Abstract
The paper aimed to compare how factors previously identified as predictive factors for cognitive decline and dementia related to cognitive performance on the one hand and brain health on the other. To that aim, multiple linear regression was applied to the AGES-Reykjavik study epidemiological data. Additionally, a regression analysis was performed for change in cognition over 5 years, using the same exposure factors. The study ran from 2002 to 2011, and the sample analyzed included 1707 participants between the ages of 66 and 90. The data contains MR imaging, cognitive testing, background data, and physiological measurements. Overall, we conclude that risk factors linked to dementia relate differently to cognition and brain health. Mobility, physical strength, alcohol consumption, coronary artery disease, and hypertension were associated with cognition and brain volume. Smoking, depression, diabetes, and body fat percentage were only associated with brain volume, not cognitive performance. Modifiable factors previously linked to cognitive reserve, such as educational attainment, participation in leisure activities, multilingualism and good self-reported health, were associated with cognitive function but did not relate to brain volume. These findings show that, within the same participant pool, cognitive reserve proxy variables have a relationship with cognitive performance but have no association with relative brain volume measured simultaneously.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00642-z.
Keywords: Cognitive aging, Brain health, Brain pathology, Cognitive performance, Cognitive reserve, AGES-Reykjavik study
An essential aspect of healthy and successful aging is the trajectory of cognitive aging [1, 2]. Cognitive aging affects individuals’ quality of life and well-being, and retaining good cognition as you age is considered very important by many [3, 4]. Cognitive decline can substantially affect the daily functioning of those who experience it, and these individuals become very dependent on the care of others [5].
Numerous factors affect the rate of cognitive aging, including genetics, physical health, and lifestyle choices, and it has been suggested that modifiable risk factors contribute to up to 50% of dementia risk [6–8]. Among the risk and protective factors that have been identified are alcohol consumption [9], smoking [9], depression [9], physical activity [10, 11], body composition [12], sleep [13], hypertension [14], diabetes [15], atrial fibrillation [16], coronary artery disease [17], level of education [18], participation in leisure activities [19, 20], multilingualism [21], self-reported health [22], healthy diet [19], and mobility and strength [23, 24].
Cognition and brain pathology
Much of the research into cognitive aging and dementia has been focused on brain pathology, which is understandable since, in theory, it could lead researchers to the root of what causes dementia and closer to a cure [25]. There is no doubt that there is a relationship between cognitive performance and parameters of brain pathology, such as brain volume, brain infarcts, and white matter hyperintensities [26–28]. Unfortunately, all of this work, identifying the physiological underpinnings of dementia, has not led us much closer to a cure [29].
When cognition and brain pathology has been studied in parallel, for example, in Alzheimer’s disease studies, results have shown that having extensive pathology does not automatically translate into the individual experiencing cognitive impairment [30]. This suggests that brain pathology is not the only deciding factor when it comes to the symptoms of dementia. Other avenues should therefore be considered. Even though this is the case, dementia research is increasingly focused on biomarkers and less on cognitive abilities [31–33].
Cognitive reserve
A considerable amount of literature is available that attempts to explain why some people who, by a physiological definition, would be considered to have a dementing disease are more resistant to the cognitive symptoms of dementia than others [34–36]. The problem with the literature on the topic is that many terms have been used to describe the phenomenon, and some use the same terms while defining them in different ways. This is the case even though many attempts have been made to reach a consensus [34–36]. In an ambitious white paper, Stern et al. [36] suggested that the term cognitive reserve should refer to the adaptableness of cognitive processes that explain, to some extent, the differential susceptibility of cognitive processes to brain aging. Brain reserve should correspondingly refer to neurobiological structure, the number of neurons or synapses, or other structural characteristics of the brain that enable some people to withstand the effects of brain aging better than others. In another consensus paper, Cabeza et al. [34] stated that they believed that the term reserve, defined as brain resources accumulated throughout the lifespan, should be used instead of using the terms cognitive reserve and brain reserve separately. According to the paper, since cognition is dependent on the brain, a distinction should not be made between the types of reserve, and reserve should be used to discuss both cognitive and structural aspects that allow individuals to resist the effects of brain aging. This suggests that influential researchers in the field are still conceptually going in different directions, which does not simplify the discussion on the topic.
Although researchers have not been able to agree on how to define the concept of reserve, most agree that having a high reserve, most often referred to as cognitive reserve, can predict cognitive performance and the risk of incident dementia [35, 37]. Cognitive reserve is usually measured through proxy measures that are quantifiable concepts related to cognitive reserve. They include education level, occupational status, and participation in cognitively stimulating leisure activities [18, 35, 36, 38]. These factors account for some individual differences in susceptibility to age-related changes in brain pathology [36] and can be considered modifiable, both early on and throughout life [6, 7].
Present study
Cognitive performance is a key component of the health of the older population. There is a connection between the pathology of the brain and cognitive performance, albeit imperfect. We know that factors contributing to cognition and brain pathology are correlated. We can, however, not assume, a priori, that factors that have been shown to predict brain pathology will predict cognitive performance in the same way and vice versa. Therefore, it is vital to study these concepts further to identify variables that contribute to both cognition and brain pathology and pinpoint the factors that potentially contribute uniquely to each of them.
The main aim of the present study was to identify exposure factors that relate differentially to cognitive performance and brain pathology. Without focusing on what can be contributed to cognitive reserve and what can be contributed to brain reserve, we wanted to establish whether factors associated with cognitive performance relate to brain pathology similarly. We tried to identify factors that similarly relate to the two measurements and factors that relate to the two differently. Cognitive change was also considered to gain further insight into how cognitive performance develops among the older population. Therefore, an additional aim of the study was to examine whether those same exposure factors were associated to change in cognition over 5 years.
Materials and methods
Study population
The Reykjavik study was initiated by the Icelandic Heart Association (IHA) in 1967 and included individuals born from 1907 to 1935 that lived in and around the Reykjavik area in Iceland. The Age Gene/Environment Susceptibility-Reykjavik study (AGES-Reykjavik study) was a continuation of the Reykjavik study. It included 5764 randomly selected survivors from the Reykjavik study cohort that were asked to participate in further testing. The AGES-Reykjavik study was designed to examine the older population’s risk factors related to disease and disability. Measurements were carried out between 2002 and 2011. Participants were included in the data analysis whether they fulfilled the study’s dementia criterion or not [39, 40]. Measurements were taken twice, approximately 5 years apart, and 3316 participants participated in the second wave of data collection. At baseline, the average Mini-Mental State Examination (MMSE) score for participants included in the data analysis was 27.51 (SD = 2.02), ranging from 18 to 30.
