Abstract
In plants, homospermidine synthase (HSS) is a pathway‐specific enzyme initiating the biosynthesis of pyrrolizidine alkaloids (PAs), which function as a chemical defense against herbivores. In PA‐producing Convolvulaceae (“morning glories”), HSS originated from deoxyhypusine synthase at least >50 to 75 million years ago via a gene duplication event and subsequent functional diversification. To study the recruitment of this ancient gene duplicate to PA biosynthesis, the presence of putative hss gene copies in 11 Convolvulaceae species was analyzed. Additionally, various plant parts from seven of these species were screened for the presence of PAs. Although all of these species possess a putative hss copy, PAs could only be detected in roots of Ipomoea neei (Spreng.) O'Donell and Distimake quinquefolius (L.) A.R.Simões & Staples in this study. A precursor of PAs was detected in roots of Ipomoea alba L. Thus, despite sharing high sequence identities, the presence of an hss gene copy does not correlate with PA accumulation in particular species of Convolvulaceae. In vitro activity assays of the encoded enzymes revealed a broad spectrum of enzyme activity, further emphasizing a functional diversity of the hss gene copies. A recently identified HSS specific amino acid motif seems to be important for the loss of the ancestral protein function—the activation of the eukaryotic initiation factor 5A (eIF5A). Thus, the motif might be indicative for a change of function but allows not to predict the new function. This emphasizes the challenges in annotating functions for duplicates, even for duplicates from closely related species.
Keywords: deoxyhypusine synthase, Distimake, gene duplication, homospermidine synthase, Ipomoea, molecular evolution, pyrrolizidine alkaloids
List of Abbreviations
- cDNA
complementary DNA
- DHS
deoxyhypusine synthase
- FPNI‐PCR
fusion primer and nested integrated PCR
- GC
gas chromatography
- gDNA
genomic DNA
- HSS
homospermidine synthase
- MS
mass spectrometry
- NAD
nicotinamide adenine dinucleotide
- PAs
pyrrolizidine alkaloids
- PCR
polymerase chain reaction
- RACE
rapid amplification of cDNA ends
- SEC‐MALS
size‐exclusion chromatography coupled to multi‐angle light scattering
1. INTRODUCTION
Convolvulaceae, also known as “morning glories” or the “sweet potato” family, is a diverse group of flowering plants, with 60 genera and approximately 1800 species, widely distributed across the tropics, with fewer representatives in temperate regions (WCVP, 2021). This family has great economic importance, especially as food (“sweet potato” and “water spinach”) and ornamental plants (“bindweeds” and “morning glories”) and also for medicinal purpose (Chen et al., 2018). Based on molecular phylogenetic studies, the family has been classified into six monophyletic subfamilies (Figure 1a), among which Convolvuloideae is the most taxonomically diverse and economically important, including the tribes Ipomoeeae, Merremieae and Convolvuleae (Stefanović et al., 2003). Many species of Convolvulaceae form a symbiosis with fungal endosymbionts (Periglandula U. Steiner, E. Leistner & Leuchtm.), leading to the accumulation of ergot alkaloids in their seeds (Beaulieu et al., 2021; Leistner & Steiner, 2018). In consequence, the seeds of some species such as Argyreia nervosa (Burm.f.) Bojer are well known natural psychotropics (Paulke et al., 2013). Furthermore, a number of species of the Convolvuloideae subfamily are known to produce pyrrolizidine alkaloids (PAs, Figure 1b; Eich, 2008). PAs are specialized metabolites produced in plants as part of their chemical defense against insect herbivores (Dreyer et al., 1985; Hartmann, 1999; Hartmann & Ober, 2008; Reinhard et al., 2009). PAs are also toxic to mammals including humans (Wiedenfeld & Edgar, 2011). Therefore, they have an economic impact as they can cross‐contaminate food products such as honey, herbal drugs, and tea (Kaltner et al., 2018). PAs are also produced by other plant families, like the Asteraceae, Boraginaceae, Fabaceae, and Orchidaceae (Langel et al., 2011). To date, more than 400 PAs have been identified (Langel et al., 2011). Despite this structural diversity, all PAs share a common base structure, namely a nitrogen‐containing bicyclic ring system called necine base that is esterified with one or more “necic” acids (Figure 1d). Homospermidine is the precursor for the biosynthesis of the necine base (Böttcher et al., 1994) and is synthesized from putrescine and spermidine, which are part of the polyamine pool of plants. In PA‐producing plants, homospermidine is formed by the homospermidine synthase (HSS, EC 2.5.1.45), which is considered to be the first pathway‐specific enzyme of PA biosynthesis (Ober & Hartmann, 1999b; Ober, Harms, et al., 2003). In the presence of NAD+, HSS catalyzes the transfer of the aminobutyl moiety from spermidine to putrescine to form homospermidine (Ober & Hartmann, 1999b; Ober, Harms, et al., 2003, Figure 1e). Sequence‐based homology studies have shown that HSS evolved via a gene duplication from deoxyhypusine synthase (DHS), which is involved in the first step of the post‐translational activation of the eukaryotic translation initiation factor 5A (eIF5A, Ober & Hartmann, 1999b). DHS is a ubiquitous enzyme in eukaryotes and is essential for cell growth and viability (Chattopadhyay et al., 2008). Enzymatically, DHS and HSS perform an analogous reaction by transferring the aminobutyl group from spermidine to an acceptor. The difference lies in the specificity toward their respective aminobutyl acceptor. The DHS favors a specific lysine residue of the eIF5A precursor protein and catalyzes its modification to deoxyhypusine (DHS activity), while the HSS utilizes the polyamine putrescine (Figure 1e). Of note, the DHS is promiscuous and accepts various polyamines including putrescine as aminobutyl acceptor in the absence of the eIF5A precursor (Kaltenegger et al., 2021; Ober, Harms, et al., 2003; Park et al., 2003; Wątor et al., 2020; Wolff et al., 1997). Minor amounts of homospermidine, attributable to this side activity of the DHS, are found in many diverse plant species (Ober, Gibas, et al., 2003). However, in a hss knock out plant of Symphytum officinale, these low amounts of homospermidine as side‐product of DHS are not channeled into PA biosynthesis (Zakaria et al., 2021).
FIGURE 1.
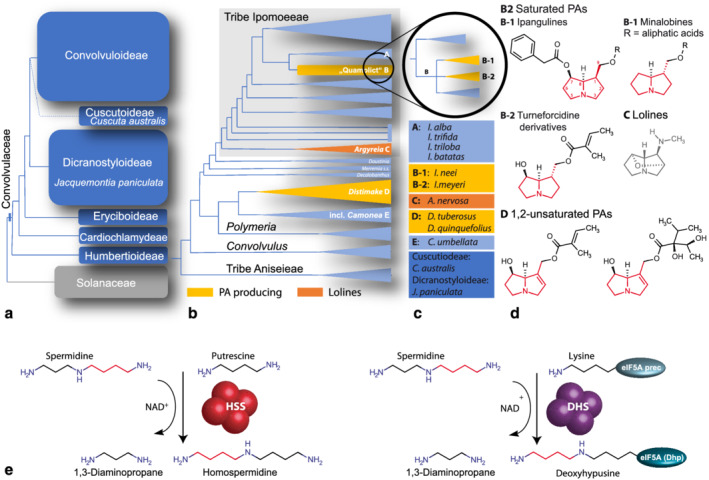
Pyrrolizidine alkaloids (PAs) occurring in the Convolvulaceae. (a) The figure illustrates the phylogenetic tree topology of the Convolvulaceae including the Solanaceae being its sister family. Species analyzed in this study all belong to the Convolvuloideae subfamily, except Cuscuta australis (Cuscutoideae), and Jacquemontia paniculata (Dicranostyloideae). (b) Tree topology of the Convolvuloideae. Groups within the Convolvuloideae, which comprise single species that produce PAs and lolines, are highlighted. Abbreviations: incl. = including. (c) Species included in this study and their affiliation to the phylogenetic groups given in (a) and (b). (d) Structures of PAs and lolines, which have been detected in individual species. The characteristic core structure of PAs, the necine base, is given in red. Ipangulines and Minalobines with a saturated necine base occur in species that belong to the “B2‐1”‐clade within the “Quamoclit” clade (B2) that includes species previously assigned to subgenus Quamoclit, or closely related to it (sensu Austin, 1979), as demonstrated by recent molecular phylogenetic results (Muñoz‐Rodríguez et al., 2019). In Ipomoea meyeri , which belongs to a sister group within this clade (B2‐2), unsaturated turneforcidine derivatives have been found (Eich, 2008; Tofern, 1999). Lolines have been described to occur in Argyreia mollis (Tofern et al., 1999). And finally, alkaloids that possess a 1,2‐unsaturated necine base occur in Distimake quinquefolius and Distimake cissoides (Eich, 2008; Mann, 1997). (e) Main reactions catalyzed by homospermidine synthase (HSS) and deoxyhypusine synthase (DHS). While HSS preferentially catalyzes the transfer of an aminobutyl moiety (highlighted in red) from spermidine to putrescine, DHS favors a specific lysine of the eIF5A precursor as aminobutyl acceptor to form deoxyhypusine.
HSS in PA producing species lost the ability to use the eIF5A precursor protein (Kaltenegger et al., 2013, 2021; Ober, Harms, et al., 2003). This change of function of HSS has been explained as an evolutionary adaptation of one of the gene copies and as a prerequisite for the recruitment to PA biosynthesis (Reimann et al., 2004). Interestingly, the recruitment of HSS from DHS occurred multiple times after the independent gene duplication events in PA‐producing plant lineages (Reimann et al., 2004). However, Convolvulaceae represent a special case, since the PAs detected in Convolvuloideae subfamily can be assigned to two different types. First, PAs with a saturated necine base including unique types of PAs known as ipangulines, minalobines and turneforcidine derivatives (Figure 1d, Jenett‐Siems et al., 1993; Jenett‐Siems et al., 1998; Jenett‐Siems et al., 2005; Tofern, 1999; Eich, 2008) occur in several species of the “Quamoclit” clade of Ipomoea L. (Figure 1b, clade B2‐1) and in Ipomoea meyeri (Spreng.) G. Don, located in the sister clade of the “Quamoclit” clade (Figure 1b, clade B2‐2, Muñoz‐Rodríguez et al., 2019). Second, 1,2‐unsaturated PAs (Figure 1d) have been described for Distimake quinquefolius (L.) A.R.Simões & Staples (syn. Merremia quinquefolia) and Distimake cissoides (Lam.) A.R.Simões & Staples (syn. Merremia cissoides) (Figure 1b, clade G, Eich, 2008; Mann, 1997). Noteworthy is also the occurrence of lolines in Argyreia mollis (Burm. f.) Choisy (Tofern et al., 1999). Lolines (Figure 1d), though having a backbone structure that is similar to the necine base, are biogenetically unrelated to PAs (Hartmann & Witte, 1995; Schardl et al., 2007).
The occurrence of two different structural types of PAs in Ipomoea L. and Distimake Raf. suggests their independent evolutionary origin. Surprisingly, the HSS in both lineages originated from a single gene duplication (Kaltenegger et al., 2013), which occurred prior to the divergence of the Ipomoeeae and the clade that includes Distimake and allied genera, roughly 75 to 50 million years ago (mya) (Eserman et al., 2014). As the last common ancestor of both groups possessed a putative hss gene copy, it could theoretically have been inherited by all of its descendants. We have thus hypothesized that the hss gene copy is widespread in species of the entire Convolvuloideae and that it might have been recruited for PA biosynthesis in further species. Indeed, an ortholog of HSS has also been found in Convolvulus arvensis L., but this ortholog shows clear signs of being a pseudogene (Kaltenegger et al., 2013). Additionally, Ipomoea alba L., which belongs to a sister clade of the “Quamoclit” clade of Ipomoea (Figure 1b), possesses an ortholog of HSS, but its function is unknown (Kaltenegger et al., 2013).
To gain more insight, we studied the presence of putative hss genes in further species of subfamily Convolvuloideae and in parallel screened for the occurrence of PAs (Figure 1c and Table S1), namely, in a selection of representatives of Ipomoeeae (A. nervosa, I. alba) and the genera Distimake [Distimake tuberosus (syn. Merremia tuberosa)] and Camonea Raf. [Camonea umbellata (syn. Merremia umbellata)], which were previously assigned to the genus Merremia Dennst. ex. Endl., but have now been recognized as distinctive based on molecular, morphological and biogeographic data (Simões et al., 2015; Simões & Staples, 2017). As positive control, we also analyzed Ipomoea neei and D. quinquefolius (syn. M. quinquefolia) for PAs. For comparison reasons, we screened representatives of the subfamilies Dicranostyloideae (Jacquemontia paniculata (Burm.f.) Hallier f.) and Cuscutoideae (Cuscuta australis Hook.f.) for the presence of hss gene copies. Due to its use as crop plant, we also included Ipomoea batatas and its ancestral species Ipomoea trifida and Ipomoea triloba in this screen.
