Abstract
Dietary fiber is fermented by the human gut microbiota, producing beneficial microbial metabolites, such as short-chain fatty acids. Over the last few centuries, dietary fiber intake has decreased tremendously, leading to detrimental alternations in the gut microbiota. Such changes in dietary fiber consumption have contributed to the global epidemic of obesity, type 2 diabetes, and other metabolic disorders. The responses of the gut microbiota to the dietary changes are specific to the type, amount, and duration of dietary fiber intake. The intricate interplay between dietary fiber and the gut microbiota may provide clues for optimal intervention strategies for patients with type 2 diabetes and other noncommunicable diseases. In this review, we summarize current evidence regarding dietary fiber intake, gut microbiota modulation, and modification in human health, highlighting the type-specific cutoff thresholds of dietary fiber for gut microbiota and metabolic outcomes.
Keywords: dietary fiber, gut microbiota, diabetes, modulation
1. Introduction
Dietary fiber is a carbohydrate in plant foods, such as whole grains, vegetables, fruit, and legumes, which have been dominant in human diets for millions of years. From the Paleolithic era, when the hunter-gatherers mainly ate fruit and wild grains, to the agricultural era, when crops began to be cultivated, the ancients consumed more than 100 g of various digestible and indigestible dietary fiber from plants per day [1,2]. During the million years, the human gut microbiota has provided vital nutritional services through digesting lactose and cellulose, degrading toxins, and biosynthesizing vitamins, signal molecules, and other essential substances [3].
In the industrialization age, however, people consumed much less fiber from diets, posing a big challenge for humans to adapt to the profoundly altered dietary pattern and environments. Relative to the highly conserved human genomes, the flexibility of gut microbes enabled their rapid responses to the changed dietary behaviors and guaranteed the establishment of a new symbiosis with humans [3]. Although the alterations in the gut microbiome may facilitate human adaptation to the changing environment from the perspective of evolution, the new human–microbiome symbiosis, much different from that maintained for millions of years, may elicit profound impacts on human health [4]. Alterations in the human gut microbiome have been implicated in a wide range of complex and chronic conditions, including obesity, diabetes, cancers, and cardiovascular disease [5,6,7,8], and may account for the increasing burden of noncommunicable diseases globally.
To better understand the intricate interplay of dietary fiber intake with gut microbiota in human health, we reviewed the bacterial fermentation of dietary fiber in humans and its role in health maintenance. Aspects considered include the type and intake levels of dietary fiber, the fermentation of dietary fiber by the gut microbiota, the impacts of dietary fiber on the gut microbiota, particularly the fiber type-specific effects and respective cutoff thresholds, and the modulations of dietary fiber on gut microbiota and metabolic outcomes of diabetes patients.
2. Dietary Fiber and Its Main Types
The proper definition of dietary fiber was highly debated during the last few decades. The controversy focused on oligosaccharides, a type of resistant carbohydrate with 3 to 9 monomeric units (MU). According to the officially published Guidelines on Nutrition Labelling (amended in 2009), dietary fiber refers to “carbohydrate polymers with ten or more MUs, which are resistant to hydrolysis by endogenous enzymes and absorption in the small intestine of humans” [9]. However, subsequent investigations observed homogeneous fermentation and physiological activities of indigestible oligosaccharides and polysaccharides that contain similar monosaccharides, providing supportive evidence for oligosaccharides as one type of dietary fiber [10]. Many countries, including China, Japan, the US, Canada, Brazil, and France, and the European Union have accepted the inclusiveness of oligosaccharides as dietary fiber in their official guidelines or standards [11,12].
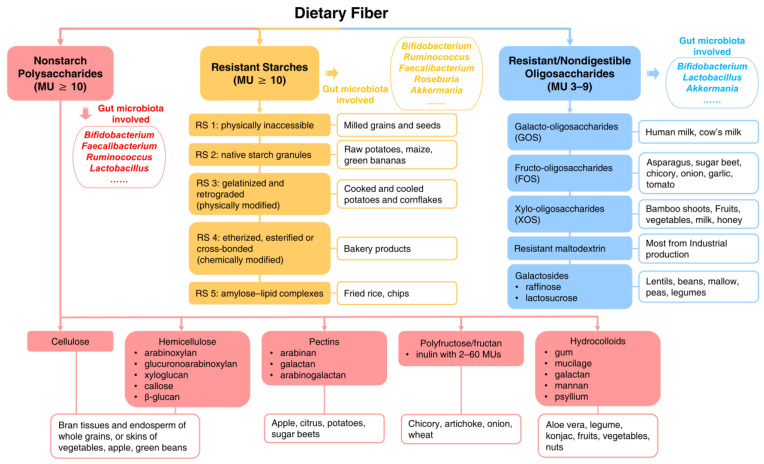
Dietary fiber can be obtained from diets as edible carbohydrate polymers naturally occurring in the food, or carbohydrate polymers extracted from food raw material by physical, enzymatic or chemical means, or synthetic carbohydrate polymers having a physiological effect of benefit to health [9]. As shown in Figure 1, dietary fiber can be classified into three types based on the physiological properties of their MU polymerization: 1) nonstarch polysaccharides (NSPs) (MU ≥ 10); 2) resistant starches (RS) (MU ≥ 10), and 3) resistant/nondigestible oligosaccharides (ROS) (MU: 3–9) [13,14]. The NSPs mainly include cellulose, hemicellulose, pectins, inulin, and various hydrocolloids [15,16]. Inulin is a fructan containing 2–60 fructose units. When MU <10, inulin is also recognized as fructo-oligosaccharides (FOS) [17], well-documented prebiotics [14]. RS can be further classified into RS 1 to RS 5, which can be derived from milled grains and seeds (RS 1), raw potatoes, maize and green bananas (RS 2), cooked and cooled potatoes and cornflakes (RS 3), bakery products (RS 4) and fried rice chips (RS 5) [18]. ROS consist of 3–9 MUs, many of which were named after polymerized monosaccharides, such as galacto-oligosaccharides (GOS), xylo-oligosaccharides (XOS), and galactosides [19].
Figure 1.
Type of dietary fiber. MU: monomeric unit.
The unified recognition of oligosaccharides as dietary fiber greatly broadens the range of healthy indigestible carbohydrates available to humans. This may help to improve the accuracy of measurements in sustainable nutritional surveillance [20] and promote intake of foods rich in fiber naturally or artificially for filling up on fiber for health [10,21].
3. Average Levels and Recommended Amounts of Dietary Fiber Intake
Table 1 summarizes the updated average levels and recommended amounts of dietary fiber intake worldwide. Generally, the global average levels range from 15 to 26 g/day, lower than the recommended 20 to 35 g/day in most countries. As shown in Table 1, the highest levels of dietary fiber intake were observed in Northern Europe (e.g., Denmark [22], Norway [23]) Central Europe (e.g., Germany [24]), and Australia [25]), probably due to widespread consumption of whole grain rye, oat, and wheat in these countries [26].
Table 1.
Global intake and recommendations of dietary fiber.
| Region | Intake Level (g/day) | Reference | Recommendation (g/day) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Overall | Men | Women | Overall | |||
| Asia | ||||||||
| China | 19.4 | 17.6 | -- | China Health and Nutrition Survey (2011) [27] | -- | -- | 25–30 | Chinese Dietary Reference Intake (2017) [28] |
| Japan | 19.9 | 18.0 | 18.8 | National Health and Nutrition Survey in Japan (2019) [29] | 21 | 18 | 10 g/kcal | Dietary Reference Intake for Japanese (2020 Edition) [30] |
| North America | ||||||||
| USA | 18.1 | 15.2 | 16.6 | What We Eat in America (2017–2020) [31] | 38 | 25 | 14 g/kcal | Dietary Guidelines for Americans (2020–2025) [32] |
| Canada | 18.4 | 16.2 | -- | Canadian Community Health Survey (2015) [33] | 38 | 25 | -- | Canada’s Dietary Guidelines (2019) [34] |
| Oceania | ||||||||
| Australia | 24.8 | 21.1 | -- | Australian Health Survey (2011–2012) [25] | 38 | 28 | -- | Australian Dietary Guidelines (2013) [35] |
| Europe | ||||||||
| European Union | -- | -- | 25 | Scientific Opinion on Dietary Reference Values (EFSA-2010) [13] | ||||
| UK | -- | -- | 19.7 | UK National Diet and Nutrition Survey (2016–2019) [36] | -- | -- | 30 | Eatwell Guide (2016) [37] |
| France | 21.6 | 17.7 | 19.6 | National Individual Food Consumption 3 (2014–2015) [38] | -- | -- | 30 | Guidelines: development of nutritional references (2016) [39] |
| Denmark | -- | -- | 22 | Dietary habits in Denmark (2011–2013) [22] | 30 | 25 | 30 g/10MJ | Nordic Nutrition Recommendations (2012) [40] |
| Norway | 26 | 22 | 24 | Norkost 3 (2010–2011) [23] | ||||
| Germany | 24.8 | 23.1 | -- | National Consumption Research II (2005–2007) [24] | -- | -- | 30 or 14.6 g/kcal | The D-A-CH reference for nutrient intake (2021) [41] |
| Russia | 15 | 12 | -- | Russia Longitudinal Monitoring Survey (2018) [42] | -- | -- | 30 | Center for hygienic education of the population [43] |
Discrepancies in body size and tolerance to high-fiber diets across populations may also account for differences in average levels of intake and recommendations. For example, in Japan, the average dietary fiber intake is 18.0 g/day in women and 19.9 g/day in men, very close to the domestic recommended intake (18 g/day for women and 21 g/day for men) [29], but much lower than the average levels in Western populations. This may be explained by the relatively smaller bodies of the Japanese [44,45] and their habits of eating rice (refined grain) and seafood [46]. Many countries have adopted energy-adjusted levels of dietary fiber as the recommended amounts. As presented in Table 1, the daily recommended intake of dietary fiber is 14 g/kcal in the US [32], 14.6 g/kcal in Germany [41], and 10 g/kcal in Japan [30].
