Abstract
Background
Chronic noncancer pain (CNCP) is a major health problem, for which opioids provide one treatment option. However, evidence is needed about side effects, efficacy, and risk of misuse or addiction.
Methods
This meta-analysis was carried out with these objectives: to compare the efficacy of opioids for CNCP with other drugs and placebo; to identify types of CNCP that respond better to opioids; and to determine the most common side effects of opioids. We searched MEDLINE, EMBASE, CENTRAL (up to May 2005) and reference lists for randomized controlled trials of any opioid administered by oral or transdermal routes or rectal suppositories for CNCP (defined as pain for longer than 6 mo). Extracted outcomes included pain, function or side effects. Methodological quality was assessed with the Jadad instrument; analyses were conducted with Revman 4.2.7.
Results
Included were 41 randomized trials involving 6019 patients: 80% of the patients had nociceptive pain (osteoarthritis, rheumatoid arthritis or back pain); 12%, neuropathic pain (postherpetic neuralgia, diabetic neuropathy or phantom limb pain); 7%, fibromyalgia; and 1%, mixed pain. The methodological quality of 87% of the studies was high. The opioids studied were classified as weak (tramadol, propoxyphene, codeine) or strong (morphine, oxycodone). Average duration of treatment was 5 (range 1– 16) weeks. Dropout rates averaged 33% in the opioid groups and 38% in the placebo groups. Opioids were more effective than placebo for both pain and functional outcomes in patients with nociceptive or neuropathic pain or fibromyalgia. Strong, but not weak, opioids were significantly superior to naproxen and nortriptyline, and only for pain relief. Among the side effects of opioids, only constipation and nausea were clinically and statistically significant.
Interpretation
Weak and strong opioids outperformed placebo for pain and function in all types of CNCP. Other drugs produced better functional outcomes than opioids, whereas for pain relief they were outperformed only by strong opioids. Despite the relative shortness of the trials, more than one-third of the participants abandoned treatment.
Chronic non–cancer-related pain (CNCP) includes chronic pain of a nociceptive or neuropathic nature with variable influence by psychological and socioenvironmental factors. Opioids are the most potent analgesics available and are well established for the treatment of severe acute,1 surgical2 and cancer pain.3 However, their use to ameliorate CNCP is still controversial because of the side effects of opioids, the physical tolerance they build up (with the related withdrawal reactions and possibility of addiction) and anxiety over disapproval by regulatory bodies.4
The prevalence of CNCP varies according to the type of pain and the population studied. A study conducted in the United Kingdom in a community in the greater London area to quantify the prevalence of chronic pain found that 46.5% of the general population reported chronic pain; low-back problems and arthritis were the leading causes.5 A recent epidemiological study in Denmark6 found that nearly 130 000 adults, corresponding to 3% of the Danish population, regularly used opioids. CNCP had a prevalence of 19%, and 12% of those who had CNCP used opioid medications.
The objectives of this review were 4-fold: to determine the efficacy of opioids for CNCP compared with placebo; to compare the effectiveness of opioids for CNCP with that of other drugs; to identify categories of CNCP with better response to opioids; and to determine the most common side effects and complications of opioid therapy for CNCP, including incidences of opioid addiction and sexual dysfunction.
Methods
We followed the QUOROM guidelines for reporting meta-analyses of randomized controlled trials.7 We searched the literature up to May 2005 through the OVID interface: MEDLINE (from 1960), EMBASE (from 1988), the Cochrane Database of Systematic Reviews, the Cochrane Controlled Trials Register (CENTRAL), the ACP Journal Club and DARE. We also reviewed the reference lists in the articles, reviews and textbooks retrieved. Our search strategies for MEDLINE and EM BASE are available online as Appendix 1 and Appendix 2, respectively (all appendices for this article are available at www.cmaj.ca/cgi/content/full/174/11/1589/DC1). A single reviewer (J.A.S.) ran the electronic searches and entered the data into Reference Manager 10, removing all duplicates.
Each of 2 independent reviewers (A.D.F., J.A.S.) screened all titles and abstracts for studies that might meet the following inclusion criteria.
• Study characteristics: randomized controlled trials published in English, French or Spanish (the languages that could be read by the members of our team). Studies published only as abstracts were excluded.