Further description of the study design has previously been published [39]. The Icelandic National Bioethics Committee and the Institutional Review Board of the US National Institute on Aging, National Institutes of Health, approved the AGES-Reykjavik study (VSN 00–063). All participants signed informed consent before taking part in the study, and participants were informed about their right to withdraw consent at any point in time.
Research design and measures
For the primary analysis, results from a baseline measurement were used as exposure variables. In contrast, results from a follow-up measurement 5 years after the baseline measurement were used as outcome variables. An additional analysis was also performed where changes in cognitive performance between measurements were used as an outcome variable. Both cognitive performance and brain volume were measured at baseline and follow-up.
Assessment of cognitive function
An extensive cognitive test battery comprised of eight cognitive tests was used to assess cognition. They included the California Verbal Learning Test [41]; Digits Forward [42]; Figure Comparison [43]; Digit Symbol Substitution Test [42]; a modified Stroop Test, Parts I, II and III [44]; and Digits Backwards [42]. Results from the eight cognitive tests were combined into one variable to create a general cognitive ability variable [45]. The composite scores for general cognitive ability were based on z-scores of the raw scores for each test averaged over all eight variables. Further description of these calculations can be found in previously published material [46]. According to previous research into the AGES-Reykjavik study dataset, the tests were divided into three cognitive domains, a memory domain, a speed of processing domain, and an executive function domain [46]. Analyses corresponding to those performed for general cognitive ability, relating to each cognitive domain, are provided as supplementary material.
Change in general cognitive ability
Changes in general cognitive ability were calculated by subtracting the baseline measurement for each cognitive test from a follow-up measurement collected 5 years later. The change in general cognitive ability variable was calculated by averaging the z-scores of the raw scores for change in cognition for each of the eight tests.
Brain imaging
Magnetic resonance (MR) brain images were collected during both rounds of data collection. The image acquisition and image processing pipeline have previously been described [47]. This study aimed not to identify possible brain regions that could influence specific types of cognitive performance; instead, the goal was to get a general sense of whether the same exposure factors would be associated with cognitive health and brain health. Since studies have shown that brain atrophy, loss of brain cells or a loss in the number of connections between brain cells, is indicative of cognitive decline, brain volume was chosen as a representative of brain pathology [48, 49]. The goal was to utilize a robust indicator of brain health that could be compared to cognitive health without differentiating between individuals considered cognitively healthy and individuals living with dementia. The variables used for data analysis were relative white matter volume (RWMV) and relative gray matter volume (RGMV) [47]. The RWMV was calculated by dividing the white matter volume (ml) by the intracranial volume (ICV) (the sum of gray matter volume, white matter volume, white matter lesion volume, and cerebral spinal fluid volume). The RGMV was calculated the same way. The calculations resulted in a percentage score that shows the volume of white matter and gray matter relative to the volume of the ICV.
Exposure variables
A search was performed within the AGES-Reykjavik study dataset for variables identified in the literature as predictors of brain aging and cognitive aging. These variables were included in the initial analysis. Only variables that had a significant relationship (before applying a Benjamini–Hochberg correction [50]) to either cognitive health or brain health (RWMV or RGMV) were included in the final analysis. Sex was incorporated as a control variable since it has been established that cognitive aging differs between males and females [51]. Age was also included as a control variable since it is a strong predictor of cognitive aging [52]. Descriptions of each of the sixteen variables included in the models and information about how they were measured can be found in Table 1.
Table 1.
Description of exposure variables
| Sex | Male, female |
|---|---|
| Age | Subject age at first measurement |
| Mobility | Timed up and go test (TUG) measured in seconds a. A lower score represents better mobility |
| Leisure activities | Average of days per month engaged, reported for mental and social leisure activities (movies, lectures, church, crossword puzzles, board/card games, and computer games) |
| Foreign languages | Number of foreign languages spoken |
| Education | Education level completed: primary school, secondary, college, university (primary and secondary school were combined into one group) |
| Self-reported health | Self-estimation of general health: excellent, very good, good, fair, poor (two categories: 1, poor and fair; 2, good to excellent) |
| Physical strength | Maximum strength value in leg in newtons |
| Smoking | Smoking status: never smoked, previous smoker, current smoker |
| Coronary artery disease | Coronary artery disease diagnosis based on rose angina, MI ECG, and use of nitrates: yes, possible case, no |
| Alcohol consumption | Grams of alcohol per week consumed |
| Depression | Geriatric Depression Scale scoreb |
| Diabetes | Diagnosed as diabetes by self-report, fasting glucose, or medication use: yes, no |
| Hypertension | Hypertension, derived from physiological measurements (systolic blood pressure, diastolic blood pressure): yes, pre-hypertension, no |
| Body fat percentage | Bioelectric Impedance (BIA): percent body fat |
| ApoE carrier | Apolipoprotein E (ApoE) genotype positive carrier: yes, no |
Analytical sample
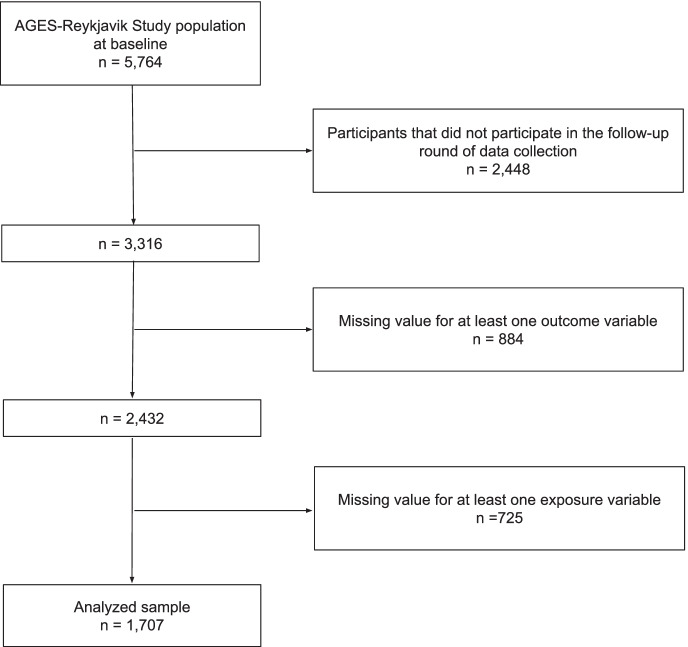
Figure 1 shows a flowchart of the participant selection process for the primary analysis. The sample consisted of 1707 participants. Since the brain volume variables were not included in the analysis of cognitive change, participants that were only missing data for brain volume were included in the study. Therefore 1901 participants were included in the analysis of cognitive change.
