Abstract
With the aging world population, the prevalence of aging‐related disorders is on the rise. Diseases such as Alzheimer's, type 2 diabetes mellitus (T2DM), Parkinson's, atherosclerosis, hypertension, and osteoarthritis are age‐related, and most of these diseases are comorbidities or risk factors for AD; however, our understandings of molecular events that regulate the occurrence of these diseases are still not fully understood. Brain and muscle Arnt‐like protein‐1 (Bmal1) is an irreplaceable clock gene that governs multiple important physiological processes. Continuous research of Bmal1 in AD and associated aging‐related diseases is ongoing, and this review picks relevant studies on a detailed account of its role and mechanisms in these diseases. Oxidative stress and inflammation turned out to be common mechanisms by which Bmal1 deficiency promotes AD and associated aging‐related diseases, and other Bmal1‐dependent mechanisms remain to be identified. Promising therapeutic strategies involved in the regulation of Bmal1 are provided, including melatonin, natural compounds, metformin, d‐Ser2‐oxyntomodulin, and other interventions, such as exercise, time‐restricted feeding, and adiponectin. The establishment of the signaling pathway network for Bmal1 in aging‐related diseases will lead to advances in the comprehension of the molecular and cellular mechanisms, shedding light on novel treatments for aging‐related diseases and promoting aging‐associated brain health.
Keywords: Alzheimer disease, ARNTL transcription factors, atherosclerosis, diabetes mellitus, type 2, osteoarthritis, Parkinson disease
Bmal1 deficiency promotes Alzheimer's disease, Parkinson's disease, type 2 diabetes mellitus, atherosclerosis, hypertension, and osteoarthritis.
These aging‐related diseases are contributors to Alzheimer's disease.
Oxidative stress and inflammation may be the common trigger when Bmal1 is lost in the brain, pancreas, blood vessels, and articular cartilage.
Abbreviations
- AD
Alzheimer's disease
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motif
- AMPK
adenosine 5′‐monophosphate (AMP)‐activated protein kinase
- AS
atherosclerosis
- BBB
blood–brain barrier
- bHLH‐PAS
helix–loop–helix/Per‐ARNT‐SIM
- BMAL1
brain and muscle Arnt‐like protein‐1
- BMP
bone morphogenetic protein
- CA
cornu ammonis
- cGAS‐STING
cyclic GMP‐AMP synthase (cGAS) and stimulator of interferon genes
- CLOCK
circadian locomotor output cycles kaput
- CRY
cryptochrome
- EndMT
endothelial‐to‐mesenchymal transition
- ER
endoplasmic reticulum
- ERK
extracellular signal‐regulated kinase
- FoxOs
forkhead box O
- GABA
gamma‐aminobutyric acid
- GLP‐1R/GCGR
glucagon‐like peptide‐1 receptor/glucagon receptor
- GSIS
glucose‐stimulated insulin secretion
- GST
glutathione transferase
- GTP
guanosine triphosphate
- HAECs
human aortic endothelial cells
- HIF1α
hypoxia‐inducible factor 1alpha
- IAPP
islet amyloid polypeptide
- iBRB
inner blood–retina barrier
- IL
interleukin
- MAPK
mitogen‐activated protein kinases
- MMPs
matrix metalloproteinases
- MuSC
muscle stem cell
- NAD (+)
nicotinamide adenine dinucleotide oxidase
- NAMPT
nicotinamide phosphoribosyltransferase
- NFATC
nuclear factor of activated T cells
- NF‐κB
nuclear factor‐kappaB
- NMN
nicotinamide mononucleotide
- NR
nicotinamide riboside
- NRF2
nuclear factor erythroid 2‐related factor 2
- OA
osteoarthritis
- Oxy
D‐Ser2‐oxyntomodulin
- PD
Parkinson's disease
- PER
period
- PGC‐1α
peroxisome proliferator‐activated receptor‐gamma coactivator‐1alpha
- PI3K/AKT/GSK3β
phosphatidylinositol‐3‐kinase/protein Kinase B/glycogen synthase kinase‐3beta
- P. gingivalis
porphyromonas gingivalis
- REV‐ERB
reverse erythroblastosis virus
- RPE
retinal pigment epithelium
- RORα
retinoid‐related orphan receptor‐α
- ROS
reactive oxygen species
- SCN
suprachiasmatic nucleus
- SIRT1
Sirtuin1
- SMAD
Drosophila similar to mothers against decapentaplegic
- STAT
signal transducer and activator of transcription
- TGF‐β
transforming growth factor‐beta
- TMJ‐OA
temporomandibular joint osteoarthritis
- TRF
time‐restricted feeding
- TTFL
transcriptional–translational feedback loop
- T2DM
type 2 diabetes mellitus
- VEGF
vascular endothelial‐derived growth factor
- WAT
white adipose tissue
1. INTRODUCTION
Aging, a complex biological process, is characterized by decreased physiological function with increased age; often aging is associated with increased susceptibility to diseases, including type 2 diabetes mellitus (T2DM), cardiovascular and musculoskeletal diseases, and neurological diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) (Zhuang et al., 2019). AD is the foremost cause of impaired cognition in the elderly and has become the fifth leading cause of death worldwide (Cortes‐Canteli & Iadecola, 2020). Studies have found that many aging‐related diseases such as PD (Cibulka et al., 2022), diabetes mellitus, atherosclerosis (AS) (Xie, Shi, et al., 2020), hypertension (Shih et al., 2018), osteoarthritis (OA) (Innes & Sambamoorthi, 2020), and age‐related macular degeneration (AMD) (Wen et al., 2021) are closely related to AD, which may be comorbidities or risk factors for AD. This important commonality is why AD and these diseases are explored in the present study. It is known that PD and AD are common neurologic diseases of old age (Fang et al., 2018). Diabetes mellitus, hypertension, and OA have been reported to be common comorbidities in AD (Kao et al., 2021; Wang, Wu, et al., 2018). AS (Xie, Shi, et al., 2020), and AMD (Wen et al., 2021) had an increased risk of their condition advancing to AD. According to the World Health Organization (WHO) estimates, by 2050, the proportion of the global population with age > 60 years will ascend to 22% (Mahjoob & Stochaj, 2021). Emerging evidence now suggests an increased trend of patients with AD and associated aging‐related diseases. For example, in the United States, the number of patients aged ≥65 years with AD and related dementias is growing and is projected to reach 13.9 million by 2060 (Matthews et al., 2019). The number of adults aged ≥65 and 65–99 years with diabetes mellitus is expected to increase to 0.253 and 1.42 billion, respectively, over the next 23 years globally (Bellary et al., 2021); nearly half of these adults are expected with T2DM. These epidemiological studies indicate a serious threat to global health with reduced quality of life of older adults amidst increased risk of AD and associated aging‐related diseases.
Brain and muscle Arnt‐like protein‐1 (Bmal1), one of the families of basic helix–loop–helix/Per‐ARNT‐SIM (bHLH‐PAS) domain‐containing transcription factors (Majumdar et al., 2017), is the core regulator of the circadian clock, driving rhythmic expression of circadian clock genes. Bmal1 is essential for regulating the circadian rhythms and maintaining the physiological functions of cells and organs. Evidence indicates that the deletion of Bmal1 can accelerate aging. For example, symptoms of premature aging were observed in Bmal1 deletion animal models, such as organ atrophy, sarcopenia, and cataract; these animals also had shorter lifespans (Kondratov et al., 2006). During natural aging, Bmal1 expression attenuation was noticed in animal models (Duncan et al., 2013). These studies show that the decreased expression of Bmal1 is closely associated with aging. Alterations of Bmal1 during aging, along with other systemic stimulators such as hormones and the changed microenvironment of tissues, may have differential impacts on the brain and peripheral tissues, promoting the progression of aging‐related diseases. The underlying mechanisms of the possible role of Bmal1 dysfunction in AD and associated aging‐related diseases remain unclear; increased understanding of effects of abnormal Bmal1 expression may give rise to the evolvement of new diagnostic and therapeutic approaches for better management of these diseases.
To provide an integrated picture of the role and mechanism of Bmal1 in AD and associated aging‐related diseases, we performed a comprehensive search in PubMed and Google Scholar for relevant studies. Based on existing evidence from the in vivo, in vitro, and clinical studies, we tried to summarize Bmal1‐dependent mechanisms in AD and associated aging‐related diseases and pay attention to therapeutic interventions involved in the regulation of Bmal1.
2. THE BIOLOGICAL FUNCTION OF Bmal1
Bmal1, also called MOP3, is a core driver of the circadian clock in mammals and is considered the only irreplaceable clock gene that regulates rhythmic behaviors (Bunger et al., 2000; Welz et al., 2019). The molecular mechanism, that drives nearly 24 h autonomous circadian oscillations, involves the transcriptional–translational feedback loop (TTFL). Here, Bmal1 and circadian locomotor output cycles kaput (CLOCK) heterodimers form the positive limb that binds to the E‐box motifs and drives the expression of the period (PER1/2/3), cryptochrome (CRY1/2), reverse erythroblastosis virus α (REV‐ERBα), and retinoid‐related orphan receptor‐α (RORα); subsequently, PER and CRY, these two proteins interact and form heterodimers in the cytoplasm, which then translocate to the nucleus to suppress the expression of the positive limb (Richards & Gumz, 2013). Further, REV‐ERBα and RORα, which form additional feedback loops, facilitate and restrain the expression of Bmal1, respectively (Peng et al., 2022). In mammals, the molecular clock based on circadian rhythmicity exists in nearly all fully differentiated cells (Reinke & Asher, 2019). The master clock, situated in the suprachiasmatic nucleus (SCN) of the hypothalamus, receives an immediate projection from the retina via light‐dark cues from the environment (Reinke & Asher, 2019). The SCN synchronizes peripheral clocks located in the non‐SCN brain areas and peripheral tissues such as muscle, liver, adipose tissue, pancreas, and gut through neural, endocrine, temperature, and behavioral signals (Stenvers et al., 2019). The circadian system regulates various physiological processes, including food‐intake behavior, rest‐activity cycle, sleep‐wake cycle, and glucose metabolism (Stenvers et al., 2019). Bmal1 is not only an important regulator of the circadian system but also involves in preserving redox homeostasis (Chhunchha et al., 2020; Xie, Tang, et al., 2020). Furthermore, Bmal1 plays a crucial role in regulating inflammatory responses (Liu et al., 2020), insulin sensitivity (Shi et al., 2013), and mitochondrial functions (E. Li, Li, et al., 2020). Bmal1 deletion abolishes 24 h activity patterns (Ray et al., 2020), leading to circadian rhythm disorders and aging‐related diseases, such as glycolipid metabolism disorders including T2DM (Marcheva et al., 2010), and neurodegenerative diseases (Musiek & Holtzman, 2016), as presented in Figure 1. Besides, Bmal1 alterations among patients with aging‐related diseases are summarized in Table 1.
FIGURE 1.
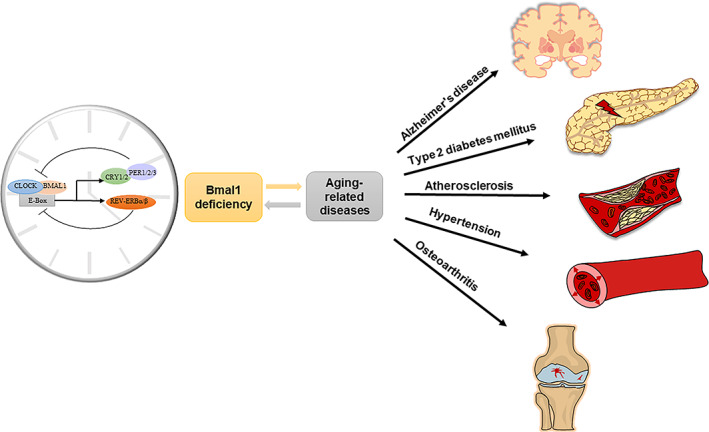
Bmal1 deficiency promotes Alzheimer's disease and associated aging‐related diseases. The mammalian circadian clock exists in nearly all fully differentiated cells, which consists of a transcription‐translation feedback loop (TTFL). The CLOCK‐BMAL1 transcriptional activators bind to the E‐box, driving the expression of CRY‐PER and REV‐ERBs transcriptional repressors. BMAL1 is in an irreplaceable position in TTFL, governing multiple important physiological processes. Bmal1 expression attenuation was observed during aging, and Bmal1 deficiency is an important contributor to aging‐related diseases such as Alzheimer's disease, T2DM, atherosclerosis, hypertension, and osteoarthritis.
TABLE 1.
