Abstract
Ischaemia–reperfusion (I–R) injury, initiated via bursts of reactive oxygen species produced during the reoxygenation phase following hypoxia, is well known in a variety of acute circumstances. We argue here that I–R injury also underpins elements of the pathology of a variety of chronic, inflammatory diseases, including rheumatoid arthritis, ME/CFS and, our chief focus and most proximally, Long COVID. Ischaemia may be initiated via fibrin amyloid microclot blockage of capillaries, for instance as exercise is started; reperfusion is a necessary corollary when it finishes. We rehearse the mechanistic evidence for these occurrences here, in terms of their manifestation as oxidative stress, hyperinflammation, mast cell activation, the production of marker metabolites and related activities. Such microclot-based phenomena can explain both the breathlessness/fatigue and the post-exertional malaise that may be observed in these conditions, as well as many other observables. The recognition of these processes implies, mechanistically, that therapeutic benefit is potentially to be had from antioxidants, from anti-inflammatories, from iron chelators, and via suitable, safe fibrinolytics, and/or anti-clotting agents. We review the considerable existing evidence that is consistent with this, and with the biochemical mechanisms involved.
Keywords: amyloid microclots, ischaemia–reperfusion injury, Long COVID
Introduction
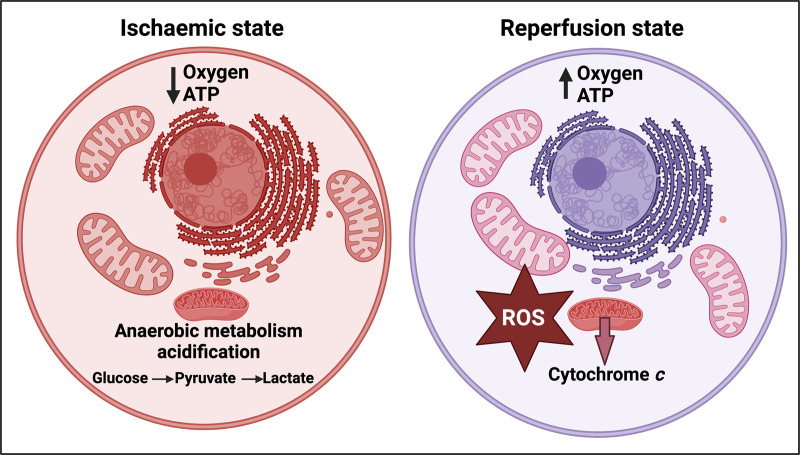
If the supply of oxygen to a normally aerobic tissue is restricted (hypoxia, often caused by ischaemia), and then is more or less rapidly restored (‘reperfusion’), that tissue may be damaged. This damage is variously known as ischaemia–reperfusion injury, hypoxia–reperfusion injury, or reoxygenation injury. It is widely observed acutely [1], for instance following an acute mycocardial infarction [2–4], stroke [5,6], in emergency medicine [7], and during the ex vivo incubation of organs as part of transplant surgery [8–11]. It is considered (see below) that the main mechanisms involve the production, especially during the reperfusion, of various partially reduced reactive oxygen species (ROS) [12,13], not least by an over-reduced mitochondrial respiratory chain that was formed during the hypoxic phase (see Figure 1 for a simplified representation of ischaemia–reperfusion injury (I–R I)). These initiate a variety of other processes such as inflammation that we discuss later.
Figure 1. Simplified representation of the initial stages of ischaemia–reperfusion injury (I–R I [7]).
Created with BioRender (https://biorender.com/).
A considerable number of chronic disorders or syndromes seem to occur following the acute stages of an infection [14–16], and include rheumatoid arthritis, various forms of more classical chronic fatigue (idiopathic chronic fatigue ICF and myalgic encephalomyelitis/chronic fatigue syndrome ME/CFS), and, most recently, Long COVID [17–23]. They are characterised by a multiplicity of symptoms, that, while seemingly (but not) unrelated, also exhibit two particular features: (i) a variety of manifestations of exceptional levels of fatigue (e.g. [18,24–35] and see below) and (ii) episodic disability [29,36–39], i.e. periods of unwellness that are relatively benign interspersed with periods (‘relapses’ or ‘crashes’) characterised by acute, severe, and often debilitating symptoms. Another striking feature of these diseases is that they predominantly affect women [40–42].
By and large, these diseases (especially ME/CFS and Long COVID) have not been well managed [29,43] because their aetiologies have been uncertain and even their organic (rather than psychological) nature has been questioned. Some of the management practices, such as graded exercise therapy, have even been harmful [43], especially since post-exertional malaise (PEM) (a worsening of symptoms following exertion) is even a hallmark of such diseases [44]. Indeed, we consider (and argue) that periodic ischaemia–reperfusion injury is largely responsible for the relapsing observed.
Thus, the main purpose of this review is to bring together the evidence that a chief cause of such crashes or relapses, that also serves to contribute to the chronic nature of such diseases, is probably a kind of chronic in vivo hypoxia/reperfusion activity that seems to have been almost completely [45] unremarked. The recognition of this leads to a variety of promising therapeutic opportunities; in most cases, there is already evidence for their benefits. We take such evidence to be evidence for the mechanisms that we propose.
A multiplicity of symptoms
Depending on the precise scoring, some 10–30% or more of individuals infected with SARS-CoV-2 and exhibiting acute COVID go on to manifest Long COVID [46–54]. This said, Long COVID has over 200 different symptoms, a subset of which (identifiable as subtypes [55]) can occur in any individual [34,56–59], likely with some consequences that may not manifest for years [60,61] or even decades [62]. These include [19,26,27,30,50,56,63–71] breathlessness [72,73], fatigue [17,18,22,25,26,31,34,35,49,74], cardiovascular issues [61,75], chest pain [74], myalgia [17,22,24,25], cognitive dysfunction [26,63,64,74,76–79], innate and cell-mediated immune responses coupled to inflammatory cytokine production [80,81], a variety of coagulopathies [82,83] including fibrin amyloid microclots [84–89], and, in particular, postural tachycardia syndrome (PoTS) [25,26,90,91] and PEM [22,25,31,92].
The great majority of these also occur in both ME/CFS [18,19,43,93–96] (also likely most commonly a post-viral disease [16,19,97,98]) and in rheumatoid arthritis [14,23,99] (where bacterial infection, especially by Proteus spp., is strongly implicated [14,100–105]). To retain focus, we do not really discuss the many other chronic, inflammatory diseases for which similar phenomena may be observed (see e.g. [15,106]), though we would comment that ischaemia–reperfusion injury has been suggested to be significant in the vascular disease pre-eclampsia [107,108], that also exhibits many of the other hallmarks (necessarily for a more limited time) of these syndromes [109,110]. Indeed, aspects of COVID-19 bear many similarities to pre-eclampsia in pregnant women [111,112]. To this end, the likely ubiquity of an infectious origin for more or less all chronic inflammatory diseases [15,106] is exemplified by the recent recognition that multiple sclerosis originates with an Epstein–Barr virus (EBV) infection [113–115].
Gene expression levels can vary very widely between different tissues (e.g. [116,117]). Thus, although in acute COVID the SARS-2-CoV virions tend to be most accumulated where their receptors are most prevalent (as were the α and δ-variants in the lungs [118,119]), there is evidence for a wide distribution between tissues [118,120,121], including in Long COVID [19,77,122]. This can help to explain particular differences in symptomology; however, we consider that there are mechanisms (particularly amyloid microclot formation [85] and, as introduced here, regular ischaemia–reperfusion injury) that are also general enough to help to understand the unusual breadth of the pathology of Long COVID.
A systems approach
Given the complexity of the potential actors and symptoms, and the fact that in one sense everything is connected to everything else, often with complex feedback loops, the task of the systems biologist is to identify, and ultimately to quantify, the pathways and elements most strongly contributing to the phenomena of interest. This will initially take the form of qualitative network diagrams [123–126]. To this end, Figure 2 indicates some of the features that we consider likely to contribute to these chronic, inflammatory diseases, along with an attempt (Figure 3) to indicate some evident causal relationships.
Figure 2. Systems that are likely to be affected by, and contribute to, chronic, inflammatory diseases.
Created with BioRender (https://biorender.com/).
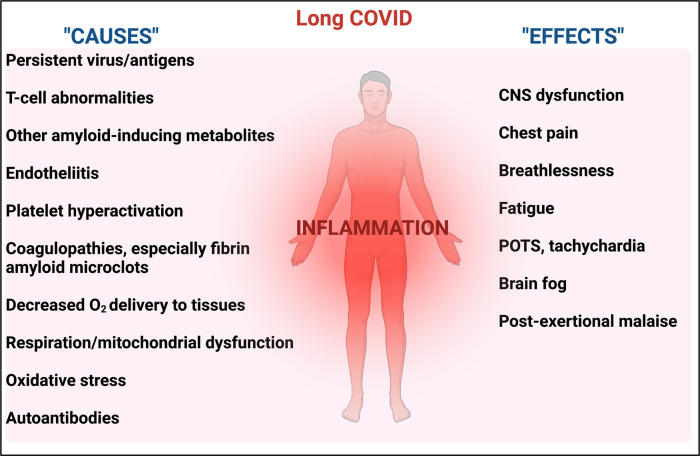
Figure 3. Some causes and effects of inflammation.
In many cases there is positive feedback in which A causes B and B causes A (e.g. inflammation ßà endotheliitis, inflammation ßà coagulopathies). Created with BioRender (https://biorender.com/).
Many the following sections summarise the mechanistic evidence for these phenomena. Given the heterogeneity of symptoms alluded to above, it is clear that some pathways will be more significant than others in particular individuals, so this is necessarily to be seen as a high-level view. In particular, to understand how one state of relatively benign conditions can morph into another of much greater severity, it is to be recognised that normally an internal or external trigger of some kind is necessary. This could be hormonal (e.g. the female menstrual cycle) or more or less any kind of stress or trauma. Our focus here is on the involvement of a kind of chronic ischaemia–reperfusion injury in these processes, and the role of fibrin amyloid microclots therein.
Ischaemia–reperfusion injury and ROS production
Under normal circumstances, the reduction in dioxygen by the mitochondrial respiratory chain is a four-electron reduction (leading to water), occurring at cytochrome c oxidase. If the respiratory chain is over-reduced, however, as a result of hypoxia or the ischaemia that causes it, O2 when readmitted (‘reperfusion’) can undergo a two-electron reduction at the level of cytochrome b (complex III) forming peroxide, or a one-electron reduction at complex I forming superoxide . A variety of other oxygen-reducing enzymes can also lead directly to the production of such ‘reduced’ forms of dioxygen in vivo (e.g. [1,127–129]), with H2O2 from xanthine oxidase being especially implicated in ischaemia/reperfusion injury (e.g. [128,130–135]).
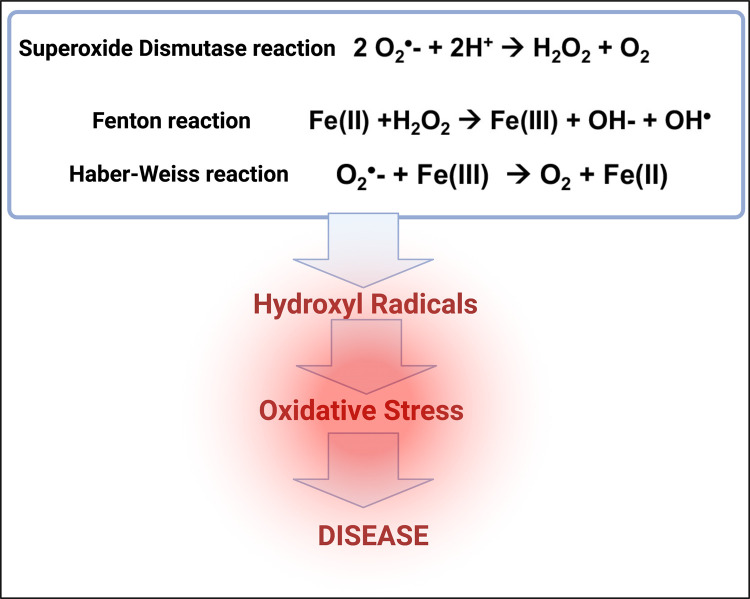
Superoxide dismutase [136] can serve to equilibrate superoxide and peroxide:
| 1 |
As reviewed in detail previously [106], although classed as ‘reactive oxygen species’, neither peroxide nor superoxide are normally excessively toxic at low concentrations (indeed they can be used as signalling molecules); what does cause major toxicity is their presence at higher levels and in addition the reaction of hydrogen peroxide with (free or poorly liganded) Fe(II) in the Fenton reaction [137], leading to the very reactive and damaging hydroxyl radical ()
| 2 |
Superoxide can also react with ferric iron in the Haber–Weiss reaction [138,139] to produce Fe(II) again, thereby effecting redox cycling:
| 3 |
It is to be stressed that the combination of these two reactions means that unliganded iron can act catalytically to produce hydroxyl radicals.
Hydroxyl radicals are exceptionally reactive, and react within nanoseconds with anything that is nearby. Thus it is they that are especially responsible for all the trouble connected with oxidative stress (Figure 4).
Figure 4. Some of the chemical reactions contributing to oxidative stress and disease.
Created with BioRender (https://biorender.com/).
Note that ascorbate can replace within the cell for reducing the Fe(III) to Fe(II) [140]. Thus, although ascorbate is ‘reducing’ and an ‘antioxidant’, its reaction with O2, especially when catalysed by Fe(II), produces superoxide and thence radicals that may be pro-oxidant [141]. Indeed, a variety of clinical trials using ascorbate in diseases considered to be accompanied by oxidative stress have found that it is actually less good than was the control/placebo in terms of all-cause mortality [142,143].
The importance of liganding free ‘iron’
This was discussed in much more detail previously [106] (see also [15]), so only a brief summary is given here. Specifically, while iron is familiar as normally existing mainly in Fe(II) or Fe(III) states, this describes only part of the picture, since the Fe atom contains six potentially ligandable sites (two polar, four equatorial) that also affect its reactivity, and only when all six sites are occupied (liganded) is its reactivity controlled. Liganding say four sites actually increases its reactivity, and for instance the reduction by ascorbate of Fe–EDTA complexes (that ligand only the equatorial sites) is in fact a potent means of producing hydroxyl radicals in the laboratory [144–147]. Thus it is vital to ensure that free iron, and especially poorly liganded iron, is absent [148]. Note, in this context, that cell death is accompanied by the release of ferritin, and that this ferritin (which should not normally be in plasma at all) liberates free iron when it enters the bloodstream [149].
Dormancy in bacteria and viruses
Although textbooks of microbiology focus on the kinetics and behaviour of microbes when they are (or have recently been) actively growing, this state is not at all the norm in nature. If an organism can grow it will do so, but if it lacks a necessary nutrient or signalling molecule it cannot (by definition). Unsurprisingly, evolution long ago selected against organisms that simply die when unable to grow, and instead selected for starvation survival [150–153] that required that they entered a state of dormancy [154,155], from which they must in time become resuscitable [156–159]. In clinical microbiology (where large segments of the human population harbour dormant forms of Mycobacterium tuberculosis [160,161] and/or Helicobacter pylori [162,163] without manifesting overt infection), such dormant forms are often referred to as ‘persisters’ [164–174].
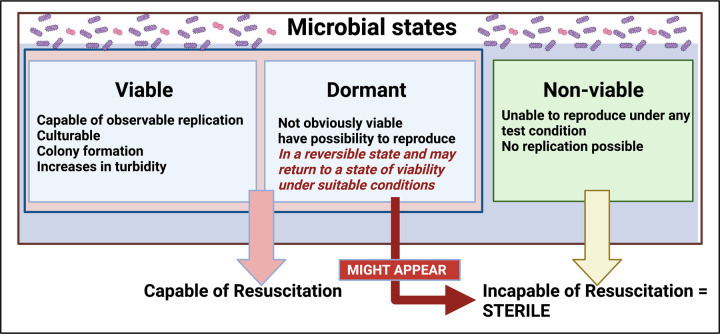
Dormant bacteria represent a conundrum for classical bacteriology, since they do not obey the classical Koch's postulates that classically allow one to state that organism X causes disease Y [175–182]; specifically, they do not necessarily adopt replicating forms, that might be assessed as axenic colonies on petri dishes. Notwithstanding claims of reagent contamination, the presence of dormant (non-replicating) bacteria also underpins the existence of (often resuscitable) microbial cells in blood [183–193] and tissues (e.g. [109,110,194]) that are normally considered to be necessarily sterile, where they may also be detected by molecular methods or using imaging techniques. In general terms, the invasion of host cells by microbes (‘intracellular pathogens’) that are known to lie in a dormant or quiescent state and reactivate at later times is actually a commonplace (e.g. [195–222]), so should not, in fact, be perceived as ‘controversial’ at all (Figure 5).
Figure 5. Microbial states and their viability and culturability, adapted from [15].
Created with BioRender (https://biorender.com/).
Viruses, especially herpes viruses, but also enteroviruses [223], can also remain latent for years [224–226], a well-known example being the activation of herpes zoster (manifesting as shingles) decades after a typically childhood infection with chickenpox [227]. Their reactivation has also been strongly implicated in Alzheimer's disease [228–231]. The prevalence of latent herpesviruses such as cytomegalovirus may be as great as 90% [232,233]. Most pertinently, there are strong indications for a role of periodic viral (and/or bacterial) reactivation in both ME/CFS [234] and Long COVID [16,19,22,43,47,220,235–239].
Triggers of viral reactivation
A chief means by which viruses are suppressed, or maintained in a latent state, involves the interferon system (e.g. [240–246]). For present purposes, it is not necessary to go into all the molecular details; sufficient here is to recognise that infection with SARS-CoV-2 (as with certain other viruses [242,247–249]) can effectively lower the normal interferon responses [250], and it is this that effectively unleashes existing latent viruses. Vaccination can sometimes elicit a similar effect [251–253].
Triggers of bacterial reactivation
With the possible exception of Borrelia burgdorferi (the causative agent of Lyme disease) [254], all microbes need a source of iron. Normally, within human hosts, free iron is strictly regulated and is not available to assist the growth of pathogens [15,255–263]. This is (in part) why typical pathogens cannot replicate in vivo, why higher iron levels correlate with infection [15], and why genes encoding iron uptake mechanisms are virulence genes [264,265]. Thus, any kind of trauma that leads to cell death can liberate free iron [15,266], which then initiate replication (and the potential shedding of inflammagens such as lipopolysaccharide (LPS) [15,267] and lipoteichoic acid [268]).
Endotheliitis
It is by now well established that endotheliopathies, commonly measured using flow-mediated dilation [269,270], are of great significance in the pathological response of hosts to SARS-CoV-2 infection [271–284]. They are initially caused by the viral infection, but likely both lead to and are caused by the coagulopathies and strong inflammatory responses that characterise the disease.
Coagulopathies
The term coagulopathies is used to describe any kind of dysregulation of the blood coagulation system. Coagulopathies [82,285–302] are a hallmark of both acute COVID [82,84,86,303–319] and Long COVID [88,89,320], and spike protein may be activated by clotting factors [321]. Acute COVID-19 is associated with both a hypercoagulable state and with bleeding; the resolution of the apparent paradox is temporal [82] since the hypercoagulation can use up elements such as von Willebrand factor (VWF) that are then insufficient for normal coagulation to occur. Hypercoagulation can be caused by any number of traumas or shocks to the system, from the more obviously acute kind of trauma [322–324], to the presence of molecules such as free iron [325–330], bacterial LPS [267,268,331–333], and lipoteichoic acid [268], or, most pertinently, the S1 spike protein of SARS-CoV-2 [84]. Coagulopathies are also a feature of rheumatoid arthritis [14,334–337] and indeed all kinds of inflammatory diseases [326,338–345], including of course COVID-19 [346–349] and ME/CFS [350–352]. However, although these links are well established, there is an important twist that makes the kind of coagulation we are talking about anomalous because it leads to fibrin amyloid-containing clots that are more resistant than usual to fibrinolysis.
Fibrin amyloid microclots triggered by bacterial and viral components
Since we are not normally talking about overt infection here, in which large numbers of proliferating organisms overwhelm the host and create sepsis [353], we need a mechanism (or set of mechanisms) by which more-or-less tiny amounts of viral or bacterial product can amplify their effects. In this case, we believe that the clotting of blood into an anomalous, amyloid-type form, is a major contributor [85]. The terminal stages of blood clotting involve the self-organised polymerisation of fibrinogen (a protein of dimensions ca 5 × 45 nm) into fibrin fibrils of great length, and diameters of say 50–100 nm. What we discovered was that highly substoichiometric amounts of the Gram-negative bacterial cell wall component LPS , viz 1 molecule LPS per 100 000 000 fibrinogen molecules, could induce the clotting to be into a thermodynamically more stable, protease-resistant amyloid form [354–356]. This could also be effected by the Gram-positive equivalent, lipoteichoic acid [268], and, importantly for present purposes, by the spike protein of SARS-CoV-2 [84,85,87–89,306,357]. (We have not yet tested surface antigens from other viruses.) Because the conversion of normal forms of proteins to their amyloid equivalents, with no change in primary sequence, is often triggered by the amyloid form itself, much as with prions [355,358–361], it is unsurprising that there is cross-reactivity between amyloid types [362–365], and amyloid deposits of various kinds are also a hallmark of SARS-CoV-2 in COVID-19 [85,87,89,306,366–368], as well as with other viruses [369] and diseases [364]. One feature of the amyloid form is that by representing a conformation different from that of normal fibrin it necessarily displays novel epitopes, which can of course select for the production of autoantibodies; the fibrinaloid clots also trap a variety of other molecules, including plasmin-inhibiting agents [88] that mechanistically may contribute to their stability. However, probably the most important consequence of the resistance of these fibrin amyloid or ‘fibrinaloid’ [85] microclots to fibrinolysis is that they are able to pass into microcapillaries and block them up [370]. Such fibrinaloid microclots have now recently been observed in the plasma of ME/CFS, at levels some 10-fold those of healthy controls [352].
We also note that in vitro studies have shown that various other molecules can induce fibrin amyloid formation (referred to in our early literature as ‘dense matted deposits’); these include oestrogen [371] (which we consider also reflects the prevalence of these diseases in women [17,40,72] and variations in the severity of Long COVID during the female monthly cycle) and unliganded iron [326,330,372,373]. In contrast, magnesium ions are protective against fibrin amyloid microclotting [374]. We recognise, however, that men also produce oestrogens, so it is not a straightforward correlation, and many elements might underlie the significant bias towards women in these and other chronic, inflammatory diseases.
Evidence for capillary blockage from venous O2 levels
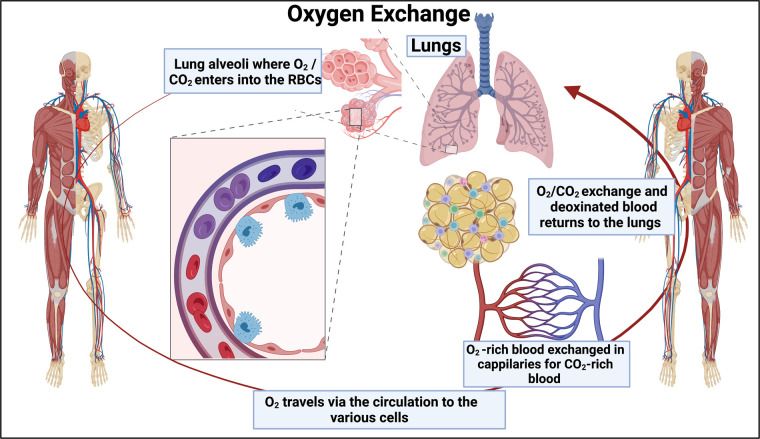
Tissue hypoxia can be caused by a variety of mechanisms, including poor O2 uptake in the first place or poor exchange with tissues. Similarly, mitochondrial respiration, whose control is distributed across many steps [375], may also be limited by a variety of causes, including the number of mitochondria and, of course, the actual availability of O2. To rehearse, arteries take O2-poor blood from the heart to the lungs where it is oxygenated, capillaries distribute it throughout tissues, and veins return the less-oxygenated blood to the heart (Figure 6). Nowadays the venous blood saturation is measured via a central venous catheter in the superior vena cava via the jugular of the subclavian vein, rather than in the pulmonary artery as previously [376]. It reflects both O2 transport to the tissues and usage by them [377], so while a low value is indicative of problems a normal value may not entirely reflect them [376]. Specifically, the evidence that in many of these diseases tissue hypoxia is a major cause of problems due to the capillaries not doing their job properly comes from the measurement of central or mixed venous (vs arterial O2) saturation [378,379]. In one study [378], central venous oxygen saturation (ScvO2) was 66% for healthy controls, 33% for acute COVID-19 survivors, and 18% for those who died from COVID-19. We note that conventional fingertip-type pulse oximeters, while reasonably accurate, mostly estimate arterial O2 levels [380], so would not necessarily pick up this kind of phenomenon; indeed this may account for the discordance [381,382] between pulse oximetry and arterial blood gas measurements. Cerebral oximetry [383] may provide a truer assessment if non-invasive measures are desired.
Figure 6. Simplified diagram of oxygen exchange, showing arteries (red) and veins (blue) linked by capillaries.
Created with BioRender (https://biorender.com/).
One typical consequence of a blockage of a flexible pipe is that it swells. While it is not so easy to detect this, one organ where this is indeed visible is the eye. Consistent with capillary blockage, the diameters of blood vessels in the eye increase during COVID-19 [384–386]. Specifically, the likelihood of retinal microvasculopathy in subjects with COVID-19 was massively higher compared with that of controls (with an odds ratio and 95% confidence interval of 8.86 and 2.54–27.53, respectively [387]). In some cases, even ocular vein thrombosis has been observed [388].
Heart-rate variability
If — as argued here — capillary blockage induced by fibrin amyloid microclots is important, especially as output demand is increased, it is reasonable that heart-rate variability might be increased in individuals suffering from Long COVID. This very much turns out to be the case [389–391], and is presumably related to postural orthostatic tachycardia syndrome (POTS) [392], another common symptom of Long COVID [21,90,393–395] with likely a similar cause. Specifically, as well as more complex physiological contributions, low O2 is a standard signal (as occurs during exercise) for the heart to beat faster (e.g. [396]); in the cases of interest here, we simply posit that this occurs at far lower levels of exercise effort because of the impaired ability to deliver O2 to tissues.
Size of fibrin amyloid microclots and capillary diameters
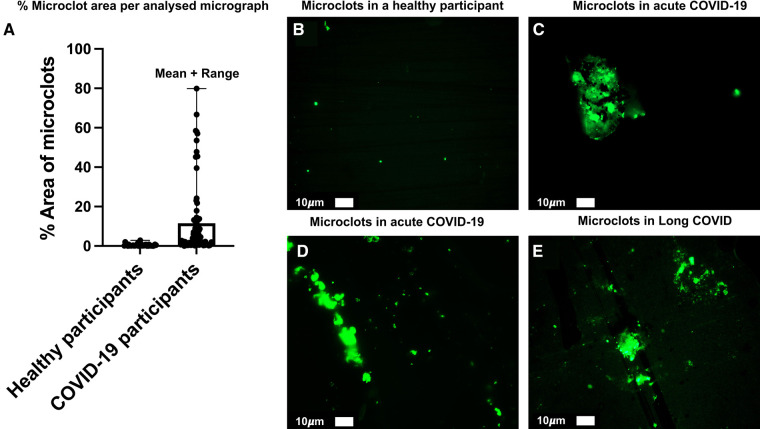
Most capillaries (depending on the individual under study [397]) are just 5–10 μm in diameter [398–406], which means that erythrocytes, with a discoid shape of 6–8 μm diameter × 2–2.5 μm [407], largely pass through them in single file (and their viscosity is here at a minimum [408]). Others are more like 15–50 μm diameter [409,410]. (There is thus a size discrepancy between the diameter of the white blood cells (6–8 μm or more) and that of the smallest capillaries (∼5.5 μm) and this size discrepancy forces the white blood cells, to deform in order to transit the capillary bed [411].) The transition into the tissue over the blood vessel walls will occur in the response to a biologically harmful stimulus (e.g. inflammation or infection) [412]. This white blood cell migration process is called transendothelial migration (TEM) or diapedesis. The fibrin amyloid microclots that we discovered are commonly 5 to even 200 μm in diameter, so it is not surprising that they can block up capillaries. See Figure 7 for representative examples of microclots in healthy participants (exposed to neither SARS-CoV-2 nor vaccine), those found in acute COVID-19 and in Long COVID. Also, note the % area distribution of microclots in acute COVID (data published in [87]). In addition, the erythrocytes themselves lose deformability in these kinds of disease [413]. Improved imaging methods coupled to advanced computer vision algorithms for assessing such capillary diameters (e.g. [398,399,409,414–421]) and their relation to blood flow represent important areas of research.
Figure 7.
(A) % area distribution of microclots in plasma from participants with acute COVID-19 (taken from raw data as in [87]). (B) Representative micrograph of microclots in plasma from a healthy individual. (C,D) Representative micrographs of microclots in plasma from acute COVID-19 participants (taken from raw data as in [87]). (E) Representative micrograph of microclots in plasma from participants with Long COVID (taken from raw data in [422]).
Two other major points pertain to this question about the relationship between the size of capillaries and the likelihood of insoluble circulating material clogging them: the first is that if microclots of whatever composition are ‘already’ trapped in capillaries for a greater or lesser time they will not be observable in standard venous plasma samples, and so these measurements may underestimate the problem. The second is that this general mechanism of capillary blockage is likely true for other kinds of debris including those from endothelial cell damage; to this end, it is worth recognising that the presence of extracellular vesicles is part of many chronic diseases (e.g. [423–427]), including rheumatoid arthritis [428–433]. In particular, extracellular vesicles are seen as a highly discriminating accompaniment to ME/CFS [434], and are being observed in both acute [435] and Long COVID-19 [436] patients.
Erythrocyte (RBC) deformability and eryptosis
The size, shape, and deformability of erythrocytes contributes strongly to their function, and there is evidence that these too are abnormal in the diseases of present interest, viz rheumatoid arthritis, ME/CFS, acute and Long COVID. The extent to which these are caused via fibrin amyloid microclots or by other means (such as reaction with ROSs [437,438]) is unknown, but this is also likely to contribute to the oxidative stress observed. In particular, RBCs from patients with ME/CFS were significantly non-discocytic [439] and stiffer [413] than those from healthy controls. Differences are also found for erythrocyte sedimentation rates (ESR) [413,440] (as it was in the case of delayed cerebral ischaemia following subarachnoid haemorrhage [441]) and also for the easily measured red cell distribution width (RDW) [442]. ESR values are changed in Long COVID [443], while RDW is raised substantially (and increasingly with severity) in acute COVID [444–448]. We are not aware of studies of RDW and Long COVID, but the ease of measurement of this variable implies that much more use might be made of it.
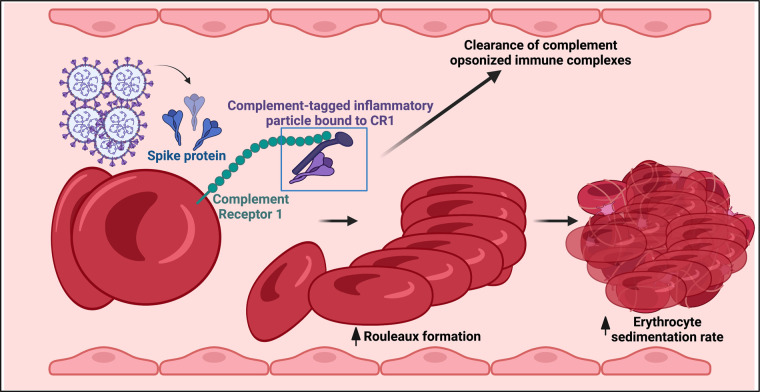
It was also found that in acute COVID-19 infection, complement deposition on erythrocytes occurs [449]. Erythrocytes have complement receptor 1 (CR1) (also known as C3b/C4b receptor or CD35) on their membranes and this binds complement activation products C3b and iC3b [449] and C4d [450]. The complement system promotes clearance of pathogens but pathological complement activation may cause microvascular thrombosis. In COVID-19 ICU patients, a reduced CR1 erythrocyte density was observed. Furthermore, deposits of C4 fragments on erythrocytes and virus spikes or C3 on erythrocytes among COVID-19 patients may be of significance to the clearance of immune complex or complement fragment-coated cell debris during COVID-19 infection [450] (see Figure 8). All of these aspects can contribute to impaired blood flow and hence lowered tissue oxygenation.
Figure 8. Immune complex formation on erythrocytes during COVID-19 and Long COVID: a hypothesis.
Created with BioRender (https://biorender.com/).
An extreme endpoint of oxidative stress-inducing erythrocyte stiffness and altered morphology is eryptosis [451–455], in which erythrocytes undergo a specific form of programmed cell death. However, since it can also be induced by a variety of drugs sometimes used to treat the relevant diseases (e.g. [456]), it may not be the best biomarker for diagnosing them precisely. Erythrocyte pathology is nonetheless a clear indicator of problems with microcirculation [453,457–459], and as such speaks clearly to our proposals that an inadequate microcirculation, leading to I–R injury, lies at the heart of these diseases.
Thromboses
While amyloid microclots in capillaries have been our focus, we should recognise that anything disposing to their formation might, statistically, tend to lead to the formation of larger clots. When the blockage by such a clot of a larger blood vessel such as a vein is effectively complete, one consequence is a venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT). It is, therefore, worth enquiring as to whether the likelihood of these is raised as part of the diseases we are considering. The answer is that individuals with rheumatoid arthritis [460–467] and acute [287,468–479] and Long COVID [370,480,481] are indeed significantly more prone to such VTEs. The extent to which these thrombi are amyloid in character seems not to have been investigated, and there is also an intriguing link with MAST cell contents [482]. Here, we should also mention the prevalence of microthrombi and VTEs as part of sepsis (e.g. [353,483–485]), and the similarities between some of the symptoms observed in Long COVID or ME/CFS and what is coming to be recognised [486–489] as post-sepsis syndrome. These events provide further mechanistic evidence for the kinds of microcapillary blockage we believe to be caused by fibrinaloid microclots.
Aneurysms
An aneurysm is a bulge in a blood vessel caused by a weakness in the blood vessel wall, commonly where it branches. Clearly, the blockage of a blood vessel by a microclot is likely to exert pressure on the vessel's walls, potentially weakening it, and so it is reasonable that aneurysms might be expected to accompany diseases involving microclots. This turns out to be the case in rheumatoid arthritis [490], acute COVID [491,492] (which bears similarities to Kawasaki disease [493]) and Long COVID [17]. The association with ME/chronic fatigue syndrome is seemingly weaker, and is usually seen as an association of fatigue, for example, driven by a subarachnoid haemorrhage [494,495].
COVID toes
Chilblains (pernio) commonly occur when the extremities get cold (restricting blood flow — ischaemia) followed by a period of rewarming (with reperfusion) leading to what might be seen as almost a classical and observable form of ischaemia–reperfusion injury. An unusual and striking feature that has been observed in both acute [496–502] and Long COVID [503–505] is just such a set of ‘chilblain-like lesions’ commonly known as ‘COVID toes’. They seem to reflect thromboembolic events, as well as a strong interferon response [506], and may represent (and certainly provide evidence for) one of the most visible sequelae (both macroscopically and via capillaroscopy [507–509]) of the kinds of ischaemia–reperfusion injury that we are positing here.
Evidence of hypoxia leading to oxidative stress
There is no doubt that hypoxia leading to ROS is the cause of ‘oxidative stress’ [510]. Hypoxia can have a variety of causes [511], and was of course one of the chief hallmarks of early variants of SARS-CoV-2 in acute COVID-19 [370,512–521], and it is reasonable that this is in part due to impaired capillary flow [370,522]. It can only be modelled if perfusion defects are included [523]. It is similarly a characteristic of Long COVID [17] and also, perhaps less well known, of rheumatoid arthritis [524]. Rheumatoid arthritis is also correlated with ischaemia as judged by a comorbidity with ischaemic heart disease [525], and indeed with VTE [461] and anomalous amyloid clotting [14,336,526]. Many positron emission tomography (PET) probes for detecting hypoxia in vivo do exist [527], and of course for research purposes one may use suitable optical probes [528] or the assessment of gene expression profiles as modulated by the transcription factor hypoxia-inducible factor (HIF-1) [529–531]. Such evidence for hypoxia is an important part of the narrative we bring together here.
Oxidative stress resulting from ischaemia–reperfusion injury
Oxidative stress refers to an imbalance between the rate of production of reactive oxygen and nitrogen species (many of which are free radicals) and their elimination via antioxidants (e.g. [106,532–545]). As discussed above, any ischaemia or hypoxia followed by a return to normoxia runs the risk of ischaemia–reperfusion injury, since ROSs are the inevitable consequence. This manifests as a combination of oxidative stress, inflammation, and (synonymously) inflammatory cytokine production. This is almost so well known that it needs no rehearsal, so we merely give a few reviews as they pertain to rheumatoid arthritis [14], to ME/CFS [16,18,19,29,43,44,97,546–548], and to Long COVID [17,19,25,27]. For us, the key question is: how is this most obviously manifest in something relatively easily measurable (that thus provides evidence for them)?
Lactate as a measure of ischaemia
If the supply of O2 to tissues is inadequate, cells must rely on non-respiratory reactions for their ATP formation, meaning that lactate is likely to accumulate. To this end, elevated lactate accumulation has indeed been observed in rheumatoid arthritis [549–551], in ME/CFS [552–557], and in acute [558–561] and long [562] COVID.
Measurable hallmarks or biomarkers of ischaemia–reperfusion injury
As rehearsed above, and in much more detail previously [15,106], while peroxide and superoxide are intermediates, and at high levels are cytotoxic, it is also the hydroxyl radical that causes many or most of the manifestations of oxidative stress and, in particular, of ischaemia–reperfusion injury. Because it is so reactive, it is not observable directly; however, the products of the effective reaction of ROSs with proteins (nitrotyrosine [563–567]), lipids (malondialdehyde [568,569]), and DNA (8-oxoguanine [570–574]) are. Consequently, if we wish to know that I–R injury is a contributor to the kinds of syndromes we are discussing here, we ‘simply’ need to check whether they manifest these oxidative biomarkers. 8-isoprostane is another, and it (and the related 15-isoprostane [575]) is raised in rheumatoid arthritis [576,577], ME/CFS [546], and COVID-19 [578–581]. Malondialdehyde and related compounds from lipid peroxidation are also easily measured in the ‘thiobarbituric acid reactive substances’ (TBARS) assay. Note, however, that being ‘endpoint assays’ they can measure determinands that can accumulate, but since the degradation rate of such molecules is normally unknown they cannot alone be used to determine the recency of any oxidative stress.
This said, there is plenty of evidence for the presence of nitrotyrosine in acute COVID [582,583], rheumatoid arthritis [584–587], and ME/CFS [588]. It does not yet seem to have been looked for in Long COVID but has been anticipated [583]. TBARS levels are also raised in rheumatoid arthritis [589–591], acute COVID [580], ME/CFS [592–595], and idiopathic chronic fatigue [94], and are similarly anticipated in Long COVID [547]. These measurements provide powerful evidence for the role of ischaemia–reperfusion phenomena in ME/CFS and Long COVID.
Post-exertional malaise
‘Fatigue’ in syndromes such as ME/CFS and Long COVID is commonly used to refer to an inability at a given moment to perform what would normally be seen as simple and very light mechanical, exercise or even cognitive tasks. In contrast, PEM refers to a state that follows such activities, commonly 24–48 h later, in which many of the symptoms of ME/CFS are exacerbated, and may be accompanied by burning sensations. Such events are often referred to as ‘crashes’. According to the ideas developed here, PEM is precisely a consequence of ischaemia–reperfusion injury, because the kinetics of cell death are such that this does not happen instantaneously but follow the flood of ROS contingent on the reperfusion events.
Inflammation
There is a certain circularity in the definition of inflammation, since inflammatory cytokines (such as IL-1β, IL-6, and TNF-α) are those that rise during inflammation, while inflammation is decreed when inflammatory cytokines are raised. Many are controlled by the transcription factors NF-κB [596] and Nrf2 [597,598], both of which are sensitive to redox state/oxidative stress [599–606], thereby providing a straightforward link between hypoxia, oxidative stress, and inflammation [607–609]. However, most measurements of these transcription factors are performed on ‘static’ samples, while it is their oscillatory nature that controls gene expression [610–614]. Consequently, beyond the qualitative statement of a linkage between these transcription factors and redox state (i.e. oxidative stress) the interpretation of most available data is not straightforward. What is also well established, however (e.g. [9,615–620]), is that inflammation is absolutely a consequence of ischaemia–reperfusion injury and ROS production, and vice versa, providing a ‘vicious cycle’.
