Abstract
‘Regulators of G protein Signalling’ (RGSs) accelerate the activation and deactivation kinetics of G protein-gated inwardly rectifying K+ (GIRK) channels. In an apparent paradox, RGSs do not reduce steady-state GIRK current amplitudes as expected from the accelerated rate of deactivation when reconstituted in Xenopus oocytes. We present evidence here that this kinetic anomaly is dependent on the degree of G protein-coupled receptor (GPCR) precoupling, which varies with different Gαi/o-RGS complexes. The gating properties of GIRK channels (Kir3.1/Kir3.2a) activated by muscarinic m2 receptors at varying levels of G protein expression were examined with or without the co-expression of either RGS4 or RGS7 in Xenopus oocytes. Different levels of specific m2 receptor-Gα coupling were established by uncoupling endogenous pertussis toxin (PTX)-sensitive Gαi/o subunits with PTX, while expressing varying amounts of a single PTX-insensitive subunit (Gαi1(C351G), Gαi2(C352G), Gαi3(C351G), GαoA(C351G), or GαoB(C351G)). Co-expression of each of the PTX-insensitive Gαi/o subunits rescued acetylcholine (ACh)-elicited GIRK currents (IK,ACh) in a concentration-dependent manner, with Gαo isoforms being more effective than Gαi isoforms. Receptor-independent ‘basal’ GIRK currents (IK,basal) were reduced with increasing expression of PTX-insensitive Gα subunits and were accompanied by a parallel rise in IK,ACh. These effects together are indicative of increased Gβγ scavenging by the expressed Gα subunit and the subsequent formation of functionally coupled m2 receptor-G protein heterotrimers (Gα(GDP)βγ). Co-expression of RGS4 accelerated all the PTX-insensitive Gαi/o-coupled GIRK currents to a similar extent, yet reduced IK,ACh amplitudes 60-90 % under conditions of low Gαi/o coupling. Kinetic analysis indicated the RGS4-dependent reduction in steady-state GIRK current was fully explained by the accelerated deactivation rate. Thus kinetic inconsistencies associated with RGS4-accelerated GIRK currents occur at a critical threshold of G protein coupling. In contrast to RGS4, RGS7 selectively accelerated Gαo-coupled GIRK currents. Co-expression of Gβ5, in addition to enhancing the kinetic effects of RGS7, caused a significant reduction (70-85 %) in steady-state GIRK currents indicating RGS7-Gβ5 complexes disrupt Gαo coupling. Altogether these results provide further evidence for a GPCR-Gαβγ-GIRK signalling complex that is revealed by the modulatory affects of RGS proteins on GIRK channel gating. Our functional experiments demonstrate that the formation of this signalling complex is markedly dependent on the concentration and composition of G protein-RGS complexes.
G protein-gated inwardly rectifying K+ (GIRK) channels mediate slow inhibitory post-synaptic potentials (sIPSPs) in the central and peripheral nervous system and are activated by a variety of G protein-coupled receptors (GPCRs) that selectively interact with pertussis toxin (PTX)-sensitive Gαi/o proteins (for recent reviews see (Dascal, 1997; Yamada et al. 1998; Mark & Herlitze, 2000). Both the amplitude and the distinguishing slow time course for GIRK-mediated sIPSPs are inherently coupled to the kinetics of the G protein activation-deactivation cycle. The time course for GIRK channel activation is dependent on the receptor catalysed production of free Gβγ dimers that interact directly with the channel and increase open probability (Logothetis et al. 1987; Krapivinsky et al. 1995). Deactivation of GIRK currents is determined by the reaction rate for Gβγ sequestration, a process rate limited by the GTPase activity of associated Gαi/o proteins, Gα(GTP) → Gα(GDP), with a rate constant for GTP hydrolysis of kGTPase (Breitwieser & Szabo, 1988).
Given the large diversity and ubiquitous expression of heterotrimeric G proteins (Simon et al. 1991), several studies have begun investigating whether functional differences exist among the various Gαβγ subunits that may participate in GPCR → GIRK channel signalling (5 Gαi/o isoforms, 5 Gβ genes, and 12 Gγ genes) (Lledo et al. 1992; Wickman et al. 1994; Schreibmayer et al. 1996; Sowell et al. 1997; Takano et al. 1997; Valenzuela et al. 1997; Fernandez-Fernandez et al. 1999; Greif et al. 2000; Leaney et al. 2000; Leaney & Tinker, 2000; Lei et al. 2000; Blake et al. 2001; Fernandez-Fernandez et al. 2001). Adding to this molecular complexity is the recently identified ‘Regulators of G protein Signalling’ (RGSs) that accelerate the termination of G protein signalling by increasing kGTPase through a direct interaction with Gα subunits (for recent reviews see De Vries et al. 2000; Ross & Wilkie, 2000). Several RGS proteins, of which there are now more than 20 mammalian genes identified, are capable of accelerating receptor-dependent GIRK activation and deactivation kinetics in heterologous expression systems (Doupnik et al. 1997; Saitoh et al. 1997; Granneman et al. 1998; Herlitze et al. 1999; Saitoh et al. 1999; Kovoor et al. 2000; Burgon et al. 2001). RGS-accelerated GIRK currents more closely resemble the properties of neuronal and cardiac GIRK currents, indicating native RGS proteins are likely to be important determinants of GIRK-mediated synaptic signalling.
Initial studies of RGS-accelerated GIRK channel kinetics revealed an apparent paradox where the RGS-accelerated activation phase, an expected consequence of accelerated deactivation according to standard kinetic concepts, was not accompanied with a reduction in steady-state current amplitude (Doupnik et al. 1997; Saitoh et al. 1997). These findings suggested that RGS proteins have actions on GPCR → GIRK signalling beyond the well-established GTPase-activating function of the RGS domain on Gα(GTP) subunits (Zerangue & Jan, 1998). To further explore the effects of RGS proteins on receptor-dependent GIRK channel gating, we examined the actions of two distinct RGS proteins (RGS4 and RGS7) on GIRK channels (Kir3.1/Kir3.2a heteromers) activated by muscarinic m2 receptors coupled to varying concentrations and compositions of Gαi/oβγ heterotrimers in Xenopus oocytes. Muscarinic m2 receptor-Gαβγ-GIRK channel coupling was established by expressing varying levels of PTX-insensitive Gαi/o subunits (Wise et al. 1997) while uncoupling oocyte Gαi/o subunits (xGαi/o) with PTX. Our findings indicate that GPCR-G protein-RGS-GIRK precoupling is a critical determinant in the anomalous channel gating, and is dependent on the concentration and composition of Gαi/o-RGS proteins.
Methods
Isolation of Xenopus oocytes
All procedures for the use and handling of Xenopus laevis (Xenopus One, Ann Arbor, MI, USA) were approved by the University of South Florida Institutional Animal Care and Use Committee in accordance with NIH guidelines. Oocytes were isolated from ovarian tissue surgically removed during hypothermia and 0.2% tricaine (MS-222)-induced anaesthesia. After recovery from anaesthesia, frogs were returned to their tanks in the institutional animal housing facility where they were monitored daily. Frogs were killed after a second procedure by exsanguination while under anaesthesia. The time between surgeries was 1-3 weeks.
The oocytes were enzymatically dissociated by a 50 min collagenase A (Boehringer Mannheim) digestion (1.8 %) at room temperature on a rocker platform in Ca2+-free oocyte Ringer (OR) solution. The OR solution was composed of 82.5 mmNaCl, 2.5 mm KCl, 1.0 mm CaCl2, 1.0 mm MgCl2, 1.0 mm NaHPO4 and 5.0 mm Hepes, at pH 7.5 (NaOH). Isolated stage V-VI oocytes were then maintained in oocyte culture medium (OCM) at 19 °C in 35 mm dishes on an orbital shaker. OCM was composed of OR solution containing 2.5 mm sodium pyruvate and 5 % heat-inactivated horse serum. OCM was changed 1-2 times daily.
Heterologous expression in Xenopus oocytes
Linearized cDNA-containing vectors (Table 1) were used to transcribe cRNA in vitro using the appropriate RNA polymerase (T7 or T3) as described by the manufacturer (mMessage mMachine, Ambion, Austin, TX, USA). Concentrations and quality of the cRNAs were determined by spectrophotometric absorbance at 260 nm and denatured (formaldehyde) agarose gel electrophoresis. On the first day after enzymatic isolation (Day 1), oocytes were injected with a mixture of cRNAs dissolved in DEPC-treated H2O at a final injection volume of 50 nl (Nanoliter2000, World Precision Instruments). All oocytes were injected with cRNAs for Kir3.1 (0.5 ng), Kir3.2a (0.5 ng), and the m2 receptor (0.5 ng). The amounts of other cRNAs, including PTX-insensitive Gα subunits and RGSs, were varied as described in the Results section. The PTX-insensitive Gα cDNAs were all constructed by PCR (kindly provided by Stephen Ikeda, NIAAA, Bethesda, MD, USA), having a 5′ Kozak sequence followed by the Gα coding region containing the C-terminal C→G mutation. The 5′ and 3′ untranslated regions of the Gα cDNAs were not included in the constructs, and therefore ribosomal binding and translation initiation of each PTX-insensitive Gα cRNA are expected to be equivalent.
Table 1.
Heterologously expressed proteins in Xenopus oocytes
| cDNA | GenBank accession# | Cloning vector |
|---|---|---|
| Human muscarinic | X15264 | pGEM3(Promega) |
| m2 receptor | ||
| Rat Kir3.1 | U01071 | pBS-MXT* |
| Mouse Kir3.2a | U37253 | pBS-MXT* |
| Rat Gαi1(C351G) | M17527 | pCI (Promega) |
| Rat Gαi2(C352G) | M17528 | pCI (Promega) |
| Rat Gαi3(C353G) | M20713 | pCI (Promega) |
| Mouse GβoA(C351G) | M36777 | pCI (Promega) |
| Mouse GβoB(C351G) | M36778 | pCI (Promega) |
| Bovine Gβ1 | M13236 | pBS-MXT* |
| Mouse Gβ5 | U69145 | pcDNA3 (Invitrogen) |
| Rat RGS4 | U27767 | pcDNA3.1(Invitrogen) |
| Bovine RGS7 | AF011359 | pcDNA3 (Invitrogen) |
| PTX-S1 | M13223 | pGEMHE* |
The pBS-MXT and pGEMHE vectors are modified pBluescript II (KS) (Stratagene) and –3Z (promega) vectors, each containing 5’ and 3’ untranslated regions of the Xenopus β–globin gene that flank the cloned cDNA and enhance RNA stability in Xenopus oocytes (Liman et al. 1992).
