Abstract
Introduction
Exercise has many benefits for people with Parkinson’s disease (PD) and has been suggested to modify PD progression, but robust evidence supporting this is lacking.
Objective
This systematic review (PROSPERO registration: CRD42020169999) investigated whether exercise may have neuroplastic effects indicative of attenuating PD progression.
Methods
Six databases were searched for randomized controlled trials (RCTs) that compared the effect of exercise to control (no or sham exercise) or to another form of exercise, on indicators of PD progression (eg, brain-derived neurotrophic factor [BDNF], brain activation, “off” Unified Parkinson’s Disease Rating Scale [UPDRS] scores). Trial quality was assessed using the Physiotherapy Evidence Database Scale. Random-effects meta-analyses were performed where at least 3 comparable trials reported the same outcome; remaining results were synthesized narratively.
Results
Forty-nine exercise trials involving 2104 PD participants were included. Compared to control, exercise improved “off” UPDRS motor scores (Hedge’s g −0.39, 95% CI: −0.65 to −0.13, P = .003) and BDNF concentration (Hedge’s g 0.54, 95% CI: 0.10-0.98, P = .02), with low to very low certainty of evidence, respectively. Narrative synthesis for the remaining outcomes suggested that compared to control, exercise may have neuroplastic effects. The exercise versus exercise comparisons were too heterogenous to enable pooling of results.
Discussion
This review provides limited evidence that exercise may have an attenuating effect on potential markers of PD progression. Further large RCTs are warranted to explore differential effects by exercise type, dose and PD stage, and should report on a core set of outcomes indicative of PD progression.
Keywords: Parkinson’s disease, exercise, disease course, systematic review, neuroprotection
Introduction
Parkinson’s disease (PD) is a neurodegenerative condition characterized by progressively worsening motor and non-motor impairments. 1 Disease progression is associated with activity limitations, increased falls risk, reduced quality of life, and care-partner burden. 2 Although pharmacotherapy can relieve PD symptoms, it does not alter disease progression. 3 Exercise therapy has been identified as a safe and effective intervention to improve physical function for people with PD. 4 It has been suggested to possibly have a disease-modifying effect on PD, with some evidence that exercise can lead to neurorestoration and neuroplasticity in people with PD. 5
Evidence from small randomized controlled trials (RCTs) and single group studies suggest that moderate to high intensity aerobic exercise or multimodal training including aerobic components can improve cortical inhibition, 6 dopamine receptor function, 7 and neurotrophic factor concentration,8,9 as well as reduce inflammation. 9 One meta-analysis showed that exercise interventions increased plasma brain-derived neurotrophic factor (BDNF) among people with PD. 10 Another systematic review showed altered functional connectivity as measured by functional magnetic resonance imaging (fMRI) in people with PD following motor training, 11 though inclusion of case-control and non-randomized studies reduces the certainty of the findings. 12
Although such changes may provide insights into the pathophysiology of PD and could reflect disease trajectory, 13 the disease-modifying effects of exercise remains unclear because there is no definitive biomarker for disease progression. There are, however, numerous potential indicators of disease progression. Neurotrophic factors (eg, BDNF) are suggested to promote survival and function of dopaminergic neurons14,15; antioxidative factors to reduce cell damage and death caused by excess build-up of oxidative stress factors, 16 one of the postulated mechanisms for the development of PD 17 ; and anti-inflammatory cytokines to alleviate neurodegeneration induced by chronic, systemic inflammation. 18 Cortical inhibition is thought to limit overactivation of the corticospinal pathway, 19 the main conduit of commands from the motor cortex to muscles to produce voluntary movements. Reduced cortical inhibition (ie, overactivation of the corticospinal pathway, common in PD 19 ) may contribute to movement deficits characteristic of PD, such as bradykinesia and hypokinesia. 1 Bradykinesia arises from basal ganglia dysfunction and manifests as slowed movements from delayed muscle activation and/or reduced muscular force production. 20 Presynaptic inhibition and disynaptic reciprocal inhibition of spinal interneurons also modulate agonist and antagonist muscle activity, 21 further contributing to bradykinesia. These muscle activation patterns underpinning bradykinesia 20 are measured using electromyography (EMG). In contrast, cortical synchrony, which enables the cortex to receive and process a stimulus then react efficiently, is suggested to reduce PD motor symptoms. 22 MRI has been used to explore brain structure and connectivity within or across networks. 13 As PD results in depletion of dopaminergic neurons in the striatum, changes in the putamen of the basal ganglia results in changes in other brain networks, such as the motor cortices. 23 There are also measures of dopaminergic function. 1 Changes in these various outcome measures toward levels seen in healthy people would indicate potential disease modification.
Assessing the effect of exercise on the Unified Parkinson’s Disease Rating Scale (UPDRS) 24 or the Movement Disorder Society-sponsored version of the UPDRS (MDS-UPDRS) 25 scores when “off” medication may also provide insights into underlying changes. Most studies examining the effects of exercise on various motor and non-motor outcomes measured using MDS/UPDRS scores have done so while “on” medication. This makes it unclear whether the improvements in MDS/UPDRS scores were due to neurochemical changes, improved motor performance due to dopaminergic medication or exercise training, or interactions between any or all of these factors. Measuring MDS/UPDRS scores “off” medication reveals unmasked PD symptoms, which may more closely reflect disease severity compared to “on” medication scores which is influenced by medication effectiveness. A recent meta-analysis found that long-term physiotherapy (ie, ≥6 months) led to improvements in “off” UPDRS scores compared to no intervention or different types of exercise. 26 However, that meta-analysis pooled studies comparing different forms of exercise together with studies comparing exercise versus no intervention, 26 making it difficult to interpret whether exercise truly modifies disease severity in people with PD. Moreover, by only including trials of exercise interventions ≥6 months duration, 26 the review may have overlooked other relevant trials.
To date, no systematic review has been done to summarize the overall effects of exercise on disease progression outcomes by synthesizing results from neurochemical, neuroimaging, neurophysiological, and clinical outcomes. Previous RCTs6,8 and systematic reviews10,11 that suggested a disease modifying effect of exercise in PD have reported a variety of exercise types and dosages. It is therefore unclear whether exercise in general or only specific types of exercise may have potential disease modifying effects, or whether any disease modifying effects of exercise are dose dependent. Additionally, some RCTs have suggested that potential disease modifying effects of exercise are most noticeable in early stage PD,7,27,28 but no study has analyzed whether effects vary by disease severity. Therefore, the aim of this systematic review was to synthesize findings from RCTs investigating the effectiveness of exercise compared to no, sham, or other forms of exercise on potential indicators of disease progression in people with PD. The specific research questions were, in people with PD:
Does exercise attenuate measures indicative of PD progression?
Do any effects of exercise on measures indicative of PD progression vary with the type or dose of exercise?
Do any effects of exercise on measures indicative of PD progression vary with participants’ disease severity?
Methods
This systematic review with meta-analysis (PROSPERO registration: CRD42020169999) was conducted according to the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 29
Identification and Selection of Trials
Comprehensive searches of the Medline, EMBASE, CINAHL, AMED, Cochrane Central, Scopus, and Physiotherapy Evidence Database (PEDro) databases were conducted to identify RCTs published between 1 January 1995 and 30 September 2022. The search was restricted to RCTs published from 1995 onward as higher quality exercise evidence has been generated since then. Search terms included those related to the population (PD), the intervention and comparator (different types of exercise or physical therapy), and the outcome (related to disease progression and neuroprotection). Terms within each category were combined with OR, and then categories were combined with AND (see Supplemental File 1 for the full search strategies).
For the population, trials including adults with idiopathic PD were included. If the trial included a mixed sample of participants, it was included if separate data were available for participants with idiopathic PD. The intervention of interest was exercise, defined as planned and structured physical activity with the purpose of improving or maintaining 1 or more components of physical fitness. Trials were included if the exercise intervention was of at least 4 weeks’ duration and a minimum of 60 minutes/week, as this is the exercise dosage likely to produce sustained physiological change. 30 Interventions in both supervised and non-supervised settings were included. Trials involving multidisciplinary or other allied health interventions in which exercise was <50% of the total intervention, or where both the intervention and comparator groups received the same type and dosage of exercise, were excluded. Trials were included if the comparator group was a control group that received no intervention, sham exercise or usual care, or another exercise group. Outcomes of interest were potential indicators of disease progression in people with PD from baseline to post-test and/or follow-up. These included clinical (MDS/UPDRS scores), neurochemical (eg, BDNF, tumor necrosis factor α), neuroimaging (eg, dopamine receptor binding, brain structure, functional activation, functional connectivity), and neurophysiological (eg, cortical inhibition, muscle activation patterns underlying bradykinesia) outcomes. Clinical outcomes were included if trials assessed MDS/UPDRS scores “off” medication. Due to the anticipated small number of studies reporting neurochemical, neuroimaging, or neurophysiological outcomes, trials reporting these outcomes were included regardless of medication status.
Title and abstract screening, followed by full text screening, was completed by pairs of reviewers using Covidence software (Veritas Health Innovation, Melbourne, VIC, Australia). Any conflicts were resolved by consensus with a separate reviewer.
Data Extraction
Data for relevant trials were extracted independently by pairs of reviewers, with discrepancies resolved by consensus with a third reviewer. A record was made of the following participant characteristics for each trial: number of participants, gender, age, disease duration, and disease severity as per the Hoehn and Yahr or MDS/UPDRS scales. Data extracted for each intervention and control group included exercise type, intensity, and dosage (ie, duration of each session in minutes, number of sessions per week, total number of weeks). Data about relevant outcome measures used, their measurement timepoints, and whether they were measured “on” or “off” medication were also extracted. Outcome data pre- and post-intervention were extracted as mean and SD where available, otherwise these were calculated where possible using standard methods. 31 If only change scores were reported post-intervention then these data were used. If data were presented in a graph, they were extracted using WebPlotDigitizer software. 32
Authors were contacted if data were missing or when clarification about data was required. Data were provided by 6 trial authors33-38; if no response was received missing data were reported as such.
Assessment of Trial Characteristics, Quality, and Certainty of the Evidence
The quality of included trials was assessed using the PEDro scale. 39 A rating out of 10 is given to each trial, with scores of 9 to 10 considered excellent methodologic quality, 6 to 8 high, 4 to 5 moderate, and 0 to 3 poor quality. 39 Scores were obtained from the PEDro database (https://pedro.org.au/). No trials were excluded from the analysis based on PEDro ratings. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were also used to provide clarity on the certainty of the findings. 40
Data Synthesis and Analyses
Separate analyses were conducted for trials comparing exercise with control (ie, no exercise or sham exercise) and trials comparing 2 exercise interventions. Random-effects meta-analyses were performed for outcomes that had sufficient data (ie, reported in ≥3 trials) and where the interventions were considered sufficiently similar to enable pooling of data. Pooled results were reported as standardized mean difference using Hedges’ g with 95% confidence intervals (CI). Heterogeneity was evaluated using the I 2 and Q statistics. We planned to report prediction intervals (ie, the true effect size in 95% of comparable studies) where at least 10 trials were included in a meta-analysis. 31
Subgroup analysis based on exercise types for BDNF and “off” MDS/UPDRS outcomes were conducted; however, there were insufficient studies for such analysis for other outcomes. Planned subgroup analyses based on exercise dosage and PD severity were unable to be performed, the former owing to exercise dosages essentially differing by exercise type, making this analysis redundant; the latter owing to studies including a mix of participants across mild to moderate disease severities.
Meta-analyses were conducted using Comprehensive Meta-Analysis (CMA) software (v3, Biostat, Englewood, NJ, USA).
