Abstract
A microscopic study of functional disorder–order folding transitions coupled to binding is performed for the p27 protein, which derives a kinetic advantage from the intrinsically disordered unbound form on binding with the phosphorylated cyclin A-cyclin-dependent kinase 2 (Cdk2) complex. Hierarchy of structural loss during p27 coupled unfolding and unbinding is simulated by using high-temperature Monte Carlo simulations initiated from the crystal structure of the tertiary complex. Subsequent determination of the transition-state ensemble and the proposed atomic picture of the folding mechanism coupled to binding provide a microscopic rationale that reconciles the initiation recruitment of p27 at the cyclin A docking site with the kinetic benefit for a disordered α-helix in the unbound form of p27. The emerging structural polarization in the ensemble of unfolding/unbinding trajectories and in the computationally determined transition-state ensemble is not determined by the intrinsic folding preferences of p27 but rather is attributed to the topological requirements of the native intermolecular interface to order β-hairpin and β-strand of p27 that could be critical for nucleating rapid folding transition coupled to binding. In agreement with the experimental data, the disorder–order folding transition for p27 is largely determined by the functional requirement to form a specific intermolecular interface that ultimately dictates the folding mechanism and overwhelms any local folding preferences for creating a stable α-helix in the p27 structure before overcoming the major free energy barrier.
It has been recently realized that a significant amount of protein domains and even entire proteins can lack intrinsic globular structure under physiological conditions suggesting a reappraisal of the conventional structure–function paradigm (1, 2). These proteins, termed intrinsically unstructured (1, 3), intrinsically disordered (2), or natively unfolded (4), can largely be composed of disordered segments in their functional state. The intrinsic plasticity and functional disorder–order folding transitions coupled to binding (5) can provide for these protein systems an important prerequisite for effective molecular recognition, including high specificity coupled with low affinity, the ability to bind with several different targets, a precise control and simple regulation of the binding thermodynamics, and the increased rates of specific macromolecular association (1–4).
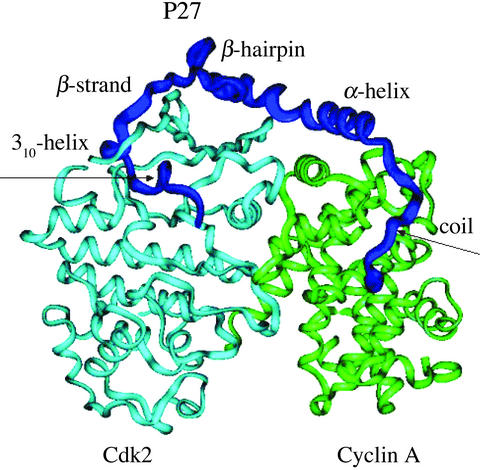
Coupling of folding and binding accompanied by a disorder–order transition has been experimentally detected for p27Kip1 (6–8), a member of the Kip/Cip protein family involved in cell cycle regulation by inhibiting cyclin-dependent kinase (Cdk) activity (6–9). The crystal structure of the tertiary complex with the 69-aa N-terminal inhibitory domain of p27 bound to the phosphorylated cyclin A-Cdk2 complex (6) has revealed an ordered conformation of p27 comprising a coil, α-helix, β-hairpin, β-strand, and 310-helix (Fig. 1). According to the proposed binding mechanism (6, 7), substrate recruitment to Cdk2 is carried out by the “hot-spot” hydrophobic docking site on cyclin A that forms the initial tertiary contacts with the conserved Leu-Phe-Gly motif of p27 serving as an initial anchor in the complex formation. Instead of contributing directly to folding of p27, the hydrophobic residues of the coil, β-hairpin, and β-strand make extensive interfacial contacts with the complex that are accompanied by the specific intermolecular contacts with the N-terminal lobe of Cdk2, including six intermolecular backbone–backbone hydrogen bonds, formed by the β-strand and stabilizing interactions of the 310-helix, which protrudes deeply into the catalytic cleft of Cdk2 (Fig. 1).
Figure 1.
The crystal structure of the tertiary complex with the bound 69-aa p27 protein (residues 25–93) comprising sequentially the coil (residues from 25 to 34), α-helix (residues from 35 to 60), β-hairpin (residues from 61 to 71), β-strand (residues 75 to 81), and 310-helix (residues from 85 to 90). Schematic drawing shows p27 in blue, cyclin A in green, and Cdk2 in light blue.
