Abstract
Most COVID-19 patients recovered with low mortality; however, some patients experienced long-term symptoms described as “long-COVID” or “Post-COVID syndrome” (PCS). Patients may have persisting symptoms for weeks after acute SARS-CoV-2 infection, including dyspnea, fatigue, myalgia, insomnia, cognitive and olfactory disorders. These symptoms may last for months in some patients. PCS may progress in association with the development of mast cell activation syndrome (MCAS), which is a distinct kind of mast cell activation disorder, characterized by hyper-activation of mast cells with inappropriate and excessive release of chemical mediators. COVID-19 survivors, mainly women, and patients with persistent severe fatigue for 10 weeks after recovery with a history of neuropsychiatric disorders are more prone to develop PCS. High D-dimer levels and blood urea nitrogen were observed to be risk factors associated with pulmonary dysfunction in COVID-19 survivors 3 months post-hospital discharge with the development of PCS. PCS has systemic manifestations that resolve with time with no further complications. However, the final outcomes of PCS are chiefly unknown. Persistence of inflammatory reactions, autoimmune mimicry, and reactivation of pathogens together with host microbiome alterations may contribute to the development of PCS. The deregulated release of inflammatory mediators in MCAS produces extraordinary symptoms in patients with PCS. The development of MCAS during the course of SARS-CoV-2 infection is correlated to COVID-19 severity and the development of PCS. Therefore, MCAS is treated by antihistamines, inhibition of synthesis of mediators, inhibition of mediator release, and inhibition of degranulation of mast cells.
Keywords: COVID-19, Post-COVID syndrome, Mast cell activation syndrome, Pathogenesis
Key summary points
Post-COVID (PCS) syndrome may progress in association with the development of mast cell activation syndrome (MCAS).
High D-dimer levels and blood urea nitrogen were observed to be risk factors associated with pulmonary dysfunction in COVID-19 survivors 3 months post-hospital discharge with the development of PCS.
Persistence of inflammatory reactions, autoimmune mimicry, and reactivation of pathogens together with host microbiome alterations may contribute to the development of PCS.
MCAS is treated by antihistamines, inhibition of synthesis of mediators, inhibition of mediator release, and inhibition of degranulation of mast cells.
Introduction
The current devastating coronavirus disease 2019 (COVID-19) pandemic leads to a worldwide impact with high morbidity and relative mortality in high-risk group patients [1]. The COVID-19 is caused by a novel single-strand RNA virus named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2, 3]. The clinical feature of COVID-19 is asymptomatic or mild flu-like illness in the majority of cases [4, 5]. However, about 15% of COVID-19 patients experienced features of acute viral pneumonia due to the propagation of acute lung injury (ALI). Approximately 5% of COVID-19 patients need intensive care unit admission and ventilation support due to the development of acute respiratory distress syndrome (ARDS) [6, 7].
SARS-CoV-2 exploits and bind specific cell membrane receptor named angiotensin converting enzyme 2 (ACE2) [8]. ACE2 is highly distributed and expressed in many organs, including the intestine, kidney, lung, brain, heart, testis, and some immune cells [8, 9]. Severe SARS-CoV-2 infection induces an exaggeration of immune response and release of pro-inflammatory cytokines with the progression of hypercytokinemia and cytokine storm [10, 11].
Most COVID-19 patients recovered with a low mortality rate of 3–5%. However, some patients experienced long-term symptoms described as long-COVID or Post-COVID syndrome (PCS) [12, 13]. PCS was initially identified by a scientific group in early 2020 following a strict survey of long-term symptoms in recovered COVID-19 patients. They found that COVID-19 patients may have persisting symptoms for weeks after acute SARS-CoV-2 infection, including dyspnea, fatigue, myalgia, insomnia, cognitive and olfactory disorders [14, 15]. However, these symptoms may last for months in some patients, which may affect their daily activities [16, 17].
It has been shown that PCS may progress in association with the development of mast cell activation syndrome (MCAS) [18]. MCAS is a distinct kind of mast cell activation disorder characterized by hyper-activation of mast cells with excessive and inappropriate release of chemical mediators [19]. The primary manifestations of MCAS are cardio-pulmonary, gastrointestinal, dermatological, and neurological problems [19]. MCAS differs from mastocytosis, which is characterized by an increase in the number and abnormal shape of mast cells. In MCAS, the number and shape of mast cells are normal [20]. However, symptoms and presentations of MCAS and systemic mastocytosis are intermingled with the development of extensive tissue damage and can be treated by receptor blockade of relevant mediators including histamine, leukotrienes, and prostaglandin, or by inhibiting mediator synthesis [21]. Diagnosis of MCAS depends on the presence of symptoms consistent with mediator release, increased mast cell mediators and clinical improvement with mediator blockers [20] (Fig. 1). Therefore, this critical review aimed to find the crucial association between PCS and MCAS with appropriate therapeutic modalities.
Fig. 1.
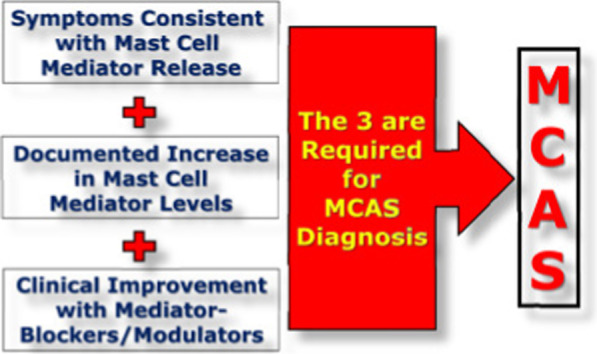
Diagnostic criteria of mast cell activation syndrome
Post-COVID syndrome
Imam Ali, cousin of the Messenger of God in the sixth century, said: “And considers what has passed for what is to come.” Now, what passed, I think, will appear.
PCS was initially defined in relation to post-acute COVID-19 as persistence symptoms for > 3 weeks from the onset of COVID-19 or chronic COVID-19 as persistence symptoms for > 12 weeks from the onset of COVID-19 [22, 23]. Yong defined PCS or long-term COVID-19 as symptoms that persist for more than 3 months after the onset of COVID-19 [24, 25]. However, different studies reported that patients with PCS had persistence symptoms in contrasting frequencies and durations of COVID-19 survivors. This might be due to dissimilar characteristics of sample size and methods for data collection [26–28]. As a result of large studies, there are different descriptions of PCS according to the duration of symptoms (Table 1).
Table 1.
Descriptions of Post-COVID syndrome
| Duration of symptoms | Description/terms | References |
|---|---|---|
| > 3 months | Post-COVID syndrome | [22, 29, 30] |
| Long COVID | ||
| Chronic COVID-19 | ||
| 1–3 months | On-going COVID-19 | [22] |
| Post-acute COVID-19 | ||
| > 24 weeks | Persistent post-COVID symptoms | [31] |
| 12–24 weeks | Long post-COVID symptoms | [32] |
| 5–12 weeks | Acute post-COVID symptoms | [33] |
| > 4 weeks (from symptoms onset) | Post-acute COVID-19 syndrome | [34] |
| > 4 weeks (from diagnosis time) | Long-COVID | [35–37] |
| Long-haulers | ||
| Late sequelae of SARS-CoV-2 infection | ||
| > 2 months | Long-COVID | [38] |
| > 100 days | Long-haul COVID | [39] |
The clinical presentations of PCS are still elusive, comprising different phenotypes and subtypes [40]. Of interest, PCS may develop in patients with mild-moderate COVID-19 and even in asymptomatic patients [41, 42]. As well, PCS may develop in asymptomatic children with COVID-19, resulting in persistent dyspnea and fatigue [43]. PCS may be similar to post-viral syndrome as in SARS and Middle East Respiratory Syndrome (MERS) [44, 45]. Prolonged signs and symptoms could be evident for 7–15 years following previous SARS, mainly in younger age groups [46]. Therefore, PCS may persist for years following the onset of COVID-19.
Risk factors for Post-COVID syndrome
It has been shown that COVID-19 survivors, mainly women, and patients with persistent severe fatigue for 10 weeks after recovery with a history of neuropsychiatric disorders are more prone to developing PCS [47]. Thus, female sex is regarded as a potential risk factor for the development of PSC due to higher immunological response and hormonal changes [48]. However, some studies found no gender difference in the risk of developing PCS after an acute SARS-CoV-2 infection [49, 50]. Furthermore, co-morbidities, advanced age, and the severity of the initial disease are associated with an increased risk of developing PCS [51]. Staven et al. found that follow-up of COVID-19 survivors after 1–6 months of their first SARS-CoV-2 infection revealed the presence of 10 symptoms during the initial acute SARS-CoV-2 infection increased their risk for development of PCS [52, 53]. In addition, patients with severe initial SARS-CoV-2 infection are regarded as a high-risk group for the development of PCS [54]. Townsend et al. observed that persistent fatigue and development of PCS are common and independent of initial COVID-19 severity [47, 55]. As well, COVID-19 survivors with initial SARS-CoV-2 infection who needed hospitalization and ICU admission with a requirement for supported ventilation were highly predisposed to develop PCS 3 months later [56]. Of note, acute disease-induced extensive tissue injury may lead to the development of post-intensive care syndrome, which is characterized by long-term neuropsychiatric disorders and physical impairment [57]. Stam et al. [58] give a call for risk of development of post-intensive care syndrome in COVID-19 patients admitted in ICU. These findings suggest that COVID-19 could have an additive impact on the severity of post-intensive care syndrome and vice versa.
On the other hand, biomarkers of COVID-19 severity are linked with the progression of PCS. For example, high D-dimer level and blood urea nitrogen were observed to be risk factors associated with pulmonary dysfunction in COVID-19 survivors 3 months post-hospital discharge with the development of PCS [59, 60]. Similarly, high levels of CRP, D-dimer, and IL-6 are related to pulmonary dysfunction and the advancement of PCS [61]. In addition, Raman et al. revealed that high systemic biomarkers of inflammation and lymphopenia are also linked with radiological lesions of various organs within 3 months in post-discharge COVID-19 survivors [62]. Surprisingly, high troponin levels are associated with the development of fatigue, whereas lymphopenia is associated with an increased risk of tachycardia in PCS patients [63]. Therefore, persistent symptoms in COVID-19 survivors must be correlated with inflammatory biomarkers and lymphocyte counts to predict the development of PCS.
Clinical presentation of Post-COVID syndrome
PCS includes a plethora of conditions and symptoms, and the incidence of explicit symptoms may vary according to the severity, duration, and nature of acute infection [44]. Fatigue represents the most common symptom of PCS and is found in 17–72% of critically ill COVID-19 patients [64]. Respiratory symptoms are more common in PCS patients, including chest pain (22%), dyspnea (10–40%), and exercise intolerance (10–40%), though worsening dyspnea in PCS patients has been reported to exaggerate by up to 65% during ICU hospitalization [65]. Arrhythmias, postural hypotension, and persistently high blood pressure with hypertension develop due to endothelial dysfunction and cardiac injury [66]. Gastrointestinal symptoms such as nausea, vomiting, diarrhea, change bowel habit, appetite disorders may remain in 30% of COVID-19 patients for more than two months following hospital discharge [67, 68].
Furthermore, patients with PCS may have neuropsychiatric disorders such as olfactory/gustatory dysfunction, which occurs in 9–11% of patients and can last 6–8 months after mild COVID-19 [69]. As well, PCS patients may experience anxiety in 26% and depression in 40% of their cases within or after six months from the onset of acute COVID-19 [70, 71]. Psychiatric complications in PCS patients, including aggression, cognitive deficit, reduction of social activity, and obsessive–compulsive disorders, have been reported [72]. Furthermore, post-traumatic stress disorders have been shown to be found in recovered COVID-19 patients by up to 43% [73, 74]. Other neurological disorders observed in PCS patients are Guillain–Barre syndrome, seizures, ischemic stroke, cerebral vasculitis, and transverse myelitis [75].