Two methods were used to inactivate endogenous oocyte Gαi/o subunits by PTX-mediated ADP ribosylation. Initially we injected the PTX holotoxin (containing S1, S2, S3, S4 and S5 subunits from Bordetella pertussis, Sigma-Aldrich Chemical) at 1 ng/oocyte on Day 2 of culture (1 day after cRNA injection), and then recorded GIRK currents on Day 4. This method inhibited > 80 % of the m2 receptor-activated GIRK currents coupled to endogenous Gαi/o subunits that was determined for each batch of oocytes to monitor the efficiency of endogenous G protein uncoupling. In subsequent experiments we included cRNA (1 ng/oocyte) encoding the catalytically active PTX-S1 subunit (kindly provided by Eitan Reuveny, Weizmann Institute, Israel) with the mixture of other cRNAs in the initial Day 1 cRNA injection (Vivaudou et al. 1997). This produced a much more effective > 95 % uncoupling of endogenous Gαi/o subunits which also was determined for each batch of oocytes tested.
Electrophysiological recordings
Macroscopic GIRK currents were measured using a two-electrode voltage clamp amplifier (GeneClamp 500, Axon Instruments) and standard recording techniques (Stuhmer & Parekh, 1995). Electrodes were fabricated from borosilicate glass tubes (1.5 outside diameter, 0.86 inside diameter, GC150F-10, Warner Instruments) by a programmable microelectrode puller (P-97, Sutter Instruments). Electrodes were filled with 3 m KCl and had tip resistances of 0.8-1.0 MΩ. Membrane currents from voltage clamped oocytes were digitized using a Digidata 1200 acquisition system (Axon Instruments) and a Dell PC computer running pCLAMP 7.0 software (Axon Instruments).
Oocytes were placed in a recording chamber continuously perfused with a minimal Ringer solution composed of 98 mmNaCl, 1 mm MgCl2 and 5 mm Hepes at pH 7.5 (NaOH). After electrode impalement and clamping the membrane potential to −80 mV, the perfusion solution was changed to a high K+ solution composed of 20 mm KCl, 78 mmNaCl, 1 mm MgCl2, and 5 mm Hepes at pH 7.5 (NaOH). The resulting increase in inward current represents a ‘basal’ K+ current (IK,basal) that is due primarily to receptor-independent GIRK channel activity (Dascal et al. 1993). Rapid application and washout of acetylcholine (ACh, Sigma-Aldrich Chemical) produced the receptor-dependent GIRK current (IK,ACh), and was performed with a computer controlled perfusion system (SF-77B, Warner Instruments) that rapidly switched the position of two perfusion barrels (barrel A and barrel B) located next to the oocyte. The perfusion barrels contained high K+ solution (barrel A) and high K+ solution plus ACh (barrel B). For barrel B, a range of ACh concentrations was tested for each oocyte via a manifold that connected multiple reservoirs containing different ACh concentrations. Flow through the perfusion barrels was gravity driven, and the time constant for solution exchange was ≈1 s as determined by the time course change in receptor-independent GIRK current with switching between 20 and 40 mm external K+. All recordings were performed at room temperature (21-23 °C).
Electrophysiological data analysis
Time-dependent GIRK current kinetics were analysed using non-linear curve fitting software that fit single exponential functions to derive activation time constants (τact) and deactivation time constants (τdeact) (Clampfit software, Axon Instruments). Dose-response relations were analysed by fitting peak GIRK current amplitudes with the following Hill function:
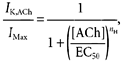 |
where the effective concentration producing a 50 % response (EC50) and Hill coefficient value (nH) were derived from the best fit (Origin 6.0 software, OriginLab Corp., Northampton, MA, USA). In some cases where the GIRK current did not reach a steady-state level at the end of the agonist application period (i.e. low agonist concentrations), the steady-state current amplitude was estimated by the fit of the activation time course to an extrapolated steady-state value.
Statistical comparisons between the various experimental groups were performed by one-way ANOVA where P < 0.05 was considered significant. Experiments were each replicated in oocytes from 2-4 separate batches (dissections) of oocytes.
Radiolabelling, immunoprecipitation and Western blotting of Gαi/o proteins
To compare the relative expression levels of PTX-insensitive Gαi/o proteins, [35S]Met/Cys (Pro-mix, Amersham Pharmacia, Piscataway, NJ, USA) was added to OCM (0.5 mCi ml−1) 1-2 h after cRNA injection to initiate radiolabelling of proteins. Fresh OCM containing [35S]Met/Cys was added on a daily basis, and 3 days after cRNA injection ≈20 oocytes from each experimental group were washed with label-free OR solution and homogenized in the following lysis buffer (150 mmNaCl, 50 mm Tris-Cl, 1 mm dithiothreitol (DTT), 1.0 % Trition X-100, and protease inhibitor cocktail (Boehringer Mannheim), pH 7.5) at 50 μl/oocyte. The oocyte lysate was then centrifuged at 10 000 g for 10 min to clear insoluble debris.
Gαi/o subunits were immunoprecipitated from oocyte lysates using a rabbit polyclonal antibody that recognizes an internal epitope (GAGESGKSTIVKQMK) identical among Gαi/o isoforms (SA-126, BIOMOL Research Laboratories, Plymouth Meeting, PA, USA). To expose the internal epitope, the supernatant from the equivalent of five oocytes (250 μl) was diluted in SDS denaturing buffer (50 mm NaPO4, 2 mm EDTA, 1 mm DTT, 0.5 % SDS, pH 8.0) and boiled for 3 min. The denaturated supernatants were then diluted and solubilized further in RIPA buffer (150 mmNaCl, 50 mm NaPO4, 2 mm EDTA, 1 mm DTT, 1.0 % Triton X-100, 1.0 % deoxycholate, 0.5 % SDS, pH 7.2). Denatured oocyte lysates (≈700 μl each) were then pre-cleared with 10 μl of non-immune rabbit serum (1 h incubation at 4 °C) and 20 μl Protein A/G-agarose beads (Santa Cruz Biotechnology, Inc.). Gαi/o proteins were immunoprecipitated in an overnight incubation at 4 °C using the SA-126 antibody precoupled to Protein A/G-agarose beads. We initially tested different amounts of oocyte lysate (0.5, 1, 2 and 4 oocytes) with a fixed amount of SA-126 antibody (2 μl) and Protein A/G-agarose (20 μl) to establish non-saturating binding conditions, which was determined to be ≤ 1 oocyte. All immunoprecipitations were then performed using the lysate equivalent of a single oocyte. At the end of the overnight incubation period, beads were washed three times with lysis buffer and the immunoprecipitated proteins eluted by boiling in 40 μl of SDS loading buffer (62.5 mm Tris-Cl pH 6.8, 10 % glycerol, 5 % β-mercaptoethanol, 2 % SDS, 0.05 % bromophenol blue).
Immunoprecipitated proteins were separated by SDS-polyacrylamide (8 %) gel electrophoresis and then transferred to a polyvinylidene difluoride (PVDF) membrane by overnight electrophoretic transfer at 4 °C. 35S-labelled proteins were resolved by autoradiography. Western blot analysis was performed using a polyclonal goat antibody that recognizes a conserved region in Gαi/o/t/z proteins (sc-12798, Santa Cruz Biotechnology). A ≈41 kDa band corresponding to Gαi/o proteins was readily detected by an HRP-conjugated donkey anti-goat secondary antibody (sc-2033, Santa Cruz Biotechnology) and enhanced chemiluminescence. Relative radiolabelling and Gαi/o protein levels were quantified by densitometric analysis (GS-700 Imaging Densitometer, Bio-Rad) of the 41 kDa band following normalization to a sample control.
Results
Conferring specific m2 receptor-Gα-coupling in Xenopus oocytes
To assess the properties of receptor-dependent GIRK channel gating elicited by specific Gα-coupled m2 receptors, oocyte Gαi/o subunits were inactivated by PTX while expressing one of five different PTX-insensitive Gαi/o subunits (Gαi1(C351G), Gαi2(C352G), Gαi3(C351G), GαoA(C351G), or GαoB(C351G)) (Wise et al. 1997). As reported previously, expression of PTX-S1 blocked > 95 % of IK,ACh and effectively abolished endogenous Gαi/o protein coupling (Vivaudou et al. 1997). Shown in Fig. 1, co-expression of Gαi2(C352G) rescued the m2 receptor-coupled GIRK currents and was dependent on the level of Gαi2(C352G) expression (Fig. 1B). Injection of 5 or 10 ng of Gαi2(C352G) cRNA per oocyte produced ACh-evoked GIRK currents that were ≈50 and 95 %, respectively, the peak amplitude of GIRK currents recorded from paired oocytes utilizing endogenous Gαi/o subunits (i.e. no PTX-S1 or Gαi2(C352G) cRNA). The Gαi2(C352G)-dependent GIRK currents represent channels activated by m2 receptors coupled specifically to Gαi2(C352G) subunits and endogenous Gβγ dimers. Gαi2(C352G)-dependent IK,ACh was also associated with a parallel 50-60 % decrease in IK,basal (Fig. 1D). The reduction in IK,basal by Gαi2(C352G) is likely due to the sequestration of free Gβγ dimers that mediate receptor-independent basal GIRK channel activity (Lim et al. 1995; Vivaudou et al. 1997; Jeong & Ikeda, 1999).
Figure 1. Specific coupling of Gαi2(C352G) to m2 receptors and GIRK channel activation in Xenopus oocytes.
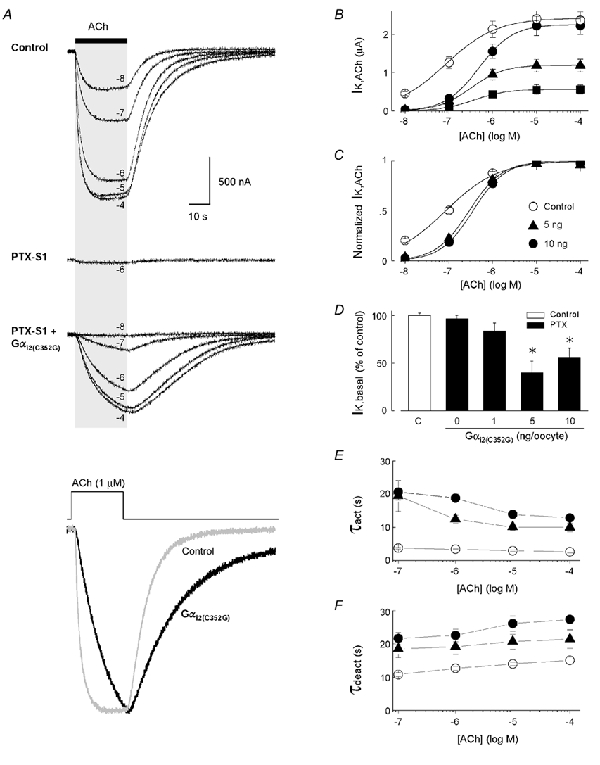
A, typical ACh-evoked GIRK currents (IK,ACh) recorded from different oocytes from three separate experimental groups. Upper traces: IK,ACh from a ‘control’ oocyte expressing muscarinic m2 receptors and Kir3.1/Kir3.2a channel subunits, utilizing endogenous Gαi/o proteins for receptor activation. Middle trace: co-expression of PTX-S1 (1 ng cRNA/oocyte) effectively uncouples ACh-evoked GIRK currents utilizing oocyte Gαi/o proteins. Lower traces: expression of the PTX-insensitive Gαi2(C352G) subunit (5 ng cRNA) with PTX-S1 rescues m2 receptor-coupled GIRK currents. All GIRK currents were elicited by a 25 s application of different concentrations of ACh as indicated. Bottom traces: superimposed IK,ACh elicited by 1 μm ACh from the control oocyte (grey trace) and the Gαi2(C352G)-coupled oocyte (black trace). Peak amplitudes are normalized to illustrate the kinetic differences in the activation and deactivation time courses. B, ACh dose-response relations for GIRK activation via m2 receptors coupled to oocyte Gαi/o subunits (○, control) and Gαi2(C352G) at different levels of expression (▪ 1 ng, ▴ 5 ng, and • 10 ng cRNA/oocyte). C, ACh-dose-response curves from B normalized to maximal IK,ACh. D, comparison of receptor-independent basal GIRK currents (IK,basal) with varying levels of Gαi2(C352G) expression. IK,basal is expressed as the percentage change in the ‘control group’ mean value determined for each batch of oocytes. E, activation time constants (τact) and F, deactivation time constants (τdeact) for GIRK currents coupled to varying levels of Gαi2(C352G) expression and different concentrations of ACh. ○ control; ▴ 5 ng; and • 10 ng of Gαi2(C352G) cRNA/oocyte. Data in B-F represent the means ±s.e.m. from at least 3 batches of oocytes with the number of oocytes indicated. * P < 0.05.