• Study populations: people with CNCP, defined as pain for longer than 6 months, including neuropathic pain, osteoarthritis, rheumatoid arthritis, fibromyalgia, and back and musculoskeletal pain. Migraines, dental pain, abdominal pains (from chronic pancreatitis, kidney stones, etc.) and ischemic pain from vascular disease were excluded because they are usually not classified as CNCP.
• Interventions: any opioid administered via an oral, transdermal or rectal route for 7 days or more. We excluded comparisons of different opioids. We included tramadol, a centrally acting, synthetic opioid analgesic with 2 complementary mechanisms of action: binding of parent and M1 metabolite to μ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.8,9 In our review we classified the opioids studied as weak (propoxyphene, codeine, tramadol) or strong (oxycodone, morphine).10
• Outcomes: the data extracted were those quantifying pain (intensity or relief), function and side effects.
Hard copies of potential studies were retrieved and the same 2 independent reviewers met to reach consensus on the studies to be included. When in doubt, the study authors were contacted; if this was not possible, a third reviewer (A.M.G.) was consulted.
Methodological quality was assessed by the same 2 independent reviewers (unmasked to authors, journals or results), who met to reach consensus. In cases of disagreement, a third reviewer was consulted. We scored the studies from 0 to 5 with the instrument developed by Jadad and colleagues,11 which has 3 questions about a study's methods of randomization and double-blinding, and numbers of withdrawals. Studies scoring 3, 4 or 5 were considered to be of high quality; 0, 1 or 2, of low quality. The sensitivity analysis was repeated, but with the upper limit for low quality changed to 3, to see if this changed the main conclusion.
Meta-analyses were conducted with Revman 4.2.7 software, with standardized mean differences (SMDs) for pain relief and functional outcomes. For side effects, absolute risk differences (RDs) were calculated. Statistical heterogeneity was tested by Q test (χ2) and reported with the I2 statistic (in which higher values indicate higher heterogeneity). All meta-analyses were carried out with use of a random effects model. Sensitivity analyses were calculated within subgroups of studies (decided a priori) to assess the robustness of the main conclusions. Cumulative meta-analysis was conducted with STATA. The clinical significance of side effects was considered when the incidence was 10% or higher in the opioid or reference group.
Results
Data was abstracted from 41 randomized trials that met the inclusion criteria (Appendix 3). The characteristics of all the trials included for meta-analysis are summarized in Appendix 4 (www.cmaj.ca/cgi/content/full/174/11/1589/DC1). We found that 90% were either funded by or had 1 or more coauthors affiliated with the pharmaceuticals industry.
Although all trials included were described as randomized, patient assignment was judged adequate to be called random in only 17. The remainder did not report the randomization method, and its adequacy could could not be gauged. Thirty-nine trials were described as double-blinded (the exceptions were Jamison12 and Gobel13 and their respective coinvestigators); the majority of these (30 trials) were judged as having adequate methods of double-blinding, for example the double-dummy technique, the capsule-in-capsule technique, or just identical appearance of active and control medications. The average dropout rate in the opioid groups was 33%: 15% left because pain relief was inadequate and 21% withdrew because of side effects (some patients dropped out for both reasons). In the control groups, the average dropout rate was 38%: 30% because pain relief was inadequate and 10% because of side effects.
A total of 6019 patients with CNCP were included in this systematic review: 80% classified as having nociceptive pain (osteoarthritis, rheumatoid arthritis and back pain without radiculopathy); 12%, neuropathic pain (including diabetic neuropathy, postherpetic neuralgia, phantom limb pain and regional cervicobrachial pain syndrome); 7%, fibromyalgia; and 1%, mixed nociceptive and neuropathic pain. The average age of the people involved was 58.1 (range 40–71) years; 63% of participants were female and 85%, white.
