Abstract
Recent research has been uncovering the role of the gut microbiota for brain health and disease. These studies highlight the role of gut microbiota on regulating brain function and behavior through immune, metabolic, and neuronal pathways. In this review we provide an overview of the gut microbiota axis pathways to lay the groundwork for upcoming sessions on the links between the gut microbiota and neurogenerative disorders. We also discuss how the gut microbiota may act as an intermediate factor between the host and the environment to mediate disease onset and neuropathology. Based on the current literature, we further examine the potential for different microbiota-based therapeutic strategies to prevent, to modify, or to halt the progress of neurodegeneration.
Keywords: gut microbiota–brain axis, neurodegeneration, inflammation, microbial metabolism, gut microbiota
Introduction
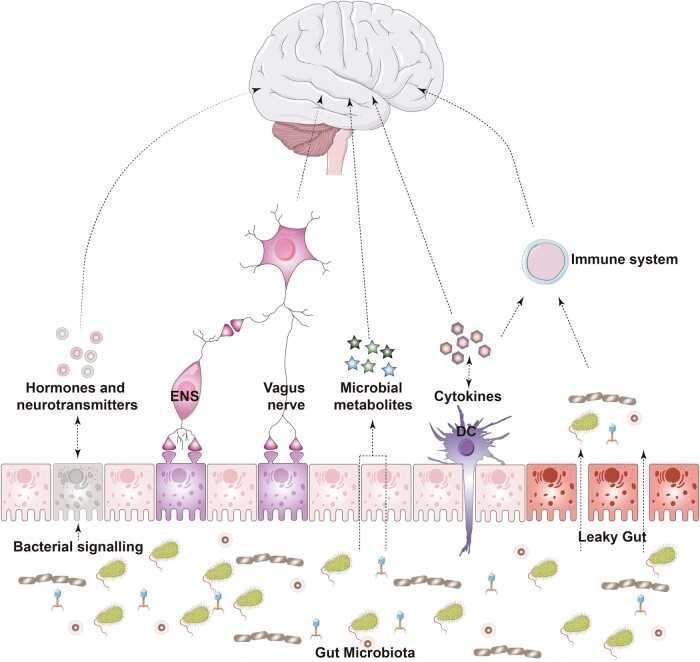
Since the discovery of the enteric nervous system (ENS) in the 1900s, the scientific community has been trying to explain the mechanisms underlying the connections between the gut and the central nervous system (CNS) that may explain behavior [1]. Over the past few decades, the gut microbiota—the trillion symbiotic microbial cells that reside in our gut—has emerged as a key player in this bidirectional communicatory pathway. The gut microbiota is capable of interacting with various physiological systems, the ENS to the CNS [1–3] (Figure 1). Most scientific evidence to date comes from preclinical studies involving animal models (Box 1), which are limited in nature but had enabled the field to move forward by demonstrating that the gut microbiota is essential for proper neurodevelopment and neuronal function [4–8]. The connection between the gut microbiota and the CNS is emphasized by studies that implicate alterations in the gut microbiota composition in neurodegenerative conditions [9].
Figure 1.
The gut microbiota–brain axis and neurodegenerative disorders. The gut microbiota–brain axis encompasses neuronal, immune, and metabolism pathways that mediate gut-to-brain communication. The gut microbiota may act as an intermediate factor between the host and the environment to mediate disease onset and neuropathology. ENS, enteric nervous system; DC, dendritic cell. (The images were purchased and adapted from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License.)
Box 1. Animal models of neurodegenerative diseases.
Animal models are important tools for investigating the role of gut microbiota on cellular dysfunction and degeneration [256]. The most common disease models are rodents and non-human primates. They include transgenic lines, knockin/knockout animals, viral-vector injections of purified recombinant proteins, toxins, and drug-induced models, among others. Animal models of neurodegenerative disorders are engineered to reproduce key behavioral and molecular aspects of disease pathology. There are several well-established behavioral tests designed to evaluate cognition, learning abilities, and motor function that are based on evolutionary adaptive traits that may be similar between humans and animals. Some genetic-based models of Alzheimer’s disease (AD) and Parkinson’s disease (PD) exhibit robust proteinopathy; however, the behavioral deficits and neuronal loss can be subtle. As no animal model is complete, the research question should guide the choice of which animal model to use [257]. Studies performed in model organisms should be carefully interpreted and the inherited microbiota, genetics, lifespan, and cellular development differences between animals and humans considered. Great efforts are being made to improve animal models of neurodegenerative diseases. In the meantime, researchers in the gut microbiota–brain field should attempt to replicate their findings in multiple disease models and species [256]. This careful approach will ultimately increase the chances of microbiota-based treatments reaching the clinic [3].
Neurodegenerative conditions are characterized by progressive degeneration of selective neuronal populations [10]. The origins of neurodegenerative disorders are mostly unknown, with both genetic and environmental factors contributors [11–14]. There is evidence that potential key factors, including aging, diet, lifestyle choices, and exposure to xenobiotics, can influence the likelihood of developing neurodegenerative disorders. Interestingly, the gut microbiota is capable of interacting with these factors, acting as an intermediate element between the host and the environment in the etiology of neurodegenerative disorders (Figure 2).
Figure 2.
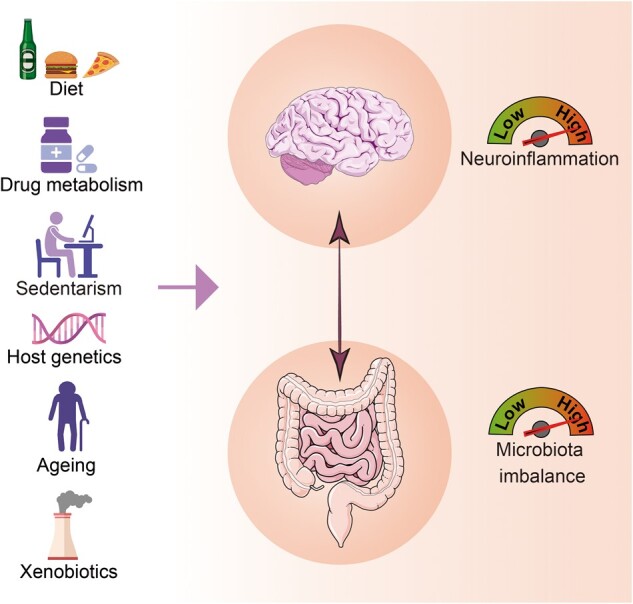
Factors associated with the gut–brain axis and the development of central nervous system diseases. (The images were purchased and adapted from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License, and created with BioRender.com.)
The most prevalent neurodegenerative disorders are characterized by the accumulation of aggregated forms of proteins, e.g. alpha-synuclein (α-syn) and amyloid-beta (Aβ), that have a major impact on disease pathology [15]. Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and more recently Huntington’s disease (HD) are the most widely studied in the context of microbiota gut–brain axis research (Table 1).
Table 1.
Key features of neurodegenerative conditions
| Neurodegenerative condition | Features |
|---|---|
| Alzheimer’s disease (AD) | AD is the most common cause of dementia worldwide. AD is a multifactorial disorder with most cases being sporadic and only 5% of cases are early-onset familial AD. The classic neuropathological hallmarks of AD consist of amyloid-β (Aβ) peptides for plaques and tau for tangles. Drug search efforts have focused on reducing Aβ load in the brains of patients with AD; however, these treatments have failed in slowing mild cognitive impairment or dementia in AD. |
| Amyotrophic lateral sclerosis (ALS) | ALS is a rare neurodegenerative disorder that affects motor neurons that extend from the brain to the spinal cord and to muscles throughout the body. Mutations in the SOD1 gene have been implicated in some cases of familial ALS. There is no treatment that reverses the disease and approved drugs can only slower the progression and ameliorate the symptoms |
| Huntington’s disease (HD) | HD is a genetic neurodegenerative disorder with an autosomal dominance inheritance, caused by the expansion of the trinucleotide (CAG) tandem repeat in the huntingtin (HTT) gene, encoding an expanded polyglutamine tract in the huntingtin protein. There are no disease-modifying therapies so far and the progression of the disease is ∼15 years from diagnosis to death. The symptomatology is complex with the presence of progressive cognitive, psychiatric, and motor impairments |
| Multiple sclerosis (MS) | MS is considered an autoimmune disease that affects the central nervous system (CNS) including brain and spinal cord cells. Here, autoreactive T-cells enter the CNS from the peripheral circulation and induce an inflammatory cascade leading to demyelination. There is no cure for MS. However, FDA-approved medications alleviate exacerbations and accelerate recovery |
| Parkinson’s disease (PD) | PD is the second most common neurodegenerative disorder after AD. The cause of PD is not known, but several genetic and environmental risk factors have been shown to be associated with disease etiology. PD is characterized by classic motor symptoms and the neurodegeneration of dopaminergic neurons of the substantia nigra and reduction of dopaminergic content in the striatum. Another pathological hallmark is the accumulation of aggregates of α-synuclein, in the form of Lewy bodies and Lewy neurites |
Here we will discuss some of the convergent mechanisms by which the gut microbiota may interact with the host to influence the development and progression of neurodegenerative conditions.
The pathways between gut microbiota and nervous system
The gut microbiota synthesizes and sends chemical messengers to its mammalian host [16]. These signals can trigger not only cellular events such as expression of enzymes, receptors, and transcription factors, but also the release of bioactive substances that are critical for regulating the host’s physiology and health [9]. Recently, the impact of the gut microbiota on physiological systems was extended to include their effects on the nervous system [17, 18]. Communication between the gut microbiota and nervous system may be driven by gut–brain axis pathways that include the ENS, vagus nerve neuronal connections, the immune system, and metabolism [1, 3] (Figure 1).
ENS
The ENS is a neuronal network composed of ∼100 million motor neurons, interneurons, and sensory neurons that spread throughout the gastrointestinal tract. These neurons are autonomous and can control motility, fluid homeostasis, and blood flow, in addition to exchanging information in real time between cells of the intestinal epithelium, the immune system, and the CNS [19, 20]. The ENS belongs to an intrinsic part of the autonomic nervous system. In this context, primary intrinsic neuronal afferents of the ENS were shown to propagate stimuli triggered by the gut microbiota to the brain using the vagus nerve and the sympathetic nervous system [21]. Intestinal epithelial cells are laid out in sheets that grow as monolayers and are responsive to gut microbiota in different ways [22]. Among these cells, enteroendocrine cells are believed to be the key cell type connecting the gut microbiota to the nervous system as they act as luminal sensors monitoring the availability of nutrients and the presence of non-nutrient chemicals, toxins, and microorganisms [23]. Furthermore, these cells can form synapses with linked nodose neurons, propagating intrinsic signals within milliseconds [24]. The gut microbiota can directly signal to enteroendocrine cells and induce secretion of circulatory hormones that may cross the blood–brain barrier (BBB) and subsequently affect CNS cells. This communication between the gut microbiota and the ENS is facilitated by the synthesis and release of a number of molecules, including neuropeptides and neurotransmitters (e.g. gamma–aminobutyric acid (GABA), glutamate, active bioamines, catecholamines), cytokines [25], and microbial metabolites [21].
Vagus nerve
The vagus nerve is linked to enteroendocrine cells and may represent the most direct link between the gut microbiota and the brain [23, 26]. Sensory vagal inputs arrive to the nucleus of the solitary tract and are widely spread to areas of the CNS, including the cerebral cortex and medulla oblongata [27]. Roughly 80% of vagus fibers transmit signals to the CNS via afferent pathways and 20% of vagus fibers transmit signals from the CNS to peripheric organs via efferent fibers [28]. The latter is associated with anti-inflammatory reflex, gastric secretions, and gut motility. Vagotomy, a surgery procedure that removes part of the nerve, is a valid tool used in the field to investigate communication between the gut and the brain [27]. In animal models, the use of vagotomy demonstrated that specific bacterial strains use the vagus nerve to communicate with the brain, thereby altering behavior performance [29, 30]. In mice, Lactobacillus rhamnosus JB1 modulates the GABAergic system in the brain and improves anxiety-related and depressive-like behavior via vagus nerve pathways [29]. These findings contextualize the importance of the vagus nerve as a conduit for propagation of the sensory information from the gut microbiota to the brain. In humans, surgical vagotomy is extensively used to treat refractory peptic ulcer disease [31]. In the context of brain health, epidemiological studies involving patients who underwent vagotomy have provided insights into the relative contribution of the vagus nerve to the neuropathology of neurodegenerative disorders, as will be discussed later.