CD spectroscopy experiments have shown that the unbound form of the p27 inhibitory domain is intrinsically disordered but not entirely unfolded with only barely stable helical structure that presages the α-helix (8). Reducing or increasing the stability of the α-helix in the unbound p27 with proline or alanine substitutions has a marginal effect on binding thermodynamics (8). However, although the disruption of the α-helix in the unbound form by proline mutations does not affect the rate of p27 binding, enhancing the α-helix propensity on engineering alanine substitutions in this region hinders binding in kinetic terms by slowing down considerably the rate of formation for the inhibited complex (8). Consequently, a partially disordered α-helix is kinetically advantageous during coupled folding and binding of the intrinsically unstructured p27 protein.
The process of complex formation when one or both binding partners is highly flexible or even completely disordered can evolve over a vast configurational space and on hierarchical time scales that makes the complexity of molecular recognition for these systems analogous to the protein-folding problem (10, 11). Protein-folding and molecular-recognition phenomena, which share a number of universal aspects, including the existence of a thermodynamically stable native structure, a large number of conformational states available to the system, and the complex nature of interactions, can be united from a dynamic perspective of the energy landscape theory (10–13). A balance between the loss of conformational entropy and the energy gain on the native structure formation is a universal aspect in folding and binding that ultimately determines the thermodynamic free energy barrier of the reaction (14, 15). In folding, the interplay between these opposing thermodynamic contributions at an early stage of the process can encourage ordering of simple topological motifs with local interactions rather than favoring many nonlocal interactions. These physical arguments have been used to infer the important role of the native structure in determining protein folding mechanisms discovered from comprehensive experimental (16, 17) and theoretical studies (18–22), in which striking similarities in the folding transition states (TS) and hierarchy of folding were found for different, small, and fast-folding protein sequences with a common native topology. The energy landscape approach and free energy functional methods that explicitly describe the interplay between energetics and topology in folding (14) have led to an elegant flycasting mechanism of molecular recognition (15), proposed to explain kinetic advantages of unstructured proteins in binding. By analogy with the folding funnel mechanism (10), the unfolded protein would bind weakly at relatively long distances, followed by coupled folding and a binding mechanism as it “reels in” on the binding partner, using a greater “capture radius” than a folded protein to provide enhanced binding rates (15).
Balancing the desired realism of all-atom computer simulations of folding and binding (13, 23, 24) with the underlying stochastic nature of these phenomena, which requires averaging over a large number of independent simulations, can be addressed by the use of equilibrium free energy methods (25) and nonequilibrium kinetic temperature-induced unfolding simulations (26–28). In this work, a microscopic study of the p27 binding mechanism is conducted by simulating hierarchy of structural loss during p27 coupled unfolding and unbinding from the crystal structure of the tertiary complex using high-temperature Monte Carlo simulations and a simplified yet all-atom energetic model (29–31). We propose a multistage mechanism of molecular recognition for p27 that proceeds first through the formation of an encounter intermediate, corresponding to the ensemble of nonspecific largely unstructured conformations weakly bound to the complex. At the second, rate-limiting stage of the coupled folding and binding reaction, this intermediate undergoes a disorder–order transition to the ensemble of ordered native-like states. The determination of the TS ensemble (TSE) using a rigorous kinetic analysis (32, 33) and the proposed atomic picture of the folding mechanism coupled to binding provide a microscopic rationale of the disorder–order transition for the p27 protein that agrees with the available experimental data and supports the flycasting model of molecular recognition for unstructured proteins.
Molecular Recognition Energy Model.
The knowledge-based simplified energetic model has been described in detail (29–31) and includes intramolecular energy terms for the ligand, given by torsional and nonbonded contributions of the DREIDING force field (34), and intermolecular ligand–protein steric and hydrogen bond interaction terms calculated from a simplified piecewise linear potential summed over all protein and ligand heavy atoms (see detailed information in Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). The parameters of the pairwise potential depend on the following different atom types: hydrogen-bond donor, hydrogen-bond acceptor, both donor and acceptor, carbon-sized nonpolar, and large nonpolar. Electronegative atoms with attached hydrogen are defined as donors, whereas oxygen and nitrogen atoms with no bound hydrogens are defined as acceptors. Hydroxyl groups are defined in this model to be both donor and acceptor types. A hydrogen bond interaction term is assigned to interactions between donor and acceptor, a repulsive interaction contribution is computed for donor–donor and acceptor–acceptor contacts, and a steric intermolecular term is assigned for all other contacts. The steric and hydrogen bond-like potentials have the same functional form, with an additional three-body contribution to the hydrogen bond term and to the repulsive interactions. Both the hydrogen bond interaction energy and the repulsive interaction contribution between donor–donor and acceptor–acceptor close contacts are modulated by an approximate angular dependence, which is determined by the relative orientation of the protein and ligand atoms (more detailed information is published as supporting information on the PNAS web site).
