Abstract
This article describes the public health impact of Alzheimer's disease (AD), including prevalence and incidence, mortality and morbidity, use and costs of care and the ramifications of AD for family caregivers, the dementia workforce and society. The Special Report discusses the larger health care system for older adults with cognitive issues, focusing on the role of caregivers and non‐physician health care professionals. An estimated 6.9 million Americans age 65 and older are living with Alzheimer's dementia today. This number could grow to 13.8 million by 2060, barring the development of medical breakthroughs to prevent or cure AD. Official AD death certificates recorded 119,399 deaths from AD in 2021. In 2020 and 2021, when COVID‐19 entered the ranks of the top ten causes of death, Alzheimer's was the seventh‐leading cause of death in the United States. Official counts for more recent years are still being compiled. Alzheimer's remains the fifth‐leading cause of death among Americans age 65 and older. Between 2000 and 2021, deaths from stroke, heart disease and HIV decreased, whereas reported deaths from AD increased more than 140%. More than 11 million family members and other unpaid caregivers provided an estimated 18.4 billion hours of care to people with Alzheimer's or other dementias in 2023. These figures reflect a decline in the number of caregivers compared with a decade earlier, as well as an increase in the amount of care provided by each remaining caregiver. Unpaid dementia caregiving was valued at $346.6 billion in 2023. Its costs, however, extend to unpaid caregivers’ increased risk for emotional distress and negative mental and physical health outcomes. Members of the paid health care and broader community‐based workforce are involved in diagnosing, treating and caring for people with dementia. However, the United States faces growing shortages across different segments of the dementia care workforce due to a combination of factors, including the absolute increase in the number of people living with dementia. Therefore, targeted programs and care delivery models will be needed to attract, better train and effectively deploy health care and community‐based workers to provide dementia care. Average per‐person Medicare payments for services to beneficiaries age 65 and older with AD or other dementias are almost three times as great as payments for beneficiaries without these conditions, and Medicaid payments are more than 22 times as great. Total payments in 2024 for health care, long‐term care and hospice services for people age 65 and older with dementia are estimated to be $360 billion. The Special Report investigates how caregivers of older adults with cognitive issues interact with the health care system and examines the role non‐physician health care professionals play in facilitating clinical care and access to community‐based services and supports. It includes surveys of caregivers and health care workers, focusing on their experiences, challenges, awareness and perceptions of dementia care navigation.
Keywords: Alzheimer's dementia, Alzheimer's disease, Biomarkers, Caregivers, Care navigation, Care navigator, COVID‐19, Dementia, Dementia care navigation, Dementia workforce, Diagnostic criteria, Family caregiver, Health care costs, Health care expenditures, Health care utilization, Long‐term care utilization, Home and community‐based services, Health care professional, Incidence, Long‐term care costs, MCI due to Alzheimer's disease, Medicaid spending, Medicare spending, Mild cognitive impairment, Morbidity, Mortality, Navigator, Prevalence, Primary care physician, Risk factors
1. ABOUT THIS REPORT
2024 Alzheimer's Disease Facts and Figures is a statistical resource for U.S. data related to Alzheimer's disease, the most common cause of dementia. Background and context for interpretation of the data are contained in the Overview. Additional sections address prevalence, mortality and morbidity, caregiving, the dementia care workforce, and the use and costs of health care and services. The Special Report provides a comprehensive look into dementia care navigation, revealing significant insights into the experiences and challenges faced by caregivers and health care workers in navigating the health care system.
The statistics, facts, figures, interpretations and statements made in this report are based on currently available data and information as cited in this report, all of which are subject to revision as new data and information become available.
1.1. Specific information in this report
Specific information in this year's Alzheimer's Disease Facts and Figures includes:
Brain changes that occur with Alzheimer's disease.
Risk factors for Alzheimer's dementia.
Number of Americans with Alzheimer's dementia nationally and for each state.
Lifetime risk for developing Alzheimer's dementia.
Proportion of women and men with Alzheimer's and other dementias.
Number of deaths due to Alzheimer's disease nationally and for each state, and death rates by age.
Number of family caregivers, hours of care provided, and economic value of unpaid care nationally and for each state.
The impact of caregiving on caregivers.
The impact of COVID‐19 on dementia caregiving.
Members of the paid workforce involved in diagnosing, treating, and caring for people with Alzheimer's or other dementias.
Expected home health and personal care aide job growth, 2020‐2030.
National cost of care for individuals with Alzheimer's or other dementias, including costs paid by Medicare and Medicaid and costs paid out of pocket.
Medicare payments for people with dementia compared with people without dementia.
Care navigator services that would be valuable to dementia caregivers.
When possible, specific information about Alzheimer's disease is provided; in other cases, the reference may be a more general one of “Alzheimer's or other dementias.” This report keeps the racial, ethnic and other population identifiers used in source documents when describing findings from specific studies.
2. OVERVIEW
Alzheimer's disease is a type of brain disease, just as coronary artery disease is a type of heart disease. It is caused by damage to nerve cells (neurons) in the brain. The brain's neurons are essential to all human activity, including thinking, talking and walking.
In Alzheimer's disease, the neurons damaged first are those in parts of the brain responsible for memory, language and thinking, which is why the first symptoms tend to be memory, language and thinking problems. Although these symptoms are new to the individual affected, the brain changes that cause them are thought to begin 20 years or more before symptoms start. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 When symptoms become severe enough to interfere with a person's ability to perform everyday tasks, a person is said to have Alzheimer's dementia.
Individuals with mild symptoms often may continue to work, drive and participate in their favorite activities, with occasional help from family members and friends. However, Alzheimer's disease is a progressive disease, meaning it gets worse with time. How quickly it progresses and what abilities are affected vary from person to person. As time passes, more neurons are damaged and more areas of the brain are affected. Increased help from family members, friends and professional caregivers is needed to carry out everyday activities. Eventually, people may need help with activities of daily living. These are activities a person typically performs without assistance, including getting into and out of a bed or chair, bathing, dressing, using the toilet, eating and grooming.
Individuals living with Alzheimer's dementia may develop changes in mood, personality or behavior. One behavior of special concern is wandering. For the person with dementia, wandering is likely an intentional effort to reach a destination. However, they may not be able to retrace their steps and may become lost. Wandering puts individuals at risk of significant injury and death. 9
Eventually, the neuronal damage of Alzheimer's extends to parts of the brain that enable basic bodily functions such as walking and swallowing. Because of mobility limitations, individuals may spend most of their time in a wheelchair or on a bed. This loss of mobility, along with cognitive limitations, means they often require around‐the‐clock care. Ultimately, Alzheimer's disease is fatal, although many people die of other conditions before Alzheimer's becomes fatal. Studies indicate that people age 65 and older survive an average of four to eight years after a diagnosis of Alzheimer's dementia, yet some live as long as 20 years. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Many factors influence how long individuals live after receiving a diagnosis. They include age at diagnosis, how far the disease has progressed at diagnosis, whether the individual has other health conditions such as diabetes or kidney disease that may limit remaining lifespan and complicate care and treatment, and whether the individual has mixed dementia — the brain changes of not only Alzheimer's disease but also another type of dementia.
There is no proven way to prevent Alzheimer's disease, and there is currently no cure. However, because of the large number of people living with Alzheimer's and other dementias worldwide (more than 55 million) 19 and the devastating effect of dementia on individuals, families, communities and health care systems, finding ways to prevent, slow, better manage and cure Alzheimer's and other dementias is a top priority for research centers around the globe.
2.1. Alzheimer's disease or dementia?
Many people wonder what the difference is between Alzheimer's disease and dementia.
Dementia is an overall term for a particular group of symptoms. The characteristic symptoms of dementia are difficulties with memory, language, problem‐solving and other thinking skills that affect a person's ability to perform everyday activities. Changes to the brain cause dementia, and many different brain changes can lead to dementia (see Table 1).
TABLE 1.
Common causes of dementia.*
| Cause | Brain changes | Percentage of dementia cases | Symptoms |
|---|---|---|---|
| Alzheimer's disease | Accumulation of the protein beta amyloid outside neurons and twisted strands of the protein tau inside neurons are hallmarks. They are accompanied by the death of neurons and damage to brain tissue. Inflammation and atrophy of brain tissue are other changes. | Alzheimer's is the most common cause of dementia, accounting for an estimated 60% to 80% of cases. Most individuals also have the brain changes of one or more other causes of dementia. 21 , 22 This is called mixed pathologies and if recognized during life is called mixed dementia. | Difficulty remembering recent conversations, names or events; apathy; and depression are often early symptoms. Communication problems, confusion, poor judgment and behavioral changes may occur next. Difficulty walking, speaking and swallowing are common in the late stages the disease. |
| Cerebrovascular disease | Blood vessels in the brain are damaged and/or bran tissue is injured from not receiving enough blood, oxygen or nutrients. People with these changes who develop dementia symptoms are said to have vascular dementia. | About 5% to 10% of individuals with dementia show evidence of vascular dementia alone. 21 , 22 However, it is more common as a mixed pathology with most people showing the brain changes of cerebrovascular disease and Alzheimer's disease. 21 , 22 | Slowed thoughts or impaired ability to make decisions, plan or organize may be the initial symptoms, but memory may also be affected. People with vascular dementia may become less emotional and have difficulty with motor function, especially slow gait and poor balance. |
| Frontotemporal degeneration (FTD) | Nerve cells in the front and temporal (side) lobes of the brain die and the lobes shrink. Upper layers of the cortex soften. Abnormal amounts or forms of tau or transactive response DNA‐binding protein (TDP‐43) are present. | About 60% of people with FTD are ages 45 to 60. 23 In a systematic review, FTD accounted for about 3% of dementia cases in studies that included people 65 and older and about 10% of dementia cases in studies restricted to those younger than 65. 24 | Typical early symptoms include marked changes in personality and behavior and/or difficulty with producing or comprehending language. Unlike Alzheimer's, memory is typically spared in the early stages of disease. |
| Hippocampal sclerosis (HP) | HS is the shrinkage and hardening of tissue in the hippocampus of the brain. The hippocampus plays a key role informing memories. HS brain changes are often accompanied by accumulation of the misfolded protein TDP‐43. | HS is present in about 3% to 13% of people with dementia. 25 It often occurs with the brain changes of other causes of dementia. An estimated 0.4% to 2% of dementia cases are due to HS alone. 25 | The most pronounced symptom of HS is memory loss, and individuals are often misdiagnosed as having Alzheimer's disease. HS is a common cause of dementia in individuals age 85 or older. |
| Lewy body disease | Lewy bodies are abnormal aggregations (or clumps) of the protein alpha‐synuclein in neurons. When they develop in a part of the brain called the cortex, dementia can result. This is called dementia with Lewy bodies or DLB. | About 5% of older individuals with dementia show evidence of DLB alone, but most people with DLB also have the brain changes of Alzheimer's disease. 26 | Early symptoms include sleep disturbances, well‐formed visual hallucinations and visuospatial impairment. These symptoms may change dramatically throughout the day or from day to day. Problems with motor function (similar to Parkinson's disease) are common. Memory loss may occur at some point in the disease. |
| Mixed pathologies | When an individual shows the brain changes of more than one cause of dementia, "mixed pathologies" are considered the cause. When these pathologies result in dementia symptoms during life, the person is said to have mixed dementia or mixed etiology dementia. | More than 50% of people diagnosed with Alzheimer's dementia who were studied at Alzheimer's Disease Research Centers had mixed dementia. 22 In community‐based studies, the percentage is considerably higher. 21 Mixed dementia is most common in people age 85 or older. 27 , 28 | Symptoms vary depending on the combination of brain changes present. |
| Parkinson's disease (PD) | Clumps of the protein alpha‐synuclein appear in an area deep in the brain called the substantia nigra. These clumps are thought to cause degeneration of the nerve cells that produce the chemical dopamine. 29 As PD progresses, alpha‐synuclein can also accumulate in the cortex. | A systematic review found that 3.6% of dementia cases were due to PD and 24.5% of people with PD developed dementia. 30 | Problems with movement (slowness, rigidity, tremor and changes in gait) are common symptoms of PD. Cognitive symptoms may develop later in the disease, typically years after movement symptoms. |
This table describes the most common causes of dementia. Emerging causes such as limbic predominant age related TDP‐43 encephalopathy (LATE) are under active investigation.
Alzheimer's disease is one cause of dementia. The brain changes of Alzheimer's disease include the excessive accumulation of the protein fragment beta‐amyloid and an abnormal form of the protein tau, as well as damage to and destruction of neurons. The brain changes of Alzheimer's disease are the most common contributor to dementia. Dementia caused by Alzheimer's disease is called Alzheimer's dementia.
2.2. Brain changes of Alzheimer's disease
A healthy adult brain has billions of neurons, each with long, branching extensions. These extensions enable individual neurons to form connections with other neurons. At such connections, called synapses, information flows in tiny bursts of chemicals that are released by one neuron and taken up by another neuron. The brain contains trillions of synapses. They allow signals to travel rapidly through the brain. These signals are the basis of memories, thoughts, sensations, emotions, movements and skills.
Over the years, researchers have identified many changes in the brain that may interfere with chemical signaling and lead to problems with thinking, learning and everyday function that arise as a result of Alzheimer's disease. The accumulation of the protein fragment beta‐amyloid into clumps (called beta‐amyloid plaques) outside neurons and the accumulation of an abnormal form of the protein tau (called tau tangles) inside neurons are two of several brain changes associated with Alzheimer's disease.
Beta‐amyloid and tau have different roles in Alzheimer's. Plaques and smaller accumulations of beta‐amyloid may damage neurons by interfering with neuron‐to‐neuron communication at synapses. Inside neurons, tau tangles block the transportation of nutrients and other molecules essential for the normal function and survival of neurons while harming connections between neurons.
Beta‐amyloid and tau accumulation is followed by damage to and destruction of neurons (called neurodegeneration) and other brain cells. Neurodegeneration, along with beta‐amyloid and tau accumulation, is a key feature of Alzheimer's disease. The presence of toxic beta‐amyloid and tau proteins is believed to activate immune system cells in the brain called microglia. Microglia try to clear the toxic proteins and debris from dead and dying cells. Chronic inflammation may set in when the microglia can't keep up with all that needs to be cleared.
Another brain change associated with Alzheimer's disease is atrophy (decreased brain volume) resulting from neurodegeneration and other factors. While some degree of brain atrophy is common in older age, even in people who are cognitively healthy, atrophy is accelerated in people with Alzheimer's dementia. 20 Normal brain function is further compromised by decreases in the brain's ability to metabolize glucose, its main fuel.
2.2.1. Timing of brain changes
Researchers have gained insight into the timing of these brain changes. Among people with rare genetic mutations that cause Alzheimer's disease for whom long‐term data have been collected, researchers have found that levels of beta‐amyloid significantly increased starting 22 years before symptoms were expected to develop (individuals with these genetic mutations usually develop symptoms at the same or nearly the same age as their parent with Alzheimer's). 5 In another study, abnormal levels of the neurofilament light chain protein, a biomarker of neurodegeneration, were also found to start 22 years before symptoms were expected to develop. 7 A third group of researchers found that levels of different types of tau protein increase when beta‐amyloid clumps together as amyloid plaques, and that levels of these types of tau increase as early as two decades before the characteristic mature tau tangles of Alzheimer's disease appear. 8 Researchers also found that glucose metabolism starts decreasing 18 years before expected symptom onset, and brain atrophy begins 13 years before expected symptom onset. 5
2.2.2. Brain changes as biomarkers
These brain changes are biomarkers of Alzheimer's disease. Biomarkers are biological changes that can be measured to indicate the presence or absence of a disease or the risk of developing a disease. For example, the level of glucose in blood is a biomarker of diabetes, and cholesterol level is a biomarker of cardiovascular disease risk. Great progress has been made in measuring Alzheimer's disease biomarkers. For example, we can now identify abnormal levels of beta‐amyloid and tau in cerebrospinal fluid (CSF, the fluid surrounding the brain), and an imaging technique known as positron emission tomography (PET) can produce pictures showing where beta‐amyloid and tau have accumulated in the brain. In addition, many research groups are working on blood tests for Alzheimer's disease. If these blood tests prove effective they could simplify and greatly speed‐up diagnosis of Alzheimer's.
2.3. Mixed dementia
Many people with dementia have brain changes associated with more than one cause. 21 , 31 , 32 , 33 , 34 , 35 , 36 This is called mixed dementia. Some studies report that the majority of people with the brain changes of Alzheimer's disease also have the brain changes of a second cause of dementia on autopsy. 21 , 22 One autopsy study showed that of 447 older people who were believed to have Alzheimer's dementia when they died, only 3% had the brain changes of Alzheimer's disease alone, while 15% had the brain changes of an entirely different cause of dementia, and 82% had the brain changes of Alzheimer's disease plus at least one other cause of dementia. 21 Studies suggest that mixed dementia is the norm, not just for those diagnosed with Alzheimer's dementia but also for those diagnosed with other types of dementia. 37 , 38
Mixed dementia is especially common at advanced ages. 31 , 39 For example, those age 85 or older are more likely than those younger than 85 to have evidence of two or more causes of dementia. 27 , 28 Having Alzheimer's brain changes plus brain changes of another type of dementia increases one's chances of having dementia symptoms in one's lifetime compared with having Alzheimer's brain changes alone. 21 , 31 Mixed dementia may also account for the wide variety of memory and thinking problems experienced by people living with dementia. It is currently not possible to determine with certainty which symptoms are due to which dementia.
2.4. Alzheimer's disease continuum
The progression of Alzheimer's disease from brain changes that are unnoticeable by the person affected to brain changes that cause problems with memory and thinking, and eventually physical disability, is called the Alzheimer's disease continuum.
On this continuum, there are three broad phases: preclinical Alzheimer's disease, mild cognitive impairment (MCI) due to Alzheimer's disease and dementia due to Alzheimer's disease, also called Alzheimer's dementia (see Figure 1). 40 , 41 , 42 , 43 The Alzheimer's dementia phase is further broken down into mild, moderate and severe dementia.
FIGURE 1.
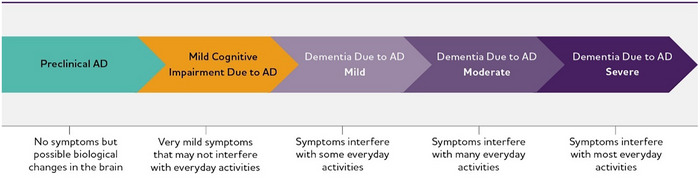
Alzheimer's disease (AD) continuum. Although these arrows are of equal size, the components of the AD continuum are not equal in duration.
While we know the Alzheimer's disease continuum starts with preclinical Alzheimer's disease (no symptoms) and ends with severe Alzheimer's dementia (severe symptoms), how long individuals spend in each part of the continuum varies. The length of each part of the continuum is influenced by age, genetics and other factors. 44
2.4.1. Preclinical Alzheimer's disease
In this phase, individuals may have measurable brain changes that indicate the earliest signs of Alzheimer's disease, but they have not yet developed symptoms such as memory loss or difficulty thinking. Examples of Alzheimer's brain changes that might be detected in this phase include abnormally increased levels and distribution of beta‐amyloid and tau and decreased metabolism of glucose as shown on PET scans, as well as changes in tau protein in cerebrospinal fluid (CSF). 45 , 46 , 47 When the early changes of Alzheimer's disease occur, the brain compensates for them, enabling individuals to continue to function normally.
Although research settings have the tools and expertise to identify some of the early brain changes of Alzheimer's disease, additional research is needed to fine‐tune the tools’ accuracy before they become available for widespread use in hospitals, doctors’ offices and other clinical settings. It is important to note that not all individuals with evidence of Alzheimer's‐related brain changes go on to develop symptoms of MCI or dementia due to Alzheimer's disease before their deaths, even if they live for many years or decades after these biomarkers are detected. 48 , 49 , 50
2.4.2. MCI due to Alzheimer's disease
People with MCI due to Alzheimer's disease have biomarker evidence of Alzheimer's brain changes plus new but subtle symptoms such as problems with memory, language and thinking. These cognitive problems may be noticeable to the individual, family members and friends, but not to others, and they may not interfere with the individual's ability to carry out everyday activities.
Everyone who develops Alzheimer's dementia first experiences MCI, although it might not be recognized or diagnosed because of the subtlety of symptoms. Among those with MCI, about 15% develop dementia after two years. 51 About one‐third develop dementia due to Alzheimer's within five years. 52 However, some individuals with MCI do not have additional cognitive decline or revert to normal cognition. Among population‐based studies, a systematic review and meta‐analysis reported a reversion rate of 26%. 53 Identifying which individuals with MCI are more likely to develop dementia is a major goal of current research.
2.4.3. Dementia due to Alzheimer's disease
Dementia due to Alzheimer's disease is characterized by noticeable memory, language, thinking or behavioral symptoms that impair a person's ability to function in daily life, combined with biomarker evidence of Alzheimer's‐related brain changes. As Alzheimer's disease progresses, individuals commonly experience multiple types of symptoms that change with time. These symptoms reflect the degree of damage to neurons in different parts of the brain. The pace at which symptoms of dementia advance from mild to moderate to severe differs from person to person.
Mild Alzheimer's dementia
In the mild stage of Alzheimer's dementia, most individuals are able to function independently in many areas but are likely to require assistance with some activities to maximize independence and remain safe. They may still be able to drive, work and participate in their favorite activities. They may need more time to complete common daily tasks.
Paying bills and making financial decisions may be especially challenging. The U.S. Social Security Administration notes that people living with dementia are at an especially high risk of becoming victims of fraud and financial abuse. 54 This may be because handling finances is a particularly complex cognitive activity made even harder by declines in executive function. Executive function comprises the higher‐level cognitive skills used to control and coordinate other cognitive abilities and behaviors. 55 Declines in executive function can play out as difficulty planning, organizing and carrying out tasks, as well as poor judgment, socially inappropriate behavior, and inability to understand how one's behavior or choices affect others. 56 Impaired executive function not only makes it challenging for individuals with Alzheimer's dementia to manage finances, but may also make them especially vulnerable to financial abuse and scams because their ability to discern between well‐intentioned and ill‐intentioned behavior and language in others may be diminished.
Moderate Alzheimer's dementia
In the moderate stage of Alzheimer's dementia, which is often the longest stage, individuals experience more problems with memory and language, are more likely to become confused, and find it harder to complete multistep tasks such as bathing and dressing. They may become incontinent at times, begin to have problems recognizing loved ones, and start showing personality and behavioral changes, including suspiciousness and agitation.
Severe Alzheimer's dementia
In the severe stage of Alzheimer's dementia, individuals’ ability to communicate verbally is greatly diminished, and they are likely to require around‐the‐clock care. Because of damage to areas of the brain involved in movement, individuals may be unable to walk. As a result, they may spend most of their time in a wheelchair or on a bed. This loss of mobility increases their vulnerability to physical complications including blood clots, skin infections and sepsis (a condition that triggers body‐wide inflammation that can result in organ failure). Damage to areas of the brain that control swallowing makes it difficult to eat and drink. This can result in individuals swallowing food into the trachea (windpipe) instead of the esophagus (food pipe). As a result, food particles may be deposited in the lungs and cause a type of lung infection called aspiration pneumonia. Aspiration pneumonia is a contributing cause of death among many individuals with Alzheimer's dementia (see Mortality and Morbidity section).
2.5. When dementia‐like symptoms are not dementia
It is important to note that some individuals have dementia‐like symptoms without the progressive brain changes of Alzheimer's or other degenerative brain diseases. Causes of dementia‐like symptoms include depression, untreated sleep apnea, delirium, side effects of medications, Lyme disease, thyroid problems, head injury, blood clots or tumors in the brain, certain vitamin deficiencies and excessive alcohol consumption. Unlike Alzheimer's and other dementias, the dementia caused by these conditions often may be reversed with treatment.
The differences between normal age‐related cognitive changes and the cognitive changes of Alzheimer's disease can be subtle (see Table 2). People experiencing cognitive changes should seek medical help to determine if the changes are normal for their age, are reversible, or may be a symptom of Alzheimer's or another dementia.
TABLE 2.
Signs of Alzheimer's dementia compared with typical age‐related changes.*
| Signs of Alzheimer's dementia | Typical age‐related changes |
|---|---|
| Memory loss that disrupts daily life: One of the most common signs of Alzheimer's dementia, especially in the early stage, is forgetting recently learned information. Others include asking the same questions over and over, and increasingly needing to rely on memory aids (for example, reminder notes or electronic devices) or family members for things that used to be handled on one's own. | Sometimes forgetting names or appointments, but remembering them later. |
| Challenges in planning or solving problems: Some people experience changes in their ability to develop and follow a plan or work with numbers. They may have trouble following a familiar recipe or keeping track of monthly bills. They may have difficulty concentrating and take much longer to do things than they did before. | Making occasional errors when managing finances or household bills. |
| Difficulty completing familiar tasks: People with Alzheimer's often find it hard to complete daily tasks. Sometimes, people have trouble driving to a familiar location, organizing a grocery list or remembering the rules of a favorite game. | Occasionally needing help to use microwave settings or record a television show. |
| Confusion with time or place: People living with Alzheimer's can lose track of dates, seasons and the passage of time. They may have trouble understanding something if it is not happening immediately. Sometimes they forget where they are or how they got there. | Getting confused about the day of the week but figuring it out later. |
| Trouble understanding visual images and spatial relationships: For some people, having vision problems is a sign of Alzheimer's. They may also have problems judging distance and determining color and contrast, causing issues with driving. | Vision changes related to cataracts. |
| New problems with words in speaking or writing: People living with Alzheimer's may have trouble following or joining a conversation. They may stop in the middle of a conversation and have no idea how to continue or they may repeat themselves. They may struggle with vocabulary, have trouble naming a familiar object or use the wrong name (e.g., calling a watch a “hand clock”). | Sometimes having trouble finding the right word. |
| Misplacing things and losing the ability to retrace steps: People living with Alzheimer's may put things in unusual places. They may lose things and be unable to go back over their steps to find them. They may accuse others of stealing, especially as the disease progresses. | Misplacing things from time to time and retracing steps to find them. |
| Decreased or poor judgment: Individuals may experience changes in judgment or decision‐making. For example, they may use poor judgment when dealing with money or pay less attention to grooming or keeping themselves clean. | Making a bad decision or mistake once in a while, such as neglecting to schedule an oil change for a car. |
| Withdrawal from work or social activities: People living with Alzheimer's disease may experience changes in the ability to hold or follow a conversation. As a result, they may withdraw from hobbies, social activities or other engagements. They may have trouble keeping up with a favorite sports team or activity. | Sometimes feeling uninterested in family and social obligations. |
| Changes in mood, personality and behavior: The mood and personalities of people living with Alzheimer's can change. They can become confused, suspicious, depressed, fearful or anxious. They may be easily upset at home, at work, with friends or when out of their comfort zones. | Developing very specific ways of doing things and becoming irritable when a routine is disrupted. |
For more information about the symptoms of Alzheimer's, visit alz.org.
2.6. Treatments
2.6.1. Drug treatments
A total of eight drugs are available for the treatment of Alzheimer's disease. Two of these drugs change the underlying biology of Alzheimer's and slow cognitive and functional decline in some individuals. A third such drug was under review by the FDA for potential approval at press time. Six additional drugs have been approved that treat the symptoms of Alzheimer's dementia.
2.6.1.1. Treatments to Slow Alzheimer's Disease
The drugs aducanumab and lecanemab change the underlying biology of Alzheimer's disease and delay disease progression. They do this by helping remove plaques and a form of beta‐amyloid called protofibrils that plays a role in the development of beta‐amyloid plaques. Earlier this year, the manufacturer of aducanumab announced that the drug was being discontinued. 57 The manufacturer said aducanumab was being discontinued in order for the company “to reprioritize its resources in Alzheimer's disease.” Aducanumab is not being discontinued for reasons related to safety or efficacy. People who are now receiving the drug as part of a clinical trial will continue to have access to aducanumab until May 1, 2024, and aducanumab will continue to be available until November 1, 2024, for people who are now receiving it by prescription.
Focusing on lecanemab, clinical trials of the drug showed moderate slowing of cognitive and functional decline in individuals with mild cognitive impairment (MCI) or mild dementia due to Alzheimer's. 58 Only individuals with MCI or mild dementia due to Alzheimer's and evidence of beta‐amyloid buildup in the brain based on brain imaging or CSF analysis were eligible to participate in clinical trials of lecanemab. Lecanemab is not a cure for Alzheimer's disease and not appropriate for all individuals living with Alzheimer's. Safety and effectiveness have only been established in individuals living with MCI due to Alzheimer's disease and mild dementia due to Alzheimer's disease.
It's important to note that while clinical trials showed statistically significant differences in cognitive outcomes between people randomized to receive lecanemab and those randomized to receive placebo, the benefits of lecanemab in the short term may be imperceptible to those receiving it. Because lecanemab has been approved fairly recently, we don't know its effectiveness over the long term, although extension studies, in which people who volunteer for a clinical trial continue to receive treatment after a trial is completed, are underway.
Anti‐amyloid treatments such as aducanumab and lecanemab can have side effects. They can cause serious allergic reactions as well as amyloid‐related imaging abnormalities (ARIA), infusion‐related reactions, headaches and falls. ARIA is a common side effect that does not usually cause symptoms but can be serious. It is typically a temporary swelling in areas of the brain and usually resolves over time. Some people may also have small spots of bleeding in or on the surface of the brain along with swelling. Most people with swelling who experience ARIA do not have symptoms. Those who do experience symptoms of ARIA may have headache, dizziness, nausea, confusion or vision changes. Management of ARIA may include discontinuation of the medication either temporarily or indefinitely.
Individuals with two copies of the APOE‐e4 gene are at increased risk of developing ARIA. 59 The FDA encourages APOE‐e4 testing before starting treatment with anti‐amyloid medications. Prior to testing, doctors should discuss with patients the risk of ARIA and the implications of genetic testing results.
These are not all the possible side effects. Individuals should talk with their doctors to develop a treatment plan that is right for them, including weighing the benefits and risks of all approved therapies.
Appropriate use recommendations have been developed to guide physicians in determining which individuals should and should not receive treatment with lecanemab. 59 The recommendations also discuss ARIA monitoring and management, key points to share with individuals living with dementia and their care partners, and incorporating treatments into clinical practice.
A variety of other treatments targeting the underlying biology of Alzheimer's disease are being developed. They address many of the known brain changes associated with Alzheimer's disease, including but not limited to tau accumulation, inflammation, altered cell metabolism and oxidative stress (damage from toxic oxygen molecules). 59 , 60 , 61 As of January 1, 2023, 156 clinical trials were underway testing additional disease‐modifying therapies. 62
2.6.1.2. Treatments to Address Cognitive and Behavioral Symptoms
Five of these eight drugs — donepezil, rivastigmine, galantamine, memantine and memantine combined with donepezil — are aimed at treating cognitive symptoms. They do not affect the underlying brain changes that cause Alzheimer's, nor do they slow or stop the course of the disease. With the exception of memantine, they treat symptoms by increasing the amount of chemicals called neurotransmitters in the brain. Neurotransmitters help brain cells communicate with each other. Memantine protects the brain from excessive levels of a neurotransmitter called glutamate, which overstimulates neurons and can damage them. These five drugs may have side effects, such as headaches and nausea. These are not all the possible side effects. As with lecanemab, individuals should talk with their doctors to develop a treatment plan that is right for them, including weighing the benefits and risks of all approved therapies.
One of the eight drugs, brexpiprazole, has been approved by the FDA to treat agitation that can occur in Alzheimer's. Agitation is common in Alzheimer's disease, with 60% of people with MCI and 76% of people with Alzheimer's dementia experiencing agitation. 63 Brexpiprazole is thought to lessen agitation through its effects on dopamine and serotonin receptors in the brain. Brexpiprazole is also FDA‐approved for the treatment of major depressive disorder. It's important to note that brexpiprazole is an atypical antipsychotic drug. Atypical antipsychotics have been associated with an increased risk of stroke and death in older people with dementia‐related psychosis. 64 , 65 Non‐drug interventions should be tried first.
In addition to these eight drugs, the drug suvorexant, approved for insomnia, has been shown in clinical trials to be effective in treating problems with falling asleep and staying asleep that can occur in people with mild to moderate Alzheimer's disease. Suvorexant inhibits the activity of orexin, a type of neurotransmitter involved in the sleep‐wake cycle. Possible side effects include, but are not limited to, impaired alertness and motor coordination (including impaired driving), worsening of depression or suicidal thinking, complex sleep behaviors (such as sleep‐walking and sleep‐driving), sleep paralysis and compromised respiratory function.
Why insomnia and other sleeping problems occur in people living with Alzheimer's is unclear. However, researchers have found that Alzheimer's brain changes disrupt the sleep‐wake cycle, leading to increased sleep fragmentation and wakefulness and decreased slow‐wave sleep. 66 Researchers have also found that sleep abnormalities accelerate the accumulation of beta‐amyloid and release of toxic tau in the brain, increasing the risk of dementia. In this way, sleep problems may be bidirectional, with Alzheimer's disease increasing the risk of sleep disturbances and sleep disturbances increasing the risk of Alzheimer's. 66 , 67 More research is needed to better understand the relationship between sleep abnormalities and Alzheimer's. About one‐quarter of people with dementia have problems sleeping, and the problems can worsen as the disease progresses. 68
As of January 1, 2023, 31 clinical trials were underway testing additional agents to treat Alzheimer's cognitive, behavioral and neuropsychiatric symptoms. 62
2.6.2. Non‐drug treatments
There are also non‐drug treatments for the symptoms of Alzheimer's disease. Non‐drug treatments do not change the underlying biology of the disease. They are often used with the goals of maintaining or improving cognitive function, overall quality of life and engagement, and the ability to perform activities of daily living.
Non‐drug treatments include physical activity, memory and orientation exercises, music‐ and art‐based therapies, and many others. Non‐drug treatments may be used with a more specific goal of reducing behavioral and psychological symptoms such as depression, apathy, wandering, sleep disturbances, agitation and aggression. For example, a review and analysis of nonpharmacologic treatments for agitation and aggression in people with dementia concluded that non‐drug interventions seemed to be more effective than pharmacologic interventions for reducing aggression and agitation. 69
2.7. Actions to proactively manage dementia
Proactive management of Alzheimer's and other dementias can improve the quality of life of affected individuals and their caregivers. Proactive management includes actions by the person living with dementia and their caregivers and actions by health care providers and other members of the health care workforce.
2.7.1. Actions for the person living with dementia and their caregivers
These actions include:
Becoming educated about dementia.
-
Maintaining a sense of self and relationships with others.
Identifying and participating in activities that are meaningful and bring purpose to one's life.
Identifying opportunities to connect with others living with dementia and their caregivers and participating in related activities.
Planning for the future, including future health care needs, changes in employment and financial changes.
2.7.2. Actions for health care providers and other members of the health care workforce
These actions include:
Appropriate use of available treatment options.
Effective management of coexisting conditions.
Coordination of care among health care providers, other health care professionals and lay caregivers.
Directing family caregivers to resources to help them learn how to manage the day‐to‐day needs of the individual living with dementia.
To learn more, see the Caregiving and Workforce sections of this report. Visit alz.org to learn more about Alzheimer's disease, as well as practical information for living with Alzheimer's and being a caregiver.
2.8. Risk factors for Alzheimer's dementia
The vast majority of people who develop Alzheimer's dementia are age 65 or older. This is called late‐onset Alzheimer's dementia. Experts believe that Alzheimer's dementia, like other common chronic diseases and conditions, develops as a result of multiple factors rather than a single cause. Exceptions are cases of Alzheimer's related to trisomy 21 in Down syndrome and rare cases of Alzheimer's disease related to specific genetic mutations.
2.8.1. Age, genetics and family history
The greatest risk factors for Alzheimer's dementia are older age, 70 , 71 genetics — especially the e4 form of the apolipoprotein E (APOE) gene 72 , 73 — and having a family history of Alzheimer's dementia. 74 , 75 , 76 , 77
Age
Age is the greatest of these three risk factors. The percentage of people with Alzheimer's dementia increases dramatically with age. Five percent of people age 65 to 74, 13.2% of people age 75 to 84, and 33.4% of people age 85 or older have Alzheimer's dementia (see Prevalence section). The aging of the population, including the baby‐boom generation, will significantly increase the number of people in the United States with Alzheimer's dementia. 78 However, it is important to note that Alzheimer's dementia is not a normal part of aging, and older age alone is not sufficient to cause Alzheimer's dementia. 79
Genetics
Researchers have found many genes that increase or decrease the risk of Alzheimer's dementia. In fact, in 2022 researchers identified 31 new genes that appear to affect biological processes known to be at play in Alzheimer's disease. 80 Of the many genes that increase risk, APOE‐e4 has the strongest impact on risk of late‐onset Alzheimer's dementia. APOE provides the blueprint for a protein that transports cholesterol in the bloodstream. Everyone inherits one of three forms (alleles) of the APOE gene — e2, e3 or e4 — from each parent, resulting in six possible APOE pairs: e2/e2, e2/e3, e2/e4, e3/e3, e3/e4 and e4/e4.
Having the e4 form of APOE increases one's risk of developing Alzheimer's dementia compared with having the e3 or e2 forms but does not guarantee that an individual will develop Alzheimer's dementia. Having the e2 form may decrease one's risk compared with having the e3 or e4 form. Individuals with the e2 form who develop Alzheimer's dementia generally do so later in life than those without the e2 form. The e3 form is thought to have a neutral effect on Alzheimer's dementia risk.
In general, the risk of developing Alzheimer's dementia increases with inheriting one copy of the e4 form and increases further still with inheriting two copies of the e4 form, compared with inheriting only copies of the e2 or e3 forms. 79 , 80 , 81 For example, based on data from a study in Europe and a study in the United States, of people age 65‐69, the risk of developing dementia by the early to mid‐80s was 5% to 7% among those with no copies of the e4 form, 15% to 16% among those with one copy, and 31% to 40% among those with two copies. 82 In addition, those with the e4 form are more likely to have beta‐amyloid accumulation and Alzheimer's dementia at a younger age than those with the e2 or e3 forms of the APOE gene. 83
A meta‐analysis including 20 published articles describing the frequency of the e4 form among people in the United States who had been diagnosed with Alzheimer's dementia found that 56% had one copy of the APOE‐e4 gene, and 11% had two copies of the APOE‐e4 gene. 84 Another study found that among 1,770 diagnosed individuals from 26 Alzheimer's Disease Research Centers across the United States, 65% had at least one copy of the APOE‐e4 gene. 85
Most of the research to date associating APOE‐e4 with increased risk of Alzheimer's dementia has studied White individuals. Studies of this association in Black and Hispanic populations have had inconsistent results. For example, some have found that having the e4 allele did not increase risk among Black people, 86 , 87 , 88 while other studies have found that it significantly increased risk. 89 , 90 , 91 , 92 In addition, researchers have found differences in the frequency of APOE pairs among racial and ethnic groups. For instance, data show that a higher percentage of African Americans have at least one copy of the e4 allele (see Table 3) than European American and American Indian people. 86 , 87 , 93 , 94 Among individuals of African ancestry who have one copy of e3 and one of e4, those with a particular variant of e3 called R145C are at increased risk of developing Alzheimer's dementia compared with those who do not have this variant. 95 Researchers have also found that another genetic factor, the ATP‐binding cassette transporter (ABCA7) protein, doubles the risk of Alzheimer's dementia in Black people with ABCA7 compared with Black people without ABCA7. 90
TABLE 3.
Percentage of African Americans, European Americans and American Indians with specified APOE pairs.*
| APOE Pair | African Americans | European Americans | American Indians † |
|---|---|---|---|
| e3/e3 | 45.2 | 63.4 | 71.6–73.2 |
| e3/e4 | 28.6 | 21.4 | 22.7–23.9 |
| e3/e2 | 15.1 | 10.2 | 2.6–3.0 |
| e2/e4 | 5.7 | 2.4 | 0.5 |
| e4/e4 | 4.5 | 2.4 | 1.0–1.2 |
| e2/e2 | 0.7 | 0.2 | 0.0–0.1 |
Data for APOE pairs in other populations are not available. Racial and ethnic identifiers reflect the terms used in the source studies.
Percentages do not total 100 due to rounding.
Study provided a percentage for women and a percentage for men.
Percentages represent the range for the two.
To better understand inconsistencies in the effect of APOE‐e4 in Hispanic/Latino groups, one research team analyzed the effect of APOE‐e4 in 4,183 individuals from six Latino backgrounds: Central American, Cuban, Dominican, Mexican, Puerto Rican and South American. 96 They found that the effect of APOE‐e4 on cognitive decline differed among groups, suggesting that factors related to geographic background and genetic ancestry may alter the extent to which APOE‐e4 contributes to cognitive decline.
These inconsistencies point to the need for more research to better understand the genetic mechanisms involved in Alzheimer's risk among different racial and ethnic groups.
Trisomy in Down Syndrome
In Down syndrome, an individual is born with three copies of chromosome 21 (called trisomy 21) instead of two. People with Down syndrome have an increased risk of developing Alzheimer's dementia, and this is believed to be related to trisomy 21. Chromosome 21 includes the gene that encodes for the production of the amyloid precursor protein (APP), which in people with Alzheimer's is cut into beta‐amyloid fragments that accumulate into plaques. Having an extra copy of chromosome 21 may increase the production of beta‐amyloid fragments in the brain.
Overall, people with Down syndrome develop Alzheimer's dementia at an earlier age than people without Down syndrome. By age 40, most people with Down syndrome have significant levels of beta‐amyloid plaques and tau tangles in their brains. 97 According to the National Down Syndrome Society, about 30% of people with Down syndrome who are in their 50s, and about 50% of those in their 60s, have Alzheimer's disease dementia. 98 Emerging research suggests that Alzheimer's brain changes in people with Down syndrome may be even more common than these percentages indicate. 99 , 100
As with all adults, advancing age increases the likelihood that a person with Down syndrome will exhibit symptoms of Alzheimer's dementia. Life expectancy of people with Down syndrome has more than doubled in the last 70 years, which corresponds to a growing population of adults living with both this condition and dementia. Dementia is the leading cause of death for adults with Down syndrome. 101 Care for people with Down syndrome and dementia is especially challenging due to the intellectual, cognitive and communication impairments associated with Down syndrome that are present in addition to the cognitive impairments of dementia. Making advances in the care of people living with Down syndrome and dementia is stymied by the common exclusion of people with Down syndrome from research studies.
Genetic Mutations
An estimated 1% or less of people living with Alzheimer's dementia develop the disease as a result of mutations to any of three specific genes. 102 (A genetic mutation is an abnormal change in the sequence of chemical pairs that make up genes.) This is called dominantly inherited or autosomal dominant Alzheimer's dementia. These mutations involve the amyloid precursor protein gene and the genes for the presenilin 1 and presenilin 2 proteins. Symptoms tend to develop before age 65 and sometimes as young as age 30. Because of this, individuals with these mutations may also be referred to as having younger‐onset Alzheimer's dementia. People who inherit an Alzheimer's mutation to these genes are virtually guaranteed to develop the disease if they live a normal life span. 103 However, rare cases of individuals who have one of these mutations and do not develop dementia symptoms until late life have recently been reported. 104 , 105 The experiences of these individuals highlight the possibility of being resilient to Alzheimer's dementia despite genetic mutations, and point to new areas of investigation to better understand resilience.
Family history
A family history of Alzheimer's dementia is not necessary for an individual to develop Alzheimer's. However, individuals who have or had a parent or sibling (first‐degree relative) with Alzheimer's dementia are more likely to develop Alzheimer's than those who do not have a first‐degree relative with Alzheimer's dementia. 74 , 81 Those who have more than one first‐degree relative with Alzheimer's dementia are at even higher risk. 77 A large, population‐based study found that having a parent with Alzheimer's dementia increases risk independent of known genetic risk factors such as APOE‐e4. 106 When diseases run in families, heredity (genetics) and shared non‐genetic factors (for example, access to healthy foods and habits related to physical activity) may play a role.
2.8.2. Modifiable risk factors
Although age, genetics and family history cannot be changed, some risk factors can be changed or modified to reduce the risk of cognitive decline and dementia. Examples of modifiable risk factors are physical activity, smoking, education, staying socially and mentally active, blood pressure and diet. In fact, The Lancet Commission report on dementia prevention, intervention and care suggests that up to 40% of dementia cases may be attributable to modifiable risk factors. 107 A 2022 study found that nearly 37% of cases of dementia in the United States were associated with eight modifiable risk factors, the most common being midlife obesity, followed by physical inactivity and low educational attainment. 108
In addition to The Lancet Commission report, a number of other influential reports point to the promising role of addressing these factors to reduce risk of dementia and cognitive decline. These include the 2019 World Health Organization (WHO) recommendations to reduce risk of cognitive decline and dementia and a report from the National Academy of Medicine. 109 , 110 There are many potentially modifiable risk factors for Alzheimer's disease — too many to discuss in a single report. This section focuses on some of the modifiable risk factors with substantial supportive evidence identified in The Lancet Commission report, the WHO recommendations and the National Academy of Medicine report.
As mentioned earlier, most people living with dementia have the brain changes of Alzheimer's disease as well as another form of dementia (see mixed dementia), and it can be difficult to tell which brain changes are the cause of dementia. As a result, research linking risk factors to dementia is often assumed to support a link between risk factors and Alzheimer's disease. However, additional research is needed to disentangle risk factors that are specific to Alzheimer's disease from those that are specific to other causes of dementia. 111
Cardiovascular health factors
Brain health is affected by the health of the heart and blood vessels. Although the brain makes up just 2% of body weight, it consumes 20% of the body's oxygen and energy supplies. 112 A healthy heart ensures that enough blood is pumped to the brain, while healthy blood vessels enable the oxygen‐ and nutrient‐rich blood to reach the brain so it can function normally. One of the clearest examples of this relationship is how stroke, which occurs when a blood vessel in the brain is blocked or bursts, markedly increases dementia risk. 113
Many factors that increase the risk of cardiovascular disease are also associated with a higher risk of dementia. 114 These factors include hypertension, 91 , 115 , 116 , 117 diabetes 118 , 119 , 120 and smoking. 121 , 122 Likewise, factors that decrease risk of cardiovascular disease are associated with decreased risk of dementia. Physical activity is an example of a modifiable factor that reduces risk of cardiovascular disease and may also reduce risk of dementia. 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 Although researchers have studied a wide variety of physical activities, they do not know if specific types of physical activity are more effective at decreasing risk, or how the frequency or duration of physical activity may influence the effectiveness of physical activity in reducing risk.
In addition to physical activity, many but not all studies suggest that consuming a heart‐healthy diet may be associated with reduced dementia risk. 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 A heart‐healthy diet emphasizes fruits, vegetables, whole grains, fish, chicken, nuts, legumes and healthy fats such as olive oil while limiting saturated fats, red meat and sugar. Examples of heart‐healthy diets include but are not limited to the Mediterranean, DASH (Dietary Approaches to Stop Hypertension) and MIND (Mediterranean‐DASH Intervention for Neurodegenerative Delay) diets. 143 , 144 , 145 However, a recent trial of the MIND diet did not show a difference in cognitive change for people following the diet compared with a control group over three years. 141 It's possible that dietary changes take many years to influence dementia risk. No single food, beverage, ingredient, vitamin or supplement has been proven to prevent or cure Alzheimer's or any other dementia. 146
The risk of developing dementia in later life can be influenced by health factors present years and decades earlier. For example, midlife obesity, 115 , 147 , 148 hypertension, 91 , 115 , 116 , 117 and high cholesterol 149 are among the midlife factors associated with an increased risk of dementia in later life.
Today, researchers are looking at potential risk factors present even earlier in life, such as in young adulthood, to understand how health factors experienced throughout one's life span may affect later life cognitive health. 150 , 151 , 152 , 153 , 154 This life course approach offers the potential to inform preventive measures across multiple stages of life.
Education
Researchers have long reported that people with more years of formal education are at lower risk for Alzheimer's and other dementias than those with fewer years of formal education. 86 , 155 , 156 , 157 , 158 , 159 , 160 Much of the research linking formal education to decreased risk of Alzheimer's dementia was conducted without the benefit of technological advances such as PET imaging of the brain that might shed light on whether education affects Alzheimer's biomarkers such as beta‐amyloid and tau accumulation that lead to dementia symptoms. More recent research incorporating these technological advances suggests that rather than reducing the risk of developing Alzheimer's brain changes, formal education may help sustain cognitive function in mid‐ and late life and delay the development of symptoms. 161 , 162
To that point, some researchers believe that having more years of education builds “cognitive reserve.” Cognitive reserve refers to the brain's ability to make flexible and efficient use of cognitive networks (networks of neuron‐to‐neuron connections) to enable a person to continue to carry out cognitive tasks despite brain changes. 163 , 164 The number of years of formal education is not the only determinant of cognitive reserve. Having a mentally stimulating job and engaging in other mentally stimulating activities may also help build cognitive reserve. 165 , 166 , 167 , 168 , 169 , 170
Other researchers emphasize the indirect effects of the number of years of formal education, such as its effects on dementia risk through socioeconomic status (SES). SES typically is defined as access to economic resources, including income, education, employment and occupation, but also includes factors such as financial security and perceived social standing. Having fewer years of formal education is associated with lower median income and lower SES. 171 SES has many effects on one's health that are relevant to dementia risk. Researchers report that lower SES is associated with being less physically active, 172 having a higher risk of diabetes, 173 , 174 , 175 and being more likely to have hypertension 176 and to smoke 177 — all of which are risk factors for dementia. In fact, in 2022 researchers reported that SES is associated with changes in brain anatomy, including gray matter volume, that may affect overall cognitive ability. 178
In addition, lower SES may decrease one's access to and ability to afford heart‐healthy foods that support brain health; decrease one's ability to afford health care or medical treatments, such as treatments for cardiovascular risk factors that are closely linked to brain health; and limit one's access to physically safe housing and employment. Housing and employment conditions can also influence brain health–promoting activities and health care, as well as influence one's exposure to substances that are toxic to the nervous system such as air pollution, 179 lead 180 and pesticides. 181
It's important to realize that SES is not a biological entity, but rather a social construct reflecting inequities in how individuals and populations are treated and have been treated over time. It also reflects inequities in the perceived social standing of individuals and populations based on factors largely outside of their control.
Social and cognitive engagement
Additional studies suggest that remaining socially and cognitively active throughout life may support brain health and possibly reduce the risk of Alzheimer's and other dementias. 123 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 Socially and cognitively stimulating activity might help build cognitive reserve. However, it is also possible that undetected cognitive impairment decreases one's interest in and ability to participate in activities involving social and cognitive skills. In this case, the association may reflect the effect of cognitive impairment on social and cognitive engagement rather than the effect of engagement on dementia risk. 188 More research is needed to better understand the mechanisms that link social and cognitive engagement to dementia risk, along with types of activities that provide benefit.
Traumatic Brain Injury (TBI)
TBI is a head injury caused by an external force that results in disruption of normal brain function. 191 TBI is associated with an increased risk of dementia. 192 , 193 , 194
According to the Centers for Disease Control and Prevention (CDC), in 2020, people age 75 and older had the highest numbers and rates of TBI‐related hospitalizations and deaths, accounting for about 32% of TBI‐related hospitalizations and 28% of TBI‐related deaths. 195 In 2018 and 2019, falls were the leading cause of TBI‐related deaths among those 75 and older. 191
Two ways to classify the severity of TBI are by the duration of loss of consciousness or post‐traumatic amnesia 196 and by the individual's initial score on the 15‐point Glasgow Coma Scale. 197
Mild TBI (also known as a concussion) is characterized by loss of consciousness or post‐traumatic amnesia lasting 30 minutes or less, or an initial Glasgow score of 13 to 15; about 75% of TBIs are mild. 198
Moderate TBI is characterized by loss of consciousness or post‐traumatic amnesia lasting more than 30 minutes but less than 24 hours, or an initial Glasgow score of 9 to 12.
Severe TBI is characterized by loss of consciousness or post‐traumatic amnesia lasting 24 hours or more, or an initial Glasgow score of 8 or less.
Moderate and severe TBIs increase risk of dementia between 2‐ and 4‐fold compared with risk among individuals without a history of moderate or severe TBI. 199 In this case, the cause of dementia is almost always brain damage attributable to the TBI, not Alzheimer's disease. The risk of dementia increases with the number of TBIs sustained. 192 , 193 , 194 In addition, studies have found that people with a history of TBI who develop dementia do so at a younger age than those without a history of TBI. 200 , 201 Whether TBI causes dementia, other conditions that lead to dementia, or both, is being investigated.
The relationship between TBI and chronic traumatic encephalopathy (CTE) is a growing area of research. Like Alzheimer's disease, CTE is characterized by tangles of an abnormal form of the protein tau in the brain. Beta‐amyloid plaques may also be present, with one study indicating that more than 50% of individuals with CTE had beta‐amyloid plaques. 202 , 203 The brain changes of CTE can only be identified at autopsy. The greatest risk factor for developing CTE‐related brain changes is repetitive brain trauma — repeated, forceful blows to the head that do not, individually, result in symptoms. 204 Among former amateur and professional football players, the odds of developing CTE increased 30% per year played. 205 A review of published articles examining CTE suggests that the relationship between these repeated impacts and CTE is likely causal. 206
Other risk factors
As mentioned earlier, there are many potentially modifiable risk factors for dementia. Among those with growing supportive evidence are the following.
Sleep
Among the many factors being studied is inadequate sleep or poor sleep quality. 207 , 208 , 209 Researchers have found that an important function of sleep is the removal of toxic beta‐amyloid and other substances from the brain. 210 , 211 Inadequate or poor sleep may compromise the brain's ability to remove beta‐amyloid and other toxins, enabling levels of toxins to remain elevated. In addition, poor sleep quality such as that caused by obstructive sleep apnea may increase risk by interfering with blood flow to the brain and normal patterns of brain activity that promote memory and attention. 212 , 213 As discussed earlier, many researchers believe that the relationship between sleep and Alzheimer's disease is bidirectional, meaning that not only may poor sleep increase one's risk of Alzheimer's, but also that the brain changes of Alzheimer's may increase the risk of poor sleep. 214 , 215 , 216 For example, increases in beta‐amyloid and tau may interrupt the sleep‐wake cycle, 217 leading to increased sleep fragmentation and wakefulness and decreased slow‐wave sleep. 63 Poor sleep may have similar bidirectional relationships with other causes of dementia, including poor cerebrovascular health. 218
Air Pollution
There is also rapidly emerging evidence on how exposure to toxicants in the environment, especially air pollution, may be related to dementia risk. A number of different air pollutants have been studied in relation to cognition, cognitive decline and dementia itself. The most consistent and rigorous results concern fine particulate matter (PM) air pollution. PM consists of tiny solid particles and liquid droplets generated by fuel combustion, fires and processes that produce dust. PM2.5, particulate matter that is 2.5 microns in diameter or smaller, is small enough to be inhaled deeply into the lungs. This subset of PM particles has been shown to have the greatest health impact and is the focus in most studies. Based on its sweeping review in 2019, the U.S. Environmental Protection Agency judged long‐term exposure to PM2.5 as “likely to be causal” in relation to “nervous system effects.” 219 Studies specific to dementia and related outcomes have reported that higher long‐term exposure to PM2.5 is associated with worse cognitive decline, 179 , 220 reduced brain volumes 179 and increased rates of incident (newly onset) dementia. 220 , 221 , 222 Whether air pollution promotes the brain changes of Alzheimer's or other dementias is unclear.
Critical illness in older adults
A growing body of evidence suggests that critical illness and medical encounters such as hospitalization in older people increase their risk of long‐term cognitive impairment and dementia. 223 , 224 , 225 , 226 , 227 There are a number of ways that critical illness and aspects of the hospital experience may affect the brain. 228 One example is that experiencing hospitalization may make older adults more vulnerable to the existing brain changes of dementia. 229 This is not to suggest that hospitalization should be avoided if one is ill; rather, researchers are focusing on specific aspects of hospitalization, such as prolonged sedation, immobilization, and lack of family engagement that may increase risk of cognitive impairment. 228 Furthermore, experiencing delirium — a sudden and transient state of confusion common in hospitalized older adults — has been linked to long‐term cognitive decline and dementia. 228 , 230 Modifying these aspects of hospitalization may decrease risk of cognitive decline. In addition, better preventive health measures and improved and expanded health care coordination may help to prevent critical illness and subsequent hospitalization and the negative cognitive outcomes that may follow.
Additional research is needed to build the evidence for these and other risk factors being investigated and, importantly, to determine how such risk factors may vary for different causes of dementia, across the lifecourse, and among different racial and ethnic groups.
2.9. Looking to the future
2.9.1. Importance of biomarkers
The discovery that Alzheimer's disease begins 20 years or more before the onset of symptoms suggests that there is a substantial window of time in which we may be able to intervene in the progression of the disease. Scientific advances are already helping the field to make progress in these presymptomatic years. For example, advances in the identification of biomarkers for Alzheimer's disease make it possible to identify individuals who may qualify for clinical trials of treatments to return those biomarkers to normal levels and in doing so prevent or delay the onset of symptoms. Biomarkers also enable earlier detection of the brain changes of Alzheimer's disease, giving those affected the opportunity to address modifiable risk factors that may slow or delay cognitive decline. Biomarkers are already accelerating the development of new treatments by making it possible for clinical trials to specifically recruit individuals with the brain changes that experimental therapies target. In addition, biomarker, basic science and other research advances offer the potential to expand the field's understanding of which therapies or combination of therapies may be most effective at which points in the Alzheimer's disease continuum.
When validated biomarker tests become available for routine use in health care providers’ offices and other clinical settings, it will be important to provide educational materials to help individuals and their families understand the risks and benefits of biomarker tests, make informed decisions about whether to have biomarker testing, and know what to expect in care after testing. 231 , 232 On a broader scale, biomarker disclosure may have social and societal implications. For example, biomarker results that are positive for increased dementia risk and that are shared with others may result in individuals experiencing the social stigma and discrimination so often experienced by people living with dementia, even though individuals with increased risk may never develop dementia. 233 In addition, disclosure may well highlight the need for reform in societal areas such as health insurance coverage and costs, the capacity of the health care workforce, and health equity. 233
2.9.2. The need for increased diversity in research participation
A fuller understanding of Alzheimer's — from its causes to how to prevent, manage and treat it — depends on crucial factors outside of biomarker, basic science and other research advances. Among these is the inclusion of participants from diverse racial and ethnic groups in research. The lack of inclusion has several consequences. First, without adequate data from diverse racial and ethnic groups, the current and future burden of Alzheimer's disease and Alzheimer's dementia in the United States cannot be accurately measured. 234 Such data is necessary because the populations of older adults from these groups make up nearly a quarter of the nation's older adult population, and that share is projected to grow. 235 Second, current data indicate that, compared with non‐Hispanic White older adults, Black and Hispanic older adults are at increased risk for Alzheimer's dementia (see Prevalence section). Alzheimer's research with too few Black and Hispanic participants to reflect the proportion of these groups in the overall population largely ignores populations who bear the greatest risk. As a result, risk factors common in these populations but less common in non‐Hispanic White older adults are likely to be poorly understood. In addition, lack of inclusion limits our ability to understand whether and how dementia risk factors and interventions work in populations that carry different baseline susceptibility to Alzheimer's disease including those with Down syndrome.
Inclusion is more than a matter of enrolling more participants from underrepresented groups. Increasing diversity among researchers and engaging with and seeking input from marginalized communities are also important. Improving inclusion in all of these ways expands the range of lived experiences among participants and the extent to which those experiences are known and become topics of investigation. 236 Only by improving representation in the participation and leadership of clinical trials, observational studies and other investigations will everyone have the potential to benefit from advances in dementia research.
3. PREVALENCE
Millions of Americans are living with Alzheimer's or other dementias. As the size of the U.S. population age 65 and older continues to grow, so too will the number and proportion of Americans with Alzheimer's or other dementias.
This section reports on the number and proportion of people with Alzheimer's dementia to describe the magnitude of the burden of Alzheimer's dementia on communities, health care systems and social safety nets. The prevalence of Alzheimer's dementia refers to the number and proportion of people in a population who have Alzheimer's dementia at a given point in time. Incidence refers to the number or rate of new cases per year, often expressed as the number of people per 100,000 who newly develop the condition in a year. This section reports estimates from several studies of the number of people and proportion of the population with Alzheimer's or other dementias. Those estimates vary depending on how each study was conducted.
The number and proportion of Americans with Alzheimer's or other dementias is expected to continue to grow in coming years because the risk of dementia increases with advancing age. The population of Americans age 65 and older is projected to grow from 58 million in 2022 to 82 million by 2050. 237 By 2030, all members of the of the baby‐boom generation (Americans born between 1946 and 1964) will be age 65 or older, 238 the age range of greatest risk of Alzheimer's dementia; 239 in fact, the oldest members of the baby‐boom generation turned age 75 in 2021. A number of recent studies have reported that the incidence rate of Alzheimer's and other dementias appears to have declined in recent decades (see “Trends in the Prevalence and Incidence of Alzheimer's Dementia Over Time” in this section). This decline in incidence has been attributed to improvements over the 20th century in modifiable risk factors for dementia, such as increased prevention and treatment of hypertension and greater educational attainment. 240 It is unknown how COVID‐19, including infection with SARS‐CoV‐2 (the virus that causes COVID‐19), mortality from COVID‐19, and changes in health care access resulting from the COVID‐19 pandemic will influence the number and proportion of people in the U.S. with Alzheimer's dementia in years to come. However, even with this potentially lower incidence rate and the impact of COVID on people at risk of dementia, the absolute number of people with Alzheimer's and other dementias is expected to continue growing because of the large increase in the number of adults age 65 and older, the age group that is at increased risk of Alzheimer's and many other dementias. 241
3.1. Prevalence of Alzheimer's and other dementias in the United States
An estimated 6.9 million Americans age 65 and older are living with Alzheimer's dementia in 2024.A2 , 241 Seventy‐four percent are age 75 or older (Figure 2). 241
FIGURE 2.
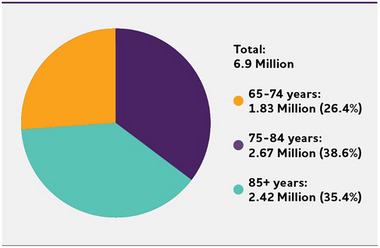
Number and ages of people 65 or older with Alzheimer's dementia, 2024. Percentages do not total 100 due to rounding. Created from data from Rajan et al.A2 , 241
Of the total U.S. population:
About 1 in 9 people (10.9%) age 65 and older has Alzheimer's dementia.A2 , 241
The percentage of people with Alzheimer's dementia increases with age: 5.0% of people age 65 to 74, 13.2% of people age 75 to 84, and 33.4% of people age 85 and older have Alzheimer's dementia.A2 , 241 People younger than 65 can also develop Alzheimer's dementia. Although prevalence studies of younger‐onset dementia in the U.S. are limited, researchers believe about 110 of every 100,000 people age 30 to 64 years, or about 200,000 Americans in total, have younger‐onset dementia. 242
The estimated number of people age 65 and older with Alzheimer's dementia comes from an updated study using the latest data from the 2024 population projections from the U.S. Census Bureau and the Chicago Health and Aging Project (CHAP), a population‐based study of chronic health conditions of older people. 241
Whereas CHAP generated estimates specific to Alzheimer's dementia, national estimates of the prevalence of all dementias combined are available from other population‐based studies, including the Health and Retirement Study (HRS), a nationally representative sample of older adults. Based on newly available estimates from HRS's Harmonized Cognitive Assessment Protocol (HCAP), 10% of people age 65 and older in the U.S. had dementia in 2016.A3 , 160
3.2. Prevalence estimates
The prevalence numbers included in this report are based on estimates of how many people in the U.S. are living with Alzheimer's dementia; that is, the number of people living with the clinical symptoms described in the “Dementia Due to Alzheimer's Disease” (mild, moderate, or severe) portion of the “Alzheimer's Disease Continuum” described in the Overview.
The estimate of 6.9 million older adults who have Alzheimer's dementia comes from a single longitudinal study in which participants were systematically evaluated and then re‐evaluated on a regular basis; those who exhibited the clinical symptoms of Alzheimer's dementia were classified as having Alzheimer's dementia.A2 , 241 A major advantage of this approach is that it attempts to capture all individuals living with the condition and does not rely on the diagnosis of people living with Alzheimer's by the health care system, a process that has resulted in a substantial undercount (i.e., “underdiagnosis”) of the Alzheimer's population. The disadvantage is that the longitudinal study is located in a single, small geographic area and may not be nationally representative (although the estimation process attempted to account for the demographics of the entire U.S. population). In the future, Facts and Figures could report estimates of Alzheimer's dementia prevalence from multiple longitudinal studies or using different symptom‐based diagnostic criteria; these differences in criteria could result in different prevalence estimates from what we report here.A3 , 160
Almost all existing Alzheimer's dementia prevalence studies are based on the identification of clinical symptoms to classify an individual as having Alzheimer's dementia; they do not rely on the brain changes believed to be responsible for Alzheimer's disease across the continuum of the disease. As data sources, methods and scientific knowledge improve, estimates of prevalence may incorporate these brain changes using biomarkers. This addition could lead to very different prevalence estimates for a number of reasons, which are discussed below.
3.2.1. Prevalence estimates of dementia due to Alzheimer's disease based on biomarkers and dementia symptoms
Prevalence estimates of dementia due to Alzheimer's disease based on Alzheimer's brain changes, as well as overt clinical dementia symptoms, are likely to be lower than the 6.9 million figure reported here. This is because autopsy‐ and biomarker‐based studies 21 , 79 , 243 , 244 , 245 indicate that some individuals counted as having Alzheimer's dementia based on symptoms do not have the biological brain changes defined as Alzheimer's disease; that is, their dementia is caused by something other than Alzheimer's disease. Both autopsy studies and clinical trials have found that 15% to 30% of individuals who meet the criteria for clinical Alzheimer's dementia based on symptoms did not have Alzheimer's‐related brain changes. Thus, these studies indicate that estimates using biomarkers of Alzheimer's disease could be up to 30% lower than prevalence estimates based only on symptoms. This would translate to roughly 4.8 million Americans age 65 and older being classified as having dementia due to Alzheimer's disease in 2024.A3 , 160
3.2.2. Prevalence estimates of MCI due to Alzheimer's disease based on biomarkers and mild cognitive symptoms
For decades it has been recognized that all individuals with dementia pass through a precursor stage frequently referred to as mild cognitive impairment (MCI; see Overview). With the recent advent of biomarkers that detect the brain changes believed to characterize Alzheimer's disease, it may now be possible to determine which individuals diagnosed with MCI have MCI due to Alzheimer's disease. The number and proportion of older adults who have MCI due to Alzheimer's disease are currently difficult to estimate because they require studies with both population‐based prevalence measures of MCI and tests of Alzheimer's biomarkers, and this line of research is in its infancy. Furthermore, there is variation across studies in both the threshold of cognitive impairment required for an MCI diagnosis and the level of biomarker burden that defines the presence of Alzheimer's disease. However, we can roughly estimate this prevalence indirectly using multiple data sources. A systematic review of more than 30 studies of all‐cause MCI reported that about 17% of people age 65 and older had MCI. 51 The HRS HCAP study more recently estimated the prevalence of MCI in people age 65 and older to be 22%. 160 Meanwhile, studies assessing biomarkers for Alzheimer's disease with PET scans have reported that about half of people with MCI have Alzheimer's‐related brain changes. 246 , 247 Therefore, roughly 8% to 11% of the 63 million Americans who are age 65 and older in 2024 — or approximately 5 to 7 million older Americans — may have MCI due to Alzheimer's disease. 248 This broad prevalence estimate needs to be refined with population‐based studies involving biomarkers and more precise estimates from narrower age ranges.
3.2.3. Prevalence estimates of Alzheimer's disease based on biomarkers and any cognitive symptoms (MCI or dementia)
Combining the estimates of the prevalence of dementia due to Alzheimer's disease and the prevalence of MCI due to Alzheimer's disease provides an estimate of people living with the brain changes of Alzheimer's disease and some form of cognitive impairment. This estimate would include older adults with the earliest detectable stages of cognitive impairment who have the brain changes of Alzheimer's but may or may not have the overt symptoms of dementia that interfere with their ability to carry out everyday activities. Combining the estimates of roughly 4.8 million Americans age 65 and older with dementia due to Alzheimer's disease based on Alzheimer's brain changes and the 5 to 7 million older Americans with MCI due to Alzheimer's disease translates to approximately 10 to 12 million older Americans with Alzheimer's disease and some form of cognitive impairment in 2024. Furthermore, because MCI develops years before dementia onset and can affect individuals younger than 65, there are likely more than 5 to 7 million people of any age with MCI due to Alzheimer's disease, and thus the 10 to 12 million estimate could be even higher if we consider Americans of all ages, not just those 65 or older.
3.2.4. Prevalence estimates of Alzheimer's disease across the entire cognitive spectrum
Finally, as measurements of the brain changes of Alzheimer's disease become more widely available in research, we will be able to estimate how many people have Alzheimer's disease regardless of the presence or absence of dementia or any form of cognitive impairment. The total number of people living with the brain changes of Alzheimer's disease is likely to be much larger than the number with MCI or dementia due to Alzheimer's disease given that there is an incipient and silent (i.e., “preclinical”) stage of Alzheimer's disease before the emergence of cognitive symptoms of either MCI or dementia (see Overview). 249 While this is still the subject of ongoing research, estimates are emerging of the prevalence of preclinical Alzheimer's disease in the population. 250 , 251 More research is needed to validate preclinical Alzheimer's and determine how to measure it with biomarkers that conclusively represent Alzheimer's disease, as opposed to other dementia‐causing diseases. We also need to further understand if this preclinical stage is a valid representation of people who may go on to develop dementia due to Alzheimer's disease. When a conclusive connection is shown between biomarkers and the preclinical stage, and when epidemiological studies include biomarker‐based diagnoses, it will be possible to estimate the number of individuals throughout the entire continuum of Alzheimer's disease (i.e., those with biomarker‐confirmed Alzheimer's dementia, those with biomarker‐confirmed MCI due to Alzheimer's disease and those with biomarker‐confirmed preclinical Alzheimer's disease). The resulting estimated prevalence will be even higher than any estimates presented in the current report.
