Abstract
Oxytocin is a peptide molecule with a multitude of physiological and behavioral functions. Based on its association with reproduction - including social bonding, sexual behavior, birth and maternal behavior - oxytocin also has been called “the love hormone.” This essay specifically examines association and parallels between oxytocin and love. However, many myths and gaps in knowledge remain concerning both. A few of these are described here and we hypothesize that the potential benefits of both love and oxytocin may be better understood in light of interactions with more ancient systems, including specifically vasopressin and the immune system. Oxytocin is anti-inflammatory and is associated with recently evolved, social solutions to a variety of challenges necessary for mammalian survival and reproduction. The shared functions of oxytocin and love have profound implications for health and longevity, including the prevention and treatment of excess inflammation and related disorders, especially those occurring in early life and during periods of chronic threat or disease.
Keywords: Oxytocin, Vasopressin, Immune system, Social behavior, Stress, Love
Highlights
-
•
Oxytocin is a peptide molecule with functions that support a sense of safety, sociality, as well as survival and reproduction.
-
•
Oxytocin is associated with social and neuroimmune solutions to chronic stress.
-
•
The related, but more primitive, peptide vasopressin supports more individualistic survival strategies.
-
•
Controversies and myths surround the properties of oxytocin and love.
1. Overview
Oxytocin was the first peptide molecule to be biochemically identified [1]. The oxytocin molecule has properties and functions essential to mammalian behavior and to understanding human origins [2] which are only now being described [3,4]. Interest in oxytocin has increased exponentially, especially over the last two decades. At present approximately 30,000 research papers listed on Pubmed deal in some way with oxytocin. However, despite this massive effort, the study of oxytocin remains associated with myths and mysteries.
This perspective essay considers a series of questions which are fundamental to understanding oxytocin, but which also have been sources of repeated controversy and misunderstandings: What is oxytocin and why is this molecule difficult to measure? Is there a unique receptor for oxytocin? Is oxytocin a stress hormone? Are the actions of oxytocin sexually-dimorphic? Why is awareness of vasopressin and the immune system essential to understanding oxytocin? What is social behavior and how does oxytocin influence sociality? And of course, why is oxytocin sometimes called “the hormone of love”?
Oxytocin has effects on social connection, a perception of safety, and also immunology and inflammation. Of particular importance in understanding oxytocin are its interactions with the immune system [5], as well as the anti-inflammatory effects of mitochondria [6,7]. Oxytocin regulates and is regulated by glia [8] and the immune system [9]. Furthermore, oxytocin mediates behavioral and immune consequences that are attributed to the microbiota [10,11].
All of these have consequences for health and wellbeing. As described below, recent studies are helping to explain some of the myths and controversies associated with oxytocin. The mechanisms linking love and oxytocin to each other remain both metaphorical and mysterious, but a mystery worth exploring.
2. So, what exactly is oxytocin?
The “what is oxytocin” question should be easy to answer. The use of the word oxytocin is not new. The website of the National Institutes of Mental Health states that the term oxytocin was already in the use in the 1800s and perhaps as early as the 1500s. https://www.nimh.nih.gov/research/research-conducted-at-nimh/research-areas/clinics-and-labs/lcmr/snge/vpot/some-selected-history-of-oxytocin-and-vasopressin. As originally used, the word oxytocin appears to have referred to a process of “swift birth.” However, in current usage oxytocin most often describes a specific molecule.
Although modern dictionaries usually translate the derivation of the word oxytocin as “swift birth,” as described in an email from Roger Guillemin, “oxytocin,” the English spelling, may have been a “mistaken translation”? (Box 1). Dr. Guillemin is a Nobel laurate and sometimes described as father of the field of neuroendocrinology. Guillemin suggested that oxytocin translates to “sharp” birth and proposed that “ocytocin” (still used in French) would have been a more accurate translation of the Greek symbols intended to describe the functional capacity of this molecule to induce a quick birth.
BOX 1. THE MYSTERIOUS ORIGINS OF THE WORD “OXYTOCIN. (An historical aside).
This excerpt is from an email correspondence with Roger Guillemin. It came following a talk I had given at the Salk Institute. In that presentation I showed a portrait of Antoine Lavoisier (father of modern chemistry), his wife, Marie-Anne, and instruments for collecting oxygen – a picture I sometimes used to symbolize the notion that loving relationships are based on biochemistry.
…. As per Lavoisier, the word oxygen comes from the Greek ὀξύς/oxys, acid, sharp and γενής/genês, generates, because Lavoisier thought that the substance oxygen was involved in producing acids in combination with other substances (still true though not exclusive …). Oxytocin is a totally different story. It is actually a mistake for ocytocin.
The etymology is ὠκύς/ocys fast and τόκος/birth, delivery. Note the κ (kappa) always translates as c (ocytocin) different from the ξ (ksi) always translated as x as in oxygen. I went over on and on with texts in Greek, Herodotes, Hippocrates, where I found the words ὠκύς τόκος, as in 'ωκυτοκιος ′ωκυτοκιοv ὠκυτόκου all in phrases referring to speedy birth, never as ὀξύς as for oxygen (acid generating) So the correct word is OCYTOCIN.
Well maybe here's the last word in a story told me, many years ago by Roger Acher whose name you recognize of course... At some meeting duVigneaud was talking about his early work on isolation of oxytocin when this French participant (?) got up and said …
“ Sir, with all due respect please note that the correct word is ocytocin and I can show the etymology” to which duVigneaud is supposed to have answered. “ listen I don't care about all your grammar... all I know is that stuff comes from the pituitary of oxen... so that's oxytocin by me..." I knew duVigneaud quite well and I bet the story is true …
I still have not been able to find out who first originated OXYTOCIN.
Roger Guillemin May 29, 2013.
Alt-text: BOX 1
Genomic studies, tracing the evolutionary origins of these molecules, further question the accuracy of this name [12]. Theofanopoulou and her colleagues have suggested the need for a “universal nomenclature for oxytocin-vasotocin ligand and receptor families.” One family, that they propose to call “vasotocin,” would consist of the molecules that are currently called vasotocin and vasopressin (Table 1). A second chemical family they propose to call “oxytocin,” would consist of oxytocin, mesotocin and isotocin. These authors argue that a more accurate naming system would be based on the relative affinity of ligands to receptors. Following this system, the molecules now called mesotocin and isotocin, would be called oxytocin. Of course, calling these three structurally different molecules “oxytocin,” offers opportunity for other sources of confusion and controversy.
Table 1.
Amino acid sequences in the vasotocin-oxytocin family of peptides.a
| Amino acid position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Expressed in (among others)a |
|---|---|---|---|---|---|---|---|---|---|---|
| Vasotocin | Cys- | Tyr- | Ile- | Gln- | Asn- | Cys- | Pro- | Arg- | Gly (NH2) | Non-mammalian vertebrates and fetuses |
| Vasopressin | Cys- | Tyr- | Phe- | Gln- | Asn- | Cys- | Pro- | Arg- | Gly (NH2) | Mammals |
| Oxytocin | Cys- | Tyr- | Ile- | Gln- | Asn- | Cys- | Pro- | Leu- | Gly (NH2) | Mammals |
| Mesotocin | Cys- | Tyr- | Ile- | Gln- | Asn- | Cys- | Pro- | Ile- | Gly (NH2) | Non-eutherian tetrapods & birds |
| Isotocin | Cys- | Tyr- | Ile- | Ser- | Asn- | Cys- | Pro- | Ile- | Gly (NH2) | Ray-finned fishes |
[15].
Oxytocin and its targets have consequences that remain essential to coordinating mammalian behavior and reproduction with environmental demands, including functions such as defense in the face of pathogens [13], immunological responses, mitochondrial functions and basic cellular metabolism [6]. Oxytocin also can function as a neurotransmitter and neuromodulator [14]. Perhaps even calling oxytocin a “hormone” is a misnomer?
3. The variable chemistry of the molecule called oxytocin
In 1953 oxytocin and vasopressin, were identified biochemically [1]. The gene for oxytocin in mammals was sequenced in 1984 and the genes for receptors for oxytocin and vasopressin were published in 1992 [15,16]. It would appear that the chemical structures of oxytocin and the oxytocin receptor have been well understood for several decades. But again, the story is not that simple.
