Keywords: chemosensation, fat taste, obesity, oleogustus, olfaction
Abstract
Taste and smell play a key role in our ability to perceive foods. Overconsumption of highly palatable energy-dense foods can lead to increased caloric intake and obesity. Thus there is growing interest in the study of the biological mediators of fat taste and associated olfaction as potential targets for pharmacologic and nutritional interventions in the context of obesity and health. The number of studies examining mechanisms underlying fat taste and smell has grown rapidly in the last 5 years. Therefore, the purpose of this systematic review is to summarize emerging evidence examining the biological mechanisms of fat taste and smell. A literature search was conducted of studies published in English between 2014 and 2021 in adult humans and animal models. Database searches were conducted using PubMed, EMBASE, Scopus, and Web of Science for key terms including fat/lipid, taste, and olfaction. Initially, 4,062 articles were identified through database searches, and a total of 84 relevant articles met inclusion and exclusion criteria and are included in this review. Existing literature suggests that there are several proteins integral to fat chemosensation, including cluster of differentiation 36 (CD36) and G protein-coupled receptor 120 (GPR120). This systematic review will discuss these proteins and the signal transduction pathways involved in fat detection. We also review neural circuits, key brain regions, ingestive cues, postingestive signals, and genetic polymorphism that play a role in fat perception and consumption. Finally, we discuss the role of fat taste and smell in the context of eating behavior and obesity.
CLINICAL HIGHLIGHTS.
Taste and smell play an integral role in maintaining health, including mental health and nutritional status.
Both taste and smell contribute to flavor perception.
Taste and smell screening is important for early diagnosis of disease processes and management of the adverse effects of chemosensory disturbances.
Fats are sources of essential fatty acids and are important mediators of energy balance and cellular homeostasis.
Both animals and humans exhibit a preference for high-fat foods.
In addition to texture, taste and smell are integral to fat perception.
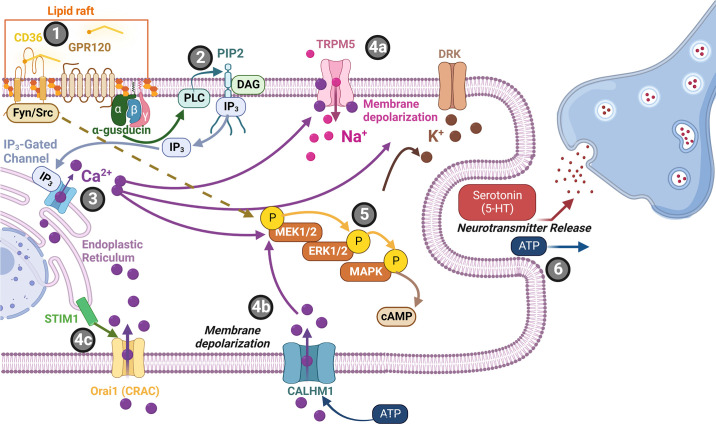
Current candidates for fat taste and smell receptors are CD36 and GPR120.
Fat taste transduction involves Ca2+ signaling as well as secondary messenger cascades, such as MAP kinases.
The most frequently studied genetic polymorphisms associated with fat chemosensation are CD36 polymorphisms. The rs1761667, rs1527483, rs2312018, and rs3840546 SNPs of CD36 are reportedly associated with fat perception.
Individuals with obesity reportedly display fat chemosensory dysfunction.
1. INTRODUCTION
1.1. Health and Clinical Implications of Taste and Smell (Olfaction): COVID-19 and Beyond
Taste and smell (i.e., the chemical senses) play an integral role in maintaining health, including mood, social behaviors, nutritional intake, detecting hazards (e.g., smell of smoke or contaminated foods), and other essential survival/physiological mechanisms. Taste and smell dysfunction are known to have a negative impact on emotional well-being and mental health (1, 2) and are positively correlated with increased anxiety, depression, and reductions in health-related quality of life (1). Despite the well-documented negative impact of taste and smell dysfunction, the chemical senses have often been overlooked in the context of health and disease. The COVID-19 pandemic highlighted the importance of taste and smell impairments, with millions of people world-wide reporting COVID-19-related taste and smell dysfunction (1, 3). For systematic reviews on COVID-19-related taste and smell dysfunction, see Refs. 4–6. COVID-19-related taste and smell dysfunction was soon accompanied by patients reporting depression, anxiety, and even detachment from reality. For example, a 2020 qualitative study captured a patient’s experience “I feel sad and depressed and distanced to the world as it used to be. Not being able to smell the most mundane things, like the rain or my boyfriend’s perfume. Not being able to participate socially like I used to” (7). While taste and smell symptoms were reported, acutely, persistent smell impairment was associated with more COVID-19 symptoms, and some have postulated that it may be a key marker for long-COVID illness (8). In COVID-19-related smell loss, SARS-CoV-2 may target sustentacular cells (rather than olfactory sensory neurons or the olfactory bulb) and others have shown that there is also a downregulation of olfactory receptors and signaling molecules (9–12). It has been recently suggested that COVID-19-related taste loss may arise from a direct infection of tastebud cells by SARS-CoV-2, which impairs taste receptor stem-cell activity (13). Importantly, taste and smell dysfunction are prevalent in other disease processes beyond COVID-19 (e.g., Parkinson’s and Alzheimer’s). In fact, the identification of chemosensory dysfunction in other illnesses can aid in early diagnosis. Thus screening for chemosensory dysfunction is important for health diagnosis and management.
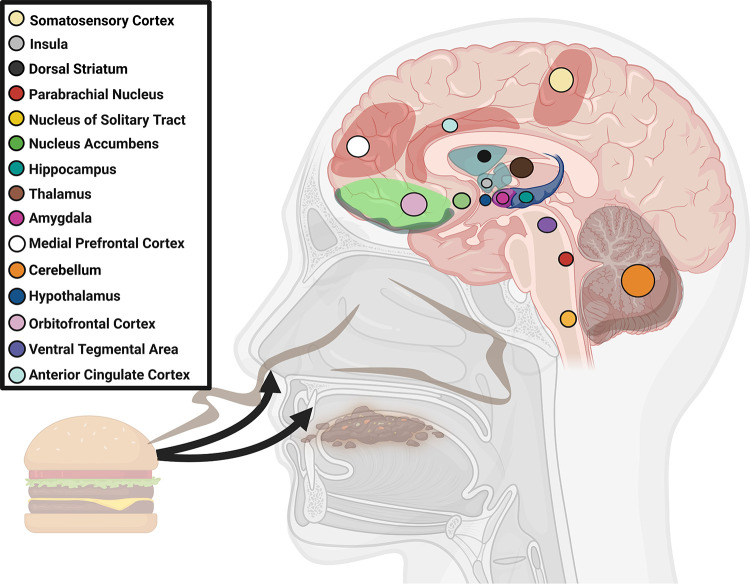
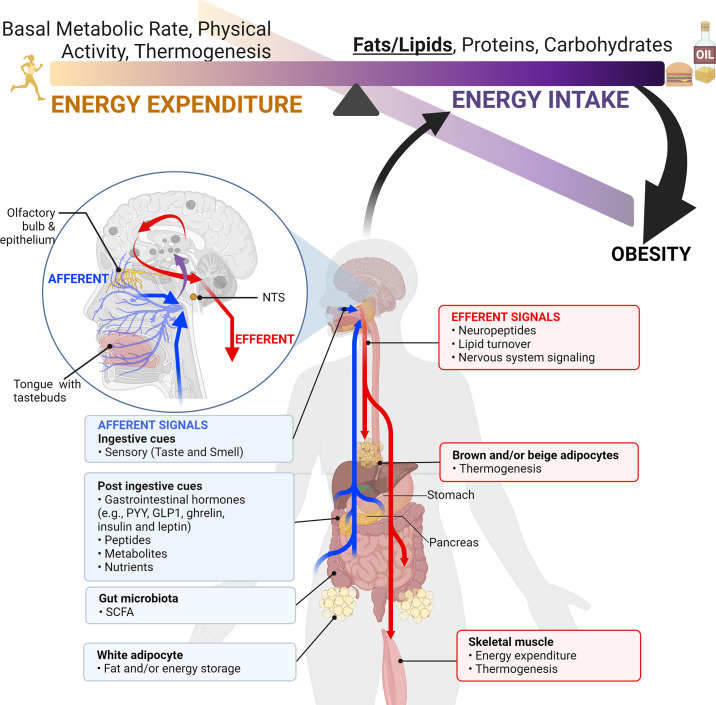
Taste and smell also play an integral role in our ability to identify, find, and preferentially consume nutrients necessary to maintain health, including fats. Taste and smell are the first sensory signals along the gut-brain axis to detect appetitive food cues, including the perception of fat (14, 15). This is exemplified by our predilection for energy-dense foods, including fats. However, while fats and other energy-dense nutrients are essential for survival, chronic overconsumption of these foods can lead to obesity. Thus there is a growing interest in the role of fat taste (also known as oleogustus) and smell in the pathogenesis of obesity, including the study of the biological mediators of fat taste and smell as potential targets in pharmacologic and nutritional interventions. Fats are the most energy-dense macronutrient (16). They are sources of essential fatty acids and important mediators of energy balance and cellular homeostasis (17). Palatable cues associated with fat perception activate reward circuitry in the brain [such as the nucleus accumbens (NAc) and prefrontal cortex (PFC), among others], motivating eating behavior (18). Deciphering the biological mechanisms of fat taste and smell constitutes an important step in addressing obesity as a public health challenge.
1.2. Measurement and Assessment of Taste and Smell Function
There are several methods used to measure taste and smell. Chemosensory screening can be used to assess smell or taste disorders. For example, smell/olfactory and taste screening tools include the NIH Toolbox (which assesses sensory function), NHANES Pocket Smell Test (PST), the SCENTinel rapid smell test, Sniffin’ Sticks 12-item Screening test, The University of Pennsylvania Smell Identification Test (UPSIT), and the Global Consortium for Chemosensory Research (GCCR) Smell and Taste Challenge. Other tests measure odor/tastant identification, discrimination, and detection threshold. For a comprehensive summary of tools and measures, see Refs. 19, 20. In the context of eating behavior, studies examining the role of fat taste and smell often use a combination of psychophysical methods to measure taste and smell (e.g., sensitivity, preference/liking, and intensity). Taste and olfactory sensitivity are measured by determining threshold: the concentration at which a smell or taste is detected or recognized (19–22). Preference is a measure of an individuals’ most preferred concentration of a tastant (taste-invoking chemical molecule) or olfactory cue (23). In addition to preferred concentrations, a taste or odor intensity may also contribute to what an individual perceives as pleasant foods. Sensitivity, intensity, and preference for tastants and odors can impact food choices in normal weight and individuals with obesity (24, 25).
1.3. Anatomy and Physiology of Taste and Smell
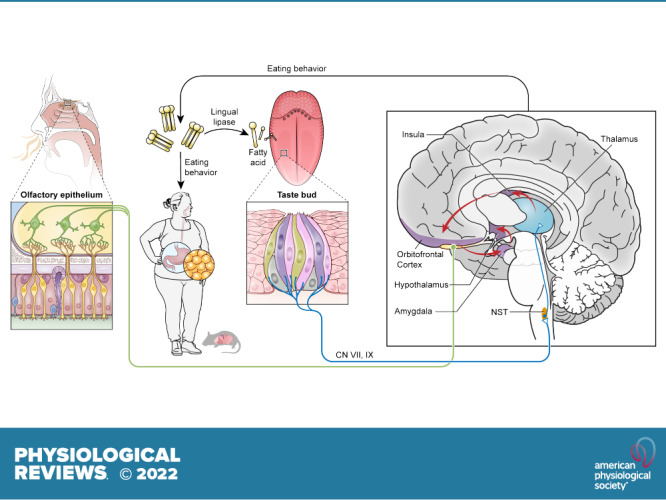
Importantly, what humans perceive as flavor encompasses more than taste; olfaction also plays an essential role in our perception of foods and beverages. Stimulation of chemoreceptor cells in both the mouth [e.g., taste bud cells (TBCs)] and nose (e.g., olfactory receptor cells) are processed by our central nervous system to contribute to flavor perception (26). Taste receptor cells are found in onion-shaped structures called taste buds, which are found on the surface of the tongue and form taste papillae (FIGURE 1). The olfactory epithelium lines the nasal cavity and is made up of olfactory receptor cells, basal, and supporting cells (FIGURE 2). Both taste and smell contribute to flavor perception, making it difficult for most people to discern what is exclusively taste and/or smell. For example, smell impairments induced by inflammation of olfactory tissue following a respiratory infection are often reported as a loss of taste (27, 28). In turn, flavor (rather than taste or smell alone) contributes to pleasurable qualities associated with food.
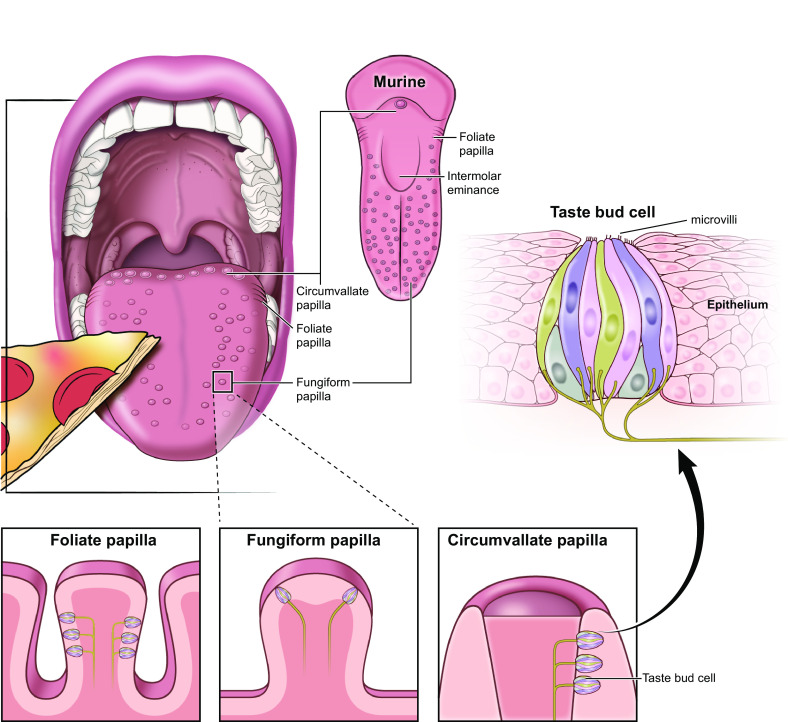
FIGURE 1.
Taste bud cell. Taste buds are onion-shaped clusters of taste receptor cells. They are found in the tongue, the soft palate, the pharynx, and the esophagus. There are three different kinds of papillae that contain taste bud cells: foliate, fungiform, and circumvallate papillae. Food particles that dissolve in liquids and/or saliva (tastants) bind to microvilli to stimulate taste receptors and initiate transduction cascades that give rise to tate.
FIGURE 2.
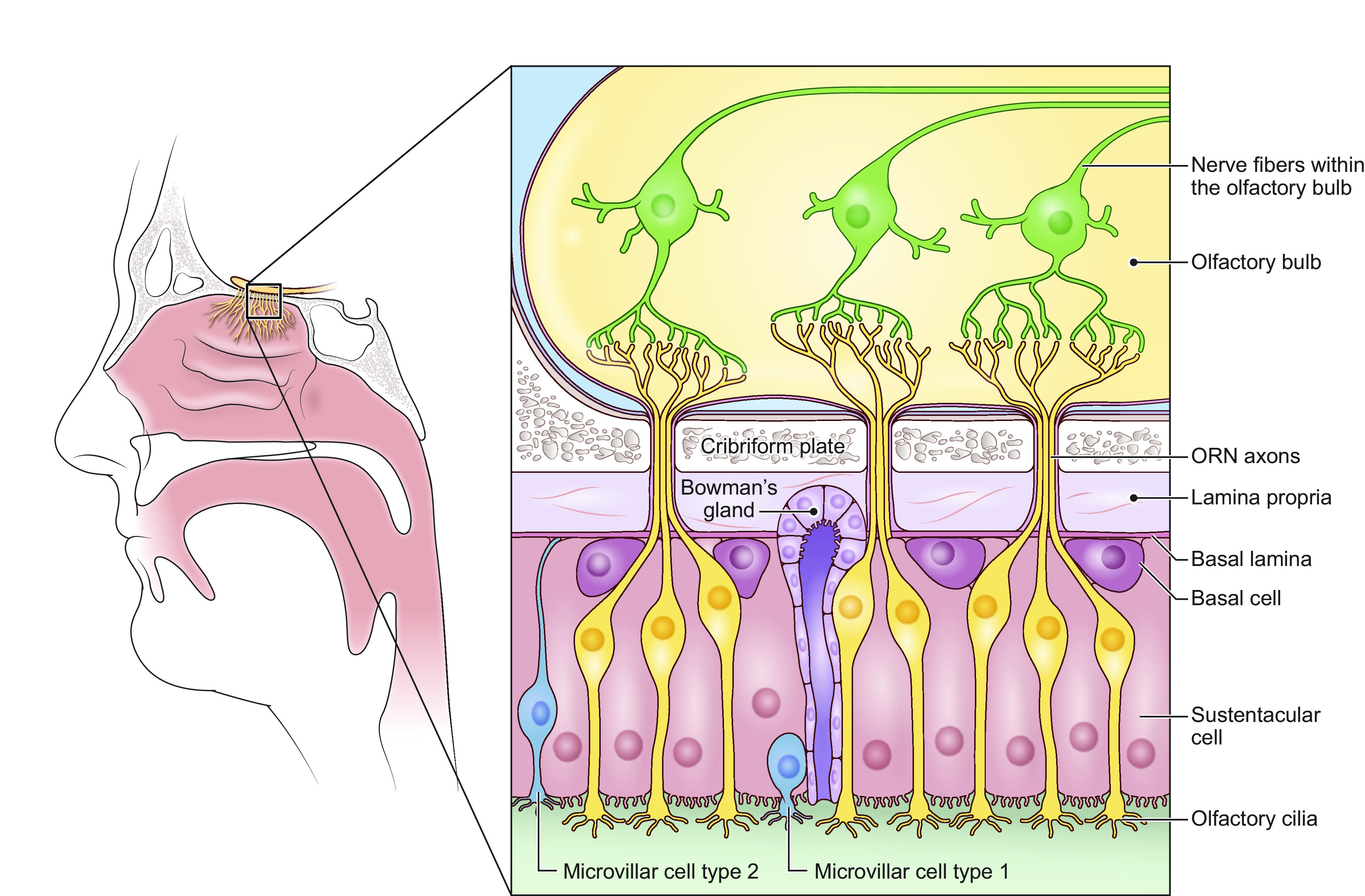
Olfactory epithelium and olfactory bulb. The olfactory epithelium is located within the nasal cavity and is comprised of olfactory sensory neurons (ORNs), basal cells, and supporting cells (i.e., sustentacular and microvillar cells). ORN dendrites project to the mucus layer that lines the olfactory epithelium. Smell/odors stimulate the olfactory cilia initiating olfactory signal transduction.
In addition to taste and olfaction, other sensory information contributes to our experience of flavor, such as food texture, irritation, and temperature (e.g., heat or spiciness). Somatosensory receptors such as mechanoreceptors, nociceptors, and thermoreceptors are responsible for our ability to discern or perceive these additional qualities, respectively (29–32). Fatty foods often have unique textural properties (e.g., creaminess) and can dissolve capsaicin and other spicy ingredients that do not easily dissolve in water. Thus it is crucial to study and understand how taste, smell, and associated chemical properties interact and contribute to fat perception and eating behavior.
1.4. Fat Taste as an Emerging Taste Modality
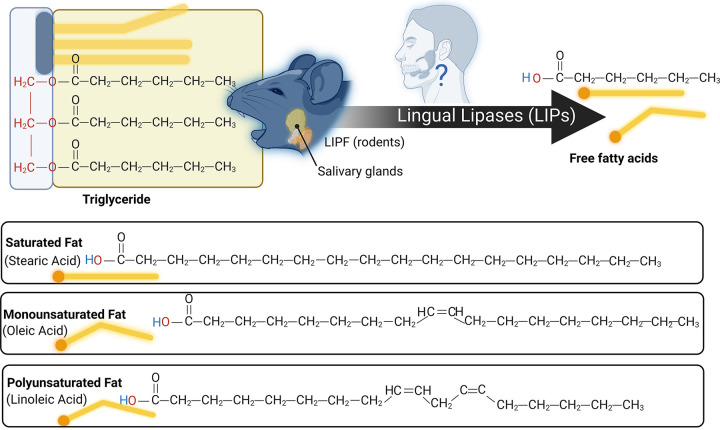
Literature exploring taste has primarily focused on five taste modalities: sweet, bitter, salty, sour, and umami (e.g., fish sauce, meat extract, and other savory foods). Each taste modality is characterized by specific criteria, including having an effective stimulus, a unique combination of taste-bud cell receptors specific to that taste modality (FIGURE 3), and taste perception that is independent of other taste modalities. There is emerging literature examining fat taste as an additional taste modality (for reviews, see Refs. 18, 33–35). Preclinical studies strongly suggest that free fatty acids (FFAs) (36) are the effective stimuli responsible for fat taste (18, 34). There are three types of FFAs (differing in degree of saturation): monosaturated FFAs (e.g., oleic acid), polyunsaturated FFAs (e.g., linoleic acid), and saturated FFAs (e.g., stearic acid) (FIGURE 4). Rodents specifically detect FFAs independently of other taste modalities, textural, and postingestive cues (37, 38). In contrast, the role of FFAs in human fat taste perception is not well established, as naïve subjects cannot easily distinguish FFAs (39). FFAs are also poorly soluble in saliva (compared to other tastants), and there are individual variations in fatty acid perception (34, 39, 40). Nonetheless, humans can be trained to detect FFAs (39, 40). Humans also possess salivary lipase isoforms (LIPK, LIPM, and LIPN), which break down triglycerides into FFAs (41) and can detect long-, medium-, and short-chain fatty acids (39, 40). Thus FFA detection may play a role in fat perception. However, the mechanisms of FFA-mediated activation of taste-related cells are not fully understood, as it may involve other transduction mechanisms, genetic, and environmental factors.
FIGURE 3.
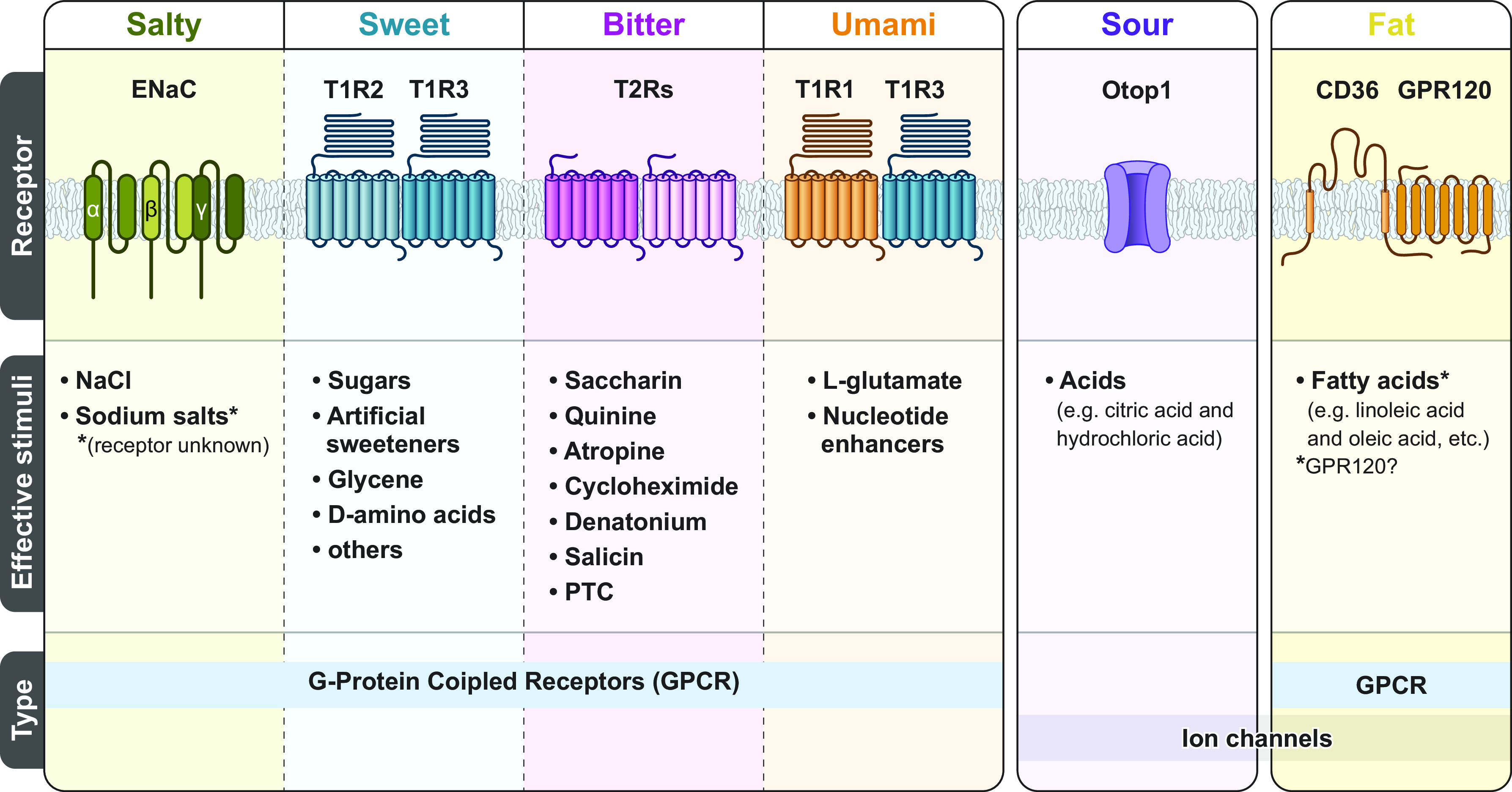
Taste receptors by taste modality. There are five commonly recognized primary taste modalities: salty, sweet, bitter, sour, and umami. However, there is growing evidence suggesting fat may be an additional taste modality. Each primary taste modality is characterized by multiple elements including having dedicated receptors [i.e., G protein-coupled receptors (GPCRs) and ion channels] and a defined class of effective stimuli. CD36, cluster of differentiation 36; ENaC, epithelial sodium channel; PTC, phenylthiocarbamide. Figure adapted from Ref. 26, with permission from Springer Nature.
FIGURE 4.
Triglyceride breakdown and fatty acid types. Triglycerides are a common type of dietary fat, composed of three fatty acids joined to glycerol. Lingual lipase can breakdown dietary triglycerides into fatty acids and glycerol. Rodents synthesize salivary lipase (LIPF). Humans also have salivary lipase forms (e.g., LIPK, LIPM, LIPN). Studies selected in this review often studied human and animal mode responses to common fatty acids, including oleic acid and linoleic acid. Image created with BioRender.com, with permission.
The growing interest in fat chemosensation and the underlying mechanisms in the context of obesity has led to a significant and rapid rise in studies examining the biological mediators of fat taste and smell. For example, since 2016, 88 articles discussing “fat taste” (in the title and abstract) have been indexed in PubMed alone. (For reviews, see Refs. 18, 35, 42–45.) While previous reviews have examined the biological mediators of fat taste, few reviews have examined both fat taste and smell. However, as mentioned above, taste and smell collectively contribute to flavor perception. Furthermore, there are few systematic reviews examining fat perception.
Systematic reviews provide a comprehensive and reproducible summary of available literature that examines a specific topic (46). Systematic reviews are conducted using a specific methodology that includes a succinct and clearly defined research question; predefined eligibility criteria and reproducible methods to select relevant articles; a comprehensive and systematic search of the published scholarly literature (and sometimes gray literature) to identify potentially relevant studies; study selection (i.e., screening) process conducted independently in pairs using the predefined eligibility criteria to minimize bias; an assessment of the internal and external validity (e.g., risk for bias) of the included articles; and a narrative synthesis of the findings from the included studies (46). If the specified methods are followed to ensure rigor and minimize bias, systematic reviews can serve as a valuable tool for researchers and clinicians to inform future research and shape clinical practice and guidelines. Therefore, as the number of studies examining fat chemosensation grows, conducting a systematic review of this field is increasingly important to provide a comprehensive overview of how these studies are contributing and expanding what we know about fat taste and smell.
Thus this systematic review will discuss studies that examine potential associations between taste and smell measures, biological mediators of fat taste and smell, and eating behavior in normal weight and individuals with obesity. The aim of this systematic review is to identify, analyze, and integrate the findings of emerging literature (2014–2021) to build on our understanding of the biological mechanisms of fat taste and olfaction. This knowledge can ultimately contribute to identifying potential therapeutic targets in nutritional and pharmacological interventions of fat-chemosensory dysfunction, as observed in obesity.
2. METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was used for reporting this review (47).
2.1. Eligibility Criteria
Our inclusion criteria were as follows: an original research article published in a peer-reviewed journal in English within the past 8 years (2014–2021) on biological mechanisms associated with fat taste and olfaction in human adults (individuals 18 years of age and older) and animals. Although taste and olfactory receptors exist outside the mouth and nose, this review focused on oral taste and nasal and retronasal olfaction. Studies had to discuss biological mechanisms associated with fat taste and olfaction, such as taste and olfactory threshold and/or preference, in relation to potential biological mediator(s) (e.g., fat taste preference in relation to brain activity, protein expression, or genetic polymorphisms). Exclusion criteria were articles published before 2014, studies including pediatric populations (17 years of age and younger) or nonadult animals (e.g., mice: postnatal day >35 days), descriptive studies, conference abstracts, case studies, studies focused only on a disease condition, nonprimary data articles (methods papers, opinions, proceedings), and any secondary data articles or reviews of any kind.
2.2. Information Sources and Search Strategy
Four databases: Embase (Elsevier), PubMed (US National Library of Medicine), Scopus (Elsevier), and Web of Science: Core Collection (Clarivate Analytics) were searched by a biomedical librarian (AAL). Search terms used were a combination of keywords and controlled vocabulary terms (e.g., MeSH in PubMed and EMTREE in Embase) for each concept of interest (i.e., fat, taste, smell, obesity), and search strategies were developed by the biomedical librarian in consultation with the review team. Searches were limited by publication year (2014–2021) and language (English). See final search strategies captured in Supplemental File S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.20415975.v1). The bibliographies of included articles were manually searched and screened by authors R. B. Jaime-Lara, B. E. Brooks, R. S. E. Ortiz-Figueroa, C. Vizioli, M. Chiles, and N. Nawal.
2.3. Study Selection
Before beginning, a pilot of 10 articles was conducted to test the eligibility criteria and screening process at both title and abstract and full text levels with all reviewers. After completing the pilot at both levels, all reviewers met to compare results, discuss the process, address questions and problems, and make final revisions to the criteria.
The titles and abstracts were first reviewed followed by the full text for those included after title and abstract screening. Two rounds of screening were conducted at each level (i.e., two rounds at title abstract level and two rounds at full text level. For both rounds, two reviewers (R.B.J.-L., B.E.B., R.S.E.O.-F., M.C., and N.N.) independently screened each article using the eligibility criteria and a third reviewer compared the results. Disagreements during screening were resolved by discussion between the two reviewers that screened and the third reviewer that checked initial screens to reach a consensus on whether to include or exclude the article. EndNote 20 (Clarivate Analytics) was used for the study selection process.
2.4. Data Collection and Data Items
A pilot of the data collection step was conducted on a sample set of three articles wherein the reviewers (R.B.J.-L., C.V., B.E.B., R.S.E.O-F., M.C., N.N., and N.I.) practiced extracting the specified data points and discussed questions or problems. For all articles included after full text screening, data collection was conducted using Microsoft Word and organized using Microsoft Excel. For each included study, sample size, participant/animal subject characteristics (e.g., age and sex), methods (intervention and/or test administered, biological samples collected, materials/fat sources used, etc.), and key findings about the biological mediators of fat taste or smell were collected. The extracted data is summarized in TABLES 1 and 2 and throughout the discussion. Two reviewers independently extracted the specified data points, and a third reviewer compared the data to identify discrepancies or errors, which were discussed by the third reviewer with the two reviewers who collected the data until a consensus was reached regarding the correct data to collect.
Table 1.
