Abstract
Renewed interest in the effects of psychedelics in the treatment of psychiatric disorders warrants a better understanding of the neurobiological mechanisms underlying the effects of these substances. During the past two decades, state-of-the-art studies of animals and humans have yielded new important insights into the molecular, cellular, and systems-level actions of psychedelic drugs. These efforts have revealed that psychedelics affect primarily serotonergic receptor subtypes located in cortico-thalamic and cortico-cortical feedback circuits of information processing. Psychedelic drugs modulate excitatory-inhibitory balance in these circuits and can participate in neuroplasticity within brain structures critical for the integration of information relevant to sensation, cognition, emotions, and the narrative of self. Neuroimaging studies showed that characteristic dimensions of the psychedelic experience obtained through subjective questionnaires as well as alterations in self-referential processing and emotion regulation obtained through neuropsychological tasks are associated with distinct changes in brain activity and connectivity patterns at multiple-system levels. These recent results suggest that changes in self-experience, emotional processing, and social cognition may contribute to the potential therapeutic effects of psychedelics.
Key words: psychedelics, psilocybin, whole-brain models, neuroplasticity, LSD
Introduction
Classic psychedelics, or serotonergic hallucinogens, comprise three main chemical classes: the indoleamines such as N,N-Dimethyltryptamine (DMT) contained in plants 1 , psilocybin and its active metabolite psilocin contained in several mushroom species 2 , the phenylalkylamines such as mescaline contained in several cacti 3 , and synthetic “amphetamines” such as 2,5-Dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-bromoamphetamine, and the semisynthetic ergolines such as lysergic acid diethylamide(LSD) 4 . They produce profound alterations in perception, cognition, emotion, and self-consciousness 5 6 7 8 . Given these intense mind-altering properties, naturally-occurring psychedelics have been used by humans for millennia for spiritual and medicinal purposes 3 9 .
During the 1950s and 1960s, the clinical potential of LSD and psilocybin was extensively investigated for the treatment of different psychiatric disorders including depression and alcohol use disorder. Although these early clinical studies had serious methodological flaws by current standards, systematic reviews suggest that repeated low doses of psychedelics in combination with psychotherapy (psycholytic or “mind loosening” model) or a few high doses with psychological support (psychedelic or “mind-manifesting” model) resulted in impressive improvement rates in the treatment of various forms of depression, anxiety, and alcohol dependence 10 11 12 . The association of psychedelics with the counterculture and concerns over misuse led to the placement of LSD and related drugs in a restrictive regulated drug category (Schedule I) in 1976 in the United States and most other countries. Hence, human research with psychedelics declined, leaving many questions about the mechanism of action and clinical efficacy of classic psychedelics unexplored 13 .
However, in the early 1990s, human psychedelic research with psilocybin, mescaline, and DMT resumed in healthy volunteers by employing different new brain imaging techniques and concepts borrowed from cognitive neurosciences 14 . Since then, an increasing number of molecular and neurophysiological underpinnings of various psychological effects of psilocybin, LSD, and DMT have been identified in healthy volunteers that allow firmer inferences about the potential mechanisms of psychedelic drug action.
Recent behavioral and neuroimaging studies demonstrated that psychedelics produce their psychological effects primarily via agonist action at 5-HT2A receptors in the brain 15 16 , although modulatory downstream effects upon the gamma-aminobutyric acid (GABA)ergic 17 , dopaminergic 18 and glutamatergic 17 systems also seem to be implicated 19 . Current psychological and cognitive studies of psychedelics drug effects in combination with functional human brain imaging in healthy volunteers suggest that psychedelics can profoundly change the sense of self, often experienced as a dissolution of the ordinary boundaries between the self and the world, enhance mood and shift emotion processing to the positive, and facilitate prosocial behavior 19 20 which is accompanied by modulation of neural circuits that are implicated in mood and affective disorders 21 22 23 24 25 . Psychedelics have also been shown to increase glutamate-driven neuroplastic adaptations in animals 26 which may provide a novel mechanism for the lasting beneficial outcomes reported in non-clinical and clinical populations 27 .
In this review, we first outline the phenomenology and key psychological dimensions of psychedelic-induced altered states of consciousness as measured by standardized psychometric scales and then review potential state and trait predictors of the acute responses to psychedelics. We have summarized the potential mechanism of action of classic psychedelic drugs at the molecular, cellular, and circuitry levels. Then, neural correlates of psychedelic-induced alterations of self-consciousness and emotion regulation have been reviewed and the relevance of these findings for the treatment of affective disorders has been discussed. A better understanding of the biological and neurocognitive mechanisms underlying the psychedelic experience and their long-term impact on the mind and brain shall help to develop more specific intervention strategies for improving well-being in health and disease.
Phenomenology and Predictors of Psychedelic States
Classic psychedelics produce multifaceted altered states of consciousness, characterized by profound changes in self-consciousness and interrelated psychological functions: altered perception, including visual illusion, (pseudo-)hallucinations, and synesthesia, alterations in mood and cognitive capacities, and transcendence of time and space 6 .
The profound transient alteration in self-consciousness, experienced as a dissolution of the sense of self/ego and a breakdown of the boundaries between the self and the world, appears to be one of the core features of the psychedelic experiences (the term self and ego are used synonymously in these studies) 28 29 . However, the phenomenon of ego-dissolution is neither an all or nothing affair nor does it occur on its own 30 31 . The experience of ego-dissolution arises dose-dependently along a perception-hallucination continuum associated with increased sensory and emotional arousal, distinct changes in cognitive functions, the release of emotions, often with the recall of emotionally loaded autobiographic memories, and increased capacity for introspection 6 . Empirical research has repeatedly shown that in a supportive and controlled setting, medium to high doses of psychedelics (i. e., psilocybin<25 mg, LSD<200 µg) can trigger with relatively high incidence a pleasurable self-dissolution associated with bliss, feelings of oneness, and insightfulness 32 33 34 35 36 . Such unitive experiences can sporadically also occur during deep mediative states or spontaneously in religious exaltation and have been referred to as states of selflessness 37 or mystical-type experiences, respectively 38 39 40 . Although in this dose range the sense of being a self, or “I” distinct from the world, is diminished or briefly abolished, some remnant “self-observer” (self-awareness) remains preserved in most, if not all, psychedelic states 6 . In fact, memories of such experiences can apparently be formed and reported 41 42 . However, at larger doses, the same dose of a given psychedelic (e. g., psilocybin 30 mg) might induce a pleasurable “mystical-type” experience, or under certain circumstances, a more psychologically challenging or psychotic-like response characterized by fear of losing control over thinking and one’s autonomy, delusions of grandeur, impairment of reasoning, and anxiety or panic 42 43 44 . This clinical observation is underscored by a recent placebo-controlled dose-response study with psilocybin demonstrating that a 30 mg/70 kg psilocybin dosage compared to 20 mg/70 kg markedly increased the incidence for fear and paranoid thinking 33 . Likewise, when comparing dosages of LSD, the ratings for pleasurable “oceanic” self-dissolution increased with dosages of 25, 50, and 100 µg of LSD but plateaued at the highest dose of 200 µg, which also substantially increased the ratings for anxious ego-dissolution 36 .
Although the intensity of the psychedelic experience depends most critically on the dosage 32 33 36 45 46 , it is generally thought that several non-pharmacological factors categorized as the “set” (i. e., the individual’s expectations, personality traits) and the “setting” (i. e., the therapeutic interventions, the physical and social environment) are important in shaping the quality of the acute psychedelics experience 32 42 47 48 49 50 .
To date, however, only a few prospective studies including controlled conditions 47 48 49 51 52 53 54 55 and a meta-analysis pooling data from 23 controlled studies involving 409 psilocybin administrations to 261 healthy volunteers 32 , have investigated the impact of non-pharmacological predictors of the acute response to psychedelics. In most of these studies, the well-validated Altered State of Consciousness Questionnaire (5D-ASC) was employed to measure the broad spectrum of the psychedelic experiences along the five core dimensions (factors): ‘‘oceanic self-boundlessness’’(OB), “dread of ego dissolution’’(DED) ‘‘visionary restructuralisation’’(VIS), ‘‘auditory alterations’’(AA), and ‘‘vigilance reductions’’ (VR). The dimension OB assesses the blissfully experienced self-dissolution including feelings of oneness and insightfulness, while the dimension DED assesses the more distressing reaction associated with thought disorders, fear of losing control, and anxiety. The dimension VIS measures altered perception, changed meaning, and facilitated recall of memories and imagination. The dimensions OB, DED, and VIS can be further described along 11 second-order scales (11-ASC) 31, 56 .
The OB-related blissful “mystical-type” experience can also be measured by the Mysticism Scale (M-Scale) 57 or by the Mystical Experience Questionnaire (MEQ30) 58 . Both scales yield a total score for mysticism comprising various subscale scores, such as a sense of unity, ego-loss, transcendence of space and time, ineffability, deeply-felt positive mood, feelings of sacredness, and noetic insight 57 58 59 60 . Notably, both the M-scale and MEQ30 total scores correlate highly with the OB score of the 5D-ASC scale 48 61 suggesting that these scales assess an overall similar experience.
The results of these studies suggest that scoring high on the personality traits including openness to experience 47 48 , trait absorption 32 54 , optimism towards life 47 48 , being well and relaxed the day(s) before and during drug intake 32 47 48 , and using a mindful attention and emotion regulation strategy involving a non-judgmental orientation of acceptance towards all emotions and thoughts arising in the present moment predicted the magnitude of positive self-dissolution (OB) or mystical-type experience (M-total score) 48 ( Fig. 1a ). Pre-experience with altered states 47 48 , older age 32 47 , and a pleasant environment and the application of music during drug intake were also found to contribute to a blissful experience of OB 32 62 or beneficial outcomes 51 53 . Finally, scoring high on cognitive-emotional re-appraisal capacity seems to buffer from distressing aspects of psychedelic experiences indexed as DED in mediation experts during a group retreat, which may arise with higher doses of psilocybin 48 . However, further research in mediation novices is needed to disentangle the interaction of mindfulness training and group setting. On the other hand, high neuroticism, younger age, and an impersonal laboratory setting predicted unpleasant and anxious reactions to psilocybin 32 . A high absorption capacity also predicted heightened visual perception 54 , reduced stimulus-color consistency during synesthetic-like experiences 55 , and together with esthetic sensibility VIS, included facilitated imagination and changed meaning 32 47 ( Fig. 1a ). Notably, absorption has also been identified as a predisposing trait for hallucinatory and mystical-type experiences 63 and linked to the binding potential (BP) of the 5-HT2A receptor 64 , suggesting that the assessment of 5-HT2A BP may provide a predictor of the overall psychedelic drug effects 65 66 .
Fig. 1.
