Abstract
Background
Inflammatory bowel disease (IBD), comprised of Crohn's disease (CD) and ulcerative colitis (UC), is characterized by chronic mucosal inflammation, frequent hospitalizations, adverse health economics, and compromised quality of life. Diet has been hypothesised to influence IBD activity.
Objectives
To evaluate the efficacy and safety of dietary interventions on IBD outcomes.
Search methods
We searched the Cochrane IBD Group Specialized Register, CENTRAL, MEDLINE, Embase, Web of Science, Clinicaltrials.gov and the WHO ICTRP from inception to 31 January 2019. We also scanned reference lists of included studies, relevant reviews and guidelines.
Selection criteria
We included randomized controlled trials (RCTs) that compared the effects of dietary manipulations to other diets in participants with IBD. Studies that exclusively focused on enteral nutrition, oral nutrient supplementation, medical foods, probiotics, and parenteral nutrition were excluded.
Data collection and analysis
Two review authors independently performed study selection, extracted data and assessed bias using the risk of bias tool. We conducted meta‐analyses where possible using a random‐effects model and calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. We assessed the certainty of evidence using GRADE.
Main results
The review included 18 RCTs with 1878 participants. The studies assessed different dietary interventions for active CD (six studies), inactive CD (seven studies), active UC (one study) and inactive UC (four studies). Dietary interventions involved either the consumption of low amounts or complete exclusion of one or more food groups known to trigger IBD symptoms. There was limited scope for data pooling as the interventions and control diets were diverse. The studies were mostly inadequately powered. Fourteen studies were rated as high risk of bias. The other studies were rated as unclear risk of bias.
The effect of high fiber, low refined carbohydrates, low microparticle diet, low calcium diet, symptoms‐guided diet and highly restricted organic diet on clinical remission in active CD is uncertain. At 4 weeks, remission was induced in: 100% (4/4) of participants in the low refined carbohydrates diet group compared to 0% (0/3) of participants in the control group (RR 7.20, 95% CI 0.53 to 97.83; 7 participants; 1 study; very low certainty evidence). At 16 weeks, 44% (23/52) of participants in the low microparticle diet achieved clinical remission compared to 25% (13/51) of control‐group participants (RR 3.13, 95% CI 0.22 to 43.84; 103 participants; 2 studies; I² = 73%; very low certainty evidence). Fifty per cent (16/32) of participants in the symptoms‐guided diet group achieved clinical remission compared to 0% (0/19) of control group participants (RR 20.00, 95% CI 1.27 to 315.40; 51 participants ; 1 study; very low certainty evidence) (follow‐up unclear). At 24 weeks, 50% (4/8) of participants in the highly restricted organic diet achieved clinical remission compared to 50% (5/10) of participants in the control group (RR 1.00, 95% CI 0.39 to 2.53; 18 participants; 1 study; very low certainty evidence). At 16 weeks, 37% (16/43) participants following a low calcium diet achieved clinical remission compared to 30% (12/40) in the control group (RR 1.24, 95% CI 0.67 to 2.29; 83 participants; 1 study; very low certainty evidence).
The effect of low refined carbohydrate diets, symptoms‐guided diets and low red processed meat diets on relapse in inactive CD is uncertain. At 12 to 24 months, 67% (176/264) of participants in low refined carbohydrate diet relapsed compared to 64% (193/303) in the control group (RR 1.04, 95% CI 0.87 to 1.25; 567 participants; 3 studies; I² = 35%; low certainty evidence). At 6 to 24 months, 48% (24/50) of participants in the symptoms‐guided diet group relapsed compared to 83% (40/48) participants in the control diet (RR 0.53, 95% CI 0.28 to 1.01; 98 participants ; 2 studies; I² = 54%; low certainty evidence). At 48 weeks, 66% (63/96) of participants in the low red and processed meat diet group relapsed compared to 63% (75/118) of the control group (RR 1.03, 95% CI 0.85 to 1.26; 214 participants; 1 study; low certainty evidence). At 12 months, 0% (0/16) of participants on an exclusion diet comprised of low disaccharides / grains / saturated fats / red and processed meat experienced clinical relapse compared to 26% (10/38) of participants on a control group (RR 0.11, 95% CI 0.01 to 1.76; 54 participants; 1 study; very low certainty evidence).
The effect of a symptoms‐guided diet on clinical remission in active UC is uncertain. At six weeks, 36% (4/11) of symptoms‐guided diet participants achieved remission compared to 0% (0/10) of usual diet participants (RR 8.25, 95% CI 0.50 to 136.33; 21 participants; 1 study; very low certainty evidence).
The effect of the Alberta‐based anti‐inflammatory diet, the Carrageenan‐free diet or milk‐free diet on relapse rates in inactive UC is uncertain. At 6 months, 36% (5/14) of participants in the Alberta‐based anti‐inflammatory diet group relapsed compared to 29% (4/14) of participants in the control group (RR 1.25, 95% CI 0.42 to 3.70; 28 participants; 1 study; very low certainty evidence). Thirty per cent (3/10) of participants following the carrageenan‐free diet for 12 months relapsed compared to 60% (3/5) of the participants in the control group (RR 0.50, 95% CI 0.15 to 1.64; 15 participants; 1 study; very low certainty evidence). At 12 months, 59% (23/39) of milk free diet participants relapsed compared to 68% (26/38) of control diet participants (RR 0.83, 95% CI 0.60 to 1.15; 77 participants; 2 studies; I² = 0%; low certainty evidence).
None of the included studies reported on diet‐related adverse events.
Authors' conclusions
The effects of dietary interventions on CD and UC are uncertain. Thus no firm conclusions regarding the benefits and harms of dietary interventions in CD and UC can be drawn. There is need for consensus on the composition of dietary interventions in IBD and more RCTs are required to evaluate these interventions. Currently, there are at least five ongoing studies (estimated enrollment of 498 participants). This review will be updated when the results of these studies are available.
Plain language summary
Diets for inducing and maintaining remission in inflammatory bowel disease (IBD)
What is the aim of the review?
The aim was to find out what diets can be used to induce or maintain remission in people with IBD.
What is IBD?
IBD involves inflammation of the gastrointestinal tract. Ulcerative colitis (UC) and Crohn’s disease (CD) are the most common types of IBD. Symptoms include abdominal pain, diarrhea and rectal bleeding. IBD is characterized by periods of relapse where people experience symptoms of active disease and periods of remission when the symptoms stop. While some foods may provoke IBD symptoms, little is known about whether diets help to induce or maintain remission in IBD.
How up to date is the review?
We searched for studies up to 31 January 2019.
What are the main results of the review?
We found 18 studies including 1878 participants. The diets studied included reduction or exclusion of foods believed to provoke IBD symptoms. These diets were compared with 'usual' diets. The studies assessed dietary interventions for active CD (six studies), inactive CD (seven studies), active UC (one study) and inactive UC (four studies). One study recruited children, while the rest included adults. The studies were poorly designed and had few participants. As a result the overall quality of the evidence was very low.
The effect of high fiber, low refined carbohydrates, low microparticle, low calcium, symptoms‐guided diet and highly restricted organic diet on clinical remission in active CD is uncertain. In one study, remission was achieved at 4 weeks in 100% (4/4) of low refined carbohydrates participants compared to 0% (0/3) of usual diet participants. In a pooled analysis of two studies, 44% (23/52) of low microparticle participants achieved remission at 16 weeks compared to 25% (13/51) of usual diet participants. One study found that 50% (16/32) of symptoms‐guided participants achieved remission compared to 0% (0/19) of usual diet participants. One study found that 50% (4/8) of highly‐restricted organic diet participants achieved remission at 24 weeks compared to 50% (5/10) of usual diet participants. One study found that 37% (16/43) of low‐calcium participants achieved remission at 16 weeks compared to 30% (12/40) of usual diet participants.
The effect of low refined carbohydrate, symptoms‐guided and low red processed meat diets on relapse in inactive CD is uncertain. In a pooled analysis of three studies, 67% (176/264) of low refined carbohydrate participants relapsed at 12 to 24 months compared to 64% (193/303) of usual diet participants. In a pooled analysis of two studies, 48% (24/50) of symptoms‐guided participants relapsed at 6 to 24 months compared to 83% (40/48) of usual diet participants. One study found that 66% (63/96) of low red and processed meat participants relapsed at 48 weeks compared to 63% (75/118) of usual diet participants. One study showed that 0% (0/16) of exclusion diet participants (i.e. low disaccharides, grains, saturated fats, red and processed meat) relapsed at 12 months compared to 26% (10/38) of usual diet participants.
The effect of a symptoms‐guided diet on clinical remission in active UC is uncertain. In one study, 36% (4/11) of symptoms‐guided participants achieved remission at six weeks compared to 0% (0/10) in the usual diet group.
The effect of the Alberta‐based anti‐inflammatory diet, the Carrageenan‐free diet and the milk‐free diet on relapse in inactive UC is uncertain. In one study, 36% (5/14) of Alberta‐based diet participants relapsed at 6 months compared to 29% (4/14) of usual diet participants. In one study, 30% (3/10) of carrageenan‐free participants relapsed at 12 months compared to 60% (3/5) of usual diet participants. At 12 months, 59% (23/39) of milk‐free diet participants relapsed compared to 68% (26/38) in the usual diet group.
None of the included studies reported on diet‐related side effects.
Conclusions
The effects of dietary interventions on CD and UC are uncertain. Thus no firm conclusions regarding the benefits and harms of dietary interventions in CD and UC can be drawn. There is need for consensus on the composition of dietary interventions in IBD and more studies are required to evaluate these interventions. Currently, there are five ongoing studies (estimated enrollment of 498 participants). This review will be updated when the results of these studies are available.
Summary of findings
Background
Description of the condition
Inflammatory bowel disease (IBD), predominantly comprised of Crohn's disease (CD) and ulcerative colitis (UC), is characterized by chronic mucosal inflammation, frequent hospitalizations, adverse health economics, and compromised quality of life. Common symptoms of IBD include abdominal pain, diarrhoea, and rectal bleeding. Inflammation in UC is limited to the colonic mucosa. Inflammation in CD is a non‐uniform transmural disease process that can occur anywhere along the alimentary tract and can lead to complications including intestinal strictures, fistulization to surrounding tissues or organs, and abscesses. Other complications that can arise in both CD and UC include intestinal cancer, nutrient malabsorption, malnutrition, and extra‐intestinal manifestations (e.g. arthralgias, dermatologic lesions, uveitis).
The incidence of CD is approximately 20 per 100,000 person‐years in North America and 13 per 100,000 person‐years in Europe (Molodecky 2012). Higher disease incidence and prevalence are seen in North America and Europe compared to lower rates in Asia and the Middle East. Nonetheless, the incidence and prevalence of IBD have more recently been rising in Asia and the Middle East, and individuals from these geographic regions experience an increased risk of developing IBD when immigrating to North America or Europe (Benchimol 2015; Pinsk 2007). This overall increase in IBD among populations not traditionally associated with IBD has been hypothesized to stem from the Westernization of lifestyles and diets (Foster 2013; Ooi 2016). For example, immigrants from Latin American to South Florida develop IBD at a later age; however, first‐generation US‐born Hispanics develop IBD at an age similar to non‐Hispanic whites (Damas 2013). The development of IBD in this generation of immigrants is also occurring sooner than previously documented (Damas 2017).
Factors that contribute to the development of IBD are unclear, although the current paradigm of pathogenesis involves the interaction of disease‐susceptibility genes, inappropriate immune response, gut microbiota, and environmental factors (Abraham 2009). Some potential environmental factors include gastrointestinal infections, antibiotics, tobacco use, and oral contraceptives (Birrenbach 2004; Cornish 2008; Garcia Rodriguez 2006; Gradel 2009; Ungaro 2014). Epidemiologic studies have implicated diet in IBD pathogenesis (Chapman‐Kiddell 2010). Increased intake of refined sugars has been associated with an increased risk of CD in several small cohort studies (Bianchi 1985; Hansen 2011; Jakobsen 2013; Martini 1976; Silkoff 1980). Other studies have associated dietary fiber consumption with a reduced risk of CD (Amre 2007; Persson 1992; Thornton 1979). An analysis of the Nurses' Health Study that included 170,776 adult women, who were prospectively followed over 26 years, revealed that long‐term consumption of dietary fiber was associated with a reduced incidence of CD (Ananthakrishnan 2013). Compared with the lowest quintile of energy‐adjusted cumulative average intake of dietary fiber, intake of the highest quintile (median of 24.3 g/day of dietary fiber) was associated with a 40% reduction in risk of CD. Fiber derived from fruits was significantly associated with a reduced risk of CD, while fiber from vegetables, cereals, and whole grains was not associated with a reduced risk of IBD. A separate analysis of the Nurses’ Health Study revealed that higher intakes of fruits, vegetables, and fish in high school were associated with a 53% lower risk of developing CD with fish having the greatest impact (Ananthakrishnan 2015). Dietary fat may also play a role in CD pathogenesis, although this relationship appears less clear. Some studies have associated an increased fat intake with CD risk (Hou 2011; Reif 1997; Sakamoto 2005). However, an analysis of the large prospective Nurses' Health Study did not find an association between the intake of total fat, saturated fats, unsaturated fats, omega‐6 polyunsaturated fatty acids (PUFAs), or omega‐3 PUFAs and CD risk (Ananthakrishnan 2014). A greater intake of omega‐3 PUFAs and higher ratio of n‐3:n‐6 PUFAs were associated with a lower risk of UC. Furthermore, high long‐term intake of trans‐unsaturated fatty acids was associated with a trend towards an increased incidence of UC. Table 5 summarizes the influence of some dietary components on the risk of IBD.
1. Dietary components can influence risk of inflammatory bowel disease.
| Dietary Component | Effect on IBD Risk | References |
| Animal Protein | Increased | Jantchou 2010 |
| Heme iron, sulfur | Increased | Ananthakrishnan 2015 |
| Refined sugars | Increased | Janerot 1983 |
| High trans‐fat | Increased | Ananthakrishnan 2014 |
| Fiber | Decreased | Ananthakrishnan 2015 |
| Fruit | Decreased | Hou 2011 |
| Vegetables | Decreased | Hou 2011 |
| High omega‐3 fatty acids | Decreased | Chan 2014 |
Adpated from Mullin 2016.
Given its potential effects on disease pathogenesis, dietary intake has similarly been hypothesized to influence disease activity. For instance, exclusive enteral nutrition may be effective for the induction and maintenance of remission in pediatric CD (Akobeng 2018; Critch 2012; Narula 2018). A high intake of red and processed meat or alcoholic beverages may increase the risk of a UC flare among adults (Jowett 2004). Diet‐derived micronutrients, such as zinc, iron, and vitamin D, may have modifying effects on immunity, barrier function, and oxidative load with a downstream potential to impact the course of CD (Brown 2011; Lih‐Brody 1996; Limketkai 2016). There may be a role of nutritional therapies for the induction and maintenance of remission in IBD, although the potential efficacy may vary according to diet composition, disease type, and age group (pediatric or adult).
Description of the intervention
The intervention is a controlled manipulation of the subject’s oral diet by a deliberate change in the consumption of food (i.e. no formulas or supplements used) for a specified period of time.
How the intervention might work
The mechanisms that drive the benefits or harms of diets in IBD are unclear, although studies on dietary macronutrients may provide some insight. For instance, omega‐6 PUFAs are pro‐inflammatory mediators, while omega‐3 PUFAs, medium‐chain oils, and a family of diverse plant‐derived flavonoids (e.g. phytonutrients) have anti‐inflammatory properties (Kono 2010; Papada 2014). Dietary fiber can be converted by intestinal bacteria to short‐chain fatty acids, which have anti‐inflammatory properties (Galvez 2005). In IBD mouse models, high‐fat diets promote further intestinal inflammation by disrupting gut barrier function and the resident microbiome (Devkota 2012; Gruber 2013; van der Logt 2013). Similarly, one of the theories underlying the efficacy of exclusive enteral nutrition or elimination diets for induction of remission in IBD relates to an avoidance of dietary triggers. These pro‐ or anti‐inflammatory nutrients are thus suspected to confer respective pro‐ or anti‐inflammatory properties of diets. Others have hypothesized that people with IBD may possess individualized food sensitivities and disease activity could improve with the personalized exclusion of foods that provoke symptoms or cause abnormal increases in food‐specific IgG antibodies (Bentz 2010; Gunasekeera 2016; Rajendran 2011), although food‐specific IgG antibodies have not been found to correlate with gastrointestinal symptom severity (Zuo 2007). The elimination of foods that are high in short‐chain carbohydrates (i.e. FODMAP: Fermentable Oligo‐, Di‐, Monosaccharides, And Polyols) may improve CD symptoms through several possible mechanisms including reduction of gaseous byproducts of bacterial fermentation, gaseous distention, osmotic diarrhoea, and shifts in the gut microbiome (Gearry 2009; Gibson 2015; Halmos 2015; Halmos 2016; Prince 2016; See Figure 1).
1.
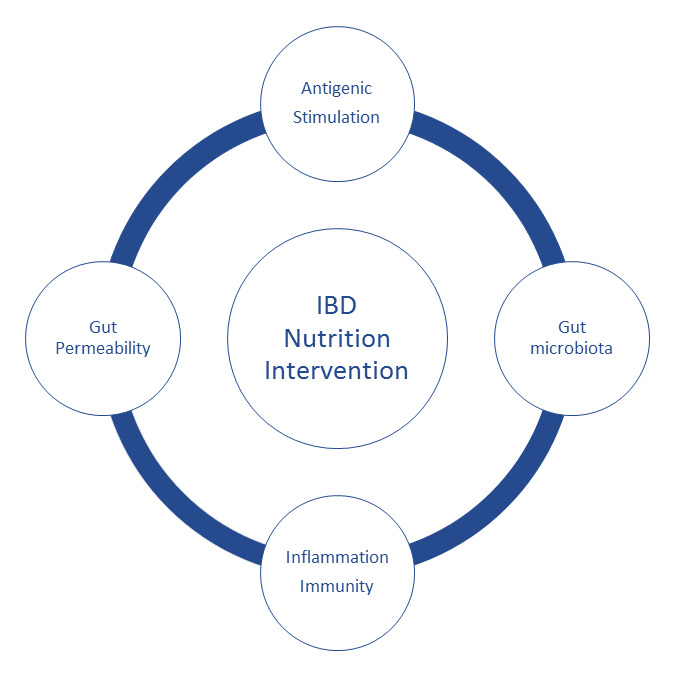
Source: Mullin 2016.
Why it is important to do this review
Patients and clinicians have long sought guidance on the dietary management of IBD. A prospective evaluation of 400 consecutive IBD patients at a tertiary‐care center reported that approximately half felt that diet could be the initiating factor in their disease and the majority cited food provocation of IBD symptoms (57%) and disease flares (60%) (Limdi 2016). Several 'brand' diets (e.g. Specific Carbohydrate Diet, gluten‐free diet, Anti‐Inflammatory Diet, Gut and Psychology Diet) are promoted on the internet by healthcare practitioners and even non‐licensed individuals, often without supporting evidence. These diet programs restrict, exclude, or promote the intake of differing food types to achieve purported improvements in IBD symptoms. Several clinical trials and observational cohort studies have studied the effects of diverse diets on clinical endpoints in IBD. Nonetheless, the individual studies are often limited by small sample sizes, suboptimal study design, and inconsistent findings. Despite several opinion papers and reviews on the issue of dietary management of IBD, there is still no consensus or clear guidance in the literature on optimal dietary therapies for induction or maintenance of remission in IBD. A systematic review is lacking and could potentially benefit both clinicians and patients to guide dietary management of IBD based on the best available evidence.
Objectives
The objective of this systematic review is to evaluate the efficacy and safety of dietary interventions on IBD outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) were considered for inclusion.
Types of participants
Adults or children with established IBD (CD, UC) were considered for inclusion. For studies that only reported on IBD, we contacted the authors to request a breakdown of results for participants with CD and UC. Trials conducted in all settings (e.g. single‐center, multi‐center) with any established method used to confirm disease diagnosis were included. Studies were not included unless stratified results for IBD (CD and UC) were provided.
Types of interventions
Interventions of interest included all defined oral diets compared to a different or unrestricted oral diet. Studies that exclusively focused on enteral nutrition, oral nutrient supplementation, medical foods, probiotics, and parenteral nutrition were excluded.
Types of outcome measures
Primary outcomes
Primary outcomes were induction and maintenance of remission as defined by the included studies.
Induction of remission involves the therapeutic reduction of intestinal symptoms below a clinical threshold as measured by CD and UC symptom scores, including the Pediatric Crohn’s Disease Activity Index (PCDAI), the Crohn’s Disease Activity Index (CDAI), the Harvey‐Bradshaw Index (HBI), the Mayo score, modified Mayo score or Colitis Activity Index (CAI).
Maintenance of remission involves the continual abatement of symptoms over time attributable to a therapeutic modality (in this case, diet). Maintenance of remission will be assessed based on available fixed time intervals (e.g. six months, one year) and as variable time contributions (e.g. person‐years). A clinical relapse is defined as the transition from a state of clinical remission to active disease, based on symptom scores (i.e. PCDAI, CDAI, HBI, Mayo score, or CAI).
Although symptom scores are validated indices routinely used to assess disease activity in IBD clinical trials, a potential limitation is the inability to differentiate between IBD or irritable bowel syndrome (IBS)‐associated mediators of non‐specific gastrointestinal symptoms.
Secondary outcomes
Secondary outcomes (when available) were the following:
Clinical improvement as defined by the included studies;
Corticosteroid‐free remission;
Surrogate biomarkers of inflammation (i.e., erythrocyte sedimentation rate [ESR] and C‐reactive protein [CRP]), fecal biomarkers (i.e., calprotectin);
Endoscopic endpoints of improvement and remission;
Histologic endpoints of improvement and remission;
Health‐related quality of life as measured by the Inflammatory Bowel Disease Questionnaire (IBDQ), Short Inflammatory Bowel Disease Questionnaire (SIBDQ), or related surveys;
Hospitalizations;
Need for surgery;
Progression of disease from a state of inflammation‐only disease to stricturing/obstructing to penetrating/fistulizing disease;
Escalation of therapy including the need to add or modify pharmacologic therapy due to lack of efficacy at inducing or maintaining remission after enrollment in the trial;
Adverse events;
Withdrawal due to adverse events; and
Serious adverse events.
Search methods for identification of studies
Electronic searches
We conducted a comprehensive and systematic search to identify RCTs and non‐randomized studies (i.e. cohort or case‐control) from inception to 31 January 2019 using the following databases:
CENTRAL;
Cochrane IBD Group Specialized Trials Register;
Embase (Ovid);
MEDLINE (Ovid); and
Web of Science.
We searched databases using controlled vocabulary and keywords (details in appendices). No restrictions were placed on publication dates (after 1966) or language. Note that the searches were designed to include interventional and observational studies on adults and children but exclude those using oral nutrition supplements (enteral nutrition drinks, tube feeds), medical foods, probiotics, parenteral nutrition or a combination of these modalities. We report the detailed search strategies in Appendix 1.
Searching other resources
We searched reference lists from included articles and any existing relevant reviews. We also scanned proceedings from Digestive Disease Week (2005 to date), Advances in Inflammatory Bowel Disease (2005 to date), Clinical Nutrition Week (2005 to date), European Crohn's and Colitis Organisation (2005 to date), and United European Gastroenterology Week (2005 to date). We also searched ongoing trials registered in ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform portal.
Data collection and analysis
This review has been carried out according to methods presented in the published protocol (Limketkai 2017), which are based on the Cochrane Handbook (Higgins 2011).
