Abstract
Background:
Descriptive epidemiological data on incidence rates (IRs) of asthma with recurrent exacerbations (ARE) are sparse.
Objective:
We hypothesized that IRs for ARE would vary by time, geography, age, race and ethnicity, irrespective of parental asthma history.
Methods:
We leveraged data from 17246 children born after 1990 enrolled in 59 U.S. and one Puerto Rican cohort in the Environmental Influences on Child Health Outcomes consortium to estimate IRs for AREs.
Results:
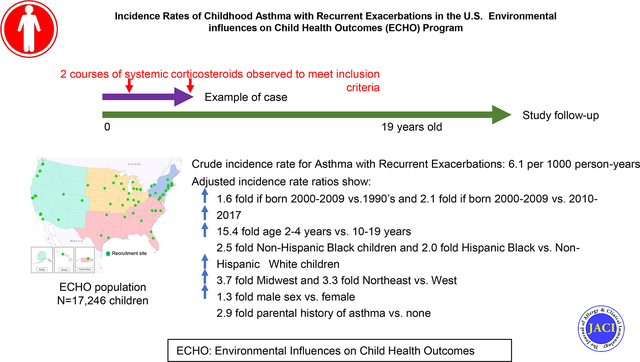
The overall crude IR for ARE was 6.07/1000 person-years (95% confidence intervals (CI) 5.63, 6.51) and was highest for children age 2–4 years, for Hispanic and non-Hispanic Black children and for those with a parental history of asthma. ARE IRs were higher for 2–4 year olds in each race and ethnicity category and for both sexes. Multi-variable analysis confirmed higher adjusted ARE IRs (aIRR) for children born 2000–2009 compared to 1990–1999 and 2010–2017, 2–4 versus 10–19 years old (aIRR=15.36; CI 12.09, 2.99), and for males versus females (aIRR=1.34; CI 1.16, 1.55). Black children (non-Hispanic and Hispanic) had higher rates than non-Hispanic White children (aIRR=2.51; CI 2.10, 2.99 and aIRR=2.04; CI 1.22, 3.39, respectively). Children born in the Midwest, Northeast and South had higher rates than the West (p<0.01 for each comparison). Children with a parental history of asthma had rates nearly three times higher than those without such history (aIRR=2.90; CI 2.43–3.46).
Conclusions:
Factors associated with time, geography, age, race and ethnicity, sex and parental history appear to influence the inception of ARE among children and adolescents.
Keywords: Asthma, Recurrent exacerbations, Incidence rates, Environmental and social determinants of asthma
Capsule Summary:
Incidence rates of ARE may vary over time and by demographic factors in addition to the influence of a parental asthma history.
Graphical Abstract.
Introduction
Childhood asthma is a heterogeneous disease.1 Some children are prone to an asthma phenotype associated with experiencing multiple exacerbations,2–6 and therefore represent a group with a higher morbidity and greater medical care costs.7–10 Despite the substantial health care and family burden, the causal factors driving asthma with recurrent exacerbations (ARE) are not well-characterized. Indeed, there are a lack of even basic descriptive epidemiological data.11 Biological influences such as molecular endotype and airway physiology contribute to exacerbation risk.3, 12, 13 Physical, environmental, social and behavioral risk factors including adherence to treatment recommendations and timely access to optimal medical care are important as well.2, 3, 14–16
While prevalence ratios for asthma have been reported widely in the U.S. and elsewhere,17–20 incidence rates (IRs) for asthma and its phenotypes across the childhood age range that better inform on potential risk factors related to disease inception have been sparse.10, 11, 21–26 Recent novel work under the auspices of the national Environmental Influences on Child Health Outcomes (ECHO) consortium demonstrated that U.S. asthma IRs were related to multiple demographic factors.27 In addition to parental history of asthma, the year of surveillance, decade of birth, geographical region of residence at birth, age, race and ethnicity were associated with asthma incidence rates. These findings suggested that environmental exposures that have changed over time have impacted population sub-groups differentially and contributed substantially beyond an inherited risk. However, an understanding of the IR specifically for ARE persists as a substantial research gap.
We leveraged ECHO’s large and diverse sample size with harmonized data from childhood cohorts across the U.S. to overcome limitations from analyses of individual or small groups of birth cohorts to determine the IRs for ARE among the ECHO pediatric population. We applied recommended criteria for defining an asthma exacerbation, namely an asthma episode that is treated with systemic corticosteroids, or, for patients on a stable maintenance dose, an increase in the use of systemic corticosteroids.28 Our objective was to provide and compare incidence rates of ARE, serving as an initial critical step for identifying potential influential factors and causal pathways. We hypothesized that IRs for ARE would vary by time of surveillance as well as by decade of birth, geographic region, age, race and ethnicity. Although not typically collected in a public health surveillance system, we also included the established risk factor parental history of asthma so that we could understand the extent to which these other factors acted independently of parental history. Identifying the descriptive epidemiological patterns associated with ARE should allow for both an improved understanding of ARE as well as point towards research directions to understand etiology and eventually intervene against this disease outcome.
Methods
Study Population:
All children in this study were enrolled in the ECHO consortium, a nationwide research platform combining data from established cohorts using extant and prospectively collected data with the aim of achieving demographic and geographic diversity and a large sample size to address hypotheses related to child health, including airway diseases.29, 30 Children meeting the following criteria were included in the analyses: a) born 1990–2017, and b) had relevant data throughout follow-up, including information on reported healthcare provider-diagnosed asthma22 and date of diagnosis and any corticosteroid use. Children were followed until age 20 years, the date of last visit or loss to follow-up, or the end of the study follow up period. Data for analyses were locked on 8/31/2022.
All reports of systemic corticosteroid use were ascertained. An ARE outcome was determined based on at least two reports of systemic corticosteroid use as described by Fuhlbrigge et. al28 at any time during the entire follow-up period, and counted as a case of ARE at the time of the second occurrence. To exclude children solely with bronchiolitis,31, 32 a corticosteroid event prior to age 2 years was counted as an asthma exacerbation but the second event needed to occur after age 2 years. Use of systemic corticosteroids were required to be at least 30 days apart to be counted as separate events; in 46 cases (6.1%) a reported use was therefore combined with the previous occurrence. There were only 16 children treated with corticosteroids at least twice before age 2 years who did not have evidence of an asthma diagnosis during follow-up and were therefore not classified as a child with ARE.
To be ascertained as having ARE, a child also had to have a reported healthcare provider diagnosis of asthma during follow-up. The date of asthma diagnosis could be at any time, irrespective of the ascertained date of ARE. Asthma diagnoses before age 5 years were required to have confirmation after 5 years, either by parent/caregiver or adolescent self-report of asthma symptoms, hospitalization/emergency department (ED)/urgent care visit for asthma, provider visits due to asthma, or asthma medication use.22
We calculated incidence rates of ARE for the study population overall and by standard surveillance demographics: calendar time of surveillance, decade of birth (1990–1999, 2000–2009, 2010–2017), child age and sex, self-reported race and ethnicity and the census region of residence at birth, as well as by the established risk factor of parental history of asthma (from either or both biological parents). We categorized age at ARE into 3 strata: ≥2–4 years, ≥5–9 years and ≥10–19 years. Race and ethnicity were categorized as non-Hispanic White, Hispanic White, non-Hispanic Black, Hispanic Black and Other (including multiple races, unknown race or ethnicity). Geographic region was identified as the location of the enrollment site, categorized by regions as defined by the U.S. Census.33
The institutional review boards of records for each cohort site as well as the host institution of the Data Analysis Center (Johns Hopkins Bloomberg School of Public Health) approved all activities associated with this study.