Bmal1 alterations among patients with aging‐related diseases
| First Author, Year | Participants | age | Cells or tissues | Bmal1 alterations |
|---|---|---|---|---|
| Hulme et al. (2020) | 96 brains of donors who were participants of a large prospective cognitive aging cohort known as The University of Manchester Longitudinal Study of Cognition in Normal Healthy Old Age cohort (UMLCHA) | Age at death = 88.6 ± 5.75 years | Frontal cortex tissues | The positive correlation between DNA methylation at the BMAL1 gene with Braak stage and CERAD score stages, suggests a reduced activity of BMAL1 with increased AD pathology, specifically tau and neurofibrillary tangles |
| Chen, Peng et al. (2015) | AD patients (n = 296) and control subjects (n = 423) |
AD: 77.84 ± 3.97 years (range: 65 to 85 years) Control: 76.92 ± 3.76 years (range: 65 to 85 years) |
Whole blood drawn from the antecubital vein | AD patients showed a higher prevalence of T carriers in BMAL1 rs.2278749 T/C (30.91 vs. 24.82%, p < 0.0001) than was observed in controls |
| Cermakian et al. (2011) | AD patients (N = 26, 13 females, 13 males) and controls (N = 30, 6 females, 24 males) |
AD:79.69 ± 1.64 Control: 74.43 ± 2.16 years |
99 samples (29 BNST, 39 cingulate cortex, 31 pineal gland) from 56 donors (26 AD patients, 30 controls) | In AD patients, the phase of the 24‐h harmonic of BMAL1 expression in the pineal and the phases of the 24‐h rhythms of BMAL1 expression in the BNST were significantly different from controls |
| Yoo et al. (2020) |
A total of 12 paraffin embedded brain tissues from 3 donors with AD. A total of 4 paraffin embedded adult normal brain tissues |
NA |
AD: frontal cortex, occipital cortex, temporal cortex and parietal cortex Control: frontal cortex, occipital cortex, temporal cortex and parietal cortex |
The protein levels of BMAL1 were significantly elevated in impaired astrocytes of the cerebral cortex from patients with AD (every individual patient) |
| Ando et al. (2009) | Patients with type 2 diabetes (n = 12) and healthy individual (n = 14) |
T2DM: 58 ± 6 years Control: 59 ± 6 years |
Peripheral leucocytes | T2DM patients expressed significantly lower transcript levels of BMAL1. BMAL1 mRNA levels were inversely correlated with HbA1c levels (r = −0.47, p < 0.05) |
| Pappa et al. (2013) | Gestational diabetes mellitus (GDM) (n = 185) and pregnant women with normal glucose tolerance (n = 161) |
GDM: 33.63 ± 5.14 years Control: 30.71 ± 9.56 years |
Peripheral blood leukocytes from 20 GDM and 20 control. Patients are selected according to their polymorphism of Bmal1 gene | Bmal1 is a crucial susceptibility gene for GDM in Greek women. The expression levels of BMAL1 mRNA were significantly lower in GDM patients than in controls |
| Gunton et al. (2005) | Type 2 diabetic subjects (n = 5) and normoglycemic controls (n = 7) | Average age was 47 years in both groups. The mean duration of T2DM was 5.8 ± 2.1 years | Diabetic human pancreatic islets | Bmal1 expression was significantly downregulated |
| Yu et al. (2019) | T2D patients (n = 36) and non‐diabetic volunteers (n = 14) |
T2D: 49.47 ± 7.88 years Control: 45.93 ± 7.16 years |
Peripheral blood leucocytes | The BMAL1 mRNA levels were decreased in the diabetic patients. In addition, HbA1c levels were negatively correlated with BMAL1 mRNA levels |
| Cai et al. (2010) | PD patients (n = 17) and age‐matched controls (n = 16) | PD: 62.2 ± 2.8 years Control: 57.8 ± 1.1 years | Peripheral leukocytes. Blood samples were collected during 21:00–09:00 | Expression of BMAL1 was reduced in PD. The expression levels of BMAL1 in PD patients were correlated with their disease severity and sleep quality |
| Li et al. (2021) | PD patients (n = 326) and healthy controls (n = 314) |
PD: 67.43 ± 9.71 years Control:66.03 ± 9.24 years |
Peripheral blood mononuclear cells (PBMCs) | BMAL1 expression was significantly decreased in PD. The severity of pRBD, the severity of daytime sleepiness, and the self‐reported sleep quality were inversely associated with the expression levels of the BMAL1 gene |
| Breen et al. (2014) | Parkinson disease cohort (N = 239), subgroup of these patients (n = 30) and healthy age‐ and sex‐matched controls (n = 15) | PD: age at diagnosis = 68 ± 9 years | Peripheral blood | PD patients had a lack of time‐dependent variation in Bmal1 expression |
| Akagi et al. (2017) | OA patients (n = 14) and normal controls (n = 14) |
OA: 10 females (mean age = 69 years, range:61–82) and 6 males (mean age = 71 years, range: 66–84) Control: 5 females (mean age = 39 years range:26–57) and 18 males (mean age = 30, range:18–44) |
Human knee cartilage | BMAL1 mRNA and protein levels were significantly reduced in OA compared with normal cartilage |
| Dudek et al. (2016) | Non‐OA or mild OA samples (n = 6), grade 0/1; grade 2/3, moderate OA samples (n = 6); and grade 4, OA (n = 6) | Age range, 45–60 years | Human cartilage | The number of BMAL1‐positive chondrocytes and the protein levels of BMAL1 were progressively reduced in OA cartilage with increasing severity compared with the numbers detected in non‐OA human tissue |
| Wu et al. (2019) | Atherosclerosis patients (n = 31) and healthy controls (n = 15) | More than 40 years, from both sexes | Plasma from blood specimens | The expression of Bmal1 mRNA was decreased in the plasma of patients with atherosclerosis |
Abbreviations: AD, Alzheimer's disease; BMAL1, brain and muscle Arnt‐like protein‐1; BNST, bed nucleus of the stria terminalis; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; GDM, gestational diabetes mellitus; OA, osteoarthritis; PD, Parkinson's disease; pRBD, rapid eye movement sleep behavior disorder; T2D, type 2 diabetes.
3. THE ROLE AND MECHANISM OF Bmal1 IN AD
AD is the most prevalent aging‐associated neurodegenerative disorder globally. It is characterized by the accumulation of extracellular β‐amyloid (Aβ) plaques and intracellular hyperphosphorylated tau protein, forming neurofibrillary tangles in the brain. These factors promote the progressive destruction of dendritic spines, synapses, and loss of nerve cells, impaired neurotransmission, progressive isolation of remaining nerve cells, and eventually brain atrophy (Scheltens et al., 2016). The loss of the normal circadian rhythm is a common symptom of AD and is characterized by the increased sleep and awakening during the day and night, respectively (Mattis & Sehgal, 2016). AD‐related circadian dysfunction has been widely explored by disrupting Bmal1 to understand the related mechanism, suggesting an important regulatory role of Bmal1 in AD. The contribution of Bmal1 deficiency to the neurodegeneration associated with AD and other aging‐related dementia is depicted in Figure 2.
FIGURE 2.
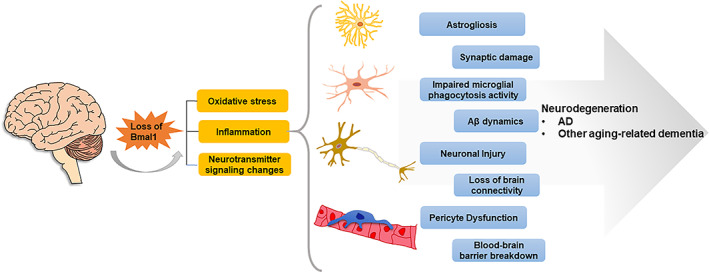
Loss of Bmal1 contributes to the neurodegeneration associated with Alzheimer's disease and other aging‐related dementia. The loss of Bmal1 in the brain has adverse effects on the astrocytes, microglia cells, neurons, and pericyte cells, leading to an increased level of oxidative stress and inflammation, as well as neurotransmitter signaling changes. The above alterations may play a contributory role in astrogliosis, impaired microglial phagocytosis activity, neuronal injury, and pericyte dysfunction, further leading to synaptic damage, Aβ dynamics, loss of brain connectivity, and disruption of the blood–brain barrier, which constitutes the pathological basis of Alzheimer's disease and other aging‐related dementias.
3.1. Abnormal expression of Bmal1 promoted Aβ accumulation and hyperphosphorylation of tau protein
Many studies have shown that the Bmal1 function loss and Aβ deposition are mutually promoting scenarios. Aβ can incur Bmal1 degradation in AD mouse models (Song et al., 2015). Further, the aberrant expression of Bmal1 mRNA and protein in the mouse hippocampus can be induced by Aβ (Wang, Lv, et al., 2018), while it has also been reported that the Bmal1 loss can in turn accelerates the amyloid plaques accumulation (Kress et al., 2018). These studies indicated that the altered Bmal1 levels might be the cause or a consequence of AD pathology. The effect of Bmal1 deletion on Aβ dynamics and amyloid plaque deposition depends on the scope of the deletion; the global deletion including the SCN generates an aberrant diurnal fluctuation of Aβ in the hippocampal interstitial fluid and speeds up the amyloid plaque accumulation, whereas targeted Bmal1 deletion in the hippocampus does not alter interstitial fluid Aβ rhythms (Kress et al., 2018). Recently, it has also been found that the loss of Bmal1 from astrocytes does not interpret the increased plaque burden in whole‐brain Bmal1‐knockout mice, suggesting that the status of astrocyte activation in AD brains has complex effects; further studies are needed to explore its non‐Aβ‐associated contribution in AD (McKee et al., 2022). Interestingly, the inhibition of REV‐ERBs can increase the transcription of BMAL1 and enhance microglial Aβ phagocytic activity in AD animal models, which may be a strategy to clear and reduce the amyloid plaque deposition (Lee et al., 2020). In addition, deficiency of Bmal1 may lead to sleep disorders (Akladious et al., 2018; Qiu et al., 2019), preventing the removal of Aβ and increasing the inflammatory cytokines and formation of Aβ plaques (Ettcheto et al., 2019). Furthermore, Bmal1 deletion induced pericyte dysfunction and decreased the integrity of the blood–brain barrier (BBB) in an age‐dependent manner, which promoted the decreased cerebral blood flow rate and accumulation of blood‐derived neurotoxins (Nakazato et al., 2017). Additionally, the link between Bmal1 and tau protein has been confirmed in several studies. Disturbances in normal circadian rhythms also contribute to more disruptive daily oscillations of Bmal1 and other clock genes and aggravate the hyperphosphorylation levels of soluble tau protein. These observations provide a link between Bmal1 expression rhythms and AD pathology (Huang et al., 2021). Abnormal tau phosphorylation correlates with alterations in Bmal1 protein levels (Niu et al., 2021), while in patients with AD, methylation of Bmal1, indicating Bmal1 activity reduction, was correlated with tau pathology assessed by CERAD score, night wake, and alterations in cognitive function of some dimensions (Hulme et al., 2020).
3.2. Bmal1 deficiency aggravated neuropathology and synaptic degeneration
Astrocytes are the most abundant cell type in the central nervous system (CNS), having close structural and functional interactions with neurons and synapses (Dallérac et al., 2018). Further, reactive astrogliosis is a hallmark of AD (Reichenbach et al., 2019). Since it is difficult to select regulatory elements (to target Bmal1 for deletion) in all the astrocytes in vivo using the available tools, the deletion of Bmal1 in astrocytes in many studies refers to either the Bmal1 deficiency in astrocytes specific to certain brain regions such as the SCN, or the astrocytes in the brain of Bmal1 global knockout mice (Barca‐Mayo et al., 2017). Mounting evidence shows that the influence of Bmal1 deficiency on astrocytes is closely related to pathological factors that promote AD. In an in vitro study, Bmal1 −/− astrocytes showed a changed morphology (shorter actin filaments), lower expression of cortactin, and lower levels of Rho‐GTP, impairing the actin cytoskeleton dynamics and formation of the distal astrocyte processes which led to an adverse effect on the synaptic integrity (Ali et al., 2020). In vivo, very fine astrocytic processes were lacking, and the synaptic coverage of astrocytes in CA3 was reduced in Bmal1 −/− mice (Ali et al., 2020). These altered synaptic functions are related to cognitive deficits caused by chronodisruption. In addition, the deletion of Bmal1 in astrocytes affects neurons; astrocytic Bmal1 is essential to prevent the accumulation of extracellular gamma‐aminobutyric acid (GABA) that mediates astrocyte‐to‐neuron communication (McKee et al., 2022). A study found that the administration of GABA receptor antagonists restored the cognitive functions of Bmal1cKO mice, demonstrating that the Bmal1 deficiency in astrocytes may be associated with significant inhibition of learning and memory‐related circuits through altered GABA levels (Barca‐Mayo et al., 2017). Moreover, astrocyte‐specific Bmal1 deletion promotes neuronal death and astrogliosis and induces inflammatory gene expression in vitro (Lananna et al., 2018). Consistently, inflammatory gene expression and astrocyte activation are also induced in vivo (Lananna et al., 2018). Additionally, when Bmal1 is abolished in both neurons and astrocytes, these mice show much more remarkable astrogliosis in the whole brain. This particular study also demonstrated that Bmal1 regulates astrogliosis mainly via a cell‐autonomous mechanism mediated by altered glutathione transferase‐mediated protein glutathionylation (Lananna et al., 2018). Musiek et al. found that when Bmal1 was partially knockdown in primary neurons, these cells exhibited spontaneous neurodegeneration, revealing another cellular mechanism by which diminished Bmal1 expression contributed to neurodegenerative diseases (Musiek et al., 2013); further, Bmal1 deletion contributes to the impaired structure and dysfunction of synapses, as well as damaged cortical functional connectivity in brain regions that are seriously influenced in animal models of brain aging and AD (Musiek et al., 2013).
3.3. Signaling pathway for Bmal1 deficiency in AD
Nicotinamide phosphoribosyltransferase (NAMPT) expression is controlled by the BMAL1/CLOCK complex (Ramsey et al., 2009); therefore, the inhibition of nicotinamide adenine dinucleotide (NAD+) biosynthesis is closely associated with Bmal1 deficiency. Sirtuin1 (SIRT1) is an NAD+ ‐dependent enzyme that regulates important physiological process, such as glucose metabolism, mitochondrial homeostasis (Bonkowski & Sinclair, 2016), and immune responses (Chang & Guarente, 2014). It has been reported that the expression of SIRT1 diminishes with the aging (Chen, Zhou, et al., 2020). The correlation of SIRT1 with the molecules participating in various signaling networks (NF‐κB, P53, PGC1α, and FoxOs) has been comprehensively reviewed in the literature (Chen, Zhou, et al., 2020; Gomes et al., 2018). SIRT1 is a positive regulator of Bmal1 and clock genes in SCN (Chang & Guarente, 2013). The results of multiple evidence have indicated that the NAD+/SIRT1 dysfunction contributes to AD (Julien et al., 2009; Koo et al., 2017; Lautrup et al., 2019). Mechanistic studies of the aging brain in PD also showed the involvement of the BMAL1/SIRT1 pathway (Wang, Lv, et al., 2018). Furthermore, NAD+ depletion‐mediated activation of cyclic GMP‐AMP synthase and stimulator of interferon genes (cGAS‐STING) have been reported to play an indispensable role in neuroinflammation and cellular senescence in AD (Hou, Wei, et al., 2021). However, the evidence of pathological progression of AD caused by NAD+ decline caused directly by Bmal1 deficiency is not available, and further studies are needed to reveal the underlying mechanism. Thus, we describe this indirect link in light gray in Figure 3.
FIGURE 3.
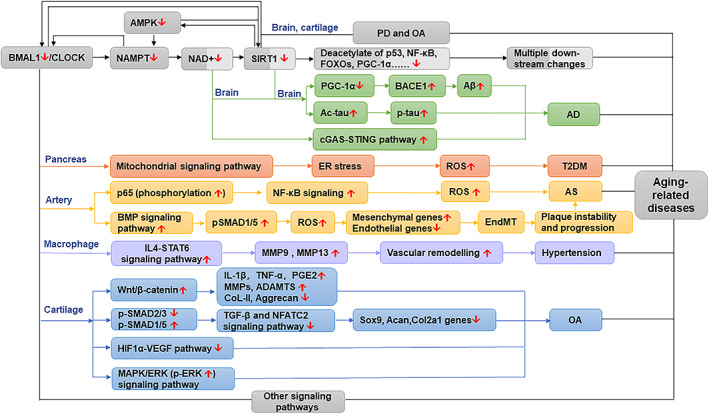
Signaling pathways related to Bmal1 deficiency in AD and associated aging‐related diseases. Dark gray represents the direct link from Bmal1 to NAD+ and SIRT1. Light gray represents the indirect link between Bmal1 to NAD+ and SIRT1. Direct evidence suggested that the SIRT1‐BMAL1 pathway was involved in PD and OA, and indirect evidence indicated that the SIRT1‐BMAL1 pathway may involve in other aging‐related diseases such as AD. Bmal1 deficiency in the pancreas contributed to T2DM via accumulated ROS mediated by the mitochondrial signaling pathway. In the artery, ROS accumulation was aggravated by Bmal1 deficiency through NF‐κB signaling and BMP‐mediated signaling, leading to the progression of AS. Bmal1 loss in macrophages enhanced hypertensive vascular remodeling in hypertension through the IL4‐STAT6 signaling pathway. Bmal1 deficiency in cartilage exacerbated OA through interaction with multiple signaling pathways, which included the Wnt/β‐catenin signaling pathway, TGF‐β and NFATC2 signaling pathway, HIF1α‐VEGF pathway, and MAPK/ERK signaling pathway. There may exist other potential Bmal1‐dependent signaling pathways involved in aging‐related diseases.
