Abstract
Argonaute proteins use nucleic acid guides to find and bind specific DNA or RNA target sequences. Argonaute proteins have diverse biological functions and many retain their ancestral endoribonuclease activity, cleaving the phosphodiester bond between target nucleotides t10 and t11. In animals, the PIWI proteins—a specialized class of Argonaute proteins—use 21–35 nucleotide PIWI-interacting RNAs (piRNAs) to direct transposon silencing, protect the germline genome, and regulate gene expression during gametogenesis1. The piRNA pathway is required for fertility in one or both sexes of nearly all animals. Both piRNA production and function require RNA cleavage catalysed by PIWI proteins. Spermatogenesis in mice and other placental mammals requires three distinct, developmentally regulated PIWI proteins: MIWI (PIWIL1), MILI (PIWIL2) and MIWI22–4 (PIWIL4). The piRNA-guided endoribonuclease activities of MIWI and MILI are essential for the production of functional sperm5,6. piRNA-directed silencing in mice and insects also requires GTSF1, a PIWI-associated protein of unknown function7–12. Here we report that GTSF1 potentiates the weak, intrinsic, piRNA-directed RNA cleavage activities of PIWI proteins, transforming them into efficient endoribonucleases. GTSF1 is thus an example of an auxiliary protein that potentiates the catalytic activity of an Argonaute protein.
Subject terms: RNA, RNAi
The evolutionarily conserved RNA-binding protein GTSF1 and its homologues interact with members of the PIWI class of Argonaute proteins, increasing the efficiency of the RNA-cleaving activity of PIWI proteins, an essential function across the animal kingdom.
Main
In animals, PIWI-interacting RNAs (piRNAs) 21 to 35 nucleotides in length direct PIWI proteins to silence transposons and regulate gene expression1. Invertebrates produce piRNAs and PIWI proteins in both the soma and the germline but mammalian piRNAs act only in the germline. Mice that lack any of their three PIWI proteins—MIWI2, MILI and MIWI—or other proteins required for piRNA production are invariably male-sterile1–4. As in other animals, mouse piRNA production requires catalytically active PIWI proteins, MILI and MIWI5,6. Transposon silencing is the ancestral function of piRNAs; uniquely, placental mammals also produce pachytene piRNAs, which first appear shortly after the onset of meiosis I13–18 and reach a peak abundance in spermatocytes rivalling that of ribosomes19. Pachytene piRNAs tune the abundance of mRNAs required for spermiogenesis, the process by which round spermatids become sperm20–23. Pachytene piRNAs have been proposed to direct MIWI and MILI to cleave specific mRNAs, ensuring appropriate levels of their protein products20,23–25. Testing this idea has been thwarted by the absence of a cell-free system in which MIWI or MILI can be loaded with synthetic piRNAs of defined sequence.
Recombinant MIWI loaded with a defined piRNA
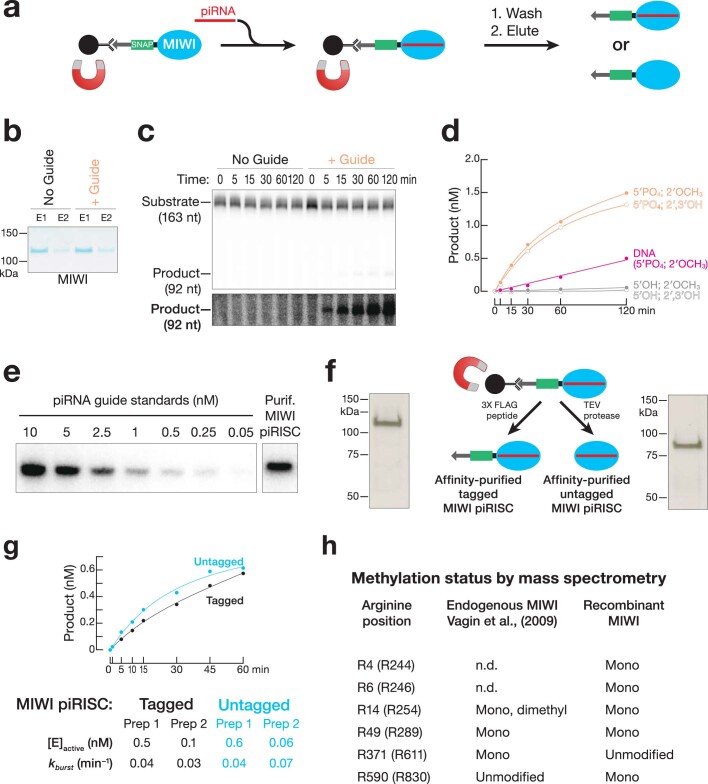
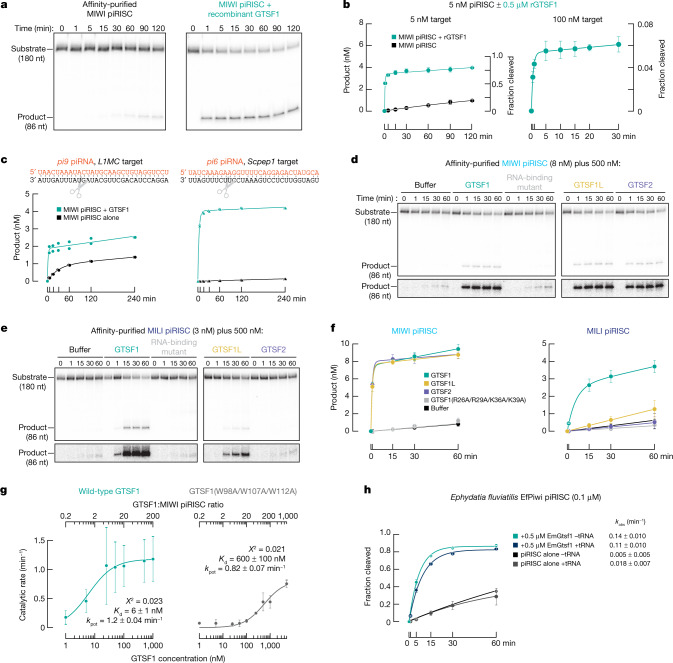
We used lentiviral transduction to engineer a stable HEK293T cell line over-expressing epitope-tagged MIWI. Tagged MIWI was captured from cell lysate using anti-Flag antibody coupled to paramagnetic beads, incubated with a synthetic piRNA bearing a monophosphorylated 5′ terminus and 2′-O-methylated 3′ end, and eluted from the magnetic beads using 3×Flag peptide. MIWI loaded with a synthetic piRNA (MIWI piRISC) (Fig. 1a), but not unloaded apo MIWI (Extended Data Fig. 1a), cleaved a 5′ 32P-radiolabelled target RNA fully complementary to the synthetic guide (Extended Data Fig. 1b,c and Supplementary Data Fig. 1).
Fig. 1. A component of mouse testis lysate potentiates piRNA-directed target RNA cleavage by MIWI.
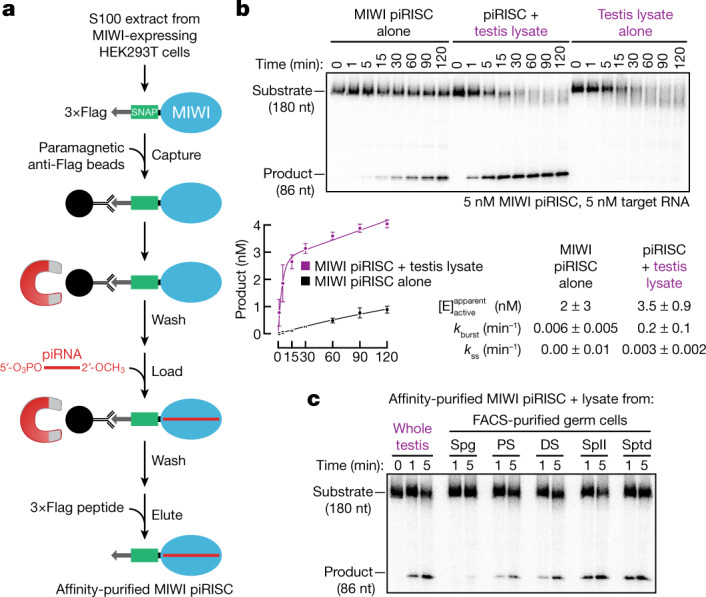
a, Strategy for programming recombinant MIWI with synthetic piRNA. b, Top, representative denaturing polyacrylamide gel electrophoresis showing target RNA cleavage by MIWI piRISC with and without added testis lysate. Bottom, product formed as a function of time by MIWI piRISC (mean ± s.d., n = 3). Rate constants were determined by fitting the data to the burst-and-steady-state equation (Methods, equation (1)). , the apparent concentration of active MIWI piRISC estimated from data fitting. c, Target RNA cleavage by MIWI piRISC in the presence of lysate from either whole testis or FACS-purified germ cells. DS, diplotene spermatocytes; PS, pachytene spermatocytes; SpII, secondary spermatocytes; Spg, spermatogonia; Sptd, spermatids. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 1. Recombinant MIWI programmed with guide piRNA cleaves complementary target RNA.
a, Purification of apo-MIWI and of MIWI loaded with a synthetic piRNA guide (piRISC). b, Coomassie-stained SDS-PAGE of purified apo-MIWI and MIWI piRISC. E1, E2: first and second eluates from sequential incubation of the paramagnetic beads with 3XFLAG peptide. E1 was used for the cleavage assay. c, Representative denaturing polyacrylamide gel of target cleavage assay using apo-MIWI or MIWI piRISC. A digital overexposure is shown below the gel. d, MIWI piRISC loaded with synthetic guides differing at their 5′ or 3′ termini was assayed for the ability to cleave a complementary target RNA. e, Northern blot to estimate the yield of purified MIWI piRISC. f, Silver-stained gel showing purified MIWI piRISC with and without an amino-terminal epitope tag. g, Target cleavage assay comparing the activity of piRISC with and without an amino-terminal epitope tag. Data were fit to Equation 1 (see Methods); error of fit (SD) is reported. h, Mass spectrometry was used to determine arginine methylation status for recombinant, affinity-purified MIWI (n = 3); arginine methylation status of endogenous MIWI from mouse testis is provided for comparison76. Arginine position reports the amino acid number in endogenous mouse MIWI and in parentheses, the corresponding residue in the epitope-tagged recombinant protein. Only modified arginine residues detected in all three independent preparations are reported. N.D., not detected. For gel source data, see Supplementary Fig. 1.
Recombinant MIWI bound stably to an RNA guide bearing a 5′ monophosphate but not to a guide with a 5′ hydroxyl group (Extended Data Fig. 1d). The MID domain of Argonaute proteins contains a 5′ monophosphate-binding pocket that anchors the RNA guide to the protein26–30. Mutations predicted to disrupt 5′ monophosphate-binding perturb PIWI function28,31–35. We immobilized MIWI on paramagnetic beads via its 3×Flag tag, incubated it with guide RNA, washed the beads, eluted the MIWI piRISC with 3×Flag tag peptide, and tested its ability to cleave a fully complementary target RNA. Incubation with a 5′ monophosphorylated but not an otherwise identical 5′ hydroxy guide yielded MIWI piRISC that cleaved target RNA (Extended Data Fig. 1d). In vivo, the methyltransferase HENMT1 adds a 2′-O-methyl group to the 3′ ends of piRNAs. Terminal 2′-O-methyl modification likely stabilizes small RNAs against degradation by cellular ribonucleases rather than secures the guide to the protein1. Consistent with this function for 2′-O-methylation, piRNAs bearing a 3′ terminal 2′ hydroxyl or 2′-O-methyl were equally functional in directing target cleavage, provided that the piRNA was 5′ monophosphorylated (Extended Data Fig. 1d).
Purified MIWI piRISC is a slow-acting enzyme
Although affinity-purified MIWI loaded with a piRNA bearing 5′ monophosphorylated and 3′, 2′-O-methylated termini specifically cleaved a fully complementary target RNA at the phosphodiester bond that links target nucleotides t10 to t11, the rate of cleavage (0.01 min−1) was more than 300 times slower than that catalysed by mouse AGO2 RISC36,37 (≥3 min−1). At physiological temperature (37 °C), mouse AGO2 RISC catalyses multiple rounds of target cleavage38. By contrast, 5 nM MIWI piRISC cleaved only around 15% of the target RNA (5 nM) after 1 h (Fig. 1b, Extended Data Fig. 1e and Supplementary Fig. 1). Inefficient target cleavage by MIWI piRISC was not caused by the presence of the amino-terminal 3×Flag–SNAP tandem tag: removing the tandem tags using tobacco etch virus protease generated an untagged protein (Extended Data Fig. 1f and Supplementary Data Fig. 1) whose target-cleavage kinetics were identical to that of piRISC assembled with the tagged MIWI (Extended Data Fig. 1g).
The ubiquitously expressed arginine methyltransferase PRMT5 modifies the amino terminus of PIWI proteins, allowing it to bind Tudor domain-containing proteins, many of which are required for piRNA biogenesis, gametogenesis and fertility39–41. A potential explanation for the sluggish activity of recombinant MIWI piRISC is that it lacks arginine methylation. We used mass spectrometry to map the positions of methyl arginine in our affinity-purified MIWI. All of the arginine residues previously shown to be methylated in endogenous MIWI immunoprecipitated from mouse testis39 were methylated in recombinant MIWI produced in HEK293T cells (Extended Data Fig. 1h).
