Abstract
Immunological aging is strongly associated with the observable deleterious effects of human aging. Our understanding of the causes, effects, and therapeutics of aging immune cells has long been considered within the sole purview of immunosenescence. However, it is being progressively realized that immunosenescence may not be the only determinant of immunological aging. The cellular senescence-centric theory of aging proposes a more fundamental and specific role of immune cells in regulating senescent cell (SC) burden in aging tissues that has augmented the notion of senescence immunotherapy. Now, in addition, several emerging studies are suggesting that cellular senescence itself may be prevalent in aging immune cells, and that senescent immune cells exhibiting characteristic markers of cellular senescence, similar to non-leucocyte cells, could be among the key drivers of various facets of physiological aging. The present review integrates the current knowledge related to immunosenescence and cellular senescence in immune cells per se, and aims at providing a cohesive overview of these two phenomena and their significance in immunity and aging. We present evidence and rationalize that understanding the extent and impact of cellular senescence in immune cells vis-à-vis immunosenescence is necessary for truly comprehending the notion of an ‘aged immune cell’. In addition, we also discuss the emerging significance of dietary factors such as phytochemicals, probiotic bacteria, fatty acids, and micronutrients as possible modulators of immunosenescence and cellular senescence. Evidence and opportunities related to nutritional bioactive components and immunological aging have been deliberated to augment potential nutrition-oriented immunotherapy during aging.
Keywords: Aging, Immunity, Inflamm-aging, Cellular senescence, Immunosenescence, Nutrition
Introduction
The mammalian immune system has evolved not only as a central instrument to protect against the invading pathogens, but is also essential for tissue repair and regeneration (Ding et al. 2019), as well as identification and removal of damaged host cells (Rock et al. 2011). The diverse cells of the immune system along with its allied components such as the complement proteins, are present throughout the body and are critical to preserving the parenchymal tissue homeostasis. The immune system is also one of the major regulatory systems to be affected by the deleterious process of aging, and age-related immune dysfunctions are often associated with increased risk of diseases and weakened vaccine response in the elderly (Allen et al. 2020; Pereira et al. 2019). Understanding the development and progression of immunological aging is thus central to our mitigatory strategies against a variety of age-dependent disorders and infectious diseases as also aptly highlighted by the ongoing COVID-19 pandemic (Bartleson et al. 2021). Immunosenescence is an umbrella term that refers to a myriad of age-dependent qualitative and quantitative changes in the immune system such as shrinkage in the thymus gland output, decreased cell-mediated and humoral immune responses, development of chronic systemic inflammation (inflamm-aging), changes in T cell subset population, and loss of T cell differentiation which are together accounted for increased risk of morbidity and mortality in the elderly (Barnes 2015; Fulop et al. 2018; Goronzy and Weyand 2017; Sadighi Akha 2018; Xu et al. 2020). Considering these multifaceted deleterious effects, an immune system-oriented theory of aging was initially formulated which postulated that aging in organisms is pathologically linked to the impaired immune functions (immunosenescence), and that the loss of self and non-self recognition in immune cells due to immunogenetic diversification leads to the development of age-dependent auto-immune disorders and inflammation (Walford 1964). The original immunosenescence theory was then further integrated with perturbed age-associated cellular oxidative and inflammatory homeostasis, and an updated oxi-inflamm-aging theory of aging was proposed (De la Fuente and Miquel 2009). Recent advances in our understanding of the molecular basis of aging are now revealing even novel role(s) of the immune system in impacting aging. This is specifically aligned with the process of ‘cellular senescence’ that is rapidly emerging as the central and arguably the fundamental process governing both aging and age-related diseases (Borghesan et al. 2020; Gil 2019; McHugh and Gil 2018). The critical significance of the immune system in regulating senescent cells (SC) survival and accumulation has gained a particular attention, and cellular senescence-associated immunotherapy is emerging as a desirable approach for targeting aging (Burton and Stolzing 2018; Kale et al. 2020).
The different facets of the immune system including its development, maturation, and activation are tightly regulated. However, dietary nutritional components including bioactive phytochemicals and probiotic microorganisms can strongly influence multiple aspects of the immune system. Studies have demonstrated that modulation of the immune functions by dietary factors can favorably influence the proliferation, activation, and efficacy of the immune system (Barrea et al. 2021; Childs et al. 2019; Tourkochristou et al. 2021). In particular, the role of food components in modulating the immune response for the mitigation of infectious agents including COVID-19 (Mrityunjaya et al. 2020; Tomas et al. 2022), food-borne contaminants (Pan et al. 2020), inflammatory disorders (Sung et al. 2018), and cancer immunotherapy (Soldati et al. 2018; Spencer et al. 2021) have been documented. Considering this and given the fact that immunological aging essentially involves impaired effector immune functions; the application of dietary factors in alleviating at least some of these deleterious aspects seems plausible. This is also reasonable since nutrition and exercise are currently at the forefront of developing anti-aging therapies and in fact, a novel discipline called ‘nutrigerontology’ was emphasized for achieving successful ageing and longevity (Aiello et al. 2016; Verburgh 2015). Therefore, in the present paper, we first discuss the emerging advances in our understanding of immunological aging in terms of immunosenescence and cellular senescence, and then deliberate the available evidence of nutritional and bioactive dietary factors-mediated modulation of immunity and aging. Future research directions and lacunae have been discussed aimed to truly understand immunological aging as well as the potential of diet in impacting immunity, aging, and diseases.
Immunosenescence and immunological aging
Immunosenescence refers to widespread age-dependent changes in the immune system including the lymphoid organs that ultimately manifest as impaired immune responses in the elderly. Despite its name, immunosenescence does not explicitly imply decreased or attenuated immune functions, but is rather best defined as immune-remodeling wherein certain cellular functions may decline while others may be exaggerated (Xu et al. 2020). Moreover, the one-dimensional detrimental consideration of immunosenescence has recently been challenged, and it is argued that immunosenescence actually represents a continuum of adaptation and maladaptation to lifelong aggressions and insults which ultimately defines the course of organismal aging and health (Fulop et al. 2020). This also provides a rationale as to why healthy centenarians (≥ 100 years age) are able to preserve essential immune functions and are less prone to chronic age-related pathologies (Santoro et al. 2021). Regardless, modulation of the different aspects of immunosenescence has traditionally been considered a viable strategy for improving the quality of life in elderly (Borgoni et al. 2021; Stahl and Brown 2015; Weyand and Goronzy 2016). Although the exact underlying causes of immunosenescence are still not completely understood, the concept of immunosenescence has vastly contributed to our present knowledge of aging in both innate and adaptive immune functions, and are briefly discussed below (Table 1).
Table 1.
Major immunosenescence markers in the innate and adaptive immune cells
| S. no. | Innate immune cells | Adaptive immune cells |
|---|---|---|
| 1 | Decreased identification and stimulation in response to pathogens (Boehmer et al. 2005; Loyer et al. 2022) | Reduced stimulatory response and effector functions (Haynes and Eaton 2005; Nikolich-Žugich 2014) |
| 2 | Changes in circulatory numbers (Jing et al. 2009; Gounder et al. 2018) | Decreased synaptic activity with APCs (Marko et al. 2007) |
| 3 | Impaired chemotaxis (Brubaker et al. 2013) | Characteristic changes in the expression of cell surface markers (Rodriguez et al. 2021; Zhang et al. 2021) |
| 4 | Attenuated antigenic presentation (Wong and Goldstein 2013) | Increased memory T cells pool and reduced diversity (Saule et al. 2006) |
| 5 | Impaired effector functions (Gomez et al. 2010; Sharma et al. 2014b; Zacca et al. 2015) | Reduced activation of B cells and conversion to plasma cells (Pritz et al. 2015) |
| 6 | Impaired resolution of inflammation (Zhang et al. 2020a) | Decreased antigen-specific antibodies production output (Maue and Haynes 2009) |
Innate immune system
Neutrophils are amongst the first innate immune cells to respond to growing infectious agents and inflammation. These short-lived cells are produced in vast numbers by the bone marrow, and studies have shown that circulatory neutrophil numbers in the blood or bone marrow precursors do not decline with age suggesting minimal quantitative impact on hemopoiesis (Butcher et al. 2000; Chatta et al. 1993). However, deterioration of several functional aspects of neutrophils has been documented. We and others have reported that neutrophils exhibit impaired chemotaxis, reduced respiratory oxidative burst, and phagocytic potential with age in both experimental animals and humans (Brubaker et al. 2013; Mege et al. 1988; Perskin and Cronstein 1992; Sapey et al. 2014; Sharma et al. 2014b; Wenisch et al. 2000). Changes in neutrophil receptors and intracellular signaling have also been observed which may explain some of the apparent age-associated functional defects in neutrophils (Fülöp et al. 1984, 1985; Gasparoto et al. 2021; Sapey et al. 2014). Monocytes/macrophages are another critical component of the innate immune response. Several age-related changes have been documented in macrophages. It has been demonstrated that macrophages show reduced expression of toll-like receptors (TLRs) (Boehmer et al. 2005; Renshaw et al. 2002; Sharma et al. 2014b), decreased phagocytosis and respiratory burst (Linehan et al. 2014; Wong et al. 2017), altered cytokine production (Gomez et al. 2010; Roubenoff et al. 1998), diminished response to pathogen identification (Boehmer et al. 2004; Ding et al. 1994), impaired antigenic presentation (Vĕtvicka et al. 1985), skewed M1/M2 macrophage polarization (Becker et al. 2018; Cui et al. 2019), and delayed resolution of inflammation and injury (Zhang et al. 2020a) suggesting multifaceted deleterious effects of age on macrophage functions.
Similarly, studies have shown a decrease in circulatory numbers of dendritic cells (DC), as well as functional deterioration in all DC subsets with age (Della Bella et al. 2007; Gardner et al. 2017; Jing et al. 2009). There is strong evidence that DC lose their potency to accurately process and present antigens which may result in failure of the activation of immune cells and inadequate effector functions (Grolleau-Julius et al. 2008; Guo et al. 2014; Wong and Goldstein 2013; Zacca et al. 2015). Further, DCs have been shown to exhibit impaired cytokine production (Della Bella et al. 2007; Grolleau-Julius et al. 2006), decreased expression of co-stimulatory molecules such as CD86 and CD40 (Varas et al. 2003), and altered intracellular signaling in response to activation signals (Agrawal et al. 2007) which ultimately results in poor response to infections and vaccines in elderly. Contrary to other immune cells, studies have consistently demonstrated that peripheral NK cell numbers increase with age (Gounder et al. 2018; Le Garff-Tavernier et al. 2010). However, a parallel decline in the proliferative and cytolytic capacity of NK cells as well as a decrease in cytokine production and NK cell migration has also been reported which is often associated with increased incidences of viral infection in the elderly (Almeida-Oliveira et al. 2011; Fang et al. 2010; Gounder et al. 2018; Hazeldine et al. 2012; Mariani et al. 2002).
Unlike neutrophils, the role of remaining granulocytes, i.e., eosinophils and basophils during aging is less understood. It has been reported that eosinophil deregulation response to IL5 stimulation and superoxide production decreases with age in human subjects (Mathur et al. 2008). A recent study has shown that eosinophils are critical for maintaining the adipose tissue functions and inflammatory homeostasis during aging (Brigger et al. 2020). Regarding basophils, some studies have demonstrated that absolute basophil numbers decrease with age (Song et al. 1999; Valiathan et al. 2016), and there is also evidence that basophils accumulate with age in tissues (van Beek et al. 2018). Age-associated impaired Th2 response of basophils has also been reported which can predispose elderly to parasitic infections (Nel et al. 2011; Smith et al. 2001). It is interesting to note that in addition to adaptive immunity, innate immune cells are now also emerging to be associated with immunological memory (Bulut et al. 2021). This phenomenon has been dubbed as ‘trained immunity’ or ‘innate immune memory’ that can augment host innate immune response to secondary microbial infections mediated by the activation of pathogen recognition receptors and epigenetic changes in the cells of the innate immune system (Netea et al. 2016; Sherwood et al. 2022). However, the impact of aging on innate immune memory as well as the role of innate immune memory in influencing recurring infections and vaccine response in the elderly is yet to be explored. Nonetheless, a recent clinical trial has reported that trained immunity can be effectively induced in the elderly on account of BCG vaccination which suggests novel methods and mechanisms of vaccine effects (Giamarellos-Bourboulis et al. 2020).
Adaptive immune system
Most of our knowledge regarding immunosenescence and aging is attributed to studies in the adaptive immune cells such as T and B cells and is covered in detail elsewhere (Goronzy and Weyand 2005; Minato et al. 2020; Zhang et al. 2021). Although the numbers of T cells generally remain constant over the lifespan, several characteristic compositional changes in T cell subsets with age have been documented. Typically, the proportion, activation, and differentiation of naïve T cells decline with age presumably due to thymus involution and loss of thymic output that strongly affects the peripheral T cell homeostasis. (Appay and Sauce 2014; Čičin-Šain et al. 2007; Goronzy and Weyand 2005; Lazuardi et al. 2005). However, it important to consider that the effects of thymic involution are species-specific and are much more pronounced in experimental animals as compared to humans. This is likely attributed to homeostatic proliferation potential of peripheral T cells in humans that results in their self-renewal thereby compensating for loss of naïve T cells population due to gradual thymic involution (Goronzy and Weyand 2019; Thome et al. 2016). Memory T cells subsets remain constant through the adulthood but show a decline in numbers as well as functions with advancing age (Salam et al. 2013; Zhou and McElhaney 2011). Further, an age-dependent shift of naïve T cell subsets towards central memory T cells and effector memory T cells has also been observed (Saule et al. 2006). The cytolytic CD8 T cells show a clear age-associated numerical decline as well as compromised effector functions with age (Čičin-Šain et al. 2007; Nikolich-Žugich 2014), while the CD4 helper T cells also develop progressive changes in functions such as reduced stimulatory response (Haynes and Eaton 2005), impaired immunological synaptic activity with APCs (Marko et al. 2007), and attenuated B cell antibody response which decreases downstream antigen specific responses (Maue and Haynes 2009). In addition, aged T cells exhibit a distinct phenotype of cell surface markers often characterized by decreased expression of genes such as CD28 and CD154 while an increase in the expression of certain receptors such as CD57 and CD95 are also reported which are often considered hallmarks of immunosenescence in T cells (Rodriguez et al. 2021; Zhang et al. 2021). It is pertinent to note here that age-dependent changes in CD8 T cells effector functions can be compensated by the acquisition of innate like immune phenotype in these cells that express markers of both TCR and NK cell lineage (Pereira and Akbar 2016). These innate-like αβCD8 + T cells likely represent a beneficial adaptation to gradual age-dependent loss of CD8 T cell numbers and activity, and therefore are significantly considered for understanding the ‘restructuring’ nature of immunosenescence.
Naturally occurring Tregs (CD4 + CD25 + Tregs+) appear to increase with age in humans (Bryl and Witkowski 2004; Gregg et al. 2005), and studies in mice revealed increased suppressive functions of Tregs during aging (Garg et al. 2014) with possible implications in age-dependent diseases (Deng et al. 2021). Similar to T cells, naïve B cells output has been shown to decline presumably due to age-related changes in the bone marrow (Colonna-Romano et al. 2009; Lin et al. 2016), and B cell diversity is also reported to decrease with age with a direct impact on health status (Gibson et al. 2009). Aged B cells exhibit compromised immune functions such as differentiation to plasma cells on antigenic challenge as well as a general decline in antigen-specific antibody production with age (Howard et al. 2006; Pritz et al. 2015). Collectively, these changes in B cells populations and their functional capacity result in diminished antibody production and poor vaccine response in the elderly which is also implicated in the COVID-19 pandemic (Collier et al. 2021).
