Abstract
Background:
Metastatic prostate cancer (MPC) includes metastases detected at diagnosis (de novo) and those occurring after diagnosis with early-stage disease (recurrent). Cancer registries collect data only on de novo MPC, providing a partial picture of the burden of MPC. We use cancer registry data to estimate the number of men living with MPC in the US including both de novo and recurrent cases.
Methods:
We apply a back-calculation method to estimate MPC incidence and prevalence from US prostate cancer (PC) mortality and de novo MPC relative survival for cases diagnosed between 2000–2017 in 18 Surveillance, Epidemiology, and End Results registries. We hold overall PC mortality and MPC survival constant for future prevalence projections.
Results:
On January 1, 2018, we estimated 120,400 US men living with MPC (45% de novo, 55% recurrent). The age-adjusted prevalence in 2018 for Black men was over double that of White men (137.1 vs. 62.2 per 100,000 men). By 2030, 192,500 men are expected to be living with MPC, with the increase being driven by population growth projections.
Conclusions:
The number of men living with MPC in the US exceeds 100,000 and represents a small fraction of the >3 million men living with a prior diagnosis of PC.
Impact:
Relatively similar fractions of de novo and recurrent MPC among prevalent cases highlight opportunities for early detection and management of localized disease in reducing the MPC burden. Changes in diagnostic technologies could lead to greater growth in MPC cases in the US than projected.
Keywords: Metastatic prostate cancer, prevalence, de novo, recurrence, prostate cancer
Introduction
The vast majority of prostate cancer (PC) patients present with clinically localized disease. However, 6.8% of cases in the United States [1], representing over 18,000 men, are expected to be diagnosed with metastatic disease each year, with only 31% surviving at least five years [1]. Incidence in Black men is approximately double that in White men for localized disease (126.3 vs. 75.7 per 100,000 men/year), slightly higher for regional disease (18.5 vs. 15.2 per 100,000 men/year), and approximately double for distant disease (18.3 vs. 8.9 per 100,000 men/year), where distant disease is defined as disease that is metastatic at diagnosis [1]. Across stages, post-diagnosis survival is similar for Black and White men [1]. From 2010 to 2017, the incidence of metastatic prostate cancer (MPC) increased in the general population and among Black men [2], and the increase is more pronounced among younger men [3]. With improved survival and aging of the male population, it is likely that the number of men living with MPC is growing.
MPC represents the most advanced form of disease when resource utilization tends to be continuous and intensive and quality of life is severely impacted. There is an acute awareness of and interest in studying the needs of the population with MPC [4], which includes those presenting with metastatic disease at diagnosis (de novo) and those diagnosed with early-stage disease who later advance to metastasis. While cancer registry data can be used to estimate the number of men in the population who were initially diagnosed with MPC, they do not capture those who advance to MPC as registries do not collect longitudinal data providing information on recurrence or progression.
In this study, we estimate the number of men living with MPC including both those initially diagnosed with de novo MPC and those who advance to metastatic disease after being diagnosed with early-stage disease. Hereafter, we refer to this overall count of prevalent MPC cases as simply the “MPC prevalence.” In the absence of empirical data on the incidence of recurrent or progressive MPC, we employ a modeling approach that has previously been used in breast cancer [5] to estimate the size of the population living with metastatic disease. In addition, we separately calculate the de novo MPC prevalence using a counting method for Surveillance, Epidemiology, and End Results (SEER) registry data [6]. Together, our calculations permit partitioning MPC prevalence (i.e. those alive and coping with metastasis) into the proportions attributable to de novo versus non-de novo cases. We compare these proportions by race to explore disparities in the metastatic disease burden between White and Black men. We also provide projections of future MPC prevalence assuming continuation of current incidence rates and diagnostic and treatment modalities and based on population size projections through 2030.
Materials and Methods
Overview
Recurrence is generally considered to imply the return of a cancer after initial treatment and a disease-free period. However, identification of cases recurrent by this definition is challenging both clinically and in terms of data collection. It can be difficult to clinically identify a cancer-free period as a patient may have microscopic metastases that are not detectable and are therefore not documented. Thus, for the purposes of this study, “recurrent MPC” is used to designate men initially diagnosed with in situ, localized, or regional PC whose disease later advanced to metastasis in distant organs or tissues.
We estimate 1) the overall prevalence of MPC (i.e. all men alive with a prior PC diagnosis) and 2) the prevalence of de novo MPC. For the first prevalence, we use a back-calculation method to estimate first incidence and then prevalence from mortality and survival [7]. We previously applied this approach to estimate the prevalence of metastatic breast cancer [5, 7]. Because the back calculation method cannot distinguish between de novo and recurrent prevalent cases, we use a method encoded in the SEER*Stat software [6, 8] to estimate the de novo MPC prevalence. The difference between the overall and de novo MPC prevalence is the recurrent MPC prevalence. We then partition the overall MPC prevalence into the portions that arise from de novo versus recurrent presentation. The methods and data sources used for each calculation are described below.