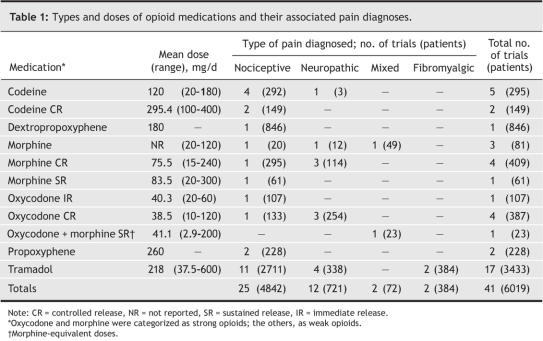
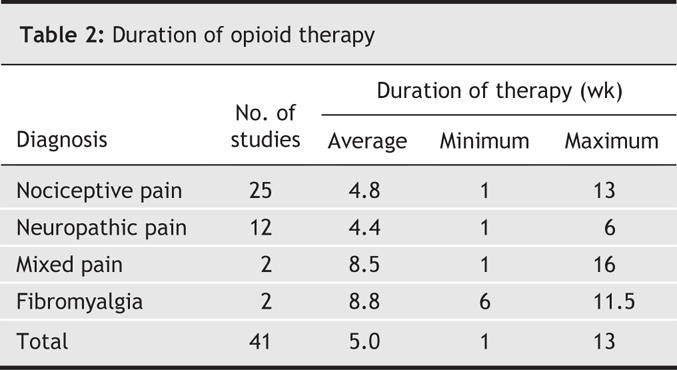
Table 1 shows the 5 different opioids prescribed in these 41 trials: codeine, morphine, oxycodone, tramadol and propoxyphene. All were administered orally; the range of doses used were reported in all 41 studies; the average dose, only in 28. Trials with parallel groups were longer than those of crossover design (5.6 v. 3.8 weeks, on average). Treatment duration also varied according to type of pain (Table 2): Durations of opioid therapy during the studies of fibromyalgia and mixed types of pain (mean lengths 8.8 and 8.5 weeks, respectively) were about twice as long as those involving patients with nociceptive and neuropathic pain (4.8 and 4.3 weeks, respectively).
Table 1
Table 2
Efficacy of opioids compared with placebo
Although we found 30 placebo-controlled trials of opioids for pain relief, only 28 reported data that could be meta-analyzed. Meta-analysis of these 28 studies showed results in favour of opioids (SMD –0.60, 95% confidence interval [CI] –0.69 to –0.50; Appendix 5); the 2 trials not included in the meta-analyses14,15 also had findings in favour of opioids. The sensitivity analysis (Appendix 6) showed no change in the conclusions with the type of opioid, methodological quality of the study (with cut-off points of either 2 or 3 points) or study design. Only for the patient category of “mixed pain” was the difference between opioids and placebo statistically nonsignificant, and this was a single trial with a small patient sample. Cumulative meta-analysis of these 28 trials (Appendix 7) revealed that the efficacy of opioids compared with placebo reached a stable effect size in 2002; in other words, the additional 8 trials published in 2003 and 2004 did not change the conclusions.
Similarly, the meta-analysis of the 20 trials that had data on functional outcomes showed results in favour of opioids (SMD –0.31, 95% CI –0.41 to –0.22; Appendix 8). The sensitivity analysis (Appendix 6) showed that in all cases the benefit of opioids compared with placebo for functional outcomes was statistically significant except for long-acting morphine, patients with “mixed pain” and “low quality” studies (defined as having 2 or fewer points in the Jadad scale). In these 3 cases, the overall effect was in favour of opioids, but the confidence interval included the null effect. Our cumulative meta-analysis of these 20 trials (Appendix 9, www.cmaj.ca/cgi/content/full/174/11/1589/DC1) corroborated the results of the cumulative meta-analysis of pain-relief outcomes.
Effectiveness of opioids compared with other drugs
Meta-analysis of the 8 trials with suitable data available that compared opioids and other analgesics for pain relief (which are summarized in Appendix 4D) showed that the difference between was statistically nonsignificant (SMD –0.05, 95% CI –0.32 to 0.21; Appendix 10). Sensitivity analysis (Appendix 11) showed that this conclusion did not change with the type of comparison group (nonsteroidal anti-inflammatory drugs [NSAIDs] or tricyclic antidepressants [TCAs]) or with the study's methodological quality (high or low). However, when sensitivity analysis was conducted based on the type of opioids, the strong opioids (oxycodone, morphine) were significantly more effective than other drugs for pain relief (SMD –0.34, 95% CI –0.67 to –0.01). One trial16 not included in the meta-analysis showed that the addition of codeine to a regimen of acetaminophen was superior to acetaminophen alone at 7 days of follow-up, but not afterward.
