Abstract
Muscle glucose transport activity increases with an acute bout of exercise, a process that is accomplished by the translocation of glucose transporters to the plasma membrane. This process remains intact in the skeletal muscle of individuals with insulin resistance and type 2 diabetes mellitus (T2DM). Exercise training is, therefore, an important cornerstone in the management of individuals with T2DM. However, the acute systemic glucose responses to carbohydrate ingestion are often augmented during the early recovery period from exercise, despite increased glucose uptake into skeletal muscle. Accordingly, the first aim of this review is to summarize the knowledge associated with insulin action and glucose uptake in skeletal muscle and apply these to explain the disparate responses between systemic and localized glucose responses post-exercise. Herein, the importance of muscle glycogen depletion and the key glucoregulatory hormones will be discussed. Glucose uptake can also be stimulated independently by hypoxia; therefore, hypoxic training presents as an emerging method for enhancing the effects of exercise on glucose regulation. Thus, the second aim of this review is to discuss the potential for systemic hypoxia to enhance the effects of exercise on glucose regulation.
Keywords: Glucose, Insulin resistance, Hypoxia, Glucose metabolism, Exercise
Introduction
Maintaining blood glucose concentration within a narrow range is not trivial, with postprandial glycaemic excursions overlaying the fasting blood glucose concentrations. This is particularly evident in type 2 diabetes mellitus (T2DM), where insulin resistance and altered glucose metabolism are a natural consequence of the clinical history. In T2DM, poor glycaemic control is associated with increased risks of cardiovascular disease (Bassuk and Manson 2005; Gayda et al. 2008), renal disease (Ismail et al. 1999), non-traumatic amputations (Reiber et al. 1995) and all-cause mortality (Laukkanen et al. 2013).
Glycaemic control is improved with chronic exercise training (Umpierre et al. 2013; Boulé et al. 2003; Burgomaster et al. 2007; Mikus et al. 2012; Richards et al. 2010; Whyte et al. 2010). Improved glycaemic control in response to exercise training is primarily attributed to improved insulin-mediated glucose disposal (Babraj et al. 2009; Richards et al. 2010; Whyte et al. 2010) rather than lower fasting glucose concentration (MacLeod et al. 2013). Given that the skeletal muscle is the predominant site for insulin-mediated glucose disposal, regular exercise training is thus an important factor for glycaemic control. Importantly, these effects of exercise on glucose transport remain intact in individuals with T2DM. However, whilst the benefits of chronic exercise training on glycaemic control (commonly assessed via glycated haemoglobin [HbA1c]) are well established (Tsukui et al. 2000; Thomas et al. 2006; Umpierre et al. 2011), there is large variability in the magnitude of the response (Boulé et al. 2005). Potential factors contributing to this variability include individual differences (e.g. duration of T2DM; dietary intake), and differences in exercise-related factors such as the volume, type and intensity of exercise. Increases in insulin-stimulated skeletal muscle glucose uptake with exercise is associated with the magnitude of muscle glycogen depletion during exercise (Ivy et al. 1985; Bogardus et al. 1983). Indeed, both insulin- and contraction-mediated glucose transport are influenced by skeletal muscle glycogen concentration (Host et al. 1998; Derave et al. 2000; Derave et al. 1999; Kawanaka et al. 1999; Kawanaka et al. 2000). However, glycogen utilization is dependent on exercise being performed at a sufficiently high intensity and/or duration, which is challenging in clinical populations. As such, alternative exercise interventions are continually sought.
Exercising in hypoxia may represent an effective strategy to enhance muscle glycogen utilization and glucose uptake. The relative intensity of exercise is substantially higher when performed in hypoxia than in normal ambient conditions (i.e., normoxia). As such, increases in exercise intensity, which is important for improving glucose regulation, can be undertaken without a substantial increase in workload. Furthermore, hypoxia may stimulate the activation of signalling molecules responsible for glucose transport activity, similar to high-intensity exercise. Exercising in hypoxia may thus promote a synergistic effect on glucose regulation that is safe and effective for individuals with T2DM.
The purpose of this review is to first summarize the existing knowledge related to insulin action garnered from classic in vitro and in situ glucose uptake studies. The effects of exercise and insulin on cellular glucose uptake and the physiological consequences on systemic glucose concentration are then reviewed. Finally, this review aims to explain factors that may influence the mismatch between cellular glucose uptake and systemic glucose concentration following exercise. In this context, systemic changes in plasma glucose post-exercise in relation to the effects of key glucoregulatory hormones will be discussed. Finally, the review will discuss the potential for hypoxia to enhance the effects of exercise on systemic glucose regulation.
Glucose transport
Cellular uptake of glucose occurs through facilitated diffusion using a carrier protein from the glucose transporter (GLUT) family. There are 14 facilitative glucose transporter proteins encoded in the human genome (Mueckler and Thorens 2013). Of these, glucose transporter type 1 (GLUT-1) is ubiquitously distributed and does not change in response to hormonal or other stimuli (Douen et al. 1990; Holloszy and Hansen 1996). For this reason, GLUT-1 is primarily responsible for glucose transport under basal conditions.
Glucose transporter type 4 (GLUT-4), is present primarily in adipose cells (Garvey et al. 1991; Hussey et al. 2011) and striated (skeletal and cardiac) muscle cells (Olson and Pessin 1996). Of the several glucose transporters including GLUT-1, GLUT-5 and GLUT-12, GLUT-4 is the predominant protein expressed in the skeletal muscle (Stuart et al. 2006). That said, the GLUT-4 protein content varies between muscle fibre types (Henriksen et al. 1990), but can be increased with exercise training, even in individuals with T2DM (O'Gorman et al. 2006). In this instance, GLUT-4 displays membrane trafficking capability (Huang and Czech 2007) that is highly responsive to insulin, muscle contraction and other stimuli (e.g. hypoxia) (Richter et al. 2001). Accordingly, GLUT-4 is likely responsible for the bulk of glucose uptake into the muscle and is, therefore, an important determinant of glucose homeostasis.
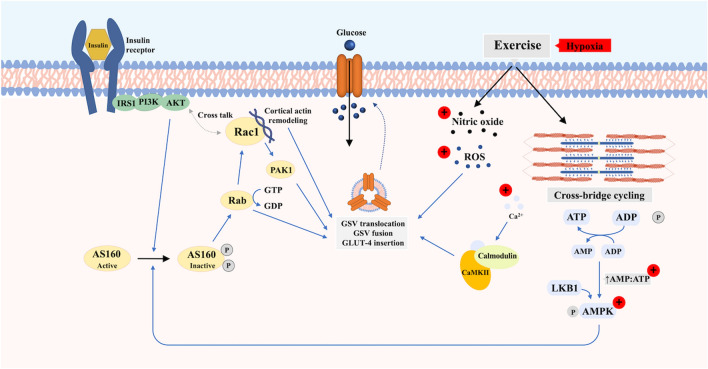
Insulin plays a central role in glucose homeostasis through its direct effect on insulin-sensitive tissues, namely, the liver, adipose tissues and skeletal muscle (Petersen and Shulman 2018). The skeletal muscle comprising ~ 40% of total body mass, accounts for ~ 85% of insulin-stimulated glucose disposal (DeFronzo et al. 1981). Insulin binding (Wardzala and Jeanrenaud 1981) and/or muscle contraction (Hirshman et al. 1990; Goodyear et al. 1990a) initiates the translocation of GLUT-4 from its intracellular compartments to the sarcolemma and t-tubules. In this instance, an increased GLUT-4 protein content at the cell membrane ultimately increases the rate of glucose transport (Constable et al. 1988; Goodyear et al. 1990b). Specifically, the increase in GLUT-4 content and glucose transport parallels the increased contraction rates (Lund et al. 1995). Combining maximal insulin stimulation (1 mU/ml) and muscle contractions (10 Hz for 5 min) has also been shown to result in a 9.3-fold increase in the GLUT-4 plasma membrane expression in rat soleus muscle (Lund et al. 1995). This additive effect in skeletal muscle may be due to the existence of discrete intracellular GLUT-4 pools, which are mobilized via distinct molecular mechanisms (Coderre et al. 1995; Hansen et al. 1998; Richter et al. 1987) (Fig. 1). That said, the molecular mechanisms stimulating GLUT-4 translocation during insulin stimulation and contraction do appear to partially converge at the Rab GTPase-activating protein (GAP) AKT substrate of 160 kDa (AS160 or also known as TBC1D4) and GAP TBC1D1 (Mackenzie and Watt 2016; Sylow et al. 2021). Phosphorylation of TBC1D4/TBC1D1 inactivates Rab-GAP activity regulating vesicle trafficking which in turn, increase the concentration of additional signalling molecules (e.g. Rac1) (Sylow et al. 2013).
Fig. 1.
Proposed pathways mediating translocation of GLUT-4 to the cell membrane of skeletal myocyte in response to insulin and exercise/contraction with and without additional hypoxic stimulus. Upon insulin binding, the activated insulin receptor initiates downstream metabolic signalling that recruits diverse substrates, which ultimately leads to the translocation and fusion of the glucose transporter storage vesicle to the cell membrane and insertion of GLUT-4. Exercise and muscle contraction stimulate GLUT-4 translocation and glucose uptake through a distinct mechanism independent of insulin. This mechanism involves changes in cellular energy status, intracellular Ca2+ concentration, reactive oxygen species (ROS) and nitric oxide (NO). Hypoxia activates GLUT-4 translocation via similar or overlapping pathways to that of contraction-mediated glucose uptake. The (+) indicates the proposed potentiation of the hypoxia stimulus
Increased rates of glucose transport have been shown to persist into recovery following exhaustive exercise, with the rate of glucose transport reported to be ~ 5.6-, ~ 3.3-, ~ 2.7-, and ~ 1.5-fold higher than pre-exercise (Wallberghenriksson et al. 1988) at 10, 30, 60 and 180 min post-exercise. These findings in rat epitrochlearis muscle provided early evidence that glucose uptake after exercise occurs in two phases. An additive effect of insulin and exercise on glucose uptake during the early stages of recovery; followed by an increase in insulin sensitivity and responsiveness during the latter stages of recovery when contraction-mediated glucose uptake had largely been reversed (Wallberghenriksson et al. 1988).
Insulin sensitivity and responsiveness
Insulin sensitivity refers to the concentration of insulin required to achieve half of its maximal effect on glucose transport (Holloszy 2005). Conversely, insulin responsiveness refers to the rate of glucose transport associated with a maximally effective insulin. In this instance, the measurement of insulin sensitivity post-exercise requires careful interpretation to distinguish between increased insulin action (i.e., latter stage of recovery) and exercise-induced increase in glucose transport that is additive to insulin (early stage of recovery) (Table 1).
Table 1.