Clinical study characteristics and findings
| Author (Reference), Year, Country | Sample Size | Participant Characteristics | Methods | Key Findings |
|---|---|---|---|---|
| Andersen et al. (48), 2020, Denmark | N = 24 adults | Age = 26 ± 3.5 years | Analysis of neural taste responses to fat using EEG | Neural responses to the three fatty tastants (skim milk, whole milk, and cream) were significant and highly uniform. Neural activation occurred shortly (0.0 s) after the fatty stimulus was applied. They identified a neural correlate to fat taste. |
| Sex = female (n = 15) and male (n = 9) | ||||
| BMI = mean 23.5 ± 2.7 kg/m2 | ||||
| Appelqvist et al. (49), 2016, Australia | N = 10 adults | Age = mean age of 44.5 ± 7.2 years | Oil-in-water emulsions (50%, 10%, and 2% fat) were used as model food systems. | There was an enhancement of fatty after feel intensity for 50% fat emulsions containing the more lipophilic aroma ethyl hexanoate compared to ethyl butanoate, indicating a cross-modal interaction. |
| Sex = male (n = 2) and female (n = 8) | Particle size distribution was measured by laser light scattering. | |||
| Groups = solutions consisted of 2, 10, and 50% fat, with either HEX or BUT | ||||
| Bajit et al. (50), 2020, Morocco | N = 100 Moroccan adults | Age = mean 32.37 years ± 9.52 years | Anthropometric measurements | Analysis of the CD36 rs1761667 SNP in obese and normal weight subjects found a higher frequency of the AA genotype in obese subjects compared to the GG genotype. Obese subjects also had a higher OA detection threshold. The results for the OA detection threshold in the obese group, however, were very dispersed and did not correlate to a specific level of concentration, making the results inconclusive. |
| Sex = female (n = 72) and male (n = 28) | Oleic acid sensitivity test | |||
| Groups = obese (mean BMI 34.97 ± 4.02 kg/m2) and normal weight (mean BMI 22.16 ± 1.81 kg/m2) | SNP analysis | |||
| Besnard et al. (51), 2018, France | N = 38 Caucasian adults | Age = age-matched adults | Oral lipid detection threshold tests | Salivary flow and CA-IV were suspected to decrease oral sensitivity of fat in the obese nontaster group. Ultimately, specific microbial and salivary environments surrounding circumvallate papillae are involved in fat taste sensitivity. |
| Oral microbiota analysis | ||||
| Sex = all male | 16S-targeted metagenomics | |||
| Bioinformatics pipeline | ||||
| Groups = normal weight BMI <25.0 kg/m2 (n = 21) and obese BMI ≥30 kg/m2 (n = 17) | Saliva collection and analysis (flow, protein concentration, enzymatic activities, total antioxidant capacity, CA-VI levels, and LPS levels) | |||
| Bricio-Barrios et al. (52), 2019, Mexico | N = 27 Mestizo adults | Age = 18–25 years old | Oral fatty acid detection threshold test | BMI positively correlates with the oral sensitivity threshold of fatty acids and negatively correlates with serum sCD36 levels. Obesity can lower fat taste sensitivity and impact serum CD36 levels. |
| Sex = female (n = 51) and male (n = 21) | Anthropometric measures | |||
| BMI = normal weight (BMI 18.5 to 24.9 kg/m2) (n = 52) and overweight (BMI 25 to 29.9 kg/m2) (n = 20) | Serum CD36 quantification | |||
| Burgess et al. (53), 2018, USA | N = 68 Caucasian (n = 36) and East Asian adults (n = 32) | Age = mean age 25.3 ± 0.8 years for Caucasians and 25.0 ± 0.9 for East Asians | Buccal swab-DNA extraction | Oral perception of fat was found to vary by CD36 genotype between Caucasians and East Asians. There was no main effect of CD36 genotype on oral detection of fatty acids and no interaction between CD36 rs1761667 genotype and ethnicity on threshold detection. East Asians who had the GG genotype gave higher fattiness and creaminess ratings to the samples compared to those who had the AA genotype. No effect of this SNP was observed on the perception of fat-associated attributes among Caucasian participants. Heightened ratings of fat perception among East Asians who were GG carriers may be due to increased CD36 protein expression although it is presently unclear why the same relationship was not observed in GG Caucasians in this study. |
| Sex = 25 females, 11 males for Caucasians and 24 females, 8 males for East Asians | PCR amplification for CD36 target | |||
| BMI = BMIs ranged from 24.8 to 26.2 kg/m2 (Caucasians) and from 21.8 to 22.8 kg/m2 (East Asians) | Taste threshold: 3-Alternative Forced Choice (AFC) | |||
| Labeled Magnitude Scale (LMS) | ||||
| Chamoun et al. (54), 2021, Canada | N = 49 adults | Age = 25 ± 3 years | SNP genotyping | CD36 SNPs rs1527483 and rs3211908 were found to have significant associations with fat taste sensitivity. |
| Sex = 35 females, 14 males | Fat taste sensitivity test with OA | |||
| BMI = 22.3 ± 2.6 kg/m2 | ||||
| Chmurzynska et al. (55), 2020, Poland | N = 421 adults | Age = 20–40 years | Oral fat discrimination analysis | Participants with the GG CD36 genotype were more likely to be fat discriminators than were carriers of the A allele (P < 0.05). Polymorphisms of FFAR1, FFAR3, or CA6 were not related to fat discrimination. Fat discrimination was not associated with fat intake and polymorphisms of CD36, FFAR1, FFAR4, or CA6 were not associated with frequency of HF food consumption. |
| Sex = 207 females, 214 males | Genotyping of SNPs | |||
| BMI = controls <25 kg/m2 (n = 208), experimental group >25 kg/m2 (n = 213) | Assessment of food intake and high-fat-food consumption | |||
| Costanzo et al. (56), 2018, Australia | N = 88 (44 twin pairs) Australian adults | Age = mean age 43.7 ± 15.5 years | Three 2-h laboratory sessions 4 weeks apart. | The 8-wk consumption of an LF diet increases sensitivity to FT and the same period with an HF diet attenuates sensitivity, regardless of body weight. There is little indication of genetic contribution to FT. |
| Measured: 1) taste detection threshold to oleic acid (FATT), 2) ability to rank the amount of fat in food, 3) liking ratings for high-fat and reduced-fat foods, and 4) intensity ratings to 5 prototypical tastants | ||||
| Dietary intervention: participants given and taught about the HF or LF diet and nutrition labels to identify which foods for their diet. | ||||
| Sex = 33 female pairs, 10 male pairs, and 1 gender discordant pair. | Detection threshold of OA: 3-AFC test | |||
| FT (fat taste) rank | ||||
| BMI = BMIs at baseline ranged from 21.2 to 31.4 kg/m2 (LF diet) and from 21 to 32.6 kg/m2 (HF diet) | TG (triglyceride) ranking task | |||
| Fatty food liking: LMS | ||||
| Costanzo et al. (56), 2019, Australia | N = 18 (9 pairs) Australian adults | Age = mean age 41·6 ± 16.5 years | Participants attended two tasting sessions | No significant time-diet interactions were observed for CD36, GPR84, FFAR2, and KCNA2 expression. |
| Two biopsy sessions | A significant negative association was observed between Δ FATT and Δ FFAR4. There was a positive association between Δ FATT and Δ GPR84. Significant negative associations were observed between Δ FFAR4 and Δ fat intake, Δ saturated fat intake, Δ monosaturated fat intake, and Δ polyunsaturated fat intake. There was a significant positive association between Δ FFAR2 and Δ dietary fiber intake. | |||
| Sex = 16 females, 2 males | A diet booklet for each diet was created with the aid of an accredited practicing dietitian | No significant associations were observed for CD36 or GPR84. | ||
| Food recall and records were analyzed for carbohydrate, protein, fat, and fiber intake (g and % of energy) using computer software FoodWorks (version 8; Xyris) | There was a statistical trend for a negative association between KCNA2 and polyunsaturated fat intake. | |||
| BMI = BMIs ranged from 20.6 to 33.2 kg/m2 | Fungiform papillae biopsy | |||
| The gene expression of FAT receptors: RT-PCR | ||||
| Eldeghaidy et al. (57), 2016, United Kingdom | N = 16 Adults | Age = mean age 25 ± 2 years | gLMS | Compared to the WL, consuming HFM led to decreased anterior insula taste activation in response to fat-related satiety. HFM caused reduced amygdala activation in response to the FS compared to the CS. Baseline cerebral blood flow significantly reduced in taste, homeostatic, and reward areas after the HFM. An individual’s plasma CCK concentration correlated negatively with brain activation in taste and oral somatosensory and reward areas. |
| Sex = 6 females, 11 males | Two emulsion stimuli were delivered during fMRI | |||
| BMI = BMIs ranged from 21.6 to 23.2 kg/m2 | A randomized two-way crossover design to assess the effect of the prior consumption of fat either a high-caloric high-fat-diet meal (HFM) or noncaloric water load (WL) on the BOLD response to the oral control (OC) and fat stimuli (FS) | |||
| Visual analog scale | ||||
| CCK measurement | ||||
| MRI scanning | ||||
| Shapiro-Wilk normality test | ||||
| Frank-Podlech et al. (58), 2019, Germany | N = 15 healthy adult males | Age = 24.6 years ± 2.4 SD | Visual analog scale (VAS) | Oral fat sensitivity was positively correlated with functional connectivity between homeostatic regions and limbic areas in the high-fat condition but negatively correlated with functional connectivity between the dorsal striatum and somatosensory regions in the low-fat condition. |
| Sex = 15 adult males | Functional MRI (fMRI) | |||
| BMI = 23.1 kg/m2 ± 2.0 SD | Seedbased functional connectivity (FC) maps | |||
| Grabenhorst et al. (59), 2014, United Kingdom | N = 14 Adults | Age = mean age of 24 years | Fat flavor stimuli consisted of vanilla and strawberry-flavored dairy drinks delivered through Teflon tubes and held between the lips during fMRI | The activity in somatosensory cortex (SSC) was more strongly correlated with the orbitofrontal cortex (OFC) during the consumption of a high-fat food with a pleasant (vanilla) flavor compared to a low-fat food with the same flavor. |
| Sex = 5 females, 9 males | fMRI data acquisition and analysis | |||
| BMI = not specified | Psychophysiological interaction (PPI) for differential FC | |||
| Graham et al. (60), 2021, United Kingdom | N = 48 adults | Age = 32.7 ± 11.4 years | Fat taste sensitivity assessment | The BDNF rs6265 SNP and the TNNI3K rs1514175 SNP were analyzed to find differences in fat taste sensitivity in an ethnically similar cohort. The rs6265 SNP was found to have lower fat taste threshold for the CT/TT genotype compared to the CC genotype. The rs1514175 SNP AA/AG genotype was found to have a moderately higher fat taste threshold. |
| Sex = all females | Anthropomorphic measurements | |||
| SNP genotyping | ||||
| Han et al. (61), 2020, Germany | N = 38 adults | Age = mean 25.9 years | Sweet taste sensitivity test | High sensitivity was associated with: higher preference for carbs, higher liking of sweet foods, lower liking for protein-dominated foods, higher level of frontal inferior operculum activity in response to sweet vs. savory food odors, and stronger insular activations to high-fat vs. low-fat food odors. Additionally, individual sweetness sensitivity was positively correlated with insula activation in response to a high-fat odorant. |
| Sex = 21 females, 17 males | Macronutrient and taste preference task | |||
| BMI = 18.5–29.4 | fMRI study with odor stimuli | |||
| Macronutrient and energy density questionnaire | ||||
| Kadouh et al. (62), 2019, USA | N = 40 liraglutide (n = 19) and Placebo (n = 21) obese American adults | Age = mean age of 42 years for liraglutide and 37 years for placebo | Standardized nutrient drink test | Compared to placebo group, liraglutide group had significant reductions in MTV; prospective food consumption score; desire to eat something sweet, salty, savory, or fatty; and an increase in perceived fullness. Postprandial plasma levels of GLP-1 decreased and PYY levels increased with liraglutide relative to baseline. Significant reductions in total body, trunk, and upper and lower body fat without reduction in lean body mass were observed. |
| Sex = all females | VAS | |||
| BMI = BMIs ranged from a baseline BMI of 37.2 kg/m2 for liraglutide and 34.6 kg/m2 for the placebo group. | Plasma gastrointestinal hormone measurement | |||
| Karmous et al. (24), 2018, Tunisia | N = 104 NW (n = 52) and OB (n = 52) Tunisian adults | Age = normal weight (NW) mean age 35.3 ± 4.10 years and OB mean age 35.0 ± 5.43 years | Taste preference test for linoleic acid (LA) | There was a positive correlation between BMI and PROP oral detection thresholds in obese participants. There was no association between rs10246939 and obesity. |
| 3-AFC method | ||||
| Sex = NW 29 females, 23 males and OB 42 females, 10 males. | Venous blood collection | |||
| Column chromatography | ||||
| BMI = BMIs ranged from 21.78 to 24.66 kg/m2 (NW) and from 28.98 to 39.6 kg/m2 (OB) | ELISA | |||
| Genomic DNA extraction RFLP method and gel electrophoresis: analyze rs10246939 (Val296Ile) and rs1726866 (Ala262Val) | ||||
| Karthi et al. (63), 2021, India | N = 444 adults | Age = not specified | LA oral detection threshold test | The AA genotype at rs1761667 (of CD36 gene) had a higher LA detection threshold (lower sensitivity to fat taste). |
| Sex = 234 males, 210 females | Genotyping for genetic polymorphisms | |||
| BMI = group 1 (NW) ranged 20–24.9 kg/m2, group 2 (OW) ranged 25–29.9 kg/m2, and group 3 (obese) ranged 30–35 kg/m2 | Measurement of PYY hormone | |||
| Kulkarni and Mattes (64), 2014, USA | N = 15 healthy adults | Age = range: 18–50 years | Salivary nonsteroidal fatty acids (NEFA) measures | Lingual lipase was active during oral processing of almond and coconut. No activity of lingual lipase was detected during processing of almond butter. There was only weak evidence lingual lipase is a determinant of oral fat detection. Lingual lipase may only contribute to NEFA generation and oral fat detection. |
| Sex = 11 females, 4 males | Sensory ratings for almond butter with and without lipase inhibitor oralist | |||
| BMI = mean: 18.5–25 kg/m2 | NEFA present in 5 HF foods varying in physical states and fatty acid composition (almond, almond butter, olive oil, walnut, and coconut) | |||
| Liu et al. (65), 2018, Australia | N = 36 | Cohort 1 and 2 | Fungiform papillae collection | qRT-PCR and Western blotting indicated that mRNA and protein of CD36, FFAR4, FFAR2, GPR84, and delayed rectifying K+ channels are expressed in human fungiform taste buds. The expression level of CD36 was associated with the liking difference score between high-fat and low-fat food. |
| Age = between 20 and 42 years | qPCR | |||
| Sex = 6 females, 4 males | Phenotyping test | |||
| BMI = between 20 and 33 kg/m2 | Western blots | |||
| Cohort 3 | Immunohistochemistry | |||
| Age = 8 pairs of female twins | ||||
| Sex = age ranged between 20 and 62 years | ||||
| BMI = between 17 and 35 kg/m2 | ||||
| Méjean et al. (66), 2015, France | N = 216 French adults | Age = mean age 49.6 ± 13.5 years | Questionnaires: dietary intake, physical activity, anthropometric measures, lifestyle, and socioeconomic and health status | Salivary flow was positively associated with liking for fat. Proteolysis was positively associated with liking for saltiness and for fat. |
| Sex = males and females | Sensory tests: liking for salty, sweet, and fat sensations | |||
| BMI = not specified | Saliva for: protein concentration, enzyme activity, lipolysis, proteolysis, amylolysis, Carbonic anhydrase 6 and cystatin SN and sodium quantification, and antioxidant capacity | |||
| 24-h food records | ||||
| Food products assessment for sensory liking | ||||
| Melis et al. (67), 2015, Italy | N = 64 Caucasian adults | Age = mean age 27.6 years | PROP taster status (super taster, medium taster, or nontaster) | Subjects homozygous for GG of the rs1761667 polymorphism showed higher sensitivity to oleic acid than AA subjects. The capability to detect oleic acid was directly associated with TAS2R38 or PROP responsiveness. PROP nontasters had a lower papilla density than tasters, and those with genotype GG of the rs1761667 polymorphism had lower oleic acid thresholds than PROP nontasters with genotype AA. |
| Sex = 41 females, 23 males | LMS | |||
| BMI = BMIs ranged from 18.6 to 25.3 kg/m2 | Oral perception (for fatty acid) using 3-AFC procedure | |||
| DNA extraction and PCR for CD36 SNPs genotyping = rs1761667 (G/A) and rs1527483 (C/T) | ||||
| TAS2R38 genotyping for three SNPs at basepairs 145 (C/G), 785 (C/T), and 886 (G/A) were performed using PCR | ||||
| Melis et al. (68), 2017, Italy | N = 126 Caucasian volunteers from Italy | Age = not specified | Blood samples and saliva collection for DNA extraction | The A/G allele of the rs1761667 polymorphism of CD36 was associated with distinct metabolic patterns in NW and obese subjects. The G allele of the CD36 gene rs1761667 was associated with increased endocannabinoid plasma levels and a trend for increased waist/hip ratio in obese subjects, even though exhibited decreased BMI with respect to those with AA genotype. |
| Sex = 80 females, 46 males | HPLC analysis: aliquot of the lipid fraction, from erythrocytes | |||
| BMI = 64 participants with BMIs ranging from 18 to 25 kg/m2 and 62 obese participants with BMIs 30–50 kg/m2 | SAFA were analyzed as fatty acid methyl esters by a gas chromatography with FID | |||
| Fisher method: genotype distribution and allele frequencies of CD36 SNP | ||||
| Melis et al. (69), 2018, Italy | N = 46 Caucasian adults | Age = mean age 26.6 ± 0.79 years | PROP-taster status classification: LMS | rs1761667 in CD36 indicate that the effectiveness of l-Arg supplementation in increasing the perception of oleic acid is directly related to the presence of allele A in rs1761667 polymorphism of CD36 gene, which has been associated with a lower expression of CD36 scavenger receptor, with respect to that of allele G. |
| Sex = 38 females, 8 males | Saliva samples: DNA extraction | |||
| BMI = BMIs ranged from 18.6 to 25.3 kg/m2 | rs713598, rs1726866, and rs10246939 genotyping of TAS2R38 locus, rs1761667 of CD36 and rs2590498 of OBPIIa gene polymorphism | |||
| PCR | ||||
| DFT method LA oral perception with l-Arg | ||||
| Mounayar et al. (70), 2014, France | N = 73 French adults | Age = mean age of 43 ± 15.4 years, for sensitive+ subjects and 40 ± 13.1 years for sensitive− subjects | The screening procedure to determine fatty acid sensitivity. Low concentration of C18:1 was considered ‘‘sensitive+,’’ while subjects who detected least frequently the sample containing the high concentration of C18:1 were considered ‘‘sensitive−’’ | Fatty acid sensitivity was associated with changes in saliva composition induced by C18:1 stimulation. |
| Sex = 73 males | Saliva collection | |||
| BMI = BMIs ranged from 22.3 to 26.7 kg/m2 for “sensitive+” subjects,and 21.6 to 27.8 kg/m2 for “sensitive−” subjects | Protein content measurement and two-dimensional electrophoresis analysis | |||
| 1H-NMR analysis | ||||
| Mrizak et al. (71), 2015, Tunisia | N = 203 Tunisian adults | Age = mean age 38.4 ± 11.4 years | Plasma and serum from fasting venous blood samples were collected | The A allele of cluster of differentiation 36 (CD36) SNP 1761667 is associated with decreased lipid taste perception in obese Tunisian women. Women with the CD36 GG genotype exhibited oral detection thresholds for oleic acid that were more than three times lower than those with the CD36 AA genotype. |
| Enzymatic methods were used to determine blood parameters (serum TAG and total and free cholesterol concentrations) | ||||
| Sex = 203 females | Taste emulsions containing food grade oleic acid (Sigma) were prepared | |||
| 3-AFC | ||||
| BMI = BMIs ranged from 30.4 to 38.8 kg/m2 | Genomic DNA extraction | |||
| PCR amplification | ||||
| Ong et al. (72), 2017, Malaysia | N = 313 Chinese (n = 293) and Indian (n = 20) adults | Age = mean age 20.73 ± 1.55 (males) and 20.74 ± 1.49 (females) years | Sensory stimuli and rating test | CD36 rs1761667 and rs1527483 are not associated with obesity and adiposity. CD36 rs1527483 plays a role in OFP. |
| Sex = 195 females, 118 males | Participants were presented with four increasing oil (fat) content by-weight custards and low-fat/regular versions of commercially available milk, mayonnaise, and cream crackers. | |||
| BMI = 88% of participants had a BMI ≥25 kg/m2, 12% had a BMI <25 kg/m2 | VAS | |||
| DNA extraction | ||||
| PCR-RFLP-CD36 rs1761667 and rs1527483 SNPs genotype | ||||
| Plesník et al. (73), 2018, Czech Republic | N = 116 young Caucasian adults | Age = mean age 21.84 ± 0.22 years | Food craving inventory (FCI) | Participants with the CC genotype of the rs1527483 polymorphism of the CD36 gene had lower BMI, waist circumference, waist:height ratio, and higher sensitivity for LA than the participants with the CT and TT genotypes. No association was found between the rs32TLCA18 polymorphism and LA detection threshold or BMI, waist circumference, and waist:height ratio. |
| Sex = 73 females, 43 males | Oral LA detection thresholds | |||
| BMI = BMIs ranged from 22.92 to 23.76 kg/m2 | Genomic DNA extraction | |||
| Proserpio et al. (74), 2016, Italy | N = 103 adults | Age = less than 65 years of age (not specified) | Taste threshold OA was used to elicit fat sensation tastes, 7 concentrations of each compound prepared 3-AFC test | Obese subjects had higher threshold values (for fat, sweet, bitter, salty, and sour tastants) and a reduced number of fungiform papillae. Obese subjects had significantly higher liking ratings for high energy dense foods. Food neophobia was not associated with BMI or taste sensitivity. |
| Sex = 28 obese females, 23 obese males; 27 normal weight females, 25 normal weight males | Fungiform papillae density | |||
| BMI = 27.76 ± 7.10 | FNS | |||
| Food liking: 26-item liking questionnaire | ||||
| Ramos-Lopez et al. (75), 2019, Spain | N = 474 Spanish and Chilean adults | Age = mean age 47.2 ± 14.1 years | Nutriepigenomic analysis | 15 CpG were correlated with BMI (FDR adjusted-linear regression). No relationships between methylation status of olfactory genes and metabolic markers and blood pressure. Pathway enrichment analysis revealed a significant contribution of genes involved in the regulation of the olfactory transduction network, such as odor detection and signal processing in the nervous system. These genes included the olfactory receptors OR4D2, OR51A7, OR2T34, and OR2Y1 and several downstream effectors, such as SLC8A1, ANO2, PDE2A, CALML3, GNG7, CALML6, PRKG1, and CAMK2D. |
| Sex = 303 females, 171 males | Associations between taste receptors polymorphisms, dietary intakes, lipid disorders, and liver disease in Mexican subjects were reported | |||
| BMI = BMIs ranged from 24.5 to 35.7 kg/m2 | Data normality was screened by the Kolmogorov-Smirnov test. | |||
| Running et al. (76), 2017, USA | N = 78 adults for Stearic acid test | Age = range 18–55 years | Three fatty acids with different degrees of saturation were tested (stearic, oleic, and linoleic acid) | This study demonstrates that degree of unsaturation influences rejection of a chocolate with added FFA. With polyunsaturated FAs being rejected by both taste and aroma at lower concentrations than the monounsaturated (oleic) acid. No rejection observed for the flavor of saturated fatty acids. |
| N = 69 adults for oleic acid test | Sex = stearic acid test: 57 females, 21 males; for oleic acid test: 48 females, 21 males; linoleic acid test: 57 females, 18 males | Paired preference tests were conducted for 10 concentrations (0.04% to 2.25%) of added FFAs compared with the control chocolate without added FFAs. | ||
| N = 75 linoleic acid test | BMI = not specified | Stearic acid was tested for flavor (tasting and nares open), whereas the unsaturated fatty acids were tested for both aroma (orthonasal only and no tasting) and taste (tasting with nares blocked to eliminate retro nasal odor) | ||
| Shen et al. (77), 2017, United Kingdom | N = 136 UK adults | Age = 65% of participants were 18–29, 35% 30–55 years of age; range 18–55 years | Two visits 2 weeks apart. First visit: prestudy questionnaires, buccal cell swab, anthropometric measurements, and FPD measurements. Between visits 1 and 2 the FFQ and TFEQ were conducted. Second visit: fat liking rating and PROP sensitivity test. | Liking for ice cream was significantly affected by the fat content of the sample, and by demographic factors (gender, ethnicity, age) but no associations were found between CD36 rs1761667 or CA6 rs2274333 genotypes, PROP taster status, nor FPD. |
| LMS-PROP taster status | ||||
| Blue dye: FPD | ||||
| Sex = 95 females, 41 males | DNA extraction from buccal cells | |||
| Trained sensory panel: for sensory profile | ||||
| Nine-point hedonic category scale-fat liking | ||||
| BMI = 17–43.5 kg/m2, mean of 22.9 ± 0.34 kg/m2; 74% of participants were in the normal weight range (18.5–25 kg/m2) | Oxford FFQ: participants diet assessment | |||
| TFEQ: to categorize eating behavior | ||||
| Tanita body composition analyzer: for BMI | ||||
| Solakivi et al. (78), 2015, Finland | N = 736 Finnish adults (314 with hypertension and 422 controls) | Age = 50 years (50-year-old cohort of TAMARISK study) | Interview-structured questionnaire about health and health-related behavior | CD36 rs1761667 was associated with BMI in the TAMRISK study. Considering the multitude of roles of CD36 in processes related to fatty acid metabolism and sensing in the body, it is plausible that genetic variation in human fatty acid transporter CD36 can have effects on regulation of energy homeostasis. |
| Sex = 129 females with hypertension; 185 males with hypertension, 160 females and 262 males in control group | Buccal swabs- DNA extraction | |||
| BMI = 28.8 ± 5.2 (hypertension group) and 25.5 ± 3.6 (control group) | Genotyping: CD36 SNP rs1761667 | |||
| Physical examination | ||||
| Serum cholesterol and glucose | ||||
| Sun et al. (79), 2016, USA | N = 33 right-handed American subjects | Age = mean = 26.9; range 18–40 | gLMS used for internal state ratings and perceptual quality ratings | These findings demonstrate that the effect of a meal on suprathreshold odor intensity perception is associated with metabolic measures such as body weight and total ghrelin reactivity, supporting endocrine influences on olfactory perception. |
| Sex = 17 females, 16 males | Plasma levels of FFAs, triglycerides, ghrelin, insulin | |||
| BMI = 13/33 had BMI >25.0 | fMRI with MRI compatible olfactometer and portable gustometer | |||
| Watanabe et al. (80), 2019, Japan | N = 53 young Japanese adults | Age = mean age 24.3 ± 1.5 years; range, 20–38 years | Genotype Trp64 (Trp64trp and Trp63Arg) | When divided into two groups based on greasy food preference, the Trp64Arg had higher preference for HF foods (vs. Trp64Trp), suggesting that Arg substitution might genetically enhance HF preference. Understanding the relationship between ADRB3 Trp64Arg substitution and fat preference could be valuable for obesity prevention. |
| Sex = 28 females, 25 males | Buccal swab: DNA extraction | |||
| BMI = BMIs ranged from 17.1 to 28.2 kg/m2 | ||||
| Voigt et al. (41), 2014, Germany | N = 12 trained adult participants | Age = 26−40 years | Oral fatty acid sensitivity test (using oleic acid) | Lipases (LIPs), different from LIPF (as observed in rodents), are present in human salivary glands. Oral perception of triglycerides is associated with differential LIP activities on individual threshold concentrations. |
| Sex = 4 males 8 females | Oral lipolytic activity | |||
| BMI = not specified | Hyman circumvallate papillae biopsy for RT-PCR and in situ hybridization | |||
| In vitro lipolysis | ||||
| Zhou et al. (81), 2021, United Kingdom | N = 94 health adults | Age = range 18−70 years | Fatty acid taste sensitivity test | Higher fungiform papillae density was correlated with higher fat taste sensitivity. Authors hypothesize that this is due to a larger amount of fatty acid taste receptors on the tongue. |
| Sex = 64 females, 30 males | Measure of fungiform papillae density | |||
| BMI = mean 22.7 kg/m2 female, mean 24.1 kg/m2 male | Tactile sensitivity measurement | |||
| Biscuit mouthfeel perception and texture measurements |
AFC, alternative-forced choice; BMI, body mass index; BOLD, blood-oxygen-level-dependent; DFT, density functional theory; HEX, ethyl hexanoate; but, ethyl butanoate; FT, fat taste; FAT, fatty acid taste; FID, flame ionization detector; FFQ, food frequency questionnaire; FCI, food craving inventory; FNS, food neophobia scale; fMRI, functional magnetic resonance imaging; FPD, fungiform papillae density; GLP-1, glucagon-like-peptide-1; HF, high fat; HPLC, high performance liquid chromatography; LMS/gLMS, labeled magnitude/general labeled magnitude scales; LF, low fat; OA, oleic acid; 1H-NMR, proton nuclear magnetic resonance; MTV, maximal tolerated volume; PPI, psychophysiological interaction; PROP, 6-n-propylthiouracil; RFLP, restriction fragment-length polymorphism; qRT-PCR, quantitative real-time-polymerase chain reaction; SAFA, saturated fatty acid; SNP, single-nucleotide polymorphism; TFEQ, three-factor eating questionnaire; TC, total cholesterol; TG, triglycerides; CCK, cholecystokinin; VAS, visual analog scales; NEFA, nonesterified fatty acids; CD36, cluster of differentiation 36.
Table 2.