3.2.5. Future Facts and Figures prevalence estimates
What does all this mean for future prevalence estimates? Future Facts and Figures reports will continue to include the estimated prevalence of individuals in the Alzheimer's dementia stage, defined according to clinical symptoms, currently estimated at 6.9 million Americans, in addition to the best available estimated prevalence of MCI due to Alzheimer's disease. Accurate, up‐to‐date estimates of the number of people living with these conditions will remain essential to understanding the demands on affected families, health systems, social and health safety nets, and, of course, the people living with these conditions. When biomarker‐based prevalence estimates become available, Facts and Figures will also report the estimated prevalence of individuals with any clinical cognitive impairment and Alzheimer's disease to reflect both those in the dementia phase and those in the MCI phase of Alzheimer's. Facts and Figures will not include prevalence estimates of the preclinical Alzheimer's disease stage until (1) there is convincing evidence of a connection between biomarkers in this silent stage and the development of MCI due to Alzheimer's disease and (2) epidemiologic studies have estimated the number of individuals in this stage. In addition, as the evidence and epidemiological data warrant, future reports may also include estimates of the prevalence of dementia from all causes. It should be noted that both symptom‐based prevalence estimates of Alzheimer's dementia and biomarker‐based prevalence estimates of Alzheimer's disease are expected to increase in the future due to growth in the population of Americans age 65 and older, the group most at risk for developing cognitive symptoms.
3.2.6. Underdiagnosis of Alzheimer's and other dementias in the primary care setting
Prevalence studies such as CHAP and HRS are designed so that everyone in the study undergoes evaluation for dementia. But outside of research settings, a substantial portion of those who would meet the diagnostic criteria for Alzheimer's and other dementias are not diagnosed with dementia by a physician. 252 , 253 , 254 , 255 , 256 , 257 , 258 , 259 , 260 , 261 Furthermore, only about half of Medicare beneficiaries who have a diagnosis of Alzheimer's or another dementia in their Medicare billing records report being told of the diagnosis. 262 , 263 , 264 , 265 , 266 Because dementia is often underdiagnosed — and if it is diagnosed by a clinician, some people appear to be unaware of their diagnosis — a large portion of Americans with dementia may not know they have it. 267 Some studies indicate that underdiagnosis is higher in Black and Hispanic older adults. 260 , 261 , 268 A number of potential harms may result from a missed or delayed dementia diagnosis. These include delayed access to treatment, less time for care planning, higher costs of care, and negative impact on the individual's physical or mental health or even the mental health of their family members and potential caregivers; more research is needed to better understand the potential harms of delayed or lack of diagnosis. 269 Underdiagnosis is most pronounced at the earliest stages of dementia when symptoms are mild. 268 Even fewer people living with mild cognitive impairment (MCI), a precursor to dementia (see Overview), receive a diagnosis despite this being a stage where treatment and planning may be most effective. 270 One recent study estimates that only 8% of older Americans living with MCI receive a diagnosis. 271
3.2.7. Prevalence of subjective cognitive decline
Subjective cognitive decline refers to an individual's perception that their memory or other thinking abilities are worsening, independent of cognitive testing or a physician's diagnosis. Subjective cognitive decline is one of the earliest warning signs of dementia and may be a way to identify people who are at high risk of developing Alzheimer's or other dementias, as well as MCI. 272 , 273 , 274 , 275 , 276 Not all those who experience subjective cognitive decline go on to develop MCI or dementia, but many do. 277 , 278 , 279 Subjective cognitive decline often prompts medical attention, and a formal diagnosis can help distinguish experiences potentially related to higher dementia risk from experiences less likely to be related, such as other underlying health conditions. 280 One study showed those who consistently reported subjective cognitive decline that they found worrisome were at higher risk for developing Alzheimer's dementia. 281 The Behavioral Risk Factor Surveillance System survey, a large cross‐sectional, telephone‐based survey of community‐dwelling people across the U.S. that includes questions on subjective cognitive decline, found that 10% of Americans age 45 and older reported subjective cognitive decline, but 54% of those who reported it had not consulted a health care professional. 282 Individuals concerned about declines in memory and other cognitive abilities should consult a health care professional.
3.3. Estimates of the prevalence of Alzheimer's dementia by state and county
Recently, an analysis was conducted using the same data sources that generated the national prevalence estimate in this report that provides estimates of the prevalence of Alzheimer's dementia by state and, for the first time, by county. 283 As shown in Figure 3, states and counties in the eastern and southeastern U.S. have the highest prevalence of Alzheimer's dementia; eight of the 10 counties (with at least 10,000 older adults) with the highest prevalence are in the East and Southeast. In these regions, older people and Black and Hispanic residents — groups that are at higher risk of Alzheimer's dementia — comprise larger percentages of the population (see Racial and Ethnic Differences in the Prevalence of Alzheimer's and Other Dementias). Table 4 displays the prevalence (both number and percentage) of Alzheimer's dementia for each state. Understanding these regional differences can help guide the allocation of resources to public health programs for Alzheimer's in the U.S.
FIGURE 3.
Prevalence of Alzheimer's disease in the 50 U.S. states, including the 10 counties with the highest prevalence, 2020. Created from data from Dhana et al. 283
TABLE 4.
Estimated prevalence (number and percentage) of Alzheimer's dementia in the 50 U.S. states and District of Columbia among adults age 65 years and older in 2020.
| State | Number (in thousands) | % |
|---|---|---|
| Alabama | 103.6 | 11.8 |
| Alaska | 8.4 | 8.8 |
| Arizona | 151.5 | 11 |
| Arkansas | 60.4 | 11.3 |
| California | 719.7 | 12 |
| Colorado | 90.8 | 10.4 |
| Connecticut | 76.8 | 11.9 |
| Delaware | 22.3 | 11.3 |
| District of Columbia | 15.1 | 16.8 |
| Florida | 579.9 | 12.5 |
| Georgia | 188.3 | 12 |
| Hawaii | 31.2 | 11.3 |
| Idaho | 29.9 | 9.8 |
| Illinois | 250.6 | 12 |
| Iowa | 62.1 | 11 |
| Indiana | 121.3 | 10.9 |
| Kansas | 54.5 | 11.2 |
| Kentucky | 80.5 | 10.4 |
| Louisiana | 94.7 | 12.4 |
| Maine | 29.6 | 10.1 |
| Maryland | 127.2 | 12.9 |
| Massachusetts | 135.2 | 11.3 |
| Michigan | 202.8 | 11.2 |
| Minnesota | 101.9 | 10.7 |
| Mississippi | 62.5 | 12.5 |
| Missouri | 122.3 | 11.2 |
| Montana | 21 | 9.8 |
| Nebraska | 35.1 | 11 |
| Nevada | 54.9 | 10.6 |
| New Hampshire | 26.5 | 10.1 |
| New Jersey | 185.3 | 12.3 |
| New Mexico | 46 | 11.8 |
| New York | 426.5 | 12.7 |
| North Carolina | 210.5 | 11.6 |
| North Dakota | 13.7 | 11.1 |
| Ohio | 236.2 | 11.3 |
| Oklahoma | 70.5 | 10.8 |
| Oregon | 79.1 | 10 |
| Pennsylvania | 282.1 | 11.5 |
| Rhode Island | 22 | 11.4 |
| South Carolina | 112.5 | 11.5 |
| South Dakota | 16.5 | 10.5 |
| Tennessee | 129.2 | 10.9 |
| Texas | 459.3 | 11.9 |
| Utah | 38.3 | 10 |
| Vermont | 12.8 | 9.9 |
| Virginia | 164 | 11.7 |
| Washington | 126.7 | 10.2 |
| West Virginia | 38.1 | 10.2 |
| Wisconsin | 110.9 | 10.6 |
| Wyoming | 10.3 | 9.9 |
Created from data from Dhana et al. 283
3.4. Incidence of Alzheimer's dementia
While prevalence refers to existing cases of a disease in a population at a given time, incidence refers to new cases of a disease that develop in a given period in a defined population — for example, the number of people who develop Alzheimer's dementia during 2024 among U.S. adults who are age 65 or older. Incidence provides a measure of risk for developing a disease. According to estimates using data from the CHAP study and the U.S. Census Bureau, approximately 910,000 people age 65 or older developed Alzheimer's dementia in the U.S. in 2011, a number that would be expected to be even higher in 2024 if CHAP estimates were available for that year. 284 The rate at which new cases of Alzheimer's develop increases dramatically with age: according to estimates from CHAP, in 2011 the average annual incidence in people age 65 to 74 was 0.4% (meaning four of every 1,000 people age 65 to 74 developed Alzheimer's dementia in 2011); in people age 75 to 84, the annual incidence was 3.2% (32 of every 1,000 people); and in people age 85 and older, the incidence was 7.6% (76 of every 1,000 people). 284 A 2015 study using data from the Adult Changes in Thought Study, a cohort of members of a health care delivery system in the Seattle area, reported similar incidence rates to the CHAP study.10 Because of the increasing number of people age 65 and older in the U.S., particularly those age 85 and older, the annual number of new cases of Alzheimer's and other dementias is projected to double by 2050. 285
3.5. Lifetime risk of Alzheimer's dementia
Lifetime risk is the probability that someone of a given age who does not have a particular condition will develop the condition during that person's remaining life span. Data through 2009 from the Framingham Heart Study were used to estimate lifetime risk of Alzheimer's dementia by age and sex.A4 , 286 As shown in Figure 4, the study estimated that the lifetime risk for Alzheimer's dementia at age 45 was approximately 1 in 5 (20%) for women and 1 in 10 (10%) for men. The risks for both sexes were slightly higher at age 65. 286
FIGURE 4.
Estimated lifetime risk for Alzheimer's dementia, by sex, at ages 45 and 65. Created from data from Chene et al. 286
3.6. Differences between women and men in the prevalence and risk of Alzheimer's and other dementias
Almost two‐thirds of Americans with Alzheimer's are women. 241 Of the 6.9 million people age 65 and older with Alzheimer's dementia in the United States, 4.2 million are women and 2.7 million are men. 241 This represents 11% of women and 9% of men age 65 and older in the United States. 287
Women live longer than men on average, and older age is the greatest risk factor for Alzheimer's. 286 , 288 , 289 This survival difference contributes to higher prevalence of Alzheimer's and other dementias in women compared with men. However, it is not clear that the risk of developing Alzheimer's or other dementias differs between men and women of the same age. Most studies of incidence in the United States have found no meaningful difference between men and women in the proportion who develop Alzheimer's or other dementias at any given age, 10 , 86 , 289 , 290 , 291 while some European studies have reported a higher incidence among women at older ages, 292 , 293 or higher incidence among men. 294 Therefore, differences in the risk of dementia between men and women may depend in part on age, birth cohort and/or geographic region. 295 , 296
Other studies have provided evidence that any observed difference in dementia risk between men and women may be an artifact of who is more or less likely to die of other health factors before developing dementia. A study using Framingham Heart Study data suggested that men in the study appeared to have a lower risk for dementia due to “survival bias,” in which the men who survived to age 65 or beyond and were included in the study were the ones with a healthier cardiovascular risk profile (men have a higher rate of death from cardiovascular disease in middle age than women) and thus a lower risk for dementia. 288 Recent studies have supported the notion that selection bias contributes to reports of sex and gender differences in Alzheimer's dementia risk. 285 , 297 More research is needed to support this interpretation.
Although differences in the rates at which men and women develop Alzheimer's or other dementias do not appear to be large or consistent, the reasons men and women develop dementia may vary. These differences may be based in biology, such as chromosomal or hormonal differences related to reproductive history 298 (i.e., sex differences), or in how social and cultural factors are distributed among or are experienced by men and women (i.e., gender differences), or a combination of the two. 295 , 299 , 300 Gender differences may exist in the distribution of or even the effect of known risk factors for dementia, such as education, occupation, cardiovascular disease and health behaviors. For example, lower educational attainment in women than in men born in the first half of the 20th century may contribute to elevated risk in women, as limited formal education is a risk factor for dementia. 301 This possibility requires more research, but evidence supports that greater educational attainment over time in the United States — the gains in which have been more substantial for women than men — has led to decreased risk for dementia. 302 In addition to differences in educational attainment relating to dementia risk differences in men and women, the same level of education affect men's and women's dementia risk differently. European studies have found that the association of lower educational attainment with dementia outcomes may be stronger in women than men. 303 , 304
Other societal gender differences may also be at play, such as differences in occupational attainment between men and women, with a recent study showing that women who participated in the paid workforce earlier in life had better cognitive outcomes after age 60 than women who were not part of the paid workforce. 297 , 305 , 306 More recently, gender differences during the lockdown phase in the early part of the COVID‐19 pandemic included increased child care and job loss in sectors where women were more likely to be employed. 307 , 308 , 309 It is unclear how these differential impacts on women may affect their brain health in the future. Researchers have begun exploring how mental health challenges, lost job opportunities, and decreased employment earnings experienced during the pandemic may affect women's ability to maintain brain health. 308
It is unclear whether genetic risk operates differently in women and men in the development of, or susceptibility to, Alzheimer's pathology. 310 A number of studies have indicated that the APOE‐e4 genotype, the best known common genetic risk factor for Alzheimer's dementia, may have a stronger association with Alzheimer's dementia 311 , 312 and neurodegeneration 313 in women than in men. However, a meta‐analysis found no difference between men and women in the association between APOE‐e4 and Alzheimer's dementia overall, although age played an interesting interactive role. That is, APOE‐e4 was related to higher Alzheimer's risk in women than men between ages 55 and 70, when APOE is thought to exert its largest effects. 314 It is unclear whether the influence of APOE‐e4 may depend on the sex hormone estrogen. 315 , 316
It should be recognized that sex and gender identities cannot be reduced to binary categories. Individuals who identify with nonbinary sex or gender identities may have different risks for Alzheimer's disease (see “Risk for Alzheimer's and Other Dementias in Sexual and Gender Minority Groups” in this section).
3.7. Racial and ethnic differences in the prevalence and risk of Alzheimer's and other dementias
The risk of Alzheimer's and other dementias appears to vary by race and ethnicity in the U.S. While risk is poorly characterized in smaller racial and ethnic groups in the U.S., multiple studies have reported on differences in risk across non‐Hispanic Black, non‐Hispanic White, and Hispanic Americans. In the U.S., non‐Hispanic Black and Hispanic older adults are more likely than White older adults to have Alzheimer's or other dementias. 317 , 318 , 319 , 320 , 321 , 322 , 323 Data from the CHAP study indicates 19% of Black and 14% of Hispanic adults age 65 and older have Alzheimer's dementia compared with 10% of White older adults. 241 In line with these observations, most other prevalence studies indicate that Black older adults are about twice as likely to have Alzheimer's or other dementias as White older adults. 160 , 284 , 324 , 325 Some other studies indicate Hispanic older adults are about one and one‐half times as likely to have Alzheimer's or other dementias as White older adults, 325 , 326 , 327 though others have shown similar prevalences among Hispanic older adults and White older adults.160 The population of Hispanic people comprises very diverse groups with different cultural histories and health profiles, and there is evidence that prevalence may differ from one specific Hispanic ethnic group to another (for example, Mexican Americans compared with Caribbean Americans). 328 , 329
The higher prevalence of Alzheimer's dementia in Black and Hispanic populations compared with the White population appears to be due to a higher risk of developing dementia in these groups compared with the White population of the same age. 330 , 331 Race is a social construct with little to no genetic or biological basis. Instead, race is an idea created and used throughout history by groups in power to justify their control and dominance over other groups, and genetic factors do not account for the large differences in prevalence and incidence among racial groups. 330 , 332 While there is some research into how the influence of genetic risk factors on Alzheimer's and other dementias may differ by race — for example, the influence of the APOE‐e4 allele on Alzheimer's risk may be stronger for White Americans than Black Americans 88 , 89 , 90 , 91 , 92 , 333 — these small differences in genetic influence do not account for the large differences in dementia risk across racial groups. Instead, research suggests that the historic and continued marginalization of Black and Hispanic people in the U.S. has produced disparities between older Black and Hispanic populations and older White populations in life experiences, socioeconomic indicators and, ultimately, health conditions. It is these disparities that most likely explain the difference in risk for Alzheimer's and other dementias among racial and ethnic groups. 334 These health and socioeconomic disparities are rooted in the history of discrimination against Black individuals and other people of color in the U.S., not only during interpersonal interactions, but also as codified in the rules, practices and policies of U.S. banks, laws, health care and other systems — that is, structural racism. 335 , 336 Structural racism pervades many aspects of life that may directly or indirectly alter dementia risk, including where people can live, the quality of schools in their communities, exposure to harmful toxicants and pollutants, access to quality health care, employment prospects, occupational safety, the ability to pass wealth to subsequent generations, treatment by the legal system and exposure to violence. 337 , 338 , 339 , 340
The cumulative stress imparted by the effects of structural racism and the resulting differences in social and physical environments may directly influence dementia risk among historically marginalized and socially disadvantaged racial and ethnic groups. Further, structural racism leads to disparities by race and ethnicity in a wide range of health outcomes including increased risk for chronic conditions that are themselves associated with higher dementia risk, such as cardiovascular disease and diabetes. These health conditions, which disproportionately affect Black and Hispanic populations, are believed to explain much of the elevated risk of dementia among Black and Hispanic populations. 88 , 334 , 341 , 342 Many studies suggest that racial and ethnic differences in dementia risk do not persist in rigorous analyses that account for health and socioeconomic factors. 156 , 330 , 343
The influence of structural racism on health and dementia risk may cascade and compound across the course of a person's life. For example, some studies indicate that early life experiences with residential and school segregation can have detrimental effects on the cognitive health of Black Americans in later life. 337 , 338 , 339 This points to a need for health disparities researchers to employ a life course perspective and to seek the insights of race equity scholars to account for the cumulative interplay of many environmental and sociopolitical factors that may put some groups of people at increased risk for Alzheimer's and other dementias. 334 , 342
Many of the social processes that influence disparities in the development of Alzheimer's could also influence whether and when a diagnosis of dementia occurs. There is evidence that missed or delayed diagnoses of Alzheimer's and other dementias are more common among Black and Hispanic older adults than among White older adults. 254 , 256 , 259 , 344 , 345 Based on data from Medicare beneficiaries age 65 and older, it has been estimated that Alzheimer's or another dementia has been diagnosed in 10.3% of White older adults, 12.2% of Hispanic older adults and 13.8% of Black older adults. 346 Although these percentages indicate that the dementia burden is greater among Black and Hispanic older adults than among White older adults, the percentages should be even higher according to prevalence studies that detect all people who have dementia irrespective of their use of health care systems.
Population‐based cohort studies regarding the prevalence and incidence of Alzheimer's and other dementias in racial and ethnic groups other than White, Black and Hispanic populations are relatively sparse. 331 Among the few studies, one examined electronic medical records of members of a large health plan in California. Its findings indicated that dementia incidence — determined by the first presence of a dementia diagnosis in members’ medical records — was highest among African American older adults (the term used in the study for those who self‐reported as Black or African‐American); intermediate for Latino older adults (the term used in the study for those who self‐reported as Latino or Hispanic), American Indian and Native Alaskan older adults, Pacific Islander older adults and White older adults; and lowest among Asian American older adults. 347 A follow‐up study with the same cohort showed differences across Asian American subgroups, but all subgroups studied had lower dementia incidence than the White population. 348 A systematic review of the literature found that Japanese Americans were the only Asian American subgroup with reliable prevalence data, and that they had the lowest prevalence of dementia compared with all other ethnic groups. 328 We have limited understanding of Alzheimer's disease as experienced by people of Middle Eastern and North African descent, 349 those who identify with more than one race or ethnicity, and subgroups of origin within racial or ethnic groups. 346 More studies, especially those involving community‐based cohorts and those that focus on racial/ethnic groups historically not included in Alzheimer's research, are necessary to draw conclusions about the prevalence of Alzheimer's and other dementias in different racial and ethnic groups and subgroups.
3.8. Risk for Alzheimer's and other dementias in sexual and gender minority groups
There are other groups with shared social identities and characteristics that may experience different risks of Alzheimer's and other dementias. This includes members of sexual and gender minority (SGM) groups. SGM refers to individuals who identify as lesbian, gay, bisexual (sexual minorities), and/or transgender or gender nonbinary, as well as people with a gender identity, gender expression or reproductive development that varies from traditional, societal, cultural or physiological norms (gender minorities).
SGM older adults may face an increased dementia risk, through pervasive exposure to systematic discrimination, marginalization, disadvantage and/or exclusion from social organizations and enterprises. Those enterprises include Alzheimer's research, and, until recently, little has been known about the dementia risks of people who self‐identify as SGM. Although few studies have been designed to investigate whether SGM older adults are at greater risk for dementia than non‐SGM older adults, a growing body of preliminary evidence suggests that this may be the case. In a study of adults living in any of 25 U.S. states, SGM older adults reported experiencing more cognitive problems than non‐SGM older adults. 350 Two population‐based studies found higher rates of cognitive impairment among SGM older adults than among non‐SGM older adults, 351 , 352 yet a third study reported that the risks for dementia and mild cognitive impairment were similar for people in same‐sex relationships and people in another‐sex relationships. 353 Two studies found indications of potentially elevated dementia risk among transgender adults. One study of Medicare beneficiaries estimated that dementia was present among 18% of transgender adults age 65 years and older, compared with 12% among cisgender (not transgender) adults. 354 A second study of adults in Florida reported that transgender adults were more likely than cisgender adults to have a diagnosis of Alzheimer's and other dementias in their electronic medical records. 355 A recent review of the evidence found that most studies examining subjective cognitive decline as an outcome showed higher prevalence among SGM older adults, while those examining objective measures of cognitive performance showed more mixed results. 356
More research is necessary to establish whether SGM older adults face elevated dementia risk and if so, to understand reasons for it. Researchers have hypothesized that stressors experienced by SGM older adults, such as discrimination and marginalization, may elevate their risk for Alzheimer's and other dementias. 300 These stressors could take a toll on the physical and mental health of SGM older adults. 357 One study showed that SGM older adults who were experiencing depression were more likely to report subjective cognitive decline than SGM older adults without depression. 358 SGM older adults experience disparities in other health‐related factors that elevate the risk of Alzheimer's and other dementias, including higher rates of alcohol and tobacco use, obesity and other cardiovascular risk factors compared with non‐SGM older adults. SGM older adults also have lower rates of health care access and preventive health screenings, in part due to experiencing barriers such as discrimination and heterosexist attitudes in health care settings. 359 Finally, the history of HIV/AIDS and its burden of illness, mortality and social stigma has been tied to the SGM population, particularly gay and bisexual men and transgender people. HIV/AIDS is now a chronic condition that can be managed successfully with medication, and many people with HIV/AIDS survive into older ages. In addition to any effects of this history on aforementioned social stressors and health care access, HIV/AIDS itself is a risk factor for dementia. 360 The elevated prevalence of HIV/AIDS in gay and bisexual men and transgender people puts them at higher risk for dementia due to HIV/AIDS than non‐SGM older adults.
There is increasing recognition that historically marginalized groups — whether defined by gender, sexual orientation, race, ethnicity or other traits — are not monolithic when it comes to their identities and experiences. These identities and experiences intersect, and belonging to more than one of these groups may be particularly consequential for health, including dementia risk. For example, a recent study showed that transgender adults from minoritized ethnoracial groups are more likely to report subjective cognitive decline than other transgender adults. 361 This “intersectionality” framework is important for developing more informative dementia research and more effective and compassionate dementia care in these communities. 362 It is important that research and care efforts consider how systems of oppression based on gender, race, ethnicity, class, sexual orientation and HIV status may intersect and influence dementia. 363 , 364
3.9. Trends in the prevalence and incidence of Alzheimer's dementia over time
A growing number of studies indicate that the prevalence (i.e., proportion) 239 , 259 , 290 , 345 , 346 , 347 , 348 , 349 , 365 , 366 , 367 and incidence 294 , 365 , 366 , 367 , 368 , 369 , 370 , 371 , 372 , 373 , 374 of Alzheimer's and other dementias in the U.S. and other high income countries may have declined in the past 25 years, 294 , 302 , 365 , 366 , 367 , 368 , 369 , 370 , 371 , 372 , 373 , 375 , 376 , 377 , 378 , 379 though results are mixed. 70 , 284 , 380 , 381 One recent systematic review found that incidence of dementia has decreased worldwide over the last four decades while incidence of Alzheimer's dementia, specifically, has held steady, but more research on this distinction is needed, especially in low‐income and middle‐income countries. 382 Declines in dementia risk have been attributed to increasing levels of education and improved control of cardiovascular risk factors. 302 , 368 , 371 , 375 , 383 , 384 Such findings are promising and suggest that identifying and reducing risk factors for dementia may be effective — whether interventions occur person by person (such as obtaining treatment for one's blood pressure) or are integrated into the fabric of communities (such as changes in education policies). Although these findings indicate that a person's risk of dementia at any given age may be decreasing slightly, the total number of people with Alzheimer's or other dementias in the U.S. and other high‐income countries is expected to continue to increase dramatically because of the increase in the number of people at the oldest ages.
It is unclear whether these encouraging declines in incidence will continue. For example, worldwide increases in diabetes and obesity, which are risk factors for dementia, among people younger than 65 may lead to a rebound in dementia risk in coming years. 366 , 385 , 386 , 387 , 388 It is also not clear that the encouraging trends pertain to all racial and ethnic groups. 284 , 323 , 383 , 384 , 389 , 390 Thus, while recent findings are promising, the social and economic burden of Alzheimer's and other dementias will continue to grow. Moreover, 68% of the projected increase in the global prevalence and burden of dementia by 2050 will take place in low‐ and middle‐income countries, where current evidence does not support a decline in the risk of Alzheimer's and other dementias. 391 Finally, it is not known how COVID‐19 will influence the prevalence and incidence of Alzheimer's dementia. For example, the neurologic effects of COVID‐19 392 and the pandemic's disruptions to general and brain‐related health care may increase the incidence of Alzheimer's and other dementias. Some researchers have surmised that factors such as social isolation from lockdowns, no‐visitor policies in long‐term care facilities, and increased intensive hospitalizations may increase dementia risk at the population level, but research in coming years will be necessary to confirm this and examine whether the impact is time‐limited or long term. On the other hand, the number of people living with Alzheimer's dementia could be influenced in the opposite direction by increased mortality due to COVID‐19 and other causes of death during the pandemic in 2020‐2023, which may have resulted in death prior to the onset of Alzheimer's dementia, or death with fewer years lived with Alzheimer's dementia. 393
3.10. Looking to the future
3.10.1. Continued population aging
In 2011, the largest ever demographic generation of the American population — the baby‐boom generation — started reaching age 65. By 2030, the segment of the U.S. population age 65 and older will have grown substantially, and the projected 71 million older Americans will make up over 20% of the total population (up from 17% in 2022). 237 Additionally, the size of the older adult population is expected to continue to increase relative to the population age 64 and younger — a shift known as population aging — due to a projected decline in fertility, as well as to mortality improvements at older ages. Fertility, the average number of children per woman, has decreased since 1960 in the United States. 394 With fewer babies born each year, older adults will make up a larger proportion of the population. Because increasing age is the predominant risk factor for Alzheimer's dementia, as the number and proportion of older Americans grows rapidly, so too will the numbers of new and existing cases of Alzheimer's dementia, as shown in Figure 5.A5 , 241 By 2060, the number of people age 65 and older with Alzheimer's dementia is projected to reach 13.8 million, barring the development of medical breakthroughs to prevent or cure Alzheimer's disease.A5 , 241
FIGURE 5.
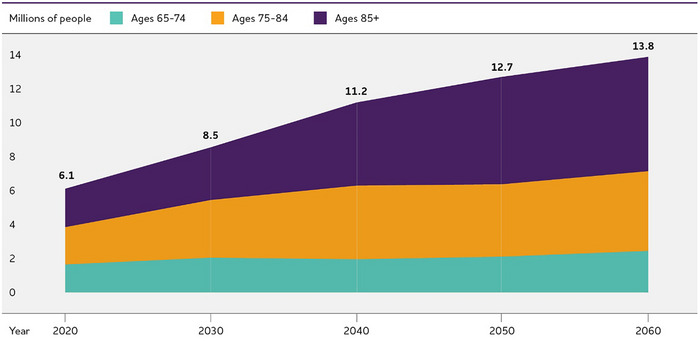
Projected number of people age 65 and older (total and by age) in the U.S. population with Alzheimer's dementia, 2020 to 2060. Created from data from Rajan et al.A5 , 241
3.10.2. Growth of the age 85 and older population
The number of Americans in their 80s, 90s and beyond is expected to grow dramatically due to population aging. 237 This will lead to an increase in the number and percentage of Americans 85 and older. This age group is expected to comprise 11% of the population age 65 and older in 2025 and 21% of the population age 65 and older in 2050. 395 This will result in an additional 10 million people age 85 and older — individuals at the highest risk for developing Alzheimer's dementia. 395
In 2024, about 2.4 million people living with Alzheimer's dementia are expected to be age 85 or older, accounting for 34% of all people with Alzheimer's dementia. 241
By 2060, 6.7 million people age 85 and older are expected to have Alzheimer's dementia, accounting for about half (48%) of all people 65 and older with Alzheimer's dementia. 241
3.10.3. Increased diversity of older adults
The group of older adults who will be at risk for Alzheimer's in the coming years will be socially, culturally and economically different from previous groups of older U.S. adults. For example, between 2018 and 2040, projections for older adults show increases in the American Indian population of 75%, in the Black population of 88%, in the Asian population of 113% and in the Hispanic population of 175% compared with an increase of 32% in the White population. 396
In addition, in the coming decades women age 65 and older will be among the first generations of women to have widely worked outside the home, and they will have more years of formal education than previous generations of women. 397 In parallel these generations of women came of age during a decrease in the birth rate, resulting in smaller family size. 398 Whether and how these social and economic experiences influence women's risk of and resilience to Alzheimer's will become clearer in the decades ahead.
Since the 1970s, the gap in income in the U.S. between lower‐income, middle‐income, and upper‐income households has been widening. 399 This means that the many people who are age 65 and over experienced their adulthood during this trend, which may have influenced health and health behaviors prior to age 65. In older adulthood, income inequality may influence a wealth gap, which may have implications for health care, health behaviors, and social determinants of health that influence Alzheimer's risk in particular among low‐income households.
Given the different life experiences of future older adult populations, it is unclear what the accompanying changes will be to dementia incidence and prevalence, both at the population level and within racial/ethnic, socioeconomic, and sex and gender groups. A birth cohort perspective, which considers how a certain group of people has passed through different stages of life in particular years, will be increasingly important for understanding factors of risk and resilience that may be unique to the groups of people at risk for dementia in the coming decades. 374 , 400 , 401
4. MORTALITY AND MORBIDITY
Alzheimer's disease was officially listed as the sixth‐leading cause of death in the United States in 2019 402 ; in the years 2020 and 2021, when COVID‐19 became the third‐leading cause of death, Alzheimer's disease was the seventh‐leading cause of death. Official counts for more recent years are still being compiled. 403
Alzheimer's disease was the fifth‐leading cause of death among individuals age 65 and older in 2021. 402 Alzheimer's disease may cause even more deaths than official sources recognize. It is also a leading cause of disability and poor health (morbidity) in older adults. 404 Before a person with Alzheimer's dies, they are likely to live through years of morbidity as the disease progresses.
4.1. Deaths from Alzheimer's disease
The data presented in this section are through 2021, the latest year for which finalized death data are available. Starting in 2020, the COVID‐19 pandemic had a dramatic effect on deaths in the United States (see the box “The Effect of the COVID‐19 Pandemic on Deaths from Alzheimer's Disease” for a discussion of the effect of the pandemic on Alzheimer's mortality). In 2021, Alzheimer's mortality trends started to more closely resemble the year‐by‐year trends from before the COVID‐19 pandemic.
In this section, “deaths from Alzheimer's disease” refers to what is officially reported on death certificates. Note that while death certificates use the term “Alzheimer's disease,” the determination is made based on clinical symptoms in almost every case, and thus more closely aligns with “Alzheimer's dementia” as we have defined it in previous sections of this report; to remain consistent with the CDC terminology for causes of death, we use the term “Alzheimer's disease” for this section when referring to officially reported statistics gleaned from death certificates.
It is difficult to determine how many deaths are caused by Alzheimer's disease each year because of the way causes of death are recorded. According to data from the Centers for Disease Control and Prevention (CDC), 119,399 people died from Alzheimer's disease in 2021. 402 The CDC considers a person to have died from Alzheimer's if the death certificate lists Alzheimer's as the underlying cause of death, defined as “the disease or injury which initiated the train of events leading directly to death.” 405
The number of deaths from dementia of any type is much higher than the number of reported Alzheimer's deaths. In 2021, some form of dementia was the officially recorded underlying cause of death for 279,704 individuals (this includes the 119,399 from Alzheimer's disease). 402 Therefore, the number of deaths from all causes of dementia, even as listed on death certificates, is more than twice as high as the number of reported Alzheimer's deaths alone.
Severe dementia frequently causes complications such as immobility, swallowing disorders and malnutrition that significantly increase the risk of acute conditions that can cause death. One such condition is pneumonia (infection of the lungs), which is the most commonly identified immediate cause of death among older adults with Alzheimer's or other dementias. 406 , 407 , 408 , 409 One pre‐COVID‐19 autopsy study found that respiratory system diseases were the immediate cause of death in more than half of people with Alzheimer's dementia, followed by circulatory system disease in about a quarter. 407 Death certificates for individuals with Alzheimer's often list acute conditions such as pneumonia as the primary cause of death rather than Alzheimer's. 407 , 408 As a result, people with Alzheimer's dementia who die due to these acute conditions may not be counted among the number of people who die from Alzheimer's disease, even though Alzheimer's disease may well have caused the acute condition listed on the death certificate. This difficulty in using death certificates to determine the number of deaths from Alzheimer's and other dementias has been referred to as a “blurred distinction between death with dementia and death from dementia.” 410
Another way to determine the number of deaths from Alzheimer's dementia is through calculations that compare the estimated risk of death in those who have Alzheimer's dementia with the estimated risk of death in those who do not have Alzheimer's dementia. A study using data from the Rush Memory and Aging Project and the Religious Orders Study estimated that 500,000 deaths among people age 75 and older in the United States in 2010 could be attributed to Alzheimer's dementia (estimates for people age 65 to 74 were not available), meaning that those deaths would not be expected to occur in that year if the individuals did not have Alzheimer's dementia. 406 A more recent study using data from the nationally representative Health and Retirement Study (HRS) estimated that about 14% of deaths among Americans age 70 and older from 2000‐2009 were attributable to dementia, while only 5% of death certificates listed dementia as the underlying cause of death for this age group, indicating underreporting on death certificates. 411 According to 2019 Medicare claims data, about one‐third of all Medicare beneficiaries who die in a given year have been diagnosed with Alzheimer's or another dementia. 412 Based on data from the Chicago Health and Aging Project (CHAP) study, in 2020 an estimated 700,000 people age 65 and older in the United States had Alzheimer's dementia at death. 413 Although some undoubtedly died from causes other than Alzheimer's, it is likely that many died from Alzheimer's disease itself or from conditions for which Alzheimer's was a contributing cause, such as pneumonia. Thus, taken together, the specific number of deaths caused by Alzheimer's is unknown.
Adding further complexity, the vast majority of death certificates listing Alzheimer's disease as an underlying cause of death are not verified by autopsy, and research has shown that 15% to 30% of those diagnosed with Alzheimer's dementia during life do not have the brain changes of Alzheimer's disease but instead have the brain changes of another cause of dementia (see Table 1). 21 , 79 , 243 , 244 , 245 Therefore, an underlying cause of death listed as Alzheimer's disease may not be accurate. Irrespective of the cause of death, among people age 70, 61% of those with Alzheimer's dementia are expected to die before age 80 compared with 30% of people without Alzheimer's dementia. 414
4.2. The effect of the COVID‐19 pandemic on deaths from Alzheimer's disease
In 2020 and 2021, COVID‐19 was the third‐leading cause of death in the United States, pushing Alzheimer's disease from the sixth‐ to the seventh‐leading cause of death. 403 Data for more recent years were still being compiled as of the time this report was written. Despite the change in rankings on the list of causes of death, the total number of deaths from Alzheimer's disease recorded on death certificates increased 10.5% between 2019 and 2020 to 134,242. 402 COVID‐19 was likely a significant contributor to the large increase in deaths from Alzheimer's. Data from the Centers for Disease Control and Prevention (CDC) show that excess mortality (the difference between the observed number of deaths and the expected number of deaths during a given period) from any cause has been very high during the height of the pandemic, especially among older adults. 415 Many of these excess deaths were in vulnerable older adults with Alzheimer's disease and other dementias. Among Medicare beneficiaries age 65 and over with Alzheimer's disease and other dementias, overall mortality increased 26% between 2019 and 2020, which is twice as high as the increase among beneficiaries without Alzheimer's disease and other dementias. 416 Furthermore, increased mortality between 2019 and 2020 among Medicare beneficiaries with Alzheimer's disease and related dementia was greater among Black, Hispanic and Asian beneficiaries than among White beneficiaries and the nursing home population. 416 As shown in Figure 6, compared with the average annual number of deaths in the five years before 2020, there were 13,925 more deaths from Alzheimer's disease and 44,729 more deaths from all dementias, including Alzheimer's, in 2020. This is, respectively, 12% and 17% more than expected. 415 In 2021, there were 1,082 more deaths from Alzheimer's disease and 20,449 more deaths from all dementias compared with the average of the five years before 2020. 402 The number of people dying from Alzheimer's has been increasing over the last two decades, but the number of excess deaths from Alzheimer's disease in 2020 far exceeded what would have been expected from this pre‐pandemic trend. The number for 2021, by contrast, is closer to the pre‐pandemic trend. Data for more recent years are still being compiled, but one study found that deaths due to all dementias, including Alzheimer's, decreased between March 2021 and February 2022, in particular among residents of nursing homes and long‐term care facilities. 417
FIGURE 6.
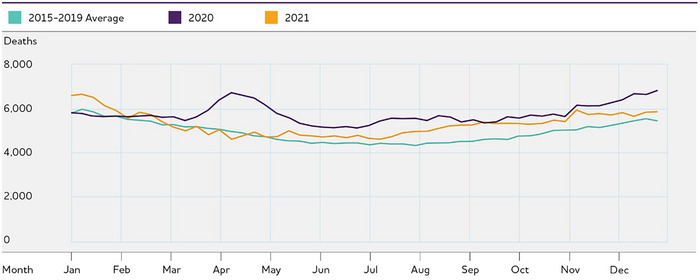
Deaths due to Alzheimer's and other dementias in the United States in 2020 and 2021 compared with previous years. Data for 2021 are as of February 7, 2022. Created from data from the National Center for Health Statistics. 415
The impact of COVID‐19 can also be seen when examining the number of deaths from COVID‐19 for which death certificates also listed Alzheimer's or another dementia as a cause of death (referred to as a “multiple cause of death”). In 2020 and 2021, 1 in every 10 death certificates listing COVID‐19 as the primary cause of death also listed Alzheimer's disease or another dementia as a multiple cause of death. Among people age 85 or older who died of COVID‐19 in 2020 or 2021, Alzheimer's disease or another dementia was listed as a multiple cause of death on almost a quarter of death certificates. 403
The COVID‐19 pandemic had a dramatic effect on mortality from Alzheimer's and other dementias. Nursing homes and other long‐term care facilities were the site of major outbreaks in the early stages of the pandemic, and residents with Alzheimer's and other dementias were particularly vulnerable. What remains unclear is whether and how this will affect the longer‐term trend in deaths from Alzheimer's now that the COVID‐19 pandemic has subsided. As the pandemic has progressed and COVID‐19 is no longer as fatal for most people, the question of “dying with” or “dying from” COVID‐19 is getting harder to parse. In many ways this echoes the discussion about dying with or from Alzheimer's disease discussed in this section. What is clear is that for at least the first years of the pandemic, having Alzheimer's or another dementia made older adults more vulnerable to COVID‐19 and increased the risk of dying from COVID‐19.
4.3. Public health impact of deaths from Alzheimer's disease
In the past two decades, although the number of deaths from other major causes decreased significantly or remained approximately the same, official records indicate that deaths from Alzheimer's disease increased significantly. Between 2000 and 2021, the number of deaths from Alzheimer's disease as recorded on death certificates more than doubled, increasing 141%, while deaths from the number‐one cause of death (heart disease) decreased 2.1% (Figure 7). 402 , 418 The increase in the number of death certificates listing Alzheimer's as the underlying cause of death probably reflects two trends: first, Alzheimer's has become a more common cause of death as the population ages; and second, over time, physicians, coroners and others who assign causes of death may be increasingly likely to report Alzheimer's on death certificates. 419
FIGURE 7.
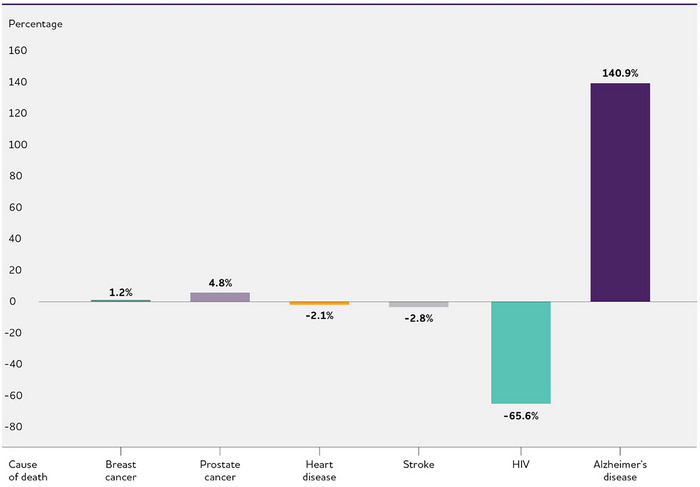
Percentage changes in selected causes of death (all ages) between 2000 and 2021. Created from data from the National Center for Health Statistics. 402 , 418
4.4. State‐by‐state deaths from Alzheimer's
Table 5 provides information on the number of deaths due to Alzheimer's by state in 2021, the most recent year for which state‐by‐state data are available. This information was obtained from death certificates and reflects the condition identified by the physician or other medical personnel who filled out the death certificate as the underlying cause of death. The table also provides annual mortality rates by state, computed with the death certificate data, to compare the risk of death due to Alzheimer's disease across states with varying population sizes. For the United States as a whole, in 2021, the mortality rate for Alzheimer's disease was 36 deaths per 100,000 people.A6 , 402
TABLE 5.
Number of deaths and annual mortality rate (per 100,000 people) due to Alzheimer's disease by state, 2021.
| State | Number of Deaths | Mortality Rate |
|---|---|---|
| Alabama | 2,725 | 54.1 |
| Alaska | 135 | 18.4 |
| Arizona | 2,754 | 37.8 |
| Arkansas | 1,559 | 51.5 |
| California | 16,911 | 43.1 |
| Colorado | 1,778 | 30.6 |
| Connecticut | 1,049 | 29.1 |
| Delaware | 381 | 38.0 |
| District of Columbia | 66 | 9.9 |
| Florida | 6,716 | 30.8 |
| Georgia | 4,378 | 40.5 |
| Hawaii | 562 | 39.0 |
| Idaho | 789 | 41.5 |
| Ilinois | 4,025 | 31.8 |
| Indiana | 2,238 | 32.9 |
| lowa | 1,185 | 37.1 |
| Kansas | 805 | 27.4 |
| Kentucky | 1,632 | 36.2 |
| Louisiana | 2,121 | 45.9 |
| Maine | 539 | 39.3 |
| Maryland | 1,129 | 18.3 |
| Massachusetts | 1,558 | 22.3 |
| Michigan | 4,198 | 41.8 |
| Minnesota | 2,251 | 39.4 |
| Mississippi | 1,694 | 57.4 |
| Missouri | 2,517 | 40.8 |
| Montana | 341 | 30.9 |
| Nebraska | 687 | 35.0 |
| Nevada | 804 | 25.6 |
| New Hampshire | 422 | 30.4 |
| New Jersey | 2,399 | 25.9 |
| New Mexico | 634 | 30.0 |
| New York | 3,582 | 18.1 |
| North Carolina | 4,260 | 40.4 |
| North Dakota | 325 | 41.9 |
| Ohio | 4,947 | 42.0 |
| Oklahoma | 1,580 | 39.6 |
| Oregon | 2,047 | 48.2 |
| Pennsylvania | 4,109 | 31.7 |
| Rhode Island | 445 | 40.6 |
| South Carolina | 2,419 | 46.6 |
| South Dakota | 396 | 44.2 |
| Tennessee | 2,879 | 41.3 |
| Texas | 10,437 | 35.3 |
| Utah | 998 | 29.9 |
| Vermont | 337 | 52.2 |
| Virginia | 2,582 | 29.9 |
| Washington | 3,644 | 47.1 |
| West Virginia | 851 | 47.7 |
| Wisconsin | 2,371 | 40.2 |
| Wyoming | 208 | 35.9 |
| Total | 119,399 | 36.0 |
4.5. Alzheimer's death rates
As shown in Figure 8, the annual rate of deaths due to Alzheimer's — that is, the number of Alzheimer's deaths per 100,000 people in the population — has risen substantially since 2000. 402 Table 6 shows that the annual rate of death from Alzheimer's increases dramatically with age, especially after age 65.A6 , 402 The increase in the Alzheimer's death rate over time has disproportionately affected people age 85 and older. 418 Between 2000 and 2021, the death rate from Alzheimer's increased 41% for people age 65 to 74, 54% for people age 75 to 84 and 86% for people age 85 and older. 402 A report by the CDC determined that even after adjusting for changes over time in the specific ages of people within these age groups, the annual Alzheimer's death rate in the U.S. increased substantially between 1999 and 2014. 419 Therefore, the advancing average age of the older adult population in the U.S. is not the only explanation for the increase in Alzheimer's death rates. Other possible reasons include fewer deaths from other common causes of death in old age such as heart disease and stroke; increased clinical recognition of and formal diagnosis of Alzheimer's dementia; and increased reporting of Alzheimer's as a cause of death by physicians and others who complete death certificates. 419
FIGURE 8.
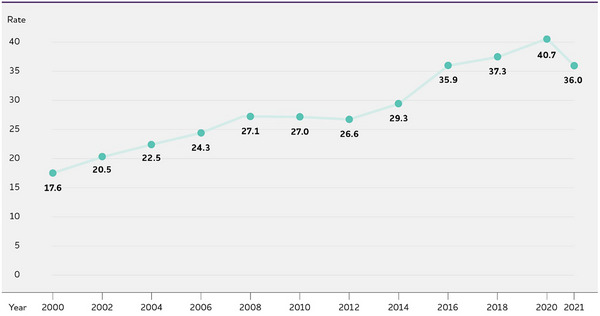
U.S. annual Alzheimer's death rate (per 100,000 people) by year. Created from data from the National Center for Health Statistics. 402
TABLE 6.
U.S. annual Alzheimer's death rates (per 100,000) by age and year.
| Age | 2000 | 2002 | 2004 | 2006 | 2008 | 2010 | 2012 | 2014 | 2016 | 2018 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45‐54 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 |
| 55‐64 | 2.0 | 1.9 | 1.8 | 2.1 | 2.2 | 2.1 | 2.2 | 2.1 | 2.7 | 2.9 | 3.3 | 3.2 |
| 65‐74 | 18.7 | 19.6 | 19.5 | 19.9 | 21.1 | 19.8 | 17.9 | 19.6 | 23.6 | 24.7 | 28.6 | 26.4 |
| 75‐84 | 139.6 | 157.7 | 168.5 | 175.0 | 192.5 | 184.5 | 175.4 | 185.6 | 214.1 | 213.9 | 229.3 | 214.3 |
| 85+ | 667.7 | 790.9 | 875.3 | 923.4 | 1,002.2 | 987.1 | 936.1 | 1,006.8 | 1,216.9 | 1,225.3 | 1,287.3 | 1,243.6 |
Created from data from the National Center for Health Statistics. 402
4.6. Duration of illness from diagnosis to death and time spent in nursing home
Studies indicate that people age 65 and older survive an average of four to eight years after a diagnosis of Alzheimer's dementia, yet some live as long as 20 years with Alzheimer's dementia. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 This reflects the slow, insidious and uncertain progression of Alzheimer's. A person who lives from age 70 to age 80 with Alzheimer's dementia will spend an average of 40% of this time in the severe stage. 414 Much of this time will be spent in a nursing home (see Use and Costs section). At age 80, approximately 75% of people with Alzheimer's dementia live in a nursing home compared with only 4% of the general population age 80. 414 In all, an estimated two‐thirds of those who die of dementia do so in nursing homes, compared with 20% of people with cancer and 28% of people dying from all other conditions. 420
4.7. The burden of Alzheimer's disease
The long duration of illness before death contributes significantly to the public health impact of Alzheimer's disease because much of that time is spent in a state of severe disability and dependence. Scientists have developed measures that compare the burden of different diseases on a population in a way that takes into account not only the number of people with the condition, but also the number of years of life lost due to that disease and the number of healthy years of life lost by virtue of being in a state of disability. One measure of disease burden is called disability‐adjusted life years (DALYs), which is the sum of the number of years of life lost (YLLs) due to premature mortality and the number of years lived with disability (YLDs), totaled across all those with the disease or injury. These measures indicate that Alzheimer's is a very burdensome disease, not only to the individuals with the disease, but also to their families, informal caregivers and communities at large. In recent years, the burden of Alzheimer's has increased more dramatically in the United States than the burden of other diseases. According to the most recent Global Burden of Disease classification system, Alzheimer's disease rose from the 12th most burdensome disease or injury in the United States in 1990 to the sixth in 2016 in terms of DALYs. 404 In 2016, Alzheimer's disease was the fourth highest disease or injury in terms of YLLs and the 19th in terms of YLDs. 404
These estimates should be interpreted with consideration of the comparability of data across time and 421 and how disability is incorporated. These Alzheimer's burden estimates use different sources for each state in a given year, and data sources for states may differ over the years. Estimates do not account for the context in which disability is experienced, including social support and economic resources, 422 which vary widely. Estimates may not fully account for variation in disability levels between individuals and along the stages of Alzheimer's dementia. These variations in data sources and consideration of disability may limit the value of these metrics and the comparability of estimates across states and across years.
4.8. Looking to the future
Taken together, these statistics indicate that not only is Alzheimer's disease responsible for the deaths of more and more Americans, but also that Alzheimer's and other dementias are contributing to more and more cases of poor health and disability in the U.S. With population aging, the percentage of deaths related to Alzheimer's and other dementias will likely continue to increase. The health and well‐being of people with Alzheimer's and other dementias should continue to be prioritized. Thus, it will remain important to develop a comprehensive understanding of how Alzheimer's and other dementias contribute to poor health, disability and mortality. That understanding requires innovation in research methods that are more inclusive and that fully capture the lived experience of disability of people living with dementia and of their families and caregivers.
5. CAREGIVING
Caregiving refers to attending to another person's health needs and well‐being. When supporting a person living with Alzheimer's dementia, caregiving often includes assistance with one or more activities of daily living (ADLs), such as bathing and dressing, as well as multiple instrumental activities of daily living (IADLs), such as paying bills, shopping and using transportation. 423 , 424 Caregivers also provide emotional support to people with Alzheimer's dementia, help them manage health conditions, and communicate and coordinate care with other family members and health care providers (see Table 7). In addition to providing descriptive information about caregivers of people with Alzheimer's or other dementias, this section characterizes caregivers of people with dementia in comparison with either caregivers of people with other medical conditions or, if that comparison is not available, with people who are not caregivers (referred to here as non‐caregivers).
TABLE 7.
Dementia caregiving tasks.
| Helping with instrumental activities of daily living (IADLs), such as household chores, shopping, preparing meals, providing transportation, arranging for doctor's appointments, managing finances and legal affairs, and answering the telephone. |
| Helping the person take medications correctly, either via reminders or direct administration of medications. |
| Helping the person adhere to treatment recommendations for dementia or other medical conditions. |
| Assisting with personal activities of daily living (ADLs), such as bathing, dressing, grooming and feeding, and helping the person walk, transfer from bed to chair, use the toilet and manage incontinence. |
| Managing behavioral symptoms of the disease such as aggressive behavior, wandering, depressive mood, agitation, anxiety, repetitive activity and nighttime disturbances. |
| Finding and using support services such as support groups and adult day service programs. |
| Making arrangements for paid in‐home, nursing home or assisted living care. |
| Hiring and supervising others who provide care. |
| Assuming additional responsibilities that are not necessarily specific tasks, such as: |
|
5.1. Unpaid caregivers
Eighty‐three percent of the help provided to older adults in the United States comes from family members, friends or other unpaid caregivers. 425 Nearly half of all caregivers (48%) who provide help to older adults do so for someone with Alzheimer's or another dementia. 426 More than 11 million Americans provide unpaid care for people with Alzheimer's or other dementias.A7 Table 8 provides details about unpaid caregivers.
TABLE 8.
Who are the caregivers?
| Sex/gender | |
| Race/ethnicity | |
| Living status |
|
| Caring for parents | |
| Income |
|
| Education | |
| Age | |
| Caring for spouse |
|
In 2023, caregivers of people with Alzheimer's or other dementias provided an estimated 18.4 billion hoursA8 of informal — that is, unpaid — assistance, a contribution valued at $346.6 billion.A9 This is approximately 57% of the net value of Walmart's total revenue in fiscal year 2023 ($611.3 billion) 427 and nearly 15 times the total revenue of McDonald's in 2022 ($23.3 billion). 428 The total lifetime cost of care for someone with dementia was estimated at almost $400,000 in 2023 dollars. Seventy percent of this lifetime cost of care is borne by family caregivers in the forms of unpaid caregiving and out‐of‐pocket expenses for items ranging from medications to food for the person with dementia. The remaining costs encompass payments by Medicare and Medicaid (see the Use and Costs of Health Care, Long‐Term Care and Hospice section). 429 , 430 Current estimates of the lifetime costs of care may underestimate the financial impact of a relative's dementia on family caregivers’ health and workplace productivity, as other potential costs such as home modifications, respite service use, and health/work productivity challenges are not always considered in cost estimates. 431
Among the reasons shared by caregivers for providing assistance to a person with Alzheimer's or another dementia are the desire to keep a family member or friend at home (65%), close proximity to the person with dementia (48%), and the caregiver's perceived obligation to the person with dementia (38%).A10 In addition, caregivers often indicate love and a sense of duty when describing what motivates them to assume care responsibilities for a relative or friend living with dementia. 432
Individuals with dementia living in the community are more likely than older adults without dementia to rely on multiple unpaid caregivers (often family members); 30% of older adults with dementia rely on three or more unpaid caregivers, whereas 23% of older adults without dementia do so. 433 Only a small percentage (8%) of older adults with dementia do not receive help from family members or other informal care providers. Of these individuals, nearly half live alone, perhaps making it more difficult to ask for and receive informal care. 433 Among caregivers of spouses with dementia who are at the end of life, close to half provide care without the help of other family or friends. 434
Living alone with dementia may be a particular challenge for certain subgroups, such as lesbian, gay, bisexual and transgender (LGBT) individuals, who may experience greater isolation due to potential social stigma and a diminished social network of available family or friend caregivers. 435 , 436 , 437 , 438 ,
5.1.1. Caregiving and women
The responsibilities of caring for someone with dementia often fall to women. Approximately two‐thirds of dementia caregivers are women.A10 , 439 , 440 , 445 Findings from the 2018 National Health and Wellness survey indicated that more dementia caregivers in the United States are women (61.5%) than in Japan (51.9%) or five European countries/ regions (56.3%: France, Germany, the United Kingdom, Italy and Spain). 447 Over one‐third of dementia caregivers in the United States are daughters caring for a parent. 425 , 433 It is more common for wives to provide informal care for a husband than vice versa. 448 On average, female caregivers spend more time caregiving than male caregivers. 433 The 2015‐2017 BRFSS surveys found that of all dementia caregivers who spend more than 40 hours per week providing care, 73% were women. 442 Two and a half times as many women as men reported living with the person with dementia full time. 449 Of those providing care to someone with dementia for more than five years, 63% were women. 442 Similarly, caregivers who are women may experience slightly higher levels of burden, impaired mood, depression and impaired health than do caregivers who are men, with evidence suggesting that these differences arise because female caregivers tend to spend more time caregiving, assume more caregiving tasks, and care for someone with more cognitive, functional and/or behavioral problems. 450 , 451 , 452 Among dementia caregivers who indicated a need for individual counseling or respite care, the large majority were women (individual counseling, 85%, and respite care, 84%). 442
5.1.2. Race, ethnicity and dementia caregivingA1
Only recently have population‐based studies examined racial disparities in dementia caregiving. Close to half of Black and Hispanic individuals with dementia live with adult children (47.1%), compared with less than a quarter of White individuals with dementia (24.6%). 453 Compared with White caregivers, Black caregivers are more likely to provide more than 40 hours of care per week (54.3% versus 38.6%) and care for someone with dementia (31.7% versus 11.9%). Black dementia caregivers are also more likely to provide help with ADLs than White dementia, White non‐dementia and Black non‐dementia caregivers. 454 , 455 Black male dementia caregivers are 3.3 times more likely to experience financial burdens when compared with Black female and White male and female dementia caregivers, whereas Black and White male dementia caregivers are 37% to 71% less likely than White female dementia caregivers to indicate emotional burden. 456 Black dementia caregivers were found to be 69% less likely than White caregivers to use respite services, although the need for dementia care relief is considerable among Black families. 457 , 458 Hispanic, Black and Asian American dementia caregivers indicate greater care demands, less outside help/formal service use and greater depression compared with White caregivers. 459 , 460 , 461 In a nationally representative study, 462 Black and Hispanic participants had poorer health prior to becoming a caregiver for a spouse with dementia than those of similar race/background who did not become caregivers; such differences were not apparent among White caregivers. Discrimination is also linked with depressive symptoms among African American dementia caregivers. 463
Black caregivers are more likely than White caregivers to report positive aspects of caregiving. 454 A meta‐analysis found that Black dementia caregivers indicate slightly higher psychological well‐being than White dementia caregivers. Hispanic dementia caregivers, however, reported slightly lower physical well‐being than White dementia caregivers. 464 Other research has examined variations in self‐rated health among dementia caregivers of diverse racial and ethnic backgrounds. Support from family and friends is associated with better self‐rated health for Black dementia caregivers but not for White or Hispanic caregivers. 459 Having a more positive perceived relationship between the caregiver and person with dementia was associated with better self‐rated health among Black and White caregivers. 459 Non‐Hispanic Black dementia family caregivers are less likely to exercise and live with diabetes than non‐Hispanic White and non‐Hispanic Asian dementia family caregivers. 465
The need for culturally informed theories, research frameworks, and services for people living with dementia and their caregivers is pronounced. 466 , 467 , 468 , 469 , 470 Cultural values (e.g., familismo: the Latino cultural value of placing family needs and loyalty to one's family above one's own needs) may influence disparities in perceptions and use of support among caregivers across diverse racial and ethnic contexts. 471 , 472 Underutilization of needed services on the part of Latino dementia caregivers may be due to culturally incongruent expectations on the part of health care systems and providers that assume that families are the predominant/only support network for Latino individuals with dementia. 473 Black/African‐American dementia caregivers' needs include greater education about dementia treatment, diagnosis and care strategies; navigating what is often perceived as a “broken” health care system; improved access to affordable transportation and health care services; greater education about navigation of family conflict; increased availability of respite support; better communication about dementia within the Black/African‐American community; and increased availability of financial/legal planning. 458 , 474 , 475 , 476
Dementia caregiving is experienced by many, regardless of race or ethnicity. The comparisons above suggest that the experience of caregiving often varies depending on racial and ethnic context. Studies of caregivers often lack sufficient numbers of diverse participants to confirm these findings or delve deeper into them for important insights. Recent reviews and national summits have emphasized the need to revise recruitment strategies to capture the range of dementia care experiences among caregivers of diverse racial and ethnic identity. 461 If representation in dementia care research is not improved, our ability to generalize findings or determine whether findings vary by diverse subgroups is not possible. This hinders the progress of all dementia caregiving research. Furthermore, if individuals continue to lack representation in dementia research, they will not receive the benefits of racially and ethnically sensitive prevention, treatment or care innovations. 459 , 461 Establishing stronger relationships with existing organizations and resources in Black communities, Indigenous communities and other communities of color offers the potential for research‐based partnerships to enhance representation in dementia research and result in more culturally appropriate and effective services. 467 , 473 , 477 , 478 , 479 , 480 , 481 , 482 , 483 , 484 , 485 , 486 , 487 , 488 , 489
5.1.3. Caregiving tasks
The care provided to people with Alzheimer's or other dementias is wide‐ranging and in some instances all‐encompassing. Table 7 summarizes some of the most common types of dementia care provided.
Although the care provided by family members of people with Alzheimer's or other dementias can be similar to that provided by caregivers of people with other conditions, dementia caregivers tend to provide more extensive assistance. 490 Family caregivers of people with dementia are more likely to monitor the health of the care recipient than are caregivers of people without dementia (79% versus 66%). 491 Data from the 2011 National Health and Aging Trends Study indicated that caregivers of people with dementia are more likely than caregivers of people without dementia to provide help with self‐care and mobility (85% versus 71%) and health or medical care (63% versus 52%). 426 , 439 Figure 9 illustrates how caregivers of people with dementia are more likely than caregivers of other older people to assist with ADLs. 441
FIGURE 9.
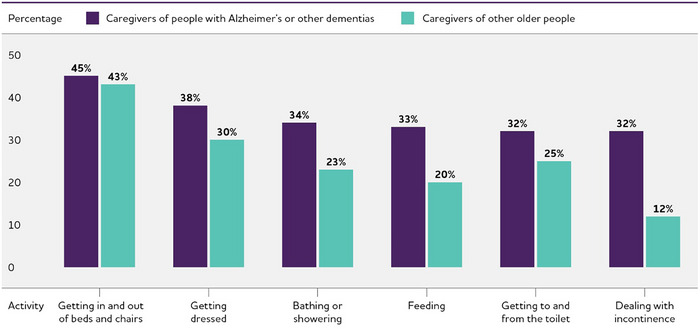
Proportion of caregivers of people with Alzheimer's or other dementias versus caregivers of other older people who provide help with specific activities of daily living, United States, 2015. Created from data from the National Alliance for Caregiving in partnership with the Alzheimer's Association. 441
People with dementia tend to have larger networks of family and friends involved in their care compared with people without dementia. More family members and friends in dementia care networks tend to provide help for household activities, mobility and functional needs, and transportation than family members and friends in non‐dementia care networks. 492
When a person with Alzheimer's or another dementia moves to an assisted living residence or a nursing home, the help provided by his or her family caregivers usually changes from the comprehensive care summarized in Table 7 to providing emotional support, interacting with residential care staff and advocating for appropriate care. However, some family caregivers continue to help with bathing, dressing and other ADLs. 493 , 494
5.1.4. Duration of caregiving
One national poll found that 86% of dementia caregivers provided assistance for at least the past year.A10 According to another study, well over half (57%) of family caregivers of people with Alzheimer's or other dementias living in the community had provided care for four or more years. 433
5.1.5. Hours of unpaid care and economic value of caregiving
In 2023, the 11.5 million family and other unpaid caregivers of people with Alzheimer's or other dementias provided an estimated 18.4 billion hours of unpaid help. This number represents an average of nearly 31 hours of care per caregiver per week, or 1,612 hours of care per caregiver per year.A8 With this care valued at the average of the state minimum wage and the median hourly cost of a home health aide (a conservative estimate),A9 the estimated economic value of care provided by family and other unpaid caregivers of people with dementia across the United States was $346.6 billion in 2023. Table 9 shows the total hours of unpaid care as well as the value of care provided by family and other unpaid caregivers for the United States and each state. Unpaid caregivers of people with Alzheimer's or other dementias provided care valued at more than $5 billion in each of 25 states. Unpaid caregivers provided care valued at more than $18 billion in each of the four most populous states — California, Texas, Florida and New York. A longitudinal study of the monetary value of family caregiving for people with dementia found that the overall value of daily family care increased 18% with each additional year of providing care, and that the value of this care further increased as the care recipient's cognitive abilities declined. 495 In contrast, family care costs are reduced up to 24% in situations where caregivers indicated they were emotionally close to the person with dementia. 496 More research is needed to estimate the future value of family care for people with Alzheimer's disease and other dementias as the U.S. population continues to age, particularly since many existing economic studies only consider primary caregivers when there are often multiple relatives and others helping an older person with dementia. 433 , 497
TABLE 9.
Number of caregivers of people with Alzheimer's or other dementias, hours of unpaid care and economic value of unpaid care by state, 2023.*
| State | Number of caregivers (in thousands) | Hours of unpaid care (in millions) | Value of unpaid care (in millions of dollars) |
|---|---|---|---|
| Alabama | 217 | 387 | 5,310 |
| Alaska | 25 | 39 | 796 |
| Arizona | 292 | 483 | 10,228 |
| Arkansas | 155 | 270 | 4,448 |
| California | 1.373 | 1,864 | 44,272 |
| Colorado | 177 | 307 | 7,249 |
| Connecticut | 128 | 201 | 4,331 |
| Delaware | 31 | 46 | 909 |
| District of Columbia | 14 | 15 | 343 |
| Florida | 840 | 1,321 | 24,437 |
| Georgia | 374 | 755 | 11,417 |
| Hawaii | 60 | 91 | 1,907 |
| Idaho | 66 | 105 | 1,875 |
| Ilinois | 311 | 480 | 9,840 |
| Indiana | 216 | 322 | 5,186 |
| lowa | 98 | 125 | 2,284 |
| Kansas | 89 | 125 | 1,989 |
| Kentucky | 157 | 302 | 4,869 |
| Louisiana | 168 | 256 | 3,428 |
| Maine | 51 | 87 | 1,911 |
| Maryland | 247 | 405 | 8,144 |
| Massachusetts | 213 | 246 | 5,668 |
| Michigan | 380 | 872 | 17,044 |
| Minnesota | 164 | 225 | 5,276 |
| Mississippi | 93 | 175 | 2,380 |
| Missouri | 223 | 350 | 6,478 |
| Montana | 17 | 25 | 478 |
| Nebraska | 40 | 62 | 1,188 |
| Nevada | 84 | 142 | 2,681 |
| New Hampshire | 48 | 77 | 1,529 |
| New Jersey | 272 | 494 | 10,882 |
| New Mexico | 67 | 118 | 2,142 |
| New York | 543 | 879 | 18,996 |
| North Carolina | 373 | 723 | 10,939 |
| North Dakota | 19 | 25 | 465 |
| Ohio | 414 | 624 | 11,427 |
| Oklahoma | 108 | 189 | 3,099 |
| Oregon | 170 | 229 | 5,285 |
| Pennsylvania | 465 | 822 | 13,668 |
| Rhode Island | 36 | 51 | 1,132 |
| South Carolina | 219 | 361 | 5,550 |
| South Dakota | 27 | 34 | 716 |
| Tennessee | 369 | 499 | 7,804 |
| Texas | 1,016 | 1,532 | 23,937 |
| Utah | 112 | 132 | 2,465 |
| Vermont | 19 | 28 | 615 |
| Virginia | 342 | 662 | 12,572 |
| Washington | 247 | 378 | 9,499 |
| West Virginia | 65 | 115 | 1,585 |
| Wisconsin | 205 | 297 | 5,528 |
| Wyoming | 16 | 21 | 385 |
| U.S. Total | 11,457 | 18,376 | $346,585 |
Apart from its long duration, caregiving involves demands that are time‐intensive. Caregivers of people with dementia report providing 27 hours more care per month on average (92 hours versus 65 hours) than caregivers of people without dementia. 439 An analysis of national caregiving trends from 1999 to 2015 found that among dementia caregivers the average hours of care per week increased from 45 in 1999 to 48 in 2015; among non‐dementia caregivers, weekly hours of care decreased from 34 to 24. 498 The amount of time required for caregiving increases as dementia progresses; one study showed that people with dementia required 151 hours of caregiving per month at the outset of dementia and this increased to 283 hours per month eight years later. This is an increase from approximately 5 hours a day to 9 hours a day (it is important to note that some family members/caregivers provide assistance to someone due to cognitive issues before a formal diagnosis of Alzheimer's disease or a related dementia). 499 , 500 Each instance of a decrease in ADL or IADL function in someone with dementia results in nearly five more hours of monthly caregiving compared with a similar functional decrease for someone without dementia. 501 Over a two‐year period, one national study found that impairment in one additional self‐care activity (e.g., bathing, dressing, eating and using the toilet) for those with dementia resulted in 28 additional hours of family care required per month; for those without dementia, an additional self‐care need was associated with an increase in 15 hours of family care per month. 502
5.1.6. Health and economic impacts of Alzheimer's caregiving
Caring for a person with Alzheimer's or another dementia poses special challenges. For example, people in the moderate to severe stages of Alzheimer's dementia experience losses in judgment, orientation, and the ability to understand and communicate effectively. Family caregivers must often help people with dementia manage these issues. The personality and behavior of a person with dementia are affected as well, and these changes are often among the most challenging for family caregivers. 503 , 504 , 505 Individuals with dementia also require increasing levels of supervision and personal care as the disease progresses. As the person with dementia's symptoms worsen, caregivers can experience increased emotional stress and depression; new or exacerbated health problems; and depleted income and finances due in part to disruptions in employment and paying for health care or other services for both themselves and the person living with dementia. 506 , 507 , 508 , 509 , 510 , 511 , 512 , 513
Caregiver emotional and social well‐being
The intimacy, shared experiences and memories that are often part of the relationship between a caregiver and person living with dementia may be threatened due to memory loss, functional impairment and psychiatric/behavioral disturbances that can accompany the progression of dementia. In the 2017 National Poll on Healthy Aging, however, 45% of caregivers of people with dementia indicated that providing help to someone with cognitive impairment was very rewarding. 445 In the 2011 National Study of Caregiving, greater satisfaction from dementia caregiving was associated with more emotional support from family members and friends. 514 Although caregivers report positive feelings about caregiving, such as family togetherness and the satisfaction of helping others,A10 , 515 , 516 , 517 , 518 , 519 , 520 , 521 , 522 , 523 they also frequently report higher levels of burden and stress; depression or other adverse mental health outcomes; strain; and problems with navigating care transitions when compared with other caregivers or non‐caregivers.
