Abstract
Rheumatoid arthritis (RA) is a common autoimmune disease that afflicts the synovium of diarthrodial joints. The pathogenic mechanisms inciting this disease are not fully characterized, but may involve the loss of tolerance to posttranslationally modified (citrullinated) antigens. We have demonstrated that this modification leads to a selective increase in antigenic peptide affinity for major histocompatibility complex (MHC) class II molecules that carry the RA-associated shared epitope, such as HLA-DRB1*0401 (DR4). We describe the induction of arthritis in DR4-IE transgenic (tg) mice with citrullinated fibrinogen, a protein commonly found in inflamed synovial tissue and a frequent target of autoantibodies in RA patients. The disease induced in these mice was characterized by synovial hyperplasia followed by ankylosis, but lacked a conspicuous polymorphonuclear cell infiltrate. Immunological analysis of these mice through T cell epitope scanning and antibody microarray analysis identified a unique profile of citrulline-specific reactivity that was not found in DR4-IE tg mice immunized with unmodified fibrinogen or in wild-type C57BL/6 mice immunized with citrullinated fibrinogen, two conditions where arthritis was not observed. These observations directly implicate citrullinated fibrinogen as arthritogenic in the context of RA-associated MHC class II molecules.
Rheumatoid arthritis (RA) is a chronic disease affecting the peripheral joints in which abnormalities in the synovium precipitate a destructive process that often leads to cartilage and bone erosion. The autoimmune nature of this disease has been defined, in part, through the presence of IgG autoantibodies such as rheumatoid factor and a tight genetic association with MHC class II molecules that contain a motif known as the shared epitope (SE) (1, 2). This SE forms one of the major MHC class II anchoring pockets (known as P4) and imparts the ability to preferentially interact with certain amino acid side chains from antigenic peptides for subsequent presentation to CD4 T cells (3). Because of these properties, the adaptive arm of the immune system has been implicated in driving disease pathogenesis through autoantigen recognition.
Although many candidate autoantigens have been investigated in RA, a frequent target of the immune response found predominantly in this patient population has been lacking until recently. The discovery of serum IgG autoantibodies from RA patients that bind posttranslationally modified arginine (citrulline) within the context of certain proteins/peptides has provided an excellent diagnostic tool due in large part to their disease specificity (4–7). The propensity to develop anti-citrulline antibodies is also associated with the expression of the SE, suggesting that an MHC class II–restricted mechanism may initiate this immune response (8–10). We have shown that the conversion of arginine to citrulline at the peptide side chain position that interacts with the P4 pocket formed by the SE leads to a profound increase in MHC–peptide affinity and to the subsequent activation of CD4 T cells (11). This phenomenon is caused by the different charge interactions made between the MHC class II P4 pocket (positively charged because of arginine or lysine at position 71 of the β chain) and either peptide-bound arginine (positively charged because of the terminal amino group) or citrulline (polar and uncharged because of the terminal carbonyl group), where the latter interaction is preferred. These observations suggest that MHC class II–restricted CD4 T cells may propagate the autoimmune response to citrullinated self-antigens found in RA patients.
Although the substrate of anti-citrulline antibodies was initially identified as citrullinated filaggrin (a protein that is found in the cornified layer of the skin, but not the joint), further investigation determined that citrullinated fibrinogen is a synovial-derived target (12). Because the expression of peptidylarginine deiminase, the enzyme responsible for converting protein-bound arginine to citrulline, has been found to colocalize with fibrin deposits and other intracellular citrullinated proteins (possibly vimentin) within RA synovial tissue (13–15), it is likely that these autoantigens can be generated in the rheumatoid lesion. This, in addition to fact that autoantibodies that bind citrullinated fibrinogen are frequently and specifically found in RA patients, implicate this autoantigen in disease etiology (16–18).
We provide evidence that citrullinated fibrinogen is arthritogenic in mice made tg for the RA-associated MHC class II molecule DRB1*0401 (DR4-IE tg mice). Immunization of DR4-IE tg mice with citrullinated, but not unmodified, human fibrinogen (hFib) induced a progressive arthritic condition characterized by synovial fibroblast-like cell hyperplasia and the transient appearance of citrullinated proteins in the joints, but lacked significant inflammatory cell infiltration. Notably, wild-type C57BL/6 (B6) mice expressing murine H-2b were not susceptible to this disease, potentially owing to the fact that distinct differences in the immune response were found to be mediated by the HLA transgene. Although these results implicate citrullinated fibrinogen as an arthritogenic antigen in the context of the RA-associated MHC class II molecule DRB1*0401, they also suggest that this HLA-restricted immune response may provoke arthritis in the absence of a robust and persistent polymorphonuclear cell infiltrate.
RESULTS
Induction of arthritis in DR4-IE tg mice
To determine the MHC class II–restricted arthritogenicity of citrullinated antigens, we chose to explore the immune response to citrullinated fibrinogen, a protein that is found in the diseased synovium of RA patients and is a target of autoantibodies in this disease (12, 14–18). DR4-IE tg mice were immunized with either citrullinated hFib (CithFib) or unmodified hFib and assessed for clinical signs of arthritis. Although DR4-IE tg mice immunized with hFib did not develop arthritis during an extended observation period (200 d), immunization with CithFib induced arthritis in ∼35% of tg mice. Joint swelling typically ensued 10 wk after primary immunization (Fig. 1 and Fig. 2 A) and was almost completely restricted to the ankles (one arthritic mouse also developed dactylitis in one forepaw). The disease had a progressive and persistent course, beginning with mild swelling followed by severe erythema, eventually leading to ankylosis. Clinically evident swelling lasted for up to 6 wk, and restricted plantarflexion persisted in arthritic mice that were observed for an extended period (200 d).
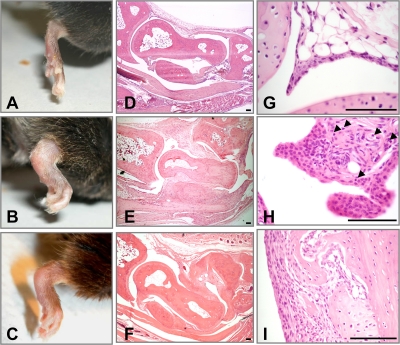
Figure 1.
Clinical and histological evaluation of arthritis induced by CithFib in DR4-IE tg mice. (A) Normal ankle joint of a DR4-IE tg mouse immunized with hFib (day 70). (B) Maximal swelling in an arthritic DR4-IE tg mouse immunized with CithFib (day 70). (C) Progression of arthritis showing reduced erythema in an arthritic DR4-IE tg mouse immunized with CithFib (day 150). Corresponding HE-stained histological sections of tibiotalar joints from nonarthritic and arthritic DR4-IE tg mice (D–F from mice shown in A–C, respectively). (D) Normal histological appearance of synovial membrane and cartilage surfaces. (E) Hyperplastic synovium showing some areas of cartilage damage, but lacking conspicuous inflammatory cell infiltration. (F) Ankylosed joint showing fibrotic changes in the tissues spanning the talus and navicular bones. (G) High-powered magnification of normal synovial lining from a nonarthritic DR4-IE tg mouse immunized with hFib (day 70). (H) Synovial hyperplasia of the lining layer with subtle lymphocyte infiltration (arrows). This is representative of the earliest histological abnormality seen in arthritic DR4-IE tg mice immunized with CithFib. (I) Pannus tissue in an arthritic DR4-IE tg mouse highlighting areas of cartilage degradation. Bar, 100 μm.
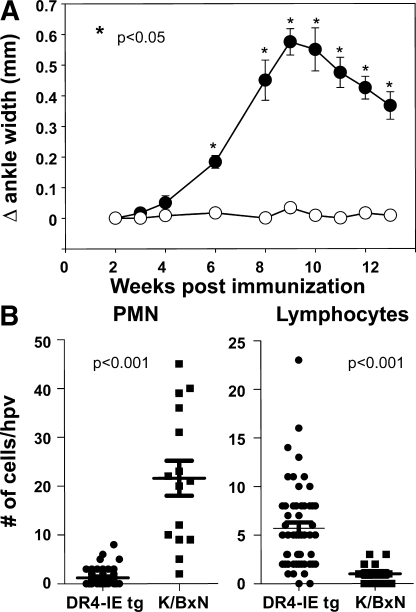
Figure 2.
