Abstract
Background
Clinical trials allow development of innovative treatments and ameliorate the quality of clinical care in oncology. Data show that only a minority of patients are enrolled in clinical trials. We assessed enrolment in clinical trials and its correlates among women with early breast cancer.
Methods
We included 9516 patients with stage I-III breast cancer from the multicenter, prospective CANTO study (NCT01993498), followed-up until year 4 (Y4) post-diagnosis. We assessed factors associated with enrolment using multivariable logistic regression. In exploratory, propensity score matched analyses, we used multiple linear regression to evaluate the relationship of enrolment in clinical trials with the European Organisation for Research and Treatment of Cancer Quality Of Life (QoL) questionnaire (EORTC QLQ-C30) Summary Score and described clinical outcomes (distant disease event, invasive disease event, and death by any cause) according to enrolment.
Results
Overall, 1716 patients (18%) were enrolled in a clinical trial until Y4 post-diagnosis of breast cancer. Socioeconomic factors were not associated with enrolment. Centres of intermediate volume were most likely to enrol patients in clinical trials [versus low volume, odds ratio 1.45 (95% confidence interval (CI) 1.08-1.95), P = 0.0124]. Among 2118 propensity score matched patients, enrolment was associated with better QoL at Y4 (adjusted mean difference versus not enrolled 1.37, 95% CI 0.03-2.71, P = 0.0458), and clinical outcomes (enrolled versus not enrolled, distant disease event 7.3% versus 10.1%, P = 0.0206; invasive disease event 8.2% versus 10.5%, P = 0.0732; death by any cause 2.8% versus 3.7%, P = 0.2707).
Conclusions
In this large study, one in five patients enrolled on a clinical trial until Y4 after diagnosis of early breast cancer. Geographical and centre-related factors were significantly associated with enrolment in clinical trials. Inclusion in clinical trials seemed associated with improved QoL and clinical outcomes. Access to innovation for early-stage breast cancer patients should be encouraged and facilitated by overcoming organizational and geographical barriers to recruitment.
Key words: breast neoplasms, clinical trial, survivorship, quality of life
Highlights
-
•
The proportion of patients who access innovation through participation in clinical trials is generally limited.
-
•
Rate of enrolment in clinical trials among women with early breast cancer exceeded what previously found in other settings.
-
•
Clinical and geographical factors were associated to access to innovation in clinical trials.
-
•
Enrolment in clinical trials is associated with better quality of life and clinical outcomes.
Introduction
Clinical trials are the backbone and foundation of modern evidence-based oncology, as they represent a fundamental instrument to develop innovative treatments and test strategies that can optimize the quality of clinical care. Literature suggests how being enrolled in clinical trials may improve survival and reduce morbidity in patients with cancer of all types, thanks to early access to the latest and most promising investigational interventions, and close clinical monitoring.1, 2, 3, 4 Participation in clinical trials and equal access to innovation represent, therefore, key aspects in modern oncology care. According to the National Comprehensive Cancer Network, ‘the best management for any patient with cancer is in a clinical trial’.5 Nevertheless, it has been estimated that only 3%-5% of eligible adult cancer patients in the United States6 and 2%-11% in Australia7 are enrolled in clinical trials, mostly at the time of cancer recurrence. Trends regarding clinical trial enrolment seem not to have changed substantially over time, suggesting that barriers to trial participation are still numerous and sometimes difficult to overcome, and that access to innovation may be still suboptimal.6,8 Several studies tried to elucidate the reasons for low recruitment in clinical trials, usually giving emphasis to patient-related barriers, and evaluating patients’ attitude and motivations about enrolment.9,10 Fear of delay of initiation of antineoplastic drugs, or fear of being enrolled in the placebo arm due to randomization procedures were identified as major drivers of non-participation.11 In addition, ∼85% of patients receive treatment in community or in small-volume facilities of care, whereas only ∼15% are treated in larger, urban academic centres, where the number of available protocols is generally higher.12 Socioeconomic and demographic issues can also influence patients’ decision making regarding clinical trial participation. For example, participation in a clinical trial usually requires an increased frequency of follow-up visits, more testing, and more procedures, possibly leading to concerns about travel costs, particularly among patients that have longer travel times to their facility of care or lower income.13 On the contrary, some factors were found to be associated with higher rates of enrolment in clinical trials, including younger age, male sex, Caucasian race, and having later-stage cancer.14 Although some factors that may act as barriers or facilitators to clinical trial enrolment were identified, limitations of prior studies include the lack of an extensive characterization of clinical, socioeconomic, tumour, and treatment-related information that may represent relevant determinants of enrolment. Furthermore, previous studies were often single-institution experiences.2,4,15,16 In addition, evidence regarding determinants of enrolment in clinical trials among patients with early-stage breast cancer is still limited.
In the present analysis, we specifically aimed to examine factors associated with enrolment in clinical trials among women with early breast cancer, using data from the CANcer TOxicity cohort (CANTO). In addition, we assessed the relationship of enrolment in clinical trials with long-term (i) patient-reported functional health and symptom burden, and (ii) survival outcomes.
