Abstract
We examined the protective effect of the apigeninidin (API)-enriched fraction from Sorghum bicolor sheaths extracts (SBE-05, SBE-06, and SBE-07) against aflatoxin B1 (AFB1)-induced dysregulation of male rat’s reproductive system that may trigger infertility. Male rats (160 ± 12 g) were treated with AFB1 (50 µg/kg) along with 5 or 10 mg/kg of SBE-05, SBE-06, and SBE-07 for 28 days. Subsequently, we assessed the reproductive hormone—prolactin, FSH, LH, testosterone levels, and testicular function enzymes. Moreover, we examined rats’ testes, epididymis, and hypothalamus for oxidative and inflammatory stress biomarkers, caspase-9 activity and tissues pathology. We observed that comparative to AFB1 alone treated rats, API co-treatment significantly (p < 0.05) abated the AFB1-mediated decrease in prolactin and antioxidant defenses and lessened lipid peroxidation (LPO) and reactive oxygen and nitrogen species levels in the examined organs—testes, epididymis, and hypothalamus. API abated AFB1-induced hormone decreases—testosterone, FSH, and LH; and caused improvement in sperm quantity and quality. API lessened AFB1-mediated increase in pro-inflammatory cytokine, increased interleukin-10 level, an anti-inflammatory cytokine and reduced caspase-9 activities. In addition, API reduced alterations in the examined tissue histology. Our findings suggest that S. bicolor API-enrich extracts have active antioxidative, antiapoptotic, and anti-inflammatory activities, which can protect against AFB1-induced dysfunction of the hypothalamic–pituitary–gonadal axis.
Keywords: Aflatoxin B1, Sorghum bicolor, male reproductive toxicity, hypothalamic–pituitary–gonadal axis, oxidative–inflammatory biomarkers, antioxidant activity
Impact Statement
Aflatoxin B1 (AFB1), a dietary mycotoxin widespread in inadequately stored grains, elicits reproductive derangement experimentally in rodents and infertility. Childlessness stemming from reproductive insufficiency is traumatic. AFB1 poisonousness is centered on oxidative epoxide formation; hence we assessed the probable advantageous effect of apigeninidin (API)-enrich extract from Sorghum bicolor (API) with pharmacological benefit against oxidative–inflammation-related alterations in rats’ hypothalamic–pituitary–gonadal axis. In a bid to extend novel proof advancing cheap nutriceutical derived from plant waste as a source of chemopreventive phytochemicals. Our findings here offer the field of chemoprevention by reporting new data on API. Our results demonstrate that (1) API eased inflammatory and apoptotic responses and blocked oxidative damages; (2) API enhanced sperm functionality and hormonal levels pertinent to reproductive function; and (3) API offered protection against histological alterations in the examined organs, all brought about by accidental exposure to AFB1.
Introduction
The incidence of infertility affects over 70 million people globally. 1 There is an increase in reports indicating a continuous decline in human fertility in both developed and developing countries. Male infertility can be caused by several factors, including genetics, underlying disorders, lifestyle choices, and drug-related effects. 1 Previous findings from our lab and others have implicated mycotoxins, including aflatoxin B1 (AFB1) and zearalenone, infertility impairment through damage to sex organs, gametes, and disruption of steroidogenesis.2–4
AFB1 is a common contaminant found in food crops, predominant in the developing countries’ essential diets. AFB1 is biosynthesized under favorable conditions of high temperature (between 24 and 35°C) and high humidity (7–10%). Hence, subtropical/tropical regions, including Asian and Sub-Saharan Africa, often experience more significant contamination. 5 AFB1 and closely related analogues are secondary metabolites synthesized by fungi of the Aspergillus species—flavus and parasiticus during production, harvest, storage, and food processing. In developing countries, the inability to store food in a dry and temperature-controlled environment increases contamination. 5 AFB1 is categorized as an inevitable food contaminant by the US Food and Drug Administration (FDA). 6
AFB1 happens to be the most common and toxic among the AFs in nature, as it is teratogenic, hepatocarcinogenic, and hepatotoxic. 5 Upon ingestion of AFB1-contaminated food, cytochrome P450 (CYP450) enzymes mediate AFB1 transformation into a range of metabolites, including AFB1-epoxide (AFBO), aflatoxins M1(AFM1), P1 (AFP1), Q1 (AFQ1), and aflatoxicol. 7 AFBO is a highly reactive and unstable AFB1 metabolite that reacts with cellular macromolecules, including genetic materials—DNA, RNA, proteins, and membrane lipids, mediating lipid peroxidation (LPO) and cell damage. 8
Primarily, AFB1 is hepatotoxic, yet, AFB1 equally affects the reproductive system. Prior animal studies have revealed that sublethal doses of AFB1 cause testicular degeneration. 9 Mukumu and Macharia 10 reported that AFB1 could cause notable histopathological alterations to rat testis and epididymis, necrosis, and atrophy of seminiferous tubules and decrease spermatogenesis. Other published reports have shown that treatment with AFB1 significantly reduced organ—hypothalamus, testes, epididymis—to body weight ratio and weight gain of treated rats. 4 AFB1 impairs spermatogenesis and steroidogenesis in rats by initiating significant decreases in testicular steroidogenic enzymes deteriorates sperm quality and quantity in rats.4,11 AFB1 has also been reported to be an endocrine disruptor. 12 By binding with steroidogenic regulatory (StAR) protein, AFB1 impedes cholesterol import into the mitochondria resulting in decreased steroidogenesis. 4 Owumi et al. reported disruption of serum hormone levels in AFB1-treated rats, and it caused prolactinemia while reducing follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone.2,13,14 In addition, AFB1 reduces the activity of antioxidant enzymes and levels of endogenous antioxidants, elevates inflammation biomarkers, and induces pathological lesions in rats’ testicular and epididymal tissues, 15 ultimately rendering male animals infertile.
Currently, there is no antidote for AFs. However, plant-derived natural compounds have been proposed as a promising strategy to control AFB1 contamination. 16 Natural compounds that minimize but do not eliminate AFB1 exposure have been identified.17–19 Several of these compounds function by reducing the levels of AFB1 in foods.2,20–22 However, acute toxicity due to AFB1 can cause damage to organs. Therefore, the need arises to explore other alternatives, natural products, including dietary constituents, to manage aflatoxicosis better and restore organs to normal functioning status.
The use of plants to prevent and treat ailments has been in practice since time immemorial. 23 In this regard, sorghum species have been extensively used as components of phytomedicine and are a staple food in several tropical and sub-tropical countries. S. bicolor (L.) Moench is one of many sorghum grass species grown primarily for grain in Africa and other tropical countries, but many are also used as pasture fodder plants. 23 The inhabitants of southwest Nigeria ferment the grains to make gruels, used as baby weaning food. 23 Furthermore, sorghum extracts are used as an infusion to manage anemia resulting from sickle cell disease. The extracts are also antimalarial, antihelminthic, colorants, and dyes.24,25 Sorghum is abundant in phenolic acids and flavonoids, such as anthocyanins and 3-deoxyanthocyanidins. These flavonoids and phenolic acids provide the functional antioxidant capability for plants and confer cellular protection in vivo via the antioxidative processes. 26 API, luteolinidin, apigeninidin-5-glucoside, and luteolinidin 5-glucoside are some of the 3-deoxyanthocyanidins presents in Sorghum.23,27 Intriguingly, the non-grain tissues of the West African Sorghum variety, such as the leaf sheath, contain extraordinarily high levels of 3-deoxyanthocyanin pigments. 25 The leaf sheath and glumes offer an accessible and helpful way to obtain valuable amounts of 3-deoxyanthocyanin pigments that are stable. 25
Sorghum extracts’ beneficial medicinal roles are associated with its phytochemical components, including anthocyanins and 3-deoxyanthocyanidins.23,26,28 These phytochemicals protect the plant from pests and diseases, making the grain a very potent and rich antioxidant source. 28 The antioxidant capacity of the grain is responsible for its health benefits, including protection from diseases like cardiovascular diseases, obesity, and cancer. 29 These phytochemicals have been shown to have anti-inflammatory 28 and vasoprotective characteristics, lessen cellular LPO, and scavenge free radicals.27,30 Anthocyanins and 3-deoxyanthocyanidins also exhibit antineoplastic, anticancer, and chemoprotective properties. 23
The mechanism of AFB1-mediated toxicity has been extensively studied.4,31–33 Moreover, several research groups have investigated the beneficial effects of S. bicolor extracts and concluded that the health benefits of these extracts are due to the presence of phytochemicals25,28 such as API. From our point of view, no investigation has addressed S. bicolor extract’s role in protecting the male reproductive system against AFB1-induced toxicity. To bridge this knowledge gap, we thought it necessitous to examine API’s role in averting male reproductive toxicity brought about by AFB1 exposure. This study can implicitly elucidate API’s mechanism(s) of action, particularly in the male reproductive system. In addition, results from the study could proffer a potential phytomedicine-based solution to the problem of male infertility that may occur due to exposure to AFB1.
Materials and methods
Chemicals, reagents, and kits
AFB1, TBA: thiobarbituric acid; DTNB: 5′, 5′-dithiobis-2-nitrobenzoic acid; epinephrine; CDNB: 1-chloro-2,4-dinitrobenzene; H2O2: hydrogen peroxide; and GSH: glutathione, was purchased from Sigma-Aldrich Chemical (St Louis, USA). Caspase-3 and IL-10—Interleukin 10 ELISA—Enzyme-Linked Immunosorbent Assay Kits were purchased from Elabscience Biotech (Wuhan, China). LH (BXE0651A), FSH (BXE0631A), prolactin (PR234F), and testosterone (BXE0862A) ELISA kits were purchased from Fortress Diagnostics (Antrim, UK). All other reagents and chemicals used were commercially sourced and were of analytical grade.
Animal model, husbandry, and care
Adult male Wistar rats (ages: 10 weeks old; strain: albino; weight: 160 ± 12 g; n = 60) were procured from the University of Ibadan Primate colony and housed in polycarbonate cages. The experimental rats were fed with nutritionally balanced rat pellets (BreedwellTM Feeds, Ibadan, Nigeria) and had free access to water in a well-ventilated vivarium. Also, the rats were subjected to a 12 h light:12 h dark cycle photoperiod, and allowed to acclimatize to their new environment for 1 week preceding experimentation. All the rats were healthy and amply tended for as specified by “Guide for the Care and Use of Laboratory Animals, United States National Institute of Health.” This study was carried out following the approval (UI-ACUREC/032-0521/7) of the University of Ibadan Ethical Committee.