4. Fermentation of Dietary Fiber by the Gut Microbiota
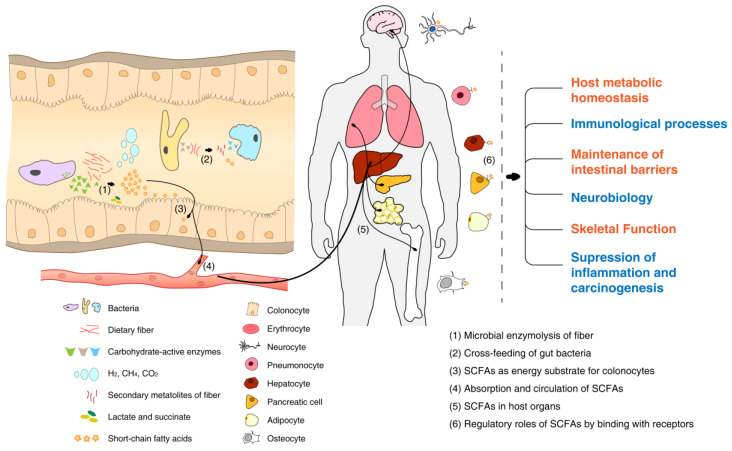
Dietary fibers escape digestion in the upper gastrointestinal tract and are fermented by bacteria in the colon. The degree of polymerization, particle size, solubility, viscosity, and other dietary fiber features may influence fiber fermentability and the bacteria specificity [47,48]. Fibers with low-degree polymerization can be degraded into small molecules in the gut with fast fermentation [47]; small particles are more likely to be exposed to microbial enzymes; while soluble, viscous fibers, with a high capacity for water retention and stool formation and thus limited exposure to microbes, are resistant to fermentation [47]. The various interactions between chains of monomers and enzymes influence the growth of bacteria, leading to the fiber-specific gut microbiota [48]. Generally, the saccharolytic degradation of fiber in the gut depends on the specific microbes rich in carbohydrate-active enzymes (CAZymes), mainly glycoside hydrolases (GHs) and polysaccharide lyases (PLs) [49], the two enzymes not existing in humans. As profiled in Figure 2, dietary fibers can be shared by some strains synergistically through cross-feeding, i.e., the breakdown products of polysaccharides partially hydrolyzed by the primary degraders can be used as substrates for the secondary degraders [50]. For example, inulin-type fructans (ITF) were found to be extracellularly hydrolyzed by Bifidobacteria in the human colon, liberating monosaccharides and/or oligosaccharides accessible to the butyrate producers, the secondary degraders [51,52,53]. During the utilization and metabolism of polysaccharides by bacteria, multiple metabolites were generated, including gases (e.g., H2, CH4, CO2), lactate, succinate, and short-chain fatty acids (SCFAs) [54,55].
Figure 2.
Fermentation of dietary fiber by gut microbiota.
The most abundant SCFAs are acetate, propionate, and butyrate. SCFAs can be used by gut mucosal cells as energy sources, with butyrate the preferred energy substrate for colonocytes [56,57]. The absorbed SCFAs, on the other hand, are transferred to the circulation via the hepatic portal vein to act as signaling molecules [58]. By binding to free fatty acid receptors (FFARs) or G protein-coupled receptors (GPCRs: GPR41/FFAR3, GPR43/FFAR2, GPR109A) of host cells [59], SCFAs can activate the complicated downstream molecular pathways in liver, brain, lung, pancreas, bones, adipose tissue, and other organs [55,60,61,62,63,64]. SCFAs play crucial regulatory roles in host metabolic homeostasis, immunological processes, maintenance of intestinal barriers, neurobiology, skeletal functions, and suppression of inflammation and carcinogenesis [55], and have been proved to be beneficial for human health. SCFAs were believed to be the crucial molecules that alleviated diabetes via greater postprandial glucagon-like peptide 1 (GLP-1) and fasting peptide YY (PYY) [65].
5. Impacts of Dietary Fiber on Gut Microbial Community Structure and Diversity
The fermentation of fiber in the colon is driven by the gut microbiota, and in return, the nutritional substrate of intestinal flora can modulate the structure and diversity of the microbiome. Results are consistent regarding the relationship between dietary fiber intake and the beta diversity of gut microbiota. People on fiber-rich diets (rural/unindustrialized diet, Mediterranean diet, or vegetarian diet) were consistently found to harbor a dramatically different microbial community structure from their counterparts living in developed areas [66,67,68]. Recently, a Chinese adult cohort study revealed a significant correlation between the intake of whole grains and vegetables in the habitual diet and the changes in beta diversity of the gut microbiome [69]. Similar shifts in bacterial composition were consistently observed in most intervention trials administering high-fiber diets [70,71,72,73,74,75,76].
As a diverse ecosystem with microbes functionally compensating for each other is more robust against environmental influences [77], higher microbial richness and evenness (i.e., alpha diversity) may represent a healthy community [78]. However, results are inconsistent on the association between dietary fiber and alpha diversity of gut microbiota. In a comparative study, adults from unindustrialized regions in Papua New Guinea accustomed to a plant-based diet were found to have a higher alpha diversity compared with American adults on Western diets [79]. While agrarian diets high in fruit/legume fiber were beneficial for microbial richness [80], the rapidly decreased dietary fiber in Western diets was believed to cause the loss of intestinal biodiversity [81]. However, among two independent populations from Washington DC and New York, Lin et al. [82] did not observe significant associations of total fiber intake with the Shannon index or evenness index. Long-term diet quality (with more fruit and vegetables) in Chinese adults was positively associated with microbiome alpha diversity [83], but frequent habitual intake of whole grains and vegetables was not found to increase the Shannon index in another Chinese population [69].
Results derived from trials were less consistent. In a trial in healthy Swedish volunteers, no significant intervention effect was observed for a 3-month vegetarian diet on the Shannon index compared with a normally omnivorous diet [84]. In randomized trials administering interventions with specific dietary fibers, the alpha diversity was observed to decrease in some target healthy populations [70,73,74,85,86,87,88,89,90], but was found to remain stable in some others [75,91,92,93]. In our previous study, we also observed reduced alpha diversity among diabetes patients taking a higher level of dietary fiber [94]. It seems that high-fiber diets may lead to the enrichment of specific fiber-digested strains: most are beneficial SCFA producers, which may inhibit the residence or growth of detrimental species and thereby demonstrate a temporary loss of alpha diversity [77].
6. Influences of Dietary Fiber on Different Gut Microbes
Dietary fiber intake improved the richness of SCFA producers, but demonstrated type-specific roles in microbial proliferation [72,74,85,88,91,95]. Interventions using inulin [72,87,96,97], guar gum [98], resistant starch [88,91,99], GOS [75,76,95,100], FOS [76], or arabinoxylan oligosaccharide (a kind of FOS) [85,101,102] consistently resulted in an increased abundance of Bifidobacterium, while intake of a specific fiber type led to the promotion of Faecalibacterium, Ruminococcus (particularly for RS), Lactobacillus (particularly for fibers containing galactose or fructose units), Akkermania, or Roseburia (Figure 1). Most microbial changes could be detected after 1- to 2-week interventions, but were found to remain stable throughout the whole period of interventions [75,96,98,101].
Theoretically, the increase in SCFA producers by fiber fermentation in the human colon should be followed by an increase in fecal SCFA concentrations [88,91,100,102]. However, numerous studies demonstrated the opposite result [70,72,75,76,85,86,88,89,90,96,97,103]. The increased fecal bulk due to high-fiber intake may dilute the concentration of SCFAs [104].
6.1. Bifidobacterium
The bifidogenic effect of dietary fiber determines its ability to increase various species belonging to the Bifidobacterium genus. Healey et al. [72] found that a supplement of inulin increased the relative abundance of Bifidobacterium from 6.69% to 15.07%. Kiewiet et al. [96] found that inulin mainly increased the abundance of Bifidobacterium adolescentis and raised Bifidobacterium angulatum and Bifidobacterium ruminantium to a detectable level in the treatment group. The consumption of inulin-rich food was also found to increase the Bifidobacterium longum level by threefold [87]. In addition, participants consuming 2-week partially hydrolyzed guar gum had an increased Bifidobacterium abundance to 12% from 8% at baseline, while those taking a placebo did not [98]. RS from potato led to a 6.5-fold elevated level of Bifidobacterium faecale/adolescentis/stercoris sequences [91].
GOS was reported to remarkably increase the relative abundance of Bifidobacterium from 7.0% to 34.8% [75]. Another study also observed a GOS-induced elevated bifidobacteria count from 7.2 to 7.8 (log 10 CFU/g). Adults treated with GOS and FOS had more abundant Bifidobacterium, with linear discriminant analysis effect sizes (LDA effect size) of around 4.0 compared to the baseline level [76]. Müller et al. [85] found that AXOS significantly increased two OTUs of Bifidobacterium, the leading drivers of the microbial deviations between pre- and postintervention.
6.2. Faecalibacterium
Many long-chain types of fiber demonstrated effects to enhance the abundance of Faecalibacterium. Inulin was found to increase the relative abundance of Faecalibacterium from 0.41% to 0.61% [72]. Partially hydrolyzed guar gum persistently caused an increment of Faecalibacterium during intervention and washout periods [74]. The consumption of whole-grain wheat rich in RS and total fiber induced more than a doubling of Faecalibacterium spp. [88]. Similar results were found by Hughes et al. [90]: an RS2-enriched wheat intervention was related to a significant elevation in Faecalibacterium compared to baseline and the control group.
