Abstract
Among the premenopausal women, Polycystic Ovary Syndrome (PCOS) is the most prevalent endocrinopathy affecting the reproductive system and metabolic rhythms leading to disrupted menstrual cycle. Being heterogeneous in nature it is characterized by complex symptomology of oligomennorhoea, excess of androgens triggering masculine phenotypic appearance and/or multiple follicular ovaries. The etiology of this complex disorder remains somewhat doubtful and the researchers hypothesize multisystem links in the pathogenesis of this disease. In this review, we attempt to present several hypotheses that tend to contribute to the etiology of PCOS. Metabolic inflexibility, aberrant pattern of gonadotropin signaling along with the evolutionary, genetic and environmental factors have been discussed. Considered a lifelong endocrinological implication, no universal treatment is available for PCOS so far however; multiple drug therapy is often advised along with simple life style intervention is mainly advised to manage its cardinal symptoms. Here we aimed to present a summarized view of pathophysiological links of PCOS with potential therapeutic strategies.
Keywords: PCOS, Etiology, Genetics, Environmental toxins, Neuroendocrine, Potential therapeutics
Introduction
Polycystic ovary syndrome (PCOS) is a gender specific disorder and is documented as the most common endocrine disorder-affecting women of childbearing age across the globe. It typically involves impairment in the reproduction and estrogen controlled organs in females. Rotterdam criteria hold the most accepted and classical diagnostic criteria by world scientific and health regulatories for defining the disease [1]. PCOS represents broad spectrum of symptoms, which chiefly involves hyperandrogenism (HA), anovulation and or polycystic ovarian morphology (PCOM) [1, 2]. The consequent features mainly result in the hirsutism, acne, androgenic alopecia and or raised serum level free androgens, clitoromegaly (enlargement of clitoris), seborrhea (oily skin) and acanthosisnigricans (dark patches near the skin creases) [3]. Moreover, constellation of metabolic impairment such as insulin resistance (I.R), increased serum level triglycerides, obesity, glucose intolerance, increased risk of developing type 2 diabetes mellitus (T2D), obstructive sleep apnea, hypertension and cardiovascular disease associated comorbidities can be encountered by PCOS women in later period of their life [4–6]. It has been observed that PCOS women suffers from psychological disorders like low self-esteem, anxiety, depression possibly because of negative body image perception eventually further degrading their quality of life [7, 8]. PCOS was first reported by Stien and Leventhal in 1935 as a condition associated with infertility, amenorrhoea, hirsutism and enlarged ovaries [9]. However, till then it remained as the most controversial medical disorder to be studied and diagnosed. No definite diagnostic test can categorically identifies this disease, however, the varying presence of the three defining elements i.e., clinical or biochemical hyperandrogenism, disrupted menstrual cycles (anovulation) and polymorphic ovaries stands considerable [10]. Broadly, speaking three different diagnostic criteria have been put forward from time to time to define the disease state [11] (Table 1). Recently, in 2018 International evidence-based guideline for the assessment and management of PCOS approved Rotterdam criteria for defining the disease [12]. PCOS in the adolescents is not well outlined. However, the epidemiologists and researchers diagnose the disorder based upon well-defined phenotypic features, which mainly include Oligoamenorrhoea / amenorrhoea for atleast 2 years after menarche, ovarian volume > 10cm3, increased serum level androgens and progressive hirsutism as per modified Ferriman-Gallwey scale [13, 14].
Table 1.
Diagnostic criteria and corresponding prevalence rates of PCOS
| Diagnostic criteria | Parameters | Diseases prevalence rate (%) | References |
|---|---|---|---|
| NIH (1990) | HA, OD | 5–10 | [205, 19, 206] |
| Rotterdam (2003) | HA, OD and PCOM (at least 2 parameters out of 3) | 2–21 | [205, 19, 206] |
| AE and PCOS society (2006) | HA defining feature and (OD or PCOM) | 2–17 | [205, 19, 206] |
| NIH (2012) |
HA, OD and PCOM (at least 2 parameters out of 3) with specific phenotypes Phenotype A: HA + OD + PCOM Phenotype B: HA + OD Phenotype C: HA + PCOM Phenotype D: OD + PCOM |
2–21 | [205, 19, 206] |
NIH National Institutes of Health, HA hyperandrogenism, OD ovarian dysfunction, PCOM polycystic ovarian morphology, AE Androgen Excess-PCOS Society
Despite being so common, PCOS presents an enigmatic state, as the etiologies of this disease remain undefined. Evidences from the scientific data suggest that PCOS is multivariative in nature [15]. The molecular pathomechanism of PCOS chiefly revolves around the underlying hormonal imbalance, impairing the normal ovarian environment leading to disrupted menstrual cycles, cutaneous manifestation and defective metabolic rhythms [5]. To underpin its etiological roots, numerous hypotheses have been suggested from time to time and are being continuously evolving with new research [16]. Although much has been clarified on its pathogenesis, diagnostic criteria and treatment options; it is a noteworthy health condition with no exclusive cure. Currently this disorder continuously evade us, therefore it is important to underpin its pathophysiological links which ultimately can pave for the mechanism based direct intervention to tackle this disease state.
Burden of PCOS
PCOS presents a sharp rise with respect to its prevalence [17, 18]. Understanding its prevalence and phenotype, two important considerations should be taken care of; first geographic variation and second is ethnicity [19]. The overall picture is quiet variable; reason being the various diagnostic criteria employed to validate this disorder [20, 21]. It is reported that more than, 68–75% of PCOS cases remain undiagnosed adding to the ambiguity with respect to its overall prevalence [20, 22]. The first study to determine its prevalence among the reproductive aged women of U.S was carried out by Aziz and colleagues and they documented its prevalence as 4–6.6% as per the NIH, 1990 criteria [23, 24]. Talking about the overall prevalence of this disorder, it is estimated that about 21% of U.S female population are diagnosed with it [24]. Global prevalence of PCOS is not consistent, so far it is generally taken as 5–20%, and changes as per the diagnostic criteria employed [18, 25] (Table 1). Recently Liu et al., [26] reported the global burden of PCOS and associated disability-adjusted life-years (DALYs) between the year 2007–2017 and communicated 1.55 million age-standardized incident cases of PCOS, and 0.43 million associated DALYs. Furthermore, there has been an increase of 1.45% and 1.91% for age-standardized incidence rate of PCOS and DALYs respectively. The study further added that revealed that the incidence rate with respect to the age-standardized PCOS incidence and DALYs was highest in Tropical Latin America compared to southern Latin America, North Africa, and the Middle East at regional level owing to their poor health system and reduced female awareness programs [27]. At national level, the China and the Ethiopia showed the highest increase in the incidence rates of PCOS and Afghanistan observed the lowest incidence rates probably due to the fragile socioeconomic condition of the nation and unawareness among women [26]. The prevalence of PCOS within European population using the data from Global Burden of Disease Study 2016 estimated the overall prevalence rate as 276.4 cases per 100,000 (95% UI: 207.8–363.2) with the highest rates observed in Czech Republic (460.6 per 100,000; 95% UI: 346.2–602.1) and the lowest in Sweden (34.1 per 100,000; 95% UI: 24.59–45.77) [28]. Recent study conducted by et al. communicated the pooled prevalence of PCOS in India was close to 10% using Rotterdam's criteria and AES criteria, while it was 5.8% using the NIH criteria [29]. Sharma et al. studied the overall the prevalence of various menstrual issues related to PCOS women in the Malwa region of Punjab, India and confirmed that 60.61% of women suffered menstrual dysregulation with dysmenorrhea accounting for the majority of issues (50.64%). The study further furnished that the only 3.30% subjects were aware about PCOS [30]. Undoubtedly, women with PCOS have higher lifetime risks of T2DM, metabolic disorders, and metabolic syndrome than weight-matched controls due to their higher rates of insulin resistance. Studies have revealed that 10% of women with PCOS have Type 2 Diabetes Mellitus (T2D), 70% have dyslipidaemia, there is a 5- to tenfold greater chance of developing obstructive sleep apnea, and the incidence of metabolic syndrome ranges from 34 to 46% [31, 32]. Study by Long et al., concluded that the overall prevalence of PCOS in women with T2DM was approximately 21%. It further confirmed that the prevalence of PCOS among women with T2DM was highest in Oceania, followed by Europe and Asia, and was lowest in North America [33]. Assessing the economical burden of this disease (initial diagnosis and treatment of menstrual dysfunction, infertility, and hirsutism) it estimates approximately $3.7 billion annually in 2020 USD [34].
Pathogenesis and Etiology of PCOS
PCOS is marked by abnormal cellular pathways regulating steroid synthesis, body’s metabolic processes and proper ovarian function. In the normal cycling ovaries, follicles are present at developmental stages. The immature follicle known as primodial follicle develops into preovulatory follicle and the process is termed as folliculogenesis [35]. There are finite numbers of follicles present in each ovary containing single oocyte. By the end of folliculogenesis, primodial follicle develops into antral follicle, major source of ovarian steroids. Upon the stimulation of luteinizing hormone (LH) antral follicle, releases the mature oocyte, resulting in the ovulation and what remains of antral follicle after ovulation under goes luteinization forming corpus luteum (important source of progesterone) necessary for the maintenance of pregnancy. Disruption in the major pathways governing the folliculogenesis, steroidogenesis or ovulation consequently results in the adverse conditions like amenorrhea, ovarian dysfunction, infertility, hyperandrogenism or disrupted ovarian morphology.
Broadly, speaking androgens are mainly produced in the ovaries and in the adrenal glands under the stimulation of their tropic hormones luteinizing hormone and adrenocorticotropic hormone (LH and ACTH respectively). Undoubtly, modest androgen excess can disrupt the major pathways associated with the negative feedback mechanism in relation to female sex hormone [36] (Fig. 1). The steroidogenic pathway in the ovaries have been described by two-cell, two-gonadotropin theory [37]. As per this theory, ovarian theca cells and granulosa cells are the major sites involved together in the biosynthesis of all the sex steroids. Cholesterol acts as the major precursor in the synthesis process. It is recruited in the theca cells via lipoprotein receptors or is synthesized de novo. Furthermore, steroidogenic acute regulatory protein (StAR) delivers it to the mitochondria [38] where cytochrome P450 cholesterol side-chain cleavage (cyp450scc; CYP11a1) results in its conversion to pregnenolone [39]. Then 17a-hydroxylase-17, 20-desmolase (CYP17a1 or CYP45017α) catalyze the conversion of pregnenolone to dehydroepiandrosterone (DHEA) and 3β-hydroxysteroid dehydrogenase (HSD3B) to progesterone [40]. DHEA and progesterone is further converted to androstenedione by HSD3B and CYP17a1 respectively [41]. It is pertinent to mention here that CYP17a1 enzyme activity depends on the LH stimulation in a dose-dependent manner [36]. Furthermore, androstenedione is the major precursor for testosterone and estrogen synthesis in ovaries [42]. It is either converted to testosterone via 17β-hydroxysteroid dehydrogenase (HSD17B) in the theca cells or gets diffused to granulosa cells where it is either converted directly to estrone by aromatase (CYP450arom; CYP19A1) [41] or again to testosterone by HSD17B from which it is finally converted to 17β- estradiol mainly by aromatase (CYP450arom; CYP19A1) [43–45]. The androgen synthesis and conversion to estrogens needs to be properly coordinated for the optimized ovarian function, which seems to be disturbed in PCOS women. Simplifying the abovementioned pathway, it can be concluded that the androgens act as obligate substrate for the synthesis of estrogen as well as plays important role in the follicular development [46, 47]. The rate limiting enzyme for the synthesis of androgens is CYP17a1, which on the other hand is dependent upon LH [48] in a dose-dependent manner. LH acts on theca cells via its receptors and induces the production androgens; concomitantly, FSH acts on granulosa cells via its receptors and converts the androgens formed in theca cells into estrogens (estradiol) via aromatase (CYP19A1). The transcription of CYP19A1 is known to be promoted by FSH and hyperandrogenic follicular environment is reported to be hazardous factor leading to the down-regulation of aromatase. Conversely, in PCOS women imbalance in the hypothalamic–pituitary–ovarian axis, results in the impaired androgen synthesis and folliculogenesis. An increase in the hypothalamic GnRH favors the synthesis of β subunit of LH over β subunit of FSH, leading in the production of LH over FSH (increased LH/FSH ratio), hallmark feature of PCOS [49]. Also, increased LH surge results in the follicular arrest at preantral and antral stages leading to hyperplasia of theca cells and the development of multiple follicles along the periphery of ovary [50]. Of note, it can be generalized that the over activity of the steroidogeneic cascade and LH/FSH surge may trigger the ovarian hyperesponsivness, ultimately leading to hyperandrogenism, disrupted ovarian morphology and anovulation.
