Abstract
The global prevalence of myopia, or nearsightedness, has increased at an alarming rate over the last few decades. An eye is myopic if incoming light focuses prior to reaching the retinal photoreceptors, which indicates a mismatch in its shape and optical power. This mismatch commonly results from excessive axial elongation. Important drivers of the myopia epidemic include environmental factors, genetic factors, and their interactions, e.g., genetic factors influencing the effects of environmental factors. One factor often hypothesized to be a driver of the myopia epidemic is environmental light, which has changed drastically and rapidly on a global scale.
In support of this, it is well established that eye size is regulated by a homeostatic process that incorporates visual cues (emmetropization). This process allows the eye to detect and minimize refractive errors quite accurately and locally over time by modulating the rate of elongation of the eye via remodeling its outermost coat, the sclera. Critically, emmetropization is not dependent on post-retinal processing. Thus, visual cues appear to influence axial elongation through a retina-to-sclera, or retinoscleral, signaling cascade, capable of transmitting information from the innermost layer of the eye to the outermost layer.
Despite significant global research interest, the specifics of retinoscleral signaling pathways remain elusive. While a few pharmacological treatments have proven to be effective in slowing axial elongation (most notably topical atropine), the mechanisms behind these treatments are still not fully understood. Additionally, several retinal neuromodulators, neurotransmitters, and other small molecules have been found to influence axial length and/or refractive error or be influenced by myopigenic cues, yet little progress has been made explaining how the signal that originates in the retina crosses the highly vascular choroid to affect the sclera.
Here, we compile and synthesize the evidence surrounding three of the major candidate pathways receiving significant research attention — dopamine, retinoic acid, and adenosine. All three candidates have both correlational and causal evidence backing their involvement in axial elongation and have been implicated by multiple independent research groups across diverse species. Two hypothesized mechanisms are presented for how a retina-originating signal crosses the choroid — via 1) all-trans retinoic acid or 2) choroidal blood flow influencing scleral oxygenation. Evidence of crosstalk between the pathways is discussed in the context of these two mechanisms.
Keywords: Myopia, retinoscleral signaling, dopamine, retinoic acid, adenosine, retina, RPE, choroid, sclera, hypoxia, hif1a
1. Introduction
Myopia, commonly known as “nearsightedness”, describes a mismatch between an eye’s optical power and its geometry where the eye is too long for its optics. This mismatch results in light focusing in front of the retina instead of directly on it (i.e., a refractive error) and is typically the result of excessive axial elongation of the eye rather than improper development of optical power (Flitcroft et al., 2019). While myopic refractive errors are commonly treated by correcting the optical power of the eye via spectacles or contact lenses, these optical interventions do not slow axial elongation or myopia progression (Wildsoet et al., 2019). In the United States, myopia prevalence increased from about 25% in 1970 to 40% in 2000 (Vitale, 2009), and in Singapore, myopia reportedly affects more than 80% of young adults (Morgan et al., 2012). Predictions estimate that about 50% of the global population will be myopic by 2050 unless more effective preventative interventions or treatments of the underlying biological cause are identified (Holden et al., 2016). With excessive elongation being a risk factor for many additional sight-threatening disorders (Grytz et al., 2020), this epidemic requires additional study of the mechanisms of myopigenesis to develop interventions capable of resolving the underlying biological mismatch.
The rapid rise in prevalence is thought to be real, not due to increased identification, and driven primarily by environmental factors rather than drifting population genetics (Dolgin, 2015; Goldschmidt and Jacobsen, 2014; Vitale, 2009). While polygenic risk scores obtained from genome-wide association studies (GWAS) can be modestly predictive for severe myopia, they do not perform well for mild and moderate myopia (Ghorbani Mojarrad et al., 2020), which make up the vast majority of cases. Instead, the pathogenesis of myopia is thought to be environmentally driven by “myopigenic” cues, a hypothesis dating back to at least the late 16th century, when near work was first proposed as causing myopia (de Jong, 2018). In the last century, the types of environmental visual cues presented to an eye during adolescence have been found to be highly influential over its mature size, with the homeostatic process linking vision and eye size being termed emmetropization (Troilo et al., 2019). Thus, the global increase in myopia throughout the 20th century occurring during a period of increasing urbanization and widespread adoption of artificial electric lighting is likely not coincidental. Indoor environments are known to contain very different visual cues than outdoor, natural environments, e.g., different luminance, spatial, and spectral properties (Flitcroft et al., 2020; French et al., 2013).
The rise in time spent indoors and in urban environments is a likely driver of the myopia epidemic (Flitcroft, 2013), supported by the often-repeated finding that increasing time spent outdoors displays protective effects against myopigenesis. A study of monozygotic twins in the UK (n=64 pairs), where each pair had a ≥2 diopters (D) difference in refractive error between them, found that the less myopic of the pair tended to spend more time outdoors or doing outdoor sports, while the more myopic individuals more often lived in an urban area, performed more near work, and had greater professional status (Ramessur et al., 2015). Other studies have shown that children who spent more time outdoors (assessed via questionnaire or objective light measurement) showed modest, but significant, slowing of myopia progression (Jin et al., 2015; Read et al., 2014; Rose et al., 2008; Wu et al., 2013). The protective effect also appears to be quite variable. A study of monozygotic twins in China (n=490 pairs) found that near work and time outdoors only explained roughly 3% of the discordance in refractive error between twins (Ding et al., 2018). These discrepancies could possibly be explained by low sensitivity of questionnaire data for exposure measurements; however, it is also possible that variation in outdoor visual environments (outdoors in a city around buildings versus outdoors in a forest) and/or genetic factors that influence susceptibility to myopigenic cues could influence the amount of protection conferred.
Significant progress has been made in not only identifying myopigenic/protective visual cues, but also interventions capable of slowing myopigenesis, including optical, behavioral, and pharmacological methods (Smith and Walline, 2015; Wildsoet et al., 2019). Despite this, our understanding of the signaling mechanisms underlying myopigenesis is still limited. This is exemplified with the clinical use of topical atropine; atropine has been the most common pharmacological intervention for myopia control for over a century now, yet little consensus has been reached regarding its mechanism of action in the eye (Galvis et al., 2016; Mathis et al., 2020; Upadhyay and Beuerman, 2020).
Nevertheless, much has still been learned about general characteristics and requirements of myopigenic signaling. The mature eye size is influenced by visual cues detected by the retina. Remodeling of the sclera, the white outermost layer of the posterior eye, is causal to modulating eye size and occurs during myopigenesis (Boote et al., 2020; Grytz, 2018). The signaling does not appear to require post-retinal processing of the visual stimulus and thus is largely contained to the posterior eye wall (McFadden and Wildsoet, 2020; Norton et al., 1994; Troilo et al., 1987; Troilo and Wallman, 1991; Wildsoet, 2003; Wildsoet and Pettigrew, 1988; Wildsoet and Wallman, 1995). Myopigenic visual cues presented to the retina therefore result in a signal, likely chemical in nature (e.g., signaling molecules), capable of 1) propagating across the retinal pigment epithelium (RPE) and vascular choroid and 2) influencing remodeling processes in the sclera. How environmental visual cues interact with genetics to induce myopigenesis in some people but not others and what molecules are involved in the retina-to-sclera (retinoscleral) signaling pathways are currently open questions and the subject of active research (de Jong, 2018; Dirani et al., 2009; Huang et al., 2015; Jones-Jordan et al., 2012; Rose et al., 2008).
This review aims to summarize and contextualize recent findings related to a subset of signaling molecules and pathways (dopamine, retinoic acid, and adenosine), as opposed to serving as a fully comprehensive review of every molecule involved in retinoscleral signaling (for a broader review, see (Summers et al., 2021)). These signaling pathways were selected as the most likely to directly regulate myogenesis due to each having been correlated with and causally linked to axial length and myopia numerous times across many different research groups and species. Additionally, each has been suggested to act in multiple tissues throughout the retinoscleral cascade.
2. Emmetropization and homeostasis of eye size
The size of a mature eye is determined by a combination of growth and elongation, both of which appear to involve the sclera. Here, growth is defined as an increase in scleral tissue volume, which leads to an overall increase in ocular dimensions. In contrast, axial elongation is a process that modulates the axial length of the eye without increasing scleral tissue volume, which in mammals is believed to be determined primarily by remodeling of the sclera (Lim et al., 2011). This remodeling includes both the reorganization of existing tissue components (primarily collagen) and altered synthesis/degradation of others (collagen and proteoglycans/glycosaminoglycans). During remodeling, the sclera is more extensible, permitting elongation with either no change or a small decrease in overall volume (Backhouse and Gentle, 2018) and leading to a thinner sclera (for recent reviews, see (Boote et al., 2020; Grytz, 2018)).
In the human prenatal period, eye size increases rapidly via growth, closely matching the general growth curve of the body (Mutti et al., 2005; Watanabe et al., 1999). Upon birth, infants typically have mismatches in the power and geometry of their eyes (i.e., refractive errors), biased towards the eyes being too short for their optical power, a state termed hyperopia (Chakraborty et al., 2020; Mutti et al., 2005; Watanabe et al., 1999). Growth of the eye may only occur for about two years (Shen et al., 2016); however, eyes continue to elongate for many years, initially quite rapidly but slowing exponentially, reaching mature size around the teenage years (Fledelius et al., 2014; Fledelius and Christensen, 1996). By the age of 6, significant emmetropization of the population has generally occurred (characterized by a significant decrease in variance of refractive errors) (Chakraborty et al., 2020); however, eyes that will continue on to be emmetropic later in life are still on average hyperopic by about 1 diopter (Figure 1A). The remaining hyperopic refractive errors are corrected over the next decade while the eye continues to elongate and the optical components mature (Gordon and Donzis, 1985; Hagen et al., 2019; Jones et al., 2005; Mutti et al., 2005). Thus, throughout the first nearly two decades of life, eye development occurs in a complex yet highly coordinated manner, evidenced by the fact that only a minority (albeit a rapidly growing one) develop significant refractive errors.
Figure 1:
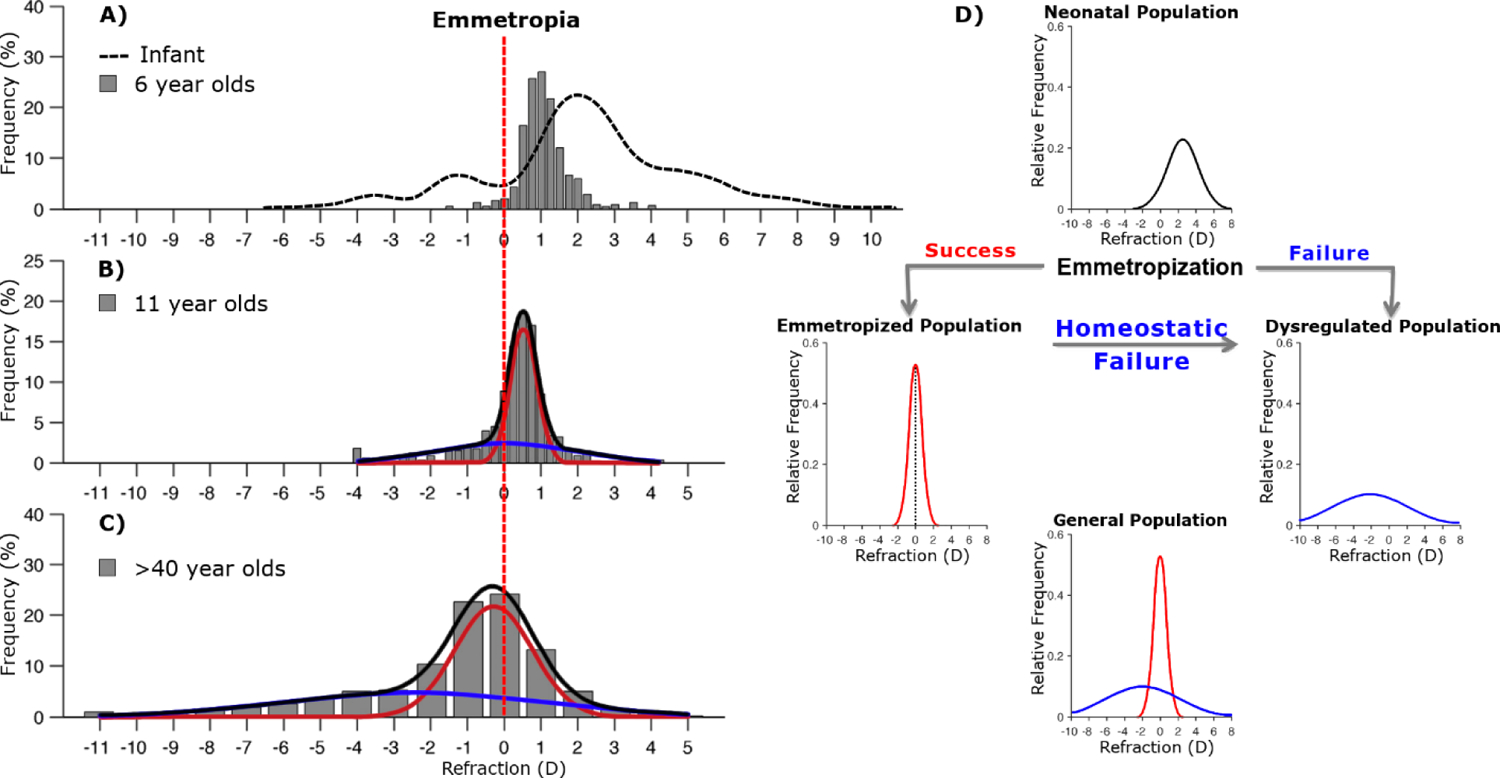
(A-C) Real distributions of refractive errors in various populations. Vertical red dashed line indicates no refractive error. A) Infant populations show approximately Gaussian distributions of refractive error, the majority being hyperopic on average (dashed line). By age 6, emmetropization has reduced the variance. (B,C) As populations age, refractive errors become increasingly leptokurtic and can be well described by fitting two separate Gaussian distributions, representing properly emmetropized (red) and dysregulated (blue) populations. D) Hypothetical paths of refractive development. Dysregulated populations could be explained by a combination of failure to initially emmetropize (A), or failure maintain emmetropia (B,C). Modified from Flitcroft, 2013 and Chakraborty et al, 2020.
The homeostatic process responsible for this coordinated development is called emmetropization, and it appears to function via controlling the rate of axial elongation. Emmetropic eyes can properly coordinate their rate of axial elongation such that the length of the eye matches the optical power and results in little to no refractive error. In most adult human populations, emmetropes are overrepresented, leading to a non-Gaussian leptokurtic distribution of refractive errors (Figure 1B,C) (Flitcroft, 2013). While refractive errors follow a mostly Gaussian distribution at birth and through early adolescence (Flitcroft, 2013), the number of myopes continue to increase. These statistical features, among others, reflect a tightly controlled homeostatic mechanism that is now understood to incorporate visual signals, primarily, blur.
Interestingly, many features that differ between indoor and outdoor visual environments have been demonstrated to be disruptive to achieving or maintaining emmetropia (for in-depth review see (Lingham et al., 2020; Norton, 2016; Rucker, 2019; Schaeffel and Feldkaemper, 2015)). Sunlight is significantly brighter than most indoor environments, and this increased luminance has been shown to be protective in chicks (Ashby et al., 2009; Ashby and Schaeffel, 2010; Chen et al., 2017), tree shrews (Norton and Siegwart, 2013), guinea pigs (Li et al., 2014), and macaques (Smith et al., 2013, 2012) compared to typical indoor lighting. Additionally, emmetropic children have been observed to spend more time in these bright light levels (Read et al., 2014). However, a recent re-analysis of the study by Read et al. found that children with myopia spent less time in both bright and dim light (Landis et al., 2018), and both luminance conditions were demonstrated to be protective in mice (Landis et al., 2021). Additionally, when chicks were exposed to sunlight daily, they had shorter myopic axial elongation (Ashby et al., 2009), but when they were reared outdoors, acute myopic protection did not last (Stone et al., 2016).
Sunlight also contains a broader spectrum of wavelengths than indoor light, including a larger proportion of shorter wavelengths (Krutmann et al., 2014; Thorne et al., 2009). Visual stimulation with violet light slowed myopia progression in human clinical studies (Torii et al., 2017), and short-wavelength light protected mice (Jiang et al., 2021; Strickland et al., 2020), guinea pigs (Yu et al., 2021), and chicks (Torii et al., 2017; Wang et al., 2018) from induced myopia. However, in tree shrews and rhesus monkeys, long-wavelength red light was protective, suggesting potential species differences (Gawne et al., 2017; Smith et al., 2015; Ward et al., 2018).
The protective effect of the outdoors could also be related to the spatial frequency content of the environment since outdoor environments contain more mid-level (~10cpd) and higher spatial frequencies than artificial environments (Flitcroft et al., 2020). Manipulating the visual experience to degrade (Howlett and McFadden, 2006; Norton and Rada, 1995; Pardue et al., 2013; Shen et al., 2005; Troilo and Nickla, 2005; Wallman et al., 1978) or reduce mid-high spatial frequencies leads to axial elongation in chicks (Bartmann and Schaeffel, 1994; Tran et al., 2008), guinea pigs (Bowrey et al., 2015), mice (Pardue et al., 2008), and monkeys (Smith and Hung, 2000), and evidence exists that extends this effect to humans. Adolescents with cataracts, which significantly reduce sensitivity to high spatial frequencies (Lewis and Maurer, 2005), often develop severe myopia (He et al., 2017). More directly, it has recently been shown that movies carefully low-pass filtered to match spatial frequency spectra of imposed optical defocus can acutely influence axial length in emmetropic and myopic humans (Swiatczak and Schaeffel, 2021). Thus, axial blur, luminance, chromaticity, and spatial frequency content have all been demonstrated to be myopigenic cues. Most animal studies of myopia impose one or more of these visual cues on the animal, commonly axial blur via powered lenses (lens induced myopia, LIM) or diffuser goggles (form-deprivation myopia, FDM).
Myopic eyes have failed to either properly emmetropize or maintain emmetropia (Figure 1D), and it is now well established that these failures cannot be traced back to a single source. The variable age of myopia onset in adolescents and the much larger variance in the distribution of refractive errors in myopic populations (Flitcroft, 2013) (Figure 1) supports many distinct origins of a disrupted homeostatic process. Visual environments contain many types of myopigenic visual cues, some of which may drive myopigenesis to differing degrees. However, the retinal circuitry used to sense these cues is highly complex, involving many distinct cell types and signaling pathways and thus has many points at which one’s genetics could drive myopia or influence susceptibility to myopigenic cues.
While many genetic abnormalities have been demonstrated to disrupt emmetropization and confer a degree of heritability to myopia (with estimates ranging from 15% to 98%) (Tedja et al., 2019), the types and durations of visual cues presented to the eye appear especially critical to emmetropization and its maintenance. Thus, it is important to study the mechanisms by which these myopigenic cues signal to increase axial elongation.