Patients and methods
Data source
CANTO (ClinicalTrials.gov/NCT01993498) is a prospective observational cohort study that includes patients with stage I-III breast cancer across 26 participating French cancer centres since 2012. CANTO recruiting centres include 20 comprehensive cancer centres, two university hospitals in Paris, two public non-teaching hospitals and two private hospitals (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100513). UNICANCER, the National Cooperative Group of French Cancer Centers, coordinates CANTO. The CANTO study was approved by the national regulatory authorities on 14 September 2011 (ref 2011-A011095-36) and the central ethical committee for human subjects (CPP - Ile de France 7, on 14 October 2011—ref 11-039). All patients were age ≥18 years old and provided written informed consent at inclusion. Patients were assessed at diagnosis of breast cancer, and follow-up data were available through year 4 (Y4) after diagnosis at the time of the present analysis. Patients could choose to enrol in concomitant observational or interventional studies at any time during the follow-up period. CANTO study procedures were previously published.17 We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for observational studies18 to assess the quality of our report.
Study cohort
For this analysis, we had access to information from 9597 patients with early breast cancer from the CANTO cohort. We excluded 81 patients with unknown participation status in concomitant research studies and those who were enrolled in concomitant studies but study information was unavailable or inaccessible. Overall, 9516 patients were included in the final analytic cohort (Figure 1). The aim of this study was to find associations between baseline cohort characteristics (collected at diagnosis of breast cancer) and likelihood of subsequent enrolment in clinical trials. Particularly, we assessed whether patients were enrolled in a clinical trial at any time over the course of their participation to the CANTO cohort study.
Figure 1.
CONSORT diagram of patient population.
Variables of interest
Clinical trials enrolment variables
Clinical trials enrolment variables were: (i) clinical trials enrolment rate [e.g. whether patients enrolled or not in a clinical trial over the course of their participation in the CANTO study (from breast cancer diagnosis through Y4 after diagnosis)]; (ii) the number, and (iii) the type of clinical trial in which patients enrolled. Enrolment in a clinical trial was defined as participation in a concomitant interventional study comparing a new therapeutic approach to the standard of care, or testing a new clinical strategy through a direct intervention on the patient.19 Being included only in observational studies did not account as enrolment in clinical trials.
Other variables
Clinical variables
Clinical, sociodemographic and socioeconomic (educational level, income, and occupation), tumour and treatment-related characteristics, and data regarding cancer centre of care [including geographical region and patient volume (expressed as the number of patients included in the CANTO cohort by centre)] were available at breast cancer diagnosis and categorized as in Table 1. Anxiety and depression were assessed through the Hospital Anxiety and Depression Scale.20
Table 1.
Baseline cohort characteristics, overall and according to clinical trials enrolment
| Whole cohort | According to clinical trial enrolment |
|||
|---|---|---|---|---|
| N (%) | Yes | No | P∗ | |
| Total | 9516 | 1716 (18.0) | 7800 (82.0) | |
| Age at diagnosis, years | ||||
| Median (Q1-Q3) | 57.0 (48.5-65.5) | 57.7 (48.1-66.3) | 56.8 (48.6-65.4) | 0.1080 |
| Missing | — | — | — | |
| Age at diagnosis, years | ||||
| <40 | 672 (7.1) | 136 (20.2) | 536 (79.8) | 0.0021 |
| 40-50 | 2145 (22.5) | 384 (17.9) | 1761 (82.1) | |
| 50-65 | 4154 (43.7) | 688 (16.6) | 3466 (83.4) | |
| ≥65 | 2545 (26.7) | 508 (20.0) | 2037 (80.0) | |
| Missing | — | — | — | |
| BMI at diagnosis | ||||
| Median (Q1-Q3) | 24.8 (22.0-28.7) | 25.3 (22.3–29.1) | 24.7 (21.9-28.7) | 0.0012 |
| Missing | 55 | 4 | 51 | |
| BMI at diagnosis | ||||
| <25 | 4832 (51.1) | 815 (16.9) | 4017 (83.1) | 0.0015 |
| ≥25 | 4629 (48.9) | 897 (19.4) | 3732 (80.6) | |
| Missing | 55 | 4 | 51 | |
| Menopausal status | ||||
| Premenopausal | 3514 (37.2) | 617 (17.6) | 2897 (82.4) | 0.3161 |
| Postmenopausal | 5925 (62.8) | 1089 (18.4) | 4836 (81.6) | |
| Missing | 77 | 10 | 67 | |
| Charlson comorbidity index | ||||
| 0 | 7049 (80.8) | 1202 (17.0) | 5849 (83.0) | 0.0210 |
| 1+ | 1672 (19.2) | 325 (19.4) | 1347 (80.6) | |