Immune system
The gut microbiota has a crucial role in guiding the development of the immune system and priming the host immune response [32]. Immune-signaling mediators, including cytokines, chemokines, and microbial-associated molecular patterns, can facilitate communication between the gut microbiota and the brain via direct and indirect pathways. As an example, microbial-associated molecular patterns, which include bacterial peptidoglycan and lipopolysaccharide (LPS), components of gram-negative cell walls, are candidates mediating this communication. The host immune system recognizes bacterial LPS through Toll-like receptor 4 (TLR4). Among other cells, TLRs are expressed in microglia, the primary resident-immune cells of the brain. In pathological conditions, LPS can reach the brain via blood circulation and act on central TLRs, initiating a cascade of neuroinflammatory processes [33]. The gut microbiota was also shown to influence microglia maturation and function via bacterial-derived short-chain fatty acids (SCFAs), i.e. acetate, butyrate, and propionate. Compared to conventionally colonized controls, germ-free mice (GF) have increased the numbers of immature microglia in several brain regions [17]. Further, microbial-derived acetate was demonstrated to modulate the microglial metabolic state and histone acetylation in mice [34]. Microbial influences on microglia maturation and function are dependent on host sex [35], which can further explain sex differences in the predisposition for developing certain CNS conditions [36]. The relationship between gut microbiota and the immune system in neurodegeneration will be discussed in detail in the following sequence.
Microbial metabolism
Products of microbial metabolism serve as chemical signals to the host’s cells, affecting cellular function. The gut microbiota influences the metabolism of key molecules that are important for proper neuronal function [29, 34, 37]. In this review, we will focus on SCFAs and tryptophan (Trp) metabolism, which are among the most widely studied microbial metabolites.
SCFAs (i.e. butyrate, propionate, and acetate) are the main products of the bacterial fermentation of indigestible polysaccharides and fibers. They can act locally by communicating with epithelial cells and the ENS or be released into the blood circulation and target the immune and neuronal cells and SCFAs. Within the ENS, SCFAs are involved in epithelium cell proliferation and differentiation, and gene expression among other functions [38]. SCFAs are known for inducing epigenetic modifications, influencing neurotransmission and regulating inflammation [39].
Dietary-derived Trp can be used in the synthesis of serotonin (5-HT) via Trp hydroxylase 1 (TPH1), expressed in enterochromaffin cells. Alternatively, Trp can be metabolized and used as a substrate for the kynurenine pathway to produce the coenzyme nicotinamide adenine dinucleotide (NAD+) [40]. The gut microbiota can also catabolize Trp into kynurenine, kynurenic acid, quinolinate, indole, and indole derivatives, which are key molecules connecting the gut with the immune and nervous systems [41].
Leaky gut
The gut is formed by an epithelial barrier that allows the selective entry of nutrients and metabolites while retaining microorganisms, conferring protection to the host against infections. Thus, the integrity of the epithelial barriers is fundamental for health [25]. The gut microbiota regulates the development and function of the intestinal epithelium barrier using different mechanisms including the expression of epithelial tight junctions and proteins that regulate paracellular diffusion of substances. Microbiota depletion using antibiotics is shown to decrease tight junction protein expression [42]. The intestinal epithelium is dynamically renewed within days and the mucus layer within hours [43, 44]. This capacity for and speed of regeneration are vital because the intestinal epithelium is constantly exposed to environmental challenges, including high amounts of xenobiotic substances [45, 46]. Damage to intestinal barrier properties can increase intestinal permeability, the so-called “leaky gut,” which can lead to the infiltration of pathobionts, or toxic substances that can lead to detrimental effects on the host [47]. For example, gut barrier disruption increases systemic LPS, which in turn activates astrocytes and damages the BBB [45, 48–50]. In certain neurodegenerative disorders, increased intestinal permeability may lead to translocation of bacterial products and/or pro-inflammatory cytokines into systemic circulation, which has the potential to affect the brain [51].
The microbiota gut–brain axis mechanisms in neurodegenerative disorders
The role of the gut microbiota on modulating inflammation in neurodegenerative disorders
Despite different etiologies, AD, PD, MS, ALS, and HD (Table 1) share a common pathological feature about the presence of inflammation—both systemic inflammation and neuroinflammation [52, 53]. Due to particularities of each neurodegenerative disease mentioned, their courses, gravities, and roles of inflammation can vary; however, there is a convergence in the mechanisms responsible for the inflammatory process in these diseases. Furthermore, a tight connection between the gut microbiota and inflammation has been widely demonstrated [54]. The regulation of the immune system by the host microbiota is the most widely studied microbiota gut–brain pathway [2, 55] (Figure 1) and, unsurprisingly, gut inflammation has an impact on brain disorders [56].
The inflammatory pathways involved in AD and the potential for gut microbiota mediation have been explored [57–60]. It has been suggested that inflammation induced by gut microbiota dysbiosis can be a critical driving point for AD pathogenesis [57, 58]. Activation of NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which plays a crucial role in innate immune response, is a rising mechanism of neuroinflammation in AD. More recently, a study in which gut microbiota was transplanted from AD patients to the APP/PS1 mouse model of AD—a transgenic mice line that expresses a chimeric mouse/human amyloid precursor protein—showed that the expression of NLRP3 was increased in the gut of the recipient mice, which was associated with peripheral inflammation, cognitive decline, and neuroinflammation. Following antibiotic treatment and gut microbiota transplantation from healthy donors, the described outcomes were improved [61]. More studies are needed, but there is a potential for therapies targeting the gut microbiota to attenuate neuroinflammation in patients with AD.
Similarly to that of AD, a gut microbiota–brain axis model with an inflammation-driven component has been proposed for PD. This model states that inflammatory triggers could initiate an immune response in susceptible individuals, impacting the gut microbiota, leading to leaky gut and the expression and aggregation of α-syn, which in turn could reach the brain via the vagus nerve. This profile could be associated with systemic inflammation and an increase in BBB permeability along with neuroinflammation and neurodegeneration [62]. A recent meta-analysis of 10 existing 16S microbiota data sets revealed alterations in the gut microbiota composition of PD patients that may be linked to systemic inflammation [63]. Interestingly, the same study found similarities between the differences in the taxa abundance of PD patients and controls with those in other inflammatory conditions, such as MS and inflammatory gastrointestinal diseases. Conversely, a study including 55 PD patients and 56 controls showed that although there was an intestinal inflammatory response in PD, which was correlated with the gut microbiota and disease onset, their systemic inflammatory profile remained the same [64]. A few hypotheses for the mediation of the gut microbiota–brain axis in PD have been raised recently, including the role of microbiota-induced T-cell-driven inflammation on the regulation of α-syn in the gut mucosa and later affecting the brain [65]. Also, a role for the TLR4-mediated inflammation has been pointed out, as a study has demonstrated attenuated gut and brain inflammation, gut and motor dysfunction, and neurodegeneration in rotenone-treated TLR4-knockout mice [66]. Taken together, these studies call for further investigation on the complex communication between the gut microbiota and the immune system in PD.
The link between the gut microbiota and ALS is also being investigated. A recent clinical study involving 19 ALS patients and 9 cohabiting controls (sex-matched and age-aligned) showed differential systemic and intestinal inflammatory profiles in ALS patients as well as the presence of gut dysbiosis with a decrease in butyrate-producing bacteria and the Firmicutes/Bacteroidetes ratio [67]. While interesting and promising, these findings should be interpreted cautiously due to a limited sample size and design limitations. Interestingly, it was shown that mice with a chromosome 9 open reading frame 72 (C9orf72) mutation (the most common genetic variation observed in ALS) had a longer lifespan and less severe disease phenotype depending on their housing facility conditions, explicitly having a more diverse gut microbiota with fewer inflammatory molecules and consequently less systemic and central inflammation, compared with mice without the C9orf72 mutation [68]. Thus, they showed that gut microbiota and associated inflammatory markers could modulate how an ALS-related phenotype manifests in C9orf72-mutated mice. In addition, a long-term study using the transgenic SOD1G93A mouse model of ALS (a mouse line that contains mutations at superoxide dismutase 1 and displays neurodegeneration of spinal motor neurons and motor impairments) identified that gut dysbiosis precedes motor dysfunction, muscle atrophy, and peripheral and central inflammation—all characteristics of the ALS phenotype, with positive correlations between the gut microbiota profile and the immune system [69].
Potential roles for gut microbiota and immune system interactions have also been considered in MS [70, 71]. The administration of antibiotics from weaning to adulthood in an experimental-autoimmune encephalomyelitis (EAE) mouse model induced by administration of myelin oligodendrocyte glycoprotein peptide—which is relevant to MS—delayed the onset of the disease phenotype and decreased systemic inflammation. Conversely, the antibiotic treatment decreased levels of hippocampal brain-derived neurotrophic factor (BDNF), and increased hippocampal inflammatory cytokines, anxiety and depression behavior, and cognitive impairment in the EAE-induced mice [72]. It is important to note that the cocktail of antibiotics used in this study may have crossed the BBB, having a central action per se independent of the effects on the gut microbiota. The relationship between the gut microbiota, inflammation, and MS is promising but very complex and further studies with better and more controlled models of gut microbiota modulation are needed. Central and systemic inflammation markers in HD pathology appear before the onset of classic symptoms [73]. Recently, a study enrolling 33 HD patients and 33 sex-matched and age-matched healthy individuals detected differences in microbiota diversity and composition in HD patients. Further this study revealed positive correlations between Intestinimonas spp. abundance with total functional capacity scores and plasma interleukin 4 (IL-4) levels. As plasma IL-4 levels were decreased in patients with HD, these data suggest a potential role for the gut microbiota and inflammatory response in HD [74]. Further studies should be performed to confirm this relationship and to investigate the presence of gut inflammation in patients with HD.
More studies are needed to clarify the role of inflammation and the position that the gut microbiota plays in neurodegenerative diseases. The potential applications for new and adjunctive therapeutics are tremendous since they expand the therapeutic target possibilities for these devastating diseases.
Microbial–host metabolism interactions in the neurodegenerative disorders
λ SCFAs
The gut microbiota was shown to influence the metabolism of several neuroactive compounds that have been implicated in neurodegenerative disorders, including SCFAs [9]. The contribution of SCFAs to neurodegenerative conditions in clinical settings remains relatively unexplored with only a few human studies examining this relationship. For instance, in PD, individuals’ fecal SCFAs concentrations seem to be lower compared with those of healthy controls [75, 76], which may reflect the decrease in SCFA-producing bacteria (i.e. Faecalibacterium prausnitzii) in their gut microbiota [77]. In MS patients, the concentration of SCFAs producers is depleted in comparison to that of healthy individuals [78]. In line with this, the levels of circulating SCFAs are also lower [79]. A recent study demonstrated that individuals with MS have fewer species and lower levels of genes involved in butyrate production [80]. However, compared with healthy controls, MS patients have increased acetate levels, which correlated with greater disability and increased T helper 17 (TH17) cells [81]. The therapeutic potential of SCFAs to MS was also investigated in a cohort of 300 MS patients and it was demonstrated that treatment with propionate for 14 days was able to restore regulatory T-cell (Treg)/TH17 cells imbalance, normalize Tregs mitochondrial respiration, and improve long-term clinical symptoms [78]. These proof-of-concept studies strengthen the link between the gut microbiota and neurological diseases and highlight the potential use of microbial-based therapies in the management of neurodegenerative disorders. In ALS, the abundance of SCFAs producers in the fecal microbiota is also reduced; however, this does not result in decreased fecal SCFAs concentrations [82]. These discrepancies may be due to genetic heterogeneity of the subjects or differences in dietary habits. Thus, future studies should consider the role of host genetics and diets when investigating microbial–host metabolism interactions.
