Abstract
Introduction
Asthma is a heterogeneous disease with poorly defined phenotypes. Patients with severe asthma often receive multiple treatments including oral corticosteroids (OCS). Treatment may modify the observed metabotype, rendering it challenging to investigate underlying disease mechanisms. Here, we aimed to identify dysregulated metabolic processes in relation to asthma severity and medication.
Methods
Baseline urine was collected prospectively from healthy participants (n=100), patients with mild-to-moderate asthma (n=87) and patients with severe asthma (n=418) in the cross-sectional U-BIOPRED cohort; 12–18-month longitudinal samples were collected from patients with severe asthma (n=305). Metabolomics data were acquired using high-resolution mass spectrometry and analysed using univariate and multivariate methods.
Results
A total of 90 metabolites were identified, with 40 significantly altered (p<0.05, false discovery rate <0.05) in severe asthma and 23 by OCS use. Multivariate modelling showed that observed metabotypes in healthy participants and patients with mild-to-moderate asthma differed significantly from those in patients with severe asthma (p=2.6×10−20), OCS-treated asthmatic patients differed significantly from non-treated patients (p=9.5×10−4), and longitudinal metabotypes demonstrated temporal stability. Carnitine levels evidenced the strongest OCS-independent decrease in severe asthma. Reduced carnitine levels were associated with mitochondrial dysfunction via decreases in pathway enrichment scores of fatty acid metabolism and reduced expression of the carnitine transporter SLC22A5 in sputum and bronchial brushings.
Conclusions
This is the first large-scale study to delineate disease- and OCS-associated metabolic differences in asthma. The widespread associations with different therapies upon the observed metabotypes demonstrate the need to evaluate potential modulating effects on a treatment- and metabolite-specific basis. Altered carnitine metabolism is a potentially actionable therapeutic target that is independent of OCS treatment, highlighting the role of mitochondrial dysfunction in severe asthma.
Short abstract
Metabolomics identified a urinary metabotype of asthma driven by lower carnitine levels in an oral corticosteroid-independent manner. The carnitine transporter SLC22A5 was also decreased, suggesting carnitine metabolism as a potential therapeutic target. https://bit.ly/3BJfvT0
Introduction
Asthma is a heterogeneous inflammatory disease consisting of multiple phenotypes [1, 2]. Research has focused on identifying molecular descriptors of subgroups in relation to clinical outcomes with the aim of stratifying individuals for appropriate treatment strategies [3]. While it is ideal to interrogate the disease in the organ of manifestation, it is not feasible to perform routine sampling in the lung, especially in individuals with severe disease or at the population level. Accordingly, there is a need to identify molecular signatures in accessible biofluids (e.g. blood, urine, exhaled breath condensate) that indicate pathophysiologically driven biochemical perturbations. Urine has been successfully used to investigate local physiology in the lung [4] and is well suited to clinical applications owing to accessibility and ease of collection.
Mass spectrometry-based metabolomics in blood and urine has identified molecular signatures associated with both adult [5] and paediatric [6] asthma. In particular, metabolomics has detected metabolic signatures associated with aspirin-exacerbated respiratory disease [7], disease severity [8–10], bronchodilator response [11], pulmonary function [12], exacerbation [13] and corticosteroid resistance [14]. While these investigations have provided insight into metabolic dysregulation in association with disease, they have generally focused on smaller cohorts with little information on the longitudinal stability of the observed metabotypes [5, 14]. Furthermore, the potential modulating effects of asthma treatment on the observed metabotypes have not been evaluated. Oral corticosteroid (OCS) treatment is of particular interest because long-term OCS use is associated with multiple side effects including osteoporosis, adrenal suppression, metabolic disorders, psychiatric disorders and infection [15].
We hypothesise that systemic therapies such as OCSs as well as disease severity are reflected in the urinary metabolome. Using the Unbiased Biomarkers for the Prediction of Respiratory Disease outcomes (U-BIOPRED) study [16], we demonstrate that urinary metabotypes of severe asthma are dysregulated relative to healthy individuals, stable for 12–18 months and susceptible to associations with asthma medication on a metabolite-specific basis.
Methods
Study subjects and design
Urine samples were prospectively collected for the cross-sectional U-BIOPRED study [16]; all available samples were included in the present study (table 1). Participants were classified according to international guidelines into the following groups: healthy participants (n=100), patients with mild-to-moderate asthma (n=87), non-smoking patients with severe asthma (n=310) and smoking/ex-smoking patients with severe asthma (n=108) [16]. Non-smokers were defined as participants who were never smokers or non-smokers for at least 12 months prior to recruitment with a smoking history of <5 pack-years. Participants provided a urine sample within 28 days of initial screening (baseline visit). An additional urine sample was provided by 305 participants with severe asthma at a 12–18-month longitudinal visit. An overview of participant characteristics is shown in table 1, with a detailed description provided elsewhere [16]. Ethics approval was obtained from each clinical institution and all participants provided written informed consent (ClinicalTrials.gov identifier: NCT01976767).
TABLE 1.
Study characteristics of U-BIOPRED participants used for urinary metabolomics
| Baseline | Longitudinal | |||||
| Healthy controls | Mild-to-moderate asthma | Severe asthma non-smokers # | Severe asthma ex/smokers | Severe asthma non-smokers | Severe asthma ex/smokers | |
| Subjects (n) | 100 | 87 | 310 | 108 | 225 | 80 |
| Age, years | 35 (27–49) | 43 (28–53) | 53 (43–62) | 55 (48–61) | 55 (44–62) | 55 (49–63) |
| Females | 38 (38%) | 43 (49%) | 204 (68%) | 56 (52%) | 146 (65%) | 37 (46%) |
| Body mass index, kg/m2 | 24.9 (22.8–27.5) | 24.8 (23.0–28.8) | 27.7 (24.6–33.6) | 28.9 (25.1–32.6) | 27.7 (24.5–33.3) | 28.5 (25.1–32.5) |
| FEV1 % pred (pre-salbutamol) | 101.8 (93.6–110.3) | 91.6 (76.0–100.3) | 67.4 (50.7–84.8) | 66.2 (52.4–78.2) | 67.85 (50.0–84.8) | 60.4 (52.2–75.7) |
| FEV1/FVC (pre-salbutamol) | NA | 72.7 (65.6–77.5) | 63.4 (54.1–73.4) | 60.1 (52.7–69.3) | 62.2 (52.4–72.0) | 60.8 (50.2–67.2) |
| FEV1/FVC (post-salbutamol) | NA | 77.7 (72.0–83.1) | 66.9 (56.9–77.4) | 63.5 (54.8–72.6) | 66.1 (54.4–76.0) | 60.5 (53.8–68.9) |
| Exacerbations in previous year (n) | NA | 0 (0–1) | 2 (1–3) | 2 (1–4) | 2 (0–4) | 1 (0–4) |
| Smoking history, pack-years | 0.9 (0.3–3.5) | 4 (0.7–4.6) | 2 (1–4) | 17.1 (10–26) | NR | NR |
| Serum IgE, IU/mL | 23 (9–62) | 89 (50–244) | 117 (40–347) | 122 (60–328) | NR | NR |
| Blood eosinophils, ×10–3/µL | 100 (90–200) | 200 (100–300) | 220 (110–405) | 200 (100–405) | 209 (100–401) | 255 (148–450) |
| Sputum eosinophils, % | 0.4 (0.2–0.9) | 1.3 (0.7–3.9) | 4.5 (1.2–13.7) | 4.1 (1.3–26.5) | 1.8 (0.4–8.8) | 3.2 (0.8–16.5) |
| FeNO, ppb | 19.5 (13.8–29.0) | 25.5 (18.0–55.0) | 22.5 (12.0–42.0) | 26.5 (15.9–47.6) | 24.0 (15.0–42.5) | 20.8 (13.4–36.9) |
| Serum periostin, ng/mL | 49.7 (44.1–57.6) | 48.3 (40.9–54.5) | 43.8 (36.3–59.3) | 49.7 (41.9–60.1) | 51.7 (43.4–63.0) | 48.8 (39.6–64.7) |
| Human C-reactive protein, mg/µL | 0.8 (0.4–1.6) | 0.8 (0.4–2.1) | 2.3 (1.0–4.8) | 2.1 (0.9–4.8) | 2 (0.8–4.9) | 3.5 (1.4–6.0) |
| Interleukin-13, pg/mL | 0.39 (0.28–0.62) | 0.59 (0.4–0.86) | 0.52 (0.3–1.11) | 0.61 (0.31–1.14) | 0.61 (0.31–1.23) | 0.73 (0.36–1.20) |
| Atopy test positive | 36/89 (40.4%) | 68/77 (88.3%) | 178/239 (74.5%) | 46/78 (58.9%) | NR | NR |
| Participants prescribed OCS | NA | NA | 160/310 (52%) | 50/108 (46%) | 85/225 (37.8%) | 28/80 (32.9%) |
| Prescribed OCS dose (mg prednisolone equivalent) | NA | NA | 12 (9–20) | 16 (10–21) | 15 (9–29) | 16 (9–29) |
| Prednisolone detected in urine | NA | NA | 101/310 (32.6%) | 31/108 (28.7%) | 61/225 (27.1%) | 24/80 (28.2%) |
| Confirmed OCS users ¶ | NA | NA | 66/310 (21.3%) | 25/108 (23.1%) | 29/225 (12.9%) | 12/80 (15%) |
| Prescribed OCS dose (mg prednisolone equivalent) in confirmed OCS users | NA | NA | 10 (7.5–15) | 10 (10–20) | 10 (5–15) | 10 (10–20) |
| Confirmed OCS non-users + | NA | NA | 123/310 (39.7%) | 49/108 (45.3%) | 100/225 (44.4%) | 40/80 (50%) |
| Theophylline users § | NA | NA | 54/310 (17.4%) | 22/108 (20.3%) | 39/225 (17.3%) | 16/80 (20%) |
| Theophylline non-users ƒ | NA | NA | 245/310 (79.0%) | 85/108 (78.7%) | 178/225 (79.1%) | 64/80 (80%) |
| Omalizumab users | NA | NA | 39/310 (12.5%) | 13/108 (12.0%) | 33/225 (14.7%) | 10/80 (12.5%) |
| Serum IgE-matched omalizumab non-users ## | NA | NA | 71/310 (22.9%) | 26/108 (24.1%) | 54/225 (24.0%) | 26/80 (32.5%) |
| Anticholinergic users § | NA | NA | 68/310 (21.9%) | 31/108 (28.7%) | 54/225 (24.0%) | 23/80 (28.8%) |
| Anticholinergic non-users ƒ | NA | NA | 230/310 (74.2%) | 75/108 (69.4%) | 152/225 (67.6%) | 51/80 (63.8%) |
| Leukotriene modifier users § | NA | NA | 131/310 (42.3%) | 42/108 (38.9%) | 87/225 (38.7%) | 28/80 (35%) |
| Leukotriene modifier non-users ƒ | NA | NA | 169/310 (54.5%) | 62/108 (57.4%) | 117/225 (52.0%) | 48/80 (60%) |
Data are presented as n (%), median (IQR) or n/N (%), unless otherwise indicated. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; IgE: immunoglobulin E; FeNO: exhaled nitric oxide fraction; OCS: oral corticosteroids; NA: not applicable; NR: not reported. #: non-smoking status was defined as being never smokers or non-smokers for at least the last 12 months with <5 pack-year smoking history; ¶: reported at least daily use of OCS and positive detection of the presence of prednisolone or prednisone, methylprednisolone, 16α-OH-prednisolone, 20β-dihydroprednisolone or desacetyl deflazacort in urine; +: reported no prior use of OCS, and OCS metabolites were not detected in urine; §: reported at least daily use; ƒ: reported no prior use; ##: serum IgE-matched individuals with no prior omalizumab use.
