Abstract
Background
Although randomized controlled trials (RCT) are the reference standard of evidence in allergen immunotherapy (AIT), nonrandomized studies (NRS) are needed to confirm their results in more representative populations, particularly for treatment duration and persistence. However, when discrepancies are observed between RCT and NRS, NRS reliability decreases because these discrepant results are generally attributed to the methodologic flaws of NRS.
Objective
We compared the benefit of sublingual AIT (SLIT) for allergic rhinoconjunctivitis in NRS versus RCT focusing on a single product/allergen to reduce heterogeneity.
Methods
For meta-analysis, house dust mite (HDM) SLIT liquid formulation studies were sourced from computerized (Medline, Web of Science, and LILACS databases, to January 2023) and manual literature searches. Populations, treatments, and outcome data were combined (DerSimonian-Laird method). Noncomparative NRS were compared to RCT’ SLIT arm before and after treatment. Efficacy was determined as the standardized mean difference (SMD) in symptom score (SS) and medication score (MS).
Results
Data from 12 NRS (682 patients) and 8 RCT (176 patients) were analyzed. The benefit with index of reactivity (IR)-HDM SLIT liquid formulation was found significant for, first, SS in both NRS (SMD = −1.27; 95% confidence interval [CI], −1.64, −0.90) and RCT (SMD = −0.56; 95% CI, −0.90, −0.21), and second, MS with SMD equal to −1.35 (95% CI, −1.77, −0.93) and −0.46 (95% CI, −0.67, −0.25), respectively. Metaregression showed that symptom improvement was correlated with treatment duration with consistent results in NRS and RCT with 12-month SS data: −0.87 (interquartile range, −1.02, −0.77) and −0.75 (interquartile range, −0.93, −0.41), respectively.
Conclusion
This meta-analysis showed comparable clinical benefit of IR-HDM SLIT liquid formulation increasing over time in both NRS and RCT, suggesting that NRS may reliably integrate RCT results and be considered for guidelines.
Key words: Guidelines, nonrandomized studies, randomized controlled trials, real-life, rhinoconjunctivitis, study quality, sublingual immunotherapy
Randomized controlled trials (RCT) have proven the efficacy of allergen immunotherapy (AIT) in reducing symptoms and medication use in allergic patients.1, 2, 3, 4, 5 However, a variety of factors, which are tightly controlled in RCT, can play an important role in modulating AIT effectiveness in real life, such as adherence or treatment persistence. Furthermore, despite the recommendation of treating patients for at least 3 years to achieve disease modification and long-term tolerance with AIT, very few RCT have this duration.6
Hence, even though RCT with low risk of bias are the most trustworthy in estimating the effect of an intervention, nonrandomized studies (NRS) of representative populations in real life can provide evidence that better reflects the treatment effects achievable at the population level—that is, the environment in which the intervention is used. This explains the advisability of incorporating evidence from NRS in systematic reviews when they provide complementary, sequential, or replacement evidence to RCT.7
A recent Grades of Recommendation Assessment, Development, and Evaluation (GRADE) article, based on previous published works7,8 and surveys of experts and members of Cochrane and the Guidelines International Network, provides guidance for optimizing the integration of randomized and NRS of intervention in evidence syntheses.9 According to the guidance, including NRS in a health synthesis may be recommended when RCT findings do not provide high-certainty evidence.
There are few comparative NRS in AIT, and their quality is insufficient to be considered in guidelines.10,11 In contrast, a considerable number of single-arm cohort studies and case series are available. Despite the lack of control groups, these studies are a valuable source of information, particularly studies with a long treatment duration (eg, 3 years) and follow-up after treatment cessation, which is usually not feasible in RCT for practical reasons.
Focusing on a single manufacturer product and a single allergen extract (house dust mite [HDM]) to reduce the possible heterogeneity associated with different products, we undertook a systematic review of the literature and meta-analysis to compare single-arm cohort studies or case series to RCT using a specific sublingual immunotherapy (SLIT) liquid formulation in order to assess possible difference in direction or magnitude of effect between relevant NRS and RCT; to explain possible differences in the results of the 2 types of studies; and to assess study quality and possible integration of RCT findings with findings from NRS.
Methods
Search strategy and selection criteria
We undertook and reported this systematic review and meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA);12,13 GRADE;14,15 and Cochrane guidelines.16
This study was registered at International Prospective Register of Systematic Reviews (PROSPERO; no. CRD42023376265).
We searched the Medline, LILACS, and Web of Science databases from inception to January 30, 2023, for published and unpublished observational studies and RCT assessing the effectiveness of index of reactivity (IR)-HDM SLIT liquid formulation in patients with allergic rhinoconjunctivitis (ARC). To make RCT results comparable to observational study (single-arm cohort studies, case series, and before-and-after studies) results, only the treatment arm of RCT was analyzed, and data before and after AIT treatment were extracted.
A full list of the search terms is available in the protocol and in this article’s Methods section in the Online Repository at www.jaci-global.org. Studies were included in the meta-analysis if they (1) included patients with ARC to HDM with or without mild to moderate asthma, (2) included patients who were prescribed HDM SLIT liquid formulation from a single manufacturer (Staloral, Stallergenes Greer, Antony, France) for ARC, and (3) assessed the relevant outcome measures of the treatment effect, regardless of whether these were the primary end points. Studies were excluded if they did not report the required information.
We did not use any language restrictions. We checked all reference lists and articles citing included studies and recent reviews or meta-analyses for any additional relevant studies. We also asked Stallergenes Greer to help provide a complete list of observational studies on IR-HDM SLIT liquid (Staloral) for ARC for additional data.
Data collection
We screened titles and abstracts, reviewed full texts, extracted data, and assessed risk of bias/study quality independently in duplicate (P.C. and F.S.) using a standardized prepiloted form (www.rayyan.ai). We resolved disagreements by consensus adjudication or discussion with a third reviewer (D.D.B.). We collected characteristics of studies, setting, eligibility criteria, population studied, intervention, and outcomes.
