ABSTRACT
Candida albicans is the most prevalent cause of vulvovaginal candidiasis (“yeast infection” or VVC) and recurrent vulvovaginal candidiasis (RVVC), although the incidence of non-albicans yeast species is increasing. The azole fluconazole is the primary antifungal drug used to treat RVVC, yet isolates from some species have intrinsic resistance to fluconazole, and recurrent infection can occur even with fluconazole-susceptible populations. The second-line broad-spectrum antimicrobial drug, boric acid, is an alternative treatment that has been found to successfully treat complicated VVC infections. Far less is known about how boric acid inhibits growth of yeast isolates in different morphologies compared to fluconazole. We found significant differences in drug resistance and drug tolerance (the ability of a subpopulation to grow slowly in high levels of drug) between C. albicans, Candida glabrata, and Candida parapsilosis isolates, with the specific relationships dependent on both drug and phenotype. Population-level variation for both susceptibility and tolerance was broader for fluconazole than boric acid in all species. Unlike fluconazole, which neither prevented hyphal formation nor disrupted mature biofilms, boric acid inhibited C. albicans hyphal formation and reduced mature biofilm biomass and metabolic activity in all isolates in a dose-dependent manner. Variation in planktonic response did not generally predict biofilm phenotypes for either drug. Overall, our findings illustrate that boric acid is broadly effective at inhibiting growth across many isolates and morphologies, which could explain why it is an effective treatment for RVVC.
KEYWORDS: antifungal resistance, antifungal tolerance, biofilms, hyphal development, vulvovaginal candidiasis
INTRODUCTION
Vulvovaginal candidiasis (VVC), a pathological condition of the lower female reproductive tract, affects ~75% of females at least once in their life (1–3). Although patients usually respond well to the first-line treatment, ~8% of females globally will experience recurrent VVC, which can have a significant negative impact on quality of life (4, 5). Candida albicans has been the primary cause (75 to 90%) of VVC, yet the frequency of other species, particularly Candida glabrata followed by Candida parapsilosis, is increasing (6–8). In light of the transition to rename C. glabrata as Nakaseomyces glabrata (9), we use the acronym “NAC” to refer to “non-albicans clinical” species, rather than “non-albicans Candida” species, as has been done historically.
In North America, the azole drugs clotrimazole and fluconazole (FLC) are the first-line treatments recommended by the Infectious Diseases Society of America and the Society of Gynecologists and Obstetricians of Canada (3, 4). When recurrence occurs on first-line treatment or when NAC species are implicated, boric acid (BA) intravaginal suppositories are one of the recommended treatments (3, 4, 10, 11). Despite this success, BA remains a second-line treatment because its exact mechanism of action is unknown, and much less work has evaluated its broad efficacy and possible long-term effects (12, 13). In other jurisdictions (e.g., Germany), BA is not permitted due to concerns regarding toxicity by transdermal reabsorption. However, studies have shown that using one or two 600-mg BA intravaginal suppositories daily for 1 to 2 weeks led to blood concentrations of <1 μg/mL (blood concentrations <200 μg/mL are considered safe), suggesting there is little absorption of BA across the vaginal wall (11). The minimum inhibitory concentration (MIC) of BA was previously measured in a relatively small number of C. albicans, C. glabrata, and C. parapsilosis isolates (13, 14); however, unlike FLC (and many other antifungal drugs), there are no standard clinical methods for drug resistance testing nor have breakpoints been established for BA planktonic resistance. Furthermore, variation among isolates in fungal drug tolerance, the ability of a subpopulation of drug-susceptible isolates to grow slowly at inhibitory drug concentrations (15–18), has largely been ignored, yet recent studies are beginning to implicate tolerance as a factor in predicting drug efficacy (16–18).
Morphological plasticity is an important virulence trait in many contexts for Candida species (19). The yeast-to-hyphal transition is critical for biofilm stability, penetration of host epithelial cells, and escape from host phagocytes (20, 21). Hyphal formation is also important for biofilm formation in C. albicans (C. glabrata do not form hyphae, and C. parapsilosis form pseudohyphae) (22). The involvement of biofilms in RVVC is still being debated (23, 24). However, hyphal forms were recently detected in vaginal lavage fluid from an RVVC patient (25). In vivo mice models showed that C. albicans can form biofilms on the vaginal mucosa (23, 24), and transcripts from genes involved in hyphal morphogenesis biofilm formation were detected in vaginal lavage (25). FLC was shown previously not to inhibit hyphal formation (26). However, low BA concentrations (0.02 mg/mL) can disrupt the cytoskeleton of hyphae by changing the actin distribution from the apical to the isotropic pattern (27), and higher BA concentrations (10 or 50 mg/mL) were shown to inhibit hyphal formation in two C. albicans isolates (13). BA has also been found to reduce the biomass of mature biofilms relative to biofilms growing without drugs (13); it was unclear, however, whether this reflected the drug simply stopping further growth, or whether boric acid acted to reduce the biofilm below pre-drug treatment levels.
To quantify the diversity of BA phenotypic responses among different species, we compared FLC and BA planktonic susceptibility and tolerance among 235 clinical isolates of C. albicans, C. glabrata, and C. parapsilosis. We also quantify the impact of FLC and BA on C. albicans yeast-to-hyphal transition (leading to biofilm formation) and on C. albicans mature biofilms. We found significant differences among species for drug resistance and tolerance and a consistent increase in the variance among isolates for both drug response phenotypes in FLC compared to BA. We also found that unlike FLC, BA is effective at inhibiting the yeast-to-hyphal transition and thus biofilm formation and can effectively break apart mature biofilms. Combined, this demonstrates multiple pathways where BA is more effective at inhibiting Candida isolate growth compared to fluconazole.
RESULTS
Variation in drug susceptibility and tolerance.