3. Retinoscleral signaling
In mammals, myopigenic visual cues appear to influence eye size primarily by increasing the rate of axial elongation through altering scleral remodeling (Troilo et al., 2019), as opposed to the avian model in which the cartilaginous layer of the sclera grows. The influence of visual cues on the sclera occurs quite locally — myopigenic visual cues can influence eye size unilaterally (if only one eye is presented the stimulus), without a functioning connection to the visual cortex (Norton et al., 1994; Troilo et al., 1987; Wildsoet, 2003; Wildsoet and Pettigrew, 1988), and regionally within an eye (if presented a spatially nonuniform myopigenic stimulus, e.g., hemi-diffusers) (Diether and Schaeffel, 1997; Norton and Siegwart, 1995; Smith et al., 2014, 2010, 2009; Wallman et al., 1987). Thus, the retina senses and encodes visual cues and initiates a signaling cascade that propagates to regions of the sclera located posterior to the region of the retina that sense the cue, ultimately leading to scleral remodeling and modulation of eye size.
While this general framework for retinoscleral signaling is well supported, the specifics of the process are still unknown. Many types of retinal signaling molecules and pathways have been associated with myopigenesis, and the signaling dynamics of some have been studied in detail (most notably, dopamine). However, efforts to incorporate retinal research into a pathway spanning the entire ocular wall have been much less successful. Additionally, different myopigenic cues may have differing retinal mechanisms, evidenced by retinal interventions that protect against lens-induced myopia but not form-deprivation (or vice versa) (Bitzer et al., 2000; Dong et al., 2011; Wang et al., 2014). However, there is no evidence to date showing different myopigenic cues result in different outcomes in scleral remodeling. Thus, various types of retinal signaling may converge at or before the choroid, resulting in a graded signal that combines various myopigenic cues and determines if the eye will increase, maintain, or slow its axial elongation (sometimes referred to as a GO, STAY, STOP signal (Guo et al., 2019)).
In the following sections, we focus on the roles and influence of dopamine, retinoic acid, and adenosine in myopigenesis (Figure 2). While these represent only a subset of the many signaling molecules reported in the literature, we focus on these three due to 1) robust reports of causal influence on and correlations with refractive development, 2) localization of the signaling molecule/receptor in multiple ocular tissues, 3) evidence from a variety of species (especially mammalian), and 4) corroboration by multiple independent research groups. While little direct evidence currently exists linking these three pathways in the eye, we suggest that these pathways likely represent key components of the signaling cascade that warrant future study. Additionally, two possible points of signal convergence are discussed alongside supporting evidence.
Figure 2:
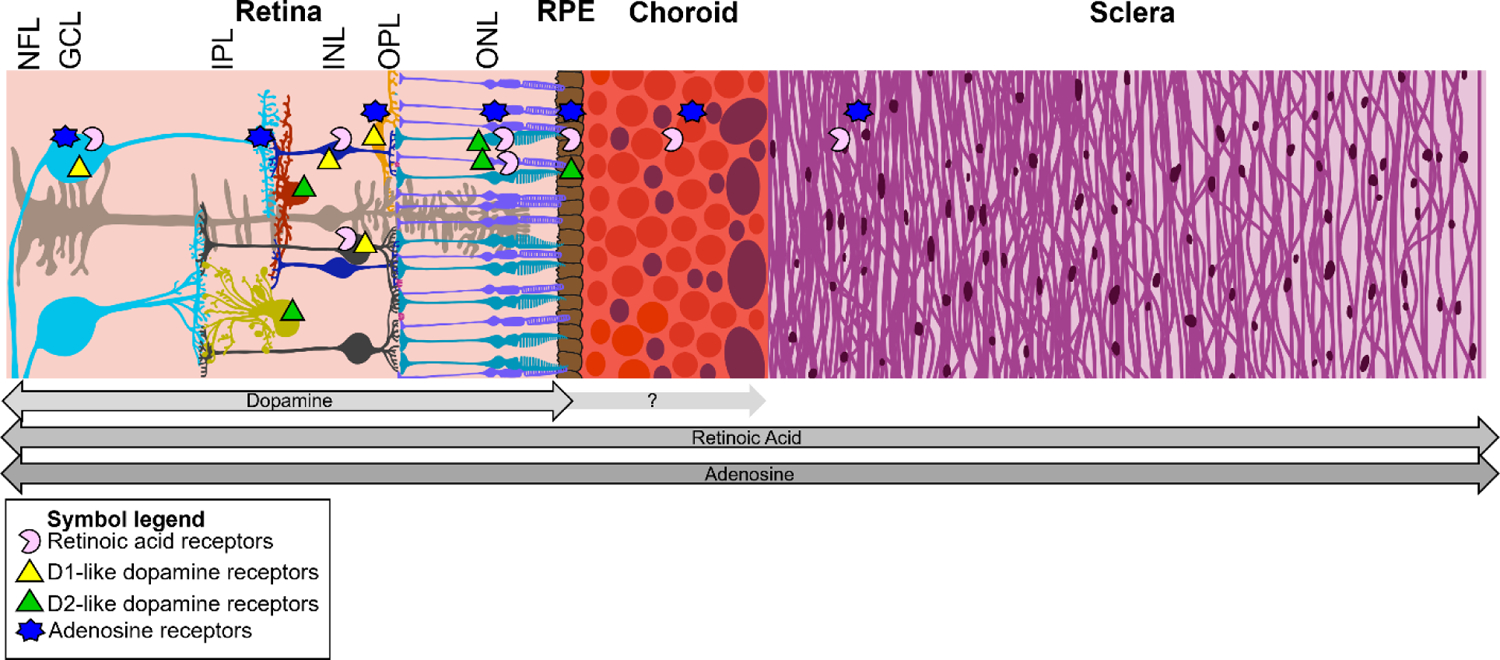
Cartoon representation of the human posterior eye wall and the path of a hypothetical retinoscleral signal. Arrows below the diagram signify where each molecule has been implicated as a potential signaling molecule. The proportions of each layer are approximately to scale. NFL: nerve fiber layer, GCL: ganglion cell layer, IPL: inner plexiform layer, INL: inner nuclear layer, OPL: outer plexiform layer, ONL: outer nuclear layer, RPE: retinal pigment epithelium.
3.1. Dopamine
The role of retinal dopamine (DA) in myopigenesis has been an active area of research for the last 30 years since the first report that DA may link visual signals with myopic axial elongation (Stone et al., 1989). In this report, Stone and colleagues discovered that levels of DA and its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), were significantly reduced in retinas of chickens with experimental myopia (FDM). This study suggested that retinal dopaminergic (DAergic) signaling could be a principal modulator of myopic changes in the retina. Since then, extensive effort has been spent investigating DAergic signaling (Table 1), mostly through measurements of DA and its metabolites, in retinas of myopic eyes or those exposed to myopigenic stimulus (for a recent in-depth review, see (Zhou et al., 2017)). In this section, we will present evidence for DA as the starting point for the retinoscleral signaling cascade that leads to myopic axial elongation.
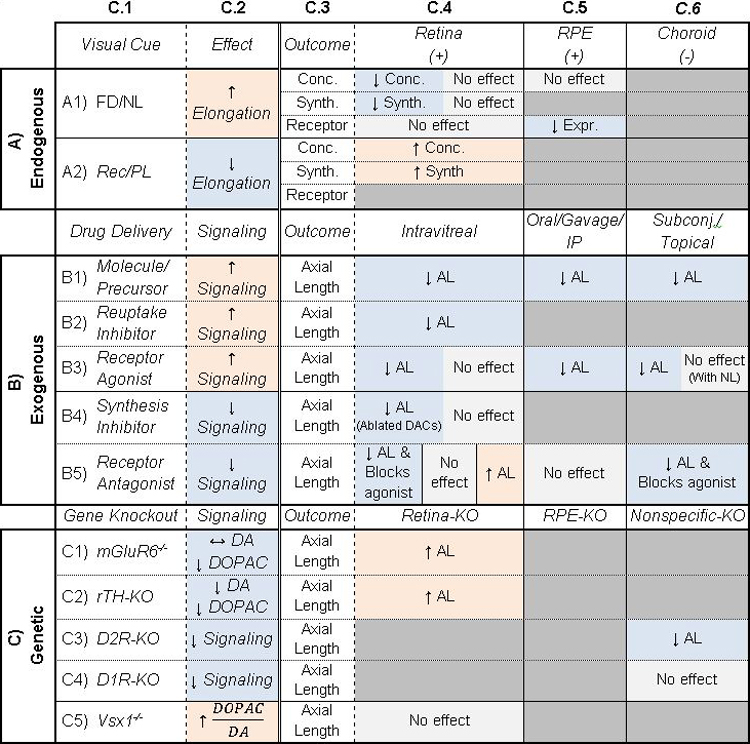
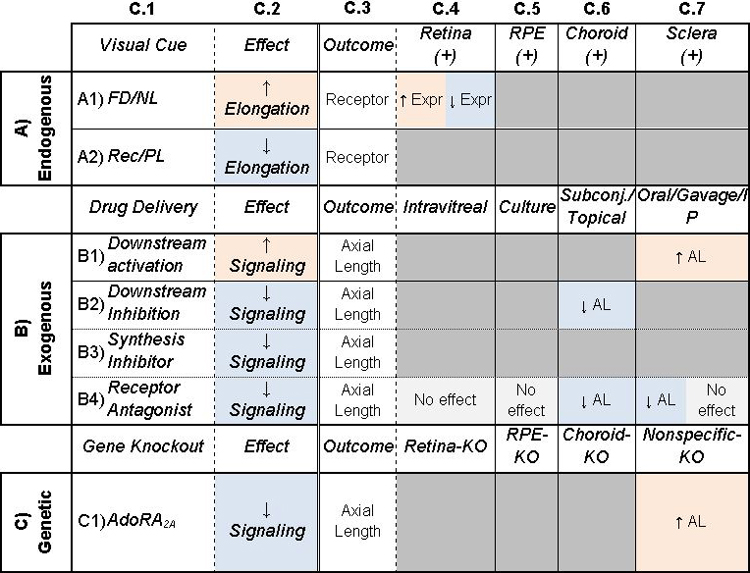
Table 1: Potential of dopamine to be a retinoscleral signal in refractive eye growth.
Column C.1: Treatments given to animals. C.2: General description of main effect of treatment. Columns C.3–6: Additional outcomes (C.3) studied with corresponding treatments. In A) Endogenous, C.4–6 are locations of measurements, and (+/−) represent whether receptors are expressed. In B) Exogenous and C) Genetic, C.4–6 are different locations of the treatment. Cells are shaded to highlight trends. Orange/blue shading indicate an increase/decrease in signaling or elongation. Gray indicates inconclusive or no effect. Table cells are split when studies came to different conclusions. AL: Axial Length, Conc: Concentration, Expr: Expression, Synth: Synthesis, FD: Form-Deprivation, NL: Negative Lens, PL: Positive Lens, Rec: Recovery from myopia, DA: Dopamine, DOPAC: 3,4-Dihydroxyphenylacetic acid, DAC: Dopaminergic amacrine cell. See Supplemental Table 1 for associated references.
|
3.1.a. Dopamine signaling pathway
Retinal DA is produced and released by a subset of DAergic amacrine cells (DACs) that are sparsely tiled across the retina (Witkovsky, 2004). Despite their low numbers, DACs have long dendrites that can span millimeters of the retina and can extend from the inner plexiform layer to the outer plexiform layer and ganglion cell layer (Witkovsky, 2004). DA can act through local synaptic release from DAC varicosities and dendritic spines (Dacey, 1990) or extra-synaptically through paracrine diffusion (Puopolo et al., 2001) across the retina to activate multiple types of postsynaptic and extra synaptic receptors. DA receptors are located on almost all retinal cells and fall into two families, D1-like receptors (D1 and D5) and D2-like receptors (D2, D3, and D4), which respectively increase and decrease cyclic adenosine monophosphate (cAMP) (Witkovsky, 2004). D1 receptors are located on bipolar, horizontal, amacrine, and ganglion cells, D2 receptors on DACs, D4 receptors on rod and cone photoreceptors, and D1, D2, D4, and D5 receptors on the RPE (Baba et al., 2017; Witkovsky, 2004). After DA binds to its receptor, it is metabolized to DOPAC (Cohen et al., 1983) which reflects DA release and turnover (Megaw et al., 2001).
DA release and synthesis is light-dependent, increasing with continuous diffuse and flickering light stimulation (Bauer et al., 1980; Boelen et al., 1998; Cohen et al., 2012; Godley and Wurtman, 1988; Iuvone et al., 1978; Kramer, 1971; Proll et al., 1982) and regulated by circadian rhythms (Doyle et al., 2002). Consequently, DACs are activated by light onset visual signals through three potential pathways: 1) rod photoreceptor-mediated input through the rod-rod bipolar-AII amacrine-ON cone bipolar circuit 2) direct cone photoreceptor-ON cone bipolar cell connection, and 3) melanopsin retinal ganglion cell (mRGC) axon collateral input (Boelen et al., 1998; Dumitrescu et al., 2009; Prigge et al., 2016; Zhang et al., 2012, 2008; Zhao et al., 2017). Therefore, DACs can release DA from stimulation of multiple ON pathways (Qiao et al., 2016) that are active under different environmental conditions. The actions of DA on retinal signaling is widespread and occurs at multiple levels from photoreceptors to ganglion cells (Roy and Field, 2019). Therefore, the extensive functions that DA has in retinal signaling supports its potential role in the development of myopia due to altered visual input, as was first noted by Stone et al. (Stone et al., 1989).
3.1.b. Evidence for dopamine signaling in experimental myopia
DA is generally understood to be a ‘stop’ signal in myopic axial elongation (Feldkaemper and Schaeffel, 2013). In chickens, guinea pigs, and monkeys, DA and DOPAC levels are reduced after form-deprivation (Dong et al., 2011; I. Papastergiou et al., 1998; Iuvone et al., 1989; Stone et al., 1989; Sun et al., 2018) and lens defocus (Guo et al., 1995; Ohngemach et al., 1997). Interestingly, the reduction in DOPAC was specific to only the region of the retina deprived of form vision (Ohngemach et al., 1997; Stone et al., 2006), matching the property of regional specificity observed in visually-mediated axial elongation. However, altered DA metabolism associated with myopigenic stimuli may not be conserved across species, as form-deprivation did not alter retinal DA levels in mice (Chakraborty et al., 2015, 2014; Park et al., 2014; Wu et al., 2015). However, mice, as well as rabbits and guinea pigs, show inhibited FDM (Gao et al., 2006; Junfeng et al., 2010; Landis et al., 2020; Mao et al., 2016; Mao and Liu, 2017) following injections of L-DOPA, the precursor to DA, or other DAergic activators. These findings suggest that while direct measurements of DA levels in mice were not different in FDM, activation of the whole DAergic system could still slow myopic eye growth.
Furthermore, in multiple species, DA receptor agonists prevent FDM, but do not appear to be as effective for LIM (Ashby et al., 2007; Dong et al., 2011; Iuvone et al., 1991; McCarthy et al., 2007; Rohrer et al., 1993; Schmid and Wildsoet, 2004; Stone et al., 1989; Yan et al., 2015), and it is still unclear the reason for this difference. One explanation could be that the visual cues that cause FDM and LIM initiate two distinct pathways that are differently influenced by dopamine; however, this cannot yet be concluded. Direct comparisons between the two models may be confounded by other unmatched features of the visual cues, e.g., the spatial frequency spectra or optical aberrations introduced by the lenses.
Surprisingly, reduced axial elongation associated with increased DA is only effective when visual input is disrupted (e.g., form deprivation, lens defocus) and not during normal vision (Dong et al., 2011; Junfeng et al., 2010; Landis et al., 2020; Rohrer et al., 1993; Yan et al., 2015). These findings suggest that the activation of DA pathways is important but not sufficient to affect eye growth. One hypothesis is that visual environments deficient in certain features change activation of pathways that drive DAergic signaling (e.g., ON pathways). Recent work shows that stimuli which overstimulate ON pathways lead to anti-myopigenic outcomes, e.g., choroid thickening (Aleman et al., 2018) and increased vitreal DA (Wang et al., 2019). However, the direct connections between environmental stimulation of ON pathways, DA, and myopia susceptibility are still not fully elucidated.
Unlike activation of DA pathways, depletion of DA pathways prevents FDM but has no effect on LIM in chickens and fish (Kröger et al., 1999; Li et al., 1992; Schaeffel et al., 1995, 1994) (Table 1). In contrast, decreasing DA with 6-hydroxydopamine in mice results in a myopic refractive shift with normal vision and increased FDM (Wu et al., 2016). Additionally, when retinal tyrosine hydroxylase, the rate-limiting enzyme in DA synthesis, is conditionally knocked out, mice have reduced DA levels and develop spontaneous myopia in normal conditions, while responding like wild-type mice to FDM (Bergen et al., 2016).
Interestingly, the dopamine receptor pathways involved in the protective effects of dopamine and bright light in myopia depends on the animal model (Zhou et al., 2017). For example, in chickens, D2 receptor activation inhibited FDM and LIM (Nickla et al., 2010; Rohrer et al., 1993; Schmid and Wildsoet, 2004; Stone et al., 1989) while blocking D2 receptors inhibited the protective effect of light on FDM and LIM (Ashby and Schaeffel, 2010; McCarthy et al., 2007). Additionally, blocking D2, but not D1, receptors attenuated the inhibitory effects of levodopa and dopamine on FDM and LIM (Thomson et al., 2020a, 2020c). Likewise, activation of primarily D2 receptor pathways reduced FDM in tree shrews (Ward et al., 2017). However, in the guinea pig, D1 receptor activation inhibited myopic refractive development and FDM while D2 receptor activation tended to do the opposite; however, this modulation varied with the DA receptor agonist (Zhang et al., 2018). In the mouse, it appears to be more complicated. Blocking D1 receptors attenuated the protective effect of bright light exposure during FDM (Chen et al., 2017). Additionally, activation of all DA receptors in wild-type mice and D2, but not D1, receptor knockout mice inhibited FDM (Huang et al., 2018) supporting a D1 receptor mechanism for myopigenesis in the mouse model. However in other studies, D2 receptor agonists and antagonists altered FDM in a dose-dependent manner and the protective effects of a D2 antagonist disappeared in D2 receptor knockout mice (Huang et al., 2022, 2020, 2014). Lastly, a D1 receptor mechanism is further supported in human genetic studies; a GWAS utilizing data from the Consortium for Refractive Error and Myopia (CREAM) cohort and the 23andMe, Inc. participants reported that a single nucleotide polymorphism (SNP) located near the Dopamine Receptor 1 gene (DRD1), a type of D1-like receptor, was significantly associated with refractive error (untransformed spherical equivalent) (Tedja et al., 2018).
3.1.c. Influence of dopamine signaling on ocular tissues
There is strong evidence showing a clear but complicated relationship between DAergic pathways and myopia. Retinal DA appears to have a profound influence over axial length under certain conditions. For example, in chicks, topical or intravitreal application of DA, DA receptor agonists, or levodopa reduced axial length in form-deprived or negative lens-treated eyes compared to control eyes (Nickla et al., 2019; Thomson et al., 2021, 2020b, 2020a, 2020c). Moreover, in mice, intravitreal injections of the DA agonist apomorphine attenuated axial length changes after form-deprivation (Huang et al., 2018). Additionally, D1 receptor antagonist blocked the protective effect of bright light on axial length changes in FDM (Chen et al., 2017). Likewise, intravitreal injections of high doses of D2 DA receptor agonists or antagonists reduced vitreous chamber elongation in the form-deprived tree shrew eye (Ward et al., 2017). However, modulation of DAergic signaling by DA, DA receptor agonists or antagonists, and levodopa did not affect axial length in otherwise untreated eyes (Thomson et al., 2020a, 2020c; Zhang et al., 2018), further supporting the notion that DA effects on axial elongation and myopia require altered visual input.