6.3. Ruminococcus
The specific effect of RS2 on the proliferation of Ruminococcus was consistently demonstrated in previous studies. Baxter et al. [91] found that RS2 derived from native maize led to a 2.5-fold increase in the relative abundance of Ruminococcus bromii, a specific taxon that performed as a primary degrader of RS. Martínez et al. [99] noticed that subjects consuming maize RS2 had a significantly higher proportion of Ruminococcus bromii (average 4.1%) than at baseline (average 1.0%) and those taking placebo (average 2.6%) or chemically modified RS4 (average 1.2%). RS2-enriched wheat/whole-grain wheat was found to induce increases in the Ruminococcus genus and Ruminococcus bromii [88,90].
A similar Ruminococcus-induced effect was observed for other types of fiber. For examples, arabinogalactan supplementation resulted in a nearly eightfold increase in an uncultured Ruminococcus spp. [86]. Both Yasukawa et al. [98] and Reider et al. [74] observed an association of partially hydrolyzed guar gum with a bloom in Ruminococcus. Regarding inulin, a negative correlation was observed between the consumption and the richness of Ruminococcus bromii [91]. A randomized trial of 34 healthy participants demonstrated a decreased relative abundance of Ruminococcus (from 2.11% to 1.15%) by intervention with an ITF prebiotic [72]. Administration of yeast mannan, another type of gum, also contributed to a lower abundance of Ruminococcus compared with the control group [103].
6.4. Lactobacillus
Fibers containing fructose or galactose units such as inulin, fructo-oligosaccharides and galacto-oligosaccharides could particularly lead to a higher fecal abundance of Lactobacillus. An intervention using very-long-chain inulin, a special inulin with an average number of fructose units between 50 and 103, was found to increase lactobacilli levels by 2.42-fold compared to baseline and by 5.88-fold relative to the placebo group [97]. Another inulin-induced increment of Lactobacillus was observed in 34 healthy subjects (0.26% to 1.26%), and the effect was more pronounced (from 0.6% to 3.0%) in subjects with initial low habitual dietary fiber intake [72]. Deshipu stachyose granules, a mixture of GOS derived from the dietary roots of Lycopus lucidus turcz, were found to increase the mean number of lactobacilli from 6.8 to 7.5 (log 10 CFU/g) after 14-day treatment [95]. A lactobacilli-promoted effect was also observed for gum arabic [105] and arabinoxylan oligosaccharides (AXOS) [85].
6.5. Prevotella
Observational studies consistently identified a specific higher Prevotella in unindustrialized or rural populations consuming high-fiber diets, just like Hadza hunter-gatherers in Tanzania [106] and adults from Papua New Guinea [79], than those from industrialized regions or urban areas [66,79,106,107]. Therefore, Wu et al. [108] proposed the concept of gut microbial enterotypes, based on which gut microbiomes can be clustered as Prevotella enterotype (corresponding to dietary carbohydrates) and Bacteroides enterotype (corresponding to dietary protein and animal fat).
However, many intervention trials did not find that Prevotella could be induced by dietary fiber [108,109]. The discrepancy might be due to the ability of Prevotella to ferment complicated carbohydrates, not only indigestible fiber but also digestible carbohydrates, digested oligosaccharides, and monosaccharides [108], all of which were rich in the typical starchy plant food in unindustrialized areas [110]. It is the long-term intake of various carbohydrates in habitual diets but not the short-term single-fiber intervention that could construct a Prevotella-dominated microbial community [109,111]. The microbial differences could also be explained by the discrepancies in geography, ethnicity, and lifestyles across populations [112].
In summary, low-fiber diets have been suggested to influence the richness of the gut microbiome in healthy individuals, disrupt the symbiotic relationship between the gut microbiota and the intestine, and may increase the risk of diseases. High-fiber diets, on the other hand, have been used to modify the microbiota to achieve improved health outcomes.
7. Cutoff Threshold of Dietary Fiber Intake on Gut Microbiota
A dose-dependent bacterial proliferation with levels of fiber was demonstrated in vitro with significant growth, but only with detected metabolic promotion at a low level [113]. The relationship was also observed in population studies. Among 80 adults with an average of 14.1 ± 5.11 g/day intake of dietary fiber, Dominianni et al. [114] observed different compositions of gut microbiota in subjects with more than 11.7 g/day dietary fiber intake and found linear negative associations of fiber intake with Coprococcus and Porphyromonadaceae. Gaundal et al. [115] observed an inverse linear association of Alistipes with dietary fiber among subjects consuming more than 30 g/day dietary fiber and a positive correlation of Bacteroides stercoris with a total intake of healthy food components, such as fiber and grain products. In our previous study on diabetes patients, a mediating effect was observed for Desulfovibrio in the habitual dietary fiber–A1c associations among patients taking more than 7.2 g/day dietary fiber, but not in those taking less [94].
In intervention trials, administering multiple dosages of dietary fiber facilitate the evaluation of the type-specific dose-dependent microbial effects of dietary fiber and enabled the identification of cutoff thresholds. Summarized in Table 2 are the trials implementing multiple-dosage inulin, gum arabic, RS4, FOS, AXOS, or resistant maltodextrin. These trials demonstrated various altered taxa in gut microbiota induced by dietary fiber, but consistently observed an increased abundance of bifidobacteria, albeit with varied cutoff thresholds of fiber intake.
Table 2.
Cutoff thresholds of dietary fiber on gut microbiota in intervention trials.
| Author (Year) Region |
Participants N | F:M a Mean Age (Years) |
RCT Design |
Dietary Fiber |
Type of Fiber |
Administrated Dosage (g/day) |
Duration of Intervention |
Changes in Gut Microbiota b | Cutoff Threshold (g/day) |
|---|---|---|---|---|---|---|---|---|
| Kolida, S. [116] (2007) UK |
30 | 15:15 26.5 ± 3.1 |
double-blind crossover |
Inulin | NSP | 0 g 5 g 8 g |
2-week intervention 1-week washout |
↑: Bifidobacteria * Depending on initial abundance |
NO |
| Reimer, R. A. [117] (2020) Canada |
50 | 28:22 around 31 |
double-blind crossover |
Inulin-type fructans (ITF) | NSP | 0 g or 7 g (n = 25) 0 g or 3 g (n = 25) |
4-week intervention 4-week washout |
↔: α-diversity (Shannon index) ↔: β-diversity (Bray–Curtis distance) ↑: Bifidobacterium, Cellulomonas, Nesterenkonia, Brevibacterium ↓: Lachnospira, Oscillospira ↔: Fecal SCFAs |
7 g for Bifidobacterium, Cellulomonas, Nesterenkonia, Brevibacterium, Lachnospira, Oscillospira |
| Calame, W. [105] (2008) Netherlands |
48 | -- 30.9 ± 12.8 |
double-blind | Gum arabic | NSP | 0 g (placebo-control, n = 8) 5 g (n = 8) 10 g (n = 8) 20 g (n = 8) 40 g (n = 8) 10 g inulin (positive control, n = 8) |
4-week intervention |
↑: Bifidobacteria, lactobacilli, bacteroides | 10 g for bifidobacteria, lactobacilli, bacteroides |
| Deehan, E. C. [118] (2020) Canada |
40 | 20:20 28.4 ± 8.1 |
double-blind | RS4 | RS | Placebo (n = 10) Maize RS4 (n = 10) Potato RS4 (n = 10) Tapioca RS4 (n = 10) All 10 g for 1 week →20 g for 1 week →35 g for 1 week →50 g for 1 week |
4-week intervention |
In maize group: ↓: α-diversity (Pielou and Shannon index) ↕: β-diversity (Bray-Curtis) ↑: Bifidobacterium ↑: Eubacterium rectale, Oscillibacter, Anaeromassilibacillus, Ruminococcus ↓: Agathobaculum butyriciproducens, Adlercreutzia equolifaciens ↑: Fecal butyrate In tapioca group: ↓: α-diversity (Pielou and Shannon index) ↕: β-diversity (Bray-Curtis) ↑: Bifidobacterium ↑: Parabacteroides distasonis, Faecalibacterium, Eisenbergiella ↓: Eubacterium hallii and Clostridium viride ↑: Fecal propionate *No effect of potato RS4 detected |
In maize group: 20 g for α-diversity, β-diversity, Eubacterium rectale, Oscillibacter, Anaeromassilibacillus, Ruminococcus; 35 g for fecal butyrate; In tapioca group: 35 g for α-diversity, β-diversity, fecal propionate |
| Bouhnik, Y. [119] (1999) France |
40 | 22:18 29.6 |
Short-chain fructo-oligosaccharides (SC-FOS) |
ROS | 0 g (n = 8) 2.5 g (n = 8) 5 g (n = 8) 10 g (n = 8) 20 g (n = 8) |
7-day intervention |
↑: Bifidobacteria | 5 g for bifidobacteria | |
| Bouhnik, Y. [120] (2006) France |
40 | 22:18 29 ± 1.3 |
Short-chain fructo-oligosaccharides (SC-FOS) |
ROS | 0 g (n = 8) 2.5 g (n = 8) 5 g (n = 8) 7.5 g (n = 8) 10 g (n = 8) |
7-day intervention |
↑: Bifidobacteria (linear correlation) ↑: Total anaerobes |
10 g for total anaerobes | |
| Tandon, D. [121] (2019) India |
69 | 35:34 around 30 |
double-blind | Fructo-oligosaccharides (FOS) |
ROS | 0 g (n = 17) 2.5 g (n = 16) 5 g (n = 18) 10 g (n = 18) |
90-day intervention |
↑: α-diversity (Chao) ↑: Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcus, Sutterella, Oscillospira *Reversal impact of prebiotics postdiscontinuation *Inconsistent results of analyses performed on data at two distinct levels of taxonomy (OTUs or genus) |
10 g for α-diversity, Bifidobacterium, Lactobacillus, Faecalibacterium, Ruminococcus, Sutterella, Oscillospira |
| Maki, K. C. [122] (2012) The US |
55 | -- 53.1 ± 12.6 |
double-blind crossover |
Arabinoxylan-oligosaccharide (AXOS) |
ROS | 0 g 2.2 g 4.8 g |
3-week intervention 2-week washout |
↑: Bifidobacterium spp. | 4.8 g for Bifidobacterium spp. |
| François, I. E. [123] (2012) Belgium |
57 | 27:30 42 ± 17 |
double-blind crossover |
Arabinoxylan oligosaccharides (AXOS) | ROS | 0 g 2.4 g 8 g |
3-week intervention 2-week washout |
↑: Bifidobacteria ↑: Fecal SCFAs (acetic acid, propionic acid) |
8 g for bifidobacteria, fecal SCFAs |
| Fastinger, N. D. [104] (2008) The US |
38 | 19:19 around 27 |
double-blind | Resistant maltodextrin | ROS | 0 g (n = 12) 7.5 g (n = 13) 15 g (n = 13) |
3-week intervention |
↑: Bifidobacterium (nonsignificant) ↑: Proportion of propionic acid (linear) |
NO |
| Lefranc-Millot, C. [124] (2012) France |
48 | 24:24 28 |
double-blind | Resistant dextrin | ROS | 0 g(n = 12) 10 g (n = 13) 15 g (n = 12) 20 g (n = 11) |
2-week intervention |
↔: Bifidobacterium spp., Lactobacillus spp. ↑: Bacteroides ↓: Clostridium perfringens ↓: Colonic pH |
10 g for Bacteroides; 15 g for Clostridium perfringens; 20 g for colonic pH |
| Burns, A. M. [125] (2018) The US |
49 | 28:21 26.3 ± 6.8 |
double-blind cross-over |
Resistant maltodextrin | ROS | 0 g (n = 16) 15 g (n = 17) 25 g (n = 16) |
3-week intervention 2-week washout |
↑: Bifidobacteria | 25 g for bifidobacteria |
| Mai, V. [126] (2022) The US |
49 | 28:21 26.3 ± 6.8 |
double-blind cross-over |
Resistant maltodextrin | ROS | 0 g (n = 16) 15 g (n = 17) 25 g (n = 16) |
3-week intervention 2-week washout |
↑: Fusicatenibacter saccharivorans ↑: Akkermansia muciniphila, Faecalibacterium prausnitzii (in individuals with low baseline counts) |
25 g for Akkermansia muciniphila, Faecalibacterium prausnitzii |
a: Ratio of female subjects to male subjects; -- data not available. b: ↑ increased; ↓ decreased; ↔ unchanged; ↕ changed β-diversity.