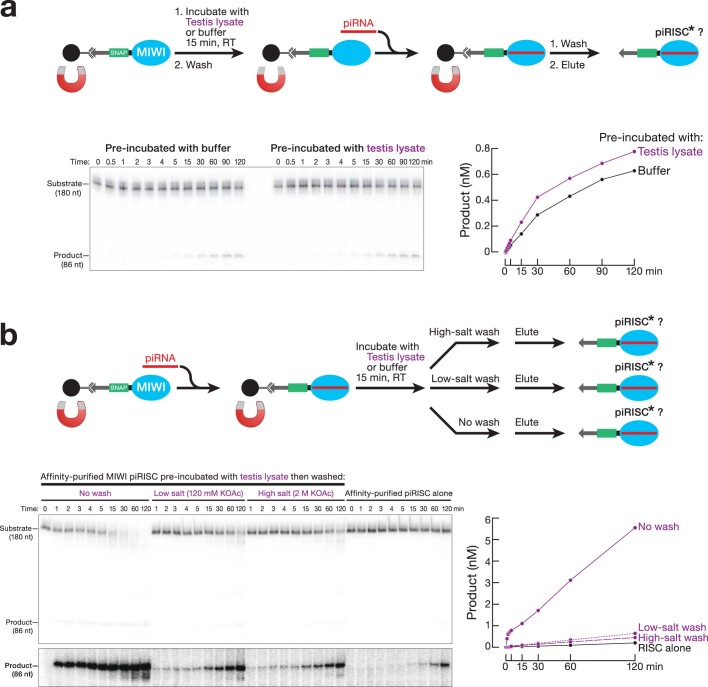
Another possible explanation for the inefficiency of target cleavage by MIWI piRISC is that the recombinant protein, although properly arginine methylated, lacks other post-translational modifications. To test this idea, we immobilized apo MIWI, incubated it with wild-type (C57BL/6) mouse testis lysate in the presence of an ATP-regenerating system at 25 °C for 15 min, removed the testis lysate by washing, and then loaded MIWI with a synthetic piRNA and eluted the resulting piRISC. Pre-incubation of MIWI with testis lysate either before or after loading with a piRNA did not increase its target-cleaving activity (Extended Data Fig. 2a,b). We conclude that neither a missing post-translational modification nor a tightly associated protein partner is likely to explain the low activity of MIWI piRISC compared to AGO2 RISC.
Extended Data Fig. 2. The target-cleavage potentiating component in testis lysate component transiently interacts with MIWI.
a, Immobilized, unloaded apo-MIWI was preincubated with testis lysate, purified as depicted, and then assayed for target cleavage activity. b, Immobilized MIWI piRISC was preincubated with testis lysate. purified as depicted, and then assayed for target cleavage activity. A digital overexposure is shown below the gel. For gel source data, see Supplementary Fig. 1.
MIWI piRISC requires an auxiliary factor
Adding testis lysate increased the rate of target cleavage by affinity-purified MIWI piRISC around 20-fold (Fig. 1b and Extended Data Fig. 2b). This effect cannot be attributed to the lysate contributing additional piRISC, because testis lysate alone did not generate detectable cleavage product (Fig. 1b).
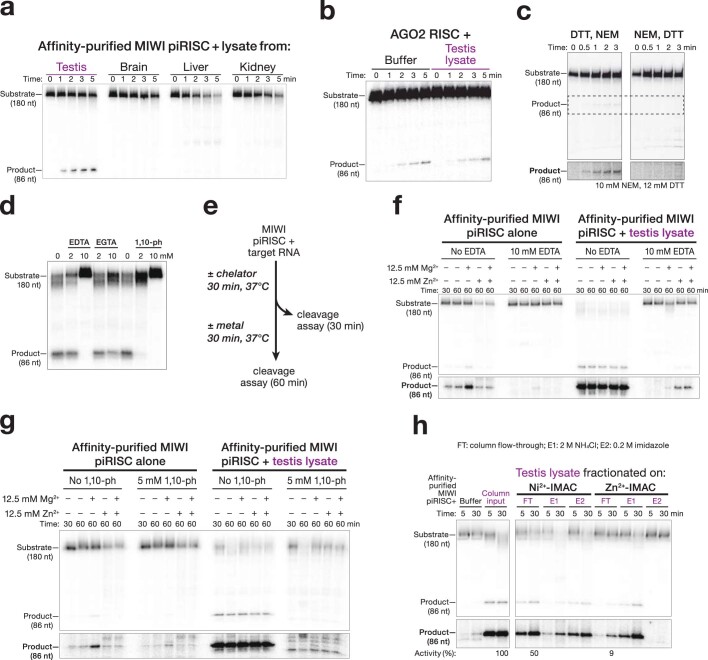
MIWI is first produced as spermatogonia enter meiosis and differentiate into spermatocytes3,42. To determine whether the MIWI-potentiating factor is differentially expressed during spermatogenesis, we supplemented the target-cleavage assay with lysate prepared from stage-specific germ cells purified using fluorescence-activated cell sorting (FACS). piRISC-potentiating activity was greatest in lysate from secondary spermatocytes (Fig. 1c), a cell type in which pachytene piRNA-directed target cleavage is readily detected in vivo20,23. Moreover, the potentiating activity was testis-specific: lysates from brain, liver or kidney did not enhance piRNA-directed target cleavage by MIWI (Extended Data Fig. 3a). Finally, the potentiating activity was specific for PIWI proteins and had no effect on the rate of target cleavage by mouse AGO2 (Extended Data Fig. 3b).
Extended Data Fig. 3. Biochemical properties of the MIWI potentiating component in testis lysate.
a, The MIWI-potentiating factor is specific to testis lysate. b, Testis lysate does not enhance target cleavage by purified mouse AGO2. c, The MIWI-potentiating factor is sensitive to alkylation by N-ethylmaleimide. d, Effect of divalent cation chelators on MIWI-potentiating activity. For 0 nM chelator, water was added for EDTA and EGTA and ethanol for 1,10-phenanthroline. e, Strategy to test metal rescue after incubation of testis lysate with divalent cation chelators. f, g, Metal rescue experiments were performed as in (e) using EDTA (f) or 1,10-phenanthroline (g). h, Fractionation of testis lysate using Zn2+ and Ni2+ immobilized metal affinity chromatography (IMAC). In c and f–h, a digital overexposure is shown below each gel. Numbers below the gel indicate relative product produced in each lane. For gel source data, see Supplementary Fig. 1.
Three lines of evidence suggest that the MIWI-potentiating activity contains one or more structural Zn2+ ions. First, pre-treating testis lysate with the sulfhydryl alkylating agent N-ethylmaleimide inactivated the potentiating activity (Extended Data Fig. 3c), indicating that reduced cysteine residues, which often bind divalent metal cations43, are essential. Second, the MIWI-potentiating activity was unaltered by EGTA, which chelates Ca2+, but was irreversibly inactivated by EDTA, which chelates many divalent metals, and by 1,10-phenanthroline, which specifically chelates Zn2+ (Extended Data Fig. 3d). Adding additional metal ions did not rescue the activity (Extended Data Fig. 3e–g), suggesting that loss of Zn2+ irreversibly denatures the MIWI-potentiating factor, a characteristic of zinc-finger proteins44,45. Third, the MIWI-potentiating activity bound more tightly to an immobilized metal affinity resin charged with Zn2+ than to resin charged with Ni2+ (Extended Data Fig. 3h).
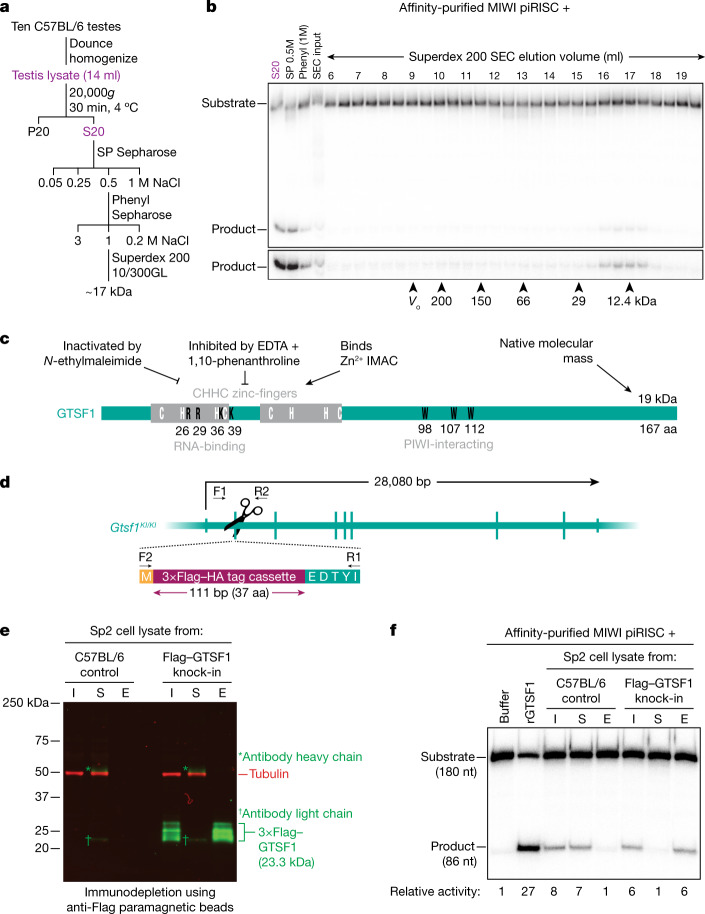
To identify the MIWI-potentiating activity, we developed a chromatographic purification scheme using cation-exchange, hydrophobic-interaction and size-exclusion chromatography (Fig. 2a). Notably, the activity eluted from a Superdex 200 size-exclusion column as a single peak at approximately 17 kDa (Fig. 2b). Together, our data suggest that the MIWI-potentiating activity corresponds to a small, testis-specific, Zn2+-binding protein abundantly expressed in meiotic and post-meiotic male germ cells (Fig. 2c).
Fig. 2. The testis protein GTSF1 potentiates target RNA cleavage by MIWI piRISC.
a, Scheme to purify the MIWI-potentiating factor from mouse testis lysate. P20, pellet; S20, supernatant. b, Target-cleavage assay to estimate the apparent molecular mass of the MIWI-potentiating activity by size-exclusion chromatography (SEC). Arrowheads indicate the peak concentration of the molecular mass standards, and their peak elution volumes. V0, void volume; phenyl (1M), 1 M NaCl eluate from hydrophobic-interaction chromatography; SP 0.5M, 0.5 M NaCl eluate from cation-exchange chromatography. c, Schematic representing known attributes of GTSF1 and the corresponding properties of the MIWI-potentiating activity in testis lysate. IMAC, immobilized metal affinity chromatography. d, Strategy for creating knock-in mouse expressing 3×Flag–HA–GTSF1 (Gtsf1Flag/Flag). F1, F2, R1 and R2 represent sequencing primers used to validate the insertion. The exon encodes an incomplete isoleucine codon, which is completed upon splicing. e, Western blotting showing immunodepletion of 3×Flag–HA–GTSF1 from secondary spermatocyte lysate using anti-Flag paramagnetic beads. I, input; S, supernatant; E, 3×Flag peptide eluate. f, Product formed by MIWI piRISC in the presence of the indicated components. Numbers below the gel indicate relative amount of product formed in each condition. For gel source data, see Supplementary Fig. 1.
GTSF1 potentiates catalysis by MIWI piRISC
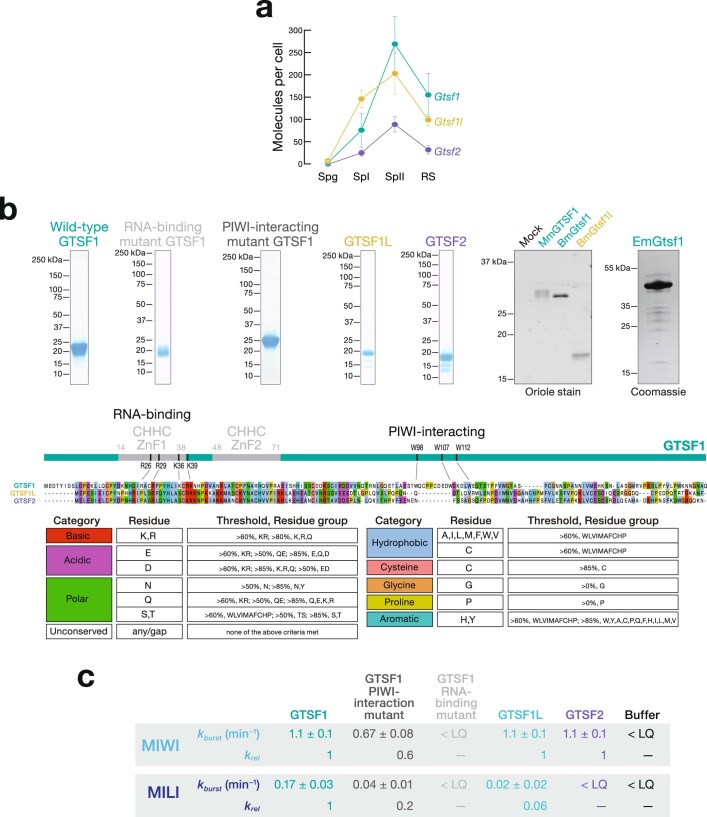
Gametocyte specific factor 1 (GTSF1) is a conserved, 19,083 Da, tandem CHHC-type zinc-finger protein essential for piRNA function and fertility in flies7,8,10, silk moths11, worms46 and mice9,12. Similar to Miwi (also known as Piwil1) and Mili (also known as Piwil2), the mRNA abundance of Gtsf1, as well as its paralogues Gtsf1l and Gtsf2, rises sharply as male germ cells enter meiosis I, peaks in secondary spermatocytes, and then declines in round spermatids (Extended Data Fig. 4a). Immobilized mouse GTSF1 captures all three PIWI proteins from testis lysate, but GTSF1 and PIWI proteins co-expressed in HEK293T cells do not co-immunoprecipitate, leading to the suggestion that the GTSF1–PIWI interaction requires additional protein or RNA components9. MIWI2-bound piRNAs are absent in Gtsf1−/− male mice9, which are sterile12, probably because loss of GTSF1 causes loss of MIWI2-directed retrotransposon promoter methylation47. Although RNA cleavage by MIWI2 is not required for piRNA biogenesis or function, production of MIWI2 piRISC requires MILI-dependent piRNA amplification, a process that requires the endonuclease activity of MILI6. In flies, piRNA-directed transcriptional silencing of transposons by Piwi, but not Piwi piRISC assembly, requires the GTSF1 orthologue Asterix7,8.