Despite the significant effects of DA on axial length changes, DA has not been associated with scleral signaling, and there is no evidence that DA itself reaches the sclera to affect structural remodeling. However, choroidal thickness is well established to change with myopia and correlated with axial elongation (for a recent review see (Liu et al., 2021), and there is strong evidence that dopamine also affects choroidal thickness. In chickens, choroidal thickness is regulated through D2 receptors, where D2 receptor agonists increase thickness after brief periods of unaltered vision following LIM and D2 receptor antagonists inhibit this increase after FDM (Nickla et al., 2010; Nickla and Totonelly, 2011). However, in rabbits, dopamine-induced vasodilation of the choroid was mediated through D1 receptors (Reitsamer et al., 2004). Based on the previous evidence, it is likely that the interaction between DA and choroidal thickness occurs outside of the neural retina and not within the choroid. While tyrosine hydroxylase has been localized to the choroid (Klooster et al., 1996), no evidence to date shows significant choroidal expression of DA receptors. This leaves the RPE as a tissue potentially able to translate dopaminergic signaling to choroidal changes, that ultimately may influence axial elongation.
The RPE is a single layer of pigmented cells whose apical surface is embedded between photoreceptor outer segments and whose basal surface is lined with Bruch’s membrane, separating it from the vascular choroid. The RPE carries out diverse and important set of roles for visual function, but most relevant is its role in ionic transport (Strauss, 2005), which 1) may affect fluid flow into the choroid/choroidal thickness (Zhang and Wildsoet, 2015) and 2) is altered during myopigenesis. Both the gene for chloride channels and expression of chloride channels and transporters were down-regulated after lens defocus (Seko et al., 2000; Zhang et al., 2011). Moreover, during recovery from FDM in chickens, sodium and chloride ions decrease in the RPE and increase in the choroid, which correlates temporally with a thickening of the choroid (Liang et al., 2004). Ionic flow across the retina, particularly with regards to chloride, sodium, and potassium, have been implicated in and are affected by experimental myopia (Crewther et al., 2008, 2006) and hypothesized to regulate fluid flow that could change the size of various ocular layers. Since changes in cell size and transporters can affect fluid balance across cells, regulation at the RPE provides an intriguing potential link between the retina and the classical changes in choroidal thickness associated with myopia reported more than 20 years ago (Wallman et al., 1995; Wildsoet and Wallman, 1995).
DA is a promising signal that could link the RPE to changes in the choroid due to RPE expression of D1, D2, D4, and D5 DA receptors in chicks and mice, likely on both the apical and basolateral membranes (Baba et al., 2017; Rymer and Wildsoet, 2005). When DA is applied to the basolateral surface, chicken RPE cells depolarize, due to the opening of chloride channels (Gallemore and Steinberg, 1990). This response is dependent on DA concentration and likely involves interaction effects with other receptors, possibly adrenergic receptors. For example, with low DA concentrations, DA receptors and B-adrenergic receptors are active, reducing the probability of chloride channels opening. However, when DA concentrations are high, alpha-adrenergic receptors become active which may also contribute to chloride channel opening (Rymer and Wildsoet, 2005). These findings on chloride channel activity in vitro are supported by in vivo ERG recordings using intravitreal injections of DA or DA receptor antagonists and measuring the chloride channel-mediated ERG component (Gallemore and Steinberg, 1990; Sato et al., 1987; Wioland et al., 1990). Regardless of the specific pathway, these data suggest that DAergic activity has the potential to regulate ionic flow and thus fluid transfer across the RPE into the choroid. However, whether or not this effect is large enough to significantly impact choroidal thickness and myopigenesis has not been directly shown.
3.1.d. Summary
In summary, DA has long been known to be causally involved in myopigenesis and is likely the starting point linking at least some types of myopigenic cues to scleral remodeling. However, its direct influence via receptor binding appears to be limited to the retina and RPE. It is possible that the RPE serves as the link between myopigenic stimuli, DA, and choroidal thickness by regulating choroidal swelling via dopamine receptors. Additional research is necessary to elucidate signaling occurring at the RPE and how it may propagate and influence the choroid.
3.2. Retinoic acid
Retinoic acid (RA) is a metabolite of vitamin A and a critical signaling molecule involved in numerous autocrine and paracrine developmental and physiological processes (Cvekl and Wang, 2009), regulating the transcription of over 100 genes (Balmer and Blomhoff, 2002). With advances in retinoid measurement technologies, evidence suggests that all-trans RA (atRA) is important in postnatally regulating the growth of many organ systems, including the eye (Summers, 2019). It is a promising candidate in signaling myopic axial elongation for many reasons: it is present in all eye wall tissues, bidirectionally modulated by the direction of blur (Mao et al., 2012; McFadden et al., 2004), and is known to stimulate catabolic remodeling processes (Bonassar et al., 1997; Mertz and Wallman, 2000). However, despite significant evidence linking RA to myopia development, several discrepancies remain, complicated by the breadth of its biological roles and the spatial and temporal variability in its action.
3.2.a. RA signaling pathway
Retinal RA is thought to be synthesized primarily by RPE cells as a byproduct of phototransduction (Weiler et al., 1998); however, subpopulations of amacrine (Milam et al., 1997; Saari et al., 1995) and Müller cells (Edwards et al., 1992; Milam et al., 1990) have been shown to either synthesize or express enzymes that synthesize RA. Currently, the canonical pathway by which RA appears to exert its effects involves the all-trans form (Balmer and Blomhoff, 2002). The synthesis of atRA occurs in two steps: all-trans retinaldehyde is synthesized from all-trans retinol (vitamin A) via alcohol dehydrogenases (ADHs), which is subsequently oxidized into atRA via cytosolic retinaldehyde dehydrogenases (RALDH1/2/3). A dimer of a nuclear RA receptor (RAR) and retinoid-X receptor (RXR) binds atRA, subsequently binding DNA and influencing transcription (Balmer and Blomhoff, 2002). After synthesis, atRA can be bound by one of many retinoid binding proteins (RBPs) to facilitate intra- or extracellular transport or metabolized by CYP26 (Summers, 2019).
In vertebrates, retinal atRA concentration and synthesis have been demonstrated to be visually mediated. It is increased in response to both myopigenic cues (Mao et al., 2012; McFadden et al., 2004; Seko et al., 1998; Troilo et al., 2006) and greater luminance (McCaffery et al., 1996), although these increases may occur through different pathways. Together, this implies that the regulation of many retinal processes by light may occur at the transcriptional level via atRA, including light adaptation of horizontal cells (Pottek and Weiler, 2000; Weiler et al., 2000, 1999, 1998) and the transcription of arrestin, a critical phototransduction protein (Wagner et al., 1997).
3.2.b. Evidence for RA signaling in experimental myopia
Retinal atRA has been demonstrated to be increased after FDM and LIM in the guinea pig (Huang et al., 2011; Mao et al., 2012; McFadden et al., 2004) and chicken (Bitzer et al., 2000; Seko et al., 1998). It has also been observed to decrease with positive defocus (either via powered lenses or recovery from myopia) (McFadden et al., 2004). The significance of this bidirectional modulation of atRA in the retina is still not known. While recent genomics studies suggest that the sign of blur may be encoded through two distinct sets of genes (Tkatchenko et al., 2018), this does not imply distinct signaling pathways between the retina and the RPE.
Recently, atRA has been demonstrated to modulate the protective effect of short wavelength light against myopigenesis. A study in guinea pigs exposed to different wavelengths of light showed a retinal atRA dependence on chromatic content, with the retinal atRA concentrations decreasing in the shorter wavelength (blue) light. Additionally, guinea pigs given unilateral LIM and reared in white light had significantly more retinal atRA in the myopic eyes than the contralateral eyes. However, when reared in blue light, there was no difference between the treated and control eyes, and both eyes had less atRA than those reared in white light (Yu et al., 2021). Finally, inhibition of RA-synthesizing enzymes in the retina has also been demonstrated as protective against myopia development (Bitzer et al., 2000; Yu et al., 2021), suggesting that the influence of atRA on axial elongation may begin in the retina.
As has been found in the retina, choroidal atRA concentrations appear to be bidirectionally influenced by the direction of blur imposed on the retina (Table 2). This choroidal atRA is likely choroidally-derived, as it has been demonstrated that the choroid synthesizes a large amount of atRA, more than the retina and liver (Mertz and Wallman, 2000). Three sets of co-culture experiments have important implications regarding atRA and retinoscleral signaling. When eyes from chickens were exposed to hyperopic defocus/diffusers or myopic defocus (conditions that influence choroidal thickness) and the choroids subsequently dissected and cultured, atRA production was bidirectionally altered (Mertz and Wallman, 2000) (similar bidirectional modulation of atRA synthesis, although opposite trends, were found in marmosets (Troilo et al., 2006)). Second, a more complex experiment was carried out which exposed chicken eyes to various visual cues and co-cultured different combinations of tissues, assessing scleral remodeling when cultured with choroids from different visual conditions (form-deprived or recovering from myopia) (Marzani and Wallman, 1997). Scleral remodeling (quantified by glycosaminoglycan incorporation) was influenced by the visual condition from which the choroid came. Finally, Mertz and Wallman also showed that when sclera and choroid were cultured together, the choroid would rapidly release atRA into medium, and the sclera had the tendency to concentrate significantly more atRA than would be predicted by diffusion (the scleral tissue accumulated over 50% of the atRA released by the choroid, despite only making up 1% of the volume in culture) (Mertz and Wallman, 2000). Together, these studies make a compelling case that atRA can be produced in the choroid, has the tendency to be transported to the sclera, and can influence scleral remodeling. However, the exact source of the atRA is still unclear. A recent study aiming to characterize cells positive for RALDH2, an atRA synthesis enzyme, in the choroid of both humans and chicks found that choroidal atRA concentrations were partially controlled by proliferation of RALDH2+ cells (Summers et al., 2020). Yet, protein levels of RALDH2 were only found to be altered in chicks recovering from myopia, not in those with myopia (Rada et al., 2012; Summers et al., 2020).
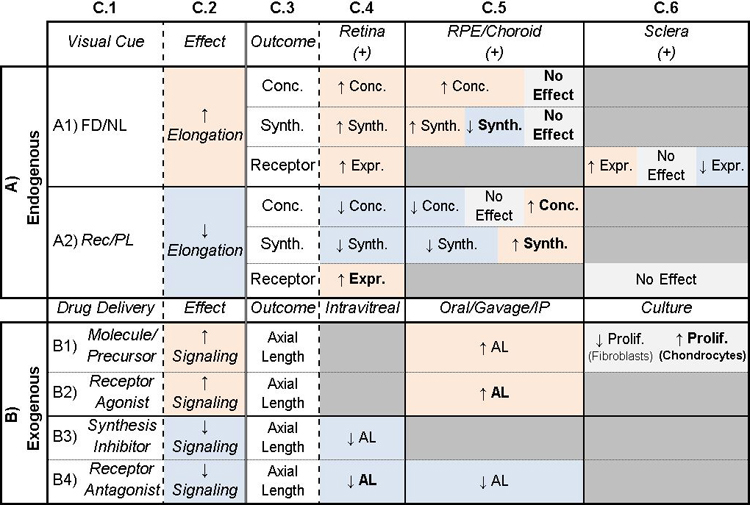
Table 2: Potential of atRA to be a retinoscleral signal in refractive eye growth.
Column C.1: Treatments given to animals. C.2: General description of main effect of treatment. Columns C.3–6: Additional outcomes (C.3) studied with corresponding treatments. In A) Endogenous, C.4–6 are locations of measurements, and (+/−) represent whether receptors are expressed. In B) Exogenous, C.4–6 are different locations of the treatment. Cells are shaded to highlight trends. Orange/blue shading indicate an increase/decrease in signaling or elongation. Gray indicates inconclusive or no effect. Table cells are split when studies came to different conclusions. Bolded text indicates non-mammalian species used in study. AL: Axial Length, Conc: Concentration, Expr: Expression, Prolif: Proliferation, Synth: Synthesis, FD: Form-Deprivation, NL: Negative Lens, PL: Positive Lens, Rec: Recovery from myopia. See Supplemental Table 2 for associated references.
|
The association of retinoids with specific RBPs that influence transport may be another means by which atRA could reach the sclera. Recently, Summers and colleagues showed that apolipoprotein A-1 (apoA-1) may act as a RBP in the eye that can traverse the choroid. apoA-1 is produced by the chick choroid and is also upregulated by atRA (Summers et al., 2016). In the same study, it was found that despite cultured sclera not synthesizing apoA-1 at detectable levels, cultured sclera releases significant amounts of apoA-1 into the medium, implying transport from choroid to sclera (Summers et al., 2016) and possibly explaining the previously discussed finding from Mertz and Wallman showing the tendency for the sclera to concentrate choroidally-derived atRA. Together, these results suggest a directionality to the transport of atRA and may overcome one of the primary limiting factors of retinoscleral signaling.
In humans, multiple GWAS studies of refractive error and myopia have also identified SNPs in genes related to atRA signaling. For example, multiple studies have reported associations between refractive error and the retinol dehydrogenase 5 (RDH5) gene (Kiefer et al., 2013; Tedja et al., 2018; Verhoeven et al., 2013), which encodes the enzyme 11-cis retinol dehydrogenase and is expressed in the RPE. GWAS findings have also implicated the RPE-retinal G protein-coupled receptor (RGR) (Kiefer et al., 2013; Tedja et al., 2018), expressed in the RPE and Müller glia (Pandey et al., 1994), and the atRA receptor-related orphan receptor beta (RORB) (Tedja et al., 2018), expressed in retinal tissue (Jia et al., 2009) and a component of the circadian clock system (André et al., 1998).
While retinoscleral signaling by atRA appears to be well supported, many nuances remain to be studied. Perhaps the greatest discrepancy is in the opposite effects of axial elongation and atRA in mammals and chickens. In both, retinal atRA increases with myopigenic stimuli while choroidal and scleral atRA concentrations are negatively correlated with axial elongation in the chick but positively correlated in mammals (Table 2). A likely factor contributing to this are the different scleral compositions of the two species: mammals have a fibrous sclera and chicks have both a cartilaginous and fibrous sclera. The different resident cell types of the two tissues have been demonstrated to respond differently to atRA in the presence of TGF-β, arresting the proliferation of fibroblasts but not chondrocytes (Y. Seko et al., 1996). Additionally, co-culturing the choroid with fibrous sclera and cartilaginous sclera also lead to opposite outcomes on scleral remodeling (Marzani and Wallman, 1997). Thus, it is possible that there are significant differences in the physiological function of atRA as a retinoscleral signal. For example, in guinea pigs, RALDH2 was not found to be expressed in the choroid (Mao et al., 2012). Additional study of the function of atRA in mammalian models is required to determine the extent of species differences.
3.2.c. Influence of RA on ocular tissues
Unlike DA, atRA displays the ability to affect scleral remodeling processes that underly myopic axial elongation. While atRA is not produced in the sclera, scleral fibroblasts have atRA receptors and remodeling processes are influenced by atRA (Li et al., 2010; Troilo et al., 2006). In marmosets, RA concentration in retina, choroid, and sclera were found to be positively correlated with rate of axial elongation and negatively correlated to the rate of proteoglycan synthesis (Troilo et al., 2006). Systemic treatment of guinea pigs with atRA also led to axial elongation and an altered scleral microstructure with similarities to the outcome of myopigenic visual cues (McFadden et al., 2004), and treatment of mice led to myopic refractive errors, axial elongation, and altered scleral biomechanics (Brown et al., 2021).
However, exogenous atRA treatment has also been observed to lead to significant ocular growth without refractive errors in both chickens and guinea pigs, proportionally growing the eye and causing the lens of these animals to thicken instead of just causing axial elongation (McFadden et al., 2006, 2004). One possible explanation could be a dual role for atRA in signaling proportional eye growth and axial elongation, with younger animals more susceptible to growth rather than elongation.
3.2.d. Summary
In summary, atRA is one of the few chemical signals with evidence supporting the capacity to cross the choroid. However, its role in myopigenesis is still unclear. While exogenous atRA causes myopia to develop in some cases, in others it leads to excessive proportional growth of the eye. Additional mammalian research is required to make more general conclusions due to previous work being avian-specific, such as the role of apoA-1 as an RBP. However, with multiple genes related to atRA signaling and myopia implicated in GWAS studies, this is a promising pathway for further investigation.
3.3. Adenosine
Adenosine (Ado) is one of the most widely occurring organic compounds. It is a purine and is a basic building block of life as one of four nucleosides of DNA and RNA with a role in energy transport. Ado’s role as an extracellular signaling molecule was discovered in the early 20th century (Drury and Szent-Györgyi, 1929). It has since been observed to modulate many cellular processes and act as a neuromodulator, exerting its actions through four types of membrane-bound, G-protein coupled receptors (AdoRs) - AdoRA1, AdoRA2A, AdoRA2B, AdoRA3 (Eltzschig, 2013). Similar to DA, AdoRs have opposing effects on cAMP production, with AdoA1 and AdoRA3 decreasing cAMP and AdoRA2A, AdoRA2B increasing cAMP by inhibiting or activating adenyl cyclase (AC), respectively (Spinozzi et al., 2021).
3.3.a. Adenosine signaling pathway
The exact mechanisms by which Ado reaches extracellular targets are complex and multivariate. Cells and interstitial fluids in tissues have basal concentrations of Ado in the nanomolar range (Fredholm, 2007). Many types of cells, including neurons and glial cells, have been demonstrated to release some amount of Ado from intracellular stores into the extracellular space (Brundege and Dunwiddie, 1998; Cotrina et al., 1998; Pascual et al., 2005), which appears to be increased by glutamate binding to N-methyl-D-aspartate (NMDA) receptors (Brambilla et al., 2005).
In general, extracellular Ado tends to dramatically increase with tissue activity, hypoxia, and other pathological states and biological stressors, increasing to the micromolar range (Fredholm, 2014; Haskó et al., 2018). However, this increase appears to be due to leakage or controlled release of adenosine triphosphate, which is subsequently degraded into adenosine monophosphate and then Ado (Antonioli et al., 2013; Eltzschig, 2013). Additionally, the extent to which these changes affect signaling is not clear, as Ado signaling is highly dynamic. Ado is rapidly metabolized to inosine and hypoxanthine, exhibiting a half-life of only ~1.5 seconds (Spinozzi et al., 2021), and equilibrative nucleoside transporters (ENTs) rapidly equilibrate imbalances in intra- and extracellular Ado (Lovatt et al., 2012).
In many cases, AdoRs are significantly activated by basal levels of Ado (Fredholm, 2007) and significantly blocked by commonly consumed amounts of caffeine, a nonselective inhibitor of AdoRs (Fredholm et al., 1999). However, the sensitivity of the cell or tissue to the ligands are highly dependent on the expression of the receptor, which has also been noted to vary significantly with diseased states and presence of stressors, most notably of which are related to the immune system and hypoxia. Hypoxic conditions have been shown to increase expression of AdoRA2B (Kong et al., 2006) without affecting AdoRA2A (Fredholm et al., 2007) and may influence trafficking of the receptors to the membrane (Arslan et al., 2002).