Mast cell activation
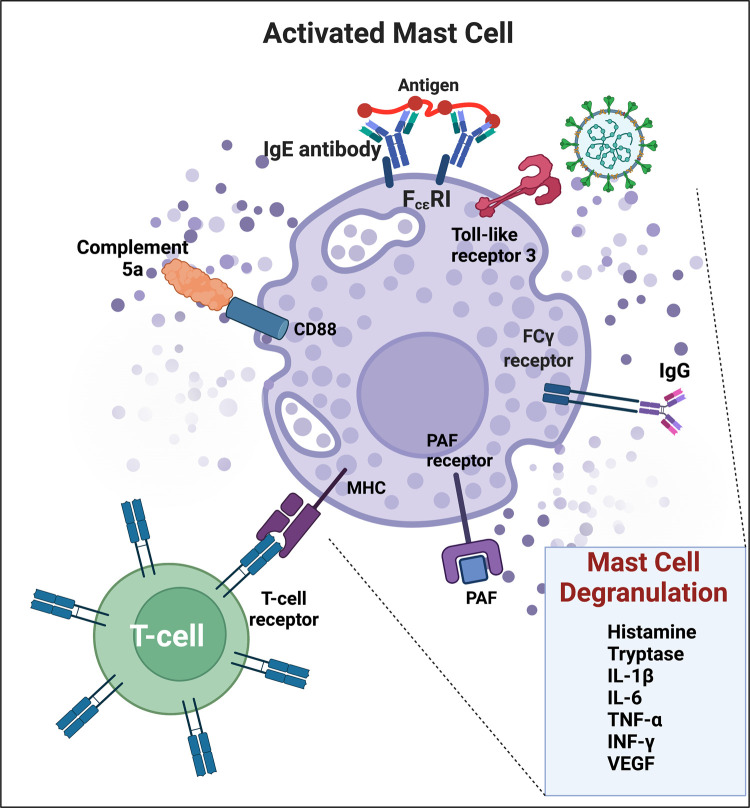
Another mechanism mediating hyperinflammation is mast cell activation [621]. It occurs during acute COVID [622–624], and the symptoms [625] of those with known mast cell activation syndrome significantly mirror those of individuals with Long COVID [626,627]. The elevation of IL-6 levels, as well as proteases such as carboxypeptidase A3 and tryptase, is a hallmark of this and these are also observed in PASC patients [628]. Importantly for the present focus, mast cells are also activated during (and exacerbate) ischaemia–reperfusion injury [629–634], and the inhibition of mast cell degranulation [634] might consequently be of benefit in Long COVID. Indeed, these findings are consistent with the known beneficial effects of antihistamines in PASC [80] (see Figure 9).
Figure 9. Mast cell activation during COVID-19 and the resulting inflammation and coagulation pathology via mast cell degranulation.
SARS-CoV-2 can activate mast cells through TLR(3) to release proinflammatory mediators. TLR, Toll-like receptor; FcεRI, high affinity IgE receptor; FcγR, IgG receptor; PAF, platelet-activating factor; MHC, major histocompatibility complex. Adapted from [635–637]. Created with BioRender (https://biorender.com/).
Platelet hyperactivation
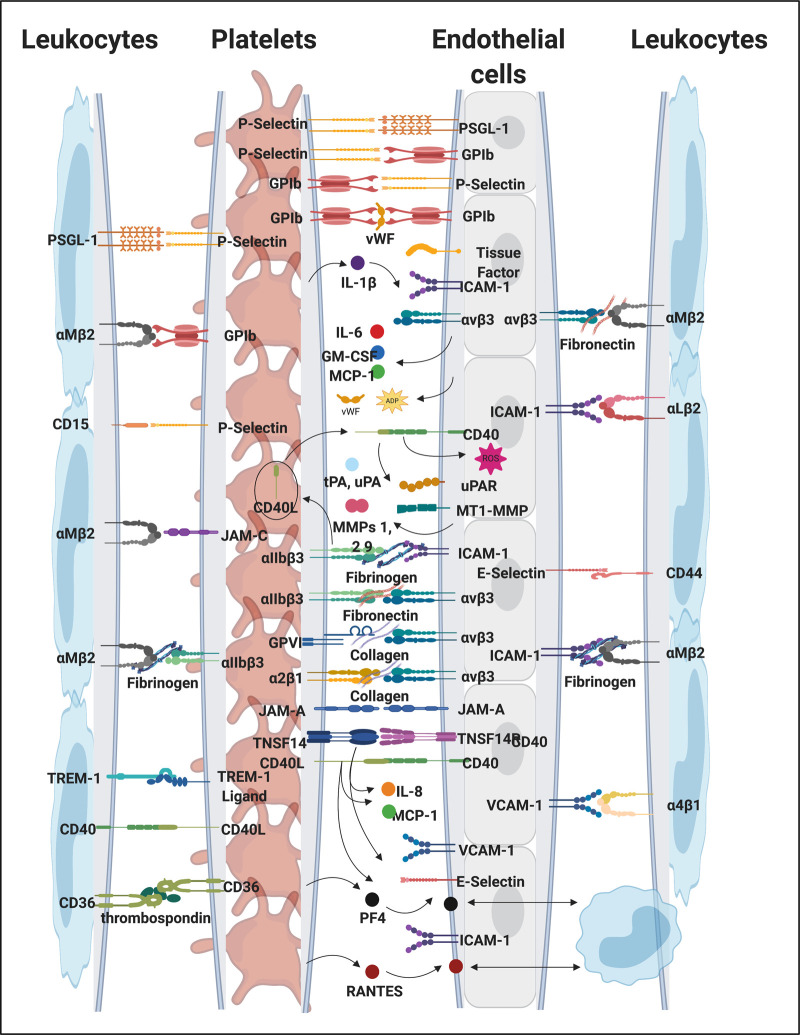
Platelet hyperactivation is one of the observables of both acute [638,639] and Long COVID [89], and one of its causes can certainly be oxidative stress [640], as well as circulating inflammatory molecules that may trigger platelet hyperactivation, spreading, and significant platelet clumping [86,301]. It is also part of ME/CFS [641]. Platelets have numerous receptors on their membranes that may interact with circulating inflammatory molecules, including viral and bacterial inflammagens [82,642–647]. In addition, platelets and their membrane receptors may also interact with each other, with endothelial cells and with other immune cells, forming platelet complexes. These interactions not only drive pathological clotting, but can also perpetuate endothelial damage and immunothrombosis [648,649], not only in COVID-19 as Long COVID, but also in all inflammatory diseases [647,650–660]. P-selectin is a well-known inflammatory molecule that may be found inside the granules of healthy platelets, on the membrane of the activated platelet where it acts as a binding receptor, or in circulation, as a soluble inflammatory molecule [82,301,646,661,662], Levels were raised in both participants in a small study [663]. Figure 10 shows selected platelet receptors and Figure 11 examples of platelet hyperactivation in individuals with Long COVID. Platelets are well-known for their storage of serotonin [664] and platelet factor 4 (PF4) and serotonin are stored in α- and δ-granules [665]. Circulating immune complexes may also activate platelets via receptor–receptor binding followed by the release of serotonin from platelet granules [665]. A subpopulation of platelets (the COAT-platelets) activated with collagen and thrombin express functional α-granule factor V. These COAT-platelets can bind fibrinogen, VWF, thrombospondin, fibronectin, and α2-antiplasmin [666]. In addition, COAT-platelets use serotonin conjugation to bind pro-coagulant proteins on their cell surface through a serotonin receptor [666]. As platelet serotonin is the main source of serotonin in the blood, if a significant proportion of the circulating platelet population is hyperactivated, these platelets will shed their serotonin content and it will, in addition, provide a pro-coagulant surface, allowing these platelets to form platelet complexes, bind to fibrin(ogen), and also damaged endothelial cells (see Figure 10). Platelet hyperactivation has recently also been observed in ME/CFS [352].
Figure 10. Platelet receptors and interactions with inflammatory molecules, endothelium and leucocytes during inflammation and clotting pathologies (adapted from [652]).
Created with BioRender (https://biorender.com/).
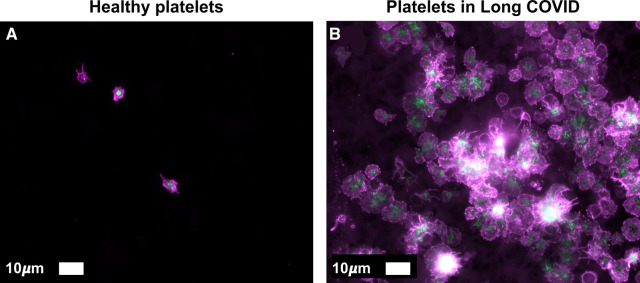
Figure 11. Platelet hyperactivation noted in a healthy individual (A) and an individual with Long COVID with severe platelet hyperactivation (B).
Haematocrit samples were exposed to the two fluorescent markers, CD62P (PE-conjugated) (platelet surface P-selectin) (IM1759U, Beckman Coulter, Brea, CA, U.S.A.) and PAC-1 (FITC-conjugated) (340507, BD Biosciences, San Jose, CA, U.S.A.). CD62P is a marker for P-selectin that is either on the membrane of platelets or found inside them. PAC-1 identifies platelets through marking the glycoprotein IIb/IIIa (gpIIb/IIIa) on the platelet membrane. Samples were viewed using a Zeiss Axio Observer 7 fluorescent microscope with a Plan-Apochromat 63x/1.4 Oil DIC M27 objective (Carl Zeiss Microscopy, Munich, Germany). (Unpublished data; Ethics from Stellenbosch University Human Ethics Committee (HREC) number 9521.).
Interestingly, serotonin receptor antagonists reverse serotonin-mediated pulmonary vasoconstriction, lessen pulmonary platelet trapping, inhibit platelet activation and aggregation, and normalise increased respiratory drive, in severe COVID-19 [667,668]. It was also noted that there is an inverse association between serotonin antagonist medication usage and mortality in severe COVID-19 mortality [669].
Autoimmunity
Autoimmunity is of course a chief feature of rheumatoid arthritis, and the details are in principle reasonably well understood [14,102–105,670]. It seems that autoimmunity is also a significant contributor to the after-effects of many viral infections [671], including Long COVID [672,673]. The microclots entrap a great many proteins [88], and of course any protein whose conformation is changed may present a new epitope that appears as ‘non-self’ and thus elicits autoantibodies. In addition, the nitration of proteins — as occurs during and following oxidative stress — also leads to the production of autoantibodies [674] and autoantibodies for actin lead to muscle weakness [587]. Autoantibodies are a well-known element in ME/CFS [20,98,548,675,676], and we consider they are likely to play an increasing role as the duration of Long COVID extends, since various autoantibodies share elements of epitope with the SARS-CoV-2 virus [677], and some of the viral sequences are in fact amyloidogenic themselves [678,679]. Since there is significant evidence for viral persistence during Long COVID (e.g. [680], and below), if they and autoantibodies also possess the ability to stimulate fibrin amyloid microclots, this provides one straightforward mechanism for continuing microclot persistence.
A gut microbiome reservoir for SARS-CoV-2?
In some cases, we are aware that there seems to be a continuation of Long COVID symptoms in spite of all kinds of treatments, and one explanation involves a unusual extent of viral persistence [681], for which there is increasing evidence (e.g. [19,682]). To this end, the suggestion of Brogna et al. [683] that SARS-CoV-2 could act like a bacteriophage and use bacteria as replication hosts is of especial interest. The gut microbiome does of course contain many trillions of organisms [684,685], contributes massively to immunity and inflammation [686], provides a clear pathway between diet and health [687], and would be the most logical place for such a reservoir to persist. The composition of the microbiome is also highly influenced by all kinds of drugs (including [688] but far beyond antibiotics [689–691]). Certainly, the gut and oral [692] microbiomes are highly dysregulated in both acute [693,694] and Long COVID [19,695,696], though the extent to which susceptibility to the disease is a cause or a consequence or both (or involving attendant medications) is largely unclear [697]. This said, one study [695] showed clear predictability between the microbiome and the nature and likelihood of PASC symptoms, while another showed it tracked recovery [698]. Overall it would be astonishing if improvements in the gut microbiome were not accompanied by improvements in the symptoms of PASC, and that certain unfavourable organisms might serve as intermediate hosts for viral replication in the gut. If this is the case, a reset using antibiotics followed by pre- and pro-biotics would seem to be the correct strategy. Persistence through cell–cell viral transmission without release [699] may also occur.
Candidate treatments: drugs
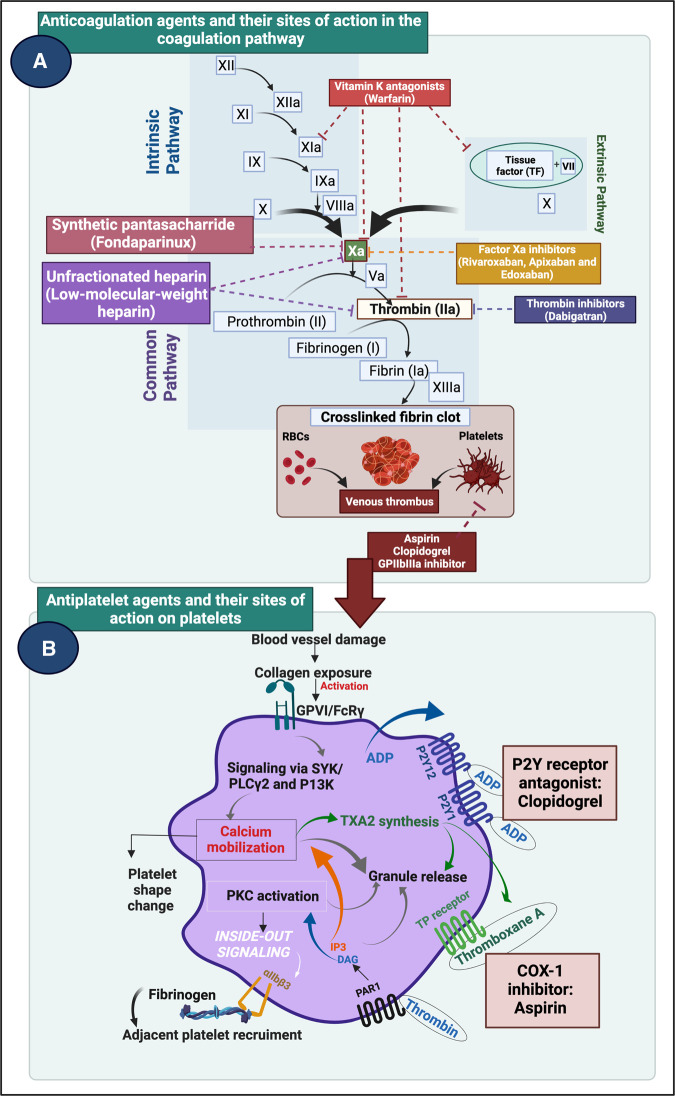
Based on what we have seen above and now know, it is reasonable to rehearse known pharmaceutical drugs [548] for which there is evidence of benefit for those in various stages of ME/CFS or Long COVID. Of course, the efficacy in treating rheumatoid arthritis of various small molecules (‘DMARDs’, which were in fact originally isolated as antibiotics) and anti-inflammatory molecules such as antibodies against TNF-α [700,701], is very well known, giving credence to the importance of such kinds of processes in Long COVID. Antiplatelet and anticoagulation medication may work on different parts of the clotting cascade by blocking platelet activation or by preventing new clots from forming by blocking the enzymatic pathway [86,702–704]. See Figure 12 that shows selected direct oral anticoagulants (DOAC) and dual antiplatelet therapy (DAPT) medication on clotting and platelet function.
Figure 12. Effects of selected direct oral anticoagulants (DOAC) and dual antiplatelet therapy (DAPT) medication on clotting and platelet function.
Created with BioRender (https://biorender.com/).
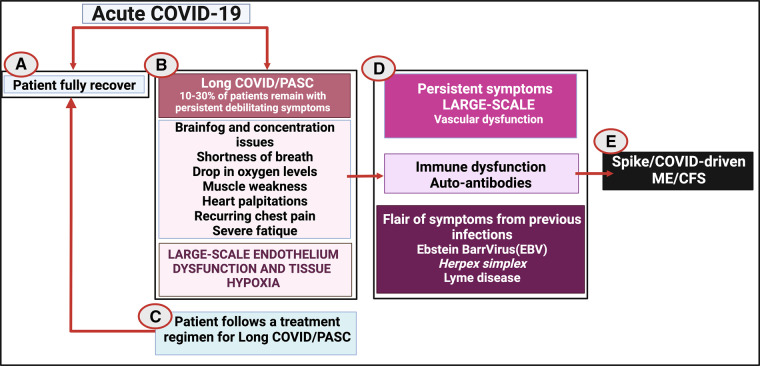
If it is confirmed that individuals with Long COVID do indeed have platelet hyperactivation and microclot presence in their circulation, and these pathologies are not sufficiently treated, we hypothesise that a few scenarios might then develop (see Figure 13):
Figure 13. Possible Long COVID disease progression if left untreated.
Disease/recovery progression developing the acute disease to (A) full recovery; (B) developing Long COVID/PASC; (C) treatment for Long COVID/PASC and subsequent recovery; (D) no treatment or positive treatment outcomes and the eventual development of spike/COVID-driven ME/CFS. Created with BioRender (https://biorender.com/).
Patients recover spontaneously where their fibrinolytic system returns to healthy clotting and lysis cycles.
Patients do not spontaneously recover, but instead develop a persistent hypercoagulable state with the persistent triggering of hyperactivated platelets and persistent endotheliitis, that may lead to more widespread endothelial damage.
Microclots continuously entrap inflammatory molecules and will eventually cause immune dysfunction and even autoimmunity.
Some individuals, who previously might have suffered from EBV, Herpes simplex virus or Lyme disease might suffer from a flare of those original symptoms, caused by reinfection of even by the vaccine.
In some individuals the persistent microclots, and widespread endothelial pathology may culminate in eventually COVID triggering ‘spike/COVID-driven ME/CFS’.
Antivirals
Given that viruses tend to persist (whether in dormant or more active forms) [680], antivirals seem like a logical component of any therapy for ME/CFS [705], though the evidence of strong benefits of any individual drug is still weak [706]. As with any complex system, it is likely that multiple targets will need to be modulated simultaneously [548]. In the case of Long COVID, it is still too early to know what benefits, if any, will come from the deployment of antivirals (which are often quite toxic); the antiparasitic drug ivermectin was advocated as an antiviral in some quarters, but seems not to be effective [707].
Anticoagulants, platelet inhibitors, and the triple treatment
Heparin and Fondaparinux
Heparin is a well-known regulator of the coagulation cascade that is also a potent inhibitor of angiogenesis [708] (see Figure 12). Heparin, therefore, directly modulates the coagulation cascade and is an excellent anticoagulant in diseases where hypercoagulation is prevalent. There are two types of heparins are that are widely used in prophylactically and as treatment regimes: unfractionated heparin (UFH) and low molecular mass heparin (LMWH). UFH can bind to antithrombin (SERPINC1) via a pentasaccharide, catalysing the inactivation of thrombin and other clotting factors. UFH also binds endothelial cells, PF4, and platelets [709]. Antithrombin is an essential regulator of the coagulation cascade and of proteolytic activity, as it acts to inactivate several enzymes of the coagulation cascade. It is well-known to inhibit thrombin and multiple other coagulation factors e.g. FIXa, Xa, XIa, and XIIa. It acts in both the intrinsic and extrinsic pathways [710]. The Heparin of choice is currently LMWH, as it lacks the nonspecific binding affinities of UFH, and has more predictable pharmacokinetic and pharmacodynamic properties [709]. Fondaparinux (a synthetic heparin pentasaccharide with a sequence identical with that found in anticoagulant heparin) is a well-known antithrombotic agent for the prevention and treatment of VTE and in ischaemic heart disease without significant bleeding risk [711]. It has also been suggested that Fondaparinux should be used in the treatment of COVID-19 coagulopathies [712]. As well as its role as an anticoagulant in decreasing mortality with acute COVID-19 [713] it was recognised early in the piece [714] that heparin binds to the SARS-CoV-2 spike protein and can thus inhibit its entry into cells [715]. The usefulness of antithrombotics was also recently discussed in a paper that investigated outcomes of antithrombotic use in patients with atrial fibrillation who subsequently developed COVID-19 [716]. It was found that individuals that were on antithrombotic therapies before they developed COVID-19 were less likely to die from the infection.
In 2020, Viecca and co-workers reported on a single-centre, investigator initiated, proof of concept, case-control study, conducted in Italy. Specifically, the effects of antiplatelet therapy on arterial oxygenation and clinical outcomes in patients with severe COVID-19 with hypercoagulability were investigated in a phase IIb trial (NCT04368377) [717]. Patients received 25 μg/kg body weight tirofiban as bolus infusion, followed by a continuous infusion of 0.15 μg/kg body weight per minute for 48 h. Before tirofiban, patients received acetylsalicylic acid 250 mg infusion and oral clopidogrel 300 mg; both were continued at a dose of 75 mg daily for 30 days. Fondaparinux 2.5 mg/day sub-cutaneous was given for the duration of the hospital stay. The study found that antiplatelet therapy might be effective in improving the ventilation and perfusion ratio in COVID-19 patients with severe respiratory failure and that the therapy prevented clot formation in lung capillary vessels. A recent 2022 JAMA paper [718] discussed the outcome of a trial [719] where moderately ill hospitalised patients with COVID-19, who were given the P2Y12 inhibitor Ticagrelor (in addition to therapeutic doses of heparin) did not improve their health outcome. Data were compared with a group of patients who received only a therapeutic dose of heparin.
Biologics as anti-inflammatories
Inflammation is strongly associated with (and virtually defined as) the production of inflammatory cytokines such as IL-6 and TNF-α (see Figure 9). Any means of lowering either the cause or such effects of inflammation is likely to be beneficial, and so it has proven, with the anti-TNF-α antibodies such as adalimumab (Humira), etanercept (Enbrel), and infliximab (Remicade) demonstrating huge benefits against rheumatoid arthritis, as well as other automimmune diseases. As biosimilars begin to come in (e.g. [720–722]), the substantial costs of the originals [723] may be expected to fall considerably. While there are grounds for optimism that these drugs can assist in the treatment of both acute [724–726] and Long COVID [727], it is too early yet to have the numbers to tell if they will [728]. However, one might have expected them to have been trialled more often in patients with ME/CFS [729], where a variety of inflammatory cytokine levels are also raised [730–732]. Consequently, we consider that such drugs might have considerable benefits in the treatment of both ME/CFS and Long COVID. We note in particular that RA patients taking anti-TNF-α therapies experienced major improvements in their fatigue symptoms [733].
Other drugs
Colchicine has long been used in various inflammatory diseases, and has shown promise in acute COVID-19 [734]. Other drugs being studied for use in ME/CFS or Long COVID include metformin [735–737], fenofibrate (which is somewhat protective against reperfusion injury [738,739]), and low-dose naltrexone [740–742]. While we are no experts, and the mechanisms are normally not well understood [743], but as a segue to the following section on nutraceuticals, we also note the effective use of certain traditional Chinese and other traditional medicines in acute COVID-19 [744–749] and their potential for use in Long COVID [748–750]. This seems like an area well worth further study by those qualified to do so.
Candidate treatments: nutraceuticals
Given the mismatch between the time taken to get a new drug approved and the urgency of the long COVID pandemic, both the literature and social media have turned to the use of nutraceuticals [751–753], at least for treating the symptoms of ME/CFS, Long COVID, and related disorders. It is inconceivable [754] that any single one will work for all individuals, but we consider it worthwhile to rehearse the kinds of nutraceuticals and less mainstream approaches that people have tried in the past for chronic, inflammatory diseases, particularly those whose efficacy may provide evidence the role of ischaemia and I–R injury. This allows us to assess them within the framework of the significance of microclots and oxidative stress caused by chronic ischaemia–reperfusion events that we consider to be a substantial part of these syndromes. Such agents include anti-clotting agents, iron chelators, and antioxidants more generally [3,540,545,755–758] (although the latter two are heavily bound up with each other [106]).
N-acetyl cysteine
N-acetyl cysteine is a well established and widely used antioxidant and anti-inflammatory molecule [759–762], which acts both to increase the intracellular levels of glutathione and to decreases the downstream activities of NF-κB. It has shown benefits in rheumatoid arthritis [763–765], in various viral diseases [766], and in abating the cytokine storm in acute COVID [767,768].
Curcumin
Curcumin is a polyphenol antioxidant and the active constituent (and main colouring agent) of the spice turmeric. It has shown benefits in a variety of diseases involving oxidative stress [542,769–774], including ME/CFS [234,775], rheumatoid arthritis [776–780], and acute COVID-19 [781–783]. While curcumin is a well-established antioxidant, it should be noted that it also may have antiplatelet activities [784–786]. In consequence, it is contra-indicated (i.e. not recommended for) use with other anticoagulants or blood thinners.
Ergothioneine
Ergothioneine is a major antioxidant [787–794] considered sufficiently important to the host during evolution that a natural transporter (SLC22A4 in humans) has been selected to ensure its uptake [795–797]. As we originally proposed [798], its anti-inflammatory potency has been established in a model of pre-eclampsia [799]. It is strongly protective against diseases of oxidative stress affecting the heart [793,800], liver [801,802], kidney [802], CNS [803,804], and other tissues (e.g. [794,805]. Consequently, it has been proposed as a suitable antioxidant for use in COVID-19 amelioration [806]. Most pertinently to the present analysis, it has been shown to be of benefit in preventing ischaemia–reperfusion injury [794,801,807], and so could have real value in chronic diseases that exhibit it. It is not easily obtained in pure form (though biotechnological processes are starting to make it [808–817]), but its availability via mushrooms can provide a convenient supply [818–820]. Indeed mushrooms themselves have been shown to be highly protective against mild cognitive impairment [821], as well as other diseases involving oxidative stress [787], and are themselves under consideration and trial as anti-COVID-19 agents [822,823].
Flavonoids
Flavonoids of various kinds, often referred to as polyphenols [824], are widely recognised as antioxidants with the potential in ameliorating inflammatory diseases involving oxidative stress (e.g. [606,825–832]). Since much of their bioavailability depends on suitable transporters [827,833] it would seem wise to use a cocktail. They have shown benefits in rheumatoid arthritis [778,826,834–842], in ME/CFS [843–845], and even activity against coronaviruses [846] such as the SARS-CoV-2 responsible for acute COVID-19 [847–858] and Long COVID [859].
Iron chelation
As noted above, and reviewed e.g. in [15,106], free iron can contribute massively to oxidative stress. Ferritin is the main intracellular storage molecule for iron, and serum ferritin is a marker of cell death [149]; it is, therefore, unsurprising that it is raised massively in acute SARS-CoV-2 infection, and especially so in non-survivors [512,860–866]. Consequently, molecules that chelate iron fully (i.e. via all six of its chelation sites, see above) can serve to relieve oxidative stress [862,865,867–870] and inhibit SARS-CoV-2 effects [871]. Other nutraceutical iron chelators such as green tea catechins (epigallocatechin-3-gallate, also a polyphenol) [872–881] were discussed in detail previously [106].
Lactoferrin
As commented in the previous section, iron dysregulation is another important element of all these chronic, inflammatory diseases, both through its behaviour in catalysing ROS formation [106,266] and hypercoagulation [326], and its ability to awaken dormant microbes [15]. Since it binds iron effectively, as well as various cell surface receptors used by SARS-CoV-2, oral lactoferrin has been proposed as a suitable treatment for (and indeed preventive of) COVID-19 [372,868,869,882–894].
Magnesium
Although blood levels of magnesium ions are more-or-less tightly regulated, ‘magnesium’ was experimentally one of the earliest substances that we found to inhibit fibrin amyloid microclotting (then known as dense matted deposit formation) [374]. Intriguingly, populations exhibiting low magnesium ion levels were found to be more susceptible to COVID-19 [895], and magnesium supplementation has shown benefits in SARS-CoV-2 therapy [896], ME/CFS [93,897], and in maintaining endothelial cell function [898].
Melatonin
Melatonin is a natural small molecule (a tryptophan derivative) produced in the pineal gland and involved in the induction of sleep [899]; it has been strongly promoted for its anti-oxidative and anti-nitrosative properties [900–902], including in COVID [903–911], and I–R injury [912], and is a useful ligand for free iron [106].
Thrombolytics: nattokinase, serrapeptase, lumbrokinase, and bromleain
Clots are normally removed by plasmin, a serine protease, but some clots (such as fibrin amyloid microclots) contain antiplasmin compounds [88]; the plasma of acute COVID patients also contains anti-thrombolytic compounds [913–915], despite raised levels of tissue plasminogen activator (tPA) [915]. However, a variety of other enzymes have thrombolytic activity [916–919]. Considered less potent and safer than post-stroke ‘clotbusters’ such as tPA [920], nattokinase is a fibrinolytic [921–923] (and amyloid-degrading [924]), orally available despite having to pass through the gut wall [925–931], safe [932,933], serine protease enzyme from Bacillus subtilis (an organism which may itself be of value [934]). It is found naturally in the Japanese fermented food nattō [751,928,935–939], (which is also a source of vitamin K, and has antiviral properties directly [940]). Its structure is known [941,942], and it may also be produced recombinantly [943–950]. It also has antiplatelet [951], anti-inflammatory [952], and anti-hypertensive [953] properties, and along with pycnogenol [954] was active in preventing DVT on longhaul flights [955]. Serrapeptase (serratiopeptidase) [934,956–959] has a similar activity (as well as others such as mucolytic behaviour [958]) and comes from a Serratia marcescens strain that originates in the guts of silkworms, where it has a natural role in helping the worms emerge from their cocoons. Lumbrokinases are another orally active [960] set of fibrinolytic enzymes that have been found in earthworms [961–965], and may also be produced recombinantly [966,967]; they may also have some tPA activity [965]. Each has been proposed as of value in acute and/or Long COVID treatment [751,958,968]. With two (positive) exceptions [968,969], and another planned [970], randomised controlled trials are awaited. In the plant kingdom, bromelain (from pineapples) is a cysteine protease found in pineapple tissue [971–974]. Multiple effects imply its utility in preventing or treating SARS-CoV-2 infection and acute COVID-19 [975–978]. Given the significance of fibrin amyloid microclots; however, it would seem of value (i) to stress the importance of quality control in nutraceutical production and (ii) to assess the comparative activities of these enzymes in removing fibrin amyloid microclots in vitro.
Vitamins
There is little doubt that many individuals may be deficient in their dietary supply of one or more vitamins [979]. In particular, there is evidence for the importance of vitamins B12 [980–983], D3 [906,983–986], and K2 [987–990] in benefitting outcome from acute COVID. However, in some cases where ‘vitamin D’ is not explicitly measured as D3 the evidence is equivocal [991–993]. As mentioned above, vitamin C [994] is to be recommended only if one is sure that free or poorly liganded iron is absent. Niacin (vitamin B3) may also be of value in a tapered-up strategy, since NAD+ levels are known to be lowered in COVID-19 [995] and other viral infections [996]; its metabolite 1-methylnicotinamide has also shown promise in Long COVID [997].
Overall, there is a considerable body of evidence that nutraceuticals active as antioxidants, iron chelators, or fibrinolytics, as well as other targets, may be of benefit in the sets of diseases under consideration here, consistent with the role of ischaemia–reperfusion injury therein.
Other, non-pharmacological methods
H.E.L.P.: apheresis
Apheresis refers to the specific extracorporeal removal of particular substances from blood, such as lipids [998,999]. Originally introduced for the lowering of low-density lipoproteins (LDLs) (e.g. [1000,1001]), Heparin-mediated LDL precipitation (H.E.L.P.) apheresis has come to the fore as a means for assisting Long COVID patients, since it too seems to remove fibrin amyloid microclots with high efficiency. Alternative aphereses [1002,1003] also be of value.
Hyperbaric oxygen therapy
If ischaemia is an important part of Long COVID, then preventing it (while not mimicking reperfusion) should be of value [1004,1005]. To this end, hyperbaric oxygen therapy [1006–1008] has been recommended, and in some cases found useful in rheumatoid arthritis [1009–1011], acute [1012,1013] and Long COVID [1006,1014]. An alternative involves O2 nanobubbles [1015].
Concluding remarks
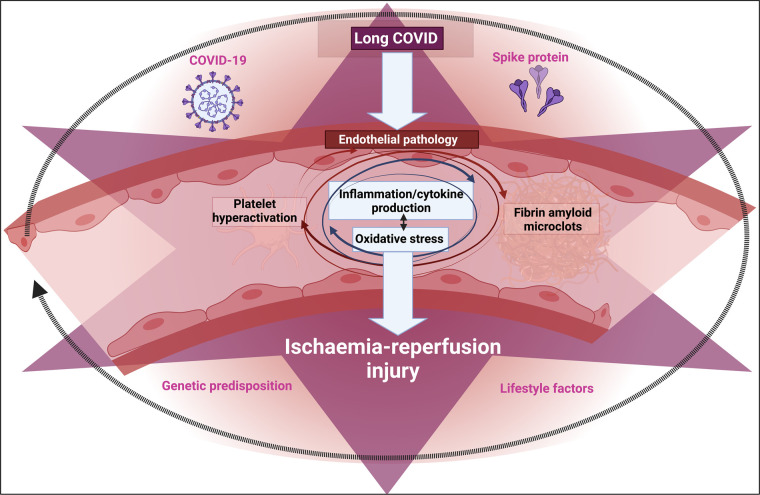
We have sought to bring together what is the very considerable evidence that many of the features of Long COVID resemble those observed in ME/CFS, and to some degree those in rheumatoid arthritis, and that a common denominator may be fibrin amyloid microclots and damaged cell structures that block up capillaries and can consequently lead to oxidative stress and ischaemia–reperfusion injury (Figure 14).
Figure 14. Some of the major elements of Long COVID and related diseases that we have highlighted here.
They illustrate the complexity of ischaemia–reperfusion injury driven by platelet hyperactivation, fibrin amyloid microclots, circulating inflammatory molecules/cytokine production. Created with BioRender (https://biorender.com/).
Many of the predicted sequelae have indeed been observed, and thus provide evidence for this general mechanism of chronic illness. If the analysis is correct it implies that considerable therapeutic benefits are to be had from strategies that inhibit the formation of the microclots and that act — especially the use of antioxidants — to diminish the effects of ROS by mopping them up. A variety of further predictions remain to be tested, but we hope that we have set them out clearly enough to enable others to do so.
Consent for publication
All authors approved the submission of the paper.
Acknowledgements
D.B.K. thanks Rachel Stark for a useful conversation. D.B.K. thanks the Novo Nordisk Foundation for financial support (grant no. NNF20CC0035580). E.P. thanks the South Africa MRC (SIR grant 2022–2024) and the NRF from South Africa: Competitive Programme for Rated Researchers (grant no. 142142) for grant support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the discussants at the twitter hashtag #TeamClots for sharing many useful insights.
Abbreviations
- CR1
complement receptor 1
- DVT
deep vein thrombosis
- EBV
Epstein–Barr virus
- ESR
erythrocyte sedimentation rates
- I–R
ischaemia–reperfusion
- LDLs
low-density lipoproteins
- LMWH
low molecular mass heparin
- PE
pulmonary embolism
- PEM
post-exertional malaise
- PF4
platelet factor 4
- RDW
red cell distribution width
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances
- tPA
tissue plasminogen activator
- UFH
unfractionated heparin
- VTE
venous thromboembolism
- VWF
von Willebrand factor
Competing Interests
E.P. is the managing director of BioCODE Technologies. D.B.K. holds shares in PhenUTest Ltd. These companies are developing or contemplating developing diagnostics for Long COVID.
CRediT Author Contribution
Douglas Kell: Conceptualization, Funding acquisition, Writing — original draft, Writing — review and editing. Etheresia Pretorius: Conceptualization, Funding acquisition, Writing — original draft, Writing — review and editing.