Experimental design
Post acclimatization, the experimental rats were sorted randomly into eight cohorts of six rats each. The rats were dosed by gavage in mg/kg body weight or mL/kg as required for four uninterrupted weeks, as shown in Table 1.
Table 1.
Show the experimental design and the various dosing regimen for the rats.
| Groups | Treatment |
|---|---|
| Control | 0.05% carboxymethyl cellulose (CMC) |
| Aflatoxin B1 alone | Aflatoxin B1 50 µg/kg |
| AFB1 + SBE-05-D1 | Aflatoxin B1 (50 µg/kg) + SBE-05-D1 (5 mg/kg) |
| AFB1 + SBE-05-D2 | Aflatoxin B1 (50 µg/kg) + SBE-05-D2 (10 mg/kg) |
| AFB1 + SBE-06-D1 | Aflatoxin B1 (50 µg/kg) + SBE-06-D1 (5 mg/kg) |
| AFB1 + SBE-06-D2 | Aflatoxin B1 (50 µg/kg) + SBE-06-D2 (10 mg/kg) |
| AFB1 + SBE-07-D1 | Aflatoxin B1 (50 µg/kg) + SBE-07-D1 (5 mg/kg) |
| AFB1 + SBE-07-D2 | Aflatoxin B1 (50 µg/kg) + SBE-07-D2 (10 mg/kg) |
CMC: carboxymethyl cellulose; AFB1: aflatoxin B1; SBE: Sorghum bicolor extract.
The dose of AFB1 (50 µg/kg), body weight20,34 applied in this study was established on previously published articles. Carboxymethyl cellulose (CMC, 0.05%) was used as a vehicle for the extracts. The control animals received 0.32 mL of 0.05% CMC, and Sorghum bicolor extract (SBE) was prepared by dissolving in 0.05% CMC. 35 A total of 0.16 mL and 0.32 mL of SBE were administered to the 5 and 10 mg/kg cohorts, respectively, and 0.16 mL of AFB1 was administered to the animals. Between 9:30 and 11:00 h, experimental rats were consistently treated with SBE daily and thrice weekly with AFB1, during the study, with the doses specified above.
We measured the terminal body weights of the experimental rat on day 29, after the last treatment. The rats were exsanguination into labeled plain tubes via the retro-orbital veins. The rats were subsequently sacrificed via cervical vertebrae dislocating. Whole blood was left for 30 min at room temperature to clot, and the serum was obtained by centrifugation (condition: 3000 g, 10 min, at 4°C) using an Eppendorf 5417R centrifuge (Hamburg, Germany). Subsequently, the serum obtained was aliquoted and preserved at −20°C in a Bosch Freezer (Stuttgart, Germany), awaiting analysis of specific reproduction hormones. Furthermore, the organs of interest, that is, epididymis, hypothalamus, and testis, were instantaneously harvested, weighed, and processed in phosphate-buffered formalin/Bouin’s solution and homogenizing buffers for histological and biochemical tests, respectively.
Preparation of tissues homogenates for biochemical analysis
The testes, epididymis, and hypothalamus were finely homogenized in homogenizing buffers—50 mM Tris-HCl; pH 7.4. The homogenates were subsequently centrifuged (condition: 12,000 rpm, 15 min, at 4°C) to obtain the supernatants used for biochemical assays. Following Lowry’s method, 36 the total protein concentrations of the tissues were biochemically estimated.
Assessment of sperm function characteristics
Sperm motility was estimated following the established methods Zemjanis, 37 by recording the quantity of non-motile sperm, non-progressive, and progressive sperm under a microscopic field of view Carl Zeiss Axio light microscope (Gottingen, Germany). The data generated were expressed as percentages of progressive sperm motility. Morphological aberrations and viability of the spermatozoa were estimated by staining—with eosin (1%) and nigrosin (5%) stains—according to Wells and Awa. 38 Epididymal sperm count was evaluated following the World Health Organization 39 protocol to determine sperm count.
Assessment of hormones of the pituitary and testicle
Testicular and pituitary hormones were determined by immunoassay kits purchased for testosterone (catalog #: EIA-5179) (DRG Diagnostics GmbH, Marburg, Germany); LH (catalog #: RPN 2562), and FSH (catalog #: RPN 2560) (Amersham, UK). The hormones were assayed for following the manufacturer’s guide. All the samples were assessed on the same day to minimize inter-assay variation. LH sensitivity = 0.06 ng at 90%; FSH sensitivity = 0.05 ng at 94%. While the intra-assay coefficients of variations (CoV) were LH (CoV: 3.7%) and FSH (CoV: 3.9%). Testosterone assay sensitivity = 0.08 ng/mL, with negligible cross-reactivity with other androgens—5α-dihydrotestosterone, methyltestosterone, and androstenedione derivatives. Testosterone intra-assay CoV = 3.4%.
Assessment of testicular function enzymes: glucose-6-phosphate dehydrogenase, acid and alkaline phosphatase, and lactate dehydrogenase
Biomarkers of testicular function were evaluated from the prepared supernatant of the testes. Testicular glucose-6-phosphate dehydrogenase (G6PD) was assayed according to Wolf et al. 40 The activities of acid 41 and alkaline 42 phosphatase (ACP and ALP) were assayed following methods based on the degradation of p-nitrophenyl phosphate in acid and alkaline milieu, respectively. Testis lactate dehydrogenase (LDH) activity was estimated according to the method of Vassault. 43
The assessment of antioxidant status: superoxide dismutase, catalase, glutathione-S-transferase, and glutathione peroxidase and glutathione, and oxidative damage malondialdehyde
Antioxidant enzyme activities, including superoxide dismutase (SOD) were assayed for by Misra and Fridovich method; 44 catalase (CAT) by Claiborne’s technique; 45 glutathione-S-transferase (GST) and glutathione peroxidase (GPx) were measured by Habig’s 46 and Rotruck’s 47 methods, respectively. Furthermore, the antioxidant GSH—glutathione and a biomarker of oxidative lipid damage—malondialdehyde (MDA) levels were determined by the methods of Ellman 48 and Okhawa, 49 respectively.
Measurement of reactive oxygen and nitrogen species level
Reactive oxygen and nitrogen species (RONS) generation in the examined organs was determined by the oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to 2′,7′-dichlorofluorescein (DCF).50,51 This reaction is dependent on the RONS present in the probed sample homogenates. With minimal exposure to air, 10 µL of the samples are briefly incubated with 150 µL of potassium phosphate buffer, 35 µL of distilled water and 5 µL DCFH-DA. The ensuing DCF fluorescence emission from the oxidation of DCFH-DA was evaluated at 488 nm (excitation) and 525 nm (emission) wavelengths. The readings were monitored for 10 min at 30-s intervals and recorded with a Multi-modal Plate Reader SpectraMaxTM M384 (Molecular Devices, San Jose, USA). Compared to the control group, the fraction of DCF produced was represented as a percentage fold.
Estimation of inflammatory biomarkers
Nitrites/nitrates levels as a measure of nitric oxide (NO) in the examined samples were assessed by Green method 52 since NO rapidly dissociates into its metabolites. Griess reagent and the probed samples mixed in equal volume were incubated for 15 min at 27°C. Subsequently, the absorbance was recorded at 540 nm. NO level was estimated from a known sodium nitrite solution curve. Spectrophotometrically, myeloperoxidase (MPO) activity was assessed by the previously reported method of Trush.53,54 MPO catalyzes the oxidation of dianisidine to a brown-colored product when hydrogen peroxide is present. This product exhibits absorbance of 470 nm. Furthermore, testicular, epididymal, and hypothalamic caspase-9 activity, and concentrations of IL-10 were measured by ELISA following the manufacturer’s protocol. The readings were obtained using the same Plate reader.
Histological examination
The testes, epididymis, and hypothalamus were fixed in 10% formalin for 3 days. Subsequently, the samples were embedded in paraffin after a sequential dehydration process. Afterwards, 4–5 µm tissue sections on charged glass slides were stained with hematoxylin and eosin (H&E). 55 Pathological examination of the stained tissue sections was carried out under a light microscope (Leica DM 500, Germany) by a pathologist blinded to the study slides. Tissue pathological abnormalities were scored, and representative images were captured with a digital camera (Leica ICC50 E, Germany).
Statistical analysis
The data generated were analyzed by the one-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test in GraphPad Prism v-8.3 (www.graphpad.com; GraphPad, LaJolla, USA). Significant differences among treatment cohorts were peg at p ⩽ 0.05. All results are expressed as the mean (SD) of the replicates.
Results
Effect of API on the body weight gain, organ to weight ratio, and survivability of rats treated with AFB1
As indicated in the Kaplan–Meier curve, no mortality was recorded in the experimental rats treated with the various doses of SBE and AFB1 during the study (Supplementary material S1). The untreated and treated groups presented total survivability of 1 (100%). The effects of AFB1 and API-enriched SBE on experimental rats’ mean weight change, and organ and relative organ weights are displayed in Table 2. Relative to the control, the body weight of rat cohorts treated with AFB1 alone decreased non-significantly. On average, the mean weight change of AFB1 is 2.23 g less than in the untreated control cohort. Rats co-treated with AFB1 + SBE-05-D1, AFB1 and AFB1 + SBE-05-D2, AFB1 + SBE-06-D1, and AFB1 + SBE-06-D2 showed decreased mean weight change. Our results further revealed that the mean weight change was significantly lowered in the group of animals treated with AFB1, AFB1 + SBE-05-D1 compared to other experimental groups. This observation was non-significantly higher in the group treated with AFB1 + SBE-07-D1 and AFB1 + SBE-07-D2. Furthermore, the results disclosed no significant alterations (p > 0.05) in the mean organ and relative organ weight of the examined organs—testis, epididymis, and hypothalamus. These non-invasive measures are not complete markers but contribute to an index of toxicity; hence, we further probe for other biomarkers of toxicities.