Preclinical study characteristics and findings
| Author (Reference), Year, Country | Sample Size | Animal Characteristics | Design/Methods | Key Findings |
|---|---|---|---|---|
| Ackroff and Sclafani (82), 2014, USA | N = 43 C57BL/6J (B6) Mus musculus (mice) | Age = 10 weeks old | Intralipid intragastric infusion | Intragastric (IG) administered self-infusions of fat produces concentration-dependent increases in the intake of and preference for a flavored solution in C57BL/6J mice. IG fat rapidly generates concentration dependent postoral signals that stimulate intake and enhance preferences for energy-dense foods. |
| Sex = all male | Licking tests | |||
| Groups = infusions of 1.6% (n = 11), 3.2% (n = 10), 6.4% (n = 11), or 12.8% (n = 11) intralipid | Two-bottle preference tests | |||
| Ahn et al. (83), 2017, USA | N = 22–124 Drosophila melanogaster (fruit fly) | Age = 3–7 days | Proboscis extension reflex (PER) assay | A novel role for IR25a and IR76b in fatty acid taste was established. These two subunits are not only critically important to elicit PER responses in flies when challenged with fatty acids but are also necessary for fatty acid induced Ca2+ increases in tarsal sweet gustatory receptor neurons (GRNs). |
| Sex = all female flies | Immunofluorescence | |||
| Groups = control, IR25a, and IR76b mutant | Calcium imaging | |||
| Ancel et al. (84), 2015, France | N = 10–12 C57Bl/6 mice | Age = young mice | Two-bottle preference tests | GPR120 disruption is not associated with fat preference or CD36 expression in circumvallate papillae. However, GPR120 agonist, grifolic acid, triggered a rise in [Ca2+]i which was drastically decreased when GPR120 was disrupted. These data suggest that although GPR120 is expressed in lingual tissue, it is not required for oral fat detection. |
| Sex = all male | Licking tests | |||
| Groups = wild type (WT) and GPR120−/− | Conditioned taste aversion tests | |||
| Measurement of Ca2+-signaling in taste bud cells (TBC)s | ||||
| Avalos et al. (85), 2020, USA | N = 5–8 (per group) C57BL/6Tac male mice and CB1R-deficient mice | Age = 8–10 weeks | Western diet preference test | Preference for the Western high-fat diet (HFD) was significantly decreased after pharmacological blockade of CB1R. HFD preference was also decreased for CB1R−/− mice, showing that CB1 in the intestinal epithelium plays a role in fat preference. |
| Sex = all male | Pharmacological blockade of cannabinoid receptor type 1 (CB1R) | |||
| Groups = WT and CB1R−/− | Utilizing CB1R−/− mice | |||
| Avau et al. (86), 2015, Belgium | N = 32 C57BL/6J mice | Age = adult mice | Comparing WT with knockout mice on HFD | α-Gustducin knockout mice did not gain as much weight as WT mice on HFD. Intragastric administration of bitter agonists caused further weight loss via α-gustducin pathway. Therefore, α-gustducin is involved in induction of obesity during HFD. |
| Sex = not specified | Comparing food intake after administration of bitter tastants vs. control | |||
| Groups = WT and α-gustducin−/− | Respiratory quotient/heat production measurements | |||
| Bensalem et al. (87), 2020, France | N = 24 C57B1/6 mice | Age = 12- to 14-week-old mice | Comparing WT with knockout mice on standard/HFD | The TGR5−/− obese mice exhibited high daily food/energy intake, fat mass and inflammatory status. TGR5−/− obese mice maintained an attraction for lipids. In TBCs, the fatty acid-triggered Ca2+ signaling was increased in TBC from TGR5−/− obese mice. TGR5 may modulate fat eating behavior and obesity. |
| Analysis of lean/fat mass | ||||
| Sex = all male mice | Two-bottle preference test | |||
| LPS assay | ||||
| Groups = WT and TRG−/−; standard chow and high-fat-chow mice | Taste bud isolation from CV | |||
| Measurement Ca2+ signaling | ||||
| Boone et al. (88), 2021, USA | N = 5–22 (per group) C57BL/6J mice | Age = >8 weeks | Olfactory bulb ablation and anosmia screening | Anosmic mice (following complete removal of the olfactory bulb) and sham mice (with intact olfactory bulbs) both displayed comparable HFD intake. HFD smell (in the absence of consumption) did not alter feeding or devaluation of standard food. Thus, while the olfactory bulb may play a role in fat-olfaction it may not be necessary for the development a HFD preferential consumption. |
| Sex = both: male and female | Inaccessible and short accessibility food experiments | |||
| Groups = standard diet (SD), SD + HFD, and SD + inaccessible HFD | Fast-refeed tests | |||
| Measuring SD and HFD consumption of control and anosmic mice | ||||
| Braymer et al. (89), 2017, USA | N = 5–9 (per group) obesity-prone (OP) and obesity-resistant (OR) S5B/P1 rats | Age = 8–9 weeks old | Linoleic acid (LA) preference testing | Lingual CD36 mRNA levels increased in OR rats, but not in OP rats. Lingual application of CD36 siRNA decreased LA preference in OR rats, but not in OP rats. OP rats did not show other effects of CD36 siRNA on HFD preference or HFD or LFD intake. |
| Sex = all male | Administration of lingual CD36 siRNA | |||
| Experiment 1: effect of fasting- 16-h fast (OP n = 8; OR n = 7) + standard chow (OP n = 8; OR n = 8) | Effect of fasting: 16-h fast + chow | |||
| Experiment 2: effect of HFD on LA preference (OP n = 5; OR n = 5) | Effect of HFD: HFD vs. chow fed | |||
| Experiment 3: effect of fasting lingual CD36 mRNA expression + standard chow (OP n = 6; OR n = 6), fasted overnight (OP n = 9; OR n = 6), refed for 2 h (OP n = 7, OP n = 7) | ||||
| Brown et al. (90), 2021, USA | N = 13–41 (per trial) IR56d Drosophila melanogaster | Age = 7–9 days | Aversive taste memory | Flies were able to discriminate medium-chain fatty acids (MCFAs) from short-chain fatty acids (SCFAs) and long-chain fatty acids (LCFAs). They were not able to discriminate different MCFAs from each other. Similar discrimination abilities were exhibited in both males and females. |
| Sex = all female | ||||
| Buttigieg et al. (91), 2014, Chile | N = 5–12 (per group) Swiss CD1 mice | Age = weaned mice | RD vs. HFD chow preference tests | After 18 days (short term) exposure to HFD, mice developed preference for high fat chow, indicating that high fat preference is not spontaneous in CD1 mice, but can be acquired after short term exposure to HFD. Development of preference for HFD dependent on NMDA receptor signaling. |
| Sex = all male | HF preference tests after 18-day exposure to either RD or HFD | |||
| Groups = Exp1 group (n = 12); Exp2: regular diet (n = 9) and high-fat diet (n = 9); Exp3: IP injections of ketamine, ifenprodilMK-801, or PBS (control), (n = 5 per group) | NMDA antagonist injections during HFD with preference tests | |||
| Calder et al. (92), 2021, USA | N = 66 C57BL/6J WT and Growth Hormone Secretagogue Receptor knockout (Ghsr -/-) mice | Age = 6 weeks old | Conditioned taste aversion assay | GHSR expression within the taste system- with GHSR being largely present in type II cells. Additionally, HFD-fed female GHSR-/- exhibited reduced responsiveness to LA (compared to WT). Ghrelin signaling may play a critical role in the recognition of fatty acids in female mice, and this may contribute to ingestive behaviors. |
| Sex = both: female (n = 33) and male (n = 34) | ||||
| Groups = WT (n = 37) and Ghsr-/- (n = 29) | ||||
| Camandola and Mattson (93), 2017, USA | N = 5–19 mice per group | Age = adult mice | Two-bottle preference test | TLR4 knockout mice show low preference for fat. TRPM5 and G-protein dependent phospholipase Cb2 significantly decreased in TLR4 knockout. Overall, TLR4 promotes fat ingestion (FA endocytosis) and preference for fat intake. |
| Sex = all male | Comparing standard diet and obese diet | |||
| Groups =WT and TLR4 knockout mice; standard diet and high-fat, high-sugar diet | Tongue epithelium PCR | |||
| De la Cruz et al. (94), 2015, USA | N = 37 Sprague-Dawley rats (260−300 g) | Weight = 260- to 300-g rats | Feed rats test solutions | Corn oil caused dopaminergic signaling, measured by c-Fos-like immunoreactivity, in the ventral tegmental area, infralimbic/prelimbic prefrontal cortex, dorsal striatum, nucleus accumbens core, and basolateral/central-cortico-medial amygdala. This suggests that these brain regions may form a distributed network to help mediate fat intake. |
| Sex = all male | Brain tissue collection | Consumption of corn oil solutions, isocaloric to glucose and fructose, significantly increased FLI in all sites except for the NAc shell. | ||
| Groups = water (n = 7), cherry-flavored saccharin (0.2%) (n = 7), corn oil in xanthan gum (3.5%) (n = 5), fructose (8%) (n = 7), glucose (8%) (n = 7), and saccharin, xanthan gum (0.3%) (n = 4) | Immunoreactive c-Fos quantification | |||
| Devineni et al. (95), 2019, USA | N = 6–18 (per trial group/set) Drosophila melanogaster | Age = 3–6 days old | PER experiments were conducted by taste stimulation of the labellum | Fed flies show taste aversion to acetic acid, whereas starved flies show a robust appetitive response. These opposing responses are mediated by two different classes of taste neurons, the sugar- and bitter-sensing neurons. |
| Sex = mated all females | Taste stimuli | |||
| Groups = Gr64f-Gal4; Gr66a-Gal4; ppk28-Gal4; Gr98d-Gal4, Gr22f-Gal4, Gr59c-Gal4, and Gr47a-Gal4; UAS-Kir2.1; UAS-GCaMP6f, UAS-norpARNAi,, poxnΔM22-B5 and poxn ΔM22-B5+ SuperA rescue; Δ8Grs (R1, ΔGr5a; ΔGr61a, ΔGr64a-f) and Δ8Grs with transgenes for GCaMP imaging (R1, ΔGr5a; Gr61a-Gal4, UAS-GCaMP6m; ΔGr61a, ΔGr64a-f); IR25a1 and IR25a2; IR76b1 and IR76b2 | Surgery was performed prior to starvation, and after surgery flies were given∼30 min to recover in food vials before starvation | |||
| Calcium imaging | ||||
| Djeziri et al. (96), 2018, France | N = 6 (per group) C57B1/6J mice | Age = 6–10 weeks | Two-bottle preference test | HFD mice showed decreased CD36 expression. OLA-treated obese mice showed increased CD36 mRNA in TBC. They exhibited higher preference for fat and more sensitive orosensory detection of OLA. Oleic acid triggered an increase in intracellular calcium in mTBCs. Oleic acid-induced increases in Ca2+ were abolished completely in the presence of SSO, a selective CD36 inhibitor. |
| Sex = all female | Two-bottle preference test | |||
| Groups: WT (n = 6), HFD (n = 6), HFD + OLA (n = 6) | Blood glucose tolerance test | |||
| Plasma LPS, insulin, liver lipids analyses | ||||
| Fatty acid analysis | ||||
| Measurement of Ca2+ signaling | ||||
| Isolation of mTBCs | ||||
| Espitia-Bautista and Escobar (97), 2019, Mexico | N = 80 (10 per group) Wistar rats | Age = not specified | Assessment of binge-type eating, food anticipatory activity, and effort behavior to obtain the diet. | After an acute exposition, rats ate more SRD than FRD, but FDR stimulated higher c-Fos. After chronic administration, the FDR group exhibited higher levels of BTE and FAA; this was associated with higher c-Fos and accumulation of ΔFosB in the corticolimbic system. |
| Sex = all male | Immunohistochemistry | |||
| Groups = (n = 10) | ||||
| Experiment 1: sugar-rich diet (SRD) and fat-rich diet (FRD) 8 groups: SRD = 10%, 25%, 50%, 75% FRD = 10% 25%, 50%, 75% | ||||
| Experiment 2: 3 groups: chow; 50% SRD, 50% FRD rats | ||||
| Experiment 3: groups: chow, Daily 1 h restricted access to 50% SRD, or to 50% FRD | ||||
| Experiment 4: 3 groups: chow; 50% SRD; 50% FRD | ||||
| Fardone et al. (98), 2019, USA | N = 72 M72-IRES-tauGFP mice with mixed agouti/C57BL6/J | Age = not specified | Odor (Olfr160 ligand) exposure | Neuronal excitability of juxtaglomerular (JG) cells is significantly reduced in moderate HFD (MHF) and HFD mice when stimulated by a preferred odorant, whereas control animals showed normal activation. This is mainly seen in interneurons surrounding the lateral but not medial glomerulus. Diet-induced obesity (DIO) causes deleterious effects on OSN survival that extends to a reduced neuronal activity of JG cells surrounding the genetically identified glomerulus for that class of ORs. |
| Sex = all male | c-Fos immediate-early gene expression for neuronal activity mapping | |||
| Groups = control food (n = 24), moderately high-fat diet (n = 19), high-fat diet (n = 29) | IPGTT | |||
| Gaudet et al. (99), 2019, USA | N = 39 Sprague-Dawley rats | Age = 8–10 weeks | Comparing expression levels of taste-related genes between standard chow diet vs. continuous, daily, and intermittent HFD access | Expression levels of the fat taste-sensing markers, CD36, SERT, and TPH2 mRNA in the circumvallate papillae were higher in the continuous HFD group. |
| Sex = all male | ||||
| Groups = chow diet (n = 14), continuous HFD (n = 9), daily (n = 8), intermittent (n = 8) | ||||
| Olvera Hernández et al. (100), 2021, France | N = 48 Wistar rats | Age = 3 months | Evaluation of preferences for fatty and sugary foods | Adult males and females born to undernourished dams exhibited increased expression of Cd36, Trpm5, Plc-b2 in the hypothalamus. The severity was greater in females. Only males from undernourished dams consumed more standard and sweetened food and had higher AgRP NPY in hypothalamus and increased dopamine transporter and dopamine receptor d2 in VTA. |
| Sex = both: female (n = 24) and male (n = 24) | Tissue collection from the tongue, nucleus accumbens, and ventral tegmental area (VTA) in the brain | |||
| Iskhakov et al. (101), 2019, USA | N = 30 inbred BALB/c, C57BL/6 and SWR mice | Age = 6 weeks | Scopolamine injections and comparing intralipid intake | Scopolamine (muscarinic receptor antagonist) reduced fat intake in all 3 strains and eliminated the ability to learn fat-CFP in the 3 strains. Therefore, muscarinic receptor signaling mediates learning and to a lesser degree maintenance of fat-CFP while maximally inhibiting fat intake in the 3 strains. |
| Sex = all male | Fat-conditioned flavor preference (CFP) tests | |||
| Groups = (n = 10 per group) BALB/c, C57BL/6, and SWR | ||||
| Jung et al. (102), 2018, Korea | N = 20 Canton-S wild-type Drosophila melanogaster | Age = adult flies | Life span assays | A HFD reduced DmOrco gene expression by 70% in olfactory neurons and decreased olfactory sensitivity to short-chain fatty-acids. This suggests HFD leads to olfactory dysfunction in homeostatic processing in Drosophila. |
| Sex = all male | Climbing assays | |||
| Groups = (n = 20 per vial on HFD and SD) | Odor stimulation | |||
| Behavioral assay | ||||
| Electrophysiological readings | ||||
| Khan et al. (103), 2017, France | N = 7 per group C57BL/6J WT and Erk1-/- mice | Age = <9 weeks | Comparing standard diet vs. HFD | Erk1-/- exhibited a low preference for dietary fatty acids and developed obesity. They also showed higher phosphorylation of MEK, an upstream regulator of ERK1/2 and exhibited high ERK2 phosphorylation, high lipogenesis, and low fatty acid oxidation. Overall, ERK1 and ERK2 have different but key roles in obesity. |
| Sex = all male | OGTT | |||
| Groups = (n = 7 per group) WT-normal diet (ND), Erk1-/- ND, WT-HFD, Erk1-/- HFD | Lipid analysis | |||
| Western blots | ||||
| mRNA RT-qPCR | ||||
| Kim et al. (104), 2018, Korea | N = 3–25 Drosophila melanogaster per trial group | Age = 3–5 days old | CRISPR/Cas9 Gr64 cluster deletion | Gr64e, a gustatory receptor, is required for the behavioral and electrophysiological responses to fatty acid detection. It functions as an ion-gated ligand channel for glycerol detection and acts downstream of phospholipase C signaling. |
| Sex = both: mated males and females | Proboscis extension reflex assay | |||
| Groups = multiple Gr64e mutant groups | ||||
| Kraft et al. (105), 2017, USA | N = 35 BALB/c and SWR mice | Age = 6 weeks old | Two-bottle conditioned stimuli (CS) choice test | Preference response for flavor associated with higher intralipid content was eliminated in mice treated with 100ug/kg MK-801. Therefore, NMDA receptor signaling must be needed in the formation of major triggers toward fat preference learning. |
| Sex = all male | Systemic NMDA antagonist (MK-801) injections | |||
| Groups = vehicle control (BALB/c, n = 8; SWR, n = 9) and MK-801 (BALB/c, n = 9; SWR, n = 9) | ||||
| Lacroix et al. (106), 2015, France | N = 19 OR and OP Sprague-Dawley rats | Age = 4 weeks old | Weight recorded | In OP rats, 1) decreased odor threshold, but 2) poor olfactory performances, associated with learning/memory deficits, 3) decreased influence of fasting, and 4) impaired insulin control on food-seeking behavior were reported. Modulation of metabolism-related factors implicated in 1) electrical olfactory signal regulation (insulin receptor), 2) cellular dynamics (glucocorticoids receptors, pro- and antiapoptotic factors), and 3) homeostasis of the olfactory mucosa and bulb (monocarboxylate and glucose transporters). |
| Food intakes were recorded during the diurnal and nocturnal phases of the day | ||||
| Insulin tolerance test | ||||
| Sex = all male | Concentrations of glucose, triglycerides, insulin, and leptin were measured | |||
| Tea-ball (odor) test | ||||
| Conditioned odor aversion test | ||||
| Groups = OR (n = 9) and OP (n = 10) | Hidden cookie test | |||
| Western blot analysis | ||||
| Quantitative real-time RT-PCR | ||||
| Lee et al. (107), 2015, Japan | N = (not specified) C57BL6/J WT and CD36-knockout mice | Age = 8–12 weeks old | Two-bottle choice test | WT mice avoided solutions with KOdiA-PC (CD36 ligand), an irritant phospholipid species CD36. Knockout effects are only seen at low levels of KOdiA-PC, suggesting that CD36 contributes to lipid recognition, but may not be the sole receptor. Mice that had olfactory nerve transected could not perceive KOdiA-PC. This implies that CD36 may operate in a nasal capacity and contribute to olfactory lipid detection. |
| Sex = not specified | Licking test | |||
| Groups = control, CD36 knockout; surgical control, olfactory nerve transected | Olfactory nerve transection | |||
| Lee et al. (108), 2017, Japan | N = 18 C57BL/6J and CD36 knockout mice | Age = 8–12 weeks | Two-bottle choice test | WT mice discriminated a sucrose solution with oleic aldehyde from sucrose solution alone in the two-bottle choice test. CD36 knockout mice did not discriminate the differences in solutions and fed on both bottles equally. WT mice also exhibited increased exploratory behavior (including sniffing) for an oleic aldehyde vehicle compared to the control, while CD36 knockout mice did not. These behavioral tests display the role of CD36 in fat taste and olfaction. |
| Sex = all female | Exploration test to assess sniffing behavior | |||
| Groups = WT (n = 8) and CD36 knockout (n = 10) | ||||
| Liu et al. (109), 2021, USA | N = 5–10 mice for nerve analysis, 16 mice for CTA assays | Age = 2–6 months | Calcium imaging | GPR84 mRNA expression was found in mouse fungiform and CV papillae. MCFAs were found to activate mouse TBCs via increases in intracellular calcium concentration. Gpr84−/− mice also exhibited significantly reduced taste nerve response to MCFAs and reduced taste responsiveness in the controlled taste aversion assay compared to WT. GPR84 is therefore implicated in the detection of MCFAs. |
| Sex = all male | CT nerve recording | |||
| Groups = WT and Gpr84−/− | Conditioned taste aversion assay | |||
| Makarova et al. (110), 2021, Russia | N = 37 C57Bl/6J diet-induced obese mice 24 mice in experimental groups | Age = 12–26 weeks | Administration of HFD to induce obesity | In females, they found that FGF21 administration reduced the preference for fatty food. However, food intake was not significantly different. |
| Sex = both: females (n = 10) and males (n = 14) | Injection of PBS control or FGF21 | |||
| Groups = control PBS (n = 12) and FGF21 (n = 12) | Real-time PCR | |||
| Mathes et al. (111), 2015, USA | N = 40 (2 groups of 20) Sprague-Dawley rats | Age = 2 weeks apart. First set: 2 months and second set: 1.5 months of age | Progressive ratio (PR) behavioral task | When tested before surgery while nondeprived, HFD rats had lower PR breakpoints (number of operant responses in the last reinforced ratio) for sucrose, but not for Ensure, than CHOW rats. After surgery, at no time did rats given RYGB show lower breakpoints than SHAM rats for Ensure, sucrose, or when 5% Intralipid served postoperatively as the reinforcer. |
| Surgery and recovery procedure | ||||
| Sex = all male | Two-bottle preference test | |||
| Food-deprived testing | ||||
| Groups = chow group (average body weight = 262 g) and HFD group (average initial body weight = 225 g) | Kruskal-Wallis tests | |||
| Friedman tests | ||||
| Murtaza et al. (112), 2017, France | N = 20 C57B1/6J mice | Age = 12 weeks | Zizyphin extraction and purification | Preference for LA solution was significantly increased when zizyphin was added to a LA solution (compared to LA alone), suggesting it is involved in modulating fatty acid perception. Zizyphin trigger opening of Ca2+ channels in hTBC. Zizyphin does not act on fatty acid receptors. |
| Sex = all male | Measurement of calcium signaling in human taste bud cells (hTBCs) | |||
| Groups: WT (n = 10), Gpbar1 -/- (n = 10) | Two-bottle preference test | |||
| Murtaza et al. (113), 2020, France | N = not specified wild-type C57BL/6J mice | Age = 2 months old | Isolation and culture of mTBCs | In cultured mouse and human TBCs, TUG891 induced a rapid increase in Ca2+ by acting on GPR120. LA, also recruited Ca2+ via GPR120 in human and mouse TBCs. Both TUG891 and LA induced ERK1/2 phosphorylation and enhanced in vitro release of glucagon-like peptide-1 from cultured human and mouse TBCs. Mice exhibited a spontaneous preference for solutions containing either TUG891 or LA instead of a control. However, addition of TUG891 to a solution containing LA significantly curtailed fatty acid preference. |
| Sex = all male | Ca2+ signaling measurement | |||
| Groups = one group received the test solution and the other a control solution | Western blot analysis | |||
| GLP-1 measurement | ||||
| ELISA | ||||
| Licking test | ||||
| Two-bottle test | ||||
| Murtaza et al. (114), 2021, France | N = 3–5 mice/condition | Age = 2 months | Measuring of Ca2+ signaling in TBC | TRPC3 was found to play a role in the orosensory detection of dietary lipids. Inactivation of TRPC3 in mTBCs caused a decreased in fatty acid induced Ca2+ signaling. Preference for a dietary LCFA was also abolished in TRPC3 KO mice. The same effect was seen in mice where TRPC3 was blocked via lingual application of an siRNA. |
| Sex = all male | Two-bottle preference test | |||
| Groups = WT C57BL/6J and TRPC3−/− | TRPC3 knockdown by siRNA | |||
| Ozdener et al. (115), 2014, USA | N = 20–40 cells per experiment/run human and C57BL/6J mice taste bud cells (TBCs) | Age = not applicable (isolated cells) | siRNA transfection | High concentrations of LA induced Ca2+ signaling via CD36 and GPR120 in human and mice TBC; low concentrations induced Ca2+ signaling via only CD36. Incubation of human and mice fungiform TBC with linoleic down-regulated CD36 and up-regulated GPR120 in membrane lipid rafts. Fungiform TBC from obese mice had reduced levels of CD36 and increased levels of GPR120 in lipid rafts. Therefore, CD36 is necessary for fat detection, while GPR120 only amplifies response of high concentrations of LA, acting downstream of long-chain fatty acid receptors. |
| Sex = not specified | Isolation of CD36−/− | |||
| Groups = CD36 and GPR120 siRNA-transfected human TBC, LA-human TBC, Grifolic acid-human TBC, CD36-/-, WT lean and WT obese TBC | Measurement of Ca2+ signaling in TBC | |||
| Serotonin and GLP1 secretion measurement | ||||
| Peterschmitt et al. (116), 2018, France | N = 6 mice per group | Age = 6–10 weeks old | Addition of LA to circumvallate papillae | LA induced a significant increase in c-Fos expression in the nucleus of the solitary tract (NTS), parabrachial nucleus (PBN), and ventroposterior medialis parvocellularis (VPMPC) of the thalamus, which are the regions known to be activated by gustatory signals. LA also triggered c-Fos expression in the central amygdala and VTA, involved in food reward, in conjunction with emotional traits. |
| Sex = all male | Immunocytochemical localization of c-Fos | |||
| Groups = lingual application of LA group vs. no application | mRNA expression of BDNF, Sif-268, and Glut-1 | |||
| Ricci et al. (117), 2018, Italy | N = 7–10 (per group) C57BL/6J Prep1i/+ mice | Age = 6 months | Macromorphological analysis of brain structural alterations | Prep1 deficiency alters olfactory morpho-functional integrity and olfaction-mediated eating behavior by affecting BDNF-TrkB signaling. Prep1 could play an important role in behavioral dysfunction associated with responsiveness to BNDF. |
| Sex = all male | Hemalum and COX Staining | |||
| Groups = macromorphological analysis and COX staining- C57BL/6J (n = 7) Prep1i/+ (n = 7) for behavioral-C57BL/6J (n = 9) and Prep1i/+ (n = 9) | Immunofluorescence | |||
| Behavioral (open field, olfactory preference, food preference) | ||||
| Western blotting | ||||
| Real-time (RT-PCR) | ||||
| Cell viability assay | ||||
| Sakamoto et al. (118), 2015, Japan | N = 8–12 (per group) BALB/c mice | Age = 8 weeks old | Two-bottle choice test | The opioid system seems to have a greater role in determining the palatability of high-fat foods unlike the contribution of olfactory and glossopharyngeal nerves. |
| Sex = all male | Olfactory nerve transection (ONX) | |||
| Groups = water vs. intralipid (n = 12), water vs. intralipid +/- naltrexone (0.5 or 2 mg/kg, n = 8 Sham vs. ONX (n = 8) Sham vs. GLX (n = 8) ONX and GLX +/- naltrexone (n = 12) | Glossopharyngeal nerve transection (GLX) | |||
| Operant lever-press paradigm: progressive (PR) schedule | ||||
| Sakamoto et al. (119), 2015, Japan | N = 7–10 (per group) BALB/c mice | Age = 8 week old | Two-bottle choice test | In mice, preference of fat relies strongly on the opioid system, while that of sucrose is regulated by other mechanisms in addition to the opioid system. |
| Preference between sucrose and intralipids in naive mice + opioid receptor antagonists | ||||
| Sex = all male | Preference between sucrose and intralipids following naltrexone | |||
| Preference between sucrose and intralipids following food deprivation in naive mice | ||||
| Groups = saline, naloxanazine, naltrindole, Nor-BNI (n = 8) saline vs. naltrexone (n = 8) food deprivation (n = 7) Saline vs. naltrexone compared to water in naïve (n = 8) Saline vs. naltrexone in licking behavior (n = 10) | Preference between sucrose and intralipids compared to water in naive mice | |||
| Licking behavior for sucrose and intralipids in naive mice | ||||
| Sasaki et al. (120), 2017, Japan | N = 3–10 (per group) C57BL/6J | Age = 8–10 weeks old; except for in CTA experiment mice were 12–14 weeks | d-serine IP injection effect on HFD consumption | IP-injected d-serine inhibited HFD intake and acquisition of an HFD preference. Individual mice with the same genetic background showed different sensitivities to d-serine; thus d-serine sensitivity may be associated with unidentified traits. |
| Sex = all male | d-serine IP injection effect on CTA | |||
| Groups = HFD vs. NC + saline or IP d-serine day 0 (n = 6), day 1 (n = 7), day 2 (n = 8), and day 4 (n = 7) IP d-serine (n = 9) vs. LiCl (n = 8) brain d-serine (n = 3), and L-serine (n = 3) post-IP D-serine IP D-serine vs. VEH (n = 6/group) IP D-serine + water or lipid emulsion (n = 6/group) | d-serine and l-serine levels pre- and post-IP d-serine | |||
| Single IP d-serine injection effect on HFD preference effect of intraperitoneally injected d-serine under the single-food access paradigm using liquid meals (water or lipid emulsion) | ||||
| Schreiber et al. (121), 2020, USA | N = 4–10 (per group) OP and OR S5B/PI rats | Age = 8–9 weeks old | Transection of glossopharyngeal nerves | OR rats had a higher fungiform papillae density than OP rats. Transection of glossopharyngeal nerves decreased HFD intake in OR rats and had no change in OP rats. |
| Sex = all male | Comparing the effect of GLX/CTX on quinine intake | |||
| Groups = fungiform papillae assessment: OP (n = 6), OR (n = 5) | Comparing the effect of GLX/CTX on HFD and LFD intake | |||
| Effect of GLX/CTX on quinine intake: OR-Sham (n = 5), OR-GLX/CTX (n = 5), OP-Sham (n = 4), OP-GLX/CTX (n = 4) | ||||
| Effect of GLX/CTX on HFD and LFD intake: OR-SHAM-LFD (n = 10), OR-GLX/CTX-LFD (n = 8), OR-SHAM-HFD (n = 11), OR-GLX/CTX-HFD (n = 10), OP-SHAM-LFD (n = 11), OP-GLX/CTX-LFD (n = 8), OP-SHAM-HFD (N = 10), OP-GLX/CTX-HFD (n = 9) | ||||
| Sclafani and Ackroff (122), 2014, USA | N = 11 (per group) P2X2/P2X3 double knockout mice (P2X DoKO) | Age = 11–14 weeks old | Utilize P2X DoKO to examine maltodextrin preference for polycose solutions (vs. water) | Preference of mice for maltodextrin and fat are dependent on adenosine triphosphate taste cell signaling. With experience, however, P2X DoKO mice develop strong preferences for the nontaste flavor qualities of maltodextrin and fat conditioned by postoral actions of these nutrients. |
| Sex = all male | Utilizes P2X DoKO to examine fat preference to intralipid (vs. water) | |||
| Group = P2X DoKO (n = 11), WT (n = 11) | ||||
| Sclafani et al. (123), 2015, USA | N = 12 per group GPR40/120 double knockout and C57BL/6J wild-type mice | Age = 15 weeks old | Utilizes DoKO GPR40/120 to examine if GPR40 and GPR120 play a role in intralipid and glucose preference | Postoral GPR40/120 signaling is not required to process IG fat infusions in food-baited spout training sessions but contributes to postoral fat reinforcement in empty spout tests and flavor conditioning tests. |
| Sex = all male | ||||
| Group = GPR40/120 double knock out (n = 12), C57BL/6J WT (n = 12) | ||||
| Sclafani et al. (123), 2015, USA | N = WT C57BL/6J and GHSR null mice | Age = adult-age not specified | Utilizes GHSR-null mice | Ghrelin receptor signaling is not required for flavor preferences conditioned by the oral or postoral action of sugar and fat. |
| Sex = both: | ||||
| Exp1: 9 females, 10 males | Flavor conditioning | |||
| Exp2: male | ||||
| Exp3: 9 female, 13 males | Intragastric feeding | |||
| Exp4–9: female, 13 males | ||||
| Exp5–11: female, 11 males | ||||
| Exp6–12: female, 12 males | ||||
| Groups: | ||||
| Exp1: GHRS-null vs. WT+/− CS+/CS- | ||||
| Exp2: CS+/glucose, CS+ S + S, CS-/S + S | ||||
| Exp3: GHRS-null mice vs. WT+ CS+/CS- +GHSR antagonist | ||||
| Exp4: GHRS-null mice vs. WT+ CS+/CS- +IG intralipid/IG water | ||||
| Exp5: food-deprived GHRS-null mice vs. WT+ CS+/CS- +IG intralipid/IG water Exp6- GHRS-null mice vs. WT | ||||
| Sclafani and Ackroff (124), 2018, USA | N = CAST/EiJ (n = 10) and C57BL/6J mice (n = 10) | Age = 9 weeks old | Compare the preferences of CAST and B6 mice for fat vs. sugar and maltodextrin vs. water in 2-day choice tests | CAST/EiJ mice strongly prefer fat to isocaloric carbohydrate (sucrose, maltodextrin). C57BL/6 J mice show the opposite preference profile. CAST/EiJ mice show weaker fat preferences in fat vs. water tests compared to C57BL/6 J. CAST/EiJ mice, like C57BL/6 J mice strongly prefer sweetened fat to maltodextrin. Taste rather than postoral factors is implicated in the low-fat preferences of CAST/EiJ mice. |
| Sex = all male | ||||
| Groups = CAST & B6 | ||||
| Sclafani and Ackroff (125), 2018, USA | N = not specified CD36 KO mice | Age = 11 weeks | Use of CALHM1 knockout (KO) to evaluate the primary role of CD36 as a taste receptor mediating fat preference | Naïve CD36 KO mice displayed reduced preferences for soybean oil emulsions (intralipid) at low concentrations (0.1–1%). CALHM1 KO mice displayed even greater Intralipid preference deficits compared with WT and CD36 KO mice. This suggests there may be other taste receptors other than CD36 (but also through CALHM1). After experience with concentrated fat (2.5–5%), CD36 KO and CALHM1 KO mice displayed normal preferences for 0.1–5% fat, the experience-induced rescue of fat preferences in KO mice can be attributed to postoral fat conditioning. |
| Sex = all male | ||||
| Groups = CAST & B6 | ||||
| Subramaniam et al. (126), 2016, France | N = 164 C57B1/6J mice, X Erk-1-/- C57B1/6J mice, X calhm1-/- C57B1/6J mice | Age = 22.2 ± 1.8 | Measurement of calcium signaling | Fat preference is decreased by downregulation of ERK1/2. LA induces MAPK activation in hTBCs. Src-kinases and raft integrity are involved in LA-induced ERK1/2 activation in hTBCs. LA induces ERK1/2 phosphorylation via CD36 in hTBCs. CALHM1 channels are upstream regulators of LA-induced ERK1/2 phosphorylation. LA-induced Ca2+ signaling and ERK1/2 phosphorylation are impaired in Calhm1−/− TBCs. Preference for fat is abolished in Calhm1−/− mice. |
| Sex = both: male (n = 2), female (n = 17) | Lipid raft isolation | |||
| Groups = orosensory detection of LA | Licking and 2-bottle preference tests | |||
| Tauber et al. (127), 2017, USA | N = 9–49 Drosophila flies per experiment | Age = 7–9 days old | Measured proboscis extension reflex | Neurons expressing IR56d are necessary and sufficient for reflexive feeding response to FAs in Drosophila. IR56d/Gr64f neurons are activated by medium-chain FAs and are sufficient for reflexive feeding response to FAs. Flies can discriminate between sugar and FAs in an aversive taste memory assay, indicating that FA taste is a unique modality in Drosophila. |
| Sex = all female | Uses Ca2+ sensor GCAMPS under control of, e.g., Gr64g-GAL4, IR56d-GAL4, etc. | |||
| Groups = not specified | Selective silencing and expression | |||
| Examination of neuronal activity | ||||
| Tsuzuki et al. (128), 2016, Japan | N = not specified | Sex = not specified | oxLDL-CD36 binding/inhibition assays | Z,Z-TTD is an odor-active fatty aldehyde that is recognized by CD36 in the (nose). Aldehyde functional groups on fatty acid chains are especially important for CD36 lipid recognition. This suggests that CD36 in the mucus layer of olfactory tissue binds to specific lipids and relays them to olfactory receptors. |
| Groups = control and CD36 peptide residues | Fluorescence measurement | |||
| Weiss et al. (129), 2019, USA | N = 78 male Sprague-Dawley rats | Age = 8 weeks | Body composition monitoring | A high-energy diet produces blunted, but more prevalent, responses in the nucleus of the solitary tract (NTS), and weaker association of taste responses with ingestive behavior. |
| Sex = all male | Taste stimuli and odor stimuli testing | |||
| Groups = diet-induced obese (n = 39 ); lean (n = 39) | Electrophysiology testing in operant chamber | |||
| Wu et al. (130), 2017, Korea | N = xC57BL/6J WT, ob/ob, and Olf544−/− mice | Age = 8 weeks old | Microarray + qPCR of olfactory receptors in liver and adipose tissue | Azelaic acid (a FA) is a ligand of Olfactory receptor 544 (Olfr544). Olfr544 orchestrates the metabolic interplay between the liver and adipose tissue, mobilizing stored fats from adipose tissue and shifting the fuel preference to fats in the liver and BAT. |
| CRISPR/Cas9 to generate Olfr544-/- | ||||
| Sex = all male | Administration of 60% HFD + 50 mg/kg of Olfr544 agonist AzA | |||
| OGTT + ITT | ||||
| Groups = HFD + azelaic acid (AzA), Olf544−/−, and ob/ob | Micro-CT for adipocyte tissue measures | |||
| Histological analysis of BAT | ||||
| Quantification of hepatic triglycerides | ||||
| Indirect calorimetry | ||||
| Xavier et al. (131), 2016, Brazil | N = 6 Cd36obl/Mmucd mice, n = 6 Olfr17tm7Mom MomJ mice, and n = 4 C57BL/6JB WT mice | Age = 4 weeks old | RT-PCR for Cd36 transcripts in olfactory epithelium | The Cd36 receptor is highly expressed in a small subset of mature olfactory sensory neurons in the main olfactory epithelium. In addition, the main olfactory epithelium expresses distinct populations of sensory neurons, typically dependent on the receptor they express. Cd36-deficient mice show normal general olfactory behaviors but do not show a preference for a lipid-odor mixture compared to WT mice. |
| Sex = all male | In situ hybridization and immunofluorescence staining to determine which cells Cd36 is present in | |||
| Groups = Cd36obl/Mmucd and wild-type C57BL/6J and Olfr17tm7Mom/MomJ (P2-IRES-tauGFP) mice | Behavioral assays to study olfactory behavior upon lipid exposure | |||
| Yasumatsu et al. (132), 2018, Japan | N = 7–9 C57BL/6JB WT and GPR120-KO mice per group | Age = 8–20 weeks | Single-fiber nerve response recordings from CT nerve | Pharmaceutical blockade of GPR120 caused suppression of CT nerve responses to fatty acids in WT mice. GPR120-KO mice also exhibited a higher threshold for LA detection than WT mice. |
| Sex = all male | CTA experiments on WT and GPR120-KO mice | |||
| Groups = WT and GPR120-KO | Chemical stimulation of the tongue with GPR120 antagonist |
WT, wild type; HFD, high-fat diet; CS, conditioned stimuli; OLA, oleic acid; TBC/LPS, taste bud cells/mouse tas; lipopolysaccharide; CV, circumvallate; LA, linoleic acid; IP, intraperitoneal; CFP, conditioned flavor preference; RD, regular diet; NMDA, N-Methyl-d-aspartate; RT-qPCR, quantitative polymerase chain reaction/reverse transcription polymerase chain reaction; PER, proboscis extension reflex; IPGTT, intraperitoneal glucose tolerance test; OGTT, oral glucose tolerance test; CRISPR, clustered regularly interspaced short palindromic repeats; CS, conditioned stimulus; GLP-1, glucagon-like peptide 1; ELISA, enzyme-linked immunosorbent assay; FGF21, fibroblast growth factor 21; BDNF, brain-derived neurotrophic factor; OP, obesity prone; OR, obesity resistant; COX, cytochrome c oxidase; OSNs, olfactory sensory neurons; ONX, olfactory nerve transection; GLX, glossopharyngeal nerve transection; GHSR, growth hormone secretagogue receptor; OGTT, oral glucose tolerance test; ITT, insulin tolerance test; CT, computed tomography; BAT, Bayesian analysis toolkit; TRPC, transient receptor potential canonical; CD36, cluster of differentiation 36.