The simplified piecewise linear energy function, which has no singularities at interatomic distances and allows an enhanced sampling of the conformational space, has provided robust structure prediction for a variety of ligand–protein complexes, while faithfully reproducing structural and thermodynamic properties of ligand–protein binding, including stable native structure and appropriate energetics for the binding transitions (29–31). The used energy model, which is adequate in describing nonpolar and hydrogen bond intermolecular interactions, can follow the shape of the “true” potential and detect the density of low-energy states in the regions surrounding favorable ligand–protein-bound states. However, this function does not include an explicit electrostatic component that may lead to certain inaccuracies in quantifying the exact magnitude of ligand–protein interactions in the native structure.
Monte Carlo Simulations of Coupled Folding and Binding.
The cyclin A-Cdk2 portion of the tertiary complex is held fixed in its minimized crystallographic conformation, whereas rigid body degrees of freedom and a total of 169 rotatable angles of the p27 ligand are treated as independent variables during simulations. p27 conformations and orientations are simulated in a parallelepiped that encompasses the crystallographic structure of the p27 tertiary complex with a large 10.0-Å cushion added to every side of this box surrounding the complex, which guarantees a sufficiently unbiased conformational search. Monte Carlo simulations dynamically optimize the step sizes by taking into account the inhomogeneity of the molecular system (35). The acceptance ratio method is used to update the step sizes every cycle consisting of 1,000 sweeps. For equilibrium simulations, we equilibrated the system for 1,000 cycles, and then collected data during 5,000 cycles at each temperature. A sweep is defined as a single trial move for each degree of freedom of the system. The rms deviation (rmsd) from the native-bound p27 structure is the order parameter that measures the unfolding progress for a given state. The total number of intermolecular hydrogen bonds formed by p27 in the tertiary complex is another order parameter that monitors unbinding.
Results and Discussion
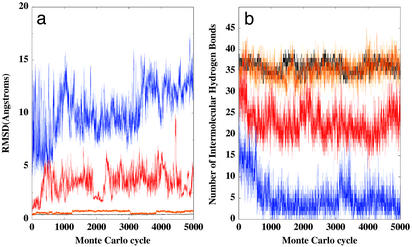
Hierarchy of structural loss during p27 coupled unfolding and unbinding is simulated by using 20 independent temperature-induced Monte Carlo simulations initiated at each temperature from the crystal structure of the tertiary complex at T = 400, 500, and 600 K. Although equilibrium simulations at T = 300 and 400 K generate stable, native-like p27 structures, temperature-induced motions at T = 500 K produce considerably larger fluctuations among distorted p27 conformations that are still characteristic of an expanded form of the native-bound structure and largely bound to the complex (Fig. 2). Only at T = 600 K is coupled unfolding and unbinding seen on the simulation time scale as p27 and undergoes an order–disorder transition to the ensemble of largely unstructured conformations that are weakly bound to the complex (Fig. 2). Rather than analyzing a typically diverse behavior of individual trajectories, we focus on the recurrent features in the hierarchy of structural loss observed in the ensemble of trajectories that could mimic the average effect seen in the experiment. The most persistent interactions of p27 at the intermolecular interface are formed by the β-hairpin and β-strand elements that maintain their structural integrity considerably longer during unbinding/unfolding than other p27 elements (Figs. 2 and 3). The shape of the distribution for p27 and its β-hairpin and β-strand components deviates considerably from the concerted picture: as the number of intermolecular hydrogen bonds progressively decreases and p27 gradually dissociates from the bound complex, these structural elements continue to maintain their native-bound conformation intact until the late stage of the process. Only after the majority of the intermolecular contacts are lost does the structural integrity of the β-hairpin and the β-strand elements begin to fade, accompanied by the depletion of the most stable intermolecular specific interactions, particularly six backbone–backbone hydrogen bonds formed by β-strand with the cyclin A-Cdk2 complex (Fig. 3).
Figure 2.