These findings suggest that PCS has systemic manifestations which might be mild or severe. However, Moreno-Perez et al. illustrated that the majority of persisting symptoms in PCS are resolved with time with no further complications [76]. Though the final results of PCS are largely unknown.
Category of Post-COVID syndrome
It has been proposed that PCS symptoms are classified into residual symptoms (persist after recovery from acute SARS-CoV-2 infection), organ dysfunction symptoms (persist following recovery) and new symptoms (developed after mild SARS-CoV-2 infection) [27]. However, PCS was classified according to the onset, type, and duration of symptoms into 5 types: [22] (Table 2).
Table 2.
Types of Post-COVID syndrome
| Types | Initial symptoms | Duration of symptoms | Quiescence period | Delayed symptoms |
|---|---|---|---|---|
| Type I | Variable | Variable | Negative | Negative |
| Type II | Mild | > 6 weeks | Negative | Negative |
| Type IIIA | Mild | 3–6 months | Present | Negative |
| Type IIIB | Mild | > 6 months | Present | ………… |
| Type IVA | Negative | Variable | Negative | > 3 months |
| Type IVB | Negative | Variable | Negative | > 6 months |
| Type V | Negative | ………… | ………… | Present |
Type I: PCS patients have varying durations of recovery, and the symptoms are linked to the severity and complications of the SARS-CoV-2 infection.
Type II: PCS patients have persisting symptoms for 6 weeks from the onset of the SARS-CoV-2 infection.
Type III: PCS patients show a recovery period followed by a re-appearance of symptoms.
Type IIIA: symptoms persist for 3 months. Type IIIB: symptoms persist for 6 months.
Type IV: PCS patients were initially asymptomatic at the time of diagnosis but later became symptomatic.
Type IVA: become symptomatic within 1–3 months, Type IVB: become symptomatic after 3 months.
Type V: PCS patients were initially asymptomatic at time of diagnosis and die within 12 months.
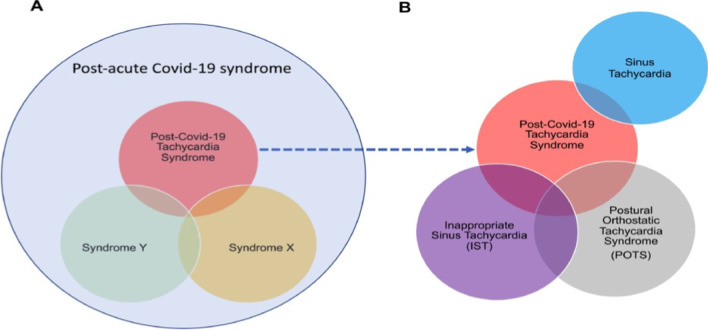
Of interest, post-COVID POTS is regarded as a distinct type of PCS characterized by sinus tachycardia, postural tachycardia, and inappropriate sinus tachycardia (Fig. 2).
Fig. 2.
Post-COVID tachycardia syndrome [77]
Pathogenesis of Post-COVID syndrome
Systemic inflammatory response syndrome (SIRS) could be the potential cause for the development of organ dysfunction and tissue injury in PCS. Due to the development of an exaggerated immune response and a high pro-inflammatory response in COVID-19, a counter-balanced anti-inflammatory response is developed, leading to a state of immunosuppression to maintain immunological homeostasis [78, 79]. However, prolonged immunosuppression may cause propagation catabolism syndrome and the development of PCS [80]. It has been reported that post-septic patients are susceptible to latent viral infections and reactivations [81] and a relapse of SARS-CoV-2 infection in recovered COVID-19 with the development of secondary infections [82, 83]. Further, Russell et al., exemplified that transforming growth factor β (TGF-β) which is an immunosuppressive, profibrotic, and anti-inflammatory cytokine, is increased during and after SARS-CoV-2 infection to dampen an exaggerated pro-inflammatory response [84]. TGF-β is associated with the development of pulmonary interstitial fibrosis in COVID-19 patients [85]. As a result, targeting TGF-β in COVID-19 patients may have a therapeutic value in reducing the fibrotic changes in PCS [86].
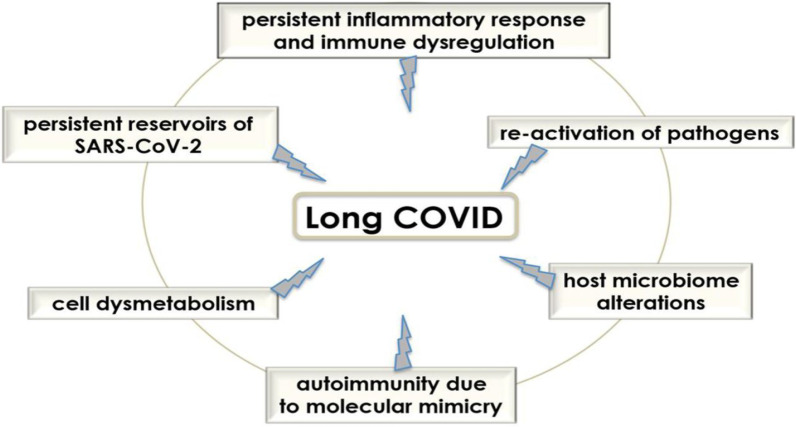
Several studies [87, 88] have reported the persistence of subclinical and/or symptomatic SARS-CoV-2 infection for up to 3 months after infection. Several studies [89, 90] have found SARS-CoV-2 shedding in both lungs for 4 months and in the GI tract for 2 months. Persistence SARS-CoV-2 infection triggers long-term immune stimulation and development of PCS. Persistent SARS-CoV-2 infection activates autoreactive T cells through presentation of antigens by antigen-presenting cells as a bystander effect in MIS syndrome [91]. Of interest, MIS may develop in children and adults within 2–6 weeks and is correlated with pro-inflammatory cytokine levels, but it was not related to the severity of the initial SARS-CoV-2 infection [91]. The delayed onset of MIS following SARS-CoV-2 infection may be due to the deregulation of adaptive immune responses [92]. The stimulation of T cell-mediated immunity in PCS is evident by the development of autoimmune-mediated thyroid dysfunction [93]. Likewise, B cell activation and the production of antiphospholipid autoantibodies were detected in 52% of PCS patients [94]. Similarly, autoantibodies were detected in up to 50% of COVID-19 patients and PCS, indicating a link with the development of autoimmune diseases including systemic lupus erythematosus [95]. Prolonged lymphopenia is associated with PCS patients' chronic immune activation and hyperinflammation [76]. It has been shown that lymphopenia is related to persistent SARS-CoV-2 infection, abnormal immune response, and persistent COVID-19 patient’s symptoms in PCS [96]. Taken together, immune dysregulation, persistence of inflammatory reactions, autoimmune mimicry, and reactivation of pathogens together with host microbiome alterations may contribute to the development of PCS (Fig. 3).
Fig. 3.
Pathogenesis of Post-COVID syndrome
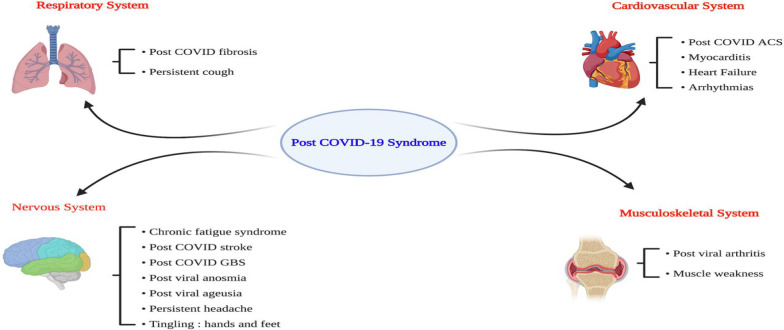
Therefore, unresolved lymphopenia and protracted high pro-inflammatory cytokine levels are linked with the development of headaches, joint pain, and fatigue, the cardinal symptoms of PCS [44]. As well, fatigue is also related to the development of cerebral hypoperfusion, muscle channelopathy, and dysautonomia [44]. In turn, PCS leads to systemic effects involving the cardiovascular, respiratory, nervous system, and musculoskeletal systems (Fig. 4).
Fig. 4.
Systemic effects of Post-COVID syndrome
Pulmonary complications
Pulmonary complications Organic or functional impairments have been reported in SARS-CoV-2 survivor COVID-19 patients following acute pneumonia [97]. Most COVID-19 patients experience mild-moderate respiratory complications, and about 5% of them develop ARDS [6]. It has been reported that ARDS has three pathological phases (exudative, proliferative, and fibrotic) [98].
In a follow-up study of 55 survivor COVID-19 patients, symptoms related to SARS-CoV-2 infection were detected in 35 patients, and radiological abnormalities in 39 patients that correlated with high blood urea nitrogen (OR = 7.14, 95% CI = 1.03–49.21, P = 0.04). Functional pulmonary disorders were found in 14 patients that correlated with D-dimer (OR = 1.066, 95% CI = 1.006–1.129, P = 0.03) [97]. In 42% of the COVID-19 patients' survivors 3 months after hospital discharge, there was a significant reduction in lung diffusion capacity [99]. Remarkably, persistent symptoms and pulmonary radiological changes may persist for 6 months in 50% of COVID-19 patients' survivors [100].
Of note, radiological abnormalities and lung fibrosis may persist up to 6 months following an acute SARS-CoV-2 infection [101]. Besides, pulmonary dysfunction with impairment of gas exchange was detected in patients with moderate COVID-19 even with a normal lung computed tomography (CT) scan [102]. Likewise, maximal aerobic capacity was reduced in follow-up COVID-19 patients compared with controls [103].
Disruption of endothelial integrity and alveolar injury due to the release of pro-inflammatory cytokines and infiltration of immune cells are highly evident in the exudative phase [98]. In the fibro-proliferative phase, deposition of collagen and fibronectin in the alveolar space occurred due to accumulation of fibrocytes, myofibroblasts, and fibroblasts [104]. These pathological changes trigger the release of TGF-β, inhibition of the collagenase enzyme, and further deposition of collagen [104]. Most of the survivor patients from COVID-19 with ARDS managed by mechanical ventilation develop lung fibrosis and pulmonary dysfunction [105]. It has been shown that survivors of ARDS may have exercise intolerance with a profound reduction in life quality for about 5 years [106].
Indeed, imbalance between helper T cells and regulatory T cells as well as augmentation of CD8 + T cells were observed in PCS, resulting in the progression of an autoimmune response that persisted for a long time [107]. An imbalance of T cell immune response was detected to be associated with pulmonary complications, as mature T cells have the ability to produce granzyme B, which is elevated in COVID-19 survivors [108].
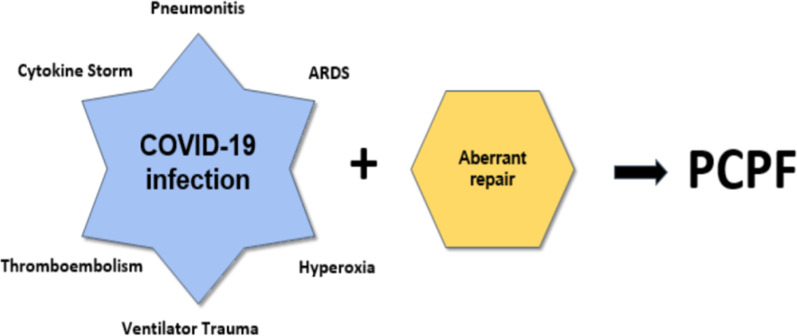
Overall, pathological changes, including cytokine storm, ALI, ARDS, ventilation injury, hypoxia, and hyperoxia, may interact together to induce an aberrant repair with the development of post-COVID pulmonary fibrosis (Fig. 5).