For functional outcomes, the other analgesics were significantly more effective than were opioids (SMD 0.16, 95% CI 0.03 to 0.30; Appendix 12, www.cmaj.ca/cgi/content/full/174/11/1589/DC1). This is primarily explained by the findings of 1 study17 that accounted for 74% of our meta-analysis, in which the authors compared dextropropoxyphene (a weak opioid) with diclofenac. In the other 2 comparisons of tramadol versus diclofenac18 and controlled-release morphine versus nortriptyline19, the differences were not statistically significant.
Side effects and other problematic outcomes
There were 6 side effects that occurred significantly more often among those taking opioids than those in the placebo groups: constipation (RD 16%, 95% CI 10%–22%); nausea (RD 15% (11%–19%); dizziness or vertigo (RD 8% (5%–12%); somnolence or drowsiness (RD 9% (5%–13%); vomiting (RD 5% (2%–7%); and dry skin, itching or pruritus (RD 4% (1%–6%). Risk differences for the other side effects noted (diarrhea, appetite loss, abdominal pain, dry mouth, headache, fatigue, blurred vision or accommodation disturbance, sleeplessness or insomnia, confusion, and sweating) were all statistically nonsignificant.
Compared with other drugs, only 3 side effects occurred significantly more frequently with opioids: the RD for nausea was 14% (95% CI 4%–25%); constipation, 9% (1%–17%); and somnolence or drowsiness, 6% (0–11%). One side effect, diarrhea (RD –2%, 95% CI –3% to 0), occurred less often with opioids than with other drugs. Risk differences for the other 12 side effects (vomiting, dizziness, dry skin, loss of appetite, abdominal pain, dry mouth, headache, fatigue, vision disturbance, insomnia, confusion and sweating) were not statistically significant.
Patients with history of addiction (alcohol or drugs) were excluded from 25 trials.12,14,18–40 In the others, this information was unreported. With regard to the incidence of opioid addiction developed during the trials, only 313,33,41 asked participants about symptoms and signs of addiction. Of these, 2 used indirect questioning; the other33 inquired if the patients experienced “drug craving,” and reported that 8.7% in the morphine and 4.3% in the placebo group developed drug craving.
Only 4 studies29,32,33,42 inquired about sexual activity by using the Pain Disability Index (PDI). This index consists of 7 self-reported disability subscales, one of which refers to sexual activity; each scale is graded from 0 to 10, where 0 = no disability and 10 = total disability. Only 2 studies give a specific score on sexual activity. In the first,32 with 46 patients randomly assigned to receive controlled-release codeine or placebo, the score was 4.1 and 6.3, respectively. In the other,29 which involved 45 patients, the score was 3.4 for controlled-release oxycodone and 4.5 for placebo. Both studies, therefore, suggested that patients taking opioid medications self-reported better sexual function than those taking placebo.
Interpretation
This systematic review demonstrated that, based on the available trials analyzed:
• Opioids were effective in the treatment of CNCP overall; they reduced pain and improved functional outcomes better than placebo.
• Opioids were more effective than placebo for both nociceptive and neuropathic pain syndromes.
• Tramadol reduced pain and improved functional outcomes in patients with fibromyalgia.
• Strong opioids (oxycodone and morphine) were significantly superior, statistically, to naproxen and nortriptyline (respectively) for pain relief but not for functional outcomes.
• Weak opioids (propoxyphene, tramadol and codeine) did not significantly outperform NSAIDs or TCAs for either pain relief or functional outcomes.
• Clinically (> 10%) and statistically, only constipation and nausea were significantly more common with opioids.
• Although recent studies43,44 have indicated that endocrinological abnormalities and erectile dysfunction can be experienced by patients taking opioid medication for chronic conditions, most researchers did not ask participants about sexual dysfunction. The few studies in our review that collected such data were relatively short for the observation of any endocrinological abnormalities. The only 2 studies29,32 that reported data on sexual function showed that patients taking opioids actually perceived themselves as doing better in terms of sexual behaviour compared with those in the control groups. Improvement of well-being secondary to better pain control may account for this result: the PDI is a patient-rated global rating of function and does not measure variables such as libido, sexual dysfunction or gonadal function, and cannot be used to estimate the risk of hypogonadism.