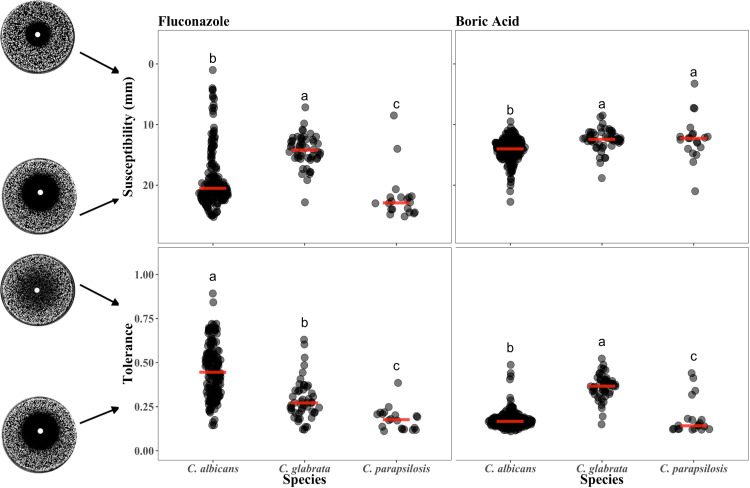
Fluconazole (FLC) and boric acid (BA) drug susceptibility and tolerance were measured for 235 Candida isolates (165 C. albicans, 50 C. glabrata, and 20 C. parapsilosis) using diskImageR, a computational analysis tool that quantifies drug response from images of disk diffusion assays (15). Susceptibility was measured as the radius where 20% of growth reduction occurs (RAD20), while tolerance was measured as the fraction of the population that is able to grow above RAD20 after 48 h (FoG20). C. albicans isolates on average had higher susceptibility than C. glabrata but lower susceptibility than C. parapsilosis and higher tolerance than either in FLC (Fig. 1, left panels; Kruskal-Wallis rank-sum test; susceptibility: χ2 = 63.43 df = 2, P < 0.0001, species differences determined by post hoc pairwise Wilcoxon tests with the P adjustment [28]; tolerance: χ2 = 82.04, df = 2, P < 0.0001). In BA, C. albicans isolates had higher susceptibility than both NAC species and lower tolerance than C. glabrata (susceptibility: χ2 = 39.8, df = 2, P < 0.0001; tolerance: χ2 = 97.9, df = 2, P < 0.0001). The magnitude of isolate variation in BA was significantly less than in FLC for both C. albicans and C. glabrata, the two species with a sufficiently large sample size (Fligner-Killeen test of homogeneity; susceptibility: C. albicans, χ2 = 23.4, df = 1, P < 0.0001; C. glabrata, χ2 = 7.8, df = 1, P = 0.005; tolerance: C. albicans, χ2 = 116.59, df = 1, P < 0.0001; C. glabrata, χ2 = 4.94, df = 1, P = 0.03).
FIG 1.
Susceptibility and tolerance for fluconazole and boric acid was quantified for 165 C. albicans, 50 C. glabrata, and 20 C. parapsilosis isolates. Note that susceptibility is measured as radius where 20% of growth reduction occurs (RAD20), and the y axis is reversed so that the less susceptible (more resistant) isolates are toward the top. Tolerance is measured as the fraction of the population that is able to grow above RAD20 after 48 h (FoG20) with diskImageR analysis. Letters indicate the statistical differences among species in each panel from a post hoc pairwise Wilcox test with the Benjamini and Hochberg (28) P-value adjustment; when species do not share a letter, they are significantly different from each other (P < 0.05).
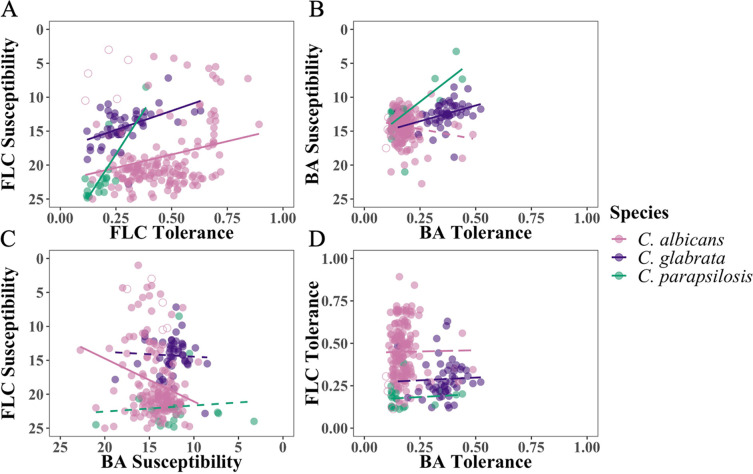
There was a significant positive correlation for FLC susceptibility and tolerance within isolates of the same species: isolates that had lower susceptibility (i.e., higher resistance levels) also tended to have higher tolerance (Fig. 2A, Spearman’s rank correlation, for C. albicans, ρ = −0.207, S = 824,123, P = 0.009; C. glabrata, ρ = −0.49, S = 29,249, P < 0.0001; C. parapsilosis, ρ = −0.73, S = 2,295, P < 0.0001). In BA, the correlation was significant for C. glabrata and C. parapsilosis (Fig. 2B; C. glabrata, ρ = −0.43, S = 29,758, P = 0.002; C. parapsilosis, ρ = −0.63, S = 2,173, P = 0.003) but not C. albicans (ρ = 0.112, S = 720,453, P = 0.119). Looking between drugs, there was a significant negative correlation between FLC susceptibility and BA susceptibility: isolates that are less susceptible to FLC tend to be more susceptible to BA (ρ = −0.20, S = 982,263, P < 0.009). This was also seen as a significant difference in BA RAD20 when we separated the data set into FLC-resistant C. albicans isolates (zone of inhibition in the disk assay < 14 mm, N = 33) (29) and FLC-susceptible isolates (zone ≥14 mm, N = 137; Welch’s t test: t44.0 = 3.60, P = 0.0008; we note, however, that the effect size is small: a difference of 1.5 mm between groups). There were no other significant between-drug correlations for susceptibility or tolerance for other species comparisons (Fig. 2C and D; Spearman’s rank correlation, susceptibility; C. glabrata: S = 20,266, P = 0.85; C. parapsilosis: S = 975, P = 0.26; tolerance, C. albicans: S = 623,346, P = 0.28; C. glabrata: S = 15,401, P = 0.14; C. parapsilosis: S = 1,332, P = 0.995). Combined, this indicates that the mode of action for BA differs from that of FLC.
FIG 2.
Association within and between drug response parameter and drugs. (A) Susceptibility and tolerance in fluconazole (FLC). (B) Susceptibility and tolerance in boric acid (BA). (C) Susceptibility in FLC and BA. (D) Tolerance in FLC and BA. Solid lines indicate a significant correlation (P < 0.05; dashed lines indicate P > 0.05) from a Spearman’s rank correlation test.