4. THE ROLE AND MECHANISM OF Bmal1 IN AD‐ASSOCIATED AGING‐RELATED DISEASES
4.1. The role and mechanism of Bmal1 in T2DM
T2DM is a prevalent chronic metabolic disease characterized by hyperglycemia, relative insulin deficiency, insulin resistance, and pancreatic beta‐cell dysfunction (Yaribeygi et al., 2020). Bmal1 appears to be highly relevant to the pancreatic islet's function, regulation of insulin secretion, and glucose metabolism. For instance, it was suggested that the expression of Bmal1 in beta cells is a feature of postnatal islet cell maturation and contributes to the establishment of circadian control of glucose‐stimulated insulin secretion (GSIS) (Rakshit et al., 2018). Mice adopt a high‐fat diet undergo compensatory increased beta‐cell mass to preserve normal beta‐cell function and glucose homeostasis (Rakshit et al., 2016); however, this response is diminished in mice with beta‐cell‐specific deletion of Bmal1; increased apoptosis was observed additionally, suggesting a regulating role of Bmal1 in beta‐cell compensatory proliferation and function (Rakshit et al., 2016). Furthermore, Bmal1 in the hypothalamic paraventricular nucleus was significant in regulating insulin secretion and glucose tolerance by regulating the expression and release of vasopressin, a humoral factor that stimulates insulin secretion (Nakata et al., 2021).
Disruption of Bmal1 in beta cells plays a notable role in the onset of diabetes. Marcheva et al. suggested that pancreas‐specific Bmal1 KO mice displayed reduced glucose tolerance and lessened pancreatic islets size, proliferation, and insulin release that worsened with age (Marcheva et al., 2010). Lee et al. found that deletion of Bmal1 in beta cells results in cell dysfunction and diabetes; this was owing to the increased reactive oxygen species (ROS) accumulation, consequent mitochondrial uncoupling, and decreased antioxidant regulatory factor (Lee et al., 2018, 2013). Another study found that the loss of Bmal1 can impair beta‐cell function through the mitochondrial signaling pathway, as disruptive mitochondrial morphologies such as swelling, fracture, and disappearance of mitochondrial cristae can be observed in Bmal1‐deficient beta cells (Ye et al., 2020). Importantly, beta‐cell‐specific Bmal1 overexpression at the mRNA and protein levels enhances islet's circadian clock amplitude as well as GSIS, meanwhile, preventing obesity‐induced glucose intolerance and reducing the cellular oxidative and endoplasmic reticulum (ER) stress (Rakshit & Matveyenko, 2021). These findings indicate that the downregulation of Bmal1 may be related to T2DM by promoting mitochondrial dysfunction and oxidative stress in beta cells. Genetic variations in Bmal1 have also been linked with susceptibility to T2DM in humans (Pappa et al., 2013; Woon et al., 2007). Consistently, patients with T2DM and animal models of T2DM show disrupted Bmal1 expression in several tissues. For example, downregulated Bmal1 expression was found in diabetic human islets and peripheral blood leukocytes from T2DM patients (Gunton et al., 2005; Yu et al., 2019). Decreased Bmal1 expression in T2DM rats was associated with suppressed bone marrow mesenchymal stem cell osteogenesis (Li et al., 2017). Dampened oscillations of Bmal1 were found in diabetic mice aortas (Su et al., 2008). Decreased level of Bmal1 protein was found in white adipose tissue (WAT) of db/db mice (Caton et al., 2011). Overall, the findings have revealed that Bmal1 expression is impaired in T2DM.
4.2. PD
PD is the second most common age‐related neurodegenerative disorder and is manifested through the motor and non‐motor symptoms (Wang, Lv, et al., 2018). Many clinical manifestations of PD suggest circadian rhythm dysfunction, such as disturbances of rest and activity cycles, aberrant blood pressure fluctuation patterns, and an abnormal rhythm of melatonin secretion (Wang, Lv, et al., 2018). These observations indicate that disrupted circadian rhythm is one of the most common non‐motor symptoms in PD. The clinicopathological features of PD are characterized by the dopaminergic neuronal loss in the substantia nigra pars compacta and the formation of Lewy bodies and neuritis (Gómez‐Benito et al., 2020). Evidence in human studies and animal models showed that decreased level of Bmal1 is involved in PD. Relatively lower Bmal1 levels were found in leukocytes of PD patients, which may be a reflection of the severity of PD (Cai et al., 2010). Bmal1 expression levels in the peripheral blood mononuclear cells and plasma melatonin levels were decreased in patients with PD (Li et al., 2021). Moreover, a lack of time‐dependent variation in Bmal1 expression was observed (Breen et al., 2014). One potential explanation for the alteration of Bmal1 expression is that dopamine can regulate the activity of the BMAL1/CLOCK complex, and hence, a lack of dopamine in PD affects this central component of the molecular clock (Breen et al., 2014). After melatonin treatment, the levels of Bmal1 were increased in PD patients (Delgado‐Lara et al., 2020), reinforcing a close link between Bmal1 and PD. In a rat model of PD, the expression of Bmal1 in the striatum was significantly lower than that in the sham group at some timepoints (Yang et al., 2021). Further, in another rat model of PD induced by lipopolysaccharide combined with rotenone, the mRNA and protein expressions of Bmal1 also decreased (Li et al., 2019).
Additionally, Bmal1 was shown to be involved in regulating PD pathogenesis. In the 1‐methyl‐4‐phenyl‐1,2,4,5‐tetrahydropyridine‐induced PD mouse model, inactivation of Bmal1 can lead to distinct motor dysfunction, dopaminergic neuronal injury in the substantia nigra pars compacta, deficiency in dopamine transmitters, and aggravation of the neuroinflammatory response, suggesting that Bmal1 may exert a beneficial effect on survival of dopaminergic neurons by regulating the microglia‐mediated neuroinflammation response (Liu et al., 2020). The increase in oxidative stress (and loss of antioxidative defense) is critically related to the onset of PD (Johnson et al., 2012); treatment with 6‐hydroxydopamine, in an animal model of PD, caused a reduction and elevation in the levels of SIRTI and acetylated Bmal1, respectively, causing abnormal antioxidative activity. These findings suggest that the SIRT1‐BMAL1 pathway is involved in the regulation of abnormal antioxidative responses in PD (Wang, Lv, et al., 2018).
4.3. Atherosclerosis
Several studies indicate a direct link between Bmal1 and atherosclerosis (AS). For instance, Bmal1 downregulation is involved in Porphyromonas gingivalis (P. gingivalis) induced atherosclerosis by exacerbating the development of oxidative stress (Xie, Tang, et al., 2020). In the indirect co‐culture model of P. gingivalis and human aortic endothelial cells, Bmal1 upregulation inhibited monocyte recruitment, reduced the levels of pro‐inflammatory cytokines, and decreased cell apoptosis, demonstrating the antagonistic role of Bmal1 in oxidative stress (Xie, Tang, et al., 2020). The loss of Bmal1 in macrophages could give rise to increased trafficking of Ly6chi monocytes to atherosclerotic lesions, which resulted in an increase in macrophage content and lesion size in the carotid arteries and promoted atherosclerosis (Huo et al., 2017). Bmal1 deficiency in the human carotid aggravated intracellular ROS accumulation and endothelial‐to‐mesenchymal transition through the bone morphogenetic protein‐mediated signaling, demonstrating the central role of Bmal1 loss in atherosclerosis progression (Zhu et al., 2018). Liang et al. found that the upregulated expression of microRNA‐155 (miR‐155) was positively associated with the downregulation of Bmal1 in the aorta, leading to an increased atherosclerotic plaque area and weakened aortic diastolic function, and these changes were reversed after downregulation of miR‐155 or an increase in Bmal1 (Liang et al., 2020). Pan et al. found that both the global and hepatic Bmal1 deficiency enhances atherosclerosis in Apoe−/− mice and demonstrated that Bmal1 is an anti‐atherogenic transcription factor for its important contribution to lipoprotein and cholesterol metabolism (Pan et al., 2016). These studies supported that Bmal1 deficiency plays an important role in AS.
4.4. Hypertension
The clock gene Bmal1 is reported to be highly correlated with the etiology of hypertension. Analysis of single‐nucleotide polymorphisms (SNPs) has elucidated that BMAL1 is associated with susceptibility to hypertension (Woon et al., 2007). In a genetic animal model of hypertension, the spontaneously hypertensive (SHR) rats, the expression of Bmal1 was decreased (Tharmalingam et al., 2020). A significant rhythm imbalance of the Bmal1 mRNA levels was found in SHR liver tissues (Hou, Zhang, et al., 2021). Hypertension in db/db mice is associated with attenuated Bmal1 oscillations in the vasculature (Su et al., 2008). Recently, Huo et al. suggested that BMAL1 deletion has a tonic effect on vascular remodeling, leading to the promoted blood pressure increase (Huo et al., 2021). They indicated that loss of Bmal1 enhanced the phosphorylation and nuclear translocation of STAT6 triggered by IL4, which promoted the activation of STAT6 and its target gene transcription, leading to increased expression of MMP9 and MMP13 in the vascular wall, contributing to vascular remodeling (Huo et al., 2021).
4.5. Osteoarthritis
Osteoarthritis (OA) is the most widespread degenerative joint disease and is characterized by cartilage degeneration in articulating joints, with a higher risk in older people. The OA disease affects >30 million people in the United States (Alliston et al., 2018). Bmal1 is essential for the development of hard tissues such as bones, cartilage, and teeth (Chen, Tang, et al., 2020). A growing body of studies suggest that Bmal1 loss can inhibit osteogenesis and promote osteoclastogenesis, playing a significant role in the pathogenesis of OA. Akagi et al. found that Bmal1 is reduced in OA cartilage, and alterations in Bmal1 expression affect transforming growth factor‐beta (TGF‐β) downstream gene expression in chondrocytes, which may accelerate cartilage injury through inflammation‐related pathways (Akagi et al., 2017). Consistently, Dudek et al. found that the expression of Bmal1 was reduced in the articular cartilage of human knees with OA (Dudek et al., 2016). In the Bmal1‐cKO mouse model used in their study, targeted Bmal1 ablation abolished circadian rhythm as well as resulted in progressive degeneration of articular cartilage, which may be related to the disturbance of cartilage homeostasis caused by Bmal1 dysregulation; reduced TGF‐β and NFATC2 pathway signaling was identified in Bmal1‐cKO chondrocyte (Dudek et al., 2016). It is known that the increase in matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motif (ADAMTS) downstream induced by the extracellular signal‐regulated kinase (ERK) lead to cartilage decomposition (Ma et al., 2014). Chen et al. observed that Bmal1 expression was decreased in rats with circadian rhythm disruption, along with increased P‐ERK/MMPs/ADAMTS expression (Chen, Zhao, et al., 2020). IL‐6‐induced MMP3/13 and ADAMTS5 upregulation was retracted by Bmal1 overexpression in vitro; temporomandibular joint osteoarthritis (TMJ‐OA) was reversed in Bmal1‐overexpressing rats, suggesting that Bmal1 is involved in the regulation of OA through the mitogen‐activated protein kinase (MAPK)/ERK signaling pathway (Chen, Zhao, et al., 2020). Evidence in vivo and in vitro found that the deletion of Bmal1 could lead to inhibited chondrocyte proliferation and elevated apoptosis, along with decreased expression of hypoxia‐inducible factor 1alpha (HIF1α) and vascular endothelial‐derived growth factor (VEGF); consistently, the recovery of Bmal1 expression improved the function of chondrocytes (Ma et al., 2019). Moreover, Bmal1 takes part in the regulation of cartilage homeostasis through SIRT1, which is affected by the level of NAD+ oxidase (Yang et al., 2016). Bmal1 also participates in the regulation of matrix synthesis and catabolic metabolism in cartilage and chondrocytes through the Wnt/β‐catenin signaling pathway (Song, Ma, et al., 2021). These findings suggest that Bmal1 deletion of chondrocytes results in disruptive critical pathways that impair cartilage homeostasis and exacerbate a catabolic state, thus resulting in cartilage degradation.
4.6. Age‐related macular degeneration
Age‐related macular degeneration (AMD) is a leading cause of visual impairment and severe irreversible blindness and is classified as early‐stage (medium‐sized drusen and retinal pigmentary changes) to late‐stage (neovascular and atrophic) in clinical settings (Mitchell et al., 2018). In patients older than 75 years, the risk of developing early AMD and late AMD is 25% and 8%, respectively, and the number of cases is expected to grow as the population ages (Stahl, 2020). By 2040, the global number of people suffering from AMD is expected to be nearly 300 million (Mitchell et al., 2018). The inner blood–retina barrier (iBRB) is exceedingly dynamic, and claudin‐5, a tight junction protein, is abundantly expressed. Persistent suppression of claudin‐5 induces remarkable retinal pigment epithelium (RPE) cell atrophy (Hudson et al., 2019). Hudson et al. found that disruption of Bmal1 expression results in dysregulated claudin‐5 cycling and adverse effects on the integrity of endothelial cells, suggesting that the Bmal1 dysfunction may strongly implicate in drusen accumulation and subsequent RPE atrophy (Hudson et al., 2019). Therefore, it is necessary to explore a more comprehensive and in‐depth mechanism of Bmal1 in AMD.
5. SUMMARY AND DISCUSSION OF THE ROLE AND MECHANISM OF Bmal1 IN AD AND ASSOCIATED AGING‐RELATED DISEASES
The above studies explored the role of Bmal1 in AD and associated aging‐related diseases, which suggested the dysfunction of Bmal1 in many important organs: the brain, pancreas, blood vessels, and articular cartilage. Some ideas can be extracted from these studies. First, oxidative stress may be the common trigger when Bmal1 is lost in these organs, driving the aging‐related loss of function in organs; and inflammation may be another important trigger. This idea was consistent with the main idea of another study discussing oxidative stress in aging and related chronic diseases (Mas‐Bargues et al., 2022). Here are some issues worth considering. At the organ‐tissue level, it would be interesting to know whether Bmal1 loss in one organ affects other organs and whether the start of oxidative stress in one organ may be the trigger of oxidative stress for another organ, contributing to the organ‐organ communication involved in the development of the aging‐related diseases and cognition dysfunction. On a cellular level, it remains unclear whether Bmal1 deficiency in different organs shares a common signal pathway before the initiation of the oxidative stress‐related signal pathway.
Second, evidence showed that Bmal1 was associated with other ischemia‐related diseases such as myocardial infarction (Liu, Xiao, et al., 2021), stroke (Beker et al., 2018), and peripheral vascular disease (Xu et al., 2021), which are highly prevalent in aging populations (Hadjipanayi & Schilling, 2013); and some of these diseases have a close relationship with hypertension and atherosclerosis and may promote AD (Vijayan & Reddy, 2016; Zhang & Luo, 2020). There is an interesting link between vascular damage and Bmal1 deficiency when putting these diseases together, suggesting that a mechanism related to vascular pathology may be a common trigger. Cumulative evidence has demonstrated that the lack of Bmal1 would impair angiogenesis, and endothelial cell functions (Xu et al., 2021), accelerating microvascular and macrovascular damage (Lee et al., 2021). Endothelial dysfunction from the Bmal1‐knockout mice was associated with enhanced superoxide levels and endothelial NO synthase uncoupling in blood vessels (Anea et al., 2012). In part, the mechanisms underlying these impairments may involve oxidative stress and that the Bmal1 exerts on key pathways in endothelial cell signaling.