The acute and chronic hormonal responses during exercise with and without hypoxia
| Hormones | Protocol | Observations |
|---|---|---|
| Insulin | Exercise in NOR |
Acute response Insulin concentration decreases (Gyntelberg et al. 1977) during acute prolonged cycling (60% ), relative to baseline Insulin concentration increases significantly (and rapidly) during post-exercise recovery (Kjaer et al. 1990; Marliss et al. 1991) Training response Decrease in insulin during exercise is attenuated following exercise training compared to prior training at the same absolute intensity (Gyntelberg et al. 1977; Mendenhall et al. 1994) |
| Exercise in NOR vs. Exercise in HYP |
Acute response Insulin response to glucose at rest is similar between hypoxia and normoxia (Kelly et al. 2010) Similar insulin response to exercise in hypoxia (~ 2000–4300 m) relative to normoxia (Bouissou et al. 1986; Bailey et al. 2015; Engfred et al. 1994; Matu et al. 2017), whereas insulin response during post-exercise recovery is elevated in severe hypoxia (4300 m) compared to moderate hypoxia (2100 m) and normoxia (Matu et al. 2017) Training response Similar decrease in insulin response during exercise in normoxia following 5 weeks of training in normoxia or hypoxia (Engfred et al. 1994) |
|
| Glucagon | Exercise in NOR |
Acute response ~ Threefold increase from baseline during prolonged exhaustive treadmill running (i.e., volitional exhaustion) (Galbo et al. 1975) Increase in catecholamine is associated with the rise in glucagon concentration but does not fully account for the glucagon response to prolonged exercise (Galbo et al. 1975, 1976) Training response Increase in glucagon during exercise is blunted following a 10-week (4 d/wk) exercise training (Gyntelberg et al. 1977) The attenuated increase in glucagon is observed during exercise performed at the same relative (Gyntelberg et al. 1977) and absolute intensity (Gyntelberg et al. 1977; Mendenhall et al. 1994; Winder et al. 1979) as pre-training |
| Exercise in NOR vs. Exercise in HYP |
Acute response No significant differences in glucagon response during a maximal graded exercise that was performed in normoxia and hypoxia at similar relative intensity (Bouissou et al. 1986) Training response Glucagon response to exercise (in normoxia) following 5 weeks of training in normoxia or hypoxia was similar (Engfred et al. 1994) |
|
| Epinephrine | Exercise in NOR |
Acute responses Plasma epinephrine concentration increases with exercise intensity (Kjaer et al. 1990; Galbo et al. 1975) ~ Threefold and ~ 14 fold increase from baseline during acute exhaustive cycling at 75% (Hartley et al. 1972b) and 100% (Marliss et al. 1991), respectively Exaggerated epinephrine response to maximal exercise in T2DM compared to healthy individuals (Kjaer et al. 1990) Training responses Attenuated increase in epinephrine response to exercise at the same absolute intensity (as pre-training) following short-term training (10 days, 6d/wk) with minimal changes after further training (12 weeks) (Mendenhall et al. 1994) |
| Exercise in NOR vs. Exercise in HYP |
Acute responses Resting plasma epinephrine AUC after glucose ingestion is ~ twofold greater in hypoxia compared to normoxia (Kelly et al. 2010) Similar increase in epinephrine response during a maximal graded exercise that was performed in normoxia and hypoxia at similar relative intensity (Bouissou et al. 1986) Training response 5-week exercise training in hypoxia at the same relative or absolute intensity to normoxia did not alter epinephrine response (albeit blunted increase) to exhaustive exercise performed at the same absolute workload before training (Engfred et al. 1994) |
|
| Norepinephrine | Exercise in NOR |
Acute response ~ Sevenfold and ~ ninefold increase in norepinephrine following continuous (15 min) and intermittent running (3 × 300 m), respectively, (Näveri et al. 1985) compared to baseline ~ 18-fold increase from baseline during exhaustive high-intensity (100% ) cycling (Marliss et al. 1991) Exaggerated norepinephrine response to maximal exercise in T2DM compared to healthy individuals (Kjaer et al. 1990) Training response Attenuated increase in epinephrine response to exercise at the same absolute intensity (as pre-training) following short-term training (10 days, 6d/wk), with minimal changes after further training (12 weeks) (Mendenhall et al. 1994) |
| Exercise in NOR vs. Exercise in HYP |
Acute response Similar norepinephrine response during submaximal exercise in hypoxia relative to normoxia (performed at same relative intensity), but lower response at maximal aerobic capacity (100% ) (Bouissou et al. 1986) Training response 5-week exercise training in hypoxia at the same relative and absolute intensity to normoxia did not alter epinephrine response (albeit blunted increase) to exhaustive exercise that was performed at the same absolute workload before training (Engfred et al. 1994) |
|
| Growth hormone | Exercise in NOR |
Acute response Growth hormone concentration increased in response to exercise (Kjaer et al. 1990; Kindermann et al. 1982) ~ 14-fold increase following prolonged exercise (50 min at the anaerobic threshold of 4 mmol/L) (Kindermann et al. 1982) Training response Growth hormone response to an acute bout of exercise is not altered following 10-week training (at similar relative intensity) of different modalities (running, resistance training, and combined) (Craig et al. 1991) |
| Exercise in NOR vs. Exercise in HYP |
Acute response Hypoxia exercise (60 min at 60% of VO2max) induces a similar increase in growth hormone compared to exercise in normoxia at the same relative intensity (Schmidt et al. 1995) Elevated growth hormone concentration immediately after exercise following repeated sprint exercise (30 s efforts) in hypoxia compared to normoxia (Kon et al. 2015) Training response 5-week exercise training in hypoxia at the same relative and absolute intensity as normoxia did not alter growth hormone response to exhaustive exercise that was performed at the same absolute workload before training (Engfred et al. 1994) |
|
| Cortisol | Exercise in NOR |
Acute response ~ 1.3- and ~ 1.5-fold increase in plasma cortisol concentration following aerobic (50 min of treadmill running at 4 mmol/L) and anaerobic (all-out run to exhaustion at 20 km/h) exercise, respectively (Kindermann et al. 1982) Greater cortisol response with increasing exercising intensity (> ~ 60% ), whereas lower exercise intensity elicits minimal changes compared to baseline (Hill et al. 2008) Cortisol response to exercise, when expressed as absolute workload, is lower in trained individuals compared to untrained and moderately trained individuals (Luger et al. 1987) Training response Cortisol response to exhaustive exercise (at the same absolute intensity as pre-training) remains largely unchanged following 7 weeks of exercise training (Hartley et al. 1972b) |
| Exercise in NOR vs. Exercise in HYP |
Acute response Elevated acute cortisol response in hypoxia compared to normoxia during exercise performed at similar absolute intensity (Sutton 1977), whereas similar response during exercise performed at the same relative intensity (Bouissou et al. 1986) Training response Increase in cortisol in response to exhaustive exercise at 85% following 5 weeks of exercise training in hypoxia at the same relative and absolute intensity to normoxia (Engfred et al. 1994) |
|
| Incretins (postprandial responses) | ||
| GLP-1 | Exercise in NOR |
Acute response Prior exercise (60 min of treadmill walking at 55–60% ) did not alter GLP-1 response to a mixed meal (Heden et al. 2013) or oral fat tolerance test (Dekker et al. 2010) measured 12 and 16 h post-exercise Training response Short-term exercise training (7 consecutive days) decreased fasting plasma GLP-1 concentration and increased acute GLP-1 response to glucose in obese individuals with non-alcoholic fatty liver disease (Kullman et al. 2016) |
| Exercise in NOR vs. Exercise in HYP |
Acute response Resting and postprandial GLP-1 response were not altered by hypoxia exposure (FiO2 ~ 0.15) (Morishima and Goto 2016) GLP-1 response following continuous moderate or high-intensity interval exercise were identical between hypoxia and normoxia (Bailey et al. 2015) Postprandial GLP-1 response was not altered following exercise in hypoxia (compared to normoxia) (Bailey et al. 2015) Training response Postprandial GLP-1 response following exercise training was elevated following 4-week training (3d/wk) in normoxia, but unchanged in hypoxia (Morishima et al. 2014) |
|
| GIP | Exercise in NOR |
Acute response GIP response remains relatively unchanged during 120 min of running at 60% (O’Connor et al. 2006), and 3-h cycling at 40% (Hilsted et al. 1980) Single bout of running 120 min at 60% (O’Connor et al. 2006) or exercise at 75% (Blom et al. 1985) until exhaustion attenuates increase in postprandial plasma GIP concentration during the immediate postexercise recovery phase Training response Effect of exercise training on postprandial GIP response remains unclear, partly due to factors including dietary restriction (Kelly et al. 2009; Kahle et al. 1986) and weight loss (Kelly et al. 2009; Solomon et al. 2010) |
| Exercise in NOR vs. Exercise in HYP | NA | |
AUC area under curve, Ex exercise, GLP-1 glucagon-like peptide-1, GIP gastric inhibitory polypeptide, HYP hypoxia, NA not applicable, NOR normoxia, T2DM type 2 diabetes mellitus
The duration of increased insulin sensitivity following exercise may persist from 3 to 48 h and is dependent on the dietary status (Cartee et al. 1989; Gulve et al. 1990; Richter et al. 1982). In rats fed a carbohydrate-free diet post-exercise, the exercise-stimulated increases in glucose uptake (7.8-fold above non-exercised levels) were still largely present 18 h later (3.5-fold increase above non-exercised levels). However, in rats fed a 60% carbohydrate diet, the exercise-stimulated glucose uptake was no longer present at the same time-point (1.1-fold increase above non-exercise levels) (Young et al. 1983). These findings support the observations that insulin-mediated glucose uptake and GLUT4 translocation in the skeletal muscle of rats, are directly associated with muscle glycogen concentration (Host et al. 1998; Derave et al. 2000; Derave et al. 1999; Kawanaka et al. 1999; Kawanaka et al. 2000).
Glucose transport measurement in humans
The in vivo measurement of glucose transport in humans is challenging and relies on tracer-labelled glucose (such as [13C]glucose, [2H]glucose) (Zinker et al. 1993a, b). The tracer-labelled glucose is coupled with either tissue biopsies (Roussel et al. 1998), measures of arterio-venous glucose difference (Wahren et al. 1971), or imaging techniques such as dynamic positron emission tomography (Bertoldo et al. 2005) and magnetic resonance spectroscopy (Roden and Shulman 1999) which allow assessment of tissue glucose uptake rates. Using arterio-venous glucose differences, skeletal muscle glucose transport was shown to increase with intensity as well as duration during knee extensions in humans (Kjaer et al. 1990; Holloszy and Narahara 1965; Helge et al. 2003). For instance, glucose uptake rates of 0.05, 0.3, 0.75 and 1.07 mmol/min/thigh were reported at rest, 25%, 65% and 85% of maximal work capacity (Helge et al. 2003). The greater recruitment of total muscle fibres, and in particular fast-twitch fibres, likely underpins the higher rates of glucose uptake at higher work capacities (Katz et al. 1986; Ploug et al. 1984).
The role of exercise intensity on glucose regulation
Increasing exercise intensity results in greater recruitment of muscle fibres as well as an increased reliance on plasma glucose and muscle glycogen (Coggan 1991; Jeukendrup 2011; Sahlin 1992; Vollestad and Blom 1985) for energy. Furthermore, increased utilization and reduction of muscle glycogen are associated with improved glucose uptake in the skeletal muscle of rats (Host et al. 1998; Derave et al. 2000; Kawanaka et al. 2000) and glucose tolerance in humans (Kang et al. 1996). Hence, it is tenable to expect that high-intensity exercise enhances glucose tolerance. However, when matched for total work, low-moderate intensity exercise (40–50% ) resulted in a similar GLUT-4 recruitment to high intensity (80% ) exercise in healthy untrained individuals (Kraniou et al. 2006). Similarly, there were no differences in plasma glucose response to an oral glucose tolerance test (OGTT) performed 1 h and 24 h after a moderate- or high-intensity exercise bout in healthy, middle-aged individuals (Bonen et al. 1998). In contrast, in prediabetic adults the plasma glucose and insulin response to an OGTT were improved 1 h post an isocaloric moderate- or high-intensity exercise bout, although the high-intensity exercise improved insulin sensitivity to a greater degree than the moderate-intensity exercise bout (Rynders et al. 2014).
High-intensity interval exercise/training (HIIE/HIIT) protocols are defined as brief periods of near-maximal efforts (≤ 4 min) that elicit ≥ 80% of maximal heart rate or interspersed with a short period of rest or lower-intensity exercise (Callahan et al. 2021; MacInnis and Gibala 2017). HIIE results in significant muscle glycogen depletion (Hermansen and Vaage 1977), which would be expected to elicit increased skeletal muscle glucose uptake. Post-meal glucose AUC and ‘time in hyperglycaemia’ during the 24 h following HIIE were reduced in overweight/obese individuals with T2DM (Gillen et al. 2012; Little et al. 2011). In contrast, a continuous moderate-intensity (45 min at 75% ) cycling has been shown to elicit greater improvements in insulin sensitivity 24 h post-exercise when compared to an interval-based exercise (five 30-s cycling bout performed at 125% interspersed with 4–5 min of rest or 50 W cycling) in overweight/obese individuals (Brestoff et al. 2009). This was due largely to reduced fasting plasma insulin concentration (rather than changes in glucose concentration) and insulin AUC during the OGTT (Brestoff et al. 2009), indicating an improvement in muscle insulin action.
Changes in systemic plasma glucose post-exercise: a balance between the rate of glucose appearance and disappearance
While acute exercise may increase the uptake of glucose, it should be noted that systemic plasma glucose concentration ultimately reflects the balance between the rate of glucose appearance (Ra) or entry, and the rate of glucose disappearance (Rd) or exit from the circulation. Glucose Ra in the fasted state is principally governed by total hepatic glucose release. Glucose Ra in the postprandial period is governed by total hepatic glucose release and the glucose remaining in the portal vein (dietary glucose absorbed from the small intestine) following hepatic first-pass. The Rd is the sum of glucose uptake by all cells of the body. In this instance, the balance between Ra and Rd becomes more complex during exercise since both Ra and Rd are affected. Specifically, acute exercise may (i) increase—in orders of magnitude—glucose uptake into the exercised/contracted muscles (Sylow et al. 2017); (ii) decrease in insulin response and glucose uptake in tissues other than the contracting musculature (i.e., reduced Rd in inactive tissues); (iii) increase net hepatic glucose release which remains elevated for approximately 30 min after exercise; and (iv) decrease the rate of glucose absorption from the small intestine (Knudsen et al. 2014). Furthermore, during high-intensity exercise, a host of glucoregulatory hormones (described below), rather than insulin per se, become the key regulators of glucose regulation.
