Abstract
Purpose:
Older patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) may be considered ineligible for curative-intent therapy including high-dose chemotherapy with autologous stem-cell transplantation (HDT-ASCT). Here, we report outcomes of a preplanned subgroup analysis of patients ≥65 years in ZUMA-7.
Patients and Methods:
Patients with LBCL refractory to or relapsed ≤12 months after first-line chemoimmunotherapy were randomized 1:1 to axicabtagene ciloleucel [axi-cel; autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy] or standard of care (SOC; 2–3 cycles of chemoimmunotherapy followed by HDT-ASCT). The primary endpoint was event-free survival (EFS). Secondary endpoints included safety and patient-reported outcomes (PROs).
Results:
Fifty-one and 58 patients aged ≥65 years were randomized to axi-cel and SOC, respectively. Median EFS was greater with axi-cel versus SOC (21.5 vs. 2.5 months; median follow-up: 24.3 months; HR, 0.276; descriptive P < 0.0001). Objective response rate was higher with axi-cel versus SOC (88% vs. 52%; OR, 8.81; descriptive P < 0.0001; complete response rate: 75% vs. 33%). Grade ≥3 adverse events occurred in 94% of axi-cel and 82% of SOC patients. No grade 5 cytokine release syndrome or neurologic events occurred. In the quality-of-life analysis, the mean change in PRO scores from baseline at days 100 and 150 favored axi-cel for EORTC QLQ-C30 Global Health, Physical Functioning, and EQ-5D-5L visual analog scale (descriptive P < 0.05). CAR T-cell expansion and baseline serum inflammatory profile were comparable in patients ≥65 and <65 years.
Conclusions:
Axi-cel is an effective second-line curative-intent therapy with a manageable safety profile and improved PROs for patients ≥65 years with R/R LBCL.
Translational Relevance.
Older patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) may be considered ineligible for curative-intent treatments such as high-dose chemotherapy with autologous stem-cell transplantation due to age and/or the presence of comorbidities that increase the risk of intolerable adverse events. In this preplanned subgroup analysis of patients aged ≥65 years enrolled in the ZUMA-7 trial of axicabtagene ciloleucel [axi-cel; an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy] versus standard of care (SOC) in second-line R/R LBCL, CAR T-cell expansion was comparable, with patients aged ≥65 years and axi-cel having significantly improved event-free survival and health-related quality of life over SOC. Although the pharmacodynamic (serum proinflammatory and immune-modulatory analytes, including cytokines and chemokines) profile of axi-cel was elevated post axi-cel infusion in patients ≥65 years versus patients <65 years, adverse events were manageable. Together, these results support axi-cel as a viable and effective curative-intent second-line treatment option for older patients with R/R LBCL.
Introduction
Age can be a determining factor when using curative-intent therapy, in part due to increased toxicity (1–3). Historically, patients were frequently deemed transplant-ineligible based upon age due to an inability to tolerate the toxicities of high-dose chemotherapy, and recommended therapies for older patients were described as palliative with a very poor chance of long-term disease control (4). Older patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) are at risk of worse outcomes and an inability to tolerate second-line standard-of-care (SOC) chemotherapy-based treatment (1–3). Given the increased risk for toxicities with SOC treatment, including nausea, fatigue, and infections, quality of life (QoL) is significantly decreased after SOC-based regimens (5). Considering the median age at LBCL diagnosis is 66 years (2), there remains a large unmet need for effective and tolerable curative-intent therapies in older patients with R/R LBCL.
Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved for the treatment of R/R LBCL in adult patients after ≥2 lines of systemic therapy. In addition, in the United States and the European Union, axi-cel is approved for LBCL that is refractory to or relapsed ≤12 months after first-line chemoimmunotherapy, based on the phase III ZUMA-7 (NCT03391466) study comparing axi-cel to SOC as second-line treatment in patients with R/R LBCL (6, 7). In the primary analysis of ZUMA-7, axi-cel significantly improved event-free survival (EFS) versus SOC (HR, 0.398, P < 0.0001), with a longer median EFS (8.3 vs. 2.0 months) and higher estimated 24-month EFS rate (40.5% vs. 16.3%; ref. 8). The adverse event (AE) profile of axi-cel was consistent with a prior axi-cel study in refractory LBCL (9).
Quality of life (QoL) is an important consideration for patients when selecting a therapeutic approach, and may be a determining factor for older patients. Second-line SOC is often associated with poor health-related QoL (10), and QoL further declines following chemotherapy (11, 12). Patient-reported outcome (PRO) instruments, including the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) and EuroQoL 5-dimension questionnaire using a 5-level scale (EQ-5D-5L), are used in oncology clinical trials to assess cancer and/or cancer therapies’ impact on health-related QoL. PRO data from clinical trials are considered by regulatory agencies during evaluation of new drug applications and development of product labeling (13–15). However, despite the potential usefulness of PRO data to inform treatment decisions, there is a lack of published literature on health-related QoL in R/R LBCL, especially in older patients. In ZUMA-7, axi-cel patients demonstrated clinically meaningful QoL improvement versus SOC patients (8).
Here, we report the results from a preplanned subgroup analysis of patients ≥65 years with R/R LBCL assessing clinical outcomes and PROs of second-line axi-cel versus SOC in the ZUMA-7 trial. In addition, we report levels of CAR T cells and serum markers of inflammation in patients ≥65 years compared with patients <65 years.
Patients and Methods
Patients
Full ZUMA-7 details were previously reported (8). Briefly, eligible patients were ≥18 years (no upper age limit), had LBCL confirmed by histology according to World Health Organization 2016 classification criteria (16), and were refractory to first-line treatment or had relapsed ≤12 months after completing first-line chemoimmunotherapy. Patients were intended to proceed to high-dose chemotherapy with autologous stem-cell transplantation (HDT-ASCT).
Trial design
All patients provided written informed consent. The trial was conducted after institutional review board approval of the protocol and in compliance with the Declaration of Helsinki. Patients were randomized 1:1 to receive axi-cel or investigator-selected SOC chemoimmunotherapy, stratified by response to first-line therapy and second-line age-adjusted International Prognostic Index (sAAIPI; ref. 8). Axi-cel patients underwent leukapheresis followed by conditioning chemotherapy with cyclophosphamide (500 mg/m2/day) and fludarabine (30 mg/m2/day) 5, 4, and 3 days before receiving an axi-cel infusion (target dose, 2 × 106 CAR T cells/kg). Optional bridging therapy was limited to glucocorticoids to isolate the effects of CAR T-cell therapy as second-line therapy (8). SOC patients received 2 to 3 cycles of protocol-defined, investigator-selected platinum-based chemoimmunotherapy. Patients who had a complete or partial response following chemoimmunotherapy proceeded to HDT-ASCT. Disease assessments per Lugano classification (17) occurred at time points specified from randomization. Although trial crossover between treatment groups was not planned, patients who did not respond to SOC could receive off-protocol treatment, including cellular immunotherapy.
Endpoints and assessments
The primary endpoint was EFS [time from randomization to the earliest date of disease progression according to the Lugano classification (17), new lymphoma therapy, death from any cause, or a best response of stable disease up to and including the response on day 150 assessment after randomization, per blinded central review]. Key secondary endpoints were objective response rate (ORR) and overall survival (OS). Other secondary endpoints were progression-free survival (PFS) and incidence of AEs, including cytokine release syndrome (CRS; ref. 18), neurologic events (19), and QoL.
Peak CAR T-cell levels and change over time were exploratory endpoints (8). Pharmacokinetic (PK) analysis of CAR T cells was performed by qPCR, as previously described (8, 20). Peak of anti-CD19 CAR T cells (cells/μL blood) was calculated as previously described (20). Serum cytokines, chemokines, and other inflammatory markers were analyzed with validated Meso Scale Discovery methods at MedPace. Peak of cytokine levels post baseline was defined as the maximum level of cytokines in serum attained after baseline up to week 4 postinfusion. AUC of cytokine levels from baseline to week 4 postinfusion was defined as the AUC in a plot of levels of cytokines against scheduled visits (from baseline to week 4 postinfusion). This AUC measured the total levels of cytokines over time. The trapezoidal rule was used to estimate AUC (21).
PRO instruments (EORTC QLQ-C30 and EQ-5D-5L; Supplementary Table S1) were administered at baseline, day 50, day 100, day 150, month 9, and every 3 months thereafter from randomization up to 24 months but were not required after an EFS event. PRO instrument analyses were conducted as previously reported for the full study population.