BA inhibits C. albicans hyphal formation.
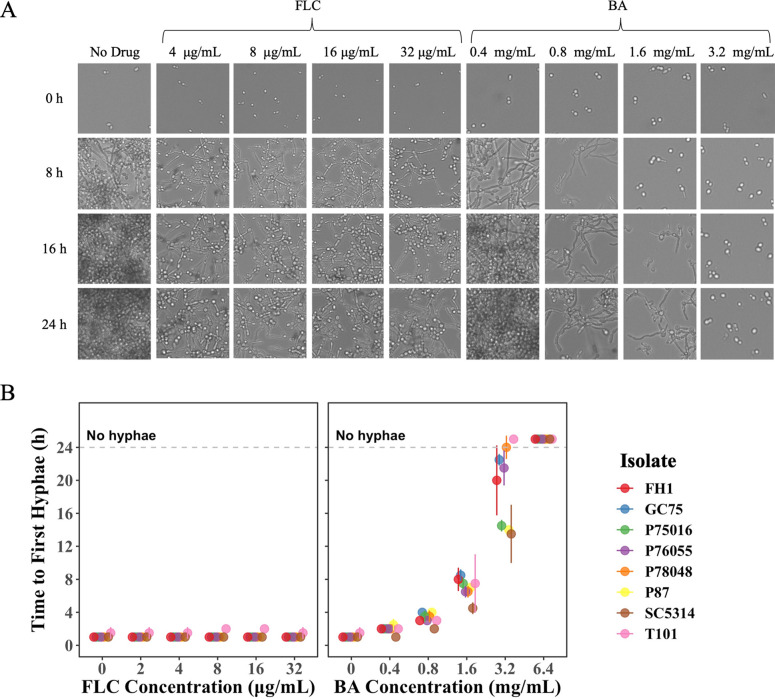
We used time-lapse microscopy to examine the ability of eight phylogenetically diverse C. albicans isolates to form hyphae and begin biofilm formation in the presence of drug (Fig. 3). Visual inspection of images indicated that it took only 1 to 2 h for all isolates to form hyphae regardless of the FLC concentration (Fig. 3B). In contrast, BA affected hyphal formation in a highly dose-dependent manner. In low levels of BA (0.4 and 0.8 mg/mL), there was very little variation in the time to first hyphal formation among isolates. As the level of BA increased, the time to hyphal formation, as well as the variation among isolates, increased. No hyphae were observed by 24 h at the highest level of BA (3.2 mg/mL).
FIG 3.
Biofilm formation drug responses of eight C. albicans isolates. Fungal cells were initially cultured in liquid YPD at 30°C, washed, and standardized to an optical density at 600 nm (OD600) of 0.01 in RPMI medium. The cells were inoculated into various concentrations of FLC or BA. The plate was incubated at 37°C and (A) manually taken out every 1 h for 24 h for scan using Evos FL Auto 2 inverted microscopy. (B) Time to the first hyphal formation was measured by manually going through the images and identifying the hour when the first hypha was observed. The values presented for each isolate are the means of two biological replicates ± SD.
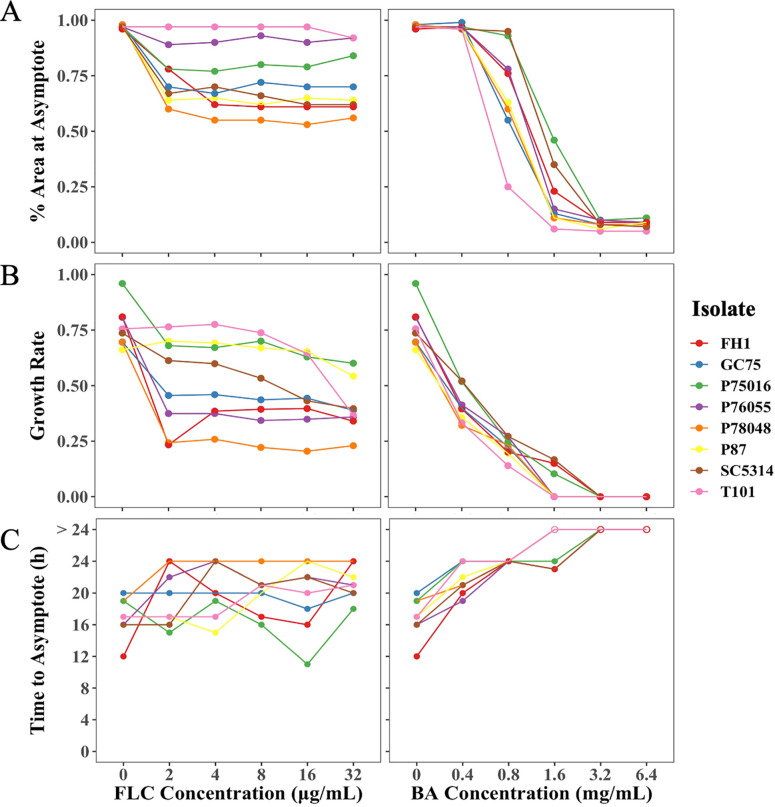
To further quantify biofilm formation from the time-lapse images, we used a computational pipeline that we recently developed that uses machine learning in the Orbit Image Analysis program (30) and custom R scripts. The pipeline computationally quantifies the percentage of area covered by cells in each image and uses this to calculate the biofilm growth rate, the time to reach the growth asymptote (i.e., growth plateau), and the percentage of area covered at the asymptote. At the highest FLC concentration, populations retained 50 to 100% of percentage of area covered by cells at the asymptote relative to no drug (Fig. 4A) and had a reduced but still relatively high growth rate (Fig. 4B). There was not a clear trend among isolates in the time required to reach the asymptote (Fig. 4C). Consistent with the identified variation by eye in the time to hyphal formation, BA reduced the percentage of area covered by cells at the growth asymptote, decreased the growth rate, and increased the time to reach the growth asymptote in a dose-dependent manner (Fig. 4A to C). Interestingly, there was more variation in percentage of area at the growth asymptote, growth rate, and time to reach the growth asymptote among isolates in FLC compared to BA. There was no correlation between FLC drug responses and BA drug responses (Spearman’s rank correlation, percentage of area covered by cells: S = 1,399,899, P = 0.48; time to reach the growth asymptote S = 1,346,887, P = 0.89; growth rate: S = 1,275,866, P = 0.5447). Overall, unlike FLC, BA effectively inhibits hyphal formation and biofilm formation in a dose-dependent manner.