Glucoregulatory effects of key hormones associated with exercise: epinephrine, glucagon, growth hormone and cortisol
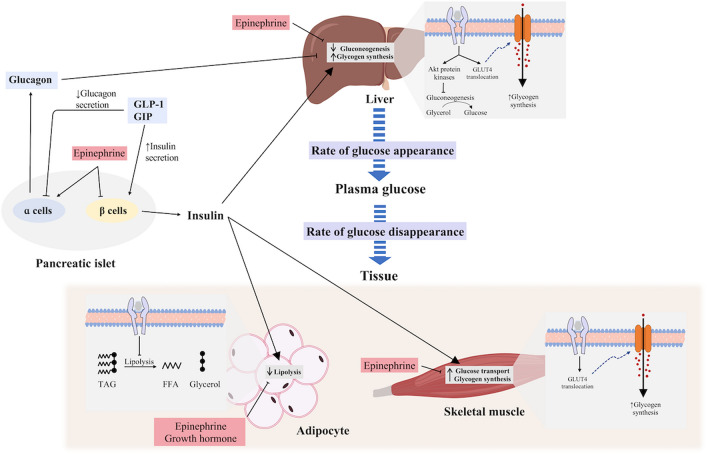
Increased hepatic glucose output is essential to sustaining prolonged exercise capacity and preventing hypoglycaemia (Trefts et al. 2015; Wasserman 2009), with the increased glucose output continuing for up to 30–60 min into the recovery period (Knudsen et al. 2014). This increased hepatic glucose output coincides with increases in glucagon, catecholamine and cortisol concentrations and decreases in insulin concentration (Wasserman 2009; Kindermann et al. 1982). Of these hormones, single infusions of epinephrine and glucagon during resting conditions are associated with increases in plasma glucose, with glucagon increasing hepatic glucose output to the greatest level, while cortisol appears to have negligible effect in isolation (Eigler et al. 1979; Kjaer et al. 1990; Kjaer 1998) (Fig. 2). Glucagon, in concert with the associated decrease in insulin concentration, likely plays the greatest role in increasing hepatic glucose release during exercise (Hirsch et al. 1991; Wasserman et al. 1989). In contrast, the influences of catecholamines, and specifically epinephrine on increased hepatic glucose output, appear minimal (Wasserman et al. 1990; Coker et al. 2000; Moates et al. 1988). Several hormones appear to act synergistically on hepatic glucose production, with the addition of epinephrine and glucagon being greater than either alone, and the addition of cortisol prolonging the action of these hormones on the liver (Eigler et al. 1979).
Fig. 2.
Glucoregulatory effects of key hormones controlling plasma glucose concentration. Effect of insulin on the liver, adipose tissue and skeletal muscle pathways are demonstrated, along with counter-regulatory effects of epinephrine and norepinephrine, growth hormone and glucagon
While the effects of glucagon may be largely limited to the liver, epinephrine can play important roles in Rd (Sherwin et al. 1980). In perfused rat muscles, epinephrine (25 nM) significantly increased GLUT-4 content and glucose transport (22–48%), with the greatest increase occurring in oxidative muscle (red gastrocnemius and soleus) (Han and Bonen 1998). However, infusion of epinephrine (25 nM) has been shown to partially inhibit the insulin-stimulated glucose transport when compared to insulin infusion (20 mU/ml) alone, despite the persistent increase in GLUT-4 plasma membrane content (Han and Bonen 1998). Subsequent work found that the inhibition of glucose transport by epinephrine (24 nM) persists at moderate (50 mU/ml), but not at a high physiological concentration of insulin (100 mU/ml), even when the concentration of epinephrine was increased to 500 nM (Hunt and Ivy 2002). The underlying mechanisms associated with the downregulation of insulin-stimulated glucose transport with epinephrine is likely associated with a decreased activity of insulin receptor substrate-1 associated phosphatidylinositol 3-kinase (PI3K) (Hunt and Ivy 2002).
Circulating levels of growth hormone (GH) are increased with exercise (Hirsch et al. 1991), with the magnitude of change being dependent on exercise duration and intensity (Hartley et al. 1972a; Kindermann et al. 1982). The role of GH in glucose regulation is complex and appears to occur in two phases with an initial, transient, insulin-like response followed by an anti-insulin response in the hours thereafter (Rizza et al. 1982; Smith et al. 1997; Svensson et al. 2002). The anti-insulin effects of GH include the promotion of gluconeogenesis and hepatic glucose release (Schwarz et al. 2002; Svensson et al. 2002), although these effects seem minimal (Kjaer 1998). GH was shown to reduce glucose uptake in an in vivo adipocyte model (Kilgour et al. 1995) and this was due to reduced expression of both GLUT1 and GLUT4 in the plasma membrane (Smith et al. 1997; Takano et al. 2001). Muscle glucose uptake also decreases (Jessen et al. 2005) and could be due to the direct effect of GH on insulin action, or an indirect consequence of increased free fatty acid (FFA) flux (Møller and Jørgensen 2009). Increases in growth hormone, as well as epinephrine, also increase lipolysis, and consequently increased FFA concentrations (Schwarz et al. 2002; Kindermann et al. 1982; Rizza et al. 1982). The increase in circulating FFA interferes with glucose uptake via both insulin-dependent (Stefan et al. 2001; Segerlantz et al. 2003) and independent effects, which can act synergistically (Boden et al. 1994) by decreasing cellular glucose utilization (Ferrannini et al. 1983; Randle et al. 1963, 1964).
Given the complex interactions of systemic glucoregulatory factors governing glucose Ra and Rd in response to exercise, it may come as no surprise that there are discrepancies in studies assessing postprandial glucose tolerance in response to acute exercise. Some studies found glucose tolerance to remain unchanged (Roberts et al. 2013; Venables et al. 2007), while others reported glucose tolerance to be either increased (Mikines et al. 1988; Oberlin et al. 2014) or decreased (King et al. 1995; Rose et al. 2001; O'Connor et al. 2006). Whilst longer-term improvement in glycaemic control with exercise training is firmly established (Umpierre et al. 2013; Burgomaster et al. 2007; Mikus et al. 2012), there is also variability in the magnitude of the response (Boulé et al. 2005).
Mediator to simulate or enhance the effects of exercise on glucose regulation
While duration (total training duration; acute session duration) and intensity are important considerations, findings from longer-term training studies (6 months) suggest that total work or energy expenditure is likely more important than either intensity or duration alone (Houmard et al. 2004). Increases in exercise “dose” (i.e., combination of exercise volume and intensity), however, can be difficult to achieve in some individuals due to (i) reduced adherence rates (Wadden et al. 1998); and (ii) increased risk for injuries (Girard et al. 2017; Fernández Menéndez et al. 2018). Not surprisingly, efforts have been made to identify the potential stimulus that may bypass the defects in insulin signalling in skeletal muscle. Alternative strategies, which may potentiate the effects of exercise at a reduced dose, have, therefore, been explored. One such strategy is exercise training in hypoxia (i.e., decreased oxygen concentration at the tissue level). Specifically, when exercise is performed in hypoxia at the same absolute intensity as in normoxia, the relative intensity (in relation to ) of exercise is higher compared to exercise in normoxia; which subsequently contributes to increased muscle glycogen utilization/depletion (Wadley et al. 2006; Parolin et al. 2000) and glucose Rd (Cooper et al. 1986). This is of particular relevance since skeletal muscle glycogen concentration is a potent regulator of insulin sensitivity and therefore, systemic glucose homeostasis (Shearer and Graham 2004). Furthermore, hypoxia may also stimulate the activation of signalling molecules (De Groote and Deldicque 2021), increasing glucose uptake similar to high-intensity exercise. As such, hypoxic exercise may represent an alternate strategy to enhance glycaemic control (via enhanced glucose metabolism). Accordingly, the following sections attempt to synthesize the effects of systemic hypoxia, with and without exercise on glucose homeostasis.
Stimulation of glucose uptake by hypoxia
Although insulin and muscle contraction are the primary means to facilitate GLUT-4 translocation and increase glucose uptake, additional physiological stimuli including hypoxia can also increase glucose uptake (Fig. 1). For example, Cartee et al. (1991) showed that 3-methylglucose uptake in rat epitrochlearis muscle incubated under anoxic conditions (95% N2–5% CO2) for 40 min were approximately sixfold higher relative to baseline glucose uptake in oxygenated muscle (Cartee et al. 1991). Similarly, rectus abdominis muscle obtained from lean, obese and individuals with T2DM resulted in a twofold increase in glucose uptake relative to baseline when incubated under anoxic conditions (95% N2–5% CO2) (Azevedo et al. 1995). Several mechanisms may mediate GLUT4 trafficking in response to hypoxia, including an increase in intracellular Ca2+ concentration and activation of the downstream Ca2+/calmodulin-dependent protein kinase (CaMK); increase in reactive oxygen species; increased phosphorylation of AMPK via an increased AMP:ATP ratio (Mackenzie and Watt 2016; Sylow et al. 2021). These findings indicate that hypoxia stimulates glucose uptake via similar mechanisms to contraction-simulated pathways. Additionally, hypoxia-inducible factors (HIFs) are activated upon cellular exposure to hypoxia (Semenza 2011, 2012). HIF1ɑ has been implicated in the regulation of AKT activity (in human HepG2 cells) (Dongiovanni et al. 2008) and AMPK-mediated AS160 phosphorylation (Sakagami et al. 2014). These findings showing that hypoxia (similar to exercise) may stimulate glucose transport via pathways distinct from insulin imply that hypoxia may be a relevant strategy to improve glucose tolerance in individuals with insulin resistance.
Hypoxia exposure under resting conditions on glucose tolerance and insulin sensitivity
Although in vitro experiments have shown that hypoxia stimulates glucose transport via activation of GLUT-4 translocation in the myocytes, it remains unclear if similar mechanistic pathways regulating glucose uptake and GLUT-4 translocation will be activated in vivo by hypoxia in humans. In one of the first studies to examine the effects of acute hypoxia (for 4 h) on intramuscular insulin signalling following a high glycaemic meal in healthy humans, D'Hulst et al. (2015) showed that (1) GLUT-4 in the sarcolemmal membrane was 30% higher in hypoxia compared to normoxia; (2) Rac1 and PAK1 were activated in normoxia but not hypoxia; and (3) hypoxia resulted in lower glucose response. Together, this result suggested that hypoxia may reduce glucose response to a high glycaemic meal by increasing the abundance of GLUT-4 at the sarcolemmal through insulin-independent pathways (D'Hulst et al. 2015).
However, while in vivo and in vitro studies have shown that hypoxia may elicit an increase in muscle glucose uptake, studies examining the effects of hypoxia on systemic glucose regulation have been inconsistent (Braun et al. 2001; Oltmanns et al. 2004; Mackenzie et al. 2011; D'Hulst et al. 2015). For instance, relative to normoxia, reduced glucose tolerance was observed when healthy men were exposed to hypoxia (by decreasing arterial oxygen saturation to 75%) for 30 min. Similarly, reduced insulin sensitivity has been reported in healthy men (Larsen et al. 1997) and women (Braun et al. 2001) following 48 h and 16 h of passive hypobaric hypoxic exposure (at 4559 m and 4300 m), respectively. In contrast, improved glucose tolerance has been observed following 60 min of passive hypoxia (FiO2 ~ 0.14), which was associated with a decrease in insulin AUC relative to passive normoxia. While some methodological differences exist between studies, these conflicting findings highlight the complex nature of the processes regulating the systemic glucose Ra and Rd.
In this instance, evidence indicates that acute hypoxic exposure increases the hormonal responses of several glucoregulatory hormones that may augment Ra and Rd, especially in individuals who are unacclimatized to a hypoxic environment (Moncloa et al. 1968). Most prominent among these glucoregulatory hormones is epinephrine; several studies have reported significant increases in epinephrine during hypoxic exposure which coincide with the development of insulin resistance (Larsen et al. 1997; Braun et al. 2001). In contrast, changes in glucagon, growth hormone and cortisol do not seem to be influenced by hypoxia (Larsen et al. 1997). However, studies have also observed an increase in cortisol (Woods et al. 2012; Moncloa et al. 1968); although cortisol concentration (being a stress hormone) may also be influenced by physiological stress when individuals ascent to high altitudes. As such, the dissociation between studies showing increased muscle glucose uptake and decreased systemic glucose tolerance following hypoxia could in part, be explained by changes in glucoregulatory hormones.