Treatment use and stratification
Treatment use and stratification protocols are presented in detail in the supplementary material and have been reported previously [4]. Briefly, all patients with mild-to-moderate asthma were on ≤500 µg inhaled fluticasone equivalents/day (ICS), while patients with severe asthma received ≥1000 µg fluticasone equivalents/day [16]. Reliever medication, such as short/long-acting β2 agonists (SABA/LABA) or combination therapy, was used by all asthmatic patients. Non-smoker patients with severe asthma were stratified based upon treatment where use could be confirmed (OCS, omalizumab) or where existing literature provides evidence for a confounding effect on the metabolome (OCS, theophylline) [8, 9, 17–19], or where a sufficient proportion of the individuals received treatment to render stratification meaningful (anticholinergics, leukotriene modifiers).
Mass spectrometry analysis
Metabolomics data were acquired by liquid chromatography–high-resolution mass spectrometry (LC-HRMS) using previously published methods [20] that enabled detection of hydrophilic metabolites. Urine dilution was normalised to specific gravity prior to data acquisition [21]. Detailed methods are provided in the supplementary material. Tryptophan and six of its metabolites were quantified by reversed-phase liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) as described in the supplementary material.
Transcriptomics and genotyping
Bronchial brushings [22], sputum [23] and peripheral blood mononuclear cells (PBMCs) [24] were collected and transcriptomics analyses performed as previously described. Bronchial bushings and sputum samples were genotyped on the Affymetrix Axiom UK Biobank array [25].
Statistical analysis
Metabolomics data were not normally distributed (Lilliefors test); non-parametric univariate statistical tests were subsequently used. The Storey positive false discovery rate (FDR) [26] was calculated for all univariate analyses. Median fold-changes and confidence intervals were estimated using bootstrap resampling [8].
To identify similarities between metabolites, hierarchical cluster analysis was performed. The means of the log-transformed and z-scaled data of the resulting clusters were plotted against clinical groups to qualitatively visualise metabolite patterns across clinical groups. Multivariate principal components–canonical variate analysis (PC-CVA) was performed [8] to assess the multifactorial and correlated discrimination between clinical groups. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway mapping was performed using gene set variation analysis (GSVA) enrichment score (ES) determination of the genes identified as being part of the fatty acid metabolism pathway. The type-2 patient stratification was based on the ES of the interleukin (IL)-13-induced gene expression patterns in human bronchial epithelial cells using GSVA [23, 27], and transcriptome-associated cluster (TAC) membership was assigned based upon previous work [23]. Participant clinical and biochemical data were collected from the U-BIOPRED TranSMART platform (eTRIKS). All statistical analyses were performed using MATLAB (Mathworks, Natick, MA, USA).
Results
The identities of 90 urinary metabolites were confirmed against an in-house metabolite library. Using an FDR of 0.05, 40 metabolites were significantly (p<0.05) altered between the four study groups based on univariate analysis. Fold-changes compared to healthy participants and all statistics (including post hoc pairwise group comparisons) are presented in supplementary table E1.
Clustering of correlated metabolites
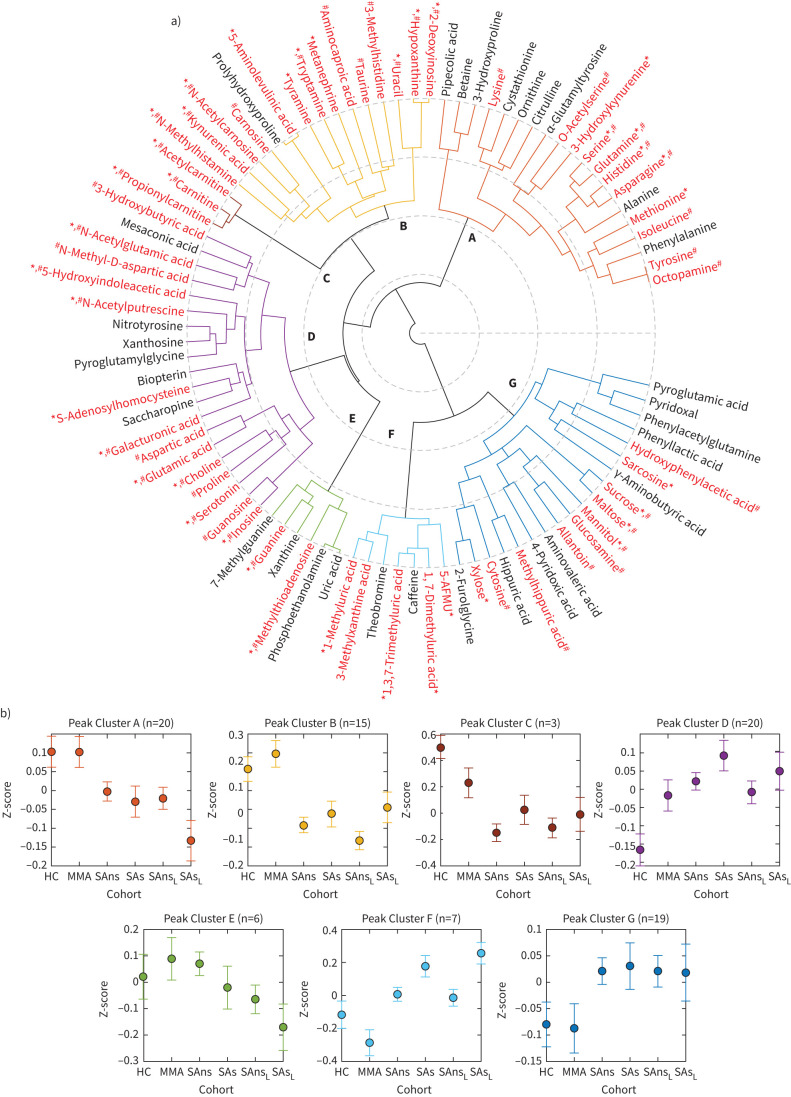
Hierarchical cluster analysis identified seven metabolite clusters, revealing different metabolite abundance patterns across the four study groups (figure 1). Cluster A comprised 20 metabolites, six of which were significant by univariate analysis (k=20; k=6, p<0.05). Both Cluster A and Cluster B (k=15; k=10, p<0.05) consisted primarily of amino acid metabolites and showed lower abundances in the patients with severe asthma than the healthy participants and patients with mild-to-moderate asthma. Cluster C (k=3, p<0.05) included carnitines, which decreased linearly with disease severity in the non-smoking groups. In patients with severe asthma, smokers showed elevated carnitine levels compared to non-smokers. Cluster D (k=20; k=9, p<0.05) included the diverse metabolite classes amino acid metabolites, organic acids, biogenic amines and purine nucleosides, which increased with disease severity and smoking status in severe asthma. Cluster E (k=6; k=2, p<0.05) consisted of purine metabolites, methylthioadenosine and phosphoethanolamine; all groups exhibited similar abundances. In Clusters F (k=7; k=5, p<0.05) and G (k=19; k=5, p<0.05), healthy participants and patients with mild-to-moderate asthma had similar levels, while patients with severe asthma showed higher abundances. These clusters represented dietary and drug metabolites, with Cluster F consisting of caffeine metabolites and Cluster G containing sugars, gut microbial metabolites and other dietary products.