Results
Trial Selection, Quality, and Characteristics of Included Trials
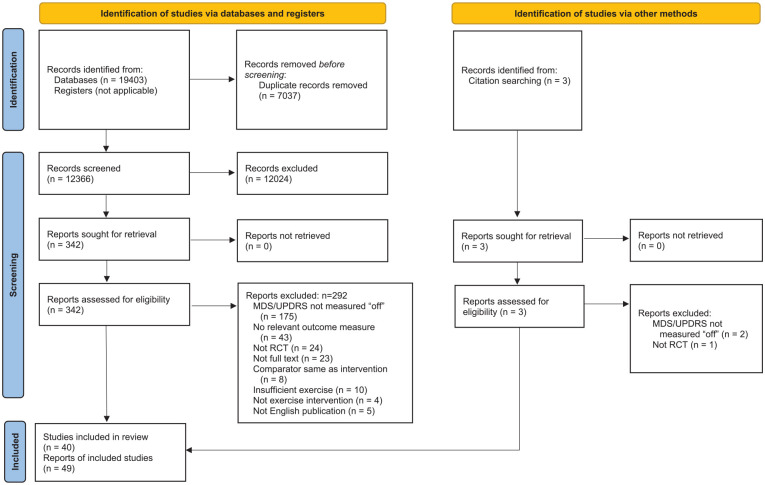
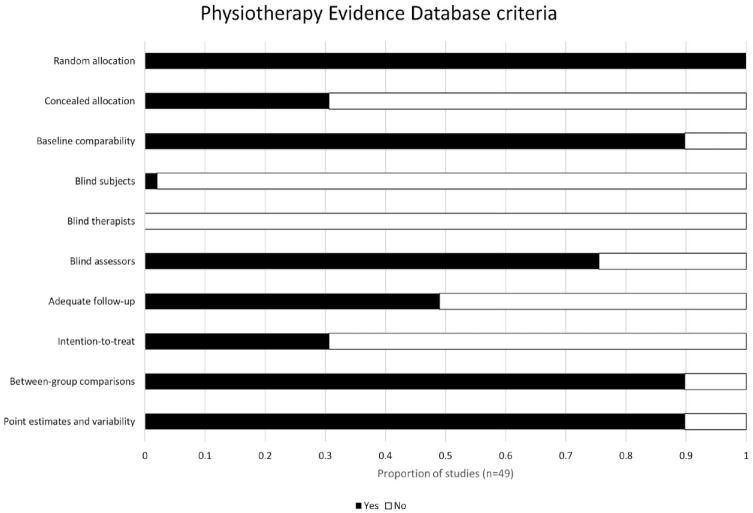
The searches identified 19 406 records. After removing duplicates, then screening titles and abstracts, 345 full text records were retrieved. Of these, 49 records met the inclusion criteria (Figure 1). As 7 trials were reported across 17 records, and 1 record reported 2 trials, a total of 40 trials were included in this review. The mean PEDro score for the included trials was 5.6 (range 2-8), with 23 trials (47%) of high quality,8,27,28,37,41-59 25 (51%) of moderate quality,6,7,33-36,38,60-77 and 1 (2%) of low quality 78 (Figure 2, Supplemental File 2).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources.
Figure 2.
Quality of included trials based on the Physiotherapy Evidence Database (PEDro) criteria and scores.
This review included 40 trials with 2104 PD participants (range 4 7 to 130 28 across studies). Participants had mild-moderate PD (mean HY score 1.6 when “on” and 2.2 when “off,” range 1-4 within a trial) and a mean PD duration of 6.1 years (range 0.5 27 to 12.1 6 across trials). Twenty trials (29 pairwise comparisons) involving 1225 participants compared exercise with a control group (ie, no intervention or sham exercise).6-8,27,28,35-38,43,46-48,53,56,60,63,64,66,68,71,73,74,77 Twenty-four trials (31 comparisons) involving 1044 participants compared 2 different exercise groups.6,27,33,34,41,42,44,45,49-52,54,55,57-59,61,62,65,67,69-72,75-78
Exercise was categorized according to the primary nature of the intervention: aerobic,6,7,27,28,33,42,48,52,53,55,58,63,65,67,69,70,72,77,78 strength,35,38,44,62,71 balance,43,56,59,60,73,74,77 dance,46,66 aquatic,51,76 or multimodal,6,8,34,36-38,41,42,44,45,47,49,50,54,55,57,59,61,62,64,68,71,72,75 the latter including a combination of aerobic, strength, balance, flexibility, and/or functional exercises where no single component was ≥50% of the entire intervention. The dosage of exercise varied among the 60 pairwise comparisons: session duration ranged from 3055,63,72 to 165 41 minutes, frequency ranged from 235,43-46,51,56,59,60,62-64,66,71,76,77 to 15 8 times per week and total program duration ranged from 48,57,58,76 to 10444,62,66 weeks, with the majority of trials (n = 38, 78%) reporting intervention durations no longer than 12 weeks. Participants completed between 8 76 and 286 55 exercise sessions throughout the intervention period.
Included trials reported diverse outcomes indicative of PD progression. Fourteen trials reported clinical outcomes, that is, “off” medication MDS/UPDRS scores27,28,36,37,44-47,51,52,55,61,66,67,74,76; all trials reported motor scores except 1 76 which reported total scores. Thirteen trials reported neurochemical outcomes, that is, trophic factors,8,34,38,49,56,63,72,73,77 oxidative stress,35,43,49,63,64 and/or inflammatory markers,34,38,49,72,73,77 with most not reporting medication status when outcomes were taken. Ten trials reported neuroimaging outcomes, that is, brain functional activation,53,54,56,69,70,75 connectivity,48,57,58,65,68,78 structure,48,60 or volume48,60 measured through fMRI or functional near-infrared spectroscopy in 1 trial, 69 mostly “on” medication54,56-58,60,65,69,70,75; and dopamine receptor binding7,53 measured “off” medication through positron emitted tomography. Neuroimaging trials examined different brain networks and used different imaging techniques and analysis methods. Nine trials reported neurophysiological outcomes, that is, cortical inhibition6,33,59 and cortical synchrony41,42 measured “on” medication, and bradykinesia measured through EMG50,62,67,71 “off” medication except for 1 trial. 71 Outcomes were assessed at baseline and the end of the intervention period in all trials. Nine trials (18%) reported follow-up outcomes following a period of no exercise (range 2.5-96 weeks).33,35,47,51,52,61,67,76,78 Owing to the heterogeneity of comparisons, we did not analyse effects at follow-up.
Effects of Exercise Compared to Control
Data were pooled from 6/6 trials (7 pairwise comparisons)27,28,37,46,47,74 that reported “off” MDS/UPDRS motor scores, and from 4/5 trials (5 comparisons)8,38,56,73 that reported BDNF. The remaining outcomes were synthesized narratively because data were unable to be pooled due to insufficient comparable interventions or outcomes (Table 1).
Table 1.
Studies Comparing the Effect of Exercise vs Control (ie, No or Sham Exercise) on Outcomes Indicative of Parkinson’s Disease Progression. Studies Are Organized by Outcome Measures.
| Study | Participants † | Intervention group | Control group | Between group differences ‡ |
|---|---|---|---|---|
| Clinical outcomes (“off” MDS/UPDRS scores) | ||||
| Duncan and Earhart (2012) 46 | n = 62 Age: 69.9 ± 9.0 Sex (M/F): 35/27 PD duration: 6.1 ± 5.1 Disease severity (off): HY 2.6 ± 0.5, MDS-UPDRS-III 46.3 ± 10.5 |
Dance: Tango 60 min × 2/wk × 52 wk |
No exercise |
MDS-UPDRS “off” - Reduced MDS-UPDRS-III scores at 3, 6, and 12 mo |
| Duncan and Earhart (2014)66,d | n = 10 Age: 67.8 ± 9.1 Sex (M/F): 8/2 PD duration: 8.8 ± 6.0 Disease severity (off): HY 2.4 ± 0.4, MDS-UPDRS-III 48.4 ± 19.6 |
Dance: Tango 60 min × 2/wk × 104 wk |
No exercise |
MDS-UPDRS “off” - Reduced MDS-UPDRS-III scores at 12 and 24 mo |
| Frazzitta et al. (2015) 47 | n = 40 (31) Age: 68.5 ± 7.1 Sex (M/F): 21/19 PD duration: NR Disease severity (off): HY 1.2 ± 0.3, MDS-UPDRS-III 13.6 ± 4.6 |
Multimodal exercise: Multidisciplinary intensive rehabilitation (stretching, balance, gait, and aerobic treadmill training) 60 min × 10/wk × 8 wk |
No exercise |
UPDRS “off” - Reduced UPDRS-III scores at 6 and 24 mo |
| Jung et al. (2020),37,a Hasegawa et al. (2020)36,a,e | n = 93 (86) Age: 68.8 ± 7.6 Sex (M/F): 58/28 PD duration: 6.5 ± 5.0 Disease severity (off): HY 2.3 ± 0.5, MDS-UPDRS-III 42.3 ± 12.2 |
Multimodal exercise: Agility boot camp (gait training, strength training, functional exercises, Tai Chi, and boxing) 90 min × 3/wk × 6 wk |
No exercise |
MDS-UPDRS “off” - No difference in MDS-UPDRS-III scores |
| Schenkman et al. (2018) 27 | n = 128 Age: 63.6 ± 9.7 Sex (M/F): 73/55 PD duration: 0.5 ± 0.7 Disease severity (off): HY 1.7 ± 0.4, UPDRS-III 16.6 ± 7.0 |
Aerobic exercise: High intensity treadmill training (80%-85% heart rate maximum) Aerobic exercise: Moderate intensity treadmill training (60%-65% heart rate maximum) 45 min × 4/wk × 26 wk |
No exercise |
UPDRS “off” - Smaller increase in UPDRS-III scores at 6 mo UPDRS “off” - No difference in increased UPDRS-III scores at 6 mo |
| van der Kolk et al. (2019) 28 | n = 130 Age: 59.4 ± 8.8 Sex (M/F): 80/50 PD duration: 3.9 ± 4.2 Disease severity (off): HY 2.0 ± 0.2, MDS-UPDRS-III 28.4 ± 15.3 |
Aerobic exercise: Moderate-high intensity cycling (50%-80% heart rate reserve) 30-45 min × 3/wk × 26 wk |
Sham exercise: Stretching 30-45 min × 3/wk × 26 wk |
MDS-UPDRS “off” - Smaller increase in MDS-UPDRS-III scores at 6 mo |
| Vergara-Diaz et al. (2018)74,a | n = 32 Age: 63.9 ± 6.1 Sex (M/F): 16/16 PD duration: 2.9 ± 2.3 Disease severity (off): HY 2.2 ± 0.2, UPDRS-III 23.5 ± 9.2 |
Balance exercise: Tai Chi 55 min × 3/wk × 26 wk |
No exercise |
UPDRS “off” - Smaller increase in UPDRS-III scores at 6 mo |
| Neurochemical outcomes (trophic factors, inflammatory markers, oxidative stress factors) | ||||
| Bloomer et al. (2008)35,a,c | n = 16 Age: 59.0 ± 2.5 Sex (M/F): 8/8 PD duration: NR Disease severity (on): HY 1.5 ± 0.5 |
Strength exercise 2×/wk × 8 wk |
No exercise |
Oxidative stress (blood essay) - No differences in concentration of all antioxidative factors (GPx, SOD, catalase, TEAC) - Decreased concentration of oxidative factor (H2O2) |
| Cheung et al. (2018)43,a | n = 20 Age: 64.7 ± 7.6 Sex (M/F): NR PD duration: 4.8 ± 2.9 Disease severity (on): HY 2.0 ± 0.8; UPDRS-III 25.0 ± 7.0 |
Balance exercise: Hatha yoga 60 min × 2/wk × 12 wk |
No exercise |
Oxidative stress (blood essay) - No difference in concentration of antioxidative factors (catalase, SOD, GPX, GSH, GSH:GSSG ratio) - No difference in concentration of oxidative factors (MDA, protein carbonylation) |
| Dias Belchior et al. (2017)63,a | n = 22 (18) Age: 68.1 ± 11.7 Sex (M/F): 11/7 PD duration: 5.6 ± 4.1 Disease severity (off): HY 2.5 ± 0 |
Aerobic exercise: Treadmill gait training 30 min × 2/wk × 8 wk |
No exercise |
Trophic factor (blood essay) - No difference in serum BDNF concentration Oxidative stress (blood essay) - No difference in concentration of antioxidative factors (GSH) |
| DiFrancisco-Donoghue et al. (2012) 64 | n = 41 (36) Age: 68.0 ± 7.0 Sex (M/F): 20/17 PD duration: 8.2 ± 5.2 Disease severity (on): HY 2.0 ± 0 |
Multimodal exercise: Moderate-high intensity aerobic exercises (60%-70% VO2 max), and strength exercises (50%-80% 1RM) 40 min × 2/wk × 6 wk |
No exercise |
Oxidative stress (blood essay) - Increased concentration of antioxidative factors (GSH, and GSH:GSSG ratio) |
| Frazzitta et al. (2014) 8 | n = 25 (24) Age: 66.2 ± 4.6 Sex (M/F): NR PD duration: 8.0 ± 4.1 Disease severity (on): UPDRS-III 16.1 ± 3.0 |
Multimodal exercise: Low-moderate intensity aerobic treadmill exercises at (≤60% heart rate reserve), flexibility, balance and functional exercises. 60 min × 15/wk × 4 wk |
No exercise |
Trophic factor (blood essay) - Increased serum BDNF concentration |
