Abstract
ME/CFS is a debilitating chronic condition that often develops after viral or bacterial infection. Insight from the study of Long COVID/Post Acute Sequelae of COVID-19 (PASC), the post-viral syndrome associated with SARS-CoV-2 infection, might prove to be useful for understanding pathophysiological mechanisms of ME/CFS. Disease presentation is similar between the two conditions, and a subset of Long COVID patients meet the diagnostic criteria for ME/CFS. Since Long COVID is characterized by significant vascular pathology – including endothelial dysfunction, coagulopathy, and vascular dysregulation – the question of whether or not the same biological abnormalities are of significance in ME/CFS arises. Cardiac abnormalities have for a while now been documented in ME/CFS cohorts, with recent studies demonstrating major deficits in cerebral blood flow, and hence vascular dysregulation. A growing body of research is demonstrating that ME/CFS is accompanied by platelet hyperactivation, anomalous clotting, a procoagulant phenotype, and endothelial dysfunction. Endothelial damage and dysregulated clotting can impair substance exchange between blood and tissues, and result in hypoperfusion, which may contribute to the manifestation of certain ME/CFS symptoms. Here we review the ME/CFS literature to summarize cardiovascular and haematological findings documented in patients with the condition, and, in this context, briefly discuss the potential role of previously-implicated pathogens. Overall, cardiac and haematological abnormalities are present within ME/CFS cohorts. While atherosclerotic heart disease is not significantly associated with ME/CFS, suboptimal cardiovascular function defined by reduced cardiac output, impaired cerebral blood flow, and vascular dysregulation are, and these abnormalities do not appear to be influenced by deconditioning. Rather, these cardiac abnormalities may result from dysfunction in the (autonomic) nervous system. Plenty of recently published studies are demonstrating significant platelet hyperactivity and endothelial dysfunction in ME/CFS, as well as anomalous clotting processes. It is of particular importance to determine to what extent these cardiovascular and haematological abnormalities contribute to symptom severity, and if these two systems can be targeted for therapeutic purposes. Viral reservoirs of herpesviruses exist in ME/CFS, and most likely contribute to cardiovascular and haematological dysfunction directly or indirectly. This review highlights the potential of studying cardiac functioning, the vasculature, and coagulation system in ME/CFS.
Keywords: Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Haematological system, Endothelial dysfunction
1. Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex, debilitating, multisystem disease that is characterized by profound fatigue and an inability to withstand certain forms of physiological insult (e.g., physical and cognitive exertion) without symptom exacerbation [1,2]. Viruses, including herpes viruses such human herpesvirus (HHV)-4 (also known as Epstein-Barr virus (EBV)), HHV-6, and HHV-7, are implicated in ME/CFS [3], and many cases begin with an initial infection of some sort [4]. Hence, ME/CFS is viewed as a post-viral illness.
In 2021, it was estimated that 1.5 million (low-end value) individuals suffer from ME/CFS in the USA alone, which is accompanied by a national economic burden of more than $35 billion per year [5]. The prevalence figures are predicted to increase to between 5 and 9 million [6]. This expected surge in the prevalence of ME/CFS can be mostly attributed to the virus responsible for the COVID-19 pandemic, as a subset of patients who contract COVID-19 go on to develop chronic symptoms that meet the diagnostic criteria for ME/CFS [[6], [7], [8], [9], [10]].
As of yet, biomarkers associated with disease are absent for clinical use. Furthermore, an FDA-approved treatment is lacking, leaving patients and clinicians to focus on symptomatic alleviation and symptom management. As a consequence of the symptomatic burden, many ME/CFS patients lose a great deal of function and eventually become disabled. Using the EQ-5D-3 L based health-related quality of life assessment [11], researchers have shown that ME/CFS patients experience a quality of life that is considerably worse than that accompanying other diseases, including cardiovascular disease, certain cancers, mental illness, and more. Patients suffer severely, and often without clinical aid, especially since a large proportion of afflicted patients are undiagnosed [12,13].
Symptom expression amongst ME/CFS patients vary considerably, and some symptoms overlap with many other conditions including Gulf War illness, fibromyalgia, infection, and depression [[14], [15], [16]]. Post exertional symptom exacerbation (PESE) has been deemed a hallmark symptom for the disease [17], although there still exist difficulties when using PESE for diagnostic conclusions [18]. PESE is described as the worsening of ME/CFS symptoms (often referred to as a ‘crash’) following physical activity (including ostensibly undemanding daily functions), or even after cognitive exertion and emotional distress [1]. PESE typically lasts over 24 h, and can even induce flu-like symptoms [18].
Patients predominantly experience unresolved fatigue (independent of and aggravated by PESE) that is considered pathological due to resulting impairments. Other symptoms include joint and muscle pain, intolerance towards exercise, cognitive impairments, headaches, gastrointestinal issues, flu-like symptoms, and sleeping difficulties [19,20]. Common comorbidities of ME/CFS are orthostatic intolerance (OI) and postural orthostatic tachycardia syndrome (POTS) [20,21], which is thought to be a result of underlying autonomic defects [22]. The severity of orthostatic symptoms influences the functional capabilities of patients [23] and is thus seen as a favoured target for therapy.
Underlying mechanisms are not fully defined, but there is clear evidence that multiple physiological systems are affected, including the neurological, immunological, muscular, gastrointestinal, cardiovascular, and endocrine system; defects in metabolism, cellular bioenergetics, and ion transport systems are also common findings in this disease population [[24], [25], [26], [27], [28]]. In relation to microbes, ME/CFS might be maintained by the persistence of certain viral and bacterial pathogens in patient tissue and the associated shedding of virulent proteins or products. It has previously been shown that enterovirus infection can persist in intestinal tissue and lead to the development of ME/CFS symptoms [29]. Other viruses implicated in ME/CFS include HHV-6, HHV-7, cytomegalovirus, and EBV [3,30,31], of which EBV and HHV-6 were recently discovered in the central nervous system of deceased ME/CFS individuals [32]. Relevantly, viral persistence of SARS-CoV-2 in tissue is becoming increasingly implicated as a central factor of Long COVID pathology [33], due to a growing number of studies that show SARS-CoV-2 RNA or antigens in tissue collected from Long COVID patients [[34], [35], [36]].
The symptoms associated with ME/CFS hint towards an energy- and/or oxygen-deficit problem, which can either be explained by defects associated with perfusion and the exchange of substances between blood and tissues, by neurological failure, or by defects associated with mitochondria and cellular bioenergetics, or all of the above. Both perfusion and mitochondrial issues have been implicated in ME/CFS [[37], [38], [39], [40], [41], [42], [43]], but elucidation and proof of mechanisms are still required. Hypotheses for ME/CFS being a disease governed by suboptimal tissue perfusion have been published [44,45]. With regards to mitochondrial dysfunction, certain viruses can hijack host systems to further their survival [46]. Indeed, the viruses previously implicated in ME/CFS have the ability to modulate mitochondrial activity of the cells that they infect [3], and may play a relevant role in pathology. Functional abnormalities in the nervous system – which have been demonstrated in ME/CFS cohorts [[47], [48], [49]] – also have potential to explain the manifestation of certain symptoms [50,51].
This systemic disease is indeed complex, hence the lack of clinical success and the long list of unanswered questions. Some of the answers for ME/CFS might come from the COVID-19 pandemic as many similarities are shared between ME/CFS and the post-viral syndrome associated with acute COVID-19 [52,53]. This overlap brings into focus the involvement of the circulatory system in ME/CFS as COVID-19 and its post-viral syndrome are strongly characterized by vascular and haematological pathology [44,[54], [55], [56], [57], [58], [59]]. In this article, we wish to review the cardiovascular and haematological findings associated with ME/CFS as these two systems are becoming more implicated in this disease. Because of recent findings of viral RNA in the brain and spinal cord of ME/CFS patients post-mortem [32], as well as other indications of viral influence [60,61], we also discuss how viral reservoirs might contribute to pathology. Whilst the haematological system is indeed a part of the cardiovascular system, they are discussed separately here. A haematological emphasis focuses on the cells and cell-like structures (leukocytes, erythrocytes and platelets), and the free plasma proteins, specifically those related to coagulation (fibrin(ogen), clotting factors, etc.); whereas the cardiovascular emphasis concentrates on cardiac and vessel structure and function. Finally, we aim to tie findings together with a focus on the endothelium, and a commentary on the role of viruses.
2. Cardiovascular findings
Although ME/CFS is not considered as a primary cardiovascular disorder, there are certainly indications of cardiovascular dysfunction in this disease population. ME/CFS individuals exhibit an increased risk for premature heart failure and earlier all-cause mortality [[62], [63], [64], [65]], as do Long COVID suffers [66]. The exact mechanistic path leading from ME/CFS to heart failure is not yet elucidated, but oxidative and nitrosative stress (O&NS) as well as dysregulated inflammatory function are thought to play a major role [67]. Indeed, ME/CFS is accompanied by increases in O&NS and a reduced antioxidant capacity [[68], [69], [70], [71], [72], [73]], as well as a proinflammatory phenotype [68,[74], [75], [76], [77], [78], [79], [80]]. With that being said, it must be noted that the ME/CFS-induced lifestyle – one that is defined by reduced physical activity and disablement – is one that itself likely increases cardiovascular risk, and hence needs to be accounted for when interpreting data [81].
Related to the association between ME/CFS and heart failure, ME/CFS patients exhibit increases in arterial wave reflection when compared to controls [75]. Arterial wave reflection is inversely associated with left ventricular systolic function (as determined by tissue Doppler imaging techniques) and is involved in the pathogenesis of heart failure [82]. Abnormalities in left ventricular function were described in an ME/CFS population in the early 1990s [83].
In a study involving 56 ME/CFS participants who were subdivided into severe (n=30) and non-severe (n = 26) groups, the severe ME/CFS group presented with a 10.2% reduction in stroke volume and mild decreases in contractility in comparison to controls [84]. Furthermore, both ME/CFS groups had reduced total blood volume, plasma volume, and red blood cell volume in relation to the control groups. The authors inferred that the lower cardiac volume observed in the ME/CFS cohorts is most likely a result of a hypovolemic comorbidity instead of a cardiac-contractile issue. In a comment article published the following year [85], it was emphasized that the aforementioned results do not point to classical cardiovascular disease, but rather highlight impairments in circulation and cardiovascular function. The ME/CFS-induced lifestyle – of which a consequence is physical deconditioning – was also mentioned as a reason for the observed results, as chronic physical inactivity is related to decreased stroke volume [86].
Reduced stroke volume in ME/CFS has since been corroborated, and decreases in end-diastolic volume and cardiac output have also been reported [[87], [88], [89]]. van Campen and Visser (2018) performed a tilt table test on 150 ME/CFS individuals who were subdivided into three groups based on disease severity – mild, moderate, and severe – and 37 controls [90]. Suprasternal aortic Doppler imaging was used to measure stroke volume index (SVI) and cardiac index (CI), and determined that decreases in SVI and CI were significantly greater in the ME/CFS group than controls. Importantly, the researchers showed that these cardiac shortcomings were not statistically different between the mild, moderate, and severe ME/CFS groups. This suggests that deconditioning due to the ME/CFS-induced lifestyle does not account in significant part for the cardiovascular abnormalities observed in this disease population, and increases the plausibility of cardiac involvement in ME/CFS pathogenesis and symptom manifestation.
To further investigate the role of deconditioning in ME/CFS symptomology, van Campen and colleagues assessed the relation between the extent of reduction in peak oxygen consumption during cardiopulmonary exercise testing and the degree of reduction in cerebral blood flow during head-up tilt tests in 199 ME/CFS and 22 control participants [91]. Again, there were no differences observed between the ME/CFS groups (no, mild, or severe deconditioning), which implies that deconditioning does not govern orthostatic symptoms in ME/CFS. Other studies have reached the same inferences [92,93]. With the evidence at hand, it seems that cardiac dysfunction present in ME/CFS is not a result of physical inactivity, and hence is not something that should be brushed off as a consequence of ME/CFS – it might be (intimately or modestly) involved in pathology.
Using magnetic resonance imaging and cardiac tagging techniques, Hollingsworth and colleagues investigated the cardiac morphology of 12 ME/CFS and 10 control subjects and determined that left ventricular mass was significantly decreased in the ME/CFS group [87]. This has since been corroborated [88,94]. Contrastingly, the study by Hurwitz and colleagues found no significance in left ventricular mass [84]; the same goes for a more recent study [95], where cardiac function was also deemed normal.
A significant reduction (P < .001) in coenzyme Q10 (CoQ10) plasma levels has been observed in an ME/CFS cohort [63]. CoQ10 is an essential component of the respiratory chain, and exerts anti-inflammatory and antioxidant effects [96]. Reductions in CoQ10 are associated with cardiovascular pathology and reduced mitochondrial biogenesis [97], and supplementation offers cardio-protection [98,99]. CoQ10 is also an independent risk predictor of mortality in chronic heart failure [100].
Decreases in CoQ10 reduce antioxidant capacity and might account in part for the increases in O&NS observed in ME/CFS [67]. Furthermore, CoQ10 might hold potential as a useful predictor of heart failure in ME/CFS patients. CoQ10 supplementation increases cellular resistance to lipid peroxidation [[101], [102], [103]], and may therefore prove useful against the lipid peroxidation observed in ME/CFS [68,104]. Indeed, it has been shown that CoQ10 and selenium supplementation in ME/CFS subjects increased antioxidant capacity and decreased lipid peroxidation, cytokine levels, and symptom severity [105]. ME/CFS is also accompanied by reduced plasma levels of omega-3 fatty acids [106], which is associated with chronic inflammation [107] and also confers an increased cardiovascular risk [108,109]. On that note, metabolic abnormalities are common findings amongst ME/CFS cohorts [28,[110], [111], [112]].
Significantly increased levels of fibroblast growth factor 21 (FGF21) and the N-terminal prohormone of brain natriuretic peptide (NT-proBNP) have been revealed in an ME/CFS cohort [68]. Positive correlations between NT-proBNP concentrations and that of proinflammatory cytokines (namely, IL-1β and IL-6) were noted. NT-proBNP is positively and independently associated with cardiovascular risk [113] and FGF21 is involved in glucose and lipid metabolism [114]. Supplementation of CoQ10 and selenium did not alter levels of these two proteins in ME/CFS subjects [105].
2.1. Orthostatic intolerance
One of the more consistent findings amongst ME/CFS populations is the presence of OI and POTS [27,[115], [116], [117], [118]], which is not surprising considering the evidence of reduced venous return and cardiac function [87,88,93,119], and reduced ambulatory blood pressure [120]. OI forms part of the ME/CFS diagnosis [2,121] and significantly reduces one's functional capabilities and quality of life [122].
On the upper end of the scale, some studies that have employed the 10 min stand-up test have reported prevalence figures for OI above 95% in ME/CFS cohorts [88,123]. In another study, symptoms of light-headedness and dizziness were present in 72% (32/39) of standing and 41% (16/39) of recumbent patients; ME/CFS patients without POTS scored higher in orthostatic measures upon standing than those with POTS, suggesting that orthostatic tachycardia does not account for the symptoms of OI in ME/CFS [124]. From a therapeutic perspective, compression stockings have shown benefit for orthostatic symptoms and cardiac measurements [125].
