Abstract
Given that available antidepressant pharmacotherapies are not optimally effective, there is a need for alternative treatment options that are rooted in a comprehensive understanding of the illness's pathophysiology. Major depressive disorder (MDD) has been historically attributed to monoamine, i.e., serotonin (5-hydroxytryptamine, 5-HT) imbalance and some brain morphological pathologies that have directed treatment towards particular medications that are only minimally effective. MDD pathophysiologies have now been regarded as linked to chronic inflammation and MDD can be treated with compounds that have anti-inflammatory properties. Individuals vulnerable to MDD have increased baseline neuroinflammatory response that is exacerbated by psychogenic stress. When pro-inflammatory mechanisms are chronically hyperactive, dysfunction of brain-related processes occur. We propose that inflammation is one of the primary mechanisms that trigger biological changes leading to MDD. Inflammatory resolution occurs when homeostasis is achieved after an inflammatory response. However, cascading biological events are likely to prevent resolution from occurring and worsen both inflammation and MDD. Novel and alternative pharmacotherapies—e.g., ketamine, cannabinoids, and psychedelics—provide a richer mechanistic perspective on the role of neuroinflammation and neuroprogression by means of rapid, short-term, and long-term symptom relief potentially based on their anti-inflammatory properties. These drugs ultimately decrease proinflammatory cytokine levels that correspond with improved symptoms. However, it is unclear what differentiates these compounds from others in their mechanistic efficacy. Thus, a closer investigation into their anti-inflammatory effects is imperative in order to better elucidate the link between MDD and inflammation, as well as uncover the mechanisms involved in long-term symptom reduction of MDD.
Keywords: Inflammation, Cytokine, Depression, Major depressive disorder (MDD), Neuroplasticity, Neuroprogression, Serotonin, Treatment
Highlights
-
•
Chronic and hyperactive inflammation is heavily implicated in the development of depression.
-
•
Dysfunction of inflammatory mechanisms induces perpetual inflammation linked to the onset and duration of depression.
-
•
Treatment of inflammation also treats some depression (e.g., SSRI's).
-
•
Effective long-term treatment of depression is likely rooted in inflammatory resolve.
-
•
Propose alternative treatments for depression provide novel insights into the role of inflammation in depression.
1. Introduction
Major depression disorder (MDD) is a prevalent and debilitating mental illness that comes with cognitive and affective impairments and decreased quality of life (Tran et al., 2021). Its core diagnostic features are depressed mood and the blunting of reward sensitivity (anhedonia). Other symptoms include hopelessness, loss of motivation, issues with sleep, feelings of worthlessness, comorbid anxiety; and in its severe form, suicidality (Mayo Clinic, 2018). Almost 800,000 people die of suicide each year (World Health Organization, 2020). MDD is the world's leading cause of disability, and over 264 million people suffer from it (World Health Organization, 2020). While there are a number of treatments available, there are several barriers that stymie many people from receiving treatment, such as low income and living in rural areas (World Health Organization, 2020). Moreover, the efficacy and outcome of conventional medications are mixed, leading to a convoluted discussion on the best treatment options for sufferers (Howland, 2008).
The pathogenesis of MDD is complex. While many hypotheses circulate the scientific community, research lacks consistency in isolating biomarkers of MDD's origin. Therefore, our understanding of treatment mechanisms and the development of conventional medications may be inadequately premised on the role of a single factor and precludes the contribution of others. One of the factors that may have been downplayed is neuroinflammation. Indeed, fairly new research has made a strong link between MDD and enhanced inflammatory processes, and it has been suggested that treating inflammation can result in significant symptom relief (Menke, 2019; Bai et al., 2020). Historically, MDD has been loosely linked to monoamine imbalance and neural morphological abnormalities not well understood (Schildkraut, 1965; Miguel-Hidalgo and Rajkowska, 2002). However, disruption of monoaminergic activity and irregular brain development have been observed to occur after chronic inflammatory responses (Miller and Raison, 2016). Thus, inflammation provides earlier mechanistic insights into the development of MDD.
This review presents a critical synthesis of findings obtained from the growing literature on several aspects of the role of neuroinflammation in the etiology of depressive disorders and how they have upended the trajectory of treatment development and refinement. The medical anthropology and history of mental health has overlooked much of the biological mechanisms and neural changes underlying depressive disorders. The neuroprogression of MDD, i.e., the progressive and protracted brain-based biochemical and neurodegenerative changes in MDD, is now apparent and gives a wider view of how the disorder affects cognitive, affective, behavioral and physiological functions. Second, there are newly understood links between important inflammatory cytokines and cognate proteins and the worsening of depressive symptoms that lends a new impetus to investigate the mechanisms of new alternative pharmacotherapies based on their inflammation-resolving properties. Third, conventional treatments may be supported and amended by these alternatives, and research in the field has opened new possible areas in the development of alternative options that have promising effects on both MDD and inflammation.
2. Linking inflammation with MDD
Throughout the past century, it has been noted that inflammation caused by injury and disease was often accompanied by depressive symptoms. For example, one condition characterized by profound neuroinflammatory changes is traumatic brain injury (TBI). A review of the literature shows that about 31% of those with TBI also exhibit MDD symptoms (Guillamondegui et al., 2011). Inflammation is also implicated in Alzheimer's disease, Parkinson's disease, and multiple sclerosis (Amor et al., 2010). High rates of depression are also seen in each of these conditions (Arnett et al., 2008; Lyketsos and Olin, 2002; Reijnders et al., 2008). Neurodegenerative and brain injury conditions come with changes in quality of life that must be considered when discussing depression. A portion of the explanatory power of depression in these conditions are likely due to decreased quality of life. However, these findings provoked a closer look at the mechanistic influence of inflammation on MDD and lead to studies that suggest a cause of MDD that could be rooted in inflammation (Miller et al., 2009). Some of the indications were that depressed people have increased peripheral levels of cytokines and decreased neurotrophic support (Miller et al., 2009). A key factor in this link was findings of how pro-inflammatory cytokines and cognate proteins behave in the body (Abbott, 2018). It was believed that cytokines were too large and hydrophilic and therefore could not cross the blood brain barrier (BBB), effectively leaving the brain unaffected by their confinement in the periphery. However, earlier animal studies have demonstrated that tumour necrosis factor alpha (TNF-α) did in fact cross the BBB (Gutierrez et al., 1993). Later, Avery et al. (2014) was able to link peripheral and central cytokine activity by demonstrating altered patterns of insula activation and vagus nerve activity as a function of cytokine levels in individuals with untreated MDD. The insula has since been recognized to play a significant role in affect and cognition (Flynn, 1999; Gasquoine, 2014). For example, anterior insular regions are believed to play a role in evaluating stress from cognition and interoception as negative emotional experiences (Reiman, 1997). In addition, other areas of the insula are responsible for self-generated emotional states and regulating homeostasis (Tataranni et al., 1999; Damasio et al., 2000). Importantly, activity of both the right-fronto-insular cortex and the default mode network (DMN) is associated with greater depressive rumination (Hamilton et al., 2011), and increased levels of the cytokine interleukin-6 (IL-6) are associated with decreased insular functional connectivity (Chen et al., 2020). Furthermore, post-stress rumination is associated with prolonged increased levels of cortisol and the hepatic inflammatory marker, C-reactive protein (CRP) (Zoccola et al., 2014), and one's rumination response can predict inflammatory reaction (Moriarity et al., 2020). It may be that the activity between the insula and other connected structures plays a role in resilience towards stressful experiences (Shao et al., 2018).
The vagus nerve is a primary component of the brain-gut axis and is a bidirectional pathway of signalling between the enteric nervous system (ENS) and the central nervous system (CNS) in which the sympathetic and parasympathetic nervous systems are moderated to maintain homeostasis and immune response (Breit et al., 2018). Treatment of the vagus nerve via stimulation results in inhibition of cytokine production and is an effective method in treatment resistant MDD (Schlaepfer et al., 2008; Breit et al., 2018; O'Keane et al., 2005; Koopman et al., 2016). Given related functions of both the insula and vagus nerve and their communication across the autonomic system and in inflammatory response, it has been suggested that MDD is associated with heightened inflammation likely due to impaired interoception via internal signalling between central and peripheral nervous circuitry important for homeostasis (Avery et al., 2014).
There are several structures and systems within the nervous system that have overlapping functions in both inflammatory processing and affect regulation. Both the default mode network (DMN) and the hypothalamic pituitary adrenal axis (HPA) function as emotional and inflammatory regulators and are disrupted in MDD (Marsland et al., 2017; Menke, 2019; Cowen, 2010; Murrough et al., 2016). One way in which hyperactivity of the HPA affects a significant proportion of depressed individuals is through reduced sensitivity of the glucocorticoid receptors (GR), which fails to provide negative feedback to the HPA and results in sustained corticotropin-releasing factor secretion (Menke, 2019; Holsboer, 2000; Pariante and Miller, 2001). Moreover, dysfunction of the HPA axis results in increased levels of pro-inflammatory cytokines (Tapp et al., 2019). This axis has an important role in integrating emotional responses to stress that are dysfunctional in MDD, leading to increased cortisol and pro-inflammatory cytokines (Iob et al., 2020). Glucocorticoids are believed to be anti-inflammatory under most circumstances and are used as anti-inflammatory treatment (Cruz-Topete and Cidlowski, 2015). Thus, dysfunction of GR, or glucocorticoid receptor resistance (GCR), in MDD likely results in a runaway effect of inflammation. Moreover, GCR is associated with high levels of pro-inflammatory cytokine and can be caused by life stress (Cohen et al., 2012; Perrin et al., 2019). Overall, HPA axis dysfunction is seen in MDD and can be an indication of its prognosis (Varghese and Brown, 2001; Keller et al., 2017). Cytokine presence activates the HPA axis, and if already dysfunctional, results in increased inflammatory response (Dunn, 2006; Tapp et al., 2019).
The DMN is a neural circuitry that is known to be passively active when one is not engaged in externally directed activity (Buckner, 2013). Depressive rumination is correlated with DMN activity, particularly with the dorsal medial prefrontal cortex (dmPFC; Zhou et al., 2020; Hamilton et al., 2015). The medial prefrontal cortex (mPFC) is one of the main structural components of the DMN and is consistently shown to have a decreased volume in MDD, suggested to be caused by HPA axis dysfunction and increased inflammation (Belleau, Treadway, &1 Pizzagalli, 2019). Moreover, psychological stress can induce increased levels of inflammatory cytokines that impact the function and structure of the hippocampus, amygdala, and mPFC (Vecchiarelli et al., 2016; Belleau et al., 2019). Areas of the mPFC have been shown to integrate emotional processes and regulate the HPA axis in response to stress (Diorio et al., 1993; Figueiredo et al., 2003; Spencer et al., 2005; Radley et al., 2006). In MDD, and as a response to life stressors, impairments and global dysfunctions in connectivity occurs in the mPFC, ventral striatum, hippocampus, amygdala, and insular cortex, and is associated with increased C-reactive protein (CRP) levels (Murrough et al., 2016; Zeev-Wolf et al., 2019; Sripada et al., 2014; Yin et al., 2019). Moreover, depressed individuals show increased resting state activity of the DMN and greater activity increase in the DMN in response to negative stimuli (Grimm et al., 2009; Sheline et al., 2009). Depressed individuals failed to regulate heightened DMN reactivity (Sheline et al., 2009).
System and structure dysfunction may lead to increased inflammatory response and has been measured as per cytokine levels. However, there are other biomarkers now used to indicate inflammatory response in MDD. It may be argued that as the body uses inflammation as an indication of insult or injury, it responds via stress and immune activity that could manifest as MDD (Singhal and Baune, 2017; Bierhaus et al., 2003; Raison et al., 2010). Microglial cells are responsible for a range of complex functions, but primarily respond by modulating the expression of pro- and anti-inflammatory cytokines (Singhal and Baune, 2017). Translocator protein's (TSPO) are proteins that sit on the outer membrane wall of mitochondria of microglia and astrocyte cells (Lee et al., 2016). Microglia and astrocytes respond to damage and inflammation and TSPO activity increases as a response (Lee et al., 2016; Setiawan et al., 2015). Microglia also induce the production of important neurotrophins such as brain-derived neurotrophic factor (BDNF; Singhal and Baune, 2017). The interaction between inflammation and BDNF has been shown to affect different aspects of neuronal integrity such as neurogenesis, long-term potentiation, and dendritic sprouting, and increased inflammatory responses may result in decreased BDNF and increased excitotoxicity (Miller and Raison, 2016). Ligands that bind to TSPO have been shown to supress production of cytokines (Lee et al., 2016). Moreover, serotonergic signaling is an important mechanism for TSPO to produce anxiolytic effects (Yao et al., 2020). A TSPO ligand (i.e., YL-IPA08) was showed to elevate 5-HT levels in the hippocampus of rats (Yao et al., 2020). Furthermore, another TSPO ligand (i.e., ZBD-2) was shown to produce antidepressant effects in mice (Li et al., 2018, Li et al., 2018). Li et al., 2018, Li et al., 2018 assessed TSPO binding via the [18F]FEPPA ligand and found elevated binding in MDD participants in the frontal and temporal cortex that was reduced in response to therapy treatment. Setiawan et al. (2015) used [18F]FEPPA and found a significant increase in TSPO volume of depressed participants in all brain regions examined: PFC, anterior cingulate cortex (ACC), insula, dorsal putamen, ventral striatum, thalamus, and hippocampus. TSPO volume was increased, on average, by 30% in the prefrontal cortex, ACC and insula (Setiawan et al., 2015). Moreover, greater TSPO volume in the ACC correlated with greater MDD severity (Setiawan et al., 2015). A later study found that total duration of untreated MDD was a significant independent predictor of TSPO volume in the prefrontal cortex, anterior cingulate cortex, and insula, while duration of antidepressant exposure was a negative predictor of TSPO volume in these regions (Setiawan et al., 2018). Moreover, years of untreated MDD was a significant predictor of TSPO volume across all sampled grey-matter regions (Setiawan et al., 2018). TSPO volume increased at a rate of 14–18% per decade of untreated illness and could be a biomarker for duration and/or diagnosis of MDD (Setiawan et al., 2018).