2.5. Risk of Bias Assessment
To assess the risk of bias in the clinical studies, we used three JBI (formerly known as Joanna Briggs Institute) Critical Appraisal Tools: Checklist for Analytical Cross-Sectional Studies (8 questions) (133); the Checklist for Randomized Controlled Trials (13 questions) (134); and the Checklist for Cohort Studies (11 questions) (135). All JBI checklists included questions and guided instructions to systematically assess each study based on multiple criteria (e.g., reliability and validity). To assess preclinical animal intervention studies, we utilized the SYstematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk of bias tool (RoB) (10 questions) (136). The checklists and scoring we used are included as supplementary materials (Supplemental File S2–3).
To quantitatively score each JBI and SYRCLE RoB checklist, each of the questions received a score between zero and two; zero (cannot tell), one (no), and two (yes). Total possible scores for the Checklist for Analytical Cross-Sectional Studies could range from 0 to 16; 0 to 26 for the Checklist for Randomized Controlled Trials; 0 to 22 for the Checklist for Cohort Studies; and 0 to 20 for the SYRCLE RoB. Higher scores for each checklist indicated a lower risk for bias.
A sample of four articles was used by all reviewers (R.B.J.-L., C.V., B.E.B., R.S.E.O-F., M.C., N.N., and P.V.J.) participating to pilot test the selected checklist tools and discuss problems and questions with the tools and risk of bias assessment step.
Two reviewers independently assessed the risk of bias for each included study, and a third reviewer checked the completed checklists for discrepancies. Any errors or discrepancies were resolved by discussion between the two reviewers who completed the checklist and the third reviewer until a consensus was reached for the specific question.
Studies were not excluded from the analyses based on their risk of bias score. Any uncertainty about a study’s risk of bias was discussed as a group.
2.6. Effect Measures and Synthesis Methods
The effect measures collected are shown in TABLE 1. The age, sex, and body mass index (BMI) were collected as reported in the article. If it was possible to calculate the mean for age and BMI only based on the data provided in the article, this was done. Descriptive statistics were calculated, and a narrative synthesis of the findings was completed.
2.7. Certainty Assessment
No assessment of the overall certainty or confidence in the included studies was completed as we included both human clinical studies and animal studies, which were not comparable, and the animal studies overall had a very low risk of bias scores.
3. RESULTS
3.1. Selected Studies
The initial search resulted in a total of 4,059 articles retrieved and manual searching of reference lists of included studies resulted in 7 additional articles (FIGURE 5). After duplicates were removed (n = 1,484), the remaining 2,582 article titles and abstracts were assessed for relevance, and studies that did not meet inclusion criteria were excluded. Studies that did not discuss biological mediators of fat taste or smell were excluded (n = 2,189). Of the 393 remaining articles, 304 were excluded after screening the full text. Studies not included due to the exclusion criteria are summarized in FIGURE 5. After each study was assessed using exclusion criteria, 89 articles were included in this systematic review. The heterogeneity and diverse methods between the studies prevented the conduct of a meta-analysis.
FIGURE 5.
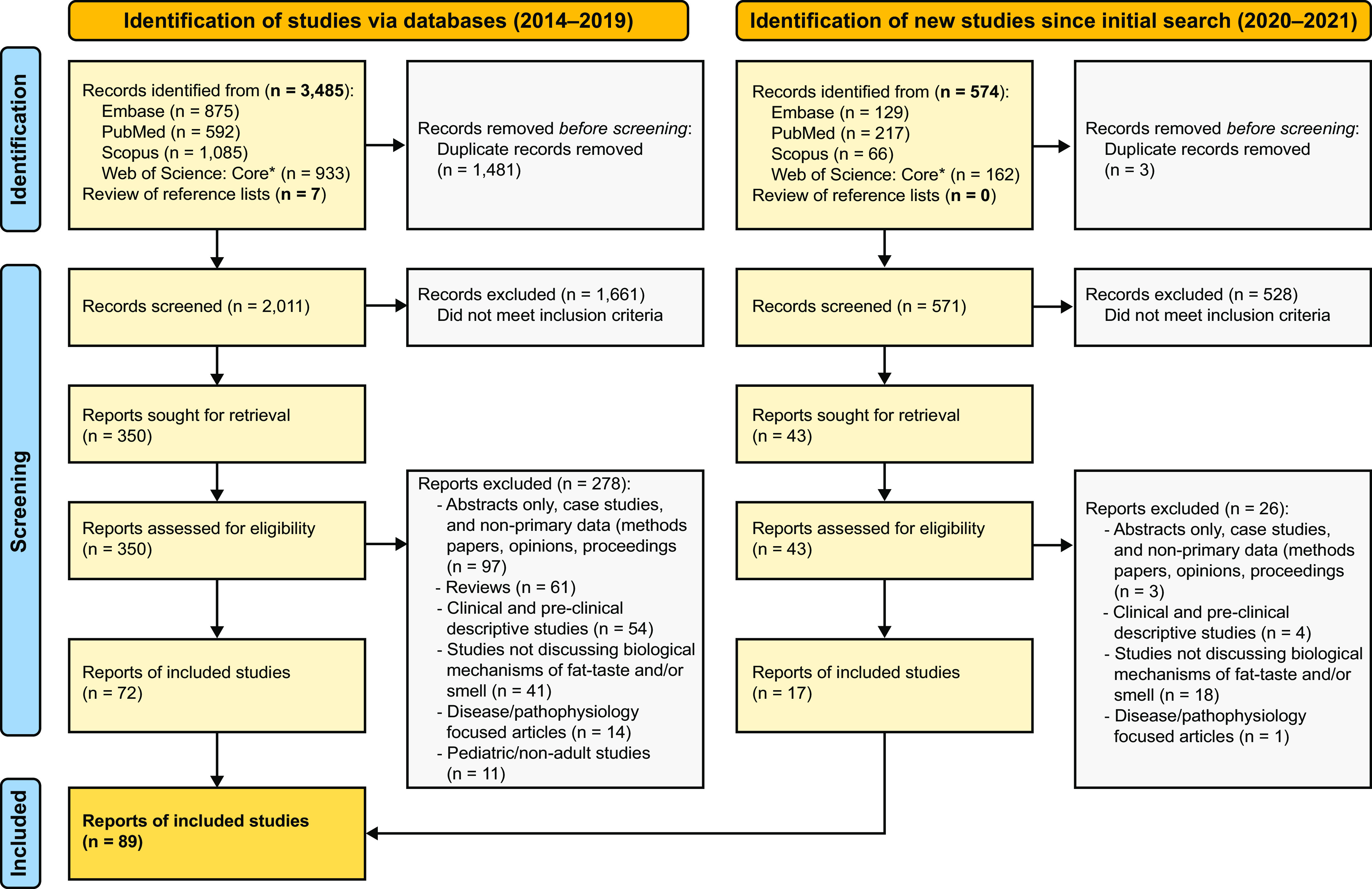
PRISMA flow diagram. The diagram shows the selection of reports included in this systematic review.
3.2. Study Characteristics
This review included 37 clinical (TABLE 1) and 52 preclinical (TABLE 2) studies. Clinical studies had sample sizes ranging from 10 to 736 participants. 83.78% of clinical studies included both male and female participants. However, three studies included only females and three included only males. Study designs encompassed: randomized control, cross-sectional, crossover design, case-control, and cohort (prospective observational) studies. Clinical study methods included: psychometric tests such as taste preference [Alternative-Forced Choice (AFC)], detection threshold [e.g., general Label Magnitude Scale (gLMS)], and nutritional assessments [e.g., Food Frequency Questionnaire (FFQ)]. Articles in this review were set in 22 countries and the United Kingdom. Clinical studies were conducted in Australia (n = 4), Canada (n = 1), Czech Republic (n = 1), Denmark (n = 1), Finland (n = 1), France (n = 3), Germany (n = 3), Italy (n = 4), Japan (n = 1), India (n = 1), Malaysia (n = 1), Mexico (n = 1), Morocco (n = 1), Poland (n = 1), Spain (n = 1), Tunisia (n = 2), United Kingdom (n = 5), and the United States (n = 5). Preclinical studies were conducted in Belgium (n = 1), Brazil (n = 1), Chile (n = 1), France (n = 11), Italy (n = 1), Japan (n = 7), Korea (n = 3), Mexico (n = 1), Russia (n = 1), and the United States (n = 25).
Seven preclinical studies included both sexes, 35 were conducted in males, 6 in females, and 4 were not specified. Study characteristics are summarized in TABLE 2. Preclinical studies were conducted on animal and in vitro models, including Mus musculus (mice; n = 36), Rattus sp. (rats; n = 9), Drosophila melanogaster (fruit flies; n = 6), and Homo sapiens (human cells; n = 1). The preclinical study sample size varied by group and total sample size. TABLE 2 summarizes group and total sample size for each study.
3.3. Risk of Bias Assessment
Total score for the JBI Checklist for Analytical Cross-Sectional Studies ranged from 9 to 16 (n = 29) (highest score = 16); 11 to 19 for the Checklist for Randomized Controlled Trials (n = 5) (highest score = 26); 10 to 20 for the Checklist for Cohort Studies (n = 3) (highest score = 20); and 1 to 15 for the SYRCLE RoB (n = 52) (highest score = 20). See Supplemental File S3 for a final risk of bias scores for the clinical and preclinical/animal included studies.
4. DISCUSSION
Studies included in this systematic review demonstrate advances in understanding the biological mediators of fat taste and smell. Studies focus on five themes: molecules and their biological pathways, neuroanatomical regions, ingestive cues, postingestive cues, and genetic variability associated with fat chemosensation. Finally, we will discuss the role of fat taste and smell in the context of eating behavior and obesity. In this section, we will discuss each theme. First, we will summarize and describe the molecules involved in fat taste and smell.
4.1. Molecules, Transduction Pathways, and Ingestive Cues Mediating Fat Taste and Smell
Taste and olfactory receptors play a key role in the detection of chemical stimuli in the environment, including fat and other taste and smell stimuli. Different taste modalities and olfactory receptors are important in initiating transduction pathways that ultimately relay information about the tastes and smells we perceive. There are three different types of taste cells, with each taste modality being characterized by a unique combination of taste receptors and transduction pathways (summarized in FIGURE 6). Multiple molecules have been implicated in fatty acid detection, including cluster of differentiation 36 (CD36) and G protein-coupled receptor (GPCR) 120 (GPR120). CD36 is one of the most studied proteins as it displays a high affinity for long-chain fatty acids. For reviews of CD36’s role in fatty acid detection, please see Degrace-Passily et al. (232) and Pepino et al. (141). GPCRs, including GPR120, were also proposed as potential fatty acid detector proteins. For a review that examines GPR120’s role in fatty acid detection, please see Besnard et al. (18). In this systematic review, we summarize emerging literature exploring CD36 and GPR120. We will also discuss other proteins that have been studied in the context of fat chemosensation, including Toll-like receptor 4 (TLR4), calcium homeostasis modulator 1 (CALHM1), Takeda-G-protein-receptor-5 (TGR5), gustatory receptor (GR), ionotropic receptor (IR) proteins, and beta-adrenergic receptor (ADRB3). Many of these proteins act in the TBCs after initial activation by CD36 and GPR120. The role of each protein in fat chemosensation is summarized in TABLE 3 and FIGURE 7.
FIGURE 6.
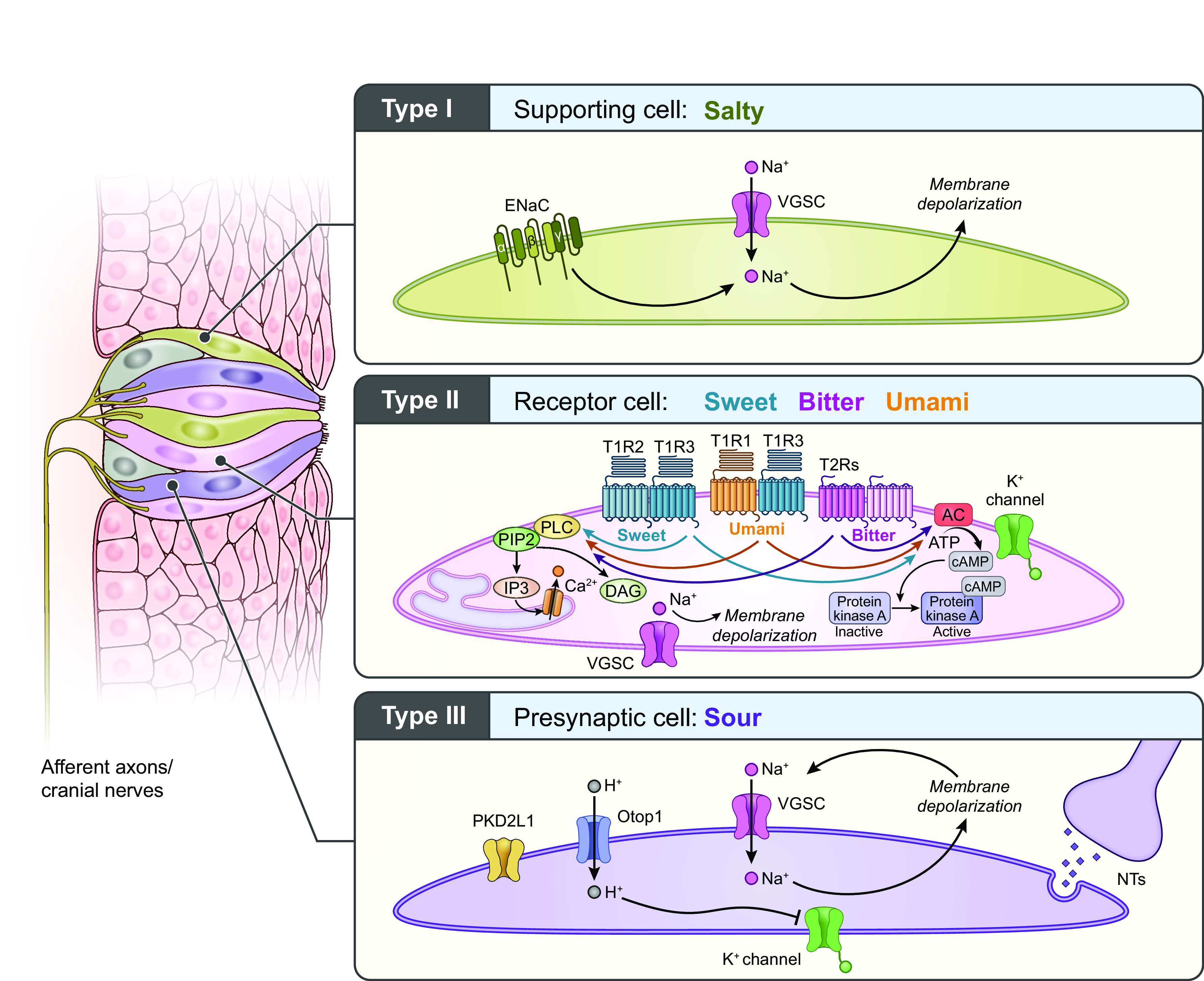
Taste transduction by taste modality. Taste buds contain three cell types specific to taste modalities: type I, type II, and type III cells. Type I cells are glial-like cells and are involved in salt detection. Type II cells are involved in sweet, bitter, and umami. Presynaptic type III cells detect sour stimuli and potentially salty stimuli. Each cell type and each taste modality are characterized by their own combination of receptors and transduction pathways. Taste information is then relayed to the brain via afferent nerves (cranial nerves VII, IX, and X). ENaC, epithelial sodium channel; IP3, inositol (1,4,5)-triphosphate; NTs, neurotransmitters; PIP2, phosphatidylinositol 4,5-bisphophate; VGSC, voltage-gated sodium channel. Figure adapted from Ref. 26, with permission from Springer Nature.
Table 3.
Proteins involved in fat chemosensation
| Protein | Study (Reference), Year | Findings |
|---|---|---|
| α-Gustducin | Avau et al. (86), 2015 | α-Gustducin is involved in fat intake and obesity. |
| Watanabe et al. (80), 2019 | ADRB3 is an essential mediator of fat perception and metabolism in the body. The Trp64Arg variant of this gene is associated with high-fat preference, indicating the structure of the adrenergic receptor protein may play a role in oral fat perception. | |
| ADRB3 | Sclafani et al. (124), 2018 | CALHM1 KO mice displayed even greater intralipid preference deficits compared with WT and CD36 KO mice. Suggesting that non-CD36 taste receptors also contribute to fat detection and preference. CALHM1 KOs can still develop normal preferences after multiple exposures (not in naïve) attributed to post-oral fat conditionin |
| CALHM1 | Subramaniam et al. (126), 2016 | CALHM1 channels are upstream regulators of LA-induced ERK1/2 phosphorylation. LA-induced Ca2+ signaling and ERK1/2 phosphorylation are impaired in Calhm1-/- TBCs. Preference for fat is abolished in Calhm1-/- mice. |
| Braymer et al. (89), 2017 | CD36 mRNA levels were increased in lean rats. | |
| Lingual application of CD36 siRNA decreased fat preference in lean, obesity-resistant rats. | ||
| CB1R | Avalos et al. (85), 2020 | CB1R knockout (KO) mice displayed an attenuated preference for HFD for the first 6 hours of a preference test compared to WT mice. |
| CD36 | Bricio-Barrios et al. (52), 2019 | BMI was associated with low serum CD36 and lower fat sensitivity. |
| Djeziri et al. (96), 2018 | CD36 inhibition prevented lipid-induced intracellular calcium increases. CD36 expression was decreased with HFD alone but increased with oleic acid. | |
| Gaudet et al. (99), 2019 | Continuous access to HFD increased lingual CD36 expression in rats. | |
| Lee et al. (107), 2015 | CD36 contributes to lipid recognition in mice. | |
| Lee et al. (108), 2017 | Wild-type, but not CD36-knockout mice, were able to detect oleic aldehyde, providing evidence for the involvement in CD36 in in the perception of dour-active volatile compounds in the nasal cavity. | |
| Ozdener et al. (115), 2014 | High concentrations of linoleic acid induced Ca2+ signaling via CD36 and GPR120 in human and mice TBC, as well as in STC-1 cells, and low concentrations induced Ca2+ signaling via only CD36. CD36 and GPR120 have nonoverlapping roles in TBC signaling during orogustatory perception of dietary lipids; these are differentially regulated by obesity. | |
| Sclafani et al. (124), 2018 | CD36 KO reduced preference for lipids (in naïve CD36 KO). | |
| Subramaniam et al. (126), 2016 | CD36 is involved in FA induction of ERK1/2 phosphorylation. LA induced phosphorylation of MEK1/2-ERK1/2ETS-like transcription factor-1 cascade via CD36 in human TBCs. | |
| Tsuzuki et al. (128), 2016 | CD36 may be expressed in the nasal cavity and binds to fatty aldehyde. Nasal CD36 can signal to olfactory neurons. | |
| Xavier et al. (131), 2016 | CD36-deficient mice did not demonstrate changes in the organization of the olfactory epithelium but showed impaired preference for a lipid mixture odor. CD36-expressing neurons represent a distinct population of OSNs, which may have specific functions in olfaction. | |
| FFAR4 (GPR120) | Costanzo et al. (144), 2019 | FFAR4 in fungiform papillae may play a role in fat perception. FFAR4 expression was positively associated with FAT sensitivity. Increases in FFAR4 may also increase intestinal satiety signals, leading to reduced further fat intake. |
| GPR120 | Ancel et al. (84), 2015 | GPR120 is not necessary for fat-taste detection. |
| Murtaza et al. (113), 2020 | Select GPR120 agonist can trigger intracellular Ca2+ increases, induce MAPK phosphorylation, and modulate fatty acid preference. Therefore, GPR120 is involved in fat-taste pathway. | |
| Ozdener et al. (115), 2014 | GPR120 is involved in amplifying transduction response. CD36 and GPR120 have nonoverlapping roles in TBC signaling during orogustatory perception of dietary lipids | |
| Sclafani et al. (123), 2015 | Post-oral GPR40/120 signaling is not required to process IG fat infusions in food-baited spout training. | |
| Yasumatsu et al. (132), 2018 | GPR120 antagonist caused suppressed CT nerve signaling and the reduction of maximal nerve responses in WT mice. | |
| GPR84 | Liu et al. (109), 2021 | GPR84 is involved in taste detection of MCFAs in TBCs by triggering intracellular Ca2+ increases and membrane depolarization. |
| Gr64e, Gr64f, IR56d | Kim et al. (104), 2018 | Gr64e is involved in fat chemosensation, but not direct receptor; another Gustatory receptor required for the behavioral and electrophysiological responses to FA detection. |
| Tauber et al. (127), 2017 | IR56d/Gr64f neurons are activated by medium-chain FAs and are necessary and sufficient for reflexive feeding response to FAs. | |
| Olfr544 | Wu et al. (130), 2017 | Olfr544 orchestrates the metabolic interplay between liver and adipose tissue, mobilizing stored fats from adipose tissue and shifting fat preference. |
| OR4D2, OR51A7, OR2T34, OR2Y1 | Ramos-Lopez et al. (75), 2019 | OR4D2, OR51A7, OR2T34, and OR2Y1, along with several downstream signaling molecules (SLC8A1, ANO2, PDE2A, CALML3, GNG7, CALML6, PRKG1, and CAMK2D) regulate odor detection and signal transduction processes within the complete olfactory cascade. |
| Prep1 | Ricci et al. (117), 2018 | Prep1 deficiency alters olfactory morphofunctional integrity and olfaction-mediated eating behavior. |
| P2X2/P3X3 | Bensalem et al. (87), 2020 | TGR5 KOs show changes in fat preference and calcium signaling |
| TGR5 | Camandola and Mattson (93), 2017 | TLR4 promotes fat ingestion (FA endocytosis) and fat taste preference |
| TRPC3 | Murtaza et al. (114), 2021 | TRPC3 KO mice TBCs showed significantly curtailed Ca2+ signaling in response to LA. |
BMI, body mass indes; HFD, high-fat diet; WT, wild type; CD36, cluster of differentiation 36; GPR120, G protein-coupled receptor 120; TLR4, Toll-loke receptor 6; FAs, fatty acids; LA, linoleic acid; TBCs, taste bud cells; MCFAs, medium-chain fatty acids.
FIGURE 7.
Fat Transduction signaling in taste bud cells. In taste bud cells: (1) free fatty acids (FFAs) binds to cluster of differentiation 36 (CD36), and CD36 interacts with GPR120. This triggers a signaling cascade involving α-gustducin which activates Ca2+, dependent phospholipase (PLC). (2) PLC cleaves phosphatidylinositol 4,5-bisphophate (PIP2; bound to the membrane) into diacylglycerol (DAG) (stays in membrane) and inositol (1,4,5)-triphosphate (IP3) (free in cytosol). DAG can phosphorylate protein kinase C (PKC), which helps to activate the extracellular signal-regulated kinases (ERK) pathway. (3) IP3 leaves the membrane and binds to IP3-gated calcium ion channels on the endoplasmic reticulum (ER) membrane. This opens the IP3 channels and releases Ca2+ stores from the ER into the cytosol, increasing intracellular calcium levels ([Ca2+]i). (4) The increased [Ca2+]i triggers several events that lead to membrane depolarization of the cell. (4a) TRPM5 channels (a Ca2+-gated sodium channel) open, allowing Na+ to come into the cell. Additionally, Delayed rectifying K+ (DRK) channels close, preventing potassium from leaving the cell. (4b) The calcium release from the ER requires that stromal interaction molecule 1 (STIM1) replenish the ER’s calcium stores. (4c) STIM1 activates Orai1 (calcium release-activated calcium channel) to enable the influx of calcium into the cytosol. Subsequent opening of calcium homeostasis modulator 1 (CALHM1) channels allows for additional calcium influx into the cell. (5) Increased [Ca2+]i and CD36-induced Fyn/Src kinase activation contribute to phosphorylation of the ERK1/2 pathway. This can activate cAMP signaling, which promotes transcription of cellular regulation factors. (6) Depolarization throughout the cell leads to the release of serotonin (5-HT) and ATP, which act as neurotransmitters. Serotonin binds to its receptors, and ATP binds to purinergic P2X2 and P2X3 receptors that cause neuronal excitability, further relaying fat chemosensory signals to the brain. CD36, cluster of differentiation 36; GPR120, G protein-coupled receptor 120. Image created with BioRender.com, with permission.
4.1.1. Proteins associated with fat detection: CD36, GPR120, and fat taste and smell.
4.1.1.1. cd36.
CD36 is a transmembrane protein with a high affinity for FFAs, such as linoleic acid (LA) and oleic acid (OLA) (43, 69, 70, 126, 137), and a hydrophobic region that acts as a binding for these ligands (138, 139). Extra-orally, CD36 is expressed in multiple tissues (e.g., the nasal epithelium, brain, and cardiovascular tissue) (131), and it plays a role in immune response, inflammation, and angiogenesis (140). In the mouth, CD36 is located on the apical surface of TBCs (43, 137) where it binds to FFAs. For example, a 2016 study by Subramaniam et al. (126) demonstrated that in human TBCs, LA binds to CD36, which activates downstream pathways. This triggers GPCR-dependent secondary signaling cascades that ultimately depolarize cells and/or contribute to neurotransmitter release (43). These signaling cascades are implicated in sweet and bitter taste, including cyclic adenosine monophosphate (cAMP), inositol triphosphate (IP3), and phospholipase C (transduction signaling cascades are discussed in sect. 3) (141). The role of CD36 in fat taste and olfaction is examined in 20 studies, summarized in TABLES 3 and 5. Ten studies discussed the effect of CD36 polymorphisms (described in sect. 4) on fat perception. This section will discuss 10 studies that examined the function and expression of CD36 in the context of fat perception (89, 96, 99, 107, 108, 115, 124, 126, 128, 131). Together, these studies highlight the role of CD36 as a fat-detection protein that initiates fat taste transduction.
Table 5.
CD36 polymorphisms associated with oral fat perception
| SNIP | Study (Reference), Year | Population | Findings |
|---|---|---|---|
| rs1527483 | Plesník et al. (73)., 2018 | Czech young adults | The participants with the CC genotype of the rs1527483 polymorphism had lower BMI (P = 0·011), waist circumference (P = 0.005), waist-to-height ratio (P = 0.010), and higher sensitivity for linoleic acid (LA) (P = 0·037) than the participants with the CT and TT genotypes. |
| N = 116; 73 females, 43 males | |||
| Mean age: 21.84 ± 0.22 years | |||
| Ong et al. (72), 2016 | Malaysian adults (293, ethnic Chinese, 20 ethnic Indians) | The overall minor allele frequency for rs1527483 was 0.26. Females and individuals with rs1527483 TT genotype significantly perceived greater creaminess of 10% fat-by-weight custard. Also, individuals with rs1527483 TT genotype and T allele significantly perceived greater fat content of cream crackers, independent of fat concentration. Variants were not associated with obesity. | |
| N = 313; 195 females, 118 males | |||
| Mean age: 20.73 ± 1.55 (males) and 20.74 ± 1.49 (females) years | |||
| rs2312018 | Plesník et al. (73), 2018 | Czech young adults | No association was found between the rs3212018 polymorphism and LA detection threshold or anthropometric parameters (i.e., BMI, waist circumference, waist-to-height ratio). |
| N = 116; 73 females, 43 males | |||
| Mean age: 21.84 ± 0.22 years | |||
| rs1761667 | Bajit et al. (50), 2020 | Moroccan adults | There was a higher AA genotype frequency of the SNP in obese subjects, and obese subjects had a significantly higher OA threshold. However, OA thresholds in obese subjects were very dispersed, skewing the results. |
| N = 100; 72 females and 28 males, mean age 32.37 ± 9.52 years (both) | |||
| Burgess et al. (53), 2018 | Caucasian | Supplementation of oleic acid did not enhance fattiness and creaminess perception for the cohort. However, East Asians carrying the GG genotype perceived more overall fattiness and creaminess than their AA genotype counterparts (P < 0.001). No differences were observed for the Caucasians. Thus, variation at rs1761667 may have ethnic‐specific effects on fat-taste perception. | |
| N = 36; 25 females, 11 males) | |||
| Mean age: 25.3 years | |||
| East Asian | |||
| N = 32; 24 females, 8 males | |||
| Mean age: 25 years | |||
| Karmous et al. (24), 2017 | Tunisian adults | A higher AA genotype frequency of rs1761667 was observed in obese subjects compared to normal weight ones (P = 0.012). Also, AA genotype (which encodes alanine) of rs1726866 was more abundant in the obese group (P = 0.017). | |
| N = 104; 71 females, 33 males | |||
| Mean age: 35.3 years | |||
| Karthi et al. (63), 2021 | Indian adults | The AA/AG genotype was associated with a higher LA detection threshold. | |
| N = 444; 234 males and 210 females | |||
| Ong et al. (72), 2016 | Malaysian adults (293, ethnic Chinese, 20 ethnic Indians) | The rs1761667 SNP did not significantly affect oral fat perception, except for cream crackers. Variants were not associated with obesity. | |
| N = 313; 195 females, 118 males | |||
| Mean age: 20.73 ± 1.55 (males) and 20.74 ± 1.49 (females) years | |||
| Melis et al. (67), 2017 | Caucasian Italian adults | The A/G allele of the rs1761667 polymorphism of CD36 was found associated to a distinct metabolic pattern in NW and obese subjects. | |
| N = 126 | The G allele of the CD36 gene rs1761667 was associated with increased endocannabinoid plasma levels and a trend for increased waist-to-hip ratio in obese subjects, even though exhibited decreased BMI with respect to those with AA genotype. | ||
| Age not specified | |||
| Mrizak et al. (71), 2015 | Tunisian women | The A allele of cluster of differentiation 36 (CD36) SNP 1761667 is associated with decreased lipid taste perception in obese Tunisian women. Women with the CD36 GG genotype exhibited oral detection thresholds for oleic acid that were more than three times lower than those with the CD36 AA genotype. | |
| N = 203 | |||
| Mean age: 38.4 years | |||
| Shen et al. (77), 2017 | UK adults | No associations were found between CD36 rs1761667 and liking of ice-cream. | |
| N = 136; 95 females, 41 males | |||
| Age = 50 years (50–year-old cohort of TAMARISK study) | |||
| Solakivi et al. (78), 2015 | Finish adults | CD36 SNP rs1761667 variant AA was significantly associated with lower BMI, compared to variants AG and GG at ages of 40, 45, and 50 years. There was no association CD36 variation with hypertension. | |
| N = 736; 289 females, 447 males | |||
| Age = 1 cohort at 40, 45, and 50 years of age |
BMI, body mass index; SNP, single-nucleotide polymorphism; NW, normal weight.