ACh dose-response curves derived from oocytes expressing different levels of Gαi2(C352G) were not significantly different with respect to their EC50 value as shown in Fig. 1C, and this is consistent with previous studies where EC50 values were shown to be relatively unaffected by overexpressed Gα subunits, but most sensitive to levels of GPCR expression (Henry et al. 1995). Since the same amount of m2 receptor cRNA was injected for all experimental groups (0.5 ng cRNA/oocyte), m2 receptor protein levels were assumed to be roughly equivalent across groups and this was supported by the equivalent EC50 values with varying Gαi2(C352G) expression levels. The ACh dose-response curves for Gαi2(C352G)-coupled m2 receptors, however, were notably shifted rightward compared to the ACh dose-response relation from m2 receptor coupling to endogenous Gαi/o subunits (Fig. 1B). The higher EC50 value for Gαi2(C352G)versus endogenous Gαi/o-coupled receptors may be attributable to the mixed coupling of oocyte Gαo and Gαi subunits, and/or a lower level of m2 receptors precoupled to heterotrimeric G proteins (Shea & Linderman, 1997; Shea et al. 2000) (see Results below and Discussion).
One readily apparent feature of the m2 receptor- Gαi2(C352G)-coupled GIRK current was the significantly slower activation and deactivation kinetics as compared to GIRK currents coupled to endogenous xGαi/o subunits (Fig. 1A). The time constants for IK,ACh activation and deactivation over a range of ACh concentrations are shown in Fig. 1E and F. The slower kinetics for m2 receptor-Gαi2(C352G)-coupled GIRK currents were not attributable to the level of Gαi2(C352G) expression since increasing amounts of Gαi2(C352G) cRNA, which nearly doubled maximal IK,ACh amplitudes, did not result in significantly different GIRK activation or deactivation time constants. The slower GIRK gating properties may be caused by altered coupling properties of the C→G mutated Gαi2 subunit, or alternatively the faster properties in the absence of overexpressed Gα subunits may reflect the influence of endogenous RGS proteins (Saitoh et al. 2000).
GIRK channel gating properties via Gαo- and Gαi-coupled m2 receptors
The gating properties of GIRK currents elicited by m2 receptors coupled to each of the PTX-insensitive Gαi/o subunits are shown in Fig. 2. Since there were no noticeable differences in GIRK current kinetics at different levels of Gαi2(C352G) expression, comparisons between the five different Gαi/o isoforms were initially made using 5 ng cRNA/oocyte for each Gα isoform. The three Gαi isoforms (Gαi1(C351G), Gαi2(C352G) and Gαi3(C351G)) each produced ACh-elicited GIRK currents that were indistinguishable from each other with regard to the five parameters analysed (maximal IK,ACh, IK,basal, EC50, τact and τdeact) (Fig. 2). GαoA(C351G) and GαoB(C351G) also produced equivalent GIRK responses but were distinct from Gαi-coupled GIRK currents in several respects. First, Gαo-coupled GIRK currents were significantly larger than Gαi-coupled currents, with maximal IK,ACh amplitudes being ≈2-fold greater (Fig. 2B). Second, expression of GαoA(C351G) and GαoB(C351G) more prominently inhibited IK,basal compared to Gαi1(C351G), Gαi2(C352G), or Gαi3(C351G) (Fig. 2B). Third, the EC50 values for GαoA(C351G) and GαoB(C351G) were more similar to the EC50 values with endogenous xGαi/o coupling than with Gαi1(C351G), Gαi2(C352G), or Gαi3(C351G) (Fig. 2C). And finally, the activation of Gαo-coupled GIRK currents was faster compared to Gαi-coupled currents and had a more prominent sigmoidal time course (Fig. 2D). The deactivation time course for IK,ACh was not significantly different among all five Gαi/o isoforms (Fig. 2D).
Figure 2. Properties of ACh-evoked GIRK currents activated by m2 receptors coupled to five different Gαi/o subunits.
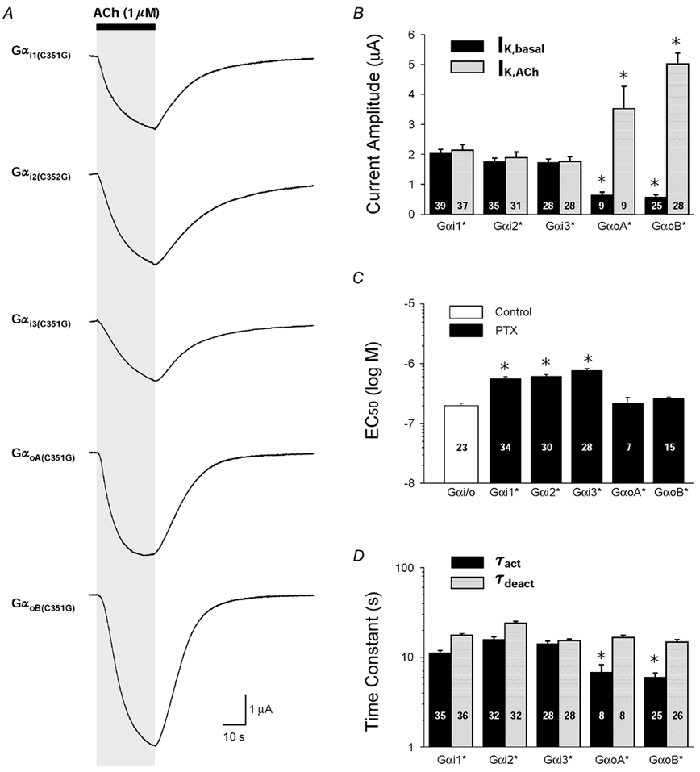
A, typical ACh-evoked GIRK currents for each PTX-insensitive Gαi/o subunit examined (Gαi1(C351G), Gαi2(C352G), Gαi3(C351G), GαoA(C351G) and GαoB(C351G)). Currents were elicited by a 25 s application of 1 μm ACh. B, maximal amplitude of receptor-dependent GIRK currents (IK,ACh, grey bars) and receptor-independent GIRK currents (IK,basal, black bars) for each expressed PTX-insensitive Gα subunit (Gα*). Gα* expression was produced by 5 ng cRNA/oocyte. Maximal IK,ACh responses are to 10 μm ACh. C, EC50 values for control (open bar) and each PTX-insensitive Gα* subunit, derived from ACh dose-response relations for IK,ACh activation. D, activation (black bars) and deactivation time constants (grey bars) derived from exponential fits of the IK,ACh time course in response to rapid ACh application and washout. For B-D, data represent the means + s.e.m. from at least 3 batches of oocytes with the number of oocytes indicated (* P < 0.05). In B and D, statistical comparisons were with Gαi1(C351G), and in C comparisons were with oocyte Gαi/o coupling (open bar).
The differences between Gαi and Gαo-coupled GIRK currents were not attributable to cDNA/vector construction (i.e. untranslated regions), cRNA quantification, or batch-to-batch oocyte variability (see Methods). Several factors could conceivably contribute to these differences and include (1) differences in Gαi/o biosynthesis or degradation (Li et al. 1996), (2) preferential Gαo coupling to m2 receptors (Leaney & Tinker, 2000), (3) preferential Gαo association with oocyte Gβγ subunits (Fernandez-Fernandez et al. 2001), and (4) different Gαi/o subcellular distributions (Devic et al. 1996).
Biochemical analysis of PTX-insensitive Gαi/oprotein expression levels
To determine whether PTX-insensitive Gαi/o subunits are differentially expressed following cRNA injection (5 ng cRNA/oocyte, as in Fig. 2), Gαi/o proteins were immunoprecipitated from oocytes following metabolic labelling with [35S]Met/Cys. The PTX-insensitive Gαi/o proteins each contain an equivalent number of Met/Cys sites (19) for potential radiolabelling. As shown in Fig. 3A, a prominent ≈41 kDa band that corresponds to Gαi/o proteins was radiolabelled in each experimental group. A significant portion represents radiolabelling of endogenous xGαi/o proteins over the 3 day incubation period as indicated by the oocyte groups that were not injected with PTX-insensitive Gαi/o cRNA. Quantitative comparisons of the 35S-labelled 41 kDa band via densitometric analysis indicate a small 10-20 % elevation in oocytes injected with PTX-insensitive Gαi/o cRNAs (Fig. 3B). The observed changes in 35S labelling were also paralleled by densitometric changes in the bands detected by Western blot analysis (Fig. 3B). Since the peak IK,ACh amplitudes for Gαo-coupled m2 receptors were ≈2 times higher compared to Gαi-coupled m2 receptors under equivalent expression conditions (cf. Fig. 2B), the ≈2-fold difference in GαoB(C351G)versus Gαi2(C352G) or Gαi3(C351G) protein expression could potentially account for the differences in m2 receptor-GIRK channel coupling. GαoA(C351G)-elevated protein levels, however, were similar to Gαi2(C352G) and Gαi3(C351G) levels (Fig. 3B), and resolving small expression changes was clearly limited by the biochemical approach. Thus despite equivalent cRNA injections (5 ng cRNA/oocyte each), differences in Gαi/o biosynthesis and/or degradation may result in different steady-state Gαi/o protein levels that account for the distinct gating properties of Gαo- versus Gαi-coupled GIRK currents.
Figure 3. Biochemical analysis of PTX-insensitive Gαi/o protein levels in Xenopus oocytes.