Fig. 1.
Flowchart of the participant selection for the primary analysis
Statistical analysis
Means and standard deviations for continuous variables and counts and percentages for discrete variables were displayed for all variables included in the models.
For the primary analysis, linear regression models were used to analyze how exposure variables related to outcome variables. Each model included the same exposure variables but had different outcome variables related to cognition or brain health (MR brain imaging parameters). As an additional analysis, a linear regression model was also used to analyze how exposure variables related to changes in cognitive performance.
The Benjamini–Hochberg correction was used for all linear regression models to control for false discovery rate caused by multiple comparisons [50].
Results
Descriptive statistics
Exposure variables
Table 2 shows both the means and standard deviations and counts and percentages, for every exposure variable. Sex and age were included in the models as control variables.
Table 2.
Descriptive statistics for exposure variables showing means, standard deviations, counts and percentages
| M | SD | |
| Age | 74.24 | 4.47 |
| Mobility (sec) | 11.31 | 2.61 |
| Leisure activity (days/month) | 5.62 | 3.67 |
| Foreign languages (languages spoken) | 2.10 | 1.51 |
| Physical strength (Newtons) | 345.41 | 114.83 |
| Alcohol consumption (grams/week) | 17.18 | 33.36 |
| Depression (Geriatric Depression Scale score) | 1.93 | 1.90 |
| Body fat percentage (%) | 29.08 | 8.08 |
| n (%) | ||
| Male | 724 (42.4) | |
| Education (Primary and secondary school as reference) | 1220 (71.5) | |
| College | 285 (16.7) | |
| University | 202 (11.8) | |
| Self-reported health—good to excellent | 1326 (77.7) | |
| Smoking (Never as reference) | 754 (44.2) | |
| Previously | 782 (45.8) | |
| Current | 171 (10.0) | |
| Coronary artery disease (No as reference) | 1309 (76.7) | |
| Possible case | 116 (6.8) | |
| Yes | 282 (16.5) | |
| Diabetes—diagnosed with | 154 (9.0) | |
| Hypertension (No as reference) | 195 (11.4) | |
| Pre-Hypertension | 689 (40.4) | |
| Yes | 823 (48.2) | |
| ApoE carrier | 454 (26.6) | |
Outcome variables
Three outcome variables were modeled, general cognitive ability, relative white matter volume, and relative gray matter volume. The means and standard deviations for those variables for both sexes are displayed in Table 3. Results from t-tests comparing males and females on these outcome variables showed that women had better outcomes on all measurements.
Table 3.
Descriptive statistics for outcome variables and statistical difference between males and females
| Overall | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | M | SD | n | M | SD | n | p | |
| General cognitive ability score | < 0.001 | 0.62 | 1707 | -0.12 | 0.64 | 724 | 0.09 | 0.60 | 983 | < .001 |
| Relative white matter volume (%) | 24.71 | 0.03 | 1707 | 24.58 | 2.01 | 724 | 24.81 | 1.97 | 983 | .017 |
| Relative gray matter volume (%) | 45.24 | 0.02 | 1707 | 43.78 | 2.94 | 724 | 46.32 | 2.94 | 983 | < .001 |
Regression models comparing cognitive performance and brain health
Regression analysis was applied to test which variables had an association with cognitive performance and brain health using exposure variables that have previously been identified as factors that contribute to cognitive aging and dementia risk. The exposure variables were entered into the model simultaneously after it was ascertained that no assumption was violated, including the assumptions of normality and multicollinearity.
Table 4 shows regression models for general cognitive ability, relative white matter volume, and relative gray matter volume, including the same exposure variables. All three regression models were significant (p < 0.001).
Table 4.
Linear regression models for general cognitive ability, relative white matter volume, and relative gray matter volume with the same exposure variables included in the models
| General cognitive ability | Relative white matter volume | Relative grey matter volume | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | p | B | β | p | B | β | p | ||||||||
| Male a | 0.346 | .275 | < .001* | 0.007 | .175 | < .001* | 0.021 | .331 | < .001* | |||||||
| Age a | − 0.036 | − .258 | < .001* | − 0.001 | − .298 | < .001* | − 0.002 | − .268 | < .001* | |||||||
| Mobility b | − 0.027 | − .114 | < .001* | − 0.001 | − .078 | .001* | − 0.001 | − .109 | < .001* | |||||||
| Leisure activities | 0.023 | .134 | < .001* | < 0.001 | .000 | .991 | < 0.001 | .006 | .778 | |||||||
| Foreign languages | 0.106 | .257 | < .001* | < 0.001 | .028 | .332 | − 0.001 | − .053 | .052 | |||||||
| Education (Primary or secondary school education as reference) | ||||||||||||||||
| College education | 0.154 | .093 | < .001* | − < 0.001 | − .008 | .735 | − 0.002 | − .019 | .416 | |||||||
| University education | 0.072 | .037 | .127 | − 0.003 | − .050 | .074 | − 0.005 | − .052 | .048** | |||||||
| Self-reported health | 0.095 | .063 | .003* | 0.001 | .012 | .618 | 0.002 | .032 | .160 | |||||||
| Physical strength | 0.001 | .124 | < .001* | < 0.001 | .096 | .003* | < 0.001 | .035 | .248 | |||||||
| Smoking (Never having smoked as reference) | ||||||||||||||||
| Previously smoked | − 0.011 | − .009 | .688 | − 0.001 | − .014 | .560 | − 0.004 | − .068 | .003* | |||||||
| Current smoker | − 0.062 | − .030 | .152 | − 0.005 | − .077 | .001* | − 0.011 | − .104 | < .001* | |||||||
| Coronary artery disease (No coronary artery disease as reference) | ||||||||||||||||
| Possible case of coronary artery disease | − 0.048 | − .019 | .322 | 0.001 | .013 | .556 | − 0.003 | − .023 | .278 | |||||||
| Confirmed case of coronary artery disease | − 0.078 | − .047 | .021* | − 0.005 | − .092 | < .001* | − 0.008 | − .091 | < .001* | |||||||
| Alcohol consumption | 0.001 | .058 | .004* | − < 0.001 | − .044 | .059 | − < 0.001 | − .087 | < .001* | |||||||
| Depression | − 0.015 | − .045 | .039** | − 0.001 | − .101 | < .001* | − < 0.001 | − .009 | .705 | |||||||
| Diabetes | − 0.011 | − .005 | .788 | − 0.005 | − .075 | .001* | − 0.004 | − .035 | .088 | |||||||
| Hypertension (No hypertension as reference) | ||||||||||||||||
| Pre-hypertension | − 0.087 | − .068 | .031** | − 0.001 | − .032 | .380 | − 0.004 | − .059 | .086 | |||||||
| Hypertension | − 0.126 | − .101 | .002* | − 0.004 | − .106 | .004* | − 0.003 | − .047 | .171 | |||||||