Fig. 1.
Two-cell, two-gonadotropin theory depicting ovarian sterodgenesis; theca cells produces androgens in response to LH which are later converted into estrogens in granulosa cells under the action of FSH, governed by the series of various enzymes. The androgen synthesis and conversion to estrogens needs to be properly coordinated for the optimized ovarian function, which seems to be disturbed in PCOS women resulting in hyperandrogenism and anovulation [39]
The major ethiopathological hypotheses that have been proposed mainly include:
-
2.1
Evolutionary hypothesis
-
2.2
Genetic hypothesis
-
2.3
Insulin hypothesis
-
2.4
Environmental hypothesis
-
2.5
Neuroendocrine hypothesis
Evolutionary Hypothesis
An evolutionary hypothesis suggest, that during past times humans were subjected to extreme physical activities also they have to with stand the challenging and stressful environmental conditions. The diet constituents were rich in proteins and carbohydrates with lesser fat content. Also the long periods of famine and disease epidemics might have favored in the development of thrifty genotypes and phenotypes [47]. However, with the scientific revolution and modern lifestyle the life expectancy has dramatically increased. There is no threat of famine and majority of countries have unrestricted accessibility to food with sedentary lifestyle, the need of thrifty genotype and phenotype are no longer beneficial resulting in the dramatic increase in the prevalence of obesity, T2DM and may also have contributed to the development of PCOS [47, 51].
Genetic Hypothesis
Elucidating the genetic basis of PCOS is quiet challenging task. The PCOS presents constellation of symptoms therefore the genes associated with major pathways mainly, steroidogenesis, I.R (I.R), inflammation, glucose homeostasis and those associated with the proper functioning of ovaries could be linked [52, 53]. The familial aggregation of this syndrome also validates its genetic roots [54, 55]. Number of candidate gene association studies has been carried out in relation to PCOS. Walterworth in 1997, studied the role of insulin gene, INS in the pathogenesis of PCOS in revealed its role in the familial transmission of the syndrome [56]. The role of insulin receptor gene in PCOS was demonstrated by Taylor 1992 [57] and the results showed its role in hyper insulinemia and I.R. the role of fat and obesity associated gene (FTO) and childhood obesity are strongly associated particularly in the European population and thus could be possibly linked with PCOS [58]. The association between the genetic mutation in FTO gene and increased risk of PCOS was studied by [59] and they concluded that the SNP rs9939609 is linked withincreased risk of PCOS and can be attributed to increased adiposity. The sex hormone binding globulin (HSBG) is a glycoprotein and specifically binds with the androgens thus regulates their bioavailability. The penta repeat nucleotide polymorphism (TAAAA)n was observed with decreased serum level concentration of HSBG thus marking the increased serum free androgens and consequently increased the risk of PCOS [60]. The GWAS study of DENND1A gene (rs10818854, rs2479106, and rs10986105 SNPs) have been reported to be associated to the high risk of PCOS in Chinese and mixed Asian ancestry respectively [61–63]. Whereas, SNP rs9696009 have been linked with PCOS in European ancestry [64]. SNPs in the INS-VNTR, which are linked to the development of T2DM, were also reported to implicate in PCOS related traits [63, 65]. Shi et al. reported the SNP rs2059807 in INSR associated with PCOS [61], which was further confirmed by Feng et al., [65], who furnished multiple SNP’s association with PCOS development. Studies conducted by by Chen et al., and Shi et al., discovered important FSHR gene loci (rs2268361 and rs2349415) were associated with PCOS and could be related to FSH and LH imbalances and PCOM morphology [61, 62]. The locus THADA represent another intriguing candidate gene in the aetiology of PCOS that has been reproduced across various ancestries [66, 67]. A study conducted by Tian et al., 2020 communicated the role of rs13429458 in THADA, rs10818854 in DENND1A, rs2059807 in INSR and rs4784165 in TOX3a significantly associated in the pathogenesis of PCOS chiefly via disturbing the metabolic rhythms [68]. Several other studies reported the role of GATA4/NEIL2 (rs804279), ZBTB16 (also known as PLZF), MAPRE1 (involved in adipogenesis) may mediate ovarian angiogenesis and follicle development [64, 69]. The variants at the ERBB4, YAP1, C9orf3, DENND1A, RAB5B and ZBTB16 loci were strongly associated with OD and PCOM, and therefore, might be more important for links to menstrual cycle regularity and fertility [64]. The detailed summary of candidate gene studies along with the GWAS studies in relation to PCOS is presented in Tables 2, 3, and 4 respectively.
Table 2.
Candidate gene studies and the associated pathways involved in the pathogenesis of PCOS
| Associated pathway | Candidate gene | References |
|---|---|---|
| Insulin secretion and metabolism | INS (Insulin gene) | [56, 207] |
| INSR (Insulin receptor gene) | [57, 208] | |
| CAPN10 | [209] | |
| IRS1 (Insulin receptor substrate 1) | [210] | |
| Fat metabolism | FTO gene | [58, 59] |
| MC4R | [211] | |
| ADIPOQ | [212] | |
| Steroidogenic pathway | CYP11a | [213, 214] |
| CYP17 | [215, 216] | |
| CYP19 | [217] | |
| CYP21 | [218] | |
| INSL3 (Insulin-like factor 3) | [219] | |
| HSD17B5 (17β-hydroxysteroid dehydrogenase type 5) | [220] | |
| Regulation of gonadotropin | FSHR | [221] |
| FSHβ | [222] | |
| AMHR | [223] | |
| LH | [224] | |
| AMH | [225] | |
| Androgen bioavailability | SHBG | [60, 226] |
| Inflammation | TNF-alpha | [227] |
| IL-6 gene | [228] | |
| Vitamin D | VDR | [229] |
Table 3.
Genome-Wide Association Studies (GWAS) and the SNPs identified in relation to Polycystic Ovary Syndrome (PCOS
| Nearest gene | Gene locus | SNPs | References |
|---|---|---|---|
| THADA | 2p21 | rs12468394 | [62, 64, 68] |
| rs13429458 | |||
| rs12478601 | |||
| rs7563201 | |||
| DENND1A | 9q33.3 | rs10818854 | [62, 64, 68, 230] |
| rs2479106 | |||
| rs10986105 | |||
| rs9696009 | |||
| LHCGR, STON1, GTF2A1L | 2p16.3 | rs13405728 | [64, 230] |
| FSHR | 2p16.3 | rs2349415 | [61] |
| rs2268361 | |||
| C9orf3 | 9q22.32 | rs4385527 | [61, 62] |
| rs3802457 | |||
| rs10818854 | |||
| rs10986105 | |||
| rs10993397 | |||
| YAP1 | 11q22.1 | rs1894116 | [61, 230, 231] |
| rs11225154 | |||
| RAB5B, SUOX | 12q13.2 | rs705702 | [61, 64] |
| HMGA2 | 12q14.3 | rs2272046 | [61, 232] |
| TOX3 | 16q12.1 | rs4784165 | [61, 64, 68] |
| rs8043701 | |||
| INSR | 19p13.3 | rs2059807 | [61, 68] |
| rs1799817 | |||
| SUMO1P1 | 20q13.2 | rs6022786 | [61, 233] |
| ERBB4 | 2q.34 | rs2178575 | [61] |
| IRF1/RAD50 | 5q31.1 | rs13164856 | [64] |
| PEX5L | 3q26.33 | rs7652876 | [223] |
| ERBB3/RAB5B | 12q13.2 | rs2271194 | [64] |
| HIPK3 | 11p13 | rs4755571 | [223] |
| MAPRE1 | 20q11.21 | rs853854 | [64] |
| GATA4, NEIL2 | 8p32.1 | rs804279 | [64] |
| GYS2 | rs7485509 | [234] | |
| rs10841843 | |||
| rs6487237 |
Table 4.
Genome-wide significant associations with PCOS subtypes
| Subtype | Gene | Locus | SNPs | References |
|---|---|---|---|---|
| Reproductive subtype | PRDM2/KAZN | 1p36.21 | rs78025940 | [235] |
| IQCA1 | 2q37.3 | rs76182733 | [235] | |
| BMPR1B/ UNC5C | 4q22.3 | rs17023134 | [235] | |
| CDH10 | 5p14.2–p14.1 | rs7735176 | [235] | |
| Metabolic subtype | KCNH7/FIGN | 2q24.2–q24.3 | rs55762028 | [235] |
| Intermediate subtype | FSHB | 11p14.1 | rs10835638 | [235] |
Insulin Hypothesis
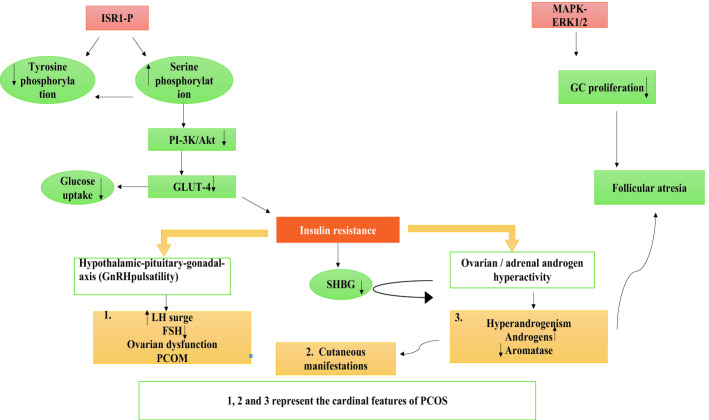
Insulin is mainly secreted by the beta cells of pancreas. Its role is well established in ovarian growth and in the modulation of androgens by ovaries [70]. PCOS women are often hyperinsulinemic (50–70% of PCOS patients) [71], thus representing a unique characteristic of this disorder (Fig. 1). I.R simply involves the reduced sensitivity to the normal insulin levels resulting in the irregular glucose metabolism primarily in skeletal muscle and adipose tissue [72]. Studies have revealed that I.R promotes PCOS by disrupting two major pathways- phosphatidylinositol 3-kinase (PI-3 K)/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway [73–75] [Fig. 2]. Different studies have supported the fact that the cross talk between insulin and LH results in the over expression of StAR and Cyp-17 genes in the theca cells thus establishing their potent link in governing the androgen biosynthesis [76]. I.R is a result of disrupted signaling pathways involved in activation of GLUT4 transporter [77]. In trophoblastic cells of humans, I.R results in the decreased expression of sex hormone-binding globulin (SHBG) consequently affecting the expressions of Insulin Receptor sensitive 1 (IRS1), Insulin Receptor Sensitive 2 (IRS2), PI3Kp85 and finally GLUT4 [78] suggesting its active role in inducing PI3K/Akt pathway-mediated systemic I.R. In addition to this, more insulin levels tend to diminish the synthesis of SHBG so to reduce the binding with testosterone thereby increasing the bioavailability of the serum androgens [79]. We can conclude that in PCOS women I.R and hyperandrogenism can be regarded as “partners in crime” in delivering the pathogenesis [Fig. 2]. Burghen et al. [80] firstly described the role of insulin in the ovarian function and proposed a link between hyperinsulinemia and hyperandrogenism. Till then various studies have explained the possible role of I.R in the pathogenesis of PCOS [73, 81]. Thus, I.R and compensatory hyperinsulinism can induce hyperandrogenism in number of ways as:
Insulin acts as cogonadotropin on ovaries thus, contributing to the excess androgens [73, 82].