FIG 4.
Quantitative image analysis of time-lapse images of eight C. albicans isolates. Orbit Image Analysis was used to calculate the percentage of area covered by cells for all the images, and custom R scripts were used to calculate the percentage of area at the asymptote, growth rate, and time to reach the asymptote. BA affected biofilm formation in a dose-dependent manner; it reduced the percentage of area at the asymptote (A), decreased the growth rate (B), and increased time to reach the asymptote (C), unlike FLC. The values presented are the means of two biological replicates.
BA eradicates C. albicans mature biofilms.
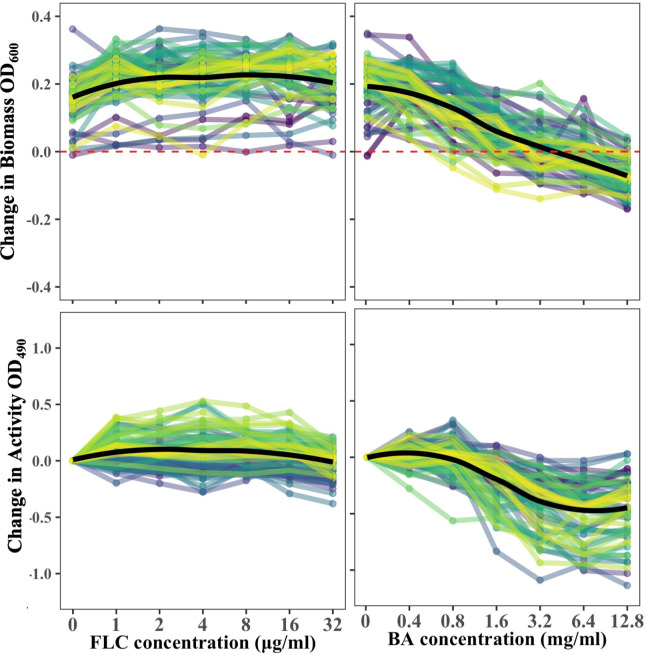
The ability of drugs to break apart mature C. albicans biofilms formed in the absence of drug was evaluated by quantifying both biomass and activity. As previously shown by others (24, 31, 32), mature biofilms were largely impervious to FLC; postdrug biomass was higher than the measured predrug biomass for all levels of FLC (Fig. 5; linear mixed-effect model implemented in the lmer R package [33], with change in biomass as the response variable, level of the drug as the predictor, and isolate as a random effect; P value was obtained through the analysis of variance test with Satterthwaite’s method for degrees of freedom; F1,342 = 2.98, P = 0.09). There was a negative association between FLC concentration and biofilm activity (linear mixed-effect model with change in activity as the response variable, level of the drug as the predictor, and isolate as a random effect, F1,342 = 32.46, P < 0.0001), driven by reduced activity at the highest level of FLC (model with FLC 32 μg/mL removed from the data set: F1,285 = 0.0937, P = 0.76).
FIG 5.
Biofilms of drug responses of 63 C. albicans isolates. In each panel, the black line indicates the mean across all isolates at each concentration. The line colors are arbitrary and used to visualize different isolates. Biofilms were insensitive to FLC regardless of the FLC concentration. BA eradicated mature biofilms in a dose-dependent manner and reduced activity. The values presented are the means of three biological replicates.
Similar to biofilm formation, BA significantly affected biofilm biomass and activity in a dose-dependent manner (Fig. 5; biomass, F6,342 = 249.94, P < 0.0001; activity, F6,342 = 249.94, P < 0.0001). Interestingly, the biomass of 48% of isolates and the activity of 57% of isolates increased at 0.4 mg/mL BA relative to no drug, suggesting that BA can stimulate growth at a low concentration. The biofilm biomass at high BA concentrations relative to a biofilm grown without drugs was reduced for all isolates. The activity of the remaining biofilm at the highest BA levels for some isolates increased above that of the preceding lower drug level, likely because BA degraded the biofilm matrix, allowing 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) to better penetrate the cells.
Planktonic drug responses were partially predictive of biofilm responses, albeit in an inconsistent manner. Isolates with higher planktonic growth tended to have a higher biofilm activity but not higher biomass, while lower susceptibility (higher resistance) did not predict either biofilm biomass or activity (Supplemental Materials Fig.S1 and S4; linear mixed-effect model with the FLC planktonic response as the predictor variables and isolate as a random effect; change in biomass as the response variable: susceptibility, F1, 57 = 1.59, P = 0.21 and tolerance, F1, 54 = 1.28, P = 0.26; change in activity as the response variable, susceptibility, F1, 57 = 2.51, P = 0.12 and tolerance, F1, 54 = 5.76, P = 0.02). In contrast, reduced BA planktonic susceptibility was associated with higher biofilm activity but not biomass, while tolerance was associated with neither (susceptibility, change in biomass, F1, 57 = 1.04, P = 0.31 and change in activity, F1, 57 = 5.55, P = 0.02; tolerance, change in biomass, F1, 57 = 0.0004, P = 0.98 and change in activity, F1, 57 = 0.07, P = 0.79). Hence, isolate differences in planktonic drug responses are likely not a good predictor for how a specific isolate will respond to drug when in a biofilm.
DISCUSSION
We screened isolates of Candida species to enhance our understanding of how diverse isolates in multiple morphologies respond to boric acid (BA), an effective drug for treating complicated vulvovaginal candidiasis (VVC). We directly compared responses against fluconazole (FLC), the first-line treatment. Consistent with previous work, compared to C. albicans, we found that C. glabrata on average had lower susceptibility to both FLC (34, 35) and BA (13, 14). In C. albicans, we found a negative correlation between FLC and BA; isolates that were less susceptible to FLC tended to be more susceptible to BA. Although FLC resistance does not seem to be the major driver of complicated VVC, azole resistance can be a factor (36). Here, we show that FLC resistance and BA resistance are negatively correlated (C. albicans) or not correlated (C. glabrata and C. parapsilosis) from disk assays. Our work is in agreement with previous studies that found that the mode of action of FLC is different from that of BA, including studies that found no correlation between FLC and BA susceptibility in 76 C. albicans isolates measured in liquid assay (27) and no interaction between FLC and BA in checkerboard assay (37). Thus, our work demonstrates that boric acid is likely to be a good alternative therapy if FLC-resistant isolates are involved.