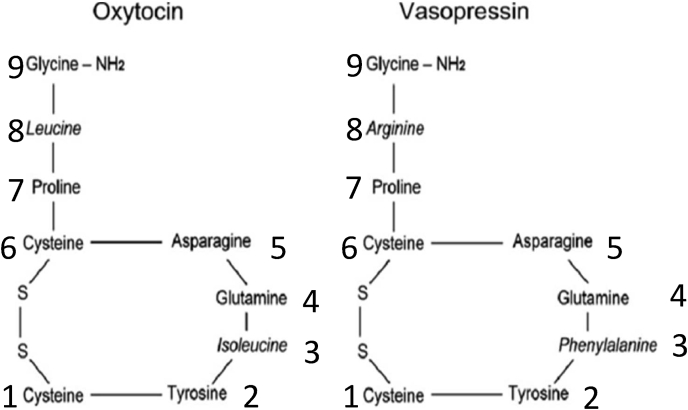
Canonical forms of oxytocin and vasopressin consist of peptide molecules composed of nine amino acids, with a three amino acid tail (implicated in ligand binding to the receptor) and a six amino acid ring (implicated in coupling with G-coupled protein receptors (GCPR) [17]. The ring in this structure is formed by the disulfide bonds in two cysteines (positions 1 and 6). (Fig. 1; Table 1). Vasopressin differs from oxytocin by two amino acids, one at position 3 (in the ring) and one at position 8 (in the tail). Vasotocin, considered the ancestral peptide from which both evolved, differs from vasopressin by one amino acid at position 3 and from oxytocin by one amino acid at position 8.
Fig. 1.
Canonical structures of oxytocin and vasopressin.
One interesting difference between the structure of oxytocin versus other major peptides in this family (Table 1) is the unique sequence of three amino acids in the tail at positions 7–9 (PLG: proline-leucine-glycine), which is also known as melanocyte-inhibiting factor (MIF-1). MIF-1 has pain-modulating effects via interactions with opioid and dopaminergic systems (reviewed [3]). The structure of MIF-1 is not found in vasopressin or mesotocin. This may be just one of many clues regarding what makes oxytocin unique.
A common feature shared by canonical oxytocin and other members of this chemical family is the presence of the disulfide bonds connecting the two cysteines and creating the ring structure of these molecules. The rings in these peptides are capable of opening to create linear molecules. It has been proposed that the linear form of oxytocin or fragments of oxytocin may be of particular importance to its anti-inflammatory and anti-oxidant effects [18].
Oxytocin with leucine (at position 3) and isoleucine (at position 8) is found in most, but not all, mammals. However, species-specific variants in the amino acid sequences for oxytocin have been discovered recently and associated with different patterns of sociality. For example, amino acid substitutions at position 8 (and in some cases also position 3) exist in New World versus Old World monkeys. Substitutions of one or more amino acid also may produce not only different binding affinities, but also differential capacities to desensitize the oxytocin receptor, and thus potentially allow longer durations or strengths of action.
It has been suggested that in comparison to canonical oxytocin, the oxytocin-like variants found in highly social New World monkeys may be not only better able to bind to the oxytocin receptor, but also less likely to bind to the vasopressin V1a receptor [19]. Associated with these novel variants is male parental behavior, a defining behavioral trait of many species of New World monkeys. Remarkably, after receiving one novel form of oxytocin, described as Pro8-OXT and previously associated with parental behavior and social monogamy in New World monkeys, nonparental male rats were more likely to show parenting behavior toward infants [20]. Findings of this sort continue to support the notion that, irrespective of nomenclature, the exact chemistry of oxytocin-like molecules matters and small structural differences can have functional consequences.
4. Are measurements of oxytocin meaningful?
The structural characteristics and especially the “sticky” sulfur bonds in oxytocin influence its functional availability within tissues. The capacity of oxytocin to bind to other biological entities also has contributed to controversy regarding the quantification of oxytocin. Current antibody-based methodologies provide snapshots of hormone levels, and are most often used in plasma or saliva. These have proven useful when considering correlations with behavioral patterns and responses to external stimuli. For example, levels of oxytocin and vasopressin rise after cold-stressors or music [21] and oxytocin levels have been individually correlated with electrical activity in the brain specifically in response to social cues [22]. These measurements are of most value within a given study [23]. Furthermore, as described below other molecules capable of transporting oxytocin, including RAGE (“receptor for advanced glycation end-products”), may create biochemical interference in antibody-based assays [24,25]. This in turn is likely to contribute to variation in estimates of the amount of endogenous oxytocin in bodily fluids.
Perhaps, rather than dealing with the dynamic and variable characteristics of the oxytocin system as controversies or myths [26], it may be more helpful to conceptualize bodily fluids as dynamic delivery and/or storage systems. These in turn function as components of the body's active defense systems, providing local availability of oxytocin, especially in response to threatening conditions such as infection or inflammation [27].
5. Is oxytocin a “pituitary” hormone?
Sir Henry Dale identified the presence of a substance in pituitary extracts that could facilitate uterine contraction. Dale is usually given credit for discovering oxytocin, although in his original 1906 paper he did not specifically call this molecule “oxytocin” [28]. Oxytocin does have classical hormonal functions, since it is stored in the posterior pituitary, released into the blood stream, and carried to target tissues throughout the body. However, oxytocin is not actually synthesized in the pituitary gland, but in fact is produced in the hypothalamus.
Hypothalamic neurons which synthesize oxytocin and vasopressin do extend processes to the posterior pituitary gland; there these peptides are released into the systemic circulation, coordinating birth, lactation, sexual responses, water and mineral homeostasis, cardiovascular function and metabolism in bones and muscles, to name only a few. Hypothalamic cells also release both oxytocin and vasopressin directly into the brain with local effects on specific tissues and functions [29]. For these reasons oxytocin is now commonly described as a “hypothalamic neuropeptide,” rather than a pituitary hormone. But perhaps even that distinction is not totally accurate?
6. Is oxytocin primarily a hypothalamic hormone?
Although the hypothalamus is often cited as the primary source of oxytocin, the oxytocin molecule is synthesized in other parts of the body. In fact, based on gene expression for oxytocin, in the female rat uterus near the time of parturition oxytocin levels are reportedly 70 fold higher than any other place in the body, including the hypothalamus. As originally explained by Lefebvre, Zingg and their colleagues, “endogenous circulating OT appears not to participate in the induction of labor… and the finding of OT messenger RNA and peptide in the uterus suggests a solution for this paradox. During parturition, OT may act primarily as a local mediator and not as a circulating hormone” [30].
Oxytocin also appears in extremely high levels at sites of inflammation. In ovarian cancer tumors the levels of oxytocin were up to 200 times greater than in plasma [27]. Oxytocin levels measured in these tumors were negatively associated with an inflammatory cytokine, interleukin-6 (IL-6). A higher level of oxytocin within tumors also was correlated with mortality in advanced cases of ovarian cancer, an effect that was mediated by IL-6. This research further implicates locally synthesized oxytocin as an anti-inflammatory agent and an important component of the immune response [31].
7. Is there a “unique” oxytocin receptor and why does this matter?
In a comprehensive review of the oxytocin receptor Jurek and Neumann [32] state… “The many facets of OXT are, on a molecular basis, brought about by a single receptor.” When compared to related peptides, oxytocin generally binds most readily to the oxytocin receptor and GPCRs are an important mechanism for oxytocin's action. GPCRs have the capacity to respond to different ligands and transduce information across cell membranes, triggering subcellular processes [4] and thus influencing downstream transcription. Differences in subcellular signaling can have an array of functions that may include the expression of social behavior [33].
A separate set of GCPRs are described as vasopressin receptors. Of these the vasopressin V1a and V1b receptors found in the nervous system, can be targets for both oxytocin and vasopressin [34,35]. In the genetic absence of oxytocin the vasopressin system may assume some, but not all, of the functions of oxytocin, acting in part via sensitized vasopressin receptors [36]. As described below interactions between oxytocin and vasopressin are most obvious under threatening conditions.
Receptors responsive to oxytocin are abundant in the nervous, reproductive and immune systems including on microglia and astrocytes [9]. These receptors are cell-type specific [14]. Awareness of local effects of oxytocin is providing critical information regarding tissue-specific actions of oxytocin, especially in the nervous system [4]. In one of many recent examples, oxytocin receptors on astrocytes in the amygdala have been implicated in a neural circuit that can reduce anxiety [37]. Studies such as these provide additional evidence for interactions between oxytocin, components of the immune system and neural function.
The crystal structure of the GCPR oxytocin receptor was recently described [38], offering fresh clues to the specific capacity of different kinds of molecules to bind to the oxytocin receptor and moderate its function. This research revealed that the functions of the oxytocin receptor are affected via positive allosteric stimulation by magnesium (Mg++) and other divalent cations, as well as negative allosteric regulation by sodium (Na+).
Some of the short-term consequences of oxytocin act via ion channel receptors [39]. Gaseous molecules, including hydrogen sulfide (H2S) and nitric oxide (NO), also have been implicated in the rapid actions of oxytocin, helping to explain the role of oxytocin in the regulation of both the autonomic and cardiovascular systems [40]. Clearly, GPCRs - even those originally described as oxytocin receptors - are only one mechanism through which oxytocin functions. The availability of multiple mechanisms for responding to oxytocin, often with different time courses, has consequences for the effects of oxytocin throughout the body.