Autonomic receptors, such as adrenergic and cholinergic G-protein-coupled receptors (GPCRs), relay sympathetic and parasympathetic signals involved in the regulation of blood vessels. Elevated levels of autoantibodies against adrenergic and cholinergic receptors have been found in ME/CFS individuals [[126], [127], [128]], and also correlate with autonomic dysfunction and symptom severity [22]. Yamamoto et al (2012) demonstrated that these autoantibodies have an impact on the central muscarinic cholinergic receptor system as inferred by positron emission tomography [129], and may therefore interfere with cell signalling. Attenuated β2 adrenergic receptor activation has also been noticed [130]. An explanation for ME/CFS pathology with a focus on the dysfunction of these autonomic receptors has been published [17]. With that being said, it must be noted that herpes viruses, which are significantly implicated in ME/CFS pathology [31], produce GPCRs that share homology with human GPCRs [131]. Hence, molecular mimicry between herpes and human GPCRs might underlie what is seen as ‘autoimmunity involving GPCRs’ in ME/CFS.
2.2. Cerebral blood flow
There are data that indicate that cerebral blood flow is significantly reduced in ME/CFS patients compared to controls [27,91,[132], [133], [134], [135], [136]]. Out of 429 ME/CFS participants, 90% (384/429) exhibited reductions in cerebral blood flow measurements that surpassed a defined cut-off value (13%) during orthostatic testing (but not in the supine position) [27]. The mean reduction for the control and ME/CFS group was 7% and 26%, respectively. Not unexpectedly, 100% of patients with POTS and 98% with delayed orthostatic hypotension presented with values greater than 13%; unexpectedly, in ME/CFS patients without heart rate or blood pressure problems, 82% exceeded the cut-off value. Markedly reduced cerebral blood flow during 30-min head-up tilt testing was observed in those ME/CFS participants with and without heart rate and blood pressure abnormalities, meaning that cerebral blood flow is perturbed even in those without orthostatic pathophysiology. Deficits in cerebral blood flow have potential to account for ME/CFS symptom manifestation and have even been associated with symptom severity [134] – further follow-up is essential.
Related to the findings of reduced cerebral blood flow, hypoperfusion of particular brain regions have been documented in ME/CFS participants, including the brainstem [37], cerebral cortex [137], anterior cingulate [138,139], lingual gyrus [139], superior temporal gyri [140], and regions associated with the limbic system [141]. There are also differences in regional cerebral blood flow between ME/CFS and control subjects following mental exertion [140], as well as neuro-functional differences [142].
The abnormalities in cardiovascular dysfunction discussed thus far may result from neuronal issues. Functional and structural defects of the nervous system have been reported in ME/CFS [49,[143], [144], [145], [146]], and have been proposed as drivers of the disease [50,147]. Autonomic dysfunction is a common finding in ME/CFS cohorts [[148], [149], [150], [151]], and recent studies have shown that a subset of patients exhibit signs of small fibre neuropathy [152,153]. In a systematic review, it was inferred that the autonomic defects underlie the cardiovascular abnormalities observed in ME/CFS patients [154]. Pyridostigmine, an acetylcholinesterase inhibitor which aims to ameliorate impaired autonomic signalling, improves cardiac performance in ME/CFS patients [153]. Further study of pyridostigmine in ME/CFS individuals with cardiovascular abnormalities/orthostatic symptoms is therefore warranted.
3. Haematological findings
Components of the haematological system are susceptible to influence from pathology in different physiological systems, and are thus useful and relatively convenient markers for health and pathology assessments. Erythrocytes, leukocytes, platelets, and clotting proteins are exposed to and affected by O&NS, inflammation, and metabolites. In diabetes, excessive glycation is noticeable in red blood cells and plasma proteins [155]; inflammatory cytokines can induce changes in platelets, erythrocytes, and coagulation [156,157]; hormones modulate viscoelastic changes [158]; and microbes can influence the activity of platelets and upregulate or inhibit clotting processes [[159], [160], [161]]. Whilst a fair amount of study has been conducted on cardiovascular function and leukocytes in ME/CFS, there is much less literature regarding erythrocytes, platelets and clotting proteins.
3.1. Erythrocytes, platelets, and clotting proteins
An early study noticed that ME/CFS individuals have a significantly lower number of normal, discocytic erythrocytes, and instead possessed, what seems to be, high levels of stomatocytes [162]. There are also indications of an increased erythrocyte sedimentation rate [[163], [164], [165], [166], [167]], although there are conflicting results [168]. Red blood cells from ME/CFS individuals exhibit reduced deformability, accompanied by diminished membrane fluidity [168]. This makes erythrocytes less pliable and stiffer, hindering efficient traversal through microcapillaries. This will impact the supply of oxygen to and retrieval of carbon dioxide from tissues and hinder blood flow (especially in capillaries where erythrocytes flow in a single file), which in turn might give rise to some symptoms associated with ME/CFS. Low erythrocyte volume might also contribute to shortcomings in circulation and oxygen delivery to tissues in ME/CFS [84]. Defective erythrocytes also have the ability to induce endothelial dysfunction [169].
With regards to the coagulation system, a significant hypercoagulable state was recognised in ME/CFS patients with active herpes infection [43]. However, over 80% of patients possessed hereditary risk factors for thrombosis (a finding which has not been exclusively identified in ME/CFS cohorts since), thereby limiting interpretive power. A different study demonstrated elevated fibrinogen levels, platelet hyperactivation, and hypercoagulability [170]. The authors also alluded to the notion of anomalous clot deposition on the endothelium which can impair substance exchange between blood and tissues, and subsequently give rise to ME/CFS symptoms. Bonilla and colleagues determined that extracellular vesicles from ME/CFS patients contained significantly elevated levels of the platelet marker, CD41a [171]. CD41a is a component of GPIIb/IIIa complex – the platelet receptor that binds fibrinogen and von Willebrand factor, and mediates platelet adhesion and aggregation, and clotting. Further indications of platelet hyperactivity come from a recent study using transmission electron microscopy, where significant platelet spreading and aggregation was documented in ME/CFS samples [172]. In contrast, a study from 2006 found neither platelet hyperactivation nor hypercoagulation in their ME/CFS cohort [173].
Recently, more attention has been directed towards ME/CFS due to the insights obtained from the COVID-19 pandemic. Some patients who become infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop a post-viral syndrome called Long COVID (or post-acute sequelae of COVID-19) [[174], [175], [176], [177]], of which the symptoms include: chronic fatigue that is unresolved with rest; PESE; cognitive dysfunction; sleep difficulties; OI; POTS; muscle and joint pain; headaches; flu-like symptoms; gastrointestinal issues; sensory impairments; and respiratory defects [[178], [179], [180]]. Long COVID patients respond negatively to exercise therapy, exhibiting adverse reactions much in the same manner as ME/CFS individuals [181]. Additionally, females are also more affected than males [182], as is observed in ME/CFS populations [183]. The effect of sex-specific physiology in the context of these two post-viral diseases is acknowledged [[184], [185], [186]], but requires further study and elucidation. The two syndromes exhibit striking similarities, so much so that many Long COVID patients meet the diagnostic criteria for ME/CFS [8,9].
It is widely accepted that COVID-19 is associated with severe micro- and macro-clotting pathology [[187], [188], [189], [190]], which is a major target of therapy in acute cases [191]. We have published findings of small amyloid fibrin clots, called fibrinaloids or microclots, as well as hypercoagulation and hyperactivated platelets, in Long COVID patients [180,192]. SARS-CoV-2 spike protein is sufficient to induce the anomalous clotting, that has an amyloid character [193], and can be induced with small amounts of a variety of initiators that bind to fibrinogen molecules [[194], [195], [196]].
Fibrinaloids are amyloid in nature and more resistant to fibrinolysis, and can be larger than the lumen of the smallest capillary [59,[193], [194], [195],197]. Hence, fibrinaloids have the potential to block microcapillaries [198] and impair oxygen delivery to tissues [59]. There is also evidence that anomalous clots formed by SARS-CoV-2 spike protein exhibit increased proinflammatory activity [197]. Using proteomics, we discovered that inflammatory molecules – including α(2)-antiplasmin and SAA – were ‘trapped within’/associated with fibrinaloids. α(2)-antiplasmin might be important for the persistence of fibrinaloids as its activity inhibits plasmin, an enzyme essential for fibrinolysis [199]. To add, the molecular phenotype of amyloid clots decreases the ability of fibrinolytic proteins to degrade fibrin as the interior of clots are less accessible [59,195,[200], [201], [202], [203]].
Because of the similarities between Long COVID and ME/CFS, we sought to investigate whether the clotting pathology observed in Long COVID patients is also present in individuals with ME/CFS. We recruited 25 individuals with ME/CFS and 15 age-matched controls, and obtained blood samples for both whole-blood (WB) and platelet-poor plasma (PPP) analyses. Our results show that ME/CFS PPP samples contain significantly greater levels of fibrinaloids when compared to controls [42]. The load of fibrinaloids in ME/CFS, however, seems to be lower than that of Long COVID. Fibrin networks – formed by the addition of thrombin to PPP – from the ME/CFS group contained significant amyloid fibrinogen, indicating pathology of terminal fibrin networks. The latter may interfere with hemostasis, leading to prolonged endothelial/vessel repair as a result of amyloid fibrin deposition on the endothelium and subsequent inflammation. Platelet hyperactivation, as determined by the degree of spreading and clumping, was also present in the ME/CFS population, although the degree of activation varied across participants. Thromboelastography (TEG) analysis of both WB and PPP samples demonstrated a high prevalence of hypercoagulability in the ME/CFS group. Together, these results demonstrate pathology in the coagulation system of individuals with ME/CFS, and that this pathology is mirrored in Long COVID. We have recently published a review proposing that ME/CFS and Long COVID pathology is a result of ischaemia-reperfusion injury [44], where fibrinaloids play a central role. Pertinently, Long COVID patients who were treated with antiplatelet and anticoagulant drugs (Clopidogrel, Aspirin, and Apixiban) experienced symptom relief [204] – whether this is a possibility or not for a majority of ME/CFS patients with such clotting pathology remains to be determined.
Over 20 years ago, Berg and colleagues reported hypercoagulability and platelet hyperactivation in an ME/CFS cohort, and alluded to the idea that small (anomalous) fibrin aggregates adhere to the endothelium and impair the exchange of substances between the blood and tissue, subsequently giving rise to symptoms [170]. The latter – in light of our findings [42] – can now be interpreted as fibrinaloids. Fibrin(gen) binds to several endothelial receptors and hence can modulate their activity [[205], [206], [207]] – it is of interest to determine the mechanistic differences between normal fibrin and fibrinaloids/amyloid fibrinogen in an endothelial context. Amyloid-type molecules have a tendency to damage lipid membranes [208] and might account for lipid peroxidation [104] and endothelial dysfunction [24,166,209] observed in ME/CFS. Furthermore, fibrinaloids are proinflammatory and persist more than normal fibrin matter due to their fibrinolytic resistance [192,197]. These are characteristics that can exaggerate any pathological impact on the endothelium. It is known that ME/CFS plasma induces endothelial dysfunction in healthy cells [209,210] – further analysis is required to determine if fibrinaloids are largely responsible for this phenomenon.
4. The endothelium - An interface for symptom manifestation?
Endothelial dysfunction is a prominent component of cardiovascular disease [211,212], and is prompted by an increase in O&NS and inflammation [[213], [214], [215]]. Endothelial cells are important for vessel regulation whereby nitric oxide and endothelin synthesis and release modulate constriction and dilation activity. Hence, changes in the regulation of vessel modulators from endothelial cells are implicated in cardiovascular disease [[216], [217], [218]]. Furthermore, the endothelium is responsible for enabling the transfer of substances across the vessel wall; a damaged endothelium is expected to lead to impairments in substance exchange in localised areas [219,220].
In Long COVID, endothelial dysfunction is a common finding amongst patients and has been centralized in disease hypotheses [44,58,59,[221], [222], [223], [224]]. Reduced tissue perfusion and oxygenation at the capillary level, resulting from coagulopathy and endotheliopathy [192,225], is one of the proposed mechanisms for symptom manifestation in Long COVID [59,198,226]. Targeting endotheliopathy in Long COVID has shown benefit in some cases [224,227,228], and is therefore receiving further exploration. This highlights the question of whether or not endothelial dysfunction is involved in ME/CFS pathology and symptom manifestation.
A study examining endothelial function in ME/CFS has revealed peripheral endothelial dysfunction in 51% (18/35) of subjects [335]. Patients with endothelial dysfunction also reported worse symptom scores than those without. Studies using flow-mediated dilation (FMD) and post-occlusive reactive hyperaemia have described endothelial dysfunction in both large and small vessels in ME/CFS cohorts [24,229]. MicroRNA markers associated with endothelial dysfunction have also been implicated [166].
Human umbilical vein endothelial cells exposed to plasma from ME/CFS individuals show significant reductions in synthesized and secreted nitric oxide in the absence or presence of endothelial nitric oxide synthase (eNOS) stimulators [209], therefore pointing to a defective enzymatic function of eNOS. Furthermore, inhibitory phosphorylation of eNOS at Thr495 was greater as a result of ME/CFS plasma than it was with control plasma. Identifying the plasma constituent responsible for the aforementioned effects is of utmost importance, as it may lead to the identification and annotation of pathological mechanisms involved in ME/CFS, and, hopefully, biomarker establishment. Furthermore, targeting (or replacing) this unknown molecule(s) in a therapeutic manner might offer symptomatic relief. These studies emphasize the potential of investigating the haematological system in ME/CFS.
Similarly, another study that exposed ME/CFS sera to healthy endothelial cells reported the release of molecules that inhibit nitric oxide pathways, as well as the downregulation of endothelial activation markers [210]. The researchers also revealed an increase in autoantibody binding to endothelial surfaces in the ME/CFS group, which they sought to investigate due to evidence of autoimmunity in ME/CFS [230]. Whether antibody-dependent cellular cytotoxicity is mounted against the healthy endothelial cells is still to be determined, but the finding suggests that autoimmune processes and impairments of the endothelium are linked. It might also be a possibility that this autoantibody binding to the endothelium induces procoagulant cascades, perhaps via the complement system or interaction with platelets [170,231].
Endothelin-1, a potent vasoconstrictor that is released by endothelial cells and involved in cardiovascular pathology [232], was shown to be significantly increased in 5/14 COVID-19 patients who have ME/CFS [58]. The dysregulation of these vaso-modulators – endothelins and nitric oxide – might contribute to impairments in blood flow in ME/CFS. To add, regional cerebral blood flow and endothelial dysfunction have been linked [233]. Whether endothelial dysfunction underlies the abnormalities of cerebral perfusion in ME/CFS remains to be determined. A recent study published in 2023 has further corroborated endothelial dysfunction in ME/CFS [234].
Endothelial dysfunction and the consequences that follow, including reduced substance delivery to tissues and hypoxia [220,235], might lead to systemic defects that bring about symptom manifestation. Indeed, endothelial dysfunction and reduced tissue perfusion has recently been implicated in hypotheses for ME/CFS pathology [44,45,236]. More research is required to elucidate the status and role of the endothelium in ME/CFS.
5. Viruses: How are they involved, and where are they hiding?
The overlap between ME/CFS and Long COVID brings into the spotlight (if not already in the spotlight) the role of viral infection in ME/CFS initiation and maintenance. The etiology of Long COVID can be confidently attributed to SARS-CoV-2 infection, and since Long COVID clinically presents much in the same way as ME/CFS, the two diagnoses may share similar etiology. There is now even more reason to believe that viruses cause, or at least have a major role to play in pathogenesis of post-viral, fatigue-like illnesses like ME/CFS. An elucidation of the mechanisms whereby SARS-CoV-2 contributes to Long COVID pathology may consequently inform the ME/CFS disease process.
Although multiple viral species have been implicated in ME/CFS [3,29,31,61,237], a single pathogenic specie has not yet been identified in all ME/CFS patients within a particular cohort. However, few teams have studied persistent infection in ME/CFS with advanced technologies capable of identifying low biomass organisms in tissue and/or associated gene expression patterns. To add, the viruses implicated in ME/CFS – predominantly the herpes viruses (EBV, human cytomegalovirus, HHV-6, and HHV-7) [3,31] – are common infectious agents within the general population, which increases the difficulty of identifying a causative role for these pathogens in ME/CFS individuals.
