize for the best PhD Thesis in the field of technical sciences by Tekniikan Akateemisten Liitto and Tekniska Föreningen in Finland (TEK/TFiF) (2001), IUPAC Prize for Young Chemists – Honourable Mention Award (2001).
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Kavindra Kumar Kesari, Email: kavindra.kesari@aalto.fi.
Janne Ruokolainen, Email: janne.ruokolainen@aalto.fi.
References
- 1.Rao M., Gershon M.D. Enteric nervous system development: what could possibly go wrong? Nat Rev Neurosci. 2018;19(9):552–565. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morais L.H., Schreiber H.L., Mazmanian S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 3.Sender R., Fuchs S., Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabke K., Hendrick G., Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins J., Borojevic R., Verdu E.F., Huizinga J.D., Ratcliffe E.M. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26(1):98–107. doi: 10.1111/nmo.2013.26.issue-1. [DOI] [PubMed] [Google Scholar]
- 8.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vich Vila A., Collij V., Sanna S., Sinha T., Imhann F., Bourgonje A.R., et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11(1) doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Cuesta-Zuluaga J, Kelley ST, Chen Y, Escobar JS, Mueller NT, Ley RE, et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. MSystems 2019;4. https://doi.org/10.1128/msystems.00261-19. [DOI] [PMC free article] [PubMed]
- 11.Catanzaro J.R., Strauss J.D., Bielecka A., Porto A.F., Lobo F.M., Urban A., et al. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-49923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y., Palm N.W. Immunoglobulin A and the microbiome. Curr Opin Microbiol. 2020;56:89–96. doi: 10.1016/j.mib.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Fasano A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020;9. https://doi.org/10.12688/f1000research.20510.1. [DOI] [PMC free article] [PubMed]
- 14.Huizinga J.D., Lammers W.J.E.P. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol - Gastrointest Liver Physiol. 2009;296(1):G1–G8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 15.De Punder K., Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:15. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Needham B.D., Kaddurah-Daouk R., Mazmanian S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci. 2020;21(12):717–731. doi: 10.1038/s41583-020-00381-0. [DOI] [PubMed] [Google Scholar]
- 17.Dinan T.G., Cryan J.F. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489–503. doi: 10.1113/tjp.2017.595.issue-210.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B., Yao M., Lv L., Ling Z., Li L. The Human Microbiota in Health and Disease. Engineering. 2017;3(1):71–82. doi: 10.1016/J.ENG.2017.01.008. [DOI] [Google Scholar]
- 19.Luca M., Di Mauro M., Di Mauro M., Luca A. Gut microbiota in Alzheimer’s disease, depression, and type 2 diabetes mellitus: The role of oxidative stress. Oxid Med Cell Longev. 2019;2019:1–10. doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raval U., Harary J.M., Zeng E., Pasinetti G.M. The dichotomous role of the gut microbiome in exacerbating and ameliorating neurodegenerative disorders. Expert Rev Neurother. 2020;20(7):673–686. doi: 10.1080/14737175.2020.1775585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheon M.-J., Lim S.-M., Lee N.-K., Paik H.-D. Probiotic properties and neuroprotective effects of lactobacillus buchneri ku200793 isolated from korean fermented foods. Int J Mol Sci. 2020;21(4):1227. doi: 10.3390/ijms21041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirin S., Aslim B. Characterization of lactic acid bacteria derived exopolysaccharides for use as a defined neuroprotective agent against amyloid beta1–42-induced apoptosis in SH-SY5Y cells. Sci Rep. 2020;10:1–18. doi: 10.1038/s41598-020-65147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J., Lee J., Yeom Z., Heo D., Lim Y.H. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-15163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung I.-H., Jung M.-A., Kim E.-J., Han M.J., Kim D.-H. Lactobacillus pentosus var. plantarum C29 protects scopolamine-induced memory deficit in mice. J Appl Microbiol. 2012;113(6):1498–1506. doi: 10.1111/jam.2012.113.issue-610.1111/j.1365-2672.2012.05437.x. [DOI] [PubMed] [Google Scholar]
- 25.Woo J.Y., Gu W., Kim K.A., Jang S.E., Han M.J., Kim D.H. Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a d-galactose-induced accelerated aging mouse model. Anaerobe. 2014;27:22–26. doi: 10.1016/j.anaerobe.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Harwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans., vol. 35, Biochem Soc Trans; 2007, p. 1147–50. https://doi.org/10.1042/BST0351147. [DOI] [PubMed]
- 27.Sun Y.u., Lu Y., Saredy J., Wang X., Drummer IV C., Shao Y., et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020;37:101696. doi: 10.1016/j.redox.2020.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp Neurobiol. 2015;24(4):325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voet D, Voet J, Pratt C. Fundamentals of biochemistry: life at the molecular level. 2016.
- 31.Wang W., Zhao F., Ma X., Perry G., Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol Neurodegener. 2020;15:1–22. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoccarato F, Cavallini L, … SB-B, 2007 U. Succinate modulation of H2O2 release at NADH:ubiquinone oxidoreductase (Complex I) in brain mitochondria. PortlandpressCom 2007;406:125–9. [DOI] [PMC free article] [PubMed]
- 33.Dykens J.A. Isolated Cerebral and Cerebellar Mitochondria Produce Free Radicals when Exposed to Elevated Ca2+ and Na+: Implications for Neurodegeneration. J Neurochem. 1994;63(2):584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 34.Smaga I., Niedzielska E., Gawlik M., Moniczewski A., Krzek J., Przegaliński E., et al. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Reports. 2015;67(3):569–580. doi: 10.1016/j.pharep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Nagatsu T. Progress in monoamine oxidase (MAO) research in relation to genetic engineering. Elsevier. 2004;25(1-2):11–20. doi: 10.1016/S0161-813X(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 36.Riederer P, Konradi C, Schay V, … EK-A in, 1987 U. Localization of MAO-A and MAO-B in human brain: a step in understanding the therapeutic action of L-deprenyl. Adv Neurol 1987;45:111–8. [PubMed]
- 37.Forstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju Kim M., Shin K.-S., Chung Y.-B., Jung K.W., Cha C.I., Hoon Shin D. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040(1-2):178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 39.Tejada-Simon M.V., Serrano F., Villasana L.E., Kanterewicz B.I., Wu G.-Y., Quinn M.T., et al. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29(1):97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bréchard S., Tschirhart E.J. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008;84(5):1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarafdar A., Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19:3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51(7):1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nanda B., Nataraju A., Rajesh R., Rangappa K., Shekar M., Vishwanath B. PLA2 Mediated Arachidonate Free Radicals: PLA2 Inhibition and Neutralization of Free Radicals by Anti-Oxidants – A New Role as Anti-Inflammatory Molecule. Curr Top Med Chem. 2007;7:765–777. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- 44.Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592:692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z., Zhang L.u., Zhou L.i., Lei Y., Zhang Y., Huang C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019;25:101047. doi: 10.1016/j.redox.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules. 2019;24(8):1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta - Mol Basis Dis. 2017;1863(5):1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito F., Ammendola R., Faraonio R., Russo T., Cimino F. Redox Control of Signal Transduction, Gene Expression and Cellular Senescence. Neurochem Res. 2004;29(3):617–628. doi: 10.1023/B:NERE.0000014832.78725.1a. [DOI] [PubMed] [Google Scholar]
- 49.Forsberg K., Wuttke A., Quadrato G., Chumakov P.M., Wizenmann A., Di Giovanni S. The tumor suppressor p53 fine-tunes reactive oxygen species levels and neurogenesis via PI3 kinase signaling. J Neurosci. 2013;33(36):14318–14330. doi: 10.1523/JNEUROSCI.1056-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickinson B.C., Peltier J., Stone D., Schaffer D.V., Chang C.J. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol. 2011;7(2):106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giorgi C., Agnoletto C., Baldini C., Bononi A., Bonora M., Marchi S., et al. Redox control of protein kinase C: Cell-and disease-specific aspects. Antioxidants Redox Signal. 2010;13(7):1051–1085. doi: 10.1089/ars.2009.2825. [DOI] [PubMed] [Google Scholar]
