Abstract
Osteoarthritis (OA) is the most common joint disease characterized by degeneration of articular cartilage and causes severe joint pain, physical disability, and impaired quality of life. Recently, it was found that mitochondria not only act as a powerhouse of cells that provide energy for cellular metabolism, but are also involved in crucial pathways responsible for maintaining chondrocyte physiology. Therefore, a growing amount of evidence emphasizes that impairment of mitochondrial function is associated with OA pathogenesis; however, the exact mechanism is not well known. Moreover, the AMP-activated protein kinase (AMPK)–Sirtuin (SIRT) signaling pathway, long non-coding RNA (lncRNA), and microRNA (miRNA) are important for regulating the physiological and pathological processes of chondrocytes, indicating that these may be targets for OA treatment. In this review, we first focus on the importance of mitochondria metabolic dysregulation related to OA. Then, we show recent evidence on the AMPK-SIRT mediated pathway associated with OA pathogenesis and potential treatment options. Finally, we discuss current research into the effects of lncRNA and miRNA on OA progression or inhibition.
Keywords: osteoarthritis, mitochondria, AMP-activated protein kinase (AMPK), sirtuins (SIRT), long non-coding RNA (lncRNA), microRNA (miRNA)
1. Introduction
Osteoarthritis (OA) is a degenerative and debilitating joint disease associated with the symptoms and signs of joint pain, swelling, stiffness, mobility limitation, and joint deformity [1]. According to data from the United States Centers for Disease Control and Prevention in 2015, OA affected 54.4 million adults in the U.S., and the number is expected to reach 78.4 million by 2040, which indicates the large and growing problems for clinical and public health systems [2,3]. OA is a complicated disease with multifactorial etiologies, including aging, obesity, genetic predisposition, and mechanical overloading [4]. Cartilage degeneration, chondrocyte senescence and apoptosis, degradation of the extracellular matrix, inflammation of the synovium, and subchondral bone dysfunction are the core pathological changes [4,5,6,7]. Clinical symptoms and radiographic changes include chronic pain, joint instability, tenderness, stiffness, joint deformities, thinning of the cartilage, subchondral bone sclerosis, and radiographic joint space narrowing [8]. At present, there are no effective interventions or disease-modifying treatments for osteoarthritis, and pharmacological treatments are confined to symptom relief and function restoration rather than stopping the progression of the disease. The only end-point treatment for advanced-stage OA is joint replacement surgery [9,10]. We still need a better understanding of the molecular mechanisms related to OA pathogenesis in order to design potential molecular targets for prevention and treatment.
Mitochondria are double-membrane organelles that convert organic molecules (e.g., glucose, amino acids, and fatty acids) into adenosine triphosphate (ATP) via the electron transport chain (ETC) and oxidative phosphorylation [11,12]. Hence, mitochondria are traditionally described as a cell’s power factory. In addition, articular chondrocytes have been regarded as highly glycolytic cells in past studies [13,14]. However, recent studies have found that aerobic respiration seems to be an important energy source in articular chondrocytes, accounting for approximately 25% of their ATP production [15,16]. Consequently, mitochondrial aerobic respiration is one of the essential energy-producing pathways in articular chondrocytes. Besides energy production, mitochondria also play important roles in many cellular functions, including cell differentiation, senescence, and cell death. Recent investigations demonstrated that the alteration of mitochondrial function is associated with OA pathogenesis [16,17,18]. In addition, more and more evidence suggests that mitochondrial dysfunction is broadly involved in multiple molecular mechanisms of OA progression, including excess oxidative stress, energetic metabolism change, respiratory chain dysfunction, cell senescence, autophagy and apoptosis, and calcium homeostasis [19,20].
In addition, recent studies have shown that the AMP-activated protein kinase (AMPK) and sirtuin (SIRT) pathways are important for signal transduction to regulate mitochondrial physiological function. They are also related to the normal metabolism of chondrocytes, including extracellular matrix (ECM) production, cytoskeleton rearrangement, autophagy, and apoptosis, which are the core pathogenesis of OA. Furthermore, increasing evidence shows that long non-coding RNA (lncRNA) and microRNA (miRNA) are closely related to it. LncRNA and miRNA regulate the expression levels of downstream genes and transmit the biological information to the target cells. Each has different regulatory effects on ECM degradation, chondrocyte apoptosis and proliferation, inflammatory responses, and autophagy activity, which indicates their great potential as treatment options for OA.
Therefore, in this review, we first discuss how mitochondrial metabolism dysfunction can lead to OA pathogenesis. Then, we unravel the AMPK-SIRT pathway, lncRNA, and microRNA in association with OA pathogenesis, and discuss potential therapeutic strategies.
2. Mitochondrial Metabolism Dysfunction to OA Pathogenesis
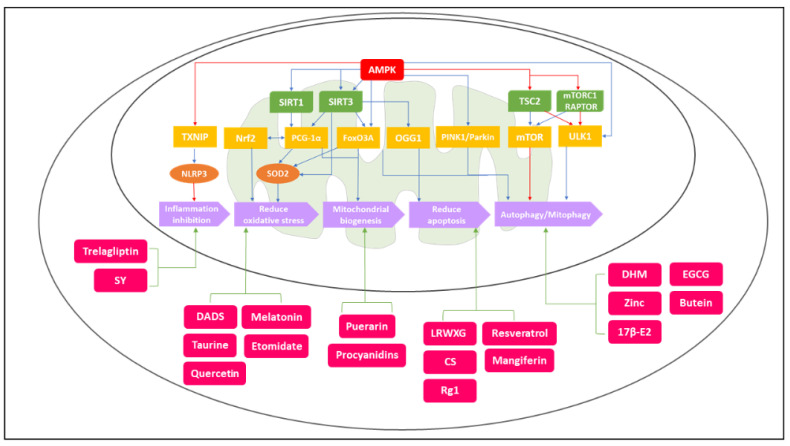
Mitochondria are known to be dynamic and complex organelles responsible for numerous physiological processes in chondrocytes related to energy production and cell growth and proliferation. Recent studies have also reported that mitochondrial metabolism dysfunction could affect several pathways, causing the generation of reactive oxygen species (ROS), inflammation regulation, chondrocyte senescence, matrix catabolism, apoptosis induction, and calcium homeostasis [15,21,22,23]. Consequently, alterations in mitochondrial metabolic function are associated with OA pathological processes as mentioned above. Furthermore, recent investigations showed that AMPK-SIRT functions as important biosensors that regulate a variety of physiological processes via targeting downstream regulators, as shown in Figure 1. These downstream regulatory factors then lead to different metabolic responses, including inflammation inhibition, oxidative stress reduction, mitochondrial biogenesis, apoptosis prohibition, autophagy, and mitophagy [24,25,26]. All of these metabolic responses regulated by AMPK-SIRT pathways are closely related to physiological mitochondrial functions. Therefore, the imbalance or disruption of these important signaling pathways may further lead to mitochondrial dysfunction, and eventually, evolve into OA.
Figure 1.
The diagram shows the AMPK–SIRT signaling pathways related to OA pathogenesis and the potential treatment options for OA targeting at mitochondrial pathways. The outer circle is the cell membrane, and the inner circle is the mitochondrial outer membrane. The red, green, yellow, and orange blocks represent the regulatory molecules and the purple blocks represent the final metabolic responses in the AMPK–SIRT signaling pathways. The light-red blocks represent potential treatment drugs. The blue arrow means activation, and the red arrow means inhibition in the signaling pathways. The activation of AMPK–SIRT1/SIRT3–PCG–1α/FoxO3A, AMPK–SIRT3–OGG1, AMPK–PINK1/Parkin, and AMPK-ULK1/mTOR could reduce oxidative stress, promote mitochondrial biogenesis, reduce apoptosis, enhance autophagy/mitophagy, and inhibit inflammatory responses. In addition, the targeting metabolic pathways of potential treatment options—DADS, Taurine, Quercetin, Melatonin, LRWXG, CS, Rg1, DHM, Puerarin, Zinc, 17β-E2, Trelagliptin, Etomidate, SY, EGCG, Resveratrol, Procyanidins, Butein, and Mangiferin—are shown above. The green arrows indicate the metabolic effects of these potential treatment drugs.
In addition to AMPK-SIRT regulation, recent studies have yielded evidence on the interaction between lncRNA and miRNA in relation to OA pathogenesis. LncRNA, which binds to miRNAs to inhibit their combining with downstream genes, is crucial for metabolic reactions and homeostasis of articular chondrocytes [27]. Together with miRNA, it focuses on promoting or inhibiting ECM degradation, chondrocyte apoptosis and proliferation, and inflammation responses, which are all related to OA pathogenesis [28,29,30]. Therefore, LncRNA and miRNA are integral to the metabolic regulation of chondrocytes, and their downstream targets may interconnect with AMPK-SIRT signaling pathways. Hence, their abnormal expression in chondrocytes could cause metabolic dysfunction and lead to pathological progression of OA.
2.1. Mitochondrial Respiratory Chain (MRC) in Osteoarthritis
The mitochondrial membrane potential is generated by proton pumps (Complexes I, III, and IV), which are located on the inner membrane and generate transmembrane potential energy in the form of hydrogen ions that create an electrochemical gradient during oxidative phosphorylation (OXPHOS) [31]. Adenosine triphosphate (ATP), which functions as the primary energy source for several physiological reactions in human tissue cells, is mainly generated by the mitochondrial respiratory chain (MRC). However, in chondrocytes, the ATP generation mostly relies on glycolysis because metabolism in chondrocytes occurs under a relatively low oxygen supply, which ranges from 5 to 10% at the superficial zone to <1% in the deep zone [13,32].
However, in OA cartilage, due to degradation (thinning, micro-cracks, and even disappearance), chondrocytes in the deep zone receive more oxygen stimulation. In a relatively high oxygen concentration environment, the dominant chondrocyte metabolic state switches from glycolysis to aerobic respiration, resulting in the production of more ROS byproducts [17,33,34]. Meanwhile, other studies hypothesized that chondrocytes, which have an anti-inflammatory effect on oxidative phosphorylation, turn pro-inflammatory when glycolysis is used for energy production, which leads to the progression of OA [35,36,37]. Other studies also demonstrated that the abnormal glycolytic process may lead to chondrocyte dysfunction via the downregulation of glycolytic enzymes. Qu, J et al. found that restoring glycolysis can reduce the expression of endoplasmic reticulum (ER) stress-associated genes and matrix metalloproteinase-13 (MMP-13), which are induced by inflammatory factors such as tumor necrosis factor-α (TNF-α) or IL-1β. [38,39]. Therefore, the link between impaired glycolysis and OXPHOS dysfunction contributory to OA needs further study.
Currently, the standard evaluation of mitochondrial function is the analysis of respiratory chain enzyme complexes, citrate synthase (CS), and changes in mitochondrial membrane potential (ΔΨm). Recent studies have shown that chondrocytes in OA reduced activities of complexes II and III and impaired ΔΨm compared with normal chondrocytes. These findings suggest that malfunction of the mitochondrial respiratory chain is involved in the pathophysiology of OA chondrocytes [11,40]. López-Armada, M. J. et al. revealed that tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) significantly decreased the activity of complex I and the production of ATP, and caused depolarization of the mitochondria, which play a key role in cartilage degradation [41]. Furthermore, Cillero-Pastor et al. reported that mitochondrial respiratory chain (MRC) dysfunction modulates matrix metalloproteinases (MMPs) expression, and leads to ECM degradation of the articular cartilage, which is a crucial component in OA pathogenesis [42].
Recent studies have reported that mitochondria are critical mechanotransducers, which connect the extracellular mechanical signals with intracellular signaling pathways [43,44]. Bartell et al. reported that mechanical overloading will induce mitochondrial dysfunction including mitochondrial depolarization and rapid structural changes in mitochondria, within a few minutes after injury, and finally cause cartilage damage [45]. The mechanically induced distortion of mitochondrial membrane polarity, the ETC, and the cristae structure can be related to the physical linkage between the cartilage ECM, cytoskeleton, and mitochondria, resulting in compromised respiratory capacity, release of ROS, and reduced cell viability [16,46,47].
2.2. Reactive Oxygen Species (ROS) in Osteoarthritis
ROS are free radicals formed by the normal cellular metabolism of oxygen. The main ROS produced by chondrocytes are nitric oxide (NO), superoxide anion (O2−), and their derivative radicals, including peroxynitrite (ONOO-) and hydrogen peroxide (H2O2) [17,18,23]. Because of unpaired electrons, ROS are short-lived, highly reactive, and unstable. They interact with lipids, proteins, and DNA leading to increased oxidative stress [22]. Recent research revealed two major processes involved in the generation of ROS: the mitochondrial and non-mitochondrial pathways. In the former, mitochondria, which are considered to be a cell’s powerhouses, produce ROS during OXPHOS, which are involved in cartilage degradation and chondrocyte cell death [48,49]. Under normal physiological conditions, ROS act as second messengers for intracellular signaling pathways, regulating gene expression, immune responses, and metabolism homeostasis [18]. However, under pathological conditions, excessive ROS contribute to joint inflammation by inducing cytokine production. They also cause chondrocyte apoptosis, cartilage degradation from reducing matrix synthesis and activation of MMPs, and mtDNA damage [23,50]. Therefore, the ROS produced by mitochondria play an important role in OA pathogenesis.