Inflamm-aging
Although the acute inflammatory response against pathogens decreases with age, surprisingly, elderly also report higher levels of circulatory pro-inflammatory cytokines such as TNF-α and IL-6 (Myśliwska et al. 1998, 1997), indicating the presence of a chronic, sterile, and low-grade systemic inflammation referred to as inflamm-aging (Ferrucci et al. 2005; Franceschi et al. 2000; Franceschi and Campisi 2014; Fulop et al. 2021). The increased levels of cytokines and chemotactic proteins during inflamm-aging are often associated with predisposition of the elderly to increased risk of inflammatory disorders such as arthritis and diabetes (Franceschi and Campisi 2014). In addition, inflamm-aging can have adverse effects on the immune response itself. For instance, increased serum TNF-α levels with age are negatively correlated with T cell functions (Parish et al. 2009), while age-related increase in IL-6 levels can suppress macrophage functions (Gomez et al. 2010). Thus, inflamm-aging impairs the acute phase response to pathogens while the chronic presence of inflammatory proteins augments systemic damage. The precise factors augmenting inflamm-aging are yet unclear, but are likely to be multifactorial and could be attributed to lifelong exposure to antigens (including latent viruses such as CMV), accumulation of SC and their inflammatory secretome, increased macromolecular damage and release of damage-associated molecular patterns (DAMPs) that chronically activate the innate immune cells (Garb-aging), pro-inflammatory microRNAs, age-related gut dysbiosis and leaky gut, as well as certain disorders such as adiposity (Santoro et al. 2021). Specifically, the double-stranded DNA (ds DNA) sensor cyclic-GMP-AMP synthase (cGAS) and the downstream stimulator of interferon genes (STING) pathways (cGAS/STING) is emerging as an important regulator and therapeutic target of sterile inflammation and its unwarranted effects (Decout et al. 2021; Huang et al. 2020). Cytosolic dsDNA is recognized as a universal DAMP that acts as a ligand to activate cGAS/STING pathway, and the stress-induced cytosolic leakage of mitochondrial DNA (mt DNA), including in immune cells such as macrophages, has been implicated in augmented inflamm-aging that contributes to immunosenescence (Atayik and Çakatay 2022b; Conte et al. 2020; Lv et al. 2022; Zhong et al. 2022). Accumulating evidence suggests that inflamm-aging is essentially an age-dependent remodeling of the immune response due to an imbalance between anti-inflammatory and pro-inflammatory networks. This adaptation of the immune system to proinflammatory environment due to a weakening of anti-inflammatory state is considered a driving force of age-dependent morbidity (Fulop et al. 2016, 2017; Santoro et al. 2021). Thus, strategies decreasing the proinflammatory stimulus as well as those enhancing the anti-inflammatory cellular attributes are desirable for negating inflamm-aging and thus promoting healthy aging. As a result, characterization of inflamm-aging is an important parameter of potential anti-aging therapies and understanding disease pathology during aging. It is prudent to consider here that the significance of compromised functions of the immune system during aging in augmenting organismal risk of infections and delayed immune response appears contentious. On one hand, there is mounting evidence that age-dependent functional and phenotypic changes in immune cells can contribute to reduced vaccine response and increased risk of infections during aging (He et al. 2021; Loyer et al. 2022; Sabbatinelli et al. 2022; Simmons et al. 2022); on the other hand, there are also reports indicating that aspects of immunosenescence, such as inflamm-aging, could be positively corelated with increased vaccine immunogenicity in older adults (Picard et al. 2022). These conflicting observations corroborate the recent efforts of Pawelec et al. that highlighted the limitations and lacunae of the prevailing one-dimensional view of immunosenescence (Pawelec et al. 2020).
Cellular senescence and immunological aging
Unlike other aspects of organismal growth and development, aging is not considered to be programmed but is rather argued as a quasi-programmed phenomenon (Blagosklonny 2013) that also appears to be a classic case of antagonistic pleiotropy (Austad and Hoffman 2018). Organismal aging begins in cells themselves (biological aging) and represents a culmination of progressive increase in cellular and molecular damage owing to several intrinsic and extrinsic cellular stressors (DiLoreto and Murphy 2015; Gil del Valle 2011; Liguori et al. 2018). Hayflick and Moorhead reported the phenomenon of ‘cellular senescence’ which indicated that primary human cells are not immortal as previously thought, but rather have a finite replicative lifespan in vitro (Hayflick and Moorhead 1961). Identification of replicative senescence was a seminal discovery as it provided a hint of a direct correlation between cells and organismal aging. The recent decade has seen tremendous improvements in our understanding of cellular senescence and its relevance to the causes and effects of aging (Di Micco et al. 2021). SC are characterized by increased expression of cell cycle inhibitors (p53/p16Ink4a/p21WAF1), activation of senescence-associated β-galactosidase activity (SA-β-gal), enlarged and heterogenous morphology, persistent stress, DNA damage, telomere attrition, chromatin remodeling, apoptotic resistance, and altered metabolic and energetic homeostasis (Ovadya and Krizhanovsky 2018; van Deursen 2014). Although SC develop naturally and their presence is considered essential for certain processes such as wound healing and even embryonic development; the role of accumulating tissue SC in augmenting the aging phenotype across vertebrate species is also becoming evident (Childs et al. 2015; Mylonas and O’Loghlen 2022). An increased SC burden in several aging tissues has been reported (Idda et al. 2020; Yousefzadeh et al. 2020) and further landmark studies also identified their causative role in promoting age and age-related pathologies (Aguayo-Mazzucato et al. 2019; Baker et al. 2016). SC accumulation in tissues is particularly deleterious due to the chronic presence of the senescence-associated secretory phenotype (SASP) which is a milieu of characteristic cytokines and growth factors that augments proinflammatory and pro-tumorigenic environment in healthy cells surrounding SC through paracrine effects (Birch and Gil 2020). As a result, cellular senescence appears to be a common denominator for a variety of human inflammatory diseases and it is argued that pathophysiologically distinct but age-dependent disorders should be considered within the purview of cellular senescence itself (Prašnikar et al. 2021). As the cellular senescence-centric view of aging is being rapidly acknowledged; novel therapies aimed at mitigating or selectively removing SC (through senolytics) are of considerable interest for lifespan extension despite their potential pitfalls and apprehensions (Dolgin 2020; Owens et al. 2021; Pils et al. 2021; Sharma 2021a; Thirumurugan 2022).
Although most of our understanding regarding immunity and aging is often associated with immunosenescence, yet, immunosenescence has often been criticized for the lack of universal biomarkers, its causal relationship with organismal aging, and its association with inflamm-aging (Pawelec et al. 2020; Xu et al. 2020). The past decade has seen rapid transformation in our understanding of immunological aging, and as a result, previously unknown or less emphasized functions and phenomenon of the immune system that may impact aging have been discovered. In this regard, the role of immune system in the development and progression of cellular senescence in non-leucocyte cells as well as its impact on immune cells themselves is being greatly recognized (Budamagunta et al. 2021; Burton and Stolzing 2018; Sharma 2021b). It is becoming evident that immunosenescence may not be the only player in regulating immunological aging, and that cellular senescence in immune cells per se could be sufficient to drive organismal aging including immunosenescence (Yousefzadeh et al. 2021). Therefore, a mutual interrelationship between immunosenescence and cellular senescence can be envisaged and elucidating this association is critical for understanding immunological as well as organismal aging. At this point, it is also prudent to consider that although certain effects of cellular senescence and immunosenescence in immune cells may appear to overlap, yet the etiology of both these processes is fundamentally distinct (Burton and Stolzing 2018). Subsequent to the initial identification of cellular senescence in fibroblasts by Hayflick and Moorhead (Hayflick and Moorhead 1961), several other cell types were also reported to undergo cellular senescence in vitro. However, scientific interest in identifying cellular senescence in immune cells remained subdued until rather recently primarily due to the fact that T cells were initially observed to propagate indefinitely following an exposure to T cell growth factor (Gillis and Smith 1977). It was later discovered that similar to other cell types, replicative senescence in T cells can be induced by multiple rounds of antigenic stimulation which can also impair T cell effector functions (Callender et al. 2018; Dunne et al. 2005; Spaulding et al. 1999). However, the extent and significance of cellular senescence in different immune cells as well as the role of senescent immune cells in promoting cellular senescence and accumulation of non-leucocyte SC is only beginning to be understood. Emerging studies are suggesting a more intricate, bidirectional, and dynamic role of cellular senescence in governing aging in both immune as well as in non-leucocyte cells. As a result, an emphasis on understanding the relationship between immunosenescence and cellular senescence, and its impact and relevance in the context of organismal aging, has been recently advocated (Budamagunta et al. 2021; Burton and Stolzing 2018).
Cellular senescence in immune cells: characteristics and impact
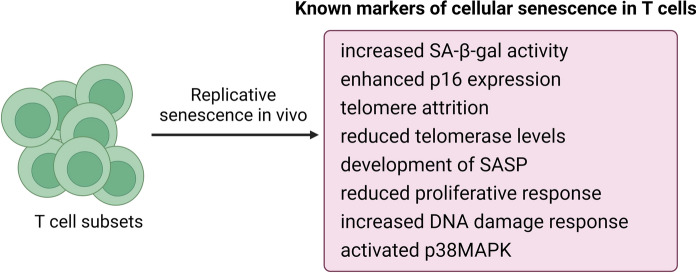
Although often overlooked, but similar to other cells, immune cells are also liable to undergo cellular senescence. This is especially relevant since we now understand that cellular senescence is not simply a feature of proliferative cells, but even post-mitotic tissues have prevalent senescence program (von Zglinicki et al. 2021). Although replicative senescence was earlier established in T cells, characterization of cellular senescence in various immune cells has largely remained in obscurity and incompletely understood. However, given the emerging significance of cellular senescence, recent studies have demonstrated that cellular senescence could indeed be more prevalent and significant in immune cells, especially in T cells (Fig. 1). For instance, it was reported that similar to other somatic cells, peripheral blood mononuclear cells (PBMCs) in humans strongly display classical markers of cellular senescence such as high SA-β-gal activity, p16Ink4a overexpression, telomere dysfunction, and impaired proliferative response (Martínez-Zamudio et al. 2021). Interestingly, the authors noted that the senescent CD8 + T cells population (including TEM and TEMRA subsets) developed unique SASP-associated gene expression profile as compared to senescent fibroblasts, and such T cells reached average levels of 64% in aging subjects thereby suggesting that T cell senescence substantially overlaps with replicative senescence in non-leucocyte cells and that such senescent T cells could be far more abundant in circulation than previously thought (Martínez-Zamudio et al. 2021). Another recent work demonstrated that the development of p16Inka4a mediated cellular senescence and suppressed proliferative response is a characteristic of aging human T cells which could be a potential therapeutic target (Janelle et al. 2021). Similarly, a high percentage of CD4 + and CD8 + T cells expressing SA-β-gal activity, increased transcripts of p21, p16INK4a, and inflammatory cytokines, as well as DNA damage were observed in aged human PBMCs as compared to the cells isolated from younger subjects (Dewald et al. 2020). Using a murine model of spontaneous genotoxic damage as well as natural aging, senescent peripheral blood lymphocytes were identified that were characterized by over 15 folds increase in p16Ink4a and p21Cip1 mRNA expression suggesting prevalent cellular senescence (Yousefzadeh et al. 2020). Further, senescent CD8 + T cells exhibited p38MAPK-dependent SASP-like features characterized by the secretion of proteases and cytokines similar to non-leucocyte senescent cells (Callender et al. 2018; Henson et al. 2014). Similarly, p16Ink4a expression increased exponentially with chronological age in human peripheral blood T-lymphocytes suggesting the systemic presence of senescent T cells (Liu et al. 2009; Shen et al. 2020). Similar to other senescent cells, expression of telomerase reverse transcriptase gene declines in aging T cells which directly contributes to shortened telomeres and development of senescence features (Matthe et al. 2022; Röth et al. 2003). Flow cytometry analyses of T cell subsets in elderly population have revealed that among CD8 + T-lymphocytes, CD28 + CD57 + T cells represented a subset with strong senescent features such as increased expression of p16Ink4a, p21, Bcl-2, CD95, CD45RO, CCR5 and PD-1 (Onyema et al. 2015). A positive relationship between increased circulatory senescent CD8 + T cells and the development of Behçet's disease was also reported (Yang et al. 2018). Moreover, induction of T cell senescence has been observed to be a key mechanism governing the pathophysiology of human infectious agents including COVID-19 (Covre et al. 2018; De Biasi et al. 2020; Witkowski et al. 2022).
Fig. 1.
Identified markers of replicative senescence in T cells
On the other hand, studies identifying senescent innate immune cells in vivo are limited. Few reports have assessed tissue-associated macrophages for the presence of cellular senescence but with controversial findings. It was observed that characteristic markers of cellular senescence such as increased p16Ink4a expression, SA-β-gal activity, and activation of SASP are prevalent in aging macrophages (Hall et al. 2016; Kumar et al. 2020b; Liu et al. 2019; Prattichizzo et al. 2018; Wang et al. 2021). A recent study further reported that irradiated macrophages exhibited several features of senescence, including increased expression of p16Ink4A and p21, SA-β-gal activity, SASP, and impaired efferocytosis in vitro, and when transferred to mice, they exacerbated inflammation in vivo (Sadhu et al. 2021). However, curiously, it was also revealed that the apparent senescent phenotype in macrophages might be a reversible phenomenon in response to physiological stimuli (Hall et al. 2017), while another study observed distinct differences between in vitro and in vivo aged microglia cells (Stojiljkovic et al. 2019). In addition to macrophages, NK cells have also been reported to show increased markers of age-dependent cellular senescence although such studies are limited (Dewald et al. 2020). It is also interesting to note that in addition to endogenous factors, immune cells, especially tissue resident cells such as macrophages and memory T cells, which persist in the microenvironment of non-leucocyte tissue cells, can be chronically exposed to the SASP of tissue SC that may undermine their functional activities and potentially contribute to premature immunosenescence and increased accumulation of SC (Fig. 2). For instance, it was observed that acute exposure to the SASP of senescent fibroblasts impaired the phagocytic ability of macrophages and ultimately accelerated the accumulation of SC in the dermis (Ogata et al. 2021). Similarly, peritoneal macrophages isolated from old mice exhibited significant characteristics of cellular senescence which were aggravated by ex vivo exposure to the SASP of senescent preadipocyte cells (Kumar et al. 2020b). In vitro exposure to the SASP of hepatocytes induced migration of inflammatory macrophages (but not of non-inflammatory macrophages) that could contribute to a proinflammatory microenvironment (Irvine et al. 2014).
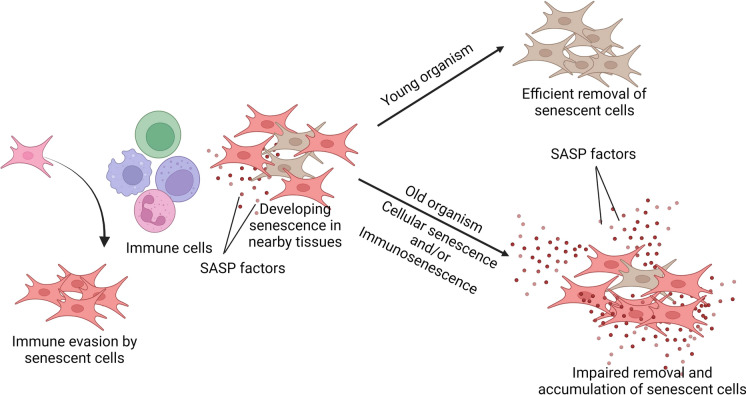
Fig. 2.
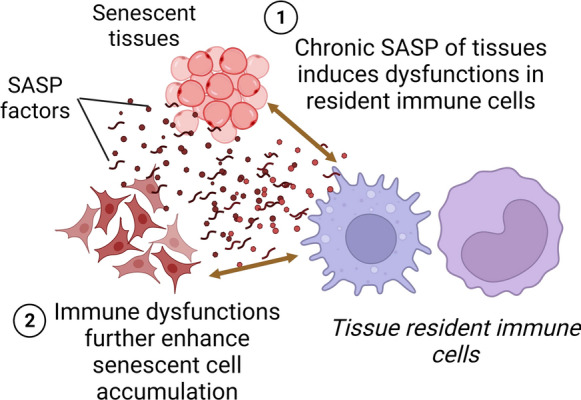
Overview of interactions between tissue resident immune cells and senescent non-leucocyte cells with implications in immunological and systemic aging. (1) Chronic exposure to the SASP of nearby senescent cells can induce immunological dysfunctions in resident immune cells and (2) these changes can impair immune cells’ ability of immunosurveillance and efferocytosis that may ultimately result in inefficient removal of senescent cells and thus augmentation of senescent cell burden
The immune system and tissue senescent cell burden
The relationship between immunity and cellular senescence appears to be bidirectional. While aging may augment levels of senescent immune cells, accumulation of various non-leucocyte SC in the body seems to be strongly regulated by immune cell functions. Several independent studies have demonstrated that SC secrete certain chemokines and cytokines that attract immune cells such as macrophages, NK cells, and CD8 + T cells which then identify SC through upregulated SC-specific ligands such as MICA/B, ULBP1-6, and lysophosphatidylcholines (Antonangeli et al. 2016; Narzt et al. 2021) resulting in their targeted cytolysis. Thus, it is perceived that immune cells can identify and remove SC from the body thereby indicating their pivotal role in regulating tissue SC turnover with age. The exact causes for the apparent increase in SC numbers in the aging tissues is yet incompletely understood but it could be attributed to either the increased development of SC in vivo or due to their decreased removal probably due to impaired immune functions with age. In a breakthrough study, it was demonstrated that if immune cells have defective cytolytic properties, it results in higher SC burden in tissues, chronic inflammation, multiple age-related disorders, and ultimately decreased lifespan in experimental mice (Ovadya et al. 2018). This clearly indicated that defective immunosurveillance and effector immune functions (as observed during immunological aging) could be key mechanisms governing increased SC burden with age. In another significant study, it was demonstrated that mouse hematopoietic cells defective in DNA damage repair response resulted in induction of premature immunosenescence and cellular senescence in not only immune cells, but also in non-lymphoid cells thereby strongly indicating that senescence in immune cells is sufficient to drive systemic senescence (Yousefzadeh et al. 2021). Interestingly, transplantation of young immune cells significantly reduced systemic senescence suggesting that an aging immune system can be critical augmenter of SC development and accumulation (Yousefzadeh et al. 2021). Similarly, dysfunctional mitochondria in T cells were causatively related to premature systemic senescence and multimorbidity characterized by neurological, metabolic, muscular, and cardiovascular impairments, as well as inflamm-aging in mice (Desdín-Micó et al. 2020). Another emerging and interesting aspect of SC accumulation and immune cells is related to their immune evasive attributes. It was observed that senescent dermal fibroblasts exhibit heightened expression of non-classical MHC molecule HLA-E which inhibit immune responses against SC (Pereira et al. 2019). This study thus showed that similar to tumor cells, SC may develop strategies to circumvent the immune system thereby indicating that the relationship between immune cells and SC could be even more intricate. Further, whether and how the immune evasive attributes of SC could be facilitated by the presence of immunosenescence or cellular senescence in immune cells remains to be deciphered. Taken, together, these observations suggest that cellular senescence may be prevalent in immune cells (especially in adaptive immune cells), and although its significance is yet to be completely delineated, however, when coupled with immunosenescence, it can be envisaged that age-dependent deregulation of the immune system could be a significant contributor to the systemic increase in tissue SC population (Fig. 3).