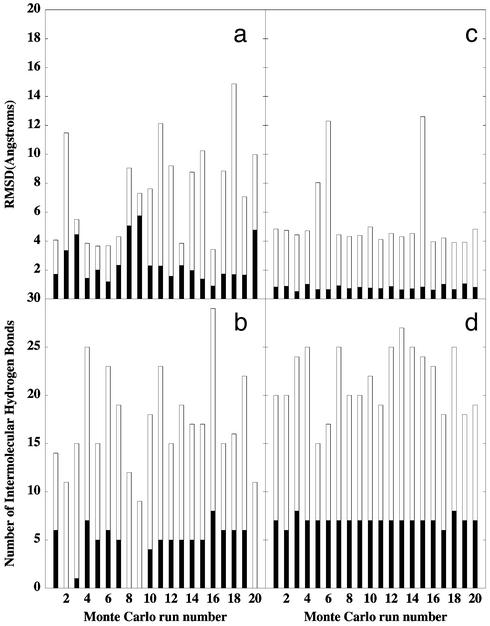
Time-dependent history of the rmsd from the crystal structure for the p27 protein (a) and the total number of the intermolecular hydrogen bonds formed by p27 (b) during simulations at T = 300 K (black), T = 400 K (orange), T = 500 K (red), and T = 600 K (blue).
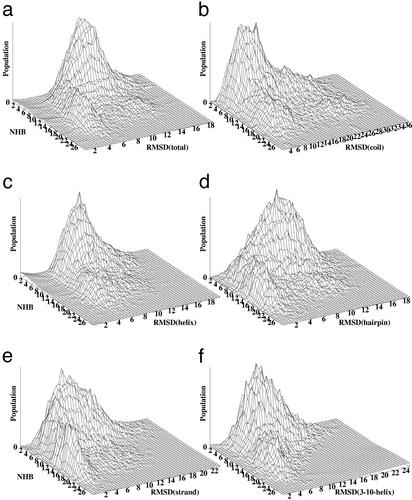
Figure 3.
Occupancy histograms monitor the hierarchy of structural loss for p27 (a), coil (b), α-helix (c), β-hairpin (d), β-strand (e), and 310-helix (f). The histograms are generated by averaging the results of 20 independent unfolding/unbinding simulations for p27 at T = 600 K and are represented as a function of the total number of the intermolecular hydrogen bonds (NHB) formed by p27 in the complex versus the rmsd from the crystal structure for p27 and for each of the p27 elements.
The α-helix and 310-helix are the first structural elements that begin to unbind/unfold in simulations and rarely populate their native orientations, which suggests that these structural elements of p27 may be the last ones to be ordered, after overcoming the rate-limiting free energy barrier. The shape of distributions for the α-helix and the 310-helix reveals a more symmetric picture, with a concerted loss in the native structure and the total number of intermolecular contacts (Fig. 3), which is more consistent with a simple funnel description when the interactions stabilizing the native-bound structure also stabilize partially folded conformations. Strikingly, after the vast majority of the intermolecular contacts are lost and p27 becomes largely unbound, the distribution of structural loss for the coil is still shifted toward rather moderate rmsd values from the native-bound state and reveals a noticeable occupancy of distorted yet native-like bound coil orientations (Fig. 3). Consequently, a significant population of unstructured p27 conformations can bind weakly at the cyclin A docking site, in agreement with the hypothesis suggesting this region as an initial anchor in the recognition process.
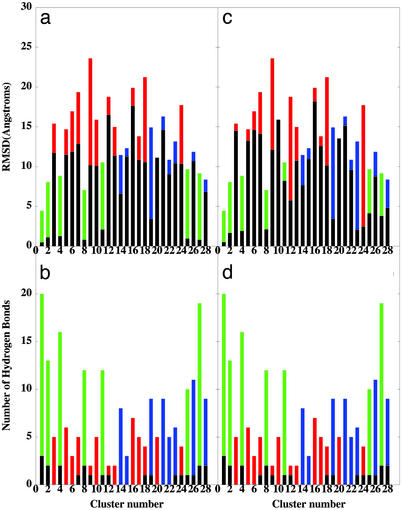
To characterize the nature of conformational ensembles represented and to select the putative TS conformations for a subsequent kinetic analysis, all generated conformational samples at T = 600 K were subjected to structural similarity analysis. Structures that are equivalent with 85% structural similarity threshold are grouped into the same cluster by comparing the intermolecular similarity coefficient (36). A total of 28 structurally different conformational clusters (Fig. 4) represent three major conformational families: the ensemble of largely unstructured and weakly bound states (clusters 3, 5, 6, 7, 9, 10, 12, 13, 16, 17, 18, 20, and 24), the ensemble of partially unfolded/unbound conformations (clusters 14, 15, 19, 21, 22, 23, 26, and 28), and the ensemble of native-like bound conformations (clusters 1, 2, 4, 8, 11, 25, and 27). We have found that the conformations from clusters 14, 19, 21, and 28 often, but not always, correspond to the states from the high-temperature unfolding/unbinding trajectories during the time interval when the character of the conformation rapidly changes from one state to another, i.e., may represent putative TS. Furthermore, these states may exhibit quite moderate rmsd values for the β-strand and β-hairpin segments of the protein, coexisting with the lack of intermolecular hydrogen bonds formed in these regions (Fig. 4).