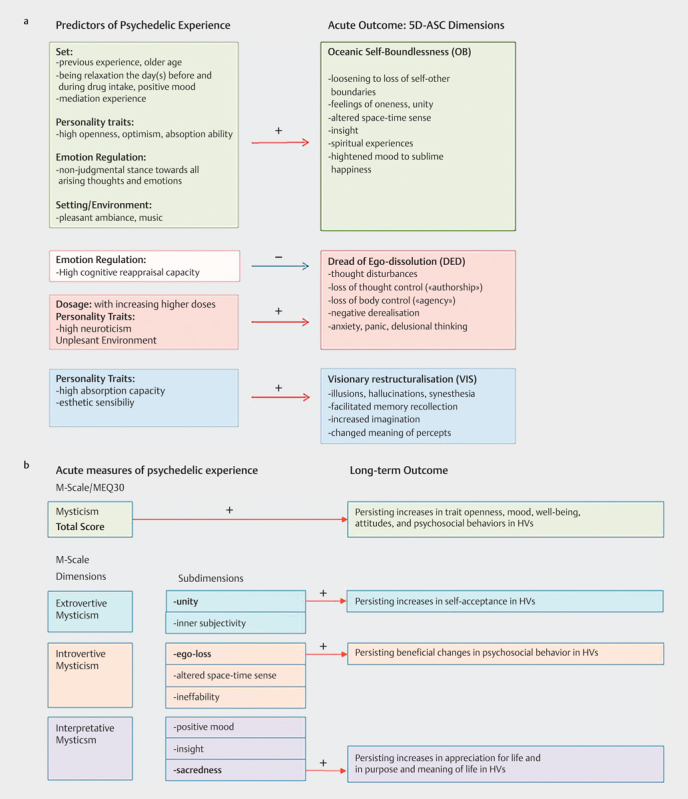
Empirical described predictors of acute and long-term effects of Psychedelics. The Altered State of Consciousness Questionnaire (5D-ASC) 31 79 and the Mysticism Scale (M-Scale) 58 59 are usually administered shortly after the acute psychedelic experience. Red arrows=positive correlations; blue arrows=negative correlations: a : Scoring high on trait openness 47 48 , absorption 32 54 , and optimism about life 47 48 , being relaxed the day(s) before drug intake intake 32 47 48 , using a non-judgmental emotion regulation strategy 48 , pre-experience with ASCs 47 48 , older age 47 , and a pleasant ambiance 32 , supportive music 51 53 62 , and meditation practice 49 were predictive for a positive psychedelic experience (e. g., “Oceanic Boundlessness”) in healthy volunteers (HVs). High emotional re-appraisal capacity reduced the occurrence of distressing experiences (e. g., “Dread of ego-dissolution”) 48 . On the other hand, high neuroticism, young age, and an impersonal laboratory setting predicted unpleasant and anxious reactions to psilocybin in healthy volunteers 32 . In addition, high absorption capacity and esthetic sensibility predicted changes in visual perception and altered meaning of percepts (VIS) 32 47 . b : Mysticism Total Score (M-Scale or MEQ30) predicted persisting increases in trait openness 67 68 , mood, well-being, attitudes and psychosocial behaviors in healthy volunteers 48 67 69 . The M-Scale subdimensions “unity” and “sacredness” predicted persisting increases in self-acceptance and appreciation for life in healthy volunteers 48 , while “ego dissolution” predicted lasting increases in openness and mood 80 .
However, given the current experimental limitations (e. g., small sample sizes, homogenous samples) further studies yet need to replicate these findings by using well-power, placebo-controlled designs, and more diverse populations. The impact of other important potential predictors, such as the participant’s expectations, the experimenter’s mindset, the number and quality of preparation sessions, or the influence of the psychological interventions during drug intake, is not known and needs to be empirically investigated.
A better understanding of the influence of non-pharmacological variables seems not only to be crucial for the fine-tuning of the acute experience but also for producing enduring beneficial effects after drug intake 48 . A few recent studies have emphasized that the mystical-type experience (MEQ30, M-total or OB score) mediates the persisting positive changes in trait openness 67 68 , mood, well-being, attitudes, and psychosocial behaviors in healthy volunteers 48 67 69 as well as the enduring antidepressant effects in patients with major depression 70 and terminal cancer patients 71 72 ( Fig. 1b ). However, not every study found an increase in openness as a personality trait 73 or a correlation between the overall mystical experience and the enduring therapeutic effects in patients 74 75 . A recent prospective study with psilocybin reported that the M-Scale subscale scores for ‘unity’ and ‘sacredness’ were the strongest predictors of the increases in “self-acceptance” and “appreciation for life” at a four-month follow-up in healthy meditation experts 48 ( Fig. 1b ). Thus, the specific contribution of the different dimensions of the psychedelic experience, including the release and working-through of distressing emotions, to the long-term outcomes, remains to be systematically investigated 41 70 76 77 78 .
Neurobiology of Psychedelics
Receptor activation and pharmacological effects of psychedelics
Classic psychedelics such as psilocybin, DMT, or LSD act as partial agonists upon 5-HT1, 5-HT2, 5-HT6, and 5-HT7 receptors 4 . LSD and other ergolines also act upon dopaminergic (D1, D2) and adrenergic receptors 4 , while mescaline and DOI are selective agonists at 5-HT2A, 5-HT2B, and 5-HT2C sites 81 . Activation of 5-HT2A receptors located in cortical and subcortical structures seems to be a key mechanism in mediating many of the behavioral and psychological effects of psychedelics in animals 82 and humans 15 16 . Blocking 5-HT2A/5-HT2C receptors with ketanserin abolished virtually all of the subjective effects of psilocybin, LSD, and DMT in humans 6 15 16 83 84 85 86 87 . The intensity of psilocybin-induced subjective effects correlated with 5-HT2A receptor occupancy in the prefrontal cortex and other cortical regions 66 88 . In addition, pre-treatment with the 5-HT1AR agonist buspirone significantly reduced the visual effects of psilocybin in healthy volunteers 89 , while the 5-HT1AR antagonist pindolol significantly increased the psychological responses to DMT 90 , suggesting a modulatory effect of the 5-HT1AR system on 5-HT2-mediated psychedelic effects. The 5-HT1AR has also been thought to contribute to the attention-disrupting effects of psilocybin in humans 91 .
Psilocybin was also found to increase striatal dopamine concentrations, which correlate with euphoria and depersonalization phenomena in humans 18 . Blocking of D2 receptors with haloperidol partially diminished the psilocybin-induced positively experienced depersonalization but not the visual alterations and working memory impairments, and even increased anxious derealization phenomena in healthy volunteers 15 . While psilocybin does not act directly on dopamine receptors, LSD shows high intrinsic activity at dopamine D2 receptors which may be responsible for the more psychotic-like effects in humans 4 . However, studies specifically blocking dopaminergic receptors after LSD administration are currently lacking. A recent animal study showed that high doses of LSD known to produce psychotic-like behavioral effects in rodents 92 , but not low doses, modulated dopaminergic activity in the ventral tegmental area via activation of trace amine-associated receptors 1 (TAAR1) 93 . Hence, TAAR1 receptors may provide a novel target for the treatment of LSD-induced psychotic-like symptoms. In addition, hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists such LSD and lisuride differentially activate intracellular signaling pathways in cortical neurons 94 , and only hallucinogenic agonists such as LSD and DOI increased the expression of the early genes EGR1 and EGR-2 95 . This functional selectivity remains to be further investigated and maybe a reference for the development of novel compounds with specific therapeutic properties.
Neuroplastic effects of psychedelics
Several preclinical studies have shown that LSD and DOI increase cortical glutamate levels and layer 5 pyramidal cell activity in the prefrontal cortex 96 . The increase in glutamate is due to recurrent network activity triggered by activation of postsynaptic 5-HT2A receptors located in deep layer 5 or 6 pyramidal neurons that project to layer 5 pyramidal neurons. This glutamate release subsequently activates postsynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors located in the apical dendrites in the same neurons which in turn is suggested to increase the gene expression of brain-derived neurotrophic factor (BDNF), a protein known to promote neuronal growth and neuroplasticity. DOI administration was found to increase BDNF expression in the prefrontal cortex and hippocampus in rodents 97 . In recent, DOI, LSD, psilocybin, and DMT produced both structural and functional neuronal plasticity in prefrontal cortical neurons in vitro and in vivo 98 99 100 102 . The increased synaptogenesis appears to be mediated through activation of 5-HT2A, tropomyocin receptor kinase B (TrkB), and mTOR signaling pathways 98 , given that the spine remodeling in cortical layer V pyramidal neurons was abolished by antagonism of TrkB, BDNF’s primary target, and activator or mTOR, or by blocking 5-HT2A receptors with ketanserin 98 . However, a recent study conducted in mice 102 showed that blocking of 5-HT2A receptors with a ketanserin dose (1 mg/kg), sufficient to completely abolish head twitch responses, did not block psilocybin-induced structural plasticity 102 . Another study similarly found that ketanserin (2 mg/kg) almost completely reduces the ability of psilocybin to induce head twitches but not its neuroplastic and antidepressant-like behavioral effects in mice 103 . The findings suggest that the spine remodeling and antidepressant-like effects of psychedelics in animals may not or only partially depend on 5-HT2A receptor activation but may also involve the activation of other serotonin receptors and signaling pathways [4]. However, given that different routes of administration and dosage were used in these two studies, further dose-response research may be necessary to clarify the role of the 5-HT2A receptor in these processes. Whether psychedelics exert their neuroplastic effects and potentially associated therapeutic consequences via 5-HT2A receptor agonism and/or polypharmacological action remains to be investigated.
To date, only a few studies have investigated the relevance of this psychedelic-induced increase of glutamate-driven AMPA receptor throughput and associated neuroplastic adaptations for the behavioral effects in animals and humans. In mice, low-dose psilocybin has been shown to facilitate the extinction of fear memory associated with a tendency to increase hippocampal neuroplasticity 104 . Similarly, DOI administration in mice led to fast-acting dendritic spine structural plasticity in prefrontal pyramidal neurons and acceleration of fear-extinction via the 5-HT2A receptor 100 . In another in vivo study, DOI produced a long-lasting depression of evoked AMPA excitatory postsynaptic currents in layer V pyramidal neurons in mice as an index of synaptic plasticity 105 . In a recent study on mice, repeated LSD administration (but not a single dose) selectively enhanced prosocial behavior without eliciting antidepressant effects by increasing medial prefrontal cortex (mPFC) excitatory neurotransmission through activation of 5-HT2A/AMPA receptors and mTOR signaling 106 . The inactivation of the mPFC excitatory neurons inhibited social interactions and nullified the social effects of LSD 106 . Using multiple measures of behavior, a recent study found that psilocybin produced fast antidepressant-like behaviors accompanied by strengthened synaptic transmission in the hippocampus of mice 103 . Intriguingly, neither the behavioral nor the electrophysiological responses were prevented by pretreatment with the 5-HT2A/C antagonist ketanserin, suggesting that the behavioral and synaptic effects of psilocybin are independent of 5-HT2A receptor activation, at least in these paradigms tested so far. The authors concluded that psilocybin may promote restoration of synaptic connectivity in cortico-mesolimbic circuits processing reward and emotions without involving 5-HT2AR-dependent psychedelic effects, which has to be confirmed in further studies. With regards to neuroplasticity effects in humans, one clinical trial of ayahuasca for depression found a correlation between BDNF plasma levels 48 hours post-treatment and symptom improvements 107 . However, in a recent study, 200 µg LSD significantly increased plasma BDNF levels 6 hours post-treatment, while there were only nonsignificant increases in plasma BDNF after 25, 50, and 100 µg LSD or after ketanserin with LSD treatment in healthy volunteers 36 108 . A crucial limitation of such studies is that BNDF concentration cannot be directly assessed in the brain. Further studies including alternative approaches to brain plasticity are needed to investigate if and how the neuroplastic effects seen in animals relate to the long-lasting symptom improvements reported in recent clinical studies 27 .
Functional Network Models of Psychedelic States
Recent human neuroimaging studies into psychedelic-induced changes in brain activity and connectivity patterns during resting state gave rise to various hypotheses regarding the neural underpinnings and widespread functional network disruptions underlying acute psychedelic states. Empirical evidence supports changes in thalamic gating, signal diversity of cortical activity, between- and within functional network integration, and temporal dynamics induced by psychedelic compounds.