8. Is oxytocin a “female” hormone?
The first myth concerning oxytocin's functions arose when the molecule was identified as a female reproductive hormone, without known effects in males. Male and female mammals do use different biological and behavioral strategies and functions both for reproduction and for survival. Patterns of sex differences are reported in the production of oxytocin, the oxytocin receptor and their functional consequences [41]. Dozens of studies also reveal sex differences in response to exogenous peptides, including oxytocin and vasopressin [42,43]. These sex differences are managed in part by steroid-peptide interactions [44,45], and also may reflect sex differences in the functions of mitochondria and the immune system [8,46], in turn relevant to managing inflammation and reproductive demands, as well as sexual differentiation [47].
Sex differences in oxytocin and vasopressin are especially likely to be detected in response to stressful experiences during the perinatal period [48]. Under these conditions vasopressin has been more often implicated in males [49,50], while CRF and adrenergic systems (including norepinephrine) may be critical to managing chronic stressors in females [51]. However, a role for oxytocin in coping with stress also has been identified in male rodents [52]. This involves the capacity of estrogens and androgenic metabolites (specifically 3 β diol) to differentially stimulate estrogen receptors, including the estrogen receptor (ER) β. The ER β found in the hypothalamus can affect the release of oxytocin, and reduce measures of anxiety and inflammation. Thus, although usually identified with females, steroidal systems including estrogen receptors and oxytocin have important consequences for stress management in males.
Sex differences in physiology and behavior also appear during reactions to stressful experience during the perinatal period and there is increasing evidence implicating inflammation in sexual differentiation [47]. Given the importance of oxytocin as an anti-inflammatory molecule, it is likely that oxytocin moderates certain aspects of masculinization, possibly reducing the neural consequences of androgens. Differential roles for sex steroids and the oxytocin-vasopressin system also can have major consequences for species differences in social behavior [44].
Complex interactions among genetic sex, gonadal steroids, stress and immune factors and lifespan development are difficult to parse experimentally. However, these are very important pathways to consider since they help to sculpt the connections among social support and wellbeing.
9. Is oxytocin an “anti-stress” hormone?
Oxytocin has been described as a stress hormone [53], a stress-coping molecule [54], or both [29]. In general, oxytocin has central roles in coping with challenge. Oxytocin modulates stress reactivity and facilitates restoration following periods of challenge and has many interactions with the hypothalamic-pituitary-adrenal (HPA) axis, immune and autonomic systems [3,32]. In addition, time is a critical factor in understanding this aspect of oxytocin's functions. Oxytocin and vasopressin are both released under conditions of acute demands, including sexual orgasm [55], ejaculation [56], birth [57], pair bond formation [58], intense exercise [59], severe pain or shock-trauma [40,60] and sodium challenge [61]. In all of these cases at least one of the roles for oxytocin and social connections may be to calm the organism and reduce inflammation [62], helping systems return to homeostasis, while also predicting upcoming allostatic demands [63].
Oxytocin also has the capacity to increase its own synthesis and release [64]; this feature of oxytocin accounts in part for its extensive use to facilitate parturition [57]. Even in nonpregnant animals, stimulating the release of oxytocin or administering exogenous oxytocin is capable of increasing the subsequent synthesis of endogenous oxytocin [65]. The ability of oxytocin to feed forward and increase its own synthesis could be critical to coping with chronic challenges - especially in a social context. Vasopressin also has the capacity to feed forward, an effect that can be increased by androgens [66]. However, in general the long-term effects of vasopressin are different, and in many contexts opposite from those of oxytocin (reviewed [32]).
The effects of oxytocin during responses to challenge occur against a functional background of interactions between vasopressin and corticotropin releasing hormone (CRH). Vasopressin and CRH are often co-localized and can initially amplify the effects of each other, increasing activity in the HPA axis [49]. However, during chronic stress vasopressin, versus CRH, provides a mechanism allowing “escape” from glucocorticoid-regulated negative feedback [50]. Under chronic stress, oxytocin's capacity to downregulate defense systems may be especially relevant, allowing social and psychological safety to modulate both the HPA axis and emotional reactivity.
Particularly important to understanding the stress-related consequences of oxytocin is its capacity to regulate chronic pain [67]. Whether oxytocin's role in reducing pain is via the oxytocin and/or vasopressin receptor is still being debated [68,69]. There is evidence that oxytocin can induce analgesia through effects on the vasopressin V1a receptor [70]. Vasopressin may have a different time course of action, serving in the early stages of a stressful experience when a pro-inflammatory response is important. Oxytocin seems to be particularly relevant during repeated or chronic stressors, possibly through its capacity to counteract the pro-inflammatory effects of vasopressin as well as the capacity to signal social safety [8].
Positive or negative experiences can adjust reactivity in the HPA axis, as well as the oxytocin system across the lifespan. This is especially apparent around the time of birth, when oxytocin can upregulate the oxytocin receptor [71]. For example, postnatal exposure to oxytocin or parental nurture interferes with de novo methylation of the oxytocin receptor which occurs in the postpartum period. Under these conditions oxytocin and/or positive nurture is associated with upregulation of the expression of the oxytocin receptor and increasing indications of sociality in later life [72,73]. The developmental effects of vasopressin are less well studied [74]. However, research in rodents suggests that - especially in males - early exposure to vasopressin upregulates emotional reactivity and aggression, with consequences across the life cycle [75,76].
Both immediate demands and the history of each individual also can influence responses to oxytocin and vasopressin. For example, research, primarily in nonhuman animals suggests that vasopressin's effects are most obvious after a history of adversity, especially when compared to high levels of early nurture [77]. The social history of an individual can alter the threshold for responding to oxytocin, vasopressin and other stress-related molecules [74]. This likely reflects adjustments to the sensitivity of both vasopressin and oxytocin systems, with later consequences for the capacity to manage inflammation and oxidative stress, of importance in many stressful contexts.
10. Ancient molecules and the evolution of a social solution to the stress of life
Many of the properties of oxytocin are best appreciated in the context of its' evolutionary history and interactions with even more ancient molecules. Oxytocin-like molecules facilitate social and reproductive interactions in both vertebrates and invertebrates [78]. Precursors for oxytocin and the related peptide vasopressin existed prior to the preCambrian explosion when atmospheric oxygen levels on Earth increased, permitting a dramatic increase in multicellular organisms. The evolution of these peptides was associated with the transition to living on dry land, the need to regulate bodily fluids and nutrients and to deal with exposure to elevated levels of oxygen [79]. However, oxygen is potentially dangerous. Oxytocin's anti-inflammatory and anti-oxidant properties also may have helped to protect the mammalian nervous system during exposure to atmospheric oxygen.
Oxytocin evolved from mesotocin possibly as long as 400 million years ago [81], preceding and facilitating the origins of mammals and lactation [82]. Many types of organisms exhibit viviparity, but milk from a mammary gland and the genes responsible for milk production are uniquely expressed in Mammalia, including monotremes, marsupials and eutherian mammals [83]. Supporting a gradual evolutionary change, monotremes, egg-laying mammals that also lactate, produce both mesotocin and oxytocin.
Peptides structurally identified as canonical oxytocin have been identified in jawless fish and in cartilaginous dogfish [82]. However, the oxytocin found in fish does not appear to have been the ancestral source of mammalian oxytocin. It is hypothesized that oxytocin in fish arose as a function of “random genetic drift,” while “selection evolution” led to the independent emergence and maintenance of oxytocin in mammals [80].
Many other biologically active molecules, including common neurotransmitters and variations on the CRH molecule existed prior to the preCambrian explosion [84]. In that context the evolution of the specific molecule known as oxytocin is comparatively recent. Furthermore, in comparison to the “primitive” anatomy of vasopressin-secreting neurons, hypothalamic neurons producing oxytocin have very complex branching patterns [79], forming feedback networks reaching from the brainstem to the cortex [85].
Oxytocin's functions also are intertangled at many levels with the ancient immune system, helping to manage challenges across the lifespan [5,86]. For example, RAGE is a component of the immune system that serves as a carrier molecule facilitating oxytocin's movement across tissues, including placental and blood brain barriers [87]. CD 38 is another immune system molecule that regulates oxytocin. CD 38 is located on cells throughout the immune system and was initially described as an “immune cell marker”. It is now understood that CD 38 also is essential for the calcium-dependent release of oxytocin. Both CD 38 and RAGE also are implicated in maternal behavior and other forms of sociality [5]. Research on these interactions supports the importance of interactive functions of oxytocin, the immune system and positive sociality. Although CD 38 is essential to the normal functioning of oxytocin system, apparently CD 38 is not required by the vasopressin system [86], suggesting another unique feature linking the oxytocin and immune systems.