Figure 4.
(a) The distribution of the mean rmsd values from the native-bound p27 conformation for clusters representing the ensemble unstructured and weakly bound states (red filled bars), the ensemble of partially unfolded/unbound states (blue filled bars), and the ensemble of native-like bound states (green filled bars) with the rmsd values of β-strand (black filled bars) and with the rmsd values of β-hairpin (black filled bars) (c). (b) The distribution of the mean number of the intermolecular hydrogen bonds formed by p27 in clusters representing these conformations ensembles (red, blue, and green filled bars, respectively) with the distribution for the β-strand (black filled bars) and for the β-hairpin (black filled bars) (d). The mean values are obtained by averaging over all conformations in a given cluster. Conformational ensembles are ordered according to the decreasing number of conformations in a given cluster.
A computational method for identifying TS conformations is applied in which a number of short trajectories are initiated from a putative TS conformation, followed by computing the probability that these trajectories reach the native state, which, for a TS conformation, should be close to 50% probability (32, 33). Twenty short kinetic simulations at T = 350 K were performed from 10 representative conformations of each of the determined clusters. These kinetic runs are conducted at a lower temperature and on a short time scale, constituting only 2% of the total simulation time necessary to consistently observe unfolding and unbinding at high-temperature simulations. We have found that the representative conformations from clusters 14 and 19 (Fig. 4) rapidly and consistently encounter the native-like states and the ensemble of unstructured weakly bound states with a similar, close to 50%, probability (Fig. 5). Although during kinetic runs 4, 5, 6, 7, 13, and 16 a rapid convergence to the native-like states is achieved, runs 2, 3, 8, 9, and 20 quickly encounter the unstructured unbound states (Fig. 5 a and b). A fraction of structures in the immediate region of the TS may oscillate around the initial TS conformation (as seen during kinetic runs 10, 14, 15, 17, and 18), fluctuating between becoming more and less native-like, but typically with the increasing content of the native intermolecular interface in the β-strand region (Fig. 5 a and b). The realism of the used all-atom energetic model along with a very short simulation time scale and the potential ruggedness on the top of a broad activation barrier could contribute to these deviations from 100% compliance in kinetic runs.
Figure 5.
The outcome of the 20 short kinetic runs for a representative conformation in the TSE (a and b) and in the ensemble of postcritical conformations (c and d). (a and c) The distribution of the rmsd values from the native bound conformation of p27 in the tertiary complex (empty bars), and the rmsd values of the β-strand (filled bars). (b and d) The distribution of the number of the intermolecular hydrogen bonds formed with the complex by p27 (empty bars) and by the β-strand (filled bars).
Inferring the mechanism of folding coupled to binding from nonequilibrium high-temperature unfolding/unbinding simulations implies the principle of microscopic reversability (or detailed balance), which is valid only under equilibrium conditions. Despite these obvious limitations, the atomic picture of the binding mechanism is deduced from the consistent features observed in the ensemble of unfolding/unbinding pathways rather from few highly diverse individual trajectories. In principle, the TSE cannot be rigorously located directly from high-temperature unfolding/unbinding simulations, because the TS can shift significantly with temperature changes. Nevertheless, we use the results of nonequilibrium high-temperature simulations not only to uncover the important mechanistic elements of the coupled folding and binding reaction, but also to generate the ensemble of putative TS that are subjected to a subsequent rigorous analysis at lower temperature. Importantly, we have mapped the TSE not between the metastable intermediate states found only at high temperatures but rather between the native structure of the tertiary complex and the ensemble of unfolded/unbound p27 conformations.
The determined TSE can be broadly described as an expanded form of the native-bound structure and consists of the states with the native-like topology in the β-hairpin and β-strand regions of the intermolecular interface and considerable flexibility in the coil, α-helix, and 310-helix regions (Figs. 4 and 6). The emerging structural polarization of the unfolding/unbinding trajectories and TSE is not determined by the intrinsic folding preferences of p27 but rather can be viewed as a consequence of the topological requirements of binding to minimize free energy cost associated with ordering the β-hairpin and β-strand intermolecular contacts, which could be critical for nucleating rapid folding transition coupled to binding.
Figure 6.