Among the included studies, four examined the necessity of CD36 in fat taste. These studies consistently found that CD36 played a role in fat preference and/or sensitivity (89, 107, 115, 124). These studies also suggested that CD36 works in conjunction with other proteins to mediate fat chemosensation. Lee et al. (107) determined that CD36 contributes to lipid recognition of irritant1-(palmitoyl)-2-(5-keto-6-octanedioyl)-phosphatidylcholine (KOdiA-PC) (a CD36 ligand) triggering a taste aversion response. Cd36 knockout mice (KO) mice had a diminished ability to detect this phospholipid. However, Cd36 KO mice were still able to detect KOdiA-PC at specific concentrations (i.e., 7.5 μM), suggesting CD36 is not the sole KOdiA-PC receptor (107). This is supported by Sclafani and Ackroff (124), who found that Cd36 KO mice demonstrated lower preference for lipids than wild-type mice. Additionally, naïve Cd36 KO mice showed reduced taste preference for fat emulsions solutions compared to wild-type (WT) mice. However, Cd36 KOs developed normal preference following exposure to fat. This is consistent with the hypothesis that CD36 is not the sole fat detection receptor and that plasticity occurs with fat exposure (107, 124).
The role of CD36 in fat detection was also examined in rats. Braymer et al. (89) found that interfering with lingual Cd36 expression using small interfering RNA (siRNA) in obesity-resistant rats reduced the preference for LA. This suggests that interfering with Cd36 expression reduces orosensory detection of FFAs. The role of CD36 and its interaction with other proteins in fat detection was also examined in human cells. Ozdener et al. (115) determined that knocking down lingual CD36 (using siRNA) suppressed calcium signaling in human TBCs. Moreover, concomitant siRNA knock down of GPR120 and CD36 further suppressed calcium signaling in human TBCs, suggesting these are parallel mechanisms (115). The interaction of CD36 and GPR120 is further discussed below.
Additionally, three studies examined the relationship between Cd36 expression and fat preference. Djeziri et al. (96) reported that chronic (16 weeks) ad libitum access to a high-fat diet (HFD) altered fat preference and Cd36 expression in female mice TBCs. They found that HFD-fed mice had significantly lower lingual Cd36 mRNA expression and decreased preference for fat. However, Gaudet et al. (99) reported that short-term (2 weeks) continuous access to HFD increased lingual Cd36 expression in male rat’s TBCs and limited intermittent access to a HFD did not change Cd36 expression. This discrepancy may be due to differences in sex, length of the HFD exposure, and/or species-related differences. Recently, Olvera Hernández et al. (100) also examined fat preference and Cd36 expression in lingual tissue (specifically in circumvallate papillae) and the hypothalamus of female and male mice. Specifically, this study examined intergenerational and sex-related effects of maternal undernutrition (UN) on Cd36 expression and fat preference in adult offspring of UN dams. They found female and male mice born to UN dams exhibited increased expression of Cd36 in taste buds. Further, female mice displayed a higher preference for fat than their male counterparts. Additionally, while males of undernourished dams showed decreased fat preference compared to control males, no differences in preference were observed between UN and control females (100). Thus this study brings to light the importance of sex and transgenerational nutrition on Cd36 expression and fat preference. Further studies are needed to clarify the relationship between Cd36 expression and fat preference and whether these effects differ by sex.
While the study of CD36 in fat detection has focused on lingual tissue, recent studies have examined the role of CD36 in the olfactory perception of lipids. Lee et al. (107) observed that transection of the olfactory nerve led to the inability to perceive KOdiA-PC (a CD36 ligand). Although it is unclear if this reduction is CD36-dependent, the authors postulate that CD36 may play a role in the olfactory detection of KOdiA-PC on the surface of the olfactory epithelium (107). Tsuzuki et al. (128) observed that odor-active fatty acids with aldehyde domains bind to CD36 on tissue within the nasal cavity, supporting CD36’s role in nasal fat perception. Aldehyde-containing FFA binding to CD36 sends signals to olfactory neurons and can affect further fat ingestion and eating behavior. Xavier et al. (131) found that CD36-KO mice exhibited an impaired preference for a lipid mixture odor compared to wild-type mice, yet there was no impairment in general odorant detection of predator pheromones or food in CD36-KO mice. A study by Lee et al. (108) compared the detection of oleic aldehyde, an odor-active and volatile fatty aldehyde that binds to CD36, in WT and CD36 KO. They found WT mice, but not CD36 KO, displayed increased exploratory behavior (including sniffing oleic aldehyde). This suggests that CD36 KO mice could have reduced sensitivity to oleic aldehyde. The authors suggest that this supports the involvement of CD36 in olfactory detection of this fatty aldehyde (108). These studies are consistent with previous literature suggesting that CD36 plays an essential role in fat chemosensation.
4.1.1.2. gpr120.
GPR120, also known as free fatty acid receptor 4 (FFAR4), is an important transmembrane GPCR in fat taste transduction. After binding to FFAs, CD36 and GPR120 work together to initiate signaling cascades that ultimately lead to depolarization of TBCs. To begin understanding the role of GPR120 in fat taste perception, studies utilized Gpr120 KOs. However, although some studies have reported that GPR120 knockout mice demonstrated decreased fatty acid preference (141, 142), others have found that while Gpr120 plays a role in fat chemosensation, it is not necessary for fat taste perception (84, 143). Six studies included in this review discussed the role of GPR120 in fat perception (84, 113, 115, 123, 132, 144). Gpr120 KO mice demonstrated decreased, but not eliminated, preference for fat (84). This suggests that GPR120 modulates but is not completely necessary in mediating fat taste sensitivity (84). Furthermore, GPR120 activation in TB100 with TUG891, a GPR120 agonist, leads to increased intracellular calcium and MAPK phosphorylation in mice (discussed in sect. 3) (113). Costanzo et al. (144) investigated the role of fat receptor genes, including GPR120, and showed that GPR120 expression in human fungiform papillae was associated with fat taste sensitivity. Furthermore, Ozdener et al. (115) found that GPR120 interacts with downstream proteins to amplify the cellular response to high dietary fat concentrations in human and mouse TBCs. However, the role of GPR120 in oral fat detection was challenged in later studies that suggest that GPR120 may mediate postingestive cues that reinforce fat consumption but is not essential for oral fat detection (84, 123). GPR120 has also been implicated in fat taste sensation via nerve responses. After examining chorda tympani nerve responses to fatty acids in WT mice, Yasumatsu et al. (132) found that 17.9% of nerve fibers showed maximal responses to fatty acids, subsequently naming them F-type fibers. GPR120 antagonists in WT mice suppressed the response to LA in F-type fibers, and the percentage of F-type fibers greatly decreased to 4.0%. In addition, the generalization threshold for linoleic acid in GPR120-KO mice was higher than that in WT mice (132). The results from all six studies indicate that GPR120 plays a facilitator role in TB100 signal transduction, alongside CD36, and reinforces fat preferences via postoral mechanisms. GPR120 and CD36 may operate to influence calcium signaling, which is discussed in sect. 2. GPR120 in the gastrointestinal (GI) tract and other postoral actions are discussed further in sect. 3.
4.1.2. Other molecules involved in fat taste and smell.
4.1.2.1. adrb3.
β-3-Adrenergic receptor (ADRB3) is a protein in the GPCR family that mediates fat breakdown and metabolism (80). Previous literature primarily linked ADRB3 to its function in regulating overall energy expenditure, as its mutations are associated with reduced lipolysis and visceral obesity (145–149). Watanabe et al. (80) found that ADRB3 Trp64Arg substitution is highly correlated with high-fat food preference in young Japanese women. This polymorphism is discussed in sect. 5. The association between ADRB3 polymorphisms and fat preference indicates that this receptor may play a role in oral fat perception.
4.1.2.2. cb1r.
Cannabinoid subtype-1 receptors, belonging to the endocannabinoid system, have been found to play a role in fat taste perception and preference. Avalos et al. (85) investigated the impact of CB1Rs in the upper small-intestinal epithelium on preference for a Western-style HFD in mice. The study elucidated that acute preferences for the HFD were inhibited by the global pharmacological blockade of CB1Rs by an antagonist, AM251. They also found that CB1R+ control mice displayed robust preferences for the HFD compared to the standard diet and ate significantly more kilocalories from the HFD throughout the 24-h preference test. CB1R– mice did not display a preference for HFD for the first 6 hours of the test and ate fewer kilocalories from the HFD. Similarly, Brissard et al. (150) found that CB1R KOs and WT mice treated with rimonabant (a CB1R blocker) displayed a significant decrease in their preference for fatty solutions (rapeseed and LA) compared to untreated WT mice. These results provide evidence of a crucial role for CB1Rs in the rodent upper intestinal epithelium in acute preferences for food containing high levels of fat (85).
4.1.2.3. fgf21.
Fibroblast growth factor 21 (FGF21) is a protein secreted by the liver in response to metabolic stress (151). It plays a role in coordinating metabolic responses from adipose tissue. In mice, FGF21 reduces body weight and sugar intake but not fat (152). However, human studies have found an association between single-nucleotide polymorphisms in FGF21 and decreased fat intake (153). The FGF21 single-nucleotide polymorphisms are discussed in sect. 4. Thus FGF21’s ability to aid in healthier macronutrient intake is unclear. Therefore, Makarova et al. (110) examined the effect of FGF21 administration on taste preference for HFD. In FG21-treated males, there was a significant reduction in preference for fatty food (compared to control-treated males). However, in FGF21-treated females, there was a small, but not significant, preference for HFD. These data suggest that FGF21’s effect on fat preference may be sex dependent.
4.1.2.4. gpr84.
Another protein implicated in fat sensing in TBCs is G protein-coupled receptor 84 (GPR84). Although GPR84 has previously been identified as a receptor of medium-chain fatty acids (MCFAs) in the immune system via hematopoietic cells, it was found to be a receptor of MCFAs in the mouth by Liu et al. (109). They identified extensive Gpr84 mRNA in the fungiform papillae and CVP cells of mice. Cells induced to express Gpr84 exhibited robust intracellular increases in Ca2+ in response to five different MCFAs, including capric and lauric acid. In addition, Gpr84-deficient mouse TBCs had a significantly reduced ability to respond to MCFAs via intracellular Ca2+ rise and membrane depolarization. Gpr84-deficient mice also showed loss of chorda tympani (CT) nerve activity during capric acid stimulation, while nerve responses to all other taste modalities remained intact in the absence or presence of GPR84. These results suggest that GPR84 has a new role in the oral cavity as a gustatory receptor of MCFAs (109).
4.1.2.5. olfr544.
Olfactory receptor 544 (Olfr544) has been studied in the context of fatty acid detection and metabolism. Olfactory receptors in the nasal epithelium, such as Olfr544, initiate depolarizing transduction mechanisms representing the detection and recognition of odors (154). Additionally, Olfr544 is highly expressed in extra-olfactory tissues such as adipose and hepatic tissue of mice (130). Wu et al. (130) found that azelaic acid (FA) is a ligand of Olfr544. Results showed that Olfr544 mediates the metabolic interplay between the liver and the adipose tissue. Specifically, Olfr544 mobilized stored fats from adipose tissue and shifted the fuel preference to fats in these tissues. Thus this olfactory receptor can detect fatty acids and regulate cellular energy and metabolism.
4.1.2.6. prep1.
Prep1 has also been studied in the context of fat perception. Prep1 is a transcription factor involved in metabolic homeostasis and is highly expressed in the mouse olfactory bulb (155). Ricci et al. (117) found that Prep1 deficiency reduced the preference for high-fat foods via olfaction deficits. Prep1 hypomorphic heterozygous mice display a scant ability to distinguish odors, which significantly impacts feeding behavior. Furthermore, Prep1 deficiency was also associated with decreased activation of ERK1/2 and decreased brain-derived neurotrophic factor (BDNF) levels (117). ERK1/2 involved in fat transduction signaling via Prep1 is discussed below. Previous studies suggested that BDNF influences olfactory behavior (156, 157). These findings suggest that reduced fat preference observed in Prep1-deficient mice may be mediated by impaired responsiveness to BDNF-induced ERK1/2 phosphorylation.
4.1.2.7. serotonin.
Serotonin (5-hydroxytryptamine) also has an implication in fat taste transduction. Serotonin is a neurotransmitter that is highly expressed in brain tissue and is involved in many mechanisms influencing energy balance, mood, and sleep (70, 158). Increases in serotonin levels can cause abnormal regulation of metabolic processes such as lipolysis and gluconeogenesis (159). Supporting previous research, Ozdener et al. (115) and El-Yassimi (160) also found that FAs triggered serotonin release from TBCs (grifolic acid more so than LA). The authors indicated that TBCs release serotonin downstream of calcium signaling; as a neurotransmitter, it communicates fat taste stimuli to afferent gustatory nerve fibers (115). Confirming serotonin’s involvement in fat taste, Gaudet et al. (99) found increases in serotonin gene (SERT) expression in circumvallate papillae TB100 in response to several days of HFD. These results together support serotonin’s role in fat taste transduction, as a TB100 neurotransmitter.
4.1.2.8. tgr5.
TGR5 is a GPCR, more traditionally known as a bile acid receptor in the GI tract, recently found in TBCs. Tgr5 KO mice exhibited increased food intake and fat mass. Loss of TGR5 function increased calcium signaling and glucagon-like-peptide-1 (GLP-1) secretion in response to fatty acids in TBCs. Tgr5’s role in calcium signaling is discussed below. TGR5’s involvement may underlie a high preference for fat in the development of obesity (87).
4.1.2.9. tlr4.
TLR4 is another protein that was initially believed to be a fat receptor (161). TLR4 is a transmembrane protein that is an important mediator of innate immunity (162). It binds primarily to LPS (163) and several saturated FFAs (164–166). Camandola and Mattson (93) found that Tlr4 KO mice demonstrated a low preference for fat. The authors postulated that TLR4 promoted fat ingestion and preference by mediating FA endocytosis. However, a subsequent study found that TLR4 is not a fat receptor but rather mediates lipid-induced inflammation. This process is critical in developing chronic low-grade inflammation in the pathogenesis of obesity (167).
4.1.2.10. purinergic receptors.
A receptor family implicated in fat taste is purinergic receptors. While adenosine triphosphate (ATP) is a global energy source, it also acts as a signaling molecule that relays information from taste bud receptor cells to afferent neurons (168–170). Ionotropic purinergic receptors P2X2 and P2X3 are expressed in gustatory nerve fibers in fungiform papillae, and their binding to ATP is an important step in gustation (171). Several primary tastes have been associated with the release of ATP and its binding to P2X2 and P2X3 (168). Sclafani and Ackroff (122) also reported that naive P2X2/P2X3 double knockout (DoKO) mice showed significant preference deficits toward fat solutions. However, following exposure to fat, these mice did develop strong preferences for the nontaste qualities of fat, such as texture and odor. The authors suggest that the experience-induced preferences were likely due to postoral conditioning (122).
4.1.3. Drosophila proteins.
The previously mentioned proteins participate in the primary stages of fat detection in taste and smell organs. FFA binding ultimately causes depolarization of gustatory and olfactory receptor cells, which leads to neurotransmitter release at afferent nerve fibers (43). It is important to understand the proteins involved in afferent neurons’ response to dietary FAs. In Drosophila melanogaster models, gustatory receptor neurons (GRN) are housed within the gustatory sensilla, act as the primary nutrient-sensing cells, and shed light on proteins involved in rodent and human neurons (127). In the current search, five papers discussed the role of ionotropic receptor (IR) proteins, Gr64, and DmOrco in fatty acid taste in Drosophila (83, 90, 102, 104, 127).
4.1.3.1. dmorco.
Odorant receptor coreceptor (Orco), which serves as a chaperoning coreceptor for odors, is an olfactory-related molecule involved in sensory-mediated responses to fat in Drosophila. In D. melanogaster, these olfactory receptors are referred to as DmOrco, are expressed in most olfactory receptor neurons, and are involved in olfactory and nutrient-related signaling (172). A 2018 study by Jung et al. (102) found that a HFD reduced DmOrco gene expression by 70% in olfactory neurons and decreased olfactory sensitivity to short-chain fatty acids. This suggests that DmOrco may play a role in the olfactory detection of fatty acids and may be analogous to key olfactory receptors in other species.
4.1.3.2. gr64.
Previous literature describes phospholipase C (PLC) in the fat taste pathway in TB100 (43). It acts to signal inositol triphosphate (IP3)-gated calcium channels after TB100 activation (43). A 2018 study by Kim et al. (104) found that novel gustatory receptors, such as Gr64e and Gr64f, ligand-gated ion channels in glycerol detection, may act downstream of PLC in Drosophila. Results from gene deletion tests on FFA palatability show that Gr64e is involved in fat chemosensation (104). Similarly, Tauber et al. (127) reported that neurons expressing the gustatory receptor Gr64f were activated by FFA stimulation and contributed to reflexive feeding responses to fat. Additionally, Brown et al. (90) found that all FA increased GR64f neural responsiveness, but the proboscis extension response to medium-chain FA is only controlled by GR64f, which suggests that short-, medium-, and long-chain FA taste discrimination occurs through different neural channels. Although only expressed in Drosophila, the involvement of these two gustatory receptors highlights the role of PLC in fat chemosensation.
4.1.3.3 ir proteins.
In Drosophila, taste neurons express ionotropic receptors (IRs), such as IR56d, which are involved in Ca2+ signaling in and activation of gustatory neurons. GRNs expressing the IR56d gene are necessary for responding to short- and medium-chain FAs in Drosophila (83, 127). Silencing IR56d transcripts (83) and IR56d-expressing neurons (127) inhibits Drosophila’s ability to detect and respond to FAs. This effect was seen at the cellular and behavioral level (127). Similarly, Brown et al. (90) found that flies can discriminate between short-, medium-, and long-chain fatty acids, but they are unable to discriminate between different compounds of the same fatty acid chain. They found that IR76b and IR25a are required for taste response to all three fatty acid classes. Additionally, they observed a significant reduction in the proboscis extension response to medium-chain fatty acids when silencing IR56d-expressing neurons and GR64f-expression neurons indicating that IR56d-expressing neurons are required for medium-chain fatty acid taste perception. However, they did not see any significant proboscis extension response to short- and long-chain fatty acids between the GR64f-silenced group and the control group (90). Although IR proteins are not found in humans and rodents, these findings reinforce the importance of calcium signaling in fatty acid detection (discussed below).
4.1.4. Signal transduction.
FFAs released following the mechanical and chemical breakdown of foods initiate signaling cascades in gustatory and olfactory tissues. These signaling cascades impact intracellular signal transduction pathways, including calcium and glutamatergic signaling. Intracellular signal transduction pathways are summarized in FIGURE 2.
4.1.4.1. calcium signaling.
Calcium signaling plays a vital role in cellular activities that relay chemical information received by chemosensory cells to the brain (42). In TB100 and OSNs, calcium is released from the endoplasmic reticulum (ER) via IP3-gated channels. This triggers the opening and closing of ion channels/exchangers leading to cell depolarization and neurotransmitter release to afferent neurons (43, 173). Five studies included in this review highlight the role of intracellular calcium signaling in fat perception (96, 103, 112, 126, 127).
Subramaniam et al. (126) found that fatty acids (i.e., LA) impact calcium signaling via CD36. They found that CD36 contributed to the opening of calcium channels, such as CALHM1, and that genetic ablation of CALHM1 impaired preference for dietary fat. Furthermore, the ERK1/2-MAPK cascade is regulated by the opening of CALHM1 in TBCs modulating orogustatory detection of lipids in humans and mice (126). Calcium signaling can lead to activation of the ERK1/2 pathway. The impact of ERK signaling on fat taste was examined in a 2017 study by Khan et al. (103). Erk1 knockout mice (ERK1-/-) maintained on HFD exhibited a low preference for dietary fatty acids and developed obesity. Phosphorylation of ERK1 and ERK2 is an important step in the fat taste transduction pathway in TB100 and is needed for dietary fat preference (103). Similarly, a 2018 study by Djeziri et al. (96) reported an association between intracellular calcium and fat preference. They found that oleanolic acid increased intracellular Ca2+ concentrations; this change was correlated with increased CD36 mRNA (96). These studies support the role of calcium signaling (via CD36, CALHM1, and EKR1/2 phosphorylation) in fat perception.
Furthermore, a translational 2017 study by Murtaza et al. (112) examined the use of calcium to modify taste and eating behavior in humans. They tested the effects of zizyphin, a triterpenoid and steroid precursor that influences lipid signaling, by recruiting Ca2+ from the ER and the extracellular environment via the opening of store-operated calcium channels. When given in conjunction with LA, the zizyphin produced an additive effect on intracellular Ca2+ signaling. This increase in intracellular calcium was accompanied by an increase in fat preference (112). This indicates that the intermediate action of calcium signaling is needed for oral fat detection.
Calcium signaling is also involved in the brain’s transduction pathways. Sweet gustatory receptor neurons (GRN) that typically respond to sweet taste are involved in the recognition of fatty acids. Ionotropic receptors IR25a and IR76b signal fat taste recognition in Drosophila by triggering intracellular Ca2+ increases in tarsal sweet GRNs (127). These mechanisms similarly confirm that calcium signaling is the facilitator of fat taste transduction in neurons.
4.1.4.2. calhmi.
CALHM1 is a downstream calcium ion channel that facilitates the calcium uptake in TBCs and neurotransmitter release (124). Sclafani and Ackroff (124) reported that naïve CALHM1 KO mice displayed deficits in fat preference (even stronger than CD36 KOs) that were rescued following postoral conditioning. CALHM1 channels also influence LA-induced calcium signaling and extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, which ultimately leads to ATP upregulation and neurotransmitter release, including serotonin (115) release (124, 126). Together these findings suggest that CALHM1 is a key downstream calcium signaling mediator and contributes to fat detection and preference.
4.1.4.3. α-gustducin.
α-Gustducin is the α subunit of the gustatory G protein that is associated with GPR120 (116). In GPR-mediated transduction, GPR120 exchanges the inactive guanosine diphosphate (GDP) for a guanosine triphosphate (GTP) molecule on the G protein, allowing it to phosphorylate downstream proteins, including phospholipase C (PLC) (43). Avau et al. (86) investigated the involvement of α -gustducin in nutrient sensing during nutrient excess. They found that α-gustducin KO mice receiving a HFD had decreased weight gain relative to WT mice. This suggests that interfering with α -gustducin gustatory signaling may be implicated in the development of diet-induced obesity (86)
4.1.4.4. trpc3.
Transient receptor potential canonical (TRPC) channels are nonselective cation channels activated by phospholipase C and endogenous diacylglycerol, and they play a role in orosensory detection. Murtaza et al. (114) found that spontaneous liking, preference, and wanting of fatty acid solutions were significantly decreased in Trpc3, a TRPC channel subtype. LA-induced Ca2+ signaling was also significantly curtailed in Trpc3 knockout mice compared to WT. The same effect occurred for WT mice treated with a TRPC3 channel blocker. These results point to the involvement of TRPC channels in secondary signaling cascades in response to fat stimuli (114).
4.1.5. Other ingestive cues associated with fat taste and smell.
So far, we have discussed the role of proteins and the anatomy of fat taste and olfaction (70). This section will describe the role of oral chemosensory cues, such as food texture, saliva, and other chemical transduction signals in fat perception. Eating is a dynamic behavior in which food is processed and perceived via mechanical and physiological mechanisms (66). When food is brought into the mouth, it is broken down through mechanical (e.g., mastication and tongue manipulation) and chemical (e.g., salivary enzymes) processes. Altogether, the mechanical and chemical processes that lead to the release and binding of FFA contribute to fat perception (e.g., detection and liking/preference).
4.1.5.1. texture.
The recognition of food texture results from the interaction between oral surfaces and food. Early studies of fat perception proposed that texture rather than fat taste was responsible for fat perception (174–176). Later studies concluded that fat perception was independent of texture, but texture could contribute to fat perception (40) and fat texture is represented by neurons independent of viscosity (177). Three studies in the current review discussed texture in the context of fat perception (49, 59, 76). Grabenhorst and Rolls (59) found that human participants were able to distinguish HF concentrations and rated them as more pleasant in texture than LF stimuli. Similarly, Appelqvist et al. (49) found that HF content contributed to fatty mouthfeel and consumer preference. This is due to increased oral lipid-droplet coating, which can be perceived long after swallowing (49). Additionally, Running et al. (76) demonstrated that degree of saturation influences rejection of chocolate products. The taste and aroma of chocolate with higher concentrations of polyunsaturated FAs were preferred over monounsaturated acid (OLA) (76). Together these studies support that texture contributes to fat perception.
4.1.5.2. saliva.
Saliva initiates the chemical breakdown of food, contributing to oral fat taste perception (70). Saliva is a body fluid consisting of water, inorganic salts, enzymes, and other proteins synthesized and secreted from the salivary glands (178). Five studies in this systematic review examined the role of saliva in fat perception. Voigt et al. (41) found lipases (LIPs), different from LIPF (as observed in rodents), are present in human salivary glands. They also observed that oral perception of triglycerides is associated with differential LIP activities on individual threshold concentrations. Thus this study provided evidence that lingual lipolysis is important for oral perception of fat in humans (41). Méjean et al. (66) found a positive association between the salivary properties (flow, proteolysis, and composition) and liking for fat in humans. Similarly, Mounayar et al. (70) observed that fatty acid sensitivity was associated with changes in saliva composition induced by OLA. Furthermore, Melis et al. (69) found that a small surplus of l-arginine (l-arg) was sufficient to increase OLA perception, suggesting l-arg might contribute to fatty acid perception. Additionally, Besnard et al. (51) reported an association between fat taste sensitivity and the oral microbiome. This suggests that specific microbial communities (e.g., TM7 bacterial family) and salivary signatures (e.g., salivary flow, lysozyme activity, total antioxidant capacity) influence orosensory detection of dietary lipids (51). Together these studies highlight that salivary flow and composition (e.g., proteolytic enzymes, l-arg, and salivary microbiome) may contribute to fat perception.
4.1.5.3. tongue characteristics.
The tongue possesses the papillae and TBCs necessary for chemosensation of food in the oral cavity. Therefore, it is plausible to suggest that individual tongue characteristics can impact the way that one tastes flavors and the intensity of those flavors. Zhou et al. (81) investigated this by correlating fungiform papillae density on the tongues of human subjects to fat taste sensitivity. They found that participants classified as hypersensitive to OA had a higher fungiform papillae density than those classified as hyposensitive to OA. The authors hypothesized that higher fungiform papillae density results in more fat taste receptors in the mouth and increased taste sensitivity to OA (81). Additionally, Liu et al. (65) found gene expression of CD36, FFAR4, FFAR2, GPR84, and KCNA2 in their collected fungiform papillae samples. Following Western blot analysis, they also observed positive gene expression of candidate fat taste receptors and DRK channel proteins in the human fungiform papillae. While the expression level of CD36 presented a negative correlation with the preference to high-fat foods, they found that the expression of FFAR2 in fungiform papillae was related to fat intake.
4.2. Neuroanatomy and Physiology of Fat Taste and Smell
Although, as previously discussed, taste and olfaction are deeply intertwined, they are two separate senses with their own receptor organs and separate neural substrates. Information from TBCs is relayed to the brain where they are processed and give rise to our perception of taste (179). Olfactory sensory neurons (OSN) are found in the olfactory epithelium that lines the nasal cavity. Olfactory receptors in these sensory cells initiate transduction processes that result in odor perception (180). We provide a brief overview, and a summary of basic gustatory anatomy is provided in FIGURE 8. In general, the perception of taste and smell involves the activation of multiple brain regions. In this section, we discuss advancements in our understanding of the anatomy and physiology specific to fat taste and olfaction (TABLE 4) (57, 59, 79, 87, 94, 97–99, 116).
FIGURE 8.
Brain regions involved in fat taste and smell. The perception of fat taste and smell activates of multiple brain regions involved in fat chemosensation. Image created with BioRender.com, with permission.
Table 4.
Brain regions involved in fat chemosensation
| Region | Study (Reference), Year | Finding |
|---|---|---|
| Arcuate nucleus | Peterschmitt et al. (116), 2018 | When adding LA linoleic acid (LA) on circumvallate papillae on the mouse brain, mRNA expression of Zif-268, brain-derived neuroptrophic factor (BDNF), and Glut-1 occurs on the arcuate nucleus. |
| Amygdala | De la Cruz et al. (94), 2015 | Corn oil induced c-Fos activation on different subareas (basolateral, central-cortico-medial) of the amygdala. |
| Eldeghaidy et al. (57), 2016 | A high-fat meal caused a reduction on the activation of the amygdala in response to the fat stimulus compared to the no-fat control stimulus (fat-related satiety). | |
| Cerebellum | Sun et al. (79), 2016 | Change in odor intensity perception and ghrelin reactivity associated with a negative association in the cerebellum with less cerebellar response. |
| Cortical (corticolimbic area) | Espitia-Bautista et al. and Escobar (97), 2019 | A rat brain on a diet rich on fat promoted high levels of free fatty acids, leading c-Fos to be found higher in ΔFosB in all the corticolimbic areas on comparison with rat brains on a standard diet. |
| Dorsal striatum | De la Cruz et al. (94), 2015 | c-Fos activation was increased on the exposure of corn oil on the dorsal striatum. |
| Hippocampus | Peterschmitt et al. (116), 2018 | On the hippocampus of the mouse brain, mRNA expression increases of Zif-268, BDNF, and Glut-1 when LA is applied on circumvallate papillae. |
| Hypothalamus | Gaudet et al. (99), 2019 | A high-fat diet increased the expression of CD36, serotonin, and variations of tryptophan hydroxylase isoenzymes (TPH1 and TPH2). It also downregulated the expression of the orexigenic hypothalamic neuropeptideY (NPY), which is involved on feeding behavior in the mediobasal hypothalamus (MBH). |
| Eldeghaidy et al. (57), 2016 | After a high-fat diet, cerebral blood flow (CBF) on baseline resulted on a reduction on the hypothalamus taste. | |
| Insular cortex | Espitia-Bautista and Escobar (97), 2019 | A diet rich in fat leads an increase in the number of c-Fos on the insular cortex. |
| mPFC | De la Cruz et al. (94), 2015 | An increased c-Fos expression in the areas of infralimbic and prelimbic mPFC by corn oil intake. |
| Nucleus accumbens (NAc) | De la Cruz et al. (94), 2015 | Corn oil intake increased c-Fos on NAc core but not on the NAc shell. |
| Espitia-Bautista and Escobar (97), 2019 | A rat brain with a diet rich on fat promoted high levels of free fatty acids, leading c-Fos activation to be found higher in the core and shell NAc regions, than the brains on a standard-rich diet. | |
| Peterschmitt et al. (116), 2018 | There is no significant difference in c-Fos expression after the application of LA seen on a mouse brain. | |
| Nucleus of the solitary tract (NTS) | Peterschmitt et al. (116), 2018 | On the mouse brain, c-Fos, Zif-268, and Glut-1 mRNA increases its expression on the NTS with the addition of LA on CV. |
| Nucleus of the solitary tract (NTS) | Weiss et al. (129), 2019 | A high-energy diet produces blunted, but more prevalent, responses in the NTS, and weaker association of taste responses with ingestive behavior. |
| Orbitofrontal cortex (OFC) | Grabenhorst et al. (59), | Human somatosensory cortex (SSC) activity was strongly correlated with the OFC during consumption of high-fat food. |
| Parabrachial nucleus (PBN) | Peterschmitt et al. (116), 2018 | Increase in c-Fos expression in the mouse brain after LA application. |
| Ventral tegmental Area (VTA) | De la Cruz et al. (94), 2015 | c-Fos activation was observed in the VTA following consumption of corn oil in rats. |
mPFC, medial prefrontal cortex.
4.2.1. Neuroanatomy of taste perception of fat.
Taste afferent pathways converge at the nucleus of the solitary tract (NTS) and innervate multiple brain regions. Fat taste is associated with the activation of the gustatory cortex, thalamus, hypothalamus, amygdala, striatum, ventral tegmental area (VTA), and hippocampus (181). The involvement of these regions emphasizes the role of energy balance, reward, emotion, and memory in fat chemosensation. Studies included in this systematic review support the involvement of these anatomical regions in fat perception.