Sufferers of MDD are often more vulnerable to adverse effects of stressful life events, and this leads to neurological changes (Yang et al., 2015; Felsten, 2004). Entrapment in a negative mental state, e.g., rumination over negative personal events curtail the capacity to pragmatically address and actively resolve significant sources of life stressors (Morrison and O’Connor, 2010). Studies show a deficit in executive control over rumination in response to stressors, suggesting that those with MDD are less able to control their mental state post-stressor (Quinn and Joormann, 2015). Negative thinking/rumination is an early cognitive symptom of MDD, and is as a strong predictor of relapse rate, chronicity, long-term outcome and severity of MDD (Spinhoven et al., 2018; Michalak et al., 2011; Vanderhasselt et al., 2016). Bierhaus et al. (2003) refers to the consequent somatic activation by stress as psychogenic conversion into cellular activation. Changes in nuclear factor kappa B (NF-κB), an important transcription factor for immune response is instigated by psychosocial stressors, precipitating a cascade of cellular changes in the neuroendocrine axis (Bierhaus et al., 2003). Moreover, increased pro-inflammatory proteins in those with MDD may be linked to suicidal behavior (González-Castro et al., 2021). Psychological stress has been consistently shown to increase inflammatory response and as a key factor in inflammatory disease (Wirtz and Kanel, 2017; Peters et al., 2012; Mawdsley and Rampton, 2006; Maes et al., 1998; de Pablos et al., 2006). A population-based study by Bremmer et al. (2008) has linked MDD in older adults to significantly higher levels of inflammatory markers. Harrison et al. (2009) induced depressive symptoms in healthy volunteers with a typhoid vaccine and showed a significant increase in IL-6 and a decrease in cognitive performance consistent with cognitive issues seen in patients with MDD.
Pace et al. (2006) found that increased IL-6 and NF-κB binding correlated with MDD severity and may be linked to early life stress. Similar results were shown in a 2012 study that found higher baseline levels of IL-6, TNF-α, and soluble interleukin-2 receptors (sIL-2R) in depressed participants compared to healthy controls (Liu et al., 2012). Chen et al. (2019) found that grey matter volume of the orbitofrontal cortex, lingual gyrus, inferior frontal cortex, middle frontal cortex, and planum temporale were reduced in bipolar disorder as compared to unipolar depression. This reduction was negatively correlated with soluble IL-6 receptor levels (Chen et al., 2019). Rajkowska et al. (1999) discovered a decrease in cortical thickness, neuronal sizes, and neuronal and glial density in a number of areas in the PFC of depressed brains. These structural changes are also seen in inflammation and implicate a neuroplastic responses in MDD (Aktas et al., 2007; Fleischman et al., 2010). CRP has been proposed as a potential biomarker for MDD (Chamberlain et al., 2019). Levels of CRP in patients with MDD have been shown to be elevated as compared to healthy controls (Chamberlain et al., 2019). Moreover, the greatest elevation of CRP is seen in treatment resistant MDD (Chamberlain et al., 2019). Normal activity of cytokines is important for neuroplasticity, and lower levels play a vital role in modulating metabolic and molecular pathways to promote long-term potentiation, neuroplastic changes, neuron health, and learning/memory consolidation (Bourgognon and Cavanagh, 2020). However, excessive presence can cause abnormalities in the structure and behavior of neurons (Barrientos et al., 2003; Ben Menachem-Zidon et al., 2008; Miller et al., 2009; Tilleux and Hermans, 2007; Bourgognon and Cavanagh, 2020). Pro-inflammatory cytokines can reduce the availability of monoamines by increasing the expression and function of presynaptic reuptake pumps leading to decreased synaptic availability of 5-HT and increased depressive-like behaviour (Miller and Raison, 2016). Cytokines can also decrease monoamine precursors by activating the enzyme that breaks them down and have been linked to decreased levels of BDNF (Calabrese et al., 2014). BDNF and 5-HT are crucially involved in neural plasticity and development, and the disruption of these systems especially during critical stages of development can lead to drastic structural/morphological changes (Yu and Chen, 2011; Daubert and Condron, 2010). Therefore, chronic inflammatory-induced monoamine and neurotrophin reduction likely leads to morphological changes seen in MDD. It still remains unclear whether abnormal changes as regards neuroplasticity occur prior to, or after, the onset of MDD symptoms, and if changes are completely reversible (Palazidou, 2012).
The link between inflammation and MDD may also have its evolutionary and adaptive origins. Miller and Raison (2016) gave support to the pathogen-host-defence hypothesis of depression that incorporates the need of inflammatory processes in relation to depressed states that are adaptations for survival. For example, depression-related symptoms and could promote survivability and reproduction in the highly pathogenic environments humans once lived in (Miller and Raison, 2016). This hypothesis is supported by a number of observations and findings. Cross cultural hunter-gatherer history data show many humans died of infectious disease before adulthood, enforcing genetic selectiveness in immune defence (Gurven and Kaplan, 2007). Pathogens increase inflammatory activity associated with depressed states, and this increased depressed behavior promotes survivability (Fumagalli et al., 2011; Kuningas et al., 2009). However, the same increase in inflammation risks increased mortality in non-pathogenic environments (Fumagalli et al., 2011; Kuningas et al., 2009). As such, society is now in a relatively low risk environment in which pathogenic host defense is less important. Thus, humans are currently genetically and biologically prepared to defend against a number of pathogens, however this increased defense has been used less as our environment is now cleaner. As a result, humans are now dealing with the consequences of being on high alert in a low-risk environment. However, in light of recent pandemic-related issues, this hypothesis may stand to be advantageous. Biological preparedness creates a hypervigilant system that increases inflammatory production that can be dangerous in a non-useful environment (Fumagalli et al., 2011; Kuningas et al., 2009).
Within the last decade or so, Serhan (2011) has coined the term “resolution” in the context of a complete return to homeostasis after the inflammatory response (Panigrahy et al., 2020; Serhan, 2017; Serhan and Savill, 2005; Serhan et al., 2008). This may be the missing link that completes the termination of prolonged inflammation. It is likely that resolution confirms the end of the inflammatory process, and while anti-inflammation gives us a glimpse into symptom relief, it does not provide proper resolution. Moreover, it is likely that the complexity of the issue is beyond our current understanding and that treatments that provide long-term relief after a small or single dose may hold some of the answers to acquiring treatment induced resolution. One possibility maybe that there is a systematic dysfunction between the DMN, the hypothalamic-pituitary-adrenal axis (HPA) axis, and negative cognition (i.e., rumination) that interferes with resolution. Psychogenic stress induces activation of numerous pathways, and chronic activation results in maladaptive functional and structural changes. Notably, the mPFC sees both a decrease in volume and an increase in activity in MDD. Furthermore, the HPA axis becomes dysfunctional in response to increased inflammation from chronic stress (Tapp et al., 2019; Silverman and Sternberg, 2012; Guilliams and Edwards 2010). Thus, the mPFC fails to provide appropriate modulation and signaling to the HPA axis, as a result of dysfunction due to chronic stress, which in turn increases inflammation, leading to the dysfunction of the HPA axis. This is a vicious cycle that causes perpetual effects, likely resulting in neurodegeneration.
3. Environmental and genetic risks of inflammation and MDD
Biological contribution to MDD manifestation is only one of many factors involved. It is understood that there is a complex interaction of factors that include environmental and genetic risks and can cause neurological changes to occur in individuals (van den Bosch and Meyer-Lindenberg, 2019; World Health Organization, 2020). Moreover, there is a link between these risks of MDD and increased inflammatory activity (van den Bosch and Meyer-Lindenberg, 2019).
Known environmental risk factors of MDD include substance abuse, lack of peer support, marital problems, low socioeconomic status, low education, obesity/diet, exercise, and stressful life events and all of which have been linked to increased inflammatory activity, further linking the relationship between inflammation and MDD (Dobson and Dozois, 2008; Muscatell et al., 2020; Martinez et al., 2018; Lacey et al., 2014; Fagundes et al., 2011; Friedman and Herd, 2010; Ljungberg et al., 2020). It is likely that these less-than-ideal circumstances exert stressful events and biological changes that are converted into increased inflammatory response. Altering some aspects of one's environment, such as lifestyle choices, can cause neurological changes corresponding with symptom relief (Lopresti et al., 2013). It is likely that similar types of neuroplastic changes are seen in cognitive behavioral therapy and may explain why it has increasingly become popular and effective for the treatment of MDD (Driessen and Hollon, 2011).
Poor diet is one factor of which poses increased risk of MDD and inflammation (Ljungberg et al., 2020). The prevalence of MDD has been increasing along side rates of obesity and sugar consumption, which are also linked to inflammation (Ellulu et al., 2017; Greenberg et al., 2021; Stierman et al., 2021; Bentley et al., 2020). The link between gut biome and neuroscience has been a common topic relevant to behavioural consequences and psychological disorders for roughly the past decade (Cryan and O’Mahony, 2011; Lyte, 2013; Moulton et al., 2019). Hsu et al. (2015) used a rat model to find that overconsumption of sugars, specifically high fructose corn syrup (HFCS-55), induced hippocampal and metabolic dysfunction, as well as significant increases in IL-6 and interleukin-1β (IL-1β); especially when consumed during the adolescent period. Other studies show increased neuroinflammatory markers TNF-α and IL-6, and anxiety and depression behaviors as a result of high carbohydrate induced obesity in mice (Gomes et al., 2020). Daneshzad et al. (2020) studied women with type 2 diabetes and compared their carbohydrate dietary intake and found a negative relationship between incidences of depressive symptoms, anxiety, and sleep quality with level of carbohydrate diet (Daneshzad et al., 2020). This evidence base can give us important cues about why we have widespread simultaneous increases in mental disorder instances and obesity rates, with MDD and dysthymia being the most prevalent disorders globally (Avila et al., 2015; Baxter et al., 2013).
Inverse-diet studies provide evidence of how dietary adjustment can influence psychological health. Xu et al. (2021) published findings on the ketogenic diet in a mouse model of Alzheimer's disease and found that it ameliorated cognitive impairment and significantly reduced neuroinflammation. Koh et al. (2020) describe the improved immunomodulatory effects of the ketogenic diet. Results indicated powerful anti-inflammatory and neuroprotective effects on the nervous system shown in the improved outcomes after application of the diet on neurological disorder models and patients. Improvements resulting from the immunomodulation and improved polyunsaturated fatty acid intake may be supported by, or result from, favourable gut microbiota modifications due to this dietary change.
Certain types of sedentary lifestyles are linked with MDD and risk the development of increased inflammation, diabetes, and obesity (Hallgren et al., 2020; Lira et al., 2010). Physical exercise has been shown to induce the production of testosterone and attenuate cortisol production, mitigating the effects of perpetual inflammation (Brownlee et al., 2005). Testosterone has neuroprotective properties and is associated with neuroplasticity and synaptogenesis (Camacho-Arroyo et al., 2020). Excess cortisol is a major factor in the onset and maintenance of MDD and is a major contributing factor of inflammation (Jia et al., 2019; Furtado and Katzman, 2015). Further, Gold et al. (2008) demonstrated the neuroprotective effects of testosterone in male multiple sclerosis patients. They found significant increases in BDNF and platelet-derived growth factor (PDGF) via improved immunomodulation (Gold et al., 2008). Testosterone supplementation in diagnosed hypogonadal men has been shown to result in extreme and rapid recovery from treatment-resistant MDD with even low supplementation dosage which resulted in low-normal serum testosterone (Seidman and Rabkin, 1998).
