Review discusses the chemistry of NO and ROS in the context of anti-pathogen activity and immunoregulation.
Keywords: oxidative nitrosative stress, signaling cascade
Abstract
The role of redox molecules, such as NO and ROS, as key mediators of immunity has recently garnered renewed interest and appreciation. To regulate immune responses, these species trigger the eradication of pathogens on the one hand and modulate immunosuppression during tissue-restoration and wound-healing processes on the other. In the acidic environment of the phagosome, a variety of RNS and ROS is produced, thereby providing a cauldron of redox chemistry, which is the first line in fighting infection. Interestingly, fluctuations in the levels of these same reactive intermediates orchestrate other phases of the immune response. NO activates specific signal transduction pathways in tumor cells, endothelial cells, and monocytes in a concentration-dependent manner. As ROS can react directly with NO-forming RNS, NO bioavailability and therefore, NO response(s) are changed. The NO/ROS balance is also important during Th1 to Th2 transition. In this review, we discuss the chemistry of NO and ROS in the context of antipathogen activity and immune regulation and also discuss similarities and differences between murine and human production of these intermediates.
Introduction
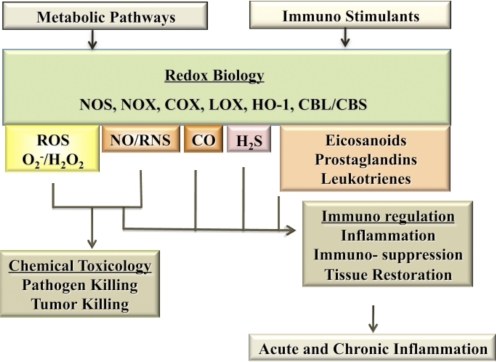
Over the course of the last three decades, the term “oxidative stress” has become a household word. The concept that excess oxidation leads to aging and to many diseases has been thoroughly implanted into the psyches of our culture. Molecules such as ROS, similar to those produced from ionizing radiation, can be generated in the body and when uncontrolled, can lead to cellular and tissue damage [1, 2]. Antioxidants have frequently been touted as the antidote to oxidative stress but are only part of the complex chemical story. Although oxidation can lead to toxicity, it is important to realize that redox-based molecules are essential mediators of critical functions in physiological systems and are essential to immunity against disease. In fact, redox reactions are key signal transduction mechanisms and should be included in the pantheon of interwoven cell regulatory systems along with phosphorylation reactions mediated by kinases/phosphatases and other types of cellular signaling pathways [3–5]. Redox molecules such as peroxide, superoxide, NO, and RNS, once thought to be only toxic, are essential in tissue repair [6, 7]. More recently, other toxic gases, such as CO and H2S, have emerged as therapies to correct dysregulated cell functions [8, 9]. As indicated in Fig. 1, there are two major roles for these reactive small molecules in the immune response: direct participation in the eradication of pathogens or regulation of immune pathways. However, it is only NO/RNS and ROS that serve “double duty” by participating as immunotoxins as well as immunomodulators. Not only does the incoming immune signal initiate the production of NO and ROS to intercept and kill pathogens, but downstream signaling pathways that lead to the full expression of the immune response are modulated by NO and ROS in complex ways. Overall, the complexity of these reactions is a reflection of the diverse nature of redox chemistry within a biological setting.
Figure 1. The immunotoxic and immunoregulatory aspects of redox-based biology.
The redox species and sources are: NO and RNS derived from NOS; ROS derived from NOX; arachidonic acid products derived from COX and lipoxygenase (LOX); CO derived from HO-1; and H2S from CBS and cystathionine β-lyase (CBL). Of the multiple redox species involved in immunity, NO/RNS and ROS are critical to defense against pathogens and for regulation of the response.
EARLY STUDIES CONNECTING NO AND IMMUNITY
The discovery of NO as an endothelial-derived relaxation factor and as the active ingredient in nitroglycerin (a potent vasodilator) clearly demonstrated that redox-reactive molecules, normally thought to be toxic, were endogenously generated within the cardiovascular system [10–12]. Furthermore, these molecules served important, normal physiological functions [11]. Concurrently, NO was found to be an important component of the immune system. Hibbs and coworkers [13, 14] showed that a substance that was released by macrophages and exhibited antipathogen and antitumor activity also required arginine for its production. These data supported the earlier observation by Tannenbaum and coworkers [15], who showed that plasma levels of nitrite and nitrates increased upon infection, suggesting an increase in endogenous production of NOs. This group went on to show that nitrosoproline was formed in vivo, demonstrating for the first time that not only did nitrosative chemistry occur beyond the gastrointestinal tract but also that humans generated high levels of NOs in response to illness [16, 17]. Hibbs and coworkers [18, 19] further connected immunity and NO when they showed that IL-2-mediated immune activation increased NO levels in patients and promoted tumor eradication in mice. Direct evidence that macrophages made nitrite and nitrate, as well as nitrosamines, was shown in the late 1980s by a number of groups [20–22]. Miwa et al. [23] showed that nitrosocompounds were detected in animals that were treated with LPS. Stuehr and Nathan [24] went on to show that NO generated by macrophages could kill leukemia cells. Finally, Lancaster and Hibbs [25] demonstrated the formation of iron-NO complexes within macrophages. In total, these data provided strong evidence that NO plays a critical role in the immune response and opened the door to understanding the participation of redox molecules and their reactions in immunity and to a greater appreciation of their importance.
NO AND ROS ARE THE PRINCIPLE REDOX MOLECULES IN IMMUNITY
The primary source of NO is the NOS enzyme, which has three isoforms; two are constitutively expressed, and one is inducible. eNOS (NOS3) and nNOS (NOS1) are constitutively expressed and in general, release short bursts of NO in a calcium-dependent manner [26]. More recent studies have found that post-transcriptional modification of eNOS can convert short, calcium-dependent NO bursts to sustained, calcium-independent production of NO [27]. In the case of eNOS, this post-transcriptional regulation plays a fundamental role in the health of endothelium during angiogenesis, as well as regulation of immune activation. The inducible form of iNOS (NOS2) is generally associated with the immune system and produces NO for prolonged periods of time in a calcium-independent manner. Levels of NO produced by iNOS in the microenvironment of the cell can range from as low as 10 nM to μM amounts for days [28]. By producing high levels of NO, iNOS activity can orchestrate varied NO-modulated microenvironments within a tissue, each with potentially different functions. Thus induction of iNOS is not solely defined by high, local amounts of NO, as iNOS activity can generate a wide range of levels of NO for variable periods of time [29]. Thus, iNOS provides unique flexibility in dealing with an immune challenge. In contrast, the low flux of NO produced by eNOS restricts its actions. To be effective, eNOS or nNOS must be targeted and anchored/sequestered in close proximity to its target. An additional level of immune regulation by iNOS is provided by the ability of the NOS enzyme to make products other than NO. These include NOHA and O2–. By generating NOHA, an inhibitor of AG activity, the iNOS system can affect pathways that mediate cell growth (ornithine to polyamines) or tissue matrices (ornithine to proline) [30]. This diversity of NOS activities can produce different temporal and concentration profiles of NO as well as other products to facilitate and broaden the functional versatility of these enzymes during immune challenge.
The second basic biological redox system involved with immunity is comprised of O2–, H2O2, and other ROS, known collectively as ROS. This system can function independently of NO to carry out specific oxidation events that modify intracellular signaling pathways, including regulation of migration [31], cell mitogenic potential [32], or host defense. The primary cellular sources of ROS are oxidases that generate O2– by the transfer of a single electron to oxygen from NADPH (reduced form). Further single electron reduction to H2O2 or other ROS is catalyzed by a series of enzymes that includes SOD and MPO through interactions with transition metals or through reactions with NO.