Back calculation method to estimate overall prevalence of MPC (de novo and recurrent)
We use the Mortality Incidence Approach Model (MIAMOD), a computer package that implements a back-calculation method to estimate chronic disease incidence and prevalence from mortality and survival [7]. We assume that each observed PC death is the result of either de novo or recurrent MPC. The incidence of MPC can then be inferred from PC mortality and MPC survival. For example, if MPC mortality in year Y is 5 per 100,000 men, 60% of MPC cases survive 1 year, and the other 40% survive 2 years, then the incidence of MPC in year Y − 1 is 3 per 100,000 men/year and the incidence of MPC in year Y − 2 is 2 per 100,000 men/year. MIAMOD is equipped to handle multiple years of mortality data and considerably more complex mortality and survival distributions.
The prevalence in any given year, i.e., the number of people alive with MPC, is calculated from the estimated MPC incidence and overall survival as
where the sum is over all years t prior to Y. MPC prevalence is calculated and reported by race (All races, White, Black), age group, and years since diagnosis (0-<2 years, 2-<5 years, 5-<10 years, 10+ years). MIAMOD does not produce prevalence for the open age class 85+. To project prevalence for men 85+, we first calculate the prevalence for men in the 80–84 age group and then multiply by the size of the 85+ population to estimate the number of men living with MPC aged 85+. Prevalence projections from 2019 to 2030 assume constant PC mortality rates at 2018 levels, constant MPC survival, and dynamic population size projections for these years via the “Dynamic cohort, Linear period” projection option in MIAMOD. We also calculate prevalence projections assuming improving MPC survival for years 2019–2030; details are provided in the Supplementary Material and Methods.
MIAMOD software can be downloaded from (www.eurocare.it/MiamodPiamod/tabid/60/Default.aspx) [9].
Data sources for back-calculation method
The SEER Program (https://seer.cancer.gov/) collects clinical, demographic, and survival information on all cancer cases diagnosed in defined geographic areas within the US. We include data from the SEER-18 registries (November 2020 submission, RRID:SCR_006902) covering approximately 28% of the US population. SEER-18 provides incidence and survival data from 2000–2018.
The inputs to the back-calculation method are cancer deaths, all-cause deaths, population sizes, and MPC survival. We obtain US male deaths due to PC and all causes from 1990 to 2018 from the National Center for Health Statistics (NCHS) and US male populations from 1990 to 2018 from the US Census Bureau by single calendar year, single ages 0–84, and age group 85+ using the SEER*Stat software [8]. Future population projections are based on July 1, 2016 population estimates, which provide projections of the population for the years 2017 to 2060 (https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html).
MPC survival is estimated using data from men diagnosed with distant stage prostate cancer between 2000 and 2017 in the SEER-18 registries. For survival analysis, we include only invasive PCs that were the first primary tumor (sequence number 0 or 1). Thus, our prevalence estimates pertain only to men living with a prior prostate cancer diagnosis that was their first cancer diagnosis. We exclude men diagnosed through death certificate or autopsy due to uncertainty in the diagnosis date and also those with no follow-up information. Stage at diagnosis is defined using summary stage 2000, which is available for cancers diagnosed between 1998 and 2017. De novo MPC is defined as distant summary stage, which includes only tumors with distant metastasis. We estimate MPC relative survival by race (All races, White, Black), age group (0–49, 50–64, 65–74, 75–84), and year of diagnosis (2000–2005, 2006–2011, 2012–2017) via SEER*Stat. Relative survival is a proxy for disease-specific survival that is based on a comparison of all-cause deaths among men with de novo MPC and the age-matched population. Relative survival for men aged 85+ is assumed equal to that of men aged 75–84. In MIAMOD, we use the tabulated relative survival option, which uses relative survival grouped by age, race, and period of diagnosis data as input.
Our primary prevalence calculations assume equal survival for de novo and recurrent disease. For a sensitivity analysis, we recalculate prevalence under different assumptions for MPC survival. Scenarios with either 1) worse survival for recurrent disease or 2) better survival for recurrent disease are considered (details are provided in the Supplementary Material and Methods).
Prevalence of de novo MPC using the counting method
The prevalence of de novo MPC in SEER-18 areas is calculated directly using the SEER counting method and incidence data from the SEER-18 areas [6]. The counting method counts all men alive on December 31, 2018 with a previous diagnosis of distant PC (1998–2017) in the SEER-18 areas and adjusts for cases lost to follow-up. To estimate de novo MPC prevalence counts in the United States, we apply the SEER-18 prevalences by 5-year age group and race to the respective male US populations.
Pre- and post-processing of data was done in R version 4.0.5. All prevalence numbers reported below are rounded to the nearest 100.
Data Availability
The data used in this study are available upon request from the corresponding author. Data files used in the MIAMOD analysis are provided in the Supplementary Material and Methods.
Results
Relative survival
Relative survival calculations included 46,461 men diagnosed with distant PC from 2000 to 2017 (Table 1). Median survival and 5-year relative survival for men aged 50+ generally increased over time, though increases were modest. Median relative survival increased from 2.4 to 2.9 months and five-year relative survival increased from 29% to 31% comparing men diagnosed during the periods 2000–2005 and 2012–2017. The percentage of men who survived 10 years or more after de novo MPC diagnosis was similar for men diagnosed between 2000 and 2005 and men diagnosed between 2006 and 2011 (Table 1). Relative survival among Black men at five and ten years was generally similar to that among White men (Supplementary Table S1), although median survival was consistently slightly higher for White men than Black men across calendar intervals and for most age groups.