5-HT related gene polymorphisms have also been shown to disrupt 5-HT signalling as well as increase inflammatory cytokine levels. Polymorphism specifically in the SERT gene (SLC6A4), particularly the 5-HT-transporter-linked promoter region (5-HTTLPR) of depressed individuals increases activity of the amygdala, while reducing the connectivity between the amygdala and the subgenual region of the anterior cingulate cortex, the dorsal region of the anterior cingulate cortex, and the dorsolateral prefrontal cortex (Pezawas et al., 2005; Costafreda et al., 2013). Moreover, 5-HTTLPR is responsible for regulating IL-6ß during acute stress, and polymorphisms result in increased cytokine levels (Yamakawa et al., 2015). Carriers of the RS6295 gene has been shown to reduce 5-HT transmission in way of altered 5-HT1A autoreceptor activity and induce anxiety and depressive like behaviors (Albert, 2012). Even after recovery from MDD, cortical 5-HT1A binding potential is lowered, likely causing a trait abnormality that puts people at risk of subsequent major depressive episodes (Bhagwagar et al., 2004). Carriers of a specific gene variant that reduces the expression of SERT are more sensitive to stress-induced activation of the amygdala (Hariri et al., 2002). Epigenetic changes, e.g., significantly lower methylation of SLC6A4, has been found in depressed patients, which correlate with decreased amygdala reactivity (Schneider et al., 2018).
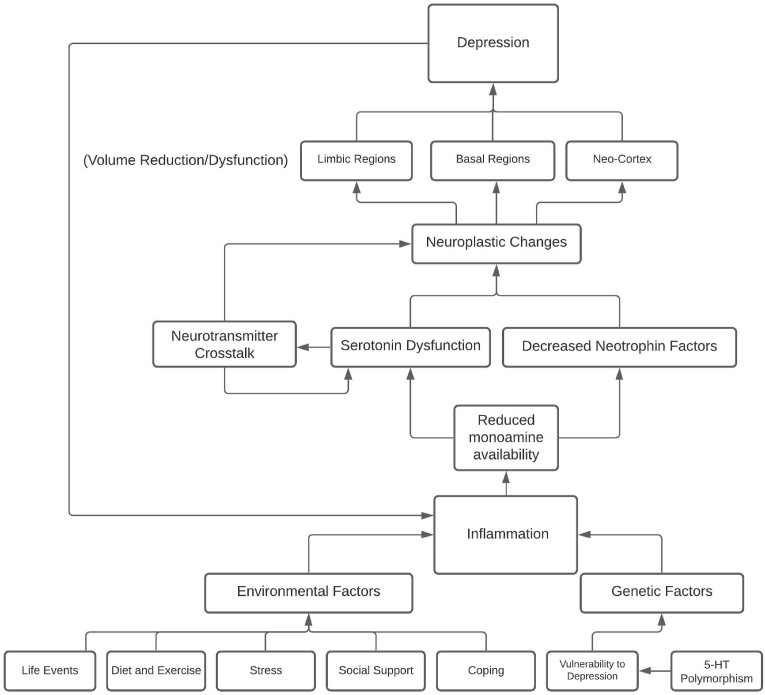
MDD is deeply associated with environmental and genetic risk factors that are now known to influence inflammatory proteins, furthering the link between inflammation and MDD. See Fig. 1 for a concept map synthesis of the hypothesized relationship of the risk factors of MDD and inflammation. Greater exposure to environmental risks should increase the prevalence of both MDD and inflammation. Finding new ways to prevent exposure to these factors could provide better outcomes for individuals, as well as a path for researchers to further investigate the relationship between inflammation and MDD.
Fig. 1.
This concept map visualizes the hypothesized complexity of the relationship between multiple factors of depression and inflammation. Summarized; Environmental and genetic factors predispose individuals to a level of vulnerability to inflammatory response of which reduces monoamine availability and leads to cascading effects of neurotransmitter dysfunction and decreased neurotrophin factors. This perpetual process provides chronic neuroplastic changes in the affected regions and systems that result in depressive symptoms. Finally, depression results in a cyclic circumstance in which inflammation is increased and resumes the aforementioned effects. Effective treatment would be one that breaks this cycle, providing anti-inflammatory and anti-depressant effects.
4. Antidepressant's effect on inflammation
The most common treatment administered is the prescription of selective 5-HT reuptake inhibitor (SSRI) antidepressants. SSRI's target 5-HT transporter (SERT) protein as its mechanism of prolonged 5-HT neurotransmitter exposure in the synaptic cleft. While SSRI's do provide relief for some, research shows that activity involving SERT degrades over the course of prolonged treatment and may become less effective (Descarries and Riad, 2012). Meta-analyses typically report antidepressants as being 20%–30% more effective than placebo but indicate that effectiveness varies as a function of symptom severity, with greatest efficacy in severe MDD (Penn and Tracey, 2012). Historically it has been believed that SSRI's efficacy relied on restoring the balance of 5-HT in the brain. However, new evidence suggests that SSRIs have modest anti-inflammatory effects that may explain their limited effectiveness.
It is now apparent that there is a tight relation between inflammation, 5-HT, and MDD (Catena-Dell'Osso et al., 2013). One study compared the mechanisms of action of anti-inflammatories and antidepressants on enzymes responsible for the metabolism of tryptophan, the precursor to 5-HT (Regan et al., 2018). This study found that increased inflammation due to exposure to lipopolysaccharide (LPS), an endotoxin frequently used to induce inflammatory animal models, and the cytokine interferon alpha (IFN-α) upregulated the expression of genes responsible for the production of tryptophan enzymes (Regan et al., 2018). Moreover, anti-inflammatories, but not antidepressants, mitigated the effects of gene expression (Regan et al., 2018). Concluding that inflammation can cause a depletion of tryptophan that can be protected against through the use of some anti-inflammatory compounds (Regan et al., 2018). The fact that there was no effect from anti-depressants suggests either a more potent anti-inflammatory is required, or there was no direct mechanistic effect on the specific gene expression investigated. Limited efficacy of antidepressants alludes to the likely maximum effect antidepressants have on inflammation. Conversely, some common anti-inflammatory drugs have positive antidepressant effects (Schmidt et al., 2016). Indeed, common antidepressants of different classes have been found to have acute anti-inflammatory effects (Tomaz et al., 2020). This contributes to the explanation of how antidepressants work for some individuals. However, there is a concerning issue with efficacy of antidepressants that is likely attributed to the diffuse nature of antidepressants and the complex physiological behaviors of individuals. For instance, SSRI's can worsen rumination which then leads to worsened inflammatory and neurological processes and rumination is a predictor of relapse in MDD (Andrews et al., 2015; Michalak et al., 2011).
It has been shown that antidepressants may induce neurogenesis and neuroplasticity (Duman et al., 2016; Serafini, 2012). Further, SSRIs have been shown to increase BDNF related processes (Martinowich and Lu, 2008). BDNF plays a role in neurogenesis, neuroplasticity, as well as promotes the survival of serotonergic neurons (Martinowich and Lu, 2008). The association is then that neuroprogression of MDD is accompanied and worsened by inflammation, and antidepressants work to mitigate the negative affects of inflammation such as decreased spine density, less dendrites, loss of synapses, and loss of glia cells (Serafini, 2012). Given that many antidepressants affect both the serotonergic and glutamatergic system, there are implications that antidepressants may in fact promote neuroplasticity (Azmitia, 1999). Manipulating certain aspects of 5-HT neurons’, such as the activity pattern and microstructure, can prevent the loss of synapses (Azmitia, 1999). Neural adaptation to environmental changes relies on the involvement of serotonergic plasticity (see more in Figure, 7 of Azmitia, 1999). There is a consensus on the role of 5-HT as it is related to plasticity and its implications in MDD (Liu et al., 2017).
5-HT dysfunction has been historically linked to MDD, and treatment commonly focuses on manipulating 5-HT. New evidence sheds light on the relationship between 5-HT-function and inflammatory regulation and may explain common antidepressant efficacy. The 5-HT system as plays an important role in inflammation regulation. Thus, dysfunction would cause increased inflammatory activity and 5-HT related medications would likely provide some assistance in re-establishing regulation. However, low-rate efficacy suggests that SSRI's may not sufficiently suppress inflammatory-induced dysfunction and that additional mechanisms are involved. Thus, it is imperative that researchers turn their focus to investigating mechanisms of compounds or interventions that show high-rate long-term efficacy.
5. Alternative treatments
Alternative treatments may provide greater efficacy in treating MDD symptoms and may commonly exhibit potent anti-inflammatory properties. See Table 1 for a summary of pharmacological effects of alternative treatments on inflammation and MDD.
Table 1.
Anti-inflammatory properties of alternative antidepressant treatments.
| Drug Classification | Mood/Affect | Inflammation | Sources |
|---|---|---|---|
| Ketamine/NMDA receptor antagonists | - rapid onset (30 min) of antidepressant effects, especially in treatment resistant depression - decreased rumination, depressive behaviors, and depression scores - Antidepressant effects persist for up to 7 days |
- Decreased pro-inflammatory cytokines (i.e., TNF-α) that are correlated with decreased depression | Human
|
| Cannabis/Cannabinoid | - THC, CBD, and HU-580 show antidepressant-like effects in rodent behavioral tests - UCS decreases eCB signalling - FAAH enzyme inhibition leads to anti-depressant effects |
- Decreased inflammatory cytokines (e.g., IL-6, TNF-α, IFN-ß) - Decreased PGE2, COX activity, oxygen-derived free radicals, and nitric oxide - Chronic stress induces inflammation which is rescued by cannabinoid treatment |
HumanAnimal |
| Psychedelics | - Decreased stress and anxiety-related behaviors - Rapid and long-term antidepressant effects in MDD lasting up to 6 months |
- Decreased CRP that correlates with decreased depression - 5-MeO-DsMT increased cortisol and decreased IL-6 |
Human
|
Note: Acronyms: UCS – unpredictable chronic stress, eCB – endocannabinoids, FAAH - Fatty acid amide hydrolase, TNF-α - Tumor necrosis factor alpha, IFN-ß – Tumor necrosis factor beta, PGE2 - Prostaglandin E2, COX – cyclooxygenase, CRP – C-reactive protein, 5-MeO-DMT - 5-methoxy-N,N-dimethyltryptamine, IL-6 – Interleukin-6, HU-580 – cannabidiolic acid methyl ester, MDD – major depressive disorder.
5.1. Ketamine
Serotonergic manipulation plays one role in antidepressant and anti-inflammatory action, however, mechanisms involved in MDD continue to be investigated revealing new and complex processes. Huang et al. (2017) proposed that new treatment options based on the glutamatergic system may yield better outcomes related to MDD relief and neuroplasticity. Ketamine treatment has been accredited for having promising fast-acting results in relieving depressive symptoms and inducing plastic changes and mood improvement (Huang et al., 2017). Ketamine, as a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, blocks transmission of glutamate at the NMDA receptor (Molero et al., 2018). Ketamine has anti-inflammatory properties (Loix et al., 2011; Cathomas et al., 2022; Chen et al., 2021; Kopra et al., 2021). Moreover, ketamine modulates the DMN and reduces self-directed negative cognition (e.g., rumination; Lehmann et al., 2016). Its primary use has been as a dissociative anesthesia, but studies found that it reduced inflammatory response to surgery (Dale et al., 2012). Ketamine has been shown to reduce TNF-a and IL-6 expression in immune cells such as macrophages and glial cells (Chang et al., 2009, 2010; Yuhas et al., 2015). Single-dose monotherapy has shown great efficacy in treating MDD and can rapidly reduce suicidal thoughts (Molero et al., 2018). Indeed, ketamine has been shown to produce antidepressant effects as quickly as 30-min after administration (Liebrenz et al., 2009). In animal models, this rapid antidepressant effect has been linked with down-regulating pro-inflammatory cytokines in the hippocampus (Wang et al., 2015), without any sex differences in its neurobiological/physiological effects despite apparent sex differences in antidepressant behavioural phenotype (Chang et al., 2018). Since the efficacy of ketamine is tied with mammalian target of rapamycin (mTOR) activation and the production of BDNF, Su et al. (2017) assessed the efficacy of ketamine in treatment-resistant MDD participants who carried a low functioning BDNF gene. This study found that ketamine was not effective in low severity MDD but was increasingly effective as depression severity increased (Su et al., 2017). Chen et al. (2018) specifically looked at providing low-dose ketamine infusions in treatment-resistant MDD patients as well. They found rapid reduction in the proinflammatory cytokine TNF-α that correlated with decreased scores on a depression scale (Chen et al., 2018). Moreover, a dose-dependent effect was seen, and as dose increased from control to 0.2 mg/kg to 0.5 mg/kg, depression scores decreased accordingly (Chen et al., 2018). Low-dose efficacy of ketamine has been recently shown in animal models of depression as well, and researchers link its effects to anti-inflammatory pathways (Zhao et al., 2020). Zhan et al. (2020) investigated a large number of cytokines in MDD patients and found that there were 14 different inflammatory factors downregulated after 12 days of treatment with ketamine. Moreover, interleukin-17A (IL-17A) and IL-6 were both positively correlated with improved symptoms.