Fig. 5.
COVID-19 and the risk of Post-COVID pulmonary fibrosis (PCPF)
These findings suggest that lung fibrosis/scarring could be the possible causes of chronic cough and dyspnea in patients with PCS. However, results from a cohort-prospective study revealed that respiratory symptoms may persist in patients with PCS even with enhancement of pulmonary function and resolution of radiological abnormalities [109]. This verdict suggests that other pathophysiological mechanisms might be responsible for dyspnea in patients with PCS.
Neurological complications
In general, SARS-CoV-2 infection is associated with neurological manifestations and complications like dysgeusia and anosmia due to the neurotropic effect of SARS-CoV-2 [110]. It has been shown that neurological manifestations are present in 36.4% of COVID-19 patients, including central and peripheral neurological complications as well as skeletal muscle disorders [111, 112]. The most common neurological symptoms in COVID-19 are dizziness (16.8%), headache (13.1%), and fatigue (13.0%) [113]. In addition, stroke, seizure, ataxia, and confusion were also documented as central neurological manifestations/complications in COVID-19 [113].
Of interest, fatigue in PCS is developed due to autoantibodies against muscarinic and adrenergic receptors, resulting in an association between fatigue and dysautonomia [114]. An association between anxiety and fatigue proposed the development of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), which is characterized by chronic fatigue, cognitive impairment, dysautonomia, and endocrinopathies [115]. It has been reported that ME/CFS is linked with the production of autoantibodies that injure phospholipids, gangliosides, and 5-hydroxytryptamine [116]. ME/CFS is more common in women, and has been reported to develop after 6 months of the initial SARS-CoV-2 infection [117].
Similarly, neuropsychiatric disorders including depression, psychosis, and anxiety have been reported following COVID-19 [118]. Mazza and colleagues reported that depression and anxiety in COVID-19 survivors were correlated with high inflammatory burden [119], since hyper-active immune response and neuroinflammation increase the risk of development of neuropsychiatric complications in COVID-19 [118]. In an MRI-based study for the assessment of neurological changes in COVID-19 survivors 3 months following discharge, there were significant structural changes which interrelated with prolonged neurological symptoms such as cognitive deficits and anosmia [120]. In a prospective study involving 60 COVID-19 survivors compared to 39 matched controls, there were neurological dysfunctions in 55% of the COVID-19 survivors compared to healthy controls (P < 0.05). Some of these structural brain changes were correlated with high LDH levels [120]. Therefore, the long-term effects of SARS-CoV-2 infection, even a mild-moderate one, may affect functional and micro-structural brain integrity, resulting in neurological consequences in patients with PCS.
Furthermore, Paterson et al., illustrated a high frequency of acute disseminated encephalomyelitis in COVID-19 survivors that was not correlated with the initial severity of COVID-19 [121]. This finding proposes that SARS-CoV-2 infection, regardless of its severity, may cause long-term neurological complications, and this may explain neuropsychiatric manifestations in patients with PCS.
In addition, delirium was reported in hospitalized patients with severe COVID-19 and may be present in patients with PCS [122]. It has been observed that early presentation of delirium in SARS-CoV-2 infection may predict the development of cognitive dysfunction, mainly in elderly survivors [123]. A meta-analysis study of 20 studies revealed that delirium symptoms in COVID-19 patients at the time of admission were linked with poor neurological outcomes (OR = 2.36, 95% CI = 1.80–3.09, P < 0.00001) and high mortality [123].
Rogers and colleagues revealed that neuropsychiatric symptoms in the acute phase of SARS-CoV-2 infection may lead to fatigue, cognitive impairment, and other neuropsychiatric squeals due to brain dysfunction [124]. Besides, a retrospective cohort study involving 236,379 COVID-19 survivors six months following acute SARS-CoV-2 infection illustrated that 56% of COVID-19 survivors developed various neuropsychiatric spectrums, mainly with ICU admission [125].
Indeed, brainstem injury in acute SARS-CoV-2 infection may cause cardio-respiratory dysfunction due to injury to respiratory and vasomotor centers [126]. Brainstem dysfunction may persist for a long time after acute SARS-CoV-2 infection, leading to dyspnea and neurological dysfunction in patients with PCS [127]. Higher expression of ACE2 in the brainstem increases its susceptibility to SARS-CoV-2 neurotropism and subsequent inflammatory reaction-induced dysfunction [127]. In this state, post-mortem studies demonstrated that SARS-CoV-2 proteins and genes were detected in COVID-19 victims [128, 129].
The underlying mechanism of neuropsychiatric disorders in COVID-19 could be due to cytokine storm-induced disruption of the blood brain barrier (BBB), neuroinflammation, and peripheral neuronal injury, or due to direct SARS-CoV-2 neurotropism [130]. Notably, exaggerated inflammatory response with high TGF-β might be the proposed mechanism for the development of neuropsychiatric and other neurological disorders in COVID-19 [131]. The underlying proposed mechanism for neurological involvement in COVID-19 could be linked with the development of demyelination disorders since previous coronavirus infections have caused neurodegeneration and demyelination [132]. As well, the high distribution of ACE2 in specific brain regions such as the substantia nigra and limbic system may increase the interaction between SARS-CoV-2 and neurons, with subsequent neurological complications [133, 134].
These observations suggest that SARS-CoV-2 infections may lead to long-term neurological complications which participate in the development of PCS.
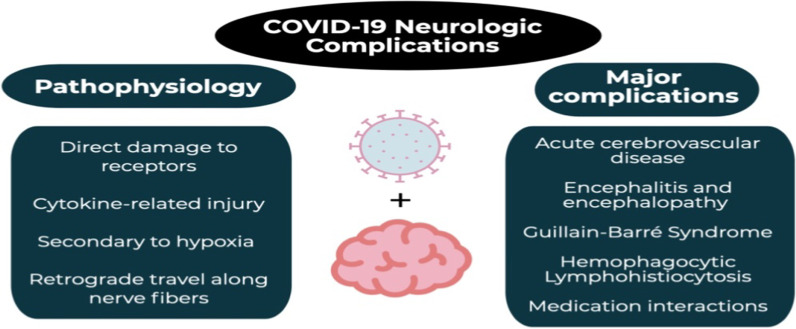
Neurological manifestations in PCS, mainly fatigue and cognitive deficits, are related to the presence of autoantibodies against β2 adrenoceptors, α1-adrenoceptors, AT1R, MAS receptors, muscarinic type 2 receptors, and nociceptin-like opioid receptors, leading to dysautonomia, POTS, and other neurological dysfunctions [135]. The underlying pathophysiology of neurological manifestations and complications in COVID-19 with the possibility of development of PCS is complex (Fig. 6).
Fig. 6.
Post-COVID syndrome and neurological complications
Cardiovascular complications
It has been shown that cardiac fibrosis, endothelial dysfunction, and other cardiovascular complications are evident in patients with PCS [136]. In severe SARS-CoV-2 infections, myocarditis and acute cardiomyocyte injury may lead to the development of heart failure in COVID-19 patients [137]. The potential mechanisms of cardiomyocyte injury in COVID-19 include direct cardiomyocyte injury by SARS-CoV-2, hypoxia, cytokine storm, increased pulmonary vascular-induced pulmonary hypertension, augmentation of RAS, endothelial dysfunction, coagulopathy, and acute coronary syndrome [137, 138]. These changes lead to myocardial remodeling and cardiac fibrosis through the induction of expression and upregulation of TGF-β [139]. Nalbandian et al., observed that cardiac fibrosis and resultant cardiomyopathy and heart failure from SARS-CoV-2 infection in COVID-19 survivors can lead to tachyarrhythmia and exertional dyspnea in patients with PCS [140].
Indeed, SARS-CoV-2-induced autonomic dysfunction and dysautonomia in COVID-19 survivors can cause sinus tachycardia and postural orthostatic tachycardia syndrome (POTS) [12, 141]. POTS is more common in women and is caused by an autoimmune reaction to SARS-CoV-2 infection [142].Furthermore, hypercytokinemia's exaggerated catecholaminergic state may cause cardiac action potential prolongation by modulating the expression of cardiomyocyte ion channels [143].
In a study of 100 COVID-19 survivors, Puntman et al., discovered that 60% of them have myocarditis and 78% have other cardiac abnormalities [144]. Interestingly, these cardiac pathological changes were not related to the initial severity of COVID-19 [144]. A longitudinal multicenter echocardiographic study involving COVID-19 survivors three months after previous COVID-19 pneumonia showed that 29% of them had some degree of myocardial remodeling [145]. Furthermore, even in mild or asymptomatic patients, asymptomatic COVID-19 may be associated with myocardial inflammation, as evidenced by cardiac magnetic resonance in athletes recovered from SARS-CoV-2 infection [146].
Long-term cardiac complications COVID-19 survivors need further long-term prospective studies. Nevertheless; cardiac symptoms like palpitations and chest pain were reported in patients with PCS [147]. Of note, silent and progressive cardiac injury in the course of SARS-CoV-2 infection could contribute to the progression of cardiovascular complications, including heart failure, following complete recovery [147]. Persistent cardiac injury was observed in 78% of 100 recovered COVID-19 survivors after 3 months, regardless of initial COVID-19 severity [147].
Furthermore, persistent endothelial dysfunction (ED) in patients with PCS is correlated with the initial severity of SARS-CoV-2 infection [142]. A cohort, case-controlled study comprised of 133 recovered COVID-19 patients 3 months from previous infection compared with matched 133 controls illustrated that flow-mediated dilatation was reduced in recovered COVID-19 patients compared to the controls (P < 0.001) [142]. After controlling for major confounders, COVID-19 was identified as an independent risk factor for the development of ED [142]. Similarly, a prospective study involving 70 COVID-19 patients 3 months after infection showed that markers of ED were evident, suggesting that ED in COVID-19 survivors could be implicated in cardiovascular complications in recovered COVID-19 patients [148].
In this state, ED and cardiovascular complications in recovered COVID-19 patients may increase the risk of development of new-onset systemic hypertension [149]. A study of 153 post-COVID-19 patients revealed that both systolic and diastolic blood pressures were higher at post-COVID-19 than at the time of admission [149]. Over-activation of RAS with reduction of Ang1-7, ED, and coagulopathy could be the major causes for the development of hypertension in patients with PCS [149]. Besides, ED and pulmonary thromboembolic disorders may increase the risk of the development of pulmonary hypertension [150].
These observations indicate that cardiovascular complications in patients with PCS are linked with the development of cardiac injury and new onset hypertension (Fig. 7).
Fig. 7.
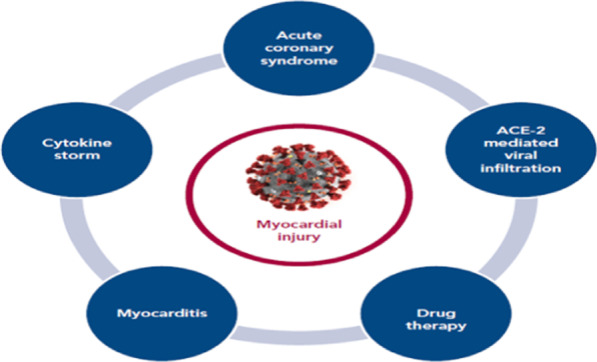
Post-COVID syndrome and cardiovascular complications
Renal complications
Acute kidney injury (AKI) may develop in 5% of hospitalized COVID-19 patients and up to 31% of severely ill COVID-19 patients with mechanical injury in acute SARS-CoV-2 infection [151, 152]. Huang et al. in a cohort study disclosed that 13% of recovered COVID-19 patients developed a significant reduction in the estimated glomerular filtration rate at 6 months after acute SARS-CoV-2 infection when renal function was normal during the initial acute phase [100]. AKI in acute COVID-19 increases the risk of developing AKI within 3 months of the COVID-19 [153].