Insulin and glucose response following acute exercise bout in hypoxia
Exercising in hypoxia (compared to normoxia) reduces oxygen availability and therefore induces a proportional shift in metabolic pathway flux (Davison et al. 2018). To compensate for the incomplete oxidation of glucose and reduced ATP-generating efficiency, exercise in hypoxia increases reliance on glucose and glycogen utilization (Larsen et al. 1997; Péronnet et al. 2006; Parolin et al. 2000). Although acute exercise in hypoxia induces a shift towards glucose and glycogen utilization, the subsequent effects on glycaemic control remain unclear (De Groote et al. 2021; Mackenzie et al. 2011, 2012a). Mackenzie et al. (2011) reported that an acute cycling bout (at an absolute intensity of 90% lactate threshold determined in normoxia) for 60 min in normobaric hypoxia (FiO2 ~ 0.146) enhanced insulin sensitivity in T2DM individuals to a greater extent when compared to exercise in normoxia (Mackenzie et al. 2011). In a subsequent study, improved insulin sensitivity was observed immediately following exercise in normoxia and hypoxia, although the effects appeared to be sustained 24 h post-exercise only after continuous cycling in hypoxia (Mackenzie et al. 2012b). In contrast, De Groote et al. (2021) showed that 60 min of continuous cycling (at a relative intensity based on heart rate corresponding to 55% ) in normobaric hypoxia (FiO2 ~ 0.14) increased (worsened) glucose AUC relative to baseline during an OGTT in both prediabetic and healthy individuals (De Groote et al. 2021). Despite exercising at the same relative intensity, cortisol levels during exercise in hypoxia were higher than in normoxia. Key regulators of the glucose transport pathways (e.g. AMPK, TBC1D1) were not reduced immediately after exercise by hypoxia (De Groote et al. 2021). These results suggest that hypoxia does not impair muscle insulin sensitivity locally, and the acute decrease in systemic glucose tolerance after exercise in hypoxia may be attributed to changes in the glucoregulatory hormones.
Given the systemic effects of hypoxia, it is possible that the interplay of glucoregulatory hormones, including glucagon, catecholamines, GH and incretin hormones could be disrupted, thereby altering glucose homeostasis. The extent of these increases in glucoregulatory hormones, however, depends on the severity and duration of hypoxia as well as the intensity of exercise in hypoxia relative to normoxia (absolute vs. relative intensity). In particular, exercise performed in hypoxia at the same absolute intensity as in normoxia has been shown to exaggerate the increase in epinephrine (Cooper et al. 1986). In contrast, submaximal exercise in hypoxia does not seem to alter the responses of catecholamines, glucagon and insulin compared to exercise in normoxia when performed at a similar relative intensity (Bouissou et al. 1986; Bailey et al. 2015; Engfred et al. 1994). Altogether, the identification of a single candidate hormone (e.g. epinephrine) that is altered by the addition of hypoxia to exercise, has so far been unable to account for the differences in acute systemic glucose tolerance (Larsen et al. 1997; Braun et al. 2001). Rather, it is more likely that several glucoregulatory hormones interact to induce the changes in systemic glucose tolerance (Eigler et al. 1979).
Exercise in hypoxia amplifies many intracellular processes associated with glucose uptake [e.g. GLUT-4 translocation, increased utilization of glycogen and glucose-derived metabolites Cooper et al. 1986; Cartee et al. 1991)]. As such, the glucose Rd, at least into the contracting musculature, is expected to be increased. However, the localized increase in glucose uptake may not be reflected in the systemic glucose concentrations since a concomitant increase in the glucose Ra via hepatic glucose production and release are expected. Indeed, a nearly two-fold increase in the glucose Ra has been observed in hypoxia versus normoxia (Cooper et al. 1986). In addition to the effects of glucoregulatory hormones, the glucose Ra may also be influenced by circulating metabolites due to a shift in metabolic flux. Specifically, under hypoxic conditions, plasma triglyceride levels remain unchanged (De Groote et al. 2021) but increases when exercise is performed at the same relative intensity in normoxia. Additionally, the conversion of pyruvate to lactate is increased (Lundby and Van Hall 2002; Wadley et al. 2006), indicating an increase in glycolytic flux. Lactate, being a gluconeogenic substrate, constitutes a prime carbon source for the repletion of blood glucose and consequently muscle glycogen via the Cori cycle, while a proportion of accumulated lactate is also oxidized (Fournier et al. 2002, 2004). Further research is required to examine the contribution of lactate to glucose Ra following exercise in hypoxia. While the regulation of these metabolic pathways may reflect a transient acute response in maintaining metabolic balance to a perturbation (induced by hypoxia), longer-term training studies in hypoxia are needed to provide a greater understanding of any underlying metabolic changes.
Effects of exercise training in hypoxia on insulin sensitivity
Findings from studies assessing the effects of exercise training in hypoxia on insulin sensitivity have been inconsistent (Haufe et al. 2008; De Groote et al. 2018; Wiesner et al. 2010; Klug et al. 2018). Haufe et al. (2008) showed that four weeks of training (60 min running, 3 days/week, at 3 mmol/L lactate value) in normobaric hypoxia (FiO2 0.15) resulted in improvements in insulin sensitivity (assessed via HOMA index) and insulin AUC in healthy men. Additionally, De Groote et al. (2018) showed that six weeks of training (aerobic and resistance exercise, 3 days/week) in normobaric hypoxia (FiO2 0.15), but not normoxia, reduced insulin and glucose AUC during an OGTT in obese adolescents. Importantly, whilst the absolute aerobic workload (power output during cycling multiplied by the duration of exercise) was lower in hypoxia, both normoxia and hypoxia training-induced similar improvements in insulin sensitivity (assessed via HOMA index). In contrast, six weeks of running (60 min running, 3 days/week, at 60% of ) did not alter glucose and insulin AUC in normobaric hypoxia (FiO2 0.15) and normoxia in men with metabolic syndrome (Klug et al. 2018). Similarly, eight weeks of training (45 min cycling, 3 days/week, at 75% of maximal HR) in normobaric hypoxia (individually adjusted to SpO2 of 80%) and normoxia did not alter insulin sensitivity (assessed via HOMA index) in sedentary, overweight or obese individuals (Chacaroun et al. 2020); although was significantly increased only following hypoxic training (~ + 10% vs ~ + 1%). However, it should be highlighted that individuals naïve to exercise training may elicit exaggerated responses to novel stimuli (hypoxia and/or exercise). Whether the larger improvements in insulin sensitivity following short-term (i.e., ≤ 6 weeks) hypoxia training in some studies was due to faster upregulation of selected metabolic markers, or whether the difference would have remained with longer training durations, remains to be determined.
Of note, studies reporting a comparable effect of exercise training on glucose tolerance in hypoxia and normoxia have employed similar relative exercise intensities (De Groote et al. 2018; Haufe et al. 2008). Accordingly, training in hypoxia is performed at a lower absolute intensity than in normoxia, which could be beneficial for individuals who are unable to tolerate high loads imposed on their locomotor system, including comorbidities such as knee osteoarthritis (Girard et al. 2017). However, given that the intensity (absolute vs. relative) of exercise largely influences skeletal muscle AMPK signalling (Wadley et al. 2006), muscle glycogen use (Wadley et al. 2006), as well as hormonal responses (catecholamines (Kjaer et al. 1988)), further investigation is required to determine the optimal method of implementing exercise intensity in hypoxia on insulin sensitivity.
A key concern associated with hypoxia training is the associated inflammatory response (Hosogai et al. 2007), given the widely established link between pro-inflammatory markers and insulin resistance (Petersen and Shulman 2018). An alteration in oxygen tension in the cells results in the activation of HIF, a key regulator of inflammation and immunity; whether the activation of HIF is pro- or anti-inflammatory in vivo is, however, dependent on the internal environment (Scholz and Taylor 2013). Additionally, it has also been suggested that hypoxia may trigger endoplasmic reticulum stress (Hosogai et al. 2007). That said, acute inflammatory responses are an important physiologic response that functions to restore tissue homeostasis and are required for beneficial adaptations (Medzhitov 2008). Accordingly, it is chronic or excessive inflammatory responses, that may impair metabolic regulation and that is associated with insulin resistance (Petersen and Shulman 2018). Further research will need to determine the role of hypoxic training in regulating the pro- and anti-inflammatory responses and the subsequent management of glycaemia in individuals with insulin resistance or T2DM.
Hypoxic training—an integrated physiology approach
The hypothesis that hypoxic training may potentiate the effect of exercise on glucose tolerance is based on the findings that hypoxia activates glucose transport via pathways similar to muscle contraction (Kang et al. 1996), as well as the characterization of HIF1ɑ linking transcriptional responses to metabolic adaptation (Kierans and Taylor 2021). However, such a framework does not cover the conflicting effects of exercise in hypoxia on glycaemic control (Mackenzie et al. 2011; De Groote et al. 2021). As such, the role of the multiple, integrated systemic responses which regulate glucose tolerance need to be considered. Specifically, the balance between the net tissue glucose uptake and endogenous glucose production and release, are systemically regulated via the central nervous system (Güemes and Georgiou 2018), the endocrine system (Röder et al. 2016) and various inflammatory markers (Bruce and Dyck 2004); these factors sit on the backdrop of the individual (e.g. level of insulin sensitivity), the exercise variables (type, duration, intensity and frequency) as well as the hypoxic exposure (duration, severity of hypoxia, method of implementation [normobaric vs. hypobaric; intermittent vs. continuous]).
A clinical phenotype of insulin resistance and T2DM is a metabolic dysregulation of lipids, amino acids, and glucose. As such, analysis of the quantitative complement of metabolites in a biological system (i.e., metabolome) (Dunn et al. 2011) is a useful method to further the understanding of insulin resistance and T2DM. Indeed, metabolomics (i.e., the comprehensive study of all relevant metabolites in a biological system) has been extensively used in the study of T2DM (Milburn and Lawton 2013; Suhre 2014).
Molecular phenotyping has emerged as an essential tool in exploring, characterizing, and understanding the dynamic interactions between our genes and environment (diet, lifestyle) and their phenotypic expression across diverse human populations. While a wide array of biofluids, tissues and cells can be used in metabolic phenotyping studies, the collection of a blood sample is most frequently performed since it is minimally invasive. Analytical platforms for deep phenotyping of biofluids such as plasma, urine and cerebrospinal fluid based on nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) can generate metabolic “fingerprints” that contain latent information relating to physiological or pathological status, informing on disease mechanisms, diagnosis and assessment of biological function (Belhaj et al. 2021; Dunn et al. 2011). As such, accurate measurement of hundreds of metabolites from a small amount of blood or urine sample (typically < 50 uL) can facilitate the identification of metabolites associated with T2DM, with metabolic profiling research already looking into branched-chain amino acids (Würtz et al. 2012; Tai et al. 2010), diacylglycerol (Wittenbecher et al. 2022), acylcarnitines (Sun et al. 2016) and ceramides (Haus et al. 2009). Accordingly, this has led to the discovery of biomarkers that may predict the onset and severity of T2DM. More recently, there has been an increased interest in the use of metabolic profiling approaches to identify molecular transducers mediating the metabolic benefits of exercise (Li et al. 2022). Importantly, the identification of mechanistic biomarkers could be used to determine the efficacy of hypoxic training stimulus. For instance, intermediate biomarkers could be used to predict the effectiveness of shorter-term training studies (6 weeks) instead of commonly used clinical endpoints (that determine the effectiveness of hypoxia training) such as , HbA1c and glucose tolerance which may require a longer time frame to observe significant changes.
Another possible application of metabolomics is the analysis of metabolites associated with glycogen metabolism. As highlighted in this review, the depletion of glycogen following high-intensity exercise is an important factor promoting glucose transport and improving insulin sensitivity (Shearer and Graham 2004; Jensen et al. 2011). Within this context, the discovery of multiple proteins containing glycogen binding domains (regions within proteins that allow interactions with glycogen) has added increased complexity to the structure and processes regulating glycogen depletion and synthesis (McBride and Hardie 2009; Shearer and Graham 2004; Philp et al. 2012). The application of metabolomics and proteomics will likely progress our understanding of the processes regulating glycogen depletion and synthesis, including the apparent preservation of a minimum glycogen level in the skeletal muscle, and the link with insulin sensitivity.