A functional magnetic resonance imaging (fMRI) study found that oral fat texture was represented in the somatosensory insula, the anterior cingulate cortex, and the orbitofrontal cortex (OFC) (174). Building on these studies’ findings, Grabenhorst and Rolls (59) determined that human somatosensory cortex (SSC) is involved in oral fat processing via functional coupling to the OFC, a brain region associated with fat texture. SSC activity was strongly correlated with the OFC during the consumption of HF food. This effect was concentration dependent, as a higher correlation between the SSC and the OFC was observed during consumption of high-fat foods compared to low-fat (LF) foods. SSC activity was also correlated with subjective ratings of fattiness but not of texture pleasantness or flavor. This suggests that the SSC is not involved in hedonic processing but may relay information to the OFC to contribute to valuation of fat taste. Andersen et al. (48) found that significant discrimination in the subject’s electroencephalography (EEG) responses when their anterior tongue was stimulated with skim milk, whole milk, and the intermediate between the two while controlling for confounding somatosensory variables. In these EEG recordings, they observed consistent negative polarizations to the right and left frontotemporal regions 0.1 s after stimulus was applied. Additionally, 0.4 s poststimulus, they observed negative deflections merging at the frontal lobe, and 0.7 s after, it separated back to the frontotemporal regions. This indicated that taste alone can be responsible for changes in the neural circuitry. More recently, Han et al. (61) found that there is stronger insular activation in response to high-fat versus slow-fat odors in individuals with high sensitivity (HS) to sweetness. They also found that individual sweetness sensitivity was positively correlated with insula activation. This suggests that the sweet taste sensitivity may interact with fat chemosensation and that the insula may play a role in fat-odor perception in individuals with HS to sweetness (61). Collectively, these studies highlight the involvement of brain regions involved in higher cognitive functions during fat chemosensation.
Other clinical studies have examined the role of limbic and homeostatic regions in fat chemosensation. Frank-Podlech et al. (58) observed a positive association between oral fat sensitivity and functional connectivity (FC) between homeostatic (hypothalamus) and limbic areas (hippocampus and amygdala) following a HFD. Conversely, they found a negative correlation between the FC between dorsal striatum and somatosensory regions following low-fat stimuli. Finally, they reported that fat ingestion disrupts the connection between the reward and gustatory networks. These data provide evidence for the involvement of brain networks involved in hunger and satiety regulation in oral fat perception.
Preclinical studies have also examined brain regions involved in fat taste, including the insula amygdala, striatum, and VTA. De la Cruz et al. (94) found activation of c-Fos, an indirect marker of neuronal activity, in the striatum, VTA, and amygdala following the consumption of corn oil in rats. Similarly, Peterschmitt et al. (116) also reported increased c-Fos expression in the central amygdala and VTA in response to lingual fatty acid application. Additionally, Espitia-Bautista and Escobar (97) reported that a fat-rich diet stimulated c-Fos activation in rat corticolimbic areas, including the striatum (NAc core and shell) and the insular cortex. Notably, acute HFD exposure induced higher c-Fos activation than sugar-rich diets. Chronic HFD increased binge-type eating behavior, and anticipatory activity associated with increased c-Fos in the corticolimbic system (97). This suggests that fat perception may engage brain regions involved in reward and emotion. Gaudet et al. (99) found that a HFD affected the expression of the fat taste receptor CD36 in TBCs, the hypothalamus, and the ventral striatum (NAc). Peterschmitt et al. (116) also found c-Fos expression in response to lingual fatty acids within the NTS, parabrachial nucleus (PBN), and ventroposterior medialis parvocellularis (VPMPC) of the thalamus, and other regions known to be activated by gustatory signals. Weiss et al. (129) also examined NTS activity. They collected electrophysiological recordings from NTS cells and found that taste response in NTS cells of HFD-induced obese rats was smaller in magnitude, shorter in duration, and occurred at longer latencies compared with that in lean rats. However, a larger proportion of taste-responsive cells in NTS was observed in the HFD-induced rats than in the lean rats, which most likely relates to compensation for the weakened taste response effect of the HFD (129). Together, preclinical studies suggest that the amygdala, VTA, PBN, VPMPC, NTS, striatum, and anterior insula are important in fat chemosensation.
4.2.2 Neuroanatomy of the olfactory perception of fat.
In olfaction, sensory neurons project to the olfactory bulb, the cortex (e.g., OFC), energy balance centers (e.g., the thalamus and the hypothalamus), the limbic system (e.g., the amygdala) and memory-associated regions (e.g., the hippocampus) (180). This is supported by studies included in this systematic review.
One fMRI study examined brain activity in response to fat stimuli in humans. Sun et al. (79) found that in humans, fMRI blood-oxygen-level-dependent responses in the cerebellum were negatively correlated with fat and sugar (e.g., strawberry and cream) odor intensity perception. The results from this study indicated the cerebellum may be associated with fat olfactory perception (79). A 2019 study by Fardone et al. (98) found that mice receiving a moderate HFD (MHF) or HFD showed decreased neuron excitation in the olfactory bulb and lateral glomerulus in response to fatty acid odors. The findings from both studies suggest that neuronal activity in the cerebellum, olfactory bulb, and lateral glomerulus contribute to olfactory-related fat detection. Further studies are needed to elucidate the neurophysiology of fat-related olfaction.
4.2.3. Neuromodulators of fat taste and smell.
4.2.3.1. glutamatergic signaling.
As a palatable macronutrient, fat stimulates reward pathways via glutamatergic circuits. Glutamate is a major excitatory neurotransmitter (182), and in the hypothalamus, glutamatergic signaling can modulate feeding responses (183). N-methyl-d-aspartate (NMDA) receptors are glutamate receptors, and previous studies have found that NMDA antagonism reduced food intake and sucrose preference (184). NMDA signaling may be involved in maintaining learned preferences for specific nutrients (e.g., sucrose, fat, and other flavors) (91). However, the role of NMDA receptors in fat perception and their mechanisms are not fully understood. In this review, three studies examined the role of glutamatergic signaling in fat perception (91, 105, 120).
Buttigieg et al. (91) reported that the acquisition of fat preference in young Swiss CD-1 mice is mediated by NMDA signaling. Mice that received systemic MK-801, an NMDA receptor antagonist, demonstrated blunted fat preference. Kraft et al. (105) confirmed these findings in Balb/c and SWR mice. Kraft et al. also found that MK-801 eliminated preference for an intralipid-flavored solution. This suggests that NMDA receptor signaling is needed for the development of fat-conditioned flavor preference. Furthermore, Sasaki et al. (120) determined that intraperitoneal administration of d-serine, an NMDA coagonist in C57BL6/J, inhibited HFD intake and acquisition of HFD preference. Overall, these studies confirm the key role of glutamatergic circuits in fat preference.
4.2.3.2. acetylcholine signaling.
Acetylcholine is a neurotransmitter and an important neuromodulator that regulates synaptic plasticity, synaptic transmission, and neuronal excitability (185). Like glutamate, cholinergic signaling in the hypothalamus can regulate food intake and eating behavior (185, 186). Hence, acetylcholine neurotransmitter function has been studied in the context of fat taste. Iskhakov et al. (101) examined the effect of cholinergic inhibition on intralipid intake in mice. Scopolamine, a rodent-specific cholinergic receptor antagonist, reduced intralipid consumption and prevented the development of fat-condition flavor preferences in three strains of mice (inbred C57BL/6 and BALB/c, and SWR). These results suggest that cholinergic receptor signaling is essential for regulating fat intake and the development of fat taste preference (101).
4.2.3.3. glucocorticoid signaling.
Glucocorticoids are part of the corticosteroid family involved in inflammatory function (187). Glucocorticoid receptors are expressed in mature olfactory neurons (188). Glucocorticoid signaling may be influenced by fat consumption and impact olfactory function. Lacroix et al. (106) examined glucocorticoid receptor (GR) expression in olfactory mucosa and the olfactory bulb. Results showed that obesity-prone rats fed a HFD demonstrated significantly decreased GR expression in the olfactory mucosa and slightly decreased expression in the olfactory bulb. The authors interpret that these decreases in GR expression may induce inflammation (189), apoptotic changes, and reduced olfactory mucosa renewal (190). In turn, these changes may impair olfaction sensitivity and reduce the olfactory perception of fat stimuli (106). Obesity’s effect on fat taste and smell is discussed in sect. 5.
4.2.4. Reward pathways.
Fat is a particularly palatable nutrient and commonly contributes to foods traditionally perceived as enjoyable. Many energy-dense HF foods (e.g., ice cream, fried foods, baked goods) are widely accepted as palatable, activating brain reward circuitry (191). Reward circuits are responsible for conveying pleasure in response to gustatory stimuli. As discussed in sect. 2, brain regions such as the VTA, NAc, and the amygdala are involved in food response and reward processing (192, 193). The following three studies discuss the role of neural reward circuits in fat perception (94, 99, 116).
De la Cruz et al. (94) used corn oil and sugars to measure immunoreactivity in various brain areas that may help mediate fat intake. Corn oil-induced activation in the VTA, PFC, dorsal striatum, NAc, and amygdala; these areas are strongly implicated in reward processing (94). Similarly, a 2018 study by Peterschmitt et al. (116) showed that the application of LA on the circumvallate papillae increased activation in the VTA and central amygdala compared to control. Both areas are associated with emotional processing and food reward (116). Hence, fat can reinforce reward by triggering neural pleasure centers.
Further, a 2019 study by Gaudet et al. (99) showed that chronic access to HFD alters eating behavior and affects the expression of genes related to fat perception (CD36, dopamine transporters, serotonin transporters) in CV, HYP, and NAc. This suggests that HF feeding patterns significantly affect markers of hedonic eating. Therefore, these studies highlight the strong relationship between fat taste and reward. This relationship may propagate via a positive-feedback mechanism, where activated reward systems promote further fat consumption (99).
4.2.4.1. opioid signaling.
The endogenous opioid system within the brain has implications in the reinforcement of palatable food taste. Opioid agonists and antagonists are involved in stimulatory and inhibitory effects of food intake and affect food reward and food preferences (194–196); for reviews, see Refs. 197, 198. In the current search, two papers discussed the involvement of the opioid system in fat preference (118, 119). Sakamoto et al. (119) looked at the effects of nonselective opioid antagonist naltrexone and mu-selective antagonist naloxonazine on both intralipid and sucrose intake. While more significant results were seen in sucrose intake, both naltrexone and naloxonazine antagonists reduced intralipid intake. The nonselective naltrexone also reduced intralipid preference (119). This indicates that the opioid system, and particularly mu-opioid receptors, play a supportive role in fat intake and the development of fat preference. Building on these findings, in 2015 the same group compared the effect of naltrexone and olfactory (ONX) and glossopharyngeal (GSX) nerve transections. Sakamoto et al. (118) found that naltrexone’s inhibitory effect on fat taste preference was primarily seen at higher concentrations of fat solutions, whereas transecting the ONX and GLX nerves reduced fat intake and fat taste preference at lower fat concentrations. This suggests that the opioid receptor system has a significant impact on fat perception at high concentrations (e.g., HF solutions).
4.3. Gut-Brain Axis: Postingestive Cues and Fat Taste and Smell
Fat perception is not only mediated by the orosensory cues discussed above (e.g., mechanical, and chemical processes) but also by postingestive cues. Postingestive cues are comprised of postoral feedback loops that drive or inhibit food intake (199) (FIGURE 9). These signals are essential for maintaining energy balance. Both the absence and presence of nutrients in the gastrointestinal tract induce the transmission of signals to the brain and other organs (200). This section includes six studies (82, 85, 95, 111, 124, 125) that discuss several postingestive signals, such as insulin, GLP-1, PYY, ghrelin, GPR120, GPR40, and CB1R. Here we examine how these postingestive cues (e.g., nutrient-sensing and reward signals) affect fat taste and olfaction.
FIGURE 9.
Postingestive and ingestive cues mediate energy intake and energy balance. The brain receives afferent signals including ingestive cues (e.g., taste and smell), and postingestive cues (e.g., hunger and satiety hormones, peptides, metabolites, and nutrient sensing). Together these afferent signals are processed by the brain to inform homeostatic status/energy balance. In response to these cues, efferent signals can stimulate physiological events, including lipid turnover and energy expenditure. Importantly, postingestive cues, can also trigger efferent signals that impact future ingestive cues. For example, an animal or human who may not initially prefer a fatty substance may develop a preference following rewarding postingestive cues. Altogether, these afferent and efferent signals can impact energy intake and excessive energy intake can lead to obesity. NTS, nucleus of the solitary tract; GLP-1, glucagon-like-peptide-1; SCFA, short-chain fatty acid. Image created with BioRender.com, with permission.
The importance of postingestive cues in fat perception is captured by studies examining the role of gastrointestinal signals following fat consumption. Mathes et al. (111) found that gastric signals can impact fat preference. They observed that chow-fed rats that underwent Roux-en-Y gastric bypass (RYGB) surgery showed less preference for palatable solutions (e.g., Ensure and intralipid solution) than rats that underwent control surgery. This indicates that the stomach plays a role in fat detection (111). Additionally, gastrointestinal signals can reinforce the drive to consume fat. Ackroff and Sclafani (82) used intragastric (IG) infusions to bypass the oral cavity and isolate the postoral gastrointestinal responses to nutrients in mice. Results showed that IG infusions of lipid solutions generated concentration-dependent enhancement of fat intake and preference (82). While the exact mechanism of these postoral signals was uncertain, the authors proposed that localized suppression of cholecystokinin (activates digestion) by GPR40, GPR120, and other endocannabinoid receptors may promote fat intake (82, 143, 201). Sclafani et al. (82, 143, 201) also observed that postingestive cues played a role in fat preference. They found that prior exposure to fat rescued fat preference in CD36 and CALHM1 knockout mice. In addition, Avalos et al. (85) found that the knockout of CB1R endocannabinoid receptors in the upper intestinal epithelium of mice caused an extinction in preference for a HFD compared to control mice. This indicates that postingestive mechanisms were sufficient to develop fat preference (124).
Some studies focused on the importance of specific digestive actions in fat. Dietary fat content is commonly found in the form of triglycerides, composed of a glycerol molecule and three fatty acid tails. In both the mouth and intestine, lipases break down triglycerides into their components so that the FFAs can be easily recognized by receptors (202). Sclafani and Ackroff (125) examined the role of lipolysis in oral and postoral fat perception. They used orlistat, a drug that prevents fat breakdown with (oral and intestinal) lipases. Mice learned a preference for intragastric oil solutions over those paired with orlistat (125). Additionally, these effects were more substantial in intragastric experiments than in oral administration. This indicates that postingestive lipolysis is a necessary pathway to develop fat preference. In a clinical study on 15 adults, Kulkarni and Mattes (64) investigated the role of lingual lipase in oral fat detection for almond butter with and without orlistat. The authors did not find that lingual lipase contributed to oral fat detection. They hypothesized that oral fat detection of fatty foods requires stronger oral processing effort.
Additionally, other studies have shown that postingestive signals’ effect on fat intake and preference is also mediated by satiation. For example, in Drosophila, Devineni et al. (95) observed that starving flies showed a robust appetitive response to free fatty acids (i.e., acetic acid), likely reflecting a compensatory mechanism following a state of negative energy balance. Meanwhile, fed flies showed aversion (95). Although satiation signals can acutely inhibit fat intake and preference, prolonged exposure can promote increased fat intake and preferences (203). Ultimately, this suggests that acute postingestive cues are sufficient to impact fat perception based on a fed/fasted state.
4.3.1. Insulin.
As mentioned above, hormones play an integral role in energy balance by eliciting satiation and mediating hunger signals. Insulin regulates the metabolism of fats and proteins by initiating glucose absorption from the blood (204). After ingestion, insulin mediates the conversion of glucose into glycogen or fats (205). Studies included in this review examined the association between insulin, insulin-related proteins, and fat perception. Lacroix et al. (106) found that insulin did not affect obese rats’ olfactory perception of fat. Obesity-resistant rats displayed insulin-dependent changes in odor-sniffing activity; however, intraperitoneal insulin injections (mimicking a meal-induced insulin surge) in obese rats did not alter odor sniffing activity of high-fat diet. This suggests that insulin signaling is involved in the olfactory detection of fats. Additionally, obesity-induced decreased insulin sensitivity may lead to an impaired olfactory perception of fats, leading to disinhibited eating (106).
4.3.2. GLP-1 and PYY.
Additionally, studies examined the role of glucagon-like-peptide-1 (GLP-1), a peptide that reduces blood glucose by enhancing insulin secretion. GLP-1 is an incretin hormone that influences energy intake by reducing glucagon production and slowing gastric emptying after a meal (206). While it primarily acts in the gastrointestinal tract, GLP-1 and its receptor are also expressed in TB100 (207). Studies by Shin et al. (207) and Martin et al. (208) reported that GLP-1 is involved in the general taste pathways and specifically modulates sensitivity. These studies examined traditional modalities such as sweet, sour, and umami; however, GLP-1’s role in fat taste still required investigation. Following this, GLP-1 knockout mouse studies showed that GLP-1 activity could influence fat taste (209). The current search included two studies that provided more specific details on GLP-1’s role in fat taste perception (62, 115).
Ozdener et al. (115) reported that GLP-1 plays an important role in fat taste transduction. Results showed that LA and grifolic acid triggered GLP-1 release from mouse TB100. The authors indicated that GPR120 might initiate this release from lipid rafts (115). These data also suggest that GLP-1 signaling may perpetuate transduction amplification needed specifically for fat taste sensitivity (209). Kadouh et al. (62) examined the influence of liraglutide, a GLP-1 analog, on fat perception. They found that liraglutide (3 mg subcutaneous) modulates taste preference. There was a significant difference between placebo and liraglutide groups in fat taste perception score (determined using the visual analog scale). This suggests that GLP-1 signaling and insulin regulation can mediate fat perception. GLP-1 also was associated with lower levels of plasma peptide YY (210). A previous study by La Sala et al. (211) found that augmenting salivary PYY rescued taste responsiveness to lipids. However, the role of peripheral PYY on fat perception is not fully understood.
4.3.3. Ghrelin.
Ghrelin is a hormone that has been examined in the context of fat taste. Ghrelin is an orexigenic hormone mainly secreted by the stomach during fasting and increases appetite. It promotes meal initiation, influences nutrient sensing, and usually declines after feeding (212, 213). Research in animals has shown that olfactory sensitivity is enhanced in a fasted state (214). However, human research has generated inconsistent results. The following two studies investigated the influence of ghrelin on fat chemosensation (79, 215).
Sclafani et al. (215) reported null findings on ghrelin signaling and postoral effects of fat intake. Results showed that ghrelin receptor signaling was not involved in fat-conditioned flavor preferences (215). A recent paper by Calder et al. (92) also found that male ghrelin receptor Ghsr knockouts (Ghsr-/-) mice did not display a reduced aversion to LA following conditioned taste aversion. However, Ghsr-/- females showed a reduction in LA taste responsiveness. These data suggest that ghrelin-GHS-R effects on fatty acid taste may be sex specific.
Furthermore, ghrelin has also been studied in the olfactory system. However, Sun et al. (79) focused on the effect of fat perception on metabolic changes. Obese subjects experienced stronger suprathreshold odor intensity of fatty foods (e.g., “chocolate cookie” and “strawberry and cream”) when hungry than when full (79). This was associated with more robust postprandial ghrelin level suppressions and differential cerebellar olfactory responses. (216). These results indicate that ghrelin signaling may impact fatty acid taste and olfactory pathways.
4.4. Genetic Polymorphisms
This section will discuss the 10 studies that specifically examined genetic variability associated with fat taste and olfaction (24, 53, 67, 68, 71–73, 77, 78, 80).
4.4.1. CD36.
Single nucleotide polymorphisms (SNPs), variations to a single nucleotide (basepair) within a gene, are the most common type of genetic variation (217). The most frequently studied genetic polymorphisms associated with fat chemosensation are CD36 polymorphisms. The rs1761667, rs1527483, rs2312018, and rs3840546 SNPs of CD36 are reportedly associated with fat perception (TABLE 5). Of these SNPs, the rs1761667 CD36 polymorphism is the most studied in the context of fat taste and has been examined across multiple populations. It has been suggested that the A allele in this SNP is associated with lower oral fat sensitivity, and the G allele is associated with higher oral fat sensitivity (218, 219). However, this association is not fully understood, and a few studies examining the association between the rs1761667 SNP and oral fat perception have conflicting findings (24, 53, 67, 68, 71, 72, 77). The effect of this polymorphism on CD36 function is not fully understood.
Studies included in this review were somewhat consistent with the hypothesis that the G allele of rs1761667 is associated with higher oral fat taste sensitivity than the A allele in specific populations. Melis et al. (67) found that Caucasian adults with the G allele of rs1761667 displayed higher sensitivity to OLA than participants with the AA genotype. Similarly, Burgess et al. (53) found that East Asians homozygous for the G allele of the rs1761667 polymorphism perceived more overall fattiness and creaminess compared to participants with the AA genotype. These GG subjects showed a higher sensitivity to OLA than homozygous AA subjects. However, no differences in fat perception were observed for Caucasians. Thus the authors suggested that the variation at rs1761667 may have ancestry-specific effects on fat perception (53). Similar results were observed by Karthi et al. (63) in a population of Indian adults. Participants with the AA/AG genotype exhibited a significantly higher LA detection threshold and higher BMI than those with the GG genotype (63). Therefore, there is growing evidence that the A allele of the rs1761667 SNP is associated with lower fat taste sensitivity.
However, other studies have reported conflicting findings. A study by Ong et al. (72), which included individuals of East Asian ancestry, also examined the association between rs1761667 and oral fat perception. Malaysian subjects of Chinese and Indian ethnicity were presented with custards of varying fat contents as well as commercially available high-fat foods (i.e., milk mayonnaise, and cream crackers). Ong et al. (72) found a significant association between the rs1761667 SNP and fat taste for cream crackers in the Malaysian subjects. Individuals with the AA genotype of rs1761667 SNP had a lower sensitivity to fat and oiliness of cream crackers. However, they did not find a significant association between the rs1761667 SNP and any other HF tastants (72). A study by Bajit et al. (50) on Moroccan subjects did find an association between the AA and AG genotypes of the rs1761667 SNP and a higher fat sensitivity threshold in subjects with obesity. However, there were large variations in fat sensitivity threshold concentrations for this group, and thus no significant associations were observed. The authors noted that this result was likely due to a small sample size, variations in habitual food consumption, or unfamiliarity with the OA threshold test (50).
Similarly, Shen et al. (77) found no association between CD36 rs1761667 and liking of ice cream in European adults from the United Kingdom. They utilized ice cream as a tastant, which includes a high concentration of fat but also includes a high concentration of sugar and other ingredients (77). Further research comparing individuals of different ancestry is needed to clarify the role of rs1761667 in oral fat perception. Additionally, tastants used have widely varied, so it would be important to utilize more standard tastants across studies.
There is also no consensus regarding the role of CD36 polymorphisms and fat taste in the context of obesity. Mrizak et al. (71) reported an association between the GG genotype of CD36 rs1761667 SNP and decreased lipid taste perception in Tunisian women with obesity. These women had lower (more than 3 times lower) oral detection threshold (higher sensitivity) for OLA compared to women with obesity with the AA genotype. This indicates that those with the AA genotype had lower fat taste sensitivity than the GG genotype and highlights the importance of the rs1761667 SNP in women with obesity. However, as both groups were comprised of women with obesity, it was difficult to determine whether sex or body weight affected oral fat perception (71). A follow-up study by Karmous et al. (24) found that the frequency of the AA genotype of rs1761667 was higher in obese Tunisian men and women compared to their normal-weight counterparts. This further supports the association between rs1761667 and obesity across Tunisian adults (24). This is supported by a study by Melis et al. (68), which found that the G allele of the rs1761667 SNP, compared to the A allele, was associated with a trend for increased waist-to-hip ratio in obese subjects, even though they exhibited decreased BMI (68). This is consistent with a study by Solakivi et al. (78), which found that the AA genotype of rs1761667 was significantly associated with lower BMI, compared to AG and GG variants in middle-aged adults. Additionally, Chmurzynska et al. (55) found that participants with the GG CD36 genotype were more likely to be fat discriminators than participants who were carriers of the A allele, indicating that fat discrimination is associated with CD36 polymorphism. However, they found that fat discrimination was not associated with fat intake and polymorphisms of CD36, FFAR1 (rs1573611), FFAR4 (rs17108973), or CA6 (rs2274333) were not associated with the frequency of high-fat-food consumption (55). However, the Ong et al. (72) study discussed above did not find an association between the rs1761667 SNP, adiposity, or oral fat perception in Malaysian participants (72). Thus the relationship between the CD36 rs1761667 SNP and fat taste remains to be elucidated (68). The role of oral fat perception and obesity is further discussed in sect. 5.
In addition to rs1761667, other CD36 SNPs, such as rs1527483 and rs2312018, have been examined in the context of fat perception. Plesník et al. (73) examined the association between LA detection and rs2312018. No association was found between rs2312018 polymorphisms and LA detection or other anthropometric variables (BMI, waist circumference, and waist-to-height ratio). This suggests that rs2312018 may not be associated with oral fat perception of LA in this population (73). However, further studies should be conducted to better understand the role of rs231018 in oral fat perception.
The CD36 SNP rs1527483 may play a role in oral fat perception and has been linked to high nonesterified fatty acids plasma levels. A previous study was the first to suggest that rs1527483 may play a role in oral fat perception in African Americans (220). Additionally, Chamoun et al. (54) investigated the relationship between single nucleotide polymorphisms in taste receptor genes and psychophysical measures including detection threshold, suprathreshold sensitivity, and fat taste preference. They found that the CD36 SNPs rs1527483 and rs3211908 have significant associations with fat taste sensitivity and preference (54). A later study by Melis et al. (67) found associations between bitter and fat taste sensitivity. They reported that Caucasian participants with a CC genotype of rs1527483, who were also homozygous for the PAV taster variant [associated with super taste perception of 6-n-propylthiouracil (PROP)] of the bitter taste receptor TAS2R38, displayed a 5-fold lower threshold (higher sensitivity) for fat. PROP super tasters with the CC genotype of rs1527483 also showed a 4-fold lower OLA threshold (higher sensitivity) than PROP nontasters with the same genotype. However, there was no association (independent of PAV or PROP status) between rs1527483 and OLA detection (67). Ong et al. (72) found that rs1527483 played a more dominant role in fat perception than rs1761667. Ong et al. reported that females with the TT genotype of rs1527483 significantly perceived greater creaminess of a fat-containing custard (10% fat-by-weight). Also, individuals with the TT genotype of rs1527483 significantly perceived greater fat content of cream crackers, independent of fat concentration. This suggests that the TT genotype of rs1527483 is associated with increased oral fat taste sensitivity (72). However, a study by Plesník et al. (73) found that participants with the CC genotype of rs1527483 had higher sensitivity for LA than participants with either a TT or CT genotype. Additionally, unlike Ong et al. (72), who did not find an association between the CC genotype and obesity, Plesník et al. (73) found that rs1527483 was also associated with lower BMI (P = 0.011), waist circumference (P = 0.005), and waist-to-height ratio (P = 0.010). However, Ong et al. (72) and Plesník et al. (73) examined fat sensitivity in different populations (Czech young adults and Malaysian adults, respectively) and utilized different fat-containing tastants (fat-containing foods versus a LA solution). Fat taste sensitivity concerning rs1527483 may vary across populations of different genetic ancestry. Additionally, the combination of fat and other ingredients (e.g., custards and cream) may be perceived differently than fat alone. Thus the association between rs1527483, fat sensitivity, different fat tastants, and ancestry remains to be clarified.
4.4.2. ADRB3.
A study by Watanabe et al. (80) in Japanese young adults found that the TC genotype of the ADRB3 Trp64Arg polymorphism is associated with a higher preference for high-fat sweet food. These individuals also demonstrated an increased preference for higher degrees of greasiness. This variant is associated with a reduction in noradrenalin-dependent lipolysis activity. The 64th amino acid (Trp) is located on the first intracellular loop of ADRB3 and is essential for the effector and movement function of this protein (80). Therefore, the Trp64Arg variant can lead to protein conformational changes impairing the function of ADRB3. However, the mechanism through which ADRB3 impacts fat perception is not fully understood.
4.4.3. BDNF.
A study by Graham et al. (60) in a UK female cohort looked at the brain-derived neurotrophic factor (BDNF) gene, known to be a regulator of appetite. The aim of their study was to determine whether known genetic polymorphisms related to fat taste and dietary intake would lead to differences in fat taste sensitivity. The study found that participants with the CT/TT genotype of SNP rs6265 BDNF had a lower fat taste threshold than the CC genotype, highlighting an association between BDNF and fat taste sensitivity.
4.4.4. CA6.
Polymorphisms of other genes, including the CA6 gene that codes for the salivary trophin factor Gustin/CA6, have also been examined in the context of fat taste. Shen et al. (77) found a significant association between the GG genotype of CA6 SNP rs2274333 and liking of ice cream. Subjects with the GG genotype of rs2274333 had a significantly lower liking of the taste of ice cream (a high-fat food) than subjects with the AA or AG genotype. This decreased preference for high-fat food suggests that the GG genotype of rs2274333 may reduce oral fat preference. However, since ice cream also contains high levels of sugar and other ingredients, this preference change may be associated with another ingredient (77). The association between CA6 polymorphisms and other taste modalities has been examined, and studies have reported an association between CA6 and salt taste but not bitter taste (221, 222).
4.4.5. TNNI3K.
A study by Graham et al. (60) in a UK female cohort examined the association between troponin I-interacting protein kinase (TNNI3K) gene and obesity. Participants with the AA/AG genotype of SNP rs1514175 TNN13K exhibited a higher fat taste threshold compared to the GG genotype. Participants with the AA/AG genotype also exhibited higher energy intake, total fat intake, and saturated fatty acid intake (60). This suggests TNN13K may play a role in obesity via mediation of fat taste sensitivity.
4.5. Implications of Obesity on Fat Taste and Smell
As discussed above, the sensory characteristics of food, i.e., smell and taste detection, intensity, quality, and hedonic valence, help determine its palatability (223). Additionally, there are documented taste and olfactory changes in individuals with obesity and following weight changes. In turn, taste and smell dysfunction can impact eating behavior. Chronic hyperphagia, alongside altered hedonics and genetic expression, can disrupt energy homeostasis and promote obesity. Individuals with obesity display a lack of postingestion adaptations, unlike their normal weight counterparts. Similarly, a predisposition for fat liking/preference has been observed in overweight individuals and animals. While normal weight individuals demonstrate a decrease in fat preference following food consumption and decreased hunger, individuals with obesity do not display decreased preference even in the absence of hunger (89). However, the relationship between fat taste threshold and intensity is unclear. A recent systematic review and meta-analysis found no significant differences in fatty acid taste threshold or intensity between lean and obese adults (224). Olfactory detection of fat in individuals with obesity compared to lean controls has also been studied, with varying results. While some studies have found an association between obesity and decreased olfactory sensitivity (225), other studies have reported that individuals with obesity had a stronger hedonic response to odors. Thus the association between olfactory sensitivity and obesity is unclear. Nine studies included in this section discuss the influence of obesity on fat chemosensation (52, 56, 74, 75, 89, 96, 98, 106, 226).
4.5.1. Obesity and taste.
Multiple studies have examined the association between fat taste threshold/sensitivity and obesity. For a review of fat taste sensitivity and obesity, see Tucker et. al. (224). In this review, we examine only studies that examined potential biological mechanisms underlying fat taste threshold in lean and obese individuals. Two studies examined potential biological mediators of fat taste threshold. Proserpio et al. (74) found that obese subjects exhibited higher threshold values for fat tastants than normal weight controls and significantly higher liking ratings for high-fat foods. Obese subjects also had a reduced number of fungiform papillae on the tongue. Thus the authors postulated that lower sensitivity may play a causal role in alterations to food preference in obesity (74). Costanzo et al. (56) conducted a randomized controlled trial with twin dyads to examine the effect of dietary fat intake and genetics on fat taste sensitivity. In two groups, 8 wk of LFD consumption increased fat taste sensitivity, while HFD attenuated fat taste sensitivity, regardless of body weight. Overall, the authors suggested that environmental factors and dietary composition, and not genetics, primarily drive dietary fat intake and sensitivity (56). These studies suggest that fungiform papillae density and nongenetic environmental factors may play a role in fat taste threshold in humans.
This review also identified studies that have examined the relationship between fat taste preference and intake and obesity. Djeziri et al. (96) looked at oleanolic acid’s (OLN) effects on obesity and fat preference in mice. They found that HFD-fed mice showed a significantly decreased preference for fat. They further reported that adding OLN to the HFD given to obese mice restored proper orosensory detection threshold for OLA. The authors suggested that improved fat sensitivity was likely due to increased CD36 mRNA expression and intracellular calcium concentration in TBCs (96). This suggests that OLN can help regulate fat taste sensitivity by mediating fatty acid detection. Schreiber et al. (121) examined the role of lingual input on obesity-resistant (OR) and obesity-prone (OP) rats. They found that while in OR rats, fungiform papillae density was higher, and a transection of glossopharyngeal nerves decreased HFD intake. Meanwhile, OP rats had lower papillae density and did not alter their HFD intake following nerve transection. Thus OP rats demonstrated dysregulated orosensory perception of high fat foods. These studies both report and highlight fat taste dysregulation in mouse models of obesity.