Statistical analysis
The primary efficacy analysis was previously reported (8), and analyses conducted for this preplanned subset analysis were similar. Multivariate analyses of EFS, ORR, OS, and PFS (per investigator assessment) were conducted to adjust for multiple covariates [gender, disease type per investigator, molecular subgroup per investigator, lactate dehydrogenase, tumor burden (sum of product diameters per investigator, mm2; ref. 22), and age (year)]. Reported here is an OS interim analysis that occurred at primary analysis of EFS; a prespecified sensitivity analysis of OS (rank preserving structure failure time model; ref. 23) was conducted to adjust for the confounding effect of treatment switching from SOC to cellular immunotherapy.
Prespecified hypotheses for PRO domains [EORTC QLQ-C30 Physical Functioning, EORTC QLQ-C30 Global Health Status/QoL, and EQ-5D-5L visual analog scale (VAS)] were tested as previously reported (24, 25). A mixed-effects model with repeated measures at day 100 and at subsequent time points conditional on statistical significance at the previous time point was used. A clinically meaningful change was defined as 10 points for each EORTC QLQ-C30 score, 7 points for each EQ-5D-5L VAS score, and 0.06 point for EQ-5D-5L index (26, 27). Sensitivity analyses were conducted to control for patterns of missingness (Model 2) and patterns of missingness with additional covariates (Model 3) to account for attrition over time.
Efficacy analyses were conducted according to the intention-to-treat principle and included randomized ZUMA-7 patients ≥65 years, with additional analyses conducted in patients ≥70 years. Safety analyses included randomized patients ≥65 years who received axi-cel or ≥1 dose of SOC therapy per protocol, analyzed by protocol therapy received. The QoL subgroup included patients ≥65 years who had a baseline PRO and ≥1 post-baseline measure completed (24, 25). CAR T-cell level analyses included axi-cel infused patients with ≥1 evaluable blood sample collected ≤1 month postinfusion.
Kaplan–Meier estimates for time-to-event endpoints were provided. Estimated HRs with two-sided 95% confidence intervals (CI) were calculated from a stratified Cox proportional-hazards model. Stratified log-rank P values (one-sided) were calculated for time-to-event endpoints. Response was evaluated with stratified Cochran–Mantel–Haenszel test. All reported P values are descriptive.
Data availability statement
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
Results
Patients
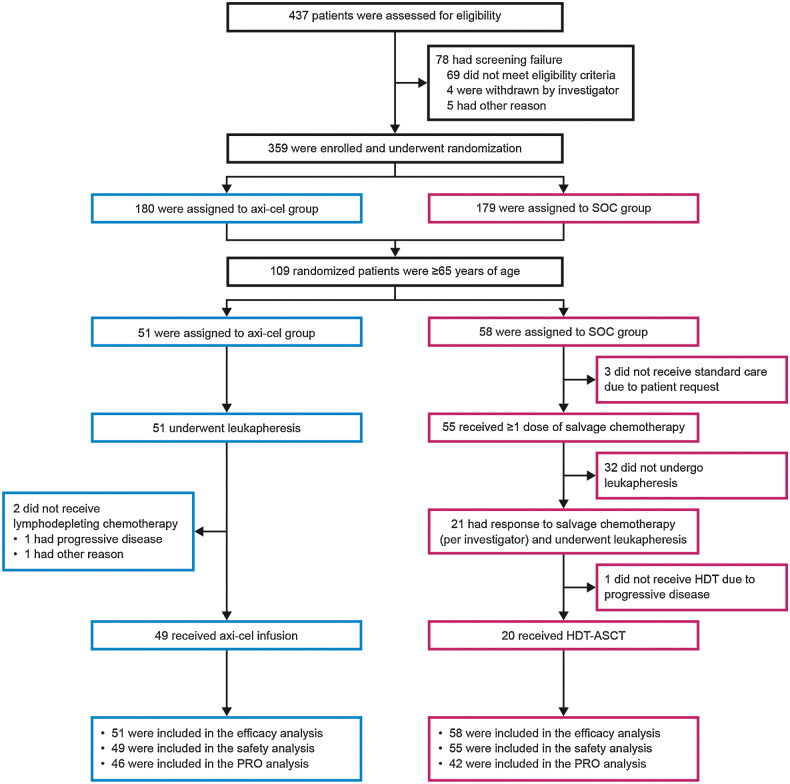
Of 359 patients randomized in the ZUMA-7 primary analysis, 109 patients were ≥65 years (axi-cel: 51 and SOC: 58; Fig. 1). Of those patients, 53 were ≥70 years (axi-cel: 26 and SOC: 27). As of March 18, 2021, the median follow-up from randomization was 24.3 months. The median age of all patients ≥65 years was 69 years (range, 65–81). Overall, 70% of patients had primary refractory disease. Baseline characteristics were generally balanced between the axi-cel and SOC arms, although more axi-cel versus SOC patients had high-risk features, including high sAAIPI 2–3 (53% vs. 31%), elevated lactate dehydrogenase (61% vs. 41%), and high-grade B-cell lymphoma (per investigator; 33% vs. 14%; Table 1).
Figure 1.
Randomization, treatment, and follow-up of patients ≥65 years. Figure shows the disposition of patients ≥65 years randomized to axi-cel and SOC arms.
Table 1.
Baseline characteristics in patients ≥65 years.a
| Characteristic | Axi-Cel, N = 51 | SOC, N = 58 | Overall, N = 109 |
|---|---|---|---|
| Median age (range), years | 70 (65–80) | 69 (65–81) | 69 (65–81) |
| Male sex, n (%) | 28 (55) | 39 (67) | 67 (61) |
| Race or ethnic group, n (%)b | |||
| American Indian or Alaska Native | 0 | 1 (2) | 1 (1) |
| Asian | 2 (4) | 2 (3) | 4 (4) |
| White | 47 (92) | 54 (93) | 101 (93) |
| Other | 2 (4) | 1 (2) | 3 (3) |
| Hispanic or Latino ethnic group, n (%)b | |||
| Yes | 3 (6) | 4 (7) | 7 (6) |
| No | 47 (92) | 53 (91) | 100 (92) |
| Not reported | 1 (2) | 1 (2) | 2 (2) |
| ECOG performance status score of 1, n (%)c | 26 (51) | 22 (38) | 48 (44) |
| Disease stage, n (%) | |||
| I-II | 9 (18) | 14 (24) | 23 (21) |
| III-IV | 42 (82) | 44 (76) | 86 (79) |
| sAAIPI of 2–3, n (%)d,e | 27 (53) | 18 (31) | 45 (41) |
| Molecular subgroup according to central laboratory, n (%)f | |||
| Germinal center B-cell–like | 32 (63) | 34 (59) | 66 (61) |
| Activated B-cell–like | 3 (6) | 3 (5) | 6 (6) |
| Unclassified | 4 (8) | 3 (5) | 7 (6) |
| Not applicable | 7 (14) | 7 (12) | 14 (13) |
| Missing data | 5 (10) | 11 (19) | 16 (15) |
| Disease type according to central laboratory, n (%) | |||
| DLBCL not otherwise specified/without further classification possibleg | 33 (65) | 42 (72) | 75 (69) |
| HGBL, including rearrangement of MYC with BCL2 or BCL6 or both | 12 (24) | 7 (12) | 19 (17) |
| Not confirmed or missing data | 5 (10) | 7 (12) | 12 (11) |
| Other | 1 (2) | 2 (3) | 3 (3) |
| Disease type according to the investigator, n (%) | |||
| DLBCL not otherwise specified | 27 (53) | 40 (69) | 67 (61) |
| T-cell/histiocyte-rich LBCL | 0 | 1 (2) | 1 (1) |
| Large cell transformation from follicular lymphomah | 7 (14) | 9 (16) | 16 (15) |
| HGBL with/without MYC and BCL2 and/or BCL6 rearrangement | 17 (33) | 8 (14) | 25 (23) |
| Prognostic marker according to central laboratory, n (%) | |||
| HGBL, double or triple hit | 12 (24) | 7 (12) | 19 (17) |
| Double-expressor lymphoma | 20 (39) | 23 (40) | 43 (39) |
| MYC rearrangement | 4 (8) | 2 (3) | 6 (6) |
| Not applicable | 15 (29) | 21 (36) | 36 (33) |
| Missing data | 0 | 5 (9) | 5 (5) |
| Response to 1L therapy, n (%)e | |||
| Primary refractory | 37 (73) | 39 (67) | 76 (70) |
| Relapse ≤12 months after initiation or completion of 1L therapy | 14 (27) | 19 (33) | 33 (30) |
| Bone marrow involvement, n (%)i | 1 (2) | 4 (7) | 5 (5) |
| Elevated LDH level, n (%)j | 31 (61) | 24 (41) | 55 (50) |
| Median tumor burden (range), mm2,k | 1826 (181–22538) | 1722 (252–16649) | 1775 (181–22538) |
Abbreviations: 1L, first-line; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HGBL, high-grade B-cell lymphoma; LDH, lactate dehydrogenase; sAAIPI, second-line age-adjusted International Prognostic Index.