Herpes viruses are capable of establishing life-long latency in human tissue and reactivate spontaneously or when immune function is impaired [[238], [239], [240]]. EBV, HHV-6, HHV-7, and human cytomegalovirus can remain dormant in mononuclear cells, including monocytes, T-cells, and B-cells, and reactivate to infect other cells or hosts [240]. EBV expresses a particular affinity for B-cells, with latency in this cell-type well characterized [[241], [242], [243]]. It must be noted that reactivation of herpesviruses is not always associated with disease, and often occurs without any noticeable symptoms. Hence, this emphasizes the complexity associated with herpesvirus reactivation and diseased states, such as ME/CFS. The specifics of herpesvirus latency, reactivation, and therapeutics is reviewed elsewhere [240,[244], [245], [246]].
Incidentally, SARS-CoV-2 infection leads to the reactivation of herpesviruses, specifically EBV [[247], [248], [249]]. Reactivation of these viruses and the subsequent maladaptation of physiological systems, including the immune, endocrine, and nervous system, are believed to be important steps in ME/CFS pathology [3,60,250]. In a study from 2019, 38% of ME/CFS patients exhibited an upregulation of the Epstein–Barr virus (EBV) induced gene 2 (EBI2) in PBMCs [251], suggestive of EBV reactivation [252]. A follow-up study provided further indications that this gene is upregulated as a result of EBV activity in ME/CFS [237]. Furthermore, B-cells – the cell-type favoured by EBV – have been noted to be dysfunctional in ME/CFS studies [[253], [254], [255]] – the thinking goes that latent (or active) EBV infection is responsible for this B-cell dysfunction. Furthermore, autoimmunity driven by defective B-cell functioning is suspected to bring about autoreactivity which in turn contributes to symptom manifestation [127,230,256,257]. There are many other studies demonstrating increased antibody levels directed at herpesviruses (not only EBV) in ME/CFS [30,[258], [259], [260], [261], [262], [263]]. More recent studies have identified herpesvirus nucleic acids and antigens, in significant concentrations, in ME/CFS tissue [32,60,263,264]. Rasa- Dzelzkaleja et al (2023) showed that 45% of ME/CFS individuals within their study were experiencing reactivation of herpesviruses (HHV-6 and HHV-7), and that these individuals expressed higher proinflammatory markers (IL-6, TNF-α) than those ME/CFS individuals with latent infection. While more work is needed to effectively define the mechanisms associated with herpesvirus-related ME/CFS pathology, there is a general acceptance that herpesviruses play an important role in the pathogenesis of this disease. ME/CFS patients with reactivated herpesvirus (HHV-6 and HHV-7) infection were treated with antiviral drugs which resulted in some degree of success (less than 50% within each group of patients presented with negative PCR results following treatment) [265].
Infection with herpesviruses leads to the production of proinflammatory cytokines and impairment of immune cell function [[266], [267], [268], [269], [270]]. In ME/CFS, both an increase in proinflammatory cytokines [271] and a reduction in natural killer (NK) cell activity (cytotoxicity) are present [272]. Well known is it that chronic inflammation is central to a variety of diseases, including cardiovascular disease, cancer, diabetes, and psoriasis [196,[273], [274], [275]]. However, the cardiovascular abnormalities observed in ME/CFS patients do not seem to be ‘classical’, i.e. atherosclerotic in nature, but rather manifest as reduced cardiac function (as inferred from findings of reduced stroke volume and cardiac output) [88,119,152]. Defects in autonomic control of the heart and blood vessels are believed to underlie these cardiac abnormalities [149,154]. Relevantly, the role of pathogens (including herpesviruses) in the induction of autonomic dysfunction, predominantly in a cardiovascular context, has been reviewed [276] and might influence cardiac function, indirectly, in ME/CFS.
Infection of nervous tissue by herpes viruses can lead to inflammation, cellular dysfunction, and sometimes severe complications like meningitis [[277], [278], [279], [280]]. With regards to the autonomic nervous system, EBV infection has previously been shown to exist alongside acute autonomic neuropathy [[281], [282], [283], [284], [285]], as well as orthostatic symptoms [281,286]. Furthermore, infectious mononucleosis – a disease predominantly caused by EBV – is accompanied by long-lasting autonomic symptoms [287], suggestive of persistent infection or neurological maladaptation. A pathogenic protein produced by EBV, deoxyuridine triphosphate nucleotidohydrolase (dUTPase), can alter gene expression in glial and endothelial cells in a manner which can promote neuroinflammation and potentially symptom manifestation [288], and has been implicated (immunologically) in an ME/CFS cohort [30]. Interestingly, a hypothesis involving neuroglia dysfunction in ME/CFS has recently been published [147].
Defective autonomic functioning, neuropathy, and orthostatic symptoms have also been observed following human cytomegalovirus infection [289,290], HHV-6 infection [291], and SARS-CoV-2 infection [292,293]. To further implicate herpes viruses in neuronal tissue, and specifically in ME/CFS, a recent study found significant levels of EBV and HHV-6 microRNA in brain and spinal cord tissue from deceased ME/CFS individuals [32]. Ultimately, there is evidence to suggest that (herpes) viruses might, via the infection of nervous tissue and subsequent impairment of autonomic functioning, account, in part, for the cardiac abnormalities observed in ME/CFS individuals; although more research is required with regards to this topic.
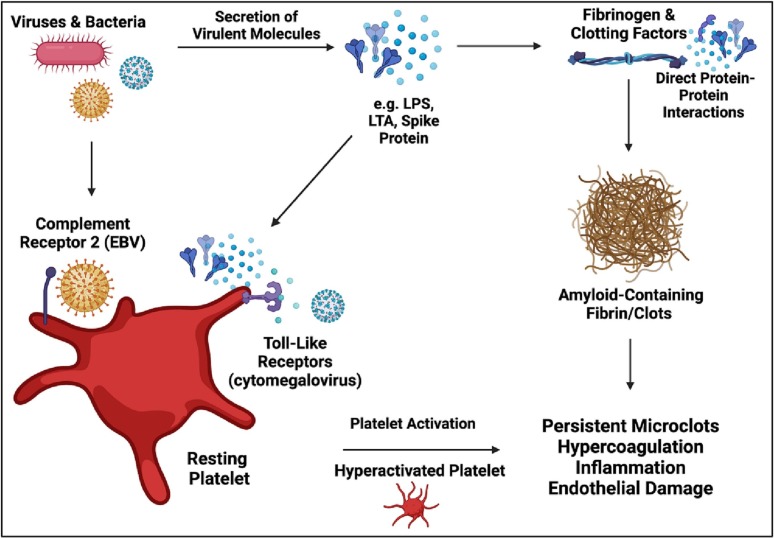
In the context of the coagulation system, molecular products from viruses and bacteria have the ability to influence platelets and clotting proteins (refer to Fig. 1 ) [161,194,195,200,[294], [295], [296], [297]]. Both lipopolysaccharide and lipoteichoic acid can induce anomalous, amyloid-containing clots that are distinctly different from healthy clots [161,193,195,200]. Furthermore, both of these bacterial inflammagens can directly interact with platelet receptors, modulate platelet activity, and induce hypercoagulability, ultimately leading to a prothrombotic state [159,200,294,298,299]. Conversely, gingipain R1, a protease from a periodontal pathogen named Porphyromonas gingivalis, can degrade clots and inhibit enzymatic formation of fibrin networks [161].
Fig. 1.
The influence of microbes on the coagulation system. Microbes and their secreted molecules can directly interact with platelets and clotting proteins (clotting factors and fibrinogen) to induce platelet activation, hypercoagulation, inflammation, anomalous clotting (amyloid containing clots and fibrinaloids), and subsequent endothelial damage. Herpes viruses, including EBV and cytomegalovirus, interact with platelets via toll-like and complement receptors. Created using Biorender.com.
For long it has been known that viruses can influence coagulation, via direct interaction with clotting proteins and platelets [[300], [301], [302], [303], [304]]. SARS-CoV-2 causes severe clotting pathology, reflected by hyperactivated platelets, hypercoagulability, and fibrinaloid microclots [192,305,306]. We assessed whether the spike protein S1 subunit from SARS-CoV-2 virus can induce fibrinaloid formation [194], from which it was confirmed that spike protein induces fibrinaloid formation in control plasma samples which lack fibrinaloids in their naïve state – this finding has been corroborated [197]. Specifically, the spike protein S1 subunit induced structural modifications in the β and γ fibrinogen chains, as well as prothrombin. The latter may lead to activation of the zymogen, and subsequent (defective) conversion of fibrinogen into fibrinaloids. We note too that spike itself is potentially amyloidogenic [307]. As fibrinaloids are present in ME/CFS individuals [42], albeit to a lesser extent than observed in Long COVID cohorts, the question of which agent or agents are responsible for the induction of fibrinaloids in ME/CFS arises.
It is plausible to hypothesize that viruses (and potentially other microbes) are contributing to the clotting pathology observed in ME/CFS individuals [42,43,[170], [171], [172]]. Herpes viruses, the virus types most implicated in ME/CFS, are known to influence coagulation in a prothrombotic manner [[308], [309], [310]]. EBV infection has been associated with disseminated intravascular coagulation [311,312], and cytomegalovirus can induce hypercoagulation [[313], [314], [315], [316], [317]]. These two herpes viruses also interact with platelets via a number of platelet receptors, including toll-like receptors and complement receptors [[318], [319], [320], [321]]. Hence, there is reason to hypothesize that herpes viruses are responsible, to a certain extent, for clotting dysfunction observed in ME/CFS individuals.
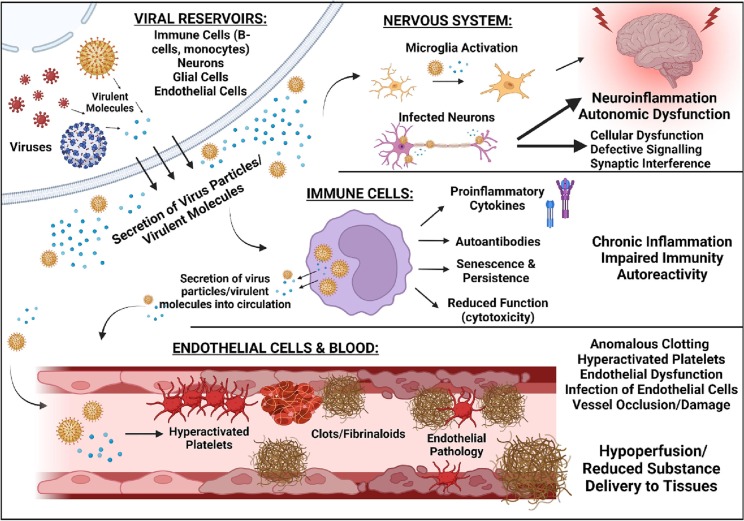
Next, where are the viruses? In Long COVID, they are widely distributed [34,35]. ME/CFS harbours viral reservoirs, where reactivation and virulent molecule secretion might underlie pathology. A recent study has identified significant levels of EBV and HHV-6 microRNA in the central nervous system of deceased ME/CFS individuals [32], which points at active infection in the brain and spinal cord. It is known that EBV favours B-cells for infection [241,243]; the finding of EBV infection in the brain might be indicative of EBV reactivation in and shedding of virus particles and proteins from B-cells – perhaps EBV viruses have ‘spilled over’ into other physiological systems. Microglial activation and inflammatory sequelae can ensue following herpes infection [[322], [323], [324]], and may contribute to neuroinflammation and autonomic dysfunction observed in ME/CFS. The endothelium is an infection site for herpes viruses [313,317,325], and constitutes a site from which clotting pathology can be easily orchestrated. Monocytes, a reservoir site from which the coagulation system can also be influenced from, act as viral reservoirs for herpes viruses too [326,327], as they do for SARS-CoV-2 proteins in Long COVID patients [35]. These monocytes exhibit senescence, which is believed to enable their persistence in circulation [35]. Furthermore, the gut microbiome is not exempt from scrutiny [328]. Whilst further research on viral persistence in ME/CFS is required, we aim to emphasize the idea that viral reservoirs and their subsequent influence on host physiology might be responsible for the maintenance of ME/CFS, Long COVID, and other post-viral syndromes. In light of what has been discussed, Fig. 2 represents a depiction of viral persistence and subsequent influence of various physiological systems in ME/CFS.
Fig. 2.
Possible roles of viral persistence in ME/CFS, with an emphasis on the haematological, immune, and neurological system. Virus particles and/or virulent molecules are secreted from viral reservoirs (immune cells like B-cells and monocytes, endothelial cells, and neurons). Infection of neurological tissue might lead to autonomic dysfunction and subsequently the cardiovascular abnormalities observed in ME/CFS. Infection of immune cells can result in cellular senescence, reduced cell function (e.g. cytotoxicity of NK cells), and inflammatory signalling which might prompt chronic inflammation; furthermore, infected immune cells can release virus particles and/or virulent molecules into circulation. The endothelium might also act as a reservoir, where subsequent endothelial dysfunction will ensue. Secretion of virus particles and molecules into circulation can interfere with platelets and clotting machinery to influence coagulation. Hyperactivated platelets, hypercoagulability, anomalous clotting, inflammation, and O&NS stress induced by viral activity will induce further endothelial damage, possibly to the extent whereby substance exchange between blood and tissues is impaired. Created using Biorender.com.
6. Conclusions and future directions
ME/CFS is a complex disease intimately related to viral infection [3,31] and is accompanied by abnormalities in multiple physiological systems with specific defects in metabolism and cellular energetics [1,329]. Whilst ME/CFS has been studied for many decades, clinical success is underwhelming. Insight can be borrowed from the COVID-19 pandemic, as there are many similarities between the post-viral syndrome associated with SARS-CoV-2, Long COVID, and ME/CFS [52,53,179,330]. Long COVID is a disease characterized by vascular pathology [59,132,178,192,197,204,331,332]. With that in mind, and the fact that some individuals who contract COVID-19 develop chronic symptoms that meet the diagnostic criteria for ME/CFS [[7], [8], [9]], there is reason to suspect that vascular pathology is also involved in ME/CFS. Prompted by the overlap between Long COVID and ME/CFS, in this review, we sought to summarize the cardiovascular and haematological findings associated with ME/CFS.
The literature suggests cardiovascular dysfunction and haematological abnormalities are present in ME/CFS populations, although certain studies require further corroboration with larger sample sizes. Whilst primary cardiovascular disease is not considered as a component of ME/CFS, research has revealed that afflicted individuals have weakened and dysregulated cardiovascular function, as indicated by findings of reduced stroke volume and cerebral blood flow, and vascular dysregulation. Deconditioning appears to not be the cause of the cardiovascular abnormalities observed in ME/CFS. Neurological defects, particularly in the autonomic branch of the nervous system, are believed to underlie this cardiovascular dysfunction [154]. It might be true that the shortcomings in cardiac function and blood flow regulation contribute to functional deficits and the manifestation of symptoms in patients. Pyridostigmine can improve cardiac function in ME/CFS individuals [153], and hence awaits further determination of its efficacy.
There are also indications of platelet hyperactivity, defects in erythrocyte biomechanics, endothelial dysfunction, and clotting dysregulation in ME/CFS. Novel findings of fibrinaloids (anomalous, amyloid microclots) in ME/CFS plasma samples, which tie Long COVID and ME/CFS even closer together, demand further corroboration and study. Such coagulopathy and associated endothelial damage, along with shortcomings in cardiovascular function, might be responsible for symptom manifestation via inefficient transportation of substances between capillaries and tissue, and perhaps via ischemia-reperfusion injury [44,220,236]. Indeed, there are signs of reduced oxygen delivery in ME/CFS [152,333,334]. Treatment of fibrinaloids and other clotting pathology is an option [204], but further corroboration and detailing of the clotting pathology in ME/CFS are required before this sort of therapy is considered.