In both C. albicans and C. glabrata, we found that the distribution of susceptibility values was broader in FLC than BA. Rosenberg et al. (16) measured susceptibility in 19 C. albicans isolates in 7 antifungal drugs and similarly found FLC had the broadest distribution. Our work suggests that BA-resistant isolates may be rare as only 1 C. parapsilosis isolate out of 240 C. albicans, C. glabrata, and C. parapsilosis isolates had considerably higher resistance than other isolates. Additional screening with a larger isolate collection is required to establish clinical breakpoints of BA resistance. We also found significant variation among species and a wider breadth of responses in FLC compared to BA for drug tolerance. There is a growing acknowledgment that fungal drug tolerance may be associated with treatment failure and infection persistence. One study showed that C. albicans isolates from patients with persistent candidemia had a higher tolerance to FLC than isolates from patients with nonpersistent candidemia (16). Increased tolerance was also found to be a good predictor of FLC efficiency in patients with C. albicans bloodstream infections (18).
Interestingly, using the Clinical and Laboratory Standards Institute (CLSI) disk diffusion assay protocol (29), we found that FLC drug susceptibility and tolerance were significantly correlated for all three species, while BA drug susceptibility and tolerance were significantly correlated for C. glabrata and C. parapsilosis. This result differs from a previous screen of 219 C. albicans isolates that found no correlation between FLC susceptibility and tolerance when isolates were grown on yeast-peptone-dextrose (YPD) and tested on casitone plates at 30°C (16). The mechanisms of tolerance have not yet been well resolved, although assay medium and temperature have previously been shown to significantly influence fungal drug susceptibility and can dramatically affect drug tolerance (15). This result suggests there may be also species-specific differences in the molecular basis of tolerance. The drivers and clinical importance of fungal drug tolerance is an area of active interest and research.
The involvement of morphological changes in symptomatic vulvovaginal candidiasis is also an area of current interest. Hyphal formation is an important virulence trait in many infection contexts, and compounds that inhibit hyphal formation in C. albicans significantly reduce virulence in multiple contexts (38, 39). Our results show that C. albicans forms hyphae in the first 2 h regardless of FLC concentration, in alignment with previous research (26). Through the new quantitative platform we developed, we similarly found that the percentage of area covered at the asymptote, biofilm growth rate, and time to reach the growth asymptote are also not correlated with FLC concentration, although we did uncover significant variation among isolates for these phenotypes. BA is clearly superior to FLC at the inhibition of hyphal formation, with the speed of morphological transition and subsequent biofilm formation influenced by BA in a dose-dependent manner. Hyphal formation was previously reported to be responsible for vaginal inflammation (40, 41), and hyphae contribute to the invasive growth of C. albicans (20), suggesting that inhibiting the yeast-to-hyphal transition could help explain why BA is an effective treatment against complicated VVC.
At low BA concentrations (0.4 and 0.8 mg/mL), approximately half of the C. albicans isolates tested had higher biomass and higher activity compared to biofilms grown without drugs. Low concentrations (185 μmol/liter) of BA were previously shown to be strongly mitogenic, and it has been shown to stimulate growth in planktonic Saccharomyces cerevisiae (42). If a biofilm has formed in the absence of a drug (e.g., prior to a symptomatic infection), penetration of drugs through the extracellular matrix is critical for eradication (43). We found that C. albicans biofilms are insensitive to FLC, consistent with past work (31, 32). Conversely, we found that BA can reduce biomass and activity of mature biofilms in a dose-dependent manner and therefore is likely capable of penetration. Approximately 40% of the extracellular matrix of C. albicans is composed of polysaccharides, and the major carbohydrates present is glucose (~40%) (22, 44). Saccharides (especially glucose) have a strong affinity for borate (45); thus, we think that BA likely penetrates the extracellular matrix of the biofilm by breaking the matrix apart. Compared to planktonic cells, C. albicans cells in a biofilm have a significantly higher β-1,3-glucan content in their cell wall, which has been implicated in FLC impermeability (46). We observed an increase in activity at high BA concentrations, even though the biomass was decreasing. We think that this increase in activity is artificial and due to how the XTT activity assay works. Mitochondrial succinoxidase, cytochrome P450 systems, and flavoprotein oxidases convert XTT to a colored formazan (47). If high concentrations of BA degrade the extracellular matrix, then more XTT will get inside the cells, which is read out as an increase in activity. Our future work will thus directly investigate the ability of BA to penetrate the cell wall of different Candida morphologies.
Conclusion.
Our work provides baseline data for multiple ways boric acid is an effective drug against diverse isolates of Candida species. We find that planktonic susceptibility and tolerance are more similar among isolates in boric acid than fluconazole. Boric acid is also effective at slowing or stopping the yeast-to-hyphal transition and biofilm growth in C. albicans and is able to penetrate and degrade mature biofilms. Planktonic responses in fluconazole and boric acid were not correlated; nor were planktonic and biofilm phenotypes. Combined, this work advances our understanding of multiple biological fronts about why boric acid may be an effective treatment against vulvovaginal candidiasis.
MATERIALS AND METHODS
Isolates.
A total of 235 Candida spp. isolates were used in this study (Table S1). Of these 235 isolates, 155 (85 C. albicans, 50 C. glabrata, and 20 C. parapsilosis) were provided by the clinical microbiology lab in the Health Sciences Centre (HSC) in Winnipeg, Canada. We also examined 80 additional C. albicans isolates from our lab freezer inventory that have been discussed in previous publications. To look for C. albicans correlation in drug responses, we also added in five additional FLC-resistant isolates. These isolates were used for the correlation analysis but not the distribution analysis.
Disk diffusion assay.