aPatients were randomly assigned to receive axi-cel or SOC. Percentages may not total 100 because of rounding.
bRace and ethnic group were determined by the investigator.
cEastern Cooperative Oncology Group (ECOG) performance status scores are assessed on a 5-point scale, with a score of 0 indicating no symptoms and higher scores indicating greater disability. A score of 1 indicates that the patient is ambulatory but restricted from strenuous activity. Only patients with an ECOG performance status score of 0–1 were included in the study.
dValues are the sAAIPI at randomization, which were like the sAAIPI according to the investigator as entered into the clinical database. The sAAIPI is used to assess prognostic risk based on various factors after adjustment for patient age and extranodal status at the time of diagnosis of refractory disease; risk categories are assessed as low (0 factors), intermediate (1 factor), or high (2 or 3 factors).
eAs reported by investigator at time of randomization via Interactive Voice/Web Response System.
fThe molecular subgroup as assessed by the investigator was as follows: germinal center B-cell–like in 28 patients (55%) in the axi-cel arm, 24 (41%) in the SOC arm, and 52 (48%) overall; non–germinal center B-cell–like in 15 (29%), 21 (36%), and 36 (33%), respectively. The molecular subgroup was not assessed in 8 patients (16%) in the axi-cel arm, 13 (22%) in the SOC arm, and 21 (19%) overall.
gThe definition of DLBCL according to the central laboratory included cases of incomplete evaluation that were due to inadequate sample amount or sample type, for which further classification of the subtype was not possible. DLBCL, not otherwise specified, according to the World Health Organization 2016 definition (16), is also included.
hTransformation was defined as the presence of large cells noted anywhere in the biopsy sample.
iThe data shown were as collected on the diagnosis history case-report form.
jAn elevated lactate dehydrogenase level was defined as a level that was above the upper limit of the normal range per local laboratory reference range.
kTumor burden was determined on the basis of the sum of product diameters of the target lesions, according to the Cheson criteria (54) and was assessed by the central laboratory.
The manufacturing success rate of axi-cel for patients ≥65 years was 100%. Of 51 patients randomized to axi-cel, 49 (96%) received axi-cel. Among 58 patients randomized to SOC, 55 (95%) initiated second-line chemoimmunotherapy and 20 (34%) reached HDT-ASCT (Fig. 1). For patients who received axi-cel, median time from leukapheresis to product release (when the product passed quality testing and was made available to the investigator) was 12 days (range, 10–19) and median time from leukapheresis to delivery of axi-cel at study site was 18 days (range, 13–49; Supplementary Table S2). In patients ≥70 years, 24 (92%) axi-cel patients received axi-cel, and 6 (22%) SOC patients reached HDT-ASCT.
Efficacy
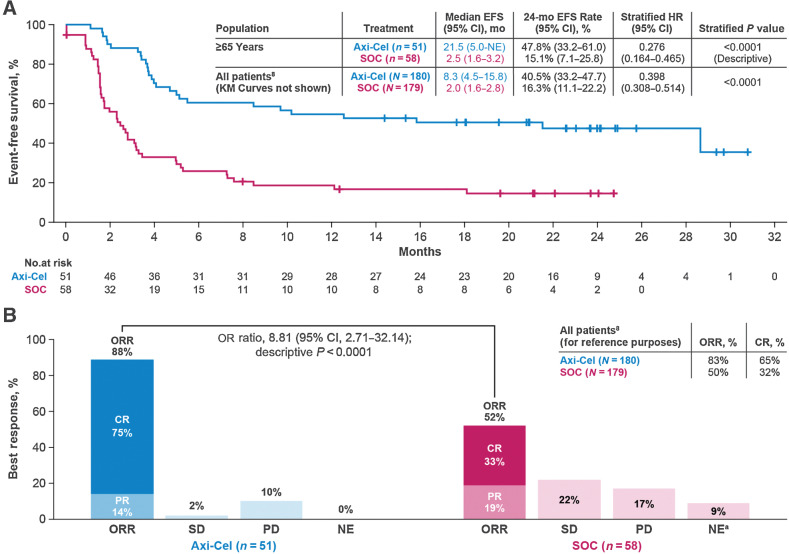
The primary endpoint of EFS in patients ≥65 years was significantly longer in the axi-cel versus the SOC arm (HR, 0.276; descriptive P < 0.0001; Fig. 2A) with a median EFS of 21.5 months [95% CI, 5.0–not estimable (NE)] vs. 2.5 months (95% CI, 1.6–3.2), respectively. The Kaplan–Meier estimate of the EFS rate at 24 months was 47.8% (95% CI, 33.2–61.0) in the axi-cel and 15.1% (95% CI, 7.1–25.8) in the SOC arm. The Kaplan–Meier estimate of the EFS rate at 12 months in patients ≥70 years was 42.3% (95% CI, 23.5–60.0) in the axi-cel and 3.7% (95% CI, 0.3–15.9) in the SOC arm (Supplementary Fig. S1A). Multivariate analyses showed similar EFS results when adjusted for baseline characteristics differences (HR, 0.23; 95% CI, 0.12–0.45; descriptive P < 0.0001). ORR was significantly higher in the axi-cel versus the SOC arm [88% vs. 52%; OR, 8.81 (95% CI, 2.71–32.14; descriptive P < 0.0001) in patients ≥65 years; Fig. 2B] and in patients ≥70 years (88% vs. 41%; descriptive P < 0.001; Supplementary Fig. S1B). After adjusting for differences in baseline characteristics among patients ≥65 years, odds of achieving objective response continued to favor axi-cel (OR, 9.61; 95% CI, 2.54–36.32; descriptive P < 0.001). Complete response (CR) rate was higher with axi-cel versus SOC [CR: 75% vs. 33%; OR, 8.95; 95% CI, 2.78–25.02; descriptive P < 0.0001) in patients ≥65 years; similar results were seen in patients ≥70 years (69% vs. 22%; descriptive P < 0.01).
Figure 2.
Event-free survival per central review and ORR in patients ≥65 years. A, The Kaplan–Meier estimate of EFS by blinded central review in patients ≥65 years. EFS was defined as the time from randomization to the earliest date of disease progression according to the Lugano classification (17), new lymphoma therapy, death from any cause, or a best response of stable disease up to and including the response on day 150 assessment after randomization, per blinded central review. Tick marks indicate patients who did not meet the criteria for an event and were censored. B, Summary of best response by blinded central review in patients ≥65 years. a In the SOC arm, 1 patient had undefined disease, and 4 did not have response assessments completed. EFS, event-free survival; PD, progressive disease; PR, partial response; SD, stable disease.
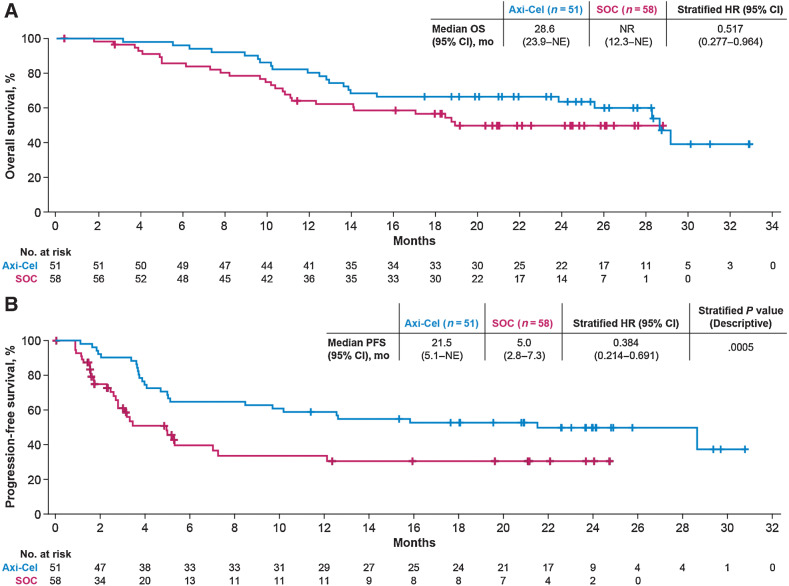
In patients ≥65 years, OS, evaluated as a preplanned interim analysis, was prolonged in the axi-cel versus the SOC arm (HR, 0.517; 95% CI, 0.277–0.964; Fig. 3A). Similar results were seen in multivariate analysis after adjusting for differences in baseline characteristics (HR, 0.40; 95% CI, 0.18–0.90), and among patients ≥70 years (HR, 0.260; 95% CI, 0.097–0.698; Supplementary Fig. S2A). In patients ≥65 years, the Kaplan–Meier estimate of OS at 2 years was 64% and 51% in the axi-cel and SOC arm, respectively. Thirty-three (57%) SOC patients received commercially available or investigational CAR T-cell therapy off protocol as subsequent treatment. A sensitivity analysis adjusting for subsequent CAR T-cell therapy in the SOC arm also suggested an OS benefit for axi-cel versus SOC (HR, 0.364; 95% CI, 0.183–0.723; Supplementary Fig. S3).