Selection of studies
The stages of article selection included the following: (i) title screening; (ii) abstract screening; and (iii) full‐text review. Two authors (BNL and GEM) independently reviewed each article at each stage of selection. Included and excluded studies were recorded.
Title screening involved selection of articles that reported studies with even a minor possibility of inclusion. Articles that are clearly unrelated were excluded. Adjudication did not occur at the title screening stage and ambiguous studies were included by default.
Abstract screening involved the selection of articles that reported studies with a reasonable possibility of inclusion. Differences in assessment for inclusion were resolved by discussion between the two independent investigators. Adjudication did not occur at the abstract screening state and studies that were ambiguous were included by default.
Full‐text review involved selection of articles based on careful examination of the full report. Differences in assessment for inclusion were resolved by discussion between the two independent investigators. Adjudication was performed as needed by a third author (AP).
Data extraction and management
Two authors (TH and ZIE) independently performed data extraction from each included study. Any discrepancies were resolved by discussion between the two independent investigators. Adjudication was performed as needed by a third author (MG). Extracted data included the study design, population characteristics, intervention, comparator, duration of interventions and follow‐up, outcomes, timing, setting, the method of handling missing data, funding source, and potential conflicts of interest.
Assessment of risk of bias in included studies
Two authors (TH and ZIE) independently assessed the study quality of each included RCT using the Cochrane risk of bias tool. Adjudication was performed as needed by a third author (MG). Domains of interest included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias (e.g. baseline imbalance). Each domain was assessed as having a low, moderate, high, or unclear risk of bias. Based on the aggregate assessment of these items, study quality was rated as good (low risk of bias), fair, or poor (high or unclear risk of bias). Each domain followed standard definitions used for Cochrane systematic reviews (Higgins 2011).
We considered trials which were classified as having a low risk of bias for sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, and selective reporting as low bias‐risk trials. All other trials were considered to be at high risk of bias. We tabulated the risk of bias in the 'Risk of bias' table as part of the 'Table of characteristics of included studies'. We also illustrated the risk of bias of each trial using the 'Risk of bias summary' and cross‐tabulated all the judgement of risk on a 'Risk of bias graph'.
The overall strength of evidence supporting the primary outcome and selected secondary outcomes was assessed using the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) criteria (Guyatt 2008; Schünemann 2011). Evidence from RCTs starts as high quality and evidence from observational studies starts as low quality. The quality of the evidence can be downgraded due to risk of bias, indirect evidence, inconsistency (unexplained heterogeneity), imprecision; and publication bias. GRADE also allows for the potential of rating up the overall quality of evidence from methodologically sound observational studies (Guyatt 2011). For example, evidence could be rated up if high quality observational studies show a two‐ to five‐fold reduction or increase in risk (Guyatt 2011). Taking all of these factors into account, we rated the overall quality of evidence as follows:
High. We are very confident that the true effect lies close to that of the estimate of the effect;
Moderate. We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
Low. Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect; or
Very low. We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Measures of treatment effect
For binary outcomes, we calculated the risk ratio (RR) with corresponding 95% confidence interval (CI). For nominal or ordinal outcomes, we planned to calculate the RR with corresponding 95% CI for each category relative to a reference category. For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI.
Unit of analysis issues
The unit of analysis was the individual participant. We planned to include cross‐over trials if data were available from the first phase of the study (i.e. before cross‐over occurred). For outcomes where events recur (e.g. clinical relapses, adverse events), we calculated the proportion of patients who experienced at least one event, individual events were not counted separately. The studies were otherwise not anticipated to have repeated observations of outcomes or multiple treatment events. Ecologic studies that did not include individual‐level intervention and analyses were to be excluded. For studies with multiple treatment arms, we only included single pair‐wise comparisons as appropriate.
Dealing with missing data
We collected information on how each trial handled missing data. When a study appeared to collect and not report all primary outcomes of interest, the original investigators were contacted to request missing data. If the original investigators did not provide the data, this would be noted in the systematic review. For studies with missing dichotomous data, a separate intention‐to‐treat analysis was performed where participants with missing data were assumed to have been treatment failures. For studies with missing continuous data, we used of available cases and imputation with the last observation carried forward. Multiple imputation was to be applied to missing data.
Assessment of heterogeneity
Heterogeneity was first assessed qualitatively considering the study populations (e.g. adults, children, age, sex, race), research setting, methods of dietary interventions, duration of interventions, and definitions and thresholds for remission. For studies that had qualitative homogeneity, statistical heterogeneity was assessed using the Chi² test (P value < 0.10 was considered statistically significant heterogeneity). The degree of heterogeneity across studies was estimated using the I² statistic. An I² of 25% or less was considered low heterogeneity, 26 to 50% was considered moderate heterogeneity, and 50% and greater was considered substantial heterogeneity. We planned to only report on summary effect estimates from meta‐analyses of groups of studies with clinical, methodologic, and statistical homogeneity (i.e. I² < 50%). Additionally, we visually inspected the forest plots and planned to perform a sensitivity analysis excluding any obvious outliers.
Assessment of reporting biases
The total number of registered trials that could qualify for inclusion if published were to be compared against the number of peer‐reviewed publications. Study contacts for registered trials without a peer‐reviewed publication were to be contacted to assess reasons for the absence of publication. If 10 of more studies were included in the meta‐analysis, a funnel plot would have been used to assess for potential publication bias.
Data synthesis
This systematic review qualitatively reported on the included study characteristics and outcomes. We conducted a meta‐analysis of studies where at least two studies with similar interventions, participants and reported outcomes were present (to be determined by consensus). Analyses were performed separately according to disease type (CD or UC), population (adult or pediatric), and type of diet. For dichotomous outcomes, we calculated the pooled RR and corresponding 95% CI. For continuous outcomes, we calculated the pooled MD and corresponding 95% CI. Studies were pooled using a random‐effects model. Studies were grouped according to disease state (active or inactive) and type (UC or CD).
Subgroup analysis and investigation of heterogeneity
We planned to qualitatively evaluate the usage patterns of concurrent IBD‐specific therapies (e.g. antibiotics, aminosalicylates, immunomodulators, biologics) in the study populations. Where possible, subgroup analyses were to be performed based on therapy classes.
Sensitivity analysis
We planned to conduct sensitivity analyses that exclude studies with high risk of bias. However, as over 70% of the studies were at high risk of bias, there would have been little or no data to assess in a sensitivity analysis
Results
Description of studies
Results of the search
The literature search identified 8097 records which was reduced to 7166 unique records following the removal of duplicates. Titles and abstracts were screened and we initially identified 60 studies which appeared to meet the inclusion criteria. Full text copies of these 60 studies were obtained and further scrutinised. After reviewing full text articles, we excluded 35 studies which had the wrong study design, participants, interventions or outcomes. We included 18 studies with a total of 1878 participants in our systematic review. We also identified five ongoing studies with an estimated enrollment of 498 participants (See Characteristics of ongoing studies) and two studies awaiting classification (Bodini 2018; Tapete 2018). The results of the search are reported in the PRISMA flow diagram (See Figure 2). Full details of the included and excluded studies are available in the Characteristics of included studies and Characteristics of excluded studies tables and are summarised below.
2.
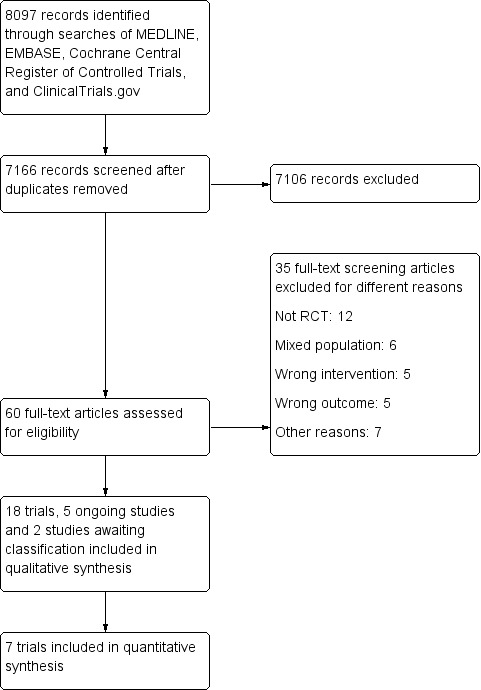
Study flow diagram.
Included studies
Study design and setting
The included studies were conducted in single or multi centers across eight different countries and were published between 1965 and 2018. Thirty‐five per cent of the studies were conducted in the UK (Jones 1985; Lomer 2001; Lomer 2005; Riordan 1993; Ritchie 1987; Wright 1965), 23% in the USA (Albenberg 2018; Bhattacharyya 2017; Brotherton 2014; Mutlu 2016), 11% in Germany (Brandes 1981; Lorenz‐Meyer 1996), 11% in Italy (Levenstein 1985; Strisciuglio 2013) and the rest were conducted in Austria (Bartel 2008), Canada (Keshteli 2016), Israel (Dariel 2007), and South Africa (Candy 1995).
Participants
The 18 studies included a total of 1878 randomized participants with sample sizes ranging between 7 and 659 participants. Disease severity was reported as mild, or mild to moderate in two studies (Bartel 2008; Dariel 2007). The other studies did not report on disease severity. The use of medication was apparent in some or all participants in almost half of the studies regardless of disease type or state. However, this information was not reported in the rest of the studies. The age of participants was reported in all except two studies (Albenberg 2018; Wright 1965), and ranged between an average of 11.2 years to 48 years across 12 studies. Other studies reported age as median (Candy 1995), or range (Bhattacharyya 2017; Jones 1985; Ritchie 1987). One study recruited only paediatric patients (Strisciuglio 2013), and the rest of the studies appear to have included mainly adults. The studies looked at people with the following disease states and types:
Active Crohn's disease (Bartel 2008; Brotherton 2014; Dariel 2007; Levenstein 1985; Lomer 2001; Lomer 2005);
Inactive Crohn's disease (Albenberg 2018; Brandes 1981; Jones 1985; Lorenz‐Meyer 1996; Mutlu 2016; Riordan 1993; Ritchie 1987);
Active ulcerative colitis (Candy 1995); and
Inactive ulcerative colitis (Bhattacharyya 2017; Keshteli 2016; Strisciuglio 2013; Wright 1965).
Interventions
All the included studies had two trial arms, except four studies with more than two trial arms (Lomer 2005; Lorenz‐Meyer 1996; Mutlu 2016;Wright 1965). The studies compared intervention diets with control diets. For the purpose of this review, 'intervention diet' has been used to describe diets involving the consumption of low levels or complete exclusion of one or more food groups that are thought to trigger symptoms of IBD (see Description of the condition). Control diets involved normal amounts these food groups which were restricted in the intervention group, other diet modifications or advice. From Table 6 it is apparent that whilst some dietary modifications were centred around single food groups, other diets seemed to involve multiple food groups. These interventions and controls are summarised as follows:
2. Summary of interventions and outcomes.
| Study ID | Group 1 (n) | Group 2 (n) | Group 3 (n) | Group 4 (n) | Remission | Relapse |
| Induction of Remission in Crohn's Disease | ||||||
| Bartel 2008 | Restricted organic diet (5) | Low‐fat, high‐carbohydrate, low‐fiber, no red meat diet (9) | 4/5 vs. 7/9 (week 6) PP:4/5 vs. 5/9 (week 24) ITT: 4/8 vs. 5/10 |
N/A | ||
| Brotherton 2014 | High‐fiber, reduced refined carbohydrate diet (4) | Low‐fiber diet (3) | Remission: 4/4 vs. 0/3 (week 4) Change in pHBI: 5.8 to 0.5 vs. 5.2 to 3.5 (P = 0.008) |
N/A | ||
| Dariel 2007 | Sequential elimination diets for 30 food components (32) | Conventional nutritional advice (19) | Response: 16/32 vs. 1/19 Remission: 16/32 vs. 0/19 |
N/A | ||
| Levenstein 1985 | Low fiber diet | Normal diet (with gradual fiber introduction) | Not reported | N/A | ||
| Lomer 2001 | Diet low in microparticles. Fibrous fruit and vegetables were excluded (10) | Foods containing dietary microparticles were not discouraged. Fibrous fruit and vegetables were excluded (10) | PP: 7/9 vs. 0/9 (month 4) ITT: 7/10 vs. 0/10 |
N/A | ||
| Lomer 2005 | Low calcium and low microparticle diet (22) | Low calcium and normal microparticle diet (21) | Normal calcium and low microparticle diet (20) | Normal calcium and normal microparticle diet (20) | Low vs. normal microparticle groups. Response: 16/42 vs. 17/41 (week 16) Remission: 16/42 vs. 13/41 (week 16) Low versus normal calcium Remission: 16/43 versus 13/40 |
N/A |
| Maintenance of Remission in Crohn's Disease | ||||||
| Albenberg 2018 | Low red and processed meats (96) | Moderate red and processed meats (118) | N/A | 54/87 vs. 72/115 (week 48) | ||
| Brandes 1981 | Low carbohydrate diet with increased intake of protein and fat | High carbohydrate diet with reduced intake of protein and fat | N/A | 1/5 vs. 1/6 | ||
| Jones 1985 | Exclusion of foods that provoked symptoms (10) | Unrefined carbohydrate fiber‐rich diet (10) | N/A | 3/10 vs. 10/10 (month 6) | ||
| Lorenz‐Meyer 1996 | Low‐carbohydrate diet of less than 84 g/day (69) | Omega‐3 fatty acid capsules and general nutrition guidelines (70) | Placebo and general nutrition guidelines (65) | N/A | 45/69 vs. 50/70 vs. 46/65 | |
| Mutlu 2016 | Anti‐IBD diet and placebo supplement (16) | Fructooligosaccharide supplement and "placebo diet" (19) | "Placebo diet" and placebo supplement (19) | N/A | 0/16 vs. 6/19 vs. 4/19 (month 12) | |
| Riordan 1993 | Elemental diet followed by reintroduction of single food each day and exclusion of symptom‐provoking foods (40) | General dietary advice and prednisolone taper (38) | N/A | PP: 12/40 vs. 25/38 (month 24) ITT: 21/40 vs. 30/38 |
||
| Ritchie 1987 | Unrefined, fiber‐rich diet (190) | Refined carbohydrate‐rich diet and unrestricted sugar intake (162) | N/A | 130/190 vs. 96/162 (month 24) | ||
| Induction of Remission in Ulcerative Colitis | ||||||
| Candy 1995 | Systematic exclusion of symptoms‐provoking foods (11) | Usual diet (10) | Response: 9/11 vs. 1/7 (week 6) Remission: 4/11 vs. 0/7 (week 6) |
N/A | ||
| Maintenance of Remission in Ulcerative Colitis | ||||||
| Bhattacharyya 2017 | Carrageenan‐free diet + placebo (10) | Carrageenan‐free diet + carrageenan‐containing capsules (5) | N/A | PP: 0/7 vs. 3/5 (month 12) ITT: 3/10 vs. 3/5 |
||
| Keshteli 2016 | Alberta‐based Anti‐inflammatory Diet (14) | Diet based on Canada's Food Guide (14) | N/A | 5/14 vs. 4/14 (month 6) | ||
| Strisciuglio 2013 | Cow’s milk protein elimination diet (14) | Usual diet (15) | N/A | 5/13 vs. 4/15 (month 6) 7/13 vs. 8/15 (month 12) |
||
| Wright 1965 | Milk‐free, low‐roughage diet (26) | Exclusion diet and liberal consumption of milk and milk products (24) | N/A | 16/26 vs. 18/23 (month 12) | ||
Abbreviations: ITT, Intention‐to‐treat; N/A, not applicable; pHBI, partial Harvey‐Bradshaw Index; PP, per‐protocol
Low refined carbohydrate diets (Brandes 1981; Brotherton 2014; Lorenz‐Meyer 1996; Ritchie 1987). The control diets were either intentionally rich in refined carbohydrates (Brandes 1981; Ritchie 1987), or provided no guidance on carbohydrate intake (Brotherton 2014; Lorenz‐Meyer 1996).
Low microparticle diets (Lomer 2001; Lomer 2005). The control diets included a sham diet that avoided other food additives (i.e., sulphur dioxide and sulphites) and added 5 mg/day titanium dioxide (TiO2). Toothpaste that was free of TiO2, but not particulate silicates, was provided.
Low calcium diet (Lomer 2005). The control diet included a calcium supplement of 400 mg/day.
Low red, processed meat diet (Albenberg 2018). The control diet included a minimum of two servings per week of red meat.
Low disaccharides, grains, saturated fats, red and processed meat diet (Mutlu 2016). The control diet was undefined, but presumably included the opposite composition of the intervention.
Symptoms‐guided diets (Candy 1995; Dariel 2007; Jones 1985; Riordan 1993). The controls arms included a high fiber diet (Jones 1985), undefined 'conventional' dietary advice (Dariel 2007), no dietary modification (Candy 1995), and corticosteroids (Riordan 1993).
Highly restricted organic diet (Bartel 2008). The control diet included a low‐fat, low‐fiber, high‐carbohydrate diet.
Milk‐free diets (Strisciuglio 2013; Wright 1965). The control diets included an unrestricted diet (Strisciuglio 2013), or the exclusion of certain food items, such as fried foods, condiments, and ice cream (Wright 1965).
Alberta‐based anti‐inflammatory diet (Keshteli 2016). The control diet included recommendations from the Canada Food Guide.
Carrageenan‐free diet (Bhattacharyya 2017). The control diet included 100 mg of encapsulated food‐grade carrageenan with each meal (Bhattacharyya 2017).
After randomisation, dietary instruction was provided by a dietitian or other research personnel in most studies (Bartel 2008; Bhattacharyya 2017; Brandes 1981; Candy 1995; Keshteli 2016; Lomer 2001; Lomer 2005; Riordan 1993; Ritchie 1987; Wright 1965). Dietary instruction was primarily provided by written materials in three studies (Brotherton 2014; Lorenz‐Meyer 1996; Strisciuglio 2013), and advice was provided through unclear mechanisms in four studies (Albenberg 2018; Dariel 2007; Jones 1985; Mutlu 2016).
In studies which provided some description, the intervention regimen varied and there was very little information on food groups which participants were exposed to other than the study intervention. The specific proportions or concentrations of macro‐ and micronutrients consumed at baseline or after randomisation were not reported. Nonetheless, adherence to dietary recommendations was monitored through periodic interviews in most studies (Bartel 2008; Bhattacharyya 2017; Brandes 1981; Candy 1995; Keshteli 2016; Lomer 2001; Lomer 2005; Lorenz‐Meyer 1996; Riordan 1993; Ritchie 1987). The method for assessing dietary adherence was not reported in six studies (Albenberg 2018; Brotherton 2014; Dariel 2007; Jones 1985; Mutlu 2016; Strisciuglio 2013).
The use of concomitant treatments was discussed in eight studies with six studies reporting the use of medication (Bartel 2008; Lomer 2001; Lomer 2005; Lorenz‐Meyer 1996; Strisciuglio 2013; Wright 1965), one study indicating that drug treatment was omitted 14 days before the study commenced (Brandes 1981), and one study which administered prednisolone in the control arm which was gradually withdrawn over the course of the study (Riordan 1993). There was no mention of concomitant treatments in the rest of the studies.
Outcomes
Participants were followed up for 1 to 24 months. Outcomes of interest reported in the studies were:
Induction of remission (Bartel 2008; Brotherton 2014; Candy 1995; Dariel 2007; Lomer 2001; Lomer 2005);
Clinical relapse (Albenberg 2018; Bhattacharyya 2017; Brandes 1981; Jones 1985; Keshteli 2016; Lorenz‐Meyer 1996; Mutlu 2016; Riordan 1993; Ritchie 1987; Strisciuglio 2013; Wright 1965);
Surrogate biomarkers of inflammation (Bartel 2008; Bhattacharyya 2017; Brotherton 2014; Jones 1985; Lomer 2005; Riordan 1993; Strisciuglio 2013);
Endoscopic improvement (Bartel 2008; Candy 1995; Strisciuglio 2013);
Histologic improvement (Candy 1995; Strisciuglio 2013);
Health‐related quality of life (Bartel 2008; Bhattacharyya 2017; Brotherton 2014; Dariel 2007; Keshteli 2016; Lomer 2005);
Need for surgery (Brandes 1981; Lomer 2001; Ritchie 1987; Levenstein 1985);
Progression of disease (Bartel 2008; Brandes 1981; Levenstein 1985); and
Escalation of therapy (Levenstein 1985).
Funding and declaration of interest
Seventy‐two per cent of the included studies reported no information on both funding sources and declarations of interest (Albenberg 2018; Brandes 1981; Candy 1995; Dariel 2007; Jones 1985; Keshteli 2016; Levenstein 1985; Lorenz‐Meyer 1996; Mutlu 2016; Riordan 1993; Ritchie 1987; Strisciuglio 2013; Wright 1965). In two studies, authors had no conflicts of interest, however, funding was not reported (Bhattacharyya 2017; Lomer 2005). Three studies were funded by a stipend from a University (Bartel 2008), or grants from government organizations (Brotherton 2014; Lomer 2001), however, the authors of these studies did not declare any financial interests.
Excluded studies
Thirty‐five studies were excluded for reasons which are detailed in the Characteristics of included studies tables and summarised below:
Twelve excluded studies had the wrong study design (Barnes 2016; Beattie 1994; Brandes 1982; Castro 1995; Ciccimarra 1998; Cohen 2012; Davies 1978; Halmos 2016, NCT02345733; NCT02922881; NCT03171246; Pituch‐Zdanowska 2018).
Six studies either assessed a mixed population and did not report outcomes by sub‐population (Gunasekeera 2016; Pedersen 2017; Stange 1990), or assessed IBS (Vincenzi 2016), healthy participants (NCT02426567) or failed to provide sufficient information on baseline disease activity (Kyaw 2014).
Five studies assessed the wrong interventions (Boneh 2017; Dunn 2017; El‐Tahir 1998; NCT02231814; Strohm 1981).
Five of the excluded studies failed to assess outcomes of interest (Bentz 2010; Mikolaitis 2013; NCT02469220; Pedersen 2014; Svolos 2016).
Seven studies were excluded for other reasons such as lack of response from authors who were contacted for additional information (NCT02093780; NCT02213835; NCT02357537; NCT02610101; NCT02930564), study abandonment (NCT02945488) and study termination (NCT01749813).
Risk of bias in included studies
Fourteen included studies were rated as high risk of bias for one or more items. Four studies were rated as unclear risk of bias for two or more items. Details of the risk of bias assessment have been presented in the Characteristics of included studies table and are summarised below (See Figure 3).
3.
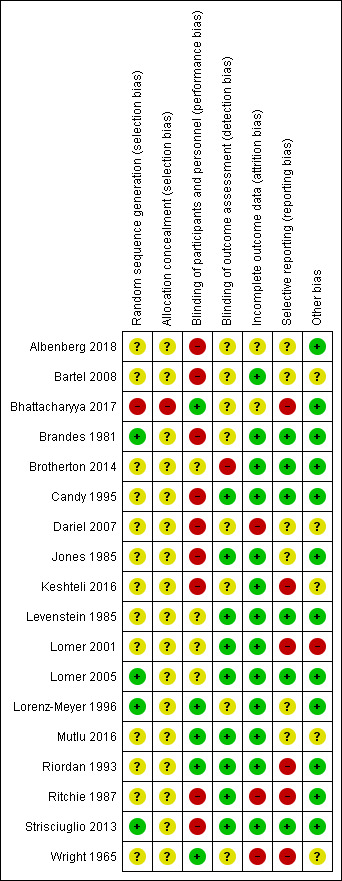
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen of the included studies were rated as unclear risk of bias for failing to report methods used for random sequence generation and allocation concealment (Albenberg 2018; Bartel 2008; Brotherton 2014; Candy 1995; Dariel 2007; Jones 1985; Keshteli 2016; Levenstein 1985; Lomer 2001; Mutlu 2016; Riordan 1993; Ritchie 1987; Wright 1965). One study was judged as being at high risk of bias for random sequence generation because a quasi‐randomized procedure (date of birth) was used to assign participants at one of the study centers (Bhattacharyya 2017). Since allocation concealment is not feasible in quasi‐randomized trials, we also judged the study as being at high risk of bias for allocation concealment. Four studies were judged as being at low risk of bias for both sequence generation and unclear risk of bias for allocation concealment (Brandes 1981; Lomer 2005; Lorenz‐Meyer 1996; Strisciuglio 2013).