• Addiction or opioid abuse in patients with chronic pain cannot be assumed not to exist (despite popular statements), because the existing randomized trials are not designed to evaluate it; the duration of the trials was too short to allow for the development or detection of aberrant drug use, even if appropriate screening tools for addiction had been used. An adequate measure of “diagnosis of addiction” is also lacking in every study. For example, it is hazardous to equate reported “drug craving” or “reported symptoms and signs of addiction” with addiction. At best, this analysis suggests that only in a minority of comparative trials have investigators even attempted to approach this question. Furthermore, none of the studies have been methodologically sound enough to allow for conclusions about opioid addiction or abuse.
In regard to the contentious issue of whether opioids for pain patients can improve function, this meta-analysis depends on standardized measures of function that were adopted in the respective studies. Such instruments are often self-reported measures, such as the Pain Disability Index. The specific functional change is measured narrowly in terms of the functional measure used. For example, one cannot assume that functional improvement should be interpreted to mean improvement in any and all functions.
Most trials that compare opioids with other drugs were not adequately designed as equivalence or noninferiority trials.45,46 We therefore have some reservations about declaring any equivalence between opioids and these other drugs. There is a need for well-designed equivalence trials to compare opioids and other drugs.
Chronic pain is a long-term disorder. The studies included in this meta-analysis had various follow-up periods; most trials were not long enough to estimate the duration of efficacy of opioids in chronic pain, the potential for opioid tolerance, or long-range adverse effects such as hypogonadism or opioid abuse.
The majority of the studies included in this review were funded by the pharmaceutical industry. However, there is insufficient information to determine whether or not pharmaceutical-industry funding might introduce publication bias by not publishing small or unfavourable studies.
The results of our review were similar to those of others recently conducted. In 2004, Kalso and colleagues47 systematically reviewed studies of World Health Organization step 3 opioids for CNCP and found the mean decrease with opioids in pain intensity in most studies to be at least 30%, with comparable effects on neuropathic and musculoskeletal pain. Their review did not include evidence from studies of weak opioids (tramadol or codeine), nor did it assess the effectiveness of opioids compared with other analgesics. A Cochrane systematic review by Duhmke and associates48 published in 2004 showed that tramadol is an effective treatment for neuropathic pain. Eisenberg and coworkers' 2005 systematic review49 of 8 randomized controlled trials of opioid agonists (excluding tramadol) for neuropathic pain demonstrated opioid efficacy for spontaneous neuropathic pain with intermediate-term follow-up. Moore and McQuay50 recently published a systematic review of the side effects of opioids for chronic nonmalignant pain; they found the most common adverse effects to be dry mouth (25%), nausea (21%) and constipation (15%).
Our cumulative meta-analyses for placebo-controlled trials of orally administered opioids in regard to pain relief and functional outcomes showed that additional placebo-controlled trials of these outcomes are desirable only for other-than-oral routes of administration. For example, we found no reports of placebo-controlled trials of transdermal or rectal routes of administration of opioids, nor infusion programs for chronic pain. More refined experimental strategies will be required to assess other outcomes such as opioid abuse or addiction, sexual dysfunction and hypogonadism. Solid conclusions about the relative effectiveness and risk or benefit of opioids compared with other nonopioid drugs are still to be determined in adequately designed equivalence trials. Future trials of opioids for CNCP should consistently have well-defined methods and follow-up periods adequate in length to assess long-term complications such as sexual dysfunction or addiction. More attention should be paid to factors affecting methodological rigour, such as success of blinding, avoidance of dropouts, and adequate intention-to-treat analysis.
Supplementary Material
Acknowledgments
Andrea Furlan is funded by grants from the Canadian Institute of Health Research (CIHR) and by the University of Toronto Centre for the Study of Pain. We thank Cynthia Chen for her assistance with the cumulative meta-analyses.
Footnotes
This article has been peer reviewed.
Contributors: All authors participated in the conception and writing of the paper, and approved the final version for publication. Andrea Furlan wrote the protocol, performed the data extraction and was responsible for quality assessment, statistical analyses and report writing. Juan Sandoval was responsible for literature searches, data extraction and quality assessment. Angela Mailis-Gagnon was the principal investigator and oversaw the project and the writing of protocols and the final paper. Eldon Tunks was consulted during the protocol, analysis and interpretation phases as a content expert.
Competing interests: None declared.