Mechanistic insights towards understanding how SCFAs participate in neurodegenerative disorders are coming from in vivo animal studies. SCFA concentrations are altered in animal models of AD induced by injection of Aβ [83] and are restored following the administration of Bifidobacterium breve A1—a bacterium that is known to produce SCFAs. One important mechanism by which SCFAs seem to induce neuroprotection is via epigenetic modulation. Sodium butyrate improved motor behavior and altered global H3 histone acetylation and BDNF levels in the neurotoxin-based 6-hydroxidopamine (6-OHDA) model of PD. Similar effects were observed in mice administered with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxic compound known to induce dopaminergic neurodegeneration and PD model [84]. In the G93A mouse model of ALS, treatment with butyrate restored alterations in the gut microbiota composition and intestinal barrier deficits, decreased SOD1G93A protein aggregation in the intestine, and prolonged mice lifespan [85]. The influence of SCFAs in HD is less explored; however, administration of exogenous phenylbutyrate also induced neuroprotection and increased survival in the N171-82Q transgenic mouse model of HD via regulation of epigenetic markers [86]. SCFAs were also shown to modulate neuroinflammation and protein aggregation in animal models of neurodegenerative diseases. For example, SCFAs regulate motor deficits in the Thy1-α-Syn transgenic mouse model of PD via microglia activation and α-syn aggregation [87]. Further, SCFAs influence Aβ plaque formation and the microglial transcriptomic profile, including upregulation of apolipoprotein E in a GF amyloidosis mouse model (APPPS1) relevant to AD [88]. Acetate was also shown to affect microglial metabolism, function, and mitochondria number in the 5xFAD mice model of AD, which is based on the accumulation of high levels of neuronal Aβ42 [34].
These studies show that SCFAs can modulate disease or induce neuroprotection in different animal models of neurodegenerative conditions. Identification of cell-type-specific effects of microbial metabolites is essential for accessing the contribution of the gut microbiota to cell vulnerability to neurodegeneration.
λ Tryptophan metabolism
Several Trp-derived metabolites have been shown to regulate inflammation, with implications for autoimmune and neurodegenerative disorders [41]. One of the largest integrated metabolomics-metagenomic studies revealed that plasma indolelactate, indolepropionate, and its producing bacteria are lower in patients with MS compared with controls [80]. In the EAE mouse model, dietary Trp is required for induction of autoimmune neuroinflammation as mice fed a free-Trp diet have decreased numbers of circulating myelin oligodendrocyte glycoprotein (MOG) 35–55 reactive CD4+ T-cells—a finding that was dependent on the presence of an intact gut microbiota as antibiotics-treated mice do not exhibit the phenotype [89]. In a separate study using the EAE mouse model, supplementation with indoxyl-3-sulfate, a microbial metabolite produced by dietary Trp, activated microglia and elevated transforming growth factor alpha (TGF-α) and vascular endothelial growth factor beta (VEGF-β) production, which in turn modulated astrocyte activity and CNS inflammation through a mechanism mediated by the aryl hydrocarbon receptor (AhR) [90]. AhRs signaling is largely involved in peripheral immune system activation, maintenance of the gut barrier integrity, and host defense against pathogens and inflammatory insults [91]. In the CNS, the AhR pathway impacts microglia and the function of astrocytes, and participates in adult neurogenesis [91]. Alterations in the Trp metabolism have been linked to cognitive decline in AD patients [92]. A recent study that investigated alterations in fecal metabolites showed a decrease in hydroxytryptophan and DL-5-methoxytryptophan in AD patients’ samples in comparison to healthy controls’ samples [93]. In the same study, the abundance of indole derivatives that participate in AhR signaling were also decreased in the AD fecal metabolome whereas kynurenine levels were unchanged [93]. In line, indolamine 2,3-dioxygenase (IDO), a rate-limiting enzyme in the conversion of Trp to kynurenine, is increased in AD and inhibition of this IDO ameliorated anxiety and depression-like behaviors in 3xTg-AD mice, a triple-transgenic mouse line that exhibit both Aβ and tau pathology [94]. Similarly, Trp metabolism is altered in PD individuals and in animal models of disease [95]. Analysis of a publicly available microbiota data set of PD patients and healthy controls revealed an increase in indole-producing bacteria (i.e. Akkermansia, Alistipes, and Porphyromonas) in PD patients [96–98]. In a rat model of PD induced by rotenone, dietary Trp improved motor deficits and attenuated neurodegeneration and neuroinflammation via AhR activation [99]. Thus, the gut microbiota effect on Trp metabolism may have a significant impact on neurodegenerative disorders and warrants further investigation.
The vagus nerve at the interface of the microbiota and neurodegeneration
The vagus nerve is strategically located in the body; thus, it has become an emerging focus of studies that attempt to explain the origins of neurodegenerative disorders. In this context, Braak’s hypothesis postulates that an unknown pathogen (virus or bacterium) present in the gut could trigger the propagation of α-syn pathology from the gut to the brain, implicating the gut in the origins of sporadic PD and other synucleinopathies [100]. PD is associated with bilateral atrophy of the vagus nerve where viscero-afferent and viscero-efferent vagal fibers, but not of the spinal accessory, are affected [101]. Two epidemiological cohort studies demonstrated that patients undergoing complete truncal vagotomy have a reduced risk of developing PD, highlighting the critical involvement of the vagus nerve in this pathology [100, 102, 103].
Gut-to-brain propagation of pathogenic forms of α-syn via the vagus nerve was demonstrated in different animal models of PD [104–106]. In a study, injection of α-syn fibrils into the duodenal and pyloric muscularis layers in mice allowed pathogenic forms of alpha-synuclein to reach the lower brainstem and other brain areas that control motor behavior function via the vagus nerve [105]. The Braak staging hypothesis of PD is still a matter of extensive debate and investigation, and disease spread may occur in different ways depending on the PD subtype. Given that the gut microbiota signals the brain via the vagus nerve and microbiota perturbations may be implicated in PD, it is plausible that this dynamic interplay has an impact on PD’s onset in some cases, but not in others. However, this hypothesis needs to be further investigated.
Although autonomic nervous system alterations have been described in other neurodegenerative conditions, there is scarce evidence demonstrating the role of the vagus nerve in AD, ALS, MS, and HD. Interestingly, vagus nerve stimulation is approved for treating refractory depression and anxiety [107, 108]. Further, vagus nerve stimulation has shown beneficial effects on improving cognition—a finding that is particularly relevant to AD, as this is the most prominent cognitive disorder in aging. In this sense, in a small cohort of patients with AD, vagus nerve stimulation was shown to ameliorate cognitive deficits [109] and/or prevent cognitive decline [110]. Likewise, vagus nerve stimulation showed beneficial effects for MS patients [111]. A study involving 10 patients with ALS detected atrophy in the cervical nerve roots and peripheral nerves [112]. Although preliminary, these studies call for an investigation into the role of the gut microbiota on mediating gut–vagus nerve–brain communication in neurodegeneration.
The gut microbiota during aging
Aging is one of the major contributors of neurodegenerative disorders [113]. To illustrate this, it is estimated that one in nine individuals aged ≥65 years has AD [113]. Among other factors, aging can be influenced by host genetics, lifestyle, diet, environment, and the gut microbiota [114]. Several independent studies using 16S sequencing and metagenomics analysis demonstrate that the gut microbiota structure undergoes significant transformation during aging. For example, in a study that investigated the gut microbiota composition of semi-supercentenarians (≥105 years old) and compared it to adult (22–48 years old), elderly (65–75 years old), and centenarian (99–104 years old) microbiota, it was found that longevity is linked to changes in the microbial ecosystem with enrichment of certain taxa [115]. The gut microbiota of centenarians and supercentenarians is characterized by high abundance of health-associated microorganisms such as Bifidobacteria and Christensenella [116]. In contrast, others observed a decrease in Clostridiales and Bifidobacterium, and enrichment in Proteobacteria and Enterobacteriaceae in frail, aged individuals [117]. Although these findings seem to be replicated in independent cohorts, the microbial signature seems to change according to geographical and other individual factors [116, 118].
The gut microbiota composition varies according to lifestyle and diet as individuals age. This concept has been further investigated using different approaches [116]. A study that evaluated the microbiota composition of ELDERMET cohort individuals revealed that diets rich in sugars and fats are linked to a frailer microbiota [116]. In a more recent study investigating the gut microbial patterns of 9,000 individuals from three independent cohorts aging from 18 to 101 years, it was observed that a more unique compositional gut microbiota status was associated with a decrease in low-density lipoprotein cholesterol, higher vitamin D, and lower triglycerides [118]. Altogether, these studies show that the maintenance of healthy aging may depend on the interplay between gut microbes, host dietary habits, and metabolism. Aging is also associated with chronic low-grade inflammation, known as “inflammaging” [119]. Age-related changes in microbial composition affect the senescence of immune cells and the entire cellular immune network [116]. It is important to note that a wide range of chronic and metabolic diseases is associated with aging. These comorbid conditions can have an impact on the gut microbiota and on longevity per se. A recent study compared the gut microbiota and metabolism profiles of young (20–29 years old), middle-aged (40–59 years old), and older individuals (≥60 years old) with five diseases (inflammatory bowel disease, type II diabetes, intestinal polyps, colorectal cancer, and liver cirrhosis) with those of healthy individuals with similar ages. The authors demonstrated that the gut microbiota of older individuals with type II diabetes, colorectal cancer, and intestinal polyps was the most affected. Taken together, these studies demonstrate that diet and disease status are important co-variates for aging-linked microbiota associations [120].
The gut microbiota can influence both the immune system and the metabolome in aged mice. For instance, microbiota transplants from young (3–4 months) mice to aged hosts (19–20 months) reversed aging-associated differences in the hippocampus metabolome and attenuated peripheral and CNS inflammation [121]. The maturation of microfold cells (M-cells) in the intestine is important for appropriate immunoglobulin A (IgA) response and can deteriorate with aging [122]. This line of defense helps to protect us against invading pathogens [122]. Importantly, the decline in M-cell maturation during aging can be reversed by housing aged mice on used bedding of young mice or exposing them to bacterial flagellin [123]. A better understanding of the role of the gut microbiota during healthy aging and frailty may help in preventing the development and progression of neurodegenerative disorders.
Xenobiotics exposure, gut microbiota, and neurodegenerative disorders
The gut microbiota is viewed as one of the main surfaces of contact with the environment and is constantly receiving stimulus from it. Recent research demonstrated that exposure to xenobiotics (exogenous substances that are not recognized by the body including pesticides, pollutants, pathogens, and drugs) can alter the gut microbiota composition [124]. In return, gut bacteria can bio-transform and metabolize exogenous substances [124]. There is growing concern that exposure to xenobiotics may affect human health and lead to neurodegenerative conditions [125, 126]. Exposure to xenobiotic substances alters the gut microbiota composition in animal models of neurodegenerative conditions. For instance, prolonged exposure to rotenone, a commonly used pesticide known to induce mitochondrial dysfunction and PD in animals [127], was found to increase Bifidobacterium spp., mucosal thickening, and goblet cell hyperplasia in adult rats [128]. Nonetheless, the detrimental effects of pesticides and other xenobiotics to the microbiota and to the brain may depend on the dose and age of exposure [129]. Early-life exposure to chlorpyrifos, an organophosphorus insecticide that inhibits acetylcholinesterase thereby increasing the concentration of acetylcholine, is linked to AD [130] and can alter the relative abundance of several bacterial taxa including Anaerobranca, Borrelia, Brevundimonas, Nitrincola, Paracoccus, and Vogesella, while changing the cholinergic system and the GABAergic systems in the mouse brain [131]. Thus, the gut microbiota may contribute to chemical transformation of environmental toxins modulating its effects. As the use of xenobiotics substances is increasing worldwide, more research is needed to fully understand the impact they could have to brain health to help in informing policy makers.
Exposure to pathogens, including bacteria, viruses, and even fungi that trigger the immune system, is associated with increased risk of neurodegenerative diseases [132–135]. For example, intestinal infection with gram-negative bacteria including Citrobacter rodentium in a genetic model of PD, Pink1−/− mice, engages mitochondrial antigen presentation and autoimmune mechanisms that elicit the establishment of CD8+ T-cells in the periphery and in the brain [136]. The epsilon toxin produced by Clostridium perfringens, a gram-positive, spore-forming enteric pathogen, was found to be significantly higher in the serum of MS patients than in that of healthy individuals [137]. Interestingly, common MS treatments such as Fingolimod-related compounds can inhibit the in vitro growth of C. perfringens [138]. More recently, a large longitudinal study involving US military recruits demonstrated that Epstein-Barr virus infection is strongly associated with MS as it increases the chances of developing MS by 32-fold. It is plausible to think that certain gastrointestinal infections may affect the gut–brain axis, influencing neuropathology. However, the long-term effects of infections to neurodegenerative disorders are difficult to disentangle and remain to be fully elucidated.