Blinding
We judged two studies as being at low risk of performance and detection bias (Mutlu 2016; Riordan 1993). Whilst Mutlu 2016 was referred to as double blind and further details were not provided, we considered the information sufficient to make this judgement.
Performance bias
Nine studies which were not referred to as blinded were judged as being at high risk of performance bias. Four studies only provided information on the blinding of either the participants or study personnel but not both (Brotherton 2014; Levenstein 1985; Lomer 2001; Lomer 2005). We judged five studies as being at low risk of bias for using some sort of 'dummy' diet or tablet (Bhattacharyya 2017; Lorenz‐Meyer 1996; Mutlu 2016; Riordan 1993; Wright 1965).
Detection bias
When assessing for detection bias, nine studies were found to be at low risk of bias (Candy 1995; Jones 1985; Levenstein 1985; Lomer 2001; Lomer 2005; Mutlu 2016; Riordan 1993; Ritchie 1987; Strisciuglio 2013). Eight were judged to be at unclear risk of bias (Albenberg 2018; Bartel 2008; Bhattacharyya 2017; Brandes 1981; Dariel 2007; Keshteli 2016; Lorenz‐Meyer 1996; Wright 1965). Outcome assessors were not blinded in Brotherton 2014.
Incomplete outcome data
In two studies, attrition rates were sufficiently different enough to induce clinically relevant bias in intervention effect estimates (Dariel 2007; Wright 1965), and in one study four times more patients from the intervention group withdrew due to non‐compliance compared to the control group (Ritchie 1987). These studies were assessed as being at high risk of attrition bias. We found 13 studies to be at low risk of attrition bias as attrition rates were low and balanced across groups (Bartel 2008; Brandes 1981; Candy 1995; Levenstein 1985; Lomer 2001; Lomer 2005; Lorenz‐Meyer 1996; Riordan 1993), and all participants were accounted for (Brotherton 2014; Jones 1985; Keshteli 2016; Mutlu 2016; Strisciuglio 2013). Bhattacharyya 2017 was found to be at unclear risk of attrition bias.
Selective reporting
None of the included studies had a protocol or trial registration with the exception of Bhattacharyya 2017. Six studies were judged as being at low risk of bias for reporting all outcomes prespecified in the methods section (Brandes 1981; Brotherton 2014; Candy 1995; Levenstein 1985; Lomer 2005, Strisciuglio 2013). Five studies were judged to be at high risk of bias due to a study not reporting on an outcome which was prespecified in the trial protocol (Bhattacharyya 2017), or for reporting outcomes as not statistically significant (or reporting P values) without reporting any further data (Keshteli 2016; Lomer 2001; Riordan 1993; Ritchie 1987). The rest of the studies were judged to be at unclear risk of bias.
Other potential sources of bias
Most of the studies (65%) were judged as being at low risk of bias for other sources of bias as there was no indication of other biases occurring. Five studies were at unclear risk of other bias for not reporting information sufficient to determine whether there were other biases (Bartel 2008; Dariel 2007; Keshteli 2016; Mutlu 2016; Wright 1965), and one study was judged as being at high risk of bias for including participants with significantly higher CDAI scores in one group (Lomer 2001).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Intervention diet compared to control diet for induction of remission in active Crohn's disease.
| Intervention diet compared to control diet in active Crohn's disease for induction of remission in active Crohn's disease | ||||||
| Patient or population: people with active Crohn's disease Setting: home Intervention: intervention diet (various) Comparison: control diet (various) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control diet | Risk with intervention diet | |||||
| Induction of remission at 4 weeks ‐ High‐fiber, low refined carbohydrates diet | Study population | RR 7.20 (0.53 to 97.83) | 7 (1 study) | ⊕⊝⊝⊝ VERY LOW1,2 | We were unable to calculate absolute effects. Remission was induced in 100% (4/4) of participants in the low refined carbohydrates diet group compared to 0% (0/3) in the control group Clinical remission was defined as CDAI ≤150 |
|
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Induction of remission at 16 weeks ‐ Low microparticle diet | Study population | RR 3.13 (0.22 to 43.84) | 103 (2 studies) | ⊕⊝⊝⊝ VERY LOW1,3 | Clinical remission was defined as CDAI ≤150 | |
| 255 per 1,000 | 798 per 1,000 (56 to 1,000) | |||||
| Induction of remission at 16 weeks ‐ Low calcium diet | Study population | RR 1.24 (0.67 to 2.29) |
83 (1 study) | ⊕⊝⊝⊝ VERY LOW1,4 | Clinical remission was defined as CDAI ≤150 | |
| 300 per 1000 | 372 per 1000 (201 to 687) | |||||
| Induction of remission (timeframe not reported) ‐ Symptoms‐guided diet | Study population | RR 20.00 (1.27 to 315.40) | 51 (1 study) | ⊕⊝⊝⊝ VERY LOW1,5 | We were unable to calculate absolute effects. Remission was induced in 50% (16/32) of participants in the symptoms‐guided diet group compared to 0% (0/19) in the control Clinical remission was defined as CDAI ≤150 |
|
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Induction of remission at 6 weeks ‐ Highly restricted, organic diet | Study population | RR 1.00 (0.39 to 2.53) | 18 (1 study) | ⊕⊝⊝⊝ VERY LOW1,6 | Clinical remission was defined as CDAI ≤150 | |
| 500 per 1,000 | 500 per 1,000 (195 to 1,000) | |||||
| Health‐related quality of life (timeframe not reported) ‐ IBDQ ‐ Symptoms‐guided diet | The mean health related quality of life ‐ IBDQ ‐ was 0 | MD 23.75 higher (7.12 higher to 40.38 higher) |
‐ | 51 (1 study) | ⊕⊕⊝⊝ LOW1,7 | |
| Health‐related quality of life at 6 weeks ‐ IBDQ ‐ Highly restricted, organic diet | The mean health related quality of life ‐ IBDQ was 0 | MD 4 higher (17.86 lower to 25.86 higher) |
‐ | 14 (1 study) | ⊕⊝⊝⊝ VERY LOW1,8 | |
| Adverse events | None reported in any of the studies | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio; CDAI: Crohn's disease activity index; IBDQ: Inflammatory Bowel Disease Questionnaire; MD: Mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high or unclear risk of bias
2 Downgraded two levels due to serious imprecision (4 events)
3 Downgraded two levels due to serious imprecision (36 events)
4 Downgraded two levels due to serious imprecision (28 events)
5 Downgraded two levels due to serious imprecision (16 events)
6 Downgraded two levels due to serious imprecision (9 events)
7 Downgraded one level due to imprecision (51 participants)
8 Downgraded two levels due to serious imprecision (14 participants)
Summary of findings 2. Intervention diet compared to control diet for maintenance of remission in inactive Crohn's disease.
| Intervention diet compared to control diet for maintenance of remission in inactive Crohn's disease | ||||||
| Patient or population: people with inactive Crohn's disease Setting: home Intervention: intervention diet (various) Comparison: control diet (various) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control diet | Risk with intervention diet | |||||
| Clinical relapse at 12 to 24 months ‐ Low refined carbohydrate diet | Study population | RR 1.04 (0.87 to 1.25) | 567 (3 studies) | ⊕⊕⊝⊝ LOW1,2 | Relapse was defined as CDAI > 150 | |
| 637 per 1,000 | 662 per 1,000 (554 to 796) | |||||
| Clinical relapse at 6 to 24 months ‐ Symptoms‐guided diet | Study population | RR 0.53 (0.28 to 1.01) | 98 (2 studies) | ⊕⊕⊝⊝ LOW1,3 | Relapse was defined as CDAI > 150 | |
| 833 per 1,000 | 442 per 1,000 (233 to 842) | |||||
| Clinical relapse at 48 weeks ‐ Low red, processed meat diet | Study population | RR 1.03 (0.85 to 1.26) | 214 (1 study) | ⊕⊕⊝⊝ LOW1,4 | Relapse was defined as CDAI > 150 | |
| 636 per 1,000 | 655 per 1,000 (540 to 801) | |||||
| Clinical relapse at 12 months ‐ Exclusion diets (low disaccharides, grains, saturated fats, red and processed meats) | Study population | RR 0.11 (0.01 to 1.76) | 54 (1 study) | ⊕⊝⊝⊝ VERY LOW1,5 | ||
| 263 per 1,000 | 29 per 1,000 (3 to 463) | |||||
| Health‐related quality of life | None reported in any of the studies | |||||
| Adverse events | None reported in any of the studies | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; CDAI: Crohn's disease activity index. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high or unclear risk of bias
2 Downgraded one level due to imprecision (369 events)
3 Downgraded one level due to imprecision (64 events)
4 Downgraded one level due to imprecision (138 events)
5 Downgraded two levels due to serious imprecision (10 events)
Summary of findings 3. Intervention diet compared to control diet for induction of remission in active ulcerative colitis.
| Intervention diet compared to control diet for the induction of remission in active ulcerative colitis | ||||||
| Patient or population: people with active ulcerative colitis Setting: home Intervention: intervention diet (various) Comparison: control diet (various) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control diet | Risk with intervention diet | |||||
| Induction of remission ‐ Symptoms‐guided diet | Study population | RR 8.25 (0.50 to 136.33) | 21 (1 study) | ⊕⊝⊝⊝ VERY LOW1,2 | We were unable to calculate absolute effects. Remission was induced in 36% (4/11) of symptoms‐guided diet participants compared to 0% (0/10) of the control group Remission was defined as passage of normal stools with absence of rectal bleeding |
|
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Health‐related quality of life | Not reported in any of the studies | |||||
| Adverse events | Not reported in any of the studies | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high or unclear risk of bias
2 Downgraded two levels due to serious imprecision (4 events)
Summary of findings 4. Intervention diet compared to control diet for maintenance of remission in inactive ulcerative colitis.
| Intervention diet compared to control diet for maintenance of remission in inactive ulcerative colitis | ||||||
| Patient or population: Participants with inactive ulcerative colitis Setting: home Intervention: Intervention diet (various) Comparison: control diet (various) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control diet in inactive ulcerative colitis | Risk with Intervention diet | |||||
| Clinical relapse at 6 months ‐ Anti‐inflammatory diet (Alberta‐based) | Study population | RR 1.25 (0.42 to 3.70) | 28 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,2 | Relapse was defined by a Mayo score > 3 | |
| 286 per 1,000 | 357 per 1,000 (120 to 1,000) | |||||
| Clinical relapse at 12 months ‐ Carrageenan‐free diet | Study population | RR 0.50 (0.15 to 1.64) | 15 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,3 | Relapse was defined by an increase in the Simple Clinical Colitis Activity Index of > 2 points | |
| 600 per 1,000 | 300 per 1,000 (90 to 984) | |||||
| Clinical relapse at 12 months ‐ Milk free diet | Study population | RR 0.83 (0.60 to 1.15) | 77 (2 RCTs) | ⊕⊕⊝⊝ LOW1,4 | Relapse was defined by a Pediatric Ulcerative Colitis Activity Index score > 10 points or by clinical symptoms (4 or more diarrhea movements per day with visible blood) | |
| 684 per 1,000 | 568 per 1,000 (411 to 787) | |||||
| Health related quality of life at 12 months ‐ SIBDQ ‐ Carrageenan‐free diet | The mean health related quality of life ‐ SIBDQ was 0 | MD 1.7 higher (4.83 lower to 8.23 higher) | ‐ | 12 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,5 | |
| Adverse events | Not reported in any of the studies | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; SIBDQ: Short Inflammatory Bowel Disease Questionnaire; MD: Mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high or unclear risk of bias
2 Downgraded two levels due to serious imprecision (9 events)
3 Downgraded two levels due to serious imprecision (6 events)
4 Downgraded one level due to imprecision (49 events)
5 Downgraded two levels due to very serious imprecision (12 participants)
Intervention diet versus control diet for the induction of remission in active CD
Six studies compared various exclusion diets with control diets in participants with active Crohn's disease (Bartel 2008; Brotherton 2014; Dariel 2007; Levenstein 1985; Lomer 2001; Lomer 2005). The exclusion diets that were assessed included low refined carbohydrates, low microparticle, low fiber, low calcium, symptoms‐guided and a highly restricted organic diet.
Induction of remission
The effect of low refined carbohydrates, low microparticle diet, low calcium diet, symptoms‐guided diet, and a highly restricted organic diet on inducing remission in active CD is uncertain as the certainty of the evidence was assessed as very low (See Analysis 1.1; Table 1).
1.1. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 1 Induction of remission.
At 4 weeks, remission was achieved by all participants (4/4) in the high fiber, low refined carbohydrates diet group compared with none of the participants (0/3) in the control group (RR 7.20, 95% CI 0.53 to 97.83; 7 participants; 1 study; very low certainty evidence). Forty‐four per cent (23/52) of patients in the low microparticle diet group achieved remission at 16 weeks compared to 25% (13/51) of control‐group patients (RR 3.13, 95% CI 0.22 to 43.84; 103 participants; 2 studies; I² = 73%; very low certainty evidence). Fifty per cent (16/32) of participants in the symptoms‐guided diet group achieved remission (unclear when remission was measured) compared to 0% (0/19) of participants in the control group (RR 20.00, 95% CI 1.27 to 315.40; 51 participants; 1 study; very low certainty evidence). Fifty per cent (4/8) of patients in the highly restricted organic diet group achieved remission at 6 weeks (50%; 4/8) compared to 50% (5/10) of the control group (RR 1.00, 95% CI 0.39 to 2.53; 18 participants; 1 study; very low certainty evidence). Thirty‐seven per cent (16/43) of participants in the low calcium diet group achieved remission at 16 weeks compared to 30% (12/40) of the control group (RR 1.24, 95% CI 0.67 to 2.29; 83 participants; 1 study; very low certainty evidence).
Need for surgery
The effect of a low microparticle diet or a low fiber diet on the need for surgery is uncertain (Analysis 1.6). At 4 months, the proportion of patients who needed surgery in the low microparticle group was 10% (1/10) compared to 0% (0/10) in the control group (RR 3.00, 95% CI 0.14 to 65.90; 20 participants ; 1 study). After 24 months, the need for surgery was reported among an equal proportion of patients on both low fiber diet (14%; 5/30) and control diet (14%; 4/28) diet (RR 1.17, 95% CI 0.35 to 3.91; 58 participants; 1 study).
1.6. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 6 Need for surgery.
Health related quality of life (IBDQ)
It is uncertain whether symptoms‐guided or highly restricted organic diets improve health related quality of life as the certainty of the evidence was assessed as very low (Analysis 1.5; Table 1). The mean difference in the IBDQ‐score between the symptoms‐guided diets (mean 175.9 +/‐ 28.8; 32 participants) and the control diet (mean 152.15 +/‐ 29.6; 19 participants) (follow‐up period unclear) was 23.75 (95% CI 7.12 to 40.38; 51 participants; 1 study; very low certainty evidence). The mean IBDQ score among the followers of the highly restricted organic diet after 24 weeks was 196 (+/‐ 20) compared to a mean score of 192 (SD = 20) found among the control group (MD 4.00, 95% CI ‐17.86 to 25.86; 14 participants; 1 study; very low certainty evidence).
1.5. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 5 Health related quality of life ‐ IBDQ.
Two studies also reported on IBDQ but did not report sufficient information to allow for meta‐analysis. Lomer 2005 measured IBDQ scores among patients in both the low and normal microparticle diet (reported P = 0.2; 83 participants; no specific data for either group reported) and the low versus normal calcium diet (reported P = 0.7; 83 participants; no specific data for either group reported) groups after 52 weeks. However, data were not sufficiently reported for a meta‐analysis. Brotherton 2014 reported higher scores on the IBDQ in the intervention group over time than those in control group during the 4‐week period (reported P = 0.028; 7 participants). Change in mean scores were reported in the low refined carbohydrate group (44.25 points) and the control diet (19 points). When both groups were compared, reported P = 'n.s'.
Surrogate biomarkers of inflammation
One study with 14 participants and a follow‐up of 24 weeks reported on CRP and ESR. It is uncertain whether highly restricted organic diets lead to a difference in CRP (Analysis 1.2) or ESR (Analysis 1.3). At 24 weeks, the mean CRP among the followers of the highly restricted organic diet was 1.1 mg/dL (+/‐ 1) compared to a mean of 0.7 mg/dL (+/‐ 0.4) in the control group (MD 0.40, 95% CI ‐0.51 to 1.31; 14 participants; 1 study); while the mean ESR was 15 mm/h (+/‐ 3) in the highly restricted organic diet compared to a mean of 20 mm/h (+/‐ 20) found among the control group (MD ‐5.00, 95% CI ‐15.15 to 5.15; 14 participants; 1 study).
1.2. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 2 Surrogate inflammatory biomarker ‐ CRP.
1.3. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 3 Surrogate inflammatory biomarker ‐ ESR.
CRP, ESR and fecal calprotectin data in the low microparticle (42 participants) versus normal microparticle (41 participants) diet (reported P = 0.2, 0.5 and 0.07 respectively) and among the low calcium (43 participants) versus normal (40 participants) calcium diet (reported P = 0.6, 0.6 and 0.9 respectively) at week 52 were reported in Lomer 2005, however, the data were not shown.
Brotherton 2014 also measured CRP (reported P = 0.125) and ESR (reported P = 0.788) at 4 weeks. No further details were provided.
Endoscopic improvement
The effect of highly restricted organic diets on endoscopic improvement is uncertain (Analysis 1.4). At 24 weeks 60% (3/5) of participants in the highly restricted organic diet group reported endoscopic improvement versus 11% (1/9) in the control group (RR 5.40, 95% CI 0.74 to 39.17; 14 participants; 1 study).
1.4. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 4 Endoscopic improvement.
Disease progression
Disease progression was reported in two studies which compared highly restricted organic diet and low fiber diet with control diets (Bartel 2008; Levenstein 1985). The effect of highly restricted organic diets and low fiber diet on disease progression is uncertain (Analysis 1.7). At 24 weeks, progression of disease was reported in 20% (1/5) of participants in the highly restricted organic diet compared to 33% (3/9) of participants in the control group (RR 0.60, 95% CI 0.08 to 4.35; 14 participants; 1 study). After 24 months, progression of disease was reported in 37% (11/30) participants in the low fiber diet group versus 28% (8/28) in the control (RR 1.28, 95% CI 0.61 to 2.72; 58 participants; 1 study).
1.7. Analysis.
Comparison 1 Intervention diet versus control diet in active Crohn's disease, Outcome 7 Disease progression.
Intervention diet versus control diet for the maintenance of remission in inactive CD
Seven studies compared various exclusion diets with control equivalent in inactive CD (Albenberg 2018; Brandes 1981; Jones 1985; Lorenz‐Meyer 1996; Mutlu 2016; Riordan 1993; Ritchie 1987). The exclusion diets studied included low refined carbohydrate diet, symptoms‐guided diet, low red processed meat diet and low disaccharides / grains / saturated fats / red and processed meat diet.
Clinical relapse
There is no clear difference in clinical relapse rates when low refined carbohydrate diets, symptoms‐guided diets and low red processed meat diets are compared with control diet. The certainty of the evidence is judged as low (Analysis 2.1; Table 2).
2.1. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 1 Clinical relapse.
At 12 to 24 months, the proportion of participants with clinical relapse in the low refined carbohydrate group was 67% (176/264) compared to 64% (193/303) in the control group (RR 1.04, 95% CI 0.87 to 1.25; 567 participants; 3 studies; I² = 35%; low certainty evidence). In the symptoms guided diet clinical relapse was reported in 48% (24/50) of participants compared to 83% (40/48) of participants in the control diet at 6 to 24 months (RR 0.53, 95% CI 0.28 to 1.01; 98 participants; 2 studies; I² = 54%; low certainty evidence). At 48 weeks, 66% (63/96) of participants in the low red processed meat diet group relapsed compared to 63% (75/118) in the control group (RR 1.03, 95% CI 0.85 to 1.26; 214 participants; 1 study; I² = 0%; low certainty evidence). At 12 months, an exclusion diet of low disaccharides / grains / saturated fats / red and processed meat resulted in no clinical relapse (0/16) compared to 26% (10/38) among the control group (RR 0.11, 95% CI 0.01 to 1.76; 54 participants; 1 study; very low certainty evidence).
We carried out sensitivity analyses based on per‐protocol data and fixed‐effect model. In both instances we found that the effect of symptoms‐guided diets on clinical relapse was uncertain.
Need for surgery
It is uncertain whether low refined carbohydrate diets reduce the need for surgery (Analysis 2.4). After 24 months, surgery appeared to be necessary for 4% (8/200) of the participants following a low refined carbohydrate diet compared to 9% (16/172) of control group participants (RR 0.44, 95% CI 0.19 to 1.00; 372 participants; 2 studies; I² = 0%).
2.4. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 4 Need for surgery.
Escalation of therapy
Riordan 1993 reported on escalation of therapy and it is uncertain whether low refined carbohydrate diet reduces the incidence of escalation of therapy (Analysis 2.6). Escalation of therapy was reported for 10% (1/10) of the participants in the low refined carbohydrate group compared to none (0/10) in the control group (RR 3.00, 95% CI 0.14 to 65.90; 20 participants; 1 study).
2.6. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 6 Escalation of therapy.
Withdrawals due to adverse events
Withdrawals due to adverse events were reported in one study with 78 participants during 24 months of follow‐up. We are uncertain whether symptoms‐guided diets reduce withdrawals due to adverse events (Analysis 2.7). None (0/40) of the participants in the symptoms‐guided diet group withdrew from the study due to adverse events compared to 5% (2/38) of the participants from the control diet (RR 0.19, 95% CI 0.01 to 3.84; 74 participants; 1 study). Both of the patients in the control group were withdrawn due to steroid side effects.
2.7. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 7 Withdrawals due to adverse events.
Surrogate markers of inflammation
Riordan 1993 assessed the effect of symptoms‐guided diets on CRP ( Analysis 2.2). After 24 months, participants in the symptoms‐based diets had mean CRP scores of 2.71 mg/dL (+/‐ 6.58) compared to 2.42 mg/dL (+/‐ 2.9) in the control group (MD 0.29, 95% CI ‐1.95 to 2.53; 78 participants; 1 study).
2.2. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 2 Surrogate inflammatory biomarker ‐ CRP.
Evidence from two studies shows that the effect of symptoms‐guided diets on ESR is uncertain (Analysis 2.3). The mean ESR value during a follow‐up of 6 to 24 months, was 27.6 (SD = 24.7) mm/h among the symptoms‐guided diet followers and 33.2 (SD = 26.6) mm/h in the control diet group (MD ‐7.29, 95% CI ‐17.22 to 2.64; I² = 0%; 95 participants; 2 studies; low certainty evidence).
2.3. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 3 Surrogate inflammatory biomarker ‐ ESR.