Extended Data Fig. 4. Gtsf1, Gtsf1l, and Gtsf2 mRNA abundance and recombinant protein expression.
a, For each germ cell type, mRNA abundance (from publicly available data; see Methods) is reported as mean ± SD (n = 3) in molecules/cell. Spg: spermatogonia; SpI: primary spermatocytes; SpII: secondary spermatocytes; RS: round spermatids. b, Right: Coomassie-stained SDS-PAGE of purified, recombinant wild-type GTSF1, GTSF1 mutants, and wild-type GTSF1Like and GTSF2. Left: Oriole protein stain detection of purified, recombinant, C-terminal V5SBP-tagged GTSF1 orthologs. Mm: Mus musculus (mouse); Bm: Bombyx mori (silkmoth). Coomassie protein stain detection of purified, recombinant Ephydatia muelleri Gtsf1. Bottom: Clustal Omega amino acid sequence alignment of the three mouse GTSF1 paralogs. c, Pre-steady-state rates (kburst) of target cleavage by MIWI and MILI determined by fitting the data in Fig. 3d–f (n = 3) to the burst-and-steady-state equation. LQ: limit of quantification. For gel source data, see Supplementary Fig. 1.
To test whether the MIWI-potentiating activity in the testis lysate corresponded to GTSF1, we used CRISPR–Cas9 to engineer a mouse strain with a 3×Flag–HA epitope tag inserted into the endogenous Gtsf1 coding sequence (C57BL6/J-Gtsf1em1(Flag)Pdz, hereafter referred to as Gtsf1Flag; Fig. 2d). Because the MIWI-potentiating activity was greatest in secondary spermatocytes, we prepared lysate from FACS-purified secondary spermatocytes from homozygous Gtsf1Flag/Flag male mice. Incubation with anti-Flag antibody coupled to paramagnetic beads depleted the lysate of both epitope-tagged GTSF1 (Fig. 2e) and the MIWI-potentiating activity (Fig. 2f). The activity was recovered from the beads by elution with 3×Flag peptide (Fig. 2f). By contrast, lysate from C57BL/6 secondary spermatocytes retained the MIWI-potentiating factor after incubation with anti-Flag antibody, and no activity was eluted from the beads after incubation with Flag peptide. Thus, GTSF1 is necessary to potentiate the catalytic activity of MIWI.
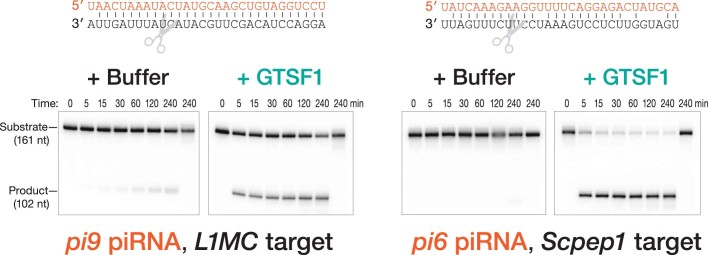
GTSF1 is also sufficient to potentiate the catalytic activity of MIWI. Adding purified recombinant GTSF1 (Extended Data Fig. 4b) to MIWI piRISC increased the rate of target cleavage by MIWI 19–100-fold for three different piRNA sequences (Fig. 3a–c and Extended Data Fig. 4c). First, the addition of 500 nM GTSF1 to MIWI programmed with an artificial, 30-nt piRNA (5 nM) caused the pre-steady-state rate (kburst) of target cleavage to increase from 0.010 min−1 to 1.1–1.5 min−1 (Fig. 3), a rate similar to that of AGO236,37 (≥3 min−1). Second, programming MIWI with an endogenous mouse piRNA sequence from the 9-qC-10667.1 locus (pi9) that is antisense to the L1MC transposon increased the pre-steady-state rate of cleavage of a fully complementary target RNA ~19-fold: kburst = 0.033 min−1 for piRISC alone and 0.62 min−1 with GTSF1 added (Fig. 3c and Extended Data Fig. 5). Finally, for a 6-qF3–28913(−),8009(+) locus (pi6) piRNA that directs cleavage at a partially complementary site in the Scpep1 mRNA in diplotene spermatocytes20, GTSF1 increased kburst 80-fold, from 0.0040 min−1 to 0.32 min−1 (Fig. 3c and Extended Data Fig. 5).
Fig. 3. GTSF1 paralogues can distinguish between MIWI and MILI.
a, Representative denaturing polyacrylamide gel electrophoresis showing that purified GTSF1 recapitulates the effect of testis lysate on MIWI catalysis. b, Amount of product generated as a function of time (mean ± s.d., n = 3). Data were fit to the burst-and-steady-state equation. Data for MIWI piRISC alone are from Fig. 1. c, Product formed as a function of time by MIWI programmed with pi6 and pi9 piRNAs for two independent trials. The mean values of the two trials were fit to the burst-and-steady-state equation. d,e, Representative denaturing polyacrylamide gel images of the assay to test GTSF1 mutants and paralogues in target cleavage by MIWI (d) or MILI (e) piRISC. f, Product formed as a function of time (mean ± s.d., n = 3) fit to the burst-and-steady-state equation. g, Pre-steady-state first-order rate constants of target cleavage by MIWI piRISC in the presence of either wild-type GTSF1 or the PIWI-interacting mutant GTSF1(W98A/W107A/W112A) were plotted as a function of GTSF1 concentration. Data are mean ± s.d., n = 3. kpot, maximum observable rate. h, Single-turnover rate of target cleavage by EfPiwi piRISC in the presence or absence of EmGtsf1 or yeast tRNA (mean ± s.d., n = 3). For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 5. Efficient target cleavage by MIWI guided by endogenous piRNAs requires GTSF1.
Representative target cleavage assay using MIWI piRISC programmed with an abundant mouse pachytene piRNA antisense to the L1MC transposon (left) or targeting the Scpep1 mRNA (right). piRNA sequences are in orange; below each piRNA sequence, the target in the RNA sequence is in black. Scissors indicates the cleavage site of the target RNA. The last lane in each gel shows the extent of cleavage using the reciprocal, non-cognate target, incubated with piRISC for 240 min as a negative control. Where data points from the two trials overlap, jitter has been introduced for clarity. Curves show the fit of the burst-and-steady equation to the mean of the two trials. For gel source data, see Supplementary Fig. 1.
Our data suggest that GTSF1 does not promote product release or turnover: the steady-state rate (kss < 0.005 min−1) of target cleavage under multiple-turnover conditions (that is, GTSF1 ≫ target RNA ≫ MIWI) was essentially unchanged from the rate in the absence of GTSF1 (Fig. 3b). MIWI might remain bound to the cleaved products, preventing it from catalysing multiple rounds of target cleavage. Supporting this idea, at incubation times greater than 15 min, 3′-to-5′ exonucleases present in testis lysate degrade the uncut, full-length target RNA, but the 5′ cleavage product remains stable, consistent with its being protected by MIWI bound to its 3′ end (Fig. 1b). In vivo, product release has been proposed to be facilitated in insects and mammals by the RNA-stimulated ATPase Vasa48–50 (also known as DDX4) and the Vasa-like protein DDX4351.
RNA binding is essential for GTSF1 function
Only three eukaryotic proteins are known to contain CHHC zinc-fingers: the spliceosomal RNA-binding protein U11-48K, the TRM13 tRNA methyltransferase, and GTSF1 and its paralogues52. In vitro, the first zinc-finger of GTSF1 binds RNA directly; RNA binding requires four basic surface residues53 (R26, R29, K36 and K39). Potentiation of target cleavage required RNA binding by GTSF1: purified mutant GTSF1(R26A/R29A/K36A/K39A) (Extended Data Fig. 4b) did not detectably increase the rate of catalysis by either MIWI or MILI (Fig. 3d–f and Extended Data Fig. 4c), suggesting that GTSF1 must interact with RNA to function.
GTSF1 function requires PIWI binding
In flies and mice, GTSF1 interacts with PIWI proteins through conserved aromatic residues in its central region7,9 (Fig. 2c). Mutating these residues in the recombinant protein—W98A, W107A and W112A for mouse GTSF1—reduced the stimulatory effect of mouse GTSF1 on MIWI in a concentration-dependent manner, supporting the idea that GTSF1 binds MIWI directly when potentiating target cleavage (Fig. 3g and Extended Data Fig. 4c) and suggesting that W98, W107, and W112 define a conserved surface of GTSF1 that interacts with PIWI proteins. We measured kburst as a function of GTSF1 concentration (Supplementary Data Fig. 2). Fitting the data to a hyperbolic function revealed that GTSF1 binds MIWI piRISC more than 60-fold more tightly than GTSF1(W98A/W107A/W112A) (GTSF1: dissociation constant (Kd) = 8 nM, 95% confidence interval 6–9 nM; GTSF1(W98A/W107A/W112A): Kd = 500 nM, 95% confidence interval 100–900 nM) (Fig. 3g). Moreover, PIWI binding appears to be the sole defect in GTSF1(W98A/W107A/W112A), since the first-order rates of target cleavage by MIWI at saturating concentrations of either wild-type or mutant GTSF1 were essentially indistinguishable (wild-type: 1.1 min−1, 95% confidence interval 0.7–2 min−1; mutant GTSF1: 0.8 min−1, 95% confidence interval 0.6–0.9 min−1).
GTSF1 function is evolutionarily conserved
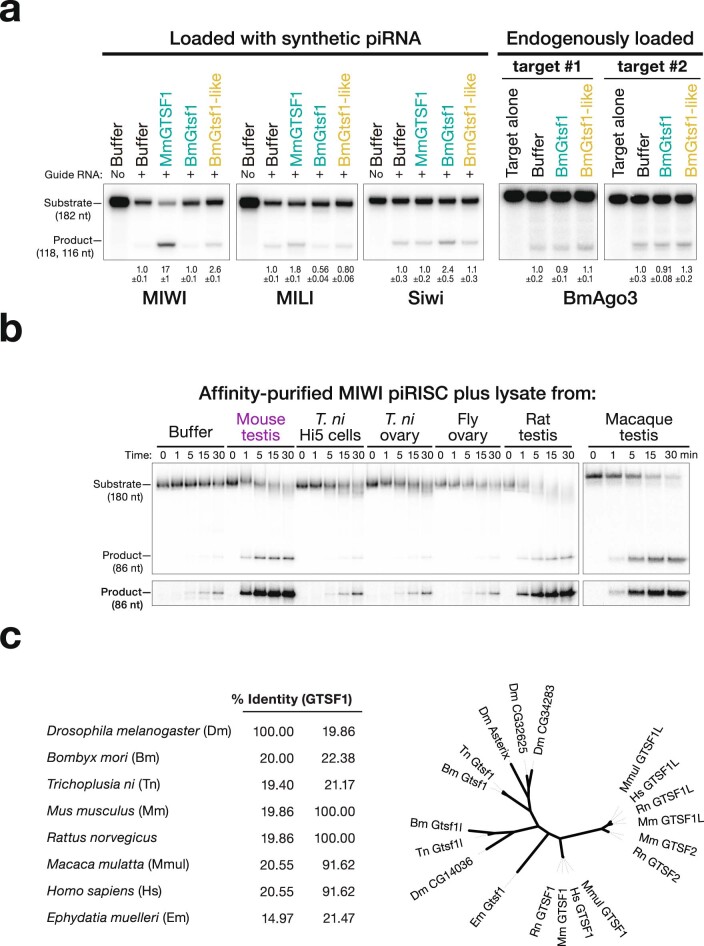
GTSF1 orthologues are found in many metazoan genomes52, suggesting that GTSF1 may potentiate target cleavage by PIWI proteins in many animals. Supporting this idea, purified recombinant Gtsf1 from the arthropod Bombyx mori (Extended Data Fig. 4b) potentiated the catalytic activity of the B. mori PIWI protein, Siwi (Extended Data Fig. 6a).
Extended Data Fig. 6. GTSF proteins are PIWI selective.
a, MIWI piRISC target cleavage assay in the presence of lysates from different animals. T.ni: Trichoplusia ni. Macaque: rhesus macaque. A digital overexposure is shown below the gel. b, Representative target cleavage assay using the indicated PIWI protein in the presence of different GTSF1 orthologs (100 nM). Numbers below the gel lanes report fraction target cleaved (mean ± SD; n = 3). c, Left: Percent identity of different GTSF1 orthologs Right: Unrooted tree of GTSF1 orthologs. For gel source data, see Supplementary Fig. 1.
The structure and kinetics of the sponge Ephydatia fluviatilis (freshwater sponge) Piwi (EfPiwi) were recently described35. Like MIWI, EfPiwi possesses inherently weak catalytic activity, a feature common to all PIWI proteins examined to date. Although the E. fluviatilis genome is yet to be sequenced, the closely related, fully sequenced genome of Ephydatia muelleri contains a readily identifiable GTSF1 orthologue. Purified recombinant EmGtsf1 (Extended Data Fig. 4c) stimulated the single-turnover catalytic rate of EfPiwi piRISC by around 28-fold (Fig. 3h). Sponges (Porifera) are the sister group to all other animals, the Eumetazoa, having separated around 900 million years ago. Thus, the last common ancestor of all animals probably required GTSF1 to potentiate target cleavage by PIWI proteins.