Joint swelling and histological analysis of inflammatory infiltrate in arthritic DR4-IE tg mice. (A) Caliper measurements of DR4-IE tg mice immunized with CithFib were assessed every 7 d over 13 wk. Data are presented as mean Δ ankle width (± the SEM), which was calculated by caliper measurements at the indicated time point, minus the preimmunization ankle width, from 12 individual arthritic mice (filled circles) and 12 nonarthritic mice (open circles). P values were calculated by Student's t test. (B) High-powered view (hpv) histological analysis of inflammatory infiltrate in the joints of arthritic DR4-IE tg mice (day 70 postimmunization) and K/BxN mice (8 wk old). Lymphocyte and polymorphonuclear cell content was determined by nuclear morphology in HE-stained sections from hyperplastic synovial tissue. Points represent the number of cells identified in each high power view (63x objective), with 54 individual views from 5 arthritic DR4-IE tg mice and 15 views from 2 arthritic K/BxN mice. P values were obtained by Mann-Whitney test.
To determine whether this arthritis was restricted by expression of the human DR4 transgene, wild-type C57BL/6 (B6) mice were also immunized with these antigens and observed for arthritis. Clinical signs of disease did not develop in B6 mice after immunization with these proteins. Arthritis did not develop in either strain after immunization with citrullinated KLH, citrullinated mFib (CitmFib), or unmodified mFib (Table I). The development of arthritis was therefore dependent on the posttranslational modification (citrullination) of hFib and the expression of the RA-associated DRB1*0401 MHC class II molecule.
Table I.
Summary of immunizations and incidence of arthritis in DR4-IE tg and B6 mice
| Mouse strain | Immunizing antigen |
Mice immunized | Arthritic mice |
|---|---|---|---|
| % | |||
| DR4-IE tg | CithFib | 135 | 48 (35.5) |
| DR4-IE tg | hFib | 24 | 0 |
| DR4-IE tg | CitmFib | 14 | 0 |
| DR4-IE tg | mFib | 14 | 0 |
| DR4-IE tg | CitKLH | 8 | 0 |
| B6 | CithFib | 14 | 0 |
| B6 | hFib | 14 | 0 |
| B6 | CitmFib | 8 | 0 |
| B6 | mFib | 8 | 0 |
Immunizations were conducted as outlined in Materials and methods. All mice were observed for at least 70 d after primary immunization, with the maximum observation period for some groups being 200 d.
Histological and immunohistochemical assessment of joint pathology
Histological analysis of tissues from arthritic mice revealed prominent synovial hyperplasia, with some pannus formation at bone and cartilage interfaces (Fig. 1 and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1). Surprisingly, although synovial sublining inflammatory cells were evident in arthritic DR4-IE tg mice, their presence was less conspicuous compared with other disease models, such as streptococcal cell wall–induced arthritis (unpublished data) (19) or the arthritis seen in K/BxN tg mice (20). To accurately quantitate the degree and type of infiltrate in the synovial tissue, we compared arthritic DR4-IE tg mice with the well-characterized K/BxN model. Contrasting cellular content by high-power view quantification revealed a stark difference in the degree of polymorphonuclear cell infiltration that dominated the infiltrate in K/BxN mice, whereas arthritic DR4-IE tg mice showed a small but significant increase in lymphocytes (Fig. 2 B).
Histological abnormalities persisted in the ankle joints of arthritic DR4-IE tg mice assessed at later stages of disease (>150 d after primary immunization; Fig. 1 F) and showed fibrotic synovial tissue in joint spaces, which is consistent with the clinical appearance of ankylosis. Other histological abnormalities were rarely seen outside of the ankle joint of arthritic mice, as the spine and major organs appeared normal (unpublished data).
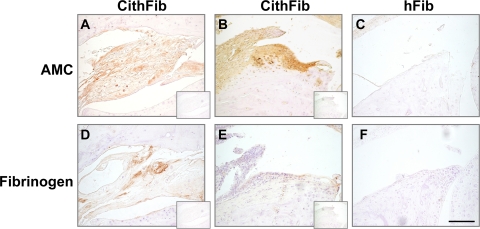
We next addressed whether citrullinated protein was evident in the joints of arthritic mice by immunohistochemistry using an anti-modified citrulline (AMC) antibody (Fig. 3, A–C). The most intense staining was found intracellularly in synovial fibroblast-like cells from hyperplastic tissue of mice with arthritis of recent onset. Some chondrocytes in the superficial zone of articular surfaces were also found to stain with AMC; however, no citrullinated protein was identified at sites of cartilage or bone erosion or in ankylosed joints (unpublished data). Detection of fibrin in serial sections (Fig. 3, D–F) showed mainly perivascular deposition in synovial tissues, but minimal colocalization with citrullinated protein, suggesting that other intracellular citrullinated proteins (possibly citrullinated vimentin) could be present in the joints of these arthritic mice.
Figure 3.
Immunohistochemical localization of citrullinated proteins and fibrinogen deposition in arthritic DR4-IE tg mice. Citrullinated protein was identified in arthritic mice 70 d after immunization with CithFib by staining with AMC antibodies in areas of synovial hyperplasia (A), with the most intense staining identifying intracellular proteins in synovial fibroblast cells (B). (C) Nonarthritic mice immunized with hFib did not stain positive for AMC in these regions. Although fibrin deposition was evident in perivascular regions of synovial tissue in arthritic mice (D), colocalization with citrullinated protein was minimal and virtually absent in areas of intense AMC staining (E). (F) No fibrin deposition was detected in joints of nonarthritic mice immunized with hFib. Control staining without the primary antibody for each section is shown in the insets. Bar, 100 μm.
Assessment of T cell responses
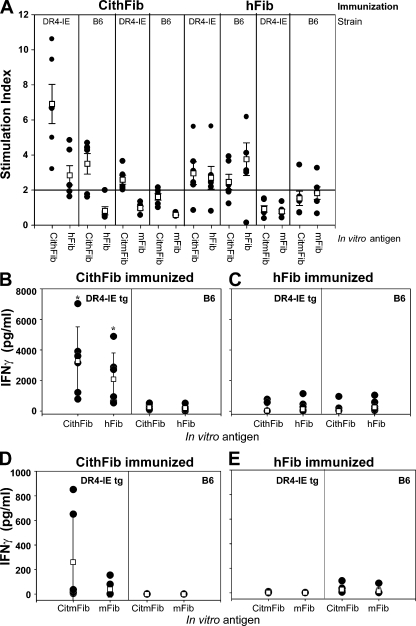
Splenic T cell responses to various forms of fibrinogen were assessed by proliferation and cytokine production in both DR4-IE tg and B6 mice 70 d after primary immunization. Antigen-specific proliferation in response to CithFib in DR4-IE tg mice immunized with this antigen was prominent and consistently higher than the response to hFib in individual mice (Fig. 4 A). B6 mice also showed citrulline-specific reactivity in this context; however, proliferation was approximately twofold lower than that seen in DR4-IE tg mice. Recall proliferative responses were detected in both strains after immunization with hFib, but in this circumstance, augmented reactivity to CithFib was not detected.
Figure 4.
Antigen-specific recall proliferation and cytokine production in DR4-IE tg and B6 mice immunized with CithFib or hFib (day 70). (A)Splenocytes were cultured in the presence of 50 μg/ml of the indicated in vitro antigens and proliferation (shown as stimulation index) was determined by [3H]thymidine incorporation. (B–E) Supernatant from these cultures were removed at 72 h and tested for the presence of IFN-γ by ELISA. Individual (filled circles) and mean (open boxes) responses ± the SEM (proliferation) or the SD (IFN-γ) are shown (n = 6 mice/strain/immunizing antigen). * indicates significant difference in cytokine production between DR4-IE tg and B6 mice. P < 0.05, paired Student's t test.
Clear differences in cytokine production were also evident, and paralleled responses identified by proliferation. After immunization with CithFib, DR4-IE tg mice produced high levels of IFN-γ after in vitro challenge with CithFib; again, much of this response appeared to be citrulline specific (Fig. 4 B). These levels of IFN-γ production were also increased ∼10-fold compared with B6 mice immunized with the same antigen or with either strain after immunization with hFib (Fig. 4 C). A similar trend in cytokine production was also observed for IL-10 (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1), suggesting that a highly polarized Th1 response occurred (21, 22). IL-4 production was not detectable in these cultures (unpublished data).