It can act on adrenal glands and facilitates its further secretion [83].
It affects the LH pulse frequency thus disturbing the delicate balance and driving increased pituitary LH secretion more than FSH secretion, thereby inducing hyperandrogenism and PCOM [84].
I.R can suppress the production of SHBG, which intrun can further exaggerate with the insulin related signaling pathways and favors hyperandrogenism.
Fig. 2.
Depiction of the possible mechanism involved in triggering the hyperandogenism via defective insulin signaling pathway. Increased serine phosphorylation of IRS-1 hampers the activation of PI3K leading to reduced uptake of glucose by GLUT4 favoring I.R. I.R interferes with the SHBG, acts on theca cells and provokes LH excess to aggravate hyperandrogenism. On the other hand, the picture becomes further exaggerated when HA downregulates the aromatase activity leading to follicular atresia. MAPK-ERK1/2 impairment inhibits GCs proliferation resulting in follicular atresia [73–75]
The abovementioned picture becomes more exacerbated in PCOS obese women because of adiposity. The increased phosphorylation of the serine residue of the insulin receptor substrate-1 molecule (IRS-1ser 312) has been witnessed in the obese PCOS women, consequently leading to impaired Insulin receptor signaling (impaired regulation of IRS-1 and PI3K activity) leading to hyperinsulinemia, favoring hyperandrogenism [85]. Additionally, downregulation of MAPK-ERK1/2 caused enhanced rate of apoptosis and diminished proliferation of granulosa cells in PCOS women. Studies suggested that disruption in mTORC1 autophagy pathway results in mitochondrial impairment and reduction in uptake of glucose, leading to I.R in skeletal muscles due to hyperandrogenism [86, 87]. The Vitamin D also plays a significant role in governing the insulin signaling pathway in PCOS women however, the deficiency of the former modifies the entire picture. Vitamin D deficiency triggers IR [88]. Vitamin D also serves very important precursor for the adipose cells development and is involved in the activation of enzyme system important for lipid and carbohydrate metabolism thus playing an important role in glucose metabolism [89]. In addition, the genetic variation in the MTNR1B gene has been witnessed in PCOS women resulting in the impairment in insulin secretion thus indicating its possible role in PCOS and IR cross talk [90]. Furthermore the role of insulin like growth factors 1&2, their receptors and ILGFBPs have been well studied for their role in antral follicle development [91, 92]. Studies have revealed that hyperinsulinemia can alter synthesis of IGF- binding proteins thus increase the levels of free IGF-1[93]. IGF-1 directly affects the miRNA-323-3p regulation [94] consequently leading to accelerated granulose cell apoptosis via IGF-1, thus obstructs follicular development [94]. Moreover, the role of vascular endothelial growth factor (VEGF) has also been documented in the pathogenesis of PCOS [95]. However, more research is needed to simplify how I.R actually alters the follicular development and hinders the ovulation.
Environmental Hypothesis
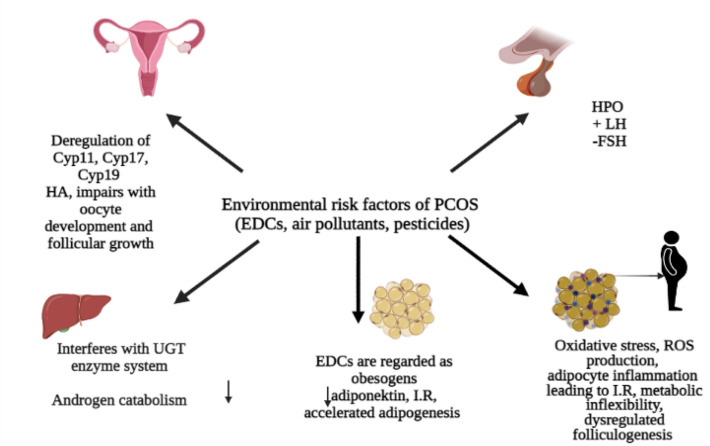
There has been growing evidences in the recent years that environmental toxins could contribute to the pathogenesis of PCOS. In the current industrial era, the indiscriminate use of chemicals has significantly affected the health status and has potentially altered the reproductive health by directly interfering with the normal ovarian morphology as well as function. Researches throughout the world are taking pains to understand the possible link between environmental toxins and pathophysiology of PCOS. The most well studied environmental risk factor in the pathogenesis of PCOS includes Endocrine disrupting chemicals (EDCs). EDCs are the group of heterogeneous group of compounds containing a phenolic group in their chemical structure making them to mimic the body’s endogenous hormones (e.g., steroid hormones, and thyroid hormones) eventually interfering with their receptors trigging altered molecular mechanism. Not only this, these chemicals alter the delivery of natural blood borne hormones by mingling with their transport proteins (like sex hormone binding globulin) consequently disturbing body’s homeostasis and impairing with developmental, metabolic and reproduction processes [96, 97]. However, the toxicity of such chemicals is determined by the time of exposure (fetal, adulthood), variety and concentration as it is likely that the cocktail of chemicals might result in more complex effects on the individual’s health [98]. More than 800 chemicals are documented as EDC’s and some of them possesses lipophilic properties can bioaccumlate in the adipose tissue (pesticides, POPs) leading to more adverse health effects [99, 100]. Humans are consuming packed foods in plastic containers, using cosmetics, plastic baby bottles, sanitary napkins, diapers, thermal papers, PVC pipes; these are found to be loaded with plasticizers which is considered to be the chief source of EDCs (BPA, BPF, phthalates). Also the pesticides are studied as the persistent toxicants with similar properties [101, 102]. EDC’s can lead to the disruption of various pathways potentially leading to PCOS and its consequences (Fig. 3). Some of the ways are summarized as under:
Environmental toxins chiefly EDC’s can directly affect the hormonal homeostasis thus leading to anovulation and reproductive dysfunction. EDC’S can either directly target ovaries or indirectly impact the HPO axis (hypothalamic pituitary ovarian axis) [103, 104] leading to hormonal disruption. BPA possess weak affinity towards variety of receptors (classical NRs α and β) [105], non-classical membrane ERs [106] thus favoring hormonal destabilization. Furthermore, studies have revealed that the expression of aromatase and E2 synthesis in the granulosa cells was downregulated in PCOS women vis-à-vis with higher levels of BPA in their follicular fluid sighting role of such toxins in disrupting the intrafollicular environment [107].
EDC’s can interfere with the steroidogenic pathways in multiple ways. A vast literature supports that BPA as well as phthalate interferes agonistically or antagonistically with the steroid binding proteins, steroid receptors or somehow modulates steroidogenic enzymes in the ovaries. BPA modulates the expression of various steroidogenic enzymes chiefly CYP17a1 and Cyp11a1, involved in the synthesis of androgens [107]. Additionally EDC’s may also interfere with the androgen catabolism thereby increasing the total free androgen index in PCOS women [108, 109]. This on the other hand downregulates the activity of hepatic UGT enzymes, key phase II players involved in the metabolism and clearance of BPA [110], thus exacerbating this vicious cycle in PCOS women.
EDC’s can alter the metabolic pathways as well which is been already entangled among the PCOS women thus making them more vulnerable. Obesity, I.R and compensatory hyperinsulinemia witnessed in majority of PCOS women; EDC’s can find their way in disturbing these delicate metabolic pathways. EDC’s are regarded as obesogens as they promote fat storage [111]. Animal models have revealed that EDC’s like mono-isobutyl phthalate, tributyltin and BPA act via various receptors (PPARγand ER) thus modulating different molecular pathways involved in adipogenisis and accumulation of lipids [112–114]. The role of various EDC’s in inducing hyperinsulinimia has been well documented in various toxicological studies [115]. EDC’s can directly alter the glucose metabolism [116] or indirectly may decrease the secretion of adiponectin which may later on lead to I.R [117].
Apart from plasticizers (BPA, phthalate) number of air pollutants have been studied for their role in causing the defects related to reproductive health [118]. Some of these are enlisted as EDC’s (PHA, heavy metals- Pb, Cu, Zn) [118, 119]. Studies suggest that these chemicals may induce compromised oxidative state or can lead to DNA abrasion leading to epigenetic modifications [120]. PCOS has been linked to the decreased antioxidant concentration and is termed as an oxidative state [121]. Oxidative stress developed in response to the excess ROS production have been seen to cause impaired folliculogenesis [122]. Elevated levels of oxidative stress biomarkers were reported from PCOS women indicating their role in diseases development [123]. A study conducted by Lin et al., [124] concluded that the women exposed to air pollutants chiefly- SO2, NO2, NO and PM2.5 are at high risk in developing PCOS. Sun and colleagues studied the role of particulate matter (< 2.5 μm; PM2.5) in diet-induced I.R, adipose inflammation, and visceral adiposity in C57BL/6 mice. They communicated that the inflammatory state because of oxidative stress and Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), E-selectin, intracellular adhesion molecule-1 (ICAM-1), plasminogen activator inhibitor-1, and resistin were main culprits in resulting this compromised metabolic state. Another study carried out by González et al., [125] revealed that the proinflmmatory response could stimulate the ovarian theca cells for the over production of androgens leading to PCOS. Thus, we can conclude that the inflammatory condition in response to the oxidative stress may result in the creation of hostile redox environment favoring metabolic inflexibility and hyperandrogenism consequently leading to PCOS progression [102, 126]. Furthermore, defective menstrual and ovarian functions were reported in relation to the numerous organic pollutants such as Tetrachlorodibenzo-p-dioxin, Dichlorodiphenyltrichloroethane, polychlorinated biphenyl (TCDD, DDT, and PCBs) [127–129].
Fig. 3.