The gut microbiota was also shown to affect the metabolism of drugs that are used for ameliorating the symptoms of PD. For example, Levodopa (L-dopa), the primary medication used to treat PD, is decarboxylated into dopamine by tyrosine decarboxylase of Enterococcus faecalis [139] and also converted into meta-tyramine by dopamine dehydroxylase of Eggerthella lenta A2 [140]. Additionally, L-dopa accumulates in the surface of Helicobacter pyloriin vitro [141], which may be linked to a lower concentration of L-dopa in PD patients and to motor symptom fluctuations [142]. The complex interactions between gut microorganisms and drugs that are used for the management of neurodegenerative conditions need further investigation. Perhaps, in the future, the gut microbiome could potentially be explored for increasing therapeutical effects of pharmaceutical drugs.
Diet and gut microbiota
Diet is one of the major modulators of the gut microbiota composition, directly interacting with the gut microbial population to modulate the gut community within days of intervention [143, 144]. Diet can affect microbiota diversity and the abundance of different types of bacteria that will reflect changes in the production of metabolites, protect the intestinal barrier, and ultimately influence the host health [145]. The effects of diet on the gut microbiota have been investigated not only in healthy individuals [146], but also in the context of aging [147] and various diseases, including neurodegenerative diseases [148, 149]. A promising role for diet intervention in neurodegenerative diseases has been hypothesized, especially for AD and PD [150–155].
Amongst the different diets, the Mediterranean diet is considered one of the most balanced options, with no caloric restriction and increased intake of fibers, antioxidants, polyunsaturated fatty acids (PUFAs), and polyphenols (found in fruits, vegetables, legumes, cereals, olive oil, nuts), and low consumption of meat, saturated fatty acids, low or moderate intake of fish, dairy products, and alcohol [156]. The Mediterranean diet positively modulates the gut microbiota [157] and a systematic review and meta-analysis found that the Mediterranean diet associates inversely with cognitive disorders [158], with great potential for neurodegenerative diseases. A study involving a large cohort of non-frail or pre-frail with 602 individuals from five European countries demonstrated that anti-inflammatory effects of the Mediterranean diet paralleled the increased SCFAs production by the gut microbiota [159], with great potential for neurodegenerative diseases. A randomized, double-blind, cross-over pilot study in older adults at risk for AD (11 subjects with and 6 subjects without cognitive impairment) comparing a modified Mediterranean-ketogenic diet (MMKD) with the American heart association diet (AHAD) found that MMKD modulated the gut microbiota and SCFAs associated with AD markers in subjects with mild cognitive impairment [160]. A ketogenic diet is characterized by high fats, moderate proteins, and very low carbohydrates [161]. Similarly, in another pilot study, the same group showed that the combination of these two diets (MMKD and AHAD) was able to modulate the gut mycobiome in association with AD markers and uncovered an association between mild cognitive impairment with a fungal–bacterial co-regulation network [162].
Polyphenols (phytochemicals found in fruits and vegetables) are not only a significant part of the Mediterranean diet, but have also been reported to have their own beneficial effects on aging and on several diseases, including neurodegenerative diseases [163, 164]. The gut microbiota plays a central role in the conversion of dietary polyphenols into phenolic acids [165] and these were shown to increase gut microbiota diversity, decrease the Firmicutes/Bacteroidetes ratio, and modulate metabolic activity, leading the gut microbiota to a healthier profile [166, 167]. The administration of lemon polyphenols in drinking water improved aging-related scores and locomotor activity, and increased the ratio of Bacteroidetes/Firmicutes senescence-accelerated mouse prone 1 (SAMP1) [168]. Curcumin, a polyphenolic substance, improved cognition and reduced amyloid plaque in APP/PS1 mice while modulating the relative abundance of bacteria involved in AD development [169]. Mediated by the gut microbiota, polyphenols were already shown to effectively attenuate AD Aβoligomerization [170] and the gut microbiota has been hypothesized as one of the potential contributions for resveratrol in mediating neuroprotection and promoting cognition. While the impact of the gut microbiota on ALS and the potential benefits of polyphenols seem promising [171], so far, no study has directly investigated them.
The omega-3 PUFAs include eicosapentaenoic acid (EPA), alpha-linolenic acid (ALA), and docosahexaenoic acid (DHA). Besides their well-known effects of contributing to membrane integrity, cell signaling, an anti-inflammatory state, and neurotransmission [172], they have also been shown to influence the human gut microbiota towards a healthier profile by promoting commensal and SCFA-producing bacteria, decreasing inflammation, and by modulating the intestinal–blood barrier [173, 174]. In mice, plant-derived ALA attenuated aging-linked thrombosis by modulating trimethylamine-N-oxide (TMAO; an important gut microbial metabolite) and SCFAs [175]. Interestingly, a decrease in peripheral and central omega-3 fatty acid levels is observed in patients with AD, which was shown to be linked to disease progression [176, 177]. A systematic review described beneficial effects of omega-3 supplementation to mild cognitive impairments at the prodromal AD stage [174]. While promising, the direct effect of the gut microbiota in this process has not been investigated.
A preliminary small case–control study assessed the gut microbiota of 54 PD patients (16 of them submitted to a bowel-cleansing procedure for 8 days plus an ovo-lacto vegetarian intervention for 14 days and 6 of them submitted to the diet intervention only) and 32 healthy individuals, and found an effect of the combined intervention on the clinical PD outcomes and in the gut microbiota profiles of the patients [178]. However, this study must be interpreted cautiously due to the small sample size, the significant age difference between the groups, and the lack of proper controls, including an enema-only group. The beneficial effects of diet mediated through the microbiota must be confirmed in both preclinical and clinical studies.
Recently, a study found that differences in gut microbiota composition between patients with autism spectrum disorder and neurotypical individuals were mostly due to reduced dietary diversity [179]. As neurodegenerative disorders are debilitating conditions that can have drastic effects on diet, it is important to consider dietary data when analysing and interpreting findings in this field. There is a critical need for randomized, double-blind, controlled clinical trials, with adequate sample sizes, adequate controls, and gut microbiota analysis for the diet interventions.
Exercise and gut microbiota
Physical exercise has been demonstrated to have positive effects on general health and on preventing and improving a wide range of diseases, including brain disorders [180]. Exercise acts as whole-body therapeutics, with its benefits targeting various organs and biological systems, including the gut microbiota [181]. A systematic review including 25 studies found that exercise was associated with changes in gut microbial composition, with a specific increase in butyrate-producing bacteria and fecal butyrate levels in humans and rodents [182]. Additionally, a more recent systematic review included 18 clinical studies with healthy adults and identified a positive association between high levels of exercise and fecal bacterial alpha diversity and fecal SCFAs concentrations [183]. Of the beneficial effects of exercise on health and disease, the impact on the brain is remarkable, with prominent cognitive enhancement induced by exercise observed in a wide range of brain disorders [181]. Not surprisingly, the gut microbiota was already suggested as the possible link between exercise and neurodegenerative diseases [184].
Recently, a direct link between exercise and microbiota gut–brain communication has been proposed, with the immune system acting as an essential modulator [184]. This is a topic of increased relevance for AD [185]. Using the Aβ1–40 mouse model of AD, mice were subjected to a 4-week treadmill exercise, which could prevent depressive-like behavior, gut dysfunction, and the increase in the Firmicutes/Bacteroidetes ratio induced by the AD model [186]. Interestingly, young (but not middle-aged) APP/PS1 mice exposed to 16 weeks of running wheels exhibited an increase in cognition and an improvement in their gut microbiota profiles, indicating a need to start exercise intervention earlier, in a pre-symptomatic phase, to prevent AD-associated decline [187]. Another possibility of intervention is the combination of exercise and probiotics. Rats injected with Aβ1–42 as an AD model were subjected to a probiotic mixture and moderate-intensity interval training using a rodent treadmill for 8 weeks and the study demonstrated neuroprotective effects of the combined intervention on cognition neurodegeneration synaptic plasticity [188]. Similarly, in the APP/PS1 mouse model, a mixture of probiotics and an interval treadmill running protocol was administered for 20 weeks; this study showed that the combination of interventions improved cognition and markers of neurodegeneration, and that exercise was able to shift the gut microbiota profile observed in the AD mice, mainly towards butyrate-producing species [189].
Exercise in PD, ALS, MS, or HD has been widely explored and its promising beneficial effects have been demonstrated [190–197]. A systematic review and meta-analysis of randomized–controlled trials including 15 studies indicated that exercise could improve muscle strength in patients with PD and has the potential to improve movement and quality of life [198]. Exercise has also been shown to improve motor and non-motor outcomes of PD in both human and rodent models, including neuroplasticity and cognition [199, 200]. In ALS, a systematic review and meta-analysis including 322 patients showed increased functionality and vital capacity with no effect on muscle strength or quality of life [195]. A more recent systematic review corroborated the effect of exercise on functional abilities in patients with MS in the short, medium and long terms [201]. In the context of HD, a preclinical model of HD exercise was able to delay the onset of the disease and cognitive impairment [202]. Recently, a study revealed the main bacterial signature (the orders of Bacteroidales, Lachnospirales, and Oscillospirales) that discriminates the effect of exercise and environmental enrichment in wild-type and HD mice, suggesting a promising role for the gut microbiome in mediating the positive effects of these interventions in HD [203].
For MS, a systematic review including 54 studies indicated that exercise could increase aerobic capacity and muscular strength as well as improve health-related quality of life, mobility, and fatigue [204]. The effects of exercise on brain function in patients with MS are also promising [205], including on cognition [206]. A few studies have investigated the role of the gut microbiota in mediating the beneficial effects of exercise in MS. A study investigating the effect of five sessions of home-based exercise (or no exercise) for 6 months in 42 people with MS showed that the exercise intervention eased anxiety and depression scores as well as modulated the gut microbiota profile, especially towards increasing Prevotella and decreasing Akkermansia muciniphila [207]. Still, in an animal model of EAE, 4 weeks of strength exercise training before the immunization and 7 days after showed that the exercise intervention increased the gut microbiota abundance and diversity, decreased the Firmicutes/Bacteroidetes ratio, intestinal permeability, and SCFA-producing bacteria, and reduced the TH17 and Treg immune responses. Also, when mice received fecal microbiota transplantation from exercised mice, lower disease severity and neuropathology phenotype were observed [208].
While some mechanisms for the positive effect of exercise on these neurodegenerative diseases have been pointed out in the studies mentioned above, the role of the gut microbiota in mediating this effect is still poorly explored.
Microbiota-based interventions
Probiotics
Probiotics are live microorganisms (e.g. Lactobacillus spp., Bifidobacterium spp.) that, when administered in adequate amounts, confer a health benefit to the host [209]. While they can be delivered in food as supplements or drugs, their efficacy or ability to successfully engraft has been a topic of concern [145, 210]. Their proposed mechanisms of action include modulation of the immune system, support of microbiota stability, production of SCFAs, and improvement in the gut barrier function, amongst others [211]. Several studies have analysed the role of probiotics in human health. The compilation of 313 trials, including 43,826 participants, has identified an overall positive benefit, from gastrointestinal to cardiometabolic parameters [145]. Not surprisingly, the role of probiotics in the context of neurodegenerative disorders has been explored and neurological factors, endocrine pathways, and immune signals have been suggested as their main potential mechanisms of action [212, 213].
A recent review summarizing the clinical data has revealed a promising role in using probiotic interventions for cognition, inflammation, and oxidative stress in AD patients and individuals with middle cognitive impairment [214]. A randomized, double-blind, and controlled trial with 60 AD patients (30 receiving milk and 30 receiving a mixture of probiotics in milk for 12 weeks) showed a positive effect of probiotics on cognitive and metabolic outcomes [215]. In a smaller, uncontrolled randomized trial, 20 AD patients received a mixture of probiotics for 28 days and cognitive, microbial, and inflammatory outcomes were tested in comparison with their baselines before the intervention, leading to the discovery of changes in the gut microbiota and the Trp metabolism in serum [216]. On the other hand, a randomized, double-blind, and controlled trial with 46 AD patients administered a probiotic mixture for 12 weeks did not find any significant difference in any of the outcomes measured that included cognitive, inflammatory, and oxidative biomarkers [217]. Still, a recent systematic review and meta-analysis revealed an effect of probiotics on cognitive function in people with mild cognitive impairment and to a lesser extent in AD patients, associated with the number of microorganisms added to the probiotic mixture, the dosage and duration of the intervention, and the severity of the disease [218].