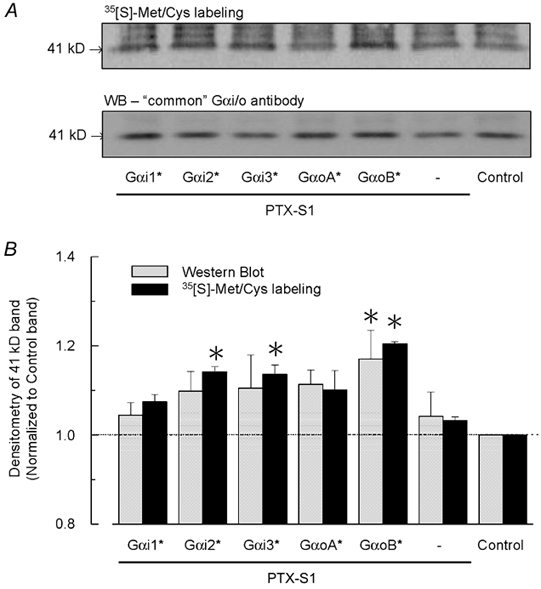
A, radiolabelling (upper panel) and Western blot analysis (lower panel) of oocytes injected with cRNAs encoding five different PTX-insensitive Gαi/o subunits (*) as described in Methods and Fig. 2. Oocytes were incubated for 3 days in OCM containing 0.5 mCi ml−1 [35S]Met/Cys. All groups were injected with cRNAs for the m2 receptor and GIRK channel subunits Kir3.1 and Kir3.2a (0.5 ng each/oocyte). PTX-insensitive Gαi/o cRNAs (5 ng/oocyte) were injected with PTX-S1 cRNA (1 ng/oocyte). Both endogenous and heterologously expressed Gα proteins were immunoprecipitated from the lysate equivalent of one oocyte using a ‘common’ Gα antibody, then separated by SDS-PAGE and transferred to a PVDF membrane for autoradiography and Western blotting. The 41 kDa band corresponding to Gαi/o proteins is indicated. B, quantitative analysis of Gαi/o proteins detected by radiolabelling and Western blot analysis. The 41 kDa band from the control group (no PTX-insensitive Gαi/o cRNA) served as an internal reference and was used to normalize the band intensity among the different experimental groups in each autoradiogram and Western blot. The Western blot results are the means + s.e.m. obtained from 3 independent experiments (separate batches of injected oocytes that were immunoprecipitated and immunostained as described in Methods). The radiolabelling data are the means + s.e.m. obtained from 2 of the Western blot experiments. * P < 0.05.
The GIRK activation time course is dependent on free Gβγ levels
A sigmoidal time course for GIRK activation, as observed with Gαo expression (Fig. 2), is well documented in cardiomyocytes and neurons and indicative of a multi-step process in channel opening (Inomata et al. 1989; Sodickson & Bean, 1996). Tetrameric GIRK channels can bind up to four Gβγ dimers, and functional studies indicate at least two Gβγ dimers are necessary for channel opening (Nemec et al. 1999; Corey & Clapham, 2001). We questioned whether the sigmoidal GIRK activation time course via Gαo-coupled receptors was a consequence of a markedly reduced free Gβγ concentration as indicated by the low IK,basal level. As illustrated in Fig. 4A, conditions favouring high free Gβγ concentrations (high IK,basal) would yield a single exponential time course for receptor activation, where the binding of a single Gβγ dimer leads to channel opening. At low free Gβγ concentrations (i.e. low IK,basal), GIRK channels would instead be occupied by fewer Gβγ dimers (less than 3 Gβγ) and require multiple Gβγ binding events during receptor activation (2-4 Gβγ). According to this hypothesis, reducing Gαo expression levels should increase IK,basal and promote a single exponential time course for IK,ACh activation.
Figure 4. Activation kinetics of Gαo-coupled GIRK currents at different levels of GαoB(C352G) expression.
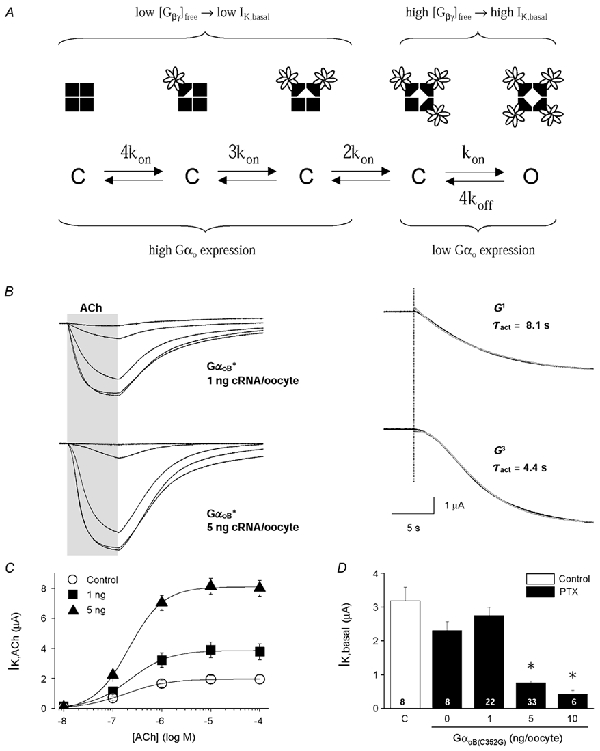
A, kinetic scheme for GIRK channel activation via Gβγ binding to each of the four GIRK channel subunits. Four closed states are depicted with 0 to 3 bound Gβγ dimers. Channel opening (O) occurs with the binding of 4 Gβγ dimers. The level of free Gβγ dimers then determines the channel-bound state that precedes receptor activation. B, left panel: ACh-evoked GIRK currents from oocytes injected with either 1 or 5 ng GαoB(C351G) cRNA. Right panel: the activation phase for the 1 μm ACh-evoked GIRK currents are displayed at higher temporal resolution. Red lines represent non-linear fits to the data. A single exponential function (G1) best described the IK,ACh time course with low GαoB(C351G) expression (1 ng RNA/oocyte), having a time constant of 8.1 s. A third-order exponential function (G3) best described the activation time course at high GαoB(C351G) expression (5 ng RNA/oocyte), having a time constant of 4.4 s. C, ACh dose-response curves for m2 receptor-activated GIRK currents from control oocytes (○) and oocytes expressing GαoB(C351G) at two different levels (▪ 1 ng and ▴ 5 ng cRNA/oocyte). D, effects of GαoB(C351G) expression levels on receptor-independent GIRK channel activity (IK,basal). The open bar is from control oocyte responses, and black bars are from oocytes injected with varying amount of GαoB(C351G) cRNA and PTX-S1 cRNA (1 ng/oocyte). Data represent the means + s.e.m. from 4 batches of oocytes with the number of oocytes indicated.
To test this, we compared the activation time course of IK,ACh at two different levels of GαoB(C351G) expression (Fig. 4). As expected, lower expression of GαoB(C351G) (1 ng GαoB(C351G) cRNA/oocyte) produced smaller IK,ACh amplitudes compared to high GαoB(C351G) expression, and had significantly higher receptor-independent basal GIRK activity (Fig. 4C and D). The activation time course for IK,ACh was clearly affected by the level of GαoB(C351G) expression and was consistent with the gating scheme shown in Fig. 4A. Low GαoB(C351G) expression yielded GIRK currents whose activation time course was well described by a single exponential function. In contrast, high GαoB(C351G) expression yielded GIRK currents whose activation time course had a significant delay and were best described by a 3rd order exponential function, suggesting the binding of three Gβγ subunits in receptor-dependent GIRK activation (see Fig. 4A). The τact was smaller for high GαoB(C351G) expression versus low GαoB(C351G) expression, and single exponential fits to the rising phase of the sigmoidal time course underestimate the activation rate constant (1/τact) under these conditions. Comparisons of τdeact and EC50 values from ACh dose-response curves indicated the these properties were not significantly affected by these levels of GαoB(C351G) expression (data not shown).
RGS4 reduces GIRK currents at low Gαi/o coupling levels
We next evaluated the effects of RGS4 at saturating expression levels (10 ng RGS4 cRNA/oocyte; Doupnik et al. 1997;
 |
Keren-Raifman et al. 2001) on both Gαo- and Gαi-coupled m2 receptors as described in Fig. 2. As shown in Fig. 5, RGS4 accelerated the receptor-dependent GIRK activation and deactivation kinetics for each Gαi/o isoform, consistent with the non-selective GTPase activating properties of RGS4 on Gαi/o subunits in vitro (Berman et al. 1996; Watson et al. 1996). Interestingly, however, RGS4 suppressed 60-70 % of the IK,ACh amplitude for Gαi-coupled m2 receptors, yet had no effect on the peak IK,ACh amplitude of Gαo-coupled receptors. RGS4 did not significantly affect the EC50 for channel activation via Gαi-coupled receptors, but did cause a small shift the EC50 value for GαoA(C351G)-coupled GIRK currents towards a higher ACh concentration, which more closely corresponded to the EC50 values of Gαi-coupled GIRK currents (Fig. 5F). Kinetic analysis indicated the RGS4-mediated reduction in Gαi-coupled IK,ACh (Fig. 5E) was fully explained by the RGS4-accelerated GIRK deactivation rate (see Table 2 and Discussion).
Figure 5. Effects of RGS4 on GIRK currents coupled to specific PTX-insensitive Gαi/o subunits.
