Abstract
Microraptor gui, a four-winged dromaeosaur from the Early Cretaceous of China, provides strong evidence for an arboreal-gliding origin of avian flight. It possessed asymmetric flight feathers not only on the manus but also on the pes. A previously published reconstruction shows that the hindwing of Microraptor supported by a laterally extended leg would have formed a second pair of wings in tetrapteryx fashion. However, this wing design conflicts with known theropod limb joints that entail a parasagittal posture of the hindlimb. Here, we offer an alternative planform of the hindwing of Microraptor that is concordant with its feather orientation for producing lift and normal theropod hindlimb posture. In this reconstruction, the wings of Microraptor could have resembled a staggered biplane configuration during flight, where the forewing formed the dorsal wing and the metatarsal wing formed the ventral one. The contour feathers on the tibia were positioned posteriorly, oriented in a vertical plane for streamlining that would reduce the drag considerably. Leg feathers are present in many fossil dromaeosaurs, early birds, and living raptors, and they play an important role in flight during catching and carrying prey. A computer simulation of the flight performance of Microraptor suggests that its biplane wings were adapted for undulatory “phugoid” gliding between trees, where the horizontal feathered tail offered additional lift and stability and controlled pitch. Like the Wright 1903 Flyer, Microraptor, a gliding relative of early birds, took to the air with two sets of wings.
Keywords: arboreal origin of flight, Chinese feathered dinosaurs, phugoid gliding, Wright 1903 Flyer
The evolution of powered flight in birds from theropod dinosaurs is recognized as the key adaptive breakthrough that contributed to the biological success of this group. The transformation of wing design from nonavian dinosaurs to early birds is beginning to unravel in recent times from a wealth of fossil record from China. Hundreds of small, exquisitely preserved, feathered theropods were discovered in the Early Cretaceous Jehol Group of northeastern China as they died some 125 million years ago, smothered in the “Cretaceous Pompeii.” Both anatomy and phylogeny strongly suggest that these theropods, including Sinosauropteryx (1), Caudipteryx and Protarchaeopteryx (2), Microraptor (3), Sinornithosaurus (4), Cryptovolans (5), and the early bird Confuciusornis (6), show constructions ranging from small winged, arboreal theropods to fully winged, active flying birds. They offer new insights into the origins of feathers and flight, favoring the arboreal (“trees-down”) over the cursorial (“ground-up”) hypothesis (3–5, 7–11).
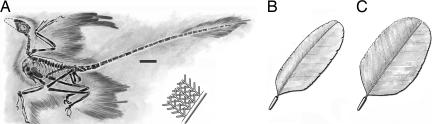
Among these recent finds, Microraptor gui offers the best evidence that arboreal dromaeosaurs might have acquired powered flight through a gliding stage where both forelimbs and hindlimbs were involved (12). With two sets of winged limbs, each having long, asymmetric flight feathers at the distal segments of both forelimb and hindlimb, Microraptor developed broad airfoil surfaces and was probably an efficient glider. There are ≈12 manual and ≈14 pedal primary feathers, which are long and asymmetric to create aerodynamic forces for lift. The longest primary on the metatarsus would be 19 cm (Fig. 1 A). The hooked, interlocking barbs gave strength and flexibility to the asymmetric feathers and prevented air from passing through it in flight (Fig. 1 B). In the proximal part of the wing and hindleg, the contour feathers have symmetric vanes (Fig. 1 C).
Fig. 1.
Feathers of M. gui. (A) Holotype of M. gui [Institute of Vertebrate Paleontology and Paleoanthropology (IVPP) V13352] as preserved [modified from Xu et al. (12)]. (Scale bar, 5 cm.) (B) The long feathers on the hand and metatarsal sections had evolved for flight; they were asymmetric with interlocking barbules. (C) In the rest of the wing and hindleg, the feathers are symmetric (10).
A typical contour feather is composed of a long, tapering central rachis with a broad, flexible vane on either side. Vanes are asymmetrical in flight feathers in relation to the central rachis, where the leading edge is narrower and stronger than the trailing edge. This asymmetry provides an airfoil-shaped cross-section of the feather because air pressure is greater along the leading edge. The aerodynamic function of the asymmetric feather is attributable to aeroelastic stability in the lifting feather. The forward location of the rachis suppresses a tendency for the rachis to twist elastically in response to the aerodynamic force on the lifting feather because lift is concentrated in the forward quarter of the feather's area. The asymmetry is a sure sign that the feather has been adapted for lifting.