Thalamic gating model
Alteration of information processing within cortico–striato–thalamo-cortical (CSTC) feedback loops is one mechanism that has been proposed to underly the psychedelic experience. The thalamus within this circuit is crucial in gating external and internal information to the cortex and, thereby, in the regulation of the level of consciousness and attention 109 110 111 ). Thalamic gating is under the control of glutamatergic cortico–striatal and cortico-thalamic pathways that project to specific and nonspecific nuclei of the thalamus and under the modulatory influence of serotonergic and dopaminergic projections arising from the raphe and ventral tegmentum to several components of the CSTC loops. The CSTC model proposes that psychedelics disrupt thalamocortical information flow through the stimulation of 5-HT2A receptors located on cortical pyramidal cells and/or GABA interneurons in several parts of the CSTC loop, resulting in an information overload of the cortex and subsequent disruption of cortico-cortical integration of distributed neuronal activity. This could ultimately cause the increased sensory perception, cognitive disturbances, and ego-dissolution that arise in psychedelic experiences 109 112 ( Fig. 2 ). This hypothesis is also compatible with the suggested increase of bottom-up information flow and relaxed priors proposed in the relaxed beliefs under psychedelics (REBUS) model described below 113 .
Fig. 2.
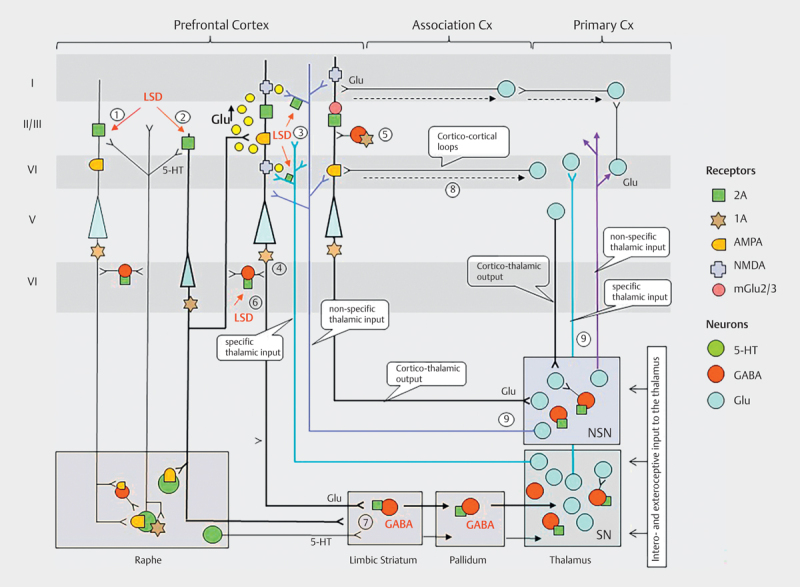
Working hypothesis of psychedelic drug effects on cortico-striato-thalamo-cortical and cortico-cortical circuits of information flow: The schema in Fig. 2 comprises central brain networks on the effects of psychedelic drugs responsible for bottom-up sensory input via the thalamus to the cortex and top-down cortico-striato-thalamic, cortico-thalamic and/or cortico-cortical control of information processing. The model is based mostly on data obtained on the action of LSD and DOI in animals as well as from some studies with LSD and psilocybin in humans. The 5-HT2A receptors are highly expressed in the apical dendrites of layer 5 pyramidal (L5p) neurons in the cortex and are particularly enriched in the prefrontal cortex (PFC) 129 130 131 . A smaller proportion is located pre-synaptically on thalamocortical afferents projecting to the neocortex 96 . 5-HT2ARs are also expressed on GABAergic interneurons in the cortex and subcortical structures 131 . LSD and DOI both increase extracellular glutamate levels via activation of post-synaptic 5-HT2A receptors on deep layers 5 and 6 pyramidal neurons (L5p) (stage 1) and on Lp6 (stage 2) neurons projecting to L5p neurons 96 132 133 as well as via activation of pre-synaptic 5-HT2A receptors on specific (SP) and non-specific (NSP) thalamocortical afferents 96 134 . Psychedelics such as LSD can also stimulate 5-HT1A receptors on the hillock on Lp5 and Lp6 neurons (stage 4) and cortical GABAergic interneurons (stage 5) resulting in both inhibition and disinhibition of prefrontal pyramidal cell activity 132 135 136 . Furthermore, LSD or DOI are also potent partial agonists at cortical (stage 6) and subcortical (striatal, pallidal or thalamic) (stage 7) 5-HT2A receptors in GABAergic interneurons 137 138 . Despite this partially inhibitory mechanisms, this LSD- or DOI-induced increased glutamate release produces a striking net-excitatory effect on L5p neurons 139 140 141 and promotes synaptic plasticity via AMPA and NMDA receptor-dependent mechanisms 98 105 106 133 142 . L5P neurons affect both thalamic and cortical processing and have the unique ability to couple thalamo-cortical (stage 8) and cortico-cortical loops (stage 9) of information streams with each other 143 . This is thought to provide a mechanism through which the state and content of consciousness are functionally coupled 134 . Psychedelics appear to affect this extended thalamic-cortical broadcasting system and thus consciousness as a whole, by simultaneously producing sensory flooding and arousal via reduced thalamic gating of interoceptive and exteroceptive stimuli and by altering the meaning and attachment of percepts due to disrupted cortico-cortical interactions 19 109 . In this model, thalamic gating is thought to be under the control of glutamatergic cortico-striatothalamic and cortico-thalamic loops projecting back to the cortex, in addition to being under the modulatory influence of serotonergic (and dopaminergic) projections from the raphe (and the VTA) to several parts of the CSTC.
The CSTS model is supported by the recent finding that LSD dose-dependently reduced the firing activity of reticular thalamus GABAergic neurons accompanied by disinhibition of mediodorsal thalamus relay neurons and increased firing activity of infralimbic prefrontal pyramidal neurons in mice 114 . Infusion of DOI into the dorsal pallidum in rodents and systemic administration of psilocybin, LSD, and DMT in humans disrupts sensorimotor gating and is associated with cognitive impairments in a 5-HT2A -dependent manner 34 115 116 117 . Two neuroimaging studies reported that LSD increased functional connectivity between the thalamus and sensory-somatomotor cortical regions in healthy volunteers 118 119 . LSD increased directed excitatory connectivity from the thalamus to the posterior cingulate cortex (PCC) and concomitantly decreased functional connectivity to the temporal cortex 120 . In line with the CTSC model, LSD also decreased control of the ventral striatum over the thalamus 120 . These results indicate that LSD differentially affects thalamo-cortical connectivity and does not lead to an undifferentiated increase in cortical information flow 120 . According to the hypothesis that disruption of thalamic gating may result in a sensory overload of the frontal cortex (“hyperfrontality”) 121 , two positron emission tomography studies reported increased prefrontal glucose metabolism after psilocybin administration in healthy volunteers 121 122 which also remained evident after normalizing for global effects of psilocybin 123 . Similar frontal-dominated effects were shown with DMT and mescaline measuring cerebral blood flow (CBF) with single-photon emission computed tomography 124 125 . However, using arterial spin labeling to investigate changes in brain perfusion, LSD was found to increase CBF in the visual cortex 126 while psilocybin produced brain-wide hypoperfusion in healthy subjects 127 . This latter result was replicated by Lewis et al. 128 , but after adjusting for unspecific global effects, psilocybin was found to increase CBF in frontal and temporal regions and decrease CBF in subcortical and occipital regions. These findings are consistent with the hypothesis that reduced thalamic gating leads to overactivity of prefrontal brain regions, and also illustrate that the interpretation of such changes depends on the analytical methods used. It is also conceivable that modern imaging techniques are unable to determine the delay between changes in brain activity and signal acquisition and how temporally dynamic thalamic gating may be. Future studies are warranted to investigate whether differential effects on thalamic subregions or other subcortical structures may provide a more detailed model and their linkage with specific psychological alterations of psychedelic states.
The functional state of CSTC loops can be inferred by perturbational imaging (e. g., electroencephalography, EEG combined with transcranial magnetic stimulation, TMS) to assess drug-induced changes in brain state in real-time 146 . Perturbational imaging reveals the synchronized neuronal firing mediated by receptor kinetics 147 and can be used to describe the functional state of the brain. TMS-pulses induce a phase-reset of several endogenous cortical oscillations and can therefore also be used as a biomarker of the physiological state and to compare across physiological conditions [146]. TMS-EEG is currently being used to probe psychedelic-induced changes in cortico-thalamo-cortical dynamics in humans (unpublished). This unique approach to characterizing the effect of psychedelics on regional interactions at millisecond resolution is expected to clarify the relationship between phenomenological state and brain-state. The role of receptor kinetics in the TMS-evoked response, in combination with the ability to infer cortico-thalamo-cortical interactions using this technique, offers the possibility to model the relationship between pharmacodynamics and psycho-physiological responses.
Neural Entropy model
The “entropic brain hypothesis” (EBH) proposes that the variety of altered states of consciousness can be indexed through the information-theoretic measure of the entropy of key parameters of brain activity 113 148 . The EBH together with the ‘free-energy principle’ has recently been integrated to formulate the “REBUS and the anarchic brain” model 113 . In brief, this model states that psychedelics increase the entropy of spontaneous cortical activity and consequently reduce the precision of high-level priors (expectations or beliefs about the world), and thereby liberating bottom-up information flow 113 . This renders recurrent cortical information processing more sensitive to the ascending information flow resulting in increased entropy or complexity of the underlying neuronal activity. Recent empirical research has identified increased entropy or signal diversity as a signature of psychedelic states 149 . Recent neuroimaging studies with magnetoencephalography (MEG) and EEG showed that LSD, psilocybin, and DMT increased the Lempel-Ziv complexity, a measure of signal diversity and approximation to entropy, which correlated with the overall intensity of the psilocybin and DMT-induced psychedelic experience 150 151 . Furthermore, in an fMRI study, LSD increased sample entropy in sensory and some higher-order networks 80 . An increased repertoire of different brain states including rapid brain dynamics and functional connectivity was reported after the administration of psilocybin and LSD in the same set of healthy individuals 148 152 . Increased Shannon entropy, broadly defined as the amount of information in a variable, was also reported in seven participants after ayahuasca 153 . A recent mechanistic simulation model of the entropic effects of LSD suggests that 5-HT2A receptor activation leads to an increase in the overall entropy of the neural signals, but also that the entropy changes are not uniform across brain regions. Entropy increased in some cortical regions and decreased in some subcortical regions as a result of 5-HT2A receptor activation, suggesting a reconfiguration of the topographical distribution of entropy. Intriguingly, at the whole-brain level, this change was poorly explained by 5-HT2A receptor density, but correlated strongly with local connectivity strength 154
The REBUS model proposes that the increase in entropy under psychedelics reflects a relaxation of the precision weighting of high-level priors, leading to decreased top-down and increased bottom-up information flow. In support of this view, LSD reduced the electrophysiological responses to surprising stimuli in an auditory mismatch paradigm 155 . Analysis by Dynamic Causal Modeling (DCM) revealed that this effect was best explained by reduced top-down information flow from the frontal cortex 155 . However, other studies with psilocybin 156 157 158 or DMT 159 did not reveal reductions in auditory mismatch processing. On the other hand, a recent fMRI-EEG study using a tactile mismatch paradigm revealed that psilocybin reduced the blood oxygenation level dependent (BOLD) signal responses to surprising tactile stimuli in frontal cortex regions, visual cortex, and cerebellum, as well as the electrophysiological responses in frontal cortical regions correlating with the experience of disembodiment and altered meaning of percepts 160 . Hence, it is conceivable that increased bottom-up information flow, presumably by altered cortico-thalamo-cortical gating and impaired top-down cortical integration 109 113 149 , may underlie the reduced sensation of body touch and thus the experience of disembodiment 160 . Further research is needed to unravel the extent to which alterations in bottom-up and top-down information transfer contribute to the topology of entropy changes and signal diversity observed in psychedelic states.