FIGURE 1.
Hierarchical cluster analysis (HCA) of metabolite abundances. HCA was performed using multivariate Spearman correlation distance metric and Ward's group linkage. a) Resulting metabolite clusters are presented as a polar dendrogram (differentially coloured and labelled as a to g). Black text: metabolites not significant in either univariate or multivariate analysis; red text: metabolites significant in univariate and/or multivariate analysis. *: p<0.05 univariate analysis; #: p<0.05, canonical variate 1 (CV1) (supplementary figure E1C). b) The mean (95% CI) of log-transformed and z-scaled data of the resulting clusters plotted against the clinical groups. HC: healthy controls; MMA: mild-to-moderate asthma; SAns: severe asthma non-smokers; SAs: severe asthma ex/smokers; L: longitudinal data.
All clusters, except for Cluster E, qualitatively demonstrated temporal metabolic stability of non-smoking severe asthma, with metabolite levels at the 12-to-18-month longitudinal time point unchanged relative to the baseline values. Univariate analysis showed that four Cluster E metabolites (7-methylguanine, phosphoethanolamine, uric acid, xanthine) had a significantly different distribution (p<0.05) between baseline and longitudinal time points (supplementary table E2).
Multivariate analysis
PC-CVA supported the hierarchical clustering and univariate findings (supplementary figure E1a) and identified correlated metabolic drivers of severe asthma not revealed by these analyses. The first canonical variate (CV1) showed that the severe asthma groups were highly significantly different from the healthy participants and mild-to-moderate asthma groups (p=2.6×10−20). A total of 46 metabolites significantly (p<0.05) contributed to this mean group difference (supplementary figure E1c), 27 of which were also univariately significant (p<0.05, supplementary table E1). Metabolites that most strongly drove this separation included short-chain acylcarnitines, histidine, taurine, uracil, 2-deoxyinosine, kynurenic acid (decreased in severe asthma), sugars, proline, serotonin, N-methyl-D-aspartate (NMDA), glutamate, N-acetylputrescine and 5-hydroxyindole acetic acid (increased in severe asthma) (supplementary figure E1c). When projected through the model, the mean (95% CI) scores of the longitudinal groups overlapped with the respective baseline groups (supplementary figure E1b), further demonstrating temporal metabolic stability at the 12-to-18-month longitudinal visit.
Treatment effects upon the urinary metabolome
To delineate between metabolic dysregulation associated with disease and treatment (OCS, theophylline, leukotriene modifiers, anti-cholinergics and omalizumab), patients with severe asthma who were non-smokers were stratified based upon treatment (table 1). Of the 90 metabolites reported, 23 (25%; table 2, supplementary table E3) were significantly different (p<0.05, FDR<0.05) between OCS-treated and non-treated patients within this group. Of the 27 metabolites dysregulated in association with severe asthma by both univariate and multivariate analysis, nine were altered in OCS-treated individuals (33%). Metabolite abundances in Clusters A (k=3), B (k=3), D (k=6), E (k=2) and G (k=9) were different between OCS-treated and non-treated individuals, while metabolites in Clusters C and F were not significantly affected.
TABLE 2.
Metabolites associated with OCS
| Metabolite | Cluster # | SAns¶ (n=123) | SAns+OCS¶ (n=66) | p-value + | FDR |
| Cystathionine | A | 0.87 (0.71–1.14) | 1.10 (0.85–1.53) | 0.005 | 0.003 |
| Histidine | A | 0.87 (0.73–0.98) | 0.77 (0.61–0.85) | 0.005 | 0.003 |
| Isoleucine | A | 1.10 (0.92–1.20) | 0.92 (0.77–1.06) | 0.029 | 0.010 |
| 5-Aminolevulinic acid | B | 0.91 (0.76–1.12) | 0.76 (0.58–0.92) | 0.046 | 0.015 |
| Kynurenic acid | B | 0.89 (0.78–1.04) | 0.82 (0.73–0.95) | 0.047 | 0.014 |
| Uracil | B | 0.86 (0.73–1.01) | 0.66 (0.52–0.78) | 2.16×10−4 | 0.001 |
| 5-Hydroxyindoleacetic acid | D | 1.06 (0.96–1.19) | 1.19 (1.04–1.34) | 0.022 | 0.008 |
| Aspartic acid | D | 1.17 (0.98–1.35) | 0.86 (0.73–1.07) | 0.001 | 0.001 |
| Glutamic acid§ | D | 1.16 (1.04–1.37) | 1.04 (0.90–1.20) | 0.058 | 0.015 |
| N-Acetylputrescine | D | 1.06 (0.94–1.25) | 1.24 (1.04–1.42) | 0.035 | 0.012 |
| N-Methyl-D-aspartic acid | D | 0.92 (0.82–1.00) | 1.16 (1.05–1.30) | 7.52×10−5 | 0.001 |
| S-Adenosylhomocysteine | D | 1.00 (0.83–1.16) | 1.15 (1.04–1.38) | 0.004 | 0.003 |
| Serotonin | D | 1.23 (1.06–1.44) | 1.41 (1.17–1.83) | 0.017 | 0.007 |
| Methylthioadenosine | E | 1.25 (1.07–1.65) | 0.90 (0.72–1.26) | 0.003 | 0.003 |
| Xanthine | E | 1.16 (0.97–1.39) | 0.96 (0.69–1.18) | 0.008 | 0.004 |
| 2-Furoylglycine | G | 0.68 (0.47–0.90) | 2.05 (1.24–3.15) | 0.003 | 0.003 |
| 4-Pyridoxic acid | G | 0.94 (0.84–1.05) | 1.06 (0.88–1.36) | 0.048 | 0.014 |
| Allantoin | G | 1.02 (0.84–1.26) | 1.17 (0.95–1.47) | 0.019 | 0.008 |
| Aminovaleric acid | G | 1.19 (0.71–1.78) | 0.83 (0.44–1.21) | 0.014 | 0.006 |
| Glucosamine | G | 0.87 (0.77–1.06) | 1.18 (0.97–1.43) | 0.003 | 0.003 |
| Maltose | G | 1.09 (0.91–1.49) | 1.84 (1.19–2.86) | 0.003 | 0.003 |
| Methylhippuric acid | G | 0.89 (0.71–1.16) | 1.18 (0.94–1.49) | 0.014 | 0.006 |
| Sucrose | G | 1.13 (0.83–1.51) | 1.78 (1.34–2.35) | 2.35×10−4 | 0.001 |
| Xylose | G | 1.00 (0.82–1.16) | 1.44 (1.01–2.04) | 0.002 | 0.003 |
SAns: non-smoking severe asthmatics; #: cluster assignment as shown in figure 1; ¶: all fold-change estimates are in comparison to healthy participants and displayed as the mean (95% CI); +: Wilcoxon Rank-Sum test between OCS-treated and non-treated groups; §: glutamic acid was included due to its high magnitude of effect on the corresponding multivariate principal components–canonical variate analysis model (figure 2).