Table 2.
The effect of apigeninidin-enriched Sorghum bicolor extract on body weight gains and organ weight of rats treated with AFB1 for 28 days.
| Control | AFB1 | AFB1 + SBE-05-D1 | AFB1 + SBE-05-D2 | AFB1 + SBE-06-D1 | AFB1 + SBE-06-D2 | AFB1 + SBE-07-D1 | AFB1 + SBE-07-D2 | |
|---|---|---|---|---|---|---|---|---|
| Body weight gain (g) | 65.40 ± 6.80 | 62.17 ± 9.41 | 29.00 ± 19.15 # | 51.50 ± 24.69 | 55.83 ± 14.78 | 65.00 ± 16.29 | 70.50 ± 0.88 | 54.67 ± 12.82 |
| Testis weight (g) | 2.54 ± 0.16 | 2.43 ± 0.19 | 2.30 ± .0.24 | 2.25 ± 0.05 | 2.38 ± 0.16 | 2.48 ± 0.10 | 2.50 ± 0.21 | 2.48 ± 0.13 |
| Relative testes weight (%) | 1.12 ± 0.13 | 1.10 ± 0.06 | 1.24 ± 0.29 | 1.04 ± 0.05 | 1.10 ± 0.14 | 1.09 ± 0.13 | 1.06 ± 0.12 | 1.16 ± 0.13 |
| Epididymis weight (g) | 0.43 ± 0.09 | 0.36 ± 0.07 | 0.37 ± 0.04 | 0.37 ± 0.03 | 0.39 ± 0.04 | 0.39 ± 0.03 | 0.50 ± 0.15 | 0.41 ± 0.05 |
| Relative epididymis weight (%) | 0.19 ± 0.05 | 0.16 ± 0.02 | 0.20 ± 0.03 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.02 | 0.21 ± 0.06 | 0.19 ± 0.02 |
| Hypothalamus weight (g) | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.05 ± 0.03 |
| Relative hypothalamus weight (%) | 0.033 ± 0.014 | 0.031 ± 0.002 | 0.031 ± 0.004 | 0.028 ± 0.008 | 0.028 ± 0.008 | 0.020 ± 0.001 | 0.024 ± 0.005 | 0.028 ± 0.015 |
AFB1: aflatoxin B1; SBE: Sorghum bicolor extract.
AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Values are expressed as mean (SD) for six rats per group.
Values differ significantly from control (p ⩽ 0.05).
Values differ significantly from AFB1 alone (p ⩽ 0.05).
API alleviated impaired testicular function and lessened hormone levels in AFB1 only treated rats
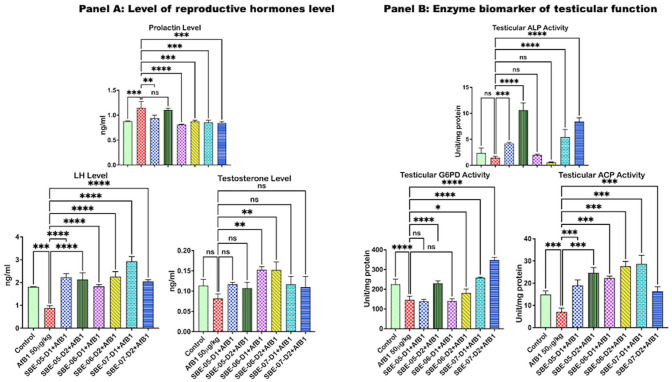
API effect on prolactin, testosterone, and LH concentrations in the serum AFB1 treated rats are represented in Figure 1(A) and FSH concentration in Supplementary material S2a. Compared with the control rats, exposure to AFB1 alone significantly lessened the serum FSH by 19.4% and LH by 51.5%. It increased prolactin by 30.5%, while the testosterone level was reduced by 27.6%. However, the level of LH was significantly increased by treatment with 5 and 10 mg/kg of SBE-05 (153.2 and 142.8%) and SBE-06 (108.0 and 156.8%). At the same time, 5 mg/kg of SBE-07 (9.8%), 10 mg/kg of SBE-05 (8.3%), and SBE-06 (5.0%) increased the level of FSH, while 5 and 10 mg/kg of SBE-06 increased (p < 0.05) the level of testosterone by 81.4 and 81.3%, respectively. Furthermore, treatment with SBE-05 (5 mg/kg), SBE-06 and SBE-07 (5 and 10 mg/kg) caused a 17.8, 29.0, 23.7, 24.4, and 26.2% significant decrease in the level of serum prolactin, respectively. Also, compared to the control rats, ACP (52.3%), LDH (26.9%), and G6PD (35.1) activities were decreased significantly in the AFB1 alone treated rats. In contrast, ALP activity (38.9) was reduced in AFB1 alone treated rats. Nevertheless, not significantly Figure 1(B), see Supplementary material 2b for LDH data. In contrast, treatment with SBE-05 (167.7 and 247%), SBE-06 (214.4 and 289.7%), and SBE-07 (303.7 and 130.1%) significantly increased the activities of ACP. In addition, 5 and 10 mg/kg of SBE-05 and SBE-07 treatment significantly increased the activities of ALP (190.5, 636.3, 278.7, and 486.1%, respectively). The activity of LDH was significantly increased by 10 mg/kg of SBE-05 (72.0%) and 5 mg/kg of SBE-07 (42.1%). As for G6PD, its activity was upregulated by 10 mg/kg of SBE-05 and SBE-06 (57.2% and 23.4%), and 5 and 10 mg/kg of SBE-07 (76.7 & 137.9%).
Figure 1.
Panel A: Effect of API on serum LH and FSH, prolactin, and testosterone levels in AFB1-treated rats. AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. FSH follicle-stimulating hormone; LH: luteinizing hormone. Panel B: Effect of API on testicular activities of ACP, ALP, and G6PD in AFB1-treated rats similarly treated as indicated above. (A color version of this figure is available in the online journal.)
ns: not significant; ACP: acid phosphatase; ALP: alkaline phosphatase; G6PD: glucose-6-phosphate dehydrogenase.
*All values differ significantly from control (p < 0.05).
**All values differ significantly from AFB1 alone (p < 0.05).
API improved altered sperm morphology and functional parameters in cohorts of rats treated with AFB1
The protective effect of API on sperm morphology and functional parameters is shown in Table 3. Rats treated with AFB1 only exhibited reductions (p < 0.05) in their sperm number (testicle: 5.1%), sperm count (epididymis: 26.1%), and overall sperm motility (48.0%) compared with the untreated control. Also, increased sperm morphological aberrations and reduced viability were evident in rats treated with AFB1 only. Conversely, the administration of 5 and 10 mg/kg of SBE-05, SBE-06, and SBE-07 reversed the AFB1-induced deficits in sperm parameters, testicular sperm numbers, and reduced sperm morphological aberrations.
Table 3.
Sperm analysis and sperm abnormalities of rats following exposure to AFB1 for 28 days.
| *Total rats per grouping | Control (6) |
AFB1
(6) |
AFB1 + SBE-05-D1 (6) | AFB1 + SBE-05-D2 (6) |
AFB1 + SBE-06-D1 (6) |
AFB1 + SBE-06-D2 (6) |
AFB1 + SBE-07-D1 (6) |
AFB1 + SBE-07-D2 (6) |
|---|---|---|---|---|---|---|---|---|
| Sperm functional analysis | ||||||||
| Motility | 93.00 ± 2.74 | 48.33 ± 2.58* | 65.00 ± 5.48 # | 67.50 ± 5.00 # | 68.33 ± 4.08 # | 62.00 ± 4.47 # | 61.67 ± 4.08 # | 56.67 ± 5.16 |
| Viability | 95.80 ± 3.49 | 92.50 ± 2.74 | 96.50 ± 1.64 | 96.50 ± 1.72 | 96.50 ± 1.64 # | 96.80 ± 1.64 | 96.50 ± 1.64 | 96.50 ± 1.64 |
| Testicular sperm count | 5.18 ± 0.04 | 4.92 ± 0.04* | 5.18 ± 0.04 # | 5.17 ± 0.05 # | 5.16 ± 0.05 # | 5.18 ± 0.04 # | 5.16 ± 0.05 # | 5.16 ± 0.05 # |
| Epididymal sperm count | 116.60 ± 2.88 | 86.20 ± 8.14* | 98.83 ± 10.53 | 99.75 ± 8.88 | 99.17 ± 6.94 | 89.60 ± 11.80 | 92.83 ± 7.08 | 123.20 ± 5.31 # |
| Sperm abnormalities | ||||||||
| Abnormality of the head (%) | 2.22 ± 0.30 | 2.43 ± 0.48 | 2.30 ± 0.29 | 2.53 ± 0.45 | 2.27 ± 0.53 | 2.14 ± 0.40 | 2.35 ± 0.41 | 2.18 ± 0.29 |
| Abnormality of the mid-piece (%) | 4.10 ± 0.39 | 6.20 ± 0.35* | 5.31 ± 0.39 # | 5.28 ± 0.41 | 5.41 ± 0.43 | 5.16 ± 0.18 # | 5.41 ± 0.35 | 5.65 ± 0.40 |
| Abnormality of the tail (%) | 5.23 ± 0.49 | 6.67 ± 0.39* | 6.25 ± 0.53 | 6.25 ± 0.73 | 6.08 ± 0.27 | 5.97 ± 0.63 | 6.33 ± 0.52 | 6.36 ± 0.36 |
| Total abnormality (%) | 116.60 ± 2.88 | 123.20 ± 5.30 | 98.83 ± 10.53 | 99.75 ± 8.88 | 99.17 ± 6.94 | 89.60 ± 11.80 | 92.83 ± 7.08 | 88.17 ± 8.72 |
AFB1: aflatoxin B1; SBE: Sorghum bicolor extract.
AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Values are expressed as mean ± SD for six rats per group.
Values differ significantly from control (p < 0.05).
Values differ significantly from AFB1 alone (p < 0.05).