Models of this nature are an important tool for interpreting neurophysiological changes induced by psychedelic drugs. They act as a conceptual lens for explaining how the induced psychological states may be causally linked with physiological states. Distinguishing correlation from causation remains a challenge for neuroscience in general 161 . Approaches like the REBUS model, which conceptualize the brain as having properties of a Bayesian process (i. e., updating high-level priors), have proven to be predictive in many areas of cognitive research 162 . However, confidently mapping Bayesian objects, such as priors, onto the dynamic activity of neuronal populations remains ongoing research 163 . As models of information processing and neuronal population coding are developed and aligned, our understanding of psychedelic states will continue to expand. When borrowing nomenclature from other disciplines, such as “entropy”, “complexity”, “information” and “noise”, it is important to anchor terminology to signal properties and the experimental paradigm used. Experiments that investigate brain activity can either deliver a controlled stimulus and record the (causally known/trial-invariant) signals elicited, or they can record unconstrained (spontaneous) brain activity. Properties such as “signal” and “noise” are easier to define in the context of controlled stimuli because activity can be parsed based on stimulus invariance. Spontaneous activity, on the other hand, used to support the REBUS model, is the preferred terminology, and could be considered by these criteria to be structured noise and therefore a measure of signal diversity such as entropy. Entropy may be the most appropriate description for scenarios when brain activity changes in unpredictable directions and the only consistent outcome following drug administration is unconstrained change.
Effects of Psychedelics on Brain Network Integration
Several neuroimaging studies have investigated the impact of psychedelics on brain network dynamics by measuring resting-state functional connectivity changes between and within intrinsic networks 119 126 127 164 165 166 167 168 . Two studies exploring the effect of LSD and psilocybin on global brain connectivity (GBC) using a graph-based measure of intrinsic whole-brain network connectivity and global signal correction (GSR) found that both drugs increased connectivity of brain region in sensory and somatomotor networks and decreased connectivity of brain regions in associative networks including the Default Mode Network (DMN) 119 169 . The DMN is a large-scale network – consisting of brain regions such as the medial prefrontal cortex, posterior cingulate cortex, precuneus, and angular gyrus - that is activated when one is awake, but not involved in any specific mental exercise 170 . Moreover, the regional GBC changes correlated significantly with the topography of HTR2A gene expression 119 . These results are in line with the finding that psilocybin decreased expression of the frontoparietal control network (that is, a decreased probability of the occurrence of a recurrent phase-locking of BOLD signal over time), and concomitantly increased occurrence of a globally coherent brain state 171 . However, two other studies investigating the effects of LSD on GBC, although without using GSR, reported no overlapping results 118 172 , except for increased functional thalamic connectivity 118 164 172 . The decision to use or forgo GSR to adjust for shared variance across brain regions as well as for physiological-, movement- and scanner-related artifacts 173 remains a point of contention, and there is essentially no single "right" way to process resting-state data 173 . Together, these findings suggest that increased (bottom-up) sensory processing and reduced top-down integration capacity due to diminished associative network integrity may underlie psychedelic experiences. Notably, a recent whole-brain model using the dynamical mean-field quantitative description of excitatory and inhibitory neuronal populations as well as the associated synaptic gain function suggests that the effect of LSD on global brain connectivity can be best explained by the regional distribution and density of 5-HT2A receptors located on cortical pyramidal neurons 174 . A similar approach employing a transcriptomics-informed large-scale cortical model, including the expression level of various serotonergic and dopaminergic genes also found that modulation of pyramidal cell gain by 5-HT2A receptor activation accurately captures the LSD-induced GBC changes 119 144, 145 . In addition, fitting to GBC in individual subjects revealed that the model also captures patterns of individual differences in LSD response that predict different aspects of the psychedelic experience 144 . Thus, it appears that the integration of bio-physical modeling and empirical neuroimaging data provides a promising framework to further unravel circuit mechanisms through which psychedelics alter cortical functional topography. Future work may also incorporate 5-HT2A receptors located in high density within the claustrum 175 and to a lesser extent in subcortical structures 138 , but may also include other types of neuroreceptors such as the dopamine D2 93 138 176 , AMPA 98 105 106 133 or NMDA receptor 142 , all of which have been shown to contribute to the emotional and cognitive effects of psychedelics as described above.
Some studies also reported that psychedelics alter functional network connectivity which is the strength of typical anticorrelations between the DMN and other intrinsic networks 168 . Although psilocybin decreased DMN – Task Positive Network orthogonality 165 , this finding was not replicated in another study after the administration of the DMT-containing drink Ayahuasca 167 . Psilocybin 166 and LSD 126 168 were also found to induce widespread changes in between-network connectivity, although no uniform pattern of changes has emerged so far 168 .
A consistent finding of several studies that have explored within-network functional connectivity (FC) is that psilocybin, LSD, and DMT decrease FC in or between structures of the DMN, 119 126 127 167 168 177 . For example, psilocybin 69 127 and LSD 126 decoupled FC between the medial prefrontal (mPFC) and the posterior cingulate cortices (PCC) - two major hubs of the DMN that have been implicated in self-other distinction, self-related cognition, and inward- versus outward-directed mentalizing 178 . Notably, a recent study found that the decrease in mPFC – PCC FC, two days after psilocybin administration, correlated with the intensity of the acutely experienced self-dissolution (OB) and predicted positive changes in psychosocial functioning in healthy volunteers four months later 69 . However, similar decreases in FC between the nodes of the DMN have also been reported after the administration of selective serotonin reuptake inhibitors 179 and the serotonin-releaser N-Methyl-3, 4-methylenedioxyamphetamine (MDMA) 180 . Changes in DMN activity have been reported for several conditions, including meditation 181 and task-positive behaviors 182 . Hence, identifying DMN changes specific to psychedelic drugs and the contribution of decreased DMN FC to the subjective effects of psychedelics remains to be further investigated.
The concerted interaction of brain networks and brain regions is also reflected by brain oscillations 183 184 . They are characteristic features of the cortical dynamics implicated in the modulation of perception and cognitive functions, which can be measured via resting-state MEG/EEG recordings 185 . MEG/EEG studies reveal that psilocybin, LSD, and DMT reduce spontaneous oscillatory power of low-frequency signals including the delta, theta, beta, and alpha (1–12.5 Hz) frequency bands. The reduction of alpha power in the DMN including the ACC and PCC 177 186 , in parahippocampal regions 186 , and parieto-occipital and posterior association cortices 177 186 187 188 189 was the most consistent finding after administration of psychedelics. Alpha oscillations reflect cortical inhibition of neuronal ensembles 190 . Thus, the decrease in alpha power may indicate a bias of the cortical excitation/inhibition balance towards excitation. DCM applied to MEG data suggests that the reduction in PCC alpha power after psilocybin administration is consistent with increased L5p neuron activity, which also correlated with ego-dissolution 127 . In another study, lagged phase synchronization of delta oscillations between the orbitofrontal cortex, the parahippocampus, and the retrosplenial cortex correlated with the psilocybin-induced spiritual experience and insightfulness 186 . Psilocybin and DMT also increased low gamma oscillations in the PCC 186 as well as low and high gamma power in frontal, temporal, and parieto-occipital cortices 189 . However, decreases in gamma power in prefrontal, sensory and somatomotor areas have also been reported 177 . Gamma oscillations are thought to provide a neuronal mechanism to bind coherently distributed cooperating neuronal assemblies for representation, storage, and retrieval of information 184 191 . The range of cognitive processes in which gamma synchronization has been implicated suggests that its presence may reflect an array of simultaneous processes at work 192 . Changes in gamma synchronization would then reflect changes in information processing, including endogenously-generated states. Hence, alterations in gamma synchronization may well contribute to changes in processes like autobiographical memory retrieval 193 and awareness of one’s own internal state during the psychedelic experience 194 195 .
Neural Correlates of Altered Self- and Emotion-Processing in the Psychedelic States
Early clinical observations in psychedelic research suggested that psychedelics induce regression of the self, lowering of rational thinking, increased affectivity, and facilitated recall of memory blocks. This gave rise to the hypothesis that these are important psychological mechanisms that contribute to the restructuring of the self and self-related functions, as well as the changes in emotion regulation, and thus to the clinical efficacy of psychedelic-assisted psychotherapy 41 76 196 197 198 . Building upon these findings, several studies have investigated the neural correlates of psychedelic-induced alterations in self-experience, cognition, mood, and emotion processing.
Self-Processing
Psychedelics profoundly alter various aspects of the ordinary coherent self-experience 6 . This is often described as a loosening of self-boundaries, an experience of oneness, disembodiment, a loss of authorship of thought, emotions, and actions, and dissolution or disintegration of the experiential “I” or “ego” 6 199 . To date, several studies have attempted to capture the neural correlates of these phenomena by correlating psychometrically assessed subjective alterations in self-experience with brain imaging data. So far, different constructs – ranging from dimensional (e. g. “oceanic boundlessness” 119 200 to sub-dimensional (e. g. “unity” 6 201 and to single item-based approaches (e. g., “I experienced a disintegration of my self or ego” 126 172 177 202 203 – have been used to measure the complex multi-layered alterations of self-experience in psychedelic states.
In an fMRI study, the LSD-induced loss of self-boundaries correlated with increased global brain connectivity in the somatomotor network 119 , while in another study the subjective reports of ego-dissolution correlated with increased global FC in the angular gyrus and the insula 172 . In a subsequent analysis of the later study 172 , the LSD-induced ego-dissolution also correlated with decreased seed-based functional connectivity within the DMN and between the parahippocampus and retrosplenial cortex as measured by fMRT and with decreased delta and alpha power as measured by MEG 126 . By focusing on the time-dependent effects of LSD on functional connectivity, another analysis of this study 172 revealed that the feeling of ego dissolution correlated with the increased weighted small-world propensity (organization) during the dynamic sub-state of high global integration 203 . Concerning the effect of psilocybin, one study noted that the self-reported ego-dissolution correlated with decreased alpha power in the PCC 177 , and in the same participants, with a reduction of FC between the medial temporal lobe (MTL) and high-level cortical regions, a “disintegration” of the salience network (SLN), and a reduction of interhemispheric communication 202 . In another study, the Psilocybin-induced spiritual experience and insightfulness – two subdimensions of OB – correlated with the lagged phase synchronization of delta oscillations between the retrosplenial cortex, the parahippocampus, and the orbitofrontal cortex 186 .
Recent neurocognitive approaches to the self suggest that self-referential processing of internal and sensory stimuli in cortical midline structures constitute a core concept of one’s self 204 205 206 , a phenomenal self as the subject of experience, also referred to as a self-model 7 28 . The representation of the self as a solid entity includes in parallel the processing of internal stimuli from one’s own body with emotions and cognition which are also examined through self-reference and bound to that entity 7 207 . This complex multi-layered representation of the self has various features such as a sense of being, ownership of a body, temporal order, spatial location, ownership and authorship of thoughts, emotions and actions, and a history 208 . Along this line, a recent EEG-ERP study using an auditory self-monitoring task found that psilocybin abolished self-stimuli encoding via a P300 mechanism associated with current source density changes in the supragenual anterior cingulate cortex and right insula 209 . Notably, the extent of the P300 effect significantly correlated with the intensity of the experience of unity (‘oneness with the surroundings’) and changed the meaning of percepts, assessed psychometrically. Moreover, in accordance with predictive coding principles 210 , psilocybin also reduced tactile mismatch processing in prefrontal cortex regions that correlated with the extent of disembodiment and changed meaning in a combined EEG-fMRT study in healthy volunteers 160 . The phenomenon of reduced mismatch processing has been interpreted as reflecting an impairment of predictive coding or, more generally, the “Bayesian brain” notion that the brain continuously updates a hierarchical model to infer the causes of its sensory inputs 162 . Thus, this study provides the first evidence to the hypothesis that the profound alteration of the bodily-self as an aspect of self-dissolution during psychedelic states is due to a dysfunctional integration of bodily states and sensory inputs with prior beliefs 211 . However, more research is warranted to further investigate the detailed hierarchical and temporal dynamics of psychedelics-induced disruption of belief updating within the framework of predictive coding 212 .