The first NOX to be identified as a participant in the innate immune response was found in phagocytic cells. Originally, the activity of this enzyme was described as the “respiratory burst” and was associated with intracellular killing of bacteria or other invaders [33]. It is now known that this mechanism generates a rapid increase and high level of superoxide when phagocytes are exposed to bacteria through a heteromeric assembly of multiple protein components. These components include a membrane-bound cytochrome b558 complex made up of gp91phox and p22phox catalytic subunits and four cytosolic proteins (p47phox, p67phox, p40phox, and GTPase Rac1). During immune stimulation, the cytosolic proteins assemble into a complex and translocate to the membrane, joining with the gp91phox-p22phox complex. This step results in full activation of the enzyme complex and the production of O2– [34–36]. gp91phox is also now known as NOX2 and is a member of the Nox gene family of NOX that includes homologs of NOX2 (NOX1, NOX3, NOX4, and NOX5) and related DUOXs, termed DUOX1 and DUOX2. NOX1, NOX3–5, and DUOX1 and -2 are most commonly found in nonphagocytic cells, such as smooth muscle or endothelial cells, where they produce limited levels of O2– (or H2O2 in the case of DUOX1 and -2) in a tightly regulated, temporal-spatial pattern [31].
Activation of the NOX is initiated by the interaction of immunogens with specific membrane receptors such as complement or FcRs. NOX is also stimulated by treatment with agents that activate calcium entry or by treatment with cellular products such as arachidonic acid [34–36]. Additional exposure to “priming” agents such as IFN-γ, TNF-α, or IL-1β significantly increases the levels of O2– produced during the immune response. In some cases, low levels of superoxide and H2O2 are constitutively produced, primarily by NOX4 or DUOX activation [37, 38]. Although the activation process has been well described, less is known about the mechanisms that limit NADPH-oxidase activity and superoxide production or help to tightly regulate its outcomes. Potential deactivation mechanisms range from dephosphorylation of complex components by phosphatases to membrane depolarization or loss of charge counterbalance mechanisms (e.g., loss of proton or chloride channels associated with oxidase activity) and loss of PLA2 and arachidonic acid production [35, 39–44]. Many of these mechanisms are modulated by NO, where NO plays a key role in controlling levels of ROS and in limiting the reactivity of O2– and H2O2 to specific cellular sites. Restricted delivery of superoxide under specific conditions and locations helps minimize the collateral damage that may be initiated by ROS. Compartmentalization of superoxide/H2O2 production is also used to control signaling processes. For example, endothelial cell migration and endothelial growth factor signaling during processes such as wound repair are dependent on NADPH-oxidase activity [31, 45, 46]. For migration, the NOX complex is located to specific membrane regions associated with directed movement of the cell [31].
REDOX CHEMISTRY OF THE IMMUNE RESPONSE
To understand how NO and redox molecules carry out their diverse immune functions, it is important to understand the type of chemical reactions that produces those outcomes. In general, there are two classes of redox chemistry based on NO or O2– as the starting molecular species. The first class involves NO itself and RNS made from the interaction of NO with ROS. These are frequently called RNS. The second class involves ROS as the starting molecule species. NO/RNS and ROS can be used separately or in combination in immune responses and participate in “killing” and immune regulation. As discussed above, NO is produced as a primary product of the enzymatic action of iNOS [47]. NO is relatively nonreactive and only reacts directly with transition metals in heme or cobalamine, with nonheme iron [48], or with reactive radicals such as hydroperoxide radicals formed during lipid peroxidation [48]. This latter reaction exemplifies the potent antioxidant capability of NO. It is now well established that NO acts directly as a powerful antioxidant in mammalian cells and prevents ROS injury initiated by reactions with metals, with superoxide, or with lipid radicals [49]. In contrast, NO does not react with thiols or other nucleophiles directly but requires activation with superoxide or O2– to generate RNS such as ONOO–, NO2, and N2O3 [48]. ONOO– and NO2 can oxidize substrate, and N2O3 is a primary source of nitrosation of substrate. Thus, these RNS participate in the generation of conditions leading to nitrosative or oxidative stress [48].
There are several key chemical concepts that are important for understanding the role of RNS and ROS intermediates in vivo. The first is that NO and NO2 are lipophilic, and both can migrate through cells, broadening the potential profile of targets and reactivity. As a RNS, ONOO– reacts rapidly with CO2 to form CO2-OONO–, which shortens its lifetime to <10 ms [50]. The anionic character and short lifetime drastically limit its mobility, thereby effectively prohibiting migration across membranes. As a result of the short lifetime, the location of superoxide formation dictates the location of ONOO– formation. When NO levels are higher than superoxide levels, the CO2-OONO– intermediate is converted to NO2 and N2O3 and changes the redox profile from an oxidative to a nitrosative microenvironment [51]. The balance between NO and superoxide then determines not only the bioavailability of NO but also can generate RNS in the close proximity of a superoxide source, consequently producing a different reaction profile. Another important consequence of ROS and NO interaction is that peroxidase activity leads to consumption of NO and the formation of nitrite [52]. The same peroxidase and Fenton reactions that consume NO can also oxidize nitrite to NO2 to increase oxidation and nitration further [28]. Thus, ROS consumes NO to generate NO2 and N2O3, as well as nitrite, in specific locations.
REDOX CHEMISTRY OF THE IMMUNE RESPONSE: ROLES IN ERADICATION OF BACTERIA, PARASITES, AND VIRUSES
The phagosome “cauldron”
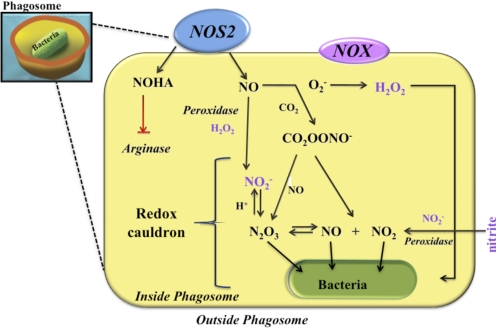
Studies on bacterial killing by the NOX clearly show the compartmentalization of ROS production to phagosomes. Delivery of superoxide under specific conditions and at specific locations helps to minimize the collateral damage that may be initiated by ROS to other subcellular organelles within close vicinity of the phagosome. However, the phagosome is a uniquely restricted space (Fig. 2). As NOX2 is a plasma membrane protein, which upon uptake of pathogens, directs O2– release into the phagosome, it is likely that any oxidizing species produced by NOX2 is restricted to the phagosome interior. In contrast, iNOS is not commonly, spatially restricted, and the readily diffusible nature of NO and RNS allows these redox species to participate in pathogen killing in a widespread and in an interesting concentration-dependent manner. The level of NO within phagosomes will depend on the specific NOS isoform as well as the level of NOS activity and thus, can be varied by a number of cellular conditions. The presence of NOX and superoxide in the phagosome combines to scavenge NO, resulting in increased nitrite within the phagosome. Nitrite under acidic conditions also contributes to the cauldron effect. Like NO, under specific conditions, nitrite can generate N2O3 and NO2/NO similar to the NO/superoxide and NO/O2– reactions described previously. This is particularly important during phagocytosis of pathogens. As phagosomes have an acidic environment, these conditions are compatible with the generation of NO, NO2, and/or N2O3 [53, 54]. Thus, the phagosome may be thought of as a cauldron of redox reactions, which uses RNS and ROS to kill pathogens.
Figure 2. The NO chemistry of the phagosome.
This diagram depicts the different nitrogen oxide and ROS chemistry that can occur within the phagosome to fight pathogens. The presence of NOX2 in the phagosomes serves two purposes: one is to focus the nitrite accumulation through scavenging mechanisms, and the second provides peroxide as a source of ROS or FA generation. The nitrite (NO2−) formed in the acidic environment provides nitrosative stress with NO/NO2/N2O3. The combined acidic nature and the ability to form multiple RNS and ROS within the acidic environment of the phagosome provide the immune response with multiple chemical options with which it can combat bacteria.