| Missing | 795 | 189 | 606 | |
| Depression, categorical | ||||
| Normal | 7087 (81.6) | 1301 (18.4) | 5786 (81.6) | 0.3774 |
| Doubtful case/case | 1602 (18.4) | 279 (17.4) | 1323 (82.6) | |
| Missing | 827 | 136 | 691 | |
| HADS depression scorea | ||||
| Mean (SD) | 4.2 (3.7) | 4.1 (3.6) | 4.3 (3.7) | 0.0647 |
| Missing | 827 | 136 | 691 | |
| Anxiety, categorical | ||||
| Normal | 3434 (39.5) | 661 (19.2) | 2773 (80.8) | 0.0394 |
| Doubtful case/case | 5250 (60.5) | 919 (17.5) | 4331 (82.5) | |
| Missing | 832 | 136 | 696 | |
| HADS anxiety scorea | ||||
| Mean (SD) | 9.0 (4.2) | 8.7 (4.2) | 9.0 (4.2) | 0.0205 |
| Missing | 832 | 136 | 696 | |
| Marital status | ||||
| In a relationship | 6786 (77.9) | 1236 (18.2) | 5550 (81.8) | 0.8015 |
| Not in a relationship | 1928 (22.1) | 356 (18.5) | 1572 (81.5) | |
| Missing | 802 | 124 | 678 | |
| Having a child | ||||
| Yes | 8313 (96.5) | 1524 (18.3) | 6789 (81.7) | 0.8324 |
| No | 303 (3.5) | 57 (18.8) | 246 (81.2) | |
| Missing | 900 | 135 | 765 | |
| Familiar history of breast cancer | ||||
| Positive | 2122 (22.7) | 379 (17.9) | 1743 (82.1) | 0.8419 |
| Negative | 7230 (77.3) | 1305 (18.0) | 5925 (82.0) | |
| Missing | 164 | 32 | 132 | |
| Personal history of previous cancer | ||||
| Yes | 735 (7.9) | 118 (16.0) | 617 (84.0) | 0.1607 |
| No | 8613 (92.1) | 1561 (18.1) | 7052 (81.9) | |
| Missing | 168 | 37 | 131 | |
| Previous breast surgery | ||||
| Yes | 694 (7.3) | 94 (13.5) | 600 (86.5) | 0.0014 |
| No | 8776 (92.7) | 1615 (18.4) | 7161 (81.6) | |
| Missing | 46 | 7 | 39 | |
| Highest education level | ||||
| Primary or lower | 1285 (14.8) | 255 (19.8) | 1030 (80.2) | 0.0605 |
| High school | 4029 (46.6) | 751 (18.6) | 3278 (81.4) | |
| College graduate or higher | 3341 (38.6) | 571 (17.1) | 2770 (82.9) | |
| Missing | 861 | 139 | 722 | |
| Monthly household income (euros/month) | ||||
| <2000 | 2288 (28.6) | 430 (18.8) | 1858 (81.2) | 0.0763 |
| 2000-4000 | 3730 (46.6) | 710 (19.0) | 3020 (81.0) | |
| ≥4000 | 1990 (24.8) | 332 (16.7) | 1658 (83.3) | |
| Missing | 1508 | 244 | 1264 | |
| Occupational class | ||||
| Chief executives, managers, and intellectual professions | 3925 (45.1) | 668 (17.0) | 3257 (83.0) | 0.0020 |
| Middle-class workersb | 3249 (37.4) | 612 (18.8) | 2637 (81.2) | |
| Self employed and manual workersc | 1365 (15.7) | 270 (19.8) | 1095 (80.2) | |
| Unemployed, retired | 160 (1.8) | 43 (26.9) | 117 (73.1) | |
| Missing | 817 | 123 | 694 | |
| Region of residency | ||||
| Île-de-France | 2632 (27.7) | 391 (14.9) | 2241 (85.1) | <0.0001 |
| Centre/North of Franced | 4989 (52.4) | 940 (18.8) | 4049 (81.2) | |
| South of Francee | 1895 (19.9) | 385 (20.3) | 1510 (79.7) | |
| Missing | — | — | — | |
| Anonymized COC | ||||
| High volume | 2320 (24.4) | 373 (16.1) | 1947 (83.9) | 0.0004 |
| Intermediate volume | 6454 (67.8) | 1232 (19.1) | 5222 (80.9) | |
| Low volume | 742 (7.8) | 111 (15.0) | 631 (85.0) | |
| Missing | — | — | — | |
| COC and patients region of residence | ||||
| COC in the same region | 8591 (90.3) | 1550 (18.0) | 7041 (82.0) | 0.9424 |
| COC in a different region/foreign patients | 925 (9.7) | 166 (17.9) | 759 (82.1) | |
| Missing | — | — | — | |
| Tumour stage | ||||
| I | 4582 (48.7) | 644 (14.0) | 3938 (86.0) | <0.0001 |
| II | 3911 (41.6) | 864 (22.1) | 3047 (77.9) | |
| III | 912 (9.7) | 188 (20.6) | 724 (79.4) | |
| Missing | 111 | 20 | 91 | |
| Tumour subtype | ||||
| HR+/HER− | 7186 (76.0) | 1318 (18.3) | 5868 (81.7) | 0.0951 |
| HR±/HER2+ | 1317 (13.9) | 248 (18.8) | 1069 (81.2) | |
| HR−/HER2− | 954 (10.1) | 149 (15.6) | 805 (84.4) | |
| Missing | 59 | 1 | 58 | |
| Chemotherapy | ||||
| Yes | 5041 (53.0) | 997 (19.8) | 4044 (80.2) | <0.0001 |
| No | 4475 (47.0) | 719 (16.1) | 3756 (83.9) | |
| Missing | — | — | — | |
| Surgery | ||||
| Mastectomy | 2539 (26.7) | 456 (18.0) | 2083 (82.0) | 0.9100 |
| Conservative surgery | 6971 (73.3) | 1259 (18.1) | 5712 (81.9) | |
| Missing | 6 | 1 | 5 | |
| Hormone therapy | ||||
| Yes | 7679f (81.0) | 1442 (18.8) | 6237 (81.2) | 0.0003 |
| No | 1797g (19.0) | 272 (15.1) | 1525 (84.9) | |
| Missing | 40 | 2 | 38 | |
| Radiotherapy | ||||
| Yes | 8661 (91.2) | 1630 (18.8) | 7031 (81.2) | <0.0001 |
| No | 831 (8.8) | 82 (9.9) | 749 (90.1) | |
| Missing | 24 | 4 | 20 | |
| Anti-HER2 therapy | ||||
| Yes | 1158 (12.2) | 230 (19.9) | 928 (80.1) | 0.0879 |
| No | 8341 (87.8) | 1485 (17.8) | 6856 (82.2) | |
| Missing | 17 | 1 | 16 | |
BMI, body mass index; COC, centre of care; HADS, Hospital Anxiety and Depression Scale; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; Q1-Q3, interquartile range; SD, standard deviation.
Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS).20
Includes clerks, service and sales workers, technicians, and associates.
Includes farmers, workers, freelancers, artisans, and merchants.
Centre/North of France includes Brittany, Normandy, Hauts de France, Pays de la Loire, Grand Est, Bourgogne-Franche-Comtè, Centre-Val de Loire, and other regions or foreign countries.
South of France includes Provence-Alpes-Cote d'Azur, Occitaine, Corsica, Novelle Aquitaine, and Auvergne-Rhône-Alpes.
Some 87.3% had an HR+ HER2− early breast cancer and 12.7% an HR+ HER2+ early breast cancer.
Some 6.6% had an HR+ HER2− early breast cancer, 29.2% an HR+ HER2+ early breast cancer, and 95.2% a triple-negative breast cancer.
Chi square for categorical variables, Wilcoxon rank sum test for continuous variables.
Patient-reported outcomes
Patient-reported outcomes (PROs) were collected using the European Organisation for Research and Treatment of Cancer (EORTC) quality-of-life (QoL) questionnaires QLQ-C30,21 BR23,22 and FA12.23 A higher-order QoL metric, the C30 Summary Score (C30 SumScore), was used to assess overall QoL. The C30 SumScore incorporates 13 (functional: physical, emotional, social, cognitive, and role; symptoms: fatigue, pain, nausea/vomiting, sleep disturbance, dyspnea, appetite loss, constipation, diarrhoea) of the 15 domains from EORTC QLQ-C30 (global health status and financial difficulties excluded).24,25 PROs were available in CANTO at diagnosis, and at Y1, Y2, and Y4 after diagnosis.
Survival outcomes
(i) A distant disease event (dDE) was defined as a distant breast cancer recurrence or death by any cause; (ii) invasive disease event (iDE) was defined as any invasive breast cancer recurrence (ipsilateral, regional, contralateral, or distant), second primary invasive cancer, or death by any cause; (iii) death by any cause (following standard DATECAN definitions).26
Statistical analysis
Primary analyses
First, we used descriptive statistics to summarize clinical trials enrolment variables. Then, cohort characteristics at diagnosis were tabulated, overall and by enrolment in clinical trials. Chi-square tests and Wilcoxon rank sum tests were used to compare characteristics according to enrolment for categorical and continuous variables, as appropriate. A multivariable logistic regression model was used to assess factors associated with clinical trials enrolment at any time over the course of the participation to the CANTO cohort study. All patients who had information about enrolment status at any point over post-diagnosis follow-up were included.
Exploratory analyses
We assessed the relationship of enrolment in clinical trials with PROs and clinical outcomes. A propensity score matched analysis was carried out to reduce the potential influence of confounding factors and balance characteristics between patients enrolled and not enrolled in clinical trials. This propensity score analysis was carried out following standard guidelines previously reported in literature.27 In order to calculate the propensity scores, we included in a logistic regression model all the variables assessed at baseline, including year of breast cancer diagnosis (as in Table 1), and we obtained the predicted probability of enrolment in clinical trials based on each patient characteristic. Once the propensity scores were calculated, subjects were matched by single score. Among patients selected and matched by propensity scores, the longitudinal evolution of PRO (EORTC QLQ-C30, BR23, and FA12) from diagnosis to Y4 was described according to clinical trials enrolment. A multiple linear regression model evaluated the association between clinical trials enrolment and C30 SumScore at Y4 after diagnosis, adjusting by C30 SumScore and covariates at baseline. The rates of dDE, iDE, and death by any cause were compared by enrolment in clinical trials using chi-square tests.
Sensitivity analyses
To confirm the robustness of our findings, we carried out the analyses of factors associated with enrolment among a subset of patients diagnosed with breast cancer from 2012 to 2015, who had complete follow-up available 4 years after diagnosis at the time of the most recent database update (n = 7810).
All tests were two-sided with a 95% confidence interval (CI) and a P value of <0.05 was considered statistically significant. Statistical analysis was carried out using SAS statistical software Version 9.4 (University of Bordeaux, Bordeaux, France).
Results
Cohort characteristics
Patients’ characteristics are reported in Table 1. Overall, median age at diagnosis was 57.0 years [interquartile range (Q1-Q3) 17.0 years]. The majority of patients included in the cohort were postmenopausal women (62.8%), with a Charlson comorbidity score of 0 (80.8%), and reported at least doubtful symptoms of anxiety (60.5%). More than half of patients were diagnosed with stage II (41.6%) or III (9.7%) breast cancer, 76.0% of them had a hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer, 81.0% received hormonal therapy, and 91.2% radiotherapy.
Enrolment in clinical trials and associated factors
The rate of enrolment in clinical trials was 18.0 % in the overall cohort (1716/9516 patients); 89% of patients were recruited in only one clinical trial, 10% concomitantly in two clinical trials, whereas ∼1% were recruited in three or four clinical trials. The majority of patients were enrolled in phase III drug evaluation studies (n = 641), phase III not-drug evaluation studies (n = 549), and phase II drug evaluation studies (n = 398), whereas only a few patients (n = 59) were recruited in early-phase clinical trials (Table 2).
Table 2.