Table 1:
Number of men, median overall and relative survival in months, and 5-year relative survival in percentage (95% confidence interval) for men diagnosed with de novo metastatic prostate cancer (MPC) in the SEER-18 areas by grouped age (15–84) and year at diagnosis. Relative survival is presented in terms of de novo only survival.
| Year | Age, y | N | Median Relative Survival (in months) |
5-yr De novo Relative Survival (95% CI) | 10-yr De novo Relative Survival (95% CI) |
|---|---|---|---|---|---|
| 2000–2005 | 15–49 | 310 | 2.5 | 29% [24%, 34%] | 20% [15%, 24%] |
| 50–64 | 2986 | 2.8 | 32% [30%, 33%] | 19% [18%, 21%] | |
| 65–74 | 3689 | 2.9 | 34% [32%, 36%] | 18% [16%, 19%] | |
| 75–84 | 4073 | 2.1 | 26% [25%, 28%] | 15% [13%, 17%] | |
| 15+ | 12794 | 2.4 | 29% [28%, 30%] | 17% [16%, 17%] | |
| 2006–2011 | 15–49 | 349 | 2.7 | 28% [23%, 33%] | 13% [9%, 17%] |
| 50–64 | 3896 | 3.0 | 34% [33%, 36%] | 18% [17%, 20%] | |
| 65–74 | 3826 | 2.9 | 34% [33%, 36%] | 20% [18%, 22%] | |
| 75–84 | 3741 | 2.2 | 28% [26%, 30%] | 14% [12%, 16%] | |
| 15+ | 13812 | 2.5 | 30% [30%, 31%] | 16% [16%, 17%] | |
| 2012–2017 | 15–49 | 351 | 2.9 | 26% [20%, 32%] | ─ |
| 50–64 | 5404 | 3.4 | 35% [33%, 37%] | ─ | |
| 65–74 | 6136 | 3.3 | 35% [33%, 37%] | ─ | |
| 75–84 | 5002 | 2.5 | 29% [27%, 32%] | ─ | |
| 15+ | 19855 | 2.9 | 31% [30%, 32%] | ─ | |
Prevalence of MPC for all races
In 2018, we estimate that there are 120,400 men living with MPC in the US (MPC prevalence; Table 2), representing approximately 3.7% of the 3,245,400 men living in the US with a prior diagnosis of prostate cancer [10]. We project that by January 1, 2030, there will be 192,500 men living with MPC in the US, representing a 60% increase from 2018 (Table 2). Projected prevalence under improving MPC survival is estimated to be 208,500 in 2030 (Supplementary Table S2).
Table 2:
Estimates (January 1, 2018) and projections (January 1, 2030) of prostate cancer (PC) mortality, and incidence and prevalence in terms of numbers of cases of metastatic prostate cancer (MPC) including de novo and recurrent disease in the United States. Prevalence is estimated for all races assuming same survival for men with de novo and recurrent disease.
| Estimates on January 1, 2018 | |||||||
|
PC Deaths
(counts) |
MPC Incidence
(counts) |
MPC Prevalence
(counts) |
|||||
| Age | US Male Population | Observed | Estimated |
De novo
(Observed) |
De novo and recurrent (Estimated) |
De novo
(Observed) |
De novo and recurrence (Estimated) |
| 15–39 | 55,858,246 | 6 | 7 | 13 | 15 | 29 | 43 |
| 40–49 | 20,031,221 | 112 | 116 | 227 | 272 | 434 | 565 |
| 50–59 | 20,923,318 | 1,250 | 1,262 | 1,854 | 2,731 | 4,951 | 6,602 |
| 60–69 | 17,744,019 | 5,158 | 5,223 | 5,568 | 9,102 | 15,559 | 27,154 |
| 70–79 | 10,335,773 | 9,238 | 9,094 | 5,687 | 13,289 | 18,340 | 45,145 |
| 80–99 | 4,920,115 | 10,407 | 10,179 | 5,120 | 14,978 | 14,880 | 40,860 |
| 15–99 | 129,812,692 | 26,171 | 25,881 | 18,469 | 40,388 | 54,192 | 120,368 |
| Projections on January 1, 2030 | |||||||
|
PC Deaths
(counts) |
MPC Incidence
(counts) |
MPC Prevalence
(counts) |
|||||
| Age | Projected US Male Population | Observed | Estimated |
De novo
(Observed) |
De novo and recurrent (Estimated) |
De novo
(Observed) |
De novo and recurrence (Estimated) |
| 15–39 | 58,136,855 | ─ | 4 | ─ | 9 | ─ | 22 |
| 40–49 | 22,856,668 | ─ | 113 | ─ | 272 | ─ | 573 |
| 50–59 | 19,513,317 | ─ | 1,302 | ─ | 2,920 | ─ | 7,208 |
| 60–69 | 19,067,422 | ─ | 6,446 | ─ | 11,989 | ─ | 38,072 |
| 70–79 | 15,281,722 | ─ | 13,262 | ─ | 21,302 | ─ | 78,558 |
| 80–99 | 8,012,516 | ─ | 14,161 | ─ | 22,874 | ─ | 68,107 |
| 15–99 | 142,868,500 | ─ | 35,288 | ─ | 59,366 | ─ | 192,540 |
Of the 120,400 men in 2018 living with MPC, we estimate that 66,200 (55%) are non-distant-stage diagnoses who later progressed to MPC and are therefore not captured as MPC cases in cancer registry data (Table 2). The remaining 54,200 (45%) are cases who were initially diagnosed with de novo distant disease.