Statistical Analyses:
Data from all participating cohorts were pooled prior to analysis. IRs were calculated and then stratified by the above-mentioned demographic variables using a Poisson regression model with a fixed effect to account for the clustering of research site location. For analyses by calendar time of surveillance, a bootstrapped confidence interval that included a cluster term for site location was used for the 1990–1999 stratum due to model convergence issues. We examined the demographic variation in the underlying population over time to identify heterogeneity that we subsequently accounted for using stratified analyses. Finally, we implemented a multivariable Poisson model, also controlling for clustering on research site location by the inclusion of a fixed effect, to calculate incidence rate ratios (IRRs) and adjusting for birth decade, age, race and ethnicity, census region, and parental history of asthma. Sociodemographic data were evaluated for missingness (30% threshold) where multiple imputation may be considered. Where determined not reasonable to multiply impute, covariates with >30% missingness included a separately coded “missing” category to allow for comparison to the referent group. No missingness was resolved using multiple imputation.
All analyses were performed using Stata Version 16 (College Station, TX, USA).
Results
Of the 62,165 children in ECHO, 17,246 children from 59 geographically diverse ECHO cohorts from the U.S. and Puerto Rico met study eligibility criteria and provided 121,340 person-years of follow-up. (Figures 1 and 2, Table 1 and Supplemental Table 1). Of the 4,114 children ever diagnosed with asthma, there were 2,061 children with at least one asthma exacerbation and 734 children with ≥2 exacerbations who met the definition of ARE. The latter represents 1.2% of the studied ECHO population, almost 18% of those with an asthma diagnosis and 35% of those with at least 1 exacerbation. Supplemental Figure 1a displays the number of asthma exacerbation episodes among the 2,061 children with at least one episode, showing the distribution of up to eleven episodes. Supplemental Figure 1b displays the variable time in between exacerbations for children with ARE, with a median (IQR) time between their first two exacerbations of 18.4 months (11.76, 34.99).
Figure 1:
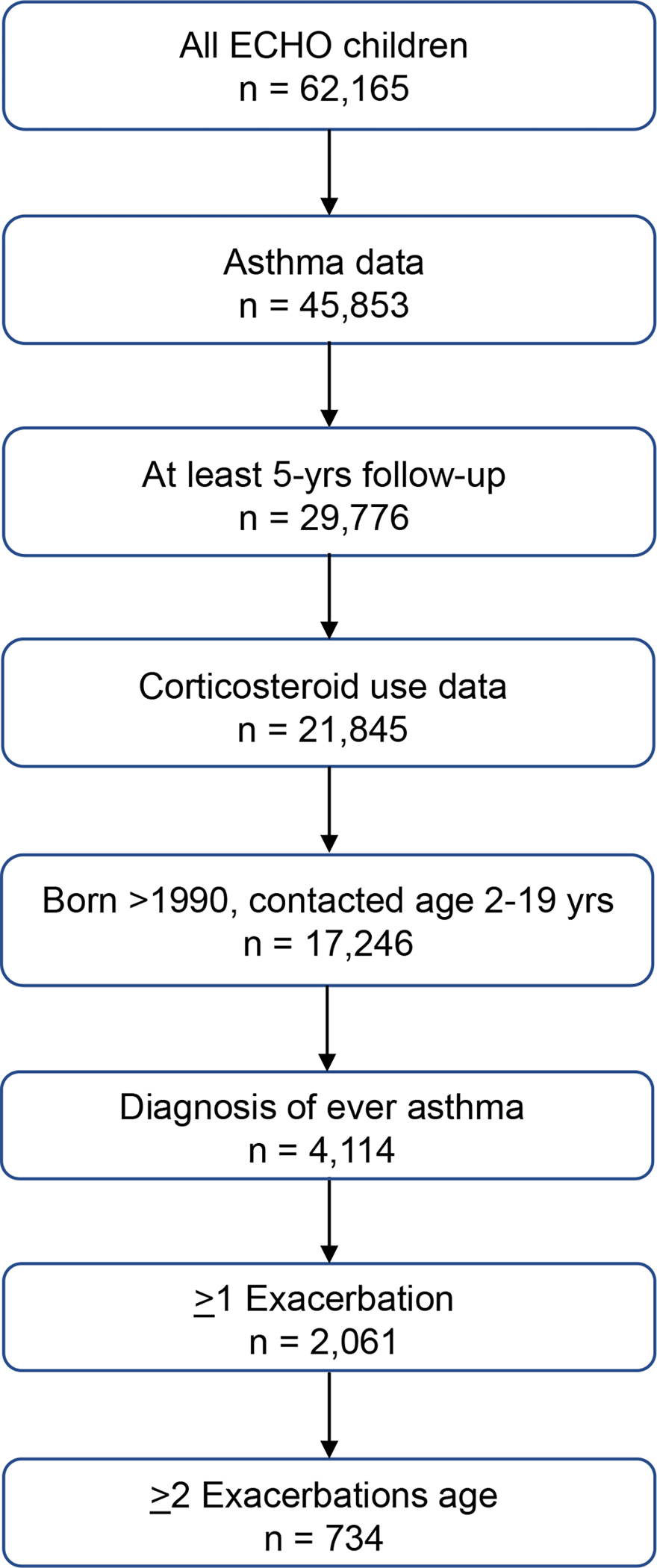
Consort diagram.
N=4,114 children from the general ECHO population of n=17,246 who were determined to report healthcare provider diagnosis of ever asthma, and among these children, n=2,061 had at least one use of corticosteroids. N=278 children received corticosteroid prescriptions without determination of an asthma diagnosis prior to age 5 years.