Riordan 1993 indicated that participants in the intervention group had improved CRP concentrations. No further details were provided.
Disease progression
It is uncertain whether low refined carbohydrate diets reduce disease progression (Analysis 2.5). At the end of the 24‐month follow‐up period, disease progression was reported in 20% (1/5) of the participants on low refined carbohydrate compared to 17% (1/6) on the control diet (RR 1.20, 95% CI 0.10 to 14.69; 11 participants; 1 study).
2.5. Analysis.
Comparison 2 Intervention diet versus control diet in inactive Crohn's disease, Outcome 5 Disease progression.
Intervention diet versus control diet for the induction of remission in active UC
One study assessed a symptoms‐guided diet in participants with active UC (Candy 1995).
Induction of remission
It is uncertain whether symptoms‐guided diets improve the induction of remission as the certainty of the evidence has been assessed as very low (Analysis 3.1; Table 3). After six weeks, symptoms‐guided diet led to induction of remission among 36% ( 4/11) of symptoms‐guided diet participants compared to none (0/10) of the participants in the control dietary arm (RR 8.25, 95% CI 0.50 to 136.33; 21 participants; 1 study; very low certainty evidence).
3.1. Analysis.
Comparison 3 Intervention diet versus control diet in active ulcerative colitis, Outcome 1 Induction of remission.
Clinical improvement
It is uncertain whether symptoms‐guided diets lead to clinical improvement (Analysis 3.2). After six weeks of symptoms‐guided diets, 45% (5/11) of participants achieved clinical improvement versus 10% (1/10) in the control group (RR 4.55, 95% CI 0.63 to 32.56; 21 participants; 1 study).
3.2. Analysis.
Comparison 3 Intervention diet versus control diet in active ulcerative colitis, Outcome 2 Clinical improvement.
Endoscopic improvement
Whether symptoms‐guided diets lead to endoscopic improvement remains uncertain (Analysis 3.3). At 6 weeks, endoscopic improvement was achieved by 45% (5/11) of patients on a symptoms‐guided diet compared to 10% (1/10) of the control group (RR 3.64, 95% CI 1.00 to 13.23; 21 participants; 1 study).
3.3. Analysis.
Comparison 3 Intervention diet versus control diet in active ulcerative colitis, Outcome 3 Endoscopic improvement.
Histologic improvement
The impact of symptoms‐guided diets on histologic improvement is uncertain (Analysis 3.4). At 6 weeks, 27% (3/11) of participants in the symptoms‐guided diet group improved histologically compared to 30% (3/10) of the control group (RR 0.91, 95% CI 0.24 to 3.51; 21 participants; 1 study).
3.4. Analysis.
Comparison 3 Intervention diet versus control diet in active ulcerative colitis, Outcome 4 Histologic improvement.
Intervention diet versus control diet for the maintenance of remission in inactive UC
Four studies compared various exclusion diets with control diets in participants with inactive UC (Bhattacharyya 2017; Keshteli 2016; Strisciuglio 2013; Wright 1965). The exclusion diets studied included the Alberta‐based anti‐inflammatory diet (Bhattacharyya 2017), a Carrageenan‐free diet (Keshteli 2016), and a milk‐free diet (Strisciuglio 2013; Wright 1965).
Clinical relapse
All four studies reported on clinical relapse, but it is uncertain whether the Alberta‐based anti‐inflammatory diet, the Carrageenan‐free diet or the milk free diet reduces clinical relapse, as the certainty of the evidence was assessed as very low or low (Analysis 4.1; Table 4). At 6 months, the Alberta‐based anti‐inflammatory diet led to clinical relapse in 36% (5/14) of patients in comparison to 29% (4/14) of control participants (RR 1.25, 95% CI 0.42 to 3.70; 28 participants; 1 study; very low certainty evidence). Thirty per cent (3/10) of patients following the carrageenan‐free diet for 12 months reported clinical relapse compared to 60% (3/5) of the control group participants (RR 0.50, 95% CI 0.15 to 1.64; 15 participants; 1 study; very low certainty evidence). At 12 months, 59% (23/39) of milk free diet participants relapsed compared to 68% (26/38) of control diet participants (RR 0.83, 95% CI 0.60 to 1.15; 77 participants; 2 studies; I² = 0%; low certainty evidence).
4.1. Analysis.
Comparison 4 Intervention diet versus control diet in inactive ulcerative colitis, Outcome 1 Clinical relapse.
Endoscopic relapse
Mean endoscopic Matt colonoscopy grading scores (0 to 4; higher score = increased severity; Matt 1961) were reported by in Strisciuglio 2013 (mean change score: reported P = 0.5; 29 participants) after 12 months for the milk ‐free diet and control diet, however, data were not sufficient for a statistical analysis.
Histologic relapse
Mean histologic Matt scores at 12 months were reported in Strisciuglio 2013 (mean change score: reported P = 0.4; 29 participants) for the milk ‐free and control dietary arms, however, data were not sufficiently reported for a statistical analysis.
Surrogate inflammatory biomarkers
One study with 12 participants (Bhattacharyya 2017), reported on fecal calprotectin (Analysis 4.2), IL‐6 (Analysis 4.4), IL‐8 (Analysis 4.5) and TNF‐α (Analysis 4.3) and it is uncertain whether carrageenan‐free diets improve these parameters. At 12 months, the mean difference in the levels of these parameters between the carrageenan and the control group were as follows: fecal calprotectin 60.00 g/gm (95% CI ‐59.24 to 179.24); IL‐6 1.94 pg/ml (95% CI ‐0.35 to 4.23); IL‐8 38.00 pg/ml (95% CI ‐139.24 to 215.24) and TNF‐α ‐4.50 pg/ml (95% CI ‐8.92 to ‐0.08). After 12 months, median and range of CRP (reported P = 0.6), ESR (reported P = 0.3) and FC (reported P = 0.3) were reported in Strisciuglio 2013, however, as measures of variance were not reported, we were unable to analyse the results further.
4.2. Analysis.
Comparison 4 Intervention diet versus control diet in inactive ulcerative colitis, Outcome 2 Fecal calprotectin.
4.4. Analysis.
Comparison 4 Intervention diet versus control diet in inactive ulcerative colitis, Outcome 4 Surrogate inflammatory biomarker ‐ IL‐6.
4.5. Analysis.
Comparison 4 Intervention diet versus control diet in inactive ulcerative colitis, Outcome 5 Surrogate inflammatory biomarker ‐ IL‐8.
4.3. Analysis.
Comparison 4 Intervention diet versus control diet in inactive ulcerative colitis, Outcome 3 Surrogate inflammatory biomarker ‐ TNF‐α.
Health related quality of life (SIBDQ)
It is uncertain whether carrageenan‐free diets improve health related quality of life as the certainty of the evidence has been assessed as very low (Analysis 4.6).The mean difference in the SIBDQ between the carrageenan group and the non‐carrageenan group at 12 months was ‐1.70 (95% CI ‐8.23 to 4.83; 12 participants; 1 study; very low certainty evidence).
4.6. Analysis.
Comparison 4 Intervention diet versus control diet in inactive ulcerative colitis, Outcome 6 Health related quality of life ‐ SIBDQ.
Discussion
Summary of main results
We included 18 randomized controlled trials (1878 randomized participants) that assessed dietary interventions for the induction and maintenance of remission in IBD. The trials were subdivided into groups based on disease state and type: active CD, inactive CD, active UC and inactive UC. The dietary interventions studied involved some dietary restriction or exclusion of single or multiple food components which are believed to trigger IBD symptoms or inflammation. The diets were so varied that there was little scope for pooling data.
Induction and maintenance of remission in Crohn's disease
There is insufficient evidence to determine whether exclusion diets (low refined carbohydrates, low microparticle, symptoms‐guided diet, highly restricted organic diet and low calcium diet) improve clinical remission rates in active Crohn's disease as the certainty of the evidence was assessed as very low.
When we looked at the evidence for inactive Crohn's disease, there was little or no difference in clinical relapse rates when exclusion diets (low refined carbohydrates, symptoms‐guided and low red/processed meat diet) were compared with control diets. The certainty of the evidence was low due to risk of bias and imprecision. On the other hand, we found insufficient evidence to determine whether low disaccharides/low grain/low saturated fats/low red and processed meat diet reduces clinical relapse as the certainty of evidence was assessed as very low.
Other than two participants being withdrawn for steroid‐related side effects in a small maintenance of remission study (Riordan 1993), none of the studies assessing diet in CD reported on adverse events.
Induction and maintenance of remission in ulcerative colitis
There was insufficient evidence on whether symptoms‐based diets improve clinical remission rates in active ulcerative colitis (very low certainty).
We found very low certainty evidence from two trials which was insufficient to determine whether the Alberta‐based anti‐inflammatory diet, or a Carrageenan‐free diet reduce clinical relapse rates in inactive ulcerative colitis. We found low certainty evidence from two studies which was insufficient to determine if a milk‐free diet reduces clinical relapse rates in inactive ulcerative colitis.
None of the studies assessing diets in UC reported on adverse events.
Overall completeness and applicability of evidence
The evidence was very limited due to the initial clinical design, specific interventions and choice of populations. In particular, several studies do not describe in much detail the actual dietary manipulations used. As this almost excludes any form of true dissemination and replication, the applicability is severely hampered. Examples include high fiber + reduced refined carbohydrate, low carbohydrate + high protein and fat, milk‐free + low‐roughage, low disaccharides + low grains + low saturated fats + low red and low processed meat. This is further exemplified in the results of one intervention which showed a potential efficacious outcome in the context of symptoms‐guided diet. As this is a very subjective dietary therapy and no national or international consensus or evidence‐based method, framework or evidence was cited in the individual studies, further local research or practice in this area is also severely hampered. All the studies in this review except one, are based on an adult population with little or no information on severity of disease.
It is a real concern that no adverse events related to the interventions were discussed in any of the studies. Patients who access information from different media which promote dietary interventions based on these studies and try these diets, need to be informed of any adverse effects yet this vital piece of information is absent.
Quality of the evidence
The included studies lacked homogeneity of any sort and were methodologically flawed resulting in very heterogenous data that were difficult to analyse or summarise. This resulted in our downgrading the evidence to low or very low certainty. Another contributing factor was the lack of information to assess risk of bias and high risk of bias resulting from the difficulty in blinding participants and personnel to dietary interventions. Most of the studies had small sample sizes and sparse data with the smallest study having as few as seven participants. We found that having inadequately powered heterogenous studies with limited scope for pooling, resulted in mostly imprecise results.
There was no indirectness as the included studies were all within the scope of the review. Having a limited number of studies precluded our assessment of publication bias.
Potential biases in the review process
We acknowledge that there are certain decisions which were made during the review process which may have introduced bias in the results. For instance, some studies reported results which we had to estimate visually from graphs. Using this method may have led to inaccuracies; however, having counter measures, such as extracting the data in duplicate coupled with additional checking from other members of the author team, is likely to have minimised error. Again, the differences in dietary interventions may have led to the misclassification of diets resulting in erroneous pooling of clinically heterogenous study data. This has been a consequence of the plurality, lack of consensus and insufficient reporting of trial interventions among study investigators. These obvious differences in interventions and controls highlighted meant that we rarely downgraded evidence for unexplained heterogeneity (inconsistency). However, as most of the evidence was of very low certainty and obtained from single trials, this may not be a source of concern as a further downgrade of the certainty of evidence would not change the interpretation of the results.
Agreements and disagreements with other studies or reviews
Although it was recommended in the NICE guideline that people with Crohn's disease be given information on diet and nutrition, no details were provided on what the diets should be (NICE 2016). There is also no indication that this recommendation is based on systematic review evidence. There are currently no other systematic reviews assessing dietary interventions for induction and maintenance of remission in IBD. Compared to other studies, it is important to note that RCTs which have evaluated symptoms‐guided diets in active and inactive CD (Dariel 2007; Jones 1985; Riordan 1993), when analyzed individually, all show that the diet offers an advantage for the induction and maintenance of remission in CD. However, the evidence from our review was insufficient to support the use of symptoms‐guided diets. This could be an area for future research as more studies are needed.
Authors' conclusions
Implications for practice.
The effects of dietary interventions on CD and UC are uncertain. Thus no firm conclusions regarding the benefits and harms of dietary interventions in CD and UC can be drawn. This evidence was of very low certainty due to sparse data from heterogenous studies which had limited scope for pooling. Adverse effects of the interventions were not reported.
Implications for research.
There is need for consensus on the composition of dietary interventions in IBD and more RCTs are required to evaluate these interventions. Around 15% of the excluded studies which otherwise would have met the inclusion criteria had mixed populations of participants with UC and CD and did not report separate results for each group. As a result, these studies did not contribute to the sum of synthesised evidence. Future studies must treat these populations separately to allow meaningful interpretations to take place.
We need researchers to agree to a standardized, dietetically informed, framework or protocol for such diets being explored in RCTs to allow the wider research community to collaboratively and iteratively build on each others' research. Future RCTs need to report sufficient details on randomization, allocation concealment, blinding of outcome assessors and outcomes including adverse events.
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
Funding for ZIE, TH, and partial funding for MG was provided through a larger NIHR Cochrane Programme Grant in the UK.
Appendices
Appendix 1. Medline Search Strategy
MEDLINE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. randomized controlled trial/
14. or/1‐13
15. Exp Inflammatory bowel disease/
16. Crohn*.tw.
17. Ulcerative colitis.tw.
18. IBD.tw.
19. Inflammatory bowel disease.tw.
20. Regional ileitis.tw.
21. Colitis.tw.
22. or/15‐21
23. Exp Diet/
24. Diet therapy.tw.
25. Diet*.tw.
26. Regimen.tw.
27. Nutrition*.tw.
28. Elimination.tw.
29. Eliminat*.tw.
30. (food* OR oligosaccharides OR oligofructose OR fructooligosaccharide* OR monosaccharide*). tw.
31. (maker* diet OR fodmap* OR gluten* OR polyols OR omega* OR sugar* OR carbo* OR fruit* OR vegetable* OR sodium OR fatty acid* OR dairy OR fiber OR fibre OR protein*).tw.
32. (Vegetarian OR vegan OR macro* OR keto* OR paleo OR dissacharide* OR lactose OR sucrose OR fructose OR bran* OR solbitol OR xylitol OR psyllium OR Metamucil OR plantaglucide OR ispaghula OR isogel OR reguval OR plantago seed OR ispaghule gum ).tw.
33. Or/23‐32
35. 14 and 22 and 33
Embase
1. random$.mp.
2. factorial$.mp.
3. (crossover$ or cross over$ or cross‐over$).mp.
4. placebo$.mp.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).mp.
9. (double$ adj blind$).mp.
10. (tripl$ adj blind$).mp.
11. assign$.mp.
12. allocat$.mp.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. Exp Inflammatory bowel disease/
20. Crohn*.tw.
21. Ulcerative colitis.tw.
22. IBD.tw.
23. Inflammatory bowel disease.tw.
24. Regional ileitis.tw.
25. Colitis.tw.
26. or/19‐25
27. Exp Diet/
28. Diet therapy.tw.
29. Diet*.tw.
30. Regimen.tw.
31. Nutrition*.tw.
32. Elimination.tw.
33. Eliminat*.tw.
34. (Food* OR oligosaccharides OR oligofructose OR fructooligosaccharide* OR monosaccharide*). tw.
35. (maker* diet OR fodmap* OR gluten* OR polyols OR omega* OR sugar* OR carbo* OR fruit* OR vegetable* OR sodium OR fatty acid* OR dairy OR fiber OR fibre OR protein*).tw.
36. (Vegetarian OR vegan OR macro* OR keto* OR paleo OR dissacharide* OR lactose OR sucrose OR fructose OR bran* OR solbitol OR xylitol OR psyllium OR Metamucil OR plantaglucide OR ispaghula OR isogel OR reguval OR plantago seed OR ispaghule gum ).tw.
37. Or/27‐36
39. 18 and 26 and 37
CENTRAL
#1 MeSH descriptor: [Inflammatory Bowel Diseases] explode all trees
#2 crohn* or IBD or (inflammatory bowel disease*) or (ulcerative colitis) or colitis
#3 #1 or #2
#4 MeSH descriptor: [Diet] explode all trees
#5 Diet therapy
#6 Diet*
#7 Regimen
#8 Nutrition*
#9 Elimination
#11 Food* OR oligosaccharides OR oligofructose OR fructooligosaccharide* OR monosaccharide*
#12 Maker* diet OR fodmap* OR gluten* OR polyols OR omega* OR sugar* OR carbo* OR fruit* OR vegetable* OR sodium OR fatty acid* OR dairy OR fiber OR fibre OR protein*
#13 Vegetarian OR vegan OR macro* OR keto* OR paleo OR dissacharide* OR lactose OR sucrose OR fructose OR bran* OR solbitol OR xylitol OR psyllium OR Metamucil OR plantaglucide OR ispaghula OR isogel OR reguval OR plantago seed OR ispaghule gum
#14 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#20 #3 and #14
Web of Science
#1 TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
#2 TS= ("inflammatory bowel disease" OR "inflammatory bowel diseases" OR "ibd" OR "crohn" OR "crohns" OR “terminal ileitis” OR "enteritis regionalis" OR "Regional enteritis" OR "regional enterocolitis" OR "Ileitis" OR "colitis" OR proctit* OR proctocolit* OR colitide* OR “ulcerative colorectitis”)
#3 TS=(diet OR "diets" OR "dietary" OR "oligosaccharides" OR "oligofructose" OR "raw food" OR fermented OR "Whole 30" OR "atkins" OR "south beach" OR "makers diet" OR "maker's diet" OR fodmap OR fodmaps OR fructooligosaccharide* OR polyols OR monosaccharide* OR gluten OR "fish diet" OR "fatty acid" OR "fatty acids" OR omega‐3 OR omega‐3s OR omega‐6 OR omega‐6s OR elimination OR microparticle OR "red meat" OR sugar OR sugars OR dairy OR fiber OR fibre OR low‐residue OR "low residue" OR fruit OR fruits OR vegetable OR vegetables OR “olive oil” OR "olive oils" OR “Protein diet” OR “protein diets” OR “Protein restricted” OR “Protein‐restricted” OR “Protein‐free” OR “Protein free” OR carbohydrate OR carbo OR "fad diet" OR "fad diets" OR "high fat" OR "high fats" OR Mediterranean OR Paleolithic OR paleo OR sodium OR vegetarian OR vegan OR macrobiotic OR ketogenic OR “antinflammatory diet” OR “anti‐inflammatory diet” OR “IBD AID” OR “IBD‐AID” OR dissacharide* OR lactose OR sucrose OR fructose OR “exclusion diet” OR “exclusion diets” OR SCD OR solbitol OR xylitol OR bran OR brans OR psyllium OR Metamucil OR plantaglucide OR “ispaghule gum” OR “plantago seed” OR ispaghula OR isogel OR reguval)
#4 #1 AND #2 AND #3
Clinical trials. Gov
1. Diet and Inflammatory bowel disease
2. Diet and Ulcerative colitis
3. Diet and Crohn’s Disease
IBD Group Specialized Register
1. Diet and Inflammatory bowel disease
2. Diet and Ulcerative colitis
3. Diet and Crohn’s Disease
WHO trial registry (ICTRP)
1. Diet and Inflammatory bowel disease
2. Diet and Ulcerative colitis
3. Diet and Crohn’s Disease
Data and analyses
Comparison 1. Intervention diet versus control diet in active Crohn's disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 High‐fiber, low refined carbohydrates diet | 1 | 7 | Risk Ratio (M‐H, Random, 95% CI) | 7.20 [0.53, 97.83] |
| 1.2 Low microparticle diet | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [0.22, 43.84] |
| 1.3 Symptoms‐guided diet | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 20.00 [1.27, 315.40] |
| 1.4 Highly restricted, organic diet | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.39, 2.53] |
| 1.5 Low calcium diet | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.67, 2.29] |
| 2 Surrogate inflammatory biomarker ‐ CRP | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Highly restricted, organic diet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Surrogate inflammatory biomarker ‐ ESR | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Highly restricted, organic diet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Endoscopic improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Highly restricted, organic diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Health related quality of life ‐ IBDQ | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Symptoms‐guided diet | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 23.75 [7.12, 40.38] |
| 5.2 Highly restricted, organic diet | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐17.86, 25.86] |
| 6 Need for surgery | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Low microparticle diet | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 6.2 Low fiber diet | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.35, 3.91] |
| 7 Disease progression | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Highly restricted, organic diet | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.08, 4.35] |
| 7.2 Low fiber diet | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.61, 2.72] |
Comparison 2. Intervention diet versus control diet in inactive Crohn's disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical relapse | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low refined carbohydrate diet | 3 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.25] |
| 1.2 Symptoms‐guided diet | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.28, 1.01] |
| 1.3 Low red, processed meat diet | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.26] |
| 1.4 Exclusion diets (low disaccharides, grains, saturated fats, red and processed meats) | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.76] |
| 2 Surrogate inflammatory biomarker ‐ CRP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Symptoms‐guided diet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Surrogate inflammatory biomarker ‐ ESR | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐7.29 [‐17.22, 2.64] |
| 3.1 Symptoms‐guided diet | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐7.29 [‐17.22, 2.64] |
| 4 Need for surgery | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.00] |
| 4.1 Low refined carbohydrate diet | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.00] |
| 5 Disease progression | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Low refined carbohydrate diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Escalation of therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Low refined carbohydrate diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Symptoms‐guided diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Intervention diet versus control diet in active ulcerative colitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Symptoms‐guided diet | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 8.25 [0.50, 136.33] |
| 2 Clinical improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Symptoms‐guided diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Endoscopic improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Symptoms‐guided diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Histologic improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Symptoms‐guided diet | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Intervention diet versus control diet in inactive ulcerative colitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical relapse | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Anti‐inflammatory diet (Alberta‐based) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.42, 3.70] |
| 1.2 Carrageenan‐free diet | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.15, 1.64] |
| 1.3 Milk free diet | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.60, 1.15] |
| 2 Fecal calprotectin | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Carrageenan‐free diet | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Surrogate inflammatory biomarker ‐ TNF‐α | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Carrageenan‐free diet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Surrogate inflammatory biomarker ‐ IL‐6 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Carrageenan‐free diet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Surrogate inflammatory biomarker ‐ IL‐8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Carrageenan‐free diet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Health related quality of life ‐ SIBDQ | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Carrageenan‐free diet | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albenberg 2018.
| Methods |
Study design: RCT (Abstract) Setting: USA; Recruited from CCFA (Crohn's & Colitis Foundation of America) Partners, an internet‐based cohort of IBD patients |
|
| Participants |
State of disease / disease type: Inactive / CD Inclusion: Individuals who were in remission (abbreviated Crohn’s Disease Activity Index [aCDAI] ≤ 150) and who reported consumption of red meat at least once weekly Exclusion: Not stated Age: Not stated Sex: Not stated Disease location: Not stated Medication use: Not stated Length of remission at study entry: Not stated Number randomized (n = 659): Not stated (Group 1) / Not stated (Group 2). Among randomized participants who consented (n = 214) ‐ 96 (Group 1) / 118 (Group 2) Number analyzed: Not stated Post‐randomisation exclusion (n = 445): 445 randomized subjects did not consent to participation in study |
|
| Interventions |
Group 1: Not more than 1 serving per month of red or processed meat for 48 weeks Group 2: Minimum of 2 servings per week of red or processed meat for 48 weeks All participants: Not stated |
|
| Outcomes |
Duration of follow‐up: 48 weeks 1. Relapse (a CDAI > 150 and increase in a CDAI by 70 points) 2. Moderate/severe relapse (a CDAI > 219 or need for CD surgery or new CD medication) |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were reportedly randomized to study groups The method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however, it is unlikely that blinding was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract publication ‐ dropouts were not described |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome was incompletely reported in the abstract, but additional details were obtained after contacting the authors |
| Other bias | Low risk | Quote: "Baseline characteristics were similar in each arm and among those who consented versus non‐participants" |
Bartel 2008.