The microvasculature and endothelium are becoming important focal points in ME/CFS and Long COVID research [44,59,236]. Suboptimal tissue perfusion as a result of endothelial pathology deserves further attention [220]. Implications of endothelial damage have long ago been proposed as a component of ME/CFS pathology that drives symptom manifestation [170], but is only now starting to receive due attention. The idea of endothelial dysfunction driving ME/CFS seems more plausible after acknowledging such pathology in Long COVID [58,198,210,[221], [222], [223],226,228]; not to mention the evidence of endothelial dysfunction in ME/CFS [24,45,166,209,210,229,233,236,335]. Further exploration of the vasculature, cardiac functioning, and coagulation system in ME/CFS is therefore warranted.
Lastly, the role of viruses in ME/CFS requires deeper scrutiny. Viral infections in ME/CFS is not news [3,31], but one could argue that they received less attention than deserved. It now seems that idea of viruses as causative and driving agents of ME/CFS is more likely to be true, especially when one considers the etiology of Long COVID and its symptomatic similarity to ME/CFS. Novel research has identified viral reservoir sites in both ME/CFS [32] and Long COVID [34,35], namely, the central nervous system and immune cells. The endothelium is also an infection site of herpes viruses [313], and, along with monocytes, pose as convenient locations to prompt clotting pathology from. Study is required to detail any herpesvirus-induced synthesis of fibrinaloids – as they are induced by spike protein from SARS-CoV-2 [194]. Furthermore, identifying the paths/mechanisms from acute COVID-19 infection to Long COVID might lead to substantial advancement in our understanding of ME/CFS.
7. Practice points
-
•
The evidence presented in this review resonates with the notion that ME/CFS is characterized by physiological pathology, and not psychosomatic illness. This is a biologically-driven disease characterized by vascular (including haematological) pathology.
-
•
Assessment of cardiovascular (specifically cardiac functioning) and haematological health are necessary steps in the clinical evaluation of ME/CFS patients.
-
•
Deconditioning does not seem to be responsible for the symptoms of ME/CFS.
-
•
The coagulation system and endothelium is becoming more and more implicated in ME/CFS; perhaps these systems are more involved in ME/CFS than previously suspected.
-
•
Viruses are heavily involved in ME/CFS pathology, and their role in causing in ME/CFS seems more likely when scrutinizing the etiology of the similarly-presenting Long COVID – elucidation of the mechanisms of how SARS-CoV-2 leads to Long COVID may advance ME/CFS knowledge.
8. Research agenda
-
•
Future studies need to expand on the involvement of the cardiovascular and haematological system in ME/CFS pathology, and determine to what extent these systems and dysfunction thereof contributes to symptom manifestation.
-
•
The cardiac and vascular dysfunction observed in ME/CFS individuals is atypical in the sense that it is non-atherosclerotic heart disease; it seems that neurological (autonomic) dysfunction underlies these abnormalities – mechanisms need to be unveiled, and therapeutics trialled in this neurological context, especially since orthostatic symptoms greatly affect the functional capabilities of patients.
-
•
Given the complexity of ME/CFS, research and clinical efforts will require collaborative multidisciplinary involvement, that include virologists, cardiologists, neurologists, and haematologists.
-
•
There is an urgent need for biomarker establishment in ME/CFS; further investigation of the physiological systems discussed in this review may help aid in this quest, especially since these systems (or aspects thereof) are becoming more and more implicated in ME/CFS research.
Author contributions
J.M.N.: Wrote the paper. E.P.: Edited the paper, funding, co-corresponding author; study leader; D.B.K.: Edited the paper, co-corresponding author. All authors have read and agreed to the published this version of the manuscript.
Acknowledgements and funding
D.B.K. thanks the Novo Nordisk Foundation for funding (grant NNF10CC1016517). E.P. thanks PolyBio Research Foundation for funding, the NRF of South Africa (grant number 142142) and SA MRC (self-initiated research (SIR) grant). The content and findings reported and illustrated are the sole deduction, view and responsibility of the researchers and do not reflect the official position and sentiments of the funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Cortes Rivera M., Mastronardi C., Silva-Aldana C.T., Arcos-Burgos M., Lidbury B.A. Myalgic encephalomyelitis/chronic fatigue syndrome: a comprehensive review. Diagnostics (Basel). 2019:9. doi: 10.3390/diagnostics9030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T., et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasa S., Nora-Krukle Z., Henning N., Eliassen E., Shikova E., Harrer T., et al. Chronic viral infections in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2018;16:268. doi: 10.1186/s12967-018-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underhill R.A. Myalgic encephalomyelitis, chronic fatigue syndrome: an infectious disease. Med Hypotheses. 2015;85:765–773. doi: 10.1016/j.mehy.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Jason L.A., Mirin A.A. Updating the National Academy of Medicine ME/CFS prevalence and economic impact figures to account for population growth and inflation. Fatigue: Biomedicine, Health & Behavior. 2021;9:9–13. [Google Scholar]
- 6.Mirin A.A., Dimmock M.E., Jason L.A. Updated ME/CFS prevalence estimates reflecting post-COVID increases and associated economic costs and funding implications. Fatigue: Biomedicine, Health & Behavior. 2022:1–11. [Google Scholar]
- 7.Petracek L.S., Suskauer S.J., Vickers R.F., Patel N.R., Violand R.L., Swope R.L., et al. Adolescent and Young adult ME/CFS after confirmed or probable COVID-19. Front Med (Lausanne). 2021;8 doi: 10.3389/fmed.2021.668944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kedor C., Freitag H., Meyer-Arndt L., Wittke K., Hanitsch L.G., Zoller T., et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. 2022;13:5104. doi: 10.1038/s41467-022-32507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedor C., Freitag H., Meyer-Arndt L., Wittke K., Zoller T., Steinbeis F., et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany – a first analysis of a prospective observational study. medRxiv. 2021 2021.02.06.21249256. [Google Scholar]
- 10.Tokumasu K., Honda H., Sunada N., Sakurada Y., Matsuda Y., Yamamoto K., et al. 2022. Clinical Characteristics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Diagnosed in Patients with Long COVID. Medicina (Kaunas) p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk Hvidberg M., Brinth L.S., Olesen A.V., Petersen K.D., Ehlers L. The health-related quality of life for patients with Myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) PloS One. 2015;10 doi: 10.1371/journal.pone.0132421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon L., Reeves W.C. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. 2004;164:2241–2245. doi: 10.1001/archinte.164.20.2241. [DOI] [PubMed] [Google Scholar]
- 13.Araja D., Berkis U., Lunga A., Murovska M. Shadow burden of undiagnosed Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) on society: retrospective and prospective—in light of COVID-19. J Clin Med. 2021;10:3017. doi: 10.3390/jcm10143017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannan K.L., Berg D.E., Baumzweiger W., Harrison H.H., Berg L.H., Ramirez R., et al. Activation of the coagulation system in gulf war illness: a potential pathophysiologic link with chronic fatigue syndrome. A laboratory approach to diagnosis. Blood Coagul Fibrinolysis. 2000;11:673–678. doi: 10.1097/00001721-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda S., Kuratsune H., Kajimoto O., Watanabe Y. Fatigue-related problem scale for better understanding of pathophysiology of chronic fatigue syndrome and fibromyalgia. Advances in Neuroimmune Biology. 2012;3:361–366. [Google Scholar]
- 16.Helliwell A.M., Sweetman E.C., Stockwell P.A., Edgar C.D., Chatterjee A., Tate W.P. Changes in DNA methylation profiles of Myalgic encephalomyelitis/chronic fatigue syndrome patients reflect systemic dysfunctions. Clin Epigenetics. 2020;12:167. doi: 10.1186/s13148-020-00960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth K., Scheibenbogen C. A unifying hypothesis of the pathophysiology of Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): recognitions from the finding of autoantibodies against ss2-adrenergic receptors. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102527. [DOI] [PubMed] [Google Scholar]
- 18.Chu L., Valencia I.J., Garvert D.W., Montoya J.G. Onset patterns and course of Myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. 2019;7:12. doi: 10.3389/fped.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bested A.C., Marshall L.M. Review of Myalgic encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30:223–249. doi: 10.1515/reveh-2015-0026. [DOI] [PubMed] [Google Scholar]
- 20.Clayton E.W. Beyond Myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA. 2015;313:1101–1102. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds G.K., Lewis D.P., Richardson A.M., Lidbury B.A. Comorbidity of postural orthostatic tachycardia syndrome and chronic fatigue syndrome in an Australian cohort. J Intern Med. 2014;275:409–417. doi: 10.1111/joim.12161. [DOI] [PubMed] [Google Scholar]
- 22.Freitag H., Szklarski M., Lorenz S., Sotzny F., Bauer S., Philippe A., et al. Autoantibodies to Vasoregulative G-protein-coupled receptors correlate with symptom severity, autonomic dysfunction and disability in Myalgic encephalomyelitis/chronic fatigue syndrome. J Clin Med. 2021:10. doi: 10.3390/jcm10163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costigan A., Elliott C., McDonald C., Newton J.L. Orthostatic symptoms predict functional capacity in chronic fatigue syndrome: implications for management. QJM. 2010;103:589–595. doi: 10.1093/qjmed/hcq094. [DOI] [PubMed] [Google Scholar]
- 24.Newton D.J., Kennedy G., Chan K.K., Lang C.C., Belch J.J., Khan F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int J Cardiol. 2012;154:335–336. doi: 10.1016/j.ijcard.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Cambras T., Castro-Marrero J., Zaragoza M.C., Diez-Noguera A., Alegre J. Circadian rhythm abnormalities and autonomic dysfunction in patients with chronic fatigue syndrome/Myalgic encephalomyelitis. PloS One. 2018;13 doi: 10.1371/journal.pone.0198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missailidis D., Annesley S.J., Fisher P.R. Pathological mechanisms underlying Myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics (Basel) 2019:9. doi: 10.3390/diagnostics9030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Campen C., Verheugt F.W.A., Rowe P.C., Visser F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: a quantitative, controlled study using Doppler echography. Clin Neurophysiol Pract. 2020;5:50–58. doi: 10.1016/j.cnp.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth K.J., Scheibenbogen C. Pathophysiology of skeletal muscle disturbances in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2021;19:162. doi: 10.1186/s12967-021-02833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chia J., Chia A., Voeller M., Lee T., Chang R. Acute enterovirus infection followed by Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J Clin Pathol. 2010;63:165–168. doi: 10.1136/jcp.2009.070466. [DOI] [PubMed] [Google Scholar]
- 30.Cox B.S., Alharshawi K., Mena-Palomo I., Lafuse W.P., Ariza M.E. EBV/HHV-6A dUTPases contribute to Myalgic encephalomyelitis/chronic fatigue syndrome pathophysiology by enhancing TFH cell differentiation and extrafollicular activities. JCI. Insight. 2022:7. doi: 10.1172/jci.insight.158193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ariza M.E. Myalgic encephalomyelitis/chronic fatigue syndrome: the human herpesviruses are Back! Biomolecules. 2021:11. doi: 10.3390/biom11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasimir F., Toomey D., Liu Z., Kaiping A.C., Ariza M.E., Prusty B.K. Tissue specific signature of HHV-6 infection in ME/CFS. Front Mol Biosci. 2022:9. doi: 10.3389/fmolb.2022.1044964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buonsenso D., Piazza M., Boner A.L., Bellanti J.A. Long COVID: a proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 2022;43:187–193. doi: 10.2500/aap.2022.43.220018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein S.R., Ramelli S.C., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022 doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson B.K., Francisco E.B., Yogendra R., Long E., Pise A., Rodrigues H., et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in Post-acute sequelae of COVID-19 (PASC) up to 15 months Post-infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., et al. COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abf8396. eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa D.C., Tannock C., Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. Qjm. 1995;88:767–773. [PubMed] [Google Scholar]
- 38.Li X., Julin P., Li T.Q. Limbic perfusion is reduced in patients with Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Tomography. 2021;7:675–687. doi: 10.3390/tomography7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson G., Maes M. Mitochondria and immunity in chronic fatigue syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2020;103 doi: 10.1016/j.pnpbp.2020.109976. [DOI] [PubMed] [Google Scholar]
- 40.Missailidis D., Annesley S.J., Allan C.Y., Sanislav O., Lidbury B.A., Lewis D.P., et al. An isolated complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holden S., Maksoud R., Eaton-Fitch N., Cabanas H., Staines D., Marshall-Gradisnik S. A systematic review of mitochondrial abnormalities in Myalgic encephalomyelitis/chronic fatigue syndrome/systemic exertion intolerance disease. J Transl Med. 2020;18:290. doi: 10.1186/s12967-020-02452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes J.M., Kruger A., Proal A., Kell D.B., Pretorius E. The occurrence of Hyperactivated platelets and Fibrinaloid microclots in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Pharmaceuticals. 2022;15:931. doi: 10.3390/ph15080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brewer J.H., Berg D. Hypercoaguable state associated with active human Herpesvirus-6 (HHV-6) viremia in patients with chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome. 2001;8:111–116. [Google Scholar]
- 44.Kell D.B., Pretorius E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J. 2022;479:1653–1708. doi: 10.1042/BCJ20220154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirth K.J., Scheibenbogen C., Paul F. An attempt to explain the neurological symptoms of Myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2021;19:471. doi: 10.1186/s12967-021-03143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Proal A.D., VanElzakker M.B. Pathogens hijack host cell metabolism: intracellular infection as a driver of the Warburg effect in cancer and other chronic inflammatory conditions. Immunometabolism. 2021;3 [Google Scholar]
- 47.Shan Z.Y., Kwiatek R., Burnet R., Del Fante P., Staines D.R., Marshall-Gradisnik S.M., et al. Progressive brain changes in patients with chronic fatigue syndrome: a longitudinal MRI study. J Magn Reson Imaging. 2016;44:1301–1311. doi: 10.1002/jmri.25283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakatomi Y., Kuratsune H., Watanabe Y., et al. Brain Nerve. 2018;70:19–25. doi: 10.11477/mf.1416200945. [DOI] [PubMed] [Google Scholar]
- 49.Finkelmeyer A., He J., Maclachlan L., Watson S., Gallagher P., Newton J.L., et al. Grey and white matter differences in chronic fatigue syndrome - a voxel-based morphometry study. Neuroimage Clin. 2018;17:24–30. doi: 10.1016/j.nicl.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris G., Maes M. A neuro-immune model of Myalgic encephalomyelitis/chronic fatigue syndrome. Metab Brain Dis. 2013;28:523–540. doi: 10.1007/s11011-012-9324-8. [DOI] [PubMed] [Google Scholar]
- 51.Mackay A. A neuro-inflammatory model can explain the onset, symptoms and flare-ups of Myalgic encephalomyelitis/chronic fatigue syndrome. J Prim Health Care. 2019;11:300–307. [Google Scholar]