Xu et al. (12) (Fig. 1)C) reconstructed limbs of four-winged Microraptor as tandem wings similar to those of insects and gliding fish, where all wings are spread horizontally in tetrapteryx fashion. They argued that Microraptor was clumsy on the ground; because it had these long feathers on its feet, it would have had trouble walking or running and would have been vulnerable on the ground. It was probably a tree-dweller, thus supporting the arboreal theory of flight, where gravity was the main source of flying energy.
The holotype specimen of Microraptor, with an estimated live weight of ≈1 kg and measuring ≈77 cm in length, has a long bony tail that bears asymmetric retrices on two sides that could provide additional lift and control pitch (Table 1). However, Xu et al. (12) did not discuss why Microraptor was a glider and how it used its wings during flight. The life restoration of the hindlimb of Microraptor in a laterally extended position by Xu et al. (12) (Fig. 1)C appears to be aerodynamically inefficient and so anatomically anomalous that it generated lively debate and speculation (13). In our view, the leading edge of the asymmetric flight feathers on the metatarsus should face forward against the direction of the airflow like those of the hand section, not sidewise, as they reconstructed. In all theropods (including birds), the hindlimb is held in an erect and parasagittal gait, unlike the reconstruction of Microraptor in a laterally extended position. Because of the critical importance of this fossil in the early evolutionary history of avian flight, we propose here a second restoration of the wing planform and estimate the flight performance of Microraptor§ in a manner fundamentally different from the conclusions reached by Xu et al. (12).
Table 1.
Aerodynamic data of M. gui, Nyctosaurus gracilis, and F. magnificens
| Specimen (holotype) | M, kg | Wing area (forewing plus hindwing), S (m2) | Tailwing area, S (m2) | Wingspan, b (m) | Aspect ratio, A = b2/S | WL, mg/S (N/m2) | Gliding speed (m/s) |
|---|---|---|---|---|---|---|---|
| Microraptor IVPP V13352 | 0.95 | 0.132 | 0.0136 | 0.94 | 6.69 | 70.6 | 12–15 |
| Nyctosaurus YPM 1178 | 1.86 | 0.409 | 2.72 | 18.08 | 44.6 | 9.6 | |
| Fregata | 1.5 | 0.324 | 2.86 | 25.24 | 45.42 | 10 |
M, mass; N, Newton; S, wing area; WL, wing loading; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology; YPM, Yale Peabody Museum.
Hindlimb Posture and Orientation of Metatarsal Feathers
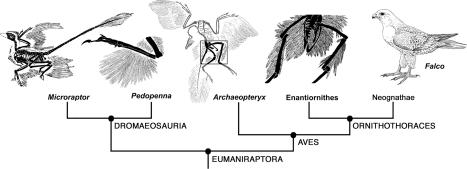
In all theropods (including birds), the hip, knee, and ankle joints are stable and fully congruent during parasagittal motion of the hindlimbs, permitting a wide range of flexion and extension but little abduction and adduction. The femur head is cylindrical, fitting into a perforated acetabulum, which allows little transverse deviation from the parasagittal plane (14). The hip joint becomes quickly incongruent as the femur is abducted horizontally from the parasagittal plane. The parasagittal hindlimb posture of Microraptor is beautifully preserved in the holotype specimen (Fig. 1)A, which differs strikingly from the horizontal restoration (12) but closely resembles that of the Berlin Archaeopteryx specimen, which also displays tibial feathers (15) (see Fig. 4).
Fig. 4.
A simple cladogram of eumaniraptoran theropods showing the distribution of leg feathers in selected taxa (modified from refs. 23, 25, and 26). In Microraptor, the contour feathers are present on the femur, tibia, and metatarsus, but only the metatarsal feathers are asymmetric and form the ventral wing of the biplane design; the feathers on the femur and tibia are symmetric (12). In Pedopenna, long metatarsal feathers are present to form the ventral wing of the biplane layout, but they appear to be symmetrical (25). In Archaeopteryx, long contour feathers are present on the femur and tibia, but they appear to be lost on the metatarsus (15). At this stage, the evolution of monoplane design probably took place. In an unnamed enantiornithine bird, long contour feathers are present on the femur and tibia but absent in the metatarsal region (26). In modern raptors such as the falcon Falco, similar contour leg feathers persist on the femur and tibia for streamlining, but metatarsal feathers are generally reduced or absent.