Two studies explored the relationship between CD36 expression with fat taste in obesity. Braymer et al. (89) examined the effect of lingual application of CD36 siRNA on fat preference. They measured LA preference in obesity-prone (OP) and lean obesity-resistant (OR) rats. While in OR rats, lingual application of CD36 siRNA decreased fat preference, OP rats did not decrease their preference for LA. This suggests that OP rats do not alter their fat preference based on nutritional status or changes in lingual CD36 levels. The relationship between CD36 and fat preference was also investigated following fasting. Fasting increased lingual CD36 mRNA in OR rats, but not in OP rats, and postfasting application of CD36 siRNA decreased LA preference in OR but not OP rats. These results support that CD36 is essential in regulating fat preference and demonstrate that obesity is associated with altered fat taste, likely mediated by CD36 dysregulation (89). The relationship between fat taste, CD36, and obesity has also been studied in humans. A 2019 cross-sectional study by Bricio-Barrios et al. (52) measured the correlation between CD36 and fat taste sensitivity, adiposity, and BMI. They showed that serum levels of the soluble form of CD36 (sCD36) are positively correlated with FFA sensitivity and negatively correlated with adiposity and BMI. Specifically, overweight subjects exhibited a higher threshold (lower sensitivity) for FAs and lower serum sCD36 levels than the normal-weight group (52). The results of both studies demonstrate that obesity can modulate the effect of CD36 on fat taste sensitivity and preference.
4.5.2. Obesity and olfaction.
Multiple studies have investigated how obesity can affect fat olfactory function. Lacroix et al. (106) compared olfactory performance and tissue homeostasis between DIO OP and lean normal chow-fed OR Sprague-Dawley rats. Results showed that OP rats experienced decreased odor threshold (higher sensitivity) but lowered olfactory performance and related memory/learning deficiency. Moreover, the authors found differences in olfactory mucosa and bulb homeostasis, electrical olfactory signaling, and cellular dynamics between the OP rats and control. The authors suggest that obese pathophysiology may significantly influence perturbed olfactory satiety signals and resulting food intake (106).
Similarly, Fardone et al. (98) looked at the impact of obesity on OSNs in mice. DIO mice fed MHF and HFD showed reduced neural activity in juxtaglomerular cells. They also identified an asymmetry in the responsiveness of the ‘mirror image’ glomerular map for the M72 receptor that shows greater sensitivity of the lateral versus medial glomerulus toward fatty diets. DIO also led to decreased OSNs that expressed olfactory marker protein (OMP) (98). OMP is important in OSN development, signaling transduction, and responsiveness to and discrimination of odors (227, 228). While the sensitivity results from LaCroix et al. (106) differ from these findings, their olfactory performance results support the mechanistic changes found in this study (98). These results indicate that DIO and high-fat intake significantly impairs OSN function and survival, ultimately dampening olfactory sensitivity to fats and other odors (98). Thus Lacroix et al. (106) and Fardone et al. (98) observed differences in the olfactory bulb and neurons projecting to the olfactory bulb in DIO mice, highlighting the importance of the olfactory bulb to olfactory perception of fat.
Meanwhile, Boone et al. (88) found that anosmic mice (following complete removal of the olfactory bulb) and sham mice (with intact olfactory bulbs) both displayed comparable HFD intake. The authors also observed that a HFD smell (in the absence of consumption) did not alter feeding or devaluation of standard food. Thus, while the olfactory bulb may play a role in fat olfaction, it may not be necessary for the development of a HFD preferential consumption (88). It is important to note that while Boone et al., postulated that olfaction is not necessary for fat preference or devaluation of standard food, this does not entail that the olfactory bulb and olfaction do not play a role in fat preference or detection. Rather, these findings highlight how much emphasis the body places on fat chemosensation; multiple systems have developed in parallel to ensure the consumption of this important macronutrient. As mentioned in the preceding sections, a predilection for fat can develop in response to other nonolfactory cues, such as oral cues and postingestive cues.
Besides transduction pathways, genetic and epigenetic changes can regulate olfactory function, impacting eating behavior and dietary intake. Ramos-Lopez et al. (75) used roughly 500 human subjects from the Methyl Epigenome Network Association to understand the relationships between olfactory genes and clinical variables indicative of obesity. Their results identified several associations between olfactory pathway gene methylation patterns, dietary intake, and anthropometric measures (BMI and waist circumference), independently of age and sex. These genes included olfactory receptors (e.g., OR4D2, OR51A7, OR2T34, OR2Y1) and downstream signaling molecules (e.g., SLC8A1, ANO2, PDE2A, CALML3, GNG7, CALML6, PRKG1, CAMK2D). Pathway enrichment analyses revealed that these genes were significantly involved in odor perception and olfactory cascade signal transduction. Specifically, methylation patterns of OR4D2 and OR2Y1 were strongly correlated with daily energy and macronutrient intakes. These data signify that dietary habits and weight status may significantly impact olfactory gene methylation and olfactory function (75). Olfactory and taste-related genes are also associated with transgenerational nutrient sensing. Ng et al. (226) found that paternal HFD caused downregulation of 187 olfactory transduction Olr genes in retroperitoneal white adipose tissue and pancreatic islets of female rats. This suggests that olfaction pathways may be significantly impacted by paternal obesity. The genes susceptible to epigenetic modification may lead to odor perception dysfunction, ultimately leading to changes in eating behavior and body weight regulation (75). In turn, these genetic modifications could increase propensity for obesity and its comorbidities. Further studies in clinical populations are needed to examine the heritability of olfactory genes and their association with eating behavior and obesity.
4.5.3. Limitations.
This systematic review’s search strategy was formulated by experienced chemosensory researchers and a library specialist with expertise in systematic reviews. Our results are limited by our inclusion and exclusion criteria. This includes the sole inclusion of studies published in English, which would fail to capture non-English literature and would be subject to bias against studies contributed from specific nations or regions. Furthermore, our search strategy yielded a wide variation of clinical and preclinical studies, with heterogeneous variables of interest, methodologies, and populations. Thus, although we collected rich data through this comprehensive systematic review, we were unable to conduct a meta-analysis.
Importantly, we observed a lack of standardized reporting of methodology and results across many of the animal studies. For example, many did not report randomization and/or blinding and/or provide detailed statistical methods. The risk of bias is reflected in the SYRCLE scores, with 78% of the studies scoring below 10 (out of a total possible score of 20). However, we chose to include all the animal studies, despite their low SYRCLE scores, because it may reflect reporting inconsistencies across animal studies rather than the quality of the science. Thus this should be considered when interpreting the preclinical results reported in the present systematic review. The observed limitations in animal studies have been identified, highlighted, and reported in multiple systematic reviews (229–231). This has led to journals requiring the use of reporting guidelines, such as the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. However, it is important that authors, journals, and reviewers adhere and ensure adherence, respectively, to ARRIVE or similar reporting guidelines. This is important to improve transparency in experimental design, reporting, and statistical analyses of animal studies to increase reproducibility and facilitate systematic reviews and meta-analyses in the future. This would strengthen not only the validity and rigor of the studies but would also be an important step toward better interpreting and translating preclinical studies into clinical efforts.
5. CONCLUSIONS
The present systematic review highlights a growing interest in fat chemosensation in the context of eating behavior and obesity. Multiple studies examined the role of several proteins in fat chemosensation, including CD36 and GPR120, which act together to detect and/or mediate fat detection by TBCs and OSNs. The involvement of CD36 in fat chemosensation is supported by studies that have found that genetic variations of CD36 (e.g., rs1761667, rs1527483, and rs2312018) can significantly impact individuals’ fat sensitivity and preference. On a cellular level, studies have begun to identify and map signal transduction pathways involved in fat chemosensation. These pathways are initiated by the binding of FFAs to fat chemoreceptors and activate downstream signaling cascades, involving calcium signaling, and leading to cellular depolarization of TBCs and olfactory-related cells. Furthermore, multiple brain regions (e.g., mPFC, OFC, striatum, amygdala, VTA, and somatosensory cortex), neural signaling pathways, and neuromodulators regulate the hedonic properties of fat. Subsequently, hormones, ingestive, and postingestive signals (e.g., insulin, GLP-1, PYY, ghrelin, and serotonin) can reinforce or inhibit the hedonic properties of fatty acids and contribute to fat perception and consumption. As more studies examining fat taste and smell emerge, we hope to learn more about the biological mechanisms underlying fat chemosensation. Understanding these complex interactions is important as we continue to better understand the multifactorial nature of eating behavior and obesity. Identifying and investigating these mechanisms can be important steps in developing management strategies and/or interventions to treat obesity and associated comorbid conditions.
SUPPLEMENTAL INFORMATION
Supplementary material available at DOI: https://doi.org/10.6084/m9.figshare.20415975.v1.
GRANTS
P.V.J. is supported by National Institute of Alcohol Abuse and Alcoholism Grant Z01AA000135, the National Institute of Nursing Research, and the Rockefeller University Heilbrunn Nurse Scholar Award. P.V.J. and H.A.T. are supported by the Office of Workforce Diversity, National Institutes of Health Distinguished Scholar Program. H.A.T. is supported by the National Institute on Mental Health and the Brain and Behavior Foundation Young Investigator Award. R.B.J., C.V., and K.A. received postdoctoral Intramural Research Training Awards, Office of Intramural Training & Education. R.S.E.O.-F., B.E.B., M.C., and N.N. received Postbaccalaureate Intramural Research Training Awards, Office of Intramural Training & Education, and N.I. received a summer fellowship award, Office of Intramural Training & Education, National Institutes of Health, Department of Health and Human Services.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B.J.-T., C.V., A.A.L., H.A.T., and P.V.J. and conceived and designed research; R.B.J.-T. and A.A.L. analyzed data; R.B.J., B.E.B., C.V., M.C., N.N., R.S.E.O.-F., A.A.L., K.A., C.C.-P., N.I., A.H., H.A.T., and P.V.J., interpreted results of experiments; R.B.J.-T., B.E.B., C.V., K.A., C.C.-P., and N.I. prepared figures; R.B.J.-T., B.E.B., C.V., M.C., N.N., R.S.E.O.-F., A.A.L., K.A., C.C.-P., N.I., H.A.T., and P.V.J. drafted manuscript; R.B.J.-T., B.E.B., C.V., M.C., N.N., A.A.L., K.A., C.C.-P., A.H., H.A.T., and P.V.J., edited and revised manuscript; R.B.J.-T., C.V., M.C., N.N., R.S.E.O.-F., A.A.L., K.A., C.C.-P., N.I., A.H., H.A.T., and P.V.J. approved final version of manuscript.
ACKNOWLEDGEMENTS
The authors thank Erina He and Alan Hoofring for assistance in creating the graphical abstract and FIGURES 1–3 and FIGURE 6. FIGURES 3 and 6 are adapted from Figures 1.6 and 1.7 in Ref. 26. FIGURES 4 and 7–9 were created with BioRender.com.
REFERENCES
- 1. Dudine L, Canaletti C, Giudici F, Lunardelli A, Abram G, Santini I, Baroni V, Paris M, Pesavento V, Manganotti P, Ronchese F, Gregoretti B, Negro C. Investigation on the loss of taste and smell and consequent psychological effects: a cross-sectional study on healthcare workers who contracted the COVID-19 infection. Front Public Health 9: 666442, 2021. doi: 10.3389/fpubh.2021.666442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hur K, Choi JS, Zheng M, Shen J, Wrobel B. Association of alterations in smell and taste with depression in older adults. Laryngoscope Investig Otolaryngol 3: 94–99, 2018. doi: 10.1002/lio2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, , et al. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses 45: 609–622, 2020. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, Lin C, Joseph PV, Reed DR. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chemical Senses 47: bjac001, 2022. doi: 10.1093/chemse/bjac001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Hannum ME, Ramirez VA, Lipson SJ, Herriman RD, Toskala AK, Lin C, Joseph PV, Reed DR. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19-positive patients compared to subjective methods: a systematic review and meta-analysis. Chem Senses 45: 865–874, 2020. doi: 10.1093/chemse/bjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu N, Yang D, Zhang T, Sun J, Fu J, Li H. Systematic review and meta-analysis of olfactory and gustatory dysfunction in COVID-19. Int J Infect Dis 117: 155–161, 2022. doi: 10.1016/j.ijid.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker JK, Kelly CE, Smith BC, Kirkwood AF, Hopkins C, Gane S. Patients’ perspectives on qualitative olfactory dysfunction: thematic analysis of social media posts. JMIR Form Res 5: e29086, 2021. doi: 10.2196/29086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohla K, Veldhuizen MG, Green T, Hannum ME, Bakke AJ, Moein ST, Tognetti A, Postma EM, Pellegrino R, Hwang DL, Albayay J, Koyama S, Nolden AA, Thomas-Danguin T, Mucignat-Caretta C, Menger NS, Croijmans I, Öztürk L, Yanık H, Pierron D, Pereda-Loth V, Nunez-Parra A, Martinez Pineda AM, Gillespie D, Farruggia MC, Cecchetto C, Fornazieri MA, Philpott C, Voznessenskaya V, Cooper KW. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology 60: 207–217, 2022. doi: 10.4193/Rhin21.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan M, Yoo SJ, Clijsters M, Backaert W, Vanstapel A, Speleman K, Lietaer C, Choi S, Hether TD, Marcelis L, Nam A, Pan L, Reeves JW, Van Bulck P, Zhou H, Bourgeois M, Debaveye Y, De Munter P, Gunst J, Jorissen M, Lagrou K, Lorent N, Neyrinck A, Peetermans M, Thal DR, Vandenbriele C, Wauters J, Mombaerts P, Van Gerven L. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 184: 5932–5949.e15, 2021. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, McArthur NG, Moeller R, Uhl S, Omer AD, Gottesman ME, Firestein S, Gong Q, Canoll PD, Goldman JE, Roussos P, tenOever BR, Lomvardas S., Overdevest JB. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 185: 1052–1064.e12, 2022. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 6: eabc5801, 2020. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, Albers MW, Barlow LA, Datta SR, Di Pizio A. COVID-19 and the chemical senses: supporting players take center stage. Neuron 107: 219–233, 2020. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doyle ME, Appleton A, Liu QR, Yao Q, Mazucanti CH, Egan JM. Human type II taste cells express angiotensin-converting enzyme 2 and are infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J Pathol 191: 1511–1519, 2021. doi: 10.1016/j.ajpath.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boesveldt S, de Graaf K. The differential role of smell and taste for eating behavior. Perception 46: 307–319, 2017. doi: 10.1177/0301006616685576. [DOI] [PubMed] [Google Scholar]
- 15. Lundström JN, Boesveldt S, Albrecht J. Central processing of the chemical senses: an overview. ACS Chem Neurosci 2: 5–16, 2011. doi: 10.1021/cn1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zohoori FV. Chapter 1: nutrition and diet. Monogr Oral Sci 28: 1–13, 2020. doi: 10.1159/000455365. [DOI] [PubMed] [Google Scholar]
- 17. Carreiro AL, Dhillon J, Gordon S, Higgins KA, Jacobs AG, McArthur BM, Redan BW, Rivera RL, Schmidt LR, Mattes RD. The macronutrients, appetite, and energy intake. Annu Rev Nutr 36: 73–103, 2016. doi: 10.1146/annurev-nutr-121415-112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: a sixth taste modality? Physiol Rev 96: 151–176, 2016. doi: 10.1152/physrev.00002.2015. [DOI] [PubMed] [Google Scholar]
- 19. Parma V, Boesveldt S. Measurement of olfaction: screening and assessment. In: Sensory Science and Chronic Diseases: Clinical Implications and Disease Management, edited by Joseph PV, Duffy VB.. Cham, Germany: Springer International Publishing, 2021, p. 45–63. [Google Scholar]
- 20. Duffy VB, Rawal S, Hayes JE. Measurement of gustation: from clinical to population-based methods. In: Sensory Science and Chronic Diseases: Clinical Implications and Disease Management, edited by Joseph PV, Duffy VB.. Cham, Germany: Springer International Publishing, 2021, p. 65–102. [Google Scholar]
- 21. Bartoshuk LM. The psychophysics of taste. Am J Clin Nutr 31: 1068–1077, 1978. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- 22. Joseph PV, Mennella JA, Cowart BJ, Pepino MY. Psychophysical tracking method to assess taste detection thresholds in children, adolescents, and adults: the taste detection threshold (TDT) test. J Vis Exp 170: 10.3791/62384, 2021. doi: 10.3791/62384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mennella JA, Bobowski NK. Psychophysical tracking method to measure taste preferences in children and adults. J Vis Exp 113: 10.3791/54163, 2016. doi: 10.3791/54163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karmous I, Plesník J, Khan AS, Šerý O, Abid A, Mankai A, Aouidet A, Khan NA. Orosensory detection of bitter in fat-taster healthy and obese participants: genetic polymorphism of CD36 and TAS2R38. Clin Nutr 37: 313–320, 2018. doi: 10.1016/j.clnu.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 25. Fernández-Aranda F, Agüera Z, Fernández-García JC, Garrido-Sanchez L, Alcaide-Torres J, Tinahones FJ, Giner-Bartolomé C, Baños RM, Botella C, Cebolla A, de la Torre R, Fernández-Real JM, Ortega FJ, Frühbeck G, Gómez-Ambrosi J, Granero R, Islam MA, Jiménez-Murcia S, Tárrega S, Menchón JM, Fagundo AB, Sancho C, Estivill X, Treasure J, Casanueva FF. Smell-taste dysfunctions in extreme weight/eating conditions: analysis of hormonal and psychological interactions. Endocrine 51: 256–267, 2016. doi: 10.1007/s12020-015-0684-9. [DOI] [PubMed] [Google Scholar]
- 26. Jaime-Lara RB, To L, Joseph PV. Anatomy, physiology, and neurobiology of olfaction, gustation, and chemesthesis. In: Sensory Science and Chronic Diseases: Clinical Implications and Disease Management, edited by Joseph PV, Duffy VB.. Cham, Germany: Springer International Publishing, 2021, p. 3–20. [Google Scholar]
- 27. Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg 117: 519–528, 1991. doi: 10.1001/archotol.1991.01870170065015. [DOI] [PubMed] [Google Scholar]
- 28. Goodspeed RB, Gent JF, Catalanotto FA. Chemosensory dysfunction. Clinical evaluation results from a taste and smell clinic. Postgrad Med 81: 251–257, 1987. doi: 10.1080/00325481.1987.11699680. [DOI] [PubMed] [Google Scholar]
- 29. Roper SD. TRPs in taste and chemesthesis. Handb Exp Pharmacol 223: 827–871, 2014. doi: 10.1007/978-3-319-05161-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aroke E, Powell-Roach K, Jaime-Lara R, Tesfaye M, Roy A, Jackson P, Joseph P. Taste the pain: the role of TRP channels in pain and taste perception. Int J Mol Sci 21: 5929, 2020. doi: 10.3390/ijms21165929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simons CT, Klein AH, Carstens E. Chemogenic subqualities of mouthfeel. Chem Senses 44: 281–288, 2019. doi: 10.1093/chemse/bjz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Green BG. Chemesthesis: pungency as a component of flavor. Trends Food Sci Technol 7: 415–420, 1996. doi: 10.1016/S0924-2244(96)10043-1. 16617338 [DOI] [Google Scholar]
- 33. Mattes RD. Is there a fatty acid taste? Annu Rev Nutr 29: 305–327, 2009. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Running CA, Craig BA, Mattes RD. Oleogustus: the unique taste of fat. Chem Senses 40: 507–516, 2015. doi: 10.1093/chemse/bjv036. [DOI] [PubMed] [Google Scholar]
- 35. Keast RS, Costanzo A. Is fat the sixth taste primary? Evidence and implications. Flavour 4: 5, 2015. doi: 10.1186/2044-7248-4-5. [DOI] [Google Scholar]
- 36. Garneau NL, Nuessle TM, Mendelsberg BJ, Shepard S, Tucker RM. Sweet liker status in children and adults: consequences for beverage intake in adults. Food Qual Prefer 65: 175–180, 2018. doi: 10.1016/j.foodqual.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fukuwatari T, Shibata K, Iguchi K, Saeki T, Iwata A, Tani K, Sugimoto E, Fushiki T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol Behav 78: 579–583, 2003. doi: 10.1016/S0031-9384(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 38. Takeda M, Sawano S, Imaizumi M, Fushiki T. Preference for corn oil in olfactory-blocked mice in the conditioned place preference test and the two-bottle choice test. Life Sci 69: 847–854, 2001. doi: 10.1016/S0024-3205(01)01180-8. [DOI] [PubMed] [Google Scholar]
- 39. Tucker RM, Mattes RD. Influences of repeated testing on nonesterified fatty acid taste. Chem Senses 38: 325–332, 2013. doi: 10.1093/chemse/bjt002. [DOI] [PubMed] [Google Scholar]
- 40. Mattes RD. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses 34: 145–150, 2009. doi: 10.1093/chemse/bjn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Voigt N, Stein J, Galindo MM, Dunkel A, Raguse JD, Meyerhof W, Hofmann T, Behrens M. The role of lipolysis in human orosensory fat perception. J Lipid Res 55: 870–882, 2014. doi: 10.1194/jlr.M046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu D, Archer N, Duesing K, Hannan G, Keast R. Mechanism of fat taste perception: association with diet and obesity. Prog Lipid Res 63: 41–49, 2016. doi: 10.1016/j.plipres.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 43. Reed DR, Xia MB. Recent advances in fatty acid perception and genetics. Adv Nutr 6: 353S–60S, 2015. doi: 10.3945/an.114.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DiPatrizio NV. Is fat taste ready for primetime? Physiol Behav 136: 145–154, 2014. doi: 10.1016/j.physbeh.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tucker RM, Mattes RD, Running CA. Mechanisms and effects of “fat taste” in humans. Biofactors 40: 313–126, 2014. doi: 10.1002/biof.1162. [DOI] [PubMed] [Google Scholar]
- 46. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 350: g7647, 2015. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 47. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372: n160, 2021. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andersen CA, Nielsen L, Møller S, Kidmose P. Cortical response to fat taste. Chem Senses 45: 283–291, 2020. doi: 10.1093/chemse/bjaa019. [DOI] [PubMed] [Google Scholar]
- 49. Appelqvist IA, Poelman AA, Cochet-Broch M, Delahunty CM. Impact of model fat emulsions on sensory perception using repeated spoon to spoon ingestion. Physiol Behav 160: 80–86, 2016. doi: 10.1016/j.physbeh.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 50. Bajit H, Ait Si Mohammed O, Guennoun Y, Benaich S, Bouaiti E, Belghiti H, Mrabet M, Elfahime EM, El Haloui NE, Saeid N, El Kari K, Hichami A, Khan NA, Benkirane H, Aguenaou H. Single-nucleotide polymorphism rs1761667 in the CD36 gene is associated with orosensory perception of a fatty acid in obese and normal-weight Moroccan subjects. J Nutr Sci 9: e24, 2020. doi: 10.1017/jns.2020.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Besnard P, Christensen JE, Brignot H, Bernard A, Passilly-Degrace P, Nicklaus S, Pais de Barros JP, Collet X, Lelouvier B, Servant F, Blasco-Baque V, Verges B, Lagrost L, Feron G, Burcelin R. Obese subjects with specific gustatory papillae microbiota and salivary cues display an impairment to sense lipids. Sci Rep 8: 6742, 2018. doi: 10.1038/s41598-018-24619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bricio-Barrios JA, Del Toro-Equihua M, Huerta M, Ríos-Silva M, Cárdenas Y, López M, Saavedra-Molina A, Urzúa Z, Ortiz-Mesina M, Andrade-Urzúa F, García-Contreras JA, Trujillo X. Oral fatty acid taste sensitivity in healthy young individuals of both sexes is related to body mass index and soluble CD36 serum levels. Nutr Hosp 36: 1133–1138, 2019. doi: 10.20960/nh.02419. [DOI] [PubMed] [Google Scholar]
- 53. Burgess B, Melis M, Scoular K, Driver M, Schaich KM, Keller KL, Tomassini Barbarossa I, Tepper BJ. Effects of CD36 genotype on oral perception of oleic acid supplemented safflower oil emulsions in two ethnic groups: a preliminary study. J Food Sci 83: 1373–1380, 2018. doi: 10.1111/1750-3841.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chamoun E, Liu AS, Duizer LM, Feng Z, Darlington G, Duncan AM, Haines J, Ma DW. Single nucleotide polymorphisms in sweet, fat, umami, salt, bitter and sour taste receptor genes are associated with gustatory function and taste preferences in young adults. Nutr Res 85: 40–46, 2021. doi: 10.1016/j.nutres.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 55. Chmurzynska A, Mlodzik-Czyzewska MA, Galinski G, Malinowska AM, Radziejewska A, Mikołajczyk-Stecyna J, Bulczak E, Wiebe DJ. Polymorphism of CD36 determines fat discrimination but not intake of high-fat food in 20- to 40-year-old adults. J Nutr 150: 2016–2022, 2020. doi: 10.1093/jn/nxaa136. [DOI] [PubMed] [Google Scholar]
- 56. Costanzo A, Nowson C, Orellana L, Bolhuis D, Duesing K, Keast R. Effect of dietary fat intake and genetics on fat taste sensitivity: a co-twin randomized controlled trial. Am J Clin Nutr 107: 683–694, 2018. doi: 10.1093/ajcn/nqy022. [DOI] [PubMed] [Google Scholar]
- 57. Eldeghaidy S, Marciani L, Hort J, Hollowood T, Singh G, Bush D, Foster T, Taylor AJ, Busch J, Spiller RC, Gowland PA, Francis ST. Prior consumption of a fat meal in healthy adults modulates the brain’s response to fat. J Nutr 146: 2187–2198, 2016. doi: 10.3945/jn.116.234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frank-Podlech S, Heinze JM, Machann J, Scheffler K, Camps G, Fritsche A, Rosenberger M, Hinrichs J, Veit R, Preissl H. Functional connectivity within the gustatory network is altered by fat content and oral fat sensitivity–a pilot study. Front Neurosci 13: 725, 2019. doi: 10.3389/fnins.2019.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grabenhorst F, Rolls ET. The representation of oral fat texture in the human somatosensory cortex. Hum Brain Mapp 35: 2521–2530, 2014. doi: 10.1002/hbm.22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Graham CA, Pilic L, King A, Nixon JE, Pipe J, Holton J, Tamba K, Hearne G, Pedlar CR, Lorente-Cebrián S, González Muniesa P, Mavrommatis Y. Genetic differences in fat taste sensitivity and dietary intake in a UK female cohort. Food Quality Prefer 92: 104202, 2021. doi: 10.1016/j.foodqual.2021.104202. [DOI] [Google Scholar]
- 61. Han P, Mohebbi M, Seo HS, Hummel T. Sensitivity to sweetness correlates to elevated reward brain responses to sweet and high-fat food odors in young healthy volunteers. Neuroimage 208: 116413, 2020. doi: 10.1016/j.neuroimage.2019.116413. [DOI] [PubMed] [Google Scholar]
- 62. Kadouh H, Chedid V, Halawi H, Burton DD, Clark MM, Khemani D, Vella A, Acosta A, Camilleri M. GLP-1 analog modulates appetite, taste preference, gut hormones, and regional body fat stores in adults with obesity. J Clin Endocrinol Metab 105: 1552–1563, 2020. doi: 10.1210/clinem/dgz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karthi M, Deepankumar S, Vinithra P, Gowtham S, Vasanth K, Praveen Raj P, Senthilkumar R, Selvakumar S. Single nucleotide polymorphism in CD36: correlation to peptide YY levels in obese and non-obese adults. Clin Nutr 40: 2707–2715, 2021. doi: 10.1016/j.clnu.2021.02.044. [DOI] [PubMed] [Google Scholar]
- 64. Kulkarni BV, Mattes RD. Lingual lipase activity in the orosensory detection of fat by humans. Am J Physiol Regul Integr Comp Physiol 306: R879–R885, 2014. doi: 10.1152/ajpregu.00352.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu D, Costanzo A, Evans MD, Archer NS, Nowson C, Duesing K, Keast R. Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br J Nutr 120: 64–73, 2018. doi: 10.1017/S0007114518001265. [DOI] [PubMed] [Google Scholar]
- 66. Méjean C, Morzel M, Neyraud E, Issanchou S, Martin C, Bozonnet S, Urbano C, Schlich P, Hercberg S, Péneau S, Feron G. Salivary composition is associated with liking and usual nutrient intake. PLoS One 10: e0137473, 2015. doi: 10.1371/journal.pone.0137473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Melis M, Sollai G, Muroni P, Crnjar R, Barbarossa IT. Associations between orosensory perception of oleic acid, the common single nucleotide polymorphisms (rs1761667 and rs1527483) in the CD36 gene, and 6-n-propylthiouracil (PROP) tasting. Nutrients 7: 2068–2084, 2015. doi: 10.3390/nu7032068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Melis M, Carta G, Pintus S, Pintus P, Piras CA, Murru E, Manca C, Di Marzo V, Banni S, Tomassini Barbarossa I. Polymorphism rs1761667 in the CD36 gene is associated to changes in fatty acid metabolism and circulating endocannabinoid levels distinctively in normal weight and obese subjects. Front Physiol 8: 1006, 2017. doi: 10.3389/fphys.2017.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Melis M, Mastinu M, Arca M, Crnjar R, Tomassini Barbarossa I. Effect of chemical interaction between oleic acid and L-arginine on oral perception, as a function of polymorphisms of CD36 and OBPIIa and genetic ability to taste 6-n-propylthiouracil. PLoS One 13: e0194953, 2018. doi: 10.1371/journal.pone.0194953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mounayar R, Morzel M, Brignot H, Tremblay-Franco M, Canlet C, Lucchi G, Ducoroy P, Feron G, Neyraud E. Nutri-metabolomics applied to taste perception phenotype: human subjects with high and low sensitivity to taste of fat differ in salivary response to oleic acid. Omics 18: 666–672, 2014. doi: 10.1089/omi.2014.0108. [DOI] [PubMed] [Google Scholar]
- 71. Mrizak I, Šerý O, Plesnik J, Arfa A, Fekih M, Bouslema A, Zaouali M, Tabka Z, Khan NA. The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br J Nutr 113: 1330–1337, 2015. doi: 10.1017/S0007114515000343. [DOI] [PubMed] [Google Scholar]
- 72. Ong HH, Tan YN, Say YH. Fatty acid translocase gene CD36 rs1527483 variant influences oral fat perception in Malaysian subjects. Physiol Behav 168: 128–137, 2017. doi: 10.1016/j.physbeh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 73. Plesník J, Šerý O, Khan AS, Bielik P, Khan NA. The rs1527483, but not rs3212018, CD36 polymorphism associates with linoleic acid detection and obesity in Czech young adults. Br J Nutr 119: 472–478, 2018. doi: 10.1017/S0007114517003981. [DOI] [PubMed] [Google Scholar]
- 74. Proserpio C, Laureati M, Bertoli S, Battezzati A, Pagliarini E. Determinants of obesity in italian adults: the role of taste sensitivity, food liking, and food neophobia. Chem Senses 41: 169–176, 2016. doi: 10.1093/chemse/bjv072. [DOI] [PubMed] [Google Scholar]
- 75. Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Zulet MA, Santos JL, Martinez JA; MENA project. Associations between olfactory pathway gene methylation marks, obesity features and dietary intakes. Genes Nutrition 14: 11, 2019. doi: 10.1186/s12263-019-0635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Running CA, Hayes JE, Ziegler GR. Degree of free fatty acid saturation influences chocolate rejection in human assessors. Chem Senses 42: 161–166, 2017. doi: 10.1093/chemse/bjw116. [DOI] [PubMed] [Google Scholar]
- 77. Shen Y, Kennedy OB, Methven L. The effect of genotypical and phenotypical variation in taste sensitivity on liking of ice cream and dietary fat intake. Food Quality Prefer 55: 79–90, 2017. doi: 10.1016/j.foodqual.2016.08.010. [DOI] [Google Scholar]
- 78. Solakivi T, Kunnas T, Nikkari ST. Contribution of fatty acid transporter (CD36) genetic variant rs1761667 to body mass index, the TAMRISK study. Scand J Clin Lab Invest 75: 254–258, 2015. doi: 10.3109/00365513.2014.1003596. [DOI] [PubMed] [Google Scholar]
- 79. Sun X, Veldhuizen MG, Babbs AE, Sinha R, Small DM. Perceptual and brain response to odors is associated with body mass index and postprandial total ghrelin reactivity to a meal. Chem Senses 41: 233–248, 2016. doi: 10.1093/chemse/bjv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Watanabe K, Hong G, Tominami K, Izumi S, Hayashi Y, Kudo TA. Association between beta3-adrenergic receptor Trp64Arg polymorphism and fat preference in healthy young Japanese women. Tohoku J Exp Med 248: 181–192, 2019. doi: 10.1620/tjem.248.181. [DOI] [PubMed] [Google Scholar]
- 81. Zhou X, Yeomans M, Thomas A, Wilde P, Linter B, Methven L. Individual differences in oral tactile sensitivity and gustatory fatty acid sensitivity and their relationship with fungiform papillae density, mouth behaviour and texture perception of a food model varying in fat. Food Quality Prefer 90: 104116, 2021. doi: 10.1016/j.foodqual.2020.104116. [DOI] [Google Scholar]