In the report of Kolida et al. [116], 25 of 30 individuals taking 8 g/day inulin responded positively and only 20 of 30 in the group taking 5 g/day. In Reimer et al.’s study [117], subjects on 7 g/day ITF had higher levels of Bifidobacterium, Cellulomonas, Nesterenkonia, and Brevibacterium and lower Lachnospira and Oscillospira, with a cutoff threshold of 3 g/day for Bifidobacterium only. Calame et al. [105] used multiple dosages of gum arabic (0, 5, 10, 20, 40 g/day for 4 weeks) and found significantly increased bifidobacteria, lactobacilli and bacteroides in the volunteer group consuming 10 g/d gum arabic only, but not those on a higher dosage. Deehan et al. [118] observed remarkable microbial modulations by RS4 derived from maize and tapioca, particularly for the dosages of 20 g/day for maize and 35 g/day for tapioca.
The dose-dependent effect of fructo-oligosaccharides (FOS), a prebiotic supplement with bifidogenic capabilities, has been well evaluated. Bouhnik et al. [119] administrated 0 to 20 g/day short-chain FOS (SC-FOS) in healthy volunteers, and observed a dose-dependent increase in fecal bifidobacteria with a cutoff value of 5 g/day. Considering the tolerance of humans, the optimal dose of SC-FOS is 10 g/d for significantly elevated fecal bifidobacteria in healthy volunteers consuming their usual diet. In a subsequent trial, Bouhnik et al. [120] observed a linear correlation of bifidobacteria, with SC-FOS ranging from 0 to 10 g/day. Tandon et al. [121] identified multiple beneficial microbes promoted by FOS, including Bifidobacterium, Lactobacillus (predominated by Lactobacillus ruminis, which was newly classified as Ligilactobacillus ruminis [127]), Faecalibacterium, and Ruminococcus, with relatively higher increases at 10 g/day than the other three levels (0, 2.5, 5 g/day). The microbial effects of AXOS were evaluated in healthy men and women by Maki et al. [122] and François et al. [123], respectively. Bifidobacterium spp. and postprandial plasma ferulic acid were found to be significantly higher in subjects consuming 4.8 g/d AXOS than those on 2.2 g/d or 0 g/d (control) [122]. In comparison, bifidobacteria and SCFAs (acetic acid and propionic acid) increased after consumption of 8 g/day AXOS (not significant at 2.4 g/day) [123].
The microbiological properties of resistant maltodextrin were incompletely understood. No significant boost in Bifidobacterium but a linearly increased proportion of propionic acid in stool samples were observed in a trial administering 0, 7.5, 15 g/day resistant maltodextrin [104]. Lefranc-Millot et al. [124] did not find altered abundance of Bifidobacterium spp. or Lactobacillus spp., but apparent dose-dependent effects of resistant dextrin on Bacteroides (increased at 10 g/d only), Clostridium perfringens (decreased at 15 g/d only), and colonic pH (changed at 20 g/d only). Based on the same database, two studies obtained different results. In one study, only 25 g/day resistant maltodextrin consumption resulted in a positive change in bifidobacteria [125], while another analysis observed a slightly increased abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii among subjects taking 25 g/d resistant maltodextrin, but only among those with low baseline abundance of the bacteria [126]. It seems that the initial abundance of microbes resident in the human colon may determine the effects of fiber interventions [72,101,116]. Cloetens et al. [101] found that—regardless of the dosage used and the duration of intervention—AXOS supplementation succeeded in increasing Bifidobacterium in subjects with a low initial abundance, but failed in those without any detected fecal Bifidobacterium at baseline, further supporting the influences of the initial abundance of microbes.
8. Modulation of Dietary Fiber on Gut Microbiota in Diabetes Patients
Alterations in the human gut microbiota can be implicated in a variety of complex and chronic diseases, such as diabetes, obesity, cancers, and cardiovascular disease [5,6,7,8]. Type 2 diabetes is a metabolic disease that may affect and can be affected by gut microbiota [128], which represents a good example to show how dietary fiber induces modulation of the gut microbiota and improves clinical outcomes.
Table 3 presents selected trials administering fiber interventions among diabetes patients. The first trial focused on the effects of GOS by administering an intervention with low-dose GOS (5.5 g/day for 12 weeks) among 29 men with well-controlled type 2 diabetes [129]. As a result, a slightly elevated abundance of Bifidobacterium was observed, but without significant overall changes in gut microbiota or any improvement in glucose metabolisms. In 52 Japanese patients with type 2 diabetes, Gonai et al. [130] observed markedly restored abundance (taxa at the family level only) of Bifidobacteriaceae by GOS, but found reduced OTUs, Lachnospiraceae, Ruminococcaceae, Peptostreptococcaceae, Erysipelotrichaceae, and Porphyromonadaceae. Although no significant improvements in metabolic outcomes were observed in this study, the negative correlations of HbA1c levels with abundance of Bifidobacteriaceae and Peptostreptococcaceae, fasting plasma glucose (FPG) with Peptostreptococcaceae, and triglyceride (TG) with Ruminococcaceae, and the positive correlations of aspartate transaminase (AST) and alanine transaminase (ALT) with Lachnospiraceae indicate the roles of gut microbiota in metabolic homeostasis among diabetes patients.
Table 3.
Modulation of dietary fiber on gut microbiota and metabolic outcomes in patients with type 2 diabetes.