The most unusual feature in Microraptor is the presence of long, asymmetric flight feathers on the entire length of the metatarsus, which are unknown in Archaeopteryx, feathered dromaeosaurs, and modern birds. The orientation of the metatarsal feathers as reconstructed by Xu et al. (12) is problematic because these feathers extend below the level of the feet, thus hindering terrestrial locomotion. In this reconstruction, the leading edge of these primary feathers on the metatarsus would face sidewise during gliding without producing any lift. Because they are asymmetric flight feathers like those of the forewings, the narrow, leading edge should face forward against the direction of airflow to gain lift. We suggest that these feathers were oriented in life in a transverse horizontal plane like those of the hand section, to be an effective airfoil during flight, but that they collapsed backward during fossilization. The symmetrical tibial feathers also are preserved in a backward fashion. In life, these contour feathers should be projected posteriorly for streamlining.
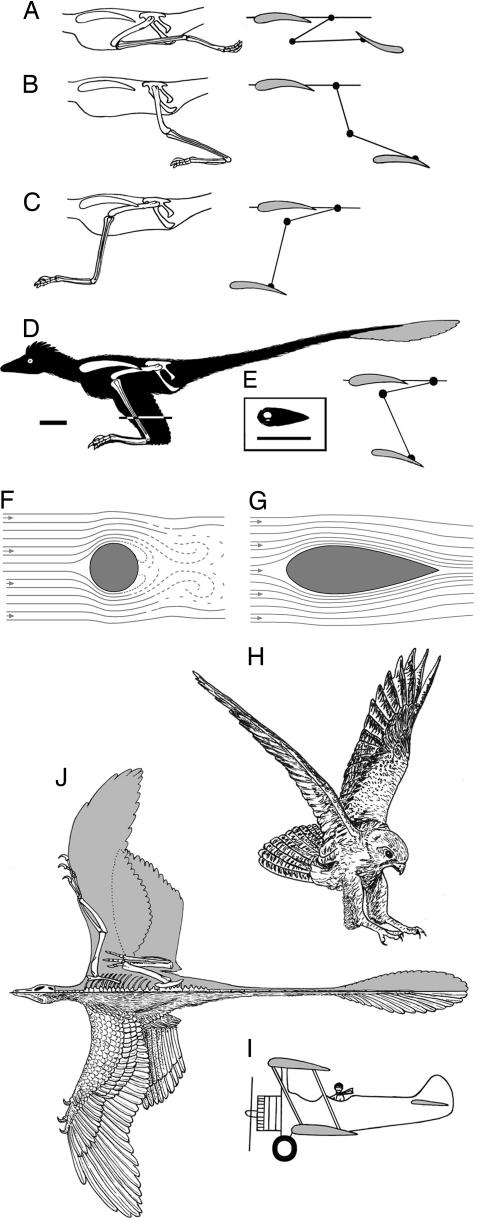
Three biological and aerodynamic constraints provide important clues to the hindwing design of Microraptor: (i) The hindlimb should be oriented in a parasagittal plane as in all theropods (including birds) and could not be splayed sidewise. (ii) The hindlimb wings should be folded neatly into a compact package during walking in such a fashion that metatarsal feathers would not project ventrally beyond the foot to prevent damage to the feathers. (iii) The leading edge of the primary feathers on the metatarsus should face forward as in the manus; this arrangement makes each feather capable of acting as an individual airfoil and also is needed to maintain the entire wing's camber and to carry the aerodynamic load. The first two constraints contradict the original hindlimb reconstruction of Xu et al. (12) (Fig. 1)C.
We present several possible hindlimb orientations (Fig. 2 A–D) in Microraptor during flight. We have discarded the bird-like pedal configuration in flight (Fig. 2 A) because in that pose, the primaries would face backward to produce lift in an unproductive direction (ventrally) that would interfere with gliding. Among other alternatives, we prefer (Fig. 2 D, a reconstruction in which the hindlimb is held in a z-fashion resembling the pouncing posture of modern raptors when catching prey from the air and carrying it (Fig. 2 H). In this pose, the femur would be kept in a subhorizontal position close to the body, directed forward, with its feathers oriented backward and merging with the body contour to form a continuous surface, allowing this airfoil to move smoothly through air with the least drag. We propose that the symmetric feathers on the tibia were arranged like those in modern raptors, streamlining the circular shaft of the tibia by stretching it backward to maintain a smooth flow of air (Fig. 2 E–G). Without tibial streamlining, the cylindrical leading edge of the vertical tibia could increase the total drag by nearly 40%.