| Methods |
Study design: RCT Setting: Austria |
|
| Participants |
State of disease / disease type: Active / CD Inclusion: Mild‐to‐moderate active CD (CDAI 150 ‐ 220) with ulceration of the left‐sided colon or a significant lesion of the small bowel or right‐sided colon that was assessable by means of MRI Exclusion: Signs or symptoms that needed operation within 1 week, obvious lack of compliance with any of the diet regimens (e.g., vegetarians), BMI < 18, accidental weight loss of > 5% in the last 3 months, therapy with prednisone > 25 mg or any dose equal or below that had been adjusted during the previous 4 weeks, budesonide or mesalamine that had been adjusted during the same period, azathioprine or anti‐TNF therapy that had been initiated or changed during the previous 3 months, or concomitant antibiotic or probiotic therapy Age: 48 ± 14.7 years Sex (M:F): 9:5 Disease location: Not stated Medication use: Mesalazine (6); Prednisolone (1); Budesonide (4); Azathioprine (4); Infliximab (1) Length of remission at study entry: Not applicable Number randomized (n = 18): 8 (Group 1) / 10 (Group 2) Number analyzed (n = 14): 5 (Group 1) / 9 (Group 2) Post‐randomisation exclusion (n = 4): Reluctant to follow strict diet (3 in Group 1); ineffective diet (1 in Group 2) |
|
| Interventions |
Group 1: Restricted organic diet ‐ highly restricted diet composed of red meat, certain sourdough bread, and small amounts of rape oil (for frying the meat) and fresh butter, all from intensively monitored organic farming (avoidance of chemical fertilisation, pesticides, genetically manipulated crops, food processing, gamma irradiation, chemical preservation, or industrial food additives). Only plain tap water and organic tea were allowed for drinking and rock salt (halite) was allowed for spicing. Fruits and vegetables were excluded from the diet. Baking soda toothpaste was given for dental care. Participants were told to avoid the use of dishwasher, limit dishwashing‐soap, and rinse places with water. Between weeks 6 and 24, participants were instructed to add other food items and drinks, derived from organic farming (local fruits and vegetables, dairy products, beer, wine, honey, etc.). Refined sugar and ready‐made canned or frozen food were not allowed Group 2: Low‐fat, high‐carbohydrate diet that is complete in nutrients. No fiber‐rich fruits, vegetables, or red meat were allowed. From weeks 6 to 24, participants were instructed to eat red meat but still to avoid fiber‐rich and hard fibrous fruits and vegetables All participants: Both groups received 3 intramuscular multivitamin injections (vitamin B complex and vitamin C) at weeks 0, 3, and 6. All participants received dietary counselling by a single dietician. No change in concomitant therapy was made during the study. Where symptoms of CD deteriorated (CDAI increase by > 70 points from baseline for 2 weeks) or a change in medication was necessary, the patient was withdrawn from the study. Both groups were contacted by the dietician 1 week after the initial counselling and at weeks 3 and 6. Participants had 2 more dietary counsels at weeks 12 and 24 |
|
| Outcomes |
Duration of follow‐up: 24 weeks 1. Improvements in MRI, endoscopy, and transabdominal bowel sonography scores 2. CDAI 3. Inflammatory parameters (CRP, ESR, α‐1‐acid glycoprotein) 4. IBDQ 5. Nutritional status assessed using BMI, total protein, albumin, cholesterol, triglycerides, ferritin, transferrin |
|
| Notes |
Funding source: G.B. received a student stipend from the Medical University of Vienna Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eighteen patients were randomly assigned to receive either the active or the control diet at a 1:1 ratio" Comment: Insufficient information on how this was performed |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only the study coordinator and the dietician were aware of the group assignment There is no information to suggest that participants where blinded to the intervention they received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. However, outcomes using MRI were assessed blindly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were balanced across group |
| Selective reporting (reporting bias) | Unclear risk | Trial registration/protocol not available. It was not specified in the Methods section that remission would be reported |
| Other bias | Unclear risk | Baseline characteristics partially reported and did not account for the participants who were excluded post‐randomisation |
Bhattacharyya 2017.
| Methods |
Study design: RCT Setting: USA |
|
| Participants |
State of disease / disease type: Inactive / UC Inclusion: A biopsy‐confirmed diagnosis of ulcerative colitis in patients >18; previous need for corticosteroids to obtain remission; in clinical remission off corticosteroids for at least one month; SCCAI score ≤ 2; on no medications or stable dose of maintenance medication; and willingness to follow a carrageenan‐free diet for 12 months. Exclusion: Active UC on corticosteroids; unable to read labels on food products; inability to make choices about diet Age: 34 ‐ 65 years Sex (M:F): 8:4 Disease location: Not stated Medication use: None (2); Mesalamine (5); Sulfasalazine (2); Thiopurine (4); Adalimumab (3) Length of remission at study entry: Not stated Number randomized (n = 15): 10 (Group 1) / 5 (Group 2) Number analyzed (n = 12): 7 (Group 1) / 5 (Group 2) Post‐randomisation exclusion (n = 3): 3 patients in Group 1 did not receive allocated intervention, citing reluctance to comply |
|
| Interventions |
Group 1: Carrageenan‐free diet + placebo (dextrose) Group 2: Carrageenan‐free diet + carrageenan‐containing capsules ‐ initially one capsule daily (100 mg), and increased intake to two capsules (200 mg) daily after finding that one capsule daily was well‐tolerated All participants: All participants were instructed by study investigators and/or participating dieticians in the carrageenan‐free diet |
|
| Outcomes |
Duration of follow‐up: 12 months (or study was curtailed when statistical significance in relapses between the two groups was demonstrated 1. Relapse ‐ defined as an increase of 2 or more points on the SCCAI in association with an increase in treatment (either an increase in maintenance medication dose or addition of new therapies for flare) by the participant’s personal physician for manifestations of UC 2. Inflammatory biomarkers (serum Interleukin‐8, interleukin‐6, tumor necrosis factor‐α, monocyte chemoattractant protein‐1, leukocyte nuclear factor‐kappaB, B‐cell leukemia/lymphoma 10, fecal calprotectin 3. SIBDQ |
|
| Notes |
Funding source: Not stated Conflict of interest: Authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Two randomization schemes were used due to convenience. Randomization at UIC was performed by a UIC investigational pharmacist who had no contact with the study participants. Allocation to study tablets #1 or #2 was performed by using a randomization table (www.randomization.com) in which the next available treatment was assigned to the next study enrollee. Within a block of ten subjects, an equal number of assignments to #1 or #2 was allocated. Randomization at U of C was performed by a blinded investigator, who allocated assignment based on the participant’s year of birth [odd or even; #1 or #2]." Comment: Both centres used different methods of randomisation. The University of Illinois at Chicago (UIC) used an adequate method of randomisation, while participants at the University of Chicago (U of C) were quasi‐randomized |
| Allocation concealment (selection bias) | High risk | Quote: "Randomization at U of C was performed by a blinded investigator, who allocated assignment based on the participant’s year of birth [odd or even; #1 or #2]" Comment: Quasi‐randomisation where allocation could have been predicted Quote: "Randomization at UIC was performed by a UIC investigational pharmacist who had no contact with the study participants" Comment: Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were randomized to receive either capsules containing 100 mg of food‐grade carrageenan or similar appearing dextrose‐containing capsules" Comment: Placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Similar to participants, there was insufficient information on whether outcome assessors were sufficiently blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three participants in the intervention group did not wish to comply with the diet and dropped out before receiving the intervention |
| Selective reporting (reporting bias) | High risk | Protocol available (NCT01065571). However, the prespecified primary outcome of time to relapse was not reported in the publication |
| Other bias | Low risk | Groups well‐balanced with no statistical differences in any parameter at baseline |
Brandes 1981.
| Methods |
Study design: RCT Setting: Germany |
|
| Participants |
State of disease / disease type: Active and inactive / CD* Inclusion: Patients with radiographic, endoscopic, and/or histologically confirmed CD who could not be included in another European multi‐center CD study and has had CD > 2 years; at least 14 days after discontinuation of all medications Exclusion: Patients with CDAI > 200 points; treatment failures (i.e., patients who have undergone surgery or whose activity index increased due to recurrence of disease > 250 points or with persistent duration of > 3 months between 200 to 250 points) Age: 32 years (Group 1) / 35 (Group 2) Sex (M:F): 13:7 Disease location: Ileal: Ileum only (10); colon only (3); ileum and colon (7) Medication use: Sulphasalazine (1); Corticosteroids (2); Sulphasalazine + corticosteroids (8); Corticosteroids + azathioprine (9); Length of remission at study entry: Not stated Number randomized (n = 20): 10 (Group 1) / 10 (Group 2); stratified by disease activity CDAI 0 ‐ 100: 5 (Group 1) / 6 (Group 2); CDAI 101 ‐ 200: 5 (Group 1) / 4 (Group 2) Number analyzed (n = 17): 10 (Group 1) / 10 (Group 2) Post‐randomisation exclusion (n = 3): 3 patients no longer followed the diet |
|
| Interventions |
Group 1: Low carbohydrate diet with a relative increase in protein and fat intake, with macronutrient nutrient ratio approx 45% carbohydrates, 25% protein, and 30% fat. Foods and beverages with a high content of refined carbohydrates were forbidden. The residual content of carbohydrates contained largely natural, non‐refined carbohydrates Group 2: Carbohydrate‐rich diet with relative reduction in protein and fat intake. The nutrient ratios were approximately 60% carbohydrates, 15% protein, and 25% fat. The difference in carbohydrate content from Group 1 consisted exclusively of foods and drinks with high levels of refined carbohydrates All participants: Drug treatment was omitted 14 days before commencement of the study. Depending on body weight, a high‐calorie or low‐calorie diet was prescribed. All patients were advised in detail by a dietician. Each patient received a recipe for the diet with several days' worth of cost‐cutting suggestions and a list of permitted and prohibited foods. For each ambulatory visit, a targeted survey was performed to assess adherence to the assigned diet. Patients who did not adhere to the prescribed diet for at least 12 months were withdrawn from the study |
|
| Outcomes |
Duration of follow‐up: 24 months (average of 18 months) 1. CDAI (patients withdrawn from the study if they had to undergo surgery or whose CDAI > 250 due to a relapse of the disease and persisted between 200 ‐ 250 for longer than three months) 2. Blood markers |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated *Participants were stratified by disease activity CDAI 0 ‐ 100 and CDAI 101 ‐ 200. While those with CDAI 0 ‐ 100 were regarded as having inactive CD (remission), those with CDAI 101 ‐ 200 were classed as moderate. Due to lack of clarity we focused only on data from those with inactive remission (11 participants) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation in two diet groups was carried out with random numbers" |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. However, it is unlikely that blinding was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information on blinding of outcome assessors not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low and balanced across groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods section assessed and reported |
| Other bias | Low risk | Groups well‐balanced with no statistical differences in any parameter at baseline. No other apparent sources of bias detected |
Brotherton 2014.
| Methods |
Study design: RCT Setting: USA |
|
| Participants |
State of disease / disease type: Active / CD Inclusion: Diagnosed with CD through colonoscopy and biopsy; aged 18 to 64 years; partial HBI (pHBI) score ≥3 and at least 4 weeks of stable pharmacologic therapy Exclusion: Short bowel syndrome, diverticulitis, or any other major diagnosis that affects the GI tract; special dietary restrictions; mental, emotional, cognitive, or other disorders that might interfere with the ability to independently follow detailed dietary instructions over time; cancer; pregnancy; unstable or uncontrolled kidney or cardiovascular disease; decompensated liver disease; penetrating CD; clinically significant stricturing CD; a pHBI score >9; use of biologic drugs Age: 29.5 ± 13.6 Sex (M:F): 1:6 Disease location: Not stated Medication use: Not stated Number randomized (n = 7): 4 (Group 1) / 3 (Group 2) Number analyzed (n = 7): 4 (Group 1) / 3 (Group 2) Post‐randomisation exclusion: None |
|
| Interventions |
Group 1: Specific instructions and general tips to increase whole wheat bran and reduced refined carbohydrate intake. Examples of specific instructions were (a) to eat one packet of whole wheat bran cereal (½ cup) each day (supplied by the study coordinator) and (b) to drink at least 48 ounces of unsweetened fluids each day. Examples of general tips included (a) ideas for saving money while purchasing nutritious whole foods and (b) how to recognize added sugar in commercial food products Group 2: Specific instructions and general tips suggested by experiences of individuals who have used trigger identification for CD symptom control and who have avoided consumption of dietary fiber Examples of specific instructions were (a) to avoid whole grains, dairy products, and spicy foods on symptomatic days and (b) to drink at least 48 ounces of fluid each day, but limit fluids to sips within 30 minutes of meals. Examples of general tips were (a) how to recognize whole grain food products and (b) how to calculate grams of fiber consumed each day All participants: Each participant received dietary instructions in the form of a take‐home sheet that was printed in the front of the Daily Diary |
|
| Outcomes |
Duration of follow‐up: 4 weeks 1. Remission (defined as IBDQ ≥170 points) and clinical improvement (IBDQ score change ≥ 32 is considered to be a clinically significant response) 2. CRP 3. ESR 4. IBDQ 5. pHBI |
|
| Notes |
Funding source: Grant number 5‐F31‐NRO11121 from the National Institute of Nursing Research (NINR) at the National Institutes of Health Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Each enrolled participant was randomized to receive either the dietary fiber instruction or the control diet instruction Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "participants were not informed that there were two groups because the researchers sought to reduce the chance of differing expectations of improvement between groups at baseline. If the two groups had differed in expectation of improvement during the study, such a difference could have been a confounding variable in the outcomes analyses" Comment: The trial was referred to as single blinded and study participants were blinded. However, it is unclear whether the unblinding of study personnel would have introduced bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized participants were accounted |
| Selective reporting (reporting bias) | Low risk | Trial registration not available, however, all primary and secondary outcomes specified in the methods section were reported |
| Other bias | Low risk | No significant differences between groups at baseline. No other apparent biases |
Candy 1995.
| Methods |
Study design: RCT Setting: South Africa |
|
| Participants |
State of disease / disease type: Active / UC Inclusion: Patients with active UC, not undergone surgery for their UC, and were willing to embark on a diet. Active disease was defined as the presence of diarrhoeal stools and/or rectal bleeding Exclusion: Systemic complications; on rectal or oral steroids in the past week Age: Median 37 (Group 1) / 41 (Group 2) Sex (M:F): 9:9 Disease location: Proctitis (4); Left‐sided (10); Total colitis (4) Medication use: Sulphasalazine (16) Length of remission at study entry: n/a Number randomized (n = 21): 11 (Group 1) / 10 (Group 2) Number analyzed (n = 18): 11 (Group 1) / 7 (Group 2) Post‐randomisation exclusion (n = 3): 3 patients in Group 2 were found to have insufficient symptoms to warrant admission and were therefore excluded before intervention |
|
| Interventions |
Group 1: Diets which were systematically manipulated to exclude foods that appeared to provoke symptoms. On day 1, all subjects were given a similar menu. A selection of fruits, vegetables, grains, meat, and fish prepared by boiling, grilling, roasting, baking, or microwave were included. Frying of food was prohibited. In the first week, only one food item was allowed at breakfast and lunch. Supper comprised two foods. No food was repeated more than once. No two foods of same type were allowed within a 48‐hour period. A brief summary of commonly available fruits and vegetables and their respective "families", based on botanical phylogenetic classification, was provided. Dairy products were excluded from the first week's diet but were introduced over the rest of the trial period in the following order: skim milk, yoghourt, skim‐milk cheese, full‐cream milk, cream and finally full cream cheeses. Permitted foods could be consumed ad libitum. If hungry between meals, participants could only eat foods allocated to the previous or next meal. The menu was expanded over 6 weeks to include a greater variety of foods based on individual tolerance. Refined sugars, additives, preservatives, all condiments and spices (other than salt) and drinks (other than boiled water) were prohibited. If/when the participant became asymptomatic, the offending food was reintroduced. If symptoms recurred upon reintroduction, the food was excluded for the remainder of the trial Group 2: No alteration to usual diet. All foods and drinks consumed were recorded All participants: Patients were weighed, interviewed, and examined clinically at entry to the study and weekly for 6 weeks |
|
| Outcomes |
Duration of follow‐up: 6 weeks 1. Remission (defined as the passage of normal stools with absence of rectal bleeding) 2. Improvement (defined as a decrease in the number of diarrhoeal stools and/or a diminution of rectal bleeding) 3. Sigmoidoscopy improvement 4. Biopsy improvement |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomized into either diet (11) or control (10) subjects" Comment: Participants were reportedly randomized. However, no further details were provided |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. However, highly unlikely due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "Sigmoidoscopy was performed at entry and completion on all subjects by one of the investigators (J.P.W.) who was unaware of their status in the study ... each sigmoidoscopic examination a biopsy was taken and histological evaluation was subsequently undertaken by a pathologist unaware of both the clinical and macroscopic findings." Comment: The outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attirition rate was low and balanced across groups |
| Selective reporting (reporting bias) | Low risk | Trial registration/protocol not available. However, outcomes specified in the methods section were reported |
| Other bias | Low risk | The controls had less severe disease than the experimental group. However, the difference was not statistically significant. No other apparent risk of biases |
Dariel 2007.
| Methods |
Study design: RCT (Abstract) Setting: Israel |
|
| Participants |
State of disease / disease type: Active / CD Inclusion: Patients with mild CD (CDAI 150 ‐ 250) not receiving corticosteroids and on stable therapy for at least 4 weeks Exclusion: Not stated Age: 36.1 ± 15 Sex (M:F): 24:27 Disease location: Not stated Medication use: Not stated Length of remission at study entry: Not applicable Number randomized (n = 51): 32 (Group 1) / 19 (Group 2) Number analyzed (n = 51): 32 (Group 1) / 19 (Group 2) Post‐randomisation exclusion (n = 12): 12 participants from Group 1 did not adhere to the study protocol and complete the study |
|
| Interventions |
Group 1: Sequential elimination diets. Elimination diets for 30 food components were prepared using a specially designed computer program Group 2: Conventional nutritional advice All participants: Not stated |
|
| Outcomes |
Duration of follow‐up: 2 weeks period per each diet rotation (however, the number of different diet rotations were not stated) 1. Remission (defined as CDAI < 150) and clinical improvement > 70 CDAI points) 2. IBDQ |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Patients were randomized (3:2) to receive sequential elimination diets (32 patients) for 2‐week periods for each diet rotation or conventional nutritional advice (19 patients)" Comment: Insufficient information provided |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Insufficient information provided. However, blinding of patients seems highly unlikely due to the nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis seems to have been applied. However the number of patients in remission is partially reported while IBDQ reported per protocol. Very high attrition rate only in Group 1 compared to Group 2 (12 vs 0) |
| Selective reporting (reporting bias) | Unclear risk | Study was published as an abstract, therefore not sufficiently reported to determine whether all measured outcomes were reported |
| Other bias | Unclear risk | Quote: "patients requiring a median of 5 different diet protocols per patient" Comment: Diet protocols appeared to have varied within Group 1. Insufficient information provided to determine whether this may have been a source of bias |
Jones 1985.
| Methods |
Study design: RCT Setting: England |
|
| Participants |
State of disease / disease type: Inactive / CD Inclusion: Patients with active Crohn’s disease (CDAI >150) who entered remission (CDAI < 150) after induction with parenteral nutrition or an elemental diet Exclusion: Not stated Age: 3 < 19 years and 17 between 19 to 49 years Sex (M:F): 2:18 Disease location: Ileum (6); Terminal ileum (17); Colon (14); Rectum (2) Medication use: None (5); Coirtcosteroids (14); Sulfasalazine (11); Azathioprine (2); Antibiotics (3) Length of remission at study entry: N/A Number randomized (n = 20): 10 (Group 1) / 10 (Group 2) Number analyzed (n = 20): 10 (Group 1) / 10 (Group 2) Post‐randomisation exclusion: None |
|
| Interventions |
Group 1: Investigated for specific food intolerances. Patients introduced a single food each day, starting with those such as chicken and fish, leaving until later those such as cereals and dairy products. A food that provoked symptoms was subsequently avoided. During the first fortnight of food testing, an elemental diet was taken to maintain a nutritionally adequate diet Group 2: Unrefined carbohydrate fiber‐rich diet All participants: All patients were followed up in the outpatient clinic for 6 months and by the dietitian as often as was thought necessary to give them adequate guidance and encouragement in keeping to their diets |
|
| Outcomes |
Duration of follow‐up: 6 months 1. Relapse (CDAI > 150 were considered to be treatment failures) 2. Orosomucoid 3. ESR |
|
| Notes |
Funding source:not stated Conflict of interest:not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "When patients entered remission (CDAI <150) they were randomly allocated either to take an unrefined carbohydrate fiber rich (UCFR) diet or to be investigated for specific food intolerances.". Comment:insufficient information, It is unclear how the codes were generated |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not stated and is highly unlikely due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "They were also seen separately every month by R. J. D., who assessed the activity of the disease without knowledge of the diet" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients assessed |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not available and there was insufficient information about the methods to determine whether all outcomes were reported |
| Other bias | Low risk | Baseline characteristics similar, ERS higher in IG albeit not statistically significant |
Keshteli 2016.
| Methods |
Study design: RCT (Abstract) Setting: Canada |
|
| Participants |
State of disease / disease type: Inactive / UC Inclusion: Adult UC patients in clinical remission (partial Mayo score ≤ 2) who had a clinical relapse during the previous 18 months Exclusion: Not stated Age: 37.7 ± 15.0 Sex (M:F): 12:16 Disease location: Not stated Medication use: Not stated Length of remission at study entry: Not stated Number randomized (n = 28): 14 (Group 1) / 14 (Group 2) Number analyzed (n = 28): 14 (Group 1) / 14 (Group 2) Post‐randomisation exclusion: Not stated |
|
| Interventions |
Group 1: Alberta‐based Anti‐inflammatory Diet (anti‐inflammatory diet designed to increase patients' intakes of probiotics, prebiotics, soluble fibers, and omega‐3 polyunsaturated fatty acids and decrease red meat intake) Group 2: Diet based on Canada's Food Guide All participants: Dietary counselling |
|
| Outcomes |
Duration of follow‐up: 6 months 1. Relapse (defined as partial Mayo of 3 or more) 2. CRP 3. SIBDQ 4. Fecal calprotectin |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was referred to as an RCT, however, no further details on random sequence generation were provided |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, however, it is unlikely that blinding was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All were assessed at baseline and at month 6/or time of flare up Comment: All participants appear to have been accounted |
| Selective reporting (reporting bias) | High risk | All outcomes mentioned in the methods section of the abstract were reported, albeit only partially reported as not significant without further information |
| Other bias | Unclear risk | Insufficient information to determine whether there were other sources of bias, as study was published as an abstract |
Levenstein 1985.