API improved antioxidant enzyme and increased thiol-containing molecule levels but reduced oxidative damage in rats co-treated with AFB1
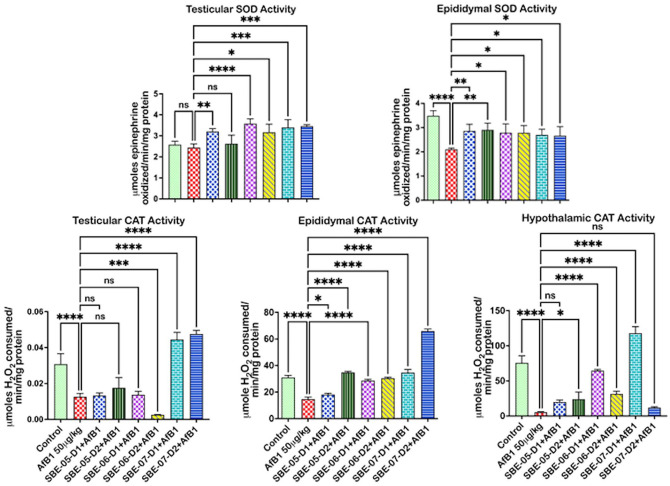
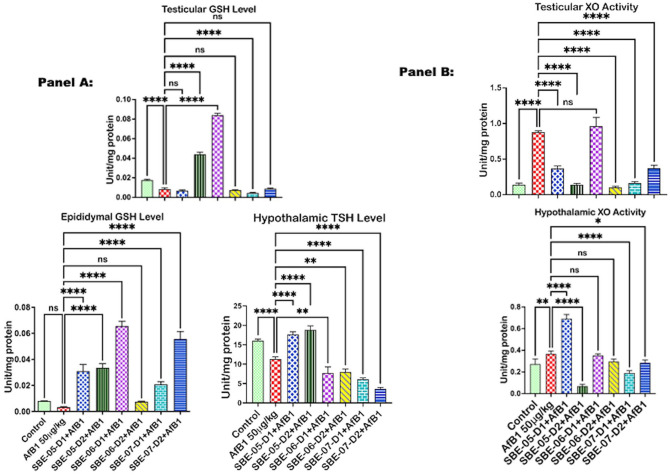
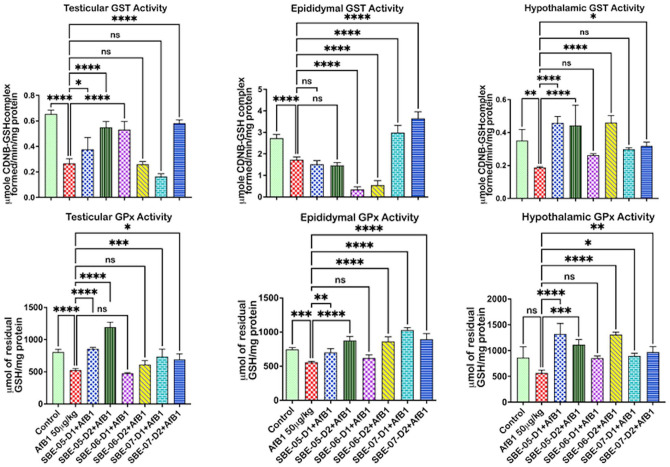
API effect on antioxidant and thiol levels in the examined organs of rats treated with AFB1 are exemplified in Figures 2 to 4. Relative to the untreated control, the activity of SOD was reduced (p < 0.05) (Figure 2) in the epididymis by 39.7%, but not in the hypothalamus (see Supplementary material 2c), and the testes of AFB1 alone treated rats. The antioxidant enzyme CAT, GPx, and GST levels (Figures 2 and 3) decreased (p < 0.05) in the testes by 59.16, 34.8, and 59.2%, respectively, in the epididymis by 52.6, 25.4, and 36.9%, and in the hypothalamus of rats administered AFB1 alone by 92.9, 34.5, and 46.7%, respectively. Furthermore, rats administered AFB1 alone were observed to have reduced GSH and TSH levels (Figure 4(A)). Specifically, significant reductions in testicular GSH by 52.2% and hypothalamic TSH by 29.4% were observed. However, SOD activity was significantly increased by 10 mg/kg of SBE-06 in rats’ testes, epididymis, and hypothalamus by 46.6, 32.5, and 69.8%, respectively. In the testes, epididymis, and hypothalamus of rats, the activity of CAT was increased (p < 0.05) by 10 mg/kg of SBE-06 (80.2, 107.0, and 491.9%). In comparison, 5 mg/kg of SBE-07 (253.6, 73.6, and 59.4%), 10 mg/kg of SBE-4 (38.3, 50.4, and 67.9%) and SBE-07 (278.4, 111.7, and 69.9%) significantly increased the activity of GST, respectively. The activity of GPx was increased by 10 mg/kg of SBE-05 by 126.9, 17.2, and 97.1%, and 5 and 10 mg/kg of SBE-07 39.8 and 31.7%, 37.6 and 19.9%, 58.4 and 71.5%, in the testes, epididymis, and hypothalamus, respectively. Conversely, the activity of CAT was significantly reduced by 10 mg/kg of SBE-06 in the testes by 80.2%. Also, 5 and 10 mg/kg of SBE-06 decreased GST activity in the epididymis of rats by 80.1 and 68.2%, respectively. In addition, treatment with 10 mg/kg of SBE-05 and 5 mg/kg of SBE-06 significantly raised the levels of GSH in the testes by 422.6 and 898.3%, respectively, and in the epididymis of rats by 887.6 and 1829.9%, respectively. In contrast, 5 mg/kg of SBE-05 increased (p < 0.05) hypothalamus GSH level by 180.8%; 5 and 10 mg/kg of SBE-05 increased (p < 0.05) the level of TSH in the epididymis (23.5 and 39.3%) (see Supplementary material 2f), and hypothalamus (56.5 and 66.7%) of rats, respectively. However, reductions (p < 0.05) in TSH and GSH levels were observed in rat cohorts co-treated with API. Notably, 5 mg/kg of SBE-07 significantly reduced the level of GSH in the testes by 46.9%, while 10 mg/kg of SBE-06 significantly reduced TSH level in the same organ by 35.0% (see Supplementary material 2e). In addition, 5 and 10 mg/kg of both SBE-06 (31.8 and 29.4%) and SBE-07 (45.9 and 67.9%) decreased (p < 0.05) TSH level in the hypothalamus.
Figure 2.
Effect of API on the activities of SOD and CAT in the testes, epididymis, and hypothalamus of AFB1-exposed rats. AFB1, 50 µg/kg; AFB1 + SBE-05-D1 0.05 + 5 mg/kg; AFB1 + SBE-5-D2 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Values are expressed as mean (SD) for six rats per group. (A color version of this figure is available in the online journal.)
ns: not significant; SOD: superoxide dismutase; CAT: catalase.
*Values differ significantly from control (p < 0.05).
**Values differ significantly from AFB1 alone (p < 0.05).
Figure 4.
Panel A: Effect of API on GSH and TSH levels in the testes, epididymis, and hypothalamus of AFB1-exposed rats. AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Panel B: The effect of API on testis, epididymal, and hypothalamic activities of XO n AFB1—rats similarly treated as indicated above. All values are expressed as mean (SD) for six rats per group. (A color version of this figure is available in the online journal.)
ns: not significant; GSH: glutathione-S-transferase; TSH: total sulfhydryl groups; XO: xanthine oxidase.
*Values differ significantly from control (p < 0.05).
**Values differ significantly from AFB1 alone (p < 0.05).
Figure 3.
Effect of API on the activities of GST and GPx in the testes, epididymis, and hypothalamus of AFB1-exposed rats. AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Values are expressed as mean (SD) for six rats per group. (A color version of this figure is available in the online journal.)
ns: not significant; GST: glutathione-S-transferase; GPx: glutathione peroxidase.
*Values differ significantly from control (p < 0.05).
**Values differ significantly from AFB1 alone (p < 0.05).
API regulated oxidative stress biomarkers in rats treated with AFB1 only
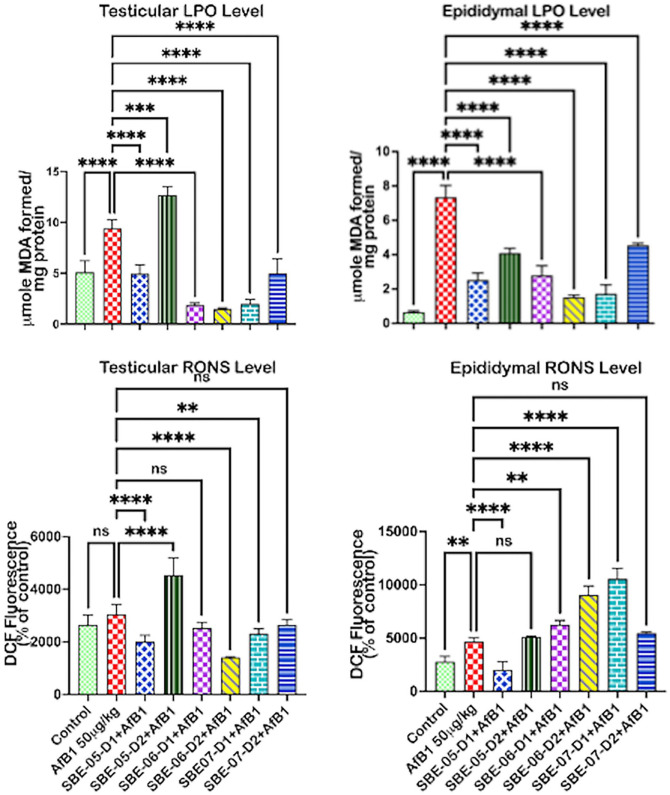
The impact of API on the epididymal, testicular, and hypothalamic biomarkers of oxidative stress in rats is depicted in Figure 4(B). The activity of xanthine oxidase (XO) increased (p < 0.05) by 530.9, 132.2, and 34.7% in the testes, epididymis (see Supplementary material 3c), and hypothalamus, respectively, in rats treated with AFB1 alone. However, treatment with API significantly decreased XO activity in the testes by 10 mg/kg of SBE-05 (84.1%), 10 mg/kg of SBE-06 (88.5%), and 5 and 10 mg/kg of SBE-07 (81.6 and 57.8%), while in the hypothalamus by 10 mg/kg of SBE-05 (81.4%), 10 mg/kg of SBE-06 (23.9%), and 5 and 10 mg/kg of SBE-07 (48.2 & 22.5%). The quantity of RONS produced in the epididymis (67.7%) of rats treated with AFB1 alone was higher (p < 0.05) compared to the untreated control Figure 5. Testicular RONS level also increased by 15.4% and that of the hypothalamus by 27.2% (see Supplementary material 3a). LPO level was significantly higher in the testes (84.3%) and epididymis (1026.8%) in rats administered AFB1 alone, while it was increased by 34.4% in the hypothalamus (see Supplementary material 3b). However, 5 mg/kg of SBE-05 and SBE-07, and 10 mg/kg of SBE-06 significantly reduced the level of RONS in the testes by 99.0, 24.1, and 53.7%, respectively. SBE-05 (5 mg/kg) significantly reduced the level of RONS in the co-treated rats’ epididymis (56.7%). In contrast, in the hypothalamus of rats, RONS level was significantly reduced by 5 mg/kg of SBE-06 by 46.6%, and 5 and 10 mg/kg of SBE-07 by 46.0 and 34.2%, respectively. However, SBE-05 (10 mg/kg) in the testes, SBE-06 (10 mg/kg) in the epididymis, and SBE-05 (10 mg/kg) in the hypothalamus significantly increased the RONS level in the co-administered rats. Furthermore, the level of LPO was decreased considerably in the co-treated rats’ testes and epididymis by all fractions of the extract except SBE-05 (10 mg/kg), which increased the LPO level in the testes. Conversely, 10 mg/kg of SBE-05 increased (p < 0.05) the level of RONS by 48.5% in the testes and by 34.0% in the hypothalamus. In comparison, 5 and 10 mg/kg of SBE-06 and 5 mg/kg of SBE-07 increased the level of RONS in the epididymis by 34.5, 94.0, and 126.5%, respectively. Furthermore, 10 mg/kg of SBE-05 increased (p < 0.05) LPO level in the testes (34.8%), while the LPO level was raised considerably in the hypothalamus (96.1%) by 5 mg/kg of SBE-05.