One promise of ketamine's potential is that it requires infrequent administration and is likely easier to maintain long-term. For example, Zarate et al. (2006) showed that the antidepressant effects of a single administration persisted for up to 7 days. Drug adherence is an issue in effective long-term treatment in MDD (Dell’Osso et al., 2020). Thus, ketamine may provide a treatment plan that is more forgiving. Ketamine also has a good safety profile with minimal adverse effects and has been frequently used in children (Green et al., 1996; Dolansky et al., 2008).
5.2. Cannabinoids
Research and proper regulation of cannabis products for medicinal purposes as a treatment option for MDD is scarce. The use of cannabis has been historically associated with worsening or initiating the onset of MDD; however, recent investigations have begun to explore the potential of an inverse relationship (Feingold and Weinstein, 2021). The relationship between cannabis and MDD has not been well elucidated, and there are now indications that it may be an effective alternative treatment for MDD (Martin et al., 2021). For example, while it has been found that those who used cannabis had worsened MDD, this was only the case for heavy users and was a modest effect (Degenhardt et al., 2003). Reviews have been able to show a high comorbidity between cannabis use disorder and MDD (Onaemo et al., 2021), however the perspective of this relationship may be biased by the historical prohibition of cannabis products. For instance, cannabis may fare better as an effective treatment in which those who have MDD use it to self-medicate, explaining a high rate of cannabis consumption among those with MDD (Wieckiewicz et al., 2022). Delta(9)-tetrahydrocannabinol (THC) and cannabidiol (CBD) are two well-known constituents of cannabis that continue to show anti-depressant and anti-inflammatory effects (Henshaw et al., 2021; Sales et al., 2018; El-Alfy et al., 2010). While there are many other constituents of cannabis, research on these is still fairly new. Recently, Faouzi et al. (2022) showed anti-inflammatory effects of cannabigerolic acid by blocking nuclear factor of activated T-cells and interleukin-2 (IL-2) production. In 2005, Hill et al. (2005) revealed that unpredictable stress induced dysfunction of the cannabinoid system of which was rescued via exogenous cannabinoid treatment. It was later showed that chronic stress reduced the production of endocannabinoids and led to depressive-like behavior (Haj-Dahmane and Shen, 2014).
The endocannabinoid system modulates serotonergic neurotransmission via the CB1 receptor to produce antidepressant effects (Gobbi et al., 2016; Bambico et al., 2016). Fatty acid amide hydrolase (FAAH) is an enzyme responsible for degradation of endocannabinoids, and when genetic expression is removed effects appear to be anxiogenic and anti-depressant in mice (Bambico et al., 2010a, Bambico et al., 2010b; Gobbi et al., 2016). Moreover, by providing more availability of endogenous cannabinoids, serotonergic transmission is altered (Bambico et al., 2010, 2016; Gobbi et al., 2016). Bambico et al. (2016) was able show a connection between the endocannabinoid system, BDNF, and antidepressant and anxiolytic effects. Using single and repeated dose of the endocannabinoid enhancer drug URB597, animal models showed reduced depressed- and anxious-like behaviors in the forced swim test, elevated plus maze, and novelty-supressed feeding test (Bambico et al., 2016). URB597 is an inhibitor of the enzyme fatty acid amide hydrolase (FAAH) that degrades the endocannabinoid anandamide (AEA; Bambico et al., 2016). Repeated URB597 treatment was associated with increased BDNF and showed profound antidepressant-like and anxiolytic-like effects compared to a single dose (Bambico et al., 2016). In addition, non-steroidal anti-inflammatory drugs (NSAIDs, e.g., oleamide, arachidonamide and stearoylamide) are linked to FAAH inhibition (Dongdem et al., 2022). URB597 has also been linked to anti-inflammatory effects across animal models of inflammation (Jayamanne et al., 2009; Murphy et al., 2012). A recent lipopolysaccharide (LPS)-induced inflammation mouse model study showed that inhibiting enzymes responsible for the degradation of endocannabinoids using URB597 and JZL184 can decrease inflammatory response and pro-inflammatory cytokines (Abohalaka et al., 2020). Moreover, these inhibitors decreased 5-HT hyperactivity and it was suggested that inhibition of endocannabinoid enzymes prevented inflammation-induced 5-HT hyperactivity.
CBD is increasingly used for medical purposes, and is known to be neuroprotective, cardioprotective, and anti-inflammatory (Britch et al., 2021). CBD's neuroprotective potential has strong implications in helping reduce symptoms of neurodegenerative diseases such as ALS, Parkinson's disease, Huntington's disease, Alzheimer's disease, and multiple sclerosis (Iuvone et al., 2009). CBD has a low affinity for cannabinoid receptor 2 (CB2), and it activates 5-HT1A and transient receptor potential vanilloid (TRPV) receptor subtypes, which may implicate its medicinal use in neuroplasticity and inflammation (Britch et al., 2021; Bujak et al., 2019). Rodent models and human clinical trials reveal antidepressant and anxiolytic effects of CBD (García-Gutiérrez et al., 2020). A synthetic analogue precursor of CBD, cannabidiolic acid methyl ester (HU-580), has also been shown to rescue animals from depressive-like behaviors (Hen-Shoval et al., 2018). CBD and THC have been shown to be anti-inflammatory and can be effective in symptom relief in conditions such as gastrointestinal inflammation, ulcerative colitis, ocular hypertension and glaucoma, graft-versus-host disease, and diabetes (Britch et al., 2021; Nagarkatti et al., 2009). While CBD has been shown to affect many mechanisms with indirect action on inflammation, there are few studies directly testing its effect on pro-inflammatory cytokines (Atalay et al., 2019). Costa et al. (2007) found that CBD was effective in reducing hyperalgesia in an inflammatory model of rats and showed that a single-dose CBD injection decreased edema in carrageenan-induced inflammation (Costa et al., 2004). After three doses of CBD, rodents showed decreased prostaglandin E2 (PGE2) plasma levels, tissue COX activity, production of oxygen-derived free radicals, and nitric oxide; all of which play a role in maintenance of inflammatory processing (Costa et al., 2004). Petrosino et al. (2018) tested CBD's effect on pro-inflammatory cytokines and AEA levels in vitro. They found that CBD increase AEA and decreased IL-6, interleukin-8 (IL-8), and TNF-α. This was also blocked by CB2 and TRPV1 antagonism revealing the mechanisms involved for pro-inflammatory reduction. Jean-Gilles et al. (2015) were able to show an association between peripheral immune cell CB1/CB2 upregulation and pro-inflammatory cytokines IL-1ß, IL-6, and TNF-α in patients with multiple sclerosis, primarily an inflammatory disease. Two randomized controlled trials were able to show CBD decreased inflammatory cytokines (Morissette et al., 2021; Verrico et al., 2020), however, to our knowledge there is no findings of this nature in the context of MDD. Thus, it is imperative to further investigate the possible link between the anti-inflammation and anti-depressant effects of CBD.
In 2012, a study was conducted using rats to assess behavioral outcomes of chronic THC administration. The results indicated that THC increased BDNF and had antidepressant-like behavioral changes (Elbatsh et al., 2012). Motwani et al. (2018) discovered powerful anti-inflammatory properties of delta(8)-tetrahydocannabinol (THC)-11-oic acid (anabasum), a synthetic analog of THC, in humans. Yekhtin et al. (2022) tested both THC and CBD in an inflammatory model using mice and found a reduction in the pro-inflammatory cytokines IL-6, TNF-α, and interferon-beta (IFN-ß). Notably, they tested the effects of both forms of pure cannabinoids and cannabis extract and found stronger effects from cannabis extract.
Karoly et al. (2018) conducted a preliminary study to assess the anti-inflammatory potential of cannabis in people who use alcohol and cannabis. Alcohol is a frequently used substance that also tends to cause increased inflammation making it a prime target for this study. Although this study was based on self-report data, the results showed an interaction effect in that IL-6 levels were strongly associated with non-cannabis users who drank alcohol (Karoly et al., 2018). Moreover, there was a negative correlation between cannabis and IL-1ß. It was concluded that cannabis may mitigate the inflammatory effects of alcohol (Karoly et al., 2018).
5.3. Psychedelics
Psychedelics as a classification have been identified to mediate inflammation via the 5-HT2A receptor, but there have been very little specific investigations on psychedelic compounds (Flanagan and Nichols, 2018). In 2008, Yu et al. showed that psychedelics could inhibit TNF-α inflammatory processes via the 5-HT2A receptor subtype, and this was considered groundbreaking and sparked future research. Flanagan and Nichols hypothesized that psychedelics halted perpetual neural processes by resetting resting state functional connectivity (RFSC; Flanagan and Nichols, 2018). Thus, psychedelics likely reduce neuroinflammation and stop cascading processes in the brain from reoccurring, causing lasting therapeutic effects (Flanagan and Nichols, 2018). Indeed, psychedelics have recently taken new light as a therapeutic treatment option for MDD and inflammation, and there is a great push for more clinical research to further link this relationship (Szabo, 2019). Flanagan et al. (2021) confirmed the hypothesis that anti-inflammatory action of a number of psychedelics occurred via 5-HT2A receptor activity and found the compound 2C-H produced the strongest anti-inflammatory action. Galvão-Coelho et al. (2020) conducted a double-blind placebo-controlled trial with treatment-resistant MDD patients to assess the efficacy and anti-inflammatory effects of ayahuasca; treatment resistant patients were compared to healthy controls. Results showed that treatment resistant patients had higher levels of baseline C-reactive protein (Galvão-Coelho et al., 2020). Both healthy and treatmen-resistant patients seen a reduction in CRP in response to ayahuasca treatment, and greater reduction of CRP in treatment-resistant patients was correlated with lower depression scores (Galvão-Coelho et al., 2020).
Another recent study assessed the effects of 5-MeO-DMT on inflammatory biomarkers and behavioral measures (Uthaug et al., 2020). Participants were measured at baseline, about 1 h directly after their inhalation, and during a seven-day follow up. This study found that inhaling a synthetic 5-MeO-DMT resulted in increased cortisol and decreased IL-6 (Uthaug et al., 2020). Stress and anxiety as per survey measures were significantly lower 7 days later and correlations were found between participants' ratings of the psychedelic experiences and improved affect (Uthaug et al., 2020). The quality of a psychedelic experience is important in one's perception of the experience and thus affects the efficacy of treatment (Yaden and Griffiths, 2021). Indeed, subjective experience and psychological state do affect one's physiology (Bierhaus et al., 2003). Thus, while negative psychological experiences influence inflammation and respective cascading biological events, it is likely that positive psychological experiences provide an opposing effect. It turns out that positive affective traits do in fact predict later lower levels of IL-6 (Stellar et al., 2015). This is likely a contributing factor in the efficacy of treatments that also induce positive experiences (e.g., psychedelics).
In a 2016 breakthrough randomized double-blind trial, Griffiths et al. (2016) provided psilocybin to patients with terminal cancer. Intuitively, dealing with a life-threatening illness would induce depressed mood and anxiety. This study concluded that psilocybin produced large decreases in depression and anxiety, as well as specifically death anxiety (Griffiths et al., 2016). Further, patients seen an increase in quality of life, particularly optimism (Griffiths et al., 2016). Six months later, it has been found that 80% of patients sustained these effects. One important follow up study by Davis et al. (2021) showed outstanding results in the antidepressant effect of psilocybin. This randomized clinical trial study provided two psychotherapy-assisted psilocybin treatment sessions for participants who were diagnosed with MDD. Results of this study showed a rapid onset of antidepressant effects that persisted for up to four weeks (Davis et al., 2021). Moreover, 71% of the participants at weeks one and four had a clinically significant reduction in depressive symptoms, and 58% were in full remission (Davis et al., 2021).
Barrett et al. (2020) ran a pilot study to investigate the effect of psilocybin on brain structure activity in response to depression and anxiety provoking stimuli. Results showed that negative affect and amygdala activity in response to facial stimuli were reduced, suggesting that reactivity to emotionally charged stimuli is decreased (Barrett et al., 2020). Further, when participants responded to emotionally conflicting stimuli, positive affect and dorsal lateral prefrontal and medial orbitofrontal cortex activity were increased (Barrett et al., 2020). This suggests that participants are more able to employ executive functions (e.g., rational and logical thinking) when processing emotionally relevant stimuli due to their psilocybin experience. This study also found lasting effects up to one month after consuming psilocybin. Trait anxiety was reduced, and functional connectivity was better increased after one month of treatment than after one week of treatment (Barrett et al., 2020). Researchers concluded that psilocybin likely increases functional brain plasticity in response to emotional stimuli (Barrett et al., 2020).