| Methods |
Study design: RCT Setting:Inflammatory Bowel Disease Clinic, Italy |
|
| Participants |
State of disease / disease type: active and inactive randomized, only active analyzed / CD Inclusion:Patients with non‐stenosing CD considered by their physicians to be following a low residue diet as defined below Exclusion:not stated Age: mean 38.2 (Group 1) / 42 (Group 2) years Sex (M:F): 36:24 Disease location: Ieocolitis ‐ 17; Fistulas ‐ 16; Extra‐intestinal complications ‐ 9 Medication use: Steroids ‐ 27 Length of remission at study entry: not stated Number randomized (n = 71): 36 (Group 1) / 35 (Group 2) Number analyzed (n = 58): 30(Group 1) / 28 (Group 2) Post‐randomisation exclusion (n = 13): excluded after the first 3 months as free of radiological/clinical evidence of recurrence after intestinal resection ‐ 12; diagnosis change ‐ 1 |
|
| Interventions |
Group 1: a low residue diet specifically forbidding legumes, whole grains, nuts, ordinary juices (containing some pulp) and all fruits and vegetables with the exception of ripe bananas and skinned potatoes.Patients were encouraged to buy a centrifuge in order to prepare solid free extracts of fruits and vegetables Group 2: gradually normalising Italian diet with a graded plan of gradual fiber reintroduction for patients previously following a low residue diet All participants: Patients asked to eliminate any foods that proved to cause pain or diarrhoea. Coffee, spices, simple sugars, alcohol, and dairy products were permitted to patients in both groups as tolerated.Non‐dietary medical and surgical therapy kept. Multivitamin was prescribed by the research physician for patients in low residue diet group. Patients were asked to discuss all questions concerning diet with the research physician only |
|
| Outcomes |
Duration of follow‐up: approximately 24 months (range 23‐34 months) 1. NCDAI (New CDAI) 2. Index developed by authors (5 point scale for pain, severity of diarrhoea, and global assessment on how the patient has done since the start) 3. CDAI modified by Brest 4. Number of hospitalisations and surgeries required 5. Steroid therapy |
|
| Notes |
Funding source: not stated Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient information on how the random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Non‐dietary medical and surgical therapy, which was managed by two 'treating physicians' (CP, CL) kept blind to the diet assignment of each patient, was not affected by the study" Comment: Unclear whether bias was sufficiently eliminated given that there was no mention of participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:"All outcome criteria were evaluated by the two 'blinded' treating physicians, who rated pain and diarrhoea on a five point scale at each clinic visit" Comment: All outcomes were blindly assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "All participants with active CD were accounted for and those with inactive CD were not analyzed due to lack of compliance Comment: However attrition rates were balanced across groups |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in the methods section were reported |
| Other bias | Low risk | Quote: "At randomisation, experimental and control groups were virtually identical in age, sex distribution, disease duration, history of fistulae, NCDAI, and previous adherence to a low residue diet" Comment: Baseline characteristics were balanced and there were no other apparent biases |
Lomer 2001.
| Methods |
Study design: RCT Setting: England |
|
| Participants |
State of disease / disease type: Active / CD Inclusion: Patients with active ileal or ileocolonic disease (CDAI >150) and were prepared to accept full dietary advice and cease vitamin/mineral supplements Exclusion: Patients were excluded if they were pregnant or lactating, or if they were unable to understand written and verbal instructions Age: 36.2 ± 11.8 Sex (M:F): 3:17 Disease location: Ileum only (6); Ileum and colon (14) Medication use: Not stated Length of remission at study entry: N/A Number randomized (n = 20): 10 (Group 1) / 10 (Group 2) Number analyzed (n = 18): 9 (Group 1) / 9 (Group 2) Post‐randomisation exclusion (n = 2): Lack of compliance (2). Two additional patients only completed 3 of 4 months, but had the last value extended. One was withdrawn due to gastrointestinal bleeding, iron deficiency anaemia, and unintentional weight loss of 9%, and another was lost to careful follow‐up |
|
| Interventions |
Group 1: Diet low in microparticle (foods that could contain the dietary microparticle titanium dioxide and aluminosilicates were excluded). Fibrous fruit and vegetables were avoided. Toothpaste that did not contain titanium dioxide was recommended, while filtered bottled water was supplied for all drinks, cooking, teeth cleaning, and washing of fruits/vegetables. Fresh fruit and vegetables were peeled and washed (minimizing soil contamination) Group 2: Designed to match the trial diet, except that foods containing dietary microparticle were not especially discouraged. Fibrous fruit and vegetables, which are known to cause symptoms in stricturing Crohn's disease, were excluded All participants: Advice was given to achieve the dietary reference values for the UK adult population. Dietary follow‐up was always by the same dietitian, initially by telephone at week 1, and then by interview at monthly intervals. Patients who were unable to comply following the first follow‐up interview at month 1 were excluded. At the end of the trial all patients were encouraged to return to their usual diet. Prednisolone (up to 30 mg/day), and in three patients (one trial, two controls) budesonide (up to 9 mg/day), were prescribed as required according to normal clinical management, and their doses were adjusted over the next few months with the aim of controlling symptoms while reducing corticosteroid usage. Aminosalicylates were given equally in 4 versus 4 participants. No other anti‐inflammatory or immunosuppressive drugs were given through the trial and any other medication was monitored. The only other drugs used were loperamide and ferrous sulphate in four and two different control patients, respectively |
|
| Outcomes |
Duration of follow‐up: 4 months 1. Remission (defined as CDAI <150) 2. Corticosteroid use |
|
| Notes |
Funding source: NHS Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Comment: Unclear whether the envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Other investigators and all patients were blinded to the study randomization" Comment: Unclear whether bias was sufficiently eliminated given that the dieticians were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: "Medical management was continued routinely by clinicians unaware of the randomization." and "Patients were also assessed, independently of the dietitian, pre‐treatment and at monthly intervals, by a research nurse and doctor unaware of the randomization." Comment: Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low and balanced across groups |
| Selective reporting (reporting bias) | High risk | All outcomes prespecified in the method section were reported, however, outcome data were reported as not significant (n.s) without further information |
| Other bias | High risk | Quote: "At entry, the two groups were comparable with respect to clinical history and demographic parameters, although the trial group had a higher mean CDAI than the control group (392 ±25 vs 302 ± 28; mean SEM; P < 0.03)" Comment: Participants in the intervention group had higher CDAI scores |
Lomer 2005.
| Methods |
Study design: RCT Setting: England |
|
| Participants |
State of disease / disease type: Active / CD Inclusion: Patients (18 – 70 years) with active Crohn’s disease of the ileum and/or colon with a diagnosis confirmed by standard criteria according to histological and/or radiologic findings, with a current CDAI ≥ 150 and who could be treated as outpatients Exclusion: Patients that had been treated within 3 months prior to recruitment (or had concurrent treatment) with cyclosporine, methotrexate, azathioprine, mercaptopurine or anti‐tumour necrosis factor therapy. They were also excluded if they had external fistulae or stoma; previous small bowel resection > 100 cm; previous colectomy or proctocolectomy; isolated active peri‐anal disease, proctitis or rectosigmoid disease; symptomatic stenosis or strictures; taking part in another clinical trial; pregnancy or lactation or planned pregnancy; unintentional weight loss > 10% body weight in the last 3 months; nutritional supplementation, such as enteral feeds or vitamin/mineral supplements that could not be stopped Age: 36 ± 13 Sex (M:F): 40:43 Disease location: Ileum only (30); Ileum and colon (28); Colon only (25) Medication use: Not stated Length of remission at study entry: N/A Number randomized (n = 83): 22 (Group 1 ‐ Low Calcium Low Microparticle) / 21 (Group 2 ‐ Low Calcium Normal Microparticle) / 20 (Group 3 ‐ Normal Calcium Low Microparticle) / 20 (Group 4 ‐ Normal Calcium Normal Microparticle) Number analyzed (n = 83): 22 (Group 1) / 21 (Group 2) / 20 (Group 3) / 20 (Group 4) Post‐randomisation exclusion (n = 13): Dietary non‐compliance (4); commencement of azathioprine (3); Surgery (5); Error (1) |
|
| Interventions |
Group 1: Low calcium (400 mg/day) and low microparticle diet. Low microparticle diets exclude foods that contain dietary microparticle (titanium dioxide and particulate silicates). For more details, see characteristics of Lomer 2001 Group 2: Low calcium (400 mg/day) and normal microparticle diet. Based on a sham manipulated diet that avoided a different group of food additives, namely sulphur dioxide and the sulphites, which are preservatives used to prolong the shelf life of certain foods. Typically, these included sausages and similar processed meats, dried fruits and shellfish. Foods containing dietary microparticle were not discouraged. Bottled water was supplied for all cold drinks, while it was recommended that tap water was used for cooking and hot drinks such as tea and coffee. Titanium dioxide‐free, but not particulate silicilate‐free toothpaste was provided. However, 5 mg/day of titanium dioxide was added back into the diet with the supplement Group 3: Normal calcium (800 mg/day) and low microparticle diet Group 4: Normal calcium (800 mg/day) and normal microparticle diet All participants: Avoidance of fibrous fruits and vegetables, which can cause symptoms in stricturing Crohn's disease At entry, prednisolone tablets, but not other corticosteroids, were prescribed for all patients starting at 30 mg/day and the daily dose was reduced by 5 mg each week to reach zero by week 6; patients receiving extra corticosteroids were then considered failures in the primary analysis. Aminosalicylates which contain large particulate silicates (mean diameter 10 mm) but not microparticle were prescribed according to normal clinical practice and the dose was maintained throughout the intervention time. For those patients taking a different aminosalicylate at entry, the prescription was changed to Pentasa using dose equivalents. No other medication was allowed. All patients were also advised on avoidance of fibrous fruit and vegetables and all received similarly detailed written dietary information. Follow‐up was initially by telephone at week 1, and then by interview at weeks 4, 8, 12 and 16. Dietary compliance was monitored by dietary recall and patients unable to comply following the first follow‐up interview at week 4 were excluded. At the end of the intervention period (i.e., 16 weeks) all patients were advised to return to their usual eating pattern with advice on meeting recommended nutritional requirements (especially for calcium). Patients continued to be monitored by their gastroenterologist at weeks 20, 28, 40 and 52 |
|
| Outcomes |
Duration of follow‐up: intervention 16 weeks (follow up weeks 16‐52) 1. Clinical remission and response (remission defined as a CDAI <150, and clinical response as a decrease in CDAI from baseline by ≥60 points) 2. Van Hees Index 3. IBDQ 4. ESR 5. CRP 6. Fecal calprotectin 7. Intestinal permeability |
|
| Notes |
Funding source: Not stated Conflict of interest: Authors reported none conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were referred to only one dietitian (M.C.E.L.), who then randomly allocated the patients to dietary treatment based upon schemes given in independently sealed envelopes in permuted blocks at each centre" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were referred to only one dietitian (M.C.E.L.), who then randomly allocated the patients to dietary treatment based upon schemes given in independently sealed envelopes in permuted blocks at each centre" Comment: Unclear whether envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were not informed which dietary group they were in" Comment: Apart from dietitian, all personnel were blinded. However, it is unclear whether bias was completely eliminated and remained unbroken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were seen by only one local physician (gastroenterologist) throughout the trial period who was blinded to the dietary treatment" and "Patients were assessed independently at pre‐treatment and at weeks 4, 8, 12 and 16 by their physician who was unaware of the study randomization" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analyses were based on intention to treat, with no account taken of variations in corticosteroid weaning. Missing data for patients who were withdrawn were extended to the end of the 16 week treatment phase, but not the follow‐up phase, according to the last value extended principle" |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the method section were reported |
| Other bias | Low risk | "At entry all groups were comparable for demographic parameters and there were no significant differences between the groups for baseline CDAI or distribution of Crohn’s disease" Comment: Baseline characteristics were balanced across groups and there were no other apparent biases |
Lorenz‐Meyer 1996.
| Methods |
Study design: RCT Setting: Germany |
|
| Participants |
State of disease / disease type: Inactive / CD Inclusion: Patients with active Crohn’s disease (CDAI > 200) were considered for admission to the trial and were included once they reached remission (CDAI > 150) under conventional steroid therapy over a 3‐month period. Disease localization determined within the last 2 years Exclusion: Unwillingness to give written informed consent; questionable ability to cooperate; concomitant intake of salicylates or nonsteroidal antiphlogistics; intake of drugs commonly used for treatment of Crohn’s disease, such as metronidazole, azathioprine, 5‐aminosalicylic acid, sulfasalazine, total parenteral nutrition, and elemental diet; short bowel syndrome; proven steatorrhea; pregnancy. Age: 30.7 ± 10.3 Sex (M:F): 67:135 Disease location: Small bowel (31); Large bowel (35); Both (136) Medication use: Not stated Length of remission at study entry: Not stated Number randomized (n = 204): 69 (Group 1) / 70 (Group 2) / 65 (Group 3) Number analyzed (n = 202): 69 (Group 1) / 70 (Group 2) / 63 (Group 3) Post‐randomisation exclusion (n = 2): No baseline data due to non‐compliance immediately after randomisation (2) |
|
| Interventions |
Group 1 (Diet): Patients in the diet group were instructed to adhere to a low‐carbohydrate diet of less than 84 g/day Group 2 (Verum): Verum consisted of two gelatine capsules three times a day containing 1 g of an ethylester fish oil concentrate with 55% eicosapentanoic acid (C20:5n3) and 30% docosahexanoic acid (C22:6n3) as their major omega‐3 fatty acid components. Patients were to follow general nutrition guidelines from the German Society of Nutrition. Most carbohydrates were to be ingested in a fiber‐rich form Group 3 (Placebo): Placebo capsules (two, three times a day) contained the same amount of corn oil as the Group 2 verum. Double‐blind conditions were intended for the verum‐placebo comparison Patients were to follow general nutrition guidelines from the German Society of Nutrition. Most carbohydrates were to be ingested in a fiber‐rich form All participants: During the first 7 weeks all patients were treated daily with 8 mg methylprednisolone. During the 8th week steroids were tapered to 6, 4, 2, and, finally, 0 mg/day.Patients were urged to seek the investigator’s immediate advice in case of substantial deterioration of their state of health |
|
| Outcomes |
Duration of follow‐up:12 months 1. Relapse (increase of the CDAI above 200 points and by at least 60 points above base line plus an increase of CRP serum level two standard deviations above the mean of the healthy population) |
|
| Notes |
Funding source: not stated Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Quote: "block size blinded to the centers" Comment: Insufficient information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind conditions were intended for the verum‐placebo comparison" Comment: Patients received placebo pills. We assume the placebo was identical to the active intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "The intention‐to‐treat approach was chosen as the essential method of inference." and "Dropouts and withdrawals were prospectively documented in the Case Report Forms" Coimment: Low attrition rates and reasons were balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes appear to have been reported except CRP data which was measured as part of the definition for relapse. Trial registration not available to confirm whether CRP was an outcome of interest |
| Other bias | Low risk | Quote: "The three randomized groups were comparable on the basis of age, sex, Broca index, body mass index (BMI), duration,and localization of disease, indices of active phases, peak CDAI before entry, CDAI at entry, numbers of external fistulas, and registrations of a palpable abdominal mass; no noticeable difference in laboratory findings at study entry (confirmed by chi‐square tests, H‐tests, and analyses of variance not showing any extreme P values." Comment: Similar baseline characteristics and no additional sources of bias were identified |
Mutlu 2016.
| Methods |
Study design: RCT (Abstract) Setting: USA |
|
| Participants |
State of disease / disease type: Inactive / CD Inclusion: Subjects with quiescent CD, with medical induction of remission from two sites located in Illinois and Georgia Exclusion: Not stated Age: 45 ± 14.5 years Sex (M:F): 21:33 Disease location: Not stated Medication use: Not stated Length of remission at study entry: Not stated Number randomized (n = 54): 16 (Group 1) / 19 (Group 2) / 19 (Group 3) Number analyzed: Not stated Post‐randomisation exclusion: Not stated |
|
| Interventions |
Group 1: Anti‐IBD diet and placebo supplement Group 2: Fructooligosaccharide supplement and 'placebo diet' Group 3: 'Placebo diet' and placebo supplement All participants:The subjects were followed until either they had a flare or up to 12 months. Biopsy samples were collected from the sigmoid colon before and after the study interventions, and were analyzed for bacterial composition |
|
| Outcomes |
Duration of follow‐up: 12 months 1. Relapse (defined as the need for a new medication for treatment or a 100‐point rise in the CDAI) 2. Biopsy (colon) 3. Microbiome |
|
| Notes |
Funding source: Not reported Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were reportedly randomized to three study groups. However, it is unclear how this was done |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "In a randomized, placebo‐controlled, double‐blinded study, we enrolled 54 subjects with quiescent CD" Comment: The study appears to be double blinded. Whilst there is insufficient information to make a judgement, the review authors consider this adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In a randomized, placebo‐controlled, double‐blinded study, we enrolled 54 subjects with quiescent CD..." Comment: The study appears to be double blinded. Whilst there is insufficient information to make a judgement, the review authors consider this adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to have been accounted for |
| Selective reporting (reporting bias) | Unclear risk | The study was published as an abstract with insufficient information to determine whether there was selective reporting |
| Other bias | Unclear risk | Quote: "The study groups were not different in regards to age (mean = 45 ± 14.5 years), gender (F/M=33/21), race, or education" Comment: There was no information regarding other baseline characteristics |
Riordan 1993.
| Methods |
Study design: RCT Setting: England |
|
| Participants |
State of disease / disease type: Inactive / CD Inclusion: Patients with active CD confirmed by standard radiological and histological tests within 2 months of entry; HBI > 6; permanent residents of the health districts where the hospitals were situated Patients who achieved remission with an elemental diet were then included in the trial Exclusion: Pregnancy, lactation, surgical complications (such as intestinal obstruction, abscesses, and symptomatic fistulae), and severe complications necessitating corticosteroids, such as uveitis Patients with CD of the rectum only were excluded, as were those with perianal disease more severe than simple fissures or skin tags Age: 33.7 ± 12.2 Sex (M:F): 26:52 Disease location: Small bowel (31); Large bowel (21); Small and large bowel (26) Medication use: Azathioprine (5) Length of remission at study entry: Not stated Number randomized (n = 78): 40 (Group 1) / 38 (Group 2) Number analyzed (n = 78): 40 (Group 1) / 38 (Group 2) Post‐randomisation exclusion (n = 14): Non‐compliance (7); Intercurrent illness (3); Steroid side‐effects (diabetes mellitus and severe furunculosis) (2); Pregnancy (2) |
|
| Interventions |
Group 1: Elemental diet with instruction to reintroduce a single food each day and to exclude any food that provoked symptoms such as diarrhoea and pain. They were also given placebo tablets identical to the prednisolone and instructed to reduce the dose in the same way Group 2: Prednisolone 40 mg/day. If they remained in remission, the prednisolone dose was reduced to 30 mg after 1 week, to 20 mg after 1 month, and to 10 mg after 2 months, and withdrawn after 3 months. They received general dietary advice from a dietitian All participants: Both groups saw the dietitians at every clinic visit and were free to telephone for advice if necessary. Patients in both groups were told that they had entered a trial of diet in CD and that the tablets might be corticosteroids or a harmless placebo |
|
| Outcomes |
Duration of follow‐up: 24 months or until relapse 1. Relapse / Failure (HBI > 6 was taken as a relapse. Other criteria for treatment failure included: unwillingness by the patient to continue; a diet found on computer analysis to be deficient in energy, protein, or any other nutrient that could not be replaced by simple supplements; surgery for CD; serious medical complications; and steroid side‐effects severe enough to warrant withdrawal of therapy 2. ESR 3. CRP |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation codes were separate for each participating centre and were stratified for the extent of the disease" Comment: Authors did not indicate how the randomisation codes were generated |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients in both groups were told that they had entered a trial of diet in CD and that the tablets might be corticosteroids or a harmless placebo [...] The group assignment was known to the dietitians who advised the patients but not to the physicians who assessed their progress" Comment: The intervention diet and corticosteroid groups received placebo tablets and general (dummy) dietary advice respectively. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The group assignment was known to the dietitians who advised the patients but not to the physicians who assessed their progress" Comment: The outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low and reasons were balanced across groups |
| Selective reporting (reporting bias) | High risk | Trial registration not available, however, outcome results were described as not statistically significant without providing any data |
| Other bias | Low risk | Quote: "there were no differences in these variables between the 38 patients assigned corticosteroid treatment and the 40 patients assigned diet treatment" Comment: Baseline characteristics were balanced across groups and there were no other apparent biases |
Ritchie 1987.
| Methods |
Study design: RCT Setting: England |
|
| Participants |
State of disease / disease type: Inactive / CD Inclusion: Patients with Crohn's disease who were well, either with known structural disease or after resection of all apparently diseased intestine, and who were receiving no treatment other than nutritional supplements, antidiarrhoeal drugs, or maintenance sulphasalazine Exclusion: Patients with a stoma and those with disease limited to the stomach or duodenum, or both, were excluded, as were patients with anal disease only Age: 14.4 ‐ 77.7 years Sex (M:F): 130:222 Disease location: Small bowel (68); Small and large bowel (37); Large bowel (55); No macroscopic disease after resection (134) Medication use: Sulphasalazine (64) Length of remission at study entry: Not stated Number randomized (n = 352): 190 (Group 1) / 162 (Group 2) Number analyzed (n = 352): 190 (Group 1) / 162 (Group 2) Post‐randomisation exclusion (n = 56): Non‐compliance (24); Unknown (14); Unrelated (18) |
|
| Interventions |
Group 1: Advised to eat carbohydrate in its natural unrefined state only, avoiding all products containing sugar or white flour Group 2: Diet with carbohydrate in its refined form (e.g. white flour and rice) and avoiding unrefined carbohydrate foods. Sugar intake was unrestricted All participants: Both types of diets were accompanied by a booklet; patients given a list of "acceptable" and "unacceptable" foods. At every visit the dietitian reviewed the patient's diet and strongly reinforced the advice given |
|
| Outcomes |
Duration of follow‐up: 24 months 1. Clinical deterioration (defined as the need for surgical or medical treatment in hospital; need for corticosteroid, sulphasalazine [if not already being taken], antibiotic, or immunosuppressive drug treatment for intestinal disease as an outpatient; or as a worsening of symptoms attributable to the disease and severe enough to warrant withdrawal from the trial) 2. Wellbeing at end of the trial was assessed in terms of symptoms, body weight, and laboratory findings |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were referred to the dietitian, who held the randomization code as a consecutive series of sealed envelopes” Comment: It is unclear how the codes were generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were referred to the dietitian, who held the randomization code as a consecutive series of sealed envelopes" Comment: It is unclear whether the envelopes were opaque and numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A further explanation of the principles of each diet was given by the dietician, and if the patient agreed to accept whichever of the two diets was allocated the next envelope was opened and the trial diet disclosed. The clinician was not informed of the diet allocated and, as far as possible, remained blind to the advice given” Comment: Blinding appears to have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A further explanation of the principles of each diet was given by the dietitian, and if the patient agreed to accept whichever of the two diets was allocated the next envelope was opened and the trial diet disclosed. The clinician was not informed of the diet allocated and, as far as possible, remained blind to the advice given” Comment: Clinician was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates were high(30%) and generally balanced across groups, however, there were more withdrawals from the unrefined carbohydrate group due to non‐compliance compared to the refined carbohydrate group (10.5% versus 2.5%) |
| Selective reporting (reporting bias) | High risk | Data on outcomes related to clinical improvement were collected and described as showing no significant change without full reporting of data |
| Other bias | Low risk | Baseline characteristics were balanced. No other apparent biases |
Strisciuglio 2013.