To screen for drug susceptibility and tolerance, the CLSI document M44 guidelines for antifungal disk diffusion susceptibility testing were followed with slight modifications (29). Briefly, isolates were streaked from frozen glycerol stocks into Sabouraud dextrose agar (SDA) and incubated for 48 h at 37°C. The colonies were resuspended in 200 μL of phosphate-buffered saline (PBS) and were standardized to an optical density at 600 nm (OD600) of 0.01 in 1 mL PBS. 100 μL of the standardized cells were spread in duplicate onto 15-mL Muller Hinton (MH) plates using sterile beads, and 5-mg BA disks were prepared by transferring 10 μL of preheated 500 mg/mL BA in dimethyl sulfoxide (DMSO) stock to blank antimicrobial disks (6 mm, Fisher Scientific). Single disks containing either 5 mg BA or 25 μg FLC were placed in the center of the MH plates. The plates were then incubated at 37°C for 48 h, and individual photographs were taken of each plate every 24 and 48 h. The images were edited using ImageJ (48) according to recommendations specified in diskImageR vignette V2 (49). Disk diffusion analysis to measure drug susceptibility and drug tolerance was done using the diskImageR package and the R script available at https://github.com/acgerstein/diskImageR/blob/master/inst/walkthrough.R (15). Susceptibility was measured from 48-h images as the radius where 20% growth of reduction occurs (RAD20), while tolerance was measured as the fraction of growth above RAD20 (FoG20).
Boric acid levels.
We picked the concentrations of BA by measuring the MIC for the isolates used in biofilm formation and eradication assays using the CLSI M27-A2 guidelines with some modifications. Briefly, isolates were streaked from frozen stocks on SDA plates and were incubated overnight at 37°C. Colonies were suspended in PBS and were standardized to an OD600 of 0.01 in 1 mL of RPMI 1640. A total of 100 μL of the standardized culture were transferred to a 96-well plate (Greiner Bio-One) containing 2-fold dilutions of BA with the maximum concentration of 50 mg/mL (maximum solubility of BA in RPMI medium) in column 1, no drug in column 11, and just RPMI 1640 in column 12. MIC50 were determined by taking OD600 after 24 and 48 h. The MIC50 of all isolates was either 0.39 or 0.78 mg/mL, and the MIC was 1.56 mg/mL. We decided to pick two levels above and below the MIC and to round the concentrations to one decimal place (i.e., 0.4, 0.8, 1.6, 3.2, and 6.4 mg/mL).
Biofilm formation drug response.
The ability to form a biofilm in the presence of a drug of eight C. albicans isolates was measured as previously described (50). Briefly, 100 μL of RPMI 1640 was added to all the wells of columns 1 to 9 and 12 of a flat-bottomed 96-well microtiter plate (Greiner Bio-One). 200 μL of RPMI 1640 + 12.8 mg/mL BA was added to all wells of column 11, and 200 μL of RPMI 1640 + 64 μg/mL FLC was added to all wells of column 10. A 2-fold serial dilution for each drug was done so that odd-numbered columns (columns 3, 5, 7, 9, and 11) contain dilutions of BA, even-numbered columns (columns 2, 4, 6, 8, and 10) contain dilutions of FLC, and columns 1 and 12 contain no drug. C. albicans isolates SC5314 (ATCC MYA-2876), FH1 (51), P87 (52), GC75 (52), P78048 (52), P75016 (52), P76055 (52), and T101 (53) isolates were grown overnight at 30°C in liquid YPD from frozen glycerol stocks. These isolates were picked because they span the phylogenetic diversity of C. albicans. Overnight cultures were washed with PBS and standardized to OD600 of 0.005 in 1.5 mL RPMI 1640, and 100 μL of the standardized cultures were inoculated into the 96-well microtiter plate that contains the drugs. The plate was sealed with a clear seal (Thermo Scientific) and polystyrene microplate lid (Greiner Bio-One), and parafilm was used to seal the sides of the plate. The plate was incubated at 37°C, and images were taken manually every 1 h for 24 h using an Evos FL Auto 2 inverted microscope (Thermo Scientific).
Microscopic image analysis.
Time to form hyphae was recorded manually by going through the images and taking note of the time of the first hypha that was observed. Important parameters (percentage of area at the asymptote, growth rate, and time to reach the asymptote) that correlate with biofilm formation ability were extracted using a high-throughput automated method (50). Briefly, Orbit Image Analysis (30) was trained on 14 images, and a detection model with 99.3% correctly classified instances was created. This detection model was used to calculate the percentage of area covered by cells for all of the images. Custom R scripts (50) were used to extract the percentage of area at the asymptote, growth rate, and time to reach the asymptote from Orbit output.
Biofilm eradication.
Biofilms were prepared as described previously with slight modifications (54). Overnight cultures of 68 different C. albicans isolates were prepared by inoculating 10 μL of frozen glycerol stocks in 500 μL YPD liquid medium in a microplate shaker (about 200 rpm) at 30°C overnight. Isolates were then standardized to OD600 of 0.01 in 1.5 mL RPMI 1640 broth, and aliquots of 100 μL of the standardized culture were inoculated into flat-bottomed 96-well microtiter plates (Fisher Scientific). The plates were then covered with a sealing membrane and sterilized lids, and the sides of the plates were sealed with parafilm. The plates were incubated statically at 37°C for 24 h. Mature biofilms were washed three times with phosphate-buffered saline (PBS) to remove the nonadherent cells, and OD600 was determined to quantify the predrug biomass of the biofilms.
The examined drug concentrations ranged from 0.4 to 12.8 mg/mL for BA and from 1 to 32 μg/mL for FLC. The biofilm antifungal susceptibility testing was done according to Pierce et al. (54) with slight modifications. Briefly, the mature biofilms were treated with either BA or FLC and incubated statically with the drug for 24 h at 37°C. The drug-treated biofilms were washed three times with PBS, and OD600 was taken to quantify the postdrug biomass of the biofilms. The biomass of the biofilms was normalized by subtracting the biomass of the biofilms before drug exposure from the biomass of the biofilms at each concentration. The metabolic activity was determined using the calorimetric XTT assay, which measures mitochondria reduction of the tetrazolium salt reagent; mature biofilms were incubated with 100 μL of XTT for 2 h in 37°C, and OD490 was taken to quantify the metabolic activity. Biofilm activity was normalized by subtracting the activity at no drug concentration from the activity at each drug concentration. We excluded four isolates from our analysis because they failed to form biofilms during the pre-drug exposure phase.
Statistical analysis.