Outcomes
We prioritized outcomes that were patient-important events of ARC, consistent with the established approach for AIT as informative of treatment efficacy/effectiveness and safety.17
The critical/important outcomes were as follows: symptom severity, assessed as SS or visual analog score (VAS); decrease in receipt of antisymptomatic drugs, assessed as MS or VAS; and adverse events (AEs).
Data analysis
The effect of IR-HDM SLIT liquid was assessed by comparing outcomes before treatment (a 12-month observation period before starting treatment) and after treatment (during a 6-month to 36-month observation period after starting treatment).
We pooled summary measures using DerSimonian-Laird random effects, estimating heterogeneity using the Mantel-Haenszel model.18 We combined continuous outcomes across studies (SS, MS, VAS) using standardized mean difference (SMD) because the outcomes were measured with different scales.
There were studies not reporting standard deviations (SDs). For these, we estimated SDs using methods based on summary statistics (minimum, maximum, lower quartile, upper quartile, median, P values).16,19 When standard error (SE) was reported, SD was obtained using the following formula: SD = SE√n. In some studies, means and SE were obtained from the graphs.
We used the Quality Appraisal of Case Series Studies Checklist developed by the Institute of Health Economics (IHE; www.ihe.ca/research-programs/rmd/cssqac/cssqac-about) for longitudinal studies with responses as “yes,” “unclear/partial,” or “no.” We classified studies as being of acceptable quality (low to moderate risk of bias) if ≥70% were “yes” responses.20,21 We also assessed RCT with this tool because we used only the treatment arm of these studies. We also evaluated the risk of bias of RCT using a specific tool, RoB2.22
We evaluated the certainty (quality) of evidence using the GRADE approach (see the Methods section in the Online Repository).14
Prespecified descriptive subgroup analysis was performed for the study outcomes. Metaregression was also used to predict the size of the outcome variable according to the values of 1 or more explanatory variables. The selection of characteristics defining subgroups/explanatory variables was motivated by clinical and methodologic hypotheses (see the Methods section in the Online Repository).
Sensitivity analyses were performed to test the robustness of the findings (see the Methods section in the Online Repository). We then excluded each study in turn to ensure that no single study would be solely responsible for the significance of any result (influential analysis).
We tested between-study heterogeneity by the chi-square test (threshold P = .10) and quantified it using the I2 statistic, which describes the percentage of variability due to heterogeneity rather than sampling error.23
We assessed publication bias by inspecting funnel plots, Egger linear regression test,24 fail-safe calculation, and trim-and-fill analysis.16 We also assessed it qualitatively by applying GRADE guidance.14
We used GRADEpro GDT software (gradepro.org) to create the summary of finding tables. All meta-analyses and statistical analyses were performed by ProMeta 3.0 software.25
Results
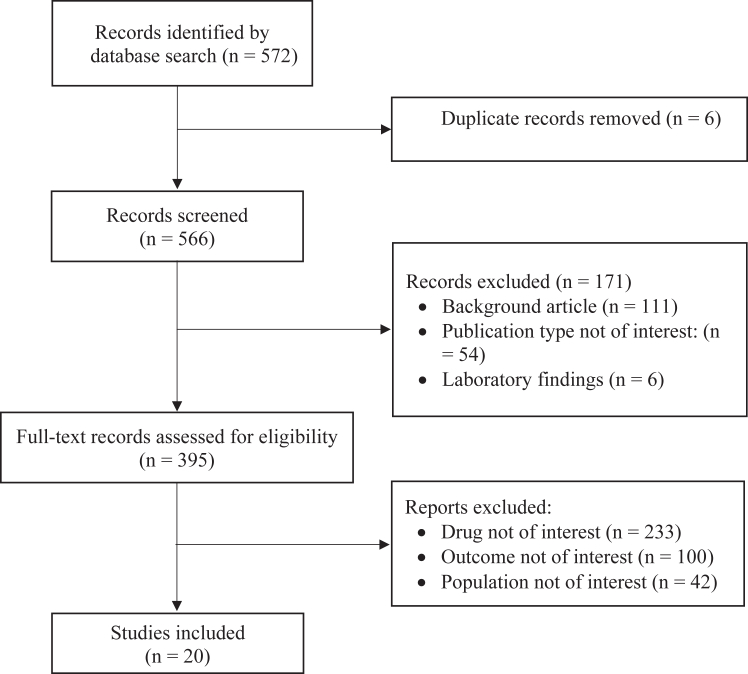
Our bibliographic searches yielded 572 records. After initial screening, we reviewed 395 studies and included 20 studies, 12 NRS and 8 RCT. Data on the SS were available in all 20 studies including a total of 858 patients.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Data on MS were available in 6 NRS (252 patients) and 7 RCT (160 patients) (Fig 1).
Fig 1.
Study selection.
The characteristics of the 20 qualifying studies are summarized in Table I. Thirteen studies were conducted in Europe, 5 in Asia (3 in South Korea, 1 in Singapore, 1 in Taiwan), 1 in Australia, and 1 in South Africa. The study completion rate ranged from 26%37 to 100%;26,28,31,32,34,35,38,40,44 and was higher in RCT (88% on average) than NRS (77.2% on average).
Table I.