Fig. 2.
Wing planform of Microraptor. (A–D) Different possible hindlimb postures during flight. (A) Hindlimb backwardly directed as in modern birds. (B–D) Biplane configuration. (B) Hindlimb backwardly sloping position. (C) Hindlimb forwardly sloping in predatory strike position. (D) Hindlimb in z-fashion with a body silhouette showing the animal in lateral view with an upwardly tilted tail for pitch control. (E) Cross-section of the tibia–fibula showing a streamlining and stretching effect of the cylindrical tibia by adding feathers caudally. (F) Cylindrical structure offers maximum resistance to the airstream as the airflow behind it becomes broken up into eddies, creating turbulence. (G) Filling the spaces in a cylindrical structure in front and behind improves streamlining, as in the case of the feathered tibia of raptors. (H) Pouncing posture of a raptor, Falco. (I) A typical staggered biplane (Stearman 75) for comparison with Microraptor; in biplane aircraft of the 1920s, there was a large additional drag of wires, struts, etc. between the two wings, which eventually made the biplane obsolete except for a niche application; such drag-induced structures were absent in Microraptor. (J) Life reconstruction of M. gui (IVPP V13352) in dorsal view showing the morphology and distribution of hindlimb feathers (Left) and orientation of the hindlimb (Right) during gliding, based on Fig. 1 A; proximal feathers on the humerus and femur are inferred (data are from ref. 12). (Scale bar, 5 cm.)
Biplane Wing Configuration
Because Microraptor could not extend its hindwings directly behind the forewings in the same plane, it probably held its feet lower than its arms, a more anatomically and aerodynamically stable configuration. Once the parasagittal posture and feather orientation of the hindlimb are corrected based on both anatomical and aerodynamic modeling, the wings of Microraptor resemble those of a staggered biplane from the side, where the forewing forms the dorsal wing and the metatarsal wing forms the ventral one (Fig. 2) I and J). The metatarsal wing of Microraptor is unique among vertebrates and needs further comment. In the z-shaped orientation of the hindlimb, the feathers on the metatarsus become horizontal, laterally extended, and form the ventral wing. The ventral wing tilts slightly upward from the horizontal position, allowing the lower wing to have a few degrees greater incidence (upward angle of attack) than the upper wing, known as decalage in biplane theory (an unstable situation, which is compensated by the long tail) (16) (Fig. 2)D). The lower wing is placed somewhat posterior and ventral in relation to the dorsal wing (on the hand) like a staggered biplane (Fig. 2)G) with unequal wing sections and different chords (16) and a ventral wing area (0.042 m2) approximately one-half the extent of the dorsal wing (0.089 m2) (Fig. 2)J). Merely by stretching both of its wings, Microraptor would have been able to glide in much the same way as a mechanical glider without muscle power. The stiff mesotarsal ankle joint would prevent torsion of the ventral wing on the metatarsus. A ligament, analogous to the avian vinculum, could hold the feathers of the hindwing in place during flight and aid in folding when not in use. Using Fig. 2 D as a guide, we reconstruct the dorsal view of Microraptor in a gliding pose to show the biplane configuration of the wing planform (Fig. 2)J).
Flight Performance
Microraptor displays several anatomical features that suggest it could become airborne (12): elongate and asymmetric vanes in the flight feathers (17) at the distal segment of each limb; a scapulocoracoid whose ends are oriented at an acute angle to each other (18); a laterally facing glenoid for gentle dorsoventral movement of the wing (19); a single, enlarged sternum for attachment of the flight muscles; ossified sternal ribs and well developed uncinate processes for resisting compression force on the thoracic cavity imposed during downstroke; strongly bowed outer metacarpal; and a flattened central digit for attachment of primaries.