| Freidle et al. (2022)56,d | n = 95 Age: 71.0 ± 6.1 Sex (M/F): 60/35 PD duration: 4.3 ± 5.7 Disease severity (on): HY 2.2 ± 0.4, MDS-UPDRS-III 31.5 ± 11.1 |
Balance exercise: Progressive balance training including dual-tasking 60 min × 2/wk × 10 wk |
No exercise |
Trophic factor (blood essay) - No difference in serum BDNF concentration |
| Li et al. (2022) 77 | n = 95 Age: 62.2 ± 6.0 Sex (M/F): 58/37 PD duration: 4.7 ± 2.9 Disease severity (on): HY 2.0 ± 0.4, UPDRS-III 20.7 ± 8.8 |
Balance exercise: Tai Chi Aerobic exercise: Brisk walking (50%-60% heart rate maximum) 60 min × 2/wk × 52 wk |
No exercise |
Trophic factor (blood essay) - Increased PDGF-BB - No difference in VEGF or basic FGF Inflammatory markers (blood essay) - No difference in most pro-inflammatory (TNF-α, and 71% of interleukins) or anti-inflammatory cytokines - Some pro-inflammatory markers were downregulated (29% of interleukins, MCP-1, MIP-1a, and MIP-1β) - One anti-inflammatory marker (GM-CSF) was upregulated Trophic factor (blood essay) - No difference in VEGF, basic FGF, or PDGF-BB Inflammatory markers (blood essay) - No difference in pro-inflammatory cytokines (TNF-α, interleukins, other factors) |
| O’Callaghan et al. (a) (2020)38,b | n = 32 (27) Sex (M/F): 17/10 Age: 67.4 ± 7.9 PD duration: NR Disease severity (on): HY 2.0 ± 0.2 |
Multimodal exercise: Moderate-high intensity aerobic and resistance exercises (60%-80% heart rate maximum) 45-60 min × 3/wk × 12 wk |
No exercise |
Trophic factor (blood essay) - No difference in serum BDNF concentration Inflammatory markers (blood essay) - Reduction in some pro-inflammatory cytokines |
| O’Callaghan et al. (b) (2020)38,b | n = 20 (17) Sex (M/F): 9/8 Age: 68.9 ± 7.3 PD duration: NR Disease severity (on): HY 2.3 ± 0.5 |
Strength exercise: High intensity interval power training (≥85% maximum heart rate) 45-60 min × 3/wk × 12 wk |
No exercise |
Trophic factor (blood essay) - Increased serum BDNF concentration |
| Szymura et al. (2020) 73 | n = 29 Sex (M/F): 19/10 Age: 65.7 ± 7.5 PD duration: NR Disease severity (on): HY 2.4 ± 0.5 |
Balance exercise: Moderate intensity balance training (60%-70% maximum heart rate) 60 min × 3/wk × 12 wk |
No exercise |
Trophic factor (blood essay) - Increased serum BDNF concentration - No between group differences in other circulating trophic factors (IGF-1, β-NGF) Inflammatory markers (blood essay) - Increased concentration of some anti-inflammatory cytokines (TGF-β1, fractalkine) - No difference in other anti-inflammatory cytokines (IL-6, IL-10, CD200) - No difference in pro-inflammatory cytokines (TNF-α) |
| Neuroimaging outcomes (brain structure, volume, activation and functional connectivity, dopamine receptor binding) | ||||
| Albrecht et al. (2021)60,d | n = 95 (65) Age: 70.4 ± 6.0 Sex (M/F): 40/25 PD duration: 5.1 ± 4.1 Disease severity (on): HY 2.2 ± 0.4, MDS-UPDRS-III 29.9 ± 12 |
Balance exercise: Progressive balance training including dual-tasking 60 min × 2/wk × 10 wk |
No exercise |
Brain structure (structural MRI: volume-based morphometry and structural covariance network analysis) - Increased connectivity between the bilateral thalami and right cerebellum in the structural covariance network Brain volume - No difference in gray matter volume |
| Fisher et al. (2013) 7 | n = 4 Age: 55.0 ± 4.0 Sex (M/F): 3/1 PD duration: NR Disease severity (off): UPDRS-III 15.0 ± 2.5 |
Aerobic exercise: Moderate-high intensity treadmill training at (>3 METs and/or >75% age-adjusted heart rate maximum) 60 min × 3/wk × 8 wk |
No exercise |
Dopamine receptor binding potential (PET scan) - Increased dopamine-D2 receptor binding potential |
| Freidle et al. (2022)56,d | n = 95 Age: 71.0 ± 6.1 Sex (M/F): 60/35 PD duration: 4.3 ± 5.7 Disease severity (on): HY 2.2 ± 0.4, MDS-UPDRS-III 31.5 ± 11.1 |
Balance exercise: Progressive balance training including dual-tasking 60 min × 2/wk × 10 wk |
No exercise |
Brain activation (t-fMRI: activation detection) - No difference in activation in the striatum, primary motor cortex, premotor cortex, supplementary motor area, anterior cingulate cortex, or dorsolateral prefrontal cortex |
| Johansson et al. (2022)48,d | n = 57 Age: 59.4 ± 9.6 Sex (M/F): 37/20 PD duration: 3.8 ± 3.0 Disease severity (off): MDS-UPDRS-III 28.3 ± 14.0 |
Aerobic exercise: Moderate-high intensity cycling (50%-80% heart rate reserve) 38 min × 3/wk × 26 wk |
Sham exercise: Stretching + relaxation 38 min × 3/wk × 26 wk |
Brain connectivity (rs-fMRI: [a] seed-based connectivity analysis, [b] intra-network connectivity analysis) - [a] Right corticostriatal-sensorimotor connectivity shifted anteriorly - [b] Improved right frontoparietal-dorsolateral prefrontal connectivity (ie, improved cognitive control) Brain volume and structure ([c] structural and [d] diffusion MRI) - [c] Reduced loss of global brain volume - [c] No change in localized gray matter volume - [d] No change in substantia nigra tissue integrity |
| King et al. (2020)68,a,d | n = 46 Age: 68.6 ± 7.5 Sex (M/F): NR PD duration: 8.4 ± 5.1 Disease severity (off): HY 2.5 ± 0.8, MDS-UPDRS-III 47.2 ± 13.0 |
Multimodal exercise: Agility boot camp (gait training, strength training, functional exercises, Tai Chi, and boxing) 80 min × 3/wk × 6 wk |
No exercise |
Brain connectivity (rs-fMRI: region to region connectivity analysis) - Reduced right pedunculopontine-supplementary motor area connectivity (ie, reduced maladaptive response associated with FOG) |
| Sacheli et al. (2019)53,c | n = 35 Age: 67.2 ± 7.2 Sex (M/F): 22/13 PD duration: 4.5 ± 3.5 Disease severity (off): MDS-UPDRS-III 24.6 ± 12.0 |
Aerobic exercise: High intensity cycling (60%-80% VO2 max) 50 min × 3/wk × 12 wk |
Sham exercise: Stretching 3×/wk × 12 wk |
Dopamine release (PET scan) - Increased dopamine release only in the caudate in the better hemisphere - No difference in dopamine release in the caudate of the worse hemisphere, or in the putamen of both hemispheres. Dopamine receptor binding potential (PET scan) - Increased D2/3 receptor availability in the middle and posterior putamen of both hemispheres. Brain activation (t-fMRI: activation detection) - Significant higher BOLD signal increment in the ventral striatum of both hemispheres when performing a reward task with 75% probability of winning (ie, increased responsivity of the mesolimbic reward pathways). |
| Neurophysiological outcomes (cortical inhibition, muscle activation patterns underlying bradykinesia) | ||||
| Fisher et al. (2008) 6 | n = 30 Age: 62.9 ± 12.1 Sex (M/F): 19/11 PD duration: 1.1 ± 0.9 Disease severity (on): HY 1.9 ± 0.4; UPDRS-III 28.6 ± 8.9 |
Aerobic exercise: Moderate-high intensity treadmill training (>3 METs and/or >75% age-adjusted heart rate maximum) Multimodal exercise: Low intensity flexibility, balance, resistance, and functional exercises (≤3 METs and/or ≤50% age-adjusted heart rate maximum) 45 min × 3/wk × 8 wk |
No exercise |
Cortical inhibition (TMS with EMG-measured MEP) - Increased maximum CSP in both brain hemispheres (ie, greater cortical inhibition indicative of more normal cortical function) Cortical inhibition (TMS with EMG-measured MEP) - No difference in maximum CSP in both brain hemispheres (ie, no change in cortical inhibition) |
| Silva-Batista et al. (2017) 71 | n = 37 Age: 64.5 ± 9.4 Sex (M/F): 29/8 PD duration: 10.2 ± 4.5 Disease severity (on): HY 2.5 ± 0.4, UPDRS-III 44.6 ± 10.3 |
Strength exercise: Progressive resistance training (8-12 RM of 5 upper and lower limb muscle groups) Multimodal exercise: Progressive resistance training (8-12 RM of 5 upper and lower limb muscle groups) performed on unstable surfaces 50 min × 2/wk × 12 wk |
No exercise |
EMG measures of bradykinesia - Increased presynaptic inhibition (ie, reduced bradykinesia) - No change in disynaptic reciprocal inhibition (ie, no change in rigidity) EMG measures of bradykinesia - Increased presynaptic inhibition and disynaptic reciprocal inhibition (ie, improved muscle coordination), to levels similar to healthy people |
Abbreviations: BDNF, brain-derived neurotrophic factor; β-NGF, beta nerve growth factor; CD200, cluster of differentiation-200; CSP, cortical silent period; EMG, electromyography; FGF, fibroblast growth factor; GM-CSF, granulocyte macrophage colony stimulating factor; GPX, glutathione peroxidase; GSH, glutathione; GSH:GSSG ratio, glutathione and oxidized glutathione ratio; H2O2, hydrogen peroxide; HY, Hoehn and Yahr scale; IGF-1, insulin-like growth factor 1; IL-6, interleukin 6; IL-10, interleukin 10; MDA, malondialdehyde; MDS-UPDRS-III, Movement Disorder Society Unified Parkinson’s Disease Rating Scale motor section; MEP, motor evoked potential; MET, metabolic equivalent; NR, not reported; PDGF-BB, platelet derived growth factor BB; PET, positron emitted tomography; rs-fMRI, resting state functional magnetic resonance imaging; SOD, superoxide dismutase; t-fMRI, task-based functional magnetic resonance imaging; TEAC, trolox equivalent antioxidant capacity; TGF-β1, transforming growth factor beta 1; TMS, transcranial magnetic stimulation; TNF-α, tumor necrosis factor alpha; UPDRS-III, Unified Parkinson’s Disease Rating Scale motor section; VEGF, vascular endothelial growth factor.
n: number randomized. If participant characteristics are reported only for those who completed, this n is reported in brackets. Age and PD duration were measured in years. Values are reported as number, or mean ± SD.