Figure 1 shows trends in the estimated MPC incidence and the calculated MPC prevalence including future projections to 2030. The estimated number of men living with MPC decreased approximately 20% from 1990 to 2000 and 13% from 2000 to 2010 and increased approximately 15% from 2010 to 2018. The projected increase in prevalence after 2018 is a function of the projected increase in MPC incidence along with the projected increase in population size.
Figure 1:
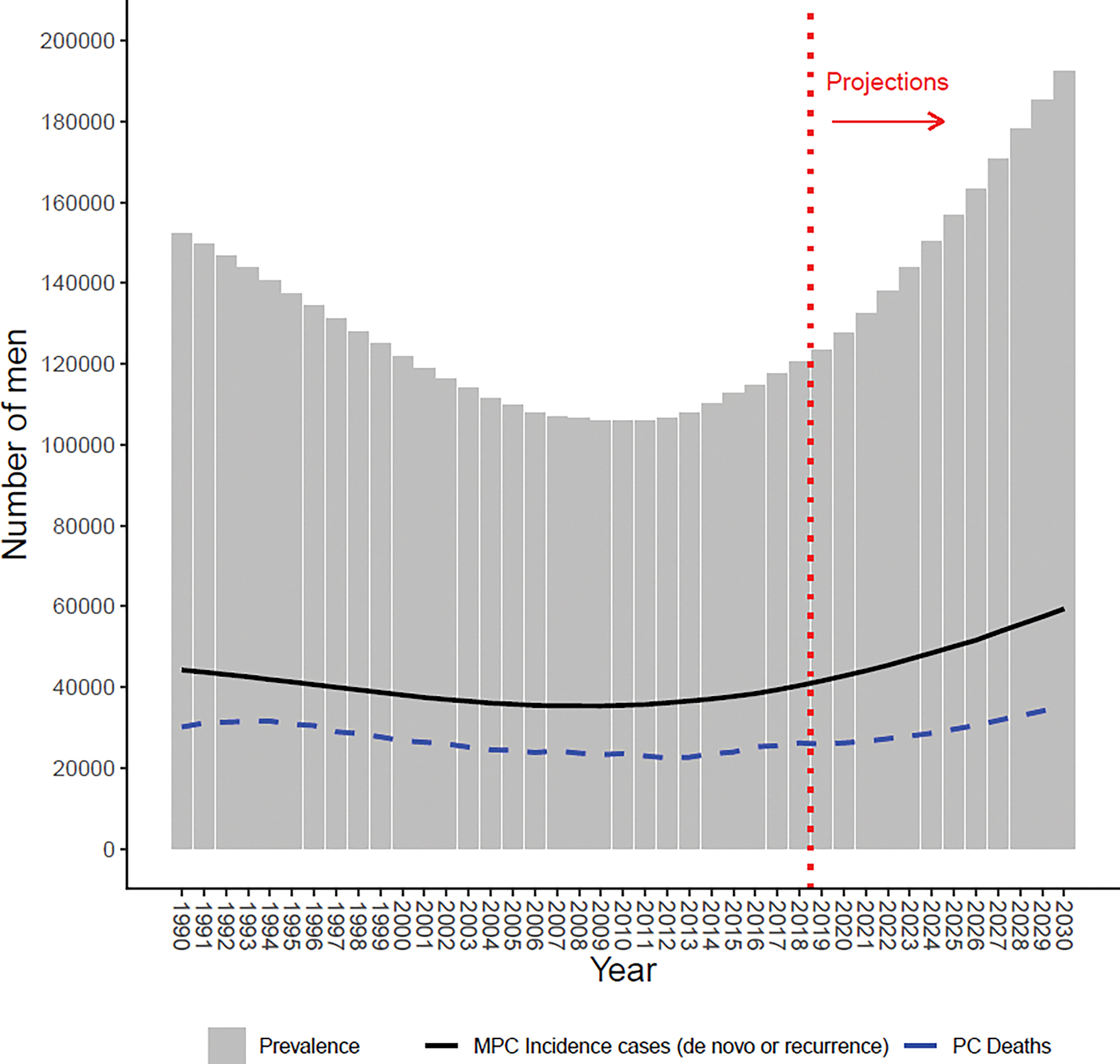
Prevalence over time for all races, assuming equal de novo and recurrent survival. Solid black line represents estimated number of new metastatic prostate cancer (MPC) cases; dashed blue line represents observed number of prostate cancer (PC) deaths; dotted red line indicates projections after 2018.