A key challenge in hypoxic training is the inter-individual variation in response to hypoxia (Soo et al. 2020; Lawler et al. 2019). There is also heterogeneity in the metabolic profile (typically measured under fasting condition) of individuals with prediabetes (Chen et al. 2019). This is further complicated by the amplification of inter-individual variations in the metabolic profile when healthy humans are subjected to physiological stress (e.g. exercise, cold) (Krug et al. 2012). Accordingly, it is tenable to expect inter-individual variations in physiological responses when individuals with prediabetes undergo hypoxic training. In this instance, the use of metabolic profiling (using biomarkers that are known to be associated with T2DM) may help to discriminate individuals with prediabetes who are at higher risk of developing T2DM, that may require a more intensified training intervention (Stefan et al. 2015; Fritsche et al. 2021; Chen et al. 2019). Additionally, the hypoxia-induced metabolomic response can be measured to evaluate changes in metabolic profile due to the hypoxic training which may provide an early indicator of possible therapeutic benefits or harm of hypoxia.
Conclusion
Systemic glucose regulation is intricately linked to cellular glucose transport, which is mediated by the translocation of glucose transport (i.e., GLUT-4) in response to a stimulus. The increase in glucose transport is not only influenced by insulin but can also be stimulated by muscle contraction. Importantly, glucose transport appears to be influenced by the exercise intensity. Unsurprisingly, high-intensity exercise is now widely accepted and recommended for individuals with T2DM (Mendes et al. 2016). While glucose uptake increases in the exercised skeletal muscle tissue, there remains a discrepancy between studies regarding the effects of acute exercise on systemic postprandial glucose tolerance. This may in part, be explained by different responses in glucoregulatory hormones (e.g. epinephrine, growth hormone, cortisol), which are in turn influenced by the intensity and duration of exercise. The increased rates of glucose uptake (disappearance) into the skeletal muscle with exercise, may thus have been negated by an increased rate of glucose appearance resulting in either unchanged or worsened systemic glucose concentration. Accordingly, the role of the multiple, integrated systemic responses which regulate glucose tolerance needs careful consideration to progress current knowledge.
The finding that contraction-induced increase in glucose uptake is not limited to exercise but can also be stimulated by hypoxia (Cartee et al. 1991) suggest that the exercise stimulus could be potentiated by hypoxia. The relative intensity of exercise (in reference to ) is substantially higher in hypoxia compared to normoxia at the same absolute workload. In this instance, current findings seem to indicate that exercise in hypoxia performed at the same relative intensity (i.e., lower absolute intensity) as in normoxia induces comparable effects on glucose tolerance. Hypoxic training may thus be an exercise intervention for individuals who are unable to tolerate high loads. However, further research is required to determine the optimal intensity during hypoxic training. Inter-individual variability in response to hypoxia may influence the outcomes of hypoxia training. The application of metabolomics presents as a promising approach to optimize hypoxic training by enabling the quantification of the individual metabolic responses to the training intervention and hypoxic stimuli.
Abbreviations
- AMP
Adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ATP
Adenosine triphosphate
- AUC
Area under curve
- CaMK
Ca2+/calmodulin-dependent protein kinase
- FFA
Free fatty acid
- FiO2
Fraction of inspired oxygen
- GAP
GTPase-activating protein
- GH
Growth hormone
- GLUT
Glucose transporters
- HbA1c
Glycated haemoglobin
- HIFs
Hypoxia-inducible factors
- HIIE
High-intensity interval exercise
- HIIT
High-intensity interval training
- HOMA
Homeostasis model assessment
- HOMA-IR
Homeostasis model assessment of insulin resistance
- HR
Heart rate
- kDa
Kilodalton
- PI3K
Phosphatidylinositol 3-kinase
- Ra
Rate of glucose appearance
- Rd
Rate of glucose disappearance
- SpO2
Arterial oxygen saturation
- T2DM
Type 2 diabetes mellitus
Maximal oxygen uptake
Peak oxygen uptake
Author contributions
JS, AR and TF conceived the idea for the article. JS, AR and TF drafted the manuscript. All authors critically edited, reviewed and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
The data generated in the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/27/2023
Missing Open Access funding information has been added in the Funding Note
References
- Azevedo JL, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes. 1995;44(6):695–698. doi: 10.2337/diab.44.6.695. [DOI] [PubMed] [Google Scholar]
- Babraj J, Vollaard N, Keast C, Guppy F, Cottrell G, Timmons J. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9(1):3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DP, Smith LR, Chrismas BC, Taylor L, Stensel DJ, Deighton K, Douglas JA, Kerr CJ. Appetite and gut hormone responses to moderate-intensity continuous exercise versus high-intensity interval exercise, in normoxic and hypoxic conditions. Appetite. 2015;89:237–245. doi: 10.1016/j.appet.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99(3):1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- Belhaj MR, Lawler NG, Hoffman NJ. Metabolomics and lipidomics: expanding the molecular landscape of exercise biology. Metabolites. 2021;11(3):151. doi: 10.3390/metabo11030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldo A, Price J, Mathis C, Mason S, Holt D, Kelley C, Cobelli C, Kelley DE. Quantitative assessment of glucose transport in human skeletal muscle: dynamic positron emission tomography imaging of [O-methyl-11C]3-O-methyl-d-glucose. J Clin Endocrinol Metab. 2005;90(3):1752–1759. doi: 10.1210/jc.2004-1092. [DOI] [PubMed] [Google Scholar]
- Blom P, Høstmark A, Flaten O, Hermansen L. Modification by exercise of the plasma gastric inhibitory polypeptide response to glucose ingestion in young men. Acta Physiol Scand. 1985;123(3):367–368. doi: 10.1111/j.1748-1716.1985.tb07602.x. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Investig. 1994;93(6):2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C, Thuillez P, Ravussin E, Vasquez B, Narimiga M, Azhar S. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Investig. 1983;72(5):1605–1610. doi: 10.1172/JCI111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Ball-Burnett M, Russel C. Glucose tolerance is improved after low-and high-intensity exercise in middle-age men and women. Can J Appl Physiol. 1998;23(6):583–593. doi: 10.1139/h98-033. [DOI] [PubMed] [Google Scholar]
- Bouissou P, Peronnet F, Brisson G, Helie R, Ledoux M. Metabolic and endocrine responses to graded exercise under acute hypoxia. Eur J Appl Physiol. 1986;55(3):290–294. doi: 10.1007/BF02343801. [DOI] [PubMed] [Google Scholar]
- Boulé N, Kenny G, Haddad E, Wells G, Sigal R. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003;46(8):1071–1081. doi: 10.1007/s00125-003-1160-2. [DOI] [PubMed] [Google Scholar]
- Boulé NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28(1):108–114. doi: 10.2337/diacare.28.1.108. [DOI] [PubMed] [Google Scholar]
- Braun B, Rock PB, Zamudio S, Wolfel GE, Mazzeo RS, Muza SR, Fulco CS, Moore LG, Butterfield GE. Women at altitude: short-term exposure to hypoxia and/or α1-adrenergic blockade reduces insulin sensitivity. J Appl Physiol. 2001;91(2):623–631. doi: 10.1152/jappl.2001.91.2.623. [DOI] [PubMed] [Google Scholar]
- Brestoff JRBJ, Clippinger BCB, Spinella TST, Duvillard SPVDSV, Nindl BNB, Arciero PJAP. An acute bout of endurance exercise but not sprint interval exercise enhances insulin sensitivity. Appl Physiol Nutr Metab. 2009;34(1):25–32. doi: 10.1139/H08-126. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-α. Am J Physiol-Endocrinol Metab. 2004;287(4):E616–E621. doi: 10.1152/ajpendo.00150.2004. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A, Gibala MJ. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1970–R1976. doi: 10.1152/ajpregu.00503.2006. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Parr EB, Hawley JA, Camera DM. Can high-intensity interval training promote skeletal muscle anabolism? Sports Med. 2021;51(3):405–421. doi: 10.1007/s40279-020-01397-3. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy J. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy J. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70(4):1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- Chacaroun S, Borowik A, Gonzalez V-EY, Doutreleau S, Wuyam B, Belaidi E, Tamisier R, Pepin J-L, Flore P, Verges S. Hypoxic exercise training to improve exercise capacity in obese individuals. Med Sci Sports Exerc. 2020;52:1641–1649. doi: 10.1249/MSS.0000000000002322. [DOI] [PubMed] [Google Scholar]
- Chen Z-Z, Liu J, Morningstar J, Heckman-Stoddard BM, Lee CG, Dagogo-Jack S, Ferguson JF, Hamman RF, Knowler WC, Mather KJ. Metabolite profiles of incident diabetes and heterogeneity of treatment effect in the diabetes prevention program. Diabetes. 2019;68(12):2337–2349. doi: 10.2337/db19-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre L, Kandror KV, Vallega G, Pilch PF. Identification and characterization of an exercise-sensitive pool of glucose transporters in skeletal muscle. J Biol Chem. 1995;270(46):27584–27588. doi: 10.1074/jbc.270.46.27584. [DOI] [PubMed] [Google Scholar]
- Coggan AR. Plasma glucose metabolism during exercise in humans. Sports Med. 1991;11(2):102–124. doi: 10.2165/00007256-199111020-00003. [DOI] [PubMed] [Google Scholar]
- Coker RH, Lacy DB, Williams PE, Wasserman DH. Hepatic alpha- and beta-adrenergic receptors are not essential for the increase in R(a) during exercise in diabetes. Am J Physiol Endocrinol Metab. 2000;278(3):E444–451. doi: 10.1152/ajpendo.2000.278.3.E444. [DOI] [PubMed] [Google Scholar]
- Constable SH, Favier RJ, Cartee GD, Young D, Holloszy J. Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. J Appl Physiol. 1988;64(6):2329–2332. doi: 10.1152/jappl.1988.64.6.2329. [DOI] [PubMed] [Google Scholar]
- Cooper D, Wasserman DH, Vranic M, Wasserman K. Glucose turnover in response to exercise during high-and low-FIO2 breathing in man. Am J Physiol Endocrinol Metab. 1986;251(2):E209–E214. doi: 10.1152/ajpendo.1986.251.2.E209. [DOI] [PubMed] [Google Scholar]
- Craig BW, Lucas J, Pohlman R, Stelling H. The effects of running, weightlifting and a combination of both on growth hormone release. J Strength Cond Res. 1991;5(4):198. [Google Scholar]
- Davison G, Vinaixa M, McGovern R, Beltran A, Novials A, Correig X, McClean C. Metabolomic response to acute hypoxic exercise and recovery in adult males. Front Physiol. 2018 doi: 10.3389/fphys.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote E, Deldicque L. Is physical exercise in hypoxia an interesting strategy to prevent the development of type 2 diabetes? A narrative review. Diabetes Metab Syndr Obes Targets Ther. 2021;14:3603. doi: 10.2147/DMSO.S322249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote E, Britto FA, Bullock L, François M, De Buck C, Nielens H, Deldicque L. Hypoxic training improves normoxic glucose tolerance in adolescents with obesity. Med Sci Sports Exerc. 2018;50(11):2200–2208. doi: 10.1249/MSS.0000000000001694. [DOI] [PubMed] [Google Scholar]
- De Groote E, Britto FA, Balan E, Warnier G, Thissen J-P, Nielens H, Sylow L, Deldicque L. Effect of hypoxic exercise on glucose tolerance in healthy and prediabetic adults. Am J Physiol Endocrinol Metab. 2021;320(1):E43–E54. doi: 10.1152/ajpendo.00263.2020. [DOI] [PubMed] [Google Scholar]
- DeFronzo R, Jacot E, Jequier E, Maeder E, Wahren J, Felber J. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Dekker MJ, Graham TE, Ooi TC, Robinson LE. Exercise prior to fat ingestion lowers fasting and postprandial VLDL and decreases adipose tissue IL-6 and GIP receptor mRNA in hypertriacylglycerolemic men. J Nutr Biochem. 2010;21(10):983–990. doi: 10.1016/j.jnutbio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Derave W, Lund S, Holman GD, Wojtaszewski J, Pedersen O, Richter EA. Contraction-stimulated muscle glucose transport and GLUT-4 surface content are dependent on glycogen content. Am J Physiol. 1999;277(6):E1103–1110. doi: 10.1152/ajpendo.1999.277.6.E1103. [DOI] [PubMed] [Google Scholar]