- 52.Bedogni B., Pani G., Colavitti R., Riccio A., Borrello S., Murphy M., et al. Redox regulation of cAMP-responsive element-binding protein and induction of manganous superoxide dismutase in nerve growth factor-dependent cell survival. J Biol Chem. 2003;278(19):16510–16519. doi: 10.1074/jbc.M301089200. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Jope R.S. Oxidative stress differentially modulates phosphorylation of ERK, p38 and CREB induced by NGF or EGF in PC12 cells. Neurobiol Aging. 1999;20(3):271–278. doi: 10.1016/S0197-4580(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 54.Crossthwaite A.J., Hasan S., Williams R.J. Hydrogen peroxide-mediated phosphorylation of ERK1/2, AKt/PKB and JNK in cortical neurones: Dependence on Ca2+ and PI3-kinase. J Neurochem. 2002;80(1):24–35. doi: 10.1046/j.0022-3042.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 55.Massaad C.A., Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxidants Redox Signal. 2011;14(10):2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knapp L.T., Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: Contributory or inhibitory? J Neurosci Res. 2002;70(1):1–7. doi: 10.1002/(ISSN)1097-454710.1002/jnr.v70:110.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 57.Knapp L.T., Klann E. Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J Biol Chem. 2000;275(31):24136–24145. doi: 10.1074/jbc.M002043200. [DOI] [PubMed] [Google Scholar]
- 58.Ji G., Li Z., Neugebauer V. Reactive oxygen species mediate visceral pain-related amygdala plasticity and behaviors. Pain. 2015;156:825–836. doi: 10.1097/j.pain.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bear M.F. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93(24):13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.HUGHES JR. Post-tetanic potentiation. Physiol Rev 1958;38:91–113. https://doi.org/10.1152/physrev.1958.38.1.91. [DOI] [PubMed]
- 61.Castellani G.C., Quinlan E.M., Cooper L.N., Shouval H.Z. A biophysical model of bidirectional synaptic plasticity: Dependence on AMPA and NMDA receptors. Proc Natl Acad Sci U S A. 2001;98(22):12772–12777. doi: 10.1073/pnas.201404598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab 2014;19:49–57. https://doi.org/10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed]
- 63.Bailey Damian Miles, Bärtsch Peter, Knauth Michael, Baumgartner Ralf W. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: From the molecular to the morphological. Cell Mol Life Sci. 2009;66(22):3583–3594. doi: 10.1007/s00018-009-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukuto Jon M., Carrington Samantha J., Tantillo Dean J., Harrison Jason G., Ignarro Louis J., Freeman Bruce A., et al. Small molecule signaling agents: The integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem Res Toxicol. 2012;25(4):769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kishida KT, Hoeffer CA, Hu D, Pao M, Holland SM, Klann E. Synaptic Plasticity Deficits and Mild Memory Impairments in Mouse Models of Chronic Granulomatous Disease. Mol Cell Biol 2006;26:5908–20. https://doi.org/10.1128/mcb.00269-06. [DOI] [PMC free article] [PubMed]
- 66.Gauron Carole, Meda Francesca, Dupont Edmond, Albadri Shahad, Quenech’Du Nicole, Ipendey Eliane, et al. Hydrogen peroxide (H2O2) controls axon pathfinding during zebrafish development. Dev Biol. 2016;414(2):133–141. doi: 10.1016/j.ydbio.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Franco M.C., Ye Y., Refakis C.A., Feldman J.L., Stokes A.L., Basso M., et al. Nitration of Hsp90 induces cell death. Proc Natl Acad Sci U S A. 2013;110(12):E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hernández D.E., Salvadores N.A., Moya-Alvarado G., Catalán R.J., Bronfman F.C., Court F.A. Axonal degeneration induced by glutamate excitotoxicity is mediated by necroptosis. J Cell Sci. 2018;131 doi: 10.1242/jcs.214684. [DOI] [PubMed] [Google Scholar]
- 70.Baxter Paul S., Bell Karen F.S., Hasel Philip, Kaindl Angela M., Fricker Michael, Thomson Derek, et al. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun. 2015;6(1) doi: 10.1038/ncomms7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Telling Neil D., Everett James, Collingwood Joanna F., Dobson Jon, van der Laan Gerrit, Gallagher Joseph J., et al. Iron Biochemistry is Correlated with Amyloid Plaque Morphology in an Established Mouse Model of Alzheimer’s Disease. Cell Chem Biol. 2017;24(10):1205–1215.e3. doi: 10.1016/j.chembiol.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Garza-Lombó Carla, Posadas Yanahi, Quintanar Liliana, Gonsebatt María E., Franco Rodrigo. Neurotoxicity Linked to Dysfunctional Metal Ion Homeostasis and Xenobiotic Metal Exposure: Redox Signaling and Oxidative Stress. Antioxidants Redox Signal. 2018;28(18):1669–1703. doi: 10.1089/ars.2017.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burbulla Lena F., Song Pingping, Mazzulli Joseph R., Zampese Enrico, Wong Yvette C., Jeon Sohee, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science (80-) 2017;357(6357):1255–1261. doi: 10.1126/science:aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LIU Hua-qing, ZHU Xing-zu, WENG En-qi. Intracellular dopamine oxidation mediates rotenone-induced apoptosis in PC12 cells. Acta Pharmacol Sin. 2005;26(1):17–26. doi: 10.1111/aphs.2005.26.issue-110.1111/j.1745-7254.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 75.Chen S., An F.-m., Yin L., Liu A.-r., Yin D.-k., Yao W.-b., et al. Glucagon-like peptide-1 protects hippocampal neurons against advanced glycation end product-induced tau hyperphosphorylation. Neuroscience. 2014;256:137–146. doi: 10.1016/j.neuroscience:2013.10.038. [DOI] [PubMed] [Google Scholar]
- 76.Takeuchi Masayoshi, Bucala Richard, Suzuki Takako, Ohkubo Tadatoshi, Yamazaki Matsumi, Koike Takao, et al. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000;59(12):1094–1105. doi: 10.1093/jnen/59.12.1094. [DOI] [PubMed] [Google Scholar]
- 77.Rabbani N, Thornalley PJ. Dicarbonyls linked to damage in the powerhouse: Glycation of mitochondrial proteins and oxidative stress. Biochem. Soc. Trans., vol. 36, Portland Press; 2008, p. 1045–50. https://doi.org/10.1042/BST0361045. [DOI] [PMC free article] [PubMed]
- 78.Williams Taufika Islam, Lynn Bert C., Markesbery William R., Lovell Mark A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27(8):1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Shi, Eitan Erez, Mattson Mark P. Early involvement of lysosome dysfunction in the degeneration of cerebral cortical neurons caused by the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 2017;140(6):941–954. doi: 10.1111/jnc.2017.140.issue-610.1111/jnc.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim Yu Rim, Kim Young Min, Lee Jaeho, Park Joohyun, Lee Jong Eun, Hyun Young-Min. Neutrophils Return to Bloodstream Through the Brain Blood Vessel After Crosstalk With Microglia During LPS-Induced Neuroinflammation. Front Cell Dev Biol. 2020;8 doi: 10.3389/fcell.2020.613733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirkman Henry N., Gaetani Gian F. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32(1):44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Bell Karen F.S., Al-Mubarak Bashayer, Martel Marc-André, McKay Sean, Wheelan Nicola, Hasel Philip, et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun. 2015;6(1) doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dixon Scott J, Stockwell Brent R. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 84.Wang Xiaoying, Mori Tatsuro, Sumii Toshihisa, Lo Eng H. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: Caspase activation and oxidative stress. Stroke. 2002;33(7):1882–1888. doi: 10.1161/01.STR.0000020121.41527.5D. [DOI] [PubMed] [Google Scholar]
- 85.Chen-Roetling Jing, Ma Sheng-Kai, Cao Yang, Shah Aishwarya, Regan Raymond F. Hemopexin increases the neurotoxicity of hemoglobin when haptoglobin is absent. J Neurochem. 2018;145(6):464–473. doi: 10.1111/jnc.2018.145.issue-610.1111/jnc.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez Frank J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat Res - Fundam Mol Mech Mutagen. 2005;569(1-2):101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Salech F., Ponce D.P., Paula-Lima A.C., SanMartin C.D., Behrens M.I. Nicotinamide, a Poly [ADP-Ribose] Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer’s Disease. Front Aging Neurosci. 2020;12:255. doi: 10.3389/fnagi.2020.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hwang J.Y., Aromolaran K.A., Zukin R.S. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci. 2017;18(6):347–361. doi: 10.1038/nrn.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho I., Blaser M.J. The human microbiome: At the interface of health and disease. Nat Rev Genet. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sgritta Martina, Dooling Sean W., Buffington Shelly A., Momin Eric N., Francis Michael B., Britton Robert A., et al. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron. 2019;101(2):246–259.e6. doi: 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaglin Mathilde, Rhimi Moez, Philippe Catherine, Pons Nicolas, Bruneau Aurélia, Goustard Bénédicte, et al. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front Neurosci. 2018;12 doi: 10.3389/fnins.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian P., Wang G., Zhao J., Zhang H., Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J Nutr Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. https://doi.org/10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed]
- 95.Luck B., Engevik M.A., Ganesh B.P., Lackey E.P., Lin T., Balderas M., et al. Bifidobacteria sshape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci Rep. 2020;10:1–18. doi: 10.1038/s41598-020-64173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:263ra158-263ra158. https://doi.org/10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed]