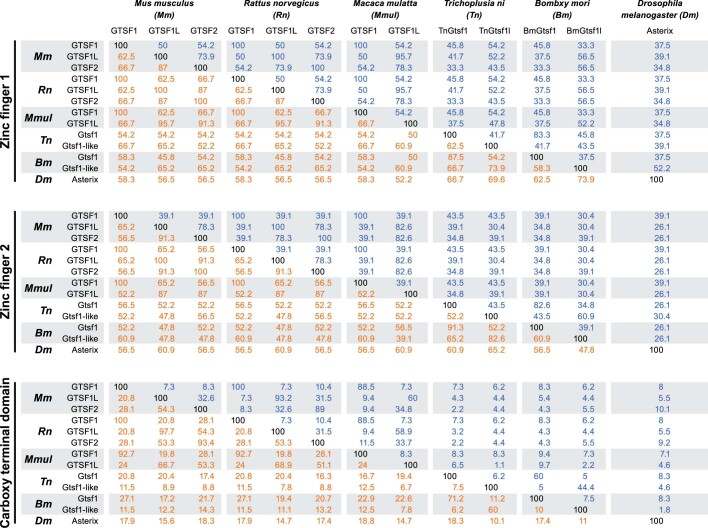
The GTSF1 tandem zinc-finger domains are conserved across phyla, whereas the GTSF1 central and carboxy-terminal sequence diverges substantially between mammals and arthropods (Extended Data Table 1). For example, the sequences of the mouse and rhesus macaque first and second zinc-fingers are 100% identical, whereas their C-terminal domains share 88.5% identity. The first zinc-finger of mouse GTSF1 is 37.5% and 45.8% identical to its fly and moth orthologues, but the mouse protein shares just 8% and 8.3% identity with the central and C-terminal domains of the fly and moth proteins, respectively. Consistent with the evolutionary divergence of their central and C-terminal domains, testis lysate from rat or rhesus macaque enhanced target cleavage by MIWI piRISC, whereas lysate from Drosophila melanogaster or Trichoplusia ni ovaries, T. ni Hi5 cells or purified recombinant B. mori BmGtsf1 did not (Extended Data Fig. 6b,c and Supplementary Data Fig. 1).
Extended Data Table 1.
Pairwise comparison of percent identity (blue) or similarity (orange) for domain sequence of GTSF1-related proteins
GTSF1 paralogues can distinguish PIWI paralogues
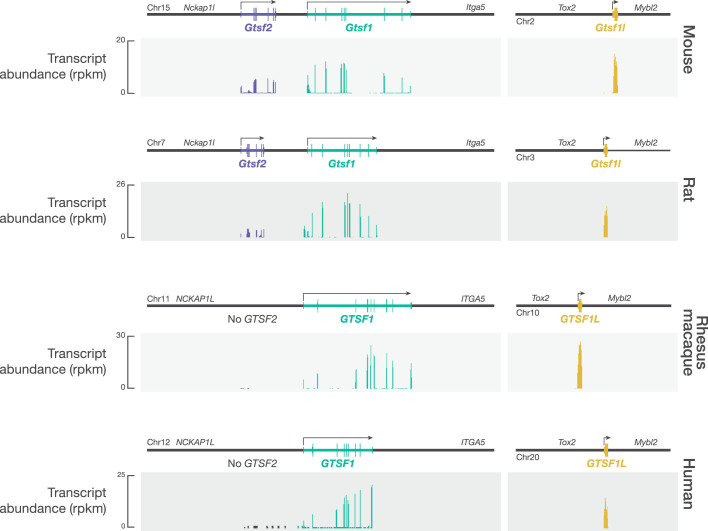
Many animal genomes encode more than one GTSF protein52 (Extended Data Fig. 6c). For example, D. melanogaster has four GTSF paralogues. The D. melanogaster OSC and OSS cell lines, which are derived from somatic follicle cells that support oogenesis, express Piwi but lack the PIWI paralogues Aub or Ago3. Piwi-mediated, piRNA-guided transposon silencing in these cells requires Asterix, a GTSF1 orthologue7,8,10. In vivo, asterix mutants phenocopy piwi mutants and are female sterile, even though Piwi is successfully loaded with piRNAs and transits to the nucleus in the absence of Asterix7,8. Whether the other fly GTSF paralogues have a function in vivo, perhaps as auxiliary factors for Aub or Ago3, remains to be tested. Similar to fly Asterix, mouse GTSF1 is essential for piRNA function and fertility. In mice, two GTSF1 paralogues, GTSF1L and GTSF2 are also expressed during spermatogenesis and interact with PIWI proteins54. Unlike GTSF1, single and double Gtsf1l- and Gtsf2-knockout males are fertile54. Genes encoding the Gtsf paralogues are syntenic in mammals, whereas Gtsf2 is lost in primates (Extended Data Fig. 7).
Extended Data Fig. 7. Expression of GTSF1 paralogs in mouse, rat, rhesus macaque, and human whole testes.
Gtsf genes are syntenic in mice, rats, macaques, and humans. Primates, including humans, do not express GTSF2. RNA-seq from total testis RNA of the indicated animals sourced from previously published datasets (see Methods).
The central and C-terminal domains of GTSF orthologues are more similar among closely related species than among GTSF paralogues within the same species (Extended Data Figs. 4b and 6c and Extended Data Table 1), further supporting the view that this domain has evolved to bind specific PIWI proteins. Consistent with this idea, mouse GTSF1, GTSF1L and GTSF2 differ in their ability to potentiate target cleavage by MIWI and MILI. Whereas GTSF1 accelerated target cleavage by both MIWI and MILI, purified recombinant GTSF1L and GTSF2 (Extended Data Fig. 4b) efficiently potentiated target cleavage by MIWI but not by MILI (Fig. 3d–f and Extended Data Fig. 4c). GTSF2 was unable to detectably increase the rate of target cleavage by MILI. Although GTSF1L had a modest effect on the rate of target cleavage by MILI piRISC, this enhancement was less than one-sixteenth that provided by GTSF1 and half that of the PIWI binding mutant GTSF1(W98A/W107A/W112A) (Fig. 3d–f and Extended Data Fig. 4c). We conclude that GTSF1L and GTSF2 are specialized to potentiate target cleavage by MIWI piRISC.
Silk moth BmGtsf1-like is more similar to mouse GTSF1 than to BmGtsf1 (Extended Data Fig. 6c and Extended Data Table 1). BmGtsf1-like increased the amount of target cleaved by MIWI piRISC by 2.6-fold (s.d. = 0.1; P = 0.0027 by ANOVA with Dunnett’s post-hoc test) but had no detectable effect on MILI (Extended Data Fig. 6a), further supporting the idea that the GTSF central domain determines the affinity of the protein for specific PIWI proteins. In vivo, BmGtsf1 interacts with BmSiwi and is required for transposon silencing and sex determination11. BmGtsf1 increased the rate of target cleavage by affinity-purified Siwi but not that of the other silk moth PIWI protein, BmAgo3 (Extended Data Fig. 6a).
MIWI and MILI require extensive base pairing
piRNA–target RNA complementarity from g2–g22 (that is, 21 base pairs) is required for efficient target cleavage directed by endogenous piRNAs loaded into MIWI piRISC immunoprecipitated from adult mouse testis5. However, GTSF1 does not detectably co-immunoprecipitate with MIWI piRISC from mouse testis5,39. A requirement for 21-base-pair complementarity between target and guide is, to our knowledge, unique among Argonaute proteins: fly Ago2 slices a target with as few as 11 contiguous base pairs55; mammalian AGO2 requires only 11 contiguous base pairs for detectable cleavage56; and the eubacterial DNA-guided DNA endonuclease TtAgo57 requires as few as 14.
Affinity-purified MIWI, programmed with either of two different synthetic 30-nt piRNAs, readily cleaved target RNA complementary to guide nucleotides g2–21 but not a target complementary to g2–g16 (Extended Data Fig. 8a,b). Under multiple-turnover conditions with saturating amounts of purified, recombinant GTSF1 ([GTSF1] ≫ [target] > [piRISC]), MIWI readily cleaved a target RNA with 19 nucleotides (g2–g20) complementary to its synthetic piRNA guide (Fig. 4a, top). The lower background of single-turnover experiments ([GTSF1] ≫ [piRISC] > [target]) enabled longer incubation times. Under these conditions, we could detect GTSF1-stimulated cleavage of a target RNA with as few as 15 complementary nucleotides (g2–g16; Fig. 4a, bottom). We note that the pachytene stage of meiosis in mouse spermatogenesis lasts about 175 h, and the pachytene piRNA pathway components are expressed until at least the round spermatid stage, a time interval spanning more than 400 h.
Extended Data Fig. 8. Complementarity and GTSF1 requirements for target cleavage by AGO2, MIWI, and MILI.
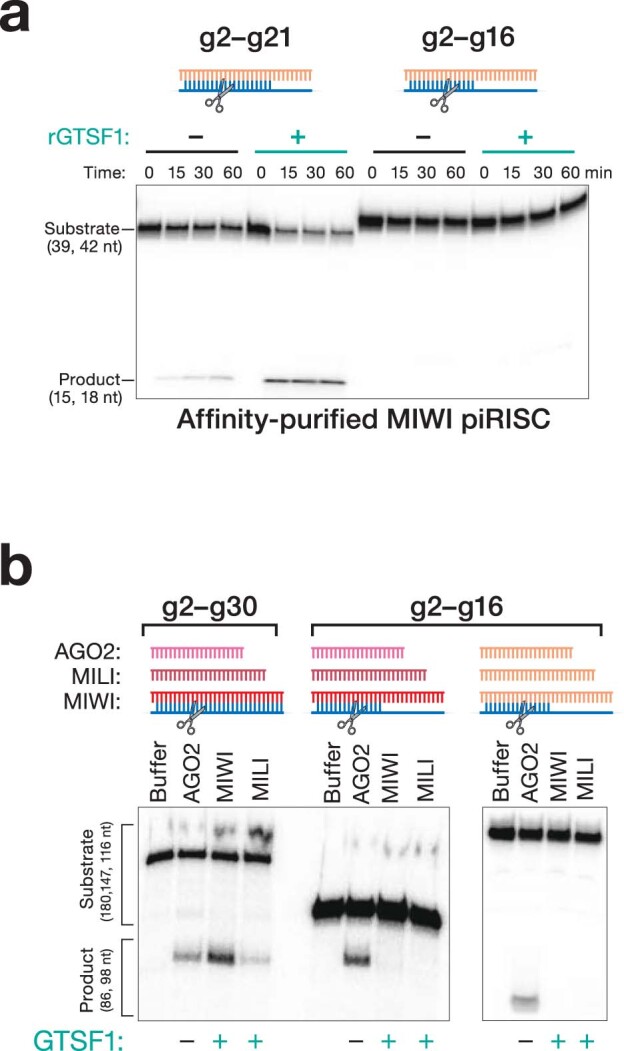
a, MIWI target cleavage ± GTSF1 using synthetic piRNA guide 1. b, Target cleavage by AGO2 siRISC compared with MIWI or MILI piRISC loaded with synthetic piRNA guide 1 or 2, for targets with complete (g2–g30) or partial (g2–g16) complementarity to the piRNA. For gel source data, see Supplementary Fig. 1.
Fig. 4. Cleavage by MIWI piRISC is sensitive to the complementarity between the piRNA and target.
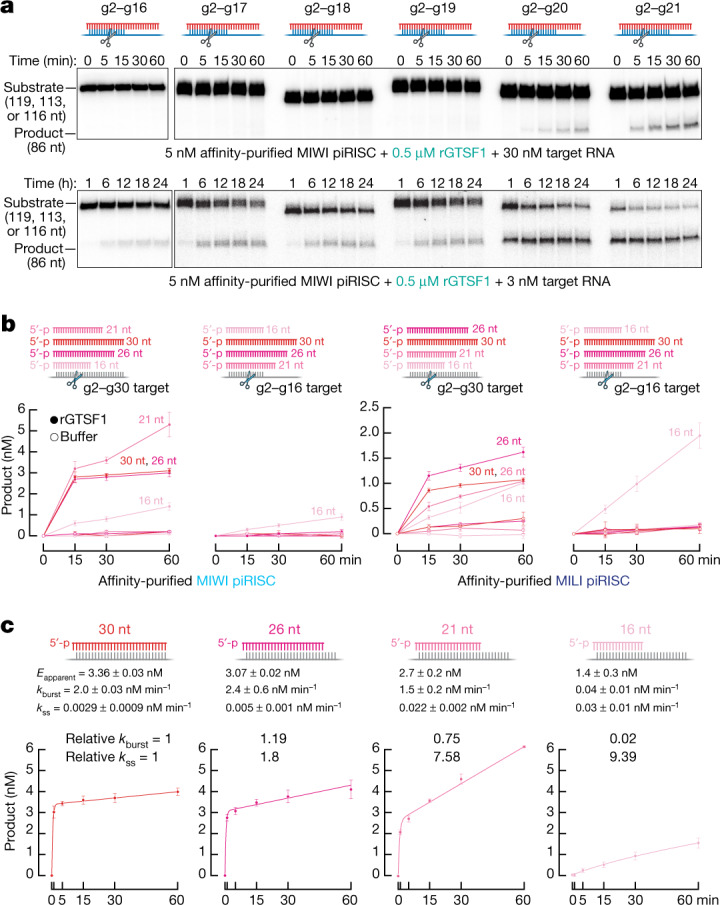
a, MIWI piRISC target cleavage in the presence of GTSF1 for targets with varying complementarity to synthetic piRNA guide. Top, multiple-turnover conditions; bottom, single-turnover conditions. All reactions contained saturating amounts of GTSF1. b, Target-cleavage assay using targets complementary to piRNA guide nucleotides g2–g16 or g2–g30 and MIWI or MILI loaded with piRNAs of the indicated lengths, with or without GTSF1 (mean ± s.d., n = 3). c, Absolute and relative pre-steady-state and steady-state rates of cleavage of the g2–g30 target by MIWI loaded with piRNAs of the indicated lengths in the presence of GTSF1 (mean ± s.d., n = 3), Eapparent, apparent active enzyme concentration estimated from fitting data to equation (1). For gel source data, see Supplementary Fig. 1.