2. RESULTS
2.1. Identifying homologs of dhs and hss in Convolvulaceae
For every species, except J. paniculata, we were able to amplify two dhs homologs from genomic or cDNA (Table S2). The amplified sequences varied in length and covered the partial open reading frame (ORF) or, in five out of nine sequences, the complete ORF (Table S4). Additionally, we obtained sequences homologous to dhs from the genomes of I. trifida (Kunth) G. Don, I. triloba L., and I. batatas L. (Table S4). The average length of the ORF was about 1140 bp. In pairwise sequence comparisons, including the previously published DHS‐ and HSS‐coding sequences from I. neei, I. alba, and D. quinquefolius (Kaltenegger et al., 2013), the sequences showed a high degree of identity from 83.6% to 99.7% (Table 1). Highest pairwise sequence identity was found between the putative DHS sequences from the two D. quinquefolius individuals. GenBank accession numbers are given in Table S4.
TABLE 1.
Pairwise sequence identity among DHS and HSS sequences (cDNA) from the Convolvulaceae
| No. | Species | 1 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DHS Cuscuta australis | 2 | ||||||||||||||||||||
| 2 | DHS Justica paniculata | 87.1 | 3 | |||||||||||||||||||
| 3 | DHS Camonea umbellata | 86.8 | 88.4 | 4 | ||||||||||||||||||
| 4 | DHS Argyreia nervosa | 86.5 | 87.6 | 94.7 | 5 | |||||||||||||||||
| 5 | DHS Ipomoea batatas | 86.7 | 87.6 | 95.7 | 96.8 | 6 | ||||||||||||||||
| 6 | DHS Ipomoea trifida | 86.2 | 87 | 95.1 | 96.2 | 99 | 7 | |||||||||||||||
| 7 | DHS Ipomoea triloba | 87.2 | 87.6 | 95.7 | 96.3 | 98.8 | 98.4 | 8 | ||||||||||||||
| 8 | DHS Ipomoea alba | 86.4 | 87.4 | 94.6 | 96.3 | 98.1 | 97.6 | 97.4 | 9 | |||||||||||||
| 9 | DHS Ipomoea neei | 86.2 | 87 | 94.7 | 95.5 | 97.6 | 96.9 | 97.2 | 97.2 | 10 | ||||||||||||
| 10 | DHS Dichotomosiphon tuberosus | 88.7 | 93.4 | 96.6 | 95.4 | 96.4 | 95.7 | 96.1 | 95.4 | 95.4 | 11 | |||||||||||
| 11 | DHS Distimake quinquefolius 1 | 87.2 | 87.9 | 96.2 | 94.5 | 95.4 | 95.1 | 95.2 | 94.5 | 94 | 96.6 | 12 | ||||||||||
| 12 | DHS Distimake quinquefolius 2 | 87 | 87.9 | 96.2 | 94.3 | 95.2 | 94.9 | 95 | 94.3 | 93.8 | 96.4 | 99.7 | 13 | |||||||||
| 13 | HSS C. umbellata | 84.5 | 85.9 | 90.3 | 88.8 | 89.9 | 89.3 | 89.8 | 89.7 | 89.5 | 91.9 | 90.4 | 90.4 | 14 | ||||||||
| 14 | HSS A. nervosa | 83.2 | 82.8 | 88.1 | 87.5 | 87.6 | 87.2 | 87.9 | 88 | 87.6 | 89.6 | 87.8 | 87.6 | 89.6 | 15 | |||||||
| 15 | HSS I. trifida | 82.5 | 82.7 | 86.7 | 86.8 | 86.9 | 86.4 | 86.8 | 86.9 | 86.3 | 88.9 | 86.9 | 86.7 | 90.3 | 92.7 | 16 | ||||||
| 16 | HSS I. triloba | 82.4 | 81.2 | 86.4 | 86.2 | 86.5 | 86 | 86.5 | 86.3 | 86 | 89.1 | 86.3 | 86.1 | 89.6 | 92.5 | 97.8 | 17 | |||||
| 17 | HSS I. alba | 82.7 | 81.9 | 86 | 86.3 | 86.2 | 85.9 | 86.6 | 86.2 | 85.8 | 89.2 | 86 | 85.8 | 89.2 | 91.7 | 93.4 | 93.5 | 18 | ||||
| 18 | HSS I. neei | 81.9 | 81.6 | 85.8 | 85.6 | 85.8 | 85.4 | 86 | 85.9 | 85.5 | 88.6 | 85.9 | 85.7 | 89.5 | 92.4 | 94.5 | 94.1 | 95.1 | 19 | |||
| 19 | HSS D. tuberosus | 86.2 | 89.9 | 85.1 | 84.2 | 85.4 | 84.8 | 85.2 | 84.6 | 84.7 | 90.9 | 85 | 84.8 | 87.1 | 85.4 | 85.3 | 84.2 | 85.4 | 84.8 | 20 | ||
| 20 | HSS D. quinquefolius 1 | 82.8 | 82.5 | 86.5 | 85.4 | 86.5 | 86 | 86.5 | 85.7 | 85.7 | 88.2 | 86.5 | 86.3 | 90 | 87.8 | 88.4 | 88.1 | 87.7 | 88 | 88.3 | 21 | |
| 21 | HSS D. quinquefolius 2 | 81.8 | 81.6 | 84.7 | 84.1 | 84.8 | 84.4 | 84.8 | 84.3 | 84.1 | 86.8 | 84.7 | 84.7 | 87.8 | 86.3 | 86.9 | 86.8 | 85.5 | 86.7 | 86.8 | 92.6 |
Note: The sequences were identified as deoxyhypusine synthase (DHS) and homospermidine synthase (HSS) according to their position in the phylogenetic tree. Percentage of positions are shown where two sequences have an identical base or residue. Colors highlight the pairwise sequence identities of DHS versus DHS (gray) and of HSS versus HSS (orange).
However, based on sequence data alone, DHS‐ and HSS‐coding sequences cannot be differentiated. Thus, to further distinguish the newly identified sequences in dhs and hss orthologs, their phylogeny was reconstructed by calculating a maximum likelihood tree. Only the coding regions were used for the analysis. The DHS‐encoding sequences of representatives of Solanaceae (sister family to Convolvulaceae in the order Solanales)—Nicotiana tabacum L. and Solanum lycopersicum L. (syn. Lycopersicon esculentum)—were included as outgroup. The resulting phylogenetic tree showed a clear bifurcation with two main clades (Figure 2a), and the branching pattern within both clades was mainly consistent with the topology of the currently published species trees of the Convolvulaceae (Eserman et al., 2014; Muñoz‐Rodríguez et al., 2019; Simões et al., 2015; Stefanovic et al., 2002). Based on the previously biochemically characterized sequences from I. neei, I. alba, and D. quinquefolius, the two main clades could be classified as DHS‐ and HSS‐coding clades (Figure 2a). Thus, the phylogeny added evidence to the previously described single gene duplication event of the dhs in the Convolvulaceae (Kaltenegger et al., 2013). Furthermore, as the DHS from C. australis and J. paniculata are sister to Convolvuloideae DHS and HSS, the phylogeny suggests that the gene duplication occurred within the Convolvuloideae. The pairwise Ks distances between the paralogous DHS/HSS pairs range from 0.2 to 0.4 (Table S6).
FIGURE 2.
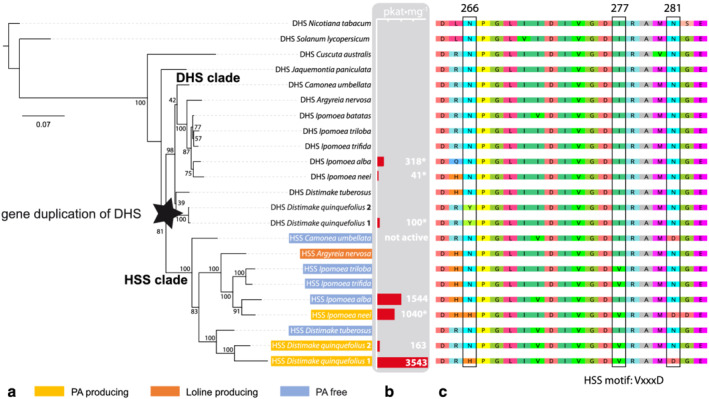
Phylogenetic analyses of deoxyhypusine synthase (DHS)‐ and homospermidine synthase (HSS)‐encoding sequences. (a) A maximum likelihood tree was built from the cDNA sequences of HSS and DHS from the Convolvulaceae family and two DHS sequences from Solanaceae family. Percentages of bootstrap support values (for 1000 replicates) are indicated. The ancient duplication is highlighted, and the DHS and HSS clades are indicated. (b) Selected sequences were chosen for biochemical analyses of the encoded enzymes. The calculated specific activity (pkat·mg) of homospermidine production of these enzymes in glycine‐based assay buffer is given (see Table 3). Specific activities marked with an asterisk were taken from a previous study (Kaltenegger et al., 2013) (c) Alignment of the functionally characterized “HSS motif” (H‐V‐D)
In contrast to the highly conserved DHS clade, a longer branch length that indicated an increased nucleotide substitution rate between the individual hss sequences was observed in the HSS clade (Figure 2a) as previously described for the HSS sequences of the Boraginaceae and Asteraceae (Reimann et al., 2004).
Of note, all species of subfamily Convolvuloideae that were selected for this study possessed an ortholog in both clades, providing them with a putative HSS encoding gene copy to catalyze the first step in PA biosynthesis; while in species of the other subfamilies—J. paniculata (subfamily Dicranostyloideae) and C. australis (subfamily Cuscutoideae)—only a dhs gene copy was detected. As the sequences identified in this study were amplified from cDNA, except for D. tuberosus (Table S4), the genes also seem to be actively transcribed. Also, transcriptome profiles retrieved from the “Sweetpotato Genomics Resource” platform support that both genes, dhs as well as the putative hss, are transcribed in I. triloba as well as I. trifida. In detail, the dhs gene in I. triloba and I. trifida was found to be transcribed in flowers, flower buds, roots, leaves, and stems. The putative hss gene transcription profile differs only slightly, as the hss ortholog is supported to be strongest transcribed in flower buds but not in roots.
2.2. Presence of PAs in the Convolvulaceae
Of the Convolvulaceae species studied here, I. neei and D. quinquefolius have been previously described as producing PAs (Eich, 2008; Jenett‐Siems et al., 2005, 1998; Mann, 1997). Leaves, stems, and roots of these species, grown in the greenhouses of Kiel Botanic gardens, were analyzed in this study and served as positive control. In I. neei, PAs were mostly detected in the roots (Suppl. Table S5). In shoots, only traces of PAs were detected. The exact pattern of PAs was slightly different from that of previous studies (Jenett‐Siems et al., 2005, 1998), as ipanguline C3 was identified to be the main component in the roots in this study and so far was not described to occur in I. neei. However, all PAs found in the roots belonged to the ipanguline/isoipanguline type (Figure 1a). Slight variations in the species‐specific bouquet of PAs have previously been observed (Jenett‐Siems et al., 2005, 1998). In roots of the two individuals of D. quinquefolius, 16 different retronecine‐based PAs (Table 2) were detected. In agreement with Mann (1997), 9‐angeloylretronecine was the main alkaloid. Further prominent compounds that have also been described by Mann (1997) were creatonotine B, and lycopsamine. Interestingly, creatonotine was first isolated and described from adults of the moth Creatonotos transiens Walker, which as larvae received retronecine or ester alkaloids in their diet (Hartmann et al., 1990) and is usually not found in plants itself. The specific bouquet of PAs in the roots of the two individuals is very similar; however, the total amount differs considerably, with individual 2 accumulating only half of the PAs compared to individual 1 (.58 versus 1.28 mg·g−1 dry weight, Table 2).
TABLE 2.