Changes indicate the effect of exercise for the intervention group/s relative to the control group. Outcomes were measured at post-test upon completion of the intervention period, unless otherwise specified.
Studies which did not specify exercise intensity.
Two trials were reported in O’Callaghan et al. 38 Each trial was conducted at different time points with different groups of PD participants.
Studies which did not report session duration for the intervention and/or control group/s.
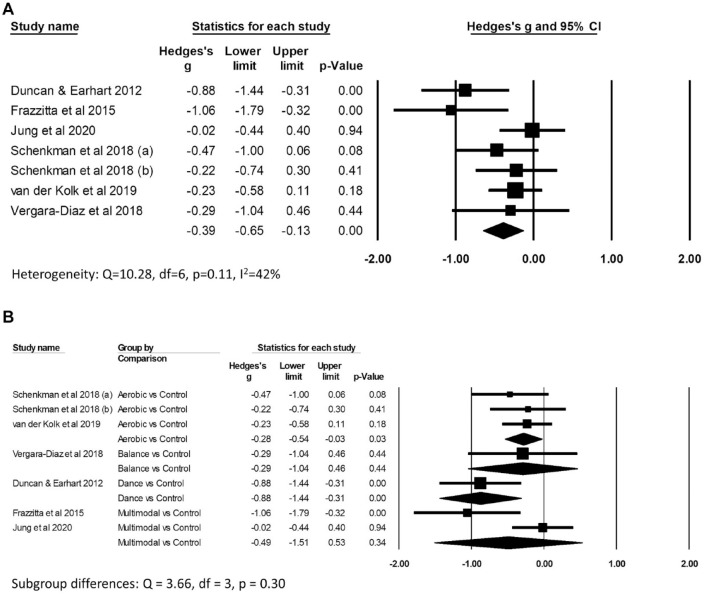
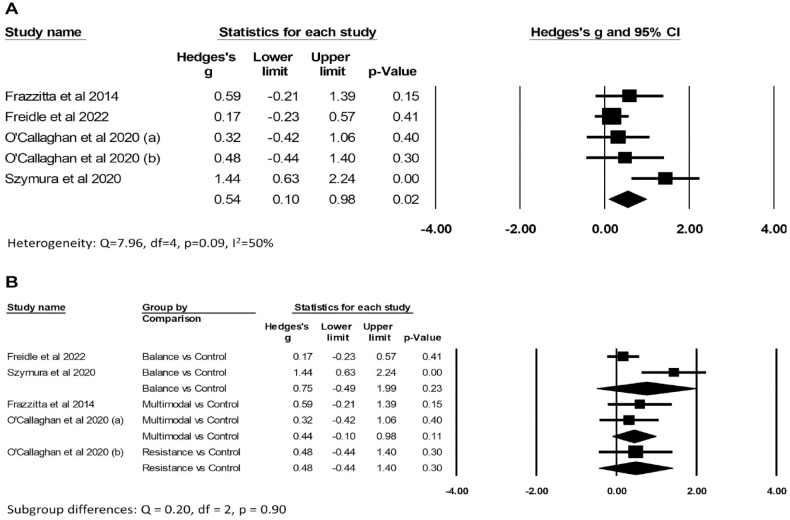
There was low certainty evidence (Supplemental File 3A) of benefit of exercise on improving “off” MDS/UPDRS motor scores compared to control (Hedges’ g −0.39, 95% CI: −0.65 to −0.13, P = .003; I2 42%; 469 participants from 7 comparisons; Figure 3A). There was very low certainty evidence (Supplemental File 3B) of benefit of exercise on improving BDNF levels compared to control (Hedges’ g 0.54, 95% CI: 0.10-0.98, P = .02; I2 50%; 192 participants from 5 comparisons; Figure 4A). There was no evidence of subgroup differences according to type of exercise for either “off” MDS/UPDRS scores (Q = 3.66, df = 3, P = .30; Figure 3B) or BDNF (Q = 0.20, df = 2, P = .90; Figure 4B), however there were insufficient trials across different types of exercise to find a subgroup difference if one exists. Although pooled results from studies of aerobic exercise suggest a positive effect on reducing “off” MDS/UPDRS (Hedges’ g −0.28, 95% CI: −0.54 to −0.03, P = .03; 258 participants from 3 comparisons), this result needs to be interpreted with caution given the limited data available.
Figure 3.
Forest plots of the effects of exercise versus control on Movement Disorders Society/Unified Parkinson’s Disease Rating Scale (MDS/UPDRS) “off” motor scores. Panel (A) shows the overall results and (B) the subgroup analysis.
Figure 4.
Forest plots of the effects of exercise versus control on brain-derived neurotrophic factor (BDNF) concentration. Panel (A) shows the overall results and (B) the subgroup analysis.
Overall, there was no evidence of effects across other neurochemical outcomes (ie, inflammatory cytokines38,73,77 or oxidative stress35,43,63). Individual trials reported that exercise improved very few anti-inflammatory73,77 or pro-inflammatory 38 cytokines. Findings for glutathione were equivocal.43,63,64
For neuroimaging outcomes, 4 trials (5 comparisons) reported fMRI outcomes and examined aerobic,48,53 balance,56,60 and multimodal 68 exercise. Findings from these trials suggest that exercise generally had a neuroplastic effect, by improving activation 53 and network connectivity 60 toward normal function or by improving the efficiency of compensatory brain networks.48,68 These effects were generally associated with improved behavioral performance (eg, mobility). In contrast, there was no evidence that exercise altered gray matter volume,48,60 although 1 trial found that exercise reduced global brain volume loss 48 in PD. Additionally, 2 trials (2 comparisons) found increased dopamine receptor binding potential 7 and receptor availability in the posterior putamen 53 following moderate to high intensity aerobic exercise.
For neurophysiological outcomes, 1 trial (2 comparisons) 6 found that aerobic exercise increased cortical inhibition, but this effect was not apparent following low intensity multimodal exercise. In another trial (2 comparisons), 71 strength and multimodal exercise were found to improve bradykinesia by increasing presynaptic inhibition, but only multimodal exercise improved disynaptic reciprocal inhibition to better improve task performance.
Effects of Exercise Compared to Another Type of Exercise
In trials reporting pairwise comparisons of different exercises, the groups differed based on exercise type, exercise intensity and/or cognitive engagement. Within each outcome category, trials were too heterogenous to enable pooling of results in meta-analyses (Table 2).
Table 2.
Studies Comparing the Effect of Exercise vs Exercise of a Different Type and/or Dose on Outcomes Indicative of Parkinson’s Disease Progression. Studies Are Organized by Outcome Measures.
| Study | Participants † | Intervention group 1 | Intervention group 2 | Between group differences ‡ |
|---|---|---|---|---|
| Clinical outcomes (“off” MDS/UPDRS scores) | ||||
| Beck et al. (2018)61,b | n = 47 (39) Age: 70.8 ± 8.9 Sex (M/F): 31/8 PD duration: 6.9 ± 4.6 Disease severity (off): MDS-UPDRS-III 31.4 ± 10.9 |
Multimodal exercise: Walking, balance, stretching, and coordination exercises, with external focus of attention 60 min × 3/wk × 11 wk |
Multimodal exercise: Walking, balance, stretching, and coordination exercises, with internal focus of attention 60 min × 3/wk × 11 wk |
UPDRS “off” - Reduced UPDRS-III scores at 6 and 24 mo |
| Corcos et al. (2013) 44 | n = 48 Age: 58.8 ± 5.1 Sex (M/F): 28/20 PD duration: 6.5 ± 4.4 Disease severity (off): HY 2.3 ± 0.5, UPDRS-III 34.6 ± 11.7 |
Strength exercise: Alternating bimonthly between 3 sets of 8 or 2 sets of 12 for 11 upper (30%-40% 1RM) and lower (50%-60% 1RM) limb muscle groups 75 min × 2/wk × 104 wk |
Multimodal exercise: Balance exercise, low intensity strength training, stretching 75 min × 2/wk × 104 wk |
UPDRS “off” - No difference in the reduction in UPDRS-III scores at 6 mo - Reduced UPDRS-III scores at 12, 18, and 24 mo |
| Dibble et al. (2015) 45 | n = 41 Age: 68.4 ± 12.2 Sex (M/F): 25/16 PD duration: 6.8 ± 4.4 Disease severity (off): UPDRS-III 25.0 ± 9.2 |
Multimodal exercise: Strength and fitness training at RPE of 13 (ie, “somewhat hard”), and lower limb eccentric resistance training 60 min × 2/wk × 12 wk |
Multimodal exercise: Strength and fitness training at RPE of 13 (ie, “somewhat hard”) 60 min × 2/wk × 12 wk |
UPDRS “off” - No difference in UPDRS-III scores at 6 mo |
| Jansen et al. (2021) 67 | n = 29 Age: 62.8 ± 9.8 Sex (M/F): 14/15 PD duration: 3.5 ± 4.5 Disease severity (off): HY 2.0 ± 0, MDS-UPDRS-III 34.5 ± 10.6 |
Aerobic exercise: Cycling (60%-80% heart rate reserve) at forced high cadence 55 min × 3/wk × 8 wk |
Aerobic exercise: Cycling (60%-80% heart rate reserve) at preferred cadence 55 min × 3/wk × 8 wk |
MDS-UPDRS “off” - No difference in reduced MDS-UPDRS-III scores at 8 wk |
| Perez de la Cruz (2017)51,b | n = 30 Age: 67.2 ± 7.9 Sex (M/F): 13/16 PD duration: 6.5 ± 2.9 Disease severity (off): HY 2.8 ± 0.7, UPDRS-III 15.2 ± 7.3 |
Aquatic exercise: Aquatic Ai Chi (includes balance and flexibility exercises) 45 min × 2/wk × 10 wk |
Multimodal exercise: Strength, aerobic, balance, and stretching exercises on land 45 min × 2/wk × 10 wk |
UPDRS “off” - Reduced UPDRS-III scores at 10 and 14 wk - Reduced UPDRS-total scores at 14 wk |
| Ridgel et al. (2009)52,b | n = 10 Age: 61.2 ± 6.0 Sex (M/F): 8/2 PD duration: 6.2 ± 5.7 Disease severity (off): UPDRS-III 48.7 ± 14.1 |
Aerobic exercise: Cycling at forced high cadence (30% faster than preferred cadence) 60 min × 3/wk × 8 wk |
Aerobic exercise: Cycling at preferred cadence 60 min × 3/wk × 8 wk |
UPDRS “off” - Reduced UPDRS-III scores at 8 wk |
| Schenkman et al. (2018)27,a | n = 88 Age: 63.5 ± 9.5 Sex (M/F): 49/39 PD duration: 0.5 ± 0.7 Disease severity (off): HY 1.7 ± 0.5, UPDRS-III 16.5 ± 7.0 |
Aerobic exercise: High intensity treadmill training (80%-85% heart rate maximum) 45 min × 4/wk × 26 wk |
Aerobic exercise: Moderate intensity treadmill training at (60%-65% heart rate maximum) 45 min × 4/wk × 26 wk |
UPDRS “off” - NR. Appears that there are no differences in UPDRS-III scores |
| Vivas et al. (2011)76,b | n = 12 Age: 67.0 ± 5.5 Sex (M/F): 7/5 PD duration: 6.0 ± 2.9 Disease severity (off): HY 2.5 ± 0.5, UPDRS-total 41.1 ± 12.7 |
Aquatic exercise: Trunk mobility, balance, and transfer training in the pool 45 min × 2/wk × 4 wk |
Multimodal exercise: Trunk mobility, balance, and transfer training on land 45 min × 2/wk × 4 wk |
UPDRS “off” - Reduced UPDRS-total scores at 1 mo |
| Xiao and Zhuang (2016)55,b | n = 96 Age: 67.3 ± 15.2 Sex (M/F): 67/29 PD duration: 5.8 ± 0.5 Disease severity (off): HY 2.2 ± 1.6, UPDRS-III 27.2 ± 15.9 |
Aerobic exercise: Walking 30 min × 7/wk × 26 wk |
Multimodal exercise: Qigong and walking Qigong: 45 min × 4/wk; Walking 30 min × 7/wk; x26 wk |
UPDRS “off” - Reduced UPDRS-III scores at 6 mo |
| Neurochemical outcomes (trophic factors, inflammatory markers, oxidative stress factors) | ||||
| Landers et al. (2019) 49 | n = 27 Age: 64.0 ± 8.9 Sex (M/F): 19/8 PD duration: 4.8 ± 4.6 Disease severity (off): HY 2 ± 0.4, MDS-UPDRS-III 36.4 ± 13.7 |
Multimodal exercise: Moderate to high intensity aerobic exercises (70%-80% estimated heart rate maximum), resistance exercises (50%-80% 1RM), balance exercises, and flexibility exercises. 90 min × 3-4/wk × 8 wk |
Multimodal exercise: Low to moderate intensity aerobic exercises (50%-65% estimated heart rate maximum), resistance exercises (<50% 1RM), balance exercises, and flexibility exercises. 60 min × 3-4/wk × 8 wk |
Trophic factor - No difference in BDNF at 8 wk and at 6-mo follow-up. - BDNF concentration returned to baseline levels in both groups at 6-mo follow-up. Inflammatory markers - No differences in anti-inflammatory cytokines (IL10, IL10: TNF-α, IL6) at 8 wk and at 6-mo follow-up. - No differences in pro-inflammatory cytokines (TNF-α) 8 wk and at 6-mo follow-up. - All anti-inflammatory cytokines returned to baseline levels, whereas pro-inflammatory cytokines (TNF-α) continued to increase to a higher level in both groups at 6-mo follow-up. Oxidative stress - No differences in the concentration of antioxidative factor (SOD1) at 8 wk and at 6-mo follow-up. - Antioxidative factor (SOD1) returned to baseline levels in both groups at 6-mo follow-up. |