Bacteria
The variety of chemical species generated from NO/RNS and ROS provides an arsenal that can be used by the immune system to combat pathogens. Rather than killing by nitrosative and oxidative modification of critical bacterial macromolecules as once thought, NO and the formation of RNS provide a multifaceted pathogen eradication program [55, 56]. The requirement for the combination of RNS and ROS, rather than NO or superoxide/H2O2 by themselves, is shown in the response to Escherichia coli [57]. Little bactericidal activity against E. coli is observed with bolus treatment of NO or of H2O2 [57]. However, when combined together, H2O2 plus NO mediate a dramatic, three-log increase in cytotoxicity, as opposed to 50% killing by NO alone or H2O2 alone. This indicates that although E. coli are resistant to NO/RNS and H2O2 separately, these bacteria are highly susceptible to their synergistic actions. The mixture of NO/RNS and ROS effectively provides a potent killing combination that increases bactericidal activity and protects the host cell from ROS-mediated toxicity by the antioxidant and other protective actions of NO. Interestingly, the resistance of E. coli to NO/RNS or ROS alone is consistent with studies that show E. coli can increase the O2– stress regulon, a key gene regulator for the adaptive/antioxidant response to H2O2 and superoxide [58].
The synergistic redox action, in this case, acts as a “one-two” punch. Unlike mammalian systems, bacteria use iron sulfur clusters in electron transport. These clusters are located in the periplasmic space and are particularly susceptible to NO's attack. The result is the release of iron into the interior of the cell where is binds to DNA [57]. Unlike mammalian cells, NO does not penetrate easily to the interior of the bacteria and thus, peroxide, via Fenton chemistry, is required to interact with the bound iron. This process oxidizes DNA and leads to its cleavage. In contrast, NO can diffuse to most parts of mammalian cells, where it serves as an antioxidant [59]. These two basic differences make NO/H2O2 a perfect killing combination for E. coli and at the same time, protects mammalian cells from ROS-mediated toxicity.
Other bacterial pathogens such as Staphylococcus exhibit a different response to redox-mediated attack. The simultaneous presence of NO and peroxide in staphylococcal infections leads to a protective effect, where NO reduces ROS-mediated toxicity to the bacteria [60]. Yet, when Staphylococcus bacteria are exposed first to peroxide followed by NO, increased toxicity is observed [60]. In this case, it is the sequential exposure to a burst of superoxide/ROS production followed by NO that is critical for the maximum killing effect by these redox molecules. This combination is extremely effective in eradicating Staphylococcus bacteria.
The NO/RNS and ROS redox arsenal, however, does not kill all bacteria. For example, the gram-positive bacteria, Listeria, is resistant to redox-mediated killing, most likely because of adaptive mechanisms developed by their propagation under harsh environmental conditions [61]. Regardless, high NO levels lead to cytostasis and imprisonment of the bacteria within the infected cell. Nitrosation of the thiol in the pore-forming protein listeriolysin O (which is a cysteine protease) prevents the bacteria from exiting the cells it has infected [61]. As a consequence, the infectivity of the bacteria is reduced. In addition, imprisonment in the cytoplasm allows other immune constituents to eradicate the infection. Thus, for Listeria, although resolution of the infection is slow, generation of NO by NOS remains an important event [62–65].
Another bacterium that is susceptible to RNS is Mycobacterium tuberculosis. Like other bacterium, M. tuberculosis is taken up by lung alveolar macrophages. These bacterium are sensitive to NO and RNS, but in this case, NO2 is the toxic species. Although μM ONOO– or 90 ppm NO are toxic, NO2 at <1 ppm exposure is considerably more toxic [66, 67]. As discussed above, the macrophage can use various conditions to generate NO2 in the phagosome (Fig. 2). Where anions such as ONOO–/CO2OONO– cannot diffuse through the cell wall, NO and NO2 will penetrate. Thus, the acidification of the phagosome in the presence of nitrite and NO provides an optimal condition(s) to generate the RNS and ROS required to kill M. tuberculosis. Interestingly, oxidation of methionine groups is a major factor in eradication of this pathogen, and M. tuberculosis is protected by the activity of methionine sulfoxide reductase. This finding is confirmed by studies showing that M. tuberculosis lacking the reductase are killed more readily [68]. Another role of macrophage-derived NO is that it prevents M. tuberculosis-induced apoptosis of T cells, thus aiding in the clearance of the disease via adaptive immune mechanisms [69, 70]. Like Listeria, the eradication of tuberculosis in murine models requires iNOS, demonstrating that the chemistry of RNS provides a tool for an aggressive defense [71]. In the phagosome, nitrosative and oxidative conditions exist that are functionally hypoxic, carbohydrate-poor, and capable of perturbing the cell envelope of the pathogen [72]. The ROS is delivered in the first 3 h, followed by FA from 0 to 48 h and then NO from 4 to 72 h [73]. Iron depletion of the organism forces M. tuberculosis to undergo anaerobic metabolism, which retards growth. Bacteria also evade macrophage killing by neutralizing reactive nitrogen intermediates [74]. With respect to ROS, it has been shown that hypochlorous acid/hypobromous acid are more effective in eradicating M. tuberculosis, supporting the additional and critical contribution of peroxidases to the eradication of this pathogen [73]. In the presence of nitrite, these peroxidases can also enhance NO2 formation. Thus, a phagosome microenvironment consisting of ROS combined with acidic nitrite generates NO2/N2O3/NO, which is essential for pathogen eradication by the alveolar macrophage. Overall, NO has a dual function; it participates directly in killing an organism, and/or it disarms a pathway used by that organism to elude other immune responses. This illustrates the requirement for redundancy of redox mechanisms that can generate ROS, NO2, and RNS in bactericidal mechanisms.
Protozoan/parasites
Many common human parasites have been shown to elicit host iNOS induction and the subsequent initiation of immune mechanisms, resulting in the expulsion of the parasite. The list includes species such as Plasmodia (malaria), Leishmania (leishmaniasis), and Toxoplasma (toxoplasmosis), which are responsible for infections in many parts of the world. Among these parasitic, human infestations, malaria is one of the most prevalent. It is also an example of a parasitic infestation that activates host responses regulated by the innate and adaptive immune systems. Studies have shown that severe cases of human malaria are accompanied by increased iNOS activity and the subsequent production of NO [75, 76]. Increased levels of NO were found to be beneficial, as NO was shown to kill parasites [77, 78]. However, it is also interesting to note that elevated NO levels in the blood of malaria patients correlated with reduced parasitic adhesion to endothelial cells, maintenance of microvascular perfusion, and reduced deleterious cytokine production [79]. Kun, Weinberg, and colleagues [80] demonstrated that the increased NO levels and the resulting protection against malaria were linked to a single nucleotide polymorphism in the promoter of the NOS2 gene, termed the NOS2Lambaréné (G-954C) mutation. The exact impact that Plasmodium falciparum components have on NOS2 and NOS metabolite production in human monocytes remains unclear, as neither of these redox species was induced by hemozoin, a malarial pigment that is known to act as an immunogen. As discussed in a later section, the lack of NOS2 induction, despite simultaneous treatment of hemozoin with IFN-γ or a cytokine-LPS mix, is typical of human monocytes [81]. On the other hand, mouse monocytes responded strongly to this stimulus by producing significant quantities of NO and NOS metabolites. These results highlight the species-specific differences between human and mouse redox systems.
Plasmodium berghei is a similar malarial species that affects rodents, where ookinetes are susceptible to NO-induced, apoptosis-like programmed cell death while still in the gut of the mosquito [82]. Studies showed that ingested blood and mosquito tissues were the likely sources of NO and postulated that manipulating NO levels in the mosquito's gut could provide a method for controlling Plasmodium transmission [83]. Interestingly, the induction of TGF-β under these nitrosative stress conditions leads to down-regulation of the NO defense system [84], suggesting a potential counter-control of malaria in the mosquito.