Third, most studies suggested that decreased level of Bmal1 was a contributor to aging‐related disease; there are also a few studies linking elevated Bmal1 to certain diseases. For instance, the elevated level of Bmal1 was also found in the pineal of streptozotocin‐induced type 1 diabetes (Peschke et al., 2008), astrocytes of the cerebral cortex in AD (Yoo et al., 2020), tissue samples of follicular and papillary thyroid carcinoma (Mannic et al., 2013), indicating that Bmal1 may exhibit a tissue‐specific expression representing an additional regulation. This specific role in AD and associated aging‐related disease and the potential mechanisms remain to be further clarified. Besides, in addition to contributing to the development of aging‐related diseases, the deletion of Bmal1 in specific tissue may offer new treatment strategies for some diseases. For example, microglia‐specific knockdown of Bmal1 improved memory in the animal model, and the authors proposed that microglial Bmal1 may be a potential therapeutic target for metabolic and cognitive disorders related to psychiatric disease (Wang et al., 2021).
The present study identified important role and mechanism of Bmal1 in AD and associated aging‐related diseases such as PD, T2DM, hypertension, AS, OA, and AMD, suggesting that Bmal1 deficiency was implicated in the etiology of these diseases. These AD‐associated diseases can also promote the initiation or progression of AD through numerous pathways, and the pathology of one of the diseases may be a risk factor for another. Thus, it is interesting to explore the role of Bmal1 in this relationship. Unfortunately, there was rare evidence supporting the role of Bmal1 in the contributing effects of AD‐associated diseases on AD. For example, the deposition of islet amyloid polypeptide (IAPP) in the pancreas is one of the pathological hallmarks of T2DM. IAPP was detected in brain tissues and was associated with cognitive decline and AD development; its interaction with Aβ contributed to a synergistic toxic activity (Zhang & Song, 2017). Besides, IAPP was a crucial mediator of tau pathology in AD (Zhang et al., 2022). However, we found no evidence about the role of Bmal1 in the above pathological links, which suggested that the role of Bmal1 in the mechanisms connecting these aging‐related diseases to AD is poorly understood (Figure 4).
FIGURE 4.
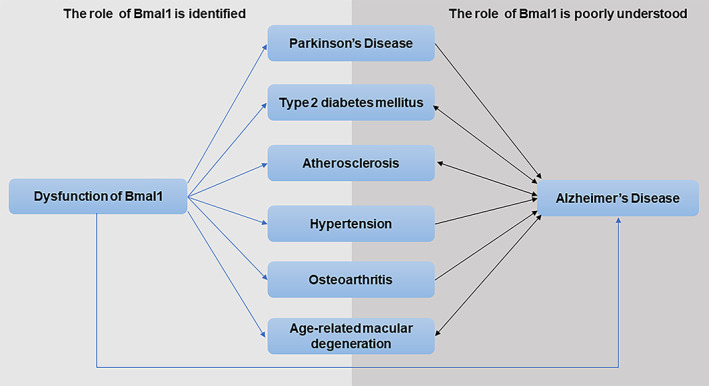
The role of Bmal1 in the mechanisms connecting aging‐related diseases to AD is poorly understood. The role of Bmal1 in AD, PD, T2DM, hypertension, AS, OA and AMD is identified (blue lines). These AD‐associated diseases can also promote AD or have close interaction with AD through numerous pathways (black lines), and the role of Bmal1 in the mechanisms connecting these aging‐related diseases to AD is poorly understood.
6. THERAPEUTIC PERSPECTIVES
Therapeutics tailored to regulate molecular Bmal1 could be the key to preventing the progression of aging‐related diseases. Several relevant treatments targeting Bmal1 in aging‐related diseases are discussed in this section and summarized in Table 2. Oxidative stress is a consensus mechanism of aging, and the antioxidant N‐acetyl‐L‐cysteine has been shown to ameliorate signs of premature aging related to Bmal1 deficiency (Kondratov et al., 2009). Antioxidants that boost nuclear factor erythroid 2‐related factor 2 (NRF2) activity are available to rescue the excessive pro‐oxidant and pro‐inflammatory phenotype of Bmal1 −/− macrophages (Early et al., 2018). Therefore, treatments involving antioxidative effects may have anti‐aging effects, and we focused on those involved in the regulation of Bmal1.
TABLE 2.
Therapeutic strategies targeting Bmal1 in aging‐related diseases in different models
| First Author, Year | Design and subjects | Age a | Treatment | Bmal1 alterations | Results |
|---|---|---|---|---|---|
| Ikeno and Nelson (2015) | Siberian hamsters | 16‐18 was | Melatonin, 20 μg, subcutaneous injections 4 h before killed | Increased | Remodeling of hippocampal neurons, altered the synaptic structure in the CA1 region and the dentate gyrus region |
| Beker et al. (2019) | N2A cells mimic focal cerebral ischemia in vitro | NA | Melatonin (MEL), 1 μM | Increased | Increased cellular survival under normoxic conditions and after oxygen–glucose deprivation |
| Delgado‐Lara et al. (2020) | A double‐blind, cross‐over, placebo‐controlled RCT, 26 patients with stage 1–3 PD | Median age = 55.5 years |
First group: MEL (25 mg, twice a day for 3 months)‐ 4 days of washout period ‐Placebo (25 mg, twice a day for 3 months) Second group: Placebo‐ washout period ‐ MEL. At the same way |
The levels of BMAL1 genes increased from 0.56 to 2.2 | The global perception of sleep comfort was increased |
| Kondratov et al. (2009) | Bmal1−/− mice | 4‐45 w | Antioxidant N‐acetyl‐L‐cysteine (NAC), starting from prenatal development, ending in spontaneous death | NA | The age‐dependent weight loss (40 w) was delayed; the incidence of cataracts (phenotype of premature aging) was decreased, and the lifespan was extended |
| Kim et al. (2021) | APP/PS1 mice, 3 months | 19–22 months | Regular diets containing equivalent macronutrients with Purina 5053 with or without 0.1% nobiletin (NOB), for 16‐18 months | The mRNA levels of Bmal1 at ZT6 was higher compared with ZT18 | The amounts of full‐length APP proteins (APP‐FL) were significantly reduced by NOB at ZT6, but not at ZT18. The expression of clock‐controlled metabolic genes involved in insulin signaling and mitochondrial function were enhanced. The Aβ plaques in the cortex were reduced |
| He et al. (2016) |
C57BL/6J mice, 6 week. HFD for 10 weeks. Clock Δ19/Δ19 C57BL/6J mice. db/db mice. db/dbClock Δ19/Δ19 mutant mice |
16 w | Nobiletin (200 mg/kg body weight) via oral gavage every other day, in the time window of ZT8‐10, for 10 weeks | Increased Bmal1 promoter‐driven luciferase reporter expression with wild‐type, but not mutant | NOB improved glucose and lipid homeostasis in diet‐induced obesity (DIO) mice and db/db mice but not in ClockΔ19/Δ19 C57BL/6J mice, and improved glucose homeostasis in db/db ClockΔ19/Δ19 mutant mice |
| Qi et al. (2017) | Human neuroblastoma SH‐SY5Y cell line | 5–8 generations | Pretreated with tea polyphenols (TP, purity of >98%) (40 μg/ml) for 12 h and then with H2O2 (100 μM) for 12 h after a washing with PBS | TP functions in a Bmal1‐dependent manner |
Result1:TP suppressed H2O2‐triggered apoptosis and loss of cell viability and ameliorated oxidative stress and mitochondrial dysfunction. Result2: The neuroprotective effects of TP were abolished by silencing Bmal1 expression with siRNA |
| Quezada‐Fernández et al. (2019) | A double‐blind, placebo‐controlled RCT, 20 patients with T2DM, mean age = 53.2 years | Green tea extract (n = 10, mean age = 50.2), placebo (n = 141, mean age = 56.1) | 400 mg of decaffeinated green tea extract (polyphenols ≥90%) or placebo for 12 weeks | NA | cAIx75 (75 bpm heart rate adjusted central augmentation index) was decreased. Arterial stiffness was improved |
| Zhao et al. (2016) | T2DM rats (high‐sucrose‐fat diets for 4 weeks, then intraperitoneally injected with 0.5% STZ solution at the dose of 50 mg/kg weights) | >8 w | LBP‐4a treatment group at high dose (10 mg/kg·d), and the LBP‐4a treatment group at low dose (5 mg/kg·d), intragastric administration for 4 weeks | Increased BMAL1 expression in pancreatic islet cells in high dose group | Blood glucose and insulin were decreased in both high dose and low dose groups. Melatonin was increased in the high dose group. Disruption of pancreatic architecture was meliorated in both high dose (more obvious) and low dose groups |
| Caton et al. (2011) | Db/db, Db/+ mice, 7 w | 8 w | Metformin, (250 mg/kg/day for 7 days), by oral gavage | Increased Bmal1 mRNA expression by 2.1‐fold | The BMAL1 protein expression was increased in tWAT of db/db mice. The fasting glucose and fasting insulin were decreased |
| Alex et al. (2020) | Db/db, Db/+ mice, 8–10 w | 10‐12 w | Metformin (164 ± 40 mg/kg/day for 14 days) | Increased BMAL1 protein | Body mass and Glycated hemoglobin were decreased. The retinal Kir4.1 level in Müller cells was increased |
| Wang, Zhao, et al. (2020) | C57BL/6 mice received Aβ31‐35 via hippocampal injection, 9‐11 w | 9‐11 w | D‐Ser2‐Oxyntomodulin (Oxy) (30 nmol/kg), via hippocampal injection, 15 min before Aβ31‐35 was injected | The rhythmic mRNA and protein expression of Bmal1 in the hippocampus were improved | The Aβ31‐35‐induced disruption of the circadian rhythm (The sleep–wake cycle, free‐running period, locomotor activity, and ratio of the subjective night activity/total activity) was recovered. The abnormal expression of Bmal1 and Per2 was improved in the mice hippocampus and HT22 cells |
| Erickson et al. (2020) | 26 adults with prediabetes and obesity provided with isoenergetic diets | Mean age = 66 years | 12‐week exercise intervention (5 days per week at 85% of heart rate max on a treadmill for 60 min) | BMAL1 gene expression in skeletal muscle was increased | Body weight, BMI, and total abdominal adiposity were decreased. Exercise capacity and peripheral insulin sensitivity were improved. The fold change in BMAL1 was positively correlated with insulin sensitivity and negatively correlated with BMI |
| Chaix et al. (2019) | Liver‐specific Bmal1 knockout mice, 12 w | 24 w | 12 weeks on the time‐restricted feeding (TRF) | NA | TRF prevented whole‐body fat accumulation and serum hyperlipidemia |
| Regmi et al. (2021) | C57BL/6J mice, 8 w, high‐fat diet ad libitum for 4 w | 20 w | A further 8 weeks on TRFe (initiated at lights off) or TRFd (initiated at 4‐h after lights off) | The amplitude of Bmal1mRNA levels in liver was increased | TRF reduced weight and fat mass (greater reduction in TRFe), improved glucose tolerance, and protected mice from high‐fat diet‐induced hepatosteatosis (no difference in TRFe vs TRFd) |
Abbreviations: Aβ, amyloid β‐protein; APP, amyloid precursor protein; BMAL1, brain and muscle Arnt‐like protein‐1; CA, cornu ammonis; DIO, diet‐induced obesity; MEL, melatonin; NAC, antioxidant N‐acetyl‐L‐cysteine; NOB, nobiletin; N2a, neuroblastoma cell lines; Oxy, D‐Ser2‐oxyntomodulin; PD, Parkinson's disease; RCT, randomized controlled trial; STZ, streptozotocin; TP, tea polyphenols; TRF, time‐restricted feeding; T2DM, type 2 diabetes mellitus; WAT, white adipose tissue; ZT, Zeitgeber time.
The age of mice was sacrificed, or the time point of relative parameters w collected.
6.1. Melatonin
Melatonin is an amine hormone and an effective endogenous antioxidant (Tan et al., 2015), synthesized and secreted primarily by the pineal gland under normal light/dark conditions at night. Numerous studies have suggested that melatonin plays an important role in regulating Bmal1 and exhibits beneficial physiological influences in the intervention of aging‐associated diseases such as AD (Li, Zhang, et al., 2020), PD (Delgado‐Lara et al., 2020), T2DM (Abdulwahab et al., 2021), AS (Xie, Tang, et al., 2020), and OA (Hosseinzadeh et al., 2016). Melatonin strengthens the expression of Bmal1 protein and regulates the expression of Bmal1 via PI3K/AKT signaling (Beker et al., 2019). A study showed that melatonin treatment increased Bmal1 expression in the hippocampus, induced rapid remodeling of hippocampal neurons, and altered the synaptic structure in the CA1 and dentate gyrus regions, suggesting that melatonin can regulate the structural changes in hippocampal neurons through the circadian clock molecule Bmal1 (Ikeno & Nelson, 2015). Intermittent administration of melatonin can reset the daily pattern of Bmal1 expression (Furtado et al., 2020). Furthermore, endogenous and exogenous melatonin ameliorates diabetes and related metabolic dysfunction by regulating insulin secretion and protecting against ROS (Espino et al., 2019). A study reported that melatonin increased the expression of SIRT1‐BMAL1 pathway‐related proteins in a dose‐dependent manner and protected against cerebral ischemia–reperfusion‐induced brain damage in diabetic mice (Liu, Cao, et al., 2021). The above evidence reveals that melatonin may be a prospective therapeutic agent for aging‐related diseases.
6.2. Natural compounds
Nobiletin, a citrus flavonoid, was shown to improve cognitive deficits and pathological features and motor and cognitive deficits in AD and PD, respectively, in animal model studies (Nakajima & Ohizumi, 2019). Nobiletin increases diurnal changes in Bmal1 expression and decreases Aβ plaques in the cortex of APP/PS1 mice (Kim et al., 2021). A previous study showed that nobiletin directly binds and activates RORs, while the RORs enhance the activity of nobiletin on Bmal1 transcription. It was also postulated that nobiletin is a natural compound correlated with the enhancement of molecular clock and counteracts metabolic syndrome in a clock‐dependent manner (He et al., 2016). Nobiletin can promote GSIS and prevent ER stress‐induced beta‐cell apoptosis, thereby exhibiting its anti‐diabetic effect (Kaneko et al., 2020). The anti‐diabetic and anti‐inflammatory effects of nobiletin were also determined in an in vitro human model (Nguyen‐Ngo et al., 2020).