| Author Year Region |
Subjects | F:M a Mean Age (years) |
RCT Design |
Fiber Type or Sources | Amount of Fiber (g/day) |
Duration of Intervention |
Change in Gut Microbiota b | Metabolic Effects b | Microbiome and metabolic Indicators |
|---|---|---|---|---|---|---|---|---|---|
| Pedersen, C. [129] (2016) UK |
29 well-controlled men | All men around 57 |
Double-blind | galacto-oligosaccharide (GOS) | 5.5 (n = 14) placebo (n = 15) |
12 weeks | ↑: α-diversity (Shannon and Simpson indices) ↑: Bifidobacterium (close to significance) |
No significant effect on glucose, insulin, or C-peptide fasting concentrations | Bifidobacterium positively correlated with total AUC of glucose and IL-6 |
| Gonai, M. [130] (2017) Japan |
52 patients | -- 50 ± 10 |
Double-blind | galacto-oligosaccharide (GOS) | 10 (n = 27) placebo (n = 25) |
4 weeks | ↓: α-diversity (Observed OTUs) ↑: Bifidobacteriaceae ↓: Lachnospiraceae, Ruminococcaceae, Peptostreptococcaceae, Erysipelotrichaceae, Porphyromonadaceae |
No clinical parameters changed significantly | Negative correlations of A1c with Bifidobacteriaceae and Peptostreptococcaceae; FPG with Peptostreptococcaceae; TG with Uminococcaceae; positive correlations of AST and ALT with Lachnospiraceae |
| Zhao, L. [65] (2018) China |
43 patients | -- 35–70 |
Open-label | fiber in diet | high-fiber diet (n = 27) usual care (n = 16) |
84 days | ↕: β-diversity (Bray-Curtis) ↑: Bifidobacterium spp. and other SCFA producers ↑: CAZyme-encoding genes for starch and inulin degradation ↑: fhs for acetate and but for butyrate formation pathway ↑: acetate and butyrate |
↓: A1c ↑: Postprandial insulin |
Higher acetate and butyrate coincided with a significantly greater AUC of postprandial glucagon-like peptide-1 and a higher level of fasting peptide YY, which partly improve A1c level |
| Birkeland, E. [131] (2020) Norway |
25 patients | 10:15 63.1 |
Double-blinded, crossover | inulin-type fructans | 16 placebo |
6-week intervention 4-week washout |
↔: α-diversity (observed OTUs) ↕: β-diversity ↑: Bifidobacterium adolescentis, Bacteroides ovatus, Faecalibacterium prausnitzii ↓: Ruminococcus ↑: SCFAs (acetic acid and propionic acid) |
Bifidobacterium adolescentis negatively related to fecal butyric acid | |
| Mitchell, C. M. [132] (2021) US |
22 adults at risk for T2D |
14:8 54.4 ± 8.3 |
Double-blind | Inulin | 10 (n = 13) placebo (n = 9) |
6 weeks | ↑: Bifidobacteria | ↓: Fasting insulin, HOMA-IR | No significant correlation between changes in bifidobacteria and any outcome variables. |
| Reimer, R. A. [133] (2021) Canada |
290 adult patients with overweight/obesity |
198:92 around 55 |
Double-blind | soluble viscous fiber | 15–20 (n = 147) isocaloric placebo (n = 143) |
52 weeks | ↑: Collinsella, Parabacteroides, Roseburia ↓: Faecalibacterium, Lactobacillus, Oscillibacter |
↓: A1c, BMI, WC, LDL | |
| Mateo-Gallego, R. [134] (2021) Spain |
14 patients with overweight or obesity | -- 56.1 ± 6.27 |
Double-blinded, crossover | isomaltulose + resistant dextrin | 16.5 + 5.28 placebo |
10-week intervention 6–8 weeks’ washout |
↔: α-diversity (Shannon, Pielou, Observed features) ↔: β-diversity (weighted Unifrac) ↑: Parabacteroides ↓: Bacteroides, Odoribacter, Butyricimonas, Oscillospira |
↓: BMI, Blood glucose, HOMA-IR |
a: Ratio of female subjects to male subjects; -- data not available. b: ↑ increased; ↓ decreased; ↔ unchanged; ↕ changed β-diversity.
In a milestone intervention trial conducted in Chinese diabetes patients, Zhao et al. [65] found that a diet with a high mixture of fibers selectively promoted SCFA-producing strains, particularly fecal Bifidobacterium spp., leading to enriched CAZyme-encoding genes for starch and inulin degradation and activated pathways for acetate and butyrate formation, and thus achieved a better improvement in HbA1c levels and postprandial insulin concentrations. More trials were subsequently designed to evaluate the intervention effects of specific types of dietary fiber. In a pilot study of 22 high-risk adults for type 2 diabetes, Mitchell et al. [132] observed significantly increased bifidobacteria and reduced fasting insulin level and homeostatic model of assessment for insulin resistance (HOMA-IR) after a 6-week inulin (10 g/day) intervention, but did not find a significant correlation of changes in bifidobacteria with any metabolic outcomes. In another pilot study, Mateo-Gallego et al. [134] added isomaltulose (16.5 g/day) and resistant maltodextrin (5.28 g/day) into alcohol-free beer for 14 diabetes subjects for 10 weeks, and observed higher Parabacteroides and lower Bacteroides, Odoribacter, Butyricimonas and Oscillospira accompanying decreased body mass index, blood glucose level and HOMA-IR.
In recent years, well-designed RCTs have supported beneficial alternations in gut microbiota induced by specific types of fiber in diabetes patients. A one-year intervention using a soluble viscous fiber product containing 15–20 g/day dietary fiber achieved significantly increased Collinsella, Parabacteroides and Roseburia, but decreased Faecalibacterium, Lactobacillus, and Oscillibacte, and thus improved metabolic outcomes of diabetes patients, including decreased levels of body mass index, waist circumference, HbA1c and LDL [133]. Birkeland et al. [131] evaluated the prebiotic effect of ITF on the fecal microbiota and produced SCFAs in 25 patients with type 2 diabetes. A prominent bifidogenic effect was observed, with the highest positive effect on OTUs of Bifidobacterium adolescentis, followed by OTUs of Bacteroides. Analogous effects were also observed among prediabetes individuals administered beta glucan, inulin, RS and GOS [135,136].
Evidently, altering the gut microbiota of diabetes patients has great potential to improve glycemic control status, and can be used as an effective intervention for patients [137]. However, alpha diversity of gut microbiota was observed to remain unchanged or decrease in patients placed on a high-fiber diet. Bifidobacterium was the only beneficial fiber-enriched microbe consistently induced by fiber interventions. It is possible that—due to prevalent diabetes or related conditions—the gut microbiome may have been altered, leading to serious depletion of beneficial bacteria and microbial dysbiosis that could not be easily promoted by dietary interventions [128,138,139]. As the key roles of SCFAs in metabolic enhancement have been highlighted, more approaches should be developed and validated to improve the abundance of microbial SCFAs producers.
9. Conclusions and Future Prospects
Evidence is accumulating on the beneficial effects of dietary fiber intake on human health. The mechanisms involved have been better understood in recent years, indicating the critical role of the gut microbiota in this process via the production of SCFAs and other functional metabolites. The decreasing dietary fiber intake over the centuries has fostered a gut microbiota detrimental to human health, leading to a global epidemic of diabetes, cancers, and other noncommunicable diseases. The responses of the gut microbiota to increased availability of dietary fiber may differ by type, level, and duration of intake, demonstrating dietary fiber type-specific cutoff thresholds. Understanding the intricate interplay between dietary fiber and the gut microbiota may help to develop effective intervention strategies to prevent and control noncommunicable diseases. Further studies are warranted to further elucidate this complex relationship in human health and identify targets for effective interventions.
Acknowledgments
We are grateful to the staff and graduate students of the Department of Epidemiology, Fudan University School of Public Health for their helpful comments and suggestions.
Author Contributions
Conceptualization and supervision, W.X.; writing—original draft preparation, J.F.; writing—review and editing, W.X., Y.Z. and Y.G. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant 81773504.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrews P., Johnson R.J. Evolutionary basis for the human diet: Consequences for human health. J. Intern. Med. 2020;287:226–237. doi: 10.1111/joim.13011. [DOI] [PubMed] [Google Scholar]
- 2.Eaton S.B., Eaton S.B., 3rd, Konner M.J. Paleolithic nutrition revisited: A twelve-year retrospective on its nature and implications. Eur. J. Clin. Nutr. 1997;51:207–216. doi: 10.1038/sj.ejcn.1600389. [DOI] [PubMed] [Google Scholar]
- 3.Amato K.R., Jeyakumar T., Poinar H., Gros P. Shifting Climates, Foods, and Diseases: The Human Microbiome through Evolution. BioEssays News Rev. Mol. Cell. Dev. Biol. 2019;41:e1900034. doi: 10.1002/bies.201900034. [DOI] [PubMed] [Google Scholar]
- 4.Malesza I.J., Malesza M., Walkowiak J., Mussin N., Walkowiak D., Aringazina R., Bartkowiak-Wieczorek J., Mądry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10:3164. doi: 10.3390/cells10113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Z., Zhang L., Yang L., Chu H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022;13:1025706. doi: 10.3389/fendo.2022.1025706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadopoulos P.D., Tsigalou C., Valsamaki P.N., Konstantinidis T.G., Voidarou C., Bezirtzoglou E. The Emerging Role of the Gut Microbiome in Cardiovascular Disease: Current Knowledge and Perspectives. Biomedicines. 2022;10:948. doi: 10.3390/biomedicines10050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye J., Wu Z., Zhao Y., Zhang S., Liu W., Su Y. Role of gut microbiota in the pathogenesis and treatment of diabetes mullites: Advanced research-based review. Front. Microbiol. 2022;13:1029890. doi: 10.3389/fmicb.2022.1029890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joint FAO/WHO Food Standards Programme . Secretariat of the CODEX Alimentarius Commission: CODEX Alimentarius (CODEX) Guidelines on Nutrition Labeling CAC/GL 2-1985 as Last Amended 2010. FAO; Rome, Italy: 2010. [Google Scholar]
- 10.de Menezes E.W., Giuntini E.B., Dan M.C.T., Sardá F.A.H., Lajolo F.M. Codex dietary fibre definition–Justification for inclusion of carbohydrates from 3 to 9 degrees of polymerisation. Food Chem. 2013;140:581–585. doi: 10.1016/j.foodchem.2013.02.075. [DOI] [PubMed] [Google Scholar]
- 11.Dai F.J., Chau C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017;25:37–42. doi: 10.1016/j.jfda.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephen A.M., Champ M.M., Cloran S.J., Fleith M., van Lieshout L., Mejborn H., Burley V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017;30:149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 13.EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010;8:1462. [Google Scholar]
- 14.Rezende E.S.V., Lima G.C., Naves M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition. 2021;89:111217. doi: 10.1016/j.nut.2021.111217. [DOI] [PubMed] [Google Scholar]
- 15.Kumar V., Sinha A.K., Makkar H.P., de Boeck G., Becker K. Dietary roles of non-starch polysaccharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012;52:899–935. doi: 10.1080/10408398.2010.512671. [DOI] [PubMed] [Google Scholar]
- 16.Tungland B.C., Meyer D. Nondigestible Oligo- and Polysaccharides (Dietary Fiber): Their Physiology and Role in Human Health and Food. Compr. Rev. Food Sci. Food Saf. 2002;1:90–109. doi: 10.1111/j.1541-4337.2002.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 17.Roberfroid M.B. Inulin-type fructans: Functional food ingredients. J. Nutr. 2007;137((Suppl. 11)):2493s–2502s. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- 18.Raigond P., Ezekiel R., Raigond B. Resistant starch in food: A review. J. Sci. Food Agric. 2015;95:1968–1978. doi: 10.1002/jsfa.6966. [DOI] [PubMed] [Google Scholar]
- 19.Mussatto S.I., Mancilha I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007;68:587–597. doi: 10.1016/j.carbpol.2006.12.011. [DOI] [Google Scholar]
- 20.Miller K.B. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr. Rev. 2020;78((Suppl. 1)):29–36. doi: 10.1093/nutrit/nuz052. [DOI] [PubMed] [Google Scholar]
- 21.Jones J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014;13:34. doi: 10.1186/1475-2891-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agnes N.P., Christensen T., Matthiessen J., Knudsen V.K., Rosenlund-Sørensen M., Biltoft-Jensen A., Hinsch H., Ygil K.H., Kørup K., Saxholt E., et al. Dietary Habits in Denmark 2011–2013: Main Results. 2015. [(accessed on 25 October 2022)]. Available online: https://www.food.dtu.dk/publikationer/ernaering-og-kostvaner/de_nationale_kostundersoegelser.