We also tested for T cell reactivity to the endogenous antigens CitmFib and mFib and found that only DR4-IE tg mice that were immunized with CithFib produced an immune response to CitmFib (Fig. 4, A, D, and E). Of particular note, although T cell reactivity to unmodified hFib was frequently detected in DR4-IE tg mice, no responses to unmodified mFib occurred. These results show that in contrast to murine H-2b, the HLA-DRB1 transgene can generate a strong citrulline-specific T cell response characterized by the production of high levels of IFN-γ and IL-10 and mediates cross-reactivity to CitmFib.
To understand the pathogenic potential of this citrulline-specific T cell response in DR4-IE tg mice, we established a cell transfer system. Here, we immunized and boosted DR4-IE tg mice with CithFib, harvested splenocytes at day 31, and activated them in vitro with CithFib for an additional 4 d. At the end of this culture, viable cells were isolated (of which >85% were CD4+ by FACS) and transferred IP to DR4-IE tg recipients that received an intraarticular injection of CithFib, hFib, CitmFib, mFib, or BSA (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1). In this transfer system, only recipients that received an intraarticular injection of CithFib developed arthritis, suggesting that CithFib-specific T cells can drive disease, but only when the cognate antigen is found within the joint.
Identification of DR4-restricted T cell epitopes
Using a predictive model for identifying DR4-binding epitopes (23), we selected all peptides within the α, β, and γ chain of hFib that would contain the required P1 anchor (aliphatic or aromatic amino acid), an arginine or citrulline at the P4 SE position, and a noninhibitory amino acid at the P6 anchor. This resulted in the identification of 10 peptides, 7 originating from the α chain, 2 from the β chain, and 1 from the γ chain of fibrinogen. To monitor the immune response to heterologous regions of hFib, we also synthesized a DR4-binding peptide (Fibα371-383) that is found exclusively in the α chain of hFib and lacks arginine (Fig. 5 C). These peptides were used to monitor T cell recall responses in the two strains of mice at day 70 after immunization with CithFib or hFib.
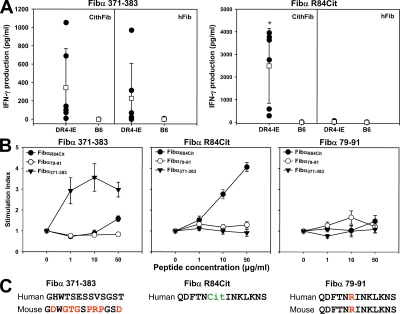
Figure 5.
T cell responses to fibrinogen derived peptides in DR4-IE tg and B6 mice. (A) Splenocytes from DR4-IE tg and B6 mice immunized with CithFib or hFib (day 70) were cultured in the presence of 50 μg/ml of either Fibα 371–383 (left) or Fibα R84Cit (right). Supernatants from these cultures were removed at 72 h and tested for the production of IFN-γ by ELISA. Individual (filled circles) and mean (open boxes) responses ± the SD are shown (n = 6 mice/strain/immunizing antigen). Labels above boxes indicate the antigen used for in vitro challenge, and labels within boxes indicate the immunizing antigen. * indicates significant difference in cytokine production between DR4-IE tg immunized with CithFib and hFib (P < 0.05, paired Student's t test). (B) Confirmation of peptide immunogenicity by antigen-specific T cell proliferation. DR4-IE tg mice were immunized with either Fibα 371–383 (left), Fibα R84Cit (middle), or Fibα 79–91 (right); 10 d later, draining lymph node cells were cultured with various concentrations of peptide (Fibα R84Cit, closed circle; Fibα 79–91, open circle; Fibα 371–383, inverted closed triangle). Data represent the mean response ± the SEM of six mice per immunizing antigen. (C) Peptides corresponding to human α chain sequences used in A and B for determining T cell recall responses. Amino acids differing between mouse Fibα 371–383 are indicated in red. P4 amino acid from Fibα R84Cit and Fibα 79–81 predicted to be positioned at the SE are indicated in green and red, respectively, with the corresponding sequence from the α chain of mFib.
As expected, HLA-DRB1*0401–restricted T cell reactivity to Fibα371-383 (measured by proliferation and IFN-γ production) was evident in DR4-IE tg mice after immunization with either CithFib or hFib, whereas no recall response to this peptide was seen in B6 mice (Fig. 5 A). Of the 10 fibrinogen peptides containing citrulline at the P4 position, only one peptide from the α chain (FibαR84Cit) consistently induced strong IFN-γ production in DR4-IE tg mice immunized with CithFib, whereas no response was seen against the corresponding arginine-containing peptide Fibα79-91 (Fig. 5 A and Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1). Notably, this particular sequence is identical in both the human and mFib α chain (Fig. 5 C). T cell reactivity to FibαR84Cit was absent in DR4-IE tg mice immunized with hFib and in B6 mice immunized with either antigen. The HLA-restricted response to the α chain peptide in CithFib immunized DR4-IE tg mice was further confirmed by incubating splenocytes in the presence of anti-DR antibody and FibαR84Cit, which completely inhibited proliferation (Fig. S4).
The immunogenicity of the peptides Fibα371-383, FibαR84Cit, and Fibα79-91 was also assessed by immunizing DR4-IE tg mice with each peptide and characterizing the recall proliferative response to these antigens. Both Fibα371-383 and FibαR84Cit induced T cell proliferation after in vitro challenge with the cognate peptide, whereas Fibα79-91 did not (Fig. 5 B). Although T cell reactivity to Fibα371-383 and FibαR84Cit was evident in DR4-IE tg mice after peptide immunization, neither peptide induced arthritis (observed until day 70). No response was seen in B6 mice. These data clearly confirm the DR4-restricted T cell response to heterologous regions of hFib and identify a citrulline-specific epitope restricted by this human MHC class II molecule.
Assessment of antibody responses
To determine if anti-citrulline antibodies were produced in the two strains of mice, we tested for serum IgG reactivity to citrullinated and unmodified forms of mFib by ELISA. These antigens were chosen because initial studies using hFib and CithFib showed extremely high titers (>1:64,000) with no discernable difference in antibody reactivity between either antigen.
In DR4-IE tg mice immunized with CithFib, a significant increase in IgG antibody reactivity to CitmFib was seen (Fig. 6 A). Weaker IgG reactivity to mFib was also evident, but was not significantly different from that seen in DR4-IE tg or B6 mice immunized with hFib. Although antibody reactivity to CitmFib was significantly higher in DR4-IE tg vs. B6 mice after immunization with CithFib, a weak but clearly evident citrulline-specific response could be detected in mice that lacked the SE sequence (confirmed by inhibition; Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1).
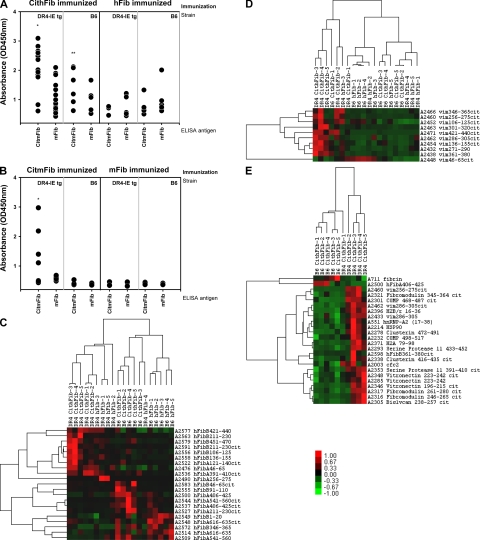
Figure 6.