On the other hand, the non-mitochondrial pathway mainly refers to nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Some studies showed that in pathological conditions, including mechanical overloading and inflammatory mediator stimulation, chondrocytes produce abnormal levels of ROS through NADPH oxidase, which exceeds the antioxidant capacities of the cell, leading to structural and functional cartilage damage such as cell death and matrix degradation [51,52,53]. In addition, Grange, L. et al. reported that NADPH oxidase, which responds to interleukin-1β, is significantly involved in ROS production in chondrocytes, leading to MMP production, which mediates the progressive degradation of cartilage [54,55].
Oxidative stress damage may contribute to chronic and persistent mitochondrial dysfunction because overproduction of ROS impairs mitochondrial respiration, which further increases ROS production, thus forming a vicious cycle. In addition, oxidative stress is essential for the development of certain characteristics of OA, including synovial inflammation, cell senescence and apoptosis, impaired autophagy, and ECM degradation [56]. Lepetsos, P. et al. reported that ROS overproduction contributed to decreasing ECM synthesis by inhibiting the OXPHOS process, which impaired mitochondrial ATP formation, and increased ECM degradation by upregulating the expression of genes encoding MMPs, such as MMP-1, MMP-3, and MMP-13 [57]. Reed et al. also demonstrated that oxidative stress resulting from mitochondria could modulate ECM destruction through the upregulation of the MMP level [50]. The contributions of ROS to autophagy, inflammation, cell senescence, and apoptosis will be discussed later.
On the other hand, antioxidant systems balance the production and elimination of ROS. These systems, the first line of defense, consist of enzymatic and non-enzymatic antioxidants, including glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), and catalase (CAT). In particular, superoxide dismutase (SOD) is crucial for catalyzing the dismutation of the superoxide radical (O2−) into harmless molecular oxygen (O2) and hydrogen peroxide (H2O2), thereby protecting cells from oxidative damage [58,59]. Ruiz-Romero, C. et al. reported that the expression of superoxide dismutase 2 (SOD2) is significantly downregulated in OA chondrocytes at both the gene and protein levels. This causes a global alteration of the redox balance in the bone, cartilage, and synovial fluid and further increases the accumulation of ROS [60]. Koike, M. et al. also revealed that the intra-articular injection of a permeable antioxidant effectively suppressed mechanical loading-induced mitochondrial superoxide generation and cartilage degeneration in mice [61]. Consequently, the imbalance between ROS production and antioxidant defense leads to intracellular oxidative stress damage, which raises the possibility that the mitochondrial superoxide balance may be a promising target for the treatment of cartilage degeneration.
The contents in Table 1 represent the mechanism and biological functions of traditionally potential drugs in OA. The metabolic effects of these drugs include reduction in ROS, inhibition of ECM degradation, reduced inflammatory responses, and apoptosis inhibition. On the other hand, the contents in Table 2 represent the classification and biological functions of polyphenol, which is a newly emerging potential drug for OA. There are many kinds of polyphenol, and its physiological effects include autophagy activation, apoptosis inhibition, inflammation attenuation, and mitophagy promotion. All of the metabolism responses are key factors in OA pathogenesis, and we discuss these potential drugs in each chapter.
Some research into drugs that target ROS production is shown in Table 1 and Table 2. Hosseinzadeh, A. et al. reported that diallyl disulfide (DADS), which has antioxidant and anti-inflammatory properties, can increase nuclear transcription factor erythroid-2-like factor 2 (Nrf2) nuclear translocation, and the gene expression of antioxidant enzymes, and it reduced the IL-1β-induced elevation of ROS [62]. Kim, Y. S. et al. revealed that taurine increased cell viability by protecting cells from ROS-induced by H2O2 exposure [63]. In addition, Qiu, L. et al. showed that LRWXG, a flavonol-type flavonoid ubiquitously present in vegetables, attenuated ROS generation and promote glutathione (GSH) and glutathione peroxidase (GSH-PX) expression levels in an OA rat [64]. Furthermore, Lim, H. D. et al. reported that melatonin, an amine hormone produced by the pineal gland, reduces oxidative stress and inflammatory responses by inhibiting H2O2 cytotoxicity, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) gene expression in chondrocytes [65]. According to the studies shown above, antioxidants including DADS, taurine, quercetin, and melatonin are potential drugs for the treatment of OA.
Table 1.
Classifications and biological functions of drugs in osteoarthritis.
| Drugs | Mechanism | Function | Ref. |
|---|---|---|---|
| DADS | Increase Nrf2 nuclear translocation and gene expressions of antioxidant enzymes | Reduce the production of ROS | [62] |
| Taurine | Scavenge excessive ROS | Decrease the H2O2-induced ROS production | [63] |
| Quercetin | Promote the expression of GSH and GSH-PX | Attenuate the generation of ROS | [64] |
| AMPK/SIRT1 signaling pathway | Inhibit cartilage ECM degradation | ||
| Melatonin | Inhibit H2O2 cytotoxicity, iNOS, and COX-2 gene expression | Reduce the oxidative stress and inflammatory responses | [65] |
| LRWXG | Upregulate the expressions of Bcl-2 and downregulate the expressions of caspase-9, caspase-3, and Bax | Inhibit the apoptosis of chondrocytes | [66] |
| CS | Inhibit the expressions of caspase-3 and caspase-9 | Inhibit the apoptosis of chondrocytes | [67] |
| Rg1 | PI3K/Akt/mitochondrial signaling pathway | Inhibit IL-1β-induced chondrocyte apoptosis | [68] |
| DHM | AMPK/SIRT3/PGC-1α signaling pathway | Inhibit cartilage degeneration | [69] |
| Upregulate SIRT3 | Promote mitophagy activity | ||
| Puerarin | AMPK/PGC-1α signaling pathway | Increase mitochondrial biogenesis and attenuate mitochondrial dysfunctions | [70] |
| Zinc | PINK1-dependent mitophagy pathway | Promote mitophagy activity | [71] |
| 17β-E2 | AMPK/SIRT1/mTOR signaling pathway | Induce mitophagy upregulation | [72] |
| Trelagliptin | AMPK/SOX-9 | Ameliorate oxidative stress and inflammatory responses | [73] |
| Etomidate | Upregulate AMPK signaling | Decrease oxidative stress, degradation of ECM, and chondrocyte senescence | [74] |
| SY | AMPK/SIRT1/NF-κB pathway | Inhibit degradation of cartilage ECM and inflammatory responses | [75] |
Table 2.
Classifications and biological functions of polyphenol in osteoarthritis.
| Drugs | Mechanism | Function | Ref. |
|---|---|---|---|
| EGCG | Decrease the expressions of mTOR | Reduce the chondrocyte apoptosis and activate autophagy | [76] |
| Decrease expressions of COX-2 and MMP-13 | Attenuate the inflammation on cartilage | ||
| Resveratrol | Suppression of IL-1β, ROS, and p53 production | Inhibit IL-1β-induced degradation of mitochondria and chondrocytes apoptosis | [77,78] |
| Inhibit mitochondrial membrane depolarization, PGE2 synthesis, and ATP depletion | Reduce IL-1β-induced catabolic metabolism and chondrocytes apoptosis | ||
| Activate SIRT1 signaling pathway | Inhibit NO-induced apoptosis | ||
| Procyanidins | AMPK/SIRT1/PGC-1α signaling | Promote mitochondrial biogenesis and proteoglycan homeostasis in chondrocytes | [79] |
| Butein | AMPK/TSC2/ULK1/mTOR signaling pathway | Activate autophagy and inhibit inflammatory responses in OA chondrocytes | [80] |
| Mangiferin | Activate AMPK signaling pathway | Inhibit apoptosis, ECM degradation and enhance autophagy in OA chondrocytes | [81] |
2.3. Chondrocyte Senescence and Mitochondrial Autophagy in Osteoarthritis
Recent research showed that cell studies and animal models point to aging-related and stress-induced oxidative stress as important factors related to chondrocyte senescence [82,83,84]. Senescent cells, which are cell-cycle arrested, are actually metabolically active and associated with the overproduction of cytokines (e.g., interleukins 1 and 6), growth factors (e.g., epidermal growth factor, transforming growth factor β), as well as matrix-degrading enzymes such as the MMPs (e.g., MMP-3, MMP-13), collectively known as the senescence-associated secretory phenotype (SASP) [83,85]. Furthermore, apart from the senescent cell itself, SASP can also induce paracrine senescence in neighboring healthy chondrocytes [86]. Jeon et, al. demonstrated that eliminating senescent cells could diminish the development of post-traumatic OA and increase the growth of articular cartilage [87]. In addition, Brian et al. reported that repeated intra-articular injection of senolytic cells, which target anti-apoptotic pathways, mitigate the development of OA [88]. Furthermore, Xu et al. revealed that senescent cells transplanted into the knee could cause clinical characteristics similar to OA, suggesting that targeting senescent cells could be a strategy for treating OA [89]. Overall, the SASP is highly regulated by mitochondria, and dysfunctional mitochondria can induce cellular senescence associated with SASP presentation [90]. The profile of inflammatory and catabolic mediators in senescent cells contributes to OA pathogenesis and may be the potential target for OA treatment [91].
Autophagy is a cellular process regarded as a mechanism for cell survival when cells become stressed, degrading dysfunctional proteins and macromolecules, and then recycling them to produce materials for protein synthesis [91,92]. Increasing evidence shows that autophagy’s role in OA can be modulated by mitochondria [93,94,95,96]. Some studies showed that limited autophagy is a major characteristic of OA chondrocytes, which cause excessive cellular apoptosis and cartilage degradation, thereby implying that autophagy and apoptosis are mutually regulated [94,97,98]. In addition, Zhong et al. revealed that defects in the activation of mitophagy, a specific form of autophagy that selectively eliminates depolarized and dysfunctional mitochondria, cause pronounced accumulation of damaged mitochondria and excessive IL-1β-dependent inflammation in macrophages [99]. Furthermore, Figueroa et al. reported that MRC complex V inhibition will attenuate autophagy, which protects against mitochondrial dysfunction and enhances chondrocyte apoptosis with increased ROS production and decreased ΔΨm. This suggests that enhancing autophagy may have a chondroprotective effect on OA cartilage degradation [100]. Regarding treatment, Huang, H. T. et, al. showed that (−)-Epigallocatechin 3-gallate (EGCG), the most plentiful bioactive polyphenol of green tea with anti-arthritic, anti-inflammatory, and antioxidant effects, could reduce chondrocyte apoptosis by activating autophagy through a reduction in the expression of the mammalian target of rapamycin (mTOR) and attenuation of cartilage inflammation through decreased expression of COX-2 and MMP-13 [76]. These findings suggested that EGCG may be a disease-modifying drug for preventing OA progression.
2.4. Chondrocyte Apoptosis and Calcium Homeostasis Regulation in Osteoarthritis
Apoptosis is the most common form of cell death. It is characterized by a series of morphological and biochemical changes that lead to the formation of apoptotic bodies that are phagocytosed by macrophages [96]. It is well known that apoptosis can be initiated by a variety of microenvironmental perturbations in which mitochondria play a central role (intrinsic apoptosis), or by specific ligand binding to death receptors, including the Fas cell surface death receptor (FAS) and the tumor necrosis factor (TNF) receptor (extrinsic apoptosis) [96,101]. The critical step for intrinsic apoptosis is the irreversible mitochondrial outer membrane permeabilization (MOMP), which leads to the release of cytochrome c and promotes the formation of the supramolecular complex known as apoptosome with apoptotic peptidase activating factor 1 (APAF1), which is responsible for caspase-9 activation. Concerning extrinsic apoptosis, the binding of a specific ligand to death receptors induces a conformational change at their intracellular tails and recruits several proteins, such as the FAS-associated death domain (FADD), and further activates caspase-8, which leads to the proteolytical activation of downstream effector caspases [96,101].
There are a large number of apoptotic chondrocytes in OA cartilage, and mitochondrial-induced chondrocytes apoptosis is mediated by the reduction in ΔΨm, overexpression of ROS, mechanical stress, aging, and the inflammatory factors [102]. Maneiro, E. reported that the collapse of ΔΨm is associated with mitochondrial swelling, disruption of the outer mitochondrial membrane, and the release of pro-apoptotic factors from the intermembrane space: cytochrome c, and pro-caspases such as caspase-9, which might lead to a series of events culminating in apoptosis [11,102]. Furthermore, they also revealed that sodium nitroprusside (SNP), an NO donor and a pro-apoptotic stimulus, induces depolarization of the mitochondrial membrane along with reduced activity of MRC complex IV, which eventually causes chondrocyte apoptosis [103]. The mechanical stress on the induction of chondrocyte apoptosis is also involved in mitochondrial-induced chondrocytes apoptosis and is highly connected with calcium homeostasis regulation, which is discussed in the next paragraph [104,105].