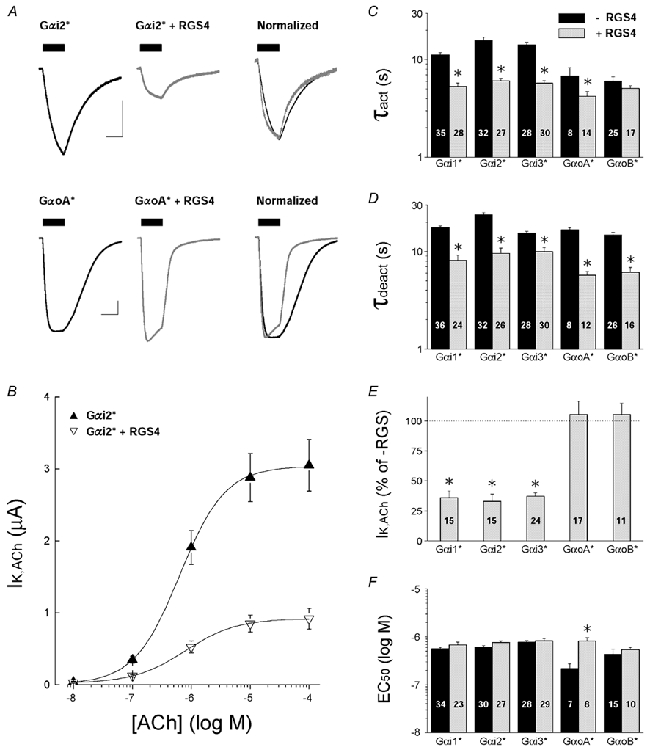
All data are from oocytes injected with 5 ng Gα* cRNA/oocyte, with or without 10 ng RGS4 cRNA/oocyte. Endogenous PTX-sensitive Gαi/o subunits were inactivated by either PTX injection or PTX-S1 expression as described in Methods. Data are means and s.e.m.; * P < 0.05. A, representative ACh-evoked GIRK currents from oocytes expressing either Gαi2(C352G) (upper traces) or GαoA(C351G) (lower traces) with and without RGS4 coexpression. Scale bars indicate 1 μA and 10 s. B, ACh dose-response relations for m2 receptor-coupled GIRK currents from oocytes expressing Gαi2(C352G) in the absence (▴) or presence of RGS4 (▿). C, IK,ACh activation time constants (τact) from oocytes expressing PTX-insensitive Gαi/o subunits alone (black bars) or with co-expressed RGS4 (grey bars). Time constants were derived from a single exponential fit to the rising phase of IK,ACh elicited by 1 μm ACh. D, deactivation time constants (τdeact) derived from ACh-elicited GIRK currents recorded from oocytes expressing PTX-insensitive Gαi/o subunits alone (black bars) or with co-expressed RGS4 (grey bars). Time constants were derived from a single exponential fit to the decay of IK,ACh after rapid washout of 1 μm ACh. E, effects of RGS4 on maximal IK,ACh responses elicited by m2 receptors coupled to PTX-insensitive Gαi/o subunits. IK,ACh amplitudes (10 μm ACh) from RGS4-expressing oocytes are expressed as a percentage of the mean IK,ACh amplitude from oocytes expressing the PTX-insensitive Gαi/o subunit alone (RGS-). F, effects of RGS4 on the EC50 for ACh activation of GIRK currents coupled to m2 receptors and PTX-insensitive Gαi/o subunits. Black bars are without RGS4 expression (-RGS4), grey bars are with RGS4 co-expression (+RGS4).
Table 2.
‘Expected’ versus ‘Observed’ gating parameters for RGS-accelerated GIRK currents
| Expressed proteins | Observed Tdeact (S) | Expected Mean Tact (S) | Observed Tact (S) | Expressed amplituted (% of control) | Observed amplituted (% of control) |
|---|---|---|---|---|---|
| Gαi1* | 17.6 ± 4.6 | 11.5 | 11.2 ± 3.8 | — | — |
| Gαi1*+ RGS4 | 8.1 ± 5.0 | 6.5 | 5.3 ± 2.2 | 56 ± 28 | 36 ± 5 |
| Gαi1*+ RGS7 | 12.5 ± 6.1 | 9.1 | 8.8 ± 2.9 | 79 ± 25 | 74 ± 6 |
| Gαi1*+ RGS7 + Gβ1 | 17.5 ± 2.9 | 11.5 | 13.0 ± 2.6 | — | — |
| Gαi1*+ RGS7 + Gβ5 | 13.9 ± 2.1 | 9.8 | 11.4 ± 2.5 | 85 ± 9 | 88 ± 17 |
| Gαi2* | 23.8 ± 7.1 | 13.9 | 15.7 ± 7.1 | — | — |
| Gαi2*+ RGS4 | 9.6 ± 6.3 | 7.5 | 6.1 ± 2.1 | 54 ± 23 | 33 ± 6 |
| Gαi2*+ RGS7 | 17.1 ± 6.4 | 11.3 | 10.8 ± 4.3 | 81 ± 18 | 90 ± 8 |
| Gαi2*+ RGS7 + Gβ1 | 20.3 ± 3.6 | 12.6 | 14.8 ± 2.9 | — | — |
| Gαi2*+ RGS7 + Gβ5 | 18.6 ± 2.9 | 11.9 | 15.7 ± 7.5 | 95 ± 9 | 100 ± 18 |
| Gαi3* | 15.4 ± 3.4 | 10.5 | 14.1 ± 5.2 | — | — |
| Gαi3*+ RGS4 | 10.0 ± 5.3 | 7.7 | 5.7 ± 2.0 | 73 ± 27 | 37 ± 3 |
| Gαi3*+ RGS7 | 13.5 ± 4.4 | 9.6 | 10.5 ± 3.6 | 91 ± 20 | 61 ± 4 |
| GαoA* | 16.7 ± 2.8 | 11.1 | 6.8 ± 4.1 | — | — |
| GαoA*+ RGS4 | 5.7 ± 1.9 | 4.9 | 4.2 ± 1.9 | 44 ± 12 | 105 ± 11† |
| GαoA*+ RGS7 | 3.3 ± 1.7 | 3.0 | 3.1 ± 1.8 | 27 ± 12 | 79 ± 8† |
| GαoA*+ RGS7 + Gβ1 | 6.3 ± 2.2 | 5.3 | 2.9 ± 1.5 | — | — |
| GαoA*+ RGS7 + Gβ5 | 2.2 ± 1.3 | 2.0 | 0.9 ± 0.5 | 39 ± 21 | 17 ± 2 |
| Gαob* | 14.8 ± 4.9 | 10.3 | 6.0 ± 3.4 | — | — |
| Gαob*+ RGS4 | 6.1 ± 2.9 | 5.2 | 5.0 ± 1.4 | 50 ± 19 | 105 ± 9† |
| Gαob*+ RGS7 | 4.0 ± 1.5 | 3.6 | 3.1 ± 0.7 | 35 ± 11 | 91 ± 13† |
| Gαob*+ RGS7 + Gβ1 | 2.7 ± 1.0 | 2.5 | 2.4 ± 0.7 | — | — |
| Gαob*+ RGS7 + Gβ5 | 1.4 ± 0.9 | 1.3 | 1.7 ± 1.0 | 54 ± 32 | 32 ± 3 |
denotes deviation from expected steady—state GIRK current amplitude.
To determine whether the higher degree of Gαoversus Gαi coupling (cf. Fig. 2) could account for the RGS4-dependent effects on steady-state GIRK currents, we examined the effects of RGS4 at a lower level of GαoA expression (0.5 ng cRNA/oocyte). At low GαoA expression levels, RGS4 significantly reduced ≈90 % of both IK,ACh (GαoA alone, 0.76 ± 0.11 μA, n = 17; GαoA + RGS4, 0.07 ± 0.03 μA, n = 9; P < 0.05) and IK,basal (GαoA alone, 2.34 ± 0.18 μA, n = 19; GαoA + RGS4, 0.25 ± 0.07 μA, n = 14; P < 0.05). Thus the effect of RGS4 on steady-state GIRK current amplitudes is dependent on the level of Gαi/o expression and GPCR precoupling. At high levels of m2 receptor-Gαo coupling (i.e. Fig. 5), RGS4 accelerates GIRK activation and deactivation without significantly reducing steady-state GIRK current amplitudes and resembles the effects of RGS4 with endogenous xGαi/o coupling (Doupnik et al. 1997).
RGS7 selectively accelerates Gαo-coupled GIRK currents
RGS7 belongs to a distinct subfamily of RGS proteins that contain a Gγ-like (GGL) domain that specifically binds Gβ5 subunits (Snow et al. 1998; Sondek & Siderovski, 2001). RGS7 has relatively weak effects on GIRK current kinetics when expressed without Gβ5 in Xenopus oocytes (Saitoh et al. 1999), but is significantly enhanced by Gβ5 co-expression (Kovoor et al. 2000; Keren-Raifman et al. 2001). We examined whether RGS7 (10 ng cRNA/oocyte), with or without co-expressed Gβ5 subunits, differentially affected Gαo- versus Gαi-coupled GIRK currents. In the absence of coexpressed Gβ5, RGS7 preferentially accelerated Gαo-coupled GIRK currents having little effect on Gαi-coupled GIRK kinetics (Fig. 6), which is consistent with other studies indicating RGS7 selectively interacts with Gαo subunits (Posner et al. 1999; Lan et al. 2000; Rose et al. 2000). Interestingly, RGS7 acceleration of Gαo-coupled IK,ACh was greater than that caused by RGS4 (cf. Fig. 5C and D) and was evident for both the IK,ACh activation and deactivation time course as well as the desensitization rate. As observed with RGS4, RGS7 did not cause a significant reduction in the maximal Gαo-coupled GIRK current despite accelerating the GIRK channel gating kinetics (Fig. 6E), but did cause a small shift in the ACh dose-response curve (Fig. 6B and F). Yet in contrast to RGS4, RGS7 did not reduce steady-state GIRK currents when GαoA expression was markedly reduced (0.5 ng cRNA/oocyte): IK,basal (GαoA alone, 2.34 ± 0.18 μA, n = 19; GαoA + RGS7, 2.18 ± 0.20 μA, n = 18) and IK,ACh (GαoA alone, 0.76 ± 0.11 μA, n = 17; GαoA + RGS7, 1.25 ± 0.21 μA, n = 17).
Figure 6. Effects of RGS7 on GIRK currents coupled to specific PTX-insensitive Gαi/o subunits.
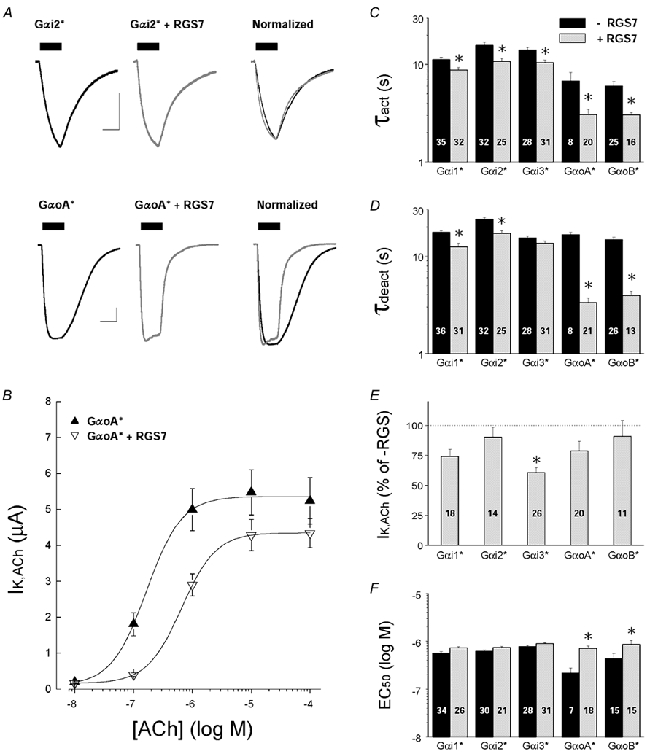
All data are from oocytes injected with 5 ng Gα* cRNA/oocyte, with or without 10 ng RGS7 cRNA/oocyte. Endogenous PTX-sensitive Gαi/o subunits were inactivated by either PTX injection or PTX-S1 expression as described in Methods. Data are means and s.e.m.; * P < 0.05. A, representative ACh-evoked GIRK currents from oocytes expressing either Gαi2(C352G) (upper traces) or GαoA(C351G) (lower traces) with and without RGS7 coexpression. Scale bars indicate 1 μA and 10 s. B, ACh dose-response relations for m2 receptor-coupled GIRK currents from oocytes expressing Gαi2(C352G) in the absence (▴) or presence of RGS7 (▿). C, IK,ACh activation time constants (τact) from oocytes expressing PTX-insensitive Gαi/o subunits alone (black bars) or with co-expressed RGS7 (grey bars). Time constants are from GIRK currents evoked by 1 μm ACh. D, deactivation time constants (τdeact) from ACh-elicited GIRK currents recorded from oocytes expressing PTX-insensitive Gαi/o subunits alone (black bars) or with co-expressed RGS7 (grey bars). Time constants were derived after rapid washout of 1 μm ACh. E, effects of RGS7 on maximal IK,ACh responses elicited by m2 receptors coupled to PTX-insensitive Gαi/o subunits. IK,ACh amplitudes (10 μm ACh) from RGS4-expressing oocytes as a percentage of the mean IK,ACh amplitude from oocytes expressing the PTX-insensitive Gαi/o subunit alone (no RGS7). F, effects of RGS7 on the EC50 for ACh activation of GIRK currents coupled to m2 receptors and PTX-insensitive Gαi/o subunits. Black bars are without RGS7 expression, grey bars are with RGS7 co-expression.