Tea polyphenols contain powerful antioxidant properties and ameliorate mitochondrial dysfunction in a Bmal1‐dependent manner, playing an important role in the recovery of neurodegenerative diseases (Qi et al., 2017). Evidence has shown that tea polyphenols also improve glucose metabolism (Egbuna et al., 2021) and memory decline in aging models of rats (Song, Zhang, et al., 2021). Another natural product, LBP‐4a prepared from Lycium barbarum, can improve hyperglycemia and insulin resistance by regulating biological rhythms, which are related to the increased expression of Bmal1 in pancreatic islet cells to improve impaired pancreatic islets (Zhao et al., 2016).
6.3. Metformin and D‐Ser2‐oxyntomodulin
In db/db mice, decreased AMP‐activated protein kinase (AMPK) activity, NAMPT expression, and SIRT1 expression in WAT were associated with suppressed expression of CLOCK and Bmal1 (Caton et al., 2011). Metformin treatment restored AMPK levels together with evidently increased expression of NAMPT, SIRT1, CLOCK, and Bmal1, suggesting that metformin may regulate glycolipid metabolism partly through AMPK‐NAMPT‐SIRT1‐mediated alterations in the clock components (Caton et al., 2011). Metformin protects against the principal inwardly rectifying potassium‐conducting channel (Kir4.1) in Müller cells; since, Bmal1 plays an intermediary role in the AMPK‐mediated increase in Kir4.1 protein expression, which makes Bmal1 supposed to be a potential therapeutic target to prevent Müller cell dysfunction in diabetic retinopathy (Alex et al., 2020). Although several findings support the therapeutic potential of metformin for age‐related diseases, including AD (Farr et al., 2019), PD (Mor et al., 2020), AS (Wu et al., 2021), and OA (Feng et al., 2020), involvement of Bmal1 is still poorly understood.
D‐Ser2‐oxyntomodulin (Oxy), a new glucagon‐like peptide‐1 receptor/glucagon receptor (GLP‐1R/GCGR) dual receptor agonist, can rescue Aβ31‐35‐induced circadian rhythm disturbance and increase the expression of Bmal1 (Wang, Zhao, et al., 2020). It has also been illustrated that Oxy acts a part in the improvement of synaptic plasticity, reduction of Aβ, and restoration of phosphatidylinositol‐3‐kinase/protein kinase B/glycogen synthase kinase‐3beta (PI3K/AKT/GSK3β) cell signaling in the hippocampus, and hence, it may be used for the treatment of AD (Wang, Han, et al., 2020).
6.4. Other interventions
Bmal1 gene expression in skeletal muscle was found to elevate after nearly three months of exercise intervention, along with enhanced peripheral insulin sensitivity and reduced BMI and body weight, demonstrating that metabolic benefits from long‐term exercise are partly mediated by the clock molecule Bmal1 (Erickson et al., 2020). A normal chow‐fed, ad libitum, circadian mutant mice (including mice with liver‐specific KO of Bmal1) developed some aspects of metabolic dysfunction that were exacerbated with age, while the time‐restricted feeding (TRF) prevented the whole‐body fat accumulation and serum hyperlipidemia, suggesting that TRF contributes to sustaining metabolic health in the condition of circadian rhythm/molecular clock defects such as Bmal1 deletion (Chaix et al., 2019). Another study found that TRF was related to increased Bmal1 mRNA levels in the liver and improved metabolic health (Regmi et al., 2021). Adiponectin, an adipocyte‐derived hormone, ameliorates the aberrant expression of the Bmal1 mRNA/protein induced by Aβ31‐35 via inhibition of GSK3β activity, and exogenous administration of adiponectin may be a promising treatment for AD (Yuan et al., 2021). NAD+ deficiency in diverse tissues with age contributes to the progress of multiple age‐related diseases such as T2DM, AD, vascular dysfunction (de Picciotto et al., 2016), and cerebral ischemia. Accumulating evidence suggests that supplementation with nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR) to increase NAD+ levels is a promising therapy for diverse aging‐related diseases (Hong et al., 2020). Recently, Zhu et al. found that increased cytosolic NAD+ could restore hypoxic cell proliferation and myofiber formation in Bmal1‐deficient myoblasts (Zhu et al., 2022). Hence, exercise, TRF, adiponectin, and supplementation with NMN and NR might also be promising treatment strategies for aging‐related diseases that are dependent on the modulation of Bmal1.
7. CONCLUSIONS AND FUTURE DIRECTIONS
Mounting evidence supports the existence of a significant interaction between Bmal1 deficiency/abnormality and aging‐related diseases. Some mechanisms related to Bmal1 deficiency in AD and associated aging‐related diseases (Figures 2 and 3) and interventions have been reviewed in the present study. Future studies are required to further understand the physiological intricacies of Bmal1 in the context of aging, with the following main to‐be‐explored aspects: (1) the signaling pathway network of Bmal1 mechanisms in aging‐related diseases needs to be established, (2) several studies have shown that Bmal1 deficiency is involved in the occurrence and development of many other diseases not discussed in this review such as dilated cardiomyopathy (Lefta et al., 2012; Li, Li, et al., 2020) and cancer (Stokes et al., 2021; Wang et al., 2019). Therefore, the relationship of Bmal1 with other key diseases remains to be explored, (3) third, the effects of different Bmal1‐modulating interventions on aging‐related diseases have been analyzed in animal and cell culture model studies. Patients‐oriented studies are lacking, and hence, interventions with sufficient evidence of efficacy should be selected to confirm their therapeutic potential using randomized controlled trials.
ACKNOWLEDGMENTS
Special thanks to Hao Duan for his assistance with some image drawings.
AUTHOR CONTRIBUTIONS
RF and YY drafted the manuscript. XP, LX, KD, DM, WX, XS, SZ, JC, XY, and YY revised the manuscript. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This work was funded by grants from the National Nature Science Foundation of China (Grant number: 81974114) and the Jie Chu Jing Ying foundation (grant number 2018076).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Fan, R. , Peng, X. , Xie, L. , Dong, K. , Ma, D. , Xu, W. , Shi, X. , Zhang, S. , Chen, J. , Yu, X. , & Yang, Y. (2022). Importance of Bmal1 in Alzheimer's disease and associated aging‐related diseases: Mechanisms and interventions. Aging Cell, 21, e13704. 10.1111/acel.13704
DATA AVAILABILITY STATEMENT
No data were created or analyzed in this study. Data sharing is not applicable to this review article.
REFERENCES
- Abdulwahab, D. A. , El‐Missiry, M. A. , Shabana, S. , Othman, A. I. , & Amer, M. E. (2021). Melatonin protects the heart and pancreas by improving glucose homeostasis, oxidative stress, inflammation and apoptosis in T2DM‐induced rats. Heliyon, 7(3), e06474. 10.1016/j.heliyon.2021.e06474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi, R. , Akatsu, Y. , Fisch, K. M. , Alvarez‐Garcia, O. , Teramura, T. , Muramatsu, Y. , Saito, M. , Sasho, T. , Su, A. I. , & Lotz, M. K. (2017). Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF‐β signaling in chondrocytes. Osteoarthritis and Cartilage, 25(6), 943–951. 10.1016/j.joca.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akladious, A. , Azzam, S. , Hu, Y. , & Feng, P. (2018). Bmal1 knockdown suppresses wake and increases immobility without altering orexin A, corticotrophin‐releasing hormone, or glutamate decarboxylase. CNS Neuroscience & Therapeutics, 24(6), 549–563. 10.1111/cns.12815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex, A. , Luo, Q. , Mathew, D. , Di, R. , & Bhatwadekar, A. D. (2020). Metformin corrects abnormal circadian rhythm and Kir4.1 channels in diabetes. Investigative Ophthalmology & Visual Science, 61(6), 46. 10.1167/iovs.61.6.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, A. A. H. , Schwarz‐Herzke, B. , Rollenhagen, A. , Anstötz, M. , Holub, M. , Lübke, J. , Rose, C. R. , Schnittler, H. J. , & von Gall, C. (2020). Bmal1‐deficiency affects glial synaptic coverage of the hippocampal mossy fiber synapse and the actin cytoskeleton in astrocytes. Glia, 68(5), 947–962. 10.1002/glia.23754 [DOI] [PubMed] [Google Scholar]
- Alliston, T. , Hernandez, C. J. , Findlay, D. M. , Felson, D. T. , & Kennedy, O. D. (2018). Bone marrow lesions in osteoarthritis: What lies beneath. Journal of Orthopaedic Research, 36(7), 1818–1825. 10.1002/jor.23844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, H. , Takamura, T. , Matsuzawa‐Nagata, N. , Shima, K. R. , Eto, T. , Misu, H. , Shiramoto, M. , Tsuru, T. , Irie, S. , Fujimura, A. , & Kaneko, S. (2009). Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia, 52(2), 329–335. 10.1007/s00125-008-1194-6 [DOI] [PubMed] [Google Scholar]
- Anea, C. B. , Cheng, B. , Sharma, S. , Kumar, S. , Caldwell, R. W. , Yao, L. , Ali, M. I. , Merloiu, A. M. , Stepp, D. W. , Black, S. M. , Fulton, D. J. , & Rudic, R. D. (2012). Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1‐knockout mice. Circulation Research, 111(9), 1157–1165. 10.1161/CIRCRESAHA.111.261750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca‐Mayo, O. , Pons‐Espinal, M. , Follert, P. , Armirotti, A. , Berdondini, L. , & de Pietri Tonelli, D. (2017). Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nature Communications, 8, 14336. 10.1038/ncomms14336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beker, M. C. , Caglayan, B. , Caglayan, A. B. , Kelestemur, T. , Yalcin, E. , Caglayan, A. , Kilic, E. , Baykal, A. T. , Reiter, R. J. , & Kilic, E. (2019). Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Scientific Reports, 9(1), 19082. 10.1038/s41598-019-55663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beker, M. C. , Caglayan, B. , Yalcin, E. , Caglayan, A. B. , Turkseven, S. , Gurel, B. , Kelestemur, T. , Sertel, E. , Sahin, Z. , Kutlu, S. , Kilic, U. , Baykal, A. T. , & Kilic, E. (2018). Time‐of‐day dependent neuronal injury after ischemic stroke: Implication of circadian clock transcriptional factor Bmal1 and survival kinase AKT. Molecular Neurobiology, 55(3), 2565–2576. 10.1007/s12035-017-0524-4 [DOI] [PubMed] [Google Scholar]
- Bellary, S. , Kyrou, I. , Brown, J. E. , & Bailey, C. J. (2021). Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nature Reviews Endocrinology, 17(9), 534–548. 10.1038/s41574-021-00512-2 [DOI] [PubMed] [Google Scholar]
- Bonkowski, M. S. , & Sinclair, D. A. (2016). Slowing ageing by design: The rise of NAD and sirtuin‐activating compounds. Nature Reviews Molecular Cell Biology, 17(11), 679–690. 10.1038/nrm.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, D. P. , Vuono, R. , Nawarathna, U. , Fisher, K. , Shneerson, J. M. , Reddy, A. B. , & Barker, R. A. (2014). Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurology, 71(5), 589–595. 10.1001/jamaneurol.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger, M. K. , Wilsbacher, L. D. , Moran, S. M. , Clendenin, C. , Radcliffe, L. A. , Hogenesch, J. B. , Simon, M. C. , Takahashi, J. S. , & Bradfield, C. A. (2000). Mop3 is an essential component of the master circadian pacemaker in mammals. Cell, 103(7), 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Y. , Liu, S. , Sothern, R. B. , Xu, S. , & Chan, P. (2010). Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. European Journal of Neurology, 17(4), 550–554. 10.1111/j.1468-1331.2009.02848.x [DOI] [PubMed] [Google Scholar]
- Caton, P. W. , Kieswich, J. , Yaqoob, M. M. , Holness, M. J. , & Sugden, M. C. (2011). Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically‐obese db/db mice. Diabetes, Obesity & Metabolism, 13(12), 1097–1104. 10.1111/j.1463-1326.2011.01466.x [DOI] [PubMed] [Google Scholar]
- Cermakian, N. , Lamont, E. W. , Boudreau, P. , & Boivin, D. B. (2011). Circadian clock gene expression in brain regions of Alzheimer 's disease patients and control subjects. Journal of biological rhythms, 26(2), 160–170. 10.1177/0748730410395732 [DOI] [PubMed] [Google Scholar]
- Chaix, A. , Lin, T. , Le, H. D. , Chang, M. W. , & Panda, S. (2019). Time‐restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metabolism, 29(2), 303–319.e4. 10.1016/j.cmet.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H.‐C. , & Guarente, L. (2013). SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell, 153(7), 1448–1460. 10.1016/j.cell.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H.‐C. , & Guarente, L. (2014). SIRT1 and other sirtuins in metabolism. Trends in Endocrinology and Metabolism, 25(3), 138–145. 10.1016/j.tem.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Peng, X. D. , Huang, C. Q. , Hu, X. Y. , & Zhang, X. M. (2015). Association between ARNTL (BMAL1) rs2278749 polymorphism T>C and susceptibility to Alzheimer disease in a Chinese population. Genetics and molecular research: GMR, 14(4), 18515–18522. 10.4238/2015.December.23.39 [DOI] [PubMed] [Google Scholar]
- Chen, G. , Tang, Q. , Yu, S. , Xie, Y. , Sun, J. , Li, S. , & Chen, L. (2020). The biological function of BMAL1 in skeleton development and disorders. Life Sciences, 253, 117636. 10.1016/j.lfs.2020.117636 [DOI] [PubMed] [Google Scholar]
- Chen, C. , Zhou, M. , Ge, Y. , & Wang, X. (2020). SIRT1 and aging related signaling pathways. Mechanisms of Ageing and Development, 187, 111215. 10.1016/j.mad.2020.111215 [DOI] [PubMed] [Google Scholar]