- 23.Totland T.H., Melnæs B.K., Lundberg-Hallén N., Helland-Kigen K.M., Lund-Blix N.A., Myhre J.B., Johansen A.M.W., Løken E.B., Andersen L.F. Norkost 3–En Landsomfattende Kostholdsundersøkelse Blant Menn og Kvinner i Norge i Alderen 18–70 år 2010–11. 2012. [(accessed on 25 October 2022)]. Available online: https://www.helsedirektoratet.no/search?searchquery=Norkost%203.
- 24.Max Rubner-Institut . Die Nationale Verzehrsstudie II Abschlussbericht Teil 2 (Diet-History-Interviews) Max Rubner-Institut; Karlsruhe, Germany: 2008. [Google Scholar]
- 25.Australian Bureau of Statistics Australian Health Survey: Nutrition First Results-Foods and Nutrients. [(accessed on 25 October 2022)]; Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-nutrition-first-results-foods-and-nutrients/2011-12/Table%201%20Mean%20daily%20energy%20and%20nutrient%20intake.xls.
- 26.Frølich W., Aman P., Tetens I. Whole grain foods and health–A Scandinavian perspective. Food Nutr. Res. 2013:57. doi: 10.3402/fnr.v57i0.18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H.J., Wang Z.H., Zhang J.G., Du W.W., Su C., Zhang J., Zhai F.Y., Zhang B. Trends in dietary fiber intake in Chinese aged 45 years and above, 1991–2011. Eur. J. Clin. Nutr. 2014;68:619–622. doi: 10.1038/ejcn.2014.24. [DOI] [PubMed] [Google Scholar]
- 28.National Health and Family Planning Commission Chinese Dietary Reference Intakes— Part 1: Macronutrient. [(accessed on 25 October 2022)];2017 Available online: http://www.nhc.gov.cn/wjw/yingyang/201710/fdade20feb8144ba921b412944ffb779.shtml.
- 29.Ministry of Health, Labour and Welfare The National Health and Nutrition Survey in Japan, 2019. 2019. [(accessed on 25 October 2022)]. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html.
- 30.Ministry of Health, Labour and Welfare Dietary Reference Intakes for Japanese, (2020 Edition) 2020. [(accessed on 25 October 2022)]. Available online: https://www.mhlw.go.jp/stf/newpage_08517.html.
- 31.U.S. Department of Agriculture, Agricultural Research Service Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Gender and Age. [(accessed on 25 October 2022)];2022 Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables.
- 32.U.S. Department of Agriculture. U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020–2025, 9th ed.; 2020. [(accessed on 25 October 2022)]; Available online: https://www.dietaryguidelines.gov.
- 33.Ahmed M., Praneet Ng A., L’Abbe M.R. Nutrient intakes of Canadian adults: Results from the Canadian Community Health Survey (CCHS)-2015 Public Use Microdata File. Am. J. Clin. Nutr. 2021;114:1131–1140. doi: 10.1093/ajcn/nqab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Health Canada Canada’s Dietary Guidelines: For Health Professionals and Policy Makers. 2019. [(accessed on 25 October 2022)]. Available online: https://food-guide.canada.ca/en/guidelines.
- 35.National Health and Medical Research Council, Department of Health and Aged Care Australian Dietary Guidelines-Providing the Scientific Evidence for Healthier Australian Diets. [(accessed on 25 October 2022)];2013 Available online: https://www.eatforhealth.gov.au/guidelines.
- 36.Public Health England NDNS: Results from Years 9 to 11 (Combined)–Statistical Summary. [(accessed on 25 October 2022)];2020 Available online: https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019/ndns-results-from-years-9-to-11-combined-statistical-summary.
- 37.Public Health England Government Dietary Recommendations. [(accessed on 25 October 2022)];2016 Available online: https://www.gov.uk/government/publications/the-eatwell-guide.
- 38.INCA 3: Evolution des Habitudes et Modes de Consommation, de Nouveaux Enjeux en Matière de Sécurité Sanitaire et de Nutrition. [(accessed on 25 October 2022)]. Available online: https://www.anses.fr/fr/content/inca-3-evolution-des-habitudes-et-modes-de-consommation-de-nouveaux-enjeux-en-mati%C3%A8re-de.
- 39.Agence Nationale de Sécurité Sanitaire Actualisation des Repères du PNNS: Élaboration des Références Nutritionnelles. 2016. [(accessed on 25 October 2022)]. Available online: https://www.anses.fr/en/system/files/NUT2012SA0103Ra-2.pdf.
- 40.Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity. 5th ed. Nordisk Ministerråd; Copenhagen, Denmark: 2014. p. 627. [Google Scholar]
- 41.Ballaststoffe (Nahrungsfasern): Richtwerte für die Zufuhr. [(accessed on 25 October 2022)]. Available online: https://www.dge.de/wissenschaft/referenzwerte/ballaststoffe/
- 42.Baturin A.K., Martinchik A.N., Kambarov A.O. The transit of Russian nation nutrition at the turn of the 20th and 21st centuries. Vopr. Pitan. 2020;89:60–70. doi: 10.24411/0042-8833-2020-10042. [DOI] [PubMed] [Google Scholar]
- 43.Federal Public Health Institution, Centre for Hygiene Education Nutrients/Fiber. [(accessed on 25 October 2022)]. Available online: http://cgon.rospotrebnadzor.ru/content/sostav-pitaniya/pishevaya-kletchatka.
- 44.Grasgruber P., Hrazdíra E. Nutritional and socio-economic predictors of adult height in 152 world populations. Econ. Hum. Biol. 2020;37:100848. doi: 10.1016/j.ehb.2020.100848. [DOI] [PubMed] [Google Scholar]
- 45.Ihira H., Sawada N., Iwasaki M., Yamaji T., Goto A., Noda M., Iso H., Tsugane S. Adult height and all-cause and cause-specific mortality in the Japan Public Health Center-based Prospective Study (JPHC) PLoS ONE. 2018;13:e0197164. doi: 10.1371/journal.pone.0197164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuyama S., Sawada N., Tomata Y., Zhang S., Goto A., Yamaji T., Iwasaki M., Inoue M., Tsuji I., Tsugane S. Association between adherence to the Japanese diet and all-cause and cause-specific mortality: The Japan Public Health Center-based Prospective Study. Eur. J. Nutr. 2021;60:1327–1336. doi: 10.1007/s00394-020-02330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill S.K., Rossi M., Bajka B., Whelan K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021;18:101–116. doi: 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- 48.Tuncil Y.E., Thakkar R.D., Arioglu-Tuncil S., Hamaker B.R., Lindemann S.R. Subtle Variations in Dietary-Fiber Fine Structure Differentially Influence the Composition and Metabolic Function of Gut Microbiota. mSphere. 2020;5:e00180-20. doi: 10.1128/mSphere.00180-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndeh D., Gilbert H.J. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol. Rev. 2018;42:146–164. doi: 10.1093/femsre/fuy002. [DOI] [PubMed] [Google Scholar]
- 50.Falony G., De Vuyst L. Ecological Interactions of Bacteria in the Human Gut. In: Charalampopoulos D., Rastall R.A., editors. Prebiotics and Probiotics Science and Technology. Springer; New York, NY, USA: 2009. pp. 639–679. [Google Scholar]
- 51.Turroni F., Milani C., Duranti S., Mahony J., van Sinderen D., Ventura M. Glycan Utilization and Cross-Feeding Activities by Bifidobacteria. Trends Microbiol. 2018;26:339–350. doi: 10.1016/j.tim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 52.De Vuyst L., Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 2011;149:73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Rivière A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 56.Zeng H., Umar S., Rust B., Lazarova D., Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019;20:1214. doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar J., Rani K., Datt C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020;47:6229–6237. doi: 10.1007/s11033-020-05611-3. [DOI] [PubMed] [Google Scholar]
- 60.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 61.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 62.Mandaliya D.K., Seshadri S. Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes. Pancreatol. Off. J. Int. Assoc. Pancreatol. (IAP) 2019;19:280–284. doi: 10.1016/j.pan.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 63.Lucas S., Omata Y., Hofmann J., Böttcher M., Iljazovic A., Sarter K., Albrecht O., Schulz O., Krishnacoumar B., Krönke G., et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018;9:55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X., Fu H., Xue X., Lu C., Ma J., et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science (New York N.Y.) 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 66.De Filippo C., Di Paola M., Ramazzotti M., Albanese D., Pieraccini G., Banci E., Miglietta F., Cavalieri D., Lionetti P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017;8:1979. doi: 10.3389/fmicb.2017.01979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merra G., Noce A., Marrone G., Cintoni M., Tarsitano M.G., Capacci A., De Lorenzo A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020;13:19–25. doi: 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruengsomwong S., La-Ongkham O., Jiang J., Wannissorn B., Nakayama J., Nitisinprasert S. Microbial Community of Healthy Thai Vegetarians and Non-Vegetarians, Their Core Gut Microbiota, and Pathogen Risk. J. Microbiol. Biotechnol. 2016;26:1723–1735. doi: 10.4014/jmb.1603.03057. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Chen H., Lu M., Cai J., Lu B., Luo C., Dai M. Habitual Diet Pattern Associations with Gut Microbiome Diversity and Composition: Results from a Chinese Adult Cohort. Nutrients. 2022;14:2639. doi: 10.3390/nu14132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.An R., Wilms E., Smolinska A., Hermes G.D.A., Masclee A.A.M., de Vos P., Schols H.A., van Schooten F.J., Smidt H., Jonkers D., et al. Sugar Beet Pectin Supplementation Did Not Alter Profiles of Fecal Microbiota and Exhaled Breath in Healthy Young Adults and Healthy Elderly. Nutrients. 2019;11:2193. doi: 10.3390/nu11092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandeputte D., Falony G., Vieira-Silva S., Wang J., Sailer M., Theis S., Verbeke K., Raes J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66:1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Healey G., Murphy R., Butts C., Brough L., Whelan K., Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018;119:176–189. doi: 10.1017/S0007114517003440. [DOI] [PubMed] [Google Scholar]