Preclinical studies have corroborated this promising effect of probiotics on cognition in AD animal models (reviewed in [84]) [219]. Using an intracerebroventricular injection of the Aβ AD rat model, a mixture of probiotics was administered orally for 56 days and was shown to alleviate spatial cognitive performance, restore synaptic plasticity, and balance the antioxidant/oxidant biomarkers [220]. APP/PS1 double-transgenic mice fed standard chow or a high-fat diet received A. muciniphila or vehicle orally for 6 months. Apart from the metabolic improvement that A. muciniphila showed, reduction of Aβ deposits in the cortex of APP/PS1 mice and improved spatial cognitive outcomes and anxiety-related behavior of these mice were also demonstrated [221]. Using the same AD animal model, a study recently showed that 12 weeks of co-treatment of Lactobacillus plantarum with memantine potentiated the effect of the latter by decreasing hippocampal Aβ deposits, improving neuronal plasticity, ameliorating neuroinflammation, attenuating cognitive impairments, and promoting the reduction in TMAO [222]. Also, supplementation with SLAB51 probiotic formulation for 10 weeks was able to counteract the cognitive decline observed in these mice as well as brain damage and gut hormone plasma levels in 3xTg-AD mice [222]. When administered for 16 weeks, this formulation showed a reduction in oxidative stress in AD mice via the activation of Sirtuin 1 (SIRT1)-dependent mechanisms [223] and ≤48 weeks of administration could restore impaired glucose metabolism [224]. In addition, probiotics (VSL#3) have also been shown to alleviate intestinal pathophysiology [225] and to increase SCFA levels in the AppNL-G-F mouse, a knockin mouse model of AD [226].
A role for probiotics in PD has also been suggested and recently reviewed [227–229]. A randomized placebo-controlled trial recruiting 48 PD patients (22 receiving a multi-strain probiotic and 26 receiving placebo over 8 weeks) demonstrated an improvement induced by probiotics in gut motility and constipation in PD patients, which are common and very incapacitating non-motor symptoms of PD [230]. Still, a randomized, double-blind, placebo-controlled trial administered either a mix of probiotics (30 PD patients) or placebo (30 PD patients) for 12 weeks. It showed a decrease in motor and non-motor implications of PD as well as in metabolic outcomes related to PD, including oxidative stress, insulin levels, and resistance [231].
In the context of the effects of probiotics in MS, a systematic review (five animal and two human studies included) has pointed to an overall beneficial effect of probiotics in MS, mainly on the immune system, with ultimate alleviation, prevention, and ability to delay the onset of the disease [232]. More recently, a meta-analysis of three clinical studies (173 MS patients receiving probiotics) has found an overall effect on mental health, insulin resistance, and inflammatory and oxidative stress markers [233]. They performed a systematic review of 22 preclinical studies revealing positive effects of probiotics in the progression of MS, including decreasing incidence, and ameliorating the severity of the disease and motor impairment [233]. This study also identified that formulation SLAB51 administered for 2 weeks prior and 3 weeks after 6-OHDA injection in mice protected dopaminergic neurons and improved behavior outcomes relevant to PD [228].
Similarly, a mixture of probiotics was administered for 4 weeks prior to the MPTP and rotenone injection. Probiotics prevented dopaminergic neurodegeneration and behavioral impairments while attenuating glial activation induced by both PD models [234]. Using the MPTP mouse model of PD, Clostridium butyricum was administered for 4 weeks after the MPTP treatment and was also able to improve motor deficits, dopaminergic neurodegeneration, and glial activation. Importantly, this study showed that probiotic interventions could reverse MPTP-induced dysbiosis and suggested a role for the glucagon-like peptide-1 (GLP-1) pathway [235].
In addition, a comprehensive review systematically evaluated 6 human and 22 animal studies of probiotics in MS and has pointed to VSL#3, Lactobacillus paracasei, Bifidobacterium animalis, Escherichia coli Nissle 1917, and Prevotella histicola as the most promising candidates [236]. The administration of P. histicola showed an inverse correlation with the severity of MS and potential ability to suppress the phenotype in preclinical models [237].
As previously mentioned, there are numerous benefits of the use of probiotics for neurodegenerative diseases; however, there is still not a consensus regarding formulation, the use of multi-strain probiotic vs one strain only, the dose, or even the treatment length. The field would greatly benefit from research focused on clarifying these points.
Prebiotics and symbiotics
A prebiotic is defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit,” usually carbohydrate-based, and other substances such as polyphenols and PUFAs when converted to their respective conjugated fatty acids [238]. Their mechanisms of action include microbial growth and improvement in bowel habit, production of SCFAs, and reduction in pH, altogether leading to the production of antimicrobial agents and consequent defense against pathogens, reduced inflammation, and increased satiety [211]. The general health benefits promoted by prebiotics vary from alleviating constipation, irritable bowel syndrome, and metabolic disease to psychiatric and neurodegenerative disorders [213, 238, 239]. Symbiotics, in turn, are a combination of prebiotic and probiotic ingredients [240] that act synergically with the specific prebiotic compound(s), favoring the probiotic organism(s) implantation, survival, and ultimately overall beneficial effect [241]. However, less is known about the impact of these combined compounds on host physiological markers of health compared with those of probiotics and prebiotics separately.
A few studies have investigated the effect of prebiotics on AD [219]. An oligosaccharide from Morinda officinalis was administered to APP/PS1 transgenic mice for 6 months, demonstrating positive effects on cognitive impairment, neurodegeneration, and Aβ deposits while also modulating the gut microbiota [242]. Using the same APP/PS1 AD-like mice model, fructooligosaccharides were administered for 6 weeks. Through the GLP-1 pathway, fructooligosaccharides ameliorated the observed cognitive deficits and pathological processes related to AD and the gut microbiota profile [243]. Lactulose and trehalose were administered to mice before a bilateral intrahippocampal CA1 injection of oligomeric Aβ25−35, which attenuated cognitive impairment induced by the model as well as neuroinflammation, with lactulose showing promising neuroprotective effects of relevance to AD [244]. Still, Aβ1−42 AD-like mice were treated with the yeast beta-glucan, which modulated the gut microbiota and ameliorated cognitive deficits, neuroinflammation, and brain insulin resistance [245]. In addition, 8 weeks of treatment with the prebiotic mannan oligosaccharide was successful in positively modulating several outcomes observed in the 5xFAD transgenic AD-like mouse model, including brain-related effects such as cognition, anxiety, Aβ accumulation, neuroinflammation, oxidative stress, the hypothalamic–pituitary–adrenal axis, and gut microbiota associated with improvement in the gut microbiota profile, intestinal barrier integrity, and the production of SCFAs [246].
In PD studies, prebiotics targeting butyrogenic bacteria, modulating the inflammatory response and improving gut integrity, have been proposed to have promising potential to alleviate the disease [247]. However, only a few studies have addressed this point and it is essential to note that butyrate also acts as a histone deacetylase inhibitor, having an epigenetic role [248]. While oral administration of sodium butyrate was already shown to positively modulate the PD motor and non-motor phenotypes [249, 250], this delivery method does not explicitly affect the gut microbiota since it is mostly absorbed in upper segments of the gut tract [251]. Among the non-motor symptoms of PD, constipation has been one of the most explored symptoms, with a systematic review pointing to the use of prebiotics as being promising for patients’ quality of life [252]. A randomized, double-blind, placebo-controlled trial was performed in PD patients with constipation who received either fermented milk (80 patients), containing both probiotics and prebiotics, or a placebo (40 patients) for 4 weeks, showing a positive effect on constipation outcomes [253]. Using the preclinical rat model of 6-OHDA, the probiotic AP-32 (considered prebiotics by the authors but also containing other molecules including SCFAs), the residual medium of the probiotics, and the symbiotic effect of both were evaluated over 8 weeks. They show a significant prevention of dopaminergic loss, improvement of motor function, restoration of fecal SCFAs levels, and mitochondrial and energy metabolism, with the probiotic AP-32 intervention being superior to the general outcomes [254].
In the context of ALS, the prebiotic oligosaccharide was administered in the SOD1G93A transgenic mouse model of ALS for 10 weeks and this showed an effect in delaying the onset of the disease and prolonging the lifespan of the ALS mouse [255]. Preclinical and clinical evidence on the beneficial activities of prebiotics and/or symbiotics in neurodegenerative diseases is still limited. Similarly to probiotics, there is no consensus on prebiotics and symbiotic formulation, dose, or treatment length.
Conclusions and future perspectives
Despite huge research efforts, the cause of the various neurodegenerative disorders is mostly unknown with genetic and environmental contributors. As noted in this review, the gut microbiota may act as an intermediate factor between the host and the environment, affecting key aspects in the neurodegeneration process such as inflammation and protein homeostasis. However, a causal role for the gut microbiota in neurodegeneration is missing. Many of these challenges are rooted in the limitation of translating animal findings to humans (Box 1). Several animal models have been used to study neurodegenerative conditions, yet they fail to capture the complexity of multifactorial disorders. Neurodegenerative disorders result from progressive damage and loss of neuronal function. Thus, human studies involving larger longitudinal cohorts are needed to understand the relative contribution of the gut microbiota to neuropathology. A better understanding of the role of the gut microbiota in neurodegenerative disorders is essential and may lead to the discovery of new therapeutical targets and/or disease-modifying strategies.
Authors’ Contributions
All the authors contributed substantially to discussions of the article content and to the writing. L.H.M. conceived of the idea and coordinated the study. All authors read and approved the final manuscript.
Funding
C.G. is a Hereditary Disease Foundation (HDF) Fellow. L.H.M. is a postdoctoral scholar at Caltech and recipient of the American Parkinson’s Disease Association postdoctoral fellowship.
Conflict of Interest
None declared.