- 97.Dalile Boushra, Van Oudenhove Lukas, Vervliet Bram, Verbeke Kristin. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 98.Strandwitz Philip, Kim Ki Hyun, Terekhova Darya, Liu Joanne K., Sharma Anukriti, Levering Jennifer, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zorov D.B., Plotnikov E.Y., Silachev D.N., Zorova L.D., Pevzner I.B., Zorov S.D., et al. Microbiota and mitobiota. Putting an equal sign between mitochondria and bacteria. Biochem. 2014;79(10):1017–1031. doi: 10.1134/S0006297914100046. [DOI] [PubMed] [Google Scholar]
- 100.Migeotte I., Communi D., Parmentier M. Formyl peptide receptors: A promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17(6):501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 101.Tiso Mauro, Schechter Alan N., Jourd'heuil David. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE. 2015;10(3):e0119712. doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sellge Gernot, Kufer Thomas A. PRR-signaling pathways: Learning from microbial tactics. Semin Immunol. 2015;27(2):75–84. doi: 10.1016/j.smim.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 103.Holzer P., Farzi A., Hassan A.M., Zenz G., Jacan A., Reichmann F. Visceral inflammation and immune activation stress the brain. Front Immunol. 2017;8:22. doi: 10.3389/fimmu.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vargas-Caraveo Alejandra, Sayd Aline, Maus Sandra R., Caso Javier R., Madrigal José L.M., García-Bueno Borja, et al. Lipopolysaccharide enters the rat brain by a lipoprotein-mediated transport mechanism in physiological conditions. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-13302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Jiayi, Bi Wei, Xiao Shu, Lan Xin, Cheng Xiaofeng, Zhang Jiawei, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-42286-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caputi Valentina, Giron Maria. Microbiome-gut-brain axis and toll-like receptors in parkinson’s disease. Int J Mol Sci. 2018;19(6):1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiu Raymond, Hall Lindsay J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018;7(1):1–15. doi: 10.1038/s41426-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agata N., Ohta M., Mori M., Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett. 1995;129(1):17–19. doi: 10.1016/0378-1097(95)00119-P. [DOI] [PubMed] [Google Scholar]
- 109.Hu DL, Zhu G, Mori F, Omoe K, Okada M, Wakabayashi K, et al. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell Microbiol 2007;9:2267–77. https://doi.org/10.1111/j.1462-5822.2007.00957.x. [DOI] [PubMed]
- 110.Leschelle Xavier, Goubern Marc, Andriamihaja Mireille, Blottière Hervé M., Couplan Elodie, Gonzalez-Barroso Maria-del-Mar, et al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim Biophys Acta - Gen Subj. 2005;1725(2):201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 111.Beaumont Martin, Andriamihaja Mireille, Lan Annaïg, Khodorova Nadezda, Audebert Marc, Blouin Jean-Marc, et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic Biol Med. 2016;93:155–164. doi: 10.1016/j.freeradbiomed.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 112.Donertas Ayaz Basak, Zubcevic Jasenka. Gut microbiota and neuroinflammation in pathogenesis of hypertension: A potential role for hydrogen sulfide. Pharmacol Res. 2020;153:104677. doi: 10.1016/j.phrs.2020.104677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Braak Heiko, de Vos Rob A.I., Bohl Jürgen, Del Tredici Kelly. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 114.Friedland Robert P., Chapman Matthew R., Bliska James B. The role of microbial amyloid in neurodegeneration. PLOS Pathog. 2017;13(12):e1006654. doi: 10.1371/journal.ppat.100665410.1371/journal.ppat.1006654.g00110.1371/journal.ppat.1006654.g002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement 2020;16:391–460. https://doi.org/10.1002/alz.12068. [DOI] [PubMed]
- 116.Sato Chihiro, Barthélemy Nicolas R., Mawuenyega Kwasi G., Patterson Bruce W., Gordon Brian A., Jockel-Balsarotti Jennifer, et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018;97(6):1284–1298.e7. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanseeuw B.J., Betensky R.A., Jacobs H.I.L., Schultz A.P., Sepulcre J., Becker J.A., et al. Association of Amyloid and Tau with Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. 2019;76(8):915. doi: 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Veitch D.P., Weiner M.W., Aisen P.S., Beckett L.A., Cairns N.J., Green R.C., et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Dement. 2019;15(1):106–152. doi: 10.1016/j.jalz.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Almeida JR, Almeida MC, Cunha ME, Silva AGC. Risk factors and interventions for Alzheimer’s disease: A systematic review. Alzheimer’s Dement 2020;16. https://doi.org/10.1002/alz.037688.
- 120.Tönnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimer’s Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Persson Torbjörn, Popescu Bogdan O., Cedazo-Minguez Angel. Oxidative stress in alzheimer’s disease: Why did antioxidant therapy fail? Oxid Med Cell Longev. 2014;2014:1–11. doi: 10.1155/2014/427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bhatia Vandana, Sharma Saurabh. Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer’s disease. J Neurol Sci. 2021;421:117253. doi: 10.1016/j.jns.2020.117253. [DOI] [PubMed] [Google Scholar]
- 123.Uddin M.S., Tewari D., Sharma G., Kabir M.T., Barreto G.E., Bin-Jumah M.N., et al. Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol Neurobiol. 2020;57(7):2902–2919. doi: 10.1007/s12035-020-01929-y. [DOI] [PubMed] [Google Scholar]
- 124.Birla Hareram, Minocha Tarun, Kumar Gaurav, Misra Anamika, Singh Sandeep Kumar. Role of Oxidative Stress and Metal Toxicity in the Progression of Alzheimer’s Disease. Curr Neuropharmacol. 2020;18(7):552–562. doi: 10.2174/1570159X18666200122122512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simpson D.S.A., Oliver P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants. 2020;9:743. doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Michalska P., León R. When It Comes to an End: Oxidative Stress Crosstalk with Protein Aggregation and Neuroinflammation Induce Neurodegeneration. Antioxidants. 2020;9:740. doi: 10.3390/antiox9080740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abramov A.Y., Potapova E.V., Dremin V.V., Dunaev A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life. 2020;10:101. doi: 10.3390/life10070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Le Page Aurélie, Dupuis Gilles, Frost Eric H., Larbi Anis, Pawelec Graham, Witkowski Jacek M., et al. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp Gerontol. 2018;107:59–66. doi: 10.1016/j.exger.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 129.Vogt Nicholas M., Kerby Robert L., Dill-McFarland Kimberly A., Harding Sandra J., Merluzzi Andrew P., Johnson Sterling C., et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dodiya H.B., Frith M., Sidebottom A., Cao Y., Koval J., Chang E., et al. Synergistic depletion of gut microbial consortia, but not individual antibiotics, reduces amyloidosis in APPPS1-21 Alzheimer’s transgenic mice. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-64797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Xinyi, Sun Guangqiang, Feng Teng, Zhang Jing, Huang Xun, Wang Tao, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29(10):787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bonfili Laura, Cecarini Valentina, Berardi Sara, Scarpona Silvia, Suchodolski Jan S., Nasuti Cinzia, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin Y.N., Yu Q.F., Fu N., Liu X.W., Lu F.G. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16:3394–3401. doi: 10.3748/wjg.v16.i27.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bo Ting-bei, Wen Jing, Zhao Yuan-chun, Tian Shuang-jie, Zhang Xue-ying, Wang De-hua. Zhang X ying, Wang D hua. Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food. J Steroid Biochem Mol Biol. 2020;198:105602. doi: 10.1016/j.jsbmb.2020.105602. [DOI] [PubMed] [Google Scholar]
- 135.Yunes R.A., Poluektova E.U., Dyachkova M.S., Klimina K.M., Kovtun A.S., Averina O.V., et al. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe. 2016;42:197–204. doi: 10.1016/j.anaerobe.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 136.Tiwari Vivek, Patel Anant B. Impaired glutamatergic and GABAergic function at early age in AβPPswe-PS1dE9 mice: Implications for Alzheimer’s disease. J Alzheimer’s Dis. 2012;28(4):765–769. doi: 10.3233/JAD-2011-111502. [DOI] [PubMed] [Google Scholar]
- 137.Patterson E., Ryan P.M., Wiley N., Carafa I., Sherwin E., Moloney G., et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-51781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodgers Kenneth J., Main Brendan J., Samardzic Kate. Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity. Neurotox Res. 2018;33(1):168–177. doi: 10.1007/s12640-017-9757-2. [DOI] [PubMed] [Google Scholar]
- 139.Jaunmuktane Z., Brandner S. Invited Review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol Appl Neurobiol. 2020;46(6):522–545. doi: 10.1111/nan.v46.610.1111/nan.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daly B., Thompsell A., Sharpling J., Rooney Y.M., Hillman L., Wanyonyi K.L., et al. Evidence summary: The relationship between oral health and dementia. Br Dent J. 2017;223(11):846–853. doi: 10.1038/sj.bdj.2017.992. [DOI] [PubMed] [Google Scholar]