| Body Fat Percentage | − 0.002 | − .029 | .366 | − < 0.001 | − .144 | < .001* | < 0.001 | .010 | .777 | |||||||
| ApoE carrier | − 0.052 | − .037 | .060 | − 0.002 | − .044 | .045** | − 0.003 | − .045 | .031** | |||||||
| R2 | .381 | .198 | .291 | |||||||||||||
aSex and age are control variables. bThe TUG test for mobility has a negative relationship with the outcome variables. A low score represents more mobility. Better mobility is therefore related to better outcomes
*Variables marked with one asterisk have a significant p value after a Benjamini–Hochberg correction [50]. **Variables marked with two asterisks have a p value < 0.05 but are considered non-significant after the Benjamini–Hochberg correction
Cognitive health
Nine exposure variables were associated with general cognitive ability (Table 4). Seven variables were positively related to general cognitive ability (mobility, leisure activities, foreign languages, college education, self-reported health, physical strength, and alcohol consumption). These results indicate that, for example, an individual’s general cognitive ability was likely to be better if he spoke more languages and participated in more leisure activities. It should be noted that although mobility appears to have a negative association with general cognitive ability, that is only because the test that measures mobility measures the time it takes a person to stand up. Therefore a negative association implies that an individual that stands up in less time has a better general cognitive ability. Two variables were negatively associated with general cognitive ability (confirmed case of coronary artery disease and hypertension). This indicates that having coronary artery disease or hypertension makes an individual less likely to score high on general cognitive ability.
Corresponding results for the three cognitive domains, memory, speed of processing, and executive function, can be found in Supplementary Table 1. Most of the exposure variables associated with general cognitive ability were associated with at least one of the cognitive domains. Coronary artery disease was, however, not associated with any domains, even though it was associated with general cognitive ability. Having a university education, having depressive symptoms, and having diabetes were associated with one of the domains, but those variables were not associated with general cognitive ability.
Brain health
Relative white matter volume
Eight exposure variables were associated with relative white matter volume (Table 4). Two variables were positively related to RWMV (mobility and physical strength), meaning that having more mobility and physical strength made an individual more likely to have greater RWMV. Six variables were negatively associated with RWMV (currently smoking, confirmed case of coronary artery disease, depression, diabetes, hypertension, and body fat percentage). This indicates, for example, that smoking or having a higher body fat percentage made an individual less likely to have greater RWMV.
Relative gray matter volume
Five exposure variables were associated with relative gray matter volume (Table 4). One variable was positively related to RGMV (mobility), suggesting that with better mobility, an individual was more likely to have greater RGMV. Four variables were negatively associated with RGMV (former smoker, current smoker, confirmed case of coronary artery disease, and alcohol consumption). This means that smoking, having coronary artery disease, or consuming more alcohol makes an individual less likely to have greater RGMV.
Regression model for cognitive change
Using the same exposure variables as before, linear regression analysis was also applied to predict change in cognitive performance over 5 years. Table 5 shows a regression model for change in general cognitive ability. The exposure variables were entered into the model simultaneously after it was ascertained that no assumption was violated, including the assumptions of normality and multicollinearity. In addition to sex and age, cognitive performance at baseline was included in the model as a control variable. The model was significant with p < 0.001.
Table 5.
Linear regression model for change in general cognitive ability
| Change in general cognitive ability | |||
|---|---|---|---|
| B | β | p | |
| Male a | 0.183 | .209 | < .001* |
| Age a | − 0.013 | − .134 | < .001* |
| Mobility b | − 0.011 | − .063 | .011* |
| Leisure activities | 0.002 | .018 | .463 |
| Foreign languages | 0.014 | .050 | .104 |
| Education (Primary or secondary school education as reference) | |||
| College education | 0.022 | .019 | .458 |
| University education | 0.032 | .024 | .393 |
| Self-reported health | 0.058 | .057 | .021** |
| Physical strength | < 0.001 | .096 | .004* |
| Smoking (Never having smoked as reference) | |||
| Previously smoked | − 0.006 | − .007 | .775 |
| Current smoker | 0.007 | .005 | .837 |
| Coronary artery disease (No coronary artery disease as reference) | |||
| Possible case of coronary artery disease | 0.035 | .020 | .370 |
| Confirmed case of coronary artery disease | − 0.019 | − .016 | .485 |
| Alcohol consumption | 0.001 | .048 | .043** |
| Depression | − 0.008 | − .034 | .174 |
| Diabetes | 0.024 | .016 | .467 |
| Hypertension (No hypertension as reference) | |||
| Pre-hypertension | − 0.052 | − .059 | .111 |
| Hypertension | − 0.056 | − .065 | .083 |
| Body Fat Percentage | − 0.002 | − .031 | .393 |
| ApoE carrier | − 0.076 | − .078 | .001* |
| R2 | .074 | ||
aSex and age are control variables. bThe TUG test for mobility has a negative relationship with the outcome variables. A low score represents more mobility. Better mobility is therefore related to a better outcome
*Variables marked with one asterisk have a significant p value after a Benjamini–Hochberg correction [50]. **Variables marked with two asterisks have a p value < 0.05 but are considered non-significant after the Benjamini–Hochberg correction
Three exposure variables were associated with a change in general cognitive ability (Table 5). Mobility and physical strength were positively related to change in general cognitive ability meaning that general cognitive ability was less likely to deteriorate over the 5 years between measurements if an individual had good mobility and physical strength at baseline. Being an ApoE carrier was negatively associated with general cognitive ability making an ApoE carrier more likely to deteriorate in the five years between measurements.