References
- 1. Margolis KG, Cryan JF, Mayer EA.. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 2021;160:1486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cryan JF, O'Riordan KJ, Cowan CSM. et al. The microbiota-gut-brain axis. Physiol Rev 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 3. Morais LH, Schreiber HL, Mazmanian SK.. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol 2021;19:241–55. [DOI] [PubMed] [Google Scholar]
- 4. Kim E, Paik D, Ramirez RN. et al. Maternal gut bacteria drive intestinal inflammation in offspring with neurodevelopmental disorders by altering the chromatin landscape of CD4+ T cells. Immunity 2022;55:145–58.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morais LH, Golubeva AV, Moloney GM. et al. Enduring behavioral effects induced by birth by caesarean section in the mouse. Curr Biol 2020;30:3761–74.e6. [DOI] [PubMed] [Google Scholar]
- 6. Sgritta M, Dooling SW, Buffington SA. et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019;101:246–59.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharon G, Cruz NJ, Kang D-W. et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in Mice. Cell 2019;177:1600–18.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vuong HE, Pronovost GN, Williams DW. et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020;586:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Needham BD, Kaddurah-Daouk R, Mazmanian SK.. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 2020;21:717–31. [DOI] [PubMed] [Google Scholar]
- 10. Dugger BN, Dickson DW.. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 2017;9:a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobs BM, Noyce AJ, Bestwick J. et al. Gene-environment interactions in multiple sclerosis: a UK Biobank study. Neurol Neuroimmunol Neuroinflamm 2021;8:e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon DK, Tanner CM, Brundin P.. Parkinson disease epidemiology, pathology, genetics and pathophysiology. Clin Geriatr Med 2020;36:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spires TL, Hannan AJ.. Nature, nurture and neurology: gene–environment interactions in neurodegenerative disease. Febs J 2005;272:2347–61. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Yang F, Zhang S. et al. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Parkinsons Dis 2021;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soto C, Pritzkow S.. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 2018;21:1332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fischbach MA, Segre JA.. Signaling in host-associated microbial communities. Cell 2016;164:1288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erny DH, de Angelis AL, Jaitin D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoban AE, Stilling RM, Ryan FJ. et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016;6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joly A, Rousset R.. Tissue adaptation to environmental cues by symmetric and asymmetric division modes of intestinal stem cells. IJMS 2020;21:6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Philip V, Bercik P.. Gastrointestinal microbiota and the neural system. In: MH Floch, Y Ringel, W Allan Walker (eds). The Microbiota in Gastrointestinal Pathophysiology. Boston: Academic Press, 2017, 243–7. [Google Scholar]
- 21. Muller PA, Schneeberger M, Matheis F. et al. Microbes modulate sympathetic neurons via a gut-brain circuit. Nature 2020;583:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dutton JS, Hinman SS, Kim R. et al. Primary cell-derived intestinal models: recapitulating physiology. Trends Biotechnol 2019;37:744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Latorre R, Sternini C, Giorgio R D. et al. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 2016;28:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaelberer MM, Bohórquez DV.. Where the gut meets the brain. Brain Res 2018;1693:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chelakkot C, Ghim J, Ryu SH.. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 2018;50:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fülling C, Dinan TG, Cryan JF.. Gut microbe to brain signaling: what happens in Vagus…. Neuron 2019;101:998–1002. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Forsythe P, Vagotomy and insights into the microbiota-gut-brain axis. Neurosci Res 2021;168:1 20–7. [DOI] [PubMed] [Google Scholar]
- 28. Bonaz B, Bazin T, Pellissier S.. The Vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018;12:49, 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bravo JA, Forsythe P, Chew MV. et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011;108:16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buffington SA, Di Prisco GV, Auchtung TA. et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016;165:1762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lagoo J, Pappas TN, Perez A.. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014;207:120–6. [DOI] [PubMed] [Google Scholar]
- 32. Fujimura KE, Sitarik AR, Havstad S. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016;22:1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Aidy S, Dinan TG, Cryan JF.. Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin Ther 2015;37:954–67. [DOI] [PubMed] [Google Scholar]
- 34. Erny D, Dokalis N, Mezö C. et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab 2021;33:2260–76.e7. [DOI] [PubMed] [Google Scholar]
- 35. Thion MS, Low D, Silvin A. et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018;172:500–16.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jaggar M, Rea K, Spichak S. et al. You’ve got male: sex and the microbiota-gut-brain axis across the lifespan. Front Neuroendocrinol 2020;56:100815. [DOI] [PubMed] [Google Scholar]
- 37. Yano JM, Yu K, Donaldson GP. et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parada Venegas D, De la Fuente MK, Landskron G. et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases . Front Immunol 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dalile B, Van Oudenhove L, Vervliet B. et al. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol 2019;16:461–78. [DOI] [PubMed] [Google Scholar]
- 40. Platten M, Nollen EAA, Röhrig UF. et al. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov 2019;18:379–401. [DOI] [PubMed] [Google Scholar]
- 41. Roager HM, Licht TR.. Microbial tryptophan catabolites in health and disease. Nat Commun 2018;9:3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feng Y, Huang Y, Wang Y. et al. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One 2019;14:e0218384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Crouch LI, Liberato MV, Urbanowicz PA. et al. Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nat Commun 2020;11:4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider H, Pelaseyed T, Svensson F. et al. Study of mucin turnover in the small intestine by in vivo labeling. Sci Rep 2018;8:5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang S, Wu X, Jin F.. Gut-brain psychology: rethinking psychology from the microbiota-gut-brain axis. Front Integr Neurosci 2018;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Luissint A-C, Parkos CA, Nusrat A.. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016;151:616–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019;68:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Braniste V, Al-Asmakh M, Kowal C. et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:263ra158. 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gasparotto J, Ribeiro CT, Bortolin RC. et al. Anti-RAGE antibody selectively blocks acute systemic inflammatory responses to LPS in serum, liver, CSF and striatum. Brain Behav Immun 2017;62:124–36. [DOI] [PubMed] [Google Scholar]
- 50. Thoo L, Noti M, Krebs P.. Keep calm: the intestinal barrier at the interface of peace and war. Cell Death Dis 2019;10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelly JR, Kennedy PJ, Cryan JF. et al. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stephenson J, Nutma E, van der Valk P. et al. Inflammation in CNS neurodegenerative diseases. Immunology 2018;154:204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Amor S, Puentes F, Baker D. et al. Inflammation in neurodegenerative diseases. Immunology 2010;129:154–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Glass CK, Saijo K, Winner B. et al. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140:918–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blander JM, Longman RS, Iliev ID. et al. Regulation of inflammation by microbiota interactions with the host. Nat Immunol 2017;18:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Serra D, Almeida LM, Dinis TCP.. The impact of chronic intestinal inflammation on brain disorders: the microbiota-gut-brain axis. Mol Neurobiol 2019;56:6941–51. [DOI] [PubMed] [Google Scholar]
- 57. Goyal D, Ali SA, Singh RK.. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2021;106:110112. [DOI] [PubMed] [Google Scholar]
- 58. Sochocka M, Donskow-Łysoniewska K, Diniz BS. et al. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease: a critical review. Mol Neurobiol 2019;56:1841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin L, Zheng LJ, Zhang LJ.. Neuroinflammation, gut microbiome, and Alzheimer’s disease. Mol Neurobiol 2018;55:8243–50. [DOI] [PubMed] [Google Scholar]
- 60. Leblhuber F, Ehrlich D, Steiner K. et al. The immunopathogenesis of Alzheimer’s disease is related to the composition of gut microbiota. Nutrients 2021;13:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shen H, Guan Q, Zhang X. et al. New mechanism of neuroinflammation in Alzheimer’s disease: the activation of NLRP3 inflammasome mediated by gut microbiota. Prog Neuropsychopharmacol Biol Psychiatry 2020;100:109884. [DOI] [PubMed] [Google Scholar]
- 62. Houser MC, Tansey MG.. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis 2017;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romano S, Savva GM, Bedarf JR. et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. Npj Parkinsons Dis 2021;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aho VTE, Houser MC, Pereira PAB. et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol Neurodegeneration 2021;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Campos-Acuña J, Elgueta D, Pacheco R.. T-cell-driven inflammation as a mediator of the gut-brain axis involved in Parkinson’s. Front Immunol 2019;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Perez-Pardo P, Dodiya HB, Engen PA. et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 2019;68:829–43. [DOI] [PubMed] [Google Scholar]
- 67. Rowin J, Xia Y, Jung B. et al. Gut inflammation and dysbiosis in human motor neuron disease. Physiol Rep 2017;5:e13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Burberry A, Wells MF, Limone F. et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020;582:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Figueroa-Romero C, Guo K, Murdock BJ. et al. Temporal evolution of the microbiome, immune system and epigenome with disease progression in ALS mice. Dis Model Mech 2019;13:dmm041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haase S, Haghikia A, Wilck N. et al. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 2018;154:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Adamczyk-Sowa M, Medrek A, Madej P. et al. Does the gut microbiota influence immunity and inflammation in multiple sclerosis pathophysiology? J Immunol Res 2017;2017:7904821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zeraati M, Enayati M, Kafami L. et al. Gut microbiota depletion from early adolescence alters adult immunological and neurobehavioral responses in a mouse model of multiple sclerosis. Neuropharmacology 2019;157:107685. [DOI] [PubMed] [Google Scholar]
- 73. Valadão PAC, Santos KBS, Ferreira e Vieira TH. et al. Inflammation in Huntington’s disease: a few new twists on an old tale. J Neuroimmunol 2020;348:577380. [DOI] [PubMed] [Google Scholar]
- 74. Du G, Dong W, Yang Q. et al. Altered gut microbiota related to inflammatory responses in patients with Huntington’s. Front Immunol 2020;11:603594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Unger MM, Spiegel J, Dillmann K-U. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 2016;32:66–72. [DOI] [PubMed] [Google Scholar]
- 76. Aho VTE, Houser MC, Pereira PAB. et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol Neurodegener 2021;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Keshavarzian A, Green SJ, Engen PA. et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord 2015;30:1351–60. [DOI] [PubMed] [Google Scholar]
- 78. Duscha A, Gisevius B, Hirschberg S. et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020;180:1067–80.e16. [DOI] [PubMed] [Google Scholar]
- 79. Olsson A, Gustavsen S, Nguyen TD. et al. Serum short-chain fatty acids and associations with inflammation in newly diagnosed patients with multiple sclerosis and healthy controls. Front Immunol 2021;12:1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Levi I, Gurevich M, Perlman G. et al. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiome-metabolome analysis. Cell Rep Med 2021;2:100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pérez-Pérez S, Domínguez-Mozo MI, Alonso-Gómez A. et al. Acetate correlates with disability and immune response in multiple sclerosis. PeerJ 2020;8:e10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Niccolai E, Di Pilato V, Nannini G. et al. The gut microbiota-immunity axis in ALS: a role in deciphering disease heterogeneity? Biomedicines 2021;9:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kobayashi Y, Sugahara H, Shimada K. et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep 2017;7:13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hou Y, Li X, Liu C. et al. Neuroprotective effects of short-chain fatty acids in MPTP induced mice model of Parkinson’s disease. Exp Gerontol 2021;150:111376. [DOI] [PubMed] [Google Scholar]
- 85. Zhang Y, Wu S, Yi J. et al. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther 2017;39:322–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gardian G, Browne SE, Choi D-K. et al. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington’s disease. J Bio Chem 2005;280:556–63. [DOI] [PubMed] [Google Scholar]
- 87. Sampson TR, Debelius JW, Thron T. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016;167:1469–80.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Colombo AV, Sadler RK, Llovera G. et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. Elife 2021;10:e59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sonner JK, Keil M, Falk-Paulsen M. et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat Commun 2019;10:4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rothhammer V, Borucki DM, Tjon EC. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018;557:724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scott SA, Fu J, Chang PV.. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 2020;117:19376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Porter RJ, Lunn BS, Walker LL. et al. Cognitive deficit induced by acute tryptophan depletion in patients with Alzheimers disease. Am J Psychiatry 2000;157:638–40. [DOI] [PubMed] [Google Scholar]