IgG antibody responses detected by ELISA and synovial proteome microarray. (A) IgG reactivity to either CitmFib or mFib in DR4-IE tg or B6 mice 70 d after immunization with either CithFib or hFib. Mean OD of duplicates for individual serum are shown (n = 12 for DR4-IE tg mice immunized with CithFib and n = 6 for all other groups). (B) Total IgG reactivity to either CitmFib or mFib in DR4-IE tg or B6 mice 100 d after immunization with either CitmFib or mFib (n = 7 for DR4-IE tg mice immunized with CitmFib and n = 5 for all other groups). * indicates significant difference in antibody reactivity (P < 0.05, paired Student's t test) between all groups, whereas ** indicates significant difference in antibody reactivity to CitmFib in B6 mice immunized with CithFib versus reactivity to CitmFib in B6 or DR4-IE tg mice immunized with hFib (P < 0.05, paired Student's t test). Analysis of serum IgG reactivity to fibrinogen- (C) or vimentin-derived (D) peptides identified by SAM and grouped by Cluster based on relationships. (E) Pairwise analysis of significantly different IgG antibody reactivity between DR4-IE tg and B6 mice immunized with CithFib (P < 0.05, Student's t test). Antigens shown were stratified based on having greater than fourfold increase in reactivity compared with immunization with hFib. Values shown are median subtracted and row normalized with relative expression shown in relation to scale bar. Raw data and peptide sequences can be found in Table S1–S3, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1.
Because T cells reactive with epitopes unique to hFib (and thus foreign) could provide help to citrulline-specific B cells in B6 mice, we also tested IgG antibody reactivity in both strains after immunization (day 100) with citrullinated or unmodified autologous mFib. Under these conditions, the production of citrulline-specific IgG was clearly confined to mice that carried the SE (Fig. 6 B). In fact, the antibody reactivity was similar to that seen in human RA patients, as significant reactivity to self- (autologous) unmodified fibrinogen was not observed (18).
IgG profiling using synovial proteome microarray
To gain further insight into IgG specificity, we assessed serum reactivity to hundreds of unique protein and peptide antigens implicated in RA pathogenesis using antibody microarray. These arrays contained overlapping peptides spanning the full length of the α and β chains of fibrinogen in both citrullinated and unmodified forms, in addition to peptides from other candidate citrullinated autoantigens, notably vimentin. Serum samples from DR4-IE tg and B6 mice immunized with CithFib or hFib (day 70) were used to identify patterns of epitope recognition within and between strains.
We initially looked for differential reactivity to fibrinogen-derived antigens contained on the array and found that the HLA transgene, indeed, skewed the antibody response to multiple epitopes (Fig. 6 C and Table S1, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1). This is highlighted by the fact that both strains responded to unique clusters of peptides after immunization with either form of antigen. The microarray platform also confirmed the increased reactivity to CitmFib that was seen in DR4-IE tg mice by ELISA. In general, a moderate degree of variability was seen in the IgG response to any particular fibrinogen-derived antigen from individual mice. As a result, the only significantly different IgG antibody response between B6 and DR4-IE tg mice immunized with CithFib was the targeting of the hFib α chain peptide 406–425 by B6 mice (Fig. 6 E and Table S2).
We next addressed IgG reactivity to vimentin in these sera because autoantibodies to this citrullinated antigen (also known as the Sa antigen) are a specific marker of disease in RA patients (8, 15, 24). Differential responses were noted for multiple citrullinated vimentin peptides, with increased antibody reactivity clearly biased in favor of DR4-IE tg mice after immunization with CithFib (Fig. 6 D and Table S3, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1). Vimentin-derived targets that were significantly different between DR4-IE tg and B6 mice immunized with CithFib were vim286-305cit and vim256-275cit. Additional analysis through peptide-based ELISA assay with vim256-275cit confirmed the specific HLA-restricted antibody reactivity seen by microarray (unpublished data).
Finally, pairwise comparison between DR4-IE tg and B6 mice immunized with CithFib was conducted to identify all significant differences in antibody reactivity. Several epitopes outside of vimentin and fibrinogen were highlighted (Fig. 6 E). Of the epitopes specifically targeted in DR4-IE tg mice, >50% were citrullinated, whereas the two targets of antibodies in B6 mice were derived from unmodified fibrinogen.
These results provide evidence that the HLA transgene mediates a distinct skewing in anti-citrulline antibody reactivity, and suggests that immunization with CithFib can perpetuate a wide range of IgG target recognition, which is indicative of broad cross-reactivity or epitope spreading.
Xenoreactive antibody responses are evident to CithFib
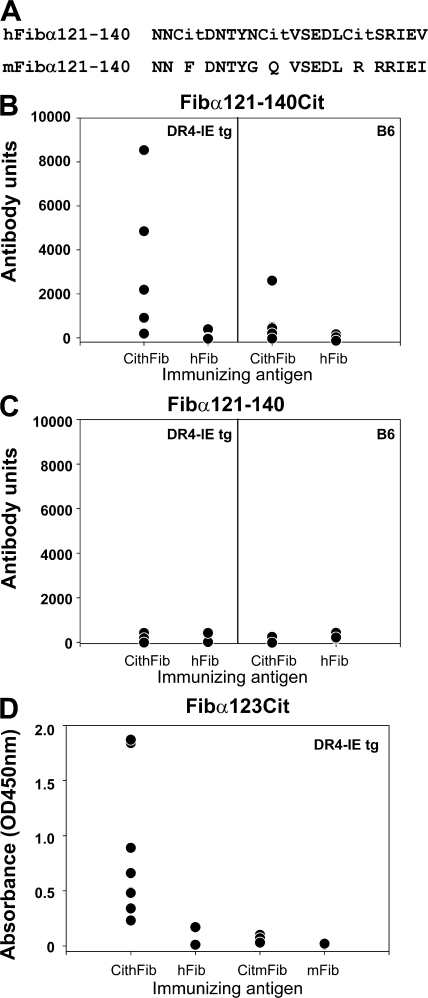
Because arthritis was found after immunization with citrullinated human, but not mouse, fibrinogen in DR4-IE tg mice, we further characterized aspects of the xenoreactive immune response that may be important in this process by using antibody microarray data in combination with mass spectrometry sequencing of citrullinated fibrinogen that was previously described (25). We first aligned the human and mFib sequences to identify heterologous regions where arginine substitutions occur. In total, there were 16 arginine residues from the α chain and 4 from the β chain of hFib that were not present in mouse. Many of these arginines were shown to be citrullinated in vitro by PAD2 or 4 (Table S4, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1, and unpublished data) (25). We next searched for citrulline-specific antibody reactivity to these regions (found in CithFib- but not hFib-immunized DR4-IE tg mice), and then stratified for antibody reactivity that was higher in DR4-IE tg versus B6 mice immunized with CithFib. From the antibody microarray data, and using the preceding selection criteria, we identified the α chain sequence 121-140cit (Fig. 7 A) as a candidate target for a xenoreactive antibody response in DR4-IE tg mice (Fig. 7, B and C, and Table S4). To confirm the species specificity in regard to the immunizing antigen, we synthesized a region of this peptide to test serum from DR4-IE tg mice immunized with CitmFib or mFib by ELISA. As predicted from the sequencing data, antibody reactivity to the Fibα123Cit peptide was only found in DR4-IE tg mice immunized with CithFib, but not with CitmFib (Fig. 7 D). These results identify a xenospecific citrullinated B cell epitope that is targeted by the antibody response in HLA-DR4-IE tg mice immunized with CithFib.
Figure 7.
IgG antibody responses to heterologous regions of CithFib and CitmFib. (A) Amino acid substitutions between human and mFib are found in the α chain peptide 121–140 at arginine residues. Previous mass spectrometry analysis on in vitro CithFib identified αR123 and αR129 as sites of citrullination (25). (B) Increased antibody reactivity is evident in DR4-IE tg mice immunized with CithFib compared with B6 mice or either strain after immunization with hFib. Values represent normalized arbitrary antibody units from microarray analysis with n = 5 mice/strain/immunization. (C) Antibody reactivity to Fibα121-140Cit is citrulline specific because no reactivity is found to Fibα121-140. Values represent normalized arbitrary antibody units from microarray analysis with n = 5 mice/strain/immunization. (D) Antibody reactivity to Fibα123Cit in DR4-IE tg mice immunized with CithFib (n = 9), hFib (n = 5), CitmFib (n = 4), and mFib (n = 5) detected by ELISA. Mean OD of duplicates for individual serum are shown.