Most prevalent cases (72.8%) have been living with metastatic disease for less than five years, with approximately 44% of cases living less than two years with metastatic disease (Supplementary Table S3). Nearly one-quarter have been living with metastatic disease for more than five years (Supplementary Table S3).
These results assume equal disease-specific survival for men with de novo and recurrent MPC. Results assuming worse and better survival for recurrent MPC compared to de novo MPC are included in Supplementary Tables S4–S9 and Supplementary Figures S1–S2. Assuming worse recurrent survival leads to a reduction in the prevalence of MPC, and conversely assuming better recurrent survival results in an increase in the prevalence of MPC. In 2018, we estimate 88,900 men living with MPC assuming worse recurrent survival (Supplementary Table S5) and 169,000 men living with MPC assuming better recurrent survival (Supplementary Table S6).
Comparison of White and Black men
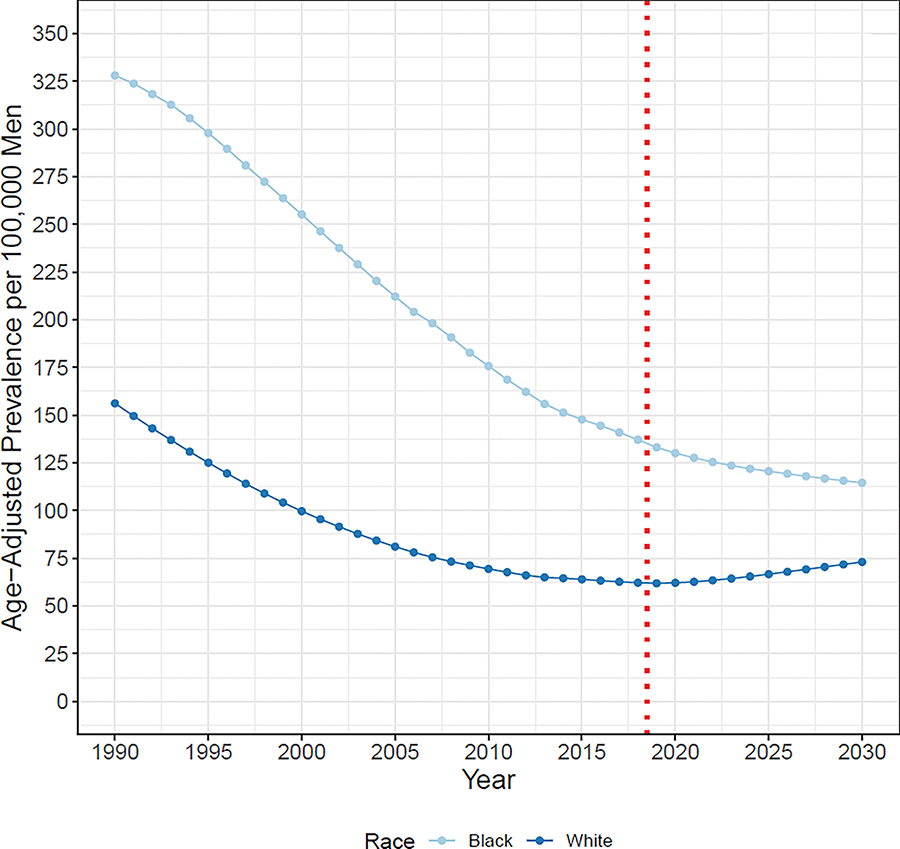
In 2018, we estimate 92,500 White men and 21,200 Black men living with MPC in the US (Supplementary Table S10). In 2018, 2,635,300 White men and 445,200 Black men are estimated to be living with PC [9]. Thus, nearly 3.5% of White and 4.8% of Black prevalent PC cases are metastatic. By 2030, we project 150,900 White men and 28,000 Black men will be living with MPC in the US, representing increases in prevalence of 63% and 32%, respectively, after 2018 (Figure 2, Supplementary Table S11). The differences in the projected prevalence after 2018 are due to differences in the projected MPC incidence trends along with the differences in population growth projections by race. When projections are expressed as prevalences per 100,000 men, the projected increase after 2018 is considerably less pronounced (Figure 3). Based on this figure, we estimate the prevalence in 2018 among Black men (137) to be approximately double that among White men (62), with the projected difference in prevalences for Black and White men narrowing between 2018 and 2030.