Potential impact of environmental toxins on female reproductive health linked to PCOS phenotypes and adverse health effects
Neuroendocrine Hypothesis
PCOS considered to be an ovarian dysfunction but accumulating various clinical and pre-clinical evidence indicates the role of master gland “pituitary-gland”, located in the fore brain as the major suspect in the pathophysiology of the disease [130–132]. Numerous findings have suggested that any alteration in the specific neuronal network in the brain controlling fertility can results in the downstream ovarian dysfunction. Gonadotropin Releasing Hormone (GnRH) neuronal network is comprised of the small population of GnRH neurons. This portion is the fertility governing circuit scattered mainly in the fore brain and around glial cells, transmitting normal hormonal, metabolic and stress related signals to the reproductive axis [133]. GnRH neurons stimulate the release of tropical peptide hormone GnRH, consequently governs the release of two important gonadotropins; LH and FSH from the anterior pituitary. GnRH is released in a pulsatile manner and the frequency of the pulses determines which gonadotropin is secreted. A faster GnRH pulse favors the release of LH on contrary a weak pulse drives FSH from the master gland [134, 135]. Of note, the balanced LH/FSH ratio downstream regulates important ovarian events typically ssteroidogenesis and folliculogenesis. The ovarian cycle comprises of three basic phases, (1) Follicular phase, (2) Ovulatory phase and (3) Leutal phase. There exists a balanced neuroendocrine loop between the ovaries and hypothalamus pituitary axis governing the release of GnRH, LH and FSH. Over most of the ovarian cycle, the estradiol and progesterone send a negative feedback to the GnRH neuron activity favoring FSH surge and thus regulating the folliculogenesis. A positive feedback is mediated during the mid follicular phase driving GnRH/LH surge triggering ovulation. This delicate loop is somewhat disrupted in PCOS women resulting in the ovarian dysregulation. It is well recognized that increased LH frequency favors the hyperproduction of androgens by the theca cells leading to hyperandrogenism [136, 137]. Moreover, the decreased FSH frequency does not allow the follicular development thus arresting the folliculogenesis eventually resulting in the formation of tiny follicular cysts and anovulation [136, 138] [Fig. 4. Studies have showed that more than 95th percentile of the elevated LH at a single time measurement has been witnessed in 75% of PCOS women [139]. Scientists worldwide are proposing various mechanisms to explain this neuroendocrine impairment of PCOS. The upstream neuron network mostly regulates GnRH activity [140]. Karolina et al., [141] reported that the kisspeptin network plays significant role in reproductive processes. Kisspeptin directly regulates GnRH function via its receptors, located on the GnRH neurons. Administration of kisspeptin can directly regulate the LH secretion [142]. Studies have revealed that the invitro kisspeptin exposure can increase the firing rate of GnRH and stimulates the secretion of GnRH in hypothalamic explants [143, 144]. In addition, to underpin its role in the release of LH, it was observed that using Kisspeptin antagonist the pulsatile release of GnRH was affected consequently affecting the LH/FSH pulse which was further demonstrated by number of studies [145, 146]. Taking the advantage of optogenetics and chemogenetics techniques, the arcuate nucleus population of kisspeptin neurons (ARNKISS) has been identified as the extrinsic GnRH pulse generator [147, 148]. Studies have further confirmed that the inactivating mutations in kiss genes or its receptors resulted in hypogonadotropic hypogonadism and delayed puberty [149, 150], whist its activating mutations was related to precocious puberty [151, 152], suggesting that kisspeptin modulates GnRH pulsatility. It is pertinent to mention here that the classical neurotransmitter GABA is seen to play a significant role in the maintaining proper steroid hormone feedback [153]. The role of these neuronal population in GnRH/LH pulse patterns and PCOS pathophysiology have also been reported in several studies [154–158]. In addition, several animal models revealed that the exposure to the exogenous androgens or anti-Müllerian hormone (AMH) during the various developmental stages may induce PCOS like symptoms latter on [159–162]. The prenatal animal model (PNA) supports the fact that the prenatal exposure to the excessive androgens results in the PCOS-like neuroendocrine dysfunction [132, 161, 163, 164]
Fig. 4.
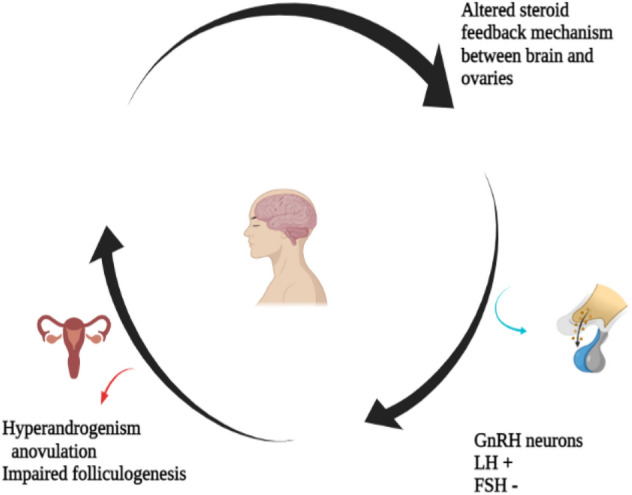
Neuroendocrine circuit disruption leading to PCOS
Potential Drugs and Therapeutics
PCOS is turning out to be monstrous in nature with increasing prevalence in both developing and developed countries. Unfortunately, no universal treatment is currently available to cure it completely but the current treatments are mostly symptom oriented, thus managing this disorder seems to be the only promising approaches available [165]. The present strategy of the researchers and pharmacologist is to target I.R, hyperandrogenism and oligomenorrehea. The long-term management to check the associated commorbities should include life style counselling to prevent obesity, T2DM, CVD or other metabolic complication [166].
Managing Hyperandrogenism
More than 80% of PCOS women suffer from hyperandrogenism i.e., hyperproduction of androgens leading to the elevated levels of circulating testosterone and androsterodione It represents to be the most burdensome characteristics as it triggers masculine phenotypic appearance besides having health consequences. Cholesterol serves as the chief precursor for the synthesis of androgens via series of various enzymatic reactions. The upreguation of various steroidogenic enzymes chiefly CYP17a1, Cyp11a1, 3βHSD, AKRIC1 are seen to be the major hyperandrogenic culprits in PCOS women [36, 167]. Thus, the simple approach that can be manifested to manage hyperandrogenism is to attenuate the expression of aforementioned enzymes. Moreover studies have confirmed that the downregulation of the expression of GnRH can lessen down LH pluse thus balancing LH/FSH ratio [165]; proving helpful in managing hyperandrogenism. A variety of drugs mainly oral contraceptive pills (OCPs) and anti-androgens are advised to PCOS women who are not attempting to conceive [168]. Anti-androgenic drugs mainly eflornithine, finastride can prove teratogenic [169, 170] therefore a combined therapy of OCPs with neutral or anti-progestin and/or anti-androgens (androgen receptors blockers) namely spironolactone, cyproterone acetate or flutamide acting as antagonists of androgen receptors proves highly effective for hirsutism [168]. Dopamine and dopamine agonists have long been known to decrease females' production of LH [171]. Bromocriptine, a dopamine agonist, may be helpful for PCOS patients by lowering LH hypersecretion. For acne retinoids[172], and for alopecia minoxidil [173] proves to be drug of choice. Cosmetic techniques are also advised along with the oral drugs depending upon the severity and patient’s requirement. For managing hirsutism waxing, bleaching, shaving, plucking and laser treatment are employed [168]. For acne dermocosmetics [174], light therapy [175] or cosmetic surgery can be helpful. Alopecia can be resolved by hair transplantation [176] or inducing growth factors from platelet rich plasma [177]. Statins are the most recent therapies that have been incorporated into PCOS treatment methods. They are helpful in the treatment of PCOS because they decrease the synthesis of sex hormones, alleviate dyslipidemia, and lessen the development of ovarian androgen by preventing the creation of androgen by thecal cells[171, 178]. Studies have shown that women with PCOS specifically have higher levels of bad bacteria (Escherichia and Shigella) and lower levels of good bacteria (Lactobacilli and Bifidobacteria) [179, 180]. The metabolic profile in PCOS has been demonstrated to benefit from probiotic administration [181, 182]. When compared to placebo, PCOS patients showed a statistically significant decrease in weight, BMI, plasma glucose, serum insulin levels, and a reduction in androgen levels [181, 183, 184]
Managing I.R
I.R appears to be the most common metabolic consequences related to PCOS. Hyperinsulinemia can pave to various secondary comorbidities in PCOS women in future of which T2DM and CVD appears to be most vulnerable. The basic strategy to manage this is life style modifications i.e. maintaining proper diet plan and physical activities. Studies have revealed that life style modifications not only improve hyperinsulinimia but also prove to be effective against hyperandrogenism in PCOS women [185]. A variety of anti-obesity drugs are advised to improve weight loss in PCOS women but their effectiveness remains to be established [186]. The bariatric surgery proves to be fruitful option for PCOS obese women [187]. The mainstay of pharmacotherapy of PCOS against hyperinsulinimia proves to be metformin, insulin sensitizer drug. It proves to be a mutihand drug which not only improves weight loss but also reduces hyperandrogenism and related symptoms [188–190]. Inositols, another insulin sensitizer drug have also proven effective in attenuating the PCOS symptoms [191, 192]. Berberine, a plant based alkaloid also showed promising results in animal based models against I.R by modulating expression of GLUT type 4 (glucose transport proteins) [193] but it needs to be further elucidated [194]. The class of anti-diabetic medications known as GLP-1 receptor agonists imitates the effects of incretin. In both diabetic and PCOS patients, GLP-1 agonists provide a dual control by lowering body fat and hyperglycemia thus showing promising results in managing I.R. [195]. DPP-4 inhibitors are seen to increase the levels of incretin in the body [196] there by regulating the normal blood glucose levels. SGLT2 inhibitors have also proven impactful in managing I.R by regulating blood glucose levels in the body through glycosuria and natriuresis. SGLT2 inhibitors increase urinary glucose excretion, weight loss and improve cardiovascular risks in patients with type 2 diabetes. Only one drug of SGLT2 inhibitors (Empagliflozin) have been used in PCOS patients with markedly effective results to manage I.R when compared to metformin [197]. Supplementing with vitamin D and calcium may have therapeutic effects on I.R, menstrual regularity, hyperandrogenism, and follicular development in women with PCOS [198, 199]. Vitamin D therapy normalizes the serum AMH levels, which may lead to improved folliculogenesis [200].
Managing Oligomennnorhea
Oligoovulation or oligomennorhea represents another common feature of PCOS. It is associated with menstrual dysfunction where ovaries seldom ovulate leading to subfertility. PCOS women experiencing oligomennorhea are at higher risk of endometrial hyperplasia, canacer and infertility [1]. Two basic approaches could be put into action based on patient’s requirement. PCOS women not attempting for pregnancy should be prescribed OCPs [201], cyclic or continuous progestin administration [201], or levonorgestrel –releasing intra utride device [202] can be employed as per the requirement and severity of her symptoms. Women expressing desire to get pregnant it is important to know the severity of oligoanovulation. If only mild menstrual dysfunction is present, proper counseling and suggestions regarding their most fertile days will suffice their desire. In contrast when the symptoms are more severe with acute menstrual dysfunction or when ovulation is unpredictable, ovulation needs to be induced [1] and accordingly clomiphene citrate or letrozole remain drug of choice [203]. Metformin may also be used as the data suggests that it can improve ovulation and pregnancy rates [204].
Conclusion and Future Perspectives
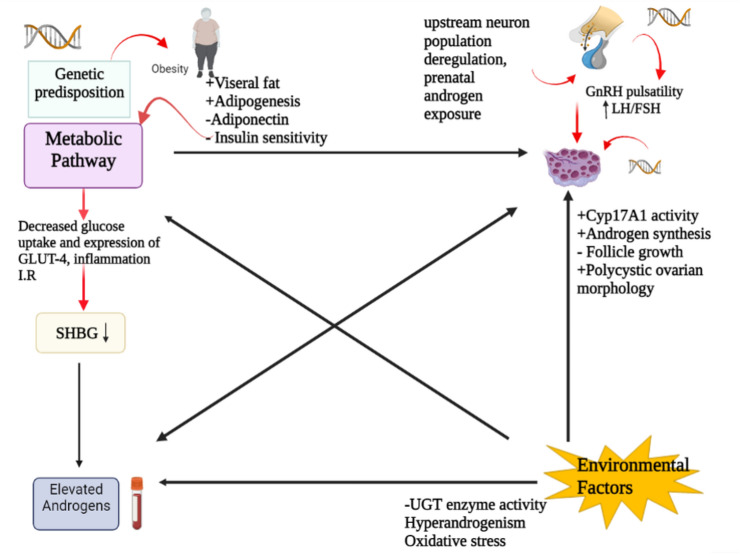
PCOS represents the most prevalent female endocrine disorder affecting the ovulation consequently leading to sub-fecundity in the industrialized population. The disease represent spectrum of symptoms thus making it difficult to understand and diagnose. In this review, we tried to discuss its major etiological factors and pathological links (Fig. 5). Plentiful researches suggests that the PCOS is no longer restricted as an ovarian diseases, rather numerous pathways—neuroendocrine, metabolic dysregulation, defective steroid state, environmental factors and genetic predisposition may induce and paves way for disease development. So taking consideration its increasing prevalence, it is need of the hour to understand the complex interplay among the potential pathways inducing HA, IR, metabolic aberration in shaping this disease. Molecular biology provides a promising approach to simplify this multifactorial paradox by exploring a common denominator that may trigger abnormal regulation of steroidogenesis, homologous desensitization to LH, folliculogenesis and metabolic dysregulation thus ultimately kick starts this vicious cycle. Meanwhile, unraveling the diverse pathological links will help to deduce a proper treatment opportunities of PCOS at early stages.