Guide length limits target cleavage by MIWI
In vivo, piRNAs are trimmed to a length characteristic of the PIWI protein in which they reside: around 30 nt for MIWI and 26–27 nt for MILI13,14,58. An attractive hypothesis is that these piRNA lengths are optimal for target cleavage catalysed by the specific PIWI protein. Our data suggest a more complex relationship between piRNA length, PIWI protein identity and target complementarity. In the presence of GTSF1, MIWI loaded with a 30-nt piRNA and MILI loaded with a 26-nt piRNA readily cleaved a fully complementary target RNA in a 60-min reaction (Fig. 4b). By contrast, neither piRISC cleaved a target complementary to piRNA positions g2–g16 (Fig. 4a,b and Extended Data Fig. 8a,b). Similarly, both MIWI and MILI loaded with a 26- or 21-nt guide produced little cleavage for a target complementary to positions g2–g16, although both guide lengths supported cleavage of a fully complementary target (Fig. 4b). In fact, for MIWI, a 21mer was more active than a 30-nt guide (Fig. 4b). Without GTSF1, MIWI or MILI loaded with any of these guide lengths produced little cleaved target in 60 min (Fig. 4b). Of note, MIWI or MILI loaded with a 16-nt guide RNA, a piRNA length that is not present in vivo, readily cleaved the RNA target (Fig. 4b).
GTSF1 accelerates the rate of pre-steady-state target cleavage (kburst) by MIWI and MILI but has little effect on their slow rates of steady-state cleavage (kss), suggesting that piRISC remains bound to its cleavage by-products (Fig. 3b). We incubated GTSF1 and MIWI—loaded with a 30-, 26-, 21- or 16-nt guide—with a target RNA fully complementary to each guide and measured kburst and kss (Fig. 4c). As the guide length decreased, kburst decreased and kss increased. Compared with its native 30-nt guide length, the 16-nt guide increased kss approximately ninefold and decreased kburst by more than 50-fold. These data suggest that as the guide was shortened, the cleaved products were released more rapidly.
We estimated the binding affinity (ΔG) of the piRNA for its target using the nearest-neighbour rules for base pairing at 37 °C. As the strength of the piRNA base pairing to the target increased, the rate of pre-steady-state cleavage increased (Fig. 5a), consistent with base pairing serving to extract the 3′ end of the piRNA from the PAZ domain, facilitating the transition to a more catalytically competent piRISC conformation59. Conversely, decreased base-pairing strength increased the steady-state rate of target cleavage, supporting the view that for biologically relevant piRNA lengths, product release is the rate-determining step for MIWI-catalysed target cleavage (Fig. 5a).
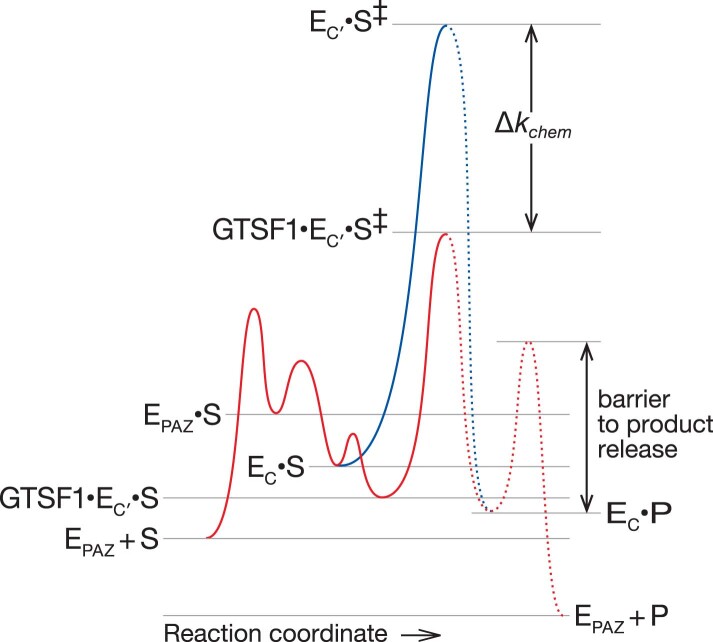
Fig. 5. A model for the function of guide length and GTSF1 in target cleavage by PIWI proteins.
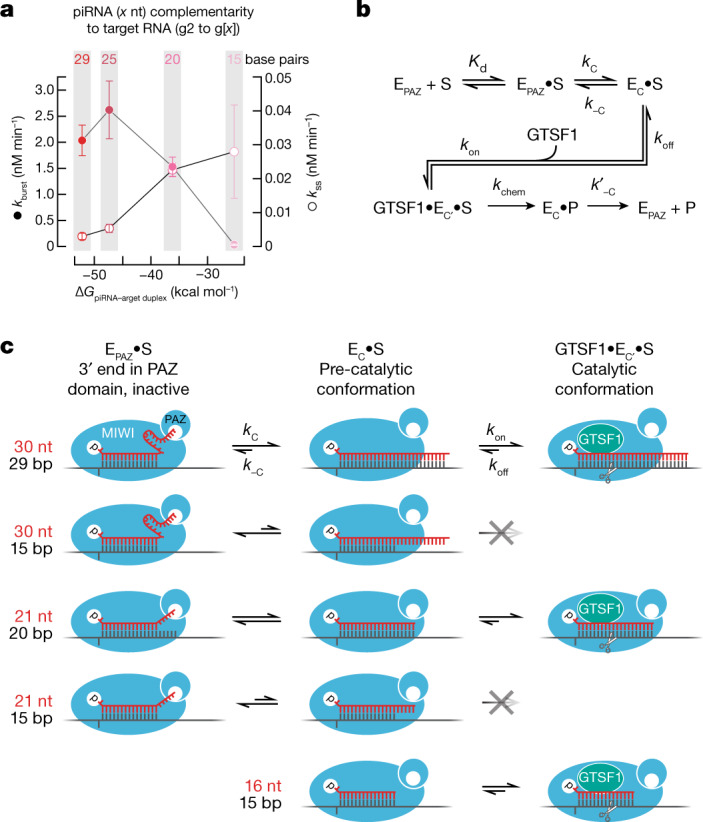
a, The energy of base pairing, estimated by standard nearest-neighbour methods versus pre-and steady-state rates of GTSF1-potentiated target cleavage (mean ± s.d., n = 3), directed by piRNAs of different lengths loaded into MIWI. The same target, which was fully complementary to each piRNA, was used in all experiments. b, piRNA-directed, MIWI-catalysed target cleavage is envisioned to require two sequential conformational changes: (1) a target-dependent conformational change in piRISC (EPAZ) in which the piRNA 3′ end leaves the PAZ domain, allowing extensive base pairing of the piRNA with the target substrate (S); and (2) GTSF1-dependent conversion of this piRISC pre-catalytic state (EC) to the fully competent catalytic state (EC′). P, cleaved target products. Kd, dissociation constant; kc and k−c, rate constants for pre-catalytic state; kon and koff, rate constants for GTSF1 binding; kchem, rate constant for the endoribonucleolytic chemistry step; k′−c, rate constant for piRISC (EPAZ) regeneration. c, Proposed effects of different piRNA lengths, extent of guide–target complementarity and GTSF1 on the forward and reverse rates of the two conformational rearrangements. The wide, central cleft, as observed in the cryo-electron microscopy structure of E. fluviatilis Piwi-A35, is envisioned to allow the central region of a 30 nt piRNA to be mobile and exposed to solvent when its 3′ end is secured to the PAZ domain (top right).
Discussion
In nearly all animals, both piRNA biogenesis and piRNA function require the PIWI endoribonuclease activity. Yet purified mouse MIWI and MILI are intrinsically slow to cleave complementary target RNAs. Our data show that unlike Argonaute proteins, PIWI proteins require an auxiliary factor, GTSF1, to efficiently cleave their RNA targets.
The ability of GTSF1 to potentiate PIWI-catalysed target cleavage provides a biochemical explanation for the genetic requirement for this small zinc-finger protein in the piRNA pathway. GTSF1 function requires that it bind both RNA and the PIWI protein, and differences among C-terminal domains restrict individual GTSF1 paralogues to specific PIWI proteins.
We propose a testable kinetic scheme for target cleavage by MIWI (Fig. 5b,c and Extended Data Fig. 9) that incorporates the requirement for GTSF1 and the observation that a 16-nt guide changes the rate-determining step of target cleavage catalysed by MIWI. As originally proposed for fly Ago259, piRISC bound to a target is presumed to exist in two states: one in which the piRNA 3′ end is secured in the PAZ domain and a competing, pre-catalytic conformation in which the piRNA is fully base paired to its target. Structures of Piwi-A from the freshwater sponge E. fluviatilis show that extensive pairing between a piRNA and its target induces a catalytically competent conformation in which the PAZ domain is rotated away from the piRNA 3′ end35. We propose that the PAZ-bound state is more favourable for guides bound to PIWI proteins than to AGOs, requiring a high degree of complementarity between guide and target to extract the piRNA 3′ terminus from the PAZ domain. Our data also suggest that a slow rate of product release after cleavage results directly from the extensive piRNA–target RNA complementarity required for this conformational change. A 16 nt piRNA allows MIWI to more easily adopt a pre-catalytic state, perhaps because the 3′ end of the short guide cannot reach the PAZ domain. Notably, single-stranded siRNA guides as short as 14 nt allow mammalian AGO3, initially believed to have lost its endonuclease activity, to efficiently cleave RNA targets60. In golden hamsters, piRNAs bound to PIWIL1 are initially around 29 nt long, but a shorter population of approximately 23 nt piRNAs appears at metaphase II and predominates in 2-cell embryos61. piRNAs bound to PIWIL3, a female-specific PIWI protein that is absent from mice, are around 19 nt long in hamster and around 20 nt long in human oocytes61,62. We speculate that these short piRNAs enable PIWIL1 and PIWIL3 to function as multiple-turnover endonucleases.
Extended Data Fig. 9. Model of the free-energy landscape of GTSF1-induced potentiation of target cleavage by PIWI.
Two conformational changes in piRISC (E) are proposed to be required for efficient target cleavage catalyzed by PIWI proteins. EPAZ: piRISC with the 3′ end of the piRNA bound to the PAZ domain; EC: piRISC with the piRNA fully paired to the target RNA, i.e., the pre-catalytic conformation; EC′: EC in the catalytically competent conformation; S‡: transition-state. For piRNAs of biologically relevant length, extensive complementarity is proposed to promote the first conformational change; GTSF1 is proposed to promote the second. Blue: energy barrier to catalysis without GTSF1, i.e., the spontaneous conversion of EC to EC′.
Our model proposes that GTSF1 recognizes the pre-catalytic piRISC state, facilitating a second conformational change in PIWI proteins that may occur spontaneously in Argonaute clade proteins. GTSF1 binding probably stabilizes the catalytically active conformation, facilitating target cleavage, but has no detectable effect on subsequent release of the cleaved products. Slow product release may be a general property of PIWI proteins: purified E. fluviatilis PIWI similarly catalyses only a single round of target cleavage35.
Potentiation of the endonuclease activity of PIWI proteins by GTSF1 probably represents its ancestral function. It remains unknown why GTSF1 is required for the function of PIWI proteins such as mouse MIWI26,9 and fly Piwi7,10, which silence transcription rather than cleave target RNAs. We propose that GTSF1 stabilizes the functional conformation of non-catalytic PIWI proteins, enabling them to bind the downstream proteins required for transcriptional silencing.
Methods
Plasmids and cell lines
Supplementary Data Table 1 provides the sequences of all oligonucleotides used. To create pScalps_Puro_eGFP, an IRES-driven eGFP was inserted downstream of the multiple cloning site and upstream of the puromycin coding sequence of the lentivirus transfer vector pScalps_Puro. MIWI cDNA was obtained from Mammalian Gene Collection (https://genecollections.nci.nih.gov/MGC/). MILI cDNA was amplified by RT–PCR from mouse testis total RNA. Gibson assembly and restriction cloning were used to clone the MILI and MIWI coding sequences into pScalps_Puro_eGFP, fusing them in-frame with N-terminal 3×Flag and SNAP tags. Lentivirus transfer vectors were packaged by co-transfection with psPAX2 and pMD2.G (4:3:1) using TransIT-2020 (Mirus Bio) in HEK293T cells. Supernatant containing lentivirus was used to transduce HEK293T cells in the presence of 16 µg ml−1 polybrene (Sigma) to obtain stable PIWI-expressing cell lines. Three sequential transductions were performed to maximize recombinant protein production. The transduced cells were selected in the presence of 2 µg ml−1 Puromycin for two weeks, then the cells expressing the 5–10% highest eGFP fluorescence were selected by FACS (UMASS Medical School Flow Cytometry Core). Selected cells stably expressing the recombinant PIWI proteins were expanded, collected, and cell pellets flash-frozen and stored at −80 °C.