Contrary to most literature, Porr et al. (2020) found that SSRIs in conjunction with environmental changes had better outcomes for decreasing depressed behaviors as compared to psychedelics. While the results for psychedelics were mixed in this study, it should be noted that this study used animal subjects and the subjective experience may be a key factor in treatment efficacy (Yaden and Griffiths, 2021). Biologically, psychedelics modulate the DMN and decrease its resting activity, and this alteration has been linked to their efficacy (Palhano-Fontes et al., 2015; Smigielski et al., 2019; Ruban and Kołodziej, 2018). Psychologically, psychedelics provide alternative perspectives and understandings of the connections between the self and the outside world. Several hypothesizes are being developed on the way in which psychedelics tend to disrupt one's way of thinking and provide new positive beliefs (Carhart-Harris, 2019). This highlights a likely important cognitive factor of one's own beliefs and the risk of relapse of depressive rumination (Michalak et al., 2011). However, current research outcomes are lacking explanatory power on the mechanisms involved in the treatment efficacy of psychedelics. Therefore, it is paramount that future research investigates the precise mechanisms of psychedelics in the context of MDD and inflammation to further elucidate this relationship and provide insights into long term relief.
6. Conclusions
This review was set out to describe the importance of the impact of inflammation on MDD by elucidating the relationship. Inflammation impacts monoamine systems, neural structures, and system functions related to MDD. The presented literature depicts a very complex and dynamic picture of the important functions of many biological processes in relation to MDD that are also influenced by inflammatory activity. Moreover, given the complex biopsychosocial nature of MDD, it is even more challenging to find consistent data on these processes. Through the presence of and changes in cytokine levels, we can now see that inflammation is greater in those with MDD than those without. Moreover, individuals with MDD also show higher immune-related activities that are known to respond to inflammation. There are a number of environmental risks of MDD that can alter neurological and inflammatory activity, and changes in these can decrease inflammation and depressive symptoms.
Common treatment for MDD can be effective for some, while not being effective for others. This discrepancy in effectiveness has provided the motivation to find new alternative drug treatments that can be more effective than common treatment and provide long-term relief. The proposed alternatives provide a promising future for the treatment of MDD by potentially influencing a inflammatory resolution mechanism. We propose that through the effects of its own presence at a threshold, inflammatory cytokines influence a perpetual process that is increasingly difficult to bring to resolution, and the aforementioned alternatives provide insights of a potential resolution not yet understood. Specifically, psychedelics have breakthrough treatment efficacy in MDD long term. Thus, it is imperative that researchers investigate the anti-inflammatory natures of these compounds to further elucidate the mechanisms involved in their resolution potential. This would provide individuals with a potential treatment more efficacious than current options and would greatly alleviate the pressure on health care professionals. Moreover, having several alternatives available provides the client with more options to fit their comfort level.
There are limitations to some of the presented findings, however. Most importantly, many results are based on correlations. For this reason, causality and generalizability can still be improved by future investigations, especially with the use of preclinical experimental models. Given the nature of the topic, sample sizes are often small, and controllability and validity of measures are sometimes limited. Despite this, there seems to be some degree of replicability. This research is still in its infancy, and because of this, much of the proposed relationships are theoretical. At this time, animal models continue to offer invaluable information despite issues with translatability. Future clinical instigations on early diagnoses of MDD and prodromal markers that align with preclinical models would enrich and improve longitudinal and experimental designs. This would allow researchers to track inflammatory processes, neurological changes, and MDD phenotype over time. Alternatively, more research is required on each of the mentioned drug treatment alternatives, especially in the context of DMN and HPA axis functioning. To further substantiate the inflammation hypothesis of MDD, clinical investigations should be conducted on related and/or comorbid disorders (e.g., anxiety, TBI) that may be linked to inflammation. A major factor in causality and/or exacerbation of MDD is increased inflammatory processes that can be mediated and treated by multiple treatment options that have both antidepressant and anti-inflammation properties.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Abbott A. Depression revisited. Nature. 2018;557:633–634. [Google Scholar]
- Abohalaka R., Bozkurt T.E., Nemutlu E., Onder S.C., Sahin-Erdemli I. The effects of fatty acid amide hydrolase and monoacylglycerol lipase inhibitor treatments on lipopolysaccharide-induced airway inflammation in mice. Pulm. Pharmacol. Therapeut. 2020;62 doi: 10.1016/j.pupt.2020.101920. [DOI] [PubMed] [Google Scholar]
- Aktas O., Ullrich O., Infante-Duarte C., Nitsch R., Zipp F. Neuronal damage in brain inflammation. Arch. Neurol. 2007;64(2):185–189. doi: 10.1001/archneur.64.2.185. [DOI] [PubMed] [Google Scholar]
- Albert P.R. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos. Trans. R. Soc. B. 2012;367(1601):2402–2415. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S., Puentes F., Baker D., Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.W., Bharwani A., Lee K.R., Fox M., Thomson J.A., Jr. Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neurosci. Biobehav. Rev. 2015;51:164–188. doi: 10.1016/j.neubiorev.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Arnett P., Barwick F., Beeney J. Depression in multiple sclerosis: review and theoretical proposal. J. Int. Neuropsychol. Soc. 2008;14(5):691–724. doi: 10.1017/S1355617708081174. [DOI] [PubMed] [Google Scholar]
- Atalay S., Jarocka-Karpowicz I., Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2019;9(1):21. doi: 10.3390/antiox9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J.A., Drevets W.C., Moseman S.E., Bodurka J., Barcalow J.C., Simmons W.K. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatr. 2014;76(3):258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila C., Holloway A.C., Hahn M.K., Morrison K.M., Restivo M., Anglin R., Taylor V.H. An overview of links between obesity and mental health. Curr. Obesity Rep. 2015;4(3):303–310. doi: 10.1007/s13679-015-0164-9. [DOI] [PubMed] [Google Scholar]
- Azmitia E.C. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology. 1999;21(2):33–45. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Bai S., Guo W., Feng Y., Deng H., Li G., Nie H., Guo G., Yu H., Ma Y., Wang J., Chen S., Jing J., Yang J., Tang Y., Tang Z. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatr. 2020;91(1):21–32. doi: 10.1136/jnnp-2019-320912. [DOI] [PubMed] [Google Scholar]
- Bambico F.R., Nguyen N., Katz N., Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol. Dis. 2010;37(3):641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Bambico F.R., Cassano T., Dominguez-Lopes S., Katz N.S., Walker C.D., Piomelli D., Gobbi G. Genetic deletion of fatty acid amide hydrolase alters emotional behaviour and serotonergic transmission in the dorsal raphe, prefrontal cortex and hippocampus. Neuropsychopharmacology. 2010;35(10):2083–2100. doi: 10.1038/npp.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico F.R., Duranti A., Nobrega J.N., Gobbi G. The fatty acid amide hydrolase inhibitor URB597 modulates serotonin-dependent emotional behaviour, and serotonin1A and serotonin2A/C activity in the hippocampus. Eur. Neuropsychopharmacol. 2016;26(3):578–590. doi: 10.1016/j.euroneuro.2015.12.027. [DOI] [PubMed] [Google Scholar]
- Barrett F.S., Doss M.K., Sepeda N.D., Pekar J.J., Griffiths R.R. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 2020;10:2214. doi: 10.1038/s41598-020-59282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., Sprunger D.B., Campeau S., Higgins E.A., Watkins L.R., Rudy J.W., Maier S.F. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Baxter A.J., Patton G., Scott K.M., Degenhardt L., Whiteford H.A. Global epidemiology of mental disorders: what are we missing? PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau E.L., Treadway M.T., Pizzagalli D.A. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol. Psychiatr. 2019;85(6):443–453. doi: 10.1016/j.biopsych.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O., Goshen I., Kreisel T., Ben Menahem Y., Reinhartz E., Ben Hur T., Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Bentley A.R., Ruck D.J., Fouts H.N. U.S. obesity as delayed effect of excess sugar. Econ. Hum. Biol. 2020;36 doi: 10.1016/j.ehb.2019.100818. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z., Rabiner E.A., Sargent P.A., Grasby P.M., Cowen P.J. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol. Psychiatr. 2004;9(4):386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Bierhaus A., Wolf J., Andrassy M., Rohleder N., Humpert M., Petrov D.…Nawroth P.P. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgognon J.M., Cavanagh J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020;4 doi: 10.1177/2398212820979802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S., Kupferberg A., Rogler G., Hasler G. Vagus nerve as modulator of the brain–gut Axis in psychiatric and inflammatory disorders. Front. Psychiatr. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer M.A., Beekman A.T.F., Deeg D.J.H., Penninx B.W.J.H., Dik M.G., Hack C.E., Hoogendijk W.J.G. Inflammatory markers in late-life depression: results from a population-based study. J. Affect. Disord. 2008;106(3):249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Britch S.C., Babalonis S., Walsh S.L. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology. 2021;238(1):9–28. doi: 10.1007/s00213-020-05712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee K.K., Moore A.W., Hackney A.C. Relationship between circulating cortisol and testosterone: influence of physical exercise. J. Sports Sci. Med. 2005;4(1):76–83. [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. The brain's default network: origins and implications for the study of psychosis. Dialogues Clin. Neurosci. 2013;15(3):351–358. doi: 10.31887/DCNS.2013.15.3/rbuckner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujak J.K., Kosmala D., Szopa I.M., Majchrzak K., Bednarczyk P. Inflammation, cancer and immunity-implication of TRPV1 channel. Front. Oncol. 2019;9:1087. doi: 10.3389/fonc.2019.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F., Rossetti A.C., Racagni G., Gass P., Riva M.A., Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014;8:430. doi: 10.3389/fncel.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I., Piña-Medina A.G., Bello-Alvarez C., Zamora-Sánchez C.J. Sex hormones and proteins involved in brain plasticity. Vitam. Horm. 2020;114:145–165. doi: 10.1016/bs.vh.2020.04.002. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R.L. How do psychedelics work? Curr. Opin. Psychiatr. 2019;32:16–21. doi: 10.1097/YCO.0000000000000467. 10.1097.YCO.0000000000000467. [DOI] [PubMed] [Google Scholar]
- Catena-Dell'Osso M., Rotella F., Dell'Osso A., Fagiolini A., Marazziti D. Inflammation, serotonin and major depression. Curr. Drug Targets. 2013;14(5):571–577. doi: 10.2174/13894501113149990154. [DOI] [PubMed] [Google Scholar]
- Cathomas F., Bevilacqua L., Ramakrishnan A., Kronman H., Costi S., Schneider M., Chan K.L., Li L., Nestler E.J., Shen L., Charney D.S., Russo S.J., Murrough J.W. Whole blood transcriptional signatures associated with rapid antidepressant response to ketamine in patients with treatment resistant depression. Transl. Psychiatry. 2022;12(1):12. doi: 10.1038/s41398-021-01712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S.R., Cavanagh J., De Boer P., Mondelli V., Jones D.N.C., Drevets W.C.…Bullmore E.T. Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry. 2019;214(1):11–19. doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Lee J.J., Hsieh C.Y., Hsiao G., Chou D.S., Sheu J.R. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediat. Inflamm. 2009 doi: 10.1155/2009/705379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.C., Lin K.H., Tai Y.T., Chen J.T., Chen R.M. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating Toll-like receptor 2-mediated activation oF ERK1/2 and NFκB. Shock. 2010;33(5):485–492. doi: 10.1097/SHK.0b013e3181c3cea5. [DOI] [PubMed] [Google Scholar]
- Chang L., Toki H., Qu Y., Fujita Y., Mizuno-Yasuhira A., Yamaguchi J.…Hashimoto K. No sex-specific differences in the acute antidepressant actions of (R)-ketamine in an inflammation model. Int. J. Neuropsychopharmacol. 2018;21(10):932–937. doi: 10.1093/ijnp/pyy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.H., Li C.T., Lin W.C., Hong C.J., Tu P.C., Bai Y.M., Cheng C.M., Su T.P. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatr. Res. 2018;269:207–211. doi: 10.1016/j.psychres.2018.08.078. [DOI] [PubMed] [Google Scholar]
- Chen M.H., Chang W.C., Hsu J.W., Huang K.L., Tu P.C., Su T.P., Li C.T., Lin W.C., Bai Y.M. Correlation of proinflammatory cytokines levels and reduced gray matter volumes between patients with bipolar disorder and unipolar depression. J. Affect. Disord. 2019;245:8–15. doi: 10.1016/j.jad.2018.10.106. [DOI] [PubMed] [Google Scholar]