Correspondence to: Dr. Angela Mailis-Gagnon, Medical Director, Comprehensive Pain Program, Toronto Western Hospital, 399 Bathurst St., Rm. 4F811, Toronto ON M5T 2S8; fax 416 603-5725; angela.mailis@uhn.on.ca
REFERENCES
- 1.Gordon DB, Dahl J, Phillips P, et al. The use of “as-needed” range orders for opioid analgesics in the management of acute pain: a consensus statement of the American Society for Pain Management Nursing and the American Pain Society. Pain Manag Nurs 2004;5:53-8. [DOI] [PubMed]
- 2.Walder B, Schafer M, Henzi I, et al. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain: a quantitative systematic review. Acta Anaesthesiol Scand 2001;45:795-804. [DOI] [PubMed]
- 3.Bruera E, Kim HN. Cancer pain. JAMA 2003;290:2476-9. [DOI] [PubMed]
- 4.Collett BJ. Chronic opioid therapy for non-cancer pain. Br J Anaesth 2001;87:133-43. [DOI] [PubMed]
- 5.Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet 1999;354:1248-52. [DOI] [PubMed]
- 6.Eriksen J, Jensen MK, Sjogren P, et al. Epidemiology of chronic non-malignant pain in Denmark. Pain 2003;106:221-8. [DOI] [PubMed]
- 7.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900. [DOI] [PubMed]
- 8.Pozos-Guillen AJ, Aguirre-Banuelos P, Arellano-Guerrero A, et al. Evidence of self-synergism in the antinociceptive effect of tramadol in rats. Proc West Pharmacol Soc 2004;47:117-9. [PubMed]
- 9.Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an “atypical” opioid analgesic. J Pharmacol Exp Ther 1992;260:275-85. [PubMed]
- 10.Repchinsky C, editor. Table 2: opioid analgesics. Approximate analgesic equivalences. In: Compendium of pharmaceuticals and specialties. Ottawa: Canadian Pharmacists Association; 2004. p. 457.
- 11.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed]
- 12.Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain: a randomized prospective study. Spine 1998;23:2591-600. [DOI] [PubMed]
- 13.Gobel H, Stadler TH. Treatment of pain due to postherpetic neuralgia with tramadol: results of an open, parallel pilot study vs clomipramine with and without levomepromazine. Clin Drug Invest 1995;10:208-14.
- 14.Ruoff GE. Slowing the initial titration rate of tramadol improves tolerability. Pharmacotherapy 1999;19:88-93. [DOI] [PubMed]
- 15.Schnitzer TJ, Gray WL, Paster RZ, et al. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol 2000;27:772-8. [PubMed]
- 16.Kjaersgaard-Andersen P, Nafei A, Skov O, et al. Codeine plus paracetamol versus paracetamol in longer-term treatment of chronic pain due to osteoarthritis of the hip: a randomised, double-blind, multi-centre study. Pain 1990;43:309-18. [DOI] [PubMed]
- 17.Parr G, Darekar B, Fletcher A, et al. Joint pain and quality of life: results of a randomised trial. Br J Clin Pharmacol 1989;27:235-42. [DOI] [PMC free article] [PubMed]
- 18.Pavelka K, Peliskova Z, Stehlikova H, et al. Intraindividual differences in pain relief and functional improvement in osteoarthritis with diclofenac or tramadol. Clin Drug Invest 1998;16:421-9. [DOI] [PubMed]
- 19.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2002;59:1015-21. [DOI] [PubMed]
- 20.Glowinski J, Boccard E. Placebo-controlled study of the analgesic efficacy of a paracetamol 500 mg/codeine 30 mg combination together with low-dose vs high-dose diclofenac in rheumatoid arthritis. Clin Drug Invest 1999;18:189-97.