Taken together, these disparate findings regarding the neuronal correlates of altered self-experience or ego-dissolution appear to reflect different facets and layers of the dynamics of self-loss in psychedelic states, but may also be due to the different methods, metrics, and doses used across studies. The participant’s widely different understanding of ambiguous terms such as “ego”, “sacredness” or “spiritual experience” may also have contributed to the variability of present results 213 214 . More differentiated operationalization and fine-grained psychometric instruments are needed to conclusively identify specific neural correlates of altered self-experiences across future studies. However, the present data also suggest that the self should be understood as a complex matrix of representations involving different structures and functions rather than a single entity that could be readily abandoned in psychedelic states. The investigation of self-referential processing may offer a promising alternative operationalized approach to unravel the neural correlates of altered self and ego-dissolution.
Beyond the scope of getting a deeper insight into the different organizing principles and processing levels that constitute our self, these studies are important because alterations in self-processing are considered to be crucial for the efficacy of psychedelic-assisted therapy 41 197 198 . So far, positively experienced self-dissolution (e. g., OB) or mystical-type experiences were correlated with the treatment success in an open-label study of major depression 215 and two controlled studies of depression and anxiety in palliative care 71 72 . Clinical observations suggest that the transient dissolution of self-boundaries and the reduction of self-referential processing leads to decentering 41 48 216 217 218 , which is a process of stepping outside of one’s own immediate experience enabling a person to realize that their thoughts and emotions are not unchangeable facts, but only a constructed reality of the self 219 220 . This shift in perspective facilitates more appropriate reactions and adaptions to one’s own cognitions and negative attribution of emotions and reduces dysfunctional attitudes towards the self 48 219 . Increased self-focus and reduced attention to others and the environment are characteristic features of depression - presumably due to increased DMN resting-state activity and altered balance between DMN and executive network activity 24 221 – therefore, it is conceivable that the transient self-dissolution associated with reduced DMN activity leads to reduced and more flexible cognitive reactions, especially in depressed patients suffering from negative self-attribution and ruminative thinking 22 23 . Consistently, LSD and psilocybin acutely increased emotional empathy 222 223 , facilitated social adaptation 224 , and reduced rejection sensitivity and feelings of social exclusion 20 201 . Hence, reduced self-processing may also promote improvements in social cognition 20 , thereby contributing to the emotional attunement which is important in (psychedelic-assisted) psychotherapy of depressed patients 11 41 198 . However, these hypotheses remain to be tested in clinical studies.
Emotional Processing
Several studies in healthy volunteers have shown that psychedelics also acutely alter emotion processing, and particularly reduce the response to negative emotional stimuli. For example, psilocybin and LSD dose-dependently attenuated the recognition of negative facial expression in healthy volunteers 84 223 225 226 . Intriguingly, psilocybin reduced both non-conscious and conscious structural encoding of fearful faces, although somewhat more pronounced during conscious processing 226 , suggesting that emotional awareness may enhance psychedelic-mediated emotion regulation (for an opposite view see 103 ). Furthermore, psilocybin and LSD reduced the neuronal response to negative stimuli in the amygdala correlated with the increase in the positive mood 227 228 . Subsequent correlation analyses revealed that psilocybin reduced directed connectivity from the amygdala to the primary visual cortex during threat processing 229 and decreased the functional connectivity between the amygdala and the striatum during angry face discrimination 230 . In a recent study, reduced amygdala response to negative stimuli was observed one week after psilocybin administration but returned to baseline one month after administration 231 .
Negative cognitive and emotional biases, as well as increased amygdala reactivity to negative stimuli, are characteristic features of depression 22 . Hence, it is conceivable that psychedelics may acutely abolish this negative cognitive and emotional bias by reducing amygdala reactivity, allowing a cognitive-emotional re-adaption from a decentered stance. To what extent this process on its own or in combination with psychotherapy may contribute to the enduring symptom reductions reported in psychedelic-assisted therapy is hardly understood. So far, a recent open-label study in depression reported increased amygdala reactivity in response to fearful stimuli and decreased amygdala-prefrontal cortex FC one day after psilocybin administration 215 232 . This discrepancy may be because increased amygdala reactivity in these depressed patients was measured prior to psychological integration work 215 . Further longitudinal studies are needed to explore the long-term effects of psychedelics on amygdala reactivity and its clinical relevance.
Conclusion
Elucidating the biological mechanisms of classical psychedelic drugs, while hindered by a complex socio-political history, persists as a promising research endeavor for neuropsychopharmacology. Realizing the full potential of psychedelic compounds as an effective and reliable clinical tool will require a continuous understanding of their interdependent effects at many biophysical and psycho-social levels. Psychedelic experiences have broadly defined phenomenological trajectories, which makes their contents accessible to researchers via psychometrics. Standardized questionnaires currently exist to quantify aspects of these drug-induced alterations in consciousness, which lends to quantification and correlation with biomarkers and measures of physiological state. The diversity of receptor targets is well-characterized, affecting primarily serotonergic receptor subtypes and mediated by several other neuromodulatory receptors. Through these known mechanisms, aspects of psychedelic experiences in humans can be modified by administering other drugs concomitantly to target specific receptor subtypes. The affected receptor sites are situated within neuronal pathways necessary for cortico-thalamic and cortico-cortical feedback circuits. By modulating excitatory-inhibitory balance in these circuits, psychedelic drugs can participate in neuroplasticity within structures critical for information processing in the brain. These insights have laid a foundation for several, non-conflicting, theories about the multi-system-level changes induced by psychedelic drugs. They predict altered pathways in brain structures associated with the integration of information relevant to sensation, cognition, emotions, and the narrative of self. These theories also converge to explanations inspired by biological (statistical) thermodynamics, using the concepts of entropy and information, in combination with the necessary receptor-mediated dynamics. Moreover, modern approaches to causal inference applied to imaging techniques (like DCM and perturbational imaging) are helping to forge models directly relevant to neuropsychiatry which will hopefully become prognostic tools. Neurophenomenological metrics for transient and long-lasting effects of psychedelic drugs on healthy volunteers and patients continue to be discovered, and those which best predict clinical outcomes of psychedelic-assisted therapies are an ever-expanding field and show great promise.
Acknowledgements
This work was supported by a grant from Swiss Neuromatrix Foundation (2021–0104) given to FXV and JS.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.McKenna D, Riba J. New world tryptamine hallucinogens and the neuroscience of Ayahuasca. Curr Top Behav Neurosci. 2018;36:283–311. doi: 10.1007/7854_2016_472. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann A, Heim R, Brack A et al. Psilocybin und psilocin, zwei psychotrope wirkstoffe aus Mexikanischen rauschpilzen. Helv chim Acta. 1959;42:1557–1572. [Google Scholar]
- 3.Schultes R E, Hofmann A.The botany and chemistry of hallucinogens2 edn.Springfield: Charles C. Thomas; 1980 [Google Scholar]
- 4.Nichols D E. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Dittrich A, von Arx S, Staub S. International study on altered states of consciousness (ISASC). Summary of the results. Germ J Psych. 1985;9:319–339. [Google Scholar]
- 6.Preller K H, Vollenweider F X. Phenomenology, structure, and dynamic of psychedelic States. Curr Top Behav Neurosci. 2018;36:221–256. doi: 10.1007/7854_2016_459. [DOI] [PubMed] [Google Scholar]
- 7.Letheby C, Gerrans P. Self unbound: Ego dissolution in psychedelic experience. Neurosci Conscious. 2017;2017:nix016. doi: 10.1093/nc/nix016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milliere R. Looking for the self: Phenomenology, neurophysiology and philosophical significance of drug-induced ego dissolution. Front Hum Neurosci. 2017;11:245. doi: 10.3389/fnhum.2017.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann A.Psychotomimetic Agents. In: Burger A, ed. Chemical constitution and pharmacodynamic actions2 edn.New York: M.Dekker; 1968169–235. [Google Scholar]
- 10.Rucker J J, Jelen L A, Flynn S et al. Psychedelics in the treatment of unipolar mood disorders: A systematic review. J Psychopharmacol. 2016;30:1220–1229. doi: 10.1177/0269881116679368. [DOI] [PubMed] [Google Scholar]
- 11.Leuner H.Hallucinogens as an Aid in Psychotherapy: Basic Principles and Results. In: Pletscher A, Ladewig D, eds. 50 years of LSD: Current status and perspectives of hallucinogens a symposium the Swiss Academy of Medical Sciences1 edn.New York: The Parthenon Publishing Group; 1994175–189. [Google Scholar]
- 12.Abuzzahab F S, Anderson B J. A review of LSD treatment in alcoholism. Int Pharmacopsychiatry. 1971;6:223–235. doi: 10.1159/000468273. [DOI] [PubMed] [Google Scholar]
- 13.Pletscher A, Ladewig D. New York, London: The Parthenon Publishing Group; 1994. 50 Years of LSD: Current Status and Perspectives of Hallucinogens. A Symposium the Swiss Academy of Medical Sciences. [Google Scholar]
- 14.Vollenweider F X, Kometer M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 15.Vollenweider F X, Vollenweider-Scherpenhuyzen MF I, Babler A et al. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 16.Preller K H, Herdener M, Pokorny T et al. The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2A receptor activation. Curr Biol. 2017;27:451–457. doi: 10.1016/j.cub.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Mason N L, Kuypers KP C, Muller F et al. Me, myself, bye: Regional alterations in glutamate and the experience of ego dissolution with psilocybin. Neuropsychopharmacology. 2020 doi: 10.1038/s41386-020-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollenweider F X, Vontobel P, Hell D et al. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man - A PET study with [C-11]raclopride. Neuropsychopharmacology. 1999;20:424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]
- 19.Vollenweider F X, Preller K H. Psychedelic drugs: Neurobiology and potential for treatment of psychiatric disorders. Nat Rev Neurosci. 2020;21:611–624. doi: 10.1038/s41583-020-0367-2. [DOI] [PubMed] [Google Scholar]
- 20.Preller K H, Vollenweider F X. Modulation of social cognition via hallucinogens and “Entactogens”. Front Psychiatry. 2019;10:881. doi: 10.3389/fpsyt.2019.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northoff G, Wiebking C, Feinberg T et al. The ‘resting-state hypothesis’ of major depressive disorder – a translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35:1929–1945. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Disner S G, Beevers C G, Haigh E A et al. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 23.Disner S G, Shumake J D, Beevers C G. Self-referential schemas and attentional bias predict severity and naturalistic course of depression symptoms. Cogn Emot. 2017;31:632–644. doi: 10.1080/02699931.2016.1146123. [DOI] [PubMed] [Google Scholar]
- 24.Brakowski J, Spinelli S, Dorig N et al. Resting state brain network function in major depression - Depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res. 2017;92:147–159. doi: 10.1016/j.jpsychires.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Scalabrini A, Vai B, Poletti S et al. All roads lead to the default-mode network-global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. 2020;45:2058–2069. doi: 10.1038/s41386-020-0785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks M I, Zahid Z, Jones N T et al. Catalysts for change: The cellular neurobiology of psychedelics. Mol Biol Cell. 2021;32:1135–1144. doi: 10.1091/mbc.E20-05-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dos Santos R G, Hallak JE C. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci Biobehav Rev. 2020;108:423–434. doi: 10.1016/j.neubiorev.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Wozniak M. “I” and “Me”: The self in the context of consciousness. Front Psychol. 2018;9:1656. doi: 10.3389/fpsyg.2018.01656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metzinger T.The ego tunnel. The science of the mind and the myth of the self1 edn.New York: Basic Books; 2009 [Google Scholar]