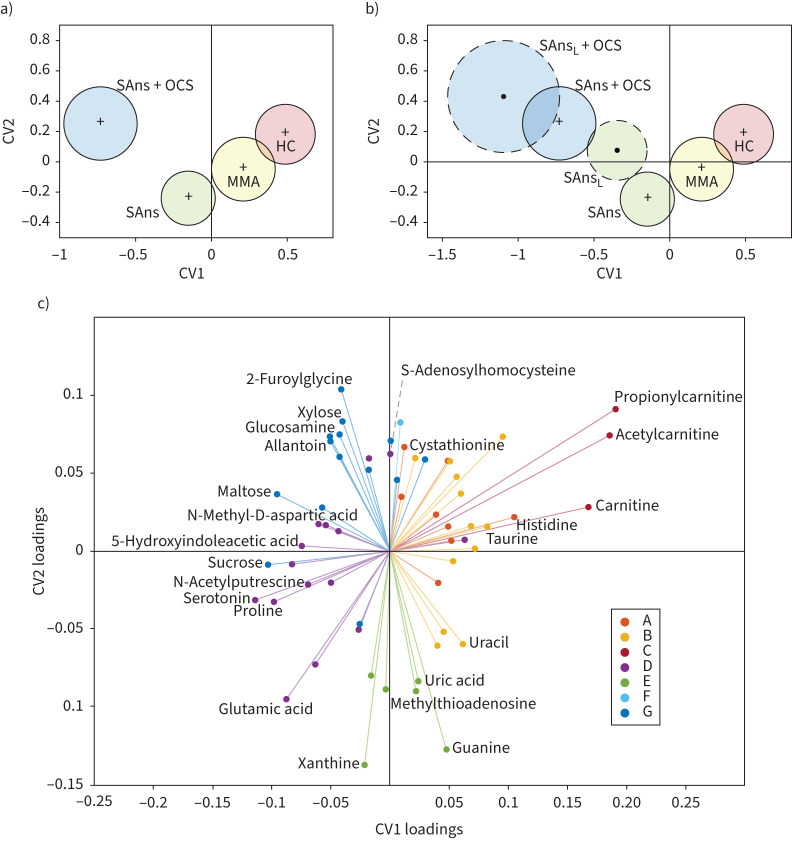
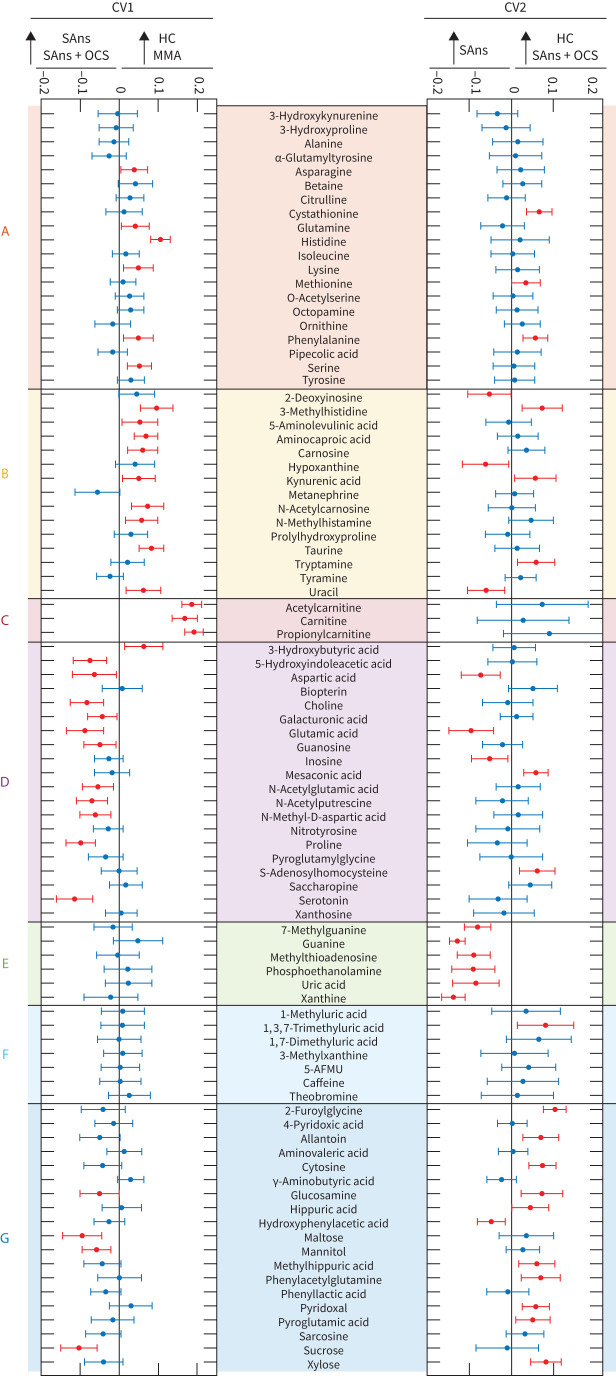
Multivariate analysis using PC-CVA (figure 2) corroborated the univariate findings. CV1 described a significant mean difference between healthy participants and those with severe asthma (p=7.8×10−12). CV2 described a significant mean difference between OCS-treated and OCS-not treated patients with severe asthma who were non-smokers (p=9.5×10−4). Metabolites from Clusters A, B and C were less abundant in patients with severe asthma (figures 2c and 3). Four of the six Cluster A and B metabolites were further reduced in OCS users. The carnitines (Cluster C) had the largest effect on the PC-CVA model (figures 2c and 3) and were not altered in the OCS-treated group. While metabolites in Cluster D were increased in patients with severe asthma (figure 2c), further inspection revealed that the increases for all metabolites except for glutamate were associated with OCS treatment, independent of asthma diagnosis (table 2). Conversely, the Cluster D metabolite glutamate and all Cluster E metabolites were elevated in patients with severe asthma (figure 2c) and decreased in the OCS-treated individuals (table 2). The combined multivariate response showed that Cluster G metabolites were uniquely elevated in OCS-treated individuals (figure 2c); univariate analysis showed varied associations with disease and OCS use (table 2). Projection of longitudinal metabolite profiles into the multivariate model (figure 2b) showed temporal stability for the OCS-treated group, but less stability for the non-treated group. Two Cluster E metabolites (phosphoethanolamine, p=0.0043; uric acid, p=0.016) were significantly different between the two time points.
FIGURE 2.
Principal components–canonical variate analysis (PC-CVA) with non-smoking patients with severe asthma stratified by oral corticosteroid (OCS) use. Cross validation showed that five principal components were the optimal number to use in the CVA model (supplementary figure E2). a) Scores plot of baseline data, labelled by clinical class and b) longitudinal data for severe asthma groups projected into the baseline model. Red: healthy controls (HC); yellow: mild-to-moderate asthma (MMA); green: severe asthma non-smokers (SAns); blue: severe asthma non-smokers taking OCS treatment (SAns+OCS); L: longitudinal data; black cross: mean of each baseline group; black dot: mean of each longitudinal group; solid circles: 95% CI of the mean of baseline groups; dashed circles: 95% CI of the mean of longitudinal groups. c) Loadings plot displaying metabolites that significantly (p<0.05) contributed to the model. Metabolite position displays the magnitude and direction of effect in canonical variate (CV) 1 (x-axis) and CV2 (y-axis). The quadrant positions of metabolites are related to those of the clinical groups in the scores plots. In other words, metabolites are most abundant in the clinical groups with which they share a quadrant. Metabolites are colour-coded based on the corresponding cluster as identified in figure 1 and according to the figure legend.
The abundances of four metabolites were significantly different (p<0.05, FDR<0.05) between theophylline-treated and non-treated patients with severe asthma who were non-smokers (supplementary table E4); these included metabolites from Clusters A (k=1), B (k=1) and F (k=2). PC-CVA multivariate analysis showed that while there was a mean difference between theophylline-treated and non-treated individuals, it was less pronounced than OCS effects (supplementary figures E3 and E4). Omalizumab treatment was associated with few differences in urinary metabotypes, with only two metabolites (3-methylxanthine, ornithine) being significantly different (p<0.05, FDR<0.05) between omalizumab-treated and immunoglobulin E-matched non-omalizumab-treated patients with severe asthma who were non-smokers (supplementary table E5). Anticholinergics (supplementary table E6) and leukotriene modifiers (supplementary table E7) were not associated with any significant differences (p<0.05, FDR<0.05) between users and non-users. Supplementary table E8 provides fold-change analysis for all metabolites significantly affected by at least one treatment or smoking.
Carnitine metabolism
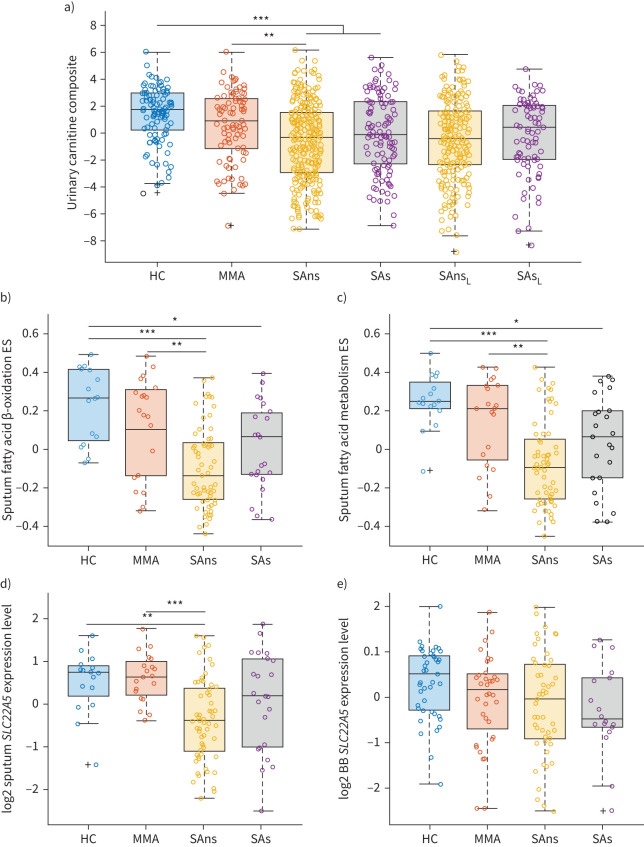
The strongest observed metabolic shift in association with asthma severity was in the carnitines (Cluster C). Given that this alteration was independent of OCS treatment, it was explored in more detail. While urinary carnitine levels were lower in females relative to males, this effect was independent of asthma diagnosis (supplementary figure E5). Confounder correction, which included sex, age and body mass index as predictor variables (supplementary table E9), showed that the significantly reduced carnitine abundances observed in the severe asthmatic non-smoking group were independent of these potential confounders. In addition, the carnitine species were also evaluated for recruitment site bias and found to be independent of collection centre (p>0.8, supplementary figure E6). The three carnitine species were then z-scaled and concatenated (figure 4a), which further demonstrated a strong decrease in association with asthma severity (p=4.0×10−9).
FIGURE 4.