The calcium ion (Ca2+), one of the most important intracellular second messengers, is responsible for biological functions, such as cell adhesion, metabolism, secretion, proliferation, and apoptosis [106]. Particularly, the calcium stored in mitochondria helps maintain intracellular Ca2+ homeostasis [107]. Studies reported that when MRC was directly suppressed, the matrix-vesicle-mediated mineralization of chondrocytes was promoted, and these are present in OA cartilage [15,108,109]. In addition, mechanical stimulation significantly promoted increased cytosolic Ca2+ concentration, which mainly relies on two mechanisms: Ca2+ influx from the extracellular environment and Ca2+ release from intracellular stores such as the endoplasmic reticulum (ER) [106,110]. Furthermore, research revealed that calcium is released from the endoplasmic reticulum via the ryanodine receptor and is taken up by the mitochondria via the uniport transporter following a single-impact load. This causes mitochondrial depolarization and caspase 9 activation, which eventually leads to chondrocyte apoptosis and OA progression [111].
Under physiological cell stress, mitochondria can take up Ca2+ from cytosolic Ca2+ or the ER, and store it transiently to maintain homeostasis and regulate mitochondrial function [105,112]. Wann et al. reported that under physiological stress, elevated intracellular Ca2+ is beneficial for matrix synthesis [113]. However, with supra-physiological elevations of intracellular Ca2+, mitochondrial Ca2+ uptake can increase 10- to 1000-fold and begin to shape Ca2+ dynamics [105]. Huser and Davies showed that impact-induced chondrocyte apoptosis and mitochondrial depolarization were significantly decreased when the amount of calcium available to chondrocytes was reduced, suggesting that calcium homeostasis appeared to be involved in articular chondrocyte apoptosis following excessive mechanical stimulation in the single-impact-loading model [111]. Moreover, the accumulation of excessive calcium in the mitochondrial matrix leads to the formation of the permeability transition pore (PTP), which in turn causes the collapse of the mitochondrial transmembrane potential. The result is the release of Ca2+ pro-caspases such as caspase-9, and mitochondrial proteins such as cytochromes, as well as mitochondrial swelling, and cell apoptosis, as the abovementioned intrinsic pathway [114,115].
Several studies are concerned with drugs that focus on the mitochondrial apoptotic pathway, and these are listed in Table 1 and Table 2. Shao, X. et al. showed that a lower range of the molecular weight of xanthan gum (LRWXG) could inhibit the apoptosis of chondrocytes by upregulating the expression of B-cell lymphoma 2 (bcl-2) and downregulating the expression of active caspase-9 and caspase-3, and Bcl-2-associated X protein (bax) [66]. Liu, Q. et al. reported that chondroitin sulfate (CS), an important component of cartilage ECM, could increase cell survival and decrease chondrocyte apoptosis by inhibiting expression of caspase-3 and caspase-9 [67]. Huang, Y. et al. revealed that ginsenoside Rg1 (Rg1) could protect chondrocytes from IL-1β-induced apoptosis via targeting the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling pathway by inhibiting caspase-3 activation [68]. Csaki, C. et al. indicated that resveratrol, a natural polyphenolic compound with anti-inflammatory and antioxidative properties, inhibited IL-1β-induced degradation of mitochondria and apoptosis in chondrocytes via suppression of IL-1β, ROS, and production of tumor suppressor protein p53 [77]. Dave, M. et al. also reported that resveratrol could protect against IL-1β-induced catabolic effects and prevent chondrocyte apoptosis by inhibiting mitochondrial membrane depolarization, COX-2 derived PGE2 synthesis, and ATP depletion [78]. Based on these results, we concluded that anti-apoptosis drugs may be potential treatments for treating OA.
2.5. mtDNA Haplogroups in Osteoarthritis
Mitochondria, which is well known as “maternal inheritance”, have their own genomic DNA [116]. Mitochondrial DNA (mtDNA) encodes 13 polypeptide subunits essential for the OXPHOS process, 2 ribosomal RNAs (rRNAs), and 22 transfer RNAs (tRNAs) in human cells [117,118]. The mitochondrial genome, maternally inherited, has very high rates of mutation and sequence evolution; therefore, after the sequential accumulation of mtDNA mutations, they further establish stable polymorphic sites in various regions of the mtDNA, which define mitochondrial haplogroups [119,120]. Blanco et al. reported that mtDNA, which persists with high-frequency, continent-specific polymorphisms, enables humans to adapt to different climates and influences health [121]. In addition, studies revealed that mtDNA haplogroups are associated with the prevalence, incidence, and progression of OA in various populations, and with comorbidities that are closely related to various OA phenotypes [19,121].
In recent studies, European mtDNA haplogroup J was notably correlated with a decreased risk of knee OA in Spain [122,123], and individuals from the United Kingdom (UK) carrying the haplogroup T showed a decreased risk of knee OA [124]. In addition, a recent meta-analysis, which included 3217 subjects, showed that the mtDNA haplogroup J was associated with a lower incidence of knee OA over an 8-year period [125]. Furthermore, in a study from southern China, mtDNA haplogroup G exhibited an increased risk of OA occurrence, and mtDNA haplogroup B seemed to be a protective factor for OA occurrence [126]. Moreover, mtDNA haplogroup cluster TJ showed a lower proportion in OA cases, and both mtDNA haplogroup cluster TJ and haplogroup T revealed a reduced risk of radiographic knee OA progression [127,128]. Finally, some research has reported that patients carrying mtDNA haplogroup H are apt to require total joint replacement surgery and have a greater risk of radiographic OA progression compared with non-H patients [129].
There are different viewpoints to explain the associations between mtDNA haplogroups and OA. First, the associations could reside in the subtle functional differences between haplogroups, which eventually influence ROS formation, ATP production, heat generation, and apoptosis in the mitochondria [130]. Second, some studies reported that the protective role of these haplogroups may be attributed to a lower rate of aerobic respiration, decreased production of inflammatory cytokines, and reduced expression of a mitochondrially related pro-apoptotic gene [125,131]. Fernández-Moreno et al. observed that haplogroup J is an uncoupled haplogroup, which presents a protective role against ROS production and inflammatory response; on the other hand, haplogroup H is more efficiently coupled and results in more ROS generation [125]. Martínez-Redondo et al. also showed that haplogroup H presented a higher maximal oxygen uptake than haplogroup J, and had a positive correlation with greater ROS production and mitochondrial oxidative damage [132]. As not all patients carrying the same haplogroups respond in the same way and there are still many parameters that can influence the biological function of mitochondrial haplogroups, precise identification of molecular mechanisms between each haplogroup and OA pathogenesis need further research [20,133].
3. AMPK and SIRT Pathway in Related to OA Pathogenesis
AMP-activated protein kinase (AMPK) and sirtuins (SIRT) are important biosensors that are activated in response to high AMP/ATP and ADP/ATP ratios [134]. AMPK activation restores ATP levels by inhibiting ATP-consuming biosynthetic pathways while simultaneously activating pathways that regenerate ATP through the breakdown of macromolecules, including upregulating glucose uptake, fatty acid oxidation, and glycolysis [135,136]. Some studies have reported that AMPK regulates energy metabolism through activating downstream SIRT activity and inhibiting mTOR-activated pathways, which further enhances oxidative metabolism, mitochondrial biogenesis, and lifespan prolongation [134,137,138]. In addition, recent studies have also revealed that one significant function of AMPK is to promote mitochondrial health, and multiple newly discovered targets of AMPK are involved in various aspects of mitochondrial homeostasis, including autophagy and mitophagy, which are involved in OA pathogenesis [139].
Sirtuins, which are a family of highly conserved protein modifying enzymes, detect fluctuations in the NAD+/NADH ratio and regulate mitochondrial physiology and cell metabolism [140]. There are seven mammalian sirtuins, SIRT1-7, which function to regulate cell metabolism and modulate stress responses in many tissues [141]. Among these sirtuins, the SIRT3, SIRT4, and SIRT5 are localized in mitochondria, and regulate basic mitochondrial biology, including energy production, metabolism, apoptosis, and intracellular signaling [141,142,143].
As mentioned above, AMPK and SIRT together regulate a variety of cellular physical activities. Here, we summarize the mechanism of action of AMPK and SIRT on ECM production, mitochondrial biogenesis, autophagy, mitophagy, oxidative stress, and inflammation inhibition, which is shown in Figure 1. A thorough understanding of the functions and downstream targets of AMPK and SIRT are necessary not only to understand their part in cellular metabolism but also to search for therapeutic intervention in many diseases, including OA.
3.1. AMPK and SIRT Regulate ECM (Extracellular Matrix) Production and Mitochondrial Biogenesis in Chondrocytes
AMPK activation can increase ATP generation, which is beneficial for synthesizing ECM components. Some studies reported that physiological dynamic compression loading alters central metabolism in chondrocytes, in which AMPK has an important role in promoting the production of amino acid precursors and glycosaminoglycan (GAG) for ECM synthesis [144,145]. In addition, the previous study showed that upregulation of SIRT1 expression may inhibit OA chondrocyte apoptosis and ECM degradation by increasing Bcl-2 expression and decreasing Bax, MMP1, and MMP13 expression [146].
AMPK and SIRT regulate mitochondrial biosynthesis in chondrocytes via the AMPK-SIRT1/SIRT3-PCG-1α (peroxisome proliferator-activated receptor-γ coactivator 1 alpha) signaling pathway [147,148]. Wang et al. reported that the activated AMPK–SIRT1–PGC-1α pathway increases mitochondrial biogenesis and reverses pro-catabolic responses in chondrocytes, consequently limiting OA progression [148]. Other studies also showed that the impaired activation of AMPK is a key factor in OA chondrocytes and that activated AMPK upregulates the expression of PGC-1α and Forkhead box O3A (FoxO3A) in human chondrocytes, which promotes mitochondrial biogenesis and decreases oxidative stress [70,140]. AMPK and SIRT regulate the activity of the downstream target, such as PGC1α, by phosphorylation and deacetylation [137,150].
PGC-1α is a coactivator with nuclear respiratory factors (NRFs) in transcription and regulates the mitochondrial transcription factor A (TFAM), which is responsible for the mtDNA duplication, mitochondrial biogenesis, and mtDNA stability; moreover, it regulates transcription factor B (TFB), which enhances mtDNA transcription and strengthens mitochondrial biogenesis [151,152]. Nuclear transcription factor erythroid-2-like factor 2 (Nrf2) also has a chondroprotective function in OA, which suppresses MMP expression induced by IL-1β [153]. Nrf2 is a redox-sensitive transcription factors that positively regulates the expression of antioxidants, including Heme oxygenase 1 (HO-1), SOD, GSH-PX, and CAT [62,154]. Additionally, Nrf2 regulates the antioxidant response element (ARE) signal transduction, one of the crucial antioxidant systems that maintain the redox state, and has been regarded as a strategy for eliminating excessive ROS production [155,156]. As a result, PGC-1α and Nrf2 are highly important for mitochondrial biogenesis and reduction in ROS. A study further indicated that PGC-1α activity decreased in OA chondrocytes, which may be a potential target for OA treatment [149].
Some studies that focus on drugs targeting AMPK–SIRT pathways related to ECM production and mitochondrial biogenesis are shown in Table 1 and Table 2. Dihydromyricetin (DHM), which has anti-inflammation and antioxidation properties, protected rat chondrocytes from TNF-α-induced cartilage degeneration and maintained mitochondrial homeostasis via the AMPK/SIRT3/PGC-1α signaling pathway [69]. In addition, quercetin suppressed the IL-1β-induced accumulation of nitric oxide (NO), MMP-3, and MMP-13, and maintained the integrity of cartilage ECM by targeting the AMPK–SIRT1 signaling pathway [64]. Moreover, Wang, L. reported that puerarin, which has several biological functions—antioxidation, anti-inflammation, and anti-apoptosis—increased mitochondrial biogenesis, attenuated mitochondrial dysfunctions, and alleviated cartilage damage in OA rats by upregulating AMPK–PGC-1α signaling pathways [157]. Furthermore, Masuda, I. et al. revealed that procyanidins, a class of polyphenols found in apples that has radical scavenging activity, could promote mitochondrial biogenesis and proteoglycan homeostasis in chondrocytes by targeting AMPK/SIRT1/PGC-1α signaling, suggesting that apple polyphenols might be potential drugs for treating OA [79].
3.2. AMPK and SIRT Regulate Autophagy and Mitophagy in Chondrocytes
Autophagy is a conserved intracellular process that delivers cytoplasmic elements (e.g., proteins, organelles, and pathogens) to lysosomes for degradation via double-membrane autophagosomes [158]. On the other hand, mitophagy is a specific form of autophagy that selectively eliminates dysfunctional and depolarized mitochondria, which is a core mechanism for controlling mitochondrial quality and quantity [159].