It is critical to note that these effects are very much cell type-dependent, and only a small fraction of the work on Ado signaling has occurred in ocular tissues. In the retina, it has been demonstrated that extracellular Ado signaling is controlled by light intensity and circadian rhythms, both of which have been implicated in myopigenesis (Ribelayga and Mangel, 2005).
3.3.b. Evidence for adenosine signaling in experimental myopia
The first connection of purinergic Ado signaling to myopigenesis was made with the discovery that 7-methylxanthine (7-MX), a metabolite of caffeine and a nonselective inhibitor of AdoRs, influenced scleral collagen and proteoglycan content in rabbits, opposite to that occurring with myopigenesis (Trier et al., 1999). Following this finding, many additional studies have also found a causal role of Ado on refractive state, axial elongation, and scleral remodeling (see 3.3.c and Table 3).
Table 3: Potential of adenosine to be a retinoscleral signal in refractive eye growth.
Column C.1: Treatments given to animals. C.2: General description of main effect of treatment. Columns C.3–7: Additional outcomes (C.3) studied with corresponding treatments. In A) Endogenous, C.4–7 are locations of measurements, and (+/−) represent whether receptors are expressed. In B) Exogenous and C) Genetic, C.4–7 are different locations of the treatment. Cells are shaded to highlight trends. Orange/blue shading indicate an increase/decrease in signaling or elongation. Gray indicates inconclusive or no effect. Table cells are split when studies came to different conclusions. AL: Axial Length, Expr: Expression, FD: Form-Deprivation, NL: Negative Lens, PL: Positive Lens, Rec: Recovery from myopia. See Supplemental Table 3 for associated references.
|
Despite significant experimental evidence, most details of the role of purinergic signaling in myopigenesis are still unclear. All four subtypes of AdoRs have been found to be expressed in all layers of the posterior eye wall of rhesus monkeys and guinea pigs (Beach et al., 2018; Cui et al., 2010) and in human scleral fibroblasts (Cui et al., 2008). A small epigenetic study of youth with high myopia (n=18 cases, 18 controls) reported hypermethylation of the AdoA2A receptor gene (ADORA2A) in peripheral blood samples (Vishweswaraiah et al., 2019), suggesting Ado signaling may be influenced by myopia. Additionally, when guinea pigs were deprived of form vision, protein levels of AdoA1 / AdoA2B in the retina were elevated (Cui et al., 2010).
The poor ability of other methylated xanthines like 7-MX to pass the blood-brain/blood-retina barrier (Hung et al., 2018; Shi and Daly, 1999) would suggest that the target tissue of these systemic treatments is not likely to be the retina. Neither AdoA2A gene expression (in tree shrew) nor protein levels (in guinea pigs) are affected in choroid or sclera (Cui et al., 2010; He et al., 2014). However, scleral cAMP, known to influence collagen remodeling, has been demonstrated to be influenced by visual cues (Tao et al., 2013), and pharmacologically activating AC led to myopic refractive errors, and inhibiting AC led to attenuation of myopigenesis in guinea pigs (Tao et al., 2013). Yet, it is not clear if altered collagen metabolism is critical to myopigenesis, or if it occurs in response to the elongated eye, since significant myopia can develop in tree shrews with little change in collagen remodeling (McBrien et al., 2001).
3.3.c. Influence on ocular tissues
7-MX treatment has been demonstrated to reduce the progression of myopia in children (Trier et al., 2008) and in rabbits, chickens, guinea pigs, and macaques subjected to myopigenic visual stimuli (Cui et al., 2011; Hung et al., 2018; Nie et al., 2012; Wang et al., 2014). Despite these findings, oral 7-MX had only a minor protective effect with lens defocus and no effect on form-deprivation in chickens and tree shrews (Khanal et al., 2020; Liu et al., 2020; Wang et al., 2014) (Table 3). Together, these data may indicate significant species differences in myopigenic signaling and/or bioavailability of the 7-MX. Additionally, AdoR inhibition often only leads to partial protection against myopia development, which may suggest Ado influences myopia development either through a separate parallel and opposing pathway to myopigenic signaling or a pathway that contributes to retinoscleral signaling. In contrast to the pharmacological findings, transgenic mice lacking AdoA2A developed relative myopia, both in terms of refractive error and axial length, compared to littermate wildtype controls (Zhou et al., 2010).
3.3.d. Summary
Ado is a highly dynamic and ubiquitous type of signaling with clear connections to scleral remodeling. While present in all tissues of the posterior eye wall, there is little evidence suggesting it connects any two layers and relatively little evidence showing altered signaling with myopigenesis, both in animal and human GWAS studies. However, thus far scleral Ado has not been ruled out as integral to myopigenesis, if influenced by another pathway capable of transmitting information through the choroid.
3.4. Other signals
It is known that retinoscleral signaling involved in emmetropization and myopigenesis involves many distinct signaling molecules and pathways. In addition to the signaling molecule candidates featured above, a few other signaling pathways are of particular interest due to their bidirectional modulation with visual stimulus (retinal glucagon) and successful clinical use as a pharmacological treatment for slowing myopia (atropine, a nonselective metabotropic muscarinic acetylcholine receptor antagonist). For in depth review of these and other chemical signaling molecules, see the recent review (Troilo et al., 2019).
Beyond these commonly studied signaling molecules, the thickness of the choroid has long been hypothesized to be involved in directing eye growth. It is bidirectionally modulated by visual cues, thinning with myopigenic cues and thickening in response to positive lenses or recovery from myopia. The consequences of these changes have been hypothesized to influence the scleral oxygen content and thus potentially function as a signal (Wu et al., 2018).
3.4.1. Hypoxia-inducible factor 1-alpha (Hif-1α)
It is well established that the choroid rapidly thins in response to myopigenic cues. It has been proposed that this thinning may be the result of decreased blood flow, and thus may decrease the oxygenation of the sclera (Liu et al., 2021). Retinoscleral signaling via oxygenation appears to be potentially viable, requiring that: 1) visual cues presented to the retina are able to influence the choroid, 2) decreased blood flow in the choroid underlies the choroidal thinning and results in graded degrees of scleral hypoxia, and 3) hypoxia in the sclera affects remodeling processes. Additionally, if oxygenation of the sclera explains myopigenesis, choroidal thickening should either elicit an opposite response in the sclera, or an additional pathway is required to signal slowing of axial elongation.
In support of this hypothesis, recent studies have found scleral Hif-1α to be correlated to myopigenesis. Hif-1α protein levels in the sclera, but not the retina, were associated with FDM in mice and guinea pigs and LIM in guinea pigs (Pan et al., 2021; Wu et al., 2018). Additionally, analysis of human gene databases revealed a moderate association with scleral Hif-1α signaling pathway in individuals with high myopia (Wu et al., 2018; Zhao et al., 2020). However, in tree shrews, scleral Hif-1α mRNA expression was not altered with form-deprivation, lens defocus, or recovery from lens-defocus (Guo et al., 2019, 2013). And in contrast to all, one study reported scleral Hif-1α mRNA expression decreased with lens defocus in guinea pigs (Guo et al., 2013).
Furthermore, experimentally manipulating hypoxia signaling appears to influence refractive state. Simultaneous treatment of guinea pigs with lens defocus and anti-hypoxia drugs resulted in reduced Hif-1α levels and partially suppressed myopic refractive error development and axial elongation (Wu et al., 2018). Additionally, two treatments used to experimentally reduce choroidal blood perfusion (and presumably decrease oxygenation of the sclera) were found to also induce myopia in guinea pigs (Zhou et al., 2021). These studies suggest a relationship between hypoxia, Hif-1α and development of myopia; however, the strength of this connection and whether scleral hypoxia is solely responsible for trans-choroidal propagation of myopigenic signaling is not clear. Additionally, whether the signal can encode both signs of defocus is not clear; the choroid has been shown to thicken with positive defocus and recovery from myopia, but how this translates to oxygen diffusion to the sclera and Hif-1α is not known.
4. Pathway crosstalk
Each of the primary signaling molecules discussed in the previous section are implicated to some degree in myopigenic retinoscleral signaling (Figure 2). Currently, the only retina-derived signal with any evidence to reach and act on the sclera is atRA, and more evidence suggests atRA acting on the sclera may be derived from the choroid. Thus, DA and other retinal signaling molecules known to affect myopigenesis acting anterior to the choroid, suggest that the choroid is a likely point of crosstalk between various pathways. Two mechanisms by which information can traverse the choroid have been presented: 1) changes to choroidal thickness/blood flow 2) a RA-based signal propagated across the choroid, possibly involving RBPs.
Many visual cues and retinal signaling molecules are known to influence choroidal thickness. Retina-originating signals (DA, Ado, atRA, glucagon, insulin, vasoactive intestinal peptide, monamines) could interact in the retina. While the genetic evidence for DA and Ado in human cohorts is limited, one of the most robust and repeated GWAS findings are the associations between refractive error and the gap junction delta-2 protein (GJD2) gene (Cheng et al., 2013; Fan et al., 2016; Hysi et al., 2020; Miyake et al., 2015; Solouki et al., 2010; Tedja et al., 2018; Verhoeven et al., 2013), which encodes connexin-36. As connexin-36 is dephosphorylated by DA and phosphorylated by Ado (Li et al., 2013), connexin-36 may be a conduit for these neuromodulators to affect eye growth. While not yet evaluated in the context of myopia, DA has also been found to act as an epigenetic histone marker in a process termed “dopaminylation” (Lepack et al., 2020), which may provide a future explanation linking the influence of the visual environment on genetic contribution to eye growth.
It is likely that many retina-originating signals are relayed by the RPE, due to the presence of many receptor types. With many distinct myopigenic cues similarly affecting choroidal thickness, and choroidal thickness possibly influenced by the RPE, many distinct retinal signals could be summed and influence choroidal thickness in a graded manner at the RPE. If choroidal thickness does in fact influence the oxygen environment of the sclera, further study into the role of the RPE on choroidal thickness could help elucidate these connections.
apoA-1 expression is known to be at least partially regulated by peroxisome proliferator-activated receptors (PPARs) (Gervois et al., 2000), which may be downregulated under hypoxic conditions. Intravitreal injections of the PPARα agonist GW6747, upregulated retinal and scleral apoA-I expression and suppressed experimental myopia in chickens (Bertrand et al., 2006). This increased protein expression of apoA-1 in the sclera could be from the choroid, since the sclera has not been shown to synthesize apoA-1 (Summers et al., 2016). However, GW6747 had no effect on choroidal apoA-1 mRNA expression (Summers et al., 2016).
Evidence suggests that hypoxic conditions may inhibit PPAR subtypes, possibly linking the effects of hypoxia to apoA-1 and atRA. When guinea pigs were treated with PPAR α/γ antagonists, Hif-1α increased and the animals developed myopic refractive errors and axial elongation (Pan et al., 2018). The opposite effect was found for animals treated with agonists (Pan et al., 2018). This bidirectionality of the influence of PPAR would further support the possibility of hypoxia as a cue for signaling increased/decreased axial elongation. However, PPAR activation has not yet been demonstrated to occur with choroidal thickening/hyperoxia or recovery from myopia. It is also not clear if scleral hypoxia and PPAR expression would influence apoA-1 that has presumably been transported from the choroid.
5. Conclusions and Future Directions
All of the major signaling pathways identified in this paper contribute in some way to myopigenesis and retinoscleral signaling. While DAergic signaling appears to be primarily restricted to the retina and RPE, the consequences of the signaling are known to influence both choroidal thickness and axial length. Ado is ubiquitous through the posterior eye wall and influences scleral remodeling; however, whether it is involved in signaling between tissues is not clear. RA is a promising candidate, ubiquitous to all posterior eye wall tissues, bidirectionally modulated with positive/negative defocus and has a plausible hypothesized transport mechanism by which it could cross the choroid. However, much of the work surrounding this molecule has been performed in the chicken model, which displays significant differences in scleral composition compared to mammalian species and necessitates further study in mammalian models. While many pathways play a role in myopigenesis, signaling across the choroid appears to be a bottleneck before which crosstalk likely occurs. Identified here are two main mechanisms by which information originating in the retina may reach the sclera: via an atRA-driven mechanism and/or oxygenation of the sclera. Future studies should be performed in mammalian models in order to better understand which findings are general mechanisms of myopigenesis. Additionally, directing research effort towards the use of inducible knockout mouse models could help to elucidate additional details of some of these difficult to study signaling mechanisms, such as: the role of adenosine in the retina, how dopamine influences the RPE, whether myopigenesis can occur in apoA-1 deficient animals, and how the various pathways influence choroidal apoA-1.
Supplementary Material
Funding
This project was supported by the National Institutes of Health [NIH R01 EY016435 (MTP), NIH P30 EY006360 (MTP), T32 EY007092 (D.M.B.), T32 HL007901 (D.C.T.), F31 HD097918 (D.C.T.), T32 ES012870 (D.C.T.)], Department of Veterans Affairs [Rehabilitation R&D Service Research Career Scientist Award IK6 RX003134 (MTP)].
Abbreviations/acronyms
- GWAS
Genome-wide association study
- D
Diopters
- RPE
Retinal Pigement Epithelium
- LIM
Lens induced myopia
- FDM
Form-deprivation myopia
- DA
Dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- DAergic
dopaminergic
- DAC
Dopaminergic amacrine cell
- cAMP
cyclic adenosine monophosphate
- mRGC
melanopsin retinal ganglion cell
- CREAM
Consortium for Refractive Error and Myopia
- SNP
single nucleotide polymorphism
- DRD1
Dopamine Receptor 1 gene
- RA
Retinoic Acid
- atRA
all-trans retinoic acid
- ADH
alcohol dehydrogenase
- RALDH
Retinaldehyde Dehydrogenase
- RAR
retinoic acid receptor
- RXR
Retinoid-X receptor
- RBP
retinoid binding protein
- apoA-1
apolipoprotein A-1
- RDH5
retinol dehydrogenase 5
- RGR
retinal G protein-coupled receptor
- RORB
receptor-related orphan receptor beta
- Ado
Adenosine
- AdoR
Adenosine receptor
- AC
adenyl cyclase
- NMDA
N-methyl-D-aspartate
- ENT
equilibrative nucleoside transporters
- 7-Mx
7-methylxanthine
- Hif-1α
Hypoxia-inducible factor 1-alpha
- GJD2
gap junction delta-2 protein
References
- Aleman AC, Wang M, Schaeffel F, 2018. Reading and Myopia: Contrast Polarity Matters. Sci. Rep 8, 10840. 10.1038/s41598-018-28904-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-André M, 1998. Disruption of retinoid-related orphan receptor β changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 17, 3867–3877. 10.1093/emboj/17.14.3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli L, Pacher P, Vizi ES, Haskó G, 2013. CD39 and CD73 in immunity and inflammation. Trends Mol. Med 19, 355–367. 10.1016/j.molmed.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan G, Kull B, Fredholm BB, 2002. Anoxia redistributes adenosine A(2A) receptors in PC12 cells and increases receptor-mediated formation of cAMP. Naunyn. Schmiedebergs Arch. Pharmacol 365, 150–157. 10.1007/s002100100456 [DOI] [PubMed] [Google Scholar]
- Ashby R, McCarthy CS, Maleszka R, Megaw P, Morgan IG, 2007. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp. Eye Res 85, 15–22. 10.1016/j.exer.2007.02.019 [DOI] [PubMed] [Google Scholar]
- Ashby R, Ohlendorf A, Schaeffel F, 2009. The Effect of Ambient Illuminance on the Development of Deprivation Myopia in Chicks. Investig. Opthalmology Vis. Sci 50, 5348. 10.1167/iovs.09-3419 [DOI] [PubMed] [Google Scholar]
- Ashby RS, Schaeffel F, 2010. The Effect of Bright Light on Lens Compensation in Chicks. Investig. Opthalmology Vis. Sci 51, 5247. 10.1167/iovs.09-4689 [DOI] [PubMed] [Google Scholar]
- Baba K, DeBruyne JP, Tosini G, 2017. Dopamine 2 Receptor Activation Entrains Circadian Clocks in Mouse Retinal Pigment Epithelium. Sci. Rep 7, 5103. 10.1038/s41598-017-05394-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhouse S, Gentle A, 2018. Scleral remodelling in myopia and its manipulation: a review of recent advances in scleral strengthening and myopia control. Ann. Eye Sci 3, 5–5. 10.21037/aes.2018.01.04 [DOI] [Google Scholar]
- Balmer JE, Blomhoff R, 2002. Gene expression regulation by retinoic acid. J. Lipid Res 43, 1773–1808. 10.1194/jlr.R100015-JLR200 [DOI] [PubMed] [Google Scholar]
- Bartmann M, Schaeffel F, 1994. A simple mechanism for emmetropization without cues from accommodation or colour. Vision Res. 34, 873–876. 10.1016/0042-6989(94)90037-x [DOI] [PubMed] [Google Scholar]
- Bauer B, Ehinger B, Aberg L, 1980. [3H]-Dopamine release from the rabbit retina. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 215, 71–78. 10.1007/BF00414464 [DOI] [PubMed] [Google Scholar]