Figure 3.
OS (interim) and PFS in patients ≥65 years. A, Kaplan–Meier estimate of OS in patients ≥65 years. B, Kaplan–Meier estimate for PFS as assessed by investigator in patients ≥65 years. NR, not reached.
The median PFS was 21.5 months (95% CI, 5.1–NE) for the axi-cel and 5.0 months (95% CI, 2.8–7.3) for the SOC arm (HR, 0.384; 95% CI, 0.214–0.691; descriptive P = 0.0005; Fig 3B) for patients ≥65 years. Multivariate analyses showed similar PFS results when adjusted for baseline characteristics differences (HR, 0.31; 95% CI, 0.15–0.64; descriptive P < 0.002). The estimated PFS at 2 years was 50% in the axi-cel and 30% in the SOC arm. In patients ≥70 years, median PFS was 11.4 months (95% CI, 4.1–NE) for the axi-cel and 2.7 months (95% CI, 1.7–5.0) for the SOC arm (HR, 0.206; 95% CI, 0.078–0.547; descriptive P < 0.001; Supplementary Fig. S2B).
Axi-cel significantly improved efficacy outcomes versus SOC in patients ≥65 years, which was comparable with the overall ZUMA-7 primary analysis population (Supplementary Table S3).
Safety
In the safety analysis population, all patients ≥65 years had ≥1 any grade AE. Grade ≥3 treatment-emergent AEs occurred in 46/49 (94%) axi-cel and 45/55 (82%) SOC patients. The most commonly reported grade ≥3 AE was neutropenia, which occurred in 39 of 49 (80%) axi-cel and 24 of 55 (44%) SOC patients (Table 2). Serious AEs occurred in 29 of 49 (59%) axi-cel and 26 of 55 (47%) SOC patients (Supplementary Table S4). Grade ≥3 prolonged cytopenias present ≥90 days after definitive therapy initiation occurred in 6 of 49 (12%) axi-cel and 2 of 20 (10%) SOC patients (Supplementary Table S5). Fatal events occurred in 21 axi-cel and 26 SOC patients (Supplementary Table S6). Grade 5 treatment-related AEs occurred in 0 axi-cel and in 1 (cardiac arrest) SOC patient.
Table 2.
Most common AEs, CRS, and neurologic events in patients ≥65 years.
| Axi-Cel, N = 49 | SOC, N = 55 | |||
|---|---|---|---|---|
| n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Any AE | 49 (100) | 46 (94) | 55 (100) | 45 (82) |
| Pyrexia | 47 (96) | 4 (8) | 14 (25) | 0 |
| Neutropeniaa | 39 (80) | 39 (80) | 24 (44) | 24 (44) |
| Nausea | 23 (47) | 1 (2) | 37 (67) | 3 (5) |
| Anemia | 22 (45) | 19 (39) | 32 (58) | 25 (45) |
| Thrombocytopeniab | 21 (43) | 14 (29) | 37 (67) | 35 (64) |
| Leukopeniac | 19 (39) | 18 (37) | 10 (18) | 10 (18) |
| Fatigue | 17 (35) | 2 (4) | 31 (56) | 1 (2) |
| Diarrhea | 17 (35) | 1 (2) | 24 (44) | 0 |
| Decreased appetite | 18 (37) | 2 (4) | 19 (35) | 2 (4) |
| Hypokalemia | 16 (33) | 7 (14) | 17 (31) | 5 (9) |
| Hypotension | 23 (47) | 6 (12) | 8 (15) | 1 (2) |
| Constipation | 9 (18) | 0 | 21 (38) | 0 |
| Headache | 14 (29) | 0 | 14 (25) | 1 (2) |
| Hypophosphatemia | 20 (41) | 15 (31) | 8 (15) | 7 (13) |
| Cough | 18 (37) | 0 | 9 (16) | 0 |
| Edema peripheral | 9 (18) | 0 | 15 (27) | 1 (2) |
| Vomiting | 5 (10) | 0 | 18 (33) | 1 (2) |
| Chills | 18 (37) | 0 | 4 (7) | 0 |
| Hypocalcemia | 16 (33) | 0 | 6 (11) | 0 |
| Sinus tachycardia | 18 (37) | 1 (2) | 4 (7) | 0 |
| Confusional state | 19 (39) | 6 (12) | 2 (4) | 0 |
| Hypoxia | 15 (31) | 8 (16) | 6 (11) | 4 (7) |
| Hypomagnesemia | 7 (14) | 1 (2) | 13 (24) | 1 (2) |
| Acute kidney injury | 7 (14) | 1 (2) | 12 (22) | 3 (5) |
| Lymphocyte count decreased | 10 (20) | 10 (20) | 7 (13) | 7 (13) |
| Alanine aminotransferase increased | 10 (20) | 1 (2) | 4 (7) | 1 (2) |
| Hypoalbuminemia | 10 (20) | 1 (2) | 4 (7) | 0 |
| Encephalopathy | 12 (24) | 7 (14) | 1 (2) | 0 |
| Tremor | 12 (24) | 0 | 1 (2) | 0 |
| Febrile neutropenia | 0 | 0 | 12 (22) | 12 (22) |
| Hypogammaglobulinemia | 10 (20) | 0 | 1 (2) | 0 |
| Aphasia | 10 (20) | 3 (6) | 0 | 0 |
| CRS | 48 (98) | 4 (8) | — | — |
| Pyrexia | 47 (98) | 3 (6) | — | — |
| Hypotension | 21 (44) | 5 (10) | — | — |
| Sinus tachycardia | 16 (33) | 1 (2) | — | — |
| Chills | 13 (27) | 0 | — | — |
| Hypoxia | 12 (25) | 6 (13) | — | — |
| Headache | 8 (17) | 0 | — | — |
| Neurologic events | 32 (65) | 13 (27) | 14 (25) | 1 (2) |
| Confusional state | 19 (39) | 6 (12) | 2 (4) | 0 |
| Encephalopathy | 12 (24) | 7 (14) | 1 (2) | 0 |
| Tremor | 12 (24) | 0 | 1 (2) | 0 |
| Aphasia | 10 (20) | 3 (6) | 0 | 0 |
| Somnolence | 5 (10) | 3 (6) | 2 (4) | 0 |
| Delirium | 1 (2) | 1 (2) | 4 (7) | 1 (2) |
| Paresthesia | 0 | 0 | 5 (9) | 0 |
Note: Shown are any adverse events of any grade that occurred in at least 20% of the patients in either the axi-cel arm or the SOC arm, as well as events of the CRS that occurred in at least 15% of the patients in the axi-cel arm and neurologic events of any grade that occurred in at least 15% of the patients in the axi-cel arm or at least 3% of those in the SOC arm. The severity of the CRS was graded according to Lee and colleagues (18). Neurologic events were identified with the use of a prespecified search list of preferred terms in the Medical Dictionary for Regulatory Activities, version 23.1, on the basis of known neurotoxic effects associated with anti-CD19 immunotherapy, and were specifically identified with the use of methods that were based on the phase II study of blinatumomab (19). The severity of all adverse events, including neurologic events and symptoms of the CRS, was graded with the use of the Common Terminology Criteria for Adverse Events, version 4.03, of the NCI.
aNeutropenia refers to the combined preferred terms of neutropenia and neutrophil count decreased.
bThrombocytopenia refers to the combined preferred terms of thrombocytopenia and platelet count decreased.
cLeukopenia refers to the combined preferred terms of leukopenia and white-cell count decreased.
In patients ≥65 years, CRS occurred in 48 of 49 (98%) axi-cel patients, with grade ≥3 CRS occurring in 4 of 49 (8%) patients (Table 2). Tocilizumab was administered to 67%, glucocorticoids to 29%, and vasopressors to 6% of axi-cel patients for CRS management. The median time to onset of CRS was 3 days (range, 1–10), and the median duration was 8 days (range, 3–22). No deaths related to CRS occurred.