Some studies reported that AMPK activates autophagy and mitophagy to remove damaged or defective mitochondria with impaired OXPHOS, whereas mTORC1, the mechanistic target of rapamycin complex 1, blocks this protective process [130,135]. In addition, AMPK triggers the initiation of autophagy by directly phosphorylating and activating ULK1 (unc-51-like autophagy-activating kinase 1); on the other hand, AMPK inhibits mTOR by means of directly phosphorylating the mTOR upstream regulator, which strongly suppresses autophagy by directly phosphorylating and inhibiting ULK1. Studies have reported that AMPK directly phosphorylates the mTOR upstream regulator including TSC2 and the mTORC1 subunit RAPTOR, which results in reducing mTOR activity under conditions of energy stress and inhibiting the phosphorylation on ULK1 that activates autophagy [160,161]. Moreover, Egan, D. et al. reported that cells expressing ULK1 mutants appear to accumulate defective mitochondria, suggesting that the AMPK–ULK1 axis is important not only for general autophagy, but also specifically for the selective removal of damaged mitochondria [162]. Consequently, AMPK promotes autophagy not only by directly activating ULK1, but also by negatively regulating mTORC1. Therefore, the direct links between AMPK and ULK1 are vital for autophagy and mitophagy, which are core events in OA pathogenesis [135,139,163].
AMPK also directly regulates downstream mitophagy in chondrocytes via the SIRT1/SIRT3-FoxO3A-PINK1/Parkin signaling pathway, which is involved in clearing damaged mitochondria, limiting ROS production, and improving OA chondrocyte survival under pathological conditions [69,164,165]. Research has shown that AMPK directly phosphorylates FOXO3, which regulates the genes involved in autophagy, and stimulates its transcriptional activity under conditions of energy stress [166,167]. On the contrary, FOXK1 and FOXK2, which are members of the FOX transcription factor family, are phosphorylated by mTOR under a high nutrient environment and compete with FOXO3 to repress genes involved in autophagy [168]. Another important pathway controlling mitophagy is the PINK1–Parkin-mediated removal of depolarized mitochondria. Parkin, an E3 ubiquitin ligase located on the mitochondrial outer membrane, operates in conjunction with PTEN-induced kinase 1 (PINK1), and the phosphorylation of Parkin by PINK1 transforms it into an active E3 ligase that eliminates damaged mitochondria in response to the loss of ΔΨm [169,170]. Ansari, M. Y. et al. reported that the PINK1–Parkin-mediated removal of damaged mitochondria limited ROS generation and further prevented the induction of oxidative stress in OA chondrocytes [171]. Martini, H. also reported that the PINK1-Parkin pathway was activated when mitochondria were damaged or dysfunctional. Then, E3 ubiquitin ligase Parkin from the cytosol further ubiquitinated numerous proteins on the outer mitochondrial membrane, which mediated autophagosome formation and the lysosomal elimination of dysfunctional mitochondria.
Recent research has also reported that autophagy declines with age, which further leads to chondrocyte dysfunction and OA progression. Some studies have indicated that basal autophagy decreases with age, contributing to the accumulation of protein aggregates and ultimately an aging-related articular cartilage dysregulation disease such as OA [100,172]. In addition, the activation of autophagy has chondroprotective effects, indicating that mitophagy is essential for preventing oxidative stress by eliminating damaged mitochondria. This will be discussed later [164,172,173].
There are some potential drugs that focus on the AMPK–SIRT pathways related to mitophagy in OA chondrocytes, and these are shown in Table 1 and Table 2. Huang, L. W. reported that zinc treatment ameliorates the negative effects on energy metabolism and upregulates the PINK1-dependent mitophagy pathway [71]. In addition, Wang, J. indicated that DHM promotes mitophagy by upregulating SIRT3 in rat chondrocytes. This suggests that SIRT3 might be a potential therapeutic target against chondrocyte degeneration and OA [69]. Moreover, Mei, R. et al. showed that 17β-estradiol (17β-E2), which is associated with articular cartilage metabolism and postmenopausal OA, could induce mitophagy upregulation to protect chondrocytes via targeting the AMPK–SIRT1–mTOR signaling pathway [72]. Ansari, M. Y. et al. further indicated that butein, a polyphenol produced by several plants with anti-inflammatory activity, could activate autophagy, blocking the IL-1β-induced expression of IL-6 in OA chondrocytes, by targeting the AMPK/TSC2/ULK1/mTOR pathway [80]. Additionally, Li, Y. et al. revealed that mangiferin, a natural polyphenol with anti-inflammatory and antioxidative effects, protects chondrocytes from apoptosis and ECM degradation and also enhances autophagy in rat OA chondrocytes by activating the AMPK signaling pathway. This suggests that mangiferin might serve as an OA treatment option [81].
3.3. AMPK and SIRT Regulate Oxidative Stress and Inflammation Inhibition in Chondrocytes
There is increasing evidence suggesting that AMPK and SIRT may be redox-sensing proteins in the management of ROS levels [135,174]. Some studies have indicated that AMPK and SIRT regulate chondrocyte catabolism and reduce mitochondrial ROS levels via the AMPK–SIRT3–PGC-1α/FoxO3A signaling pathway, and then prevent chondrocyte senescence and death that may inhibit the progression of cartilage damage in OA [149,175]. Olmos, Y. et al. reported that both PGC-1α and FoxO3A reduced cellular oxidative stress by upregulating antioxidant enzymes, including SOD2 (superoxide dismutase 2) and catalase [176]. Kincaid, B et al. revealed that the activation of SIRT3 by AMPK directly activates SOD2 via deacetylation to scavenge ROS, which maintains the intracellular redox balance [177]. In addition, recent research has indicated that SIRT3 contributes to the regeneration of reduced GSH, the major antioxidant responsible for preventing ROS damage, via the deacetylation of isocitrate dehydrogenase 2 (IDH2), a critical component of the mitochondrial antioxidant pathway because of its ability to generate NADPH to regenerate GSH [178,179]. Furthermore, studies have reported that SIRT3 plays a critical role in repairing mtDNA damage, protecting mitochondrial integrity, and preventing cell apoptosis under oxidative stress. It does so by upregulating the acetylation of 8-oxoguanine-DNA glycosylase 1 (OGG1), which is a newly identified target protein that functions in DNA repair [174,175,180].
Apart from regulating oxidative stress, some studies also showed that AMPK and SIRT inhibit inflammation [70,140]. Salminen, A. and Kaarniranta, K. reported that inhibition of NF-κB signaling by AMPK suppresses inflammatory responses, which is significant because inflammation inhibition and autophagic clearance decline with age [181]. Wei, H. et al. also reported that AMPK phosphorylation contributes to the degeneration of TXNIP, which promotes the activation of the NLRP3 inflammasome in cardiomyocytes [182]. In addition, autophagy, which is a negative regulator of NLRP3 inflammasome activation, can be upregulated by AMPK and confer anti-inflammatory effects by improving mitochondrial quality control and removing molecules such as mitochondrial ROS and cytosolic mtDNA, which may be a potential OA treatment target [183].
Several research works discussed the drugs that focus on the AMPK–SIRT pathways related to oxidative stress regulation and inflammation inhibition in OA chondrocytes, as shown in Table 1 and Table 2. Liu J. et al. demonstrated that trelagliptin, a selective inhibitor of dipeptidyl peptidase 4 (DPP-4) used for the treatment of type 2 diabetes mellitus (T2DM), ameliorated IL-1β-induced oxidative stress and mitigated IL-1β-induced inflammatory responses via the AMPK/SOX-9 pathway [73]. Furthermore, Yin, M. and Xu, Y. reported that etomidate, an intravenous anesthetic with antioxidant and anti-inflammatory effects, protected chondrocytes from IL-1β-induced oxidative stress, degradation of ECM, and chondrocyte senescence by upregulating AMPK signaling [74]. Moreover, Wang, C. et al. showed that safflower yellow (SY), the main active ingredient in safflowers, which has antioxidant and anti-arthritic properties, prevented degradation of cartilage ECM and inhibited inflammatory responses by regulating the AMPK/SIRT1/NF-κB pathways [75]. Furthermore, Takayama, K. et al. indicated that resveratrol reduced the amount of Bax and increased the amount of Bcl-2 in the mitochondrial fraction, and activated SIRT1 to inhibit NO-induced apoptosis. This indicated that resveratrol and the SIRT1 pathway might contribute to ending the pathogenesis of OA [184].
4. Long Non-Coding RNA (lncRNA) and MicroRNA (miRNA) in Related to OA Pathogenesis
The human genome is estimated to consist of around 2% protein-coding RNA (pcRNA); however, the majority of the genome consists of non-coding RNA (ncRNA) [185]. The most common are tRNA, and rRNA, which are essential for protein synthesis. Other ncRNAs, including long and small ncRNAs, regulate gene expression through epigenetic pathways and function as signaling molecules to regulate crucial cellular and metabolic processes [186,187]. In recent years, with the development of molecular biology technology, researchers have gradually discovered the important roles of long non-coding RNA (lncRNA) and MicroRNA (miRNA). Both lncRNA and miRNA are involved in important pathways related to OA pathogenesis, and they can be used as therapeutic biomarkers for evaluating the progression and prognosis of OA.
4.1. Relationship between lncRNA and OA Pathogenesis
LncRNA is an ncRNA that transcribes more than 200 nucleotides in length and modulates mRNA stability [188]. Previous studies have reported that, compared with healthy cartilage tissue, the expression levels of different lncRNA—homeobox transcript antisense RNA (HOTAIR), metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), growth arrest-specific transcript 5 (GAS5), PMS1 homolog 2 mismatch repair system component pseudogene 2 (PMS2P2), RP11 445H22.4, H19, CTD 2574D22.4, maternal expression gene 3 (MEG3), and FOXD2-adjacent opposite strand RNA 1 (FOXD2-AS1)—may be upregulated or downregulated in OA cartilage, suggesting that various lncRNAs may modulate the pathological progression of OA. The specific mechanisms are shown in Table 3 [188,189].
Previous studies have shown that HOTAIR, which serves important for cancer progression, is expressed in normal human cartilage [190,191]. Research has also reported that HOTAIR and alpha-1, 2 fucosyltransferase 2 (FUT2) are upregulated in OA cartilage compared with healthy cartilage, resulting in aggravated ECM degradation, chondrocyte apoptosis, and OA progression through the Wnt/β-catenin pathway [192,193]. In addition, Dou, P. et al. reported that HOTAIR strongly promotes the expression of a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) in human OA articular chondrocytes, which aggravates ECM degradation and promotes OA [194]. Jiang, M. et al. found that the expression of lncRNA PACER is low in OA chondrocytes and decreases cell apoptosis. The inverse correlation between the expression of lncRNA HOTAIR and lncRNA PACER suggested that there was a mutual regulation mechanism between lncRNAs [195].
MALAT1 is expressed in many tissues and participates in numerous biological processes [196]. Zhang, Y. et al. reported that MALAT1 levels in OA cartilage were upregulated with AKT3 compared with those in healthy cartilage, whereas the levels of miR-150-5p were downregulated, suggesting that overexpression of MALAT1 inhibited the expression of miR-150-5p and promoted that of AKT3 [197]. In addition, the study showed that overexpression of MALAT1 reduced the expression of MMP13 and ADAMTS 5, promoted the expression of type II collagen in chondrocytes, and inhibited apoptosis and ECM degradation. Consequently, these suggested that lncRNA MALAT1 plays an important role in OA and may participate in OA pathogenesis via the miR-150-5p/AKT3 axis [197]. Studies also reported that Knockdown of MALAT1 in OA synovial fibroblasts increased the expressions of interleukin-8 (IL-8), and also increased the expressions of genes that control cell growth, cell proliferation, and the inflammatory response [198].
Regarding other lncRNAs involved in OA pathogenesis, Song, J. et al. reported that upregulated GAS5 levels in OA chondrocytes negatively regulated miR-21, which increased the expression of MMPs, stimulated apoptosis, and inhibited autophagy [199]. In addition, research showed that H19 lncRNA, which is upregulated in OA chondrocytes, induced chondrocyte damage by promoting the expression of miR-675, and enhanced LPS-induced C28/I2 cell injury by inhibiting miR-130a [200,201]. Previous studies have also reported that lncRNA MEG3 inhibited ECM degradation in cartilage and delayed the progression of OA by modulating the miR-93–TGFBR2 axis. It also attenuated LPS-induced inflammatory damage by targeting the miR-203 that mediates the Sirt1/PI3K/AKT pathways [202,203,204]. Furthermore, previous research demonstrated that FOXD2-AS1, levels of which are upregulated in OA compared with those in healthy cartilage, induced ECM degradation, inflammation response, and promoted OA progression by targeting miR-27a 3p, which upregulated toll-like receptor 4 (TLR4) expression levels [205,206]. In addition, Cao et al. reported that lncRNA FOXD2-AS1 promoted the survival and development of chondrocytes by inhibiting expression of miR-206 and upregulating expression of Cyclin D1 (CCND1), indicating that FOXD2-AS1/miR-206/CCND1 are important for OA pathogenesis [207].
Table 3.