- Beach KM, Hung L-F, Arumugam B, Smith EL, Ostrin LA, 2018. Adenosine receptor distribution in Rhesus monkey ocular tissue. Exp. Eye Res 174, 40–50. 10.1016/j.exer.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen MA, Park H. na, Chakraborty R, Landis EG, Sidhu C, He L, Iuvone PM, Pardue MT, 2016. Altered Refractive Development in Mice With Reduced Levels of Retinal Dopamine. Investig. Opthalmology Vis. Sci 57, 4412. 10.1167/iovs.15-17784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E, Fritsch C, Diether S, Lambrou G, Müller D, Schaeffel F, Schindler P, Schmid KL, van Oostrum J, Voshol H, 2006. Identification of apolipoprotein A-I as a “STOP” signal for myopia. Mol. Cell. Proteomics MCP 5, 2158–2166. 10.1074/mcp.M600073-MCP200 [DOI] [PubMed] [Google Scholar]
- Bitzer M, Feldkaemper M, Schaeffel F, 2000. Visually Induced Changes in Components of the Retinoic Acid System in Fundal Layers of the Chick. Exp. Eye Res 70, 97–106. 10.1006/exer.1999.0762 [DOI] [PubMed] [Google Scholar]
- Boelen MK, Boelen MG, Marshak DW, 1998. Light-stimulated release of dopamine from the primate retina is blocked by l −2-amino-4-phosphonobutyric acid (APB). Vis. Neurosci 15, 97–103. 10.1017/S0952523898151040 [DOI] [PubMed] [Google Scholar]
- Bonassar LJ, Sandy JD, Lark MW, Plaas AHK, Frank EH, Grodzinsky AJ, 1997. Inhibition of Cartilage Degradation and Changes in Physical Properties Induced by IL-1β and Retinoic Acid Using Matrix Metalloproteinase Inhibitors. Arch. Biochem. Biophys 344, 404–412. 10.1006/abbi.1997.0205 [DOI] [PubMed] [Google Scholar]
- Boote C, Sigal IA, Grytz R, Hua Y, Nguyen TD, Girard MJA, 2020. Scleral structure and biomechanics. Prog. Retin. Eye Res 74, 100773. 10.1016/j.preteyeres.2019.100773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowrey HE, Metse AP, Leotta AJ, Zeng G, Mcfadden SA, 2015. The relationship between image degradation and myopia in the mammalian eye. Clin. Exp. Optom 98, 555–563. 10.1111/cxo.12316 [DOI] [PubMed] [Google Scholar]
- Brambilla D, Chapman D, Greene R, 2005. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron 46, 275–283. 10.1016/j.neuron.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Brown D, Ethier CR, Pardue MT, 2021. Oral All-trans Retinoic Acid Treatment Induces Myopia and Alters Scleral Biomechanics in Mice. Invest. Ophthalmol. Vis. Sci 62, 2876–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundege JM, Dunwiddie TV, 1998. Metabolic regulation of endogenous adenosine release from single neurons. Neuroreport 9, 3007–3011. 10.1097/00001756-199809140-00016 [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Park H. na, Aung MH, Tan CC, Sidhu CS, Iuvone PM, Pardue MT, 2014. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol. Vis 20, 1318–1327. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Park H. na, Hanif AM, Sidhu CS, Iuvone PM, Pardue MT, 2015. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp. Eye Res 137, 79–83. 10.1016/j.exer.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Vincent SJ, 2020. Understanding Myopia: Pathogenesis and Mechanisms, in: Ang M, Wong TY (Eds.), Updates on Myopia: A Clinical Perspective. Springer, Singapore, pp. 65–94. 10.1007/978-981-13-8491-2_4 [DOI] [Google Scholar]
- Chen S, Zhi Z, Ruan Q, Liu Q, Li F, Wan F, Reinach PS, Chen J, Qu J, Zhou X, 2017. Bright Light Suppresses Form-Deprivation Myopia Development With Activation of Dopamine D1 Receptor Signaling in the ON Pathway in Retina. Investig. Opthalmology Vis. Sci 58, 2306. 10.1167/iovs.16-20402 [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Schache M, Ikram MK, Young TL, Guggenheim JA, Vitart V, MacGregor S, Verhoeven VJM, Barathi VA, Liao J, Hysi PG, Bailey-Wilson JE, Pourcain B St., Kemp JP, McMahon G, Timpson NJ, Evans DM, Montgomery GW, Mishra A, Wang YX, Wang JJ, Rochtchina E, Polasek O, Wright AF, Amin N, van Leeuwen EM, Wilson JF, Pennell CE, van Duijn CM, de Jong PTVM, Vingerling JR, Zhou Xin, Chen P, Li R, Tay W-T, Zheng Y, Chew M, Rahi JS, Hysi PG, Yoshimura N, Yamashiro K, Miyake M, Delcourt C, Maubaret C, Williams C, Guggenheim JA, Northstone K, Ring SM, Davey-Smith G, Craig JE, Burdon KP, Fogarty RD, Iyengar SK, Igo RP, Chew E, Janmahasathian S, Iyengar SK, Igo RP, Chew E, Janmahasathian S, Stambolian D, Wilson JEB, MacGregor S, Lu Y, Jonas JB, Xu L, Saw S-M, Baird PN, Rochtchina E, Mitchell P, Wang JJ, Jonas JB, Nangia V, Hayward C, Wright AF, Vitart V, Polasek O, Campbell H, Vitart V, Rudan I, Vatavuk Z, Vitart V, Paterson AD, Hosseini SM, Iyengar SK, Igo RP, Fondran JR, Young TL, Feng S, Verhoeven VJM, Klaver CC, van Duijn CM, Metspalu A, Haller T, Mihailov E, Pärssinen O, Wedenoja J, Wilson JEB, Wojciechowski R, Baird PN, Schache M, Pfeiffer N, Höhn R, Pang CP, Chen P, Meitinger T, Oexle K, Wegner A, Yoshimura N, Yamashiro K, Miyake M, Pärssinen O, Yip SP, Ho DWH, Pirastu M, Murgia F, Portas L, Biino G, Wilson JF, Fleck B, Vitart V, Stambolian D, Wilson JEB, Hewitt AW, Ang W, Verhoeven VJM, Klaver CC, van Duijn CM, Saw S-M, Wong T-Y, Teo Y-Y, Fan Q, Cheng C-Y, Zhou Xin, Ikram MK, Saw S-M, Teo Y-Y, Fan Q, Cheng C-Y, Zhou Xin, Ikram MK, Saw S-M, Wong T-Y, Teo Y-Y, Fan Q, Cheng C-Y, Zhou Xin, Ikram MK, Saw S-M, Wong T-Y, Teo Y-Y, Fan Q, Cheng C-Y, Zhou Xin, Ikram MK, Saw S-M, Tai E-S, Teo Y-Y, Fan Q, Cheng C-Y, Zhou Xin, Ikram MK, Saw S-M, Teo Y-Y, Fan Q, Cheng C-Y, Zhou Xin, Ikram MK, Mackey DA, MacGregor S, Hammond CJ, Hysi PG, Deangelis MM, Morrison M, Zhou Xiangtian, Chen W, Paterson AD, Hosseini SM, Mizuki N, Meguro A, Lehtimäki T, Mäkelä K-M, Raitakari O, Kähönen M, Burdon KP, Craig JE, Iyengar SK, Igo RP, Lass JH, Reinhart W, Belin MW, Schultze RL, Morason T, Sugar A, Mian S, Soong HK, Colby K, Jurkunas U, Yee R, Vital M, Alfonso E, Karp C, Lee Y, Yoo S, Hammersmith K, Cohen E, Laibson P, Rapuano C, Ayres B, Croasdale C, Caudill J, Patel S, Baratz K, Bourne W, Maguire L, Sugar J, Tu E, Djalilian A, Mootha V, McCulley J, Bowman W, Cavanaugh HD, Verity S, Verdier D, Renucci A, Oliva M, Rotkis W, Hardten DR, Fahmy A, Brown M, Reeves S, Davis EA, Lindstrom R, Hauswirth S, Hamilton S, Lee WB, Price F, Price M, Kelly K, Peters F, Shaughnessy M, Steinemann T, Dupps BJ, Meisler DM, Mifflin M, Olson R, Aldave A, Holland G, Mondino BJ, Rosenwasser G, Gorovoy M, Dunn SP, Heidemann DG, Terry M, Shamie N, Rosenfeld SI, Suedekum B, Hwang D, Stone D, Chodosh J, Galentine PG, Bardenstein D, Goddard K, Chin H, Mannis M, Varma R, Borecki I, Chew EY, Haller T, Mihailov E, Metspalu A, Wedenoja J, Simpson CL, Wojciechowski R, Höhn R, Mirshahi A, Zeller T, Pfeiffer N, Lackner KJ, Donnelly P, Barroso I, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Deloukas P, Duncanson A, Jankowski J, Markus HS, Mathew CG, Palmer CNA, Plomin R, Rautanen A, Sawcer SJ, Trembath RC, Viswanathan AC, Wood NW, Spencer CCA, Band G, Bellenguez C, Freeman C, Hellenthal G, Giannoulatou E, Pirinen M, Pearson R, Strange A, Su Z, Vukcevic D, Donnelly P, Langford C, Hunt SE, Edkins S, Gwilliam R, Blackburn H, Bumpstead SJ, Dronov S, Gillman M, Gray E, Hammond N, Jayakumar A, McCann OT, Liddle J, Potter SC, Ravindrarajah R, Ricketts M, Waller M, Weston P, Widaa S, Whittaker P, Barroso I, Deloukas P, Mathew CG, Blackwell JM, Brown MA, Corvin A, Spencer CCA, Bettecken T, Meitinger T, Oexle K, Pirastu M, Portas L, Nag A, Williams KM, Yonova-Doing E, Klein R, Klein BE, Hosseini SM, Paterson AD, Genuth S, Nathan DM, Zinman B, Crofford O, Crandall J, Reid M, Brown-Friday J, Engel S, Sheindlin J, Martinez H, Shamoon H, Engel H, Phillips M, Gubitosi-Klug R, Mayer L, Pendegast S, Zegarra H, Miller D, Singerman L, Smith-Brewer S, Novak M, Quin J, Dahms W, Genuth Saul, Palmert M, Brillon D, Lackaye ME, Kiss S, Chan R, Reppucci V, Lee T, Heinemann M, Whitehouse F, Kruger D, Jones JK, McLellan M, Carey JD, Angus E, Thomas A, Galprin A, Bergenstal R, Johnson M, Spencer M, Morgan K, Etzwiler D, Kendall D, Aiello LP, Golden E, Jacobson A, Beaser R, Ganda O, Hamdy O, Wolpert H, Sharuk G, Arrigg P, Schlossman D, Rosenzwieg J, Rand L, Nathan DM, Larkin M, Ong M, Godine J, Cagliero E, Lou P, Folino K, Fritz S, Crowell S, Hansen K, Gauthier-Kelly C, Service J, Ziegler G, Luttrell L, Caulder S, Lopes-Virella M, Colwell J, Soule J, Fernandes J, Hermayer K, Kwon S, Brabham M, Blevins A, Parker J, Lee D, Patel N, Pittman C, Lindsey P, Bracey M, Lee K, Nutaitis M, Farr A, Elsing S, Thompson T, Selby J, Lyons T, Yacoub-Wasef S, Szpiech M, Wood D, Mayfield R, Molitch M, Schaefer B, Jampol L, Lyon A, Gill M, Strugula Z, Kaminski L, Mirza R, Simjanoski E, Ryan D, Kolterman O, Lorenzi G, Goldbaum M, Sivitz W, Bayless M, Counts D, Johnsonbaugh S, Hebdon M, Salemi P, Liss R, Donner T, Gordon J, Hemady R, Kowarski A, Ostrowski D, Steidl S, Jones B, Herman WH, Martin CL, Pop-Busui R, Sarma A, Albers J, Feldman E, Kim K, Elner S, Comer G, Gardner T, Hackel R, Prusak R, Goings L, Smith A, Gothrup J, Titus P, Lee J, Brandle M, Prosser L, Greene DA, Stevens MJ, Vine AK, Bantle J, Wimmergren N, Cochrane A, Olsen T, Steuer E, Rath P, Rogness B, Hainsworth D, Goldstein D, Hitt S, Giangiacomo J, Schade DS, Canady JL, Chapin JE, Ketai LH, Braunstein CS, Bourne PA, Schwartz S, Brucker A, Maschak-Carey BJ, Baker L, Orchard T, Silvers N, Ryan C, Songer T, Doft B, Olson S, Bergren RL, Lobes L, Rath PP, Becker D, Rubinstein D, Conrad PW, Yalamanchi S, Drash A, Morrison A, Bernal ML, Vaccaro-Kish J, Malone J, Pavan PR, Grove N, Iyer MN, Burrows AF, Tanaka EA, Gstalder R, Dagogo-Jack S, Wigley C, Ricks H, Kitabchi A, Murphy MB, Moser S, Meyer D, Iannacone A, Chaum E, Yoser S, Bryer-Ash M, Schussler S, Lambeth H, Raskin P, Strowig S, Zinman B, Barnie A, Devenyi R, Mandelcorn M, Brent M, Rogers S, Gordon A, Palmer J, Catton S, Brunzell J, Wessells H, de Boer IH, Hokanson J, Purnell J, Ginsberg J, Kinyoun J, Deeb S, Weiss M, Meekins G, Distad J, Van Ottingham L, Dupre J, Harth J, Nicolle D, Driscoll M, Mahon J, Canny C, May M, Lipps J, Agarwal A, Adkins T, Survant L, Pate RL, Munn GE, Lorenz R, Feman S, White N, Levandoski L, Boniuk I, Grand G, Thomas M, Joseph DD, Blinder K, Shah G, Boniuk Burgess, Santiago J, Tamborlane W, Gatcomb P, Stoessel K, Taylor K, Goldstein J, Novella S, Mojibian H, Cornfeld D, Lima J, Bluemke D, Turkbey E, van der Geest RJ, Liu C, Malayeri A, Jain A, Miao C, Chahal H, Jarboe R, Maynard J, Gubitosi-Klug R, Quin J, Gaston P, Palmert M, Trail R, Dahms W, Lachin J, Cleary P, Backlund J, Sun W, Braffett B, Klumpp K, Chan K, Diminick L, Rosenberg D, Petty B, Determan A, Kenny D, Rutledge B, Younes N, Dews L, Hawkins M, Cowie C, Fradkin J, Siebert C, Eastman R, Danis R, Gangaputra S, Neill S, Davis M, Hubbard L, Wabers H, Burger M, Dingledine J, Gama V, Sussman R, Steffes M, Bucksa J, Nowicki M, Chavers B, O’Leary D, Polak J, Harrington A, Funk L, Crow R, Gloeb B, Thomas S, O’Donnell C, Soliman E, Zhang ZM, Prineas R, Campbell C, Ryan C, Sandstrom D, Williams T, Geckle M, Cupelli E, Thoma F, Burzuk B, Woodfill T, Low P, Sommer C, Nickander K, Budoff M, Detrano R, Wong N, Fox M, Kim L, Oudiz R, Weir G, Espeland M, Manolio T, Rand L, Singer D, Stern M, Boulton AE, Clark C, D’Agostino R, Lopes-Virella M, Garvey WT, Lyons TJ, Jenkins A, Virella G, Jaffa A, Carter R, Lackland D, Brabham M, McGee D, Zheng D, Mayfield RK, Boright A, Bull S, Sun L, Scherer S, Zinman B, Natarajan R, Miao F, Zhang L, Chen Z, Nathan DM, Makela K-M, Lehtimaki T, Kahonen M, Raitakari O, Yoshimura N, Matsuda F, Chen LJ, Pang CP, Yip SP, Yap MKH, Meguro A, Mizuki N, Inoko H, Foster PJ, Zhao JH, Vithana E, Tai E-S, Fan Q, Xu L, Campbell H, Fleck B, Rudan I, Aung T, Hofman A, Uitterlinden AG, Bencic G, Khor C-C, Forward H, Pärssinen O, Mitchell P, Rivadeneira F, Hewitt AW, Williams C, Oostra BA, Teo Y-Y, Hammond CJ, Stambolian D, Mackey DA, Klaver CCW, Wong T-Y, Saw S-M, Baird PN, 2013. Nine Loci for Ocular Axial Length Identified through Genome-wide Association Studies, Including Shared Loci with Refractive Error. Am. J. Hum. Genet 93, 264–277. 10.1016/j.ajhg.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Hadjiconstantinou M, Neff NH, 1983. Activation of dopamine-containing amacrine cells of retina: light-induced increase of acidic dopamine metabolites. Brain Res. 260, 125–127. 10.1016/0006-8993(83)90771-0 [DOI] [PubMed] [Google Scholar]
- Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS, 2012. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp. Eye Res 103, 33–40. 10.1016/j.exer.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M, 1998. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U. S. A 95, 15735–15740. 10.1073/pnas.95.26.15735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther SG, Liang H, Junghans BM, Crewther DP, 2006. Ionic control of ocular growth and refractive change. Proc. Natl. Acad. Sci. U. S. A 103, 15663–15668. 10.1073/pnas.0607241103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther SG, Murphy MJ, Crewther DP, 2008. Potassium channel and NKCC cotransporter involvement in ocular refractive control mechanisms. PloS One 3, e2839. 10.1371/journal.pone.0002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Trier K, Chen X, Zeng J, Yang X, Hu J, Ge J, 2008. Distribution of adenosine receptors in human sclera fibroblasts. Mol. Vis 14, 523–529. [PMC free article] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J, Wu K, Yu M, Ge J, 2010. Adenosine receptor protein changes in guinea pigs with form deprivation myopia. Acta Ophthalmol. (Copenh.) 88, 759–765. 10.1111/j.1755-3768.2009.01559.x [DOI] [PubMed] [Google Scholar]
- Cui D, Trier K, Zeng J, Wu K, Yu M, Hu J, Chen X, Ge J, 2011. Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol. (Copenh.) 89, 328–334. 10.1111/j.1755-3768.2009.01688.x [DOI] [PubMed] [Google Scholar]
- Cvekl A, Wang W-L, 2009. Retinoic acid signaling in mammalian eye development. Exp. Eye Res 89, 280–291. 10.1016/j.exer.2009.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, 1990. The dopaminergic amacrine cell. J. Comp. Neurol 301, 461–489. 10.1002/cne.903010310 [DOI] [PubMed] [Google Scholar]
- de Jong PTVM, 2018. Myopia: its historical contexts. Br. J. Ophthalmol 102, 1021–1027. 10.1136/bjophthalmol-2017-311625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diether S, Schaeffel F, 1997. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 37, 659–668. 10.1016/s0042-6989(96)00224-6 [DOI] [PubMed] [Google Scholar]
- Ding X, Hu Y, Guo Xinxing, Guo Xiaobo, Morgan I, He M, 2018. Possible Causes of Discordance in Refraction in Monozygotic Twins: Nearwork, Time Outdoors and Stochastic Variation. Invest. Ophthalmol. Vis. Sci 59, 5349–5354. 10.1167/iovs.18-24526 [DOI] [PubMed] [Google Scholar]
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, Rose KA, Mitchell P, Saw S-M, 2009. Outdoor activity and myopia in Singapore teenage children. Br. J. Ophthalmol 93, 997–1000. 10.1136/bjo.2008.150979 [DOI] [PubMed] [Google Scholar]
- Dolgin E, 2015. The myopia boom. Nature 519, 276–278. 10.1038/519276a [DOI] [PubMed] [Google Scholar]
- Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen J-F, Willcox MDP, Qu J, Zhou X, 2011. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol. Vis 17, 2824–2834. [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIVOR W, Menaker M, 2002. Circadian rhythms of dopamine in mouse retina: The role of melatonin. Vis. Neurosci 19, 593–601. 10.1017/S0952523802195058 [DOI] [PubMed] [Google Scholar]
- Drury AN, Szent-Györgyi A, 1929. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol 68, 213–237. 10.1113/jphysiol.1929.sp002608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM, 2009. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: Contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J. Comp. Neurol 517, 226–244. 10.1002/cne.22158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RB, Adler AJ, Dev S, Claycomb RC, 1992. Synthesis of retinoic acid from retinol by cultured rabbit Müller cells. Exp. Eye Res 54, 481–490. 10.1016/0014-4835(92)90126-d [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, 2013. Extracellular Adenosine Signaling in Molecular Medicine. J. Mol. Med. Berl. Ger 91, 141–146. 10.1007/s00109-013-0999-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, Verhoeven VJM, Wojciechowski R, Barathi VA, Hysi PG, Guggenheim JA, Höhn R, Vitart V, Khawaja AP, Yamashiro K, Hosseini SM, Lehtimäki T, Lu Y, Haller T, Xie J, Delcourt C, Pirastu M, Wedenoja J, Gharahkhani P, Venturini C, Miyake M, Hewitt AW, Guo X, Mazur J, Huffman JE, Williams KM, Polasek O, Campbell H, Rudan I, Vatavuk Z, Wilson JF, Joshi PK, McMahon G, St Pourcain B, Evans DM, Simpson CL, Schwantes-An T-H, Igo RP, Mirshahi A, Cougnard-Gregoire A, Bellenguez C, Blettner M, Raitakari O, Kähönen M, Seppälä I, Zeller T, Meitinger T, Ried JS, Gieger C, Portas L, van Leeuwen EM, Amin N, Uitterlinden AG, Rivadeneira F, Hofman A, Vingerling JR, Wang YX, Wang X, Tai-Hui Boh E, Ikram MK, Sabanayagam C, Gupta P, Tan V, Zhou L, Ho CEH, Lim W, Beuerman RW, Siantar R, Tai E-S, Vithana E, Mihailov E, Khor C-C, Hayward C, Luben RN, Foster PJ, Klein BEK, Klein R, Wong H-S, Mitchell P, Metspalu A, Aung T, Young TL, He M, Pärssinen O, van Duijn CM, Jin Wang J, Williams C, Jonas JB, Teo Y-Y, Mackey DA, Oexle K, Yoshimura N, Paterson AD, Pfeiffer N, Wong T-Y, Baird PN, Stambolian D, Wilson JEB, Cheng C-Y, Hammond CJ, Klaver CCW, Saw S-M, 2016. Meta-analysis of gene–environment-wide association scans accounting for education level identifies additional loci for refractive error. Nat. Commun 7, 11008. 10.1038/ncomms11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkaemper M, Schaeffel F, 2013. An updated view on the role of dopamine in myopia. Exp. Eye Res 114, 106–119. 10.1016/j.exer.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Fledelius HC, Christensen AC, 1996. Reappraisal of the human ocular growth curve in fetal life, infancy, and early childhood. Br. J. Ophthalmol 80, 918–921. 10.1136/bjo.80.10.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledelius HC, Christensen AS, Fledelius C, 2014. Juvenile eye growth, when completed? An evaluation based on IOL-Master axial length data, cross-sectional and longitudinal. Acta Ophthalmol. (Copenh.) 92, 259–264. 10.1111/aos.12107 [DOI] [PubMed] [Google Scholar]