| Li et al. (2022)77,a | n = 63 (54) Age: 62.3 ± 5.6 Sex (M/F): 39/24 PD duration: 4.9 ± 3.2 Disease severity (on): HY 1.9 ± 0.5, UPDRS-III 21.4 ± 10.2 |
Balance exercise: Tai Chi 60 min × 2/wk × 52 wk |
Aerobic exercise: Brisk walking (50%-60% heart rate maximum) 60 min × 2/wk × 52 wk |
Trophic factor - No difference in VEGF, basic FGF, or PDGF-BB Inflammatory markers - No difference in most pro-inflammatory (TNF-α, 93% of interleukins) or anti-inflammatory cytokines - A few pro-inflammatory markers were downregulated (7% of interleukins, MCP-1, MIP-1a) - Two anti-inflammatory markers (GM-CSF, eotaxin) were upregulated |
| Sajatovic et al. (2017)34,b | n = 30 Sex (M/F): 19/11 Age: 70.0 ± 7.9 PD duration: 6.8 ± 5.3 Disease severity (on): HY 2.5 ± 0.7 MDS-UPDRS-III 33.8 ± 9.1 |
Multimodal exercise: Aerobic and resistance exercises with supervision 40 min × 3/wk × 24 wk |
Multimodal exercise: Written instruction for the same aerobic and resistance exercises as Intervention-1, performed without supervision 40 min × 3/wk × 24 wk |
Trophic factor - No differences in BDNF concentration at 12 wk (midway through intervention) or 24 wk. Inflammatory markers - No differences in pro-inflammatory cytokines (TNF-α), and anti-inflammatory cytokines (IL-6) at 12 wk (midway through intervention) and 24 wk. |
| Soke et al. (2021) 72 | n = 40 (29) Age: 57.6 ± 8.6 Sex (M/F): 19/10 PD duration: 7.9 ± 4.4 Disease severity (off): HY 2.3 ± 0.5, UPDRS-III 31.2 ± 11.3 |
Multimodal exercise: Treadmill training (60%-80% heart rate maximum) and task-oriented circuit training 83 min × 3/wk × 8 wk |
Aerobic exercise: Treadmill training (60%-80% heart rate maximum) 30 min × 3/wk × 8 wk |
Trophic factors - No differences in BDNF, GDNF, IGF-1, or VEGF concentrations Inflammatory markers - No differences in pro-inflammatory cytokines (TNF-α) and anti-inflammatory cytokines (IL-1b) |
| Neuroimaging outcomes (brain structure, volume, activation and functional connectivity, dopamine receptor binding) | ||||
| Droby et al. (2020)65,c | n = 42 (37) Age: 73.2 ± 6.6 Sex (M/F): 22/15 PD duration: 9.0 ± 6.3 Disease severity (on): HY 2.5 ± 0.4, UPDRS-III 27.8 ± 12.4 |
Aerobic exercise: Treadmill training in virtual reality environment (dual tasking) at 80% of overground speed 45 min × 3/wk × 6 wk |
Aerobic exercise: Treadmill training without virtual reality at 80% of overground speed 45 min × 3/wk × 6 wk |
Brain connectivity (rs-fMRI: intra-network connectivity analysis) - Improved functional connectivity in the sensorimotor and cerebellar networks (ie, improved attentional resource allocation for maintaining automated motor sequences) - No difference in functional connectivity in the dorsal attention, lateral motor, executive control, salience, frontostriatal, or basal ganglia networks (ie, no change in gait control via higher motor and cognitive pathways which bypass the striatum, or in the striatum itself) |
| Hajebrahimi et al. (2022) 57 | n = 30 (24) Age: 65.9 ± 9.1 Sex (M/F): 19/5 PD duration: NR Disease severity (on): UPDRS-III 14.5 ± 8.2 |
Multimodal exercise: Balance and leg strengthening exercises using Nintendo Wii™ 60 min × 3/wk × 4 wk |
Multimodal exercise: Stretching, balance and leg strengthening exercises 60 min × 3/wk × 4 wk |
Brain connectivity (rs-fMRI: intra-network connectivity analysis) - Increased activation of precuneus in the DMN network |
| Kim et al. (2022) 58 | n = 44 Age: 68.1 ± 8.2 Sex (M/F): 13/31 PD duration: 9.0 ± 5.2 Disease severity (on): HY 3.0 ± 0, MDS-UPDRS-III 36.6 ± 11.9 |
Aerobic exercise: Treadmill walking with exoskeleton, auditory cues and feedback on performance 45 min × 3/wk × 4 wk |
Aerobic exercise: Treadmill walking 45 min × 3/wk × 4 wk |
Brain connectivity (rs-fMRI: intra-network and inter-network connectivity analysis) - Increased activation of the precuneus within the default mode network (intra-network) - Decreased coupling between the visual and dorsal attention, the bilateral central executive, and the auditory and medial temporal, networks (ie, less reliance on compensatory attentional pathways) (inter-networks analysis) |
| Maidan et al. (2018)69,b,c | n = 64 Sex (M/F): 45/19 Age: 71.7 ± 1.2 PD duration: 9.3 ± 1.1 Disease severity (on): UPDRS-III 33.2 ± 2.6 |
Aerobic exercise: Treadmill training in virtual reality environment (dual tasking) at 80% of overground speed 45 min × 3/wk × 6 wk |
Aerobic exercise: Treadmill training without virtual reality at 80% of overground speed 45 min × 3/wk × 6 wk |
Brain activation (fNIRS: activation detection) - Improved cortical inhibition in the right prefrontal cortex (ie, improved automaticity). - No differences in cortical inhibition in the left prefrontal cortex. |
| Maidan et al. (2017)70,c | n = 34 Age: 71.4 ± 1.6 Sex (M/F): 23/11 PD duration: 9.8 ± 1.5 Disease severity (on): MDS-UPDRS-III 28.9 ± 3.5 |
Aerobic exercise: Treadmill training in virtual reality environment (dual tasking) at 80% of overground speed 45 min × 3/wk × 6 wk |
Aerobic exercise: Treadmill training without virtual reality at 80% of overground speed 45 min × 3/wk × 6 wk |
Brain activation (t-fMRI: activation detection) - Reduced activation of right Brodmann area 10 and inferior frontal gyrus (ie, increased efficiency of cognitive networks) - Greater activation of left anterior cerebellum and middle temporal gyrus (ie, worse sensory integration for gait control) |
| Shah et al. (2016) 78 | n = 27 Age: 56.9 ± 8.3 Sex (M/F): 17/10 PD duration: 3.7 ± 4.1 Disease severity (off): MDS-UPDRS-III 24.8 ± 9.8 |
Aerobic exercise: Cycling (60%-80% heart rate reserve) at forced high cadence 60 min × 3/wk × 8 wk |
Aerobic exercise: Cycling (60%-80% heart rate reserve) at preferred cadence 60 min × 3/wk × 8 wk |
Brain connectivity (t-fMRI and rs-fMRI: seed based connectivity analysis) - No difference in thalamus-cortical connectivity (ie, no change in sensory integration to modulate motor output) |
| Silva-Batista et al. (2020) 54 , Vieira-Yano et al. (2021)75,d | n = 40 (32) Age: 65.6 ± 9.8 Sex (M/F): 21/11 PD duration: 8.8 ± 4.8 Disease severity (on): HY 3.1 ± 0.4, UPDRS-III 48.7 ± 11.0 |
Multimodal exercise: Progressive resistance training (of 7 upper and lower limb muscle groups) performed on unstable surfaces 85 min × 3/wk × 12 wk |
Multimodal exercise: Gait and balance training, resistance training with free weights, stretching 85 min × 3/wk × 12 wk |
Brain activation (t-fMRI: activation detection) - Increased BOLD activation of right middle-inferior temporal gyrus, mesencephalic locomotor region (which correlated to reduced NFOG-Q scores) and cerebellar locomotor region (ie, increased compensation for lack of gait automaticity) - No difference in SMA activation - Increased activation of right cerebellar locomotor region (which correlated to improved automaticity of stride length) |
| Neurophysiological outcomes (cortical inhibition, muscle activation patterns underlying bradykinesia) | ||||
| Calabrò et al. (2019)41,b | n = 50 Age: 71.5 ± 8 Sex (M/F): NR PD duration: 9.7 ± 3 Disease severity (on): UPDRS-III 30 ± 4 |
Multimodal exercise: Treadmill walking with auditory cues, overground gait, daily activity, and biomechanical training 195 min × 5/wk × 8 wk |
Multimodal exercise: Treadmill walking without auditory cues, overground gait, daily activity, and biomechanical training 195 min × 5/wk × 8 wk |
Cortical synchrony (EEG) - Improved ERS and ERD during gait (ie, greater cortical synchrony) - Increased TRCoh estimations (ie, greater inter-regional cortical connectivity) |
| Cheng et al. (2018)42,a,b | n = 18 Sex (M/F): 14/4 Age: 67.7 ± 7.8 PD duration: 5.8 ± 2.8 Disease severity (on): HY 1.4 ± 0.4 |
Aerobic exercise [a]: High intensity treadmill walking at RPE 14-15 (ie, “hard”), and overground walking training Multimodal exercise [b]: High intensity balance and strengthening exercises at RPE 14-15 (ie, “hard”), and overground walking training 40 min × 2-3/wk × 5 wk |
Multimodal exercise: Seated upper limb resistance exercise and overground walking training 40 min × 2-3/wk × 5 wk |
Cortical synchrony (EEG) - Improved β-ERD (ie, reduced cortical desynchrony) - No difference in α-ERD Cortical synchrony (EEG) - Improved β-ERD (ie, reduced cortical desynchrony) - No difference in α-ERD Cortical synchrony (EEG; [a] vs [b]) - [a vs b] No difference in β-ERD or α-ERD |
| David et al. (2016) 62 | n = 48 Age: 58.8 ± 5.1 Sex (M/F): 28/20 PD duration: 6.5 ± 4.4 Disease severity (off): HY 2.3 ± 0.5, UPDRS-III 34.6 ± 11.7 |
Strength exercise: Progressive resistance training: alternating bimonthly between 3 sets of 8 or 2 sets of 12 for 11 upper (30%-40% 1RM) and lower (50%-60% 1RM) limb muscle groups 75 min × 2/wk × 104 wk |
Multimodal exercise: Balance exercise, low intensity strength training, stretching 75 min × 2/wk × 104 wk |
EMG measures of bradykinesia - Increased peak velocity, increased first agonist burst duration and magnitude, and fewer agonist bursts prior to peak velocity (ie, improved bradykinesia) - No difference in antagonist burst magnitude |
| Fisher et al. (2008)6,a | n = 20 Age: 62.8 ± 12.4 Sex (M/F): 11/9 PD duration: 1.0 ± 0.8 Disease severity (on): HY 1.9 ± 0.4; UPDRS-III 29.1 ± 9.5 |
Aerobic exercise: Moderate-high intensity treadmill training at (>3 METs and/or >75% age-adjusted heart rate maximum) 45 min × 3/wk × 8 wk |
Multimodal exercise: Low intensity flexibility, balance, resistance, and functional exercises at (≤3 METs and/or ≤50% age-adjusted heart rate maximum) 45 min × 3/wk × 8 wk |
Cortical inhibition (TMS with EMG-measured MEP) - Increased maximum CSP in both brain hemispheres (ie, greater cortical inhibition indicative of more normal cortical function) |
| Jansen et al. (2021) 67 | n = 29 Age: 62.8 ± 9.8 Sex (M/F): 14/15 PD duration: 3.5 ± 4.5 Disease severity (off): HY 2.0 ± 0, MDS-UPDRS-III 34.5 ± 10.6 |