Type of clinical trials among patients who enrolled on a clinical trial
| Type of study | No. patients enrolled N = 1716a (%) |
|---|---|
| Phase III drug assessing | 641 (37.3) |
| Phase III not drug assessing | 549 (32.0) |
| Phase III supportive care | 136 (7.9) |
| Phase II-III | 3 (0.2) |
| Phase II-III supportive care | 19 (1.1) |
| Phase II drug assessing | 398 (23.2) |
| Phase II not drug assessing | 20 (1.2) |
| Phase II supportive care | 21 (1.2) |
| Phase I-II | 57 (3.3) |
| Phase I | 2 (0.1) |
Numbers do not add up to 1716 as patients could be enrolled in more than 1 type of clinical trial at the same time. Note, among patients enrolled in clinical trials, there were 90 patients who also participated in concomitant observational studies.
After multivariable adjustment, factors independently associated with enrolment in clinical trials were: age at diagnosis [40-50 versus >65 years, odds ratio (OR) 0.79 (95% CI 0.64-0.98), P = 0.0300; 50-65 versus >65 years, OR 0.75 (95% CI 0.63-0.90), P = 0.0021], not reporting anxiety symptoms at diagnosis [normal versus doubtful case/case OR 1.17 (95% CI 1.01-1.35), P = 0.0363], region of residency [centre/North versus Ile de France OR 1.26 (95% CI 1.05-1.51) P = 0.0145; South versus Ile de France OR 1.51 (95% CI 1.20-1.89) P = 0.0004], being followed up in an intermediate volume centre of care [intermediate versus low volume centre OR 1.43 (95% CI 1.07-1.92), P = 0.0161] tumour stage [stage II versus stage I OR 1.56 (95% CI 1.33-1.84), P < 0.0001; stage III versus stage I OR 1.32 [95% CI 1.01-1.73], P = 0.0451], and adjuvant treatments such as hormonal therapy [yes versus no OR 1.34 (95% CI 1.11-1.62), P = 0.0021] and radiotherapy [yes versus no OR 1.98 (95% CI 1.43-2.76), P < 0.0001]. Full results are displayed in Table 3 and Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100513; details regarding rates of enrolment in clinical trials by different regions are displayed in Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100513.
Table 3.
Multivariable logistic regression of factors associated with enrolment in clinical trials (N = 9516)
| Effect | Adjusteda OR | 95% Confidence intervals | P value | |
|---|---|---|---|---|
| Age <40 versus >65 | 0.96 | 0.70 | 1.31 | 0.7902 |
| Age 40-50 versus >65 | 0.79 | 0.64 | 0.98 | 0.0300 |
| Age 50-65 versus >65 | 0.75 | 0.63 | 0.90 | 0.0021 |
| BMI ≥25 versus <25 | 1.05 | 0.91 | 1.21 | 0.5268 |
| Charlson ≥1 versus 0 | 1.09 | 0.91 | 1.29 | 0.3527 |
| Depression normal versus doubtful case/case | 1.00 | 0.83 | 1.21 | 0.9994 |
| Anxiety normal versus doubtful case/case | 1.17 | 1.01 | 1.35 | 0.0363 |
| Relationship no versus yes | 0.98 | 0.80 | 1.19 | 0.8295 |
| Children no versus yes | 1.13 | 0.79 | 1.60 | 0.5068 |
| BC familiar history negative versus positive | 0.99 | 0.84 | 1.17 | 0.9134 |
| Previous cancer no versus yes | 1.09 | 0.82 | 1.44 | 0.5395 |
| Previous breast surgery no versus yes | 1.18 | 0.89 | 1.56 | 0.2421 |
| High school versus primary school | 1.13 | 0.90 | 1.42 | 0.3031 |
| College or higher versus primary school | 1.09 | 0.82 | 1.44 | 0.5580 |
| Income <2000 versus 2000-4000 | 0.91 | 0.75 | 1.09 | 0.3045 |
| Income >4000 versus 2000-4000 | 0.92 | 0.77 | 1.10 | 0.3761 |
| Middle-class workers versus executives, managers | 1.04 | 0.86 | 1.24 | 0.6899 |
| Unemployed, retired, versus executives, managers | 1.49 | 0.89 | 2.49 | 0.1281 |
| Manual workers and self employed versus executive, managers | 1.04 | 0.82 | 1.32 | 0.7409 |
| Residency centre/north versus Ile de France | 1.26 | 1.05 | 1.51 | 0.0145 |
| Residency south versus Ile de France | 1.51 | 1.20 | 1.89 | 0.0004 |
| Centre volume intermediate (200-1000) versus low (<200) | 1.45 | 1.08 | 1.95 | 0.0124 |
| Centre volume high (>1000) versus low (<200) | 1.17 | 0.84 | 1.63 | 0.3597 |
| Different region versus same region between residence and centre of care | 0.98 | 0.77 | 1.23 | 0.8365 |
| Stage II versus stage I | 1.56 | 1.33 | 1.84 | <0.0001 |
| Stage III versus stage I | 1.32 | 1.01 | 1.73 | 0.0451 |
| Chemotherapy yes versus no | 1.12 | 0.94 | 1.33 | 0.2167 |
| Conservative surgery versus mastectomy | 1.07 | 0.89 | 1.28 | 0.4740 |
| Hormone therapy yes versus no | 1.34 | 1.11 | 1.62 | 0.0021 |
| Radiotherapy yes versus no | 1.98 | 1.43 | 2.76 | <0.0001 |
| Anti-HER2 therapy yes versus no | 1.14 | 0.92 | 1.40 | 0.2286 |
BC, breast cancer; BMI, body mass index; HER2, human epidermal growth factor receptor 2; OR, odds ratio.