Fig. 3.
Interrelationship between immune cells, cellular senescence, and aging. (1) In young organisms, SASP mediated activation of immune cells such as macrophages and NK cells identify and clear senescent cells efficiently. (2) However, in old organisms, due to cumulative effects of both cellular senescence and immunosenescence, immune cells lose the potency to manage senescent cell turnover that gradually decreases with age. (3) Inherent immune evasive attributes of senescent cells are also indicated that further highlight the complex interplay between immunity and senescent cells
Dietary bioactive factors and immunological aging
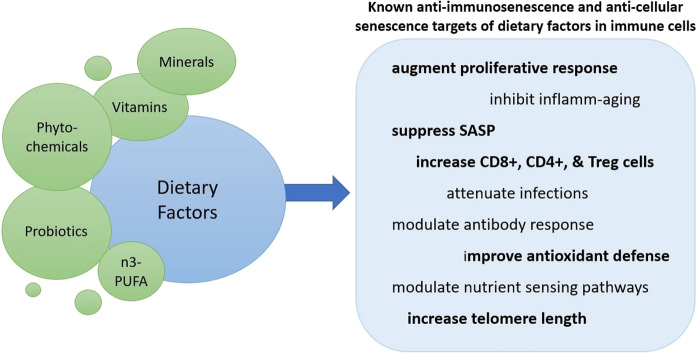
In addition to essential functions for growth and development, dietary constituents can dynamically influence the immune system including its maturation and effector immune responses (Ponton et al. 2011; Saeed et al. 2016). The human immune system undergoes characteristic changes and maturation with different phases of life. For example, pregnancy is characterized by downregulation of cell mediated immune responses, while post-birth, exposure to food (including milk antigens), benign environmental factors, as well as colonization of commensal bacteria in the gut induces tolerance in the immune system resulting in overall immunological maturation and management of inflammation (Calder et al. 2006; Gil and Rueda 2002). Thus, nutritional exposure in early life is considered critical for immunological competence in later life against pathogens and the development of immunological disorders. Similarly, modulation of the aging immune system through various dietary factors has also been observed that may have implications for improving the deleterious effects of immunological aging (Fig. 4).
Fig. 4.
An overview of identified anti-cellular senescence and anti-immunosenescence effects of various dietary factors specific to immunological aging
Phytochemicals
Food-based phytochemicals are a major source of immunomodulators including immunostimulators and immunosuppressants (Behl et al. 2021). Phytochemicals describe a collection of various categories of plant metabolites such as polyphenols, alkaloids, carotenoids, carbohydrates, and lipids. These chemicals are abundant in fruits and vegetables, and in addition to immunomodulation, they have been recognized for various cell signaling modulatory and cytoprotective attributes (Pham et al. 2020). As such, the modulatory effects of phytochemicals on different aspects of organismal healthspan and lifespan have also been documented (Corrêa et al. 2018; Si and Liu 2014). In terms of immunological aging, several studies have shown that dietary polyphenols can influence multiple facets of immunosenescence although their impact on cellular senescence in immune cells is little understood. For instance, we previously observed that ex vivo exposure to green tea catechin EGCG reversed SASP-induced markers of cellular senescence in murine macrophages including SA-β-gal activity, expression of cell cycle inhibitory genes, and oxi-inflammatory stress (Kumar et al. 2020b). In a further study, chronic consumption of green tea catechin EGCG influenced multiple markers of cellular senescence and immunosenescence in experimental mice (Sharma et al. 2022). Similarly, long term consumption of the phytochemical resveratrol in Sprague–Dawley rats improved neurocognitive performance through the downregulation of inflammatory and oxidative stress pathways in the innate immune system (Garrigue et al. 2021). Resveratrol consumption also attenuated surgery-induced cognitive impairment and hippocampal neuroinflammation in aged rats through the downregulation of hippocampal microglial activity (Locatelli et al. 2018). Dietary supplementation with polyphenol-rich plant extract enhanced the median lifespan of obese mice by improved lipid metabolism and restriction in activation and infiltration of tissue macrophages in the adipose tissue (Aires et al. 2019). Consumption of the polyphenol syringaresinol to middle-aged mice delayed markers of immunosenescence by enhancing the numbers of total CD3 + T cells and naïve T cells, enhanced humoral immunity against influenza vaccination to the level of young control mice, and also attenuated inflamm-aging (Cho et al. 2016). An increase in the number of cytotoxic T-lymphocyte associated protein 4-positive cells and in the gene expression levels of CTLA-4, FoxP3, IL-10 and TGF-β was recorded after dietary supplementation with arachidin-1 and resveratrol in aged mice suggesting their role in successful aging of regulatory immune cells (Weng et al. 2016). Further, age-dependent stimulatory effects on T cell cytokine production were observed after in vitro exposure to the polyphenol-Oenothein B (Ramstead et al. 2015). Administration of Brazilian green propolis induced positive effects on innate and adaptive immune functions in aged mice characterized by enhanced phagocytosis and antibodies production (Gao et al. 2014). Consumption of Pu-erh tea extracts by senescence accelerated mice reversed elements of immunosenescence by significantly increasing the fractions of naïve T lymphocytes, CD8(+)CD28(+) T lymphocytes and NK cells in the peripheral blood, and concomitant decrease in the levels of proinflammatory IL-6 (Zhang et al. 2012). We also observed that oral administration of green tea catechin EGCG enhanced immune functions in aging mice by suppressing inflamm-aging, enhancing CD8 + T cell population, and modulation of antibody response (Sharma et al. 2017). Resveratrol supplementation in aged mice significantly increased the T helper cells (CD4(+)) population and delayed-type hypersensitivity response, and promoted the production of IgG without disturbing immunological homeostasis and therefore leading to immune rejuvenation (Yuan et al. 2012). Consumption of polyphenols derived from Cassia auriculata flowers by aged rats increased T and B cells percentage accompanied by an elevation of CD4+, CD8+, and CD4 + CD25 + regulatory cells along with enhanced proliferation of splenocytes in both resting and LPS-stimulated cells (John et al. 2011). Supplementation with polyphenol-rich biscuits in aged mice conferred immunomodulatory effects on both the innate and adaptive immune functions as compared to the control (De la Fuente et al. 2011). In another study, ovariectomy accelerated the age-related impairment of immune functions in old mice as well as the oxidative and proinflammatory imbalance, which was reversed by the administration of soybean isoflavones and green tea (Baeza et al. 2010).
Not only polyphenols, consumption of other bioactive phytochemicals, such as carotenoids, has also demonstrated potential modulatory effects in d-galactose induced aging rats by attenuating cellular and humoral markers of immunosenescence (Chen et al. 2020). Similarly, administration of the lignan Anwulignan reversed the effects of d-galactose induced immunosenescence in aged mice by suppressing circulatory levels of inflammatory cytokines, enhancing immunoglobulins production, reducing oxidative stress in spleen, and augmenting antioxidant defenses (Li et al. 2020). Further, when aged animals were fed with a novel wheat–lentil bread, a significant decrease in inflamm-aging and an increase in CD8 + T cells was observed (Carcea et al. 2019). Consumption of Chrysanthemum indicum plant extracts ameliorated the effects of d-galactose induced senescence in mice through decreased oxidative stress, inflammation, and apoptosis in various animal tissues (Zhang et al. 2019). Oral administration of β-1,3-glucans to aged male mice modulated immunosenescence characterized by a significant increase in T helper cells, the delayed-type hypersensitivity response, and immunoglobulin production (Song et al. 2020). The plant melatonin, i.e., phytomelatonin, is also emerging as a potential nutraceutical with pharmacological effects against cellular senescence and immunosenescence in experimental animals (Arnao and Hernández-Ruiz 2018; Atayik and Çakatay 2022a; Cruciani et al. 2022; Fernández-Ortiz et al. 2022; Srinivasan et al. 2005). The mechanisms governing the apparent cellular senescence and immunosenescence modulatory effects of phytochemicals are multifaceted but are linked to the longevity nutrient sensing pathways such as the Sirtuins and mTOR as well as transcription factors such as NRF-2 and NF-κB (Mannick et al. 2014; Micó et al. 2017; Robledinos-Antón et al. 2019). As such, pharmacological natural modulators of these pathways are considered important intermediates to extend longevity including through immunomodulation (Sharma 2021a).
Although preclinical studies using mice and other animals have been greatly useful in biomedical research; however, their association and translation with respect to humans is not always linear due to the evolutionary distance between species. This is starkly evident from the failure of a large percentage (up to 85%) of clinical trials despite promising results in preclinical animal studies (Ledford 2011; Mak et al. 2014). While this could be attributed to multiple reasons including dosage, route, and concerned disease; however, the differences in the physiologies of experimental animals and humans cannot be overlooked. It has been reported that despite having the same genes, species-dependent differences in cellular functions exist that contribute to considerable heterogeneity in cellular responses (Hodge et al. 2019). In terms of the immune system too, several characteristics changes in the innate and adaptive immune system, even during immunosenescence, have been identified which warrant a cautious approach in using and interpreting studies, especially related to immune modulatory preclinical work, for human translation (Goronzy and Weyand 2013; High et al. 2012; Mestas and Hughes 2004). Nonetheless, similar to preclinical reports, few clinical trials in elderly have directly demonstrated the immunosenescence modulatory effects of phytomolecules. For instance, a recent double-blind, randomized controlled pilot trial observed that consumption of non-digestible polysaccharide preparations significantly improved the humoral immune response of healthy seniors (50–79 years aged) against influenza vaccination (Laue et al. 2021). Similarly, administration of a fermented green banana-derived acidic glycoconjugate to 30 elderly bed-ridden patients for 8 weeks increased the antibody responses to influenza vaccination thereby countering the deleterious effects of immunosenescence (Horie et al. 2021). A randomized clinical trial on sixty-six old subjects (aged ≥ 60 years) revealed that 8-week long consumption of a polyphenol-rich diet significantly attenuated systemic inflamm-aging by improving gut dysbiosis (Del Bo et al. 2021). A Japanese case study observed preventive effects of coffee consumption on the occurrence of pneumonia in the elderly (Kondo et al. 2021). Another clinical trial reported that diet supplementation with combinations of resveratrol, pterostilbene, morin hydrate, quercetin, δ-tocotrienol, riboflavin, and nicotinic acid reduced cardiovascular risk factors and inflammation in healthy senior subjects (Qureshi et al. 2012).
Probiotics
The impact and relevance of commensal gut microbes in the development, maturation and regulation of immune response as well as maintenance of immune homeostasis is well recognized (Belkaid and Hand 2014; Zheng et al. 2020). The composition of the gut microbiota strongly influences the type of immune response, and the presence of dysbiotic gut microbiota is implicated in several inflammatory and immunological disorders including immunosenescence (Al-Rashidi 2022; DeJong et al. 2020; Shimizu et al. 2021; Toor et al. 2019). On the other hand, application of probiotics is considered useful for the maintenance of gut eubiosis and improved immunological response and homeostasis (Gagliardi et al. 2018; Maldonado Galdeano et al. 2019; Martin Manuel et al. 2017; Unno et al. 2015). Not only the gut, probiotics are known to influence several distal organs and functions through the generation of novel secretory metabolites that can enter circulation. Studies have also demonstrated that probiotics are particularly effective in altering different aspects of immunosenescence. For example, a recent study reported that consumption of probiotic Lactobacillus casei CRL 431 in aged mice not only improved the cellular and functional markers of immunosenescence but also restored the age-related loss in the thymus medulla (Balcells et al. 2022). Probiotic Lactobacillus plantarum JBC5 enhanced the lifespan of Caenorhabditis elegans through the modulation of multiple markers related to oxidative stress and innate immunity (Kumar et al. 2022). Application of a probiotic cocktail containing 5 Lactobacillus and 5 Enterococcus strains to old mice prevented the leaky gut by improving the expression of tight junction proteins that ultimately prevented unwarranted aggravation of intestinal immune cells and thus inflammation (Ahmadi et al. 2020). Supplementation of milk fermented with probiotic microbes improved the redox state and functions of peritoneal immune cells such as macrophages and NK cell in aged mice (Hunsche et al. 2019). Administration of Lactobacillus acidophilus DDS-1 to aging mice attenuated the proinflammatory profile in serum and colonic explants as compared to age match controls (Vemuri et al. 2019). Feeding accelerated aging Ercc1−/Δ7 mice with probiotic Akkermansia muciniphila for 10 weeks modulated the colonic immune profile by inhibiting B-cell migration, expression of genes related to inflammation along with a reduction in peritoneal resident macrophages thereby suggesting its anti-inflammatory effects for healthy aging (van der Lugt et al. 2019). In our previous study, we also observed that consumption of a synbiotic formulation containing green tea EGCG and probiotic Lactobacillus fermentum alleviated various aspects of immunosenescence as evidenced by increased proliferation and activation of CD3 + T cells as well as improved Th1/Th2 cytokines ratio in splenic culture supernatants (Sharma et al. 2019). Further, oral consumption of milk fermented with probiotic Lactobacillus rhamnosus to aged mice attenuated age-related Th1/Th2 cytokine imbalance, inflamm-aging, IgG1/IgG2a antibodies ratio, and also improved the immune response against pathogenic E. coli (Sharma et al. 2014a). Similarly, administration of probiotic fermented dahi increased the phagocytic potential and oxidative burst capacity in aging mice (Kaushal and Kansal 2014). When supplemented with probiotic Lactobacillus reuteri BM36301, aged mice exhibited gender-specific effects wherein male mice experienced less weight gain and higher testosterone level while females maintained a lower serum TNF-α levels as well as healthy skin with active folliculogenesis and hair growth (Lee et al. 2016).