- 141.Nishiwaki H., Ito M., Ishida T., Hamaguchi T., Maeda T., Kashihara K., et al. <scp>Meta-Analysis</scp> of Gut Dysbiosis in Parkinson’s Disease. Mov Disord. 2020;35:1626–1635. doi: 10.1002/mds.28119. [DOI] [PubMed] [Google Scholar]
- 142.Tysnes Ole-Bjørn, Storstein Anette. Epidemiology of Parkinson’s disease. J Neural Transm. 2017;124(8):901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 143.Parkinson James. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. 2002;14(2):223–236. doi: 10.1176/jnp.14.2.223. [DOI] [PubMed] [Google Scholar]
- 144.Chai Xin‐yi, Diwakarla Shanti, Pustovit Ruslan V., McQuade Rachel M., Di Natale Madeleine, Ermine Charlotte M., et al. Investigation of nerve pathways mediating colorectal dysfunction in Parkinson’s disease model produced by lesion of nigrostriatal dopaminergic neurons. Neurogastroenterol Motil. 2020;32(9) doi: 10.1111/nmo.v32.910.1111/nmo.13893. [DOI] [PubMed] [Google Scholar]
- 145.Svensson Elisabeth, Horváth-Puhó Erzsébet, Thomsen Reimar W., Djurhuus Jens Christian, Pedersen Lars, Borghammer Per, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.v78.410.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 146.Hauser D.N., Hastings T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Braak Heiko, Del Tredici Kelly. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J Parkinsons Dis. 2017;7(s1):S71–S85. doi: 10.3233/JPD-179001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cardoso S.M., Empadinhas N. The Microbiome-Mitochondria Dance in Prodromal Parkinson’s Disease. Front Physiol. 2018;9:471. doi: 10.3389/fphys.2018.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grassi Diego, Howard Shannon, Zhou Minghai, Diaz-Perez Natalia, Urban Nicolai T., Guerrero-Given Debbie, et al. Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease. Proc Natl Acad Sci U S A. 2018;115(11):E2634–E2643. doi: 10.1073/pnas.1713849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sampson T.R., Challis C., Jain N., Moiseyenko A., Ladinsky M.S., Shastri G.G., et al. A gut bacterial amyloid promotes a-synuclein aggregation and motor impairment in mice. Elife. 2020;9 doi: 10.7554/eLife.53111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang X., Qian Y., Xu S., Song Y., Xiao Q. Longitudinal Analysis of Fecal Microbiome and Pathologic Processes in a Rotenone Induced Mice Model of Parkinson’s Disease. Front Aging Neurosci. 2018;9:441. doi: 10.3389/fnagi.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I, et al. Inflammatory Bowel Disease and Parkinson’s Disease: A Nationwide Swedish Cohort Study. Inflamm Bowel Dis 2019;25:111–23. https://doi.org/10.1093/ibd/izy190. [DOI] [PubMed]
- 153.Matheoud Diana, Cannon Tyler, Voisin Aurore, Penttinen Anna-Maija, Ramet Lauriane, Fahmy Ahmed M., et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1 −/− mice. Nature. 2019;571(7766):565–569. doi: 10.1038/s41586-019-1405-y. [DOI] [PubMed] [Google Scholar]
- 154.Bedarf J.R., Hildebrand F., Coelho L.P., Sunagawa S., Bahram M., Goeser F., et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9(1) doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rekdal VM, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science (80-) 2019;364:1055. https://doi.org/10.1126/science.aau6323. [DOI] [PMC free article] [PubMed]
- 156.van Kessel S.P., Frye A.K., El-Gendy A.O., Castejon M., Keshavarzian A., van Dijk G., et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun. 2019;10:1–11. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ostojic Sergej M. Inadequate Production of H2 by Gut Microbiota and Parkinson Disease. Trends Endocrinol Metab. 2018;29(5):286–288. doi: 10.1016/j.tem.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 158.Ohta Shigeo. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol Ther. 2014;144(1):1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 159.Sun Meng-Fei, Zhu Ying-Li, Zhou Zhi-Lan, Jia Xue-Bing, Xu Yi-Da, Yang Qin, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 160.Choi Jin Gyu, Kim Namkwon, Ju In Gyoung, Eo Hyeyoon, Lim Su-Min, Jang Se-Eun, et al. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-19646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hyder Adnan A., Wunderlich Colleen A., Puvanachandra Prasanthi, Gururaj G., Kobusingye Olive C., Neufeld Jacob A. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2007;22(5):341–353. doi: 10.3233/NRE-2007-22502. [DOI] [PubMed] [Google Scholar]
- 162.Greve Mark W., Zink Brian J. Pathophysiology of traumatic brain injury. Mt Sinai J Med. 2009;76(2):97–104. doi: 10.1002/msj.v76:210.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 163.Sundman Mark H., Chen Nan-kuei, Subbian Vignesh, Chou Ying-hui. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 2017;66:31–44. doi: 10.1016/j.bbi.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 164.Kharrazian D. Traumatic Brain Injury and the Effect on the Brain-Gut Axis. 2015. [PubMed]
- 165.Hang C.H., Shi J.X., Li J.S., Wu W., Yin H.X. Alterations of intestinal mucosa structure and barrier function following traumatic brain injury in rats. World J Gastroenterol. 2003;9:2776–2781. doi: 10.3748/wjg.v9.i12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Houlden A., Goldrick M., Brough D., Vizi E.S., Lénárt N., Martinecz B., et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma Elise L., Smith Allen D., Desai Neemesh, Cheung Lumei, Hanscom Marie, Stoica Bogdan A., et al. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun. 2017;66:56–69. doi: 10.1016/j.bbi.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoban A.E., Stilling R.M., Moloney G., Shanahan F., Dinan T.G., Clarke G., et al. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry. 2018;23(5):1134–1144. doi: 10.1038/mp.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Winek Katarzyna, Engel Odilo, Koduah Priscilla, Heimesaat Markus M., Fischer André, Bereswill Stefan, et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke. 2016;47(5):1354–1363. doi: 10.1161/STROKEAHA.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chu Coco, Murdock Mitchell H., Jing Deqiang, Won Tae Hyung, Chung Hattie, Kressel Adam M., et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574(7779):543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Angoa-Pérez Mariana, Zagorac Branislava, Anneken John H., Briggs Denise I., Winters Andrew D., Greenberg Jonathan M., et al. Repetitive, mild traumatic brain injury results in a progressive white matter pathology, cognitive deterioration, and a transient gut microbiota dysbiosis. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-65972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Simon Dennis W., Rogers Matthew B., Gao Yuan, Vincent Garret, Firek Brian A., Janesko-Feldman Keri, et al. Depletion of gut microbiota is associated with improved neurologic outcome following traumatic brain injury. Brain Res. 2020;1747:147056. doi: 10.1016/j.brainres.2020.147056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kigerl K.A., Hall J.C.E., Wang L., Mo X., Yu Z., Popovich P.G. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213:2603–2620. doi: 10.1084/jem.20151345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krych Lukasz, Hansen Camilla H.F., Hansen Axel K., van den Berg Frans W.J., Nielsen Dennis S., Bereswill Stefan. Quantitatively Different, yet Qualitatively Alike: A Meta-Analysis of the Mouse Core Gut Microbiome with a View towards the Human Gut Microbiome. PLoS ONE. 2013;8(5):e62578. doi: 10.1371/journal.pone.0062578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nicholson SE, Watts LT, Burmeister DM, Merrill D, Scroggins S, Zou Y, et al. Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock 2019;52:240–8. https://doi.org/10.1097/SHK.0000000000001211. [DOI] [PubMed]
- 176.Treangen T.J., Wagner J., Burns M.P., Villapol S. Traumatic brain injury in mice induces acute bacterial dysbiosis within the fecal microbiome. Front Immunol. 2018;9:2757. doi: 10.3389/fimmu.2018.02757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Howard Benjamin M, Kornblith Lucy Z, Christie Sabrinah A, Conroy Amanda S, Nelson Mary F, Campion Eric M, et al. Characterizing the gut microbiome in trauma: Significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2017;2(1):e000108. doi: 10.1136/tsaco-2017-000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Celorrio Marta, Abellanas Miguel A., Rhodes James, Goodwin Victoria, Moritz Jennie, Vadivelu Sangeetha, et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol Commun. 2021;9(1) doi: 10.1186/s40478-021-01137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cobley J.N., Fiorello M.L., Bailey D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503. doi: 10.1016/j.redox.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 181.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr J. 2016;15:1–22. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Elsayed A, Azab AE. Oxidative stress and antioxidant mechanisms in human body Schistosomiasis View project Oxidative stress and antioxidant capacity of natural products View project. Artic J Biotechnol 2019. https://doi.org/10.15406/jabb.2019.06.00173.