- 30.Studerus E.Psilocybin-Induced Altered States of Consciousness Tolerability, Assessment, and Prediction. Südwestdeutscher Verlag für Hochschulschriften 20131–180.. ISBN 978-3-8381-3663-9 [Google Scholar]
- 31.Studerus E, Gamma A, Vollenweider F X. Psychometric evaluation of the altered states of consciousness rating scale (OAV) PLoS One. 2010;5:e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Studerus E, Gamma A, Kometer M et al. Prediction of psilocybin response in healthy volunteers. Plos One. 2012;7:e30800. doi: 10.1371/journal.pone.0030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths R R, Johnson M W, Richards W A et al. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011;218:649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid Y, Enzler F, Gasser P et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78:544–553. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Dolder P C, Schmid Y, Steuer A E et al. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin Pharmacokinet. 2017;56:1219–1230. doi: 10.1007/s40262-017-0513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holze F, Vizeli P, Ley L et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology. 2021;46:537–544. doi: 10.1038/s41386-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dambrun M, Ricard M. Self-centeredness and selflessness: A theory of self-based psychological functioning and its consequences for happiness. Rev Gen Psychol. 2011;15:138–157. doi: 10.1037/a0023059. [DOI] [Google Scholar]
- 38.Stace W T.Mysticism and philosophy1 edn.London: Macmillan; 1961 [Google Scholar]
- 39.Hood R W. Theory and methods in the psychological study of mysticism. Int J Psychol Relig. 2013;23:294–306. doi: 10.1080/10508619.2013.795803. [DOI] [Google Scholar]
- 40.Yaden D B, Le Nguyen K D, Kern M L et al. Of roots and fruits: A comparison of psychedelic and nonpsychedelic mystical experiences. J Humanist Psychol. 2017;57:338–353. doi: 10.1177/0022167816674625. [DOI] [Google Scholar]
- 41.Leuner H.Halluzinogene. Psychische grenzzustände in forschung und therapie1 edn.Bern: Hans Huber; 1981 [Google Scholar]
- 42.Leuner H.Die experimentelle psychose1 edn.Berlin: Springer; 1962 [Google Scholar]
- 43.Leuner H, Holfeld H. Results and problems of psychotherapy with adjuvant LSD-25 and related substances. Psychiatr Neurol (Basel) 1962;143:379–391. [PubMed] [Google Scholar]
- 44.Griffiths R R, Richards W A, McCann U et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 45.Studerus E, Kometer M, Hasler F et al. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol. 2011;25:1434–1452. doi: 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- 46.Hirschfeld T, Schmidt T T. Dose–response relationships of psilocybin-induced subjective experiences in humans. J Psychopharmacol. 2021:2.69881121992676E14. doi: 10.1177/0269881121992676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dittrich A.Psychological Aspects of Altered States of Consciousness of the LSD Type: Measurements of Their Basic Dimensions and Prediction of Individual Differences. In: Pletscher A, Ladewig D, eds. 50 years of LSD: Current status and perspectives of hallucinogens a symposium the Swiss Academy of Medical Sciences1 edn.New York: Parthenon Publishing; 1994101–118. [Google Scholar]
- 48.Smigielski L, Kometer M, Scheidegger M et al. Characterization and prediction of acute and sustained response to psychedelic psilocybin in a mindfulness group retreat. Sci Rep. 2019;9:14914. doi: 10.1038/s41598-019-50612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffiths R R, Johnson M W, Richards W A et al. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol. 2018;32:49–69. doi: 10.1177/0269881117731279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartogsohn I. Set and setting, psychedelics and the placebo response: An extra-pharmacological perspective on psychopharmacology. J Psychopharmacol. 2016;30:1259–1267. doi: 10.1177/0269881116677852. [DOI] [PubMed] [Google Scholar]
- 51.Kaelen M, Giribaldi B, Raine J et al. The hidden therapist: Evidence for a central role of music in psychedelic therapy. Psychopharmacology (Berl) 2018;235:505–519. doi: 10.1007/s00213-017-4820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaelen M, Roseman L, Kahan J et al. LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol. 2016;26:1099–1109. doi: 10.1016/j.euroneuro.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Strickland J C, Garcia-Romeu A, Johnson M W. Set and setting: A randomized study of different musical genres in supporting psychedelic therapy. ACS Pharmacol Transl Sci. 2021;4:472–478. doi: 10.1021/acsptsci.0c00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haijen E, Kaelen M, Roseman L et al. Predicting responses to psychedelics: A prospective study. Front Pharmacol. 2018;9:897. doi: 10.3389/fphar.2018.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terhune D B, Luke D P, Kaelen M et al. A placebo-controlled investigation of synaesthesia-like experiences under LSD. Neuropsychologia. 2016;88:28–34. doi: 10.1016/j.neuropsychologia.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Studerus E, Hasler F, Vollenweider F X. The factorial structure of the altered states of consciousness rating scale (OAV) Neuropsychobiology. 2009:70–71. [Google Scholar]
- 57.Hood R W, Morris R J, Watson P S. Further factor-analysis of Hoods Mysticism Scale. Psychol. Rep. 1993;73:1176–1178. doi: 10.2466/pr0.1993.73.3f.1176. [DOI] [Google Scholar]
- 58.Barrett F S, Johnson M W, Griffiths R R. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol. 2015;29:1182–1190. doi: 10.1177/0269881115609019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hood R W. The construction and preliminary validation of a measure of reported mystical experience. J Sci Stud Relig. 1975;14:29–41. doi: 10.2307/1384454. [DOI] [Google Scholar]
- 60.Hood R W, Ghorbani N, Watson P J et al. Dimensions of the mysticism scale: Confirming the three-factor structure in the United States and Iran. J Sci Stud Relig. 2001;40:691–705. [Google Scholar]
- 61.Liechti M E, Dolder P C, Schmid Y. Alterations of consciousness and mystical-type experiences after acute LSD in humans. Psychopharmacology (Berl) 2017;234:1499–1510. doi: 10.1007/s00213-016-4453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaelen M, Barrett F S, Roseman L et al. LSD enhances the emotional response to music. Psychopharmacology (Berl) 2015;232:3607–3614. doi: 10.1007/s00213-015-4014-y. [DOI] [PubMed] [Google Scholar]
- 63.Glicksohn J, Barrett T R. Absorption and hallucinatory experience. Appl Cogn Psychol. 2003;17:833–849. doi: 10.1002/acp.913. [DOI] [Google Scholar]
- 64.Ott U, Reuter M, Hennig J et al. Evidence for a common biological basis of the absorption trait, hallucinogen effects, and positive symptoms: Epistasis between 5-HT2a and COMT polymorphisms. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:29–32. doi: 10.1002/ajmg.b.30197. [DOI] [PubMed] [Google Scholar]
- 65.Stenbaek D S, Madsen M K, Ozenne B et al. Brain serotonin 2A receptor binding predicts subjective temporal and mystical effects of psilocybin in healthy humans. J Psychopharmacol. 2021;35:459–468. doi: 10.1177/0269881120959609. [DOI] [PubMed] [Google Scholar]
- 66.Quednow B, Geyer M A, Halberstadt A L. Amsterdam: Elsevier BV; 2010. Serotonin and Schizophrenia. In: Müller C, B. J, eds. Handbook of behavioral neurobiology of serotonin; pp. 585–619. [DOI] [Google Scholar]
- 67.Griffiths R R, Richards W, Johnson M et al. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22:621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacLean K A, Johnson M W, Griffiths R R. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol. 2011;25:1453–1461. doi: 10.1177/0269881111420188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smigielski L, Scheidegger M, Kometer M et al. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. 2019;196:207–215. doi: 10.1016/j.neuroimage.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Roseman L, Nutt D J, Carhart-Harris R L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol. 2017;8:974. doi: 10.3389/fphar.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffiths R R, Johnson M W, Carducci M A et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol. 2016;30:1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross S, Bossis A, Guss J et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J Psychopharmacol. 2016;30:1165–1180. doi: 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid Y, Liechti M E. Long-lasting subjective effects of LSD in normal subjects. Psychopharmacology (Berl) 2018;235:535–545. doi: 10.1007/s00213-017-4733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palhano-Fontes F, Barreto D, Onias H et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol Med. 2019;49:655–663. doi: 10.1017/S0033291718001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agin-Liebes G I, Malone T, Yalch M M et al. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol. 2020;34:155–166. doi: 10.1177/0269881119897615. [DOI] [PubMed] [Google Scholar]
- 76.Leuner H. Halluzinogene in der psychotherapie. Pharmakopsychiatr Neuropsychopharmakol. 1971;4:333–351. [Google Scholar]
- 77.Roseman L, Haijen E, Idialu-Ikato K et al. Emotional breakthrough and psychedelics: Validation of the emotional breakthrough inventory. J Psychopharmacol. 2019;33:1076–1087. doi: 10.1177/0269881119855974. [DOI] [PubMed] [Google Scholar]
- 78.Carbonaro T M, Bradstreet M P, Barrett F S et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30:1268–1278. doi: 10.1177/0269881116662634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31:80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- 80.Lebedev A V, Kaelen M, Lovden M et al. LSD-induced entropic brain activity predicts subsequent personality change. Hum Brain Mapp. 2016;37:3203–3213. doi: 10.1002/hbm.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halberstadt A L. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Halberstadt A L, Chatha M, Klein A K et al. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933. doi: 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kometer M, Cahn B R, Andel D et al. The 5-HT2A/1A agonist psilocybin disrupts modal object completion associated with visual hallucinations. Biol Psychiatry. 2011;69:399–406. doi: 10.1016/j.biopsych.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Kometer M, Schmidt A, Bachmann R et al. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Barrett F S, Preller K H, Herdener M et al. Serotonin 2A receptor signaling underlies LSD-induced alteration of the neural response to dynamic changes in music. Cereb Cortex. 2018;28:3939–3950. doi: 10.1093/cercor/bhx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kraehenmann R, Pokorny D, Aicher H et al. LSD increases primary process thinking via serotonin 2A receptor activation. Front Pharmacol. 2017;8:814. doi: 10.3389/fphar.2017.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valle M, Maqueda A E, Rabella M et al. Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol. 2016;26:1161–1175. doi: 10.1016/j.euroneuro.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Madsen M K, Fisher P M, Burmester D et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. 2019;44:1328–1334. doi: 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pokorny T, Preller K H, Kraehenmann R et al. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol. 2016;26:756–766. doi: 10.1016/j.euroneuro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Strassman R J. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73:121–124. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 91.Carter O L, Burr D C, Pettigrew J D et al. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 92.Halberstadt A L, Geyer M A. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 2013;227:727–739. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Gregorio D, Comai S, Posa L et al. d-Lysergic acid diethylamide (LSD) as a model of psychosis: Mechanism of action and pharmacology. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez-Maeso J, Weisstaub N V, Zhou M et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Maeso J, Ang R L, Yuen T et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marek G J. Interactions of hallucinogens with the glutamatergic system: Permissive network effects mediated through cortical layer V pyramidal neurons. Curr Top Behav Neurosci. 2018;36:107–135. doi: 10.1007/7854_2017_480. [DOI] [PubMed] [Google Scholar]