Human randomized controlled studies have also reported immunosenescence alleviating effects of probiotics. A recent study observed that consumption of combination of the probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 in elderly subjects for 12 months enhanced seasonal influenza vaccination response despite limited effects on other immune functions (Castro-Herrera et al. 2021). A randomized, double-blind, placebo-controlled trial was conducted with 98 elderly subjects (aged 84.6 ± 7.8 years), supplemented for 30 days with a biscuit containing a probiotic mixture of Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 resulted in increased naive, activated memory, regulatory T cells, B cells, and NK cell activity compared with placebo suggesting strong influence on prevalent immunosenescence (Finamore et al. 2019). Several other studies have reported that consumption of probiotic bacteria in elderly subjects can protect against incidences of acute upper respiratory tract infections and augment vaccine response (Boge et al. 2009; Davidson et al. 2011; Fonollá et al. 2019; Jespersen et al. 2015; Pu et al. 2017). Further, immune cell subpopulations also appeared to change in elderly subjects supplemented with heat-killed Lactobacillus gasseri wherein CD8(+) T cells not only significantly increased in PBMCs, but expression of the co-stimulatory molecule CD28 did not exhibit age-dependent decline in expression as observed for the placebo group (Miyazawa et al. 2015). Similarly, increased levels of CD4 + T cells and reduced markers of inflammation were observed after ingestion of Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2 in older adults (Spaiser et al. 2015). Supplementation of a synbiotic composed of probiotic Lactobacillus rhamnosus GG and prebiotic corn fiber in healthy elderly enhanced NK cell response while decreasing systemic inflamm-aging (Costabile et al. 2017). In contrast, some studies have also observed limited or complete lack of immunomodulation by probiotic bacteria in elderly subjects which could be related to the efficacy of the particular type of probiotic strain used (Maruyama et al. 2016; Van Puyenbroeck et al. 2012). Studies assessing the role of probiotics in influencing cellular senescence and SASP are limited but emerging. It has been demonstrated that probiotic bacteria and their metabolites can directly suppress cellular senescence at least in vitro (Kumar et al. 2020a) while previous studies indicated that probiotic treatment can inhibit colonic senescence by downregulating the expression of cell cycle markers p53/p16 (Jeong et al. 2015a, b). However, to the best of our knowledge, there is no information whether probiotic treatment can also suppress cellular senescence in immune cells. Nonetheless, given the known immunosenescence modulatory as well as emerging anti-cellular senescence effects of probiotics, it seems prudent to assess their cellular senescence modulatory attributes in senescent immune cells.
Polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs), especially n-3 PUFA such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are important constituents of a healthy diet and are implicated in a myriad of health beneficial effects including against cardiovascular diseases, chronic inflammation, diabetes, and age-related cognitive decline (Shahidi and Ambigaipalan 2018). Fish and sea food are particularly rich sources of PUFAs and studies have demonstrated that PUFAs can impact certain features of cellular senescence and immunosenescence in immune cells per se. For instance, clinical trials have shown that consumption of n-3 PUFAs is associated with increased telomere length of blood leucocytes in elderly that results in improved proliferative response and attenuation of several markers of immunosenescence (Ali et al. 2022). A study on Chinese population concluded that higher plasma n6:n3 PUFA ratio, and lower EPA and DHA n-3 PUFAs were associated with shorter leucocyte telomere length and increased coronary artery disease (Chang et al. 2020). Another study observed that age-related telomere shortening in leucocytes can be attenuated in elderly subjects with mild cognitive impairments by PUFA supplementation for 6 months (O'Callaghan et al. 2014). A double-blind 4-month trial on older adults revealed that supplementation with n-3 PUFA increased telomere length in leucocytes which correlated with decreasing n-6:n-3 ratios and decreased oxidative and inflammatory stress thereby indicating that n-3 PUFAs can impact immune cell aging (Kiecolt-Glaser et al. 2013). Supplementation with EPA and DHA (2.5 g/day) in elderly subjects for 8 weeks reduced the circulatory markers of inflamm-aging (Tan et al. 2018). Similarly, consumption of PUFA by middle aged older adults reduced systemic inflamm-aging as evident by a decrease in markers of pro-inflammatory cytokines (Kiecolt-Glaser et al. 2012). Another randomized clinical trial observed that even very low consumption of marine oil (600 mg/day) for 6 weeks by elderly subjects can significantly improve the immune response by enhancing the proliferative potential and antioxidant capacity of lymphocytes (Bechoua et al. 2003). Animal studies have also demonstrated that consumption of fish oil and other PUFAs can attenuate several markers of age-related immunosenescence, inflamm-aging, and Th1/Th2 cytokine imbalance (de Gomes et al. 2018; Gheorghe et al. 2017; Jolly and Karri 2009). Despite these compelling observations, few studies have also reported the lack of any significant effect of n-3 PUFA administration on frailty and infection rates in the elderly (Bischoff-Ferrari et al. 2020; Orkaby et al. 2022). However, taken together, there appears to be enough data to suggest that PUFA may be effective in modulating aging immune cell functions including both immunosenescence and cellular senescence, and thus could be useful sources of potential anti-aging foods although their further characterization in terms of anti-cellular senescence effects is desirable.
Vitamins and minerals
Micronutrients such as vitamins and minerals are essential for immunocompetence and their nutritional deficiency is associated with inadequate immunological response (Alpert 2017; Djukic et al. 2014). Micronutrients can influence multiple aspects of immune functions such as activation of phagocytes, regulation of inflammation, antigen presentation, as well as humoral antibody response (Gombart et al. 2020). Studies have also shown that supplementation with certain vitamins and minerals can counter immunosenescence and boost the immune response in elderly. For instance, oral application of vitamins C and E to elderly subjects improved blood neutrophils and lymphocytes functions which were maintained even after 6 months of treatment (De la Fuente et al. 2020). Ex vivo application of vitamins C and E to lymphocytes isolated form healthy elderly significantly enhanced the proliferative response and attenuated oxidative stress (Bouamama et al. 2017). Similarly, long-term high-dose intake of vitamin C (200 mg/kg/day) in senescence marker protein-30 knockout mice for 1 year significantly enhanced the circulatory leucocyte profile and splenic T cell differentiation while also suppressing thymic involution with age (Uchio et al. 2015). Vitamin C application to old mice prevented age-related depletion of memory T cells in bone marrow while also augmenting the activation of antigen presenting cells (Meryk et al. 2020). In addition to vitamins C and E, clinical trials of vitamin D have observed improved response to vaccines and enhanced ability to resist respiratory infections in old age adults (Ginde et al. 2017; Goncalves-Mendes et al. 2019; Sadarangani et al. 2016). Using vitamin A, it was observed that retinoic acid had no significant effect on the leukocyte subpopulations or on the functions of PBMCs but enhanced the spontaneous migration and adhesion of neutrophils in elderly subjects (Minet-Quinard et al. 2010). Similarly, vitamin A levels were correlated with preserved neutrophil functions during aging in humans characterized by increased cellular migration and phagocytic activity (Farges et al. 2012).
Among minerals, the role of zinc is recognized as of particular significance in countering immunological aging (Baarz and Rink 2022). That elderly are often deficient in zinc suggesting a direct correlation between chronic inflammation, immunosenescence, and increased susceptibility to infections (Cabrera 2015). Studies have demonstrated that dietary supplementation with zinc can reverse several facets of immunosenescence as evident by increased naïve T-cell subset (Wong et al. 2020, 2021), improved immune cell functions (Barnett et al. 2016; Varin et al. 2008), increased thymopoiesis (Wong et al. 2009), attenuated inflamm-aging (Wong et al. 2013), and modulated Th1/Th2 immune homeostasis (Uciechowski et al. 2008) during aging. In addition to zinc, copper (Giacconi et al. 2017; Malavolta et al. 2015) and iron (Handono et al. 2021; Macciò and Madeddu 2012) deficiency is also correlated with aging and health, including immunosenescence. Further, there is sporadic evidence indicating that impaired metabolism of specific minerals in senescent non-leucocyte cells could be a prevalent phenomenon (Killilea and Ames 2008; La Fata et al. 2015; Masaldan et al. 2018a, b), and that supplementation with certain vitamins can delay cellular senescence (Chen et al. 2019; Jeong et al. 2017; La Fata et al. 2015; Ricciarelli et al. 2020). However, such information in immune cells is completely lacking and needs to be pursued. Further, it is important to consider that although elderly often have dietary deficiency of minerals such as zinc, copper, selenium, and iodine (Vural et al. 2020) as well as vitamins including vitamins D, K, and B (Fabian et al. 2012; Wei et al. 2019); indiscriminate supplementation of vitamins and minerals in individuals with no clinical deficiency is considered controversial, especially related to non-communicable diseases (Zhang et al. 2020b), and could even be harmful (Hamishehkar et al. 2016). Moreover, supplementation with multivitamins and minerals is not always sufficient to alter immune status in the elderly population as evidenced recently (Fantacone et al. 2020), and thus a cautious approach in this regard is warranted. Table 2 summarizes selected studies demonstrating nutritional modulators of immunological aging.
Table 2.
Representative examples of modulation of immunological aging through major dietary bioactive factors
| S. no. | Dietary factors | Reported effects | Experimental model | References |
|---|---|---|---|---|
| 1 | EGCG | Anti-SASP and anti-cellular senescence effects ex vivo | Murine macrophages | Kumar et al. (2020b) |
| 2 | Resveratrol | Suppression of inflamm-aging and oxidative stress in immune cells in vivo | Sprague-Dawley rats | Garrigue et al. (2021) |
| 3 | Lignan | Anti-immunosenescence effects in vivo | ICR mice | Li et al. (2020) |
| 4 | Carotenoids | Improved immunoglobulins profile during aging in vivo | Rats | Chen et al. (2020) |
| 5 | Polysaccharide preparations | Improved humoral immune response against influenza vaccine during aging | Clinical trial | Laue et al. (2021) |
| 6 | Polyphenol-rich diet | Anti-inflammaging effects and gut eubiosis during aging | Clinical trial | Del Bo et al. (2021) |
| 7 | Lactobacillus casei CRL 431 | Anti-immunosenescence effects including on thymus | Aged mice | Balcells et al. (2022) |
| 8 | Probiotic Akkermansia muciniphila | Modulation of colonic B cell migration and inflammation | Aging Ercc1-/Δ7 mice | van der Lugt et al. (2019) |
| 9 | Synbiotic preparation | Increased proliferation and activation of CD3+ T cells | Aged mice | Sharma et al. (2019) |
| 10 | n-3 PUFAs | Increased telomere length of blood leucocytes | Clinical trial | Ali et al. (2022) |
| 11 | EPA and DHA | Anti-inflamm-aging | Clinical trial | Tan et al. (2018) |
| 12 | Vitamins C&E | Improved neutrophils and lymphocyte functional markers | Clinical trial | De la Fuente et al. (2020) |
| 13 | Zinc | Increased naïve T-cell subset | Aged mice | Wong et al. (2020) |
Conclusions and future prospects
How to define an aged immune cell? The answer to this deceptively simple question is challenging since, as discussed in this manuscript, the immune cells are unique to undergo two different and mutually inclusive age-dependent processes, i.e., cellular senescence and immunosenescence. Besides, understanding aging in immune cells has always remained ambiguous due to the limitations and contradictions associated with immunosenescence (Pawelec et al. 2020), and the emerging significance of cellular senescence in immune cells only adds to this conundrum. The relevance of the emerging immunoadaptation view of immunosenescence in cellular senescence in immune cells is also not known. However, in this regard, it is interesting to note that unlike non-leucocyte cells, contradictions among characteristic effects of cellular senescence in immune cells such as macrophages have already been reported (Hall et al. 2017; Stojiljkovic et al. 2019), which perhaps suggest a similar plasticity akin to immunosenescence that, however, needs further exploration. Nonetheless, the emerging cellular senescence-based perspective in understanding of the aging immune system as well as developing mitigative nutritional therapies has prompted more questions than answers. There are several niche research areas that need immediate attention:
The extent and depth of cellular senescence in immune cells is not fully understood, particularly in innate immune cells including macrophages and DCs, which is important considering their heterogeneity and tissue-specific niches. Although such attempts have been made recently, but elucidation of cellular senescence in immune cells is still much limited as compared to immunosenescence. Moreover, several available reports are contradictory which possibly indicate an unexpected role of cellular senescence in these cells.
More significantly, the impact and biological relevance of cellular senescence in immune cells in vivo is little understood and lacks conclusive evidence in natural aging settings. Besides, since immune cells constantly interact with tissue SC (and SASP thereof), how tissue SC can impact aging in resident immune cells (such as macrophages) is only beginning to be understood.
Delineating the dichotomy of cellular senescence and immunosenescence in aging immune cells should be the ultimate goal. Studies focused on the molecular etiology of these processes are thus highly desirable for specifying the functional features of cellular senescence and immunosenescence.
The relationship between accumulation of SC in tissues and the aging immune system is still developing and unclear. There seems to be causative association, but is yet to be verified in vivo during natural aging. The effects of adaptive immune system aging and increased SC turnover with age is even less understood.
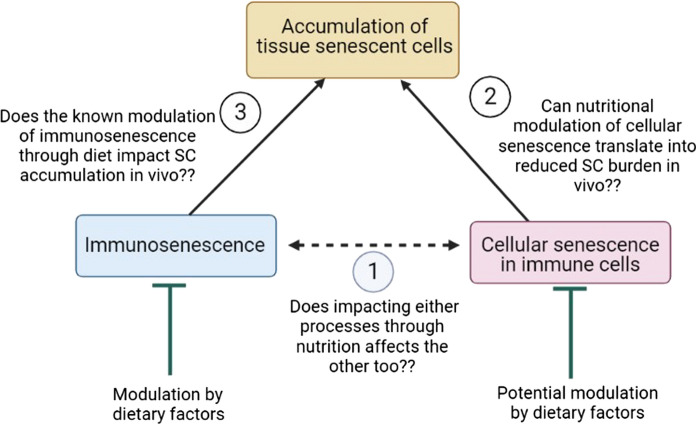
Functional foods and especially curated diets, such as the Mediterranean diet or plant-based diets, have shown the potential to suppress inflamm-aging as well as cellular senescence (Canudas et al. 2020; Crous-Bou et al. 2019; García-Calzón et al. 2015; Sharma and Diwan 2022). However, studies identifying nutritional targets of cellular senescence in immune cells per se are severely limited and thus efforts should be made to assess the immune modulating potential of natural bioactive compounds and/or healthy diets within the purview of cellular senescence (Fig. 5).
Similarly, whether the apparent immunosenescence modulatory effects of dietary factors also result in improved immune functions with regard to clearance of natural SC in vivo is not known (Fig. 5). This is of significance as it could unravel a direct correlation between improved immune functions and senescence immunotherapy. It is therefore desirable that future investigations study the immune rejuvenation potential of putative nutraceuticals within the purview of cellular senescence for a more meaningful and integrative understanding.
Fig. 5.
Dietary factors can influence different aspects of aging by impacting both cellular senescence (both in immune and non-leucocyte cells) and immunosenescence (to a relatively larger extent). However, whether their known anti-immunosenescence or anti-cellular senescence effects can also translate into enhanced removal of tissue senescent cells in vivo and thus systemic improvements in organismal senescent cell burden remains to be elucidated
In conclusion, cellular senescence is emerging as an impactful phenomenon in immune cell aging which should be considered not only for truly comprehending the aging physiology in the immune system but also for implementing nutritional strategies aimed at potentially rejuvenating the aging immune system.
Acknowledgements
RS acknowledges a grant from the Department of Science and Technology, Government of India under the INSPIRE Faculty scheme (Grant No. IFA17-LSPA79). JW acknowledges the Polish Ministry of Science and Higher Education statutory Grants No. 02-0058/07/262.
Authors' contributions
RS conceived the idea, wrote the initial draft, and edited the manuscript. BD curated data and prepared figures. AS and JW edited and reviewed the manuscript. All authors read and approved the final manuscript.
Data availability
The manuscript has no associated data.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rohit Sharma, Email: rohit25sharma@gmail.com.