Classifications and biological functions of lncRNAs in osteoarthritis.
| lncRNA | Target/Signaling Pathway | Function | Ref. |
|---|---|---|---|
| HOTAIR | FUT2/Wnt/β-catenin | Increase ECM degradation, chondrocyte apoptosis | [192,193] |
| ADAMTS-5 | Increase ECM degradation, promoting OA progression | [194] | |
| MALAT1 | miR-150-5p/AKT3 | Inhibit apoptosis and ECM degradation, promote chondrocyte proliferation | [197] |
| IL-8 | Promote chondrocyte proliferation, inhibit inflammatory responses | [198] | |
| GAS5 | miR-21/MMPs | Stimulate apoptosis, inhibit autophagy | [199] |
| H19 lncRNA | miR-675 | Increase inflammatory responses, aggravate chondrocyte injury | [200,201] |
| miR-130a/PTEN/PI3K/Akt | |||
| MEG3 | miR-93/TGFBR2 | Inhibit ECM degradation | [202,203] |
| miR-203/Sirt1/PI3K/AKT | Attenuate inflammatory damage | [204] | |
| FOXD2-AS1 | miR-27a-3p/TLR4 | Increase ECM degradation and inflammation responses | [205,206] |
| miR-206/CCND1 | Promote chondrocyte proliferation | [207] |
4.2. Relationship between miRNA and OA Pathogenesis
In the pathogenesis of OA, miRNA has certain biological functions that mediate ECM metabolism, chondrocyte proliferation and apoptosis, and inflammatory responses. These are shown in Table 4 [208,209]. Recent studies demonstrated that an abundance of miRNAs is involved in ECM metabolism. Wang et al. found that miR-483-5p promotes chondrocyte hypertrophy, ECM degradation, and accelerated the process of OA by directly targeting matrilin 3 (Matn3) and the tissue inhibitor of metalloproteinase 2 (Timp2) [210]. Hu, G. et al. reported that miR-145 alleviated the TNF-α triggered cartilage degradation by inhibiting the phosphorylation of mitogen-activated protein kinase kinase 4 (MKK4) [211]. Li, L. et al. found that the expression of miR-16-5p, which is significantly higher in OA cartilage than in healthy cartilages, reduced expression of type II collagen and aggrecan while inducing expression of MMPs and ADAMTS via the Smad3 pathway [212]. Zhang, Y. revealed that overexpression of miR-21 attenuated the process of chondrogenesis by directly targeting growth differentiation factor 5 (GDF-5) [213].
Chondrocyte proliferation and apoptosis play a key role in OA pathogenesis, and many miRNAs regulate these important pathways. Research has demonstrated that miR-146a targets Smad4 to promote chondrocyte apoptosis, and miR-146b upregulated the activities of proteolytic enzymes, promoted cell apoptosis, and accelerated the development of OA by inhibiting the expression of alpha-2-macroglobulin (A2M) [214,215]. Zhao et al. reported that miR-181a accelerated the apoptosis of chondrocytes by inhibiting the expression of Glycerol-3-Phosphate Dehydrogenase 1-Like Protein (GPD1L) [216]. Li, Z. et al. showed that miR-210 increased chondrocyte proliferation and prompted ECM deposition by inhibiting the expression of hypoxia-inducible factor (HIF)-3α [217].
Regarding the inflammatory response, previous studies revealed that several miRNAs are involved in inflammation regulation. Jones, S. revealed that miR-146, which is downregulated in OA cartilage, reduced interleukin-1 beta (IL-1β)-induced TNF-α production [218]. In addition, Bazzonia F. et al. reported that TLR4-activated NF-κB rapidly increased the expression of miR-9, which functions as a negative feedback control of the NF-κB-dependent responses [219]. Furthermore, Park, S. et al. showed that miR-558, which was significantly lower in OA cartilage compared with normal human articular cartilage, controlled cartilage homeostasis by directly targeting COX-2, a major prostaglandin E2 (PGE2) synthetic enzyme involved in chronic inflammation, and the regulation of IL-1β-stimulated catabolic effects in human chondrocytes [220].
Table 4.
Classifications and biological functions of mi-RNAs in osteoarthritis.
| mi-RNA | Target/Signaling Pathway | Function | Ref. |
|---|---|---|---|
| miR-483-5p | Matn3 | Promote ECM degradation and chondrocyte hypertrophy | [210] |
| TIMP2 | |||
| miR-145 | MKK4 | Alleviate cartilage degradation | [211] |
| miR-16-5p | Smad3 | Increase ECM degradation | [212] |
| miR-21 | GDF-5 | Inhibit chondrocyte proliferation | [213] |
| miR-146a | Smad4 | Promote chondrocyte apoptosis | [214] |
| miR-146b | A2M | Promote chondrocyte apoptosis | [215] |
| miR-181a | GPD1L | Promote chondrocyte apoptosis | [216] |
| miR-210 | HIF-3α | Promote chondrocyte proliferation, increase ECM deposition | [217] |
| miR-146 | TNF-α | Inhibit inflammatory responses | [218] |
| miR-9 | NF-κB | Inhibit inflammatory responses | [219] |
| miR-558 | COX-2 | Inhibit inflammatory responses, inhibit ECM degradation | [220] |
5. Conclusions
In this review, we first presented evidence that mitochondria not only function as “energy powerhouses”, but also regulate several physiological activities of chondrocytes, including oxidative stress, autophagy, apoptosis, chondrocyte senescence, and calcium homeostasis in OA. In addition, more and more evidence has revealed the connection involving the AMPK–SIRT signaling pathways, mitochondria dysfunction, and OA pathogenesis. The AMPK–SIRT signaling pathways help to regulate mitochondrial bioactivities and chondrocyte viability, including ECM production, mitochondrial biogenesis, autophagy, mitophagy, oxidative stress, and inflammation inhibition, suggesting that the AMPK–SIRT signaling pathways could be potential targets for developing new OA therapeutic strategies. Furthermore, a growing number of findings have shown that lncRNA and miRNA have great potential to be the options for OA treatment because of their involvement in several important pathways related to OA pathogenesis, including the regulation of ECM degradation, chondrocyte apoptosis, chondrocyte proliferation, inflammatory responses, and autophagy. Based on the above findings, we found a lot of drugs that target different signaling pathways. However, we need further research to better understand the downstream mechanisms of the AMPK–SIRT signaling pathways, lncRNA, and miRNA to pursue new pharmacologic targets for OA pathogenesis.
Acknowledgments
We appreciate the support from members of our orthopedic research center and the Department of Physiology, Kaohsiung Medical University, Kaohsiung City, Taiwan.
Author Contributions
Conceptualization, H.-Y.L., C.-F.C., C.-C.L., S.-C.W., B.H., C.-W.W., L.K. and C.-H.C.; methodology, H.-Y.L., C.-F.C., C.-C.L., S.-C.W., B.H., T.-L.C., S.-Y.L., C.-J.H. and M.-J.L.; software, S.-C.W., B.H., T.-L.C., S.-Y.L., C.-J.H., M.-J.L., C.-D.Y., Y.-C.W., J.-Y.L. and P.-C.L.; validation, H.-Y.L., C.-F.C., C.-C.L., S.-C.W., B.H., T.-L.C., S.-Y.L., C.-J.H., M.-J.L., C.-D.Y., Y.-C.W., J.-Y.L., P.-C.L., C.-W.W., L.K. and C.-H.C.; data curation, H.-Y.L., C.-F.C., C.-C.L., S.-C.W., B.H., T.-L.C., S.-Y.L., C.-J.H., M.-J.L., C.-D.Y., Y.-C.W., J.-Y.L., P.-C.L. and C.-W.W.; writing—original draft preparation, H.-Y.L., C.-F.C., C.-C.L. and S.-C.W.; writing—review and editing, C.-W.W., L.K. and C.-H.C.; project administration, C.-W.W., L.K. and C.-H.C.; funding acquisition, C.-H.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This study was supported in part by the National Health Research Institute (NHRI-EX101-9935EI) of Taiwan, Kaohsiung Medical University (NPUST-KMU-109-P002, NCTUKMU108-BIO-04, KMU-TC108A02-1, KMU-DK105009, KMU-DK(B) 110002 and KMU-111-P005), National Cheng Kung University (NCKUH-11102060), Kaohsiung Municipal Ta-Tung Hospital (KMTTH-109-R014, KMTTH-DK(B) 110002-1 and KMTTH-110-R008), and the Minister of Science and Technology of Taiwan (MOST 110-2314-B-006-037-MY3, 108-2314-B-037-059-MY3 and 110-2314-B-037-029-MY3). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.He Y., Wu Z., Xu L., Xu K., Chen Z., Ran J., Wu L. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell. Mol. Life Sci. 2020;77:3729–3743. doi: 10.1007/s00018-020-03497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.Hootman J.M., Helmick C.G., Barbour K.E., Theis K.A., Boring M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016;68:1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D., Shen J., Zhao W., Wang T., Han L., Hamilton J.L., Im H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas Aigner M., Kurz B., Fukui N., Sandell L. Roles of chondrocytes in the pathogenesis of osteoarthritis. Curr. Opin. Reumatol. 2002;14:578–584. doi: 10.1097/00002281-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Funck-Brentano T., Cohen-Solal M. Subchondral bone and osteoarthritis. Curr. Opin. Rheumatol. 2015;27:420–426. doi: 10.1097/BOR.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 7.Malemud C.J. Matrix Metalloproteinases and Synovial Joint Pathology. Prog. Mol. Biol. Transl. Sci. 2017;148:305–325. doi: 10.1016/bs.pmbts.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Xia B., Chen D., Zhang J., Hu S., Jin H., Tong P. Osteoarthritis Pathogenesis: A Review of Molecular Mechanisms. Calcif. Tissue Res. 2014;95:495–505. doi: 10.1007/s00223-014-9917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martel-Pelletier J. Osteoarthritis. Nat. Rev. Dis. Primers. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 10.Sacitharan P.K. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123–159. doi: 10.1007/978-981-13-3681-2_6. [DOI] [PubMed] [Google Scholar]
- 11.Maneiro E., Martín M.A., De Andres M.C., López-Armada M.J., Fernández-Sueiro J.L., Del Hoyo P., Galdo F., Arenas J., Blanco F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Care Res. 2003;48:700–708. doi: 10.1002/art.10837. [DOI] [PubMed] [Google Scholar]
- 12.Sousa J.S., D’Imprima E., Vonck J. Mitochondrial Respiratory Chain Complexes. Subcell Biochem. 2018;87:167–227. doi: 10.1007/978-981-10-7757-9_7. [DOI] [PubMed] [Google Scholar]
- 13.Marcus R.E., Sokoloff L. The effect of low oxygen concentration on growth, glycolysis, and sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Care Res. 1973;16:646–656. doi: 10.1002/art.1780160509. [DOI] [PubMed] [Google Scholar]
- 14.Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z. Rheumatol. 1991;50:304–312. [PubMed] [Google Scholar]
- 15.Blanco F.J., Rego I., Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 16.Liu H., Li Z., Cao Y., Cui Y., Yang X., Meng Z., Wang R. Effect of chondrocyte mitochondrial dysfunction on cartilage degeneration: A possible pathway for osteoarthritis pathology at the subcellular level. Mol. Med. Rep. 2019;20:3308–3316. doi: 10.3892/mmr.2019.10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepetsos P., Papavassiliou A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016;1862:576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Li D., Wang W., Xie G. Reactive Oxygen Species: The 2-Edged Sword of Osteoarthritis. Am. J. Med Sci. 2012;344:486–490. doi: 10.1097/MAJ.0b013e3182579dc6. [DOI] [PubMed] [Google Scholar]
- 19.Blanco J.F., June R.K., 2nd Cartilage Metabolism, Mitochondria, and Osteoarthritis. J. Am. Acad. Orthop. Surg. 2020;28:e242–e244. doi: 10.5435/JAAOS-D-19-00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y., Makarczyk M.J., Lin H. Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci. 2020;263:118602. doi: 10.1016/j.lfs.2020.118602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phaniendra A., Jestadi D.B., Periyasamy L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henrotin Y.E., Bruckner P., Pujol J.-P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003;11:747–755. doi: 10.1016/S1063-4584(03)00150-X. [DOI] [PubMed] [Google Scholar]
- 24.Quiros P.M., Mottis A., Auwerx J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 25.Kim J., Kundu M., Viollet B., Guan K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes L.C., Di Benedetto G., Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun H., Peng G., Ning X., Wang J., Yang H., Deng J. Emerging roles of long noncoding RNA in chondrogenesis, osteogenesis, and osteoarthritis. Am. J. Transl. Res. 2019;11:16–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Aigner T., Söder S., Gebhard P.M., McAlinden A., Haag J. Mechanisms of disease: Role of chondrocytes in the pathogenesis of osteoarthritis—Structure, chaos and senescence. Nat. Clin. Pract. Rheumatol. 2007;3:391–399. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- 29.Le L.T.T., Swingler T.E., Clark I.M. Review: The Role of MicroRNAs in Osteoarthritis and Chondrogenesis. Arthritis Care Res. 2013;65:1963–1974. doi: 10.1002/art.37990. [DOI] [PubMed] [Google Scholar]
- 30.Swingler E.T., Niu L., Smith P., Paddy P., Le L., Barter M.J., Young D., Clark I.M. The function of microRNAs in cartilage and osteoarthritis. Clin. Exp. Reumatol. 2019;37:40–47. [PubMed] [Google Scholar]
- 31.Zorova L.D. Mitochondrial membrane potential. Anal. Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou S., Cui Z., Urban J.P.G. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: A modeling study. Arthritis Care Res. 2004;50:3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 33.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:14708728. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terkeltauba R.J.K., Murphy A., Ghoshb S. Invited review the mitochondrion in osteoarthritis. Mitochondrion. 2002;1:301–319. doi: 10.1016/S1567-7249(01)00037-X. [DOI] [PubMed] [Google Scholar]