References
- 1.Wu, M.Y., Yiang, G.T., Liao, W.T., Tsai, A.P., Cheng, Y.L., Cheng, P.W.et al. (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 46, 1650–1667 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy, D.J. and Yellon, D.M. (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100 10.1172/JCI62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daiber, A., Andreadou, I., Oelze, M., Davidson, S.M. and Hausenloy, D.J. (2021) Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Rad. Biol. Med. 163, 325–343 10.1016/j.freeradbiomed.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 4.Murphy, E. and Steenbergen, C. (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 88, 581–609 10.1152/physrev.00024.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enzmann, G., Kargaran, S. and Engelhardt, B. (2018) Ischemia-reperfusion injury in stroke: impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 11, 1756286418794184 10.1177/1756286418794184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu, M., Gu, X. and Ma, Z. (2021) Mitochondrial quality control in cerebral ischemia-reperfusion injury. Mol. Neurobiol. 58, 5253–5271 10.1007/s12035-021-02494-8 [DOI] [PubMed] [Google Scholar]
- 7.Naito, H., Nojima, T., Fujisaki, N., Tsukahara, K., Yamamoto, H., Yamada, T.et al. (2020) Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 7, e501 10.1002/ams2.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aigner, F., Maier, H.T., Schwelberger, H.G., Wallnofer, E.A., Amberger, A., Obrist, P.et al. (2007) Lipocalin-2 regulates the inflammatory response during ischemia and reperfusion of the transplanted heart. Am. J. Transplant. 7, 779–788 10.1111/j.1600-6143.2006.01723.x [DOI] [PubMed] [Google Scholar]
- 9.Jaeschke, H. and Woolbright, B.L. (2012) Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant. Rev. 26, 103–114 10.1016/j.trre.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Situmorang, G.R. and Sheerin, N.S. (2019) Ischaemia reperfusion injury: mechanisms of progression to chronic graft dysfunction. Pediatr. Nephrol. 34, 951–963 10.1007/s00467-018-3940-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai, Y., Petrowsky, H., Hong, J.C., Busuttil, R.W. and Kupiec-Weglinski, J.W. (2013) Ischaemia–reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 10, 79–89 10.1038/nrgastro.2012.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouchani, E.T., Pell, V.R., Gaude, E., Aksentijevic, D., Sundier, S.Y., Robb, E.L.et al. (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger, D.N. and Kvietys, P.R. (2015) Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 6, 524–551 10.1016/j.redox.2015.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretorius, E., Akeredolu, O.-O., Soma, P. and Kell, D.B. (2017) Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp. Biol. Med. 242, 355–373 10.1177/1535370216681549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kell, D.B. and Pretorius, E. (2018) No effects without causes. The iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. 93, 1518–1557 10.1111/brv.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasa, S., Nora-Krukle, Z., Henning, N., Eliassen, E., Shikova, E., Harrer, T.et al. (2018) Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J. Transl. Med. 16, 268 10.1186/s12967-018-1644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalbandian, A., Sehgal, K., Gupta, A., Madhavan, M.V., McGroder, C., Stevens, J.S.et al. (2021) Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komaroff, A.L. and Lipkin, W.I. (2021) Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 27, 895–906 10.1016/j.molmed.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proal, A.D. and VanElzakker, M.B. (2021) Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 12, 698169 10.3389/fmicb.2021.698169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sukocheva, O.A., Maksoud, R., Beeraka, N.M., Madhunapantula, S.V., Sinelnikov, M., Nikolenko, V.N.et al. (2022) Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J. Adv. Res. Still in press. 10.1016/j.jare.2021.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Campen, C.L.M.C., Rowe, P.C. and Visser, F.C. (2021) Orthostatic symptoms and reductions in cerebral blood flow in long-Haul COVID-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina (Kaunas) 58, 28 10.3390/medicina58010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong, T.L. and Weitzer, D.J. (2021) Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas) 57, 418 10.3390/medicina57050418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sapkota, H.R. and Nune, A. (2022) Long COVID from rheumatology perspective - a narrative review. Clin. Rheumatol. 41, 337–348 10.1007/s10067-021-06001-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agergaard, J., Leth, S., Pedersen, T.H., Harbo, T., Blicher, J.U., Karlsson, P.et al. (2021) Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 132, 1974–1981 10.1016/j.clinph.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbarialiabad, H., Taghrir, M.H., Abdollahi, A., Ghahramani, N., Kumar, M., Paydar, S.et al. (2021) Long COVID, a comprehensive systematic scoping review. Infection 49, 1163–1186 10.1007/s15010-021-01666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis, H.E., Assaf, G.S., McCorkell, L., Wei, H., Low, R.J., Re'em, Y.et al. (2021) Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshee, S., Vatti, N. and Chang, C. (2022) Long-term effects of COVID-19. Mayo Clin. Proc. 97, 579–599 10.1016/j.mayocp.2021.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladds, E., Rushforth, A., Wieringa, S., Taylor, S., Rayner, C., Husain, L.et al. (2020) Persistent symptoms after COVID-19: qualitative study of 114 "long COVID" patients and draft quality principles for services. BMC Health Serv. Res. 20, 1144 10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nacul, L., O'Boyle, S., Palla, L., Nacul, F.E., Mudie, K., Kingdon, C.C.et al. (2020) How myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) progresses: the natural history of ME/CFS. Front. Neurol. 11, 826 10.3389/fneur.2020.00826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raveendran, A.V., Jayadevan, R. and Sashidharan, S. (2021) Long COVID: an overview. Diabetes Metab. Syndr. 15, 869–875 10.1016/j.dsx.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twomey, R., DeMars, J., Franklin, K., Culos-Reed, S.N., Weatherald, J. and Wrightson, J.G. (2021) Chronic fatigue and post-exertional malaise in people living with long COVID. medRxiv 2021.2006.2011.21258564 [Google Scholar]
- 32.Vehar, S., Boushra, M., Ntiamoah, P. and Biehl, M. (2021) Post-acute sequelae of SARS-CoV-2 infection: caring for the 'long-haulers'. Cleve Clin. J. Med. 88, 267–272 10.3949/ccjm.88a.21010 [DOI] [PubMed] [Google Scholar]
- 33.Walitt, B. and Bartrum, E. (2021) A clinical primer for the expected and potential post-COVID-19 syndromes. Pain Rep. 6, e887 10.1097/PR9.0000000000000887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yong, S.J. and Liu, S. (2021) Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 32, e2315 10.1002/rmv.2315 [DOI] [PubMed] [Google Scholar]
- 35.Ceban, F., Ling, S., Lui, L.M.W., Lee, Y., Gill, H., Teopiz, K.M.et al. (2022) Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown, D.A. and O'Brien, K.K. (2021) Conceptualising long COVID as an episodic health condition. BMJ Glob. Health 6, e007004 10.1136/bmjgh-2021-007004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien, K.K., Brown, D.A., Bergin, C., Erlandson, K.M., Vera, J.H., Avery, L.et al. (2022) Long COVID and episodic disability: advancing the conceptualisation, measurement and knowledge of episodic disability among people living with long COVID - protocol for a mixed-methods study. BMJ Open 12, e060826 10.1136/bmjopen-2022-060826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nune, A., Durkowski, V., Titman, A., Gupta, L., Hadzhiivanov, M., Ahmed, A.et al. (2021) Incidence and risk factors of long COVID in the UK: a single-centre observational study. J. R. Coll. Physicians Edinb. 51, 338–343 10.4997/jrcpe.2021.405 [DOI] [PubMed] [Google Scholar]
- 39.Salmon-Ceron, D., Slama, D., De Broucker, T., Karmochkine, M., Pavie, J., Sorbets, E.et al. (2021) Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J. Infect. 82, e1–e4 10.1016/j.jinf.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart, S., Newson, L., Briggs, T.A., Grammatopoulos, D., Young, L. and Gill, P. (2021) Long COVID risk - a signal to address sex hormones and women's health. Lancet Reg. Health Eur. 11, 100242 10.1016/j.lanepe.2021.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortona, E., Buonsenso, D., Carfi, A., Malorni, W. and Long COVID kids study group (2021) Long COVID: an estrogen-associated autoimmune disease? Cell Death Discov. 7, 77 10.1038/s41420-021-00464-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortona, E. and Malorni, W. (2022) Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur. Respir. J. 59, 2102245 10.1183/13993003.02245-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bateman, L., Bested, A.C., Bonilla, H.F., Chheda, B.V., Chu, L., Curtin, J.M.et al. (2021) Myalgic encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin. Proc. 96, 2861–2878 10.1016/j.mayocp.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 44.Carruthers, B.M., van de Sande, M.I., De Meirleir, K.L., Klimas, N.G., Broderick, G., Mitchell, T.et al. (2011) Myalgic encephalomyelitis: international consensus criteria. J. Internsive Med. 270, 327–338 10.1111/j.1365-2796.2011.02428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green, M.A. and Shearman, C.P. (1994) Reperfusion injury in peripheral vascular disease. Vasc. Med. Rev. 5, 97–106 10.1177/1358863X9400500202 [DOI] [Google Scholar]
- 46.Chen, C., Haupert, S.R., Zimmermann, L., Shi, X., Fritsche, L.G. and Mukherjee, B. (2021) Global prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID: a meta-analysis and systematic review. medRxiv 2021.2011.2015.21266377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gold, J.E., Okyay, R.A., Licht, W.E. and Hurley, D.J. (2021) Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens 10, 763 10.3390/pathogens10060763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall, M. (2021) The four most urgent questions about long COVID. Nature 594, 168–170 10.1038/d41586-021-01511-z [DOI] [PubMed] [Google Scholar]
- 49.Arjun, M.C., Singh, A.K., Pal, D., Das, K., Gajjala, A., Venkateshan, M.et al. (2022) Prevalence, characteristics, and predictors of long COVID among diagnosed cases of COVID-19. medRxiv 2022.2001.2004.21268536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taquet, M., Dercon, Q., Luciano, S., Geddes, J.R., Husain, M. and Harrison, P.J. (2021) Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 18, e1003773 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yomogida, K., Zhu, S., Rubino, F., Figueroa, W., Balanji, N. and Holman, E. (2021) Post-acute sequelae of SARS-CoV-2 infection among adults aged ≥8 years - long beach, California, April 1-December 10, 2020. MMWR Morb. Mortal. Wkly Rep. 70, 1274–1277 10.15585/mmwr.mm7037a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitaker, M., Elliott, J., Chadeau-Hyam, M., Riley, S., Darzi, A., Cooke, G.et al. (2022) Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat. Commun. 13, 1957 10.1038/s41467-022-29521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen, C., Haupert, S.R., Zimmermann, L., Shi, X., Fritsche, L.G. and Mukherjee, B. (2022) Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J. Infect. Dis. jiac136 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasserie, T., Hittle, M. and Goodman, S.N. (2021) Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw. Open. 4, e2111417 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reese, J., Blau, H., Bergquist, T., Loomba, J.J., Callahan, T., Laraway, B.et al. (2022) Generalizable long COVID subtypes: findings from the NIH N3C and RECOVER programs. medRxiv 2022.2005.2024.22275398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayoubkhani, D., Bermingham, C., Pouwels, K.B., Glickman, M., Nafilyan, V., Zaccardi, F.et al. (2021) Changes in the trajectory of long COVID symptoms following COVID-19 vaccination: community-based cohort study. medRxiv 2021.2012.2009.21267516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caspersen, I.H., Magnus, P. and Trogstad, L. (2021) Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. medRxiv 2021.2010.2015.21265038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munblit, D., Nicholson, T.R., Needham, D.M., Seylanova, N., Parr, C., Chen, J.et al. (2022) Studying the post-COVID-19 condition: research challenges, strategies, and importance of core outcome Set development. BMC Med. 20, 50 10.1186/s12916-021-02222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desai, A.D., Lavelle, M., Boursiquot, B.C. and Wan, E.Y. (2022) Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 322, C1–C11 10.1152/ajpcell.00375.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie, Y. and Al-Aly, Z. (2022) Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 10, 311–321 10.1016/S2213-8587(22)00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie, Y., Xu, E., Bowe, B. and Al-Aly, Z. (2022) Long-term cardiovascular outcomes of COVID-19. Nat. Med. 28, 583–590 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martínez-Salazar, B., Holwerda, M., Studle, C., Piragyte, I., Mercader, N., Engelhardt, B.et al. (2022) COVID-19 and the vasculature: current aspects and long-term consequences. Front. Cell Dev. Biol. 10, 824851 10.3389/fcell.2022.824851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graham, E.L., Clark, J.R., Orban, Z.S., Lim, P.H., Szymanski, A.L., Taylor, C.et al. (2021) Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 "long haulers". Ann. Clin. Transl. Neurol. 8, 1073–1085 10.1002/acn3.51350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deer, R.R., Rock, M.A., Vasilevsky, N., Carmody, L., Rando, H., Anzalone, A.J.et al. (2021) Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine 74, 103722 10.1016/j.ebiom.2021.103722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michelen, M., Manoharan, L., Elkheir, N., Cheng, V., Dagens, A., Hastie, C.et al. (2021) Characterising long COVID: a living systematic review. BMJ Glob. Health 6, e005427 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bell, M.L., Catalfamo, C.J., Farland, L.V., Ernst, K.C., Jacobs, E.T., Klimentidis, Y.C.et al. (2021) Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS One 16, e0254347 10.1371/journal.pone.0254347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blomberg, B., Mohn, K.G., Brokstad, K.A., Zhou, F., Linchausen, D.W., Hansen, B.A.et al. (2021) Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 27, 1607–1613 10.1038/s41591-021-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Logue, J.K., Franko, N.M., McCulloch, D.J., McDonald, D., Magedson, A., Wolf, C.R.et al. (2021) Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open. 4, e210830 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sudre, C.H., Murray, B., Varsavsky, T., Graham, M.S., Penfold, R.S., Bowyer, R.C.et al. (2021) Attributes and predictors of long COVID. Nat. Med. 27, 626–631 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandal, S., Barnett, J., Brill, S.E., Brown, J.S., Denneny, E.K., Hare, S.S.et al. (2021) 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 76, 396–398 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nurek, M., Rayner, C., Freyer, A., Taylor, S., Jarte, L., MacDermott, N.et al. (2021) Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br. J. Gen. Pract. 71, e815–e825 10.3399/BJGP.2021.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sigfrid, L., Drake, T.M., Pauley, E., Jesudason, E.C., Olliaro, P., Lim, W.S.et al. (2021) Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg. Health Eur. 8, 100186 10.1016/j.lanepe.2021.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenhalgh, T., Knight, M., A'Court, C., Buxton, M. and Husain, L. (2020) Management of post-acute COVID-19 in primary care. BMJ 370, m3026 10.1136/bmj.m3026 [DOI] [PubMed] [Google Scholar]
- 74.Rando, H.M., Bennett, T.D., Byrd, J.B., Bramante, C., Callahan, T.J., Chute, C.G.et al. (2021) Challenges in defining Long COVID: striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv 10.1101/2021.1103.1120.21253896 [DOI] [Google Scholar]
- 75.Tereshchenko, L.G., Bishop, A., Fisher-Campbell, N., Levene, J., Morris, C.C., Patel, H.et al. (2021) Risk of cardiovascular events after COVID-19: a double-cohort study. medRxiv 2021.2012.2027.21268448 [Google Scholar]
- 76.Asadi-Pooya, A.A., Akbari, A., Emami, A., Lotfi, M., Rostamihosseinkhani, M., Nemati, H.et al. (2021) Long COVID syndrome-associated brain fog. J. Med. Virol. 94, 979–984 10.1002/jmv.27404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefanou, M.I., Palaiodimou, L., Bakola, E., Smyrnis, N., Papadopoulou, M., Paraskevas, G.P.et al. (2022) Neurological manifestations of long-COVID syndrome: a narrative review. Ther. Adv. Chronic. Disord. 13, 20406223221076890 10.1177/20406223221076890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hampshire, A., Trender, W., Chamberlain, S.R., Jolly, A.E., Grant, J.E., Patrick, F.et al. (2021) Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 39, 101044 10.1016/j.eclinm.2021.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marshall, M. (2021) COVID and the brain: researchers zero in on how damage occurs. Nature 595, 484–485 10.1038/d41586-021-01693-6 [DOI] [PubMed] [Google Scholar]
- 80.Glynne, P., Tahmasebi, N., Gant, V. and Gupta, R. (2021) Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J. Investig. Med. 70, 61–67 10.1136/jim-2021-002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montes, N., Domènech, È, Guerrero, S., Oliván-Blázquez, B. and Magallón-Botaya, R. (2021) Analysis of cell-mediated immunity in people with long COVID. medRxiv 2021.2006.2009.21258553 [Google Scholar]
- 82.Grobler, C., Maphumulo, S.C., Grobbelaar, L.M., Bredenkamp, J., Laubscher, J., Lourens, P.J.et al. (2020) COVID-19: the rollercoaster of fibrin(ogen), D-dimer, von willebrand factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int. J. Mol. Sci. 21, 5168 10.3390/ijms21145168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katsoularis, I., Fonseca-Rodríguez, O., Farrington, P., Lindmark, K. and Fors Connolly, A.M. (2021) Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 398, 599–607 10.1016/S0140-6736(21)00896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grobbelaar, L.M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G.J., Lourens, P.J.et al. (2021) SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci. Rep. 41, BSR20210611 10.1042/BSR20210611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kell, D.B., Laubscher, G.J. and Pretorius, E. (2022) A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem. J. 479, 537–559 10.1042/BCJ20220016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laubscher, G.J., Lourens, P.J., Venter, C., Kell, D.B. and Pretorius, E. (2021) TEG®, microclot and platelet mapping for guiding early management of severe COVID-19 coagulopathy. J. Clin. Med. 10, 5381 10.3390/jcm10225381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pretorius, E., Venter, C., Laubscher, G.J., Lourens, P.J., Steenkamp, J. and Kell, D.B. (2020) Prevalence of readily detected amyloid blood clots in ‘unclotted’ type 2 diabetes mellitus and COVID-19 plasma: a preliminary report. Cardiovasc. Diabetol. 19, 193 10.1186/s12933-020-01165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J.A., Laubscher, G.J., Steenkamp, J.et al. (2021) Persistent clotting protein pathology in long COVID/ post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 20, 172 10.1186/s12933-021-01359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pretorius, E., Venter, C., Laubscher, G.J., Kotze, M.J., Oladejo, S., Watson, L.R.et al. (2022) Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Research Square 10.21203/rs.21203.rs-1205453/v1205452 [DOI] [PMC free article] [PubMed]
- 90.Kavi, L. (2022) Postural tachycardia syndrome and long COVID: an update. Br. J. Gen. Pract. 72, 8–9 10.3399/bjgp22X718037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Novak, P., Mukerji, S.S., Alabsi, H.S., Systrom, D., Marciano, S.P., Felsenstein, D.et al. (2022) Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann. Neurol. 91, 367–379 10.1002/ana.26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh, I., Joseph, P., Heerdt, P.M., Cullinan, M., Lutchmansingh, D.D., Gulati, M.et al. (2022) Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest 161, 54–63 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Afari, N. and Buchwald, D. (2003) Chronic fatigue syndrome: a review. Am. J. Psychiatry 160, 221–236 10.1176/appi.ajp.160.2.221 [DOI] [PubMed] [Google Scholar]
- 94.Lee, J.S., Kim, H.G., Lee, D.S. and Son, C.G. (2018) Oxidative stress is a convincing contributor to idiopathic chronic fatigue. Sci. Rep. 8, 12890 10.1038/s41598-018-31270-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mantovani, E., Mariotto, S., Gabbiani, D., Dorelli, G., Bozzetti, S., Federico, A.et al. (2021) Chronic fatigue syndrome: an emerging sequela in COVID-19 survivors? J. Neurovirol. 27, 631–637 10.1007/s13365-021-01002-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joseph, P., Arevalo, C., Oliveira, R.K.F., Faria-Urbina, M., Felsenstein, D., Oaklander, A.L.et al. (2021) Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest 160, 642–651 10.1016/j.chest.2021.01.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rowe, P.C., Underhill, R.A., Friedman, K.J., Gurwitt, A., Medow, M.S., Schwartz, M.S.et al. (2017) Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: a primer. Front. Pediatr. 5, 121 10.3389/fped.2017.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deumer, U.S., Varesi, A., Floris, V., Savioli, G., Mantovani, E., Lopez-Carrasco, P.et al. (2021) Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): an overview. J. Clin. Med 10, 4786 10.3390/jcm10204786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karaarslan, F., Demircioğlu Guneri, F. and Kardeş, S. (2022) long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin. Rheumatol. 41, 289–296 10.1007/s10067-021-05942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ebringer, A., Khalafpour, S. and Wilson, C. (1989) Rheumatoid arthritis and Proteus: a possible aetiological association. Rheumatol. Int. 9, 223–228 10.1007/BF00271885 [DOI] [PubMed] [Google Scholar]
- 101.Ebringer, A. and Wilson, C. (2000) HLA molecules, bacteria and autoimmunity. J. Med. Microbiol. 49, 305–311 10.1099/0022-1317-49-4-305 [DOI] [PubMed] [Google Scholar]
- 102.Ebringer, A. and Rashid, T. (2009) Rheumatoid arthritis is caused by Proteus: the molecular mimicry theory and Karl Popper. Front. Biosci. (Elite Ed.) 1, 577–586 10.2741/e56 [DOI] [PubMed] [Google Scholar]
- 103.Ebringer, A., Rashid, T. and Wilson, C. (2010) Rheumatoid arthritis, proteus, anti-CCP antibodies and Karl Popper. Autoimmun. Rev. 9, 216–223 10.1016/j.autrev.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 104.Ebringer, A. (2012) Rheumatoid arthritis and Proteus, Springer, London [Google Scholar]
- 105.Ebringer, A. and Rashid, T. (2014) Rheumatoid arthritis is caused by a Proteus urinary tract infection. APMIS 122, 363–368 10.1111/apm.12154 [DOI] [PubMed] [Google Scholar]
- 106.Kell, D.B. (2009) Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2, 2 10.1186/1755-8794-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hung, T.H., Skepper, J.N. and Burton, G.J. (2001) In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 159, 1031–1043 10.1016/S0002-9440(10)61778-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hung, T.H., Charnock-Jones, D.S., Skepper, J.N. and Burton, G.J. (2004) Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am. J. Pathol. 164, 1049–1061 10.1016/S0002-9440(10)63192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kell, D.B. and Kenny, L.C. (2016) A dormant microbial component in the development of pre-eclampsia. Front. Med. Obs. Gynecol. 3, 60 10.3389/fmed.2016.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kenny, L.C. and Kell, D.B. (2018) Immunological tolerance, pregnancy and pre-eclampsia: the roles of semen microbes and the father. Front. Med. Obs. Gynecol. 4, 239 10.3389/fmed.2017.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jayaram, A., Buhimschi, I.A., Aldasoqi, H., Hartwig, J., Owens, T., Elam, G.L.et al. (2021) Who said differentiating preeclampsia from COVID-19 infection was easy? Pregnancy Hypertens. 26, 8–10 10.1016/j.preghy.2021.07.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mendoza, M., Garcia-Ruiz, I., Maiz, N., Rodo, C., Garcia-Manau, P., Serrano, B.et al. (2020) Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG 127, 1374–1380 10.1111/1471-0528.16339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lanz, T.V., Brewer, R.C., Ho, P.P., Moon, J.S., Jude, K.M., Fernandez, D.et al. (2022) Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 603, 321–327 10.1038/s41586-022-04432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wekerle, H. (2022) Epstein-Barr virus sparks brain autoimmunity in multiple sclerosis. Nature 603, 230–232 10.1038/d41586-022-00382-2 [DOI] [PubMed] [Google Scholar]
- 115.Mullard, A. (2022) The quest to prevent MS - and understand other post-viral diseases. Nature 603, 784–786 10.1038/d41586-022-00808-x [DOI] [PubMed] [Google Scholar]
- 116.O'Hagan, S., Wright Muelas, M., Day, P.J., Lundberg, E. and Kell, D.B. (2018) Genegini: assessment via the Gini coefficient of reference ‘‘housekeeping’’ genes and diverse human transporter expression profiles. Cell Syst. 6, 230–244 10.1016/j.cels.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wright Muelas, M., Mughal, F., O'Hagan, S., Day, P.J. and Kell, D.B. (2019) The role and robustness of the Gini coefficient as an unbiased tool for the selection of Gini genes for normalising expression profiling data scientific reports. Sci. Rep. 9, 17960 10.1038/s41598-019-54288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deinhardt-Emmer, S., Wittschieber, D., Sanft, J., Kleemann, S., Elschner, S., Haupt, K.F.et al. (2021) Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and the correlation with tissue damage. eLife 10, e60361 10.7554/eLife.60361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jackson, C.B., Farzan, M., Chen, B. and Choe, H. (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23, 3–20 10.1038/s41580-021-00418-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baig, A.M., Khaleeq, A., Ali, U. and Syeda, H. (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 11, 995–998 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 121.Shen, X.R., Geng, R., Li, Q., Chen, Y., Li, S.F., Wang, Q.et al. (2022) ACE2-independent infection of T lymphocytes by SARS-CoV-2. Signal. Transduct. Target. Ther. 7, 83 10.1038/s41392-022-00919-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung, C.C.L., Goh, D., Lim, X., Tien, T.Z., Lim, J.C.T., Lee, J.N.et al. (2022) Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 71, 226–229 10.1136/gutjnl-2021-324280 [DOI] [PubMed] [Google Scholar]
- 123.Palsson, BØ. (2006) Systems biology: properties of reconstructed networks, Cambridge University Press, Cambridge [Google Scholar]
- 124.Klipp, E., Herwig, R., Kowald, A., Wierling, C. and Lehrach, H. (2005) Systems biology in practice: concepts, implementation and clinical application, Wiley/VCH, Berlin [Google Scholar]
- 125.Kell, D.B. and Knowles, J.D. (2006) The role of modeling in systems biology. In System Modeling In Cellular Biology: From Concepts to Nuts And Bolts (Szallasi, Z., Stelling, J. and Periwal, V., eds), pp. 3–18, MIT Press, Cambridge [Google Scholar]
- 126.Alon, U. (2006) An introduction to systems biology: design principles of biological circuits, Chapman and Hall/CRC, London [Google Scholar]
- 127.Babior, B.M. (2000) Phagocytes and oxidative stress. Am. J. Med. 109, 33–44 10.1016/S0002-9343(00)00481-2 [DOI] [PubMed] [Google Scholar]
- 128.Cave, A.C., Brewer, A.C., Narayanapanicker, A., Ray, R., Grieve, D.J., Walker, S.et al. (2006) NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signal. 8, 691–728 10.1089/ars.2006.8.691 [DOI] [PubMed] [Google Scholar]
- 129.Bedard, K. and Krause, K.H. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- 130.Brown, J.M., Terada, L.S., Grosso, M.A., Whitmann, G.J., Velasco, S.E., Patt, A.et al. (1988) Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J. Clin. Invest. 81, 1297–1301 10.1172/JCI113448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Granger, D.N. (1988) Role of xanthine oxidase and granulocytes in ischemia reperfusion injury. Am. J. Physiol. 255, H1269–H1275 10.1152/ajpheart.1988.255.6.H1269 [DOI] [PubMed] [Google Scholar]
- 132.Linas, S.L., Whittenburg, D. and Repine, J.E. (1990) Role of xanthine oxidase in ischemia reperfusion injury. Am. J. Physiol. 258, F711–F716 10.1152/ajpcell.1990.258.5.C849 [DOI] [PubMed] [Google Scholar]
- 133.Thompson-Gorman, S.L. and Zweier, J.L. (1990) Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J. Biol. Chem. 265, 6656–6663 10.1016/S0021-9258(19)39200-2 [DOI] [PubMed] [Google Scholar]
- 134.Adkins, W.K. and Taylor, A.E. (1990) Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury in rabbit lung. J. Appl. Physiol. 69, 2012–2018 10.1152/jappl.1990.69.6.2012 [DOI] [PubMed] [Google Scholar]
- 135.Müller, M.J., Vollmar, B., Friedl, H.P. and Menger, M.D. (1996) Xanthine oxidase and superoxide radicals in portal triad crossclamping-induced microvascular reperfusion injury of the liver. Free Rad. Biol. Med. 21, 189–197 10.1016/0891-5849(96)00028-7 [DOI] [PubMed] [Google Scholar]
- 136.Fridovich, I. (1995) Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64, 97–112 10.1146/annurev.bi.64.070195.000525 [DOI] [PubMed] [Google Scholar]
- 137.Wardman, P. and Candeias, L.P. (1996) Fenton chemistry: an introduction. Rad. Res. 145, 523–531 10.2307/3579270 [DOI] [PubMed] [Google Scholar]
- 138.Kehrer, J.P. (2000) The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50 10.1016/S0300-483X(00)00231-6 [DOI] [PubMed] [Google Scholar]
- 139.Koppenol, W.H. (2001) The Haber-Weiss cycle--70 years later. Redox Rep. 6, 229–234 10.1179/135100001101536373 [DOI] [PubMed] [Google Scholar]
- 140.Hershko, C. and Weatherall, D.J. (1988) Iron-chelating therapy. Crit. Rev. Clin. Lab. Sci. 26, 303–345 10.3109/10408368809105894 [DOI] [PubMed] [Google Scholar]
- 141.Hininger, I., Waters, R., Osman, M., Garrel, C., Fernholz, K., Roussel, A.M.et al. (2005) Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Rad. Biol. Med. 38, 1565–1570 10.1016/j.freeradbiomed.2005.02.016 [DOI] [PubMed] [Google Scholar]
- 142.Miller, III, E.R., Pastor-Barriuso, R., Dalal, D., Riemersma, R.A., Appel, L.J. and Guallar, E. (2005) Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 142, 37–46 10.7326/0003-4819-142-1-200501040-00110 [DOI] [PubMed] [Google Scholar]
- 143.Bjelakovic, G., Nikolova, D., Gluud, L.L., Simonetti, R.G. and Gluud, C. (2008) Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2008, CD007176 10.1002/14651858.CD007176 [DOI] [PubMed] [Google Scholar]
- 144.Feierman, D.E. and Cederbaum, A.I. (1983) The effect of EDTA and iron on the oxidation of hydroxyl radical scavenging agents and ethanol by rat liver microsomes. Biochem. Biophys. Res. Commun. 116, 765–770 10.1016/0006-291X(83)90590-9 [DOI] [PubMed] [Google Scholar]
- 145.Graf, E., Mahoney, J.R., Bryant, R.G. and Eaton, J.W. (1984) Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 259, 3620–3624 10.1016/S0021-9258(17)43139-5 [DOI] [PubMed] [Google Scholar]
- 146.Uchida, K., Enomoto, N., Itakura, K. and Kawakishi, S. (1989) The hydroxyl radical generated by an iron(II)/EDTA/ascorbate system preferentially attacks tryptophan residues of the protein. Agr. Biol. Chem. 53, 3285–3292 10.1080/00021369.1989.10869852 [DOI] [Google Scholar]
- 147.Miller, C.J., Rose, A.L. and Waite, T.D. (2016) Importance of iron complexation for fenton-mediated hydroxyl radical production at circumneutral pH. Front. Mar. Sci. 3, 134 10.3389/fmars.2016.00134 [DOI] [Google Scholar]
- 148.Smith, J.K., Carden, D.L., Grisham, M.B., Granger, D.N. and Korthuis, R.J. (1989) Role of iron in postischemic microvascular injury. Am. J. Physiol. 256, H1472–H1477 10.1152/ajpheart.1989.256.5.h1472 [DOI] [PubMed] [Google Scholar]
- 149.Kell, D.B. and Pretorius, E. (2014) Serum ferritin is an important disease marker, and is mainly a leakage product from damaged cells. Metallomics 6, 748–773 10.1039/C3MT00347G [DOI] [PubMed] [Google Scholar]
- 150.Matin, A., Auger, E.A., Blum, P.H. and Schultz, J.E. (1989) Genetic basis of starvation survival in nondifferentiating bacteria. Annu. Rev. Microbiol. 43, 293–316 10.1146/annurev.mi.43.100189.001453 [DOI] [PubMed] [Google Scholar]
- 151.Morita, R.Y. (1988) Bioavailability of energy and its relationship to growth and starvation survival in nature. Can. J. Microbiol. 34, 346–441 10.1139/m88-076 [DOI] [Google Scholar]
- 152.Thorsen, B.K., Enger, Ø, Norland, S. and Hoff, K.A. (1992) Long-term starvation survival of Yersinia ruckeri at different salinities studied by microscopical and flow cytometric methods. Appl. Environ. Microbiol. 58, 1624–1628 10.1128/aem.58.5.1624-1628.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bergkessel, M. and Delavaine, L. (2021) Diversity in starvation survival strategies and outcomes among heterotrophic proteobacteria. Microb. Physiol. 31, 146–162 10.1159/000516215 [DOI] [PubMed] [Google Scholar]
- 154.Kaprelyants, A.S., Gottschal, J.C. and Kell, D.B. (1993) Dormancy in non-sporulating bacteria. FEMS Microbiol. Rev. 10, 271–286 10.1111/j.1574-6968.1993.tb05871.x [DOI] [PubMed] [Google Scholar]
- 155.Kell, D.B., Kaprelyants, A.S., Weichart, D.H., Harwood, C.L. and Barer, M.R. (1998) Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek 73, 169–187 10.1023/A:1000664013047 [DOI] [PubMed] [Google Scholar]
- 156.Mukamolova, G.V., Kaprelyants, A.S., Young, D.I., Young, M. and Kell, D.B. (1998) A bacterial cytokine. Proc. Natl Acad. Sci. U.S.A. 95, 8916–8921 10.1073/pnas.95.15.8916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mukamolova, G.V., Yanopolskaya, N.D., Kell, D.B. and Kaprelyants, A.S. (1998) On resuscitation from the dormant state of Micrococcus luteus. Antonie Leeuwenhoek 73, 237–243 10.1023/A:1000881918216 [DOI] [PubMed] [Google Scholar]
- 158.Shleeva, M., Goncharenko, A., Kudykina, Y., Young, D., Young, M. and Kaprelyants, A. (2013) Cyclic AMP-dependent resuscitation of dormant mycobacteria by exogenous free fatty acids. PLoS One 8, e82914 10.1371/journal.pone.0082914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nikitushkin, V.D., Demina, G.R., Shleeva, M.O., Guryanova, S.V., Ruggiero, A., Berisio, R.et al. (2015) A product of RpfB and RipA joint enzymatic action promotes the resuscitation of dormant mycobacteria. FEBS J. 282, 2500–2511 10.1111/febs.13292 [DOI] [PubMed] [Google Scholar]
- 160.Houben, R.M.G.J. and Dodd, P.J. (2016) The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 13, e1002152 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Knight, G.M., McQuaid, C.F., Dodd, P.J. and Houben, R. (2019) Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect. Dis. 19, 903–912 10.1016/S1473-3099(19)30307-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reshetnyak, V.I. and Reshetnyak, T.M. (2017) Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J. Gastroenterol. 23, 4867–4878 10.3748/wjg.v23.i27.4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li, J. and Perez-Perez, G.I. (2018) Helicobacter pylori the latent human pathogen or an ancestral commensal organism. Front. Microbiol. 9, 609 10.3389/fmicb.2018.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lewis, K. (2010) Persister cells. Annu. Rev. Microbiol. 64, 357–372 10.1146/annurev.micro.112408.134306 [DOI] [PubMed] [Google Scholar]
- 165.Zhang, Y., Yew, W.W. and Barer, M.R. (2012) Targeting persisters for tuberculosis control. Antimicrob. Agents Chemother. 56, 2223–2230 10.1128/AAC.06288-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wood, T.K., Knabel, S.J. and Kwan, B.W. (2013) Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol. 79, 7116–7121 10.1128/AEM.02636-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holden, D.W. (2015) Persisters unmasked. Science 347, 30–32 10.1126/science.1262033 [DOI] [PubMed] [Google Scholar]
- 168.Kell, D.B., Potgieter, M. and Pretorius, E. (2015) Individuality, phenotypic differentiation, dormancy and ‘persistence’ in culturable bacterial systems: commonalities shared by environmental, laboratory, and clinical microbiology. F1000Research 4, 179 10.12688/f1000research.6709.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rowe, S.E., Conlon, B.P., Keren, I. and Lewis, K. (2016) Persisters: methods for isolation and identifying contributing factors--a review. Methods Mol. Biol. 1333, 17–28 10.1007/978-1-4939-2854-5_2 [DOI] [PubMed] [Google Scholar]
- 170.Fisher, R.A., Gollan, B. and Helaine, S. (2017) Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 15, 453–464 10.1038/nrmicro.2017.42 [DOI] [PubMed] [Google Scholar]
- 171.Van den Bergh, B., Fauvart, M. and and Michiels, J. (2017) Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 41, 219–251 10.1093/femsre/fux001 [DOI] [PubMed] [Google Scholar]
- 172.Salcedo-Sora, J.E. and Kell, D.B. (2020) A quantitative survey of bacterial persistence in the presence of antibiotics: towards antipersister antimicrobial discovery. Antibiotics 9, 508 10.3390/antibiotics9080508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Song, S. and Wood, T.K. (2020) Persister cells resuscitate via ribosome modification by 23S rRNA pseudouridine synthase RluD. Environ. Microbiol. 22, 850–857 10.1111/1462-2920.14828 [DOI] [PubMed] [Google Scholar]
- 174.Yamasaki, R., Song, S., Benedik, M.J. and Wood, T.K. (2020) Persister cells resuscitate using membrane sensors that activate chemotaxis, lower cAMP levels, and revive ribosomes. iScience 23, 100792 10.1016/j.isci.2019.100792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Antonelli, G. and Cutler, S. (2016) Evolution of the Koch postulates: towards a 21st-century understanding of microbial infection. Clin Microbiol Infect. 22, 583–584 10.1016/j.cmi.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 176.Byrd, A.L. and Segre, J.A. (2016) Adapting Koch's postulates. Science 351, 224–226 10.1126/science.aad6753 [DOI] [PubMed] [Google Scholar]
- 177.Falkow, S. (1988) Molecular Koch's postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10, S274–S276 10.1093/cid/10.Supplement_2.S274 [DOI] [PubMed] [Google Scholar]
- 178.Falkow, S. (2004) Molecular Koch's postulates applied to bacterial pathogenicity - a personal recollection 15 years later. Nat. Rev. Microbiol. 2, 67–72 10.1038/nrmicro799 [DOI] [PubMed] [Google Scholar]
- 179.Fredricks, D.N. and Relman, D.A. (1996) Sequence-based identification of microbial pathogens - a reconsideration of Koch's postulates. Clin. Micr. Rev. 9, 18–33 10.1128/CMR.9.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lowe, A.M., Yansouni, C.P. and Behr, M.A. (2008) Causality and gastrointestinal infections: Koch, Hill, and Crohn's. Lancet Infect. Dis. 8, 720–726 10.1016/S1473-3099(08)70257-3 [DOI] [PubMed] [Google Scholar]
- 181.Miklossy, J. (2011) Alzheimer's disease - a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J. Neuroinflammation 8, 90 10.1186/1742-2094-8-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Segre, J.A. (2013) What does it take to satisfy Koch's postulates two centuries later? Microbial genomics and Propionibacteria acnes. J. Investig. Dermatol. 133, 2141–2142 10.1038/jid.2013.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Castillo, D.J., Rifkin, R.F., Cowan, D.A. and Potgieter, M. (2019) The healthy human blood microbiome: fact or fiction? Front. Cell. Infect. Microbiol. 9, 148 10.3389/fcimb.2019.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Damgaard, C., Magnussen, K., Enevold, C., Nilsson, M., Tolker-Nielsen, T., Holmstrup, P.et al. (2015) Viable bacteria associated with red blood cells and plasma in freshly drawn blood donations. PLoS One 10, e0120826 10.1371/journal.pone.0120826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Damgaard, C., Saekmose, S.G., Nilsson, M., Kilian, M., Nielsen, C.H. and Holmstrup, P. (2021) Periodontitis increases risk of viable bacteria in freshly drawn blood donations. Blood Transfus. 19, 376–383 10.2450/2021.0336-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kowarsky, M., Camunas-Soler, J., Kertesz, M., De Vlaminck, I., Koh, W., Pan, W.et al. (2017) Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc. Natl Acad. Sci. U.S.A. 114, 9623–9628 10.1073/pnas.1707009114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Panaiotov, S., Filevski, G., Equestre, M., Nikolova, E. and Kalfin, R. (2018) Cultural isolation and characteristics of the blood microbiome of healthy individuals. Adv. Microbiol. 8, 406–421 10.4236/aim.2018.85027 [DOI] [Google Scholar]