Aerobic exercise: Cycling at (60%-80% heart rate reserve) at forced high cadence 55 min × 3/wk × 8 wk |
Aerobic exercise: Cycling at (60-80% heart rate reserve) at preferred cadence 55 min × 3/wk × 8 wk |
EMG measures of bradykinesia - Improved maximal rate of force production of the moving hand (ie, reduced bradykinesia) - Improvements in the maximal rate of force production disappeared at follow-up 2 mo later - No difference in grip force delay, load force delay or total task time (ie, no change in interlimb coordination) |
| Liu et al. (2022) 59 | n = 28 Age: 67.9 ± 6.6 Sex (M/F): 16/12 PD duration: 6.9 ± 5.2 Disease severity (on): HY 1.8 ± 0.7, UPDRS-III 26.0 ± 10.9 |
Balance exercise: Square stepping in a smooth pattern 60 min x2/wk × 8 wk |
Multimodal exercise: Upper and lower limb strength training, postural education, and hand-eye coordination exercises 60 min x2/wk × 8 wk |
Cortical inhibition (TMS with EMG-measured MEP) - Increased maximum CSP (ie, greater cortical inhibition indicative of more normal cortical function) - Reduced SICI (ie, greater intracortical inhibition indicative of more normal function) |
| Marusiak et al. (2019) 50 | n = 20 Age: 73.0 ± 9.5 Sex (M/F): 9/11 PD duration: 8.5 ± 4.5 Disease severity (off): HY 2.3 ± 0.6 |
Multimodal exercise: Moderate intensity interval training on cycle ergometer (60%-75% heart rate maximum), and physiotherapy 60 min × 3/wk × 8 wk |
Multimodal exercise: Physiotherapy NR |
EMG measures of bradykinesia - Reduced muscle onset time, and increased rate of force development of the moving hand (ie, improved automatic adjustments) - Reduced delay between grip force development in both hands (ie, improved automatic bimanual coordination) |
| Pelosin et al. (2020)33,b,c | n = 24 Sex (M/F): 12/12 Age: 71.8 ± 3.6 PD duration: NR Disease severity (on): UPDRS-III 30.6 ± 10.3 |
Aerobic exercise: Treadmill training in virtual reality environment (dual tasking) at 80% of overground speed 45 min × 3/wk × 6 wk |
Aerobic exercise: Treadmill training without virtual reality at 80% of overground speed 45 min × 3/wk × 6 wk |
Cortical inhibition (TMS with EMG-measured MEP) - Improved cortical inhibition (greater SAI in M1 cortex) at 6 wk (ie, indicative of more normal cortical function) - No difference in cortical inhibition at 6-mo follow-up |
| Silva-Batista et al. (2017)71,a | n = 26 Age: 64.2 ± 9.9 Sex (M/F): 20/6 PD duration: 10.1 ± 4.0 Disease severity (on): HY 2.5 ± 0.5, UPDRS-III 44.4 ± 11.1 |
Multimodal exercise: Progressive resistance training (8-12 RM of 5 upper and lower limb muscle groups) performed on unstable surfaces 50 min × 2/wk × 12 wk |
Strength exercise: 8-12 RM of 5 upper and lower limb muscle groups 50 min × 2/week × 12 wk |
EMG measures of bradykinesia - Increased presynaptic inhibition and disynaptic reciprocal inhibition (ie, improved muscle coordination), the latter to levels similar to healthy people |
Abbreviations: 1RM, 1-repetition maximum; BDNF, brain-derived neurotrophic factor; EEG, electroencephalography; EMG, electromyography; ERD, event-related desynchronization; ERS, event-related synchronization; fNIRS, functional near-infrared spectroscopy; HY, Hoehn and Yahr scale; IL-6, interleukin 6; IL-10, interleukin 10; MDS-UPDRS-III, Movement Disorder Society Unified Parkinson’s Disease Rating Scale motor section; NR, not reported; RPE, rating of perceived exertion; rs-fMRI, resting state functional magnetic resonance imaging; SAI in M1 cortex, short-latency afferent inhibition in primary motor cortex; SICI, short-interval intracortical inhibition; SMA, supplementary motor area; SOD, superoxide dismutase; t-fMRI, task-based functional magnetic resonance imaging; TNF-α, tumor necrosis factor-alpha; TRCoh, task-related coherence; UPDRS-III, Unified Parkinson’s Disease Rating Scale motor section.
n: number randomized. If participant characteristics were reported only for those who completed, this n was reported in brackets. Age and PD duration were measured in years. Values are reported as number, or mean ± SD.
Changes indicate the effect of intervention group/s 1 relative to intervention group 2. If specific timepoints of outcome measures were not specified, the outcomes were measured upon completion of the intervention. Outcomes were measured at post-test upon completion of the intervention period, unless otherwise specified.
Studies with 3 groups. Study characteristics and results for the exercise vs exercise comparisons only are presented in this table; for all studies except 1, 42 the study characteristics were a subset of the entire study sample. If the study included a control group, study characteristics for the entire sample and results for exercise vs control comparisons were presented in Table 1.
Studies which did not specify exercise intensity.
Non-pooled forest plots of the effects of different types of exercise did not show any evidence that 1 type of exercise was superior to another for decreasing “off” MDS/UPDRS scores (9 pairwise comparisons from 9 trials,27,44,45,51,52,55,61,67,76Supplemental File 4A). Similarly, there was no evidence favoring 1 type of exercise over another for changing the concentration of trophic (BDNF levels: 3 comparisons from 3 trials,34,49,72Supplemental File 4B) or inflammatory factors (interleukin-6 or tumor necrosis factor-α: 3 comparisons each from 3 trials,34,49,77Supplemental File 4C and 4D, respectively).
For neuroimaging outcomes, 5 trials (7 comparisons) investigated the effects of different types of exercise on brain activation54,69,70,75 and connectivity57,58,65,78 and found that effects of exercise were localized to specific brain regions (eg, the medial temporal gyrus54,70 or precuneus57,58), depending on the type of exercise investigated and the networks underlying the behaviors of interest to the investigators (eg, freezing of gait 54 ). These trials generally found that the exercise involving greater cognitive engagement was associated with greater improvements.57,58,65,69,70,78
Eight trials reported neurophysiological outcomes. Two trials (4 comparisons) measuring cortical synchrony generally found that the exercise involving greater cognitive engagement 41 or higher training intensity 42 resulted in greater improvements in cortical synchrony,41,42 with no difference between exercises of similarly intensity. 42 Similarly, across 3 trials (3 comparisons) measuring cortical inhibition, exercise involving greater cognitive engagement33,59 or higher training intensity 6 showed greater cortical inhibition post training. Four trials50,62,67,71 (4 comparisons) investigated the effect of different forms of exercise on muscle activation patterns underlying bradykinesia and showed higher intensity exercise50,62 or multimodal exercise combining balance and resistance training 71 reduced bradykinesia, primarily through improvements in agonist muscle force production.50,62,67 Improvements in muscle coordination50,71 also contributed to reduced bradykinesia, though 1 trial found no differences in muscle coordination between exercise groups. 67
Discussion
This systematic review provides evidence that exercise may have an attenuating effect on disease progression in PD. The meta-analyses showed medium or small effects in favor of exercise on reducing “off” MDS/UPDRS motor scores and improving BDNF levels, respectively, compared to control. Nonetheless, the certainty of this evidence was low to very low and needs to be interpreted with caution. Findings from the narrative synthesis indicated that compared to control, exercise normalized brain activation and functional connectivity and/or improved the efficiency of compensatory brain networks, improved dopamine receptor binding, and reduced bradykinesia. However, we did not find evidence that exercise reduced inflammatory or oxidative stress markers, or improved brain volume. Overall, no one type of exercise was found to be consistently superior to another in attenuating PD progression across the various outcome measures investigated, with no evidence of subgroup differences based on type of exercise. However, there were limited data available from each subgroup. Exercise versus exercise comparisons were too heterogenous to enable pooling of results.
The effects of exercise on reducing “off” motor severity scores and bradykinesia may indicate attenuation of PD progression but we cannot discount that such improvements may partially reflect improved motor performance. Nonetheless, outcomes taken during “off” medication are more likely to reflect underlying symptom severity that is otherwise masked by PD medications. Effects of exercise on trophic and oxidative stress factors, and dopamine receptor binding, are of particular interest as alterations to the concentrations of trophic13,14 and oxidative stress 17 factors are thought to contribute to the development of PD, while reduced dopamine receptor binding is a hallmark of PD. 81 Although the moderate increase in BDNF levels following exercise found in this study is in line with previous meta-analyses in people with PD,10,82,83 the very low certainty of evidence means that this effect is uncertain. Our rigorous approach to meta-analysis extends previous findings, as prior reviews either pooled trials of PD with other neurodegenerative diseases 82 which limits the applicability of findings to people with PD, or imputed change scores 10 which is likely to underestimate baseline variability and therefore exaggerate findings. 31 In contrast to the positive effects of exercise on BDNF levels, the results of this review did not show an effect of exercise in altering the concentration of oxidative stress factors or inflammatory factors.