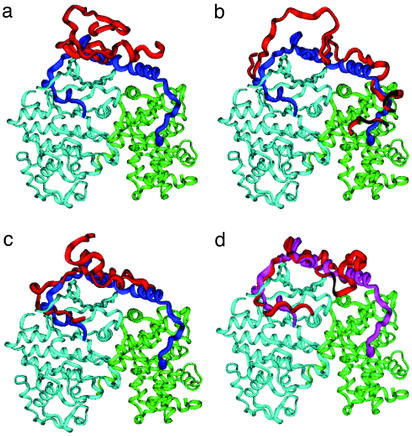
A schematic representation of the proposed p27 binding mechanism: (a) the ensemble of nonspecific, largely unbound conformations; (b) initiation recruitment of p27 at the cyclin A docking site; (c) the TSE; and (d) the ensemble of postcritical states. The major conformational ensembles (red) are superimposed onto the crystal structure of the bound p27 (blue), cyclin A (green), and Cdk2 (light blue).
We have also identified the ensemble of postcritical states (11) that are defined as the first stable structures that appear immediately after the TS is overcome and lead to a consistent and rapid refolding/rebinding to the native-bound structure during short kinetic runs (Fig. 4, clusters 4 and 11). The principal difference between the TSE (Fig. 5 a and b) and postcritical conformations (Fig. 5 c and d), which have a similar topology of the intermolecular interface, yet may diverge dramatically during kinetic runs, is determined by a considerable structural consolidation of the β-hairpin and β-strand intermolecular contacts for the postcritical states, in particular, by strengthening six backbone–backbone hydrogen bonds established by β-strand with the complex (Figs. 4 and 6). The differences in structural integrity of the β-hairpin and β-strand are reflected in larger rmsd fluctuations from the native structure for the conformations in the TSE (Fig. 4, clusters 14 and 19), as compared with the postcritical conformations (Fig. 4, clusters 4 and 11). Hence, structural polarization of the TSE may also reflect the importance of specific hydrogen bonds in stabilizing the TS, because these interactions are the last to break during unfolding/unbinding simulations and may contribute to the nucleation of coupled refolding and rebinding. Importantly, significant structural changes in the Cdk2 portion of the intermolecular interface on binding with p27, which are not modeled in our simulations, are localized in the Cdk2 regions interacting with the β-hairpin and β-strand of p27. Hence, the topological requirements for a stable intermolecular interface combined with the importance of specific interactions in the β-hairpin and β-strand regions may indeed contribute to the rate-limiting step of the reaction. A detailed experimental characterization of the binding mechanism and structural mapping of the TSE for p27 and related proteins is required to rigorously quantify the role of specific interactions and topological determinants in folding and binding. By using protein engineering and binding kinetics experiments, similar to the φ-value analysis method developed in protein folding (37, 16–22), it should be possible to validate the predicted details of the TSE and the binding mechanism.
According to the proposed binding mechanism, a large entropic cost of forming nonlocal interactions early in the process may encourage nonspecific largely unstructured p27 conformations (Fig. 6a) to probe collisions with the rigid cyclin A using the conserved Leu-Phe-Gly motif of p27 coil (Fig. 6b). At this stage, p27 exploits the intrinsic disorder in the α-helix segment until thermal fluctuations allow the system to wrap around the binding partner, with the β-hairpin starting to form hydrophobic interactions and the β-strand adopting the native structure topology at the intermolecular interface. The intrinsic folding preferences to form a stable α-helix in p27 are discouraged at this stage by insufficient gain in the intermolecular interactions that would not compensate for the loss of entropy. Furthermore, the entropy loss caused by probing nonlocal contacts simultaneously at the remote regions of the interface may not be fully compensated by insufficient strength in these interactions, which are yet to be consolidated. Indeed, the specific interactions formed with the complex by the β-hairpin and β-strand have considerably stronger orientational requirements than the hydrophobic intermolecular contacts and are not likely to be stabilizing unless they are fully formed. Consequently, p27 may find it more advantageous at this stage to partially release contacts with the cyclin A in favor of further ordering the β-hairpin and β-strand segments and formation of a network of specific intermolecular hydrogen bonds in this region that could be important for nucleating rapid folding and binding (Fig. 6c). Only after the TS is passed do the α-helix and 310-helix form their native structure concomitantly with the formation of the native intermolecular contacts (Fig. 6d).