- 188.Loohuis, O., Mangul, L.M., Ori, S., Jospin, A.P.S., Koslicki, G., Yang, D.et al. (2018) Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl. Psychiatry 8, 96 10.1038/s41398-018-0107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Potgieter, M., Bester, J., Kell, D.B. and Pretorius, E. (2015) The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 39, 567–591 10.1093/femsre/fuv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thwaites, G.E. and Gant, V. (2011) Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 9, 215–222 10.1038/nrmicro2508 [DOI] [PubMed] [Google Scholar]
- 191.Joura, M.I., Brunner, A., Nemes-Nikodém, É, Sárdy, M. and Ostorházi, E. (2021) Interactions between immune system and the microbiome of skin, blood and gut in pathogenesis of rosacea. Acta Microbiol. Immunol. Hung. 68, 1–6 10.1556/030.2021.01366 [DOI] [PubMed] [Google Scholar]
- 192.D'Aquila, P., Giacconi, R., Malavolta, M., Piacenza, F., Burkle, A., Villanueva, M.M.et al. (2021) Microbiome in blood samples from the general population recruited in the MARK-AGE project: a pilot study. Front. Microbiol. 12, 707515 10.3389/fmicb.2021.707515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Emery, D.C., Cerajewska, T.L., Seong, J., Davies, M., Paterson, A., Allen-Birt, S.J.et al. (2020) Comparison of blood bacterial communities in periodontal health and periodontal disease. Front. Cell. Infect. Microbiol. 10, 577485 10.3389/fcimb.2020.577485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aagaard, K., Ma, J., Antony, K.M., Ganu, R., Petrosino, J. and Versalovic, J. (2014) The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mysorekar, I.U. and Hultgren, S.J. (2006) Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl Acad. Sci. U.S.A. 103, 14170–14175 10.1073/pnas.0602136103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rosen, D.A., Hooton, T.M., Stamm, W.E., Humphrey, P.A. and Hultgren, S.J. (2007) Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4, e329 10.1371/journal.pmed.0040329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Casadevall, A. (2008) Evolution of intracellular pathogens. Annu. Rev. Microbiol. 62, 19–33 10.1146/annurev.micro.61.080706.093305 [DOI] [PubMed] [Google Scholar]
- 198.Shin, S. and Roy, C.R. (2008) Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol. 10, 1209–1220 10.1111/j.1462-5822.2008.01145.x [DOI] [PubMed] [Google Scholar]
- 199.Hunstad, D.A. and Justice, S.S. (2010) Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 64, 203–221 10.1146/annurev.micro.112408.134258 [DOI] [PubMed] [Google Scholar]
- 200.Martirosyan, A., Moreno, E. and Gorvel, J.P. (2011) An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 240, 211–234 10.1111/j.1600-065X.2010.00982.x [DOI] [PubMed] [Google Scholar]
- 201.Fraunholz, M. and Sinha, B. (2012) Intracellular Staphylococcus aureus: live-in and let die. Front. Cell. Infect. Microbiol. 2, 43 10.3389/fcimb.2012.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Silva, M.T. (2012) Classical labeling of bacterial pathogens according to their lifestyle in the host: inconsistencies and alternatives. Front. Microbiol. 3, 71 10.3389/fmicb.2012.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Amano, A. and Furuta, N. (2012) Cell entry and exit by periodontal pathogen Porphyromonas gingivalis. J. Oral Biosci. 54, 54–57 10.1016/j.job.2011.03.001 [DOI] [Google Scholar]
- 204.Kozarov, E. (2012) Bacterial invasion of vascular cell types: vascular infectology and atherogenesis. Future Cardiol. 8, 123–138 10.2217/fca.11.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pulliainen, A.T. and Dehio, C. (2012) Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation. FEMS Microbiol. Rev. 36, 563–599 10.1111/j.1574-6976.2012.00324.x [DOI] [PubMed] [Google Scholar]
- 206.von Bargen, K., Gorvel, J.P. and Salcedo, S.P. (2012) Internal affairs: investigating the Brucella intracellular lifestyle. FEMS Microbiol. Rev. 36, 533–562 10.1111/j.1574-6976.2012.00334.x [DOI] [PubMed] [Google Scholar]
- 207.Horsley, H., Malone-Lee, J., Holland, D., Tuz, M., Hibbert, A., Kelsey, M.et al. (2013) Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. PLoS One 8, e83637 10.1371/journal.pone.0083637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Silva, M.T. and Pestana, N.T. (2013) The in vivo extracellular life of facultative intracellular bacterial parasites: role in pathogenesis. Immunobiology 218, 325–337 10.1016/j.imbio.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 209.Blango, M.G., Ott, E.M., Erman, A., Veranic, P. and Mulvey, M.A. (2014) Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLos One 9, e93327 10.1371/journal.pone.0093327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buchacher, T., Wiesinger-Mayr, H., Vierlinger, K., Ruger, B.M., Stanek, G., Fischer, M.B.et al. (2014) Human blood monocytes support persistence, but not replication of the intracellular pathogen C. pneumoniae. BMC Immunol. 15, 60 10.1186/s12865-014-0060-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Flores-Mireles, A.L., Walker, J.N., Caparon, M. and Hultgren, S.J. (2015) Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Knodler, L.A. (2015) Salmonella enterica: living a double life in epithelial cells. Curr. Opin. Microbiol. 23C, 23–31 10.1016/j.mib.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 213.Reniere, M.L., Whiteley, A.T., Hamilton, K.L., John, S.M., Lauer, P., Brennan, R.G.et al. (2015) Glutathione activates virulence gene expression of an intracellular pathogen. Nature 517, 170–173 10.1038/nature14029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ribet, D. and Cossart, P. (2015) How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 17, 173–183 10.1016/j.micinf.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 215.Leatham-Jensen, M.P., Mokszycki, M.E., Rowley, D.C., Deering, R., Camberg, J.L., Sokurenko, E.V.et al. (2016) Uropathogenic Escherichia coli metabolite-dependent quiescence and persistence may explain antibiotic tolerance during urinary tract infection. mSphere 1, e00055-15 10.1128/mSphere.00055-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kortebi, M., Milohanic, E., Mitchell, G., Péchoux, C., Prevost, M.C., Cossart, P.et al. (2017) Listeria monocytogenes switches from dissemination to persistence by adopting a vacuolar lifestyle in epithelial cells. PLoS Pathog. 13, e1006734 10.1371/journal.ppat.1006734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McClure, E.E., Chavez, A.S.O., Shaw, D.K., Carlyon, J.A., Ganta, R.R., Noh, S.M.et al. (2017) Engineering of obligate intracellular bacteria: progress, challenges and paradigms. Nat. Rev. Microbiol. 15, 544–558 10.1038/nrmicro.2017.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pucciarelli, M.G. and García-Del Portillo, F. (2017) Salmonella Intracellular Lifestyles and Their Impact on Host-to-Host Transmission. Microbiol. Spectr. 5, 1–18 10.1128/microbiolspec.MTBP-0009-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim, W.J., Shea, A.E., Kim, J.H. and Daaka, Y. (2018) Uropathogenic Escherichia coli invades bladder epithelial cells by activating kinase networks in host cells. J. Biol. Chem. 293, 16518–16527 10.1074/jbc.RA118.003499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Proal, A. and Marshall, T. (2018) Myalgic encephalomyelitis/chronic fatigue syndrome in the era of the human microbiome: persistent pathogens drive chronic symptoms by interfering with host metabolism, gene expression, and immunity. Front. Pediatr. 6, 373 10.3389/fped.2018.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Forrester, J.V., McMenamin, P.G. and Dando, S.J. (2018) CNS infection and immune privilege. Nat. Rev. Neurosci. 19, 655–671 10.1038/s41583-018-0070-8 [DOI] [PubMed] [Google Scholar]
- 222.Proal, A.D. and VanElzakker, M.B. (2021) Pathogens hijack host cell metabolism: intracellular infection as a driver of the Warburg effect in cancer and other chronic inflammatory conditions. Immunometabolism 3, e210003 10.20900/immunometab20210003 [DOI] [Google Scholar]
- 223.O'Neal, A.J. and Hanson, M.R. (2021) The enterovirus theory of disease etiology in myalgic encephalomyelitis/chronic fatigue syndrome: a critical review. Front. Med. (Lausanne) 8, 688486 10.3389/fmed.2021.688486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Speck, S.H. and Ganem, D. (2010) Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8, 100–115 10.1016/j.chom.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Traylen, C.M., Patel, H.R., Fondaw, W., Mahatme, S., Williams, J.F., Walker, L.R.et al. (2011) Virus reactivation: a panoramic view in human infections. Future Virol. 6, 451–463 10.2217/fvl.11.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Grinde, B. (2013) Herpesviruses: latency and reactivation - viral strategies and host response. J. Oral Microbiol. 5. 10.3402/jom.v5i0.22766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Le, P. and Rothberg, M. (2019) Herpes zoster infection. BMJ 364, k5095 10.1136/bmj.k5095 [DOI] [PubMed] [Google Scholar]
- 228.Itzhaki, R.F., Lathe, R., Balin, B.J., Ball, M.J., Braak, H., Bearer, E.L.et al. (2016) Microbes and Alzheimer's disease. J. Alzheimers Dis. 51, 979–984 10.3233/JAD-160152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Itzhaki, R.F. and Lathe, R. (2018) Herpes viruses and senile dementia: first population evidence for a causal link. J. Alzheimers Dis. 64, 363–366 10.3233/JAD-180266 [DOI] [PubMed] [Google Scholar]
- 230.Itzhaki, R.F. (2018) Corroboration of a major role for herpes simplex virus type 1 in Alzheimer's disease. Front. Aging Neurosci. 10, 324 10.3389/fnagi.2018.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Itzhaki, R.F., Golde, T.E., Heneka, M.T. and Readhead, B. (2020) Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 16, 193–197 10.1038/s41582-020-0323-9 [DOI] [PubMed] [Google Scholar]
- 232.Manicklal, S., Emery, V.C., Lazzarotto, T., Boppana, S.B. and Gupta, R.K. (2013) The "silent" global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 26, 86–102 10.1128/CMR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Diggins, N.L. and Hancock, M.H. (2018) HCMV miRNA targets reveal important cellular pathways for viral replication, latency, and reactivation. Noncoding RNA 4, 29 10.3390/ncrna404002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jason, L.A., Porter, N., Herrington, J., Sorenson, M. and Kubow, S. (2009) Kindling and oxidative stress as contributors to myalgic encephalomyelitis/chronic fatigue syndrome. J. Behav. Neurosci. Res. 7, 1–17 https://pubmed.ncbi.nlm.nih.gov/21253446/ [PMC free article] [PubMed] [Google Scholar]
- 235.Chapenko, S., Krumina, A., Logina, I., Rasa, S., Chistjakovs, M., Sultanova, A.et al. (2012) Association of active human herpesvirus-6, -7 and parvovirus b19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv. Virol. 2012, 205085 10.1155/2012/205085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shikova, E., Reshkova, V., Kumanova, А., Raleva, S., Alexandrova, D., Capo, N.et al. (2020) Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic small ie, cyrillicncephalomyelitis/chronic fatigue syndrome. J. Med. Virol. 92, 3682–3688 10.1002/jmv.25744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Friedman, K.J., Murovska, M., Pheby, D.F.H. and Zalewski, P. (2021) Our evolving understanding of ME/CFS. Medicina (Kaunas) 57, 200 10.3390/medicina57030200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee, J.S., Lacerda, E.M., Nacul, L., Kingdon, C.C., Norris, J., O'Boyle, S.et al. (2021) Salivary DNA loads for human herpesviruses 6 and 7 are correlated with disease phenotype in myalgic encephalomyelitis/Chronic fatigue syndrome. Front. Med. (Lausanne) 8, 656692 10.3389/fmed.2021.656692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ruiz-Pablos, M., Paiva, B., Montero-Mateo, R., Garcia, N. and Zabaleta, A. (2021) Epstein-Barr virus and the origin of myalgic encephalomyelitis or chronic fatigue syndrome. Front. Immunol. 12, 656797 10.3389/fimmu.2021.656797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu, T., Khanna, K.M., Carriere, B.N. and Hendricks, R.L. (2001) Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75, 11178–11184 10.1128/JVI.75.22.11178-11184.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Katze, M.G., He, Y. and Gale, Jr., M. (2002) Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675–687 10.1038/nri888 [DOI] [PubMed] [Google Scholar]
- 242.Haller, O., Kochs, G. and Weber, F. (2006) The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344, 119–130 10.1016/j.virol.2005.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Decman, V., Kinchington, P.R., Harvey, S.A. and Hendricks, R.L. (2005) Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79, 10339–10347 10.1128/JVI.79.16.10339-10347.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kang, S., Brown, H.M. and Hwang, S. (2018) Direct antiviral mechanisms of interferon-gamma. Immune Netw. 18, e33 10.4110/in.2018.18.e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Van der Sluis, R.M., Zerbato, J.M., Rhodes, J.W., Pascoe, R.D., Solomon, A., Kumar, N.A.et al. (2020) Diverse effects of interferon alpha on the establishment and reversal of HIV latency. PLoS Pathog. 16, e1008151 10.1371/journal.ppat.1008151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Schuhenn, J., Meister, T.L., Todt, D., Bracht, T., Schork, K., Billaud, J.N.et al. (2022) Differential interferon-alpha subtype induced immune signatures are associated with suppression of SARS-CoV-2 infection. Proc. Natl Acad. Sci. U.S.A. 119, e2111600119 10.1073/pnas.2111600119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Weber, F. and Haller, O. (2007) Viral suppression of the interferon system. Biochimie 89, 836–842 10.1016/j.biochi.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.García-Sastre, A. (2017) Ten strategies of interferon evasion by viruses. Cell Host Microbe 22, 176–184 10.1016/j.chom.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rojas, J.M., Alejo, A., Martin, V. and Sevilla, N. (2021) Viral pathogen-induced mechanisms to antagonize mammalian interferon (IFN) signaling pathway. Cell. Mol. Life Sci. 78, 1423–1444 10.1007/s00018-020-03671-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lei, X., Dong, X., Ma, R., Wang, W., Xiao, X., Tian, Z.et al. (2020) Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 11, 3810 10.1038/s41467-020-17665-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Triantafyllidis, K., Giannos, K., Mian, P., Kyrtsonis, I.T., and Kechagias, G. and S, K. (2021) Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel) 9, 1013 10.3390/vaccines9091013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Maldonado, M.D. and Romero-Aibar, J. (2021) The Pfizer-BNT162b2 mRNA-based vaccine against SARS-CoV-2 may be responsible for awakening the latency of herpes varicella-zoster virus. Brain Behav. Immun. Health 18, 100381 10.1016/j.bbih.2021.100381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Psichogiou, M., Samarkos, M., Mikos, N. and Hatzakis, A. (2021) Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel) 9, 572 10.3390/vaccines9060572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Posey, J.E. and Gherardini, F.C. (2000) Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 10.1126/science.288.5471.1651 [DOI] [PubMed] [Google Scholar]
- 255.Armitage, A.E. and Drakesmith, H. (2014) The battle for iron. Science 346, 1299–1300 10.1126/science.aaa2468 [DOI] [PubMed] [Google Scholar]
- 256.Carver, P.L. (2018) The battle for iron between humans and microbes. Curr. Med. Chem. 18, 25–36 10.2174/0929867324666170720110049 [DOI] [PubMed] [Google Scholar]
- 257.Haley, K.P. and Skaar, E.P. (2012) A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes Infect. 14, 217–227 10.1016/j.micinf.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.McDermid, J.M. and Prentice, A.M. (2006) Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMP1 (SLC11A1) on host-pathogen interactions in tuberculosis and HIV. Clin. Sci. (Lond.) 110, 503–524 10.1042/CS20050273 [DOI] [PubMed] [Google Scholar]
- 259.Miethke, M. (2013) Molecular strategies of microbial iron assimilation: from high-affinity complexes to cofactor assembly systems. Metallomics 5, 15–28 10.1039/C2MT20193C [DOI] [PubMed] [Google Scholar]
- 260.Segond, D., Abi Khalil, E., Buisson, C., Daou, N., Kallassy, M., Lereclus, D.et al. (2014) Iron acquisition in Bacillus cereus: the roles of IlsA and bacillibactin in exogenous ferritin iron mobilization. PLoS Pathog. 10, e1003935 10.1371/journal.ppat.1003935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nairz, M., Schroll, A., Sonnweber, T. and Weiss, G. (2010) The struggle for iron - a metal at the host-pathogen interface. Cell Microbiol. 12, 1691–1702 10.1111/j.1462-5822.2010.01529.x [DOI] [PubMed] [Google Scholar]
- 262.Skaar, E.P. (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6, e1000949 10.1371/journal.ppat.1000949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Subashchandrabose, S. and Mobley, H.L.T. (2015) Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection. Metallomics 7, 935–942 10.1039/C4MT00329B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Do, J., Zafar, H. and Saier, Jr., M.H. (2017) Comparative genomics of transport proteins in probiotic and pathogenic Escherichia coli and Salmonella enterica strains. Microb. Pathog. 107, 106–115 10.1016/j.micpath.2017.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tang, F. and Saier, Jr., M.H. (2014) Transport proteins promoting Escherichia coli pathogenesis. Microb. Pathog. 71–72, 41–55 10.1016/j.micpath.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kell, D.B. (2010) Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson's, Huntington's, Alzheimer's, prions, bactericides, chemical toxicology and others as examples. Arch. Toxicol. 577, 825–889 10.1007/s00204-010-0577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kell, D.B. and Pretorius, E. (2015) On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr. Biol. 7, 1339–1377 10.1039/C5IB00158G [DOI] [PubMed] [Google Scholar]
- 268.Pretorius, E., Page, M.J., Hendricks, L., Nkosi, N.B., Benson, S.R. and Kell, D.B. (2018) Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J. R. Soc. Interface 15, 20170941 10.1098/rsif.2017.0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ghiadoni, L., Salvetti, M., Muiesan, M.L. and Taddei, S. (2015) Evaluation of endothelial function by flow mediated dilation: methodological issues and clinical importance. High Blood Press. Cardiovasc. Prev. 22, 17–22 10.1007/s40292-014-0047-2 [DOI] [PubMed] [Google Scholar]
- 270.Chia, P.Y., Teo, A. and Yeo, T.W. (2020) Overview of the assessment of endothelial function in humans. Front. Med. (Lausanne) 7, 542567 10.3389/fmed.2020.542567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Dirican, A., Ildir, S., Uzar, T., Karaman, I. and Ozkaya, S. (2021) The role of endotheliitis in COVID-19: real-world experience of 11 190 patients and literature review for a pathophysiological map to clinical categorisation. Int. J. Clin. Pract. 75, e14843 10.1111/ijcp.14843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Huertas, A., Montani, D., Savale, L., Pichon, J., Tu, L., Parent, F.et al. (2020) Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 56, 2001634 10.1183/13993003.01634-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kirschenbaum, D., Imbach, L.L., Rushing, E.J., Frauenknecht, K.B.M., Gascho, D., Ineichen, B.V.et al. (2021) Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 47, 454–459 10.1111/nan.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lei, Y.Y., Zhang, J., Schiavon, C.R., He, M., Chen, L.L., Shen, H.et al. (2021) SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 128, 1323–1326 10.1161/CIRCRESAHA.121.318902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Maccio, U., Zinkernagel, A.S., Shambat, S.M., Zeng, X., Cathomas, G., Ruschitzka, F.et al. (2021) SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine 63, 103182 10.1016/j.ebiom.2020.103182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Teuwen, L.A., Geldhof, V., Pasut, A. and Carmeliet, P. (2020) COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 20, 389–391 10.1038/s41577-020-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kaur, S., Tripathi, D.M. and Yadav, A. (2020) The enigma of endothelium in COVID-19. Front. Physiol. 11, 989 10.3389/fphys.2020.00989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Varga, Z., Flammer, A.J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A.S.et al. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Amersfoort, J., Eelen, G. and Carmeliet, P. (2022) Immunomodulation by endothelial cells — partnering up with the immune system? Nat. Rev. Immunol. in press. 10.1038/s41577-022-00694-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ackermann, M., Verleden, S.E., Kuehnel, M., Haverich, A., Welte, T., Laenger, F.et al. (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 383, 120–128 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Calabretta, E., Moraleda, J.M., Iacobelli, M., Jara, R., Vlodavsky, I., O'Gorman, P.et al. (2021) COVID-19-induced endotheliitis: emerging evidence and possible therapeutic strategies. Br. J. Haematol. 193, 43–51 10.1111/bjh.17240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Charfeddine, S., Ibn Hadj Amor, H., Jdidi, J., Torjmen, S., Kraiem, S., Hammami, R.et al. (2021) Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front. Cardiovasc. Med. 8, 745758 10.3389/fcvm.2021.745758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Fogarty, H., Townsend, L., Morrin, H., Ahmad, A., Comerford, C., Karampini, E.et al. (2021) Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 19, 2546–2553 10.1111/jth.15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Goshua, G., Pine, A.B., Meizlish, M.L., Chang, C.H., Zhang, H., Bahel, P.et al. (2020) Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 7, e575–e582 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Altschul, D.J., Unda, S.R., de La Garza Ramos, R., Zampolin, R., Benton, J., Holland, R.et al. (2020) Hemorrhagic presentations of COVID-19: risk factors for mortality. Clin. Neurol. Neurosurg. 198, 106112 10.1016/j.clineuro.2020.106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iba, T., Levy, J.H., Levi, M. and Thachil, J. (2020) Coagulopathy in COVID-19. J. Thromb. Haemost. 18, 2103–2109 10.1111/jth.14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Klok, F.A., Kruip, M., van der Meer, N.J.M., Arbous, M.S., Gommers, D., Kant, K.M.et al. (2020) Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 191, 148–150 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Malas, M.B., Naazie, I.N., Elsayed, N., Mathlouthi, A., Marmor, R. and Clary, B. (2020) Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 29, 100639 10.1016/j.eclinm.2020.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Thachil, J., Tang, N., Gando, S., Falanga, A., Cattaneo, M., Levi, M.et al. (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 18, 1023–1026 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gómez-Mesa, J.E., Galindo-Coral, S., Montes, M.C. and Muñoz Martin, A.J. (2021) Thrombosis and coagulopathy in COVID-19. Curr. Probl. Cardiol. 46, 100742 10.1016/j.cpcardiol.2020.100742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Leentjens, J., van Haaps, T.F., Wessels, P.F., Schutgens, R.E.G. and Middeldorp, S. (2021) COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 8, e524–e533 10.1016/S2352-3026(21)00105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lorini, F.L., Di Matteo, M., Gritti, P., Grazioli, L., Benigni, A., Zacchetti, L.et al. (2021) Coagulopathy and COVID-19. Eur. Heart J. Suppl. 23, E95–E98 10.1093/eurheartj/suab100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Polimeni, A., Leo, I., Spaccarotella, C., Mongiardo, A., Sorrentino, S., Sabatino, J.et al. (2021) Differences in coagulopathy indices in patients with severe versus non-severe COVID-19: a meta-analysis of 35 studies and 6427 patients. Sci. Rep. 11, 10464 10.1038/s41598-021-89967-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sarkar, M., Madabhavi, I.V., Quy, P.N. and Govindagoudar, M.B. (2021) COVID-19 and coagulopathy. Clin. Respir. J. 15, 1259–1274 10.1111/crj.13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Vincent, J.L., Levi, M. and Hunt, B.J. (2021) Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir. Med. 10, 214–220 10.1016/S2213-2600(21)00455-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ortega-Paz, L., Capodanno, D., Montalescot, G. and Angiolillo, D.J. (2021) Coronavirus disease 2019-associated thrombosis and coagulopathy: review of the pathophysiological characteristics and implications for antithrombotic management. J. Am. Heart Assoc. 10, e019650 10.1161/JAHA.120.019650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wang, Z., Gao, X., Miao, H., Ma, X. and Ding, R. (2021) Understanding COVID-19-associated coagulopathy: from PIC to SIC or DIC. J. Intensive Med. 1, 35–41 10.1016/j.jointm.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Douillet, D., Riou, J., Penaloza, A., Moumneh, T., Soulie, C., Savary, D.et al. (2021) Risk of symptomatic venous thromboembolism in mild and moderate COVID-19: a comparison of two prospective European cohorts. Thromb. Res. 208, 4–10 10.1016/j.thromres.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Levi, M. and Thachil, J. (2020) Coronavirus disease 2019 coagulopathy: disseminated intravascular coagulation and thrombotic microangiopathy-either, neither, or both. Semin. Thromb. Hemost. 46, 781–784 10.1055/s-0040-1712156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Thachil, J. (2021) Lessons learnt from COVID-19 coagulopathy. EJHaem 2, 577–584 10.1002/jha2.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Venter, C., Bezuidenhout, J.A., Laubscher, G.J., Lourens, P.J., Steenkamp, J., Kell, D.B.et al. (2020) Erythrocyte, platelet, serum ferritin and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int. J. Mol. Sci. 21, 8234 10.3390/ijms21218234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fogarty, H., Townsend, L., Ni Cheallaigh, C., Bergin, C., Martin-Loeches, I., Browne, P.et al. (2020) COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 189, 1044–1049 10.1111/bjh.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Bray, M.A., Sartain, S.E., Gollamudi, J. and Rumbaut, R.E. (2020) Microvascular thrombosis: experimental and clinical implications. Transl. Res. 225, 105–130 10.1016/j.trsl.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Levi, M., Thachil, J., Iba, T. and Levy, J.H. (2020) Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7, e438–e440 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Willyard, C. (2020) Coronavirus blood-clot mystery intensifies. Nature 581, 250 10.1038/d41586-020-01403-8 [DOI] [PubMed] [Google Scholar]
- 306.Pretorius, E., Venter, C., Laubscher, G.J., Lourens, P.J., Steenkamp, J. and Kell, D.B. (2020) Prevalence of amyloid blood clots in COVID-19 plasma. medRxiv 2020.2007.2028.20163543v20163541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Asakura, H. and Ogawa, H. (2021) COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 113, 45–57 10.1007/s12185-020-03029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wygrecka, M., Birnhuber, A., Seeliger, B., Michalick, L., Pak, O., Schultz, A.S.et al. (2021) Altered fibrin clot structure and dysregulated fibrinolysis contribute to thrombosis risk in severe COVID-19. Blood Adv. 6, 1074–1087 10.1182/bloodadvances.2021004816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wool, G.D. and Miller, J.L. (2021) The impact of COVID-19 disease on platelets and coagulation. Pathobiology 88, 15–27 10.1159/000512007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Iba, T., Levy, J.H., Connors, J.M., Warkentin, T.E., Thachil, J. and Levi, M. (2020) The unique characteristics of COVID-19 coagulopathy. Crit. Care 24, 360 10.1186/s13054-020-03077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Loo, J., Spittle, D.A. and Newnham, M. (2021) COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax 76, 412–420 10.1136/thoraxjnl-2020-216243 [DOI] [PubMed] [Google Scholar]
- 312.Manolis, A.S., Manolis, T.A., Manolis, A.A., Papatheou, D. and Melita, H. (2021) COVID-19 Infection: viral macro- and micro-vascular coagulopathy and thromboembolism/prophylactic and therapeutic management. J. Cardiovasc. Pharmacol. Ther. 26, 12–24 10.1177/1074248420958973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Poor, H.D. (2021) Pulmonary thrombosis and thromboembolism in COVID-19. Chest 160, 1471–1480 10.1016/j.chest.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.McGonagle, D., O'Donnell, J.S., Sharif, K., Emery, P. and Bridgewood, C. (2020) Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2, e437–e445 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z.et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gorog, D.A., Storey, R.F., Gurbel, P.A., Tantry, U.S., Berger, J.S., Chan, M.Y.et al. (2022) Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the international COVID-19 thrombosis biomarkers colloquium. Nat. Rev. Cardiol. 19, 475–495 10.1038/s41569-021-00665-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wichmann, D., Sperhake, J.P., Lütgehetmann, M., Steurer, S., Edler, C., Heinemann, A.et al. (2020) Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 173, 268–277 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Panigada, M., Bottino, N., Tagliabue, P., Grasselli, G., Novembrino, C., Chantarangkul, V.et al. (2020) Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 18, 1738–1742 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Aktaa, S., Wu, J., Nadarajah, R., Rashid, M., de Belder, M., Deanfield, J.et al. (2021) Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: multi-sourced population-based health records cohort study. Thromb. Res. 202, 17–23 10.1016/j.thromres.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zuin, M., Engelen, M.M., Barco, S., Spyropoulos, A.C., Vanassche, T., Hunt, B.J.et al. (2021) Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: a systematic review and meta-analysis. Thromb. Res. 209, 94–98 10.1016/j.thromres.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kastenhuber, E. R., Mercadante, M., Nilsson-Payant, B., Johnson, J. L., Jaimes, J. A., Muecksch, F.et al. (2022) Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. eLife 11, e77444 10.7554/eLife.77444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hess, J.R., Brohi, K., Dutton, R.P., Hauser, C.J., Holcomb, J.B., Kluger, Y.et al. (2008) The coagulopathy of trauma: a review of mechanisms. J. Trauma 65, 748–754 10.1097/TA.0b013e3181877a9c [DOI] [PubMed] [Google Scholar]
- 323.Curry, N., Stanworth, S., Hopewell, S., Doree, C., Brohi, K. and Hyde, C. (2011) Trauma-induced coagulopathy--a review of the systematic reviews: is there sufficient evidence to guide clinical transfusion practice? Transfus. Med. Rev. 25, 217–231.e212 10.1016/j.tmrv.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 324.Simmons, J.W., Pittet, J.F. and Pierce, B. (2014) Trauma-induced coagulopathy. Curr. Anesthesiol. Rep. 4, 189–199 10.1007/s40140-014-0063-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Jankun, J., Landeta, P., Pretorius, E., Skrzypczak-Jankun, E. and Lipinski, B. (2014) Unusual clotting dynamics of plasma supplemented with iron(III). Int. J. Mol. Med. 33, 367–372 10.3892/ijmm.2013.1585 [DOI] [PubMed] [Google Scholar]
- 326.Kell, D.B. and Pretorius, E. (2015) The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integr. Biol. 7, 24–52 10.1039/c4ib00173g [DOI] [PubMed] [Google Scholar]
- 327.Lipinski, B. and Pretorius, E. (2012) Novel pathway of iron-induced blood coagulation: implications for diabetes mellitus and its complications. Pol. Arch. Med. Wewn 122, 115–122 [PubMed] [Google Scholar]
- 328.Pretorius, E., Vermeulen, N., Bester, J., Lipinski, B. and Kell, D.B. (2013) A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicol. Mech. Methods 23, 352–359 10.3109/15376516.2012.762082 [DOI] [PubMed] [Google Scholar]
- 329.Pretorius, E. and Lipinski, B. (2013) Differences in morphology of fibrin clots induced with thrombin and ferric ions and its pathophysiological consequences. Heart Lung Circ. 22, 447–449 10.1016/j.hlc.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 330.Pretorius, E., Bester, J., Vermeulen, N., Lipinski, B., Gericke, G.S. and Kell, D.B. (2014) Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents. PLoS One 9, e85271 10.1371/journal.pone.0085271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pretorius, E., Mbotwe, S., Bester, J., Robinson, C. and Kell, D.B. (2016) Acute induction of anomalous blood clotting by highly substoichiometric levels of bacterial lipopolysaccharide (LPS). bioRxiv version. bioRxiv 2016-053538v053531 [DOI] [PMC free article] [PubMed]
- 332.Pretorius, E., Page, M.J., Mbotwe, S. and Kell, D.B. (2018) Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson's disease. PLoS One 13, e0192121 10.1371/journal.pone.0192121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pretorius, E., Bester, J., Page, M.J. and Kell, D.B. (2018) The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Front. Aging Neurosci. 10, 257 10.3389/fnagi.2018.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Conn, D.L., Mcduffie, F.C., Kazmier, F.J., Schroeter, A.L. and Sun, N.C.J. (1976) Coagulation abnormalities in rheumatoid disease. Arthritis Rheum. 19, 1237–1243 10.1002/art.1780190602 [DOI] [PubMed] [Google Scholar]
- 335.Bezuidenhout, J., Venter, C., Roberts, T., Tarr, G., Kell, D. and Pretorius, E. (2020) Detection of citrullinated fibrin in plasma clots of RA patients and its relation to altered structural clot properties, disease-related inflammation and prothrombotic tendency. Front. Immunol. 11, 577523 10.3389/fimmu.2020.577523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pretorius, E., Oberholzer, H.M., van der Spuy, W.J., Swanepoel, A.C. and Soma, P. (2012) Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol. Int. 32, 1611–1615 10.1007/s00296-011-1805-2 [DOI] [PubMed] [Google Scholar]
- 337.Zhang, P., Liu, J. and Tan, B. (2015) Hypercoagulation in patients with rheumatoid arthritis correlates with activation of Act1/NF-Κb signaling pathway. J. Rheum. Dis. Treat. 1, 4 10.23937/2469-5726/1510024 [DOI] [Google Scholar]
- 338.Dusse, L.M., Rios, D.R.A., Pinheiro, M.B., Cooper, A.J. and Lwaleed, B.A. (2011) Pre-eclampsia: relationship between coagulation, fibrinolysis and inflammation. Clin. Chim. Acta 412, 17–21 10.1016/j.cca.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 339.Esmon, C.T. (2005) The interactions between inflammation and coagulation. Br. J. Haematol. 131, 417–430 10.1111/j.1365-2141.2005.05753.x [DOI] [PubMed] [Google Scholar]
- 340.Jennewein, C., Paulus, P. and Zacharowski, K. (2011) Linking inflammation and coagulation: novel drug targets to treat organ ischemia. Curr. Opin. Anaesthesiol. 24, 375–380 10.1097/ACO.0b013e3283489ac0 [DOI] [PubMed] [Google Scholar]
- 341.Schouten, M., Wiersinga, W.J., Levi, M. and van der Poll, T. (2008) Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 83, 536–545 10.1189/jlb.0607373 [DOI] [PubMed] [Google Scholar]
- 342.Tantry, U.S., Bliden, K.P., Suarez, T.A., Kreutz, R.P., Dichiara, J. and Gurbel, P.A. (2010) Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the thrombotic risk progression (TRIP) study. Platelets 21, 360–367 10.3109/09537100903548903 [DOI] [PubMed] [Google Scholar]
- 343.van der Poll, T., de Boer, J.D. and Levi, M. (2011) The effect of inflammation on coagulation and vice versa. Curr. Opin. Infect. Dis. 24, 273–278 10.1097/QCO.0b013e328344c078 [DOI] [PubMed] [Google Scholar]
- 344.van der Poll, T. and Levi, M. (2012) Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr. Vasc. Pharmacol. 10, 632–638 10.2174/157016112801784549 [DOI] [PubMed] [Google Scholar]
- 345.Zia, A., Russell, J., Sarode, R., Veeram, S.R., Josephs, S., Malone, K.et al. (2017) Markers of coagulation activation, inflammation and fibrinolysis as predictors of poor outcomes after pediatric venous thromboembolism: a systematic review and meta-analysis. Thromb. Res. 160, 1–8 10.1016/j.thromres.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Magro, G. (2020) COVID-19: review on latest available drugs and therapies against SARS-CoV-2. coagulation and inflammation cross-talking. Virus Res. 286, 198070 10.1016/j.virusres.2020.198070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Bhattacharyya, R., Iyer, P., Phua, G.C. and Lee, J.H. (2020) The interplay between coagulation and inflammation pathways in COVID-19-associated respiratory failure: a narrative review. Pulm. Ther. 6, 215–231 10.1007/s41030-020-00126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kowalewski, M., Fina, D., Slomka, A., Raffa, G.M., Martucci, G., Lo Coco, V.et al. (2020) COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit. Care 24, 205 10.1186/s13054-020-02925-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hulshof, A.M., Brüggemann, R.A.G., Mulder, M.M.G., van de Berg, T.W., Sels, J.E.M., Olie, R.H.et al. and Dutch COVID-19 thrombosis consortium (DCTC) (2021) Serial EXTEM, FIBTEM, and tPA rotational thromboelastometry observations in the maastricht intensive care COVID cohort-persistence of hypercoagulability and hypofibrinolysis despite anticoagulation. Front. Cardiovasc. Med. 8, 654174 10.3389/fcvm.2021.654174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Berg, D., Berg, L.H., Couvaras, J. and Harrison, H. (1999) Chronic fatigue syndrome and/or fibromyalgia as a variation of antiphospholipid antibody syndrome: an explanatory model and approach to laboratory diagnosis. Blood Coagul. Fibrinolysis 10, 435–438 10.1097/00001721-199910000-00006 [DOI] [PubMed] [Google Scholar]
- 351.Brewer, J.H. and Berg, D. (2011) Hypercoagulable state associated with active human herpesvirus-6 (HHV-6) viremia in patients with chronic fatigue syndrome. J. Chron. Fatigue Syndr. 8, 111–116 10.1300/J092v08n03_10 [DOI] [Google Scholar]
- 352.Nunes, J.M., Kruger, A., Proal, A., Kell, D.B. and Pretorius, E. (2022) The occurrence of hyperactivated platelets and fibrinaloid microclots in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Res. Sq. 10.21203/rs.3.rs-1727226/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Kell, D.B. and Pretorius, E. (2018) To what extent are the terminal stages of sepsis, septic shock, SIRS, and multiple organ dysfunction syndrome actually driven by a toxic prion/amyloid form of fibrin? Semin. Thromb. Hemost. 44, 224–238 10.1055/s-0037-1604108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Pretorius, E., Mbotwe, S., Bester, J., Robinson, C.J. and Kell, D.B. (2016) Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J. R. Soc. Interface 123, 20160539 10.1098/rsif.2016.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Kell, D.B. and Pretorius, E. (2017) Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr. Biophys. Mol. Biol. 123, 16–41 10.1016/j.pbiomolbio.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 356.Pretorius, E., Mbotwe, S. and Kell, D.B. (2017) Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular comorbidities. Sci. Rep. 7, 9680 10.1038/s41598-017-09860-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J.A., Laubscher, G.J., Steenkamp, J.et al. (2021) Persistent clotting protein pathology in Long COVID/ post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. medRxiv 2021.2005.2021.21257578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Prusiner, S.B. (1982) Novel proteinaceous infectious particles cause scrapie. Science 216, 136–144 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- 359.Aguzzi, A., Heikenwalder, M. and Polymenidou, M. (2007) Insights into prion strains and neurotoxicity. Nat. Rev. Mol. Cell Biol. 8, 552–561 10.1038/nrm2204 [DOI] [PubMed] [Google Scholar]