DISCUSSION
In this study, we show that citrullinated fibrinogen, an antigen that is frequently targeted by autoantibodies in RA patients, can induce arthritis in mice that carry the RA-associated MHC class II SE allele DRB1*0401. This disease was restricted by both the posttranslational modification of the antigen and the HLA transgene, as arthritis was not seen in mice administered unmodified fibrinogen or in wild-type (B6) mice administered either form of fibrinogen. Citrulline-specific T and B cell responses were prominent in DR4-IE tg mice, but they were not absolutely restricted by the SE, as antibody reactivity to some citrullinated antigens could be detected by ELISA and antibody microarray profiling in B6 mice. Further, although the arthritis in DR4-IE tg mice led to joint damage, and like RA, was accompanied by synovial cell hyperplasia, we did not find a robust and persistent polymorphonuclear cell infiltrate within joint tissues, suggesting that in this model, HLA-restricted responses to citrullinated fibrinogen can recapitulate some, but not all, aspects of the rheumatoid pathology.
Evidence that, in large part, the SE regulates the production of anti-citrulline antibodies in RA patients implies a causal relationship with disease pathogenesis mediated through CD4 T helper cell activation (26, 27). We have shown previously that the increased affinity of peptide-bound citrulline versus arginine for RA-associated MHC class II molecules correlates with CD4 T cell activation, but whether these peptides can be processed from citrullinated fibrinogen was not known. In this study, we addressed this question by assessing T cell recall responses in DR4-IE tg and B6 mice after immunization with CithFib. The presence of the HLA SE transgene afforded citrulline-specific T cell proliferation accompanied by IFN-γ production after in vitro challenge with CithFib and CitmFib. We also assessed peptide-specific recall responses to predicted DR4-binding epitopes and confirmed that a strong HLA SE-restricted T cell response was generated against both heterologous regions of hFib, and to a region of the α chain that was identical between species. The later peptide (Fibα R84Cit) contained citrulline at the critical P4 SE position and T cell responses in DR4-IE tg mice were restricted by this posttranslational modification. Notably, this was the only peptide within fibrinogen that we identified with this stimulatory property after conversion from arginine to citrulline at P4. The significance of T cell reactivity to this epitope is not known, but sequencing data from in vitro citrullination shows that modification of arginine at position 84 of the α chain is variable for hFib, whereas the same amino acid is consistently converted to citrulline after in vitro modification of mFib (unpublished data) (25). Site-specific citrullination within antigens highlights another level of variability that could impact the development of autoimmune responses. Moreover, whereas epitope specificity of the PAD enzymes have been assessed in vitro, naturally occurring citrullination sites within fibrinogen (or other antigens) have not been determined in vivo, or in other disease conditions besides RA.
In regard to HLA-restricted antibody responses, we found that characteristics of RA serum reactivity toward citrullinated antigens were paralleled in these mice. First, as in human patients (28), anti-citrulline antibodies were not completely restricted by the MHC class II SE, but instead levels were significantly higher in DR4-IE tg mice. This was evident by ELISA and antibody microarray profiling when assessing reactivity to CitmFib. Second, antibody reactivity to citrullinated antigens was broad, targeting sequences not only within fibrinogen, but also antigens such as vimentin (the target of anti-Sa antibodies in RA patients), fibromodulin, cartilage oligomeric protein (COMP), vitronectin, biglycan, and clusterin. Some of these proteins remain to be confirmed as targets of PAD in vivo, but all have been implicated either in RA pathogenesis or synovial/cartilage homeostasis. An unresolved issue with respect to anti-citrulline antibody specificity and evolution in RA patients is whether this broad response is the result of epitope spreading or merely cross-reactivity, which is a question that is not directly addressed in this study, but one that is currently being pursued. If epitope spreading is the cause, it would suggest that many of the proteins targeted by anti-citrulline antibodies are functionally and/or physically associated. This may be true for some of the citrullinated aforementioned antigens, as fibrinogen, vimentin, and vitronectin have been shown to associate during platelet activation, whereas fibromodulin, COMP, and biglycan are integral components of cartilage (29–31). The polyreactive nature of this antibody response also confounds the interpretation of the potential arthritogenicity of autoantibodies solely directed against citrullinated fibrinogen in this model and in RA.
It was interesting that several DR4-IE tg mice immunized with CithFib responded uniquely to citrullinated peptides from vimentin. The reactivity to Cit vimentin peptides derived from the C-terminal region of vimentin downstream of a caspase 6/8 cleavage site of the protein (e.g., vim 256-275cit) was noteworthy. Autoantibodies to citrullinated vimentin or Sa are strongly associated with disease severity and SE carriage in RA patients (8), and a role in disease pathogenesis has been speculated for years. Vimentin's role as an intracellular intermediate filament protein has been extensively studied; however, it is now evident that this protein is also present extracellularly, and could therefore interact directly with autoantibodies. Extracellular vimentin has been detected on platelets, macrophages, neutrophils, and T cells (30, 32–35). It is not known if extracellular vimentin is citrullinated, but vimentin is seen in situations of cellular activation and apoptosis, two conditions where intracellular calcium fluxes occur, a process necessary to activate peptidylarginine deiminase.
A distinct feature of the arthritis seen in DR4-IE tg mice was pronounced synoviocyte dysregulation, but a relative paucity of polymorphonuclear cells within the joints compared with other mouse models of arthritis. The reason for this is not known, but it is possible that a transient and self-limiting inflammatory infiltrate (not captured at the time points of our histological examination) could have provided a stimulus for synovial hyperplasia. A discordant relationship between anti-citrulline immune responses and synovial inflammatory cell infiltration has, however, been reported in RA patients. Baeten et al. have shown that anti-citrulline antibody titers do not correlate with histological parameters of synovial inflammation, nor does local inflammation correlate with SE carriage (14). Although not formally addressed in the current study, autoantibodies in this model could perpetuate disease by directly altering synovial fibroblast or chondrocyte cellular homeostasis, as has been shown previously with IgG (36, 37). Alternatively, a persistent and robust inflammatory cell infiltrate in the joint could be facilitated by additional genetic insults (possibly in PTPN22 or FcγRIII) that are independent of the SE (38–41).
This is the first description of citrulline-dependent arthritis in mice. Previous studies in mice have shown that the state of citrullination can influence disease severity in collagen-induced arthritis and that citrullination can break tolerance, leading to the development of citrulline-specific antibodies (42–44). Recent work has also shown that murine monoclonal antibodies specific for citrullinated fibrinogen can augment arthritis through passive transfer with anti–collagen II antibodies (45). Our studies show that the influence of the HLA transgene in citrulline-specific immune responses influences the magnitude and diversity of reactivity rather than the mere presence or absence of a response. These factors likely influence disease expression, as thresholds for T cell reactivity and antibody titers, as well as IgG epitope specificity, are limiting factors in other murine models of arthritis (46–49). Threshold effects may also be relevant to human disease, as prospective analysis of anti–cyclic citrullinated peptide antibodies in healthy individuals who later go on to develop RA show a marked increase in titer before disease onset (50, 51).
There are still unresolved issues pertaining to the model of arthritis presented in this study that require further exploration. For instance, it is not known why <40% of the mice develop disease, and we have not identified any 1 parameter of the immune response that significantly differentiates arthritic and nonarthritic DR4-IE tg mice immunized with CithFib (although arthritic mice did show a trend to higher cytokine responses and antibody production to certain antigens). In preliminary studies, we have induced a transient arthritis in DR4-IE tg mice after the transfer of citrulline-specific CD4+ T cells or anti-citrulline antibodies, directly implicating these mediators in the disease process (Fig. S3 and unpublished data) (52).
We have also found that CitmFib, an antigen that can evoke a citrulline-specific immune responses in DR4-IE tg mice, is not arthritogenic. Our examination of heterologous regions of fibrinogen that have been found to be citrullinated by mass spectrometry (25), coupled with IgG antibody microarray and ELISA data, does suggest that reactivity to Fibα121-140Cit may be important to this species-specific response. It is possible that reactivity to this sequence provides a platform for cross-reactivity to other proteins (such as vimentin), but a role in pathogenesis requires further examination.
In conclusion, these results indicate that in genetically susceptible DR4-IE tg mice, citrullinated fibrinogen can drive an autoimmune response that is associated with the development of arthritis. Further exploration of this model will help elucidate the role and contribution of SE-restricted T and B cell responses to citrullinated antigens in the pathogenesis of RA.
MATERIALS AND METHODS
Mice.