11. The effects of oxytocin and vasopressin are hierarchical and context-dependent
In healthy individuals who have experienced high levels of early nurture, oxytocin can have immediate benefits in response to stressors, including allowing a return to homeostasis [33], more accurate appraisals of threat versus safety and future risks [88] and prediction of future allostatic demands [63]. However, under extreme or repeated stress, and especially in individuals that have experienced high levels of adversity in early life, the consequences of high levels of oxytocin are less predictable. In part this could be because under these conditions vasopressin receptors are triggered [74,89]. Interactions among oxytocin and vasopressin probably help to explain what have been called “paradoxical” effects of oxytocin [90], including negative responses to exogenous oxytocin [35].
During social encounters oxytocin potentially inhibits the defensive and more primitive actions of vasopressin and other stress-related pathways. Oxytocin, may reduce the perception of threat, allowing animals to engage in prosocial interactions and in some cases develop selective relationships. However, under conditions of adversity or trauma across the lifespan, pathways dependent on vasopressin may dominate, even when oxytocin is administered or otherwise abundant. For example, during a difficult childbirth, maternal physiological systems may default to mechanisms that are regulated by vasopressin [91] and/or effects of oxytocin on the vasopressin receptor [69,70]. In another example, after receiving exogenous oxytocin individuals with a history of early life adversity or mood disorders may show negative emotional reactions, resembling those that might be expected following vasopressin [92,93].
In summary, oxytocin both regulates and is regulated by components of the immune system including glia [89]. Evidence is growing for other relationships between oxytocin and the immune system [5] as well as the capacity to affect the anti-inflammatory actions of mitochondria [67]. Oxytocin also has consequences for the release of gaseous transmitters, such as H2S or NO, with potential protective effects throughout the body [40]. Furthermore, oxytocin apparently mediates behavioral and immune consequences that are attributed to the microbiota [10,11].
12. What does it mean to be called a “social neuropeptide”?
It has become common to described oxytocin as a social neuropeptide and hundreds of studies describe the role of oxytocin and vasopressin in the neurobiology of social behavior. However, exactly what this means needs additional consideration. States of perceived threat, versus safety, influence the capacity for and expression of positive social behaviors (reviewed [89]). Constructs like social behavior [94], social recognition [95], social salience [96] and even maternal behavior [97] are difficult to differentiate from the general tendency of mammals to avoid danger and seek safety, especially in response to novel social stimuli.
Awareness of emotional context and the history of each individual, including prior experiences of safety versus threat, are particularly critical in understanding the consequences of oxytocin and vasopressin [98]. However, the capacity of oxytocin to influence responses to social cues may not be readily apparent until the individual is faced with a challenge, including for example a newborn infant. As another example, cues of sickness can be powerful in eliciting social avoidance [99] [8]. Oxytocin can override social avoidance [100] promoting social interactions even in the face of biological threat [13]. Here again, cross-reactivity among oxytocin, vasopressin and the immune system offers mechanisms for adaptive responses to specific social stimuli [101].
13. Is oxytocin “the hormone of love?”
Single molecules – even ones as versatile as oxytocin - cannot explain the intricacies of a behavioral construct like love. A host of neural systems, neurotransmitters and neuromodulators, especially catecholamines, indoleamines, acetylcholine, endogenous opioids, sex steroids, molecules of the HPA axis, including CRF and corticosterone, and inflammatory cytokines, interact in the regulation of the oxytocin system [102]. For example, of particular importance are GABA [103], serotonin [104], dopamine [105], and opioids [106,107]. All of these chemicals are more ancient than oxytocin and vasopressin [84]. However, most of these molecules and their evolved interactions with mammalian biochemistry have been implicated in the neurobiology of love [108], as well as physiological reactions to threat.
Oxytocin does support physically intimate forms of sociality and nurture and plays a critical role in infant feeding. Unique properties of oxytocin also were involved in the evolution of the capacity to form lasting attachments [109]. Acting on various target tissues and neuroendocrine pathways oxytocin helps to regulate emotional states including those that are experienced by the human nervous system as love. In this context, oxytocin became known as “the hormone of love” [110].
Mammalian maternal behavior, acting through oxytocin, was originally suggested as a model for “love” [111]. However, studies of genetically mutant mice unexpectedly revealed that maternal care continued in the absence of oxytocin and its receptor [112]. Parental behaviors are the product of a complex and potentially redundant neurobiology. In a strict sense oxytocin is not “the” hormone of mother's love [113,114].
Young mammals are especially salient stimuli, with the potential to attract attention and elicit care, apparently even in the absence of oxytocin. In some contexts, young animals, including offspring, also can serve as threats, inducing avoidance or attack. To engage in parenting, mammals must selectively overcome cues of threat, while permitting defense against possible dangers. For example, early research showed that responses to infants increased in anosmic virgin female rats, suggesting the olfactory cues from pups inhibited maternal-like behaviors [115].
However, oxytocin may serve mammals as a kind of insurance policy against over reaction to their young or other environmental stressors [116]. For example, a postpartum surge in oxytocin helps to expel the placenta and could support initial bonding between the mother and child [117]. Whether the mechanisms through which oxytocin serves to influence approach behavior are specific to parental-young interactions, or perhaps represent a more general reduction in reactivity to threats, deserves additional research [118,119].
14. Is oxytocin a metaphor for love and safety?
The word “love” has many meanings and interpretations [120,121]. Love can be described as a metaphor, albeit with physical and psychological manifestations [122,123]. Of course, even repeated associations across different levels of analysis do not prove causation. However, within science most definitions of love involve selective behaviors and attachments [109]. Using this limited definition, remarkable parallels can be identified between the functions and properties of oxytocin and love (Table 2). The features and functions of oxytocin and social attachments suggest that these apparently diverse constructs have shared roots. As described here, it can be argued that oxytocin is a component of an embodied biological system that supports the benefits of secure relationships.
Table 2.
Parallels between functions and properties of OXYTOCIN and LOVE.
| FUNCTIONS (among many) | LOVE | OXYTOCIN |
|---|---|---|
| MODERN (evolutionarily recent) | + | + |
| Associated with SELECTIVE sociality & bonds | + | + |
| Supporting parental investment | + | + |
| Metaphor for SAFETY | + | + |
| Selectively rewarding | + | + |
| Anti-inflammatory/Anti-oxidant | + | + |
| Anxiolytic/Analgesic | + | + |
| Allows immobilization without fear | + | + |
| Sexually dimorphic | + | + |
| Epigenetically tuned and Context dependent | + | + |
Both oxytocin and love are linked to the health benefits of safety including emotional and physical support [124]. Both also have adaptive interactions with more primitive and individualistic systems, including processes needed to manage threat, inflammation and disease. These more archaic mechanisms support individual survival in the short-term, but prolonged activation of the immune system can be dangerous for long-term health.
Oxytocin and vasopressin interact with other defensive systems, including the autonomic and immune systems, and also regulate the emotional states necessary for perceptions of safety [63,125]. These coordinate the capacity of an organism to selectively exhibit behavioral and emotional states necessary for active versus passive defense, including the alertness and mobility needed for escape from threat, danger or pain [126]. Peptide-mediated behavioral and autonomic functions also can influence the capacity for the voluntary immobility without fear, required for social interactions including maternal and sexual behaviors [127].
Of particular importance to mammalian survival are both early nurture and lasting and secure relationships [128] which may be facilitated by oxytocin [129]. Oxytocin has an essential role in lactation, facilitates the capacity to give birth and may allow mammals to become attached to their infants even under conditions of extreme challenge, such as those associated with human birth and child rearing [116]. Lactating women are uniquely able to manage stressors [130]. A postpartum surge in oxytocin helps to expel the placenta and could support initial bonding between the mother and child [117]. The development of offspring also is modulated by oxytocin [131], with effects that can be protective after a surgical birth [132]. Furthermore, infants reared on human milk receive a cocktail containing optimal nutrition, as well as oxytocin itself and probiotic bacteria [7,11].
The oxytocin system is an intrinsic component in the capacity of experiences, including both nurture and adversity, to manage adaptations in the mammalian body. The epigenetic consequences associated with modifying the oxytocin system can be detected across the lifespan, but especially around the time of birth when newborns must anticipate the demands of their future environments [7,103]. Early life is a period when mammals are particularly sensitive to the need for nurture and when the effects of oxytocin are most likely to create epigenetic change [[71], [72], [73]].
Oxytocin and positive social experiences play a critical role in brain development [94,133]. Early experiences also are implicated in the capacity to create lasting attachments [134]. As discussed elsewhere, oxytocin was permissive in the evolution of the human nervous system [2], with the resultant large neocortex necessary for consciousness, language and even spirituality. This capacity in modern humans is required for relationships to be experienced and expressed [135]. Furthermore, accurate appraisal of future threats [136], the relevance of social stimuli [119], and the development of a complex brain and autonomic nervous system demand oxytocin [2].
Both the health benefits of oxytocin and the capacity for love rely on interactions with more ancient physiological systems, including the immune and vasopressin systems [3,81,137]. The patterns of behavior regulated by vasopressin also are adaptive and in the functional absence of oxytocin the effects of vasopressin may be associated with fear, self-defense and in some cases aggression [89]. Understanding the factors that regulate these reactions provides novel insight into the complexity, power and possible dangers associated with love or its absence.