- 360.Aguzzi, A. and Calella, A.M. (2009) Prions: protein aggregation and infectious diseases. Physiol. Rev. 89, 1105–1152 10.1152/physrev.00006.2009 [DOI] [PubMed] [Google Scholar]
- 361.Aguzzi, A. and Lakkaraju, A.K.K. (2016) Cell biology of prions and prionoids: a status report. Trends Cell Biol. 26, 40–51 10.1016/j.tcb.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 362.Meng, X., Munishkina, L.A., Fink, A.L. and Uversky, V.N. (2009) Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry 48, 8206–8224 10.1021/bi900506b [DOI] [PubMed] [Google Scholar]
- 363.Cortes-Canteli, M., Paul, J., Norris, E.H., Bronstein, R., Ahn, H.J., Zamolodchikov, D.et al. (2010) Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron 66, 695–709 10.1016/j.neuron.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hill, J.M. and Lukiw, W.J. (2015) Microbial-generated amyloids and Alzheimer's disease (AD). Front. Aging Neurosci. 7, 9 10.3389/fnagi.2015.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Ahn, H.J., Chen, Z.L., Zamolodchikov, D., Norris, E.H. and Strickland, S. (2017) Interactions of beta-amyloid peptide with fibrinogen and coagulation factor XII may contribute to Alzheimer's disease. Curr. Opin. Hematol. 24, 427–431 10.1097/MOH.0000000000000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Jana, A.K., Greenwood, A.B. and Hansmann, U.H.E. (2021) Presence of a SARS-CoV-2 protein enhances amyloid formation of serum amyloid A. J. Phys. Chem. B 125, 9155–9167 10.1021/acs.jpcb.1c04871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Michiels, E., Rousseau, F. and Schymkowitz, J. (2021) Mechanisms and therapeutic potential of interactions between human amyloids and viruses. Cell. Mol. Life Sci. 78, 2485–2501 10.1007/s00018-020-03711-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Nyström, S. and Hammarström, P. (2021) Amyloidogenesis of SARS-CoV-2 spike protein. bioRxiv 2021.2012.2016.472920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ezzat, K., Pernemalm, M., Palsson, S., Roberts, T.C., Järver, P., Dondalska, A.et al. (2019) The viral protein corona directs viral pathogenesis and amyloid aggregation. Nat. Commun. 10, 2331 10.1038/s41467-019-10192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Østergaard, L. (2021) SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 9, e14726 10.14814/phy2.14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Swanepoel, A.C., Lindeque, B.G., Swart, P.J., Abdool, Z. and Pretorius, E. (2014) Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: a qualitative investigation. Microsc. Res. Tech. 77, 594–601 10.1002/jemt.22378 [DOI] [PubMed] [Google Scholar]
- 372.Kell, D.B., Heyden, E.L. and Pretorius, E. (2020) The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 11, 1221 10.3389/fimmu.2020.01221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Pretorius, E. and Kell, D.B. (2014) Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr. Biol. 6, 486–510 10.1039/C4IB00025K [DOI] [PubMed] [Google Scholar]
- 374.Lipinski, B. and Pretorius, E. (2013) The role of iron-induced fibrin in the pathogenesis of Alzheimer's disease and the protective role of magnesium. Front. Hum. Neurosci. 7, 735 10.3389/fnhum.2013.00735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Groen, A.K., Wanders, R.J., Westerhoff, H.V., van der Meer, R. and Tager, J.M. (1982) Quantification of the contribution of various steps to the control of mitochondrial respiration. J. Biol. Chem. 257, 2754–2757 10.1016/S0021-9258(19)81026-8 [DOI] [PubMed] [Google Scholar]
- 376.van Beest, P., Wietasch, G., Scheeren, T., Spronk, P. and Kuiper, M. (2011) Clinical review: use of venous oxygen saturations as a goal - a yet unfinished puzzle. Crit. Care 15, 232 10.1186/cc10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Nelson, L.D. (1986) Continuous venous oximetry in surgical patients. Ann. Surg. 203, 329–333 10.1097/00000658-198603000-00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Oliynyk, O.V., Rorat, M. and Barg, W. (2021) Oxygen metabolism markers as predictors of mortality in severe COVID-19. Int. J. Infect. Dis. 103, 452–456 10.1016/j.ijid.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Elezagic, D., Johannis, W., Burst, V., Klein, F. and Streichert, T. (2021) Venous blood gas analysis in patients with COVID-19 symptoms in the early assessment of virus positivity. J. Lab. Med. 45, 27–30 10.1515/labmed-2020-0126 [DOI] [Google Scholar]
- 380.Philip, K.E.J., Bennett, B., Fuller, S., Lonergan, B., McFadyen, C., Burns, J.et al. (2020) Working accuracy of pulse oximetry in COVID-19 patients stepping down from intensive care: a clinical evaluation. BMJ Open Respir. Res. 7, e000778 10.1136/bmjresp-2020-000778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Rubano, J.A., Maloney, L.M., Simon, J., Rutigliano, D.N., Botwinick, I., Jawa, R.S.et al. (2021) An evolving clinical need: discordant oxygenation measurements of intubated COVID-19 patients. Ann. Biomed. Eng. 49, 959–963 10.1007/s10439-020-02722-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wilson-Baig, N., McDonnell, T. and Bentley, A. (2021) Discrepancy between Sp O2 and Sa O2 in patients with COVID-19. Anaesthesia 76, 6–7 10.1111/anae.15228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Ferrari, M. and Quaresima, V. (2020) Hypoxemia in COVID-19: cerebral oximetry should be explored as a warning indicator for mechanically ventilated adults with COVID-19. Respir. Res. 21, 261 10.1186/s12931-020-01530-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Invernizzi, A., Torre, A., Parrulli, S., Zicarelli, F., Schiuma, M., Colombo, V.et al. (2020) Retinal findings in patients with COVID-19: results from the SERPICO-19 study. EClinicalMedicine 27, 100550 10.1016/j.eclinm.2020.100550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Invernizzi, A., Schiuma, M., Parrulli, S., Torre, A., Zicarelli, F., Colombo, V.et al. (2021) Retinal vessels modifications in acute and post-COVID-19. Sci. Rep. 11, 19373 10.1038/s41598-021-98873-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Invernizzi, A. (2022) The impact of COVID-19 on the retina: clinical features and management considerations. Expert. Rev. Ophthalmol. 17, 53–60 10.1080/17469899.2022.2021877 [DOI] [Google Scholar]
- 387.Teo, K.Y., Invernizzi, A., Staurenghi, G. and Cheung, C.M.G. (2022) COVID-19-related retinal micro-vasculopathy - a review of current evidence. Am. J. Ophthalmol. 235, 98–110 10.1016/j.ajo.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Le Ng, X., Betzler, B.K., Testi, I., Ho, S.L., Tien, M., Ngo, W.K.et al. (2021) Ocular adverse events after COVID-19 vaccination. Ocul. Immunol. Inflamm. 29, 1216–1224 10.1080/09273948.2021.1976221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Barizien, N., Le Guen, M., Russel, S., Touche, P., Huang, F. and Vallee, A. (2021) Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 11, 14042 10.1038/s41598-021-93546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Asarcikli, L.D., Hayiroglu, M.I., Osken, A., Keskin, K., Kolak, Z. and Aksu, T. (2022) Heart rate variability and cardiac autonomic functions in post-COVID period. J. Interv. Card. Electrophysiol. 63, 715–721 10.1007/s10840-022-01138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Shah, B., Kunal, S., Bansal, A., Jain, J., Poundrik, S., Shetty, M.K.et al. (2022) Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol. J. 22, 70–76 10.1016/j.ipej.2022.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Chadda, K.R., Blakey, E.E., Huang, C.L. and Jeevaratnam, K. (2022) Long COVID-19 and postural orthostatic tachycardia syndrome- is dysautonomia to be blamed? Front. Cardiovasc. Med. 9, 860198 10.3389/fcvm.2022.860198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Varanasi, S., Sathyamoorthy, M., Chamakura, S. and Shah, S.A. (2021) Management of long-COVID postural orthostatic tachycardia syndrome with enhanced external counterpulsation. Cureus 13, e18398 10.7759/cureus.18398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Monaghan, A., Jennings, G., Xue, F., Byrne, L., Duggan, E. and Romero-Ortuno, R. (2022) Orthostatic intolerance in adults reporting long COVID symptoms was not associated with postural orthostatic tachycardia syndrome. Front. Physiol. 13, 833650 10.3389/fphys.2022.833650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Larsen, N.W., Stiles, L.E. and Miglis, M.G. (2021) Preparing for the long-haul: autonomic complications of COVID-19. Auton. Neurosci. 235, 102841 10.1016/j.autneu.2021.102841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Kay, G.N., Ashar, M.S., Bubien, R.S. and Dailey, S.M. (1995) Relationship between heart rate and oxygen kinetics during constant workload exercise. Pacing Clin. Electrophysiol. 18, 1853–1860 10.1111/j.1540-8159.1995.tb03832.x [DOI] [PubMed] [Google Scholar]
- 397.Emblem, K.E., Scheie, D., Due-Tonnessen, P., Nedregaard, B., Nome, T., Hald, J.K.et al. (2008) Histogram analysis of MR imaging-derived cerebral blood volume maps: combined glioma grading and identification of low-grade oligodendroglial subtypes. AJNR Am. J. Neuroradiol. 29, 1664–1670 10.3174/ajnr.A1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Bhargava, A., Monteagudo, B., Kushwaha, P., Senarathna, J., Ren, Y., Riddle, R.C.et al. (2022) Vascuviz: a multimodality and multiscale imaging and visualization pipeline for vascular systems biology. Nat. Methods 19, 242–254 10.1038/s41592-021-01363-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Epah, J., Palfi, K., Dienst, F.L., Malacarne, P.F., Bremer, R., Salamon, M.et al. (2018) 3D imaging and quantitative analysis of vascular networks: a comparison of ultramicroscopy and micro-computed tomography. Theranostics 8, 2117–2133 10.7150/thno.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Kirst, C., Skriabine, S., Vieites-Prado, A., Topilko, T., Bertin, P., Gerschenfeld, G.et al. (2020) Mapping the fine-scale organization and plasticity of the brain vasculature. Cell 180, 780–795.e725 10.1016/j.cell.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 401.Chaigneau, E., Oheim, M., Audinat, E. and Charpak, S. (2003) Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc. Natl Acad. Sci. U.S.A. 100, 13081–13086 10.1073/pnas.2133652100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Smith, A.F., Doyeux, V., Berg, M., Peyrounette, M., Haft-Javaherian, M., Larue, A.E.et al. (2019) Brain capillary networks across species: a few simple organizational requirements are sufficient to reproduce both structure and function. Front. Physiol. 10, 233 10.3389/fphys.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Gould, I.G., Tsai, P., Kleinfeld, D. and Linninger, A. (2017) The capillary bed offers the largest hemodynamic resistance to the cortical blood supply. J. Cereb. Blood Flow Metab. 37, 52–68 10.1177/0271678X16671146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.McMahan, C.A., Maxwell, L.C. and Shepherd, A.P. (1986) Estimation of the distribution of blood vessel diameters from the arteriovenous passage of microspheres. Biometrics 42, 371–380 10.2307/2531057 [DOI] [PubMed] [Google Scholar]
- 405.Müller, B., Lang, S., Dominietto, M., Rudin, M., Schulz, G., Deyhle, H.et al. (2008) High-resolution tomographic imaging of microvessels. Proc. SPIE 7078, 70780B 10.1117/12.794157 [DOI] [Google Scholar]
- 406.Razavi, M.S., Shirani, E. and Kassab, G.S. (2018) Scaling laws of flow rate, vessel blood volume, lengths, and transit times with number of capillaries. Front. Physiol. 9, 581 10.3389/fphys.2018.00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Diez-Silva, M., Dao, M., Han, J., Lim, C.T. and Suresh, S. (2010) Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull./Mater. Res. Soc. 35, 382–388 10.1557/mrs2010.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Pries, A.R., Neuhaus, D. and Gaehtgens, P. (1992) Blood viscosity in tube flow: dependence on diameter and hematocrit. Am. J. Physiol. 263, H1770–H1778 10.1152/ajpheart.1992.263.6.H1770 [DOI] [PubMed] [Google Scholar]
- 409.Kelch, I.D., Bogle, G., Sands, G.B., Phillips, A.R.J., LeGrice, I.J. and Dunbar, P.R. (2015) Organ-wide 3D-imaging and topological analysis of the continuous microvascular network in a murine lymph node. Sci. Rep. 5, 16534 10.1038/srep16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wang, L., Yuan, J., Jiang, H., Yan, W., Cintrón-Colón, H.R., Perez, V.L.et al. (2016) Vessel sampling and blood flow velocity distribution with vessel diameter for characterizing the human bulbar conjunctival microvasculature. Eye Contact Lens 42, 135–140 10.1097/ICL.0000000000000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Downey, G.P., Doherty, D.E., Schwab, III, B., Elson, E.L., Henson, P.M. and Worthen, G.S. (1990) Retention of leukocytes in capillaries: role of cell size and deformability. J. Appl. Physiol. (1985) 69, 1767–1778 10.1152/jappl.1990.69.5.1767 [DOI] [PubMed] [Google Scholar]
- 412.Schimmel, L., Heemskerk, N. and van Buul, J.D. (2017) Leukocyte transendothelial migration: a local affair. Small GTPases 8, 1–15 10.1080/21541248.2016.1197872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Saha, A.K., Schmidt, B.R., Wilhelmy, J., Nguyen, V., Abugherir, A., Do, J.K.et al. (2019) Red blood cell deformability is diminished in patients with chronic fatigue syndrome. Clin. Hemorheol. Microcirc. 71, 113–116 10.3233/CH-180469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Winkelmann, J.A., Eid, A., Spicer, G., Almassalha, L.M., Nguyen, T.Q. and Backman, V. (2019) Spectral contrast optical coherence tomography angiography enables single-scan vessel imaging. Light-Sci. Appl. 8, article no. 7 10.1038/s41377-018-0117-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Hilty, M.P., Guerci, P., Ince, Y., Toraman, F. and Ince, C. (2019) Microtools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun. Biol. 2, 217 10.1038/s42003-019-0473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Behrens, F., Holle, J., Kuebler, W.M. and Simmons, S. (2020) Extracellular vesicles as regulators of kidney function and disease. Intensive Care Med. Exp. 8, 22 10.1186/s40635-020-00306-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Chen, J., Sivan, U., Tan, S.L., Lippo, L., De Angelis, J., Labella, R.et al. (2021) High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging. Sci. Adv. 7, eabd7819 10.1126/sciadv.abd7819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Abdou, M.A.H., Truong, T.T., Dykky, A., Ferreira, P. and Jul, E. (2022) Capillarynet: an automated system to quantify skin capillary density and red blood cell velocity from handheld vital microscopy. Artif. Intell. Med. 127, 102287 10.1016/j.artmed.2022.102287 [DOI] [PubMed] [Google Scholar]
- 419.Lugo-Hernandez, E., Squire, A., Hagemann, N., Brenzel, A., Sardari, M., Schlechter, J.et al. (2017) 3D visualization and quantification of microvessels in the whole ischemic mouse brain using solvent-based clearing and light sheet microscopy. J. Cereb. Blood Flow Metab. 37, 3355–3367 10.1177/0271678X17698970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Basser, P. (2022) Detection of stroke by portable, low-field MRI: a milestone in medical imaging. Sci. Adv. 8, eabp9307 10.1126/sciadv.abp9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yuen, M.M., Prabhat, A.M., Mazurek, M.H., Chavva, I.R., Crawford, A., Cahn, B.A.et al. (2022) Portable, low-field magnetic resonance imaging enables highly accessible and dynamic bedside evaluation of ischemic stroke. Sci. Adv. 8, eabm3952 10.1126/sciadv.abm3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Pretorius, E., Venter, C., Laubscher, G.J., Kotze, M.J., Oladejo, S., Watson, L.R.et al. (2022) Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/ post-acute sequelae of COVID-19 (PASC) cardiovasc diabetol 21, 148 10.1186/s12933-022-01579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Robbins, P.D., Dorronsoro, A. and Booker, C.N. (2016) Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Invest. 126, 1173–1180 10.1172/JCI81131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Benedikter, B.J., Wouters, E.F.M., Savelkoul, P.H.M., Rohde, G.G.U. and Stassen, F.R.M. (2018) Extracellular vesicles released in response to respiratory exposures: implications for chronic disease. J. Toxicol. Environ. Health B Crit. Rev. 21, 142–160 10.1080/10937404.2018.1466380 [DOI] [PubMed] [Google Scholar]
- 425.Shetty, A.K. and Upadhya, R. (2021) Extracellular vesicles in health and disease. Aging Dis. 12, 1358–1362 10.14336/AD.2021.0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Trappe, A., Donnelly, S.C., McNally, P. and Coppinger, J.A. (2021) Role of extracellular vesicles in chronic lung disease. Thorax 76, 1047–1056 10.1136/thoraxjnl-2020-216370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Gomez, N., James, V., Onion, D. and Fairclough, L.C. (2022) Extracellular vesicles and chronic obstructive pulmonary disease (COPD): a systematic review. Respir. Res. 23, 82 10.1186/s12931-022-01984-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Withrow, J., Murphy, C., Liu, Y., Hunter, M., Fulzele, S. and Hamrick, M.W. (2016) Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis. Res. Ther. 18, 286 10.1186/s13075-016-1178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Burbano, C., Rojas, M., Muñoz-Vahos, C., Vanegas-García, A., Correa, L.A., Vásquez, G.et al. (2018) Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci. Rep. 8, 17917 10.1038/s41598-018-36335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Schioppo, T., Ubiali, T., Ingegnoli, F., Bollati, V. and Caporali, R. (2021) The role of extracellular vesicles in rheumatoid arthritis: a systematic review. Clin. Rheumatol. 40, 3481–3497 10.1007/s10067-021-05614-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Wang, W., Deng, Z., Liu, G., Yang, J., Zhou, W., Zhang, C.et al. (2021) Platelet-derived extracellular vesicles promote the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes via CXCR2 signaling. Exp. Ther. Med. 22, 1120 10.3892/etm.2021.10554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Alghamdi, M., Alamry, S.A., Bahlas, S.M., Uversky, V.N. and Redwan, E.M. (2022) Circulating extracellular vesicles and rheumatoid arthritis: a proteomic analysis. Cell. Mol. Life Sci. 79, 25 10.1007/s00018-021-04020-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Miao, H.B., Wang, F., Lin, S. and Chen, Z. (2022) Update on the role of extracellular vesicles in rheumatoid arthritis. Expert. Rev. Mol. Med. 24, e12 10.1017/erm.2021.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.González-Cebrián, A., Almenar-Pérez, E., Xu, J., Yu, T., Huang, W.E., Giménez-Orenga, K.et al. (2022) Diagnosis of myalgic encephalomyelitis/chronic fatigue syndrome with partial least squares discriminant analysis: relevance of Blood extracellular vesicles. Front. Med . 9, 842991 10.3389/fmed.2022.842991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Wai Yim, K.H., Borgoni, S. and Chahwan, R. (2022) Serum extracellular vesicles trace COVID-19 progression and immune responses. medRxiv 2022.2001.2019.22269529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Sun, B., Tang, N., Peluso, M.J., Iyer, N.S., Torres, L., Donatelli, J.L.et al. (2021) Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells 10, 386 10.3390/cells10020386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Richards, R.S., Wang, L. and Jelinek, H. (2007) Erythrocyte oxidative damage in chronic fatigue syndrome. Arch. Med. Res. 38, 94–98 10.1016/j.arcmed.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 438.Thomas, T., Stefanoni, D., Dzieciatkowska, M., Issaian, A., Nemkov, T., Hill, R.C.et al. (2020) Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J. Proteome Res. 19, 4455–4469 10.1021/acs.jproteome.0c00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Simpson, L.O. (1989) Nondiscocytic erythrocytes in myalgic encephalomyelitis. N. .Z Med. J. 102, 126–127 [PubMed] [Google Scholar]
- 440.Baklund, I.H., Dammen, T., Moum, TÅ, Kristiansen, W., Duarte, D.S., Castro-Marrero, J.et al. (2021) Evaluating routine blood tests according to clinical symptoms and diagnostic criteria in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. J. Clin. Med 10, 3105 10.3390/jcm10143105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.McMahon, C.J., Hopkins, S., Vail, A., King, A.T., Smith, D., Illingworth, K.J.et al. (2013) Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J. Neurointerv. Surg. 5, 512–517 10.1136/neurintsurg-2012-010386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Broadbent, S. and Coutts, R. (2015) Irregularities in red cell distribution width and lymphocyte concentration in individuals with chronic fatigue syndrome. Int. J. Health Sci. 3, 71–78 10.15640/ijhs.v3n4a7 [DOI] [Google Scholar]
- 443.Pasini, E., Corsetti, G., Romano, C., Scarabelli, T.M., Chen-Scarabelli, C., Saravolatz, L.et al. (2021) Serum metabolic profile in patients with long-COVID (PASC) syndrome: clinical implications. Front. Med. 8, 714426 10.3389/fmed.2021.714426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Zinellu, A. and Mangoni, A.A. (2021) Red blood cell distribution width, disease severity, and mortality in hospitalized patients with SARS-CoV-2 infection: a systematic review and meta-analysis. J. Clin. Med. 10, 286 10.3390/jcm10020286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Russo, A., Tellone, E., Barreca, D., Ficarra, S. and Lagana, G. (2022) Implication of COVID-19 on erythrocytes functionality: red blood cell biochemical implications and morpho-Functional aspects. Int. J. Mol. Sci. 23, 2171 10.3390/ijms23042171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wang, Z.H., Fu, B.Q., Lin, Y.W., Wei, X.B., Geng, H., Guo, W.X.et al. (2022) Red blood cell distribution width: a severity indicator in patients with COVID-19. J. Med. Virol. 94, 2133–2138 10.1002/jmv.27602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Karampitsakos, T., Akinosoglou, K., Papaioannou, O., Panou, V., Koromilias, A., Bakakos, P.et al. (2020) Increased Red cell distribution width Is associated with disease severity in hospitalized adults with SARS-CoV-2 infection: an observational multicentric study. Front. Med. (Lausanne) 7, 616292 10.3389/fmed.2020.616292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Guaní-Guerra, E., Torres-Murillo, B., Muñoz-Corona, C., Rodríguez-Jiménez, J.C., Macias, A.E., Scavo-Montes, D.A.et al. (2022) Diagnostic accuracy of the RDW for predicting death in COVID-19. Medicina (Kaunas) 58, 613 10.3390/medicina58050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lam, L.K.M., Reilly, J.P., Rux, A.H., Murphy, S.J., Kuri-Cervantes, L., Weisman, A.R.et al. (2021) Erythrocytes identify complement activation in patients with COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 321, L485–L489 10.1152/ajplung.00231.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Kisserli, A., Schneider, N., Audonnet, S., Tabary, T., Goury, A., Cousson, J.et al. (2021) Acquired decrease of the C3b/C4b receptor (CR1, CD35) and increased C4d deposits on erythrocytes from ICU COVID-19 patients. Immunobiology 226, 152093 10.1016/j.imbio.2021.152093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Lang, F., Abed, M., Lang, E. and Föller, M. (2014) Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 21, 138–153 10.1089/ars.2013.5747 [DOI] [PubMed] [Google Scholar]
- 452.Pretorius, E., du Plooy, J.N. and Bester, J. (2016) A comprehensive review on eryptosis. Cell. Physiol. Biochem. 39, 1977–2000 10.1159/000447895 [DOI] [PubMed] [Google Scholar]
- 453.Qadri, S.M., Bissinger, R., Solh, Z. and Oldenborg, P.A. (2017) Eryptosis in health and disease: a paradigm shift towards understanding the (patho)physiological implications of programmed cell death of erythrocytes. Blood Rev. 31, 349–361 10.1016/j.blre.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 454.Repsold, L. and Joubert, A.M. (2018) Eryptosis: an erythrocyte's suicidal type of cell death. Biomed. Res. Int. 2018, 9405617 10.1155/2018/9405617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Brun, J.F., Varlet-Marie, E., Myzia, J., de Mauverger, E.R. and Pretorius, E. (2022) Metabolic influences modulating erythrocyte deformability and eryptosis. Metabolites 12, 4 10.3390/metabo12010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Alzoubi, K., Egler, J., Abed, M. and Lang, F. (2015) Enhanced eryptosis following auranofin exposure. Cell. Physiol. Biochem. 37, 1018–1028 10.1159/000430228 [DOI] [PubMed] [Google Scholar]
- 457.Lang, F., Lang, E. and Foller, M. (2012) Physiology and pathophysiology of eryptosis. Transfus. Med. Hemother. 39, 308–314 10.1159/000342534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Lang, E. and Lang, F. (2015) Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin. Cell Dev. Biol. 39, 35–42 10.1016/j.semcdb.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 459.Lang, E., Qadri, S.M. and Lang, F. (2012) Killing me softly - suicidal erythrocyte death. Int. J. Biochem. Cell Biol. 44, 1236–1243 10.1016/j.biocel.2012.04.019 [DOI] [PubMed] [Google Scholar]
- 460.Mameli, A., Barcellona, D. and Marongiu, F. (2009) Rheumatoid arthritis and thrombosis. Clin. Exp. Rheumatol. 27, 846–855 [PubMed] [Google Scholar]
- 461.Holmqvist, M.E., Neovius, M., Eriksson, J., Mantel, Ä, Wållberg-Jonsson, S., Jacobsson, L.T.H.et al. (2012) Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. J. Am. Med. Assoc. 308, 1350–1356 10.1001/2012.jama.11741 [DOI] [PubMed] [Google Scholar]
- 462.Choi, H.K., Rho, Y.H., Zhu, Y., Cea-Soriano, L., Avina-Zubieta, J.A. and Zhang, Y. (2013) The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann. Rheum. Dis. 72, 1182–1187 10.1136/annrheumdis-2012-201669 [DOI] [PubMed] [Google Scholar]
- 463.Kim, S.C., Schneeweiss, S., Liu, J. and Solomon, D.H. (2013) Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res. (Hoboken) 65, 1600–1607 10.1002/acr.22039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Chung, W.S., Peng, C.L., Lin, C.L., Chang, Y.J., Chen, Y.F., Chiang, J.Y.et al. (2014) Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann. Rheum. Dis. 73, 1774–1780 10.1136/annrheumdis-2013-203380 [DOI] [PubMed] [Google Scholar]
- 465.Ungprasert, P., Srivali, N., Spanuchart, I., Thongprayoon, C. and Knight, E.L. (2014) Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin. Rheumatol. 33, 297–304 10.1007/s10067-014-2492-7 [DOI] [PubMed] [Google Scholar]
- 466.Ketfi, C., Boutigny, A., Mohamedi, N., Bouajil, S., Magnan, B., Amah, G.et al. (2021) Risk of venous thromboembolism in rheumatoid arthritis. Joint Bone Spine 88, 105122 10.1016/j.jbspin.2020.105122 [DOI] [PubMed] [Google Scholar]
- 467.Molander, V., Bower, H., Frisell, T. and Askling, J. (2021) Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann. Rheum. Dis. 80, 169–175 10.1136/annrheumdis-2020-218419 [DOI] [PubMed] [Google Scholar]
- 468.Cui, S., Chen, S., Li, X., Liu, S. and Wang, F. (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 18, 1421–1424 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Goldman, I.A., Ye, K. and Scheinfeld, M.H. (2020) Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology 297, E263–E269 10.1148/radiol.2020202348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Sakr, Y., Giovini, M., Leone, M., Pizzilli, G., Kortgen, A., Bauer, M.et al. (2020) Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann. Intensive Care 10, 124 10.1186/s13613-020-00741-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Gómez, C.A., Sun, C.K., Tsai, I.T., Chang, Y.P., Lin, M.C., Hung, I.Y.et al. (2021) Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci. Rep. 11, 16025 10.1038/s41598-021-95512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Hesam-Shariati, S., Fatehi, P., Abouzaripour, M., Fathi, F., Hesam-Shariati, N. and Hesam Shariati, M.B. (2021) Increased pulmonary embolism in patients with COVID-19: a case series and literature review. Trop. Dis. Travel. Med. Vaccines 7, 16 10.1186/s40794-021-00145-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Husser, D., Hohenstein, S., Pellissier, V., Ueberham, L., Konig, S., Hindricks, G.et al. (2021) Potential contributors to increased pulmonary embolism hospitalizations during the COVID-19 pandemic: insights from the German-wide Helios Hospital Network. Front. Cardiovasc. Med. 8, 715761 10.3389/fcvm.2021.715761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Jevnikar, M., Sanchez, O., Chocron, R., Andronikof, M., Raphael, M., Meyrignac, O.et al. (2021) Prevalence of pulmonary embolism in patients with COVID-19 at the time of hospital admission. Eur. Respir. J. 58, 2100116 10.1183/13993003.00116-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Miró, Ó, Jiménez, S., Mebazaa, A., Freund, Y., Burillo-Putze, G., Martin, A.et al. (2021) Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur. Heart J. 42, 3127–3142 10.1093/eurheartj/ehab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Oba, S., Hosoya, T., Amamiya, M., Mitsumura, T., Kawata, D., Sasaki, H.et al. (2021) Arterial and venous thrombosis complicated in COVID-19: a retrospective single center analysis in Japan. Front. Cardiovasc. Med. 8, 767074 10.3389/fcvm.2021.767074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Parekh, Y.H., Altomare, N.J., McDonnell, E.P., Blaser, M.J. and Parikh, P.D. (2021) Recurrence of upper extremity deep vein thrombosis secondary to COVID-19. Viruses 13, 878 10.3390/v13050878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Porfidia, A., Mosoni, C., Talerico, R., Porceddu, E., Lupascu, A., Tondi, P.et al. (2021) Pulmonary embolism in COVID-19 patients: which diagnostic algorithm should we use? Front. Cardiovasc. Med. 8, 714003 10.3389/fcvm.2021.714003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Riyahi, S., Dev, H., Behzadi, A., Kim, J., Attari, H., Raza, S.I.et al. (2021) Pulmonary embolism in hospitalized patients with COVID-19: a multicenter study. Radiology 301, E426–E433 10.1148/radiol.2021210777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Lund, L.C., Hallas, J., Nielsen, H., Koch, A., Mogensen, S.H., Brun, N.C.et al. (2021) Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect. Dis. 21, 1373–1382 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Yan, Z., Yang, M. and Lai, C.L. (2021) Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines 9, 966 10.3390/biomedicines9080966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Ponomaryov, T., Payne, H., Fabritz, L., Wagner, D.D. and Brill, A. (2017) Mast cells granular contents are crucial for deep vein thrombosis in mice. Circ. Res. 121, 941–950 10.1161/CIRCRESAHA.117.311185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Levi, M. and van der Poll, T. (2017) Coagulation and sepsis. Thromb. Res. 149, 38–44 10.1016/j.thromres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 484.Connors, J.M. and Levy, J.H. (2020) COVID-19 and its implications for thrombosis and anticoagulation. Blood 135, 2033–2040 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Whyte, C.S., Morrow, G.B., Mitchell, J.L., Chowdary, P. and Mutch, N.J. (2020) Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 18, 1548–1555 10.1111/jth.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.van der Slikke, E.C., An, A.Y., Hancock, R.E.W. and Bouma, H.R. (2020) Exploring the pathophysiology of post-sepsis syndrome to identify therapeutic opportunities. EBioMedicine 61, 103044 10.1016/j.ebiom.2020.103044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Mostel, Z., Perl, A., Marck, M., Mehdi, S.F., Lowell, B., Bathija, S.et al. (2019) Post-sepsis syndrome - an evolving entity that afflicts survivors of sepsis. Mol. Med. 26, 6 10.1186/s10020-019-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Huang, C.Y., Daniels, R., Lembo, A., Hartog, C., O'Brien, J., Heymann, T.et al. (2019) Life after sepsis: an international survey of survivors to understand the post-sepsis syndrome. Int. J. Qual. Health Care 31, 191–198 10.1093/intqhc/mzy137 [DOI] [PubMed] [Google Scholar]
- 489.Zhu, X., Wu, Y., Lv, X., Liu, Y., Du, G., Li, J.et al. (2022) Combining CRISPR-Cpf1 and recombineering facilitates fast and efficient genome editing in Escherichia coli. ACS Synth. Biol. 11, 1897–1907 10.1021/acssynbio.2c00041 [DOI] [PubMed] [Google Scholar]
- 490.Shovman, O., Tiosano, S., Comaneshter, D., Cohen, A.D., Amital, H. and Sherf, M. (2016) Aortic aneurysm associated with rheumatoid arthritis: a population-based cross-sectional study. Clin. Rheumatol. 35, 2657–2661 10.1007/s10067-016-3372-0 [DOI] [PubMed] [Google Scholar]
- 491.Fiani, B., Fowler, J.B., Figueras, R.A., Hessamian, K., Mercado, N., Vukcevich, O.et al. (2021) Ruptured cerebral aneurysms in COVID-19 patients: a review of literature with case examples. Surg. Neurol. Int. 12, 187 10.25259/SNI_214_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Richardson, K.L., Jain, A., Evans, J. and Uzun, O. (2021) Giant coronary artery aneurysm as a feature of coronavirus-related inflammatory syndrome. BMJ Case Rep. 14, e238740 10.1136/bcr-2020-238740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Wang, L., Zhang, S., Ma, J., Ni, J., Wang, J., Li, X.et al. (2021) Kawasaki disease- management strategies given symptoms overlap to COVID-19: a review. JNMA J. Nepal. Med. Assoc. 59, 417–424 10.31729/jnma.5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Western, E., Sorteberg, A., Brunborg, C. and Nordenmark, T.H. (2020) Prevalence and predictors of fatigue after aneurysmal subarachnoid hemorrhage. Acta Neurochir. (Wien) 162, 3107–3116 10.1007/s00701-020-04538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Western, E., Nordenmark, T.H., Sorteberg, W., Karic, T. and Sorteberg, A. (2021) Fatigue after aneurysmal subarachnoid hemorrhage: clinical characteristics and associated factors in patients with good outcome. Front. Behav. Neurosci. 15, 633616 10.3389/fnbeh.2021.633616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Massey, P.R. and Jones, K.M. (2020) Going viral: a brief history of Chilblain-like skin lesions ("COVID toes") amidst the COVID-19 pandemic. Semin. Oncol. 47, 330–334 10.1053/j.seminoncol.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Micevic, G., Morris, J., Lee, A.I. and King, B.A. (2020) Perniolike lesions and coagulopathy in a patient with COVID-19 infection. JAAD Case Rep. 6, 1294–1296 10.1016/j.jdcr.2020.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Discepolo, V., Catzola, A., Pierri, L., Mascolo, M., Della Casa, F., Vastarella, M.et al. (2021) Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw. Open. 4, e2111369 10.1001/jamanetworkopen.2021.11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Rocha, K.O., Zanuncio, V.V., de Freitas, C., and Lima, B.A. and M, L. (2021) "COVID toes": a meta-analysis of case and observational studies on clinical, histopathological, and laboratory findings. Pediatr. Dermatol. 38, 1143–1149 10.1111/pde.14805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Kashetsky, N., Mukovozov, I.M. and Bergman, J. (2021) Chilblain-like lesions (CLL) associated with COVID-19 ("COVID toes"): a systematic review. J. Cutan Med. Surg. 25, 627–633 10.1177/12034754211004575 [DOI] [PubMed] [Google Scholar]
- 501.Yilmaz, M.M., Szabolcs, M.J., Geskin, L.J. and Niedt, G.W. (2021) An autopsy review: "COVID toes". Am. J. Dermatopathol. 43, 554–555 10.1097/DAD.0000000000001827 [DOI] [PubMed] [Google Scholar]
- 502.Kolivras, A., Thompson, C., Pastushenko, I., Mathieu, M., Bruderer, P., de Vicq, M.et al. (2022) A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J. Cutan. Pathol. 49, 17–28 10.1111/cup.14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Mehta, P., Bunker, C.B., Ciurtin, C., Porter, J.C., Chambers, R.C., Papdopoulou, C.et al. (2021) Chilblain-like acral lesions in long COVID-19: management and implications for understanding microangiopathy. Lancet Infect. Dis. 21, 912 10.1016/S1473-3099(21)00133-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.André, R., Hsieh, A. and Trellu, L.T. (2022) Chronic acral lesions ("COVID toes"): to add to long post- COVID-19 syndrome? Angiology 33197211068938 10.1177/00033197211068938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Nirenberg, M.S., Requena, L., Santonja, C., Smith, G.T. and McClain, S.A. (2022) Histopathology of Persistent long COVID Toe: a case report. J. Cutan. Pathol. 9, 791–794 10.1111/cup.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Frumholtz, L., Bouaziz, J.D., Battistella, M., Hadjadj, J., Chocron, R., Bengoufa, D.et al. (2021) Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br. J. Dermatol. 185, 1176–1185 10.1111/bjd.20707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Çakmak, F., Demirbuga, A., Demirkol, D., Gümüş, S., Torun, S.H., Kayaalp, G.K.et al. (2021) Nailfold capillaroscopy: a sensitive method for evaluating microvascular involvement in children with SARS-CoV-2 infection. Microvasc. Res. 138, 104196 10.1016/j.mvr.2021.104196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Natalello, G., De Luca, G., Gigante, L., Campochiaro, C., De Lorenzis, E., Verardi, L.et al. (2021) Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement. Microvasc. Res. 133, 104071 10.1016/j.mvr.2020.104071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Sulli, A., Gotelli, E., Bica, P.F., Schiavetti, I., Pizzorni, C., Aloe, T.et al. (2022) Detailed videocapillaroscopic microvascular changes detectable in adult COVID-19 survivors. Microvasc. Res. 142, 104361 10.1016/j.mvr.2022.104361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Madamanchi, N.R., Vendrov, A. and Runge, M.S. (2005) Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 25, 29–38 10.1161/01.ATV.0000150649.39934.13 [DOI] [PubMed] [Google Scholar]
- 511.Kane, A.D., Kothmann, E. and Giussani, D.A. (2020) Detection and response to acute systemic hypoxia. BJA Educ. 20, 58–64 10.1016/j.bjae.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Cavezzi, A., Troiani, E. and Corrao, S. (2020) COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin. Pract. 10, 1271 10.4081/cp.2020.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Negri, E.M., Piloto, B.M., Morinaga, L.K., Jardim, C.V.P., Lamy, S.A.E., Ferreira, M.A.et al. (2020) Heparin therapy improving hypoxia in COVID-19 patients - a case series. Front. Physiol. 11, 573044 10.3389/fphys.2020.573044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Somers, V.K., Kara, T. and Xie, J. (2020) Progressive hypoxia: a pivotal pathophysiologic mechanism of COVID-19 pneumonia. Mayo Clin. Proc. 95, 2339–2342 10.1016/j.mayocp.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Alvis, B., Vaughn, L., Schmeckpeper, J., Huston, J., Case, M., Semler, M.et al. (2021) Respiratory non-invasive venous waveform analysis for assessment of respiratory distress in coronavirus disease 2019 patients: an observational study. Crit. Care Explor. 3, e0539 10.1097/CCE.0000000000000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Böning, D., Kuebler, W.M. and Bloch, W. (2021) The oxygen dissociation curve of blood in COVID-19. Am. J. Physiol. Lung Cell Mol. Physiol. 321, L349–L357 10.1152/ajplung.00079.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Brouqui, P., Amrane, S., Million, M., Cortaredona, S., Parola, P., Lagier, J.C.et al. (2021) Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int. J. Infect. Dis. 102, 233–238 10.1016/j.ijid.2020.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Mejia, F., Medina, C., Cornejo, E., Morello, E., Vásquez, S., Alave, J.et al. (2020) Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One 15, e0244171 10.1371/journal.pone.0244171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Nechipurenko, Y.D., Semyonov, D.A., Lavrinenko, I.A., Lagutkin, D.A., Generalov, E.A., Zaitceva, A.Y.et al. (2021) The role of acidosis in the pathogenesis of severe forms of COVID-19. Biology (Basel) 10, 852 10.3390/biology10090852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Páez-Franco, J.C., Torres-Ruiz, J., Sosa-Hernández, V.A., Cervantes-Díaz, R., Romero-Ramírez, S., Pérez-Fragoso, A.et al. (2021) Metabolomics analysis reveals a modified amino acid metabolism that correlates with altered oxygen homeostasis in COVID-19 patients. Sci. Rep. 11, 6350 10.1038/s41598-021-85788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Rahman, A., Tabassum, T., Araf, Y., Al Nahid, A., Ullah, M.A. and Hosen, M.J. (2021) Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol. Biol. Rep. 48, 3863–3869 10.1007/s11033-021-06358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Østergaard, L. (2020) Blood flow, capillary transit times, and tissue oxygenation: the centennial of capillary recruitment. J. Appl. Physiol. 129, 1413–1421 10.1152/japplphysiol.00537.2020 [DOI] [PubMed] [Google Scholar]