- 97.Vaidya V A, Marek G J, Aghajanian G K et al. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ly C, Greb A C, Cameron L P et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–3182. doi: 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raval N R, Johansen A, Donovan L L et al. A single dose of psilocybin increases synaptic density and decreases 5-HT(2A) receptor density in the pig brain. Int J Mol Sci. 2021;22:835. doi: 10.3390/ijms22020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de la Fuente Revenga M, Zhu B, Guevara C et al. Prolonged epigenetic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021;37:109836. doi: 10.1101/2021.02.24.432725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de la Fuente Revenga M, Shin J M, Vohra H Z et al. Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Sci Rep. 2019;9:14247. doi: 10.1038/s41598-019-49913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shao L X, Liao C, Gregg I et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–2544 e2534. doi: 10.1016/j.neuron.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hesselgrave N, Troppoli T A, Wulff A B et al. Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci U S A. 2021:118. doi: 10.1073/pnas.2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Catlow B J, Song S, Paredes D A et al. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res. 2013;228:481–491. doi: 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- 105.Berthoux C, Barre A, Bockaert J et al. Sustained activation of postsynaptic 5-HT2A receptors gates plasticity at prefrontal cortex synapses. Cereb Cortex. 2019;29:1659–1669. doi: 10.1093/cercor/bhy064. [DOI] [PubMed] [Google Scholar]
- 106.De Gregorio D, Popic J, Enns J P et al. Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc Natl Acad Sci U S A. 2021:118. doi: 10.1073/pnas.2020705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Almeida R N, Galvao AC M, da Silva F S et al. Modulation of serum brain-derived neurotrophic factor by a single dose of ayahuasca: Observation from a randomized controlled trial. Front Psychol. 2019;10:1234. doi: 10.3389/fpsyg.2019.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holze F, Vizeli P, Muller F et al. Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology. 2020;45:462–471. doi: 10.1038/s41386-019-0569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geyer M A, Vollenweider F X. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 110.Alkire M T, Hudetz A G, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferrarelli F, Massimini M, Sarasso S et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vollenweider F X, Geyer M A. A systems model of altered consciousness: Integrating natural and drug-induced psychoses. Brain Research Bulletin. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 113.Carhart-Harris R L, Friston K J. REBUS and the anarchic brain: Toward a unified model of the brain action of psychedelics. Pharmacol Rev. 2019;71:316–344. doi: 10.1124/pr.118.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inserra A, De Gregorio D, Gobbi G. Psychedelics in psychiatry: Neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacol Rev. 2021;73:202–277. doi: 10.1124/pharmrev.120.000056. [DOI] [PubMed] [Google Scholar]
- 115.Vollenweider F X, Csomor P A, Knappe B et al. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- 116.Quednow B B, Kometer M, Geyer M A et al. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riba J, Rodriguez-Fornells A, Barbanoj M J. Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacology (Berl) 2002;165:18–28. doi: 10.1007/s00213-002-1237-5. [DOI] [PubMed] [Google Scholar]
- 118.Muller F, Lenz C, Dolder P et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand. 2017;136:648–657. doi: 10.1111/acps.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Preller K H, Burt J B, Ji J L et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018:7. doi: 10.7554/eLife.35082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Preller K H, Razi A, Zeidman P et al. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci U S A. 2019;116:2743–2748. doi: 10.1073/pnas.1815129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vollenweider F X, Leenders K L, Scharfetter C et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 122.Gouzoulis-Mayfrank E, Thelen B, Habermeyer E et al. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology (Berl) 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- 123.Vollenweider F X. Advances and pathophysiological models of hallucinogen drug actions in humans: A preamble to schizophrenia research. Pharmacopsychiat. 1998;31:92–103. doi: 10.1055/s-2007-979353. [DOI] [PubMed] [Google Scholar]
- 124.Hermle L, Funfgeld M, Oepen G et al. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: Experimental psychosis as a tool for psychiatric research. Biol Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 125.Riba J, Romero S, Grasa E et al. Increased frontal and paralimbic activation following ayahuasca, the pan-Amazonian inebriant. Psychopharmacology (Berl) 2006;186:93–98. doi: 10.1007/s00213-006-0358-7. [DOI] [PubMed] [Google Scholar]
- 126.Carhart-Harris R L, Muthukumaraswamy S, Roseman L et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A. 2016;113:4853–4858. doi: 10.1073/pnas.1518377113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carhart-Harris R L, Erritzoe D, Williams T et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lewis C R, Preller K H, Kraehenmann R et al. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. Neuroimage. 2017;159:70–78. doi: 10.1016/j.neuroimage.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 129.Hall H, Farde L, Halldin C et al. Autoradiographic localization of 5-HT(2A) receptors in the human brain using [(3)H]M100907 and [(11)C]M100907. Synapse. 2000;38:421–431. doi: 10.1002/1098-2396(20001215)38:4<421::AID-SYN7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 130.Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42:2039–2057. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- 131.Celada P, Puig M V, Artigas F.Serotonin modulation of cortical neurons and networks Front Integr Neurosci 2013725; 1–20. https://doi.org/10.3389/fnint.2013.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Llado-Pelfort L, Celada P, Riga M S et al. Effects of hallucinogens on neuronal activity. Curr Top Behav Neurosci. 2018;36:75–105. doi: 10.1007/7854_2017_473. [DOI] [PubMed] [Google Scholar]
- 133.Zhang C, Marek G J. AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:62–71. doi: 10.1016/j.pnpbp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 134.Aru J, Suzuki M, Rutiku R et al. Coupling the state and contents of consciousness. Front Syst Neurosci. 2019;13:43. doi: 10.3389/fnsys.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: Rationale and current status of research. CNS Drugs. 2013;27:703–716. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- 136.Llado-Pelfort L, Santana N, Ghisi V et al. 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex. 2012;22:1487–1497. doi: 10.1093/cercor/bhr220. [DOI] [PubMed] [Google Scholar]
- 137.Marek G J, Aghajanian G K. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- 138.Inserra A, De Gregorio D, Rezai T et al. Lysergic acid diethylamide differentially modulates the reticular thalamus, mediodorsal thalamus, and infralimbic prefrontal cortex: An in vivo electrophysiology study in male mice. Journal of Psychopharmacology. 2021:2.69881121991569E14. doi: 10.1177/0269881121991569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lambe E K, Aghajanian G K. Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology. 2006;31:1682–1689. doi: 10.1038/sj.npp.1300944. [DOI] [PubMed] [Google Scholar]
- 140.Martin-Ruiz R, Puig M V, Celada P et al. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Puig M V, Celada P, az-Mataix L et al. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: Relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- 142.Barre A, Berthoux C, De B D et al. Presynaptic serotonin 2A receptors modulate thalamocortical plasticity and associative learning. Proc Natl Acad Sci U S A. 2016;113:E1382–E1391. doi: 10.1073/pnas.1525586113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larkum M E, Zhu J J, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- 144.Burt J B, Preller K H, Demirtas Met al. Transcriptomics-informed large-scale cortical model captures topography of pharmacological neuroimaging effects of LSD Elife 202110DOI: ARTN e6932010.7554/eLife.69320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ji JL S, Helmer M, Fonteneau Cet al. Mapping brain-behavior space relationships along the psychosis spectrum Elife 202110DOI: ARTN e6696810.7554/eLife.66968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Farzan F, Vernet M, Shafi M M et al. Characterizing and modulating brain circuitry through transcranial magnetic stimulation combined with electroencephalography. Front Neural Circuits. 2016;10:73. doi: 10.3389/fncir.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tremblay S, Rogasch N C, Premoli I et al. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol. 2019;130:802–844. doi: 10.1016/j.clinph.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 148.Carhart-Harris R L, Leech R, Hellyer P J et al. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20. doi: 10.3389/fnhum.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carhart-Harris R L. The entropic brain - revisited. Neuropharmacology. 2018;142:167–178. doi: 10.1016/j.neuropharm.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 150.Schartner M M, Carhart-Harris R L, Barrett A B et al. Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep. 2017;7:46421. doi: 10.1038/srep46421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Timmermann C, Roseman L, Schartner M et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. 2019;9:16324. doi: 10.1038/s41598-019-51974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Atasoy S, Roseman L, Kaelen M et al. Connectome-harmonic decomposition of human brain activity reveals dynamical repertoire re-organization under LSD. Sci Rep. 2017;7:17661. doi: 10.1038/s41598-017-17546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Viol A, Palhano-Fontes F, Onias H et al. Shannon entropy of brain functional complex networks under the influence of the psychedelic Ayahuasca. Sci Rep. 2017;7:7388. doi: 10.1038/s41598-017-06854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Herzog R, Mediano PA M, Rosas F E et al. A mechanistic model of the neural entropy increase elicited by psychedelic drugs. Sci Rep. 2020;10:17725. doi: 10.1038/s41598-020-74060-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Timmermann C, Spriggs M J, Kaelen M et al. LSD modulates effective connectivity and neural adaptation mechanisms in an auditory oddball paradigm. Neuropharmacology. 2018;142:251–262. doi: 10.1016/j.neuropharm.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 156.Umbricht D, Vollenweider F X, Schmid L et al. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: Implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology. 2003;28:170–181. doi: 10.1038/sj.npp.1300005. [DOI] [PubMed] [Google Scholar]
- 157.Schmidt A, Bachmann R, Kometer M et al. Mismatch negativity encoding of prediction errors predicts S-ketamine-induced cognitive impairments. Neuropsychopharmacology. 2012;37:865–875. doi: 10.1038/npp.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bravermanova A, Viktorinova M, Tyls F et al. Psilocybin disrupts sensory and higher order cognitive processing but not pre-attentive cognitive processing-study on P300 and mismatch negativity in healthy volunteers. Psychopharmacology (Berl) 2018;235:491–503. doi: 10.1007/s00213-017-4807-2. [DOI] [PubMed] [Google Scholar]
- 159.Heekeren K, Daumann J, Neukirch A et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duerler P, Brem S, Fraga-Gonzalez G et al. Psilocybin induces aberrant prediction error processing of tactile mismatch responses-a simultaneous EEG-FMRI study. Cereb Cortex. 2021 doi: 10.1093/cercor/bhab202. [DOI] [PubMed] [Google Scholar]
- 161.Grasso M, Albantakis L, Lang J P et al. Causal reductionism and causal structures. Nat Neurosci. 2021;24:1348–1355. doi: 10.1038/s41593-021-00911-8. [DOI] [PubMed] [Google Scholar]
- 162.Friston K. The free-energy principle: A unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 163.Keller G B, Mrsic-Flogel T D. Predictive processing: A canonical cortical computation. Neuron. 2018;100:424–435. doi: 10.1016/j.neuron.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Muller F, Dolder P C, Schmidt A et al. Altered network hub connectivity after acute LSD administration. Neuroimage Clin. 2018;18:694–701. doi: 10.1016/j.nicl.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carhart-Harris R L, Leech R, Erritzoe D et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2013;39:1343–1351. doi: 10.1093/schbul/sbs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roseman L, Leech R, Feilding A et al. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci. 2014;8:204. doi: 10.3389/fnhum.2014.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Palhano-Fontes F, Andrade K C, Tofoli L F et al. The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS One. 2015;10:e0118143. doi: 10.1371/journal.pone.0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muller F, Liechti M E, Lang U E et al. Advances and challenges in neuroimaging studies on the effects of serotonergic hallucinogens: Contributions of the resting brain. Prog Brain Res. 2018;242:159–177. doi: 10.1016/bs.pbr.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 169.Preller K H, Duerler P, Burt J B et al. Psilocybin induces time-dependent changes in global functional connectivity. Biol Psychiatry. 2020 doi: 10.1016/j.biopsych.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 170.Raichle M E. The brain's default mode network. Annu Rev Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 171.Lord L D, Expert P, Atasoy S et al. Dynamical exploration of the repertoire of brain networks at rest is modulated by psilocybin. Neuroimage. 2019;199:127–142. doi: 10.1016/j.neuroimage.2019.05.060. [DOI] [PubMed] [Google Scholar]