RGS7-Gβ5 disrupts Gαo-coupled GIRK currents
Gβ5 binds with high affinity to the GGL domain of RGS7, enhancing the GAP activity and protein stability of RGS7 (Levay et al. 1999; Kovoor et al. 2000; Keren-Raifman et al. 2001). We examined the effects of Gβ5 expression on the RGS7- mediated regulation of both Gαo- and Gαi-coupled GIRK currents, with comparisons made using Gβ1 as a negative control which does not bind to RGS7 (Levay et al. 1999; Kovoor et al. 2000). As shown in Fig. 7, co-expression of Gβ5 with RGS7 caused a significant reduction (70-80 %) in Gαo-coupled GIRK current amplitudes, yet had no effect on Gαi-coupled currents. The lack of effect of Gβ5 on Gαi-coupled GIRK currents indicates the level of Gβ5 (or Gβ5γ formation) does not cause a direct inhibition of GIRK currents as observed in transfected HEK-293 cells (Lei et al. 2000). In addition to reducing Gαo-coupled GIRK currents, Gβ5 enhanced the RGS7-accelerated GIRK kinetics consistent with RGS7-Gβ5 complexes having greater GAP activity compared to RGS7 alone (Kovoor et al. 2000; Keren-Raifman et al. 2001). Thus the formation of RGS7-Gβ5 complexes apparently disrupts Gαo coupling (either to m2 receptors and/or GIRK channels) and is supported by the effects of Gβ5 on RGS7-Gαo interactions in vitro (Levay et al. 1999).
Figure 7. Effects of Gβ5 on RGS7-accelerated GIRK currents coupled to specific PTX-insensitive Gαi/o subunits.
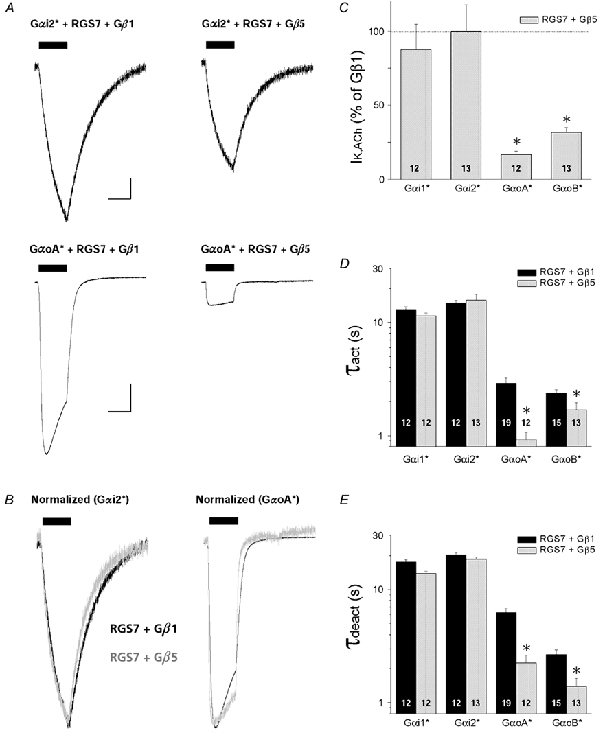
All data are from oocytes injected with 5 ng Gα* cRNA/oocyte and 10 ng RGS7 cRNA/oocyte, with either 5 ng Gβ5 cRNA/oocyte or 5 ng Gβ1 cRNA/oocyte as a negative control. Oocytes also received Kir3.1 and Kir3.2a cRNA (0.5 ng/oocyte each), and m2 receptor cRNA (0.5 ng/oocyte), and endogenous PTX-sensitive Gαi/o subunits were inactivated by PTX-S1 expression (1.0 ng/oocyte). Data are means + s.e.m.; * P < 0.05. A, representative ACh-evoked GIRK currents from oocytes expressing RGS7 and Gαi2(C352G) with either Gβ1 or Gβ5 (upper traces), or GαoA(C351G) with either Gβ1 or Gβ5 (lower traces). Scale bars indicate 1 μA and 10 s. B, GIRK currents from A, normalized to peak amplitude and superimposed to highlight their temporal features (black traces, RGS7+Gβ1; grey traces, RGS7+Gβ5). C, Gβ5 selectively suppresses RGS7/ Gαo-coupled GIRK currents. IK,ACh amplitudes are expressed as a percentage of the mean IK,ACh amplitude from oocytes expressing the negative control Gβ1. D, GIRK activation time constants (τact) derived from oocytes expressing different PTX-insensitive Gαi/o subunits with either RGS7 + Gβ1 (black bars) or RGS7 + Gβ5 (grey bars). Time constants were derived from a single exponential fit to the rising phase of IK,ACh elicited by 1 μm ACh. E, deactivation time constants (τdeact) derived from ACh-elicited GIRK currents as in D. Time constants were derived from a single exponential fit to the decay of IK,ACh after rapid washout of 1 μm ACh.
Discussion
The goal of this study was to determine whether functional differences exist in the GPCR-dependent gating properties of GIRK channels coupled to specific Gαi/o subunits and RGS proteins. Our findings reveal that m2 receptors coupled to Gαi and Gαo subunits are differentially regulated by RGS4 and RGS7. A significant finding of our study is the identification of G protein coupling levels as a critical determinant in mediating the anomalous kinetic effects of RGS proteins on GIRK channel gating (Doupnik et al. 1997; Saitoh et al. 1997; Zerangue & Jan, 1998; Kovoor & Lester, 2002). Incremental increases in Gαoβγ coupling by increased Gαo expression revealed a transition in the RGS4-accelerated GIRK currents from ‘expected’ steady-state properties, characterized by a large reduction in maximal GIRK current amplitude, to ‘anomalous’ gating kinetics which displayed accelerated activation and deactivation kinetics with no effect on steady-state GIRK amplitude. We propose that this transition in steady-state GIRK channel kinetics is a functional indicator of the formation of a GPCR-G protein-GIRK channel signalling complex (Huang et al. 1995; Slesinger et al. 1995) that is precipitated at a critical level of Gαi/o expression and revealed by the modulatory actions of co-expressed RGS proteins.
RGS proteins reveal GPCR-G protein-GIRK coupling levels via steady-state gating properties
RGS proteins were originally identified as ‘negative regulators’ of G protein signalling causing reduced GPCR signalling in yeast, fungi, nematodes and mammals (Dohlman et al. 1995, 1996; Druey et al. 1996; Koelle & Horvitz, 1996; Yu et al. 1996). These findings were readily explained by RGS proteins behaving as GTPase-activating proteins and accelerating the termination of G protein signalling (Berman et al. 1996; Chen et al. 1996; Hunt et al. 1996; Watson et al. 1996). Initial studies of the effects of RGS proteins on GPCR-evoked GIRK currents revealed the anticipated acceleration in current deactivation, which was well explained by the RGS-accelerated GTPase activity of Gαi/o subunits causing a more rapid sequesteration of channel-activating Gβγ dimers (Doupnik et al. 1997; Saitoh et al. 1997). Unexpectedly, however, the accelerated deactivation was also accompanied with an accelerated activation phase in the absence of reduced current amplitude.
We analysed the temporal and steady-state GIRK current kinetics for the various conditions tested in this study using a simplified two-state gating scheme (Doupnik et al. 1997):
 |
where the forward rate constant for GIRK channel opening (kopen) is dependent on the rate of Gβγ production during GPCR activation (Breitwieser & Szabo, 1988; Yamada et al. 1998), and the closing rate constant (kclose) is dependent on Gβγ clearance, which is rate-limited by the GTP hydrolysis rate of Gα subunits. Accordingly, the time constant for GIRK channel deactivation (τdeact) is equal to kclose−1, and the time constant for GIRK activation (τact) is equal to (kopen +kclose)−1 (Doupnik et al. 1997). Using these relations and an empirically derived kopen value of 0.03 s−1 (held constant to simulate a saturating agonist concentration), the experimentally derived τdeact values were used to calculate ‘expected’ values of τact and steady-state GIRK current amplitudes (kopen/[kopen +kclose]) in the absence and presence of RGS expression according to the kinetic model. The ‘expected’ values were then compared to the experimentally ‘observed’ values (Table 2).
As seen in Table 2, the ‘observed’ effects of RGS4 on τact and steady-state amplitude for Gαi-coupled GIRK currents closely correlate with the ‘expected’ consequences of the RGS4-accelerated deactivation rate, but not so for the Gαo-coupled GIRK currents. Similarly, a correlation was seen for the effects of Gβ5 on RGS7-accelerated GIRK currents activated by Gαo-coupled receptors. Thus during reduced levels of G protein coupling, GIRK channels behave according to standard kinetic concepts in response to RGS modulation and are consistent with the collision coupling model of G protein activation (Shea & Linderman, 1997; Shea et al. 2000). In contrast, RGS4 and RGS7 modulate Gαo-coupled GIRK currents in a manner similar to previous descriptions of the anomalous kinetic effects of RGS proteins on GIRK channel gating which do not correlate with the kinetic model (Table 2) (Doupnik et al. 1997; Saitoh et al. 1997). We propose that these expression conditions promote the formation of a precoupled m2 receptor-Gαoβγ-GIRK channel complex (Huang et al. 1995; Slesinger et al. 1995), having altered activation properties that are revealed by the modulatory effects of RGS proteins and effectively preserve maximal GIRK current amplitudes (see Fig. 8).
Figure 8. RGS modulation of GPCR → GIRK signal transduction at varying levels of G protein coupling.