Burden and Stress
Compared with caregivers of people without dementia, caregivers of those with dementia indicate more substantial emotional, financial and physical difficulties. 439 , 490
Fifty‐nine percent of family caregivers of people with Alzheimer's or other dementias rated the emotional stress of caregiving as high or very high (Figure 10).A10
Spousal dementia caregivers are more likely than non‐spousal dementia caregivers to experience increased burden over time. This increased burden also occurs when the person with dementia develops behavioral changes and decreased functional ability. 524
Many people with dementia have co‐occurring chronic conditions, such as hypertension or arthritis, which may complicate caregiving. For example, a national study found that caregivers of people with dementia who had a diagnosis of diabetes or osteoporosis were 2.6 and 2.3 times more likely, respectively, to report emotional difficulties with care compared with caregivers of people with dementia who did not have these co‐occurring conditions. 525
FIGURE 10.
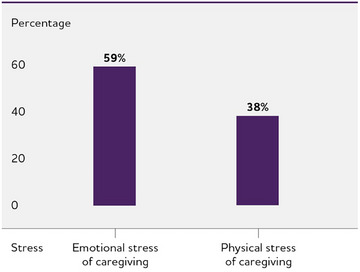
Percentage of caregivers who report high to very high stress due to caregiving. Created from data from the Alzheimer's Association.A10
Depression and Mental Health (see Table 10 )
A meta‐analysis reported that caregivers of people with dementia were significantly more likely to experience depression and anxiety than non‐caregivers. 452 Dementia caregivers also indicate more depressive symptoms than non‐dementia caregivers. 526
The prevalence of depression is higher among dementia caregivers (30% to 40% as reported in multiple studies) than other caregivers, such as those who provide help to individuals with schizophrenia (20%) or stroke (19%). 527 , 528
Caring for a spouse with dementia is associated with a 30% increase in depressive symptoms compared with spousal caregivers of partners without dementia. 529
In a meta‐analysis, the type of relationship was the strongest predictor of caregiver depression; caregivers of spouses with dementia had two‐and‐a‐half times higher odds of having depression than caregivers of people with dementia who were not spouses. 527
The prevalence of anxiety among dementia caregivers is 44%, which is higher than among caregivers of people with stroke (31%). 527
Dementia caregivers in the United States were more likely to have experienced depression (32.5%) or anxiety (26%) when compared with dementia caregivers from Japan (16.8% and 12.9%, respectively) or those from across Germany, Italy, Spain, France and the United Kingdom (29.3% for depression and 22.4% for anxiety). 447
Caregivers of individuals with Alzheimer's report more subjective cognitive problems (for example, problems with memory) and experience greater declines in cognition over time than non‐caregivers matched on age and other characteristics. 530 , 531
Caring for people with dementia who have four or more behavioral and psychological symptoms (for example, aggression, self‐harm and wandering) represents a “tipping point,” as these caregivers are more likely to report clinically meaningful depression and burden. 532
A systematic review found the prevalence of suicidal ideation (thinking about or making plans for suicide) in dementia caregivers with a mean age of 64 was 32% compared with 2.7% in U.S. adults age 56 and older (please note that an exact age comparator is not available). 533 , 534
Providing physical and medical care is associated with worse mental health among dementia caregivers than non‐dementia caregivers. 490
TABLE 10.
Percentage of dementia caregivers reporting health conditions by state, 2016‐2022.
| State | Percentage of caregivers reporting at least one chronic condition | Percentage of caregivers reporting depression | Percentage of caregivers reporting frequent poor physical health |
|---|---|---|---|
| Alabama | 57.5 | 30.9 | 15.0 |
| Alaska | 53.7 | 27.7 | 15.2 |
| Arizona | 66.7 | 27.7 | 15.5 |
| Arkansas | 72.8 | 38.0 | 25.0 |
| California | 61.0 | 18.6 | 13.1 |
| Colorado | 58.0 | 36.7 | 15.5 |
| Connecticut | 64.0 | 27.9 | 9.4 |
| Delaware | 61.8 | 23.3 | † |
| District of Columbia * | 65.1 | † | † |
| Florida | 66.4 | 28.6 | 13.6 |
| Georgia | 64.9 | 33.2 | 15.1 |
| Hawaii | 49.6 | 16.5 | 8.1 |
| Idaho | 57.5 | 31.1 | 13.4 |
| Ilinois | 64.2 | 29.0 | † |
| Indiana | 57.3 | 34.1 | 18.2 |
| lowa | 60.5 | 27.4 | 13.8 |
| Kansas | 60.6 | 33.8 | 18.7 |
| Kentucky | 65.5 | 39.8 | 21.4 |
| Louisiana | 62.4 | 37.2 | 15.9 |
| Maine | 60.8 | 38.0 | 12.8 |
| Maryland | 55.7 | 24.8 | 8.4 |
| Massachusetts | 54.2 | 20.2 | † |
| Michigan | 66.0 | 30.6 | 22.1 |
| Minnesota | 53.1 | 29.8 | 8.4 |
| Mississippi | 57.0 | 25.9 | 22.2 |
| Missouri | 59.5 | 28.1 | 20.2 |
| Montana * | 56.9 | 22.8 | † |
| Nebraska | 57.6 | 25.4 | 13.2 |
| Nevada | 54.2 | 31.1 | † |
| New Hampshire | 66.2 | 28.4 | 14.7 |
| New Jersey | 62.3 | 27.9 | 12.8 |
| New Mexico | 64.8 | 31.3 | 12.6 |
| New York | 59.0 | 24.7 | 12.0 |
| North Carolina | 58.8 | 41.0 | 18.1 |
| North Dakota | 60.1 | 30.4 | 8.6 |
| Ohio | 63.7 | 27.8 | 17.4 |
| Oklahoma | 68.2 | 39.6 | 17.2 |
| Oregon | 57.4 | 33.6 | 8.5 |
| Pennsylvania | 76.6 | 32.5 | 16.0 |
| Rhode Island | 54.2 | 41.0 | 11.5 |
| South Carolina | 60.6 | 31.0 | 15.2 |
| South Dakota | 61.0 | 22.2 | † |
| Tennessee * | 66.7 | 29.8 | † |
| Texas | 59.0 | 26.7 | 11.2 |
| Utah | 59.3 | 34.6 | 14.9 |
| Vermont | 61.5 | 35.4 | 10.7 |
| Virginia | 64.1 | 31.2 | 15.1 |
| Washington | 61.1 | 39.0 | 18.0 |
| West Virginia | 63.5 | 32.2 | 12.0 |
| Wisconsin | 62.9 | 27.8 | 18.9 |
| Wyoming | 59.8 | 22.8 | † |
Data are for caregivers of individuals whose main reason for needing care is Alzheimer's or other dementia, which is not necessarily all caregivers of people with dementia.
Data not included because the sample size was less than 50 or the relative standard error was greater than 30%.
Created from data from the Behavioral Risk Factor Surveillance System Survey. 442
Other Key Findings About the Challenges of Dementia Caregiving
Caregivers of people with Alzheimer's or other dementias are twice as likely as caregivers of individuals without dementia (22% compared with 11%) to report that completing medical/nursing‐related tasks (for example, injections, tube feedings and catheter/colostomy care) was difficult. 491
Dementia caregivers often experience challenges managing medications for individuals with dementia, such as non‐adherence. 535 , 536 , 537 , 538
Compared with non‐dementia caregivers, dementia caregivers indicate a greater decrease in their social networks (e.g., other relatives, friends, acquaintances). 539
According to a national Alzheimer's Association poll of caregivers, respondents often believed they had no choice in taking on the role of caregiver.A10
The poll also found that more than half (53%) of women with children under age 18 felt that caregiving for someone with dementia was more challenging than caring for children.A10
Non‐heterosexual dementia caregivers are significantly younger and more likely to be employed than heterosexual dementia caregivers and indicate greater difficulty when paying for necessities while also reporting higher family quality of life than their heterosexual peers. 540
Many caregivers of people with Alzheimer's or other dementias are at risk of social isolation. 541 Forty‐one percent of dementia caregivers in the 2014 Alzheimer's Association poll reported that no one else provided unpaid assistance.A10
Among dementia caregivers of care recipients who have experienced severe psychiatric symptoms (e.g., aggression, anxiety), those who live in low‐ or medium‐income neighborhoods indicate higher distress than those caregivers living in high‐income neighborhoods. 542
In a survey of caregivers from a large health care system, fewer than 4 in 10 respondents (39.2%) agreed that their primary care providers help them with managing symptoms of a care recipient with dementia. 543
Stress of Care Transitions
Caregivers who helped someone with a formal diagnosis of dementia indicated more emotional difficulty and family disagreement than caregivers of individuals without a formal diagnosis. However, those caregivers of individuals with a formal dementia diagnosis were also more engaged in communication during doctors’ visits and also more likely to receive caregiver training than those who assisted someone without a diagnosis of dementia, suggesting the importance of linking support to dementia diagnostic procedures. 544
Admitting a relative to a residential care facility has mixed effects on the emotional and psychological well‐being of dementia family caregivers. Some studies suggest that distress remains unchanged or even increases for some caregivers (such as spouses), but other studies have found that distress decreases. 494 , 545 , 546 , 547
The demands of caregiving may intensify as people with dementia approach the end of life. 548 In the year before the death of the person living with dementia, 59% of caregivers felt they were “on duty” 24 hours a day, and many felt that caregiving during this time was extremely stressful. 549 The same study found that 72% of family caregivers experienced relief when the person with Alzheimer's or another dementia died. 549
In the last 12 months of life, people with dementia relied on more hours of family care (64.5 hours per week) than people with cancer (39.3 hours per week). 550
Caregiver physical health and health conditions
For some caregivers, the demands of caregiving may cause declines in their own health. Evidence suggests that the stress of providing dementia care increases caregivers’ susceptibility to disease and health complications. 551 As shown in Figure 10, 38% of Alzheimer's and other dementia caregivers indicate that the physical stress of caregiving is high to very high.A10 Dementia caregivers are 1.5 times more likely to indicate substantial physical difficulty providing assistance to their care recipients compared with non‐dementia caregivers. 552 The distress associated with caring for a relative with Alzheimer's or another dementia has also been shown to negatively influence the quality of family caregivers’ sleep. 553 , 554 , 555 , 556 Compared with those of the same age who were not caregivers, caregivers of people with dementia are estimated to lose between 2.4 hours and 3.5 hours of sleep a week. 554
Tables 10 and 11 present data on caregiver physical and mental health. Table 10 presents state‐by‐state data on the health status of dementia caregivers, and Table 11 compares the percentages of dementia caregivers, non‐dementia caregivers and non‐caregivers who report having a specific chronic health condition.
TABLE 11.
Percentage of dementia caregivers who report having a chronic health condition compared with caregivers of people without dementia or non‐caregivers.
| Condition | Dementia caregivers | Non‐dementia caregivers | Non‐caregivers |
|---|---|---|---|
| Stroke | 5.2 | 3.4 | 3.2 |
| Coronary heart disease | 8.3 | 7.2 | 6.6 |
| Cardiovascular disease* | 11.8 | 9.5 | 8.6 |
| Diabetes | 12.8 | 11.1 | 11.3 |
| Cancer | 14.3 | 13.3 | 11.5 |
| Obesity | 32.7 | 34.6 | 29.5 |
Combination of coronary heart disease and stroke.
Table includes caregivers age 18 and older.
Created from data from the Behavioral Risk Factor Surveillance System survey. 442
General Health
Seventy‐four percent of caregivers of people with Alzheimer's or other dementias reported that they were “somewhat concerned” to “very concerned” about maintaining their own health since becoming a caregiver.A10 A 2017 poll found that 27% of dementia caregivers delayed or did not do things they should to maintain their own health. 445 , 557 , 558 Data from the Health and Retirement Study showed that dementia caregivers who provided care to spouses were much more likely (41% increased odds) than other spousal caregivers of similar age to become increasingly frail during the time between becoming a caregiver and their spouse's death. 559 , 560 , 561 , 562
Physiological Changes
The chronic stress of caregiving may be associated with an increased incidence of hypertension and a number of physiological changes that could increase the risk of developing chronic conditions, including high levels of stress hormones, impaired immune function, slow wound healing and coronary heart disease. 563 , 564 , 565 , 566 , 567 , 568 , 569 , 570 A recent meta‐analysis of studies examining the associations between family caregiving, inflammation and immune function suggests that dementia caregivers had slight reductions in immune function and modestly elevated inflammation. 571 However, a study of physiological changes before and after the start of caregiving found no change in six biomarkers of inflammation among dementia caregivers. 572
Health Care
When people with dementia also have depression, behavioral disturbances or low functional status, their caregivers face a higher risk of emergency department visits and hospitalization compared with caregivers of people with dementia without these challenges. 573 , 574 Increased depressive symptoms among caregivers are linked to more frequent caregiver doctor visits, increased outpatient tests and procedures, and greater use of over‐the‐counter and prescription medications. 574 Dementia caregivers also have twice the odds of experiencing an overnight hospitalization than non‐caregivers. 575
Mortality
Studies of how the health of people with dementia affects their caregivers’ risk of dying have had mixed findings. 576 , 577 For example, spouses of hospitalized care recipients with dementia were more likely to die in the following year than caregivers whose spouses were hospitalized but did not have dementia (after accounting for differences in caregiver age). 578 In addition, caregivers who perceived higher strain due to care responsibilities were at higher risk for death than caregivers who perceived little or no strain. 579 In contrast, a longitudinal analysis of the Health and Retirement Study found that dementia caregivers were less likely to die than non‐caregivers of similar age over a 12‐year period. These results are consistent with a protective effect of dementia care, at least as it pertains to mortality. 576 The findings are also consistent with the possibility that individuals who assume dementia care roles do so in part because their initial health allows them to do so. Eighteen percent of spousal caregivers die before their partners with dementia. 580
Caregiver employment and finances
Six in 10 caregivers of people with Alzheimer's or another dementia were employed or had been employed in the prior year while providing care. 441 These individuals worked an average of 35 hours per week while caregiving. 441 Among people who were employed in the past year while providing care to someone with Alzheimer's or another dementia, 57% reported sometimes needing to go in late or leave early compared with 47% of non‐dementia caregivers. Eighteen percent of dementia caregivers reduced their work hours due to care responsibilities, compared with 13% of non‐dementia caregivers. In particular, adult daughters with less than a high school degree were most likely to reduce work hours when compared with other dementia caregivers. Other work‐related changes among dementia and non‐dementia caregivers who had been employed in the past year are summarized in Figure 11. 441 In the 2018 National Health and Wellness Survey, close to 13% of dementia caregivers in the United States indicated absence from work in the past seven days due to a health problem compared with 6% of dementia caregivers in Japan and 10% of dementia caregivers across France, Germany, Italy, Spain and the United Kingdom. 447 In addition, caregivers living with a family member with dementia pay for 64% of total care costs (e.g., total health care spending and out‐of‐pocket costs) incurred during their relatives' last seven years of life. 581
FIGURE 11.
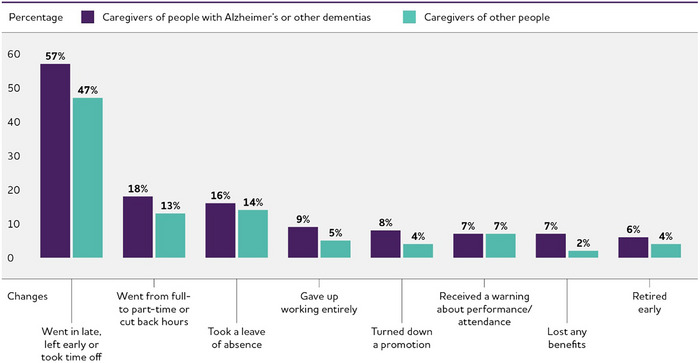
Work‐related changes among caregivers of people with Alzheimer's or other dementias who had been employed at any time since they began caregiving. Created from data from the National Alliance for Caregiving in partnership with the Alzheimer's Association. 441
In 2021, it was estimated that dementia caregivers bore nearly twice the average out‐of‐pocket costs of non‐dementia caregivers ($12,388 versus $6,667). 430 , 582 Examples include costs of medical care, personal care and household expenses for the person with dementia, and personal expenses and respite services for the caregiver. Caregivers of a spouse with dementia indicate higher home health care expenditures but lower outpatient expenditures than those who do not have a spouse with dementia, which suggests a possible “substitution” effect and greater referrals to home health care by providers for patients with dementia. 583 , 584 National survey data among “care contributors” (or, a friend or relative who paid for dementia expenses and/or provided care for someone with dementia at least once a month in the prior year) revealed that 48% cut back on other spending and 43% cut back on savings due to the out‐of‐pocket costs of providing help to someone with dementia. 512 Due to care responsibilities, close to 4 in 10 care contributors indicated that the “food they bought just didn't last, and they didn't have money to get more,” and 3 in 10 ate less because of care‐related costs. 512
One in five caregivers of people with Alzheimer's or other dementias (22%) report problems dealing with a bank or credit union when helping to manage the finances of people living with dementia, compared with 9% of caregivers of people without dementia. 441
Effects of stress and other caregiving factors on people with dementia
Research has documented the effects of caregiver stress on people with dementia and their use of health care services. For example, distress on the part of family caregivers is associated with increased odds of residential care entry for the person with dementia, exacerbated behavioral and psychological challenges in the person with dementia, and increased likelihood of someone with dementia being abused. 585 Individuals with dementia are more likely to be hospitalized if their caregiver has less than one year of caregiving experience when compared with caregivers who have provided assistance for more than one year. 586 In addition, care recipients with dementia whose caregivers indicate greater distress are also more likely to experience hospitalization. 586 , 587 A synthesis of available qualitative studies found that “personhood,” or the extent to which others value, support and establish meaningful relationships with someone with dementia, is enhanced through personal interactions with family, friends, other people with dementia and professional caregivers as well as through opportunities for ongoing engagement in social and occupational activities/roles. 588 Some meta‐analyses suggest that care coordination/case management and psychoeducational and multi‐component programs delivered to dementia caregivers may improve important care recipient outcomes, including improvements in behavior, mood and quality of life and delayed placement in a residential care home. However, effects sizes are small. 589
5.1.7. Interventions designed to assist caregivers
For more than 35 years, strategies to support family caregivers of people with dementia have been developed and evaluated. The types and focus of these strategies (often called “interventions”) are summarized in Table 12. 510 , 590
TABLE 12.
Type and focus of caregiver interventions.
| Type | Focus |
|---|---|
| Case management | Provides assessment, information, planning, referral, care coordination and/or advocacy for family caregivers. |
| Psychoeducational approaches | Include structured programs that provide information about the disease, resources and services, and about how to expand skills to effectively respond to symptoms of the disease (for example, cognitive impairment, behavioral symptoms and care‐related needs). Include lectures, discussions and written materials and are led by professionals with specialized training. |
| Counseling | Aims to resolve pre‐existing personal problems that complicate caregiving to reduce conflicts between caregivers and care recipients and/or improve family functioning. |
| Psychotherapeutic approaches | Involve the establishment of a therapeutic relationship between the caregiver and a professional therapist (for example, cognitive‐behavioral therapy for caregivers to focus on identifying and modifying beliefs related to emotional distress, developing new behaviors to deal with caregiving demands, and fostering activities that can promote caregiver well‐being). |
| Respite | Provides planned, temporary relief for the caregiver through the provision of substitute care; examples include adult day services and in‐home or institutional respite care for a certain number of weekly hours. |
| Support groups | Are less structured than psychoeducational or psychotherapeutic interventions. Support groups provide caregivers the opportunity to share personal feelings and concerns to overcome feelings of isolation. |
| Multicomponent approaches | Are characterized by intensive support strategies that combine multiple forms of interventions, such as education, support and respite, into a single, long‐term service (often provided for 12 months or more). |
In general, the goal of interventions is to improve the health and well‐being of dementia caregivers by relieving the negative aspects of caregiving. Some also aim to delay nursing home admission of the person with dementia by providing caregivers with skills and resources (emotional, social, psychological and/or technological) to continue helping their relatives or friends at home. Specific approaches used in various interventions include providing education to caregivers, helping caregivers manage dementia‐related symptoms, improving social support for caregivers and providing caregivers with respite from caregiving duties.
According to a publication on dementia caregiver interventions that reviewed seven meta‐analyses and 17 systematic reviews of randomized controlled trials, the following characteristics distinguish interventions that are effective: family caregivers are actively involved in the intervention, in contrast to passively receiving information; the intervention is tailored and flexible to meet the changing needs of family caregivers during the course of a relative's dementia; and the intervention meets the needs not only of caregivers but of people living with dementia as well. 591 A meta‐analysis examining the components of dementia caregiver interventions that are most beneficial found that interventions that initially enhance caregiving competency, gradually address the care needs of the person with dementia, and offer emotional support for loss and grief when needed appeared most effective. 592 A prior report examined randomized, controlled studies of caregiver interventions and identified 44 interventions that benefited individuals with dementia as well as caregivers, and more such interventions are emerging each year. 593 , 594 , 595 , 596 , 597 , 598 Although several national reports have suggested that the available scientific evidence does not provide clear suggestions as to which intervention types benefit dementia caregivers consistently, 599 other recent meta‐analyses report that specific intervention types (such as psychoeducation; see Table 12) may result in a small reduction in burden for caregivers, with other meta‐analyses indicating broader effects of various interventions across multiple dementia caregiver outcomes. 589 , 600 , 601 , 602 , 603 , 604 A meta‐review of over 60 meta‐analyses and systematic reviews of dementia caregiver interventions indicate that although various interventions may have positive effects on depression and other measures of caregiver well‐being, challenges related to how interventions are reported and classified has made it difficult to ascertain what works and why for dementia caregivers. 605
Interventions for dementia caregivers that have demonstrated efficacy in scientific evaluations have been gradually implemented in the community, but are still not widespread or available to all family caregivers. 606 , 607 , 608 When interventions are implemented, they are generally successful at improving how caregiver services are delivered and have the potential to reach a large number of families while also helping caregivers cope with their responsibilities (this includes the Alzheimer's Association 24/7 Helpline). 609 , 610 , 611 , 612 In one example, researchers utilized an “agile implementation” process to more rapidly select, locate, evaluate and replicate a collaborative care model for dementia care. This care model has successfully operated for over a decade in an Indianapolis health care system. 613 Other efforts have attempted to broaden the reach and accessibility of interventions for dementia caregivers through the use of technologies (for instance, video‐phone delivery and online training), 614 , 615 , 616 , 617 , 618 , 619 , 620 , 621 , 622 while others have disseminated evidence‐based dementia care interventions into community‐based programs and health care systems. 609 , 623 , 624 Dissemination efforts, such as Best Practice Caregiving, have attempted to provide tools and resources to providers and others to facilitate the implementation of successful interventions into community‐based organizations, health care systems and other “real‐world” settings. 625
Because caregivers and the settings in which they provide care are diverse, more studies are required to define which interventions are most effective for specific situations and how these interventions are successful. 626 , 627 , 628 , 629 , 630 Improved tools and measures to personalize services for caregivers to maximize their benefits represent an emerging area of research. 631 , 632 , 633 , 634 , 635 , 636 More studies are also needed to adapt proven interventions or develop new intervention approaches for families from different racial, ethnic and socioeconomic backgrounds and in different geographic settings. 436 , 461 , 637 , 638 , 639 , 640 , 641 , 642 , 643 , 644 Additional research on interventions focused on disease stages is also required, as is research on specific intervention needs for LGBT caregivers for whom a lack of inclusive practices on the part of health care professionals, stigma, and a reluctance to seek support may result in greater unmet needs compared with non‐LGBT dementia caregivers. 438 , 645 , 646
In 2019, the National Institute on Aging (NIA) awarded funding to create the NIA Imbedded Pragmatic AD/ADRD Clinical Trials (IMPACT) Collaboratory. The Collaboratory includes experts from more than 30 research universities/ centers and supports pilot trials and larger studies that test non‐drug, care‐based interventions for people living with dementia. The goal of IMPACT is to expedite the timeline of research implementation in real‐world settings to improve care for people living with dementia and their caregivers. In 2020, the CDC established three Public Health Centers of Excellence on dementia to disseminate best practices and tools to local, tribal and state public health organizations throughout the United States; one of those Centers focuses on dementia caregiving.
The Alzheimer's Association has also undertaken several efforts to improve dementia care interventions and services. Its dementia care practice recommendations 647 place individuals with dementia and their caregivers at the center of how care should be delivered (see Figure 12). Essential to this model is the need to reconsider how care for people with dementia is measured and designed by moving away from an approach that focuses on loss of abilities to one that emphasizes the individual's unique needs, personal experiences and strengths. This person‐centered care philosophy not only values and respects the individual with dementia but also promotes well‐being and health. 588 , 648 Frameworks such as the Alzheimer's Association dementia care practice recommendations are designed to shift how researchers and care providers think about dementia and may point the way to a greater understanding of the resilience, adaptability and possibilities of maintenance or even improvement of skills and abilities when living with dementia. 649 , 650 A core element of these frameworks is ensuring that every experience and interaction is seen as an opportunity to have authentic and meaningful engagement, which in turn helps create a better quality of life for the person with dementia and their caregivers.
FIGURE 12.
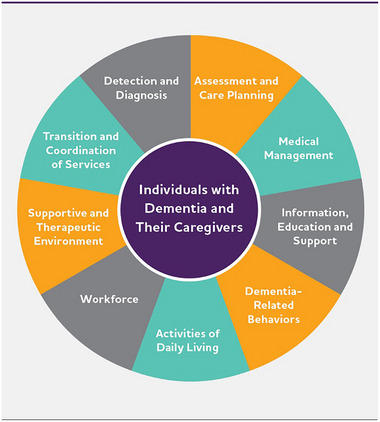
Person‐centered care delivery. Created from data from the Alzheimer's Association. 647
5.2. Trends in dementia caregiving
There is some indication that families have greater capacity to manage the care they provide to relatives with dementia than in the past. Compared with dementia caregivers in 1999, dementia caregivers in 2015 were significantly less likely to report physical difficulties (from 30% in 1999 to 17% in 2015) and financial difficulties (from 22% in 1999 to 9% in 2015) related to care provision. In addition, use of respite care by dementia caregivers increased substantially (from 13% in 1999 to 27% in 2015). 498 However, as noted earlier, more work is needed to ensure that interventions for dementia caregivers are available and accessible to those who need them. A 2016 study of the Older Americans Act's National Family Caregiver Support Program found that over half (52%) of Area Agencies on Aging did not offer evidence‐based family caregiver interventions. 651 In addition, there is some indication that the number of family members available to provide care to older relatives with health needs is likely to decrease due to a range of sociodemographic and health trends in the U.S. (e.g., the aging of the U.S. population, a lower birth rate and adult children's geographic mobility/dispersion over the prior several decades). 652 The need to bridge this impending “family care gap” and other dementia caregiving challenges and concerns through new policies, services and research is a growing public health concern. 653 , 654
5.3. COVID‐19 and dementia caregiving
Existing reports indicate that the COVID‐19 pandemic has posed significant social, psychological, emotional and physical challenges to family members and friends who provide care for people with dementia. 655 , 656 , 657 , 658 , 659 , 660 , 661 , 662 , 663 , 664 , 665 Fatigue and burnout among dementia caregivers and their lack of access to services and supports for themselves and for the people for whom they provide care are common themes in research on the wide‐ranging effects of the COVID‐19 pandemic. 666 For these reasons, the pandemic has created a crisis for dementia caregivers. 655 , 667 , 668
Telephone interviews with family caregivers in rural Virginia following the governor's stay‐at‐home order in 2020 found that those who were more concerned about the COVID‐19 pandemic and those who received less help from family and friends experienced greater feelings of emotional exhaustion and fatigue related to dementia care. 669 In the earlier stages of the pandemic, caregivers were limited in or completely barred from visiting and communicating with relatives who lived in long‐term care residences due to COVID‐19 lockdown procedures. The inability to visit or engage with relatives resulted in distress as well as significant concerns about the health of relatives living in residential long‐term care during the pandemic. 670 , 671 Studies of end‐of‐life care during the pandemic indicated that dementia caregivers felt that enforced social isolation was prevalent and adversely influenced the death and dying experience of relatives during the pandemic. 672 Adult day programs and other community‐based services in many states were interrupted or closed. 673 These and other factors shaped by the COVID‐19 pandemic have caused emotional distress and other negative outcomes among caregivers. 622 , 674 In addition, staff and directors of adult day service programs in the United States reported perceived declines in cognition, function and well‐being among clients due to state closures during the pandemic. 674 Together, this suggests the need for improved support of long‐term programs that serve community‐residing people with dementia and their caregivers as well as strategies/policies to maintain links between family caregivers and residents of congregate care settings (assisted living, nursing homes) during future public health emergencies. 675 , 676
Studies have shown that family caregivers who were able to engage in more direct phone and email contact with relatives in long‐term care residences during COVID‐19 lockdowns indicated greater emotional well‐being for themselves and their relatives, whereas relying on residential care staff to engage in communication resulted in lower perceived well‐being among family caregivers and their relatives. 677 Other studies suggested that some dementia family caregivers adjusted during the pandemic by relying more heavily on other sources of family/unpaid help as well as technologies to maintain social connection with relatives. 678 , 679 , 680 , 681 In studies of dementia caregivers of relatives living in nursing homes or similar residential settings, caregivers indicated a number of challenges during the COVID‐19 pandemic, including severely limited contact with relatives due to visitation restrictions, a lack of transparent information and communication from care residences, fears of relatives dying alone and concerns about overburdened staff at care residences. 671 , 682 In addition, caregivers highlighted a number of resources and practices that were helpful during COVID‐19, including effective infection control measures adopted by care residences, robust communication with staff, and the need for creativity when remaining socially connected with relatives in nursing homes or similar residential settings. 671 There is also evidence of racial and gender differences in dementia care provision during the pandemic. Compared with White dementia and non‐dementia caregivers as well as Black non‐dementia caregivers, Black dementia caregivers provided greater ADL care to relatives with dementia. 455 Providing telehealth support to dementia caregivers that was culturally appropriate, delivering COVID‐19 safety education, and offering compassionate listening appeared to benefit social connections and reduce distress. 683 , 684 In a survey, women dementia caregivers were more likely to indicate needs related to carrying out caregiving responsibilities during the pandemic, whereas men indicated more needs for health and social resources. Men were also more likely to report psychological distress. 685
At the outset of the pandemic, the National Institutes of Health and other federal agencies issued multiple requests for rapid grant applications to study and design interventions to mitigate the effects of COVID‐19 on people with dementia and their caregivers. 686 The Alzheimer's Association also provided regularly updated guidance for dementia caregivers and professional care providers as the pandemic unfolded. In addition, the challenges of the pandemic have motivated some service providers to transition their support programs toward remote/virtual care delivery, which has helped to extend the reach and accessibility of dementia care innovations. 687 , 688 Concerns remain, however, about the “digital divide” facing caregivers who do not have reliable broadband access or do not regularly use the internet.
5.4. A national strategy to support family caregivers
The Recognize, Assist, Include, Support, and Engage (RAISE) Family Caregivers Act, which was signed into law in January 2018, authorized the Secretary of Health and Human Services to develop the first national strategy to support family caregivers. To advance the development of this strategy, a 30‐member Family Caregiving Advisory Council was established to provide key recommendations, guidance and best practices that support family caregivers. In September 2022 the Advisory Council delivered its National Strategy to Support Family Caregivers to Congress. It features nearly 350 actions that 15 federal agencies will adopt and 150 actions that states, communities and others can take. The four core principles that drive these many supportive actions include: 1) placing the family and person at the center of all interactions; 2) addressing trauma and its impact on families; 3) advancing equity, accessibility and inclusion for family caregivers in underserved communities; and 4) elevating direct care workers as family caregiving partners. 689
On July 31, 2023, the Centers for Medicare & Medicaid Services (CMS) announced that beginning July 2024 it will support the Guiding an Improved Dementia Experience (GUIDE) Model until 2032. The GUIDE Model features the provision of comprehensive dementia care coordination and management, caregiver education and support, and respite services. Individuals living with dementia and their caregivers will also have access to a 24/7 support line. The GUIDE Model is unique in that it incentivizes providers to incorporate both the person with dementia and the caregiver (or caregivers) into the collaborative, multidisciplinary service approach. Critically, CMS will include policies to ensure that underserved communities have equal access to GUIDE Model services to address disparities in access to and quality of dementia care. 690
6. WORKFORCE
As the prevalence of Alzheimer's disease and other dementias increases, so does the need for more members of the paid workforce to be knowledgeable and skillful about working with a diverse population of people living with dementia as well as with their families. 691 , 692 A dementia‐capable workforce addresses the full arc of care — from identifying a concern to screening, detecting and diagnosing within clinical settings, to treating, monitoring and caring for those living with these diseases in residential or home and community‐based settings. This workforce includes, but is not limited to, primary care physicians (PCPs) and advanced practice clinicians; specialists, such as geriatricians, neurologists and psychiatrists; other licensed providers, such as registered nurses, psychologists, therapists and social workers; members of the direct care workforce, including personal care aides, home health aides and nursing assistants; and the broader community‐based workforce who interact with the public and help meet the needs of people living with dementia, such as police officers, bank tellers, librarians, hairdressers, bus drivers and others.
6.1. Screening, detecting and diagnosing workforce
Improving dementia screening, detection and diagnosis is a high priority. 267 , 693 , 694 , 695 A recent study of Medicare beneficiaries found that only about 8% of expected mild cognitive impairment (MCI) cases are diagnosed on average, suggesting there may be as many as 8 million people with undiagnosed MCI (acknowledging that not all individuals with MCI develop dementia; see Overview). 271 , 696 Among over 200,000 clinicians and practices surveyed, only 0.1% had diagnosis rates within the expected range, likely due to limited expertise and confidence and other factors discussed on the following pages. 271 See the Special Report from 2022 Alzheimer's Disease Facts and Figures that examines consumers’ and primary care physicians' perspectives on awareness, diagnosis and treatment of MCI, including MCI due to Alzheimer's disease. 695
With early detection of cognitive impairment comes opportunities for individuals and their families to plan for future care and to participate in clinical trials or be treated with FDA‐approved disease‐modifying therapies. Suboptimal detection and diagnosis of dementia, conversely, reduces the ability of individuals and their families to make informed decisions, access appropriate medical care, create financial and legal plans, and pursue services and support. Timely and accurate detection is particularly important considering that dementia is progressive. While more evidence is needed to support screening of asymptomatic individuals, 697 it is generally accepted that clinically significant cognitive concerns that arise in the primary care setting should be followed by an evaluation for cognitive impairment using a standardized and validated assessment. 698 , 699 , 700 See the Special Report from 2019 Alzheimer's Disease Facts and Figures that explores the state of cognitive assessment in the primary care setting and identifies potential solutions for existing barriers to widespread adoption of assessment in primary care settings. 267
Health care professionals who are involved in screening for, detecting, and/or diagnosing Alzheimer's and other dementias include PCPs (e.g., family medicine and internal medicine physicians), advanced practice clinicians (e.g., nurse practitioners and physician assistants), and specialists such as geriatricians (who specialize in caring for older adults), neurologists (especially geriatric and cognitive neurologists), neuropsychologists, geropsychologists and geriatric psychiatrists. However, limited skill and confidence in diagnosing dementia, 701 , 702 time constraints among PCPs during routine office visits 703 , 704 and a widespread shortage of geriatricians and other specialists has resulted in delayed screening, detection and diagnosis of Alzheimer's disease and other dementias.
6.1.1. Primary care physicians
PCPs are most likely to make the initial diagnosis of dementia. 702 , 705 A study of Medicare beneficiaries found that 85% of people living with dementia were diagnosed by providers who do not specialize in dementia (e.g., PCP, emergency medicine physician, nurse practitioner, clinical psychologist). Among the remaining 15% diagnosed by a provider who specializes in dementia, 47% were diagnosed by a psychiatrist, including geriatric psychiatrists and neuropsychiatrists, 44% by a neurologist and 9% by a geriatrician. 706
PCPs are well‐situated to detect dementia because they often have long‐standing relationships with patients and may witness clinical manifestations of cognitive decline — both overt functional and communication changes and subtle signs, such as irregularities in medication or appointment adherence, loss of control of chronic disease, weight loss, or increase in emergency room visits or hospitalizations. Even though the vast majority of initial dementia diagnoses are made by PCPs, studies have found that diagnosis is delayed until moderate or advanced stages in 50% or more patients with Alzheimer's, 707 , 708 with greater delays among individuals from racial and ethnic minority groups. 258 , 709 , 710 , 711 It is important to reiterate that many people living with dementia never receive a diagnosis in the primary care setting (see Prevalence section). During these delays or lack of diagnosis, people living with dementia could otherwise have been enrolled in potentially life‐changing clinical trials of new treatments, begun taking currently approved treatments, begun receiving emotional support through a support group of others living with dementia, and started planning for financial, caregiving and accommodation changes they may experience as their condition progresses.
If a person shows signs of cognitive impairment during a routine doctor's visit, Medicare covers a separate visit to assess the person's cognitive function and develop a care plan. 712 , 713 As of January 1, 2024, Medicare reimbursed approximately $268 to physicians and other eligible billing practitioners for providing a comprehensive clinical visit that results in a written care plan (current procedural terminology code 99483; rate may be geographically adjusted). 712 , 714 , 715 , 716 Although screening is now a reimbursable service by Medicare, 712 PCPs experience numerous barriers to detecting cognitive impairment and diagnosing dementia. 267 For instance, commonly used cognitive assessments take time and training to administer, interpret, document and follow up on, which makes them hard to use in a busy practice setting. 702 , 717 Furthermore, the next steps following detection can be seen as a barrier as many PCPs report low confidence disclosing a dementia diagnosis and providing post‐diagnostic care. 702 , 718 Even if dementia is diagnosed, providers sometimes wait to disclose this information to the patient due to diagnostic uncertainty, time constraints, stigma and fear of causing emotional distress. The U.S. Government Accountability Office (GAO) found that use of the cognitive assessment and care plan service in traditional fee‐for‐service Medicare tripled from 2018 through 2022. However, use of the service was relatively low among Medicare beneficiaries diagnosed with a cognitive impairment; GAO estimated that in 2021, the most recent year of data available, only 2.4% of beneficiaries with a dementia diagnosis received the service through traditional Medicare. 719
Among PCPs surveyed by the Alzheimer's Association in 2019, nearly 40% reported that they were “never” or “only sometimes” comfortable making a diagnosis of Alzheimer's or another dementia. 701 More than 25% of PCPs reported being “never” or “only sometimes” comfortable answering patient questions about Alzheimer's or other dementias, and 50% did not feel adequately prepared to care for individuals who had been diagnosed. Given this discomfort and uncertainty, almost one‐third of PCPs in the survey reported referring patients to specialists; however, most PCPs (55%) reported that there were not enough specialists (e.g., geriatricians) in their area to meet the demand. 701 See the Special Report from 2020 Alzheimer's Disease Facts and Figures that examines the gaps and projected shortages in specialty care for Alzheimer's and other dementias. 720
6.1.2. Geriatricians and other specialists
There is a particular need for geriatricians to screen for, detect, and diagnose possible dementia. Geriatricians are family physicians or board‐certified internists who are specially trained to evaluate and manage the unique health care needs and treatment preferences of older adults.
Up to 30% of people age 65 and older are estimated to need a geriatrician. 721 There were approximately 5,170 to 7,454 geriatricians in the United States in 2021, depending on the source of the estimate, 722 , 723 indicating a sizable and potentially consequential shortage relative to need. Indeed, the National Center for Health Workforce Analysis (NCHWA) determined that there was already a shortage of geriatricians a decade ago, 724 and the projected increase in demand for geriatricians by 2050 is expected to far exceed the supply in every region of the United States. 721 , 724 , 725
Table 13 shows state‐by‐state projections for the number of geriatricians needed in 2050, using December 2021 data from IQVIA as a starting point. 723 If things continue at the current pace, the United States will have to nearly triple the number of geriatricians who were practicing in 2021 to effectively care for the approximately 10% of those 65 and older who are projected to have Alzheimer's dementia in 2050. The number must increase nearly nine times to have enough geriatricians to care for the approximately 30% of the population age 65 and older who will need geriatrician care. 721
TABLE 13.
Number of geriatricians in 2021 and projected number of geriatricians needed in 2050 by state.
| State | Number of geriatricians in 2021 | Number of geriatricians needed in 2050 to serve 10% of those 65 and older | Number of geriatricians needed in 2050 to serve 30% of those 65 and older | State | Number of geriatricians in 2021 | Number of geriatricians needed in 2050 to serve 10% of those 65 and older | Number of geriatricians needed in 2050 to serve 30% of those 65 and older |
|---|---|---|---|---|---|---|---|
| Alabama | 33 | 228 | 684 | Montana | 9 | 59 | 177 |
| Alaska | 8 | 31 | 92 | Nebraska | 23 | 84 | 253 |
| Arizona | 92 | 363 | 1,089 | Nevada | 43 | 158 | 474 |
| Arkansas | 55 | 134 | 402 | New Hampshire | 33 | 72 | 217 |
| California | 587 | 1,676 | 5,029 | New Jersey | 206 | 398 | 1,193 |
| Colorado | 96 | 289 | 867 | New Mexico | 27 | 93 | 279 |
| Connecticut | 91 | 166 | 497 | New York | 568 | 818 | 2,454 |
| Delaware | 18 | 55 | 165 | North Carolina | 158 | 535 | 1,606 |
| District of Columbia | 36 | 28 | 83 | North Dakota | 12 | 34 | 103 |
| Florida | 362 | 1,365 | 4,096 | Ohio | 163 | 537 | 1,611 |
| Georgia | 100 | 492 | 1,476 | Oklahoma | 26 | 171 | 512 |
| Hawaii | 63 | 64 | 192 | Oregon | 69 | 232 | 695 |
| Idaho | 8 | 87 | 261 | Pennsylvania | 273 | 601 | 1,803 |
| Illinois | 212 | 517 | 1,551 | Rhode Island | 33 | 49 | 147 |
| Indiana | 66 | 299 | 897 | South Carolina | 66 | 288 | 865 |
| Iowa | 26 | 142 | 426 | South Dakota | 15 | 44 | 131 |
| Kansas | 20 | 121 | 364 | Tennessee | 37 | 343 | 1,029 |
| Kentucky | 39 | 207 | 622 | Texas | 333 | 1,255 | 3,766 |
| Louisiana | 31 | 198 | 595 | Utah | 25 | 114 | 341 |
| Maine | 36 | 71 | 213 | Vermont | 9 | 32 | 95 |
| Maryland | 146 | 288 | 865 | Virginia | 113 | 406 | 1,218 |
| Massachusetts | 214 | 347 | 1,042 | Washington | 126 | 399 | 1,198 |
| Michigan | 164 | 465 | 1,394 | West Virginia | 16 | 83 | 250 |
| Minnesota | 84 | 270 | 811 | Wisconsin | 83 | 273 | 820 |
| Mississippi | 23 | 124 | 373 | Wyoming | 3 | 26 | 79 |
| Missouri | 91 | 283 | 849 | U.S. Total | 5,170 | 15,417 | 46,252 |
The 10% column is how many geriatricians will be needed to serve only those 65 and older projected to have Alzheimer's dementia in 2050, assuming that the percentage of people age 65 and older with Alzheimer's dementia remains at approximately 10%. The 30% column is how many geriatricans will be needed to serve the 30% of people age 65 and older who need geriatrician care, regardless of whether they have dementia. The number of practicing geriatricians in 2021 was provided by IQVIA and includes physicians with geriatrics as either their primary or secondary specialty. Calculations assume that each geriatrician can care for up to 700 patients. 721 The underlying state‐by‐state estimates of the 2050 population age 65 and older were provided by Claritas Pop‐Facts 2020.
These shortages will affect states differently. For example, Hawaii and Washington, D.C., have almost enough or more than enough geriatricians (respectively) to match the approximately 10% of those age 65 and older projected to have Alzheimer's dementia in 2050. In contrast, 12 states need to at least quintuple the number of practicing geriatricians by 2050 to care for those 65 and older projected to have Alzheimer's dementia, or increase the number by at least 13 times to care for the 30% of the population age 65 and older projected to need geriatrician care. Two states, Tennessee and Idaho, will need to increase the number of geriatricians by at least nine times just to meet the care needs of those projected to have Alzheimer's dementia in 2050, or by at least 29 times to meet the needs of all those age 65 and older who are projected to need geriatrician care in 2050.
While the shortage of geriatricians and other specialists extends nationwide, it appears to be most acute in rural settings — with many rural counties facing a shortage of health care providers overall. 726 , 727 For instance, according to the 2019 Alzheimer's Association survey, 44% of PCPs in large cities and 54% in suburban areas near large cities reported that there were not enough specialists in their area, while 63% of PCPs in small cities or towns and 71% of PCPs in rural areas reported the same. 703 Another indicator of the growing shortage of geriatricians is that, in 2023, there were 411 geriatric medicine fellowship positions available, but more than half (234) went unfilled. 728
The shortage of specialists extends to neurologists as well. The National Center for Health Workforce Analysis (NCHWA) projected that there will be a shortfall of neurologists by 2025, but suggested that the growing number of physician assistants in neurology could help address this workforce gap. 729 Twenty U.S. states have already been identified as “dementia neurology deserts,” meaning they are projected to have fewer than 10 neurologists per 10,000 people with dementia in 2025. 730
The shortage of geriatricians and other relevant specialists has been attributed to a combination of factors, including growth in demand due to population aging; a smaller percentage of working aged adults; substantively lower pay for geriatricians and neurologists compared with many other specialist physicians; an inadequate number of clinician educators with relevant specialties on the faculties of health professional schools; limited availability of incentives to choose these specialties, such as loan forgiveness programs; and the insufficient respect and recognition accorded to geriatricians and related specialists. 731 , 732 Many of these factors are modifiable and must be addressed in order to increase the number of providers available to provide specialized dementia diagnosis and care. Moreover, beyond increasing the supply of dementia specialists, geriatric care principles should be integrated across all health care professionals' educational curricula to ensure widespread delivery of age‐friendly care.
6.2. Medical treatment and care team workforce
As well as screening for, detecting and diagnosing dementia, PCPs are responsible for managing treatment for people living with dementia. 733 Even so, dementia care is inadequately covered in health care training programs, both in curricula and in opportunities for clinical practice. 734
Advanced practice providers, including physician assistants and nurse practitioners, also play key roles in treatment for people living with dementia. However, there is limited specialization in caring for older adults among these advanced practice roles, likely for many of the same reasons cited for the shortages of geriatricians and neurologists. In 2022, there were 355,000 nurse practitioners licensed in the United States, up from 91,000 in 2010, but less than 1% had a certification in gerontology, 6.1% had a certification in gerontology acute care, and 8.9% had a certification in gerontology primary care. 735 , 736 Also, less than 700 geriatric psychiatrists were certified by the American Board of Psychiatry and Neurology (ABPN) in the last decade (from 2012 to 2020) 737 and over half of geriatric psychiatrists certified by the ABPN are concentrated in just seven states (2015). 738 As of 2018, less than 1% of physician assistants were certified in geriatric medical care. Although this figure represents a nearly 400% increase in the absolute number of physician assistants specializing in geriatric care since 2013 (indicating a positive trend), 739 significant gaps remain in the capacity of this workforce to support older adults living with dementia and other chronic health concerns.
Registered nurses, licensed practical nurses, licensed psychologists and licensed therapists comprise other critical segments of the dementia care workforce, providing a range of nursing, rehabilitation and supportive services in community settings, skilled nursing homes and other settings. These services include medication administration, intravenous injections, wound care, catheter care, physical therapy, occupational therapy, behavioral consultation and much more. In addition, social workers assist with care navigation and management, and licensed clinical social workers and psychologists may provide therapeutic services to people living with dementia and their caregivers.
Specialization in caring for older adults, however, remains limited across these occupational groups as well. For instance, a survey of Masters of Social Work students who graduated from 2017 to 2019 found that only 4.2% were specializing in aging or gerontology. 740 Despite these low percentages, between 20% to 48% of social work students have high interest in working with older adults. 741 , 742 , 743 , 744 , 745 Since the social work profession has a broad scope, student interest may focus on social problems rather than age‐based populations. 746 Other reports indicate that less than 1% of registered nurses are certified in geriatrics 747 , 748 and only 1.2% of psychologists specialize in geropsychology. 749
6.3. Collaborative workforce models for dementia care management
Several decades of research supports the value of collaborative primary care models that bring different health professionals together, such as social workers, registered nurses and non‐clinical care managers, in addition to physicians and advanced practice providers, to care for people living with dementia. 750 , 751 Researchers and practitioners have identified eight essential elements of comprehensive dementia care to improve outcomes and lower costs: treatment of related conditions, coordination of care, continuous monitoring and assessment, ongoing care plan, psychosocial interventions, self‐management, caregiver support and medication management. 752 These collaborative and comprehensive models have been associated with a range of benefits including reduced behavioral symptoms, improved function and quality of life, decreased caregiver burden, and lower health care costs related to hospitalizations, emergency department visits and other outpatient visits. 751 , 753 , 754
As one collaborative dementia care example, the Alzheimer's and Dementia Care (ADC) Program is a health systems‐based model in which clinical providers with extensive training in dementia care, known as dementia care specialists (DCSs), co‐manage care with the PCP. DCSs provide comprehensive care addressing medical, behavioral and social aspects of dementia through the development of care plans tailored to the needs and goals of each patient living with dementia and their caregiver. In this co‐management model, the PCP is responsible for the patient's primary care needs but shares responsibility for the dementia‐related aspects of care with the DCS, including reviewing and providing input on the dementia care plan. The care plan is then implemented by a team, led by the DCS, that includes family members, other health professionals and community‐based organizations. 624 This model was found to reduce nursing home admissions for participating Medicare beneficiaries. The findings were $601 per patient per quarter ($2,404 per year) in savings, while the cost of running the program was $317 per patient per quarter ($1,268 per year), for a net savings of $284 per patient per quarter ($1,136 per year) for Medicare. 755
As a second example, the Care Ecosystem — a collaborative, team‐based dementia care program utilizing telehealth that involved care navigators, advanced practice nurses, social workers and pharmacists — resulted in fewer ambulance rides, emergency department visits and hospitalizations and lower total cost of care compared with usual care. 756 , 757 A non‐academic health care delivery organization which adopted the Care Ecosystem found that the model can be successfully implemented and integrated into purely clinical settings. 758 With regard to cost savings, participation in the Care Ecosystem reduced the total cost of care by $3,290 from 1 to 6 months post‐enrollment and by $3,027 from 7 to 12 months post‐enrollment, corresponding to a mean monthly cost reduction of $526 across 12 months. 757 An implementation toolkit for the Care Ecosystem is publicly available at: https://memory.ucsf.edu/sites/memory.ucsf.edu/files/wysiwyg/CareEcosystemToolkit.pdf. 759
As further evidence of the cost‐saving potential of collaborative dementia care team models, an interprofessional memory care clinic called the Healthy Aging Brain Center was shown to reduce per‐person health care costs by $3,474 over a year for individuals with memory problems, compared with those whose care was overseen by a PCP only. 757 More than half of the cost savings were attributed to lower inpatient hospital costs. The average annual cost of the program was $618 per person — indicating a nearly 6‐to‐1 return on investment. See a description of the new Guiding an Improved Dementia Experience (GUIDE) Model in the Caregiving section and later in the Workforce section to learn more about efforts to disseminate collaborative dementia care widely.
6.4. Direct care workforce
The largest segment of the workforce that supports people living with dementia is the direct care workforce. 760 Direct care workers — who are formally classified as personal care aides, home health aides and nursing assistants, but known by a wide range of job titles in the field — assist older adults and people with disabilities in private homes, community‐based settings such as adult day services and residential care, skilled nursing homes and other settings such as hospitals. 761 Across these settings, direct care workers deliver the majority of day‐to‐day care to patients, clients or residents living with Alzheimer's disease and other forms of dementia.
Direct care workers provide assistance with ADLs, such as bathing, eating, toilet care and mobility. In home care settings, they also support individuals with household chores, meal preparation, attending appointments and other instrumental activities of daily living (IADLs). Under the supervision of licensed nurses or other health care professionals, home health aides and nursing assistants also perform certain clinical tasks, such as wound care, measuring vital signs and medication administration (depending on the setting and regulatory context). 762 , 763
Beyond these distinct tasks, direct care workers play a broader role in promoting nutrition, exercise, functional ability, social engagement and emotional well‐being for those living with dementia. With training in active listening, empathic response and other relevant skills, direct care workers can reduce social isolation, provide emotional support and, with additional training, help administer nonpharmacological treatments — such as music and pet therapy and person‐centered bathing — to prevent or reduce distress associated with dementia. 764 , 765 , 766 , 767
Direct care workers also support quality outcomes and cost savings. Direct care workers providing in‐home care enable individuals to continue living at home and help prevent or delay nursing home placement. 768 Across settings, they also provide care to individuals returning from a hospital stay and can help reduce the risk of readmission, as well as assist with end‐of‐life care transitions. 769 , 770 , 771 , 772 Thanks to their daily caregiving role, direct care workers are well‐placed to observe and report changes of status to clinical colleagues, thereby helping to reduce the risk of emergency department visits, avoidable hospitalizations and other adverse outcomes that are disproportionately high among people living with dementia. 773 , 774 Research suggests that with enhanced dementia‐specific training, direct care workers may also play a role in reducing inappropriate antipsychotic prescribing for individuals living with dementia in nursing homes. 775
Between 2012 and 2022, the number of direct care workers increased from 3.2 million to 4.8 million due to growing demand for long‐term care. 761 Looking ahead, just over 1 million additional direct care workers will be needed between 2021 and 2031 — more new workers than in any other single occupation in the United States. 761 This job growth is occurring primarily among personal care aides and home health aides, reflecting the overwhelming preference for “aging in place” and public policies that have expanded access to home and community‐based services. 776
This projected growth in the direct care workforce is seen across the country. As shown in Table 14, double‐digit percentage increases in the number of home health and personal care aides will be needed between 2020 and 2030 to meet demand in every state except Maine. (Unlike the national workforce projections, updated state‐specific projections will not be available until mid‐2024.) Twenty‐one states are expected to see a 30% to 40% increase in the size of this workforce, while in two states (Arizona and Nevada) the workforce is expected to increase more than 50%. Although sizable, these employment projections fall short of true workforce demand, as they do not account for the additional workers who will be needed through the “gray market,” meaning private‐pay, usually unreported employment arrangements. One study using a nationally representative sample of adults found that nearly a third of people who arrange paid care for an aging adult or person living with dementia rely on the gray market (rather than a home care agency or other formal care provider). 777
TABLE 14.
Expected home health and personal care aide job growth, 2020‐2030.
| Number in 2020 and projected number needed in 2030 | Percentage increase | ||
|---|---|---|---|
| State | 2020 | 2030 | 2020‐2030 |
| Alabama | 21,700 | 25,910 | 19.4 |
| Alaska | 6,270 | 7,130 | 13.7 |
| Arizona | 72,920 | 117,740 | 61.5 |
| Arkansas | 21,900 | 28,350 | 29.5 |
| California | 766,000 | 985,800 | 28.7 |
| Colorado | 36,890 | 49,220 | 33.4 |
| Connecticut | 44,180 | 53,250 | 20.5 |
| Delaware | 8,430 | 11,780 | 39.7 |
| District of Columbia | 12,120 | 15,180 | 25.2 |
| Florida | 76,140 | 93,270 | 22.5 |
| Georgia | 44,060 | 60,350 | 37.0 |
| Hawaii | 9,290 | 12,270 | 32.1 |
| Idaho | 17,400 | 20,640 | 18.6 |
| Illinois | 99,460 | 118,600 | 19.2 |
| Indiana | 42,200 | 55,720 | 32.0 |
| Iowa | 23,880 | 31,580 | 32.2 |
| Kansas | 25,710 | 30,110 | 17.1 |
| Kentucky | 22,230 | 30,130 | 35.5 |
| Louisiana | 37,900 | 44,160 | 16.5 |
| Maine | 17,380 | 18,710 | 7.7 |
| Maryland | 42,560 | 56,790 | 33.4 |
| Massachusetts | 109,430 | 139,560 | 27.5 |
| Michigan | 71,750 | 89,820 | 25.2 |
| Minnesota | 107,500 | 133,420 | 24.1 |
| Mississippi | 19,130 | 25,200 | 31.7 |
| Missouri | 75,960 | 86,160 | 13.4 |
| Montana | 7,190 | 9,670 | 34.5 |
| Nebraska | 12,500 | 15,210 | 21.7 |
| Nevada | 15,830 | 23,860 | 50.7 |
| New Hampshire | 8,410 | 10,970 | 30.4 |
| New Jersey | 59,610 | 76,930 | 29.1 |
| New Mexico | 32,360 | 40,750 | 25.9 |
| New York | 510,870 | 710,570 | 39.1 |
| North Carolina | 65,150 | 82,070 | 26.0 |
| North Dakota | 6,790 | 8,540 | 25.8 |
| Ohio | 95,560 | 118,540 | 24.0 |
| Oklahoma | 20,460 | 26,210 | 28.1 |
| Oregon | 32,330 | 39,960 | 23.6 |
| Pennsylvania | 175,140 | 214,740 | 22.6 |
| Rhode Island | 7,410 | 9,450 | 27.5 |
| South Carolina | 31,750 | 41,850 | 31.8 |
| South Dakota | 3,830 | 4,570 | 19.3 |
| Tennessee | 31,470 | 44,740 | 42.2 |
| Texas | 320,780 | 418,500 | 30.5 |
| Utah | 17,080 | 22,440 | 31.4 |
| Vermont | 7,770 | 10,310 | 32.7 |
| Virginia | 56,390 | 73,160 | 29.7 |
| Washington | 63,300 | 80,760 | 27.6 |
| West Virginia | 16,470 | 21,370 | 29.8 |
| Wisconsin | 77,810 | 92,320 | 18.6 |
| Wyoming | 3,750 | 5,020 | 33.9 |
| U.S. Total | 3,470,700 | 4,600,600 | 32.6 |
Created from data from Projections Managing Partnership. Projections Central: Long‐Term Occupational Projections (2020‐2030). Available at: https://www.projectionscentral.org/Projections/LongTerm. Accessed January 17, 2024.
Although more direct care workers will be needed in the years ahead, the long‐term care field is already struggling to fill existing direct care positions. Turnover rates are high in this workforce — with an estimated median rate of 77% annually for direct care workers providing home care 778 and 99% for nursing assistants in nursing homes 779 — and recruitment and retention are long‐standing challenges. 780 , 781 , 782 In turn, instability in the workforce and understaffing across care settings can lead to stress, injury and burnout among direct care workers, thereby further contributing to turnover while also compromising care access and quality. 783 , 784
Workforce challenges are driven by persistently low compensation and poor job conditions for direct care workers, which are in turn underpinned by structural racial and gender inequities (that marginalize this workforce composed predominantly of women and people of color), 761 as well as ageism and disablism (toward the individuals receiving care and, by extension, those providing it). 785 According to the most recent national data available, the median wage for direct care workers is just $15.43 per hour and, due to low wages and the high prevalence of part‐time positions, median annual earnings are less than $24,000. 761 Research shows that, despite their complex and critical role in supporting the health and well‐being of older adults and people with disabilities, direct care workers earn a lower median wage than workers in other “entry‐level” occupations with similar education and training requirements, such as janitors, retail salespersons and customer service representatives. 786
Direct care workers also receive limited training and professional development opportunities, another indicator of poor job conditions. Nursing assistants in nursing homes and home health aides employed by Medicare‐certified home health agencies are required by federal regulations to complete at least 75 hours of entry‐level training and 12 hours of annual continuing education (although many states have set higher training requirements). 782 Care for individuals with cognitive impairment is among the requisite training topics for nursing assistants, but not for home health aides. In contrast, training requirements for other direct care workers vary by state and setting. With regards to dementia‐specific training, a 2015 review found that only 13 states had established dementia care training requirements for direct care workers who provide in‐home care. According to the same review, 44 states and the District of Columbia had set dementia care training standards for assisted living staff, but those regulations only pertained to special dementia care facilities or units in 14 of those states. 787 Inadequate training for direct care workers perpetuates their mischaracterization as “low‐skill” workers, fails to prepare them for the complexity and challenges of their role, undermines job satisfaction and retention, and directly impacts the provision of dementia care.
Direct care is also physically and emotionally demanding work, which is not well‐reflected in the training standards or compensation for this workforce. As one indicator, occupational injury data from the Bureau of Labor Statistics show that nursing assistants in nursing homes were nearly eight times more likely than U.S. workers overall to experience workplace injuries in 2020 (the most recent available year of occupation‐specific data on injuries in nursing homes). 761 These data reflect the impact of the COVID‐19 pandemic on this workforce — as COVID‐19 was classified as a “workplace injury” 788 — as well as long‐standing occupational risks. 789 Comparable occupational injury data are not available for direct care workers in home and community‐based settings due to reporting limitations, but these workers are also exposed to a range of occupational risks, including unsafe physical environments, infection hazards, interpersonal violence and more. 790
6.5. Dementia‐friendly initiatives and the community‐based workforce
The term “dementia‐friendly” has become increasingly common to describe initiatives to make local communities, environments and health and social systems more supportive of people living with dementia and their caregivers. 791 Work on dementia‐friendly communities began in Japan as early as 2004, with a nationwide campaign to better understand dementia and build supportive community networks, which inspired growth of the movement worldwide. 792 In the U.S., the Dementia‐Friendly America (DFA) initiative launched in 2015 and was described as a first‐of‐its‐kind national effort that was announced at the White House Conference on Aging. 793 DFA was built on the leadership of ACT on Alzheimer's, a community‐led initiative in Minnesota that began in 2013. 794 There are other ever‐evolving dementia‐friendly efforts as well that encompass a range of settings and contexts, including dementia‐friendly care for people living in hospitals; 795 , 796 , 797 dementia‐friendly design for nursing homes, senior centers, and similar settings; 798 , 799 , 800 and dementia‐friendly neighborhood efforts to improve quality of life for local residents. 801 , 802 Research is still needed on the effectiveness of these various dementia‐friendly efforts.
To support people living with dementia in their homes and communities, as well as their family caregivers, greater dementia‐related knowledge, skills and competencies are needed in the workforce beyond health care. For instance, dementia gatekeeper programs have had some success identifying and supporting people with dementia by training postal workers, bank tellers, ministers and other personnel to identify signs of cognitive impairment in older adults and provide appropriate direction to services. 803 Additional workforces that play a role in creating dementia‐friendly environments include librarians who provide supportive services and programming; 804 architects and others who design floor plans, landscapes, soundscapes and sonic environments; 798 , 805 adult protective service workers who handle elder abuse cases; 806 , 807 police officers and law enforcement agencies that interact with the public; 808 , 809 and hairdressers, 810 bus drivers and building superintendents among others. 811
6.6. Impact of COVID‐19 on the workforce
The COVID‐19 pandemic has had a significant and enduring impact on the health care workforce and especially on the dementia care workforce, given the disproportionately high infection and death rates due to COVID‐19 among people living with Alzheimer's disease and other dementias. 812
At the onset of the pandemic, the number of people employed in health care fell from 16.2 to 14.9 million, an unprecedented decrease of more than 8%. 813 , 814 As of October 2023, the number of people employed in the health sector was 3.9% higher than in February 2020, compared with 2.9% higher in all other sectors — but health sector employment still remained below expected levels (i.e., there were nearly 482,000 fewer jobs in October 2023 than would have been expected without the pandemic). 813 , 814 Employment levels in nursing homes and community care settings — where a significant proportion of dementia care takes place — are still far below pre‐pandemic levels and direct care workforce shortages remain acute. 813 As of October 2023, employment was nearly 10% lower in nursing homes and 1.3% lower in assisted living and continuing care retirement communities than in February 2020. 813 , 814
Some of the initial job loss in health care was caused by changes in service delivery and utilization. Elective procedures were canceled, routine and preventive care visits were postponed, and admissions into congregate care settings such as nursing homes were avoided if possible. Health care workers also had to leave their jobs to safeguard their own or their families’ health because of illness, or for caregiving or other reasons. As one startling example of how COVID‐19 directly impacted health care workers, more than 1.7 million COVID‐19 cases had been confirmed among nursing home staff as of October 2023 and over 3,000 nursing home staff had died from the disease. 815
Working during the COVID‐19 pandemic has also taken a significant emotional and psychological toll on the health care workforce. 816 , 817 , 818 As stated by the U.S. Surgeon General, “COVID‐19 has been a fully and uniquely traumatic experience for the health workforce, and for their families.” 819 A systematic review of research published in the first year of the pandemic found evidence for increased levels of burnout, emotional exhaustion, depersonalization and compassion fatigue among health care workers, with nurses, women and those working directly with COVID‐19 patients most impacted. 820 One survey of nearly 21,000 U.S. health care workers in 2020 found that stress related to workload and mental health was highest among nursing assistants, medical assistants and social workers versus other occupational groups, workers in inpatient versus outpatient settings, women versus men, and Black and Latinx workers versus White workers. 821 Researchers are now assessing these outcomes over time; one example is the longitudinal COVID‐19 Study of Healthcare and Support Personnel (CHAMPS), which aims to document the effects of the pandemic on the long‐term physical and mental health of the health care workforce. 822 Participants were recruited for the initial CHAMPS study in 2020‐2021 and, if they consented to be recontacted, were surveyed at six months’ follow‐up and will be surveyed annually thereafter.
The workforce employed by home and community‐based services who assist community‐dwelling older adults, including people living with dementia and their caregivers, such as those in adult day programs or who deliver congregate meals, developed new strategies to provide services and programming to maintain safety, improve socialization and reduce isolation and loneliness during lockdown periods. These strategies included providing home‐delivered groceries or grab‐and‐go meals; facilitating virtual and remote socialization activities; engaging in vaccine education and distribution; and deploying technology, digital literacy and device support, 823 , 824 , 825 with many of these remote and virtual activities still ongoing.
For the dementia care workforce, the trauma of caring for those most vulnerable to COVID‐19 (and related challenges, such as social isolation) has likely been significant. 826 Given the preexisting shortages among different segments of this workforce, the longer‐term impact of this crisis on workforce recruitment and retention — as well as on individual health and well‐being — must be closely monitored. 822
6.7. Looking to the future
In 2020, the American Public Health Association (APHA) identified “strengthening the dementia care workforce” as a public health priority. 827 “Continued failure to strengthen the dementia care workforce,” according to the APHA, “will increasingly limit the ability of people living with dementia to access quality services and supports, adding to health, social and economic burdens for individuals, families and society.” This section outlines four emergent areas that will strengthen the dementia care workforce into the future.
6.7.1. Health care workforce development
First, the health care workforce must expand overall to meet the needs of the rapidly growing population of older adults, who are at the highest risk of developing Alzheimer's disease and other dementias (see Prevalence section). 828 More PCPs, geriatricians, physician assistants, nurse practitioners, psychologists, therapists, social workers, direct care workers, other health care workers and community‐based workers who are trained in caring for people living with dementia will be critically needed in the years ahead.
One important effort to build the health care workforce is the Geriatrics Workforce Enhancement Program (GWEP) funded by the Health Resources and Services Administration, which comprises a network of 48 GWEPs across most U.S. states and two territories. 829 The goals of this program are to educate and train the health care workforce to provide value‐based care for older adults in integrated geriatrics and primary care models and to deliver community‐based programs that improve health outcomes for older adults. One particular goal for the GWEPs is to provide dementia training to a broad range of health care professionals, educators, individuals and families.
Additionally, recognizing the need for expanded training for professionals who serve older adults, the Substance Abuse and Mental Health Services Administration (SAMHSA) has funded a Center of Excellence for Behavioral Health Disparities in Aging and a Center of Excellence for Building Capacity in Nursing Facilities to Care for Residents with Behavioral Health Conditions.
In 2023, the National Institute on Aging funded the National Dementia Workforce Study (NDWS) under the leadership of a team of experts in survey research, health workforce research, and clinical care of people living with dementia. The NDWS will build a data infrastructure to inform efforts to strengthen the workforce of clinicians and other care providers required by the growing population of people living with dementia in the United States. 830
6.7.2. Dementia training and specialization
Targeted dementia training and specialization among PCPs and across the health care workforce is also needed, 831 , 832 including training to address PCPs’ lack of confidence in diagnosing dementia and communicating that diagnosis, as discussed earlier. Training in cultural and linguistic competency is also needed to help the dementia care workforce better support individuals from diverse populations, including individuals from various racial and ethnic and sexual and gender minority groups. Moreover, language‐concordant and culturally tailored resources and referrals are important for overcoming misunderstandings, biases, misdiagnoses and related disparities experienced by people of color and other individuals in minority populations who are living with dementia and by their families. 833 , 834 , 835 , 836 , 837 , 838
One successful training model is the Alzheimer's and Dementia Care ECHO® Program, which pairs PCPs with multidisciplinary specialist teams through telementoring to develop their knowledge and confidence in dementia care. According to an evaluation of the program, which was launched in 2018 by the Alzheimer's Association, 94% of surveyed providers participating in the program reported making changes in their delivery of dementia care due to the program and 87% reported higher job satisfaction. 839
Another burgeoning program is Dementia Care Aware, a state‐wide program in California that equips PCPs with information and tools to successfully administer cognitive health assessments and determine appropriate next steps for patients. 840 Once they have completed the online training, PCPs can receive $29 for providing an annual screening for cognitive impairment (current procedural terminology code 1494F) for California Medicaid patients who are fee‐for‐service and do not have Medicare. 841 If the initial screening leads to concerns regarding cognitive impairment, providers can receive $246 for conducting a cognitive assessment and providing care plan services (current procedural terminology code 99483) for California Medicaid patients (Medicare reimburses approximately $266). 712 , 841 , 842
The Gerontological Society of America's Kickstart, Assess, Evaluate, Refer (KAER) model provides another example of how to expand the workforce to better detect and manage dementia. 843 Among other strategies, this model suggests that non‐clinical office staff are well‐positioned to participate in the primary care team's efforts to detect cognitive impairment. Receptionists or schedulers, for example, can take note when patients miss their appointments, show up at the wrong time, defer to family members while completing paperwork or answering questions, or have difficulty following care plans.