Corresponding results for changes in the three cognitive domains, memory, speed of processing, and executive function, can be found in Supplementary Table 2. Only one of the exposure variables associated with change in general cognitive ability, being an ApoE carrier, was also associated with change in a cognitive domain. However, mobility and physical strength were not associated with changes in any of the domains, even though they were associated with change in general cognitive ability. Self-reported health and alcohol consumption were associated with change in at least one of the domains, but those variables were not associated with change in general cognitive ability.
Regression models for cognition with brain volume as exposure variables
Since it is known that brain volume predicts cognition [27], additional analyses were performed where RWMV and RGMV were included as exposure variables in the models for general cognitive ability (Supplementary Table 3) and change in general cognitive ability (Supplementary Table 4). These analyses aimed to establish whether including the brain volume variables in the models for cognition would render other exposure variables insignificant.
When RWMV and RGMV were included in the models for cognitive performance as exposure variables, both RWMV and RGMV were significant and had a positive association with cognition. Having more brain volume means that an individual was more likely to score higher on general cognitive ability. The only change to the other exposure variables was that coronary artery disease became insignificant.
When RWMV and RGMV were included in the model for cognitive change as exposure variables, no other variables than brain volume were significant. Both RWMV and RGMV had a positive association to change in general cognitive ability, meaning that those with more brain volume were less likely to deteriorate over the 5 years between measurements.
Discussion
This study compared how risk factors for cognitive decline and dementia are differentially associated with cognitive performance and brain health. The findings suggest a considerable difference between risk and protective factors strongly associated with brain health and those strongly associated with cognition.
Factors only related to cognition
Leisure activities, foreign languages, education, and self-reported health only had a significant relationship to cognition, not brain volume. Education is considered an essential predictor of cognition [7, 18]. A longitudinal study that looked at the relationship between education, school performance, occupation, and dementia showed that good school performance was protective against dementia risk and that the protective effect was even more substantial if good performance in school was accompanied by occupational complexity later in life [55].
Clinical measures of brain tissue volume and brain function based on the AGES-Reykjavik study dataset suggest that conditions in utero are associated with late-life brain volume and brain function [56]. An adverse intrauterine environment was only related to poorer cognitive function for those with lower education levels. A high level of education minimizes a suboptimal intrauterine environment’s effect on cognition. Results such as these could explain why it seems that the relationship to the pathology of dementia is not as strong as the relationship to the clinical manifestations of dementia. Rather than educational attainment being directly linked to brain pathology, when these factors are introduced into the equation, it makes up for suboptimal brain structure, which would otherwise translate into cognitive performance.
The time people spend on leisure activities also has a significant relationship to cognition. The findings align with previous research showing a relationship between participating in leisure activities and maintaining cognitive function [19]. Furthermore, Saczynski et al. [20] previously suggested that leisure activity participation reduced the effect of brain lesions on cognitive performance. Like educational attainment, this would explain why leisure activities only had an association with cognitive performance, not brain volume. Participation in leisure activities can prevent brain lesions from having the effect they otherwise would have on cognition.
These two variables, education and participation in leisure activities, are often mentioned as proxy measures for cognitive reserve and are recognized as such in the literature [34–36]. However, self-reported health and multilingualism could also contribute to cognitive reserve [34]. The findings reported here are in line with other studies showing that multilingualism predicts cognitive performance in the later adulthood [57, 58]. More specifically, it seems that lifelong bilingualism is protective when it comes to the onset of symptoms of dementia [58, 59]. However, a recent randomized controlled trial suggested that foreign language learning at an older age does not improve cognition [60], which casts doubt on findings that have indicated that foreign language learning at an older age can enhance cognitive functioning [61, 62]. It, therefore, seems that the importance of multilingualism lies in language acquisition earlier in life and might operate as a protective factor through cognitive reserve, in a similar manner as educational attainment and participation in leisure activities.
The fact that self-reported health was related to cognitive performance in this study is in line with other research suggesting that self-reported health predicts dementia risk and cognitive function [22, 63]. Although it is not often mentioned as a proxy measure for cognitive reserve, it could well be that these factors work together to establish a better cognitive reserve. Studies have shown that personality traits matter in cognitive reserve [64]. Having low tendencies towards neuroticism and being open to experiences is related to better cognitive reserve. Neuroticism has also been linked to the mental aspect of the experience of health-related quality of life [65]. Based on these studies and the fact that neuroticism is also linked to increased dementia risk, how individuals view their health could be an essential contributor to cognitive reserve. This is further supported by recent studies that have established that having a positive attitude toward aging can work protectively against dementia overriding to some extent the effects of the ApoE gene [66].
It should be noted that when brain volume was added to the model as an exposure variable, the significance of other variables in the model remained. Therefore, adding brain volume to the models did not reduce the relationship between these variables and cognitive performance. Both RWMV and RGMV were also significant. This is in line with other studies that consider cognitive reserve in conjunction with brain pathologies [67]. These findings suggest that brain volume is vital for cognitive performance. At the same time, it is evident that cognitive reserve also contributes substantially to cognitive performance in an independent manner.
Change in cognition
When change in cognition was analyzed, very few of the exposure variables were associated with general cognitive ability. Only mobility and physical strength had a positive relationship with change in general cognitive ability, while being an ApoE carrier, had a negative association with change in general cognitive ability. These results are similar to the findings of other studies showing that predictors of cognitive function often do not predict cognitive decline [9–, 68–70]. When brain volume was added to the models as an exposure variable, it was associated with cognitive change while other exposure variables were not. This suggests that brain volume would better predict cognitive decline over 5 years than exposure factors that are known predictors of cognitive performance. It is possible that factors that show an association with brain volume also have a relationship with cognitive performance, mediated through brain pathology. They would likely need a longer time interval than 5 years to be able to show an association with cognitive performance.
Factors associated with brain volume
Mobility, physical strength, alcohol consumption, coronary artery disease, and hypertension were associated with cognition and brain volume. The fact that some predictors of dementia and cognitive decline show a relationship to both cognitive performance and brain pathology does not come as a surprise since many studies have established that the two are correlated [27, 71].
The variables only associated with brain volume, not cognitive performance, were smoking, depression, diabetes, and body fat percentage. Smoking or having previously smoked, having depressive symptoms, having diabetes, and having a high body fat percentage are all factors that make an individual more likely to have less brain volume. Although these variables do not show an association with cognitive performance within this dataset, that is not to say that they do not predict cognitive performance as has been reported in other studies [9, 12, 46]. It could be that more than 5 years need to pass between measurements for the relationship between these variables and cognitive performance to come to light. These variables do predict brain volume, and brain volume does have an association with cognitive performance when included as an exposure variable.