Pyrrolizidine alkaloids from the Distimake quinquefolius individuals
| Alkaloid | RI ZB1MS/TG‐5SILMS | [M]+ | D. quinquefolius | MS data: characteristic ions m/z (% relative abundance) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Ind. 1 | Ind. 2 | ||||||
| root | root | ||||||
| 1 | Supinidine | 1254/1294 | 139 (100) | 6 | 30 | 139(100), 138(15), 122(55), 120(14), 111(21), 110(20), 108(43), 94(10), 80(82), 68(7) | c , d |
| 2 | Retronecine | 1427/1484 | 155 (44) | 52 | 22 | 155(44), 111(85), 110(6), 94(21), 93(8), 82(6), 81(12), 80(100), 68(12), 53(5) | c , e |
| 3 | 7‐Angeloylretronecine | 1784/1829 | 237 (5) | 11 | 22 | 219(8), 137(28), 136(23), 124(32), 111(45), 106(40), 94(23), 83(16), 81(12), 80(100), 55(20) | c , e |
| 4 | 9‐Angeloylretronecine | 1792/1841 | 237(4) | 260 | 207 | 219(2), 154(29), 138(49), 137(59), 136(15), 94(31), 93(100), 83(5), 80(9), 55(10), 53(3) | c , e |
| 5 | 9‐Senecioylretronecine | 1838/1883 | 237 (2) | 8 | 6 | 219(1), 193 (4), 155(19), 154(20), 138(26), 137(38), 136(11), 94(26), 93(100), 83(14), 80(11), 67(3), 55(5) | c , e |
| 6 | Unknown PA | 1851/1895 | ? | tr | tr | 154(19), 138(19), 137(26), 94(25), 93(100), 80(14), 55(15) | — |
| 7 | Isocreatonotine B | 1966/2016 | 269 (6) | 50 | 8 | 251(26), 236(3), 234(3), 154(4), 138(68), 137(30), 136(17), 124(20), 120(29), 111(75), 106(56), 94(27), 93(12), 80(100), 68(13) | c , f |
| 8 | Creatonotine B | 1984/2039 | 269 (2) | 170 | 30 | 225(6), 139(10), 138(100), 137(14), 136(8), 94(23), 93(56), 80(7), 67(3), 57(2), 53(1) | c , f |
| 9 | Retronecine‐9‐ester | 2038/2084 | 297 a (.53) | 35 | 9 | 155(10), 138(31), 137(18), 136(13), 94(21), 93(100), 80(16), 67(6), 53(6) | — |
| 10 | 7‐Acetyl‐creatonotine isomer | 2083/2124 | 311 a (.60) | 54 | 39 | 197(5), 181(11), 180(100), 136(23), 120(42), 119(17), 118(15), 101(16), 94(34), 93(80), 80(20), 67(11), 57(18) | — |
| 11 | Indicine/Intermedine | 2133/2196 | 299 (2) | 210 | 130 | 156(9), 139(37), 138(100), 137(11), 136(10), 120(7), 95(10), 94(35), 93(52), 80(7), 67(5) | c , e |
| 12 | Lycopsamine | 2141/2202 | 299(2) | 119 | 47 | 254(2)n 156(9), 139(33), 138(100), 137(11), 136(9), 120(8), 95(10), 94(36), 93(49), 80(7), 67(4) | c , e |
| 13 | Acetylindicine/Acetylintermedine | 2219/2264 | 341 (.33) | tr | tr | 181(26), 180(100), 152(8), 136(23), 121(23), 120(18), 119(18), 101(14), 94(29), 93(86), 80(29), 67(5) | — |
| 14 | Unknown 220 #1 | 2288/2339 | ? | tr | tr | 237 (30), 220(19), 137(15), 136(100), 120(30), 119(26), 94(35), 93(42), 83(26), 55(39) | — |
| 15 | Unknown 220 #2 | 2352/2372 | ? | tr | tr | 237(21), 221(21), 220(100), 136(90), 120(21), 119(22), 93(20), 83(22), 57(18) | — |
| 16 | Unknown 220 #3 | 2500/2396 | 351 a (.86) | 57 | 13 | 220(100), 136(97), 120(42), 119(30), 94(41), 93(70), 83(70), 55(22) | |
| Total PAs (μg·g −1 dry weight): b | 1029 | 561 | |||||
Note: The columns show the name of the alkaloid, the linear retention index (RI) for TG‐1MS and TG‐5SILMS columns, respectively, the molecular ion [M]+, the abundance, mass spectra of the identified PA, and the spectral references.
Putative [M]+. ion, estimated based on the mass spectrum.
Heliotrine equivalents based on GC‐FID quantification.
In house database of pyrrolizidine alkaloids: MS and linear retention index data.
Kelley RB, Seiber JN. Pyrrolizidine alkaloid chemosystematics in Amsinckia. Phytochemistry. 1992; 31:2369–87.
Witte L, Rubiolo P, Bicchi C, Hartmannt T. Comparative analysis of pyrrolizidine alkaloids from natural sources by gas chromatography–mass spectrometry. Phytochemistry. 1992; 32:187–96.
Hartmann T, Theuring C, Beuerle T, Ernst L, Singer MS, Bernays EA. Acquired and partially de novo synthesized pyrrolizidine alkaloids in two polyphagous arctiids and the alkaloid profiles of their larval food‐plants. J Chem Ecol. 2004; 30:229–54.
We also screened leaves, stems and roots of the hss‐possessing Convolvulaceae species A. nervosa, I. alba, D. tuberosus, and C. umbellata for the presence of PAs. In I. alba, the free necine base (trachelanthimidine and isoretronecanol, in a ratio 20:1) was detected in roots. However, in all other species, neither the free necine base nor PAs were detected.
2.3. The ability of HSS from selected morning glories to produce homospermidine
As many Convolvulaceae possess an HSS ortholog, but do not produce PAs according to our analyses, we decided to test the ability of some of the newly identified HSS orthologs to produce homospermidine in vitro with a newly established non‐radioactive activity assay (Kaltenegger et al., 2021). We chose the HSS from the PA‐free C. umbellata (CuHSS) and the PA‐producing D. quinquefolius individuum 2 (Dq2HSS). Both differ in a previously described specific amino acid motif (“HSS motif”. 277Vxxx281D, Figure 2c, Livshultz et al., 2018; Gill et al., 2018, the numbering follows Kaltenegger et al., 2013), that is shared by many HSS enzymes. They also differ in a third site (266H, Figure 2c), which was calculated to be positively selected in the PA producing Ipomoea species (Kaltenegger et al., 2013), providing an “extended” HSS motif (H‐VxxxD) in the morning glories. We also re‐analyzed the abilities of the previously characterized HSS from D. quinquefolius individuum 1 (Dq1HSS), which possesses the extended HSS motif, and the HSS from I. alba, lacking the motif (IaHSS) (Kaltenegger et al., 2013). All four enzymes were successfully expressed and purified (Figure 3a). Size exclusion chromatography coupled with multi‐angle light scattering (MALS) and an UV detector confirmed the tetrameric state of the purified proteins (Figure 3b), which corresponds to the biological active form of DHS and its homologs (Tanaka et al., 2020; Umland et al., 2004; Wątor et al., 2020). Only CuHSS showed an additional signal to the main peak in borate buffer, which size corresponds to a dimeric state of the enzyme and indicates a misassembly. In the in vitro activity assays, the Dq2HSS (N‐VxxxN), was able to produce low amounts of homospermidine only in glycine buffer, and the specific activity of homospermidine formation was twentyfold lower (163 pkat·mg−1, Table 3) compared to the specific activity of Dq1HSS (H‐VxxxD, 3543 pkat·mg−1). In contrast, the IaHSS, lacking the motif, produced homospermidine in glycine and borate buffer (1544 pkat·mg−1, 46 pkat·mg−1, respectively). The CuHSS (N‐IxxxD) did not produce homospermidine, neither in glycine nor borate buffer.
FIGURE 3.
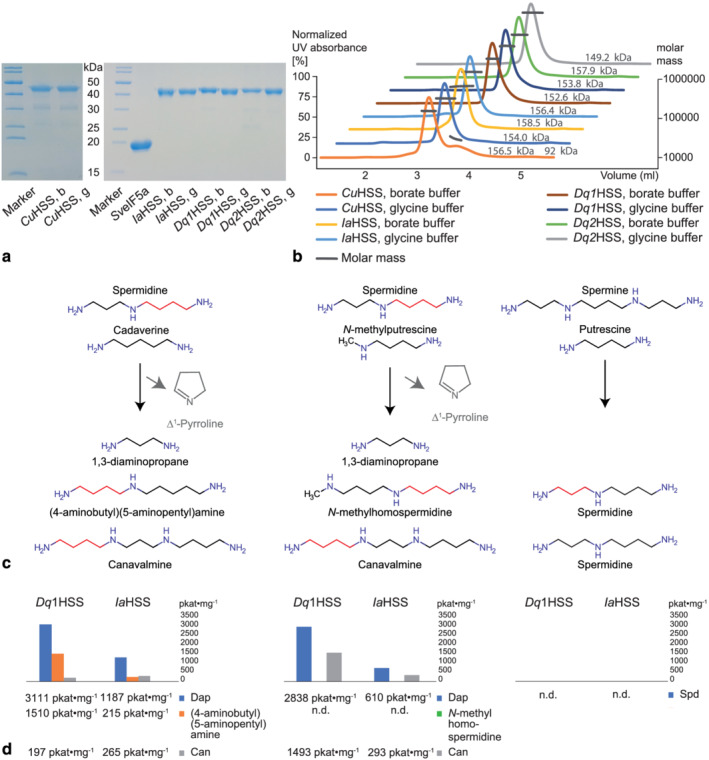
Heterologously expressed and purified HSS enzymes and their biosynthetic activity. (a) A PageBlue stained SDS‐polyacryl gel of the purified and concentrated CuHSS (41.9 kDa), Dq2HSS (42.1 kDa), Dq1HSS (41.3 kDa), IaHSS (41.5 kDa) in borate (b) and glycine (g) buffer and the Senecio vernalis eIF5A precursor protein (18.3 kDa) is shown. (b) SEC‐MALS/UV confirmed the tetrameric state (~150 kDa) of purified HSS enzymes. Only CuHSS in borate buffer shows a second signal of 92 kDa, indicating misassembly. (c) Potential reactions catalyzed by homospermidine synthase (HSS). If the polyamines cadaverine and N‐methylputrescine are accepted as aminobutyl acceptors from the donor spermidine, (4‐aminobutyl)(5aminopentyl)amine and N‐methylhomospermidine, respectively, would be formed with 1,3‐diaminopropane as byproduct. Potential side reactions include the transfer from aminobutyl to spermidine, yielding canavalmine, and the sole spermidine cleavage, yielding 1,3‐diaminopropane and Δ1‐Pyrroline. If spermine can be utilized as aminopropyl donor with putrescine as acceptor, spermidine would be formed. (d) The observed specific activities of Dq1HSS and IaHSS in in vitro activity assays with the substrates depicted in (c). Abbreviations: Can, Canavalmine; Dap, 1,3‐Diaminopropane; Spd, Spermidine
TABLE 3.
Specific activities of HSS encoding cDNA sequences from various Convolvulaceae species
| Species | Specific activity (pkat·mg−1) | PAs | PA type | |
|---|---|---|---|---|
| Glycine buffer | Borate buffer | |||
| Ipomoea neei | 1040* | n.a. | + | Ipangulines |
| Ipomoea alba | 1544 (12%) | 46 (13%) | − | Necine base |
| Distimake quinquefolius ind. 1 | 3543 (3%) | 482 (6%) | + | Lycopsamine |
| D. quinquefolius ind. 2 | 163 (23%) | Not active | + | Lycopsamine |
| Camonea umbellata | Not active | Not active | − | − |
Note: The enzymes ability to produce homospermidine in glycine‐ and borate‐based assay buffer is given. In bracket, relative standard deviation of three assays are given. Specific activities labeled with an asterisk (*) refer to Kaltenegger et al. (2013).
2.4. The HSS motif and its effect on enzyme activity
Although Dq1HSS and IaHSS differ in the HSS motif, they are both able to produce homospermidine. However, the presence of this motif might still be related to functional differences between the HSS. To get a better idea about this, their ability to use cadaverine and N‐methylputrescine as putrescine analogs as well as spermine as spermidine analog was investigated (Table S7). Cadaverine could be used by both enzymes, thereby producing (4‐aminobutyl)(5‐aminopentyl)amine (Figure 3c), but Dq1HSS showed with 1510 pkat·mg−1 higher activity compared to IaHSS (215 pkat·mg−1). Additionally, canavalmine was produced by using spermidine as aminobutyl donor and acceptor. However, 1,3‐diaminopropane increased most prominently in this assay (Dq1HSS 3,111 pkat·mg−1, IaHSS 1,187 pkat·mg−1), indicating that spermidine was cleaved to 1,3‐diaminopropane and the aminobutyl moiety. The latter is assumed to spontaneously form Δ1‐pyrroline, as described for human DHS (Wolff et al., 1990), hence could not be detected by the applied in vitro assay.