Nurse practitioners, physician assistants and other care providers can also play a greater role in dementia care delivery, particularly for rural and underserved communities. 739 , 844 With training, support and recognition, direct care workers can also provide more tailored care for people living with dementia, for example, by implementing non‐pharmacological interventions to mitigate distress; observing and recording changes to clinical team members; and educating and supporting family members. 845
Furthermore, as new therapies for Alzheimer's and other dementias develop, the composition and size of the dementia care workforce must continue to evolve. For example, the U.S. Food and Drug Administration recently approved two drugs for the treatment of Alzheimer's that are delivered through intravenous infusion and require careful monitoring of patients for a serious potential side effect called amyloid‐related imaging abnormalities, or ARIA. Ensuring the health of individuals while they receive these drugs requires an expanded workforce including infusion nurses, radiologists and radiology technicians with special training in recognizing ARIA, and specialists with expertise in managing ARIA if it occurs. Neuropsychologists and other health professionals are also needed to evaluate whether individuals are benefiting from the treatments, as those who do not experience improvements in cognitive skills and the ability to perform ADLs may be advised to discontinue treatment.
Many people living with dementia move to a nursing home or other group home setting because there are inadequate community‐based services and supports in their local area. 846 To create dementia‐friendly communities that support people to age in place, it will be important to also bolster the dementia knowledge and skills of the broader community‐based workforce — such as postal workers, bank tellers, ministers, church leaders, librarians, police officers, building superintendents, bus drivers and hair dressers. 482 , 623 , 803 , 804 , 808 , 809 , 810 , 811 For instance, financial advisors are on the frontline with their clients. Research conducted by Bank of America found that Alzheimer's dementia was the most feared condition in later life among the bank's clients across all ages and genders. Given this insight, Bank of America created training programs for their financial advisors on both Alzheimer's dementia and caregiving. 847
6.7.3. Payment models to support the dementia care workforce
Alternative payment models may be needed to scale‐up the delivery of collaborative, comprehensive and innovative dementia care. 754 , 848 , 849 One development in this area, as described earlier, is that since 2017 Medicare reimburses physicians, nurse practitioners, physician assistants and nurse specialists for health care visits that result in a comprehensive dementia care plan. Reimbursement requires cognition‐focused evaluation, identification of caregiver needs, and development, revision or review of an advance care plan. Early uptake of this benefit has been limited; a study using a 20% nationwide random sample of eligible fee‐for‐service Medicare beneficiaries’ claims data found that only 0.65% had received this benefit in the first two years. 850 The authors of this study concluded that providers may be insufficiently aware of these billing codes, especially in smaller practices and rural areas, and/or may be billing for similar services under different codes. In the future, providers could be better informed about these codes, and the codes could be revised to include other professionals such as social workers and psychologists as billing entities.
Another development in the area of payment models is the nationwide voluntary GUIDE (Guiding an Improved Dementia Experience) Model, announced by CMS in 2023. 690 , 851 Through the GUIDE Model, participating organizations will offer dementia care programs that provide ongoing, longitudinal care and support for dually eligible Medicare and Medicaid people living with dementia and their caregivers, through an interdisciplinary team. Each team must include a knowledgeable and skilled care navigator to help people living with dementia and their caregivers access clinical and non‐clinical services and supports, such as person‐centered assessments and care plans, care coordination, caregiver training and education, meals and transportation through community‐based organizations, and 24/7 access to a support line. CMS will test an alternative payment for participating organizations, who must be Medicare Part B enrolled providers/suppliers and eligible to bill for Medicare Physician Fee Schedule services. To address racial health disparities and inequities in dementia care, CMS will actively seek out safety‐net organizations that provide care to underserved communities to participate in the GUIDE Model and will provide financial and technical supports to ensure they can develop their infrastructure, improve their workforce and care delivery capabilities, and participate successfully. 851
Financing and other public policy reforms are also needed to strengthen and stabilize the direct care workforce. On a hopeful note, the federal government and states are taking unprecedented action to improve job quality and bolster this workforce, particularly through Medicaid, including by overhauling training and credentialing systems, designing new career development opportunities, implementing reimbursement rate increases tied to increased compensation, developing new recruitment campaigns and pipeline programs and more. 852 , 853 The challenge will be to sustain these investments into the future, as the need for direct care services continues to escalate.
6.7.4. Technology to augment dementia care delivery
Major advances in technology are optimizing the time and effectiveness of the dementia care workforce. As one example, e‐learning programs can greatly increase access to dementia care training, although evidence suggests that the effectiveness of such programs relies on the relevance of the content and the inclusion of interactive learning strategies. 854
Technology is also helping to improve access to care for people living with dementia, especially for those in rural areas and those with mobility limitations. 855 A randomized clinical trial of more than 1,500 individuals across urban and rural areas in California, Nebraska and Iowa to determine whether telephone‐ and internet‐based delivery of the Care Ecosystem was effective in improving outcomes found that the intervention resulted in better quality of life, reduced emergency department visits and decreased caregiver depression and burden. 756 A systematic review of telehealth for dementia care, including routine care, cognitive assessment and rehabilitation, found that telehealth delivered results similar to those of in‐person services. 856 More research is needed to identify the strengths and weaknesses of telehealth and how it can be utilized appropriately in the diagnosis and treatment of individuals living with dementia, as well as in supporting their caregivers.
Assistive, therapeutic and remote monitoring technologies, which range from smart home devices to automated medication prompts, robotic animals, and devices that support personalized activities and much more, can be used to augment the role of the dementia care workforce. 857 As with telehealth, more research is needed to understand the efficacy of these myriad different technologies and to address concerns and unintended consequences related to privacy, autonomy and interpersonal interactions. As the 2020 report of the Lancet Commission on dementia prevention, intervention, and care concluded, “technology is not a replacement for human contact.” 107
7. USE AND COSTS OF HEALTH CARE, LONG‐TERM CARE AND HOSPICE
The costs of health care and long‐term care for individuals with Alzheimer's or other dementias are substantial, and dementia is one of the costliest conditions to society. 858 Total payments in 2024 (in 2024 dollars) for all individuals with Alzheimer's or other dementias are estimated at $360 billion (Figure 13), not including the value of informal caregiving that is described in the Caregiving section. Medicare and Medicaid are expected to cover $231 billion, or 64%, of the total health care and long‐term care payments for people with Alzheimer's or other dementias. Out‐of‐pocket spending is expected to be $91 billion, or 25% of total payments.A11 For the remainder of this section, costs are reported in 2023 dollars unless otherwise indicated.A12 With the exception of the section, “The COVID‐19 Pandemic and Health Care Utilization and Costs,” data reported in this section reflect patterns of use before the pandemic. It is unclear at this point what long‐term effect the pandemic will have on these patterns.
FIGURE 13.
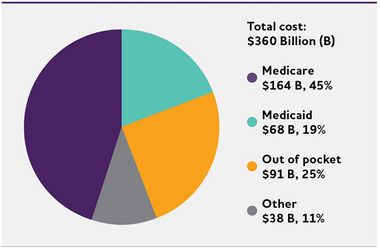
Distribution of aggregate costs of care by payment source for Americans age 65 and older with Alzheimer's or other dementias, 2023. Data are in 2023 dollars. Created from data from the Lewin Model.A11 “Other” payment sources include private insurance, health maintenance organizations, other managed care organizations and uncompensated care. The sum of individual dollar amounts does not equal the total cost due to rounding.
7.1. Total cost of health care and long‐term care
Table 15 reports the average annual per‐person payments for health care and long‐term care services for fee‐for‐service (i.e., traditional) Medicare beneficiaries age 65 and older with and without Alzheimer's or other dementias based on data from the 2018 Medicare Current Beneficiary Survey.A13 Unless otherwise noted, cost and health care utilization statistics for Medicare beneficiaries are for fee‐for‐service Medicare and do not represent those enrolled in Medicare Advantage.A14 Total per‐person health care and long‐term care payments in 2023 dollars from all sources for Medicare beneficiaries with Alzheimer's or other dementias were nearly three times as great as payments for other Medicare beneficiaries in the same age group ($43,644 per person for those with dementia compared with $14,660 per person for those without dementia).A15 , 859
TABLE 15.
Average annual per‐person payments by payment source for health care and long‐term care services, Medicare beneficiaries age 65 and older, with and without Alzheimer's or other dementias, in 2023 dollars.*
| Payment source | Beneficiaries with Alzheimer's or other dementias | Beneficiaries without Alzheimer's or other dementias |
|---|---|---|
| Medicare | $21,973 | $7,918 |
| Medicaid | 6,771 | 305 |
| Uncompensated | 192 | 240 |
| Health maintenance organization | 1,952 | 2,292 |
| Private insurance | 1,534 | 958 |
| Other payer | 933 | 419 |
| Out of pocket | 10,289 | 2,529 |
| All sources | 43,644 | 14,660 |
Payments for beneficiaries with Alzheimer's or other dementias include payments for community‐dwelling beneficiaries and beneficiaries residing in residential care facilities.
Created from unpublished data from the Medicare Current Beneficiary Survey for 2018. 859
Despite having Medicare and other sources of financial assistance, individuals with Alzheimer's or other dementias and their family members still incur high out‐of‐pocket costs. These costs are for Medicare deductibles, copayments and coinsurance; other health insurance premiums, deductibles, copayments and coinsurance; and services not covered by Medicare, Medicaid or other sources of support. On average, Medicare beneficiaries age 65 and older with Alzheimer's or other dementias paid $10,289 out of pocket annually for health care and long‐term care services not covered by other sources (Table 15). 859 One group of researchers found that out‐of‐pocket and informal caregiving costs for a family member with dementia total $203,117 in 2016 dollars ($240,046 in 2023 dollars) in the last seven years of life, compared with $102,955 in 2016 dollars ($121,674 in 2023 dollars) for those without dementia. 581 However, informal caregiving costs in the last seven years of life were considerably higher for households with a family member with dementia living in the community compared with households with a family member with dementia living in a nursing home ($231,730 versus $165,910 in 2016 dollars [$273,862 versus $196,075 in 2023 dollars]), due to Medicaid covering the cost of nursing home care for many individuals. 581
Researchers have evaluated the additional or “incremental” health care, residential long‐term care and family caregiving costs of dementia (that is, the costs specifically attributed to dementia when comparing people with and without dementia who have the same coexisting medical conditions and demographic characteristics). 430 , 858 , 860 , 861 These studies have used different time horizons, ranging from lifetime costs (i.e., costs between the time of diagnosis and death) to annual costs. The lifetime total cost of care, including out‐of‐pocket expenses, Medicare and Medicaid expenditures, and informal caregiving is estimated at $321,780 per person with Alzheimer's dementia in 2015 dollars ($394,683 in 2023 dollars), more than twice the estimated lifetime cost for individuals without Alzheimer's dementia). 429 Another group of researchers found that lifetime total costs were three times higher for women compared with men with Alzheimer's dementia, due to women having a longer duration of illness and spending more time in a nursing home. 862 Annual incremental health care and nursing home costs for individuals with dementia (that is, the additional costs compared with those for individuals without dementia) are estimated at $28,501 per person per year in 2010 dollars ($40,209 in 2023 dollars).A16 , 858 The majority of incremental costs have been attributed to informal care and out‐of‐pocket costs, rather than medical care and nursing home costs paid by Medicare or Medicaid. 429 , 862 , 863 The incremental five‐year cost of care for dementia paid by Medicare has been estimated at nearly $16,000 per person in 2017 dollars ($22,573 in 2023 dollars), with nearly half of these costs incurred in the year after diagnosis and 87% concentrated in the two years after diagnosis. 863 , 864 However, these estimates include costs for individuals who died during the five‐year time period, and the incremental costs for individuals who survive at least five years after diagnosis are even higher.
Several groups of researchers have specifically examined out‐of‐pocket costs and found that individuals with Alzheimer's or other dementias and their families incur substantially higher out‐of‐pocket costs compared with individuals without Alzheimer's. Although incremental Medicare expenditures peak in the year after diagnosis and decrease in the subsequent four years, out‐of‐pocket costs have been shown to increase over time, from $3,104 in the first two years after diagnosis to $3,730 in years three to four after diagnosis, to $3,934 in years seven to eight after diagnosis (in 2017 dollars; $3,579, $4,300 and $4,535 in 2023 dollars). 865 Higher out‐of‐pocket costs for Alzheimer's and other dementias have been attributed to nursing home care, home health care and prescription drug payments. 866 , 867 Furthermore, individuals with Alzheimer's dementia spend 12% of their annual income on out‐of‐pocket health care services on average, excluding nursing home and informal care, compared with 7% for individuals without Alzheimer's dementia. 867
Another perspective to examine incremental costs for individuals with Alzheimer's and other dementias is through end‐of‐life costs. A recent systematic review of end‐of‐life costs for individuals with dementia reported that costs were especially high during the last month of life, even compared with monthly costs over the last year of life. 868 Researchers comparing end‐of‐life costs in the last five years of life for individuals with and without dementia found that the total cost was $287,038 per person for individuals with dementia in 2010 dollars and $183,001 per person for individuals without dementia ($404,949 and $258,175, respectively, in 2023 dollars), a difference of 57%. 869 Out‐of‐pocket costs represent a substantially larger proportion of total wealth for those with dementia than for people without dementia (32% versus 11%).
7.2. Use and costs of health care services
7.2.1. Use of health care services
Unadjusted data (that is, data that don't account for differences in the characteristics of people with versus without Alzheimer's or other dementias) show that people with Alzheimer's or other dementias have more than twice as many hospital stays per year as other older people. 412 Moreover, the use of health care services by people with other serious medical conditions is strongly affected by the presence or absence of dementia. In particular, people with coronary artery disease, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, stroke or cancer who also have Alzheimer's or other dementias have higher use and costs of health care services than people with these medical conditions but no coexisting dementia.
In addition to having more hospital stays, older people with Alzheimer's or other dementias have more skilled nursing facility stays per year than other older people.
7.2.1.1.
7.2.1.1.1. Hospital
In 2019, there were 518 hospital stays per 1,000 Medicare beneficiaries age 65 and older with Alzheimer's or other dementias compared with 234 hospital stays per 1,000 Medicare beneficiaries age 65 and older without these conditions. 412 Overall, 32% of Medicare beneficiaries with Alzheimer's or other dementias have at least one hospital discharge annually compared with 15% of beneficiaries without these conditions, with average hospital lengths of stay of 5.1 days versus 4.5 days, respectively. 412 The most common reasons that people with Alzheimer's dementia are hospitalized are not due to the disease itself, but for other reasons, including syncope (fainting), fall and trauma (26%), ischemic heart disease (17%), and gastrointestinal disease (9%), 870 although the COVID‐19 pandemic may have changed the most common reasons for hospitalization starting in 2020. A study of inpatient hospitalizations of adults age 60 and older found that those with Alzheimer's dementia were at 7% greater risk of dying during the hospital stay and stayed nearly a day longer than individuals without Alzheimer's dementia. 871 Among Medicare beneficiaries with Alzheimer's or other dementias, 22% of hospital stays are followed by a readmission within 30 days. 850 Although not directly comparable, one study of a random sample of Medicare beneficiaries from 50 U.S. hospital referral regions found an overall 30‐day readmission rate of 18%. 872 The proportion of hospital stays followed by a readmission within 30 days remained relatively constant between 2008 and 2018 for Medicare beneficiaries with Alzheimer's and related dementias (23% in 2008 versus 22% in 2018). 873
7.2.1.1.2. Emergency department
There were nearly 1.8 million emergency department visits for people with Alzheimer's in 2021, representing 1.3% of all emergency department visits. 874 There are 1,545 emergency department visits per 1,000 Medicare beneficiaries with Alzheimer's or other dementias per year, including emergency department visits that result in a hospital admission. 873 Although not directly comparable, there were 640 emergency department visits per 1,000 Medicare beneficiaries per year based on a review of utilization patterns of a subset of Medicare beneficiaries. 872 Emergency department visits for people with Alzheimer's or other dementias increased 22% between 2008 and 2018 (from 1,265 to 1,545 per 1,000 Medicare beneficiaries), exceeding the increases in emergency department visits for individuals with cancer, ischemic heart disease and heart failure among others (Figure 14). 873 One group of researchers found that individuals with Alzheimer's or another dementia seen in the emergency department are more likely to be admitted to the hospital or a nursing home from the emergency department than Medicare beneficiaries without Alzheimer's or other dementias. 875 Additionally, individuals with Alzheimer's or other dementias are more likely to have at least one hospitalization, have at least one subsequent emergency department visit and be admitted to hospice in the 12 months following the initial emergency department visit.
FIGURE 14.
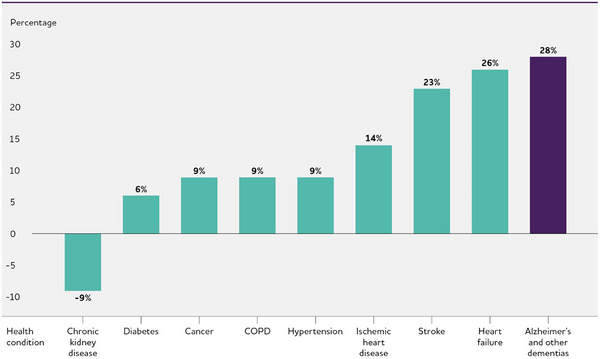
Percentage changes in emergency department visits per 1,000 fee‐for‐service Medicare beneficiaries with selected health conditions between 2008 and 2018. Includes Medicare beneficiaries with a claims‐based diagnosis of each chronic condition. Beneficiaries may have more than one chronic condition. Created from data from U.S. Centers for Medicare & Medicaid Services. 873
7.2.1.1.3. Skilled nursing facility
Skilled nursing facilities provide direct medical care that is performed or supervised by registered nurses, such as giving intravenous fluids, changing dressings, administering tube feedings and providing around‐the‐clock personal care services. 876 There are 188 skilled nursing facility stays covered by Medicare per 1,000 Medicare beneficiaries with Alzheimer's or other dementias per year compared with 40 stays per 1,000 beneficiaries without these conditions — a rate nearly five times as high. 412 Overall, 19% of Medicare beneficiaries with Alzheimer's or other dementias have at least one skilled nursing facility stay annually compared with 4% of Medicare beneficiaries without these conditions. 412
7.2.2. Costs of health care services
Average per‐person payments for health care services (hospital, physician and other medical provider, nursing home, skilled nursing facility, hospice and home health care) and prescription medications were higher for Medicare beneficiaries with Alzheimer's or other dementias than for Medicare beneficiaries without dementia in the same age group (see Table 16).A13 , 859 ,
TABLE 16.
Average annual per‐person payments by type of service for health care and long‐term care services, Medicare beneficiaries age 65 and older, with and without Alzheimer's or other dementias, in 2023 dollars.
| Payment source | Beneficiaries with Alzheimer's or other dementias | Beneficiaries without Alzheimer's or other dementias |
|---|---|---|
| Inpatient hospital | $7,580 | $2,836 |
| Outpatient events | 2,867 | 2,256 |
| Medical provider* | 5,956 | 3,844 |
| Skilled nursing facility | 3,890 | 392 |
| Nursing home | 14,347 | 555 |
| Hospice | 2,321 | 136 |
| Home health care | 1,857 | 274 |
| Prescription medications** | 5,016 | 3,383 |
“Medical provider” includes physician, other provider and laboratory services, and medical equipment and supplies.
Information on payments for prescription medications is only available for people who were living in the community, that is, not in a nursing home or an assisted living residence.
TABLE 17.
Emergency department (ED) visits, hospital readmissions and per capita Medicare payments in 2023 dollars by Medicare beneficiaries with Alzheimer's or other dementias.
| State | Number of ED visits per 1,000 beneficiaries* | Percentage of hospital stays followed by readmission within 30 days* | Per capita Medicare payments† | State | Number of ED visits per 1,000 beneficiaries* | Percentage of hospital stays followed by readmission within 30 days* | Per capita Medicare payments† |
|---|---|---|---|---|---|---|---|
| Alabama | 1,410.8 | 21.2 | $27,369 | Montana | 1,328.6 | 16.6 | $22,360 |
| Alaska | 1,477.6 | 19.3 | 29,741 | Nebraska | 1,153.6 | 18.7 | 25,745 |
| Arizona | 1,436.2 | 20.2 | 29,111 | Nevada | 1,711.5 | 25.8 | 41,608 |
| Arkansas | 1,530.4 | 21.5 | 27,092 | New Hampshire | 1,493.8 | 20.4 | 28,663 |
| California | 1,496.3 | 23.0 | 44,310 | New Jersey | 1,456.3 | 22.9 | 38,795 |
| Colorado | 1,424.8 | 18.6 | 28,687 | New Mexico | 1,563.7 | 20.6 | 27,258 |
| Connecticut | 1,635.4 | 22.7 | 34,958 | New York | 1,461.3 | 23.7 | 43,601 |
| Delaware | 1,577.6 | 21.5 | 32,413 | North Carolina | 1,683.8 | 21.5 | 27,591 |
| District of Columbia | 1,741.7 | 25.6 | 39,378 | North Dakota | 1,173.3 | 18.4 | 21,711 |
| Florida | 1,551.9 | 23.0 | 34,142 | Ohio | 1,618.7 | 22.5 | 30,661 |
| Georgia | 1,573.2 | 22.5 | 30,219 | Oklahoma | 1,692.1 | 21.6 | 32,584 |
| Hawaii | 1,248.2 | 16.0 | 24,763 | Oregon | 1,628.4 | 18.7 | 25,470 |
| Idaho | 1,389.2 | 17.2 | 25.057 | Pennsylvania | 1,470.5 | 22.0 | 31,112 |
| Illinois | 1,624.1 | 23.4 | 34,637 | Rhode Island | 1,605.6 | 23.2 | 30,782 |
| Indiana | 1,514.2 | 21.3 | 30,219 | South Carolina | 1,558.2 | 21.7 | 29,657 |
| Iowa | 1,310.7 | 18.0 | 22,067 | South Dakota | 1,200.1 | 18.6 | 27,776 |
| Kansas | 1,406.0 | 19.8 | 27,260 | Tennessee | 1,548.6 | 21.5 | 28,543 |
| Kentucky | 1,735.5 | 23.1 | 29,473 | Texas | 1,549.1 | 22.1 | 37,679 |
| Louisiana | 1,709.9 | 22.1 | 34,182 | Utah | 1,194.3 | 16.7 | 26,233 |
| Maine | 1,665.3 | 19.7 | 24,119 | Vermont | 1,528.4 | 19.6 | 23,329 |
| Maryland | 1,524.1 | 24.4 | 37,381 | Virginia | 1,621.7 | 21.6 | 27,870 |
| Massachusetts | 1,668.4 | 24.7 | 36,930 | Washington | 1,479.2 | 18.6 | 25,274 |
| Michigan | 1,691.4 | 24.0 | 30,432 | West Virginia | 1,811.4 | 24.1 | 29,240 |
| Minnesota | 1,467.1 | 21.6 | 27,300 | Wisconsin | 1,519.9 | 19.9 | 27,335 |
| Mississippi | 1,714.8 | 22.1 | 31,174 | Wyoming | 1,445.9 | 17.4 | 25,452 |
| Missouri | 1,529.6 | 22.6 | 28,324 |
7.2.3. Use and costs of health care services by state
Substantial geographic variation exists in health care utilization and Medicare payments by individuals with Alzheimer's or other dementias (see Table 17). Emergency department visits, including visits that result in a hospital admission, range from 1,154 per 1,000 beneficiaries annually in Nebraska to 1,811 per 1,000 beneficiaries annually in West Virginia, and the percentage of hospital stays followed by hospital readmission within 30 days ranges from 16% in Hawaii to 25.8% in Nevada. Medicare spending per capita ranged from $21,711 in North Dakota to $44,310 in California. 873
7.2.4. Use and costs of health care services across the Alzheimer's disease continuum
Health care costs increase with the presence of dementia. In a population‐based study of adults age 70 to 89, annual health care costs were significantly higher for individuals with dementia than for those with either mild cognitive impairment (MCI) or without cognitive impairment. 878 Annual health care costs for individuals with MCI were not significantly different, however, from costs for individuals without cognitive impairment.
Several groups of researchers have found that both health care and prescription drug spending are significantly higher for people diagnosed with Alzheimer's or other dementias in the year prior to diagnosis, 879 , 880 , 881 although the sources of increased spending differed across these studies. In one study, the largest differences in spending were due to inpatient and post‐acute care, 880 while in another study the differences in spending were primarily due to outpatient care, home care and medical day services, with only a small difference in inpatient care costs. 881
Three groups of researchers have found that spending in the year after diagnosis was substantially higher than spending for individuals who had similar characteristics but did not have Alzheimer's or dementia, by amounts ranging from $7,264 in 2017 dollars ($8,374 in 2023 dollars) 863 based on individuals with fee‐for‐service (i.e., traditional) Medicare coverage, to $17,852 in additional costs in 2014 dollars ($22,473 in 2023 dollars) 880 based on another group of individuals with fee‐for‐service Medicare. One group of researchers, however, did not find a significant difference in health care spending in the two years after diagnosis. 882
Researchers have found that health care costs remain higher beyond the year after diagnosis. One group of researchers also found the incremental costs remained higher in the second year after diagnosis ($7,327 in additional costs in 2014 dollars [$9,224 in 2023 dollars]). 880 Another research team found that health care costs remained higher in the second through fourth years after a dementia diagnosis but were not significantly different from costs for individuals without the diagnosis in the fifth year after diagnosis. 863 Incremental costs decreased over time, from $4,241 in 2014 dollars ($4,889 in 2023 dollars) in year two to $1,302 ($1,501 in 2023 dollars) in year four, although costs increase dramatically in the last year and last month of life. 850 Researchers have also found a similar increase in health care costs in the year before and two years after a diagnosis of MCI, although the additional costs were lower than costs for Alzheimer's. 880 One possible explanation for the spike in health care costs in the year immediately before and the year immediately after diagnosis of Alzheimer's or another dementia relates to delays in timely diagnosis. One group of researchers found that individuals with cognitive decline who sought care from a specialist (that is, a neurologist, psychiatrist or geriatrician) had a shorter time to diagnosis of Alzheimer's disease. 883 Additionally, individuals diagnosed with cognitive impairment by a specialist had lower Medicare costs in the year after receiving a diagnosis of Alzheimer's dementia than those diagnosed by a non‐specialist.
7.2.5. Impact of Alzheimer's and other dementias on the use and costs of health care in people with coexisting medical conditions
Nearly 9 out of 10 Medicare beneficiaries with Alzheimer's disease or other dementias have at least one other chronic condition. 412 Additionally, they are more likely than those without dementia to have other chronic conditions. 412 Overall, 2.7 times more Medicare beneficiaries with Alzheimer's or other dementias have four or more chronic conditions (excluding Alzheimer's disease and other dementias) than Medicare beneficiaries without dementia. 412 Table 18 reports the percentage of people with Alzheimer's or other dementias who had certain coexisting medical conditions. In 2019, 46% of Medicare beneficiaries age 65 and older with dementia also had coronary artery disease, 46% had chronic kidney disease, 37% had diabetes, 34% had congestive heart failure and 20% had chronic obstructive pulmonary disease. 412
TABLE 18.
Percentage of Medicare beneficiaries age 65 and older with Alzheimer's or other dementias who have specified coexisting conditions.
| Coexisting condition | Percentage |
|---|---|
| Coronary artery disease | 46 |
| Chronic kidney disease | 46 |
| Diabetes | 37 |
| Congestive heart failure | 34 |
| Chronic obstructive pulmonary disease | 20 |
| Stroke | 13 |
| Cancer | 10 |
Created from unpublished data from the National 100% Sample Medicare Fee‐for‐Service Beneficiaries for 2019. 412
Medicare beneficiaries who have Alzheimer's or other dementias and a coexisting medical condition have higher average per‐person payments for most health care services than Medicare beneficiaries with the same medical condition but without dementia. Table 19, A13 shows the average per‐person Medicare payments for seven specific medical conditions among beneficiaries who have Alzheimer's or other dementias and beneficiaries who do not have Alzheimer's or another dementia.A13 , 412 Medicare beneficiaries with Alzheimer's or other dementias have higher average per‐person payments in all categories except physician care. Additionally, one group of researchers found that individuals with dementia and behavioral disturbances, such as agitation, had more psychiatric comorbidities than individuals with dementia but without behavioral disturbances. 884 This group of researchers also found that larger proportions of individuals with dementia and behavioral disturbances used medications including antihypertensives, dementia treatments, antipsychotics, antidepressants, antiepileptics and hypnotics compared with individuals with dementia but without behavioral disturbances.
TABLE 19.
Average annual per‐person payments by type of service and coexisting medical condition for Medicare beneficiaries age 65 and older, with and without Alzheimer's or other dementias, in 2023 dollars.
| Average per‐person Medicare payments | ||||||
|---|---|---|---|---|---|---|
| Medical condition by Alzheimer's/dementia (A/D) status | Total Medicare payments | Hospital care | Physician care | Skilled nursing home care | Home health care | Hospice care |
| Coronary artery disease | ||||||
| With A/D | $28,418 | $8,462 | $4,815 | $4,362 | $2,446 | $3,799 |
| Without A/D | 17,976 | 6,119 | 4,718 | 1,353 | 938 | 421 |
| Diabetes | ||||||
| With A/D | 28,064 | 8,478 | 4,834 | 4,417 | 2,353 | 3,263 |
| Without A/D | 15,729 | 5,213 | 4,225 | 1,227 | 827 | 291 |
| Congestive heart failure | ||||||
| With A/D | 31,433 | 9,739 | 5,006 | 4,928 | 2,595 | 4,305 |
| Without A/D | 25,415 | 9,331 | 5,478 | 2,385 | 1,557 | 798 |
| Chronic kidney disease | ||||||
| With A/D | 29,151 | 8,799 | 4,791 | 4,552 | 2,471 | 3,857 |
| Without A/D | 19,733 | 6,720 | 4,918 | 1,626 | 1,082 | 470 |
| Chronic obstructive pulmonary disease | ||||||
| With A/D | 31,981 | 10,055 | 5,226 | 5,088 | 2,622 | 3,842 |
| Without A/D | 22,785 | 8,086 | 5,372 | 1,898 | 1,298 | 708 |
| Stroke | ||||||
| With A/D | 30,551 | 9,154 | 5,069 | 4,854 | 2,584 | 3,753 |
| Without A/D | 22,196 | 7,310 | 5,226 | 2,334 | 1,548 | 652 |
| Cancer | ||||||
| With A/D | 28,352 | 8,127 | 5,200 | 4,076 | 2,429 | 3,760 |
| Without A/D | 18,330 | 5,056 | 5,717 | 1,032 | 732 | 732 |
7.3. Use and costs of long‐term care services
Long‐term care services include home‐ and community‐based services and services delivered in assisted living residences and nursing homes. An estimated 65% of older adults with Alzheimer's or other dementias live in the community, compared with 98% of older adults without Alzheimer's or other dementias. 863 Of those with dementia who live in the community, 74% live with someone and the remaining 26% live alone. 859 As their disease progresses, people with Alzheimer's or other dementias generally receive more care from family members and other unpaid caregivers. Many people with dementia also receive paid long‐term care services at home; in adult day centers, assisted living residences or nursing homes; or in more than one of these settings at different times during the often long course of the disease. Medicaid is the only public program that covers the long nursing home stays that most people with dementia require in the severe stage of their illnesses.
7.3.1. Use of long‐term care services by setting
Most people with Alzheimer's or other dementias who live at home receive unpaid help from family members and friends, but some also receive paid home‐ and community‐based services, such as personal care and adult day care. People with Alzheimer's or other dementias make up a large proportion of all older adults who receive adult day services and nursing home care.
Home health services and other home‐based services. Medicare covers home health services, such as part‐time skilled nursing care; skilled therapy services; home health aide care, such as intermittent help with bathing, toileting and dressing if needed, with skilled nursing or therapy services; and medical social services in the home. Home health agencies provide the majority of home health care services. 885 Fee‐for‐service Medicare does not cover homemaker services, such as meal preparation, or personal care services, such as help with bathing, toileting and dressing, if this is the only care that is needed; however, Medicare Advantage plans (Medicare Part C) are allowed to offer these services as supplemental benefits, and 17% offered in‐home support services as a benefit in 2023. 886 Additionally, 16% of Medicare Advantage plans offered food and produce as a supplemental benefit, and 8% offered meals beyond a limited basis. 886 These supplemental benefits are more common in Medicare Advantage Special Needs Plans (i.e., plans that are designed for Medicare enrollees with specific needs, such as individuals with a chronic condition, individuals who are also enrolled in Medicaid, and institutionalized enrollees), with 41% offering a food and produce benefit, 31% offering in‐home support services, and 15% offering meals beyond a limited basis. Although Medicare Advantage Special Needs Plans can be offered to individuals with specific chronic conditions, including dementia, only 8% of Special Needs Plan enrollees are enrolled in a plan for chronic or disabling conditions, representing less than 1% of all Medicare enrollees. 887 , 888 The vast majority of Special Needs Plan enrollees are individuals also enrolled in Medicaid (i.e., Dual Eligible Special Needs Plan enrollees).
Thirty‐six percent of individuals using home health services have Alzheimer's or other dementias. 889 Of Medicare beneficiaries age 65 and older with Alzheimer's or other dementias, 26% have at least one home health visit paid by Medicare during the year, compared with 8% of Medicare beneficiaries age 65 and older without Alzheimer's or other dementias 412 and they use an average of 110 days of home care per year (including homemaker services and other services not covered by Medicare) compared with 64 days per year for individuals age 65 and older without the disease. 885 Receipt of home health services after hospital discharge has been shown to increase the likelihood of remaining in the community for at least 30 days after hospital discharge, with greater benefits from longer durations of home health care. 890
Adult day services. The fourth most common chronic condition in participants using adult day services is Alzheimer's disease or other dementias, and 25% of individuals using adult day services have Alzheimer's or other dementias. 889 Fourteen percent of adult day service centers in the United States specialized in caring for individuals with Alzheimer's disease or other dementias in 2020, up from 10% in 2016. 889 , 891 The percentage of participants with Alzheimer's or other dementias was higher in adult day service centers that provided either low‐ or moderate‐level medical services than in centers that either provided no medical services or mainly provided health or medical services. 891 ,
Residential care facilities. Forty‐two percent of residents in residential care facilities (that is, housing that includes services to assist with everyday activities, such as personal care, medication management and meals), including assisted living facilities, had Alzheimer's or other dementias in 2020, up from 34% in 2016. 889 , 892 Sixty‐one percent of residential care communities are small (four to 25 beds), 892 and these facilities have a higher percentage of residents with Alzheimer's or other dementias than larger facilities (51% in facilities with four to 25 beds compared with 47% in facilities with 26 to 50 beds and 39% in facilities with more than 50 beds). 893 Fifty‐eight percent of residential care facilities offer activities or programs for residents with Alzheimer's or other dementias. 894 Average aide staff hours per resident day in residential care communities range from 2.2 hours per day in facilities with less than 25% of residents diagnosed with dementia to 2.7 hours per day in facilities with more than 75% of residents diagnosed with dementia. 892
Nursing home care. Overall, 46% of nursing home residents have Alzheimer's or other dementias, 889 although the prevalence differs by duration of nursing home stay. While 36% of short‐stay (less than 100 days) nursing home residents have Alzheimer's or other dementias, 58% of long‐stay (100 days or longer) residents have these conditions. Twenty‐four percent of Medicare beneficiaries with Alzheimer's or other dementias reside in a nursing home, compared with 1% of Medicare beneficiaries without these conditions. 859 At age 80, approximately 75% of people with Alzheimer's dementia live in a nursing home compared with only 4% of the general population age 80. 414
Alzheimer's special care units and dedicated facilities. An Alzheimer's special care unit is a dedicated unit, wing or floor in a nursing home or other residential care facility that has tailored services for individuals with Alzheimer's or other dementias. Thirteen percent of nursing homes and 21% of assisted living and other residential care communities have a dementia special care unit. 889 Less than 1% (0.3%) of nursing homes and 11% of other residential care facilities exclusively provide care to individuals with dementia.
Long‐term care services provided at home and in the community
Overall, 70% of spending for long‐term care services and supports is covered by public payers, including Medicaid (43%), Medicare (21%) and other public payers (6%), and 15% is covered by out‐of‐pocket payments, including direct payments and deductibles and copayments for services covered by another payment source (15%). Private insurance covers only 9% of long‐term services and supports. 895 Nationally, state Medicaid programs are shifting long‐term care services from institutional care to home‐ and community‐based services as a means to both reduce unnecessary costs and meet the growing demand for these services by older adults. The federal and state governments share the management and funding of Medicaid, and states differ greatly in the services covered by their Medicaid programs. In 2020, home‐ and community‐based services represented the majority (62%) of the $199.4 billion spent by Medicaid on long‐term care services and supports, with institutional care representing the remaining 38%. 896 However, there is substantial variation across states in spending on home‐ and community‐based services, ranging from 32% of total Medicaid long‐term care services and supports in Mississippi to 84% of long‐term care services and supports spending in Oregon, despite evidence demonstrating that Medicaid spending on these services reduces costs. 897 Thirty‐three percent of Medicaid's total expenditures cover expenditures related to long‐term care services and supports.
Between 2010 and 2020, Medicaid spending on home‐ and community‐based services increased from 48% to 62% of total long‐term services and supports expenditures. 896 Additionally, total spending on home care for Medicare beneficiaries with Alzheimer's or other dementias increased dramatically between 2004 and 2018.902 Increases in spending may have been due to a variety of factors, including more people being diagnosed with Alzheimer's dementia, more people using home care, an increase in the number of coexisting medical conditions, more intensive use of home care services and an increase in Medicaid coverage for older adults. 898 In two systematic reviews of the cost‐effectiveness of enhanced home support interventions for individuals with dementia, researchers found some evidence to support occupational therapy, home‐based exercise, and some psychological and behavioral treatments as potentially cost‐effective approaches, although research that has evaluated both the costs and benefits of enhanced home support interventions is scant. 899 , 900
Transitions between care settings
Individuals with dementia often move between a nursing facility, hospital and home, rather than remaining solely in a nursing facility. In a longitudinal study of primary care patients with dementia, researchers found that individuals discharged from a nursing facility were nearly equally as likely to be discharged home (39%) as discharged to a hospital (44%). 901 Individuals with dementia may also transition between a nursing facility and hospital or between a nursing facility, home and hospital, creating challenges for caregivers and providers to ensure that care is coordinated across settings. Other researchers have shown that nursing home residents frequently have burdensome transitions at the end of life, including admission to an intensive care unit in the last month of life and late enrollment in hospice, 902 although the number of care transitions for nursing home residents with advanced cognitive impairment varies substantially across geographic regions of the United States. 903
7.3.2. Costs of long‐term care services
Home care. The median cost in 2021 for a nonmedical home health aide was $27 per hour and $5,148 per month ($28 and $5,341 in 2023 dollars). 904 Nonmedical home care costs increased 5.9% annually on average between 2017 and 2021. The cost of homemaker services was $26 per hour and $4,957 per month ($27 and $5,143 in 2023 dollars) and increased 5.4% annually on average between 2017 and 2021.
Adult day centers. The median cost of adult day services was $78 per day in 2021 ($85 in 2023 dollars). 904 The cost of adult day services increased 2.8% annually on average between 2017 and 2021.
Assisted living residences. The median cost for care in an assisted living residence was $4,500 per month, or $54,000 per year in 2021 ($4,921 and $59,047 in 2023 dollars). 904 The cost of assisted living increased 4.4% annually on average between 2017 and 2021.
Nursing homes. The 2021 average cost for a private room in a nursing home was $297 per day, or $108,405 per year ($325 and $118,536 in 2023 dollars), and the average cost of a semi‐private room was $260 per day, or $94,900 per year ($284 and $103,769 in 2023 dollars). 904 The cost of nursing home care increased 3.3% annually on average for a private room and 2.9% annually on average for a semi‐private room between 2017 and 2021.
Affordability of long‐term care services
Few individuals with Alzheimer's or other dementias have sufficient long‐term care insurance or can afford to pay out of pocket for long‐term care services for as long as the services are needed.
Medicare beneficiaries with a dementia diagnosis have lower household incomes on average than beneficiaries without a dementia diagnosis. In 2018, 23% of community‐dwelling Medicare beneficiaries with a dementia diagnosis had household incomes below the federal poverty level, and 53% had household incomes between 100% and 200% of the federal poverty level, while 15% of those without a dementia diagnosis lived below the federal poverty level and 40% had household incomes between 100% and 200% of the federal poverty level. 905
Asset data are not available for people with Alzheimer's or other dementias specifically, but 50% of Medicare beneficiaries age 65 and older had total savings of $83,850 or less in 2019 dollars ($92,188 in 2023 dollars), and 25% had savings of $9,650 or less in 2019 dollars ($10,610 in 2023 dollars). Median savings for White Medicare beneficiaries were 8.5 times higher than for Black beneficiaries and more than 15 times higher than for Hispanic beneficiaries. 906 In a 2022 survey of adults about the affordability of long‐term care, less than one‐third (31%) of adults age 65 and older reported being very confident that they would have the financial resources to pay for necessary care as they age. 907 Additionally, of adults age 50 and older, nearly two‐thirds reported feeling anxious about being able to afford nursing home or assisted living care, if they should need it. Although individuals from lower income households were more likely to report feeling anxious about the affordability of long‐term care (77% with household incomes less than $40,000 reported being anxious about the affordability of long‐term care), nearly half of individuals from households with incomes $90,000 or greater also reported being anxious about the affordability (in 2022 dollars; $41,553 and $93,495, respectively, in 2023 dollars).
Medicare does not cover long‐term care in a nursing home
Although Medicare covers care in a long‐term care hospital, skilled nursing care in a skilled nursing home and hospice care, it does not cover long‐term care (i.e., stays more than 90 days) in a nursing home. 908
Results from a 2022 survey about the affordability of long‐term care revealed that 23% of adults believed that Medicare would cover the cost of nursing home care, and 28% were not sure who would pay for nursing home care. Even more concerning, 45% of individuals age 65 and older believed that Medicare would cover the cost of nursing home care. 907 These findings suggest that Medicare beneficiaries and caregivers need more education and information about the types of services that Medicare covers. In particular, Medicare does not cover custodial care, that is, care to assist with activities of daily living, such as dressing and bathing. Most nursing home care is custodial care, and therefore is not covered by Medicare.
Medicare does cover post‐acute skilled nursing care, or nursing and therapy care that must be performed or supervised by medical professionals, such as registered or licensed nurses. 909 For Medicare to cover skilled nursing care, the Medicare beneficiary must have a qualifying hospital stay, a physician must decide that skilled care is needed, and the medical condition requiring skilled care must be related to the hospitalization. 910 Fee‐for‐service Medicare (Part A) covers the first 20 days of skilled nursing care with $0 coinsurance for each benefit period. For the next 80 days of skilled nursing care (days 21‐100), the beneficiary pays $204 per day in coinsurance. 911
A long‐term care hospital is an acute care hospital that specializes in caring for people who stay more than 25 days, on average. A long‐term care hospital provides specialized care, such as respiratory therapy, pain management and treatment for head trauma. 912 Benefits work in the same way that Medicare covers other acute care hospitalizations.
The terms “Medicare” and “Medicaid” are also often confused. Most individuals who are age 65 or older, have a permanent disability or have end‐stage kidney disease qualify for Medicare Part A, which is also referred to as hospital insurance. 913 Individuals are eligible to receive Medicare Part A at no cost if they have worked and paid Medicare taxes for at least 10 years (i.e., have a sufficient earnings history) or a spouse, parent or child has a sufficient earnings history. Medicare Part B (medical insurance) is a voluntary program that requires enrollees to pay a monthly premium. Medicare Advantage plans, also referred to as Medicare Part C, are becoming more common, with more than one‐half (51%) of Medicare beneficiaries enrolled in this type of plan in 2023. 914 Advantage plans are privately offered Medicare plans that combine Medicare Parts A and B and often also include prescription drug coverage (Medicare Part D). 915
While Medicare is a federal program, Medicaid is a joint federal and state program, and benefits vary state‐to‐state. 916 Individuals with low incomes and/or low resources may qualify for Medicaid coverage. Medicaid covers some services that Medicare either does not cover or only partially covers, such as nursing home care and home‐ and community‐based care. Individuals who are enrolled in both Medicare and Medicaid are sometimes referred to as being “dually eligible.”
For more information about Medicare, visit medicare.gov. For more information about Medicaid, visit https://www.medicaid.gov/.
Long‐term care insurance
Long‐term care insurance typically covers the cost of care provided in a nursing home, assisted living residence and Alzheimer's special care residence, as well as community‐based services such as adult day care and services provided in the home, including nursing care and help with personal care. 917
Based on data from the National Health Expenditure Account, it is estimated that private insurance covered only 9% ($38.5 billion) of the cost of long‐term services and supports in 2019. 895 Industry reports estimate that between 5.3 and 7.1 million Americans had private long‐term care insurance in 2020‐2021. 918 , 919 However, the long‐term care insurance market is shrinking, with only 57,000 new policies sold in 2018, compared with 754,000 in 2002. 920 The average premium for a long‐term care insurance policy was $155 per month in 2021. 919 The private long‐term care insurance market has consolidated since 2000. In 2000, 41% of individuals with a long‐term care policy were insured by one of the five largest insurers versus 60% in 2020. 909 , 918 Cognitive conditions are the most common final diagnosis for long‐term care insurance claims lasting more than one year, representing 49% of claims; however, these conditions are third most common (16%) for insurance claims lasting one year or less, after cancer and musculoskeletal conditions (31% and 25% of claims, respectively). 918 Medicare Advantage plans are allowed to provide supplemental benefits, such as adult day care, caregiver support and in‐home support services for chronically ill beneficiaries. However, only 17% of individual plans offered in‐home support services as a benefit in 2023, and these supplemental benefits are unlikely to offset a substantial portion of long‐term care costs. 886
To address the dearth of private long‐term care insurance options and the high out‐of‐pocket cost of long‐term care services, Washington became the first state in the country to create a public state‐operated long‐term care insurance program. 921 The Long‐Term Services and Supports Trust Program (WA Cares Fund) is funded by a payroll tax on employees of 58 cents per $100 earned that began in July 2023, and self‐employed individuals can choose to participate in the program. The program is currently structured to pay up to $36,500 in lifetime benefits beginning in July 2026. 922 Although other states have contemplated implementing a long‐term care tax to fund long‐term care insurance, none have yet passed legislation. 923
Medicaid costs
Medicaid covers nursing home care and long‐term care services in the community for individuals who meet program requirements for level of care, income and assets. 924 To receive coverage, beneficiaries must have low incomes. Beneficiaries with financial resources above Medicaid thresholds may spend down their assets and income to become eligible for coverage. Once enrolled, most nursing home residents with Medicaid must spend all of their Social Security income and any other monthly income, except for a very small personal needs allowance, to pay for nursing home care. Medicaid only makes up the difference if the nursing home resident cannot pay the full cost of care or has a financially dependent spouse. Although Medicaid covers the cost of nursing home care, its coverage of many other long‐term care and support services, such as assisted living care, home‐based skilled nursing care and help with personal care, varies by state.
Twenty‐four percent of older individuals with Alzheimer's or other dementias who have Medicare also have Medicaid coverage, compared with 10% of individuals without dementia. 859 Because Medicaid pays for nursing home and other long‐term care services, the high use of these services by people with dementia translates into high costs to Medicaid. Average annual Medicaid payments per person for Medicare beneficiaries with Alzheimer's or other dementias ($6,771) were 22 times as great as average Medicaid payments for Medicare beneficiaries without Alzheimer's or other dementias ($305) (see Table 15). 859 Much of the difference in payments for beneficiaries with Alzheimer's or other dementias compared with other beneficiaries is due to the costs associated with nursing home care.
Total Medicaid spending for people with Alzheimer's or other dementias is projected to be $68 billion in 2024.A11 Actual and estimated state‐by‐state Medicaid spending for people with Alzheimer's or other dementias in 2020 and 2025 (in 2020 dollars) is included in Table 20.
TABLE 20.
Total Medicaid payments for Americans age 65 and older living with Alzheimer's or other dementias by state.*
| State | 2020 (in millions of dollars) | 2025 (in millions of dollars) | Percentage increase | State | 2020 (in millions of dollars) | 2025 (in millions of dollars) | Percentage increase |
|---|---|---|---|---|---|---|---|
| Alabama | $925 | $1,127 | 21.8 | Montana | $166 | $203 | 22.2 |
| Alaska | 76 | 110 | 44.6 | Nebraska | 372 | 411 | 10.3 |
| Arizona | 414 | 545 | 31.7 | Nevada | 203 | 277 | 36.5 |
| Arkansas | 396 | 454 | 14.6 | New Hampshire | 254 | 335 | 31.9 |
| California | 4,197 | 5,235 | 24.7 | New Jersey | 2,186 | 2,614 | 19.6 |
| Colorado | 635 | 789 | 24.1 | New Mexico | 227 | 279 | 22.9 |
| Connecticut | 1,022 | 1,187 | 16.1 | New York | 5,453 | 6,306 | 15.6 |
| Delaware | 253 | 313 | 23.6 | North Carolina | 1,332 | 1,628 | 22.2 |
| District of Columbia | 126 | 135 | 6.8 | North Dakota | 190 | 215 | 13.2 |
| Florida | 2,689 | 3,453 | 28.4 | Ohio | 2,534 | 2,940 | 16.0 |
| Georgia | 1,265 | 1,594 | 26.0 | Oklahoma | 516 | 611 | 18.3 |
| Hawaii | 240 | 285 | 18.7 | Oregon | 253 | 317 | 25.4 |
| Idaho | 149 | 196 | 31.2 | Pennsylvania | 3,658 | 4,029 | 10.2 |
| Illinois | 1,787 | 2,199 | 23.1 | Rhode Island | 470 | 565 | 20.1 |
| Indiana | 1,054 | 1,233 | 17.1 | South Carolina | 652 | 818 | 25.4 |
| Iowa | 676 | 792 | 17.2 | South Dakota | 182 | 212 | 16.6 |
| Kansas | 473 | 543 | 14.6 | Tennessee | 1,109 | 1,377 | 24.2 |
| Kentucky | 803 | 949 | 18.2 | Texas | 3,202 | 3,949 | 23.3 |
| Louisiana | 765 | 934 | 22.1 | Utah | 185 | 235 | 27.0 |
| Maine | 212 | 274 | 29.5 | Vermont | 116 | 146 | 26.4 |
| Maryland | 1,231 | 1,535 | 24.7 | Virginia | 1,000 | 1,266 | 26.6 |
| Massachusetts | 1,753 | 2,031 | 15.9 | Washington | 547 | 689 | 26.0 |
| Michigan | 1,487 | 1,738 | 16.9 | West Virginia | 445 | 521 | 17.1 |
| Minnesota | 905 | 1,087 | 20.1 | Wisconsin | 777 | 924 | 18.9 |
| Mississippi | 606 | 729 | 20.4 | Wyoming | 86 | 111 | 28.8 |
| Missouri | 973 | 1,137 | 16.8 |
All cost figures are reported in 2020 dollars.
Created from data from the Lewin Model.A11
7.3.3. Use and costs of care at the end of life
Hospice care provides medical care, pain management, and emotional and spiritual support for people who are dying, including people with Alzheimer's or other dementias, either in a care residence or at home. Hospice care also provides emotional and spiritual support and bereavement services for families of people who are dying. The main purpose of hospice is to allow individuals to die with dignity and without pain and other distressing symptoms that often accompany terminal illness. Medicare is the primary source of payment for hospice care, but private insurance, Medicaid and other sources also pay for hospice care. Medicare beneficiaries enrolled in Medicare Part A (i.e., Medicare's hospital insurance) can choose to enroll in Medicare's hospice benefit if a hospice physician certifies that the individual is terminally ill (i.e., expected to live six months or less), and the individual accepts palliative or comfort care and forgoes curative care for the terminal illness. In this way, hospice care replaces other Medicare‐covered benefits for treating the terminal illness and related conditions. 925 Based on data from the National Hospice Survey for 2008 to 2011, nearly all hospices (99%) cared for individuals with dementia, and 67% of hospices had residents with a primary diagnosis of dementia. 926 In 2017, 4,254 U.S. companies provided hospice care in the home, assisted living communities, long‐term care residences, inpatient hospitals, and inpatient hospice and other settings. 927
Nearly two‐thirds (63%) of Medicare decedents (i.e., people who have died) with Alzheimer's or other dementias used hospice in their last six months of life in 2017 compared with 36% of Medicare decedents without Alzheimer's or other dementias. 928 In 2017, dementia, including Alzheimer's dementia, was the second most common primary diagnosis for Medicare beneficiaries using hospice care, representing 18% of Medicare beneficiaries receiving hospice care (Table 21). 927 Alzheimer's or other dementias are even more common in individuals receiving hospice care when taking into account the disease as a coexisting or secondary condition. Forty‐five percent of hospice users in 2020 had a diagnosis of Alzheimer's or other dementias. 889
TABLE 21.
Number and percentage of Medicare beneficiaries admitted to hospice with a primary diagnosis of dementia by state, 2017.
| State | Number of beneficiaries | Percentage of beneficiaries | State | Number of beneficiaries | Percentage of beneficiaries |
|---|---|---|---|---|---|
| Alabama | 5,867 | 18 | Montana | 507 | 11 |
| Alaska | 95 | 14 | Nebraska | 1,648 | 18 |
| Arizona | 7,229 | 18 | Nevada | 2,167 | 17 |
| Arkansas | 3,133 | 18 | New Hampshire | 1,007 | 17 |
| California | 30,045 | 20 | New Jersey | 8,207 | 23 |
| Colorado | 3,254 | 15 | New Mexico | 1,523 | 15 |
| Connecticut | 2,380 | 15 | New York | 7,669 | 16 |
| Delaware | 716 | 12 | North Carolina | 8,486 | 17 |
| District of Columbia | 263 | 18 | North Dakota | 468 | 18 |
| Florida | 19,897 | 15 | Ohio | 12,656 | 17 |
| Georgia | 10,435 | 21 | Oklahoma | 4,102 | 18 |
| Hawaii | 943 | 16 | Oregon | 3,565 | 17 |
| Idaho | 1,566 | 17 | Pennsylvania | 12,384 | 17 |
| Illinois | 9,795 | 18 | Rhode Island | 1,657 | 25 |
| Indiana | 5,922 | 17 | South Carolina | 6,038 | 20 |
| Iowa | 3,278 | 17 | South Dakota | 421 | 13 |
| Kansas | 2,770 | 18 | Tennessee | 6,435 | 19 |
| Kentucky | 2,895 | 15 | Texas | 26,672 | 22 |
| Louisiana | 4,786 | 19 | Utah | 2,506 | 19 |
| Maine | 1,494 | 19 | Vermont | 543 | 17 |
| Maryland | 4,072 | 17 | Virginia | 6,440 | 19 |
| Massachusetts | 7,245 | 23 | Washington | 5,459 | 20 |
| Michigan | 9,001 | 16 | West Virginia | 1,552 | 15 |
| Minnesota | 5,399 | 21 | Wisconsin | 5,086 | 16 |
| Mississippi | 3,547 | 20 | Wyoming | 89 | 7 |
| Missouri | 5,991 | 17 | U.S. Total | 278,192 | 18 |
Created from data from the U.S. Centers for Medicare & Medicaid Services. 927
Patterns of hospice use for individuals with dementia differ from patterns for individuals without dementia in at least two notable ways. The average number of days of hospice care for individuals with a primary diagnosis of dementia was 50% higher than for individuals with other primary diagnoses, based on data from the 2008 to 2011 National Hospice Survey. 926 Individuals with a primary diagnosis of dementia use an average of 112 days of hospice care versus 74 days for individuals with other primary diagnoses. Recently, researchers found that individuals with dementia as either the primary hospice diagnosis or as a secondary condition were more likely than other hospice users to be enrolled in hospice for more than six months. 929 However, long hospice stays place individuals with dementia at risk for disenrollment, and researchers have found that individuals with dementia are more likely to be disenrolled after more than six months in hospice than patients with other diagnoses. 926 , 929 Reasons for disenrollment include admission to an acute care hospital, loss of eligibility because the individual was no longer terminally ill, and failure to recertify for hospice. 930 For hospice enrollments of at least six months, hospice providers are required to assess individuals every 60 days, beginning at six months, to ensure they continue to meet eligibility requirements, and these assessments coupled with Medicare payment rates that are roughly 20% lower after the first 60 days, may contribute to disenrollment; however, more research is needed to understand the implications of these policies for individuals with dementia in hospice. 931 , 932
Overall, 12.2% of Medicare beneficiaries with Alzheimer's had at least one hospice claim in 2018, compared with 1.4% of Medicare beneficiaries without the disease, translating into per‐person hospice payments (for all beneficiaries, regardless of whether they used any hospice services) of $2,321 for individuals with Alzheimer's compared with $136 for all other Medicare beneficiaries. 859 In 2016, Medicare reimbursement for home hospice services changed from a simple daily rate for each setting to a two‐tiered approach that provides higher reimbursement for days 1 to 60 than for subsequent days and a service intensity add‐on payment for visits by a registered nurse or social worker in the last seven days of life. In fiscal year 2024, the routine home care rates are $218.33 per day for days 1 to 60 and $172.35 per day for days 61 and beyond. 931
Intensity of care at the end of life has decreased over the past two decades as hospice enrollment has increased. One group of researchers found that the number of inpatient hospital days in the last six months of life decreased from 15.3 to 11.8 between 2004 and 2017, although intensive care unit stays and number of days in a skilled nursing facility increased modestly over the same time period. 928 Expansion of hospice care is associated with fewer individuals with dementia having more than two hospitalizations for any reason or more than one hospitalization for pneumonia, urinary tract infection, dehydration or sepsis in the last 90 days of life. 933 For Medicare beneficiaries with advanced dementia who receive skilled nursing home care in the last 90 days of life, those who are enrolled in hospice are less likely to die in the hospital. 934 Additionally, those enrolled in hospice care are less likely to be hospitalized in the last 30 days of life 935 and more likely to receive regular treatment for pain. 936 Satisfaction with medical care is higher for families of individuals with dementia who are enrolled in hospice care than for families of individuals with dementia not enrolled in hospice care. 937 Despite the important role of end‐of‐life care for individuals with Alzheimer's, differences in hospice use by race/ethnicity exist. One group of researchers found substantially smaller proportions of Black and Hispanic Medicare beneficiaries with dementia enrolled in hospice in the last six months of life compared with White Medicare beneficiaries with dementia (38% and 43% versus 51%, respectively). 938 Furthermore, larger proportions of Black and Hispanic beneficiaries with dementia had at least one emergency department visit (80% and 77% respectively) and at least one hospitalization (77% for both groups) compared with White beneficiaries with dementia (71% and 68% respectively) in the last six months of life. 938 Black and Hispanic beneficiaries were also more likely to have an emergency department visit and/or a hospitalization after hospice enrollment.
Researchers have found similar reductions in hospitalizations at the end of life for individuals receiving palliative care. For nursing home residents with moderate‐to‐severe dementia, those who received an initial palliative care consultation between one and six months before death had significantly fewer hospitalizations and emergency department visits in the last seven and 30 days of life compared with those who did not receive palliative care. 939 Individuals with an initial palliative care consultation within one month of death also had significantly fewer hospitalizations in the last seven days of life compared with those who did not receive palliative care. 939 One essential component of palliative care is advance care planning (i.e., a plan for future medical care that includes the patient's goals and preferences, should the patient become unable to make their own decisions). Although Medicare reimburses physicians for visits related to advance care planning, these visits rarely occur. In 2017, less than 3% of fee‐for‐service Medicare beneficiaries had at least one claim for advance care planning. 940 However, compared with individuals without newly diagnosed conditions, Medicare beneficiaries with newly diagnosed Alzheimer's were 1.3 times as likely to have one or more claims for advance care planning. Racial/ethnic disparities in the completion of advance care planning in the last six months of life are concerning. One group of researchers found that the proportion of Black and Hispanic Medicare beneficiaries with dementia with advance care planning was less than half that of White beneficiaries. 938
Life‐sustaining interventions at the end of life
Life‐sustaining interventions, such as mechanical ventilation, tracheostomy, tube feeding and resuscitation can be especially harmful to individuals with Alzheimer's. Although these interventions may not be consistent with patient preferences, individuals with Alzheimer's may be at greater risk for receiving these treatments. One group of researchers found that Medicare beneficiaries with advanced dementia who lived in the community were 1.8 times as likely to receive life‐sustaining treatments in the last three months of life, compared with individuals without dementia living in the community. 941 Individuals with frequent transitions between health care settings are more likely to have feeding tubes at the end of life, even though feeding tube placement does not prolong life or improve outcomes. 942 The odds of having a feeding tube inserted at the end of life vary across the country and are not explained by severity of illness, restrictions on the use of artificial hydration and nutrition, ethnicity or gender. With the expansion of Medicare‐supported hospice care, the use of feeding tubes in the last three to six months of life has decreased for individuals with Alzheimer's or other dementias. 928 , 933 Finally, with the increased focus on the lack of evidence supporting feeding tube use for people with advanced dementia, the proportion of nursing home residents receiving a feeding tube in the 12 months before death decreased from nearly 12% in 2000 to less than 6% in 2014. 943 However, individuals with advanced dementia are significantly more likely to receive tube feeding in the last three months of life compared with those without dementia. 941
Place of death for individuals with Alzheimer's disease
Between 2002 and 2021, the proportion of individuals with Alzheimer's who died in a nursing home decreased from 67% to 42%, and the proportion who died in a medical facility decreased from 13% to 5%. During the same period, the proportion of individuals who died at home increased from 15% to 37% (Figure 15). 944 , 945
FIGURE 15.
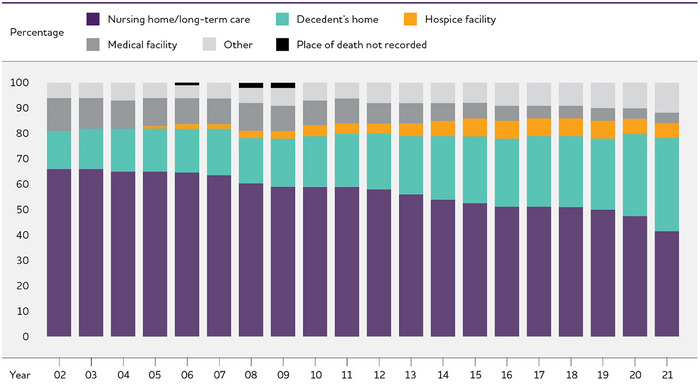
Place of death due to Alzheimer's disease, 2002 to 2021. Created from data from the National Center for Health Statistics. 944 , 945
7.4. Use and costs of health care and long‐term care services by race and ethnicity
Among Medicare beneficiaries with Alzheimer's or other dementias, Black beneficiaries had the highest unadjusted Medicare payments per person per year, while White beneficiaries had the lowest payments ($27,814 versus $22,306, respectively) (Table 22). The largest difference in payments was for hospital care, with Black Medicare beneficiaries incurring 1.6 times as much in hospital care costs as White beneficiaries ($9,006 versus $5,791). 412 White beneficiaries had the highest hospice payments, however, of all racial and ethnic groups. A study of racial and ethnic differences in health care spending using the Medical Expenditure Panel Survey found similar patterns in unadjusted total spending. 946 However, after adjusting for socioeconomic characteristics and functional status, total health care spending did not differ significantly among groups.
TABLE 22.
Average annual per‐person payments by type of service and race/ethnicity for Medicare beneficiaries age 65 and older, with Alzheimer's or other dementias, in 2023 dollars.
| Race/ethnicity | Total Medicare payments | Hospital care | Physician care | Skilled nursing home care | Home health care | Hospice care |
|---|---|---|---|---|---|---|
| White | 22,306 | 5,791 | 3,725 | 3,297 | 1,912 | 4,137 |
| Black | 27,814 | 9,006 | 4,528 | 4,338 | 1,970 | 2,910 |
| Hispanic | 25,729 | 7,836 | 4,298 | 3,763 | 2,371 | 3,416 |
| Other | 22,864 | 7,260 | 3,917 | 3,663 | 1,959 | 2,817 |
Created from unpublished data from the National 100% Sample Medicare Fee‐for‐Service Beneficiaries for 2019. 412
In a study of Medicare‐Medicaid dually eligible beneficiaries diagnosed with Alzheimer's dementia, researchers found significant differences in the costs of care by race and ethnicity. 947 These results demonstrated that Blacks had significantly higher costs of care than Whites or Hispanics, primarily due to more inpatient care and more comorbidities. These differences may be attributable to later‐stage diagnosis, which may lead to higher levels of disability while receiving care; delays in accessing timely primary care; lack of care coordination; duplication of services across providers; or inequities in access to care. However, more research is needed to understand the reasons for this health care disparity.
7.5. Use of potentially avoidable health care services
7.5.1. Preventable hospitalizations and emergency department care
Preventable hospitalizations are one common measure of health care quality. Preventable hospitalizations are hospitalizations for conditions that could have been avoided with better access to, or quality of, preventive and primary care. Unplanned hospital readmissions within 30 days are another type of hospitalization that potentially could have been avoided with appropriate post‐discharge care. In 2013, 21% of hospitalizations for fee‐for‐service Medicare enrollees with Alzheimer's or other dementias were either for unplanned readmissions within 30 days or for an ambulatory care‐sensitive condition (a condition that was potentially avoidable with timely and effective ambulatory — that is, outpatient — care). 948 The total cost to Medicare of these potentially preventable hospitalizations was $4.7 billion (in 2013 dollars; $6.1 billion in 2023 dollars). 948 Of people with dementia who had at least one hospitalization, 18% were readmitted within 30 days; and of those who were readmitted within 30 days, 27% were readmitted two or more times. 948 Ten percent of Medicare enrollees had at least one hospitalization for an ambulatory care‐sensitive condition, and 14% of total hospitalizations for Medicare enrollees with Alzheimer's or other dementias were for ambulatory care‐sensitive conditions. 948
Based on Medicare administrative data from 2013 to 2015, 23.5% of diagnosed individuals with Alzheimer's or other dementias had at least one preventable hospitalization. 949 Black older adults had a substantially higher proportion of preventable hospitalizations (31%) than Hispanic and White older adults (22% for each group).
Based on data from the Health and Retirement Study (HRS) and Medicare, after controlling for demographic variables, clinical characteristics (e.g., presence of chronic medical conditions, number of hospitalizations in the prior year) and health risk factors, individuals with dementia had a 30% greater risk of having a preventable hospitalization than those without a neuropsychiatric disorder (that is, dementia, depression or cognitive impairment without dementia). 950 Moreover, individuals with both dementia and depression had a 70% greater risk of preventable hospitalization than those without a neuropsychiatric disorder. 950 Another group of researchers found that individuals with dementia and a caregiver with depression had 73% higher rates of emergency department use over six months than individuals with dementia and a caregiver who did not have depression. 951
Medicare beneficiaries who have Alzheimer's or other dementias and a serious coexisting medical condition (for example, congestive heart failure) are more likely to be hospitalized than people with the same coexisting medical condition but without dementia (Figure 16). 412 One research team found that individuals hospitalized with heart failure were more likely to be readmitted or die after hospital discharge if they also had cognitive impairment. 952 Another research team found that Medicare beneficiaries with Alzheimer's or other dementias had more potentially avoidable hospitalizations for diabetes complications and hypertension, meaning that the hospitalizations could possibly have been prevented through proactive care management in the outpatient setting. 953 A third research team found that having depression, rheumatoid arthritis or osteoarthritis was associated with higher emergency department use in Medicare beneficiaries with possible or probable dementia and two or more other chronic conditions. 954
FIGURE 16.
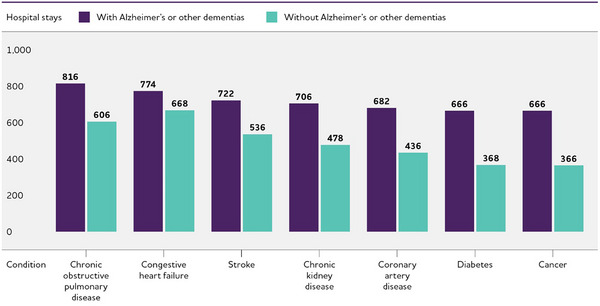
Hospital stays per 1,000 Medicare beneficiaries age 65 and older with specified coexisting medical conditions, with and without Alzheimer's or other Dementias, 2019. Created from unpublished data from the National 100% Sample Medicare Fee‐for‐Service Beneficiaries for 2019. 412
Differences in health care use between individuals with and without dementia are most prominent for those residing in the community. Based on data from the HRS, community‐residing individuals with dementia were more likely to have a potentially preventable hospitalization, an emergency department visit that was potentially avoidable, and/or an emergency department visit that resulted in a hospitalization than community‐residing individuals without dementia. 955 For individuals residing in a nursing home, there were no differences between those with and without dementia in the likelihood of being hospitalized or having an emergency department visit.
7.5.2. Health care delivery models with skilled nursing facilities
Changes in health care delivery and payment models, such as the integration of care across different health care settings and the structure of health care payments, may impact health care utilization for individuals with Alzheimer's disease or other dementias. Research has shown modest differences in outcomes for skilled nursing facilities that share providers with at least one hospital versus those that have dedicated providers within the skilled nursing facilities. An analysis of Medicare claims data for 2008 to 2016 showed that skilled nursing facilities that shared providers with at least one hospital were more likely to have an Alzheimer's unit, had fewer 30‐day readmissions, and had more patients successfully discharged to the community. The skilled nursing facilities that maintain these relationships have modestly better outcomes, 956 although there has been a decline in hospital‐skilled nursing facility linkages in the past two decades due to a shift toward dedicated hospitalists and skilled nursing facility providers.
7.6. The COVID‐19 pandemic and health care utilization and costs
The COVID‐19 pandemic has disproportionately affected Americans living with Alzheimer's and other dementias.