Patients and study characteristics of NRS and RCT included in meta-analyses
| Study, year (country) | Study type | No. of patients (enrollment→observation) | No. male (%) | Age (years), mean ± SD (range) | Mono- or polysensitization | Asthma (%) | Rhinitis (%) | Measure∗ | Treatment duration (months) | Evaluation time point (months) | Cumulative dose (IR) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NRS | |||||||||||
| Bahceciler, 2005 (Turkey)26 | O, P, U, SC | 14→14 | 8 (57.1) | 7.6 ± 2.5 | Mono | 100 | 100 | SS; MS | 12 | 12 | 7,000 (14,000/y) |
| Cadario, 2008 (Italy)27 | O, P, U, SC | 20→17 | NR | 5-50 | Mono | 0 | 100 | SMS | 12 | 12 | 40,320 |
| Ciprandi, 2010 (Italy)28 | O, P, U, MC | 16→16 | NR | 11.8 (5.2-17.7) | Mono/poly | NR | NR | VAS-SS↑, VAS-MS↑ | 12 | 12 | NR |
| Ferrés, 2011 (Spain)29 | O, R, U, SC | 78→73 | 34 (43.6) | 10.96 ± 3.0 | NR | NR | 100 | VAS-SS↓, MS | 24 | 12 | 36,000 |
| Park, 2012 (South Korea)30 | O, P, U, SC | 112→88 | 49 (55.7) | 9.6 (5-15) | Mono | NR | 100 | SS, MS | 12 | 12 | 43,200 |
| Tosca, 2014 (Italy)31 | O, R, U, SC | 39→39 | 22 (56.4) | 13 | NR | 71.8 | 84.6 | VAS-SS↑ | 36 | 36 | 46,800 |
| Cingi, 2015 (Turkey)32 | O, P, U, MC | 186→186 | 51 (27.4) | 27.04 (19-51) | NR | 0 | 100 | SS | 12 | 12 | NR |
| Soh, 2016 (Singapore)33 | O, P, U, SC | 39→33 | 31 (82.1) | 24† (7-18), 15† (19-72) | NR | 0 | 100 | SS | 24 | 12 | 43,200 |
| Novakova, 2017 (Bulgaria)34 | O, P, U, SC | 76→76 | 42 (55.3) | 26.10 ± 5.85 (18-48) | NR | 36.84 | 100 | SS† | 36 | 36 | NR |
| Novakova, 2018 (Bulgaria)35 | O, P, U, SC | 86→86 | 46 (53.5) | 26.1 ± 5.78 (18-48) | Mono | NR | 100 | VAS-SS↑ | 36 | 36 | NR |
| Kim, 2020 (South Korea)36 | O, R, U, SC | 26→15 | 20 (76.9) | 13.0 ± 9.0 | Mono | NR | 100 | SS | 12-36 (mean 24) | EOT | 37,440 |
| Jung, 2021 (South Korea)37 | O, P, U, SC | 147→39 | (68.02) | 16.5 ± 12.3 | NR | 8.3 | 100 | SS, MS | 12 | 12 | NR |
| Total | 839→682 | ||||||||||
| RCT | |||||||||||
| Mungan, 1999 (Turkey)38 | PC, SB, SC | 15→15 | 2 (13.3) | 31.67 ± 7.28 (18-41) | NR | 86 | 100 | SS; MS | 12 | 12 | 11,316 |
| Guez, 2000 (France)39 | PC, DB, SC | 36→26 | 14 (38.8) | 29.6 ± 12.4 (12-51) | Mono/poly | NR | 100 | SS; MS | 24 | 12 | 90,000 (45,000/y) |
| Bahceciler, 2001 (Turkey)40 | PC, DB, SC | 8→8 | 4 (50) | 12.4 (7.8-18) | Mono | 100 | 100 | SS; MS | 6 | 6 | 7,000 (14,000/y) |
| Tseng, 2008 (Taiwan)41 | PC, DB, MC | 30→28 | 22 (73) | 9.7 ± 3.3 | Mono | 0 | 100 | SS; MS | 6 | 6 | 37,312 (74,424) |
| O’Hehir, 2009 (Australia)42 | PC, DB→O (exten), SC | 13→9 | 3 (33.3) | 27.3 (9.3) | Mono/poly | 77.7 | 100 | SS; MS | 12 (DB) + 12 O | 24 | 85,621 (42,810/y) |
| Aydogan, 2013 (Turkey)43 | PC, DB, SC | 8→7 | 6 (85) | 8.1 ± 2.2 | Mono | 0 | 100 | SS; MS | 12 | 12 | 44,500 |
| Bozek, 2013 (Poland)44 | PC, DB, SC | 51→51 | 23 (45) | 65.78 ± 4.89 | Mono | 11.7 | 100 | SS; MS | 36 | 36 | 421,200 (140,400) |
| Potter, 2015 (South Africa)45 | PC, DB, SC | 39→32 | 14 (35.9) | 33.7 | Mono/poly | NR | 100 | SS | 24 | 24 | 96,600 (48,300) |
| Total | 200→176 |
DB, Double blind; EOT, end of treatment; exten, extension; MC, multicenter; NR, not reported; O, open; P, prospective; PC, placebo controlled; R, retrospective; SB, single blind; SC, single center; U, uncontrolled (vs standard treatment or no treatment).
SS, symptom score; MS, medication score; SMS, symptom-medication score; VAS scores are as follows: VAS-SS↑, symptom assessed by VAS ascending (0 = better; 10 = worse); VAS-SS↓, symptom assessed by VAS descending (0 = worse; 10 = better); VAS-MS↑, medication receipt assessed by VAS ascending (0 = lower; 10 = higher).
Only nasal symptoms.