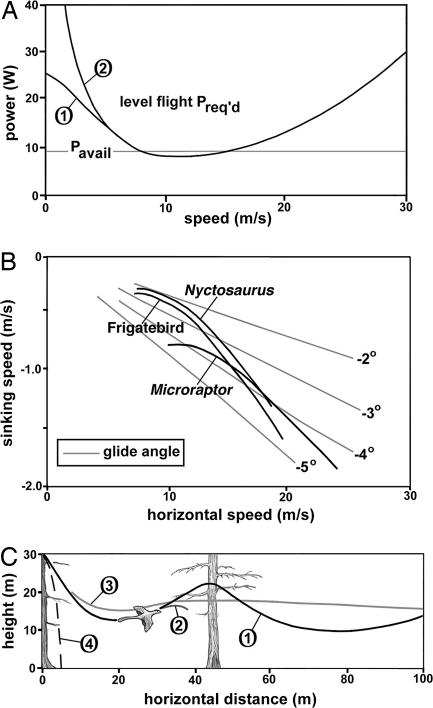
To analyze the flight performance of Microraptor, we used two computer algorithms, ANFLTPWR (animal flight power) and ANFLTSIM (animal flight simulation), which are based on the streamtube model (20) using several flight parameters (Table 1). For any flying animal or fixed-wing aircraft or helicopter, the plot of power required for level flight against airspeed is a U-shaped curve, higher at both ends than at the middle. Using the ANFLTPWR program (20), we generated the power curve of Microraptor (Fig. 3 A), where the U-shaped curve is the power required for steady level flight and the horizontal line is the estimated maximum steady power available. Because the two curves, power required and power available, nearly coincide at flight speeds of ≈9–15 m/s, limited steady flight may have been theoretically possible.
Fig. 3.
Flight performance of Microraptor. (A) Power curves (steady level flight) for Microraptor. The horizontal line represents the estimated maximum continuous power available. Two curves are shown for the level flight power required. Curve 1 is from streamtube theory (20), and curve 2 is based on the simpler aircraft theory (22). They converge for speeds of greater than ≈6 m/s. (B) Glide polars for Microraptor, compared with a seabird (frigatebird, M = 1.5 kg) and a pterosaur (Nyctosaurus, M = 1.85 kg) (ref. 22; see Table 1 for aerodynamic data). (C) Glide paths of Microraptor from a perch. Curve 1 shows phugoid gliding. Curve 2 shows a final rapid pitchup with high drag. Curve 3 shows gliding path with pitch damper on. Curve 4 shows a parachuting trajectory.
Gliding performance is shown as a polar curve, which is a plot of horizontal speed versus sinking speed. We used the ANFLTSIM program (20) to calculate these values. Fig. 3 B shows the potential gliding performance of Microraptor in terms of sinking speed versus horizontal speed. By this plot, Microraptor was possibly a moderate glider, not as efficient as high-performance gliders, such as the long-span frigatebird (Fregata magnificens, M = 1.5 kg) or a Cretaceous pterosaur (Nyctosaurus, M = 1.86 kg) (21), of comparable size.
Anatomical evidence indicates that Microraptor was not capable of ground or running takeoff, because it lacked the supracoracoideus pulley to elevate the wings. Moreover, running takeoff would damage the ventral metatarsal wings (12). We have calculated the takeoff capability of Microraptor from a perch using both streamtube (20) and aircraft (22) models (Fig. 3 C). When birds take off from a perch, they do not seem to use excess power; they lose height at first and then swoop up with a large-amplitude undulation to swing between two perches, known as phugoid gliding (8, 20). In Fig. 3 C, we have plotted several glide paths for Microraptor, starting from horizontal launches at 3 m/s. Considerable height losses are involved, as> shown in Fig. 3 C (curve 1), but they are minimized by the use of a simulated pitch damper, which may imitate the tail motion of Microraptor to control the phugoid oscillations. The pitch damper adjusts the wing lift in synchrony with speed changes to reduce or eliminate the oscillations. By using phugoid gliding, Microraptor could potentially have traveled from one tree to another tree by undulating flight covering a horizontal distance of >40 m. This mode of transportation would have been energetically very efficient for Microraptor. The long feathered tail would provide pitch damping as well as general stability. The minimum speed during the pitchup is 4.5 m/s, which may have been safe for landing.
The combined wing appears to be too small to serve as a parachute that would withstand a fatal fall. We plotted curve 4 in Fig. 3 C, a trajectory for a flat parachute descent in which Microraptor has high drag with outstretched wings, while generating no lateral force. The trajectory is not simply a vertical line because of the horizontal jump-off. The terminal velocity is ≈8.7 m/s, a potentially crashing speed against a solid surface but perhaps safe for landing on padded ground cover or on a flexible tree branch.