- Chen P., Chen F., Chen G., Zhong S., Gong J., Zhong H., Ye T., Tang G., Wang J., Luo Z., Qi Z., Jia Y., Yang H., Yin Z., Huang L., Wang Y. Inflammation is associated with decreased functional connectivity of insula in unmedicated bipolar disorder. Brain Behav. Immun. 2020;89:615–622. doi: 10.1016/j.bbi.2020.07.004. [DOI] [PubMed] [Google Scholar]
- Chen M.H., Wu H.J., Li C.T., Lin W.C., Tsai S.J., Hong C.J., Tu P.C., Bai Y.M., Mao W.C., Su T.P. Is one or two infusions better in the first week of low-dose ketamine treatment for medication-resistant depression? A post hoc pooled analysis of randomized placebo-controlled and open-label trials. J. Psychiatr. Res. 2021;144:448–454. doi: 10.1016/j.jpsychires.2021.11.006. [DOI] [PubMed] [Google Scholar]
- Clinic Mayo. Depression (major depressive disorder) 2018. https://www.mayoclinic.org/diseases-conditions/depression/symptoms-causes/syc-20356007 Available at:
- Cohen S., Janicki-Deverts D., Doyle W.J., Miller G.E., Frank E., Rabin B.S., Turner R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. U.S.A. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B., Colleoni M., Conti S., Parolaro D., Franke C., Trovato A., Giagnoni G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. N. Schmied. Arch. Pharmacol. 2004;369(3):294–299. doi: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- Costa B., Trovato A.E., Comelli F., Giagnoni G., Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007;556(1–3):75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Costafreda S.G., McCann P., Saker P., Cole J.H., Cohen-Woods S., Farmer A.E.…Fu C.H.Y. Modulation of amygdala response and connectivity in depression by serotonin transporter polymorphism and diagnosis. J. Affect. Disord. 2013;150(1):96–103. doi: 10.1016/j.jad.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Cowen P. Not fade away: the HPA axis and depression. Psychol. Med. 2010;40(1):1–4. doi: 10.1017/S0033291709005558. [DOI] [PubMed] [Google Scholar]
- Cruz-Topete D., Cidlowski J.A. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., O'Mahony S.M. The microbiome-gut-brain axis: from bowel to behavior. Neuro Gastroenterol. Motil. 2011;23(3):187–192. doi: 10.1111/j.13652982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- Dale O., Somogyi A.A., Li Y., Sullivan T., Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth. Analg. 2012;115(4):934–943. doi: 10.1213/ANE.0b013e3182662e30. [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L.B., Parvizi J., Hichwa R. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Daneshzad E., Keshavarz S.A., Qorbani M., Larijani B., Azadbakht L. Association between a low-carbohydrate diet and sleep status, depression, anxiety, and stress score. J. Sci. Food Agric. 2020;100(7):2946–2952. doi: 10.1002/jsfa.10322. [DOI] [PubMed] [Google Scholar]
- Daubert E.A., Condron B.G. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci. 2010;33(9):424–434. doi: 10.1016/j.tins.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.K., Barrett F.S., May D.G., Cosimano M.P., Sepeda N.D., Johnson M.W.…Griffiths R.R. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatr. 2021;78(5):481–489. doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablos R.M., Villarán R.F., Argüelles S., Herrera A.J., Venero J.L., Ayala A., Cano J., Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J. Neurosci. 2006;26(21):5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., Hall W., Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–1504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- Dell'Osso B., Albert U., Carrà G., Pompili M., Nanni M.G., Pasquini M., Poloni N., Raballo A., Sambataro F., Serafini G., Vigano C., Demyttenaere K., McIntyre R.S., Fiorillo A. How to improve adherence to antidepressant treatments in patients with major depression: a psychoeducational consensus checklist. Ann. Gen. Psychiatr. 2020;19:61. doi: 10.1186/s12991-020-00306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L., Riad M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT 1A receptors and SERT. Philos.Trans.Royal Soc. 2012;367(1601):2416–2425. doi: 10.1098/rstb.2011.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D., Viau V., Meaney M.J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic–pituitary–adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson K.S., Dozois D.J.A., editors. Risk Factors in Depression. Elsevier/Academic; San Diego, CA: 2008. [Google Scholar]
- Dolansky G., Shah A., Mosdossy G., Rieder M. What is the evidence for the safety and efficacy of using ketamine in children? Paediatr. Child Health. 2008;13(4):307–308. doi: 10.1093/pch/13.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongdem J.T., Helegbe G.K., Opare-Asamoah K., Wezena C.A., Ocloo A. Assessment of NSAIDs as potential inhibitors of the fatty acid amide hydrolase I (FAAH-1) using three different primary fatty acid amide substrates in vitro. BMC pharmacol. icol. 2022;23(1):1. doi: 10.1186/s40360-021-00539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen E., Hollon S.D. Cognitive behavioral therapy for mood disorders: efficacy, moderators and mediators. Psychiatr. Clin. 2011;33(3):537–555. doi: 10.1016/j.psc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Aghajanian G.K., Sanacora G., Krystal J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A.J. Cytokine activation of the HPA Axis. Ann. N. Y. Acad. Sci. 2006;917(1):608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- El-Alfy A.T., Ivey K., Robinson K., Ahmed S., Radwan M., Slade D., Khan I., ElSohly M., Ross S. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 2010;95(4):434–442. doi: 10.1016/j.pbb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbatsh M.M., Moklas M.A., Marsden C.A., Kendall D.A. Antidepressant-like effects of Δ⁹-tetrahydrocannabinol and rimonabant in the olfactory bulbectomised rat model of depression. Pharmacol. Biochem. Behav. 2012;102(2):357–365. doi: 10.1016/j.pbb.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Ellulu M.S., Patimah I., Khaza'ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes C.P., Bennett J.M., Derry H.M., Kiecolt-Glaser J.K. Relationships and inflammation across the lifespan: social developmental pathways to disease. Social and Personality Psychology Compass. 2011;5(11):891–903. doi: 10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faouzi M., Wakano C., Monteilh-Zoller M.K., Neupane R.P., Starkus J.G., Neupane J.B., Cullen A.J., Johnson B.E., Fleig A., Penner R. Acidic cannabinoids suppress proinflammatory cytokine release by blocking store-operated calcium entry. Function. 2022;3(4):zqac033. doi: 10.1093/function/zqac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D., Weinstein A. Cannabis and depression. Adv. Exp. Med. Biol. 2021;1264:67–80. doi: 10.1007/978-3-030-57369-0_5. [DOI] [PubMed] [Google Scholar]
- Felsten G. Stress reactivity and vulnerability to depressed mood in college students. Pers. Indiv. Differ. 2004;36(4):789–800. doi: 10.1016/S0191-8869(03)00152-1. [DOI] [Google Scholar]
- Figueiredo H.F., Bruestle A., Bodie B., Dolgas C.M., Herman J.P. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur. J. Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Flanagan T.W., Nichols C.D. Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatr. 2018;30(4):363–375. doi: 10.1080/09540261.2018.1481827. [DOI] [PubMed] [Google Scholar]
- Flanagan T.W., Billac G.B., Landry A.N., Sebastian M.N., Cormier S.A., Nichols C.D. Structure–activity relationship analysis of psychedelics in a rat model of asthma reveals the anti-inflammatory pharmacophore. ACS Pharmacol. Transl. Sci. 2021;4(2):488–502. doi: 10.1021/acsptsci.0c00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman D.A., Arfanakis K., Kelly J.F., Rajendran N., Buchman A.S., Morris M.C., Barnes L.L., Bennett D.A. Regional brain cortical thinning and systemic inflammation in older persons without dementia. J. Am. Geriatr. Soc. 2010;58(9):1823–1825. doi: 10.1111/j.1532-5415.2010.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn F. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999;13(1):55–78. doi: 10.1080/026870399402325. [DOI] [Google Scholar]
- Friedman E.M., Herd P. Income, education, and inflammation: differential associations in a national probability sample (The MIDUS study) Psychosom. Med. 2010;72(3):290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M., Sironi M., Pozzoli U., Ferrer-Admetlla A., Pattini L., Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 2011;7(11) doi: 10.1371/journal.pgen.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado M., Katzman M.A. Examining the role of neuroinflammation in major depression. Psychiatr. Res. 2015;229(1):27–36. doi: 10.1016/j.psychres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Galvão-Coelho N.L., de Menezes Galvão A.C., de Almeida R.N., Palhano-Fontes F., Braga I.C., Soares B.L., Maia-de-Oliveira J.P., Perkins D., Sarris J., de Araujo D.B. Changes in inflammatory biomarkers are related to the antidepressant effects of Ayahausca. J. Psychopharmacol. 2020;34(10):1125–1133. doi: 10.1177/0269881120936486. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez M.S., Navarrete F., Gasparyan A., Austrich-Olivares A., Sala F., Manzanares J. Cannabidiol: a potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules. 2020;10(11):1575. doi: 10.3390/biom10111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasquoine P.G. Contributions of the insula to cognition and emotion. Neuropsychol. Rev. 2014;24:77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- Gobbi G., Nuñez N., McLaughlin R., Bambico F.R. The Neuropathology Of Drug Addictions And Substance Misuse. 2016. Effects of delta-9-THC, synthetic cannabinoids and FAAH inhibitors on mood and serotonin neurotransmission; pp. 815–826. [Google Scholar]
- Gold S.M., Chalifoux S., Giesser B.S., Voskuhl R.R. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflammation. 2008;5(1):1–8. doi: 10.1186/1742-2094-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes J.A.S., Silva J.F., Marçal A.P., Silva G.C., Gomes G.F., De Oliveira A.C.P., Soares V.L., Oliveira M.C., Ferreira A.V.M., Aguiar D.C. High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice. J. Nutr. Biochem. 2020;77 doi: 10.1016/j.jnutbio.2019.108317. [DOI] [PubMed] [Google Scholar]
- González-Castro T.B., Tovilla-Zárate C.A., Juárez-Rojop I.E., López-Narváez M.L., Pérez-Hernández N., Rodríguez-Pérez J.M., Genis-Mendoza A.D. The role of gene polymorphisms, and analysis of peripheral and central levels of interleukins in suicidal behavior: a systematic review. J. Affect. Disord. 2021;279:398–411. doi: 10.1016/j.jad.2020.10.024. [DOI] [PubMed] [Google Scholar]
- Green S.M., Clem K.J., Rothrock S.G. Ketamine safety profile in the developing world: survey of practitioners. Acad. Emerg. Med.: Official Journal of the Society for Academic Emergency Medicine. 1996;3(6):598–604. [PubMed] [Google Scholar]
- Greenberg P.E., Fournier A.A., Sisitsky T., Simes M., Berman R., Koenigsberg S.H., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2010 and 2018) Pharmacoeconomics. 2021;39(6):653–665. doi: 10.1007/s40273-021-01019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R.R., Johnson M.W., Carducci M.A., Umbricht A., Richards W.A., Richards B.D., Cosimano M.P., Klinedinst M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J. Psychopharmacol. 2016;30(12):1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S., Boesiger P., Beck J., Schuepbach D., Bermpohl F., Walter M., Ernst J., Hell D., Boeker H., Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–943. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Guillamondegui O.D., Montgomery S.A., Phibbs F.T., McPheeters M.L., Alexander P.T., Jerome R.N., McKoy J.N., et al. Agency for Healthcare Research and Quality (US); 2011. Traumatic Brain Injury and Depression. PMID: 21938798. [PubMed] [Google Scholar]
- Guilliams T.G., Edwards L. Chronic stress and the HPA Axis: clinical assessment and therapeutic considerations. Point Institute of Nutraceutical Research. 2010;9(2):1–12. [Google Scholar]
- Gurven M., Kaplan H. Longevity among hunter-gatherers: a cross-cultural examination. Popul. Dev. Rev. 2007;33:321–365. [Google Scholar]
- Gutierrez E.G., Banks W.A., Kastin A.J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993;47(2):169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S., Shen R.Y. Chronic stress impairs α1-adrenoceptor-induced endocannabinoid-dependent synaptic plasticity in the dorsal raphe nucleus. J. Neurosci. 2014;34(44):14560–14570. doi: 10.1523/JNEUROSCI.1310-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren M., Dunstan D.W., Owen N. Passive versus mentally active sedentary behaviors and depression. Exerc. Sport Sci. Rev. 2020;48(1):20–27. doi: 10.1249/JES.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatr. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatr. 2015;78(4):224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D., Egan M.F., Weinberger D.R. 5-HT transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatr. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen-Shoval D., Amar S., Shbiro L., Smoum R., Haj C.G., Mechoulam R., Zalsman G., Weller A., Shoval G. Acute oral cannabidiolic acid methyl ester reduces depression-like behavior in two genetic animal models of depression. Behav. Brain Res. 2018;351:1–3. doi: 10.1016/j.bbr.2018.05.027. [DOI] [PubMed] [Google Scholar]
- Henshaw F.R., Dewsbury L.S., Lim C.K., Steiner G.Z. The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in vivo studies. ACS Pharmacology and Translational Cannabis Cannabinoid Res. 2021;6(3):177–195. doi: 10.1089/can.2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.N., Patel S., Carrier E.J., Rademacher D.J., Ormerod B.K., Hillard C.J., Gorzalka B.B. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30(3):508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Howland R.H. Sequenced treatment alternatives to relieve depression (STAR*D) J. Psychosocial Nurs. 2008;46(9):21–24. doi: 10.3928/02793695-20080901-06. [DOI] [PubMed] [Google Scholar]