- 523.Herrmann, J., Mori, V., Bates, J.H.T. and Suki, B. (2020) Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat. Commun. 11, 4883 10.1038/s41467-020-18672-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Olumuyiwa-Akeredolu, O.O. and Pretorius, E. (2016) Rheumatoid arthritis: notable biomarkers linking to chronic systemic conditions and cancer. Curr. Pharm. Des. 22, 918–924 10.2174/1381612822666151209153535 [DOI] [PubMed] [Google Scholar]
- 525.Mantel, Ä, Holmqvist, M., Andersson, D.C., Lund, L.H. and Askling, J. (2017) Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J. Am. Coll. Cardiol. 69, 1275–1285 10.1016/j.jacc.2016.12.033 [DOI] [PubMed] [Google Scholar]
- 526.Bezuidenhout, J., Venter, C., Roberts, T., Tarr, G., Kell, D. and Pretorius, E. (2020) The atypical fibrin fibre network in rheumatoid arthritis and its relation to autoimmunity, inflammation and thrombosis. bioRxiv 2020.2005.2028.121301v121301 [Google Scholar]
- 527.Mirabello, V., Cortezon-Tamarit, F. and Pascu, S.I. (2018) Oxygen sensing, hypoxia tracing and in vivo imaging with functional metalloprobes for the early detection of non-communicable diseases. Front. Chem. 6, 27 10.3389/fchem.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Zheng, X.C., Wang, X., Mao, H., Wu, W., Liu, B.R. and Jiang, X.Q. (2015) Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 6, 5834 10.1038/ncomms6834 [DOI] [PubMed] [Google Scholar]
- 529.Ziello, J.E., Jovin, I.S. and Huang, Y. (2007) Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 80, 51–60 [PMC free article] [PubMed] [Google Scholar]
- 530.Batie, M. and Rocha, S. (2020) Gene transcription and chromatin regulation in hypoxia. Biochem. Soc. Trans. 48, 1121–1128 10.1042/BST20191106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Frost, J., Frost, M., Batie, M., Jiang, H. and Rocha, S. (2021) Roles of HIF and 2-oxoglutarate-dependent dioxygenases in controlling gene expression in hypoxia. Cancers (Basel) 13, 350 10.3390/cancers13020350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Betteridge, D.J. (2000) What is oxidative stress? Metabolism 49, 3–8 10.1016/S0026-0495(00)80077-3 [DOI] [PubMed] [Google Scholar]
- 533.Butterfield, D.A. (2002) Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic. Res. 36, 1307–1313 10.1080/1071576021000049890 [DOI] [PubMed] [Google Scholar]
- 534.Cutler, R.G. and Rodriguez, H. (2002) Critical reviews of Oxidative Stress and Aging: Advances in Basic Science, Diagnostics And Intervention, World Scientific, Singapore [Google Scholar]
- 535.Maritim, A.C., Sanders, R.A. and Watkins, III, J.B. (2003) Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 17, 24–38 10.1002/jbt.10058 [DOI] [PubMed] [Google Scholar]
- 536.Uttara, B., Singh, A.V., Zamboni, P. and Mahajan, R.T. (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7, 65–74 10.2174/157015909787602823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T.D., Mazur, M. and Telser, J. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 538.Halliwell, B. (2011) Free radicals and antioxidants - quo vadis? Trends Pharmacol. Sci. 32, 125–130 10.1016/j.tips.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 539.Halliwell, B. and Gutteridge, J.M.C. (2015) Free Radicals in Biology and Medicine, 5th Ed., Oxford University Press, Oxford [Google Scholar]
- 540.Pisoschi, A.M. and Pop, A. (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 97, 55–74 10.1016/j.ejmech.2015.04.040 [DOI] [PubMed] [Google Scholar]
- 541.Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V.et al. (2017) Oxidative stress: harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 8416763 10.1155/2017/8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Siti, H.N., Kamisah, Y. and Kamsiah, J. (2015) The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 71, 40–56 10.1016/j.vph.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 543.Poprac, P., Jomova, K., Simunkova, M., Kollar, V., Rhodes, C.J. and Valko, M. (2017) Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 38, 592–607 10.1016/j.tips.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 544.Simunkova, M., Alwasel, S.H., Alhazza, I.M., Jomova, K., Kollar, V., Rusko, M.et al. (2019) Management of oxidative stress and other pathologies in Alzheimer's disease. Arch. Toxicol. 93, 2491–2513 10.1007/s00204-019-02538-y [DOI] [PubMed] [Google Scholar]
- 545.Pisoschi, A.M., Pop, A., Iordache, F., Stanca, L., Predoi, G. and Serban, A.I. (2021) Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 209, 112891 10.1016/j.ejmech.2020.112891 [DOI] [PubMed] [Google Scholar]
- 546.Kennedy, G., Spence, V.A., McLaren, M., Hill, A., Underwood, C. and Belch, J.J. (2005) Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic. Biol. Med. 39, 584–589 10.1016/j.freeradbiomed.2005.04.020 [DOI] [PubMed] [Google Scholar]
- 547.Paul, B.D., Lemle, M.D., Komaroff, A.L. and Snyder, S.H. (2021) Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl Acad. Sci. U.S.A. 118, e2024358118 10.1073/pnas.2024358118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Toogood, P.L., Clauw, D.J., Phadke, S. and Hoffman, D. (2021) Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): where will the drugs come from? Pharmacol. Res. 165, 105465 10.1016/j.phrs.2021.105465 [DOI] [PubMed] [Google Scholar]
- 549.Pucino, V., Certo, M., Bulusu, V., Cucchi, D., Goldmann, K., Pontarini, E.et al. (2019) Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. 30, 1055–1074.e1058 10.1016/j.cmet.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Pucino, V., Certo, M., Varricchi, G., Marone, G., Ursini, F., Rossi, F.W.et al. (2020) Metabolic checkpoints in rheumatoid arthritis. Front. Physiol. 11, 347 10.3389/fphys.2020.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Qiu, J., Wu, B., Goodman, S.B., Berry, G.J., Goronzy, J.J. and Weyand, C.M. (2021) Metabolic control of autoimmunity and tissue inflammation in rheumatoid arthritis. Front. Immunol. 12, 652771 10.3389/fimmu.2021.652771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Shungu, D.C., Weiduschat, N., Murrough, J.W., Mao, X., Pillemer, S., Dyke, J.P.et al. (2012) Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 25, 1073–1087 10.1002/nbm.2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Wallis, A., Ball, M., McKechnie, S., Butt, H., Lewis, D.P. and Bruck, D. (2017) Examining clinical similarities between myalgic encephalomyelitis/chronic fatigue syndrome and D-lactic acidosis: a systematic review. J. Transl. Med. 15, 129 10.1186/s12967-017-1229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ghali, A., Lacout, C., Ghali, M., Gury, A., Beucher, A.B., Lozac'h, P.et al. (2019) Elevated blood lactate in resting conditions correlate with post-exertional malaise severity in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Sci. Rep. 9, 18817 10.1038/s41598-019-55473-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Lien, K., Johansen, B., Veierød, M.B., Haslestad, A.S., Bøhn, S.K., Melsom, M.N.et al. (2019) Abnormal blood lactate accumulation during repeated exercise testing in myalgic encephalomyelitis/chronic fatigue syndrome. Physiol. Rep. 7, e14138 10.14814/phy2.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Nilsson, I., Palmer, J., Apostolou, E., Gottfries, C.G., Rizwan, M., Dahle, C.et al. (2020) Metabolic dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome not due to anti-mitochondrial antibodies. Front. Med. (Lausanne) 7, 108 10.3389/fmed.2020.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Murrough, J.W., Mao, X., Collins, K.A., Kelly, C., Andrade, G., Nestadt, P.et al. (2010) Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR Biomed. 23, 643–650 10.1002/nbm.1512 [DOI] [PubMed] [Google Scholar]
- 558.Bruno, R.R., Wernly, B., Flaatten, H., Fjolner, J., Artigas, A., Bollen Pinto, B.et al. (2021) Lactate is associated with mortality in very old intensive care patients suffering from COVID-19: results from an international observational study of 2860 patients. Ann. Intensive Care 11, 128 10.1186/s13613-021-00911-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Iepsen, U.W., Plovsing, R.R., Tjelle, K., Foss, N.B., Meyhoff, C.S., Ryrsø, C.K.et al. (2021) The role of lactate in sepsis and COVID-19: perspective from contracting skeletal muscle metabolism. Exp. Physiol. 107, 665–673 10.1113/EP089474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Velavan, T.P., Kieu Linh, L.T., Kreidenweiss, A., Gabor, J., Krishna, S. and Kremsner, P.G. (2021) Longitudinal monitoring of lactate in hospitalized and ambulatory COVID-19 patients. Am. J. Trop. Med. Hyg. 104, 1041–1044 10.4269/ajtmh.20-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Carpenè, G., Onorato, D., Nocini, R., Fortunato, G., Rizk, J.G., Henry, B.M.et al. (2022) Blood lactate concentration in COVID-19: a systematic literature review. Clin. Chem. Lab. Med. 60, 332–337 10.1515/cclm-2021-1115 [DOI] [PubMed] [Google Scholar]
- 562.Santinelli, L., Laghi, L., Innocenti, G.P., Pinacchio, C., Vassalini, P., Celani, L.et al. (2021) Oral bacteriotherapy reduces the occurrence of chronic fatigue in COVID-19 patients. Front. Nutr. 8, 756177 10.3389/fnut.2021.756177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Herce-Pagliai, C., Kotecha, S. and Shuker, D.E.G. (1998) Analytical methods for 3-nitrotyrosine as a marker of exposure to reactive nitrogen species: a review. Nitric Oxide-Biol. Chem. 2, 324–336 10.1006/niox.1998.0192 [DOI] [PubMed] [Google Scholar]
- 564.Bian, K., Gao, Z., Weisbrodt, N. and Murad, F. (2003) The nature of heme/iron-induced protein tyrosine nitration. Proc. Natl Acad. Sci. U.S.A. 100, 5712–5717 10.1073/pnas.0931291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Duncan, M.W. (2003) A review of approaches to the analysis of 3-nitrotyrosine. Amino Acids 25, 351–361 10.1007/s00726-003-0022-z [DOI] [PubMed] [Google Scholar]
- 566.Ryberg, H. and Caidahl, K. (2007) Chromatographic and mass spectrometric methods for quantitative determination of 3-nitrotyrosine in biological samples and their application to human samples. J. Chromatogr. B 851, 160–171 10.1016/j.jchromb.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 567.Campolo, N., Issoglio, F.M., Estrin, D.A., Bartesaghi, S. and Radi, R. (2020) 3-Nitrotyrosine and related derivatives in proteins: precursors, radical intermediates and impact in function. Essays Biochem. 64, 111–133 10.1042/EBC20190052 [DOI] [PubMed] [Google Scholar]
- 568.Janero, D.R. (1990) Malondialdehyde and thiobarbituric acid reactivity as diagnostic indexes of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 9, 515–540 10.1016/0891-5849(90)90131-2 [DOI] [PubMed] [Google Scholar]
- 569.Del Rio, D., Stewart, A.J. and Pellegrini, N. (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15, 316–328 10.1016/j.numecd.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 570.Aust, A.E. and Eveleigh, J.F. (1999) Mechanisms of DNA oxidation. Proc. Soc. Exp. Biol. Med. 222, 246–252 10.1046/j.1525-1373.1999.d01-141.x [DOI] [PubMed] [Google Scholar]
- 571.Cooke, M.S., Lunec, J. and Evans, M.D. (2002) Progress in the analysis of urinary oxidative DNA damage. Free Radic. Biol. Med. 33, 1601–1614 10.1016/S0891-5849(02)01146-2 [DOI] [PubMed] [Google Scholar]
- 572.Dizdaroglu, M., Jaruga, P., Birincioglu, M. and Rodriguez, H. (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic. Biol. Med. 32, 1102–1115 10.1016/S0891-5849(02)00826-2 [DOI] [PubMed] [Google Scholar]
- 573.Neeley, W.L. and Essigmann, J.M. (2006) Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 19, 491–505 10.1021/tx0600043 [DOI] [PubMed] [Google Scholar]
- 574.Hwang, E.S. and Bowen, P.E. (2007) DNA damage, a biomarker of carcinogenesis: its measurement and modulation by diet and environment. Crit. Rev. Food Sci. Nutr. 47, 27–50 10.1080/10408390600550299 [DOI] [PubMed] [Google Scholar]
- 575.Kageyama, Y., Takahashi, M., Ichikawa, T., Torikai, E. and Nagano, A. (2008) Reduction of oxidative stress marker levels by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 26, 73–80 [PubMed] [Google Scholar]
- 576.Pemberton, P.W., Ahmad, Y., Bodill, H., Lokko, D., Hider, S.L., Yates, A.P.et al. (2009) Biomarkers of oxidant stress, insulin sensitivity and endothelial activation in rheumatoid arthritis: a cross-sectional study of their association with accelerated atherosclerosis. BMC Res. Notes 2, 83 10.1186/1756-0500-2-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Fu, J., Schoeman, J.C., Harms, A.C., van Wietmarschen, H.A., Vreeken, R.J., Berger, R.et al. (2016) Metabolomics profiling of the free and total oxidised lipids in urine by LC-MS/MS: application in patients with rheumatoid arthritis. Anal. Bioanal. Chem. 408, 6307–6319 10.1007/s00216-016-9742-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Bowers, I. and Subedi, B. (2021) Isoprostanes in wastewater as biomarkers of oxidative stress during COVID-19 pandemic. Chemosphere 271, 129489 10.1016/j.chemosphere.2020.129489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Grossini, E., Concina, D., Rinaldi, C., Russotto, S., Garhwal, D., Zeppegno, P.et al. (2021) Association between plasma redox state/mitochondria function and a Flu-like syndrome/COVID-19 in the elderly admitted to a long-term care unit. Front. Physiol. 12, 707587 10.3389/fphys.2021.707587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Kumar, P., Osahon, O., Vides, D.B., Hanania, N., Minard, C.G. and Sekhar, R.V. (2022) Severe glutathione deficiency, oxidative stress and oxidant damage in adults hospitalized with COVID-19: implications for GlyNAC (glycine and N-acetylcysteine) supplementation. Antioxidants (Basel) 11, 50 10.3390/antiox11010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Soto, M.E., Guarner-Lans, V., Díaz-Díaz, E., Manzano-Pech, L., Palacios-Chavarria, A., Valdez-Vázquez, R.R.et al. (2022) Hyperglycemia and loss of redox homeostasis in COVID-19 patients. Cells 11, 932 10.3390/cells11060932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Dominic, P., Ahmad, J., Bhandari, R., Pardue, S., Solorzano, J., Jaisingh, K.et al. (2021) Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 43, 101982 10.1016/j.redox.2021.101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Hayden, M.R. (2020) An immediate and long-term complication of COVID-19 May be type 2 diabetes mellitus: the central role of beta-cell dysfunction, apoptosis and exploration of possible mechanisms. Cells 9, 2475 10.3390/cells9112475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Misko, T.P., Radabaugh, M.R., Highkin, M., Abrams, M., Friese, O., Gallavan, R.et al. (2013) Characterization of nitrotyrosine as a biomarker for arthritis and joint injury. Osteoarthr. Cartil. 21, 151–156 10.1016/j.joca.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 585.Khan, M.A., Alam, K., Zafaryab, M. and Rizvi, M.M.A. (2017) Peroxynitrite-modified histone as a pathophysiological biomarker in autoimmune diseases. Biochimie 140, 1–9 10.1016/j.biochi.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 586.Sá da Fonseca, L.J., Nunes-Souza, V., Goulart, M.O.F. and Rabelo, L.A. (2019) Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold regarding Novel Biomarkers and Add-On Therapies. Oxid. Med. Cell. Longev. 2019, 7536805 10.1155/2019/7536805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Steinz, M.M., Persson, M., Aresh, B., Olsson, K., Cheng, A.J., Ahlstrand, E.et al. (2019) Oxidative hotspots on actin promote skeletal muscle weakness in rheumatoid arthritis. JCI Insight 4, e126347 10.1172/jci.insight.126347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Maes, M., Mihaylova, I. and Leunis, J.C. (2006) Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol. Lett. 27, 615–621 [PubMed] [Google Scholar]
- 589.Veselinovic, M., Barudzic, N., Vuletic, M., Zivkovic, V., Tomic-Lucic, A., Djuric, D.et al. (2014) Oxidative stress in rheumatoid arthritis patients: relationship to diseases activity. Mol. Cell. Biochem. 391, 225–232 10.1007/s11010-014-2006-6 [DOI] [PubMed] [Google Scholar]
- 590.García-González, A., Gaxiola-Robles, R. and Zenteno-Savín, T. (2015) Oxidative stress in patients with rheumatoid arthritis. Rev. Invest. Clin. 67, 46–53 [PubMed] [Google Scholar]
- 591.Mititelu, R.R., Pădureanu, R., Băcănoiu, M., Pădureanu, V., Docea, A.O., Calina, D.et al. (2020) Inflammatory and oxidative stress Markers-Mirror tools in rheumatoid arthritis. Biomedicines 8, 125 10.3390/biomedicines8050125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Richards, R.S., Roberts, T.K., McGregor, N.R., Dunstan, R.H. and Butt, H.L. (2000) Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 5, 35–41 10.1179/rer.2000.5.1.35 [DOI] [PubMed] [Google Scholar]
- 593.Jammes, Y., Steinberg, J.G. and Delliaux, S. (2013) Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. Open J. Intern. Med. 272, 74–84 10.1111/j.1365-2796.2011.02488.x [DOI] [PubMed] [Google Scholar]
- 594.Fenouillet, E., Vigouroux, A., Steinberg, J.G., Chagvardieff, A., Retornaz, F., Guieu, R.et al. (2016) Association of biomarkers with health-related quality of life and history of stressors in myalgic encephalomyelitis/chronic fatigue syndrome patients. J. Transl. Med. 14, 251 10.1186/s12967-016-1010-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Brkic, S., Tomic, S., Maric, D., Novakov Mikic, A. and Turkulov, V. (2010) Lipid peroxidation is elevated in female patients with chronic fatigue syndrome. Med. Sci. Monit. 16, CR628–CR632 [PubMed] [Google Scholar]
- 596.Liu, T., Zhang, L., Joo, D. and Sun, S.C. (2017) NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther 2, 17023 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Ma, Q. (2013) Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426 10.1146/annurev-pharmtox-011112-140320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Kerins, M.J. and Ooi, A. (2018) The roles of NRF2 in modulating cellular iron homeostasis. Antioxid. Redox Signal. 29, 1756–1773 10.1089/ars.2017.7176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Schreck, R., Albermann, K. and Baeuerle, P.A. (1992) Nuclear factor kappa-B - an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Rad. Res. Commun. 17, 221–237 10.3109/10715769209079515 [DOI] [PubMed] [Google Scholar]
- 600.D'Ignazio, L. and Rocha, S. (2016) Hypoxia induced NF-kappaB. Cells 5, 10 10.3390/cells5010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Sun, Y. and Oberley, L.W. (1996) Redox regulation of transcriptional activators. Free Radic. Biol. Med. 21, 335–348 10.1016/0891-5849(96)00109-8 [DOI] [PubMed] [Google Scholar]
- 602.Flohé, L., Brigelius-Flohé, R., Saliou, C., Traber, M.G. and Packer, L. (1997) Redox regulation of NF-kappa B activation. Free Radi. Biol. Med. 22, 1115–1126 10.1016/S0891-5849(96)00501-1 [DOI] [PubMed] [Google Scholar]
- 603.Kabe, Y., Ando, K., Hirao, S., Yoshida, M. and Handa, H. (2005) Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid. Redox Signal. 7, 395–403 10.1089/ars.2005.7.395 [DOI] [PubMed] [Google Scholar]
- 604.Schmidlin, C.J., Dodson, M.B., Madhavan, L. and Zhang, D.D. (2019) Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 134, 702–707 10.1016/j.freeradbiomed.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Rathee, P., Chaudhary, H., Rathee, S., Rathee, D., Kumar, V. and Kohli, K. (2009) Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm. Allergy Drug Targets 8, 229–235 10.2174/187152809788681029 [DOI] [PubMed] [Google Scholar]
- 606.Choy, K.W., Murugan, D., Leong, X.F., Abas, R., Alias, A. and Mustafa, M.R. (2019) Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFkappaB) signaling in cardiovascular diseases: a mini review. Front. Pharmacol. 10, 1295 10.3389/fphar.2019.01295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.D'Ignazio, L., Bandarra, D. and Rocha, S. (2016) NF-kappaB and HIF crosstalk in immune responses. FEBS J. 283, 413–424 10.1111/febs.13578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.D'Ignazio, L., Batie, M. and Rocha, S. (2017) Hypoxia and inflammation in cancer, focus on HIF and NF-kappaB. Biomedicines 5, 21 10.3390/biomedicines5020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Biddlestone, J., Bandarra, D. and Rocha, S. (2015) The role of hypoxia in inflammatory disease (review). Int. J. Mol. Med. 35, 859–869 10.3892/ijmm.2015.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Nelson, D.E., Ihekwaba, A.E.C., Elliott, M., Gibney, C.A., Foreman, B.E., Nelson, G.et al. (2004) Oscillations in NF-κB signalling control the dynamics of gene expression. Science 306, 704–708 10.1126/science.1099962 [DOI] [PubMed] [Google Scholar]
- 611.Kell, D.B. (2006) Metabolomics, modelling and machine learning in systems biology: towards an understanding of the languages of cells. The 2005 Theodor Bücher lecture. FEBS J. 273, 873–894 10.1111/j.1742-4658.2006.05136.x [DOI] [PubMed] [Google Scholar]
- 612.Ashall, L., Horton, C.A., Nelson, D.E., Paszek, P., Ryan, S., Sillitoe, K.et al. (2009) Pulsatile stimulation determines timing and specificity of NFkappa-B-dependent transcription. Science 324, 242–246 10.1126/science.1164860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Xue, M., Momiji, H., Rabbani, N., Bretschneider, T., Rand, D.A. and Thornalley, P.J. (2015) Frequency modulated translocational oscillations of Nrf2, a transcription factor functioning like a wireless sensor. Biochem. Soc. Trans. 43, 669–673 10.1042/BST20150060 [DOI] [PubMed] [Google Scholar]
- 614.Xue, M., Momiji, H., Rabbani, N., Barker, G., Bretschneider, T., Shmygol, A.et al. (2015) Frequency modulated translocational oscillations of Nrf2 mediate the antioxidant response element cytoprotective transcriptional response. Antioxid. Redox Signal. 23, 613–629 10.1089/ars.2014.5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Frangogiannis, N.G., Smith, C.W. and Entman, M.L. (2002) The inflammatory response in myocardial infarction. Cardiovasc Res. 53, 31–47 10.1016/S0008-6363(01)00434-5 [DOI] [PubMed] [Google Scholar]
- 616.Marchant, D.J., Boyd, J.H., Lin, D.C., Granville, D.J., Garmaroudi, F.S. and McManus, B.M. (2012) Inflammation in myocardial diseases. Circ. Res. 110, 126–144 10.1161/CIRCRESAHA.111.243170 [DOI] [PubMed] [Google Scholar]
- 617.Slegtenhorst, B.R., Dor, F.J., Rodriguez, H., Voskuil, F.J. and Tullius, S.G. (2014) Ischemia/reperfusion injury and its consequences on immunity and inflammation. Curr. Transplant. Rep. 1, 147–154 10.1007/s40472-014-0017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Toldo, S., Mauro, A.G., Cutter, Z. and Abbate, A. (2018) Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 315, H1553–H1568 10.1152/ajpheart.00158.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Soares, R.O.S., Losada, D.M., Jordani, M.C., Évora, P. and Castro-E-Silva, O. (2019) Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int. J. Mol. Sci 20, 5034 10.3390/ijms20205034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Sánchez-Hernández, C.D., Torres-Alarcón, L.A., González-Cortés, A. and Peón, A.N. (2020) Ischemia/Reperfusion injury: pathophysiology, current clinical management, and potential preventive approaches. Mediat. Inflamm. 2020, 8405370 10.1155/2020/8405370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Afrin, L.B., Ackerley, M.B., Bluestein, L.S., Brewer, J.H., Brook, J.B., Buchanan, A.D.et al. (2021) Diagnosis of mast cell activation syndrome: a global "consensus-2". Diagnosis (Berl.) 8, 137–152 10.1515/dx-2020-0005 [DOI] [PubMed] [Google Scholar]
- 622.Conti, P., Caraffa, A., Tete, G., Gallenga, C.E., Ross, R., Kritas, S.K.et al. (2020) Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 34, 1629–1632 10.1155/2019/7536805 [DOI] [PubMed] [Google Scholar]
- 623.Theoharides, T.C. and Conti, P. (2020) COVID-19 and multisystem inflammatory syndrome, or is it mast cell activation syndrome? J. Biol. Regul. Homeost. Agents 34, 1633–1636 10.23812/20-EDIT3 [DOI] [PubMed] [Google Scholar]
- 624.Gebremeskel, S., Schanin, J., Coyle, K.M., Butuci, M., Luu, T., Brock, E.C.et al. (2021) Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: implications for an anti-Siglec-8 antibody. Front. Immunol. 12, 650331 10.3389/fimmu.2021.650331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Afrin, L.B., Butterfield, J.H., Raithel, M. and Molderings, G.J. (2016) Often seen, rarely recognized: mast cell activation disease--a guide to diagnosis and therapeutic options. Ann. Med. 48, 190–201 10.3109/07853890.2016.1161231 [DOI] [PubMed] [Google Scholar]
- 626.Afrin, L.B., Weinstock, L.B. and Molderings, G.J. (2020) COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 100, 327–332 10.1016/j.ijid.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Weinstock, L.B., Brook, J.B., Walters, A.S., Goris, A., Afrin, L.B. and Molderings, G.J. (2021) Mast cell activation symptoms are prevalent in long-COVID. Int. J. Infect. Dis. 112, 217–226 10.1016/j.ijid.2021.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Wechsler, J.B., Butuci, M., Wong, A., Kamboj, A.P. and Youngblood, B.A. (2022) Mast cell activation is associated with post-acute COVID-19 syndrome. Allergy 77, 1288–1291 10.1111/all.15188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Singh, M. and Saini, H.K. (2003) Resident cardiac mast cells and ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 8, 135–148 10.1177/107424840300800207 [DOI] [PubMed] [Google Scholar]
- 630.Yang, M.Q., Ma, Y.Y., Tao, S.F., Ding, J., Rao, L.H., Jiang, H.et al. (2014) Mast cell degranulation promotes ischemia-reperfusion injury in rat liver. J. Surg. Res. 186, 170–178 10.1016/j.jss.2013.08.021 [DOI] [PubMed] [Google Scholar]
- 631.Yang, M.Q., Ma, Y.Y., Ding, J. and Li, J.Y. (2014) The role of mast cells in ischemia and reperfusion injury. Inflamm. Res. 63, 899–905 10.1007/s00011-014-0763-z [DOI] [PubMed] [Google Scholar]
- 632.He, Z., Li, Y., Ma, S., Yang, M., Ma, Y., Ma, C.et al. (2018) Degranulation of gastrointestinal mast cells contributes to hepatic ischemia-reperfusion injury in mice. Clin. Sci. (Lond.) 132, 2241–2259 10.1042/CS20180662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.He, Z., Ma, C., Yu, T., Song, J., Leng, J., Gu, X.et al. (2019) Activation mechanisms and multifaceted effects of mast cells in ischemia reperfusion injury. Exp Cell Res. 376, 227–235 10.1016/j.yexcr.2019.01.022 [DOI] [PubMed] [Google Scholar]
- 634.Meng, S., Sun, X., Juan, Z., Wang, M., Wang, R., Sun, L.et al. (2021) Clemastine fumarate attenuates myocardial ischemia reperfusion injury through inhibition of mast cell degranulation. Front. Pharmacol. 12, 704852 10.3389/fphar.2021.704852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.DeTurk, S., Reddy, S., Pellegrino, A.N. and Wilson, J. (2019) Anaphylactic shock. In Clinical Management of Shock - The Science and Art of Physiological Restoration (Stawicki, S.P. and Swaroop, M., eds), IntechOpen, London [Google Scholar]
- 636.Demopoulos, C., Antonopoulou, S. and Theoharides, T.C. (2020) COVID-19, microthromboses, inflammation, and platelet activating factor. Biofactors 46, 927–933 10.1002/biof.1696 [DOI] [PubMed] [Google Scholar]
- 637.Kempuraj, D., Selvakumar, G.P., Ahmed, M.E., Raikwar, S.P., Thangavel, R., Khan, A.et al. (2020) COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist 26, 402–414 10.1177/1073858420941476 [DOI] [PubMed] [Google Scholar]
- 638.Zaid, Y., Puhm, F., Allaeys, I., Naya, A., Oudghiri, M., Khalki, L.et al. (2020) Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ. Res. 1494–1418 10.1161/CIRCRESAHA.120.317703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Jevtic, S.D. and Nazy, I. (2022) The COVID complex: a review of platelet activation and immune complexes in COVID-19. Front. Immunol. 13, 807934 10.3389/fimmu.2022.807934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Forde, R.C. and Fitzgerald, D.J. (1997) Reactive oxygen species and platelet activation in reperfusion injury. Circulation 95, 787–789 10.1161/01.CIR.95.4.787 [DOI] [PubMed] [Google Scholar]
- 641.Kennedy, G., Norris, G., Spence, V., McLaren, M. and Belch, J.J. (2006) Is chronic fatigue syndrome associated with platelet activation? Blood Coagul. Fibrinolysis 17, 89–92 10.1097/01.mbc.0000214705.80997.73 [DOI] [PubMed] [Google Scholar]
- 642.Bester, J. and Pretorius, E. (2016) Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 6, 32188 10.1038/srep32188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Clemetson, K.J., Clemetson, J.M., Proudfoot, A.E., Power, C.A., Baggiolini, M. and Wells, T.N. (2000) Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood 96, 4046–4054 10.1182/blood.V96.13.4046 [DOI] [PubMed] [Google Scholar]
- 644.Hottz, E.D., Bozza, F.A. and Bozza, P.T. (2018) Platelets in immune response to virus and immunopathology of viral infections. Front. Med. (Lausanne) 5, 121 10.3389/fmed.2018.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Mancuso, M.E. and Santagostino, E. (2017) Platelets: much more than bricks in a breached wall. Br. J. Haematol. 178, 209–219 10.1111/bjh.14653 [DOI] [PubMed] [Google Scholar]
- 646.Nevzorova, T.A., Mordakhanova, E.R., Daminova, A.G., Ponomareva, A.A., Andrianova, I.A., Le Minh, G.et al. (2019) Platelet factor 4-containing immune complexes induce platelet activation followed by calpain-dependent platelet death. Cell Death Discov. 5, 106 10.1038/s41420-019-0188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Olumuyiwa-Akeredolu, O.O., Page, M.J., Soma, P. and Pretorius, E. (2019) Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 15, 237–248 10.1038/s41584-019-0187-9 [DOI] [PubMed] [Google Scholar]
- 648.Bonaventura, A., Vecchie, A., Dagna, L., Martinod, K., Dixon, D.L., Van Tassell, B.W.et al. (2021) Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Bunch, C.M., Moore, E.E., Moore, H.B., Neal, M.D., Thomas, A.V., Zackariya, N.et al. (2022) Immuno-thrombotic complications of COVID-19: implications for timing of surgery and anticoagulation. Front. Surg. 9, 889999 10.3389/fsurg.2022.889999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Adams, B., Nunes, J.M., Page, M.J., Roberts, T., Carr, J., Nell, T.A.et al. (2019) Parkinson's disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front. Aging Neurosci. 11, 210 10.3389/fnagi.2019.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Page, M.J., Bester, J. and Pretorius, E. (2018) The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci. Rep. 8, 1812 10.1038/s41598-018-20220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Page, M.J. and Pretorius, E. (2020) A champion of host defense: a generic large-scale cause for platelet dysfunction and depletion in infection. Semin. Thromb. Hemost. 46, 302–319 10.1055/s-0040-1708827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Page, M.J. and Pretorius, E. (2022) Platelet behavior contributes to neuropathologies: a focus on Alzheimer's and Parkinson's disease. Semin. Thromb. Hemost. 48, 382–404 10.1055/s-0041-1733960 [DOI] [PubMed] [Google Scholar]
- 654.Pretorius, E., Oberholzer, H.M., van der Spuy, W.J. and Meiring, J.H. (2009) Macrothrombocytopenia: investigating the ultrastructure of platelets and fibrin networks using scanning and transmission electron microscopy. Ultrastruct. Pathol. 33, 216–221 10.3109/01913120903288587 [DOI] [PubMed] [Google Scholar]
- 655.Pretorius, E. (2019) Platelets as potent signaling entities in type 2 diabetes mellitus. Trends Endocrinol. Metab. 30, 532–545 10.1016/j.tem.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 656.Pretorius, E. (2021) Platelets in HIV: a guardian of host defence or transient reservoir of the virus? Front. Immunol. 12, 649465 10.3389/fimmu.2021.649465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Soma, P., Swanepoel, A.C., du Plooy, J.N., Mqoco, T. and Pretorius, E. (2016) Flow cytometric analysis of platelets type 2 diabetes mellitus reveals 'angry' platelets. Cardiovasc. Diabetol. 15, 52 10.1186/s12933-016-0373-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Soma, P. and Pretorius, E. (2015) Interplay between ultrastructural findings and atherothrombotic complications in type 2 diabetes mellitus. Cardiovasc. Diabetol. 14, 96 10.1186/s12933-015-0261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.van Rooy, M.J. and Pretorius, E. (2015) Metabolic syndrome, platelet activation and the development of transient ischemic attack or thromboembolic stroke. Thromb. Res. 135, 434–442 10.1016/j.thromres.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 660.van Rooy, M.J., Duim, W., Ehlers, R., Buys, A.V. and Pretorius, E. (2015) Platelet hyperactivity and fibrin clot structure in transient ischemic attack individuals in the presence of metabolic syndrome: a microscopy and thromboelastography study. Cardiovasc. Diabetol. 14, 86 10.1186/s12933-015-0249-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Blann, A.D., Nadar, S.K. and Lip, G.Y. (2003) The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart. J. 24, 2166–2179 10.1016/j.ehj.2003.08.021 [DOI] [PubMed] [Google Scholar]
- 662.Perkins, L.A., Anderson, C.J. and Novelli, E.M. (2019) Targeting P-selectin adhesion molecule in molecular imaging: P-selectin expression as a valuable imaging biomarker of inflammation in cardiovascular disease. J. Nucl. Med. 60, 1691–1697 10.2967/jnumed.118.225169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Tarasev, M., Mota, S., Gao, X., Ferranti, M., Zaidi, A.U., Hannan, B.et al. (2022) Possible role of P-selectin adhesion in long-COVID: a comparative analysis of a long-COVID case versus an asymptomatic post-COVID case. medRxiv 2022.2003.2009.22271297 [Google Scholar]
- 664.Lee, J., Kang, Y., Chang, J., Song, J. and Kim, B.K. (2020) Determination of serotonin concentration in single human platelets through single-entity electrochemistry. ACS Sens. 5, 1943–1948 10.1021/acssensors.0c00267 [DOI] [PubMed] [Google Scholar]
- 665.Cloutier, N., Allaeys, I., Marcoux, G., Machlus, K.R., Mailhot, B., Zufferey, A.et al. (2018) Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl Acad. Sci. U.S.A. 115, E1550–E1559 10.1073/pnas.1720553115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Dale, G.L., Friese, P., Batar, P., Hamilton, S.F., Reed, G.L., Jackson, K.W.et al. (2002) Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 415, 175–179 10.1038/415175a [DOI] [PubMed] [Google Scholar]
- 667.Jalali, F., Rezaie, S., Rola, P. and Kyle-Sidell, C. (2021) COVID-19 Pathophysiology: Are Platelets and Serotonin hiding in plain sight? SSRN preprint, https://ssrn.com/abstract=3800402
- 668.Santos, A.P., Couto, C.F., Pereira, S.S. and Monteiro, M.P. (2022) Is serotonin the missing link between COVID-19 severity observed in patients with diabetes and obesity? Neuroendocrinology 1–71 10.1159/000522115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Zimering, M.B., Razzaki, T., Tsang, T. and Shin, J.J. (2020) Inverse association between serotonin 2A receptor antagonist medication use and mortality in severe COVID-19 infection. Endocrinol. Diabetes Metab. J. 4, 1–5 [PMC free article] [PubMed] [Google Scholar]
- 670.Fert-Bober, J., Darrah, E. and Andrade, F. (2020) Insights into the study and origin of the citrullinome in rheumatoid arthritis. Immunol. Rev. 294, 133–147 10.1111/imr.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Smatti, M.K., Cyprian, F.S., Nasrallah, G.K., Al Thani, A.A., Almishal, R.O. and Yassine, H.M. (2019) Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses 11, 762 10.3390/v11080762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Rojas, M., Rodríguez, Y., Acosta-Ampudia, Y., Monsalve, D.M., Zhu, C., Quan-Zhen, L.et al. (2022) Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 20, 129 10.1186/s12967-022-03328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Winchester, N., Calabrese, C. and Calabrese, L.H. (2021) The intersection of COVID-19 and autoimmunity: what is our current understanding? Pathog. Immun. 6, 31–54 10.20411/pai.v6i1.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Malik, H.I., Mir, A.R., Abidi, M., Habib, S., Khan, F.H. and Moinuddin (2020) Preferential recognition of epitopes on peroxynitrite-modified alpha-2-macroglobulin by circulating autoantibodies in rheumatoid arthritis patients. J. Biomol. Struct. Dyn. 38, 1984–1994 10.1080/07391102.2019.1623073 [DOI] [PubMed] [Google Scholar]
- 675.Sotzny, F., Blanco, J., Capelli, E., Castro-Marrero, J., Steiner, S., Murovska, M.et al. (2018) Myalgic encephalomyelitis/chronic fatigue syndrome - evidence for an autoimmune disease. Autoimmun. Rev. 17, 601–609 10.1016/j.autrev.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 676.Riemekasten, G., Petersen, F. and Heidecke, H. (2020) What makes antibodies against G protein-coupled receptors so special? A novel concept to understand chronic diseases. Front. Immunol. 11, 564526 10.3389/fimmu.2020.564526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Liu, Y., Ebinger, J.E., Mostafa, R., Budde, P., Gajewski, J., Walker, B.et al. (2021) Paradoxical sex-specific patterns of autoantibody response to SARS-CoV-2 infection. J. Transl. Med. 19, 524 10.1186/s12967-021-03184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Charnley, M., Islam, S., Bindra, G.K., Engwirda, J., Ratcliffe, J., Zhou, J.et al. (2022) Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19. Nat. Commun. 13, 3387 10.1038/s41467-022-30932-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Nyström, S. and Hammarström, P. (2022) Amyloidogenesis of SARS-CoV-2 spike protein. J. Am. Chem. Soc. 144, 8945–8950 10.1021/jacs.2c03925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Natarajan, A., Zlitni, S., Brooks, E.F., Vance, S.E., Dahlen, A., Hedlin, H.et al. (2022) Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 3, 371–387.e9 10.1016/j.medj.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Jacobs, J.J.L. (2021) Persistent SARS-2 infections contribute to long COVID-19. Med. Hypotheses 149, 110538 10.1016/j.mehy.2021.110538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Swank, Z., Senussi, Y., Alter, G. and Walt, D.R. (2022) Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. medRxiv 2022.2006.2014.22276401 [Google Scholar]
- 683.Brogna, C., Brogna, B., Bisaccia, D.R., Lauritano, F., Marino, G., Montano, L.et al. (2022) Could SARS-CoV-2 have bacteriophage behavior or induce the activity of other bacteriophages? Vaccines (Basel) 10, 708 10.3390/vaccines10050708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Velasquez-Manoff, M. (2015) Gut microbiome: the peacekeepers. Nature 518, S3–11 10.1038/518S3a [DOI] [PubMed] [Google Scholar]
- 685.Proal, A.D. and Marshall, T.G. (2018) Re-framing the theory of autoimmunity in the era of the microbiome: persistent pathogens, autoantibodies, and molecular mimicry. Discov. Med. 140, 299–308 [PubMed] [Google Scholar]
- 686.Kamada, N., Seo, S.U., Chen, G.Y. and Núñez, G. (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- 687.Gentile, C.L. and Weir, T.L. (2018) The gut microbiota at the intersection of diet and human health. Science 362, 776–780 10.1126/science.aau5812 [DOI] [PubMed] [Google Scholar]
- 688.Maier, L., Goemans, C.V., Wirbel, J., Kuhn, M., Eberl, C., Pruteanu, M.et al. (2021) Unravelling the collateral damage of antibiotics on gut bacteria. Nature 599, 120–124 10.1038/s41586-021-03986-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Maier, L., Pruteanu, M., Kuhn, M., Zeller, G., Telzerow, A., Anderson, E.E.et al. (2018) Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 10.1038/nature25979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Vieira-Silva, S., Falony, G., Belda, E., Nielsen, T., Aron-Wisnewsky, J., Chakaroun, R.et al. (2020) Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315 10.1038/s41586-020-2269-x [DOI] [PubMed] [Google Scholar]
- 691.Forslund, S.K., Chakaroun, R., Zimmermann-Kogadeeva, M., Markó, L., Aron-Wisnewsky, J., Nielsen, T.et al. (2021) Combinatorial, additive and dose-dependent drug-microbiome associations. Nature 600, 500–505 10.1038/s41586-021-04177-9 [DOI] [PubMed] [Google Scholar]