Molecular signatures of carnitine metabolism. Scatter-overlaid boxplots stratified by clinical class. a) Urinary carnitine composite variable. Relative abundances of carnitine, acetylcarnitine and propionylcarnitine were log-transformed, z-scaled and summed (p=4×10−9). b) Sputum fatty acid β-oxidation gene set variance analysis (GSVA) enrichment score (ES) (p=8.02×10−6). c) Sputum fatty acid metabolism GSVA ES (p=6.29×10−6). d) Sputum SLC22A5 expression levels (p=5.69×10−5). e) Bronchial brushings (BB) SLC22A5 expression levels (p=0.0583). Open circles: observations; box: median and interquartile range (IQR); whiskers: range of data up to 1.5 times of IQR above Q3 or below Q1; black cross: outliers. Kruskal–Wallis p-values are reported with post hoc pairwise comparisons shown on the figure. HC: healthy controls; MMA: mild-to-moderate asthma; SAns: severe asthma non-smokers; SAs: severe asthma ex/smokers; L: longitudinal data; *: p<0.05; **: p<0.01; ***: p<0.001.
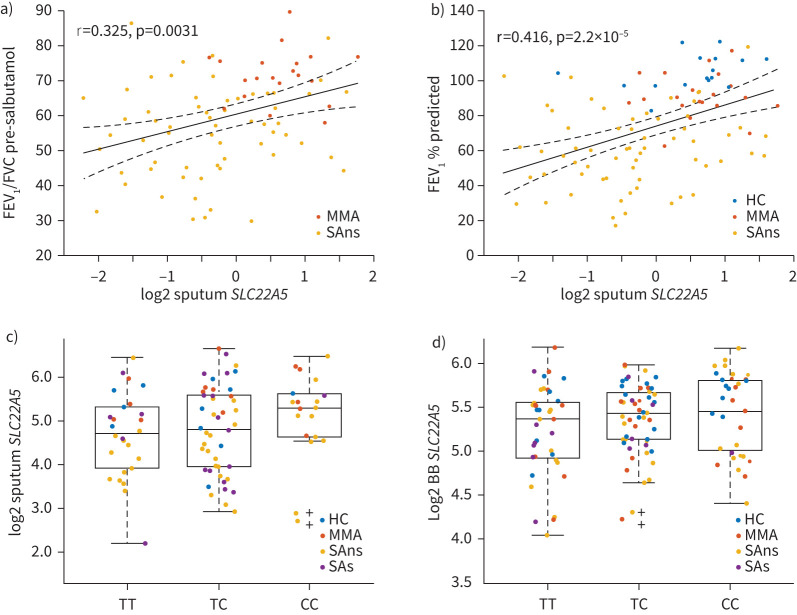
We then examined carnitine metabolism using KEGG pathway ES for fatty acid β-oxidation, which decreased with asthma severity in sputum (p=8.02×10−6, figure 4b), but did not change in bronchial brushings (p=0.82, data not shown). The ES for fatty acid metabolism also decreased with asthma severity in sputum (p=6.29×10−6, figure 4c) and bronchial brushings (p=0.08, data not shown). In addition, expression of the carnitine transporter SLC22A5 gene was evaluated owing to its known association with genetic risk of asthma [25, 28]. SLC22A5 expression decreased with asthma severity in sputum (p=5.96×10−5, figure 4d) and bronchial brushings (p=0.058, figure 4e), and correlated with lung function (forced expiratory volume in 1 s % predicted r=0.416, p=2.2×10−5; figure 5). SCL22A5 levels were lower in bronchial brushings of type-2 high individuals (p=9.7×10−4; supplementary figure E7d), as was fatty acid metabolism ES (p=0.038; supplementary figure E7b). Similar findings were observed in sputum (supplementary figure E7c). Stratification based upon previously published sputum TACs [23] found that fatty acid metabolism ES (p=2.1×10−14) and SCL22A5 levels (p=1.2×10−10) in sputum were higher in paucigranulocytic individuals with milder disease (TAC3) (supplementary figure E8). One SLC22A5 expression quantitative trait locus (rs2522051, T/C) was found in sputum (effect allele C: β=0.234, sd=0.148, p=0.119; figure 5c) and bronchial brushings (effect allele C: β=0.138, sd=0.057, p=0.028; figure 5d). With respect to allele T, the direction of rs2522051 increases the risk of asthma while decreasing the expression of SLC22A5, indicating that activating SLC22A5 may reduce the risk of asthma. However, these data should be interpreted with caution owing to the low sample number.
FIGURE 5.
Relationship of SLC22A5 gene expression levels with lung function and genotype. a) Correlation between the forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio pre-salbutamol and sputum SLC22A5 gene expression levels. b) Correlation between FEV1 % predicted and sputum SLC22A5 gene expression levels. All assumptions for parametric analysis were verified, thus Pearson correlation was used. Dots: observations; solid line: regression; dashed lines: 95% CIs of the regression. A weak linear correlation was also observed between the urinary carnitine composite and FEV1 % predicted (r=0.15, p=1.43×10−4), but not FEV1/FVC ratio pre-salbutamol (p=0.90). c) Relationship between sputum SLC22A5 gene expression levels and genotype (effect allele C: β=0.234, sd=0.148, p=0.119; n=91). d) Relationship between bronchial brushing (BB) SLC22A5 gene expression levels and genotype (effect allele C: β=0.138, sd=0.057, p=0.028; n=118). The p-value of the effect size/coefficient of genotype in the regression model was used to test if the single nucleotide polymorphism was significantly associated with gene expression. Open circles: observations; box: median and interquartile range (IQR); whiskers: range of data up to 1.5 times of IQR above Q3 or below Q1; black cross: outliers; HC: healthy controls; MMA: mild-to-moderate asthma; SAns: severe asthma non-smokers; SAs: severe asthma ex/smokers.
Discussion
We identified distinct urinary metabotypes of individuals with severe asthma that demonstrated temporal stability over at least 12–18 months; however, the metabotypes were sensitive to common treatment modalities. Cluster C, the carnitine species, displayed the largest alteration in association with severe asthma and was the only metabolite cluster unaffected by treatment. Findings in both sputum and bronchial brushings supported the observed systemic dysregulation in carnitine metabolism. Reduced fatty acid metabolism, β-oxidation (in sputum) and levels of the carnitine transporter SLC22A5 in patients with severe asthma further implicate carnitine and central energy metabolism dysregulation in asthma. Single nucleotide polymorphisms in SLC22A5 have been previously reported to affect asthma risk [25, 28] and we identified rs2522051 to be an SLC22A5 expression quantitative trait loci that increases asthma risk while decreasing SLC22A5 expression, in agreement with earlier work [29]. While the limited power warrants caution in the conclusions drawn, these findings suggest that there is a genetic component to the observed carnitine dysregulation in severe asthma and support a link between genetic determinants and systemic carnitine levels.
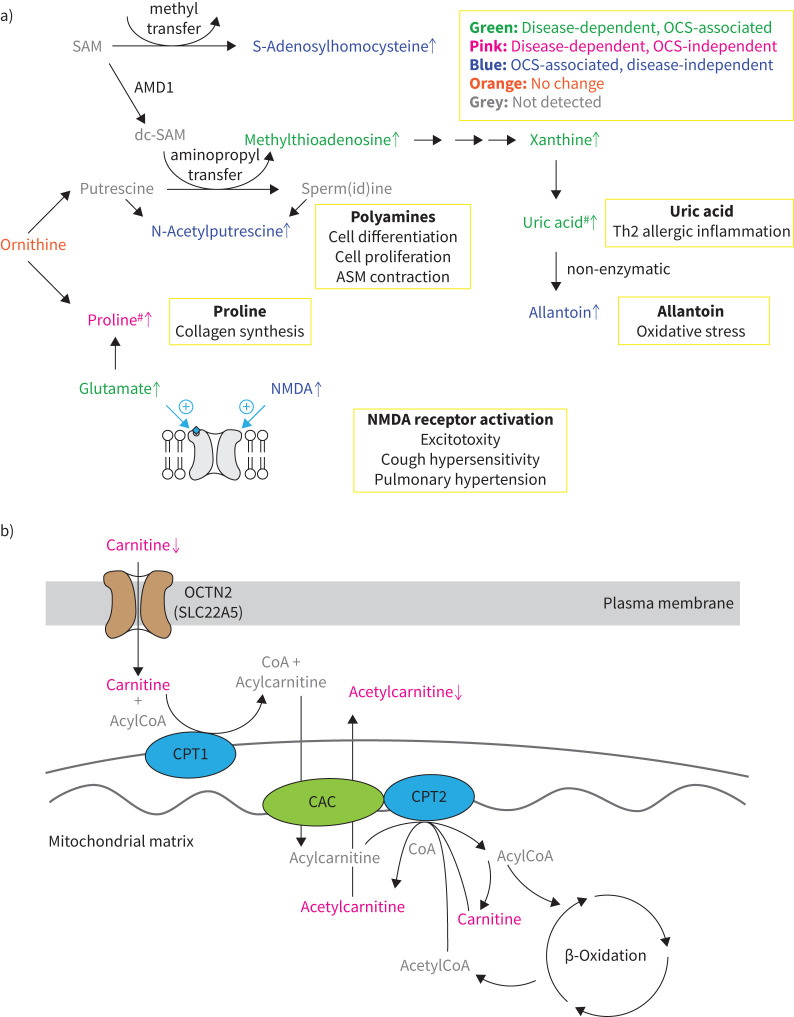
Carnitine is a small water-soluble molecule that possesses important physiological roles, including transport of fatty acids into the mitochondrial matrix for β-oxidation, while short-chain acylcarnitines (primarily acetylcarnitine) transport organic acids out of the mitochondria and peroxisomes [30, 31]. Beyond its role in β-oxidation, carnitine also acts as a free radical scavenger [32] and can reduce oxidative stress-induced apoptosis [33]. Carnitine deficiency has been reported to cause pathological symptoms [30]. For example, reduced carnitine levels can result in a concomitant decline in mitochondrial free coenzyme A (CoA) and increased acyl-CoA, which has been linked with progressive emphysema [33, 34]. Studies have also shown systemic carnitine reduction during and after paediatric asthma exacerbation [35] and plasma levels were reduced in a guinea pig model of allergic asthma [36]. Sex-specific differences in circulating carnitine levels have been previously established, with lower constitutive levels in women [37]. While we also observed this pattern, the magnitude of the decrease associated with severe asthma was similar in both sexes (supplementary figure E5c). Lower carnitine levels in women have been linked to sex hormones [38], with post-menopausal hormone replacement therapy use shown to further decrease circulating carnitine levels [39], suggesting that carnitine metabolism may play a role in the known link between female sex hormones and lung disease [40, 41].