- Flitcroft DI, 2013. Is myopia a failure of homeostasis? Exp. Eye Res 114, 16–24. 10.1016/j.exer.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Flitcroft DI, Harb EN, Wildsoet CF, 2020. The Spatial Frequency Content of Urban and Indoor Environments as a Potential Risk Factor for Myopia Development. Investig. Opthalmology Vis. Sci 61, 42. 10.1167/iovs.61.11.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, Rahi J, Resnikoff S, Vitale S, Yannuzzi L, 2019. IMI – Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Opthalmology Vis. Sci 60, M20. 10.1167/iovs.18-25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, 2014. Adenosine--a physiological or pathophysiological agent? J. Mol. Med. Berl. Ger 92, 201–206. 10.1007/s00109-013-1101-6 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, 2007. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 14, 1315–1323. 10.1038/sj.cdd.4402132 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE, 1999. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev 51, 83–133. [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M, 2007. Aspects of the general biology of adenosine A2A signaling. Prog. Neurobiol, Targeting Adenosine A2A Receptors in Parkinson’s Disease and other CNS Disorders 83, 263–276. 10.1016/j.pneurobio.2007.07.005 [DOI] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG, Rose KA, 2013. Time outdoors and the prevention of myopia. Exp. Eye Res, Josh Wallman Special Tribute Edition 114, 58–68. 10.1016/j.exer.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Gallemore RP, Steinberg RH, 1990. Effects of dopamine on the chick retinal pigment epithelium. Membrane potentials and light-evoked responses. Invest. Ophthalmol. Vis. Sci 31, 67–80. [PubMed] [Google Scholar]
- Galvis V, Tello A, Parra MM, Merayo-Lloves J, Larrea J, Julian Rodriguez C, Camacho PA, 2016. Topical Atropine in the Control of Myopia. Med. Hypothesis Discov. Innov. Ophthalmol. J 5, 78–88. [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Liu Q, Ma P, Zhong X, Wu J, Ge J, 2006. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch. Clin. Exp. Ophthalmol 244, 1329–1335. 10.1007/s00417-006-0254-1 [DOI] [PubMed] [Google Scholar]
- Gawne TJ, Siegwart JT, Ward AH, Norton TT, 2017. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp. Eye Res 155, 75–84. 10.1016/j.exer.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P, Torra IP, Fruchart JC, Staels B, 2000. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin. Chem. Lab. Med 38, 3–11. 10.1515/CCLM.2000.002 [DOI] [PubMed] [Google Scholar]
- Ghorbani Mojarrad N, Plotnikov D, Williams C, Guggenheim JA, UK Biobank Eye and Vision Consortium, 2020. Association Between Polygenic Risk Score and Risk of Myopia. JAMA Ophthalmol. 138, 7–13. 10.1001/jamaophthalmol.2019.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley BF, Wurtman RJ, 1988. Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain Res. 452, 393–395. 10.1016/0006-8993(88)90046-7 [DOI] [PubMed] [Google Scholar]
- Goldschmidt E, Jacobsen N, 2014. Genetic and environmental effects on myopia development and progression. Eye 28, 126–133. 10.1038/eye.2013.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RA, Donzis PB, 1985. Refractive Development of the Human Eye. Arch. Ophthalmol 103, 785–789. 10.1001/archopht.1985.01050060045020 [DOI] [PubMed] [Google Scholar]
- Grytz R, 2018. Scleral remodeling in myopia, in: Roberts CJ, Dupps WJ, Downs JC (Eds.), Biomechanics of the Eye. Kugler Publications, pp. 383–403. [Google Scholar]
- Grytz R, Yang H, Hua Y, Samuels BC, Sigal IA, 2020. Connective tissue remodeling in myopia and its potential role in increasing risk of glaucoma. Curr. Opin. Biomed. Eng 15, 40–50. 10.1016/j.cobme.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, He L, Siegwart JTJ, Norton TT, 2013. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest. Ophthalmol. Vis. Sci 54, 6806–6819. 10.1167/iovs.13-12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Norton TT, 2014. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol. Vis 20, 1643–1659. [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, Siegwart JTJ, Norton TT, 2019. Gene expression signatures in tree shrew sclera during recovery from minus-lens wear and during plus-lens wear. Mol. Vis 25, 311–328. [PMC free article] [PubMed] [Google Scholar]
- Guo SS, Sivak JG, Callender MG, Diehl-Jones B, 1995. Retinal dopamine and lens-induced refractive errors in chicks. Curr. Eye Res 14, 385–389. 10.3109/02713689508999936 [DOI] [PubMed] [Google Scholar]
- Hagen LA, Gilson SJ, Akram MN, Baraas RC, 2019. Emmetropia Is Maintained Despite Continued Eye Growth From 16 to 18 Years of Age. Investig. Opthalmology Vis. Sci 60, 4178. 10.1167/iovs.19-27289 [DOI] [PubMed] [Google Scholar]
- Haskó G, Antonioli L, Cronstein BN, 2018. Adenosine metabolism, immunity and joint health. Biochem. Pharmacol 151, 307–313. 10.1016/j.bcp.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, Norton TT, 2014. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp. Eye Res, Special Issue: Stem Cells 123, 56–71. 10.1016/j.exer.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JTJ, Norton TT, 2018. Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp. Eye Res 168, 77–88. 10.1016/j.exer.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Sun T, Yang J, Qin G, Wu Z, Zhu X, Lu Y, 2017. Analysis of Factors Associated with the Ocular Features of Congenital Cataract Children in the Shanghai Pediatric Cataract Study. J. Ophthalmol 2017, 1–7. 10.1155/2017/8647435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S, 2016. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 123, 1036–1042. 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Howlett MHC, McFadden SA, 2006. Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Res. 46, 267–283. 10.1016/j.visres.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Huang F, Shu Z, Huang Q, Chen K, Yan W, Wu W, Yang J, Wang Q, Wang F, Zhang C, Qu J, Zhou X, 2022. Retinal Dopamine D2 Receptors Participate in the Development of Myopia in Mice. Investig. Opthalmology Vis. Sci 63, 24. 10.1167/iovs.63.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Wang Q, Yan T, Tang J, Hou X, Shu Z, Wan F, Yang Y, Qu J, Zhou X, 2020. The Role of the Dopamine D2 Receptor in Form-Deprivation Myopia in Mice: Studies With Full and Partial D2 Receptor Agonists and Knockouts. Invest. Ophthalmol. Vis. Sci 61, 47. 10.1167/iovs.61.6.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Yan T, Shi F, An J, Xie R, Zheng F, Li Y, Chen J, Qu J, Zhou X, 2014. Activation of Dopamine D2 Receptor Is Critical for the Development of Form-Deprivation Myopia in the C57BL/6 Mouse. Investig. Opthalmology Vis. Sci 55, 5537. 10.1167/iovs.13-13211 [DOI] [PubMed] [Google Scholar]
- Huang F, Zhang L, Wang Q, Yang Y, Li Q, Wu Y, Chen J, Qu J, Zhou X, 2018. Dopamine D1 Receptors Contribute Critically to the Apomorphine-Induced Inhibition of Form-Deprivation Myopia in Mice. Investig. Opthalmology Vis. Sci 59, 2623. 10.1167/iovs.17-22578 [DOI] [PubMed] [Google Scholar]
- Huang H-M, Chang DS-T, Wu P-C, 2015. The Association between Near Work Activities and Myopia in Children—A Systematic Review and Meta-Analysis. PLOS ONE 10, e0140419. 10.1371/journal.pone.0140419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Qu X-M, Chu R-Y, 2011. Expressions of cellular retinoic acid binding proteins I and retinoic acid receptor-β in the guinea pig eyes with experimental myopia. Int. J. Ophthalmol 4, 131–136. 10.3980/j.issn.2222-3959.2011.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, Ostrin L, Patel N, Trier K, Jong M, S. EL Iii, 2018. The Adenosine Receptor Antagonist, 7-Methylxanthine, Alters Emmetropizing Responses in Infant Macaques. Investig. Opthalmology Vis. Sci 59, 472. 10.1167/iovs.17-22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysi PG, Choquet H, Khawaja AP, Wojciechowski R, Tedja MS, Yin J, Simcoe MJ, Patasova K, Mahroo OA, Thai KK, Cumberland PM, Melles RB, Verhoeven VJM, Vitart V, Segre A, Stone RA, Wareham N, Hewitt AW, Mackey DA, Klaver CCW, MacGregor S, Khaw PT, Foster PJ, Guggenheim JA, Rahi JS, Jorgenson E, Hammond CJ, 2020. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat. Genet 52, 401–407. 10.1038/s41588-020-0599-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA, 1998. Induction of axial eye elongation and myopic refractive shift in one-year-old chickens. Vision Res. 38, 1883–1888. 10.1016/S0042-6989(97)00347-7 [DOI] [PubMed] [Google Scholar]
- Iuvone P, Galli C, Garrison-Gund C, Neff N, 1978. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 202, 901–902. 10.1126/science.30997 [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Fernandes A, Tigges J, 1989. Dopamine synthesis and metabolism in rhesus monkey retina: Development, aging, and the effects of monocular visual deprivation. Vis. Neurosci 2, 465–471. 10.1017/S0952523800012360 [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM, 1991. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest. Ophthalmol. Vis. Sci 32, 1674–1677. [PubMed] [Google Scholar]
- Jia L, Oh ECT, Ng L, Srinivas M, Brooks M, Swaroop A, Forrest D, 2009. Retinoid-related orphan nuclear receptor RORβ is an early-acting factor in rod photoreceptor development. Proc. Natl. Acad. Sci 10.1073/pnas.0902425106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Pardue MT, Mori K, Ikeda S-I, Torii H, D’Souza S, Lang RA, Kurihara T, Tsubota K, 2021. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc. Natl. Acad. Sci. U. S. A 118. 10.1073/pnas.2018840118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J-X, Hua W-J, Jiang X, Wu X-Y, Yang J-W, Gao G-P, Fang Y, Pei C-L, Wang S, Zhang J-Z, Tao L-M, Tao F-B, 2015. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast china: the sujiatun eye care study. BMC Ophthalmol. 15, 73. 10.1186/s12886-015-0052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K, 2005. Comparison of Ocular Component Growth Curves among Refractive Error Groups in Children. Invest. Ophthalmol. Vis. Sci 46, 2317–2327. 10.1167/iovs.04-0945 [DOI] [PubMed] [Google Scholar]
- Jones-Jordan LA, Sinnott LT, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, Twelker JD, Zadnik Karla, 2012. Time Outdoors, Visual Activity, and Myopia Progression in Juvenile-Onset Myopes. Investig. Opthalmology Vis. Sci 53, 7169. 10.1167/iovs.11-8336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junfeng M, Shuangzhen L, Wenjuan Q, Fengyun L, Xiaoying W, Qian T, 2010. Levodopa Inhibits the Development of Form-Deprivation Myopia in Guinea Pigs: Optom. Vis. Sci 87, 53–60. 10.1097/OPX.0b013e3181c12b3d [DOI] [PubMed] [Google Scholar]
- Khanal S, Norton TT, Gawne T, 2020. 7-methylxanthine does not prevent induced myopia in tree shrews. Invest. Ophthalmol. Vis. Sci 61, 1136–1136. [Google Scholar]
- Kiefer AK, Tung JY, Do CB, Hinds DA, Mountain JL, Francke U, Eriksson N, 2013. Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia. PLOS Genet. 9, e1003299. 10.1371/journal.pgen.1003299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J, Beckers HJM, Tusscher T, Vrensen GFJM, van der Want JJL, Lamers WPMA, 1996. Sympathetic Innervation of the Rat Choroid: An Autoradiographic Tracing and Immunohistochemical Study. Ophthalmic Res. 28, 36–43. 10.1159/000267871 [DOI] [PubMed] [Google Scholar]
- Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP, 2006. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 20, 2242–2250. 10.1096/fj.06-6419com [DOI] [PubMed] [Google Scholar]
- Kramer SG, 1971. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest. Ophthalmol 10, 438–452. [PubMed] [Google Scholar]
- Kröger RHH, Hirt B, Wagner H-J, 1999. Effects of retinal dopamine depletion on the growth of the fish eye. J. Comp. Physiol. [A] 184, 403–412. 10.1007/s003590050339 [DOI] [PubMed] [Google Scholar]
- Krutmann J, Béhar-Cohen F, Baillet G, de Ayguavives T, Ortega Garcia P, Peña-García P, Remé C, Wolffsohn J, 2014. Towards standardization of UV eye protection: what can be learned from photodermatology? Photodermatol. Photoimmunol. Photomed 30, 128–136. 10.1111/phpp.12089 [DOI] [PubMed] [Google Scholar]
- Landis EG, Chrenek MA, Chakraborty R, Strickland R, Bergen M, Yang V, Iuvone PM, Pardue MT, 2020. Increased endogenous dopamine prevents myopia in mice. Exp. Eye Res 193, 107956. 10.1016/j.exer.2020.107956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis EG, Park HN, Chrenek M, He L, Sidhu C, Chakraborty R, Strickland R, Iuvone PM, Pardue MT, 2021. Ambient Light Regulates Retinal Dopamine Signaling and Myopia Susceptibility. Invest. Ophthalmol. Vis. Sci 62, 28–28. 10.1167/iovs.62.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis EG, Yang V, Brown DM, Pardue MT, Read SA, 2018. Dim Light Exposure and Myopia in Children. Investig. Opthalmology Vis. Sci 59, 4804. 10.1167/iovs.18-24415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, Smith ACW, Ramakrishnan A, Lyu Y, Bastle RM, Martin JA, Mitra S, O’Connor RM, Wang Z-J, Molina H, Turecki G, Shen L, Yan Z, Calipari ES, Dietz DM, Kenny PJ, Maze I, 2020. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science 368, 197–201. 10.1126/science.aaw8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Maurer D, 2005. Multiple sensitive periods in human visual development: Evidence from visually deprived children. Dev. Psychobiol 46, 163–183. 10.1002/dev.20055 [DOI] [PubMed] [Google Scholar]
- Li C, McFadden SA, Morgan I, Cui D, Hu J, Wan W, Zeng J, 2010. All-trans retinoic acid regulates the expression of the extracellular matrix protein fibulin-1 in the guinea pig sclera and human scleral fibroblasts. Mol. Vis 16, 689–697. [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O’Brien J, 2013. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J. Neurosci 33, 3135–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lan W, Yang S, Liao Y, Xu Q, Lin L, Yang Z, 2014. The Effect of Spectral Property and Intensity of Light on Natural Refractive Development and Compensation to Negative Lenses in Guinea Pigs. Investig. Opthalmology Vis. Sci 55, 6324. 10.1167/iovs.13-13802 [DOI] [PubMed] [Google Scholar]
- Li X-X, Schaeffel F, Kohler K, Zrenner E, 1992. Dose-dependent effects of 6-hydroxy dopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis. Neurosci 9, 483–492. 10.1017/S0952523800011287 [DOI] [PubMed] [Google Scholar]
- Liang H, Crewther SG, Crewther DP, Junghans BM, 2004. Structural and Elemental Evidence for Edema in the Retina, Retinal Pigment Epithelium, and Choroid during Recovery from Experimentally Induced Myopia. Investig. Opthalmology Vis. Sci 45, 2463. 10.1167/iovs.03-1009 [DOI] [PubMed] [Google Scholar]
- Lim LS, Yang X, Gazzard G, Lin X, Sng C, Saw S-M, Qiu A, 2011. Variations in Eye Volume, Surface Area, and Shape with Refractive Error in Young Children by Magnetic Resonance Imaging Analysis. Investig. Opthalmology Vis. Sci 52, 8878. 10.1167/iovs.11-7269 [DOI] [PubMed] [Google Scholar]
- Lingham G, Mackey DA, Lucas R, Yazar S, 2020. How does spending time outdoors protect against myopia? A review. Br. J. Ophthalmol 104, 593–599. 10.1136/bjophthalmol-2019-314675 [DOI] [PubMed] [Google Scholar]
- Liu H, Schaeffel F, Trier K, Feldkaemper M, 2020. Effects of 7-Methylxanthine on Deprivation Myopia and Retinal Dopamine Release in Chickens. Ophthalmic Res. 63, 347–357. 10.1159/000502529 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Xu Y, Pang Z, Mu G, 2021. The influence of the choroid on the onset and development of myopia: from perspectives of choroidal thickness and blood flow. Acta Ophthalmol. (Copenh.) 10.1111/aos.14773 [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M, 2012. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc. Natl. Acad. Sci. U. S. A 109, 6265–6270. 10.1073/pnas.1120997109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Liu S, 2017. Different roles of retinal dopamine in albino Guinea pig myopia. Neurosci. Lett 639, 94–97. 10.1016/j.neulet.2016.12.061 [DOI] [PubMed] [Google Scholar]
- Mao J, Liu S, Fu C, 2016. Citicoline retards myopia progression following form deprivation in guinea pigs. Exp. Biol. Med 241, 1258–1263. 10.1177/1535370216638773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Liu S, Qin W, Li F, Wu X, Tan Q, 2010. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom 87, 53–60. 10.1097/OPX.0b013e3181c12b3d [DOI] [PubMed] [Google Scholar]
- Mao J-F, Liu S-Z, Dou X-Q, 2012. Retinoic acid metabolic change in retina and choroid of the guinea pig with lens-induced myopia. Int. J. Ophthalmol 5, 670–674. 10.3980/j.issn.2222-3959.2012.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzani D, Wallman J, 1997. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest. Ophthalmol. Vis. Sci 38, 1726–1739. [PubMed] [Google Scholar]
- Mathis U, Feldkaemper M, Wang M, Schaeffel F, 2020. Studies on retinal mechanisms possibly related to myopia inhibition by atropine in the chicken. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 258, 319–333. 10.1007/s00417-019-04573-y [DOI] [PubMed] [Google Scholar]
- McBrien Neville A., Cornell LM, Gentle A, 2001. Structural and Ultrastructural Changes to the Sclera in a Mammalian Model of High Myopia. Invest. Ophthalmol. Vis. Sci 42, 2179–2187. [PubMed] [Google Scholar]
- McBrien NA, Cottriall CL, Annies R, 2001. Retinal acetylcholine content in normal and myopic eyes: a role in ocular growth control? Vis. Neurosci 18, 571–580. 10.1017/s0952523801184075 [DOI] [PubMed] [Google Scholar]
- McCaffery P, Mey J, Drager UC, 1996. Light-mediated retinoic acid production. Proc. Natl. Acad. Sci 93, 12570–12574. 10.1073/pnas.93.22.12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan IG, 2007. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp. Eye Res 84, 100–107. 10.1016/j.exer.2006.09.018 [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MHC, Mertz JR, 2004. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 44, 643–653. 10.1016/j.visres.2003.11.002 [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MHC, Mertz JR, Wallman J, 2006. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp. Eye Res 83, 949–961. 10.1016/j.exer.2006.05.002 [DOI] [PubMed] [Google Scholar]
- McFadden SA, Wildsoet C, 2020. The effect of optic nerve section on form deprivation myopia in the guinea pig. J. Comp. Neurol 528, 2874–2887. 10.1002/cne.24961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megaw P, Morgan I, Boelen M, 2001. Vitreal dihydroxyphenylacetic acid (DOPAC) as an index of retinal dopamine release: Vitreal DOPAC reflects dopamine release. J. Neurochem 76, 1636–1644. 10.1046/j.1471-4159.2001.00145.x [DOI] [PubMed] [Google Scholar]
- Mertz JR, Wallman J, 2000. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp. Eye Res 70, 519–527. 10.1006/exer.1999.0813 [DOI] [PubMed] [Google Scholar]
- Milam AH, Leeuw AMD, Gaur VP, Saari JC, 1990. Immunolocalization of cellular retinoic acid binding protein to müller cells and/or a subpopulation of GABA‐positive amacrine cells in retinas of different species. J. Comp. Neurol 296. 10.1002/cne.902960108 [DOI] [PubMed] [Google Scholar]
- Milam AH, Possin DE, Huang J, Fariss RN, Flannery JG, Saari JC, 1997. Characterization of aldehyde dehydrogenase-positive amacrine cells restricted in distribution to the dorsal retina. Vis. Neurosci 14, 601–608. 10.1017/s0952523800012256 [DOI] [PubMed] [Google Scholar]