It should be acknowledged that researchers have criticized the use of total brain volume as a measure of cognitive performance and cognitive decline since studies have shown that age-related deterioration in different brain structures does not happen simultaneously [71] and brain atrophy does not necessarily indicate neuronal loss [72]. However, the purpose of this study was to compare how known predictors of cognitive health would relate to cognitive performance on the one hand and brain pathology on the other. Therefore, relative white matter volume and relative gray matter volume were deemed acceptable as robust representatives of brain pathology for this study.
Great effort has been put into research focusing on the pathology of dementia [73, 74]. While progress has been made and available treatments can alleviate the symptoms, no cure has been found which suggests that brain pathology cannot easily be changed [29, 73]. Additionally, when considering clinical importance, cognitive performance may be regarded as more critical than neuropathological symptoms, suggesting that the focus on preventative measures should be on cognitive outcomes rather than the pathology [75]. By putting more effort into research related to factors associated with cognitive performance, alongside the emphasis on the long-term goal of curing brain pathology, a better opportunity is created to limit cognitive decline amongst those that have cognitive impairment.
An interesting outcome of the current study is that alcohol consumption had a significant relationship with cognitive performance and brain volume, but the effects were in the opposite directions. More alcohol consumption was related to better cognitive performance, but more consumption was associated with less grey matter volume. This is in line with previous findings [9, 19, 76, 77]. Some studies have even suggested that moderate alcohol consumption has a protective effect on cognitive performance [9, 19]. Why alcohol consumption has a significant relationship to so many outcome variables in this study is unknown. It does come as somewhat of a surprise, especially since the protective effect of moderate alcohol consumption had been questioned [78]. It could be, as suggested by Topiwala and Ebmeier [78], that the explanation is that alcohol consumption is highly associated with other know protective factors such as socioeconomic status and education. Given how mixed the findings are in the literature, the relationship between alcohol consumption and the outcome variables in this study should be interpreted with caution.
Strengths and limitations
An essential strength of this study is the dataset used in the analysis, the AGES-Reykjavik study dataset. The dataset is well suited for this type of analysis. It includes information from cognitive tests, MR brain imaging data, and various risk factors associated with cognitive aging, both physiological and demographical, all from the same individuals. Additionally, the subsample of participants selected for the AGES-Reykjavik study was chosen randomly from the Reykjavik study, which comprised a random sample of approximately 30,000 randomly selected individuals living in Reykjavik in 1967 [39]. This ensures that participants of all capabilities were chosen for the study, not only the most active and healthy individuals. Finally, the size of the dataset and the thorough examinations performed on participants strengthen the findings and generalizability of the results. Another strength of the study is that all exposure variables were based on information collected 5 years before the information for the outcome variables was collected. This strengthens the assumption that the exposure variables are predictors of both cognitive performance and brain health but are not only correlated with the outcome variables.
The study’s greatest strength is also one of its weaknesses. The dataset had already been collected before this research project started, so the data collection could not be amended to suit this study. Therefore, this project is limited to the information contained within the AGES-Reykjavik study and does not include all the variables considered important for cognitive aging in a format that would have made it possible to include them in the analysis. Another weakness of the dataset is that many of the variables are based on self-reported data (e.g., how many foreign languages do you speak?). This could introduce some inaccuracy to the dataset. Variables relating to diet, physical activity, and sleep were a part of the initial dataset but did not significantly correlate to cognition or brain volume. This could be because of the dataset’s limitations; for example, sleep was only measured in self-report of how many hours an individual slept each night on average and physical activity, and diet was measured by delayed self-reported information. Had the dataset not been previously collected, it could have been possible to structure the variables differently for the intended analysis.
Furthermore, due to the data analysis applied to the dataset, around half of the participants that participated in the second round of data collection were excluded from the final analysis. This was unavoidable but expected when working with so many different variables in the same model. It is not unlikely that a certain selection bias was introduced into the dataset when those who did not participate in the follow-up measurement were excluded from the data analysis. This should, however, not have a significant effect on the results of this study since the aim was to compare how exposure factors relate to two different elements, brain health and cognitive health, within the same group of participants.
Future research
Although these models give insight into how risk factors differentially relate to cognitive performance and brain health, they do not provide very accurate predictions, based on these exposure variables, of how the cognition of the participants will develop with time [79]. More accurate predictions could be reached by applying other data analysis methods, such as machine learning. Such methods could allow for an approach to tackling cognitive decline tailored to everyone. A follow-up analysis will be performed. A machine learning approach will be applied to the AGES-Reykjavik study dataset to predict the likelihood that a person has dementia based on data collected about the individual 5 years prior.
Conclusion
The study shows that exposure variables linked to dementia relate differently to cognition and brain health. Even though exposure variables, such as speaking more foreign languages, participating in leisure activities, obtaining a higher level of education, and having better self-reported health do not have a relationship to brain volume, they have an evident relationship to cognition. It has been suggested that cognitive reserve bridges the gap between an individual’s actual cognitive capabilities and brain age or brain pathology [35]. The analyses performed in this study show that, within the same participant pool, cognitive reserve proxy variables have a relationship with cognitive performance but have no association with relative brain volume measured simultaneously. These findings indicate that, without contributing to an individual’s brain pathology, being high on cognitive reserve can positively impact cognitive performance. The results of this study have implications for the definitions of reserve since they indicate that the two terms, brain reserve and cognitive reserve, are independent, a least to some extent. Therefore, a distinction should be made between the two instead of grouping them under the term reserve. Since affecting the brain would most likely require substantial intervention, these findings present an opportunity to affect cognition without relying on affecting the brain directly. Developing preventative measures that focus on these modifiable factors could allow for a significant change in the cognitive performance of the older population and, thereby, the quality of life of those individuals.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by The Foundation of St. Josef’s Hospital in cooperation with The Icelandic Gerontological Research Center, National University Hospital of Iceland. The AGES-Reykjavik study was supported by the National Institutes of Health (Intramural Research Programs of the National Institute of Aging and the National Eye Institute, ZIAEY00401), National Institutes of Health contract number N01-AG-1–2100, the Icelandic Heart Association, and the Icelandic Parliament.