Neither Dq1HSS nor IaHSS were able to use N‐methylputrescine as aminobutyl acceptor but utilized spermidine instead. In parallel, spermidine cleavage occurred. Spermine was not utilized as donor of an aminopropyl moiety, although spermine is known to bind in the human DHS similar to spermidine (Wątor et al., 2020). Thus, the presence of the HSS motif did not strongly affect the polyamine substrate spectrum of Dq1HSS and IaHSS. However, when incubated with the eIF5a precursor, only IaHSS was able to use it as an acceptor for the aminobutyl moiety of spermidine thereby producing deoxyhypusine (Figure S2). We next introduced the HSS motif to IaHSS via site‐directed mutagenesis and biochemically characterized the single mutants N266H, I277V, and N281D as well as a triple mutant with a fully introduced HSS motif for their activity with putrescine (HSS activity) as well as the eIF5a precursor. While the homospermidine producing activity was highly similar, the N281D and triple mutant showed no activity with the eIF5a precursor (Figure S2). Thus, the motif could be a diagnostic marker for the loss of the DHS activity.
2.5. The active site between DHS and HSS enzymes is highly conserved
In the course of this study, homology models of DHS and HSS of I. alba, I. neei, D quinquefolius, and C. umbellata were generated based on an available high‐resolution structure from human DHS (Figures 4b–d and S3). Due to its mobility, the N‐terminal part, which forms the so‐called ball‐and‐chain‐motif in human DHS (Umland et al., 2004), was not included. According to these homology models, HSS as well as DHS enzymes from the Convolvulaceae share a highly conserved 3D structure (Figure 4b). This is especially true for the active sites, which are located at the interface of the A‐B and C‐D homodimer and consist of residues from both subunits. All enzymes share the catalytically active Lys329 residue (numbering according to human DHS) (Figure 4c,d and S3), to which the aminobutyl moiety from spermidine is attached to form a transient enzyme‐imine intermediate (Joe et al., 1997). Additionally, the sites that form hydrogen bonds or hydrophobic interactions with spermidine and NAD according to the crystal structure of human DHS (PDB ID 6XXJ, Wątor et al., 2020) are conserved (Figure S1). Overall, in the interface region only very few residues vary between HSS enzymes and their respective DHS ancestors (Figure S3).
FIGURE 4.
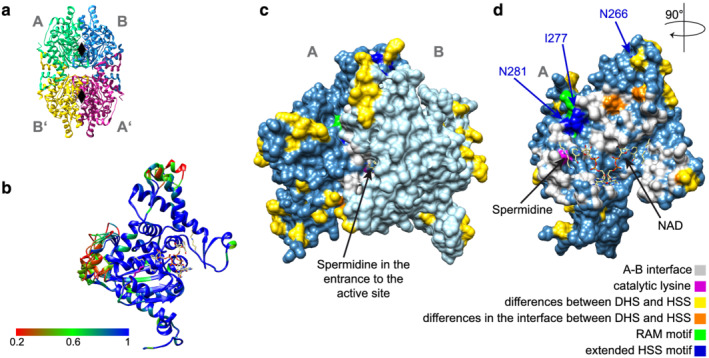
Homology models of homospermidine synthase (HSS) and deoxyhypusine synthase (DHS). Spermidine and nicotinamide adenine dinucleotide (NAD) are shown as sticks, colored by heteroatom. The N‐terminal “ball‐and‐chain” motifs are omitted. (a) Ribbon representation of the human DHS tetramer (PDB: 6XXJ) with black diamonds indicating the general location of the active sites. The tetramer consists of two dimers (A‐B, A′‐B′), which comprise two antiparallel active sites at their interfaces. (b) Overlay of chain A of all DHS and HSS models in ribbon representation from Ipomoeaalba , Ipomoea neei, Distimake quinquefolius individuum 1 and 2, Camonea umbellata , colored by residue identity from red = low identity to blue = high identity. The catalytic lysine is represented as purple stick. Spermidine and NAD from chain A and B are shown. (c,d) Surface representation of the A‐B dimer and the A monomer from the I. alba HSS model, respectively. Residues that differ between the DHS and HSS pair from I. alba are colored in yellow, and in orange, if they are additionally located in the interface of the A‐B dimer. The interface area of chain A is colored in light gray with the catalytic lysine in purple. The location of the extended HSS motif and the conserved RAM motif is highlighted.
The sites of the putative HSS motif (277Vxxx281D) are located in the interface of the A‐B dimer (Figure 4D, IaHSS and Figure S3) in close vicinity to but not as part of the active site. 266H of the extended motif is located in a loop above the interface, which is less conserved and flexible in the models. According to this location, none of the three sites seem to be directly involved in polyamine substrate or co‐factor binding. The amino acids that are flanked by the HSS motif are highly conserved (RAM motif) and buried within the protein.
The electrostatic potential distribution at the entrance to the active‐site tunnel was suggested to shape the interactions with the eIF5a precursor (Umland et al., 2004). In the morning glories, only some of the paralogous HSS/DHS pairs qualitatively differ. Dq1HSS and Dq2HSS are highly similar to their paralogous DHS counterparts but clearly show no activity with the eIF5A precursor (Figure S4). Thus, changes of the polarity might be an important factor, but there are also other, yet not identified, factors.
3. DISCUSSION
Our gene identification and phylogenetic analyses have shown that in many Convolvulaceae species a putative hss gene copy survived. According to our data, this gene originated from a gene duplication event prior to the divergence of Ipomoeeae and the clade that includes Distimake and allied genera. In the genome of parasitic plant C. australis, only a dhs gene copy was identified. However, a massive gene loss has been described for C. australis (Sun et al., 2018). Thus, the hss copy might have been secondarily lost. To exactly date the gene duplication event, further species outside Convolvuloideae have to be analyzed. However, the available data support an ancient duplication event and imply a minimal retention time of the duplicated genes of around 75 to 50 million years based on the diversification of the Convolvuloideae (Eserman et al., 2014). The Ks distances between the paralogous pairs ranged from .2 to .4 (Table S6) and are in agreement with a postulated ancient gene duplication given that Ks distances vary among different gene families (Jiao et al., 2014) and between species (Qiao et al., 2019; Rausher et al., 1999; Wang et al., 2015). Many whole genome duplications that occurred 60 to 50 mya show Ks distances from .4 to 1.2 (Vanneste et al., 2014).
Although we have established a more widespread occurrence of the hss gene copy, we were not able to detect a similarly widespread occurrence of PAs, which agrees with previous studies of PAs in Convolvulaceae (Eich, 2008). In this study, PAs have been found only in roots of D. quinquefolius and I. neei, which are already known to produce PAs (Eich, 2008; Jenett‐Siems et al., 2005; Mann, 1997). In I. alba (Figure 1b), traces of the unesterified necine base were detected in roots. One reason, why we could not detect PAs in further species might be that the individual plants that were investigated in this study lacked some necessary growing conditions or interaction partners during their cultivation. For example, a very specific regulation of PA biosynthesis by infection with rhizobial bacteria and nodulation has been reported in Crotalaria L. (Fabaceae, Irmer et al., 2015). A few studies report that tissue damage induced changes in the PA concentration like in Jacobaea vulgaris Gaertn. (syn. Senecio jacobaea) or Cynoglossum officinale L., but even without damage, high levels of PAs were present in these plants before (van Dam et al., 1993). Another explanation for the absence of PAs in the studied plants might be that not all individuals of one species produce PAs. Variations of the individual bouquet and the amount of PAs were detected in this study for I. neei and the two individuals of D. quinquefolius, but is also well documented for other PA‐producing plants (Macel et al., 2004; Wesseling et al., 2017). These problems could be addressed by the screening of various individuals of one species for the presence of PAs. Sampling from diverse habitats might also be an important factor, especially as many Convolvulaceae have a pantropical distribution. Individuals from different geographic regions might differ in their PA production, as the herbivore community in these various regions will also differ. However, in the majority of PA‐producing plant families, PAs are considered to be part of the constitutive defense system of a plant (Hartmann, 1999). Furthermore, to our knowledge, no other study has reported the presence of PAs in other than the above mentioned Convolvulaceae species (Eich, 2008). Thus, we conclude that the presence of an hss gene copy does not correlate with the ability to produce PAs in the Convolvulaceae.
3.1. Functional role of the hss gene copy
In many extant Convolvuloideae, hss orthologs were identified, but the fate of this duplicated gene might differ. In the PA‐producing species, the duplicated copy diverged from the ancestor gene and gained a different substrate preference, thereby experiencing so‐called neo‐functionalization. Of course, further steps catalyzed by downstream enzymes are necessary to convert the reaction product of the HSS, that is, homospermidine, to generate a PA. While these additional catalytic enzymes are present in the PA‐producing species, PA‐free species simply might lack them. However, this raises the question why hss copies are still present in the PA‐free species, after more than 50 million years? Several scenarios are possible. First, the gene copies are nonfunctional but have not been deleted yet. Becoming a pseudogene is considered to be the predominant fate of duplicates (Lynch & Conery, 2000). The half‐life of duplicated genes varies greatly from two (Lynch & Conery, 2000) to approximately 30 million years and is influenced by many factors such as the mode of duplication, molecular and biological functions, and structural features (Panchy et al., 2016). The pseudogenization of a gene is mainly identified based on disabling mutations that lead to a loss of function but some pseudogenes in rice and Arabidopsis Heynh. have been shown to exhibit no clear signature of pseudogenization and are indeed expressed (Panchy et al., 2016). As for hss orthologs in the Convolvulaceae, two pseudogenes of the hss gene copy have been identified in C. arvensis L. (Kaltenegger et al., 2013) that bore clear marks of non‐functionalization. The enzyme encoded by the hss gene from C. umbellata showed no activity with the tested substrates (Table 3). This gene might be a catalytically inactive paralog hence a pseudogene, but still the gene is present. Possibly, it survived solely by chance, as the presence of the redundant and thus useless gene copy was not deleterious enough for it to be efficiently purged from the genome (Koonin, 2016).
Second, PA biosynthesis might have been present in the ancestors but was secondarily lost in many extant Convolvuloideae species. hss gene copies would then be the remnants of a former functional pathway. I. alba takes an exceptional position, as it accumulates minor amounts of homospermidine and of the necine base but not complete PAs. Only recently, a copper‐containing amine oxidase was identified to catalyze the second step in PA‐biosynthesis in the PA‐producing Heliotropium indicum L. (Zakaria et al., 2022). The reaction product has already the bicyclic ring system, characteristic for the necine base. Possibly, I. alba possesses an unoptimized amine oxidase and a primitive metabolic pathway to produce the necine base but lacks the necessary downstream enzymes and/or substrates to finalize the PA biosynthesis. Such primitive metabolic pathway has also been described for morphinan biosynthesis in Papaver rhoeas L. (Yang et al., 2021). I. alba might be in a phase in which the pathway is evolving—or is in the process of losing the pathway.
A third explanation for the presence of the hss gene copy in PA‐free species is their recruitment for a yet unidentified function. For example, unique indolizidine type alkaloids have been found in I. alba (Gourley et al., 1969). Lolines, which have a backbone structure that is similar to the necine base of PAs (Figure 1d), have been detected in A. mollis (Tofern et al., 1999). Further occurrence of lolines have been described in Adenocarpus DC. (Fabaceae) and grasses (Schardl et al., 2007). In the latter, a fungal endophyte has been shown to produce lolines (Schardl et al., 2007). Whether the lolines in the Adenocarpus and Argyreia species are of plant origin, or derived from endophytic fungi, has not been determined yet (Panaccione et al., 2014).
As a fourth alternative explanation, the structural features of the encoded protein favored their retention. The biologically active unit of HSS and DHS is a homotetramer (Figure 4). After duplication, the paralogs are predicted to interfere functionally and physically at the protein level. This so‐called “paralog interference” is predicted to affect the fate of duplicates (Kaltenegger & Ober, 2015). For example, if degenerative mutations in one copy impact the activity, the gene product can still assemble with the intact subunits, and form less productive oligomers. Thus, degenerative mutations will be selected against, stabilizing both duplicates (Kaltenegger & Ober, 2015). For example, in Trypanosoma brucei DHS activity actually is dependent on heterotetramer formation between two DHS paralogs (Afanador et al., 2018).
In summary, the prediction of the function of a duplicated gene is an extremely difficult task. As we demonstrate here, even if a duplicated gene shows a dedicated function in several species, it might not have the same function (if any) in closely related species.