Neurologic events occurred in 32 of 49 (65%) axi-cel and 14 of 55 (25%) SOC patients (Table 2). Grade ≥3 neurologic events occurred in 13 of 49 (27%) axi-cel and 1 of 55 (2%) SOC patients. In the axi-cel arm, glucocorticoids were used in 45% of patients for the management of neurologic events. The median time to onset of neurologic events was 7 days (range, 2–12) in the axi-cel arm and 26 days (range, 2–108) in the SOC arm; the median duration was 9 days (range, 2–817) and 39 days (range, 1–253), respectively. Unresolved neurologic events at 30 days occurred in 6 patients in the axi-cel arm (confusional state, tremor, lethargy, mental status changes, tremor, and cognitive disorder) and 5 patients in the SOC arm (paresthesia and somnolence). Unresolved neurologic events at 90 days occurred in 4 patients in the axi-cel arm (tremor, taste disorder, hypoesthesia, and cognitive disorder) and 4 patients in the SOC arm (visual hallucination, somnolence, delirium, and paresthesia). Unresolved neurologic events at the time of death or data cutoff occurred in 3 patients in the axi-cel arm (tremor, taste disorder, and hypoesthesia) and 1 patient in the SOC arm (paresthesia). No deaths related to neurologic events occurred.
The rates of grade ≥3 CRS and grade ≥3 neurologic events were numerically higher in patients aged ≥65 years compared with the overall ZUMA-7 population (Supplementary Table S3; ref. 8), although a formal statistical analysis was not prespecified or conducted. These results are consistent with the elevated serum inflammatory profile in patients aged ≥65 years compared with patients <65 years (Supplementary Table S7). CRS occurred in 98% and 8% for any grade and grade ≥3, respectively, in patients ≥65 years compared with 92% and 6% in the overall ZUMA-7 population. In the axi-cel arm, neurologic events occurred in 65% and 27% for any grade and grade ≥3, respectively, in patients ≥65 years compared with 60% and 21% in the overall ZUMA-7 population.
Among patients ≥70 years in the safety analysis set, all had AEs, with grade ≥3 AEs occurring in 22 of 24 (92%) axi-cel and 20 of 26 (77%) SOC patients (Supplementary Table S8). Fatal events occurred in 13 axi-cel and 15 SOC patients (Supplementary Table S9). CRS occurred in 24 of 24 (100%) patients who received axi-cel, with grade ≥3 CRS occurring in 2 of 24 (8%) patients (Supplementary Table S8). Neurologic events occurred in 18 of 24 (75%) axi-cel patients and in 5 of 26 (19%) SOC patients, with grade ≥3 neurologic events occurring in 8 of 24 (33%) and 0 patients, respectively (Supplementary Table S8).
Patient-reported outcomes
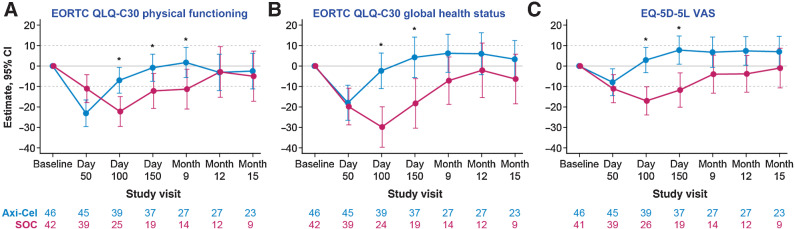
Eighty-eight patients ≥65 years met the criteria for the QoL analysis set (axi-cel, 46 patients; SOC, 42 patients). Baseline characteristics for the QoL analysis set were comparable among all patients ≥65 years (Supplementary Table S10). There was a clinically meaningful difference for patients ≥65 years in mean change of scores from baseline at day 100 in favor of axi-cel for the prespecified PRO domains EORTC QLQ-C30 Global Health (descriptive P < 0.0001), Physical Functioning (descriptive P = 0.0019), and EQ-5D-5L VAS (descriptive P < 0.0001; Fig. 4A–C) versus SOC. Similar results were observed with sensitivity analyses controlling for patterns of missingness and covariates (Supplementary Table S11). Scores also favored (P < 0.05) axi-cel over SOC at day 150 for all 3 domains and for Physical Functioning at month 9. By day 150, the mean estimated scores numerically returned to or exceeded baseline scores in the axi-cel arm. The QoL analysis set included 27 patients in the axi-cel arm and 19 patients in the SOC arm completing the questionnaires at day 150. Conversely, by month 15, the mean estimated scores never equaled or exceeded baseline scores in the SOC arm. In exploratory analyses of additional PRO domains, EORTC QLQ-C30 emotional functioning, appetite loss, and diarrhea, and EQ-5D Index at day 100; EORTC QLQ-C30 Role Functioning at day 100 and 150; and EORTC QLQ-C30 Social Functioning, Fatigue, and Dyspnea at day 100, 150, and month 9 (Supplementary Fig. S4) showed descriptive P < 0.05 for mean changes in scores in favor of axi-cel.
Figure 4.
Mixed model with repeated measures for change from baseline for prespecified patient-reported outcome endpoints in patients ≥65 years. Results were populated through month 15 due to lack of model convergence when using time points. Figures are based on Model 1. Horizontal lines, provided for clarity of interpretation, indicate the minimally important difference thresholds for clinically meaningful change. Mixed model includes variables for treatment, time, and treatment by time interaction (primary analysis) and is controlled for response to first-line therapy and age-adjusted IPI at screening. *, P < 0.05. A, The change from baseline of EORTC QLQ-C30 Physical Functioning in patients ≥65 years. B, The change from baseline of EORTC QLQ-C30 Global Health Status in patients ≥65 years. C, The change from baseline of EQ-5D-5L VAS in patients ≥65 years.
CAR T-cell levels and serum inflammatory profile
In patients ≥65 years, the median peak CAR T-cell level was 34.80 cells/μL, and median AUC from days 0–28 (AUC0–28) following axi-cel infusion was 445.11 cells/μL× days (Supplementary Fig. S5). The expansion of CAR T cells in patients ≥65 years was comparable to patients <65 years (Supplementary Fig. S5).
The serum inflammatory profiles of patients ≥65 years versus patients <65 years were comparable at baseline. However, vascular cell adhesion protein 1 (VCAM-1) and IL7 were elevated at baseline in patients ≥65 years versus patients <65 years (VCAM-1: 706.9 ng/mL vs. 589.5 ng/mL, descriptive P = 0.0005 and IL7: 21.0 pg/mL vs. 18.5 pg/mL, descriptive P = 0.049; Supplementary Table S7). Postinfusion, C-X-C motif chemokine ligand 10, ferritin, IFNγ, IL2 receptor alpha, IL15, VCAM-1, GM-CSF, and VEGF, presented significantly (P < 0.05) higher peaks and/or AUC in patients ≥65 years versus <65 years (Supplementary Table S7). Association results were exploratory in nature. Nominal P values are descriptive, and results should be interpreted with caution.
Discussion
CAR T-cell therapy has dramatically changed the treatment landscape for patients with R/R LBCL, providing a curative-intent alternative to HDT-ASCT. However, after first-line therapy, older patients with R/R LBCL face barriers that may limit curative-intent therapies. For example, patients ≥65 years may be ineligible for stem cell transplantation due to an increased risk for toxicity or mortality, or due to regional and/or institutional guidelines that limit stem cell transplantation based upon age (5, 28–31). Historically, outcomes for patients considered “transplant-ineligible” were poor and therapy was described as palliative. Patients who are transplant-ineligible have almost no chance of survival or prolonged disease control (4). Even for those who are transplant-eligible, QoL is often negatively affected by adverse events associated with treatments, which often dissuades not only physicians, but also older patients themselves, from pursuing current SOC therapy options. In this analysis, axi-cel demonstrated superior efficacy over SOC in patients ≥65 years despite greater frequency of high-risk features in the axi-cel arm versus the SOC arm. Thus, we demonstrate that axi-cel is both feasible and effective in patients ≥65 years with R/R LBCL after first-line therapy, establishing a new SOC therapeutic option (32).
In ZUMA-7, the manufacturing success rate for patients ≥65 years was 100%, which was comparable to the overall population (8). In addition, in the axi-cel arm, 96% of patients ≥65 years received axi-cel, compared with 94% in the overall population (8). Peak CAR T cells and AUC0–28 for patients ≥65 years were comparable to that of patients <65 years, demonstrating that there are no technological limitations associated with axi-cel treatment for older patients. The serum inflammatory profiles for patients ≥65 years versus patients <65 years were comparable overall at baseline. However, VCAM-1 was elevated in patients ≥65 years, which is consistent with previous reports and may be due to an increased rate of vasculatory injury in older patients (33, 34).