Jacek M. Witkowski, Email: jacek.witkowski@gumed.edu.pl
References
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178(11):6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, Andle J, Lee TB, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129–142.e124. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi S, Wang S, Nagpal R, Wang B, Jain S, Razazan A, Mishra SP, Zhu X, Wang Z, Kavanagh K, et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020 doi: 10.1172/jci.insight.132055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A, Accardi G, Candore G, Carruba G, Davinelli S, Passarino G, Scapagnini G, Vasto S, Caruso C. Nutrigerontology: a key for achieving successful ageing and longevity. Immunity Ageing. 2016;13(1):17. doi: 10.1186/s12979-016-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires V, Labbé J, Deckert V, Pais de Barros JP, Boidot R, Haumont M, Maquart G, Le Guern N, Masson D, Prost-Camus E, et al. Healthy adiposity and extended lifespan in obese mice fed a diet supplemented with a polyphenol-rich plant extract. Sci Rep. 2019;9(1):9134. doi: 10.1038/s41598-019-45600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Scapagnini G, Davinelli S. Effect of omega-3 fatty acids on the telomere length: a mini meta-analysis of clinical trials. Biomol Concepts. 2022;13(1):25–33. doi: 10.1515/bmc-2021-0024. [DOI] [PubMed] [Google Scholar]
- Allen JC, Toapanta FR, Chen W, Tennant SM. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine. 2020;38(52):8264–8272. doi: 10.1016/j.vaccine.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro Ados S, Falcão RR, Abdelhay E, Bouzas LF, Thuler LC, Ornellas MH, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72(4):319–329. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Alpert PT. The role of vitamins and minerals on the immune system. Home Health Care Manag Pract. 2017;29(3):199–202. doi: 10.1177/1084822317713300. [DOI] [Google Scholar]
- Al-Rashidi HE. Gut microbiota and immunity relevance in eubiosis and dysbiosis. Saudi J Biol Sci. 2022;29(3):1628–1643. doi: 10.1016/j.sjbs.2021.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonangeli F, Soriani A, Ricci B, Ponzetta A, Benigni G, Morrone S, Bernardini G, Santoni A. Natural killer cell recognition of in vivo drug-induced senescent multiple myeloma cells. Oncoimmunology. 2016;5(10):e1218105. doi: 10.1080/2162402x.2016.1218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Sauce D. Naive t cells: the crux of cellular immune aging? Exp Gerontol. 2014;54:90–93. doi: 10.1016/j.exger.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. The potential of phytomelatonin as a nutraceutical. Molecules. 2018 doi: 10.3390/molecules23010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayik MC, Çakatay U. Melatonin-related signaling pathways and their regulatory effects in aging organisms. Biogerontology. 2022;23(5):529–539. doi: 10.1007/s10522-022-09981-y. [DOI] [PubMed] [Google Scholar]
- Atayik MC, Çakatay U. Mitochondria-targeted senotherapeutic interventions. Biogerontology. 2022;23(4):401–423. doi: 10.1007/s10522-022-09973-y. [DOI] [PubMed] [Google Scholar]
- Austad SN, Hoffman JM. Is antagonistic pleiotropy ubiquitous in aging biology? Evol Med Public Health. 2018;2018(1):287–294. doi: 10.1093/emph/eoy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarz BR, Rink L. Rebalancing the unbalanced aged immune system—a special focus on zinc. Ageing Res Rev. 2022;74:101541. doi: 10.1016/j.arr.2021.101541. [DOI] [PubMed] [Google Scholar]
- Baeza I, De Castro NM, Arranz L, De la Fuente M. Soybean and green tea polyphenols improve immune function and redox status in very old ovariectomized mice. Rejuvenat Res. 2010;13(6):665–674. doi: 10.1089/rej.2010.1049. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. Naturally occurring p16(ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells F, Martínez Monteros MJ, Gómez AL, Cazorla SI, Perdigón G, Maldonado-Galdeano C. Probiotic consumption boosts thymus in obesity and senescence mouse models. Nutrients. 2022 doi: 10.3390/nu14030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Mechanisms of development of multimorbidity in the elderly. Eur Respir J. 2015;45(3):790. doi: 10.1183/09031936.00229714. [DOI] [PubMed] [Google Scholar]
- Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, et al. Effect of zinc supplementation on serum zinc concentration and t cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016;103(3):942–951. doi: 10.3945/ajcn.115.115188. [DOI] [PubMed] [Google Scholar]
- Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, Garcia-Velasquez E, Savastano S, Colao A. Nutrition and immune system: from the mediterranean diet to dietary supplementary through the microbiota. Crit Rev Food Sci Nutr. 2021;61(18):3066–3090. doi: 10.1080/10408398.2020.1792826. [DOI] [PubMed] [Google Scholar]
- Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. Sars-cov-2, Covid-19 and the aging immune system. Nature Aging. 2021;1(9):769–782. doi: 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechoua S, Dubois M, Véricel E, Chapuy P, Lagarde M, Prigent AF. Influence of very low dietary intake of marine oil on some functional aspects of immune cells in healthy elderly people. Br J Nutr. 2003;89(4):523–531. doi: 10.1079/bjn2002805. [DOI] [PubMed] [Google Scholar]
- Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67(5):827–836. doi: 10.1136/gutjnl-2016-312940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl T, Kumar K, Brisc C, Rus M, Nistor-Cseppento DC, Bustea C, Aron RAC, Pantis C, Zengin G, Sehgal A, et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother. 2021;133:110959. doi: 10.1016/j.biopha.2020.110959. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34(23–24):1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, Felson DT, McCloskey EV, Watzl B, Hofbauer LC, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the do-health randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging is not programmed: genetic pseudo-program is a shadow of developmental growth. Cell Cycle. 2013;12(24):3736–3742. doi: 10.4161/cc.27188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75(2):342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage tlr2- and tlr4-mediated pro-inflammatory responses without affecting the il-2-stimulated pathway. Mech Ageing Dev. 2005;126(12):1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Boge T, Rémigy M, Vaudaine S, Tanguy J, Bourdet-Sicard R, van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27(41):5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- Borghesan M, Hoogaars WMH, Varela-Eirin M, Talma N, Demaria M. A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol. 2020;30(10):777–791. doi: 10.1016/j.tcb.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Borgoni S, Kudryashova KS, Burka K, de Magalhães JP. Targeting immune dysfunction in aging. Ageing Res Rev. 2021;70:101410. doi: 10.1016/j.arr.2021.101410. [DOI] [PubMed] [Google Scholar]
- Bouamama S, Merzouk H, Medjdoub A, Merzouk-Saidi A, Merzouk SA. Effects of exogenous vitamins a, c, and e and nadh supplementation on proliferation, cytokines release, and cell redox status of lymphocytes from healthy aged subjects. Appl Physiol Nutr Metab. 2017;42(6):579–587. doi: 10.1139/apnm-2016-0201. [DOI] [PubMed] [Google Scholar]
- Brigger D, Riether C, van Brummelen R, Mosher KI, Shiu A, Ding Z, Zbären N, Gasser P, Guntern P, Yousef H, et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab. 2020;2(8):688–702. doi: 10.1038/s42255-020-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol. 2013;190(4):1746–1757. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryl E, Witkowski JM. Decreased proliferative capability of CD4(+) cells of elderly people is associated with faster loss of activation-related antigens and accumulation of regulatory t cells. Exp Gerontol. 2004;39(4):587–595. doi: 10.1016/j.exger.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Budamagunta V, Foster TC, Zhou D. Cellular senescence in lymphoid organs and immunosenescence. Aging (albany NY) 2021;13(15):19920–19941. doi: 10.18632/aging.203405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut O, Kilic G, Domínguez-Andrés J. Immune memory in aging: a wide perspective covering microbiota, brain, metabolism, and epigenetics. Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DGA, Stolzing A. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev. 2018;43:17–25. doi: 10.1016/j.arr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Butcher S, Chahel H, Lord JM. Review article: ageing and the neutrophil: No appetite for killing? Immunology. 2000;100(4):411–416. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera ÁJR. Zinc, aging, and immunosenescence: an overview. Pathobiol Aging Age Relat Dis. 2015;5:25592–25592. doi: 10.3402/pba.v5.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, Heinrich J, Garn H, Koletzko S, Lack G, et al. Early nutrition and immunity—progress and perspectives. Br J Nutr. 2006;96(4):774–790. [PubMed] [Google Scholar]
- Callender LA, Carroll EC, Beal RWJ, Chambers ES, Nourshargh S, Akbar AN, Henson SM. Human CD8(+) emra t cells display a senescence-associated secretory phenotype regulated by p38 mapk. Aging Cell. 2018 doi: 10.1111/acel.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canudas S, Becerra-Tomás N, Hernández-Alonso P, Galié S, Leung C, Crous-Bou M, De Vivo I, Gao Y, Gu Y, Meinilä J, et al. Mediterranean diet and telomere length: a systematic review and meta-analysis. Adv Nutr. 2020;11(6):1544–1554. doi: 10.1093/advances/nmaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcea M, Turfani V, Narducci V, Durazzo A, Finamore A, Roselli M, Rami R. Bread for the aging population: the effect of a functional wheat-lentil bread on the immune function of aged mice. Foods. 2019;8(10):510. doi: 10.3390/foods8100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Herrera VM, Fisk HL, Wootton M, Lown M, Owen-Jones E, Lau M, Lowe R, Hood K, Gillespie D, Hobbs FDR, et al. Combination of the probiotics lacticaseibacillus rhamnosus gg and bifidobacterium animalis subsp. Lactis, bb-12 has limited effect on biomarkers of immunity and inflammation in older people resident in care homes: results from the probiotics to reduce infections in care home residents randomized, controlled trial. Front Immunol. 2021;12:6421. doi: 10.3389/fimmu.2021.643321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Dorajoo R, Sun Y, Wang L, Ong CN, Liu J, Khor CC, Yuan JM, Koh WP, Friedlander Y, et al. Effect of plasma polyunsaturated fatty acid levels on leukocyte telomere lengths in the Singaporean Chinese population. Nutr J. 2020;19(1):119. doi: 10.1186/s12937-020-00626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatta GS, Andrews RG, Rodger E, Schrag M, Hammond WP, Dale DC. Hematopoietic progenitors and aging: alterations in granulocytic precursors and responsiveness to recombinant human g-csf, gm-csf, and il-3. J Gerontol. 1993;48(5):M207–212. doi: 10.1093/geronj/48.5.m207. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang R, Qiao W, Zhang W, Chen J, Mao L, Goltzman D, Miao D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell. 2019;18(3):e12951. doi: 10.1111/acel.12951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen Z, Xiao J, Liu H, Yao K, Hou X, Cao Y, Liu X. Astaxanthin attenuates oxidative stress and immune impairment in d-galactose-induced aging in rats by activating the nrf2/keap1 pathway and suppressing the nf-κb pathway. Food Funct. 2020;11(9):8099–8111. doi: 10.1039/d0fo01663b. [DOI] [PubMed] [Google Scholar]
- Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients. 2019;11(8):1933. doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SY, Kim J, Lee JH, Sim JH, Cho DH, Bae IH, Lee H, Seol MA, Shin HM, Kim TJ, et al. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci Rep. 2016;6:39026. doi: 10.1038/srep39026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čičin-Šain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Žugich D, Legasse A, Axthelm MK, et al. Dramatic increase in naïve t cell turnover is linked to loss of naïve t cells from old primates. Proc Natl Acad Sci. 2007;104(50):19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DA, Ferreira IATM, Kotagiri P, Datir RP, Lim EY, Touizer E, Meng B, Abdullahi A, Baker S, Dougan G, et al. Age-related immune response heterogeneity to sars-Cov-2 vaccine bnt162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna-Romano G, Bulati M, Aquino A, Pellicanò M, Vitello S, Lio D, Candore G, Caruso C. A double-negative (igd-CD27–) b cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev. 2009;130(10):681–690. doi: 10.1016/j.mad.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Conte M, Martucci M, Chiariello A, Franceschi C, Salvioli S. Mitochondria, immunosenescence and inflammaging: A role for mitokines? Semin Immunopathol. 2020;42(5):607–617. doi: 10.1007/s00281-020-00813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa RCG, Peralta RM, Haminiuk CWI, Maciel GM, Bracht A, Ferreira I. New phytochemicals as potential human anti-aging compounds: reality, promise, and challenges. Crit Rev Food Sci Nutr. 2018;58(6):942–957. doi: 10.1080/10408398.2016.1233860. [DOI] [PubMed] [Google Scholar]
- Costabile A, Bergillos-Meca T, Rasinkangas P, Korpela K, de Vos WM, Gibson GR. Effects of soluble corn fiber alone or in synbiotic combination with lactobacillus rhamnosus gg and the pilus-deficient derivative gg-pb12 on fecal microbiota, metabolism, and markers of immune function: a randomized, double-blind, placebo-controlled, crossover study in healthy elderly (saimes study) Front Immunol. 2017;8:1443. doi: 10.3389/fimmu.2017.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covre LP, Martins RF, Devine OP, Chambers ES, Vukmanovic-Stejic M, Silva JA, Dietze R, Rodrigues RR, de Matos Guedes HL, Falqueto A, et al. Circulating senescent t cells are linked to systemic inflammation and lesion size during human cutaneous leishmaniasis. Front Immunol. 2018;9:3001. doi: 10.3389/fimmu.2018.03001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous-Bou M, Molinuevo JL, Sala-Vila A. Plant-rich dietary patterns, plant foods and nutrients, and telomere length. Adv Nutr. 2019;10(Suppl_4):S296–S303. doi: 10.1093/advances/nmz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani S, Garroni G, Pala R, Barcessat ARP, Facchin F, Ventura C, Fozza C, Maioli M. Melatonin finely tunes proliferation and senescence in hematopoietic stem cells. Eur J Cell Biol. 2022;101(3):151251. doi: 10.1016/j.ejcb.2022.151251. [DOI] [PubMed] [Google Scholar]
- Cui CY, Driscoll RK, Piao Y, Chia CW, Gorospe M, Ferrucci L. Skewed macrophage polarization in aging skeletal muscle. Aging Cell. 2019;18(6):e13032. doi: 10.1111/acel.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. Lactobacillus gg as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65(4):501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi S, Meschiari M, Gibellini L, Bellinazzi C, Borella R, Fidanza L, Gozzi L, Iannone A, Lo Tartaro D, Mattioli M, et al. Marked t cell activation, senescence, exhaustion and skewing towards th17 in patients with covid-19 pneumonia. Nat Commun. 2020;11(1):3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gomes MG, Souza LC, Goes AR, Del Fabbro L, Filho CB, Donato F, Prigol M, Luchese C, Roman SS, Puntel RL, et al. Fish oil ameliorates sickness behavior induced by lipopolysaccharide in aged mice through the modulation of kynurenine pathway. J Nutr Biochem. 2018;58:37–48. doi: 10.1016/j.jnutbio.2018.05.002. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15(26):3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Medina S, Baeza I, Jiménez L. Improvement of leucocyte functions in mature and old mice after 15 and 30 weeks of diet supplementation with polyphenol-rich biscuits. Eur J Nutr. 2011;50(7):563–573. doi: 10.1007/s00394-010-0163-2. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Sánchez C, Vallejo C, Díaz-Del Cerro E, Arnalich F, Hernanz Á. Vitamin C and vitamin C plus e improve the immune function in the elderly. Exp Gerontol. 2020;142:111118. doi: 10.1016/j.exger.2020.111118. [DOI] [PubMed] [Google Scholar]
- Decout A, Katz JD, Venkatraman S, Ablasser A. The cgas–sting pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21(9):548–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28(2):180–189. doi: 10.1016/j.chom.2020.07.013. [DOI] [PubMed] [Google Scholar]
- Del Bo C, Bernardi S, Cherubini A, Porrini M, Gargari G, Hidalgo-Liberona N, González-Domínguez R, Zamora-Ros R, Peron G, Marino M, et al. A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the maple randomised controlled trial. Clin Nutr. 2021;40(5):3006–3018. doi: 10.1016/j.clnu.2020.12.014. [DOI] [PubMed] [Google Scholar]
- Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122(2):220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Deng B, Zhang W, Zhu Y, Li Y, Li D, Li B. Foxp3+ regulatory t cells and age-related diseases. FEBS J. 2021 doi: 10.1111/febs.15743. [DOI] [PubMed] [Google Scholar]
- Desdín-Micó G, Soto-Heredero G, Aranda Juan F, Oller J, Carrasco E, Gabandé-Rodríguez E, Blanco Eva M, Alfranca A, Cussó L, Desco M, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. 2020;368(6497):1371–1376. doi: 10.1126/science.aax0860. [DOI] [PubMed] [Google Scholar]
- Dewald HK, Martínez-Zamudio RI, Vasilopoulos T, Herbig U, Fitzgerald-Bocarsly P. Senescence-associated β-galactosidase activity and other markers of senescence are present in human peripheral blood mononuclear cells during healthy aging. J Immunol. 2020;204(1 Supplement):154.115. [Google Scholar]
- Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLoreto R, Murphy CT. The cell biology of aging. Mol Biol Cell. 2015;26(25):4524–4531. doi: 10.1091/mbc.E14-06-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to ifn-gamma for enhanced oxidative metabolism. J Immunol. 1994;153(5):2146–2152. [PubMed] [Google Scholar]
- Ding J, Lei L, Liu S, Zhang Y, Yu Z, Su Y, Ma X. Macrophages are necessary for skin regeneration during tissue expansion. J Transl Med. 2019;17(1):36. doi: 10.1186/s12967-019-1780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic M, Onken ML, Schütze S, Redlich S, Götz A, Hanisch U-K, Bertsch T, Ribes S, Hanenberg A, Schneider S, et al. Vitamin d deficiency reduces the immune response, phagocytosis rate, and intracellular killing rate of microglial cells. Infect Immun. 2014;82(6):2585–2594. doi: 10.1128/IAI.01814-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. Send in the senolytics. Nat Biotechnol. 2020;38(12):1371–1377. doi: 10.1038/s41587-020-00750-1. [DOI] [PubMed] [Google Scholar]
- Dunne PJ, Belaramani L, Fletcher JM, Fernandez de Mattos S, Lawrenz M, Soares MV, Rustin MH, Lam EW, Salmon M, Akbar AN. Quiescence and functional reprogramming of Epstein–Barr Virus (EBV)-specific CD8+ t cells during persistent infection. Blood. 2005;106(2):558–565. doi: 10.1182/blood-2004-11-4469. [DOI] [PubMed] [Google Scholar]
- Fabian E, Bogner M, Kickinger A, Wagner KH, Elmadfa I. Vitamin status in elderly people in relation to the use of nutritional supplements. J Nutr Health Aging. 2012;16(3):206–212. doi: 10.1007/s12603-011-0159-5. [DOI] [PubMed] [Google Scholar]
- Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207(11):2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantacone ML, Lowry MB, Uesugi SL, Michels AJ, Choi J, Leonard SW, Gombart SK, Gombart JS, Bobe G, Gombart AF. The effect of a multivitamin and mineral supplement on immune function in healthy older adults: a double-blind, randomized, controlled trial. Nutrients. 2020;12(8):2447. doi: 10.3390/nu12082447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farges MC, Minet-Quinard R, Walrand S, Thivat E, Ribalta J, Winklhofer-Roob B, Rock E, Vasson MP. Immune status is more affected by age than by carotenoid depletion-repletion in healthy human subjects. Br J Nutr. 2012;108(11):2054–2065. doi: 10.1017/s0007114512000177. [DOI] [PubMed] [Google Scholar]
- Fernández-Ortiz M, Sayed RKA, Román-Montoya Y, de Lama MÁR, Fernández-Martínez J, Ramírez-Casas Y, Florido-Ruiz J, Rusanova I, Escames G, Acuña-Castroviejo D. Age and chronodisruption in mouse heart: effect of the NLRP3 inflammasome and melatonin therapy. Int J Mol Sci. 2022 doi: 10.3390/ijms23126846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finamore A, Roselli M, Donini L, Brasili DE, Rami R, Carnevali P, Mistura L, Pinto A, Giusti A, Mengheri E. Supplementation with bifidobacterium longum bar33 and lactobacillus helveticus bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition. 2019;63–64:184–192. doi: 10.1016/j.nut.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Fonollá J, Gracián C, Maldonado-Lobón JA, Romero C, Bédmar A, Carrillo JC, Martín-Castro C, Cabrera AL, García-Curiel JM, Rodríguez C, et al. Effects of lactobacillus coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: a randomized controlled trial. Eur J Nutr. 2019;58(1):83–90. doi: 10.1007/s00394-017-1573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol Ser A. 2014;69(Suppl_1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fülöp T, Fóris G, Leövey A. Age-related changes in cAMP and cGMP levels during phagocytosis in human polymorphonuclear leukocytes. Mech Ageing Dev. 1984;27(2):233–237. doi: 10.1016/0047-6374(84)90048-4. [DOI] [PubMed] [Google Scholar]
- Fülöp T, Jr, Fóris G, Wórum I, Leövey A. Age-dependent alterations of Fc gamma receptor-mediated effector functions of human polymorphonuclear leucocytes. Clin Exp Immunol. 1985;61(2):425–432. [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Dupuis G, Baehl S, Le Page A, Bourgade K, Frost E, Witkowski JM, Pawelec G, Larbi A, Cunnane S. From inflamm-aging to immune-paralysis: a slippery slope during aging for immune-adaptation. Biogerontology. 2016;17(1):147–157. doi: 10.1007/s10522-015-9615-7. [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front Immunol. 2018;8:1960–1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Hirokawa K, Cohen AA, Witkowski JM. Immunosenescence is both functional/adaptive and dysfunctional/maladaptive. Semin Immunopathol. 2020;42(5):521–536. doi: 10.1007/s00281-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Pawelec G, Khalil A, Cohen AA, Hirokawa K, Witkowski JM, Franceschi C. Immunology of aging: the birth of inflammaging. Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi A, Totino V, Cacciotti F, Iebba V, Neroni B, Bonfiglio G, Trancassini M, Passariello C, Pantanella F, Schippa S. Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health. 2018;15(8):1679. doi: 10.3390/ijerph15081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Wu J, Wei J, Pu L, Guo C, Yang J, Yang M, Luo H. Brazilian green propolis improves immune function in aged mice. J Clin Biochem Nutr. 2014;55(1):7–10. doi: 10.3164/jcbn.13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Calzón S, Zalba G, Ruiz-Canela M, Shivappa N, Hébert JR, Martínez JA, Fitó M, Gómez-Gracia E, Martínez-González MA, Marti A. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the predimed-navarra study: cross-sectional and longitudinal analyses over 5 y. Am J Clin Nutr. 2015;102(4):897–904. doi: 10.3945/ajcn.115.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JK, Mamotte CDS, Jackaman C, Nelson DJ. Modulation of dendritic cell and t cell cross-talk during aging: the potential role of checkpoint inhibitory molecules. Ageing Res Rev. 2017;38:40–51. doi: 10.1016/j.arr.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Garg SK, Delaney C, Toubai T, Ghosh A, Reddy P, Banerjee R, Yung R. Aging is associated with increased regulatory t-cell function. Aging Cell. 2014;13(3):441–448. doi: 10.1111/acel.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigue P, Mounien L, Champion S, Mouhajir Y, Pechere L, Guillet B, Landrier JF, Seree E. Long-term administration of resveratrol at low doses improves neurocognitive performance as well as cerebral blood flow and modulates the inflammatory pathways in the brain. J Nutr Biochem. 2021;97:108786. doi: 10.1016/j.jnutbio.2021.108786. [DOI] [PubMed] [Google Scholar]
- Gasparoto TH, Dalboni TM, Amôr NG, Abe AE, Perri G, Lara VS, Vieira NA, Gasparoto CT, Campanelli AP. Fcγ receptors on aging neutrophils. J Appl Oral Sci. 2021;29:e20200770–e20200770. doi: 10.1590/1678-7757-2020-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghe A, Pérez de Heredia F, Hunsche C, Redondo N, Díaz LE, Hernández O, Marcos A, De la Fuente M. Oxidative stress and immunosenescence in spleen of obese mice can be reversed by 2-hydroxyoleic acid. Exp Physiol. 2017;102(5):533–544. doi: 10.1113/ep086157. [DOI] [PubMed] [Google Scholar]
- Giacconi R, Costarelli L, Piacenza F, Basso A, Rink L, Mariani E, Fulop T, Dedoussis G, Herbein G, Provinciali M, et al. Main biomarkers associated with age-related plasma zinc decrease and copper/zinc ratio in healthy elderly from zincage study. Eur J Nutr. 2017;56(8):2457–2466. doi: 10.1007/s00394-016-1281-2. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, Kyriazopoulou E, Gkavogianni T, Adami ME, Damoraki G, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315–323.e319. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, Nilsson BO, Wikby A, Kipling D, Dunn-Walters DK. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8(1):18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J. Cellular senescence causes ageing. Nat Rev Mol Cell Biol. 2019;20(7):388–388. doi: 10.1038/s41580-019-0128-0. [DOI] [PubMed] [Google Scholar]
- Gil A, Rueda R. Interaction of early diet and the development of the immune system. Nutr Res Rev. 2002;15(2):263–292. doi: 10.1079/nrr200248. [DOI] [PubMed] [Google Scholar]
- Gil del Valle L. Oxidative stress in aging: theoretical outcomes and clinical evidences in humans. Biomed Aging Pathol. 2011;1(1):1–7. doi: 10.1016/j.biomag.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic t cells. Nature. 1977;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, Schwartz RS. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496–503. doi: 10.1111/jgs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez CR, Karavitis J, Palmer JL, Faunce DE, Ramirez L, Nomellini V, Kovacs EJ. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators Inflamm. 2010;2010:475139. doi: 10.1155/2010/475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves-Mendes N, Talvas J, Dualé C, Guttmann A, Corbin V, Marceau G, Sapin V, Brachet P, Evrard B, Laurichesse H, et al. Impact of vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. doi: 10.3389/fimmu.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17(5):468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Successful and maladaptive t cell aging. Immunity. 2017;46(3):364–378. doi: 10.1016/j.immuni.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Mechanisms underlying t cell ageing. Nat Rev Immunol. 2019;19(9):573–583. doi: 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounder SS, Abdullah BJJ, Radzuanb NEIBM, Zain FDBM, Sait NBM, Chua C, Subramani B. Effect of aging on NK cell population and their proliferation at ex vivo culture condition. Anal Cell Pathol (amst) 2018;2018:7871814–7871814. doi: 10.1155/2018/7871814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+CD25 high regulatory t cells increases with age. Clin Exp Immunol. 2005;140(3):540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11C+CD4-CD8 alpha-dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61(10):1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Can Res. 2008;68(15):6341. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Tilburgs T, Wong B, Strominger JL. Dysfunction of dendritic cells in aged c57bl/6 mice leads to failure of natural killer cell activation and of tumor eradication. Proc Natl Acad Sci. 2014;111(39):14199. doi: 10.1073/pnas.1414780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin I, et al. Aging of mice is associated with p16(ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (albany NY) 2016;8(7):1294–1315. doi: 10.18632/aging.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin II, et al. P16(ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (albany NY) 2017;9(8):1867–1884. doi: 10.18632/aging.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamishehkar H, Ranjdoost F, Asgharian P, Mahmoodpoor A, Sanaie S. Vitamins, are they safe? Adv Pharm Bull. 2016;6(4):467–477. doi: 10.15171/apb.2016.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handono K, Wahono CS, Pratama MZ, Kalim H. Association of the premature immunosenescence with the presence and severity of anemia among patients with systemic lupus erythematosus. Lupus. 2021;30(12):1906–1914. doi: 10.1177/09612033211038057. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ t cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeldine J, Hampson P, Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11(5):751–759. doi: 10.1111/j.1474-9726.2012.00839.x. [DOI] [PubMed] [Google Scholar]
- He SWJ, van de Garde MDB, Pieren DKJ, Poelen MCM, Voß F, Abdullah MR, Hammerschmidt S, van Els CACM. Diminished pneumococcal-specific CD4+ t-cell response is associated with increased regulatory t cells at older age. Front Aging. 2021 doi: 10.3389/fragi.2021.746295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK, Tooze SA, et al. P38 signaling inhibits mtorc1-independent autophagy in senescent human CD8+ t cells. J Clin Invest. 2014;124(9):4004–4016. doi: 10.1172/jci75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Akbar AN, Nikolich-Zugich J. Translational research in immune senescence: assessing the relevance of current models. Semin Immunol. 2012;24(5):373–382. doi: 10.1016/j.smim.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573(7772):61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Hossain MS, Kim Y, Akiko I, Kon R, Yamatsu A, Kishima M, Nishikimi T, Kim M. Effects of banafine(®), a fermented green banana-derived acidic glycoconjugate, on influenza vaccine antibody titer in elderly patients receiving gastrostomy tube feeding. J Food Sci. 2021;86(4):1410–1417. doi: 10.1111/1750-3841.15675. [DOI] [PubMed] [Google Scholar]
- Howard WA, Gibson KL, Dunn-Walters DK. Antibody quality in old age. Rejuvenat Res. 2006;9(1):117–125. doi: 10.1089/rej.2006.9.117. [DOI] [PubMed] [Google Scholar]
- Huang LS, Hong Z, Wu W, Xiong S, Zhong M, Gao X, Rehman J, Malik AB. Mtdna activates CGAS signaling and suppresses the yap-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity. 2020;52(3):475–486.e475. doi: 10.1016/j.immuni.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsche C, Cruces J, De la Fuente M. Improvement of redox state and functions of immune cells as well as of behavioral response in aged mice after two-week supplementation of fermented milk with probiotics. Curr Microbiol. 2019;76(11):1278–1289. doi: 10.1007/s00284-019-01759-9. [DOI] [PubMed] [Google Scholar]
- Idda ML, McClusky WG, Lodde V, Munk R, Abdelmohsen K, Rossi M, Gorospe M. Survey of senescent cell markers with age in human tissues. Aging (albany NY) 2020;12(5):4052–4066. doi: 10.18632/aging.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KM, Skoien R, Bokil NJ, Melino M, Thomas GP, Loo D, Gabrielli B, Hill MM, Sweet MJ, Clouston AD, et al. Senescent human hepatocytes express a unique secretory phenotype and promote macrophage migration. World J Gastroenterol. 2014;20(47):17851–17862. doi: 10.3748/wjg.v20.i47.17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelle V, Neault M, Lebel M-È, De Sousa DM, Boulet S, Durrieu L, Carli C, Muzac C, Lemieux S, Labrecque N, et al. P16INK4a regulates cellular senescence in PD-1-expressing human t cells. Front Immunol. 2021 doi: 10.3389/fimmu.2021.698565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JJ, Kim KA, Jang SE, Woo JY, Han MJ, Kim DH. Orally administrated lactobacillus pentosus var. Plantarum c29 ameliorates age-dependent colitis by inhibiting the nuclear factor-kappa b signaling pathway via the regulation of lipopolysaccharide production by gut microbiota. PLoS ONE. 2015;10(2):e0116533. doi: 10.1371/journal.pone.0116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JJ, Woo JY, Ahn YT, Shim JH, Huh CS, Im SH, Han MJ, Kim DH. The probiotic mixture irt5 ameliorates age-dependent colitis in rats. Int Immunopharmacol. 2015;26(2):416–422. doi: 10.1016/j.intimp.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Jeong J-H, Kim M-B, Kim C, Hwang J-K. Inhibitory effect of vitamin C on intrinsic aging in human dermal fibroblasts and hairless mice. Food Sci Biotechnol. 2017;27(2):555–564. doi: 10.1007/s10068-017-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen L, Tarnow I, Eskesen D, Morberg CM, Michelsen B, Bügel S, Dragsted LO, Rijkers GT, Calder PC. Effect of lactobacillus paracasei subsp. Paracasei, l. Casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Am J Clin Nutr. 2015;101(6):1188–1196. doi: 10.3945/ajcn.114.103531. [DOI] [PubMed] [Google Scholar]
- Jing Y, Shaheen E, Drake RR, Chen N, Gravenstein S, Deng Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol. 2009;70(10):777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CM, Sandrasaigaran P, Tong CK, Adam A, Ramasamy R. Immunomodulatory activity of polyphenols derived from cassia auriculata flowers in aged rats. Cell Immunol. 2011;271(2):474–479. doi: 10.1016/j.cellimm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Jolly CA, Karri S. Omega-3 polyunsaturated fatty acids and immunosenescence. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, editors. Handbook on immunosenescence: basic understanding and clinical applications. Dordrecht: Springer; 2009. pp. 1423–1435. [Google Scholar]
- Kale A, Sharma A, Stolzing A, Desprez P-Y, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immunity Ageing. 2020;17(1):16. doi: 10.1186/s12979-020-00187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal D, Kansal VK. Dahi containing lactobacillus acidophilus and bifidobacterium bifidum improves phagocytic potential of macrophages in aged mice. J Food Sci Technol. 2014;51(6):1147–1153. doi: 10.1007/s13197-012-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26(6):988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Epel ES, Belury MA, Andridge R, Lin J, Glaser R, Malarkey WB, Hwang BS, Blackburn E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: a randomized controlled trial. Brain Behav Immun. 2013;28:16–24. doi: 10.1016/j.bbi.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc Natl Acad Sci USA. 2008;105(15):5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Suzuki K, Washio M, Ohfuji S, Adachi S, Kan S, Imai S, Yoshimura K, Miyashita N, Fujisawa N, et al. Association between coffee and green tea intake and pneumonia among the Japanese elderly: a case-control study. Sci Rep. 2021;11(1):5570. doi: 10.1038/s41598-021-84348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Sharma A, Gupta M, Padwad Y, Sharma R. Cell-free culture supernatant of probiotic lactobacillus fermentum protects against H2O2-induced premature senescence by suppressing ROS-Akt-mTOR axis in murine preadipocytes. Probiot Antimicrob Prot. 2020;12(2):563–576. doi: 10.1007/s12602-019-09576-z. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma A, Padwad Y, Sharma R. Preadipocyte secretory factors differentially modulate murine macrophage functions during aging which are reversed by the application of phytochemical EGCG. Biogerontology. 2020;21(3):325–343. doi: 10.1007/s10522-020-09861-3. [DOI] [PubMed] [Google Scholar]