- 35.Liu-Bryan R., Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2014;11:35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill L.A.J., Hardie D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 37.Tchetina E.V., Markova G.A. Regulation of energy metabolism in the growth plate and osteoarthritic chondrocytes. Rheumatol. Int. 2018;38:1963–1974. doi: 10.1007/s00296-018-4103-4. [DOI] [PubMed] [Google Scholar]
- 38.Nishida T. Impaired glycolytic metabolism causes chondrocyte hypertrophy-like changes via promotion of phospho-Smad1/5/8 translocation into nucleus. Osteoarthr. Cartil. 2013;21:700–709. doi: 10.1016/j.joca.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Qu J., Lu D., Guo H., Miao W., Wu G., Zhou M. PFKFB3 modulates glycolytic metabolism and alleviates endoplasmic reticulum stress in human osteoarthritis cartilage. Clin. Exp. Pharmacol. Physiol. 2016;43:312–318. doi: 10.1111/1440-1681.12537. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Guo X., Ma W., Zhang Y., Xu P., Yao J., Bai Y. Mitochondrial function is altered in articular chondrocytes of an endemic osteoarthritis, Kashin–Beck disease. Osteoarthr. Cartil. 2010;18:1218–1226. doi: 10.1016/j.joca.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Armada M.J. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr. Cartil. 2006;14:1011–1022. doi: 10.1016/j.joca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Cillero-Pastor B., Rego-Perez I., Oreiro N., Fernández-López C., Blanco F.J. Mitochondrial respiratory chain dysfunction modulates metalloproteases -1, -3 and -13 in human normal chondrocytes in culture. BMC Musculoskelet. Disord. 2013;14:235. doi: 10.1186/1471-2474-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetz J.E., Coleman M.C., Fredericks U.C., Petersen E., Martin J.A., McKinley T.O., Tochigi Y. Time-dependent loss of mitochondrial function precedes progressive histologic cartilage degeneration in a rabbit meniscal destabilization model. J. Orthop. Res. 2016;35:590–599. doi: 10.1002/jor.23327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zignego D.L., Hilmer J.K., June R.K. Mechanotransduction in primary human osteoarthritic chondrocytes is mediated by metabolism of energy, lipids, and amino acids. J. Biomech. 2015;48:4253–4261. doi: 10.1016/j.jbiomech.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartell L.R. Mitoprotective therapy prevents rapid, strain-dependent mitochondrial dysfunction after articular cartilage injury. J. Orthop. Res. 2020;38:1257–1267. doi: 10.1002/jor.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quintana-Cabrera R., Mehrotra A., Rigoni G., Soriano M. Who and how in the regulation of mitochondrial cristae shape and function. Biochem. Biophys. Res. Commun. 2018;500:94–101. doi: 10.1016/j.bbrc.2017.04.088. [DOI] [PubMed] [Google Scholar]
- 47.Sauter E., Buckwalter J., McKinley T.O., Martin J.A. Cytoskeletal dissolution blocks oxidant release and cell death in injured cartilage. J. Orthop. Res. 2011;30:593–598. doi: 10.1002/jor.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman M.C., Ramakrishnan P.S., Brouillette M.J., Martin J. Injurious Loading of Articular Cartilage Compromises Chondrocyte Respiratory Function. Arthritis Rheumatol. 2015;68:662–671. doi: 10.1002/art.39460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed K.N., Wilson G., Pearsall A., Grishko V.I. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol. Cell. Biochem. 2014;397:195–201. doi: 10.1007/s11010-014-2187-z. [DOI] [PubMed] [Google Scholar]
- 51.Drevet S., Gavazzi G., Grange L., Dupuy C., Lardy B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp. Gerontol. 2018;111:107–117. doi: 10.1016/j.exger.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Henrotin Y., Kurz B., Aigner T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthr. Cartil. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Krause K.-H. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007;42:256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Moulton P.J., Goldring M. NADPH oxidase of chondrocytes contains an isoform of the gp91phox subunit. Biochem. J. 1998;329:449–451. doi: 10.1042/bj3290449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grange L., Nguyen M.V.C., Lardy B., Derouazi M., Campion Y., Trocme C., Paclet M., Gaudin P., Morel F. NAD(P)H Oxidase Activity of Nox4 in Chondrocytes Is Both Inducible and Involved in Collagenase Expression. Antioxid. Redox Signal. 2006;8:1485–1496. doi: 10.1089/ars.2006.8.1485. [DOI] [PubMed] [Google Scholar]
- 56.Jiang W., Liu H., Wan R., Wu Y., Shi Z., Huang W. Mechanisms linking mitochondrial mechanotransduction and chondrocyte biology in the pathogenesis of osteoarthritis. Ageing Res. Rev. 2021;67:101315. doi: 10.1016/j.arr.2021.101315. [DOI] [PubMed] [Google Scholar]
- 57.Lepetsos P., Papavassiliou K.A., Papavassiliou A.G. Redox and NF-kappaB signaling in osteoarthritis. Free Radic. Biol. Med. 2019;132:90–100. doi: 10.1016/j.freeradbiomed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 58.Sen C.K. Oxygen toxicity and antioxidants: State of the art. Indian J. Physiol. Pharmacol. 1995;39:177–196. [PubMed] [Google Scholar]
- 59.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 60.Ruiz-Romero C. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. 2009;8:172–189. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koike M., Nojiri H., Ozawa Y., Watanabe K., Muramatsu Y., Kaneko H., Morikawa D., Kobayashi K., Saita Y., Sasho T., et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015;5:11722. doi: 10.1038/srep11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosseinzadeh A. Evaluating the Protective Effects and Mechanisms of Diallyl Disulfide on Interlukin-1beta-Induced Oxidative Stress and Mitochondrial Apoptotic Signaling Pathways in Cultured Chondrocytes. J. Cell. Biochem. 2017;118:1879–1888. doi: 10.1002/jcb.25907. [DOI] [PubMed] [Google Scholar]
- 63.Kim Y.-S., Kim E.-K., Hwang J.-W., Kim J.-S., Shin W.-B., Dong X., Nawarathna W.P.A.S., Moon S.-H., Jeon B.-T., Park P.-J. Neuroprotective Effect of Taurine-Rich Cuttlefish (Sepia officinalis) Extract Against Hydrogen Peroxide-Induced Oxidative Stress in SH-SY5Y Cells. Single Mol. Single Cell Seq. 2017;975:243–254. doi: 10.1007/978-94-024-1079-2_22. [DOI] [PubMed] [Google Scholar]
- 64.Qiu L., Luo Y., Chen X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018;103:1585–1591. doi: 10.1016/j.biopha.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 65.Lim H.-D., Kim Y.-S., Ko S.-H., Yoon I.-J., Cho S.-G., Chun Y.-H., Choi B.-J., Kim E.-C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012;53:225–237. doi: 10.1111/j.1600-079X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 66.Shao X., Chen Q., Dou X., Chen L., Wu J., Zhang W., Shao H., Ling P., Liu F., Wang F. Lower range of molecular weight of xanthan gum inhibits cartilage matrix destruction via intrinsic bax-mitochondria cytochrome c-caspase pathway. Carbohydr. Polym. 2018;198:354–363. doi: 10.1016/j.carbpol.2018.06.108. [DOI] [PubMed] [Google Scholar]
- 67.Liu Q., Wang J., Sun Y., Han S. Chondroitin sulfate from sturgeon bone protects chondrocytes via inhibiting apoptosis in osteoarthritis. Int. J. Biol. Macromol. 2019;134:1113–1119. doi: 10.1016/j.ijbiomac.2019.05.110. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y., Wu D., Fan W. Protection of ginsenoside Rg1 on chondrocyte from IL-1β-induced mitochondria-activated apoptosis through PI3K/Akt signaling. Mol. Cell. Biochem. 2014;392:249–257. doi: 10.1007/s11010-014-2035-1. [DOI] [PubMed] [Google Scholar]
- 69.Wang J., Wang K., Huang C., Lin D., Zhou Y., Wu Y., Tian N., Fan P., Pan X., Xu D., et al. SIRT3 Activation by Dihydromyricetin Suppresses Chondrocytes Degeneration via Maintaining Mitochondrial Homeostasis. Int. J. Biol. Sci. 2018;14:1873–1882. doi: 10.7150/ijbs.27746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salminen A., Hyttinen J.M., Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang L.-W., Huang T.-C., Hu Y.-C., Hsieh B.-S., Chiu P.-R., Cheng H.-L., Chang K.-L. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem. Biophys. Res. Commun. 2019;521:50–56. doi: 10.1016/j.bbrc.2019.10.066. [DOI] [PubMed] [Google Scholar]
- 72.Mei R., Lou P., You G., Jiang T., Yu X., Guo L. 17beta-Estradiol Induces Mitophagy Upregulation to Protect Chondrocytes via the SIRT1-Mediated AMPK/mTOR Signaling Pathway. Front. Endocrinol. 2020;11:615250. doi: 10.3389/fendo.2020.615250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J., Zuo Q., Li Z., Chen J., Liu F. Trelagliptin ameliorates IL-1β-impaired chondrocyte function via the AMPK/SOX-9 pathway. Mol. Immunol. 2021;140:70–76. doi: 10.1016/j.molimm.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Yin M., Xu Y. The protective effects of etomidate against interleukin-1beta (IL-1beta)-induced oxidative stress, extracellular matrix alteration and cellular senescence in chondrocytes. Bioengineered. 2022;13:985–994. doi: 10.1080/21655979.2021.2016085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C., Gao Y., Zhang Z., Chi Q., Liu Y., Yang L., Xu K. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-kappaB/SIRT1/AMPK signaling pathways. Phytomedicine. 2020;78:153305. doi: 10.1016/j.phymed.2020.153305. [DOI] [PubMed] [Google Scholar]
- 76.Huang H.T. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model. Antioxidants. 2020;10:8. doi: 10.3390/antiox10010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008;75:677–687. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 78.Dave M., Attur M., Palmer G., Al-Mussawir H.E., Kennish L., Patel J., Abramson S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Care Res. 2008;58:2786–2797. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 79.Masuda I., Koike M., Nakashima S., Mizutani Y., Ozawa Y., Watanabe K., Sawada Y., Sugiyama H., Sugimoto A., Nojiri H., et al. Apple procyanidins promote mitochondrial biogenesis and proteoglycan biosynthesis in chondrocytes. Sci. Rep. 2018;8:7229. doi: 10.1038/s41598-018-25348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ansari M.Y., Ahmad N., Haqqi T.M. Butein Activates Autophagy Through AMPK/TSC2/ULK1/mTOR Pathway to Inhibit IL-6 Expression in IL-1beta Stimulated Human Chondrocytes. Cell Physiol. Biochem. 2018;49:932–946. doi: 10.1159/000493225. [DOI] [PubMed] [Google Scholar]
- 81.Li Y., Wu Y., Jiang K., Han W., Zhang J., Xie L., Liu Y., Xiao J., Wang X. Mangiferin Prevents TBHP-Induced Apoptosis and ECM Degradation in Mouse Osteoarthritic Chondrocytes via Restoring Autophagy and Ameliorates Murine Osteoarthritis. Oxid. Med. Cell. Longev. 2019;2019:8783197. doi: 10.1155/2019/8783197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukuda K. Progress of research in osteoarthritis. Involvement of reactive oxygen species in the pathogenesis of osteoarthritis. Clin. Calcium. 2009;19:1602–1606. [PubMed] [Google Scholar]
- 83.Li Y.-S., Xiao W.-F., Luo W. Cellular aging towards osteoarthritis. Mech. Ageing Dev. 2017;162:80–84. doi: 10.1016/j.mad.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 84.Brandl A., Hartmann A., Bechmann V., Graf B., Nerlich M., Angele P. Oxidative stress induces senescence in chondrocytes. J. Orthop. Res. 2011;29:1114–1120. doi: 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- 85.Coppé J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.-W., Lasitschka F., Andrulis M., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeon O.H., Kim C., Laberge R.-M., DeMaria M., Rathod S., Vasserot A.P., Chung J.W., Kim D.H., Poon Y., David N., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diekman B.O., Collins J.A., Loeser R.F. Does Joint Injury Make Young Joints Old? J. Am. Acad. Orthop. Surg. 2018;26:e455–e456. doi: 10.5435/JAAOS-D-18-00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu M., Bradley E.W., Weivoda M.M., Hwang S.M., Pirtskhalava T., Decklever T., Curran G.L., Ogrodnik M., Jurk D., Johnson K.O., et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J. Gerontol. Ser. A. 2016;72:780–785. doi: 10.1093/gerona/glw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., Shirakawa K., Lim H.W., Davis S.S., Ramanathan A., et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2015;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCulloch K., Litherland G.J., Rai T.S. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16:210–218. doi: 10.1111/acel.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narita M. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srinivas V., Bohensky J., Shapiro I.M. Autophagy: A new phase in the maturation of growth plate chondrocytes is regulated by HIF, mTOR and AMP kinase. Cells Tissues Organs. 2009;189:88–92. doi: 10.1159/000151428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caramés B., Taniguchi N., Otsuki S., Blanco F.J., Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Care Res. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galluzzi L., Kepp O., Trojel-Hansen C., Kroemer G. Mitochondrial Control of Cellular Life, Stress, and Death. Circ. Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 96.Mariño G., Niso-Santano M., Baehrecke E.H., Kroemer G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carames B., Olmer M., Kiosses W.B., Lotz M.K. The Relationship of Autophagy Defects to Cartilage Damage During Joint Aging in a Mouse Model. Arthritis Rheumatol. 2015;67:1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Francisco J., Blanco R.L.O., Schwarz T.H., Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Patol. 1995;146:75. [PMC free article] [PubMed] [Google Scholar]