Figure 2:
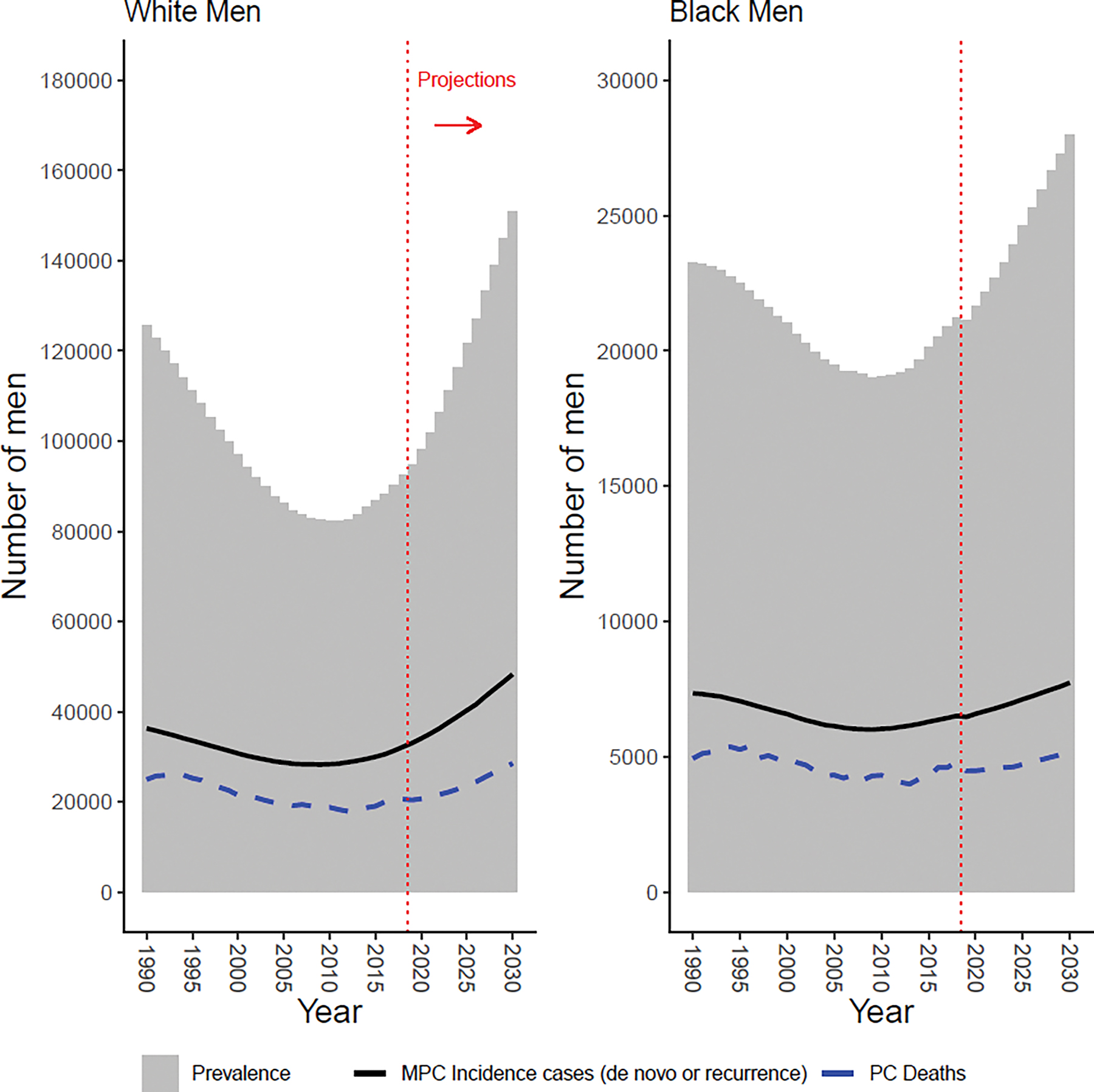
Prevalence over time for White men (left) and Black men (right), assuming equal de novo and recurrent survival. Solid black line represents estimated number of new metastatic prostate cancer (MPC) cases; dashed blue line represents observed number of prostate cancer (PC) deaths; dotted red line indicates projections after 2018.
Figure 3:
Age-adjusted MPC prevalences per 100,000 men for White and Black men. Prevalences are based on estimates of de novo and recurrent prevalence and are standardized to US 2000 standard population. Dotted red line indicates prevalences are based on projections after 2018.
Almost 75% of White and 70% of Black prevalent MPC cases have been living with metastatic disease for less than five years (Supplementary Table S3). Approximately 46% of White and 42% of Black prevalent cases have been living less than two years with metastatic disease. Nearly 10% of White and 13% of Black prevalent cases have been living with MPC for ten or more years.