| Methods |
Study design: RCT Setting: Italy |
|
| Participants |
State of disease / disease type: Active / UC Inclusion: Children with newly diagnosed UC Exclusion: Children who had received therapy‐inducing remission of UC and/or children who required surgery for complications related to UC Age: Mean 11.2 (range 4.6 ‐ 17) years Sex (M:F): 14:15 Disease location: Proctosigmoiditis (7); Left‐sided colitis (3); Extensive colitis (6); Pancolitis (13) Medication use: Steroid therapy (16) Length of remission at study entry: N/A Number randomized (n = 29): 14 (Group 1) / 15 (Group 2) Number analyzed (n = 29): 14 (Group 1) / 15 (Group 2) Post‐randomisation exclusion: None |
|
| Interventions |
Group 1: Cow’s milk protein elimination diet Group 2: Usual diet All participants: Patients with PUCAI score ≥ 35 received concomitant steroid induction treatment (oral methylprednisolone: 1 mg/kg/day, maximum 40 mg/day per 4 weeks) and oral mesalazine induction and mesalazine maintenance treatment (50 mg/kg/day), while subjects with a PUCAI score < 35 received exclusively oral mesalazine induction and mesalazine maintenance treatment (50 mg/kg/day). All children received supplemental elemental calcium 1000 mg/day and vitamin D3 0.25 mcg/day for 1 year. After 4 weeks, patients who were in remission began tapering off corticosteroids on a weekly basis on the basis of the PUCAI score. Upon induction of remission patients continued to received concomitant therapy for 1 year or until relapse. Parents and/or patients recorded in a daily diary only the amount and type of not allowed food. Available to contact investigator whenever needed. When a relapse occurred, the study protocol was stopped and the patient was treated according to the physician’s preference |
|
| Outcomes |
Duration of follow‐up: 12 months 1. Remission (Clinical remission was defined on the basis of PUCAI < 10, while clinical response to the induction treatment was identified as a PUCAI score a change of at least 20 points from baseline 2. Relapse (occurrence or worsening of symptoms accompanied by an increase of PUCAI >10 points, sufficient to require rescue treatment with corticosteroids, azathioprine/immunosuppressive agents, or surgery) 3. ESR 4. CRP 5. Fecal Calprotectin |
|
| Notes |
Funding source:not stated Conflict of interest:not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment to CMP elimination diet or free diet was determined according to a computer‐generated randomization scheme" |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The paediatric gastroenterologists (AS; EM) made all decisions regarding induction therapeutic intervention being unaware of diet group allocation" Comment: However, blinding of the participants or caregivers (parents) is unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All histologic specimens were reviewed under code by a single pathologist experienced in analysing paediatric intestinal biopsies, blinded to the patients’ clinical details, who scored biopsies according to the Matts’ histologic criteria" Comment: Assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The only withdrawals which occurred were due to relapse. All participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Trial registration not available, however, all outcomes appear to have been reported |
| Other bias | Low risk | Patients were similar at baseline. There were no other apparent biases |
Wright 1965.
| Methods |
Study design: RCT Setting: England |
|
| Participants |
State of disease / disease type: Active / UC Inclusion: All patients seen during an attack of UC confirmed by sigmoidoscopy and barium enema examination, prepared to keep to a strict diet for a period of one year and to attend for follow‐up at monthly intervals, not on corticosteroids for the attack for more than one week, and have no major complication of UC Exclusion: See Inclusion Criteria Age: Not stated Sex: Not stated Disease location: Not stated Medication use: Not stated Length of remission at study entry: N/A Number randomized (n = 77): 26 (Group 1) / 27 (Group 2) / 24 (Group 3) Number analyzed (n = 50): 26 (Group 1) / 0 (Group 2) / 24 (Group 3) Post‐randomisation exclusion: All 27 participants in Group 2 were excluded due to poor adherence and inadvertent introduction of milk protein in some brands of margarine and some articles of gluten‐free diet |
|
| Interventions |
Group 1: Milk‐free, low‐roughage diet excluded all milk/products (fresh milk, cheese, or powdered milk). Butter was permitted Group 2: Gluten‐free plus milk‐free diet. Butter was not permitted and patients were told to use margarine instead Group 3: Exclude a variety of items of diet, such as fried foods, condiments, and ice cream, but constituents of these foods which might be antigenic were included in the list of other foods which were permitted. In particular, they were advised to consume milk and milk products liberally All participants: A diet sheet was prepared for each of the diets and patients were referred to a dietitian for detailed explanation. Prednisolone by mouth in a dose of 5 mg six‐hourly for six weeks and hydrocortisone hemisuccinate 100 mg nightly by rectal infusion for two months, together with any general medical measures necessary for the particular case. If the attack was severe enough to warrant admission to hospital the dose of prednisolone was doubled and the rectal infusion used twice daily. All patients were given two tablets of Omnivite Forte for the period of the trial. A similar course of treatment was given for each relapse |
|
| Outcomes |
Duration of follow‐up: 12 months 1. Relapse (defined as diarrhoea with an average of four or more stools a day for at least a week and with macroscopic blood present, together with sigmoidoscopic evidence of diffuse inflammation) 2. Treatment failure (based on (a) the attack continued to be severe and required either surgical treatment or treatment with systemic corticosteroids for more than six weeks; or (b) three successive relapses, in addition to the initial attack during the trial period) 3. ESR |
|
| Notes |
Funding source: Not stated Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients presenting with an attack of ulcerative colitis were allocated at random to one of three dietary groups […] patients in each of these clinical categories were allotted at random to the dietary groups, employing restricted randomization to keep the numbers in the three dietary groups approximately equal” Comment: There was no mention of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | The methods used for allocation concealment were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Dummy diet used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All 27 participants randomized to one of the three trial arms were excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Quote: "The immunological findings are only mentioned briefly, as they are being reported in detail separately (Wright and Truelove, 1965 Circulating Antibodies to Dietary Proteins in Ulcerative Colitis; British Medical Journal 2:142‐144)" Comment: Upon checking the named study, it was discovered that no secondary outcome (ESR, hemoglobin) was reported |
| Other bias | Unclear risk | Insufficient information reported to make judgement on other sources of bias |
Abbreviations: BMI: body mass index; CD: Crohn's disease; CDAI: Crohn's Disease Activity Index; CRP: C‐reactive protein; ESR: erythrocyte sedimentation rate; F: female; HBI: Harvey‐Bradshaw Index; IBDQ: Inflammatory Bowel Disease Questionnaire; M: male; MRI: magnetic resonance imaging; N/A: not applicable or available; pHBI: Partial Harvey‐Bradshaw Index; PUCAI: Pediatric Ulcerative Colitis Activity Index; RCT: randomized controlled trial; SCCAI: Simple Clinical Colitis Activity Index; SIBDQ: Short Inflammatory Bowel Disease Questionnaire; TNF: tumor necrosis factor; UC: ulcerative colitis; USA: United States of America
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barnes 2016 | Not a randomized controlled trial |
| Beattie 1994 | Not a study. Editorial on Riordan 1993 |
| Bentz 2010 | Separate results were not reported for participants with active and inactive Crohn's disease. Outcomes of interest were not evaluated |
| Boneh 2017 | Study compared interventions involving enteral nutrition |
| Brandes 1982 | Not a randomized controlled trial |
| Castro 1995 | Review article. Not a study |
| Ciccimarra 1998 | Review article. Not a study |
| Cohen 2012 | Not a randomized controlled trial |
| Davies 1978 | Not a randomized controlled trial |
| Dunn 2017 | Study primarily compared oral nutrition supplements |
| El‐Tahir 1998 | Intervention was a dietary supplement: omega‐3 fatty acids |
| Gunasekeera 2016 | Separate results were not reported for participants with active and inactive Crohn's disease |
| Halmos 2016 | Not a randomized controlled trial |
| Kyaw 2014 | Disease activity (active or inactive ulcerative colitis) at baseline was not reported and unknown |
| Mikolaitis 2013 | Outcomes of interest were not evaluated |
| NCT01749813 | Study terminated with no results |
| NCT02093780 | Authors contacted on 28/01/2018 without response. Likely ClinicalTrails.gov entry of Keshteli 2016 |
| NCT02213835 | Authors contacted on 28/01/2018 without response |
| NCT02231814 | Study compared interventions involving enteral nutrition |
| NCT02345733 | Not a randomized controlled trial |
| NCT02357537 | Authors contacted on 24/01/2018 without response |
| NCT02426567 | Authors contacted. Trial involves healthy individuals |
| NCT02469220 | Outcomes of interest were not evaluated |
| NCT02610101 | Authors contacted on 28/01/2018 without response |
| NCT02922881 | Not a randomized controlled trial |
| NCT02930564 | Authors contacted on 28/01/2018 without response |
| NCT02945488 | Study abandoned |
| NCT03171246 | Not a randomized controlled trial |
| Pedersen 2014 | Separate results were not reported for participants with active and inactive Crohn's disease. Separate results were not reported for participants with active and inactive ulcerative colitis |
| Pedersen 2017 | Separate results were not reported for participants with active and inactive Crohn's disease. Separate results were not reported for participants with active and inactive ulcerative colitis |
| Pituch‐Zdanowska 2018 | Not a randomized controlled trial |
| Stange 1990 | Separate results were not reported for participants with active and inactive Crohn's disease |
| Strohm 1981 | Study compared interventions involving enteral nutrition |
| Svolos 2016 | Outcomes of interest were not evaluated |
| Vincenzi 2016 | The study made no reference to our population of interest (Crohn's disease or ulcerative colitis) |
Characteristics of studies awaiting assessment [ordered by study ID]
Bodini 2018.
| Methods | Randomized controlled trial |
| Participants | Participants with IBD including UC and CD |
| Interventions | Low FODMAP versus normal FODMAP diet for 6 weeks |
| Outcomes | Disease activity (Mayo score for UC and Harvey Bradshaw Index for CD) Quality of life (IBDQ) Adherence to diet Fecal calprotectin |
| Notes |
Tapete 2018.
| Methods | Randomized controlled trial |
| Participants | Participants with IBD (UC or CD) in remission but without complete control of intestinal symptoms |
| Interventions | Low FODMAP versus standard of care diet for 6 to 8 weeks |
| Outcomes | Intestinal, general or emotional symptoms (questionnaires), quality of life (IBDQ) and VAS to characterize the intestinal symptoms before and after diet |
| Notes |
IBD: inflammatory bowel disease; UC: ulcerative colitis; CD: Crohn's disease; FODMAP: fermentable Oligosaccharides, disaccharides, monosaccharides and polyols; IBDQ: Inflammatory Bowel Disease Questionnaire; VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
NCT02825316.
| Trial name or title | Mediterranean Diet as an add‐on Therapy for Induction of Remission in Patients With Active Crohn's Disease |
| Methods | Randomized, parallel assignment, double blinded controlled trial |
| Participants | Estimated enrollment: 100 participants, 18‐75 years, both sexes, active CD (Montreal classification: B1); Induction therapy with corticosteroids, 5‐ASA, azathioprine, 6‐mercaptopurine, methotrexate, or biologic therapy |
| Interventions | 8 weeks Mediterranean Diet versus 8 weeks Low Residue Diet |
| Outcomes | Primary: CDAI; C‐reactive protein; Calprotectin; Remission rate‐ CDAI < 150 + normal CRP / fecal calprotectin; Response rate‐ decrease in 70 points in CDAI + decreased CRP / fecal calprotectin; Microbial composition |
| Starting date | July 2016 |
| Contact information | Lihi Godny, Tel‐Aviv Sourasky Medical Center |
| Notes |
NCT02858557.
| Trial name or title | The Effect of the Mediterranean Diet and the Specific Carbohydrate Diet on Microbial Profile and Disease Outcomes in Patients With Inflammatory Bowel Diseases |
| Methods | Randomized, crossover, double blinded clinical trial |
| Participants | Estimated enrollment: 70 participants, 18‐70 years, both sexes, patients with pouch surgery because of refractory UC or Familial Adenomatous Polyposis and have a functioning pouch |
| Interventions | 7 days of Mediterranean diet with cross over to 7 days of specific carbohydrate diet vs 7 days of specific carbohydrate diet with cross over to 7 days of Mediterranean diet |
| Outcomes | Primary: Microbial diversity (Shannon α‐diversity index);Secondary: PDAI, CRP, fecal calprotectin, IBDQ, Microbial composition |
| Starting date | September 2016 |
| Contact information | Lihi Godny, Tel‐Aviv Sourasky Medical Center |
| Notes |
NCT03012542.
| Trial name or title | Randomized Trial of Diet for Crohn's Disease and Impact on Disease Activity and the Microbiome |
| Methods | Randomized, parallel assignment, quadruple masking, controlled trial |
| Participants | Estimated enrollment: 32 participants, 18 years and older, active CD, both sexes,fecal calprotectin ≥ 300, mild to moderate disease activity based upon a Harvey Bradshaw Index of 5 to 16 and on stable medication doses for ≥ 2 months |
| Interventions | Diet controlled in amount and source of carbohydrates or fiber containing foods versus Diet controlled in amount and source of carbohydrates or fiber containing foods |
| Outcomes | Primary: fecal calprotectin remission; Secondary: fecal calprotectin response, clinical response, metagenomics, microbiota correlation with clinical disease activity and inflammatory biomarkers, future use |
| Starting date | January 2017 |
| Contact information | Timothy L Zisman, MD, MPH, University of Washington Medical Center |
| Notes |
NCT03053713.
| Trial name or title | The Effect of Diet Modification on Clinical Disease Activity, the Gut Microbiome and Immune Responses in Patients With Ulcerative Colitis |
| Methods | Randomized,parallel assignment, open label clinical trial |
| Participants | Estimated enrollment: 102 participants , 18 to 64 years, UC in remission, on oral 5‐ASA, methotrexate, azathioprine, or 6‐mecaptopurine with no changes in dosage for 2 months prior to the start of the study |
| Interventions | Mediterranean diet pattern vs Habitual diet |
| Outcomes | Primary: SCCAI; Secondary: SIBDQ, fecal microbiota, fecal calprotectin, CRP, serum ferritin, |
| Starting date | April 4, 2017 |
| Contact information | Deanna L Gibson, PhD, University of British Columbia ‐ Okanagan |
| Notes |
NCT03058679.
| Trial name or title | Open Label, Randomized, Multicenter, Comparative Effectiveness Trial of Specific Carbohydrate and Mediterranean Diets to Induce Remission in Patients With Crohn's Disease |
| Methods | Randomized,parallel assignment, open label clinical trial |
| Participants | Estimated enrollment: 194 participants, 18 years and older, both sexes, active CD (sCDAI score >175), active inflammation documented by a fecal calprotectin concentration > 250 ug/g or high sensitivity C‐reactive protein (hs‐CRP) > 5 mg/L measured at screening, able to receive weekly food shipments delivered every Friday for 6 weeks |
| Interventions | Specific Carbohydrate Diet vs Mediterranean Style Diet (all food provided) |
| Outcomes | Primary: Symptomatic remission (sCDAI), reduction in bowel inflammation (calprotectin < 250 mcg/gm and > 50% reduction from baseline); Secondary: clinical remission (Harvey Bradshaw Index, reduction in systemic inflammation (hsCRP < 5mg/L > 50% reduction from baseline) |
| Starting date | September 29, 2017 |
| Contact information | James D Lewis, MD, MSCE, University of Pennsylvania |
| Notes |
Abbreviations: 5‐ASA, 5‐aminosalicylate; CD, Crohn's disease; CDAI, Crohn's Disease Activity Index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; hs‐CRP, high sensitivity C‐reactive protein; IBDQ, Inflammatory Bowel Disease Questionnaire; PDAI, Pouchitis Disease Activity Index; SCCAI, Simple Clinical Colitis Activity Index; sCDAI, Short Crohn's Disease Activity Index; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; UC, ulcerative colitis
Differences between protocol and review
Following successful publication of the protocol, support from the UK authors who were funded by an NIHR Cochrane Programme grant was added. One element of the protocol that was revisited was the consideration of including non‐randomized trials. This was an initial decision based on the perception that few studies would be discovered. However, within the very early phases of considering citations, it was clear this was not the case and a discussion between the team was held and the advice of the editorial base sought. It was decided to amend and only include randomized trials. In the protocol, we planned to calculate odds ratios for dichotomous outcomes as this effect measure is often more appropriate when including observational data. Once we decided to only include randomized trials, we decided that the risk ratio would be more appropriate effect measure to use. In the protocol we did not specify how we would deal with multi‐arm trials. For studies with multiple treatment arms, we only included single pair‐wise comparisons as appropriate.
Contributions of authors
Berkeley N. Limketkai performed screening of abstracts and titles, screening of full‐text articles, risk of bias assessments, statistical analyses, GRADE analysis, data interpretation, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Zipporah Iheozor‐Ejiofor performed data extraction, statistical analyses, data interpretation, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Teuta Gjuladin‐Hellon performed data extraction, communication with primary study authors, risk of bias assessments, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Alyssa Parian performed screening of abstracts and titles, screening of full‐text articles, data interpretation, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Laura E Matarese provided input as a dietician, reviewed and classified dietary interventions, participated in data extraction, critical revision of the manuscript, and approved the final manuscript.
Kelly Bracewell was involved with meetings at the data extraction and analysis stages, randomly reviewing data extraction, wrote the PLS, critical revision of the manuscript, and approved the final manuscript.
John K MacDonald provided methodological expertise and was involved with checking the data analyses, GRADE analysis, data interpretation, critical revision of the manuscript, and approval of the final manuscript.
Morris Gordon performed screening of abstracts and titles, screening of full‐text articles, adjudication in the screening and data extraction phases, data analyses, data interpretation, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Gerard E Mullin is the senior author and performed screening of the abstracts and titles, screening of full text articles, adjudication in the screening and data extraction phases, data interpretation, manuscript preparation, critical revision of the manuscript, and approval of the final manuscript.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Berkeley N Limketkai: None known
Zipporah Iheozor‐Ejiofor: None known
Teuta Gjuladin‐Hellon: None known
Alyssa Parian: None known
Laura E Matarese: None known
Kelly Bracewell: None known
John K MacDonald: None known
Morris Gordon has received travel fees to attend international scientific and training meeting such as DDW, Advances in IBD, ESPGHAN, BSPGHAN and Cochrane focused international events from companies including: Abbott, Nutricia, Biogaia, Ferring, Allergan, and Tillots.
Gerard E Mullin has received grants or grants pending (paid to institution) from Abbott Laboratories; and royalties from Rodale Press, Oxford University Press, and CRC Press for books written and or edited on nutrition, generically, and only a few chapters as an expert on the role of diet in IBD.