Axi-cel had a manageable safety profile in patients ≥65 years that was consistent with previous clinical trials and real-world experience in adult patients of any age. This contrasts with the increased toxicity risk with chemotherapy in advanced age (9, 35), which in this study may be underreported due to outpatient monitoring during salvage chemotherapy (with few patients receiving HDT-ASCT, likely accounting for the lower rate of AEs, in general, in the SOC versus axi-cel arm) versus inpatient monitoring after axi-cel infusion. While incidence of CRS and neurologic events was numerically higher for patients ≥65 years compared with the overall population (8), incidence of CRS events of grade ≥3 was still relatively low (8%), even among a further subgroup of patients ≥70 years. Furthermore, neurologic events of grade ≥3 occurred in 27% of patients ≥65 years and 33% of patients ≥70 years. Importantly, there were no deaths due to neurologic events or CRS in axi-cel–treated patients ≥65 years. While the incidence of neurologic events appears to increase with age, only one-third of patients ≥70 years experienced grade ≥3 neurologic events. Nevertheless, future studies should focus on implementing strategies for better toxicity management, which may improve the axi-cel therapeutic index (36, 37).
Overall, toxicity in patients ≥65 years was manageable, despite elevation in some proinflammatory and immune-modulatory cytokines and chemokines, which are known to be associated with grade ≥3 toxicities (9, 38). Therefore, although a recent meta-analysis demonstrated that older patients are at a higher risk of immune effector cell–associated neurotoxicity syndrome compared with younger patients (39), the safety profile of axi-cel in this prospective randomized trial demonstrates that toxicities associated with neurologic events are manageable and that interventions that aim to minimize higher-grade toxicity for this patient population are effective.
The risk/benefit profile of therapies for elderly patients with R/R LBCL remains favorable, given limited therapeutic options with curative intent for this patient population and the potential for long-term survival with CAR T-cell therapies (32). With two products currently approved in second-line LBCL (7, 40) and additional CAR T-cell therapies possibly approved in the future, multiple options may become available in the near future. Therapy selection may therefore be dependent on clinical risk factors and predictive biomarkers of response and toxicity (20), which have yet to be fully elucidated. In the meantime, efforts to optimize management of adverse events, especially high-grade toxicities, are ongoing (36, 37, 41–44).
Axi-cel showed meaningful improvement in QoL over SOC; axi-cel patients showed faster recovery to pretreatment QoL, indicating satisfactory symptom resolution from the patient's perspective. These data suggest that axi-cel benefit over SOC is multifaceted and that efficacy and QoL together affect patients’ overall sense of well-being. Although PRO measurements are becoming more common in oncology, including in third-line LBCL (45–48), there is a paucity of literature on health-related QoL in second-line LBCL, especially in older patients (10). In one study, patients with hematologic malignancies treated with CAR T-cell therapy reported superior short-term QoL compared with autologous or allogeneic stem cell transplantation (49). More PRO data comparing CAR T-cell therapy with SOC are expected as second-line studies are completed (50, 51).
In this preplanned subgroup analysis, axi-cel demonstrated clinical benefit over SOC in a patient population with high unmet need. Nonetheless, there are limitations to our study. As previously published in a supplementary table describing the representativeness of our study's patient population (8), there was limited racial and ethnic diversity of patients in this trial, as with many clinical trials. Generally, however, the demographics of the participants in the ZUMA-7 study, including the median patient age, the ratio of males to females, and the proportion of non-Hispanic White patients, were consistent with that observed in clinical trials in this setting; though, ZUMA-7 enrolled a greater proportion of patients ≥65 years versus most of the studies analyzed (8). Notably, real-world studies in the third-line setting showed that axi-cel provides favorable outcomes in patients with LBCL regardless of race and ethnicity (52). Furthermore, axi-cel has demonstrated real-world effectiveness among patients ≥65 years (53), suggesting that axi-cel utility may extend beyond those patients fit enough for or those included within clinical studies. In addition, although PRO data are meaningful to assess patient experience, limitations are based on the implicit nature of self-reported measurements that are often not completed following an EFS event. Therefore, cautious interpretation of results is warranted, especially at later time points, as attrition due to disease progression, new lymphoma therapy, or death was disproportionately higher on the SOC arm and could contribute to a selection bias of patients with the best outcomes, which has the potential to overestimate the QoL of the SOC patient population.
In conclusion, the results presented herein demonstrated the clinical benefit of axi-cel over SOC for second-line treatment of R/R LBCL in patients ≥65 years. In ZUMA-7, older patients had clinical outcomes similar to the overall population, and axi-cel was associated with superior clinical efficacy and QoL versus SOC, with similar, manageable toxicity. In addition, patients ≥65 years and patients <65 years had comparable CAR T-cell expansion and serum inflammatory profiles at baseline. Although a number of serum inflammatory and immune-modulatory analytes were elevated post axi-cel infusion in patients ≥65 years compared with patients <65 years, toxicity was manageable. In patients ≥65 years, nearly triple the proportion of patients randomized to axi-cel received definitive therapy compared with those randomized to SOC. Taken together, these data suggest that age should not prohibit the consideration of cellular therapy for patients with LBCL. Historically, older patients were often deemed ineligible for curative-intent therapy with transplantation due to age and/or comorbidities; therefore, they have no other curative treatment options (5, 28–31). Our results clearly demonstrate that older patients can safely receive second-line therapy with axi-cel, and together with the superior efficacy and improvements in QoL that were observed (compared with SOC), these data suggest that axi-cel should be considered as second-line therapy for patients ≥65 with R/R LBCL.
Supplementary Material
Event-free survival per central review and objective response rate in patients ≥70 years
Overall survival (interim) and progression-free survival in patients ≥70 years
Sensitivity analysis of interim overall survival in patients ≥65 years
Mixed model with repeated measures for change from baseline for prespecified patient-reported outcomes endpoints in patients ≥65 years
CAR T-cell levels in patients ≥65 years
Tabulated data supporting ZUMA-7 elderly analysisSupplementary Table S1. Patient-reported outcomes instrumentsSupplementary Table S2. Axi-cel delivery and administration timeSupplementary Table S3. Summary of efficacy and safety outcomes in patients ≥65 years versus all patients in ZUMA-7Supplementary Table S4. Serious adverse events in at least 3 patients in patients ≥65 yearsSupplementary Table S5. Summary of cytopenias present on or after 90 days from initiation of definitive therapy on protocol in patients ≥65 yearsSupplementary Table S6. Deaths in axi-cel and SOC arms for patients ≥65 years (safety analysis set)Supplementary Table S7. Summary of serum analytes in patients <65 years versus ≥65 years in the axi-cel arm (N = 170)Supplementary Table S8. Most common adverse events, cytokine release syndrome, and neurologic events in patients ≥70 yearsSupplementary Table S9. Deaths in axi-cel and SOC arms for patients ≥70 years Supplementary Table S10. Baseline characteristics for quality-of-life analysis in patients ≥65 yearsSupplementary Table S11. Mixed model with repeated measures estimated difference in change from baseline forprespecified patient-reported outcomes measures (quality-of-life analysis set) in patients ≥65 years
Acknowledgments
This work was supported by Kite, a Gilead Company. We thank the patients who participated in this trial and their families, caregivers, and friends; the trial investigators, coordinators, and health care staff at each site; Jennifer Yang, PhD, of Nexus Global Group Science, for medical writing assistance, with funding provided by Kite; and all employees of Kite involved over the course of the study for their contributions.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 1833
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