- 52.Morrow A.K., Malone L.A., Kokorelis C., Petracek L.S., Eastin E.F., Lobner K.L., et al. Long-term COVID 19 sequelae in adolescents: the overlap with orthostatic intolerance and ME/CFS. Curr Pediatr Rep. 2022:1–14. doi: 10.1007/s40124-022-00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong T.L., Weitzer D.J. 2021. Long COVID and Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas) p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang M., Li M.D., Buch K., Yoon B.C., Applewhite B.P., Leslie-Mazwi T.M., et al. Risk of acute cerebrovascular events in patients with COVID-19 infection. AJNR Am J Neuroradiol. 2020;41 doi: 10.3174/ajnr.A6796. E92-E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pretorius E., Venter C., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B. Prevalence of readily detected amyloid blood clots in ‘unclotted’ type 2 diabetes mellitus and COVID-19 plasma: a preliminary report. Cardiovasc Diabetol. 2020;19:193. doi: 10.1186/s12933-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haffke M., Freitag H., Rudolf G., Seifert M., Doehner W., Scherbakov N., et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J Transl Med. 2022;20:138. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kell D.B., Laubscher G.J., Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479:537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J.S., Lacerda E.M., Nacul L., Kingdon C.C., Norris J., O’Boyle S., et al. Salivary DNA loads for human herpesviruses 6 and 7 are correlated with disease phenotype in Myalgic encephalomyelitis/chronic fatigue syndrome. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.656692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shikova E., Reshkova V., Kumanova Capital A.C., Raleva S., Alexandrova D., Capo N., et al. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with Myalgic small ie, Cyrillicncephalomyelitis/chronic fatigue syndrome. J Med Virol. 2020 doi: 10.1002/jmv.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jason L.A., Corradi K., Gress S., Williams S., Torres-Harding S. Causes of death among patients with chronic fatigue syndrome. Health Care Women Int. 2006;27:615–626. doi: 10.1080/07399330600803766. [DOI] [PubMed] [Google Scholar]
- 63.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. 2009;30:470–476. [PubMed] [Google Scholar]
- 64.McManimen S.L., Devendorf A.R., Brown A.A., Moore B.C., Moore J.H., Jason L.A. Mortality in patients with Myalgic encephalomyelitis and chronic fatigue syndrome. Fatigue. 2016;4:195–207. doi: 10.1080/21641846.2016.1236588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bond J., Nielsen T., Hodges L. Effects of post-exertional malaise on markers of arterial stiffness in individuals with Myalgic encephalomyelitis/chronic fatigue syndrome. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18052366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43:1157–1172. doi: 10.1093/eurheartj/ehac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maes M., Twisk F.N. Why Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuro Endocrinol Lett. 2009;30:677–693. [PubMed] [Google Scholar]
- 68.Domingo J.C., Cordobilla B., Ferrer R., Giralt M., Alegre-Martín J., Castro-Marrero J. Are circulating fibroblast growth factor 21 and N-terminal prohormone of brain natriuretic peptide promising novel biomarkers in Myalgic encephalomyelitis/chronic fatigue syndrome? Antioxid Redox Signal. 2021;34:1420–1427. doi: 10.1089/ars.2020.8230. [DOI] [PubMed] [Google Scholar]
- 69.Maes M., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Increased plasma peroxides as a marker of oxidative stress in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Med Sci Monit. 2011;17 doi: 10.12659/MSM.881699. Sc11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armstrong C.W., McGregor N.R., Lewis D.P., Butt H.L., Gooley P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. 2015;11:1626–1639. [Google Scholar]
- 71.Gottschalk G., Peterson D., Knox K., Maynard M., Whelan R.J., Roy A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE) Molecular and Cellular Neuroscience. 2022;120 doi: 10.1016/j.mcn.2022.103731. [DOI] [PubMed] [Google Scholar]
- 72.Wood E., Hall K.H., Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in Myalgic encephalomyelitis/chronic fatigue syndrome: a possible approach to SARS-CoV-2 ‘long-haulers’? Chronic Diseases and Translational Medicine. 2021;7:14–26. doi: 10.1016/j.cdtm.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morris G., Maes M. Mitochondrial dysfunctions in Myalgic encephalomyelitis / chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014;29:19–36. doi: 10.1007/s11011-013-9435-x. [DOI] [PubMed] [Google Scholar]
- 74.Patarca R. Cytokines and chronic fatigue syndrome. Ann N Y Acad Sci. 2001;933:185–200. doi: 10.1111/j.1749-6632.2001.tb05824.x. [DOI] [PubMed] [Google Scholar]
- 75.Spence V.A., Kennedy G., Belch J.J., Hill A., Khan F. Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome. Clin Sci (Lond) 2008;114:561–566. doi: 10.1042/CS20070274. [DOI] [PubMed] [Google Scholar]
- 76.Maes M., Twisk F.N., Kubera M., Ringel K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. J Affect Disord. 2012;136:933–939. doi: 10.1016/j.jad.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Giloteaux L., Goodrich J.K., Walters W.A., Levine S.M., Ley R.E., Hanson M.R. Reduced diversity and altered composition of the gut microbiome in individuals with Myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4:30. doi: 10.1186/s40168-016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simonato M., Dall’Acqua S., Zilli C., Sut S., Tenconi R., Gallo N., et al. Tryptophan metabolites, cytokines, and fatty acid binding protein 2 in Myalgic encephalomyelitis/chronic fatigue syndrome. Biomedicines. 2021:9. doi: 10.3390/biomedicines9111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jason L.A., Gaglio C.L., Furst J., Islam M., Sorenson M., Conroy K.E., et al. Cytokine network analysis in a community-based pediatric sample of patients with Myalgic encephalomyelitis/chronic fatigue syndrome. Chronic Illn. 2022 doi: 10.1177/17423953221101606. 17423953221101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jonsjö M.A., Olsson G.L., Wicksell R.K., Alving K., Holmström L., Andreasson A. The role of low-grade inflammation in ME/CFS (Myalgic encephalomyelitis/chronic fatigue syndrome) - associations with symptoms. Psychoneuroendocrinology. 2020;113 doi: 10.1016/j.psyneuen.2019.104578. [DOI] [PubMed] [Google Scholar]
- 81.Lee J., Vernon S.D., Jeys P., Ali W., Campos A., Unutmaz D., et al. Hemodynamics during the 10-minute NASA lean test: evidence of circulatory decompensation in a subset of ME/CFS patients. J Transl Med. 2020;18:314. doi: 10.1186/s12967-020-02481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russo C., Jin Z., Takei Y., Hasegawa T., Koshaka S., Palmieri V., et al. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dworkin H.J., Lawrie C., Bohdiewicz P., Lerner A.M. Abnormal left ventricular myocardial dynamics in eleven patients with chronic fatigue syndrome. Clin Nucl Med. 1994;19:675–677. doi: 10.1097/00003072-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 84.Hurwitz B.E., Coryell V.T., Parker M., Martin P., Laperriere A., Klimas N.G., et al. Chronic fatigue syndrome: illness severity, sedentary lifestyle, blood volume and evidence of diminished cardiac function. Clin Sci (Lond) 2009;118:125–135. doi: 10.1042/CS20090055. [DOI] [PubMed] [Google Scholar]
- 85.Stewart J.M. Chronic fatigue syndrome: comments on deconditioning, blood volume and resulting cardiac function. Clin Sci (Lond) 2009;118:121–123. doi: 10.1042/CS20090327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spaak J., Montmerle S., Sundblad P., Linnarsson D. Long-term bed rest-induced reductions in stroke volume during rest and exercise: cardiac dysfunction vs. volume depletion. J Appl Physiol. 2005;98:648–654. doi: 10.1152/japplphysiol.01332.2003. [DOI] [PubMed] [Google Scholar]
- 87.Hollingsworth K.G., Hodgson T., Macgowan G.A., Blamire A.M., Newton J.L. Impaired cardiac function in chronic fatigue syndrome measured using magnetic resonance cardiac tagging. J Intern Med. 2012;271:264–270. doi: 10.1111/j.1365-2796.2011.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miwa K. Cardiac dysfunction and orthostatic intolerance in patients with Myalgic encephalomyelitis and a small left ventricle. Heart Vessels. 2015;30:484–489. doi: 10.1007/s00380-014-0510-y. [DOI] [PubMed] [Google Scholar]
- 89.van Campen C.M.C., Rowe P.C., Visser F.C. Blood volume status in ME/CFS correlates with the presence or absence of orthostatic symptoms: preliminary results. Frontiers in Pediatrics. 2018:6. doi: 10.3389/fped.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.CLMCV Campen, Visser F.C. 2018. The Abnormal Cardiac Index and Stroke Volume Index Changes during a Normal Tilt Table Test in Mecfs Patients Compared to Healthy Volunteers are not Related to Deconditioning. [Google Scholar]
- 91.van Campen C., Rowe P.C., Visser F.C. Deconditioning does not explain orthostatic intolerance in ME/CFS (Myalgic encephalomyelitis/chronic fatigue syndrome) J Transl Med. 2021;19:193. doi: 10.1186/s12967-021-02819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Campen C.M.C., Visser F.C. Comparison of the degree of deconditioning in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients with and without orthostatic intolerance. Medical Research Archives. 2022:10. [Google Scholar]
- 93.Newton J.L., Finkelmeyer A., Petrides G., Frith J., Hodgson T., Maclachlan L., et al. Reduced cardiac volumes in chronic fatigue syndrome associate with plasma volume but not length of disease: a cohort study. Open Heart. 2016;3 doi: 10.1136/openhrt-2015-000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olimulder M.A., Galjee M.A., Wagenaar L.J., van Es J., van der Palen J., Visser F.C., et al. Chronic fatigue syndrome in women assessed with combined cardiac magnetic resonance imaging. Neth Heart J. 2016;24:709–716. doi: 10.1007/s12471-016-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iversen P.O., von Lueder T.G., Kardel K.R., Lien K. Cardiac dimensions and function are not altered among females with the Myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare (Basel) 2020:8. doi: 10.3390/healthcare8040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gutierrez-Mariscal F.M., de la Cruz-Ares S., Torres-Peña J.D., Alcalá-Diaz J.F., Yubero-Serrano E.M., López-Miranda J. Coenzyme Q10 and cardiovascular diseases. Antioxidants. 2021;10:906. doi: 10.3390/antiox10060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Littarru G.P., Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol. 2007;37:31–37. doi: 10.1007/s12033-007-0052-y. [DOI] [PubMed] [Google Scholar]
- 98.Kumar A., Kaur H., Devi P., Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124:259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Martelli A., Testai L., Colletti A., Cicero A.F.G. Coenzyme Q(10): clinical applications in cardiovascular diseases. Antioxidants (Basel) 2020:9. doi: 10.3390/antiox9040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Molyneux S.L., Florkowski C.M., George P.M., Pilbrow A.P., Frampton C.M., Lever M., et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52:1435–1441. doi: 10.1016/j.jacc.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 101.Yalcin A., Kilinc E., Sagcan A., Kultursay H. Coenzyme Q10 concentrations in coronary artery disease. Clin Biochem. 2004;37:706–709. doi: 10.1016/j.clinbiochem.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 102.Chapidze G., Kapanadze S., Dolidze N., Bachutashvili Z., Latsabidze N. Prevention of coronary atherosclerosis by the use of combination therapy with antioxidant coenzyme Q10 and statins. Georgian Med News. 2005;20-5 [PubMed] [Google Scholar]
- 103.Dludla P.V., Orlando P., Silvestri S., Marcheggiani F., Cirilli I., Nyambuya T.M., et al. Coenzyme Q(10) supplementation improves adipokine levels and alleviates inflammation and lipid peroxidation in conditions of metabolic syndrome: a meta-analysis of randomized controlled trials. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21093247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomic S., Brkic S., Maric D., Mikic A.N. Lipid and protein oxidation in female patients with chronic fatigue syndrome. Arch Med Sci. 2012;8:886–891. doi: 10.5114/aoms.2012.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castro-Marrero J., Domingo J.C., Cordobilla B., Ferrer R., Giralt M., Sanmartín-Sentañes R., et al. Does coenzyme Q10 plus selenium supplementation ameliorate clinical outcomes by modulating oxidative stress and inflammation in individuals with Myalgic encephalomyelitis/chronic fatigue syndrome? Antioxid Redox Signal. 2022;36:729–739. doi: 10.1089/ars.2022.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Castro-Marrero J., Zaragozá M.C., Domingo J.C., Martinez-Martinez A., Alegre J., von Schacky C. Low omega-3 index and polyunsaturated fatty acid status in patients with chronic fatigue syndrome/Myalgic encephalomyelitis. Prostaglandins Leukot Essent Fatty Acids. 2018;139:20–24. doi: 10.1016/j.plefa.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 107.Lorente-Cebrián S., Costa A.G.V., Navas-Carretero S., Zabala M., Laiglesia L.M., Martínez J.A., et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71:341–349. doi: 10.1007/s13105-015-0395-y. [DOI] [PubMed] [Google Scholar]
- 108.von Schacky C. Omega-3 index and cardiovascular health. Nutrients. 2014;6:799–814. doi: 10.3390/nu6020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yanai H., Masui Y., Katsuyama H., Adachi H., Kawaguchi A., Hakoshima M., et al. An improvement of cardiovascular risk factors by Omega-3 polyunsaturated fatty acids. J Clin Med Res. 2018;10:281–289. doi: 10.14740/jocmr3362w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Germain A., Ruppert D., Levine S.M., Hanson M.R. Metabolic profiling of a Myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol Biosyst. 2017;13:371–379. doi: 10.1039/c6mb00600k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Germain A., Barupal D.K., Levine S.M., Hanson M.R. Comprehensive circulatory metabolomics in ME/CFS reveals disrupted metabolism of acyl lipids and steroids. Metabolites. 2020;10:34. doi: 10.3390/metabo10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kashi A.A., Davis R.W., Phair R.D. The IDO metabolic trap hypothesis for the Etiology of ME/CFS. Diagnostics. 2019;9:82. doi: 10.3390/diagnostics9030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bibbins-Domingo K., Gupta R., Na B., Wu A.H.B., Schiller N.B., Whooley M.A. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Staiger H., Keuper M., Berti L., Hrabě de Angelis M., Häring H.-U. Fibroblast growth factor 21—metabolic role in mice and men. Endocr Rev. 2017;38:468–488. doi: 10.1210/er.2017-00016. [DOI] [PubMed] [Google Scholar]
- 115.Schondorf R., Freeman R. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci. 1999;317:117–123. doi: 10.1097/00000441-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Lim E.-J., Son C.-G. Review of case definitions for Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2020;18:289. doi: 10.1186/s12967-020-02455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoad A., Spickett G., Elliott J., Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. Qjm. 2008;101:961–965. doi: 10.1093/qjmed/hcn123. [DOI] [PubMed] [Google Scholar]
- 118.Miwa K., Fujita M. Small heart syndrome in patients with chronic fatigue syndrome. Clin Cardiol. 2008;31:328–333. doi: 10.1002/clc.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miwa K., Fujita M. Cardiac function fluctuates during exacerbation and remission in young adults with chronic fatigue syndrome and “small heart”. J Cardiol. 2009;54:29–35. doi: 10.1016/j.jjcc.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 120.Newton J.L., Sheth A., Shin J., Pairman J., Wilton K., Burt J.A., et al. Lower ambulatory blood pressure in chronic fatigue syndrome. Psychosom Med. 2009;71:361–365. doi: 10.1097/PSY.0b013e31819ccd2a. [DOI] [PubMed] [Google Scholar]
- 121.Brown A.A., Jason L.A., Evans M.A., Flores S. Contrasting case definitions: the ME international consensus criteria vs. the Fukuda et al. CFS criteria. N Am J Psychol. 2013;15:103–120. [PMC free article] [PubMed] [Google Scholar]