Leishmania is a successful intracellular parasite that resides in mammalian macrophages. Leishmania infestation is controlled by NO produced by iNOS. This response is particularly strong in murine models of infection. A critical aspect of NO metabolism is that NOHA inhibits AG activity, thereby limiting the growth of parasites and bacteria including Leishmania, Trypanosoma, Schistosoma, Helicobacter, Mycobacterium, and Salmonella, and is distinct from the effects of RNS [85]. Therefore, NOHA is decoupled from iNOS catalysis and adds another component of the iNOS arsenal to limit bacterial and parasitic infection [86]. Type 1 and type 2 responses control the availability of arginine, from which NO is generated. The type 1 response increases conversion of arginine to NO by iNOS, and the type 2 response promotes AG induction [87]. Cytokines and ROS production are also triggered by a LPG produced by the Leishmania species [88]. Soluble and membrane LPG, obtained from Leishmania major stationary promastigotes, stimulated ROS, IL-10, and IL-12 production in human PMBCs. A subsequent study also used human PBMCs and showed that ROS and cytokine responses to L. major LPG involve TLR2, and TLR2-mediated response could be blocked using anti-TLR2 mAb, which also successfully blocked the production of ROS [89]. One class of Leishmania chemotherapeutics, pentavalent antimony complexes, such as sodium stibogluconate and SAG, is used to treat visceral leishmanias, which appear to kill the parasite through initial increases in the production of ROS, followed by NO. SAG was shown to induce ERK-P via PI3K activation, which leads to the production of ROS. NO production was induced through the effect of TNF-α [90, 91].
Toxoplasma gondii is another example of a human intracellular protozoan parasite that elicits NO-mediated responses. Its infestation is globally disseminated, affecting approximately one-third of the world's population. As with most intracellular parasites, during infestation, the attacking organism is encapsulated in a vacuole. In this case, a parasitophorous vacuole is generated when the protozoan attaches to and incorporates into the host cell [92]. Studies have demonstrated increased infectivity and severity of inflammatory lesions in the CNS of iNOS knockout mice when compared with WT animals. These results suggest that iNOS limits the progression of the disease to the CNS and that the lack of iNOS is detrimental to the host who is unable to control T. gondii replication [93].
Virus
NO is also a critical component in the eradication of viruses. Viral replication is inhibited by the induction of iNOS and the subsequent production of NO (HIV-1, coxsackievirus, influenza A and B, rhino virus, CMV, vaccinia virus, ectromelia virus, human herpesvirus-1, and human parainfluenza virus type 3) [94]. Coxsackievirus and CMV murine models show that iNOS reduces viral burden and decreases latency [95, 96]. Similarly, replication of viral influenza may also be reduced by NO; however, the symptoms may increase, suggesting a balance between an antipathogen response versus healing and resolution responses [97]. A number of viruses have been shown to increase iNOS through activation of TLR3 that increases iNOS/COX2. Interestingly, PGE2 has been shown to inhibit clearance of many viruses as a result of its immunosuppressive nature, and increased cAMP enhances viral replication [98]. In contrast, PGE2 inhibits HIV replication in infected macrophages [99].
Also, during viral clearance, elevated NO concentrations can have unwanted consequences. Influenza studies have demonstrated protection against consolidating pneumonitis in iNOS knockout mice [97]. Elevated NO has been shown in humans infected with Dengue fever. The induction of iNOS leads to retarded growth in human monocytes [100]. NO donors have been shown to inhibit the virus replication [101]. However, systemic production of NO can lead to pathological hemorrhagic fever, which is caused by increased leakiness and inhibition of platelet aggregation [102]. As discussed below, NO may play an important role in the shift from a Th1 to Th2 response but also releases a cytokine storm that participates in shock. Thus, NO is a positive and a negative factor in infectious disease [103], which poses a common problem that must be considered during therapeutic development of treatments for these diseases.
One of the main mechanisms as to how NO participates in viral eradication involves the nitrosation of critical cysteines within key proteins required for viral infection, transcription, and maturation stages. For example, viral proteases or even the host caspases that contain cysteines in their active site are involved in the maturation of the virus [104–107]. In coxsackievirus, two cysteine proteases that cleave viral polyproteins are sensitive to nitrosative-mediated inhibition, which prevents maturation of the virus [108, 109]. HIV-1 infectivity factor can be nitrosated. Reverse transcriptase can also be inhibited by nitrosation, and nitrosation of a low pKa cysteine residue in the protein limits NF-κB activity, resulting in inhibition of HIV-1 replication [110, 111]. HIV and herpes simplex viruses have critical zinc finger proteins that are also susceptible to nitrosation [112]. Proteins that participate in viral infectivity, such as integrases, can be nitrosated by NO/RNS. The nucleocaspid protein (also a redox-sensitive zinc finger containing Cys3His) is also susceptible to nitrosation. When nitrosated, this protein cannot bind to DNA and carry out its function as a topoisomerase [113]. The modification of these proteins prevents viral integration into the host DNA. NO increases host CD44 cell surface expression, where HIV-1 can bind and become internalized, suggesting that increasing CD44 may provide a viral uptake and clearance mechanism. However, in HIV infection, this process represents a dormant phase of the disease [114]. Thus, the nitrosative stress environment produced by iNOS may serve to protect against some viruses by inhibiting viral infectivity, replication, and maturation. Interestingly, the reduction of intracellular GSH may be critical to the removal of virus by increasing nitrosative stress.
REDOX REGULATION OF IMMUNE FUNCTION
Regulation of the redox immunomodulators—NO/RNS and ROS
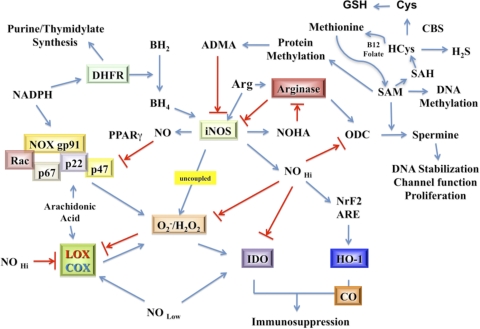
Beyond the chemical interactions of NO and ROS that combine to eradicate pathogens, these redox small molecules are effective immunomodulators that regulate cellular metabolism as well as multiple proinflammatory and repair/tissue-restoration pathways. In this section, we will examine some of the interactive intracellular pathways that regulate levels of NO/RNS and ROS that are important to the immune response. Fig. 3 provides a diagrammatic scheme of these interactive pathways. As might be expected, NOS is a nodal point with multiple inputs and outputs. Regulation of the enzymatic activity of NOS is clearly critical to the production of NO. The cellular supply of cofactors for iNOS activity, such as availability of arginine, BH4, NADPH, and superoxide, is required for NO production. However, in the absence of arginine or BH4, uncoupled NOS becomes a O2–/H2O2 generator [115, 116]. Although originally described for eNOS and nNOS, the flavin-binding sites of the reductase domain of iNOS are a source of O2– generation in the absence of arginine [117]. Therefore, metabolic pathways that control arginine and BH4 play a role in determining the NO/superoxide balance.
Figure 3. Diagrammatic view of critical interactive connections between NO and ROS-mediated metabolic pathways.
DHFR, Dihydrofolate reductase; SAH, S-adenosyl-HCys; ODC, ornithine decarboxylase.