- 82. Ackroff K, Sclafani A. Post-oral fat stimulation of intake and conditioned flavor preference in C57BL/6J mice: a concentration-response study. Physiol Behav 129: 64–72, 2014. doi: 10.1016/j.physbeh.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahn JE, Chen Y, Amrein H. Molecular basis of fatty acid taste in Drosophila. Elife 6: e30115, 2017. doi: 10.7554/eLife.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ancel D, Bernard A, Subramaniam S, Hirasawa A, Tsujimoto G, Hashimoto T, Passilly-Degrace P, Khan NA, Besnard P. The oral lipid sensor GPR120 is not indispensable for the orosensory detection of dietary lipids in mice. J Lipid Res 56: 369–378, 2015. doi: 10.1194/jlr.M055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Avalos B, Argueta DA, Perez PA, Wiley M, Wood C, DiPatrizio NV. Cannabinoid CB(1) receptors in the intestinal epithelium are required for acute western-diet preferences in mice. Nutrients 12: 2874, 2020. doi: 10.3390/nu12092874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Avau B, Bauters D, Steensels S, Vancleef L, Laermans J, Lesuisse J, Buyse J, Lijnen HR, Tack J, Depoortere I. The gustatory signaling pathway and bitter taste receptors affect the development of obesity and adipocyte metabolism in mice. PLoS One 10: e0145538, 2015. doi: 10.1371/journal.pone.0145538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bensalem A, Murtaza B, Hichami A, Khan AS, Oulamara H, Merlen G, Berrichi M, Agli AN, Tordjmann T, Khan NA. Bile acid receptor TGR5 is critically involved in preference for dietary lipids and obesity. J Nutr Biochem 76: 108298, 2020. doi: 10.1016/j.jnutbio.2019.108298. [DOI] [PubMed] [Google Scholar]
- 88. Boone MH, Liang-Guallpa J, Krashes MJ. Examining the role of olfaction in dietary choice. Cell Rep 34: 108755, 2021. doi: 10.1016/j.celrep.2021.108755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Braymer HD, Zachary H, Schreiber AL, Primeaux SD. Lingual CD36 and nutritional status differentially regulate fat preference in obesity-prone and obesity-resistant rats. Physiol Behav 174: 120–127, 2017. doi: 10.1016/j.physbeh.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brown EB, Shah KD, Palermo J, Dey M, Dahanukar A, Keene AC. Ir56d-dependent fatty acid responses in Drosophila uncover taste discrimination between different classes of fatty acids. Elife 10: e67878, 2021. doi: 10.7554/eLife.67878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Buttigieg A, Flores O, Hernández A, Sáez-Briones P, Burgos H, Morgan C. Preference for high-fat diet is developed by young Swiss CD1 mice after short-term feeding and is prevented by NMDA receptor antagonists. Neurobiol Learn Mem 107: 13–18, 2014. doi: 10.1016/j.nlm.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 92. Calder AN, Yu T, Dahir NS, Sun Y, Gilbertson TA. ghrelin receptors enhance fat taste responsiveness in female mice. Nutrients 13: 1045, 2021. doi: 10.3390/nu13041045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Camandola S, Mattson MP. Toll-like receptor 4 mediates fat, sugar, and umami taste preference and food intake and body weight regulation. Obesity (Silver Spring) 25: 1237–1245, 2017. doi: 10.1002/oby.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. De la Cruz JA, Coke T, Karagiorgis T, Sampson C, Icaza-Cukali D, Kest K, Ranaldi R, Bodnar RJ. c-Fos induction in mesotelencephalic dopamine pathway projection targets and dorsal striatum following oral intake of sugars and fats in rats. Brain Res Bull 111: 9–19, 2015. doi: 10.1016/j.brainresbull.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 95. Devineni AV, Sun B, Zhukovskaya A, Axel R. Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses. Elife 8: e47677, 2019. doi: 10.7554/eLife.47677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Djeziri FZ, Belarbi M, Murtaza B, Hichami A, Benammar C, Khan NA. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie 152: 110–120, 2018. doi: 10.1016/j.biochi.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 97. Espitia-Bautista E, Escobar C. Fat rather than sugar diet leads to binge-type eating, anticipation, effort behavior and activation of the corticolimbic system. Nutr Neurosci 24: 508–519, 2019. doi: 10.1080/1028415x.2019.1651104. [DOI] [PubMed] [Google Scholar]
- 98. Fardone E, Celen AB, Schreiter NA, Thiebaud N, Cooper ML, Fadool DA. Loss of odor-induced c-Fos expression of juxtaglomerular activity following maintenance of mice on fatty diets. J Bioenerg Biomembr 51: 3–13, 2019. doi: 10.1007/s10863-018-9769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gaudet DA, El-Desoky D, Poret JM, Braymer HD, Primeaux SD. Expression of neural markers of gustatory signaling are differentially altered by continuous and intermittent feeding patterns. Physiol Behav 212: 112719, 2019. doi: 10.1016/j.physbeh.2019.112719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Olvera Hernández S, Reyes Castro LA, Daher Abdi A, Mezo-González CE, Arredondo A, Zambrano E, Bolaños-Jiménez F. Adult rats from undernourished dams show sex-dependent impaired expression in taste papillae and hypothalamus of genes responsible for sweet and fat detection and signalling. Nutr Neurosci 25: 2011–2012, 2021. doi: 10.1080/1028415x.2021.1920678. [DOI] [PubMed] [Google Scholar]
- 101. Iskhakov B, Dohnalova P, Iskhakova J, Mustac T, Yuabov A, Macanian J, Israel E, Locurto N, Franz N, Fazilov G, Shenouda M, Bodnar RJ. Strain differences in muscarinic cholinergic receptor antagonism of fat intake and acquisition and expression of fat-conditioned flavor preferences in male BALB/c, C57BL/6 and SWR mice. Pharmacol Biochem Behav 187: 172792, 2019. doi: 10.1016/j.pbb.2019.172792. [DOI] [PubMed] [Google Scholar]
- 102. Jung J, Kim DI, Han GY, Kwon HW. The effects of high fat diet-induced stress on olfactory sensitivity, behaviors, and transcriptional profiling in Drosophila melanogaster. Int J Mol Sci 19: 2855, 2018. doi: 10.3390/ijms19102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Khan AS, Subramaniam S, Dramane G, Khelifi D, Khan NA. ERK1 and ERK2 activation modulates diet-induced obesity in mice. Biochimie 137: 78–87, 2017. doi: 10.1016/j.biochi.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 104. Kim H, Kim H, Kwon JY, Seo JT, Shin DM, Moon SJ. Drosophila Gr64e mediates fatty acid sensing via the phospholipase C pathway. PLoS Genet 14: e1007229, 2018. doi: 10.1371/journal.pgen.1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kraft TT, Huang D, LaMagna S, Warshaw D, Natanova E, Sclafani A, Bodnar RJ. Acquisition and expression of fat-conditioned flavor preferences are differentially affected by NMDA receptor antagonism in BALB/c and SWR mice. Eur J Pharmacol 799: 26–32, 2017. doi: 10.1016/j.ejphar.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 106. Lacroix MC, Caillol M, Durieux D, Monnerie R, Grebert D, Pellerin L, Repond C, Tolle V, Zizzari P, Baly C. Long-lasting metabolic imbalance related to obesity alters olfactory tissue homeostasis and impairs olfactory-driven behaviors. Chem Senses 40: 537–556, 2015. doi: 10.1093/chemse/bjv039. [DOI] [PubMed] [Google Scholar]
- 107. Lee S, Eguchi A, Sakamoto K, Matsumura S, Tsuzuki S, Inoue K, Masuda D, Yamashita S, Fushiki T. A role of CD36 in the perception of an oxidised phospholipid species in mice. Biomed Res 36: 303–311, 2015. doi: 10.2220/biomedres.36.303. [DOI] [PubMed] [Google Scholar]
- 108. Lee S, Tsuzuki S, Amitsuka T, Masuda D, Yamashita S, Inoue K. CD36 involvement in the olfactory perception of oleic aldehyde, an odour-active volatile compound, in mice. Biomed Res 38: 207–213, 2017. doi: 10.2220/biomedres.38.207. [DOI] [PubMed] [Google Scholar]
- 109. Liu Y, Xu H, Dahir N, Calder A, Lin F, Gilbertson TA. GPR84 is essential for the taste of medium chain saturated fatty acids. J Neurosci 41: 5219–5228, 2021. doi: 10.1523/JNEUROSCI.2530-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Makarova E, Kazantseva A, Dubinina A, Jakovleva T, Balybina N, Baranov K, Bazhan N. The same metabolic response to FGF21 administration in male and female obese mice is accompanied by sex-specific changes in adipose tissue gene expression. Int J Mol Sci 22: 10561, 2021. doi: 10.3390/ijms221910561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mathes CM, Bohnenkamp RA, Blonde GD, Letourneau C, Corteville C, Bueter M, Lutz TA, Le Roux CW, Spector AC. Gastric bypass in rats does not decrease appetitive behavior towards sweet or fatty fluids despite blunting preferential intake of sugar and fat. Physiol Behav 142: 179–88188, 2015. doi: 10.1016/j.physbeh.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Murtaza B, Berrichi M, Bennamar C, Tordjmann T, Djeziri FZ, Hichami A, Leemput J, Belarbi M, Ozdener H, Khan NA. Zizyphin modulates calcium signalling in human taste bud cells and fat taste perception in the mouse. Fundam Clin Pharmacol 31: 486–494, 2017. doi: 10.1111/fcp.12289. [DOI] [PubMed] [Google Scholar]
- 113. Murtaza B, Hichami A, Khan AS, Shimpukade B, Ulven T, Ozdener MH, Khan NA. Novel GPR120 agonist TUG891 modulates fat taste perception and preference and activates tongue-brain-gut axis in mice. J Lipid Res 61: 133–142, 2020. doi: 10.1194/jlr.RA119000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Murtaza B, Hichami A, Khan AS, Plesnik J, Sery O, Dietrich A, Birnbaumer L, Khan NA. Implication of TRPC3 channel in gustatory perception of dietary lipids. Acta Physiol (Oxf) 231: e13554, 2021. doi: 10.1111/apha.13554. [DOI] [PubMed] [Google Scholar]
- 115. Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36-and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 146: 995–1005, 2014. doi: 10.1053/j.gastro.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Peterschmitt Y, Abdoul-Azize S, Murtaza B, Barbier M, Khan A, Millot JL, Khan N. Fatty acid lingual application activates gustatory and reward brain circuits in the mouse. Nutrients 10: 1246, 2018. doi: 10.3390/nu10091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ricci S, Viggiano D, Cimmino I, Perruolo G, Cabaro S, Liotti A, Fiory F, Spinelli R, Di Carlo A, Beguinot F, Formisano P, Oriente F. Prep1 deficiency affects olfactory perception and feeding behavior by impairing BDNF-TrkB mediated neurotrophic signaling. Mol Neurobiol 55: 6801–6815, 2018. doi: 10.1007/s12035-018-0873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sakamoto K, Matsumura S, Okafuji Y, Eguchi A, Lee S, Adachi SI, Fujitani M, Tsuzuki S, Inoue K, Fushiki T. Mechanisms involved in guiding the preference for fat emulsion differ depending on the concentration. J Nutr Sci Vitaminol (Tokyo) 61: 247–254, 2015. doi: 10.3177/jnsv.61.247. [DOI] [PubMed] [Google Scholar]
- 119. Sakamoto K, Okahashi T, Matsumura S, Okafuji Y, Adachi SI, Tsuzuki S, Inoue K, Fushiki T. The opioid system majorly contributes to preference for fat emulsions but not sucrose solutions in mice. Biosci Biotechnol Biochem 79: 658–663, 2015. doi: 10.1080/09168451.2014.991688. [DOI] [PubMed] [Google Scholar]
- 120. Sasaki T, Yasoshima Y, Matsui S, Yokota-Hashimoto H, Kobayashi M, Kitamura T. Intraperitoneal injection of d-serine inhibits high-fat diet intake and preference in male mice. Appetite 118: 120–128, 2017. doi: 10.1016/j.appet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 121. Schreiber A, Braymer HD, Primeaux SD. Transection of gustatory nerves differentially affects dietary fat intake in obesity-prone and obesity-resistant rats. Chem Senses 45: 541–548, 2020. doi: 10.1093/chemse/bjaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sclafani A, Ackroff K. Maltodextrin and fat preference deficits in “taste-blind” P2X2/P2X3 knockout mice. Chem Senses 39: 507–514, 2014. doi: 10.1093/chemse/bju019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sclafani A, Touzani K, Ackroff K. Intragastric fat self-administration is impaired in GPR40/120 double knockout mice. Physiol Behav 147: 141–148, 2015. doi: 10.1016/j.physbeh.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sclafani A, Ackroff K. Greater reductions in fat preferences in CALHM1 than CD36 knockout mice. Am J Physiol Regul Integr Comp Physiol 315: R576–R585, 2018. doi: 10.1152/ajpregu.00015.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sclafani A, Ackroff K. Role of lipolysis in postoral and oral fat preferences in mice. Am J Physiol Regul Integr Comp Physiol 315: R434–R441, 2018. doi: 10.1152/ajpregu.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Subramaniam S, Ozdener MH, Abdoul-Azize S, Saito K, Malik B, Maquart G, Hashimoto T, Marambaud P, Aribi M, Tordoff MG, Besnard P, Khan NA. ERK1/2 activation in human taste bud cells regulates fatty acid signaling and gustatory perception of fat in mice and humans. FASEB J 30: 3489–3500, 2016. doi: 10.1096/fj.201600422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tauber JM, Brown EB, Li Y, Yurgel ME, Masek P, Keene AC. A subset of sweet-sensing neurons identified by IR56d are necessary and sufficient for fatty acid taste. PLoS Genet 13: e1007059, 2017. doi: 10.1371/journal.pgen.1007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tsuzuki S, Amitsuka T, Okahashi T, Kozai Y, Yamasaki M, Inoue K, Fushiki T. Identification of the odor-active volatile compound (Z,Z)-4,7-tridecadienal as a potential ligand for the transmembrane receptor CD36. Biomed Res 37: 335–342, 2016. doi: 10.2220/biomedres.37.335. [DOI] [PubMed] [Google Scholar]
- 129. Weiss MS, Hajnal A, Czaja K, Di Lorenzo PM. Taste responses in the nucleus of the solitary tract of awake obese rats are blunted compared with those in lean rats. Front Integr Neurosci 13: 35, 2019. doi: 10.3389/fnint.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wu C, Hwang SH, Jia Y, Choi J, Kim YJ, Choi D, Pathiraja D, Choi IG, Koo SH, Lee SJ. Olfactory receptor 544 reduces adiposity by steering fuel preference toward fats. J Clin Invest 127: 4118–4123, 2017. doi: 10.1172/JCI89344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xavier AM, Ludwig RG, Nagai MH, de Almeida TJ, Watanabe HM, Hirata MY, Rosenstock TR, Papes F, Malnic B, Glezer I. CD36 is expressed in a defined subpopulation of neurons in the olfactory epithelium. Sci Rep 6: 25507, 2016. doi: 10.1038/srep25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yasumatsu K, Iwata S, Inoue M, Ninomiya Y. Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol (Oxf) 226: e13215, 2019. doi: 10.1111/apha.13215. [DOI] [PubMed] [Google Scholar]
- 133. Moola S, Z M, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, M PF, Lisy K. Chapter 7: Systematic reviews of etiology and risk. In: Joanna Briggs Institute Reviewer’s Manual, edited by Aromataris E, Munn Z.. Adelaide, Australia: The Joanna Briggs Institute, 2017. https://reviewersmanual.joannabriggs.org/. [Google Scholar]
- 134. Tufanaru C, Z M, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: JBI Manual for Evidence Synthesis, edited by Aromataris E, Munn Z.. Adelaide, Australia: The Joanna Briggs Institute, 2020. https://synthesismanual.jbi.global. [Google Scholar]
- 135. Moola S, M Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, M PF, Lisy K. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z. (Editors). In: JBI Manual for Evidence Synthesis, edited by Aromataris E, Munn Z.. Adelaide, Australia: The Joanna Briggs Institute, 2020. https://synthesismanual.jbi.global. [Google Scholar]
- 136. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14: 43, 2014. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem 113: 839–843, 2011. doi: 10.1016/j.acthis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 138. Hao JW, Wang J, Guo H, Zhao YY, Sun HH, Li YF, Lai XY, Zhao N, Wang X, Xie C, Hong L, Huang X, Wang HR, Li CB, Liang B, Chen S, Zhao TJ. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat Commun 11: 4765, 2020. doi: 10.1038/s41467-020-18565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 268: 17665–17668, 1993. doi: 10.1016/S0021-9258(17)46753-6. [DOI] [PubMed] [Google Scholar]
- 140. Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2: re3, 2009. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pepino MY, Kuda O, Samovski D, Abumrad NA. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu Rev Nutr 34: 281–303, 2014. doi: 10.1146/annurev-nutr-071812-161220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, Le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376–8382, 2010. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sclafani A, Zukerman S, Ackroff K. GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 305: R1490–R1497, 2013. doi: 10.1152/ajpregu.00440.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Costanzo A, Liu D, Nowson C, Duesing K, Archer N, Bowe S, Keast R. A low-fat diet up-regulates expression of fatty acid taste receptor gene FFAR4 in fungiform papillae in humans: a co-twin randomised controlled trial. Br J Nutr 122: 1212–1220, 2019. doi: 10.1017/S0007114519002368. [DOI] [PubMed] [Google Scholar]
- 145. Kim-Motoyama H, Yasuda K, Yamaguchi T, Yamada N, Katakura T, Shuldiner AR, Akanuma Y, Ohashi Y, Yazaki Y, Kadowaki T. A mutation of the beta 3-adrenergic receptor is associated with visceral obesity but decreased serum triglyceride. Diabetologia 40: 469–472, 1997. doi: 10.1007/s001250050702. [DOI] [PubMed] [Google Scholar]
- 146. Sipiläinen R, Uusitupa M, Heikkinen S, Rissanen A, Laakso M. Polymorphism of the beta3-adrenergic receptor gene affects basal metabolic rate in obese Finns. Diabetes 46: 77–80, 1997. doi: 10.2337/diabetes.46.1.77,10.2337/diab.46.1.77. [DOI] [PubMed] [Google Scholar]
- 147. Tchernof A, Starling RD, Walston JD, Shuldiner AR, Dvorak RV, Silver K, Matthews DE, Poehlman ET. Obesity-related phenotypes and the beta3-adrenoceptor gene variant in postmenopausal women. Diabetes 48: 1425–1428, 1999. doi: 10.2337/diabetes.48.7.1425. [DOI] [PubMed] [Google Scholar]
- 148. Yoshida T, Sakane N. Association between beta3-adrenoreceptor polymorphism with obesity and diabetes in Japan. Intern Med 38: 207–209, 1999. doi: 10.2169/internalmedicine.38.207. [DOI] [PubMed] [Google Scholar]
- 149. Li YY, Lu XZ, Wang H, Zhou YH, Yang XX, Geng HY, Gong G, Kim HJ. ADRB3 gene Trp64Arg polymorphism and essential hypertension: a meta-analysis including 9,555 subjects. Front Genet 9: 106, 2018. doi: 10.3389/fgene.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Brissard L, Leemput J, Hichami A, Passilly-Degrace P, Maquart G, Demizieux L, Degrace P, Khan N. Orosensory detection of dietary fatty acids is altered in CB1R−/− mice. Nutrients 10: 1347, 2018. doi: 10.3390/nu10101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. BonDurant LD, Potthoff MJ. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu Rev Nutr 38: 173–196, 2018. doi: 10.1146/annurev-nutr-071816-064800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, Bernardo B, Müller CP, Tang H, Mangelsdorf DJ, Goodwin B, Kliewer SA. FGF21 regulates sweet and alcohol preference. Cell Metab 23: 344–349, 2016. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Qi Q, Curhan GC, Rimm EB, Hunter DJ, Pasquale LR, Ridker PM, Hu FB, Chasman DI, Qi L, DietGen Consortium. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet 22: 1895–1902, 2013. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Firestein S. How the olfactory system makes sense of scents. Nature 413: 211–218, 2001. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 155. Redmond L, Hockfield S, Morabito MA. The divergent homeobox gene PBX1 is expressed in the postnatal subventricular zone and interneurons of the olfactory bulb. J Neurosci 16: 2972–2982, 1996. doi: 10.1523/JNEUROSCI.16-09-02972.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hedner M, Nilsson LG, Olofsson JK, Bergman O, Eriksson E, Nyberg L, Larsson M. Age-related olfactory decline is associated with the BDNF val66met polymorphism: evidence from a population-based study. Front Aging Neurosci 2: 24, 2010. doi: 10.3389/fnagi.2010.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Simpson PJ, Miller I, Moon C, Hanlon AL, Liebl DJ, Ronnett GV. Atrial natriuretic peptide type C induces a cell-cycle switch from proliferation to differentiation in brain-derived neurotrophic factor- or nerve growth factor-primed olfactory receptor neurons. J Neurosci 22: 5536–5551, 2002. doi: 10.1523/JNEUROSCI.22-13-05536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 60: 355–366, 2009. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bamalan OA, Al Khalili Y. Physiology, Serotonin. Treasure Island, FL: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 160. El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem 283: 12949–12959, 2008. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 161. Lancaster GI, Langley KG, Berglund NA, Kammoun HL, Reibe S, Estevez E, Weir J, Mellett NA, Pernes G, Conway JR, Lee MKS, Timpson P, Murphy AJ, Masters SL, Gerondakis S, Bartonicek N, Kaczorowski DC, Dinger ME, Meikle PJ, Bond PJ, Febbraio MA. Evidence that TLR4 Is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab 27: 1096-1110.e5, 2018. doi: 10.1016/j.cmet.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 162. Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annu Rev Physiol 74: 177–198, 2012. doi: 10.1146/annurev-physiol-020911-153330. [DOI] [PubMed] [Google Scholar]
- 163. Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 4: 903–914, 2002. doi: 10.1016/S1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 164. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 165. Hwang DH, Kim JA, Lee JY. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol 785: 24–35, 2016. doi: 10.1016/j.ejphar.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DML, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29: 359–370, 2009. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rogero MM, Calder PC. Obesity, inflammation, Toll-like receptor 4 and fatty acids. Nutrients 10: 432, 2018. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci 7: 264, 2013. doi: 10.3389/fncel.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Eddy MC, Eschle BK, Barrows J, Hallock RM, Finger TE, Delay ER. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses 34: 789–797, 2009. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499, 2005. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 171. Ishida Y, Ugawa S, Ueda T, Yamada T, Shibata Y, Hondoh A, Inoue K, Yu Y, Shimada S. P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J Comp Neurol 514: 131–144, 2009. doi: 10.1002/cne.22000. [DOI] [PubMed] [Google Scholar]
- 172. Leinwand SG, Chalasani SH. Olfactory networks: from sensation to perception. Curr Opin Genet Dev 21: 806–811, 2011. doi: 10.1016/j.gde.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 173. Menini A. Calcium signalling and regulation in olfactory neurons. Curr Opin Neurobiol 9: 419–426, 1999. doi: 10.1016/S0959-4388(99)80063-4. [DOI] [PubMed] [Google Scholar]
- 174. De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci 24: 3086–3093, 2004. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mela DJ. Sensory assessment of fat content in fluid dairy products. Appetite 10: 37–44, 1988. doi: 10.1016/S0195-6663(88)80031-X. [DOI] [PubMed] [Google Scholar]
- 176. Rolls ET, Critchley HD, Browning AS, Hernadi I, Lenard L. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. J Neurosci 19: 1532–1540, 1999. doi: 10.1523/JNEUROSCI.19-04-01532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Rolls ET. The texture and taste of food in the brain. J Texture Stud 51: 23–44, 2020. doi: 10.1111/jtxs.12488. [DOI] [PubMed] [Google Scholar]
- 178. Roblegg E, Coughran A, Sirjani D. Saliva: an all-rounder of our body. Eur J Pharm Biopharm 142: 133–141, 2019. doi: 10.1016/j.ejpb.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 179. Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci 18: 485–497, 2017. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Menini A. Frontiers in neuroscience. In: The Neurobiology of Olfaction, edited by Menini A. Boca Raton, FL. CRC Press/Taylor & Francis, 2010. [Google Scholar]
- 181. Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 33: 653–661, 2009. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 92: 263–271, 2007. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 183. Stanley BG, Willett VL, Donias HW, Ha LH, Spears LC. The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res 630: 41–49, 1993. doi: 10.1016/0006-8993(93)90640-9. [DOI] [PubMed] [Google Scholar]
- 184. Stanley BG, Willett VL, Donias HW, Dee MG, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol Regul Integr Comp Physiol 270: R443–R449, 1996. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 185. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76: 116–129, 2012. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D, Diano S, De Biasi M, Horvath TL, Gao XB, Picciotto MR. Nicotine decreases food intake through activation of POMC neurons. Science 332: 1330–1332, 2011. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am 42: 15–31, 2016. doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Takanosawa M, Nishino H, Ohta Y, Ichimura K. Glucocorticoids enhance regeneration of murine olfactory epithelium. Acta Otolaryngol 129: 1002–1009, 2009. doi: 10.1080/00016480802530663. [DOI] [PubMed] [Google Scholar]
- 189. Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 24: 40–47, 2013. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Robinson AM, Kern RC, Foster JD, Fong KJ, Pitovski DZ. Expression of glucocorticoid receptor mRNA and protein in the olfactory mucosa: physiologic and pathophysiologic implications. Laryngoscope 108: 1238–1242, 1998. doi: 10.1097/00005537-199808000-00026. [DOI] [PubMed] [Google Scholar]
- 191. Arcego DM, Krolow R, Lampert C, Toniazzo AP, Garcia ED, Lazzaretti C, Costa G, Scorza C, Dalmaz C. Chronic high-fat diet affects food-motivated behavior and hedonic systems in the nucleus accumbens of male rats. Appetite 153: 104739, 2020. doi: 10.1016/j.appet.2020.104739. [DOI] [PubMed] [Google Scholar]
- 192. Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry 12: 305–320, 2004. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fallon S, Shearman E, Sershen H, Lajtha A. Food reward-induced neurotransmitter changes in cognitive brain regions. Neurochem Res 32: 1772–1182, 2007. doi: 10.1007/s11064-007-9343-8. [DOI] [PubMed] [Google Scholar]
- 194. Gosnell BA, Levine AS. Reward systems and food intake: role of opioids. Int J Obes (Lond) 33, Suppl 2: S54–S58, 2009. doi: 10.1038/ijo.2009.73. [DOI] [PubMed] [Google Scholar]
- 195. Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23: 2882–2888, 2003. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci 18: 2592–2598, 2003. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 197. Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides 25: 697–725, 2004. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 198. Meier IM, Eikemo M, Leknes S. The role of mu-opioids for reward and threat processing in humans: bridging the gap from preclinical to clinical opioid drug studies. Curr Addict Rep 8: 306–318, 2021. doi: 10.1007/s40429-021-00366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci 31: 9101–9110, 2011. doi: 10.1523/JNEUROSCI.0404-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology (Bethesda) 23: 75–83, 2008. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- 201. Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One 7: e42373, 2012. doi: 10.1371/journal.pone.0042373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol 285: R447–R454, 2003. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 203. Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol 302: R1119–R1139, 2012. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism 34: 826–831, 1985. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 205. Miller TB, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem 248: 3483–3488, 1973. doi: 10.1016/S0021-9258(19)43955-0. [DOI] [PubMed] [Google Scholar]
- 206. Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther 2: 101–121, 2011. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106: 455–463, 2008. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci 1170: 98–101, 2009. doi: 10.1111/j.1749-6632.2009.03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, Besnard P. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res 53: 2256–2265, 2012. doi: 10.1194/jlr.M025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Klevor MK, Adu-Afarwuah S, Ashorn P, Arimond M, Dewey KG, Lartey A, Maleta K, Phiri N, Pyykkö J, Zeilani M, Ashorn U. A mixed method study exploring adherence to and acceptability of small quantity lipid-based nutrient supplements (SQ-LNS) among pregnant and lactating women in Ghana and Malawi. BMC Pregnancy Childbirth 16: 253, 2016. doi: 10.1186/s12884-016-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. La Sala MS, Hurtado MD, Brown AR, Bohórquez DV, Liddle RA, Herzog H, Zolotukhin S, Dotson CD. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J 27: 5022–5033, 2013. doi: 10.1096/fj.13-228064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 213. Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci 2: 551–560, 2001. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- 214. Aimé P, Duchamp-Viret P, Chaput MA, Savigner A, Mahfouz M, Julliard AK. Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behav Brain Res 179: 258–264, 2007. doi: 10.1016/j.bbr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 215. Sclafani A, Touzani K, Ackroff K. Ghrelin signaling is not essential for sugar or fat conditioned flavor preferences in mice. Physiol Behav 149: 14–22, 2015. doi: 10.1016/j.physbeh.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kahathuduwa CN, Boyd LA, Davis T, O'Boyle M, Binks M. Brain regions involved in ingestive behavior and related psychological constructs in people undergoing calorie restriction. Appetite 107: 348–361, 2016. doi: 10.1016/j.appet.2016.08.112. [DOI] [PubMed] [Google Scholar]
- 217. Griffiths AJ, Miller JH, Suzuki DT. An Introduction to Genetic Analysis (7th ed.). New York: W. H. Freeman, 2000. [Google Scholar]
- 218. Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res 53: 561–566, 2012. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr 104: 145–152, 2010. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 220. Keller KL, Liang LC, Sakimura J, May D, van Belle C, Breen C, Driggin E, Tepper BJ, Lanzano PC, Deng L, Chung WK. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring) 20: 1066–731073, 2012. doi: 10.1038/oby.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Feeney EL, Hayes JE. Exploring associations between taste perception, oral anatomy and polymorphisms in the carbonic anhydrase (gustin) gene CA6. Physiol Behav 128: 148–154, 2014. doi: 10.1016/j.physbeh.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Barbarossa IT, Melis M, Mattes MZ, Calò C, Muroni P, Crnjar R, Tepper BJ. The gustin (CA6) gene polymorphism, rs2274333 (A/G), is associated with fungiform papilla density, whereas PROP bitterness is mostly due to TAS2R38 in an ethnically-mixed population. Physiol Behav 138: 6–12, 2015. doi: 10.1016/j.physbeh.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 223. Kershaw JC, Mattes RD. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg 4: 3–10, 2018. doi: 10.1016/j.wjorl.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Tucker RM, Kaiser KA, Parman MA, George BJ, Allison DB, Mattes RD. Comparisons of fatty acid taste detection thresholds in people who are lean vs. overweight or obese: a systematic review and meta-analysis. PLoS One 12: e0169583, 2017. doi: 10.1371/journal.pone.0169583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kindleysides S, Beck K, Walsh D, Henderson L, Jayasinghe S, Golding M, Breier B. Fat sensation: fatty acid taste and olfaction sensitivity and the link with disinhibited eating behaviour. Nutrients 9: 879, 2017. doi: 10.3390/nu9080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Ng SF, Lin RC, Maloney CA, Youngson NA, Owens JA, Morris MJ. Paternal high-fat diet consumption induces common changes in the transcriptomes of retroperitoneal adipose and pancreatic islet tissues in female rat offspring. FASEB J 28: 1830–1841, 2014. doi: 10.1096/fj.13-244046. [DOI] [PubMed] [Google Scholar]
- 227. Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, Franzen L, Richman M, Davis LM, Abbondanzo S, Stewart CL, Margolis FL. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci U S A 93: 9858–9863, 1996. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Lee AC, He J, Ma M. Olfactory marker protein is critical for functional maturation of olfactory sensory neurons and development of mother preference. J Neurosci 31: 2974–2982, 2011. doi: 10.1523/JNEUROSCI.5067-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. George RP, Semendric I, Bowley-Schubert ER, Chivonivoni CT, Warrender AP, Whittaker AL. Reporting in rodent models of “chemobrain”: a systematic review assessing compliance with the ARRIVE guidelines. Support Care Cancer 29: 7073–7084, 2021. doi: 10.1007/s00520-021-06312-8. [DOI] [PubMed] [Google Scholar]
- 230. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Gulin JE, Rocco DM, García-Bournissen F. Quality of reporting and adherence to ARRIVE guidelines in animal studies for chagas disease preclinical drug research: a systematic review. PLoS Negl Trop Dis 9: e0004194, 2015. doi: 10.1371/journal.pntd.0004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Degrace-Passilly P, Besnard P. CD36 and taste of fat. Curr Opin Clin Nutr Metab Care 15: 107–111, 2012. doi: 10.1097/MCO.0b013e32834ff19c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material available at DOI: https://doi.org/10.6084/m9.figshare.20415975.v1.