Adjusted for all factors in the table, including year of breast cancer diagnosis.
Exploratory analyses: PROs and survival outcomes by enrolment in clinical trials
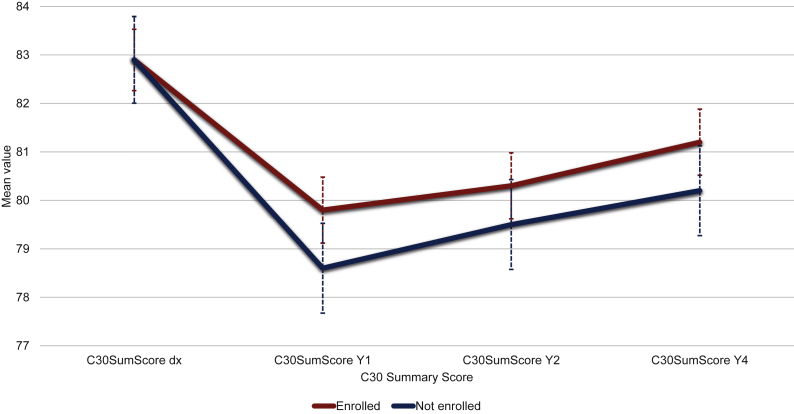
The propensity scores matching analysis led to the selection of 2118 patients with similar propensity scores. Baseline cohort characteristics of the propensity score selected population are displayed in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100513. C30 SumScore behaved similarly in patients enrolled and not enrolled in a clinical trial (Figure 2). Scores for all QLQ-C30, BR23, and FA12 domains at diagnosis, Y1, Y2, and Y4 are displayed in Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.esmoop.2022.100513. There was a significant association between the C30 SumScore at Y4 and the enrolment in clinical trials (mean difference versus not enrolled 1.37, 95% CI 0.03-2.71, P = 0.0458) (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100513). Among the propensity score selected patients, 7.3% of those enrolled in clinical trials had a dDE compared with 10.1% among those not enrolled (P = 0.0206). In addition, the rate of iDE was 8.2% among patients enrolled in clinical trials and 10.5% in those not enrolled (P = 0.0732). Lastly, death by any cause was 2.8% among patients enrolled in clinical trials and 3.7% among those not enrolled (P = 0.2707). The overall number of events by enrolment in clinical trials in the cohort of 2118 patients selected after the propensity score matched analysis is displayed in Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100513.
Figure 2.
Line graphs with mean Summary Score values [European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ) C30] over time by enrolment in clinical trials. C30 Summary Score is calculated using the mean scores for 13 of the 15 QLQ-C30 domains (the Global Health and the Financial Impact scales are not included). Higher C30 Summary Scores indicate a better quality of life. dx, diagnosis; Y1, 1 year after diagnosis; Y2, 2 years after diagnosis; Y4, 4 years after diagnosis.
Sensitivity analyses
We carried out a sensitivity analysis on a subset of patients diagnosed with breast cancer from 2012 to 2015, who had complete follow-up available 4 years after diagnosis (n = 7810). Factors associated with enrolment in clinical trials were consistent with the main analysis. Results are displayed in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2022.100513.
Discussion
Our analysis showed that among patients with early breast cancer, one in five enrolled in a clinical trial over a period of time extending from diagnosis to 4 years after, with the majority of them participating in phase III clinical trials and few patients participating in more than one study. We found some differences in clinical (age, stage of disease, anxiety) and geographical (region of residency, facilities of care) characteristics between patients enrolled in clinical trials and not. Trial participation did not seem to pose additional burden on patient-reported QoL 4 years after diagnosis, and seemed associated with improved clinical outcomes, as shown by similar PROs and lower rates of iDEs among patients who were enrolled on a clinical trial.
We believe that this study offers valuable insights into factors associated with clinical trial enrolment and expands upon the existing literature on the topic. First, we found that elderly women had the highest likelihood of enrolment in clinical trials in this cohort. Contrary to our findings, older adults are generally underrepresented in clinical trials. This was usually attributed to medical comorbidities, stringent trial eligibility criteria, and patient or physician misconceptions about the risks of enrolment among elderly individuals28,29 A possible explanation of a relatively good representation of the >65 years of age category in this study is that many patients in our cohort could access trials focused on supportive care, symptom management, and amelioration of QoL such as, for example, the ART-THERAPIE trial30 or the BEAUTY trial.31 Factors contributing to the lower enrolment rates among younger patients, however, including those aged 40-65 years in our cohort, are less well understood, including in previous literature. Among these, the physician’s failure to propose access to innovation was described as a strong barrier to enrolment in younger patients in previous studies.15,32,33
Second, some patient-related clinical factors were associated with enrolment. Particularly, an anxiety trait was associated with lower enrolment in clinical trials. As also suggested by previous studies, uncertainties, fear of potential adverse events, fear of delay of initiation of antineoplastic drugs, fear of being enrolled in the placebo arm due to randomization procedures, or possible painful procedures, may possibly reduce willingness to participate in clinical studies.34 For instance, a survey by Mancini et al.35 on 115 women enrolled in clinical trials from 21 centres in France pointed out how 43.0% of participants expressed mild regret to have chosen to participate, and 25.8% expressed moderate to strong regret after agreeing to participate in a randomized, controlled clinical trial.35 On the contrary, a strong clinical barrier to the participation in clinical trials previously reported in the literature, such as comorbidities,36 was not associated with enrolment in our cohort.