Fig. 5.
Interplay between the potential pathways that gets disrupted, eventually leading to reproductive and metabolic failure witnessed in PCOS
Acknowledgements
The authors are highly indebted to the Centre of Research for development (CORD) and Department of Environmental Sciences, University of Kashmir for providing the necessary facilities. This work didn’t receive any specific funding from any agency. However, the author KN is a recipient of MANF (MANF UGC Beneficiary Code: BININ01671381).
Abbreviations
- NIH
National Institutes of Health
- HA
Hyperandrogenism
- OD
Ovarian dysfunction
- PCOM
Polycystic ovarian morphology
- AE
Androgen Excess-PCOS Society
- I.R
Insulin resistance
- LH
Luteinizing hormone
- FSH
Follicle stimulating hormone
Author Contributions
Khair Ul Nisa and Bashir Ahmad Ganai conceived and designed this review. Khair Ul Nisa, Najeebul Tarfeen, Shahnaz Ahmad Mir, Ajaz Ahmad Waza, and Mir Bilal Ahmad collected the literature for this review. Khair Ul Nisa wrote the manuscript draft. Bashir Ahmad Ganai and Shahnaz Ahmad Mir edited this manuscript. All the authors gave final shape to this manuscript.
Funding
The work was not supported by any research grant.
Declartions
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 2.Escobar-Morreale HF. Defining PCOS: A syndrome with an intrinsic heterogeneous nature. Polycystic Ovary Syndrome: Elsevier; 2022. p. 3–13.
- 3.Yazdani, A. Polycystic ovarian syndrome. Examination Obstetrics and Gynaecology. 2010:21.
- 4.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):1–10. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. doi: 10.1016/j.molmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan M, Sultana S, Sohan M, Parvin S, Rahman MA, Hossain MJ, et al. Prevalence and associated risk factors for mental health problems among patients with polycystic ovary syndrome in Bangladesh: a nationwide cross: sectional study. PLoS ONE. 2022;17(6):e0270102. doi: 10.1371/journal.pone.0270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeks A, Gibson-Helm M, Paul E, Teede H. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod. 2011;26(6):1399–1407. doi: 10.1093/humrep/der071. [DOI] [PubMed] [Google Scholar]
- 9.Stein IF. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. doi: 10.1016/S0002-9378(15)30642-6. [DOI] [Google Scholar]
- 10.Hoeger KM, Dokras A, Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J Clin Endocrinol Metab. 2021;106(3):e1071–e1083. doi: 10.1210/clinem/dgaa839. [DOI] [PubMed] [Google Scholar]
- 11.Zawadzski J. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Polycystic Ovary Syndrome. 1992:39–50.
- 12.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertility and sterility. 2012;97(1):28–38. [DOI] [PubMed]
- 14.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Mykhalchenko K, Lizneva D, Trofimova T, Walker W, Suturina L, Diamond MP, et al. Genetics of polycystic ovary syndrome. Expert Rev Mol Diagn. 2017;17(7):723–733. doi: 10.1080/14737159.2017.1340833. [DOI] [PubMed] [Google Scholar]
- 16.Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):538–576. doi: 10.1210/endrev/bnaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barthelmess EK, Naz RK. Polycystic ovary syndrome: current status and future perspective. Front Biosci (Elite Ed) 2014;6:104. doi: 10.2741/e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azziz R. How PCOS came into its own. Elsevier; 2021.
- 19.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 20.Ding T, Hardiman PJ, Petersen I, Wang F-F, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017;8(56):96351. doi: 10.18632/oncotarget.19180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodarzi MO, Quiñones MJ, Azziz R, Rotter JI, Hsueh WA, Yang H. Polycystic ovary syndrome in Mexican-Americans: prevalence and association with the severity of insulin resistance. Fertil Steril. 2005;84(3):766–769. doi: 10.1016/j.fertnstert.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health. 2018;15(11):2589. doi: 10.3390/ijerph15112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 24.Knochenhauer E, Key T, Kahsar-Miller M, Waggoner W, Boots L, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 25.Azziz R. Introduction: determinants of polycystic ovary syndrome. Fertil Steril. 2016;106(1):4–5. doi: 10.1016/j.fertnstert.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Wu Q, Hao Y, Jiao M, Wang X, Jiang S, et al. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global Burden of Disease Study 2017. Hum Reprod. 2021;36(4):1108–1119. doi: 10.1093/humrep/deaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naal H, El Koussa M, El Hamouch M, Hneiny L, Saleh S. A systematic review of global health capacity building initiatives in low-to middle-income countries in the Middle East and North Africa region. Glob Health. 2020;16(1):1–16. doi: 10.1186/s12992-020-00585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miazgowski T, Martopullo I, Widecka J, Miazgowski B, Brodowska A. National and regional trends in the prevalence of polycystic ovary syndrome since 1990 within Europe: the modeled estimates from the Global Burden of Disease Study 2016. Arch Med Sci: AMS. 2021;17(2):343. doi: 10.5114/aoms.2019.87112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharali MD, Rajendran R, Goswami J, Singal K, Rajendran V. Prevalence of polycystic ovarian syndrome in India: a systematic review and meta-analysis. Cureus. 2022;14(12). [DOI] [PMC free article] [PubMed]
- 30.Sharma P, Kaur M, Kumar S, Khetarpal P. A cross-sectional study on prevalence of menstrual problems, lifestyle, mental health, and PCOS awareness among rural and urban population of Punjab, India. J Psychosom Obstet Gynecol. 2022;43(3):349–358. doi: 10.1080/0167482X.2021.1965983. [DOI] [PubMed] [Google Scholar]
- 31.Barber T. Why are women with polycystic ovary syndrome obese? Br Med Bull. 2022;1:11. doi: 10.1136/bmj.1.5165.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joharatnam J, Barber TM, Webber L, Conway GS, McCarthy MI, Franks S. Determinants of dyslipidaemia in probands with polycystic ovary syndrome and their sisters. Clin Endocrinol. 2011;74(6):714–719. doi: 10.1111/j.1365-2265.2011.03983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long C, Feng H, Duan W, Chen X, Zhao Y, Lan Y, et al. Prevalence of polycystic ovary syndrome in patients with type 2 diabetes: a systematic review and meta-analysis. Frontiers in Endocrinology. 2022:2091. [DOI] [PMC free article] [PubMed]
- 34.Riestenberg C, Jagasia A, Markovic D, Buyalos RP, Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J Clin Endocrinol Metab. 2022;107(2):575–585. doi: 10.1210/clinem/dgab613. [DOI] [PubMed] [Google Scholar]
- 35.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25(12):2944–2954. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- 36.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falck B. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol Scand. 1960;47(4):1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiriakidou M, Mcallister JM, Sugawara T, Strauss J., 3rd Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. J Clin Endocrinol Metab. 1996;81(11):4122–4128. doi: 10.1210/jcem.81.11.8923870. [DOI] [PubMed] [Google Scholar]
- 39.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 40.Penning TM. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev. 1997;18(3):281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 41.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43(8):779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 42.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirschner MA, Bardin CW. Androgen production and metabolism in normal and virilized women. Metabolism. 1972;21(7):667–688. doi: 10.1016/0026-0495(72)90090-X. [DOI] [PubMed] [Google Scholar]
- 44.Longcope C, Johnston C., Jr Androgen and estrogen dynamics in pre-and postmenopausal women: a comparison between smokers and nonsmokers. J Clin Endocrinol Metab. 1988;67(2):379–383. doi: 10.1210/jcem-67-2-379. [DOI] [PubMed] [Google Scholar]
- 45.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15(3):342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 46.Walters K, Allan C, Handelsman D. Androgen actions and the ovary. Biol Reprod. 2008;78(3):380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- 47.Escobar-Morreale HF, Luque-Ramírez M, San Millán JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26(2):251–282. doi: 10.1210/er.2004-0004. [DOI] [PubMed] [Google Scholar]
- 48.Anakwe OO, Payne AH. Noncoordinate regulation of de novo synthesis of cytochrome P-450 cholesterol side-chain cleavage and cytochrome P-450 17α-hydroxylase/C17-20 lyase in mouse Leydig cell cultures: Relation to steroid production. Mol Endocrinol. 1987;1(9):595–603. doi: 10.1210/mend-1-9-595. [DOI] [PubMed] [Google Scholar]
- 49.Fauser BC, Pache TD, Lamberts SW, Hop WC, de Jong FH, Dahl KD. Serum bioactive and immunoreactive luteinizing hormone and follicle-stimulating hormone levels in women with cycle abnormalities, with or without polycystic ovarian disease. J Clin Endocrinol Metab. 1991;73(4):811–817. doi: 10.1210/jcem-73-4-811. [DOI] [PubMed] [Google Scholar]
- 50.Abbott D, Barnett D, Bruns C, Dumesic D. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 51.Fernández-Real J-M, Ricart W. Insulin resistance and inflammation in an evolutionary perspective: the contribution of cytokine genotype/phenotype to thriftiness. Diabetologia. 1999;42(11):1367–1374. doi: 10.1007/s001250051451. [DOI] [PubMed] [Google Scholar]
- 52.Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;12:249. doi: 10.2147/TACG.S200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1–2):29–38. doi: 10.1016/j.mce.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper HE, Spellacy W, Prem K, Cohen W. Hereditary factors in the Stein-Leventhal syndrome. Am J Obstet Gynecol. 1968;100(3):371–387. doi: 10.1016/S0002-9378(15)33704-2. [DOI] [PubMed] [Google Scholar]
- 55.Vink J, Sadrzadeh S, Lambalk C, Boomsma D. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 56.Waterworth DM, Bennett ST, Gharani N, McCarthy MI, Hague S, Batty S, et al. Linkage and association of insulin gene VNTR regulatory polymorphism with polycystic ovary syndrome. Lancet. 1997;349(9057):986–990. doi: 10.1016/S0140-6736(96)08368-7. [DOI] [PubMed] [Google Scholar]
- 57.Taylor SI, Cama A, Accili D, Barbetti F, Quon MJ, Sierra MDLL, et al. Mutations in the insulin receptor gene. Endocr Rev. 1992;13(3):566–595. doi: 10.1210/edrv-13-3-566. [DOI] [PubMed] [Google Scholar]
- 58.Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39(6):724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 59.Barber T, Bennett A, Groves C, Sovio U, Ruokonen A, Martikainen H, et al. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia. 2008;51(7):1153–1158. doi: 10.1007/s00125-008-1028-6. [DOI] [PubMed] [Google Scholar]
- 60.Ackerman C, Garcia O, Legro R, Dunaif A, Urbanek M. SHBG (TAAAA) n is associated with serum SHBG in a PCOS case-control cohort. Endocr Rev. 2011;32:P2–66.03.