Figure 2:
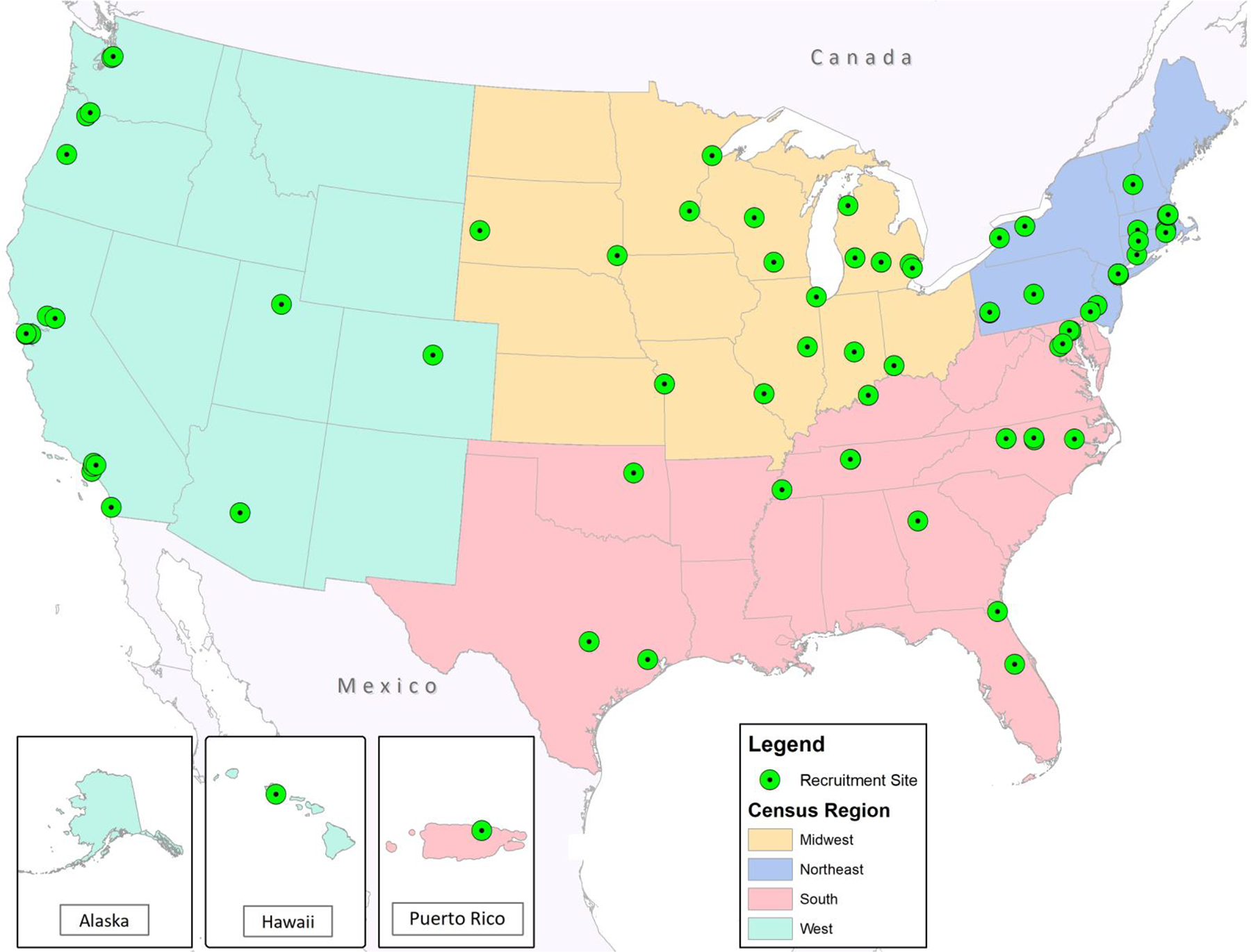
Participating ECHO cohort recruitment sites.
59 cohort recruitment sites across the United States and one from Puerto Rico, with coloring to indicate the 4 census regions, are displayed.
Table 1.
Univariate incidence rates of asthma with recurrent exacerbations (ARE) per 1000 person-years among children in the U.S. ECHO program
| Characteristic | N | Person-years | Number of cases | IR (95% CI) |
|---|---|---|---|---|
| All subjects | 17246 | 121340 | 734 | 6.07 (5.63, 6.51) |
| Time of surveillance | ||||
| 1990–1999 | 1432 | 2428 | 5 | 2.06 (0, 4.17)* |
| 2000–2009 | 5795 | 20115 | 108 | 5.35 (4.34, 6.35) |
| 2010–2022 | 14640 | 98238 | 569 | 5.84 (5.36, 6.32) |
| Decade born | ||||
| 1990–1999 | 1750 | 10966 | 47 | 4.18 (2.97, 5.40) |
| 2000–2009 | 5170 | 51426 | 311 | 5.96 (5.29, 6.62) |
| 2010–2017 | 10326 | 58948 | 376 | 6.4 (5.75, 7.04) |
| Child age (years) | ||||
| 2–4 | 17246 | 73282 | 346 | 18.55 (16.59, 20.52) |
| 5–9 | 11466 | 34891 | 277 | 4.90 (4.32, 5.47) |
| 10–19 | 3369 | 13167 | 111 | 2.41 (1.96, 2.86) |
| Child race and ethnicity | ||||
| Non-Hispanic White | 8996 | 63157 | 259 | 4.10 (3.60, 4.60) |
| Non-Hispanic Black | 3069 | 23456 | 280 | 11.99 (10.59, 13.40) |
| Hispanic White | 1703 | 10586 | 43 | 3.85 (2.69, 5.00) |
| Hispanic Black | 214 | 1412 | 16 | 11.42 (5.81, 17.03) |
| Other | 3264 | 22729 | 136 | 6.13 (5.09, 7.16) |
| Census region | ||||
| West | 5198 | 37598 | 88 | 2.27 (1.80, 2.74) |
| Northeast | 3149 | 21334 | 183 | 8.57 (7.32, 9.82) |
| Midwest | 3802 | 29877 | 224 | 7.51 (6.52, 8.49) |
| South | 3500 | 225101 | 190 | 8.17 (7.00, 9.34) |
| Child sex | ||||
| Female | 8129 | 57567 | 295 | 5.14 (4.56, 5.73) |
| Male | 9117 | 63774 | 439 | 6.90 (6.26, 7.55) |
| Parental history of asthma | ||||
| None | 6941 | 49031 | 179 | 3.69 (3.15, 4.23) |
| Either or both | 5688 | 38929 | 459 | 11.82 (10.74, 12.90) |
Abbreviations: CI=confidence interval; ECHO= Environmental influences on Child Health Outcomes; IR=incidence rates
Epidemiological characteristics were determined at birth except for Time of surveillance and Child age. N=1597 participants were missing data on Census region; N=4617 participants were missing data on Parental history of asthma. N= 3577 (63%) of the participants with either or both parents reporting history of asthma were from maternal historical data only. Child age and Time of surveillance is at time of ascertainment. Other child race and ethnicity refers to all categories that do not fit into the mutually exclusive categories described. This includes children who list race as anything other than White or Black, list multiple races, or self-select “don’t know” or “other” on their forms. Geographical representation is demonstrated in Figure 2.
Indicates bootstrapped confidence interval and no fixed effect controlling for cohort due to lack of model convergence.
As shown in Table 1, the overall crude ARE IR was 6.07 per 1000 person-years (95% confidence interval (CI) of 5.63, 6.51). Crude rates of ARE increased over calendar time from the 1990s (IR=2.06; CI 0, 4.17) to 2000–2009 (IR=5.35; CI 4.34, 6.35) and were lowest among children born 1990–1999 (IR=4.18; CI 2.97, 5.40). Crude ARE rates peaked among children aged 2–4 years (IR=18.55; CI 16.59, 20.52) and diminished with age. ARE rates were highest among non-Hispanic and Hispanic Black children (IR=11.99; CI 10.59, 13.40 and IR=11.42; CI 5.81, 17.03, respectively). ARE rates were almost 3 fold higher among non-Hispanic Black compared to non-Hispanic White children (IR=4.10; CI 3.60, 4.60), and among Hispanic Black compared to Hispanic White children (IR=3.85; CI 2.69, 5.00). Crude rates for ARE were 3.8 fold higher in the Northeast (IR=8.57; CI 7.32, 9.82) compared to the West (IR=2.27; CI 1.80, 2.74). Rates for males were approximately 1.3 fold higher than for females (IR=6.90; CI 6.26, 7.55 versus IR=5.14; CI 4.56, 5.73) and over three times greater for children with a parental history of asthma (IR=11.82; CI 10.74, 12.90) compared to no such history (IR=3.69; CI 3.15, 4.23).
Changes in crude rates over surveillance years and by birth decade would be affected by demographic changes in the underlying ECHO population as new cohorts were established and aged over time. Supplemental Figures 2 and 3 demonstrate that the percentage distribution of the ECHO population did vary over time with respect to race and ethnicity and region (both p<0.001). For example, substantially more ECHO children that were born from 2000–2009 reported Black race. Therefore, further analyses considering calendar time (year of surveillance and birth decade) accounted for these changes over time in the underlying study population.
ARE incidence rates by calendar year of surveillance.