The values for susceptibility and tolerance screening represent the average of three biological replicates with one or more technical replicates each. The values for hyphal and biofilm formation represent the average of two biological replicates with one technical replicate each. The values for biofilm eradication represent the average of three biological replicates with one technical replicate each. The error bars throughout represent standard error.
All statistical tests performed and graphs generated were done using R programming language (55). Since our data are not normally distributed, we used rank-based measures. When comparing susceptibility and tolerance among species for each drug, we used Kruskal-Wallis rank-sum test and determined significance using post hoc pairwise Wilcoxon tests with the Benjamini and Hochberg (28). We looked at homogeneity of group variances using Flinger-Killeen test of homogeneity. We used Spearman’s rank correlation when looking at the correlation between planktonic drug responses. We used linear mixed-effect models from lmerTest R package (33) to determine whether planktonic drug responses would influence biofilm drug responses. In all cases, the specific statistical test is indicated inline. Significance was assigned for P < 0.05. The raw data and scripts to generate figures and statistical analyses are available at https://github.com/acgerstein/BAFLC_Phenotypic.
ACKNOWLEDGMENTS
We thank Markus Stein at the Health Sciences Centre in Winnipeg for providing isolates from the clinical microbiology lab, as well as Judith Berman, Theodore White, Donna MacCallum, Dominique Sanglard, Peter Magee, Shawn Lockhart, Claude Pujol, and David Soll for sharing strains with the Candida community. We are grateful to Vanessa Poliquin for her critical insight and thoughtful conversations about her experience treating women with complicated vulvovaginal candidiasis and to Donald Sheppard for sharing insights about XTT and biofilm degradation. We thank Rebekah Kukurudz and Kamaldeep Chhokar for laboratory assistance and Steven Harris, Peter Pelka, Silvia Cardona, and Ayush Kumar for use of equipment.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, Reed BD, Summers PR. 1998. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 178:203–211. 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 2.Sobel JD. 2007. Vulvovaginal candidosis. Lancet 369:1961–1971. 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 3.van Schalkwyk J, Yudin MH, Infectious Disease Committee . 2015. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can 37:266–274. 10.1016/S1701-2163(15)30316-9. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. 2018. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 18:e339–e347. 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- 6.Linhares LM, Witkin SS, Miranda SD, Fonseca AM, Pinotti JA, Ledger WJ. 2001. Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by culture. Infect Dis Obstet Gynecol 9:221–225. 10.1155/S1064744901000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cetin M, Ocak S, Gungoren A, Hakverdi AU. 2007. Distribution of Candida species in women with vulvovaginal symptoms and their association with different ages and contraceptive methods. Scand J Infect Dis 39:584–588. 10.1080/00365540601148491. [DOI] [PubMed] [Google Scholar]
- 8.Okungbowa FI, Isikhuemhen OS, Dede APO. 2003. The distribution frequency of Candida species in the genitourinary tract among symptomatic individuals in Nigerian cities. Rev Iberoam Micol 20:60–63. [PubMed] [Google Scholar]
- 9.Kidd SE, Halliday CL, McMullan B, Chen SC-A, Elvy J. 2021. New names for fungi of medical importance: can we have our cake and eat it too? J Clin Microbiol 59:e02730-20. 10.1128/JCM.02730-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobel JD, Chaim W, Nagappan V, Leaman D. 2003. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol 189:1297–1300. 10.1067/s0002-9378(03)00726-9. [DOI] [PubMed] [Google Scholar]
- 11.Iavazzo C, Gkegkes ID, Zarkada IM, Falagas ME. 2011. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence. J Womens Health 20:1245–1255. 10.1089/jwh.2010.2708. [DOI] [PubMed] [Google Scholar]
- 12.Prutting SM, Cerveny JD. 1998. Boric acid vaginal suppositories: a brief review. Infect Dis Obstet Gynecol 6:191–194. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Seta F, Schmidt M, Vu B, Essmann M, Larsen B. 2009. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J Antimicrob Chemother 63:325–336. 10.1093/jac/dkn486. [DOI] [PubMed] [Google Scholar]
- 14.Otero L, Palacio V, Mendez FJ, Vazquez F. 2002. Boric acid susceptibility testing of non-C. albicans Candida and Saccharomyces cerevisiae: comparison of three methods. Med Mycol 40:319–322. 10.1080/mmy.40.3.319.322. [DOI] [PubMed] [Google Scholar]
- 15.Gerstein AC, Rosenberg A, Hecht I, Berman J. 2016. diskImageR: quantification of resistance and tolerance to antimicrobial drugs using disk diffusion assays. Microbiology 162:1059–1068. 10.1099/mic.0.000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg A, Ene IV, Bibi M, Zakin S, Segal ES, Ziv N, Dahan AM, Colombo AL, Bennett RJ, Berman J. 2018. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun 9:2470. 10.1038/s41467-018-04926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman J, Krysan DJ. 2020. Drug resistance and tolerance in fungi. Nat Rev Microbiol 18:319–331. 10.1038/s41579-019-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levinson T, Dahan A, Novikov A, Paran Y, Berman J, Ben-Ami R. 2021. Impact of tolerance to fluconazole on treatment response in Candida albicans bloodstream infection. Mycoses 64:78–85. 10.1111/myc.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble SM, Gianetti BA, Witchley JN. 2017. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol 15:96–108. 10.1038/nrmicro.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulati M, Nobile CJ. 2016. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect 18:310–321. 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson D, Naglik JR, Hube B. 2016. The missing link between Candida albicans hyphal morphogenesis and host cell damage. PLoS Pathog 12:e1005867. 10.1371/journal.ppat.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalheiro M, Teixeira MC. 2018. Candida biofilms: threats, challenges, and promising strategies. Front Med 5:28. 10.3389/fmed.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Noverr MC. 2010. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156:3635–3644. 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Zhang S, Li H, Shen L, Dong C, Sun Y, Chen H, Xu B, Zhuang W, Deighton M, Qu Y. 2020. Biofilm formation of Candida albicans facilitates fungal infiltration and persister cell formation in vaginal candidiasis. Front Microbiol 11:1117. 