- 183.Tse Joyce K.Y. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem Neurosci. 2017;8(7):1438–1447. doi: 10.1021/acschemneuro.7b00176. [DOI] [PubMed] [Google Scholar]
- 184.Liao R., Wood T.R., Nance E. Superoxide dismutase reduces monosodium glutamate-induced injury in an organotypic whole hemisphere brain slice model of excitotoxicity. J Biol Eng. 2020;14:1–12. doi: 10.1186/s13036-020-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nell Hayley J., Au Jennifer. L., Giordano Courtney R., Terlecky Stanley R., Walton Paul A., Whitehead Shawn N., et al. Targeted Antioxidant, Catalase–SKL, Reduces Beta-Amyloid Toxicity in the Rat Brain. Brain Pathol. 2017;27(1):86–94. doi: 10.1111/bpa.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yakunin Eugenia, Kisos Haya, Kulik Willem, Grigoletto Jessica, Wanders Ronald J.A., Sharon Ronit. The regulation of catalase activity by PPAR γ is affected by α-synuclein. Ann Clin Transl Neurol. 2014;1(3):145–159. doi: 10.1002/acn3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Esposito L., Raber J., Kekonius L., Yan F., Yu G.Q., Bien-Ly N., et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lovell M.A., Xie C., Gabbita S.P., Markesbery W.R. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radic Biol Med. 2000;28(3):418–427. doi: 10.1016/S0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 189.Sánchez-López F., Tasset I., Agüera E., Feijóo M., Fernández-Bolaños R., Sánchez F.M., et al. Oxidative stress and inflammation biomarkers in the blood of patients with huntington’s disease. Neurol Res. 2012;34(7):721–724. doi: 10.1179/1743132812Y.0000000073. [DOI] [PubMed] [Google Scholar]
- 190.Jones R.M., Mercante J.W., Neish A.S. Reactive Oxygen Production Induced by the Gut Microbiota: Pharmacotherapeutic Implications. Curr Med Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dumitrescu Laura, Popescu-Olaru Iulia, Cozma Liviu, Tulbă Delia, Hinescu Mihail Eugen, Ceafalan Laura Cristina, et al. Oxidative stress and the microbiota-gut-brain axis. Oxid Med Cell Longev. 2018;2018:1–12. doi: 10.1155/2018/2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bonaz B., Bazin T., Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12 doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Elsayed A, Azab AE. Oxidative stress and antioxidant mechanisms in human body Schistosomiasis View project Effects of electromagnetic fields on human and animals Health View project. Artic J Biotechnol 2019. https://doi.org/10.15406/jabb.2019.06.00173.
- 194.Teleanu, Chircov, Grumezescu, Volceanov, Teleanu. Antioxidant Therapies for Neuroprotection-A Review. J Clin Med 2019;8:1659. https://doi.org/10.3390/jcm8101659. [DOI] [PMC free article] [PubMed]
- 195.Pluta R., Ulamek-Koziol M., Januszewski S., Czuczwar S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging (Albany NY) 2020;12:5539–5550. doi: 10.18632/aging.102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grant S.M., Demorrow S. Bile acid signaling in neurodegenerative and neurological disorders. Int J Mol Sci. 2020;21:1–25. doi: 10.3390/ijms21175982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mertens K.L., Kalsbeek A., Soeters M.R., Eggink H.M. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front Neurosci. 2017;11:617. doi: 10.3389/fnins.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Parry GJ, Rodrigues CMP, Aranha MM, Hilbert SJ, Davey C, Kelkar P, et al. Safety, Tolerability, and Cerebrospinal Fluid Penetration of Ursodeoxycholic Acid in Patients With Amyotrophic Lateral Sclerosis. Clin Neuropharmacol 2010;33:17–21. https://doi.org/10.1097/WNF.0b013e3181c47569. [DOI] [PubMed]
- 199.Rodrigues C.M.P., Sola S., Nan Z., Castro R.E., Ribeiro P.S., Low W.C., et al. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A. 2003;100(10):6087–6092. doi: 10.1073/pnas.1031632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cuevas E, Burks S, Raymick J, Robinson B, Gómez-Crisóstomo NP, Escudero-Lourdes C, et al. Tauroursodeoxycholic acid (TUDCA) is neuroprotective in a chronic mouse model of Parkinson’s disease. Nutr Neurosci 2020. https://doi.org/10.1080/1028415X.2020.1859729. [DOI] [PubMed]
- 201.Wu H., Yu N., Wang X., Yang Y., Liang H. Tauroursodeoxycholic acid attenuates neuronal apoptosis via the TGR5/ SIRT3 pathway after subarachnoid hemorrhage in rats. Biol Res. 2020;53:56. doi: 10.1186/s40659-020-00323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.West R.J.H., Ugbode C., Fort-Aznar L., Sweeney S.T. Neuroprotective activity of ursodeoxycholic acid in CHMP2BIntron5 models of frontotemporal dementia. Neurobiol Dis. 2020;144 doi: 10.1016/j.nbd.2020.105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh Jashbir, Metrani Rita, Shivanagoudra Siddanagouda R., Jayaprakasha Guddadarangavvanahally K., Patil Bhimanagouda S. Review on Bile Acids: Effects of the Gut Microbiome, Interactions with Dietary Fiber, and Alterations in the Bioaccessibility of Bioactive Compounds. J Agric Food Chem. 2019;67(33):9124–9138. doi: 10.1021/acs.jafc.8b0730610.1021/acs.jafc.8b07306.s001. [DOI] [PubMed] [Google Scholar]
- 204.Nho Kwangsik, Kueider‐Paisley Alexandra, MahmoudianDehkordi Siamak, Arnold Matthias, Risacher Shannon L., Louie Gregory, et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: Relationship to neuroimaging and CSF biomarkers. Alzheimer’s Dement. 2019;15(2):232–244. doi: 10.1016/j.jalz.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hertel J, Harms A, Heinken A, Baldini F, reports CT-C, 2019 undefined. Integrated analyses of microbiome and longitudinal metabolome data reveal microbial-host interactions on sulfur metabolism in Parkinson’s disease. Elsevier n.d. [DOI] [PMC free article] [PubMed]
- 206.Golubeva A.V., Joyce S.A., Moloney G., Burokas A., Sherwin E., Arboleya S., et al. Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine. 2017;24:166–178. doi: 10.1016/j.ebiom.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bhargava P, Smith MD, Mische L, Harrington E, Fitzgerald KC, Martin K, et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J Clin Invest 2020;130:3467–82. https://doi.org/10.1172/JCI129401. [DOI] [PMC free article] [PubMed]
- 208.Vaz Ana Rita, Cunha Carolina, Gomes Cátia, Schmucki Nadja, Barbosa Marta, Brites Dora. Glycoursodeoxycholic Acid Reduces Matrix Metalloproteinase-9 and Caspase-9 Activation in a Cellular Model of Superoxide Dismutase-1 Neurodegeneration. Mol Neurobiol. 2015;51(3):864–877. doi: 10.1007/s12035-014-8731-8. [DOI] [PubMed] [Google Scholar]
- 209.Gandy K.A.O., Zhang J., Nagarkatti P., Nagarkatti M. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Sci Rep. 2019;9:1–17. doi: 10.1038/s41598-019-43356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yanguas-Casás N., Barreda-Manso M.A., Nieto-Sampedro M., Romero-Ramírez L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J Cell Physiol. 2017;232(8):2231–2245. doi: 10.1002/jcp.v232.810.1002/jcp.25742. [DOI] [PubMed] [Google Scholar]
- 211.Diotel Nicolas, Charlier Thierry D., Lefebvre d'Hellencourt Christian, Couret David, Trudeau Vance L., Nicolau Joel C., et al. Steroid transport, local synthesis, and signaling within the brain: Roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci. 2018;12 doi: 10.3389/fnins.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhu B.T., Conney A.H. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 213.Gloux K., Berteau O., El Oumami H., Béguet F., Leclerc M., Doré J. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc Natl Acad Sci U S A. 2011;108:4539–4546. doi: 10.1073/pnas.1000066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe 2011;10:324–35. https://doi.org/10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed]