- 61.Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–1025. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z-J, Zhao H, He L, Shi Y, Qin Y, Shi Y, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16. 3, 2p21 and 9q33. 3. Nat Genet. 2011;43(1):55–9. [DOI] [PubMed]
- 63.Gao J, Xue J-D, Li Z-C, Zhou L, Chen C. The association of DENND1A gene polymorphisms and polycystic ovary syndrome risk: a systematic review and meta-analysis. Arch Gynecol Obstet. 2016;294:1073–1080. doi: 10.1007/s00404-016-4159-x. [DOI] [PubMed] [Google Scholar]
- 64.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. doi: 10.1371/journal.pgen.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng C, Lv P-P, Yu T-T, Jin M, Shen J-M, Wang X, et al. The association between polymorphism of INSR and polycystic ovary syndrome: a meta-analysis. Int J Mol Sci. 2015;16(2):2403–2425. doi: 10.3390/ijms16022403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui L, Zhao H, Zhang B, Qu Z, Liu J, Liang X, et al. Genotype–phenotype correlations of PCOS susceptibility SNPs identified by GWAS in a large cohort of Han Chinese women. Hum Reprod. 2013;28(2):538–544. doi: 10.1093/humrep/des424. [DOI] [PubMed] [Google Scholar]
- 68.Tian Y, Li J, Su S, Cao Y, Wang Z, Zhao S, et al. PCOS-GWAS susceptibility variants in THADA, INSR, TOX3, and DENND1A are associated with metabolic syndrome or insulin resistance in women with PCOS. Front Endocrinol. 2020;11:274. doi: 10.3389/fendo.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210(3):807–818. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dapas M, Lin FT, Nadkarni GN, Sisk R, Legro RS, Urbanek M, et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med. 2020;17(6):e1003132. doi: 10.1371/journal.pmed.1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 72.Diamanti-Kandarakis E, Papavassiliou AG. Outstanding questions. Trends Mol Med. 2006;7(12):324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 73.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi: 10.1016/j.lfs.2019.116940. [DOI] [PubMed] [Google Scholar]
- 75.Munir I, Yen H-W, Geller DH, Torbati D, Bierden RM, Weitsman SR, et al. Insulin augmentation of 17α-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology. 2004;145(1):175–183. doi: 10.1210/en.2003-0329. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Sun X, Sun X, Meng F, Hu M, Li X, et al. Molecular characterization of insulin resistance and glycolytic metabolism in the rat uterus. Sci Rep. 2016;6(1):1–15. doi: 10.1038/srep30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Ciaraldi TP, Morales AJ, Hickman MG, Odom-Ford R, Olefsky JM, Yen SS. Cellular insulin resistance in adipocytes from obese polycystic ovary syndrome subjects involves adenosine modulation of insulin sensitivity. J Clin Endocrinol Metab. 1997;82(5):1421–1425. doi: 10.1210/jcem.82.5.3961. [DOI] [PubMed] [Google Scholar]
- 79.Feng C, Jin Z, Chi X, Zhang B, Wang X, Sun L, et al. SHBG expression is correlated with PI3K/AKT pathway activity in a cellular model of human insulin resistance. Gynecol Endocrinol. 2018;34(7):567–573. doi: 10.1080/09513590.2017.1411474. [DOI] [PubMed] [Google Scholar]
- 80.Malini N, George KR. Evaluation of different ranges of LH: FSH ratios in polycystic ovarian syndrome (PCOS)–Clinical based case control study. Gen Comp Endocrinol. 2018;260:51–57. doi: 10.1016/j.ygcen.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 81.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50(1):113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 82.Matalliotakis I, Kourtis A, Koukoura O, Panidis D. Polycystic ovary syndrome: etiology and pathogenesis. Arch Gynecol Obstet. 2006;274(4):187–197. doi: 10.1007/s00404-006-0171-x. [DOI] [PubMed] [Google Scholar]
- 83.Nestler JE, Jakubowicz DJ, Falcon de Vargas A, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83(6):2001–5. [DOI] [PubMed]
- 84.Tosi F, Negri C, Brun E, Castello R, Faccini G, Bonora E, et al. Insulin enhances ACTH-stimulated androgen and glucocorticoid metabolism in hyperandrogenic women. Eur J Endocrinol. 2011;164(2):197. doi: 10.1530/EJE-10-0782. [DOI] [PubMed] [Google Scholar]
- 85.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbould A, Kim Y-B, Youngren JF, Pender C, Kahn BB, Lee A, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol-Endocrinol Metab. 2005;288(5):E1047–E1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 87.Lan C-W, Chen M-J, Tai K-Y, Danny C, Yang Y-C, Jan P-S, et al. Functional microarray analysis of differentially expressed genes in granulosa cells from women with polycystic ovary syndrome related to MAPK/ERK signaling. Sci Rep. 2015;5(1):1–10. doi: 10.1038/srep14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song X, Shen Q, Fan L, Yu Q, Jia X, Sun Y, et al. Dehydroepiandrosterone-induced activation of mTORC1 and inhibition of autophagy contribute to skeletal muscle insulin resistance in a mouse model of polycystic ovary syndrome. Oncotarget. 2018;9(15):11905. doi: 10.18632/oncotarget.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butts SF, Seifer DB, Koelper N, Senapati S, Sammel MD, Hoofnagle AN, et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J Clin Endocrinol Metab. 2019;104(2):369–378. doi: 10.1210/jc.2018-00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wikiera B, Zubkiewicz-Kucharska A, Nocoń-Bohusz J, Noczyńska A. Metabolic disorders in polycystic ovary syndrome. Pediatr Endocrinol Diabetes Metab. 2017;23(4). [DOI] [PubMed]
- 91.Spinedi E, Cardinali DP. The polycystic ovary syndrome and the metabolic syndrome: a possible chronobiotic-cytoprotective adjuvant therapy. International journal of endocrinology. 2018;2018. [DOI] [PMC free article] [PubMed]
- 92.Monte A, Barros V, Santos J, Menezes V, Cavalcante A, Gouveia B, et al. Immunohistochemical localization of insulin-like growth factor-1 (IGF-1) in the sheep ovary and the synergistic effect of IGF-1 and FSH on follicular development in vitro and LH receptor immunostaining. Theriogenology. 2019;129:61–69. doi: 10.1016/j.theriogenology.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Martins F, Saraiva M, Celestino J, Bruno J, Almeida A, Cunha R, et al. Expression of protein and mRNA encoding Insulin Growth Factor-I (IGF-I) in goat ovarian follicles and the influence of IGF-I on in vitro development and survival of caprine preantral follicles. Anim Reprod. 2018;7(4):349–361. [Google Scholar]
- 94.Stanek MB, Borman SM, Molskness TA, Larson JM, Stouffer RL, Patton PE. Insulin and insulin-like growth factor stimulation of vascular endothelial growth factor production by luteinized granulosa cells: comparison between polycystic ovarian syndrome (PCOS) and non-PCOS women. J Clin Endocrinol Metab. 2007;92(7):2726–2733. doi: 10.1210/jc.2006-2846. [DOI] [PubMed] [Google Scholar]
- 95.Zeng X, Xie Y-j, Liu Y-t, Long S-l, Mo Z-c. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21. [DOI] [PubMed]
- 96.Ng EHY, Chan CCW, Yeung WSB, Ho PC. Comparison of ovarian stromal blood flow between fertile women with normal ovaries and infertile women with polycystic ovary syndrome. Hum Reprod. 2005;20(7):1881–1886. doi: 10.1093/humrep/deh853. [DOI] [PubMed] [Google Scholar]
- 97.Hong H, Branham WS, Ng HW, Moland CL, Dial SL, Fang H, et al. Human sex hormone-binding globulin binding affinities of 125 structurally diverse chemicals and comparison with their binding to androgen receptor, estrogen receptor, and α-fetoprotein. Toxicol Sci. 2015;143(2):333–348. doi: 10.1093/toxsci/kfu231. [DOI] [PubMed] [Google Scholar]
- 98.Sheikh IA, Turki RF, Abuzenadah AM, Damanhouri GA, Beg MA. Endocrine disruption: computational perspectives on human sex hormone-binding globulin and phthalate plasticizers. PLoS ONE. 2016;11(3):e0151444. doi: 10.1371/journal.pone.0151444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rutkowska AZ, Diamanti-Kandarakis E. Polycystic ovary syndrome and environmental toxins. Fertil Steril. 2016;106(4):948–958. doi: 10.1016/j.fertnstert.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 100.Koch CA, Diamanti-Kandarakis E. Introduction to endocrine disrupting chemicals–is it time to act? Rev Endocr Metab Disord. 2015;16(4):269–270. doi: 10.1007/s11154-016-9338-3. [DOI] [PubMed] [Google Scholar]
- 101.Dirinck E, Dirtu A, Jorens P, Malarvannan G, Covaci A, Van Gaal L. Pivotal role for the visceral fat compartment in the release of persistent organic pollutants during weight loss. J Clin Endocrinol Metab. 2015;100(12):4463–4471. doi: 10.1210/jc.2015-2571. [DOI] [PubMed] [Google Scholar]
- 102.Rehan M, Ahmad E, Sheikh IA, Abuzenadah AM, Damanhouri GA, Bajouh OS, et al. Androgen and progesterone receptors are targets for bisphenol A (BPA), 4-Methyl-2, 4-bis-(P-Hydroxyphenyl) Pent-1-Ene—a potent metabolite of BPA, and 4-Tert-Octylphenol: a computational insight. PLoS ONE. 2015;10(9):e0138438. doi: 10.1371/journal.pone.0138438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papalou O, M Victor V, Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Curr Pharm Des. 2016;22(18):2709–22. [DOI] [PubMed]
- 104.Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. 2015;93(1):20, 1–9. [DOI] [PMC free article] [PubMed]
- 105.Rutkowska A, Rachoń D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2014;30(4):260–265. doi: 10.3109/09513590.2013.871517. [DOI] [PubMed] [Google Scholar]
- 106.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 107.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102(1–5):175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 108.Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283(1–2):12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Déchaud H, Ravard C, Claustrat F, de la Perrière AB, Pugeat M. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG) 1. Steroids. 1999;64(5):328–334. doi: 10.1016/S0039-128X(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 110.Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96(3):E480–E484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- 111.Yokota H, Iwano H, Endo M, Kobayashi T, Inoue H, Ikushiro S-i, et al. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J. 1999;340(2):405–9. [PMC free article] [PubMed]
- 112.Holtcamp W. Obesogens: an environmental link to obesity. National Institute of Environmental Health Sciences; 2012. [DOI] [PMC free article] [PubMed]
- 113.Maradonna F, Evangelisti M, Gioacchini G, Migliarini B, Olivotto I, Carnevali O. Assay of vtg, ERs and PPARs as endpoint for the rapid in vitro screening of the harmful effect of Di-(2-ethylhexyl)-phthalate (DEHP) and phthalic acid (PA) in zebrafish primary hepatocyte cultures. Toxicol In Vitro. 2013;27(1):84–91. doi: 10.1016/j.tiv.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 114.Lind PM, Roos V, Rönn M, Johansson L, Ahlström H, Kullberg J, et al. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ Health. 2012;11(1):1–8. doi: 10.1186/1476-069X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84(2):319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 116.A Polyzos S, Kountouras J, Deretzi G, Zavos C, S Mantzoros C. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: a concept implicating nonalcoholic fatty liver disease. Curr Mol Med. 2012;12(1):68–82. [DOI] [PubMed]
- 117.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114(1):106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Menale C, Grandone A, Nicolucci C, Cirillo G, Crispi S, Di Sessa A, et al. Bisphenol A is associated with insulin resistance and modulates adiponectin and resistin gene expression in obese children. Pediatr Obes. 2017;12(5):380–387. doi: 10.1111/ijpo.12154. [DOI] [PubMed] [Google Scholar]