- Chen, G. , Zhao, H. , Ma, S. , Chen, L. , Wu, G. , Zhu, Y. , Zhu, J. , Ma, C. , & Zhao, H. (2020). Circadian rhythm protein bmal1 modulates cartilage gene expression in temporomandibular joint osteoarthritis the MAPK/ERK pathway. Frontiers in Pharmacology, 11, 527744. 10.3389/fphar.2020.527744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhunchha, B. , Kubo, E. , & Singh, D. P. (2020). Clock protein Bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6. Cell, 9(8), 1861. 10.3390/cells9081861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulka, M. , Brodnanova, M. , Grendar, M. , Necpal, J. , Benetin, J. , Han, V. , Kurca, E. , Nosal, V. , Skorvanek, M. , Vesely, B. , Stanclova, A. , Lasabova, Z. , Pös, Z. , Szemes, T. , Stuchlik, S. , Grofik, M. , & Kolisek, M. (2022). Alzheimer's disease‐associated SNP rs708727 in may increase risk for Parkinson's disease: Report from enlarged Slovak Study. International Journal of Molecular Sciences, 23(3), 1604. 10.3390/ijms23031604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes‐Canteli, M. , & Iadecola, C. (2020). Alzheimer's disease and vascular aging: JACC focus seminar. Journal of the American College of Cardiology, 75(8), 942–951. 10.1016/j.jacc.2019.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallérac, G. , Zapata, J. , & Rouach, N. (2018). Versatile control of synaptic circuits by astrocytes: Where, when and how? Nature Reviews Neuroscience, 19(12), 729–743. 10.1038/s41583-018-0080-6 [DOI] [PubMed] [Google Scholar]
- de Picciotto, N. E. , Gano, L. B. , Johnson, L. C. , Martens, C. R. , Sindler, A. L. , Mills, K. F. , Imai, S. , & Seals, D. R. (2016). Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell, 15(3), 522–530. 10.1111/acel.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Lara, D. L. , González‐Enríquez, G. V. , Torres‐Mendoza, B. M. , González‐Usigli, H. , Cárdenas‐Bedoya, J. , Macías‐Islas, M. A. , de la Rosa, A. C. , Jiménez‐Delgado, A. , Pacheco‐Moisés, F. , Cruz‐Serrano, J. A. , & Ortiz, G. G. (2020). Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson's disease. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 129, 110485. 10.1016/j.biopha.2020.110485 [DOI] [PubMed] [Google Scholar]
- Dudek, M. , Gossan, N. , Yang, N. , Im, H.‐J. , Ruckshanthi, J. P. D. , Yoshitane, H. , Li, X. , Jin, D. , Wang, P. , Boudiffa, M. , Bellantuono, I. , Fukada, Y. , Boot‐Handford, R. P. , & Meng, Q. J. (2016). The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. The Journal of Clinical Investigation, 126(1), 365–376. 10.1172/JCI82755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, M. J. , Prochot, J. R. , Cook, D. H. , Tyler Smith, J. , & Franklin, K. M. (2013). Influence of aging on Bmal1 and Per2 expression in extra‐SCN oscillators in hamster brain. Brain Research, 1491, 44–53. 10.1016/j.brainres.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early, J. O. , Menon, D. , Wyse, C. A. , Cervantes‐Silva, M. P. , Zaslona, Z. , Carroll, R. G. , Palsson‐McDermott, E. , Angiari, S. , Ryan, D. G. , Corcoran, S. E. , Timmons, G. , Geiger, S. S. , Fitzpatrick, D. J. , O'Connell, D. , Xavier, R. J. , Hokamp, K. , O'Neill, L. A. J. , & Curtis, A. M. (2018). Circadian clock protein BMAL1 regulates IL‐1β in macrophages via NRF2. Proceedings of the National Academy of Sciences of the United States of America, 115(36), E8460–E8468. 10.1073/pnas.1800431115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbuna, C. , Awuchi, C. G. , Kushwaha, G. , Rudrapal, M. , Patrick‐Iwuanyanwu, K. C. , Singh, O. , Odoh, U. E. , Khan, J. , Jeevanandam, J. , Kumarasamy, S. , Chukwube, V. O. , Narayanan, M. , Palai, S. , Găman, M. A. , Uche, C. Z. , Ogaji, D. S. , Ezeofor, N. J. , Mtewa, A. G. , Patrick‐Iwuanyanwu, C. C. , … Chikwendu, C. J. (2021). Bioactive compounds effective against type 2 diabetes mellitus: A systematic review. Current Topics in Medicinal Chemistry, 21(12), 1067–1095. 10.2174/1568026621666210509161059 [DOI] [PubMed] [Google Scholar]
- Erickson, M. L. , Zhang, H. , Mey, J. T. , & Kirwan, J. P. (2020). Exercise training impacts skeletal muscle clock machinery in prediabetes. Medicine and Science in Sports and Exercise, 52(10), 2078–2085. 10.1249/MSS.0000000000002368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino, J. , Rodríguez, A. B. , & Pariente, J. A. (2019). Melatonin and oxidative stress in the diabetic state: Clinical implications and potential therapeutic applications. Current Medicinal Chemistry, 26(22), 4178–4190. 10.2174/0929867325666180410094149 [DOI] [PubMed] [Google Scholar]
- Ettcheto, M. , Olloquequi, J. , Sánchez‐López, E. , Busquets, O. , Cano, A. , Manzine, P. R. , Beas‐Zarate, C. , Castro‐Torres, R. D. , García, M. L. , Bulló, M. , Auladell, C. , Folch, J. , & Camins, A. (2019). Benzodiazepines and related drugs as a risk factor in Alzheimer's disease dementia. Frontiers in Aging Neuroscience, 11, 344. 10.3389/fnagi.2019.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, L. , Tang, B.‐S. , Fan, K. , Wan, C.‐M. , Yan, X.‐X. , & Guo, J.‐F. (2018). Alzheimer's disease susceptibility genes modify the risk of Parkinson disease and Parkinson's disease‐associated cognitive impairment. Neuroscience Letters, 677, 55–59. 10.1016/j.neulet.2018.04.042 [DOI] [PubMed] [Google Scholar]
- Farr, S. A. , Roesler, E. , Niehoff, M. L. , Roby, D. A. , McKee, A. , & Morley, J. E. (2019). Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer's disease. Journal of Alzheimer's Disease, 68(4), 1699–1710. 10.3233/JAD-181240 [DOI] [PubMed] [Google Scholar]
- Feng, X. , Pan, J. , Li, J. , Zeng, C. , Qi, W. , Shao, Y. , Liu, X. , Liu, L. , Xiao, G. , Zhang, H. , Bai, X. , & Cai, D. (2020). Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging, 12(2), 1087–1103. 10.18632/aging.102635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado, A. , Astaburuaga, R. , Costa, A. , Duarte, A. C. , Gonçalves, I. , Cipolla‐Neto, J. , Lemos, M. C. , Carro, E. , Relógio, A. , Santos, C. R. A. , & Quintela, T. (2020). The rhythmicity of clock genes is disrupted in the choroid plexus of the APP/PS1 mouse model of Alzheimer's disease. Journal of Alzheimer's Disease, 77(2), 795–806. 10.3233/JAD-200331 [DOI] [PubMed] [Google Scholar]
- Gomes, B. A. Q. , Silva, J. P. B. , Romeiro, C. F. R. , dos Santos, S. , Rodrigues, C. A. , Gonçalves, P. R. , Sakai, J. T. , Mendes, P. F. S. , Varela, E. L. P. , & Monteiro, M. C. (2018). Neuroprotective mechanisms of resveratrol in Alzheimer's disease: Role of SIRT1. Oxidative Medicine and Cellular Longevity, 2018, 8152373. 10.1155/2018/8152373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Benito, M. , Granado, N. , García‐Sanz, P. , Michel, A. , Dumoulin, M. , & Moratalla, R. (2020). Modeling Parkinson's disease with the alpha‐synuclein protein. Frontiers in Pharmacology, 11, 356. 10.3389/fphar.2020.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunton, J. E. , Kulkarni, R. N. , Yim, S. , Okada, T. , Hawthorne, W. J. , Tseng, Y.‐H. , Roberson, R. S. , Ricordi, C. , O'Connell, P. J. , Gonzalez, F. J. , & Kahn, C. R. (2005). Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic‐islet dysfunction in human type 2 diabetes. Cell, 122(3), 337–349. [DOI] [PubMed] [Google Scholar]
- Hadjipanayi, E. , & Schilling, A. F. (2013). Hypoxia‐based strategies for angiogenic induction: The dawn of a new era for ischemia therapy and tissue regeneration. Organogenesis, 9(4), 261–272. 10.4161/org.25970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, B. , Nohara, K. , Park, N. , Park, Y.‐S. , Guillory, B. , Zhao, Z. , Garcia, J. M. , Koike, N. , Lee, C. C. , Takahashi, J. S. , Yoo, S. H. , & Chen, Z. (2016). The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metabolism, 23(4), 610–621. 10.1016/j.cmet.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, W. , Mo, F. , Zhang, Z. , Huang, M. , & Wei, X. (2020). Nicotinamide mononucleotide: A promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Frontiers in Cell and Developmental Biology, 8, 246. 10.3389/fcell.2020.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh, A. , Kamrava, S. K. , Joghataei, M. T. , Darabi, R. , Shakeri‐Zadeh, A. , Shahriari, M. , Reiter, R. J. , Ghaznavi, H. , & Mehrzadi, S. (2016). Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. Journal of Pineal Research, 61(4), 411–425. 10.1111/jpi.12362 [DOI] [PubMed] [Google Scholar]
- Hou, Q. , Zhang, S. , Li, Y. , Wang, H. , Zhang, D. , Qi, D. , Li, Y. , & Jiang, H. (2021). New insights on association between circadian rhythm and lipid metabolism in spontaneously hypertensive rats. Life Sciences, 271, 119145. 10.1016/j.lfs.2021.119145 [DOI] [PubMed] [Google Scholar]
- Hou, Y. , Wei, Y. , Lautrup, S. , Yang, B. , Wang, Y. , Cordonnier, S. , Mattson, M. P. , Croteau, D. L. , & Bohr, V. A. (2021). NAD supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer's disease via cGAS‐STING. Proceedings of the National Academy of Sciences of the United States of America, 118(37), e2011226118. 10.1073/pnas.2011226118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Peng, X. , Fan, R. , Dong, K. , Shi, X. , Zhang, S. , Yu, X. , & Yang, Y. (2021). Disruption of circadian clocks promotes progression of Alzheimer's disease in diabetic mice. Molecular Neurobiology, 58(9), 4404–4412. 10.1007/s12035-021-02425-7 [DOI] [PubMed] [Google Scholar]
- Hudson, N. , Celkova, L. , Hopkins, A. , Greene, C. , Storti, F. , Ozaki, E. , Fahey, E. , Theodoropoulou, S. , Kenna, P. F. , Humphries, M. M. , Curtis, A. M. , Demmons, E. , Browne, A. , Liddie, S. , Lawrence, M. S. , Grimm, C. , Cahill, M. T. , Humphries, P. , Doyle, S. L. , & Campbell, M. (2019). Dysregulated claudin‐5 cycling in the inner retina causes retinal pigment epithelial cell atrophy. JCI Insight, 4(15), e130273. 10.1172/jci.insight.130273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme, B. , Didikoglu, A. , Bradburn, S. , Robinson, A. , Canal, M. , Payton, A. , Pendleton, N. , & Murgatroyd, C. (2020). Epigenetic regulation of BMAL1 with sleep disturbances and Alzheimer's disease. Journal of Alzheimer's Disease, 77(4), 1783–1792. 10.3233/JAD-200634 [DOI] [PubMed] [Google Scholar]
- Huo, M. , Cao, X. , Zhang, H. , Lau, C. W. , Hong, H. , Chen, F. M. , Huang, Y. , Chawla, A. , & Tian, X. Y. (2021). Loss of myeloid Bmal1 exacerbates hypertensive vascular remodelling through interaction with STAT6 in mice. Cardiovascular Research, cvab336. 10.1093/cvr/cvab336 [DOI] [PubMed] [Google Scholar]
- Huo, M. , Huang, Y. , Qu, D. , Zhang, H. , Wong, W. T. , Chawla, A. , Huang, Y. , & Tian, X. Y. (2017). Myeloid deletion increases monocyte recruitment and worsens atherosclerosis. FASEB Journal, 31(3), 1097–1106. 10.1096/fj.201601030R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno, T. , & Nelson, R. J. (2015). Acute melatonin treatment alters dendritic morphology and circadian clock gene expression in the hippocampus of Siberian hamsters. Hippocampus, 25(2), 142–148. 10.1002/hipo.22358 [DOI] [PubMed] [Google Scholar]
- Innes, K. E. , & Sambamoorthi, U. (2020). The association of osteoarthritis and related pain burden to incident Alzheimer's disease and related dementias: A retrospective cohort study of U.S. medicare beneficiaries. Journal of Alzheimer's Disease, 75(3), 789–805. 10.3233/JAD-191311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, W. M. , Wilson‐Delfosse, A. L. , & Mieyal, J. J. (2012). Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients, 4(10), 1399–1440. 10.3390/nu4101399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien, C. , Tremblay, C. , Emond, V. , Lebbadi, M. , Salem, N. , Bennett, D. A. , & Calon, F. (2009). Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 68(1), 48–58. 10.1097/NEN.0b013e3181922348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, Y. K. , Kan, T. , & Ishikawa, T. (2020). Citrus flavonoids as a target for the prevention of pancreatic β‐cells dysfunction in diabetes. Nihon yakurigaku zasshi, 155(4), 209–213. 10.1254/fpj.20024 [DOI] [PubMed] [Google Scholar]
- Kao, S.‐L. , Wang, J.‐H. , Chen, S.‐C. , Li, Y.‐Y. , Yang, Y.‐L. , & Lo, R. Y. (2021). Impact of comorbidity burden on cognitive decline: A prospective cohort study of older adults with dementia. Dementia and Geriatric Cognitive Disorders, 50(1), 43–50. 10.1159/000514651 [DOI] [PubMed] [Google Scholar]