Prolonged inflammatory reactions and thrombotic disorders in COVID-19 survivors increase the risk of developing renal impairments in the high-risk group [153]. Gu et al.'s cohort study found that AKI in acute COVID-19 was related to the reduction of kidney function one year after the acute phase of COVID-19 [154]. As a result, intensive kidney function care during acute COVID-19 may prevent renal impairments in COVID-19 survivors [154].
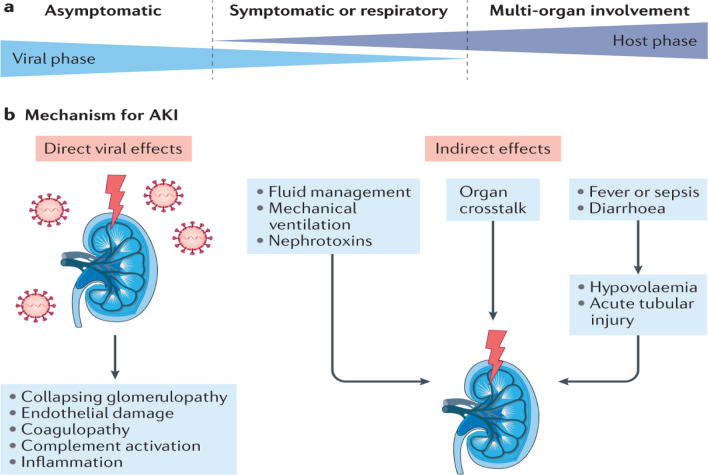
In brief, the direct cytopathic effect of SARS-CoV-2 infection or indirect effects of COVID-19 may lead to the progression of AKI (Fig. 8).
Fig. 8.
Post-COVID syndrome and renal complications
Gastrointestinal complications
In patients with PCS, there are different hepatobiliary and gastrointestinal (GIT) disorders, including abdominal pain, vomiting, nausea, diarrhea, and poor appetite due to persistent GIT inflammation [155]. Changes in gut microbiota with the development of dysbiosis by SARS-CoV-2 infection may increase the risk of the development of systemic inflammation and pulmonary dysfunction by the gut-lung axis [155]. GIT symptoms may be observed in COVID-19 survivors in about 84% of cases due to prolonged intestinal inflammation, dysbiosis, and down-regulation of intestinal ACE2 [156]. Latent intestinal inflammation may affect the liver, lungs, and brain via the gut-liver axis, gut-lung axis, and gut-brain axis, respectively [156]. These findings propose that patients with PCS had many symptoms due to GIT disorders induced by the initial SARS-CoV-2 infection. Systemic inflammatory disorders in PCS might be due to persistent SARS-CoV-2 infection in GIT and associated dysbiosis and intestinal inflammation that persist up to 30 days after COVID-19 recovery [107].
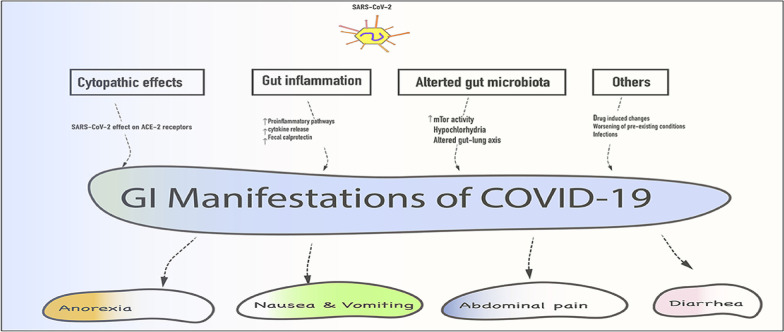
Thus, direct cytopathic effects of SARS-CoV-2 infection with associated gut inflammation and alteration of gut microbiota may lead to GIT manifestation in COVID-19 patients. These changes may induce long-term inflammatory changes with the induction of PCS (Fig. 9).
Fig. 9.
Post-COVID syndrome and gastrointestinal complications
Endocrine complications
Different endocrine disorders are addressed with the development of PCS. Diabetic ketoacidosis was reported in non-diabetic COVID-19 survivors within months of recovery from acute SARS-CoV-2 infection [157]. Furthermore, overt thyrotoxicosis, thyroiditis, and Gravis disease have been reported in recovered COVID-19 patients within weeks [158, 159]. In male COVID-19 patients, pituitary–testicular axis dysfunction was reported in recovered patients [160]. In a retrospective study, 143 COVID-19 patients were evaluated at 77 days following disease onset; a low testosterone level was detected in 28.7% of them [160]. Pituitary dysfunction has been observed after acute SARS-CoV-2 infection [161]. A case-controlled study comprised of 43 COVID-19 patients compared to 11 healthy controls illustrated that 46.5% had inadequate growth hormone response and 9.3% had low cortisol response [161]. As well, there was significant elevation in prolactin and thyroid stimulating hormone in 4.6% and 9.3% of recovered COVID-19 patients, respectively [161]. These verdicts advocate that recovered COVID-19 patients had pituitary dysfunction, mainly in the pituitary-adrenal axis and growth hormone response. Interestingly, a case-series of post-COVID hypothalamic pituitary adrenal axis dysfunction was demonstrated as post-COVID-19 perinatal depression and other neuropsychiatric manifestations [162]. This disorder may be the potential cause of post-partum psychosis in recovered pregnant women from COVID-19 [162].
Endocrinopathies in patients with PCS may be due to long-term inflammatory and immunological disturbances and direct viral injury [163]. Pre-diabetic patients may first become overt and apparent during acute SARS-CoV-2 infection and post-COVID-19 with the development of diabetic ketoacidosis due to ACE2-mediated pancreatic injury [164]. PCS has been linked to increased peripheral insulin resistance as well as decreased insulin production from pancreatic-β cells [165]. The prolonged effects of COVID-19 may exacerbate microvascular dysfunction, ED, sarcopenia, and cardiovascular complications with the induction of PCS in diabetic patients [165].
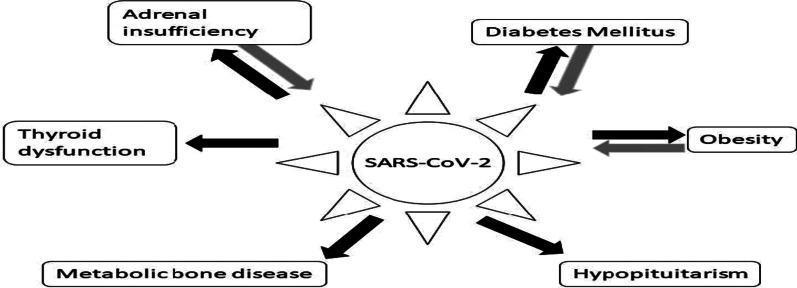
These observations indicate that initial endocrine disorders in acute SARS-CoV-2 infection may persist and contribute to the development of PCS in COVID-19 survivors (Fig. 10).
Fig. 10.
Post-COVID syndrome and endocrine complications
Thromboembolic and hematological complications
In PCS patients, there are noteworthy thromboembolic disorders with an incidence of less than 5%. As well, omitting thrombo-prophylaxis in post-COVID-19 patients may increase the risk of thrombosis with the development of cardiac thrombus and ischemic stroke [166]. The median period for the development of thromboembolic complications in PCS patients was 23 days [167]. A case-report study by Boudhabhay et al. suggested that thrombotic microangiopathy can play a crucial role in the pathophysiology of complement-mediated multi-system inflammatory syndrome in PCS patients [168].
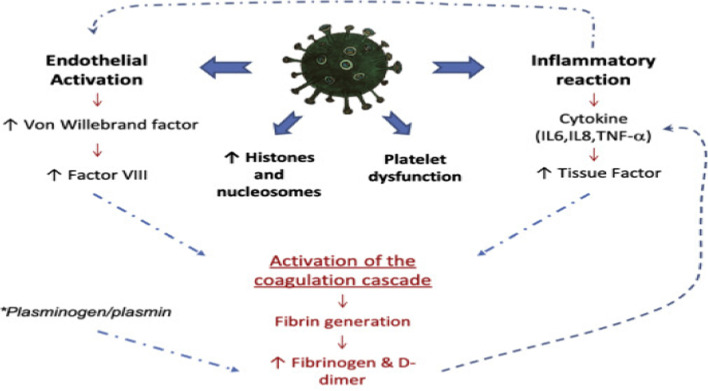
The possible mechanisms for thromboembolic complications in PCS patients could be ED, complement activation, development of neutrophil extracellular traps, platelet activation, platelet-neutrophil interactions, hypoxia, and exaggerated pro-inflammatory response [140]. These pathophysiological changes are similar to those seen in thrombotic microangiopathic syndrome in COVID-19 patients [169]. Remarkably, unlike other disorders and complications reported in PCS patients, thromboembolic complications in PCS patients are related to the hyperinflammatory state's severity and duration in the initial acute SARS-CoV-2 infection [140] (Fig. 11).
Fig. 11.
Post-COVID syndrome and thromboembolic complications
Post-COVID syndrome and mast cell activation syndrome
PCS has been hypothesized to be mediated by hyperinflammation and MCAS [170]. Deregulated release of inflammatory mediators in MCAS produces extraordinary symptoms, as evident in patients with PCS [171]. MCAS was first reported in 2007, characterized by allergic and inflammatory disorders with a prevalence of 17% [171]. Dysfunction in the behavior of mast cells following psychological or physical stress may lead to an abnormal release of inflammatory cytokines [170]. In PCS, the interactions of induced stressors may activate mast cell genes by SARS-CoV-2 infection, resulting in abnormal control of mast cell activations [172]. In SARS-CoV-2 infection, activation of toll-like receptors (TLRs) on the immune cells leads to the development of autoantibodies, which may activate mast cells through interaction with immunoglobulin receptors on the mast cells [173].
The key source of various inflammatory cytokines in COVID-19 is mast cells, which are activated by SARS-CoV-2 to release pro-inflammatory cytokines and contribute to the development of pulmonary cytokine storms and other COVID-19 pathologies [174]. Mast cells express ACE2 and can produce vasoconstrictor leukotrienes (LTs) and modulate lung RAS [175]. In general, mast cells are located in the perivascular space of the lungs where they undergo maturation under the effect of micro-environmental factors, causing the induction of hyper-responsiveness [176]. Mast cells are stimulated by allergens cross-linking with IgE Fc epsilon receptor type 1 and non-IgE stimuli through G-protein X2 receptor (GPRX2) by different neuropeptides like substance P and neurotensin [177]. The stimulation of mast cells results in the release of many inflammatory mediators, including platelet activating factor (PAF), histamine, heparin, tryptase, prostaglandins (PG), LTs, and chemokines such as IL-1β and IL-6 [178]. Of note, the release of substance P from immune cells in SARS-CoV-2 infection is augmented [179] as well; a vasoactive peptide storm caused by the increasing release of substance P and neurotensin plays a critical role in the induction of vascular permeability and lung inflammation [180]. As well, SARS-CoV-2 through its domain protein called PSD-95/Dlg/ZO-1 (PDZ), which is found in protein E and N, can activate mast cell GPRX2, resulting in mast cell activation [181]. In this state, both substance P and neurotensin with activation of mast cell GPRX2, which are induced by SARS-CoV-2 infection, could be the possible cause for the development of MCAS in COVID-19.