Cases were ascertained from 1990 to 2022. Stratifying by race and ethnicity, the ARE IRs decreased slightly over time for non-Hispanic White children but remained relatively the same for Non-Hispanic and Hispanic Black children, although the samples sizes were small in some subgroups (Supplemental Tables 2a, b). The rates over time remained consistently highest for Non-Hispanic and Hispanic Black children. IRs were highest in the Northeast during the 1990s (IR=19.84, 2.45, 37.23) and 2000’s (IR=19.61; 14.83, 24.38) compared to 2010–2022 (IR=5.90; 4.76, 7.03) calendar years of surveillance. In the South and Midwest, the IR was higher during the 2010–2022 (South: IR=8.38; CI 7,16, 9.61; Midwest: IR=7.84; 6.70, 8.98) compared to 2000–2009 decade (South: IR=1.67; 0, 4.08, Midwest: IR=3.20; 1.89, 4.51), with insufficient cases to calculate during the earlier 1990s years of surveillance. In the West, there were insufficient numbers of ARE cases to calculate IRs until after 2010 when the IR (IR=2.72; CI 2.11, 3.32) was lower than the other census regions (data not shown). Children with a parental history of asthma had higher rates across all three decades of surveillance (Supplemental Tables 2a, b).
ARE incidence rates by birth decade.
Sample size limited analyses by birth decade to Non-Hispanic White and Black children (Supplemental Table 3; Supplemental Figure 4). Results varied by age group. ARE rates increased for 2–4 year olds from the two race and ethnic groups born in the 2000–2009 decade compared to the 1990s, and then declined among those born from 2010 to 2017. For 5–9 year olds, IRs declined with successive birth decades in both groups. For those 10–19 years of age, ARE rates declined for non-Hispanic White children over the birth decades but increased for non-Hispanic Black children. For children born in the 1990s, ARE rates were higher for non-Hispanic White children age 10–19 years. For all other decades, rates were higher for non-Hispanic Black children in every age group. Non-Hispanic Black children and Hispanic Black children also had higher rates compared with children who identified as White or other races and ethnicities born from 2000–2009 (Supplemental Figure 4). The IRs for AREs displayed similar trends for census region by birth decade as years of surveillance, except that during 2010–2017 the IRs in the Northeast (IR=3.21, 2.20, 4.22) were also lower compared to the other regions (data not shown).
ARE incidence rates by age, race and ethnicity.
ARE rates were highest for non-Hispanic Black and Hispanic Black children in all age groups (Table 2). The highest rates were found for non-Hispanic Black children (IR=52.33; CI 42.70–61.97) and Hispanic Black children (IR=26.89; CI 6.96–46.82) 2–4 years of age.
Table 2.
Race and ethnicity-specific IRs of asthma with recurrent exacerbations (ARE) per 1000 person-years, stratified by child years at follow-up.
| N | Person-years | Number of cases | IR (95% CI) | |
|---|---|---|---|---|
| 2 – 4 years | 17246 | 73282 | 346 | 18.55 (16.59, 20.52) |
| Non-Hispanic White | 8996 | 38386 | 135 | 14.83 (12.31, 17.34) |
| Non-Hispanic Black | 3069 | 12829 | 123 | 52.33 (42.70, 61.97) |
| Hispanic White | 1703 | 7150 | 16 | 5.78 (2.95, 8.62) |
| Hispanic Black | 214 | 902 | 7 | 26.89 (6.96, 46.82) |
| Other | 3264 | 14015 | 65 | 15.40 (11.65, 19.16) |
| 5 – 9 years | 11466 | 34891 | 277 | 4.90 (4.32, 5.47) |
| Non-Hispanic White | 6184 | 18241 | 90 | 2.80 (2.22, 3.37) |
| Non-Hispanic Black | 2163 | 7617 | 101 | 10.88 (8.76, 13.00) |
| Hispanic White | 923 | 2503 | 22 | 4.68 (2.72, 6.64) |
| Hispanic Black | 135 | 405 | 7 | 9.80 (2.52, 17.04) |
| Other | 2061 | 6126 | 57 | 5.87 (4.34, 7.39) |
| 10 – 19 years | 3369 | 13167 | 111 | 2.41 (1.96, 2.86) |
| Non-Hispanic White | 1587 | 6530 | 34 | 1.58 (0.19, 2.96) |
| Non-Hispanic Black | 882 | 3009 | 56 | 4.82 (3.56, 6.09) |
| Hispanic White | 230 | 934 | 5 | 1.58 (0.19, 2.96) |
| Hispanic Black | 33 | 105 | <5 | 4.81 (0, 11.49) |
| Other | 637 | 2589 | <19 | 1.63 (0.77, 2.48) |
Abbreviations: CI=confidence interval; IR=incidence rates
Other child race and ethnicity refers to all categories that do not fit into the mutually exclusive categories described. This includes children who list race as anything other than White or Black, list multiple races, or self-select “don’t know” or “other” on their forms.
ARE incidence rates by sex.
Differences in ARE rates by sex were statistically significant in the 2–4 and 5–9 year old children but of small magnitude (Table 3). IRs of ARE were over 1.3 fold higher among the male 2–4 year olds (IR=20.89; CI 18.04, 23.74) and 5–9 year olds (IR=5.66; CI 4.79, 6.52) compared to females of the same ages (IR=15.84; CI 13.18, 18.50 and IR=4.09; CI 3.33, 4.85, respectively). ARE rates were 1.3– 2.1 fold higher in males compared to females for every race and ethnic group (data not shown).
Table 3.
Child sex-specific IRs of asthma with recurrent exacerbations (ARE) per 1000 person-years, stratified by child age during follow-up.
| Child age | N | Person- years |
Number of cases | IR (95% CI) |
|---|---|---|---|---|
| 2–4 years | 17246 | 73282 | 346 | 18.55 (16.59, 20.52) |
| Female | 8129 | 34744 | 137 | 15.84 (13.18, 18.5) |
| Male | 9117 | 38538 | 209 | 20.89 (18.04, 23.74) |
| 5–9 years | 11466 | 34891 | 277 | 4.90 (4.32, 5.47) |
| Female | 5496 | 16788 | 112 | 4.09 (3.33, 4.85) |
| Male | 5970 | 18103 | 165 | 5.66 (4.79, 6.52) |
| 10–19 years | 3369 | 13167 | 111 | 2.41 (1.96, 2.86) |
| Female | 1593 | 6034 | 46 | 2.13 (1.52, 2.75) |
| Male | 1776 | 7133 | 65 | 2.65 (2.00, 3.29) |
Abbreviations: CI=confidence interval; IR=incidence rates
ARE incidence rates by parental history of asthma
Children with a parental history of asthma experienced higher ARE rates. Children in all three age groups with a parental history of asthma had three times higher ARE rates (Supplemental Table 4). Parental history of asthma increased the ARE IR approximately 3 fold for both boys and girls (Supplemental Table 5). In addition, parental history of asthma increased the ARE IRs 1.6 to 7.8 fold across the race and ethnicity categories (Supplemental Table 2).
Multivariable analyses displayed in Table 4, with estimates adjusted for every other variable on the table, confirmed the stratified results. ARE rates were significantly higher for children born 2000–2009 (adjusted incidence rate ratios (aIRR)=1.62; CI 1.13, 2.32) compared to 1990–1999 and 2010–2017 (aIRR=2.12; CI 1.77–2.53), 2–4 and 5–9 compared to 10–19 years of age (aIRR=15.35; CI 12.09, 19.52 and aIRR=3.69; CI 2.89, 4.70 respectively), Non-Hispanic Black (aIRR=2.51; CI 2.10, 2.99), Hispanic Black (aIRR=2.04; CI 1.22, 3.39), and Other (race and ethnicity) children (aIRR=1.67; CI 1.36, 2.07), born in the Northeast (aIRR=3.34; CI 2.52, .43), Midwest (aIRR= 3.66; CI 2.75, 4.86) or South (aIRR=2.90; CI 2.16, 3.88) compared to West, males (aIRR=1.34, CI 1.16, 1.55) and those with a parental history of asthma (aIRR=2.90; CI 2.43, 3.46).