Cellular arginine levels depend on multiple factors, including the type of uptake mechanisms found in cells that provide arginine to various intracellular pools and the enzymatic systems that use this semiessential amino acid. Selective arginine uptake occurs via the activity of cationic amino acid (y+) transporters, also known as the SLC7A family of solute transporters, although other, less-selective uptake transporters have also been described. As shown in Fig. 3, arginine is the sole substrate for NOS, but it is also the sole substrate of AG, another key enzyme involved in immunoregulation. Two isoforms of AG have been found and are located in different intracellular compartments [118, 119]. The AG1 gene is an inducible cytosolic enzyme whose product is ornithine. In turn, ornithine is the starting point for the production of polyamine synthesis (putrescine, spermidine, and spermine), which is produced via a series of enzymatic reactions that also requires SAM. Polyamines are critical molecules supporting cell function and regulating diverse cellular processes, including DNA stabilization, ion channel transport, and cell proliferation [120, 121]. By metabolizing methionine, polyamine synthesis impacts the formation of HCys, which in turn, affects the sulfonation pathway and consequently, H2S and GSH production. The second isoform, the AG2 gene, is localized to mitochondria, where the enzymatic production of ornithine appears to be directed to the formation of proline and ultimately, collagen [122]. In turn, AG is regulated by NOS and NOX activities. NOHA, a product of NOS, inhibits AG, and O2– increases AG activity [86, 123, 124]. Importantly, high AG activity is associated with elevated ROS and low NO fluxes.
As also depicted in Fig. 3, cellular redox balance can be shifted simply by altering the effect of NO on its targets. This is commonly achieved by changing the local concentration of NO. For example, NO antagonizes NOX2 assembly through the activation of PPARγ, which in turn, inhibits expression of the p47 subunit required for NOX2 activation [125]. As a result, O2– production is suppressed when NOS activity and NO are high. NO also inhibits COX2 activity, reducing production of ROS by this enzyme [126, 127]. Thus, as NO levels decline, oxidative mechanisms increase. Oxidative and nitrosative stress can also decrease intracellular GSH (reduced form) levels, resulting in a reduced antioxidant capability of the cell. However, loss of GSH leads to the induction of the NRF2/kelch-like ECH-associated protein system with induction of the ARE to increase γ-glutamylsynthase and GSH. This safety mechanism helps to increase GSH synthesis and return antioxidant levels to normal. The ARE promoter also controls HO-1 expression, the enzyme responsible for cellular production of CO, another small redox molecule that has been shown to be immunosuppressive [128–130].
Immune-associated redox pathways regulate other important metabolic cell functions that have the potential for widespread impact on cells, organs, and organisms. For example, methionine is the principle source of methyl groups for a variety of methylation reactions within cells, including those involved in epigenetic modification of DNA [131]. The abundance of methylation equivalents is controlled by the competition between the pathways using methionine. For example, production of polyamines (Fig. 3) is controlled by the “AG to ornithine to polyamine pathway”. Polyamines are critical to multiple cell functions, such as DNA stabilization, cell proliferation, and membrane channel activity, all of which are also involved in immune-mediated repair processes. Spermine production consumes methylation equivalents, which can reduce the availability of methionine for methylation of DNA and cell proteins [132]. HCys levels may also be changed and in combination with CBS and in the presence of cysteine will affect the production of GSH via the sulfonation pathway [133]. Interestingly, this same pathway is now known to generate H2S, the least-studied of the small molecule redox immunomodulators [134, 135]. There is also a direct effect of the methylation reactions on NOS. For example, ADMA, a cleavage product of methylated proteins, is a prominent endogenous inhibitor of NOS and reduces NO levels [119].
NO levels dictate the immune signaling pathway
The ability to change the antagonistic relationship between NO and ROS plays a key role in the orchestration of inflammation and tissue restoration. In this section, we will examine how NO/RNS and ROS actively control innate and adaptive immune signaling. This aspect of redox function has been studied most intensively in cells of myeloid lineage, such as monocytes, macrophages, and neutrophils, where the NO/RNS and ROS that is produced by these cells participate in induction, maintenance, and/or termination of proinflammatory and anti-inflammatory signaling. Similar to the effect of NO on pathogen eradication, the level of NO and more precisely, the temporal and spatial concentration profiles of NO are key factors in determining immune-mediated processes.
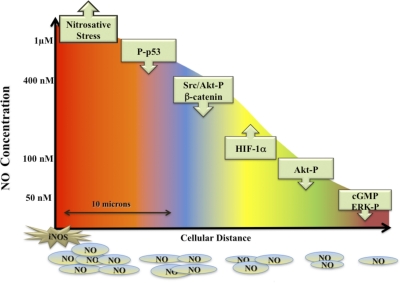
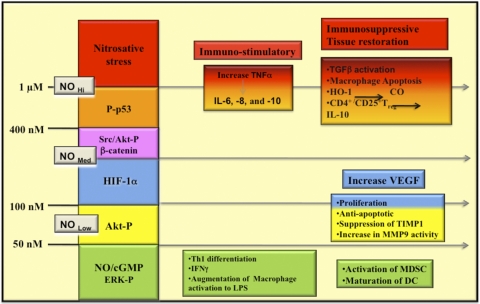
The development and use of reliable NO donors have allowed a careful and quantitative analysis of the relationship between NO/RNS concentration and the regulation of specific immune signaling pathways. Brune and coworkers [136–138] were among the first to show a relationship between increasing steady-state levels of NO and the expression of p53 in murine and human macrophage cell lines. In this case, p53 expression was associated with concentrations of NO that led to apoptosis in the macrophage. Further studies, however, have subsequently identified NO concentration profiles directly related to the expression of other key signaling proteins such as HIF-1α and Akt-P [28, 139, 140]. Stimulation of MCF-7 breast cancer cells, macrophages, or endothelial cells with NO at 10–60 nM increased ERK-P and Akt-P expression and activity, and increased HIF-1α expression was found at levels of approximately 100 nM NO [141, 142]. Fig. 4 provides a synopsis of the biological action of NO over a wide concentration range of NO and shows how this concentration-dependent profile varies with distance from the NO source. NO is highly diffusible, and its concentration will change rapidly, as it is diluted as a result of diffusion. Thus, although concentrations at the point source may reach μM levels, within one cell length (10 μ), this concentration will be reduced up to 1000-fold [143]. Time-specific changes are also observed in NO signaling. For example, NO-mediated ERK-P levels initially increased rapidly on exposure to NO donors and then decreased with continued NO exposure [141]. In contrast, HIF-1α levels remained high as long as NO levels were elevated. These data strongly suggest that the duration of the signaling response after initiation by NO may be an important regulatory factor. The transient nature of some NO signaling leads to the idea that similar to intracellular calcium signaling, NO “waves” are formed and can move through the cell. Importantly, if NOX or other ROS-generating systems are also activated, NO bioavailability changes as a result of its scavenging by superoxide, and thus, the groups of signaling pathways regulated by NO are subsequently altered [144, 145].
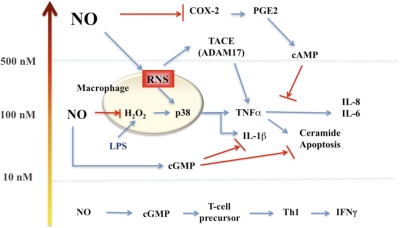
Figure 4. The effect of steady-state flux of NO on signal transduction mechanisms.
This diagram represents the level of sustained NO that is required to activate specific pathways in tumor cells. Similar effects have been seen on endothelial cells. These data were generated by treating tumor or endothelial cells with the NO donor DETANO (NOC-18) for 24 h and then measuring the appropriate outcome measures (for example, p53 activation). Various concentrations of DETANO that correspond to cellular levels of NO are: 40–60 μM DETANO = 50 nM NO; 80–120 μM DETANO = 100 nM NO; 500 μM DETANO = 400 nM NO; and 1 mM DETANO = 1 μM NO. The diagram represents the effect of diffusion of NO with distance from the point source (an activated murine macrophage producing iNOS) in vitro (Petri dish) generating 1 μM NO or more. Thus, reactants or cells located at a specific distance from the point source (i.e., iNOS, represented by star) would be exposed to a level of NO that governs a specific subset of physiological or pathophysiological reactions. The x-axis represents the different zone of NO-mediated events that is experienced at a specific distance from a source iNOS producing >1 μM. Note: Akt activation is regulated by NO at two different sites and by two different concentration levels of NO.