Drag could be dramatically reduced in a gliding Microraptor by streamlining the vertically held tibia with feathers so that the turbulent spaces behind are filled in and the front areas are rounded or tapered (Fig. 2 E–G). Without leg feathers, the cross-section of the tibia would be circular, so the airflow behind the bone would break up into eddies, producing turbulence. With the presence of tibial feathers, Microraptor could reduce 40% of drag when the legs were held in z-fashion [see supporting information (SI)].
Discussion and Conclusion
Phylogenetically, Microraptor belongs to eumaniraptorans, which also include a series of feathered theropods and Archaeopteryx (23), possessing long arms and hands for the attachment of vaned, barbed feathers (Fig. 4 A). Although Microraptor appears to have been a glider, there are two phylogenetic and biomechanical interpretations for this unusual biplane wing configuration of Microraptor: either (i) avian flight went through a Microraptor-like biplane stage to become a monoplane configuration when the hindlimb became decoupled from its gliding function (with the loss of ventral wing); or (ii) the biplane wing configuration may represent a failed or temporary experiment in the deployment of aerodynamic feathers among one branch of deinonychosaurs that dallied with gliding.
Both scenarios are equally possible. However, the former view finds support from current fossil and recent evidence in a broad phylogenetic context indicating a gradual shift in locomotory dominance from the hindlimb to forelimb during the evolution of avian flight (Fig. 4). Several Chinese maniraptorans including Caudipteryx (24), Sinornithosaurus (4), and Cryptovolans (5), as well as modern raptors, show contour feathers on the hindlimbs, where tibial feathers were probably used for streamlining. The recent discovery of Pedopenna (25) from the Middle or Late Jurassic of China, another feathered maniraptoran with long metatarsal feathers, may support the biplane wing configuration of gliding dromaeosaurs before the Archaeopteryx stage (Fig. 4). Archaeopteryx shows long contour feathers on the hindlimb, especially in the tibial region in the Berlin specimen (15); it apparently lacked the metatarsal feathers, which were compensated with larger forewing and long asymmetric retrices on the tail for additional lift. Long contour tibial feathers also are known in an unnamed Early Cretaceous enantiornithine bird from China, but metatarsal feathers appear to be absent (26). Symmetric contour feathers occur on the femur and tibia in living raptors, even on the proximal part of the tarsometatarsus (Fig. 4). Unlike other birds, raptors keep their hindlegs in a z-configuration during preparation for aerial attack and carrying prey, dangling their tibiae in a vertical plane (Fig. 2H). The feathered “trousers” are a conspicuous costume of predatory birds, keeping their prey-catching legs streamlined during aerial attack. Microraptor provided the crucial clue about the role of leg feathers in the flight of living raptors.
Aircraft designers have mimicked many of nature's flight “inventions,” usually inadvertently. Leading edge slats delay stalling, as does the alula of birds; birds' feet act as airbrakes, and streamlining reduces drag. Now, it seems likely that Microraptor invented the biplane 125 million years before the Wright 1903 Flyer.
Methodology
Various flight parameters such as wingspan (b), body length, and forewing and tailwing areas (S) were calculated from a high-fidelity cast of the holotype specimen at the National Museum of Rio de Janeiro in Brazil. The hindwing area was estimated from a modified dorsal reconstruction of Microraptor (Fig. 2)J). We have digitized the body outline in dorsal aspect with a computer program by Rohlf (http://life.bio.sunysb.edu/morph/) and estimated the combined wing area (forewing plus hindwing) as well as the area from tail feathers (Table 1). Our method of estimating the mass of Microraptor used a multivariate analysis proposed by Atanassov and Strauss.¶ The predicted mass of Microraptor was calculated to be 0.95 kg, which is approximately the mass of medium-sized extant predatory birds [i.e., the common black hawk (Buteogallus anthracinus) or the northern goshawk (Accipiter gentilis)] (27). To analyze the flight performance of Microraptor, we used two computer algorithms, ANFLTPWR and ANFLTSIM, which are based on the streamtube model (20) and also described above. In all cases, the body and wing drag coefficients are computed as functions of the Reynolds number (see §, ref. 20, and SI for detailed methodology).