- Kim, E. , Nohara, K. , Wirianto, M. , Escobedo, G., Jr. , Lim, J. Y. , Morales, R. , Yoo, S. H. , & Chen, Z. (2021). Effects of the clock modulator nobiletin on circadian rhythms and pathophysiology in female mice of an Alzheimer's disease model. Biomolecules, 11(7), 1004. 10.3390/biom11071004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov, R. V. , Kondratova, A. A. , Gorbacheva, V. Y. , Vykhovanets, O. V. , & Antoch, M. P. (2006). Early aging and age‐related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes & Development, 20(14), 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov, R. V. , Vykhovanets, O. , Kondratova, A. A. , & Antoch, M. P. (2009). Antioxidant N‐acetyl‐L‐cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging, 1(12), 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, J.‐H. , Kang, E.‐B. , Oh, Y.‐S. , Yang, D.‐S. , & Cho, J.‐Y. (2017). Treadmill exercise decreases amyloid‐β burden possibly via activation of SIRT‐1 signaling in a mouse model of Alzheimer's disease. Experimental Neurology, 288, 142–152. 10.1016/j.expneurol.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Kress, G. J. , Liao, F. , Dimitry, J. , Cedeno, M. R. , FitzGerald, G. A. , Holtzman, D. M. , & Musiek, E. S. (2018). Regulation of amyloid‐β dynamics and pathology by the circadian clock. The Journal of Experimental Medicine, 215(4), 1059–1068. 10.1084/jem.20172347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lananna, B. V. , Nadarajah, C. J. , Izumo, M. , Cedeño, M. R. , Xiong, D. D. , Dimitry, J. , Tso, C. F. , McKee, C. , Griffin, P. , Sheehan, P. W. , Haspel, J. A. , Barres, B. A. , Liddelow, S. A. , Takahashi, J. S. , Karatsoreos, I. N. , & Musiek, E. S. (2018). Cell‐autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Reports, 25(1), 1–9.e5. 10.1016/j.celrep.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrup, S. , Sinclair, D. A. , Mattson, M. P. , & Fang, E. F. (2019). NAD in brain aging and neurodegenerative disorders. Cell Metabolism, 30(4), 630–655. 10.1016/j.cmet.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Kim, D. E. , Griffin, P. , Sheehan, P. W. , Kim, D.‐H. , Musiek, E. S. , & Yoon, S.‐Y. (2020). Inhibition of REV‐ERBs stimulates microglial amyloid‐beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer's disease. Aging Cell, 19(2), e13078. 10.1111/acel.13078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Ma, K. , Moulik, M. , & Yechoor, V. (2018). Untimely oxidative stress in β‐cells leads to diabetes—Role of circadian clock in β‐cell function. Free Radical Biology & Medicine, 119, 69–74. 10.1016/j.freeradbiomed.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Moulik, M. , Fang, Z. , Saha, P. , Zou, F. , Xu, Y. , Nelson, D. L. , Ma, K. , Moore, D. D. , & Yechoor, V. K. (2013). Bmal1 and β‐cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress‐induced β‐cell failure in mice. Molecular and Cellular Biology, 33(11), 2327–2338. 10.1128/MCB.01421-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Nam, H. G. , & Kim, Y. (2021). The core circadian component, Bmal1, is maintained in the pineal gland of old killifish brain. iScience, 24(1), 101905. 10.1016/j.isci.2020.101905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefta, M. , Campbell, K. S. , Feng, H.‐Z. , Jin, J.‐P. , & Esser, K. A. (2012). Development of dilated cardiomyopathy in Bmal1‐deficient mice. American Journal of Physiology. Heart and Circulatory Physiology, 303(4), H475–H485. 10.1152/ajpheart.00238.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, E. , Li, X. , Huang, J. , Xu, C. , Liang, Q. , Ren, K. , Bai, A. , Lu, C. , Qian, R. , & Sun, N. (2020). BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein & Cell, 11(9), 661–679. 10.1007/s13238-020-00713-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Song, S. , Wang, Y. , Huang, C. , Zhang, F. , Liu, J. , & Hong, J.‐S. (2019). Low‐grade inflammation aggravates rotenone neurotoxicity and disrupts circadian clock gene expression in rats. Neurotoxicity Research, 35(2), 421–431. 10.1007/s12640-018-9968-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Cheng, C. , Jia, C. , Leng, Y. , Qian, J. , Yu, H. , Liu, Y. , Wang, N. , Yang, Y. , al‐Nusaif, M. , & Le, W. (2021). Peripheral clock system abnormalities in patients with Parkinson's disease. Frontiers in Aging Neuroscience, 13, 736026. 10.3389/fnagi.2021.736026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Liu, N. , Wang, Y. , Liu, J. , Shi, H. , Qu, Z. , du, T. , Guo, B. , & Gu, B. (2017). Brain and muscle aryl hydrocarbon receptor nuclear translocator‐like protein‐1 cooperates with glycogen synthase kinase‐3β to regulate osteogenesis of bone‐marrow mesenchymal stem cells in type 2 diabetes. Molecular and Cellular Endocrinology, 440, 93–105. 10.1016/j.mce.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zhang, J. , Wan, J. , Liu, A. , & Sun, J. (2020). Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer's disease. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 132, 110887. 10.1016/j.biopha.2020.110887 [DOI] [PubMed] [Google Scholar]
- Liang, S. , Hu, J. , Zhang, A. , Li, F. , & Li, X. (2020). miR‐155 induces endothelial cell apoptosis and inflammatory response in atherosclerosis by regulating Bmal1. Experimental and Therapeutic Medicine, 20(6), 128. 10.3892/etm.2020.9259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Cao, Q. , Gao, W. , Li, B.‐Y. , Zeng, C. , Xia, Z. , & Zhao, B. (2021). Melatonin ameliorates cerebral ischemia‐reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1‐BMAL1 pathway. FASEB Journal, 35(12), e22040. 10.1096/fj.202002718RR [DOI] [PubMed] [Google Scholar]
- Liu, W.‐W. , Wei, S.‐Z. , Huang, G.‐D. , Liu, L.‐B. , Gu, C. , Shen, Y. , Wang, X. H. , Xia, S. T. , Xie, A. M. , Hu, L. F. , Wang, F. , & Liu, C. F. (2020). BMAL1 regulation of microglia‐mediated neuroinflammation in MPTP‐induced Parkinson's disease mouse model. FASEB Journal, 34(5), 6570–6581. 10.1096/fj.201901565RR [DOI] [PubMed] [Google Scholar]
- Liu, X. , Xiao, W. , Jiang, Y. , Zou, L. , Chen, F. , Xiao, W. , Zhang, X. , Cao, Y. , Xu, L. , & Zhu, Y. (2021). Bmal1 regulates the redox rhythm of HSPB1, and homooxidized HSPB1 attenuates the oxidative stress injury of cardiomyocytes. Oxidative Medicine and Cellular Longevity, 2021, 5542815. 10.1155/2021/5542815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Wu, G. , Wang, Z. , Wang, P. , Wu, L. , Zhu, G. , & Zhao, H. (2014). Effects of chronic sleep deprivation on the extracellular signal‐regulated kinase pathway in the temporomandibular joint of rats. PLoS One, 9(9), e107544. 10.1371/journal.pone.0107544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z. , Jin, X. , Qian, Z. , Li, F. , Xu, M. , Zhang, Y. , Kang, X. , Li, H. , Gao, X. , Zhao, L. , Zhang, Z. , Zhang, Y. , Wu, S. , & Sun, H. (2019). Deletion of clock gene Bmal1 impaired the chondrocyte function due to disruption of the HIF1α‐VEGF signaling pathway. Cell Cycle, 18(13), 1473–1489. 10.1080/15384101.2019.1620572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoob, M. , & Stochaj, U. (2021). Curcumin nanoformulations to combat aging‐related diseases. Ageing Research Reviews, 69, 101364. 10.1016/j.arr.2021.101364 [DOI] [PubMed] [Google Scholar]
- Majumdar, T. , Dhar, J. , Patel, S. , Kondratov, R. , & Barik, S. (2017). Circadian transcription factor BMAL1 regulates innate immunity against select RNA viruses. Innate Immunity, 23(2), 147–154. 10.1177/1753425916681075 [DOI] [PubMed] [Google Scholar]
- Mannic, T. , Meyer, P. , Triponez, F. , Pusztaszeri, M. , le Martelot, G. , Mariani, O. , Schmitter, D. , Sage, D. , Philippe, J. , & Dibner, C. (2013). Circadian clock characteristics are altered in human thyroid malignant nodules. The Journal of Clinical Endocrinology and Metabolism, 98(11), 4446–4456. 10.1210/jc.2013-2568 [DOI] [PubMed] [Google Scholar]
- Marcheva, B. , Ramsey, K. M. , Buhr, E. D. , Kobayashi, Y. , Su, H. , Ko, C. H. , Ivanova, G. , Omura, C. , Mo, S. , Vitaterna, M. H. , Lopez, J. P. , Philipson, L. H. , Bradfield, C. A. , Crosby, S. D. , JeBailey, L. , Wang, X. , Takahashi, J. S. , & Bass, J. (2010). Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature, 466(7306), 627–631. 10.1038/nature09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas‐Bargues, C. , Alique, M. , Barrús‐Ortiz, M. T. , Borrás, C. , & Rodrigues‐Díez, R. (2022). Special issue “Oxidative stress in aging and associated chronic diseases”. Antioxidants, 11(4), 701. 10.3390/antiox11040701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, K. A. , Xu, W. , Gaglioti, A. H. , Holt, J. B. , Croft, J. B. , Mack, D. , & McGuire, L. C. (2019). Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimer's & Dementia, 15(1), 17–24. 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis, J. , & Sehgal, A. (2016). Circadian rhythms, sleep, and disorders of aging. Trends in Endocrinology and Metabolism, 27(4), 192–203. 10.1016/j.tem.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee, C. A. , Lee, J. , Cai, Y. , Saito, T. , Saido, T. , & Musiek, E. S. (2022). Astrocytes deficient in circadian clock gene Bmal1 show enhanced activation responses to amyloid‐beta pathology without changing plaque burden. Scientific Reports, 12(1), 1796. 10.1038/s41598-022-05862-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, P. , Liew, G. , Gopinath, B. , & Wong, T. Y. (2018). Age‐related macular degeneration. Lancet, 392(10153), 1147–1159. 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- Mor, D. E. , Sohrabi, S. , Kaletsky, R. , Keyes, W. , Tartici, A. , Kalia, V. , Miller, G. W. , & Murphy, C. T. (2020). Metformin rescues Parkinson's disease phenotypes caused by hyperactive mitochondria. Proceedings of the National Academy of Sciences of the United States of America, 117(42), 26438–26447. 10.1073/pnas.2009838117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek, E. S. , & Holtzman, D. M. (2016). Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science, 354(6315), 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek, E. S. , Lim, M. M. , Yang, G. , Bauer, A. Q. , Qi, L. , Lee, Y. , Roh, J. H. , Ortiz‐Gonzalez, X. , Dearborn, J. T. , Culver, J. P. , Herzog, E. D. , Hogenesch, J. B. , Wozniak, D. F. , Dikranian, K. , Giasson, B. I. , Weaver, D. R. , Holtzman, D. M. , & Fitzgerald, G. A. (2013). Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. The Journal of Clinical Investigation, 123(12), 5389–5400. 10.1172/JCI70317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, A. , & Ohizumi, Y. (2019). Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer's disease and Parkinson's disease. International Journal of Molecular Sciences, 20(14), 3380. 10.3390/ijms20143380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata, M. , Kumari, P. , Kita, R. , Katsui, N. , Takeuchi, Y. , Kawaguchi, T. , Yamazaki, T. , Zhang, B. , Shimba, S. , & Yada, T. (2021). Circadian clock component BMAL1 in the paraventricular nucleus regulates glucose metabolism. Nutrients, 13(12), 4487. 10.3390/nu13124487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato, R. , Kawabe, K. , Yamada, D. , Ikeno, S. , Mieda, M. , Shimba, S. , Hinoi, E. , Yoneda, Y. , & Takarada, T. (2017). Disruption of Bmal1 impairs blood‐brain barrier integrity via pericyte dysfunction. The Journal of Neuroscience, 37(42), 10052–10062. 10.1523/JNEUROSCI.3639-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen‐Ngo, C. , Salomon, C. , Quak, S. , Lai, A. , Willcox, J. C. , & Lappas, M. (2020). Nobiletin exerts anti‐diabetic and anti‐inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clinical Science, 134(6), 571–592. 10.1042/CS20191099 [DOI] [PubMed] [Google Scholar]
- Niu, L. , Zhang, F. , Xu, X. , Yang, Y. , Li, S. , Liu, H. , & Le, W. (2021). Chronic sleep deprivation altered the expression of circadian clock genes and aggravated Alzheimer's disease neuropathology. Brain Pathology, 32(3), e13028. 10.1111/bpa.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X. , Bradfield, C. A. , & Hussain, M. M. (2016). Global and hepatocyte‐specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nature Communications, 7, 13011. 10.1038/ncomms13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa, K. I. , Gazouli, M. , Anastasiou, E. , Iliodromiti, Z. , Antsaklis, A. , & Anagnou, N. P. (2013). The major circadian pacemaker ARNT‐like protein‐1 (BMAL1) is associated with susceptibility to gestational diabetes mellitus. Diabetes Research and Clinical Practice, 99(2), 151–157. 10.1016/j.diabres.2012.10.015 [DOI] [PubMed] [Google Scholar]
- Peng, X. , Fan, R. , Xie, L. , Shi, X. , Dong, K. , Zhang, S. , Tao, J. , Xu, W. , Ma, D. , Chen, J. , & Yang, Y. (2022). A growing link between circadian rhythms, type 2 diabetes mellitus and Alzheimer's disease. International Journal of Molecular Sciences, 23(1), 504. 10.3390/ijms23010504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschke, E. , Wolgast, S. , Bazwinsky, I. , Pönicke, K. , & Muhlbauer, E. (2008). Increased melatonin synthesis in pineal glands of rats in streptozotocin induced type 1 diabetes. Journal of Pineal Research, 45(4), 439–448. 10.1111/j.1600-079X.2008.00612.x [DOI] [PubMed] [Google Scholar]
- Qi, G. , Mi, Y. , Fan, R. , Zhao, B. , Ren, B. , & Liu, X. (2017). Tea polyphenols ameliorates neural redox imbalance and mitochondrial dysfunction via mechanisms linking the key circadian regular Bmal1. Food and Chemical Toxicology, 110, 189–199. 10.1016/j.fct.2017.10.031 [DOI] [PubMed] [Google Scholar]
- Qiu, P. , Jiang, J. , Liu, Z. , Cai, Y. , Huang, T. , Wang, Y. , Liu, Q. , Nie, Y. , Liu, F. , Cheng, J. , Li, Q. , Tang, Y. C. , Poo, M. M. , Sun, Q. , & Chang, H. C. (2019). BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. National Science Review, 6(1), 87–100. 10.1093/nsr/nwz002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada‐Fernández, P. , Trujillo‐‐Quiros, J. , Pascoe‐González, S. , Trujillo‐Rangel, W. A. , Cardona‐Müller, D. , Ramos‐Becerra, C. G. , Barocio‐Pantoja, M. , Rodríguez‐de la Cerda, M. , Nérida Sánchez‐Rodríguez, E. , Cardona‐Muñóz, E. G. , García‐Benavides, L. , & Grover‐Páez, F. (2019). Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: a randomised, double‐blind, placebo‐controlled trial. Int J Food Sci Nutr, 70(8), 977–985. 10.1080/09637486.2019 [DOI] [PubMed] [Google Scholar]
- Rakshit, K. , Hsu, T. W. , & Matveyenko, A. V. (2016). Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet‐induced obesity in mice. Diabetologia, 59(4), 734–743. 10.1007/s00125-015-3859-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit, K. , & Matveyenko, A. V. (2021). Induction of core circadian clock transcription factor bmal1 enhances β‐cell function and protects against obesity‐induced glucose intolerance. Diabetes, 70(1), 143–154. 10.2337/db20-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit, K. , Qian, J. , Gaonkar, K. S. , Dhawan, S. , Colwell, C. S. , & Matveyenko, A. V. (2018). Postnatal ontogenesis of the islet circadian clock plays a contributory role in β‐cell maturation process. Diabetes, 67(5), 911–922. 10.2337/db17-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey, K. M. , Yoshino, J. , Brace, C. S. , Abrassart, D. , Kobayashi, Y. , Marcheva, B. , Hong, H. K. , Chong, J. L. , Buhr, E. D. , Lee, C. , Takahashi, J. S. , Imai, S. , & Bass, J. (2009). Circadian clock feedback cycle through NAMPT‐mediated NAD+ biosynthesis. Science, 324(5927), 651–654. 10.1126/science.1171641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S. , Valekunja, U. K. , Stangherlin, A. , Howell, S. A. , Snijders, A. P. , Damodaran, G. , & Reddy, A. B. (2020). Circadian rhythms in the absence of the clock gene. Science, 367(6479), 800–806. 10.1126/science.aaw7365 [DOI] [PubMed] [Google Scholar]