All the NRS were prospective except 3, which were retrospective.29,31,36 There were 3 multicenter studies.28,32,41
The sample size of the studies varied greatly, ranging from 8 patients40,43 to 186 patients (NRS from Cingi et al32). The mean of the mean age of patients from the individual studies was 21 ± 13.9 years and was greater in RCT (mean age 27.3 ± 18.7 years) than NRS (16.5 ± 7.1 years). Nine studies included patients sensitized only to HDM (monosensitized),26,27,30,35,36,40,41,43,44 4 studies included both HDM-monosensitized patients and patients sensitized to allergens other than HDM (polysensitized),28,39,42,45 and 7 studies did not report whether the patients were mono- or polysensitized.29,31, 32, 33, 34,37,38 All the studies included patients with ARC (in the Tosca et al31 study, the proportion of patients with ARC was 84.6%). In the study of Ciprandi et al,28 the proportion of patients with ARC was not reported. The proportion of patients with asthma varied greatly (range, 0-100%) and was not reported in 7 studies. The treatment duration varied from 6 months40,41 to 36 months.31,34,35,44 The cumulative annualized AIT dose ranged from 11,316 IR38 to 140,400 IR (Table I).44
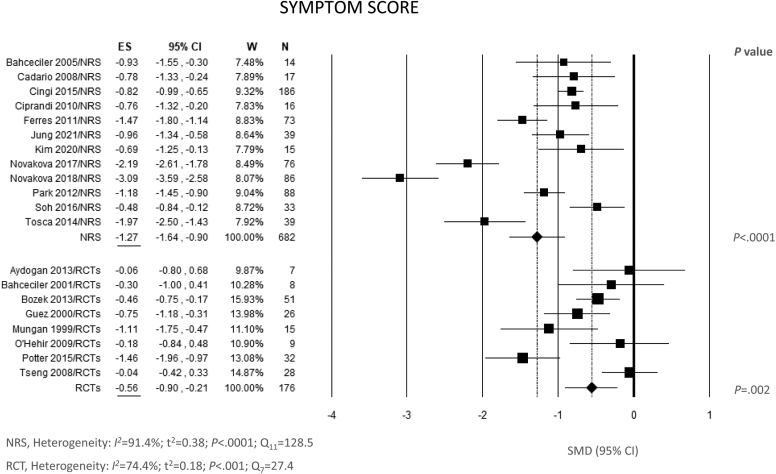
The effect of IR-HDM SLIT liquid on SS is illustrated in Fig 2. All the NRS and 4 of the 8 RCT showed a statistically significant reduction of SS at the end of treatment compared to baseline. The pooled SMD for the treatment effect was −1.27 (95% confidence interval [CI], −1.64, −0.90; P < .0001) for NRS and −0.56 (95% CI, −0.90, −0.21; P = .002) for RCT, indicating a statistically significant benefit in both NRS and RCT. A remarkable degree of heterogeneity between the results of individual studies was reported, both for NRS (Q = 128.5; df = 11; P < .0001; I2 = 91.4%) and RCT (Q = 27.4; df = 7; P < .001; I2 = 74.4%) (Fig 2). Visual inspection of funnel plots and Egger testing did not reveal substantial evidence of bias toward positive results, suggesting no selective reporting (see Fig E1 in the Online Repository at www.jaci-global.org). The fail-safe number was high enough (1439 for NRS and 71 for RCT) to confirm the robustness of these results against publication bias.
Fig 2.
Meta-analysis of 12 NRS and 8 RCT of IR-HDM SLIT liquid baseline versus end of treatment for ARC using random effects model (REM). SMD and 95% CI for effect of SLIT on SS are plotted on graph. Studies of each group are arranged in alphabetic order. ES, Estimate; W, weights.
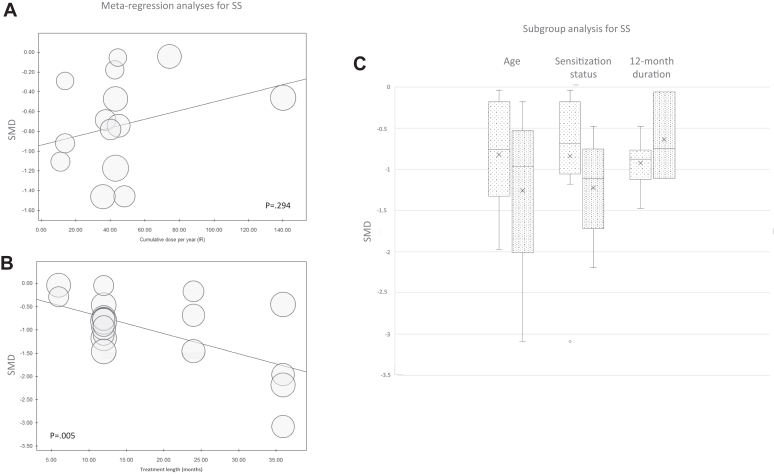
Metaregression did not show a different benefit according to the cumulative dose administered during the entire treatment course (P = .29) (Fig 3, A) but showed a positive and statistically significant correlation between treatment benefit and treatment length (P = .005) (Fig 3, B). This was confirmed by a subgroup analysis showing no substantial difference in the effect between NRS and RCT in evaluating the treatment effect at the same time point (12 months) (Fig 3, C).
Fig 3.
Metaregression analyses of SS for efficacy of IR-HDM SLIT liquid depending on cumulative dose administered per year (A) or treatment length (B). (C) Subgroup analysis box plots include middle 50% of data. Horizontal bars inside boxes represent SMD; lines to whiskers extend most extreme data points, which are no more than 1.5 times interquartile range from box.
Subgroup analyses by age and sensitization status did not show any age effect; nor was a significant difference observed between mono- and polysensitized patients (Fig 3, C).
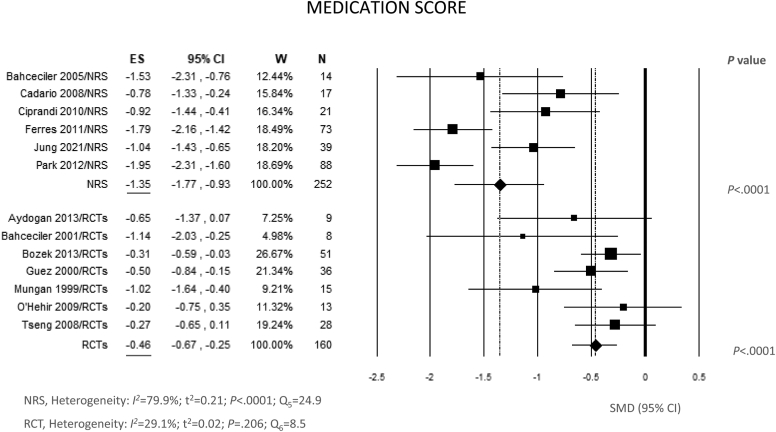
Fig 4 shows data on MS. All studies but 3 showed a statistically significant difference between end of treatment and baseline. The pooled SMD was −1.35 (95% CI, −1.77, −0.93; P < .0001) for NRS and −0.46 (95% CI, −0.67, −0.25; P = .0001) for RCT. The between-study heterogeneity was remarkable only for NRS (Q = 24.9; df = 5; P < .0001; I2 = 79.9%) (Fig 4), with no evidence of publication bias (see Fig E2 in the Online Repository at www.jaci-global.org).
Fig 4.
Meta-analysis of 6 NRS and 7 RCT of IR-HDM SLIT liquid baseline versus end of treatment for ARC using random effects model (REM). SMD and 95% CI for effect of SLIT on MS are plotted on graph. Studies of each group are arranged in alphabetic order. ES, Estimate; W, weights.