Mouse GTSF1 cDNA was synthesized at Twist Biosciences and cloned into pCold-GST (Takara Bio) bacterial expression vector by restriction cloning. GTSF1 mutants, GTSF1L, and GTSF2- expressing pCold-GST vectors were synthesized at Twist Biosciences. pIZ-Flag-6×His-Siwi was described previously63. Flag-tagged MILI and MIWI-expressing vectors were the kind gift of Shinpei Kawaoka (Kyoto University, Kyoto, Japan). MmGtsf1 cDNA was amplified by PCR with reverse transcription (RT–PCR) from mouse spermatogonial stem cell total RNA64. BmGtsf1 and BmGtsf1-like cDNAs were amplified by RT–PCR from BmN4 cell total RNA. The amplified cDNA fragment and a DNA fragment coding V5SBP were cloned into pcDNA5/FRT/TO vector (Thermo Fisher Scientific) by In-fusion cloning (Takara). The E. muelleri Gtsf1 coding sequence was obtained from65 and cloned into bacterial expression vector pSV272 (IJM).
Mice
Generation of 3×Flag–HA–tagged GTSF1 mice (C57BL6/J-Gtsf1em1(Flag)Pdz) was performed at Cyagen. The coding sequence for the tags was inserted into the endogenous locus by CRISPR–Cas9. In brief, fertilized mouse embryos were injected with the sgRNA targeting the sequence GTCTTCCATGCTGATGGCAAAGG (PAM), a 3×Flag–HA-tag cassette HDR donor, and Cas9 mRNA. Supplementary Data Table 1 provides the sequences of the HDR donor and oligonucleotide primers used for genotyping. Founder F0 mice were genotyped and bred to generate F1 mice carrying the germline-transmitted knock-in allele. Mice were maintained and used according to the guidelines of the Institutional Animal Care and Use Committee of the University of Massachusetts Chan Medical School (A201900331). C57BL/6J mice (RRID: IMSR_JAX:000664) were used as wild-type control, where indicated. Animals were housed in an AALAC-accredited barrier facility with controlled temperature (22 ± 2 °C), relative humidity (40% ± 15%), and a 12 h:12 h dark:light cycle.
Recombinant protein purification
PIWI proteins
PIWI-expressing stable cells were collected by centrifugation and stored at −80 °C until they were lysed in 10 ml lysis buffer (30 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT, 0.1% v/v Triton X-100, 20% v/v glycerol, and 1× protease inhibitor cocktail (1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Sigma, A8456), 0.3 μM aprotinin, 40 μM betanin hydrochloride, 10 μM. E-64 (Sigma, E3132), 10 μM leupeptin hemisulfate)) per g frozen cells. Cell lysis was monitored by staining with trypan blue. Crude cytoplasmic lysate was clarified at 20,000g (S20) or 100,000g (S100) for 30 min, aliquoted, flash-frozen, and stored at −80 °C. To capture PIWI proteins, clarified lysate was incubated with 20 μl anti-Flag M2 paramagnetic beads (Sigma) per ml of lysate for 2 h to overnight rotating at 4 °C. Beads were washed five times with high salt wash buffer (30 mM HEPES-KOH, pH 7.5, 2 M potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT), twice with low salt buffer (high salt wash buffer except containing 100 mM potassium acetate and 0.01% v/v Triton X-100). To assemble piRISC, beads were resuspended in low salt buffer containing 100 nM synthetic guide piRNA and incubated with rotation at 37 °C or room temperature for 30 min. MIWI piRISC or unloaded apo MIWI was eluted from the beads with 200 ng μl−1 3×Flag peptide in lysis buffer without 0.1% v/v Triton X-100 or protease inhibitors for 2 h at 4 °C. Eluate containing PIWI piRISC was aliquoted and stored at −80 °C. EfPiwi piRISC purification has been described35. AGO2 siRISC was purified using oligo-affinity purification as described66.
GTSF1, GTSF1 mutants and GTSF1 homologues
pCold-GST GTSF-expression vectors were transformed into Rosetta-Gami 2 competent cells (Novagen). Cells were grown in the presence of 1 μM ZnSO4 at 37 °C until OD600 ~0.6–0.8, then chilled on ice for 30 min to initiate cold shock. Protein expression was induced with 0.5 mM IPTG for 18 h at 15 °C. Cells were collected by centrifugation, washed twice with PBS, and cell pellets were flash frozen and stored at −80 °C. Cell pellets were resuspended in lysis/GST column buffer containing 20 mM Tris-HCl pH 7.5, 500 mM NaCl, 1 mM DTT, 5% v/v glycerol, and complete EDTA-free protease inhibitor cocktail (Roche). Cells were lysed by a single pass at 18,000 psi through a high-pressure microfluidizer (Microfluidics M110P), and the resulting lysate clarified at 30,000g for 1 h at 4 °C. Clarified lysate was filtered through a 0.2 µm low-protein binding syringe filter (Millex Durapore; EMD Millipore) and applied to glutathione Sepharose 4b resin (Cytiva) equilibrated with GST column buffer. After draining the flow-through, the resin was washed with 50 column volumes GST column buffer. To elute the bound protein and cleave the GST tag in a single step, 50 U HRV3C Protease (Novagen) was added to the column, the column was sealed and incubated for 3 h at 4 °C, following which, the column was drained to collect the cleaved protein. The protein was diluted to 50 mM NaCl and further purified using a HiTrap Q (Cytiva) anion-exchange column equilibrated with 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM DTT, and 5% v/v glycerol. The bound protein was eluted using a 100–500 mM NaCl gradient in the same buffer. Peak fractions were analysed for purity by SDS–PAGE and the purest were pooled and dialysed into storage buffer containing 30 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT, 20% v/v glycerol. To avoid precipitation of GTSF2, which has a pI of 7.3, 20 mM Tris-HCl, pH 8.8, was substituted for HEPES-KOH during purification and dialysis.
For Extended Data Fig. 6a, HEK293T cells were transfected with mouse and insect GTSF1 coding sequences cloned into pcDNA5 expression vectors using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells were collected 36 h later and homogenized in lysis buffer (20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 0.15% v/v Triton X-100, 100 μg ml−1 RNase A (Qiagen), 0.5 mM DTT, 1× Complete EDTA-free protease inhibitor (Roche)). After centrifugation at 17,000g for 20 min at 4 °C, additional NaCl (0.65 M final concentration) and 1% v/v Triton X-100 (final concentration) were added to the supernatant and the lysate was incubated with Streptavidin–Sepharose High Performance (Cytiva) beads at 4 °C for 1 h. The beads were washed with wash buffer (20 mM Tris-HCl (pH 7.4), 1 M NaCl, 1.5 mM MgCl2, 1% v/v Triton X-100, 0.5 mM DTT) five times and rinsed with lysis buffer without RNase A. SBP-tagged recombinant proteins were eluted with elution buffer (30 mM HEPES-KOH, pH 7.4, 100 mM potassium acetate, 2 mM magnesium acetate, 2.5 mM biotin, 0.5 mM DTT).
For Fig. 3h, EmGtsf1 expression vector was transformed into BL21(DE3) cells (New England Biolabs). Transformed cells were grown in LB supplemented with 1 μM ZnSO4 at 37 °C until OD600 ~0.6–0.8. The incubation temperature was lowered to 16 °C and protein expression was induced by addition of 1 mM IPTG for 4 h. Cells were collected by centrifugation and cell pellets were flash frozen in liquid nitrogen and stored at −80 °C for future use. Thawed cell pellets were resuspended in lysis buffer (50 mM Tris, pH 8, 300 mM NaCl, 0.5 mM TCEP) and passed through a high-pressure (18,000 psi) microfluidizer (Microfluidics M110P) to induce cell lysis. The resulting lysate was clarified by centrifugation at 30,000g for 20 min at 4 °C. Clarified lysate was applied to Ni-NTA resin (Qiagen) and incubated for 1 h. The resin was extensively washed with nickel wash buffer (300 mM NaCl, 20 mM imidazole, 0.5 mM TCEP, 50 mM Tris, pH 8). Protein was eluted in four column volumes of nickel elution buffer (300 mM NaCl, 300 mM imidazole, 0.5 mM TCEP, 50 mM Tris, pH 8). TEV protease was added to the eluted protein to induce cleavage and removal of the N-terminal His6 and MBP tags. The resulting mixture was dialysed against HiTrap Dialysis Buffer (300 mM NaCl, 20 mM imidazole, 0.5 mM TCEP, 50 mM Tris, pH 8) at 4 °C overnight. The dialysed protein was then passed through a 5-ml HiTrap Chelating column (Cytiva) and the unbound material collected. Unbound material was concentrated and further purified by size-exclusion chromatography using a Superdex 200 Increase 10/300 column (Cytiva) equilibrated in 50 mM Tris, pH 8, 300 mM NaCl, and 0.5 mM TCEP. Peak fractions were analysed for purity by SDS–PAGE, and the purest were pooled, concentrated to 150 µM, aliquoted, and stored at −80 °C.
Immunodepletion and western blotting
Approximately 300,000 FACS-purified secondary spermatocytes (Sp2) from wild-type (C57BL/6) or 3×Flag–HA–GTSF1-expressing (Gtsf1Flag/Flag) mice were lysed in lysis buffer (30 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT, 0.1% v/v Triton X-100, 20% v/v glycerol, and 1× protease inhibitor cocktail (1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Sigma, A8456), 0.3 μM aprotinin, 40 μM betanin hydrochloride, 10 μM E-64 (Sigma, E3132), 10 μM leupeptin hemisulfate)). Crude cytoplasmic lysate was clarified at 20,000g (S20) for 30 min at 4 °C. The S20 protein concentration was measured using Bradford assay. The S20 was incubated with anti-Flag paramagnetic beads overnight at 4 °C. The supernatant was removed, and the beads washed thrice with lysis buffer but containing 1 M potassium acetate (high salt wash) and then twice with lysis buffer. Bound proteins were eluted by incubation with 200 ng µl−1 3×Flag peptide for 2 h at 4 °C. The extent of depletion was assayed by western blotting. In brief, the samples were resolved on SDS–PAGE and transferred to nitrocellulose membrane (Amersham Protran 0.45 NC, Cytiva). The membrane was probed using mouse anti-Flag antibody (Sigma, F1804; 1:1,000 in blocking buffer, Rockland Immunochemicals, MB-070) and rabbit anti-tubulin (Cell Signaling, 2144; 1:1,000) for 2 h at room temperature. After washing 5× with TBS (+0.1% v/v Tween-20), the membrane was incubated with secondary antibodies—donkey anti-rabbit IRDye 680RD (LI-COR, 926-68073; 1:15,000) and goat anti-mouse IRDye 800CW (LI-COR, 926-32210; 1:15,000)—for 30 min at room temperature. The membrane was washed 5× with TBS (+0.1% v/v Tween-20) and imaged using Odyssey Infrared Imaging System (LI-COR).
Northern blotting
Northern blotting was performed as described67. In brief, piRNA guide standards and PIWI RISCs were first resolved on a denaturing 15% polyacrylamide gel, followed by transfer to Hybond-NX (Cytiva) neutral nylon membrane by semi-dry transfer at 20 V for 1 h. Next, crosslinking was performed in the presence of 0.16 M EDC in 0.13 M 1-methylimidazole, pH 8.0, at 60 °C for 1 h. The crosslinked membrane was pre-hybridized in Church’s buffer (1% w/v BSA, 1 mM EDTA, 0.5 M phosphate buffer, and 7% w/v SDS) at 45 °C for 1 h. Radiolabeled, 5′ 32P-DNA probe (25 pmol) in Church’s buffer was added to the membrane and allowed to hybridize overnight at 45 °C, followed by five washes with 1× SSC containing 0.1% w/v SDS. The membrane was air dried and exposed to a storage phosphor screen.
Chromatographic fractionation of the MIWI-potentiating activity
Dissected animal tissues were homogenized in lysis buffer in a Dounce homogenizer using 10 strokes of the loose-fitting pestle A, followed by 20 strokes of tight-fitting pestle B. Lysate was clarified at 20,000g, followed by 0.2 µm filtration to yield an S20 for further chromatographic purification. Lysates used without further purification were directly prepared in 30 mM HEPES-KOH, pH 7.5, 100 mM potassium acetate, 3.5 mM magnesium acetate, 1 mM DTT, and 20% v/v glycerol (‘dialysis buffer’) with 1× protease inhibitor homemade cocktail; column fractions were dialysed into this buffer before assaying. Protein concentration was measured using the BCA assay. Chromatography buffers were filtered prior to use.
For chromatography, lysate was prepared as described except using 30 mM HEPES-KOH, pH 7.5, 50 mM NaCl, 1 mM DTT, 5% v/v glycerol, and protease inhibitors. The lysate was applied to HiTrap SP column (Cytiva) equilibrated with the lysis buffer. The column was washed, and the bound proteins eluted stepwise using increasing NaCl concentrations. The NaCl content of the SP column fractions containing the peak MIWI-potentiating activity was adjusted to 2 M and applied to HiTrap Phenyl (Cytiva) equilibrated with column buffer containing 2 M NaCl. Bound proteins were eluted stepwise using decreasing NaCl concentrations. The peak MIWI-potentiating fractions elute from the HiTrap Phenyl column were pooled, concentrated (10 kDa MWCO Amicon Ultra filter), and applied to Superdex 200 Increase 10-300 GL size-exclusion chromatography column (bed volume ~24 ml) equilibrated with the dialysis buffer but containing 5% v/v glycerol. The void volume (V0) of the gel filtration column was determined with blue dextran, and all fractions (0.5 ml each), starting from just before V0 to the end of the column (Vt) were assayed for MIWI-potentiating activity. The molecular mass of the potentiating activity was determined relative to molecular mass markers (beta amylase, 200 kDa; alcohol dehydrogenase, 150 kDa; albumin, 66 kDa; carbonic anhydrase, 29 kDa; and cytochrome C, 12.4 kDa).