- 73.Clarke S.T., Brooks S.P.J., Inglis G.D., Yanke L.J., Green J., Petronella N., Ramdath D.D., Bercik P., Green-Johnson J.M., Kalmokoff M. Impact of β2-1 fructan on faecal community change: Results from a placebo-controlled, randomised, double-blinded, cross-over study in healthy adults. Br. J. Nutr. 2017;118:441–453. doi: 10.1017/S0007114517002318. [DOI] [PubMed] [Google Scholar]
- 74.Reider S.J., Moosmang S., Tragust J., Trgovec-Greif L., Tragust S., Perschy L., Przysiecki N., Sturm S., Tilg H., Stuppner H., et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota-Results from the PAGODA Trial. Nutrients. 2020;12:1257. doi: 10.3390/nu12051257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilms E., An R., Smolinska A., Stevens Y., Weseler A.R., Elizalde M., Drittij M.J., Ioannou A., van Schooten F.J., Smidt H., et al. Galacto-oligosaccharides supplementation in prefrail older and healthy adults increased faecal bifidobacteria, but did not impact immune function and oxidative stress. Clin. Nutr. (Edinb. Scotl.) 2021;40:3019–3031. doi: 10.1016/j.clnu.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 76.Liu F., Li P., Chen M., Luo Y., Prabhakar M., Zheng H., He Y., Qi Q., Long H., Zhang Y., et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017;7:11789. doi: 10.1038/s41598-017-10722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ (Clin. Res. Ed.) 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.The Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martínez I., Stegen J.C., Maldonado-Gómez M.X., Eren A.M., Siba P.M., Greenhill A.R., Walter J. The gut microbiota of rural papua new guineans: Composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–538. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 80.Simpson H.L., Campbell B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segata N. Gut Microbiome: Westernization and the Disappearance of Intestinal Diversity. Curr. Biol. CB. 2015;25:R611-3. doi: 10.1016/j.cub.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 82.Lin D., Peters B.A., Friedlander C., Freiman H.J., Goedert J.J., Sinha R., Miller G., Bernstein M.A., Hayes R.B., Ahn J. Association of dietary fibre intake and gut microbiota in adults. Br. J. Nutr. 2018;120:1014–1022. doi: 10.1017/S0007114518002465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu D., Nguyen S.M., Yang Y., Xu W., Cai H., Wu J., Cai Q., Long J., Zheng W., Shu X.O. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am. J. Clin. Nutr. 2021;113:684–694. doi: 10.1093/ajcn/nqaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang C., Björkman A., Cai K., Liu G., Wang C., Li Y., Xia H., Sun L., Kristiansen K., Wang J., et al. Impact of a 3-Months Vegetarian Diet on the Gut Microbiota and Immune Repertoire. Front. Immunol. 2018;9:908. doi: 10.3389/fimmu.2018.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Müller M., Hermes G.D.A., Emanuel E.C., Holst J.J., Zoetendal E.G., Smidt H., Troost F., Schaap F.G., Damink S.O., Jocken J.W.E., et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: A randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes. 2020;12:1704141. doi: 10.1080/19490976.2019.1704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen O., Sudakaran S., Blonquist T., Mah E., Durkee S., Bellamine A. Effect of arabinogalactan on the gut microbiome: A randomized, double-blind, placebo-controlled, crossover trial in healthy adults. Nutrition. 2021;90:111273. doi: 10.1016/j.nut.2021.111273. [DOI] [PubMed] [Google Scholar]
- 87.Hiel S., Bindels L.B., Pachikian B.D., Kalala G., Broers V., Zamariola G., Chang B.P.I., Kambashi B., Rodriguez J., Cani P.D., et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019;109:1683–1695. doi: 10.1093/ajcn/nqz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gondalia S.V., Wymond B., Benassi-Evans B., Berbezy P., Bird A.R., Belobrajdic D.P. Substitution of Refined Conventional Wheat Flour with Wheat High in Resistant Starch Modulates the Intestinal Microbiota and Fecal Metabolites in Healthy Adults: A Randomized, Controlled Trial. J. Nutr. 2022;152:1426–1437. doi: 10.1093/jn/nxac021. [DOI] [PubMed] [Google Scholar]
- 89.DeMartino P., Johnston E.A., Petersen K.S., Kris-Etherton P.M., Cockburn D.W. Additional Resistant Starch from One Potato Side Dish per Day Alters the Gut Microbiota but Not Fecal Short-Chain Fatty Acid Concentrations. Nutrients. 2022;14:721. doi: 10.3390/nu14030721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes R.L., Horn W.H., Finnegan P., Newman J.W., Marco M.L., Keim N.L., Kable M.E. Resistant Starch Type 2 from Wheat Reduces Postprandial Glycemic Response with Concurrent Alterations in Gut Microbiota Composition. Nutrients. 2021;13:645. doi: 10.3390/nu13020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baxter N.T., Schmidt A.W., Venkataraman A., Kim K.S., Waldron C., Schmidt T.M. Dynamics of Human Gut Microbiota and Short-Chain Fatty Acids in Response to Dietary Interventions with Three Fermentable Fibers. mBio. 2019;10:e02566-18. doi: 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L., Ouyang Y., Li H., Shen L., Ni Y., Fang Q., Wu G., Qian L., Xiao Y., Zhang J., et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019;9:4736. doi: 10.1038/s41598-018-38216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez J., Neyrinck A.M., Zhang Z., Seethaler B., Nazare J.A., Robles Sánchez C., Roumain M., Muccioli G.G., Bindels L.B., Cani P.D., et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes. 2020;12:1810530. doi: 10.1080/19490976.2020.1810530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu J., Xu K., Ni X., Li X., Zhu X., Xu W. Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes. Nutrients. 2022;14:1003. doi: 10.3390/nu14051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li T., Lu X., Yang X. Evaluation of clinical safety and beneficial effects of stachyose-enriched α-galacto-oligosaccharides on gut microbiota and bowel function in humans. Food Funct. 2017;8:262–269. doi: 10.1039/C6FO01290F. [DOI] [PubMed] [Google Scholar]
- 96.Kiewiet M.B.G., Elderman M.E., El Aidy S., Burgerhof J.G.M., Visser H., Vaughan E.E., Faas M.M., de Vos P. Flexibility of Gut Microbiota in Ageing Individuals during Dietary Fiber Long-Chain Inulin Intake. Mol. Nutr. Food Res. 2021;65:e2000390. doi: 10.1002/mnfr.202000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costabile A., Kolida S., Klinder A., Gietl E., Bäuerlein M., Frohberg C., Landschütze V., Gibson G.R. A double-blind, placebo-controlled, cross-over study to establish the bifidogenic effect of a very-long-chain inulin extracted from globe artichoke (Cynara scolymus) in healthy human subjects. Br. J. Nutr. 2010;104:1007–1017. doi: 10.1017/S0007114510001571. [DOI] [PubMed] [Google Scholar]
- 98.Yasukawa Z., Inoue R., Ozeki M., Okubo T., Takagi T., Honda A., Naito Y. Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial. Nutrients. 2019;11:2170. doi: 10.3390/nu11092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martínez I., Kim J., Duffy P.R., Schlegel V.L., Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vulevic J., Juric A., Walton G.E., Claus S.P., Tzortzis G., Toward R.E., Gibson G.R. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br. J. Nutr. 2015;114:586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 101.Cloetens L., Broekaert W.F., Delaedt Y., Ollevier F., Courtin C.M., Delcour J.A., Rutgeerts P., Verbeke K. Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: A randomised, placebo-controlled cross-over study. Br. J. Nutr. 2010;103:703–713. doi: 10.1017/S0007114509992248. [DOI] [PubMed] [Google Scholar]
- 102.Walton G.E., Lu C., Trogh I., Arnaut F., Gibson G.R. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr. J. 2012;11:36. doi: 10.1186/1475-2891-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tanihiro R., Sakano K., Oba S., Nakamura C., Ohki K., Hirota T., Sugiyama H., Ebihara S., Nakamura Y. Effects of Yeast Mannan Which Promotes Beneficial Bacteroides on the Intestinal Environment and Skin Condition: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2020;12:3673. doi: 10.3390/nu12123673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fastinger N.D., Karr-Lilienthal L.K., Spears J.K., Swanson K.S., Zinn K.E., Nava G.M., Ohkuma K., Kanahori S., Gordon D.T., Fahey G.C., Jr. A novel resistant maltodextrin alters gastrointestinal tolerance factors, fecal characteristics, and fecal microbiota in healthy adult humans. J. Am. Coll. Nutr. 2008;27:356–366. doi: 10.1080/07315724.2008.10719712. [DOI] [PubMed] [Google Scholar]