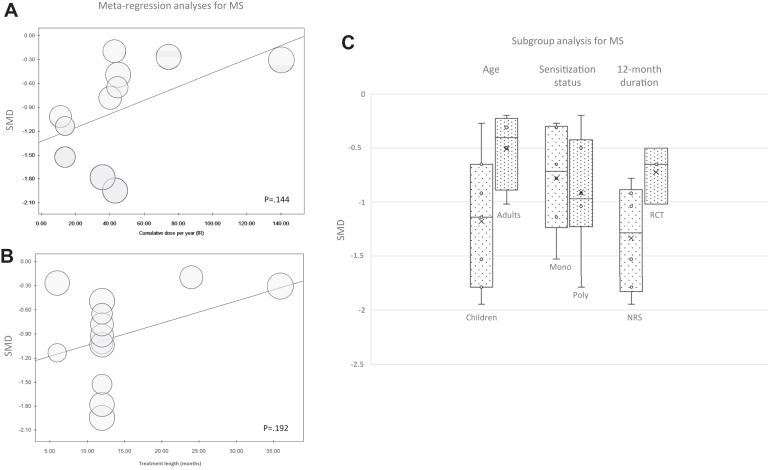
Metaregression did not show statistically significant association between MS and cumulative dose per year or treatment length. Subgroup analyses did not show significant differences between the subgroups (Fig 5).
Fig 5.
Metaregression analyses of MS for efficacy of IR-HDM SLIT liquid depending on cumulative dose administered per year (A) or treatment length (B). (C) Subgroup analysis box plots include middle 50% of data. Horizontal bars inside boxes represent SMD; lines to whiskers extend most extreme data points, which are no more than 1.5 times interquartile range from box.
All the sensitivity analyses (fixed effects model; estimated vs observed data; exclusion studies with small sample size [equal to or below the median: 39 patients]; exclusion of retrospective NRS; exclusion of NRS with consecutive enrollment; exclusion of non-European studies; metaregression by asthma prevalence; metaregression by completion rate) and influential analyses supported the overall findings (see Table E1, Fig E3, and Fig E4 in the Online Repository at www.jaci-global.org).
The quality for all outcomes across the studies was acceptable (low risk of bias) in all but 2 NRS, as in 18 of 20 studies, we reported >70% of “yes” responses (see Table E2 in the Online Repository at www.jaci-global.org). Therefore, the overall certainty of the evidence for the main outcomes (SS, MS) was judged as moderate for both RCT and NRS (see Table E3 in the Online Repository). We also assessed the quality of RCT using a specific tool, RoB2, which showed a low risk in 4 studies and some concerns in the remaining 4 studies (see Table E4 in the Online Repository).
A total of 99 (11.8%) of 839 patients included in NRS and 35 (17.5%) of 200 patients included in RCT reported AEs (see Table E5 in the Online Repository at www.jaci-global.org). Most AEs were local (57.3% of AEs for NRS; 81.3% for RCT). Most of the treatment interruptions were due to reasons other than AE. Discontinuation for AE was reported in 4.2% of the patients included in NRS and in 0.5% of those included in RCT. Discontinuation for any reason was 19.9% in NRS and 12% in RCT.
AEs were not reported in 3 of the NRS. Notably, the overall assessment was strongly influenced by the data of the study of Jung et al,37 which reported several AEs and a rate of discontinuations, including those related to AE, greater than all the other studies (patients with AE: 49 vs 50; discontinuations for any reason: 108 vs 59; discontinuations for AE: 22 vs 13 in the Jung study vs all the remaining NRS, respectively).
Discussion
This systematic review and meta-analysis of over 850 patients with ARC to HDM included in 12 observational studies and 8 RCT provides evidence that IR-HDM SLIT liquid in a real-life setting significantly reduces symptoms and the need of antisymptomatic medications, with an effect size comparable to RCT in studies with the same treatment duration. The findings were irrespective of patients’ age, annualized SLIT dose, asthma comorbidity, and sensitization status, and they were not influenced by study quality, study sample size, or publication bias. For NRS, the evidence of benefit (both SS and MS) was apparent in all the individual studies, whereas for RCT only after meta-analyses of all studies, as some single studies yielded inconclusive results when analyzed in isolation.
RCT provide higher-quality evidence than NRS, but their results may be affected by indirectness—for example, inadequate study length, lack of long-term outcome data, and lack of all relevant populations. This is the reason why not-for-profit academic organizations, such as the Respiratory Effectiveness Group (REG), the Cochrane Collaboration, and the GRADE Working Group are interested in understanding how this gap may be filled by using evidence from NRS, and how this evidence may be used to inform guideline developers.9,46 Thus, NRS, despite their inherent lower quality, cannot be discarded tout court, without any justification, especially when these studies provide information that cannot be addressed in RCT.
A comprehensive review on this issue showed that on average there is little evidence for significant effect estimate differences between observational studies and RCT, regardless of specific observational study design, heterogeneity, or inclusion of studies of pharmacologic interventions.47 Approximately 80% of the reviews analyzed (including 1583 meta-analyses covering 228 unique outcomes) found no significant difference between observational studies and RCT, while the remaining studies suggested that observational studies had larger (in most cases) or smaller effects of interest. This review showed that factors other than study design per se need to be considered when exploring reasons for a lack of agreement between results of RCT and observational studies.
We showed here that treatment benefit (SS) was positively associated with study length, with studies lasting 36 months showing the greatest effect and studies lasting 6 months showing lower effect (Fig 3, B). The subgroup analysis including only RCT and NRS with a treatment length of 12 months showed that their results were comparable (median SMD in NRS, −0.87 vs −0.75 in RCT) (Fig 3, C). This median effect size (difference from baseline to end of treatment) was large in both NRS and RCT according to the Cohen criteria (threshold of large effect, around 0.8 SMD).48 These data suggest that the differences reported in the magnitude of effect in the pooled analysis between NRS and RCT (Fig 2) are unlikely to be due to study quality or design but are likely due to different features of the studies, namely study length. This comparability we showed in the effect sizes for SS between the 2 types of studies at 12 months in a way validates the NRS, which can be considered reliable despite their inherent methodologic defects, as their results did not differ substantially from RCT that are conducted with a rigorous methodology that minimizes the risk of bias. In this light, these findings showing no substantial difference in the estimates between RCT and NRS seem particularly important because they suggest that the lack of a blinded assessment of the treatment effect in NRS, which is considered very important especially when subjective outcomes are evaluated, did not provide different results compared to the results obtained with blinded assessment in RCT.