These observations collectively suggest that carnitine metabolism may represent an actionable therapeutic target. For example, experimental allergic asthma models show mitochondrial functional changes that are reversed by an anti-IL-4 monoclonal antibody [42]. Conversely, using etomoxir to inhibit carnitine palmitoyltransferase 1, the rate-limiting enzyme for β-oxidation in the mitochondria, reduced fatty acid metabolism and enhanced IL-4 expression in a mouse model of multiple sclerosis [43]. Carnitine supplementation has shown beneficial effects in several diseases [30], with decreased C-reactive protein, IL-6 and tumour necrosis factor-α and increased superoxide dismutase reported in randomised control trials [44]. Carnitine supplementation attenuated the development of porcine pancreatic elastase-induced emphysema [33]. This study highlights the importance of carnitine and central energy biochemistry in asthma, especially given that these processes are non-responsive to OCS treatment. Central energy metabolism is known to drive immune cell activation, with glycolysis and the pentose phosphate pathway promoting pro-inflammatory responses and β-oxidation, and oxidative phosphorylation promoting anti-inflammatory responses [45]. The deterministic cause of the shifted metabolic response remains unclear, particularly as to whether the observed altered mitochondrial function is a consequence of the chronic tissue hypoxia in asthma or dysfunction at the level of the mitochondria. Because there is no definitive mechanistic insight into the aetiological role that carnitine plays in asthma, future studies are warranted to elucidate this as well as the potential therapeutic benefit of carnitine supplementation in asthma.
Because OCS treatment may modulate observed metabolite concentrations, we stratified patients with severe asthma by historical prescription of OCS and objective quantification of urinary prednisone (table 2, figures 2 and 3). Over 25% of the observed metabolites were significantly different in the OCS-treated group (table 2, figure 2), demonstrating that OCS treatment is a significant confounder in metabolomics-based investigations. We directly investigated the metabolic differences of OCS treatment alone or in association with asthma (figure 6) and identified metabolites that were dysregulated with asthma and appeared to respond to OCS treatment. Cluster E metabolites (purines including uric acid, methylthioadenosine and phosphoethanolamine) and the Cluster D metabolite glutamate increased with asthma and decreased to levels of healthy participants in association with OCS treatment (figure 2). Glutamate is a strong NMDA receptor agonist that can promote excitotoxicity, cough hypersensitivity [46] and pulmonary hypertension [47]. Polyamines promote cell differentiation and proliferation as well as airway smooth muscle contraction in asthma [48]. Methylthioadenosine is a by-product of polyamine synthesis; we recently reported its increase in the serum of patients with severe asthma [8]. Methylthioadenosine is subsequently catabolised via purine metabolism to uric acid (independent of OCS), which drives type-2-mediated inflammation in asthma [49]. These shifts agree with in vitro research showing that dexamethasone treatment reduces both polyamine and purine synthesis [50] and may provide insights into OCS mechanisms of action in treating asthma. While it remains unclear whether these metabolites responded to OCS treatment, they should be evaluated as candidate treatment efficacy biomarkers in future steroid interventional trials.
FIGURE 3.
Individual canonical variate (CV) loadings for the principal components–canonical variate analysis (PC-CVA) with non-smoking patients with severe asthma stratified by oral corticosteroid (OCS) use. Loadings plots for CV1 (left panel) and CV2 (right panel) are shown. Clinical group labels at the top of each panel reflect the group position along the CV axis, as described by the model; clinical groups were not combined for this analysis. Red: metabolites that significantly (p<0.05) contributed to separation in the CV based on 500 iterations of bootstrap resampling/remodelling; blue: metabolites that did not significantly contribute to the separation in the CV. Metabolites are ordered and colour-coded by cluster (figure 1). The cluster label is presented on the left side of the figure. SAns: severe asthma non-smokers; HC: healthy controls; MMA: mild-to-moderate asthma.
FIGURE 6.
Biochemical pathways underlying severe asthma observed in the current study. a) Metabolism associated with oral corticosteroid (OCS) use. b) Carnitine metabolism. Green: OCS-associated alteration in severe asthma; pink: OCS-independent alteration in asthma; blue: OCS-associated, disease-independent alteration; orange: no change observed; grey: metabolites not detected in the current study; black: notes on metabolic reactions; yellow boxes: known pathogenic mechanisms of asthma. Arrows indicate direction of change. SAM: S-adenosylmethionine; AMD1: adenosylmethionine decarboxylase; dcSAM: decarboxylated SAM; ASM: airway smooth muscle; NMDA: N-methyl-D-aspartate; OCTN: organic cation transporter novel; CoA: conenzyme A; CPT: carnitine palmitoyltransferase; CAC: carnitine-acylcarnitine carrier. #: shift observed via multivariate analysis only.
Several metabolites showed OCS-associated metabolic differences independent of asthma diagnosis (table 2, figure 2). N-acetylputrescine, NMDA, S-adenosylhomocysteine (Cluster D) and allantoin (Cluster G) only increased (p<0.05) in the OCS-treated group (figure 6). Allantoin is a non-enzymatic oxidation product of the purine catabolic product uric acid and marker of oxidative stress [51], while N-acetylputrescine is an intermediary breakdown product of polyamines. S-adenosylhomocysteine is the product of methyltransferase reactions and metabolically linked to the aminopropyl reactions required for polyamine synthesis (figure 6). NMDA, which is another methyltransferase product, is an alternate agonist to glutamate for NMDA receptors. It has been shown to elicit contractile responses in human airway smooth muscle cells in vitro [52] and, surprisingly, bronchorelaxation responses in the murine house dust mite model of asthma [53]. While the consequences of these observed differences are unclear, they warrant further investigation to improve our understanding of the metabolic effects of OCS treatment, as well as provide insight into possible links to treatment side effects.
A number of recent asthma metabolomics studies have reported altered caffeine metabolism [9, 17–19]. Because theophylline is a caffeine metabolite, the potential confounding effects of theophylline treatment in these interpretations remain unclear. Here we show that theophylline treatment is associated with differences in caffeine metabolism (Cluster F) as well as amino acid metabolism (Cluster A), dietary metabolites (Cluster G) and uracil (supplementary table E4). While the global metabolic differences associated with theophylline treatment are less pronounced than those with OCS treatment (supplementary figure E3), these findings suggest that metabolomics studies should be interpreted cautiously in the absence of theophylline treatment data. Conversely, the limited metabolic differences observed in association with omalizumab and no differences in association with anticholinergics or leukotriene modifier treatment demonstrate the need to evaluate potential metabolic confounders on a treatment-by-treatment basis.
There is significant interest in the potential role of tryptophan and its metabolites in multiple inflammatory diseases [54], including obstructive lung disease [55]. While the downstream metabolites of tryptophan were dysregulated with asthma severity, the magnitude of the alterations was modest (supplementary figure E9, supplementary table E10). The strongest observed changes were in the indoleamine 2,3-dioxygenase (IDO) pathway, which has previously been reported to be associated with allergic airway inflammation [55]. Interestingly, IDO1 mRNA levels were increased in bronchial brushings, sputum and PBMCs (supplementary table E11), suggesting an upregulation of this pathway that was reflected in the urinary metabotype and was temporally stable (discussed in the supplementary material). Thus, significant tryptophan dysregulation occurs in asthma, but its impact is minor.
The current study is unique and represents the only large-scale mass spectrometry-based investigation of the urinary metabolome in adult patients with asthma performed to date. However, there are limitations to this study that should be considered. While the mass spectrometry method was untargeted, reported metabolites were limited to those with identifications confirmed by chemical standards. Accordingly, the availability of additional analytical standards could increase the number of identified metabolites. Although the observed metabotypes provided insight into disease mechanisms and treatment stratification, they did not possess sufficient molecular resolution to identify unbiased endotypes of severe asthma. While a particular strength is that we confirmed adherence to OCS medication, the observed association between OCS and metabolite levels raises the question of whether ICS and reliever medication (SABA/LABA) evidence similar effects. Because current use of ICS and SABA/LABA were inclusion criteria for all U-BIOPRED subjects, it was not possible to stratify patients for these treatments. Future studies could investigate this question using a dose-dependent interventional design. Performing similar stratifications in other disease contexts (e.g. inflammatory bowel disease, rheumatoid arthritis) could help establish the effects of corticosteroid use on observed metabolism. However, it would be necessary to perform an intervention study to definitively describe the temporal and dose-response relationship between OCS treatment and observed metabotypes, and to identify metabolic biomarkers of treatment efficacy. It is also challenging to separate the effect of omalizumab on the urinary metabolome versus the effect of the qualifying (atopic) phenotype. Lastly, it is important to highlight that it remains for future studies to determine if the consistent metabolite profiles discerned in this study relate to the systemic inflammation in asthma or to more local tissue inflammation.