- Hsu T.M., Konanur V.R., Taing L., Usui R., Kayser B.D., Goran M.I., Kanoski S.E. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus. 2015;25(2):227–239. doi: 10.1002/hipo.22368. [DOI] [PubMed] [Google Scholar]
- Huang Y., Lane H., Lin C. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural Plast. 2017;2017 doi: 10.1155/2017/4605971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iob E., Kirschbaum C., Steptoe A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol. Psychiatr. 2020;25:1130–1140. doi: 10.1038/s41380-019-0501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone T., Esposito G., De Filippis D., Scuderi C., Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci. Ther. 2009;15(1):65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A., Greenwood R., Mitchell V.A., Aslan S., Piomelli D., Vaughan C.W. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br. J. Pharmacol. 2009;147(3):281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Gilles L., Braitch M., Latif M.L., Aram J., Fahey A.J., Edwards L.J., Robins R.A., Tanasescu R., Tighe P.J., Gran B., Showe L.C., Alexander S.P., Chapman V., Kendall D.A., Constantinescu C.S. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells. Acta Physiol. 2015;214(1):63–74. doi: 10.1111/apha.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Liu L., Sheng C., Cheng Z., Cui L., Li M., Zhao Y., Shi T., Yau T.O., Li F., Chen L. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J. Nerv. Ment. Dis. 2019;207(4):271–276. doi: 10.1097/NMD.0000000000000957. [DOI] [PubMed] [Google Scholar]
- Karoly H.C., Bidwell L.C., Mueller R.L., Hutchison K.E. Investigating the relationships between alcohol consumption, cannabis use, and circulating cytokines: a preliminary analysis. Alcohol Clin. Exp. Res. 2018;42(3):531–539. doi: 10.1111/acer.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J., Gomez R., Williams G., Lembke A., Lazzeroni L., Murphy G.M., Jr., Schatzberg A.F. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatr. 2017;22(4):527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S., Dupuis N., Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Res. 2020;167 doi: 10.1016/j.eplepsyres.2020.106454. [DOI] [PubMed] [Google Scholar]
- Koopman F.A., Chavan S.S., Miljko S., Grazio S., Sokolovic S.P., Schuurman P.R., Mehta A.D., Levine Y.A., Faltys M., Zitnik R., Tracey K.J., Tak P.P. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2016;113(29):8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopra E., Mondelli V., Pariante C., Nikkheslat N. Ketamine's effect on inflammation and kynurenine pathway in depression: a systematic review. J. Psychopharmacol. 2021;35(8):934–945. doi: 10.1177/02698811211026426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuningas M., May L., Tamm R., Bodegom D.V., Biggelaar A.H.J., Meij J.J., et al. Selection for genetic variation inducing pro-inflammatory responses under adverse environmental conditions in a Ghanaian population. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R.E., Kumari M., Bartley M. Social isolation in childhood and adult inflammation: evidence from the National Child Development Study. Psychoneuroendocrinology. 2014;50:85–94. doi: 10.1016/j.psyneuen.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Lee J., Nam H., Yu S. Systematic analysis of translocator protein 18 kDa (TSPO) ligands on toll-like receptors-mediated pro-inflammatory responses in microglia and astrocytes. Exp. Neurobiol. 2016;25(5):262–268. doi: 10.5607/en.2016.25.5.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Seifritz E., Henning A., Walter M., Böker H., Scheidegger M., Grimm S. Differential effects of rumination and distraction on ketamine induced modulation of resting state functional connectivity and reactivity of regions within the default-mode network. Soc. Cognit. Affect Neurosci. 2016;11(8):1227–1235. doi: 10.1093/scan/nsw034. Epub 2016 Apr 13. PMID: 27075438; PMCID: PMC4967796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Sagar A.P., Keri S. Translocator protein (18 kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018;83:1–7. doi: 10.1016/j.pnpbp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Li X.B., Liu A., Yang L., Zhang K., Wu Y.M., Zhao M.G., Liu S.B. Antidepressant-like effects of translocator protein (18 kDa) ligand ZBD-2 in mouse models of postpartum depression. Mol. Brain. 2018;11(1):12. doi: 10.1186/s13041-018-0355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrenz M., Stohler R., Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. World J. Biol. Psychiatr.: The Official Journal of the World Federation of Societies of Biological Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- Lira F.S., Rosa J.C., Pimentel G.D., Souza H.A., Caperuto E.C., Carnevali L.C., Jr., Seelaender M., Damaso A.R., Oyama L.M., de Mello M.T., Santos R.V. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010;9:82. doi: 10.1186/1476-511X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ho R.C., Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J. Affect. Disord. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Liu B., Liu J., Wang M., Zhang Y., Li L. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front. Cell. Neurosci. 2017;11:305. doi: 10.3389/fncel.2017.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T., Bondza E., Lethin C. Evidence of the importance of dietary habits regarding depressive symptoms and depression. Int. J. Environ. Res. Publ. Health. 2020;17(5):1616. doi: 10.3390/ijerph17051616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loix S., De Kock M., Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol. Belg. 2011;62(1):47–58. [PubMed] [Google Scholar]
- Lopresti A.L., Hood S.D., Drummond P.D. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J. Affect. Disord. 2013;148(1):12–27. doi: 10.1016/j.jad.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Lyketsos C.G., Olin J. Depression in Alzheimer's disease: overview and treatment. Biol. Psychiatr. 2002;52(3):243–252. doi: 10.1016/S0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- Lyte M. Microbial endocrinology in the microbiome-gut-brain Axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11) doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Song C., Lin A., De Jongh R., Van Gastel A., Kenis G., Bosmans E., De Meester I., Benoy I., Neels H., Demedts P., Janca A., Scharpé S., Smith R.S. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Kuan D.C., Sheu L.K., Krajina K., Kraynak T.E., Manuck S.B., Gianaros P.J. Systemic inflammation and resting state connectivity of the default mode network. Brain Behav. Immun. 2017;62:162–170. doi: 10.1016/j.bbi.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.L., Strickland J.C., Schlienz N.J., Munson J., Jackson H., Bonn-Miller M.O., Vandrey R. Antidepressant and anxiolytic effects of medicinal cannabis use in an observational trial. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.729800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P., Lien L., Zemore S., Bramness J.G., Neupane S.P. Circulating cytokine levels are associated with symptoms of depression and anxiety among people with alcohol and drug use disorders. J. Neuroimmunol. 2018;318:80–86. doi: 10.1016/j.jneuroim.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K., Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33(1):73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Mawdsley J.E., Rampton D.S. The role of psychological stress in inflammatory bowel disease. Neuroimmunomodulation. 2006;13:327–336. doi: 10.1159/000104861. [DOI] [PubMed] [Google Scholar]
- Menke A. Is the HPA Axis as target for depression outdated, or is there a new hope? Front. Psychiatr. 2019;10:101. doi: 10.3389/fpsyt.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak J., Hölz A., Teismann T. Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression. Psychol. Psychother. 2011;84(2):230–236. doi: 10.1348/147608310X520166. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo J.J., Rajkowska G. Morphological brain changes in depression: can antidepressants reverse them? CNS Drugs. 2002;16(6):361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatr. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero P., Ramos-Quiroga J.A., Martin-Santos R.R., Calvo-Sánchez E.E., Gutiérrez-Rojas L.L., Meana J.J. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–420. doi: 10.1007/s40263-018-0519-3. [DOI] [PubMed] [Google Scholar]
- Moriarity D.P., Ng T., Titone M.K., Chat I.K.Y., Nusslock R., Miller G., Alloy B. Reward responsiveness and ruminative styles interact to predict inflammation and mood symptomatology. Behav. Ther. 2020;51(5):829–842. doi: 10.1016/j.beth.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette F., Mongeau-Pérusse V., Rizkallah E., Thébault P., Lepage S., Brissette S., Bruneau J., Dubreucq S., Stip E., Cailhier J.F., Jutras-Aswad D. Exploring cannabidiol effects on inflammatory markers in individuals with cocaine use disorder: a randomized controlled trial. Neuropsychopharmacology. 2021;46(12):2101–2111. doi: 10.1038/s41386-021-01098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R., O'Connor R. The role of rumination, attentional biases and stress in psychological distress. Br. J. Psychol. 2010;99(2):191–209. doi: 10.1348/000712607X216080. [DOI] [PubMed] [Google Scholar]
- Motwani M.P., Bennett F., Norris P.C., Maini A.A., George M.J., Newson J., Henderson A., Hobbs A.J., Tepper M., White B., Serhan C.N., MacAllister R., Gilroy D.W. Potent anti-inflammatory and pro-resolving effects of anabasum in a human model of self-resolving acute inflammation. Clin. Pharmacol. Therapeut. 2018;104(4):675–686. doi: 10.1002/cpt.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton C.D., Pavlidis P., Norton C., Norton S., Pariante C., Hayee B., Powell N. Depressive symptoms in inflammatory bowel disease: an extraintestinal manifestation of inflammation? Clin. Exp. Immunol. 2019;197(3):308–318. doi: 10.1111/cei.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N., Cowley T.R., Blau C.W., Dempsey C.N., Noonan J., Gowran A., Tanveer R., Olango W.M., Finn D.P., Campbell V.A., Lynch M.A. The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J. Neuroinflammation. 2012;9:79. doi: 10.1186/1742-2094-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J.W., Abdallah C.G., Anticevic A., Collins K.A., Geha P., Averill L.A., Schwartz J., DeWilde K.E., Averill C., Jia-Wei Yang G., Wong E., Tang C.Y., Krystal J.H., Iosifescu D.V., Charney D.S. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum. Brain Mapp. 2016;37(9):3214–3223. doi: 10.1002/hbm.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Brosso S.N., Humphreys K.L. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatr. 2020;25(9):2189–2199. doi: 10.1038/s41380-018-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P., Pandey R., Rieder S.A., Hegde V.L., Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009;1(7):1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keane V., Dinan T.G., Scott L., Corcoran C. Changes in hypothalamic–pituitary–adrenal Axis measures after vagus nerve stimulation therapy in chronic depression. Biol. Psychiatr. 2005;58(12):963–968. doi: 10.1016/j.biopsych.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Onaemo V.N., Fawehinmi T.O., D'Arcy C. Comorbid cannabis use disorder with major depression and generalized anxiety disorder: a systematic review with meta-analysis of nationally representative epidemiological surveys. J. Affect. Disord. 2021;281:467–475. doi: 10.1016/j.jad.2020.12.043. [DOI] [PubMed] [Google Scholar]
- Pace T.W.W., Mletzko T.C., Alagbe O., Musselman D.L., Nemeroff C.B., Miller A.H., Heim C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatr. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Palazidou E. The neurobiology of depression. Br. Med. Bull. 2012;101:127–145. doi: 10.1093/bmb/lds004. [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F., Andrade K.C., Tofoli L.F., Santos A.C., Crippa J.A., Hallak J.E., Ribeiro S., de Araujo D.B. The psychedelic state induced by ayahuasca modulates the activity and connectivity of the default mode network. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortés-Puch I., Sime P.J., Phipps R.P., Serhan C.N., Hammock B.D. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020;39(2):337–340. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C.M., Miller A.H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatr. 2001;49:391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- Penn E., Tracy D.K. The drugs don't work? antidepressants and the current and future pharmacological management of depression. Therapeut.Adv. Psychopharmacol. 2012;2(5):179–188. doi: 10.1177/2045125312445469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A.J., Horowitz M.A., Roelofs J., Zunszain P.A., Pariante C.M. Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front. Psychiatr. 2019;10:423. doi: 10.3389/fpsyt.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Grunwald N., Rümmele P., Endlicher E., Lechner A., Neumann I.D., Obermeier F., Reber S.O. Chronic psychosocial stress increases the risk for inflammation-related colon carcinogenesis in male mice. Stress. 2012;15(4):403–415. doi: 10.3109/10253890.2011.631232. [DOI] [PubMed] [Google Scholar]
- Petrosino S., Verde R., Vaia M., Allarà M., Iuvone T., Di Marzo V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Therapeut. 2018;365(3):652–663. doi: 10.1124/jpet.117.244368. [DOI] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E.M., Verchinski B.A., Munoz K.E., Kolachana B.S., et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Porr B., Miller A., Trew A. An investigation into serotonergic and environmental interventions against depression in a simulated delayed reward paradigm. Adapt. Behav. 2020;28(4):241–260. doi: 10.1177/1059712319864278. [DOI] [Google Scholar]