- 99.Zhong Z. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caramés B., de Figueroa P.L., Lotz M., Blanco F. Autophagy activation protects from mitochondrial dysfunction in human chondrocytes. Osteoarthr. Cartil. 2014;22:966–976. doi: 10.1016/j.joca.2014.02.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blanco F.J., Kim H.A. Cell Death and Apoptosis in Ostearthritic Cartilage. Curr. Drug Targets. 2007;8:333–345. doi: 10.2174/138945007779940025. [DOI] [PubMed] [Google Scholar]
- 103.Maneiro E., López-Armada M.J., De Andres M.C., Caramés B., Martín M.A., Bonilla A., Del Hoyo P., Galdo F., Arenas J., Blanco F.J. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2004;64:388–395. doi: 10.1136/ard.2004.022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loening A., James I.E., Levenston M., Badger A.M., Frank E.H., Kurz B., Nuttall M.E., Hung H.-H., Blake S.M., Grodzinsky A.J., et al. Injurious Mechanical Compression of Bovine Articular Cartilage Induces Chondrocyte Apoptosis. Arch. Biochem. Biophys. 2000;381:205–212. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- 105.Williams G.S. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lv M., Zhou Y., Chen X., Han L., Wang L., Lu X.L. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J. Orthop. Res. 2017;36:730–738. doi: 10.1002/jor.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shapiro I.M., S Kakuta E.E.G., Hazelgrove J., Havery J., Chance B., Frasca P. Initiation of Endochondral Calcification Is Related to Changes in the Redox State of Hypertrophic Chondrocytes. Science. 1982;217:950–952. doi: 10.1126/science.7112108. [DOI] [PubMed] [Google Scholar]
- 108.Matsumoto H., Debolt K., Shapiro I.M. Adenine, guanine, and inosine nucleotides of chick growth cartilage relationship between energy status and the mineralization process. J. Bone Miner. Res. 1988;3:347–352. doi: 10.1002/jbmr.5650030315. [DOI] [PubMed] [Google Scholar]
- 109.Johnson K.A.J., Murphy A., Andreyev A., Dykens J., Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2000;43:1560–1570. doi: 10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 110.D’Andrea P., Calabrese A., Capozzi I., Grandolfo M., Tonon R., Vittur F. Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology. 2000;37:75–83. doi: 10.1016/S0014-5793(96)01356-7. [DOI] [PubMed] [Google Scholar]
- 111.Huser C.A.M., Davies M.E. Calcium signaling leads to mitochondrial depolarization in impact-induced chondrocyte death in equine articular cartilage explants. Arthritis Care Res. 2007;56:2322–2334. doi: 10.1002/art.22717. [DOI] [PubMed] [Google Scholar]
- 112.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M., Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. USA. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wann A.K.T., Zuo N., Haycraft C.J., Jensen C.G., Poole C.A., McGlashan S.R., Knight M.M. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2011;26:1663–1671. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duchen M.R. Contributions of mitochondria to animal physiology: From homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borutaite V., Brown C.G. Release of cytochrome c from heart mitochondria is induced by high Ca2+ and peroxynitrite and is responsible for Ca2+-induced inhibition of substrate oxidation. Biochim. Biophys. Acta Mol. Basis Dis. 1999;1453:41–48. doi: 10.1016/S0925-4439(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 116.Sato M., Sato K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim. Biophys. Acta. 2013;1833:1979–1984. doi: 10.1016/j.bbamcr.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 117.Anderson S. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 118.Lü H.B., Zhou Y., Hu J.Z. Mitochondrial DNA deletion mutations in articular chondrocytes of cartilage affected by osteoarthritis. Yi Xue Ban J. Cent. South Univ. Med. Sci. 2006;31:640–644. [PubMed] [Google Scholar]
- 119.Ruiz-Pesini E., Mishmar D., Brandon M., Procaccio V., Wallace D.C. Effects of Purifying and Adaptive Selection on Regional Variation in Human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 120.Wallace D.C. Maternal genes: Mitochondrial diseases. Birth Defects Orig. Artic. Ser. 1987;23:137–190. [PubMed] [Google Scholar]
- 121.Blanco F.J., Valdes A., Rego-Pérez I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018;14:327–340. doi: 10.1038/s41584-018-0001-0. [DOI] [PubMed] [Google Scholar]
- 122.Rego I., Fernández-Moreno M., Fernández-López C., Gómez-Reino J.J., González A., Arenas J., Blanco F.J. Role of European mitochondrial DNA haplogroups in the prevalence of hip osteoarthritis in Galicia, Northern Spain. Ann. Rheum. Dis. 2009;69:210–213. doi: 10.1136/ard.2008.105254. [DOI] [PubMed] [Google Scholar]
- 123.Rego-Pérez I., Fernández-Moreno M., Fernández-López C., Arenas J., Blanco F.J. Mitochondrial DNA haplogroups: Role in the prevalence and severity of knee osteoarthritis. Arthritis Care Res. 2008;58:2387–2396. doi: 10.1002/art.23659. [DOI] [PubMed] [Google Scholar]
- 124.Soto-Hermida A., Fernandez-Moreno M., Oreiro N., Fernández-López C., Rego-Pérez I., Blanco F. mtDNA haplogroups and osteoarthritis in different geographic populations. Mitochondrion. 2014;15:18–23. doi: 10.1016/j.mito.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 125.Fernandez-Moreno M., Soto-Hermida A., Mosquera M.E.V., Cortés-Pereira E., Relaño S., Gómez T.H., Pértega S., Oreiro-Villar N., Fernández-López C., Garesse R., et al. Mitochondrial DNA haplogroups influence the risk of incident knee osteoarthritis in OAI and CHECK cohorts. A meta-analysis and functional study. Ann. Rheum. Dis. 2016;76:1114–1122. doi: 10.1136/annrheumdis-2016-210131. [DOI] [PubMed] [Google Scholar]
- 126.Fang H., Liu X., Shen L., Li F., Liu Y., Chi H., Miao H., Lu J., Bai Y. Role of mtDNA Haplogroups in the Prevalence of Knee Osteoarthritis in a Southern Chinese Population. Int. J. Mol. Sci. 2014;15:2646–2659. doi: 10.3390/ijms15022646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao Z., Li Y., Wang M., Jin Y., Liao W., Zhao Z., Fang J. Mitochondrial DNA haplogroups participate in osteoarthritis: Current evidence based on a meta-analysis. Clin. Rheumatol. 2020;39:1027–1037. doi: 10.1007/s10067-019-04890-x. [DOI] [PubMed] [Google Scholar]
- 128.Fernandez-Moreno M., Soto-Hermida A., Mosquera M.E.V., Cortés-Pereira E., Pértega S., Relaño S., Oreiro-Villar N., Fernández-López C., Blanco F.J., Rego-Pérez I. A replication study and meta-analysis of mitochondrial DNA variants in the radiographic progression of knee osteoarthritis. Rheumatology. 2016;56:263–270. doi: 10.1093/rheumatology/kew394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Soto-Hermida A., Fernández-Moreno M., Pértega-Díaz S., Oreiro N., Fernández-López C., Blanco F.J., Rego-Pérez I. Mitochondrial DNA haplogroups modulate the radiographic progression of Spanish patients with osteoarthritis. Rheumatol. Int. 2014;35:337–344. doi: 10.1007/s00296-014-3104-1. [DOI] [PubMed] [Google Scholar]
- 130.Vaamonde-García C., López-Armada M.J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 2019;165:181–195. doi: 10.1016/j.bcp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Kenney M.C., Chwa M., Atilano S.R., Falatoonzadeh P., Ramirez C., Malik D., Tarek M., Cáceres-Del-Carpio J., Nesburn A.B., Boyer D.S., et al. Inherited mitochondrial DNA variants can affect complement, inflammation and apoptosis pathways: Insights into mitochondrial-nuclear interactions. Hum. Mol. Genet. 2014;23:3537–3551. doi: 10.1093/hmg/ddu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martínez-Redondo D., Marcuello A., Casajús J.A., Ara I., Dahmani Y., Montoya J., Ruiz-Pesini E., López-Pérez M.J., Díez-Sánchez C. Human mitochondrial haplogroup H: The highest VO2max consumer—Is it a paradox? Mitochondrion. 2010;10:102–107. doi: 10.1016/j.mito.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 133.Zole E., Ranka R. Mitochondria, its DNA and telomeres in ageing and human population. Biogerontology. 2018;19:189–208. doi: 10.1007/s10522-018-9748-6. [DOI] [PubMed] [Google Scholar]
- 134.Canto C., Auwerx J. Calorie restriction: Is AMPK a key sensor and effector? Physiology. 2011;26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Garcia D., Shaw R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cantó C., Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell. Mol. Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu J., Ji J., Yan X.-H. Cross-Talk between AMPK and mTOR in Regulating Energy Balance. Crit. Rev. Food Sci. Nutr. 2012;52:373–381. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- 139.Herzig S., Shaw R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013;45:51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang H.-C., Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2013;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Verdin E., Hirschey M., Finley L.W., Haigis M.C. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brown K., Xie S., Qiu X., Mohrin M., Shin J., Liu Y., Zhang D., Scadden D.T., Chen D. SIRT3 Reverses Aging-Associated Degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salinas D., Mumey B.M., June R.K. Physiological dynamic compression regulates central energy metabolism in primary human chondrocytes. Biomech. Model. Mechanobiol. 2018;18:69–77. doi: 10.1007/s10237-018-1068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wolff K.J., Ramakrishnan P.S., Brouillette M.J., Journot B.J., McKinley T.O., Buckwalter J.A., Martin J.A. Mechanical stress and ATP synthesis are coupled by mitochondrial oxidants in articular cartilage. J. Orthop. Res. 2013;31:191–196. doi: 10.1002/jor.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He D.-S., Hu X.-J., Yan Y.-Q., Liu H. Underlying mechanism of Sirt1 on apoptosis and extracellular matrix degradation of osteoarthritis chondrocytes. Mol. Med. Rep. 2017;16:845–850. doi: 10.3892/mmr.2017.6659. [DOI] [PubMed] [Google Scholar]
- 147.Wang C., Yang Y., Zhang Y., Liu J., Yao Z., Zhang C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends. 2019;12:605–612. doi: 10.5582/bst.2018.01263. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y., Zhao X., Lotz M., Terkeltaub R., Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha. Arthritis Rheumatol. 2015;67:2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao X., Petursson F., Viollet B., Lotz M., Terkeltaub R., Liu-Bryan R. Peroxisome proliferator-activated receptor gamma coactivator 1alpha and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014;66:3073–3082. doi: 10.1002/art.38791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jäger S., Handschin C., St-Pierre J., Spiegelman B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. 2007. Proc. Natl. Acad. Sci. USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gleyzer N., Vercauteren K., Scarpulla R.C. Control of Mitochondrial Transcription Specificity Factors (TFB1M and TFB2M) by Nuclear Respiratory Factors (NRF-1 and NRF-2) and PGC-1 Family Coactivators. Mol. Cell. Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Larsson N.G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G.S., Clayton D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in micepdf. Nat. Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 153.Poulet B., Staines K.A. New developments in osteoarthritis and cartilage biology. Curr. Opin. Pharmacol. 2016;28:8–13. doi: 10.1016/j.coph.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 154.Shan Y., Wei Z., Tao L., Wang S., Zhang F., Shen C., Wu H., Liu Z., Zhu P., Wang A., et al. Prophylaxis of Diallyl Disulfide on Skin Carcinogenic Model via p21-dependent Nrf2 stabilization. Sci. Rep. 2016;6:35676. doi: 10.1038/srep35676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Collins J.A., Diekman B., Loeser R.F. Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 2018;30:101–107. doi: 10.1097/BOR.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marchev A.S., Dimitrova P.A., Burns A.J., Kostov R.V., Dinkova-Kostova A.T., Georgiev M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can NRF2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017;1401:114–135. doi: 10.1111/nyas.13407. [DOI] [PubMed] [Google Scholar]
- 157.Wang L., Shan H., Wang B., Wang N., Zhou Z., Pan C., Wang F. Puerarin Attenuates Osteoarthritis via Upregulating AMP-Activated Protein Kinase/Proliferator-Activated Receptor-gamma Coactivator-1 Signaling Pathway in Osteoarthritis Rats. Pharmacology. 2018;102:117–125. doi: 10.1159/000490418. [DOI] [PubMed] [Google Scholar]