Interestingly, IL‐6 has been shown to increase MC proliferation and induce a more reactive phenotype providing a possible link between elevated IL‐6 levels and MCAS in PCS. While it remains unclear if MC activation is causative in PCS or simply a consequence, larger longitudinal studies to validate our findings and assess the natural history are critical. Importantly, our findings highlight MCs as potential therapeutic targets for patients with PCS, which could be targeted with agents that reduce MC‐derived mediators, engage inhibitory receptors, or attenuate inflammation [182]. Analogous to respiratory function, it cannot be excluded that the cytokine storm accompanying SARS-CoV-2 infection and MCAS during COVID-19 may indirectly affect female reproductive function, especially in more severe cases [183]. Neutralization of upstream histamine, a major mediator derived from MCs, inhibits the nuclear translocation of NF-κB, thereby preventing the release of the proinflammatory cytokines interleukin (IL-1β, TNF-α, IL-6, and IL-10). Despite the fact that COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in MCAS, the available clinical data do not provide grounds for treating this mechanism as a significant threat to female reproductive functions, including pregnancy [183]. Understandably, severe COVID-19 reduces female fertility and has been associated with impaired fetal growth during pregnancy, an approximate twofold increased risk of stillbirth, threefold increased risk of preterm birth (likely influenced by iatrogenic deliveries) and prematurity-related worse perinatal outcomes [182, 183]. It should be noted that the pathophysiological spectrum of MCAS is extremely broad and heterogeneous. SARS-CoV-2 infection induced epigenetic mechanisms may explain the increasing incidence of MCAS in PCS. Specific research in the PCS patients with MCAS may related to SARS-CoV-2 infection has been performed to date. Data continue to accumulate, including those regarding the long-term influence of COVID-19 on the course of both preexisting and SARS-CoV-2-induced MCAS. It is noteworthy that PCS with high residual cardiovascular risk and persistence of blood chemistry of inflammation and procoagulative state resembles many aspects of MCAS. For example, in both pathologies, NLRP3 inflammasome may be crucial in the consolidation of coagulation disorders.Further studies are needed to determine whether MCAS-related clotting disorders (e.g., microcoagulopathy) are subjected to long-term modulation by SARS-CoV-2 infection with possible consequences on female reproductive function [182, 183].
In addition to the induced pulmonary inflammation by SARS-CoV-2-induced mast cell activation, mast cell-derived inflammatory and vasoactive mediators can cause derangement of the BBB with the progression of brain fog [184]. In PCS, cognitive dysfunction and fatigue are mainly observed and are similar to those experienced by patients following cancer chemotherapy known as chembrain or chemofog, as well as in patients with ME/CFS or MCAS [185]. Brain fog in COVID-19 may be related to mast cell-induced neuroinflammation through activation of microglia [185]. Development of brain fog in COVID-19 could be the potential cause of persistent neuropsychiatric symptoms in patients with PCS [186]. These observations point out that MCAS may lead to deleterious central and peripheral effects in patients with acute COVID-19 and contribute to the progression of PCS.
It has been shown that the development of MCAS during the course of SARS-CoV-2 infection is correlated with COVID-19 severity and the development of PCS [18]. An observational study illustrated that cytokine storm in patients with severe COVID-19 may be rooted in the advancement of MCAS by SARS-CoV-2 infection [18]. Therefore, the development of MCAS in SARS-CoV-2 infection is linked with poor outcome in severely affected COVID-19 patients, and lung biopsies of COVID-19 patients have large numbers of activated mast cells compared with healthy controls [187]. Schofield reported a case study of POTS linked with the development of MCAS in women with COVID-19 [94]. Of note, MCAS is augmented in PCS due to the activation of mast cells by SARS-CoV-2 through different mechanisms [188].
Therefore, there are close interactions between SARS-CoV-2 infection and MCAS with the progression of PCS due to the prolonged inflammatory status (Fig. 12).
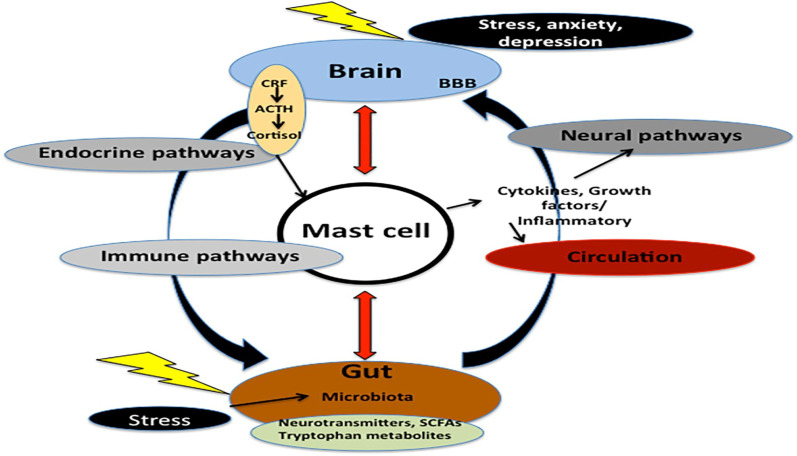
Fig. 12.
Role of mast cell activation syndrome in the development of Post-COVID syndrome: Stressful conditions during SARS-CoV-2 infection increase the release of corticotropic releasing factor (CRF), adrenocorticotropic hormone (ACTH), and cortisol. Besides, gut stress induces alteration of the gut microbiota, which affects the release of neurotransmitters, tryptophan metabolism, and the release of short-chain free fatty acids (SCFAs). These changes induce activation of mast cells, which releases inflammatory cytokines. Thus, mast cells link the brain and gut through neuronal, immune, and endocrine pathways
Treatment of MCAS in Post-COVID syndrome
In general, MCAS is treated by antihistamines (H1 and H2 blockers), inhibition of synthesis of mediators (zileuton and aspirin), inhibition of mediator release (Na-cromoglycate), and inhibition of degranulation of mast cells by anti-IgE [21].
Famotidine acts as an inverse agonist to inhibit the generation of cAMP or full antagonist to inhibit H2 receptors, thereby reducing endothelial permeability and endothelial dysfunction in Coved-19 [189]. Blocking of H2 receptors and induction of synthesis of cAMP by famotidine can decrease the pathological effects of histamine and mast cell cytokine release, respectively [189]. Therefore, famotidine could be effective against SARS-CoV-2 infection in the acute phase and relieve symptoms of MCAS in patients with PCS.
Zileuton, a 5-lipoxygenase inhibitor, inhibits LT synthesis, preventing hyperinflammation in COVID-19 patients and possibly alleviating MCAS symptoms [4].
Regarding the role of antihistamines in the management of MCAS, H1 blockers alone are not effective in treating symptoms of MCAS, thus H4 blockers could have a promising effect in this state [190]. H4R mediates mast cell activation for migration and release of pro-inflammatory cytokines through mitogen-activated protein kinase (MAPK) [191]. Activation of H4R results in recruitment of mast cells and migration of eosinophils with activation of the immune response and inflammation via activation of T cells and dendritic cells [192]. A clinical trial for the effectiveness of H4R blocker JNJ39758979 showed a notable effect in the management of different allergic disorders [193].
Of note, perturbations of T cells in PCS could be mediated by histamine-dependent mechanisms, so antihistamines could be effective in the management of PCS [194]. Therefore, use of antihistamines in PCS may reduce T cell perturbations and the development of hyperinflammation states [194]. Thus, these observations suggest that antihistamines could be of therapeutic benefit against PCS and associated MCAS.
In addition, mast cell stabilizers like Na-cromoglycate, clarithromycin, and hydrocortisone have important roles in preventing the release of histamine and pro-inflammatory cytokines [195]. Therefore, mast cell stabilizers might be effective in a dual role against PCS and linked MCAS [195].
Finally, treatments that powerfully trigger the immune system, like vaccines, must be given with strong caution as they may exacerbate symptoms of MCAS [196]. However, patients with MCAS or mastocytosis, even those with an anaphylactic history, can be vaccinated safely with COVID-19 vaccines [197].
Due to the uncontrolled release of TGF-β1 by a prolonged inflammatory state [198], pulmonary fibrosis is regarded as the main final fatal complication in PCS patients due to the uncontrolled release of TGF-β1 by a prolonged inflammatory state [198]. Therefore, TGF-β1 inhibitors like triptolide, azithromycin, and vitamin D could be effective in the attenuation of PCPF [199]. Of interest, pirfenidone, a TGF-β1 inhibitor that acts as an anti-fibrotic agent together with anti-inflammatory drugs, may reduce the risk of development of PCPF in COVID-19 survivors [200]. A case report study observed that pirfenidone was effective in preventing the development of PCPF in 40-year-old women who presented with COVID-19 pneumonia [201]. However, TGF-β1 has suppressive effects on mast cell activation both in vivo and in vitro [202]. Therefore, specific inhibitors of mast cells could be effective in the attenuation of the progression of PCS. Notably, butyrate inhibits mast cell activation by inhibiting FcR1-mediated signalling [203], ruxolitinib inhibits mast cell activation by suppressing the JAK1/JAK2 pathway [204], and monoclonal antibody against Siglec-8 inhibits airway inflammation via an IgE-independent pathway [205]. Thus, the inhibitors of mast cell activation could be effective in preventing the MCAS-induced progression of PCS in COVID-19 survivors.
Conclusions
PCS can develop within 3 months of acute COVID-19, but it can also develop after mild or asymptomatic COVID-19.The underlying pathophysiology of PCS is still unidentified, though immune dysregulation, persistence of inflammatory reactions, autoimmune mimicry, and reactivation of pathogens together with host microbiome alterations may contribute to the development of PCS. PCS may progress in association with the development of mast cell activation syndrome (MCAS). The emergence of MCAS during the course of SARS-CoV-2 infection is linked to the severity of COVID-19 and the emergence of PCS. Therefore, the use of antihistamines, inhibition of synthesis/release of mediators, and suppression of mast cell degranulation by anti-IgE may reduce MCAS-induced development of PCS. Perturbations of T cells in PCS could be mediated by histamine-dependent mechanisms, so antihistamines could be effective in the management of PCS. Taken together, the early recognition and treatment of MCAS in PCS patients may reduce systemic complications and long-term organ dysfunction.
Acknowledgements
Not applicable.
Author contributions
All authors wrote the manuscript. HMA participated in the collecting and reviewing published articles, and writing of the paper. AIA participated in designing. They contributed equally to the paper. NNW and GEB participated in writing and revision. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
All collected data are discussed in the manuscript.