On the basis of GRADE guidance, inclusion of these NRS results in the evidence synthesis can be considered appropriate because evidence from RCT was of moderate certainty. In this situation, looking at NRS may be informative.9
As for MS, we did not find a correlation between MS reduction and treatment duration like we did for SS, but data on MS were not available in 3 of the 4 studies with a 36-month treatment duration, which precluded us from making this correlation (Fig 5).
Discontinuation rate was low in both NRS (19.9%) and RCT (12%). If for RCT this finding was expected, the discontinuation rate reported in NRS appears to be much lower than that reported in the literature. A study using data from a Dutch community pharmacy database showed that in real life, the rate of SLIT (any marketed SLIT product) discontinuation 2 years after the start of treatment is about 80%, and it increases to about 90% at 3 years.49 This finding was confirmed by other studies.50,51 The low discontinuation rate reported in the studies included in this meta-analysis may suggest a bias resulting from participant selection in the studies or selective reporting. However, all but 3 studies were prospective, and no predictor of response or adherence is available so far, making these types of bias unlikely. Alternatively, we can assume that inclusion of patients in the studies could have altered the normal physician–patient relation in most NRS, increasing treatment persistence or adherence, as in the case of pragmatic trials.52
In any case, the large effect associated with low rate of discontinuation and with long-term treatment confirms that AIT must be continued for a sufficiently long time to achieve greater clinical benefit.
We showed a lack of correlation between annualized cumulative dose of IR-HDM SLIT liquid and the effect on SS and MS (Figs 3 and 5). This may suggest that dose modifications of liquid formulation may be safely done by the patient—for example, to better control AEs, as is commonly done with subcutaneous immunotherapy—without affecting the final effect on the outcomes. This may be an advantage of this formulation over AIT fixed-dose SLIT formulations, as recently shown.53
Furthermore, an analysis by the presence of asthma as comorbidity did not show differences in the AIT effect or in discontinuation due to AEs, suggesting that the treatment is not only effective but also well tolerated in patients with asthma (Fig E3, A).
Limitations and strengths
There are limitations of this meta-analysis owing to imperfections in all single studies: some NRS did not report whether patients were recruited consecutively or not; lack or incomplete reporting of missing data or loss to follow-up (in many NRS); the small sample size of some NRS and RCT; missing MS data in some studies; and missing means and/or SDs in some NRS, which led us to use estimated data for the missing values (although sensitivity analyses showed that these results were consistent with available data). Another limitation may come from the fact that we did not find studies from North and South America where IR-HDM SLIT liquid is not distributed. Nevertheless, the results seem to be generalizable: evidence was obtained from studies on other continents with similar medical practices and clinical trial conduct.
However, our study also has several strengths. The total number of patients is large enough for assessment of treatment effect. The pooled effect is large for NRS, with all the studies lying in the same side of the reference line, making questionable the importance of inconsistency observed in the estimates of individual studies. All studies were conducted with a single product from a single manufacturer and a single specific allergen, thereby limiting heterogeneity due to different product characteristics, such as allergen amount and quality. Finally, the risk of publication bias is low and unlikely to influence the final results.
Therefore, we believe that these results showing a significant clinical benefit of IR-HDM SLIT liquid in NRS, comparable to or even better than RCT, depending on treatment duration, represent a valid finding and increase the generalizability of the results. Furthermore, these results expand the findings of RCT, showing that this product is more effective in patients who continue the treatment for at least 36 months, as recommended. Finally, meta-analyses of available RCT, which show no substantial differences in the effect among different pollens2, 3, 4, 5 and between pollens and HDM,2,54 could suggest that the results provided here are generalizable to other allergens, such as pollens, molds, cat, and dog. Nonetheless, it is important to evaluate allergens extracts specifically; therefore, we are planning a study including all allergens to answer this question by providing empirical evidence.
Conclusion
The present meta-analysis of NRS and RCT enables us to draw conclusions on 2 aspects. First, from a methodologic point of view, our findings underscore the importance of NRS in AIT as source of information to complete and integrate data from RCT and may be used to inform guideline developers. Second, given the substantial evidence available from NRS and RCT, we chose as a model the IR-HDM SLIT liquid. The results of this meta-analysis contribute to reinforce this evidence through the following points: (1) NRS showed that IR-HDM SLIT liquid significantly improves rhinoconjunctivitis symptoms and reduces the use of antiallergic medications compared to baseline; (2) the overall effect in NRS is comparable to RCT with the same treatment duration; (3) prolonging treatment up to 36 months significantly increases the clinical response; and (4) a dose modulation can be performed with SLIT liquid, and this represents a relevant strategy to achieve treatment outcomes at an individual level. Further studies should explore and substantiate this individual-level effect.
Disclosure statement
Supported by Stallergenes Greer, Antony, France.