Zn2+ and Ni2+ immobilized metal affinity chromatography
HiTrap Chelating HP (Cytiva) columns were charged with 0.1 M NiSO4 or ZnSO4, washed with water, and then equilibrated in column buffer (20 mM potassium phosphate buffer, pH 7.5, 500 mM NaCl, 0.5 mM DTT, 5% v/v glycerol). S20 testis lysate was applied to the column, the flow-through was collected, and the column was washed with the column buffer until absorbance at 280 nm stabilized. Bound proteins were eluted in two steps: first, with 20 mM potassium phosphate, pH 7.5, 2 M ammonium chloride, 0.5 mM DTT, 5% v/v glycerol (elution buffer 1), and, second, with 20 mM potassium phosphate, pH 7.5, 500 mM NaCl, 200 mM imidazole, pH 8.0, 0.5 mM DTT, 5% v/v glycerol (elutionbuffer 2). The peak of each step was dialysed into the dialysis buffer and assayed for the ability to potentiate MIWI catalysis.
Target-cleavage assays
Target RNA substrates for in vitro cleavage assays were prepared as described36,68,69. In brief, piRNA target site-containing templates were amplified by PCR, in vitro transcribed with T7 RNA polymerase, purified by urea PAGE, and radiolabelled using α-32P GTP (3,000 Ci mmol−1; Perkin Elmer), S-adenosyl methionine, and vaccinia virus RNA guanylyl transferase as described69. Unincorporated α-32P GTP was removed using a G-25 spin column (Cytiva), and target RNA was gel purified. In Extended Data Fig. 8a, target cleavage was monitored using synthetic RNA oligonucleotides radiolabelled by ligating [5′-32P] cytidine 3′,5′-bisphosphate to the 3′ end of the target with T4 RNA ligase I (Ambion). The [5′-32P] cytidine 3′,5′-bisphosphate was prepared by incubating 1 mM cytidine 3′-monophosphate (Sigma) with 312.5 pmol [γ-32P] ATP (6,000 Ci mmol−1; Perkin Elmer) with 25 U T4 polynucleotide kinase (NEB) at 37 °C for 1 h, followed by 70 °C for 30 min to inactivate the kinase.
Radiolabelled target (3–100 nM final concentration) was added to purified PIWI piRISC (2–8 nM), plus ~1 μg tissue or sorted germ cell lysate per 10 μl reaction volume or 0.5 μM (final concentration) of purified GTSF protein. At the indicated times, an aliquot of a master reaction was quenched in 4 volumes 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 25 mM EDTA, 1% w/v SDS, then proteinase K (1 mg ml−1 final concentration) was added and incubated at 45 °C for 15 min. An equal volume of urea loading buffer (8 M urea, 25 mM EDTA) was added to the reaction time points, heated at 95 °C for 2 min, and resolved by 7–10% denaturing PAGE. Gels were dried, exposed to a storage phosphor screen, and imaged on a Typhoon FLA 7000 (GE Healthcare).
The raw image file was used to quantify the substrate and product bands, corrected for background. Data were fit to the reaction scheme
using the burst-and-steady-state equation:
| 1 |
The time-dependence of product formation corresponded to a pre-steady-state exponential burst (kburst = k2 + k3) followed by a linear steady-state phase, described by kcat, where kcat = kss = k2k3/(k2 + k3).
The affinity (Kd) of wild-type or GTSF1(W98A/W107A/W112A) for MIWI piRISC–target ternary complex and the maximum observable rate (kpot) were estimated by measuring the pre-steady-state rate of target cleavage (kburst) of MIWI at increasing concentrations of GTSF1 (1–5,000 nM) and fitting the data70 to equation (2):
| 2 |
All data were graphed using Igor Pro 8.
Flag–Siwi, Flag–MIWI and Flag–MILI target-cleavage assays
Vectors expressing Flag-tagged BmSiwi, MIWI or MILI were transfected into BmN4 cells with X-tremeGENE HP DNA Transfection Reagent (Sigma). Preparation of loading lysates and single-stranded RNA loading were as described71. After loading with synthetic guide RNAs in the cell lysate, piRISC was immunoprecipitated with anti-Flag antibody (Sigma) conjugated to Dynabeads protein G superparamagnetic beads (Thermo Fisher Scientific). The beads were washed 5 times with lysis buffer containing 30 mM HEPES-KOH, pH 7.4, 100 mM potassium acetate, 2 mM magnesium acetate, 0.5 mM DTT, and 0.1% v/v Empigen. Target-cleavage assays were performed at 25 °C for 3.5 h in 8 μl reaction containing 2.4 μl ‘40×’ reaction mix69, 100 nM recombinant GTSF1 protein and 0.5 nM 32P cap-radiolabelled target RNA as described72.
BmAgo3 target-cleavage assays
Naive BmN4 cells were resuspended in lysis buffer (30 mM HEPES-KOH, pH 7.4, 100 mM potassium acetate, 2 mM magnesium acetate, 0.05% v/v Triton X-100, 0.5 mM DTT, 1× Complete EDTA-free protease inhibitor (Roche)) and lysed using a Dounce homogenizer. After centrifugation at 17,000g for 20 min at 4 °C, the supernatant was incubated at 4 °C for 1 h with anti-BmAgo3 antibody as described73 conjugated to Dynabeads protein G (Thermo Fisher Scientific). The superparamagnetic beads were washed 5 times with wash buffer (30 mM HEPES-KOH, pH 7.4, 100 mM potassium acetate, 2 mM magnesium acetate, 0.5% v/v Empigen, 0.5% v/v Triton X-100, 0.5 mM DTT) and rinsed with lysis buffer. Target-cleavage assays were performed at 25 °C for 2.5 h in 8 μl reactions containing 2.4 μl 40× reaction mix, 55 nM recombinant protein, and 0.5 nM 32P cap-radiolabelled target 1 (complementary to an endogenous pi6 piRNA) or target 2 (complementary to an endogenous pi9 piRNA), prepared by in vitro transcription as described74.
EfPiwi target cleavage assays
EfPiwi piRISC was purified as described35. Purified EfPiwi piRISC (100 nM final concentration) was incubated at 37 °C with a 5′-32P-radiolabelled target RNA complementary to g2–g21 (10 nM final concentration) in reaction buffer composed of 20 mM Tris, pH 8.0, 100 mM NaCl, 2 mM MgCl2, 2 mM MnCl2, and 0.5 mM TCEP. Where indicated, purified E. muelleri GTSF1 (500 nM final concentration) or 0.1 mg ml−1 baker’s yeast tRNA were also included. Target cleavage was stopped at indicated times by mixing aliquots of each reaction with an equal volume of denaturing gel loading buffer (98% w/v formamide, 0.025% w/v xylene cyanol, 0.025% w/v bromophenol blue, 10 mM EDTA, pH 8.0). Intact and cleaved target RNAs were resolved by denaturing PAGE (15%) and visualized by phosphorimaging. Quantification of signal was performed using ImageQuant TL (GE Healthcare).
Mouse germ cell purification
Germ cells from mouse testes were sorted and purified as described19,20. In brief, freshly dissected mouse testes were decapsulated with 0.4 mg ml−1 collagenase type IV (Worthington) in 1× Gey’s balanced salt solution (GBSS) at 33 °C for 15 min. The separated seminiferous tubules were treated with 0.5 mg ml−1 trypsin and 1 μg ml−1 DNase I in 1× GBSS at 33 °C for 15 min. Trypsin was then inactivated by adding 7.5% v/v fetal bovine serum (FBS). The cell suspension was filtered through a 70-μm cell strainer, and cells pelleted at 300g at 4 °C for 10 min. Cell staining was performed at 33 °C for 15 min with 5 μg ml−1 Hoechst 33342 prepared in 1× GBSS, 5% v/v FBS, and 1 μg ml−1 DNase I, then the cells were treated with 0.2 μg ml−1 propidium iodide, followed by final pass through a 40-μm cell strainer before sorting. Sorted cells were pelleted at 100g for 5 min, the buffer was removed, and the cell pellets were flash frozen and stored at −80 °C. Cell lysates were prepared as described for HEK293T cells. Protein concentration was estimated using the BCA assay, and an equal amount of total protein from each cell type was used to assay for the ability to potentiate MIWI catalysis.
Analysis of RNA-seq data
Publicly available datasets17,75–77 were analysed. rRNA reads were removed using Bowtie 2.2.5 with default parameters78. After rRNA removal, the remaining reads were mapped to corresponding genomes (mouse, mm10; rat, rn6; macaque, rheMac8; human, hg19) using STAR 2.3 with default parameters that allowed ≤ 2 mismatches and 100 mapping locations79. Mapped results were generated in SAM format, duplicates removed and translated to BAM format using SAMtools 1.880. HTSeq 0.9.1 with default parameters was used to count uniquely mapping reads81 (steady-state transcript abundance was reported in reads per kilobase per million uniquely mapped reads (RPKM)).
Methylarginine analysis
Recombinant MIWI was immunopurified and resolved by electrophoresis on a 4–20% gradient SDS-polyacrylamide gel. The gel was fixed, stained with Coomassie G-250 (Simply Blue, Invitrogen), and the recombinant MIWI band excised and analysed at the UMASS Mass Spectrometry Core. Gel slices were chopped into ~1 mm2 pieces, 1 ml water added, followed by 20 µl 45 mM DTT in 250 mM ammonium bicarbonate. Samples were incubated at 50 °C for 30 min, cooled to room temperature, then 20 µl 100 mM iodoacetamide was added and incubated for 30 min. The solution was removed, and the gel pieces were three times washed with 1 ml water, 1 ml 50 mM ammonium bicarbonate:acetonitrile (1:1), quenched with 200 µl acetonitrile, and dried in a SpeedVac. Gel pieces were then rehydrated in 50 µl 50 mM ammonium bicarbonate containing 4 ng µl−1 trypsin (Promega, Madison, WI) and 0.01% proteaseMAX (Promega) and incubated at 37 °C for 18 h. Supernatants were collected and extracted with 200 µl an 80:20 solution of acetonitrile: 1% v/v formic acid in water. Supernatants were then combined, and the peptides lyophilized in a SpeedVac and resuspended in 25 µl 5% acetonitrile, 0.1% v/v formic acid and subject to mass spectrometry analysis. Data were acquired using a NanoAcquity UPLC (Waters Corporation) coupled to an Orbitrap Fusion Lumos Tribrid (Thermo Fisher Scientific) mass spectrometer. Peptides were trapped and separated using an in-house 100 µm internal diameter fused-silica pre-column (Kasil frit) packed with 2 cm ProntoSil (Bischoff Chromatography) C18 AQ (200 Å, 5 µm) media and configured to an in-house packed 75 µm internal diameter fused-silica analytical column (gravity-pulled tip) packed with 25 cm ProntoSil (Bischoff; 100 Å, 3 µm) media. Mobile phase A was 0.1 % v/v formic acid in water; mobile phase B was 0.1 % v/v formic acid in acetonitrile. Following a 3.8 µl sample injection, peptides were trapped at flow rate of 4 µl/min with 5% B for 4 min, followed by gradient elution at a flow rate of 300 nl min−1 from 5–35% B in 90 min (total run time, 120 min). Electrospray voltage was delivered by liquid junction electrode (1.5 kV) located between the columns and the transfer capillary to the mass spectrometer was maintained at 275 °C. Mass spectra were acquired over m/z 300–1,750 Da with a resolution of 120,000 (m/z 200), maximum injection time of 50 ms, and an AGC target of 400,000. Tandem mass spectra were acquired using data-dependent acquisition (3 s cycle) with an isolation width of 1.6 Da, HCD collision energy of 30%, resolution of 15,000 (m/z 200), maximum injection time of 22 ms, and an AGC target of 50,000.
Raw data were processed using Proteome Discoverer 2.1.1.21 (Thermo Fisher Scientific), and the database search performed by Mascot 2.6.2 (Matrix Science) using the Swiss-Prot human database (downloaded 4 September 2019; https://www.uniprot.org/). Search parameters were: semi-tryptic digestion with up to two missed cleavages; precursor mass tolerance 10 ppm; fragment mass tolerance 0.05 Da; peptide N-terminal acetylation, cysteine carbamidomethylation, methionine oxidation, N-terminal glutamine to pyroglutamate conversion, arginine methylation and arginine demethylation were specified as variable modifications. Peptide and protein validation and annotation was done in Scaffold 4.8.9 (Proteome Software, Portland, OR) employing Peptide Prophet82 and Protein Prophet83. Peptides were filtered at 1% FDR, while protein identification threshold was set to greater than 99% probability and with a minimum of two identified peptides per protein. Only arginine modification sites detected in all three replicates from separate immunoprecipitation experiments are reported in the figure.
Statistics and reproducibility
Figures 1b, 3, 4b,c and 5a and Extended Data Fig. 4a show mean ± s.d. for three independent trials. Figure 2a,b, Extended Data Figs. 1a–c,h and 6a show three independent trials with similar results. Figure 2e,f and Extended Data Figs. 1f,g, 2b, 3a,c and 5 show two independent trials with similar results. The experiments in Figs. 1c and 4a and Extended Data Figs. 1d,e, 2a, 3b,d–h, 4b, 6b and 8a,b were performed once. Protein sequences were aligned using Clustal Omega (1.2.4); unrooted tree was constructed using randomized axelerated maximum likelihood (RAxML 1.0.0) with default parameters84 and visualized in Interactive Tree of Life85.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-05009-0.
Supplementary information
Uncropped source gel images.
Individual MIWI kburst graphs using wild-type or PIWI-int mutant GTSF1.
Oligonucleotides and DNA sequences used in this study.