We demonstrate that patients with severe asthma possess a dysregulated systemic metabotype relative to healthy individuals that it is temporally stable up to 12–18 months. The observed metabolic shifts are modulated by asthma-related therapeutics on a metabolite- and treatment-specific basis. In the current study, OCS treatment was associated with a difference in 25% of the observed metabolites, further highlighting the importance of evaluating this confounder in molecular studies. Short-chain carnitines represented the strongest metabolic signature associated with asthma severity, decreased in an OCS-independent manner, and were temporally stable, providing a metabolic link to the mitochondrial dysfunction associated with severe asthma and presenting a potential therapeutic target in asthma management.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01733-2021.Supplement (2.5MB, pdf)
Supplementary tables ERJ-01733-2021.Tables (107.2KB, xlsx)
Shareable PDF
Footnotes
This article has supplementary material available from erj.ersjournals.com
Conflict of interest: S.N. Reinke reports grants from Canadian Institutes of Health Research, during the conduct of the study.
Conflict of interest: S. Naz has nothing to disclose.
Conflict of interest: R. Chaleckis has nothing to disclose.
Conflict of interest: H. Gallart-Ayala has nothing to disclose.
Conflict of interest: J. Kolmert reports personal fees for consultancy from Gesynta Pharma AB, outside the submitted work.
Conflict of interest: N.Z. Kermani has nothing to disclose.
Conflict of interest: A. Tiotiu has nothing to disclose.
Conflict of interest: D.I. Broadhurst has nothing to disclose.
Conflict of interest: A. Lundqvist has nothing to disclose.
Conflict of interest: H. Olsson is an employee and shareholder of AstraZeneca.
Conflict of interest: M. Ström has nothing to disclose.
Conflict of interest: Å.M. Wheelock has nothing to disclose.
Conflict of interest: C. Gómez has nothing to disclose.
Conflict of interest: M. Ericsson has nothing to disclose.
Conflict of interest: A.R. Sousa has nothing to disclose.
Conflict of interest: J.H. Riley works for and own shares in GlaxoSmithKline.
Conflict of interest: S. Bates is an employee of Johnson & Johnson and has previously worked for and holds stock in GlaxoSmithKline.
Conflict of interest: J. Scholfield reports grants from Innovative Medicines Initiative, during the conduct of the study; and is director and employee of TopMD Precision Medicine Ltd.
Conflict of interest: M. Loza is an employee of and owns stock in Johnson & Johnson.
Conflict of interest: F. Baribaud is a shareholder of Johnson & Johnson and a current employee of Bristol Myers Squibb.
Conflict of interest: P.S. Bakke reports personal fees for advisory board work and lectures from AstraZeneca, and personal fees for lectures from Novartis and Boehringer Ingelheim, outside the submitted work.
Conflict of interest: M. Caruso has nothing to disclose.
Conflict of interest: P. Chanez reports grants and personal fees from AstraZeneca, ALK, Boehringer Ingelheim, Chiesi, Sanofi-Aventis, Novartis and GlaxoSmithKline, outside the submitted work.
Conflict of interest: S.J. Fowler reports personal fees from AstraZeneca, Novartis, TEVA and Chiesi, outside the submitted work.
Conflict of interest: T. Geiser has nothing to disclose.
Conflict of interest: P. Howarth has nothing to disclose.
Conflict of interest: I. Horvath has nothing to disclose.
Conflict of interest: N. Krug has nothing to disclose.
Conflict of interest: P. Montuschi has nothing to disclose.
Conflict of interest: A. Behndig has nothing to disclose.
Conflict of interest: F. Singer reports personal fees from Vertex Pharmaceuticals (CH) and Novartis, outside the submitted work.
Conflict of interest: J. Musial has nothing to disclose.
Conflict of interest: D.E. Shaw has nothing to disclose.
Conflict of interest: B. Dahlén reports personal fees for advisory board work and lectures from AstraZeneca, TEVA and Sanofi, and grants from Novartis and GlaxoSmithKline, outside the submitted work.
Conflict of interest: S. Hu has nothing to disclose.
Conflict of interest: J. Lasky-Su has nothing to disclose.
Conflict of interest: P.J. Sterk reports a public private grant from the Innovative Medicines Initiative (IMI) covered by the EU and EFPIA, during the conduct of the study.
Conflict of interest: K.F. Chung has received honoraria for participating in advisory board meetings of GlaxoSmithKline, AstraZeneca, Roche, Novartis, Merck, Nocion and Shionogi regarding treatments for asthma, COPD and chronic cough and has also been remunerated for speaking engagements.
Conflict of interest: R. Djukanovic reports receiving fees for lectures at symposia organised by Novartis, AstraZeneca and TEVA, consultation for TEVA and Novartis as member of advisory boards, and participation in a scientific discussion about asthma organised by GlaxoSmithKline; and is a co-founder and current consultant, and has shares in Synairgen, a University of Southampton spin out company.
Conflict of interest: S-E. Dahlén reports personal fees for consultancy from AstraZeneca, Cayman Chemical, GlaxoSmithKline, Novartis, Merck, Regeneron, Sanofi and TEVA, outside the submitted work.
Conflict of interest: I.M. Adcock has nothing to disclose.
Conflict of interest: C.E. Wheelock has nothing to disclose.
Support statement: The U-BIOPRED consortium received funding from the European Union and from the European Federation of Pharmaceutical Industries and Associations as an Innovative Medicines Initiative Joint Undertaking funded project (number 115010). Grants were also received from the Swedish Heart Lung Foundation, Swedish Research Council (2016-02798, 2014-3281, 2016-0338), the Konsul Th C Berghs Foundation, the Centre for Allergy Research Highlights Asthma Markers of Phenotype consortium (funded by the Swedish Foundation for Strategic Research), the Karolinska Institutet, AstraZeneca and Science for Life Laboratory Joint Research Collaboration, and the Vårdal Foundation. S.N. Reinke was supported by the Canadian Institutes of Health Research (MFE-135481). C.E. Wheelock was supported by the Swedish Heart Lung Foundation (HLF 20210519). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Tyler SR, Bunyavanich S. Leveraging -omics for asthma endotyping. J Allergy Clin Immunol 2019; 144: 13–23. doi: 10.1016/j.jaci.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet 2018; 391: 783–800. doi: 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 3.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol 2019; 144: 1–12. doi: 10.1016/j.jaci.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 4.Kolmert J, Gomez C, Balgoma D, et al. Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterised by type 2 inflammation. A clinical observational study. Am J Respir Crit Care Med 2021; 203: 37–53. doi: 10.1164/rccm.201909-1869OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly RS, Dahlin A, McGeachie MJ, et al. Asthma metabolomics and the potential for integrative omics in research and the clinic. Chest 2017; 151: 262–277. doi: 10.1016/j.chest.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turi KN, Romick-Rosendale L, Ryckman KK, et al. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J Allergy Clin Immunol 2018; 141: 1191–1201. doi: 10.1016/j.jaci.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ban GY, Cho K, Kim SH, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy 2017; 47: 37–47. doi: 10.1111/cea.12797 [DOI] [PubMed] [Google Scholar]
- 8.Reinke SN, Gallart-Ayala H, Gomez C, et al. Metabolomics analysis identifies different metabotypes of asthma severity. Eur Respir J 2017; 49: 1601740. doi: 10.1183/13993003.01740-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comhair SA, McDunn J, Bennett C, et al. Metabolomic endotype of asthma. J Immunol 2015; 195: 643–650. doi: 10.4049/jimmunol.1500736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly RS, Virkud Y, Giorgio R, et al. Metabolomic profiling of lung function in Costa Rican children with asthma. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 1590–1595. doi: 10.1016/j.bbadis.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly RS, Sordillo JE, Lutz SM, et al. Pharmacometabolomics of bronchodilator response in asthma and the role of age-metabolite interactions. Metabolites 2019; 9: 179. doi: 10.3390/metabo9090179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu B, Flexeder C, McGarrah RW, III, et al. Metabolomics identifies novel blood biomarkers of pulmonary function and COPD in the general population. Metabolites 2019; 9: 61. doi: 10.3390/metabo9040061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loureiro CC, Duarte IF, Gomes J, et al. Urinary metabolomic changes as a predictive biomarker of asthma exacerbation. J Allergy Clin Immunol 2014; 133: 261–263.e5. doi: 10.1016/j.jaci.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 14.Park YH, Fitzpatrick AM, Medriano CA, et al. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J Allergy Clin Immunol 2017; 139: 1518–1524.e4. doi: 10.1016/j.jaci.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 15.Price D, Castro M, Bourdin A, et al. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev 2020; 29: 190151. doi: 10.1183/16000617.0151-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015; 46: 1308–1321. doi: 10.1183/13993003.00779-2015 [DOI] [PubMed] [Google Scholar]