Donors have also been used to show how NO affects T cell function. Treatment of T cells with low doses of NO produced by 10 μM DETANO led to increased cGMP and differentiation of Th1 cells in mice and humans [146]. These cells produce IFN-γ and appear to increase IL-12R selectively. In contrast, CD4+ CD25– Treg cells treated with NO generated by 100 to 200 μM DETANO (200–400 nM steady-state NO for 24 h [147]) mediated a cGMP-independent but p53-dependent increase in proliferation and conversion of CD4+ CD25– to CD4+/CD25+ Treg cells (called NO-Treg by the authors). These new NO-Treg cells represent an additional population of immunosuppressive Treg cells that do not express FKHD box P3 or TGF-β but instead, produce IL-10. The regulation of NO by this pathway is mediated by a p53-IL-2 pathway and acts to bypass IL-10-mediated conversion of CD4+/CD25– to CD4+/CD25+ Treg. This response is likely to occur in localized regions such as within lymphoid tissue, tumor microenvironment, or at sites of injury where NO production from immune and adjunct immune cells (particularly in humans) may occur. Importantly, the effect of high levels of NO in this setting serves to limit the M1/Th1 response.
Species-specific NO production
Much of the data that described the relationship between NO and immune signaling was derived from tumor cell lines or rodent macrophages, which are readily available sources of NO. However, in humans, although macrophages express NOS2 mRNA and iNOS protein, the levels of expression and the immune induction required to elicit expression are significantly different from rodents [148]. iNOS protein and NO production levels are remarkably lower, and induction is dependent on synergistic combinations of immune signals in human macrophagic cells. This species difference is most likely a function of the human iNOS promoter [149–152] and not the activity of iNOS itself [153]. To illustrate this point, Fig. 5 provides a comparison map of the iNOS promoter region from the human and mouse NOS2 genes. There is a significant mismatch between these promoters that is likely to account for the notable differences in the regulation of gene induction between mice and humans. In rodents and humans, NOS2 mRNA expression is not restricted to macrophagic cells, and iNOS can be found in a number of cell types, including epithelial cells [154, 155], astrocytes [156], endothelial cells [157, 158], as well as other cell types [159]. Again, multiple differences in the induction of human NOS2 compared with mouse NOS2 are observed in these adjunct immune cells. Interestingly, human hepatocytes are known to express iNOS abundantly [20, 160–165] under normal conditions, and human neurons (not commonly associated with immune responses) ectopically express iNOS in chronic neurodegenerative diseases such as Alzheimer's disease [166].
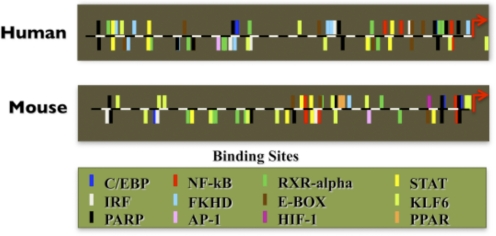
Figure 5. Comparison of the promoter region of NOS2 between human and murine.
Promoters′ information was obtained from the Genomatix Software Suite. Each bar represents a particular transcription factor binding site. Upward bars represent the bind site located on the sense strand of the DNA, and downward bars represent the binding sites located on the nonsense strand. IRF, IFN regulatory factor; PARP, poly(ADP-ribose) polymerase; RXR-α, retinoid X receptor α; E-BOX, E-box binding factors, contains binding domains bHLH-ZIP and bZIP; KLF6, Kruppel-like factor 6.
Species and tissue-specific differences are also found for mechanisms that promote ROS production and consequently, may affect NO bioavailability and the NO/RNS-ROS balance. For example, rat macrophages express the NOX2-oxidative burst system and when stimulated with phorbol esters, rapidly produce abundant O2–. The rate of release, however, is approximately tenfold slower than the rate displayed by activated human neutrophils [167]. Part of the observed difference in O2– production is likely a result of differences in cellular location of the NOX proteins. Higher levels of ROS production by immune cells including microglia have also been noted in humans compared with rodents [168, 169].
The combined data on rodent versus human NO and O2– production strongly suggest that in general, ROS production is a predominant feature of activated human macrophages, neutrophils, and monocytes, and the equivalent murine immune cells generate a combination of O2– and NO and in some cases, favor NO production. As the balance in the levels and rates of production of NO and O2– dictates oxidative versus nitrosative stress, these differences may be crucial to understanding how immune responses are regulated in a species-specific manner. Overall, the wide varieties of adjunct-immune cells plus tissue-specific requirements for varying levels of immune protection provide plasticity to the immune response. This is particularly useful, as pathogen challenges change constantly.
The impact of NO signaling on an innate immune response—classical activation
Induction of the proinflammatory phase of an innate immune response is one of the initial, functional outcomes of the immune activation process and has been defined as “classical activation”. Classically activated macrophages (also called M1 macrophages) have been well-described and are associated with the production and release of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β, of proteases (e.g., MMP-9), and of NO/RNS and O2–/ROS. In addition to a role in pathogen eradication, the early increase in ROS, followed by increasing production of NO, results in a rapidly changing NO/RNS profile within the immune cell. As discussed above, the localized level of NO/RNS will dictate the type of reactive pathway profile and signaling during this phase of the response. However, as this process continues over time, secondary factors that influence the levels of NO and ROS, as also described above, will further modify the choice of pathways that is used. In the following sections, we will address some of the pathways that may participate in the integrated signaling, with emphasis on NO/RNS-mediated regulation of cross-talk between the proinflammatory and tissue-resolution pathways (Figs. 6 and 7).
Figure 6. Mechanisms of NO that activate a Th1 phenotypic response.
For description of regulated pathways, please see text.
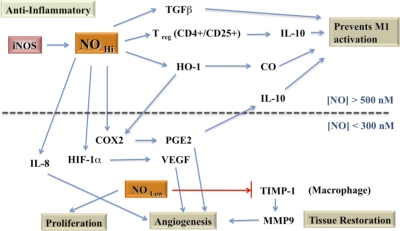
Figure 7. Mechanisms of NO in immunosuppression and tissue restoration.
For description of regulated pathways, please see text.
NO and proinflammatory genes
Microarrays have been used to characterize the types of immune genes whose regulation is modified by NO. Using human macrophage cell lines treated with NO donors for varied periods of time, ranging from 3 to 24 h, Turpaev et al. [170–172] have identified specific categories of NO-sensitive genes. These include genes encoding transcription factors (e.g., c-Fos, c-Jun, c-Maf), regulators of cell cycle (e.g., cyclin A2, p21/Cip1), mitochondrial oxidative phosphorylation (ATPase 8 and Nd4), and many other cell pathway regulators, including HO-1 and H-ferritin. Cytokine-related genes are also modified and are up-regulated as in the case of TNF-α, IL-8, and MIP-1α or down-regulated as TRAF-1-associated factor and SLC7A6 (LAT2). In some cases, however, it was not NO but rather RNS that was the critical regulatory molecule. By using a NO trapping agent, 2-phenyl-4,4,5,5-tetramethylimidazole-1-oxyl-3-oxide, which generates NO2 and ultimately N2O3, Turpaev et al. [172] showed that nitrosation chemistry is a primary driving force for expression of some proinflammatory genes. TNF-α expression is particularly increased under these conditions and to superactivated levels [172]. Activation of p38 MAPK pathways was also shown to be driven primarily by NO and RNS during classical activation. Gene regulation by nitrosative chemistry leads to additional levels of complexity. For example, a zinc thiolate ligand of the sheddase TACE (ADAM17) activates TNF-α in human monocytes, thereby increasing NF-κB and ultimately increasing expression of cytokines such as IL-8 and IL-6 [173]. Although the interconnection between IL-8 induction pathways may not be specific to nitrosation, it is interesting to speculate whether the expression of IL-8 can be used as a “surrogate” marker of nitrosative stress in diseases where high levels of NO can be generated.