- Miyake M, Yamashiro K, Tabara Y, Suda K, Morooka S, Nakanishi H, Khor C-C, Chen P, Qiao F, Nakata I, Akagi-Kurashige Y, Gotoh N, Tsujikawa A, Meguro A, Kusuhara S, Polasek O, Hayward C, Wright AF, Campbell H, Richardson AJ, Schache M, Takeuchi M, Mackey DA, Hewitt AW, Cuellar G, Shi Y, Huang L, Yang Z, Leung KH, Kao PYP, Yap MKH, Yip SP, Moriyama M, Ohno-Matsui K, Mizuki N, MacGregor S, Vitart V, Aung T, Saw S-M, Tai E-S, Wong TY, Cheng C-Y, Baird PN, Yamada R, Matsuda F, Yoshimura N, 2015. Identification of myopia-associated WNT7B polymorphisms provides insights into the mechanism underlying the development of myopia. Nat. Commun 6, 6689. 10.1038/ncomms7689 [DOI] [PubMed] [Google Scholar]
- Morgan IG, Ohno-Matsui K, Saw S-M, 2012. Myopia. The Lancet 379, 1739–1748. 10.1016/S0140-6736(12)60272-4 [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K, 2005. Axial Growth and Changes in Lenticular and Corneal Power during Emmetropization in Infants. Investig. Opthalmology Vis. Sci 46, 3074. 10.1167/iovs.04-1040 [DOI] [PubMed] [Google Scholar]
- Nickla DL, Jordan K, Yang J, Singh P, 2019. Effects of time-of-day on inhibition of lens-induced myopia by quinpirole, pirenzepine and atropine in chicks. Exp. Eye Res 181, 5–14. 10.1016/j.exer.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, 2011. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp. Eye Res 93, 782–785. 10.1016/j.exer.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, Dhillon B, 2010. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp. Eye Res 91, 715–720. 10.1016/j.exer.2010.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H-H, Huo L-J, Yang X, Gao Z-Y, Zeng J-W, Trier K, Cui D-M, 2012. Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int. J. Ophthalmol 5, 133–137. 10.3980/j.issn.2222-3959.2012.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, 2016. What Do Animal Studies Tell Us about the Mechanism of Myopia—Protection by Light?: Optom. Vis. Sci 93, 1049–1051. 10.1097/OPX.0000000000000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Essinger JA, McBrien NA, 1994. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis. Neurosci 11, 143–153. 10.1017/s0952523800011184 [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA, 1995. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res., Myopia 35, 1271–1281. 10.1016/0042-6989(94)00243-F [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, 2013. Light levels, refractive development, and myopia – A speculative review. Exp. Eye Res 114, 48–57. 10.1016/j.exer.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, 1995. Animal models of emmetropization: matching axial length to the focal plane. J. Am. Optom. Assoc 66, 405–414. [PubMed] [Google Scholar]
- Ohngemach S, Hagel G, Schaeffel F, 1997. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis. Neurosci 14, 493–505. 10.1017/S0952523800012153 [DOI] [PubMed] [Google Scholar]
- Pan M, Guan Z, Reinach PS, Kang L, Cao Y, Zhou D, Srinivasalu N, Zhao F, Qu J, Zhou X, 2021. PPARγ modulates refractive development and form deprivation myopia in Guinea pigs. Exp. Eye Res 202, 108332. 10.1016/j.exer.2020.108332 [DOI] [PubMed] [Google Scholar]
- Pan M, Jiao S, Reinach PS, Yan J, Yang Y, Li Q, Srinivasalu N, Qu J, Zhou X, 2018. Opposing Effects of PPARα Agonism and Antagonism on Refractive Development and Form Deprivation Myopia in Guinea Pigs. Invest. Ophthalmol. Vis. Sci 59, 5803–5815. 10.1167/iovs.17-22297 [DOI] [PubMed] [Google Scholar]
- Pandey S, Blanks JC, Spee C, Jiang M, Fong HKW, 1994. Cytoplasmic Retinal Localization of an Evolutionary Homolog of the Visual Pigments. Exp. Eye Res 58, 605–613. 10.1006/exer.1994.1055 [DOI] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM, 2008. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest. Ophthalmol. Vis. Sci 49, 706–712. 10.1167/iovs.07-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Stone RA, Iuvone PM, 2013. Investigating mechanisms of myopia in mice. Exp. Eye Res, Josh Wallman Special Tribute Edition 114, 96–105. 10.1016/j.exer.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. na, Jabbar SB, Tan CC, Sidhu CS, Abey J, Aseem F, Schmid G, Iuvone PM, Pardue MT, 2014. Visually-Driven Ocular Growth in Mice Requires Functional Rod Photoreceptors. Investig. Opthalmology Vis. Sci 55, 6272. 10.1167/iovs.14-14648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul J-Y, Takano H, Moss SJ, McCarthy K, Haydon PG, 2005. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. 10.1126/science.1116916 [DOI] [PubMed] [Google Scholar]
- Pottek M, Weiler R, 2000. Light-adaptive effects of retinoic acid on receptive field properties of retinal horizontal cells: Light-adaptive effects of retinoic acid. Eur. J. Neurosci 12, 437–445. 10.1046/j.1460-9568.2000.00918.x [DOI] [PubMed] [Google Scholar]
- Prigge CL, Yeh P-T, Liou N-F, Lee C-C, You S-F, Liu L-L, McNeill DS, Chew KS, Hattar S, Chen S-K, Zhang D-Q, 2016. M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina. J. Neurosci 36, 7184–7197. 10.1523/JNEUROSCI.3500-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proll MA, Kamp CW, Morgan WW, 1982. Use of liquid chromatography with electrochemistry to measure effects of varying intensities of white light on DOPA accumulation in rat retinas. Life Sci. 30, 11–19. 10.1016/0024-3205(82)90630-0 [DOI] [PubMed] [Google Scholar]
- Puopolo M, Hochstetler SE, Gustincich S, Wightman RM, Raviola E, 2001. Extrasynaptic Release of Dopamine in a Retinal Neuron: Activity Dependence and Transmitter Modulation. Neuron 30, 211–225. 10.1016/S0896-6273(01)00274-4 [DOI] [PubMed] [Google Scholar]
- Qiao S-N, Zhang Z, Ribelayga CP, Zhong Y-M, Zhang D-Q, 2016. Multiple cone pathways are involved in photic regulation of retinal dopamine. Sci. Rep 6, 28916. 10.1038/srep28916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JAS, Hollaway LR, Lam W, Li N, Napoli JL, 2012. Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest. Ophthalmol. Vis. Sci 53, 1649–1662. 10.1167/iovs.11-8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramessur R, Williams KM, Hammond CJ, 2015. Risk factors for myopia in a discordant monozygotic twin study. Ophthalmic Physiol. Opt 35, 643–651. 10.1111/opo.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SA, Collins MJ, Vincent SJ, 2014. Light Exposure and Physical Activity in Myopic and Emmetropic Children: Optom. Vis. Sci 1. 10.1097/OPX.0000000000000160 [DOI] [PubMed] [Google Scholar]
- Reitsamer HA, Zawinka C, Branka M, 2004. Dopaminergic vasodilation in the choroidal circulation by d1/d5 receptor activation. Invest. Ophthalmol. Vis. Sci 45, 900–905. 10.1167/iovs.03-0997 [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2005. A Circadian Clock and Light/Dark Adaptation Differentially Regulate Adenosine in the Mammalian Retina. J. Neurosci 25, 215–222. 10.1523/JNEUROSCI.3138-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK, 1993. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2 -receptor mechanism acting in retina or pigmented epithelium. Vis. Neurosci 10, 447–453. 10.1017/S0952523800004673 [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P, 2008. Outdoor Activity Reduces the Prevalence of Myopia in Children. Ophthalmology 115, 1279–1285. 10.1016/j.ophtha.2007.12.019 [DOI] [PubMed] [Google Scholar]
- Roy S, Field GD, 2019. Dopaminergic modulation of retinal processing from starlight to sunlight. J. Pharmacol. Sci 140, 86–93. 10.1016/j.jphs.2019.03.006 [DOI] [PubMed] [Google Scholar]
- Rucker F, 2019. Monochromatic and white light and the regulation of eye growth. Exp. Eye Res 184, 172–182. 10.1016/j.exer.2019.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer J, Wildsoet CF, 2005. The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis. Neurosci 22, 251–261. 10.1017/S0952523805223015 [DOI] [PubMed] [Google Scholar]
- Saari JC, Champer RJ, Asson-Batres MA, Garwin GG, Huang J, Crabb JW, Milam AH, 1995. Characterization and localization of an aldehyde dehydrogenase to amacrine cells of bovine retina. Vis. Neurosci 12, 263–272. 10.1017/s095252380000794x [DOI] [PubMed] [Google Scholar]
- Sato T, Yoneyama T, Kim HK, Suzuki TA, 1987. Effect of dopamine and haloperidol on the c-wave and light peak of light-induced retinal responses in chick eye. Doc. Ophthalmol. Adv. Ophthalmol 65, 87–95. 10.1007/BF00162724 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E, 1995. Studies on the role of the retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 35, 1247–1264. 10.1016/0042-6989(94)00221-7 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Feldkaemper M, 2015. Animal models in myopia research. Clin. Exp. Optom 98, 507–517. 10.1111/cxo.12312 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E, 1994. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 34, 143–149. 10.1016/0042-6989(94)90327-1 [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF, 2004. Inhibitory Effects of Apomorphine and Atropine and Their Combination on Myopia in Chicks: Optom. Vis. Sci 81, 137–147. 10.1097/00006324-200402000-00012 [DOI] [PubMed] [Google Scholar]
- Seko Y, Shimizu M, Tokoro T, 1998. Retinoic Acid Increases in the Retina of the Chick with Form Deprivation Myopia. Ophthalmic Res. 30, 361–367. 10.1159/000055496 [DOI] [PubMed] [Google Scholar]
- Seko Y, Shimokawa H, Pang J, Tokoro T, 2000. Disturbance of electrolyte balance in vitreous of chicks with form-deprivation myopia. Jpn. J. Ophthalmol 44, 15–19. 10.1016/s0021-5155(99)00177-x [DOI] [PubMed] [Google Scholar]
- Seko Y, Shimokawa H, Tokoro T, 1996. In vivo and in vitro association of retinoic acid with form-deprivation myopia in the chick. Exp. Eye Res 63, 443–452. 10.1006/exer.1996.0134 [DOI] [PubMed] [Google Scholar]
- Shen L, You QS, Xu X, Gao F, Zhang Z, Li B, Jonas JB, 2016. Scleral and choroidal volume in relation to axial length in infants with retinoblastoma versus adults with malignant melanomas or end-stage glaucoma. Graefes Arch. Clin. Exp. Ophthalmol 254, 1779–1786. 10.1007/s00417-016-3345-7 [DOI] [PubMed] [Google Scholar]
- Shen W, Vijayan M, Sivak JG, 2005. Inducing Form-Deprivation Myopia in Fish. Investig. Opthalmology Vis. Sci 46, 1797. 10.1167/iovs.04-1318 [DOI] [PubMed] [Google Scholar]
- Shi D, Daly JW, 1999. Chronic effects of xanthines on levels of central receptors in mice. Cell. Mol. Neurobiol 19, 719–732. 10.1023/a:1006901005925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Huang J, Hung L-F, Blasdel TL, Humbird TL, Bockhorst KH, 2009. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest. Ophthalmol. Vis. Sci 50, 5057–5069. 10.1167/iovs.08-3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, 2000. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 40, 371–381. 10.1016/S0042-6989(99)00184-4 [DOI] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, Arumugam B, 2014. Visual regulation of refractive development: insights from animal studies. Eye Lond. Engl 28, 180–188. 10.1038/eye.2013.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J, 2015. Effects of Long-Wavelength Lighting on Refractive Development in Infant Rhesus Monkeys. Invest. Ophthalmol. Vis. Sci 56, 6490–6500. 10.1167/iovs.15-17025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, Arumugam B, Huang J, 2013. Negative Lens–Induced Myopia in Infant Monkeys: Effects of High Ambient Lighting. Investig. Opthalmology Vis. Sci 54, 2959. 10.1167/iovs.13-11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, Huang J, 2012. Protective Effects of High Ambient Lighting on the Development of Form-Deprivation Myopia in Rhesus Monkeys. Investig. Opthalmology Vis. Sci 53, 421. 10.1167/iovs.11-8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, Huang J, Blasdel TL, Humbird TL, Bockhorst KH, 2010. Effects of Optical Defocus on Refractive Development in Monkeys: Evidence for Local, Regionally Selective Mechanisms. Invest. Ophthalmol. Vis. Sci 51, 3864–3873. 10.1167/iovs.09-4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung L-F, She Z, Beach K, Ostrin LA, Jong M, 2021. Topically instilled caffeine selectively alters emmetropizing responses in infant rhesus monkeys. Exp. Eye Res 203, 108438. 10.1016/j.exer.2021.108438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Walline JJ, 2015. Controlling myopia progression in children and adolescents. Adolesc. Health Med. Ther 6, 133–140. 10.2147/AHMT.S55834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solouki AM, Verhoeven VJM, van Duijn CM, Verkerk AJMH, Ikram MK, Hysi PG, Despriet DDG, van Koolwijk LM, Ho L, Ramdas WD, Czudowska M, Kuijpers RWAM, Amin N, Struchalin M, Aulchenko YS, van Rij G, Riemslag FCC, Young TL, Mackey DA, Spector TD, Gorgels TGMF, Willemse-Assink JJM, Isaacs A, Kramer R, Swagemakers SMA, Bergen AAB, van Oosterhout AALJ, Oostra BA, Rivadeneira F, Uitterlinden AG, Hofman A, de Jong PTVM, Hammond CJ, Vingerling JR, Klaver CCW, 2010. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat. Genet 42, 897–901. 10.1038/ng.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinozzi E, Baldassarri C, Acquaticci L, Del Bello F, Grifantini M, Cappellacci L, Riccardo P, 2021. Adenosine receptors as promising targets for the management of ocular diseases. Med. Chem. Res 30, 353–370. 10.1007/s00044-021-02704-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Cohen Y, McGlinn AM, Davison S, Casavant S, Shaffer J, Khurana TS, Pardue MT, Iuvone PM, 2016. Development of Experimental Myopia in Chicks in a Natural Environment. Investig. Opthalmology Vis. Sci 57, 4779. 10.1167/iovs.16-19310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM, 1989. Retinal dopamine and form-deprivation myopia. Proc. Natl. Acad. Sci 86, 704–706. 10.1073/pnas.86.2.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Pendrak K, Sugimoto R, Lin T, Gill AS, Capehart C, Liu J, 2006. Local Patterns of Image Degradation Differentially Affect Refraction and Eye Shape in Chick. Curr. Eye Res 31, 91–105. 10.1080/02713680500479517 [DOI] [PubMed] [Google Scholar]
- Strauss O, 2005. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev 85, 845–881. 10.1152/physrev.00021.2004 [DOI] [PubMed] [Google Scholar]
- Strickland R, Landis EG, Pardue MT, 2020. Short-Wavelength (Violet) Light Protects Mice From Myopia Through Cone Signaling. Investig. Opthalmology Vis. Sci 61, 13. 10.1167/iovs.61.2.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JA, 2019. Retinoic Acid in Ocular Growth Regulation, Vitamin A. IntechOpen. 10.5772/intechopen.84586 [DOI] [Google Scholar]
- Summers JA, Cano EM, Kaser-Eichberger A, Schroedl F, 2020. Retinoic acid synthesis by a population of choroidal stromal cells. Exp. Eye Res 201, 108252. 10.1016/j.exer.2020.108252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JA, Harper AR, Feasley CL, Van-Der-Wel H, Byrum JN, Hermann M, West CM, 2016. Identification of Apolipoprotein A-I as a Retinoic Acid-binding Protein in the Eye. J. Biol. Chem 291, 18991–19005. 10.1074/jbc.M116.725523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers JA, Schaeffel F, Marcos S, Wu H, Tkatchenko AV, 2021. Functional integration of eye tissues and refractive eye development: Mechanisms and pathways. Exp. Eye Res 209, 108693. 10.1016/j.exer.2021.108693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhao N, Liu W, Liu M, Ju Z, Li J, Cheng Z, Liu X, 2018. Study of Vesicular Monoamine Transporter 2 in Myopic Retina Using [18F]FP-(+)-DTBZ. Mol. Imaging Biol 20, 771–779. 10.1007/s11307-018-1183-1 [DOI] [PubMed] [Google Scholar]
- Swiatczak B, Schaeffel F, 2021. Emmetropic, But Not Myopic Human Eyes Distinguish Positive Defocus From Calculated Blur. Investig. Opthalmology Vis. Sci 62, 14. 10.1167/iovs.62.3.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Pan M, Liu S, Fang F, Lu R, Lu C, Zheng M, An J, Xu H, Zhao F, Chen J, Qu J, Zhou X, 2013. cAMP Level Modulates Scleral Collagen Remodeling, a Critical Step in the Development of Myopia. PLOS ONE 8, e71441. 10.1371/journal.pone.0071441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedja MS, Haarman AEG, Meester-Smoor MA, Kaprio J, Mackey DA, Guggenheim JA, Hammond CJ, Verhoeven VJM, Klaver CCW, for the CREAM Consortium, 2019. IMI – Myopia Genetics Report. Investig. Opthalmology Vis. Sci 60, M89. 10.1167/iovs.18-25965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedja MS, Wojciechowski R, Hysi PG, Eriksson N, Furlotte NA, Verhoeven VJM, Iglesias AI, Meester-Smoor MA, Tompson SW, Fan Q, Khawaja AP, Cheng C-Y, Höhn R, Yamashiro K, Wenocur A, Grazal C, Haller T, Metspalu A, Wedenoja J, Jonas JB, Wang YX, Xie J, Mitchell P, Foster PJ, Klein BEK, Klein R, Paterson AD, Hosseini SM, Shah RL, Williams C, Teo YY, Tham YC, Gupta P, Zhao W, Shi Y, Saw W-Y, Tai E-S, Sim XL, Huffman JE, Polašek O, Hayward C, Bencic G, Rudan I, Wilson JF, Joshi PK, Tsujikawa A, Matsuda F, Whisenhunt KN, Zeller T, van der Spek PJ, Haak R, Meijers-Heijboer H, van Leeuwen EM, Iyengar SK, Lass JH, Hofman A, Rivadeneira F, Uitterlinden AG, Vingerling JR, Lehtimäki T, Raitakari OT, Biino G, Concas MP, Schwantes-An T-H, Igo RP, Cuellar-Partida G, Martin NG, Craig JE, Gharahkhani P, Williams KM, Nag A, Rahi JS, Cumberland PM, Delcourt C, Bellenguez C, Ried JS, Bergen AA, Meitinger T, Gieger C, Wong TY, Hewitt AW, Mackey DA, Simpson CL, Pfeiffer N, Pärssinen O, Baird PN, Vitart V, Amin N, van Duijn CM, Bailey-Wilson JE, Young TL, Saw S-M, Stambolian D, MacGregor S, Guggenheim JA, Tung JY, Hammond CJ, Klaver CCW, 2018. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat. Genet 50, 834–848. 10.1038/s41588-018-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson K, Karouta C, Ashby R, 2020a. Form-Deprivation and Lens-Induced Myopia Are Similarly Affected by Pharmacological Manipulation of the Dopaminergic System in Chicks. Invest. Ophthalmol. Vis. Sci 61, 4. 10.1167/iovs.61.12.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson K, Karouta C, Ashby R, 2020b. Topical application of dopaminergic compounds can inhibit deprivation myopia in chicks. Exp. Eye Res 200, 108233. 10.1016/j.exer.2020.108233 [DOI] [PubMed] [Google Scholar]
- Thomson K, Morgan I, Karouta C, Ashby R, 2020c. Levodopa inhibits the development of lens-induced myopia in chicks. Sci. Rep 10, 13242. 10.1038/s41598-020-70271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson K, Morgan I, Kelly T, Karouta C, Ashby R, 2021. Coadministration With Carbidopa Enhances the Antimyopic Effects of Levodopa in Chickens. Invest. Ophthalmol. Vis. Sci 62, 25–25. 10.1167/iovs.62.4.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne HC, Jones KH, Peters SP, Archer SN, Dijk D-J, 2009. Daily and Seasonal Variation in the Spectral Composition of Light Exposure in Humans. Chronobiol. Int 26, 854–866. 10.1080/07420520903044315 [DOI] [PubMed] [Google Scholar]
- Tigges M, Iuvone PM, Tigges J, Fernandes A, Gammon JA, 1987. Presented at the Soc. Neurosci. Abstr, p. 1535.