J.R. Westin reports grants and personal fees from Kite/Gilead during the conduct of the study. J.R. Westin also reports grants and personal fees from BMS, Novartis, Genentech, MorphoSys/Incyte, AstraZeneca, ADC Therapeutics, and Janssen; personal fees from AbbVie, SeaGen, MonteRosa, and Foresight; and grants from Kymera outside the submitted work. F.L. Locke reports grants and personal fees from Kite, a Gilead Company during the conduct of the study; F.L. Locke also reports personal fees from A2, Allogene, Amgen, bluebird bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group (GLG), Iovance, Kite, a Gilead Company, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja, as well as grants to institution from Kite, a Gilead Company, Allogene, CERo Therapeutics, Novartis, bluebird bio, BMS, National Cancer Institute, and Leukemia and Lymphoma Society outside the submitted work. In addition, F.L. Locke has a patent for Double Mutant Survivin Vaccine [Frederick Locke (PI), Dmitry Gabrilovich, Dario Altieri, Scott Antonia, Claudio Anasetti; PCT/US2016/031390; Managing Entity: Moffitt Cancer Center; US010414810B2; EP1931377 (7/2014); WO2014/022138 (2/2014)] issued, a patent for CAR T Cells with Enhanced Metabolic Fitness [Frederick Locke (PI), Reginald Atkins; Managing Entity: Moffitt Cancer Center] issued, a patent for Methods of Enhancing CAR T Cell Therapies [Frederick Locke (PI), Yoga Balagurunathan; Managing Entity: Moffitt Cancer Center] issued, a patent for Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T cell Therapy [Frederick Locke (PI), Philipp Altrock; Managing Entity: Moffitt Cancer Center] issued, a patent for Method of Enhancing Immunotherapy Using ER Stress Pathway [Paulo Rodriguez (PI), Frederick L. Locke, Jose Conejo-Garcia; Managing Entity: Moffitt Cancer Center] issued, a patent for Predicting the Severity of Toxic Events During CAR T Cell Therapy [Philipp Altrock, Frederick L. Locke (PI); Managing Entity: Moffitt Cancer Center] issued, a patent for Circulating Tumor DNA Correlates With Metabolic Tumor Volume Before and After Axicabtagene-Ciloleucel in Large B-Cell Lymphoma issued, and a patent for Differential Alternative Splicing in Relapsed and Refractory Diffuse Large B-Cell Lymphoma Patients issued. M. Dickinson reports personal fees from Kite, a Gilead Company, Gilead, and BMS; grants and personal fees from Novartis, Roche, MSD, AbbVie, and Genmab; and non-financial support from AstraZeneca during the conduct of the study. A. Ghobadi reports other support from Kite, a Gilead Company, Wugen, BMS, ATARA, Amgen, and Genentech during the conduct of the study, as well as advisory board/consulting/research support from Kite, a Gilead Company. M. Elsawy reports other support from Nexus during the conduct of the study, as well as other support from Kite/Gilead, Novartis, BMS, AbbVie, and Pfizer outside the submitted work. T. van Meerten reports grants from Genentech and Celgene/BMS, as well as other support from Kite/Gilead, Celgene/BMS, and Janssen outside the submitted work. D.B. Miklos reports grants and non-financial support from Kite/Gilead during the conduct of the study; D.B. Miklos also reports grants and non-financial support from Kite/Gilead, as well as personal fees from Fosun Kite outside the submitted work. M.L. Ulrickson reports personal fees from Gilead and Stemline outside the submitted work. M.-A. Perales reports personal fees from Adicet, Allovir, Caribou Biosciences, Celgene, BMS, Equilium, Exevir, Karyopharm, Merck, MorphoSys, Omeros, Syncopation, VectivBio AG, Vor Biopharma, Cidara Therapeutics, Medigene, Sellas Life Sciences, and NexImmune; personal fees and other support from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; and other support from OrcaBio outside the submitted work. U. Farooq reports other support from Kite, a Gilead Company during the conduct of the study, other support from Caribou and MorphoSys outside the submitted work, and research funding from Checkmate for an investigator-initiated study. L. Leslie reports personal fees from Kite/Gilead, AstraZeneca, BeiGene, Pharmacyclics, Janssen/J&J, AbbVie, SeaGen, Epizyme, Karyopharm, TG Therapeutics, Eli Lilly and Company, and Celgene/BMS outside the submitted work. M.J. Kersten reports grants, personal fees, and other support from Kite/Gilead during the conduct of the study; M.J. Kersten also reports personal fees and other support from Novartis, as well as personal fees from BMS/Celgene, Adicet Bio, Miltenyi Biotec, and Roche outside the submitted work. C.A. Jacobson reports personal fees from Kite/Gilead, Novartis, BMS/Celgene, Instil Bio, Abintus Bio, Caribou Bio, ImmPACT Bio, Daiichi-Sankyo, AstraZeneca, and MorphoSys outside the submitted work. G. Wulf reports other support from Gilead Sciences, Novartis, and Miltenyi outside the submitted work. S. Filosto reports other support from Kite, a Gilead Company outside the submitted work. J. Shah reports other support from Kite, a Gilead Company during the conduct of the study, as well as other support from Kite, a Gilead Company outside the submitted work. J.T. Snider reports other support from Kite, a Gilead Company outside the submitted work. P. Cheng reports other support from Kite, a Gilead Company during the conduct of the study. C. To reports other support from Nexus Global Group Science LLC during the conduct of the study, as well as other support from Kite, a Gilead Company outside the submitted work. O.O. Oluwole reports other support from Kite, a Gilad Company, Pfizer, ADC, Nektar, Epizyme, Cargo, TGR, and Novartis during the conduct of the study. A. Sureda reports other support from Kite/Gilead during the conduct of the study, as well as other support from Novartis, BMS/Celgene, Takeda, MSD, GenMab, AbbVie, Janssen, and Sanofi outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
J.R. Westin: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. F.L. Locke: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. M. Dickinson: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. A. Ghobadi: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. M. Elsawy: Resources, data curation, formal analysis, supervision, investigation, writing–original draft. T. van Meerten: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. D.B. Miklos: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. M.L. Ulrickson: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. M.-A. Perales: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. U. Farooq: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. L. Wannesson: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. L. Leslie: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. M.J. Kersten: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. C.A. Jacobson: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. J.M. Pagel: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. G. Wulf: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. P. Johnston: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. A.P. Rapoport: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. L. Du: Formal analysis, supervision, writing–original draft, writing–review and editing. S. Vardhanabhuti: Formal analysis, supervision, writing–original draft, writing–review and editing. S. Filosto: Formal analysis, supervision, writing–original draft, writing–review and editing. J. Shah: Formal analysis, supervision, writing–original draft, writing–review and editing. J.T. Snider: Conceptualization, formal analysis, supervision, methodology, writing–original draft, writing–review and editing. P. Cheng: Conceptualization, formal analysis, supervision, methodology, writing–original draft, writing–review and editing. C. To: Conceptualization, formal analysis, supervision, methodology, writing–original draft, writing–review and editing. O.O. Oluwole: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing. A. Sureda: Resources, data curation, formal analysis, supervision, investigation, writing–original draft, writing–review and editing.
References
- 1. Di M, Huntington SF, Olszewski AJ. Challenges and opportunities in the management of diffuse large B-cell lymphoma in older patients. Oncologist 2021;26:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Surveillance Epidemiology and End Results. Cancer Stat Facts: NHL — Diffuse Large B-Cell Lymphoma (DLBCL); 2022. Available from: https://seer.cancer.gov/statfacts/html/dlbcl.html.
- 3. Neelapu SS, Jacobson CA, Oluwole OO, Munoz J, Deol A, Miklos DB, et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2020;135:2106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:498–505. [DOI] [PubMed] [Google Scholar]
- 5. Lahoud OB, Sauter CS, Hamlin PA, Dahi PB. High-dose chemotherapy and autologous stem cell transplant in older patients with lymphoma. Curr Oncol Rep 2015;17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. YESCARTA®. (axicabtagene ciloleucel). Summary of product characteristics. Amsterdam, the Netherlands: Kite Pharma EU B.V.; 2022. [Google Scholar]
- 7. YESCARTA, Kite Pharma Inc. YESCARTA® (axicabtagene ciloleucel) suspension for intravenous infusion. U.S. Prescribing Information. Santa Monica, CA: Kite Pharma Inc; 2021. [Google Scholar]
- 8. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 2022;386:640–54. [DOI] [PubMed] [Google Scholar]
- 9. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin V, Oak B, Snider J, Epstein J. Health-related quality of life (HRQOL) burden in patients with relapsed/refractory diffuse large B-cell lymphoma (RR-DLBCL) and non-Hodgkin's lymphoma (RR-NHL). J Clin Oncol 2020;38(15_suppl):e20070–e. [Google Scholar]
- 11. Hafez R, Hussein S, Ismail M. Definitive salvage chemotherapy for the treatment of refractory/relapsed non-Hodgkin lymphoma, a single center experience. Alexandria J Med 2019;54:679–83. [Google Scholar]
- 12. Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JW, Huijgens PC, et al. Health-related quality of life and persistent symptoms in relation to (R-)CHOP14, (R-)CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: results of the population-based PHAROS-registry. Ann Hematol 2014;93:1705–15. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims; 2009. [DOI] [PMC free article] [PubMed]
- 14.U.S. Food and Drug Administration. Guidance for industry: core patient-reported outcomes in cancer clinical trials guidance for industry; 2021.