Table 4.
Adjusted incidence rate ratios of asthma with recurrent exacerbations (ARE) by multivariate regression model
| aIRR (95% CI) | P value | |
|---|---|---|
| Decade born | ||
| 1990–1999 | reference | |
| 2000–2009 | 1.62 (1.13, 2.32) | <0.01 |
| 2010–2017 | 0.76 (0.54, 1.08) | 0.13 |
| Child age (years) | ||
| 10–19 | reference | |
| 5–9 | 3.69 (2.89, 4.70) | <0.01 |
| 2–4 | 15.36 (12.09, 19.52) | <0.01 |
| Child race and ethnicity | ||
| Non-Hispanic White | reference | |
| Non-Hispanic Black | 2.51 (2.10, 2.99) | <0.01 |
| Hispanic White | 1.30 (0.93, 1.82) | 0.12 |
| Hispanic Black | 2.04 (1.22, 3.39) | <0.01 |
| Other | 1.67 (1.36, 2.07) | <0.01 |
| Census region | ||
| West | reference | |
| Northeast | 3.34 (2.52, 4.43) | <0.01 |
| Midwest | 3.66 (2.75, 4.86) | <0.01 |
| South | 2.90 (2.16, 3.88) | <0.01 |
| Child sex | ||
| Female | reference | |
| Male | 1.34 (1.16, 1.55) | <0.01 |
| Parental history of asthma | ||
| None | reference | |
| Either or both | 2.90 (2.43, 3.46) | <0.01 |
Abbreviations: aIRR=adjusted incidence rate ratios; CI=confidence interval
N=17246. Model included a fixed effect for the research site location (aIRR: 1.00; 95% CI: 1.00–1.01) to control for any clustering effect. Children born during 2000–2009 had 2.12 times greater incidence of ARE than those born during 2010–2017 (95% CI: 1.77, 2.53). Other child race and ethnicity refers to all categories that do not fit into the mutually exclusive categories described. This includes children who list race as anything other than White or Black, list multiple races, or self-select “don’t know” or “other” on their forms.
Finally, we repeated the analyses using the date of outcome based on the first rather than the second exacerbation. Despite variations in the calculated IRs, the relative differences by child age on ARE did not materially differ (Supplemental Table 6). In sensitivity analyses, we excluded 6 cohorts (n=2,834; 16%) whose recruitment criteria required a positive family or personal history of asthma or bronchiolitis. This led to N = 14,412 children with an overall IR 4.31 per 1000 person-years (CI 3.90, 4.71) and a loss of all cases during the 1990s. There was a reduced but still relatively large incidence among non-Hispanic Black and Hispanic Black race and ethnic groups (IR=6.99, CI 5.75, 8.24; IR=6.96, CI 1.80, 12.12; respectively).
Discussion
ARE is a major health care burden in the U.S. but incidence rate data have been lacking for asthma in general and more so for this more burdensome outcome. By leveraging ECHO’s harmonized, diverse and large sample size of children with data from over three decades across the U.S. and in some areas of Puerto Rico,34 we calculated ARE incidence rates overall and by key demographic variables. Here we report time-dependent and demographic differences in ARE suggestive of a large influence of environmental and social factors beyond parental history of asthma. The rates we calculated are difficult to compare with other studies that have reported IRs across a wide range of 1.6 to 97 per 1000 person years as they covered less diverse populations and relied on methods that differed according to the length of follow-up, time period, determination of incidence of recurrence at the second event, and age group.10, 11, 35 Nonetheless, the increase in IRs in the U.S. in the 2000s compared to the earlier decade followed by a decline in rates suggests that shifting environmental and/or socio-behavioral exposures (such as chemical toxicants, those resulting from climate change and its consequential extreme weather events and prolonged allergy seasons) may be contributing risk factors.36 Changes in IRs of ARE over time also could have followed disproportionate access to optimal asthma care.37
IRs for ARE were highest in preschool children (IR= >15 cases per 1000 person years in adjusted analyses, Table 4) and declined by age 10–19 years, even though our ARE case definition excluded transient wheeze cases that did not develop into confirmed asthma. The early onset, high incidence rates of ARE mirrors the incidence rates for childhood asthma in general, that were highest at age 0–4 years with an IR of 39.6 (95% CI 37.2, 42.1).27 Our data also are consistent with the prevalence of emergency department (ED) and urgent care center visits for asthma, that are highest among children aged 0–4 years (34.9%), followed by those aged 5–11 years (19.3%) and 12–17 years (11.4%).38
The heightened susceptibility to ARE in preschoolers implicates environmental exposures that are more common or more damaging in that age group, such as acute respiratory tract infections, air pollution, indoor allergens, and altered diversity of the microbiome, as potential root causes of more burdensome asthma.39–41 Research from a population cohort of over 16,000 Hispanics/Latinos demonstrated that immigration to NYC, San Diego, Chicago or Miami at age 1–5 years instead of older was associated with a higher prevalence of reported ever asthma, strongly implicating early exposures to environmental factors as important.42 ED and urgent visits nationally have been more prevalent among boys in the 0–4 year old age group,38 consistent with our observation that ARE IR as well were highest among the boys age 2–4 years (Table 3). Mechanistically, the combined young age and male sex effect (1.3 fold) suggest that developmental or physiological differences between the sexes may contribute. Mismatches between the size of the airways and the lungs in relation to airway flow rates were greater in boys than girls,43 presumably increasing their susceptibility to worse symptoms when exposed to asthma triggers.
Previous research on the incidence of asthma from meta analyses of ECHO data collected 1980–2018 similarly showed that among children with any parental history of asthma, asthma incidence was not only consistently higher than among children without any parental history of asthma, but also highest in infancy (IR= 33 cases per 1000 person years) and declined (IR=10 cases per 1000 person-years) by age 17 years.22 Children with a parental history of asthma had a nearly 2 fold higher incidence rate through age 4 years (incidence rate ratio [IRR]= 1.94; 95%CI, 1.76–2.16) after which the rates converged with those without a parental history of asthma. For the latter group, the asthma rates remained relatively constant over the ages. For ARE, the higher contribution of parental asthma history reported both after meta analyses of ECHO data and also after pooled analyses over a similar time period was replicated.27 However, we report the novel findings that children with any parental history of asthma had an even higher (approximately 3 fold) IR for ARE at age 2–4 years compared to those without a parental history of asthma. This 3 fold increase by parental history persisted among children 10–19 years old, although the ARE IR decreased almost 7.8–8.3 fold since age 2–4 years (Supplemental Table 4). While the etiology of these findings is unclear, past genome-wide association studies have identified several genes associated with a higher odds of childhood asthma-related hospitalizations44 or severe asthma exacerbations.45
We also observed trends for IRs by age that varied across race and ethnicity. IRs for ARE were highest among the non-Hispanic Black and Hispanic Black children in the 2–4 year old age groups, compared to other races and ethnicities during that age. The higher incidence rates of ARE among non-Hispanic Black and Hispanic Black children are in agreement with prior reports of associations with asthma prevalence and incidence.38 46, 47 27 Race is a social construct designed to create a power hierarchy and the resulting structural racism is manifested through impact on public policies and institutional/societal practices that may influence health directly or through social, educational, or economic determinants of health. Thus, the observed racial and ethnic differences are likely mediated by multilevel socioeconomic and environmental exposures that may operate prenatally through maternal exposures that influence child development or impact access to optimal treatment for the prevention of asthma exacerbations.3, 48 These adverse exposures could include higher maternal and child stress and adversities, adverse environmental and toxicant exposures such as air pollution, allergens, nutritional inadequacies, inadequate healthcare/medication access or adherence, and comorbid mental or physical health conditions. Interestingly, the stress of systemic racism has been shown to increase bronchial hyperactivity and airway inflammation therefore modulating asthma biology.3
IRs for ARE were lowest in the West. Previously, a recent analysis of U.S. medical and pharmacy claims found that severe uncontrolled asthma was more common east of the Mississippi River.49 National Vital Statistics System (NVSS) data demonstrated asthma mortality was more prevalent in the Northeast.38 Others have noted similar geographical variations in asthma hospitalizations.50 There are many possible explanations for the geographic variation in ARE including: regional variations in socioeconomic or ethnic identity; proportions of urban, suburban and rural participants; regional differences in the density of physicians and their prescribing patterns, vitamin D status, time spent in- and outdoors related to outdoor weather, seasonal changes in air humidity, concentrations and types of allergens and pollutants. Evaluating the effect of residential location requires much more geographical precision and analysis of person-level exposure data to make inferences about causation.