- 93. Wu L, Han Y, Zheng Z. et al. Altered gut microbial metabolites in amnestic mild cognitive impairment and Alzheimer’s disease: signals in host–microbe interplay. Nutrients 2021;13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fertan E, Stover KRJ, Brant MG. et al. Effects of the novel IDO inhibitor DWG-1036 on the behavior of male and female 3xTg-AD mice. Front Pharmacol 2019;10:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Venkatesan D, Iyer M, Narayanasamy A. et al. Kynurenine pathway in Parkinson’s disease: an update. eNeurologicalSci 2020;21:100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hill-Burns EM, Debelius JW, Morton JT. et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 2017;32:739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Scheperjans F, Aho V, Pereira PAB. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 2015;30:350–8. [DOI] [PubMed] [Google Scholar]
- 98. Kaur H, Bose C, Mande SS.. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front Neurosci 2019;13:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang Y, Chen S, Tan J. et al. Tryptophan in the diet ameliorates motor deficits in a rotenone-induced rat Parkinson’s disease model via activating the aromatic hydrocarbon receptor pathway. Brain Behav 2021;11:e2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Braak H, Del Tredici K, Rüb U. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 101. Walter U, Tsiberidou P, Kersten M. et al. Atrophy of the vagus nerve in Parkinson’s disease revealed by high-resolution ultrasonography. Front Neurol 2018;9:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Svensson E, Horváth-Puhó E, Thomsen RW. et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 2015;78:522–9. [DOI] [PubMed] [Google Scholar]
- 103. Liu B, Fang F, Pedersen NL. et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 2017;88:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Challis C, Hori A, Sampson TR. et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci 2020;23:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim S, Kwon S-H, Kam T-I. et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 2019;103:627–41.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pan-Montojo F, Anichtchik O, Dening Y. et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 2010;5:e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. George MS, Ward HE, Ninan PT. et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul 2008;1:112–21. [DOI] [PubMed] [Google Scholar]
- 108. Sackeim HA, Rush AJ, George MS. et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001;25:713–28. [DOI] [PubMed] [Google Scholar]
- 109. Sjögren MJC, Hellström PTO, Jonsson MAG. et al. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psychiatry 2002;63:972–80. [DOI] [PubMed] [Google Scholar]
- 110. Merrill CA, Jonsson MAG, Minthon L. et al. Vagus nerve stimulation in patients with Alzheimer’s disease: additional follow-up results of a pilot study through 1 year. J Clin Psychiatry 2006;67:1171–8. [DOI] [PubMed] [Google Scholar]
- 111. Marrosu F, Maleci A, Cocco E. et al. Vagal nerve stimulation effects on cerebellar tremor in multiple sclerosis. Neurology 2005;65:490. [DOI] [PubMed] [Google Scholar]
- 112. Nodera H, Takamatsu N, Shimatani Y. et al. Thinning of cervical nerve roots and peripheral nerves in ALS as measured by sonography. Clin Neurophysiol 2014;125:1906–11. [DOI] [PubMed] [Google Scholar]
- 113. Hou Y, Dan X, Babbar M. et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 2019;15:565–81. [DOI] [PubMed] [Google Scholar]
- 114. Bosco N, Noti M.. The aging gut microbiome and its impact on host immunity. Genes Immun 2021;22:289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Biagi E, Franceschi C, Rampelli S. et al. Gut microbiota and extreme longevity. Cur Biol 2016;26:1480–5. [DOI] [PubMed] [Google Scholar]
- 116. Jeffery IB, Lynch DB, O'Toole PW.. Composition and temporal stability of the gut microbiota in older persons. Isme J 2016;10:170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Vangay P, Johnson AJ, Ward TL. et al. US immigration westernizes the human gut microbiome. Cell 2018;175:962–72.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wilmanski T, Diener C, Rappaport N. et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab 2021;3:274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Franceschi C, Garagnani P, Parini P. et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–90. [DOI] [PubMed] [Google Scholar]
- 120. Ghosh TS, Das M, Jeffery IB. et al. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife 2020;9:e50240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Boehme M, Guzzetta KE, Bastiaanssen TFS. et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging 2021;1:666–76. [DOI] [PubMed] [Google Scholar]
- 122. Kobayashi A, Donaldson DS, Erridge C. et al. The functional maturation of M cells is dramatically reduced in the Peyer’s patches of aged mice. Mucosal Immunol 2013;6:1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Donaldson DS, Pollock J, Vohra P. et al. Microbial stimulation reverses the age-related decline in M cells in aged mice. iScience 2020;23:101147. 10.1016/j.isci.2020.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Claus SP, Guillou H, Ellero-Simatos S.. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2016;2:16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sutherland VL, McQueen CA, Mendrick D. et al. The gut microbiome and xenobiotics: identifying knowledge gaps. Toxicol Sci 2020;176:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zaganas I, Kapetanaki S, Mastorodemos V. et al. Linking pesticide exposure and dementia: what is the evidence? Toxicology 2013;307:3–11. [DOI] [PubMed] [Google Scholar]
- 127. Sherer TB, Betarbet R, Testa CM. et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 2003;23:10756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Johnson ME, Stringer A, Bobrovskaya L.. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. NeuroToxicology 2018;65:174–85. [DOI] [PubMed] [Google Scholar]
- 129. Obata F, Fons CO, Gould AP.. Early-life exposure to low-dose oxidants can increase longevity via microbiome remodelling in Drosophila. Nat Commun 2018;9:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Voorhees JR, Remy MT, Erickson CM. et al. Occupational-like organophosphate exposure disrupts microglia and accelerates deficits in a rat model of Alzheimer’s disease. NPJ Aging Mech Dis 2019;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Perez-Fernandez C, Morales-Navas M, Guardia-Escote L. et al. Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: a locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food Chem Toxicol 2020;135:110865. [DOI] [PubMed] [Google Scholar]
- 132. Bu X-L, Wang X, Xiang Y. et al. The association between infectious burden and Parkinson’s disease: a case-control study. Parkinsonism Relat Disord 2015;21:877–81. [DOI] [PubMed] [Google Scholar]
- 133. Lövheim H, Gilthorpe J, Adolfsson R. et al. Reactivated herpes simplex infection increases the risk of Alzheimer’s disease. Alzheimers Dement 2015;11:593–9. [DOI] [PubMed] [Google Scholar]
- 134. Smeyne RJ, Noyce AJ, Byrne M. et al. Infection and risk of Parkinson’s disease. J Parkinsons Dis 2021;11:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xue YC, Feuer R, Cashman N. et al. Enteroviral infection: the forgotten link to amyotrophic lateral sclerosis? Front Mol Neurosci 2018;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Matheoud D, Cannon T, Voisin A. et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1−/− mice. Nature 2019;571:565–9. [DOI] [PubMed] [Google Scholar]
- 137. Wagley S, Bokori-Brown M, Morcrette H. et al. Evidence of clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult Scler 2019;25:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rumah KR, Vartanian TK, Fischetti VA.. Oral multiple sclerosis drugs inhibit the in vitro growth of epsilon toxin producing gut bacterium, clostridium perfringens. Front Cell Infect Microbiol 2017;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. van Kessel SP, Frye AK, El-Gendy AO. et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 2019;10:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Maini Rekdal V, Bess EN, Bisanz JE. et al. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 2019;364:eaau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Niehues M, Hensel A.. In-vitro interaction of L-dopa with bacterial adhesins of Helicobacter pylori: an explanation for clinical differences in bioavailability? J Pharm Pharmacol 2009;61:1303–7. [DOI] [PubMed] [Google Scholar]
- 142. Pierantozzi M, Pietroiusti A, Brusa L. et al. Helicobacter pylori eradication and L-dopa absorption in patients with PD and motor fluctuations. Neurology 2006;66:1824–9. [DOI] [PubMed] [Google Scholar]
- 143. David LA, Maurice CF, Carmody RN. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. O’Keefe SJD, Li JV, Lahti L. et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun 2015;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Valdes AM, Walter J, Segal E. et al. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gibiino GD, Siena M, Sbrancia M. et al. Dietary habits and gut microbiota in healthy adults: focusing on the right diet: a systematic review . IJMS 2021;22:6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Claesson MJ, Jeffery IB, Conde S. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 148. Hirschberg S, Gisevius B, Duscha A. et al. Implications of diet and the gut microbiome in neuroinflammatory and neurodegenerative diseases. IJMS 2019;20:3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tan LY, Yeo XY, Bae H-G. et al. Association of gut microbiome dysbiosis with neurodegeneration: can gut microbe-modifying diet prevent or alleviate the symptoms of neurodegenerative diseases? Life 2021;11:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bonfili L, Cecarini V, Gogoi O. et al. Microbiota modulation as preventative and therapeutic approach in Alzheimer’s disease. Febs J 2021;288:2836–55. [DOI] [PubMed] [Google Scholar]
- 151. Moreno-Arribas MV, Bartolomé B, Peñalvo JL. et al. Relationship between wine consumption, diet and microbiome modulation in Alzheimer’s disease. Nutrients 2020;12:3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Szczechowiak K, Diniz BS, Leszek J.. Diet and Alzheimer’s dementia: nutritional approach to modulate inflammation. Pharmacol Biochem Behav 2019;184:172743. [DOI] [PubMed] [Google Scholar]
- 153. Zhang M, Zhao D, Zhou G. et al. Dietary pattern, gut microbiota, and Alzheimer’s disease. J Agric Food Chem 2020;68:12800–9. [DOI] [PubMed] [Google Scholar]
- 154. Boulos C, Yaghi NE, Hayeck R. et al. Nutritional risk factors, microbiota and Parkinson’s disease: what is the current evidence? Nutrients 2019;11:1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Uyar GÖ, Yildiran H.. A nutritional approach to microbiota in Parkinson’s disease. Biosci Microbiota Food Health 2019;38:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Serra-Majem L, Román-Viñas B, Sanchez-Villegas A. et al. Benefits of the Mediterranean diet: epidemiological and molecular aspects. Mol Aspects Med 2019;67:1–55. [DOI] [PubMed] [Google Scholar]
- 157. García-Montero C, Fraile-Martínez O, Gómez-Lahoz AM. et al. Nutritional components in western diet versus Mediterranean diet at the gut microbiota–immune system interplay. implications for health and disease. Nutrients 2021;13:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wu L, Sun D.. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep 2017;7:41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ghosh TS, Rampelli S, Jeffery IB. et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020;69:1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Nagpal R, Neth BJ, Wang S. et al. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019;47:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Alharbi A, Al-Sowayan NS.. The effect of ketogenic-diet on health. FNS 2020;11:301–13. [Google Scholar]
- 162. Nagpal R, Neth BJ, Wang S. et al. Gut mycobiome and its interaction with diet, gut bacteria and Alzheimer’s disease markers in subjects with mild cognitive impairment: a pilot study. EBioMedicine 2020;59:102950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Nargeh H, Aliabadi F, Ajami M. et al. Role of polyphenols on gut microbiota and the ubiquitin-proteasome system in neurodegenerative diseases. J Agric Food Chem 2021;69:6119–44. [DOI] [PubMed] [Google Scholar]
- 164. Aryal S, Skinner T, Bridges B. et al. The pathology of Parkinson’s disease and potential benefit of dietary polyphenols. Molecules 2020;25:4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Mithul Aravind S, Wichienchot S, Tsao R. et al. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res Int 2021;142:110189. [DOI] [PubMed] [Google Scholar]
- 166. Liu Z, de Bruijn WJC, Bruins ME. et al. Microbial metabolism of theaflavin-3,3′-digallate and its gut microbiota composition modulatory effects. J Agric Food Chem 2021;69:232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Guo T, Song D, Cheng L. et al. Interactions of tea catechins with intestinal microbiota and their implication for human health. Food Sci Biotechnol 2019;28:1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shimizu C, Wakita Y, Inoue T. et al. Effects of lifelong intake of lemon polyphenols on aging and intestinal microbiome in the senescence-accelerated mouse prone 1 (SAMP1). Sci Rep 2019;9:3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sun Z-Z, Li X-Y, Wang S. et al. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl Microbiol Biotechnol 2020;104:3507–15. [DOI] [PubMed] [Google Scholar]
- 170. Wang D, Ho L, Faith J. et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res 2015;59:1025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Casani-Cubel J, Benlloch M, Sanchis-Sanchis CE. et al. The impact of microbiota on the pathogenesis of amyotrophic lateral sclerosis and the possible benefits of polyphenols. An Overview. Metabolites 2021;11:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kapoor B, Kapoor D, Gautam S. et al. Dietary polyunsaturated fatty acids (PUFAs): uses and potential health benefits. Curr Nutr Rep 2021;10:232–42. [DOI] [PubMed] [Google Scholar]
- 173. Menni C, Zierer J, Pallister T. et al. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep 2017;7:11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. La Rosa F, Clerici M, Ratto D. et al. The gut-brain axis in Alzheimer’s disease and omega-3: a critical overview of clinical trials. Nutrients 2018;10:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Saeedi Saravi SS, Bonetti NR, Pugin B. et al. Lifelong dietary omega-3 fatty acid suppresses thrombotic potential through gut microbiota alteration in aged mice. iScience 2021;24:102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Prasad MR, Lovell MA, Yatin M. et al. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem Res 1998;23:81–8. [DOI] [PubMed] [Google Scholar]