- 119.Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health. 2017;16(1):1–16. doi: 10.1186/s12940-017-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J, Xie P, Kettrup A, Schramm K-W. Inhibition of progesterone receptor activity in recombinant yeast by soot from fossil fuel combustion emissions and air particulate materials. Sci Total Environ. 2005;349(1–3):120–128. doi: 10.1016/j.scitotenv.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 121.Takeda K, Tsukue N, Yoshida S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ Sci: Int J Environ Physiol Toxicol. 2004;11(1):33–45. [PubMed] [Google Scholar]
- 122.Palacio J, Iborra A, Ulcova-Gallova Z, Badia R, Martinez P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin Exp Immunol. 2006;144(2):217–222. doi: 10.1111/j.1365-2249.2006.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10(1):1–31. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 125.Lin S-Y, Yang Y-C, Chang CY-Y, Lin C-C, Hsu W-H, Ju S-W, et al. Risk of polycystic ovary syndrome in women exposed to fine air pollutants and acidic gases: a nationwide cohort analysis. Int J Environ Res Public Health. 2019;16(23):4816. [DOI] [PMC free article] [PubMed]
- 126.González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300–305. doi: 10.1016/j.steroids.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kshetrimayum C, Sharma A, Mishra VV, Kumar S. Polycystic ovarian syndrome: environmental/occupational, lifestyle factors; an overview. J Turkish German Gynecol Assoc. 2019;20(4):255. doi: 10.4274/jtgga.galenos.2019.2018.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cooper GS, Klebanoff MA, Promislow J, Brock JW, Longnecker MP. Polychlorinated biphenyls and menstrual cycle characteristics. Epidemiology. 2005:191–200. [DOI] [PubMed]
- 129.Farr S, Cooper G, Cai J, Savitz D, Sandler D. Pesticide use and menstrual cycle characteristics among premenopausal women in the Agricultural Health Study. Am J Epidemiol. 2004;160(12):1194–1204. doi: 10.1093/aje/kwi006. [DOI] [PubMed] [Google Scholar]
- 130.Mendola P, Messer LC, Rappazzo K. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult female. Fertil Steril. 2008;89(2):e81–e94. doi: 10.1016/j.fertnstert.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 131.Caldwell AS, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci. 2017;114(16):E3334–E3343. doi: 10.1073/pnas.1616467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moore AM, Campbell RE. The neuroendocrine genesis of polycystic ovary syndrome: a role for arcuate nucleus GABA neurons. J Steroid Biochem Mol Biol. 2016;160:106–117. doi: 10.1016/j.jsbmb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Moore AM, Prescott M, Marshall CJ, Yip SH, Campbell RE. Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc Natl Acad Sci. 2015;112(2):596–601. doi: 10.1073/pnas.1415038112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hrabovszky E, Liposits Z. Afferent neuronal control of type-I gonadotropin releasing hormone neurons in the human. Front Endocrinol. 2013;4:130. doi: 10.3389/fendo.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138(3):1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- 136.Wildt L, Häusler A, Marshall G, Hutchison J, Plant T, Belchetz P, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- 137.Chang RJ. The reproductive phenotype in polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3(10):688–695. doi: 10.1038/ncpendmet0637. [DOI] [PubMed] [Google Scholar]
- 138.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- 139.Jonard S, Dewailly D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum Reprod Update. 2004;10(2):107–117. doi: 10.1093/humupd/dmh010. [DOI] [PubMed] [Google Scholar]
- 140.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 141.Campbell R. Defining the gonadotrophin-releasing hormone neuronal network: transgenic approaches to understanding neurocircuitry. J Neuroendocrinol. 2007;19(7):561–573. doi: 10.1111/j.1365-2826.2007.01561.x. [DOI] [PubMed] [Google Scholar]
- 142.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500. doi: 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193–202. doi: 10.1159/000336376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li X-F, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE. 2009;4(12):e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci. 2017;114(47):E10216–E10223. doi: 10.1073/pnas.1713897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723–3736. doi: 10.1210/en.2018-00653. [DOI] [PubMed] [Google Scholar]
- 150.Bianco SD, Kaiser UB. The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol. 2009;5(10):569–576. doi: 10.1038/nrendo.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 152.Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Silveira L, Noel S, Silveira-Neto A, Abreu A, Brito V, Santos M, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276–2280. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Labarrere CA, Woods J, Hardin J, Campana G, Ortiz M, Jaeger B, et al. Early prediction of cardiac allograft vasculopathy and heart transplant failure. Am J Transpl. 2011;11(3):528–535. doi: 10.1111/j.1600-6143.2010.03401.x. [DOI] [PubMed] [Google Scholar]
- 155.Ruddenklau A, Campbell RE. Neuroendocrine impairments of PCOS. Endocrinology. [DOI] [PubMed]
- 156.Walters KA, Gilchrist RB, Ledger WL, Teede HJ, Handelsman DJ, Campbell RE. New perspectives on the pathogenesis of PCOS: neuroendocrine origins. Trends Endocrinol Metab. 2018;29(12):841–852. doi: 10.1016/j.tem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 157.Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2013;75(4):268–274. doi: 10.1159/000350217. [DOI] [PubMed] [Google Scholar]
- 158.Wang T, Han S, Tian W, Zhao M, Zhang H. Effects of kisspeptin on pathogenesis and energy metabolism in polycystic ovarian syndrome (PCOS) Gynecol Endocrinol. 2019;35(9):807–810. doi: 10.1080/09513590.2019.1597343. [DOI] [PubMed] [Google Scholar]
- 159.Kawwass JF, Sanders KM, Loucks TL, Rohan LC, Berga SL. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with polycystic ovary syndrome. Hum Reprod. 2017;32(7):1450–1456. doi: 10.1093/humrep/dex086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tata B, Mimouni NEH, Barbotin A-L, Malone SA, Loyens A, Pigny P, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caldwell A, Middleton L, Jimenez M, Desai R, McMahon A, Allan C, et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155(8):3146–3159. doi: 10.1210/en.2014-1196. [DOI] [PubMed] [Google Scholar]
- 162.Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology. 2013;154(2):796–806. doi: 10.1210/en.2012-1954. [DOI] [PubMed] [Google Scholar]
- 163.Hogg K, Wood C, McNeilly AS, Duncan WC. The in utero programming effect of increased maternal androgens and a direct fetal intervention on liver and metabolic function in adult sheep. PLoS ONE. 2011;6(9):e24877. doi: 10.1371/journal.pone.0024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Silva MS, Prescott M, Campbell RE. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI insight. 2018;3(7). [DOI] [PMC free article] [PubMed]
- 165.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci. 2004;101(18):7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wawrzkiewicz-Jałowiecka A, Kowalczyk K, Trybek P, Jarosz T, Radosz P, Setlak M, et al. In Search of new therapeutics—molecular aspects of the PCOS pathophysiology: genetics, hormones, metabolism and beyond. Int J Mol Sci. 2020;21(19):7054. doi: 10.3390/ijms21197054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. 1999;84(6):1897–1899. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 168.McAllister JM, Legro RS, Modi BP, Strauss JF., III Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26(3):118–124. doi: 10.1016/j.tem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Escobar-Morreale H, Carmina E, Dewailly D, Gambineri A, Kelestimur F, Moghetti P, et al. Epidemiology, diagnosis and management of hirsutism: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2012;18(2):146–170. doi: 10.1093/humupd/dmr042. [DOI] [PubMed] [Google Scholar]
- 170.Squibb B-M. Bristol-Myers Squibb Labeling VANIQA. US Food & Drug Administration. 2000.
- 171.Tartagni MV, Alrasheed H, Damiani GR, Montagnani M, Maria A, De Pergola G, et al. Intermittent low-dose finasteride administration is effective for treatment of hirsutism in adolescent girls: a pilot study. J Pediatr Adolesc Gynecol. 2014;27(3):161–165. doi: 10.1016/j.jpag.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 172.Lachelin G, Leblanc H, Yen S. The inhibitory effect of dopamine agonists on LH release in women. J Clin Endocrinol Metab. 1977;44(4):728–732. doi: 10.1210/jcem-44-4-728. [DOI] [PubMed] [Google Scholar]
- 173.Leyden J, Stein-Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermatol Ther. 2017;7(3):293–304. doi: 10.1007/s13555-017-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.van Zuuren EJ, Fedorowicz Z, Schoones J. Interventions for female pattern hair loss. Cochrane Database Syst Rev. 2016(5). [DOI] [PMC free article] [PubMed]
- 175.Araviiskaia E, Dréno B. The role of topical dermocosmetics in acne vulgaris. J Eur Acad Dermatol Venereol. 2016;30(6):926–935. doi: 10.1111/jdv.13579. [DOI] [PubMed] [Google Scholar]
- 176.Gold MH, Goldberg DJ, Nestor MS. Current treatments of acne: Medications, lights, lasers, and a novel 650-μs 1064-nm Nd: YAG laser. J Cosmet Dermatol. 2017;16(3):303–318. doi: 10.1111/jocd.12367. [DOI] [PubMed] [Google Scholar]
- 177.Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2(2):189. [PMC free article] [PubMed] [Google Scholar]
- 178.Anitua E, Pino A, Martinez N, Orive G, Berridi D. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43(5):658–670. doi: 10.1097/DSS.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 179.Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ. Mevastatin inhibits ovarian theca–interstitial cell proliferation and steroidogenesis. Fertil Steril. 2004;82:1193–1197. doi: 10.1016/j.fertnstert.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 180.Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. doi: 10.3389/fmicb.2017.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, et al. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9(5):400–421. doi: 10.1080/19490976.2018.1441664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ahmadi S, Jamilian M, Karamali M, Tajabadi-Ebrahimi M, Jafari P, Taghizadeh M, et al. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hum Fertil. 2017;20(4):254–261. doi: 10.1080/14647273.2017.1283446. [DOI] [PubMed] [Google Scholar]
- 183.Heshmati J, Farsi F, Yosaee S, Razavi M, Rezaeinejad M, Karimie E, et al. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrobial Proteins. 2019;11:1236–1247. doi: 10.1007/s12602-018-9493-9. [DOI] [PubMed] [Google Scholar]
- 184.Shoaei T, Heidari-Beni M, Tehrani HG, Esmaillzadeh A, Askari G. Effects of probiotic supplementation on pancreatic β-cell function and c-reactive protein in women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Int J Prevent Med. 2015;6. [DOI] [PMC free article] [PubMed]
- 185.Gholizadeh Shamasbi S, Dehgan P, Mohammad-Alizadeh Charandabi S, Aliasgarzadeh A, Mirghafourvand M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: a randomized, triple-blind, controlled, clinical trial. Eur J Nutr. 2019;58:629–640. doi: 10.1007/s00394-018-1648-7. [DOI] [PubMed] [Google Scholar]