As data continue to emerge on the toll of the pandemic, it is increasingly clear that these individuals are more susceptible both to contracting COVID‐19 and developing severe illness due to COVID‐19. Individuals living and working in care communities have been extremely vulnerable to COVID‐19 due to the communal nature of these settings. Overall, 21% of all U.S. COVID‐19 deaths occurred in either residents or staff of long‐term care facilities. 957
Through November 2021, of all people with fee‐for‐service Medicare coverage who were hospitalized due to COVID‐19, 27% had a diagnosis of Alzheimer’ disease or another dementia. 958 Even after adjusting for demographic characteristics and other COVID‐19 risk factors (including living in long‐term care or other care communities), individuals with Alzheimer's were at higher risk for contracting and dying of COVID‐19. 959 , 960 One study using data from electronic health records and adjusting for COVID‐19 risk factors found that individuals with Alzheimer's had twice the odds of being diagnosed with COVID‐19 as individuals without Alzheimer's. The risk was even higher for Black adults with dementia, who had nearly three times the odds of contracting COVID‐19 compared with White adults with dementia. 960 Another study using Medicare claims data similarly found that beneficiaries with a diagnosis of dementia were 50% more likely to be diagnosed with COVID‐19 and 60% more likely to die of COVID‐19 than were beneficiaries without dementia, after adjusting for COVID‐19 risk factors. 959
Evidence is still emerging on how health care utilization changed during the pandemic for individuals with Alzheimer's and other dementias. For example, one area of concern is the effect of not receiving some types of health care because of service and other limitations related to COVID‐19. However, we do know that individuals diagnosed with dementia had the highest rates of hospitalization for COVID‐19 compared with individuals with any of the 20 other common chronic conditions analyzed (including chronic kidney disease, diabetes, hypertension and obesity) in 2020. 961 This risk was not limited to congregate settings such as assisted living residences and nursing homes. Individuals with a diagnosis of Alzheimer's who were living in the community were more than 3.5 times as likely to be hospitalized for COVID‐19 as individuals without Alzheimer's who were living in the community. 961
7.7. Looking to the future
Absent additional treatment breakthroughs, total annual payments for health care and long‐term care for people with Alzheimer's or other dementias are projected to increase from $360 billion in 2024 to just under $1 trillion in 2050 (in 2024 dollars). This dramatic rise includes nearly three‐fold increases both in government spending under Medicare and Medicaid and in out‐of‐pocket spending.A11 Concurrent with this large projected increase, the Medicare Hospital Insurance Trust Fund, which covers spending for Medicare Part A (hospital care), is projected to go into a deficit, based on projections of growth, overall health care spending trends and population aging. 962
7.7.1. Potential impact of changing the trajectory of Alzheimer's disease
While there are currently no treatments approved by the U.S. Food and Drug Administration (FDA) that prevent or cure Alzheimer's disease, two drugs that change the underlying biology of Alzheimer's disease (aducanumab and lecanemab) have recently been approved. They were tested in people with confirmed beta‐amyloid accumulation in the brain who were living with MCI due to Alzheimer's disease or mild dementia due to Alzheimer's. Several other treatments that target beta‐amyloid accumulation and other well‐established brain changes of Alzheimer's disease are in late‐stage development. These treatments are promising for changing the course of the disease.
Although these treatments, and others on the horizon, have the potential to improve quality of life for millions of adults and their families, there are some considerations. For example, while lecanemab demonstrated clinically significant changes in cognition and function, its effects may be imperceptible to those being treated. 963 Additionally, there is an increased risk of adverse events with lecanemab and other anti‐amyloid therapies, including amyloid‐related imaging abnormalities with edema or effusions. Another concern is the affordability of treatment to both payers, such as Medicare, and to individuals and their families, who may bear out‐of‐pocket costs due to deductibles, copayments and coinsurance. 964 Additionally, the current market price of treatment is high, at $26,500 per person per year. 965 , 966 Lack of affordability of Medicare supplemental insurance is also likely to widen disparities in access to treatment for Medicare enrollees with low incomes given these market prices.
From a societal perspective, the number of people potentially eligible and the total cost of these treatments is a potential concern. The Centers for Medicare & Medicaid Services covers Medicare beneficiaries diagnosed with MCI or Alzheimer's dementia, and has a physician participating in a registry for these treatments. 967 Although aducanumab and lecanemab are for individuals with mild Alzheimer's dementia and MCI due to Alzheimer's disease, the actual number of people who may be eligible is projected to be much smaller. One group of researchers applied the clinical trial eligibility criteria to a sample of adults with dementia or MCI and a positive brain amyloid PET scan and found that only 8% of the sample would meet the lecanemab clinical trial inclusion and exclusion criteria. 968
Before the approval of aducanumab and lecanemab, several groups of researchers had estimated the health and long‐term care cost implications of hypothetical interventions that either slow the onset of dementia or reduce the symptoms. 430 , 969 , 970 , 971 One analysis assumed a treatment that delayed onset of Alzheimer's by five years would reduce total health and long‐term care spending for people with Alzheimer's by 33%, including a 44% reduction in out‐of‐pocket payments by 2050, 969 and another study projected a 14% reduction in total health care spending for people age 70 and older with Alzheimer's from a one‐year delay, a 27% reduction from a three‐year delay and a 39% reduction from a five‐year delay by 2050. 970 Beyond the single‐year costs, the study also found that a delay in onset may increase total lifetime per capita health care spending due to longer life associated with delaying the onset of dementia, although the additional health care costs may be offset by lower informal care costs. Finally, a third study estimated that a treatment slowing the rate of functional decline among people with dementia by 10% would reduce total average per‐person lifetime costs by $3,880 in 2015 dollars ($4,759 in 2023 dollars), while a treatment that reduces the number of behavioral and psychological symptoms by 10% would reduce total average per‐person lifetime costs by $680 ($834 in 2023 dollars). 430 However, these studies did not take into account the current market price for FDA‐approved drugs.
Therapies that change the course of the disease may not be the only way to reduce health and long‐term care costs. The Alzheimer's Association commissioned a study of the potential cost savings of early diagnosis, 971 assuming that 88% of individuals who will develop Alzheimer's disease would be diagnosed in the MCI phase rather than the dementia phase or not at all. Approximately $7 trillion could be saved in medical and long‐term care costs for individuals who were alive in 2018 and will develop Alzheimer's disease. Cost savings were the result of (1) a smaller spike in costs immediately before and after diagnosis during the MCI phase compared with the higher‐cost dementia phase, and (2) lower medical and long‐term care costs for individuals who have diagnosed and managed MCI and dementia compared with individuals with unmanaged MCI and dementia.
The savings from a treatment or an earlier diagnosis may depend on structural changes to the health care system. Capacity constraints — such as a limited number of qualified providers and facilities — could severely restrict access to new treatments. 972 , 973 For example, modeling by the RAND Corporation in 2017 showed that with an anti‐amyloid therapy for people in the MCI and early dementia stages of the disease, approximately 2.1 million individuals with MCI due to Alzheimer's disease would develop Alzheimer's dementia between 2020 and 2040 while on waiting lists for treatment. This model assumed that the hypothetical treatment would require infusions at infusion centers and PET scans to confirm the presence of amyloid in the brain to support initiation of treatment with an anti‐amyloid medication.
8. SPECIAL REPORT: MAPPING A BETTER FUTURE FOR DEMENTIA CARE NAVIGATION
“Following a dementia diagnosis too many individuals and families are left on their own groping in the dark for services that can help them. I don't want others to go through what I did. I lost two to three years searching for answers. It was time I could have spent differently.” — Pamela, individual living with early‐onset Alzheimer's disease
Dementia care is a complex maze encompassing interactions with primary care providers, specialists (including those involved in managing chronic conditions coexisting with cognitive issues), social services, medication management and caregiver support (Figure 17). 974
FIGURE 17.
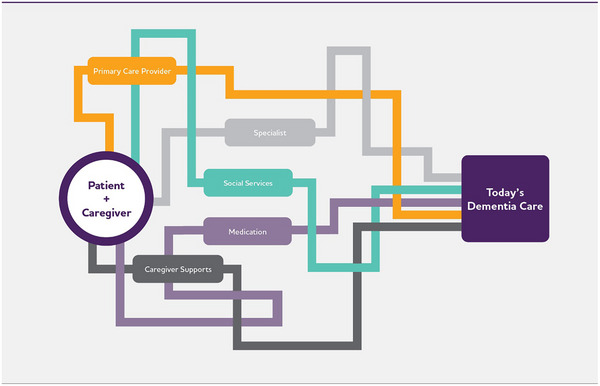
Today's dementia care. 974
Navigating this maze is difficult and often frustrating for people living with Alzheimer's or other dementia and their caregivers. Without a clear path forward, any roadblock or detour along the way can have considerable ramifications. This could materialize as delayed detection, diagnosis and treatment of early‐stage cognitive issues or mild cognitive impairment (MCI). For caregivers, a detour could cause them to miss valuable or necessary educational opportunities, miss connections with community‐based services for respite and behavioral health support or be unable to locate resources that could help reduce their stress. Breakdowns in transitions of care between health care providers and settings limit high‐quality, comprehensive and/or appropriate dementia care, as evidenced by an increase in emergency room visits and hospitalizations and decreased quality of life. 975
8.1. Caregiver burden and stress are compounded by the complexity of dementia care
Unpaid caregivers (sometimes referred to as care partners), who can be a spouse, family member or friend, provide extensive, sometimes all‐encompassing care for people living with Alzheimer's or other dementia. In 2023, 11.5 million family members and other caregivers of people living with Alzheimer's or other dementia provided an estimated 18.4 billion hours of unpaid help. On average, this represents nearly 31 hours of care per caregiver per week or 1,612 hours per caregiver per year.A8 Caregivers spend much of this time interacting with the health care system or learning more about dementia caregiving, and nearly 2 in 3 (63%) help with health or medical care. 426 , 439 Daily health care activities may include scheduling appointments with health care providers, attending doctor's visits, and scheduling social and community support for themselves and the person living with dementia, such as in‐home assistance, adult day programs or meal delivery. Collectively, performing these activities and the organization of care across multiple health care providers can be described as care coordination. 976 , 977
The effort expended trying to find their way through the health care system can add to the already high emotional and physical stress levels that caregivers experience. Caregivers need assistance to gather dementia care information, synthesize it and act upon it in a way that does not add to their stress level — support that primary care providers and health systems have historically been ill‐equipped or unprepared to provide. (For more information on supporting people living with Alzheimer's or other dementia and the impact of unpaid caregiving, see the Caregiving section.)
8.2. Nationwide movement to improve care while reducing strain on caregivers
For more than a decade, the National Plan to Address Alzheimer's Disease has included goals to improve health care quality and expand support for individuals living with Alzheimer's disease or other dementia and their families. 978 Recently, the Alzheimer's Association and the Alzheimer's Impact Movement (AIM) — a separately incorporated advocacy affiliate of the Alzheimer's Association — championed critical legislation to help unravel the health care maze. 979 This legislation, the bipartisan Comprehensive Care for Alzheimer's Act, proposed a new approach to dementia care management covering care coordination and navigation, caregiver education and support, and alternative payment models for physician reimbursement. 979 , 980
In July 2023, the Centers for Medicare & Medicaid Services (CMS) announced the culmination of policy, working group and legislative efforts like the Comprehensive Care for Alzheimer's Act with the introduction of the Guiding an Improved Dementia Experience (GUIDE) Model. 981 The new model represents a pivotal opportunity to reshape and enhance dementia care in the United States.
8.3. What is GUIDE? 982
The Guiding an Improved Dementia Experience (GUIDE) Model is an eight‐year pilot program in dementia care management designed to help dementia patients and caregivers better navigate health care and social support systems to improve dementia care. Three primary aims of GUIDE are to:
Improve quality of life for people living with dementia.
Reduce strain on their unpaid caregivers.
Enable people living with dementia to remain in their homes and communities.
Beginning in July 2024, health care providers who participate in GUIDE will deliver supportive services to people living with dementia, including comprehensive, person‐centered assessments and care plans, care coordination and 24/7 access to a support line. They will also provide access to a care navigator to help patients and caregivers access services and support.
GUIDE acknowledges that current fee‐for‐service payment structures prevent many practices from implementing sustainable dementia care management programs. 980 , 981 , 982 To overcome this challenge, GUIDE is testing an alternative payment model (APM) to incentivize health systems and increase the likelihood that smaller practices, rural practices and inner‐city health centers that traditionally do not have the financial resources of larger entities will be able to deliver this type of program. 980 , 982 The APM shifts payments from a fee for individual services to a monthly per‐patient payment for all services under the GUIDE Model umbrella, including those not typically reimbursed by Medicare. 980 , 982
Recognizing that some health care providers will face resource, staffing and capability constraints, a second GUIDE Model track will engage those who do not have experience offering comprehensive dementia care services. The Centers for Medicare & Medicaid Services will offer these organizations technical assistance, learning support and a preparatory pre‐implementation year to facilitate their participation in the model. (For more information about GUIDE and other national strategies to support caregivers, see the Caregiving and Workforce sections.)
8.4. Navigators as dementia health care wayfinders
8.4.1. What is care navigation?
Care navigation is a critical process that assists patients and caregivers with various aspects of obtaining health care, such as helping them understand and overcome the complex logistics of the health care system. 983 , 984 , 985 The concept originated in cancer clinics in the 1990s to address the overwhelming experience cancer patients faced trying to manage their care. 985 It has since expanded to support individuals with chronic diseases, including kidney disease, diabetes and dementia. 983 , 986 , 987 , 988
This assistance aims to be holistic and spans both medical and nonmedical needs. On the medical side, care navigation encompasses: 984 , 987
Scheduling appointments.
Coordinating diagnostic testing or follow‐ups.
Providing disease education.
Facilitating communication and referrals across clinical specialties and organizations.
Offering insurance and benefits assistance.
Medication management.
Beyond medical support, care navigation programs may connect patients and caregivers to nonmedical resources, such as education, social services and community support. 987
Providing both types of navigation services underscores the importance of a comprehensive approach to health care. 987 By addressing medical and nonmedical needs, care navigation programs strive to ensure better access to health care, improve health outcomes and reduce disparities in care. 986 , 987 , 988
8.4.2. What is dementia care navigation?
Dementia care navigation shares many features of care navigation programs in other specialties. However, dementia care navigation emphasizes person‐centered, empowered support throughout the dementia care journey. 986 This includes addressing nonmedical needs unique to dementia care, such as behavioral symptom management and access to community‐based services and supports for individuals with dementia and their caregivers. 983 , 986 , 988 , 989 The approach remains nimble to adapt to emerging treatments and diagnostic tests, disease progression, or other individual needs as they arise.
A key tenet of dementia care navigation is recognizing the importance of the “care dyad”— the partnership between people living with Alzheimer's disease or other dementia and their caregivers — in all aspects of dementia care. 983 , 988 As described previously, caregivers are prone to information overload and may feel lost and overwhelmed as they try to find their way to quality dementia care for the person living with dementia. Dementia care navigation programs are primed to offer assistance in this area.
“Due to high incidence, duration and medical‐social complexity, dementia is an ideal candidate for patient‐centric health care delivery models such as care navigation.” 986
In 2023, an expert workgroup convened by the Alzheimer's Association defined dementia care navigation as “a program that provides tailored, strengths‐based support to persons living with dementia and their care partners across the illness continuum and settings to mitigate the impact of dementia through collaborative problem solving and coaching.” 986
The workgroup outlined seven essential principles for dementia care navigation, which underscore person‐centered care. According to the workgroup, dementia care navigation should: 986 , 989
Be person‐ and family‐centered to ensure collaboration and enhance engagement.
Be culturally responsive and address disparities in access to health care and support services.
Include well‐defined roles and responsibilities for all members of the dementia care navigation team.
Address barriers relating to medical, legal, financial, emotional and other domains facing the person living with dementia and their care partners.
Provide coaching, education, and coordination in a manner that is empowering, solution‐focused and strengths‐based.
Focus on the family unit as defined by the person living with dementia.
Ensure processes and protocols are evidence‐based.
By adhering to these principles, dementia care navigation programs can achieve more coordinated care for patients. Health systems are already finding that dementia care navigation can improve health outcomes, decrease the number of emergency room visits, lower hospital readmissions, shorten hospital stays and minimize delays in long‐term care placement. 989
8.4.3. What is a care navigator?
Care navigators are staff who guide patients and caregivers through the health care system and help overcome barriers that prevent them from getting the care they need. 987 , 991 As integral members of interprofessional care teams, care navigators are connectors — liaising, communicating and facilitating medical and nonmedical needs. 987 , 988 , 991 Unlike other care team members, their work spans various settings, making them crucial touchpoints for care coordination. 987 , 988 Common synonyms for “care navigator” include patient navigator, care consultant and care team coordinator. 986 , 988 , 991
Qualifications, training and time dedicated to the care navigator role vary based on the care team structure and the health system. They range from paraprofessionals to licensed health care professionals, including nurses, physician assistants, social workers, community health workers or even former caregivers. 987 Care navigators, including dementia care navigators, frequently share the racial, ethnic or cultural background of those they assist, enhancing the delivery of culturally competent care and building stronger patient‐navigator relationships. 983 , 987
Dementia care navigators work with care dyads, but evidence suggests that the primary recipients of navigation services are caregivers. 983 In addition to the typical navigation services, dementia caregivers frequently look to care navigators for emotional support. 983 Through these interactions, navigators and caregivers establish trusting, long‐term relationships.
In summary, care navigators are pivotal in helping patients and caregivers find their way through an increasingly intricate dementia care landscape.
8.5. Prioritizing person‐centered care in dementia care navigation 986 , 988 , 989 , 990
Person‐centered care is the foundation of quality dementia care. It challenges the traditional medical model of care that tends to focus on processes, schedules, and staff and organizational needs. Instead, person‐centered care stresses knowing the person living with dementia, including their values, beliefs, interests, abilities, likes and dislikes — both past and present. A person‐centered approach to care assures the individual living with dementia and their caregivers that health professionals know the person, understand the person's unique needs and circumstances, and put these needs at the forefront in making decisions and directing the person's care.
Anchoring dementia care navigation in the principles of person‐centered care prioritizes the humanity of each individual living with dementia while also committing to a standard of care that elevates their dignity, autonomy and quality of life at every stage.
8.6. Awareness and understanding of dementia care navigation: caregiver and health care workforce surveys
This year's Special Report takes a deeper look into how dementia caregivers interact with the health care system and how the non‐physician health care workforce currently employs care navigation. To better understand these aspects of dementia care, the Alzheimer's Association commissioned Versta Research to conduct surveys of (1) current or recent caregivers of adults age 50 or older with cognitive issues (referred to in this report as dementia caregivers) and (2) health care workers who are likely to assume care navigation responsibilities in their role, including nurses, social workers, and community health workers (referred to in this report as the non‐physician health care workforce).
8.7. Key findings
8.7.1. Dementia caregivers
Dementia caregivers experience difficulty and stress interacting with the health care system.
Seven in 10 dementia caregivers (70%) report that coordinating care is stressful. More than half of caregivers (53%) said navigating health care is difficult. Two in 3 dementia caregivers (66%) also have difficulty finding resources and support for their needs.
Cost and care coordination are top stressors for dementia caregivers.
Two in 5 caregivers (42%) cite cost as a stressor in getting care for their recipient. More than 1 in 3 caregivers report coordinating care with multiple doctors (36%), securing appointments (35%) and getting help taking a break (35%) as leading stressors in navigating care for their recipient.
Despite these and other stressors, only half of the caregivers (51%) report ever talking with a health care professional to help address their challenges.
Care navigation is an unfamiliar term for most dementia caregivers, although many receive help akin to care navigation.
Three in 4 dementia caregivers (75%) report little or no familiarity with the term “care navigator.” Half of caregivers (50%) say they receive help with dementia health care, support and services for the care recipient from someone within their physician's office or hospital.
Nurses (42%) and social workers (35%) most often provide navigation help to dementia caregivers.
Overwhelmingly, caregivers would welcome dementia care navigator support and believe it would benefit both the person living with dementia and the caregiver.
More than 4 in 5 dementia caregivers (85%) say having access to a care navigator would influence their choice of dementia health care provider for the person they care for.
Three in 5 dementia caregivers (61%) cite improvement in quality of life for their care recipient as a benefit of having a care navigator. Two in 5 caregivers (43%) believe access to a care navigator would improve the overall health of their care recipient.
Three in 5 dementia caregivers say less stress (62%) and more peace of mind (62%) would be valuable outcomes of having a care navigator. More than half (56%) say having a care navigator could help them be better caregivers.
Top services that would be helpful to dementia caregivers include around‐the‐clock support, care coordination and help understanding their care recipient's condition.
The vast majority of dementia caregivers (97%) say they would find navigation services helpful.
Nearly 2 in 5 dementia caregivers (36%) say a 24/7 helpline would be valuable in helping navigate care for someone living with Alzheimer's or other dementia. Coordinating care and communication between different specialists (34%) and getting help in understanding their care recipient's condition (34%) are also viewed as valuable services.
Almost 1 in 3 dementia caregivers say it would be helpful to have assistance with insurance or public benefits (32%), scheduling appointments (31%), caregiver training (31%), managing behavioral symptoms (31%), understanding the health care system (30%) and finding services to help with respite care (30%).
The most helpful community‐based resources cited to help dementia caregivers include local caregiver support groups (41%), respite programs (38%) and availability of financial resources in the community (37%).
8.7.2. Non‐physician health care workforce
The following findings reflect the views of the non‐physician health care workforce currently providing navigator‐type services to patients and caregivers in addition to the other responsibilities of their role. The health care workers surveyed included medical professionals (nurse practitioners, physician assistants and registered nurses) and nonmedical professionals (health care social workers, community health workers and home health aides).
Most health care workers who provide navigator‐type services are familiar with the concept of care navigation, even if that is not their focus.
Three in 4 survey respondents (77%) are familiar with the term “care navigator.” They spend roughly half their time providing navigator‐type services, even if they do not refer to themselves as care navigators.
Nearly 2 in 3 survey respondents (62%, predominantly nonmedical professionals) help people living with Alzheimer's or other dementia and caregivers understand the health care system, and more than 1 in 2 health care workers (57%) say they coordinate care and communication with specialists.
The most frequently provided navigator services are referrals to community support services and resources (75%), helping with emotional and cultural support (68%), and screening for safety needs (66%).
Most health care workers providing navigator‐type services have experience in other medical specialties, with few focusing exclusively on dementia.
Four in 5 survey respondents (80%) have navigation experience in non‐dementia medical specialties, and fewer than 1 in 10 (7%) focus primarily on offering navigator‐type support and services to people living with dementia.
Most providing navigation services (93%) feel at least somewhat knowledgeable about MCI, Alzheimer's disease and other dementia but only 1 in 3 (36%) report they are very knowledgeable.
Nearly 9 in 10 (86%) feel knowledgeable about directing patients with dementia and caregivers to appropriate health care resources, but less than 1 in 3 (30%) feel very knowledgeable. Four in 5 (82%) feel knowledgeable about directing patients with dementia and caregivers to community resources, but only 31% say they are very knowledgeable.
Training in dementia care navigation is lacking and not standardized.
Three in 4 health care workers providing care navigation (75%) indicate they received no formal training in dementia care navigation.
Those who did receive training were predominantly nonmedical professionals, receiving a median of 30 hours of formal training. Medical professionals who were trained received a median of 20 hours of formal training.
Nonmedical professionals are viewed as best suited to help people with dementia and their caregivers navigate care.
Nine in 10 health care workers offering navigation support (92%) say social workers, community health workers or home health aides are best suited to help people living with dementia and their caregivers navigate health care.
Health care workers say more can be done to help patients and families navigate dementia care but point out current barriers.
Six in 10 survey respondents (60%) believe that the U.S. health care system is not effectively helping patients and their families navigate dementia care.
Nearly half surveyed (46%) say their organizations do not have a clearly defined process for care coordination and clinical pathways for patients with MCI, Alzheimer's disease or other dementia.
More than 3 in 4 (77%) identified a lack of community‐based resources as a barrier, and 44% viewed it as the greatest barrier. Seven in 10 (70%) called out current reimbursement as a barrier, with 41% saying this was the greatest barrier.
Nearly 9 in 10 (87%) say developing alternative payment models is important in providing future care coordination for people diagnosed with dementia.
8.8. Survey design and research methods
The surveys were designed to elicit in‐depth responses from both dementia caregivers and the non‐physician health care workforce about the current state and challenges of navigation in dementia care.
The dementia caregiver survey analyzed distinct aspects of the caregiving journey, including:
Time spent on caregiving and top stressors.
Challenges in navigating health care services.
Challenges in locating or accessing community supports and services.
Awareness of dementia care navigators and/or navigation programs.
Which health care workers help with care navigation.
Communication preferences for care navigation.
Value of navigation services and community‐based resources.
Anticipated benefits and outcomes of care navigation.
The non‐physician health care workforce survey covered various aspects of care navigation, including:
Familiarity with navigator terminology.
Focus areas for care navigation services being delivered.
Frequency and preferred method of communication.
Perceived value of care navigation.
Which health care workers deliver navigation services.
Barriers to care navigation.
Background and training in care navigation.
8.8.1. Dementia caregiver survey
A survey of 1,533 U.S. adults who were current or recent unpaid caregivers for a relative or friend age 50 or older experiencing problems with thinking, understanding, or remembering things or who sometimes have physical problems or behavioral changes was conducted from November 20, 2023, through December 20, 2023. The sample included White (n = 629), Hispanic (n = 309), Black (n = 308), Asian (n = 206) and Native American (n = 24) caregivers and caregivers who identified as belonging to other ethnic or racial groups (n = 57). While Native Americans were oversampled in an attempt to get subgroup estimates, the sample size was still insufficient; thus, Native American respondents were included in the “all caregivers” grouping. Respondents were recruited via non‐probability online panels used exclusively for research, with full population screening data weighted to match U.S. Census data on age, gender, income, education and race/ethnicity to ensure accurate representation of the caregiving population and to establish weighting benchmarks for demographic oversamples. The survey was offered in both English and Spanish. Differences noted in the report between racial and ethnic groups were tested and found to be statistically significant at the p<.05 level.
8.8.2. Non‐physician health care workforce survey
A survey of 1,204 U.S. health care workers was conducted from November 13, 2023, through December 6, 2023. The survey collected the views of medically‐trained and nonmedically‐trained professionals who perform navigation duties, regardless of whether they describe themselves as navigators or hold a formal navigator position at their organization.A17 For brevity, medically‐trained professionals are referred to as “medical professionals” and nonmedically‐trained professionals as “nonmedical professionals” throughout the remainder of the report. The report refers to the combined group of medical and nonmedical professionals as “health care workers.”
Medical professionals (n = 708) included:
Registered nurses (RN, n = 526).
Nurse practitioners (NP, n = 145).
Physician assistants (PA, n = 46).
Nonmedical professionals (n = 503) included:
Social workers (MSW, n = 458).
Community health workers (CHW, n = 32).
Home health aides (HHA, n = 14).
Health care workers are classified as both medical and nonmedical professionals if they indicate both types of training (e.g., RN with MSW degree). Because of this, the total of the numbers of medical and nonmedical professionals shown above exceeds 1,204. Likewise, if health care workers are classified as having more than one role in the medical or nonmedical category (e.g., community health worker and home health aide), they are included in the count for each role. As a result, the total of the specific roles in the medical and nonmedical categories exceeds the 708 and 503 shown above.
8.9. Dementia caregiver survey results
8.9.1. Dementia caregiving is a demanding job that can last for years
People with memory and thinking problems see an average of four different doctors every year, with more than 1 in 4 (27%) seeing five or more doctors annually. Scheduling and managing doctor's visits can be time‐consuming, and more than 1 in 3 dementia caregivers (35%) coordinate health care needs (communicating with doctors, taking care of insurance, getting appointments, picking up medication, etc.) at least once daily, with some caregivers saying they coordinated care several times per day.
Caregivers for people with Alzheimer's and other dementia provide approximately 26 hours of care per week. This is consistent with other reports that caregivers spend almost 31 hours per week on caregiving.A8 Additionally, a large majority of dementia caregivers surveyed spend years providing care, with nearly 1 in 2 acting as a caregiver for one to three years and almost 1 in 3 spending four years or more as a caregiver.
8.9.2. Black caregivers report more time on caregiving responsibilities than other groups
The need to coordinate health care is common for dementia caregivers, and this is especially true for Black and Hispanic caregivers, who are more likely to coordinate health care at least once per day than White caregivers (43%, 45%, and 31%, respectively). This likely influences the overall time spent providing care, with Black caregivers reporting the most time at 30 hours per week, followed by White caregivers (27 hours), Hispanic caregivers (25 hours) and Asian caregivers (19 hours).
8.9.3. Dementia caregivers experience difficulty and stress interacting with the health care system and addressing their own needs
A majority of caregivers surveyed (70%) indicated that coordinating care is stressful. More than half (53%) said navigating health care for the person they care for was difficult. Finding resources and support for their needs is also a challenge for 2 in 3 caregivers (66%; Figure 18).
FIGURE 18.
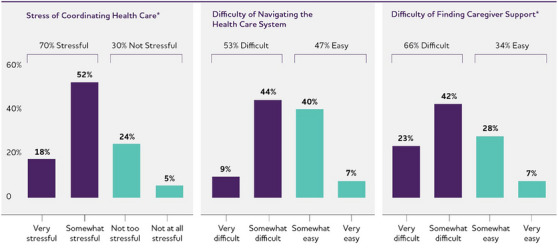
Difficulties and stressors that caregivers experience. *Percentages of bars may not total the percentages above due to rounding.
8.9.4. Black caregivers report less stress and difficulty with dementia care
Black caregivers find coordinating dementia care somewhat less difficult and stressful than all other groups. Three in 5 Black caregivers (58%) reported that coordinating care was somewhat or very stressful compared with Hispanic caregivers (71%), White caregivers (72%) and Asian caregivers (76%). When asked about difficulty coordinating health care, Asian caregivers expressed the greatest challenges, with 7 in 10 (68%) indicating that they found it somewhat or very difficult (vs. Black caregivers, 37%; White caregivers, 54%; and Hispanic caregivers, 57%). Additionally, Black caregivers have less difficulty finding support for their own needs as a caregiver than other groups (52% report somewhat or very difficult vs. White caregivers, 66%; Hispanic caregivers, 70%; and Asian caregivers, 77%).
8.9.5. Worries about costs and coordinating health care are top stressors
The most often cited worry for dementia caregivers is cost (42% of caregivers), followed by the stress of coordinating with multiple doctors (36%), securing appointments (35%) and getting help taking a break (35%; Figure 19). Finding appropriate doctors (32%) rounded out the top five stressors. When viewed together, these top five stressors underscore challenges in coordinating dementia health care without greater assistance from a care navigator.
FIGURE 19.
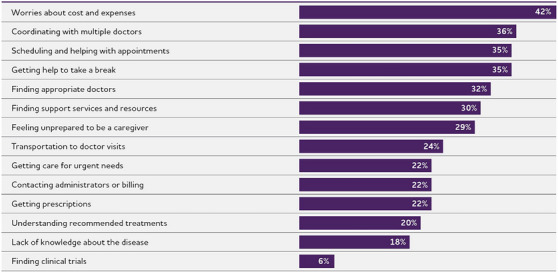
Stressors when getting health care for care recipients.
Asian caregivers report worries about costs and expenses (51%) as the top stressor, more so than other groups (Black caregivers, 37%; Hispanic caregivers, 42%; White caregivers, 41%). Asian caregivers also are more likely to report stress in finding appropriate doctors (41%) and understanding recommended treatments (30%) than other groups. Finding respite care is the top stressor for Black caregivers (39%), and concerns about cost and expenses are top of mind for Hispanic caregivers.
Despite these current stressors, only half of the dementia caregivers surveyed (51%) have ever talked with a health care professional about challenges finding their way through the health care system or asked for help with dementia care.
8.9.6. Although largely unfamiliar with the term “care navigator,” dementia caregivers receive help navigating care
Three in 4 dementia caregivers surveyed report little or no familiarity with the term “care navigator,” with 30% saying they know very little about the term and 45% reporting they have never heard of the term. Yet half of caregivers (50%) receive help with dementia health care, support and services for the care recipient from someone within their physician's office or hospital. These health care workers may or may not be serving in a formalized navigator role. Nurses (42%) or social workers (35%) most often provide navigation help to dementia caregivers, with physician assistants (18%), community health workers (14%), other caregivers (12%) or actual care navigators (7%) providing health care guidance to a lesser degree (Figure 20).
FIGURE 20.
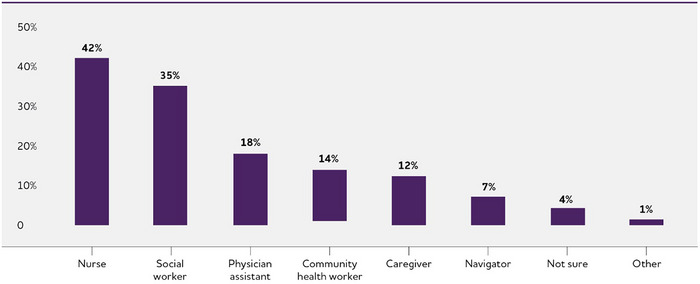
Navigator's professional role.
Nearly 7 in 10 Black caregivers (68%) report receiving help navigating care. Asian caregivers (52%), Hispanic caregivers (51%), and White caregivers (47%) also report currently receiving help navigating care.
When working with staff helping with care navigation, a majority of caregivers would prefer that they communicate via more traditional channels, with nearly 2 in 3 saying a phone call was best and 1 in 2 saying they desired in‐person communication during a visit (Figure 21). Overall, very few caregivers wanted to communicate through electronic health record (EHR) messaging or a patient portal (12%).
FIGURE 21.
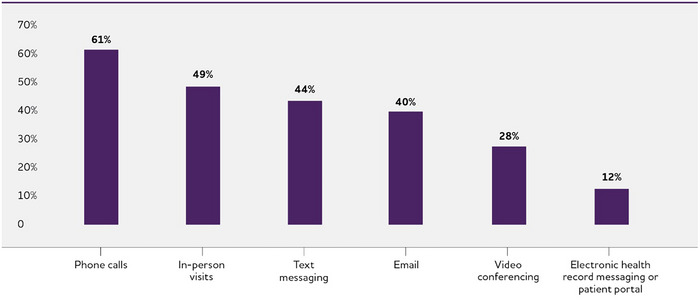
Preferred channels for communicating with a care navigator.
8.9.7. Cultural competency is fundamental for dementia care navigation
Caregivers for people living with dementia stressed that it is essential for care navigators to understand their ethnic or racial background. Significantly, 9 in 10 Asian, Black, and Hispanic caregivers felt it crucial for navigators to be aware of the background of the person they are caring for (Figure 22). In contrast, White caregivers placed less importance on this shared experience. Among White caregivers who received navigation help, 84% believed the person helping them had a good or excellent grasp of their care recipient's background, a confidence level higher than that for other racial and ethnic groups. Confidence in the cultural competency of the person providing navigation assistance was lower for all other groups, and lowest for Asian caregivers, with only 54% rating understanding of the person helping them as “good” or “excellent.”
FIGURE 22.
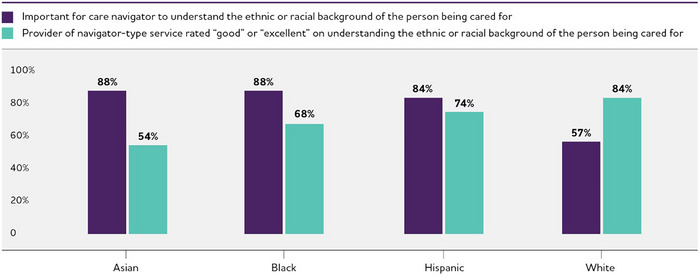
Access to care navigators with cultural competency by race/ethnicity of care recipient.
Health care workers surveyed echoed that empathy for racial, ethnic and cultural backgrounds and experiences is key. They overwhelmingly agreed that effective dementia care navigation requires cultural competence, with 99% saying that it is important for navigators to understand (Figure 23). Four in 5 health care workers believe their organization has an effective understanding of the racial, ethnic, and cultural backgrounds and experiences of people with dementia and their caregivers.
FIGURE 23.
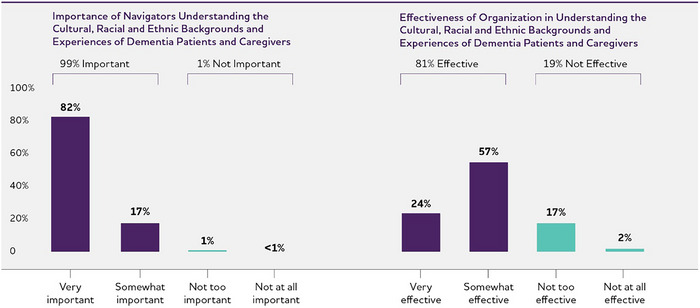
Health care professionals’ views on cultural competence.
8.9.8. Caregivers welcome dementia navigation support
Overwhelmingly, dementia caregivers surveyed would welcome navigator support, with 4 in 5 caregivers (85%) indicating that having access to a care navigator would influence their choice of a dementia health care provider for the person they care for. This sentiment was strongest among Black caregivers, with 52% saying it would influence their choice a great deal, followed by Hispanic caregivers (44%), White caregivers (43%) and Asian caregivers (38%).
8.9.9. Less stress and better outcomes are biggest benefits of working with a dementia care navigator
Caregivers see improvement in quality of life (61%) and health (43%) for the person they care for as being the greatest positives of working with a dementia care navigator (Figure 24). Other benefits for the person living with Alzheimer's or other dementia include less depression (35%), longer period of time at home (35%) and fewer behavioral symptoms (26%).
FIGURE 24.
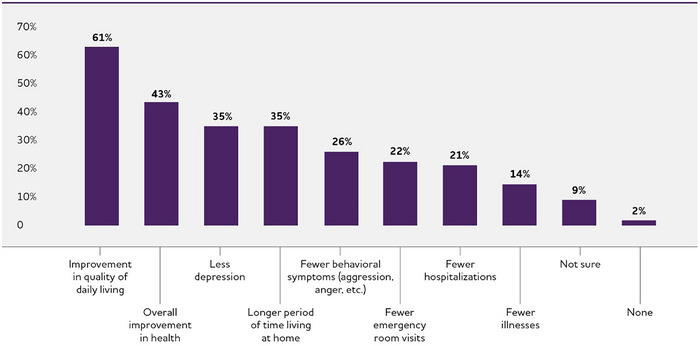
Anticipated outcomes for care recipients of a navigator program.
For themselves, 2 in 5 caregivers surveyed see less stress (62%) and more peace of mind (62%) as the most valuable outcomes of having a navigator as part of the dementia care team (Figure 25). They also think a care navigator could help them be better caregivers (56%), improve their mental health (45%) and help them find opportunities to take a break from their care responsibilities (38%). Overall, very few dementia caregivers believed a care navigator would make caregiving less expensive (18%); however, there was a clear difference in this view among caregivers from different racial and ethnic groups. Nearly twice as many Asian, Black and Hispanic caregivers thought working with a navigator could make caregiving less expensive compared with White caregivers (26%, 23% and 22%, respectively, vs. 14%)
FIGURE 25.
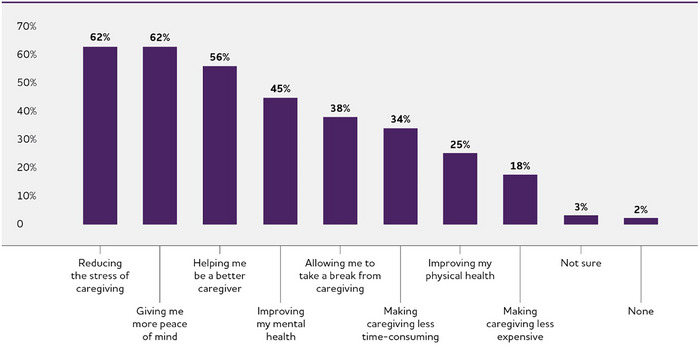
Anticipated outcomes for caregivers of a navigator program.
8.9.10. Around‐the‐clock support, care coordination and help understanding the care recipient's condition viewed as most valuable navigation services overall
Nearly all dementia caregivers (97%) say they would find navigation services helpful. Almost 2 in 5 caregivers (36%) said a 24/7 helpline to call is the top service a care navigation program should provide (Figure 26). Coordinating care and communication between different specialists (34%) and helping to understand their care recipient's condition (34%) are also viewed as valuable services. Overall, caregivers see value in a mix of medical and nonmedical navigation services. Other helpful services, according to 1 in 3 caregivers, included:
Assistance with insurance or public benefits (32%).
Help with scheduling appointments (31%).
Training on how to care for someone with thinking or memory problems (31%).
Help managing behavioral symptoms (31%).
Help understanding the health care system (30%).
Help finding services to take a temporary break from caregiving (30%).
FIGURE 26.
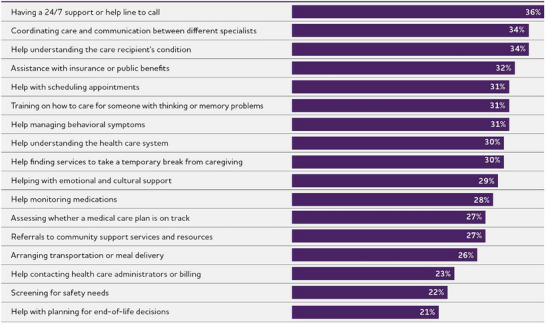
Care navigator services that would be valuable to dementia caregivers.
The findings highlight services more relevant to dementia care navigation than care navigation for other health conditions, such as respite services, managing behavioral symptoms and support for unanticipated needs outside of regular clinic hours in the form of the helpline. Typically, community‐based organizations provide these services. Although 1 in 3 caregivers (30%) have received nonmedical supports and services, such as meal delivery, home care aides, or companions to give time away from caregiving, only 17% found these resources with the help of a person providing care navigation services.
8.9.11. Community‐based resources may address some stressors
Community‐based resources and services can play an important role in supporting caregivers. While many of these services are delivered outside traditional health care settings, finding ways to connect caregivers to these resources should be viewed as an essential deliverable in dementia care navigation.
The three most helpful community‐based resources cited by caregivers in the survey were local caregiver support groups (41%), respite programs (38%) and availability of financial resources in the community (37%). These resources could alleviate some of the top stressors, like worries about costs and expenses and getting help taking a break (Figure 27).
FIGURE 27.
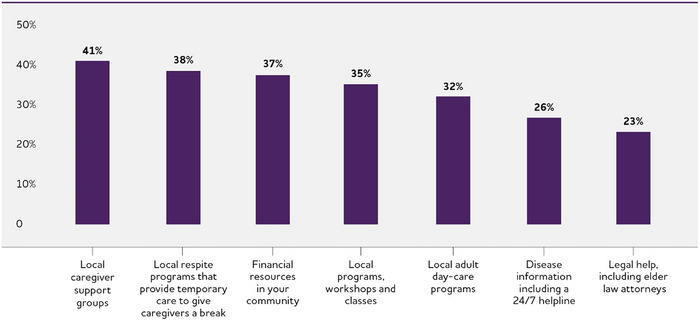
Community‐based services that would be valuable to dementia caregivers.
Caregivers of all races and ethnicities surveyed pointed to connections to local adult daycare programs as a valuable navigation service (Asian, 45%; Black, 36%; Hispanic, 35%; White, 28%).
8.10. Non‐physician health care workforce survey results
8.10.1. “Care navigator” is a known term in health care
Three in 4 health care workers who provide navigator‐type services (77%) are familiar with the term “care navigator.” Nonmedical professionals (CHW, HHA or MSW) were the most familiar, with 4 in 5 (83%) indicating they had heard this term before.
8.10.2. Many professionals already provide navigation services, but this is not their focus
Health care workers participating in the survey spend roughly half (53%) their time providing navigator‐type services, even if they do not refer to themselves as care navigators (Figure 28). Nearly 1 in 3 of the patients they provide navigation services for have cognitive issues, including MCI, Alzheimer's disease or other dementia (Figure 28). The vast majority of respondents (93%) say that caregivers or family are almost always involved in discussions of navigation‐type services — with or without the person for whom they are caring.
FIGURE 28.
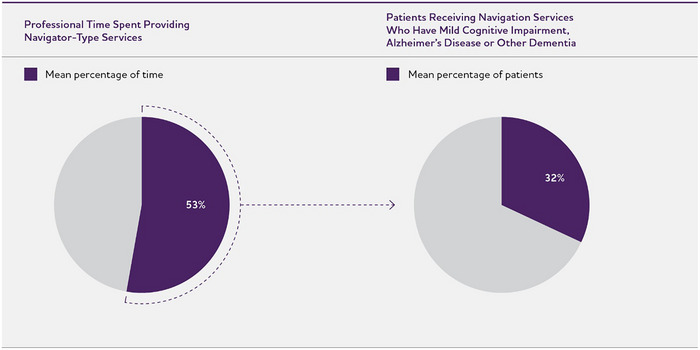
Time spent on navigator‐type services and percentage of patients with cognitive issues receiving navigation services.
Nearly all health care workers in the survey provide support for medical and nonmedical needs, with only 5% focusing exclusively on one type of need (Figure 29). The most frequently provided navigator services are referrals to community support services and resources (75%), helping with emotional and cultural support (68%) and screening for safety needs (66%) (Figure 30). Of those top navigation services, referrals to community support are more often provided by nonmedical professionals, whereas screening for safety needs is most often performed by medical professionals.
FIGURE 29.
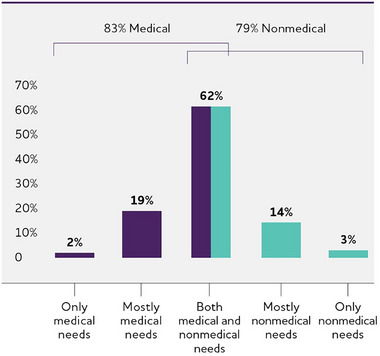
Focus of dementia care support provided.
FIGURE 30.
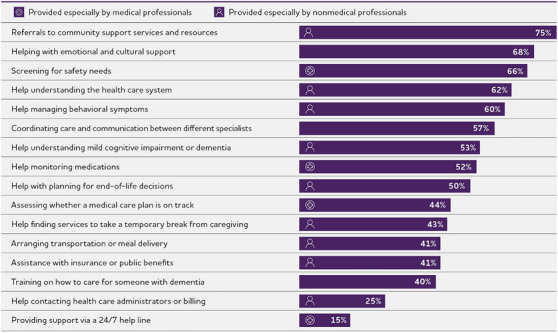
Services provided that support dementia care for patients and their families.
Unsurprisingly, medical professionals tend to offer more medically‐related navigation services, such as screening for safety, assessing if the medical plan is on track, monitoring medications and staffing helplines (Figure 30). They also viewed these services as more valuable to patients and families than nonmedical professionals did. Nonmedical professionals, on the other hand, report that they are more often involved in making referrals to community resources, disease education, assisting caregivers looking for respite care, arranging transportation or meal delivery and insurance‐related support such as working with billing or insurers (Figure 30).
Further illustrating the complicated nature of dementia care and the need for navigation as outlined earlier in the Special Report, health care workers also report that they are heavily involved in guiding patients and families through the health care system. Nearly 2 in 3 health care workers (62%; predominantly nonmedical professionals) help patients and caregivers understand the health care system, and more than 1 in 2 health care workers say they coordinate care and communication with specialists (Figure 30).
8.10.3. Greatest value from navigators is in connections to community support and services
More than 2 in 3 health care workers (68%) said the top service provided, referrals to community support services and resources, was the most valuable navigation offering (Figure 31). The top five most valuable navigation services according to survey respondents were:
Referrals to community support services and resources (68%).
Training on how to care for someone with dementia (63%).
Help managing behavioral symptoms (62%).
Helping with emotional and cultural support (59%).
Coordinating care and communication between different specialists (59%).
FIGURE 31.
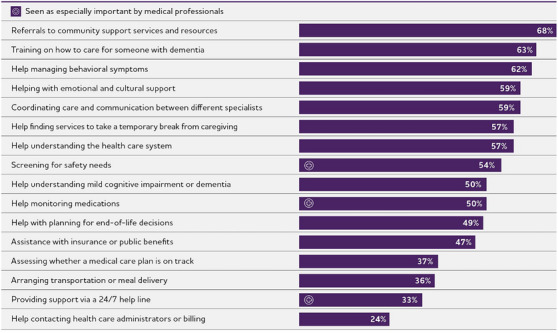
Most valuable services in supporting dementia care for patients and their families.
More than half of respondents also said that finding respite services to take a temporary break from caregiving, understanding the health care system, and screening for safety needs were valuable.
Interestingly, there were two notable disconnects between what health care workers perceive as valuable and what they deliver in the form of navigation services. The first is training for family members on how to care for someone with dementia. Whereas 63% of survey respondents rated this as valuable, only 2 in 5 (40%) provide such training. The second disconnect was in the utility of a 24/7 helpline. This feature of navigation was valued by 1 in 3 health care workers (33%), yet only 15% are currently providing this service.
8.10.4. Nonmedical professionals communicate more frequently with patients and families
Two in 5 nonmedical professionals (39%) report they connect with individuals living with dementia and their families every two weeks, and 1 in 3 (32%) make contact every month. Medical professionals typically communicate with patients every two weeks (28%) and every month (24%). However, medical professionals report that they are more likely to communicate every 3 months and 6 months than nonmedical professionals (15% vs. 7% and 5% vs. 1%). This distinction between medical and nonmedical professionals may be a result of the cadence of follow‐up visits with a physician, and medical professionals may be more likely to incorporate navigation services into a routine visit. Across groups, 1 in 4 health care workers (23%) discuss dementia care services only as needed.
On average, nonmedical professionals interact most often with patients and families:
All health care workers: 12 times per year.
All medical professionals (NP, PA or RN): 11 times per year.
All nonmedical professionals (CHW, HHA or MSW): 14 times per year.
8.10.5. Health care workers providing navigation‐type services use traditional communication channels instead of newer technologies
In‐person visits and phone calls are by far the most common channels for dementia care navigation and dramatically outpace usage of newer technologies like video conferencing, email, patient portals and text messaging. Care navigation services are 3 to 4 times more likely to be provided in‐person or by phone than other channels, and most communication still occurs at in‐person visits.
There are some distinctions in how medical and nonmedical professionals deliver navigation services. Medical professionals communicate more often via a patient portal than nonmedical professionals (19% vs. 14%). The most striking difference is how nonmedical professionals appear to have gravitated toward digital communication, possibly because they engage with patients and families more regularly. Nonmedical professionals use video conferencing and email to reach patients and families twice as often as medical professionals (29% vs. 11% and 26% vs. 12%, respectively). Nonmedical professionals also report using a phone call to communicate more often than medical professionals (76% vs. 62%).
8.10.6. Training in dementia care navigation is lacking and not standardized
Three in 4 health care workers providing navigation services indicated that they received no formal training in dementia care navigation. The 1 in 4 health care workers who did receive some kind of training were predominantly nonmedical professionals and received a median of 30 hours of formal training. On the other hand, medical professionals received a median of 20 hours of formal training.
If they received formal training, more than 1 in 2 surveyed received it from their employer (59%), not from colleges, universities or other outside programs, such as a certificate or public health program. Except for nonmedical professionals being more likely than medical professionals to have exposure to navigation training during college or university coursework (47% vs. 23%), there were no differences between the groups surveyed.
8.10.7. Dementia care is rarely the sole focus of navigation activities, but health care workers still feel knowledgeable
Four in 5 health care workers (80%) have navigation experience in non‐dementia medical specialties, and fewer than 1 in 10 (7%) focus primarily on providing navigator support and services to people living with dementia. Most providing navigation services (93%) feel at least somewhat knowledgeable about MCI, Alzheimer's disease and other dementia but only about 1 in 3 (36%) report they are very knowledgeable.
Central to effective, valuable navigation that benefits patients and families is a strong knowledge of dementia care support and resources in health care settings and the community. Nearly 9 in 10 health care workers (86%) feel knowledgeable about directing dementia patients and caregivers to appropriate health care resources, but fewer than 1 in 3 (30%) report feeling very knowledgeable. Four in 5 (82%) feel knowledgeable about directing dementia patients and caregivers to community resources, but only 31% say they are very knowledgeable.
When the group is separated into medical or nonmedical professionals, nonmedical professionals report feeling better equipped and more knowledgeable than medical professionals about health care resources (93% vs. 81%) and community‐based resources (92% vs. 75%). Understandably, nonmedical professionals feel more capable, given that they are more likely to communicate regularly with patients and caregivers and could have received formal training on delivering navigation services. Additionally, some health care workers categorized as nonmedical professionals in this survey, such as community health workers or home health aides, may have direct exposure or interaction with resources in the community.
A crucial component of dementia care today is familiarity with new and emerging treatment options and awareness of clinical trials. Nearly all health care workers surveyed believe it is important to be familiar with new treatments (98%) and with clinical trial options (93%).
8.10.8. The health care system could do more to help people navigate dementia care
Health care workers shed light on current deficits in dementia care. Sixty percent believe that the U.S. health care system is not effectively helping patients and their families navigate dementia care (Figure 32). They perceive their own organization's efforts more positively, however, with 4 in 5 saying that their organization is effective (somewhat effective [61%] or very effective [21%]) at providing dementia care; Figure 32). Nearly half surveyed (46%) say their organizations do not have a clearly defined process for care coordination and clinical pathways for patients with MCI, Alzheimer's disease or other dementia.
FIGURE 32.
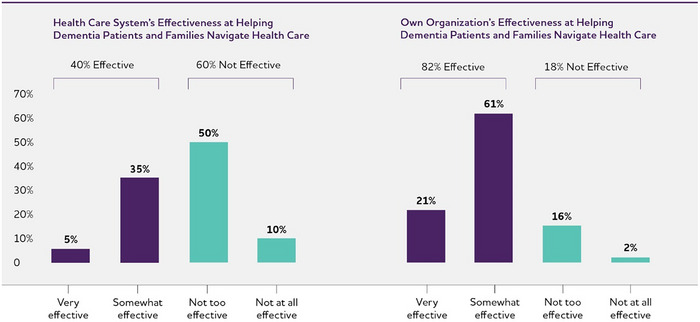
Health care workers’ views on the effectiveness of dementia care navigation.
Health care workers pointed to a lack of community‐based resources for dementia caregivers and current payment models that do not incentivize care coordination as the greatest barriers to dementia care navigation (Figure 33). More than 3 in 4 of the health care workers surveyed (77%) identified a lack of community‐based resources as a barrier, and 44% viewed it as the greatest barrier. Seven in 10 (70%) called out restrictions in current reimbursement as a barrier, with 41% saying this was the greatest barrier. Interestingly, health care workers did not identify workforce shortages as a top limitation for dementia care navigation (Figure 33). There were no differences in perceived barriers to dementia care navigation between medical and nonmedical professionals.
FIGURE 33.
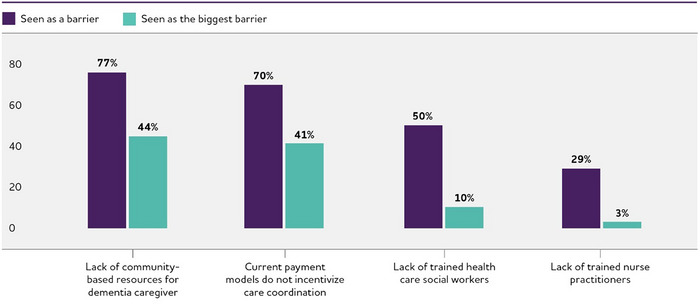
Barriers to dementia care navigation.
Almost 9 in 10 health care workers (87%) feel that developing alternative payment models is important in providing future care coordination for people diagnosed with dementia.
8.10.9. Nonmedical professionals are best suited to be dementia care navigators
Survey respondents overwhelmingly agreed that nonmedical professionals are best suited to provide navigation services (Figure 34). Within their organizations, those surveyed reported that social workers, community health workers, and home health aides are more often formally involved in care navigation, and 9 in 10 reported that these individuals are best suited to offer navigation services. The next group of individuals health care workers believed are suited for navigation roles are former caregivers or others who have lived the caregiving experience, but few reported that these individuals are formally involved in dementia care navigation at their organizations. Surprisingly, nearly 2 in 3 health care workers indicated that physicians at their organization are involved in helping patients and caregivers navigate health care but fewer than half of the health care workers surveyed think that physicians are best suited for this work.
FIGURE 34.
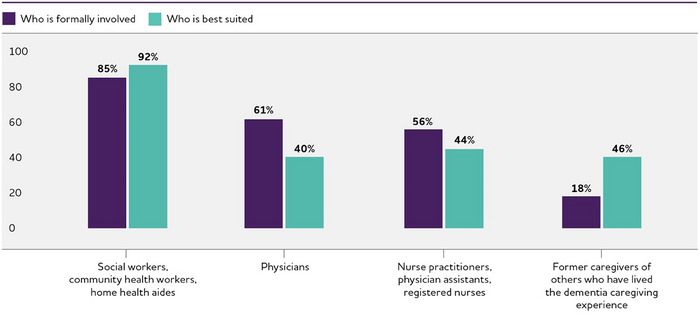
Professionals who help dementia patients and caregivers navigate health care.
8.11. A path forward: Revolutionizing dementia care with person‐centered navigation
As the complexity of health care for Alzheimer's and other dementias continues to challenge individuals living with dementia and caregivers alike, the Alzheimer's Association dementia caregiver and non‐physician health care workforce surveys call attention to the urgent need for person‐centered dementia care navigation and care delivery solutions for health systems, health care professionals, caregivers and people living with dementia.
Dementia care management is emerging as an ideal model to unravel dementia care complexity, improve outcomes and lower costs (Figure 35). 974 Care navigation is a crucial component that touches all other aspects of care management, such as caregiver education and training, care coordination, medication management, management of chronic conditions, safety assessments, and advance care planning. 974 Dementia care navigation, as part of comprehensive dementia care management, has the potential to revolutionize care if it is:
Person‐centered to meet the evolving, unique needs of all individuals living with Alzheimer's or other dementia.
Durable yet adaptable to accommodate new treatments, new diagnostics and other improvements to care.
Comprehensive to cover medical and nonmedical needs.
Coordinated to connect disparate care teams and community resources.
Feasible regardless of health system structure.
Cognizant of geographic and socioeconomic barriers.
FIGURE 35.
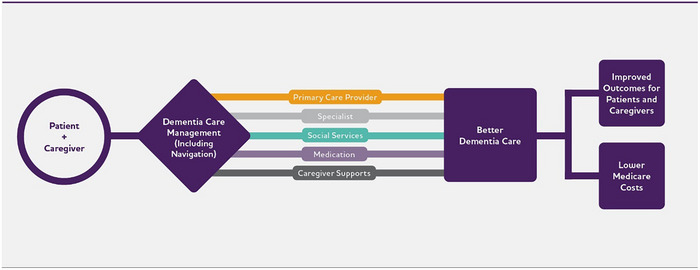
The ideal future state of dementia care. 974
Ultimately, the goal of care navigation is to improve the quality of life for people living with dementia, reduce caregiver stress and enable people living with dementia to live in their homes and communities as long as possible.
The GUIDE Model is a reason for optimism that emphasizes streamlined care coordination and robust support for caregivers — including forging a vital connection with a dedicated care navigator — and creating an alternative payment model to reimburse physicians (see What is GUIDE?). 982 However, while the GUIDE Model offers one potential approach toward enhancing dementia care navigation, it is a limited pilot program that will not be delivered by all health care providers nor available to all patients with dementia. Therefore, it is important for health systems, private insurers and other stakeholders to develop their own strategies to help people living with dementia and their caregivers navigate care.
The Special Report survey results brought to light three themes to advance dementia care navigation efforts:
Formalizing the dementia care navigator role and increasing navigator proficiency in dementia care.
Scaling and expanding access to dementia care navigation programs.
Creating direct lines to dementia care navigators.
8.11.1. Formalize the dementia care navigator role
Survey responses revealed a noteworthy trend: many health care workers are undertaking navigator duties in addition to their usual responsibilities. Ideally, practices and health systems should transition from ad hoc navigation support to formalizing dedicated dementia care navigator roles that are recognized for their vital and essential contributions to interdisciplinary, person‐centered dementia care.
As mentioned in the Workforce section, several decades of research support the value of collaborative models that bring different health professionals together, such as social workers, registered nurses and non‐clinical care managers, physicians and advanced practice providers, and direct care workers, including nurse aides, nursing assistants, home health aides and personal care aides. 750 , 751 If these individuals are the spokes in the dementia care wheel, the care navigator is the hub — supporting patients and caregivers as they find their way through the dementia care ecosystem. Given that this is a relatively new role in the dementia workforce, it's crucial for organizations to create a practice framework to seamlessly integrate dementia care navigators into existing teams and workflows to effectively coordinate longitudinal care that spans the disease course.
8.11.2. Increase dementia proficiency to cultivate specialist navigators
In identifying ideal candidates for navigator roles, medical and nonmedical professionals agreed that community health workers, social workers and home health aides are best suited to be dementia care navigators. Despite receiving some formal education in navigation, often as part of college coursework or employer‐provided training, these professionals are typically trained as generalists. Supplemental training and resources are necessary to build a solid foundation in both the practical and emotional aspects of dementia care. Their skills and compassion for the challenges caregivers and individuals living with dementia face could be enhanced with supplemental dementia‐specific training and resources, such as:
Materials for health care workers from the Health Resources & Services Administration. 992
Professional development programs in dementia care recognized by the Alzheimer's Association. 993
The Care Ecosystem Toolkit from the University of California, San Francisco (UCSF) Memory and Aging Center. 994
Home health clinician manuals from the Wisconsin Alzheimer's Institute. 995
Caregiver training videos from UCLA Health. 996
Resources, webinars, presentations and toolkits from the National Alzheimer's and Dementia Resource Center. 997
Community health worker training for participants in programs funded by the Administration for Community Living‐Alzheimer's Disease Program Initiative (ACL‐ADPI). 978
Resources and webinars on brain health and dementia from the National Association of Community Health Centers. 998
To bolster the training and resources outlined here, the Alzheimer's Association is developing a person‐centered navigator training curriculum and certification that is slated for release in late 2024. This curriculum, which incorporates the Alzheimer's Association's evidence‐based Dementia Care Practice Recommendations, has the potential to increase the proficiency of care navigators, ensuring that they are well‐equipped to meet the distinct needs of individuals living with dementia and their caregivers. Investment in navigator training and development could yield a marked improvement in the overall quality and effectiveness of dementia care.
8.11.3. Incentivize scalability of dementia care navigation to expand reach
Fee‐for‐service payment has dominated the health care market. Under these structures, health care providers are paid for individual services they perform, such as office visits or tests. 999 Experts have long argued that fee‐for‐service is inefficient because it encourages the delivery of more potentially unnecessary care while discouraging care coordination. 1000 The U.S. health care system is increasingly transitioning from fee‐for‐service structures to alternative payment models, including value‐based payment. 1000 Often called “volume to value,” the goal of value‐based payments is to restructure the approach “from one that incentivizes volume to one that rewards value.” 1001
Dementia care is not immune from financial incentives. 980 , 1002 , 1003 Health care workers in this year's survey believed that current reimbursement systems fail to incentivize dementia care and are one of the greatest barriers to dementia care navigation. They strongly believe that alternative payment models are important in providing future care coordination for people diagnosed with dementia.
8.11.4. Work to make existing and future dementia care navigation programs visible and accessible
According to this year's caregiver survey, awareness of dementia care navigators remains low despite the recognized value of navigation. This may, in part, be due to variations in terminology used by different health care providers, inconsistent definitions or that these individuals simply do not yet exist within organizations. Depending on the setting, what is defined as dementia care navigation in this Special Report may also be called memory care navigation, care navigation, a navigator program, dementia navigation or not have terminology at all, but simply be services provided to patients. 986 , 988
Future programs must focus on elevating the visibility of navigation services through targeted outreach efforts. This involves leveraging community resources, social media and health care settings to inform and educate dementia caregivers about the support available to them. A compendium that defines terms and lists programs by region could be a useful tool to empower individuals living with Alzheimer's or other dementia and their caregivers. Furthermore, integrating care navigation into primary care and specialty clinics can ensure that more patients and families benefit from these services from the onset of their dementia care journey.
Another key factor limiting access to existing dementia care navigation programs is their location. Existing dementia care navigation programs are typically housed within large health systems or academic medical centers, putting them out of reach for many individuals from rural and underrepresented communities who receive care from hospitals or clinics within their community. 988 In anticipation of the growing need for dementia care navigation programming, the Alzheimer's Association created the Dementia Care Navigation Roundtable, which will help organizations establish best practices, support implementation and increase access to navigation programs.
8.11.5. Leverage 24/7 helplines and technology to create direct lines to care navigators
Dementia caregivers reported that the most valuable service that care navigation could offer would be a 24/7 helpline. The Alzheimer's Association currently offers a 24/7 helpline that performs some navigation activities, such as assisting individuals with Alzheimer's or other dementia and their caregivers with recommendations for finding qualified care providers, general information about legal, financial and care decisions, and referrals to local programs and services. 1004 Ideally, access to 24/7 assistance would be connected directly with an individual's interdisciplinary care team. This allows the care team to manage longitudinal care, proactively assess any changes needed in care and potentially mitigate unnecessary emergencies.
While dementia caregivers and health care workers acting as navigators still prefer traditional communication methods such as phone calls and in‐person visits for everyday communication, there is an opportunity to integrate technology solutions to streamline care coordination and support; these solutions should be viewed as complementary to existing person‐centered approaches rather than replacements. Several companies are exploring on‐demand virtual and app‐based dementia care navigation, and the GUIDE Model supports contracting with suppliers to meet care delivery requirements that participants in the model wouldn't otherwise be able to meet on their own. 982 Digital platforms can offer caregivers and patients easier access to resources, appointment scheduling and direct communication with care navigators. However, any technological solution must be user‐friendly and accessible to all caregivers, regardless of their familiarity with digital tools, and compatible with any platforms used by health care providers. Navigators should be trained on how to communicate effectively through different channels.
9. CONCLUSION
The path forward for person‐centered dementia care navigation is illuminated by the insights and experiences of dementia caregivers and health care professionals. The first step on this path is to establish proficient, dedicated dementia care navigators as a new role in the interdisciplinary dementia care workforce. Then, by addressing these key areas — training, person‐centered care, accessibility, collaboration, novel payment models, and technology — future dementia care navigation programs can build on the learnings of their predecessors. Such efforts strive to improve the quality of life for individuals living with dementia and their caregivers and pave the way for a more sustainable, efficient and compassionate health care system.
ACKNOWLEDGMENTS
The Alzheimer's Association acknowledges the contributions of Joseph Gaugler, Ph.D., Bryan James, Ph.D., Tricia Johnson, Ph.D., Jessica Reimer, Ph.D., Kezia Scales, Ph.D., Sarah Tom, Ph.D., M.P.H., Jennifer Weuve, M.P.H., Sc.D., and Jarmin Yeh, Ph.D., M.P.H., M.S.S.W., in the preparation of 2024 Alzheimer's Disease Facts and Figures.
ENDNOTES
Racial and ethnic identifiers: Facts and Figures keeps the racial and ethnic terms used in source documents when describing study findings. When not referring to data from specific studies, adjectives such as “Black,” “Hispanic” and “White” may be used (for example, Black populations and Hispanic communities).
Estimated prevalence (number and proportion) of Americans age 65 and older with Alzheimer's dementia for 2024: The estimated 6.9 million individuals ages 65 years and older with Alzheimer's dementia and the estimated numbers of individuals with Alzheimer's in each age group were reported from a study that used data from the Chicago Health and Aging Project (CHAP) in combination with population projections from the U.S. Census. 241 The number, 6.9 million, is higher than estimated from previous study that also combined CHAP and U.S. Census data. This is because the more recent study used updated Census projections and incorporated information from Hispanic/Latino American individuals. The proportion of the population with Alzheimer's dementia (among people age 65 and older and by age group) is calculated using as the numerators the numbers of people with Alzheimer's dementia, as reported by the recent study in CHAP. 241 The denominators were the U.S. Census population projections for the specific age groups of interest.
Differences between CHAP and HRS‐HCAP estimates for Alzheimer's dementia prevalence: The number of people estimated to have any form of dementia in the U.S. in 2016 from the Health and Retirement Study's (HRS) Harmonized Cognitive Assessment Protocol (HCAP; 4.92 million) is lower than the CHAP estimate of how many people were living with Alzheimer's dementia only (6.07 million). 160 This is because of differences in dementia ascertainment between the two studies: both studies used scores on batteries of cognitive tests, but the HRS‐HCAP study additionally required an informant report of functional impairment (i.e. disability). Because the more stringent threshold for dementia in HRS‐HCAP may miss people with mild Alzheimer's dementia, the Association believes that the larger CHAP estimates may be a more relevant estimate of the burden of Alzheimer's dementia in the United States.
Criteria for identifying people with Alzheimer's or other dementias in the Framingham Heart Study: From 1975 to 2009, 7,901 people from the Framingham Study who had survived free of dementia to at least age 45, and 5,937 who had survived free of dementia until at least age 65 were followed for incidence of dementia. 286 Diagnosis of dementia was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria and required that the participant survive for at least 6 months after onset of symptoms. Standard diagnostic criteria (the NINCDS‐ADRDA criteria from 1984) were used to diagnose Alzheimer's dementia. The definition of Alzheimer's and other dementias used in the Framingham Study was very strict; if a definition that included milder disease and disease of less than six months’ duration were used, lifetime risks of Alzheimer's and other dementias would be higher than those estimated by this study.
Projected number of people with Alzheimer's dementia, 2020‐2060: This figure comes from the CHAP study. 241 Other projections are somewhat lower (see, for example, Brookmeyer et al. 1005 ) because they relied on more conservative methods for counting people who currently have Alzheimer's dementia.A3 Nonetheless, these estimates are statistically consistent with each other, and all projections suggest substantial growth in the number of people with Alzheimer's dementia over the coming decades.
Annual mortality rate due to Alzheimer's disease by state: Unadjusted death rates are presented rather than age‐adjusted death rates in order to provide a clearer depiction of the burden of mortality for each state. States such as Florida with larger populations of older people will have a larger burden of mortality due to Alzheimer's — a burden that appears smaller relative to other states when the rates are adjusted for age.