- 692.Haran, J.P., Bradley, E., Zeamer, A.L., Cincotta, L., Salive, M.C., Dutta, P.et al. (2021) Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight 6, e152346 10.1172/jci.insight.152346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Yeoh, Y.K., Zuo, T., Lui, G.C., Zhang, F., Liu, Q., Li, A.Y.et al. (2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70, 698–706 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Hazan, S., Stollman, N., Bozkurt, H.S., Dave, S., Papoutsis, A.J., Daniels, J.et al. (2022) Lost microbes of COVID-19: Bifidobacterium, Faecalibacterium depletion and decreased microbiome diversity associated with SARS-CoV-2 infection severity. BMJ Open Gastroenterol. 9, e000871 10.1136/bmjgast-2022-000871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Liu, Q., Mak, J.W.Y., Su, Q., Yeoh, Y.K., Lui, G.C., Ng, S.S.S.et al. (2022) Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 71, 544–552 10.1136/gutjnl-2021-325989 [DOI] [PubMed] [Google Scholar]
- 696.Wang, B., Zhang, L., Wang, Y., Dai, T., Qin, Z., Zhou, F.et al. (2022) Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal. Transduct. Target. Ther. 7, 143 10.1038/s41392-022-00986-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Hilpert, K. and Mikut, R. (2021) Is there a connection between Gut microbiome dysbiosis occurring in COVID-19 patients and post-COVID-19 symptoms? Front. Microbiol. 12, 732838 10.3389/fmicb.2021.732838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Newsome, R.C., Gauthier, J., Hernandez, M.C., Abraham, G.E., Robinson, T.O., Williams, H.B.et al. (2021) The gut microbiome of COVID-19 recovered patients returns to uninfected status in a minority-dominated United States cohort. Gut Microbes 13, 1–15 10.1080/19490976.2021.1926840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Cifuentes-Muñoz, N., Dutch, R.E. and Cattaneo, R. (2018) Direct cell-to-cell transmission of respiratory viruses: the fast lanes. PLoS Pathog. 14, e1007015 10.1371/journal.ppat.1007015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Bang, L.M. and Keating, G.M. (2004) Adalimumab: a review of its use in rheumatoid arthritis. BioDrugs 18, 121–139 10.2165/00063030-200418020-00005 [DOI] [PubMed] [Google Scholar]
- 701.Chen, Y.F., Jobanputra, P., Barton, P., Jowett, S., Bryan, S., Clark, W.et al. (2006) A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol. Assess. 10, iii–iv, xi–xiii, 1–229 [DOI] [PubMed] [Google Scholar]
- 702.Cattaneo, M. (2015) P2y12 receptors: structure and function. J. Thromb. Haemost. 13, S10–S16 10.1111/jth.12952 [DOI] [PubMed] [Google Scholar]
- 703.Eller, T., Busse, J., Dittrich, M., Flieder, T., Alban, S., Knabbe, C.et al. (2014) Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin. Chem. Lab. Med. 52, 835–844 10.1515/cclm-2013-0936 [DOI] [PubMed] [Google Scholar]
- 704.Scharf, R.E. (2012) Drugs that affect platelet function. Semin. Thromb. Hemost. 38, 865–883 10.1055/s-0032-1328881 [DOI] [PubMed] [Google Scholar]
- 705.Richman, S., Morris, M.C., Broderick, G., Craddock, T.J.A., Klimas, N.G. and Fletcher, M.A. (2019) Pharmaceutical interventions in chronic fatigue syndrome: a literature-based commentary. Clin. Ther. 41, 798–805 10.1016/j.clinthera.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Collatz, A., Johnston, S.C., Staines, D.R. and Marshall-Gradisnik, S.M. (2016) A systematic review of drug therapies for chronic fatigue syndrome/myalgic encephalomyelitis. Clin. Ther. 38, 1263–1271.e1269 10.1016/j.clinthera.2016.04.038 [DOI] [PubMed] [Google Scholar]
- 707.Reis, G., Silva, E., Silva, D.C.M., Thabane, L., Milagres, A.C., Ferreira, T.S.et al. (2022) Effect of Early treatment with ivermectin among patients with COVID-19. N. Engl. J. Med . 386, 1721–1731 10.1056/NEJMoa2115869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Bhakuni, T., Ali, M.F., Ahmad, I., Bano, S., Ansari, S. and Jairajpuri, M.A. (2016) Role of heparin and non heparin binding serpins in coagulation and angiogenesis: a complex interplay. Arch. Biochem. Biophys. 604, 128–142 10.1016/j.abb.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 709.Hirsh, J. and Raschke, R. (2004) Heparin and low-molecular-weight heparin: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126, 188S–203S 10.1378/chest.126.3_suppl.188S [DOI] [PubMed] [Google Scholar]
- 710.Rezaie, A.R. and Giri, H. (2020) Anticoagulant and signaling functions of antithrombin. J. Thromb. Haemost. 18, 3142–3153 10.1111/jth.15052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Zhang, Y., Zhang, M., Tan, L., Pan, N. and Zhang, L. (2019) The clinical use of fondaparinux: a synthetic heparin pentasaccharide. Prog. Mol. Biol. Transl. Sci. 163, 41–53 10.1016/bs.pmbts.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 712.Marongiu, F. and Barcellona, D. (2020) Fondaparinux: should it be studied in patients with COVID-19 disease? TH Open 4, e300–e302 10.1055/s-0040-1719232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Pawlowski, C., Venkatakrishnan, A.J., Kirkup, C., Berner, G., Puranik, A., O'Horo, J.C.et al. (2021) Enoxaparin is associated with lower rates of mortality than unfractionated heparin in hospitalized COVID-19 patients. EClinicalMedicine 33, 100774 10.1016/j.eclinm.2021.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Mycroft-West, C., Su, D., Elli, S., Li, Y., Guimond, S., Miller, G.et al. (2020) The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 receptor binding domain undergoes conformational change upon heparin binding. bioRxiv 2020.2002.2029.971093 [Google Scholar]
- 715.Tree, J.A., Turnbull, J.E., Buttigieg, K.R., Elmore, M.J., Coombes, N., Hogwood, J.et al. (2021) Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. Br. J. Pharmacol. 178, 626–635 10.1111/bph.15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Handy, A., Banerjee, A., Wood, A.M., Dale, C., Sudlow, C.L.M., Tomlinson, C.et al. (2022) Evaluation of antithrombotic use and COVID-19 outcomes in a nationwide atrial fibrillation cohort. Heart 108, 923–931 10.1136/heartjnl-2021-320325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Viecca, M., Radovanovic, D., Forleo, G.B. and Santus, P. (2020) Enhanced platelet inhibition treatment improves hypoxemia in patients with severe COVID-19 and hypercoagulability. A case control, proof of concept study. Pharmacol. Res. 158, 104950 10.1016/j.phrs.2020.104950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Spaetgens, B., Nagy, M. and Ten Cate, H. (2022) Antiplatelet therapy in patients with COVID-19-more is less? J. Am. Med. Assoc. 327, 223–224 10.1001/jama.2021.23866 [DOI] [PubMed] [Google Scholar]
- 719.Berger, J.S., Kornblith, L.Z., Gong, M.N., Reynolds, H.R., Cushman, M., Cheng, Y.et al. (2022) Effect of P2Y12 inhibitors on survival free of organ support among non-critically Ill hospitalized patients with COVID-19: a randomized clinical trial. J. Am. Med. Assoc 327, 227–236 10.1001/jama.2021.23605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Zhao, S., Chadwick, L., Mysler, E. and Moots, R.J. (2018) Review of biosimilar trials and data on Adalimumab in rheumatoid arthritis. Curr. Rheumatol. Rep. 20, 57 10.1007/s11926-018-0769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Huizinga, T.W.J., Torii, Y. and Muniz, R. (2021) Adalimumab biosimilars in the treatment of rheumatoid arthritis: a systematic review of the evidence for biosimilarity. Rheumatol. Ther. 8, 41–61 10.1007/s40744-020-00259-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Lu, X., Hu, R., Peng, L., Liu, M. and Sun, Z. (2021) Efficacy and safety of Adalimumab biosimilars: current critical clinical data in rheumatoid arthritis. Front. Immunol. 12, 638444 10.3389/fimmu.2021.638444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Stevenson, M., Archer, R., Tosh, J., Simpson, E., Everson-Hock, E., Stevens, J.et al. (2016) Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and Abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol. Assess . 20, 1–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Guo, Y., Hu, K., Li, Y., Lu, C., Ling, K., Cai, C.et al. (2022) Targeting TNF-alpha for COVID-19: recent advanced and controversies. Front. Public Health 10, 833967 10.3389/fpubh.2022.833967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Robinson, P.C., Liew, D.F.L., Tanner, H.L., Grainger, J.R., Dwek, R.A., Reisler, R.B.et al. (2022) COVID-19 therapeutics: challenges and directions for the future. Proc. Natl Acad. Sci. U.S.A. 119, e2119893119 10.1073/pnas.2119893119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.van de Veerdonk, F.L., Giamarellos-Bourboulis, E., Pickkers, P., Derde, L., Leavis, H., van Crevel, R.et al. (2022) A guide to immunotherapy for COVID-19. Nat. Med. 28, 39–50 10.1038/s41591-021-01643-9 [DOI] [PubMed] [Google Scholar]
- 727.Littlefield, K.M., Watson, R.O., Schneider, J.M., Neff, C.P., Yamada, E., Zhang, M.et al. (2022) SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLoS Pathog. 18, e1010359 10.1371/journal.ppat.1010359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Ledford, H. (2022) Can drugs reduce the risk of long COVID? What scientists know so far. Nature 604, 20–21 10.1038/d41586-022-00823-y [DOI] [PubMed] [Google Scholar]
- 729.Castro-Marrero, J., Sáez-Francàs, N., Santillo, D. and Alegre, J. (2017) Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br. J. Pharmacol. 174, 345–369 10.1111/bph.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.VanElzakker, M.B., Brumfield, S.A. and Lara Mejia, P.S. (2018) Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a critical review of research methods. Front. Neurol. 9, 1033 10.3389/fneur.2018.01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Morris, M.C., Cooney, K.E., Sedghamiz, H., Abreu, M., Collado, F., Balbin, E.G.et al. (2019) Leveraging prior knowledge of endocrine immune regulation in the therapeutically relevant phenotyping of women with chronic fatigue syndrome. Clin. Ther. 41, 656–674.e654 10.1016/j.clinthera.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Yang, T., Yang, Y., Wang, D., Li, C., Qu, Y., Guo, J.et al. (2019) The clinical value of cytokines in chronic fatigue syndrome. J. Transl. Med. 17, 213 10.1186/s12967-019-1948-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Druce, K.L., Jones, G.T., Macfarlane, G.J. and Basu, N. (2015) Patients receiving anti-TNF therapies experience clinically important improvements in RA-related fatigue: results from the British society for rheumatology biologics register for rheumatoid arthritis. Rheumatology (Oxford) 54, 964–971 10.1093/rheumatology/keu390 [DOI] [PubMed] [Google Scholar]
- 734.Lopes, M.I., Bonjorno, L.P., Giannini, M.C., Amaral, N.B., Menezes, P.I., Dib, S.M.et al. (2021) Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open 7, e001455 10.1136/rmdopen-2020-001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Arbab, A.A.I., Lu, X., Abdalla, I.M., Idris, A.A., Chen, Z., Li, M.et al. (2021) Metformin inhibits lipoteichoic acid-induced oxidative stress and inflammation through AMPK/NRF2/NF-kappaB signaling pathway in bovine mammary epithelial cells. Front. Vet. Sci. 8, 661380 10.3389/fvets.2021.661380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Kamyshnyi, O., Matskevych, V., Lenchuk, T., Strilbytska, O., Storey, K. and Lushchak, O. (2021) Metformin to decrease COVID-19 severity and mortality: molecular mechanisms and therapeutic potential. Biomed. Pharmacother. 144, 112230 10.1016/j.biopha.2021.112230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 737.Li, Y., Yang, X., Yan, P., Sun, T., Zeng, Z. and Li, S. (2021) Metformin in patients with COVID-19: a systematic review and meta-Analysis. Front. Med. (Lausanne) 8, 704666 10.3389/fmed.2021.704666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Bukhari, I.A., Almotrefi, A.A., Mohamed, O.Y., Al-Masri, A.A. and Sheikh, S.A. (2018) Protective effect of fenofibrate against ischemia-/reperfusion-induced cardiac arrhythmias in isolated rat hearts. Fundam. Clin. Pharmacol. 32, 141–146 10.1111/fcp.12342 [DOI] [PubMed] [Google Scholar]
- 739.Zhu, Q., He, G., Wang, J., Wang, Y. and Chen, W. (2016) Protective effects of fenofibrate against acute lung injury induced by intestinal ischemia/reperfusion in mice. Sci. Rep. 6, 22044 10.1038/srep22044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Bolton, M.J., Chapman, B.P. and Van Marwijk, H. (2020) Low-dose naltrexone as a treatment for chronic fatigue syndrome. BMJ Case Rep. 13, e232502 10.1136/bcr-2019-232502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Cabanas, H., Muraki, K., Eaton-Fitch, N., Staines, D.R. and Marshall-Gradisnik, S. (2021) Potential therapeutic benefit of low dose naltrexone in myalgic encephalomyelitis/chronic fatigue syndrome: role of transient receptor potential melastatin 3 ion channels in pathophysiology and treatment. Front. Immunol. 12, 687806 10.3389/fimmu.2021.687806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Kim, P.S. and Fishman, M.A. (2020) Low-dose naltrexone for chronic pain: update and systemic review. Curr. Pain Headache Rep. 24, 64 10.1007/s11916-020-00898-0 [DOI] [PubMed] [Google Scholar]
- 743.Xu, F., Hou, T., Shen, A., Jin, H., Xiao, Y., Yu, W.et al. (2021) Mechanism deconvolution of Qing Fei Pai Du decoction for treatment of coronavirus disease 2019 (COVID-19) by label-free integrative pharmacology assays. J. Ethnopharmacol. 280, 114488 10.1016/j.jep.2021.114488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Li, Y., Li, B., Wang, P. and Wang, Q. (2021) Traditional Chinese medicine, Qingfei Paidu decoction and Xuanfei Baidu decoction, inhibited cytokine production via NF-kappaB signaling pathway in macrophages: implications for coronavirus disease 2019 (COVID-19) therapy. Front. Pharmacol. 12, 722126 10.3389/fphar.2021.722126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Li, Z.Y., Xie, Z.J., Li, H.C., Wang, J.J., Wen, X.H., Wu, S.Y.et al. (2021) Guidelines on the treatment with integrated traditional Chinese medicine and western medicine for severe coronavirus disease 2019. Pharmacol. Res. 174, 105955 10.1016/j.phrs.2021.105955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Zhang, L.H., Zheng, X., Bai, X.K., Wang, Q., Chen, B.W., Wang, H.B.et al. (2021) Association between use of Qingfei Paidu Tang and mortality in hospitalized patients with COVID-19: a national retrospective registry study. Phytomedicine 85, 153531 10.1016/j.phymed.2021.153531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Wang, H., Xu, B., Zhang, Y., Duan, Y., Gao, R., He, H.et al. (2021) Efficacy and safety of traditional Chinese medicine in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Front. Pharmacol. 12, 609213 10.3389/fphar.2021.609213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Liu, J., Dong, F. and Robinson, N. (2022) State-of-the-art evidence of traditional Chinese medicine for treating coronavirus disease 2019. J. Tradit. Chin. Med. Sci. 9, 2–6 10.1016/j.jtcms.2022.01.005 [DOI] [Google Scholar]
- 749.Wang, J.B., Andrade-Cetto, A., Echeverria, J., Wardle, J., Yen, H.R. and Heinrich, M. (2021) Editorial: ethnopharmacological responses to the coronavirus disease 2019 pandemic. Front. Pharmacol. 12, 798674 10.3389/fphar.2021.798674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Pang, W., Yang, F., Zhao, Y., Dai, E., Feng, J., Huang, Y.et al. (2022) Qingjin Yiqi granules for post-COVID-19 condition: a randomized clinical trial. J. Evid. Based Med. 15, 30–38 10.1111/jebm.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Derosa, G., Maffioli, P., D'Angelo, A. and Di Pierro, F. (2021) Nutraceutical approach to preventing coronavirus disease 2019 and related complications. Front. Immunol. 12, 582556 10.3389/fimmu.2021.582556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Alesci, A., Aragona, M., Cicero, N. and Lauriano, E.R. (2021) Can nutraceuticals assist treatment and improve COVID-19 symptoms? Nat. Prod. Res. 1–20 36, 2672–2691 10.1080/14786419.2021.1914032 [DOI] [PubMed] [Google Scholar]
- 753.Savant, S., Srinivasan, S. and Kruthiventi, A.K. (2021) Potential nutraceuticals for COVID-19. Nutr. Dietary Suppl. 13, 25–51 10.2147/NDS.S294231 [DOI] [Google Scholar]
- 754.Williams, R.J. (1956) Biochemical Individuality, John Wiley, New York [Google Scholar]
- 755.Cuzzocrea, S., Riley, D.P., Caputi, A.P. and Salvemini, D. (2001) Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 53, 135–159 [PubMed] [Google Scholar]
- 756.Zhao, K., Zhao, G.M., Wu, D., Soong, Y., Birk, A.V., Schiller, P.W.et al. (2004) Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J. Biol. Chem. 279, 34682–34690 10.1074/jbc.M402999200 [DOI] [PubMed] [Google Scholar]
- 757.Mori, K., Lee, H.T., Rapoport, D., Drexler, I.R., Foster, K., Yang, J.et al. (2005) Endocytic delivery of lipoccalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig. 115, 610–621 10.1172/JCI23056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Kelsey, N.A., Wilkins, H.M. and Linseman, D.A. (2010) Nutraceutical antioxidants as novel neuroprotective agents. Molecules 15, 7792–7814 10.3390/molecules15117792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Tardiolo, G., Bramanti, P. and Mazzon, E. (2018) Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 23, 3305 10.3390/molecules23123305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.dos Santos Tenório, M.C., Graciliano, N.G., Moura, F.A., de Oliveira, M. and Goulart, M.O.F. (2021) N-acetylcysteine (NAC): impacts on human health. Antioxidants (Basel) 10, 967 10.3390/antiox10060967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Schwalfenberg, G.K. (2021) N-acetylcysteine: a review of clinical usefulness (an old drug with new tricks). J. Nutr. Metab. 2021, 9949453 10.1155/2021/9949453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Ye, M., Lin, W., Zheng, J. and Lin, S. (2021) N-acetylcysteine for chronic kidney disease: a systematic review and meta-analysis. Am. J. Transl. Res. 13, 2472–2485 [PMC free article] [PubMed] [Google Scholar]
- 763.Batooei, M., Tahamoli-Roudsari, A., Basiri, Z., Yasrebifar, F., Shahdoust, M., Eshraghi, A.et al. (2018) Evaluating the effect of oral N-acetylcysteine as an adjuvant treatment on clinical outcomes of patients with rheumatoid arthritis: a randomized, double blind clinical trial. Rev. Recent Clin. Trials 13, 132–138 10.2174/1574887113666180307151937 [DOI] [PubMed] [Google Scholar]
- 764.Kim, H.R., Kim, K.W., Kim, B.M., Lee, K.A. and Lee, S.H. (2019) N-acetyl-l-cysteine controls osteoclastogenesis through regulating Th17 differentiation and RANKL production in rheumatoid arthritis. Korean J. Intern. Med. 34, 210–219 10.3904/kjim.2016.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Jamali, F., Ahmadzadeh, A., Sahraei, Z. and Salamzadeh, J. (2021) Study of the effects of N-acetylcysteine on inflammatory biomarkers and disease activity score in patients with rheumatoid arthritis. Iran. J. Allergy Asthma Immunol. 20, 574–583 10.18502/ijaai.v20i5.7407 [DOI] [PubMed] [Google Scholar]
- 766.Finsterer, J., Scorza, F.A., Scorza, C.A. and Fiorini, A.C. (2022) Repurposing the antioxidant and anti-inflammatory agent N-acetyl cysteine for treating COVID-19. World J. Virol. 11, 82–84 10.5501/wjv.v11.i1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Assimakopoulos, S.F., Aretha, D., Komninos, D., Dimitropoulou, D., Lagadinou, M., Leonidou, L.et al. (2021) N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study. Infect. Dis. (Lond.) 53, 847–854 10.1080/23744235.2021.1945675 [DOI] [PubMed] [Google Scholar]
- 768.Mohanty, R.R., Padhy, B.M., Das, S. and Meher, B.R. (2021) Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: review of current evidence. Eur. Rev. Med. Pharmacol. Sci. 25, 2802–2807 10.26355/eurrev_202103_25442 [DOI] [PubMed] [Google Scholar]
- 769.Reyes-Gordillo, K., Segovia, J., Shibayama, M., Vergara, P., Moreno, M.G. and Muriel, P. (2007) Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim. Biophys. Acta 1770, 989–996 10.1016/j.bbagen.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 770.Fu, Y., Zheng, S., Lin, J., Ryerse, J. and Chen, A. (2008) Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol. Pharmacol. 73, 399–409 10.1124/mol.107.039818 [DOI] [PubMed] [Google Scholar]
- 771.Han, J., Pan, X.Y., Xu, Y., Xiao, Y., An, Y., Tie, L.et al. (2012) Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 8, 812–825 10.4161/auto.19471 [DOI] [PubMed] [Google Scholar]
- 772.Varatharajalu, R., Garige, M., Leckey, L.C., Reyes-Gordillo, K., Shah, R. and Lakshman, M.R. (2016) Protective role of dietary curcumin in the prevention of the oxidative stress induced by chronic alcohol with respect to hepatic injury and antiatherogenic markers. Oxidative Med. Cell. Longev. 2016, 5017460 10.1155/2016/5017460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Liu, Z. and Ying, Y. (2020) The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Front Cell Dev. Biol. 8, 479 10.3389/fcell.2020.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Rattis, B.A.C., Ramos, S.G. and Celes, M.R.N. (2021) Curcumin as a potential treatment for COVID-19. Front. Pharmacol. 12, 675287 10.3389/fphar.2021.675287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Morris, G., Puri, B.K., Walker, A.J., Maes, M., Carvalho, A.F., Walder, K.et al. (2019) Myalgic encephalomyelitis/chronic fatigue syndrome: from pathophysiological insights to novel therapeutic opportunities. Pharmacol. Res. 148, 104450 10.1016/j.phrs.2019.104450 [DOI] [PubMed] [Google Scholar]
- 776.Ahmed, S., Anuntiyo, J., Malemud, C.J. and Haqqi, T.M. (2005) Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid. Based Complement. Alternat. Med. 2, 301–308 10.1093/ecam/neh117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Aravilli, R.K., Vikram, S.L. and Kohila, V. (2017) Phytochemicals as potential antidotes for targeting NF-kappa B in rheumatoid arthritis. 3 Biotech 7, 253 10.1007/s13205-017-0888-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Behl, T., Mehta, K., Sehgal, A., Singh, S., Sharma, N., Ahmadi, A.et al. (2022) Exploring the role of polyphenols in rheumatoid arthritis. Crit Rev Food Sci Nutr. 62, 5372–5393 10.1080/10408398.2021.1924613 [DOI] [PubMed] [Google Scholar]
- 779.Mohammadian Haftcheshmeh, S., Khosrojerdi, A., Aliabadi, A., Lotfi, S., Mohammadi, A. and Momtazi-Borojeni, A.A. (2021) Immunomodulatory effects of curcumin in rheumatoid arthritis: evidence from molecular mechanisms to clinical outcomes. Rev. Physiol. Biochem. Pharmacol. 179, 1–29 10.1007/112_2020_54 [DOI] [PubMed] [Google Scholar]
- 780.Pourhabibi-Zarandi, F., Shojaei-Zarghani, S. and Rafraf, M. (2021) Curcumin and rheumatoid arthritis: a systematic review of literature. Int. J. Clin. Pract. 75, e14280 10.1111/ijcp.14280 [DOI] [PubMed] [Google Scholar]
- 781.Jena, A.B., Kanungo, N., Nayak, V., Chainy, G.B.N. and Dandapat, J. (2021) Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies. Sci. Rep. 11, 2043 10.1038/s41598-021-81462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Vahedian-Azimi, A., Abbasifard, M., Rahimi-Bashar, F., Guest, P.C., Majeed, M., Mohammadi, A.et al. (2022) Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials. Nutrients 14, 256 10.3390/nu14020256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Bousquet, J., Haahtela, T., Blain, H., Czarlewski, W., Zuberbier, T., Bedbrook, A.et al. (2022) Available and affordable complementary treatments for COVID-19: from hypothesis to pilot studies and the need for implementation. Clin. Transl. Allergy 12, e12127 10.1002/clt2.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Srivastava, K.C., Bordia, A. and Verma, S.K. (1995) Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot. Essent. Fat. Acids 52, 223–227 10.1016/0952-3278(95)90040-3 [DOI] [PubMed] [Google Scholar]
- 785.Shah, B.H., Nawaz, Z., Pertani, S.A., Roomi, A., Mahmood, H., Saeed, S.A.et al. (1999) Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 58, 1167–1172 10.1016/S0006-2952(99)00206-3 [DOI] [PubMed] [Google Scholar]
- 786.Kim, K. and Park, K.I. (2019) A review of antiplatelet activity of traditional medicinal herbs on integrative medicine studies. Evid. Based Complement. Alternat. Med. 2019, 7125162 10.1155/2019/7125162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Borodina, I., Kenny, L.C., McCarthy, C.M., Paramasivan, K., Pretorius, R., Roberts, T.J.et al. (2020) The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 33, 190–217 10.1017/S0954422419000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Cheah, I.K. and Halliwell, B. (2021) Ergothioneine, recent developments. Redox Biol. 42, 101868 10.1016/j.redox.2021.101868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Halliwell, B., Cheah, I.K. and Drum, C.L. (2016) Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 470, 245–250 10.1016/j.bbrc.2015.12.124 [DOI] [PubMed] [Google Scholar]
- 790.Halliwell, B., Cheah, I.K. and Tang, R.M.Y. (2018) Ergothioneine - a diet-derived antioxidant with therapeutic potential. FEBS Lett. 592, 3357–3366 10.1002/1873-3468.13123 [DOI] [PubMed] [Google Scholar]
- 791.Paul, B.D. and Snyder, S.H. (2010) The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 17, 1134–1140 10.1038/cdd.2009.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Paul, B.D. (2021) Ergothioneine: a stress vitamin with anti-aging, vascular and neuroprotective roles? Antioxid. Redox Signal. 36, 1306–1317 10.1089/ars.2021.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Servillo, L., D'Onofrio, N. and Balestrieri, M.L. (2017) Ergothioneine antioxidant function: from chemistry to cardiovascular therapeutic potential. J. Cardiovasc. Pharmacol. 69, 183–191 10.1097/FJC.0000000000000464 [DOI] [PubMed] [Google Scholar]
- 794.Fu, T.T. and Shen, L. (2022) Ergothioneine as a natural antioxidant against oxidative stress-related diseases. Front. Pharmacol. 13, 850813 10.3389/fphar.2022.850813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Gründemann, D., Harlfinger, S., Golz, S., Geerts, A., Lazar, A., Berkels, R.et al. (2005) Discovery of the ergothioneine transporter. Proc Natl Acad Sci. USA 102, 5256–5261 10.1073/pnas.0408624102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Gründemann, D. (2012) The ergothioneine transporter controls and indicates ergothioneine activity--a review. Prev. Med. 54, S71–S74 10.1016/j.ypmed.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 797.Gründemann, D., Hartmann, L. and Flögel, S. (2021) The ergothioneine transporter (ETT): substrates and locations, an inventory. FEBS Lett. 596, 1252–1269 10.1002/1873-3468.14269 [DOI] [PubMed] [Google Scholar]
- 798.Kerley, R.N., McCarthy, C., Kell, D.B. and Kenny, L.C. (2018) The potential therapeutic effects of ergothioneine in pre-eclampsia. Free Radic. Biol. Med. 117, 145–157 10.1016/j.freeradbiomed.2017.12.030 [DOI] [PubMed] [Google Scholar]
- 799.Williamson, R.D., McCarthy, F.P., Manna, S., Groarke, E., Kell, D.B., Kenny, L.C.et al. (2020) L-(+)-Ergothioneine significantly improves the clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 75, 561–568 10.1161/HYPERTENSIONAHA.119.13929 [DOI] [PubMed] [Google Scholar]
- 800.Smith, E., Ottosson, F., Hellstrand, S., Ericson, U., Orho-Melander, M., Fernandez, C.et al. (2020) Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 106, 691–697 10.1136/heartjnl-2019-315485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Bedirli, A., Sakrak, O., Muhtaroglu, S., Soyuer, I., Guler, I., Erdogan, A.R.et al. (2004) Ergothioneine pretreatment protects the liver from ischemia-reperfusion injury caused by increasing hepatic heat shock protein 70. J. Surg. Res. 122, 96–102 10.1016/j.jss.2004.06.016 [DOI] [PubMed] [Google Scholar]
- 802.Deiana, M., Rosa, A., Casu, V., Piga, R., Dessi, M.A. and Aruoma, O.I. (2004) L-Ergothioneine modulates oxidative damage in the kidney and liver of rats in vivo: studies upon the profile of polyunsaturated fatty acids. Clin. Nutr. 23, 183–193 10.1016/S0261-5614(03)00108-0 [DOI] [PubMed] [Google Scholar]
- 803.Song, T.Y., Chen, C.L., Liao, J.W., Ou, H.C. and Tsai, M.S. (2010) Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem. Toxicol. 48, 3492–3499 10.1016/j.fct.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 804.Yang, N.C., Lin, H.C., Wu, J.H., Ou, H.C., Chai, Y.C., Tseng, C.Y.et al. (2012) Ergothioneine protects against neuronal injury induced by beta-amyloid in mice. Food Chem. Toxicol. 50, 3902–3911 10.1016/j.fct.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 805.Sakrak, O., Kerem, M., Bedirli, A., Pasaoglu, H., Akyurek, N., Ofluoglu, E.et al. (2008) Ergothioneine modulates proinflammatory cytokines and heat shock protein 70 in mesenteric ischemia and reperfusion injury. J. Surg. Res. 144, 36–42 10.1016/j.jss.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 806.Cheah, I.K. and Halliwell, B. (2020) Could ergothioneine aid in the treatment of coronavirus patients? Antioxidants (Basel) 9, 595 10.3390/antiox9070595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Tang, R.M.Y., Cheah, I.K., Yew, T.S.K. and Halliwell, B. (2018) Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 8, 1601 10.1038/s41598-018-20021-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.van der Hoek, S.A., Darbani, B., Zugaj, K., Prabhala, B.K., Biron, M.B., Randelovic, M.et al. (2019) Engineering the yeast Saccharomyces cerevisiae for the production of L-(+)-ergothioneine. Front. Bioeng. Biotechnol. 7, 262 10.3389/fbioe.2019.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.van der Hoek, S.A., Rusnák, M., Jacobsen, I.H., Martínez, J.L., Kell, D.B. and Borodina, I. (2022) Engineering ergothioneine production in Yarrowia lipolytica FEBS lett 596, 1356–1364 10.1002/1873-3468.14239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.van der Hoek, S.A., Rusnák, M., Wang, G., Stanchev, L.D., de Fátima Alvez, L., Jessop-Fabre, M.M.et al. (2022) Engineering precursor supply for the high-level production of ergothioneine in Saccharomyces cerevisiae. Metab. Eng. 70, 129–142 10.1016/j.ymben.2022.01.012 [DOI] [PubMed] [Google Scholar]
- 811.Alamgir, K.M., Masuda, S., Fujitani, Y., Fukuda, F. and Tani, A. (2015) Production of ergothioneine by Methylobacterium species. Front. Microbiol. 6, 1185 10.3389/fmicb.2015.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Fujitani, Y., Alamgir, K.M. and Tani, A. (2018) Ergothioneine production using Methylobacterium species, yeast, and fungi. J. Biosci. Bioeng. 126, 715–722 10.1016/j.jbiosc.2018.05.021 [DOI] [PubMed] [Google Scholar]
- 813.Han, Y., Tang, X., Zhang, Y., Hu, X. and Ren, L.J. (2021) The current status of biotechnological production and the application of a novel antioxidant ergothioneine. Crit. Rev. Biotechnol. 41, 580–593 10.1080/07388551.2020.1869692 [DOI] [PubMed] [Google Scholar]
- 814.Kamide, T., Takusagawa, S., Tanaka, N., Ogasawara, Y., Kawano, Y., Ohtsu, I.et al. (2020) High production of ergothioneine in Escherichia coli using the sulfoxide synthase from Methylobacterium strains. J. Agric. Food Chem. 68, 6390–6394 10.1021/acs.jafc.0c01846 [DOI] [PubMed] [Google Scholar]
- 815.Osawa, R., Kamide, T., Satoh, Y., Kawano, Y., Ohtsu, I. and Dairi, T. (2018) Heterologous and high production of ergothioneine in Escherichia coli. J. Agric. Food Chem. 66, 1191–1196 10.1021/acs.jafc.7b04924 [DOI] [PubMed] [Google Scholar]
- 816.Takusagawa, S., Satoh, Y., Ohtsu, I. and Dairi, T. (2019) Ergothioneine production with Aspergillus oryzae. Biosci. Biotechnol. Biochem. 83, 181–184 10.1080/09168451.2018.1527210 [DOI] [PubMed] [Google Scholar]
- 817.Wang, L., Wang, Y., Li, J., Du, G. and Kang, Z. (2022) [Construction and optimization of ergothioneine-producing Escherichia coli]. Sheng Wu Gong Cheng Xue Bao. 38, 796–806 10.13345/j.cjb.210166 [DOI] [PubMed] [Google Scholar]
- 818.Dubost, N.J., Ou, B. and Beelman, R.B. (2007) Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 105, 727–735 10.1016/j.foodchem.2007.01.030 [DOI] [Google Scholar]
- 819.Ey, J., Schömig, E. and Taubert, D. (2007) Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 55, 6466–6474 10.1021/jf071328f [DOI] [PubMed] [Google Scholar]
- 820.Kalaras, M.D., Richie, J.P., Calcagnotto, A. and Beelman, R.B. (2017) Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem. 233, 429–433 10.1016/j.foodchem.2017.04.109 [DOI] [PubMed] [Google Scholar]
- 821.Feng, L., Cheah, I.K., Ng, M.M., Li, J., Chan, S.M., Lim, S.L.et al. (2019) The association between mushroom consumption and mild cognitive impairment: a community-based cross-sectional study in Singapore. J. Alzheimers Dis. 68, 197–203 10.3233/JAD-180959 [DOI] [PubMed] [Google Scholar]
- 822.Slomski, A. (2021) Trials test mushrooms and herbs as anti-COVID-19 agents. J. Am. Med. Assoc 326, 1997–1999 10.1001/jama.2021.19388 [DOI] [PubMed] [Google Scholar]
- 823.Phillips, J.M., Ooi, S.L. and Pak, S.C. (2022) Health-promoting properties of medicinal mushrooms and their bioactive compounds for the COVID-19 era-an appraisal: do the pro-health claims measure up? Molecules 27, 2302 10.3390/molecules27072302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Hussain, T., Tan, B., Yin, Y.L., Blachier, F., Tossou, M.C.B. and Rahu, N. (2016) Oxidative stress and inflammation: what polyphenols can do for US? Oxidative Med. Cell. Longev. 2016, 7432797 10.1155/2016/7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Middleton, E., Kandaswami, C. and Theoharides, T.C. (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52, 673–751 [PubMed] [Google Scholar]
- 826.Havsteen, B.H. (2002) The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 96, 67–202 10.1016/S0163-7258(02)00298-X [DOI] [PubMed] [Google Scholar]
- 827.Passamonti, S., Terdoslavich, M., Franca, R., Vanzo, A., Tramer, F., Braidot, E.et al. (2009) Bioavailability of flavonoids: a review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr. Drug Metab. 10, 369–394 10.2174/138920009788498950 [DOI] [PubMed] [Google Scholar]
- 828.Del Bo', C., Bernardi, S., Marino, M., Porrini, M., Tucci, M., Guglielmetti, S.et al. (2019) Systematic review on polyphenol intake and health outcomes: is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 11, 1355 10.3390/nu11020391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Bayat, P., Farshchi, M., Yousefian, M., Mahmoudi, M. and Yazdian-Robati, R. (2021) Flavonoids, the compounds with anti-inflammatory and immunomodulatory properties, as promising tools in multiple sclerosis (MS) therapy: a systematic review of preclinical evidence. Int. Immunopharmacol. 95, 107562 10.1016/j.intimp.2021.107562 [DOI] [PubMed] [Google Scholar]
- 830.Hamsalakshmi, A., Arehally Marappa, A.M., Joghee, M., and Chidambaram, S. and B, S. (2022) Therapeutic benefits of flavonoids against neuroinflammation: a systematic review. Inflammopharmacology 30, 111–136 10.1007/s10787-021-00895-8 [DOI] [PubMed] [Google Scholar]
- 831.Saeedi-Boroujeni, A. and Mahmoudian-Sani, M.R. (2021) Anti-inflammatory potential of quercetin in COVID-19 treatment. J. Inflamm. (Lond.) 18, 3 10.1186/s12950-021-00268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Aucoin, M., Cooley, K., Saunders, P.R., Cardozo, V., Remy, D., Cramer, H.et al. (2020) The effect of quercetin on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv. Integr. Med. 7, 247–251 10.1016/j.aimed.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Kell, D.B. (2021) The transporter-mediated cellular uptake and efflux of pharmaceutical drugs and biotechnology products: how and why phospholipid bilayer transport is negligible in real biomembranes. Molecules 26, 5629 10.3390/molecules26185629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Amić, D., Davidović-Amić, D., Bešlo, D., Rastija, V., Lučić, B. and Trinajstić, N. (2007) SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 14, 827–845 10.2174/092986707780090954 [DOI] [PubMed] [Google Scholar]
- 835.Lee, J.H. and Kim, G.H. (2010) Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 75, H212–H217 10.1111/j.1750-3841.2010.01755.x [DOI] [PubMed] [Google Scholar]
- 836.Lahiri, M., Morgan, C., Symmons, D.P.M. and Bruce, I.N. (2012) Modifiable risk factors for RA: prevention, better than cure? Rheumatology 51, 499–512 10.1093/rheumatology/ker299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Hughes, S.D., Ketheesan, N. and Haleagrahara, N. (2017) The therapeutic potential of plant flavonoids on rheumatoid arthritis. Crit. Rev. Food Sci. 57, 3601–3613 10.1080/10408398.2016.1246413 [DOI] [PubMed] [Google Scholar]
- 838.Kim, H.R., Kim, B.M., Won, J.Y., Lee, K.A., Ko, H.M., Kang, Y.S.et al. (2019) Quercetin, a plant polyphenol, has potential for the prevention of bone destruction in rheumatoid arthritis. J. Med. Food. 22, 152–161 10.1089/jmf.2018.4259 [DOI] [PubMed] [Google Scholar]
- 839.Sung, S., Kwon, D., Um, E. and Kim, B. (2019) Could polyphenols help in the control of rheumatoid arthritis? Molecules 24, 1286 10.3390/molecules24081589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Mateen, S., Moin, S., Zafar, A. and Khan, A.Q. (2016) Redox signaling in rheumatoid arthritis and the preventive role of polyphenols. Clin. Chim. Acta 463, 4–10 10.1016/j.cca.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 841.Christman, L.M. and Gu, L.W. (2020) Efficacy and mechanisms of dietary polyphenols in mitigating rheumatoid arthritis. J. Funct. Foods 71, 104003 10.1016/j.jff.2020.104003 [DOI] [Google Scholar]
- 842.Behl, T., Upadhyay, T., Singh, S., Chigurupati, S., Alsubayiel, A.M., Mani, V.et al. (2021) Polyphenols targeting MAPK mediated oxidative stress and inflammation in rheumatoid arthritis. Molecules 26, 6570 10.3390/molecules26216570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Kuo, Y.H., Tsai, W.J., Loke, S.H., Wu, T.S. and Chiou, W.F. (2009) Astragalus membranaceus flavonoids (AMF) ameliorate chronic fatigue syndrome induced by food intake restriction plus forced swimming. J. Ethnopharmacol. 122, 28–34 10.1016/j.jep.2008.11.025 [DOI] [PubMed] [Google Scholar]
- 844.Sathyapalan, T., Beckett, S., Rigby, A.S., Mellor, D.D. and Atkin, S.L. (2010) High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 9, 55 10.1186/1475-2891-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Martin, MÁ and Ramos, S. (2021) Impact of cocoa flavanols on human health. Food Chem. Toxicol. 151, 112121 10.1016/j.fct.2021.112121 [DOI] [PubMed] [Google Scholar]
- 846.Russo, M., Moccia, S., Spagnuolo, C., Tedesco, I. and Russo, G.L. (2020) Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 328, 109211 10.1016/j.cbi.2020.109211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Jo, S., Kim, S., Kim, D.Y., Kim, M.S. and Shin, D.H. (2020) Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J. Enzyme Inhib. Med. Chem. 35, 1539–1544 10.1080/14756366.2020.1801672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Kumari, A., Rajput, V.S., Nagpal, P., Kukrety, H., Grover, S. and Grover, A. (2020) Dual inhibition of SARS-CoV-2 spike and main protease through a repurposed drug, rutin. J. Biomol. Struct. Dyn. 40, 4987–4999 10.1080/07391102.2020.1864476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Udrea, A.M., Mernea, M., Buiu, C. and Avram, S. (2020) Scutellaria baicalensis flavones as potent drugs against acute respiratory injury during SARS-CoV-2 infection: structural biology approaches. Processes 8, 1468 10.3390/pr8111468 [DOI] [Google Scholar]
- 850.Song, J., Zhang, L., Xu, Y., Yang, D., Zhang, L., Yang, S.et al. (2021) The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 183, 114302 10.1016/j.bcp.2020.114302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Zandi, K., Musall, K., Oo, A., Cao, D., Liang, B., Hassandarvish, P.et al. (2021) Baicalein and baicalin inhibit SARS-CoV-2 RNA-dependent-RNA polymerase. Microorganisms 9, 893 10.3390/microorganisms9050893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Alzaabi, M.M., Hamdy, R., Ashmawy, N.S., Hamoda, A.M., Alkhayat, F., Khademi, N.N.et al. (2022) Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 21, 291–312 10.1007/s11101-021-09759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Kaul, R., Paul, P., Kumar, S., Busselberg, D., Dwivedi, V.D. and Chaari, A. (2021) Promising antiviral activities of natural flavonoids against SARS-CoV-2 targets: systematic review. Int. J. Mol. Sci 22, 11069 10.3390/ijms222011069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Kumar, S., Paul, P., Yadav, P., Kaul, R., Maitra, S.S., Jha, S.K.et al. (2022) A multi-targeted approach to identify potential flavonoids against three targets in the SARS-CoV-2 life cycle. Comput. Biol. Med. 142, 105231 10.1016/j.compbiomed.2022.105231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Santana, F.P.R., Thevenard, F., Gomes, K.S., Taguchi, L., Câmara, N.O.S., Stilhano, R.S.et al. (2021) New perspectives on natural flavonoids on COVID-19-induced lung injuries. Phytother. Res. 35, 4988–5006 10.1002/ptr.7131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Liskova, A., Samec, M., Koklesova, L., Samuel, S.M., Zhai, K., Al-Ishaq, R.K.et al. (2021) Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 138, 111430 10.1016/j.biopha.2021.111430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Agrawal, P.K., Agrawal, C. and Blunden, G. (2021) Pharmacological significance of hesperidin and hesperetin, two citrus flavonoids, as promising antiviral compounds for prophylaxis against and combating COVID-19. Nat. Prod. Commun. 16, 1–15 10.1177/1934578X211042540 [DOI] [Google Scholar]