- 17.McGeachie M, Kelly R, Litonjua A, et al. Network of year-3 metabolites indicative of early-life asthma. C26 pediatric asthma: epidemiology and epigenetics. Am Thorac Soc 2018; 197: A4606. [Google Scholar]
- 18.Kelly RS, Chawes BL, Blighe K, et al. An integrative transcriptomic and metabolomic study of lung function in children with asthma. Chest 2018; 154: 335–348. doi: 10.1016/j.chest.2018.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Gai XY, Chang C, et al. Metabolomic profiling differences among asthma, COPD, and healthy subjects: a LC-MS-based metabolomic analysis. Biomed Environ Sci 2019; 32: 659–672. [DOI] [PubMed] [Google Scholar]
- 20.Naz S, Gallart-Ayala H, Reinke SN, et al. Development of a liquid chromatography-high resolution mass spectrometry metabolomics method with high specificity for metabolite identification using all ion fragmentation acquisition. Anal Chem 2017; 89: 7933–7942. doi: 10.1021/acs.analchem.7b00925 [DOI] [PubMed] [Google Scholar]
- 21.Meister I, Zhang P, Sinha A, et al. High-precision automated workflow for urinary untargeted metabolomic epidemiology. Anal Chem 2021; 93: 5248–5258. doi: 10.1021/acs.analchem.1c00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo CS, Pavlidis S, Loza M, et al. A transcriptome-driven analysis of epithelial brushings and bronchial biopsies to define asthma phenotypes in U-BIOPRED. Am J Respir Crit Care Med 2017; 195: 443–455. doi: 10.1164/rccm.201512-2452OC [DOI] [PubMed] [Google Scholar]
- 23.Kuo CS, Pavlidis S, Loza M, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J 2017; 49: 1602135. doi: 10.1183/13993003.02135-2016 [DOI] [PubMed] [Google Scholar]
- 24.Bigler J, Boedigheimer M, Schofield JPR, et al. A severe asthma disease signature from gene expression profiling of peripheral blood from U-BIOPRED cohorts. Am J Respir Crit Care Med 2017; 195: 1311–1320. doi: 10.1164/rccm.201604-0866OC [DOI] [PubMed] [Google Scholar]
- 25.Shrine N, Portelli MA, John C, et al. Moderate-to-severe asthma in individuals of European ancestry: a genome-wide association study. Lancet Respir Med 2019; 7: 20–34. doi: 10.1016/S2213-2600(18)30389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B Stat Methodol 2002; 64: 479–498. doi: 10.1111/1467-9868.00346 [DOI] [Google Scholar]
- 27.Pavlidis S, Takahashi K, Ng Kee Kwong F, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J 2019; 53: 1800938. [DOI] [PubMed] [Google Scholar]
- 28.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 2010; 363: 1211–1221. doi: 10.1056/NEJMoa0906312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira MAR, Mathur R, Vonk JM, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet 2019; 104: 665–684. doi: 10.1016/j.ajhg.2019.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flanagan JL, Simmons PA, Vehige J, et al. Role of carnitine in disease. Nutr Metab (Lond) 2010; 7: 30. doi: 10.1186/1743-7075-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet 2012; 51: 553–572. doi: 10.1007/BF03261931 [DOI] [PubMed] [Google Scholar]
- 32.Selo MA, Sake JA, Ehrhardt C, et al. Organic cation transporters in the lung-current and emerging (patho)physiological and pharmacological concepts. Int J Mol Sci 2020; 21: 9168. doi: 10.3390/ijms21239168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conlon TM, Bartel J, Ballweg K, et al. Metabolomics screening identifies reduced L-carnitine to be associated with progressive emphysema. Clin Sci (Lond) 2016; 130: 273–287. doi: 10.1042/CS20150438 [DOI] [PubMed] [Google Scholar]
- 34.Halper-Stromberg E, Gillenwater L, Cruickshank-Quinn C, et al. Bronchoalveolar lavage fluid from COPD patients reveals more compounds associated with disease than matched plasma. Metabolites 2019; 9: 157. doi: 10.3390/metabo9080157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asilsoy S, Bekem O, Karaman O, et al. Serum total and free carnitine levels in children with asthma. World J Pediatr 2009; 5: 60–62. doi: 10.1007/s12519-009-0011-8 [DOI] [PubMed] [Google Scholar]
- 36.Kertys M, Grendar M, Kosutova P, et al. Plasma based targeted metabolomic analysis reveals alterations of phosphatidylcholines and oxidative stress markers in guinea pig model of allergic asthma. Biochim Biophys Acta Mol Basis Dis 2020; 1866: 165572. doi: 10.1016/j.bbadis.2019.165572 [DOI] [PubMed] [Google Scholar]
- 37.Lambert ME, Shipley K, Holbrook I, et al. Serum carnitine levels in normal individuals. JPEN J Parenter Enteral Nutr 1988; 12: 143–146. doi: 10.1177/0148607188012002143 [DOI] [PubMed] [Google Scholar]
- 38.Ruoppolo M, Campesi I, Scolamiero E, et al. Serum metabolomic profiles suggest influence of sex and oral contraceptive use. Am J Transl Res 2014; 6: 614–624. [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens VL, Wang Y, Carter BD, et al. Serum metabolomic profiles associated with postmenopausal hormone use. Metabolomics 2018; 14: 97. doi: 10.1007/s11306-018-1393-1 [DOI] [PubMed] [Google Scholar]
- 40.Han MK, Arteaga-Solis E, Blenis J, et al. Female sex and gender in lung/sleep health and disease. Increased understanding of basic biological, pathophysiological, and behavioral mechanisms leading to better health for female patients with lung disease. Am J Respir Crit Care Med 2018; 198: 850–858. doi: 10.1164/rccm.201801-0168WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam A, Churg A, Wright JL, et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 193: 825–834. doi: 10.1164/rccm.201503-0487OC [DOI] [PubMed] [Google Scholar]
- 42.Mabalirajan U, Dinda AK, Kumar S, et al. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J Immunol 2008; 181: 3540–3548. doi: 10.4049/jimmunol.181.5.3540 [DOI] [PubMed] [Google Scholar]
- 43.Morkholt AS, Oklinski MK, Larsen A, et al. Pharmacological inhibition of carnitine palmitoyl transferase 1 inhibits and reverses experimental autoimmune encephalitis in rodents. PLoS One 2020; 15: e0234493. doi: 10.1371/journal.pone.0234493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fathizadeh H, Milajerdi A, Reiner Ž, et al. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. J Diabetes Metab Disord 2020; 19: 1879–1894. doi: 10.1007/s40200-020-00627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaeloudes C, Bhavsar PK, Mumby S, et al. Role of metabolic reprogramming in pulmonary innate immunity and its impact on lung diseases. J Innate Immun 2020; 12: 31–46. doi: 10.1159/000504344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung KF. NMDA and GABA receptors as potential targets in cough hypersensitivity syndrome. Curr Opin Pharmacol 2015; 22: 29–36. doi: 10.1016/j.coph.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 47.Dumas SJ, Bru-Mercier G, Courboulin A, et al. NMDA-type glutamate receptor activation promotes vascular remodeling and pulmonary arterial hypertension. Circulation 2018; 137: 2371–2389. doi: 10.1161/CIRCULATIONAHA.117.029930 [DOI] [PubMed] [Google Scholar]
- 48.Jain V. Role of polyamines in asthma pathophysiology. Med Sci 2018; 6: 4. doi: 10.3390/medsci6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kool M, Willart MA, van Nimwegen M, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 2011; 34: 527–540. [DOI] [PubMed] [Google Scholar]
- 50.Dyczynski M, Vesterlund M, Bjorklund AC, et al. Metabolic reprogramming of acute lymphoblastic leukemia cells in response to glucocorticoid treatment. Cell Death Dis 2018; 9: 846. doi: 10.1038/s41419-018-0625-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaur H, Halliwell B. Action of biologically relevant oxidising species upon uric acid. Identification of uric acid oxidation products. Chem Biol Interact 1990; 73: 235–247. doi: 10.1016/0009-2797(90)90006-9 [DOI] [PubMed] [Google Scholar]
- 52.Anaparti V, Ilarraza R, Orihara K, et al. NMDA receptors mediate contractile responses in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2015; 308: L1253–L1264. doi: 10.1152/ajplung.00402.2014 [DOI] [PubMed] [Google Scholar]
- 53.Anaparti V, Pascoe CD, Jha A, et al. Tumor necrosis factor regulates NMDA receptor-mediated airway smooth muscle contractile function and airway responsiveness. Am J Physiol Lung Cell Mol Physiol 2016; 311: L467–L480. doi: 10.1152/ajplung.00382.2015 [DOI] [PubMed] [Google Scholar]
- 54.Yeung AW, Terentis AC, King NJ, et al. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond) 2015; 129: 601–672. doi: 10.1042/CS20140392 [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Oriss TB, Fei M, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA 2008; 105: 6690–6695. doi: 10.1073/pnas.0708809105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01733-2021.Supplement (2.5MB, pdf)
Supplementary tables ERJ-01733-2021.Tables (107.2KB, xlsx)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01733-2021.Shareable (793.6KB, pdf)