Both disruptive and stabilizing mutations in the α-helix of p27 lead to only a marginal decrease (<0.5 kcal/mol) in the thermodynamic stability of the tertiary complex (8). Furthermore, the excessive stabilization of α-helix in the unbound form of p27 on introducing multiple alanine mutations in this region is kinetically detrimental, slowing the rate of formation of the tertiary complex (8). Assuming similar binding mechanisms for the wild-type p27 and the alanine mutant, we can infer from the determined TSE that the increased stabilization of the α-helix would lower only the free energy of the unbound p27 mutant but would not greatly influence the energetics of the TSE, which is hardly populated by the conformations with the native α-helix intra- and intermolecular contacts. The proposed microscopic picture of the binding mechanism thereby rationalizes the kinetic impediment for the p27 alanine mutant during the reaction. Overall, the energetics of the intermolecular interface prevails over the local folding contributions of forming a stable α-helix in both thermodynamic and kinetic terms.
In accordance with the protein folding theory (10, 11), the TSE must have some distinctive features of the native structure that are not shared with the unbound state. Indeed, because the local β-hairpin and β-strand elements of the native structure are rarely populated in the unbound states but are present in the TSE (Fig. 6c), their further stabilization should accelerate coupled folding and the binding reaction. These results suggest the importance of the native structure topology in governing folding transitions coupled to binding and provide support to the nucleation–condensation scenario (38–40), because ordering the adjacent β-hairpin and β-strand local elements of p27 at the binding interface becomes important only in the TS rather than in the unbound states.
Conclusion
We have shown that functionally important disorder–order folding transitions coupled to binding for the largely unstructured p27 protein are not driven by the intrinsic folding preferences of p27 but are rather determined by the intermolecular requirements to form a specific complex that ultimately dictates the folding mechanism. The analysis of the unfolding/unbinding pathways and the determination of the TSE provide an atomic picture of the binding mechanism in agreement with the thermodynamic and kinetic experiments, revealing that the topological requirements of the native binding interface overwhelm any local folding preferences for creating a stable α-helix before overcoming the major free energy barrier. The results support the dimensionality reduction models of molecular recognition, in which nonspecific and weak binding at long-range distances is required to speed up association of specific binding partners. This scenario may be rather common in the binding of unstructured proteins with a functional requirement for ordered bound structures.
Supplementary Material
Abbreviations
- rmsd
rms deviation
- TS
transition state
- TSE
TS ensemble
- Cdk
cyclin-dependent kinase
References
- 1.Wright P E, Dyson H J. J Mol Biol. 1999;293:321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 2.Dunker A K, Lawson J D, Brown C J, Williams R M, Romero P, Oh J S, Oldfield C J, Campen A M, Obradovic Z. J Mol Graphics Model. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 3.Dyson H J, Wright P E. Curr Opin Struct Biol. 2002;12:54–60. doi: 10.1016/s0959-440x(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 4.Uversky V N. Protein Sci. 2002;11:739–756. doi: 10.1110/ps.4210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spolar R S, Record M T. Science. 1994;263:777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- 6.Russo A, A, Jeffrey P D, Patten A K, Massague J, Pavletich N P. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 7.Schulman B A, Lindstrom D L, Harlow E. Proc Natl Acad Sci USA. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienkiewicz E A, Adkins J N, Lumb K. Biochemistry. 2002;41:752–759. doi: 10.1021/bi015763t. [DOI] [PubMed] [Google Scholar]
- 9.Kriwacki R W, Hengst L, Tennant L, Reed S I, Wright P E. Proc Natl Acad Sci USA. 1996;93:11504–11509. doi: 10.1073/pnas.93.21.11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onuchic J N, Nymeyer H, Garcia A E, Chahine J, Socci N D. Adv Protein Chem. 2000;53:87–152. doi: 10.1016/s0065-3233(00)53003-4. [DOI] [PubMed] [Google Scholar]