- 158.Bento C.F., Renna M., Ghislat G., Puri C., Ashkenazi A., Vicinanza M., Menzies F.M., Rubinsztein D.C. Mammalian Autophagy: How Does It Work? Annu. Rev. Biochem. 2016;85:685–713. doi: 10.1146/annurev-biochem-060815-014556. [DOI] [PubMed] [Google Scholar]
- 159.Kubli D.A., Gustafsson A.B. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Inoki K., Zhu T., Guan K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 161.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W., Vasquez D.S., Joshi A., Gwinn D.M., Taylor R., et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Laker R.C., Drake J.C., Wilson R.J., Lira V.A., Lewellen B.M., Ryall K.A., Fisher C.C., Zhang M., Saucerman J.J., Goodyear L.J., et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017;8:548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Blanco F.J., Rego-Pérez I. Mitochondria and mitophagy: Biosensors for cartilage degradation and osteoarthritis. Osteoarthr. Cartil. 2018;26:989–991. doi: 10.1016/j.joca.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 165.Yu W., Gao B., Li N., Wang J., Qiu C., Zhang G., Liu M., Zhang R., Li C., Ji G., et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017;1863:1973–1983. doi: 10.1016/j.bbadis.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 166.Greer E., Oskoui P.R., Banko M.R., Maniar J.M., Gygi M.P., Gygi S.P., Brunet A. The Energy Sensor AMP-activated Protein Kinase Directly Regulates the Mammalian FOXO3 Transcription Factor. J. Biol. Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 167.Zhao J., Brault J.J., Schild A., Cao P., Sandri M., Schiaffino S., Lecker S.H., Goldberg A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 168.Bowman C.J., Ayer D., Dynlacht B.D. Foxk proteins repress the initiation of starvation-induced atrophy and autophagy programs. Nat. Cell Biol. 2014;16:1202–1214. doi: 10.1038/ncb3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sarraf S., Raman M., Guarani-Pereira V., Sowa M.E., Huttlin E., Gygi S.P., Harper J.W. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nguyen T.N., Padman B.S., Lazarou M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 171.Ansari M.Y., Khan N.M., Ahmad I., Haqqi T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthr. Cartil. 2018;26:1087–1097. doi: 10.1016/j.joca.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Choi A.M., Ryter S.W., Levine B. Autophagy in Human Health and Disease. N. Engl. J. Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 173.Caramés B., Hasegawa A., Taniguchi N., Miyaki S., Blanco F.J., Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 2011;71:575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ansari A., Rahman M.S., Saha S.K., Saikot F.K., Deep A., Kim K.-H. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell. 2017;16:4–16. doi: 10.1111/acel.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen L.-Y., Wang Y., Terkeltaub R., Liu-Bryan R. Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthr. Cartil. 2018;26:1539–1550. doi: 10.1016/j.joca.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Olmos Y., Valle I., Borniquel S., Tierrez A., Soria E., Lamas S., Monsalve M. Mutual Dependence of Foxo3a and PGC-1α in the Induction of Oxidative Stress Genes. J. Biol. Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kincaid B., Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu W., Dittenhafer-Reed K., Denu J.M. SIRT3 Protein Deacetylates Isocitrate Dehydrogenase 2 (IDH2) and Regulates Mitochondrial Redox Status. J. Biol. Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhu S., Makosa D., Miller B.F., Griffin T.M. Glutathione as a mediator of cartilage oxidative stress resistance and resilience during aging and osteoarthritis. Connect. Tissue Res. 2019;61:34–47. doi: 10.1080/03008207.2019.1665035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cheng Y., Ren X., Gowda A.S., Shan Y., Zhang L., Yuan Y.-S., Patel R., Wu H., Huber-Keener K., Yang J.W., et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013;4:e731. doi: 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 182.Wei H., Bu R., Yang Q., Jia J., Li T., Wang Q., Chen Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019;2019:8905917. doi: 10.1155/2019/8905917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nakahira K., Haspel J.A., Rathinam V.A.K., Lee S.-J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P., et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Takayama K., Ishida K., Matsushita T., Fujita N., Hayashi S., Sasaki K., Tei K., Kubo S., Matsumoto T., Fujioka H., et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Care Res. 2009;60:2731–2740. doi: 10.1002/art.24864. [DOI] [PubMed] [Google Scholar]
- 185.Mattick J.S., Makunin I.V. Non-coding RNA. Hum. Mol. Genet. 2006;15:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 186.Huang B., Zhang R. Regulatory non-coding RNAs: Revolutionizing the RNA world. Mol. Biol. Rep. 2014;41:3915–3923. doi: 10.1007/s11033-014-3259-6. [DOI] [PubMed] [Google Scholar]
- 187.Wei J.W., Huang K., Yang C., Kang C.S. Non-coding RNAs as regulators in epigenetics (Review) Oncol. Rep. 2017;37:3–9. doi: 10.3892/or.2016.5236. [DOI] [PubMed] [Google Scholar]
- 188.Yao R.-W., Wang Y., Chen L.-L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 189.Su W., Xie W., Shang Q., Su B. The Long Noncoding RNA MEG3 Is Downregulated and Inversely Associated with VEGF Levels in Osteoarthritis. BioMed Res. Int. 2015;2015:356893. doi: 10.1155/2015/356893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xing D., Liang J.-Q., Li Y., Lu J., Jia H.-B., Xu L.-Y., Ma X.-L. Identification of Long Noncoding RNA Associated with Osteoarthritis in Humans. Orthop. Surg. 2014;6:288–293. doi: 10.1111/os.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Svoboda M., Slyskova J., Schneiderová M., Makovicky P., Bielik L., Levy M., Lipska L., Hemmelova B., Kala Z., Protivankova M., et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 192.Hu J., Wang Z., Pan Y., Ma J., Miao X., Qi X., Zhou H., Jia L. MiR-26a and miR-26b mediate osteoarthritis progression by targeting FUT4 via NF-kappaB signaling pathway. Int. J. Biochem. Cell Biol. 2018;94:79–88. doi: 10.1016/j.biocel.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 193.Hu J., Wang Z., Shan Y., Pan Y., Ma J., Jia L. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/beta-catenin axis. Cell Death Dis. 2018;9:711. doi: 10.1038/s41419-018-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dou P.D.P., Hu R.H.R., Zhu W.Z.W., Tang Q.T.Q., Li D.L.D., Li H.L.H., Wang W.W.W. Long non-coding RNA HOTAIR promotes expression of ADAMTS-5 in human osteoarthritic articular chondrocytes. Pharmazie. 2017;72:113–117. doi: 10.1691/PH.2017.6649. [DOI] [PubMed] [Google Scholar]
- 195.Jiang M., Liu J., Luo T., Chen Q., Lu M., Meng D. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci. Rep. 2019;39:BSR20190404. doi: 10.1042/BSR20190404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou Q., Tang X., Tian X., Tian J., Zhang Y., Ma J., Xu H., Wang S. LncRNA MALAT1 negatively regulates MDSCs in patients with lung cancer. J. Cancer. 2018;9:2436–2442. doi: 10.7150/jca.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang Y., Wang F., Chen G., He R., Yang L. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019;9:54. doi: 10.1186/s13578-019-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nanus D.E., Wijesinghe S.N., Pearson M., Hadjicharalambous M.R., Rosser A., Davis E.T., Lindsay M.A., Jones S.W. Regulation of the Inflammatory Synovial Fibroblast Phenotype by Metastasis-Associated Lung Adenocarcinoma Transcript 1 Long Noncoding RNA in Obese Patients with Osteoarthritis. Arthritis Rheumatol. 2019;72:609–619. doi: 10.1002/art.41158. [DOI] [PubMed] [Google Scholar]
- 199.Song J., Ahn C., Chun C.-H., Jin E.-J. A long non-coding RNA, GAS5, plays a critical role in the regulation of miR-21 during osteoarthritis. J. Orthop. Res. 2014;32:1628–1635. doi: 10.1002/jor.22718. [DOI] [PubMed] [Google Scholar]
- 200.Hu Y., Li S., Zou Y. Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis. Yonsei Med. J. 2019;60:381–388. doi: 10.3349/ymj.2019.60.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Steck E., Boeuf S., Gabler J., Werth N., Schnatzer P., Diederichs S., Richter W. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. Klin. Wochenschr. 2012;90:1185–1195. doi: 10.1007/s00109-012-0895-y. [DOI] [PubMed] [Google Scholar]
- 202.Chen K., Zhu H., Zheng M.Q., Dong Q.R. LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage. 2021;13((Suppl. S2)):1274S–1284S. doi: 10.1177/1947603519855759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou H., Wu G., Ma X., Xiao J., Yu G., Yang C., Xu N., Zhang B., Zhou J., Ye Z., et al. Attenuation of TGFBR2 expression and tumour progression in prostate cancer involve diverse hypoxia-regulated pathways. J. Exp. Clin. Cancer Res. 2018;37:89. doi: 10.1186/s13046-018-0764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang Z., Chi X., Liu L., Wang Y., Mei X., Yang Y., Jia T. RETRACTED: Long noncoding RNA maternally expressed gene 3 knockdown alleviates lipopolysaccharide-induced inflammatory injury by up-regulation of miR-203 in ATDC5 cells. Biomed. Pharmacother. 2018;100:240–249. doi: 10.1016/j.biopha.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 205.Zhu J.-K., He T.-D., Wei Z.-X., Wang Y.-M. LncRNA FAS-AS1 promotes the degradation of extracellular matrix of cartilage in osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2966–2972. doi: 10.26355/eurrev_201805_15051. [DOI] [PubMed] [Google Scholar]
- 206.Wang Y., Cao L., Wang Q., Huang J., Xu S. LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019;47:1241–1247. doi: 10.1080/21691401.2019.1596940. [DOI] [PubMed] [Google Scholar]
- 207.Cao L., Wang Y., Wang Q., Huang J. LncRNA FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by acting as a sponge of miR-206 to modulate CCND1 expression. Biomed. Pharmacother. 2018;106:1220–1226. doi: 10.1016/j.biopha.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 208.Malemud C.J. MicroRNAs and Osteoarthritis. Cells. 2018;7:92. doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Al-Modawi R.N., Brinchmann J.E., Karlsen T.A. Multi-pathway Protective Effects of MicroRNAs on Human Chondrocytes in an In Vitro Model of Osteoarthritis. Mol. Ther.—Nucleic Acids. 2019;17:776–790. doi: 10.1016/j.omtn.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang H., Zhang H., Sun Q., Wang Y., Yang J., Yang J., Zhang T., Luo S., Wang L., Jiang Y., et al. Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol. Ther. 2017;25:715–727. doi: 10.1016/j.ymthe.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hu G., Zhao X., Wang C., Geng Y., Zhao J., Xu J., Zuo B., Zhao C., Wang C., Zhang X. MicroRNA-145 attenuates TNF-alpha-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8:e3140. doi: 10.1038/cddis.2017.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li L., Jia J., Liu X., Yang S., Ye S., Yang W., Zhang Y. MicroRNA-16-5p Controls Development of Osteoarthritis by Targeting SMAD3 in Chondrocytes. Curr. Pharm. Des. 2015;21:5160–5167. doi: 10.2174/1381612821666150909094712. [DOI] [PubMed] [Google Scholar]
- 213.Zhang Y., Jia J., Yang S., Liu X., Ye S., Tian H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp. Mol. Med. 2014;46:e79. doi: 10.1038/emm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li J., Huang J., Dai L., Yu D., Chen Q., Zhang X., Dai K. miR-146a, an IL-1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res. Ther. 2012;14:R75. doi: 10.1186/ar3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu X., Liu L., Zhang H., Shao Y., Chen Z., Feng X., Fang H., Zhao C., Pan J., Zhang H., et al. MiR-146b accelerates osteoarthritis progression by targeting alpha-2-macroglobulin. Aging. 2019;11:6014–6028. doi: 10.18632/aging.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhai X., Meng R., Li H., Li J., Jing L., Qin L., Gao Y. miR-181a Modulates Chondrocyte Apoptosis by Targeting Glycerol-3-Phosphate Dehydrogenase 1-Like Protein (GPD1L) in Osteoarthritis. Med. Sci. Monit. 2017;23:1224–1231. doi: 10.12659/MSM.899228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li Z., Meng D., Li G., Xu J., Tian K., Li Y. Overexpression of microRNA-210 promotes chondrocyte proliferation and extracellular matrix deposition by targeting HIF-3α in osteoarthritis. Mol. Med. Rep. 2016;13:2769–2776. doi: 10.3892/mmr.2016.4878. [DOI] [PubMed] [Google Scholar]
- 218.Jones S., Watkins G., Le Good N., Roberts S., Murphy C., Brockbank S., Needham M., Read S., Newham P. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthr. Cartil. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 219.Bazzoni F., Rossato M., Fabbri M., Gaudiosi D., Mirolo M., Mori L., Tamassia N., Mantovani A., Cassatella M.A., Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. USA. 2009;106:5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Park S.J., Cheon E.J., Kim H.A. MicroRNA-558 regulates the expression of cyclooxygenase-2 and IL-1beta-induced catabolic effects in human articular chondrocytes. Osteoarthr. Cartil. 2013;21:981–989. doi: 10.1016/j.joca.2013.04.012. [DOI] [PubMed] [Google Scholar]