Number of family and other unpaid caregivers of people with Alzheimer's or other dementias: To calculate this number, the Alzheimer's Association started with data from the Behavioral Risk Factor Surveillance System (BRFSS) survey. Since 2016, all states and the District of Columbia utilized the BRFSS caregiver module. This module identified respondents age 18 and over who had provided any regular care or assistance during the past month to a family member or friend who had a health problem, long‐term illness or disability. The module asks a series of follow‐up questions, including asking the caregiver to identify what the main health problem, long‐term illness, or disability that the person they care for has. One of the reported condition categories is “Alzheimer's disease, dementia, or other cognitive impairment.” In the BRFSS surveys conducted in 2019 and after, an additional follow‐up question was included, asking if the caregiving recipient also had dementia in addition to their main condition. Prior to 2019, the survey did not include caregivers of recipients for whom dementia was not their main condition, so these numbers were imputed using data collected in 2019 by the National Alliance for Caregiving (NAC)/AARP survey. The NAC/AARP survey asked respondents age 18 and over whether they were providing unpaid care for a relative or friend age 18 or older or had provided such care during the past 12 months. Respondents who answered affirmatively were then asked about the health problems of the person for whom they provided care: 11% of respondents reported dementia as the main condition of their care recipient, while 26% of all respondents reported the presence of dementia. Using this ratio in combination with BRFSS data, the Alzheimer's Association was able to determine the percentage of adults in all states and the District of Columbia who are caregivers for individuals living with Alzheimer's or another dementia. These percentages were applied to the estimated number of people age 18 and older in each state in July 2023, using U.S. Census Bureau data available at: https://www.census. gov/programs‐surveys/popest/data/tables.html. This resulted in a total of 11.457 million Alzheimer's and dementia caregivers across all 50 states and the District of Columbia.
Number of hours of unpaid carey: The BRFSS survey asks caregivers to identify, within five time frames, the number of hours they provide care in an average week. Using the method developed by Rabarison and colleagues, 440 the Alzheimer's Association assumed the midpoint of each time frame was the average number of hours for each caregiver within that time frame and then calculated the overall average number of hours of weekly care provided by dementia caregivers in each state. This number was then converted to a yearly average and multiplied by the number of caregivers in each stateA7 to determine the total number of hours of care provided. When added together, across all 50 states and the District of Columbia, the total number of hours provided by Alzheimer's and dementia caregivers is 18.376 billion hours.
Value of unpaid caregiving: For each state, the hourly value of care was determined as the average of the state minimum hourly wage 1006 and the most recently available state median hourly cost of a home health aide. (For Nevada, the minimum wage used was the average of the minimum wage for those who are not provided health insurance and the minimum wage for those who are provided health insurance.) 904 The average for each state was then multiplied by the total number of hours of unpaid care in that stateA8 to derive the total value of unpaid care. Adding the totals from all states and the District of Columbia resulted in an economic value of $346.585 billion for dementia caregiving in the United States in 2023.
The 2014 Alzheimer's Association Women and Alzheimer's Poll: This poll questioned a nationally representative sample of 3,102 American adults about their attitudes, knowledge and experiences related to Alzheimer's and dementia from Jan. 9, 2014, to Jan. 29, 2014. An additional 512 respondents who provided unpaid help to a relative or friend with Alzheimer's or a related dementia were asked questions about their care provision. Random selections of telephone numbers from landline and cell phone exchanges throughout the United States were conducted. One individual per household was selected from the landline sample, and cell phone respondents were selected if they were 18 years old or older. Interviews were administered in English and Spanish. The poll “oversampled” Hispanics/Latinos, selected from U.S. Census tracts with higher than an 8% concentration of this group. A list sample of Asian Americans was also utilized to oversample this group. A general population weight was used to adjust for number of adults in the household and telephone usage; the second stage of this weight balanced the sample to estimated U.S. population characteristics. A weight for the caregiver sample accounted for the increased likelihood of female and White respondents in the caregiver sample. Sampling weights were also created to account for the use of two supplemental list samples. The resulting interviews comprise a probability‐based, nationally representative sample of U.S. adults. A caregiver was defined as an adult over age 18 who, in the past 12 months, provided unpaid care to a relative or friend age 50 or older with Alzheimer's or another dementia. Questionnaire design and interviewing were conducted by Abt SRBI of New York.
Lewin Model on Alzheimer's and dementia costs: These numbers come from a model created for the Alzheimer's Association by the Lewin Group. The model estimates total payments for health care, long‐term care and hospice — as well as state‐by‐state Medicaid spending — for people with Alzheimer's and other dementias. The model was updated by the Lewin Group in January 2015 (updating previous model) and June 2015 (addition of state‐by‐state Medicaid estimates). Detailed information on the model, its long‐term projections and its methodology are available at: alz.org/trajectory. For the purposes of the data presented in this report, the following parameters of the model were changed relative to the methodology outlined at alz.org/trajectory: (1) cost data from the 2018 Medicare Current Beneficiary Survey (MCBS) were used rather than data from the 2008 MCBS; (2) prevalence among older adults was assumed to equal the prevalence levels from Rajan and colleagues 241 and included in this report (6.9 million in 2024), rather than the prevalence estimates derived by the model itself; (3) estimates of inflation and excess cost growth reflect the most recent relevant estimates from the cited sources (Centers for Medicare & Medicaid Services [CMS] actuaries and the Congressional Budget Office); and (4) 2014 state‐by‐state data from CMS on the number of nursing home residents and percentage with moderate and severe cognitive impairment were used in lieu of 2012 data. The Lewin Model's state‐specific Medicaid costs for 2020 and 2025 are based on an earlier estimate of state prevalence than reported here (Weuve J, Hebert LE, Scherr PA, Evans DA. Prevalence of Alzheimer disease in U.S. states. Epidemiology 2015;26(1):E4‐6).
All cost estimates were inflated to year 2023 dollars using the Consumer Price Index (CPI): All cost estimates were inflated using the seasonally adjusted average prices for medical care services from all urban consumers. The relevant item within medical care services was used for each cost element. For example, the medical care item within the CPI was used to inflate total health care payments; the hospital services item within the CPI was used to inflate hospital payments; and the nursing home and adult day services item within the CPI was used to inflate nursing home payments.
Average annual per‐person payments for health care and long‐term care services for Medicare beneficiaries age 65 and older with and without Alzheimer's or other dementiasy: Payments are unadjusted, and therefore, do not account for differences in patient characteristics, such as age or sex. Additionally, payments are based on health care utilization and payments in 2018, prior to the COVID‐19 pandemic, and do not reflect any pandemic‐related changes in utilization.
Enrollment in fee‐for‐service Medicare versus Medicare Part C: Individuals eligible for Medicare can enroll in traditional Medicare, also referred to as fee‐for‐service Medicare and original Medicare, or Medicare Advantage, also referred to as Medicare Part C. 1007 With traditional Medicare, beneficiaries can receive care from any doctor or hospital that accepts Medicare in the United States. Generally, beneficiaries can seek care from a specialist without a referral. Traditional Medicare has fixed cost sharing, which includes coinsurance of 20% of the Medicare‐approved amount for services covered by Part B after the deductible is met. Individuals enrolled in traditional Medicare can also enroll in Medicare Supplemental Insurance (also referred to as Medigap) to help cover the out‐of‐pocket costs. Traditional Medicare does not have an annual limit on the amount beneficiaries pay out‐of‐pocket. Benefits are the same for all individuals enrolled in traditional Medicare. Individuals enrolled in traditional Medicare can also enroll in a Medicare Part D plan to cover some of the costs of prescription drugs. Medicare Part D enrollment has a separate premium. With Medicare Advantage, individuals must enroll in a specific private plan. Premiums, benefits and out‐of‐pocket costs may vary across plans. Medicare Advantage plans have an annual limit on the amount individuals pay out‐of‐pocket. Individuals enrolled in a Medicare Advantage plan are not allowed to enroll in Medigap. Medicare Advantage plans are also allowed to offer additional benefits not included in traditional Medicare, such as vision, hearing and dental services as well as some non‐health care benefits, such as transportation costs and gym memberships. Many Medicare Advantage plans include prescription drug coverage (Medicare Part D). Individuals enrolled in a Medicare Advantage plan have a specific network of doctors and hospitals that enrollees need to use for services to be paid by the Medicare Advantage plan. Additionally, individuals enrolled in a Medicare Advantage plan may need a referral to see a specialist. Enrollment in Medicare Advantage has increased dramatically over the past decade, with 51% of all Medicare beneficiaries enrolled in a Medicare Advantage plan in 2023 compared to 29% in 2013. 914
Medicare Current Beneficiary Survey Report: These data come from an analysis of findings from the 2018 Medicare Current Beneficiary Survey (MCBS). The analysis was conducted for the Alzheimer's Association by Health Care Cost Institute. 859 The MCBS, a continuous survey of a nationally representative sample of about 15,000 Medicare beneficiaries, is linked to Medicare claims. The survey is supported by the U.S. Centers for Medicare & Medicaid Services (CMS). For community‐dwelling survey participants, MCBS interviews are conducted in person three times a year with the Medicare beneficiary or a proxy respondent if the beneficiary is not able to respond. For survey participants who are living in a nursing home or another residential care setting, such as an assisted living residence, retirement home or a long‐term care unit in a hospital or mental health facility, MCBS interviews are conducted with a staff member designated by the facility administrator as the most appropriate to answer the questions. Data from the MCBS analysis that are included in 2024 Alzheimer's Disease Facts and Figures pertain only to Medicare beneficiaries age 65 and older.
- Community‐dwelling survey participants who answered yes to the MCBS question, “Has a doctor ever told you that you had Alzheimer's disease or dementia?” Proxy responses to this question were accepted.
- Survey participants who were living in a nursing home or other residential care setting and had a diagnosis of Alzheimer's disease or dementia in their medical record
- Survey participants who had at least one Medicare claim with a diagnostic code for Alzheimer's or other dementias in 2008. The claim could be for any Medicare service, including hospital, skilled nursing facility, outpatient medical care, home health care, hospice or physician, or other health care provider visit. The diagnostic codes used to identify survey participants with Alzheimer's or other dementias are 331.0, 331.1, 331.11, 331.19, 331.2, 331.7, 331.82, 290.0, 290.1, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 291.2, 294.0, 294.1, 294.10 and 294.11.
Costs from the MCBS analysis are based on responses from 2018 and reported in 2023 dollars.
Differences in estimated costs reported by Hurd and colleagues: Hurd and colleagues 858 estimated per‐person costs using data from participants in ADAMS, a cohort in which all individuals underwent diagnostic assessments for dementia. One reason that the per‐person costs estimated by Hurd and colleagues are lower than those reported in Facts and Figures is that ADAMS, with its diagnostic evaluations of everyone in the study, is more likely than MCBS to have identified individuals with less severe or undiagnosed Alzheimer's. By contrast, the individuals with Alzheimer's registered by MCBS are likely to be those with more severe, and therefore more costly, illness. A second reason is that the Hurd et al. estimated costs reflect an effort to isolate the incremental costs associated with Alzheimer's and other dementias (those costs attributed only to dementia), while the per‐person costs in 2024 Alzheimer's Disease Facts and Figures incorporate all costs of caring for people with the disease (regardless of whether the expenditure was related to dementia or a coexisting condition).
- Spend at least 20% of professional time interacting directly with patients or caregivers.
- Report at least 10% of their patients being age 60 or older.
- Spend at least 10% of professional time performing navigation‐type services.
- Report at least 10% of patients for whom they perform these services having Alzheimer's disease, other dementia or mild cognitive impairment (MCI).
Dementia care navigation was defined as the following for this group:
- Patient education around diagnosis, treatment options and resources.
- Patient referrals to clinical specialists.
- Patient referrals to clinical trials.
- Patient referrals to social workers.
- Patient referrals to community‐based services.
- Patient scheduling (labs, care team appointments, etc.)
- Patient assistance with insurance.
REFERENCES
- 1. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid ß deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. [DOI] [PubMed] [Google Scholar]
- 2. Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: A case‐control study. Lancet Neurol. 2012;11(2):1048‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Lowe VJ, Weigand SD, et al. Serial PiB and MRI in normal, mild cognitive impairment and Alzheimer's disease: Implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: A longitudinal study. Lancet Neurol. 2018;17(3):241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960‐969. [DOI] [PubMed] [Google Scholar]
- 7. Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: A cross‐sectional and longitudinal cohort study. Lancet Neuro. 2020;19(6):513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barthelemy N, Joseph‐Mathurin N, Gordon BA, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer's disease. Nat Med. 2020;26:398‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Byard RW, Langlois NEI. Wandering dementia: A syndrome with forensic implications. J Forensic Sci. 2019;64(2):443‐445. [DOI] [PubMed] [Google Scholar]
- 10. Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer's disease in an older population: Updated incidence and life expectancy with and without dementia. Am J Public Health. 2015;105(2):408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: A 15‐year epidemiological study. Arch Neurol. 2005;62(5):779‐784. [DOI] [PubMed] [Google Scholar]
- 12. Waring SC, Doody RS, Pavlik VN, Massman PJ, Chan W. Survival among patients with dementia from a large multi‐ethnic population. Alzheimer Dis Assoc Disord. 2005;19(4):178‐183. [DOI] [PubMed] [Google Scholar]
- 13. Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Arch Neurol. 2002;59(11):1764‐1767. [DOI] [PubMed] [Google Scholar]
- 14. Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140(7):501‐509. [DOI] [PubMed] [Google Scholar]
- 15. Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: A multiethnic, population‐based study of incident cases. Neurology. 2008;71(19):1489‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie J, Brayne C, Matthews FE. Survival times in people with dementia: Analysis from a population based cohort study with 14‐year follow‐up. BMJ. 2008;336(7638):258‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brodaty H, Seeher K, Gibson L. Dementia time to death: A systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr. 2012;24(7):1034‐1045. [DOI] [PubMed] [Google Scholar]
- 18. Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: A review. Int J Geriatr Psychiatry. 2013;28(11):1109‐1124. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . Dementia: Key facts. Accessed October 10, 2023. Available at: https://www.who.int/news‐room/fact‐sheets/detail/dementia
- 20. Smith AD, Smith SM, de Jager, CA , et al. Homocysteine‐lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. Plos One. 2010;5(9):e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement. 2017;13(6):654‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute on Aging . What are frontotemporal disorders? Accessed December 16, 2023. Available at: https://www.nia.nih.gov/health/what‐are‐frontotemporal‐disorders
- 24. Hogan DB, Jette N, Fiest KM, et al. The prevalence and incidence of frontotemporal dementia: A systematic review. Can J Neurol Sci. 2016;43(suppl):S96‐109. [DOI] [PubMed] [Google Scholar]
- 25. Amador‐Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007;113(3):245‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kane JPM, Surendranathan A, Bentley A, Thomas AJ, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther. 2018. Feb 15;10(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Reuck J, Maurage CA, Deramecourt V, et al. Aging and cerebrovascular lesions in pure and in mixed neurodegenerative and vascular dementia brains: A neuropathological study. Folia Neuropathol. 2018;56(2):81‐87. [DOI] [PubMed] [Google Scholar]
- 28. James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012;307(17):1798‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stojkovska I, Krainc D, Mazzulli JR. Molecular mechanisms of α‐synuclein and GBA1 in Parkinson's disease. Cell Tissue Res. 2018;373(1):51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. 2005;20(10):1255. [DOI] [PubMed] [Google Scholar]
- 31. Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest‐old: The 90+ Study. Neurology. 2015;85(6):535‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: How to move forward? Neurology. 2009;72:368‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology. 2007;69:2197‐2204. [DOI] [PubMed] [Google Scholar]
- 34. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257(1‐2):80‐87. [DOI] [PubMed] [Google Scholar]
- 36. Jellinger KA. The enigma of mixed dementia. Alzheimers Dement. 2007;3(1):40‐53. [DOI] [PubMed] [Google Scholar]
- 37. Boyle PA, Lei Y, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person‐specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer's dementia attributed to age‐related neuropathologies. Ann Neurol. 2019;85(1):114‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest‐old: an autopsy study. Acta Neuropathol. 2010;119:421‐433. [DOI] [PubMed] [Google Scholar]
- 40. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jack CR, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vermunt L, Sikkes SAM, van den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15:888‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sperling RA, Donohue MC, Raman R, et al. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77(6):735‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: A systematic review and meta‐analysis. Lancet Neurol. 2016;15(7):673‐684. [DOI] [PubMed] [Google Scholar]
- 47. Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psych Res: Neuroimaging. 2007;155:147‐154. [DOI] [PubMed] [Google Scholar]
- 48. Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community‐based studies. Neurology. 2006;66:1837‐1844. [DOI] [PubMed] [Google Scholar]
- 49. Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087‐1095. [DOI] [PubMed] [Google Scholar]
- 50. Grontvedt GR, Schroder TN, Sando SB, White L, Brathen G, Doeller CF. Alzheimer's disease. Curr Bio. 2018;28:PR645‐PR649. [DOI] [PubMed] [Google Scholar]
- 51. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment. Neurology. 2018;90(3):126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer's disease to Alzheimer's dementia: A systematic review of the literature. Dement Geriatr Cogn Disord Extra. 2013;3(1):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Canevelli M, Grande G, Lacorte E, et al. Spontaneous reversion of mild cognitive impairment to normal cognition: A systematic review of literature and meta‐analysis. J Am Med Dir Assoc. 2016;17(10):943‐948. [DOI] [PubMed] [Google Scholar]
- 54. Social Security Administration . Minimizing the risk of scams for people living with dementia. Accessed October 11, 2023. Available at: https://blog.ssa.gov/minimizing‐the‐risk‐of‐scams‐for‐people‐living‐with‐dementia/#:~:text=People%20living%20with%20dementia%20are,struggle%20to%20make%20financial%20decisions
- 55. Memory and Aging Center, UCSF Weill Institute for Neurosciences . Executive functions. Accessed October 11, 2023. Available at: https://memory.ucsf.edu/symptoms/executive‐functions
- 56. Heerema E. How executive functioning Is affected by dementia. Verywell Health. Accessed October 30, 2023. Available at: https://www.verywellhealth.com/executive‐functioning‐alzheimers‐98596
- 57. Beshir SA, Aadithsoorya AM, Parveen A, Goh SSL, Hussain N, Menon VB. Aducanumab therapy to treat Alzheimer's disease: A narrative review. Int J Alzheimers Dis. 2022;2022:9343514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. [DOI] [PubMed] [Google Scholar]
- 59. Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: Appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: Appropriate use recommendations. J Prev Alz Dis. 2021;4(8):398‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cummings J, Rabinovici GD, Atri A. et al. Aducanumab: Appropriate Use Recommendations Update. J Prev Alzheimers Dis. 2022;9:221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development timeline: 2023. Alzheimers Dement: TRCI 2023. doi: 10.1002/trc2.12385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lim MM, Gerstner JR, Holtzman DM. The sleep‐wake cycle and Alzheimer's disease: What do we know? Neurodegener Dis Manag. 2014;4(5):351‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lloret M‐A, Cervera‐Ferri A, Nepomuceno M, Monllor P, Esteve D, Lloret A. Is sleep disruption a cause or consequence of Alzheimer's disease? Reviewing its possible role as a biomarker. Int J Mol Sci. 2020;21:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rose KM, Fagin CM, Lorenz R. Sleep Disturbances in Dementia: What They Are and What To Do. J Gerontol Nurs. 2010;36(5):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van der Mussele S, Le Bastard N, et al. Agitation‐associated behavioral symptoms in mild cognitive impairment and Alzheimer's dementia. Aging Ment Health. 2015;19(3):247‐257. [DOI] [PubMed] [Google Scholar]
- 67. Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for aggressive and agitated behaviors in dementia. Ann Internal Med. 2019;171(9):633‐642. [DOI] [PubMed] [Google Scholar]
- 68. Ralph SJ, Espinet AJ. Increased all‐cause mortality by antipsychotic drugs: Updated review and meta‐analysis in dementia and general mental health care. J Alzheimers Dis Rep. 2018;2:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72:438‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hebert LE, Bienias JL, Aggarwal NT, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Institute on Aging . Accessed December 15, 2023. Available at: https://www.nia.nih.gov/health/what‐causes‐alzheimers‐disease
- 72. Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late‐onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467‐1472. [DOI] [PubMed] [Google Scholar]
- 73. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta‐analysis. JAMA. 1997;278:1349‐1356. [PubMed] [Google Scholar]
- 74. Green RC, Cupples LA, Go R, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329‐336. [DOI] [PubMed] [Google Scholar]
- 75. Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late‐onset Alzheimer's disease: A population‐based, case‐control study. Ann Neurol. 1993;33(3):258‐266. [DOI] [PubMed] [Google Scholar]
- 76. Mayeux R, Sano M, Chen J, Tatemichi T, Stern Y. Risk of dementia in first‐degree relatives of patients with Alzheimer's disease and related disorders. Arch Neurol. 1991;48(3):269‐273. [DOI] [PubMed] [Google Scholar]
- 77. Lautenschlager NT, Cupples LA, Rao VS, et al. Risk of dementia among relatives of Alzheimer's disease patients in the MIRAGE Study: What is in store for the oldest old? Neurology. 1996;46(3):641‐650. [DOI] [PubMed] [Google Scholar]
- 78. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010‐2050) estimated using the 2010 Census. Neurology. 2013;80(19):1778‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nelson PT, Head E, Schmitt FA, et al. Alzheimer's disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121:571‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bellenguez C, Küçükali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022;54:412‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loy CT, Schofield PR, Turner AM, Kwok JBJ. Genetics of dementia. Lancet. 2014;383:828‐840. [DOI] [PubMed] [Google Scholar]
- 82. Qian J, Wolters FJ, Beiser A, et al. APOE‐related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts. PLoS Med. 2017;14(3):e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Spinney L. Alzheimer's disease: The forgetting gene. Nature. 2014;510(7503):26‐28. [DOI] [PubMed] [Google Scholar]
- 84. Ward A, Crean S, Mercaldi CJ, et al. Prevalence of apolipoprotein e4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer's disease: A systematic review and meta‐analysis. Neuroepidemiology. 2012;38:1‐17. [DOI] [PubMed] [Google Scholar]
- 85. Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer's disease. N Engl J Med. 1998;338:506‐511. [DOI] [PubMed] [Google Scholar]
- 86. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: Relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185‐189. [DOI] [PubMed] [Google Scholar]
- 87. Tang M, Stern Y, Marder K, et al. The APOE‐e4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751‐755. [DOI] [PubMed] [Google Scholar]
- 88. Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hendrie HC, Murrell J, Baiyewu O, et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int Psychogeriatr. 2014;26(6):977‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reitz C, Jun G, Naj A, et al. Variants in the ATP‐binding cassette transporter (ABCA7), apolipoprotein E epsilon 4, and the risk of late‐onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25‐year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bakulski KM, Vadari HS, Faul JD, et al. Cumulative genetic risk and APOE «4 are independently associated with dementia status in a multiethnic, population‐based cohort. Neurol Genet. 2021;7:e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rajan KB, Barnes LL, Wilson RS, et al. Racial differences in the association between apolipoprotein E risk alleles and overall and total cardiovascular mortality over 18 years. JAGS. 2017;65:2425‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kataoka S, Robbins DC, Cowan LD, et al. Apolipoprotein E polymorphism in American Indians and its relation to plasma lipoproteins and diabetes. The Strong Heart Study. Arterioscler Thromb Vasc Biol. 1996;16:918‐925. [DOI] [PubMed] [Google Scholar]
- 95. Le Guen Y, Raulin AC, Logue MW, et al. Association of African ancestry‐specific APOE missense variant R145C with risk of Alzheimer disease. JAMA. 2023;329(7):551‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Granot‐Hershkovitz E, Tarraf W, Kurniansyah N, et al. APOE alleles’ association with cognitive function differs across Hispanic/Latino groups and genetic ancestry in the study of Latinos‐investigation of neurocognitive aging (HCHS/SOL). Alzheimer's Dement. 2021;17:466‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol. 2010;9(6):623‐633. [DOI] [PubMed] [Google Scholar]
- 98. National Down Syndrome Society . Alzheimer's Disease and Down Syndrome. Accessed December 15, 2023. Available at: https://www.ndss.org/resources/alzheimers/
- 99. Fortea J, Vilaplana E, Carmona‐Iragui M, et al. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: A cross‐sectional study. Lancet. 2020;395(10242):1988‐1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fortea J, Zaman SH, Hartley S, Rafii MS, Head E, Carmona‐Iragui M. Alzheimer's disease associated with Down syndrome: A genetic form of dementia. Lancet Neurol. 2021;20(11):930‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hithersay R, Startin CM, Hamburg S, et al. Association of dementia with mortality among adults with Down syndrome older than 35 years. JAMA Neurol. 2019;76(2):152‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bekris LM, Yu CE, Bird TD, Tsuang DW. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23(4):213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Goldman JS, Hahn SE, Bird T. Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13:597‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lopera F, Marino C, Chandrahas AS, et al. Resilience to autosomal dominant Alzheimer's disease in a Reelin‐COLBOS heterozygous man. Nat Med. 2023. May;29(5):1243‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Arboleda‐Velasquez JF, Lopera F, O'Hare M, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med. 2019;25(11):1680‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wolters FJ, van der Lee SJ, Koudstaal PJ, et al. Parental family history of dementia in relation to subclinical brain disease and dementia risk. Neurology. 2017;88:1642‐1649. [DOI] [PubMed] [Google Scholar]
- 107. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020; 396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rosenwohl‐Mack A, Yaffe K, Carrasco A, Hoffmann CM, Barnes DE. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 2022;79(6):584‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. World Health Organization . Risk reduction of cognitive decline and dementia: WHO guidelines. https://www.who.int/publications/i/item/risk‐reduction‐of‐cognitive‐decline‐and‐dementia [PubMed]
- 110. Institute of Medicine . Cognitive Aging: Progress in Understanding and Opportunity for Action. Washington, D.C.: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 111. James BD, Bennett DA. Causes and patterns of dementia: An update in the era of redefining Alzheimer's disease. Ann Rev Public Health. 2019;40:65‐84. [DOI] [PubMed] [Google Scholar]
- 112. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: A systematic review and meta‐analysis. Alzheimers Dement. 2018;14(11):1416‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Samieri C, Perier MC, Gaye B, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320(7):657‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rönnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40‐year follow‐up of a population‐based cohort. Dement Geriatr Cogn Disord. 2011;31(6):460‐466. [DOI] [PubMed] [Google Scholar]
- 116. Abell JG, Kivimäki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: Role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018;39(33):3119‐3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta‐analysis of prospective observational studies. Diabetes Investig. 2013;4(6):640‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer's disease: The confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35(1):152‐160. [DOI] [PubMed] [Google Scholar]
- 120. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Johnson AL, Nystrom NC, Piper ME, et al. Cigarette smoking status, cigarette exposure, and duration of abstinence predicting incident dementia and death: A multistate model approach. J Alzheimers Dis. 2021;80(3):1013‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ogino E, Manly JJ, Schupf N, Mayeux R, Gu Y. Current and past leisure time physical activity in relation to risk of Alzheimer's disease in older adults. Alzheimers Dement. 2019;15(12):1603‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Najar J, Ostling S, Gudmundsson P, et al. Cognitive and physical activity and dementia: A 44‐year longitudinal population study of women. Neurology. 2019;92(12):e1322‐e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Buchman AS, Yu L, Wilson RS, et al. Physical activity, common brain pathologies, and cognition in community‐dwelling older adults. Neurology. 2019;92(8):e811‐e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tan ZS, Spartano NL, Beiser AS, et al. Physical activity, brain volume, and dementia risk: The Framingham Study. J Gerontol A Biol Sci Med Sci. 2017;72:789‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stephen R, Hongistro K, Solomon A, Lonnroos E. Physical activity and Alzheimer's disease: A systematic review. J Gerontol A Biol Sci Med Sci. 2017;72(6):733‐739. [DOI] [PubMed] [Google Scholar]
- 128. Blondell SJ, Hammersley‐Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta‐analysis of longitudinal studies. BMC Public Health. 2014;14:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Guure CB, Ibrahim NA, Adam MB, Said SM. Impact of physical activity on cognitive decline, dementia, and its subtypes: Meta‐analysis of prospective studies. Biomed Res Int. 2017;2017:9016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jensen CS, Simonsen AH, Siersma V, et al. Patients with Alzheimer's disease who carry the APOE e4 allele benefit more from physical exercise. TRCI. 2019;5:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Felisatti F, Gonneaud J, Palix C, et al. Role of cardiovascular risk factors on the association between physical activity and brain integrity markers in older adults. Neurology. 2022;98(20):e2023‐e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Casaletto K, Ramos‐Miguel A, VandeBunte A, et al. Late‐life physical activity relates to brain tissue synaptic integrity markers in older adults. Alzheimers Dement. 2022;18(11):2023‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nguyen S, LaCroix AZ, Hayden KM, et al. Accelerometer‐measured physical activity and sitting with incident mild cognitive impairment or probable dementia among older women. Alzheimers Dement. 2023; doi: 10.1002/alz.12908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean‐style diet and effects on cognition in adults: A qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr. 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lourida I, Soni M, Thompson‐Coon J, et al. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology. 2013;24:479‐489. [DOI] [PubMed] [Google Scholar]
- 136. Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Van den Brink AC, Brouwer‐Broisma EM, Berendsen AAM van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean‐DASH Intervention for Neurodegenerative Delay (MIND) Diets are associated with less cognitive decline and a lower risk of Alzheimer's disease: A review. Adv Nutr. 2019;10:1040‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ballarini T, Melo van Lent D, Brunner J, et al. Mediterranean diet, Alzheimer disease biomarkers and brain atrophy in old age. Neurology. 2021;96(24):e2920‐e2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Agarwal P, Leurgans SE, Agrawal S, et al. Association of Mediterranean‐DASH Intervention for Neurodegenerative Delay and Mediterranean Diets With Alzheimer Disease Pathology. Neurology. 2023;100(22):e2259‐e2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Barnes LL, Dhana K, Liu X, et al. Trial of the MIND Diet for prevention of cognitive decline in older persons. N Engl J Med. 2023;389(7):602‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Grant WB, Steven M. Diet's role in modifying risk of Alzheimer's disease: History and present understanding. J Alzheimer's Dis. 2023;96(4):1353‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Martinez‐Gonzalez MA, Gea A, Ruiz‐Canela M. The Mediterranean diet and cardiovascular health: A critical review. Circulation Res. 2019;124:779‐798. [DOI] [PubMed] [Google Scholar]
- 144. Sanches Machado d'Almeida K, Spillere SR, Zuchinali P, Souza GC. Mediterranean diet and other dietary patterns in primary prevention of heart failure and changes in cardiac function markers: A systematic review. Nutrients. 2018;10:58. doi: 10.3390/nu10010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Walker ME, O'Donnell AA, Himali JJ, et al. Associations of the Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet with cardiac remodelling in the community: The Framingham Heart Study. Br J Nutr. 2021;126(12):1888‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Butler M, Nelson VA, Davila H, et al. Over‐the‐counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer‐type dementia. Ann Intern Med. 2018;168:52‐62. [DOI] [PubMed] [Google Scholar]
- 147. Kivimaki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: Analysis of individual‐level data from 1.3 million individuals. Alzheimers Dement. 2018;14:601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;17(14):1443‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Anstey KJ, Ashby‐Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late‐life dementia: Review and meta‐analysis. J Alzheimers Dis. 2017;56(1):215‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Marden JR, Tchetgen EJ, Kawachi I, Glymour MM. Contribution of Socioeconomic Status at 3 Life‐Course Periods to Late‐Life Memory Function and Decline: Early and Late Predictors of Dementia Risk. Am J Epidemiol. 2017;186(7):805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Caunca MR, Odden MC, Glymour MM, et al. Association of Racial Residential Segregation Throughout Young Adulthood and Cognitive Performance in Middle‐aged Participants in the CARDIA Study. JAMA Neurol. 2020;77(8):1000‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chen R, Williams DR, Nishimi K, Slopen N, Kubzansky LD, Weuve J. A life course approach to understanding stress exposures and cognitive function among middle‐aged and older adults. Soc Sci Med. 2022;314:115448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. George KM, Lutsey PL, Kucharska‐Newton A, Palta P, Heiss G, Osypuk T, Folsom AR. Life‐Course Individual and Neighborhood Socioeconomic Status and Risk of Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol. 2020;189(10):1134‐4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. George KM, Gilsanz P, Peterson RL, et al. Impact of Cardiovascular Risk Factors in Adolescence, Young Adulthood, and Midlife on Late‐Life Cognition: Study of Healthy Aging in African Americans. J Gerontol A Biol Sci Med Sci. 2021;76(9):1692‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195‐204. [DOI] [PubMed] [Google Scholar]
- 156. Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59(11):1737‐1746. [DOI] [PubMed] [Google Scholar]
- 157. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sando SB, Melquist S, Cannon A, et al. Risk‐reducing effect of education in Alzheimer's disease. Int J Geriatr Psychiatry. 2008;23(11):1156‐1162. [DOI] [PubMed] [Google Scholar]
- 159. Hendrie HC, Smith‐Gamble V, Lane KA, Purnell C, Clark DO, Gao S. The Association of early life factors and declining incidence rates of dementia in an elderly population of African Americans. J Gerontol B Psychol Sci Soc Sci. 2018;16(73, suppl 1):S82‐S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Manly JJ, Jones RN, Langa KM, et al. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79(12):1242‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rawlings AM, Sharrett AR, Mosley TH, Wong DF, Knopman DS, Gottesman RF. Cognitive reserve in midlife is not associated with amyloid‐β deposition in late‐life. J Alzheimers Dis. 2019:517‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA. Education and cognitive reserve in old age. Neurology. 2019;92(10):e1041‐e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448‐460. [PubMed] [Google Scholar]
- 164. Stern Y, Arenaza‐Urquijo EM, Bartres‐Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020;16(9):1305‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Grzywacz JG, Segel‐Karpas D, Lachman ME. Workplace exposures and cognitive function during adulthood: Evidence from National Survey of Midlife Development and the O*NET. J Occup Environ Med. 2016;58(6):535‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pool LR, Weuve J, Wilson RS, Bültmann U, Evans DA, Mendes de Leon CF. Occupational cognitive requirements and late‐life cognitive aging. Neurology. 2016;86(15):1386‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Then FS, Luck T, Luppa M, et al. Association between mental demands at work and cognitive functioning in the general population: Results of the health study of the Leipzig Research Center for Civilization Diseases. J Occup Med Toxicol. 2014;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Fisher GG, Stachowski A, Infurna FJ, Faul JD, Grosch J, Tetrick LE. Mental work demands, retirement, and longitudinal trajectories of cognitive functioning. J Occup Health Psychol. 2014;19(2):231‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Coleman ME, Roessler MEH, Peng S, et al. Social enrichment on the job: Complex work with people improves episodic memory, promotes brain reserve, and reduces the risk of dementia. Alzheimers Dement. 2023;19(6):2655‐2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Soh Y, Eng CW, Mayeda ER, et al. Association of primary lifetime occupational cognitive complexity and cognitive decline in a diverse cohort: Results from the KHANDLE study. Alzheimers Dement. 2023;19(9):3926‐3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. McDowell I, Xi G, Lindsay J, Tierney M. Mapping the connections between education and dementia. J Clin Exp Neuropsychol. 2007;29(2):127‐141. [DOI] [PubMed] [Google Scholar]
- 172. Harris CD, Watson KB, Carlson SA, Fulton JE, Dorn JM, Elam‐Evans L. Adult participation in aerobic and muscle‐strengthening physical activities — United States, 2011. Morb Mortal Wkly Rep. 2013;62(17):326‐330. [PMC free article] [PubMed] [Google Scholar]
- 173. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988‐2012. JAMA. 2015;314(10):1021‐1029. [DOI] [PubMed] [Google Scholar]
- 174. Sims M, Diez Roux AV, Boykin S, et al. The socioeconomic gradient of diabetes prevalence, awareness, treatment, and control among African Americans in the Jackson Heart Study. Ann Epidemiol. 2011;21(12):892‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lee TC, Glynn RJ, Peña JM, et al. Socioeconomic status and incident type 2 diabetes mellitus: Data from the Women's Health Study. PLoS One. 2011;6(12):E27670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gillespie CD, Hurvitz KA. Prevalence of hypertension and controlled hypertension — United States, 2007–2010. MMWR. 2013;62(3):144‐148. [PubMed] [Google Scholar]
- 177. Centers for Disease Control and Prevention . Current Cigarette Smoking Among Adults in the United States. Accessed December 15, 2023. Available at: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
- 178. Kweon H, Aydogan G, Dagher A, et al. Human brain anatomy reflects separable genetic and environmental components of socioeconomic status. Sci Adv. 2022;8(20):eabm2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Weuve J, Bennett EE, Ranker L, et al. Exposure to air pollution in relation to risk of dementia and related outcomes: An updated systematic review of the epidemiologic literature. Environ Health Perspect. 2021;129(9):96001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Bernard SM, McGeehin MA. Prevalence of blood lead levels >or= 5 micro g/dL among US children 1 to 5 years of age and socioeconomic and demographic factors associated with blood of lead levels 5 to 10 micro g/dL, Third National Health and Nutrition Examination Survey, 1988‐1994. Pediatrics. 2003;112(6 Pt 1):1308‐1313. [DOI] [PubMed] [Google Scholar]
- 181. Griffith M, Tajik M, Wing S. Patterns of agricultural pesticide use in relation to socioeconomic characteristics of the population in the rural U.S. South. Int J Health Serv. 2007;37(2):259‐277. [DOI] [PubMed] [Google Scholar]
- 182. Staff RT, Hogan MJ, Williams DS, Whalley LJ. Intellectual engagement and cognitive ability in later life (the “use it or lose it” conjecture): Longitudinal, prospective study. BMJ. 2018;363:k4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Karp A, Paillard‐Borg S, Wang H‐X, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2005;21(2):65‐73. [DOI] [PubMed] [Google Scholar]
- 184. Di Marco LY, Marzo A, Muñoz‐Ruiz M, et al. Modifiable lifestyle factors in dementia: A systematic review of longitudinal observational cohort studies. J Alzheimers Dis. 2014;42(1):119‐135. [DOI] [PubMed] [Google Scholar]
- 185. James BD, Wilson RS, Barnes LL, Bennett DA. Late‐life social activity and cognitive decline in old age. J Int Neuropsychol Soc. 2011;17(6):998‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yates LA, Ziser S, Spector A, Orrell M. Cognitive leisure activities and future risk of cognitive impairment and dementia: Systematic review and meta‐analysis. Int Psychogeriatr. 2016;9:1‐16. [DOI] [PubMed] [Google Scholar]
- 187. Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sanjeev G, Weuve J, Jackson JW, et al. Late‐life cognitive activity and dementia. Epidemiology. 2016;27(5):732‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wang Z, Marseglia A, Shang Y, Dintica C, Patrone C, Xu W. Leisure activity and social integration mitigate the risk of dementia related to cardiometabolic diseases: A population‐based longitudinal study. Alzheimer's Dement. 2020;16:316‐335. [DOI] [PubMed] [Google Scholar]
- 190. Sommerlad A, Sabia S, Singh‐Manoux A, Lewis G, Livingston G. Association of social contact with dementia and cognition: 28‐year follow‐up of the Whitehall II cohort study. PLoS Med. 2019;16(8):e1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Centers for Disease Control and Prevention . Surveillance Report: Traumatic Brain Injury‐Related Deaths by Age Group, Sex, and Mechanism of Injury. Accessed December 15, 2023. Available at: https://www.cdc.gov/traumaticbraininjury/pdf/TBI‐surveillance‐report‐2018‐2019‐508.pdf
- 192. Fann JR, Ribe AR, Pedersen HS, et al. Long‐term risk of dementia among people with traumatic brain injury in Denmark: A population‐based observational cohort study. Lancet Psychiatry. 2018;5(5):424‐431. [DOI] [PubMed] [Google Scholar]
- 193. LoBue C, Munro C, Schaffert J, et al. Traumatic brain injury and risk of long‐term brain changes, accumulation of pathological markers, and developing dementia: A review. J Alzheimers Dis. 2019;70(3):629‐654. [DOI] [PubMed] [Google Scholar]
- 194. Schneider ALC, Selvin E, Latour L, et al. Head injury and 25‐year risk of dementia. Alzheimer's Dement. 2021;17:1432‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Centers for Disease Control and Prevention . TBI Data. Accessed December 15, 2023. Available at: https://www.cdc.gov/traumaticbraininjury/data/index.html
- 196. Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55(8):1158‐1166. [DOI] [PubMed] [Google Scholar]
- 197. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2(7872):81‐84. [DOI] [PubMed] [Google Scholar]
- 198. Centers for Disease Control and Prevention . Traumatic Brain Injury & Concussion. Potential Effects. Accessed December 15, 2023. Available at: https://www.cdc.gov/traumaticbraininjury/outcomes.html
- 199. Shively S, Scher AI, Perl DP, Diaz‐Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. 2012;69(10):1245‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. LoBue C, Wadsworth H, Wilmoth K, Clem M, Hart J Jr, Womack KB. Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol. 2017;31(1):85‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Schaffert J, LoBue C, White CL, et al. Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy‐confirmed Alzheimer's disease. Neuropsychology. 2018;32(4):410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Stein TD, Montenigro PH, Alvarez VE, et al. Beta‐amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130(1):21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Turk KW, Geada A, Alvarez VE, et al. A comparison between tau and amyloid‐β cerebrospinal fluid biomarkers in chronic traumatic encephalopathy and Alzheimer disease. Alz Res Therapy. 2022;14:28. Accessed December 13, 2023. Available at: 10.1186/s13195-022-00976-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Asken BM, Sullan MJ, DeKosky ST, Jaffee MS, Bauer RM. Research gaps and controversies in chronic traumatic encephalopathy: A review. JAMA Neurol. 2017;74(10):1255‐1262. [DOI] [PubMed] [Google Scholar]
- 205. Mez J, Daneshvar DH, Abdolmohammadi B, et al. Duration of American football play and chronic traumatic encephalopathy. Ann Neurol. 2020;87(1):116‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Nowinski CJ, Bureau SC, Buckland ME, et al. Applying the Bradford Hill Criteria for Causation to Repetitive Head Impacts and Chronic Traumatic Encephalopathy. Front Neurol. 2022;13:938163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Shi L, Chen S, Ma M, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta‐analysis. Sleep Med Rev. 2018;40:4‐16. [DOI] [PubMed] [Google Scholar]
- 208. Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Winer JR, Keters KD, Kennedy G, et al. Association of short and long sleep duration with amyloid‐β burden and cognition in aging. JAMA Neurol. 2021;78(10):1187‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Makin S. Deep sleep gives your brain a deep clean: Slow‐wave activity during dreamless slumber helps wash out neural detritus Scientific American Nov 1, 2019. Accessed December 15, 2023. Available at: https://www.scientificamerican.com/article/deep‐sleep‐gives‐your‐brain‐a‐deep‐clean1/
- 211. Insel PS, Mohlenhoff BS, Neylan TC, Krystal AD, Mackin RS. Association of sleep and β‐amyloid pathology among older cognitively unimpaired adults. JAMA Netw Open. 2021;4(7):e2117573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Andrade A, Bubu OM, Varga AW, Osorio RS. The relationship between obstructive sleep apnea and Alzheimer's disease. J Alzheimers Dis. 2018; 64(Suppl 1):S255‐S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bubu, OM , Andrade AA, Umasabor‐Bubu U, et al. Obstructive sleep apnea, cognition and Alzheimer's disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer's disease: Role of amyloid, tau, and other factors. Neuropsychopharmacol. 2020;45:104‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lucey BP. It's complicated: The relationship between sleep and Alzheimer's disease in humans. Neurobiol Dis. 2020:144:105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Ju Y‐ES, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lao XQ, Liu X, Deng HB, et al. Sleep quality, Sleep duration, and the risk of coronary heart disease: A prospective cohort study with 60,586 adults. J Clin Sleep Med. 2018;14(1):109‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. U.S. Environmental Protection Agency . 2019. Integrated science assessment (ISA) for particulate matter (final report, 2019). EPA/600/R‐19/188. Washington, DC. [Google Scholar]
- 220. Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis. 2019;70(s1):S145‐S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zhang B, Weuve J, Langa KM, et al. Comparison of particulate air pollution from different emission sources and incident dementia in the U.S. JAMA Intern Med. 2023. doi:10.1001/jamainternmed.2023.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Abolhasani E, Hachinski V, Ghazaleh N, Azarpazhooh MR, Mokhber N, Martin J. Air pollution and Incidence of dementia: A systematic review and meta‐analysis. Neurology. 2022. doi: 10.1212/WNL.0000000000201419 [DOI] [PubMed] [Google Scholar]
- 223. Sprung J, Knopman DS, Petersen RC, et al. Association of hospitalization with long‐term cognitive trajectories in older adults. J Am Geriatr Soc. 2021;69(3):660‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. James BD, Wilson RS, Capuano AW, et al. Cognitive decline after elective and nonelective hospitalizations in older adults. Neurology. 2019;92(7):e690‐e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Brown CH, Sharrett AR, Coresh J, et al. Association of hospitalization with long‐term cognitive and brain MRI changes in the ARIC cohort. Neurology. 2015;84:1443‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Pandharipande PP, Girard TD, Jackson JC, et al. Long‐term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mart MF, Pun BT, Pandharipande P, Jackson JC, Ely EW. ICU survivorship ‐ The Relationship of delirium, sedation, dementia, and acquired weakness. Crit Care Med. 2021;49(8):1227‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. James BD, Wilson RS, Capuano AW, et al. Hospitalization, Alzheimer's Disease and Related Neuropathologies, and Cognitive Decline. Ann Neurol. 2019;86(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Fong TG, Inouye SK. The inter‐relationship between delirium and dementia: The importance of delirium prevention. Nat Rev Neurol. 2022;18(10):579‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Kim JE, Tamres LK, Orbell SL, et al. “And does that necessarily mean absolutely Alzheimer's?” An Analysis of questions raised following amyloid PET results disclosure. Am J Geriat Psychiat. Published August 13, 2023. doi:10.1016/j.jagp.2023.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Largent EA, Bradbury AR. Bringing Alzheimer disease testing and results disclosure Into the 21st Century Cures Act. JAMA Neurol. 2022;79(3):219‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Erickson CM, Clark LR, Ketchum FB, Chin NA, Gleason CE Largent EA. Implications of preclinical Alzheimer's disease biomarker disclosure for US policy and society. Alzheimers Dement: DADM. 2022. doi:10.1002/dad2.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Barnes LL. Alzheimer disease in African American individuals: increased incidence or not enough data? Nat Rev Neurol. 2022;18(1):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Administration for Community Living . 2020 Profile of Older Americans: May 2021. Accessed December 15, 2023. Available at: https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2020ProfileOlderAmericans.Final_.pdf
- 236. Gilmore‐Bykovskyi A, Croff R, Glover CM, et al. Traversing the aging research and health equity divide: Toward intersectional frameworks of research justice and participation. Gerontologist. 2022;62(5):711‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. U.S. Census Bureau . 2023 National Population Projections: Downloadable Files. Accessed December 15, 2023. Available at: https://www.census.gov/data/tables/2023/demo/popproj/2023‐summary‐tables.html
- 238. Administration on Aging, Administration for Community Living, U.S. Department of Health and Human Services . A Profile of Older Americans: 2016. Accessed December 15, 2023. Available at: https://www.acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2016‐Profile.pdf
- 239. Guerreiro R, Bras J. The age factor in Alzheimer's disease. Genome Med. 2015;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hudomiet P, Hurd MD, Rohwedder S. Trends in inequalities in the prevalence of dementia in the United States. Proc Natl Acad Sci USA. 2022;119(46):e2212205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical AD and mild cognitive impairment in the United States (2020‐2060). Alzheimers Dement. 2021;17(12):1966‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hendriks S, Peetoom K, Bakker C, et al. Global prevalence of young‐onset dementia: A systematic review and meta‐analysis. JAMA Neurol. 2021;78(9):1080‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP‐43 stage, mixed pathologies, and clinical Alzheimer's‐type dementia. Brain. 2016;139(11):2983‐2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Serrano‐Pozo A, Qian J, Monsell SE, et al. Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol. 2014;75:597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85:528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Petersen RC, Aisen P, Boeve BF, et al. Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol. 2013;74(2):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. U.S. Census Bureau . 2023 National Populaiton Prpojections Tables: Main Series. Accessed December 15, 2023. Available at: https://www.census.gov/data/tables/2023/demo/popproj/2023‐summary‐tables.html
- 249. Jack CR Jr, Therneau TM, Weigand SD, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging‐Alzheimer's Association Research Framework. JAMA Neurol. 2019;76(10):1174‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Gustavsson A, Norton N, Fast T, Frölich L, Georges J, Holzapfel D. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2022. doi: 10.1002/alz.12694 [DOI] [PubMed] [Google Scholar]
- 251. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement. 2018;14(2):121‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kotagal V, Langa KM, Plassman BL, et al. Factors associated with cognitive evaluations in the United States. Neurology. 2015;84(1):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Taylor DH, Jr., Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. J Alzheimers Dis. 2009;17(4):807‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Gianattasio KZ, Prather C, Glymour MM, Ciarleglio A, Power MC. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimer's & Dementia. 2019;5:891‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: A systematic literature review and a meta‐analysis. BMJ Open. 2017;7(2):e011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lin PJ, Daly AT, Olchanski N, et al. Dementia diagnosis disparities by race and ethnicity. Med Care. 2021;59(8):679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: An observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33(7):1131‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nguyen HQ, Borson S, Khang P, et al. Dementia diagnosis and utilization patterns in a racially diverse population within an integrated health care delivery system. Alzheimers Dement (N Y). 2022;8(1):e12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Grodstein F, Chang C‐H, Capuano AW, Power MC, Marquez DX, Barnes LL. Identification of Dementia in Recent Medicare Claims Data, Compared With Rigorous Clinical Assessments. J Gerontol A Biol Sci Med Sci. 2022;77(6):1272‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Grodstein F, James BD, Chen Y, et al. Identification of dementia in Medicare claims compared to rigorous clinical assessments in African Americans. J Gerontol A Biol Sci Med Sci. 2023;30:glad235. doi:10.1093/gerona/glad235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: Insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Healthy People 2030 . Accessed December 15, 2023. Available at: https://health.gov/healthypeople/objectives‐and‐data/browse‐objectives/dementias/increase‐proportion‐older‐adults‐dementia‐or‐their‐caregivers‐who‐know‐they‐have‐it‐dia‐01
- 263. Barrett AM, Orange W, Keller M, Damgaard P, Swerdlow RH. Short‐term effect of dementia disclosure: How patients and families describe the diagnosis. J Am Geriatr Soc. 2006;54(12):1968‐1970. [DOI] [PubMed] [Google Scholar]
- 264. Zaleta AK, Carpenter BD, Porensky EK, Xiong C, Morris JC. Agreement on diagnosis among patients, companions, and professionals after a dementia evaluation. Alzheimer Dis Assoc Disord. 2012;26(3):232‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Amjad H, Roth DL, Samus QM, Yasar S, Wolff JL. Potentially unsafe activities and living conditions of older adults with dementia. J Am Geriatr Soc. 2016;64(6):1223‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Alzheimer's Association . 2015 Alzheimer's Disease Facts and Figures. Special report: Disclosing a diagnosis of Alzheimer's disease. Accessed December 15, 2023. Available at: https://www.alzheimersanddementia.com/article/S1552‐5260(15)00058‐8/fulltext
- 267. Alzheimer's Association. 2019 Alzheimer's Disease Facts and Figures . Special report: Alzheimer's detection in the primary care setting — connecting patients with physicians. Accessed December 15, 2023. Available at: https://www.alzheimersanddementia.com/article/S1552‐5260(19)30031‐7/fulltext
- 268. Qian Y, Chen X, Tang D, Kelley AS, Li J. Prevalence of memory‐related diagnoses among U.S. older adults with early symptoms of cognitive impairment. J Gerontol A 76(10):1846‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Power MC, Willens V, Prather C, et al. Risks and benefits of clinical diagnosis around the time of dementia onset. Gerontol Geriatr Med. 2023;9:23337214231213185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Spargo D, Zur R, Lin PJ, Synnott P, Klein E, Hartry A. Estimating prevalence of early symptomatic Alzheimer's disease in the United States. Alzheimers Dement (Amst). 2023;15(4):e12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Liu Y, Jun H, Becker A, Wallick C, Mattke S. Detection rates of mild cognitive impairment in primary care for the United States Medicare population. J Prev Alzheimers Dis. 2023. Accessed December 15, 2023. Available at: https://link.springer.com/article/10.14283/jpad.2023.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre‐mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer's disease. Int Psychogeriatr. 2008;20(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 273. Jessen F, Wolfsgruber S, Wiese B, et al. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014;10(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 274. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Buckley RF, Maruff P, Ames D, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement. 2016;12(7):796‐804. [DOI] [PubMed] [Google Scholar]
- 276. Gifford KA, Liu D, Lu Z, et al. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimers Dement. 2014;10(3):319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K. Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology. 2015;85(21):1852‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Fernandez‐Blazquez MA, Avila‐Villanueva M, Maestu F, Medina M. Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimers Dis. 2016;52(1):271‐281. [DOI] [PubMed] [Google Scholar]
- 280. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Wolfsgruber S, Kleineidam L, Wagner M, et al. Differential risk of incident Alzheimer's disease dementia in stable versus unstable patterns of subjective cognitive decline. J Alzheimers Dis. 2016;54(3):1135‐1146. [DOI] [PubMed] [Google Scholar]
- 282. Unpublished data from the 2019‐2020 Behavioral Risk Factor Surveillance System survey conducted in 46 states and the District of Columbia, analyzed and provided to the Alzheimer's Association by the Alzheimer's Disease Program, Centers for Disease Control and Prevention.
- 283. Dhana K, Beck T, Desai P, Wilson RS, Evans DA, Rajan KB. Prevalence of Alzheimer's disease dementia in the 50 US states and 3142 counties: A population estimate using the 2020 bridged‐race postcensal from the National Center for Health Statistics. Alz Dement. 2023;19(10):4388‐4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer's disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019;15(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15(4):169‐173. [DOI] [PubMed] [Google Scholar]
- 286. Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid‐adult life. Alzheimers Dement. 2015;11(3):310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. U.S. Census Bureau . 2014 National Population Projections: Downloadable Files. Accessed December 15, 2023. Available at: https://www.census.gov/data/datasets/2014/demo/popproj/2014‐popproj.html
- 288. Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498‐1504. [DOI] [PubMed] [Google Scholar]
- 289. Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol. 2001;153(2):132‐136. [DOI] [PubMed] [Google Scholar]
- 290. Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age‐specific incidence rates of Alzheimer's disease: The Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072‐2077. [DOI] [PubMed] [Google Scholar]
- 291. Zahodne LB, Schofield PW, Farrell MT, Stern Y, Manly JJ. Bilingualism does not alter cognitive decline or dementia risk among Spanish‐speaking immigrants. Neuropsychology. 2014;28(2):238‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer's disease: Incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48:132‐138. [DOI] [PubMed] [Google Scholar]
- 293. Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Matthews FE, Stephan BC, Robinson L, et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7:11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Mielke MM, Ferretti MT, Iulita MF, Hayden K, Khachaturian AS. Sex and gender in Alzheimer's disease — Does it matter? Alzheimers Dement. 2018;14(9):1101‐1103. [DOI] [PubMed] [Google Scholar]
- 296. Rocca WA. Time, Sex, gender, history, and dementia. Alzheimer Dis Assoc Disord. 2017;31(1):76‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Shaw C, Hayes‐Larson E, Glymour MM, Dufouil C, Hohman TJ, Whitmer RA. Evaluation of selective survival and sex/gender differences in dementia incidence using a simulation model. JAMA Netw Open. 2021;4(3):e211001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Gilsanz P, Lee C, Corrada MM, Kawas CH, Quesenberry CP, Jr. , Whitmer RA. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology. 2019;92(17):e2005‐e2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: Assessing sex and gender differences. Clin Epidemiol. 2014;6:37‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Mielke MM, Aggarwal NT, Vila‐Castelar C, et al. Consideration of sex and gender in Alzheimer's disease and related disorders from a global perspective. Alzheimers Dement. 2022. doi:10.1002/alz.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Rocca WA, Mielke MM, Vemuri P, Miller VM. Sex and gender differences in the causes of dementia: A narrative review. Maturitas. 2014;79(2):196‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Launer LJ, Andersen K, Dewey ME, et al. Rates and risk factors for dementia and Alzheimer's disease: Results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52(1):78‐84. [DOI] [PubMed] [Google Scholar]
- 304. Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M, Batty GD. Socioeconomic status as a risk factor for dementia death: individual participant meta‐analysis of 86 508 men and women from the UK. Br J Psychiatry. 2013;203(1):10‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Mayeda RM, Mobley TM, Weiss RE, Murchland AR, Berkman LF, Sabbath EL. Association of work‐family experience with mid‐ and late‐life memory decline in US women. Neurology. 2020; e3072‐33080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Mielke MM, James BD. Women who participated in the paid labor force have lower rates of memory decline: Working to remember. Neurolgy. 2020;95(23):1027‐1028. [DOI] [PubMed] [Google Scholar]
- 307. Population Reference Bureau . Women, Work, and the COVID Pandemic: Myths and Realities. Accessed December 15, 2023. Available at: https://www.prb.org/articles/blog‐u‐s‐women‐work‐and‐the‐covid‐pandemic‐myths‐and‐realities
- 308. Zamarro G, Prados MJ. “Gender Differences in Couples' Division of Childcare, Work, and Mental Health During COVID‐19.” Rev Econ Househ. 2021;19(1):11‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Center for American Progress . Calculating the Hidden Cost of Interrupting a Career for Child Care. Accessed December 15, 2023. Available at: https://www.americanprogress.org/article/calculating‐the‐hidden‐cost‐of‐interrupting‐a‐career‐for‐child‐care/
- 310. Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and gender differences in Alzheimer's disease: Recommendations for future research. J Womens Health. 2012;21(10):1018‐1023. [DOI] [PubMed] [Google Scholar]
- 311. Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer's Disease Neuroimaging Initiative Investigators. Sex modifies the APOE‐related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender, and Alzheimer's disease: An overlooked, but potent and promising interaction. Brain Imaging Behav. 2014;8(2):262‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex‐specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta‐analysis. JAMA Neurol. 2017;74(10):1178‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: Evidence of gene‐environment interaction. Neurology. 2000;54(10):1949‐1954. [DOI] [PubMed] [Google Scholar]
- 316. Kang JH, Grodstein F. Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging. 2012;33(7):1129‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Dilworth‐Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Diagnosis and assessment of Alzheimer's disease in diverse populations. Alzheimers Dement. 2008;4(4):305‐309. [DOI] [PubMed] [Google Scholar]
- 318. Manly JJ, Mayeux R. Ethnic differences in dementia and Alzheimer's disease. In: Anderson N, Bulatao R, Cohen B, eds. Critical perspectives on racial and ethnic differentials in health in late life. Washington, D.C.: National Academies Press; 2004: p. 95‐141. [PubMed] [Google Scholar]
- 319. Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: The South Florida Program on Aging and Health. Ann Epidemiol. 2003;13(6):472‐478. [DOI] [PubMed] [Google Scholar]
- 320. Harwood DG, Ownby RL. Ethnicity and dementia. Curr Psych Report. 2000;2(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 321. Perkins P, Annegers JF, Doody RS, Cooke N, Aday L, Vernon SW. Incidence and prevalence of dementia in a multiethnic cohort of municipal retirees. Neurology. 1997;49(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 322. Steenland K, Goldstein FC, Levey A, Wharton W. A meta‐analysis of Alzheimer's disease incidence and prevalence comparing African‐Americans and caucasians. J Alzheimers Dis. 2015;50(1):71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non‐Hispanic black and white individuals in the United States, 2000‐2016. JAMA Neurology. 2021;78(3):275‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Potter GG, Plassman BL, Burke JR, et al. Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 2009;5(6):445‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14(6):481‐493. [PubMed] [Google Scholar]
- 326. Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169‐177. [DOI] [PubMed] [Google Scholar]
- 327. Samper‐Ternent R, Kuo YF, Ray LA, Ottenbacher KJ, Markides KS, Al Snih S. Prevalence of health conditions and predictors of mortality in oldest old Mexican Americans and non‐Hispanic whites. J Am Med Dir Assn. 2012;13(3):254‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72‐83. [DOI] [PubMed] [Google Scholar]
- 329. González HM, Tarraf W, Schneiderman N, et al. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: Study of Latinos‐Investigation of Neurocognitive Aging results. Alzheimers Dement. 2019;15(12):1507‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Kornblith E, Bahorik A; Boscardin WJ; Xia F, Barnes DE, Yaffe K. Association of Race and Ethnicity With Incidence of Dementia Among Older Adults. JAMA. 2022;327(15):1488‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: The impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Froehlich TE, Bogardus ST Jr., SK Inouye. Dementia and race: Are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49(4):477‐484. [DOI] [PubMed] [Google Scholar]
- 334. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223‐254. [DOI] [PubMed] [Google Scholar]
- 335. Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works ‐ Racist Policies as a Root Cause of U.S. Racial Health Inequities. N Engl J Med. 2021;384(8):768‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: Evidence and interventions. Lancet. 2017;389(10077):1453‐1463. [DOI] [PubMed] [Google Scholar]
- 337. Caunca MR, Odden MC, et al. Association of racial residential segregation throughout young adulthood and cognitive performance in middle‐aged participants in the CARDIA study. JAMA Neurology. 2020;77(8):1000‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Lamar M, Lerner AJ, James BD, et al. Relationship of early‐life residence and educational experience to level and change in cognitive functioning: Results of the Minority Aging Research Study. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):e81‐e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Peterson RL, George KM, Barnes LL, et al. Timing of School Desegregation and Late‐Life Cognition in the Study of Healthy Aging in African Americans (STAR). JAMA Netw Open. 2021;4(10):e2129052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Bancks MP, Byrd GS, Caban‐Holt A, et al. Self‐reported experiences of discrimination and incident dementia. Alzheimers Dement. 2023;19(7):3119‐3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Lines LM, Sherif NA, Wiener JM. Racial and ethnic disparities among individuals with Alzheimer's disease in the United States: A literature review. Research Triangle Park, NC: RTI Press; 2014. [Google Scholar]
- 342. Zhang Z, Hayward MD, Yu YL. Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav. 2016;57(2):184‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1‐2):125‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Clark PC, Kutner NG, Goldstein FC, et al. Impediments to timely diagnosis of Alzheimer's disease in African Americans. J Am Geriatr Soc. 2005;53(11):2012‐2017. [DOI] [PubMed] [Google Scholar]
- 345. Fitten LJ, Ortiz F, Ponton M. Frequency of Alzheimer's disease and other dementias in a community outreach sample of Hispanics. J Am Geriatr Soc. 2001;49(10):1301‐1308. [DOI] [PubMed] [Google Scholar]
- 346. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥ 65 years. Alzheimers Dement. 2019;15(1):17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Mayeda ER, Glymour MM, Quesenberry CP, Jr. , Whitmer RA. Heterogeneity in 14‐year dementia incidence between Asian American subgroups. Alzheimer Dis Assoc Disord. 2017;31(3):181‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Ajrouch KJ, Zahodne LB, Antonucci TC. Arab American Cognitive Aging: Opportunities for Advancing Research on Alzheimer's Disease Disparities. Innov Aging. 2017;1(3):igx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Flatt JD, Cicero EC, Lambrou NH, Wharton W, Anderson JG, Bouldin ED, McGuire LC, Taylor CA. Subjective cognitive decline higher among sexual and gender minorities in the United States, 2015‐2018. Alzheimers dement. 2021;7(1):e12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Liu H, Hsieh N, Zhang Z, Zhang Y, Langa KM. Same‐sex couples and cognitive impairment: Evidence from the health and retirement study. J Gerontol B. 2021;76(7):1388‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Hsieh N, Liu H, Lai WH. Elevated risk of cognitive impairment among older sexual minorities: Do health conditions, health behaviors, and social connections matter? Gerontologist. 2021;61(3):352‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Perales‐Puchalt J, Gauthreaux K, Flatt J, Teylan MA, Resendez J, Kukull WA, Chan KCG, Burns J, Vidoni ED. Risk of dementia and mild cognitive impairment among older adults in same‐sex relationships. Int J Geriatr Psychiatry. 2019;34(6):828‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Dragon CN, Guerino P, Ewald E, Laffan AM. Transgender Medicare beneficiaries and chronic conditions: exploring fee‐for‐service claims data. LGBT health. 2017;4(6):404‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Guo Y, Li Q, Yang X, Jaffee MS, Wu Y, Wang F, Bian J. Prevalence of Alzheimer's and Related Dementia Diseases and Risk Factors Among Transgender Adults, Florida, 20122020. Am J Public Health. 2022;112(5):754‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Romanelli RJ, Rosenblatt AS, Marcum ZA, Flatt JD. Cognitive Impairment in Sexual and Gender Minority Groups: A Scoping Review of the Literature. LGBT Health. 2023. doi: 10.1089/lgbt.2023.0095 [DOI] [PubMed] [Google Scholar]
- 357. Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Flatt JD, Johnson JK, Karpiak SE, Seidel L, Larson B, Brennan‐Ing M. Correlates of Subjective Cognitive Decline in Lesbian, Gay, Bisexual, and Transgender Older Adults. J Alzheimers Dis. 2018;64(1):91‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Brennan‐Ing M, Seidel L, Larson B, Karpiak SE. Social care networks and older LGBT adults: challenges for the future. J Homosex. 2014;61(1):21‐52. [DOI] [PubMed] [Google Scholar]
- 360. Cohen RA, Seider TR, Navia B: HIV effects on age‐associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Cicero EC, Lett E, Flatt JD, Benson GP, Epps F. Transgender Adults From Minoritized Ethnoracial Groups in the U.S. Report Greater Subjective Cognitive Decline. J Gerontol B Psychol Sci Soc Sci. 2023;78(6):1051‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Hill Collins, Patricia . Intersectionality as Critical Social Theory. Duke University Press; 2019. [Google Scholar]
- 363. Correro AN, Nielson KA. A review of minority stress as a risk factor for cognitive decline in lesbian, gay, bisexual, and transgender (LGBT) elders. J Gay Lesbian Ment Health. 2020;24(1):2‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Flatt JD, Cicero EC, Kittle KR, Brennan‐Ing M. Recommendations for advancing research with sexual and gender minority older adults. J Gerontol B. 2022;77(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 365. Wolters FJ, Chibnik LB, Waziry R, et al. Twenty‐seven‐year time trends in dementia incidence in Europe and the United States. The Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519‐e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time: Current evidence. Nat Rev Neurol. 2017;13(6):327‐339. [DOI] [PubMed] [Google Scholar]
- 368. Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456‐1463. [DOI] [PubMed] [Google Scholar]
- 369. Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L. Twenty‐year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80(20):1888‐1894. [DOI] [PubMed] [Google Scholar]
- 370. Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Cerasuolo JO, Cipriano LE, Sposato LA, et al. Population‐based stroke and dementia incidence trends: Age and sex variations. Alzheimers Dement. 2017;13(10):1081‐1088. [DOI] [PubMed] [Google Scholar]
- 372. Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 2017;74(11):1345‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Ahmadi‐Abhari S, Guzman‐Castillo M, Bandosz P, et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: Modelling study. BMJ. 2017;358:j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Sullivan KJ, Dodge HH, Hughes TF, et al. Declining incident dementia rates across four population‐based birth cohorts. J Gerontol A Biol Sci Med Sci. 2019;74(9):1439‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Matthews FE, Arthur A, Barnes LE, et al. A two‐decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: Results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Wiberg P, Waern M, Billstedt E, Östling S, Skoog I. Secular trends in the prevalence of dementia and depression in Swedish septuagenarians 1976–2006. Psychol Med. 2013;43:2627‐2634. [DOI] [PubMed] [Google Scholar]
- 377. Wimo A, Sjölund BM, Sköldunger A, et al. Cohort effects in the prevalence and survival of people with dementia in a rural area in Northern Sweden. J Alzheimers Dis. 2016;50:387‐396. [DOI] [PubMed] [Google Scholar]
- 378. Hall KS, Gao S, Baiyewu O, et al. Prevalence rates for dementia and Alzheimer's disease in African Americans: 1992 versus 2000. Alzheimers Dement. 2009;5(3):227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Farina MP, Zhang YS, Kim JK, Hayward MD, Crimmins EM. Trends in dementia prevalence, incidence, and mortality in the United States (2000‐2016). J Aging Health. 2022;34(1):100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. van den Kommer TN, Deeg DJH, van der Flier WM, Comijs HC. Time trend in persistent cognitive decline: Results from the longitudinal aging study Amsterdam. J Gerontol B Psychol Sci Soc Sci. 2018;73(Suppl 1)S57‐S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Sekita A, Ninomiya T, Tanizaki Y, et al. Trends in prevalence of Alzheimer's disease and vascular dementia in a Japanese community: The Hisayama Study. Acta Psychiatr Scand. 2010;122(4):319‐325. [DOI] [PubMed] [Google Scholar]
- 382. Gao S, Burney HN, Callahan CM, Purnell CE, Hendrie HC. Incidence of Dementia and Alzheimer Disease Over Time: A Meta‐Analysis. J Am Geriatr Soc. 2019;67(7):1361‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Crimmins EM, Saito Y, Kim JK, Zhang Y, Sasson I, Hayward MD. Educational differences in the prevalence of dementia and life expectancy with dementia in the United States: Changes from 2000 to 2010. J Gerontol B Psychol Sci Soc Sci. 2018;73(Suppl 1):S20‐S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Choi H, Schoeni RF, Martin LG, Langa K M. Trends in the prevalence and disparity in cognitive limitations of Americans 55‐69 years old. J Gerontol B Psychol Sci Soc Sci. 2018;73 (Suppl 1):S29‐S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Zheng H. A New Look at Cohort Trend and Underlying Mechanisms in Cognitive Functioning. J Gerontol B Psychol Sci Soc Sci. 2021;76(8):1652‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Freedman VA, Kasper JD, Spillman BC, Plassman BL. Short‐term changes in the prevalence of probable dementia: An analysis of the 2011–2015 National Health and Aging Trends Study. J Gerontol B Psychol Sci Soc Sci. 2018;73(Suppl 1)S48‐S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Langa KM. Is the risk of Alzheimer's disease and dementia declining? Alzheimers Res Ther. 2015;7(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med. 2013;369(24):2275‐2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Sheffield KM, Peek MK. Changes in the prevalence of cognitive impairment among older Americans, 1993‐2004: Overall trends and differences by race/ethnicity. Am J Epidemiol. 2011;174(3):274‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA. Secular trends in cognitive performance in older black and white U.S. adults, 1993‐2012: Findings from the Chicago Health and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2018;73 (Suppl 1):S73‐S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Prince MJ, Wimo A, Guerchet M, Ali G‐C, Wu Y‐T, Prina M. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; 2015.