- 105.Calame W., Weseler A.R., Viebke C., Flynn C., Siemensma A.D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 2008;100:1269–1275. doi: 10.1017/S0007114508981447. [DOI] [PubMed] [Google Scholar]
- 106.Schnorr S.L., Candela M., Rampelli S., Centanni M., Consolandi C., Basaglia G., Turroni S., Biagi E., Peano C., Severgnini M., et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mancabelli L., Milani C., Lugli G.A., Turroni F., Ferrario C., van Sinderen D., Ventura M. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ. Microbiol. 2017;19:1379–1390. doi: 10.1111/1462-2920.13692. [DOI] [PubMed] [Google Scholar]
- 108.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R., et al. Linking long-term dietary patterns with gut microbial enterotypes. Science (New York N.Y.) 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clemente-Suárez V.J., Mielgo-Ayuso J., Martín-Rodríguez A., Ramos-Campo D.J., Redondo-Flórez L., Tornero-Aguilera J.F. The Burden of Carbohydrates in Health and Disease. Nutrients. 2022;14:3809. doi: 10.3390/nu14183809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fragiadakis G.K., Wastyk H.C., Robinson J.L., Sonnenburg E.D., Sonnenburg J.L., Gardner C.D. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight. Am. J. Clin. Nutr. 2020;111:1127–1136. doi: 10.1093/ajcn/nqaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu J., Zhang L., Zhai Q., Zhao J., Zhang H., Lee Y.K., Lu W., Li M., Chen W. Chinese gut microbiota and its associations with staple food type, ethnicity, and urbanization. NPJ Biofilms Microbiomes. 2021;7:71. doi: 10.1038/s41522-021-00245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sasaki D., Sasaki K., Ikuta N., Yasuda T., Fukuda I., Kondo A., Osawa R. Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci. Rep. 2018;8:435. doi: 10.1038/s41598-017-18877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dominianni C., Sinha R., Goedert J.J., Pei Z., Yang L., Hayes R.B., Ahn J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE. 2015;10:e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gaundal L., Myhrstad M.C.W., Rud I., Gjøvaag T., Byfuglien M.G., Retterstøl K., Holven K.B., Ulven S.M., Telle-Hansen V.H. Gut microbiota is associated with dietary intake and metabolic markers in healthy individuals. Food Nutr. Res. 2022:66. doi: 10.29219/fnr.v66.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kolida S., Meyer D., Gibson G.R. A double-blind placebo-controlled study to establish the bifidogenic dose of inulin in healthy humans. Eur. J. Clin. Nutr. 2007;61:1189–1195. doi: 10.1038/sj.ejcn.1602636. [DOI] [PubMed] [Google Scholar]
- 117.Reimer R.A., Soto-Vaca A., Nicolucci A.C., Mayengbam S., Park H., Madsen K.L., Menon R., Vaughan E.E. Effect of chicory inulin-type fructan-containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am. J. Clin. Nutr. 2020;111:1286–1296. doi: 10.1093/ajcn/nqaa074. [DOI] [PubMed] [Google Scholar]
- 118.Deehan E.C., Yang C., Perez-Muñoz M.E., Nguyen N.K., Cheng C.C., Triador L., Zhang Z., Bakal J.A., Walter J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe. 2020;27:389–404.e6. doi: 10.1016/j.chom.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 119.Bouhnik Y., Vahedi K., Achour L., Attar A., Salfati J., Pochart P., Marteau P., Flourié B., Bornet F., Rambaud J.C. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J. Nutr. 1999;129:113–116. doi: 10.1093/jn/129.1.113. [DOI] [PubMed] [Google Scholar]
- 120.Bouhnik Y., Raskine L., Simoneau G., Paineau D., Bornet F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: A dose-response relationship study in healthy humans. Nutr. J. 2006;5:8. doi: 10.1186/1475-2891-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tandon D., Haque M.M., Gote M., Jain M., Bhaduri A., Dubey A.K., Mande S.S. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci. Rep. 2019;9:5473. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maki K.C., Gibson G.R., Dickmann R.S., Kendall C.W., Chen C.Y., Costabile A., Comelli E.M., McKay D.L., Almeida N.G., Jenkins D., et al. Digestive and physiologic effects of a wheat bran extract, arabino-xylan-oligosaccharide, in breakfast cereal. Nutrition. 2012;28:1115–1121. doi: 10.1016/j.nut.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 123.François I.E., Lescroart O., Veraverbeke W.S., Marzorati M., Possemiers S., Evenepoel P., Hamer H., Houben E., Windey K., Welling G.W., et al. Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: A double-blind, randomised, placebo-controlled, cross-over trial. Br. J. Nutr. 2012;108:2229–2242. doi: 10.1017/S0007114512000372. [DOI] [PubMed] [Google Scholar]
- 124.Lefranc-Millot C., Guérin-Deremaux L., Wils D., Neut C., Miller L.E., Saniez-Degrave M.H. Impact of a resistant dextrin on intestinal ecology: How altering the digestive ecosystem with NUTRIOSE®, a soluble fibre with prebiotic properties, may be beneficial for health. J. Int. Med. Res. 2012;40:211–224. doi: 10.1177/147323001204000122. [DOI] [PubMed] [Google Scholar]
- 125.Burns A.M., Solch R.J., Dennis-Wall J.C., Ukhanova M., Nieves C., Jr., Mai V., Christman M.C., Gordon D.T., Langkamp-Henken B. In healthy adults, resistant maltodextrin produces a greater change in fecal bifidobacteria counts and increases stool wet weight: A double-blind, randomized, controlled crossover study. Nutr. Res. (New York N.Y.) 2018;60:33–42. doi: 10.1016/j.nutres.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 126.Mai V., Burns A.M., Solch R.J., Dennis-Wall J.C., Ukhanova M., Langkamp-Henken B. Resistant Maltodextrin Consumption in a Double-Blind, Randomized, Crossover Clinical Trial Induces Specific Changes in Potentially Beneficial Gut Bacteria. Nutrients. 2022;14:2192. doi: 10.3390/nu14112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zheng J., Wittouck S., Salvetti E., Franz C.M.A.P., Harris H.M.B., Mattarelli P., O’Toole P.W., Pot B., Vandamme P., Walter J., et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 128.Yang G., Wei J., Liu P., Zhang Q., Tian Y., Hou G., Meng L., Xin Y., Jiang X. Role of the gut microbiota in type 2 diabetes and related diseases. Metab. Clin. Exp. 2021;117:154712. doi: 10.1016/j.metabol.2021.154712. [DOI] [PubMed] [Google Scholar]
- 129.Pedersen C., Gallagher E., Horton F., Ellis R.J., Ijaz U.Z., Wu H., Jaiyeola E., Diribe O., Duparc T., Cani P.D., et al. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016;116:1869–1877. doi: 10.1017/S0007114516004086. [DOI] [PubMed] [Google Scholar]
- 130.Gonai M., Shigehisa A., Kigawa I., Kurasaki K., Chonan O., Matsuki T., Yoshida Y., Aida M., Hamano K., Terauchi Y. Galacto-oligosaccharides ameliorate dysbiotic Bifidobacteriaceae decline in Japanese patients with type 2 diabetes. Benef. Microbes. 2017;8:705–716. doi: 10.3920/BM2016.0230. [DOI] [PubMed] [Google Scholar]
- 131.Birkeland E., Gharagozlian S., Birkeland K.I., Valeur J., Måge I., Rud I., Aas A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020;59:3325–3338. doi: 10.1007/s00394-020-02282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitchell C.M., Davy B.M., Ponder M.A., McMillan R.P., Hughes M.D., Hulver M.W., Neilson A.P., Davy K.P. Prebiotic Inulin Supplementation and Peripheral Insulin Sensitivity in adults at Elevated Risk for Type 2 Diabetes: A Pilot Randomized Controlled Trial. Nutrients. 2021;13:3235. doi: 10.3390/nu13093235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reimer R.A., Wharton S., Green T.J., Manjoo P., Ramay H.R., Lyon M.R., Gahler R.J., Wood S. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur. J. Nutr. 2021;60:1237–1251. doi: 10.1007/s00394-020-02328-8. [DOI] [PubMed] [Google Scholar]
- 134.Mateo-Gallego R., Moreno-Indias I., Bea A.M., Sánchez-Alcoholado L., Fumanal A.J., Quesada-Molina M., Prieto-Martín A., Gutiérrez-Repiso C., Civeira F., Tinahones F.J. An alcohol-free beer enriched with isomaltulose and a resistant dextrin modulates gut microbiome in subjects with type 2 diabetes mellitus and overweight or obesity: A pilot study. Food Funct. 2021;12:3635–3646. doi: 10.1039/D0FO03160G. [DOI] [PubMed] [Google Scholar]
- 135.Canfora E.E., Hermes G.D.A., Müller M., Bastings J., Vaughan E.E., van Den Berg M.A., Holst J.J., Venema K., Zoetendal E.G., Blaak E.E. Fiber mixture-specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes. 2022;14:2009297. doi: 10.1080/19490976.2021.2009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Canfora E.E., van der Beek C.M., Hermes G.D.A., Goossens G.H., Jocken J.W.E., Holst J.J., van Eijk H.M., Venema K., Smidt H., Zoetendal E.G., et al. Supplementation of Diet With Galacto-oligosaccharides Increases Bifidobacteria, but Not Insulin Sensitivity, in Obese Prediabetic Individuals. Gastroenterology. 2017;153:87–97.e3. doi: 10.1053/j.gastro.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 137.Brunkwall L., Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: From current human evidence to future possibilities. Diabetologia. 2017;60:943–951. doi: 10.1007/s00125-017-4278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ojo O., Feng Q.Q., Ojo O.O., Wang X.H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2020;12:3239. doi: 10.3390/nu12113239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma S., Tripathi P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019;63:101–108. doi: 10.1016/j.jnutbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.