- 122.Moon J., Kim D.-Y., Byun J.-I., Sunwoo J.-S., Lim J.-A., Kim T.-J., et al. Orthostatic intolerance symptoms are associated with depression and diminished quality of life in patients with postural tachycardia syndrome. Health Qual Life Outcomes. 2016;14:144. doi: 10.1186/s12955-016-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roma M., Marden C.L., Flaherty M.A.K., Jasion S.E., Cranston E.M., Rowe P.C. Impaired health-related quality of life in adolescent Myalgic encephalomyelitis/chronic fatigue syndrome: the impact of core symptoms. Front Pediatr. 2019;7:26. doi: 10.3389/fped.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garner R., Baraniuk J.N. Orthostatic intolerance in chronic fatigue syndrome. J Transl Med. 2019;17:185. doi: 10.1186/s12967-019-1935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Campen C., Rowe P.C., Visser F.C. 2021. Compression stockings improve cardiac output and cerebral blood flow during tilt testing in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients: A randomized crossover trial. Medicina (Kaunas) p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tanaka S., Kuratsune H., Hidaka Y., Hakariya Y., Tatsumi K.I., Takano T., et al. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. Int J Mol Med. 2003;12:225–230. [PubMed] [Google Scholar]
- 127.Loebel M., Grabowski P., Heidecke H., Bauer S., Hanitsch L.G., Wittke K., et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav Immun. 2016;52:32–39. doi: 10.1016/j.bbi.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 128.Bynke A., Julin P., Gottfries C.G., Heidecke H., Scheibenbogen C., Bergquist J. Autoantibodies to beta-adrenergic and muscarinic cholinergic receptors in Myalgic encephalomyelitis (ME) patients - a validation study in plasma and cerebrospinal fluid from two Swedish cohorts. Brain Behav Immun Health. 2020;7 doi: 10.1016/j.bbih.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamamoto S., Ouchi Y., Nakatsuka D., Tahara T., Mizuno K., Tajima S., et al. Reduction of [11C](+)3-MPB binding in brain of chronic fatigue syndrome with serum autoantibody against muscarinic cholinergic receptor. PloS One. 2012;7 doi: 10.1371/journal.pone.0051515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hartwig J., Sotzny F., Bauer S., Heidecke H., Riemekasten G., Dragun D., et al. IgG stimulated β2 adrenergic receptor activation is attenuated in patients with ME/CFS. Brain, Behavior, & Immunity - Health. 2020;3 doi: 10.1016/j.bbih.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Slinger E., Langemeijer E., Siderius M., Vischer H.F., Smit M.J. Herpesvirus-encoded GPCRs rewire cellular signaling. Mol Cell Endocrinol. 2011;331:179–184. doi: 10.1016/j.mce.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 132.Campen CMCv, Rowe PC, Visser FC. vol. 58. 2022. Orthostatic symptoms and reductions in cerebral blood flow in Long-haul COVID-19 patients: Similarities with Myalgic encephalomyelitis/chronic fatigue syndrome. Medicina; p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Campen CMv, Rowe PC, Visser FC. Reductions in cerebral blood flow can be provoked by sitting in severe Myalgic encephalomyelitis/chronic fatigue syndrome patients. Healthcare. 2020;8:394. doi: 10.3390/healthcare8040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Campen C.M.C., Rowe P.C., Visser F.C. Cerebral blood flow remains reduced after tilt testing in Myalgic encephalomyelitis/chronic fatigue syndrome patients. Clin Neurophysiol Pract. 2021;6:245–255. doi: 10.1016/j.cnp.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Biswal B., Kunwar P., Natelson B.H. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. 2011;301:9–11. doi: 10.1016/j.jns.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boissoneault J., Letzen J., Robinson M., Staud R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav. 2019;13:789–797. doi: 10.1007/s11682-018-9897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yoshiuchi K., Farkas J., Natelson B.H. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. 2006;26:83–86. doi: 10.1111/j.1475-097X.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 138.Schmaling K.B., Lewis D.H., Fiedelak J.I., Mahurin R., Buchwald D.S. Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome. Psychosom Med. 2003;65:129–136. doi: 10.1097/01.psy.0000038942.33335.9b. [DOI] [PubMed] [Google Scholar]
- 139.Shungu D.C., Weiduschat N., Murrough J.W., Mao X., Pillemer S., Dyke J.P., et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Staud R., Boissoneault J., Craggs J.G., Lai S., Robinson M.E. Task related cerebral blood flow changes of patients with chronic fatigue syndrome: an arterial spin labeling study. Fatigue. 2018;6:63–79. doi: 10.1080/21641846.2018.1453919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li X., Julin P., Li T.-Q. Limbic perfusion is reduced in patients with Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Tomography. 2021;7:675–687. doi: 10.3390/tomography7040056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cook D.B., Light A.R., Light K.C., Broderick G., Shields M.R., Dougherty R.J., et al. Neural consequences of post-exertion malaise in Myalgic encephalomyelitis/chronic fatigue syndrome. Brain Behav Immun. 2017;62:87–99. doi: 10.1016/j.bbi.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 143.Glassford J.A.G. The neuroinflammatory etiopathology of Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mackay A., Tate W.P. A compromised paraventricular nucleus within a dysfunctional hypothalamus: a novel neuroinflammatory paradigm for ME/CFS. Int J Immunopathol Pharmacol. 2018;32 2058738418812342. [Google Scholar]
- 145.Maes M., Ringel K., Kubera M., Anderson G., Morris G., Galecki P., et al. In Myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J Affect Disord. 2013;150:223–230. doi: 10.1016/j.jad.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 146.Shan Z.Y., Barnden L.R., Kwiatek R.A., Bhuta S., Hermens D.F., Lagopoulos J. Neuroimaging characteristics of Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a systematic review. J Transl Med. 2020;18:335. doi: 10.1186/s12967-020-02506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Renz-Polster H., Tremblay M.-E., Bienzle D., Fischer J.E. The pathobiology of Myalgic encephalomyelitis/chronic fatigue syndrome: the case for neuroglial Failure. Frontiers in Cellular Neuroscience. 2022:16. doi: 10.3389/fncel.2022.888232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barnden L.R., Crouch B., Kwiatek R., Burnet R., Mernone A., Chryssidis S., et al. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. 2011;24:1302–1312. doi: 10.1002/nbm.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kemp J., Sunnquist M., Jason L.A., Newton J.L. Autonomic dysfunction in Myalgic encephalomyelitis and chronic fatigue syndrome: comparing self-report and objective measures. Clin Auton Res. 2019;29:475–477. doi: 10.1007/s10286-019-00615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Newton J.L., Okonkwo O., Sutcliffe K., Seth A., Shin J., Jones D.E.J. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM: An International Journal of Medicine. 2007;100:519–526. doi: 10.1093/qjmed/hcm057. [DOI] [PubMed] [Google Scholar]
- 151.Winkler A.S., Blair D., Marsden J.T., Peters T.J., Wessely S., Cleare A.J. Autonomic function and serum erythropoietin levels in chronic fatigue syndrome. J Psychosom Res. 2004;56:179–183. doi: 10.1016/S0022-3999(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 152.Joseph P., Arevalo C., Oliveira R.K.F., Faria-Urbina M., Felsenstein D., Oaklander A.L., et al. Insights from invasive cardiopulmonary exercise testing of patients with Myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160:642–651. doi: 10.1016/j.chest.2021.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Joseph P., Pari R., Miller S., Warren A., Stovall M.C., Squires J., et al. Neurovascular dysregulation and acute exercise intolerance in Myalgic encephalomyelitis/chronic fatigue syndrome: a randomized. Placebo-Controlled Trial of Pyridostigmine Chest. 2022;162:1116–1126. doi: 10.1016/j.chest.2022.04.146. [DOI] [PubMed] [Google Scholar]
- 154.Nelson M.J., Bahl J.S., Buckley J.D., Thomson R.L., Davison K. Evidence of altered cardiac autonomic regulation in Myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Soboleva A., Mavropulo-Stolyarenko G., Karonova T., Thieme D., Hoehenwarter W., Ihling C., et al. Multiple glycation sites in blood plasma proteins as an integrated biomarker of type 2 diabetes mellitus. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20092329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aksu K., Donmez A., Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18:1478–1493. doi: 10.2174/138161212799504731. [DOI] [PubMed] [Google Scholar]
- 157.Branchford B.R., Carpenter S.L. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Swanepoel A.C., Emmerson O., Pretorius E. Effect of progesterone and synthetic progestins on whole blood clot formation and erythrocyte structure. Microsc Microanal. 2017;23:607–617. doi: 10.1017/S1431927617000484. [DOI] [PubMed] [Google Scholar]
- 159.Lopes Pires M.E., Clarke S.R., Marcondes S., Gibbins J.M. Lipopolysaccharide potentiates platelet responses via toll-like receptor 4-stimulated Akt-Erk-PLA2 signalling. PloS One. 2017;12 doi: 10.1371/journal.pone.0186981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li C., Li J., Ni H. Crosstalk between platelets and microbial pathogens. Front Immunol. 2020;11:1962. doi: 10.3389/fimmu.2020.01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nunes J.M., Fillis T., Page M.J., Venter C., Lancry O., Kell D.B., et al. Gingipain R1 and lipopolysaccharide from Porphyromonas gingivalis have major effects on blood clot morphology and mechanics. Front Immunol. 2020;11:1551. doi: 10.3389/fimmu.2020.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Simpson L.O. Nondiscocytic erythrocytes in Myalgic encephalomyelitis. N Z Med J. 1989;102:126–127. [PubMed] [Google Scholar]
- 163.Nacul L., de Barros B., Kingdon C.C., Cliff J.M., Clark T.G., Mudie K., et al. Evidence of clinical pathology abnormalities in people with Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) from an analytic cross-sectional study. Diagnostics. 2019;9:41. doi: 10.3390/diagnostics9020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eccles J., Thompson C., Thompson B., Amato M., Themelis K., Critchley H., et al. AB1209 mechanistic factors contributing to pain and fatigue in fibromyalgia and me/CFS: autonomic and inflammatory insights from an experimental medicine study. Ann Rheum Dis. 2022;81:1719. [Google Scholar]
- 165.Eccles J., Amato M., Thompson C., Themelis K., Critchley H., Harrison N., et al. AB0949 autonomic and inflammatory mechanisms of pain and fatigue in fibromyalgia and ME/CFS: an interventional study. Ann Rheum Dis. 2020;79:1772. [Google Scholar]
- 166.Blauensteiner J., Bertinat R., León L.E., Riederer M., Sepúlveda N., Westermeier F. Altered endothelial dysfunction-related miRs in plasma from ME/CFS patients. Sci Rep. 2021;11:10604. doi: 10.1038/s41598-021-89834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cliff J.M., King E.C., Lee J.-S., Sepúlveda N., Wolf A.-S., Kingdon C., et al. Cellular immune function in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Saha A.K., Schmidt B.R., Wilhelmy J., Nguyen V., Abugherir A., Do J.K., et al. Red blood cell deformability is diminished in patients with chronic fatigue syndrome. Clin Hemorheol Microcirc. 2019;71:113–116. doi: 10.3233/CH-180469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pernow J., Mahdi A., Yang J., Zhou Z. Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res. 2019;115:1596–1605. doi: 10.1093/cvr/cvz156. [DOI] [PubMed] [Google Scholar]
- 170.Berg D., Berg L.H., Couvaras J., Harrison H. Chronic fatigue syndrome and/or fibromyalgia as a variation of antiphospholipid antibody syndrome: an explanatory model and approach to laboratory diagnosis. Blood Coagul Fibrinolysis. 1999;10:435–438. doi: 10.1097/00001721-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 171.Bonilla H., Hampton D., Marques de Menezes E.G., Deng X., Montoya J.G., Anderson J., et al. Comparative analysis of extracellular vesicles in patients with severe and mild Myalgic encephalomyelitis/chronic fatigue syndrome. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.841910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jahanbani F., Maynard R.D., Sing J.C., Jahanbani S., Perrino J.J., Spacek D.V., et al. Phenotypic characteristics of peripheral immune cells of Myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: a pilot study. PloS One. 2022;17 doi: 10.1371/journal.pone.0272703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kennedy G., Norris G., Spence V., McLaren M., Belch J.J. Is chronic fatigue syndrome associated with platelet activation? Blood Coagul Fibrinolysis. 2006;17:89–92. doi: 10.1097/01.mbc.0000214705.80997.73. [DOI] [PubMed] [Google Scholar]
- 174.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 175.Akbarialiabad H., Taghrir M.H., Abdollahi A., Ghahramani N., Kumar M., Paydar S., et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49:1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Proal A.D., VanElzakker M.B. Long COVID or Post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aiyegbusi O.L., Hughes S.E., Turner G., Rivera S.C., McMullan C., Chandan J.S., et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114:428–442. doi: 10.1177/01410768211032850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Twomey R., DeMars J., Franklin K., Culos-Reed S.N., Weatherald J., Wrightson J.G. Chronic fatigue and postexertional malaise in people living with Long COVID: an observational study. Phys Ther. 2022:102. doi: 10.1093/ptj/pzac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pretorius E., Venter C., Laubscher G.J., Kotze M.J., Oladejo S.O., Watson L.R., et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-acute sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21:148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Décary S., Gaboury I., Poirier S., Garcia C., Simpson S., Bull M., et al. Humility and acceptance: working within our limits with Long COVID and Myalgic encephalomyelitis/chronic fatigue syndrome. J Orthop Sports Phys Ther. 2021;51:197–200. doi: 10.2519/jospt.2021.0106. [DOI] [PubMed] [Google Scholar]
- 182.Sigfrid L., Drake T.M., Pauley E., Jesudason E.C., Olliaro P., Lim W.S., et al. Long Covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nkiliza A., Parks M., Cseresznye A., Oberlin S., Evans J.E., Darcey T., et al. Sex-specific plasma lipid profiles of ME/CFS patients and their association with pain, fatigue, and cognitive symptoms. J Transl Med. 2021;19:370. doi: 10.1186/s12967-021-03035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheema A.K., Sarria L., Bekheit M., Collado F., Almenar-Pérez E., Martín-Martínez E., et al. Unravelling Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): gender-specific changes in the microRNA expression profiling in ME/CFS. J Cell Mol Med. 2020;24:5865–5877. doi: 10.1111/jcmm.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Capdevila L., Castro-Marrero J., Alegre J., Ramos-Castro J., Escorihuela R.M. Analysis of gender differences in HRV of patients with Myalgic encephalomyelitis/chronic fatigue syndrome using mobile-health technology. Sensors. 2021;21:3746. doi: 10.3390/s21113746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sylvester S.V., Rusu R., Chan B., Bellows M., O’Keefe C., Nicholson S. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr Med Res Opin. 2022;38:1391–1399. doi: 10.1080/03007995.2022.2081454. [DOI] [PubMed] [Google Scholar]