- Tkatchenko TV, Troilo D, Benavente-Perez A, Tkatchenko AV, 2018. Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanism of visually guided eye growth. PLOS Biol. 16, e2006021. 10.1371/journal.pbio.2006021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, Kawashima M, Jiang X, Kondo S, Miyauchi M, Miwa Y, Katada Y, Mori K, Kato K, Tsubota Kinya, Goto H, Oda M, Hatori M, Tsubota Kazuo, 2017. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. EBioMedicine 15, 210–219. 10.1016/j.ebiom.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N, Chiu S, Tian Y, Wildsoet CF, 2008. The significance of retinal image contrast and spatial frequency composition for eye growth modulation in young chicks. Vision Res. 48, 1655–1662. 10.1016/j.visres.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier K, Munk Ribel-Madsen S, Cui D, Brøgger Christensen S, 2008. Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J. Ocul. Biol. Dis. Infor 1, 85–93. 10.1007/s12177-008-9013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier K, Olsen EB, Kobayashi T, Ribel-Madsen SM, 1999. Biochemical and ultrastructural changes in rabbit sclera after treatment with. Br. J. Ophthalmol 83, 1370–1375. 10.1136/bjo.83.12.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, Wallman J, 1987. Visual deprivation causes myopia in chicks with optic nerve section. Curr. Eye Res 6, 993–999. 10.3109/02713688709034870 [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, 2005. The Response to Visual Form Deprivation Differs with Age in Marmosets. Investig. Opthalmology Vis. Sci 46, 1873. 10.1167/iovs.04-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Mertz JR, Rada JAS, 2006. Change in the Synthesis Rates of Ocular Retinoic Acid and Scleral Glycosaminoglycan during Experimentally Altered Eye Growth in Marmosets. Invest. Ophthalmol. Vis. Sci 47, 1768–1777. 10.1167/iovs.05-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Smith EL, Nickla DL, Ashby R, Tkatchenko AV, Ostrin LA, Gawne TJ, Pardue MT, Summers JA, Kee C, Schroedl F, Wahl S, Jones L, 2019. IMI – Report on Experimental Models of Emmetropization and Myopia. Invest. Ophthalmol. Vis. Sci 60, M31–M88. 10.1167/iovs.18-25967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Wallman J, 1991. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 31, 1237–1250. 10.1016/0042-6989(91)90048-a [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Beuerman RW, 2020. Biological Mechanisms of Atropine Control of Myopia. Eye Contact Lens 46, 129–135. 10.1097/ICL.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven VJM, Hysi PG, Wojciechowski R, Fan Q, Guggenheim JA, Höhn R, MacGregor S, Hewitt AW, Nag A, Cheng C-Y, Yonova-Doing E, Zhou X, Ikram MK, Buitendijk GHS, McMahon G, Kemp JP, Pourcain BS, Simpson CL, Mäkelä K-M, Lehtimäki T, Kähönen M, Paterson AD, Hosseini SM, Wong HS, Xu L, Jonas JB, Pärssinen O, Wedenoja J, Yip SP, Ho DWH, Pang CP, Chen LJ, Burdon KP, Craig JE, Klein BEK, Klein R, Haller T, Metspalu A, Khor C-C, Tai E-S, Aung T, Vithana E, Tay W-T, Barathi VA, Consortium for Refractive Error and Myopia (CREAM), Chen P, Li R, Liao J, Zheng Y, Ong RT, Döring A, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group, Evans DM, Timpson NJ, Verkerk AJMH, Meitinger T, Raitakari O, Hawthorne F, Spector TD, Karssen LC, Pirastu M, Murgia F, Ang W, Wellcome Trust Case Control Consortium 2 (WTCCC2), Mishra A, Montgomery GW, Pennell CE, Cumberland PM, Cotlarciuc I, Mitchell P, Wang JJ, Schache M, Janmahasatian S, Janmahasathian S, Igo RP, Lass JH, Chew E, Iyengar SK, Fuchs’ Genetics Multi-Center Study Group, Gorgels TGMF, Rudan I, Hayward C, Wright AF, Polasek O, Vatavuk Z, Wilson JF, Fleck B, Zeller T, Mirshahi A, Müller C, Uitterlinden AG, Rivadeneira F, Vingerling JR, Hofman A, Oostra BA, Amin N, Bergen AAB, Teo Y-Y, Rahi JS, Vitart V, Williams C, Baird PN, Wong T-Y, Oexle K, Pfeiffer N, Mackey DA, Young TL, van Duijn CM, Saw S-M, Bailey-Wilson JE, Stambolian D, Klaver CC, Hammond CJ, 2013. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat. Genet 45, 314–318. 10.1038/ng.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishweswaraiah S, Swierkowska J, Ratnamala U, Mishra NK, Guda C, Chettiar SS, Johar KR, Mrugacz M, Karolak JA, Gajecka M, Radhakrishna U, 2019. Epigenetically dysregulated genes and pathways implicated in the pathogenesis of non-syndromic high myopia. Sci. Rep 9, 4145. 10.1038/s41598-019-40299-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S, 2009. Increased Prevalence of Myopia in the United States Between 1971–1972 and 1999–2004. Arch. Ophthalmol 127, 1632. 10.1001/archophthalmol.2009.303 [DOI] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Mey J, Farhangfar F, Applebury ML, Dräger UC, 1997. Retinoic aid increases arrestin mRNA levels in the mouse retina. FASEB J. 11, 271–275. 10.1096/fasebj.11.4.9068616 [DOI] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA, 1987. Local retinal regions control local eye growth and myopia. Science 237, 73–77. 10.1126/science.3603011 [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J, 1978. Extreme myopia produced by modest change in early visual experience. Science 201, 1249–1251. 10.1126/science.694514 [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM, 1995. Moving the retina: Choroidal modulation of refractive state. Vision Res. 35, 37–50. 10.1016/0042-6989(94)E0049-Q [DOI] [PubMed] [Google Scholar]
- Wan W, Cui D, Trier K, Zeng J, 2017. Effect of 7-methylxanthine on human retinal pigment epithelium cells cultured in vitro. Mol. Vis 23, 1006–1014. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Nava D, Trier K, Wildsoet C, 2014. Influence of Oral 7-Methylxanthine on Lens-induced and Form Deprivation Myopia in Chickens. Invest. Ophthalmol. Vis. Sci 55, 3040–3040. [Google Scholar]
- Wang M, Aleman AC, Schaeffel F, 2019. Probing the Potency of Artificial Dynamic ON or OFF Stimuli to Inhibit Myopia Development. Investig. Opthalmology Vis. Sci 60, 2599. 10.1167/iovs.18-26471 [DOI] [PubMed] [Google Scholar]
- Wang M, Schaeffel F, Jiang B, Feldkaemper M, 2018. Effects of Light of Different Spectral Composition on Refractive Development and Retinal Dopamine in Chicks. Investig. Opthalmology Vis. Sci 59, 4413. 10.1167/iovs.18-23880 [DOI] [PubMed] [Google Scholar]
- Wang S, Liu S, Mao J, Wen D, 2014. Effect of retinoic acid on the tight junctions of the retinal pigment epithelium-choroid complex of guinea pigs with lens-induced myopia in vivo. Int. J. Mol. Med 33, 825–832. 10.3892/ijmm.2014.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AH, Norton TT, Huisingh CE, Gawne TJ, 2018. The hyperopic effect of narrow-band long-wavelength light in tree shrews increases non-linearly with duration. Vision Res. 146–147, 9–17. 10.1016/j.visres.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT, Frost MR, Norton TT, 2017. Intravitreally-administered dopamine D2-like (and D4), but not D1-like, receptor agonists reduce form-deprivation myopia in tree shrews. Vis. Neurosci 34, E003. 10.1017/S0952523816000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Yamashita T, Ohba N, 1999. A longitudinal study of cycloplegic refraction in a cohort of 350 Japanese schoolchildren. Cycloplegic refraction. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom 19, 22–29. 10.1046/j.1475-1313.1999.00406.x [DOI] [PubMed] [Google Scholar]
- Weiler R, He S, Vaney DI, 1999. Retinoic Acid Modulates Gap Junctional Permeability Between Horizontal Cells Of The Mammalian Retina: Retinoic acid and gap junction coupling. Eur. J. Neurosci 11, 3346–3350. 10.1046/j.1460-9568.1999.00799.x [DOI] [PubMed] [Google Scholar]
- Weiler R, Pottek M, He S, Vaney DI, 2000. Modulation of coupling between retinal horizontal cells by retinoic acid and endogenous dopamine. Brain Res. Rev 32, 121–129. 10.1016/S0165-0173(99)00071-5 [DOI] [PubMed] [Google Scholar]
- Weiler R, Schultz K, Pottek M, Tieding S, Janssen-Bienhold U, 1998. Retinoic acid has light-adaptive effects on horizontal cells in the retina. Proc. Natl. Acad. Sci 95, 7139–7144. 10.1073/pnas.95.12.7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsoet C, 2003. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr. Eye Res 27, 371–385. 10.1076/ceyr.27.6.371.18188 [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Pettigrew J, 1988. Experimental myopia and anamalous eye growth patterns unaffected by optic nerve section in chickens: Evidence for local control of eye growth. Clin. Vis. Sci 3, 99–107. [Google Scholar]
- Wildsoet C, Wallman J, 1995. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 35, 1175–1194. 10.1016/0042-6989(94)00233-c [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, Chia A, Cho P, Guggenheim JA, Polling JR, Read S, Sankaridurg P, Saw S-M, Trier K, Walline JJ, Wu P-C, Wolffsohn JS, 2019. IMI – Interventions for Controlling Myopia Onset and Progression Report. Investig. Opthalmology Vis. Sci 60, M106. 10.1167/iovs.18-25958 [DOI] [PubMed] [Google Scholar]
- Wioland N, Rudolf G, Bonaventure N, 1990. Electrooculographic and electroretinographic study in the chicken after dopamine and haloperidol. Doc. Ophthalmol 75, 175–180. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, 2004. Dopamine and retinal function. Doc. Ophthalmol 108, 17–39. 10.1023/B:DOOP.0000019487.88486.0a [DOI] [PubMed] [Google Scholar]
- Wu H, Chen W, Zhao F, Zhou Q, Reinach PS, Deng L, Ma L, Luo S, Srinivasalu N, Pan M, Hu Y, Pei X, Sun J, Ren R, Xiong Y, Zhou Z, Zhang S, Tian G, Fang J, Zhang L, Lang J, Wu D, Zeng C, Qu J, Zhou X, 2018. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci 115, E7091–E7100. 10.1073/pnas.1721443115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P-C, Tsai C-L, Wu H-L, Yang Y-H, Kuo H-K, 2013. Outdoor Activity during Class Recess Reduces Myopia Onset and Progression in School Children. Ophthalmology 120, 1080–1085. 10.1016/j.ophtha.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Wu X-H, Li Y-Y, Zhang P-P, Qian K-W, Ding J-H, Hu G, Weng S-J, Yang X-L, Zhong Y-M, 2015. Unaltered Retinal Dopamine Levels in a C57BL/6 Mouse Model of Form-Deprivation Myopia. Invest. Ophthalmol. Vis. Sci 56, 967–977. 10.1167/iovs.13-13362 [DOI] [PubMed] [Google Scholar]
- Wu X-H, Qian K-W, Xu G-Z, Li Y-Y, Ma Y-Y, Huang F, Wang Y-Q, Zhou X, Qu J, Yang X-L, Zhong Y-M, Weng S-J, 2016. The Role of Retinal Dopamine in C57BL/6 Mouse Refractive Development as Revealed by Intravitreal Administration of 6-Hydroxydopamine. Investig. Opthalmology Vis. Sci 57, 5393. 10.1167/iovs.16-19543 [DOI] [PubMed] [Google Scholar]
- Yan T, Xiong W, Huang F, Zheng F, Ying H, Chen J-F, Qu J, Zhou X, 2015. Daily Injection But Not Continuous Infusion of Apomorphine Inhibits Form-Deprivation Myopia in Mice. Invest. Ophthalmol. Vis. Sci 56, 2475–2485. 10.1167/iovs.13-12361 [DOI] [PubMed] [Google Scholar]
- Yu M, Liu W, Wang B, Dai J, 2021. Short Wavelength (Blue) Light Is Protective for Lens-Induced Myopia in Guinea Pigs Potentially Through a Retinoic Acid–Related Mechanism. Investig. Opthalmology Vis. Sci 62, 21. 10.1167/iovs.62.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D-Q, Belenky MA, Sollars PJ, Pickard GE, McMahon DG, 2012. Melanopsin Mediates Retrograde Visual Signaling in the Retina. PLOS ONE 7, e42647. 10.1371/journal.pone.0042647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D-Q, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG, 2008. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc. Natl. Acad. Sci 105, 14181–14186. 10.1073/pnas.0803893105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wong CL, Shan SW, Li KK, Cheng AK, Lee KL, Ge J, To CH, Do CW, 2011. Characterisation of Cl− transporter and channels in experimentally induced myopic chick eyes. Clin. Exp. Optom 94, 528–535. 10.1111/j.1444-0938.2011.00611.x [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang J, Reinach PS, Wang F, Zhang L, Fan M, Ying H, Pan M, Qu J, Zhou X, 2018. Dopamine Receptor Subtypes Mediate Opposing Effects on Form Deprivation Myopia in Pigmented Guinea Pigs. Investig. Opthalmology Vis. Sci 59, 4441. 10.1167/iovs.17-21574 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wildsoet CF, 2015. RPE and Choroid Mechanisms Underlying Ocular Growth and Myopia. Prog. Mol. Biol. Transl. Sci 134, 221–240. 10.1016/bs.pmbts.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Fei, Zhang D, Zhou Q, Zhao Fuxin, He M, Yang Z, Su Y, Zhai Y, Yan J, Zhang G, Xue A, Tang J, Han X, Shi Y, Zhu Y, Liu T, Zhuang W, Huang L, Hong Y, Wu D, Li Y, Lu Q, Chen W, Jiao S, Wang Q, Srinivasalu N, Wen Y, Zeng C, Qu J, Zhou X, 2020. Scleral HIF-1α is a prominent regulatory candidate for genetic and environmental interactions in human myopia pathogenesis. EBioMedicine 57, 102878. 10.1016/j.ebiom.2020.102878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wong KY, Zhang D-Q, 2017. Mapping physiological inputs from multiple photoreceptor systems to dopaminergic amacrine cells in the mouse retina. Sci. Rep 7, 7920. 10.1038/s41598-017-08172-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Huang Q, An J, Lu R, Qin X, Jiang L, Li Y, Wang J, Chen J, Qu J, 2010. Genetic Deletion of the Adenosine A2A Receptor Confers Postnatal Development of Relative Myopia in Mice. Invest. Ophthalmol. Vis. Sci 51, 4362–4370. 10.1167/iovs.09-3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Pardue MT, Iuvone PM, Qu J, 2017. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res 61, 60–71. 10.1016/j.preteyeres.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Xuan, Zhang S, Yang F, Yang Y, Huang Q, Huang C, Qu J, Zhou Xiangtian, 2021. Decreased Choroidal Blood Perfusion Induces Myopia in Guinea Pigs. Invest. Ophthalmol. Vis. Sci 62, 30. 10.1167/iovs.62.15.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