- 392. de Erausquin GA, Snyder H, Carrillo M, Hosseini AA, Brugha TS, Seshadri S. The chronic neuropsychiatric sequelae of COVID‐19: The need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 2021;17(6):1056‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Arias E, Tejada‐Vera B, Kochanek KD, Ahmad FB. Provisional Life Expectancy Estimates for 2021. National Vital Statistics System, Report No. 23, 2022. Accessed December 15, 2023. Available at: https://www.cdc.gov/nchs/data/vsrr/vsrr023.pdf [Google Scholar]
- 394. The World Bank. Fertility, total (births per woman)—US . Accessed December 15, 2023. Available at: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?locations=US
- 395.U.S. Census Bureau . 2017 National Population Projections Tables. Accessed December 15, 2023. Available at: https://www.census.gov/data/tables/2017/demo/popproj/2017‐summary‐tables.html
- 396. Administration for Community Living . 2019 Profile of Older Americans. May 2020 Accessed December 15, 2023. Available at: https://acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2019ProfileOlderAmericans508.pdf
- 397. Bauman K. Shift Toward Greater Educational Attainment for Women Began 20 Years Ago. U.S. Census Bureau. Accessed December 15, 2023. Available at: https://www.census.gov/newsroom/blogs/random‐samplings/2016/03/shift‐toward‐greater‐educational‐attainment‐for‐women‐began‐20‐years‐ago.html [Google Scholar]
- 398. Population Reference Bureau . Why is the U.S. Birth Rate Declining? 2021. Accessed December 15, 2023. Available at: https://www.prb.org/resources/why‐is‐the‐u‐s‐birth‐rate‐declining/
- 399. Horowitz JM, Igielnik R, Kochhar R. Trends in income and wealth inequality. Pew Research Center. Accessed October 28, 2023. Available at: https://www.pewresearch.org/social‐trends/2020/01/09/trends‐in‐income‐and‐wealth‐inequality/
- 400. Tom SE, Phadke M, Hubbard RA, Crane PK, Stern Y, Larson EB. Association of Demographic and Early‐Life Socioeconomic Factors by Birth Cohort with Dementia Incidence Among US Adults Born Between 1893 and 1949. JAMA Netw Open. 2020;3(7):e2011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Skoog I. Dementia incidence: The times, they are a‐changing. Nature Rev Neurol. 2016;12:316‐368. Accessed December 15, 2023. Available at: https://www.nature.com/articles/nrneurol.2016.55 [DOI] [PubMed] [Google Scholar]
- 402. Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Mortality 2018‐2021 on CDC WONDER Online Database, released in 2021 . Data are from the Multiple Cause of Death Files, 2018‐2021, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed December 13, 2023. Accessed December 15, 2023. Available at: http://wonder.cdc.gov/ucd‐icd10‐expanded.html
- 403. U.S. Department of Health and Human Services . Centers for Disease Control and Prevention. National Center for Health Statistics. CDC WONDER online database: About Provisional Mortality Statistics, 2018 through Last Month. https://wonder.cdc.gov/mcd‐icd10‐provisional.html
- 404. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E. The State of US Health, 1990‐2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA. 2018;319(14):1444‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. World Health Organization . International Statistical Classification of Diseases and Related Health Problems. 10th revision. 2nd edition. WHO Press: Geneva, Switzerland; 2004. [Google Scholar]
- 406. Burns A, Jacoby R, Luthert P, Levy R. Cause of death in Alzheimer's disease. Age Ageing. 1990;19(5):341‐344. [DOI] [PubMed] [Google Scholar]
- 407. Brunnstrom HR, Englund EM. Cause of death in patients with dementia disorders. Eur J Neurol. 2009;16(4):488‐492. [DOI] [PubMed] [Google Scholar]
- 408. Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between nosologist and Cardiovascular Health Study review of deaths: Implications of coding differences. J Am Geriatr Soc. 2009;57(1):133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Romero JP, Benito‐Leon J, Louis ED, Bermejo‐Pareja F. Under reporting of dementia deaths on death certificates: A systematic review of population‐based cohort studies. J Alzheimers Dis. 2014;41(1):213‐221. [DOI] [PubMed] [Google Scholar]
- 410. Ganguli M, Rodriguez EG. Reporting of dementia on death certificates: A community study. J Am Geriatr Soc. 1999;47(7):842‐849. [DOI] [PubMed] [Google Scholar]
- 411. Stokes AC, Weiss J, Lundberg DJ, et al. Estimates of the association of dementia with US mortality levels using linked survey and mortality records. JAMA Neurol. 2020;77(12):1543‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Unpublished tabulations based on data from the 100% National Sample Medicare Fee‐for‐Service Beneficiaries for 2019. Prepared under contract by Health Care Cost Institute, November 2021.
- 413. Weuve J, Hebert LE, Scherr PA, Evans DA. Deaths in the United States among persons with Alzheimer's disease (2010‐2050). Alzheimers Dement. 2014;10(2):E40‐E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ. Lethality of Alzheimer disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord. 2010;24(1):90‐95. [DOI] [PubMed] [Google Scholar]
- 415. Centers for Disease Control and Prevention . National Center for Health Statistics . Excess Deaths Associated with COVID‐19. Accessed December 15, 2023. Available at: https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm
- 416. Gilstrap L, Zhou W, Alsan M, Nanda A, Skinner JS. Trends in Mortality Rates Among Medicare Enrollees With Alzheimer Disease and Related Dementias Before and During the Early Phase of the COVID‐19 Pandemic. JAMA Neurol. 2022;79(4):342‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Chen R, Charpignon M, Raquib RV, et al. Excess Mortality With Alzheimer Disease and Related Dementias as an Underlying or Contributing Cause During the COVID‐19 Pandemic in the US. JAMA Neurol. 2023;80(9):919‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Tejada‐Vera B. Mortality from Alzheimer's disease in the United States: Data for 2000 and 2010. National Center for Health Statistics Data Brief, No. 116. National Center for Health Statistics, Hyattsville, MD; 2013. [PubMed] [Google Scholar]
- 419. Taylor C, Greenlund S, McGuire L, Lu H, Croft J. Deaths from Alzheimer's Disease — United States, 1999‐2014. MMWR Morb Mortal Wkly Rep. 2017;66:521‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Mitchell SL, Teno JM, Miller SC, Mor V. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53(2):299‐305. [DOI] [PubMed] [Google Scholar]
- 421. U.S. Burden of Disease Collaborators , Mokdad AH, Ballestros K, et al. The state of U.S. health, 1990‐2016: Burden of diseases, injuries, and risk factors among U.S. states. JAMA. 2018;319(14):1444‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Barker C, Green A. Opening the debate on DALYs (disability‐adjusted life years). Health Policy Plan. 1996;11(2):179‐183. [DOI] [PubMed] [Google Scholar]
- 423. Gaugler JE, Kane RL, Kane RA. Family care for older adults with disabilities: Toward more targeted and interpretable research. Int J Aging Hum Dev. 2002;54(3):205‐231. [DOI] [PubMed] [Google Scholar]
- 424. Schulz R, Quittner AL. Caregiving through the life‐span: Overview and future directions. Health Psychol. 1998;17:107‐111. [PubMed] [Google Scholar]
- 425. Friedman EM, Shih RA, Langa KM, Hurd MD. U.S. prevalence and predictors of informal caregiving for dementia. Health Aff. 2015;34(10):1637‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Spillman B, Wolff J, Freedman VA, Kasper JD. Informal Caregiving for Older Americans: An Analysis of the 2011 National Health and Aging Trends Study. Accessed December 15, 2023. Available at: https://aspe.hhs.gov/pdf‐report/informal‐caregiving‐older‐americans‐analysis‐2011‐national‐health‐and‐aging‐trends‐study
- 427. Walmart: 2023 Annual Report . Accessed December 15, 2023. Available at: https://s201.q4cdn.com/262069030/files/doc_financials/2023/ar/Walmart‐10K‐Reports‐Optimized.pdf
- 428. McDonald's Corporation Report 2021 . Accessed December 15, 2023. Available at: https://corporate.mcdonalds.com/content/dam/sites/corp/nfl/pdf/MCD%202021%20Annual%20Report.pdf
- 429. Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B, Kuntz KM. Societal and family lifetime cost of dementia: Implications for policy. J Am Geriatr Soc. 2017;65(10):2169‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Official Data Foundation . CPI inflation calculator. Accessed December 15, 2023. Available at: http://www.in2013dollars.com/2017‐dollars‐in‐2018?amount=139765
- 431. Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing Alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res. 2017;17(2):189‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Greenwood N, Smith R. Motivations for being informal carers of people living with dementia: A systematic review of qualitative literature. BMC Geriatr. 2019;19(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Kasper JD, Freedman VA, Spillman BC, Wolff JL. The disproportionate impact of dementia on family and unpaid caregiving to older adults. Health Aff. 2015;34(10):1642‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Ornstein KA, Wolff JL, Bollens‐Lund E, Rahman OK, Kelley AS. Spousal caregivers are caregiving alone in the last years of life. Health Aff (Millwood). 2019;38(6):964‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Alzheimer's Association . Issues Brief: LGBT and Dementia. Accessed December 15, 2023. Available at https://www.alz.org/media/Documents/lgbt‐dementia‐issues‐brief.pdf
- 436. Fredriksen‐Goldsen KI, Jen S, Bryan AEB, Goldsen J. Cognitive impairment, Alzheimer's disease, and other dementias in the lives of lesbian, gay, bisexual and transgender (LGBT) older adults and their caregivers: Needs and competencies. J Appl Gerontol. 2018;37(5):545‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Candrian C, Burke ES, Kline D, Torke AM. Experiences of caregiving with Alzheimer's disease in the LGBT community. BMC Geriatr. 2023;23(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Anderson JG, Flatt JD, Jabson Tree JM, Gross AL, Rose KM. Characteristics of Sexual and Gender Minority Caregivers of People With Dementia. J Aging Health. 2021;33(10):838‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439. Kasper JD, Freedman VA, Spillman BC. Disability and Care Needs of Older Americans by Dementia Status: An Analysis of the 2011 National Health and Aging Trends Study. U.S. Department of Health and Human Services; 2014. Accessed December 15, 2023. Available at: http://aspe.hhs.gov/report/disability‐and‐care‐needs‐older‐americans‐dementia‐status‐analysis‐2011‐national‐health‐and‐aging‐trends‐study [Google Scholar]
- 440. Rabarison KM, Bouldin ED, Bish CL, McGuire LC, Taylor CA, Greenlund KJ. The economic value of informal caregiving for persons with dementia: Results from 38 states, the District of Columbia, and Puerto Rico, 2015 and 2016 BRFSS. Am J Public Health. 2018;108(10):1370‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. National Alliance for Caregiving in Partnership with the Alzheimer's Association . Dementia Caregiving in the U.S. Bethesda, MD. Accessed December 15, 2023. Available at: https://www.caregiving.org/wp‐content/uploads/2020/05/Dementia‐Caregiving‐in‐the‐US_February‐2017.pdf [Google Scholar]
- 442. Unpublished data from the 2015, 2016 and 2017 Behavioral Risk Factor Surveillance System survey, analyzed by and provided to the Alzheimer's Association by the Alzheimer's Disease and Healthy Aging Program (AD+HP), Centers for Disease Control and Prevention (CDC).
- 443. Fisher GG, Franks MM, Plassman BL, et al. Caring for individuals with dementia and cognitive impairment, not dementia: Findings from The Aging, Demographics, and Memory Study. J Am Geriatr Soc. 2011;59(3):488‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Riffin C, Van Ness PH, Wolff JL, Fried T. Family and other unpaid caregivers and older adults with and without dementia and disability. J Am Geriatr Soc. 2017;65(8):1821‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. National Poll on Healthy Aging . Dementia Caregivers: Juggling, Delaying and Looking Forward. Accessed December 15, 2023. Available at: http://www.healthyagingpoll.org/sites/default/files/2017‐10/NPHA_Caregivers‐Report‐PROOF_101817_v2.pdf
- 446. Caregiving in the U.S.: 2020 Report . Accessed December 15, 2023. Available at: https://www.aarp.org/content/dam/aarp/ppi/2020/05/full‐report‐caregiving‐in‐the‐united‐states.doi.10.26419‐2Fppi.00103.001.pdf
- 447. Ohno S, Chen Y, Sakamaki H, Matsumaru N, Yoshino M, Tsukamoto K. Burden of caring for Alzheimer's disease or dementia patients in Japan, the US, and EU: results from the National Health and Wellness Survey: a cross‐sectional survey. J Med Econ. 2021;24(1):266‐278. [DOI] [PubMed] [Google Scholar]
- 448. National Alliance for Caregiving and AARP . Caregiving in the U.S.: Unpublished data analyzed under contract for the Alzheimer's Association; 2009. [Google Scholar]
- 449. Alzheimer's Association . 2014 Alzheimer's Disease Facts and Figures. Special Report: Women and Alzheimer's Disease. Accessed December 15, 2023. Available at: https://www.alzheimersanddementia.com/article/S1552‐5260(14)00062‐4/fulltext
- 450. Xiong C, Biscardi M, Astell A, et al. Sex and gender differences in caregiving burden experienced by family caregivers of persons with dementia: A systematic review. PLoS One. 2020;15(4):e0231848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Pinquart M, Sörensen. Gender differences in caregiver stressors, social resources, and health: An updated meta‐analysis. J Gerontol B Psychol Sci Soc Sci. 2006;61(1):P33‐P45. [DOI] [PubMed] [Google Scholar]
- 452. Ma M, Dorstyn D, Ward L, Prentice S. Alzheimer's disease and caregiving: A meta‐analytic review comparing the mental health of primary carers to controls. Aging Ment Health. 2017;5:1‐11. [DOI] [PubMed] [Google Scholar]
- 453. Roberts HL, Bollens‐Lund E, Ornstein KA, Kelley AS. Caring for aging parents in the last years of life. J Am Geriatr Soc. 2023;71(9):2871‐2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Fabius CD, Wolff JL, Kasper JD. Race differences in characteristics and experiences of black and white caregivers of older Americans. Gerontologist. 2020;60(7):1244‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Moon HE, Rote SM, Sears J, Schepens Niemiec SL. Racial Differences in the Dementia Caregiving Experience during the COVID‐19 Pandemic: Findings from the National Health and Aging Trends Study (NHATS). J Gerontol B Psychol Sci Soc Sci. 2022;gbac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Liu R, Chi I, Wu S. Caregiving Burden Among Caregivers of People With Dementia Through the Lens of Intersectionality. Gerontologist. 2022;62(5):650‐661. [DOI] [PubMed] [Google Scholar]
- 457. Parker LJ, Fabius CD. Racial differences in respite use among black and white caregivers for people living with dementia. J Aging Health. 2020;32(10):1667‐1675. [DOI] [PubMed] [Google Scholar]
- 458. Ejem D, Atkins GC, Perkins M, Morhardt DJ, Williams IC, Cothran FA. Stressors and Acceptability of Services Among Black Caregivers of Persons With Memory Problems. J Gerontol Nurs. 2022;48(6):13‐18. [DOI] [PubMed] [Google Scholar]
- 459. Rote SM, Angel JL, Moon H, Markides K. Caregiving across diverse populations: New evidence from the National Study of Caregiving and Hispanic EPESE. Innov Aging. 2019;3(2):igz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Pinquart M, Sörensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: A meta‐analysis. Gerontologist. 2005;45(1):90‐106. [DOI] [PubMed] [Google Scholar]
- 461. Dilworth‐Anderson P, Moon H, Aranda MP. Dementia caregiving research: Expanding and reframing the lens of diversity, inclusivity, and intersectionality. Gerontologist. 2020;60(5):797‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Chen C, Thunell J, Zissimopoulos J. Changes in physical and mental health of Black, Hispanic, and White caregivers and non‐caregivers associated with onset of spousal dementia. Alzheimers Dement (N Y). 2020;6(1):e12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Cothran FA, Chang E, Beckett L, Bidwell JT, Price CA, Gallagher‐Thompson D. A Landscape of Subjective and Objective Stress in African‐American Dementia Family Caregivers. West J Nurs Res. 2022;44(3):239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Liu C, Badana ANS, Burgdorf J, Fabius CD, Roth DL, Haley WE. Systematic review and meta‐analysis of racial and ethnic differences in dementia caregivers' well‐being. Gerontologist. 2021;61(5):e228‐e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Baik D, Centi S, McNair B. Assessing racial and ethnic differences in cardiovascular disease in U.S. family caregivers of persons with dementia: Analysis of data from the 2015‐2020 Behavioral Risk Factor Surveillance System. Res Gerontol Nurs. 2023;16(5):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Brewster GS, Bonds K, McLennon S, Moss KO, Epps F, Lopez RP. Missing the Mark: The Complexity of African American Dementia Family Caregiving. J Fam Nurs. 2020;26(4):294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. Lewis JP, Manson SM, Jernigan VB, Noonan C. "Making Sense of a Disease That Makes No Sense": Understanding Alzheimer's Disease and Related Disorders Among Caregivers and Providers Within Alaska Native Communities. Gerontologist. 2021;61(3):363‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Parker LJ, Fabius C. Who's Helping Whom? Examination of care arrangements for racially and ethnically diverse people living with dementia in the community. J Appl Gerontol. 2022;41(12):2589‐2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Idorenyin Imoh U, Charity T. Cultural and Social Factors in Care Delivery Among African American Caregivers of Persons With Dementia: A Scoping Review. Gerontol Geriatr Med. 2023;9:23337214231152002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Ta Park VM, Ly Q, von Oppenfeld J, et al. A scoping review of dementia caregiving for Korean Americans and recommendations for future research. Clin Gerontol. 2023;46(2):223‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Moraes Balbim G, Magallanes M, Marques IG, et al. Sources of caregiving burden in middle‐aged and older Latino caregivers. J Geriatr Psychiatry Neurol. 2020;33(4):185‐194. [DOI] [PubMed] [Google Scholar]
- 472. Jaldin MA, Balbim GM, Colin SJ, et al. The influence of Latino cultural values on the perceived caregiver role of family members with Alzheimer's disease and related dementias. Ethn Health. 2023;28(4):619‐633. [DOI] [PubMed] [Google Scholar]
- 473. Martinez IL, Gonzalez EA, Quintero C, Vania MJ. The Experience of Alzheimer's Disease Family Caregivers in a Latino Community: Expectations and incongruences in support services. J Gerontol B Psychol Sci Soc Sci. 2022;77(6):1083‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Sloan DH, Johnston D, Fabius C, Pyatt T, Antonsdottir I, Reuland M. Transcending inequities in dementia care in Black communities: Lessons from the maximizing independence at home care coordination program. Dementia (London). 2022;21(5):1653‐1668. [DOI] [PubMed] [Google Scholar]
- 475. Rote SM, Moon HE, Kacmar AM, Moore S. Exploring Coping Strategies and Barriers in Dementia Care: A Mixed‐Methods Study of African American Family Caregivers in Kentucky. J Appl Gerontol. 2022;41(8):1851‐1859. [DOI] [PubMed] [Google Scholar]
- 476. Alexander K, Oliver S, Bennett SG, Henry J, Hepburn K, Clevenger C. "Falling between the cracks": Experiences of Black dementia caregivers navigating U.S. health systems. J Am Geriatr Soc. 2022;70(2):592‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Bonner GJ, Freels S, Ferrans C, et al. Advance Care Planning for African American Caregivers of Relatives With Dementias: Cluster Randomized Controlled Trial. Am J Hosp Palliat Care. 2021;38(6):547‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Meyer OL, Sun M, Do T, et al. Community‐Engaged Research with Vietnamese Americans to Pilot‐Test a Dementia Caregiver Intervention. J Cross Cult Gerontol. 2020;35(4):479‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Fields NL, Xu L, Richardson VE, Parekh R, Ivey D, Calhoun M. Utilizing the Senior Companion Program as a platform for a culturally informed caregiver intervention: Results from a mixed methods pilot study. Dementia (London). 2021;20(1):161‐187. [DOI] [PubMed] [Google Scholar]
- 480. Guest MA, Smith MP. In Our Community, Dementia Speaks: Pilot of a person‐centered training targeting African‐American caregivers of persons‐living with dementia (innovative practice). Dementia (London). 2021;20(1):391‐397. [DOI] [PubMed] [Google Scholar]
- 481. Withers M, Cortez‐Sanchez K, Herrera J, Ringman JM, Segal‐Gidan F. "My backpack is so heavy": Experiences of Latino caregivers of family with early‐onset Alzheimer's. J Am Geriatr Soc. 2021;69(6):1539‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Epps F, Heidbreder V, Alexander K, Tomlinson A, Freeman V, Williams N. A dementia‐friendly church: How can the African American church support families affected by dementia? Dementia (London). 2021;20(2):556‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Park VT, Grill JD, Zhu J, et al. Asian Americans and Pacific Islanders' perspectives on participating in the CARE recruitment research registry for Alzheimer's disease and related dementias, aging, and caregiving research. Alzheimers Dement (N Y). 2021;7(1):e12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Portacolone E, Palmer NR, Lichtenberg P, et al. Earning the Trust of African American Communities to Increase Representation in Dementia Research. Ethn Dis. 2020;30(Suppl 2):719‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Liu J, Lou Y, Wu B, Mui A. "I've been always strong to conquer any suffering:" challenges and resilience of Chinese American dementia caregivers in a life course perspective. Aging Ment Health. 2021;25(9):1716‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486. Bonds K, Song MK, Whitlatch CJ, Lyons KS, Kaye JA, Lee CS. Patterns of Dyadic Appraisal of Decision‐Making Involvement of African American Persons Living With Dementia. Gerontologist. 2021;61(3):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Epps F, Alexander K, Brewster GS, et al. Promoting dementia awareness in African‐American faith communities. Public Health Nurs. 2020;37(5):715‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Racine L, Ford H, Johnson L, Fowler‐Kerry S. An integrative review of Indigenous informal caregiving in the context of dementia care. J Adv Nurs. 2022;78(4):895‐917. [DOI] [PubMed] [Google Scholar]
- 489. Epps F, Moore M, Chester M, et al. The Alter Program: A nurse‐led, dementia‐friendly program for African American faith communities and families living with dementia. Nurs Adm Q. 2021;46(1):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Freedman VA, Patterson SE, Cornman JC, Wolff JL. A day in the life of caregivers to older adults with and without dementia: Comparisons of care time and emotional health. Alzheimers Dement. 2022;18(9):1650‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. National Alliance for Caregiving and AARP . Caregiving in the U.S. (2015 Report). Accessed December 15, 2023. Available at: https://www.aarp.org/content/dam/aarp/ppi/2015/caregiving‐in‐the‐united‐states‐2015‐report‐revised.pdf
- 492. Spillman BC, Freedman VA, Kasper JD, Wolff JL. Change over time in caregiving networks for older adults with and without dementia. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):1563‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Port CL, Zimmerman S, Williams CS, Dobbs D, Preisser JS, Williams SW. Families filling the gap: Comparing family involvement for assisted living and nursing home residents with dementia. Gerontologist. 2005;45(Special Issue 1):87‐95. [DOI] [PubMed] [Google Scholar]
- 494. Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long‐term care placement of dementia patients and caregiver health and well‐being. JAMA. 2004;292(8):961‐967. [DOI] [PubMed] [Google Scholar]
- 495. Rattinger GB, Schwartz S, Mullins CD, et al. Dementia severity and the longitudinal costs of informal care in the Cache County population. Alzheimers Dement. 2015;11(8):946‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496. Rattinger GB, Fauth EB, Behrens S, et al. Closer caregiver and care‐recipient relationships predict lower informal costs of dementia care: The Cache County Dementia Progression Study. Alzheimers Dement. 2016;12(8):917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Song M‐K, Paul S, Happ MB, Lea J, Pirkle JL, Jr. , Turberville‐Trujillo L. Informal Caregiving Networks of Older Adults With Dementia Superimposed on Multimorbidity: A Social Network Analysis Study. Innov Aging. 2023;7(4):igad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Wolff JL, Mulcahy J, Huang J, Roth DL, Covinsky K, Kasper JD. Family Caregivers of Older Adults, 1999‐2015: Trends in characteristics, circumstances, and role‐related appraisal. Gerontologist. 2018;58(6):1021‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Jutkowitz E, Gaugler JE, Trivedi AN, Mitchell LL, Gozalo P. Family caregiving in the community up to 8‐years after onset of dementia. BMC Geriatr. 2020;20(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Gaugler JE, Zarit SH, Pearlin LI. The onset of dementia caregiving and its longitudinal implications. Psychol Aging. 2003;18(2):171‐180. [DOI] [PubMed] [Google Scholar]
- 501. Jutkowitz E, Gozalo P, Trivedi A, Mitchell L, Gaugler JE. The effect of physical and cognitive impairments on caregiving. Med Care. 2020;58(7):601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Lei L, Maust DT, Leggett AN. Functional decline over time and change in family and other unpaid care provided to community‐dwelling older adults living with and without dementia. J Gerontol B Psychol Sci Soc Sci. 2023;78(10):1727‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Ornstein K, Gaugler JE. The problem with “problem behaviors”: A systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient‐caregiver dyad. Int Psychogeriatr. 2012;24(10):1536‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Vaingankar JA, Chong SA, Abdin E, et al. Psychiatric morbidity and its correlates among informal caregivers of older adults. Compr Psychiatry. 2016;68:178‐185. [DOI] [PubMed] [Google Scholar]
- 505. Feast A, Moniz‐Cook E, Stoner C, Charlesworth G, Orrell M. A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well‐being. Int Psychogeriatr. 2016;28(11):1761‐1774. [DOI] [PubMed] [Google Scholar]
- 506. Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215‐2260. [DOI] [PubMed] [Google Scholar]
- 507. Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta‐analysis. Psychol Bull. 2003;129(6):946‐972. [DOI] [PubMed] [Google Scholar]
- 508. Liu W, Gallagher‐Thompson D. Impact of dementia caregiving: Risks, strains, and growth. In: Qualls SH, Zarit SH, eds. Aging families and caregiving. Hoboken, NJ: John Wiley & Sons, Inc.; 2009: p. 85‐112. [Google Scholar]
- 509. Pinquart M, Sörensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta‐analysis. J Gerontol B Psychol Sci Soc Sci. 2003;58(2):112‐128. [DOI] [PubMed] [Google Scholar]
- 510. Sörensen S, Duberstein P, Gill D, Pinquart M. Dementia care: Mental health effects, intervention strategies, and clinical implications. Lancet Neurol. 2006;5(11):961‐973. [DOI] [PubMed] [Google Scholar]
- 511. Goren A, Montgomery W, Kahle‐Wrobleski K, Nakamura T, Ueda K. Impact of caring for persons with Alzheimer's disease or dementia on caregivers’ health outcomes: Findings from a community based survey in Japan. BMC Geriatr. 2016;16:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Alzheimer's Association . 2016 Alzheimer's Disease Facts and Figures. Alzheimer Dement. 2016;12(4):459‐509. [DOI] [PubMed] [Google Scholar]
- 513. Jones RW, Lebrec J, Kahle‐Wrobleski K, et al. Disease progression in mild dementia due to Alzheimer disease in an 18‐month observational study (GERAS): The impact on costs and caregiver outcomes. Dement Geriatr Cogn Dis Extra. 2017;20;7(1):87‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Leggett AN, Meyer OL, Bugajsky BC, Polenick CA. Accentuate the Positive: The association between informal and formal supports and caregiving gains. J Appl Gerontol. 2021l;40(7):763‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Quinn C, Toms G. Influence of positive aspects of dementia caregiving on caregivers' well‐being: A systematic review. Gerontologist. 2019;59(5):e584‐e596. [DOI] [PubMed] [Google Scholar]
- 516. Zarit SH. Positive aspects of caregiving: More than looking on the bright side. Aging Ment Health. 2012;16(6):673‐674. [DOI] [PubMed] [Google Scholar]
- 517. Cheng ST, Mak EP, Lau RW, Ng NS, Lam LC. Voices of Alzheimer caregivers on positive aspects of caregiving. Gerontologist. 2016;56(3):451‐460. [DOI] [PubMed] [Google Scholar]
- 518. Monin JK, Schulz R, Feeney BC. Compassionate love in individuals with Alzheimer's disease and their spousal caregivers: Associations with caregivers' psychological health. Gerontologist. 2015;55(6):981‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Roth DL, Dilworth‐Anderson P, Huang J, Gross AL, Gitlin LN. Positive aspects of family caregiving for dementia: Differential item functioning by race. J Gerontol B Psychol Sci Soc Sci. 2015;70(6):813‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Lloyd J, Patterson T, Muers J. The positive aspects of caregiving in dementia: A critical review of the qualitative literature. Dementia (London). 2016;15(6):1534‐1561. [DOI] [PubMed] [Google Scholar]
- 521. Yu DSF, Cheng ST, Wang J. Unravelling positive aspects of caregiving in dementia: An integrative review of research literature. Int J Nurs Stud. 2018;79:1‐26. [DOI] [PubMed] [Google Scholar]
- 522. Cheng ST. Positive aspects of caregiving attenuate the relationship between behavioral bother and anxiety and depressive symptoms in dementia family caregivers. Geriatr Gerontol Int. 2023;23(5):366‐370. [DOI] [PubMed] [Google Scholar]
- 523. Wennberg AM, Anderson LR, Cagnin A, Chen‐Edinboro LP, Pini L. How both positive and burdensome caregiver experiences are associated with care recipient cognitive performance: Evidence from the National Health and Aging Trends Study and National Study of Caregiving. Front Public Health. 2023;11:1130099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. van den Kieboom R, Snaphaan L, Mark R, Bongers I. The trajectory of caregiver burden and risk factors in dementia progression: A systematic review. J Alzheimers Dis. 2020;77(3):1107‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Polenick CA, Min L, Kales HC. Medical Comorbidities of dementia: Links to caregivers' emotional difficulties and gains. J Am Geriatr Soc. 2020;68(3):609‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Sheehan OC, Haley WE, Howard VJ, Huang J, Rhodes JD, Roth DL. Stress, Burden, and Well‐Being in Dementia and Nondementia Caregivers: Insights From the Caregiving Transitions Study. Gerontologist. 2021;61(5):670‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Sallim AB, Sayampanathan AA, Cuttilan A, Chun‐Man Ho R. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16(12):1034‐1041. [DOI] [PubMed] [Google Scholar]
- 528. Thunyadee C, Sitthimongkol Y, Sangon S, Chai‐Aroon T, Hegadoren KM. Predictors of depressive symptoms and physical health in caregivers of individuals with schizophrenia. J Nurs Health Sci. 2015;17:412‐429. [DOI] [PubMed] [Google Scholar]
- 529. Harris ML, Titler MG, Hoffman GJ. Associations between Alzheimer's disease and related dementias and depressive symptoms of partner caregivers. J Appl Gerontol. 2021;40(7):772‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530. Vitaliano PP, Ustundag O, Borson S. Objective and subjective cognitive problems among caregivers and matched non‐caregivers. Gerontologist. 2017;57(4):637‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Dassel KB, Carr DC, Vitaliano P. Does caring for a spouse with dementia accelerate cognitive decline? Findings from the Health and Retirement Study. Gerontologist. 2017;57(2):319‐328. [DOI] [PubMed] [Google Scholar]
- 532. Arthur PB, Gitlin LN, Kairalla JA, Mann WC. Relationship between the number of behavioral symptoms in dementia and caregiver distress: What is the tipping point? Int Psychogeriatr. 2018;30(8):1099‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Solimando L, Fasulo M, Cavallero S, Veronese N, Smith L, Vernuccio L. Suicide risk in caregivers of people with dementia: a systematic review and meta‐analysis. Aging Clin Exp Res. 2022;34(10):2255‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Ivey‐Stephenson AZ, Crosby AE, Hoenig JM, Gyawali S, Park‐Lee E, Hedden SL. Suicidal Thoughts and Behaviors Among Adults Aged ≥18 Years — United States, 2015–2019. MMWR Surveill Summ. 2022;71(No. SS‐1):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Gillespie R, Mullan J, Harrison L. Managing medications: The role of informal caregivers of older adults and people living with dementia: A review of the literature. J Clin Nurs. 2014;23(23‐24):3296‐3308. [DOI] [PubMed] [Google Scholar]
- 536. Alsaeed D, Jamieson E, Gul MO, Smith FJ. Challenges to optimal medicines use in people living with dementia and their caregivers: A literature review. Int J Pharm. 2016;512(2):396‐404. [DOI] [PubMed] [Google Scholar]
- 537. Polenick CA, Stanz SD, Leggett AN, Maust DT, Hodgson NA, Kales HC. Stressors and resources related to medication management: Associations with spousal caregivers' role overload. Gerontologist. 2020;60(1):165‐173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Aston L, Hilton A, Moutela T, Shaw R, Maidment I. Exploring the evidence base for how people with dementia and their informal carers manage their medication in the community: A mixed studies review. BMC Geriatr. 2017;17(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Liu C, Fabius CD, Howard VJ, Haley WE, Roth DL. Change in Social Engagement among Incident Caregivers and Controls: Findings from the Caregiving Transitions Study. J Aging Health. 2021;33(1‐2):114‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Anderson JG, Jabson Tree JM, Flatt JD, Gross AL, Williams IC, Rose KM. A comparative analysis of family quality of life between heterosexual and sexual minority caregivers of people with dementia. J Appl Gerontol. 2022;41(6):1576‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Lee J, Baik S, Becker TD, Cheon JH. Themes describing social isolation in family caregivers of people living with dementia: A scoping review. Dementia (London). 2022;21(2):701‐721. [DOI] [PubMed] [Google Scholar]
- 542. Alhasan DM, Hirsch JA, Jackson CL, Miller MC, Cai B, Lohman MC. Neighborhood Characteristics and the Mental Health of Caregivers Cohabiting with Care Recipients Diagnosed with Alzheimer's Disease. Int J Environ Res Public Health. 2021;18(3):913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Vetter M, Donelan K, Guzikowski S, et al. The needs of family caregivers of persons living with dementia cared for in primary care practices. J Eval Clin Pract. 2023;29(8):1243‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Burgdorf JG, Amjad H. Impact of diagnosed (vs undiagnosed) dementia on family caregiving experiences. J Am Geriatr Soc. 2023;71(4):1236‐1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545. Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Clinically significant changes in burden and depression among dementia caregivers following nursing home admission. BMC Medicine. 2010;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546. Mausbach BT, Chattillion EA, Ho J, et al. Why does placement of persons with Alzheimer's disease into long‐term care improve caregivers’ well‐being? Examination of psychological mediators. Psychol Aging. 2014;29(4):776‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547. Lee K, Chung J, Meyer KN, Dionne‐Odom JN. Unmet needs and health‐related quality of life of dementia family caregivers transitioning from home to long‐term care: A scoping review. Geriatr Nurs. 2022;43:254‐264. [DOI] [PubMed] [Google Scholar]
- 548. Peacock SC. The experience of providing end‐of‐life care to a relative with advanced dementia: An integrative literature review. Palliat Support Care. 2013;11(2):155‐168. [DOI] [PubMed] [Google Scholar]
- 549. Schulz R, Mendelsohn AB, Haley WE, et al. End‐of‐life care and the effects of bereavement on family caregivers of persons with dementia. N Engl J Med. 2003;349(20):1936‐1942. [DOI] [PubMed] [Google Scholar]
- 550. Kumar V, Ankuda CK, Aldridge MD, Husain M, Ornstein KA. Family Caregiving at the End of Life and Hospice Use: A national study of Medicare beneficiaries. J Am Geriatr Soc. 2020;68(10):2288‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26(5):725‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552. Parker LJ, Fabius C, Rivers E, Taylor JL. Is Dementia‐Specific Caregiving Compared With Non‐Dementia Caregiving Associated With Physical Difficulty Among Caregivers for Community‐Dwelling Adults? J Appl Gerontol. 2022;41(4):1074‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Peng H‐L, Chang Y‐P. Sleep disturbance in family caregivers of individuals with dementia: A review of the literature. Perspect Psychiatr C. 2012;49(2):135‐146. [DOI] [PubMed] [Google Scholar]
- 554. Gao C, Chapagain NY, Scullin MK. Sleep Duration and Sleep Quality in caregivers of patients with dementia: A systematic review and meta‐analysis. JAMA Netw Open. 2019;2(8):e199891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555. Liu Y, Song Y, Johnson FU, et al. Characteristics and predictors of sleep among spousal care dyads living with chronic conditions. J Gerontol B Psychol Sci Soc Sci. 2023;78(Suppl 1):S38‐S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556. Brewster GS, Wang D, McPhillips MV, Epps F, Yang I. Correlates of Sleep Disturbance Experienced by Informal Caregivers of Persons Living with Dementia: A Systematic Review. Clin Gerontol. 2022;1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557. Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11(2):217‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: Determinants of self‐rated health in spousal dementia caregivers. BMC Geriatr. 2019;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559. Dassel KB, Carr DC. Does dementia caregiving accelerate frailty? Findings from the Health and Retirement Study. Gerontologist. 2016;56(3):444‐450. [DOI] [PubMed] [Google Scholar]
- 560. Fredman L, Bertrand RM, Martire LM, Hochberg M, Harris EL. Leisure‐time exercise and overall physical activity in older women caregivers and non‐caregivers from the Caregiver‐SOF Study. Prev Med. 2006;43:226‐269. [DOI] [PubMed] [Google Scholar]
- 561. Secinti E, Wu W, Kent EE, Demark‐Wahnefried W, Lewson AB, Mosher CE. Examining Health Behaviors of Chronic Disease Caregivers in the U.S. Am J Prev Med. 2022;62(3):e145‐e158. [DOI] [PubMed] [Google Scholar]
- 562. Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the Caregiver Health Effects Study. Psychol Aging. 2000;15(2):259‐271. [DOI] [PubMed] [Google Scholar]
- 563. von Kanel R, Mausbach BT, Dimsdale JE, et al. Effect of chronic dementia caregiving and major transitions in the caregiving situation on kidney function: A longitudinal study. Psychosom Med. 2012;74(2):214‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Kiecolt‐Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345‐362. [DOI] [PubMed] [Google Scholar]
- 565. Kiecolt‐Glaser JK, Marucha PT, Mercado AM, Malarkey WB, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194‐1196. [DOI] [PubMed] [Google Scholar]
- 566. Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler I. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418‐435. [DOI] [PubMed] [Google Scholar]
- 567. Mausbach BT, Romero‐Moreno R, Bos T, et al. Engagement in pleasant leisure activities and blood pressure: A 5‐year longitudinal study in Alzheimer caregivers. Psychosom Med. 2017;79(7):735‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568. Shaw WS, Patterson TL, Ziegler MG, Dimsdale JE, Semple SJ, Grant I. Accelerated risk of hypertensive blood pressure recordings among Alzheimer caregivers. J Psychosom Res. 1999;46(3):215‐227. [DOI] [PubMed] [Google Scholar]
- 569. Mausbach BT, Roepke SK, Ziegler MG, et al. Association between chronic caregiving stress and impaired endothelial function in the elderly. J Am Coll Cardiol. 2010;55(23):2599‐2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Allen AP, Curran EA, Duggan Á, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: Focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123‐164. [DOI] [PubMed] [Google Scholar]
- 571. Roth DL, Sheehan OC, Haley WE, Jenny NS, Cushman M, Walston JD. Is family caregiving associated with inflammation or compromised immunity? A meta‐analysis. Gerontologist. 2019;59(5):e521‐e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572. Roth DL, Haley WE, Sheehan OC, et al. The transition to family caregiving and its effect on biomarkers of inflammation. Proc Natl Acad Sci USA. 2020;117(28):16258‐16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Schubert CC, Boustani M, Callahan CM, Perkins AJ, Hui S, Hendrie HC. Acute care utilization by dementia caregivers within urban primary care practices. J Gen Intern Med. 2008;23(11):1736‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. Zhu CW, Scarmeas N, Ornstein K, et al. Health‐care use and cost in dementia caregivers: Longitudinal results from the Predictors Caregiver Study. Alzheimers Dement. 2015;11(4):444‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Meyer K, Gassoumis Z, Wilber K. The Differential Effects of Caregiving Intensity on Overnight Hospitalization. West J Nurs Res. 2022;44(6):528‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576. Leggett AN, Sonnega AJ, Lohman MC. Till death do us part: Intersecting health and spousal dementia caregiving on caregiver mortality. J Aging Health. 2020;32(7‐8):871‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: A reappraisal from population‐based studies. Gerontologist. 2015;55(2):309‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578. Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. N Engl J Med. 2006;354:719‐730. [DOI] [PubMed] [Google Scholar]
- 579. Perkins M, Howard VJ, Wadley VG, et al. Caregiving strain and all‐cause mortality: Evidence from the REGARDS Study. J Gerontol B Psychol Sci Soc Sci. 2013;68(4):504‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580. Gaugler JE, Jutkowitz E, Peterson CM, Zmora R. Caregivers dying before care recipients with dementia. Alzheimers Dement (NY). 2018;4:688‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581. Kelley AS, McGarry K, Bollens‐Lund E, et al. Residential setting and the cumulative financial burden of dementia in the 7 years before death. J Am Geriatr Soc. 2020;68(6):1319‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582. AARP, Family Caregiving and Out‐of‐Pocket Costs: 2016 Report . Accessed December 15, 2023. Available at: https://www.aarp.org/content/dam/aarp/research/surveys_statistics/ltc/2016/family‐caregiving‐costs‐fact‐sheet.doi.10.26419%252Fres.00138.002.pdf
- 583. Albert SM. Are Medical Care Expenses Higher for Spouses Who Provide Dementia Care? Am J Geriatr Psychiatry. 2021;29(5):476‐477. [DOI] [PubMed] [Google Scholar]
- 584. Chu J, Benjenk I, Chen J. Incremental Health Care Expenditures of the Spouses of Older Adults With Alzheimer's Diseases and Related Dementias (ADRD). Am J Geriatr Psychiatry. 2021;29(5):462‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585. Stall NM, Kim SJ, Hardacre KA, et al. Association of informal caregiver distress with health outcomes of community‐dwelling dementia care recipients: A systematic review. J Am Geriatr So. 2019;67(3):609‐617. [DOI] [PubMed] [Google Scholar]
- 586. Amjad H, Mulcahy J, Kasper JD, et al. Do caregiving factors affect hospitalization risk among disabled older adults? J Am Geriatr Soc. 2021;69(1):129‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587. Sullivan SS, de Rosa C, Li CS, Chang YP. Dementia caregiver burdens predict overnight hospitalization and hospice utilization. Palliat Support Care. 2022;1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Hennelly N, Cooney A, Houghton C, O'Shea E. Personhood and Dementia Care: A Qualitative Evidence Synthesis of the Perspectives of People With Dementia. Gerontologist. 2021;61(3):e85‐e100. [DOI] [PubMed] [Google Scholar]
- 589. Cheng S‐K, Li K‐K, Or PPL, Losada A. Do caregiver interventions improve outcomes in relatives with dementia and mild cognitive impairment? A comprehensive systematic review and meta‐analysis. Psychol Aging. 2022;37(8):929‐953. [DOI] [PubMed] [Google Scholar]
- 590. Gaugler JE, Jutkowitz E, Shippee TP, Brasure M. Consistency of dementia caregiver intervention classification: An evidence‐based synthesis. Int Psychogeriatr. 2017;29(1):19‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591. Gitlin LN, Hodgson N. Caregivers as therapeutic agents in dementia care: The evidence‐base for interventions supporting their role. In: Gaugler JE, Kane RL, eds. Family caregiving in the new normal. Philadelphia, Pa.: Elsevier, Inc.; 2015: p. 305‐356. [Google Scholar]
- 592. Liew TM, Lee CS. Reappraising the efficacy and acceptability of multicomponent interventions for caregiver depression in dementia: The utility of network meta‐analysis. Gerontologist. 2019;16;59(4):e380‐e392. [DOI] [PubMed] [Google Scholar]
- 593. Williams F, Moghaddam N, Ramsden S, De Boos D. Interventions for reducing levels of burden amongst informal carers of persons with dementia in the community. A systematic review and meta‐analysis of randomised controlled trials. Aging Ment Health. 2019;23(12):1629‐1642. [DOI] [PubMed] [Google Scholar]
- 594. Kaddour L, Kishita N, Schaller A. A meta‐analysis of low‐intensity cognitive behavioral therapy‐based interventions for dementia caregivers. Int Psychogeriatr. 2018:1‐16. [DOI] [PubMed] [Google Scholar]
- 595. Nguyen H, Terry D, Phan H, Vickers J, McInerney F. Communication training and its effects on carer and care‐receiver outcomes in dementia settings: A systematic review. J Clin Nurs. 2019;28(7‐8):1050‐1069. [DOI] [PubMed] [Google Scholar]
- 596. Jütten LH, Mark RE, Wicherts JM, Sitskoorn MM. The effectiveness of psychosocial and behavioral interventions for informal dementia caregivers: Meta‐analyses and meta‐regressions. J Alzheimers Dis. 2018;66(1):149‐172. [DOI] [PubMed] [Google Scholar]
- 597. Maslow K. Translating Innovation to Impact: Evidence‐Based Interventions to Support People with Alzheimer's Disease and their Caregiver at Home and in the Community. Washington, D.C.: Administration on Aging; 2012. Accessed December 15, 2023. Available at: https://www.agingresearch.org/app/uploads/2017/12/50820Compliant20AoA‐White‐Paper20for20Release.pdf [Google Scholar]
- 598. Rosalynn Carter Institute for Caregiving . Accessed December 15, 2023. Available at: https://www.rosalynncarter.org/
- 599. Larson EB, Stroud C. Meeting the Challenge of Caring for Persons Living With Dementia and Their Care Partners and Caregivers: A Report From the National Academies of Sciences, Engineering, and Medicine. JAMA. 2021;325(18):1831‐1832. [DOI] [PubMed] [Google Scholar]
- 600. Cheng ST, Li KK, Or PPL, Losada A. Do caregiver interventions improve outcomes in relatives with dementia and mild cognitive impairment? A comprehensive systematic review and meta‐analysis. Psychol Aging. 2022;37(8):929‐953. [DOI] [PubMed] [Google Scholar]
- 601. Cheng S‐T, Li K‐K, Losada A, et al. The effectiveness of nonpharmacological interventions for informal dementia caregivers: An updated systematic review and meta‐analysis. Psychol Aging. 2020;35(1):55‐77. [DOI] [PubMed] [Google Scholar]
- 602. Walter E, Pinquart M. How Effective Are Dementia Caregiver Interventions? An Updated Comprehensive Meta‐Analysis. Gerontologist. 2020;60(8):609‐619. [DOI] [PubMed] [Google Scholar]
- 603. Gitlin LN, Jutkowitz E, Gaugler JE. Dementia caregiver intervention research now and into the future: Review and recommendations. Washington, D.C.: Commissioned paper for the National Academies of Science, Engineering and Medicine NIA Decadal Study. Accessed December 15, 2023. Available at: https://sites.nationalacademies.org/cs/groups/dbassesite/documents/webpage/dbasse_198208.pdf [Google Scholar]
- 604. Lee M, Ryoo JH, Chung M, Anderson JG, Rose K, Williams IC. Effective interventions for depressive symptoms among caregivers of people with dementia: A systematic review and meta‐analysis. Dementia (London). 2020;19(7):2368‐2398. [DOI] [PubMed] [Google Scholar]
- 605. Cheng S‐T, Zhang F. A comprehensive meta‐review of systematic reviews and meta‐analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatr. 2020;20(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606. Perales‐Puchalt J, Barton K, Ptomey L, et al. Effectiveness of "Reducing Disability in Alzheimer's Disease" Among Dyads With Moderate Dementia. J Appl Gerontol. 2021;40(10):1163‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607. Bass DM, Hornick T, Kunik M, et al. Findings from a real‐world translation study of the evidence‐based "Partners in Dementia Care". Innov Aging. 2019;3(3):igz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608. Hodgson N, Gitlin LN (in press). Implementing and sustaining family care programs in real world settings: Barriers and facilitators. In Gaugler J. E. (Ed.), Bridging the Family Care Gap. Academic Press: San Diego, CA. [Google Scholar]
- 609. Fauth EB, Jackson MA, Walberg DK, et al. External validity of the New York University Caregiver Intervention: Key caregiver outcomes across multiple demonstration projects. J Appl Gerontol. 2019;38(9):1253‐1281. [DOI] [PubMed] [Google Scholar]
- 610. Hodgson NA, Petrovsky DV, Finegan K, Kallmyer BA, Pike J, Fazio S. One call makes a difference: An evaluation of the Alzheimer's Association National Helpline on dementia caregiver outcomes. Patient Educ Couns. 2021;104(4):896‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611. Reuben DB, Evertson LC, Jackson‐Stoeckle R, Epstein‐Lubow G, Spragens LH, Haggerty KL. Dissemination of a successful dementia care program: Lessons to facilitate spread of innovations. J Am Geriatr Soc. 2022;70(9):2686‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612. Gitlin LN, Roth DL, Marx K, et al. Embedding caregiver support within adult day services: Outcomes of a multi‐site trial. Gerontologist. 2023:gnad107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. Boustani M, Alder CA, Solid CA. Agile implementation: A blueprint for implementing evidence‐based healthcare solutions. J Am Geriatr Soc. 2018;66(7):1372‐1376. [DOI] [PubMed] [Google Scholar]
- 614. Boots LM, de Vugt ME, van Knippenberg RJ, Kempen GI, Verhey FR. A systematic review of internet‐based supportive interventions for caregivers of patients with dementia. Int J Geriatr Psych. 2015;29(4):331‐344. [DOI] [PubMed] [Google Scholar]
- 615. Griffiths PC, Whitney MK, Kovaleva M, Hepburn K. Development and implementation of tele‐savvy for dementia caregivers: A Department of Veterans Affairs Clinical Demonstration Project. Gerontologist. 2016;56(1):145‐154. [DOI] [PubMed] [Google Scholar]
- 616. Gaugler JE, Zmora R, Mitchell LL, et al. Six‐month effectiveness of remote activity monitoring for persons living with dementia and their family caregivers: An experimental mixed methods study. Gerontologist. 2019;59(1):78‐89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Waller A, Dilworth S, Mansfield E, Sanson‐Fisher R. Computer and telephone delivered interventions to support caregivers of people with dementia: A systematic review of research output and quality. BMC Geriatr. 2017;17(1):265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Hopwood J, Walker N, McDonagh L, et al. Internet‐based interventions aimed at supporting family caregivers of people with dementia: Systematic review. J Med Internet Res. 2018;20(6):e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619. Leng M, Zhao Y, Xiao H, Li C, Wang Z. Internet‐based supportive interventions for family caregivers of people with dementia: Systematic review and meta‐analysis. J Med Internet Res. 2020;22(9):e19468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620. Pleasant M, Molinari V, Dobbs C, Meng H, Hyer K. Effectiveness of online dementia caregivers training programs: A systematic review. Geriatr Nurs. 2020;S0197‐4572(20):30209‐30213. [DOI] [PubMed] [Google Scholar]
- 621. Etxeberria I, Salaberria K, Gorostiaga A. Online support for family caregivers of people with dementia: a systematic review and meta‐analysis of RCTs and quasi‐experimental studies. Aging Ment Health. 2021;25(7):1165‐1180. [DOI] [PubMed] [Google Scholar]
- 622. Saragih ID, Tonapa SI, Porta CM, Lee BO. Effects of telehealth intervention for people with dementia and their carers: A systematic review and meta‐analysis of randomized controlled studies. J Nurs Schol. 2022;54(6):704‐719. [DOI] [PubMed] [Google Scholar]
- 623. Fortinsky RH, Gitlin LN, Pizzi LT, et al. Effectiveness of the care of persons with dementia in their environments intervention when embedded in a publicly funded home‐ and community‐based service program. Innov Aging. 2020;4(6):igaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624. Haggerty KL, Campetti R, Stoeckle RJ, Epstein‐Lubow G, Evertson LC, Spragens L. Dissemination of a successful dementia care program: Lessons from early adopters. J Am Geriatr Soc. 2022;70(9):2677‐2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625. Maslow K, Bass DM, Rentsch JH. Update on the status of effective programs to help dementia family caregivers in the United States: Observations from the search for programs to include in Best Practice Caregiving. In Gaugler JE, ed. Bridging the Family Care Gap. Academic Press; 2021:247‐302. [Google Scholar]
- 626. Gaugler JE, Potter T, Pruinelli L. Partnering with caregivers. Clin Geriatr Med. 2014;30(3):493‐515. [DOI] [PubMed] [Google Scholar]
- 627. Gitlin LN, Marx K, Stanley IH, Hodgson N. Translating evidence‐based dementia caregiving interventions into practice: State‐of‐the‐science and next steps. Gerontologist. 2015;55(2):210‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628. Wethington E, Burgio LD. Translational research on caregiving: Missing links in the translation process. In: Gaugler JE, Kane RL, eds. Family caregiving in the new normal. Philadelphia, Pa.: Elsevier, Inc; 2015: p. 193‐210. [Google Scholar]
- 629. Zarit SH. Past is prologue: How to advance caregiver interventions. Aging Ment Health. 2017;16:1‐6. [DOI] [PubMed] [Google Scholar]
- 630. Gonella S, Mitchell G, Bavelaar L, Conti A, Vanalli M, Basso I. Interventions to support family caregivers of people with advanced dementia at the end of life in nursing homes: A mixed‐methods systematic review. Palliat Med. 2022;36(2):268‐291. [DOI] [PubMed] [Google Scholar]
- 631. Kishita N, Hammond L, Dietrich CM, Mioshi E. Which interventions work for dementia family carers?: an updated systematic review of randomized controlled trials of carer interventions. Int Psychogeriatr. 2018;30(11):1679‐1696. [DOI] [PubMed] [Google Scholar]
- 632. Zarit SH, Lee JE, Barrineau MJ, Whitlatch CJ, Femia EE. Fidelity and acceptability of an adaptive intervention for caregivers: An exploratory study. Aging Ment Health. 2013;17(2):197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633. Van Mierlo LD, Meiland FJ, Van Hout HP, Dröes RM. Toward an evidence‐based implementation model and checklist for personalized dementia care in the community. Int Psychogeriatr. 2016;28(5):801‐813. [DOI] [PubMed] [Google Scholar]
- 634. Gaugler JE, Reese M, Tanler R. Care to Plan: An online tool that offers tailored support to dementia caregivers. Gerontologist. 2016;56(6):1161‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. Jennings LA, Ramirez KD, Hays RD, Wenger NS, Reuben DB. Personalized goal attainment in dementia care: Measuring what persons with dementia and their caregivers want. J Am Geriatr Soc. 2018;66(11):2120‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636. Whitlatch CJ, Orsulic‐Jeras S. Meeting the informational, educational, and psychosocial support needs of persons living with dementia and their family caregivers. Gerontologist. 2018;58(suppl_1):S58‐S73. [DOI] [PubMed] [Google Scholar]
- 637. Akarsu NE, Prince MJ, Lawrence VC, Das‐Munshi J. Depression in carers of people with dementia from a minority ethnic background: Systematic review and meta‐analysis of randomised controlled trials of psychosocial interventions. Int J Geriatr Psychiatry. 2019;34(6):790‐806. [DOI] [PubMed] [Google Scholar]
- 638. Llanque SM, Enriquez M. Interventions for Hispanic caregivers of patients with dementia: A review of the literature. Am J Alzheimers Dis Other Demen. 2012;27(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639. Napoles AM, Chadiha L, Eversley R, Moreno‐John G. Reviews: Developing culturally sensitive dementia caregiver interventions: Are we there yet? Am J Alzheimers Dis Other Dement. 2010;25:389‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640. Luchsinger JA, Burgio L, Mittelman M, et al. Comparative effectiveness of 2 interventions for Hispanic caregivers of persons with dementia. J Am Geriatr Soc. 2018;66(9):1708‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641. U.S. Department of Health and Human Services . National Research Summit on Care, Services and Supports for Persons with Dementia and their Caregivers. Accessed December 15, 2023. Available at: https://aspe.hhs.gov/national‐research‐summit‐care‐services‐and‐supports‐persons‐dementia‐and‐their‐caregivers [Google Scholar]
- 642. Young HM, Bell JF, Whitney RL, Ridberg RA, Reed SC, Vitaliano PP. Social determinants of health: Underreported heterogeneity in systematic reviews of caregiver interventions. Gerontologist. 2020;60(Suppl 1):S14‐S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643. Brewster GS, Epps F, Dye CE, Hepburn K, Higgins MK, Parker ML. The effect of the "Great Village" on psychological outcomes, burden, and mastery in African American caregivers of persons living with dementia. J Appl Gerontol. 2020;39(10):1059‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644. Demanes A, Ward KT, Wang AT, Hess M. Systematic Review of Dementia Support Programs with Multicultural and Multilingual Populations. Geriatrics (Basel). 2021;7(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645. Di Lorito C, Bosco A, Peel E, Hinchliff S, Dening T, Calasanti T. Are dementia services and support organisations meeting the needs of Lesbian, Gay, Bisexual and Transgender (LGBT) caregivers of LGBT people living with dementia? A scoping review of the literature. Aging Ment Health. 2022;26(10):1912‐1921. [DOI] [PubMed] [Google Scholar]
- 646. Kittle KR, Lee R, Pollock K, et al. Feasibility of the Savvy Caregiver Program for LGBTQ+ Caregivers of people living with Alzheimer's disease and related dementias. Int J Environ Res Pub Health. 2022;19(22):15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647. Alzheimer's Association . Alzheimer's Association Dementia Care Practice Recommendations. Accessed December 15, 2023. Available at: https://www.alz.org/media/Documents/alzheimers‐dementia‐care‐practice‐recommendations.pdf
- 648. Camp CJ. Denial of human rights: We must change the paradigm of dementia care. Clin Gerontol. 2019;42(3):221‐223. [DOI] [PubMed] [Google Scholar]
- 649. Gaugler JE, Bain LJ, Mitchell L, et al. Reconsidering frameworks of Alzheimer's dementia when assessing psychosocial outcomes. Alzheimers Dement (NY). 2019;5:388‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650. Burton A, Ogden M, Cooper C. Planning and enabling meaningful patient and public involvement in dementia research. Curr Opin Psychiatry. 2019;32(6):557‐562. [DOI] [PubMed] [Google Scholar]
- 651. The Lewin Group . Process Evaluation of the Older Americans Act Title IIIE‐National Family Caregiver Support Program: Final Report, 2016. Accessed December 15, 2023. Available at: https://acl.gov/sites/default/files/programs/2017‐02/NFCSP_Final_Report‐update.pdf [Google Scholar]
- 652. Stone RI. Factors affecting the future of family caregiving in the United States. In: Gaugler JE, Kane RL, eds. Family Caregiving in the New Normal. San Diego, CA: Elsevier, Inc; 2015: p. 57‐77. [Google Scholar]
- 653. Gaugler JE. Supporting family care for older adults: Building a better bridge. In Gaugler J. E. (Ed.). Bridging the Family Care Gap. Academic Press; 2021: p. 427‐452. [Google Scholar]
- 654. Gaugler JE, Borson S, Epps F, Shih RA, Parker LJ, McGuire LC. The intersection of social determinants of health and family care of people living with Alzheimer's disease and related dementias: A public health opportunity. Alzheimers Dement. 2023. doi:10.1002/alz.13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655. Greenberg NE, Wallick A, Brown LM. Impact of COVID‐19 pandemic restrictions on community‐dwelling caregivers and persons with dementia. Psychol Trauma. 2020;12(S1):S220‐S221. [DOI] [PubMed] [Google Scholar]
- 656. Carbone EA, de Filippis R, Roberti R, Rania M, Destefano L, Russo E. The Mental Health of Caregivers and Their Patients With Dementia During the COVID‐19 Pandemic: A Systematic Review. Front Psychol. 2021;12:782833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657. Gaigher JM, Lacerda IB, Dourado MCN. Dementia and Mental Health During the COVID‐19 Pandemic: A Systematic Review. Front Psychiatry. 2022;13:879598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658. Oliver S, Alexander K, Bennett SG, Hepburn K, Henry J, Clevenger CK. Experiences of Black American Dementia Caregivers During the COVID‐19 Pandemic. J Fam Nurs. 2022;28(3):195‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659. Perales‐Puchalt J, Peltzer J, Fracachan‐Cabrera M, Perez A, Ramirez‐Mantilla M, Greiner KA. Impact of the COVID‐19 pandemic on Latino families with Alzheimer's disease and related dementias: Perceptions of family caregivers and primary care providers. medRxiv 2022;2022.05.25.22275517.
- 660. Masoud S, Glassner AA, Mendoza M, Rhodes S, White CL. "A Different Way to Survive": The Experiences of Family Caregivers of Persons Living With Dementia During the COVID‐19 Pandemic. J Fam Nurs. 2022;28(3):243‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661. Turner RL, Reese‐Melancon C, Harrington EE, Andreo M. Caregiving during the COVID‐19 pandemic: Factors associated with feelings of caregiver preparedness. J Appl Gerontol. 2023;42(10):2089‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662. Humber MB, Yefimova M, Lessios AS, Trivedi RB, Sheffrin M, Martin M. "It isn't the same": Experiences of informal caregivers of older adults enrolled in a home‐based senior care program during COVID‐19. J Gerontol Nurs. 2023;49(3):19‐26. [DOI] [PubMed] [Google Scholar]
- 663. Shrestha P, Fick DM, Boltz M, Loeb SJ, High AC. Caregiving for persons living with dementia during the COVID‐19 pandemic: Perspectives of family care partners. J Gerontol Nurs. 2023;49(3):27‐33. [DOI] [PubMed] [Google Scholar]
- 664. Arthur P, Li CY, Southern Indiana Dementia Workgroup. Living with dementia during the COVID‐19 pandemic: A nationwide survey informing the American experience. J Alzheimers Dis Rep. 2022;6(1):733‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665. Chyu J, Cantu P, Mehta N, Markides K. Caregiving for people with dementia or cognitive impairment during the COVID‐19 pandemic: A review. Gerontol Geriatr Med. 2022;8:23337214221132369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666. Friedman EM, Kirkegaard A, Kennedy DP, Edgington S, Shih RA. Change in Caregiving to Older Adults During the COVID‐19 Pandemic: Differences by Dementia Status. J Appl Gerontol. 2023;7334648231197514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Macchi ZA, Ayele R, Dini M, et al. Lessons from the COVID‐19 pandemic for improving outpatient neuropalliative care: A qualitative study of patient and caregiver perspectives. Palliat Med. 2021;35(7):1258‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668. Hwang Y, Connell LM, Rajpara AR, Hodgson NA. Impact of COVID‐19 on Dementia Caregivers and Factors Associated With their Anxiety Symptoms. Am J Alzheimers Dis Other Demen. 2021;36:15333175211008768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669. Savla J, Roberto KA, Blieszner R, McCann BR, Hoyt E, Knight AL. Dementia caregiving during the "stay‐at‐home" phase of COVID‐19 pandemic. J Gerontol B Psychol Sci Soc Sci. 2021;76(4):e241‐e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670. Kusmaul N, Miller VJ, Cheon JH. "They Just Took Him Out of My Life": Nursing Home Care Partner Experiences During the COVID‐19 Pandemic. J Gerontol Nurs. 2022;48(2):7‐11. [DOI] [PubMed] [Google Scholar]
- 671. Mitchell LL, Albers EA, Birkeland RW, et al. Caring for a relative with dementia in long‐term care during COVID‐19. J Am Med Dir Assoc. 2022;23(3):428‐433.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Brungardt A, Cassidy J, LaRoche A, et al. End‐of‐Life Experiences Within a Dementia Support Program During COVID‐19: Context and Circumstances Surrounding Death During the Pandemic. Am J Hosp Palliat Care. 2022;10499091221116140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673. Hunt JFV, Le Caire TJ, Schroeder M, O'Toole Smith K, Marschall K, Walaszek A. The use of health care and community‐based services by people living with dementia and their caregivers during the COVID‐19 pandemic. WMJ. 2022;121(3):226‐230. [PMC free article] [PubMed] [Google Scholar]
- 674. Gaugler JE. Our vast family care system for the elderly is at risk of collapse. Accessed December 15, 2023. Available at: https://www.startribune.com/our‐vast‐family‐care‐system‐for‐the‐elderly‐is‐about‐to‐collapse/572221182/
- 675. Sadarangani T, Zhong J, Vora P, Missaelides L. "Advocating Every Single Day" so as Not to be Forgotten: Factors Supporting Resiliency in Adult Day Service Centers Amidst COVID‐19‐Related Closures. J Gerontol Soc Work. 2021;64(3):291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676. Gaugler JE, Marx K, Dabelko‐Schoeny H, et al. COVID‐19 and the Need for Adult Day Services. J Am Med Dir Assoc. 2021;22(7):1333‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677. Monin JK, Ali T, Syed S, et al. Family communication in long‐term care during a pandemic: Lessons for enhancing emotional experiences. Am J Geriatr Psychiatry. 2020;S1064‐7481(20):30478‐30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678. Pickering CEZ, Maxwell CD, Yefimova M, Wang D, Puga F, Sullivan T. Early Stages of COVID‐19 Pandemic Had No Discernable Impact on Risk of Elder Abuse and Neglect Among Dementia Family Caregivers: A Daily Diary Study. J Fam Violence. 2022;5:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679. Albers EA, Mikal J, Millenbah A, Finlay J, Jutkowitz E, Mitchell L. The Use of Technology Among Persons With Memory Concerns and Their Caregivers in the United States During the COVID‐19 Pandemic: Qualitative Study. JMIR Aging. 2022;5(1):e31552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Yoon SO, Paek EJ. Video call usage in older adults with or without dementia impacted by the COVID‐19 pandemic. Am J Alzheimers Dis Other Demen. 2023;38:15333175231160679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. O'Connor MK, Nicholson R, Epstein C, et al. Telehealth support for dementia caregivers during the COVID‐19 pandemic: Lessons learned from the NYU family support program. Am J Geriatr Psychiatry. 2023;31(1):14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682. Yan K, Sadler T, Brauner D, Pollack HA, Konetzka RT. Caregiving for older adults with dementia during the time of COVID‐19: A multi‐state exploratory qualitative study. J Appl Gerontol. 2023;42(10):2078‐2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683. Nkodo J‐A, Gana W, Debacq C, et al. The Role of Telemedicine in the Management of the Behavioral and Psychological Symptoms of Dementia: A Systematic Review. Am J Geriatr Psychiatry. 2022;30(10):1135‐1150. [DOI] [PubMed] [Google Scholar]
- 684. Liang J, Aranda MP. The use of telehealth among people living with dementia‐caregiver dyads during the COVID‐19 pandemic: Scoping review. J Med Internet Res. 2023;25:e45045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685. Lloyd SL, Caban‐Holt A, Starks TD, Clark JC, Byrd GS. Assessing gender differences on the impact of COVID‐19 on the medical and social needs of dementia caregivers. J Gerontol Soc Work. 2023 Aug 14;1‐16. [DOI] [PubMed] [Google Scholar]
- 686. National Institute on Aging . NIA COVID‐19 Response. Accessed December 4, 2023. Available at: https://www.nia.nih.gov/research/grants‐funding/nia‐covid‐19‐response
- 687. Weems JA, Rhodes S, Powers JS. Dementia Caregiver Virtual Support‐An Implementation Evaluation of Two Pragmatic Models during COVID‐19. Geriatrics (Basel). 2021;6(3):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688. Masoud SS, Meyer KN, Sweet LM, Prado PJ, White CL. "We Don't Feel so Alone": A Qualitative Study of Virtual Memory Cafés to Support Social Connectedness Among Individuals Living With Dementia and Care Partners During COVID‐19. Front Public Health. 2021;9:660144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Administration for Community Living . 2022 National Strategy to Support Family Caregivers. Accessed December 15, 2023. Available at: https://acl.gov/sites/default/files/RAISE_SGRG/NatlStrategyToSupportFamilyCaregivers.pdf
- 690. U.S. Centers for Medicare & Medicaid Services. Guiding an Improved Dementia Experience (GUIDE) Model . Accessed December 4, 2023. Available at: https://www.cms.gov/priorities/innovation/innovation‐models/guide
- 691. American Public Health Association . (2020)Strengthening the dementia care workforce: A public health priority. Available at: https://www.apha.org/policies‐and‐advocacy/public‐health‐policy‐statements/policy‐database/2021/01/13/strengthening‐the‐dementia‐care‐workforce
- 692. Alzheimer's Association and Centers for Disease Control and Prevention . Healthy Brain Initiative, State and Local Public Health Partnerships to Address Dementia: The 2018–2023 Road Map. Chicago, IL: Alzheimer's Association; 2018. https://www.cdc.gov/aging/pdf/2018‐2023‐Road‐Map‐508.pdf [Google Scholar]
- 693. Schneider J, Jeon S, Gladman JT, Corriveau RA. ADRD Summit 2019 Report to the National Advisory Neurological Disorders and Stroke Council. https://aspe.hhs.gov/collaborations‐committees‐advisory‐groups/napa/napa‐documents/napa‐research‐milestones/adrd‐summit‐2019‐prioritized‐research‐milestones
- 694. U.S. Department of Health and Human Services . National Plan to Address Alzheimer's Disease: 2020 Update. https://aspe.hhs.gov/reports/national‐plan‐address‐alzheimers‐disease‐2020‐update‐0
- 695. Alzheimer's Association . 2022 Alzheimer's Disease Facts and Figures. Special report: more than normal aging: understanding mild cognitive impairment. Accessed December 15, 2023. Available at: 10.1002/alz.12638 [DOI]
- 696. Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected and diagnosed rates of mild cognitive impairment and dementia in the U.S. Medicare population: observational analysis. Alzheimers Res Ther. 2023;15(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697. Owens DK, Davidson KW, Krist AH, et al. Screening for cognitive impairment in older adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323: 757‐763. [DOI] [PubMed] [Google Scholar]
- 698. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699. Cordell CB, Borson S, Boustani M, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9:141‐150. [DOI] [PubMed] [Google Scholar]
- 700. Galvin JE, Sadowsky CH. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25:367‐382. [DOI] [PubMed] [Google Scholar]
- 701. 2020 Alzheimer's disease facts and figures. Alzheimers Dement. 2020;doi: 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- 702. Bernstein A, Rogers KM, Possin KL, et al. Dementia assessment and management in primary care settings: A survey of current provider practices in the United States. BMC Health Serv Res. 2019;19: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703. Neprash HT, Everhart A, McAlpine D, Smith LB, Sheridan B, Cross DA. Measuring primary care exam length using electronic health record data. Med care. 2021;59(1):62‐66. [DOI] [PubMed] [Google Scholar]
- 704. Sabbagh MN, Boada M, Borson S, et al. Early Detection of Mild Cognitive Impairment (MCI) in Primary Care. J Prev Alzheimers Dis. 2020;7(3):165‐170. [DOI] [PubMed] [Google Scholar]
- 705. Liss JL, Seleri Assunção S, Cummings J, et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer's disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med. 2021;290(2):310‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706. Drabo EF, Barthold D, Joyce G, Ferido P, Chui HC, Zissimopoulos J. Longitudinal analysis of dementia diagnosis and specialty care among racially diverse Medicare beneficiaries. Alzheimers Dement. 2019;15:1402‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707. Kotagal V, Langa KM, Plassman BL, et al. Factors associated with cognitive evaluations in the United States. Neurology. 2015;84:64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708. Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care. Alzheimer Dis Assoc Disord. 2009;23:306‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709. Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of racial/ethnic disparities in timeliness and comprehensiveness of dementia diagnosis in California. JAMA Neurol. 2021;6:657‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710. Lee SA, Kim D, Lee H. Examine Race/Ethnicity Disparities in Perception, Intention, and Screening of Dementia in a Community Setting: Scoping Review. Int J Environ Res Pub Health. 2022;19(14):8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711. Davis MA, Lee KA, Harris M, et al. Time to dementia diagnosis by race: A retrospective cohort study. JAGS. 2022;70(11):3250‐3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712. U.S. Centers for Medicare & Medicaid Services . Cognitive Assessment and Care Plan Services. Accessed December 15, 2023. Available at: https://www.cms.gov/medicare/payment/fee‐schedules/physician/cognitive‐assessment#:~:text=If%20your%20patient%20shows%20signs,to%20bill%20for%20this%20service
- 713. Medicare.gov . Cognitive assessment & care plan services. Accessed December 15, 2023. Available at: https://www.medicare.gov/coverage/cognitive‐assessment‐care‐plan‐services
- 714. Centers for Medicare & Medicaid Services . Billing and Coding: Cognitive Assessment and Care Plan Service. Accessed December 15, 2023. Available at: https://www.cms.gov/medicare‐coverage‐database/view/article.aspx?articleid=59036&ver=11&
- 715. Alzheimer's Association . Cognitive Assessment and Care Planning Services: Alzheimer's Association Expert Task Force Recommendations and Tools for Implementation. Accessed December 15, 2023. Available at: https://www.alz.org/careplanning/downloads/cms‐consensus.pdf
- 716. Alzheimer's Association Fact Sheet: CPT® Code 99483, Explanatory Guide for Clinicians. Accessed December 15, 2023. Available at: https://portal.alzimpact.org/media/serve/id/5ab10bc1a3f3c
- 717. Hinton L, Franz CE, Reddy G, Flores Y, Kravitz RL, Barker JC. Practice constraints, behavioral problems, and dementia care: Primary care physicians’ perspectives. J Gen Intern Med. 2007;22:1487‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718. Bernstein A, Rogers KM, Possin KL, et al. Primary care provider attitudes and practices evaluating and managing patients with neurocognitive disorders. J Gen Intern Med. 2019;34:1691‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719. U.S. Government Accountability Office . Medicare Cognitive Assessments: Utilization Tripled between 2018 and 2022, but Challenges Remain. December 11, 2023. Accessed December 17, 2023. Available at: https://www.gao.gov/products/gao‐24‐106328
- 720. Alzheimer's Association . 2020 Alzheimer's Disease Facts and Figures. Special report: on the front lines: primary care physicians and Alzheimer's care in America. Accessed December 15, 2023. Available at: https://alz‐journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.12068
- 721. American Geriatrics Society . (n.d.) State of the geriatric workforce. Geriatrics workforce by the numbers. https://www.americangeriatrics.org/geriatrics‐profession/about‐geriatrics/geriatrics‐workforce‐numbers
- 722. American Geriatrics Society . (2022) Current number of board certified geriatricians by state. https://www.americangeriatrics.org/sites/default/files/inline‐files/Current%20Number%20of%20Board%20Certified%20Geriatricians%20by%20State%20%28July%202022%29.pdf
- 723. IQVIA (a global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry) provided initial data for the number of geriatricians (estimated at 5,170) under contract with the Alzheimer's Association on December 1, 2021.