- 187.Alam W. Hypercoagulability in COVID-19: a review of the potential mechanisms underlying clotting disorders. SAGE Open Med. 2021;9 doi: 10.1177/20503121211002996. 20503121211002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Asakura H., Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113:45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nemetski S.M., Ip A., Josephs J., Hellmann M. Clotting events among hospitalized patients infected with COVID-19 in a large multisite cohort in the United States. PloS One. 2022;17 doi: 10.1371/journal.pone.0262352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abdel-Bakky M.S., Amin E., Ewees M.G., Mahmoud N.I., Mohammed H.A., Altowayan W.M., et al. Coagulation system activation for targeting of COVID-19: insights into anticoagulants, vaccine-loaded nanoparticles, and hypercoagulability in COVID-19 vaccines. Viruses. 2022:14. doi: 10.3390/v14020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pretorius E., Vlok M., Venter C., Bezuidenhout J.A., Laubscher G.J., Steenkamp J., et al. Persistent clotting protein pathology in long COVID/Post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20:172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kell D.B., Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Prog Biophys Mol Biol. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 194.Grobbelaar L.M., Venter C., Vlok M., Ngoepe M., Laubscher G.J., Lourens P.J., et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep. 2021;41 doi: 10.1042/BSR20210611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pretorius E., Mbotwe S., Bester J., Robinson C.J., Kell D.B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface. 2016;13 doi: 10.1098/rsif.2016.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kell D.B., Pretorius E. No effects without causes: the iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol Rev Camb Philos Soc. 2018;93:1518–1557. doi: 10.1111/brv.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ryu J.K., Sozmen E.G., Dixit K., Montano M., Matsui Y., Liu Y., et al. SARS-CoV-2 spike protein induces abnormal inflammatory blood clots neutralized by fibrin immunotherapy. bioRxiv. 2021 [Google Scholar]
- 198.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9 doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miszta A., Huskens D., Donkervoort D., Roberts M.J.M., Wolberg A.S., de Laat B. Assessing plasmin generation in health and disease. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22052758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pretorius E., Page M.J., Hendricks L., Nkosi N.B., Benson S.R., Kell D.B. Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel AmytrackerTM stains. J R Soc Interface. 2018;15 doi: 10.1098/rsif.2017.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pretorius E., Mbotwe S., Kell D.B. Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular co-morbidities. Sci Rep. 2017;7:9680. doi: 10.1038/s41598-017-09860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kell D.B., Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen) Integr Biol (Camb) 2015;7:24–52. doi: 10.1039/c4ib00173g. [DOI] [PubMed] [Google Scholar]
- 203.Bester J., Soma P., Kell D.B., Pretorius E. Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia, and the possible role of bacterial lipopolysaccharides (LPS) Oncotarget. 2015;6:35284–35303. doi: 10.18632/oncotarget.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pretorius E., Venter C., Laubscher G., Kotze M., Moremi K., Oladejo S., et al. 2021. Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. [Google Scholar]
- 205.Odrljin T.M., Francis C.W., Sporn L.A., Bunce L.A., Marder V.J., Simpson-Haidaris P.J. Heparin-binding domain of fibrin mediates its binding to endothelial cells. Arterioscler Thromb Vasc Biol. 1996;16:1544–1551. doi: 10.1161/01.atv.16.12.1544. [DOI] [PubMed] [Google Scholar]
- 206.Sahni A., Francis C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- 207.Martinez J., Ferber A., Bach T.L., Yaen C.H. Interaction of fibrin with VE-cadherin. Ann N Y Acad Sci. 2001;936:386–405. doi: 10.1111/j.1749-6632.2001.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 208.Drabik D., Chodaczek G., Kraszewski S. Effect of amyloid-β monomers on lipid membrane mechanical parameters-potential implications for mechanically driven neurodegeneration in Alzheimer’s disease. Int J Mol Sci. 2020;22 doi: 10.3390/ijms22010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bertinat R., Villalobos-Labra R., Hofmann L., Blauensteiner J., Sepulveda N., Westermeier F. Decreased NO production in endothelial cells exposed to plasma from ME/CFS patients. Vascul Pharmacol. 2022;143 doi: 10.1016/j.vph.2022.106953. [DOI] [PubMed] [Google Scholar]
- 210.Flaskamp L., Roubal C., Uddin S., Sotzny F., Kedor C., Bauer S., et al. Serum of Post-COVID-19 syndrome patients with or without ME/CFS differentially affects endothelial cell function in vitro. Cells. 2022:11. doi: 10.3390/cells11152376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Corban M.T., Lerman L.O., Lerman A. Endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2019;39:1272–1274. doi: 10.1161/ATVBAHA.119.312836. [DOI] [PubMed] [Google Scholar]
- 212.Sun H.J., Wu Z.Y., Nie X.W., Bian J.S. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. 2019;10:1568. doi: 10.3389/fphar.2019.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Steyers C.M., Miller F.J. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15:11324–11349. doi: 10.3390/ijms150711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Girouard H., Park L., Anrather J., Zhou P., Iadecola C. Cerebrovascular Nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 215.Schulz E., Gori T., Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 216.Miyauchi T., Sakai S. Endothelin and the heart in health and diseases. Peptides. 2019;111:77–88. doi: 10.1016/j.peptides.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 217.Kaoukis A., Deftereos S., Raisakis K., Giannopoulos G., Bouras G., Panagopoulou V., et al. The role of endothelin system in cardiovascular disease and the potential therapeutic perspectives of its inhibition. Curr Top Med Chem. 2013;13:95–114. doi: 10.2174/1568026611313020003. [DOI] [PubMed] [Google Scholar]
- 218.Yuyun M.F., Ng L.L., Ng G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc Res. 2018;119:7–12. doi: 10.1016/j.mvr.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 219.Hasdai D., Gibbons R.J., Holmes D.R., Higano S.T., Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96 doi: 10.1161/01.cir.96.10.3390. 3390–5. [DOI] [PubMed] [Google Scholar]
- 220.Vallet B. Endothelial cell dysfunction and abnormal tissue perfusion. Crit Care Med. 2002;30 doi: 10.1097/00003246-200205001-00010. S229–34. [DOI] [PubMed] [Google Scholar]
- 221.Gavriilaki E., Eftychidis I., Papassotiriou I. Update on endothelial dysfunction in COVID-19: severe disease, long COVID-19 and pediatric characteristics. Journal of Laboratory Medicine. 2021;45:293–302. [Google Scholar]
- 222.Ambrosino P., Calcaterra I., Molino A., Moretta P., Lupoli R., Spedicato G.A., et al. Persistent endothelial dysfunction in Post-acute COVID-19 syndrome: a case-control study. Biomedicines. 2021;9:957. doi: 10.3390/biomedicines9080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Oikonomou E., Souvaliotis N., Lampsas S., Siasos G., Poulakou G., Theofilis P., et al. Endothelial dysfunction in acute and long standing COVID−19: a prospective cohort study. Vascul Pharmacol. 2022;144 doi: 10.1016/j.vph.2022.106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Charfeddine S., Ibnhadjamor H., Jdidi J., Torjmen S., Kraiem S., Bahloul A., et al. Sulodexide significantly improves endothelial dysfunction and alleviates chest pain and palpitations in patients with Long-COVID-19: insights from TUN-EndCOV study. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.866113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gu S.X., Tyagi T., Jain K., Gu V.W., Lee S.H., Hwa J.M., et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Charfeddine S., Ibn Hadj Amor H., Jdidi J., Torjmen S., Kraiem S., Hammami R., et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Frontiers in Cardiovascular Medicine. 2021:8. doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tosato M., Calvani R., Picca A., Ciciarello F., Galluzzo V., Coelho-Júnior H.J., et al. Effects of l-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with Long COVID: a single-blind randomized controlled trial. Nutrients. 2022;14:4984. doi: 10.3390/nu14234984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Trimarco V., Izzo R., Mone P., Trimarco B., Santulli G. Targeting endothelial dysfunction and oxidative stress in Long-COVID. Pharmacol Res. 2022;184 doi: 10.1016/j.phrs.2022.106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sørland K., Sandvik M.K., Rekeland I.G., Ribu L., Småstuen M.C., Mella O., et al. Reduced endothelial function in Myalgic encephalomyelitis/chronic fatigue syndrome-results from open-label cyclophosphamide intervention study. Front Med (Lausanne). 2021;8 doi: 10.3389/fmed.2021.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sotzny F., Blanco J., Capelli E., Castro-Marrero J., Steiner S., Murovska M., et al. Myalgic encephalomyelitis/chronic fatigue syndrome - evidence for an autoimmune disease. Autoimmun Rev. 2018;17:601–609. doi: 10.1016/j.autrev.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 231.Arreola-Diaz R., Majluf-Cruz A., Sanchez-Torres L., Hernandez-Juarez J. The pathophysiology of the antiphospholipid syndrome: a perspective from the blood coagulation system. Clin Appl Thromb Hemost. 2022;28 doi: 10.1177/10760296221088576. 10760296221088576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sandoval Y.H., Atef M.E., Levesque L.O., Li Y., Anand-Srivastava M.B. Endothelin-1 signaling in vascular physiology and pathophysiology. Curr Vasc Pharmacol. 2014;12:202–214. doi: 10.2174/1570161112666140226122054. [DOI] [PubMed] [Google Scholar]
- 233.Massardo T., Quintana J.C., Jaimovich R., Sáez C.G., Risco L., Liberman C., et al. Regional brain perfusion is associated with endothelial dysfunction markers in major depressive disorder. Neuropsychobiology. 2021;80:214–224. doi: 10.1159/000508110. [DOI] [PubMed] [Google Scholar]
- 234.Sandvik M.K., Sørland K., Leirgul E., Rekeland I.G., Stavland C.S., Mella O., et al. Endothelial dysfunction in ME/CFS patients. PloS One. 2023;18 doi: 10.1371/journal.pone.0280942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Levy B.I., Schiffrin E.L., Mourad J.-J., Agostini D., Vicaut E., Safar M.E., et al. Impaired tissue perfusion. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 236.Stanculescu D., Bergquist J. Perspective: drawing on findings from critical illness to explain Myalgic encephalomyelitis/chronic fatigue syndrome. Front Med (Lausanne). 2022;9 doi: 10.3389/fmed.2022.818728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kerr J. Early growth response gene upregulation in Epstein-Barr virus (EBV)-associated Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Biomolecules. 2020:10. doi: 10.3390/biom10111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stowe R.P., Kozlova E.V., Yetman D.L., Walling D.M., Goodwin J.S., Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rooney B.V., Crucian B.E., Pierson D.L., Laudenslager M.L., Mehta S.K. Herpes virus reactivation in astronauts during spaceflight and its application on earth. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cohen J.I. Herpesvirus latency. J Clin Invest. 2020;130:3361–3369. doi: 10.1172/JCI136225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kalla M., Hammerschmidt W. Human B cells on their route to latent infection – early but transient expression of lytic genes of Epstein-Barr virus. Eur J Cell Biol. 2012;91:65–69. doi: 10.1016/j.ejcb.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 242.Price A.M., Luftig M.A. In: Advances in virus research. Maramorosch K., Murphy F.A., editors. Academic Press; 2014. Chapter six - dynamic Epstein–Barr virus gene expression on the path to B-cell transformation; pp. 279–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guo R., Liang J.H., Zhang Y., Lutchenkov M., Li Z., Wang Y., et al. Methionine metabolism controls the B cell EBV epigenome and viral latency. Cell Metab. 2022;34 doi: 10.1016/j.cmet.2022.08.008. 1280–97.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Weidner-Glunde M., Kruminis-Kaszkiel E., Savanagouder M. Herpesviral latency—common themes. Pathogens. 2020;9:125. doi: 10.3390/pathogens9020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Grey F. Role of microRNAs in herpesvirus latency and persistence. J Gen Virol. 2015;96:739–751. doi: 10.1099/vir.0.070862-0. [DOI] [PubMed] [Google Scholar]
- 246.Murata T., Sugimoto A., Inagaki T., Yanagi Y., Watanabe T., Sato Y., et al. Molecular basis of Epstein–Barr virus latency establishment and lytic reactivation. Viruses. 2021;13:2344. doi: 10.3390/v13122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gold J.E., Okyay R.A., Licht W.E., Hurley D.J. Investigation of Long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. 2021;10:763. doi: 10.3390/pathogens10060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen J., Song J., Dai L., Post S.R., Qin Z. SARS-CoV-2 infection and lytic reactivation of herpesviruses: a potential threat in the postpandemic era? J Med Virol. 2022;94:5103–5111. doi: 10.1002/jmv.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Maple P.A.C. COVID-19, SARS-CoV-2 vaccination, and human herpesviruses infections. Vaccines. 2023;11:232. doi: 10.3390/vaccines11020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ruiz-Pablos M., Paiva B., Montero-Mateo R., Garcia N., Zabaleta A. Epstein-Barr virus and the origin of Myalgic encephalomyelitis or chronic fatigue syndrome. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.656797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kerr J.R. Epstein-Barr virus induced Gene-2 upregulation identifies a particular subtype of chronic fatigue syndrome/Myalgic encephalomyelitis. Front Pediatr. 2019;7:59. doi: 10.3389/fped.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Birkenbach M., Josefsen K., Yalamanchili R., Lenoir G., Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mensah F., Bansal A., Berkovitz S., Sharma A., Reddy V., Leandro M.J., et al. Extended B cell phenotype in patients with Myalgic encephalomyelitis/chronic fatigue syndrome: a cross-sectional study. Clin Exp Immunol. 2016;184:237–247. doi: 10.1111/cei.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bradley A.S., Ford B., Bansal A.S. Altered functional B cell subset populations in patients with chronic fatigue syndrome compared to healthy controls. Clin Exp Immunol. 2013;172:73–80. doi: 10.1111/cei.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nguyen C.B., Alsøe L., Lindvall J.M., Sulheim D., Fagermoen E., Winger A., et al. Whole blood gene expression in adolescent chronic fatigue syndrome: an exploratory cross-sectional study suggesting altered B cell differentiation and survival. J Transl Med. 2017;15:102. doi: 10.1186/s12967-017-1201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Steiner S., Becker S.C., Hartwig J., Sotzny F., Lorenz S., Bauer S., et al. Autoimmunity-related risk variants in PTPN22 and CTLA4 are associated with ME/CFS with infectious onset. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fluge Ø., Risa K., Lunde S., Alme K., Rekeland I.G., Sapkota D., et al. B-lymphocyte depletion in Myalgic encephalopathy/ chronic fatigue syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0129898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ablashi D.V., Eastman H.B., Owen C.B., Roman M.M., Friedman J., Zabriskie J.B., et al. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol. 2000;16:179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 259.Manian F.A. Simultaneous measurement of antibodies to Epstein-Barr virus, human herpesvirus 6, herpes simplex virus types 1 and 2, and 14 enteroviruses in chronic fatigue syndrome: is there evidence of activation of a nonspecific polyclonal immune response? Clin Infect Dis. 1994;19:448–453. doi: 10.1093/clinids/19.3.448. [DOI] [PubMed] [Google Scholar]
- 260.Lerner A.M., Beqaj S.H., Deeter R.G., Fitzgerald J.T. IgM serum antibodies to human cytomegalovirus nonstructural gene products p52 and CM2(UL44 and UL57) are uniquely present in a subset of patients with chronic fatigue syndrome. In Vivo. 2002;16:153–159. [PubMed] [Google Scholar]