10.3389/fmicb.2020.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKloud E, Delaney C, Sherry L, Kean R, Williams S, Metcalfe R, Thomas R, Richardson R, Gerasimidis K, Nile CJ, Williams C, Ramage G. 2021. Recurrent vulvovaginal candidiasis: a dynamic interkingdom biofilm disease of Candida and Lactobacillus. mSystems 6:e0062221. 10.1128/mSystems.00622-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha KC, White TC. 1999. Effects of azole antifungal drugs on the transition from yeast cells to hyphae in susceptible and resistant isolates of the pathogenic yeast Candida albicans. Antimicrob Agents Chemother 43:763–768. 10.1128/AAC.43.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pointer BR, Boyer MP, Schmidt M. 2015. Boric acid destabilizes the hyphal cytoskeleton and inhibits invasive growth of Candida albicans. Yeast 32:389–398. 10.1002/yea.3066. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 57:289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 29.Sheehan DJ, Brown SD, Pfaller MA, Warnock DW, Rex JH, Chaturvedi V, Espinel-Ingroff A, Ghannoum MA, Moore LS, Odds FC, Rinaldi MG, Walsh TJ. 2004. Method for antifungal disk diffusion susceptibility testing of yeasts: approved guideline M27-A2. Clinical and Laboratory Standards Institute. Wayne, PA. [Google Scholar]
- 30.Stritt M, Stalder AK, Vezzali E. 2020. Orbit Image Analysis: an open-source whole slide image analysis tool. PLoS Comput Biol 16:e1007313. 10.1371/journal.pcbi.1007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. 2011. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med Mycol 49:253–262. 10.3109/13693786.2010.530032. [DOI] [PubMed] [Google Scholar]
- 32.Sherry L, Kean R, McKloud E, O’Donnell LE, Metcalfe R, Jones BL, Ramage G. 2017. Biofilms formed by isolates from recurrent vulvovaginal candidiasis patients are heterogeneous and insensitive to fluconazole. Antimicrob Agents Chemother 61:e01065-17. 10.1128/AAC.01065-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J Stat Soft 82:1–26. 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 34.Bhattacharjee P. 2016. Epidemiology and antifungal susceptibility of Candida species in a tertiary care hospital, Kolkata, India. Curr Med Mycol 2:20–27. 10.18869/acadpub.cmm.2.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashemi SE, Shokohi T, Abastabar M, Aslani N, Ghadamzadeh M, Haghani I. 2019. Species distribution and susceptibility profiles of Candida species isolated from vulvovaginal candidiasis: emergence of C. lusitaniae. Curr Med Mycol 5:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanguinetti M, Posteraro B, Lass-Flörl C. 2015. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 58:2–13. 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt M, Tran-Nguyen D, Chizek P. 2018. Influence of boric acid on energy metabolism and stress tolerance of Candida albicans. J Trace Elem Med Biol 49:140–145. 10.1016/j.jtemb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Meng L, Zhao H, Zhao S, Sun X, Zhang M, Deng Y. 2019. Inhibition of yeast-to-hypha transition and virulence of Candida albicans by 2-alkylaminoquinoline derivatives. Antimicrob Agents Chemother 63:e01891-18. 10.1128/AAC.01891-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao S, Huang J-J, Sun X, Huang X, Fu S, Yang L, Liu X-W, He F, Deng Y. 2018. (1-aryloxy-2-hydroxypropyl)-phenylpiperazine derivatives suppress Candida albicans virulence by interfering with morphological transition. Microb Biotechnol 11:1080–1089. 10.1111/1751-7915.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. 2011. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One 6:e26580. 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL, Jr, Noverr MC. 2014. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun 82:532–543. 10.1128/IAI.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett A, Rowe RI, Soch N, Eckhert CD. 1999. Boron stimulates yeast (Saccharomyces cerevisiae) growth. J Nutr 129:2236–2238. 10.1093/jn/129.12.2236. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Cerdeira C, Gregorio MC, Molares-Vila A, López-Barcenas A, Fabbrocini G, Bardhi B, Sinani A, Sánchez-Blanco E, Arenas-Guzmán R, Hernandez-Castro R. 2019. Biofilms and vulvovaginal candidiasis. Colloids Surf B Biointerfaces 174:110–125. 10.1016/j.colsurfb.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Al-Fattani MA, Douglas LJ. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008. 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 45.Peters JA. 2014. Interactions between boric acid derivatives and saccharides in aqueous media: structures and stabilities of resulting esters. Coord Chem Rev 268:1–22. 10.1016/j.ccr.2014.01.016. [DOI] [Google Scholar]
- 46.Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. 2007. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother 51:510–520. 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altman FP. 1976. Tetrazolium salts and formazans. Prog Histochem Cytochem 9:1–56. 10.1016/s0079-6336(76)80015-0. [DOI] [PubMed] [Google Scholar]
- 48.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerstein A. 2016. diskImageR: a pipeline to analyze resistance and tolerance from drug disk diffusion assays. R package version 1.0.0. https://CRAN.R-project.org/package=diskImageR.
- 50.Salama OE, Gerstein AC. 2021. High-throughput computational analysis of biofilm formation from time-lapse microscopy. Curr Protoc 1:e194. [DOI] [PubMed] [Google Scholar]
- 51.Marr KA, White TC, van Burik JA, Bowden RA. 1997. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin Infect Dis 25:908–910. 10.1086/515553. [DOI] [PubMed] [Google Scholar]
- 52.Wu W, Lockhart SR, Pujol C, Srikantha T, Soll DR. 2007. Heterozygosity of genes on the sex chromosome regulates Candida albicans virulence. Mol Microbiol 64:1587–1604. 10.1111/j.1365-2958.2007.05759.x. [DOI] [PubMed] [Google Scholar]
- 53.Odds FC, Bougnoux M-E, Shaw DJ, Bain JM, Davidson AD, Diogo D, Jacobsen MD, Lecomte M, Li S-Y, Tavanti A, Maiden MCJ, Gow NAR, d’Enfert C. 2007. Molecular phylogenetics of Candida albicans. Eukaryot Cell 6:1041–1052. 10.1128/EC.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierce CG, Uppuluri P, Tummala S, Lopez-Ribot JL. 2010. A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J Vis Exp 44:2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.R Core Team. 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S4. Download aac.02406-21-s0001.pdf, PDF file, 2.5 MB (2.5MB, pdf)
Data Set S1. Download aac.02406-21-s0002.csv, CSV file, 0.01 MB (9.2KB, csv)