- 724. U.S. Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. National and Regional Projections of Supply and Demand for Geriatricians: 2013‐2025. Accessed December 15, 2023. Available at: https://bhw.hrsa.gov/sites/default/files/bureau‐health‐workforce/data‐research/geriatrics‐report‐51817.pdf
- 725. Health Resources & Services Administration . (n.d.) Workforce projections. https://data.hrsa.gov/topics/health‐workforce/workforce‐projections
- 726. Andrilla CHA, Patterson DG, Garberson LA, Coulthard C, Larson EH. Geographic variation in the supply of selected behavioral health providers. Am J Prev Med. 2018;54(6, Suppl 3):S199‐S207. [DOI] [PubMed] [Google Scholar]
- 727. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Pub health. 2015;129(6):611‐620. [DOI] [PubMed] [Google Scholar]
- 728. National Resident Matching Program . Results and Data: Specialties Matching Service. Washington, DC. 2023. Accessed December 17, 2023. Available at: https://www.nrmp.org/wp‐content/uploads/2023/04/2023‐SMS‐Results‐and‐Data‐Book.pdf [Google Scholar]
- 729. U.S. Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis . Health Workforce Projections: Neurology Physicians and Physician Assistants. Accessed December 15, 2023. Available at: https://bhw.hrsa.gov/sites/default/files/bureau‐health‐workforce/data‐research/bhw‐factsheet‐neurology.pdf
- 730. Rao A, Manteau‐Rao M, Aggarwal NT. Dementia neurology deserts: what are they and where are they located in the US? Alzheimers Dement. 2017;13(suppl 7):P509. [Google Scholar]
- 731. Majersik JJ, Ahmed A, Chen IH, et al. A Shortage of Neurologists–We Must Act Now: A Report From the AAN 2019 Transforming Leaders Program. Neurology. 2021;96(24):1122‐1134. [DOI] [PubMed] [Google Scholar]
- 732. Warshaw GA, Bragg EJ. Preparing the health care workforce to care for adults with Alzheimer's disease and related dementias. Health Aff. 2014;33(4):633‐641. [DOI] [PubMed] [Google Scholar]
- 733. Yang M, Chang CH, Carmichael D, Oh ES, Bynum JP. Who is providing the predominant care for older adults with dementia? J Am Med Dir Assoc. 2016;17(9):802‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734. Warshaw GA, Bragg EJ. The essential components of quality geriatric care. Generations. 2016;40(1):28‐37. [Google Scholar]
- 735. Auerbach DI, Buerhaus PI, Staiger DO. Implications of the rapid growth of the nurse practitioner workforce in the US: An examination of recent changes in demographic, employment, and earnings characteristics of nurse practitioners and the implications of those changes. Health Aff. 2020;39(2):273‐279. [DOI] [PubMed] [Google Scholar]
- 736. American Association of Nurse Practitioners (AANP) . NP Fact Sheet 2022. Accessed December 15, 2023. Available at: https://www.aanp.org/about/all‐about‐nps/np‐fact‐sheet
- 737. American Board of Psychiatry and Neurology . Certifications by Year ‐ Subspecialities. Accessed December 15, 2023. Available at: https://www.abpn.org/wp‐content/uploads/2023/03/Certifications‐by‐Year‐Subspecialties‐2022.pdf [Google Scholar]
- 738. Juul D, Colenda CC, Lyness JM, Dunn LB, Hargrave R, Faulkner LR. Subspecialty training and certification in geriatric psychiatry: a 25‐year overview. Am Journal Geriatr Psych. 2017;25(5):445‐453. [DOI] [PubMed] [Google Scholar]
- 739. Kozikowski A, Honda T, Segal‐Gidan F, Hooker RS. Physician assistants in geriatric medical care. BMC Geriatr. 2020;20(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740. The Social Work Profession: Findings from Three Years of Surveys of New Social Workers. Accessed December 15, 2023. Available at: https://www.cswe.org/CSWE/media/Workforce‐Study/The‐Social‐Work‐Profession‐Findings‐from‐Three‐Years‐of‐Surveys‐of‐New‐Social‐Workers‐Dec‐2020.pdf
- 741. Cummings S, Galambos C. Predictors of graduate social work students’ interest in aging‐related work. Journal of Gerontol Soc Work. 2002;39(3):77‐94. [Google Scholar]
- 742. Cummings S, Adler G, DeCoster V. Factors influencing graduate‐social‐work students’ interest in working with elders. Educational Gerontology. 2005;31(8):643‐655. [Google Scholar]
- 743. Curl A, Simons K, Larkin H. Factors affecting willingness of social work students to accept jobs in aging. J Soc Work Education. 2005;41(3):393‐406. [Google Scholar]
- 744. Kolb P. Interest of racially and ethnically diverse social work students in gerontological social work. Educational Gerontol. 2008;34(10):907‐922. [Google Scholar]
- 745. Ferguson A. Wanted: Gerontological social workers — Factors related to interest in the field. Educational Gerontology. 2012;38(10):713‐728. [Google Scholar]
- 746. Ferguson A. The future of gerontological social work: what we know and what we don't know about student interest in the field. J Evid Inf Soc Work. 2015;12(2):184‐197. [DOI] [PubMed] [Google Scholar]
- 747. Cortes T. "Estimate of geriatric specialization among RNs." Received by Kezia Scales, November 14, 2022.
- 748. Orenstein S. Geriatric Nursing and Aging . Accessed December 15, 2023. Available at: https://www.achca.org/index.php?option=com_dailyplanetblog&view=entry&year=2020&month=03&day=04&id=61:geriatric‐nursing‐and‐aging
- 749. Moye J, Karel MJ, Stamm KE, et al. Workforce Analysis of Psychological Practice With Older Adults: Growing Crisis Requires Urgent Action. Train Educ Prof Psychol. 2019;13(1):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750. Heintz H, Monette P, Epstein‐Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320‐330. [DOI] [PubMed] [Google Scholar]
- 751. Frost R, Rait G, Aw S, et al. Implementing post diagnostic dementia care in primary care: a mixed‐methods systematic review. Aging Ment Health. 2021;25(8):1381‐1394. [DOI] [PubMed] [Google Scholar]
- 752. Super N, Ahuja R, McDermott M. Scaling Comprehensive Dementia‐Care Models. Milken Institute; 2021. Accessed October 28, 2023. Available at: https://milkeninstitute.org/sites/default/files/2021‐11/Comprehensive%20Dementia%20Care%20Models.pdf [Google Scholar]
- 753. French DD, LaMantia MA, Livin LR, Herceg D, Alder CA, Boustani MA. Healthy Aging Brain Center improved care coordination and produced net savings. Health Aff. 2014;33(4):613‐618. [DOI] [PubMed] [Google Scholar]
- 754. Haggerty KL, Epstein‐Lubow G, Spragens LH, et al. Recommendations to improve payment policies for comprehensive dementia care. J Am Geriatr Soc. 2020;68(11):2478‐2485. [DOI] [PubMed] [Google Scholar]
- 755. Jennings LA, Laffan AM, Schlissel AC, et al. Health care utilization and cost outcomes of a comprehensive dementia care program for Medicare beneficiaries. JAMA Int Med. 2019;179:161‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 756. Possin KL, Merrilees JJ, Dulaney S, et al. Effect of collaborative dementia care via telephone and internet on quality of life, caregiver well‐being, and health care use: The Care Ecosystem Randomized Clinical Trial. JAMA Intern Med. 2019;179(12):1658‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 757. Guterman EL, Kiekhofer RE, Wood AJ, Allen IE, Kahn JG, Dulaneyet S. Care ecosystem collaborative model and health care costs in Medicare beneficiaries with dementia: A secondary analysis of a randomized clinical trial. JAMA Intern Med. 2023;183(11):1222‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758. Rosenbloom MH, Kashyap B, Diaz‐Ochoa A, et al. Implementation and review of the care ecosystem in an integrated healthcare system. BMC Geriatr. 2023;23(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759. Care Ecosystem . Care Ecosystem Toolkit. Accessed December 15, 2023. Accessed December 15, 2023. Available at: https://memory.ucsf.edu/sites/memory.ucsf.edu/files/wysiwyg/CareEcosystemToolkit.pdf
- 760. Scales K, Wagner LM. Direct care workers are the foundation of a dementia‐capable workforce. Generations. 2023;47(1):1‐9.38560360 [Google Scholar]
- 761. PHI . Direct Care Workers in the United States: Key Facts. New York, NY: PHI; 2023. > Accessed September 18, 2023. https://www.phinational.org/resource/direct‐care‐workers‐in‐the‐united‐states‐key‐facts‐2023/ [Google Scholar]
- 762. Campbell S, Drake ADR, Espinoza R, Scales K. Caring for the Future: The Power and Potential of America's Direct Care Workforce. Bronx, NY: PHI; 2021. https://www.phinational.org/resource/caring‐for‐the‐future‐the‐power‐and‐potential‐of‐americas‐direct‐care‐workforce/ [Google Scholar]
- 763. Reckrey JM, Tsui EK, Morrison RS, et al. Beyond functional support: the range of health‐related tasks performed in the home by paid caregivers in New York. Health Aff (Project Hope). 2019;38(6):927‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764. Lyons TL, Champion JD. Nonpharmacological interventions for management of behavioral and psychological symptoms of dementia in long‐term care facilities by direct caregivers: A systematic review. J Gerontol Nurs. 2022;48(7):18‐23. [DOI] [PubMed] [Google Scholar]
- 765. Eaton J, Cloyes KG, Paulsen B, Madden C, Ellington L. The development of knowledgeable nursing assistants as creative caregivers (KNACC). Geriatr Nurs. 2023;51:95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766. Kemp CL, Bender AA, Morgan JC, Burgess EO, Epps FR, Hill AM, Perkins MM. Understanding capacity and optimizing meaningful engagement among persons living with dementia. Dementia. 2023;22(4):854‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767. Dokos M, Schultz R, Gossner JD, Fauth EB. Supporting persons with dementia: perspectives from certified nurse's assistants. Innov Aging. 2023;7(5):igad049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768. Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: a systematic review and meta‐analysis. Int Psychogeriatr. 2017;29(2):195‐208. [DOI] [PubMed] [Google Scholar]
- 769. Carnahan JL, Slaven JE, Callahan CM, Tu W, Torke AM. Transitions From Skilled Nursing Facility to Home: The Relationship of Early Outpatient Care to Hospital Readmission. J Am Med Dir Assoc. 2017;18(10):853‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774‐784. [DOI] [PubMed] [Google Scholar]
- 771. Murtaugh CM, Deb P, Zhu C, et al. Reducing Readmissions among Heart Failure Patients Discharged to Home Health Care: Effectiveness of Early and Intensive Nursing Services and Early Physician Follow‐Up. Health Serv Res. 2017;52(4):1445‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772. Guo W, Cai S, Caprio T, Schwartz L, Temkin‐Greener H. End‐of‐Life Care transitions in assisted living: associations with state staffing and training regulations. J Am Med Dir Assoc. 2023;24(6):827‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773. Shepherd H, Livingston G, Chan J, Sommerlad A. Hospitalisation rates and predictors in people with dementia: a systematic review and meta‐analysis. BMC Med. 2019;17(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774. LaMantia MA, Stump TE, Messina FC, Miller DK, Callahan CM. Emergency department use among older adults with dementia. Alzheimer Dis Assoc Disord. 2016;30(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775. Yoon JM, Trinkoff AM, Kim M, Kim E. State‐level nursing home in‐service dementia training requirements and inappropriate psychotropic medication use. Geriatr Nursing. 2023;51:209‐214. [DOI] [PubMed] [Google Scholar]
- 776. Centers for Medicare & Medicaid Services (CMS) . Long‐term services and supports rebalancing toolkit. Accessed December 15, 2023. Available at: https://www.medicaid.gov/medicaid/long‐term‐services‐supports/downloads/ltss‐rebalancing‐toolkit.pdf
- 777. Shih RA, Friedman EM, Chen EK, Whiting GC. Prevalence and correlates of gray market use for aging and dementia long‐term care in the US. J Appl Gerontol. 2022; 41(4):1030‐1034. [DOI] [PubMed] [Google Scholar]
- 778. Donlan A. After 3‐Year Dip, Home Care Turnover Soars to 77%. Home Health Care News, May 24, 2023. Accessed September 18, 2023. Available at: https://homehealthcarenews.com/2023/05/after‐dipping‐for‐three‐years‐home‐care‐turnover‐rate‐soared‐to‐77‐in‐2022/
- 779. Gandhi A, Yu H, Grabowski DC. High nursing staff turnover in nursing homes offers important quality information. Health Aff. 2021;40(3):384‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780. Institute of Medicine . Retooling for an Aging America: Building the Health Care Workforce. Washington, D.C.: The National Academies Press; 2008. Accessed December 15, 2023. Available at: http://www.nationalacademies.org/hmd/reports/2008/retooling‐for‐an‐aging‐america‐building‐the‐health‐care‐workforce.aspx [PubMed] [Google Scholar]
- 781. Trinkoff AM, Han K, Storr CL, Lerner N, Johantgen M, Gartrell K. Turnover, staffing, skill mix, and resident outcomes in a national ample of US nursing homes. J Nurs Adm. 2013;43(12):630‐636. [DOI] [PubMed] [Google Scholar]
- 782. Scales K. Transforming direct care jobs, reimagining long‐term services and supports. J Am Med Dir Assoc. 2022;23(2):207‐213. [DOI] [PubMed] [Google Scholar]
- 783. Weller C, Almeida B, Cohen M, Stone R. Making Care Work Pay. . Accessed December 15, 2023. Available at: https://www.ltsscenter.org/wp‐content/uploads/2020/09/Making‐Care‐Work‐Pay‐Report‐FINAL.pdf
- 784. Reckrey JM, Perez S, Watman D, Ornstein KA, Russell D, Franzosa E. The need for stability in paid dementia care: family caregiver perspectives. J Appl Gerontol. 2023;42(4):607‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785. Manchha AV, Walker N, Way KA, Dawson D, Tann K, Thai M. Deeply discrediting: A systematic review examining the conceptualizations and consequences of the stigma of working in aged care. Gerontologist. 2021;61(4):e129‐e146. [DOI] [PubMed] [Google Scholar]
- 786. Khavjou O, Suarez G, Tyler D, Squillace M, Dey J, Oliveira I. Wages of direct care workers lower than other entry‐level jobs in most states (Issue brief). Washington, DC: Office of the Assistant Secretary for Planning and Evaluation (ASPE), U.S. Department of Health and Human Services. Accessed September 18, 2023. Available at: https://aspe.hhs.gov/sites/default/files/documents/7a611d901c615e5611ea095b1dcf8d08/wages‐dcw‐lower‐ib.pdf [PubMed] [Google Scholar]
- 787. Burke G, Orlowski G. Training to serve people with dementia: is our health care system ready? Accessed December 15, 2023. Available at: https://www.justiceinaging.org/wp‐content/uploads/2015/08/Training‐to‐serve‐people‐with‐dementia‐Alz1_Final.pdf
- 788. U.S. Bureau of Labor Statistics (BLS) . Occupational injuries and illnesses and fatal injuries profiles. Accessed December 15, 2023. Available at: https://www.bls.gov/iif/ [Google Scholar]
- 789. Paraprofessional Healthcare Institute (PHI) . Workplace Injuries and the Direct Care Workforce. Accessed December 15, 2023. Available at: https://phinational.org/resource/workplace‐injuries‐direct‐care‐workforce
- 790. Quinn MM, Markkanen PK, Galligan CJ, Sama SR, Lindberg JE, Edwards MF. Healthy aging requires a healthy home care workforce: the occupational safety and health of home care aides. Curr Environ Health Rep. 2021;8(3):235‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791. Hebert CA, Scales K. Dementia friendly initiatives: A state of the science review. Dementia. 2019;18(5):1858‐1895. [DOI] [PubMed] [Google Scholar]
- 792. Alzheimer's Disease International . Dementia Friendly Communities: Global Developments. 2016. Accessed December 15, 2023. Available at: https://www.alzint.org/u/dfc‐developments.pdf
- 793. The White House . Fact sheet: The White House Conference on Aging. Accessed December 17, 2023. Available at: https://obamawhitehouse.archives.gov/the‐press‐office/2015/07/13/fact‐sheet‐white‐house‐conference‐aging
- 794. ACT on Alzheimer's . About ACT on Alzheimer's. Accessed December 17, 2023. Available at: https://actonalz.org/about
- 795. Parke B, Boltz M, Hunter KF, et al. A scoping literature review of dementia‐friendly hospital design. Gerontologist. 2017;57(4):e62‐e74. [DOI] [PubMed] [Google Scholar]
- 796. Kirch J, Marquardt G. Towards human‐centred general hospitals: the potential of dementia‐friendly design. Arch Sci Rev. 2023;66(5):382‐390. [Google Scholar]
- 797. Riquelme‐Galindo J, Lillo‐Crespo M. Designing dementia care pathways to transform non dementia‐friendly hospitals: Scoping review. Int J Enviro Res Public Health. 2021;18(17):9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798. van Buuren LPG, Mohammadi M. Dementia‐friendly design: A Set of design criteria and design typologies supporting wayfinding. HERD. 2022;15(1):150‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799. Shiells K, Pivodic L, Holmerová I, Van den Block L. Self‐reported needs and experiences of people with dementia living in nursing homes: a scoping review. Aging Ment Health. 2020;24(10):1553‐1568. [DOI] [PubMed] [Google Scholar]
- 800. Scher CJ, Somerville C, Greenfield EA, Coyle C. Organizational characteristics of senior centers and engagement in dementia‐friendly communities. Innov Aging. 2023;7(5);igad050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801. Gan D, Chaudhury H, Mann J, Wister AV. Dementia‐friendly neighborhood and the built environment: A scoping review. Gerontologist. 2022;62(6):e340‐e356. [DOI] [PubMed] [Google Scholar]
- 802. Hung L, Hudson A, Gregorio M, Jackson L, Mann J, Horneet N. Creating dementia‐friendly communities for social inclusion: A scoping review. Gerontol Geriatr Med. 2021;7:23337214211013596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803. Cherry DL. HCBS can keep people with dementia at home. Generations. 2012;36(1) 83‐90. [Google Scholar]
- 804. Dickey TJ. "Public Libraries' Contribution to Sustainable Dementia‐Friendly Communities". Williams‐Cockfield, KC . and Mehra, B . (Ed.) How Public Libraries Build Sustainable Communities in the 21st Century (Advances in Librarianship, Vol. 53) , Emerald Publishing Limited, Bingley, 2023. pp. 283‐292. [Google Scholar]
- 805. Devos P, Aletta F, Thomas P, et al. Designing supportive soundscapes for nursing home residents with dementia. Int J Environ Res Public Health. 2019;16(24):4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806. Anetzberger GJ, Palmisano BR, Sanders M, Bass D, Dayton C, Eckert S, Schimer MR. A model intervention for elder abuse and dementia. Gerontologist. 2000;40(4):492‐497. [DOI] [PubMed] [Google Scholar]
- 807. Dong XQ, Chen R, Simon MA. Elder abuse and dementia: A review of the research and health policy. Health Aff (Millwood). 2014;33(4):642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808. Sun F, Gao X, Brown H, Winfree LT. Police officer competence in handling Alzheimer's cases: The roles of AD knowledge, beliefs, and exposure. Dementia. 2019;18(2):674‐684. [DOI] [PubMed] [Google Scholar]
- 809. Brown RT, Ahalt C, Rivera J, Stijacic Cenzer I, Wilhelm A, Williams BA. Good cop, bad cop: Evaluation of a geriatrics training program for police. J Am Geriatr Soc. 2017;65(8):1842‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810. Anderson KA, Cimbal AM, Maile JJ. Hairstylists’ relationships and helping behaviors with older adult clients. J Appl Gerontol. 2010;29(3):371‐380. [Google Scholar]
- 811. Che SL, Wu J, Chuang Y‐C, Van IK, Leong SM. The willingness to help people with dementia symptoms among four occupation practitioners in Macao. Am J Alzheimers Dis Other Demen. 2022;37:15333175221139172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812. Wang Q, Davis PB, Gurney ME, Xu R. COVID‐19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alz Dement. 2021;17(8):1297‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813. Health System Tracker . How has health sector employment recovered since the pandemic? Accessed December 15, 2023. Available at: https://www.healthsystemtracker.org/chart‐collection/what‐impact‐has‐the‐coronavirus‐pandemic‐had‐on‐healthcare‐employment/
- 814. Wager E, Telesford I, Hughes‐Cromwick P, Amin K, Cox C. What impact has the coronavirus pandemic had on health employment? Peterson‐KFF Health System Tracker. Accessed December 15, 2023. Available at: https://www.healthsystemtracker.org/chart‐collection/what‐impact‐has‐the‐coronavirus‐pandemic‐had‐on‐healthcare‐employment/#Cumulative%20percent%20change%20in%20health%20sector%20and%20non‐health%20sector%20employment,%20January%201990‐July%202022%C2%A0
- 815. Centers for Medicare & Medicaid Services (CMS) . COVID‐19 nursing home data. Accessed December 15, 2023. Available at: https://data.cms.gov/covid‐19/covid‐19‐nursing‐home‐data
- 816. Cutler DM. Challenges for the beleaguered health care workforce during COVID‐19. JAMA Health Forum. 2022;3(1): e220143. [DOI] [PubMed] [Google Scholar]
- 817. Hendrickson RC, Slevin RA, Hoerster KD, et al. The impact of the COVID‐19 pandemic on mental health, occupational functioning, and professional retention among health care workers and first responders. J Gen Intern Med. 2022;37(2):397‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818. White EM, Wetle TF, Reddy A, Baier RR. Front‐line Nursing Home Staff Experiences During the COVID‐19 Pandemic. J Am Med Dir Assoc. 2021;22(1):199‐203. Erratum in: J Am Med Dir Assoc. 2021 May;22(5):1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819. Office of the Surgeon General (OSG) . Addressing health worker burnout: The U.S. Surgeon General's advisory on building a thriving health workforce. Accessed December 15, 2023. Available at: https://www.hhs.gov/sites/default/files/health‐worker‐wellbeing‐advisory.pdf [PubMed] [Google Scholar]
- 820. Lluch C, Galiana L, Doménech P, Sansó N. The impact of the COVID‐19 pandemic on burnout, compassion fatigue, and compassion satisfaction in healthcare personnel: A systematic review of the literature published during the first year of the pandemic. Healthcare. 2022;10:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821. Prasad K, McLoughlin C, Stillman M, Poplau S, Goelz E, Taylor S, Nankivil N, Brown R, Linzer M, Cappelucci K, Barbouche M. Prevalence and correlates of stress and burnout among US healthcare workers during the COVID‐19 pandemic: A national cross‐sectional survey study. eClinicalMedicine. 2021;35:100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822. Kaufmann PG, Havens DS, Mensinger JL, et al. The COVID‐19 study of healthcare and support personnel (CHAMPS): Protocol for a longitudinal observational study. JMIR Res Protocols. 2021;10(10):e30757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823. National Council on Aging . (2021, March 3). The impact of COVID‐19 on community‐based organizations serving older adults. Accessed December 15, 2023. Available at: https://www.ncoa.org/article/the‐impact‐of‐covid‐19‐on‐community‐based‐organizations‐serving‐older‐adults
- 824. Gallo HB, Wilber KH. Transforming aging services: Area agencies on aging and the COVID‐19 response. Gerontologist. 2021;61(2):152‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825. Bourland DL, DeBacker L, Dotson Newman O, Loizeaux A. Frontline organizations play a vital role in movement ecosystems; let's fund them to thrive. Am J Public Health. 2022;112(1):63‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826. Numbers K, Brodaty H. The effects of the COVID‐19 pandemic on people with dementia. Nat Rev Neurol. 2021;17(2):69‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827. American Public Health Association . Strengthening the dementia care workforce: A public health priority. Accessed December 15, 2023. Available at: https://www.apha.org/policies‐and‐advocacy/public‐health‐policy‐statements/policy‐database/2021/01/13/strengthening‐the‐dementia‐care‐workforce
- 828. Vespa J, Medina L, Armstrong DM. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. Current Population Reports. 2020 P25‐1144, U.S. Census Bureau, Washington, DC, 2020. [Google Scholar]
- 829. American Geriatrics Society (AGS) . GWEP coordinating center. Accessed December 15, 2023. Available at: https://www.americangeriatrics.org/programs/gwep‐coordinating‐center
- 830. National Institutes of Health . NIH RePorter: The National Dementia Workforce Study. Accessed December 15, 2023. Accessed December 15, 2023. Available at: https://reporter.nih.gov/search/tmkzdvxu9Uaz2nxxwrpybw/project‐details/10774551
- 831. Galvin JE, Aisen P, Langbaum JB, Rodriguez E, Sabbagh M, Stefanacci R, Stern RA, Vassey EA, de Wilde A, West N, Rubino I. Early stages of Alzheimer's disease: Evolving the care team for optimal patient management. Front Neurol. 2021;11:592302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832. Lassell RK, Moreines LT, Luebke MR, Bhatti KS, Pain KJ, Brody AA, Luth EA. Hospice interventions for persons living with dementia, family members and clinicians: A systematic review. J Am Geriatr Soc. 2022;70(7):2134‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833. Alzheimer's Association . Alzheimer's facts and figures 2021 special report: Race, ethnicity and Alzheimer's in America. Available online https://www.alz.org/media/Documents/alzheimers‐facts‐and‐figures‐special‐report‐2021.pdf
- 834. Díaz‐Santos M, Yáñez J, Suarez PA. Alzheimer's disease in bilingual Latinos: clinical decisions for diagnosis and treatment planning. J Health Service Psychol. 2021;47(4):171‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835. Chejor P, Laging B, Whitehead L, Porock D. Experiences of older immigrants living with dementia and their carers: a systematic review and meta‐synthesis. BMJ open. 2022;12(5):e059783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836. Nowaskie DZ, Sewell, DD . Assessing the LGBT cultural competency of dementia care providers. Alzheimers Dement. 2021;7(1): e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837. Eller N, Belza B. Addressing Alzheimer's disease in Asian American and Pacific Islander older adults: An action guide for service providers. J Gerontol Nurs. 2019;44(4):3‐4. [DOI] [PubMed] [Google Scholar]
- 838. Abramsohn EM, Paradise KM, Glover CM, et al. CommunityRx: Optimizing a community resource referral intervention for minority dementia caregivers. J Appl Gerontol. 2022;41(1):113‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839. Alzheimer's Association . The Alzheimer's and Dementia Care ECHO® Program for clinicians. Accessed December 15, 2023. Available at: https://www.alz.org/professionals/health‐systems‐clinicians/echo‐alzheimers‐dementia‐care‐program
- 840. Dementia Care Aware . Early Detection. Better Care. Accessed December 15, 2023. Available at: https://www.dementiacareaware.org/
- 841. Medi‐Cal. Medi‐Cal Rates . Accessed December 15, 2023. Available at: https://mcweb.apps.prd.cammis.medi‐cal.ca.gov/rates?page=1&tab=rates
- 842. Dementia Care Aware . 2023. Guide to Cognitive Impairment Screening and Billing in California. Accessed December 16, 2023. Available at: https://www.dementiacareaware.org/wp‐content/uploads/2023/11/Dementia‐Care‐Aware_Guide‐to‐Cognitive‐Impairment‐Screening‐and‐Billing‐in‐California.pdf
- 843. The Gerontological Society of America . The GSA KAER Toolkit for Primary Care Teams: Supporting Conversations about Brain Health, Timely Detection of Cognitive Impairment, and Accurate Diagnosis of Dementia. Fall 2020 Edition. Accessed December 15, 2023. Available at: https://www.geron.org/images/gsa/Marketing/KAER/GSA_KAER‐Toolkit_2020_Final.pdf
- 844. Poghosyan L, Brooks JM, Hovsepian V, Pollifrone M, Schlak AE, Sadak T. The growing primary care nurse practitioner workforce: A solution for the aging population living with dementia. Am J Geriatr Psychiatry. 2021;29(6):517‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845. Gaps in the Dementia Care Workforce: Research Update and Data Needs . Committee on Population (CPOP) Semi‐Annual Meeting, May 23, 2019. Accessed December 15, 2023. Available at: https://www.nia.nih.gov/sites/default/files/2019‐11/Seminar‐Gaps‐Dementia‐Workforce‐Final‐508.pdf
- 846. Bonner A, Walsh A, Shue J, Morton G, Fulmer T. (2023). Generations: Dementia‐Friendly Initiatives for Individuals Living with Dementia, Care Partners, and Communities. Accessed October 28, 2023. Available at: https://generations.asaging.org/dementia‐friendly‐initiatives
- 847. Ty D, McDermott M. Building Workforce Capacity to Improve Detection and Diagnosis of Dementia. Milken Institute; 2021. Accessed October 28, 2023. Available at: https://milkeninstitute.org/sites/default/files/2021‐05/Building%20Dementia%20Workforce.pdf [Google Scholar]
- 848. Boustani M, Alder CA, Solid CA, Reuben D. An alternative payment model to support widespread use of collaborative dementia care models. Health Aff (Millwood). 2019;38(1):54‐59. [DOI] [PubMed] [Google Scholar]
- 849. Pizzi LT, Jutkowitz E, Prioli KM, Lu E, Babcock Z, McAbee‐Sevick H, Wakefield DB, Robison J, Molony S, Piersol CV, Gitlin LN. Cost‐benefit analysis of the COPE program for persons living with dementia: Toward a payment model. Innov Aging. 2022;6(1):igab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850. Li J, Andy C, Mitchell S. Use of Medicare's new reimbursement codes for cognitive assessment and care planning, 2017‐2018. JAMA Netw Open. 2021;4(9):e2125725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851. U.S. Centers for Medicare & Medicaid . Guiding an improved dementia experience (GUIDE) model. Accessed December 15, 2023. Available at: https://www.cms.gov/priorities/innovation/innovation‐models/guide
- 852. Winters A, Block L, Maxey H, Medlock C, Ruane K, Hockenberry S. State Strategies for Sector Growth and Retention for the Direct Care Health Workforce . 2021. Washington, DC: National Governors Association Center for Best Practices. Accessed December 15, 2023. Available at: https://www.nga.org/wp‐content/uploads/2021/10/NGA_SectorGrowth‐DirectCare_report.pdf [Google Scholar]
- 853. Scales K. State policy strategies for strengthening the direct care workforce. Accessed December 15, 2023. Available at: https://www.phinational.org/resource/state‐policy‐strategies‐for‐strengthening‐the‐direct‐care‐workforce/ [Google Scholar]
- 854. Muirhead K, Macaden L, Smyth K, Chandler C, Clarke C, Polson R, O'Malley C. Establishing the effectiveness of technology‐enabled dementia education for health and social care practitioners: a systematic review. Syst Rev. 2021;10(1):1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855. Geddes MR, O'Connell ME, Fisk JD, Gauthier S, Camicioli R, Ismail Z. Remote cognitive and behavioral assessment: Report of the Alzheimer Society of Canada Task Force on Dementia Care Best Practices for COVID‐19. Alzheimers Dement. 2020;12(1):e12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856. Yi JS, Pittman CA, Price CL, Nieman CL, Oh ES. Telemedicine and dementia care: a systematic review of barriers and facilitators. J Am Med Dir Assoc. 2021;22(7):1396‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857. Pappadà A, Chattat R, Chirico I, Valente M, Ottoboni G. Assistive technologies in dementia care: an updated analysis of the literature. Front Psych. 2021;12:644587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859. Unpublished tabulations based on data from the Medicare Current Beneficiary Survey for 2018. Prepared under contract by Health Care Cost Institute, December 2021.
- 860. Yang Z, Zhang K, Lin PJ, Clevenger C, Atherly A. A longitudinal analysis of the lifetime cost of dementia. Health Serv Res. 2012;47(4):1660‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861. Murman DL, Chen Q, Powell MC, Kuo SB, Bradley CJ, Colenda CC. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2022;59:1721‐1729. [DOI] [PubMed] [Google Scholar]
- 862. Yang Z, Levey A. Gender differences: A lifetime analysis of the economic burden of Alzheimer's disease. Women Health Iss. 2015;25(5):436‐440. [DOI] [PubMed] [Google Scholar]
- 863. White L, Fishman P, Basu A, Crane PK, Larson EB, Coe NB. Medicare expenditures attributable to dementia. Health Services Res. 2019;54(4):773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864. Coe NB, White L, Oney M, Basu A, Larson EB. Public spending on acute and long‐term care for Alzheimer's disease and related dementias. Alzheimers Dement. 2023;19(1):150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865. Oney M, White L, Coe NB. Out‐of‐pocket costs attributable to dementia: A longitudinal analysis. J Am Geriatr Soc. 2022;70(5):1538‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866. Hudomiet P, Hurd MD, Rohwedder S. The relationship between lifetime out‐of‐pocket medical expenditures, dementia and socioeconomic status in the U.S. J Econ Ageing. 2019;14:100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867. Dwibedi N, Findley AP, Wiener C, Shen C, Sambamoorthi U. Alzheimer disease and related disorders and out‐of‐pocket health care spending and burden among elderly Medicare beneficiaries. Medical Care. 2018;56:240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868. Leniz J, Yi D, Yorganci E, et al. Exploring costs, cost components, and associated factors among people with dementia approaching the end of life: A systematic review. Alzheimers Dement (NY). 2021;7(1):e12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869. Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163:729‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870. Rudolph JL, Zanin NM, Jones RN, et al. Hospitalization in community‐dwelling persons with Alzheimer's disease: Frequency and causes. J Am Geriatr Soc. 2010;58(8):1542‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871. Beydoun MA, Beydoun HA, Gamaldo AA, et al. Nationwide inpatient prevalence, predictors and outcomes of Alzheimer's disease among older adults in the United States, 2002–2012. J Alzheimers Dis. 2015;48(2):361‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872. Landon BE, Keating NL, Onnela JP, Zaslavsky AM, Christakis NA, O'Malley AJ. Patient‐sharing networks of physicians and health care utilization and spending among Medicare beneficiaries. JAMA Intern Med. 2018;178:66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873. U.S. Centers for Medicare & Medicaid Services . State Level Chronic Conditions Table: Prevalence, Medicare Utilization and Spending, 2007‐2018. Accessed December 15, 2023. Accessed October 27, 2023. Accessed December 15, 2023. Available at: https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/CC_Main.html
- 874. Cairns C, Kang K. National Hospital Ambulatory Medical Care Survey: 2021 emergency department summary tables. Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHAMCS/doc21‐ed‐508.pdf
- 875. Hill JD, Schmucker AM, Siman N, et al. Emergency and post‐emergency care of older adults with Alzheimer's disease/Alzheimer's disease related dementias. J Am Geriatr Soc. 2022;70(9):2582‐2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876. Medicare . Glossary . Medicare: The Official U.S. Government Site for Medicare. https://www.medicare.gov/glossary/a
- 877. U.S. Centers for Medicare & Medicaid Services . Mapping Medicare disparities by population. Accessed December 12, 2023. Available at: https://data.cms.gov/tools/mapping‐medicare‐disparities‐by‐population
- 878. Leibson CL, Hall Lon K, Ransom JE, et al. Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population‐based study. Alzheimers Dement. 2015;11(8):917‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879. Suehs BT, Davis CD, Alvir J, et al. The clinical and economic burden of newly diagnosed Alzheimer's disease in a Medicare Advantage population. Am J Alzheimers Dis Other Dement. 2013;28(4):384‐3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880. Lin P‐J, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare expenditures of individuals with Alzheimer's disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64:1549‐1557. [DOI] [PubMed] [Google Scholar]
- 881. Geldmacher DS, Kirson NY, Birnbaum HG, et al. Pre‐diagnosis excess acute care costs in Alzheimer's patients among a U.S. Medicaid population. Appl Health Econ Health Policy. 2013;11(4):407‐413. [DOI] [PubMed] [Google Scholar]
- 882. Zhu CW, Cosentino S, Ornstein K, et al. Medicare utilization and expenditures around incident dementia in a multiethnic cohort. J Gerontol A Biol Sci Med Sci. 2015;70(11):1448‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883. Kirson NY, Desai U, Ristovska L, et al. Assessing the economic burden of Alzheimer's disease patients first diagnosed by specialists. BMC Geriatrics. 2016;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884. Aigbogun MS, Stellhorn R, Hartry A, Baker RA, Fillit H. Treatment patterns and burden of behavioral disturbances in patients with dementia in the United States: A claims database analysis. BMC Neurology. 2019;19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885. Davis‐Ajami ML, Lu ZK, Wu J. Exploring the home healthcare workforce in Alzheimer's disease and related dementias: Utilization and cost outcomes in US community dwelling older adults. Arch Gerontol Geriat. 2022;98;104536. [DOI] [PubMed] [Google Scholar]
- 886. Ochieng N, Fuglesten Biniek J, Freed M, Damico A, Neuman T. Medicare Advantage in 2023: Premiums, out‐of‐pocket limits, cost sharing, supplemental benefits, prior authorization and star ratings. Kaiser Family Foundation. Accessed November 15, 2023. Available at: https://www.kff.org/medicare/issue‐brief/medicare‐advantage‐in‐2023‐premiums‐out‐of‐pocket‐limits‐cost‐sharing‐supplemental‐benefits‐prior‐authorization‐and‐star‐ratings/ [Google Scholar]
- 887. U.S. Centers for Medicare & Medicaid Services . SNP Comprehensive Report ‐ November 2023. Accessed November 15, 2023. Available at: https://www.cms.gov/research‐statistics‐data‐and‐systems/statistics‐trends‐and‐reports/mcradvpartdenroldata/special/snp‐comprehensive‐report‐2023‐11
- 888. U.S. Centers for Medicare & Medicaid Services . National Health Expenditures Tables: Table 12 Expenditures, Enrollment and Per Enrollee Estimates of Health Insurance. Last modified September 6, 2023. Accessed November 15, 2023. Available at: https://www.cms.gov/data‐research/statistics‐trends‐and‐reports/national‐health‐expenditure‐data/nhe‐fact‐sheet
- 889. National Center for Health Statistics . Biennial Overview of Post‐acute and Long‐term Care in the United States. Accessed November 25, 2023. Available at: https://data.cdc.gov/d/wibz‐pb5q
- 890. Knox S, Downer B, Haas A, Ottenbacher KJ. Home health utilization association with discharge to community for people with dementia. Alzheimers Dement (NY). 2022;8(1):e12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 891. Rome V, Penn Lendon J, Harris‐Kojetin L. Differences in characteristics of adult day services centers by level of medical service provision. National Center for Health Statistics. 2020;3(45):1‐28. [Google Scholar]
- 892. Sengupta M, Caffrey C. Characteristics of residential care communities by percentage of resident population diagnosed with dementia: United States, 2016. National Health Statistics Reports. 2020;148:1‐7. [PubMed] [Google Scholar]
- 893. Caffrey C, Melekin A, Lu Z, Sengupta M. Variation in residential care community resident characteristics, by size of community: United States, 2020. NCHS Data Brief, no 454. Hyattsville, MD: National Center for Health Statistics; 2022. [PubMed] [Google Scholar]
- 894. Caffrey C, Harris‐Kojetin L, Rome V, Sengupta M. Variation in operating characteristics of residential care communities by size of community: United States, 2014. NCHS Data Brief, No. 222. November 2015. [PubMed]
- 895. Colelo KJ. Who pays for long‐term services and supports? Congressional Research Service, In Focus, IF10343. August 5, 2021. Accessed December 15, 2023. Available at: https://crsreports.congress.gov/
- 896. Murray C, Eckstein M, Lipson D, Wysocki A. Medicaid Long Term Services and Supports Annual Expenditures Report: Federal Fiscal Year 2020. Chicago, IL: Mathematica, June 9, 2023. [Google Scholar]
- 897. McGarry BE, Grabowski DC. Medicaid home and community‐based services spending for older adults: Is there a "woodwork" effect? J Am Geriatr Soc. 2023;71(10):3143‐3151. [DOI] [PubMed] [Google Scholar]
- 898. Bynum J. Characteristics, Costs, and Health Service Use for Medicare Beneficiaries with a Dementia Diagnosis: Report 1: Medicare Current Beneficiary Survey. Unpublished; provided under contract with the Alzheimer's Association. Lebanon, N.H.: Dartmouth Institute for Health Policy and Clinical Care, Center for Health Policy Research, January 2009. [Google Scholar]
- 899. Clarkson P, Davies L, Jasper R, Loynes N, Challis D. Home Support in Dementia (HoSt‐D) Programme Management Group. A systematic review of the economic evidence for home support interventions in dementia. Value in Health. 2017;20:1198‐1209. [DOI] [PubMed] [Google Scholar]
- 900. Nickel F, Barth J, Kolominsky‐Rabas PL. Health economic evaluations of non‐pharmacological interventions for persons with dementia and their informal caregivers: A systematic review. BMC Geriatrics. 2018;18:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901. Callahan CM, Arling G, Tu W, et al. Transitions in care among older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902. Gozalo P, Teno JM, Mitchell SL, et al. End‐of‐life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903. Teno JM, Mitchell SL, Skinner J, et al. Churning: The association between health care transitions and feeding tube insertion for nursing home residents with advanced cognitive impairment. J Palliat Med. 2009;12(4):359‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904. Genworth . Genworth Cost of Care Survey. Genworth Financial, Inc. Accessed December 15, 2023. Accessed on December 15, 2023. Available at: https://www.genworth.com/aging‐and‐you/finances/cost‐of‐care.html [Google Scholar]
- 905. Unpublished data from the 2018 Medicare Current Beneficiary Survey (MCBS), analyzed by the Alzheimer's Association. October 2020.
- 906. Koma W, Neuman T, Jacobson G, Smith K. Medicare beneficiaries’ financial security before the coronavirus pandemic. Issue Brief. Kaiser Family Foundation. www.kff.org/medicare/issue‐brief/medicare‐beneficiaries‐financial‐security‐before‐the‐coronavirus‐pandemic/
- 907. Hamel L, Montero A. The affordability of long‐term care and support services: Findings from a KFF survey. Kaiser Family Foundation. November 14, 2023. Accessed November 25, 2023. Available at: https://www.kff.org/health‐costs/poll‐finding/the‐affordability‐of‐long‐term‐care‐and‐support‐services/
- 908. U.S. Centers for Medicare & Medicaid Services . Your Medicare Coverage. Long‐Term Care. Accessed December 15, 2023. Accessed December 15, 2023. Available at: https://www.medicare.gov/coverage/long‐term‐care.html
- 909. National Association of Insurance Commissioners and the Center for Insurance Policy and Research. The State of Long‐Term Care Insurance: The Market, Challenges and Future Innovations. CIPR Study Series 2016‐1. May 2016.
- 910. U.S. Centers for Medicare & Medicaid Services . Skilled nursing facility (SNF) care. https://www.medicare.gov/coverage/skilled‐nursing‐facility‐snf‐care
- 911. U.S. Centers for Medicare & Medicaid Services . 2024 Medicare Parts A and B premiums and deductibles. October 23, 2023. Accessed December 1, 2023. Accessed December 15, 2023. Available at: https://www.cms.gov/newsroom/fact‐sheets/2024‐medicare‐parts‐b‐premiums‐and‐deductibles?ceid=9903174&emci=19d1679d‐e769‐ee11‐9937‐00224832eb73&emdi=fa03f56d‐f869‐ee11‐9937‐00224832eb73
- 912. U.S. Centers for Medicare & Medicaid Services . What Are Long‐Term Care Hospitals? CMS Product No. 11347. . Revised June 2019. Accessed December 15, 2023. https://www.medicare.gov/Pubs/pdf/11347‐Long‐Term‐Care‐Hospitals.pdf
- 913. U.S. Centers for Medicare & Medicaid Services . Original Medicare (Part A and B) Eligibility and Enrollment. https://www.cms.gov/Medicare/Eligibility‐and‐Enrollment/OrigMedicarePartABEligEnrol
- 914. Ochieng N, Fuglesten Biniek J, Freed M, Damico A, Neuman T. Medicare Advantage in 2023: Enrollment update and key trends. Kaiser Family Foundation. August 9, 2023. Accessed November 25, 2023. Accessed December 15, 2023. Accessed December 15, 2023. Available at: https://www.kff.org/medicare/issue‐brief/medicare‐advantage‐in‐2023‐enrollment‐update‐and‐key‐trends/
- 915. U.S. Centers for Medicare & Medicaid Services . How Do Medicare Advantage Plans Work? https://www.medicare.gov/sign‐up‐change‐plans/types‐of‐medicare‐health‐plans/medicare‐advantage‐plans/how‐do‐medicare‐advantage‐plans‐work
- 916. U.S. Centers for Medicare & Medicaid Services . What's Medicare? What's Medicaid? CMS Product No. 11306. https://www.medicare.gov/Pubs/pdf/11306‐Medicare‐Medicaid.pdf
- 917. U.S. Department of Health and Human Services . What is Long‐Term Care Insurance? Accessed December 15, 2023. Available at: https://acl.gov/ltc/costs‐and‐who‐pays/what‐is‐long‐term‐care‐insurance
- 918. American Association for Long‐Term Care Insurance . Long‐term care insurance facts ‐ data ‐ statistics ‐ 2022 reports. Accessed November 15, 2023. Available at: https://www.aaltci.org/long‐term‐care‐insurance/learning‐center/ltcfacts‐2022.php
- 919. AHIP . Long‐term care insurance coverage: State‐to‐state 2023. Accessed November 15, 2023. Available at: https://www.ahip.org/documents/AHIP_LTC_State_Data_Report.pdf
- 920. U.S. Department of the Treasury . Long‐Term Care Insurance: Recommendations for Improvement of Regulation. Report of the Federal Interagency Task Force on Long‐Term Care Insurance. August 2020. Accessed December 16, 2023. Accessed December 15, 2023. Accessed December 15, 2023. Available at: https://home.treasury.gov/system/files/136/Report‐Federal‐Interagency‐Task‐Force‐Long‐Term‐Care‐Insurance.pdf
- 921. Washington State Legislature . Chapter 50B.04 RCW. Long‐Term Services and Supports Program. https://app.leg.wa.gov/RCW/default.aspx?cite=50B.04
- 922. Washington State Department of Social and Health Services . About the WA Cares Fund. https://wacaresfund.wa.gov/about‐the‐wa‐cares‐fund/
- 923. LTCNews . Multiple states considering implementing long‐term care tax. Updated July 22, 2023. Accessed November 15, 2023. Available at: https://www.ltcnews.com/articles/multiple‐states‐considering‐implementing‐long‐term‐care‐tax
- 924. U.S. Centers for Medicare & Medicaid Services . Seniors & Medicare and Medicaid enrollees. Accessed December 15, 2023. Available at: https://www.medicaid.gov/medicaid/eligibility/seniors‐medicare‐and‐medicaid‐enrollees/index.html
- 925. U.S. Centers for Medicare & Medicaid Services . Medicare and hospice benefits: Getting Started. Care and support for people who are terminally ill. CMS Product No. 11361. Revised March 2020. Accessed December 15, 2023. Available at www.medicare.gov/Pubs/pdf/11361‐Medicare‐Hospice‐Getting‐Started.pdf
- 926. De Vleminck A, Morrison RS, Meier DE, Aldridge MD. Hospice care for patients with dementia in the United States: A longitudinal cohort study. J Am Med Dir Assoc. 2018;19:633‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927. U.S. Centers for Medicare & Medicaid Services . Post‐Acute Care and Hospice Provider Data 2017. Accessed December 15, 2023. Available at: https://www.hhs.gov/guidance/document/post‐acute‐care‐and‐hospice‐provider‐data‐0
- 928. Davis MA, Chang C‐H, Simonton, S , Bynum J. Trends in US Medicare decedents' diagnosis of dementia from 2004 to 2017. JAMA Health Forum. 2022;3(4):e220346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929. Aldridge MD, Hunt L, Husain M, Li L, Kelley A. Impact of Comorbid Dementia on Patterns of Hospice Use. J Palliat Med. 2022;25(3):396‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930. Russell D, Diamond EL, Lauder B, et al. Frequency and risk factors for live discharge from hospice. J Am Geriatr Soc. 2017;65:1726‐1732. [DOI] [PubMed] [Google Scholar]
- 931. U.S. Department of Health and Human Services . U.S. Centers for Medicare & Medicaid Services. CMS Manual System. Medicare Program; FY 2024 Hospice Wage Index and Payment Rate Update, Hospice Conditions of Participation Updates, Hospice Quality Reporting Program Requirements, and Hospice Certifying Physician Provider Enrollment Requirements. Document Number 2023‐16116. Accessed November 10, 2023. Available at: https://www.federalregister.gov/documents/2023/08/02/2023‐16116/medicare‐program‐fy‐2024‐hospice‐wage‐index‐and‐payment‐rate‐update‐hospice‐conditions‐of [Google Scholar]
- 932. National Archives . Code of Federal Regulations (eCFR). Certification of terminal illness. Accessed December 15, 2023. Available at: https://www.ecfr.gov/current/title‐42/chapter‐IV/subchapter‐B/part‐418/subpart‐B/section‐418.22
- 933. Gozalo P, Plotzke M, Mor V, Miller SC, Teno JM. Changes in Medicare costs with the growth of hospice care in nursing homes. N Engl J Med. 2015;372:1823‐1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934. Miller SC, Lima JC, Looze J, Mitchell SL. Dying in U.S. nursing homes with advanced dementia: How does health care use differ for residents with, versus without, end‐of‐life Medicare skilled nursing facility care? J Palliat Med. 2012;15:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935. Miller SC, Gozalo P, Mor V. Hospice enrollment and hospitalization of dying nursing home patients. Am J Med. 2001;11(1):38‐44. [DOI] [PubMed] [Google Scholar]
- 936. Kiely DK, Givens JL, Shaffer ML, Teno JM, Mitchell SL. Hospice use and outcomes in nursing home residents with advanced dementia. J Am Geriatr Soc. 2010;58(12):2284‐2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937. Shega JW, Hougham GW, Stocking CB, Cox‐Hayley D, Sachs GA. Patients dying with dementia: Experience at the end of life and impact of hospice care. J Pain Symptom Manage. 2008;35(5):499‐507. [DOI] [PubMed] [Google Scholar]
- 938. Lin PJ, Zhu Y, Olchanski N, et al. Racial and ethnic differences in hospice use and hospitalizations at end‐of‐life among medicare beneficiaries with dementia. JAMA Netw Open. 2022;5(6):e2216260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939. Miller SC, Lima JC, Orna I, Martin E, Bull J. Hanson LC. Specialty palliative care consultations for nursing home residents with dementia. J Pain Symptom Manage. 2017;54:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940. Palmer MK, Jacobson M, Enguidanos S. Advance care planning for Medicare beneficiaries increased substantially, but prevalence remained low. Health Aff. 2021;40:613‐621. [DOI] [PubMed] [Google Scholar]
- 941. Zhu Y, Olchanski N, Cohen JT, Freund KM, Faul JD, Fillit HM, Neumann PJ, Lin PJ. Life‐sustaining treatments among Medicare beneficiaries with and without dementia at the end of life. J Alzheimers Dis. 2023;96(3):1183‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942. Bynum JPW, Meara E, Chang C‐H, Rhoads JM. Our Parents, Ourselves: Health Care for an Aging Population. A Report of the Dartmouth Atlas Project. The Dartmouth Institute for Health Policy & Clinical Practice; 2016. [PubMed]
- 943. Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube feeding in U.S. nursing home residents with advanced dementia, 2000‐2014. JAMA. 2016;316(7):769‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944. Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System, Mortality 1999‐2020 on CDC WONDER Online Database, released in 2021 . Data are from the Multiple Cause of Death Files, 1999‐2020, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed December 12, 2023. Available at: http://wonder.cdc.gov/ucd‐icd10.html
- 945. Centers for Disease Control and Prevention, National Center for Health Statistics . National Vital Statistics System, Mortality 2018‐2021 on CDC WONDER Online Database, released in 2021. Data are from the Multiple Cause of Death Files, 2018‐2021, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed December 12, 2023. Available at: http://wonder.cdc.gov/ucd‐icd10‐expanded.html
- 946. Park S, Chen J. Racial and ethnic patterns and differences in health care expenditures among Medicare beneficiaries with and without cognitive deficits or Alzheimer's disease and related dementias. BMC Geriatrics. 2020;20:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947. Gilligan AM, Malone DC, Warholak TL, Armstrong EP. Health disparities in cost of care in patients with Alzheimer's disease: An analysis across 4 state Medicaid populations. Am J Alzheimers Dis Other Dement. 2013;28(1):84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948. Lin P‐J, Zhong Y, Fillit HM, Cohen JT, Neumann PJ. Hospitalizations for ambulatory care sensitive conditions and unplanned readmissions among Medicare beneficiaries with Alzheimer's disease. Alzheimers Dement. 2017;13(10):1174‐1178. [DOI] [PubMed] [Google Scholar]
- 949. U.S. Department of Health and Human Services . Reduce the proportion of preventable hospitalizations in older adults with dementia — DIA‑02. Healthy People 2030. Accessed December 15, 2023. Available at: https://health.gov/healthypeople/objectives‐and‐data/browse‐objectives/dementias/reduce‐proportion‐preventable‐hospitalizations‐older‐adults‐dementia‐dia‐02
- 950. Davydow DS, Zibin K, Katon WJ, et al. Neuropsychiatric disorders and potentially preventable hospitalizations in a prospective cohort study of older Americans. J Gen Intern Med. 2014;29(10):1362‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 951. Guterman EL, Allen IE, Josephson SA, et al. Association between caregiver depression and emergency department use among patients with dementia. JAMA Neurol. 2019;76:1166‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini‐Cog performance: Novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8(1):8‐16. [DOI] [PubMed] [Google Scholar]
- 953. Lin PJ, Fillit HM, Cohen JT, Neumann PJ. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer's disease and related disorders. Alzheimers Dement. 2013;9(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 954. MacNeil‐Vroomen JL, Nagurney JM, Allore HG. Comorbid conditions and emergency department treat and release utilization in multimorbid persons with cognitive impairment. Am J Emerg Med. 2020;38(1):127‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955. Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff. 2014;33(4):683‐690. [DOI] [PubMed] [Google Scholar]
- 956. White EM, Kosar CM, Rahman M, Mor V. Trends in hospitals and skilled nursing facilities sharing medical providers. Health Affairs. 2020;39(8):1312‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 957. Chidambaram P, Burns A. Ten things about long‐term services and supports (LTSS). Kaiser Family Foundation. Accessed November 25, 2023. November 16, 2023. Available at: https://www.kff.org/medicaid/issue‐brief/10‐things‐about‐long‐term‐services‐and‐supports‐ltss/ [Google Scholar]
- 958. U.S. Centers for Medicare & Medicaid Services . Medicare COVID‐19 cases & hospitalizations. January 2022. Accessed December 15, 2023. Available at: https://data.cms.gov/covid‐19/medicare‐covid‐19‐cases‐hospitalizations/data
- 959. Lamont H, Samson LW, Zuckerman R, Dey J, Oliveira I, Tarazi W. The Impact of COVID‐19 on Medicare Beneficiaries with Dementia (Issue Brief). Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. April 6, 2021. [Google Scholar]
- 960. Wang Q, Davis PB, Gurney ME, Xu R. COVID‐19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021;17(8):1297‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961. Centers for Medicare & Medicaid Services . The Impact of COVID‐19 on Medicare Beneficiaries in Nursing Homes. Accessed December 15, 2023. Available at: https://www.cms.gov/files/document/medicare‐covid‐19‐nursing‐home‐analysis.pdf
- 962. Cubanski J, Neuman T. FAQs on Medicare Financing and Trust Fund Solvency. Kaiser Family Foundation, March 16, 2021. Accessed December 15, 2023. Available at: https://www.kff.org/medicare/issue‐brief/faqs‐on‐medicare‐financing‐and‐trust‐fund‐solvency/ [Google Scholar]
- 963. Burke JF, Kerber KA, Langa KM, Albin RL, Kotagal V. Lecanemab: Looking before we leap. Neurology. 2023;101(15):661‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964. Rosenthal MB. Novel Alzheimer Disease Treatments and Reconsideration of US Pharmaceutical Reimbursement Policy. JAMA. 2023;330(6):505‐506. [DOI] [PubMed] [Google Scholar]
- 965. Global Eisai. Eisai's Approach to U.S . Pricing for Leqembi (Lecanemab), a Treatment for Early Alzheimer's Disease, Sets Forth Our Concept of “Societal Value of Medicine” in Relation to “Price of Medicine.” Published January 7, 2023. Accessed October 30, 2023. Available at: www.eisai.com/news/2023/news202302.html
- 966. BIogen . BIogen Announces Reduced Price for ADUHELM(R) to Improve Access for Patients with Early Alzheimer's Disease. Published December 20, 2021. Accessed October 30, 2023. Available at: https://investors.biogen.com/news‐releases/news‐release‐details/biogen‐announces‐reduced‐price‐aduhelmr‐improve‐access‐patients
- 967. U.S. Centers for Medicare & Medicaid Services . CMS announces new details of plan to cover new Alzheimer's drugs. Published June 22, 2023. Accessed November 10, 2023. Available at: https://www.cms.gov/newsroom/fact‐sheets/cms‐announces‐new‐details‐plan‐cover‐new‐alzheimers‐drugs
- 968. Pittock RR, Aakre J, Castillo AM, et al. Eligibility for Anti‐Amyloid Treatment in a Population‐Based Study of Cognitive Aging. Neurology. 2023;101(19):e1837‐e1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969. Alzheimer's Association . Changing the Trajectory of Alzheimer's Disease: How a Treatment by 2025 Saves Lives and Dollars. Accessed December 15, 2023. Available at https://www.alz.org/help‐support/resources/publications/trajectory_report
- 970. Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2014;18(1):25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971. Alzheimer's Association . 2018 Alzheimer's Disease Facts and Figures. Alzheimers Dement. 2018;14(3):408‐411. [Google Scholar]
- 972. Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer's Treatment. Santa Monica, CA: RAND Corporation; 2017. Accessed December 15, 2023. Available at: https://www.rand.org/pubs/research_reports/RR2272.html [Google Scholar]
- 973. Mattke S, Hanson M. Expected wait times for access to a disease‐modifying Alzheimer's treatment in the United States. Alzheimer's Dement. 2021;1‐4. [DOI] [PubMed] [Google Scholar]
- 974. Alzheimer's Impact Movement. A path to better dementia care: Alzheimer's Impact Movement (AIM) and the Alzheimer's Association. Accessed January 30, 2024. Available at: https://portal.alzimpact.org/media/serve/id/6077043a225cb
- 975. Hirschman KB, Hodgson NA. Evidence‐based interventions for transitions in care for individuals living with dementia. Gerontologist. 2018;58(suppl_1):S129‐S140. [DOI] [PubMed] [Google Scholar]
- 976. Centers for Medicare & Medicaid Services . What is care coordination? Care Coordination Measures Atlas Update. Publication 14‐0037‐EF. Last reviewed June 2014. Accessed January 30, 2024. Available at: https://www.ahrq.gov/ncepcr/care/coordination/atlas/chapter2.html
- 977. Centers for Medicare & Medicaid Services. Care coordination . Accessed February 12, 2024. Available at: https://www.cms.gov/priorities/innovation/key‐concepts/care‐coordination
- 978. U.S. Department of Health and Human Services . National Plan to Address Alzheimer's Disease: 2023 Update. Accessed February 12, 2024. Available at: https://aspe.hhs.gov/sites/default/files/documents/3c45034aec6cf63414b8ed7351ce7d95/napa‐national‐plan‐2023‐update.pdf
- 979. Alzheimer's Impact Movement (AIM) . Building a path to better dementia care. Accessed January 30, 2024. Available at: https://alzimpact.org/Building‐a‐Path‐to‐Better‐Dementia‐Care
- 980. Alzheimer's Impact Movement . Comprehensive Care for Alzheimer's Act fact sheet. Alzheimer's Impact Movement (AIM) and the Alzheimer's Association. March 2023. Accessed January 30, 2024. Available at: https://alzimpact.org/sites/default/files/2023‐03/Comprehensive%20Care%20for%20Alzheimer%27s%20Act%20%281%29.pdf [Google Scholar]
- 981. Alzheimer's Association . Medicare to improve dementia care for individuals living with Alzheimer's disease, caregivers [press release]. July 31, 2023. Accessed January 30, 2024. Available at: https://www.alz.org/news/2023/cms‐dementia‐care‐caregivers‐initiative‐guide
- 982. Centers for Medicare & Medicaid Services . Guiding an Improved Dementia Experience (GUIDE) Model. Accessed January 30, 2024. Available at: https://www.cms.gov/priorities/innovation/innovation‐models/guide
- 983. Bernstein A, Harrison KL, Dulaney S, et al. The role of care navigators working with people with dementia and their caregivers. J Alzheimers Dis. 2019;71(1):45‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984. Centers for Disease Control and Prevention. Patient navigation. Last reviewed December 1, 2022. Accessed January 30, 2024. Available at: https://www.cdc.gov/cancer/community‐resources/interventions/patient‐navigation.htm
- 985. Chan RJ, Milch VE, Crawford‐Williams F, et al. Patient navigation across the cancer care continuum: An overview of systematic reviews and emerging literature. CA Cancer J Clin. 2023;73(6):565‐589. [DOI] [PubMed] [Google Scholar]
- 986. Kallmyer BA, Bass D, Baumgart M, et al. Dementia care navigation: Building toward a common definition, key principles, and outcomes. Alzheimers Dement (NY). 2023;9(3):e12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987. McBrien KA, Ivers N, Barnieh L, et al. Patient navigators for people with chronic disease: A systematic review. PLoS One. 2018;13(2):e0191980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988. Ahuja R, McDermott M, Ty D, for the Milken Institute Alliance to Improve Dementia Care . Guiding the care journey: Building dementia workforce and system capacity through care navigation. 2023. Accessed January 30, 2024. Available at: https://milkeninstitute.org/sites/default/files/2023‐03/Guiding%20the%20Care%20Journey‐%20Building%20Dementia%20Workforce%20and%20System%20Capacity%20through%20Care%20Navigation.pdf [Google Scholar]
- 989. Alzheimer's Association . Dementia care navigation guiding principles. Accessed January 30, 2024. Available at: https://www.alz.org/professionals/health‐systems‐medical‐professionals/dementia‐care‐guiding‐principles
- 990. Fazio S, Pace D, Maslow K, Zimmerman S, Kallmyer B. Alzheimer's Association Dementia Care Practice Recommendations. Gerontologist. 2018;58(suppl_1):S1‐S9. [DOI] [PubMed] [Google Scholar]
- 991. Patient navigator . National Cancer Institute (NCI) Dictionary of Cancer Terms. Accessed January 30, 2024. Available at: https://www.cancer.gov/publications/dictionaries/cancer‐terms/def/patient‐navigator
- 992. Health Resources & Services Administration (HRSA) Health Workforce . Train health care workers about dementia. Reviewed January 2023. Accessed February 2, 2024. Available at: https://bhw.hrsa.gov/alzheimers‐dementia‐training
- 993. Alzheimer's Association . Recognized dementia care training programs. Accessed February 2, 2024. Available at: https://www.alz.org/professionals/professional‐providers/dementia‐care‐training‐certification/recognized‐dementia‐care‐training‐programs
- 994. UCSF Memory and Aging Center . Care ecosystem toolkit. February 2018. Accessed February 2, 2024. Available at: https://bettercareplaybook.org/resources/care‐ecosystem‐toolkit
- 995. Wisconsin Alzheimer's Institute . Training home health clinicians to support caregivers. Accessed February 2, 2024. Available at: https://wai.wisc.edu/dementia‐capable‐wisconsin/home‐health‐caregiver‐education/
- 996. UCLA Health . Dementia care caregiver training videos. Accessed February 2, 2024. Available at: https://www.uclahealth.org/medical‐services/geriatrics/dementia/caregiver‐education/caregiver‐training‐videos
- 997. National Alzheimer's and Dementia Resource Center website. Accessed February 2, 2024. Available at: https://nadrc.acl.gov/home
- 998. National Association of Community Health Centers. Brain health. Accessed February 2, 2024. Available at: https://www.nachc.org/topic/brain‐health/
- 999. Healthcare.gov . Fee for service. Accessed February 2, 2024. Available at: https://www.healthcare.gov/glossary/fee‐for‐service/#:~:text=A%20method%20in%20which%20doctors,include%20tests%20and%20office%20visits
- 1000. Guterman S. Wielding the carrot and the stick: how to move the U.S. health care system away from fee‐for‐service payment. The Commonwealth Fund blog. August 27, 2013. Accessed February 2, 2024. Available at: https://www.commonwealthfund.org/blog/2013/wielding‐carrot‐and‐stick‐how‐move‐us‐health‐care‐system‐away‐fee‐service‐payment
- 1001. Werner RM, Emanuel EJ, Pham HH, Navathe AS. The future of value‐based payment: a road map to 2030. University of Pennsylvania Leonard Davis Institute of Health Economics. February 17, 2021. Accessed February 2, 2024. Available at: https://ldi.upenn.edu/our‐work/research‐updates/the‐future‐of‐value‐based‐payment‐a‐road‐map‐to‐2030/
- 1002. Alzheimer's Impact Movement . Dementia care management: a proposed framework for an alternative payment model. Alzheimer's Impact Movement (AIM) and the Alzheimer's Association. Accessed February 2, 2024. Available at: https://portal.alzimpact.org/media/serve/id/5f1b511b98110 [Google Scholar]
- 1003. Baumgart M. An alternative payment model could deliver better care to people with dementia ‐ and save Medicare money. Alzheimer's Impact Movement (AIM) blog. Accessed February 23, 2024. Available at: https://portal.alzimpact.org/media/serve/id/5f1b511b98110 [Google Scholar]
- 1004. Alzheimer's Association . Helpline. Accessed February 2, 2024. Available at: https://www.alz.org/help‐support/resources/helpline
- 1005. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006. U.S. Department of Labor . Changes in Basic Minimum Wages in Non‐Farm Employment Under State Law: Selected Years 1968 to 2020. Accessed December 15, 2023. Available at: https://www.dol.gov/agencies/whd/state/minimum‐wage/history
- 1007. U.S. Centers for Medicare & Medicaid Services . Your coverage options. Accessed December 11, 2023. Available at: https://www.medicare.gov/basics/get‐started‐with‐medicare/get‐more‐coverage/your‐coverage‐options