3.2. Functional meaning of the “HSS motif”
The effects of mutations on protein properties are often unpredictable and mutations located far from binding or active sites can still dramatically affect protein function (Bershtein et al., 2021). The sites of the postulated HSS motif (266H‐277Vxxx281D) are neither located in the four active sites of the tetramer nor at the entrance of the active site tunnel. Still, the presence of this HSS is functionally relevant, as it affects the activity with the eIF5a precursor. Even the single exchange of N281D in IaHSS diminishes the activity with the eIF5A precursor. This agrees with the biochemical characterization of mutated DHS from I. neei, which was rendered to bear the HSS motif (Kaltenegger et al., 2013). Mutants bearing 281D of the HSS motif showed a significantly reduced DHS activity.
Epistasis between sites might be responsible for this phenomenon, as it is described, for example, in terpene synthases, in which many combinations of mutations outside the active site achieved a shift in the biosynthetic activity (OMaille et al., 2008). Furthermore, long‐range contributions between the catalytic sites in heteromeric DHS from Trypanosomei brucei have been described (Afanador et al., 2018). Additionally, the motif might affect not only substrate specificity but also protein stability and dimer formation, as the I277 and D281 are located in the interface of the A‐B dimer (Figure 3b). CuHSS, having an intermediate motif, possibly forms less stable tetramers.
Though being crucial for ancestral activity, the HSS motif does not affect other enzymatic properties like structural stability or chemical activity as the promiscuous side activities are present in Dq1HSS (motif present) as well as in IaHSS wild type and mutant proteins (motif absent and present). Promiscuous functions can serve a starting point of evolution (Aharoni et al., 2005; DePristo, 2007). In case of DHS and its duplicates, loss of ancestral function might be an important prerequisite for acquiring or optimizing a new function in vivo.
Summarizing, the motif might be indicative for the loss of the DHS activity but it not sufficient to predict the new in vivo functions of the gene copy, exemplified by the HSS from D. quinquefolius individual 2, that lacks an HSS motif and shows a reduced HSS activity, but still PAs are produced in this individual.
3.3. Conclusions
HSS catalyzes the first step in PA biosynthesis and has been recruited for this new function after independent duplications of a dhs gene in many PA‐producing families of the angiosperms. Although putative hss gene sequences have been identified that share a common ancestry and that also show high sequence identity in eight morning glory species, PAs have only been found in two species. Thus, to our current knowledge, while a functional HSS is quintessential for PA biosynthesis, the occurrence of an hss‐like gene does not predict the presence of PAs in such species. This might be attributable to the evolutionary complexity in the recruitment of the duplicated hss gene copy into the novel function of PA biosynthesis. The functional relevance of a proposed HSS motif is far from being understood and highlights the mechanistic complexity of protein biochemistry.
4. METHODS
4.1. Plant material
All the species used in this study, the source of the seeds, and the analyzed plant parts are summarized in Table S1. The seeds of the plants were germinated on sterile wet filter paper in glass Petri dishes kept in the dark for 2–3 days at room temperature. The seedlings were cultivated in climate chambers (with 16 h light and 8 ‐h dark cycles; temperature 24°C; relative humidity 50%) for a week and then transferred to the green house in Kiel Botanical Gardens (Kiel University). Voucher specimens of D. quinquefolius individuum 1 and 2, and J. paniculata have been deposited in the Herbarium of Kiel University, accession numbers are requested. Samples for PA analyses were taken in the vegetative growth phase. Morphological characters were used to taxonomically identify the sampled plants, and taxonomic experts confirmed the identifications.
4.2. Total RNA and genomic DNA isolation
Both, genomic DNA and total RNA, were isolated either from freshly harvested plant tissues or from frozen samples stored at −80°C. Genomic DNA was extracted by using the DNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions. Total RNA was isolated by using the RNeasy Plant Mini Kit (Qiagen) or TRIzol reagent (Invitrogen) following the manufacturer's protocols (for further details, see Table S2).
4.3. cDNA synthesis
One microgram of total RNA was used to synthesize complementary DNA (cDNA) with RevertAid Reverse Transcriptase (ThermoFisher) following the manufacturer's protocol with oligo‐dT primer P28 or the oligo‐dT primer P39 (Scotto–Lavino et al., 2007a, 2007b) (for further details, see Table S2). Primer sequences are given in Table S3. All oligonucleotides were synthesized at MWG Eurofins Genomics.
4.4. Amplification of hss and dhs homologs
Various types of PCR techniques were used to amplify the homologous sequences of dhs and hss genes in the selected species. The complete amplification strategy is summarized in Table S2. First, we mined online sequence databases for previously published DHS‐ and HSS‐coding sequences of Convolvulaceae. The sequences were aligned by using MAFFT V7.0 software with default values (Katoh et al., 2002) installed in the Geneious software package (version 11.0.5, Biomatters). Based on the alignment, conserved regions were used to design the degenerate oligonucleotide primers P1‐P4, P16, P27, P29, and P30 (Table S3). These primers were employed to amplify internal fragments of the dhs and hss homologs from cDNA and/or from genomic DNA. The 5′ and 3′ regions that flank the internal fragments were amplified by using rapid amplification of cDNA ends (RACE) PCRs (Scotto–Lavino et al., 2007a, 2007b) with cDNA or by applying inverse PCR (Ochman et al., 1988) and FPNI PCR (Wang et al., 2011) techniques with genomic DNA. The resulting PCR products were purified from the PCR mix (NucleoSpin™ Gel and PCR Clean‐up, Macherey‐Nagel) or extracted after agarose gel electrophoresis (GeneJET™ Gel Extraction Kit, Thermo Scientific) and cloned into the pGEM‐T Easy vector (Promega). Resulting constructs were propagated in Escherichia coli TOP10 (Thermo Scientific) and sent out for Sanger sequencing (MWG Eurofins Genomics). Assembly of the amplified cDNA sequences and assignment of exonic/intronic regions in genomic sequences were performed with Geneious software packages based on previously characterized sequences of I. neei (HSS—HF911505.1; DHS—HF911504.1).
4.5. Site‐directed mutagenesis
The open reading frame of IaHSS, cloned in an expression vector (NovagenTM pET22a, Millipore Sigma, Billerica, MA, USA, Kaltenegger et al., 2013) with an artificial C‐terminal hexahistidine (6xHis) tag extension, was used as template for site‐directed mutagenesis guided by (Liu & Naismith, 2008). Primer pairs to introduce the single mutations N266 to H266 (numbering of the amino acids follows Kaltenegger et al., 2013), V277 to I277, and D281 to N281, are given in Table S3. The triple mutant was produced in a two‐step process. First, the single mutation N266H was introduced. Second, this plasmid was used as template for PCR with a second primer pair, which introduced the mutations V277I and D281N. PCR amplifications were performed in 25 μl reaction volume with Phusion® High‐Fidelity DNA Polymerase (Thermo‐Scientific) according to the manufacturer's instructions. Twelve amplification cycles were performed. The PCR products were treated with DpnI (Thermo‐Scientific) at 37°C for 1 hour, diluted with water (1:10), subsequently propagated in E. coli TOP10 (Thermo‐Scientific), and sent out for Sanger sequencing (MWG Eurofins Genomics) to identify successful mutants.
4.6. Gene tree reconstruction
In total, the identified DHS homologs (Table S4) plus DHS encoding sequences from N. tabacum (accession number AJ242017) and S. lycopersicum L. (syn. L. esculentum) (accession number AF296077.1) were analyzed. The latter two sequences were used as outgroup. Furthermore, the DHS encoding sequence from the genome of C. australis (accession number RAL37146.1) was included. The open reading frames (ORFs) were aligned by using the MAFFT V7.0 (Katoh et al., 2002) algorithm implemented in the Geneious software package (algorithm: G‐INS‐i; scoring matrix 200PAM/k = 2; gap opening penalty = 1.53; offset value = .123) (Kearse et al., 2012). The resulting multiple sequence alignment was evaluated by using the GUIDANCE2 server (Sela et al., 2015) to remove uncertain alignment positions (below default value of .93). The pairwise identity of nucleotides from the alignment was calculated by means of Geneious (Table 1). A maximum likelihood phylogenetic tree was constructed based on the alignment by using PhyML 3.0 (nucleotide substitution model: GTR + GAMMA; Bootstrapping with 1000 replicates, Guindon et al., 2010) installed in the Geneious software package.
4.7. Estimation of the amount of pairwise synonymous substitution (Ks)
Codon based alignment based on translated protein sequences using MAFFT V7.0 (Katoh et al., 2002) was used to estimate the amount of pairwise synonymous substitution by using the maximum likelihood method implemented in Codeml (Yang, 2007) under the basic model F3x4 (Goldman & Yang, 1994) by specifying model = 0, NSsites = 0, and runmode = −2.
4.8. PA extraction from plant tissues
Plant samples were ground to a fine powder by using a laboratory mill (Retsch MM400). 100 μl heliotrine (1 mg/ml) were added to 100 mg dried plant material as an internal standard. PAs were extracted following Kruse et al. (2017). In short, 2ml .05 M sulfuric acid was added to 100 mg ground plant material, and the mixture was incubated at room temperature for 2 h to extract PAs. After centrifugation of the solution, the supernatant was treated with zinc to reduce the PA N‐oxides to the respective tertiary alkaloids at room temperature for 3 h. The reduced PAs were extracted via Sola CX—mixed mode cation exchanger columns (Thermo‐Scientific) following the manufacturer's protocol, except for the replacement of formic acid by .05 M sulfuric acid to ensure maximum compatibility with our sample preparation. PAs were eluted from the columns with 300 μl methanol including 1% ammonia. To concentrate the PAs in the eluent, the methanol was evaporated under a hood at room temperature, and the dry residue was finally dissolved in 50 μl methanol for further analysis by GC–MS and GC‐FID. The necine bases trachelanthimidine and isoretronecanol in I. alba root extracts were identified as trimethylsilyl derivatives using MSTFA as derivatizing reagent according to Hartmann et al. (2005).
4.9. PA analysis by GC and GC–MS
GC–MS analyses were performed with Thermo TRACE GC ULTRA‐DSQ/TRACE GC1310‐TSQ Duo equipped with a TraceGOLD™ TG‐5MS (30 m × .25 mm × .25 μm; ThermoFisher Scientific, Dreieich, Germany) or TG‐1MS capillary columns (30 m × .25 mm × .25 μm; ThermoFisher Scientific, Dreieich, Germany) provided with a 5‐M Safeguard guard column. The instrumental specifications were injection volume of 1 μl and split ratio varying depending on PA concentration of the extracts from splitless (splitless time 1 min) to split 1:10; helium was used as carrier gas with a flow rate of 1 ml min−1(TRACE GC ULTRA‐DSQ) or 1.2 ml min−1(TRACE GC1310‐TSQ); injector and transfer line were set at 280°C and the ion source of the mass spectrometer was operated at 280°C and 70 eV for ionization. The temperature program used was initial 3 min at 100°C, then increasing by 6°C min−1 to 300°C, followed by 10 min at 300°C. The data processing was performed by using Xcalibur™ software (Thermo‐Scientific). The internal standard (heliotrine) and PAs were identified via comparison with in‐house and literature reference data of mass spectra (El‐Shazly & Wink, 2014; Jenett‐Siems et al., 2005, 1998; Johnson, 2014, and retention indices). The linear retention indices were calculated with a reference set of co‐injected hydrocarbons (Sigma‐Aldrich). For quantification of PAs in D. quinquefolius individuals, GC‐FID analyses with TRACE GC1310 equipped with a TG‐1MS capillary column (30 m × .25 mm × .25 μm; ThermoFisher Scientific, Dreieich, Germany) were performed. The GC conditions applied were identical as described for GC–MS. PAs were quantified via their FID signal relating to heliotrine as internal standard. The 50 μg heliotrine were added per 100 mg grinded plant powder. The PA amount was calculated as heliotrine equivalents (μg) per 1 g dry weight of plant material.