Additional grants were provided by Landspítali – University Hospital Research Fund, the Icelandic Gerontological Society, the Council on Aging in Iceland, Helga Jónsdóttir and Sigurliði Kristjánsson Memorial Fund, and the Sustainability Institute and Forum (SIF) at Reykjavik University.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vaka Valsdóttir, Email: vaka@ru.is.
Brynja Björk Magnúsdóttir, Email: brynjabm@ru.is.
Milan Chang, Email: changmilan@gmail.com.
Sigurdur Sigurdsson, Email: sigurdur@hjarta.is.
Vilmundur Gudnason, Email: v.gudnason@hjarta.is.
Lenore J. Launer, Email: launerl@nia.nih.gov
María K. Jónsdóttir, Email: mariakj@ru.is
References
- 1.Depp CA, Harmell A, Vahia IV. Successful cognitive aging. In: Behavioral neurobiology of aging. 2011. pp. 35–50. 10.1007/7854_2011_158. [DOI] [PubMed]
- 2.World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. World Health Organization, Geneva, 2019. [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/312180/9789241550543-eng.pdf?sequence=1&isAllowed=y. Accessed 16 May 2019 [PubMed]
- 3.Cutler SJ. Worries about getting Alzheimer’s: who’s concerned? Am J Alzheimers Dis Other Demen. 2015;30(6):591–598. doi: 10.1177/1533317514568889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hongisto K, et al. Quality of Life in relation to neuropsychiatric symptoms in Alzheimer’s disease: 5-year prospective ALSOVA cohort study. Int J Geriatr Psychiatry. 2018;33(1):47–57. doi: 10.1002/gps.4666. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–384. 10.1016/j.jalz.2015.02.003. [DOI] [PubMed]
- 6.Barnett JH, Hachinski V, Blackwell AD. Cognitive health begins at conception: addressing dementia as a lifelong and preventable condition. BMC Med. 2013;11(1):246. doi: 10.1186/1741-7015-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith GE, Bondi MW. Mild cognitive impairment and dementia: definitions, diagnosis, and treatment. Oxford, New York: Oxford University Press; 2013. [Google Scholar]
- 9.Zaninotto P, Batty GD, Allerhand M, Deary IJ. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J Epidemiol Community Health. 2018;72(8):685–694. doi: 10.1136/jech-2017-210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang M, et al. The effect of midlife physical activity on cognitive function among older adults: AGES—Reykjavik study. J Gerontol A Biol Sci Med Sci. 2010;65A(12):1369–1374. doi: 10.1093/gerona/glq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham C, O’Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30(5):816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 12.Won H, Abdul Manaf Z, Mat Ludin AF, Shahar S. Wide range of body composition measures are associated with cognitive function in community-dwelling older adults. Geriatr Gerontol Int. 2017;17(4):554–560. doi: 10.1111/ggi.12753. [DOI] [PubMed] [Google Scholar]
- 13.Sindi S, et al. Sleep disturbances and dementia risk: a multicenter study. Alzheimers Dement. 2018;14(10):1235–1242. doi: 10.1016/j.jalz.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinkohl I, Price JF, Strachan MWJ, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimers Res Ther. 2015;7(1):46. doi: 10.1186/s13195-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefansdottir H, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke. 2013;44(4):1020–1025. doi: 10.1161/STROKEAHA.12.679381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abete P, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. Ageing Res Rev. 2014;18:41–52. doi: 10.1016/j.arr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Aging Neuropsychol Cogn. 2016;23(1):40–60. doi: 10.1080/13825585.2015.1041450. [DOI] [PubMed] [Google Scholar]
- 19.Clare L, et al. Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS Med. 2017;14(3):e1002259. doi: 10.1371/journal.pmed.1002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saczynski JS, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63(8):848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniou M. The advantages of bilingualism debate. Annu Rev Linguist. 2019;5(1):395–415. doi: 10.1146/annurev-linguistics-011718-011820. [DOI] [Google Scholar]
- 22.Weiss J, Puterman E, Prather AA, Ware EB, Rehkopf DH. A data-driven prospective study of dementia among older adults in the United States. PLoS ONE. 2020;15(10):e0239994. doi: 10.1371/journal.pone.0239994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blankevoort CG, et al. Physical predictors of cognitive performance in healthy older adults: a cross-sectional analysis. PLoS One. 2013;8(7):e70799. doi: 10.1371/journal.pone.0070799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper R, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheltens P, et al. Alzheimer’s disease. The Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 26.Bangen KJ, et al. Baseline white matter hyperintensities and hippocampal volume are associated with conversion from normal cognition to mild cognitive impairment in the Framingham offspring study. Alzheimer Dis Assoc Disord. 2018;32(1):50–56. doi: 10.1097/WAD.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS ONE. 2013;8(6):e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigurdsson S, et al. Incidence of brain infarcts, cognitive change, and risk of dementia in the general population. Stroke. 2017;48(9):2353–2360. doi: 10.1161/STROKEAHA.117.017357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement: Transl Res Clin Interv. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and neuropathology in aging: multidimensional perspectives from the rush religious orders study and rush memory and aging project. Curr Alzheimer Res. 2011;8(4):336–340. doi: 10.2174/156720511795745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed RM, et al. Biomarkers in dementia: clinical utility and new directions. J Neurol Neurosurg Psychiatry. 2014;85(12):1426–1434. doi: 10.1136/jnnp-2014-307662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 33.Veitch DP, et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2019;15(1):106–152. doi: 10.1016/j.jalz.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Cabeza R, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettigrew C, Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep. 2019;19(1):1–12. doi: 10.1007/s11910-019-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern Y, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DH, et al. Effects of cognitive reserve in Alzheimer’s disease and cognitively unimpaired individuals. Front Aging Neurosci. 2021;13:784054. doi: 10.3389/fnagi.2021.784054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groot C, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90(2):e149–e156. doi: 10.1212/WNL.0000000000004802. [DOI] [PubMed] [Google Scholar]
- 39.Harris TB, et al. Age, gene/environment susceptibility–reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidal J-S, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik study. Stroke. 2010;41(5):891–897. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual - Adult Version (Research Edition) New York: The Psychological Corporation; 1987. [Google Scholar]