We acknowledge several limitations. First, because the ECHO consortium began after many protocols for data collection were in place, we harmonized systemic corticosteroid use and other responses to questionnaires from the data collected across cohorts with variable methodology and timing including across seasons.28 Second, our geographical categories were broad and politically defined as higher resolution residential data were not available at the time of data analysis. Future studies that consider residential addresses and use geospatial and other tools will be better suited to examine the impact of urbanicity and other environmental factors. Third, ECHO combined birth cohorts, while large and diverse in numerous respects, are not a representative sample of US children or pediatric patients. Further, the ECHO cohort race and ethnicity compositions did not permit optimal analyses for the impact of more specific Hispanic groups and additional race and ethnicity categories classified as ‘Other’. Fourth, sample sizes were more limited in some subgroups, such as Hispanic Black and adolescent age children, diminishing statistical power in analyses of these groups. Fifth, different patterns of prescribing systemic corticosteroids over time could have biased our outcome criteria.51
In conclusion, the importance of time of surveillance, decade of birth, very young age, race and ethnicity, and census region detected here all suggest substantial impacts of environment exposures that may change over time in the etiology of ARE. These do not occur independent of parental history of asthma. The susceptibility to ARE especially during the younger ages suggests that risk factors such as genetic predisposition as well as environmental exposures that are more prominent during young ages (e.g. respiratory viruses, those related to acculturation, other early lifestyle preferences) may be influential.52 Severe childhood asthma has been shown to predict asthma at age 50 years as well, suggesting long-term consequences of these early childhood morbidities.53 Preventative strategies require detailed understanding of the determinants of this complex disorder and their exposure time windows. Interventions to reduce the incidence of ARE which appear particularly needed at a very young age could improve short-term morbidity and perhaps improve long-term respiratory outcomes. These epidemiological data should be used to provide leads for studies of the etiology of ARE.
Supplementary Material
Key Messages.
The overall crude incidence rate for asthma with recurrent exacerbations from 1990–2022 was 6.07/1000 person-years (95% confidence intervals 5.63, 6.51).
Asthma with recurrent exacerbation incidence rates were highest among children born 2000–2009 compared to 1990–1999 (aIRR=1.62; CI 1.13, 2.32) and 2010–2017 (aIRR=2.12; CI 1.77, 2.53).
Asthma with recurrent exacerbations incidence rates were highest among children 2–4 years of age and among non-Hispanic Black and Hispanic Black children and males.
Incidence rates were lowest in the West and 2.9 times greater among children with a parental history of asthma compared to no parental history.
Acknowledgments
NIH Funding Acknowledgment and Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this presentation was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under ECHO components U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 with co-funding from the Office of Behavioral and Social Science Research (PRO Core), UH3OD023251 (Alshawabkeh), UH3OD023320 (Aschner), UH3OD023332 (Trasande), UH3OD023253 (Camargo), UH3OD023248 (Dabelea), UH3OD023313 (Deoni), UH3OD023328 (Duarte), UH3OD023318 (Dunlop), UH3OD023279 (Elliott), UH3OD023289 (Ferrara), UH3OD023282 (Gern), UH3OD023287 (Breton), UH3OD023365 (Hertz-Picciotto), UH3OD023244 (Hipwell), UH3OD023275 (Karagas), UH3OD023271 (Karr), UH3OD023347 (Lester), UH3OD023389 (Leve), UH3OD023268 (Weiss), UH3OD023288 (McEvoy), UH3OD023342 (Lyall), UH3OD023286 (Oken), UH3OD023348 (O’Shea), UH3OD023285 (Kerver), UH3OD023290 (Herbstman) UH3OD023272 (Schantz), UH3OD023249 (Stanford), UH3OD023337 (Wright).
ECHO Collaborators Acknowledgements:
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators:
ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL, Data Analysis Center: Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland: Jacobson LP; Research Triangle Institute, Durham, North Carolina: Catellier DJ; Person-Reported Outcomes Core: Northwestern University, Evanston, Illinois: Gershon R, Cella D.