- 21.Ruoff GE, Rosenthal N, Jordan D, et al. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin Ther 2003;25:1123-41. [DOI] [PubMed]
- 22.Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: results from a randomized, placebo-controlled, double-blind trial and an open-label extension trial. J Pain Symptom Manage 2002;23:278-91. [DOI] [PubMed]
- 23.Silverfield JC, Kamin M, Wu SC, et al. Study Group. Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther 2002;24:282-97. [DOI] [PubMed]
- 24.Schnitzer TJ, Gray WL, Paster RZ, et al. Efficacy of tramadol in treatment of chronic low back pain [comment]. J Rheumatol 2000;27:772-8. [PubMed]
- 25.Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol 2000;27:764-71. [PubMed]
- 26.Schnitzer TJ, Kamin M, Olson WH. Tramadol allows reduction of naproxen dose among patients with naproxen-responsive osteoarthritis pain: a randomized, double-blind, placebo-controlled study. Arthritis Rheum 1999;42:1370-7. [DOI] [PubMed]
- 27.Caldwell JR, Hale ME, Boyd RE, et al. Treatment of osteoarthritis pain with controlled release oxycodone or fixed combination oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double blind, randomized, multicenter, placebo controlled trial. J Rheumatol 1999;26:862-9. [PubMed]
- 28.Roth SH. Efficacy and safety of tramadol HCl in breakthrough musculoskeletal pain attributed to osteoarthritis. J Rheumatol 1998;25:1358-63. [PubMed]
- 29.Watson CP, Moulin D, Watt-Watson J, et al. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain 2003;105:71-8. [DOI] [PubMed]
- 30.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology 1998;50:1842-6. [DOI] [PubMed]
- 31.Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology 1998;50:1837-41. [DOI] [PubMed]
- 32.Arkinstall W, Sandler A, Goughnour B, et al. Efficacy of controlled-release codeine in chronic non-malignant pain: a randomized, placebo-controlled clinical trial. Pain 1995;62:169-78. [DOI] [PubMed]
- 33.Moulin DE, Iezzi A, Amireh R, et al. Randomised trial of oral morphine for chronic non-cancer pain. Lancet 1996;347:143-7. [DOI] [PubMed]
- 34.Boureau F, Boccard E. Placebo-controlled study of analgesic efficacy of 500 mg paracetamol 30 mg codeine association combined with a low dose of diclofenac versus high dose of diclofenac in rheumatoid arthritis. Acta Ther 1991;17:123-36.
- 35.Moran C. MST continus tablets and pain control in severe rheumatoid arthritis. Br J Clin Res 1991;2:1-12.
- 36.Boureau F, Legallicier P, Kabir-Ahmadi M. Tramadol in post-herpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Pain 2003;104:323-31. [DOI] [PubMed]
- 37.Emkey R, Rosenthal N, Wu S-C, et al. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double-blind, placebo-controlled trial. J Rheumatol 2004;31:150-6. [PubMed]
- 38.Russell J, Kamin M, Bennett RM, et al. Efficacy of tramadol in treatment of pain in fibromyalgia. J Clin Rheumatol 2000;6:250-7. [DOI] [PubMed]
- 39.Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology 2003;60:927-34. [DOI] [PubMed]
- 40.Peloso PM, Fortin L, Beaulieu A, et al. Analgesic efficacy and safety of tramadol/acetaminophen combination tablets (Ultracet) in treatment of chronic low back pain: a multicenter, outpatient, randomized, double blind, placebo controlled trial. J Rheumatol 2004;31:2454-63. [PubMed]
- 41.Fleischmann RM, Caldwell JR, Roth SH, et al. Tramadol for the treatment of joint pain associated with osteoarthritis: a randomized, double-blinded, placebo-controlled trial. Cur Ther Res 2001;62:113-28.
- 42.Maier C, Hildebrandt J, Klinger R, et al.; MONTAS Study Group. Morphine responsiveness, efficacy and tolerability in patients with chronic non-tumor associated pain — results of a double-blind placebo-controlled trial (MONTAS). Pain 2002;97:223-33. [DOI] [PubMed]
- 43.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med 2003;349:1943-53. [DOI] [PubMed]
- 44.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain 2002;3:377-84. [DOI] [PubMed]
- 45.McAlister FA, Sackett DL. Active-control equivalence trials and antihypertensive agents. Am J Med 2001;111:553-8. [DOI] [PubMed]
- 46.Jones B, Jarvis P, Lewis JA, et al. Trials to assess equivalence: the importance of rigorous methods [published erratum in BMJ 1996;313:550]. BMJ 1996;313:36-9. [DOI] [PMC free article] [PubMed]
- 47.Kalso E., Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372-80. [DOI] [PubMed]
- 48.Duhmke RM, Cornblath DD, Hollingshead JR. Tramadol for neuropathic pain. Cochrane Database Syst Rev 2004;(2):CD003726. [DOI] [PubMed]
- 49.Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA 2005;293:3043-52. [DOI] [PubMed]
- 50.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids [review]. Arthritis Res Ther 2005;7:R1046-51. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.