Our finding that exercise may improve dopamine receptor binding in people with PD7,53 is encouraging, particularly for people with early stage PD who have both greater preservation of dopaminergic receptors and greater rates of binding. 84 This improvement in dopamine receptor binding potential85,86 following exercise may arise in part through stimulation of the BDNF-tropomyosin related kinase B pathway. 87 Although the use of new biomarkers of PD progression such as mRNA and metabolite profiling 13 have been proposed, the only trial to date that included such targets failed to find benefits of exercise on most outcomes measured. 77 In line with the findings of a previous systematic review examining the effects of motor training on MRI outcomes, 11 our review suggested that exercise had neuroplastic effects on cortical function, connectivity between different cortical regions and connectivity between cortical and subcortical brain regions. Mostly, exercise appeared to improve compensatory pathways (eg, improved cognitive pathways and attentional allocation) to overcome the nigrostriatal deficits associated with PD 1 rather than modifying the disease process, particularly with more advanced disease. There is probably less value in examining PD attenuation among people with advanced disease, owing to the greater degeneration of brain structures with PD progression. 88 Nonetheless, exercise is still beneficial for people with more advanced PD to maintain physical function and health. 4
We did not find evidence of subgroup differences based on type of exercise, due to insufficient trials across different types of exercise. One exercise type that warrants further exploration is aerobic exercise, as a significant effect on improving “off” MDS/UPDRS motor scores when compared to control was observed. Aerobic exercise was also the exercise type consistently found in neuroimaging studies to partially normalize brain function in affected regions and networks involving links with the striatum,48,53,78 and to improve dopamine receptor binding potential.7,53 However, the limitation of the few trials and exercise modes investigated therein cannot be discounted. The potentially positive effects of aerobic exercise from this review are consistent with previous findings in animal and human studies, which have shown possible disease-modification of PD following aerobic exercise through improvements in neurochemical and neuroimaging outcomes.5,89 Nonetheless, the possibility that other types of exercise may have positive effects on potential indicators of PD progression cannot be discounted.
Some studies in the exercise versus exercise comparisons suggest that exercising at a higher intensity6,42,44,50,52,62 or with greater cognitive engagement33,41,58,65,69,70,77 may provide a greater effect on potential indicators of disease progression, however, this effect was not consistently apparent. 27 There is therefore not enough evidence from this review to provide a strong conclusion about whether higher exercise intensity or cognitive effort provides a greater effect on disease progression. Subgroup analysis of exercise dose (ie, the combination of exercise intensity and frequency) in the exercise versus control comparison may have provided useful information about this, however, we were unable to explore this as the exercise dosages in the included studies essentially differed by exercise type.
A strength of this review was the analysis of exercise versus control comparisons that allowed us to elucidate the direct effects of exercise. Previous systematic reviews with meta-analyses26,83 have combined exercise versus control and exercise versus exercise comparisons within the same meta-analysis, making it difficult to interpret the true effects of exercise on potential indicators of disease progression. Nonetheless, there were also limitations to this review. We were unable to perform planned subgroup analyses to determine whether there was a differential effect based on exercise dose or disease severity. We recommend that future RCTs examine exercise dose and disease severity across a variety of exercise types. Moreover, future RCTs should include participants from only 1 disease severity stage, or include large samples ensuring sufficient participants in each disease stage and report results for each disease stage, to enable subgroup analysis. While we included a broad range of outcomes to better understand the effects of exercise on attenuating PD progression, the large number of heterogenous outcomes examined by very few trials, many of which had small samples, limited our ability to conduct meta-analyses. Future trials should harmonize a key set of outcomes reflective of PD attenuation and report these outcomes. The results of our review suggest that assessing participants “off” medications, along with the routine inclusion of BDNF and/or dopamine receptor binding in addition to MDS/UPDRS motor scores as outcome measures, will enable future trials to better elucidate any benefits of exercise for attenuating PD progression and conduct of larger, more robust meta-analyses.
In conclusion, this systematic review provided very low to low certainty evidence that exercise may slightly improve potential indicators of disease severity and possibly slow down disease progression in PD. Further research, specifically large RCTs using a core set of outcomes reflective of disease progression in people with PD, are required to determine differential effects based on exercise type, exercise dose and disease severity.
Supplemental Material
Supplemental material, sj-docx-1-nnr-10.1177_15459683231172752 for Does Exercise Attenuate Disease Progression in People With Parkinson’s Disease? A Systematic Review With Meta-Analyses by Jiecheng A Li, Marte B. Loevaas, Catherine Guan, Lina Goh, Natalie E. Allen, Margaret K. Y. Mak, Jinglei Lv and Serene S. Paul in Neurorehabilitation and Neural Repair
Acknowledgments
The authors wish to thank Chun Man (Brian) Yiu and Darshan Nair for their assistance with title and abstract screening.
Footnotes
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Serene Sulyn Paul  https://orcid.org/0000-0002-3593-1396
https://orcid.org/0000-0002-3593-1396
References
- 1.Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J Neurochem. 2016;139(suppl 1):318-324. doi: 10.1111/jnc.13691 [DOI] [PubMed] [Google Scholar]
- 2.Hariz GM, Forsgren L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson’s disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol Scand. 2011;123(1):20-27. doi: 10.1111/j.1600-0404.2010.01344.x [DOI] [PubMed] [Google Scholar]
- 3.Reinoso G, Allen JC, Jr., Au WL, Seah SH, Tay KY, Tan LC. Clinical evolution of Parkinson’s disease and prognostic factors affecting motor progression: 9-year follow-up study. Eur J Neurol. 2015;22(3):457-463. doi: 10.1111/ene.12476 [DOI] [PubMed] [Google Scholar]
- 4.Pang MY. Physiotherapy management of Parkinson’s disease. J Physiother. 2021;67(3):163-176. doi: 10.1016/j.jphys.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 5.Segura C, Eraso M, Bonilla J, et al. Effect of a high-intensity tandem bicycle exercise program on clinical severity, functional magnetic resonance imaging, and plasma biomarkers in Parkinson’s disease. Front Neurol. 2020;11:656. doi:10.3389/fneur.2020.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7):1221-1229. doi: 10.1016/j.apmr.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher BE, Li Q, Nacca A, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport. 2013;24(10):509-514. doi: 10.1097/WNR.0b013e328361dc13 [DOI] [PubMed] [Google Scholar]
- 8.Frazzitta G, Maestri R, Ghilardi MF, et al. Intensive rehabilitation increases BDNF serum levels in parkinsonian patients: a randomized study. Neurorehabil Neural Repair. 2014;28(2):163-168. doi: 10.1177/1545968313508474 [DOI] [PubMed] [Google Scholar]
- 9.Zoladz JA, Majerczak J, Zeligowska E, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J Physiol Pharmacol. 2014;65(3):441-448. [PubMed] [Google Scholar]
- 10.Ruiz-González D, Hernández-Martínez A, Valenzuela PL, Morales JS, Soriano-Maldonado A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: a systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021;128:394-405. doi: 10.1016/j.neubiorev.2021.05.025 [DOI] [PubMed] [Google Scholar]
- 11.Baglio F, Pirastru A, Bergsland N, Cazzoli M, Tavazzi E. Neuroplasticity mediated by motor rehabilitation in Parkinson’s disease: a systematic review on structural and functional MRI markers. Rev Neurosci. 2021;33(2):213-226. doi: 10.1515/revneuro-2021-0064 [DOI] [PubMed] [Google Scholar]
- 12.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z. eds. JBI Manual for Evidence Synthesis. Joanna Briggs Institute (JBI); 2020:chap 3. [Google Scholar]
- 13.Emamzadeh FN, Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612. doi: 10.3389/fnins.2018.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira RN, de Miranda AS, Rocha NP, Simoes e, Silva AC, Teixeira AL, da Silva Camargos ER. Neurotrophic factors in Parkinson’s disease: what have we learned from pre-clinical and clinical studies? Curr Med Chem. 2018;25(31):3682-3702. doi: 10.2174/0929867325666180313101536 [DOI] [PubMed] [Google Scholar]
- 15.Rahmani F, Saghazadeh A, Rahmani M, et al. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: a systematic review and meta-analysis. Brain Res. 2019;1704:127-136. doi: 10.1016/j.brainres.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959(1):82-92. doi:10.1111/j.1749-6632.2002.tb02085.x [DOI] [PubMed] [Google Scholar]
- 17.Iarkov A, Barreto GE, Grizzell JA, Echeverria V. Strategies for the treatment of Parkinson’s disease: beyond dopamine. Front Aging Neurosci. 2020;12:4. doi: 10.3389/fnagi.2020.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mee-Inta O, Zhao ZW, Kuo YM. Physical exercise inhibits inflammation and microglial activation. Cells. 2019;8(7):691. doi: 10.3390/cells8070691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strafella AP, Valzania F, Nassetti SA, et al. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with Parkinson’s disease: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111(7):1198-202. doi: 10.1016/s1388-2457(00)00316-3 [DOI] [PubMed] [Google Scholar]
- 20.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(Part 11):2131-2146. doi:10.1093/brain/124.11.2131 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen JB. Sensorimotor integration at spinal level as a basis for muscle coordination during voluntary movement in humans. J Appl Physiol. 2004;96(5):1961-1967. doi: 10.1152/japplphysiol.01073.2003 [DOI] [PubMed] [Google Scholar]
- 22.Smith BA, Jacobs JV, Horak FB. Effects of magnitude and magnitude predictability of postural perturbations on preparatory cortical activity in older adults with and without Parkinson’s disease. Exp Brain Res. 2012;222(4):455-470. doi: 10.1007/s00221-012-3232-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herz DM, Eickhoff SB, Løkkegaard A, Siebner HR. Functional neuroimaging of motor control in Parkinson’s disease: a meta-analysis. Hum Brain Mapp. 2014;35(7):3227-3237. doi: 10.1002/hbm.22397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahn S, Elton RL, Members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Vol. 2. Macmillan Healthcare Information; 1987:153-163, 293-304. [Google Scholar]
- 25.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. doi:10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 26.Okada Y, Ohtsuka H, Kamata N, et al. Effectiveness of long-term physiotherapy in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. 2021;11(4):1619-1630. doi: 10.3233/jpd-212782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with De Novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219-226. doi: 10.1001/jamaneurol.2017.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Kolk NM, de Vries NM, Kessels RPC, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18(11):998-1008. doi: 10.1016/S1474-4422(19)30285-6 [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 30.American College of Sports Medicine. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991. doi: 10.1097/00005768-199806000-00032 [DOI] [PubMed] [Google Scholar]
- 31.Higgins J, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed.John Wiley & Sons; 2019. [Google Scholar]
- 32.Rohatgi A. WebPlotDigitizer, v4.6, 2022. Accessed September 1, 2022. https://automeris.io/WebPlotDigitizer
- 33.Pelosin E, Cerulli C, Ogliastro C, et al. A multimodal training modulates short-afferent inhibition and improves complex walking in a cohort of faller older adults with an increased prevalence of Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2020;75(4):722-728. doi:10.1093/gerona/glz072 [DOI] [PubMed] [Google Scholar]