ECHO Awardees and Cohorts— Northeastern University, Boston, MA: Alshawabkeh, A; Albert Einstein College of Medicine, Bronx, NY: Aschner J; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Merhar S; Indiana University, Riley Hospital for Children, Indianapolis, IN: Ren C; University of Buffalo, Jacobson School of Medicine and Biomedical Sciences, Buffalo, NY: Reynolds A; University of California, San Francisco, CA: Keller R; University of Rochester Medical Center, Rochester, NY: Pryhuber G; University of Texas Health Sciences Center, Houston, TX: Duncan A; Children’s Hospital and Clinic Minneapolis, MN: Lampland A; Florida Hospital for Children, Orlando, FL: Wadhawan R; Medical University of South Carolina, Charleston, SC: Wagner C; University of Arkansas for Medical Science, Little Rock, AR: Keller R; University of Florida College of Medicine, Jacksonville, FL: Hudak M; University of Washington, Seattle, WA: Mayock D; Wake Forest University School of Medicine, Winston Salem, NC: Walshburn L; Icahn School of Medicine at Mount Sinai, New York, NY: Teitelbaum SL; Cohen Children’s Medical Center, Northwell Health, New Hyde Park, New York: Stroustrup A; New York University School of Medicine, New York, NY: Trasande L; New York University, New York, NY: Blair C; Pennsylvania State University, University Park, PA; Gatzke-Kopp L; University of North Carolina, Chapel Hill, NC: Swingler M; Boston Children’s Hospital, Boston, MA: Mansbach J; Children’s Hospital of Philadelphia, Philadelphia, PA: Spergel J; Children’s Mercy, Kansas City, MO: Puls H; Norton Children’s Hospital, Louisville, KY: Stevenson M; Phoenix Children’s Hospital, Phoenix, AZ: Bauer C; Memorial Hospital of Rhode Island, Providence RI: Deoni S; New York State Psychiatric Institute, New York, NY: Duarte C; Emory University, Atlanta, GA: Dunlop A; Avera Health Rapid City, Rapid City, SD: Elliott A; Kaiser Permanente Northern California Division of Research, Oakland, CA: Croen L; Boston Medical Center, Boston MA: Bacharier L, O’Connor G; Morgan Stanley Children’s Hospital of New York Presbyterian, New York, NY: Bacharier L, Kattan M; Johns Hopkins University, School of Medicine, Baltimore, MD: Wood R, Bacharier L; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Hershey G; Henry Ford Health System, Detroit MI: Ownby D; University of California Davis Mind Institute, Sacramento, CA: Hertz-Picciotto I; University of Pittsburgh, Pittsburgh, PA: Hipwell A; Geisel School of Medicine at Dartmouth, Lebanon, NH: Karagas M; University of Washington, Department of Environmental and Occupational Health Sciences, Seattle, WA: Karr C; University of Tennessee Health Science Center, Memphis, TN: Mason A; Seattle Children’s Research Institute, Seattle, WA: Sathyanarayana S; Women & Infants Hospital of Rhode Island, Providence RI, Lester B; Children’s Mercy, Kansas City, MO: Carter B; Helen DeVos Children’s Hospital, Grand Rapids, MI: Pastyrnak S; Kapiolani Medical Center for Women and Children, Providence, RI: Neal C; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles CA: Smith L; Wake Forest University School of Medicine, Winston Salem, NC: Helderman J; Prevention Science Institute, University of Oregon, Eugene, OR: Leve L; George Washington University, Washington, DC: Ganiban J; Pennsylvania State University, University Park, PA: Neiderhiser J; Brigham and Women’s Hospital, Boston, MA: Weiss S; Boston University Medical Center, Boston, MA: O’Connor G; Kaiser Permanente, Southern California, San Diego, CA: Zeiger R; Washington University of St. Louis, St Louis, MO: Bacharier L; Indiana University, Riley Hospital for Children: Indianapolis, IN, Tepper R; Pennsylvania State University, University Park, PA: Lyall K; Johns Hopkins Bloomberg School of Public Health Kennedy Krieger Institute, Baltimore, MD: Landa R; University of California, UC Davis Medical Center Mind Institute, Sacramento, CA: Ozonoff, S; University of California, UC Davis Medical Center Mind Institute, Davis, CA: Schmidt R; University of Washington: Dager S; Children’s Hospital of Philadelphia - Center for Autism Research, Philadelphia, PA : Schultz R; University of North Carolina, Chapel Hill, NC: Piven J; Johns Hopkins Bloomberg School of Public Health, Baltimore, MD: Volk H; Baystate Children’s Hospital, Springfield, MA : Vaidya R; Beaumont Health Medical Center, Royal Oak, MI: Obeid R; Boston Children’s Hospital, Boston, MA: Rollins C; East Carolina University Brody School of Medicine, Greenville, NC: Bear K; Helen DeVos Children’s Hospital, Grand Rapids, MI: Pastyrnak S; Michigan State University College of Human Medicine, East Lansing, MI: Lenski, M; University of Chicago, Chicago IL: Msall M; University of Massachusetts Medical School, Worcester, MA: Frazier J; Wake Forest Baptist Health (Atrium Health), Winston Salem, NC: Washburn, L; Yale School of Medicine, New Haven, CT: Montgomery A; Henry Ford Health System, Detroit, MI: Barone, C; Michigan Department of Health and Human Services, Lansing, MI: McKane, P; Michigan State University, East Lansing, MI: Paneth N; University of Michigan, Ann Arbor, MI: Elliott, M; Columbia University Medical Center, New York, NY: Herbstman J; University of Illinois, Beckman Institute, Urbana, IL: Schantz S; University of Utah, Salt Lake City, UT: Porucznik C; University of Utah, Salt Lake City, UT: Silver R; University of Utah, Salt Lake City, UT: Conradt E; Boston Children’s Hospital, Boston MA: Bosquet-Enlow M; George Mason University, Fairfax, VA: Huddleston K; University of California, San Francisco, San Francisco CA: Bush N; University of Minnesota, Minneapolis, MN: Nguyen R; University of Rochester Medical Center, Rochester, NY: O’Connor, T; Massachusetts General Hospital, Boston, MA: Samuels-Kalow, M.
Abbreviations
- ARE
asthma with recurrent exacerbations
- CI
confidence interval
- ED
emergency department
- ECHO
Environmental influences on Child Health Outcomes
- IR
incidence rate
- U.S
United States
Footnotes
Conflicts of interest:
Carlos A. Camargo, Jr. reports AstraZeneca (Scientific Advisory Board in 2020); Sanofi Genzyme (Scientific Advisory Board in 2021). José F. Cordero is a consultant to Sanofi Pasteur specifically about dengue vaccine development. James E. Gern has received consulting fees from AstraZeneca and Meissa Vaccines, Inc, and has stock options in Meissa Vaccines, Inc. Tina Hartert serves on vaccine DSMB and advisory panels for Pfizer and Sanofi (without funding). Daniel J. Jackson has funding from GlaxoSmithKline and Regeneron, personal fees for DSMB from Pfizer, and personal fees for consulting from AstraZeneca, Avillion, GlaxoSmithKline, Sanofi, Regeneron. All other authors (Rachel L. Miller, Holly Schuh, Aruna Chandran, Izzuddin M. Aris, Casper Bendixsen, Jeffrey Blossom, Carrie Breton, Glorisa Canino, Kecia N. Carroll, Sarah Commodore, Dana M. Dabelea, Assiamira Ferrara, Rebecca C. Fry, Jody M. Ganiban, Frank D. Gilliland, Diane R. Gold, Rima Habre, Marion E. Hare, Robyn N. Harte, Tina Hartert, Kohei Hasegawa, Gurjit K. Khurana Hershey, Daniel J. Jackson, Christine Joseph, Jean M. Kerver, Haejin Kim, Augusto A. Litonjua, Carmen J. Marsit, Cindy McEvoy, Eneida A. Mendonça, Paul E. Moore, Flory L. Nkoy, Thomas G. O’Connor, Emily Oken, Dennis Ownby, Matthew Perzanowski, Katherine Rivera-Spoljaric, Patrick H. Ryan, Anne Marie Singh, Joseph B. Stanford, Rosalind J. Wright, Robert O. Wright, Antonella Zanobetti, Edward Zoratti and Christine C. Johnson) have nothing to disclose.
See Acknowledgments for full listing of collaborators
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol 2016; 138:1016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denlinger LC, Heymann P, Lutter R, Gern JE. Exacerbation-prone asthma. J Allergy Clin Immunol Pract 2020; 8:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federico MJ, Denlinger LC, Corren J, Szefler SJ, Fuhlbrigge AL. Exacerbation-prone asthma: a biological phenotype or a social construct. J Allergy Clin Immunol Pract 2021; 9:2627–34. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 5.Coleman AT, Jackson DJ, Gangnon RE, Evans MD, Lemanske RF, Gern JE. Comparison of risk factors for viral and nonviral asthma exacerbations. J Allergy Clin Immunol 2015; 136:1127–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.cdc.gov/asthma/most recent national asthma data.htm.].