- Regmi, P. , Chaudhary, R. , Page, A. J. , Hutchison, A. T. , Vincent, A. D. , Liu, B. , & Heilbronn, L. (2021). Early or delayed time‐restricted feeding prevents metabolic impact of obesity in mice. The Journal of Endocrinology, 248(1), 75–86. 10.1530/JOE-20-0404 [DOI] [PubMed] [Google Scholar]
- Reichenbach, N. , Delekate, A. , Plescher, M. , Schmitt, F. , Krauss, S. , Blank, N. , Halle, A. , & Petzold, G. C. (2019). Inhibition of Stat3‐mediated astrogliosis ameliorates pathology in an Alzheimer's disease model. EMBO Molecular Medicine, 11(2), e9665. 10.15252/emmm.201809665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke, H. , & Asher, G. (2019). Crosstalk between metabolism and circadian clocks. Nature Reviews Molecular Cell Biology, 20(4), 227–241. 10.1038/s41580-018-0096-9 [DOI] [PubMed] [Google Scholar]
- Richards, J. , & Gumz, M. L. (2013). Mechanism of the circadian clock in physiology. American Journal of Physiology Regulatory, Integrative and Comparative Physiology, 304(12), R1053–R1064. 10.1152/ajpregu.00066.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens, P. , Blennow, K. , Breteler, M. M. , de Strooper, B. , Frisoni, G. B. , Salloway, S. , & van der Flier, W. M. (2016). Alzheimer's disease. Lancet, 388(10043), 505–517. 10.1016/s0140-6736(15)01124-1 [DOI] [PubMed] [Google Scholar]
- Shi, S.‐q. , Ansari, T. S. , McGuinness, O. P. , Wasserman, D. H. , & Johnson, C. H. (2013). Circadian disruption leads to insulin resistance and obesity. Current Biology, 23(5), 372–381. 10.1016/j.cub.2013.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, Y.‐H. , Wu, S.‐Y. , Yu, M. , Huang, S.‐H. , Lee, C.‐W. , Jiang, M.‐J. , & Kuo, Y.‐M. (2018). Hypertension accelerates Alzheimer's disease‐related pathologies in pigs and 3xTg mice. Frontiers in Aging Neuroscience, 10, 73. 10.3389/fnagi.2018.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C. , Zhang, Y. , Cheng, L. , Shi, M. , Li, X. , Zhang, L. , & Zhao, H. (2021). Tea polyphenols ameliorates memory decline in aging model rats by inhibiting brain TLR4/NF‐κB inflammatory signaling pathway caused by intestinal flora dysbiosis. Experimental Gerontology, 153, 111476. 10.1016/j.exger.2021.111476 [DOI] [PubMed] [Google Scholar]
- Song, H. , Moon, M. , Choe, H. K. , Han, D.‐H. , Jang, C. , Kim, A. , Cho, S. , Kim, K. , & Mook‐Jung, I. (2015). Aβ‐induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer's disease. Molecular Neurodegeneration, 10, 13. 10.1186/s13024-015-0007-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X. , Ma, T. , Hu, H. , Zhao, M. , Bai, H. , Wang, X. , Liu, L. , Li, T. , Sheng, X. , Xu, X. , Zhang, X. , & Gao, L. (2021). Chronic Circadian rhythm disturbance accelerates knee cartilage degeneration in rats accompanied by the activation of the canonical Wnt/β‐catenin signaling pathway. Frontiers in Pharmacology, 12, 760988. 10.3389/fphar.2021.760988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, A. (2020). The diagnosis and treatment of age‐related macular degeneration. Deutsches Arzteblatt International, 117(29–30), 513–520. 10.3238/arztebl.2020.0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvers, D. J. , Scheer, F. A. J. L. , Schrauwen, P. , la Fleur, S. E. , & Kalsbeek, A. (2019). Circadian clocks and insulin resistance. Nature Reviews Endocrinology, 15(2), 75–89. 10.1038/s41574-018-0122-1 [DOI] [PubMed] [Google Scholar]
- Stokes, K. , Nunes, M. , Trombley, C. , Flôres, D. E. F. L. , Wu, G. , Taleb, Z. , Alkhateeb, A. , Banskota, S. , Harris, C. , Love, O. P. , Khan, W. I. , Rueda, L. , Hogenesch, J. B. , & Karpowicz, P. (2021). The circadian clock gene, Bmal1, regulates intestinal stem cell signaling and represses tumor initiation. Cellular and Molecular Gastroenterology and Hepatology, 12(5), 1847–1872.e0. 10.1016/j.jcmgh.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, W. , Guo, Z. , Randall, D. C. , Cassis, L. , Brown, D. R. , & Gong, M. C. (2008). Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. American Journal of Physiology ‐ Heart and Circulatory Physiology, 295(4), H1634–H1641. 10.1152/ajpheart.00257.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, D.‐X. , Manchester, L. C. , Esteban‐Zubero, E. , Zhou, Z. , & Reiter, R. J. (2015). Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules, 20(10), 18886–18906. 10.3390/molecules201018886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharmalingam, S. , Khurana, S. , Murray, A. , Lamothe, J. , & Tai, T. C. (2020). Whole transcriptome analysis of adrenal glands from prenatal glucocorticoid programmed hypertensive rodents. Scientific Reports, 10(1), 18755. 10.1038/s41598-020-75652-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan, M. , & Reddy, P. H. (2016). Stroke, vascular dementia, and Alzheimer's disease: Molecular links. Journal of Alzheimer's Disease, 54(2), 427–443. 10.3233/JAD-160527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Li, S. , Li, X. , Li, B. , Li, Y. , Xia, K. , Yang, Y. , Aman, S. , Wang, M. , & Wu, H. (2019). Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up‐regulating matrix metalloproteinase9 expression. Cancer Cell International, 19, 182. 10.1186/s12935-019-0902-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.‐H. , Wu, Y.‐J. , Tee, B. L. , & Lo, R. Y. (2018). Medical comorbidity in Alzheimer's disease: A nested case‐control study. Journal of Alzheimer's Disease, 63(2), 773–781. 10.3233/JAD-170786 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Zhao, J. , Wang, C.‐T. , Hou, X.‐H. , Ning, N. , Sun, C. , Guo, S. , Yuan, Y. , Li, L. , Hölscher, C. , & Wang, X. H. (2020). D‐Ser2‐oxyntomodulin ameliorated Aβ31‐35‐induced circadian rhythm disorder in mice. CNS Neuroscience & Therapeutics, 26(3), 343–354. 10.1111/cns.13211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.‐L. , Kooijman, S. , Gao, Y. , Tzeplaeff, L. , Cosquer, B. , Milanova, I. , Wolff, S. E. C. , Korpel, N. , Champy, M. F. , Petit‐Demoulière, B. , Goncalves da Cruz, I. , Sorg‐Guss, T. , Rensen, P. C. N. , Cassel, J. C. , Kalsbeek, A. , Boutillier, A. L. , & Yi, C. X. (2021). Microglia‐specific knock‐down of Bmal1 improves memory and protects mice from high fat diet‐induced obesity. Molecular Psychiatry, 26(11), 6336–6349. 10.1038/s41380-021-01169-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Lv, D. , Liu, W. , Li, S. , Chen, J. , Shen, Y. , Wang, F. , Hu, L. F. , & Liu, C. F. (2018). Disruption of the circadian clock alters antioxidative defense via the SIRT1‐BMAL1 pathway in 6‐OHDA‐induced models of Parkinson's disease. Oxidative Medicine and Cellular Longevity, 2018, 4854732. 10.1155/2018/4854732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.‐J. , Han, Y.‐F. , Zhao, F. , Yang, G.‐Z. , Yuan, L. , Cai, H.‐Y. , Yang, J. T. , Holscher, C. , Qi, J. S. , & Wu, M. N. (2020). A dual GLP‐1 and Gcg receptor agonist rescues spatial memory and synaptic plasticity in APP/PS1 transgenic mice. Hormones and Behavior, 118, 104640. 10.1016/j.yhbeh.2019.104640 [DOI] [PubMed] [Google Scholar]
- Welz, P.‐S. , Zinna, V. M. , Symeonidi, A. , Koronowski, K. B. , Kinouchi, K. , Smith, J. G. , Guillén, I. M. , Castellanos, A. , Furrow, S. , Aragón, F. , Crainiciuc, G. , Prats, N. , Caballero, J. M. , Hidalgo, A. , Sassone‐Corsi, P. , & Benitah, S. A. (2019). BMAL1‐driven tissue clocks respond independently to light to maintain homeostasis. Cell, 177(6), 1436–1447.e12. 10.1016/j.cell.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, L.‐Y. , Wan, L. , Lai, J.‐N. , Chen, C. S. , Chen, J. J.‐Y. , Wu, M.‐Y. , Hu, K. C. , Chiu, L. T. , Tien, P. T. , & Lin, H. J. (2021). Increased risk of Alzheimer's disease among patients with age‐related macular degeneration: A nationwide population‐based study. PLoS One, 16(5), e0250440. 10.1371/journal.pone.0250440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon, P. Y. , Kaisaki, P. J. , Bragança, J. , Bihoreau, M.‐T. , Levy, J. C. , Farrall, M. , & Gauguier, D. (2007). Aryl hydrocarbon receptor nuclear translocator‐like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proceedings of the National Academy of Sciences of the United States of America, 104(36), 14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Chen, L. , Zeb, F. , Li, C. , Jiang, P. , Chen, A. , Xu, C. , Haq, I. U. , & Feng, Q. (2019). Clock‐Bmal1 mediates MMP9 induction in acrolein‐promoted atherosclerosis associated with gut microbiota regulation. Environ Pollut, 252(Pt B), 1455–1463. 10.1016/j.envpol.2019.06.042 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Feng, K. , Zhang, C. , Zhang, H. , Zhang, J. , Hua, Y. , Dong, Z. , Zhu, Y. , Yang, S. , & Ma, C. (2021). Metformin attenuates atherosclerosis and plaque vulnerability by upregulating KLF2‐mediated autophagy in apoE mice. Biochemical and Biophysical Research Communications, 557, 334–341. 10.1016/j.bbrc.2021.04.029 [DOI] [PubMed] [Google Scholar]
- Xie, B. , Shi, X. , Xing, Y. , & Tang, Y. (2020). Association between atherosclerosis and Alzheimer's disease: A systematic review and meta‐analysis. Brain and Behavior, 10(4), e01601. 10.1002/brb3.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, M. , Tang, Q. , Nie, J. , Zhang, C. , Zhou, X. , Yu, S. , Sun, J. , Cheng, X. , Dong, N. , Hu, Y. , & Chen, L. (2020). BMAL1‐downregulation aggravates ‐induced atherosclerosis by encouraging oxidative stress. Circulation Research, 126(6), e15–e29. 10.1161/CIRCRESAHA.119.315502 [DOI] [PubMed] [Google Scholar]
- Xu, L. , Liu, Y. , Cheng, Q. , Shen, Y. , Yuan, Y. , Jiang, X. , Li, X. , Guo, D. , Jiang, J. , & Lin, C. (2021). Bmal1 downregulation worsens critical limb ischemia by promoting inflammation and impairing angiogenesis. Frontiers in Cardiovascular Medicine, 8, 712903. 10.3389/fcvm.2021.712903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Wan, Y. , Wu, N. , Song, L. , Liu, Z. , Zhao, J. , Liu, Y. , Liu, Z. , & Gan, J. (2021). L‐3,4‐dihydroxyphenylalanine recovers circadian rhythm disturbances in the rat models of Parkinson's disease by regulating the D1R‐ERK1/2‐mTOR pathway. Frontiers in Aging Neuroscience, 13, 719885. 10.3389/fnagi.2021.719885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Kang, X. , Liu, J. , Li, H. , Ma, Z. , Jin, X. , Qian, Z. , Xie, T. , Qin, N. , Feng, D. , Pan, W. , Chen, Q. , Sun, H. , & Wu, S. (2016). Clock gene Bmal1 modulates human cartilage gene expression by crosstalk with Sirt1. Endocrinology, 157(8), 3096–3107. 10.1210/en.2015-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaribeygi, H. , Sathyapalan, T. , Atkin, S. L. , & Sahebkar, A. (2020). Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Medicine and Cellular Longevity, 2020, 8609213. 10.1155/2020/8609213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, L. , Wu, H. , & Xu, W. (2020). Deletion of Bmal1 impairs pancreatic ‐cell function via mitochondrial signaling pathway. BioMed Research International, 2020, 9803024. 10.1155/2020/9803024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, I. D. , Park, M. W. , Cha, H. W. , Yoon, S. , Boonpraman, N. , Yi, S. S. , & Moon, J.‐S. (2020). Elevated CLOCK and BMAL1 contribute to the impairment of aerobic glycolysis from astrocytes in Alzheimer's disease. International Journal of Molecular Sciences, 21(21), 7862. 10.3390/ijms21217862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, R. , Tian, L. , Ding, Y. , Gao, Y. , Li, D. , & Tang, Y. (2019). Correlation between inflammatory markers and impaired circadian clock gene expression in type 2 diabetes mellitus. Diabetes Research and Clinical Practice, 156, 107831. 10.1016/j.diabres.2019.107831 [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Li, C. , Guo, S. , Sun, C. , Ning, N. , Hao, H. , Wang, X. , Bian, Y. , Liu, H. , & Wang, X. (2021). Adiponectin improves amyloid‐β 31‐35‐induced circadian rhythm disorder in mice. Journal of Cellular and Molecular Medicine, 25(20), 9851–9862. 10.1111/jcmm.16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G. , Meng, L. , Wang, Z. , Peng, Q. , Chen, G. , Xiong, J. , & Zhang, Z. (2022). Islet amyloid polypeptide cross‐seeds tau and drives the neurofibrillary pathology in Alzheimer's disease. Molecular Neurodegeneration, 17(1), 12. 10.1186/s13024-022-00518-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , & Luo, P. (2020). Myocardial infarction predisposes neurodegenerative diseases. Journal of Alzheimer's Disease, 74(2), 579–587. 10.3233/JAD-191225 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , & Song, W. (2017). Islet amyloid polypeptide: Another key molecule in Alzheimer's pathogenesis? Progress in Neurobiology, 153, 100–120. 10.1016/j.pneurobio.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Zhao, R. , Gao, X. , Zhang, T. , & Li, X. (2016). Effects of polysaccharide on type 2 diabetes mellitus rats by regulating biological rhythms. Iranian Journal of Basic Medical Sciences, 19(9), 1024–1030. [PMC free article] [PubMed] [Google Scholar]
- Zhu, M. , Tang, H. , Tang, X. , Ma, X. , Guo, D. , & Chen, F. (2018). BMAL1 suppresses ROS‐induced endothelial‐to‐mesenchymal transition and atherosclerosis plaque progression via BMP signaling. American Journal of Translational Research, 10(10), 3150–3161. [PMC free article] [PubMed] [Google Scholar]
- Zhu, P. , Hamlish, N. X. , Thakkar, A. V. , Steffeck, A. W. T. , Rendleman, E. J. , Khan, N. H. , Waldeck, N. J. , DeVilbiss, A. , Martin‐Sandoval, M. S. , Mathews, T. P. , Chandel, N. S. , & Peek, C. B. (2022). BMAL1 drives muscle repair through control of hypoxic NAD regeneration in satellite cells. Genes & Development, 36, 149–166. 10.1101/gad.349066.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, W. , Yue, L. , Dang, X. , Chen, F. , Gong, Y. , Lin, X. , & Luo, Y. (2019). Rosenroot (Rhodiola): Potential applications in aging‐related diseases. Aging and Disease, 10(1), 134–146. 10.14336/AD.2018.0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were created or analyzed in this study. Data sharing is not applicable to this review article.