Acknowledgements
We thank K.-E. Aryee for providing pScalps_Puro, psPAX2, pMD2.G plasmids and for guidance on lentiviral transduction; P.-H. Wu for help with mouse testis germ cell FACS and for providing sorted cells; K. Cecchini for mouse tissues; E. Norowski for rat testes; K. Orwig for rhesus macaque testes; P. Albosta and C. Tipping for guidance with T. ni and D. melanogaster dissection; L. Joshua-Tor for sharing data prior to publication; T. Gardner and K. Jouravleva for help formatting and proofreading the manuscript; the UMass Chan Flow Cytometry and Mass Spectrometry cores; and members of the Zamore laboratory for discussions and comments on the manuscript. This work was supported in part by NIGMS grants R37 GM062862 and R35 GM136275 (P.D.Z.) and R35 GM127090 (I.J.M.) and JSPS KAKENHI grants 18H05271 (Y.T.) and 19K06484 (N.I.).
Extended data figures and tables
Author contributions
A.A., P.D.Z., I.J.M. and Y.T. conceived and designed the experiments. A.A., S.B., N.I. (insect proteins), T.A.A. (sponge proteins), C.A. (bacterial culture) and I.G. (endogenous piRNAs) performed the experiments. D.M.O. and I.G. provided and analysed the sequencing data. P.D.Z. supervised the research. A.A. and P.D.Z. prepared the manuscript. All authors discussed the results and approved the manuscript.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
All data are available from the authors upon request. Mass spectrometry data are available from MassIVE using accession number MSV000089490.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-022-05009-0.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-05009-0.
References
- 1.Ozata DM, Gainetdinov I, Zoch A, O’Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 2018;20:89–108. doi: 10.1038/s41576-018-0073-3. [DOI] [PubMed] [Google Scholar]
- 2.Kuramochi-Miyagawa S, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 3.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell. 2002;2:819–830. doi: 10.1016/S1534-5807(02)00165-X. [DOI] [PubMed] [Google Scholar]
- 4.Carmell MA, et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell. 2007;12:503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Reuter M, et al. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature. 2011;480:264–267. doi: 10.1038/nature10672. [DOI] [PubMed] [Google Scholar]
- 6.De Fazio S, et al. The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature. 2011;480:259–263. doi: 10.1038/nature10547. [DOI] [PubMed] [Google Scholar]
- 7.Dönertas D, Sienski G, Brennecke J. Drosophila Gtsf1 is an essential component of the Piwi-mediated transcriptional silencing complex. Genes Dev. 2013;27:1693–1705. doi: 10.1101/gad.221150.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtani H, et al. DmGTSF1 is necessary for Piwi-piRISC-mediated transcriptional transposon silencing in the Drosophila ovary. Genes Dev. 2013;27:1656–1661. doi: 10.1101/gad.221515.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura T, et al. Mouse GTSF1 is an essential factor for secondary piRNA biogenesis. EMBO Rep. 2018;19:e42054. doi: 10.15252/embr.201642054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muerdter F, et al. A genome-wide RNAi screen draws a genetic framework for transposon control and primary piRNA biogenesis in Drosophila. Mol. Cell. 2013;50:736–748. doi: 10.1016/j.molcel.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, et al. Gtsf1 is essential for proper female sex determination and transposon silencing in the silkworm, Bombyx mori. PLoS Genet. 2020;16:e1009194. doi: 10.1371/journal.pgen.1009194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura T, et al. Gtsf1/Cue110, a gene encoding a protein with two copies of a CHHC Zn-finger motif, is involved in spermatogenesis and retrotransposon suppression in murine testes. Dev. Biol. 2009;335:216–227. doi: 10.1016/j.ydbio.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Aravin A, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 14.Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- 15.Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–1714. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau NC, et al. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 17.Özata DM, et al. Evolutionarily conserved pachytene piRNA loci are highly divergent among modern humans. Nat. Ecol. Evol. 2020;4:156–168. doi: 10.1038/s41559-019-1065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XZ, et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol. Cell. 2013;50:67–81. doi: 10.1016/j.molcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gainetdinov I, Colpan C, Arif A, Cecchini K, Zamore PD. A single mechanism of biogenesis, initiated and directed by PIWI proteins, explains piRNA production in most animals. Mol. Cell. 2018;71:775–790.e5. doi: 10.1016/j.molcel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu PH, et al. The evolutionarily conserved piRNA-producing locus pi6 is required for male mouse fertility. Nat. Genet. 2020;52:728–739. doi: 10.1038/s41588-020-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H, Wang Z, Dean J. Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA. PLoS Genet. 2021;17:e1009485. doi: 10.1371/journal.pgen.1009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo LT, et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2014;24:680–700. doi: 10.1038/cr.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh WS, et al. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev. 2015;29:1032–1044. doi: 10.1101/gad.260455.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vourekas A, et al. Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nat. Struct. Mol. Biol. 2012;19:773–781. doi: 10.1038/nsmb.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, et al. MIWI and piRNA-mediated cleavage of messenger RNAs in mouse testes. Cell Res. 2015;25:193–207. doi: 10.1038/cr.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ. Structure of the guide-strand-containing argonaute silencing complex. Nature. 2008;456:209–213. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–1040. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto N, et al. Crystal structure of silkworm PIWI-clade Argonaute Siwi bound to piRNA. Cell. 2016;167:484–497.e9. doi: 10.1016/j.cell.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Parker JS, Roe SM, Barford D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 2004;23:4727–4737. doi: 10.1038/sj.emboj.7600488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma JB, et al. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature. 2005;434:663–666. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Thomas A, Marinov GK, Aravin AA. A transgenerational process defines piRNA biogenesis in Drosophila virilis. Cell Rep. 2014;8:1617–1623. doi: 10.1016/j.celrep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi S, et al. Crystal structure of Drosophila Piwi. Nat. Commun. 2020;11:858. doi: 10.1038/s41467-020-14687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaoka S, Izumi N, Katsuma S, Tomari Y. 3′ end formation of PIWI-interacting RNAs in vitro. Mol. Cell. 2011;43:1015–1022. doi: 10.1016/j.molcel.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 34.Cora E, et al. The MID-PIWI module of Piwi proteins specifies nucleotide- and strand-biases of piRNAs. RNA. 2014;20:773–781. doi: 10.1261/rna.044701.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anzelon TA, et al. Structural basis for piRNA targeting. Nature. 2021;597:285–289. doi: 10.1038/s41586-021-03856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salomon WE, Jolly SM, Moore MJ, Zamore PD, Serebrov V. Single-molecule imaging reveals that Argonaute reshapes the binding properties of its nucleic acid guides. Cell. 2015;162:84–95. doi: 10.1016/j.cell.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker WR, et al. High-throughput analysis reveals rules for target RNA binding and cleavage by AGO2. Mol. Cell. 2019;75:741–755.e11. doi: 10.1016/j.molcel.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 39.Vagin VV, et al. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23:1749–1762. doi: 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siomi MC, Mannen T, Siomi H. How does the royal family of Tudor rule the PIWI-interacting RNA pathway? Genes Dev. 2010;24:636–646. doi: 10.1101/gad.1899210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol. Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuramochi-Miyagawa S, et al. Two mouse piwi-related genes: miwi and mili. Mech. Dev. 2001;108:121–133. doi: 10.1016/S0925-4773(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 43.Michael SF, Kilfoil VJ, Schmidt MH, Amann BT, Berg JM. Metal binding and folding properties of a minimalist Cys2His2 zinc finger peptide. Proc. Natl Acad. Sci. USA. 1992;89:4796–4800. doi: 10.1073/pnas.89.11.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matt T, Martinez-Yamout MA, Dyson HJ, Wright PE. The CBP/p300 TAZ1 domain in its native state is not a binding partner of MDM2. Biochem. J. 2004;381:685–691. doi: 10.1042/BJ20040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyborg JK, Peersen OB. That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem. J. 2004;381:e3–e4. doi: 10.1042/BJ20041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almeida MV, et al. GTSF-1 is required for formation of a functional RNA-dependent RNA Polymerase complex in Caenorhabditis elegans. EMBO J. 2018;37:e99325. doi: 10.15252/embj.201899325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ernst C, Odom DT, Kutter C. The emergence of piRNAs against transposon invasion to preserve mammalian genome integrity. Nat. Commun. 2017;8:1411. doi: 10.1038/s41467-017-01049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wenda JM, et al. Distinct roles of RNA helicases MVH and TDRD9 in PIWI slicing-triggered mammalian piRNA biogenesis and function. Dev. Cell. 2017;41:623–637.e9. doi: 10.1016/j.devcel.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiol J, et al. RNA clamping by Vasa assembles a piRNA amplifier complex on transposon transcripts. Cell. 2014;157:1698–1711. doi: 10.1016/j.cell.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Nishida KM, et al. Respective functions of two distinct Siwi complexes assembled during PIWI-interacting RNA biogenesis in Bombyx germ cells. Cell Rep. 2015;10:193–203. doi: 10.1016/j.celrep.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Murakami R, Sumiyoshi T, Negishi L, Siomi MC. DEAD-box polypeptide 43 facilitates piRNA amplification by actively liberating RNA from Ago3–piRISC. EMBO Rep. 2021;22:e51313. doi: 10.15252/embr.202051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreeva A, Tidow H. A novel CHHC Zn-finger domain found in spliceosomal proteins and tRNA modifying enzymes. Bioinformatics. 2008;24:2277–2280. doi: 10.1093/bioinformatics/btn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ipsaro JJ, O’Brien PA, Bhattacharya S, Palmer AG, III, Joshua-Tor L. Asterix/Gtsf1 links tRNAs and piRNA silencing of retrotransposons. Cell Rep. 2021;34:108914. doi: 10.1016/j.celrep.2021.108914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takemoto N, Yoshimura T, Miyazaki S, Tashiro F, Miyazaki J. Gtsf1l and Gtsf2 Are specifically expressed in gonocytes and spermatids but are not essential for spermatogenesis. PLoS ONE. 2016;11:e0150390. doi: 10.1371/journal.pone.0150390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nat. Struct. Mol. Biol. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- 56.Jackson AL, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. 2009;461:754–761. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aravin AA, et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell. 2008;31:785–799. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 60.Park MS, Sim G, Kehling AC, Nakanishi K. Human Argonaute2 and Argonaute3 are catalytically activated by different lengths of guide RNA. Proc. Natl Acad. Sci. USA. 2020;117:28576–28578. doi: 10.1073/pnas.2015026117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishino K, et al. PIWI proteins bind to piRNAs with stage-specific size variations during oocyte maturation. Nucleic Acids Res. 2021;49:2700–2720. doi: 10.1093/nar/gkab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Q, et al. Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat. Commun. 2019;10:3389. doi: 10.1038/s41467-019-11312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawaoka S, et al. The Bombyx ovary-derived cell line endogenously expresses PIWI/PIWI-interacting RNA complexes. RNA. 2009;15:1258–1264. doi: 10.1261/rna.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS ONE. 2013;8:e77715. doi: 10.1371/journal.pone.0077715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kenny NJ, et al. Tracing animal genomic evolution with the chromosomal-level assembly of the freshwater sponge Ephydatia muelleri. Nat. Commun. 2020;11:3676. doi: 10.1038/s41467-020-17397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flores-Jasso CF, Salomon WE, Zamore PD. Rapid and specific purification of Argonaute–small RNA complexes from crude cell lysates. RNA. 2013;19:271–279. doi: 10.1261/rna.036921.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pall GS, Hamilton AJ. Improved Northern blot method for enhanced detection of small RNA. Nat. Protoc. 2008;3:1077–1084. doi: 10.1038/nprot.2008.67. [DOI] [PubMed] [Google Scholar]
- 68.Wee L, Flores-Jasso C, Salomon W, Zamore P. Argonaute divides Its RNA guide into domains with distinct functions and RNA-binding properties. Cell. 2012;151:1055–1067. doi: 10.1016/j.cell.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haley B, Tang G, Zamore PD. In vitro analysis of RNA interference in Drosophila melanogaster. Methods. 2003;30:330–336. doi: 10.1016/S1046-2023(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 70.Carlson KD, Johnson RE, Prakash L, Prakash S, Washington MT. Human DNA polymerase κ forms nonproductive complexes with matched primer termini but not with mismatched primer termini. Proc. Natl Acad. Sci. USA. 2006;103:15776–15781. doi: 10.1073/pnas.0605785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawaoka S, et al. Zygotic amplification of secondary piRNAs during silkworm embryogenesis. RNA. 2011;17:1401–1407. doi: 10.1261/rna.2709411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izumi N, et al. Identification and functional analysis of the pre-piRNA 3’ trimmer in silkworms. Cell. 2016;164:962–973. doi: 10.1016/j.cell.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Izumi N, Shoji K, Suzuki Y, Katsuma S, Tomari Y. Zucchini consensus motifs determine the mechanism of pre-piRNA production. Nature. 2020;578:311–316. doi: 10.1038/s41586-020-1966-9. [DOI] [PubMed] [Google Scholar]
- 74.Yoda M, et al. ATP-dependent human RISC assembly pathways. Nat. Struct. Mol. Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merkin J, Russell C, Chen P, Burge CB. Evolutionary dynamics of gene and isoform regulation in mammalian tissues. Science. 2012;338:1593–1599. doi: 10.1126/science.1228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu T, et al. Long first exons and epigenetic marks distinguish conserved pachytene piRNA clusters from other mammalian genes. Nat. Commun. 2021;12:73. doi: 10.1038/s41467-020-20345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gainetdinov I, et al. Terminal modification, sequence, length, and PIWI-protein identity determine piRNA stability. Mol. Cell. 2021;81:4826–4842. e8. doi: 10.1016/j.molcel.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 83.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 84.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uncropped source gel images.
Individual MIWI kburst graphs using wild-type or PIWI-int mutant GTSF1.
Oligonucleotides and DNA sequences used in this study.
Data Availability Statement
All data are available from the authors upon request. Mass spectrometry data are available from MassIVE using accession number MSV000089490.