For many of the immune pathways that are regulated by NO, ROS, PGs, and the other species represented in Fig. 1, NF-κB is critical in orchestrating the innate immune response outcomes [174]. In unstimulated cells, NF-κB subunits are sequestered into the cytoplasm by inhibitory protein complexes. Multiple stimuli (e.g., LPS or TNF-α) initiate a phosphorylation cascade that causes the inhibitory proteins to be degraded proteasomally, allowing NF-κB to enter the nucleus where its modulation of gene induction dictates, in part, the pattern of immune activity. NF-κB activity is also regulated by redox-related mechanisms, particularly as NF-κB activation is biochemically regulated by intracellular thiol status [175]. ROS and RNS species have been shown to alter NF-κB signaling. For example, a short burst of NO augments the activation of NF-κB in LPS or TNF-α-stimulated cells [176, 177], and other studies show that longer duration of NO exposure led to inhibition and served as a negative-feedback signal [178–180]. Other observations with ROS show that low levels of H2O2 can similarly enhance NF-κB activity [181, 182], and higher H2O2 (>100 μM) inhibited its activity [178–180]. These redox-mediated inhibitory reactions involve critical cysteine thiolates necessary for DNA binding [183–185]. Also, oxidation of methionine-45 prevents IkBα degradation and subsequent nuclear translocation of NF-κB [186]. Excessive oxidative stress by RNS or ROS has been shown to inhibit NF-κB activity by modulating the critical cysteine thiols of NF-κB subunits [187]. Additionally, NF-κB is regulated partially by redox-sensitive phosphatases and kinases, suggesting that redox regulation of NF-κB may be multifactorial [188]. The overall effect of NO and ROS on NF-κB activity follows a biphasic pattern, where low NO/ROS levels increase NF-κB activity, but higher NO/ROS levels inhibit NF-κB-mediated gene transcription [189]. It should be noted that many of these observations are cell- and mechanism-dependent.
NO and regulation of anti-inflammatory pathways
Proinflammatory, classical activation is followed rapidly in time by an anti-inflammatory repair phase that ideally leads to wound-healing and tissue restoration, which is ultimately the desired outcome of a successful innate immune response. These time-delayed phases of the innate immune response are initiated by the original pathogens or injury but are rapidly re-enforced by the actions of anti-inflammatory cytokines released from macrophages and microglia that act in an autocrine manner. Anti-inflammatory cytokines also arise from accessory immune cells such as astrocytes or from Th2 Treg [190, 191]. The four major anti-inflammatory cytokines involved in tissue repair and wound-healing are IL-4, IL-13, IL-10, and TGF-β [191–193]. These cytokines initiate down-regulation of the proinflammatory phase and induction of tissue reconstruction and repair. NO/RNS and ROS play critical roles in regulating these pathways. Much like a traffic cop, the level of NO/RNS redirects signaling by reducing one pathway and enhancing another (Fig. 6).
An interesting example of this flexible, level-dependent regulation is found in the interaction between NO and eicosanoids. Macrophage activation by LPS or other immune stimuli leads to increased iNOS activity and NO, which suppresses COX2 enzymatic activity [194] and supports the long-term expression of COX2 mRNA [195]. However, as the activity of iNOS decreases, there is a marked increase in PGE2 by COX2 stimulation that leads to increased cAMP-mediated processes. As NO is a direct activator of soluble guanylyl cyclase and increased cGMP and as cGMP- and cAMP-dependent pathways often regulate cell processes in an antagonistic manner, the increased cAMP-based process may reflect initiation of competing pathways. This type of competition is observed in LPS-activated monocytes, where cGMP inhibits IL-1β expression, and PGE2/cAMP inhibits TNF-α [196, 197] (Fig. 6). Other studies examining phosphodiesterase inhibitors have shown that cAMP and cGMP play a key role in regulating IL-1β and TNF-α [198]. This implies that under conditions of high NO output, increased TNF-α is favored. However, when NO levels decline (such as scavenging by ROS), then COX2/PGE2 and cAMP levels increase, which may favor IL-1β production and activity. Another important aspect is that PGE2/cAMP mediates increased levels of AG, IL-10, and IL-1R antagonist and suppresses IL-12, whereas cGMP does not [199, 200]. These observations suggest that with respect to NO and ROS balance, modulation of cGMP and cAMP plays a role in polarization of the immune system.
During transition from proinflammatory to resolution phase, numerous pathways are used to down-regulate the initial killing/proinflammatory signaling mechanisms to homeostatic levels. These feedback signals include switching from cGMP- to cAMP-mediated pathways within the macrophage, as we described above, and others include the induction of decoy adaptor proteins [201] or selective ″suppressor″ proteins, such as suppressor of cytokine signaling [202]. Additionally, surface cytokine receptors modulate autocrine feedback loops, which when activated, impact NO/RNS- or ROS-mediated regulation on several levels. For example, production of O2– is linked to TNF-αR1 signaling on the macrophage plasma membrane. Kronke and colleagues [203] have recently shown that TNF-αR1 is directly coupled to a NOX by the binding of riboflavin kinase to the receptor and the p22phox subunit in the membrane. This kinase is specifically required for TNF-α-mediated ROS production but is not a component of LPS-stimulated ROS. As mentioned earlier, tethering the oxidase to specific locations helps to focus the actions of the ROS that are produced.
When high levels of NO are produced simultaneously, not only can the NO act as a local antioxidant to protect membranes against ROS, but NO can also react with the superoxide produced by TNF-αR1 activation to generate nitrite, thus reducing NO levels. This mechanism may be an important protection against TNF-α-mediated cell death and clearly demonstrates the functional significance of the NO/ROS balance. NO/RNS-mediated induction of HO-1 or of H-ferritin is an additional cellular mechanism that will further limit TNF-α-induced ROS within cells [204]. HO-1 is a microsomal enzyme that cleaves heme-containing proteins to produce the antioxidants, biliverdin and CO [205]. Part of the enzymatic action, however, is to increase cytosolic-free iron levels and consequently, H-ferritin levels [206]. H-ferritin further contributes to the antioxidant protection of the cell by mediating activation and translocation to the nucleus of the transcription factor, NRF2 [207]. Once in the nucleus, NRF2 binds to regulatory promoter elements including the ARE, which is responsible for induction of antioxidant protection mechanisms [208–210]. HO-1 further shifts the macrophage-immune function to the repair phase by increasing anti-inflammatory cytokine production, which in turn, increases HO-1 [211]. The synergistic action of HO-1 helps to re-enforce the suppression of proinflammatory factors such as iNOS, MPO, and COX by degrading these heme-containing enzymes. The lack of heme further prevents the re-insertion of heme into these critical immune enzymes, thereby blocking their function [212]. Low levels of iNOS, COX2, and MPO are associated with immune-resolution phases. In addition, CO reduces the function of TLR p38 MAPK signaling [130, 213]. Finally, H-ferritin has been shown to prevent JNK activation and TNF-α-mediated cell death by sequestration of iron [214]. Thus, HO-1 and CO-mediated pathways can play an important part in the protection of tissue as well as ameliorating inflammation.