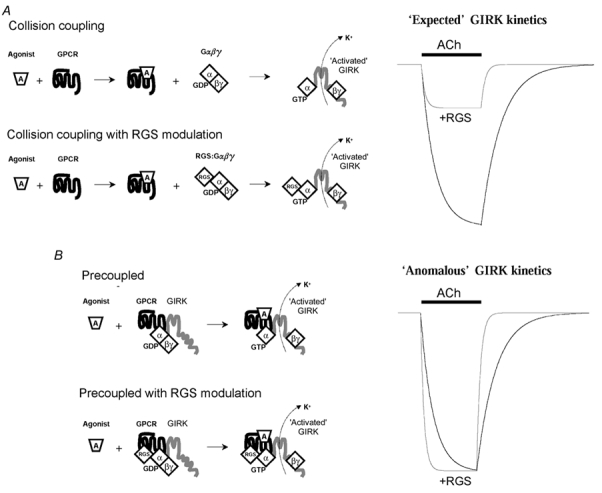
A, collision coupling model (low Gα expression) and B precoupled model (high Gα expression). The precoupled model assumes a GPCR-G protein-RGS-GIRK channel complex displaying ‘anomalous’ kinetic behaviour that was characteristic of oocytes expressing Gαo subunits with either RGS4 or RGS7, together with co-expressed m2 receptors and GIRK channel Kir3.1/3.2a subunits.
GIRK activation kinetics revealed at low levels of basal activity
GIRK channels are composed of four Kir3.0 subunits with each subunit capable of binding a single Gβγ dimer, thus a maximum of four Gβγ dimers can bind to a single GIRK channel (Corey & Clapham, 2001). Several studies indicate GIRK channel activation is a multi-step process and may represent the binding of multiple Gβγ dimers leading to channel opening (Hosoya et al. 1996; Sodickson & Bean, 1996; Nemec et al. 1999). Assuming four bound Gβγ dimers promote the channel open state, receptor-independent basal GIRK activity represents channels transiently occupied by four Gβγ dimers, excluding the potential influence of phosphatidylinositol-4,5-bisphosphate (PIP2), arachidonic acid, and intracellular Na+, Mg+2 and polyamines (Dascal, 1997; Yamada et al. 1998). IK,basal activity is directly related to the concentration of free Gβγ in the cell membrane based on the effectiveness of Gβγ scavengers to lower IK,basal amplitudes (Lim et al. 1995; Vivaudou et al. 1997; Jeong & Ikeda, 1999; Fernandez-Fernandez et al. 2001). According to the GIRK channel gating scheme shown in Fig. 4A, basal GIRK activity reflects channels transiently occupied by three to four Gβγ dimers where random association of a single free Gβγ subunit causes channel opening. Receptor activation of GIRK channels with high free Gβγ concentrations predict a single Gβγ binding event is necessary to activate the channels and IK,ACh will thus follow a single exponential time course. Conversely, receptor activation with low concentrations of free Gβγ predict multiple binding events are necessary for channel opening, producing a sigmoidal IK,ACh time course analogous to the sequential movement of multiple gates during the activation of voltage-gated ion channels (Hodgkin & Huxley, 1952). The observed time course for receptor-dependent GIRK activation at the different levels of basal activity produced by high and low levels of Gαo expression are in agreement with this and similar GIRK gating schemes (Destexhe & Sejnowski, 1995; Hosoya et al. 1996; Ivanova-Nikolova et al. 1998; Yamada et al. 1998). Different levels of GIRK-Gβγ occupancy may also influence the voltage-dependent relaxation phenomena associated with RGS4 expression, which is only observed at low agonist concentrations (Inanobe et al. 2001). Although we deliberately express low levels of m2 receptors to slow GIRK activation rates (Herlitze et al. 1999), it should be emphasized that the GIRK activation and deactivation kinetics measured in the oocyte expression system, even with RGS expression, are consistently slower than native mammalian cells and may be limited by solution exchange rates and/or other intrinsic oocyte factors.
Interestingly, overexpression of a PTX-insensitive and RGS-resistant Gαo mutant (GαoA(G184S:C351G)) in rat sympathetic neurons also reveals a prominent sigmoidal GIRK activation time course via α2-adrenergic receptor activation, and could be dramatically accelerated by coexpression of the N-terminal region of RGS8 (i.e. no RGS domain) (Jeong & Ikeda, 2001). The results of Jeong & Ikeda indicate the RGS8 N-terminal domain facilitates coupling of heterotrimeric GαoA(G184S:C351G)βγ subunits to α2-adrenergic receptors, and as a consequence greatly accelerates the GIRK activation time course. Based on our observations of RGS7 reported here, we speculate that N-terminal regions of RGS7 may similarly interact with m2 receptors and facilitate coupling to Gαo in the absence of Gβ5.
Physiological implications
GIRK channels mediate sIPSPs in the nervous system where the time course and amplitude of inhibitory synaptic events are dependent on the G protein cycle (Luscher et al. 1997). Thus RGS proteins are likely to play an important role in determining the amplitude and temporal properties of GIRK-mediated sIPSPs. Our findings demonstrate GIRK-mediated sIPSPs may be determined by specific GPCR-Gα-RGS signalling complexes that activate postsynaptic GIRK channels, where certain Gα-RGS precoupled complexes accelerate GIRK activation and deactivation kinetics without compromising amplitude. Perturbation of these signalling complexes would reduce inhibitory synaptic transmission and promote cellular excitability. The consequences of these events in the mammalian nervous system remain to be elucidated, yet the demonstrated role of RGS complexes in coordinating locomotion and egg laying behaviour in Caenorhabditis elegans highlight their potential impact on mammalian neural signalling (Chase et al. 2001; Robatzek et al. 2001).
Acknowledgments
We thank the following colleagues for generously providing cDNAs used in this study: Stephen Ikeda, NIAAA/NIH (PTX-insensitive Gαi/o subunits); Henry Lester, Caltech (Kir3.1, Kir3.2a, m2 receptor, RGS4), Vlad Slepak, University of Miami (RGS7 and Gβ 5); and Eitan Reuveny, Weizmann Institute of Science (PTX-S1 subunit). The authors also thank Ms Jenny Gulledge for technical assistance. This work was supported by an Initial Investigator Award (C.D.) and a Women and Minorities Award (M.P.) from the American Heart Association (Florida-Puerto Rico Affiliate), and a Research and Creative Scholarship Award from the University of South Florida (C.D.).
References
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Blake BL, Wing MR, Zhou JY, Lei Q, Hillmann JR, Behe CI, Morris RA, Harden TK, Bayliss DA, Miller RJ, Siderovski DP. Gβ association and effector interaction selectivities of the divergent Gγ subunit Gγ13. Journal of Biological Chemistry. 2001;276:49267–49274. doi: 10.1074/jbc.M106565200. [DOI] [PubMed] [Google Scholar]
- Breitwieser GE, Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. Journal of General Physiology. 1988;91:469–494. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgon PG, Lee WL, Nixon AB, Peralta EG, Casey PJ. Phosphorylation and nuclear translocation of a regulator of G protein signaling (RGS10) Journal of Biological Chemistry. 2001;276:32828–32834. doi: 10.1074/jbc.M100960200. [DOI] [PubMed] [Google Scholar]
- Chase DL, Patikoglou GA, Koelle MR. Two RGS proteins that inhibit Gαo and Gαq signaling in C. elegans neurons require a Gβ5-like subunit for function. Current Biology. 2001;11:222–231. doi: 10.1016/s0960-9822(01)00071-9. [DOI] [PubMed] [Google Scholar]
- Chen C-K, Wieland T, Simon MI. RGS-r, a retinal specific RGS protein, binds an intermediate conformation of transducin and enhances recycling. Proceedings of the National Academy of Sciences of the USA. 1996;93:12885–12889. doi: 10.1073/pnas.93.23.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey S, Clapham DE. The stoichiometry of Gβγ binding to G-protein-regulated inwardly rectifying K+ channels (GIRKs) Journal of Biological Chemistry. 2001;276:11409–11413. doi: 10.1074/jbc.M100058200. [DOI] [PubMed] [Google Scholar]
- Dascal N. Signalling via the G protein-activated K+ channels. Cellular Signaling. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D, Lester HA, Davidson N. Atrial G protein-activated K+ channel: Expression cloning and molecular properties. Proceedings of the National Academy of Sciences of the USA. 1993;90:10235–10239. doi: 10.1073/pnas.90.21.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Sejnowski TJ. G protein activation kinetics and spillover of γ-aminobutyric acid may account for differences between inhibitory responses in the hippocampus and thalamus. Proceedings of the National Academy of Sciences of the USA. 1995;92:9515–9519. doi: 10.1073/pnas.92.21.9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic E, Paquereau L, Rizzoti K, Monier A, Knibiehler B, Audigier Y. The mRNA encoding a β subunit of heterotrimeric GTP-binding proteins is localized to the animal pole of Xenopus laevis oocyte and embryos. Mechanisms of Development. 1996;59:141–151. doi: 10.1016/0925-4773(96)00588-6. [DOI] [PubMed] [Google Scholar]
- De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annual Review of Pharmacology and Toxicology. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- Dohlman HG, Song JP, Ma DR, Courchesne WE, Thorner J. SST2, a negative regulator of pheromone signalling in the yeast Saccharomyces cerevisae - expression, localization, and genetic interaction and physical association with GPA1 (the G-protein α-subunit) Molecular and Cellular Biology. 1996;16:5194–5209. doi: 10.1128/mcb.16.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman HR, Apaniesk D, Chen Y, Song JP, Nusskern D. Inhibition of G-protein signaling by dominant gain-of-function mutations in Sst2p, a pheromone desensitization factor in Saccharomyces cerevisae. Molecular and Cellular Biology. 1995;15:3635–3643. doi: 10.1128/mcb.15.7.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of Gβγ-activated inwardly rectifying K+ channels. Proceedings of the National Academy of Sciences of the USA. 1997;94:10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druey KM, Blumer KJ, Kang VH, Kehrl JH. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996;379:742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez JM, Abogadie FC, Milligan G, Delmas P, Brown DA. Multiple pertussis toxin-sensitive G-proteins can couple receptors to GIRK channels in rat sympathetic neurons when expressed heterologously, but only native Gi-proteins do so in situ. European Journal of Neuroscience. 2001;14:283–292. doi: 10.1046/j.0953-816x.2001.01642.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez JM, Wanaverbecq N, Halley P, Caulfield MP, Brown DA. Selective activation of heterologously expressed G protein-gated K+ channels by M2 muscarinic receptors in rat sympathetic neurones. Journal of Physiology. 1999;515:631–637. doi: 10.1111/j.1469-7793.1999.631ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Zhai Y, Zhu Z, Bannon MJ, Burchett SA, Schmidt CJ, Andrade R, Cooper J. Molecular characterization of human and rat RGS 9L, a novel splice variant enriched in dopamine target regions, and chromosomal localization of the RGS 9 gene. Molecular Pharmacology. 1998;54:687–694. [PubMed] [Google Scholar]
- Greif GJ, Sodickson DL, Bean BP, Neer EJ, Mende U. Altered regulation of potassium and calcium channels by GABAB and adenosine receptors in hippocampal neurons from mice lacking Gαo. Journal of Neurophysiology. 2000;83:1010–1018. doi: 10.1152/jn.2000.83.2.1010. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Grandy D, Lester HA, Davidson N, Lim NF, Chavkin C. κ opioid receptors couple to inwardly rectifying potassium channels when coexpressed in Xenopus oocytes. Molecular Pharmacology. 1995;47:551–557. [PubMed] [Google Scholar]