DR4-IE tg murine MHC class II–deficient mice (53) were bred at the John P. Robarts Barrier Facility and C57BL/6 (B6) mice were purchased from The Jackson Laboratory. Both strains were housed in the Animal Care and Veterinary Services Barrier Facility at the University of Western Ontario under specific pathogen–free conditions. The work with these mice was performed according to the guidelines established by the Canadian Council on Animal Care. 12–14-wk-old male mice were used in these experiments, as initial studies showed the incidence of arthritis to be higher than in females. 8-wk-old K/BxN mice used for histological analysis were maintained as previously described, and all procedures were approved by the Dana-Farber Cancer Institute Animal Care and Use Committee (20).
Immunizations.
Mice were immunized subcutaneously on the inner thigh/flank with 100 μg protein or peptide antigen in CFA (1:1 volume of antigen and IFA supplemented with 4 mg/ml Mycobacterium tuberculosis HA37) in a total volume of 100 μl. Boosting immunizations with protein antigens were conducted with the same concentration and volume of antigen in IFA 21 d later.
Protein antigens.
Purified human (Calbiochem) and mFib (Innovative Research, Inc.) were used in both citrullinated and unmodified form, whereas KLH (Sigma-Aldrich) was used in the citrullinated form. Citrullination of fibrinogen was performed as previously described (18) and confirmed by mobility shift in SDS-PAGE (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20072051/DC1), Western blotting with human RA sera, and antigen-specific ELISA.
Peptide antigens.
Peptides used in these studies were synthesized and purified by the manufacturer (Genemed Synthesis). Peptides were selected based on their predicted affinity for DRB1*0401 according to the method of Hammer et al. (23). Because citrulline is not accounted for in the predictive algorithm of Hammer et al., the value of glutamine was substituted for arginine when identifying a candidate T cell epitope (glutamine has the same terminal side chain group as citrulline). Fibrinogen-derived peptides spanning the sequences Fib α 79–91, Fib α 138–150, Fib α 173–185, Fib α 507–519, Fib α 673–685, Fib α 715–727, Fib α 738–750, Fib β 69–81, Fib β 280–292, and Fib γ 118–130 were synthesized in both arginine- and citrulline-containing forms, except for Fib α 371–383, which does not contain arginine.
Assessment of arthritis.
Mice were monitored twice weekly for clinical signs of arthritis, and caliper measurements were taken once weekly. Three clinically apparent stages of disease were apparent, beginning with mild joint swelling, followed by severe erythema, and ending with reduced swelling accompanied by plantarflexion restriction upon physical examination. These clinical observations closely followed caliper measurements. Some arthritic mice killed at day 70 that showed mild clinical swelling were further confirmed to have synovial abnormalities by histology.
Histological and immunohistological evaluation.
Mice were killed at the indicated time points, and the hind paws were dissected and fixed in 10% buffered formalin solution (VWR). Fixed tissues were decalcified for 5–7 d in 14% EDTA, followed by dehydration and paraffin embedding. Sagital sections (8 μm) were stained with hematoxylin and eosin (HE) or safranin O, or further processed for immunohistochemical staining. HE-stained sections were blindly assessed for joint abnormalities in DR4-IE tg mice immunized with CithFib or hFib at day 10, 31, and 70 after immunization (4 mice/immunizing antigen/time point). Additional arthritic mice were also assessed at day 70, 150, and 200. Sections from B6 mice immunized with CithFib or hFib (day 70) and DR4-IE tg mice immunized with CitmFib or mFib (day 100) were blindly assessed (4 mice/immunizing antigen), but did not show abnormal pathology. Joints including knee, shoulder, elbow, wrist, and spine were assessed in representative arthritic and control mice by histology, but did not show abnormal pathology. Citrullinated proteins were detected as previously described (54) using an AMC staining kit (Millipore) in combination with Vectastain ABC reagent (Vector Laboratories). Fibrinogen was detected using an anti-fibrinogen antibody (DAKO) according to the manufacturer's instructions, in combination with Vectastain ABC reagent.
T cell cultures.
Cell suspensions were prepared from the spleen or draining lymph nodes of mice killed at the indicated time points and cultured in 96-well plates at a concentration of 4 × 105 cells/well in the presence or absence of protein or peptide antigen for 4 d. 18 h before culture termination, 0.5 μCi of [3H]thymidine (ICN Biomedicals) was added to each well to assess T cell proliferation. Proliferation experiments were conducted in triplicate, and results are presented as the mean stimulation index (cpm of experimental sample/cpm of control sample) ± the SEM. Supernatants were removed from cultures after 72 h for testing cytokine production by ELISA (BD Biosciences), as previously described (55). Cytokine production was measured in duplicate and represents the mean antigen-specific cytokine production (cytokine production in control samples [no antigen in vitro] + 2 SD were subtracted from the protein- or peptide-specific cytokine production) ± the SD.
T cell transfers.
DR4-IE tg mice were immunized and boosted, as described in Immunizations. Spleens were harvested at day 31, and processed for in vitro stimulation with CithFib, as described in T cell cultures. 4 d after in vitro stimulation,viable cells were harvested by Ficoll gradient centrifugation and assessed for CD4 content by FACS (>85% positive). Cells then were transferred i.p. (10 × 106/mouse) and recipients received an intraarticular injection of 5 μg of the indicated antigen in a total volume of 5 μl. Caliper measurements were taken on the ankles every day for 2 wk, and data are presented as mean Δ ankle width (mm; ± the SEM) for the indicated number of mice.
ELISA detection of antigen-specific serum IgG.
Serum was collected from blood obtained by heart puncture from mice killed at the indicated time points. Antigen-specific ELISA was performed as previously described (18). In brief, MaxiSorp (Nunc) plates were coated with citrullinated and unmodified forms of fibrinogen, or the Fibα123Cit peptide (FSSANNCitDNTYNR), at a concentration of 10 μg/ml (100 μl/well) in carbonate coating buffer overnight at 4°C. After washing with PBS and 0.05% Tween (PBST) and blocking with PBS 0.1% BSA, serum samples were diluted 1:100 in PBST 0.1%BSA and were incubated in duplicate for 2 h at room temperature. After washing again with PBST, biotin-conjugated anti–mouse IgG secondary antibodies (Sigma-Aldrich) were diluted 1:2,000 in PBST 0.1% BSA containing streptavidin/horseradish peroxidase diluted 1:2,500 (Sigma-Aldrich) and incubated in the wells for 1h at room temperature. After further washing, wells were developed with tetramethyl benadine substrate (Sigma-Aldrich) for 10 min, after which the reaction was stopped with 2 M H2SO4 and absorbance was read at OD 450 nm. All samples were tested in parallel to standardize results and presented as the mean OD for each sample tested in duplicate.
Synovial proteome microarray analysis of sera.
Arrays were generated and probed with mouse sera as previously described (56, 57). Significance analysis of microarrays was used to identify patterns of antigen reactivity, followed by relationship arrangement with Cluster and Tree View software (58, 59). Direct Student's t test calculations were used to identify significantly different antibody reactivity between DR4-IE tg and B6 mice immunized with CithFib and stratified based on showing greater than fourfold increased reactivity compared with immunization with hFib. Raw data and peptide sequences can be found in Table S1–S3.
Online supplemental material.
Fig. S1 shows histological evaluation of arthritis in DR4-IE tg mice highlighting proteoglycan changes by safranin O staining. Fig. S2 shows that splenocytes from DR4-IE tg mice immunized with CithFib (day 70) produce IL-10 after in vitro challenge with CithFib or hFib. Fig. S3 shows that T cells from CithFib-immunized DR4-IE tg mice can induce arthritis after transfer to naive DR4-IE tg hosts that received intraarticular injection of CithFib. Fig. S4 shows the HLA-DR–restricted immune response to Fibα R84Cit in DR4-IE tg mice immunized with CithFib. Fig. S5 shows the citrulline-specific antibody reactivity determined by inhibition. Fig. S6 shows the typical mobility shift of CithFib seen by SDS-PAGE. Table S1–S3 show the raw data and peptide sequences for the synovial proteome microarray analysis presented in Fig. 6 (C–E), respectively. Table S4 shows the location of arginine substitutions between human and mFib sequences, in addition to citrullination sites (25) and antibody reactivity by synovial microarray analysis. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20072051/DC1.