- 7.Perry R, Braileanu G, Palmer T, Stevens P. The economic burden of pediatric asthma in the United States: literature review of current evidence. Pharmacoeconomics 2019; 37:155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med 2019; 200:1102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevor J, Lugogo N, Carr W, Moore WC, Soong W, Panettieri RA, Jr., et al. Severe asthma exacerbations in the United States: incidence, characteristics, predictors, and effects of biologic treatments. Ann Allergy Asthma Immunol 2021; 127:579–87 e1. [DOI] [PubMed] [Google Scholar]
- 10.Suruki RY, Boudiaf N, Ortega HG. Retrospective cohort analysis of healthcare claims in the United States characterising asthma exacerbations in paediatric patients. World Allergy Organ J 2016; 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelkes M, Janssens HM, de Ridder MA, Sturkenboom MC, de Jongste JC, Verhamme KM. Real life data on incidence and risk factors of severe asthma exacerbations in children in primary care. Respir Med 2016; 119:48–54. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick AM, Moore WC. Severe asthma phenotypes - how should they guide evaluation and treatment? J Allergy Clin Immunol Pract 2017; 5:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorkness RL, Zoratti EM, Kattan M, Gergen PJ, Evans MD, Visness CM, et al. Obstruction phenotype as a predictor of asthma severity and instability in children. J Allergy Clin Immunol 2018; 142:1090–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AH, Babineau DC, Krouse RZ, Zoratti EM, Pongracic JA, O’Connor GT, et al. Pathways through which asthma risk factors contribute to asthma severity in inner-city children. J Allergy Clin Immunol 2016; 138:1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol 2016; 138:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deliu M, Fontanella S, Haider S, Sperrin M, Geifman N, Murray C, et al. Longitudinal trajectories of severe wheeze exacerbations from infancy to school age and their association with early-life risk factors and late asthma outcomes. Clin Exp Allergy 2020; 50:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009; 123:S131–45. [DOI] [PubMed] [Google Scholar]
- 18.Hughes HK, Matsui EC, Tschudy MM, Pollack CE, Keet CA. Pediatric asthma health disparities: race, hardship, housing, and asthma in a national survey. Acad Pediatr 2017; 17:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital Signs: Asthma in children - United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018; 67:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith A, Serban N, Fitzpatrick A. Asthma prevalence among Medicaid-enrolled children. J Allergy Clin Immunol Pract 2019; 7:1207–13.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelkes M, Baan EJ, de Ridder MAJ, Svensson E, Prieto-Alhambra D, Lapi F, et al. Incidence, risk factors and re-exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol 2020; 31:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson CC, Chandran A, Havstad S, Li X, McEvoy CT, Ownby DR, et al. US Childhood Asthma Incidence Rate Patterns From the ECHO Consortium to Identify High-risk Groups for Primary Prevention. JAMA Pediatr 2021; 175:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wendt JK, Symanski E, Du XL. Estimation of asthma incidence among low-income children in Texas: a novel approach using Medicaid claims data. Am J Epidemiol 2012; 176:744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonita R, Beaglehole R, Kjellstrom T. Basic Epidemiology. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 25.MacMahon B, Pugh TF. Epidemiology: Principles and Methods. Boston, MA: Little, Brown and Company; 1970. [Google Scholar]
- 26.Rudd RA, Moorman JE. Asthma incidence: data from the National Health Interview Survey, 1980–1996. J Asthma 2007; 44:65–70. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CC, Havstad SL, Ownby DR, Joseph CLM, Sitarik AR, Biagini Myers J, et al. Pediatric asthma incidence rates in the United States from 1980 to 2017. J Allergy Clin Immunol 2021; 148:1270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012; 129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillman MW, Blaisdell CJ. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr Opin Pediatr 2018; 30:260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaisdell CJ, Park C, Hanspal M, Roary M, Arteaga SS, Laessig S, et al. The NIH ECHO Program: investigating how early environmental influences affect child health. Pediatr Res 2022; 92:1215–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plint AC, Johnson DW, Wiebe N, Bulloch B, Pusic M, Joubert G, et al. Practice variation among pediatric emergency departments in the treatment of bronchiolitis. Acad Emerg Med 2004; 11:353–60. [DOI] [PubMed] [Google Scholar]
- 32.House SA, Marin JR, Hall M, Ralston SL. Trends Over Time in Use of Nonrecommended Tests and Treatments Since Publication of the American Academy of Pediatrics Bronchiolitis Guideline. JAMA Netw Open 2021; 4:e2037356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger N The US Census and the People’s Health: Public Health Engagement From Enslavement and “Indians Not Taxed” to Census Tracts and Health Equity (1790–2018). Am J Public Health 2019; 109:1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oksel C, Granell R, Haider S, Fontanella S, Simpson A, Turner S, et al. Distinguishing wheezing phenotypes from infancy to adolescence. A pooled analysis of five birth cohorts. Ann Am Thorac Soc 2019; 16:868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med 2017; 17:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderegg WRL, Abatzoglou JT, Anderegg LDL, Bielory L, Kinney PL, Ziska L. Anthropogenic climate change is worsening North American pollen seasons. Proc Natl Acad Sci U S A 2021; 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gushue C, Miller R, Sheikh S, Allen ED, Tobias JD, Hayes D, et al. Gaps in health insurance coverage and emergency department use among children with asthma. J Asthma 2019; 56:1070–8. [DOI] [PubMed] [Google Scholar]
- 38.Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance - United States, 2006–2018. MMWR Surveill Summ 2021; 70:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murrison LB, Brandt EB, Myers JB, Hershey GKK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 2019; 129:1504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunst KJ, Ryan PH, Brokamp C, Bernstein D, Reponen T, Lockey J, et al. Timing and duration of traffic-related air pollution exposure and the risk for childhood wheeze and asthma. Am J Respir Crit Care Med 2015; 192:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol 2017; 140:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barr RG, Avilés-Santa L, Davis SM, Aldrich TK, Gonzalez F, Henderson AG, et al. Pulmonary disease and age at immigration among Hispanics. Results from the Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2016; 193:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mead J Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 1980; 121:339–42. [DOI] [PubMed] [Google Scholar]
- 44.Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46:51–5. [DOI] [PubMed] [Google Scholar]
- 45.Ahluwalia TS, Eliasen AU, Sevelsted A, Pedersen CT, Stokholm J, Chawes B, et al. FUT2-ABO epistasis increases the risk of early childhood asthma and Streptococcus pneumoniae respiratory illnesses. Nat Commun 2020; 11:6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zahran HS, Bailey CM, Damon SA, Garbe PL, PN B. Vital Signs: Asthma in Children — United States, 2001–2016. MMWR Morb Mortal Wkly Rep 2018;67:149–155. DOI: 10.15585/mmwr.mm6705e1. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nardone A, Neophytou AM, Balmes J, Thakur N. Ambient air pollution and asthma-related outcomes in children of color of the USA: a scoping review of literature published between 2013 and 2017. Curr Allergy Asthma Rep 2018; 18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsui EC, Adamson AS, Peng RD. Time’s up to adopt a biopsychosocial model to address racial and ethnic disparities in asthma outcomes. J Allergy Clin Immunol 2019; 143:2024–5. [DOI] [PubMed] [Google Scholar]
- 49.Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol 2022; 128:78–88. [DOI] [PubMed] [Google Scholar]
- 50.Shrestha P, Poudel DR, Dhital R, Karmacharya P. Seasonal and regional variation of asthma-related hospitalizations and mortality among adults in the United States. Ann Allergy Asthma Immunol 2018; 121:368–9. [DOI] [PubMed] [Google Scholar]
- 51.Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral Corticosteroid Prescribing for Children With Asthma in a Medicaid Managed Care Program. Pediatrics 2017; 139. [DOI] [PubMed] [Google Scholar]
- 52.Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med 2003; 24:160–9. [DOI] [PubMed] [Google Scholar]
- 53.Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, et al. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol 2014; 133:1572–8.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