4.10. Heterologous expression, purification and activity assays of HSS encoding cDNAs from D. quinquefolius and C. umbellata
The complete ORFs of the HSS encoding cDNAs from D. quinquefolius individuum 1 and 2, and C. umbellata, were cloned into the expression vector pET22b (Novagen), expressed in E. coli BL21(DE3) and purified according to Ober and Hartmann (Ober & Hartmann, 1999a). HSS from I. alba has already been cloned previously (Kaltenegger et al., 2013). Expression constructs were sequenced to ensure correct insertion of the insert. Protein purification was monitored via SDS‐PAGE analysis and protein quantities were estimated based on UV absorption at 280 nm and the specific extinction of the respective protein, calculated with the PROTPARAM web tool on ExPASy (Gasteiger et al., 2005). The oligomerization state was analyzed by SEC‐MALS‐UV. For biochemical characterization, the purified proteins were concentrated and rebuffered to glycine‐based (50 mM glycine‐NaOH buffer, pH 9) and borate‐based (50 mM borate‐NaOH buffer, pH 9) assay buffers. Both assay buffers included the additives 1 mM DTT and .1 mM EDTA. The in vitro assay were performed with a newly developed, non‐radioactive assay according to Kaltenegger et al. (2021).
In short, 5 to 80 μg purified recombinant protein were incubated with the acceptor polyamines putrescine, cadaverine, or N‐methylputrescine together with the donor polyamines spermine or spermidine (400 μM each), in the presence of NAD (2 mM). Product formation was quantified via derivatizing of the reaction mixture with 9‐fluorenylmethyl chloroformate (FMOC, Sigma) and subsequent analyses by HPLC coupled with UV detection. To test DHS activity, enzymes were incubated with the eIF5A precursor protein from Senecio vernalis. To detect deoxyhypusine in the in vitro assay with eIF5A precursor protein, the assays were hydrolyzed as described in (Kaltenegger et al., 2021). LC–MS analyses were performed as described in Kaltenegger et al. (2021) and used to confirm the identity of deoxyhypusine and (4‐aminobutyl)(5‐aminopentyl)amine by their [M + H]+ and [M + NH4]+ ions of: 884 and 901; 840 and 857, respectively.
4.11. Size‐exclusion chromatography coupled to multi‐angle light scattering (SEC‐MALS)
The 20 μl affinity‐purified DHS and HSS samples in glycine or borate buffer with concentrations ranging from 3–7 g·L−1 were separated on an analytical size‐exclusion chromatography (SEC) column (WTC‐015N5, equipped with guard column WTC‐015N5G; Wyatt Technology) equilibrated with PBS (10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4) and connected to an Agilent Technologies 1100 Series HPLC system (DAD G1315B, RID G1362A) and a miniDAWN TREOS multi‐angle light scattering detector (laser beam: 658.9 nm, detectors: 43.6°, 90°, 136.4°; Wyatt Technology). All analyses were run at .4 ml min−1 at room temperature (22°C) for 20 min. Data collection and SEC‐MALS analysis was performed with ASTRA V 5.3.4.10 software (Wyatt Technology).
4.12. Homology modeling of plant DHS and HSS
Homology models of Distimake quinquefolius 1 & 2, I. alba, Ipomoea neei and Camonea umbellata DHS and HSS were predicted with the SWISS‐MODEL webserver (Waterhouse et al., 2018) based on the structure of human DHS (Tanaka et al., 2020) with bound inhibitor GC7 and NAD ligands (PDB: 6P4V) and analyzed and colored with tools from the UCSF Chimera suite (v.1.15) (Pettersen et al., 2004).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
EK devised the study. EK and A‐SP prepared the plant materials for GC–MS analyses. EK and A‐SP identified and analyzed the gene sequences. A‐RGS authenticated the samples' identification and offered taxonomic support to the study in general. TB, EK, and TS analyzed and interpreted the GC–MS data. SSC analyzed and interpreted LC–MS data. CH performed and interpreted SEC‐MALS analyses and homology modeling. EK, A‐SP, A‐RGS, and DO prepared the figures and wrote, discussed, and edited the manuscript. All authors read and approved the final version. The authors also acknowledge the help of a professional editing service.
Supporting information
Table S1. Convolvulaceae species studied, sources of seeds and plant parts used for alkaloid extraction, and GC–MS analysis
Table S2. Experimental approach for amplifying dhs and hss homologs from the various Convolvulaceae species studied. Details of nucleic acid isolation, PCR amplification, and the primers used are given.
Table S3. Sequences of primers
Table S4. Length of DHS‐ and HSS‐encoding sequences of Convolvulaceae species and their accession numbers at Genbank
Table S5. Pyrrolizidine alkaloid profile from I. neei. The columns give details of the alkaloid, the Kovats retention index (RI) analyzed with ZB1MS and TG‐5SILMS columns, the molecular ion [M]+, relative abundance in various tissues according to the peak area in the total ion chromatogram, mass spectra of the identified PA, and the spectral references.
Table S6. Pairwise Ks distances between DHS and HSS sequences (cDNA) from the Convolvulaceae.
Table S7. Specific activities (pkat · mg−1) of DqHSS1 and IaHSS with various polyamine substrates.
Figure S1. Protein alignment of human DHS and DHS and HSS enzymes analyzed in this study. Sites that are predicted to form hydrogen bonds or hydrophobic interactions with spermidine and NAD are highlighted. Also, the catalytic Lys329 is highlighted.
Figure S2. Biosynthetic activity of heterologously expressed and purified IaHSS and mutated IaHSS. A. IaHSS amino acid sequence (264–283) including the sites of the HSS motif is shown and the mutated sites are highlighted. B. The specific activity of wild type IaHSS for the products 1,3‐diaminopropane and homospermidine in the HSS assay was set to 100% and the activities of the mutant enzymes relative to the wild type IaHSS are illustrated. Error bars indicate the relative standard deviation of triplicate assays. C. A PageBlue stained SDS‐polyacryl gel of the purified and concentrated IaHSS single mutants I277V, N281D, N266H, and the triple mutant (266H, I277V, N281D). D. SEC‐ UV confirmed the tetrameric state (~150 kDa) of all mutant IaHSS enzymes compared to the wild type enzyme.
Figure S3. Homology models. Shown are the surface representation of the A‐B dimer and the A monomer from Ipomoea neei DHS & HSS, Ipomoea alba DHS & HSS, Camonea umbellata DHS & HSS, and Distimake quinquefolius individuum 1 and 2, DHS & HSS. Residues that differ between the corresponding DHS and HSS pairs from one species are colored in yellow. If these residues are additionally located in the interface of the A‐B dimer, they are colored in orange. The interface area of chain A is colored in light gray with the catalytic lysine in purple. The location of the extended HSS motif and the conserved RAM motif is indicated. The substrate entrance tunnel is highlighted in slide 3.
Figure S4. Representations of the electrostatic surfaces of the homology models. Shown are the models of the A‐B dimer and the A monomer from Ipomoea neei DHS & HSS, Ipomoea alba DHS & HSS, Camonea umbellata DHS & HSS, and Distimake quinquefolius individuum 1 and 2, DHS & HSS. The substrate tunnel entrance is highlighted in the models of I. neei DHS (slide 2 and slide 12). Positively and negatively charged regions are denoted in blue and red, respectively.
ACKNOWLEDGMENTS
We thank Prof. Tatyana Livshultz for discussions, and we thank Prof. Kirsten Krause for DHS homology analyses of the Cuscuta campestris and C. australis genome. We thank Prof. Eckart Eich for providing seeds of various species of Convolvulaceae. We also thank Prof. Eckart Eich and Prof. George Staples for additional support with taxonomy. We thank Zhangjun Fei for sharing the information of the location of dhs and hss gene in synthenic blocks of I. trifida and I. triloba. We are also grateful to the staff of the Botanical Garden Kiel for their help with the cultivation of plants used in this study. Open Access funding enabled and organized by Projekt DEAL.
Prakashrao, A. S. , Beuerle, T. , Simões, A. R. G. , Hopf, C. , Çiçek, S. S. , Stegemann, T. , Ober, D. , & Kaltenegger, E. (2022). The long road of functional recruitment—The evolution of a gene duplicate to pyrrolizidine alkaloid biosynthesis in the morning glories (Convolvulaceae). Plant Direct, 6(7), e420. 10.1002/pld3.420
Funding information A‐SP was funded by the International Max Planck Research School for Evolutionary Biology, Plön, Germany. Furthermore, A‐SP was also supported by the FAZIT‐STIFTUNG (Gemeinnützige Verlagsgesellschaft mbH).
DATA AVAILABILITY STATEMENT
The DNA sequences obtained in the current study were submitted to GenBank and are available at NCBI (Table S4). The data sets supporting the conclusions of this article are included within the article (and its supporting information).
REFERENCES
- Afanador, G. A. , Tomchick, D. R. , & Phillips, M. A. (2018). Trypanosomatid Deoxyhypusine synthase activity is dependent on shared active‐site complementation between Pseudoenzyme paralogs. Structure, 26(11), 1499, e5–1512. 10.1016/j.str.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni, A. , Gaidukov, L. , Khersonsky, O. , Gould, S. M. , Roodveldt, C. , & Tawfik, D. S. (2005). The “evolvability” of promiscuous protein functions. Nature Genetics, 37(1), 73–76. 10.1038/ng1482 [DOI] [PubMed] [Google Scholar]
- Austin, D. F. (1979). An infrageneric classification for Ipomoea (Convolvulaceae). Taxon, 28(4), 359–361. [Google Scholar]
- Beaulieu, W. T. , Panaccione, D. G. , Quach, Q. N. , Smooth, K. L. , & Clay, K. (2021). Diversification of ergot alkaloids and heritable fungal symbionts in morning glories. Communications Biology, 4(1), 667–678. 10.1038/s42003-021-02870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershtein, S. , Kleiner, D. , & Mishmar, D. (2021). Predicting 3D protein structures in light of evolution. Nature Ecology & Evolution, 5(9), 1–4. 10.1038/s41559-021-01519-8 [DOI] [PubMed] [Google Scholar]
- Böttcher, F. , Ober, D. , & Hartmann, T. (1994). Biosynthesis of pyrrolizidine alkaloids: Putrescine and spermidine are essential substrates of enzymatic homospermidine formation. Canadian Journal of Chemistry, 72, 80–85. 10.1139/v94-013 [DOI] [Google Scholar]
- Chattopadhyay, M. K. , Park, M. H. , & Tabor, H. (2008). Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proceedings of the National Academy of Sciences of the United States of America, 105(18), 6554–6559. 10.1073/pnas.0710970105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G.‐T. , Lu, Y. , Yang, M. , Li, J.‐L. , & Fan, B.‐Y. (2018). Medicinal uses, pharmacology, and phytochemistry of Convolvulaceae plants with central nervous system efficacies: A systematic review. Phytotherapy Research, 32(5), 823–864. 10.1002/ptr.6031 [DOI] [PubMed] [Google Scholar]
- DePristo, M. A. (2007). The subtle benefits of being promiscuous: Adaptive evolution potentiated by enzyme promiscuity. Human Frontier Science Program, 1, 94–98. 10.2976/1.2754665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer, D. L. , Jones, K. C. , & Molyneux, R. J. (1985). Feeding deterrency of some pyrrolizidine, indolizidine, and quinolizidine alkaloids towards pea aphid (Acyrthosiphon pisum) and evidence for phloem transport of indolizidine alkaloid swainsonine. Journal of Chemical Ecology, 11(8), 1045–1051. 10.1007/BF01020674 [DOI] [PubMed] [Google Scholar]
- Eich, E. (2008). Solanaceae and Convolvulaceae: Secondary metabolites. Springer. 10.1007/978-3-540-74541-9 [DOI] [Google Scholar]
- El‐Shazly, A. , & Wink, M. (2014). Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity, 6, 188–282. 10.3390/d6020188 [DOI] [Google Scholar]
- Eserman, L. A. , Tiley, G. P. , Jarret, R. L. , Leebens‐Mack, J. H. , & Miller, R. E. (2014). Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. American Journal of Botany, 101, 92–103. 10.3732/ajb.1300207 [DOI] [PubMed] [Google Scholar]