- 42.Wechsler DW. WAIS-III: Wechsler adult intelligence scale. Manual. New York: Psychological Corporation; 1955. [Google Scholar]
- 43.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27(5):763–776. doi: 10.1037/0012-1649.27.5.763. [DOI] [Google Scholar]
- 44.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 45.Johnson W, te Nijenhuis J, Bouchard TJ. Still just 1 g: consistent results from five test batteries. Intelligence. 2008;36(1):81–95. doi: 10.1016/j.intell.2007.06.001. [DOI] [Google Scholar]
- 46.Saczynski JS, et al. Cognitive impairment: an increasingly important complication of type 2 Diabetes The Age, Gene/Environment Susceptibility-Reykjavik Study. Am J Epidemiol. 2008;168(10):1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigurdsson S, et al. Brain tissue volumes in the general population of the elderly The AGES-Reykjavik Study. Neuroimage. 2012;59(4):3862–3870. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grajauskas LA, Siu W, Medvedev G, Guo H, D’Arcy RCN, Song X. MRI-based evaluation of structural degeneration in the ageing brain: pathophysiology and assessment. Ageing Res Rev. 2019;49:67–82. doi: 10.1016/j.arr.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Harper L, Barkhof F, Scheltens P, Schott JM, Fox NC. An algorithmic approach to structural imaging in dementia. J Neurol Neurosurg Psychiatry. 2014;85(6):692. doi: 10.1136/jnnp-2013-306285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57(1):289–300. [Google Scholar]
- 51.McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31(2):166–175. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh-Manoux A, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 54.Yesavage JA, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 55.Dekhtyar S, Wang H-X, Scott K, Goodman A, Koupil I, Herlitz A. A life-course study of cognitive reserve in dementia—from childhood to old age. Am J Geriatr Psychiatry. 2015;23(9):885–896. doi: 10.1016/j.jagp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Muller M, et al. Birth size and brain function 75 years later. Pediatrics. 2014;134(4):761–770. doi: 10.1542/peds.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kavé G, Eyal N, Shorek A, Cohen-Mansfield J. Multilingualism and cognitive state in the oldest old. Psychol Aging. 2008;23(1):70–78. doi: 10.1037/0882-7974.23.1.70. [DOI] [PubMed] [Google Scholar]
- 58.Perquin M, et al. Lifelong exposure to multilingualism: new evidence to support cognitive reserve hypothesis. PLoS One. 2013;8(4):e62030. doi: 10.1371/journal.pone.0062030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bialystok E. The bilingual adaptation: how minds accommodate experience. Psychol Bull. 2017;143(3):233–262. doi: 10.1037/bul0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berggren R, Nilsson J, Brehmer Y, Schmiedek F, Lövdén M. Foreign language learning in older age does not improve memory or intelligence: evidence from a randomized controlled study. Psychol Aging. 2020;35(2):212–219. doi: 10.1037/pag0000439. [DOI] [PubMed] [Google Scholar]
- 61.Klimova B. Learning a foreign language: a review on recent findings about its effect on the enhancement of cognitive functions among healthy older individuals. Front Hum Neurosci. 2018;12. 10.3389/fnhum.2018.00305. [DOI] [PMC free article] [PubMed]
- 62.Wong PCM, et al. Language training leads to global cognitive improvement in older adults: a preliminary study. J Speech Lang Hear Res. 2019;62(7):2411–2424. doi: 10.1044/2019_JSLHR-L-18-0321. [DOI] [PubMed] [Google Scholar]
- 63.Whitley E, Deary IJ, Ritchie SJ, Batty GD, Kumari M, Benzeval M. Variations in cognitive abilities across the life course: cross-sectional evidence from Understanding Society: The UK Household Longitudinal Study. Intelligence. 2016;59:39–50. doi: 10.1016/j.intell.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karsazi H, Rezapour T, Kormi-Nouri R, Mottaghi A, Abdekhodaie E, Hatami J. The moderating effect of neuroticism and openness in the relationship between age and memory: implications for cognitive reserve. Personality Individ Differ. 2021;176:110773. doi: 10.1016/j.paid.2021.110773. [DOI] [Google Scholar]
- 65.Huang I-C, Lee JL, Ketheeswaran P, Jones CM, Revicki DA, Wu AW. Does personality affect health-related quality of life? A systematic review. PLoS ONE. 2017;12(3):e0173806. doi: 10.1371/journal.pone.0173806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levy BR, Slade MD, Pietrzak RH, Ferrucci L. When culture influences genes: positive age beliefs amplify the cognitive-aging benefit of APOE ε2. J Gerontol B Psychol Sci Soc Sci. 2020;75(8):e198–e203. doi: 10.1093/geronb/gbaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H, et al. Association of lifespan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurol. 2019;76(10):1184–1191. doi: 10.1001/jamaneurol.2019.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritchie SJ, et al. Predictors of ageing-related decline across multiple cognitive functions. Intelligence. 2016;59:115–126. doi: 10.1016/j.intell.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salthouse TA. Correlates of cognitive change. J Exp Psychol Gen. 2014;143(3):1026–1048. doi: 10.1037/a0034847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seblova D, Berggren R, Lövdén M. Education and age-related decline in cognitive performance: systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2020;58:101005. doi: 10.1016/j.arr.2019.101005. [DOI] [PubMed] [Google Scholar]
- 71.Oschwald J, et al. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev Neurosci. 2020;31(1):1–57. doi: 10.1515/revneuro-2018-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Freeman SH, et al. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008;67(12):1205–1212. doi: 10.1097/NEN.0b013e31818fc72f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. The Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 74.Ferencz B, Gerritsen L. Genetics and underlying pathology of dementia. Neuropsychol Rev. 2015;25(1):113–124. doi: 10.1007/s11065-014-9276-3. [DOI] [PubMed] [Google Scholar]
- 75.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 76.Angebrandt A, et al. Dose-dependent relationship between social drinking and brain aging. Neurobiol Aging. 2022;111:71–81. doi: 10.1016/j.neurobiolaging.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis BJK, et al. The alcohol paradox: light-to-moderate alcohol consumption, cognitive function, and brain volume. J Gerontol A Biol Sci Med Sci. 2014;69(12):1528–1535. doi: 10.1093/gerona/glu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Topiwala A, Ebmeier KP. Effects of drinking on late-life brain and cognition. Evid Based Mental Health. 2018;21(1):12–15. doi: 10.1136/eb-2017-102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yarkoni T, Westfall J. Choosing prediction over explanation in psychology: lessons from machine learning. Perspect Psychol Sci. 2017;12(6):1100–1122. doi: 10.1177/1745691617693393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.