Biologically-active molecules, such as oxytocin, are sometimes treated as if they are manifestations of a well-understood chemical reality. Of course, oxytocin is part of a larger physiological equation. Here we have examined specific examples of interactions of oxytocin with vasopressin and the immune system. These have implications for all aspects of human health, and especially therapeutic strategies for the prevention and treatment of diseases associated with stress, adversity, trauma and inflammation.
15. Gaps in knowledge and a work in progress
Oxytocin and love have shared properties and functions (Table 2). Accurately calibrating reactions in response to or in anticipation of challenge are of particular importance to survival and eventually reproduction [138]. A general feature of both oxytocin and love is the capacity to modulate more primitive defense systems including threat or fear. Many of the biological associations between oxytocin and love are only apparent under conditions of physiological challenge. Loving and secure relationships, in part through the dynamic actions of oxytocin, are especially critical in the face of threat [89]. The effects of both oxytocin and secure relationships may be most easily detected in response to stressful and chronic experiences, including sickness [13,99] and social isolation [8]. Oxytocin also may help to explain the benefits of companion animals [139], including the processes that led to canid domestication [140].
Oxytocin's role in reducing emotional threat may be of particular importance in understanding the consequences of social isolation or during disrupted relationships, and especially in women [42]. Animal research also indicates that the mechanisms through which isolation is so devastating involve dynamic and sexually-dimorphic changes in oxytocin [65,141,142]. Interactions between oxytocin, vasopressin and immune systems are important to complex “paradoxical” effects of exogenous oxytocin [89], and could help to explain why it has proven difficult to create pharmaceuticals based on these peptides [3]. However, the nature of these interactions remains at present poorly understood.
Discussed elsewhere is evidence for a major role of the autonomic nervous system in mediating social connection and emotion regulation [3,125]. The autonomic system also regulates immunity and is implicated in autoimmune disorders [143]. Oxytocin and vasopressin both affect the complex coupling of sympathetic and parasympathetic functions [63,144]. The relationship between oxytocin, vasopressin and the autonomic nervous system deserves deep analysis.
Excess inflammation and oxidative stress have been implicated in virtually every known physical and emotional disorder [145]. Oxytocin functions in part through its capacity to serve as a component of mammalian defense systems with both anti-inflammatory and anti-oxidant effects [146] [147]. The detrimental effect of oxygen also is managed in part by the anti-inflammatory and anti-oxidant properties of oxytocin. This may be a clue to an ancient and reoccurring link between oxytocin and oxygen. This link is found in responses to immune challenges and excess inflammation, including mitochondrial activity [6,148], responses to pathogenic viruses [149], and in protection of the fetus during live birth [103].
Broad metaphorical meanings have been attached to both the concept of love and the functions of oxytocin. Compared to other biologically active molecules oxytocin is recently evolved. Oxytocin links individuals to others, and also supports the benefits of sociality. Survival strategies based on connection, cooperation, the support of offspring and perceived safety are critical to emotional and physical health [150]. There is evidence that the mechanisms for the benefits of oxytocin and secure relationships offer potential, but at present largely untapped, insights into disease, cellular aging and longevity [151,152].
Myths and mysteries surround love. Despite popular assumptions that “love overcomes fear” and “love heals,” as a subject for serious study love may be considered frivolous. We argue here that oxytocin has broad consequences that resemble in part an embodied metaphor for love. However, like love, the oxytocin system also has proven difficult to identify, measure and study and the controversies associated with oxytocin are far from resolved. The same adaptive biochemical properties that make oxytocin and love difficult to study may also help to explain their potential as components of “Nature's medicine” [3].
A deeper awareness of the biology of relationships is essential to understanding what it means to be “human.” Mechanisms through which these systems are integrated has profound implications for human health [3]. However, interactions among the pieces of the peptide-immune-behavioral puzzle are only now being recognized. Embedded in the biology of love and oxytocin are secrets to the social solutions that optimize living in a dangerous world. Put simply, without oxytocin and love mammals may survive, but not thrive. How this occurs remains one of life's great mysteries.
Declaration of interests
None.
Acknowledgments
Research described here from the author's laboratory was funded by the National Institutes of Health, most recently by HD P01 07575 and HD R01 098117 and support from the Fetzer Institute. The editorial suggestions and other supportive inputs of Stephen Porges, Alex Horn, John M. Davis, Marcy Kingsbury and Robert Dantzer are gratefully acknowledged. Because of space limitations I apologize to investigators whose primary work is not fully recognized and refer the reader to additional references in earlier reviews.
References
- 1.Du Vigneaud V., Ressler C., Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J. Biol. Chem. 1953;205(2):949–957. [PubMed] [Google Scholar]
- 2.Carter C.S. Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol. 2014;65:17–39. doi: 10.1146/annurev-psych-010213-115110. [DOI] [PubMed] [Google Scholar]
- 3.Carter C.S., Kenkel W.M., MacLean E.L., et al. Is oxytocin “Nature's medicine”? Pharmacol. Rev. 2020;72(4):829–861. doi: 10.1124/pr.120.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinevich V., Ludwig M. The multiple faces of the oxytocin and vasopressin systems in the brain. J. Neuroendocrinol. June 17, 2021 doi: 10.1111/jne.13004. Published online. [DOI] [PubMed] [Google Scholar]
- 5.Higashida H., Hashii M., Tanaka Y., et al. CD38, CD157, and RAGE as molecular determinants for social behavior. Cells. 2019;9(1):E62. doi: 10.3390/cells9010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordt E.A., Smith C.J., Demarest T.G., Bilbo S.D., Kingsbury M.A. Mitochondria, oxytocin, and vasopressin: unfolding the inflammatory protein response. Neurotox. Res. 2019;36(2):239–256. doi: 10.1007/s12640-018-9962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingsbury M.A., Bilbo S.D. The inflammatory event of birth: how oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Neuroendocrinol. 2019;55:100794. doi: 10.1016/j.yfrne.2019.100794. Journal Article. [DOI] [PubMed] [Google Scholar]
- 8.Smith C.J., Bilbo S.D. Sickness and the social brain: love in the time of COVID. Front. Psychiatr. 2021;12:633664. doi: 10.3389/fpsyt.2021.633664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loth M.K., Donaldson Z.R. Oxytocin, dopamine, and opioid interactions underlying pair bonding: highlighting a potential role for microglia. Endocrinology. 2021;162(2) doi: 10.1210/endocr/bqaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar A., Harty S., Johnson K.V.A., et al. The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. Camb. Phil. Soc. 2020;95(5):1131–1166. doi: 10.1111/brv.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdman S.E. Oxytocin and the microbiome. Curr Opin Endocr Metab Res. 2021;19:8–14. doi: 10.1016/j.coemr.2021.04.006. [DOI] [Google Scholar]
- 12.Theofanopoulou C., Gedman G., Cahill J.A., Boeckx C., Jarvis E.D. Universal nomenclature for oxytocin-vasotocin ligand and receptor families. Nature. 2021;592(7856):747–755. doi: 10.1038/s41586-020-03040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavaliers M., Ossenkopp K.P., Choleris E. Pathogens, odors, and disgust in rodents. Neurosci. Biobehav. Rev. 2020;119:281–293. doi: 10.1016/j.neubiorev.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busnelli M., Chini B. Molecular basis of oxytocin receptor signalling in the brain: what we know and what we need to know. Curr Top Behav Neurosci. 2018;35:3–29. doi: 10.1007/7854_2017_6. [DOI] [PubMed] [Google Scholar]
- 15.Gwee P.C., Amemiya C.T., Brenner S., Venkatesh B. Sequence and organization of coelacanth neurohypophysial hormone genes: evolutionary history of the vertebrate neurohypophysial hormone gene locus. BMC Evol. Biol. 2008;8:93. doi: 10.1186/1471-2148-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H.J., Macbeth A.H., Pagani J., Young W.S. Oxytocin: the great facilitator of life. Prog. Neurobiol. 2009;88(2):127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierce M.L., French J.A., Murray T.F. Comparison of the pharmacological profiles of arginine vasopressin and oxytocin analogs at marmoset, macaque, and human vasopressin 1a receptor. Biomed. Pharmacother. 2020;126:110060. doi: 10.1016/j.biopha.2020.110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uvnas Moberg K., Handlin L., Kendall-Tackett K., Petersson M. Oxytocin is a principal hormone that exerts part of its effects by active fragments. Med. Hypotheses. 2019;133:109394. doi: 10.1016/j.mehy.2019.109394. [DOI] [PubMed] [Google Scholar]