Figure 5.
Effect of API on LPO, RONS levels in the testes, epididymis, and hypothalamus of AFB1-exposed rats. AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Values are expressed as mean (SD) for six rats per group. (A color version of this figure is available in the online journal.)
ns: not significant; RONS: reactive oxygen and nitrogen species; LPO: lipid peroxidation.
*Values differ significantly from control (p < 0.05).
**Values differ significantly from AFB1 alone (p < 0.05).
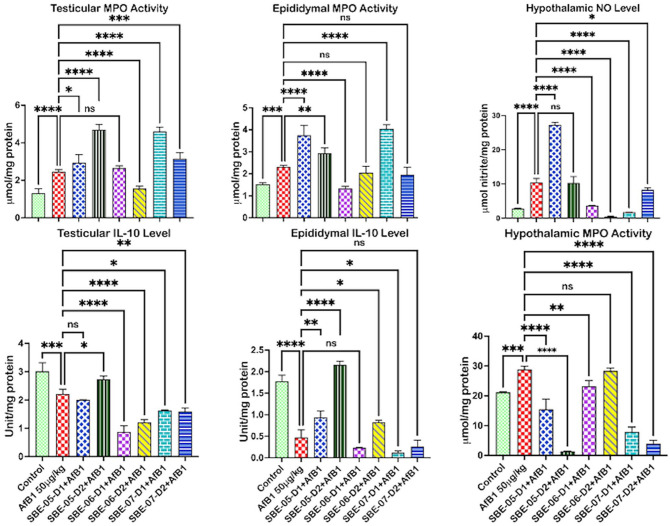
API co-treatment lessened inflammatory mediators in rats treated with AFB1
The impact of API on inflammatory biomarkers in the examined rat organs is illustrated in Figure 6. Rats treated with AFB1 have significantly higher MPO, whereas interleukin (IL)-10 decreased in the testicle (86.8 and 58.8%) and the epididymis (51.1 and 73.4%) (Figure 6), respectively. In addition, rats treated with AFB1 alone exhibited increased NO in the testicle and epididymis (15.9% and 12.6%), respectively (see Supplemental materials 3d and e, respectively). AFB1 treatment caused significantly higher NO and MPO in the hypothalamus (266.7 and 36.4%) and non-significant changes in hypothalamic IL-10 (73.4%) level (see Supplemental material 3f). Conversely, the NO level in the testes of rats was decreased by 10 mg/kg of SBE-06 (48.6%). In comparison, 5 mg/kg of SBE-05 significantly reduced the level of NO by 31.6% in the epididymis of rats, and 5 and 10 mg/kg of SBE-06 (64.5 and 95.6%) and 5 mg/kg of SBE-07 (83.2%) decreased (p < 0.5) the levels of NO in the hypothalamus of rats in the co-treated cohort. A total of 10 mg/kg of SBE-06 reduced (p < 0.5) the level of MPO in the testes by 36.4%, while 5 mg/kg of SBE-06 decreased the MPO level, respectively, by 8.0% in the epididymis. At the same time, the level of MPO was markedly (p < 0.05) reduced in the hypothalamus by all fractions except 10 mg/kg of SBE-06. In the hypothalamus, the level of IL-10 was increased (p < 0.5) by 5 and 10 mg/kg of SBE-07 by 207.8 and 294.4%, respectively. Intriguingly, the NO level was increased by 5 and 10 mg/kg of SBE-07 in the epididymis. In addition, in the testes of rats, MPO activity was significantly upregulated by 10 mg/kg of SBE-05 and 5 mg/kg of SBE-07. In contrast, 5 mg/kg of SBE-05 and 5 mg/kg of SBE-07 upregulated MPO activity in the epididymis. At the same time, in the epididymis, 5 and 10 mg/kg of SBE-05 (78.1 and 43.2%) alongside 10 mg/kg of SBE-06 (75.4%) and SBE-07 (44.2%) significantly increased the level of IL-10.
Figure 6.
Effect of API on MPO activity, NO, and IL-10 levels in the testes, epididymis, and hypothalamus of AFB1-exposed rats. AFB1, 50 µg/kg; AFB1 + SBE-05-D1, 0.05 + 5 mg/kg; AFB1 + SBE-5-D2, 0.05 + 10 mg/kg; AFB1 + SBE-06-D1, 0.05 + 5 mg/kg; AFB1 + SBE-06-D2, 0.05 + 10 mg/kg; AFB1 + SBE-07-D1, 0.05 + 5 mg/kg; and AFB1 + SBE-07-D2, 0.05 + 10 mg/kg. Values are expressed as mean (SD) for six rats per group. (A color version of this figure is available in the online journal.)
ns: not significant; NO: nitric oxide; MPO: myeloperoxidase; IL-10: interleukin-10.
*Values differ significantly from control (p < 0.05).
**Values differ significantly from AFB1 alone (p < 0.05).
Effect of API on pro-apoptotic biomarkers in AFB1 co-treated rats
API effect on caspase-9 activities in rats treated with AFB1 is shown in Supplemental material 4. Rats exposed to AFB1 alone exhibited increased (p < 0.05) caspase-9 activity in testes by 47.3% relative to the untreated control. The caspase-9 activity also increased in the epididymis (92.4%) and hypothalamus (173.1%) of the rats exposed to AFB1 alone. Conversely, caspase-9 activity was noticeably reduced in the epididymis and hypothalamus by 5 mg/kg of SBE-05 (35.7%) and 10 mg/kg of SBE-05, respectively (7.3%). However, some extracts had a pro-apoptotic effect. For example, SBE-05 (10 mg/kg) in the testes, SBE-06 (10 mg/kg) in the epididymis, (5 mg/kg) SBE-05, SBE-06 (5 and 10 mg/kg), and SBE-07 (10 mg/kg) in the hypothalamus increased (p < 0.05) the activity of caspase-9.
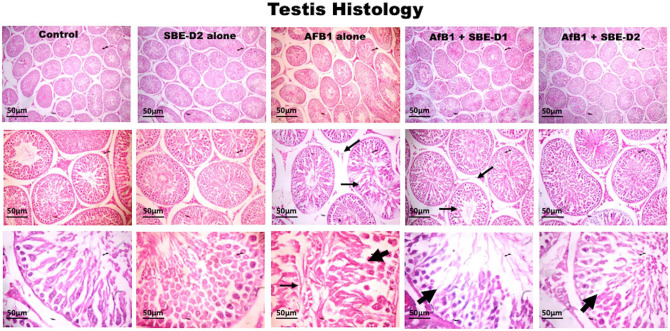
API lessened histological injury induced by AFB1 in experimental rats
The examined testis sections revealed that rats treated with SBE exhibited well-maintained testicular tissue histo-architecture (Figure 7), similar to the untreated control rats. These are characterized by the seminiferous epithelium spermatogonia, spermatocytes, spermatids, spermatozoa, and Sertoli cells. With germ cells actively dividing and drifting toward maturation, an abundance of terminally differentiated spermatozoa is evidenced. The Leydig cells appear typical in the interstitia. In contrast, rats challenged with AFB1 showed vacuolization of testicular seminiferous tubules.
Figure 7.
Representative histology characteristics of experimental rat testis. Control and SBE alone treated rats exhibited normal testicular tissue architecture. AFB1 (50 µg/kg) alone treated rats showed seminiferous tubular degeneration (tiny arrows) and evidence of vacuolization of testicular seminiferous tubules (bold arrows). AFB1 with SBE-D1 (10 mg/kg) and SBE-D2 (10 mg/kg) dose-dependently improved testicular cyto-architecture with a relatively increased number of spermatozoa. (H&E-stained tissue sections; 1.08 cm = 50 µm). (A color version of this figure is available in the online journal.)