- Herlitze S, Ruppersberg JP, Mark MD. New roles for RGS2, 5 and 8 on the ratio-dependent modulation of recombinant GIRK channels expressed in Xenopus oocytes. Journal of Physiology. 1999;517:341–352. doi: 10.1111/j.1469-7793.1999.0341t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya Y, Yamada M, Ito H, Kurachi Y. A functional model for G protein activation of the muscarinic K+ channel in guinea pig atrial myocytes. Spectral analysis of the effect of GTP on single-channel kinetics. Journal of General Physiology. 1996;108:485–495. doi: 10.1085/jgp.108.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-L, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- Hunt TW, Fields TA, Casey PJ, Peralta EG. RGS10 is a selective activator of Gαi GTPase activity. Nature. 1996;383:175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Fujita S, Makino Y, Matsushita K, Ishii M, Chachin M, Kurachi Y. Interaction between the RGS domain of RGS4 with G protein α subunits mediates the voltage-dependent relaxation of the G protein-gated potassium channel. Journal of Physiology. 2001;535:133–143. doi: 10.1111/j.1469-7793.2001.t01-1-00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata N, Ishihara T, Akaike N. Activation kinetics of the acetylcholine-gated potassium current in isolated atrial cells. American Journal of Physiology. 1989;257:C646–650. doi: 10.1152/ajpcell.1989.257.4.C646. [DOI] [PubMed] [Google Scholar]
- Ivanova-Nikolova TT, Nikolov EN, Hansen C, Robishaw JD. Muscarinic K+ channel in the heart. Modal regulation by G protein βγ subunits. Journal of General Physiology. 1998;112:199–210. doi: 10.1085/jgp.112.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR. Sequestration of G-protein βγ subunits by different G-protein α subunits blocks voltage-dependent modulation of Ca2+ channels in rat sympathetic neurons. Journal of Neuroscience. 1999;19:4755–4761. doi: 10.1523/JNEUROSCI.19-12-04755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR. Differential regulation of G protein-gated inwardly rectifying K+ channel kinetics by distinct domains of RGS8. Journal of Physiology. 2001;535:335–347. doi: 10.1111/j.1469-7793.2001.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Raifman T, Bera AK, Zveig D, Peleg S, Witherow DS, Slepak VZ, Dascal N. Expression levels of RGS7 and RGS4 proteins determine the mode of regulation of the G protein-activated K+ channel and control regulation of RGS7 by Gβ5. FEBS Letters. 2001;492:20–28. doi: 10.1016/s0014-5793(01)02220-7. [DOI] [PubMed] [Google Scholar]
- Koelle MR, Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with mammalian proteins. Cell. 1996;84:115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Chen CK, He W, Wensel TG, Simon MI, Lester HA. Co-expression of Gβ5 enhances the function of two Gγ subunit-like domain-containing regulators of G protein signaling proteins. Journal of Biological Chemistry. 2000;275:3397–3402. doi: 10.1074/jbc.275.5.3397. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Lester HA. Gi Irks GIRKs. Neuron. 2002;33:6–8. doi: 10.1016/s0896-6273(01)00572-4. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Wickman K, Clapham DE. Gβγ binds directly to the G protein-gated K+ channel, IKACh. Journal of Biological Chemistry. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- Lan KL, Zhong H, Nanamori M, Neubig RR. Rapid kinetics of Regulator of G-protein Signaling (RGS)-mediated Gαi and Gαo deactivation: Gα specificity of RGS4 and RGS7. Journal of Biological Chemistry. 2000;275:33497–33503. doi: 10.1074/jbc.M005785200. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Milligan G, Tinker A. The G protein α subunit has a key role in determining the specificity of coupling to, but not the activation of, G protein-gated inwardly rectifying K+ channels. Journal of Biological Chemistry. 2000;275:921–929. doi: 10.1074/jbc.275.2.921. [DOI] [PubMed] [Google Scholar]
- Leaney JL, Tinker A. The role of members of the pertussis toxin-sensitive family of G proteins in coupling receptors to the activation of the G protein-gated inwardly rectifying potassium channel. Proceedings of the National Academy of Sciences of the USA. 2000;97:5651–5656. doi: 10.1073/pnas.080572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein βγ subunits. Proceedings of the National Academy of Sciences of the USA. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levay K, Cabrera JL, Satpaev DK, Slepak VZ. Gβ5 prevents the RGS7-Gαo interaction through binding to a distinct Gγ-like domain found in RGS7 and other RGS proteins. Proceedings of the National Academy of Sciences of the USA. 1999;96:2503–2507. doi: 10.1073/pnas.96.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mende U, Lewis C, Neer EJ. Maintenance of cellular levels of G-proteins: different efficiencies of αs and αo synthesis in GH3 cells. Biochemistry Journal. 1996;318:1071–1077. doi: 10.1042/bj3181071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim NF, Dascal N, Labarca C, Davidson N, Lester HA. A G protein-gated K channel is activated via β2-adrenergic receptors and Gβγ subunits in Xenopus oocytes. Journal of General Physiology. 1995;105:421–439. doi: 10.1085/jgp.105.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Homburger V, Bockaert J, Vincent JD. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cells. Neuron. 1992;8:455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Golper J, Neer EJ, Clapham D. The βγ-subunit of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. European Journal of Biochemistry. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- Nemec J, Wickman K, Clapham DE. Gβγ binding increases the open time of IKACh: kinetic evidence for multiple Gβγ binding sites. Biophysical Journal. 1999;76:246–252. doi: 10.1016/S0006-3495(99)77193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner BA, Gilman AG, Harris BA. Regulators of G protein signaling 6 and 7. Purification of complexes with Gβ5 and assessment of their effects on G protein-mediated signaling pathways. Journal of Biological Chemistry. 1999;274:31087–31093. doi: 10.1074/jbc.274.43.31087. [DOI] [PubMed] [Google Scholar]
- Robatzek M, Niacaris T, Steger K, Avery L, Thomas JH. Eat-11 encodes GPB-2, a Gβ5 ortholog that interacts with Goα and Gqα to regulate C. elegans behavior. Current Biology. 2001;11:288–293. doi: 10.1016/s0960-9822(01)00074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JJ, Taylor JB, Shi J, Cockett MI, Jones PG, Hepler JR. RGS7 is palmitoylated and exists as biochemically distinct forms. Journal of Neurochemistry. 2000;75:2103–2112. doi: 10.1046/j.1471-4159.2000.0752103.x. [DOI] [PubMed] [Google Scholar]
- Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G Protein Signaling (RGS) and RGS-like proteins. Annual Review of Biochemistry. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Miyatani Y, Asano T, Nakata H. RGS8 accelerates G-protein-mediated modulation of K+ currents. Nature. 1997;390:525–529. doi: 10.1038/37385. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Kubo Y, Odagiri M, Ichikawa M, Yamagata K, Sekine T. RGS7 and RGS8 differentially accelerate G protein-mediated modulation of K+ currents. Journal of Biological Chemistry. 1999;274:9899–9904. doi: 10.1074/jbc.274.14.9899. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Odagiri M, Masuho I, Nomoto S, Kinoshita N. Molecular cloning and characterization of Xenopus RGS5. Biochemical and Biophysical Research Communications. 2000;270:34–39. doi: 10.1006/bbrc.2000.2379. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N. Inhibition of an inwardly rectifying K+ channel by G-protein α-subunits. Nature. 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- Shea L, Linderman JJ. Mechanistic model of G-protein signal transduction. Determinants of efficacy and effect of precoupled receptors. Biochemical Pharmacology. 1997;53:519–530. doi: 10.1016/s0006-2952(96)00768-x. [DOI] [PubMed] [Google Scholar]
- Shea LD, Neubig RR, Linderman JJ. Timing is everything: the role of kinetics in G protein activation. Life Sciences. 2000;68:647–658. doi: 10.1016/s0024-3205(00)00977-2. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Reuveny E, Jan YN, Jan LY. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995;15:1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- Snow BE, Krumins AM, Brothers GM, Lee SF, Wall MA, Chung S, Mangion J, Arya S, Gilman AG, Siderovski DP. A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proceedings of the National Academy of Sciences of the USA. 1998;95:13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. Journal of Neuroscience. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondek J, Siderovski DP. Gγ-like (GGL) domains: new frontiers in G-protein signaling and β-propeller scaffolding. Biochemical Pharmacology. 2001;61:1329–1337. doi: 10.1016/s0006-2952(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Sowell MO, Ye C, Ricupero DA, Hansen S, Quinn SJ, Vassilev PM, Mortensen RM. Targeted inactivation of αi2 or αi3 disrupts activation of the cardiac muscarinic K+ channel, IKACh, in intact cells. Proceedings of the National Academy of Sciences of the USA. 1997;94:7921–7926. doi: 10.1073/pnas.94.15.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhmer W, Parekh AB. Electrophysiological recordings from Xenopus oocytes. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 341–356. [Google Scholar]
- Takano K, Yasufuku-Takano J, Kozasa T, Nakajima S, Nakajima Y. Different G proteins mediate somatostatin-induced inward rectifier K+ currents in murine brain and endocrine cells. Journal of Physiology. 1997;502:559–567. doi: 10.1111/j.1469-7793.1997.559bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer EJ, Fishman MC. Gαo is necessary for muscarinic regulation of Ca2+ channels in mouse heart. Proceedings of the National Academy of Sciences of the USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, Logothetis DE. Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the KACh channel, using functional homomeric mutants. Journal of Biological Chemistry. 1997;272:31553–31560. doi: 10.1074/jbc.272.50.31553. [DOI] [PubMed] [Google Scholar]
- Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein α-subunits. Nature. 1996;383:172–177. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- Wickman KD, Inlguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Wise A, Watson-Koken MA, Rees S, Lee M, Milligan G. Interactions of the α2A-adrenoceptor with multiple Gi-family G-proteins: studies with pertussis toxin-resistant G-protein mutants. Biochemistry Journal. 1997;321:721–728. doi: 10.1042/bj3210721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacological Reviews. 1998;50:723–760. [PubMed] [Google Scholar]
- Yu JH, Wieser J, Adams TH. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO Journal. 1996;15:5184–5190. [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Jan LY. G-protein signaling: fine-tuning signaling kinetics. Current Biology. 1998;8:R313–316. doi: 10.1016/s0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]