- 177. Söderberg M, Edlund C, Kristensson K. et al. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991;26:421–5. [DOI] [PubMed] [Google Scholar]
- 178. Hegelmaier T, Lebbing M, Duscha A. et al. Interventional influence of the intestinal microbiome through dietary intervention and bowel cleansing might improve motor symptoms in Parkinson’s. Disease. Cells 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yap CX, Henders AK, Ga A. et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021;184:5916–31.e17. [DOI] [PubMed] [Google Scholar]
- 180. Nay K, Smiles WJ, Kaiser J. et al. Molecular mechanisms underlying the beneficial effects of exercise on brain function and neurological disorders. IJMS 2021;22:4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Gubert C, Hannan AJ.. Exercise mimetics: harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov 2021;20:862–79. [DOI] [PubMed] [Google Scholar]
- 182. Mitchell CM, Davy BM, Hulver MW. et al. Does exercise alter gut microbial composition? A systematic review. Med Sci Sports Exerc 2019;51:160–7. [DOI] [PubMed] [Google Scholar]
- 183. Ortiz-Alvarez L, Xu H, Martinez-Tellez B.. Influence of exercise on the human gut microbiota of healthy adults: a systematic review. Clin Transl Gastroenterol 2020;11:e00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Gubert C, Kong G, Renoir T. et al. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol Dis 2020;134:104621. [DOI] [PubMed] [Google Scholar]
- 185. Schlegel P, Novotny M, Klimova B. et al. “Muscle-gut-brain axis:” can physical activity help patients with Alzheimer’s disease due to microbiome modulation? JAD 2019;71:861–78. [DOI] [PubMed] [Google Scholar]
- 186. Rosa JM, Pazini FL, Camargo A. et al. Prophylactic effect of physical exercise on Aβ1-40-induced depressive-like behavior and gut dysfunction in mice. Behav Brain Res 2020;393:112791. [DOI] [PubMed] [Google Scholar]
- 187. Wang G, Zhou H-H, Luo L. et al. Voluntary wheel running is capable of improving cognitive function only in the young but not the middle-aged male APPSwe/PS1De9 mice. Neurochem Int 2021;145:105010. [DOI] [PubMed] [Google Scholar]
- 188. Shamsipour S, Sharifi G, Taghian F.. Impact of interval training with probiotic (L. plantarum / Bifidobacterium bifidum) on passive avoidance test, ChAT and BDNF in the hippocampus of rats with Alzheimer’s disease. Neurosci Lett 2021;756:135949. [DOI] [PubMed] [Google Scholar]
- 189. Abraham D, Feher J, Scuderi GL. et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: role of microbiome. Exp Gerontol 2019;115:122–31. [DOI] [PubMed] [Google Scholar]
- 190. Xu X, Fu Z, Le W.. Exercise and Parkinson’s disease. Int Rev Neurobiol 2019;147:45–74. [DOI] [PubMed] [Google Scholar]
- 191. Mak MKY, Wong-Yu ISK.. Exercise for Parkinson’s disease. Int Rev Neurobiol 2019;147:1–44. [DOI] [PubMed] [Google Scholar]
- 192. Halabchi F, Alizadeh Z, Sahraian MA. et al. Exercise prescription for patients with multiple sclerosis: potential benefits and practical recommendations. BMC Neurol 2017;17:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Reynolds ER, Ashbaugh AD, Hockenberry BJ. et al. Multiple sclerosis and exercise: a literature review. Curr Sports Med Rep 2018;17:31–5. [DOI] [PubMed] [Google Scholar]
- 194. Motl RW. Exercise and Multiple Sclerosis. In: Xiao J (ed.). Physical Exercise for Human Health, Vol. 1228. Singapore: Springer Singapore, 2020, 333–43. [DOI] [PubMed] [Google Scholar]
- 195. Meng L, Li X, Li C. et al. Effects of exercise in patients with amyotrophic lateral sclerosis: a systematic review and meta-analysis. Am J Phys Med Rehabil 2020;99:801–10. [DOI] [PubMed] [Google Scholar]
- 196. Lisle S, Tennison M.. Amyotrophic lateral sclerosis: the role of exercise. Curr Sports Med Rep 2015;14:45–6. [DOI] [PubMed] [Google Scholar]
- 197. Mueller SM, Petersen JA, Jung HH.. Exercise in Huntington’s disease: current state and clinical significance. Tremor Other Hyperkinet Mov (N Y) 2019;9:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Uhrbrand A, Stenager E, Pedersen MS. et al. Parkinson’s disease and intensive exercise therapy: a systematic review and meta-analysis of randomized controlled trials. J Neurol Sci 2015;353:9–19. [DOI] [PubMed] [Google Scholar]
- 199. Petzinger GM, Fisher BE, McEwen S. et al. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol 2013;12:716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Crowley EK, Nolan YM, Sullivan AM.. Neuroprotective effects of voluntary running on cognitive dysfunction in an α-synuclein rat model of Parkinson’s disease. Neurobiol Aging 2018;65:60–8. [DOI] [PubMed] [Google Scholar]
- 201. Ortega-Hombrados L, Molina-Torres G, Galán-Mercant A et al Systematic review of therapeutic physical exercise in patients with amyotrophic lateral sclerosis over time. IJERPH 2021;18:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pang TYC, Stam NC, Nithianantharajah J. et al. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 2006;141:569–84. [DOI] [PubMed] [Google Scholar]
- 203. Gubert C, Love CJ, Kodikara S. et al. Gene-environment-gut interactions in Huntington’s disease mice are associated with environmental modulation of the gut microbiome. iScience 2022;25:103687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Latimer-Cheung AE, Pilutti LA, Hicks AL. et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil 2013;94:1800–28.e3. [DOI] [PubMed] [Google Scholar]
- 205. Dalgas U. Exercise therapy in multiple sclerosis and its effects on function and the brain. Neurodegener Dis Manag 2017;7:35–40. [DOI] [PubMed] [Google Scholar]
- 206. Sandroff BM. Exercise and cognition in multiple sclerosis: the importance of acute exercise for developing better interventions. Neurosci Biobehav Rev 2015;59:173–83. [DOI] [PubMed] [Google Scholar]
- 207. Mokhtarzade M, Molanouri Shamsi M, Abolhasani M. et al. Home-based exercise training influences gut bacterial levels in multiple sclerosis. Complement Ther Clin Pract 2021;45:101463. [DOI] [PubMed] [Google Scholar]
- 208. Chen H, Shen L, Liu Y. et al. Strength exercise confers protection in central nervous system autoimmunity by altering the gut microbiota. Front Immunol 2021;12:628629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hill C, Guarner F, Reid G. et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 210. Kristensen NB, Bryrup T, Allin KH. et al. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 2016;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sanders ME, Merenstein DJ, Reid G. et al. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 2019;16:605–16. [DOI] [PubMed] [Google Scholar]
- 212. Westfall S, Lomis N, Kahouli I. et al. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci 2017;74:3769–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Peterson CT. Dysfunction of the microbiota-gut-brain axis in neurodegenerative disease: the promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics. J Evid Based Complementary Altern Med 2020;25:2515690X2095722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kesika P, Suganthy N, Sivamaruthi BS. et al. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci 2021;264:118627. [DOI] [PubMed] [Google Scholar]
- 215. Akbari E, Asemi ZD, Kakhaki R. et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: a randomized, double-blind and controlled trial. Front Aging Neurosci 2016;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Leblhuber F, Steiner K, Schuetz B. et al. Probiotic supplementation in patients with Alzheimer’s dementia: an explorative intervention study. CAR 2018;15:1106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Agahi A, Hamidi GA, Daneshvar R. et al. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol 2018;9:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zhu G, Zhao J, Zhang H. et al. Probiotics for mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Foods 2021;10:1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Arora K, Green M, Prakash S.. The microbiome and Alzheimer’s disease: potential and limitations of prebiotic, synbiotic, and probiotic formulations. Front Bioeng Biotechnol 2020;8:537847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Asl Sepehri RZ, Salami GM.. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer’s disease. Behav Brain Res 2019;376:112183. [DOI] [PubMed] [Google Scholar]
- 221. Ou Z, Deng L, Lu Z. et al. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr Diabetes 2020;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Wang Q-J, Shen Y-E, Wang X. et al. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging (Albany NY) 2020;12:628–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Bonfili L, Cecarini V, Cuccioloni M. et al. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol Neurobiol 2018;55:7987–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Bonfili L, Cecarini V, Gogoi O. et al. Gut microbiota manipulation through probiotics oral administration restores glucose homeostasis in a mouse model of Alzheimer’s disease. Neurobiol Aging 2020;87:35–43. [DOI] [PubMed] [Google Scholar]
- 225. Kaur H, Nagamoto-Combs K, Golovko S. et al. Probiotics ameliorate intestinal pathophysiology in a mouse model of Alzheimer’s disease. Neurobiol Aging 2020;92:114–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kaur H, Golovko S, Golovko MY. et al. Effects of probiotic supplementation on short chain fatty acids in the AppNL-G-F mouse model of Alzheimer’s disease. J Alzheimers Dis 2020;76:1083–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Gazerani. Probiotics for Parkinson’s disease. IJMS 2019;20:4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Castelli V, d'Angelo M, Quintiliani M. et al. The emerging role of probiotics in neurodegenerative diseases: new hope for Parkinson’s disease? Neural Regen Res 2021;16:628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Tan AH, Hor JW, Chong CW. et al. Probiotics for Parkinson’s disease: current evidence and future directions. JGH Open 2021;5:414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Ibrahim A, Ali RAR, Manaf MRA. et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: a randomised controlled trial. PLoS ONE 2020;15:e0244680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Tamtaji OR, Taghizadeh MD, Kakhaki R. et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition 2019;38:1031–5. [DOI] [PubMed] [Google Scholar]
- 232. Morshedi M, Hashemi R, Moazzen S. et al. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J Neuroinflamm 2019;16:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Jiang J, Chu C, Wu C. et al. Efficacy of probiotics in multiple sclerosis: a systematic review of preclinical trials and meta-analysis of randomized controlled trials. Food Funct 2021;12:2354–77. [DOI] [PubMed] [Google Scholar]
- 234. Srivastav S, Neupane S, Bhurtel S. et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem 2019;69:73–86. [DOI] [PubMed] [Google Scholar]
- 235. Sun J, Li H, Jin Y. et al. Probiotic clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun 2021;91:703–15. [DOI] [PubMed] [Google Scholar]
- 236. Blais LL, Montgomery TL, Amiel E. et al. Probiotic and commensal gut microbial therapies in multiple sclerosis and its animal models: a comprehensive review. Gut Microbes 2021;13:1943289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Mangalam AK, Murray J.. Microbial monotherapy with Prevotella histicola for patients with multiple sclerosis. Expert Rev Neurother 2019;19:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Gibson GR, Hutkins R, Sanders ME. et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14:491–502. [DOI] [PubMed] [Google Scholar]
- 239. Barbosa RSD, Vieira-Coelho MA.. Probiotics and prebiotics: focus on psychiatric disorders—a systematic review. Nutr Rev 2020;78:437–50. [DOI] [PubMed] [Google Scholar]
- 240. de Vrese M, Schrezenmeir J.. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 2008;111:1–66. [DOI] [PubMed] [Google Scholar]
- 241. Cencic A, Chingwaru W.. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010;2:611–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Xin Y, Diling C, Jian Y. et al. Effects of oligosaccharides from Morinda officinalis on gut microbiota and metabolome of APP/PS1 transgenic mice. Front Neurol 2018;9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Sun J, Liu S, Ling Z. et al. Fructooligosaccharides ameliorating cognitive deficits and neurodegeneration in APP/PS1 transgenic mice through modulating gut microbiota. J Agric Food Chem 2019;67:3006–17. [DOI] [PubMed] [Google Scholar]
- 244. Lee Y-S, Lai D-M, Huang H-J. et al. Prebiotic lactulose ameliorates the cognitive deficit in Alzheimer’s disease mouse model through macroautophagy and chaperone-mediated autophagy pathways. J Agric Food Chem 2021;69:2422–37. [DOI] [PubMed] [Google Scholar]
- 245. Xu M, Mo X, Huang H. et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1–42-induced AD-like mice. Int J Biol Macromol 2020;161:258–70. [DOI] [PubMed] [Google Scholar]
- 246. Liu Q, Xi Y, Wang Q. et al. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav Immun 2021;95:330–43. [DOI] [PubMed] [Google Scholar]
- 247. Cantu-Jungles TM, Rasmussen HE, Hamaker BR.. Potential of prebiotic butyrogenic fibers in Parkinson’s. Front Neurol 2019;10:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutrition 2003;133:2485S–93S. [DOI] [PubMed] [Google Scholar]
- 249. Liu J, Wang F, Liu S. et al. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci 2017;381:176–81. [DOI] [PubMed] [Google Scholar]
- 250. St. Laurent R, O’Brien LM, Ahmad ST.. ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience 2013;246:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Egorin MJ, Yuan Z-M, Sentz DL. et al. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother Pharmacol 1999;43:445–53. [DOI] [PubMed] [Google Scholar]
- 252. Pedrosa Carrasco AJ, Timmermann L, Pedrosa DJ.. Management of constipation in patients with Parkinson’s disease. NPJ Parkinsons Dis 2018;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Barichella M, Pacchetti C, Bolliri C. et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology 2016;87:1274–80. [DOI] [PubMed] [Google Scholar]
- 254. Nurrahma BA, Tsao S-P, Wu C-H. et al. Probiotic supplementation facilitates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism. Front Aging Neurosci 2021;13:668775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Song L, Gao Y, Zhang X. et al. Galactooligosaccharide improves the animal survival and alleviates motor neuron death in SOD1G93A mouse model of amyotrophic lateral sclerosis. Neuroscience 2013;246:281–90. [DOI] [PubMed] [Google Scholar]
- 256. Nagpal J, Cryan JF.. Microbiota-brain interactions: moving toward mechanisms in model organisms. Neuron 2021;109:3930–53. [DOI] [PubMed] [Google Scholar]
- 257. Dawson TM, Golde TE, Lagier-Tourenne C.. Animal models of neurodegenerative diseases. Nat Neurosci 2018;21:1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]