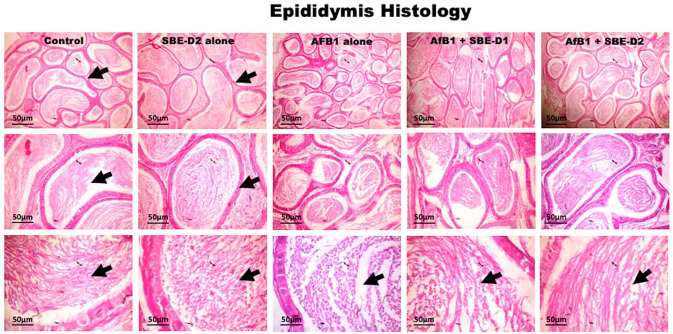
Furthermore, rats exposed to SBE in the presence of AFB1—showed a dose-related improvement in the testicular histo-architecture bordering on that of the untreated control at a higher dose of SBE. Also, the experimental rat epididymis section shows typical morphology in the control and SBE alone, depicted by normal tubules and abundant spermatozoa (Figure 8). AFB1 treatment caused a reduction in epididymal sperm cells characterized by partially sperm-filled tubules. However, AFB1 with SBE co-treatment showed dose-dependent increases of spermatozoa in the lumen, coupled with normal morphology of the epididymal tubules.
Figure 8.
Histology section from the epididymis of the control and SBE alone treated rats show typical morphology. The tubules are essentially normal with abundant spermatozoa (black arrows). AFB1 (50 µg/kg) alone treated rats’ epididymis presented reduced epididymal sperm cells. Groups treated with AFB1 and graded doses of—5 and 10 mg/kg body weight—of SBE show normal epididymis morphology. The tubules are essentially normal with SBE dose-dependent spermatozoa increases in the lumen (black arrows). (H&E-stained tissue sections; 1.08 cm = 50 µm). (A color version of this figure is available in the online journal.)
Discussion
Chronic inflammatory disorders emanating from mycotoxin exposure have been fingered in reproductive function dysregulation that culminates in infertility.56–59 Reproductive organs weight has been utilized as a sensitive androgen biomarker. 4 Rats exposed to AFB1 (50 µg/kg) alone exhibited reduced gain in weight and the testes’ weight, and epididymis, though these changes were not significant. A reduction in body weight could be due to reduced feed intake or diminished appetite. Deterioration of the testicular germinal epithelium and sperm production/storage reduction in the epididymis has been reported in AFB1 exposed rats.2,4 Co-treatment with fractions of SBE did not reverse experimental rats’ body and organ weight losses. A previous report by Tsuda on anthocyanins present in S. bicolor inhibits the body, and adipose tissue weight increases in rat 60 support our current observation. Steroidogenesis and spermatogenesis are highly intricate multistep processes, tightly regulated by enzymes and hormones, and any disturbance or disruption of any of these processes can lead to infertility. 61 The availability of androgens impacts the morphology of the testes and the functional integrity of accessory organs. The results from the current study showed that rats exposed to AFB1 alone had lower levels of circulating testosterone, implying that androgen production in the rats is inhibited. These data suggest that the reduction in the weight of testes and epididymis is due to a reduction of circulating male hormones necessary for reproductive function. Testosterone is essential in regulating the reproductive organs’ structure and function. 62 Elevated prolactin levels have been correlated with decreased sexual desire and the inability to obtain or maintain an erection, 63 which, when left untreated, eventually results in erectile dysfunction. In the AFB1-treated rats, the LH and FSH levels were notably reduced while the prolactin level increased compared to the control. However, all SBE fractions could restore prolactin, testosterone and LH to normal levels. All other SBE fractions did not significantly improve FSH levels in the presence of AFB1 co-treatment. Intriguingly, SBE-07 at the higher dose (D2: 10 mg/kg) suppressed the FSH level.
Dysregulation of the hypothalamic-testicular co-ordinating network by AFB1 adversely affects spermatogenic enzymes.15,64,65 To investigate the ameliorative effects of the SBE extracts on essential spermatogenic enzymes, we assayed for G6PD, ACP and LDH activities. We observed that G6PD, ACP, and LDH were reduced in the AFB1 alone exposed cohort. The activity of ALP was also noticeably reduced. ALP and ACP are a biomarker for rat primordial germ cells and are involved in the hydrolysis of the glycolytic intermediates 6-phosphofructose and 6-phosphoglucose to give free fructose and glucose, respectively. 66 Hence, the marked decrease in rat testicular ALP and ACP suggests that their roles in testicular glucose utilization on exposure to AFB1 is impaired. AFB1-mediated alteration of these enzymes can cause damage to reproductive tissues and reduced sperm production. A likewise reduction in testicular LDH indicates AFB1-mediated infraction on adenosine triphosphate level required by the spermatogenic cells in the testes of the rats. G6PD is necessary for the optimal function of the pentose phosphate pathway, a significant source of NADPH—nicotinamide adenine dinucleotide phosphate, a requirement for spermatogenesis in rats. 67 Decreases in testicular G6PD activity in AFB1 alone treated rats suggest AFB1-mediated reduction of NADPH supply and consequent impairment of spermatogenesis. API’s beneficial role in AFB1-induced testicular toxicity is exemplified by significantly increased testicular ACP, ALP, LDH, and G6PD activities in rats co-treated with API and AFB1. The increased levels of these enzymes caused by API-enriched SBE fractions protected the steroidogenic and spermatogenic processes in male rats’ testes.
The observed decrease in sperm functional characteristics, particularly epididymal sperm number and progressive motility and sperm viability. Increased sperm abnormalities are emblematic of AFB1 toxicity in the treated rats. This toxicity to the sperm may cause infertility due to inadequate sperm quantity and quality, and the inability of dysfunctional sperm to reach the site of fertilization. The decline in sperm functional parameters in AFB1-treated rats in the present study agrees with a previous study. 2 However, SBE extracts mediated a decrease in total sperm abnormalities and increased epididymal sperm number, progressive motility, and sperm viability, underlining the cytoprotective roles of these extracts in this model of AFB1-induced reproductive toxicity.
Herein, we also examined these extracts on rats’ antioxidant status. Specifically, SOD, CAT, GST, and GPx activity were investigated. AFB1 exposure reduced CAT, GST, and GPx activities in the testes and hypothalamus, and those of SOD, CAT, GST, and GPx in rats’ epididymis. Furthermore, non-enzymatic antioxidants, specifically TSH and GSH, were reduced in the rats’ organs examined after treatment with AFB1 alone. These findings imply dysfunction of these enzymatic and non-enzymatic antioxidants to adequately quench damaging reactive oxygen species (ROS) in the testes, epididymis, and hypothalamus.
Conversely, treatment with SBE improved these enzymes levels in the epididymis, testes, and hypothalamus. SBE (5 and 10 mg/kg) significantly increased enzymatic antioxidants in the epididymis, testes, and hypothalamus of rats co-treated with API, perhaps by the hydroxyl groups present in API, which provides radical scavenging action 68 in agreement with the previous report on the antioxidant properties of API. 69 However, certain SBE decreased the antioxidant enzyme activity, reducing GSH and TSH levels in the examined organs. Flavonoids have been shown to inhibit enzyme activity; 70 it could be that these extracts inhibited the action of the antioxidant enzymes.
Furthermore, the observed upsurge in RONS in the testes, epididymis, and hypothalamus, and associated increases of LPO in rats treated with AFB1 alone. This evidence further confirmed that AFB1 could produce an overwhelming RONS, inducing oxidative stress and peroxidation of lipids in the examined tissue. The RONS negatively affects the sperm (motility, viability) and may damage the seminiferous tubules. However, co-exposure to the SBE extracts was shown to reverse AFB1-induced oxidative stress in the reproductive system, LPO, and its damaging effects in a dose-dependent manner. The general ameliorative ability demonstrated by the SBE extracts could be due to their antioxidative and antiperoxidative properties. It is important to emphasize here that some extracts increased RONS levels in the reproductive tissues of rats at higher dosages—SBE-05 (10 mg/kg) in the testes, SBE-06 (10 mg/kg) in the epididymis, and SBE-05 (10 mg/kg) in the hypothalamus. The increase in the RONS level caused by these extracts could be due to the dose administered. It has been reported that flavonoids can be toxic to the cells at high doses when oxidation-catalyzing factors, including transition metal ions, are present. 71 Furthermore, Skibola and Smith 70 have shown that some flavonoids could function as pro-oxidants, enzyme inhibitors and mutagens.
The metalloflavoprotein enzyme xanthine oxidoreductase midwifes the oxidative production of xanthine to uric acid preceding the production of hypoxanthine to xanthine. These reactions are accompanied by the generation of ROS in the cells. 72 The significant increase in the testes, epididymis, and hypothalamus XO activities following exposure to AFB1 alone that we observed in the present study further confirms the oxidative stress-inducing ability of AFB1. Our observation in this study corroborates a previous study finding. 14 However, following treatment with the SBE extracts, the increased activity of XO was significantly reduced in the testes, epididymis, and hypothalamus, further confirming the potency of API in scavenging ROS.
Pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), are higher during an inflammatory response, while anti-inflammatory cytokines, for example, IL-10, are downregulated. When inflammation is left unchecked by cellular regulatory mechanisms, it becomes disadvantageous to systemic function, including spermatogenesis and portends a pathway to promote male infertility. 73 Therefore, the increase in the MPO activity, NO, TNF- α level, coupled with the downregulation of IL-10 in the reproductive organs of rats treated with AFB1 alone, signifies nitrosative stress induction and an evolving inflammatory milieu in the examined organs. The general decrease in the MPO activity and NO level and the corresponding increase in IL-10 levels in the epididymis testes and hypothalamus that we subsequently detected in rats co-treated with SBE could be due to the antinitrosative and inflammatory effect of these API-enriched extracts. It has been reported that API blocks the action of cyclo-oxygenase-2 and prostaglandin-E2 in peripheral blood mononuclear cells. 28
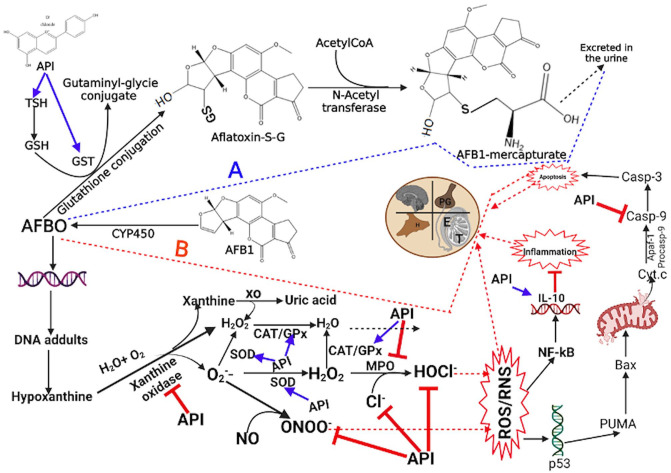
Furthermore, we investigated the protective influence of the SBE extracts against AFB1-induced caspase-9 activation -a cellular executioner protease experiencing the intrinsic/mitochondrial apoptosis pathways. AFB1 has been shown to upregulate the activity of this enzyme. 74 The increase in caspase-9 activity in the epididymis, testes, and hypothalamus ensuing from AFB1 alone treatment indicates apoptosis induction in the exposed rats. Our data substantiate earlier reports on AFB1-induced increase in caspase-9 activity. 75 Caspase-9 activity in rats co-exposed to AFB1 and the SBE extracts was significantly reduced, implying an antiapoptotic component in the extracts, which may occur through direct inactivation of caspases. It is no surprise then that SBE-05 (10 mg/kg) in the testes and SBE-06 (10 mg/kg) in the epididymis increased RONS levels and upregulated caspase-9 activity in the examined tissues. ROS has been implicated in activating the mitochondrial pathway of apoptosis. 76 Finally, histopathological findings corroborate the biochemical results from this study as we observed general protective effects of the SBE extracts, albeit at a higher dose (10 mg/kg), against AFB1-mediated derangement of testicular and epididymal tissue. AFB1 induced the dysregulation of the male rat’s reproductive system through multiple mechanisms, including the disruption of endocrine, downregulation of steroidogenic and antioxidant enzymes, reduction in endogenous antioxidants, and elevation of inflammation biomarkers. In addition, AFB1 caused upregulation of STAT3 signaling.77,78 We showed in the current manuscript that API alleviates most of the harmful effects of AFB1, possibly through the antioxidant and anti-inflammatory effects of API in the API. In a previous study, we have shown that API suppressed STAT3 activation. 79 The combination of STAT3 signaling inhibition and API’s anti-inflammatory and antioxidant effects (Figure 9) could explain its ameliorative effects on the toxicity of AFB1 to the male reproductive system.
Figure 9.
Proposed mechanism of API ameliorative effect on AFB1-mediated toxicities in the hypothalamic–testicular–epididymal axis of an experimental rat model. SBE-05, SBE-06, and SBE-07 avert AFB1-induced oxidative and nitrosative stress, inflammation, and apoptosis by attenuating the activity of inflammatory cytokines, IL-10, altering the activity of caspase 9 and inhibiting STAT3 signaling. Created by https://app.biorender.com/. (A color version of this figure is available in the online journal.)
Conclusions
In conclusion, API-enriched extracts from S. bicolor investigated in this study possessed promising antioxidative, anti-inflammatory, and antiapoptotic activities, represented in our proposed mechanism of action (Figure 9). More studies are required in the future to determine the specific mechanism(s) of each extract’s therapeutic dose that may guide the identification of safe and effective S. bicolor-derived extract for the management of male infertility.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221098060 for Apigeninidin-enriched Sorghum bicolor (L. Moench) extracts alleviate Aflatoxin B1-induced dysregulation of male rat hypothalamic-reproductive axis by Solomon E Owumi, Moses T Otunla, Uche O Arunsi and Adegboyega K Oyelere in Experimental Biology and Medicine
Acknowledgments
The authors also wish to acknowledge Mercy Olubunmi of the Department of Biochemistry, the University of Ibadan, for her technical assistance. This manuscript is dedicated to the memory of Late Professor Anthony Uwaifo 1943–2020.
Footnotes
Authors’ Contributions: All authors participated in the design, interpretation of the studies and analysis of the data and manuscript review; SEO and AKO contributed to conceptualization; MTO contributed to project administration, investigation, data curation, and analysis. SEO contributed to supervision, visualization; AKO contributed to validation; SEO, MTO, and AKO contributed to writing, reviewing, and editing manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability: The generated data from the current study are available from the corresponding author on reasonable request.
ORCID iD: Solomon E Owumi  https://orcid.org/0000-0002-4973-0376
https://orcid.org/0000-0002-4973-0376
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000Res 2019;8:F1000 Faculty Rev-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owumi SE, Otunla MT, Najophe ES, Oyelere AK. Decrease in reproductive dysfunction using aflatoxin B1 exposure: a treatment with 3-indolepropionic acid in albino Wistar rat. Andrologia 2021;54:e14248 [DOI] [PubMed] [Google Scholar]
- 3. Owumi SE, Najophe SE, Idowu TB, Nwozo SO. Protective mechanisms of gallic acid on hepatorenal dysfunction of zearalenone treated rat. Biologia 2021;76:3123–35 [Google Scholar]
- 4. Supriya C, Girish BP, Reddy PS. Aflatoxin B1-induced reproductive toxicity in male rats: possible mechanism of action. Int J Toxicol 2014;33:155–61 [DOI] [PubMed] [Google Scholar]
- 5. Benkerroum N. Chronic and acute toxicities of aflatoxins: mechanisms of action. Int J Environ Res Public Health 2020;17:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 2004;80:1106–22 [DOI] [PubMed] [Google Scholar]
- 7. Gallagher EP, Wienkers LC, Stapleton PL, Kunze KL, Eaton DL. Role of human microsomal and human complementary DNA-expressed cytochromes P4501A2 and P4503A4 in the bioactivation of aflatoxin B1. Cancer Res 1994;54:101–8 [PubMed] [Google Scholar]
- 8. Kumari S, Sharma S, Advani D, Khosla A, Kumar P, Ambasta RK. Unboxing the molecular modalities of mutagens in cancer. Environ Sci Pollut Res. Epub ahead of print 5 October 2021. DOI: 10.1007/s11356-021-16726-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egbunike GN, Emerole GO, Aire TA, Ikegwuonu FI. Sperm production rates, sperm physiology and fertility in rats chronically treated with sublethal doses of aflatoxin B1. Andrologia 1980;12:467–75 [DOI] [PubMed] [Google Scholar]
- 10. Mukumu CK, Macharia B. Effects of aflatoxin b1 on liver, testis, and epididymis of reproductively mature male pigs: histopathological evaluation. East Afr Med J 2017;94:95–9 [Google Scholar]
- 11. Mathuria N, Verma RJ. Curcumin ameliorates aflatoxin-induced toxicity in mice spermatozoa. Fertil Steril 2008;90:775–80 [DOI] [PubMed] [Google Scholar]
- 12. Rotimi OA, Onuzulu CD, Dewald AL, Ehlinger J, Adelani IB, Olasehinde OE, Rotimi SO, Goodrich JM. Early life exposure to aflatoxin B1 in rats: alterations in lipids, hormones, and DNA methylation among the offspring. Int J Environ Res Public Health 2021;18:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owumi SE, Irozuru CE, Arunsi UO, Faleke HO, Oyelere AK. Caffeic acid mitigates aflatoxin B1-mediated toxicity in the male rat reproductive system by modulating inflammatory and apoptotic responses, testicular function, and the redox-regulatory systems. J Food Biochem. Epub ahead of print 2 February 2022. DOI: 10.1111/jfbc.14090 [DOI] [PubMed] [Google Scholar]
- 14. Owumi SE, Otunla MT, Arunsi UO, Najophe ES. 3-Indolepropionic acid upturned male reproductive function by reducing oxido-inflammatory responses and apoptosis along the hypothalamic-pituitary-gonadal axis of adult rats exposed to chlorpyrifos. Toxicology 2021;463:152996. [DOI] [PubMed] [Google Scholar]
- 15. Owumi SE, Adedara IA, Akomolafe AP, Farombi EO, Oyelere AK. Gallic acid enhances reproductive function by modulating oxido-inflammatory and apoptosis mediators in rats exposed to aflatoxin-B1. Exp Biol Med 2020;245:1016–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhakal A, Sbar E. Aflatoxin toxicity. Treasure Island, FL: StatPearls Publishing, 2021 [PubMed] [Google Scholar]
- 17. Deng J, Zhao L, Zhang NY, Karrow NA, Krumm CS, Qi DS, Sun LH. Aflatoxin B1 metabolism: regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res Rev Mutat Res 2018; 778:79–89 [DOI] [PubMed] [Google Scholar]
- 18. Rasooli I, Fakoor MH, Yadegarinia D, Gachkar L, Allameh A, Rezaei MB. Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int J Food Microbiol 2008;122: 135–9 [DOI] [PubMed] [Google Scholar]
- 19. Carter AC, King JB, Mattes AO, Cai S, Singh N, Cichewicz RH. Natural-product-inspired compounds as countermeasures against the liver carcinogen aflatoxin B1. J Nat Prod 2019;82:1694–703 [DOI] [PubMed] [Google Scholar]
- 20. Owumi S, Najophe ES, Farombi EO, Oyelere AK. Gallic acid protects against aflatoxin B1-induced oxidative and inflammatory stress damage in rats kidneys and liver. J Food Biochem 2020;44:e13316 [DOI] [PubMed] [Google Scholar]
- 21. Owumi SE, Irozuru CE, Arunsi UO, Oyelere AK. Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 2022;207:1–12 [DOI] [PubMed] [Google Scholar]
- 22. Alm-Eldeen AA, Mona MH, Shati AA, El-Mekkawy HI. Synergistic effect of black tea and curcumin in improving the hepatotoxicity induced by aflatoxin B1 in rats. Toxicol Ind Health 2015;31:1269–80 [DOI] [PubMed] [Google Scholar]