Third, we did not find major differences in enrolment rates across socioeconomic strata in our cohort, as income and education level did not seem to be associated with enrolment. We acknowledge, however, that these social determinants may represent important drivers of enrolment in health care systems other than the French system. Financial issues were identified as an important barrier to clinical trial participation, especially in the United States,37 where insurances may not cover non-routine services.38 Whereas in France many health services are often accessible with no extra cost to the whole population, due to universal health coverage, in some countries, such as the United States, patients may have to deal with additional costs when participating in clinical trials, especially when this requires them to travel to their follow-up centres for additional tests or procedures that are only available locally, or cannot be obtained elsewhere.39
Fourth, centre-related features seemed to be one of the most relevant determinants of enrolment in clinical trials. This finding may relate to availability of research infrastructures that can facilitate enrolment, both for patients and for physicians. Facilitators of enrolment may indeed come from the improvement of local research infrastructures, providing facilities to physicians that can optimize the process of recruitment and trial managing.
In addition, we carried out exploratory analyses that also yielded interesting findings. We showed that enrolment in clinical trials seems to be associated with better PRO 4 years after diagnosis and lower recurrence rates. Whether the enrolment in clinical trials improves survival beside the clinical setting or trial outcomes still remains debated. Previously, one study by Chow et al.3 proved that enrolment into cancer trials could independently predict lower overall and cancer-specific mortality in patients with common cancers. Although exploratory, results of our study are consistent with these findings and provide further insight into the relationship between enrolment in trials and relapse among a specific population, such as patients with early breast cancer.
We recognize that our study has some limitations. Our cohort is selected from patients that participated in CANTO, which is per se a multicenter cohort study. Indeed, the high proportion of comprehensive cancer centres in the recruiting institutions might have led to an overestimation of the results. CANTO focuses on women diagnosed with breast cancer and followed up in France, therefore this analysis may not be generalizable to all women and health care systems of other countries. Some attrition was present with increasing missing data, particularly in PROs at later time points. CANTO did not include information about the date and duration of enrolment clinical trials, as participation could happen anytime over 4 years after diagnosis. To accommodate for the potential of premature study termination due to breast cancer recurrence or death, however, all models included breast cancer prognostic factors. CANTO includes only patients with stage I-II-III breast cancer without evidence of active disease or relapse, therefore we acknowledge potential bias from study termination for patients who develop disease progression. Analyses of clinical outcomes may be underpowered due to the limited number of events in this early-stage cohort of women with breast cancer and limited follow-up time for survival events. For these reasons, survival outcomes were summarized descriptively, as opposed to being assessed in a formal time-to-event analysis by enrolment group, and our results should be interpreted with caution as they were considered exploratory. Strengths include that this is the first multicentre prospective study to focus exclusively on the enrolment in clinical trials among women diagnosed with early breast cancer, with availability of a large amount of longitudinal PRO up to 4 years after diagnosis.
Conclusions
This study focused on the enrolment rate and factors related to enrolment in clinical trials among women diagnosed with early breast cancer. One in five women were enrolled in a clinical trial after diagnosis of early-stage breast cancer. In this population, the rate of enrolment exceeded what was previously found in different settings. Whereas patients seem adequately represented irrespective of clinical and treatment-related features, mostly clinical (age, anxiety traits) and geographical (region of residency, facilities of care) factors seem to be those associated with access to innovation in clinical trials. Enrolment did not negatively impact QoL and there were suggestions of improved clinical outcomes among women who participated in clinical trials. Access to innovation should be facilitated and encouraged by overcoming factors associated with impaired recruitment.
Acknowledgments
Funding
This work was supported by the French Government under the ‘Investment for the Future’ program managed by the National Research Agency (ANR) [grant number ANR-10-COHO-0004]. Antonio Di Meglio is supported by a Career Pathway Grant in Symptom Management from Conquer Cancer, the ASCO Foundation and Rising Tide Foundation for Clinical Cancer Research. This study was supported by grants from Odyssea (no grant number), and Foundation Gustave Roussy (no grant number).
Disclosure
BP reports personal fees from AstraZeneca, Myriad, Pfizer, Pierre Fabre, Daiichi Sankyo, and non-financial support from Daiichi Sankyo, Merus, Puma, Pfizer, AstraZeneca. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Braunholtz D.A., Edwards S.J.L., Lilford R.J. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.”. J Clin Epidemiol. 2001;54(3):217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 2.Shahar T., Nossek E., Steinberg D.M., et al. The impact of enrollment in clinical trials on survival of patients with glioblastoma. J Clin Neurosci. 2012;19(11):1530–1534. doi: 10.1016/j.jocn.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Chow C.J., Habermann E.B., Abraham A., et al. Does enrollment in cancer trials improve survival? J Am Coll Surg. 2013;216(4):774–781. doi: 10.1016/j.jamcollsurg.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arrieta O., Carmona A., Ramírez-Tirado L.A., et al. Survival of patients with advanced non-small cell lung cancer enrolled in clinical trials. Oncology. 2016;91(4):185–193. doi: 10.1159/000447404. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Available at NCCN.org. Accessed September 13, 2019.
- 6.Murthy V.H., Krumholz H.M., Gross C.P. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 7.Carey M., Boyes A.W., Smits R., Bryant J., Waller A., Olver I. Access to clinical trials among oncology patients: results of a cross sectional survey. BMC Cancer. 2017;17(1):653. doi: 10.1186/s12885-017-3644-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. 2015-2016 Global Participation In Clinical Trials Report. July 2017.