- Gasteiger, E. , Hoogland, C. , Gattiker, A. , Duvaud, S. , Wilkins, M. R. , Appel, R. D. , & Bairoch, A. (2005). Protein Identification and Analysis Tools on the ExPASy Server. In The proteomics protocols handbook (pp. 571–607). Humana Press. Available at: https://link.springer.com/protocol/10.1385/1-59259-890-0:571 [Google Scholar]
- Gill, G. P. , Bryant, C. J. , Fokin, M. , Huege, J. , Fraser, K. , Jones, C. , Cao, M. , & Faville, M. J. (2018). Low pyrrolizidine alkaloid levels in perennial ryegrass is associated with the absence of a homospermidine synthase gene. BMC Plant Biology, 18, 56. 10.1186/s12870-018-1269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, N. , & Yang, Z. (1994). A codon‐based model of nucleotide substitution for protein‐coding DNA sequences. Molecular Biology and Evolution, 11, 725–736. 10.1093/oxfordjournals.molbev.a040153 [DOI] [PubMed] [Google Scholar]
- Gourley, J. M. , Heacock, R. A. , McInnes, A. G. , Nikolin, B. , & Smith, D. G. (1969). The structure of ipalbine, a new hexahydroindolizine alkaloid, isolated from Ipomoea alba L. Journal of the Chemical Society D, (13), 709–710. 10.1039/c29690000709 [DOI] [Google Scholar]
- Guindon, S. , Dufayard, J.‐F. , Lefort, V. , Anisimova, M. , Hordijk, W. , & Gascuel, O. (2010). New algorithms and methods to estimate maximum‐likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Hartmann, T. (1999). Chemical ecology of pyrrolizidine alkaloids. Planta, 207, 483–495. 10.1007/s004250050508 [DOI] [Google Scholar]
- Hartmann, T. , Biller, A. , Witte, L. , Ernst, L. , & Boppré, M. (1990). Transformation of plant pyrrolizidine alkaloids into novel insect alkaloids by Arctiid moths (Lepidoptera). Biochemical Systematics and Ecology, 18(7–8), 549–554. 10.1016/0305-1978(90)90127-2 [DOI] [Google Scholar]
- Hartmann, T. , & Ober, D. (2008). Defense by Pyrrolizidine Alkaloids: Developed by Plants and Recruited by Insects. In Induced plant resistance to herbivory (pp. 213–231). Springer Science + Business Media B.V. 10.1007/978-1-4020-8182-8_10 [DOI] [Google Scholar]
- Hartmann, T. , Theuring, C. , Beuerle, T. , Bernays, E. A. , & Singer, M. S. (2005). Acquisition, transformation and maintenance of plant pyrrolizidine alkaloids by the polyphagous arctiid Grammia geneura . Insect Biochemistry and Molecular Biology, 35, 1083–1099. 10.1016/j.ibmb.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Hartmann, T. , & Witte, L. (1995). Chemistry, Biology and Chemoecology of the Pyrrolizidine Alkaloids. In Alkaloids: Chemical and biological perspectives (pp. 155–233). Elsevier. Available at: https://linkinghub.elsevier.com/retrieve/pii/B9780080420899500115 Accessed January 5, 2021 [Google Scholar]
- Irmer, S. , Podzun, N. , Langel, D. , Heidemann, F. , Kaltenegger, E. , Schemmerling, B. , Geilfus, C.‐M. , Zörb, C. , & Ober, D. (2015). New aspect of plant‐rhizobia interaction: Alkaloid biosynthesis in Crotalaria depends on nodulation. Proceedings. National Academy of Sciences. United States of America, 112, 4164–4169. 10.1073/pnas.1423457112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett‐Siems, K. , Kaloga, M. , & Eich, E. (1993). Ipangulines, the first pyrrolizidine alkaloids from the Convolvulaceae. Phytochemistry, 34(2), 437–440. 10.1016/0031-9422(93)80025-N [DOI] [Google Scholar]
- Jenett‐Siems, K. , Ott, S. C. , Schimming, T. , Siems, K. , Müller, F. , Hilker, M. , Witte, L. , Hartmann, T. , Austin, D. F. , & Eich, E. (2005). Ipangulines and minalobines, chemotaxonomic markers of the infrageneric Ipomoea taxon subgenus Quamoclit, section Mina. Phytochemistry, 66, 223–231. 10.1016/j.phytochem.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Jenett‐Siems, K. , Schimming, T. , Kaloga, M. , Eich, E. , Siems, K. , Gupta, M. P. , Witte, L. , & Hartmann, T. (1998). Pyrrolizidine alkaloids of Ipomoea hederifolia and related species. Phytochemistry, 47(8), 1551–1560. 10.1016/S0031-9422(97)01082-0 [DOI] [Google Scholar]
- Jiao, Y. , Li, J. , Tang, H. , & Paterson, A. H. (2014). Integrated Syntenic and Phylogenomic analyses reveal an ancient genome duplication in monocots. Plant Cell, 26(7), 2792–2802. 10.1105/tpc.114.127597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe, Y. A. , Wolff, E. C. , Lee, Y. B. , & Park, M. H. (1997). Enzyme‐substrate intermediate at a specific lysine residue is required for Deoxyhypusine synthesis. The Journal of Biological Chemistry, 272, 32679–32685. 10.1074/jbc.272.51.32679 [DOI] [PubMed] [Google Scholar]
- Johnson, S.G. (2014) NIST Standard Reference Database 1A v17. NIST, Available at: https://www.nist.gov/srd/nist-standard-reference-database-1a-v17
- Kaltenegger, E. , Eich, E. , & Ober, D. (2013). Evolution of Homospermidine synthase in the Convolvulaceae: A story of gene duplication, gene loss, and periods of various selection pressures. Plant Cell, 25(4), 1213–1227. 10.1105/tpc.113.109744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenegger, E. , & Ober, D. (2015). Paralogue interference affects the dynamics after gene duplication. Trends in Plant Science, 20, 814–821. 10.1016/j.tplants.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Kaltenegger, E. , Prakashrao, A. S. , Çiçek, S. S. , & Ober, D. (2021). Development of an activity assay for characterizing deoxyhypusine synthase and its diverse reaction products. FEBS Open Bio, 11, 10–25. 10.1002/2211-5463.13046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltner, F. , Rychlik, M. , Gareis, M. , & Gottschalk, C. (2018). Influence of storage on the stability of toxic pyrrolizidine alkaloids and their N‐oxides in peppermint tea, Hay, and honey. Journal of Agricultural and Food Chemistry, 66, 5221–5228. 10.1021/acs.jafc.7b06036 [DOI] [PubMed] [Google Scholar]
- Katoh, K. , Misawa, K. , Kuma, K. , & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , Buxton, S. , Cooper, A. , Markowitz, S. , Duran, C. , Thierer, T. , Ashton, B. , Meintjes, P. , & Drummond, A. (2012). Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin, E. V. (2016). Splendor and misery of adaptation, or the importance of neutral null for understanding evolution. BMC Biology, 14, 114. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5180405/; 10.1186/s12915-016-0338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, L. H. , Stegemann, T. , Sievert, C. , & Ober, D. (2017). Identification of a second site of pyrrolizidine alkaloid biosynthesis in comfrey to boost plant defense in floral stage. Plant Physiology, 174, 47–55. 10.1104/pp.17.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langel, D. , Ober, D. , & Pelser, P. B. (2011). The evolution of pyrrolizidine alkaloid biosynthesis and diversity in the Senecioneae. Phytochemistry Reviews, 10, 3–74. 10.1007/s11101-010-9184-y [DOI] [Google Scholar]
- Leistner, E. , & Steiner, U. (2018). The Genus Periglandula and Its Symbiotum with Morning Glory Plants (Convolvulaceae). In Anke T. & Schüffler A. (Eds.), Physiology and genetics: Selected basic and applied aspects (pp. 131–147. Available at). Springer International Publishing. 10.1007/978-3-319-71740-1_5 [DOI] [Google Scholar]
- Liu, H. , & Naismith, J. H. (2008). An efficient one‐step site‐directed deletion, insertion, single and multiple‐site plasmid mutagenesis protocol. BMC Biotechnology, 8(1), 91. 10.1186/1472-6750-8-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshultz, T. , Kaltenegger, E. , Straub, S. C. K. , Weitemier, K. , Hirsch, E. , Khrystyna, K. , Lumi, M. , & Aaron, L. (2018). Evolution of pyrrolizidine alkaloid biosynthesis in Apocynaceae: Revisiting the defence de‐escalation hypothesis. New Phytologist, 218, 762–773. 10.1111/nph.15061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, M. , & Conery, J. S. (2000). The evolutionary fate and consequences of duplicate genes. Science, 290(5494), 1151–1155. 10.1126/science.290.5494.1151 [DOI] [PubMed] [Google Scholar]
- Macel, M. , Vrieling, K. , & Klinkhamer, P. G. L. (2004). Variation in pyrrolizidine alkaloid patterns of Senecio jacobaea . Phytochemistry, 65(7), 865–873. 10.1016/j.phytochem.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Mann, P. (1997). Zur Phytochemie und Chemotaxonomie tropischer und mediterraner Convolvulaceen unter besonderer Berücksichtigung des Alkaloid‐Vorkommens. Freie Universität Berlin. [Google Scholar]
- Muñoz‐Rodríguez, P. , Carruthers, T. , Wood, J. R. I. , Williams, B. R. M. , Weitemier, K. , Kronmiller, B. , Goodwin, Z. , Sumadijaya, A. , Anglin, N. L. , Filer, D. , Harris, D. , Rausher, M. D. , Kelly, S. , Liston, A. , & Scotland, R. W. (2019). A taxonomic monograph of Ipomoea integrated across phylogenetic scales. Nature Plants, 5, 1136–1144. 10.1038/s41477-019-0535-4 [DOI] [PubMed] [Google Scholar]
- Ober, D. , & Hartmann, T. (1999a). Deoxyhypusine synthase from tobacco cDNA isolation, characterization, and bacterial expression of an enzyme with extended substrate specificity. The Journal of Biological Chemistry, 274, 32040–32047. 10.1074/jbc.274.45.32040 [DOI] [PubMed] [Google Scholar]
- Ober, D. , & Hartmann, T. (1999b). Homospermidine synthase, the first pathway‐specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proceedings. National Academy of Sciences. United States of America, 96, 14777–14782. 10.1073/pnas.96.26.14777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober, D. , Gibas, L. , Witte, L. , & Hartmann, T. (2003). Evidence for general occurrence of homospermidine in plants and its supposed origin as by‐product of deoxyhypusine synthase. Phytochemistry, 62, 339–344. 10.1016/S0031-9422(02)00553-8 [DOI] [PubMed] [Google Scholar]
- Ober, D. , Harms, R. , Witte, L. , & Hartmann, T. (2003). Molecular evolution by change of function. The Journal of Biological Chemistry, 278, 12805–12812. 10.1074/jbc.M207112200 [DOI] [PubMed] [Google Scholar]
- Ochman, H. , Gerber, A. S. , & Hartl, D. L. (1988). Genetic applications of an inverse polymerase chain reaction. Genetics, 120(3), 621–623. 10.1093/genetics/120.3.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMaille, P. E. , Malone, A. , Dellas, N. , O'Maille, P. E. , Andes Hess, B. Jr. , Smentek, L. , Sheehan, I. , Greenhagen, B. T. , Chappell, J. , Manning, G. , & Noel, J. P. (2008). Quantitative exploration of the catalytic landscape separating divergent plant sesquiterpene synthases. Nature Chemical Biology, 4(10), 617–623. 10.1038/nchembio.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaccione, D. G. , Beaulieu, W. T. , & Cook, D. (2014). Bioactive alkaloids in vertically transmitted fungal endophytes. Functional Ecology, 28, 299–314. 10.1111/1365-2435.12076 [DOI] [Google Scholar]
- Panchy, N. , Lehti‐Shiu, M. , & Shiu, S.‐H. (2016). Evolution of gene duplication in plants. Plant Physiology, 171, 2294–2316. 10.1104/pp.16.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.‐H. , Wolff, E. C. , Folk, J. E. , & Park, M. H. (2003). Reversal of the Deoxyhypusine synthesis reaction. The Journal of Biological Chemistry, 278, 32683–32691. 10.1074/jbc.M304247200 [DOI] [PubMed] [Google Scholar]
- Paulke, A. , Kremer, C. , Wunder, C. , Achenbach, J. , Djahanschiri, B. , Elias, A. , Stefan Schwed, J. , Hübner, H. , Gmeiner, P. , Proschak, E. , Toennes, S. W. , & Stark, H. (2013). Argyreia nervosa (Burm. F.): Receptor profiling of lysergic acid amide and other potential psychedelic LSD‐like compounds by computational and binding assay approaches. Journal of Ethnopharmacology, 148, 492–497. 10.1016/j.jep.2013.04.044 [DOI] [PubMed] [Google Scholar]