- 261.Blomberg J., Rizwan M., Böhlin-Wiener A., Elfaitouri A., Julin P., Zachrisson O., et al. Antibodies to human herpesviruses in Myalgic encephalomyelitis/chronic fatigue syndrome patients. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Halpin P., Williams M.V., Klimas N.G., Fletcher M.A., Barnes Z., Ariza M.E. Myalgic encephalomyelitis/chronic fatigue syndrome and gulf war illness patients exhibit increased humoral responses to the herpesviruses-encoded dUTPase: implications in disease pathophysiology. J Med Virol. 2017;89:1636–1645. doi: 10.1002/jmv.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rasa-Dzelzkaleja S., Krumina A., Capenko S., Nora-Krukle Z., Gravelsina S., Vilmane A., et al. The persistent viral infections in the development and severity of Myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2023;21:33. doi: 10.1186/s12967-023-03887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Domingues T.D., Grabowska A.D., Lee J.-S., Ameijeiras-Alonso J., Westermeier F., Scheibenbogen C., et al. Herpesviruses serology distinguishes different subgroups of patients from the United Kingdom Myalgic encephalomyelitis/chronic fatigue syndrome biobank. Front Med. 2021:8. doi: 10.3389/fmed.2021.686736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Maltsev D. A comparative study of valaciclovir, valganciclovir, and artesunate efficacy in reactivated HHV-6 and HHV-7 infections associated with chronic fatigue syndrome/Myalgic encephalomyelitis. Microbiol Immunol. 2022;66:193–199. doi: 10.1111/1348-0421.12966. [DOI] [PubMed] [Google Scholar]
- 266.De Pelsmaeker S., Romero N., Vitale M., Favoreel H.W. Herpesvirus evasion of natural killer cells. J Virol. 2018;92 doi: 10.1128/JVI.02105-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Waldman W.J., Williams M.V., Lemeshow S., Binkley P., Guttridge D., Kiecolt-Glaser J.K., et al. Epstein-Barr virus-encoded dUTPase enhances proinflammatory cytokine production by macrophages in contact with endothelial cells: evidence for depression-induced atherosclerotic risk. Brain Behav Immun. 2008;22:215–223. doi: 10.1016/j.bbi.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Krstanović F., Britt W.J., Jonjić S., Brizić I. Cytomegalovirus infection and inflammation in developing brain. Viruses. 2021;13:1078. doi: 10.3390/v13061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Caruso A., Rotola A., Comar M., Favilli F., Galvan M., Tosetti M., et al. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J Med Virol. 2002;67:528–533. doi: 10.1002/jmv.10133. [DOI] [PubMed] [Google Scholar]
- 270.Alibek K., Baiken Y., Kakpenova A., Mussabekova A., Zhussupbekova S., Akan M., et al. Implication of human herpesviruses in oncogenesis through immune evasion and supression. Infectious Agents and Cancer. 2014;9:3. doi: 10.1186/1750-9378-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Strawbridge R., Sartor M.L., Scott F., Cleare A.J. Inflammatory proteins are altered in chronic fatigue syndrome-a systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;107:69–83. doi: 10.1016/j.neubiorev.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 272.Eaton-Fitch N., du Preez S., Cabanas H., Staines D., Marshall-Gradisnik S. A systematic review of natural killer cells profile and cytotoxic function in Myalgic encephalomyelitis/chronic fatigue syndrome. Syst Rev. 2019;8:279. doi: 10.1186/s13643-019-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Krishnamoorthy S., Honn K.V. Inflammation and disease progression. Cancer and Metastasis Reviews. 2006;25:481–491. doi: 10.1007/s10555-006-9016-0. [DOI] [PubMed] [Google Scholar]
- 274.Kotas Maya E., Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2020;33:127–148. doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Carod-Artal F.J. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28:67–81. doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 277.Chen S.H., Garber D.A., Schaffer P.A., Knipe D.M., Coen D.M. Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology. 2000;278:207–216. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- 278.Steiner I., Benninger F. Update on herpes virus infections of the nervous system. Curr Neurol Neurosci Rep. 2013;13:414. doi: 10.1007/s11910-013-0414-8. [DOI] [PubMed] [Google Scholar]
- 279.Gilden D.H., Mahalingam R., Cohrs R.J., Tyler K.L. Herpesvirus infections of the nervous system. Nat Clin Pract Neurol. 2007;3:82–94. doi: 10.1038/ncpneuro0401. [DOI] [PubMed] [Google Scholar]
- 280.Volpi A. Epstein-Barr virus and human herpesvirus type 8 infections of the central nervous system. Herpes. 2004;11(Suppl. 2) 120A-7A. [PubMed] [Google Scholar]
- 281.Bennett J.L., Mahalingam R., Wellish M.C., Gilden D.H. Epstein-barr virus–associated acute autonomic neuropathy. Ann Neurol. 1996;40:453–455. doi: 10.1002/ana.410400316. [DOI] [PubMed] [Google Scholar]
- 282.Corssmit E.P.M., Leverstein-Van Hall M.A., Portegies P., Bakker P. Case report severe neurological complications in association with Epstein-Barr virus infection. J Neurovirol. 1997;3:460–464. doi: 10.3109/13550289709031193. [DOI] [PubMed] [Google Scholar]
- 283.Fujii N., Tabira T., Shibasaki H., Kuroiwa Y., Ohnishi A., Nagaki J. Acute autonomic and sensory neuropathy associated with elevated Epstein-Barr virus antibody titre. J Neurol Neurosurg Psychiatry. 1982;45:656–658. doi: 10.1136/jnnp.45.7.656-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dodig D., Ngo M., Bailey D., Bril V. Brachial plexopathy complicating Epstein-Barr virus infection in an adult. Acta Myol. 2010;29:357–359. [PMC free article] [PubMed] [Google Scholar]
- 285.Ejima M., Ota K., Yamamoto K., Sugishita Y., Maruyama S. A case of acute pandysautonomia and diffuse brain stem impairment associated with EB virus infection. Rinsho Shinkeigaku. 1994;34:1136–1141. [PubMed] [Google Scholar]
- 286.Itoh Y., Oishi T., Ohnishi A., Murai Y., Imawatari R. Acute cerebellar ataxia with sympathotonic orthostatic hypotension following Epstein-Barr virus infection--a case report. Rinsho Shinkeigaku. 1993;33:503–506. [PubMed] [Google Scholar]
- 287.Katz B.Z., Stewart J.M., Shiraishi Y., Mears C.J., Taylor R. Autonomic symptoms at baseline and following infectious mononucleosis in a prospective cohort of adolescents. Arch Pediatr Adolesc Med. 2011;165:765–766. doi: 10.1001/archpediatrics.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Williams Ph D.M., Cox B., Lafuse Ph D.W., Ariza M.E. Epstein-Barr virus dUTPase induces neuroinflammatory mediators: implications for Myalgic encephalomyelitis/chronic fatigue syndrome. Clin Ther. 2019;41:848–863. doi: 10.1016/j.clinthera.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Uchida Y., Koike H., Oguri T., Kato H., Yuasa H., Mitake S. Successful corticosteroid therapies in a case of acute motor, sensory, autonomic neuropathy after cytomegalovirus infection. Rinsho Shinkeigaku. 2015;55:339–344. doi: 10.5692/clinicalneurol.cn-000631. [DOI] [PubMed] [Google Scholar]
- 290.Nakao K., Namekawa M., Kondo S., Ono S., Nakano I. Subacute autonomic and sensory neuropathy closely related to cytomegalovirus infection preceded by frequent syncopal attacks. Rinsho Shinkeigaku. 2016;56:555–559. doi: 10.5692/clinicalneurol.cn-000863. [DOI] [PubMed] [Google Scholar]
- 291.Maes L., Theunissen K., Schepers S., Indesteege I., Delmotte K. Acute autonomic dysregulation due to HHV-6 encephalitis in an immunocompromised patient: a case report and literature review. Acta Neurol Belg. 2022;122:583–585. doi: 10.1007/s13760-021-01828-6. [DOI] [PubMed] [Google Scholar]
- 292.Shouman K., Vanichkachorn G., Cheshire W.P., Suarez M.D., Shelly S., Lamotte G.J., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Finsterer J., Scorza F.A., Scorza C., Fiorini A. COVID-19 associated cranial nerve neuropathy: a systematic review. Bosn J Basic Med Sci. 2022;22:39–45. doi: 10.17305/bjbms.2021.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Senini V., Amara U., Paul M., Kim H. Porphyromonas gingivalis lipopolysaccharide activates platelet Cdc42 and promotes platelet spreading and thrombosis. J Periodontol. 2019;90:1336–1345. doi: 10.1002/JPER.18-0596. [DOI] [PubMed] [Google Scholar]
- 295.Koch L., Hofer S., Weigand M.A., Frommhold D., Poeschl J. Lipopolysaccharide-induced activation of coagulation in neonatal cord and adult blood monitored by thrombelastography. Thromb Res. 2009;124:463–467. doi: 10.1016/j.thromres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 296.Koch A., Meesters M.I., Scheller B., Boer C., Zacharowski K. Systemic endotoxin activity correlates with clot formation: an observational study in patients with early systemic inflammation and sepsis. Crit Care. 2013;17:R198. doi: 10.1186/cc12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Grobbelaar L.M., Kruger A., Venter C., Burger E.M., Laubscher G.J., Maponga T.G., et al. Relative Hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Hemost. 2022;48:858–868. doi: 10.1055/s-0042-1756306. [DOI] [PubMed] [Google Scholar]
- 298.Knapp S., von Aulock S., Leendertse M., Haslinger I., Draing C., Golenbock D.T., et al. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. The Journal of Immunology. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- 299.Beachey E.H., Chiang T.M., Ofek I., Kang A.H. Interaction of lipoteichoic acid of group a streptococci with human platelets. Infect Immun. 1977;16:649–654. doi: 10.1128/iai.16.2.649-654.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McKay D.G., Margaretten W. Disseminated intravascular coagulation in virus diseases. Arch Intern Med. 1967;120:129–152. [PubMed] [Google Scholar]
- 301.Huang Y.-H., Liu C.-C., Wang S.-T., Lei H.-Y., Liu H.-S., Lin Y.-S., et al. Activation of coagulation and fibrinolysis during dengue virus infection. J Med Virol. 2001;63:247–251. doi: 10.1002/1096-9071(200103)63:3<247::aid-jmv1008>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 302.Mackman N., Antoniak S., Wolberg A.S., Kasthuri R., Key N.S. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Flaujac C., Boukour S., Cramer-Bordé E. Platelets and viruses: an ambivalent relationship. Cell Mol Life Sci. 2010;67:545–556. doi: 10.1007/s00018-009-0209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Assinger A. Platelets and infection – an emerging role of platelets in viral infection. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nyström S., Hammarström P. Amyloidogenesis of SARS-CoV-2 spike protein. J Am Chem Soc. 2022;144:8945–8950. doi: 10.1021/jacs.2c03925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gershom E.S., Sutherland M.R., Lollar P., ELG Pryzdial. Involvement of the contact phase and intrinsic pathway in herpes simplex virus-initiated plasma coagulation. J Thromb Haemost. 2010;8:1037–1043. doi: 10.1111/j.1538-7836.2010.03789.x. [DOI] [PubMed] [Google Scholar]
- 309.Nicholson A.C., Hajjar D.P. Herpesviruses and thrombosis: activation of coagulation on the endothelium. Clin Chim Acta. 1999;286:23–29. doi: 10.1016/s0009-8981(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 310.Sutherland M.R., Raynor C.M., Leenknegt H., Wright J.F., Pryzdial E.L. Coagulation initiated on herpesviruses. Proc Natl Acad Sci U S A. 1997;94:13510–13514. doi: 10.1073/pnas.94.25.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.van Steijn J.H.M., van Tol K.M., van Essen L.H., Gans R.O.B. Disseminated intravascular coagulation as an unusual presentation of an Epstein–Barr virus infection. Neth J Med. 2000;57:169–171. doi: 10.1016/s0300-2977(00)00047-4. [DOI] [PubMed] [Google Scholar]
- 312.O’Connor D.S., Elmariah S., Aledort L.M., Pinney S.P. Disseminated intravascular coagulation complicating Epstein-Barr virus infection in a cardiac transplant recipient: a case report. Transplant Proc. 2010;42:1973–1975. doi: 10.1016/j.transproceed.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 313.Popović M., Smiljanić K., Dobutović B., Syrovets T., Simmet T., Isenović E.R. Human cytomegalovirus infection and atherothrombosis. J Thromb Thrombolysis. 2012;33:160–172. doi: 10.1007/s11239-011-0662-x. [DOI] [PubMed] [Google Scholar]
- 314.Squizzato A., Gerdes V.E., Büller H.R. Effects of human cytomegalovirus infection on the coagulation system. Thromb Haemost. 2005;93:403–410. doi: 10.1160/TH04-08-0523. [DOI] [PubMed] [Google Scholar]
- 315.Humblot S., Martin T., Pasquali J.-L., Korganow A.-S. Blood coagulation disorders during primary cytomegalovirus infection. Arch Intern Med. 2001;161:2149–2150. doi: 10.1001/archinte.161.17.2149. [DOI] [PubMed] [Google Scholar]
- 316.Pryzdial E.L.G., Wright J.F. Prothrombinase assembly on an enveloped virus: evidence that the cytomegalovirus surface contains procoagulant phospholipid. Blood. 1994;84:3749–3757. [PubMed] [Google Scholar]
- 317.van Dam-Mieras M.C., Muller A.D., van Hinsbergh V.W., Mullers W.J., Bomans P.H., Bruggeman C.A. The procoagulant response of cytomegalovirus infected endothelial cells. Thromb Haemost. 1992;68:364–370. [PubMed] [Google Scholar]
- 318.Assinger A., Kral J.B., Yaiw K.C., Schrottmaier W.C., Kurzejamska E., Wang Y., et al. Human cytomegalovirus–platelet interaction triggers toll-like receptor 2–dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol. 2014;34:801–809. doi: 10.1161/ATVBAHA.114.303287. [DOI] [PubMed] [Google Scholar]
- 319.Ahmad A., Menezes J. Binding of the Epstein-Barr virus to human platelets causes the release of transforming growth factor-beta. J Immunol. 1997;159:3984–3988. [PubMed] [Google Scholar]
- 320.Duan X., Chen H., Zhou X., Liu P., Zhang X., Zhu Q., et al. EBV infection in epithelial malignancies induces resistance to antitumor natural killer cells via F3-mediated platelet aggregation. Cancer Res. 2022;82:1070–1083. doi: 10.1158/0008-5472.CAN-21-2292. [DOI] [PubMed] [Google Scholar]
- 321.Agbanyo F.R., Wasi S. Human cytomegalovirus interaction with platelets and adhesive glycoproteins: significance in viral pathogenesis. J Infect Dis. 1994;170:1120–1127. doi: 10.1093/infdis/170.5.1120. [DOI] [PubMed] [Google Scholar]
- 322.Katzilieris-Petras G., Lai X., Rashidi A.S., Verjans G., Reinert L.S., Paludan S.R. Microglia activate early antiviral responses upon herpes simplex virus 1 entry into the brain to counteract development of encephalitis-like disease in mice. J Virol. 2022;96 doi: 10.1128/jvi.01311-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Patrycy M., Chodkowski M., Krzyzowska M. Role of microglia in herpesvirus-related Neuroinflammation and neurodegeneration. Pathogens. 2022;11:809. doi: 10.3390/pathogens11070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hassani A., Corboy J.R., Al-Salam S., Khan G. Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PloS One. 2018;13 doi: 10.1371/journal.pone.0192109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Jacob H.S., Visser M., Key N.S., Goodman J.L., Moldow C.F., Vercellotti G.M. Herpes virus infection of endothelium: new insights into atherosclerosis. Trans Am Clin Climatol Assoc. 1992;103:95–104. [PMC free article] [PubMed] [Google Scholar]
- 326.Taylor-Wiedeman J., Sissons J.G., Borysiewicz L.K., Sinclair J.H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 327.Söderberg-Nauclér C., Fish K.N., Nelson J.A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 328.Proal A., Marshall T. Myalgic encephalomyelitis/chronic fatigue syndrome in the era of the human microbiome: persistent pathogens drive chronic symptoms by interfering with host metabolism, gene expression, and immunity. Front Pediatr. 2018;6:373. doi: 10.3389/fped.2018.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Deumer U.S., Varesi A., Floris V., Savioli G., Mantovani E., López-Carrasco P., et al. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): an overview. J Clin Med. 2021:10. doi: 10.3390/jcm10204786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Siberry V.G.R., Rowe P.C. Pediatric Long COVID and Myalgic encephalomyelitis/chronic fatigue syndrome: overlaps and opportunities. Pediatr Infect Dis J. 2022;41 doi: 10.1097/INF.0000000000003477. e139-e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022 doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.McCully K.K., Natelson B.H. Impaired oxygen delivery to muscle in chronic fatigue syndrome. Clin Sci (Lond) 1999;97 603–8; discussion 11–3. [PubMed] [Google Scholar]
- 334.McCully K.K., Smith S., Rajaei S., Leigh J.S., Jr., Natelson B.H. Muscle metabolism with blood flow restriction in chronic fatigue syndrome. J Appl Physiol. 1985;2004(96):871–878. doi: 10.1152/japplphysiol.00141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Scherbakov N., Szklarski M., Hartwig J., Sotzny F., Lorenz S., Meyer A., et al. Peripheral endothelial dysfunction in Myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020;7:1064–1071. doi: 10.1002/ehf2.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]