- 9.Castro E.M., Van Regenmortel T., Vanhaecht K., Sermeus W., Van Hecke A. Patient empowerment, patient participation and patient-centeredness in hospital care: a concept analysis based on a literature review. Patient Educ Couns. 2016;99(12):1923–1939. doi: 10.1016/j.pec.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Unger J.M., Vaidya R., Hershman D.L., Minasian L.M., Fleury M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3):245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger J.M., Cook E., Tai E., Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198. doi: 10.14694/EDBK_156686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kincaid E. Advanced cancer treatments far from big-name hospitals. The Wall Street Journal. Available at https://www.healthleadersmedia.com/strategy/advanced-cancer-treatments-far-big-name-hospitals. Accessed March 6, 2017.
- 13.Mills E.J., Seely D., Rachlis B., et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 14.Comis R.L., Miller J.D., Aldigé C.R., Krebs L., Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21(5):830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 15.Mazouni C., Deneuve J., Arnedos M., et al. Decision-making from multidisciplinary team meetings to the bedside: factors influencing the recruitment of breast cancer patients into clinical trials. Breast. 2014;23(2):170–174. doi: 10.1016/j.breast.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Besle S., Schultz E., Hollebecque A., et al. Organisational factors influencing early clinical trials enrollment: Gustave Roussy experience. Eur J Cancer. 2018;98:17–22. doi: 10.1016/j.ejca.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Vaz-Luis I., Cottu P., Mesleard C., et al. UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO) ESMO Open. 2019;4(5) doi: 10.1136/esmoopen-2019-000562. e000562-e000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.WHO International Clinical Trials Registry Platform. 2021. https://www.who.int/clinical-trials-registry-platform Available at.
- 20.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22.Sprangers M.A., Groenvold M., Arraras J.I., et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 23.Weis J., Tomaszewski K.A., Hammerlid E., et al. International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12) J Natl Cancer Inst. 2017;109(5) doi: 10.1093/jnci/djw273. [DOI] [PubMed] [Google Scholar]
- 24.Giesinger J.M., Kieffer J.M., Fayers P.M., et al. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2016;69:79–88. doi: 10.1016/j.jclinepi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Husson O., de Rooij B.H., Kieffer J., et al. The EORTC QLQ-C30 summary score as prognostic factor for survival of patients with cancer in the “Real-World”: results from the population-based PROFILES registry. Oncologist. 2020;25(4):e722–e732. doi: 10.1634/theoncologist.2019-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourgou-Bourgade S., Cameron D., Poortmans P., et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann Oncol. 2015;26(5):873–879. doi: 10.1093/annonc/mdv106. [DOI] [PubMed] [Google Scholar]
- 27.Yao X.I., Wang X., Speicher P.J., et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109(8):djw323. doi: 10.1093/jnci/djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemeny M.M., Peterson B.L., Kornblith A.B., et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21(12):2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 29.Denson A.C., Mahipal A. Participation of the elderly population in clinical trials: barriers and solutions. Cancer Control. 2014;21(3):209–214. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 30.Joly F., Pasquier D., Hanzen C., et al. Impact of art therapy (AT) on fatigue and quality of life (QoL) during adjuvant external beam irradiation (EBI) in breast cancer patients (pts): a randomized trial. Ann Oncol. 2016;27(suppl 6):vi499. [Google Scholar]
- 31.Saghatchian M., Lacas B., Charles C., et al. BEAUTY and the breast: is adjuvant chemotherapy the right time for a beauty boost? Lessons learned from a large randomized controlled trial. Qual life Res. 2022;31:723–732. doi: 10.1007/s11136-021-02947-6. [DOI] [PubMed] [Google Scholar]
- 32.Kim E.S., Bruinooge S.S., Roberts S., et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research statement. J Clin Oncol. 2017;35(33):3737–3744. doi: 10.1200/JCO.2017.73.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siembida E.J., Loomans-Kropp H.A., Trivedi N., et al. Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: identifying opportunities for intervention. Cancer. 2020;126(5):949–957. doi: 10.1002/cncr.32675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida L., Kashdan T.B., Nunes T., Coelho R., Albino-Teixeira A., Soares-da-Silva P. Who volunteers for phase I clinical trials? Influences of anxiety, social anxiety and depressive symptoms on self-selection and the reporting of adverse events. Eur J Clin Pharmacol. 2008;64(6):575–582. doi: 10.1007/s00228-008-0468-8. [DOI] [PubMed] [Google Scholar]
- 35.Mancini J., Genre D., Dalenc F., et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635–642. doi: 10.1016/j.jclinepi.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Zaorsky N.G., Zhang Y., Walter V., Tchelebi L.T., Chinchilli V.M., Gusani N.J. Clinical trial accrual at initial course of therapy for cancer and its impact on survival. J Natl Compr Cancer Netw. 2019;17(11):1309–1316. doi: 10.6004/jnccn.2019.7321. [DOI] [PubMed] [Google Scholar]
- 37.Nipp R.D., Hong K., Paskett E.D. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ B. 2019;39:105–114. doi: 10.1200/EDBK_243729. [DOI] [PubMed] [Google Scholar]
- 38.Stump T.K., Eghan N., Egleston B.L., et al. Cost concerns of patients with cancer. J Oncol Pract. 2013;9(5):251–257. doi: 10.1200/JOP.2013.000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nipp R.D., Powell E., Chabner B., Moy B. Recognizing the financial burden of cancer patients in clinical trials. Oncologist. 2015;20(6):572–575. doi: 10.1634/theoncologist.2015-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.