- 34.Sajatovic M, Ridgel AL, Walter EM, et al. A randomized trial of individual versus group-format exercise and self-management in individuals with Parkinson’s disease and comorbid depression. Patient Prefer Adherence. 2017;11:965-973. doi:10.2147/PPA.S135551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloomer RJ, Schilling BK, Karlage RE, Ledoux MS, Pfeiffer RF, Callegari J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc. 2008;40(8):1385-1389. doi: 10.1249/MSS.0b013e31816f1550 [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa N, Shah VV, Harker G, et al. Responsiveness of objective vs. Clinical balance domain outcomes for exercise intervention in Parkinson’s disease. Front Neurol. 2020;11:940. doi: 10.3389/fneur.2020.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung SH, Hasegawa N, Mancini M, et al. Effects of the agility boot camp with cognitive challenge (ABC-C) exercise program for Parkinson’s disease. NPJ Parkinsons Dis. 2020;6(1):31. doi: 10.1038/s41531-020-00132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Callaghan A, Harvey M, Houghton D, et al. Comparing the influence of exercise intensity on brain-derived neurotrophic factor serum levels in people with Parkinson’s disease: a pilot study. Aging Clin Exp Res. 2020;32(9):1731-1738. [DOI] [PubMed] [Google Scholar]
- 39.Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. 2020;66(1):59. doi: 10.1016/j.jphys.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 40.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabrò RS, Naro A, Filoni S, et al. Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson’s disease. J Neuroeng Rehabil. 2019;16(1):68. doi: 10.1186/s12984-019-0533-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng FY, Yang YR, Wu YR, Lu CF, Cheng SJ, Wang RY. Beta event-related desynchronization can be enhanced by different training programs and is correlated with improved postural control in individuals with Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2018;26(10):1957-1964. doi: 10.1109/TNSRE.2018.2868140 [DOI] [PubMed] [Google Scholar]
- 43.Cheung C, Bhimani R, Wyman JF, et al. Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson’s disease: a pilot randomized controlled trial. Pilot Feasibility Stud. 2018;4:162. doi: 10.1186/s40814-018-0355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28(9):1230-1240. doi: 10.1002/mds.25380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dibble LE, Foreman KB, Addison O, Marcus RL, laStayo PC. Exercise and medication effects on persons with Parkinson disease across the domains of disability: a randomized clinical trial. J Neurol Phys Ther. 2015;39(2):85-92. doi:10.1097/NPT.0000000000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132-143. doi: 10.1177/1545968311421614 [DOI] [PubMed] [Google Scholar]
- 47.Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. 2015;29(2):123-131. doi: 10.1177/1545968314542981 [DOI] [PubMed] [Google Scholar]
- 48.Johansson ME, Cameron IGM, Van der Kolk NM, et al. Aerobic exercise alters brain function and structure in Parkinson’s disease: a randomized controlled trial. Ann Neurol. 2022;91(2):203-216. doi: 10.1002/ana.26291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A high-intensity exercise boot camp for persons with Parkinson disease: a Phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther. 2019;43(1):12-25. doi: 10.1097/NPT.0000000000000249 [DOI] [PubMed] [Google Scholar]
- 50.Marusiak J, Fisher BE, Jaskólska A, et al. Eight weeks of aerobic interval training improves psychomotor function in patients with Parkinson’s disease—randomized controlled trial. Int J Environ Res Public Health. 2019;16(5):880. doi:10.3390/ijerph16050880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez de la Cruz S. Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson’s disease: a randomized clinical trial. Eur J Phys Rehabil Med. 2017;53(6):825-832. doi: 10.23736/S1973-9087.17.04647-0 [DOI] [PubMed] [Google Scholar]
- 52.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23(6):600-608. doi: 10.1177/1545968308328726 [DOI] [PubMed] [Google Scholar]
- 53.Sacheli MA, Neva JL, Lakhani B, et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov Disord. 2019;34(12):1891-1900. doi: 10.1002/mds.27865 [DOI] [PubMed] [Google Scholar]
- 54.Silva-Batista C, de Lima-Pardini AC, Nucci MP, et al. A randomized, controlled trial of exercise for Parkinsonian individuals with freezing of gait. Mov Disord. 2020;35(9):1607-1617. doi: 10.1002/mds.28128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao CM, Zhuang YC. Effect of health Baduanjin Qigong for mild to moderate Parkinson’s disease. Geriatr Gerontol Int. 2016;16(8):911-919. doi: 10.1111/ggi.12571 [DOI] [PubMed] [Google Scholar]
- 56.Freidle M, Johansson H, Ekman U, et al. Behavioural and neuroplastic effects of a double-blind randomised controlled balance exercise trial in people with Parkinson’s disease. NPJ Parkinsons Dis. 2022;8(1):12. doi: 10.1038/s41531-021-00269-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajebrahimi F, Velioglu HA, Bayraktaroglu Z, Helvaci Yilmaz N, Hanoglu L. Clinical evaluation and resting state fMRI analysis of virtual reality based training in Parkinson’s disease through a randomized controlled trial. Sci Rep. 2022;12(1):8024. doi:10.1038/s41598-022-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Kim E, Yun SJ, et al. Robot-assisted gait training with auditory and visual cues in Parkinson’s disease: A randomized controlled trial. Ann Phys Rehabil Med. 2022;65(3):101620. doi: 10.1016/j.rehab.2021.101620 [DOI] [PubMed] [Google Scholar]
- 59.Liu HH, Wang RY, Cheng SJ, Liao KK, Zhou JH, Yang YR. Balance training modulates cortical inhibition in individuals with Parkinson’s disease: a randomized controlled trial. Neurorehabil Neural Repair. 2022;36(9):613-620. doi: 10.1177/15459683221119761 [DOI] [PubMed] [Google Scholar]
- 60.Albrecht F, Pereira JB, Mijalkov M, et al. Effects of a highly challenging balance training program on motor function and brain structure in Parkinson’s disease. J Parkinsons Dis. 2021;11(4):2057-2071. doi:10.3233/JPD-212801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beck EN, Intzandt BN, Almeida QJ. Can dual task walking improve in Parkinson’s disease after external focus of attention exercise? A single blind randomized controlled trial. Neurorehabil Neural Repair. 2018;32(1):18-33. doi: 10.1177/1545968317746782 [DOI] [PubMed] [Google Scholar]
- 62.David FJ, Robichaud JA, Vaillancourt DE, et al. Progressive resistance exercise restores some properties of the triphasic EMG pattern and improves bradykinesia: the PRET-PD randomized clinical trial. J Neurophysiol. 2016;116(5):2298-2311. doi:10.1152/jn.01067.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias Belchior L, Santos Tomaz B, Vasconcellos Abdon AP, Ferreira Frota NA, Bucharles Mont’Alverne DG, Macêdo Gaspar D. Treadmill in Parkinson’s: influence on gait, balance, BDNF and Reduced Glutathione. Fisioter Mov. 2017;30:S93-S100. doi: 10.1590/1980-5918.030.S01.AO09 [DOI] [Google Scholar]
- 64.DiFrancisco-Donoghue J, Lamberg EM, Rabin E, Elokda A, Fazzini E, Werner WG. Effects of exercise and B vitamins on homocysteine and glutathione in Parkinson’s disease: a randomized trial. Neurodegener Dis. 2012;10(1-4):127-134. doi:10.1159/000333790 [DOI] [PubMed] [Google Scholar]
- 65.Droby A, Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Distinct effects of motor training on resting-state functional networks of the brain in Parkinson’s disease. Neurorehabil Neural Repair. 2020;34(9):795-803. doi: 10.1177/1545968320940985 [DOI] [PubMed] [Google Scholar]
- 66.Duncan RP, Earhart GM. Are the effects of community-based dance on Parkinson disease severity, balance, and functional mobility reduced with time? A 2-year prospective pilot study. J Altern Complement Med. 2014;20(10):757-763. doi: 10.1089/acm.2012.0774 [DOI] [PubMed] [Google Scholar]
- 67.Jansen AE, Koop MM, Rosenfeldt AB, Alberts JL. High intensity aerobic exercise improves bimanual coordination of grasping forces in Parkinson’s disease. Parkinsonism Relat Disord. 2021;87:13-19. doi: 10.1016/j.parkreldis.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King LA, Mancini M, Smulders K, et al. Cognitively challenging agility boot camp program for freezing of gait in Parkinson disease. Neurorehabil Neural Repair. 2020;34(5):417-427. doi: 10.1177/1545968320909331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maidan I, Nieuwhof F, Bernad-Elazari H, et al. Evidence for differential effects of 2 forms of exercise on prefrontal plasticity during walking in Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(3):200-208. doi:10.1177/1545968318763750 [DOI] [PubMed] [Google Scholar]
- 70.Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff J, Mirelman A. Disparate effects of training on brain activation in Parkinson disease. Neurology. 2017;89(17):1804-1810. doi:10.1212/WNL.0000000000004576 [DOI] [PubMed] [Google Scholar]
- 71.Silva-Batista C, Mattos ECT, Corcos DM, et al. Resistance training with instability is more effective than resistance training in improving spinal inhibitory mechanisms in Parkinson’s disease. J Appl Physiol. 2017;122(1):1-10. doi:10.1152/japplphysiol.00557.2016 [DOI] [PubMed] [Google Scholar]
- 72.Soke F, Kocer B, Fidan I, Keskinoglu P, Guclu-Gunduz A. Effects of task-oriented training combined with aerobic training on serum BDNF, GDNF, IGF-1, VEGF, TNF-alpha, and IL-1beta levels in people with Parkinson’s disease: a randomized controlled study. Exp Gerontol. 2021;150:111384. doi: 10.1016/j.exger.2021.111384 [DOI] [PubMed] [Google Scholar]
- 73.Szymura J, Kubica J, Wiecek M, Pera J. The immunomodulary effects of systematic exercise in older adults and people with Parkinson’s disease. J Clin Med. 2020;9(1):184. doi: 10.3390/jcm9010184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vergara-Diaz G, Osypiuk K, Hausdorff JM, et al. Tai Chi for reducing dual-task gait variability, a potential mediator of fall risk in Parkinson’s disease: a pilot randomized controlled trial. Glob Adv Health Med. 2018;7:2164956118775385. doi: 10.1177/2164956118775385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vieira-Yano B, Martini DN, Horak FB, et al. The adapted resistance training with instability randomized controlled trial for gait automaticity. Mov Disord. 2021;36(1):152-163. doi:10.1002/mds.28298 [DOI] [PubMed] [Google Scholar]
- 76.Vivas J, Arias P, Cudeiro J. Aquatic therapy versus conventional land-based therapy for Parkinson’s disease: an open-label pilot study. Arch Phys Med Rehabil. 2011;92(8):1202-1210. doi: 10.1016/j.apmr.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 77.Li G, Huang P, Cui S-S, et al. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl Neurodegener. 2022;11(1):6. doi:10.1186/s40035-022-00280-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah C, Beall EB, Frankemolle AMM, et al. Exercise therapy for Parkinson’s disease: pedaling rate is related to changes in motor connectivity. Brain Connect. 2016;6(1):25-36. doi: 10.1089/brain.2014.0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franzén E, Johansson H, Freidle M, et al. The EXPANd trial: effects of exercise and exploring neuroplastic changes in people with Parkinson’s disease: a study protocol for a double-blinded randomized controlled trial. BMC Neurol. 2019;19(1):280. doi: 10.1186/s12883-019-1520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirelman A, Rochester L, Maidan I, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet. 2016;388(10050):1170-1182. doi:10.1016/S0140-6736(16)31325-3 [DOI] [PubMed] [Google Scholar]
- 81.Mishra A, Singh S, Shukla S. Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J Exp Neurosci. 2018;12:1179069518779829. doi: 10.1177/1179069518779829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackay CP, Kuys SS, Brauer SG. The effect of aerobic exercise on brain-derived neurotrophic factor in people with neurological disorders: a systematic review and meta-analysis. Neural Plast. 2017;2017:4716197. doi: 10.1155/2017/4716197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirsch MA, van Wegen EE, Newman MA, Heyn PC. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: a systematic review and meta-analysis. Transl Neurodegener. 2018;7(1):7. doi:10.1186/s40035-018-0112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaasinen V, Vahlberg T, Stoessl AJ, Strafella AP, Antonini A. Dopamine receptors in Parkinson’s disease: a meta-analysis of imaging studies. Mov Disord. 2021;36(8):1781-1791. doi: 10.1002/mds.28632 [DOI] [PubMed] [Google Scholar]
- 85.Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci. 2020;21(3):1170. doi: 10.3390/ijms21031170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013;12(7):716-726. doi: 10.1016/S1474-4422(13)70123-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feter N, Alt R, Dias M, Rombaldi A. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci Sports. 2019;34(5):293-304. doi:10.1016/j.scispo.2019.02.001 [Google Scholar]
- 88.Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. 2012;8(8):435-442. doi: 10.1038/nrneurol.2012.126 [DOI] [PubMed] [Google Scholar]
- 89.Jang Y, Koo J-H, Kwon I, et al. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017;1655:186-193. doi:10.1016/j.brainres.2016.10.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-nnr-10.1177_15459683231172752 for Does Exercise Attenuate Disease Progression in People With Parkinson’s Disease? A Systematic Review With Meta-Analyses by Jiecheng A Li, Marte B. Loevaas, Catherine Guan, Lina Goh, Natalie E. Allen, Margaret K. Y. Mak, Jinglei Lv and Serene S. Paul in Neurorehabilitation and Neural Repair