- 858.Rakshit, G., Dagur, P., Satpathy, S., Patra, A., Jain, A. and Ghosh, M. (2021) Flavonoids as potential therapeutics against novel coronavirus disease-2019 (nCOVID-19). J. Biomol. Struct. Dyn. 1–13 10.1080/07391102.2021.1892529 [DOI] [PubMed] [Google Scholar]
- 859.Theoharides, T.C., Cholevas, C., Polyzoidis, K. and Politis, A. (2021) Long-COVID syndrome-associated brain fog and chemofog: luteolin to the rescue. Biofactors 47, 232–241 10.1002/biof.1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Edeas, M., Saleh, J. and Peyssonnaux, C. (2020) Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 97, 303–305 10.1016/j.ijid.2020.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Perricone, C., Bartoloni, E., Bursi, R., Cafaro, G., Guidelli, G.M., Shoenfeld, Y.et al. (2020) COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. Res. 68, 213–224 10.1007/s12026-020-09145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Bastin, A., Shiri, H., Zanganeh, S., Fooladi, S., Momeni Moghaddam, M.A., Mehrabani, M.et al. (2021) Iron Chelator or iron supplement consumption in COVID-19? The Role of iron with severity infection. Biol. Trace Elem. Res . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Girelli, D., Marchi, G., Busti, F. and Vianello, A. (2021) Iron metabolism in infections: focus on COVID-19. Semin. Hematol. 58, 182–187 10.1053/j.seminhematol.2021.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Mahroum, N., Alghory, A., Kiyak, Z., Alwani, A., Seida, R., Alrais, M.et al. (2022) Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 126, 102778 10.1016/j.jaut.2021.102778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Poonkuzhi Naseef, P., Elayadeth-Meethal, M., Salim, K.T.M., Anjana, A., Muhas, C., Vajid, K.A.et al. (2022) Therapeutic potential of induced iron depletion using iron chelators in COVID-19. Saudi J. Biol. Sci 20, 1947–1956 10.1016/j.sjbs.2021.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Taneri, P.E., Gómez-Ochoa, S.A., Llanaj, E., Raguindin, P.F., Rojas, L.Z., Roa-Díaz, Z.M.et al. (2020) Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur. J. Epidemiol. 35, 763–773 10.1007/s10654-020-00678-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Birlutiu, V., Birlutiu, R.M. and Chicea, L. (2021) Off-label tocilizumab and adjuvant iron chelator effectiveness in a group of severe COVID-19 pneumonia patients: a single center experience. Medicine (Baltimore) 100, e25832 10.1097/MD.0000000000025832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Carota, G., Ronsisvalle, S., Panarello, F., Tibullo, D., Nicolosi, A. and Li Volti, G. (2021) Role of iron chelation and protease inhibition of natural products on COVID-19 infection. J. Clin. Med. 10, 2306 10.3390/jcm10112306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Habib, H.M., Ibrahim, S., Zaim, A. and Ibrahim, W.H. (2021) The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 136, 111228 10.1016/j.biopha.2021.111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Vlahakos, V.D., Marathias, K.P., Arkadopoulos, N. and Vlahakos, D.V. (2021) Hyperferritinemia in patients with COVID-19: an opportunity for iron chelation? Artif. Organs 45, 163–167 10.1111/aor.13812 [DOI] [PubMed] [Google Scholar]
- 871.Henss, L., Auste, A., Schurmann, C., Schmidt, C., von Rhein, C., Muhlebach, M.D.et al. (2021) The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol 102, 001574 10.1099/jgv.0.001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Mandel, S., Weinreb, O., Reznichenko, L., Kalfon, L. and Amit, T. (2006) Green tea catechins as brain-permeable, non toxic iron chelators to "iron out iron" from the brain. J. Neural Transm.-Suppl. 50, 229–234 10.1007/978-3-211-33328-0_26 [DOI] [PubMed] [Google Scholar]
- 873.Mandel, S., Amit, T., Reznichenko, L., Weinreb, O. and Youdim, M.B.H. (2006) Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 50, 229–234 10.1002/mnfr.200500156 [DOI] [PubMed] [Google Scholar]
- 874.Hatcher, H.C., Singh, R.N., Torti, F.M. and Torti, S.V. (2009) Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Med. Chem. 1, 1643–1670 10.4155/fmc.09.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 875.Weinreb, O., Amit, T., Mandel, S. and Youdim, M.B. (2009) Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 4, 283–296 10.1007/s12263-009-0143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Riegsecker, S., Wiczynski, D., Kaplan, M.J. and Ahmed, S. (2013) Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 93, 307–312 10.1016/j.lfs.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Che, F., Wang, G., Yu, J., Wang, X., Lu, Y., Fu, Q.et al. (2017) Effects of epigallocatechin-3-gallate on iron metabolism in spinal cord motor neurons. Mol. Med. Rep. 16, 3010–3014 10.3892/mmr.2017.6919 [DOI] [PubMed] [Google Scholar]
- 878.Zhao, J., Xu, L., Liang, Q., Sun, Q., Chen, C., Zhang, Y.et al. (2017) Metal chelator EGCG attenuates Fe(III)-induced conformational transition of alpha-synuclein and protects AS-PC12 cells against Fe(III)-induced death. J. Neurochem. 143, 136–146 10.1111/jnc.14142 [DOI] [PubMed] [Google Scholar]
- 879.Xu, Q., Langley, M., Kanthasamy, A.G. and Reddy, M.B. (2017) Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J. Nutr. 147, 1926–1931 10.3945/jn.117.255034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880.Koonyosying, P., Tantiworawit, A., Hantrakool, S., Utama-Ang, N., Cresswell, M., Fucharoen, S.et al. (2020) Consumption of a green tea extract-curcumin drink decreases blood urea nitrogen and redox iron in beta-thalassemia patients. Food Funct. 11, 932–943 10.1039/C9FO02424G [DOI] [PubMed] [Google Scholar]
- 881.Mhatre, S., Srivastava, T., Naik, S. and Patravale, V. (2021) Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: a review. Phytomedicine 85, 153286 10.1016/j.phymed.2020.153286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Campione, E., Cosio, T., Rosa, L., Lanna, C., Di Girolamo, S., Gaziano, R.et al. (2020) Lactoferrin as protective natural barrier of respiratory and intestinal mucosa against coronavirus infection and inflammation. Int. J. Mol. Sci. 21, 4903 10.3390/ijms21144903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Cegolon, L., Javanbakht, M. and Mastrangelo, G. (2020) Nasal disinfection for the prevention and control of COVID-19: a scoping review on potential chemo-preventive agents. Int. J. Hyg. Environ. Health 230, 113605 10.1016/j.ijheh.2020.113605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.AlKhazindar, M. and Elnagdy, S.M. (2020) Can lactoferrin boost human immunity against COVID-19? Pathog. Glob. Health 114, :234-–:2235 10.1080/20477724.2020.1779514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.de Carvalho, M., da Rocha Matos, C.A., Caetano, A., de Sousa Junior, B.C., da Costa Campos, I.P., Geraldino, S.P.et al. (2020) In vitro inhibition of SARS-CoV-2 infection by bovine lactoferrin. bioRxiv 2020.2005.2013.093781 [Google Scholar]
- 886.Wang, Y., Wang, P., Wang, H., Luo, Y., Wan, L., Jiang, M.et al. (2020) Lactoferrin for the treatment of COVID-19 (review). Exp. Ther. Med. 20, 272 10.3892/etm.2020.94021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Chang, R., Ng, T.B. and Sun, W.Z. (2020) Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 56, 106118, 10.1016/j.ijantimicag.2020.106118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Campione, E., Lanna, C., Cosio, T., Rosa, L., Conte, M.P., Iacovelli, F.et al. (2021) Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence. Int. J. Environ. Res. Public Health 18, 10985 10.3390/ijerph182010985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Kucia, M., Wietrak, E., Szymczak, M., Majchrzak, M. and Kowalczyk, P. (2021) Protective action of L. salivarius SGL03 and lactoferrin against COVID-19 infections in human nasopharynx. Materials (Basel) 14, 3086 10.3390/ma14113086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Rosa, L., Tripepi, G., Naldi, E., Aimati, M., Santangeli, S., Venditto, F.et al. (2021) Ambulatory COVID-19 patients treated with lactoferrin as a supplementary antiviral agent: a preliminary study. J. Clin. Med. 10, 4276 10.3390/jcm10184276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891.Wotring, J.W., Fursmidt, R., Ward, L. and Sexton, J.Z. (2022) Evaluating the in vitro efficacy of bovine lactoferrin products against SARS-CoV-2 variants of concern. J. Dairy Sci. 105, 2791–2802 10.3168/jds.2021-21247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Zimecki, M., Actor, J.K. and Kruzel, M.L. (2021) The potential for lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int. Immunopharmacol. 95, 107571 10.1016/j.intimp.2021.107571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Gallo, V., Giansanti, F., Arienzo, A. and Antonini, G. (2022) Antiviral properties of whey proteins and their activity against SARS-CoV-2 infection. J. Funct. Foods 89, 104932 10.1016/j.jff.2022.104932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Shafqat, F., Ur Rehman, S. and Niaz, K. (2022) Lactoferrin can attenuate SARS-CoV-2: an analysis of evidential relations. Biomed. Res. Ther. 9, 4901–4919 10.15419/bmrat.v9i2.727 [DOI] [Google Scholar]
- 895.Tian, J., Tang, L., Liu, X., Li, Y., Chen, J., Huang, W.et al. (2022) Populations in Low-Magnesium areas were associated with higher risk of infection in COVID-19's early transmission: a nationwide retrospective cohort study in the United States. Nutrients 14, 909 10.3390/nu14040909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.DiNicolantonio, J.J. and O'Keefe, J.H. (2021) Magnesium and vitamin D deficiency as a potential cause of immune dysfunction, cytokine storm and disseminated intravascular coagulation in COVID-19 patients. Mo Med. 118, 68–73 [PMC free article] [PubMed] [Google Scholar]
- 897.Cox, I.M., Campbell, M.J. and Dowson, D. (1991) Red blood cell magnesium and chronic fatigue syndrome. Lancet 337, 757–760 10.1016/0140-6736(91)91371-Z [DOI] [PubMed] [Google Scholar]
- 898.Maier, J.A.M., Malpuech-Brugère, C., Zimowska, W., Rayssiguier, Y. and Mazur, A. (2004) Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta 1689, 13–21 10.1016/j.bbadis.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 899.Claustrat, B., Brun, J. and Chazot, G. (2005) The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 9, 11–24 10.1016/j.smrv.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 900.Reiter, R.J. (1998) Oxidative damage in the central nervous system: protection by melatonin. Progr. Neurobiol. 56, 359–384 10.1016/S0301-0082(98)00052-5 [DOI] [PubMed] [Google Scholar]
- 901.Reiter, R.J., Paredes, S.D., Manchester, L.C. and Tan, D.X. (2009) Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 44, 175–200 10.1080/10409230903044914 [DOI] [PubMed] [Google Scholar]
- 902.Reiter, R.J., Rosales-Corral, S., Tan, D.X., Jou, M.J., Galano, A. and Xu, B. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell. Mol. Life Sci. 74, 3863–3881 10.1007/s00018-017-2609-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Brown, G.M., Pandi-Perumal, S.R., Pupko, H., Kennedy, J.L. and Cardinali, D.P. (2021) Melatonin as an add-on treatment of COVID-19 infection: current status. Diseases 9, 64 10.3390/diseases9030064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Loh, D. (2020) The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding. Melatonin Res. 3, 380–416 10.32794/mr11250069 [DOI] [Google Scholar]
- 905.Camp, O.G., Bai, D., Gonullu, D.C., Nayak, N. and Abu-Soud, H.M. (2021) Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem. 223, 111546 10.1016/j.jinorgbio.2021.111546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Castle, R.D., Williams, M.A., Bushell, W.C., Rindfleisch, J.A., Peterson, C.T., Marzolf, J.et al. (2021) Implications for systemic approaches to COVID-19: effect sizes of remdesivir, tocilizumab, melatonin, vitamin D3, and meditation. J. Inflamm. Res. 14, 4859–4876 10.2147/JIR.S323356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Ramos, E., López-Muñoz, F., Gil-Martin, E., Egea, J., Álvarez-Merz, I., Painuli, S.et al. (2021) The coronavirus disease 2019 (COVID-19): key emphasis on melatonin safety and therapeutic efficacy. Antioxidants (Basel) 10, 1152 10.3390/antiox10071152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908.Reiter, R.J., Abreu-Gonzalez, P., Marik, P.E. and Dominguez-Rodriguez, A. (2020) Therapeutic algorithm for use of melatonin in patients with COVID-19. Front. Med. (Lausanne) 7, 226 10.3389/fmed.2020.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 909.Lan, S.H., Lee, H.Z., Chao, C.M., Chang, S.P., Lu, L.C. and Lai, C.C. (2022) Efficacy of melatonin in the treatment of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. J. Med. Virol. 94, 2102–2107 10.1002/jmv.27595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910.Ramlall, V., Zucker, J. and Tatonetti, N. (2020) Melatonin is significantly associated with survival of intubated COVID-19 patients. medRxiv [Google Scholar]
- 911.Kory, P., Meduri, G.U., Iglesias, J., Varon, J., Cadegiani, F.A. and Marik, P.E. (2022) "MATH+" multi-modal hospital treatment protocol for COVID-19 infection: clinical and scientific rationale. J. Clin. Med. Res. 14, 53–79 10.14740/jocmr4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Yang, Y., Duan, W., Jin, Z., Yi, W., Yan, J., Zhang, S.et al. (2013) JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 55, 275–286 10.1111/jpi.12070 [DOI] [PubMed] [Google Scholar]
- 913.Weiss, E., Roux, O., Moyer, J.D., Paugam-Burtz, C., Boudaoud, L., Ajzenberg, N.et al. (2020) Fibrinolysis resistance: a potential mechanism underlying COVID-19 coagulopathy. Thromb. Haemost. 120, 1343–1345 10.1055/s-0040-1713637 [DOI] [PubMed] [Google Scholar]
- 914.Maier, C.L., Sarker, T., Szlam, F. and Sniecinski, R.M. (2021) COVID-19 patient plasma demonstrates resistance to tPA-induced fibrinolysis as measured by thromboelastography. J. Thromb. Thrombolysis. 52, 766–771 10.1007/s11239-021-02438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Zuo, Y., Warnock, M., Harbaugh, A., Yalavarthi, S., Gockman, K., Zuo, M.et al. (2021) Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 11, 1580 10.1038/s41598-020-80010-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Altaf, F., Wu, S.R. and Kasim, V. (2021) Role of fibrinolytic enzymes in anti-thrombosis therapy. Front. Mol. Biosci. 8, 680397 10.3389/fmolb.2021.680397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Kumar, S.S. and Sabu, A. (2019) Fibrinolytic enzymes for thrombolytic therapy. Adv. Exp. Med. Biol. 1148, 345–381 10.1007/978-981-13-7709-9_15 [DOI] [PubMed] [Google Scholar]
- 918.Ali, M., and Bavisetty, A.M. and B, S.C. (2020) Purification, physicochemical properties, and statistical optimization of fibrinolytic enzymes especially from fermented foods: a comprehensive review. Int. J. Biol. Macromol. 163, 1498–1517 10.1016/j.ijbiomac.2020.07.303 [DOI] [PubMed] [Google Scholar]
- 919.Diwan, D., Usmani, Z., Sharma, M., Nelson, J.W., Thakur, V.K., Christie, G.et al. (2021) Thrombolytic enzymes of microbial origin: a review. Int. J. Mol. Sci 22, 10468 10.3390/ijms221910468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Zhou, X.Q., Liu, L.Z. and Zeng, X.R. (2021) Research progress on the utilisation of embedding technology and suitable delivery systems for improving the bioavailability of nattokinase: a review. Food Struct. 30, 100219 10.1016/j.foostr.2021.100219 [DOI] [Google Scholar]
- 921.Fujita, M., Hong, K., Ito, Y., Fujii, R., Kariya, K. and Nishimuro, S. (1995) Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol. Pharm. Bull. 18, 1387–1391 10.1248/bpb.18.1387 [DOI] [PubMed] [Google Scholar]
- 922.Suzuki, Y., Kondo, K., Matsumoto, Y., Zhao, B.Q., Otsuguro, K., Maeda, T.et al. (2003) Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life Sci. 73, 1289–1298 10.1016/S0024-3205(03)00426-0 [DOI] [PubMed] [Google Scholar]
- 923.Tai, M.W. and Sweet, B.V. (2006) Nattokinase for prevention of thrombosis. Am. J. Health Syst. Pharm. 63, 1121–1123 10.2146/ajhp050509 [DOI] [PubMed] [Google Scholar]
- 924.Hsu, R.L., Lee, K.T., Wang, J.H., Lee, L.Y. and Chen, R.P. (2009) Amyloid-degrading ability of nattokinase from Bacillus subtilis natto. J. Agric. Food Chem. 57, 503–508 10.1021/jf803072r [DOI] [PubMed] [Google Scholar]
- 925.Fujita, M., Hong, K., Ito, Y., Misawa, S., Takeuchi, N., Kariya, K.et al. (1995) Transport of nattokinase across the rat intestinal tract. Biol. Pharm. Bull. 18, 1194–1196 10.1248/bpb.18.1194 [DOI] [PubMed] [Google Scholar]
- 926.Sumi, H., Hamada, H., Nakanishi, K. and Hiratani, H. (1990) Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 84, 139–143 10.1159/000205051 [DOI] [PubMed] [Google Scholar]
- 927.Sumi, H., Yanagisawa, Y., Yatagai, C. and Saito, J. (2004) Natto bacillus as an oral fibrinolytic agent: nattokinase activity and the ingestion effect of Bacillus subtilis natto. Food Sci. Technol. Res. 10, 17–20 10.3136/fstr.10.17 [DOI] [Google Scholar]
- 928.Chen, H., McGowan, E.M., Ren, N., Lal, S., Nassif, N., Shad-Kaneez, F.et al. (2018) Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases. Biomark. Insights 13, 1177271918785130 10.1177/1177271918785130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Ero, M.P., Ng, C.M., Mihailovski, T., Harvey, N.R. and Lewis, B.H. (2013) A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Altern. Ther. Health Med. 19, 16–19 [PubMed] [Google Scholar]
- 930.Dabbagh, F., Negahdaripour, M., Berenjian, A., Behfar, A., Mohammadi, F., Zamani, M.et al. (2014) Nattokinase: production and application. Appl. Microbiol. Biotechnol. 98, 9199–9206 10.1007/s00253-014-6135-3 [DOI] [PubMed] [Google Scholar]
- 931.Kapoor, R., Harde, H., Jain, S., Panda, A.K. and Panda, B.P. (2015) Downstream processing, formulation development and antithrombotic evaluation of microbial nattokinase. J. Biomed. Nanotechnol. 11, 1213–1224 10.1166/jbn.2015.2071 [DOI] [PubMed] [Google Scholar]
- 932.Wu, H., Wang, H., Xu, F., Chen, J., Duan, L. and Zhang, F. (2019) Acute toxicity and genotoxicity evaluations of Nattokinase, a promising agent for cardiovascular diseases prevention. Regul. Toxicol. Pharmacol. 103, 205–209 10.1016/j.yrtph.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 933.Gallelli, G., Di Mizio, G., Palleria, C., Siniscalchi, A., Rubino, P., Muraca, L.et al. (2021) Data recorded in real life support the safety of nattokinase in patients with vascular diseases. Nutrients 13, 2031 10.3390/nu13062031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934.Metkar, S.K., Girigoswami, A., Murugesan, R. and Girigoswami, K. (2017) In vitro and in vivo insulin amyloid degradation mediated by serratiopeptidase. Mater. Sci. Eng. C Mater. Biol. Appl. 70, 728–735 10.1016/j.msec.2016.09.049 [DOI] [PubMed] [Google Scholar]
- 935.Sumi, H., Hamada, H., Tsushima, H., Mihara, H. and Muraki, H. (1987) A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese natto; a typical and popular soybean food in the Japanese diet. Experientia 43, 1110–1111 10.1007/BF01956052 [DOI] [PubMed] [Google Scholar]
- 936.Kurosawa, Y., Nirengi, S., Homma, T., Esaki, K., Ohta, M., Clark, J.F.et al. (2015) A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci. Rep. 5, 11601 10.1038/srep11601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Selvarajan, E. and Bhatnagar, N. (2017) Nattokinase: an updated critical review on challenges and perspectives. Cardiovasc. Hematol. Agents Med. Chem. 15, 126–135 10.2174/1871525716666171207153332 [DOI] [PubMed] [Google Scholar]
- 938.Weng, Y., Yao, J., Sparks, S. and Wang, K.Y. (2017) Nattokinase: an oral antithrombotic agent for the prevention of cardiovascular disease. Int. J. Mol. Sci. 18, 523 10.3390/ijms18030523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Takagaki, S., Suzuki, M., Suzuki, E. and Hasumi, K. (2020) Unsaturated fatty acids enhance the fibrinolytic activity of subtilisin NAT (nattokinase). J. Food Biochem. 44, e13326 10.1111/jfbc.13326 [DOI] [PubMed] [Google Scholar]
- 940.Oba, M., Rongduo, W., Saito, A., Okabayashi, T., Yokota, T., Yasuoka, J.et al. (2021) Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro. Biochem. Biophys. Res. Commun. 570, 21–25 10.1016/j.bbrc.2021.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Yanagisawa, Y., Chatake, T., Chiba-Kamoshida, K., Naito, S., Ohsugi, T., Sumi, H.et al. (2010) Purification, crystallization and preliminary X-ray diffraction experiment of nattokinase from Bacillus subtilis natto. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 1670–1673 10.1107/S1744309110043137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942.Yanagisawa, Y., Chatake, T., Naito, S., Ohsugi, T., Yatagai, C., Sumi, H.et al. (2013) X-ray structure determination and deuteration of nattokinase. J. Synchrotron. Radiat. 20, 875–879 10.1107/S0909049513020700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Chen, P.T., Chiang, C.J. and Chao, Y.P. (2007) Medium optimization for the production of recombinant nattokinase by Bacillus subtilis using response surface methodology. Biotechnol. Prog. 23, 1327–1332 10.1021/bp070109b [DOI] [PubMed] [Google Scholar]
- 944.Chen, P.T., Chiang, C.J. and Chao, Y.P. (2007) Strategy to approach stable production of recombinant nattokinase in Bacillus subtilis. Biotechnol. Prog. 23, 808–813 10.1002/bp070108j [DOI] [PubMed] [Google Scholar]
- 945.Cai, D., Zhu, C. and Chen, S. (2017) Microbial production of nattokinase: current progress, challenge and prospect. World J. Microbiol. Biotechnol. 33, 84 10.1007/s11274-017-2253-2 [DOI] [PubMed] [Google Scholar]
- 946.Liu, Z., Zheng, W., Ge, C., Cui, W., Zhou, L. and Zhou, Z. (2019) High-level extracellular production of recombinant nattokinase in Bacillus subtilis WB800 by multiple tandem promoters. BMC Microbiol. 19, 89 10.1186/s12866-019-1461-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Liu, Z., Zhao, H., Han, L., Cui, W., Zhou, L. and Zhou, Z. (2019) Improvement of the acid resistance, catalytic efficiency, and thermostability of nattokinase by multisite-directed mutagenesis. Biotechnol. Bioeng. 116, 1833–1843 10.1002/bit.26983 [DOI] [PubMed] [Google Scholar]
- 948.Li, C., Du, Z., Qi, S., Zhang, X., Wang, M., Zhou, Y.et al. (2020) Food-grade expression of nattokinase in Lactobacillus delbrueckii subsp. bulgaricus and its thrombolytic activity in vitro. Biotechnol. Lett. 42, 2179–2187 10.1007/s10529-020-02974-2 [DOI] [PubMed] [Google Scholar]
- 949.Wei, X., Zhou, Y., Chen, J., Cai, D., Wang, D., Qi, G.et al. (2015) Efficient expression of nattokinase in Bacillus licheniformis: host strain construction and signal peptide optimization. J. Ind. Microbiol. Biotechnol. 42, 287–295 10.1007/s10295-014-1559-4 [DOI] [PubMed] [Google Scholar]
- 950.Guangbo, Y., Min, S., Wei, S., Lixin, M., Chao, Z., Yaping, W.et al. (2021) Heterologous expression of nattokinase from B. subtilis natto using Pichia pastoris GS115 and assessment of its thrombolytic activity. BMC Biotechnol. 21, 49 10.1186/s12896-021-00708-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 951.Jang, J.Y., Kim, T.S., Cai, J., Kim, J., Kim, Y., Shin, K.et al. (2013) Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab. Anim. Res. 29, 221–225 10.5625/lar.2013.29.4.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952.Wu, H., Wang, Y., Zhang, Y., Xu, F., Chen, J., Duan, L.et al. (2020) Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 32, 101500 10.1016/j.redox.2020.101500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Kim, J.Y., Gum, S.N., Paik, J.K., Lim, H.H., Kim, K.C., Ogasawara, K.et al. (2008) Effects of nattokinase on blood pressure: a randomized, controlled trial. Hypertens. Res. 31, 1583–1588 10.1291/hypres.31.1583 [DOI] [PubMed] [Google Scholar]
- 954.Weichmann, F. and Rohdewald, P. (2020) Projected supportive effects of Pycnogenol() in patients suffering from multi-dimensional health impairments after a SARS-CoV2 infection. Int. J. Antimicrob. Agents 56, 106191 10.1016/j.ijantimicag.2020.106191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955.Cesarone, M.R., Belcaro, G., Nicolaides, A.N., Ricci, A., Geroulakos, G., Ippolito, E.et al. (2003) Prevention of venous thrombosis in long-haul flights with flite tabs: the LONFLIT-FLITE randomized, controlled trial. Angiology 54, 531–539 10.1177/000331970305400502 [DOI] [PubMed] [Google Scholar]
- 956.Bhagat, S., Agarwal, M. and Roy, V. (2013) Serratiopeptidase: a systematic review of the existing evidence. Int. J. Surg. 11, 209–217 10.1016/j.ijsu.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 957.Jadhav, S.B., Shah, N., Rathi, A., Rathi, V. and Rathi, A. (2020) Serratiopeptidase: insights into the therapeutic applications. Biotechnol. Rep. (Amst.) 28, e00544 10.1016/j.btre.2020.e00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958.Sharma, C., Jha, N.K., Meeran, M.F.N., Patil, C.R., Goyal, S.N. and Ojha, S. (2021) Serratiopeptidase, a serine protease anti-inflammatory, fibrinolytic, and mucolytic drug, can be a useful adjuvant for management in COVID-19. Front. Pharmacol. 12, 603997 10.3389/fphar.2021.603997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Vianney, Y.M., Tjoa, S.E.E., Aditama, R. and Putra, S.E.D. (2019) Designing a less immunogenic nattokinase from Bacillus subtilis subsp. natto: a computational mutagenesis. J. Mol. Model. 25, 337 10.1007/s00894-019-4225-y [DOI] [PubMed] [Google Scholar]
- 960.Yan, X.M., Kim, C.H., Lee, C.K., Shin, J.S., Cho, I.H. and Sohn, U.D. (2010) Intestinal absorption of fibrinolytic and proteolytic lumbrokinase extracted from earthworm, Eisenia andrei. Korean J. Physiol. Pharmacol. 14, 71–75 10.4196/kjpp.2010.14.2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.Mihara, H., Sumi, H., Yoneta, T., Mizumoto, H., Ikeda, R., Seiki, M.et al. (1991) A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn. J. Physiol. 41, 461–472 10.2170/jjphysiol.41.461 [DOI] [PubMed] [Google Scholar]
- 962.Cho, I.H., Choi, E.S. and Lee, H.H. (2004) Molecular cloning, sequencing, and expression of a fibrinolytic serine-protease gene from the earthworm Lumbricus rubellus. J. Biochem. Mol. Biol. 37, 574–581 10.5483/bmbrep.2004.37.5.574 [DOI] [PubMed] [Google Scholar]
- 963.Kasim, M., Kiat, A.A., Rohman, M.S., Hanifah, Y. and Kiat, H. (2009) Improved myocardial perfusion in stable angina pectoris by oral lumbrokinase: a pilot study. J. Altern. Complement. Med. 15, 539–544 10.1089/acm.2008.0506 [DOI] [PubMed] [Google Scholar]
- 964.Wang, Y.H., Chen, K.M., Chiu, P.S., Lai, S.C., Su, H.H., Jan, M.S.et al. (2016) Lumbrokinase attenuates myocardial ischemia-reperfusion injury by inhibiting TLR4 signaling. J. Mol. Cell. Cardiol. 99, 113–122 10.1016/j.yjmcc.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 965.Wang, Y.H., Li, S.A., Huang, C.H., Su, H.H., Chen, Y.H., Chang, J.T.et al. (2018) Sirt1 activation by post-ischemic treatment with lumbrokinase protects against myocardial ischemia-reperfusion injury. Front. Pharmacol. 9, 636 10.3389/fphar.2018.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 966.Dickey, A., Wang, N., Cooper, E., Tull, L., Breedlove, D., Mason, H.et al. (2017) Transient expression of lumbrokinase (PI239) in tobacco (Nicotiana tabacum) using a geminivirus-based single replicon system dissolves fibrin and blood clots. Evid. Based Complement. Alternat. Med. 2017, 6093017 10.1155/2017/6093017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967.Wang, K.Y., Tull, L., Cooper, E., Wang, N. and Liu, D. (2013) Recombinant protein production of earthworm lumbrokinase for potential antithrombotic application. Evid. Based Complement. Alternat. Med. 2013, 783971 10.1155/2013/783971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968.Rathi, A., Jadhav, S.B. and Shah, N. (2021) A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines (Basel) 8, 47 10.3390/medicines8090047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Cao, Y.J., Zhang, X., Wang, W.H., Zhai, W.Q., Qian, J.F., Wang, J.S.et al. (2013) Oral fibrinogen-depleting agent lumbrokinase for secondary ischemic stroke prevention: results from a multicenter, randomized, parallel-group and controlled clinical trial. Chin. Med. J. (Engl.) 126, 4060–4065 10.3760/cma.j.issn.0366-6999.20131332 [DOI] [PubMed] [Google Scholar]
- 970.Chen, Y., Liu, Y., Zhang, J., Zhou, K., Zhang, X., Dai, H.et al. (2022) Efficacy and safety of lumbrokinase plus aspirin versus aspirin alone for acute ischemic stroke (LUCENT): study protocol for a multicenter randomized controlled trial. Trials 23, 285 10.1186/s13063-022-06200-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Novaes, d.L., Jozala, L.C., Lopes, A.F., de Carvalho Santos-Ebinuma, A.M., Mazzola, V., G, P.et al. (2016) Stability, purification, and applications of bromelain: a review. Biotechnol. Prog. 32, 5–13 10.1002/btpr.2190 [DOI] [PubMed] [Google Scholar]
- 972.Pavan, R., Jain, S. and Shraddha and Kumar, A. (2012) Properties and therapeutic application of bromelain: a review. Biotechnol. Res. Int. 2012, 976203 10.1155/2012/976203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Hikisz, P. and Bernasinska-Slomczewska, J. (2021) Beneficial properties of bromelain. Nutrients 13, 4313 10.3390/nu13124313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974.Varilla, C., Marcone, M., Paiva, L. and Baptista, J. (2021) Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A summary. Foods 10, 2249 10.3390/foods10102249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975.Owoyele, B.V., Bakare, A.O. and Ologe, M.O. (2020) Bromelain: a review on its potential as a therapy for the management of COVID-19. Niger. J. Physiol. Sci. 35, 10–19 [PubMed] [Google Scholar]
- 976.Sagar, S., Rathinavel, A.K., Lutz, W.E., Struble, L.R., Khurana, S., Schnaubelt, A.T.et al. (2021) Bromelain inhibits SARS-CoV-2 infection via targeting ACE-2, TMPRSS2, and spike protein. Clin. Transl. Med. 11, e281 10.1002/ctm2.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Sayıner, S., Velioğlu-Öğünç, A. and Şehirli, AÖ. (2021) Bromelain: a potential therapeutic application in SARSCoV-2 infected patients. Ann. Antivir. Antriretrovir. 5, 015–018 10.17352/aaa.000011 [DOI] [Google Scholar]
- 978.Kritis, P., Karampela, I., Kokoris, S. and Dalamaga, M. (2020) The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metab. Open 8, 100066 10.1016/j.metop.2020.100066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Richardson, D.P. and Lovegrove, J.A. (2020) Nutritional status of micronutrients as a possible and modifiable risk factor for COVID-19: a UK perspective. Br. J. Nutr. 125, 678–684 10.1017/S000711452000330X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Narayanan, N. and Nair, D.T. (2020) Vitamin B12 may inhibit RNA-dependent-RNA polymerase activity of nsp12 from the SARS-CoV-2 virus. IUBMB Life 72, 2112–2120 10.1002/iub.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Jimenez-Guardeño, J.M., Ortega-Prieto, A.M., Moreno, B.M., Maguire, T.J.A., Richardson, A., Diaz-Hernandez, J.I.et al. (2021) Drug repurposing based on a quantum-inspired method versus classical fingerprinting uncovers potential antivirals against SARS-CoV-2 including vitamin B12. bioRxiv 2021.2006.2025.449609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Batista, K.S., Cintra, V.M., Lucena, P.A.F., Manhães-de-Castro, R., Toscano, A.E., Costa, L.P.et al. (2022) The role of vitamin B12 in viral infections: a comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 80, 561–578 10.1093/nutrit/nuab092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Carroll, H.A., Millar, E. and Deans, K.A. (2022) Vitamin B12 and D deficiency as cofactors of COVID-19 vaccine-induced chronic neurological adverse reactions: two cases and a hypothesis. Res. Sq. 10.3390/immuno1010003 [DOI] [Google Scholar]
- 984.Benskin, L.L. (2020) A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front. Public Health 8, 513 10.3389/fpubh.2020.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Wichniak, A., Kania, A., Sieminski, M. and Cubala, W.J. (2021) Melatonin as a potential adjuvant treatment for COVID-19 beyond sleep disorders. Int. J. Mol. Sci. 22, 8623 10.3390/ijms22168623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Varikasuvu, S.R., Thangappazham, B., Vykunta, A., Duggina, P., Manne, M., Raj, H.et al. (2022) COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials. Expert. Rev. Anti Infect. Ther. 20, 907–913 10.1080/14787210.2022.2035217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Anastasi, E., Ialongo, C., Labriola, R., Ferraguti, G., Lucarelli, M. and Angeloni, A. (2020) Vitamin K deficiency and COVID-19. Scand. J. Clin. Lab. Investig. 80, 525–527 10.1080/00365513.2020.1805122 [DOI] [PubMed] [Google Scholar]
- 988.Desai, A.P., Dirajlal-Fargo, S., Durieux, J.C., Tribout, H., Labbato, D. and McComsey, G.A. (2021) Vitamin K & D deficiencies are independently associated with COVID-19 disease severity. Open Forum. Infect. Dis. 8, ofab408 10.1093/ofid/ofab408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989.Kudelko, M., Yip, T.F., Law, G.C.H. and Lee, S.M.Y. (2021) Potential beneficial effects of vitamin K in SARS-CoV-2 induced vascular disease? Immunol. Lett. 1, 17–29 10.3390/immuno1010003 [DOI] [Google Scholar]
- 990.Linneberg, A., Kampmann, F.B., Israelsen, S.B., Andersen, L.R., Jørgensen, H.L., Sandholt, H.et al. (2021) The association of low vitamin K status with mortality in a cohort of 138 hospitalized patients with COVID-19. Nutrients 13, 1985 10.3390/nu13061985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Cara, K.C., Beauchesne, A.R., Li, R. and Chung, M. (2022) Cochrane review summary on "vitamin D supplementation for the treatment of COVID-19: a living systematic review". J. Diet. Suppl. 19, 143–145 10.1080/19390211.2022.2008601 [DOI] [PubMed] [Google Scholar]
- 992.Hu, Y., Kung, J., Cave, A. and Banh, H.L. (2022) Effects of vitamin D serum level on morbidity and mortality in patients with COVID-19: a systematic review and meta-analysis. J. Pharm. Pharm. Sci. 25, 84–92 10.18433/jpps32590 [DOI] [PubMed] [Google Scholar]
- 993.Shakeri, H., Azimian, A., Ghasemzadeh-Moghaddam, H., Safdari, M., Haresabadi, M., Daneshmand, T.et al. (2022) Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID-19. J. Med. Virol. 94, 141–146 10.1002/jmv.27277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 994.Abobaker, A., Alzwi, A. and Alraied, A.H.A. (2020) Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 72, 1517–1528 10.1007/s43440-020-00176-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995.Novak Kujundžić, R. (2022) COVID-19: are we facing secondary pellagra which cannot simply be cured by vitamin B3? Int. J. Mol. Sci. 23, 4309 10.3390/ijms23084309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Zheng, M., Schultz, M.B. and Sinclair, D.A. (2022) NAD+ in COVID-19 and viral infections. Trends Immunol. 43, 283–295 10.1016/j.it.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Chudzik, M., Kapusta, J. and Burzyńska, M. (2021) Use of 1-MNA to improve exercise tolerance and fatigue in patients after COVID-19. medRxiv 2021.2007.2014.21259081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998.Bosch, T. (1996) Lipid apheresis: from a heroic treatment to routine clinical practice. Artif. Organs 20, 414–419 10.1111/j.1525-1594.1996.tb04525.x [DOI] [PubMed] [Google Scholar]
- 999.Bornstein, S.R., Voit-Bak, K., Donate, T., Rodionov, R.N., Gainetdinov, R.R., Tselmin, S.et al. (2022) Chronic post-COVID-19 syndrome and chronic fatigue syndrome: is there a role for extracorporeal apheresis? Mol. Psychiatry 27, 34–37 10.1038/s41380-021-01148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000.Jaeger, B.R. and Seidel, D. (2001) [Hyperlipoproteinemia and LDL apheresis. Clinical experiences with the H.E.L.P. system]. Herz 26, 531–544 10.1007/PL00002058 [DOI] [PubMed] [Google Scholar]
- 1001.Jaeger, B.R., Richter, Y., Nagel, D., Heigl, F., Vogt, A., Roeseler, E.et al. (2009) Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat. Clin. Pract. Cardiovasc. Med. 6, 229–239 10.1038/ncpcardio1456 [DOI] [PubMed] [Google Scholar]
- 1002.Mattecka, S., Brunner, P., Hahnel, B., Kunze, R., Vogt, B. and Sheriff, A. (2019) Pentrasorb C-Reactive protein: characterization of the selective C-reactive protein adsorber resin. Ther. Apher. Dial. 23, 474–481 10.1111/1744-9987.12796 [DOI] [PubMed] [Google Scholar]
- 1003.Torzewski, J., Heigl, F., Zimmermann, O., Wagner, F., Schumann, C., Hettich, R.et al. (2020) First-in-man: case report of selective C-reactive protein apheresis in a patient with SARS-CoV-2 infection. Am. J. Case Rep. 21, e925020 10.12659/AJCR.925020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004.De Maio, A. and Hightower, L.E. (2020) COVID-19, acute respiratory distress syndrome (ARDS), and hyperbaric oxygen therapy (HBOT): what is the link? Cell Stress Chaperones 25, 717–720 10.1007/s12192-020-01121-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005.Feldmeier, J.J., Kirby, J.P., Buckey, J.C., Denham, D.W., Evangelista, J.S., Gelly, H.B.et al. (2021) Physiologic and biochemical rationale for treating COVID-19 patients with hyperbaric oxygen. Undersea Hyperb. Med. 48, 1–12 10.22462/01.03.2021.1 [DOI] [PubMed] [Google Scholar]
- 1006.Robbins, T., Gonevski, M., Clark, C., Baitule, S., Sharma, K., Magar, A.et al. (2021) Hyperbaric oxygen therapy for the treatment of long COVID: early evaluation of a highly promising intervention. Clin. Med. (Lond.) 21, e629–e632 10.7861/clinmed.2021-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1007.Ortega, M.A., Fraile-Martinez, O., Garcia-Montero, C., Callejón-Peláez, E., Sáez, M.A., Álvarez-Mon, M.A.et al. (2021) A general overview on the hyperbaric oxygen therapy: applications, mechanisms and translational opportunities. Medicina (Kaunas) 57, 864 10.3390/medicina57090864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1008.Paganini, M., Bosco, G., Perozzo, F.A.G., Kohlscheen, E., Sonda, R., Bassetto, F.et al. (2021) The role of hyperbaric oxygen treatment for COVID-19: a review. Adv. Exp. Med. Biol. 1289, 27–35 10.1007/5584_2020_568 [DOI] [PubMed] [Google Scholar]
- 1009.Harnanik, T., Soeroso, J., Suryokusumo, M.G. and Juliandhy, T. (2020) Effects of hyperbaric oxygen on T helper 17/regulatory T polarization in antigen and collagen-induced arthritis: hypoxia-inducible factor-1alpha as a target. Oman Med. J. 35, e90 10.5001/omj.2020.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010.Harnanik, T., Prihartono, S. and Juliandhy, T. (2020) Hyperbaric oxygen in animal model of rheumatoid arthritis: analysis Of HIF-1alpha, ACPA and IL-17a. Infect. Dis. Rep. 12, 8766 10.4081/idr.2020.8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.Sit, M.T., Schmidt, T.W., Edmonds, L.D., Kelly, J.A., Sky, K.M., Thornton, J.A.et al. (2021) The effects of hyperbaric oxygen on rheumatoid arthritis: a pilot study. J. Clin. Rheumatol. 27, e462–e468 10.1097/RHU.0000000000001540 [DOI] [PubMed] [Google Scholar]
- 1012.Oliaei, S., SeyedAlinaghi, S., Mehrtak, M., Karimi, A., Noori, T., Mirzapour, P.et al. (2021) The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): a systematic review. Eur. J. Med. Res. 26, 96 10.1186/s40001-021-00570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Yanagawa, Y. (2021) Current status of hyperbaric oxygen therapy for COVID-19. Acute Med. Surg. 8, e678 10.1002/ams2.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Bhaiyat, A.M., Sasson, E., Wang, Z., Khairy, S., Ginzarly, M., Qureshi, U.et al. (2022) Hyperbaric oxygen treatment for long coronavirus disease-19: a case report. J. Med. Case Rep. 16, 80 10.1186/s13256-022-03287-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Afshari, R., Akhavan, O., Hamblin, M.R. and Varma, R.S. (2021) Review of oxygenation with nanobubbles: possible treatment for hypoxic COVID-19 patients. ACS Appl. Nano Mater. 4, 11386–11412 10.1021/acsanm.1c01907 [DOI] [PMC free article] [PubMed] [Google Scholar]