New
References
References to studies included in this review
Albenberg 2018 {published data only}
- Albenberg L, Brensinger C, Wu Q, Gilroy E, Kappelman M, Sandler R, et al. A diet low in red and processed meats does not reduce the rate of Crohn's disease flares in a randomized controlled trial: results of the food and Crohn's disease exacerbation study (FACES). Gastroenterology 2018;154(1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartel 2008 {published data only}
- Bartel G, Weiss I, Turetschek K, Schima W, Püspök A, Waldhoer T, et al. Ingested matter affects intestinal lesions in Crohn's disease. Inflammatory Bowel Diseases 2008;14(3):374‐82. [PUBMED: 17932967] [DOI] [PubMed] [Google Scholar]
Bhattacharyya 2017 {published data only}
- Bhattacharyya S, Shumard T, Xie H, Dodda A, Varady KA, Feferman L, et al. A randomized trial of the effects of the no‐carrageenan diet on ulcerative colitis disease activity. Nutrition and Healthy Aging 2017;4:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandes 1981 {published data only}
- Brandes JW, Lorenz‐Meyer H. Sugar free diet: a new perspective in the treatment of Crohn disease? Randomized, control study. Zeitschrift fur Gastroenterologie 1981;19(1):1‐12. [PUBMED: 7013307] [PubMed] [Google Scholar]
Brotherton 2014 {published data only}
- Brotherton CS, Taylor AG, Bourguignon C, Anderson JG. A high‐fiber diet may improve bowel function and health‐related quality of life in patients with Crohn disease. Gastroenterology Nursing 2014;37(3):206‐16. [PUBMED: 24871666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Candy 1995 {published data only}
- Candy S, Borok G, Wright JP, Boniface V, Goodman R. The value of an elimination diet in the management of patients with ulcerative colitis. South African Medical Journal 1995;85(11):1176‐9. [PUBMED: 8597010] [PubMed] [Google Scholar]
Dariel 2007 {published data only}
- Dariel I, Levi Z, Fraser A, Hadad B, Niv Y, Fraser G. Elimination diets in the treatment of mildly active Crohn's disease ‐ results of a randomized controlled trial. Gastroenterology 2207;132(4):A506‐7. [Google Scholar]
Jones 1985 {published data only}
- Jones VA, Dickinson RJ, Workman E, Wilson AJ, Freeman AH, Hunter JO. Crohn's disease: maintenance of remission by diet. Lancet 1985;2(8448):177‐80. [PUBMED: 2862371] [DOI] [PubMed] [Google Scholar]
Keshteli 2016 {published data only}
- Keshteli AH, Valcheva R, Nickurak C, Halloran BP, Zanten SV, Kroeker K, et al. Adherence to an "Anti‐Inflammatory Diet" for 6 months can decrease fecal calprotectin in ulcerative colitis patients: preliminary findings of a randomized controlled trial. Gastroenterology 2016;150(4):S807‐8. [Google Scholar]
Levenstein 1985 {published data only}
- Levenstein S, Prantera C, Luzi C, D'Ubaldi A. Low residue or normal diet in Crohn's disease: a prospective controlled study in Italian patients. Gut 1985;26(10):989‐93. [PUBMED: 2996991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lomer 2001 {published data only}
- Lomer MC, Harvey RS, Evans SM, Thompson RP, Powell JJ. Efficacy and tolerability of a low microparticle diet in a double blind, randomized, pilot study in Crohn's disease. European Journal of Gastroenterology & Hepatology 2001;13(2):101‐6. [PUBMED: 11246607] [DOI] [PubMed] [Google Scholar]
Lomer 2005 {published data only}
- Lomer MC, Grainger SL, Ede R, Catterall AP, Greenfield SM, Cowan RE, et al. Lack of efficacy of a reduced microparticle diet in a multi‐centred trial of patients with active Crohn's disease. European Journal of Gastroenterology & Hepatology 2005;17(3):377‐84. [PUBMED: 15716665] [DOI] [PubMed] [Google Scholar]
Lorenz‐Meyer 1996 {published data only}
- Lorenz‐Meyer H, Bauer P, Nicolay C, Schulz B, Purrmann J, Fleig WE, et al. Omega‐3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Scandinavian Journal of Gastroenterology 1996;31(8):778‐85. [PUBMED: 8858747] [DOI] [PubMed] [Google Scholar]
Mutlu 2016 {published data only}
- Mutlu E, Mikolaitis S, Sedghi S, Chakradeo PS, Engen P, Chlipala G, et al. Dietary treatment of Crohn's disease: a randomized, placebo‐controlled, double‐blinded clinical trial. Gastroenterology 2016;150(4):S778. [Google Scholar]
Riordan 1993 {published data only}
- Riordan AM, Hunter JO, Cowan RE, Crampton JR, Davidson AR, Dickinson RJ, et al. Treatment of active Crohn's disease by exclusion diet: East Anglian multicentre controlled trial. Lancet 1993;342(8880):1131‐4. [PUBMED: 7901473] [DOI] [PubMed] [Google Scholar]
Ritchie 1987 {published data only}
- Ritchie JK, Wadsworth J, Lennard‐Jones JE, Rogers E. Controlled multicentre therapeutic trial of an unrefined carbohydrate, fibre rich diet in Crohn's disease. British Medical Journal Clinical Research Edition 1987;295(6597):517‐20. [PUBMED: 2822203] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strisciuglio 2013 {published data only}
- Strisciuglio C, Giannetti E, Martinelli M, Sciorio E, Staiano A, Miele E. Does cow's milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis?. Acta Paediatrica 2013;102(6):e273‐8. [PUBMED: 23445275] [DOI] [PubMed] [Google Scholar]
Wright 1965 {published data only}
- Wright R, Truelove SC. A controlled therapeutic trial of various diets in ulcerative colitis. British Medical Journal 1965;2(5454):138‐41. [PUBMED: 14304053] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Barnes 2016 {published data only}
- Barnes EL, Nestor MA, Onyewadume L, Silva PS, Korzenik JR. A prospective study: the role of diet in exacerbations of patients with ulcerative colitis in remission on monotherapy with mesalamine. Gastroenterology 2016;150(4):S5‐6. [Google Scholar]
Beattie 1994 {published data only}
- Beattie RM, Walker‐Smith JA. Treatment of active Crohn's disease by exclusion diet. Journal of Pediatric Gastroenterology and Nutrition 1994;19(1):135‐6. [PUBMED: 7965469] [PubMed] [Google Scholar]
Bentz 2010 {published data only}
- Bentz S, Hausmann M, Piberger H, Kellermeier S, Paul S, Held L, et al. Clinical relevance of IgG antibodies against food antigens in Crohn's disease: a double‐blind cross‐over diet intervention study. Digestion 2010;81(4):252‐64. [PUBMED: 20130407] [DOI] [PubMed] [Google Scholar]
Boneh 2017 {published data only}
- Boneh, R. S, Shabat, C. S, Yanai, H, Chermesh, I, Ben Avraham, S, Boaz, M, Levine, A. Dietary Therapy With the Crohn's Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. Journal of Crohns & Colitis Oct 2017;11(10):1205‐1212. [DOI] [PubMed] [Google Scholar]
Brandes 1982 {published data only}
- Brandes JW, Körst HA, Littman KP. Sugar‐free diet as long‐term or interval treatment in the remission phase of Crohn disease ‐ a prospective study. Leber Magen Darm 1982;12(6):225‐8. [PUBMED: 6135129] [PubMed] [Google Scholar]
Castro 1995 {published data only}
- Castro M, Lucidi V, Colombo AM, Gambarara M. Diet therapy or drug therapy in Crohn's disease. Rivista Italiana Di Pediatria 1995;21(4):439‐45. [Google Scholar]
Ciccimarra 1998 {published data only}
- Ciccimarra E, Borrelli O, Franco MT, Iula VD, Montisci A, Vizia B, et al. Nutritional therapy of Crohn's disease. Rivista Italiana Di Pediatria 1998;24(1):108‐16. [Google Scholar]
Cohen 2012 {published data only}
- Cohen SA, Stallworth AN, Koch BM, Mason DH, Blumenthal J, Gold BD. Mucosal healing with the Specific Carbohydrate Diet in pediatric Crohn's disease: preliminary results of a prospective pilot study. Gastroenterology 2012;142(5):S376. [Google Scholar]
Davies 1978 {published data only}
- Davies PS, Rhodes J. Maintenance of remission in ulcerative colitis with sulphasalazine or a high‐fibre diet: a clinical trial. British Medical Journal 1978;1(6126):1524‐5. [PUBMED: 26448] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2017 {published data only}
- Dunn KA, Boneh RS, Bielawski JP, Turner D, Limbergen JE, Levine A. Crohn's disease exclusion diet and partial enteral nutrition (CDED+PEN) vs exclusive enteral nutrition (EEN) ‐ microbiome changes of a randomized clinical trial (RCT) in pediatric CD: Remission is associated with similar structural and functional profiles. Gastroenterology 2017;152(5):S213. [Google Scholar]
El‐Tahir 1998 {published data only}
- El‐Tahir A, Heys SD, Sinclair T, Mowat NAG, Ewen S, Eremin O. Effects of dietary supplementation on disease activity in patients with ulcerative colitis: a randomized controlled clinical study. British Journal of Surgery 1998;85(11):1566. [Google Scholar]
Gunasekeera 2016 {published data only}
- Gunasekeera V, Mendall MA, Chan D, Kumar D. Treatment of Crohn's disease with an IgG4‐guided exclusion diet: a randomized controlled trial. Digestive Diseases and Sciences 2016;61(4):1148‐57. [PUBMED: 26809868] [DOI] [PubMed] [Google Scholar]
Halmos 2016 {published data only}
- E. P. Halmos, C. T. Christophersen, A. R. Bird, S. J. Shepherd, J. G. Muir, P. R. Gibson. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross‐Over Trial of Well‐Defined Diets. Clinical and Translational Gastroenterology 2016;7:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyaw 2014 {published data only}
- Kyaw MH, Moshkovska T, Mayberry J. A prospective, randomized, controlled, exploratory study of comprehensive dietary advice in ulcerative colitis: impact on disease activity and quality of life. European Journal of Gastroenterology & Hepatology 2014;26(8):910‐7. [PUBMED: 24942954] [DOI] [PubMed] [Google Scholar]
Mikolaitis 2013 {published data only}
- Mikolaitis S, Chakradeo P, Fogg L, Keshavarzian A, Mutlu E. Blinding of patients and physicians during a one‐year randomized clinical trial of dietary interventions for treatment of Crohn's disease. American Journal of Gastroenterology 2013;108:S495. [Google Scholar]
NCT01749813 {published data only}
- NCT01749813. Specific Carbohydrate Diet as Maintenance Therapy in Crohn's Disease. ClinicalTrials.gov/show/NCT01749813 (accessed 31 January 2018).
NCT02093780 {published data only}
- NCT02093780. Personalized "Alberta" Diet for Prevention of Relapse in Ulcerative Colitis. ClinicalTrials.gov/show/NCT02093780 (accessed 31 January 2018).
NCT02213835 {published data only}
- NCT02213835. Treatment With the Specific Carbohydrate Diet for Children With Active Crohns Disease and Ulcerative Colitis. ClinicalTrials.gov/show/NCT02213835 (accessed 31 January 2018).
NCT02231814 {published data only}
- NCT02231814. Pilot Study of Partial Enteral Nutrition With a Unique Diet for the Treatment of Adult Patients With Crohn's Disease. ClinicalTrials.gov/show/NCT02231814 (accessed 31 January 2018).
NCT02345733 {published data only}
- NCT02345733. Use of a Novel Diet (UC DIET) for Treatment of Mild to Moderate Active Pediatric Ulcerative Colitis. ClinicalTrials.gov/show/NCT02345733 (accessed 31 January 2018).
NCT02357537 {published data only}
- NCT02357537. Decentralized Dietary UC Pilot Trial. ClinicalTrials.gov/show/NCT02357537 (accessed 31 January 2018).
NCT02426567 {published data only}
- NCT02426567. The Impact of "Crohn's Disease‐TReatment‐with‐EATing" Diet and Exclusive Enteral Nutrition on Healthy Gut Bacteria. ClinicalTrials.gov/show/NCT02426567 (accessed 31 January 2018).
NCT02469220 {published data only}
- NCT02469220. Diet Treatment of Patients With Ulcerative Colitis in Remission. ClinicalTrials.gov/show/NCT02469220 (accessed 31 January 2018).
NCT02610101 {published data only}
- NCT02610101. Nutritional Therapy Study in Pediatric Crohn's Disease. ClinicalTrials.gov/show/NCT02610101 (accessed 31 January 2018).
NCT02922881 {published data only}
- NCT02922881. Microbiota‐targeted Diet for Pediatric UC (UCD). ClinicalTrials.gov/show/NCT02922881 (accessed 31 January 2018).
NCT02930564 {published data only}
- NCT02930564. The Challenge Study: A Dietary Personalization Protocol for Patients With Crohn's Disease and Deep Remission. ClinicalTrials.gov/show/NCT02930564 (accessed 31 January 2018).
NCT02945488 {published data only}
- NCT02945488. Exercise and Nutrition in IBD & Preconception. ClinicalTrials.gov/show/NCT02945488 (accessed 31 January 2018).
NCT03171246 {published data only}
- NCT03171246. CD‐TREAT Diet: a Novel Therapy for Active Luminal Crohn's Disease. ClinicalTrials.gov/show/NCT03171246 (accessed 31 January 2018).
Pedersen 2014 {published data only}
- Pedersen N, Vinding KK, Vegh Z, Casen C, Ankersen DV, Carlsen K, et al. Gut microbiota in IBD patients with IBS before and after 6 weeks of low FODMAP diet. Gastroenterology 2014;146(5):S241. [Google Scholar]
Pedersen 2017 {published data only}
- Pedersen N, Ankersen DV, Felding M, Wachmann H, Végh Z, Molzen L, et al. Low‐FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World Journal of Gastroenterology 2017;23(18):3356‐66. [PUBMED: 28566897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pituch‐Zdanowska 2018 {published data only}
- Pituch‐Zdanowska A, Albrecht P, Banasiuk M, Banaszkiewicz A. Dietary fiber intake in children with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition 2018;66:624‐9. [DOI] [PubMed] [Google Scholar]
Stange 1990 {published data only}
- Stange EF, Schmid U, Fleig WE, Ditschuneit H. Exclusion diet in Crohn disease: a controlled, randomized study. Zeitschrift fur Gastroenterologie 1990;28(10):561‐4. [PUBMED: 2275253] [PubMed] [Google Scholar]
Strohm 1981 {published data only}
- Strohm WD, Steinhardt HJ, Brandes JW, Jarnum S, Leonhardt H, Ehms H, et al. Low‐molecular fully balanced diets for the treatment of Crohn's disease ‐ a randomized controlled study. Zeitschrift fur Gastroenterologie 1981;19(9):525. [Google Scholar]
Svolos 2016 {published data only}
- Svolos V, Hansen R, Kughes K, Ijaz UZ, Quince C, Gaya D, et al. The impact of 'Crohn's Disease‐TReatment‐with‐EATing' diet (CD‐TREAT diet) and exclusive enteral nutrition on healthy gut bacteria. Journal of Crohn's & Colitis 2016;10(Supplement 1):S14‐5. [Google Scholar]
Vincenzi 2016 {published data only}
- Vincenzi M, Paolini B, Ciondolo I, Pasquini E, Ceccarelli L, Gennai K, et al. Effects on symptoms and on nutritional adequacy of a low FODMAPs diet and Specific Carbohydrate Diet on inflammatory bowel disease. Double blind and randomized study. Preliminary result. Gastroenterology 2016;150(4):S425. [Google Scholar]
References to studies awaiting assessment
Bodini 2018 {published data only}
- Bodini G, Giannini EGB, Savarino V, Crespi M, Lo Pumo S, Zanella C, et al. Low FODMAP diet improve disease activity and quality of life in patients with inflammatory bowel disease. Digestive and Liver Disease 2018;50:e197‐8. [Google Scholar]
Tapete 2018 {published data only}
- Tapete G, Bortoli N, Ceccarelli L, Mumolo MG, Vinci E, Albano E, et al. Low‐FODMAPs diet improves intestinal symptoms in IBD patients with disease remission: Randomized case‐control study. Digestive and Liver Disease 2018;50:e195. [Google Scholar]
References to ongoing studies
NCT02825316 {published data only}
- NCT02825316. Mediterranean Diet as an add‐on Therapy for Induction of Remission in Patients With Active Crohn's Disease. ClinicalTrials.gov/show/NCT02825316 (accessed 31 January 2018).
NCT02858557 {published data only}
- NCT02858557. The Effect of Diet on Microbial Profile and Disease Outcomes in Patients With Inflammatory Bowel Diseases. ClinicalTrials.gov/show/NCT02858557 (accessed 31 January 2018).
NCT03012542 {published data only}
- NCT03012542. Diet as Essential Therapy (DIET) for Inflammatory Bowel Disease. ClinicalTrials.gov/show/NCT03012542 (accessed 31 January 2018).
NCT03053713 {published data only}
- NCT03053713. The Effect of Diet on Disease Activity and Symptoms in Patients With Ulcerative Colitis. ClinicalTrials.gov/show/NCT03053713 (accessed 31 January 2018).
NCT03058679 {published data only}
- NCT03058679. Trial of Specific Carbohydrate and Mediterranean Diets to Induce Remission of Crohn's Disease. ClinicalTrials.gov/show/NCT03058679 (accessed 31 January 2018).
Additional references
Abraham 2009
- Abraham C, Cho JH. Inflammatory bowel disease. New England Journal of Medicine 2009;361:2066‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akobeng 2018
- Akobeng AK, Zhang D, Gordon M, MacDonald JK. Enteral nutrition for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD005984.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amre 2007
- Amre DK, D'Souza S, Morgan K, Seidman G, Lambrette P, Grimard G, et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. American Journal of Gastroenterology 2007;102:2016‐25. [DOI] [PubMed] [Google Scholar]
Ananthakrishnan 2013
- Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, Silva P, Korzenik JR, et al. A prospective study of long‐term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology 2013;145:970‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananthakrishnan 2014
- Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, Silva P, Fuchs CS, et al. Long‐term intake of dietary fat and risk of ulcerative colitis and Crohn's disease. Gut 2014;63:776‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ananthakrishnan 2015
- Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Richter JM, Nimptsch K, et al. High School Diet and Risk of Crohn's Disease and Ulcerative Colitis. Inflammatory Bowel Diseases 2015;21:2311‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benchimol 2015
- Benchimol EI, Mack DR, Guttmann A, Nguyen GC, To T, Mojaverian N, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population‐based cohort study. American Journal of Gastroenterology 2015;110:553‐63. [DOI] [PubMed] [Google Scholar]
Bianchi 1985
- Bianchi Porro G, Panza E. Smoking, sugar, and inflammatory bowel disease. British Medical Journal (Clinical Research Edition) 1985;291:971‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Birrenbach 2004
- Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflammatory Bowel Diseases 2004;10:848‐59. [DOI] [PubMed] [Google Scholar]
Brown 2011
- Brown AC, Rampertab SD, Mullin GE. Existing dietary guidelines for Crohn's disease and ulcerative colitis. Expert Review of Gastroenterology and Hepatology 2011;5:411‐25. [DOI] [PubMed] [Google Scholar]
Chan 2014
- Chan SS, Luben R, Olsen A, Tjonneland A, Kaaks R, Lindgren S, et al. Association between high dietary intake of the n‐3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn's disease. Alimentary Pharmacology and Therapeutics 2014;39(8):834‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman‐Kiddell 2010
- Chapman‐Kiddell CA, Davies PS, Gillen L, Radford‐Smith GL. Role of diet in the development of inflammatory bowel disease. Inflammatory Bowel Diseases 2010;16:137‐51. [DOI] [PubMed] [Google Scholar]
Cornish 2008
- Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta‐analysis. American Journal of Gastroenterology 2008;103:2394‐400. [DOI] [PubMed] [Google Scholar]
Critch 2012
- Critch J, Day AS, Otley A, King‐Moore C, Teitelbaum JE, Shashidhar H, et al. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. Journal of Pediatric Gastroenterology and Nutrition 2012;54:298‐305. [DOI] [PubMed] [Google Scholar]
Damas 2013
- Damas OM, Jahann DA, Reznik R, McCauley JL, Tamariz L, Deshpande AR, et al. Phenotypic manifestations of Inflammatory Bowel Disease differ between Hispanics and non‐Hispanic whites: results of a large cohort study. American Journal of Gastroenterology 2013;108(2):231‐9. [DOI] [PubMed] [Google Scholar]
Damas 2017
- Damas OM, Avalos DJ, Palacio AM, Gomez L, Quintero MA, Deshpande AR, et al. Inflammatory bowel disease is presenting sooner after immigration in more recent US immigrants from Cuba. Alimentary Pharmacology and Therapeutics 2017;46(3):303‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devkota 2012
- Devkota S, Wang Y, Musch MW, Leone V, Fehlner‐Peach H, Nadimpalli A, et al. Dietary‐fat‐induced taurocholic acid promotes pathobiont expansion and colitis in Il10‐/‐ mice. Nature 2012;487:104‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2013
- Foster A, Jacobson K. Changing incidence of inflammatory bowel disease: environmental influences and lessons learnt from the South Asian population. Frontiers in Pediatrics 2013;1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galvez 2005
- Galvez J, Rodríguez‐Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Molecular Nutrition and Food Research 2005;49:601‐8. [DOI] [PubMed] [Google Scholar]
Garcia Rodriguez 2006
- García Rodríguez LA, Ruigómez A, Panés J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006;130:1588‐94. [DOI] [PubMed] [Google Scholar]
Gearry 2009
- Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short‐chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease‐a pilot study. Journal of Crohn's and Colitis 2009;3:8‐14. [DOI] [PubMed] [Google Scholar]
Gibson 2015
- Gibson PR, Varney J, Malakar S, Muir JG. Food components and irritable bowel syndrome. Gastroenterology 2015;148:1158‐74. [DOI] [PubMed] [Google Scholar]
Gradel 2009
- Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short‐ and long‐term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 2009;137:495‐501. [DOI] [PubMed] [Google Scholar]
Gruber 2013
- Gruber L, Kisling S, Lichti P, Martin FP, May S, Klingenspor M, et al. High fat diet accelerates pathogenesis of murine Crohn's disease‐like ileitis independently of obesity. PLoS One 2013;8:e71661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [DOI] [PubMed] [Google Scholar]
Halmos 2015
- Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015;64:93‐100. [DOI] [PubMed] [Google Scholar]
Hansen 2011
- Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg M, et al. Environmental factors in inflammatory bowel disease: a case‐control study based on a Danish inception cohort. Journal of Crohn's and Colitis 2011;5:577‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Hou 2011
- Hou JK, Abraham B, El‐Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. American Journal of Gastroenterology 2011;106:563‐73. [DOI] [PubMed] [Google Scholar]
Jakobsen 2013
- Jakobsen C, Paerregaard A, Munkholm P, Wewer V. Environmental factors and risk of developing paediatric inflammatory bowel disease ‐‐ a population based study 2007‐2009. Journal of Crohn's and Colitis 2013;7:79‐88. [DOI] [PubMed] [Google Scholar]
Janerot 1983
- Jarnerot G, Jarnmark I, Nilsson K. Consumption of refined sugar by patients with Crohn’s disease, ulcerative colitis, or irritable bowel syndrome. Scandinavian Journal of Gastroenterology 1983;18(8):999‐1002. [DOI] [PubMed] [Google Scholar]
Jantchou 2010
- Jantchou P, Morois S, Clavel‐Chapelon F, Boutron‐Ruault MC, Carbonnel F. Animal protein intake and risk of Inflammatory Bowel Disease: the E3N prospective study. American Journal of Gastroenterology 2010;105(10):2195‐201. [DOI] [PubMed] [Google Scholar]
Jowett 2004
- Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 2004;53(10):1479‐84.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kono 2010
- Kono H, Fujii H, Ogiku M, Tsuchiya M, Ishii K, Hara M. Enteral diets enriched with medium‐chain triglycerides and N‐3 fatty acids prevent chemically induced experimental colitis in rats. Translational Research 2010;156:282‐91. [DOI] [PubMed] [Google Scholar]
Lih‐Brody 1996
- Lih‐Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Digestive Diseases and Sciences 1996;41:2078‐86. [DOI] [PubMed] [Google Scholar]
Limdi 2016
- Limdi JK, Aggarwal D, McLaughlin JT. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2016;22:164‐70. [DOI] [PubMed] [Google Scholar]
Limketkai 2016
- Limketkai BN, Bechtold ML, Nguyen DL. Vitamin D and the pathogenesis of Inflammatory Bowel Disease. Current Gastroenterology Reports 2016;18:52. [DOI] [PubMed] [Google Scholar]
Martini 1976
- Martini GA, Brandes JW. Increased consumption of refined carbohydrates in patients with Crohn's disease. Klinische Wochenschrift 1976;54:367‐71. [DOI] [PubMed] [Google Scholar]
Matt 1961
- Matts SF. The value of rectal biopsy in the diagnosis of ulcerative colitis. QJM 1961;30:393‐407. [PubMed] [Google Scholar]
Molodecky 2012
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46‐54. [DOI] [PubMed] [Google Scholar]
Mullin 2016
- Mullin GE. Role of diet in Inflammatory Bowel Disease. Presented at: Digestive Disease Week; May 2016; San Diego, CA.
Narula 2018
- Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD000542.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Care Excellence (2014). Crohn's disease: management. NICE guideline (CG183). [PubMed] [Google Scholar]
Ooi 2016
- Ooi CJ, Makharia GK, Hilmi I, Gibson PR, Fock KM, Ahuja V, et al. Asia Pacific consensus statements on Crohn's disease. Part 1: definition, diagnosis, and epidemiology. Journal of Gastroenterology and Hepatology 2016;31:45‐55. [DOI] [PubMed] [Google Scholar]
Papada 2014
- Papada E, Kaliora AC, Gioxari A, Papalois A, Forbes A. Anti‐inflammatory effect of elemental diets with different fat composition in experimental colitis. British Journal of Nutrition 2014;111:1213‐20. [DOI] [PubMed] [Google Scholar]
Persson 1992
- Persson PG, Ahlbom A, Hellers G. Diet and inflammatory bowel disease: a case‐control study. Epidemiology 1992;3:47‐52. [DOI] [PubMed] [Google Scholar]
Pinsk 2007
- Pinsk V, Lemberg D A, Grewal K, Barker C C, Schreiber R A, Jacobson K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. American Journal of Gastroenterology 2007;102:1077‐83. [DOI] [PubMed] [Google Scholar]
Prince 2016
- Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2016;22:1129‐36. [DOI] [PubMed] [Google Scholar]
Rajendran 2011
- Rajendran N, Kumar D. Food‐specific IgG4‐guided exclusion diets improve symptoms in Crohn's disease: a pilot study. Colorectal Disease 2011;13:1009‐13. [DOI] [PubMed] [Google Scholar]
Reif 1997
- Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre‐illness dietary factors in inflammatory bowel disease. Gut 1997;40:754‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakamoto 2005
- Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, et al. Dietary risk factors for inflammatory bowel disease: a multicenter case‐control study in Japan. Inflammatory Bowel Diseases 2005;11:154‐63. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Silkoff 1980
- Silkoff K, Hallak A, Yegena L, Rozen P, Mayberry JF, Rhodes J, et al. Consumption of refined carbohydrate by patients with Crohn's disease in Tel‐Aviv‐Yafo. Postgraduate Medical Journal 1980;56:842‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thornton 1979
- Thornton JR, Emmett PM, Heaton KW. Diet and Crohn's disease: characteristics of the pre‐illness diet. British Medical Journal 1979;2:762‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ungaro 2014
- Ungaro R, Bernstein CN, Gearry R, Hviid A, Kolho KL, Kronman MP, et al. Antibiotics associated with increased risk of new‐onset Crohn's disease but not ulcerative colitis: a meta‐analysis. American Journal of Gastroenterology 2014;109:1728‐38. [DOI] [PubMed] [Google Scholar]
van der Logt 2013
- Logt EM, Blokzijl T, Meer R, Faber KN, Dijkstra G. Westernized high‐fat diet accelerates weight loss in dextran sulfate sodium‐induced colitis in mice, which is further aggravated by supplementation of heme. Journal of Nutritional Biochemistry 2013;24:1159‐65. [DOI] [PubMed] [Google Scholar]
Zuo 2007
- Zuo XL, Li YQ, Li WJ, Guo YT, Lu XF, Li JM, et al. Alterations of food antigen‐specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clinical and Experimental Allergy 2007;37(6):823‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Limketkai 2017
- Limketkai BN, Parian A, Koretz RL, Nanavati JE, Shinohara RT, Mullin GE. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD012839] [DOI] [PMC free article] [PubMed] [Google Scholar]