Supplemental Material
Acknowledgments
This work was supported by grants from the Arthritis Society and the Canadian Institutes of Health Research and from internal research funds from the Department of Medicine, University of Western Ontario and London Health Sciences Centre. J.A. Hill was supported by a K.M Hunter/Canadian Institutes of Health Research Doctoral research award. E. Cairns is supported by an award from the Calder foundation.
The authors have no conflicting financial interests.
Abbreviations used: AMC, anti-modified citrulline; CithFib, citrullinated hFib; CitmFib, citrullinated mFib; DR4-IE tg, HLA-DRB1*0401 tg; HE, hematoxylin and eosin; hFib, human fibrinogen; mFib, mouse fibrinogen; RA, rheumatoid arthritis; SE, shared epitope; tg, transgenic.
J.A. Hill's present address is Section on Immunology and Immunogenetics, Joslin Diabetes Center, Boston, MA 02215.
References
- 1.Blass, S., J.M. Engel, and G.R. Burmester. 1999. The immunologic homunculus in rheumatoid arthritis. Arthritis Rheum. 42:2499–2506. [DOI] [PubMed] [Google Scholar]
- 2.Gregersen, P.K., J. Silver, and R.J. Winchester. 1987. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30:1205–1213. [DOI] [PubMed] [Google Scholar]
- 3.Hammer, J., F. Gallazzi, E. Bono, R.W. Karr, J. Guenot, P. Valsasnini, Z.A. Nagy, and F. Sinigaglia. 1995. Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J. Exp. Med. 181:1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schellekens, G.A., B. A.de Jong, F.H. van den Hoogen, L.B. van de Putte, and W.J. van Venrooij. 1998. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Invest. 101:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girbal-Neuhauser, E., J.J. Durieux, M. Arnaud, P. Dalbon, M. Sebbag, C. Vincent, M. Simon, T. Senshu, C. Masson-Bessiere, C. Jolivet-Reynaud, et al. 1999. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J. Immunol. 162:585–594. [PubMed] [Google Scholar]
- 6.van Venrooij, W.J., J.M. Hazes, and H. Visser. 2002. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth. J. Med. 60:383–388. [PubMed] [Google Scholar]
- 7.Vincent, C., L. Nogueira, C. Clavel, M. Sebbag, and G. Serre. 2005. Autoantibodies to citrullinated proteins: ACPA. Autoimmunity. 38:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Goldbach-Mansky, R., J. Lee, A. McCoy, J. Hoxworth, C. Yarboro, J.S. Smolen, G. Steiner, A. Rosen, C. Zhang, H.A. Menard, et al. 2000. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forslin, K., C. Vincent, G. Serre, and B. Svensson. 2000. Antifilaggrin autoantibodies in early rheumatoid arthritis. Scand. J. Rheumatol. 29:320–322. [DOI] [PubMed] [Google Scholar]
- 10.Bas, S., T.V. Perneger, E. Mikhnevitch, M. Seitz, J.M. Tiercy, P. Roux-Lombard, and P.A. Guerne. 2000. Association of rheumatoid factors and anti-filaggrin antibodies with severity of erosions in rheumatoid arthritis. Rheumatology (Oxford). 39:1082–1088. [DOI] [PubMed] [Google Scholar]
- 11.Hill, J.A., S. Southwood, A. Sette, A.M. Jevnikar, D.A. Bell, and E. Cairns. 2003. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 171:538–541. [DOI] [PubMed] [Google Scholar]
- 12.Masson-Bessiere, C., M. Sebbag, E. Girbal-Neuhauser, L. Nogueira, C. Vincent, T. Senshu, and G. Serre. 2001. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J. Immunol. 166:4177–4184. [DOI] [PubMed] [Google Scholar]
- 13.Chang, X., R. Yamada, A. Suzuki, T. Sawada, S. Yoshino, S. Tokuhiro, and K. Yamamoto. 2005. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford). 44:40–50. [DOI] [PubMed] [Google Scholar]
- 14.De Rycke, L., A.P. Nicholas, T. Cantaert, E. Kruithof, J.D. Echols, B. Vandekerckhove, E.M. Veys, F. De Keyser, and D. Baeten. 2005. Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral autoimmunity. Arthritis Rheum. 52:2323–2330. [DOI] [PubMed] [Google Scholar]
- 15.Menard, H.A., E. Lapointe, M.D. Rochdi, and Z.J. Zhou. 2000. Insights into rheumatoid arthritis derived from the Sa immune system. Arthritis Res. 2:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueira, L., S. Chapuy-Regaud, A. Constantin, C. Clavel, M. Sebbag, A. Cantagrel, C. Vincent, and G. Serre. 2003. Autoantibodies to deiminated fibrinogen are the most efficient serological criterion for early rheumatoid arthritis diagnosis. Arthritis Res. Ther. 5:18. [Google Scholar]
- 17.Nielen, M.M., A.R. van der Horst, D. van Schaardenburg, I.E. van der Horst-Bruinsma, R.J. van de Stadt, L. Aarden, B.A. Dijkmans, and D. Hamann. 2005. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann. Rheum. Dis. 64:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, J.A., J. Al Bishri, D.D. Gladman, E. Cairns, and D.A. Bell. 2006. Serum autoantibodies that bind citrullinated fibrinogen are frequently found in patients with rheumatoid arthritis. J. Rheumatol. 33:2115–2119. [PubMed] [Google Scholar]
- 19.Brintnell, W.C., J.A. Hill, C. Nadasdy, D.A. Bell, and E. Cairns. 2005. Immune response to a citrullinated peptide of fibrinogen in DR4 TG mice following intra-articular injection of streptococcal cell wall antigens. Arthritis Rheum. 52:S361. [Google Scholar]
- 20.Nigrovic, P.A., B.A. Binstadt, P.A. Monach, A. Johnsen, M. Gurish, Y. Iwakura, C. Benoist, D. Mathis, and D.M. Lee. 2007. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc. Natl. Acad. Sci. USA. 104:2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankovic, D., M.C. Kullberg, C.G. Feng, R.S. Goldszmid, C.M. Collazo, M. Wilson, T.A. Wynn, M. Kamanaka, R.A. Flavell, and A. Sher. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson, C.F., M. Oukka, V.J. Kuchroo, and D. Sacks. 2007. CD4+CD25−Foxp3− Th1 cells are the source of IL-10–mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 204:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammer, J., E. Bono, F. Gallazzi, C. Belunis, Z. Nagy, and F. Sinigaglia. 1994. Precise prediction of major histocompatibility complex class II–peptide interaction based on peptide side chain scanning. J. Exp. Med. 180:2353–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vossenaar, E.R., N. Despres, E. Lapointe, A. van der Heijden, M. Lora, T. Senshu, W.J. van Venrooij, and H.A. Menard. 2004. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. Ther. 6:R142–R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama-Hamada, M., A. Suzuki, K. Kubota, T. Takazawa, M. Ohsaka, R. Kawaida, M. Ono, A. Kasuya, H. Furukawa, R. Yamada, and K. Yamamoto. 2005. Comparison of enzymatic properties between hPADI2 and hPADI4. Biochem. Biophys. Res. Commun. 327:192–200. [DOI] [PubMed] [Google Scholar]
- 26.Berglin, E., L. Padyukov, U. Sundin, G. Hallmans, H. Stenlund, W.J. van Venrooij, L. Klareskog, and S.R. Dahlqvist. 2004. A combination of autoantibodies to cyclic citrullinated peptide (CCP) and HLA-DRB1 locus antigens is strongly associated with future onset of rheumatoid arthritis. Arthritis Res. Ther. 6:R303–R308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Gaalen, F.A., J. van Aken, T.W. Huizinga, G.M. Schreuder, F.C. Breedveld, E. Zanelli, W.J. van Venrooij, C.L. Verweij, R.E. Toes, and R. R.de Vries. 2004. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 50:2113–2121. [DOI] [PubMed] [Google Scholar]
- 28.van der Helm-van Mil, A.H., K.N. Verpoort, F.C. Breedveld, T.W. Huizinga, R.E. Toes, and R. R.de Vries. 2006. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 54:1117–1121. [DOI] [PubMed] [Google Scholar]