Declarations
Ethics approval and consent to participate
The review was written in accordance with ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
Hayder M. Al-kuraishy, Email: Hayderm36@yahoo.com
Ali I. Al-Gareeb, Email: Dr.alialgareeb78@yahoo.com
Nermeen N. Welson, Email: nermeennemr@yahoo.com
References
- 1.Al-Kuraishy HM, Al-Gareeb AI. From SARS-CoV to nCoV-2019: ruction and argument. Arch Clin Infect Dis. 2020;15(2):e102624. [Google Scholar]
- 2.Moubarak M, Kasozi KI, Hetta HF, Shaheen HM, Rauf A, Al-Kuraishy HM, et al. The rise of SARS-CoV-2 variants and the role of convalescent plasma therapy for management of infections. Life. 2021;11(8):734. doi: 10.3390/life11080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhayyat SS, Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, AboKamer AM, Batiha GE, Simal-Gandara J. Fenofibrate for COVID-19 and related complications as an approach to improve treatment outcomes: the missed key for Holy Grail. Inflamm Res. 2022;8:1–9. doi: 10.1007/s00011-022-01615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, Batiha GE. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of COVID-19: the enigmatic entity. Eur J Pharmacol. 2021;5(904):174196. doi: 10.1016/j.ejphar.2021.174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Kuraishy HM, Batiha GE, Faidah H, Al-Gareeb AI, Saad HM, Simal-Gandara J. Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals. Inflammopharmacology. 2022;31:1. doi: 10.1007/s10787-022-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type ii diabetes mellitus: the anti-inflammatory role of metformin. Front Med. 2021;19(8):110. doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE. High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology. 2022;26:1. doi: 10.1007/s10787-022-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C. Renin-Angiotensin system and fibrinolytic pathway in COVID-19: one-way skepticism. Biomed Biotechnol Res J (BBRJ) 2020;4(5):33. [Google Scholar]
- 9.Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Welson NN, Batiha GE, Conte-Junior CA. Selinexor and COVID-19: the neglected warden. Front Pharmacol. 2022;13. [DOI] [PMC free article] [PubMed]
- 10.Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE. The looming effects of estrogen in COVID-19: a rocky rollout. Front Nutr. 2021;8. [DOI] [PMC free article] [PubMed]
- 11.Babalghith AO, Al-kuraishy HM, Al-Gareeb AI, De Waard M, Sabatier JM, Saad HM, Batiha GE. The potential role of growth differentiation factor 15 in COVID-19: a corollary subjective effect or not? Diagnostics. 2022;12(9):2051. doi: 10.3390/diagnostics12092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Gyebi GA, Batiha GE. COVID-19-induced dysautonomia: a menace of sympathetic storm. ASN Neuro. 2021;13:17590914211057635. doi: 10.1177/17590914211057635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Kuraishy HM, Al-Gareeb AI, Butnariu M, Batiha GE. The crucial role of prolactin-lactogenic hormone in Covid-19. Mol Cell Biochem. 2022;11:1–2. doi: 10.1007/s11010-022-04381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCorkell L, Assaf GS, Davis HE, Wei H, Akrami A. Patient-led research collaborative: embedding patients in the long COVID narrative. Pain Rep. 2021;6(1):e913. doi: 10.1097/PR9.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Aljowaie RM, Almutairi SM, Alexiou A, Batiha GE. The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in covid-19. Inflammation. 2022;24:1–7. doi: 10.1007/s10753-022-01648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dicpinigaitis PV, Canning BJ. Is there (will there be) a post-COVID-19 chronic cough? Lung. 2020;198(6):863–865. doi: 10.1007/s00408-020-00406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Kuraishy HM, Al-Gareeb AI, Welson NN, Batiha GE. Trimetazidine and COVID-19-induced acute cardiac injury: a missed key. Int J Clin Pharm. 2022;21:1–2. doi: 10.1007/s11096-022-01408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afrin LB, Weinstock LB, Molderings GJ. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;1(100):327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126(6):1099–1104. doi: 10.1016/j.jaci.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akin C. Mast cell activation syndromes. J Allergy Clin Immunol. 2017;140(2):349–355. doi: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Castells M, Butterfield J. Mast cell activation syndrome and mastocytosis: initial treatment options and long-term management. J Allergy Clin Immunol Pract. 2019;7(4):1097–106. doi: 10.1016/j.jaip.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Greenhalgh T, Knight M, A’Court M, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 23.Al-Kuraishy HM, Al-Gareeb AI, Onohuean H, El-Saber BG. COVID-19 and erythrocrine function: the roller coaster and danger. Int J Immunopathol Pharmacol. 2022;14(36):03946320221103151. doi: 10.1177/03946320221103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, Sonbol FI, Batiha GE. Hyperviscosity syndrome in COVID-19 and related vaccines: exploring of uncertainties. Clin Exp Med. 2022;24:1. doi: 10.1007/s10238-022-00836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020;324(22):2251–2252. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- 27.Amenta EM, et al. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12):ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Kuraishy HM, Al-Gareeb AI, Fageyinbo MS, Batiha GE. Vinpocetine is the forthcoming adjuvant agent in the management of COVID-19. Future Science OA. 2022 Mar(0):FSO797. [DOI] [PMC free article] [PubMed]
- 29.Shah W, Hillman T, Playford ED, et al. Managing the long-term effects of COVID-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 30.Venkatesan P. NICE guideline on long COVID’. Lancet Respir Med. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18(5):2621. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification’. Int J Environ Res Public Health. 2021;18(5):2621. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15(3):869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendelson M, Nel J, Blumberg L, et al. Long-COVID: An evolving problem with an extensive impact. S Afr Med J. 2020;111(1):10–12. doi: 10.7196/SAMJ.2020.v111i11.15433. [DOI] [PubMed] [Google Scholar]
- 37.Sivan M, Taylor S. NICE guideline on long COVID. BMJ. 2020;371:m4938. doi: 10.1136/bmj.m4938. [DOI] [PubMed] [Google Scholar]
- 38.Davido B, et al. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. 2020;26(11):1448–1449. doi: 10.1016/j.cmi.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath A. Long-haul COVID. Neurology. 2020;95(13):559–560. doi: 10.1212/WNL.0000000000010640. [DOI] [PubMed] [Google Scholar]
- 40.Rando HM, Bennett TD, Byrd JB, et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv. 2021;383:590. doi: 10.1101/2021.03.20.21253896. [DOI] [Google Scholar]
- 41.Townsend L, Dowds J, O’Brien K, et al. Persistent poor health post-COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021 doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Saber Batiha G, Al-Gareeb AI, Saad HM, Al-Kuraishy HM. COVID-19 and corticosteroids: a narrative review. Inflammopharmacology. 2022;13:1–7. doi: 10.1007/s10787-022-00987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buonsenso D, Espuny Pujol F, Munblit D, et al. Clinical characteristics, activity levels and mental health problems in children with Long COVID: a survey of 510 children. Preprints. 2021. 10.20944/preprints202103.0271.v1 [DOI] [PMC free article] [PubMed]
- 44.Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease: a systematic review of the current data. Frontiers in medicine. 2021;8:392. doi: 10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE. COVID-19 and L-arginine Supplementations: yet to find the missed key. Curr Protein Pept Sci. 2022;23(3):166–169. doi: 10.2174/1389203723666220512104039. [DOI] [PubMed] [Google Scholar]
- 46.Zhao FC, Guo KJ, Li ZR. Osteonecrosis of the femoral head in SARS patients: seven years later. Eur J Orthop Surg Traumatol. 2013;23(6):671–677. doi: 10.1007/s00590-012-1054-4. [DOI] [PubMed] [Google Scholar]
- 47.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLOS ONE. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tleyjeh IM, Saddik B, AlSwaidan N, AlAnazi A, Ramakrishnan RK, Alhazmi D, Aloufi A, AlSumait F, Berbari E, Halwani R. prevalence and predictors of post-acute COVID-19 syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLOS ONE. 2021;16(12):e0260568. doi: 10.1371/journal.pone.0260568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simani L, Ramezani M, Darazam IA, et al. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol. 2021;27(1):154–159. doi: 10.1007/s13365-021-00949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Thomali AW, Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadiliy AK, De Waard M, Sabatier JM, Khan Khalil AA, Saad HM, Batiha GE. Role of Neuropilin 1 in COVID-19 Patients with Acute Ischemic Stroke. Biomedicines. 2022;10(8):2032. doi: 10.3390/biomedicines10082032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stavem K, Ghanima W, Olsen MK, et al. 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76(4):405. doi: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Mukerjee N, Al-Hamash SM, Al-Maiahy TJ, Batiha GE. 5-HT/CGRP pathway and Sumatriptan role in Covid-19. Biotechnol Genet Eng Rev. 2022;31:1–26. doi: 10.1080/02648725.2022.2108996. [DOI] [PubMed] [Google Scholar]
- 54.Truffaut L, Demey L, Bruyneel AV, et al. Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22(1):29. doi: 10.1186/s12931-021-01625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE. Central effects of Ivermectin in alleviation of Covid-19-induced dysautonomia. Curr Drug Targets. 2022. [DOI] [PubMed]
- 56.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 57.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Internal Med. 2017;5(2):90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stam H, Stucki G, Bickenbach J. COVID-19 and post intensive care syndrome: a call for action. J Rehabil Med. 2020;52:jrm00044. doi: 10.2340/16501977-2677. [DOI] [PubMed] [Google Scholar]
- 59.Gameil MA, Marzouk RE, Elsebaie AH, Rozaik SE. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt Liver J. 2021;11(1):1–8. doi: 10.1186/s43066-021-00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Kuraishy HM, Al-Gareeb AI, Al-Harcan NA, Alexiou A, Batiha GE. Tranexamic Acid and Plasminogen/Plasmin Glaring Paradox in COVID-19. Endoc Metab Immune Disord Drug Targets. 2022. [DOI] [PubMed]
- 61.Liao B, Liu Z, Tang L, Li L, Gan Q, Shi H, Jiao Q, Guan Y, Xie M, He X, Zhao H. Longitudinal clinical and radiographic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int J Med Sci. 2021;18(1):29. doi: 10.7150/ijms.49728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, Okell T, Sheerin F, Xie C, Mahmod M, Mózes FE. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;1(31):100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang L, Yang B, Jiang N, Fu W, He X, Zhou Y, Ma WL, Wang X. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020; 35(47). [DOI] [PMC free article] [PubMed]
- 64.Rudroff T, Fietsam AC, Deters JR, Bryant AD, Kamholz J. Post-COVID-19 fatigue: potential contributing factors. Brain Sci. 2020;10(12):1012. doi: 10.3390/brainsci10121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.COVID GA, Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;1. [DOI] [PMC free article] [PubMed]
- 66.Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carvalho-Schneider C, Laurent E, Lemaignen A, Lemaignen A, Beaufils E, Bourbao-Tournois C, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Alexiou A, Batiha GE. Calprotectin: the link between acute lung injury and gastrointestinal injury in Covid-19: Ban or boon. Curr Protein Peptide Sci. 2022. [DOI] [PubMed]
- 69.Pavli A, Theodoridou M, Maltezou HC. Post-COVID syndrome: Incidence, clinical spectrum, and challenges for primary healthcare professionals. Arch Med Res. 2021;52(6):575–581. doi: 10.1016/j.arcmed.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alemanno F, Houdayer E, Parma A, Spina A, Del Forno A, Scatolini A, et al. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: ACOVID-rehabilitation unit experience. PLOS ONE. 2021;16:e0246590. doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE. Targeting and modulation of the natriuretic peptide system in Covid-19: a single or double-edged effect?. Curr Protein Peptide Sci. 2022. [DOI] [PubMed]