- Kumar A, Joishy T, Das S, Kalita MC, Mukherjee AK, Khan MR. A potential probiotic lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants (basel) 2022;11(2):268. doi: 10.3390/antiox11020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fata G, Seifert N, Weber P, Mohajeri MH. Vitamin e supplementation delays cellular senescence in vitro. Biomed Res Int. 2015;2015:563247. doi: 10.1155/2015/563247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue C, Stevens Y, van Erp M, Papazova E, Soeth E, Pannenbeckers A, Stolte E, Böhm R, Gall SL, Falourd X, et al. Adjuvant effect of orally applied preparations containing non-digestible polysaccharides on influenza vaccination in healthy seniors: a double-blind, randomised, controlled pilot trial. Nutrients. 2021 doi: 10.3390/nu13082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve t cells and dysregulation of t-cell/b-cell interactions in human lymph nodes. Immunology. 2005;114(1):37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Garff-Tavernier M, Béziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debré P, Merle-Beral H, Vieillard V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9(4):527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477(7366):526–528. doi: 10.1038/477526a. [DOI] [PubMed] [Google Scholar]
- Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, Kim DD, Kim HS. Characterization of the anti-inflammatory lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gao J, Yu Z, Jiang W, Sun W, Yu C, Sun J, Wang C, Chen J, Jing S, et al. Regulatory effect of anwulignan on the immune function through its antioxidation and anti-apoptosis in d-galactose-induced aging mice. Clin Interv Aging. 2020;15:97–110. doi: 10.2147/cia.S237601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Kim J, Metter EJ, Nguyen H, Truong T, Lustig A, Ferrucci L, Weng NP. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing. 2016;13:24. doi: 10.1186/s12979-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13(4):699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(ink4a) in peripheral blood t-cells is a biomarker of human aging. Aging Cell. 2009;8(4):439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-Y, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, Parker JS, Sessions GA, Gudkov AV, Sharpless NE. Cells exhibiting strong p16ink4a promoter activation in vivo display features of senescence. Proc Natl Acad Sci. 2019;116(7):2603–2611. doi: 10.1073/pnas.1818313116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli FM, Kawano T, Iwata H, Aoyama B, Eguchi S, Nishigaki A, Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Yokoyama M. Resveratrol-loaded nanoemulsion prevents cognitive decline after abdominal surgery in aged rats. J Pharmacol Sci. 2018;137(4):395–402. doi: 10.1016/j.jphs.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Loyer C, Lapostolle A, Urbina T, Elabbadi A, Lavillegrand J-R, Chaigneau T, Simoes C, Dessajan J, Desnos C, Morin-Brureau M, et al. Impairment of neutrophil functions and homeostasis in covid-19 patients: association with disease severity. Crit Care. 2022;26(1):155. doi: 10.1186/s13054-022-04002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv N, Zhao Y, Liu X, Ye L, Liang Z, Kang Y, Dong Y, Wang W, Kolliputi N, Shi L. Dysfunctional telomeres through mitostress-induced cGAS/STING activation to aggravate immune senescence and viral pneumonia. Aging Cell. 2022;21(4):e13594. doi: 10.1111/acel.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macciò A, Madeddu C. Management of anemia of inflammation in the elderly. Anemia. 2012;2012:563251–563251. doi: 10.1155/2012/563251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–118. [PMC free article] [PubMed] [Google Scholar]
- Malavolta M, Piacenza F, Basso A, Giacconi R, Costarelli L, Mocchegiani E. Serum copper to zinc ratio: relationship with aging and health status. Mech Ageing Dev. 2015;151:93–100. doi: 10.1016/j.mad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Vélez E, Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019;74(2):115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, et al. Mtor inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- Mariani E, Pulsatelli L, Neri S, Dolzani P, Meneghetti A, Silvestri T, Ravaglia G, Forti P, Cattini L, Facchini A. Rantes and mip-1alpha production by t lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37(2–3):219–226. doi: 10.1016/s0531-5565(01)00187-5. [DOI] [PubMed] [Google Scholar]
- Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4+ t cells is reversed by vitamin E supplementation. J Immunol. 2007;178(3):1443. doi: 10.4049/jimmunol.178.3.1443. [DOI] [PubMed] [Google Scholar]
- Martin Manuel P, Elena B, Carolina MG, Gabriela P. Oral probiotics supplementation can stimulate the immune system in a stress process. J Nutr Intermed Metab. 2017;8:29–40. doi: 10.1016/j.jnim.2017.06.001. [DOI] [Google Scholar]
- Martínez-Zamudio RI, Dewald HK, Vasilopoulos T, Gittens-Williams L, Fitzgerald-Bocarsly P, Herbig U. Senescence-associated β-galactosidase reveals the abundance of senescent CD8+ t cells in aging humans. Aging Cell. 2021;20(5):e13344. doi: 10.1111/acel.13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Abe R, Shimono T, Iwabuchi N, Abe F, Xiao JZ. The effects of non-viable lactobacillus on immune function in the elderly: a randomised, double-blind, placebo-controlled study. Int J Food Sci Nutr. 2016;67(1):67–73. doi: 10.3109/09637486.2015.1126564. [DOI] [PubMed] [Google Scholar]
- Masaldan S, Clatworthy SAS, Gamell C, Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D, Adlard PA, Bush AI, et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaldan S, Clatworthy SAS, Gamell C, Smith ZM, Francis PS, Denoyer D, Meggyesy PM, Fontaine S, Cater MA. Copper accumulation in senescent cells: Interplay between copper transporters and impaired autophagy. Redox Biol. 2018;16:322–331. doi: 10.1016/j.redox.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008;133(2):412–419. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthe DM, Thoma O-M, Sperka T, Neurath MF, Waldner MJ. Telomerase deficiency reflects age-associated changes in CD4+ t cells. Immunity & Ageing. 2022;19(1):16. doi: 10.1186/s12979-022-00273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue AC, Haynes L. CD4+ t cells and immunosenescence–a mini-review. Gerontology. 2009;55(5):491–495. doi: 10.1159/000214842. [DOI] [PubMed] [Google Scholar]
- McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217(1):65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege JL, Capo C, Michel B, Gastaut JL, Bongrand P. Phagocytic cell function in aged subjects. Neurobiol Aging. 1988;9(2):217–220. doi: 10.1016/s0197-4580(88)80054-x. [DOI] [PubMed] [Google Scholar]
- Meryk A, Grasse M, Balasco L, Kapferer W, Grubeck-Loebenstein B, Pangrazzi L. Antioxidants n-acetylcysteine and vitamin C improve t cell commitment to memory and long-term maintenance of immunological memory in old mice. Antioxidants (basel) 2020 doi: 10.3390/antiox9111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Micó V, Berninches L, Tapia J, Daimiel L. Nutrimiraging: micromanaging nutrient sensing pathways through nutrition to promote healthy aging. Int J Mol Sci. 2017 doi: 10.3390/ijms18050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato N, Hattori M, Hamazaki Y. Physiology and pathology of t-cell aging. Int Immunol. 2020;32(4):223–231. doi: 10.1093/intimm/dxaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet-Quinard R, Farges MC, Thivat E, Deleine C, Mayot G, Brtko J, Ribalta J, Winklhofer-Roob B, Rock E, Vasson MP. Neutrophils are immune cells preferentially targeted by retinoic acid in elderly subjects. Immun Ageing. 2010;7:10. doi: 10.1186/1742-4933-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K, Kawase M, Kubota A, Yoda K, Harata G, Hosoda M, He F. Heat-killed lactobacillus Gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Benef Microbes. 2015;6(4):441–449. doi: 10.3920/bm2014.0108. [DOI] [PubMed] [Google Scholar]
- Mrityunjaya M, Pavithra V, Neelam R, Janhavi P, Halami PM, Ravindra PV. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of Covid-19. Front Immunol. 2020 doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas A, O’Loghlen A. Cellular senescence and ageing: mechanisms and interventions. Front Aging. 2022 doi: 10.3389/fragi.2022.866718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myśliwska J, Bryl E, Zorena K, Balon J, Foerster J, Myśliwski A. Overactivity of tumor necrosis factor-alpha but not interleukin 6 is associated with low natural killer cytotoxic activity in the elderly. Gerontology. 1997;43(3):158–167. doi: 10.1159/000213845. [DOI] [PubMed] [Google Scholar]
- Myśliwska J, Bryl E, Foerster J, Myśliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the ageing process are influenced by the health status. Mech Ageing Dev. 1998;100(3):313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- Narzt MS, Pils V, Kremslehner C, Nagelreiter IM, Schosserer M, Bessonova E, Bayer A, Reifschneider R, Terlecki-Zaniewicz L, Waidhofer-Söllner P, et al. Epilipidomics of senescent dermal fibroblasts identify lysophosphatidylcholines as pleiotropic senescence-associated secretory phenotype (SASP) factors. J Invest Dermatol. 2021;141(4s):993–1006.e1015. doi: 10.1016/j.jid.2020.11.020. [DOI] [PubMed] [Google Scholar]
- Nel HJ, Hams E, Saunders SP, Mangan NE, Smith P, Atzberger A, Flavell RA, Akira S, McKenzie AN, Fallon PG. Impaired basophil induction leads to an age-dependent innate defect in type 2 immunity during helminth infection in mice. J Immunol. 2011;186(8):4631–4639. doi: 10.4049/jimmunol.1002995. [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Žugich J. Aging of the t cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193(6):2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan N, Parletta N, Milte CM, Benassi-Evans B, Fenech M, Howe PR. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with ω − 3 fatty acid supplementation: a randomized controlled pilot study. Nutrition. 2014;30(4):489–491. doi: 10.1016/j.nut.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Yamada T, Hasegawa S, Sanada A, Iwata Y, Arima M, Nakata S, Sugiura K, Akamatsu H. Sasp-induced macrophage dysfunction may contribute to accelerated senescent fibroblast accumulation in the dermis. Exp Dermatol. 2021;30(1):84–91. doi: 10.1111/exd.14205. [DOI] [PubMed] [Google Scholar]
- Onyema OO, Njemini R, Forti LN, Bautmans I, Aerts JL, De Waele M, Mets T. Aging-associated subpopulations of human CD8+ t-lymphocytes identified by their CD28 and CD57 phenotypes. Arch Gerontol Geriatr. 2015;61(3):494–502. doi: 10.1016/j.archger.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Orkaby AR, Dushkes R, Ward R, Djousse L, Buring JE, Lee IM, Cook NR, LeBoff MS, Okereke OI, Copeland T, et al. Effect of vitamin D3 and omega-3 fatty acid supplementation on risk of frailty: an ancillary study of a randomized clinical trial. JAMA Netw Open. 2022;5(9):e2231206. doi: 10.1001/jamanetworkopen.2022.31206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, Krizhanovsky V. Strategies targeting cellular senescence. J Clin Invest. 2018;128(4):1247–1254. doi: 10.1172/jci95149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9(1):5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WA, Walaszczyk A, Spyridopoulos I, Dookun E, Richardson GD. Senescence and senolytics in cardiovascular disease: promise and potential pitfalls. Mech Ageing Dev. 2021;198:111540. doi: 10.1016/j.mad.2021.111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Deng Z, Shahidi F. Natural bioactive substances for the control of food-borne viruses and contaminants in food. Food Prod Process Nutr. 2020;2(1):27. doi: 10.1186/s43014-020-00040-y. [DOI] [Google Scholar]
- Parish ST, Wu JE, Effros RB. Modulation of t lymphocyte replicative senescence via TNF-{alpha} inhibition: role of caspase-3. J Immunol. 2009;182(7):4237–4243. doi: 10.4049/jimmunol.0803449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fülöp T, Gaudreau P, Gladyshev VN, Gonos ES, Gorbunova V, et al. The conundrum of human immune system “senescence”. Mech Ageing Dev. 2020;192:111357. doi: 10.1016/j.mad.2020.111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BI, Akbar AN. Convergence of innate and adaptive immunity during human aging. Front Immunol. 2016;7:445. doi: 10.3389/fimmu.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A, et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ t cell inhibition. Nat Commun. 2019;10(1):2387. doi: 10.1038/s41467-019-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perskin MH, Cronstein BN. Age-related changes in neutrophil structure and function. Mech Ageing Dev. 1992;64(3):303–313. doi: 10.1016/0047-6374(92)90086-S. [DOI] [PubMed] [Google Scholar]
- Pham DC, Shibu MA, Mahalakshmi B, Velmurugan BK. Effects of phytochemicals on cellular signaling: reviewing their recent usage approaches. Crit Rev Food Sci Nutr. 2020;60(20):3522–3546. doi: 10.1080/10408398.2019.1699014. [DOI] [PubMed] [Google Scholar]
- Picard E, Armstrong S, Andrew MK, Haynes L, Loeb M, Pawelec G, Kuchel GA, McElhaney JE, Verschoor CP. Markers of systemic inflammation are positively associated with influenza vaccine antibody responses with a possible role for ILT2(+)CD57(+) NK-cells. Immunity Ageing. 2022;19(1):26. doi: 10.1186/s12979-022-00284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pils V, Ring N, Valdivieso K, Lämmermann I, Gruber F, Schosserer M, Grillari J, Ogrodnik M. Promises and challenges of senolytics in skin regeneration, pathology and ageing. Mech Ageing Dev. 2021;200:111588. doi: 10.1016/j.mad.2021.111588. [DOI] [PubMed] [Google Scholar]
- Ponton F, Wilson K, Cotter SC, Raubenheimer D, Simpson SJ. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7(12):e1002223. doi: 10.1371/journal.ppat.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prašnikar E, Borišek J, Perdih A. Senescent cells as promising targets to tackle age-related diseases. Ageing Res Rev. 2021;66:101251. doi: 10.1016/j.arr.2020.101251. [DOI] [PubMed] [Google Scholar]
- Prattichizzo F, De Nigris V, Mancuso E, Spiga R, Giuliani A, Matacchione G, Lazzarini R, Marcheselli F, Recchioni R, Testa R, et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15:170–181. doi: 10.1016/j.redox.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritz T, Lair J, Ban M, Keller M, Weinberger B, Krismer M, Grubeck-Loebenstein B. Plasma cell numbers decrease in bone marrow of old patients. Eur J Immunol. 2015;45(3):738–746. doi: 10.1002/eji.201444878. [DOI] [PubMed] [Google Scholar]
- Pu F, Guo Y, Li M, Zhu H, Wang S, Shen X, He M, Huang C, He F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clin Interv Aging. 2017;12:1223–1231. doi: 10.2147/cia.S141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AA, Khan DA, Mahjabeen W, Papasian CJ, Qureshi N. Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J Clin Exp Cardiolog S. 2012;5:8. doi: 10.4172/2155-9880.S5-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstead AG, Schepetkin IA, Todd K, Loeffelholz J, Berardinelli JG, Quinn MT, Jutila MA. Aging influences the response of t cells to stimulation by the ellagitannin, oenothein b. Int Immunopharmacol. 2015;26(2):367–377. doi: 10.1016/j.intimp.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired toll-like receptor expression and function in aging. J Immunol. 2002;169(9):4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, Azzi A, Zingg JM. Reduction of senescence-associated beta-galactosidase activity by vitamin E in human fibroblasts depends on subjects' age and cell passage number. BioFactors. 2020;46(4):665–674. doi: 10.1002/biof.1636. [DOI] [PubMed] [Google Scholar]
- Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Lai J-J, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev. 2011;243(1):191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez IJ, Lalinde Ruiz N, Llano León M, Martínez Enríquez L, Montilla Velásquez MP, Ortiz Aguirre JP, Rodríguez Bohórquez OM, Velandia Vargas EA, Hernández ED, Parra López CA. Immunosenescence study of T cells: a systematic review. Frontiers in Immunology. 2021;11:604591. doi: 10.3389/fimmu.2020.604591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röth A, Yssel H, Jrm P, Chavez EA, Schertzer M, Lansdorp PM, Spits H, Luiten RM. Telomerase levels control the lifespan of human t lymphocytes. Blood. 2003;102(3):849–857. doi: 10.1182/blood-2002-07-2015. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci. 1998;53(1):M20–26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- Sabbatinelli J, Matacchione G, Giuliani A, Ramini D, Rippo MR, Procopio AD, Bonafè M, Olivieri F. Circulating biomarkers of inflammaging as potential predictors of Covid-19 severe outcomes. Mech Ageing Dev. 2022;204:111667. doi: 10.1016/j.mad.2022.111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani SP, Ovsyannikova IG, Goergen K, Grill DE, Poland GA. Vitamin D, leptin and impact on immune response to seasonal influenza A/H1N1 vaccine in older persons. Hum Vaccin Immunother. 2016;12(3):691–698. doi: 10.1080/21645515.2015.1097015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu S, Decker C, Sansbury BE, Marinello M, Seyfried A, Howard J, Mori M, Hosseini Z, Arunachalam T, Finn AV, et al. Radiation-induced macrophage senescence impairs resolution programs and drives cardiovascular inflammation. J Immunol. 2021;207(7):1812–1823. doi: 10.4049/jimmunol.2100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. 2018;463:21–26. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Saeed F, Nadeem M, Ahmed RS, Tahir Nadeem M, Arshad MS, Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—a review. Food Hydrocolloids. 2016;27(2):205–229. doi: 10.1080/09540105.2015.1079600. [DOI] [Google Scholar]