Supplementary Material
Acknowledgments
We thank Lynn Margulis for her suggestions, support, and sponsorship; Jeff Martz for stimulating discussions on the wing planform and for illustrations; M. Atanassov for estimating the wing area and mass of Microraptor; Alexander Kellner for the high-fidelity cast and life mount of Microraptor at the National Museum of Rio de Janeiro, Brazil; J. Martz, M. Atanassov, J. Barrick, Soumya Chatterjee, and several anonymous reviewers for insightful comments and suggestions for the improvement of the manuscript; Xu Xing for providing images of Microraptor and Pedopenna; and Thomas Moore for permission to use the sketch of Microraptor in Fig. 1. Funding was provided by Texas Tech University.
Abbreviation
- M
mass.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609975104/DC1.
Chatterjee, S., Templin, R. J. (2005) Geol Soc Am Abstr Prog 37:88 (abstr.).
Atanassov, M. N., Strauss, R. (2002) J Vertebr Paleontol 22:33A (abstr.).
References
- 1.Chen PJ, Dong Z, Zhen S. Nature. 1998;391:147–152. [Google Scholar]
- 2.Ji Q, Currie PJ, Norell MA, Ji S. Nature. 1998;393:753–761. [Google Scholar]
- 3.Xu X, Zhou Z, Wang X. Nature. 2000;408:705–708. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Wang X, Wu X. Nature. 1999;401:262–266. [Google Scholar]
- 5.Czerkas SA, Zhang D, Li J, Li Y. Mus J. 2002;1:97–126. [Google Scholar]
- 6.Hou L, Zhou Z, Martin LD, Feduccia A. Nature. 1995;377:616–618. [Google Scholar]
- 7.Chatterjee S. The Rise of Birds. Baltimore: Johns Hopkins Univ Press; 1997. [Google Scholar]
- 8.Chatterjee S, Templin RJ. Naturwissenschaften. 2003;90:27–32. doi: 10.1007/s00114-002-0385-0. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S, Templin RJ. In: Feathered Dragons: Studies on the Transition from Dinosaurs to Birds. Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Bloomington, IN: Indiana Univ Press; 2004. pp. 251–281. [Google Scholar]
- 10.Chatterjee S, Templin RJ. Nat Hist. 2005;114:54–55. [Google Scholar]
- 11.Zhang F, Zhou Z, Xu X, Wang X. Naturwissenschaften. 2002;89:394–398. doi: 10.1007/s00114-002-0353-8. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Nature. 2003;421:335–340. doi: 10.1038/nature01342. [DOI] [PubMed] [Google Scholar]
- 13.Padian K, Dial KP. Nature. 2005;438:E3–E4. doi: 10.1038/nature04354. [DOI] [PubMed] [Google Scholar]
- 14.Hotton N., III . In: A Cold Look at the Warm-Blooded Dinosaurs. Thomas RDK, Olson EC, editors. Boulder, CO: Westview; 1980. pp. 311–350. [Google Scholar]
- 15.Longrich N. Palaeobiology. 2006;32:417–431. [Google Scholar]
- 16.Munk MM. Nat Adv Aero Rep. 1923;151:475–517. [Google Scholar]
- 17.Feduccia A, Tordoff HB. Science. 1979;203:1021–1022. doi: 10.1126/science.203.4384.1021. [DOI] [PubMed] [Google Scholar]
- 18.Feduccia A. The Origin and Evolution of Birds. New Haven, CT: Yale Univ Press; 1996. [Google Scholar]
- 19.Jenkins FA., Jr Am J Sci. 1993;293A:253–267. [Google Scholar]
- 20.Templin RJ. Prog Aeronaut Sci. 2000;36:393–436. [Google Scholar]
- 21.Chatterjee S, Templin RJ. Geol Soc Am Sp Pap. 2004;376:1–64. [Google Scholar]
- 22.Pennycuick CJ. In: Mechanics of Flight in Avian Biology. Farner DS, King JR, editors. Vol 5. New York: Academic; 1975. pp. 1–75. [Google Scholar]
- 23.Holtz TR. Nat Hist. 2005;114:40–41. [Google Scholar]
- 24.Jones TD, Farlow JO, Ruben JA, Henderson DM, Hillenius WJ. Nature. 2000;406:716–718. doi: 10.1038/35021041. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Zhang F. Naturwissenschaften. 2005;92:173–177. doi: 10.1007/s00114-004-0604-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Zhou Z. Nature. 2004;431:925. doi: 10.1038/431925a. [DOI] [PubMed] [Google Scholar]
- 27.Sibley DA. The Sibley Guide to Birds. New York: Alfred A. Knopf; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.