- 172.Tagliazucchi E, Roseman L, Kaelen M et al. Increased global functional connectivity correlates with LSD-induced ego dissolution. Curr Biol. 2016;26:1043–1050. doi: 10.1016/j.cub.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 173.Murphy K, Fox M D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Deco G, Cruzat J, Cabral J et al. Whole-brain multimodal neuroimaging model using serotonin receptor maps explains non-linear functional effects of LSD. Curr Biol. 2018;28:3065–3074 e3066. doi: 10.1016/j.cub.2018.07.083. [DOI] [PubMed] [Google Scholar]
- 175.Barrett F S, Krimmel S R, Griffiths R et al. Psilocybin acutely alters the functional connectivity of the claustrum with brain networks that support perception, memory, and attention. Neuroimage. 2020:116980. doi: 10.1016/j.neuroimage.2020.116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grandjean J, Buehlmann D, Buerge M et al. Psilocybin exerts distinct effects on resting state networks associated with serotonin and dopamine in mice. Neuroimage. 2021;225:117456. doi: 10.1016/j.neuroimage.2020.117456. [DOI] [PubMed] [Google Scholar]
- 177.Muthukumaraswamy S D, Carhart-Harris R L, Moran R J et al. Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci. 2013;33:15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 179.Klaassens B L, van Gorsel H C, Khalili-Mahani N et al. Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage. 2015;122:440–450. doi: 10.1016/j.neuroimage.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 180.Muller F, Holze F, Dolder P et al. MDMA-induced changes in within-network connectivity contradict the specificity of these alterations for the effects of serotonergic hallucinogens. Neuropsychopharmacology. 2020 doi: 10.1038/s41386-020-00906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Garrison K A, Zeffiro T A, Scheinost D et al. Meditation leads to reduced default mode network activity beyond an active task. Cogn Affect Behav Neurosci. 2015;15:712–720. doi: 10.3758/s13415-015-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Elton A, Gao W. Task-positive functional connectivity of the default mode network transcends task domain. J Cogn Neurosci. 2015;27:2369–2381. doi: 10.1162/jocn_a_00859. [DOI] [PubMed] [Google Scholar]
- 183.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 184.Uhlhaas P J, Linden D E, Singer W et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lopes da Silva F. EEG and MEG: Relevance to neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 186.Kometer M, Pokorny T, Seifritz E et al. Psilocybin-induced spiritual experiences and insightfulness are associated with synchronization of neuronal oscillations. Psychopharmacology (Berl) 2015;232:3663–3676. doi: 10.1007/s00213-015-4026-7. [DOI] [PubMed] [Google Scholar]
- 187.Riba J, Anderer P, Morte A et al. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol. 2002;53:613–628. doi: 10.1046/j.1365-2125.2002.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Riba J, Anderer P, Jane F et al. Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: A functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology. 2004;50:89–101. doi: 10.1159/000077946. [DOI] [PubMed] [Google Scholar]
- 189.Schenberg E E, Alexandre J F, Filev R et al. Acute biphasic effects of ayahuasca. PLoS One. 2015;10:e0137202. doi: 10.1371/journal.pone.0137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Buschman T J, Denovellis E L, Diogo C et al. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Başar E. A review of gamma oscillations in healthy subjects and in cognitive impairment. International Journal of Psychophysiology. 2013;90:99–117. doi: 10.1016/j.ijpsycho.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 192.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 193.Griffiths B J, Parish G, Roux F et al. Directional coupling of slow and fast hippocampal gamma with neocortical alpha/beta oscillations in human episodic memory. Proc Natl Acad Sci U S A. 2019;116:21834–21842. doi: 10.1073/pnas.1914180116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vinck M, Bosman C A. More gamma more predictions: Gamma-synchronization as a key mechanism for efficient integration of classical receptive field inputs with surround predictions. Front Syst Neurosci. 2016;10:35. doi: 10.3389/fnsys.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Honkanen R, Rouhinen S, Wang S et al. Gamma oscillations underlie the maintenance of feature-specific information and the contents of visual working memory. Cerebral cortex (New York, NY: 1991) 2014:25. doi: 10.1093/cercor/bhu263. [DOI] [PubMed] [Google Scholar]
- 196.Eisner B G, Cohen S. Psychotherapy with lysergic acid diethylamide. J Nerv Ment Dis. 1958;127:528–539. doi: 10.1097/00005053-195812000-00006. [DOI] [PubMed] [Google Scholar]
- 197.Klee G D. Lysergic acid diethylamide (LSD-25) and ego functions. Arch Gen Psychiatry. 1963;8:461–474. doi: 10.1001/archpsyc.1963.01720110037005. [DOI] [PubMed] [Google Scholar]
- 198.Leuner H. Psycholytic therapy: Clinical psychotherapy with adjuvant LSD-25 and related substances. Z Psychother Med Psychol. 1963;13:57–64. [PubMed] [Google Scholar]
- 199.Hermle L, Kraehenmann R. Experimental psychosis research and schizophrenia-similarities and dissimilarities in psychopathology. Curr Top Behav Neurosci. 2017 doi: 10.1007/7854_2016_460. [DOI] [PubMed] [Google Scholar]
- 200.Vollenweider F X. Brain mechanisms of hallucinogens and entactogens. Dialogues Clin Neurosci. 2001;3:265–279. doi: 10.31887/DCNS.2001.3.4/fxvollenweider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Preller K H, Pokorny T, Hock A et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A. 2016;113:5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lebedev A V, Lovden M, Rosenthal G et al. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp. 2015;36:3137–3153. doi: 10.1002/hbm.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luppi A I, Carhart-Harris R L, Roseman L et al. LSD alters dynamic integration and segregation in the human brain. Neuroimage. 2021;227:117653. doi: 10.1016/j.neuroimage.2020.117653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Northoff G, Boeker H, Bogerts B. Subjective experience and neuronal integration in the brain: Do we need a first-person neuroscience? Fortschr Neurol Psychiatr. 2006;74:627–634. doi: 10.1055/s-2005-915610. [DOI] [PubMed] [Google Scholar]
- 205.Damasio A R. How the brain creates the mind. Sci Am. 1999;281:112–117. doi: 10.1038/scientificamerican1299-112. [DOI] [PubMed] [Google Scholar]
- 206.Araujo H F, Kaplan J, Damasio A. Cortical midline structures and autobiographical-self processes: An activation-likelihood estimation meta-analysis. Front Hum Neurosci. 2013;7:548. doi: 10.3389/fnhum.2013.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 208.Metzinger T. Self-modeling epistemic spaces and the contraction principle. Cogn Neuropsychol. 2020;37:197–201. doi: 10.1080/02643294.2020.1729110. [DOI] [PubMed] [Google Scholar]
- 209.Smigielski L, Kometer M, Scheidegger M et al. P300-mediated modulations in self-other processing under psychedelic psilocybin are related to connectedness and changed meaning: A window into the self-other overlap. Hum Brain Mapp. 2020:1–15. doi: 10.1002/hbm.25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rauss K, Pourtois G. What is bottom-up and what is top-down in predictive coding? Front Psychol. 2013;4:276. doi: 10.3389/fpsyg.2013.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ho J T, Preller K H, Lenggenhager B. Neuropharmacological modulation of the aberrant bodily self through psychedelics. Neurosci Biobehav Rev. 2020;108:526–541. doi: 10.1016/j.neubiorev.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 212.Weber L A, Diaconescu A O, Mathys C et al. Ketamine affects prediction errors about statistical regularities: A computational single-trial analysis of the mismatch negativity. J Neurosci. 2020;40:5658–5668. doi: 10.1523/JNEUROSCI.3069-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hood R W, Streib H. Cham: Springer International Publishing; 2016. “Fuzziness” or semantic diversification? Insights about the semantics of “Spirituality” in cross-cultural comparison (Conclusion). In: Streib H, Hood JRW, eds. Semantics and Psychology of Spirituality: A Cross-Cultural Analysis; pp. 153–161. [DOI] [Google Scholar]
- 214.Keller B, Klein C, Swhajor-Biesemann A et al. The semantics of 'Spirituality’ and related self-identifications: A comparative study in Germany and the USA. Arch Psychol Relig. 2013;35:71–100. doi: 10.1163/15736121-12341254. [DOI] [Google Scholar]
- 215.Roseman L, Demetriou L, Wall M B et al. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology. 2018;142:263–269. doi: 10.1016/j.neuropharm.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 216.Marrazzi A S, Meisch R A, Pew W L et al. Quantified LSD effects on ego strength. Recent Adv Biol Psychiatry. 1966;9:197–207. doi: 10.1007/978-1-4684-8228-7_14. [DOI] [PubMed] [Google Scholar]
- 217.Vago D R, Silbersweig D A. Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Front Hum Neurosci. 2012;6:296. doi: 10.3389/fnhum.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dahl C J, Lutz A, Davidson R J. Reconstructing and deconstructing the self: Cognitive mechanisms in meditation practice. Trends Cogn Sci. 2015;19:515–523. doi: 10.1016/j.tics.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bernstein A, Hadash Y, Lichtash Y et al. Decentering and related constructs: A critical review and metacognitive processes model. Perspect Psychol Sci. 2015;10:599–617. doi: 10.1177/1745691615594577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kessel R, Gecht J, Forkmann T et al. Exploring the relationship of decentering to health related concepts and cognitive and metacognitive processes in a student sample. BMC Psychol. 2016;4:11. doi: 10.1186/s40359-016-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Grimm S, Ernst J, Boesiger P et al. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12:627–637. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- 222.Pokorny T, Preller K H, Kometer M et al. Effect of psilocybin on empathy and moral decision-making. Int J Neuropsychopharmacol. 2017;20:747–757. doi: 10.1093/ijnp/pyx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dolder P C, Schmid Y, Muller F et al. LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology. 2016;41:2638–2646. doi: 10.1038/npp.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duerler P, Schilbach L, Stampfli P et al. LSD-induced increases in social adaptation to opinions similar to one’s own are associated with stimulation of serotonin receptors. Sci Rep. 2020;10:12181. doi: 10.1038/s41598-020-68899-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bershad A K, Schepers S T, Bremmer M P et al. Acute subjective and behavioral effects of microdoses of lysergic acid diethylamide in healthy human volunteers. Biol Psychiatry. 2019;86:792–800. doi: 10.1016/j.biopsych.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schmidt A, Kometer M, Bachmann R et al. The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacology (Berl) 2013;225:227–239. doi: 10.1007/s00213-012-2811-0. [DOI] [PubMed] [Google Scholar]
- 227.Mueller F, Lenz C, Dolder P C et al. Acute effects of LSD on amygdala activity during processing of fearful stimuli in healthy subjects. Transl Psychiatry. 2017;7:e1084. doi: 10.1038/tp.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kraehenmann R, Preller K H, Scheidegger M et al. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol Psychiatry. 2015;78:572–581. doi: 10.1016/j.biopsych.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 229.Kraehenmann R, Schmidt A, Friston K et al. The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. Neuroimage Clin. 2016;11:53–60. doi: 10.1016/j.nicl.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Grimm O, Kraehenmann R, Preller K H et al. Psilocybin modulates functional connectivity of the amygdala during emotional face discrimination. Eur Neuropsychopharmacol. 2018;28:691–700. doi: 10.1016/j.euroneuro.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 231.Barrett F S, Doss M K, Sepeda N D et al. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep. 2020;10:2214. doi: 10.1038/s41598-020-59282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mertens L J, Wall M B, Roseman L et al. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J Psychopharmacol. 2020;34:167–180. doi: 10.1177/0269881119895520. [DOI] [PubMed] [Google Scholar]