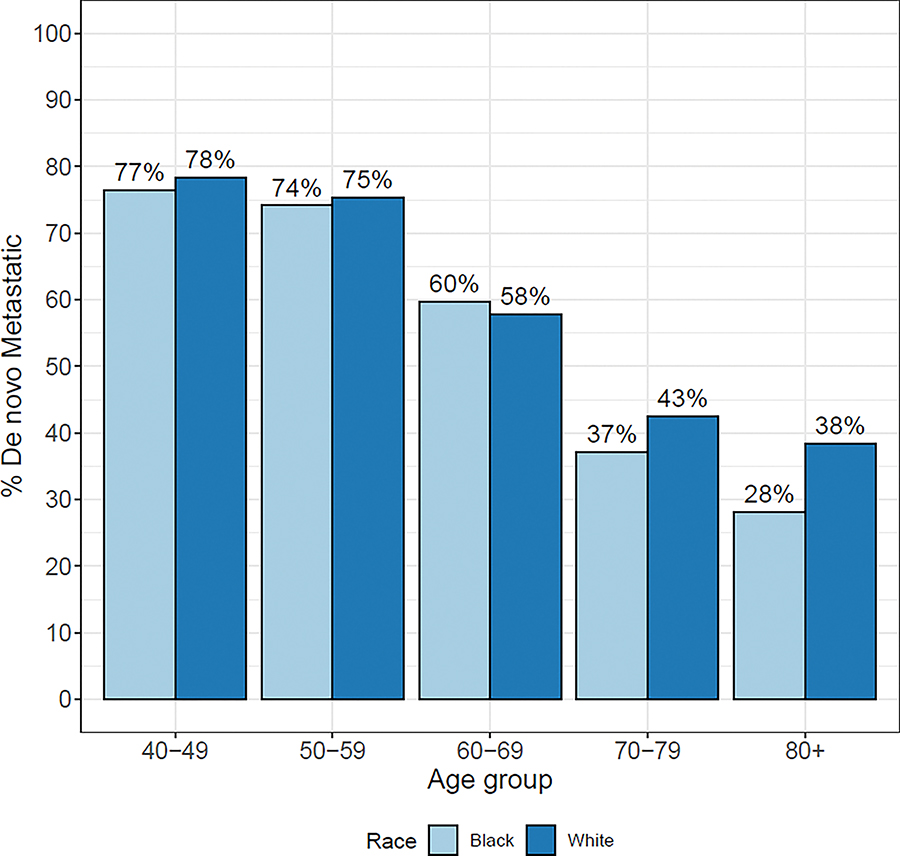
Among prevalent MPC cases in 2018, we estimate that 42,600 White men (46%) and 9,500 Black men (45%) were diagnosed de novo metastatic, and 49,800 White men (54%) and 11,800 Black men (55%) were diagnosed with early-stage PC that later progressed to metastasis (Supplementary Table S10). For men aged 40–69, the proportions of de novo metastatic disease are comparable between White and Black men (Figure 4). Among prevalent MPC cases aged 70+, Black men appear to have a lower percentage of de novo MPC than White men (Figure 4).
Figure 4:
Proportion of metastatic prostate cancer (MPC) prevalence in 2018 that is diagnosed de novo metastatic by age group and race. MPC prevalence calculated assuming equal de novo and recurrent survival.
Comparisons of White and Black prevalence by age and projected populations are presented in Supplementary Tables S10–S11 and Supplementary Figures S3–S5, respectively.
Discussion
The study presents a new estimate of the number of men living with metastatic prostate cancer in the United States. While overall prevalence of prostate cancer has been available for some time as a summary of disease burden provided by SEER, the prevalence of metastatic disease requires specialized methods to capture both newly diagnosed and progressive metastatic cases since data on progressive metastatic cases are not collected by cancer registries. Our results provide a detailed window into the burden of MPC in the population, indicating that the number of men living with MPC is substantial and likely to grow dramatically over the next decade. As a complement to our results, recent work by Gallicchio et al [11] offers a comprehensive overview of metastatic prevalence for the six most common cancer sites including prostate.
As a measure of disease burden, prevalence complements other commonly used surveillance summaries such as incidence and mortality. Prevalence provides a snapshot of the disease burden in the population at any given time. It is expressed in terms of absolute numbers of affected patients and thus more directly captures the magnitude of the problem and the resources needed than standard surveillance statistics that are expressed in terms of rates. If disease is stable, prevalence may increase solely due to increases in the size of the population or due to population aging. Prevalence that increases over time in a stable population may reflect either an increase in incidence or a lengthening of survival due to improvements in treatment, or both. Thus, increasing prevalence is not necessarily reflective of an adverse trend in disease control.
Our projections of the growth in the number of MPC cases essentially assume a stable disease process in terms of incidence and survival after 2018 and reflect only projected population growth and aging. When allowing for improved MPC survival after 2018, our projected prevalence in 2030 increased approximately 8%. Our estimates do not accommodate likely future changes in disease detection and management and are therefore likely underestimating the growth in the number of MPC cases expected to result from increasing use of effective systemic therapies, which will increase MPC survival, and dissemination of novel imaging tests, such as PSMA-PET/CT, which are far more able to identify metastatic sites than existing modalities.
Among all prevalent prostate cancer cases, those with MPC constitute a relatively small fraction, less than 5%. This does not at all indicate that the burden of MPC is inconsequential; rather it shines a light on the high frequency of prostate cancer diagnosis and the generally favorable survival in the PSA era. It further highlights the need for assessing both the overall prevalence of PC (i.e., all men alive with a prior PC diagnosis) and MPC prevalence (those alive and coping with metastasis) since these reflect quite different groups of patients.
Comparisons of MPC prevalences for Black and White men replicate known disparities in other measures of disease burden including incidence and fraction presenting with distant disease. We find that among prevalent MPC cases, the fraction de novo versus recurrent is fairly similar across race groups, but that MPC cases constitute a higher fraction of overall prevalent prostate cancer cases among Black men than White men. The disproportionate prevalence of MPC among prevalent prostate cancer cases is a disparity that has not been previously described in the literature. This translates into a higher proportion of men vulnerable to the morbidity of treatment who ultimately constitute the pool of fatal cases. Understanding what drives this MPC disparity is an important step in creating racial parity in prostate cancer outcomes.