- 23. Ademiluyi AO, Oboh G, Agbebi OJ, Boligon AA, Athayde ML. Sorghum [Sorghum bicolor (L.) Moench] leaf sheath dye protects against cisplatin-induced hepatotoxicity and oxidative stress in rats. J Med Food 2014;17:1332–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okpuzor J, Adebesin O, Ogbunugafor H, Amadi IM. The potential of medicinal plants in sickle cell disease control: a review. Int J Biomed Health Sci 2008;4:47–55 [Google Scholar]
- 25. Okubena O, Makanjuola S, Ajonuma LC, Dosunmu A, Umukoro S, Erah PO. The West African Sorghum bicolor leaf sheath extract Jobelyn® and its diverse therapeutic potentials. MOJ Drug Des Develop Ther 2018;2:20–8 [Google Scholar]
- 26. Abugri DA, Witola WH, Jaynes JM, Toufic N. In vitro activity of Sorghum bicolor extracts, 3-deoxyanthocyanidins, against Toxoplasma gondii. Exp Parasitol 2016;164:12–9 [DOI] [PubMed] [Google Scholar]
- 27. Awika JM, Rooney LW. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004;65:1199–221 [DOI] [PubMed] [Google Scholar]
- 28. Makanjuola SBL, Ogundaini AO, Ajonuma LC, Dosunmu A. Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo-oxygenase-2 and prostaglandin-E2 blockade. Int J Rheum Dis 2018;21:1487–95 [DOI] [PubMed] [Google Scholar]
- 29. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47 [DOI] [PubMed] [Google Scholar]
- 30. Vanamala JKP, Massey AR, Pinnamaneni SR, Reddivari L, Reardon KF. Grain and sweet sorghum (Sorghum bicolor L. Moench) serves as a novel source of bioactive compounds for human health. Crit Rev Food Sci Nutr 2018;58:2867–81 [DOI] [PubMed] [Google Scholar]
- 31. Rushing BR, Selim MI. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol 2019;124:81–100 [DOI] [PubMed] [Google Scholar]
- 32. Guengerich FP, Johnson WW, Shimada T, Ueng Y-F, Yamazaki H, Langouët S. Activation and detoxication of aflatoxin B1. Mutat Res 1998;402:121–8 [DOI] [PubMed] [Google Scholar]
- 33. Marchese S, Polo A, Ariano A, Velotto S, Costantini S, Severino L. Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins 2018;10:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garud MS, Kulkarni YA. Gallic acid attenuates type I diabetic nephropathy in rats. Chem Biol Interact 2018;282:69–76 [DOI] [PubMed] [Google Scholar]
- 35. Javanbakht S, Shaabani A. Carboxymethyl cellulose-based oral delivery systems. Int J Biol Macromol 2019;133:21–9 [DOI] [PubMed] [Google Scholar]
- 36. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75 [PubMed] [Google Scholar]
- 37. Zemjanis R. Collection and evaluation of semen. 2nd ed. Baltimore, MD: William and Wilkins Company/Waverly Press, Inc., 1970 [Google Scholar]
- 38. Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci 1970;53:227–32 [DOI] [PubMed] [Google Scholar]
- 39. WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization, 2010 [Google Scholar]
- 40. Wolf BH, Weening RS, Schutgens RB, van Noorden CJ, Vogels IM, Nagelkerke NJ. Detection of glucose-6-phosphate dehydrogenase deficiency in erythrocytes: a spectrophotometric assay and a fluorescent spot test compared with a cytochemical method. Clin Chim Acta 1987;168:129–36 [DOI] [PubMed] [Google Scholar]
- 41. Vanha-Perttula T, Nikkanen V. Acid phosphatases of the rat testis in experimental conditions. Acta Endocrinol 1973;72:376–90 [DOI] [PubMed] [Google Scholar]
- 42. Malymy M, Horecker BL. Alkaline phosphatase. In: Word WA. (ed.) Methods in enzymology. New York: Academic Press, 1966, pp. 639–42 [Google Scholar]
- 43. Vassault A. Lactate dehydrogenase. UV-method with pyruvate and NADH. In: Bergmeyer HU. (ed.) Methods of enzymatic analysis. 3rd ed. New York: Plenum, 1993, pp. 118–25 [Google Scholar]
- 44. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170–5 [PubMed] [Google Scholar]
- 45. Clairborne A. Catalase activity. In: Greenwald RA. (ed.) Handbook of methods for oxygen radical research. Boca Raton, FL: CRC Press, 1995, pp. 283–4 [Google Scholar]
- 46. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9 [PubMed] [Google Scholar]
- 47. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179:588–90 [DOI] [PubMed] [Google Scholar]
- 48. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–7 [DOI] [PubMed] [Google Scholar]
- 49. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8 [DOI] [PubMed] [Google Scholar]
- 50. Owumi SE, Dim UJ. Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicol Rep 2019;6:202–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pérez-Severiano F, Santamaría A, Pedraza-Chaverri J, Medina-Campos ON, Ríos C, Segovia J. Increased formation of reactive oxygen species, but no changes in glutathione peroxidase activity, in striata of mice transgenic for the Huntington’s disease mutation. Neurochem Res 2004;29:729–33 [DOI] [PubMed] [Google Scholar]
- 52. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982;126:131–8 [DOI] [PubMed] [Google Scholar]
- 53. Trush MA, Egner PA, Kensler TW. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem Toxicol 1994;32:143–7 [DOI] [PubMed] [Google Scholar]
- 54. Owumi SE, Ajijola IJ, Agbeti OM. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum Exp Toxicol 2019;38:1254–65 [DOI] [PubMed] [Google Scholar]
- 55. Bancroft JD, Gamble M. Theory and practise of histological techniques. 6th ed. Philadelphia, PA: Churchill Livingstone/Elsevier, 2008, pp. 83–134 [Google Scholar]
- 56. Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs 2016;25:S35–40 [DOI] [PubMed] [Google Scholar]
- 57. Ibeh IN, Uraih N, Ogonar JI. Dietary exposure to aflatoxin in human male infertility in Benin City, Nigeria. Int J Fertil Menopausal Stud 1994; 39:208–14 [PubMed] [Google Scholar]
- 58. Owumi SE, Bello SA, Najophe SE, Nwozo SO, Esan IO. Coadministration of gallic acid abates zearalenone-mediated defects in male rat’s reproductive function. J Biochem Mol Toxicol 2022;36:e22940 [DOI] [PubMed] [Google Scholar]
- 59. Zhou H, Wang J, Ma L, Chen L, Guo T, Zhang Y, Dai H, Yu Y. Oxidative DNA damage and multi-organ pathologies in male mice subchronically treated with aflatoxin B1. Ecotoxicol Environ Saf 2019;186:109697. [DOI] [PubMed] [Google Scholar]
- 60. Tsuda T. Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J Agric Food Chem 2008;56:642–6 [DOI] [PubMed] [Google Scholar]
- 61. El Khoury D, Fayjaloun S, Nassar M, Sahakian J, Aad PY. Updates on the effect of mycotoxins on male reproductive efficiency in mammals. Toxins 2019;11:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gurung P, Yetiskul E, Jialal I. Physiology, male reproductive system. Treasure Island, FL: StatPearls Publishing, 2021 [PubMed] [Google Scholar]
- 63. Tritos NA, Klibanski A. Hyperprolactinemia. JAMA 2015;314:1742–3 [DOI] [PubMed] [Google Scholar]
- 64. Supriya C, Reddy PS. Prenatal exposure to aflatoxin B1: developmental, behavioral, and reproductive alterations in male rats. Naturwissenschaften 2015;102:26. [DOI] [PubMed] [Google Scholar]
- 65. Zamir-Nasta T, Pazhouhi M, Ghanbari A, Abdolmaleki A, Jalili C. Expression of cyclin D1, p21, and estrogen receptor alpha in aflatoxin G1-induced disturbance in testicular tissue of albino mice. Res Pharm Sci 2021;16:182–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Berg JM, Tymoczko JL, Stryer L. Glycolysis is an energy-conversion pathway in many organisms. In: Berg JM, Tymoczko JL, Stryer L. (eds) Biochemistry. 5th ed. New York: W. H. Freeman, 2002, pp. 433–4 [Google Scholar]
- 67. Roshankhah S, Rostami-Far Z, Shaveisi-Zadeh F, Movafagh A, Bakhtiari M, Shaveisi-Zadeh J. Glucose-6-phosphate dehydrogenase deficiency does not increase the susceptibility of sperm to oxidative stress induced by H(2)O(2). Clin Exp Reprod Med 2016;43:193–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mathew S, Abraham TE, Zakaria ZA. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol 2015;52:5790–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sousa A, Araujo P, Azevedo J, Cruz L, Fernandes I, Mateus N, de Freitas V. Antioxidant and antiproliferative properties of 3-deoxyanthocyanidins. Food Chem 2016;192:142–8 [DOI] [PubMed] [Google Scholar]
- 70. Skibola CF, Smith MT. Potential health impacts of excessive flavonoid intake. Free Radic Biol Med 2000;29:375–83 [DOI] [PubMed] [Google Scholar]
- 71. Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol Pharm Bull 2005;28:253–9 [DOI] [PubMed] [Google Scholar]
- 72. Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase-derived reactive species: physiological and pathological effects. Oxid Med Cell Longev 2016;2016:3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol 2018;14:618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mughal MJ, Xi P, Yi Z, Jing F. Aflatoxin B1 invokes apoptosis via death receptor pathway in hepatocytes. Oncotarget 2017;8:8239–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Y, Wang W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim Sci J 2016;87:1490–500 [DOI] [PubMed] [Google Scholar]
- 76. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016;1863:2977–92 [DOI] [PubMed] [Google Scholar]
- 77. Zhang LY, Zhan DL, Chen YY, Wang WH, He CY, Lin Y, Lin YC, Lin ZN. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: an in vitro, ex vivo and in vivo study. Arch Toxicol 2019;93:3305–20 [DOI] [PubMed] [Google Scholar]
- 78. Zhou X, Gan F, Hou L, Liu Z, Su J, Lin Z, Le G, Huang K. Aflatoxin B1 induces immunotoxicity through the DNA methyltransferase-mediated JAK2/STAT3 pathway in 3D4/21 cells. J Agric Food Chem 2019;67:3772–80 [DOI] [PubMed] [Google Scholar]
- 79. Owumi SE, Kazeem AI, Wu B, Ishokare LO, Arunsi UO, Oyelere AK. Apigeninidin-rich Sorghum bicolor (L. Moench) extracts suppress A549 cells proliferation and ameliorate toxicity of aflatoxin B1-mediated liver and kidney derangement in rats. Sci Rep. 2022;12:7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702221098060 for Apigeninidin-enriched Sorghum bicolor (L. Moench) extracts alleviate Aflatoxin B1-induced dysregulation of male rat hypothalamic-reproductive axis by Solomon E Owumi, Moses T Otunla, Uche O Arunsi and Adegboyega K Oyelere in Experimental Biology and Medicine