- Pettersen, E. F. , Goddard, T. D. , Huang, C. C. , Couch, G. S. , Greenblatt, D. M. , Meng, E. C. , & Ferrin, T. E. (2004). UCSF chimera—A visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25(13), 1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Qiao, X. , Li, Q. , Yin, H. , Qi, K. , Li, L. , Wang, R. , Zhang, S. , & Paterson, A. H. (2019). Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biology, 20(1), 38. 10.1186/s13059-019-1650-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausher, M. D. , Miller, R. E. , & Tiffin, P. (1999). Patterns of evolutionary rate variation among genes of the anthocyanin biosynthetic pathway. Molecular Biology and Evolution, 16, 266–274. 10.1093/oxfordjournals.molbev.a026108 [DOI] [PubMed] [Google Scholar]
- Reimann, A. , Nurhayati, N. , Backenköhler, A. , & Ober, D. (2004). Repeated evolution of the pyrrolizidine alkaloid–mediated defense system in separate angiosperm lineages. Plant Cell, 16(10), 2772–2784. 10.1105/tpc.104.023176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard, A. , Janke, M. , von der Ohe, W. , Kempf, M. , Theuring, C. , Hartmann, T. , Schreier, P. , & Beuerle, T. (2009). Feeding deterrence and detrimental effects of pyrrolizidine alkaloids fed to honey bees (Apis mellifera). Journal of Chemical Ecology, 35(9), 1086–1095. 10.1007/s10886-009-9690-9 [DOI] [PubMed] [Google Scholar]
- Schardl, C. L. , Grossman, R. B. , Nagabhyru, P. , Faulkner, J. R. , & Mallik, U. P. (2007). Loline alkaloids: Currencies of mutualism. Phytochemistry, 68(7), 980–996. 10.1016/j.phytochem.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Scotto–Lavino, E. , Du, G. , & Frohman, M. A. (2007a). 3′ end cDNA amplification using classic RACE. Nature Protocols, 1(6), 2742–2745. 10.1038/nprot.2006.481 [DOI] [PubMed] [Google Scholar]
- Scotto–Lavino, E. , Du, G. , & Frohman, M. A. (2007b). 5′ end cDNA amplification using classic RACE. Nature Protocols, 1(6), 2555–2562. 10.1038/nprot.2006.480 [DOI] [PubMed] [Google Scholar]
- Sela, I. , Ashkenazy, H. , Katoh, K. , & Pupko, T. (2015). GUIDANCE2: Accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Research, 43, W7–W14. 10.1093/nar/gkv318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões, A. R. , Culham, A. , & Carine, M. (2015). Resolving the unresolved tribe: A molecular phylogenetic framework for the Merremieae (Convolvulaceae). Botanical Journal of the Linnean Society, 179, 374–387. 10.1111/boj.12339 [DOI] [Google Scholar]
- Simões, A. R. , & Staples, G. (2017). Dissolution of Convolvulaceae tribe Merremieae and a new classification of the constituent genera. Botanical Journal of the Linnean Society, 183(4), 561–586. 10.1093/botlinnean/box007 [DOI] [Google Scholar]
- Stefanović, S. , Austin, D. F. , & Olmstead, R. G. (2003). Classification of Convolvulaceae: A phylogenetic approach. Systematic Botany, 28, 791–806. [Google Scholar]
- Stefanovic, S. , Krueger, L. , & Olmstead, R. G. (2002). Monophyly of the Convolvulaceae and circumscription of their major lineages based on DNA sequences of multiple chloroplast loci. American Journal of Botany, 89(9), 1510–1522. 10.3732/ajb.89.9.1510 [DOI] [PubMed] [Google Scholar]
- Sun, G. , Xu, Y. , Liu, H. , Sun, T. , Zhang, J. , Hettenhausen, C. , Shen, G. , Qi, J. , Qin, Y. , Li, J. , Wang, L. , Chang, W. , Guo, Z. , Baldwin, I. T. , & Wu, J. (2018). Large‐scale gene losses underlie the genome evolution of parasitic plant Cuscuta australis. Nature Communications, 9(1), 2683. 10.1038/s41467-018-04721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y. , Kurasawa, O. , Yokota, A. , Klein, M. G. , Ono, K. , Saito, B. , Matsumoto, S. , Okaniwa, M. , Ambrus‐Aikelin, G. , Morishita, D. , Kitazawa, S. , Uchiyama, N. , Ogawa, K. , Kimura, H. , & Imamura, S. (2020). Discovery of novel allosteric inhibitors of Deoxyhypusine synthase. Journal of Medicinal Chemistry Available at, 63, 3215–3226. 10.1021/acs.jmedchem.9b01979 [DOI] [PubMed] [Google Scholar]
- Tofern, B. (1999). Neue und seltene Sekundärstoffe des Phenylpropan‐, Terpen‐ und Alkaloid‐Stoffwechsels aus tropischen Convolvulaceae. Freie Universität Berlin. [Google Scholar]
- Tofern, B. , Kaloga, M. , Witte, L. , Hartmann, T. , & Eich, E. (1999). Occurrence of loline alkaloids in Argyreia mollis (Convolvulaceae). Phytochemistry, 51(8), 1177–1180. 10.1016/S0031-9422(99)00121-1 [DOI] [Google Scholar]
- Umland, T. C. , Wolff, E. C. , Park, M. H. , & Davies, D. R. (2004). A new crystal structure of Deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme·NAD·inhibitor ternary complex. The Journal of Biological Chemistry, 279, 28697–28705. 10.1074/jbc.M404095200 [DOI] [PubMed] [Google Scholar]
- van Dam, N. M. , van der Meijden, E. , & Verpoorte, R. (1993). Induced responses in three alkaloid‐containing plant species. Oecologia, 95(3), 425–430. 10.1007/BF00320998 [DOI] [PubMed] [Google Scholar]
- Vanneste, K. , Baele, G. , Maere, S. , & Peer, Y. V. d. (2014). Analysis of 41 plant genomes supports a wave of successful genome duplications in association with the cretaceous–Paleogene boundary. Genome Research, 24(8), 1334–1347. 10.1101/gr.168997.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Wang, J. , Jin, D. , Guo, H. , Lee, T.‐H. , Liu, T. , & Paterson, A. H. (2015). Genome alignment spanning major Poaceae lineages reveals heterogeneous evolutionary rates and alters inferred dates for key evolutionary events. Molecular Plant, 8(6), 885–898. 10.1016/j.molp.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Wang, Z. , Ye, S. , Li, J. , Zheng, B. , Bao, M. , & Ning, G. (2011). Fusion primer and nested integrated PCR (FPNI‐PCR): A new high‐efficiency strategy for rapid chromosome walking or flanking sequence cloning. BMC Biotechnology, 11, 109. 10.1186/1472-6750-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, A. , Bertoni, M. , Bienert, S. , Studer, G. , Tauriello, G. , Gumienny, R. , Heer, F. T. , de Beer, T. A. P. , Rempfer, C. , Bordoli, L. , Lepore, R. , & Schwede, T. (2018). SWISS‐MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Research, 46(W1), W296–W303. 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wątor, E. , Wilk, P. , & Grudnik, P. (2020). Half way to Hypusine—Structural basis for substrate recognition by human Deoxyhypusine synthase. Biomolecules, 10(4), 522. 10.3390/biom10040522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WCVP . (2021) World Checklist of Vascular Plants, version 2.0. Facilitated by the Royal Botanic Gardens, Kew. Available at: http://wcvp.science.kew.org/
- Wesseling, A.‐M. , Demetrowitsch, T. J. , Schwarz, K. , & Ober, D. (2017). Variability of pyrrolizidine alkaloid occurrence in species of the grass subfamily Pooideae (Poaceae). Frontiers in Plant Science, 8, 2046. 10.3389/fpls.2017.02046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenfeld, H. , & Edgar, J. (2011). Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochemistry Reviews, 10, 137–151. 10.1007/s11101-010-9174-0 [DOI] [Google Scholar]
- Wolff, E. C. , Folk, J. E. , & Park, M. H. (1997). Enzyme‐substrate intermediate formation at lysine 329 of human Deoxyhypusine synthase. The Journal of Biological Chemistry, 272, 15865–15871. 10.1074/jbc.272.25.15865 [DOI] [PubMed] [Google Scholar]
- Wolff, E. C. , Park, M. H. , & Folk, J. E. (1990). Cleavage of spermidine as the first step in deoxyhypusine synthesis. The Role of NAD. Journal of Biological Chemistry, 265, 4793–4799. 10.1016/S0021-9258(19)34042-6 [DOI] [PubMed] [Google Scholar]
- Yang, X. , Gao, S. , Guo, L. , Wang, B. , Jia, Y. , Zhou, J. , Che, Y. , Jia, P. , Lin, J. , Xu, T. , Sun, J. , & Ye, K. (2021). Three chromosome‐scale Papaver genomes reveal punctuated patchwork evolution of the morphinan and noscapine biosynthesis pathway. Nature Communications, 12, 6030. 10.1038/s41467-021-26330-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. (2007). PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24(8), 1586–1591. 10.1093/molbev/msm088 [DOI] [PubMed] [Google Scholar]
- Zakaria, M. M. , Schemmerling, B. , & Ober, D. (2021). CRISPR/Cas9‐mediated genome editing in comfrey (Symphytum officinale) hairy roots results in the complete eradication of pyrrolizidine alkaloids. Molecules, 26(6), 1498. 10.3390/molecules26061498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria, M. M. , Stegemann, T. , Sievert, C. , Kruse, L. H. , Kaltenegger, E. , Girreser, U. , Çiçek, S. S. , Nimtz, M. , & Ober, D. (2022). Insights into polyamine metabolism: Homospermidine is double‐oxidized in two discrete steps by a single copper‐containing amine oxidase in pyrrolizidine alkaloid biosynthesis. The Plant Cell, 34(6), 2364–2382. 10.1093/plcell/koac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Convolvulaceae species studied, sources of seeds and plant parts used for alkaloid extraction, and GC–MS analysis
Table S2. Experimental approach for amplifying dhs and hss homologs from the various Convolvulaceae species studied. Details of nucleic acid isolation, PCR amplification, and the primers used are given.
Table S3. Sequences of primers
Table S4. Length of DHS‐ and HSS‐encoding sequences of Convolvulaceae species and their accession numbers at Genbank
Table S5. Pyrrolizidine alkaloid profile from I. neei. The columns give details of the alkaloid, the Kovats retention index (RI) analyzed with ZB1MS and TG‐5SILMS columns, the molecular ion [M]+, relative abundance in various tissues according to the peak area in the total ion chromatogram, mass spectra of the identified PA, and the spectral references.
Table S6. Pairwise Ks distances between DHS and HSS sequences (cDNA) from the Convolvulaceae.
Table S7. Specific activities (pkat · mg−1) of DqHSS1 and IaHSS with various polyamine substrates.
Figure S1. Protein alignment of human DHS and DHS and HSS enzymes analyzed in this study. Sites that are predicted to form hydrogen bonds or hydrophobic interactions with spermidine and NAD are highlighted. Also, the catalytic Lys329 is highlighted.
Figure S2. Biosynthetic activity of heterologously expressed and purified IaHSS and mutated IaHSS. A. IaHSS amino acid sequence (264–283) including the sites of the HSS motif is shown and the mutated sites are highlighted. B. The specific activity of wild type IaHSS for the products 1,3‐diaminopropane and homospermidine in the HSS assay was set to 100% and the activities of the mutant enzymes relative to the wild type IaHSS are illustrated. Error bars indicate the relative standard deviation of triplicate assays. C. A PageBlue stained SDS‐polyacryl gel of the purified and concentrated IaHSS single mutants I277V, N281D, N266H, and the triple mutant (266H, I277V, N281D). D. SEC‐ UV confirmed the tetrameric state (~150 kDa) of all mutant IaHSS enzymes compared to the wild type enzyme.
Figure S3. Homology models. Shown are the surface representation of the A‐B dimer and the A monomer from Ipomoea neei DHS & HSS, Ipomoea alba DHS & HSS, Camonea umbellata DHS & HSS, and Distimake quinquefolius individuum 1 and 2, DHS & HSS. Residues that differ between the corresponding DHS and HSS pairs from one species are colored in yellow. If these residues are additionally located in the interface of the A‐B dimer, they are colored in orange. The interface area of chain A is colored in light gray with the catalytic lysine in purple. The location of the extended HSS motif and the conserved RAM motif is indicated. The substrate entrance tunnel is highlighted in slide 3.
Figure S4. Representations of the electrostatic surfaces of the homology models. Shown are the models of the A‐B dimer and the A monomer from Ipomoea neei DHS & HSS, Ipomoea alba DHS & HSS, Camonea umbellata DHS & HSS, and Distimake quinquefolius individuum 1 and 2, DHS & HSS. The substrate tunnel entrance is highlighted in the models of I. neei DHS (slide 2 and slide 12). Positively and negatively charged regions are denoted in blue and red, respectively.
Data Availability Statement
The DNA sequences obtained in the current study were submitted to GenBank and are available at NCBI (Table S4). The data sets supporting the conclusions of this article are included within the article (and its supporting information).