- 72.Shanbehzadeh S, Tavahomi M, Zanjari N, Ebrahimi-Takamjani I, Amiri-Arimi S. Physical and mental health complications post-COVID-19: scoping review. J Psychosom Res. 2021;1(147):110525. doi: 10.1016/j.jpsychores.2021.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang MC, Park D. Incidence of post-traumatic stress disorder after coronavirus disease. Health Care. 2020;8:373. doi: 10.3390/healthcare8040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Kuraishy HM, Al-Gareeb AI, Al-Hamash SM, Cavalu S, El-Bouseary MM, Sonbol FI, Batiha GE. Changes in the blood viscosity in patients with SARS-CoV-2 infection. Front Med. 2022;9. [DOI] [PMC free article] [PubMed]
- 75.Scoppettuolo P, Borrelli S, Naeije G. Neurological involvement in SARS-CoV-2 infection: a clinical systematic review. Brain Behav Immun Health. 2020;5:100094. doi: 10.1016/j.bbih.2020.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moreno-Pérez O, Merino E, Leon-Ramirez JM, Prunier L, Cavelier G, Thill MP, et al. COVID19-ALC research post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J. Infect. 2021;82:378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ståhlberg M, Reistam U, Fedorowski A, Villacorta H, Horiuchi Y, Bax J, Pitt B, Matskeplishvili S, Lüscher TF, Weichert I, Thani KB. Post-COVID-19 tachycardia syndrome: a distinct phenotype of post-acute COVID-19 syndrome. Am J Med. 2021;134:1451–1456. doi: 10.1016/j.amjmed.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front Immunol. 2016;7:160. doi: 10.3389/fimmu.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cañas CA. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses. 2020;1(145):110345. doi: 10.1016/j.mehy.2020.110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walton AH, Muenzer JT, Rasche D, et al. Reactivation of multiple viruses in patients with sepsis. PLOS ONE. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang J, Zheng L, Li Z, Hao S, Ye F, Chen J, Gans HA, Yao X, Liao J, Wang S, Zeng M. Kinetics of SARS-CoV-2 positivity of infected and recovered patients from a single center. Sci Rep. 2020;10(1):1. doi: 10.1038/s41598-020-75629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Kuraishy HM, Al-Gareeb AI, Negm WA, Alexiou A, Batiha GE. Ursolic acid and SARS-CoV-2 infection: a new horizon and perspective. Inflammopharmacology. 2022;3:1–9. doi: 10.1007/s10787-022-01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancermedicalscience. 2020;14. [DOI] [PMC free article] [PubMed]
- 85.Sivashanmugam K, Kandasamy M, Subbiah R, Ravikumar V. Repurposing of histone deacetylase inhibitors: a promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci. 2021;1(266):118883. doi: 10.1016/j.lfs.2020.118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carvacho I, Piesche M. RGD-binding integrins and TGF-β in SARS-CoV-2 infections–novel targets to treat COVID-19 patients? Clin Transl Immunol. 2021;10(3):e1240. doi: 10.1002/cti2.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goërtz YM, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FV, Houben-Wilke S, Burtin C, Posthuma R, Franssen FM, van Loon N. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6(4):00542. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gombar S, Chang M, Hogan CA, Zehnder J, Boyd S, Pinsky BA, Shah NH. Persistent detection of SARS-CoV-2 RNA in patients and healthcare workers with COVID-19. J Clin Virol. 2020;1(129):104477. doi: 10.1016/j.jcv.2020.104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakajima Y, Ogai A, Furukawa K, Arai R, Anan R, Nakano Y, Kurihara Y, Shimizu H, Misaki T, Okabe N. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27(2):387–389. doi: 10.1016/j.jiac.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J, Zhang H. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kouo T, Chaisawangwong W. SARS-CoV-2 as a superantigen in multisystem inflammatory syndrome in children. J Clin Investig; 2021;131(10). [DOI] [PMC free article] [PubMed]
- 92.Koné-Paut I, Cimaz R. Is it Kawasaki shock syndrome, Kawasaki-like disease or pediatric inflammatory multisystem disease? The importance of semantic in the era of COVID-19 pandemic. RMD Open. 2020;6(2):e001333. doi: 10.1136/rmdopen-2020-001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Novak P, Mukerji SS, Alabsi HS, Systrom D, Marciano SP, Felsenstein D, Mullally WJ, Pilgrim DM. Multisystem involvement in post‐acute sequelae of COVID‐19 (PASC). Ann Neurol. 2021. [DOI] [PMC free article] [PubMed]
- 94.Schofield JR. Persistent antiphospholipid antibodies, mast cell activation syndrome, postural orthostatic tachycardia syndrome and post-COVID syndrome: 1 year on. Eur J Case Rep Internal Med. 2021;8(3). [DOI] [PMC free article] [PubMed]
- 95.Assar S, Pournazari M, Soufivand P, Mohamadzadeh D. Systemic lupus erythematosus after coronavirus disease-2019 (COVID-19) infection: case-based review. Egypt Rheumatol. 2022;44(2):145–149. doi: 10.1016/j.ejr.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varghese J, Sandmann S, Ochs K, Schrempf IM, Frömmel C, Dugas M, Schmidt HH, Vollenberg R, Tepasse PR. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep. 2021;11(1):1–8. doi: 10.1038/s41598-021-91270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 99.Van den Borst B, et al. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis. 2020; ciaa1750. [DOI] [PMC free article] [PubMed]
- 100.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei J, Yang H, Lei P, et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J Xray Sci Technol. 2020;28(3):383–389. doi: 10.3233/XST-200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H, Zhao X, Wang Y, et al. Damaged lung gas-exchange function of discharged COVID-19 patients detected by hyperpolarized (129)Xe MRI. Sci Adv. 2020;7(1):eabc8180. doi: 10.1126/sciadv.abc8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Crameri GAG, Bielecki M, Züst R, et al. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 2020;25(36):2001542. doi: 10.2807/1560-7917.ES.2020.25.36.2001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43(1):276–285. doi: 10.1183/09031936.00196412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rai DK, Sharma P, Kumar R. Post COVID 19 pulmonary fibrosis Is it real threat? Indian J Tuberc. 2021;68(3):330–333. doi: 10.1016/j.ijtb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 107.Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. 2021;12(2574):136. doi: 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Q, Yu B, Yang Y, Huang J, Liang Y, Zhou J, Li L, Peng X, Cheng B, Lin Y. Immunological and inflammatory profiles during acute and convalescent phases of severe/ critically ill COVID-19 patients. Int Immunopharmacol. 2021;97:107685. doi: 10.1016/j.intimp.2021.107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Buhadily AK, Hussien NR, Al-Niemi MS, Al-Kuraishy HM, Al-Gareeb AI. Misfortune and spy story in the neurological manifestations of COVID-19. J Pak Med Assoc. 2021;1:S157–S160. [PubMed] [Google Scholar]
- 113.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Townsend L, Moloney D, Finucane C, McCarthy K, Bergin C, Bannan C, Kenny R-A. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLOS ONE. 2021;16:e0247280. doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mackay A. A paradigm for post-COVID-19 fatigue syndrome analogous to ME/CFS. Front Neurol. 2021;12:1334. doi: 10.3389/fneur.2021.701419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryabkova VA, Churilov LP, Shoenfeld Y. Neuroimmunology: What role for autoimmunity, neuroinflammation, and small fiber neuropathy in fibromyalgia, chronic fatigue syndrome, and adverse events after human papillomavirus vaccination? Int J Mol Sci. 2019;20:5164. doi: 10.3390/ijms20205164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Komaroff AL, Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med. 2021;18(7):1132. doi: 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang SW, Helmeste D, Leonard B. Inflammatory neuropsychiatric disorders and COVID-19 neuroinflammation. Acta Neuropsychiatr. 2021;33(4):165–177. doi: 10.1017/neu.2021.13. [DOI] [PubMed] [Google Scholar]
- 119.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EM, Furlan R, Ciceri F, Rovere-Querini P, Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;1(89):594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral micro-structural changes in COVID-19 patients–an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;1(25):100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G, Raftopoulos RE, Zambreanu L, Vivekanandam V. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Hanlon S, Inouye SK. Delirium: a missing piece in the COVID-19 pandemic puzzle. Age Ageing. 2020;49:497–498. doi: 10.1093/ageing/afaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hariyanto TI, Putri C, Hananto JE, Arisa J, Situmeang RF, Kurniawan A. Delirium is a good predictor for poor outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression. J Psychiatr Res. 2021;1(142):361–368. doi: 10.1016/j.jpsychires.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bulfamante G, Chiumello D, Canevini MP, Priori A, Mazzanti M, Centanni S, Felisati G. First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem. [DOI] [PubMed]
- 127.Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12(4):573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- 128.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. Neuropathological features of COVID-19. N Engl J Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Majolo F, Silva GL, Vieira L, Anli C, Timmers LF, Laufer S, Goettert MI. Neuropsychiatric disorders and COVID-19: what we know so far. Pharmaceuticals. 2021;14(9):933. doi: 10.3390/ph14090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kumar D, Jahan S, Khan A, Siddiqui AJ, Redhu NS, Khan J, Banwas S, Alshehri B, Alaidarous M. Neurological manifestation of SARS-CoV-2 induced inflammation and possible therapeutic strategies against COVID-19. Mol Neurobiol. 2021;58(7):3417–3434. doi: 10.1007/s12035-021-02318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;19(194):145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen R, Wang K, Yu J, Howard D, French L, Chen Z, Wen C, Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2021;20(11):1860. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, Mostafa H, Caturegli M, Moghekar A, Fitzgerald KC, Neuro-COVID H. Cerebrospinal fluid in COVID-19 neurological complications: Neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. J Neurol Sci. 2021;15(427):117517. doi: 10.1016/j.jns.2021.117517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kharraziha I, Axelsson J, Ricci F, DiMartino G, Persson M, Sutton R, Fedorowski A, Hamrefors V. Serumactivityagainstg protein–coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J Am Heart Assoc. 2020;9:e015989. doi: 10.1161/JAHA.120.015989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rovere-Querini P, De Lorenzo R, Conte C, Brioni E, Lanzani C, Yacoub MR, Chionna R, Martinenghi S, Vitali G, Tresoldi M, Ciceri F. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Bio Medica: Atenei Parmensis. 2020;91(9S):22. doi: 10.23750/abm.v91i9-S.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onohuean H, Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Batiha GE. COVID-19 and development of heart failure: mystery and truth. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(10):2013–2021. doi: 10.1007/s00210-021-02147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siddiq MM, Chan AT, Miorin L, Yadaw AS, Beaumont KG, Kehrer T, Cupic A, White KM, Tolentino RE, Hu B, Stern AD. Functional effects of cardiomyocyte injury in COVID-19. J Virol. 2021;13:603. doi: 10.1128/JVI.01063-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carlson FR, Jr, Bosukonda D, Keck PC, Carlson WD. Multiorgan damage in patients with COVID-19: is the TGF-β/BMP pathway the missing link? Basic Transl Sci. 2020;5(11):1145–1148. doi: 10.1016/j.jacbts.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Desai AD, Boursiquot BC, Melki L, Wan EY. Management of arrhythmias associated with COVID-19. Curr Cardiol Rep. 2020;23:2. doi: 10.1007/s11886-020-01434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ambrosino P, Calcaterra I, Molino A, Moretta P, Lupoli R, Spedicato GA, Papa A, Motta A, Maniscalco M, Di Minno MN. Persistent endothelial dysfunction in post-acute COVID-19 syndrome: a case-control study. Biomedicines. 2021;9(8):957. doi: 10.3390/biomedicines9080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lazzerini PE, Laghi-Pasini F, Boutjdir M, Capecchi PL. Cardioimmunology of arrhythmias: the role of autoimmune and infammatory cardiac channelopathies. Nat Rev Immunol. 2019;19:63–64. doi: 10.1038/s41577-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 144.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moody WE, Liu B, Mahmoud-Elsayed HM, Senior J, Lalla SS, Khan-Kheil AM, Brown S, Saif A, Moss A, Bradlow WM, Khoo J. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr. 2021;34(5):562–566. doi: 10.1016/j.echo.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gasecka A, Pruc M, Kukula K, Gilis-Malinowska N, Filipiak KJ, Jaguszewski MJ, Szarpak L. Post-COVID-19 heart syndrome. Cardiol J. 2021;28(2):353–354. doi: 10.5603/CJ.a2021.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lambadiari V, Mitrakou A, Kountouri A, Thymis J, Katogiannis K, Korakas E, Varlamos C, Andreadou I, Tsoumani M, Triantafyllidi H, Bamias A. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur J Heart Fail. 2021;23(11):1916–1926. doi: 10.1002/ejhf.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Akpek M. Does COVID-19 cause hypertension? Angiology. 2021;10:00033197211053903. doi: 10.1177/00033197211053903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rossi R, Coppi F, Monopoli DE, Sgura FA, Arrotti S, Boriani G. Pulmonary arterial hypertension and right ventricular systolic dysfunction in COVID-19 survivors. Cardiol J. 2021;29:163–165. doi: 10.5603/CJ.a2021.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Al-Kuraishy HM, Al-Gareeb AI. COVID-19 and acute kidney injury: a new perspective. Age (years) 2021;1(30):42. [PubMed] [Google Scholar]