- 19.Mustoe A., Schulte N.A., Taylor J.H., French J.A., Toews M.L. Leu8 and Pro8 oxytocin agonism differs across human, macaque, and marmoset vasopressin 1a receptors. Sci. Rep. 2019;9(1):15480. doi: 10.1038/s41598-019-52024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parreiras-E-Silva L.T., Vargas-Pinilla P., Duarte D.A., et al. Functional New World monkey oxytocin forms elicit an altered signaling profile and promotes parental care in rats. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34):9044–9049. doi: 10.1073/pnas.1711687114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai L., Carter C.S., Ying J., Bellugi U., Pournajafi-Nazarloo H., Korenberg J.R. Oxytocin and vasopressin are dysregulated in Williams Syndrome, a genetic disorder affecting social behavior. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster K., Carter C.S., Pournajafi-Nazarloo H., et al. Plasma oxytocin explains individual differences in neural substrates of social perception. Front. Hum. Neurosci. 2015;9:132. doi: 10.3389/fnhum.2015.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLean E.L., Wilson S.R., Martin W.L., Davis J.M., Nazarloo H.P., Carter C.S. Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology. 2019;107:225–231. doi: 10.1016/j.psyneuen.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnanadesikan G.E., Hammock E.A.D., Tecot S.R., Carter C.S., MacLean E.L. Specificity of plasma oxytocin immunoassays: a comparison of commercial assays and sample preparation techniques using oxytocin knockout and wildtype mice. Psychoneuroendocrinology. 2021;132:105368. doi: 10.1016/j.psyneuen.2021.105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherepanov S.M., Gerasimenko M., Yuhi T., et al. An improved sample extraction method reveals that plasma receptor for advanced glycation end-products (RAGE) modulates circulating free oxytocin in mice. Peptides. September 17, 2021:170649. doi: 10.1016/j.peptides.2021.170649. Published online. [DOI] [PubMed] [Google Scholar]
- 26.Leng G., Ludwig M. Intranasal oxytocin: myths and delusions. Biol. Psychiatr. 2016;79(3):243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Cuneo M.G., Szeto A., Schrepf A., et al. Oxytocin in the tumor microenvironment is associated with lower inflammation and longer survival in advanced epithelial ovarian cancer patients. Psychoneuroendocrinology. 2019;106:244–251. doi: 10.1016/j.psyneuen.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale H.H. 1465771; on some physiological actions of ergot. J. Physiol. 1906;34(3):163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinevich V., Neumann I.D. Brain oxytocin: how puzzle stones from animal studies translate into psychiatry. Mol. Psychiatr. 2021;26(1):265–279. doi: 10.1038/s41380-020-0802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefebvre D.L., Giaid A., Bennett H., Larivière R., Zingg H.H. Oxytocin gene expression in rat uterus. Science. 1992;256(5063):1553–1555. doi: 10.1126/science.1598587. [DOI] [PubMed] [Google Scholar]
- 31.Cuneo M.G., Szeto A., Schrepf A., et al. Positive psychosocial factors and oxytocin in the ovarian tumor microenvironment. Psychosom. Med. 2021;83(5):417–422. doi: 10.1097/PSY.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurek B., Neumann I.D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98(3):1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 33.Jurek B., Meyer M. Anxiolytic and anxiogenic? How the transcription factor MEF2 might explain the manifold behavioral effects of oxytocin. Front. Endocrinol. 2020;11:186. doi: 10.3389/fendo.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caldwell H.K. Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist. 2017;23(5):517–528. doi: 10.1177/1073858417708284. [DOI] [PubMed] [Google Scholar]
- 35.Song Z., Albers H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018;51:14–24. doi: 10.1016/j.yfrne.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragnauth A.K., Goodwillie A., Brewer C., Muglia L.J., Pfaff D.W., Kow L.M. Vasopressin stimulates ventromedial hypothalamic neurons via oxytocin receptors in oxytocin gene knockout male and female mice. Neuroendocrinology. 2004;80(2):92–99. doi: 10.1159/000081844. [DOI] [PubMed] [Google Scholar]
- 37.Wahis J., Baudon A., Althammer F., et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021;24(4):529–541. doi: 10.1038/s41593-021-00800-0. [DOI] [PubMed] [Google Scholar]
- 38.Waltenspühl Y., Schöppe J., Ehrenmann J., Kummer L., Plückthun A. Crystal structure of the human oxytocin receptor. Sci. Adv. 2020;6(29) doi: 10.1126/sciadv.abb5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray S., Arrowsmith S. Uterine excitability and ion channels and their changes with gestation and hormonal environment. Annu. Rev. Physiol. 2021;83:331–357. doi: 10.1146/annurev-physiol-032420-035509. [DOI] [PubMed] [Google Scholar]
- 40.McCook O., Denoix N., Radermacher P., Waller C., Merz T. H2S and oxytocin systems in early life stress and cardiovascular disease. J. Clin. Med. 2021;10(16):3484. doi: 10.3390/jcm10163484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumais K.M., Veenema A.H. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor S.E., Gonzaga G.C., Klein L.C., Hu P., Greendale G.A., Seeman T.E. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom. Med. 2006;68(2):238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- 43.Rilling J.K., DeMarco A.C., Hackett P.D., et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter C.S., Perkeybile A.M. The monogamy paradox: what do love and sex have to do with it? Front. Ecol. Evol. 2018;6(202) doi: 10.3389/fevo.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jirikowski G.F., Ochs S.D., Caldwell J.D. Oxytocin and steroid actions. Curr Top Behav Neurosci. 2018;35:77–95. doi: 10.1007/7854_2017_9. [DOI] [PubMed] [Google Scholar]
- 46.Bilbo S.D., Block C.L., Bolton J.L., Hanamsagar R., Tran P.K. Beyond infection - maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018;299:241–251. doi: 10.1016/j.expneurol.2017.07.002. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy M.M. A new view of sexual differentiation of mammalian brain. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2020;206(3):369–378. doi: 10.1007/s00359-019-01376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter C.S., Boone E.M., Pournajafi-Nazarloo H., Bales K.L. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev. Neurosci. 2009;31(4):332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguilera G. Regulation of the hypothalamic-pituitary-adrenal axis by neuropeptides. Horm. Mol. Biol. Clin. Invest. 2011;7(2):327–336. doi: 10.1515/HMBCI.2011.123. [DOI] [PubMed] [Google Scholar]
- 50.Antoni F.A. Magnocellular vasopressin and the mechanism of “glucocorticoid escape. Front. Endocrinol. 2019;10:422. doi: 10.3389/fendo.2019.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bangasser D.A., Eck S.R., Ordoñes Sanchez E. Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019;44(1):129–139. doi: 10.1038/s41386-018-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuloaga D.G., Heck A.L., De Guzman R.M., Handa R.J. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol. Sex Differ. 2020;11(1):44. doi: 10.1186/s13293-020-00319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibbs D.M. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35(5):487–491. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- 54.Amico J.A., Miedlar J.A., Cai H.M., Vollmer R.R. Oxytocin knockout mice: a model for studying stress-related and ingestive behaviours. Prog. Brain Res. 2008;170:53–64. doi: 10.1016/S0079-6123(08)00405-6. [DOI] [PubMed] [Google Scholar]
- 55.Carter C.S. Oxytocin and sexual behavior. Neurosci. Biobehav. Rev. 1992;16(2):131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 56.Stadler B., Whittaker M.R., Exintaris B., Middendorff R. Oxytocin in the male reproductive tract; the therapeutic potential of oxytocin-agonists and-antagonists. Front. Endocrinol. 2020;11:565731. doi: 10.3389/fendo.2020.565731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arrowsmith S., Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014;26(6):356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 58.Cho M.M., DeVries A.C., Williams J.R., Carter C.S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 59.Jong TR de, Menon R., Bludau A., et al. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–388. doi: 10.1016/j.psyneuen.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 60.Donadon M.F., Martin-Santos R., Osório F. de L. The associations between oxytocin and trauma in humans: a systematic review. Front. Pharmacol. 2018;9:154. doi: 10.3389/fphar.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leng G., Russell J.A. The osmoresponsiveness of oxytocin and vasopressin neurones: mechanisms, allostasis and evolution. J. Neuroendocrinol. 2019;31(3) doi: 10.1111/jne.12662. [DOI] [PubMed] [Google Scholar]
- 62.Welch M.G., Ludwig R.J. Calming cycle theory and the Co-regulation of oxytocin. Psychodyn. Psychiatry. 2017;45(4):519–540. doi: 10.1521/pdps.2017.45.4.519. [DOI] [PubMed] [Google Scholar]
- 63.Quintana D.S., Guastella A.J. An allostatic theory of oxytocin. Trends Cognit. Sci. 2020;24(7):515–528. doi: 10.1016/j.tics.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Neumann I., Douglas A.J., Pittman Q.J., Russell J.A., Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J. Neuroendocrinol. 1996;8(3):227–233. doi: 10.1046/j.1365-2826.1996.04557.x. [DOI] [PubMed] [Google Scholar]