- 215.Flores Roberto, Shi Jianxin, Fuhrman Barbara, Xu Xia, Veenstra Timothy D, Gail Mitchell H, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J Transl Med. 2012;10(1) doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Soory M. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J Periodontal Res. 1995;30(2):124–131. doi: 10.1111/jre.1995.30.issue-210.1111/j.1600-0765.1995.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 217.Devendran Saravanan, Mythen Sean M., Ridlon Jason M. The desA and desB genes from Clostridium scindens ATCC 35704 encode steroid-17,20-desmolase. J Lipid Res. 2018;59(6):1005–1014. doi: 10.1194/jlr.M083949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Villa A., Vegeto E., Poletti A., Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37:372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 220.Kaliannan Kanakaraju, Robertson Ruairi C., Murphy Kiera, Stanton Catherine, Kang Chao, Wang Bin, et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome. 2018;6(1) doi: 10.1186/s40168-018-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rankin Kelsey A., Mei Feng, Kim Kicheol, Shen Yun-An A., Mayoral Sonia R., Desponts Caroline, et al. Selective estrogen receptor modulators enhance CNS remyelination independent of estrogen receptors. J Neurosci. 2019;39(12):2184–2194. doi: 10.1523/JNEUROSCI.1530-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jarras Hend, Bourque Mélanie, Poirier Andrée‐Anne, Morissette Marc, Coulombe Katherine, Di Paolo Thérèse, et al. Neuroprotection and immunomodulation of progesterone in the gut of a mouse model of Parkinson’s disease. J Neuroendocrinol. 2020;32(1) doi: 10.1111/jne.v32.110.1111/jne.12782. [DOI] [PubMed] [Google Scholar]
- 223.Fontana A., Panebianco C., Picchianti-Diamanti A., Laganà B., Cavalieri D., Potenza A., et al. Gut microbiota profiles differ among individuals depending on their region of origin: An Italian pilot study. Int J Environ Res Public Health. 2019;16(21):4065. doi: 10.3390/ijerph16214065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sasabe Jumpei, Miyoshi Yurika, Rakoff-Nahoum Seth, Zhang Ting, Mita Masashi, Davis Brigid M., et al. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat Microbiol. 2016;1(10) doi: 10.1038/nmicrobiol.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dodd Dylan, Spitzer Matthew H., Van Treuren William, Merrill Bryan D., Hryckowian Andrew J., Higginbottom Steven K., et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sperandio V., Torres A.G., Jarvis B., Nataro J.P., Kaper J.B. Bacteria-host communication: The language of hormones. Proc Natl Acad Sci U S A. 2003;100(15):8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yoshioka Yasuhiro, Negoro Ryosuke, Kadoi Hisatsugu, Motegi Toshiki, Shibagaki Fumiya, Yamamuro Akiko, et al. Noradrenaline protects neurons against H 2 O 2 -induced death by increasing the supply of glutathione from astrocytes via β 3 -adrenoceptor stimulation. J Neurosci Res. 2021;99(2):621–637. doi: 10.1002/jnr.v99.210.1002/jnr.24733. [DOI] [PubMed] [Google Scholar]
- 228.Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, et al. Plasma and Fecal Metabolite Profiles in Autism Spectrum Disorder. BioRxiv 2020:2020.05.17.098806. https://doi.org/10.1101/2020.05.17.098806. [DOI] [PMC free article] [PubMed]
- 229.Yanovsky Inessa, Finkin-Groner Efrat, Zaikin Andrey, Lerman Lena, Shalom Hila, Zeeli Shani, et al. Carbamate derivatives of indolines as cholinesterase inhibitors and antioxidants for the treatment of Alzheimer’s disease. J Med Chem. 2012;55(23):10700–10715. doi: 10.1021/jm301411g. [DOI] [PubMed] [Google Scholar]
- 230.Schwarcz Robert, Bruno John P., Muchowski Paul J., Wu Hui-Qiu. Kynurenines in the mammalian brain: When physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tanaka M., Bohár Z., Vécsei L. Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules. 2020;25(3):564. doi: 10.3390/molecules25030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rothhammer Veit, Borucki Davis M., Tjon Emily C., Takenaka Maisa C., Chao Chun-Cheih, Ardura-Fabregat Alberto, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Akasaka Naoki, Fujiwara Shinsuke. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids. 2020;52(2):181–197. doi: 10.1007/s00726-019-02720-7. [DOI] [PubMed] [Google Scholar]
- 234.Barua Sumit, Kim Jong Youl, Kim Jae Young, Kim Jae Hwan, Lee Jong Eun. Therapeutic Effect of Agmatine on Neurological Disease: Focus on Ion Channels and Receptors. Neurochem Res. 2019;44(4):735–750. doi: 10.1007/s11064-018-02712-1. [DOI] [PubMed] [Google Scholar]
- 235.Jamwal S., Kumar P. Spermidine ameliorates 3-nitropropionic acid (3-NP)-induced striatal toxicity: Possible role of oxidative stress, neuroinflammation, and neurotransmitters. Physiol Behav. 2016;155:180–187. doi: 10.1016/j.physbeh.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 236.Chai Jianshen, Luo Li, Hou Fengyan, Fan Xia, Yu Jing, Ma Wei, et al. Agmatine reduces lipopolysaccharide-mediated oxidant response via activating PI3K/Akt pathway and up-regulating Nrf2 and HO-1 expression in macrophages. PLoS ONE. 2016;11(9):e0163634. doi: 10.1371/journal.pone.0163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ahn S.K., Hong S., Park Y.M., Lee W.T., Park K.A., Lee J.E. Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res. 2011;1373:48–54. doi: 10.1016/j.brainres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 238.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fujii Y, Nguyen TTT, Fujimura Y, Kameya N, Nakamura S, Arakawa K, et al. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci Biotechnol Biochem 2019;83:2144–52. https://doi.org/10.1080/09168451.2019.1644149. [DOI] [PubMed]
- 240.Sampson Timothy R., Debelius Justine W., Thron Taren, Janssen Stefan, Shastri Gauri G., Ilhan Zehra Esra, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Frost Gary, Sleeth Michelle L., Sahuri-Arisoylu Meliz, Lizarbe Blanca, Cerdan Sebastian, Brody Leigh, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1) doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Oleskin A.V., Shenderov B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Health Dis. 2016;27:30971. doi: 10.3402/mehd.v27.30971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ho Lap, Ono Kenjiro, Tsuji Mayumi, Mazzola Paolo, Singh Risham, Pasinetti Giulio M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018;18(1):83–90. doi: 10.1080/14737175.2018.1400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liu J, Wang F, Liu S, Du J, Hu X, … JX-J of the, et al. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. Elsevier n.d. [DOI] [PubMed]
- 245.Yang Tao, Rodriguez Vermali, Malphurs Wendi L., Schmidt Jordan T., Ahmari Niousha, Sumners Colin, et al. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol Rep. 2018;6(14):e13732. doi: 10.14814/phy2.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hoyles Lesley, Snelling Tom, Umlai Umm-Kulthum, Nicholson Jeremy K., Carding Simon R., Glen Robert C., et al. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome. 2018;6(1) doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Erny Daniel, Hrabě de Angelis Anna Lena, Jaitin Diego, Wieghofer Peter, Staszewski Ori, David Eyal, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vuolo MM, Lima VS, Maróstica Junior MR. Phenolic Compounds. Bioact. Compd., Elsevier; 2019, p. 33–50. https://doi.org/10.1016/B978-0-12-814774-0.00002-5.