- Derave W, Hansen BF, Lund S, Kristiansen S, Richter EA. Muscle glycogen content affects insulin-stimulated glucose transport and protein kinase B activity. Am J Physiol Endocrinol Metab. 2000;279(5):E947–955. doi: 10.1152/ajpendo.2000.279.5.E947. [DOI] [PubMed] [Google Scholar]
- D'Hulst G, Sylow L, Hespel P, Deldicque L. Acute systemic insulin intolerance does not alter the response of the Akt/GSK-3 pathway to environmental hypoxia in human skeletal muscle. Eur J Appl Physiol. 2015;115(6):1219–1231. doi: 10.1007/s00421-015-3103-2. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Valenti L, Ludovica Fracanzani A, Gatti S, Cairo G, Fargion S. Iron depletion by deferoxamine up-regulates glucose uptake and insulin signaling in hepatoma cells and in rat liver. Am J Pathol. 2008;172(3):738–747. doi: 10.2353/ajpath.2008.070097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douen A, Ramlal T, Rastogi S, Bilan P, Cartee G, Vranic M, Holloszy J, Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin-and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265(23):13427–13430. doi: 10.1016/S0021-9258(18)77362-6. [DOI] [PubMed] [Google Scholar]
- Dunn WB, Broadhurst DI, Atherton HJ, Goodacre R, Griffin JL. Systems level studies of mammalian metabolomes: the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem Soc Rev. 2011;40(1):387–426. doi: 10.1039/B906712B. [DOI] [PubMed] [Google Scholar]
- Eigler N, Sacca L, Sherwin RS. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J Clin Invest. 1979;63(1):114–123. doi: 10.1172/JCI109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engfred K, Kjær M, Secher NH, Friedman DB, Hanel B, Nielsen OJ, Bach FW, Galbo H, Levine BD. Hypoxia and training-induced adaptation of hormonal responses to exercise in humans. Eur J Appl Physiol. 1994;68(4):303–309. doi: 10.1007/BF00571448. [DOI] [PubMed] [Google Scholar]
- Fernández Menéndez A, Saudan G, Sperisen L, Hans D, Saubade M, Millet GP, Malatesta D. Effects of short-term normobaric hypoxic walking training on energetics and mechanics of gait in adults with obesity. Obesity. 2018;26(5):819–827. doi: 10.1002/oby.22131. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Barrett E, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Investig. 1983;72(5):1737. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, Bräu L, Ferreira L-B, Fairchild T, Raja G, James A, Palmer T. Glycogen resynthesis in the absence of food ingestion during recovery from moderate or high intensity physical activity: novel insights from rat and human studies. Comp Biochem Physiol a: Mol Integr Physiol. 2002;133(3):755–763. doi: 10.1016/S1095-6433(02)00254-4. [DOI] [PubMed] [Google Scholar]
- Fournier PA, Fairchild TJ, Ferreira LD, Bräu L. Post-exercise muscle glycogen repletion in the extreme: effect of food absence and active recovery. J Sports Sci Med. 2004;3(3):139. [PMC free article] [PubMed] [Google Scholar]
- Fritsche A, Wagner R, Heni M, Kantartzis K, Machann J, Schick F, Lehmann R, Peter A, Dannecker C, Fritsche L. Different effects of lifestyle intervention in high-and low-risk prediabetes: results of the randomized controlled prediabetes lifestyle intervention study (PLIS) Diabetes. 2021;70(12):2785–2795. doi: 10.2337/db21-0526. [DOI] [PubMed] [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975;38(1):70–76. doi: 10.1152/jappl.1975.38.1.70. [DOI] [PubMed] [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ, Hilsted J. Glucagon and plasma catecholamines during beta-receptor blockade in exercising man. J Appl Physiol. 1976;40(6):855–863. doi: 10.1152/jappl.1976.40.6.855. [DOI] [PubMed] [Google Scholar]
- Garvey W, Maianu L, Huecksteadt T, Birnbaum M, Molina J, Ciaraldi T. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Investig. 1991;87(3):1072. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda M, Brun C, Juneau M, Levesque S, Nigam A. Long-term cardiac rehabilitation and exercise training programs improve metabolic parameters in metabolic syndrome patients with and without coronary heart disease. Nutr Metab Cardiovasc Dis. 2008;18(2):142–151. doi: 10.1016/j.numecd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Gillen JB, Little JP, Punthakee Z, Tarnopolsky MA, Riddell MC, Gibala MJ. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:575–577. doi: 10.1111/j.1463-1326.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- Girard O, Malatesta D, Millet GP. Walking in hypoxia: an efficient treatment to lessen mechanical constraints and improve health in obese individuals? Front Physiol. 2017 doi: 10.3389/fphys.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear LJ, Hirshman MF, King PA, Horton ED, Thompson CM, Horton ES. Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J Appl Physiol. 1990;68(1):193–198. doi: 10.1152/jappl.1990.68.1.193. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, King PA, Hirshman MF, Thompson CM, Horton ED, Horton ES. Contractile activity increases plasma membrane glucose transporters in absence of insulin. Am J Physiol. 1990;258(4 Pt 1):E667–672. doi: 10.1152/ajpendo.1990.258.4.E667. [DOI] [PubMed] [Google Scholar]
- Güemes A, Georgiou P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron Med. 2018;4:9. doi: 10.1186/s42234-018-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulve EA, Cartee GD, Zierath JR, Corpus V, Holloszy J. Reversal of enhanced muscle glucose transport after exercise: roles of insulin and glucose. Am J Physiol Endocrinol Metab. 1990;259(5):E685–E691. doi: 10.1152/ajpendo.1990.259.5.E685. [DOI] [PubMed] [Google Scholar]
- Gyntelberg F, Rennie M, Hickson R, Holloszy J. Effect of training on the response of plasma glucagon to exercise. J Appl Physiol. 1977;43(2):302–305. doi: 10.1152/jappl.1977.43.2.302. [DOI] [PubMed] [Google Scholar]
- Han X-X, Bonen A. Epinephrine translocates GLUT-4 but inhibits insulin-stimulated glucose transport in rat muscle. Am J Physiol Endocrinol Metab. 1998;274(4):E700–E707. doi: 10.1152/ajpendo.1998.274.4.E700. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol. 1998;85(4):1218–1222. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- Hartley LH, Mason J, Hogan R, Jones L, Kotchen T, Mougey E, Wherry F, Pennington L, Ricketts P. Multiple hormonal responses to graded exercise in relation to physical training. J Appl Physiol. 1972;33(5):602–606. doi: 10.1152/jappl.1972.33.5.602. [DOI] [PubMed] [Google Scholar]
- Hartley LH, Mason J, Hogan R, Jones L, Kotchen T, Mougey E, Wherry F, Pennington L, Ricketts P. Multiple hormonal responses to prolonged exercise in relation to physical training. J Appl Physiol. 1972;33(5):607–610. doi: 10.1152/jappl.1972.33.5.607. [DOI] [PubMed] [Google Scholar]
- Haufe S, Wiesner S, Engeli S, Luft FC, Jordan J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med Sci Sports Exerc. 2008;40(11):1939–1944. doi: 10.1249/MSS.0b013e31817f1988. [DOI] [PubMed] [Google Scholar]
- Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heden TD, Liu Y, Kearney ML, Park Y, Dellsperger KC, Thomas TR, Kanaley JA. Prior exercise and postprandial incretin responses in lean and obese individuals. Med Sci Sports Exerc. 2013;45(10):1897. doi: 10.1249/MSS.0b013e318294b225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol. 2003;546(1):299–305. doi: 10.1113/jphysiol.2002.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein-content and glucose-transport capacity in rat skeletal-muscles. Am J Physiol. 1990;259(4):E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Vaage O. Lactate disappearance and glycogen synthesis in human muscle after maximal exercise. Am J Physiol. 1977;233(5):E422–429. doi: 10.1152/ajpendo.1977.233.5.E422. [DOI] [PubMed] [Google Scholar]
- Hill E, Zack E, Battaglini C, Viru M, Viru A, Hackney A. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31(7):587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- Hilsted J, Galbo H, Sonne B, Schwartz T, Fahrenkrug J, de Muckadell O, Lauritsen K, Tronier B. Gastroenteropancreatic hormonal changes during exercise. Am J Physiol Gastrointest Liver Physiol. 1980;239(3):G136–G140. doi: 10.1152/ajpgi.1980.239.3.G136. [DOI] [PubMed] [Google Scholar]
- Hirsch I, Marker JC, Smith LJ, Spina RJ, Parvin C, Holloszy J, Cryer P. Insulin and glucagon in prevention of hypoglycemia during exercise in humans. Am J Physiol Endocrinol Metab. 1991;260(5):E695–E704. doi: 10.1152/ajpendo.1991.260.5.E695. [DOI] [PubMed] [Google Scholar]
- Hirshman MF, Goodyear LJ, Wardzala LJ, Horton ED, Horton ES. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990;265(2):987–991. doi: 10.1016/S0021-9258(19)40147-6. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99(1):338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- Holloszy J, Hansen P. Reviews of physiology biochemistry and pharmacology. Springer; 1996. Regulation of glucose transport into skeletal muscle; pp. 99–193. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Narahara H. Studies of tissue permeability X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem. 1965;240(9):3493–3500. doi: 10.1016/S0021-9258(18)97170-X. [DOI] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- Host HH, Hansen PA, Nolte LA, Chen MM, Holloszy JO. Glycogen supercompensation masks the effect of a traininginduced increase in GLUT-4 on muscle glucose transport. J Appl Physiol. 1998;85(1):133–138. doi: 10.1152/jappl.1998.85.1.133. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5(4):237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hunt DG, Ivy JL. Epinephrine inhibits insulin-stimulated muscle glucose transport. J Appl Physiol. 2002;93(5):1638–1643. doi: 10.1152/japplphysiol.00445.2002. [DOI] [PubMed] [Google Scholar]
- Hussey S, McGee S, Garnham A, Wentworth J, Jeukendrup A, Hargreaves M. Exercise training increases adipose tissue GLUT4 expression in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(10):959–962. doi: 10.1111/j.1463-1326.2011.01426.x. [DOI] [PubMed] [Google Scholar]
- Ismail N, Becker B, Strzelczyk P, Ritz E. Renal disease and hypertension in non–insulin-dependent diabetes mellitus. Kidney Int. 1999;55(1):1–28. doi: 10.1046/j.1523-1755.1999.00232.x. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Frishberg BA, Farrell SW, Miller WJ, Sherman WM. Effects of elevated and exercise-reduced muscle glycogen levels on insulin sensitivity. J Appl Physiol. 1985;59(1):154–159. doi: 10.1152/jappl.1985.59.1.154. [DOI] [PubMed] [Google Scholar]
- Jensen J, Rustad P, Kolnes A, Lai Y-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011 doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen N, Djurhuus CB, Jørgensen JO, Jensen LS, Møller N, Lund S, Schmitz O. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab. 2005;288(1):E194–E199. doi: 10.1152/ajpendo.00149.2004. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE. Nutrition for endurance sports: marathon, triathlon, and road cycling. J Sports Sci. 2011;29(Suppl 1):S91–99. doi: 10.1080/02640414.2011.610348. [DOI] [PubMed] [Google Scholar]
- Kahle EB, O'Dorisio TM, Walker RB, Eisenman PA, Reiser S, Cataland S, Zipf WB. Exercise adaptation responses for gastric inhibitory polypeptide (GIP) and insulin in obese children: possible extra-pancreatic effects. Diabetes. 1986;35(5):579–582. doi: 10.2337/diab.35.5.579. [DOI] [PubMed] [Google Scholar]
- Kang J, Robertson RJ, Hagberg JM, Kelley DE, Goss FL, Dasilva SG, Suminski RR, Utter AC. Effect of exercise intensity on glucose and insulin metabolism in obese individuals and obese NIDDM patients. Diabetes Care. 1996;19(4):341–349. doi: 10.2337/diacare.19.4.341. [DOI] [PubMed] [Google Scholar]
- Katz A, Broberg S, Sahlin K, Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol Endocrinol Metab. 1986;251(1):E65–E70. doi: 10.1152/ajpendo.1986.251.1.E65. [DOI] [PubMed] [Google Scholar]
- Kawanaka K, Han DH, Nolte LA, Hansen PA, Nakatani A, Holloszy JO. Decreased insulin-stimulated GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. Am J Physiol. 1999;276(5):E907–912. doi: 10.1152/ajpendo.1999.276.5.E907. [DOI] [PubMed] [Google Scholar]
- Kawanaka K, Nolte LA, Han DH, Hansen PA, Holloszy JO. Mechanisms underlying impaired GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. Am J Physiol Endocrinol Metab. 2000;279(6):E1311–1318. doi: 10.1152/ajpendo.2000.279.6.E1311. [DOI] [PubMed] [Google Scholar]
- Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab. 2009;296(6):E1269–E1274. doi: 10.1152/ajpendo.00112.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Williamson DL, Fealy CE, Kriz DA, Krishnan RK, Huang H, Ahn J, Loomis JL, Kirwan JP. Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism. 2010;59(2):200–205. doi: 10.1016/j.metabol.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599(1):23–37. doi: 10.1113/jp280572. [DOI] [PubMed] [Google Scholar]
- Kilgour E, Baldwin S, Flint D. Divergent regulation of rat adipocyte GLUT1 and GLUT4 glucose transporters by GH. J Endocrinol. 1995;145(1):27–33. doi: 10.1677/joe.0.1450027. [DOI] [PubMed] [Google Scholar]
- Kindermann W, Schnabel A, Schmitt WM, Biro G, Cassens J, Weber F. Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur J Appl Physiol Occup Physiol. 1982;49(3):389–399. doi: 10.1007/bf00441300. [DOI] [PubMed] [Google Scholar]
- King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J Appl Physiol. 1995;78(1):17–22. doi: 10.1152/jappl.1995.78.1.17. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Hepatic glucose production during exercise. Adv Exp Med Biol. 1998;441:117–127. doi: 10.1007/978-1-4899-1928-1_11. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Bangsbo J, Lortie G, Galbo H. Hormonal response to exercise in humans: influence of hypoxia and physical training. Am J Physiol Regul Integr Comp Physiol. 1988;254(2):R197–R203. doi: 10.1152/ajpregu.1988.254.2.R197. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Hollenbeck C, Frey-Hewitt B, Galbo H, Haskell W, Reaven G. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J Appl Physiol. 1990;68(5):2067–2074. doi: 10.1152/jappl.1990.68.5.2067. [DOI] [PubMed] [Google Scholar]
- Klug L, Mähler A, Rakova N, Mai K, Schulz-Menger J, Rahn G, Busjahn A, Jordan J, Boschmann M, Luft FC. Normobaric hypoxic conditioning in men with metabolic syndrome. Physiol Rep. 2018;6(24):e13949. doi: 10.14814/phy2.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen SH, Karstoft K, Pedersen BK, van Hall G, Solomon TP. The immediate effects of a single bout of aerobic exercise on oral glucose tolerance across the glucose tolerance continuum. Physiol Rep. 2014;2(8):e12114. doi: 10.14814/phy2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon M, Nakagaki K, Ebi Y, Nishiyama T, Russell AP. Hormonal and metabolic responses to repeated cycling sprints under different hypoxic conditions. Growth Hormon IGF Res. 2015;25(3):121–126. doi: 10.1016/j.ghir.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Kraniou GN, Cameron-Smith D, Hargreaves M. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. J Appl Physiol. 2006;101(3):934–937. doi: 10.1152/japplphysiol.01489.2005. [DOI] [PubMed] [Google Scholar]
- Krug S, Kastenmüller G, Stückler F, Rist MJ, Skurk T, Sailer M, Raffler J, Römisch-Margl W, Adamski J, Prehn C. The dynamic range of the human metabolome revealed by challenges. FASEB J. 2012;26(6):2607–2619. doi: 10.1096/fj.11-198093. [DOI] [PubMed] [Google Scholar]
- Kullman EL, Kelly KR, Haus JM, Fealy CE, Scelsi AR, Pagadala MR, Flask CA, McCullough AJ, Kirwan JP. Short-term aerobic exercise training improves gut peptide regulation in nonalcoholic fatty liver disease. J Appl Physiol. 2016;120(10):1159–1164. doi: 10.1152/japplphysiol.00693.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. J Physiol. 1997;504(1):241–249. doi: 10.1111/j.1469-7793.1997.241bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen JA, Mäkikallio TH, Ronkainen K, Karppi J, Kurl S. Impaired fasting plasma glucose and type 2 diabetes are related to the risk of out-of-hospital sudden cardiac death and all-cause mortality. Diabetes Care. 2013;36(5):1166–1171. doi: 10.2337/dc12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler NG, Abbiss CR, Gummer JPA, Broadhurst DI, Govus AD, Fairchild TJ, Thompson KG, Garvican-Lewis LA, Gore CJ, Maker GL, Trengove RD, Peiffer JJ. Characterizing the plasma metabolome during 14 days of live-high, train-low simulated altitude: a metabolomic approach. Exp Physiol. 2019;104(1):81–92. doi: 10.1113/EP087159. [DOI] [PubMed] [Google Scholar]
- Li VL, He Y, Contrepois K, Liu H, Kim JT, Wiggenhorn AL, Tanzo JT, Tung AS-H, Lyu X, Zushin P-JH, Jansen RS, Michael B, Loh KY, Yang AC, Carl CS, Voldstedlund CT, Wei W, Terrell SM, Moeller BC, Arthur RM, Wallis GA, van de Wetering K, Stahl A, Kiens B, Richter EA, Banik SM, Snyder MP, Xu Y, Long JZ. An exercise-inducible metabolite that suppresses feeding and obesity. Nature. 2022;606(7915):785–790. doi: 10.1038/s41586-022-04828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- Luger A, Deuster PA, Kyle SB, Gallucci WT, Montgomery LC, Gold PW, Loriaux DL, Chrousos GP. Acute hypothalamic–pituitary–adrenal responses to the stress of treadmill exercise. N Engl J Med. 1987;316(21):1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- Lund S, Holman G, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci. 1995;92(13):5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Van Hall G. Substrate utilization in sea level residents during exercise in acute hypoxia and after 4 weeks of acclimatization to 4100 m. Acta Physiol Scand. 2002;176(3):195–201. doi: 10.1046/j.1365-201X.2002.01030.x. [DOI] [PubMed] [Google Scholar]
- MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie RW, Watt P. A molecular and whole body insight of the mechanisms surrounding glucose disposal and insulin resistance with hypoxic treatment in skeletal muscle. J Diabetes Res. 2016 doi: 10.1155/2016/6934937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (SI2*) in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2011;27(1):94–101. doi: 10.1002/dmrr.1156. [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Elliott B, Maxwell N, Brickley G, Watt P. The effect of hypoxia and work intensity on insulin resistance in type 2 diabetes. J Clin Endocrinol Metab. 2012;97(1):155–162. doi: 10.1210/jc.2011-1843. [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Maxwell N, Castle P, Elliott B, Brickley G, Watt P. Intermittent exercise with and without hypoxia improves insulin sensitivity in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(4):E546–E555. doi: 10.1210/jc.2011-2829. [DOI] [PubMed] [Google Scholar]
- MacLeod S, Terada T, Chahal B, Boulé N. Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: a meta-analysis of studies using continuous glucose monitoring. Diabetes Metab Res Rev. 2013;29(8):593–603. doi: 10.1002/dmrr.2461. [DOI] [PubMed] [Google Scholar]
- Marliss EB, Simantirakis E, Miles P, Purdon C, Gougeon R, Field CJ, Halter JB, Vranic M. Glucoregulatory and hormonal responses to repeated bouts of intense exercise in normal male subjects. J Appl Physiol. 1991;71(3):924–933. doi: 10.1152/jappl.1991.71.3.924. [DOI] [PubMed] [Google Scholar]
- Matu J, Deighton K, Ispoglou T, Duckworth L. The effect of moderate versus severe simulated altitude on appetite, gut hormones, energy intake and substrate oxidation in men. Appetite. 2017;113:284–292. doi: 10.1016/j.appet.2017.02.041. [DOI] [PubMed] [Google Scholar]
- McBride A, Hardie D. AMP-activated protein kinase—a sensor of glycogen as well as AMP and ATP? Acta Physiol. 2009;196(1):99–113. doi: 10.1111/j.1748-1716.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mendenhall LA, Swanson SC, Habash DL, Coggan AR. Ten days of exercise training reduces glucose production and utilization during moderate-intensity exercise. Am J Physiol Endocrinol Metab. 1994;266(1):E136–E143. doi: 10.1152/ajpendo.1994.266.1.E136. [DOI] [PubMed] [Google Scholar]
- Mendes R, Sousa N, Almeida A, Subtil P, Guedes-Marques F, Reis VM, Themudo-Barata JL. Exercise prescription for patients with type 2 diabetes—a synthesis of international recommendations: narrative review. Br J Sports Med. 2016;50(22):1379–1381. doi: 10.1136/bjsports-2015-094895. [DOI] [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Farrell P, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol Endocrinol Metab. 1988;254(3):E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. 2012;44(2):225. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn MV, Lawton KA. Application of metabolomics to diagnosis of insulin resistance. Annu Rev Med. 2013;64(1):291–305. doi: 10.1146/annurev-med-061511-134747. [DOI] [PubMed] [Google Scholar]
- Moates JM, Lacy DB, Goldstein RE, Cherrington AD, Wasserman DH. Metabolic role of the exercise-induced increment in epinephrine in the dog. Am J Physiol. 1988;255(4 Pt 1):E428–436. doi: 10.1152/ajpendo.1988.255.4.E428. [DOI] [PubMed] [Google Scholar]
- Møller N, Jørgensen JOL. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- Moncloa F, Velasco I, Beteta L. Plasma cortisol concentration, and disappearance rate of 4–14C-cortisol in newcomers to high altitude. J Clin Endocrinol Metab. 1968;28(3):379–382. doi: 10.1210/jcem-28-3-379. [DOI] [PubMed] [Google Scholar]
- Morishima T, Goto K. Ghrelin, GLP-1, and leptin responses during exposure to moderate hypoxia. Appl Physiol Nutr Metab. 2016;41(4):375–381. doi: 10.1139/apnm-2015-0311. [DOI] [PubMed] [Google Scholar]
- Morishima T, Kurihara T, Hamaoka T, Goto K. Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin Physiol Funct Imaging. 2014;34(2):90–97. doi: 10.1111/cpf.12069. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34(2–3):121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näveri H, Kuoppasalmi K, Härkönen M. Plasma glucagon and catecholamines during exhaustive short-term exercise. Eur J Appl Physiol. 1985;53(4):308–311. doi: 10.1007/BF00422844. [DOI] [PubMed] [Google Scholar]
- Oberlin DJ, Mikus CR, Kearney ML, Hinton PS, Manrique C, Leidy HJ, Kanaley JA, Rector RS, Thyfault JP. One bout of exercise alters free-living postprandial glycemia in type 2 diabetes. Med Sci Sports Exerc. 2014;46(2):232. doi: 10.1249/MSS.0b013e3182a54d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AM, Pola S, Ward BM, Fillmore D, Buchanan KD, Kirwan JP. The gastroenteroinsular response to glucose ingestion during postexercise recovery. Am J Physiol Endocrinol Metab. 2006;290(6):E1155–E1161. doi: 10.1152/ajpendo.00500.2005. [DOI] [PubMed] [Google Scholar]
- O'Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, Glund S, Chibalin AV, Zierath JR, Nolan JJ. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia. 2006;49(12):2983–2992. doi: 10.1007/s00125-006-0457-3. [DOI] [PubMed] [Google Scholar]
- Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16(1):235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL, Peters A. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med. 2004;169(11):1231–1237. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- Parolin ML, Spriet LL, Hultman E, Hollidge-Horvat MG, Jones NL, Heigenhauser GJ. Regulation of glycogen phosphorylase and PDH during exercise in human skeletal muscle during hypoxia. Am J Physiol Endocrinol Metab. 2000;278(3):E522–E534. doi: 10.1152/ajpendo.2000.278.3.E522. [DOI] [PubMed] [Google Scholar]
- Péronnet F, Massicotte D, Folch N, Melin B, Koulmann N, Jimenez C, Bourdon L, Launay JC, Savourey G. Substrate utilization during prolonged exercise with ingestion of 13C-glucose in acute hypobaric hypoxia (4300 m) Eur J Appl Physiol. 2006;97(5):527–534. doi: 10.1007/s00421-006-0164-2. [DOI] [PubMed] [Google Scholar]
- Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp A, Hargreaves M, Baar K. More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am J Physiol Endocrinol Metab. 2012;302(11):E1343–E1351. doi: 10.1152/ajpendo.00004.2012. [DOI] [PubMed] [Google Scholar]
- Ploug T, Galbo H, Richter EA. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol. 1984;247(6 Pt 1):E726–E731. doi: 10.1152/ajpendo.1984.247.6.E726. [DOI] [PubMed] [Google Scholar]
- Randle P, Garland P, Hales C, Newsholme E. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;281(7285):785–789. doi: 10.1016/S0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P, Newsholme E, Garland P. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964;93(3):652. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. Diabetes Am. 1995;2:409–427. [Google Scholar]