- 186.Thomson RL, Buckley JD, Lim SS, Noakes M, Clifton PM, Norman RJ, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril. 2010;94(5):1812–1816. doi: 10.1016/j.fertnstert.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 187.Panidis D, Tziomalos K, Papadakis E, Vosnakis C, Chatzis P, Katsikis I. Lifestyle intervention and anti-obesity therapies in the polycystic ovary syndrome: impact on metabolism and fertility. Endocrine. 2013;44(3):583–590. doi: 10.1007/s12020-013-9971-5. [DOI] [PubMed] [Google Scholar]
- 188.Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update. 2017;23(4):390–408. doi: 10.1093/humupd/dmx012. [DOI] [PubMed] [Google Scholar]
- 189.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21(5):560–574. doi: 10.1093/humupd/dmv025. [DOI] [PubMed] [Google Scholar]
- 190.Pedersen AJ, Stage TB, Glintborg D, Andersen M, Christensen MMH. The pharmacogenetics of metformin in women with polycystic ovary syndrome: a randomized trial. Basic Clin Pharmacol Toxicol. 2018;122(2):239–244. doi: 10.1111/bcpt.12874. [DOI] [PubMed] [Google Scholar]
- 191.Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017(11). [DOI] [PMC free article] [PubMed]
- 192.Laganà AS, Rossetti P, Sapia F, Chiofalo B, Buscema M, Valenti G, et al. Evidence-based and patient-oriented inositol treatment in polycystic ovary syndrome: changing the perspective of the disease. Int J Endocrinol Metab. 2017;15(1). [DOI] [PMC free article] [PubMed]
- 193.Cabrera-Cruz H, Oróstica L, Plaza-Parrochia F, Torres-Pinto I, Romero C, Vega M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am J Physiol-Endocrinol Metab. 2020;318(2):E237–E248. doi: 10.1152/ajpendo.00162.2019. [DOI] [PubMed] [Google Scholar]
- 194.Zhang N, Liu X, Zhuang L, Liu X, Zhao H, Shan Y, et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol. 2020;110:104544. doi: 10.1016/j.yrtph.2019.104544. [DOI] [PubMed] [Google Scholar]
- 195.Niafar M, Pourafkari L, Porhomayon J, Nader N. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Arch Gynecol Obstet. 2016;293(3):509–515. doi: 10.1007/s00404-015-3976-7. [DOI] [PubMed] [Google Scholar]
- 196.Van Can J, Sloth B, Jensen C, Flint A, Blaak E, Saris W. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes. 2014;38(6):784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006;60(11):1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 198.Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol. 2019;90(6):805–813. doi: 10.1111/cen.13968. [DOI] [PubMed] [Google Scholar]
- 199.dehghani Firouzabadi R, Aflatoonian A, Modarresi S, Sekhavat L, MohammadTaheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement Ther Clin Pract. 2012;18(2):85–8. [DOI] [PubMed]
- 200.Tarfeen N, Nisa KU, Ahmad MB, Waza AA, Ganai BA. Metabolic and genetic association of vitamin D with calcium signaling and insulin resistance. Indian J Clin Biochem. 2022:1–11. [DOI] [PMC free article] [PubMed]
- 201.Irani M, Minkoff H, Seifer DB, Merhi Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J Clin Endocrinol Metab. 2014;99(5):E886–E890. doi: 10.1210/jc.2013-4374. [DOI] [PubMed] [Google Scholar]
- 202.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2(1):1–18. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 203.Andersson K. The levonorgestrel intrauterine system: more than a contraceptive. Eur J Contracept Reprod Health Care. 2001;6(sup1):15–22. doi: 10.3109/ejc.6.s1.15.22. [DOI] [PubMed] [Google Scholar]
- 204.Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. doi: 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 205.Mumusoglu S, Yildiz BO. Polycystic ovary syndrome phenotypes and prevalence: differential impact of diagnostic criteria and clinical versus unselected population. Curr Opin Endocrine Metab Res. 2020;12:66–71. doi: 10.1016/j.coemr.2020.03.004. [DOI] [Google Scholar]
- 206.Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–336. doi: 10.1097/AOG.0000000000002698. [DOI] [PubMed] [Google Scholar]
- 207.Pugliese A, Miceli D. The insulin gene in diabetes. Diabetes Metab Res Rev. 2002;18(1):13–25. doi: 10.1002/dmrr.261. [DOI] [PubMed] [Google Scholar]
- 208.Urbanek M, Woodroffe A, Ewens K, Diamanti-Kandarakis E, Legro R, Strauss Iii J, et al. Candidate gene region for polycystic ovary syndrome on chromosome 19p13. 2. J Clin Endocrinol Metab. 2005;90(12):6623–9. [DOI] [PubMed]
- 209.Gonzalez A, Abril E, Roca A, Aragón MJ, Figueroa MJ, Velarde P, et al. CAPN10 alleles are associated with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(8):3971–3976. doi: 10.1210/jcem.87.8.8793. [DOI] [PubMed] [Google Scholar]
- 210.Christopoulos P, Mastorakos G, Gazouli M, Deligeoroglou E, Katsikis I, Diamanti-Kandarakis E, et al. Study of association of IRS-1 and IRS-2 genes polymorphisms with clinical and metabolic features in women with polycystic ovary syndrome. Is there an impact? Gynecol Endocrinol. 2010;26(9):698–703. [DOI] [PubMed]
- 211.Batarfi AA, Filimban N, Bajouh OS, Dallol A, Chaudhary AG, Bakhashab S. MC4R variants rs12970134 and rs17782313 are associated with obese polycystic ovary syndrome patients in the Western region of Saudi Arabia. BMC Med Genet. 2019;20(1):1–7. doi: 10.1186/s12881-019-0876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang W, Wei D, Sun X, Li J, Yu X, Shi Y, et al. Family-based analysis of adiponectin gene polymorphisms in Chinese Han polycystic ovary syndrome. Fertil Steril. 2014;101(5):1419–23. [DOI] [PubMed]
- 213.Diamanti-Kandarakis E, Bartzis MI, Bergiele AT, Tsianateli TC, Kouli CR. Microsatellite polymorphism (tttta) n at − 528 base pairs of gene CYP11α influences hyperandrogenemia in patients with polycystic ovary syndrome. Fertil Steril. 2000;73(4):735–741. doi: 10.1016/S0015-0282(99)00628-7. [DOI] [PubMed] [Google Scholar]
- 214.Gharani N, Waterworth DM, Batty S, White D, Gilling-Smith C, Conway GS, et al. Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet. 1997;6(3):397–402. doi: 10.1093/hmg/6.3.397. [DOI] [PubMed] [Google Scholar]
- 215.Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab. 2000;85(6):2304–2311. doi: 10.1210/jcem.85.6.6631. [DOI] [PubMed] [Google Scholar]
- 216.Rosenfield RL, Barnes RB, Jose’F C, Lucky AW. Dysregulation of cytochrome P450c17α as the cause of polycystic ovarian syndrome. Fertil Steril. 1990;53(5):785–91. [PubMed]
- 217.Mehdizadeh A, Kalantar SM, Sheikhha MH, Aali BS, Ghanei A. Association of SNP rs. 2414096 CYP19 gene with polycystic ovarian syndrome in Iranian women. Int J Reprod BioMedicine. 2017;15(8):491. [PMC free article] [PubMed]
- 218.Witchel SF, Kahsar-Miller M, Aston CE, White C, Azziz R. Prevalence of CYP21 mutations and IRS1 variant among women with polycystic ovary syndrome and adrenal androgen excess. Fertil Steril. 2005;83(2):371–375. doi: 10.1016/j.fertnstert.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 219.Shaikh N, Dadachanji R, Meherji P, Shah N, Mukherjee S. Polymorphisms and haplotypes of insulin-like factor 3 gene are associated with risk of polycystic ovary syndrome in Indian women. Gene. 2016;577(2):180–186. doi: 10.1016/j.gene.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 220.Marioli DJ, Saltamavros AD, Vervita V, Koika V, Adonakis G, Decavalas G, et al. Association of the 17-hydroxysteroid dehydrogenase type 5 gene polymorphism (− 71A/G HSD17B5 SNP) with hyperandrogenemia in polycystic ovary syndrome (PCOS) Fertil Steril. 2009;92(2):648–652. doi: 10.1016/j.fertnstert.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 221.Yan J, Tian Y, Gao X, Cui L, Ning Y, Cao Y, et al. A genome-wide association study identifies FSHR rs2300441 associated with follicle-stimulating hormone levels. Clin Genet. 2020;97(6):869–877. doi: 10.1111/cge.13741. [DOI] [PubMed] [Google Scholar]
- 222.Tian Y, Zhao H, Chen H, Peng Y, Cui L, Du Y, et al. Variants in FSHB are associated with polycystic ovary syndrome and luteinizing hormone level in Han Chinese women. J Clin Endocrinol Metab. 2016;101(5):2178–2184. doi: 10.1210/jc.2015-3776. [DOI] [PubMed] [Google Scholar]
- 223.Pierre A, Peigné M, Grynberg M, Arouche N, Taieb J, Hesters L, et al. Loss of LH-induced down-regulation of anti-Müllerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod. 2013;28(3):762–769. doi: 10.1093/humrep/des460. [DOI] [PubMed] [Google Scholar]
- 224.Thathapudi S, Kodati V, Erukkambattu J, Addepally U, Qurratulain H. Association of luteinizing hormone chorionic gonadotropin receptor gene polymorphism (rs2293275) with polycystic ovarian syndrome. Genet Test Mol Biomarkers. 2015;19(3):128–132. doi: 10.1089/gtmb.2014.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zheng M-X, Li Y, Hu R, Wang F-M, Zhang X-M, Guan B. Anti-Müllerian hormone gene polymorphism is associated with androgen levels in Chinese polycystic ovary syndrome patients with insulin resistance. J Assist Reprod Genet. 2016;33(2):199–205. doi: 10.1007/s10815-015-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Deswal R, Yadav A, Dang AS. Sex hormone binding globulin-an important biomarker for predicting PCOS risk: a systematic review and meta-analysis. Syst Biol Reprod Med. 2018;64(1):12–24. doi: 10.1080/19396368.2017.1410591. [DOI] [PubMed] [Google Scholar]
- 227.Deepika M, Reddy KR, Yashwanth A, Rani VU, Latha KP, Jahan P. TNF-α haplotype association with polycystic ovary syndrome–a South Indian study. J Assist Reprod Genet. 2013;30(11):1493–1503. doi: 10.1007/s10815-013-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vural P, Değirmencioğlu S, Saral NY, Akgül C. Tumor necrosis factor α (− 308), interleukin-6 (− 174) and interleukin-10 (− 1082) gene polymorphisms in polycystic ovary syndrome. Eur J Obst Gynecol Reprod Biol. 2010;150(1):61–65. doi: 10.1016/j.ejogrb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 229.Wehr E, Trummer O, Giuliani A, Gruber H-J, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164(5):741. doi: 10.1530/EJE-11-0134. [DOI] [PubMed] [Google Scholar]
- 230.Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015;6(1):1–13. doi: 10.1038/ncomms8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Manna P, Jain SK. Vitamin D up-regulates glucose transporter 4 (GLUT4) translocation and glucose utilization mediated by cystathionine-γ-lyase (CSE) activation and H2S formation in 3T3L1 adipocytes. J Biol Chem. 2012;287(50):42324–42332. doi: 10.1074/jbc.M112.407833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Louwers YV, Stolk L, Uitterlinden AG, Laven JS. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(12):E2006–E2012. doi: 10.1210/jc.2013-2495. [DOI] [PubMed] [Google Scholar]
- 233.Zhang Y, Movva VC, Williams MS, Lee MTM. Polycystic ovary syndrome susceptibility loci inform disease etiological heterogeneity. J Clin Med. 2021;10(12):2688. doi: 10.3390/jcm10122688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dakshinamoorthy J, Jain PR, Ramamoorthy T, Ayyappan R, Balasundaram U. Association of GWAS identified INSR variants (rs2059807 & rs1799817) with polycystic ovarian syndrome in Indian women. Int J Biol Macromol. 2020;144:663–670. doi: 10.1016/j.ijbiomac.2019.10.235. [DOI] [PubMed] [Google Scholar]
- 235.Hwang J-Y, Lee E-J, Jin Go M, Sung Y-A, Lee HJ, Heon Kwak S, et al. Genome-wide association study identifies GYS2 as a novel genetic factor for polycystic ovary syndrome through obesity-related condition. J Hum Genet. 2012;57(10):660–664. doi: 10.1038/jhg.2012.92. [DOI] [PubMed] [Google Scholar]