- 11.Mirny L, Shakhnovich E. Annu Rev Biophys Biomol Struct. 2001;30:361–396. doi: 10.1146/annurev.biophys.30.1.361. [DOI] [PubMed] [Google Scholar]
- 12.Tsai C-J, Ma B, Nussinov R. Proc Natl Acad Sci USA. 1999;96:9970–9972. doi: 10.1073/pnas.96.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkhivker G M, Bouzida D, Gehlhaar D K, Rejto P, A, Freer S T, Rose P W. Curr Opin Struct Biol. 2002;12:197–202. doi: 10.1016/s0959-440x(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 14.Shoemaker B A, Wang J, Wolynes P G. J Mol Biol. 1999;287:675–694. doi: 10.1006/jmbi.1999.2613. [DOI] [PubMed] [Google Scholar]
- 15.Shoemaker B A, Portman J J, Wolynes P G. Proc Natl Acad Sci USA. 2000;97:8868–8873. doi: 10.1073/pnas.160259697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker D. Nature. 2000;405:39–42. doi: 10.1038/35011000. [DOI] [PubMed] [Google Scholar]
- 17.Riddle D S, Grantcharova V P, Santiago J V, Alm E, Ruczinski I, Baker D. Nat Struct Biol. 1999;6:1016–1024. doi: 10.1038/14901. [DOI] [PubMed] [Google Scholar]
- 18.Galzitskaya O V, Finkelstein A V. Proc Natl Acad Sci USA. 1999;96:11299–11304. doi: 10.1073/pnas.96.20.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alm E, Baker D. Proc Natl Acad Sci USA. 1999;96:11305–11310. doi: 10.1073/pnas.96.20.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz V, Eaton W A. Proc Natl Acad Sci USA. 1999;96:11311–11316. doi: 10.1073/pnas.96.20.11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai J, Levitt M, Baker D. J Mol Biol. 1999;291:215–225. doi: 10.1006/jmbi.1999.2949. [DOI] [PubMed] [Google Scholar]
- 22.Clementi C, Nymeyer H, Onuchic J. J Mol Biol. 2000;298:937–953. doi: 10.1006/jmbi.2000.3693. [DOI] [PubMed] [Google Scholar]
- 23.Duan Y, Kollman P A. Science. 1998;282:740–744. doi: 10.1126/science.282.5389.740. [DOI] [PubMed] [Google Scholar]
- 24.Snow C D, Nguyen H, Pande V S, Gruebele M. Nature. 2002;420:102–106. doi: 10.1038/nature01160. [DOI] [PubMed] [Google Scholar]
- 25.Shea J E, Brooks C L., III Annu Rev Phys Chem. 2001;52:499–535. doi: 10.1146/annurev.physchem.52.1.499. [DOI] [PubMed] [Google Scholar]
- 26.Lazaridis T, Karplus M. Science. 1997;278:1928–1931. doi: 10.1126/science.278.5345.1928. [DOI] [PubMed] [Google Scholar]
- 27.Fersht A R, Daggett V. Cell. 2002;108:573–582. doi: 10.1016/s0092-8674(02)00620-7. [DOI] [PubMed] [Google Scholar]
- 28.Pande V S, Rokhsar D S. Proc Natl Acad Sci USA. 1999;96:9062–9067. doi: 10.1073/pnas.96.16.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rejto P A, Verkhivker G M. Proc Natl Acad Sci USA. 1996;93:8945–8950. doi: 10.1073/pnas.93.17.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verkhivker G M, Bouzida D, Gehlhaar D K, Rejto P A, Arthurs S, Colson A B, Freer S T, Larson V, Rose P W. J Comput Aided Mol Des. 2000;14:731–751. doi: 10.1023/a:1008158231558. [DOI] [PubMed] [Google Scholar]
- 31.Verkhivker G M, Bouzida D, Gehlhaar D K, Rejto P, A, Schaffer L, Arthurs S, Colson A B, Freer S T, Larson V, Rose P W. Proteins Struct Funct Genet. 2001;45:456–470. doi: 10.1002/prot.10019. [DOI] [PubMed] [Google Scholar]
- 32.Du R, Pande V S, Grosberg A Y, Tanaka T, Shaknovich E I. J Chem Phys. 1998;108:334–350. [Google Scholar]
- 33.Nymeyer H, Socci N D, Onuchic J N. Proc Natl Acad Sci USA. 2000;97:634–639. doi: 10.1073/pnas.97.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayo S L, Olafson B D, Goddard W A., III J Phys Chem. 1990;94:8897–8909. [Google Scholar]
- 35.Bouzida D, Kumar S, Swendsen R H. Phys Rev A. 1992;45:8894–8901. doi: 10.1103/physreva.45.8894. [DOI] [PubMed] [Google Scholar]
- 36.Willett P, Winterman V, Bawden D. J Chem Inf Comput Sci. 1986;26:109–118. [Google Scholar]
- 37.Fersht A R, Matouschek A, Serrano L. J Mol Biol. 1992;224:771–782. doi: 10.1016/0022-2836(92)90561-w. [DOI] [PubMed] [Google Scholar]
- 38.Fersht A R. Proc Natl Acad Sci USA. 2000;97:1525–1529. doi: 10.1073/pnas.97.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fersht A R. Proc Natl Acad Sci USA. 1995;92:10869–10873. doi: 10.1073/pnas.92.24.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daggett V, Fersht A R. Trends Biochem Sci. 2003;28:18–25. doi: 10.1016/s0968-0004(02)00012-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.