- Richards JC, Johnson TK, Kuzma JN, Lonac MC, Schweder MM, Voyles WF, Bell C. Short-term sprint interval training increases insulin sensitivity in healthy adults but does not affect the thermogenic response to β-adrenergic stimulation. J Physiol. 2010;588(15):2961–2972. doi: 10.1113/jphysiol.2010.189886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Investig. 1982;69(4):785. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Cleland PJ, Rattigan S, Clark MG. Contraction-associated translocation of protein kinase C in rat skeletal muscle. FEBS Lett. 1987;217(2):232–236. doi: 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Richter EA, Derave W, Wojtaszewski JFP. Glucose, exercise and insulin: emerging concepts. J Physiol. 2001;535(2):313–322. doi: 10.1111/j.1469-7793.2001.t01-2-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich JE. Effects of growth hormone on insulin action in man: mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes. 1982;31(8):663–669. doi: 10.2337/diab.31.8.663. [DOI] [PubMed] [Google Scholar]
- Roberts S, Desbrow B, Grant G, Anoopkumar-Dukie S, Leveritt M. Glycemic response to carbohydrate and the effects of exercise and protein. Nutrition. 2013;29(6):881–885. doi: 10.1016/j.nut.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Roden M, Shulman GI. Applications of NMR spectroscopy to study muscle glycogen metabolism in man. Annu Rev Med. 1999;50:277–290. doi: 10.1146/annurev.med.50.1.277. [DOI] [PubMed] [Google Scholar]
- Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3):e219–e219. doi: 10.1038/emm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Howlett K, King DS, Hargreaves M. Effect of prior exercise on glucose metabolism in trained men. Am J Physiol Endocrinol Metab. 2001;281(4):E766–E771. doi: 10.1152/ajpendo.2001.281.4.E766. [DOI] [PubMed] [Google Scholar]
- Roussel R, Carlier PG, Robert JJ, Velho G, Bloch G. 13C/31P NMR studies of glucose transport in human skeletal muscle. Proc Natl Acad Sci USA. 1998;95(3):1313–1318. doi: 10.1073/pnas.95.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynders CA, Weltman JY, Jiang B, Breton M, Patrie J, Barrett EJ, Weltman A. Effects of exercise intensity on postprandial improvement in glucose disposal and insulin sensitivity in prediabetic adults. J Clin Endocrinol Metab. 2014;99(1):220–228. doi: 10.1210/jc.2013-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K. Metabolic factors in fatigue. Sports Med. 1992;13(2):99–107. doi: 10.2165/00007256-199213020-00005. [DOI] [PubMed] [Google Scholar]
- Sakagami H, Makino Y, Mizumoto K, Isoe T, Takeda Y, Watanabe J, Fujita Y, Takiyama Y, Abiko A, Haneda M. Loss of HIF-1α impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am J Physiol Endocrinol Metab. 2014;306(9):E1065–E1076. doi: 10.1152/ajpendo.00597.2012. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Dore S, Hilgendorf A, Strauch S, Gareau R, Brisson G. Effects of exercise during normoxia and hypoxia on the growth hormone—insulin-like growth factor I axis. Eur J Appl Physiol. 1995;71(5):424–430. doi: 10.1007/BF00635876. [DOI] [PubMed] [Google Scholar]
- Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Curr Opin Pharmacol. 2013;13(4):646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Schwarz J-M, Mulligan K, Lee J, Lo JC, Wen M, Noor MA, Grunfeld C, Schambelan M. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87(2):942–945. doi: 10.1210/jcem.87.2.8391. [DOI] [PubMed] [Google Scholar]
- Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC. Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol. 2003;149(6):511–519. doi: 10.1530/eje.0.1490511. [DOI] [PubMed] [Google Scholar]
- Semenza G. Cold Spring Harbor symposia on quantitative biology. Cold Spring Harbor Laboratory Press; 2011. Regulation of metabolism by hypoxia-inducible factor 1; pp. 347–353. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Graham TE. Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise. Exerc Sport Sci Rev. 2004;32(3):120–126. doi: 10.1097/00003677-200407000-00008. [DOI] [PubMed] [Google Scholar]
- Sherwin RS, Shamoon H, Hendler R, Sacca L, Eigler N, Walesky M. Epinephrine and the regulation of glucose metabolism: effect of diabetes and hormonal interactions. Metabolism. 1980;29(11 Suppl 1):1146–1154. doi: 10.1016/0026-0495(80)90024-4. [DOI] [PubMed] [Google Scholar]
- Smith TR, Elmendorf JS, David TS, Turinsky J. Growth hormone-induced insulin resistance: role of the insulin receptor, IRS-1, GLUT-1, and GLUT-4. Am J Physiol Endocrinol Metab. 1997;272(6):E1071–E1079. doi: 10.1152/ajpendo.1997.272.6.E1071. [DOI] [PubMed] [Google Scholar]
- Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic β-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010;33(7):1561–1566. doi: 10.2337/dc09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo J, Girard O, Ihsan M, Fairchild T. The use of the SpO2 to FiO2 ratio to individualize the hypoxic dose in sport science, exercise, and health settings. Front Physiol. 2020 doi: 10.3389/fphys.2020.570472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Wahl HG, Fritsche A, Haring H, Stumvoll M. Effect of the pattern of elevated free fatty acids on insulin sensitivity and insulin secretion in healthy humans. Horm Metab Res. 2001;33(7):432–438. doi: 10.1055/s-2001-16231. [DOI] [PubMed] [Google Scholar]
- Stefan N, Staiger H, Wagner R, Machann J, Schick F, Häring H-U, Fritsche A. A high-risk phenotype associates with reduced improvement in glycaemia during a lifestyle intervention in prediabetes. Diabetologia. 2015;58(12):2877–2884. doi: 10.1007/s00125-015-3760-z. [DOI] [PubMed] [Google Scholar]
- Stuart CA, Yin D, Howell MEA, Dykes RJ, Laffan JJ, Ferrando AA. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am J Physiol Endocrinol Metab. 2006;291(5):E1067–E1073. doi: 10.1152/ajpendo.00250.2006. [DOI] [PubMed] [Google Scholar]
- Suhre K. Metabolic profiling in diabetes. J Endocrinol. 2014;221(3):R75–R85. doi: 10.1530/joe-14-0024. [DOI] [PubMed] [Google Scholar]
- Sun L, Liang L, Gao X, Zhang H, Yao P, Hu Y, Ma Y, Wang F, Jin Q, Li H. Early prediction of developing type 2 diabetes by plasma acylcarnitines: a population-based study. Diabetes Care. 2016;39(9):1563–1570. doi: 10.2337/dc16-0232. [DOI] [PubMed] [Google Scholar]
- Sutton JR. Effect of acute hypoxia on the hormonal response to exercise. J Appl Physiol. 1977;42(4):587–592. doi: 10.1152/jappl.1977.42.4.587. [DOI] [PubMed] [Google Scholar]
- Svensson J, Fowelin J, Landin K, Bengtsson B-AK, Johansson J-O. Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab. 2002;87(5):2121–2127. doi: 10.1210/jcem.87.5.8482. [DOI] [PubMed] [Google Scholar]
- Sylow L, Jensen TE, Kleinert M, Mouatt JR, Maarbjerg SJ, Jeppesen J, Prats C, Chiu TT, Boguslavsky S, Klip A. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes. 2013;62(4):1139–1151. doi: 10.2337/db12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake—regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13(3):133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- Sylow L, Tokarz VL, Richter EA, Klip A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33(4):758–780. doi: 10.1016/j.cmet.2021.03.020. [DOI] [PubMed] [Google Scholar]
- Tai E, Tan M, Stevens R, Low Y, Muehlbauer M, Goh D, Ilkayeva O, Wenner B, Bain J, Lee J. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian–Indian men. Diabetologia. 2010;53(4):757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Haruta T, Iwata M, Usui I, Uno T, Kawahara J, Ueno E, Sasaoka T, Kobayashi M. Growth hormone induces cellular insulin resistance by uncoupling phosphatidylinositol 3-kinase and its downstream signals in 3T3-L1 adipocytes. Diabetes. 2001;50(8):1891–1900. doi: 10.2337/diabetes.50.8.1891. [DOI] [PubMed] [Google Scholar]
- Thomas D, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3(3):CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Prog Mol Biol Transl Sci. 2015;135:203–225. doi: 10.1016/bs.pmbts.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, Kobayashi I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int J Obes Relat Metab Disord. 2000;24(9):1207–1211. doi: 10.1038/sj.ijo.0801373. [DOI] [PubMed] [Google Scholar]
- Umpierre D, Ribeiro PAB, Kramer CK, Leitao CB, Zucatti ATN, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA(1c) levels in type 2 diabetes A systematic review and meta-analysis. JAMA J Am Med Assoc. 2011;305(17):1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- Umpierre D, Ribeiro P, Schaan B, Ribeiro J. Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysis. Diabetologia. 2013;56(2):242–251. doi: 10.1007/s00125-012-2774-z. [DOI] [PubMed] [Google Scholar]
- Venables MC, Shaw CS, Jeukendrup AE, Wagenmakers AJ. Effect of acute exercise on glucose tolerance following post-exercise feeding. Eur J Appl Physiol. 2007;100(6):711–717. doi: 10.1007/s00421-007-0464-1. [DOI] [PubMed] [Google Scholar]
- Vollestad NK, Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125(3):395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Vogt RA, Foster GD, Anderson DA. Exercise and the maintenance of weight loss: 1-year follow-up of a controlled clinical trial. J Consult Clin Psychol. 1998;66(2):429. doi: 10.1037/0022-006X.66.2.429. [DOI] [PubMed] [Google Scholar]
- Wadley G, Lee-Young RS, Canny BJ, Wasuntarawat C, Chen Z-P, Hargreaves M, Kemp BE, McConell GK. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab. 2006;290(4):E694–E702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Investig. 1971;50(12):2715. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberghenriksson H, Constable SH, Young DA, Holloszy JO. Glucose-transport into rat skeletal-muscle—interaction between exercise and insulin. J Appl Physiol. 1988;65(2):909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- Wardzala LJ, Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981;256(14):7090–7093. doi: 10.1016/S0021-9258(19)68926-X. [DOI] [PubMed] [Google Scholar]
- Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296(1):E11–21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman DH, Spalding JA, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am J Physiol. 1989;257(1 Pt 1):E108–117. doi: 10.1152/ajpendo.1989.257.1.E108. [DOI] [PubMed] [Google Scholar]
- Wasserman DH, Williams PE, Lacy DB, Bracy D, Cherrington AD. Hepatic nerves are not essential to the increase in hepatic glucose production during muscular work. Am J Physiol. 1990;259(2 Pt 1):E195–203. doi: 10.1152/ajpendo.1990.259.2.E195. [DOI] [PubMed] [Google Scholar]
- Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Wiesner S, Haufe S, Engeli S, Mutschler H, Haas U, Luft FC, Jordan J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity. 2010;18(1):116–120. doi: 10.1038/oby.2009.193. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hickson RC, Hagberg JM, Ehsani AA, McLane J. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol. 1979;46(4):766–771. doi: 10.1152/jappl.1979.46.4.766. [DOI] [PubMed] [Google Scholar]
- Wittenbecher C, Guasch-Ferre M, Haslam DE, Dennis C, Li J, Bhupathiraju SN, Lee C-H, Qi Q, Liang L, Eliassen AH. Changes in metabolomics profiles over ten years and subsequent risk of developing type 2 diabetes: results from the Nurses' Health Study. EBioMedicine. 2022;75:103799. doi: 10.1016/j.ebiom.2021.103799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D, Davison A, Stacey M, Smith C, Hooper T, Neely D, Turner S, Peaston R, Mellor A. The cortisol response to hypobaric hypoxia at rest and post-exercise. Horm Metab Res. 2012;44(04):302–305. doi: 10.1055/s-0032-1304322. [DOI] [PubMed] [Google Scholar]
- Würtz P, Mäkinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J, Savolainen MJ, Tammelin T, Viikari JS, Rönnemaa T, Kähönen M, Lehtimäki T, Ripatti S, Raitakari OT, Järvelin MR, Ala-Korpela M. Metabolic signatures of insulin resistance in 7098 young adults. Diabetes. 2012;61(6):1372–1380. doi: 10.2337/db11-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Garthwaite SM, Bryan JE, Cartier LJ, Holloszy JO. Carbohydrate feeding speeds reversal of enhanced glucose uptake in muscle after exercise. Am J Physiol. 1983;245(5 Pt 1):R684–688. doi: 10.1152/ajpregu.1983.245.5.R684. [DOI] [PubMed] [Google Scholar]
- Zinker BA, Lacy DB, Bracy D, Jacobs J, Wasserman DH. Regulation of glucose uptake and metabolism by working muscle: an in vivo analysis. Diabetes. 1993;42(7):956–965. doi: 10.2337/diab.42.7.956. [DOI] [PubMed] [Google Scholar]
- Zinker BA, Lacy DB, Bracy DP, Wasserman DH. Role of glucose and insulin loads to the exercising limb in increasing glucose uptake and metabolism. J Appl Physiol. 1993;74:2915–2915. doi: 10.1152/jappl.1993.74.6.2915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the current study are available from the corresponding author on reasonable request.