Disclosure of potential conflict of interest: D. Di Bona reports receipt of fees from Stallergenes Greer. G. W. Canonica reports research grants, lecturing, or advisory board fees from A. Menarini, Anallergo, Allergy Therapeutics, AstraZeneca, Chiesi Farmaceutici, Faes, Firma, Genentech, Guidotti-Malesci, GlaxoSmithKline, Hal Allergy, Innovacaremd, Novartis, OmPharma, RedMaple, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes Greer, Uriach Pharma, Thermo Fisher Scientific, and Valeas. J. Cognet-Sicé and S. Scurati are employees of Stallergenes Greer, Antony, France. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Di Bona D., Plaia A., Leto-Barone M.S., La Piana S., Di Lorenzo G. Efficacy of grass pollen allergen sublingual immunotherapy tablets for seasonal allergic rhinoconjunctivitis: a systematic review and meta-analysis. JAMA Intern Med. 2015;175:1301–1309. doi: 10.1001/jamainternmed.2015.2840. [DOI] [PubMed] [Google Scholar]
- 2.Di Bona D., Plaia A., Leto-Barone M.S., La Piana S., Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol. 2012;130:1097–1107.e2. doi: 10.1016/j.jaci.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Radulovic S., Calderon M.A., Wilson D., Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2010;2010(12):CD002893. doi: 10.1002/14651858.CD002893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderon M.A., Alves B., Jacobson M., Hurwitz B., Sheikh A., Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;2007(1):CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dretzke J., Meadows A., Novielli N., Huissoon A., Fry-Smith A., Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–1366. doi: 10.1016/j.jaci.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Penagos M., Durham S.R. Allergen immunotherapy for long-term tolerance and prevention. J Allergy Clin Immunol. 2022;149:802–811. doi: 10.1016/j.jaci.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Schunemann H.J., Tugwell P., Reeves B.C., Akl E.A., Santesso N., Spencer F.A., et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4:49–62. doi: 10.1002/jrsm.1078. [DOI] [PubMed] [Google Scholar]
- 8.Schwingshackl L., Balduzzi S., Beyerbach J., Bröckelmann N., Werner S.S., Zähringer J., et al. Evaluating agreement between bodies of evidence from randomised controlled trials and cohort studies in nutrition research: meta-epidemiological study. BMJ. 2021;374:n1864. doi: 10.1136/bmj.n1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuello-Garcia C.A., Santesso N., Morgan R.L., Verbeek J., Thayer K., Ansari M.T., et al. GRADE guidance 24 optimizing the integration of randomized and non-randomized studies of interventions in evidence syntheses and health guidelines. J Clin Epidemiol. 2022;142:200–208. doi: 10.1016/j.jclinepi.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Bona D., Paoletti G., Chu D.K., Pepys J., Macchia L., Heffler E., Canonica G.W. Allergen immunotherapy for respiratory allergy: quality appraisal of observational comparative effectiveness studies using the REal Life Evidence AssessmeNt Tool. An EAACI Methodology Committee analysis. Clin Transl Allergy. 2021;11 doi: 10.1002/clt2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Bona D., Carlucci P., Spataro F., Paoletti G., Heffler E., Pulkanen J., et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies on allergen immunotherapy. Clin Exp Allergy. 2023;53:610–625. doi: 10.1111/cea.14311. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumemann H., Brożek J., Guyatt G., Oxman A. GRADE handbook: grading of recommendations assessment, development and evaluation. GRADE Working Group. https://gdt.gradepro.org/app/handbook/handbook.html updated October 2013. Available at:
- 15.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane handbook for systematic reviews of interventions, version 6.3. Cochrane Training, updated February 2022. Available at: https://training.cochrane.org/handbook/archive/v6.3
- 17.Roberts G., Pfaar O., Akdis C.A., Ansotegui I.J., Durham S.R., Gerth van Wijk R., et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Weir C.J., Butcher I., Assi V., Lewis S.C., Murray G.D., Langhorne P., et al. Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: a systematic review. BMC Med Res Methodol. 2018;18:25. doi: 10.1186/s12874-018-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moga C., Guo B., Schopflocher D., Harstall C. Institute of Health Economics; Edmonton (AB, Canada): 2012. Development of a quality appraisal tool for case series studies using a modified Delphi technique. [Google Scholar]
- 21.Guo B, Moga C, Schopflocher D, Harstall C. Validation of a quality assessment checklist for case series studies. In: Better knowledge for better health (Un meilleur savoir pour une meilleure santé): abstracts of the 21st Cochrane Colloquium; September 19-23, 2013; Quebec City, Quebec, Canada. New York: Wiley; 2013.
- 22.Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane handbook for systematic reviews of interventions, version 6.2. Cochrane Training, updated February 2021. Available at: https://training.cochrane.org/handbook/archive/v6.2/chapter-08
- 23.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GRADEpro GDT guideline development tool [software]. McMaster University and Evidence Prime. 2021. https://methods.cochrane.org/gradeing/gradepro-gdt Available at:
- 26.Bahceciler N.N., Arikan C., Taylor A., Akdis M., Blaser K., Barlan I.B., et al. Impact of sublingual immunotherapy on specific antibody levels in asthmatic children allergic to house dust mites. Int Arch Allergy Immunol. 2005;136:287–294. doi: 10.1159/000083956. [DOI] [PubMed] [Google Scholar]
- 27.Cadario G., Ciprandi G., Di Cara G., Fadel R., Incorvaia C., Marcucci F., et al. Comparison between continuous or intermittent schedules of sublingual immunotherapy for house dust mites: effects on compliance, patients satisfaction, quality of life and safety. Int J Immunopathol Pharmacol. 2008;21:471–473. doi: 10.1177/039463200802100229. [DOI] [PubMed] [Google Scholar]
- 28.Ciprandi G., Cadario G., Di Gioacchino G.M., Gangemi S., Gasparini A., Isola S., et al. Sublingual immunotherapy in children with allergic polysensitization. Allergy Asthma Proc. 2010;31:227–231. doi: 10.2500/aap.2010.31.3337. [DOI] [PubMed] [Google Scholar]