- 15.European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man: The use of patient-reported outcome (PRO) measures in oncology studies; 2016. Available from: https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf.
- 16. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57–66. [DOI] [PubMed] [Google Scholar]
- 20. Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv 2020;4:4898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brudno JN, Lam N, Vanasse D, Shen YW, Rose JJ, Rossi J, et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med 2020;26:270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Locke FL, Chou J, Vardhanabhuti S, Perbost R, Dreger P, Hill BT, et al. Association of pretreatment (preTx) tumor characteristics and clinical outcomes following second-line (2L) axicabtagene ciloleucel (axi-cel) versus standard of care (SOC) in patients (pts) with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). J Clin Oncol 2022;40(16_suppl):7565. [Google Scholar]
- 23. Robins JM, Tsiatis AA. Correcting for non-compliance in randomized trials using rank preserving structural failure time models. Communications in Statistics - Theory and Methods 1991;20:2609–31. [Google Scholar]
- 24. Elsawy M, Chavez JC, Avivi I, Larouche JF, Wannesson L, Cwynarski K, et al. Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma. Blood 2022;140:2248–60. [DOI] [PubMed] [Google Scholar]
- 25. Elsawy M, Chavez JC, Avivi I, Larouche J-F, Cwynarski LWK, Osman K, et al. Patient-reported outcomes in a phase 3, randomized, open-label study evaluating the efficacy of axicabtagene ciloleucel (axi-cel) versus standard of care therapy in patients with relapsed/refractory large B-cell lymphoma (ZUMA-7). Blood 2021;138:430. [Google Scholar]
- 26. Maringwa JT, Quinten C, King M, Ringash J, Osoba D, Coens C, et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer 2011;19:1753–60. [DOI] [PubMed] [Google Scholar]
- 27. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med 2021;384:842–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morrison VA, Hamlin P, Soubeyran P, Stauder R, Wadhwa P, Aapro M, et al. Approach to therapy of diffuse large B-cell lymphoma in the elderly: the International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol 2015;26:1058–68. [DOI] [PubMed] [Google Scholar]
- 30. Jantunen E, Canals C, Rambaldi A, Ossenkoppele G, Allione B, Blaise D, et al. Autologous stem cell transplantation in elderly patients (>or =60 years) with diffuse large B-cell lymphoma: an analysis based on data in the European blood and marrow transplantation registry. Haematologica 2008;93:1837–42. [DOI] [PubMed] [Google Scholar]
- 31. Belete H, Burns LJ, Shanley R, Nayar M, McClune B, Lazaryan A, et al. Transplantation related toxicity and mortality in older autologous hematopoietic cell transplantation recipients. Am J Hematol 2017;92:E529–E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood 2022;139:2737–46. [DOI] [PubMed] [Google Scholar]
- 33. Tchalla AE, Wellenius GA, Travison TG, Gagnon M, Iloputaife I, Dantoine T, et al. Circulating vascular cell adhesion molecule-1 is associated with cerebral blood flow dysregulation, mobility impairment, and falls in older adults. Hypertension 2015;66:340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richter V, Rassoul F, Purschwitz K, Hentschel B, Reuter W, Kuntze T. Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology 2003;49:293–300. [DOI] [PubMed] [Google Scholar]
- 35. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castaneda-Puglianini O, Chavez JC. Assessing and management of neurotoxicity after CAR-T therapy in diffuse large B-cell lymphoma. J Blood Med 2021;12:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv 2020;4:3123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Filosto S, Vardhanabhuti S, Canales M, Poiré X, Lekakis LJ, de Vos S, et al. Product attributes of axicabtagene ciloleucel (axi-cel) that associate differentially with efficacy and toxicity in second-line large B-cell lymphoma. Cancer Res 2022;82:CT004–CT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tariq A, Katiyar V, Aijaz T. Safety of anti-CD-19 chimeric antigen receptor T-cell therapy in the older population with diffuse large B cell lymphoma: a meta-analysis. Blood 2021;138:4814. [Google Scholar]
- 40. BREYANZI® JT, Inc., a Bristol-Myers Squibb Company. BREYANZI® (lisocabtagene maraleucel) suspension for intravenous infusion. U.S. Prescribing Information. Seattle, WA. Revised June 2022. [Google Scholar]
- 41. Oluwole OO, Bouabdallah K, Munoz J, De Guibert S, Vose JM, Bartlett NL, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol 2021;194:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Topp MS, van Meerten T, Houot R, Minnema MC, Bouabdallah K, Lugtenburg PJ, et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol 2021;195:388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neelapu SS, Tummala S, Kebriaei P, Wierda WG, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila ML, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer 2020;8:e001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruark J, Mullane E, Cleary N, Cordeiro A, Bezerra ED, Wu V, et al. Patient-reported neuropsychiatric outcomes of long-term survivors after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant 2020;26:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang XS, Srour SA, Whisenant M, Subbiah IM, Chen TH, Ponce D, et al. Patient-reported symptom and functioning status during the first 12 months after chimeric antigen receptor T cell therapy for hematologic malignancies. Transplant Cell Ther 2021;27:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maziarz RT, Waller EK, Jaeger U, Fleury I, McGuirk J, Holte H, et al. Patient-reported long-term quality of life after tisagenlecleucel in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv 2020;4:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patrick DL, Powers A, Jun MP, Kim Y, Garcia J, Dehner C, et al. Effect of lisocabtagene maraleucel on HRQoL and symptom severity in relapsed/refractory large B-cell lymphoma. Blood Adv 2021;5:2245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dueck AC, Kumar SK, Lin Y, Siddiqui M, Bennani NN, Yost KJ, et al. Patient experience of chimeric antigen receptor (CAR)-T cell therapy vs. stem cell transplant: longitudinal patient reported adverse events, cognition and quality of life. Blood 2019;134:794. [Google Scholar]
- 50. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med 2022;386:629–39. [DOI] [PubMed] [Google Scholar]
- 51. Abramson JS, Solomon SR, Arnason JE, Johnston PB, Glass B, Crotta A, et al. Improved quality of life (QOL) with lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, compared with standard of care (SOC) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): results from the phase 3 TRANSFORM study. Blood 2021;138:3845. [Google Scholar]
- 52. Locke FL, Siddiqi T, Jacobson CA, Ghobadi A, Ahmed S, Miklos DB, et al. Real-world outcomes of axicabtagene ciloleucel (axi-cel) for the treatment of large B-cell lymphoma (LBCL) by race and ethnicity. J Clin Oncol 2022;40(16_suppl):7571). [Google Scholar]
- 53. Lunning MA, Wang H-L, Hu Z-H, Locke FL, Siddiqi T, Jacobson CA, et al. Outcomes of axicabtagene ciloleucel in comparison with chemoimmunotherapy (CIT) in an elderly population for treatment of relapsed or refractory (R/R) large B-cell lymphoma (LBCL) after two or more lines of prior therapy. Blood2022;140:1852–5. [Google Scholar]
- 54. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Event-free survival per central review and objective response rate in patients ≥70 years
Overall survival (interim) and progression-free survival in patients ≥70 years
Sensitivity analysis of interim overall survival in patients ≥65 years
Mixed model with repeated measures for change from baseline for prespecified patient-reported outcomes endpoints in patients ≥65 years
CAR T-cell levels in patients ≥65 years
Tabulated data supporting ZUMA-7 elderly analysisSupplementary Table S1. Patient-reported outcomes instrumentsSupplementary Table S2. Axi-cel delivery and administration timeSupplementary Table S3. Summary of efficacy and safety outcomes in patients ≥65 years versus all patients in ZUMA-7Supplementary Table S4. Serious adverse events in at least 3 patients in patients ≥65 yearsSupplementary Table S5. Summary of cytopenias present on or after 90 days from initiation of definitive therapy on protocol in patients ≥65 yearsSupplementary Table S6. Deaths in axi-cel and SOC arms for patients ≥65 years (safety analysis set)Supplementary Table S7. Summary of serum analytes in patients <65 years versus ≥65 years in the axi-cel arm (N = 170)Supplementary Table S8. Most common adverse events, cytokine release syndrome, and neurologic events in patients ≥70 yearsSupplementary Table S9. Deaths in axi-cel and SOC arms for patients ≥70 years Supplementary Table S10. Baseline characteristics for quality-of-life analysis in patients ≥65 yearsSupplementary Table S11. Mixed model with repeated measures estimated difference in change from baseline forprespecified patient-reported outcomes measures (quality-of-life analysis set) in patients ≥65 years
Data Availability Statement
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.