- 65.Grippo A.J., Pournajafi-Nazarloo H., Sanzenbacher L., et al. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress. 2012;15(2):149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong W.H., Abdulai-Saiku S., Vyas A. Arginine vasopressin in the medial amygdala causes greater post-stress recruitment of hypothalamic vasopressin neurons. Mol. Brain. 2021;14(1):141. doi: 10.1186/s13041-021-00850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ito E., Shima R., Yoshioka T. A novel role of oxytocin: oxytocin-induced well-being in humans. Biophys. Physicobiol. 2019;16:132–139. doi: 10.2142/biophysico.16.0_132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poisbeau P., Grinevich V., Charlet A. Oxytocin signaling in pain: cellular, circuit, system, and behavioral levels. Curr Top Behav Neurosci. 2018;35:193–211. doi: 10.1007/7854_2017_14. [DOI] [PubMed] [Google Scholar]
- 69.Zheng H., Lim J.Y., Kim Y., Jung S.T., Hwang S.W. The role of oxytocin, vasopressin, and their receptors at nociceptors in peripheral pain modulation. Front. Neuroendocrinol. 2021;63:100942. doi: 10.1016/j.yfrne.2021.100942. [DOI] [PubMed] [Google Scholar]
- 70.Schorscher-Petcu A., Sotocinal S., Ciura S., et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci. Off. J. Soc. Neurosci. 2010;30(24):8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danoff J.S., Connelly J.J., Morris J.P., Perkeybile A.M. An epigenetic rheostat of experience: DNA methylation of OXTR as a mechanism of early life allostasis. Compr. Psychoneuroendocrinol. 2021;8:100098. doi: 10.1016/j.cpnec.2021.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perkeybile A.M., Carter C.S., Wroblewski K.L., et al. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology. 2019;99:128–136. doi: 10.1016/j.psyneuen.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenkel W.M., Perkeybile A.M., Yee J.R., et al. Behavioral and epigenetic consequences of oxytocin treatment at birth. Sci. Adv. 2019;5(5) doi: 10.1126/sciadv.aav2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ellis B.J., Horn A.J., Carter C.S., van IJzendoorn M.H., Bakermans-Kranenburg M.J. Developmental programming of oxytocin through variation in early-life stress: four meta-analyses and a theoretical reinterpretation. Clin. Psychol. Rev. 2021;86:101985. doi: 10.1016/j.cpr.2021.101985. [DOI] [PubMed] [Google Scholar]
- 75.Stribley J.M., Carter C.S. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc. Natl. Acad. Sci. USA. 1999;96(22):12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bredewold R., Veenema A.H. Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Curr. Opin. Neurobiol. 2018;49:132–140. doi: 10.1016/j.conb.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kompier N.F., Keysers C., Gazzola V., Lucassen P.J., Krugers H.J. Early life adversity and adult social behavior: focus on arginine vasopressin and oxytocin as potential mediators. Front. Behav. Neurosci. 2019;13:143. doi: 10.3389/fnbeh.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garrison J.L., Macosko E.Z., Bernstein S., Pokala N., Albrecht D.R., Bargmann C.I. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338(6106):540–543. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grinevich V., Knobloch-Bollmann H.S., Eliava M., Busnelli M., Chini B. Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatr. 2016;79(3):155–164. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Acher R., Chauvet J., Chauvet M.T. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 1995;395:615–627. [PubMed] [Google Scholar]
- 81.Knobloch H.S., Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gwee P.C., Tay B.H., Brenner S., Venkatesh B. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol. 2009;9:47. doi: 10.1186/1471-2148-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y., Shearwin-Whyatt L., Li J., et al. Platypus and echidna genomes reveal mammalian biology and evolution. Nature. 2021;592(7856):756–762. doi: 10.1038/s41586-020-03039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lovejoy D.A., Hogg D.W. Information processing in affective disorders: did an ancient peptide regulating intercellular metabolism become Co-opted for noxious stress sensing? BioEssays News Rev. Mol. Cell Dev. Biol. 2020;42(9) doi: 10.1002/bies.202000039. [DOI] [PubMed] [Google Scholar]
- 85.Lefevre A., Benusiglio D., Tang Y., Krabichler Q., Charlet A., Grinevich V. Oxytocinergic feedback circuitries: an anatomical basis for neuromodulation of social behaviors. Front. Neural Circ. 2021;15:688234. doi: 10.3389/fncir.2021.688234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tolomeo S., Chiao B., Lei Z., Chew S.H., Ebstein R.P. A novel role of CD38 and oxytocin as tandem molecular moderators of human social behavior. Neurosci. Biobehav. Rev. 2020;115:251–272. doi: 10.1016/j.neubiorev.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 87.Yamamoto Y., Higashida H. RAGE regulates oxytocin transport into the brain. Commun. Biol. 2020;3(1) doi: 10.1038/s42003-020-0799-2. 70-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olivera-Pasilio V., Dabrowska J. Oxytocin promotes accurate fear discrimination and adaptive defensive behaviors. Front. Neurosci. 2020;14:583878. doi: 10.3389/fnins.2020.583878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carter C.S. The oxytocin-vasopressin pathway in the context of love and fear. Front. Endocrinol. 2017;8:356. doi: 10.3389/fendo.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Dreu C.K.W., Fariña A., Gross J., Romano A. Prosociality as a foundation for intergroup conflict. Curr. Opin. Psychol. 2021;44:112–116. doi: 10.1016/j.copsyc.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Fill Malfertheiner S., Bataiosu-Zimmer E., Michel H., et al. Vasopressin but not oxytocin responds to birth stress in infants. Front. Neurosci. 2021;15:718056. doi: 10.3389/fnins.2021.718056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walsh E.C., Eisenlohr-Moul T.A., Pedersen C.A., Rubinow D.R., Girdler S.S., Dichter G.S. Early life abuse moderates the effects of intranasal oxytocin on symptoms of premenstrual dysphoric disorder: preliminary evidence from a placebo-controlled trial. Front. Psychiatr. 2018;9:547. doi: 10.3389/fpsyt.2018.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindley Baron-Cohen K., Feldman R., Fearon P., Fonagy P. Intranasal oxytocin administration improves mood in new mothers with moderate low mood but not in mothers with elevated symptoms of postnatal depression: a randomised controlled trial. J. Affect. Disord. 2022 doi: 10.1016/j.jad.2021.11.062. S0165-0327(21)01292-1. [DOI] [PubMed] [Google Scholar]
- 94.Froemke R.C., Young L.J. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. April 6, 2021 doi: 10.1146/annurev-neuro-102320-102847. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferguson J.N., Young L.J., Insel T.R. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 2002;23(2):200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 96.Peled-Avron L., Abu-Akel A., Shamay-Tsoory S. Exogenous effects of oxytocin in five psychiatric disorders: a systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neurosci. Biobehav. Rev. 2020;114:70–95. doi: 10.1016/j.neubiorev.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 97.Carter C.S., Porges E.C. Parenthood, stress, and the brain. Biol. Psychiatr. 2011;70(9):804–805. doi: 10.1016/j.biopsych.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 98.Olff M., Frijling J.L., Kubzansky L.D., et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38(9):1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 99.Dantzer R. Love and fear in the times of sickness. Compr. Psychoneuroendocrinol. 2021;6:100032. doi: 10.1016/j.cpnec.2021.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gabor C.S., Phan A., Clipperton-Allen A.E., Kavaliers M., Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 2012;126(1):97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 101.Carcea I., Caraballo N.L., Marlin B.J., et al. Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596(7873):553–557. doi: 10.1038/s41586-021-03814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russell J.A., Leng G., Douglas A.J. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front. Neuroendocrinol. 2003;24(1):27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 103.Ben-Ari Y. Oxytocin and vasopressin, and the GABA developmental shift during labor and birth: friends or foes? Front. Cell. Neurosci. 2018;12:254. doi: 10.3389/fncel.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dölen G., Darvishzadeh A., Huang K.W., Malenka R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aragona B.J., Liu Y., Yu Y.J., et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 2005;9(1):133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 106.Charles S.J., Farias M., van Mulukom V., et al. Blocking mu-opioid receptors inhibits social bonding in rituals. Biol. Lett. 2020;16(10):20200485. doi: 10.1098/rsbl.2020.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gerra M.L., Gerra M.C., Tadonio L., et al. Early parent-child interactions and substance use disorder: an attachment perspective on a biopsychosocial entanglement. Neurosci. Biobehav. Rev. 2021;131:560–580. doi: 10.1016/j.neubiorev.2021.09.052. [DOI] [PubMed] [Google Scholar]