- 249.Rebas Elzbieta, Rzajew Jowita, Radzik Tomasz, Zylinska Ludmila. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr Neuropharmacol. 2020;18(5):431–445. doi: 10.2174/1570159X18666200106155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sadeghi Ekbatan Shima, Iskandar Michele, Sleno Lekha, Sabally Kebba, Khairallah Joelle, Prakash Satya, et al. Absorption and Metabolism of Phenolics from Digests of Polyphenol-Rich Potato Extracts Using the Caco-2/HepG2 Co-Culture System. Foods. 2018;7(1):8. doi: 10.3390/foods7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Marín Laura, Miguélez Elisa M., Villar Claudio J., Lombó Felipe. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res Int. 2015;2015:1–18. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang Dongjie, Ho Lap, Faith Jeremiah, Ono Kenjiro, Janle Elsa M., Lachcik Pamela J., et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res. 2015;59(6):1025–1040. doi: 10.1002/mnfr.v59.610.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mehjabeen Naushad A, Sundara Kumar Durairajan S, Kanti Bera A, Senapati S, Li M, Min Li C. Natural Compounds with Anti-BACE1 Activity as Promising Therapeutic Drugs for Treating Alzheimer’s Disease. PdfsSemanticscholarOrg n.d. https://doi.org/10.1055/a-1019-9819. [DOI] [PubMed]
- 254.Ma J., Gao S.S., Yang H.J., Wang M., Cheng B.F., Feng Z.W., et al. Neuroprotective effects of proanthocyanidins, natural flavonoids derived from plants, on rotenone-induced oxidative stress and apoptotic cell death in human neuroblastoma SH-SY5Y cells. Front Neurosci. 2018;12:369. doi: 10.3389/fnins.2018.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T. Ferulic Acid Is a Nutraceutical β-Secretase Modulator That Improves Behavioral Impairment and Alzheimer-like Pathology in Transgenic Mice. PLoS One 2013;8. https://doi.org/10.1371/journal.pone.0055774. [DOI] [PMC free article] [PubMed]
- 256.Ren Z., Li Y., Zhang R., Li Y., Yang Z., Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 2017;40:1444–1456. doi: 10.3892/ijmm.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mueller S.O., Simon S., Chae K., Metzler M., Korach K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor α (ERα) and ERβ in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 258.Filosa S., Di Meo F., Crispi S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res. 2018;13:2055–2059. doi: 10.4103/1673-5374.241429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.official MH-E journal of cancer prevention: the, 1997 undefined. Intestinal flora and endogenous vitamin synthesis. EuropepmcOrg n.d. [DOI] [PubMed]
- 260.LeBlanc Jean Guy, Milani Christian, de Giori Graciela Savoy, Sesma Fernando, van Sinderen Douwe, Ventura Marco. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 261.Merli G.J., Fink J. Vitamin K and Thrombosis. Vitam Horm. 2008;78:265–279. doi: 10.1016/S0083-6729(07)00013-1. [DOI] [PubMed] [Google Scholar]
- 262.Alisi Ludovico, Cao Roberta, De Angelis Cristina, Cafolla Arturo, Caramia Francesca, Cartocci Gaia, et al. The relationships between vitamin k and cognition: A review of current evidence. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yu Yan-xia, Li Yi-pei, Gao Feng, Hu Qing-song, Zhang Yan, Chen Dong, et al. Vitamin K2 suppresses rotenone-induced microglial activation in vitro. Acta Pharmacol Sin. 2016;37(9):1178–1189. doi: 10.1038/aps.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ramazani Elham, Fereidoni Masoud, Tayarani-Najaran Zahra. Protective effects of vitamin K2 on 6-OHDA-induced apoptosis in PC12 cells through modulation bax and caspase-3 activation. Mol Biol Rep. 2019;46(6):5777–5783. doi: 10.1007/s11033-019-05011-2. [DOI] [PubMed] [Google Scholar]
- 265.Yu Y.X., Yu X.D., Cheng Q.Z., Tang L., Shen M.Q. The association of serum vitamin K2 levels with Parkinson’s disease: From basic case-control study to big data mining analysis. Aging (Albany NY) 2020;12:16410–16419. doi: 10.18632/aging.103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Parker Aimée, Fonseca Sonia, Carding Simon R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Agnew-Blais Jessica C., Wassertheil-Smoller Sylvia, Kang Jae H., Hogan Patricia E., Coker Laura H., Snetselaar Linda G., et al. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women’s Health Initiative Memory Study. J Acad Nutr Diet. 2015;115(2):231–241. doi: 10.1016/j.jand.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Douaud G., Refsum H., De Jager C.A., Jacoby R., Nichols T.E., Smith S.M., et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A. 2013;110(23):9523–9528. doi: 10.1073/pnas.1301816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Arora K., Green M., Prakash S. The Microbiome and Alzheimer’s Disease: Potential and Limitations of Prebiotic, Synbiotic, and Probiotic Formulations. Front Bioeng Biotechnol. 2020;8:1411. doi: 10.3389/fbioe.2020.537847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Meng Guilin, Meng Xiulin, Ma Xiaoye, Zhang Gengping, Hu Xiaolin, Jin Aiping, et al. Application of Ferulic Acid for Alzheimer’s Disease: Combination of Text Mining and Experimental Validation. Front Neuroinform. 2018;12 doi: 10.3389/fninf.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nabavi S., Devi K., Malar D., Sureda A., Daglia M., Nabavi S. Ferulic Acid and Alzheimer’s Disease: Promises and Pitfalls. Mini-Rev Med Chem. 2015;15:776–788. doi: 10.2174/1389557515666150522102545. [DOI] [PubMed] [Google Scholar]
- 272.Westfall Susan, Lomis Nikita, Kahouli Imen, Dia Si Yuan, Singh Surya Pratap, Prakash Satya. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ono Kenjiro, Hirohata Mie, Yamada Masahito. Ferulic acid destabilizes preformed β-amyloid fibrils in vitro. Biochem Biophys Res Commun. 2005;336(2):444–449. doi: 10.1016/j.bbrc.2005.08.148. [DOI] [PubMed] [Google Scholar]
- 274.Bonfili Laura, Cecarini Valentina, Cuccioloni Massimiliano, Angeletti Mauro, Berardi Sara, Scarpona Silvia, et al. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol Neurobiol. 2018;55(10):7987–8000. doi: 10.1007/s12035-018-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Julien Carl, Tremblay Cyntia, Émond Vincent, Lebbadi Meryem, Salem Norman, Bennett David A., et al. Sirtuin 1 Reduction Parallels the Accumulation of Tau in Alzheimer Disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Khalili L., Alipour B., Asghari Jafarabadi M., Hassanalilou T., Mesgari Abbasi M., Faraji I. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: A randomized controlled trial. Diabetol Metab Syndr. 2019;11:5. doi: 10.1186/s13098-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 277.Mafra Denise, Lobo Julie C, Barros Amanda F, Koppe Laetitia, Vaziri Nosratola D, Fouque Denis. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. 2014;9(3):399–410. doi: 10.2217/fmb.13.165. [DOI] [PubMed] [Google Scholar]
- 278.Shapira L., Soskolne W.A., Houri Y., Barak V., Halabi A., Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996;64(3):825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cattaneo Annamaria, Cattane Nadia, Galluzzi Samantha, Provasi Stefania, Lopizzo Nicola, Festari Cristina, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 280.Doestzada Marwah, Vila Arnau Vich, Zhernakova Alexandra, Koonen Debby P.Y., Weersma Rinse K., Touw Daan J., et al. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell. 2018;9(5):432–445. doi: 10.1007/s13238-018-0547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rutsch A, Kantsjö JB, Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol 2020;11. https://doi.org/10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed]
- 282.Lu J, Claud EC. Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol., vol. 61, John Wiley and Sons Inc.; 2019, p. 739–51. https://doi.org/10.1002/dev.21806. [DOI] [PMC free article] [PubMed]
- 283.Ni Y, Yu G, Chen H, Deng Y, Wells PM, Steves CJ, et al. M2IA: A web server for microbiome and metabolome integrative analysis. Bioinformatics 2020;36:3493–8. https://doi.org/10.1093/bioinformatics/btaa188. [DOI] [PubMed]
- 284.Zeng X, Yang X, Fan J, Tan Y, Ju L, Shen W, et al. MASI: microbiota—active substance interactions database. Nucleic Acids Res 2020. https://doi.org/10.1093/nar/gkaa924. [DOI] [PMC free article] [PubMed]
- 285.O'Neill Cora. Gut microbes metabolize Parkinson’s disease drug. Science (80-) 2019;364(6445):1030–1031. doi: 10.1126/science:aax8937. [DOI] [PubMed] [Google Scholar]