- Salam N, Rane S, Das R, Faulkner M, Gund R, Kandpal U, Lewis V, Mattoo H, Prabhu S, Ranganathan V, et al. T cell ageing: effects of age on development, survival and function. Indian J Med Res. 2013;138(5):595–608. [PMC free article] [PubMed] [Google Scholar]
- Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. doi: 10.1016/j.arr.2021.101422. [DOI] [PubMed] [Google Scholar]
- Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory t cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127(3):274–281. doi: 10.1016/j.mad.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9(1):345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- Sharma R. Bioactive food components for managing cellular senescence in aging and disease: a critical appraisal and perspectives. Pharma Nutr. 2021;18:100281. doi: 10.1016/j.phanu.2021.100281. [DOI] [Google Scholar]
- Sharma R. Perspectives on the dynamic implications of cellular senescence and immunosenescence on macrophage aging biology. Biogerontology. 2021;22(6):571–587. doi: 10.1007/s10522-021-09936-9. [DOI] [PubMed] [Google Scholar]
- Sharma R, Diwan B. An update on healthspan and lifespan enhancing attributes of tea amidst the emerging understanding of aging biology. Human Nutr Metab. 2022;28:200149. doi: 10.1016/j.hnm.2022.200149. [DOI] [Google Scholar]
- Sharma R, Kapila R, Dass G, Kapila S. Improvement in TH1/TH2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic lactobacillus rhamnosus fermented milk in aging mice. Age. 2014;36(4):9686. doi: 10.1007/s11357-014-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sharma R, Kapila R, Haq MRU, Salingati V, Kapasiya M, Kapila S. Age-associated aberrations in mouse cellular and humoral immune responses. Aging Clin Exp Res. 2014;26(4):353–362. doi: 10.1007/s40520-013-0190-y. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma A, Kumari A, Kulurkar PM, Raj R, Gulati A, Padwad YS. Consumption of green tea epigallocatechin-3-gallate enhances systemic immune response, antioxidative capacity and HPA axis functions in aged male swiss albino mice. Biogerontology. 2017;18(3):367–382. doi: 10.1007/s10522-017-9696-6. [DOI] [PubMed] [Google Scholar]
- Sharma R, Kumari M, Kumari A, Sharma A, Gulati A, Gupta M, Padwad Y. Diet supplemented with phytochemical epigallocatechin gallate and probiotic lactobacillus fermentum confers second generation synbiotic effects by modulating cellular immune responses and antioxidant capacity in aging mice. Eur J Nutr. 2019;58(7):2943–2957. doi: 10.1007/s00394-018-01890-6. [DOI] [PubMed] [Google Scholar]
- Sharma R, Kumar R, Sharma A, Goel A, Padwad Y. Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and immunosenescence. J Nutr Biochem. 2022;107:109068. doi: 10.1016/j.jnutbio.2022.109068. [DOI] [PubMed] [Google Scholar]
- Shen J, Song R, Fuemmeler BF, McGuire KP, Chow WH, Zhao H. Biological aging marker p16(ink4a) in t cells and breast cancer risk. Cancers (basel) 2020 doi: 10.3390/cancers12113122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood ER, Burelbach KR, McBride MA, Stothers CL, Owen AM, Hernandez A, Patil NK, Williams DL, Bohannon JK. Innate immune memory and the host response to infection. J Immunol. 2022;208(4):785. doi: 10.4049/jimmunol.2101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Nakamura K, Kikuchi M, Ukawa S, Nakamura K, Okada E, Imae A, Nakagawa T, Yamamura R, Tamakoshi A, et al. Lower human defensin 5 in elderly people compared to middle-aged is associated with differences in the intestinal microbiota composition: the dosanco health study. Geroscience. 2021 doi: 10.1007/s11357-021-00398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Liu D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J Nutr Biochem. 2014;25(6):581–591. doi: 10.1016/j.jnutbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SR, Tchalla EYI, Bhalla M, Bou Ghanem EN. The age-driven decline in neutrophil function contributes to the reduced efficacy of the pneumococcal conjugate vaccine in old hosts. Front Cell Infect Microbiol. 2022 doi: 10.3389/fcimb.2022.849224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Dunne DW, Fallon PG. Defective in vivo induction of functional type 2 cytokine responses in aged mice. Eur J Immunol. 2001;31(5):1495–1502. doi: 10.1002/1521-4141(200105)31:5<1495::Aid-immu1495>3.0.Co;2-8. [DOI] [PubMed] [Google Scholar]
- Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16(1):75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Vandewoude M, Stevens W, De Clerck L, Van der Planken M, Whelan A, Anisman H, Dossche A, Maes M. Alterations in immune functions during normal aging and Alzheimer's disease. Psychiatry Res. 1999;85(1):71–80. doi: 10.1016/s0165-1781(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Song L, Yuan J, Ni S, Zhou Y, Wang X, Chen Y, Zhang S. Enhancement of adaptive immune responses of aged mice by dietary intake of β-glucans, with special emphasis on anti-aging activity. Mol Immunol. 2020;117:160–167. doi: 10.1016/j.molimm.2019.10.019. [DOI] [PubMed] [Google Scholar]
- Spaiser SJ, Culpepper T, Nieves C, Jr, Ukhanova M, Mai V, Percival SS, Christman MC, Langkamp-Henken B. Lactobacillus gasseri KS-13, bifidobacterium bifidum G9–1, and bifidobacterium longum MM-2 ingestion induces a less inflammatory cytokine profile and a potentially beneficial shift in gut microbiota in older adults: a randomized, double-blind, placebo-controlled, crossover study. J Am Coll Nutr. 2015;34(6):459–469. doi: 10.1080/07315724.2014.983249. [DOI] [PubMed] [Google Scholar]
- Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ t cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34(5):633–644. doi: 10.1016/s0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V, Maestroni GJ, Cardinali DP, Esquifino AI, Perumal SR, Miller SC. Melatonin, immune function and aging. Immun Ageing. 2005;2:17. doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EC, Brown BN. Cell therapy strategies to combat immunosenescence. Organogenesis. 2015;11(4):159–172. doi: 10.1080/15476278.2015.1120046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic MR, Ain Q, Bondeva T, Heller R, Schmeer C, Witte OW. Phenotypic and functional differences between senescent and aged murine microglia. Neurobiol Aging. 2019;74:56–69. doi: 10.1016/j.neurobiolaging.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Sung J, Ho C-T, Wang Y. Preventive mechanism of bioactive dietary foods on obesity-related inflammation and diseases. Food Funct. 2018;9(12):6081–6095. doi: 10.1039/C8FO01561A. [DOI] [PubMed] [Google Scholar]
- Tan A, Sullenbarger B, Prakash R, McDaniel JC. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumurugan K. Senolytic phytocompounds in redox signaling. In: Çakatay U, editor. Redox signaling and biomarkers in ageing. Cham: Springer; 2022. pp. 255–283. [Google Scholar]
- Thome JJ, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, Sempowski GD, Shen Y, Farber DL. Longterm maintenance of human naive t cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol. 2016 doi: 10.1126/sciimmunol.aah6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas M, Capanoglu E, Bahrami A, Hosseini H, Akbari-Alavijeh S, Shaddel R, Rehman A, Rezaei A, Rashidinejad A, Garavand F, et al. The direct and indirect effects of bioactive compounds against coronavirus. Food Front. 2022;3(1):96–123. doi: 10.1002/fft2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor D, Wsson MK, Kumar P, Karthikeyan G, Kaushik NK, Goel C, Singh S, Kumar A, Prakash H. Dysbiosis disrupts gut immune homeostasis and promotes gastric diseases. Int J Mol Sci. 2019;20(10):2432. doi: 10.3390/ijms20102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourkochristou E, Triantos C, Mouzaki A. The influence of nutritional factors on immunological outcomes. Front Immunol. 2021;12:665968. doi: 10.3389/fimmu.2021.665968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchio R, Hirose Y, Murosaki S, Yamamoto Y, Ishigami A. High dietary intake of vitamin C suppresses age-related thymic atrophy and contributes to the maintenance of immune cells in vitamin C-deficient senescence marker protein-30 knockout mice. Br J Nutr. 2015;113(4):603–609. doi: 10.1017/s0007114514003857. [DOI] [PubMed] [Google Scholar]
- Uciechowski P, Kahmann L, Plümäkers B, Malavolta M, Mocchegiani E, Dedoussis G, Herbein G, Jajte J, Fulop T, Rink L. Th1 and Th2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol. 2008;43(5):493–498. doi: 10.1016/j.exger.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Unno T, Choi J-H, Hur H-G, Sadowsky MJ, Ahn Y-T, Huh C-S, Kim G-B, Cha C-J. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J Dairy Sci. 2015;98(6):3568–3576. doi: 10.3168/jds.2014-8943. [DOI] [PubMed] [Google Scholar]
- Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek AA, Fransen F, Meijer B, de Vos P, Knol EF, Savelkoul HFJ. Aged mice display altered numbers and phenotype of basophils, and bone marrow-derived basophil activation, with a limited role for aging-associated microbiota. Immun Ageing. 2018;15:32–32. doi: 10.1186/s12979-018-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lugt B, van Beek AA, Aalvink S, Meijer B, Sovran B, Vermeij WP, Brandt RMC, de Vos WM, Savelkoul HFJ, Steegenga WT, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging ercc1 (–/δ7) mice. Immun Ageing. 2019;16:6. doi: 10.1186/s12979-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Puyenbroeck K, Hens N, Coenen S, Michiels B, Beunckens C, Molenberghs G, Van Royen P, Verhoeven V. Efficacy of daily intake of lactobacillus casei shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am J Clin Nutr. 2012;95(5):1165–1171. doi: 10.3945/ajcn.111.026831. [DOI] [PubMed] [Google Scholar]
- Varas A, Sacedón R, Hernandez-López C, Jiménez E, García-Ceca J, Arias-Díaz J, Zapata AG, Vicente A. Age-dependent changes in thymic macrophages and dendritic cells. Microsc Res Tech. 2003;62(6):501–507. doi: 10.1002/jemt.10411. [DOI] [PubMed] [Google Scholar]
- Varin A, Larbi A, Dedoussis GV, Kanoni S, Jajte J, Rink L, Monti D, Malavolta M, Marcellini F, Mocchegiani E, et al. In vitro and in vivo effects of zinc on cytokine signalling in human t cells. Exp Gerontol. 2008;43(5):472–482. doi: 10.1016/j.exger.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Vemuri R, Gundamaraju R, Shinde T, Perera AP, Basheer W, Southam B, Gondalia SV, Karpe AV, Beale DJ, Tristram S, et al. Lactobacillus acidophilus DDS-1 modulates intestinal-specific microbiota, short-chain fatty acid and immunological profiles in aging mice. Nutrients. 2019 doi: 10.3390/nu11061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburgh K. Nutrigerontology: why we need a new scientific discipline to develop diets and guidelines to reduce the risk of aging-related diseases. Aging Cell. 2015;14(1):17–24. doi: 10.1111/acel.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vĕtvicka V, Tlaskalová-Hogenová H, Pospísil M. Impaired antigen presenting function of macrophages from aged mice. Immunol Invest. 1985;14(2):105–114. doi: 10.3109/08820138509042005. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Wan T, Miwa S. Senescence in post-mitotic cells: A driver of aging? Antioxid Redox Signal. 2021;34(4):308–323. doi: 10.1089/ars.2020.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural Z, Avery A, Kalogiros DI, Coneyworth LJ, Welham SJM. Trace mineral intake and deficiencies in older adults living in the community and institutions: a systematic review. Nutrients. 2020 doi: 10.3390/nu12041072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL. The immunologic theory of aging1. Gerontologist. 1964;4(4):195–197. doi: 10.1093/geront/4.4.195%JTheGerontologist. [DOI] [PubMed] [Google Scholar]
- Wang Q, Nie L, Zhao P, Zhou X, Ding Y, Chen Q, Wang Q. Diabetes fuels periodontal lesions via glut1-driven macrophage inflammaging. Int J Oral Sci. 2021;13(1):11. doi: 10.1038/s41368-021-00116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Zhu A, Ji JS. A comparison study of vitamin D deficiency among older adults in china and the united states. Sci Rep. 2019;9(1):19713. doi: 10.1038/s41598-019-56297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng BB, Lin WS, Chang JC, Chiou RY. The phytogestrogenic stilbenes, arachidin-1 and resveratrol, modulate regulatory t cell functions responsible for successful aging in aged icr mice. Int J Mol Med. 2016;38(6):1895–1904. doi: 10.3892/ijmm.2016.2792. [DOI] [PubMed] [Google Scholar]
- Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67(1):40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Goronzy JJ. Aging of the immune system. Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(Supplement_5):S422–S428. doi: 10.1513/AnnalsATS.201602-095AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski JM, Fulop T, Bryl E. Immunosenescence and Covid-19. Mech Ageing Dev. 2022;204:111672. doi: 10.1016/j.mad.2022.111672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Goldstein DR. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 2013;25(4):535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CP, Song Y, Elias VD, Magnusson KR, Ho E. Zinc supplementation increases zinc status and thymopoiesis in aged mice. J Nutr. 2009;139(7):1393–1397. doi: 10.3945/jn.109.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CP, Magnusson KR, Ho E. Increased inflammatory response in aged mice is associated with age-related zinc deficiency and zinc transporter dysregulation. J Nutr Biochem. 2013;24(1):353–359. doi: 10.1016/j.jnutbio.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol. 2017;199(3):1060. doi: 10.4049/jimmunol.1700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Braun K, Bouranis J, Davis E, II, Sharpton T, Ho E. Effects of zinc status and aging on age-related immune dysfunction and chronic inflammation. Curr Dev Nutr. 2020;4(Supplement_2):1854–1854. doi: 10.1093/cdn/nzaa067_081. [DOI] [Google Scholar]
- Wong CP, Magnusson KR, Sharpton TJ, Ho E. Effects of zinc status on age-related t cell dysfunction and chronic inflammation. Biometals. 2021;34(2):291–301. doi: 10.1007/s10534-020-00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wong G, Hwang YY, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. 2020;42(5):559–572. doi: 10.1007/s00281-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, Park MJ, Park S, Lee ES. Increased senescent CD8+ t cells in the peripheral blood mononuclear cells of Behçet's disease patients. Arch Dermatol Res. 2018;310(2):127–138. doi: 10.1007/s00403-017-1802-8. [DOI] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19(3):e13094. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, Cui Y, Angelini L, Lee KA, McGowan SJ, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594(7861):100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Lu L, Zhang Z, Zhang S. Dietary intake of resveratrol enhances the adaptive immunity of aged rats. Rejuvenat Res. 2012;15(5):507–515. doi: 10.1089/rej.2012.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacca ER, Crespo MI, Acland RP, Roselli E, Núñez NG, Maccioni M, Maletto BA, Pistoresi-Palencia MC, Morón G. Aging impairs the ability of conventional dendritic cells to cross-prime CD8+ t cells upon stimulation with a tlr7 ligand. PLoS ONE. 2015;10(10):e0140672–e0140672. doi: 10.1371/journal.pone.0140672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shao WF, Yuan LF, Tu PF, Ma ZZ. Decreasing pro-inflammatory cytokine and reversing the immunosenescence with extracts of PU-ERH tea in senescence accelerated mouse (SAM) Food Chem. 2012;135(4):2222–2228. doi: 10.1016/j.foodchem.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu JZ, Lin ZX, Yuan QJ, Li YC, Liang JL, Zhan JY, Xie YL, Su ZR, Liu YH. Ameliorative effect of supercritical fluid extract of chrysanthemum indicum linnén against d-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. J Ethnopharmacol. 2019;234:44–56. doi: 10.1016/j.jep.2018.12.050. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cheng N, Qiao B, Zhang F, Wu J, Liu C, Li Y, Du J. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration. J Cachexia Sarcopenia Muscle. 2020;11(5):1291–1305. doi: 10.1002/jcsm.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FF, Barr SI, McNulty H, Li D, Blumberg JB. Health effects of vitamin and mineral supplements. BMJ. 2020;369:m2511. doi: 10.1136/bmj.m2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Weyand CM, Goronzy JJ. Hallmarks of the aging t-cell system. FEBS J. 2021 doi: 10.1111/febs.15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Rao Z, Xu J, Sun Y, Hu H, Wang P, Xia Y, Pan X, Tang W, Chen Z, et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate sting signaling during liver sterile inflammation. Aging Cell. 2022;21(6):e13622. doi: 10.1111/acel.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, McElhaney JE. Age-related changes in memory and effector t cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29(11):2169–2177. doi: 10.1016/j.vaccine.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript has no associated data.