- 108.Marazziti D., Palermo S., Mucci F. The science of love: state of the art. Adv. Exp. Med. Biol. 2021;1331:249–254. doi: 10.1007/978-3-030-74046-7_16. [DOI] [PubMed] [Google Scholar]
- 109.Carter C.S. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 110.van Anders S.M., Goodson J.L., Kingsbury M.A. Beyond “oxytocin = good”: neural complexities and the flipside of social bonds. Arch. Sex. Behav. 2013;42(7):1115–1118. doi: 10.1007/s10508-013-0134-9. [DOI] [PubMed] [Google Scholar]
- 111.Klopfer P.H. Mother love: what turns it on? Am. Sci. 1971;59(4):404–407. [PubMed] [Google Scholar]
- 112.Russell J.A., Leng G. Sex, parturition and motherhood without oxytocin? J. Endocrinol. 1998;157(3):343–359. doi: 10.1677/joe.0.1570343. [DOI] [PubMed] [Google Scholar]
- 113.Nishimori K., Young L.J., Guo Q., Wang Z., Insel T.R., Matzuk M.M. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. Unit. States Am. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Young W.S., Shepard E., Amico J., et al. Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J. Neuroendocrinol. 1996;8(11):847–853. doi: 10.1046/j.1365-2826.1996.05266.x. [DOI] [PubMed] [Google Scholar]
- 115.Fleming A.S., Rosenblatt J.S. Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J. Comp. Physiol. Psychol. 1974;86(2):233–246. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- 116.Carter C.S., Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann. N. Y. Acad. Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- 117.Nissen E., Lilja G., Widström A.M., Uvnäs-Moberg K. Elevation of oxytocin levels early post partum in women. Acta Obstet. Gynecol. Scand. 1995;74(7):530–533. doi: 10.3109/00016349509024384. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi T. Sensory stimulation of oxytocin release is associated with stress management and maternal care. Front. Psychol. 2020;11:588068. doi: 10.3389/fpsyg.2020.588068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Egito J.H., Nevat M., Shamay-Tsoory S.G., Osório A.A.C. Oxytocin increases the social salience of the outgroup in potential threat contexts. Horm. Behav. 2020;122:104733. doi: 10.1016/j.yhbeh.2020.104733. [DOI] [PubMed] [Google Scholar]
- 120.Hatfield E., Rapson R.L. Passionate love/sexual desire: can the same paradigm explain both? Arch. Sex. Behav. 1987;16(3):259–278. doi: 10.1007/BF01541613. [DOI] [PubMed] [Google Scholar]
- 121.Fisher H.E., Xu X., Aron A., Brown L.L. Intense, passionate, romantic love: a natural addiction? How the fields that investigate romance and substance abuse can inform each other. Front. Psychol. 2016;7:687. doi: 10.3389/fpsyg.2016.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jamrozik A., McQuire M., Cardillo E.R., Chatterjee A. Metaphor: bridging embodiment to abstraction. Psychon. Bull. Rev. 2016;23(4):1080–1089. doi: 10.3758/s13423-015-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Winkielman P., Coulson S., Niedenthal P. Dynamic grounding of emotion concepts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373(1752):20170127. doi: 10.1098/rstb.2017.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singer B., Friedman E., Seeman T., Fava G.A., Ryff C.D. Protective environments and health status: cross-talk between human and animal studies. Neurobiol. Aging. 2005;26(Suppl 1):113–118. doi: 10.1016/j.neurobiolaging.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 125.Porges S.W. Polyvagal Theory: a biobehavioral journey to sociality. Compr. Psychoneuroendocrinol. 2021;7:100069. doi: 10.1016/j.cpnec.2021.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grinevich V., Stoop R. Interplay between oxytocin and sensory systems in the orchestration of socio-emotional behaviors. Neuron. 2018;99(5):887–904. doi: 10.1016/j.neuron.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 127.Porges S.W. The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 2001;42(2):123–146. doi: 10.1016/S0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 128.Bowlby J. Attachment theory and its therapeutic implications. Adolesc. Psychiatr. 1978;6:5–33. [PubMed] [Google Scholar]
- 129.Shimon-Raz O., Salomon R., Bloch M., et al. Mother brain is wired for social moments. Elife. 2021;10 doi: 10.7554/eLife.59436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Altemus M., Redwine L.S., Leong Y.M., Frye C.A., Porges S.W., Carter C.S. Responses to laboratory psychosocial stress in postpartum women. Psychosom. Med. 2001;63(5):814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 131.Kenkel W. Birth signalling hormones and the developmental consequences of caesarean delivery. J. Neuroendocrinol. 2020 doi: 10.1111/jne.12912. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morais L.H., Golubeva A.V., Casey S., et al. Early-life oxytocin attenuates the social deficits induced by caesarean-section delivery in the mouse. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2021 doi: 10.1038/s41386-021-01040-3. Published online May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hammock E.A.D. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology. 2015;40(1):24–42. doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bosmans G., Bakermans-Kranenburg M.J., Vervliet B., Verhees M.W.F.T., van IJzendoorn M.H. A learning theory of attachment: unraveling the black box of attachment development. Neurosci. Biobehav. Rev. 2020;113:287–298. doi: 10.1016/j.neubiorev.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 135.Theofanopoulou C., Boeckx C., Jarvis E.D. A hypothesis on a role of oxytocin in the social mechanisms of speech and vocal learning. Proc. Biol. Sci. 2017;284(1861) doi: 10.1098/rspb.2017.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Janeček M., Dabrowska J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST) Cell Tissue Res. 2019;375(1):143–172. doi: 10.1007/s00441-018-2889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feldman R. Social behavior as a transdiagnostic marker of resilience. Annu. Rev. Clin. Psychol. 2021;17:153–180. doi: 10.1146/annurev-clinpsy-081219-102046. [DOI] [PubMed] [Google Scholar]
- 138.Sterling P. Predictive regulation and human design. Elife. 2018;7 doi: 10.7554/eLife.36133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Applebaum J.W., MacLean E.L., McDonald S.E. Love, fear, and the human-animal bond: on adversity and multispecies relationships. Compr. Psychoneuroendocrinol. 2021;7:100071. doi: 10.1016/j.cpnec.2021.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herbeck Y.E., Eliava M., Grinevich V., MacLean E.L. Fear, love, and the origins of canid domestication: an oxytocin hypothesis. Compr. Psychoneuroendocrinol. 2022;9:100100. doi: 10.1016/j.cpnec.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pohl T.T., Young L.J., Bosch O.J. Lost connections: oxytocin and the neural, physiological, and behavioral consequences of disrupted relationships. Int. J. Psychophysiol. January 9, 2018 doi: 10.1016/j.ijpsycho.2017.12.011. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cacioppo J.T., Cacioppo S., Cole S.W., Capitanio J.P., Goossens L., Boomsma D.I. Loneliness across phylogeny and a call for comparative studies and animal models. Perspect. Psychol. Sci. J. Assoc. Psychol. Sci. 2015;10(2):202–212. doi: 10.1177/1745691614564876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lv J., Ji X., Li Z., Hao H. The role of the cholinergic anti-inflammatory pathway in autoimmune rheumatic diseases. Scand. J. Immunol. 2021;94(4) doi: 10.1111/sji.13092. [DOI] [PubMed] [Google Scholar]
- 144.Yee J.R., Kenkel W.M., Frijling J.L., et al. Oxytocin promotes functional coupling between paraventricular nucleus and both sympathetic and parasympathetic cardioregulatory nuclei. Horm. Behav. 2016;80:82–91. doi: 10.1016/j.yhbeh.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buemann B., Uvnäs-Moberg K. Oxytocin may have a therapeutical potential against cardiovascular disease. Possible pharmaceutical and behavioral approaches. Med. Hypotheses. 2020;138:109597. doi: 10.1016/j.mehy.2020.109597. [DOI] [PubMed] [Google Scholar]
- 147.Nation D.A., Szeto A., Mendez A.J., et al. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE-/- mice. Psychosom. Med. 2010;72(4):376–382. doi: 10.1097/PSY.0b013e3181d74c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Naviaux R.K. Perspective: cell danger response Biology-The new science that connects environmental health with mitochondria and the rising tide of chronic illness. Mitochondrion. 2020;51:40–45. doi: 10.1016/j.mito.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 149.Imami A.S., O'Donovan S.M., Creeden J.F., et al. Oxytocin's anti-inflammatory and proimmune functions in COVID-19: a transcriptomic signature-based approach. Physiol. Genom. 2020;52(9):401–407. doi: 10.1152/physiolgenomics.00095.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leckman J.F., Ponguta L.A., Pavarini G., et al. Love and peace across generations: biobehavioral systems and global partnerships. Compr. Psychoneuroendocrinol. 2021;8:100092. doi: 10.1016/j.cpnec.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stevenson J.R., McMahon E.K., Boner W., Haussmann M.F. Oxytocin administration prevents cellular aging caused by social isolation. Psychoneuroendocrinology. 2019;103:52–60. doi: 10.1016/j.psyneuen.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Horn A.J., Carter C.S. Love and longevity: a social dependency hypothesis. Compr. Psychoneuroendocrinol. 2021;8:100088. doi: 10.1016/j.cpnec.2021.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]