- 29.Podor, T.J., S. Campbell, P. Chindemi, D.M. Foulon, D.H. Farrell, P.D. Walton, J.I. Weitz, and C.B. Peterson. 2002. Incorporation of vitronectin into fibrin clots. Evidence for a binding interaction between vitronectin and gamma A/gamma' fibrinogen. J. Biol. Chem. 277:7520–7528. [DOI] [PubMed] [Google Scholar]
- 30.Podor, T.J., D. Singh, P. Chindemi, D.M. Foulon, R. McKelvie, J.I. Weitz, R. Austin, G. Boudreau, and R. Davies. 2002. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J. Biol. Chem. 277:7529–7539. [DOI] [PubMed] [Google Scholar]
- 31.Roughley, P.J. 2001. Articular cartilage and changes in arthritis: noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage. Arthritis Res. 3:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mor-Vaknin, N., A. Punturieri, K. Sitwala, and D.M. Markovitz. 2003. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 5:59–63. [DOI] [PubMed] [Google Scholar]
- 33.Moisan, E., and D. Girard. 2006. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc. Biol. 79:489–498. [DOI] [PubMed] [Google Scholar]
- 34.Boilard, E., S.G. Bourgoin, C. Bernatchez, and M.E. Surette. 2003. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood. 102:2901–2909. [DOI] [PubMed] [Google Scholar]
- 35.Huet, D., M. Bagot, D. Loyaux, J. Capdevielle, L. Conraux, P. Ferrara, A. Bensussan, and A. Marie-Cardine. 2006. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J. Immunol. 176:652–659. [DOI] [PubMed] [Google Scholar]
- 36.Pritchard, J., S. Tsui, N. Horst, W.W. Cruikshank, and T.J. Smith. 2004. Synovial fibroblasts from patients with rheumatoid arthritis, like fibroblasts from Graves' disease, express high levels of IL-16 when treated with Igs against insulin-like growth factor-1 receptor. J. Immunol. 173:3564–3569. [DOI] [PubMed] [Google Scholar]
- 37.Amirahmadi, S.F., S. Whittingham, D.E. Crombie, K.S. Nandakumar, R. Holmdahl, I.R. Mackay, M.P. van Damme, and M.J. Rowley. 2005. Arthritogenic anti-type II collagen antibodies are pathogenic for cartilage-derived chondrocytes independent of inflammatory cells. Arthritis Rheum. 52:1897–1906. [DOI] [PubMed] [Google Scholar]
- 38.Lee, A.T., W. Li, A. Liew, C. Bombardier, M. Weisman, E.M. Massarotti, J. Kent, F. Wolfe, A.B. Begovich, and P.K. Gregersen. 2005. The PTPN22 R620W polymorphism associates with RF positive rheumatoid arthritis in a dose-dependent manner but not with HLA-SE status. Genes Immun. 6:129–133. [DOI] [PubMed] [Google Scholar]
- 39.Johansson, M., L. Arlestig, G. Hallmans, and S. Rantapaa-Dahlqvist. 2006. PTPN22 polymorphism and anti-cyclic citrullinated peptide antibodies in combination strongly predicts future onset of rheumatoid arthritis and has a specificity of 100% for the disease. Arthritis Res. Ther. 8:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieto, A., R. Caliz, M. Pascual, L. Mataran, S. Garcia, and J. Martin. 2000. Involvement of Fcgamma receptor IIIA genotypes in susceptibility to rheumatoid arthritis. Arthritis Rheum. 43:735–739. [DOI] [PubMed] [Google Scholar]
- 41.Morgan, A.W., B. Griffiths, F. Ponchel, B.M. Montague, M. Ali, P.P. Gardner, H.C. Gooi, R.D. Situnayake, A.F. Markham, P. Emery, and J.D. Isaacs. 2000. Fcgamma receptor type IIIA is associated with rheumatoid arthritis in two distinct ethnic groups. Arthritis Rheum. 43:2328–2334. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg, K., S. Nijenhuis, E.R. Vossenaar, K. Palmblad, W.J. van Venrooij, L. Klareskog, A.J. Zendman, and H.E. Harris. 2005. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res. Ther. 7:R458–R467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hida, S., N.N. Miura, Y. Adachi, and N. Ohno. 2004. Influence of arginine deimination on antigenicity of fibrinogen. J. Autoimmun. 23:141–150. [DOI] [PubMed] [Google Scholar]
- 44.Rubin, B., and G. Sonderstrup. 2004. Citrullination of self-proteins and autoimmunity. Scand. J. Immunol. 60:112–120. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn, K.A., L. Kulik, B. Tomooka, K.J. Braschler, W.P. Arend, W.H. Robinson, and V.M. Holers. 2006. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J. Clin. Invest. 116:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi, N., T. Takahashi, H. Hata, T. Nomura, T. Tagami, S. Yamazaki, T. Sakihama, T. Matsutani, I. Negishi, S. Nakatsuru, and S. Sakaguchi. 2003. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 426:454–460. [DOI] [PubMed] [Google Scholar]
- 47.Korganow, A.S., H. Ji, S. Mangialaio, V. Duchatelle, R. Pelanda, T. Martin, C. Degott, H. Kikutani, K. Rajewsky, J.L. Pasquali, et al. 1999. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 10:451–461. [DOI] [PubMed] [Google Scholar]
- 48.Burkhardt, H., T. Koller, A. Engstrom, K.S. Nandakumar, J. Turnay, H.G. Kraetsch, J.R. Kalden, and R. Holmdahl. 2002. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 46:2339–2348. [DOI] [PubMed] [Google Scholar]
- 49.Bajtner, E., K.S. Nandakumar, A. Engstrom, and R. Holmdahl. 2005. Chronic development of collagen-induced arthritis is associated with arthritogenic antibodies against specific epitopes on type II collagen. Arthritis Res. Ther. 7:R1148–R1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rantapaa-Dahlqvist, S., B.A. de Jong, E. Berglin, G. Hallmans, G. Wadell, H. Stenlund, U. Sundin, and W.J. van Venrooij. 2003. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48:2741–2749. [DOI] [PubMed] [Google Scholar]
- 51.Nielen, M.M., D. van Schaardenburg, H.W. Reesink, R.J. van de Stadt, I.E. van der Horst-Bruinsma, M.H. de Koning, M.R. Habibuw, J.P. Vandenbroucke, and B.A. Dijkmans. 2004. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50:380–386. [DOI] [PubMed] [Google Scholar]
- 52.Yue, D., W. Brintnell, S. Uniyal, B. Chan, A.M. Jevnikar, D.A. Bell, and E. Cairns. 2007. Arthritogenic CD4 T cells in citrulline-induced arthritis. Arthritis Rheum. 56:S683. [Google Scholar]
- 53.Ito, K., H.J. Bian, M. Molina, J. Han, J. Magram, E. Saar, C. Belunis, D.R. Bolin, R. Arceo, R. Campbell, et al. 1996. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 183:2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vossenaar, E.R., S. Nijenhuis, M.M. Helsen, A. van der Heijden, T. Senshu, W.B. van den Berg, W.J. van Venrooij, and L.A. Joosten. 2003. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 48:2489–2500. [DOI] [PubMed] [Google Scholar]
- 55.Hill, J.A., D. Wang, A.M. Jevnikar, E. Cairns, and D.A. Bell. 2003. The relationship between predicted peptide-MHC class II affinity and T-cell activation in a HLA-DRbeta1*0401 transgenic mouse model. Arthritis Res. Ther. 5:R40–R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hueber, W., B.A. Kidd, B.H. Tomooka, B.J. Lee, B. Bruce, J.F. Fries, G. Sonderstrup, P. Monach, J.W. Drijfhout, W.J. van Venrooij, et al. 2005. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 52:2645–2655. [DOI] [PubMed] [Google Scholar]
- 57.Robinson, W.H., P. Fontoura, B.J. Lee, H. E.de Vegvar, J. Tom, R. Pedotti, C.D. DiGennaro, D.J. Mitchell, D. Fong, P.P. Ho, et al. 2003. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21:1033–1039. [DOI] [PubMed] [Google Scholar]
- 58.Tusher, V.G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA. 98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisen, M.B., P.T. Spellman, P.O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 95:14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.