- 286.Zhuang Zhen-Qian, Shen Lin-Lin, Li Wei-Wei, Fu Xue, Zeng Fan, Gui Li, et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J Alzheimer’s Dis. 2018;63(4):1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 287.Marizzoni Moira, Cattaneo Annamaria, Mirabelli Peppino, Festari Cristina, Lopizzo Nicola, Nicolosi Valentina, et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J Alzheimer’s Dis. 2020;78(2):683–697. doi: 10.3233/JAD-200306. [DOI] [PubMed] [Google Scholar]
- 288.Cheon M.-J., Lim S.-M., Lee N.-K., Paik H.-D. Probiotic Properties and Neuroprotective Effects of Lactobacillus buchneri KU200793 Isolated from Korean Fermented Foods. Int J Mol Sci. 2020;21:1227. doi: 10.3390/ijms21041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cecarini Valentina, Bonfili Laura, Gogoi Olee, Lawrence Solomon, Venanzi Franco M., Azevedo Vasco, et al. Neuroprotective effects of p62(SQSTM1)-engineered lactic acid bacteria in Alzheimer’s disease: A pre-clinical study. Aging (Albany NY) 2020;12(16):15995–16020. doi: 10.18632/aging.103900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Labarre A, Guitard E, Tossing G, Bareke E, Labrecque M, Tétreault M, et al. Probiotic Lacticaseibacillus rhamnosus HA-114 suppresses age-dependent neurodegeneration via mitochondrial b-oxidation. n.d. [DOI] [PMC free article] [PubMed]
- 291.Sun J., Xu J., Yang B., Chen K., Kong Y., Fang N., et al. Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol Nutr Food Res. 2020;64(2):1900636. doi: 10.1002/mnfr.v64.210.1002/mnfr.201900636. [DOI] [PubMed] [Google Scholar]
- 292.Mori T, Koyama N, Tan J, Segawa T, Maeda M, Town T. Combined treatment with the phenolics -epigallocatechin-3-gallate and ferulic acid improves cognition and reduces Alzheimer-like pathology in mice. ASBMB 2019;294:2714-2731. https://doi.org/10.1074/jbc.RA118.004280. [DOI] [PMC free article] [PubMed]
- 293.Kowalski Karol, Mulak Agata. Brain-gut-microbiota axis in Alzheimer’s disease. J Neurogastroenterol Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chiroma S.M., Mohd Moklas M.A., Mat Taib C.N., Baharuldin M.T.H., Amon Z. D-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed Pharmacother. 2018;103:1602–1608. doi: 10.1016/j.biopha.2018.04.152. [DOI] [PubMed] [Google Scholar]
- 295.Nimgampalle M., Yellamma K. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J Clin Diagnostic Res. 2017;11:KC01–5. doi: 10.7860/JCDR/2017/26106.10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Georgescu D., Ancusa O.E., Georgescu L.A., Ionita I., Reisz D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin Interv Aging. 2016;11:1601–1608. doi: 10.2147/CIA.S106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tamtaji Omid Reza, Taghizadeh Mohsen, Daneshvar Kakhaki Reza, Kouchaki Ebrahim, Bahmani Fereshteh, Borzabadi Shokoofeh, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(3):1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 298.Cassani E, Privitera G, Pezzoli G, … CP-M, 2011 U. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. EuropepmcOrg 2011;57:117–21. [PubMed]
- 299.Akbari Elmira, Asemi Zatollah, Daneshvar Kakhaki Reza, Bahmani Fereshteh, Kouchaki Ebrahim, Tamtaji Omid Reza, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8 doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Alipour Nosrani Esmail, Tamtaji Omid Reza, Alibolandi Zahra, Sarkar Parichehr, Ghazanfari Mohsen, Azami Tameh Abolfazl, et al. Neuroprotective effects of probiotics bacteria on animal model of Parkinson’s disease induced by 6-hydroxydopamine: A behavioral, biochemical, and histological study. J Immunoass Immunochem. 2021;42(2):106–120. doi: 10.1080/15321819.2020.1833917. [DOI] [PubMed] [Google Scholar]
- 301.Rezaei Asl Zahra, Sepehri Gholamreza, Salami Mahmoud. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav Brain Res. 2019;376:112183. doi: 10.1016/j.bbr.2019.112183. [DOI] [PubMed] [Google Scholar]
- 302.Ni Yinhua, Yang Xin, Zheng Liujie, Wang Zhe, Wu Lianxin, Jiang Jinlu, et al. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol Nutr Food Res. 2019;63(22):1900603. doi: 10.1002/mnfr.v63.2210.1002/mnfr.201900603. [DOI] [PubMed] [Google Scholar]
- 303.Valladares Ricardo, Bojilova Lora, Potts Anastasia H., Cameron Evan, Gardner Christopher, Lorca Graciela, et al. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013;27(4):1711–1720. doi: 10.1096/fsb2.v27.410.1096/fj.12-223339. [DOI] [PubMed] [Google Scholar]
- 304.Lill Christina M., Roehr Johannes T., McQueen Matthew B., Kavvoura Fotini K., Bagade Sachin, Schjeide Brit-Maren M., et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDgene database. PLoS Genet. 2012;8(3):e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Klein Christine, Hattori Nobutaka, Marras Connie, Brundin Patrik, Langston J.W., Bloem B.R. MDSGene: Closing data gaps in genotype-phenotype correlations of monogenic Parkinson’s disease. J Parkinsons Dis. 2018;8(s1):S25–S30. doi: 10.3233/JPD-181505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kinoshita J., Clark T. Alzforum. Methods Mol Biol. 2007;401:365–381. doi: 10.1007/978-1-59745-520-6_19. [DOI] [PubMed] [Google Scholar]
- 307.Bertram Lars, McQueen Matthew B, Mullin Kristina, Blacker Deborah, Tanzi Rudolph E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 308.Book Gregory A., Anderson Beth M., Stevens Michael C., Glahn David C., Assaf Michal, Pearlson Godfrey D. Neuroinformatics database (NiDB) - A modular, portable database for the storage, analysis, and sharing of neuroimaging data. Neuroinformatics. 2013;11(4):495–505. doi: 10.1007/s12021-013-9194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gardner Daniel, Akil Huda, Ascoli Giorgio A., Bowden Douglas M., Bug William, Donohue Duncan E., et al. The neuroscience information framework: A data and knowledge environment for neuroscience. Neuroinformatics. 2008;6(3):149–160. doi: 10.1007/s12021-008-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mikula Shawn, Trotts Issac, Stone James M., Jones Edward G. Internet-enabled high-resolution brain mapping and virtual microscopy. Neuroimage. 2007;35(1):9–15. doi: 10.1016/j.neuroimage.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wheeler DW, White CM, Rees CL, Komendantov AO, Hamilton DJ, Ascoli GA. Hippocampome.org: A knowledge base of neuron types in the rodent hippocampus. Elife 2015;4. https://doi.org/10.7554/eLife.09960. [DOI] [PMC free article] [PubMed]
- 312.Tripathy SJ, Savitskaya J, Burton SD, Urban NN, Gerkin RC. NeuroElectro: A window to the world’s neuron electrophysiology data. Front Neuroinform 2014;8. https://doi.org/10.3389/fninf.2014.00040. [DOI] [PMC free article] [PubMed]
- 313.Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: A central resource for neuronal morphologies. J Neurosci 2007;27:9247–51. https://doi.org/10.1523/JNEUROSCI.2055-07.2007. [DOI] [PMC free article] [PubMed]
- 314.Teeters JL, Harris KD, Millman KJ, Olshausen BA, Sommer FT. Data sharing for computational neuroscience. Neuroinformatics, vol. 6, Springer; 2008, p. 47–55. https://doi.org/10.1007/s12021-008-9009-y. [DOI] [PubMed]
- 315.Kennedy D.N., Haselgrove C., Riehl J., Preuss N., Buccigrossi R. The Three NITRCs: A Guide to Neuroimaging Neuroinformatics Resources. Neuroinformatics. 2015 Jul;13(3):383–386. doi: 10.1007/s12021-015-9263-8. PMID: 25700675; PMCID: PMC4470758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Amunts K., Lepage C., Borgeat L., Mohlberg H., Dickscheid T., Rousseau M.-E., et al. BigBrain: An ultrahigh-resolution 3D human brain model. Science (80-) 2013;340(6139):1472–1475. doi: 10.1126/science:1235381. [DOI] [PubMed] [Google Scholar]
- 317.Sato Akira, Sekine Yukiko, Saruta Chihiro, Nishibe Hirozumi, Morita Noriyuki, Sato Yumi, et al. Cerebellar development transcriptome database (CDT-DB): Profiling of spatio-temporal gene expression during the postnatal development of mouse cerebellum. Neural Networks. 2008;21(8):1056–1069. doi: 10.1016/j.neunet.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 318.Shimogori Tomomi, Abe Ayumi, Go Yasuhiro, Hashikawa Tsutomu, Kishi Noriyuki, Kikuchi Satomi S., et al. Digital gene atlas of neonate common marmoset brain. Neurosci Res. 2018;128:1–13. doi: 10.1016/j.neures.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 319.Lein Ed S., Hawrylycz Michael J., Ao Nancy, Ayres Mikael, Bensinger Amy, Bernard Amy, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 320.Colantuoni Carlo, Lipska Barbara K., Ye Tianzhang, Hyde Thomas M., Tao Ran, Leek Jeffrey T., et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478(7370):519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]