- 29.Ferrés J., Justicia J.L., García M.P., Muñoz-Tudurí M., Alvà V. Efficacy of high-dose sublingual immunotherapy in children allergic to house dust mites in real-life clinical practice. Allergol Immunopathol (Madr) 2011;39:122–127. doi: 10.1016/j.aller.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Park I.H., Hong S.M., Lee H.M. Efficacy and safety of sublingual immunotherapy in Asian children. Int J Pediatr Otorhinolaryngol. 2012;76:1761–1766. doi: 10.1016/j.ijporl.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Tosca M., Silvestri M., Accogli A., Rossi G.A., Ciprandi G. Serum-specific IgE and allergen immunotherapy in allergic children. Immunotherapy. 2014;6:29–33. doi: 10.2217/imt.13.145. [DOI] [PubMed] [Google Scholar]
- 32.Cingi C., Bayar Muluk N., Ulusoy S., Acar M., Şirin S., Çobanoğlu B., et al. Efficacy of sublingual immunotherapy for house dust mite allergic rhinitis. Eur Arch Otorhinolaryngol. 2015;272:3341–3346. doi: 10.1007/s00405-014-3444-1. [DOI] [PubMed] [Google Scholar]
- 33.Soh J.Y., Thalayasingam M., Ong S., Loo E.X., Shek L.P., Chao S.S. Sublingual immunotherapy in patients with house dust mite allergic rhinitis: prospective study of clinical outcomes over a two-year period. J Laryngol Otol. 2016;130:272–277. doi: 10.1017/S0022215116000025. [DOI] [PubMed] [Google Scholar]
- 34.Novakova S.M., Staevska M.T., Novakova P.I., Yoncheva M.D., Bratoycheva M.S., Musurlieva N.M., et al. Quality of life improvement after a three-year course of sublingual immunotherapy in patients with house dust mite and grass pollen induced allergic rhinitis: results from real-life. Health Qual Life Outcomes. 2017;15:189. doi: 10.1186/s12955-017-0764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novakova S.M., Novakova P.I., Yakovliev P.H., Staevska M.T., Mateva N.G., Dimcheva T.D., et al. A three-year course of house dust mite sublingual immunotherapy appears effective in controlling the symptoms of allergic rhinitis. Am J Rhinol Allergy. 2018;32:147–152. doi: 10.1177/1945892418764966. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.A., Lee Y.M., Yi K.I., Kim S.D., Mun S.J., Cho K.S. Comparative analysis of sublingual immunotherapy medicines for adherence and clinical outcomes. Eur Arch Otorhinolaryngol. 2020;277:135–140. doi: 10.1007/s00405-019-05656-6. [DOI] [PubMed] [Google Scholar]
- 37.Jung J.H., Kang T.K., Kang I.G., Kim S.T. Comparison of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites in Korea. Ear Nose Throat J. 2021;100(5 suppl):505S. doi: 10.1177/0145561319882593. 12. [DOI] [PubMed] [Google Scholar]
- 38.Mungan D., Misirligil Z., Gürbüz L. Comparison of the efficacy of subcutaneous and sublingual immunotherapy in mite-sensitive patients with rhinitis and asthma—a placebo controlled study. Ann Allergy Asthma Immunol. 1999;82:485–490. doi: 10.1016/S1081-1206(10)62726-3. [DOI] [PubMed] [Google Scholar]
- 39.Guez S., Vatrinet C., Fadel R., André C. House-dust-mite sublingual-swallow immunotherapy (SLIT) in perennial rhinitis: a double-blind, placebo-controlled study. Allergy. 2000;55:369–375. doi: 10.1034/j.1398-9995.2000.00413.x. [DOI] [PubMed] [Google Scholar]
- 40.Bahçeciler N.N., Işik U., Barlan I.B., Başaran M.M. Efficacy of sublingual immunotherapy in children with asthma and rhinitis: a double-blind, placebo-controlled study. Pediatr Pulmonol. 2001;32:49–55. doi: 10.1002/ppul.1088. [DOI] [PubMed] [Google Scholar]
- 41.Tseng S.H., Fu L.S., Nong B.R., Weng J.D., Shyur S.D. Changes in serum specific IgG4 and IgG4/IgE ratio in mite-sensitized Taiwanese children with allergic rhinitis receiving short-term sublingual-swallow immunotherapy: a multicenter, randomized, placebo-controlled trial. Asian Pac J Allergy Immunol. 2008;26:105–112. [PubMed] [Google Scholar]
- 42.O’Hehir R.E., Gardner L.M., de Leon M.P., Hales B.J., Biondo M., Douglass J.A., et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180:936–947. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 43.Aydogan M., Eifan A.O., Keles S., Akkoc T., Nursoy M.A., Bahceciler N.N., et al. Sublingual immunotherapy in children with allergic rhinoconjunctivitis mono-sensitized to house-dust-mites: a double-blind-placebo-controlled randomised trial. Respir Med. 2013;107:1322–1329. doi: 10.1016/j.rmed.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Bozek A., Ignasiak B., Filipowska B., Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013;43:242–248. doi: 10.1111/cea.12039. [DOI] [PubMed] [Google Scholar]
- 45.Potter P.C., Baker S., Fenemore B., Nurse B. Clinical and cytokine responses to house dust mite sublingual immunotherapy. Ann Allergy Asthma Immunol. 2015;114:327–334. doi: 10.1016/j.anai.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Roche N., Reddel H., Martin R., Brusselle G., Papi A., Thomas M., et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(suppl 2):S99–S104. doi: 10.1513/AnnalsATS.201309-300RM. [DOI] [PubMed] [Google Scholar]
- 47.Anglemyer A., Horvath H.T., Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev. 2014;2014(4) doi: 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J. 2nd ed. Lawrence Erlbaum Associates; Hillsdale (NJ): 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 49.Kiel M.A., Röder E., Gerth van Wijk R., Al M.J., Hop W.C.J., Rutten-van Mölken M.P.M.H. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–360. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Egert-Schmidt A.M., Kolbe J.M., Mussler S., Thum-Oltmer S. Patients’ compliance with different administration routes for allergen immunotherapy in Germany. Patient Prefer Adherence. 2014;24:1475–1481. doi: 10.2147/PPA.S70326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senna G., Lombardi C., Canonica G.W., Passalacqua G. How adherent to sublingual immunotherapy prescriptions are patients? The manufacturers’ viewpoint. J Allergy Clin Immunol. 2010;126:668–669. doi: 10.1016/j.jaci.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 52.Ford I., Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 53.Thétis-Soulié M., Hosotte M., Grozelier I., Baillez C., Scurati S., Mercier V. The MaDo real-life study of dose adjustment of allergen immunotherapy liquid formulations in an indication of respiratory allergic disease: reasons, practices, and outcomes. Front Allergy. 2022;3 doi: 10.3389/falgy.2022.971155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.Y., Jang M.J., Kim D.Y., Park S.W., Han D.H. Efficacy of subcutaneous and sublingual immunotherapy for house dust mite allergy: a network meta-analysis–based comparison. J Allergy Clin Immunol Pract. 2021;9:4450–4458.e6. doi: 10.1016/j.jaip.2021.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.