- Quinn M.E., Joormann J. Stress-induced changes in executive control are associated with depression symptoms: examining the role of rumination. Clin. Psychol. Sci. 2015;3(4):628–636. doi: 10.1177/2167702614563930. [DOI] [Google Scholar]
- Radley J.J., Arias C.M., Sawchenko P.E. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci. 2006;26(50):12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Lowry C.A., Rook G.A. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatr. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J.J., Wei J., Dilley G., Pittman S.D., Meltzer H.Y.…Stockmeier C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatr. 1999;45(9):1085–1098. doi: 10.1016/S0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Regan T., Gill A.C., Clohisey S.M., Barnett M.W., Pariante C.M., Harrison N.A.…Freeman T.C. Effects of anti‐inflammatory drugs on the expression of tryptophan‐metabolism genes by human macrophages. J. Leukoc. Biol. 2018;103(4):681–692. doi: 10.1002/jlb.3a0617-261r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijnders J.S.A.M., Ehrt U., Weber W.E.J., Aarsland D., Leentjens A.F.G. A systematic review of prevalence studies of depression in Parkinson's disease. Mov. Disord. 2008;23(2):183–189. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- Reiman E.M. The application of positron emission tomography to the study of normal and pathologic emotions. J. Clin. Psychiatr. 1997;58:4–12. [PubMed] [Google Scholar]
- Ruban A., Kołodziej A. Changes in default-mode network activity and functional connectivity as an indicator of psychedelic-assisted psychotherapy effectiveness. Neuropsychiatry Neuropsychol. 2018;13(3):91–97. doi: 10.5114/nan.2018.81249. [DOI] [Google Scholar]
- Sales A.J., Crestani C.C., Guimarães F.S., Joca S. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2018;86:255–261. doi: 10.1016/j.pnpbp.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Schildkraut J.J. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatr. 1965;122(5):509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schlaepfer T.E., Frick C., Zobel A., Maier W., Heuser I., Bajbouj M., O'Keane V., Corcoran C., Adolfsson R., Trimble M., Rau H., Hoff H.J., Padberg F., Müller-Siecheneder F., Audenaert K., Van den Abbeele D., Stanga Z., Hasdemir M. Vagus nerve stimulation for depression: efficacy and safety in a European study. Psychol. Med. 2008;38(5):651–661. doi: 10.1017/S0033291707001924. [DOI] [PubMed] [Google Scholar]
- Schmidt F.M., Kirkby K.C., Lichtblau N. Inflammation and immune regulation as potential drug targets in antidepressant treatment. Curr. Neuropharmacol. 2016;14(7):674–687. doi: 10.2174/1570159x14666160115130414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I., Kugel H., Redlich R., Grotegerd D., Bürger C., Bürkner P.…Hohoff C. Association of serotonin transporter gene AluJb methylation with major depression, amygdala responsiveness, 5-HTTLPR/rs25531 polymorphism, and stress. Neuropsychopharmacology. 2018;43(6):1308–1316. doi: 10.1038/npp.2017.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman S.N., Rabkin J.G. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J. Affect. Disord. 1998;48(2):157–161. doi: 10.1016/S0165-0327(97)00168-7. [DOI] [PubMed] [Google Scholar]
- Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J. Psychiatr. 2012;2(3):49–57. doi: 10.5498/wjp.v2.i3.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N. The resolution of inflammation: the devil in the flask and in the details. Faseb. J. 2011;25(5):1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. Faseb. J. 2017;31(4):1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C., Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan C., Chiang N., Van Dyke T. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G.…Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatr. 2015;72(3):268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E., Attwells S., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L.…Meyer J.H. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatr. 2018;5(4):339–347. doi: 10.1016/s2215-0366(18)30048-8. [DOI] [PubMed] [Google Scholar]
- Shao R., Lau W.K.W., Leung M.K., Lee T.M.C. Subgenual anterior cingulate-insula resting-state connectivity as a neural correlate to trait and state stress resilience. Brain Cognit. 2018;124:73–81. doi: 10.1016/j.bandc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U.S.A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M.N., Sternberg E.M. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G., Baune B.T. Microglia: an interface between the loss of neuroplasticity and depression. Front. Cell. Neurosci. 2017;11:270. doi: 10.3389/fncel.2017.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigielski L., Scheidegger M., Kometer M., Vollenweider F.X. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. 2019;196:207–215. doi: 10.1016/j.neuroimage.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Spencer S.J., Buller K.M., Day T.A. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J. Comp. Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Spinhoven P., van Hemert A.M., Penninx B.W. Repetitive negative thinking as a predictor of depression and anxiety: a longitudinal cohort study. J. Affect. Disord. 2018;241:216–225. doi: 10.1016/j.jad.2018.08.037. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., Swain J.E., Evans G.W., Welsh R.C., Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39(9):2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar J.E., John-Henderson N., Anderson C.L., Gordon A.M., McNeil G.D., Keltner D. Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 2015;15(2):129–133. doi: 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Stierman B., Afful J., Carroll M.D., Chen T.C., Davy O., Fink S., Fryar C.D., Gu Q., Hales C.M., Hughes J.P., Ostchega Y., Storandt R.J., Akinbami L.J. National Health Statistics Reports; 2021. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. NHSR No. 158. [DOI] [Google Scholar]
- Su T.P., Chen M.H., Li C.T., Lin W.C., Hong C.J., Gueorguieva R., Tu P.C., Bai Y.M., Cheng C.M., Krystal J.H. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482–2492. doi: 10.1038/npp.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A. In: Advances in Psychedelic Medicine: State-Of-The-Art Therapeutic Applications. Winkleman M.J., Sessa B., editors. ABC-CLIO; 2019. Effects of psychedelics on inflammation and immunity; pp. 193–213. [Google Scholar]
- Tapp Z.M., Godbout J.P., Kokiko-Cochran O.G. A tilted Axis: maladaptive inflammation and HPA Axis dysfunction contribute to consequences of TBI. Front. Neurol. 2019;10:345. doi: 10.3389/fneur.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni P.A., Gautier J.F., Chen K., Uecker A., Bandy D., Salbe A.D., Pratley R.E., Lawson M., Reiman E.M., Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. U.S.A. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S., Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Tomaz V.S., Chaves Filho A., Cordeiro R.C., Jucá P.M., Soares M., Barroso P.N., Cristino L., Jiang W., Teixeira A.L., de Lucena D.F., Macedo D.S. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J. Affect. Disord. 2020;268:188–200. doi: 10.1016/j.jad.2020.03.022. [DOI] [PubMed] [Google Scholar]
- Tran T., Milanovic M., Holshausen K., Bowie C.R. What is normal cognition in depression? prevalence and functional correlates of normative versus idiographic cognitive impairment. Neuropsychology. 2021;35(1):33–41. doi: 10.1037/neu0000717. [DOI] [PubMed] [Google Scholar]
- Uthaug M.V., Lancelotta R., Szabo A., Davis A.K., Riba J., Ramaekers J.G. Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology. 2020;237(3):773–785. doi: 10.1007/s00213-019-05414-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch M., Meyer-Lindenberg A. Environmental exposures and depression: biological mechanisms and epidemiological evidence. Annu. Rev. Publ. Health. 2019;40:239–259. doi: 10.1146/annurev-publhealth-040218-044106. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., Brose A., Koster E., De Raedt R. Co-variation between stressful events and rumination predicts depressive symptoms: an eighteen months prospective design in undergraduates. Behav. Res. Ther. 2016;87:128–133. doi: 10.1016/j.brat.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Varghese F.P., Brown E.S. The hypothalamic-pituitary-adrenal Axis in major depressive disorder: a brief primer for primary care physicians. Prim. Care Companion J. Clin. Psychiatry. 2001;3(4):151–155. doi: 10.4088/pcc.v03n0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli H.A., Gandhi C.P., Gray J.M., Morena M., Hassan K.I., Hill M.N. Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain Behav. Immun. 2016;51:70–91. doi: 10.1016/j.bbi.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Verrico C.D., Wesson S., Konduri V., Hofferek C.J., Vazquez-Perez J., Blair E., Dunner K., Jr., Salimpour P., Decker W.K., Halpert M.M. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain. 2020;161(9):2191–2202. doi: 10.1097/j.pain.0000000000001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Yu H.Y., Shen X.F., Gao Z.Q., Yang C., Yang J.J., Zhang G.F. The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups. J. Med. Sci. 2015;120(4):241–248. doi: 10.3109/03009734.2015.1060281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckiewicz G., Stokłosa I., Stokłosa M., Gorczyca P., Pudlo R. Cannabidiol (CBD) in the self-treatment of depression-exploratory study and a new phenomenon of concern for psychiatrists. Front. Psychiatr. 2022;13 doi: 10.3389/fpsyt.2022.837946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz P.H., Kanel R.V. Psychological stress, inflammation, and coronary heart disease. Curr. Cardiol. Rep. 2017;19:111. doi: 10.1007/s11886-017-0919-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization Depression. 2020. https://www.who.int/news-room/fact-sheets/detail/depression Available at:
- Xu Y., Jiang C., Wu J., Liu P., Deng X., Zhang Y., Peng B., Zhu Y. Ketogenic diet ameliorates cognitive impairment and neuroinflammation in a mouse model of Alzheimer's disease. CNS Neurosci. Ther. 2021 doi: 10.1111/cns.13779. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaden D.B., Griffiths R.R. The subjective effects of psychedelics are necessary for their enduring therapeutic effects. ACS Pharmacol. ansl.Sci. 2021;4(2):568–572. doi: 10.1021/acsptsci.0c00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K., Matsunaga M., Isowa T., Ohira H. Serotonin transporter gene polymorphism modulates inflammatory cytokine responses during acute stress. Sci. Rep. 2015;5 doi: 10.1038/srep13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhao Y., Wang Y., Liu L., Zhang X., Li B., Cui R. The effects of psychological stress on depression. Curr. Neuropharmacol. 2015;13(4):494–504. doi: 10.2174/1570159x1304150831150507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Liu C., Jin Z., Liu Y., Yin Y., Fang X.…Li Y. Serotonergic transmission is required for the anxiolytic-like behavioral effects of YL-IPA08, a selective ligand targeting TSPO. Neuropharmacology. 2020;178 doi: 10.1016/j.neuropharm.2020.108230. [DOI] [PubMed] [Google Scholar]
- Yekhtin Z., Khuja I., Meiri D., Or R., Almogi-Hazan O. Differential effects of D9 tetrahydrocannabinol (THC)- and cannabidiol (CBD)-Based cannabinoid treatments on macrophage immune function in vitro and on gastrointestinal inflammation in a murine model. Biomedicines. 2022;10(8):1793. doi: 10.3390/biomedicines10081793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Xu X., Chen G., Mehta N.D., Haroon E., Miller A.H., Luo Y., Li Z., Felger J.C. Inflammation and decreased functional connectivity in a widely-distributed network in depression: centralized effects in the ventral medial prefrontal cortex. Brain Behav. Immun. 2019;80:657–666. doi: 10.1016/j.bbi.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Chen Zy. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011;(32):3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Becnel J., Zerfaoui M., Rohatgi R., Boulares A.H., Nichols C.D. Serotonin 5-hydroxytryptamine2A receptor activation suppresses tumor necrosis factor-α-induced inflammation with extraordinary potency. J. Pharmacol. Exp. Therapeut. 2008;327(2):316–323. doi: 10.1124/jpet.108.143461. [DOI] [PubMed] [Google Scholar]
- Yuhas Y., Ashkenazi S., Berent E., Weizman A. Immunomodulatory activity of ketamine in human astroglial A172 cells: possible relevance to its rapid antidepressant activity. J. Neuroimmunol. 2015;282:33–38. doi: 10.1016/j.jneuroim.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Zarate C.A., Jr., Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatr. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zeev-Wolf M., Levy J., Goldstein A., Zagoory-Sharon O., Feldman R. Chronic early stress impairs default mode network connectivity in preadolescents and their mothers. Biol. Psychiatr.: Cognit. Neurosci.Neuroimag. 2019;4(1):72–80. doi: 10.1016/j.bpsc.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Zhan Y., Zhou Y., Zheng W., Liu W., Wang C., Lan X., Deng X., Xu Y., Zhang B., Ning Y. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl. Psychiatry. 2020;10:246. doi: 10.1038/s41398-020-00933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Liu X., Chang D., Zhang X., Lian H., Du X., Gao L. Low-dose ketamine improves LPS-induced depression-like behavior in rats by activating cholinergic anti-inflammatory pathways. ACS Chem. Neurosci. 2020;11(5):752–762. doi: 10.1021/acschemneuro.9b00669. [DOI] [PubMed] [Google Scholar]
- Zhou H., Chen X., Shen Y.Q., Li L., Chen N.X., Zhu Z.C., Castellanos F.X., Yan C.G. Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. Neuroimage. 2020;206 doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- Zoccola P.M., Figueroa W.S., Rabideau E.M., Woody A., Benencia F. Differential effects of poststressor rumination and distraction on cortisol and C-reactive protein. Health Psychol. 2014;33(12):1606–1609. doi: 10.1037/hea0000019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.