- 152.Al-Kuraishy HM, Al-Gareeb AI. Acute kidney injury and COVID-19. Egypt J Internal Med. 2021;33(1):1–5. doi: 10.1186/s43162-021-00064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A, Ghazi L, Greenberg JH, Mansour SG, Moledina DG, Wilson FP. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4(3):e211095. doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gu X, Huang L, Cui D, Wang Y, Wang Y, Xu J, Shang L, Fan G, Cao B. Association of acute kidney injury with 1-year outcome of kidney function in hospital survivors with COVID-19: a cohort study. EBioMedicine. 2022;1(76):103817. doi: 10.1016/j.ebiom.2022.103817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yusuf F, Fahriani M, Mamada SS, Frediansyah A, Abubakar A, Maghfirah D, Fajar JK, Maliga HA, Ilmawan M, Emran TB, Ophinni Y. Global prevalence of prolonged gastrointestinal symptoms in COVID-19 survivors and potential pathogenesis: a systematic review and meta-analysis. F1000Research. 2021;10:301. doi: 10.12688/f1000research.52216.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sathish T, Cao Y, Kapoor N. Newly diagnosed diabetes in COVID-19 patients. Prim Care Diabetes. 2021;15(1):194. doi: 10.1016/j.pcd.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chng CL, Tan HC, Too CW, Lim WY, Chiam PP, Zhu L, Nadkarni NV, Lim AY. Diagnostic performance of ATA, BTA and TIRADS sonographic patterns in the prediction of malignancy in histologically proven thyroid nodules. Singapore Med J. 2018;59(11):578. doi: 10.11622/smedj.2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feghali K, Atallah J, Norman C. Manifestations of thyroid disease post COVID-19 illness: report of Hashimoto thyroiditis, Graves’ disease, and subacute thyroiditis. J Clin Transl Endocrinol Case Rep. 2021;1(22):100094. doi: 10.1016/j.jecr.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moreno-Perez O, Merino E, Alfayate R, Torregrosa ME, Andres M, Leon-Ramirez JM, Boix V, Gil J, Pico A. COVID19-ALC Research group. Male pituitary–gonadal axis dysfunction in post-acute COVID-19 syndrome—prevalence and associated factors: a Mediterranean case series. Clin Endocrinol. 2021;96:353–362. doi: 10.1111/cen.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Urhan E, Karaca Z, Unuvar GK, Gundogan K, Unluhizarci K. Investigation of pituitary functions after acute coronavirus disease 2019. Endocr J. 2022;69:649–658. doi: 10.1507/endocrj.EJ21-0531. [DOI] [PubMed] [Google Scholar]
- 162.Talwar D, Madaan S, Kumar S, Jaiswal A, Khanna S, Hulkoti V, Eleti MR. Post COVID hypothalamic-pituitary-adrenal axis dysfunction manifesting as perinatal depression: a case series. Med Sci. 2021;25(112):1402–1406. [Google Scholar]
- 163.Kothandaraman N, Rengaraj A, Xue B, Yew WS, Velan SS, Karnani N, Leow MK. COVID-19 endocrinopathy with hindsight from SARS. Am J Physiol Endocrinol Metab. 2021;320(1):E139–E150. doi: 10.1152/ajpendo.00480.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raveendran AV, Misra A. Post COVID-19 syndrome (“Long COVID”) and diabetes: challenges in diagnosis and management. Diabetes Metab Syndr. 2021;15(5):102235. doi: 10.1016/j.dsx.2021.102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, Bauer KA, Zwicker JI. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Salisbury R, Iotchkova V, Jaafar S, Morton J, Sangha G, Shah A, Untiveros P, Curry N, Shapiro S. Incidence of symptomatic, image-confirmed venous thromboembolism following hospitalization for COVID-19 with 90-day follow-up. Blood Adv. 2020;4(24):6230–6239. doi: 10.1182/bloodadvances.2020003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boudhabhay I, Rabant M, Roumenina LT, Coupry LM, Poillerat V, Marchal A, Frémeaux-Bacchi V, El Karoui K, Monchi M, Pourcine F. Case report: adult post-COVID-19 multisystem inflammatory syndrome and thrombotic microangiopathy. Front Immunol. 2021;23(12):2284. doi: 10.3389/fimmu.2021.680567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. 2020;16(10):581–589. doi: 10.1038/s41584-020-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tan J, Anderson D, Rathore AP, O'Neill A, Mantri CK, Saron WA, Lee C, Chu WC, Kang A, Foo R, Kalimuddin S. Signatures of mast cell activation are associated with severe COVID-19. medRxiv. 2021;586:509. [Google Scholar]
- 171.Molderings GJ, Kolck UW, Scheurlen C, Brüss M, Homann J, Von Kügelgen I. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42(9):1045–1053. doi: 10.1080/00365520701245744. [DOI] [PubMed] [Google Scholar]
- 172.Maxwell AJ, Ding J, You Y, Dong Z, Chehade H, Alvero A, Mor Y, Draghici S, Mor G. Identification of key signaling pathways induced by SARS-CoV2 that underlie thrombosis and vascular injury in COVID-19 patients. J Leukoc Biol. 2021;109(1):35–47. doi: 10.1002/JLB.4COVR0920-552RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mukherjee R, Bhattacharya A, Bojkova D, Mehdipour AR, Shin D, Khan KS, Cheung HH, Wong KB, Ng WL, Cinatl J, Geurink PP. Famotidine inhibits toll-like receptor 3-mediated inflammatory signaling in SARS-CoV-2 infection. J Biol Chem. 2021;297(2):100925. doi: 10.1016/j.jbc.2021.100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Theoharides TC. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors (Oxford, England) 2020;46:306. doi: 10.1002/biof.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Veerappan A, Reid AC, Estephan R, et al. Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci USA. 2008;105(4):1315–1320. doi: 10.1073/pnas.0709739105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fuchs B, Sjöberg L, Westerberg CM, Ekoff M, Swedin L, Dahlén SE, Adner M, Nilsson GP. Mast cell engraftment of the peripheral lung enhances airway hyperresponsiveness in a mouse asthma model. Am J Physiol Lung Cell Mol Physiol. 2012;303(12):L1027–L1036. doi: 10.1152/ajplung.00227.2012. [DOI] [PubMed] [Google Scholar]
- 177.McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Komi DE, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58(3):298–312. doi: 10.1007/s12016-019-08729-w. [DOI] [PubMed] [Google Scholar]
- 179.Mehboob R, Lavezzi AM. Neuropathological explanation of minimal COVID-19 infection rate in newborns, infants and children–a mystery so far. New insight into the role of Substance P. J Neurol Sci. 2021;420:117276. doi: 10.1016/j.jns.2020.117276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Karamyan VT. Between two storms, vasoactive peptides or bradykinin underlie severity of COVID-19? Physiol Rep. 2021;9(5):e14796. doi: 10.14814/phy2.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Caillet-Saguy C, Durbesson F, Rezelj VV, Gogl G, Tran QD, Twizere JC, Vignuzzi M, Vincentelli R, Wolff N. Host PDZ-containing proteins targeted by SARS-CoV-2. FEBS J. 2021;288(17):5148–5162. doi: 10.1111/febs.15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wechsler JB, Butuci M, Wong A, Kamboj AP, Youngblood BA. Mast cell activation is associated with post-acute COVID-19 syndrome. Allergy. 2022;77(4):1288. doi: 10.1111/all.15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Szukiewicz D, Wojdasiewicz P, Watroba M, Szewczyk G. Mast cell activation syndrome in COVID-19 and female reproductive function: theoretical background vs. accumulating clinical evidence. J Immunol Res. 2022;2022:1–22. doi: 10.1155/2022/9534163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Conti P, Ronconi G, Caraffa AL, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 185.Theoharides TC, Cholevas C, Polyzoidis K, Politis A. Long-COVID syndrome-associated brain fog and chemofog: luteolin to the rescue. BioFactors. 2021;47(2):232–241. doi: 10.1002/biof.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Krishnan K, Lin Y, Prewitt KR, Potter DA. Multidisciplinary approach to brain fog and related persisting symptoms post COVID-19. J Health Serv Psychol. 2022;2:1–8. doi: 10.1007/s42843-022-00056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. 2021;1(112):217–226. doi: 10.1016/j.ijid.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Malone RW, Tisdall P, Fremont-Smith P, Liu Y, Huang XP, White KM, Miorin L, Moreno E, Alon A, Delaforge E, Hennecker CD. COVID-19: famotidine, histamine, mast cells, and mechanisms. Front Pharmacol. 2021;23(12):216. doi: 10.3389/fphar.2021.633680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. 2018;13(9):1873. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lim HD, Van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- 192.Thurmond RL. The histamine H4 receptor: from orphan to the clinic. Front Pharmacol. 2015;6:65. doi: 10.3389/fphar.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thurmond RL, Chen B, Dunford PJ, Greenspan AJ, Karlsson L, La D, et al. Clinical and preclinical characterization of the histamine H(4) receptor antagonist JNJ-39758979. J Pharmacol Exp Ther. 2014;349:176–184. doi: 10.1124/jpet.113.211714. [DOI] [PubMed] [Google Scholar]
- 194.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70(1):61–67. doi: 10.1136/jim-2021-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kazama I. Stabilizing mast cells by commonly used drugs: a novel therapeutic target to relieve Post-COVID syndrome? Drug Discov Ther. 2020;14(5):259–261. doi: 10.5582/ddt.2020.03095. [DOI] [PubMed] [Google Scholar]
- 196.Molderings GJ, Haenisch B, Brettner S, Homann J, Menzen M, Dumoulin FL, Panse J, Butterfield J, Afrin LB. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(7):671–694. doi: 10.1007/s00210-016-1247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaakati R, Khokhar D, Akin C. Safety of COVID-19 vaccination in patients with mastocytosis and monoclonal mast cell activation syndrome. J Allergy Clin Immunol Pract. 2021;9(8):3198–9. doi: 10.1016/j.jaip.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schwensen HF, Borreschmidt LK, Storgaard M, Redsted S, Christensen S, Madsen LB. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J Clin Pathol. 2021;74(6):400–402. doi: 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- 199.Park SH, Kim JY, Kim JM, Yoo BR, Han SY, Jung YJ, Bae H, Cho J. PM014 attenuates radiation-induced pulmonary fibrosis via regulating NF-kB and TGF-b1/NOX4 pathways. Sci Rep. 2020;10(1):1–2. doi: 10.1038/s41598-020-72629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ferrara F, Granata G, Pelliccia C, La Porta R, Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2. Eur J Clin Pharmacol. 2020;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xi Z, Zhigang Z, Ting L. Post-inflammatory pulmonary fibrosis in a discharged COVID-19 patient: Effectively treated with Pirfenidone. Arch Pulmonol Respir Care. 2020;6(1):051–53. [Google Scholar]
- 202.Ryan J, Fernando J, Barnstein B. TGFb1 suppresses the mast cell response in vitro and in vivo. J Immunol. 2010;184(1):86.6. [Google Scholar]
- 203.Folkerts J, Redegeld F, Folkerts G, Blokhuis B, van den Berg MP, de Bruijn MJ, van Ijcken WF, Junt T, Tam SY, Galli SJ, Hendriks RW. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy. 2020;75(8):1966–78. doi: 10.1111/all.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schanin J, Gebremeskel S, Korver W, Falahati R, Butuci M, Haw TJ, Nair PM, Liu G, Hansbro NG, Hansbro PM, Evensen E. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal Immunol. 2021;14(2):366–376. doi: 10.1038/s41385-020-00336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hermans MA, Schrijver B, van Holten-Neelen CC, van Wijk RG, van Hagen PM, van Daele PL, Dik WA. The JAK1/JAK2-inhibitor ruxolitinib inhibits mast cell degranulation and cytokine release. Clin Exp Allergy. 2018;48(11):1412–20. doi: 10.1111/cea.13217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All collected data are discussed in the manuscript.