A key limitation of the back-calculation approach is that survival for both de novo and recurrent MPC must be provided as inputs to the MIAMOD procedure. While information on survival after de novo diagnosis is readily available from SEER registries, survival from metastatic recurrence is less well documented. A recent study [12] has suggested that survival from metastatic recurrence is less favorable than from de novo metastatic diagnosis, and our sensitivity analysis considers this possibility along with the reverse. Our results are relatively robust and qualitatively similar across these different settings. While MIAMOD accounts for other-cause mortality through the inclusion of US mortality data through 2018, we are unable to capture the impact of COVID-19 on overall survival, which may be more pronounced for older men. Thus, our prevalence projections from 2019 to 2030 may be overestimated. Additionally, we focus on the set of men with no prior diagnosis of another cancer, even if they had MPC. As a result, some men may be excluded from our prevalence calculation
Our prevalence estimates are conditional on our model inputs and should be interpreted in the context of these inputs. The input with the largest impact on the prevalence estimate is metastatic survival. Our assumption regarding how recurrent compares to de novo MPC survival has a clear impact on the resulting prevalence, as shown by our sensitivity analysis. We report our prevalence as a point estimate largely for simplicity, but it may be most accurate to report prevalence estimates such as ours as a range to emphasize the uncertainty generated by our modeling assumptions. The collection of SEER registries used to extract the survival and incidence may also modestly impact the prevalence estimate.
In conclusion, we provide estimates of the prevalence of MPC in the US population. Our future projections indicate a significant increase in the burden of MPC but likely understate how it is poised to evolve. The proportion of Black prostate cancer survivors that are coping with metastatic disease is higher than White survivors and is consistent with well-documented racial disparities in disease incidence, metastatic diagnosis, and survival. Prevalence is an absolute measure that reflects the number of cases with a specific condition and is the most directly tied to expected resource needs for disease management and control. In advocating for resources to address prostate cancer now and in the future, we therefore propose that the prevalence of metastatic disease be cited in addition to overall prevalence as a key measure of the burden of prostate cancer in the population.
Supplementary Material
FINANCIAL SUPPORT/GRANTS:
RE’s work was supported by the National Cancer Institute (grants U01CA199338, U01CA253915, U01CA425659 and P50CA423489). RE was also supported in part by the Rosalie and Harold Rea Brown Endowed Chair. YAN’s work was supported in part by the Department of Defense (W81XWH2110531) and the National Cancer Institute (P50 CA097186-17, U01CA253915, and 1L60CA274879-01).
Footnotes
CONFLICT OF INTEREST: TPD, ABM, and RE have nothing to disclose. YAN is a research consultant for Ortho-Clinical Diagnostics, which does not conflict with any of the work presented in this manuscript.
DISCLAIMER: The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. [Cited 2021 September 27]. Available from https://seer.cancer.gov/statistics-network/explorer/.
- 2.Negoita S, Feuer EJ, Mariotto A, et al. Annual Report to the Nation on the Status of Cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124(13):2801–2814. doi: 10.1002/cncr.31549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur Urol Focus. 2018;4(1):121–127. doi: 10.1016/j.euf.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollica MA, Smith AW, Tonorezos E, et al. Survivorship for Individuals Living with Advanced and Metastatic Cancers: National Cancer Institute Meeting Report. J Natl Cancer Inst 2021; doi: 10.1093/jnci/djab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809–815. doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merrill RM, Capocaccia R, Feuer EJ, Mariotto AB. Cancer prevalence estimates based on tumour registry data in the Surveillance, Epidemiology, and End Results (SEER) Program. Int J Epidemiol. 2000;29(2):197–207. doi: 10.1093/ije/29.2.197. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia A, Capocaccia R, Egidi V, Golini A. A method for the estimation of chronic disease morbidity and trends from mortality data. Stat Med. 1989;8(2):201–216. doi: 10.1002/sim.4780080207. [DOI] [PubMed] [Google Scholar]
- 8.Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 8.4.0.
- 9.De Angelis G, De Angelis R, Frova L, Verdecchia A. MIAMOD: a computer package to estimate chronic disease morbidity using mortality and survival data. Comput Method Programs Biomed. 1994;44(2):99–107. doi: 10.1016/0169-2607(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). Prevalence database: “US Estimated Complete Prevalence Counts on 1/1/2018”. National Cancer Institute, DCCPS, Surveillance Research Program, Data Analytics Branch, released April 2021, based on the November 2020 SEER data submission.
- 11.Gallicchio L, Devasia TP, Tonorezos E, Mollica MA, Mariotto A. Estimation of the numbers of individuals living with metastatic cancer in the United States [ published online ahead of print, 2022 Aug 22]. J Natl Cancer Inst. 2022;djac158. doi: 10.1093/jnci/djac158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borno HT, Cowan JE, Zhao S, Broering JM, Carroll PR, Ryan CJ. Examining initial treatment and survival among men with metastatic prostate cancer: An analysis from the CaPSURE registry. Urol Oncol. 2020;38(10):793.e1–793.e11. doi: 10.1016/j.urolonc.2020.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available upon request from the corresponding author. Data files used in the MIAMOD analysis are provided in the Supplementary Material and Methods.