The shift from NO/RNS to ROS-mediated regulation during the transition from classical activation to alternative activation involves other unique mechanisms and provides additional opportunities to understand the dual nature of these responses. Many immune receptors, including those that are activated by anti-inflammatory cytokines such as IL-4 are associated with JAK. This association requires JAK-mediated transphosphorylation for activation of the signaling pathways. Upon binding to the receptor, multiple tyrosine residues are phosphorylated and in turn, activate STAT6 or other downstream pathways. Ultimately, genes involved in tissue repair and restoration such as Ym-1 (also known as chitinase 3-like 1) or AG1 are induced, and their corresponding proteins are expressed. Part of the JAK-mediated activation process involves production of O2– by NOX5 and/or NOX1 via an insulin receptor substrate/PI3K pathway [215]. One of the targets of the ROS generated in this activation process is PTP1B. This phosphatase dephosphorylates the IL-4R and hence, is responsible for its inactivation. Other PTPs such as SHP-1 or CD45 are also critical to limiting signaling initiated by JAK-based activation [216] and are part of a general immune signaling mechanism. Interestingly, ROS play a critical role in prolonging the action of IL-4. PTPs contain cysteinyl residues in their catalytic site, which when oxidized by ROS, results in inactivation of the phosphatase [217, 218]. Consequently, kinase activity is extended, and IL-4 signaling is enhanced. The overall result is maintained suppression of proinflammatory genes and up-regulation of alternative activation genes, which favor repair and restoration of the tissue. RNS have opposing roles when compared with ROS. Nitrosation of PTPs prevents irreversible oxidation, thus allowing the phosphatase to continue to function in a normal manner [219, 220]. Interestingly, Leishmania survival within macrophages is dependent on manipulation of PTP activity; in this case, the PTP is SHP-1. Blanchette and coworkers [221] propose that continuous activation of SHP-1 leads to down-regulation of iNOS induction and decreased NO production, resulting in reduced toxicity to the pathogen. A similar effect is observed in virally infected glia cells, where NO levels are reduced by altering SHP-1 [222].
NO impact on adaptive immunity—immunosuppression and tissue-restoration response
NO is also involved in immunosuppression by regulating circulating immune cells. For example, MDSCs can be activated by NO-mediated increases in cGMP, which in turn, facilitates their binding to CTLs and reduces T cell proliferation [223]. When cell-to-cell contacts are formed, expression of AG and iNOS is required to induce apoptosis [224]. Increased iNOS activity is also found in mature DCs, where NO is associated with suppression of T cell proliferation. Furthermore, when activated by IFN-γ, TNF-α, or IL-1α/β, MSCs produce chemokines and iNOS, which leads to the immunosuppression of those T cells in the vicinity of the MSCs [225]. The resulting increase in chemokines and iNOS leads to the attenuation of T cell responsiveness. In general, T cell responses are decreased by NO. As shown in Fig. 7, the immune regulation in this case includes T cell populations with distinct immunosuppressive mechanisms (CD4+/CD25+) through the induction of HO-1 and subsequently, CO through IL-10 production and through increased activation of latent-to-active TGF-β via nitrosation [223, 226]. In this manner, NO can be viewed as switching Th1 to Th2 responses. In humans, where NOS2 activity and NO levels are low, a different story has emerged. Rather than NO as the primary factor, the tryptophan-degrading enzyme, IDO, plays the primary immunosuppressive role. Ren et al. [227] have shown that MSCs express high levels of IDO when stimulated with IFN-γ and that inhibitors of IDO blocked the MSC-mediated reduction in T cell activity. Similar IDO-mediated immunosuppression has been found for other human immune cells [228–230].
It is interesting to note that AG also plays a role in T cell regulation. An elegant study by Pesce et al. [231] recently demonstrated a NO-independent role for AG1 in alternatively activated macrophages in mice chronically infected with the parasite trematode, Schistosoma mansoni. Although AG expression in macrophages is prominently associated with Th2 responses in infected tissue and acts to promote repair processes, including fibrosis, Pesce and colleagues [231] demonstrated that part of its function is to sequester arginine away from CD4+ effector T cells within the same tissue. By competing for arginine, AG activity suppressed T cell proliferation by depleting the available levels of arginine, In this case, however, the local effector T cell population was associated with IL-4 and IL-13 production and Th2-mediated events. Thus, suppression resulted in a reduced Th2 response. When arginine was added back, the inhibition of T cell proliferation was removed, and the “brakes” on an overactive Th2 response were reapplied. Interestingly, genetic deletion of AG1 resulted in decreased survival as a result of increased liver fibrosis and portal hypertension in Schistosoma-infected liver, not proinflammatory cytokine activity, and shows unequivocally that Th2 responses can be toxic.
Overall, low NO may favor Th2, and high NO may augment Th1 responses and suggests that a balance between NO and ROS may help to determine immune polarity. During the immune repair/restoration phase, the collective activity of IL-4, IL-13, IL-10, and TGF-β helps to suppress iNOS expression and NO, shifting in favor of ROS [232, 233]. In humans, the relatively higher production of ROS and the lower production of NO, although not absolutely required for IDO action [230], clearly play a role in promoting IDO-mediated immunosuppression.
NO and revascularization
Besides regulating immune signaling pathways involved in M1 and M2 states, NO/RNS and ROS participate in tissue restoration mediated by macrophages by helping to orchestrate the clean-up of debris, the restructuring of ECM and cell proliferation, and the formation of new blood vessels [191, 234]. For example, macrophage NO production contributes to angiogenesis by increasing VEGF, a key growth factor for new blood vessels. This is accomplished through two principle immune pathways: induction and stabilization of the transcription factor, HIF-1α, and formation of PGE2. HIF-1α is regulated by NO (and in some tissues, by PGE2), cytokines including IL-1β and TNF-α, as well as growth factors. HIF-1α serves as a cellular hypoxia sensor and acts to induce genes that promote vasodilation, vascular permeability, glucose uptake, and angiogenesis [235, 235–237]. Each of these features is required to revascularize an injured tissue during immune repair. Modulation of NO levels is critical during regulation of these tissue-restoration activities. For example, binding of VEGF to endothelial cells induces eNOS serine-1179 phosphorylation, culminating in cellular proliferation [238]. MMPs, such as MMP-9, are also activated by NO (at a 50- to 500-nM range), where the increased proteolytic activity is required for restructuring the surrounding matrix for neovascularization [239]. This level of NO is associated with proliferation and antiapoptosis though activation of Akt and ERK and appears to be critical in the tissue-restoration phase [28].
Acute versus chronic inflammatory disease
NO and redox molecules are critical factors during the progression of an immune response from inflammation and immunosuppression to tissue-restoration phases in vivo (Fig. 8). Importantly, redox molecules mediate different inflammatory outcomes, depending on the specific type (e.g., chronic or acute). Thus, the effects of NO/RNS and ROS are context-dependent. This concept is particularly relevant in regard to the NO generated from iNOS and eNOS isoforms. Here, we will briefly compare and contrast some specific roles of these NOS isoforms during acute and chronic inflammation to illustrate the temporal and concentration dependence of NO with respect to outcome.
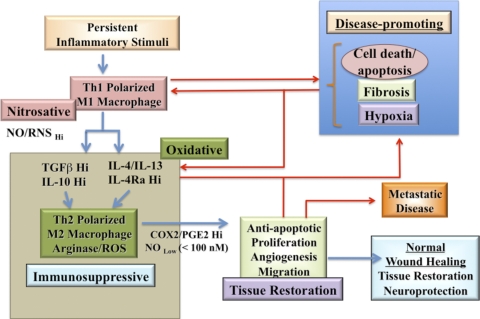
Figure 8. A flow diagram of NO in inflammation and tissue-restoration response.
Blue lines represent the linear progression from initial insult to tissue restoration. A fatal loop is found in diseases such as cancer, where the processes are restimulated, and leads to chronic conditions or a “wound that never heals”. The lines in red show pathways that contribute to perpetuation of chronic disease and failure to resolve tissue-restoration processes correctly.
During acute inflammation, eNOS and iNOS mediate different effects depending on the type of injury. In experimental arthritis models involving streptococcal cell wall injections, eNOS was found to be deleterious, and iNOS activity was protective [240–242] and suggests that striking differences in the actions of these NOS isoforms can be found under specific conditions. Similarly, Chen et al. [243] demonstrated iNOS-mediated protection against hepatic apoptotic cell death seen in models of sepsis and hepatitis. Both models show bacterial product activation of TLR, which leads to macrophage and hepatocyte activation. However, as shown in eNOS knockout mice, the cytotoxic action of eNOS most likely results from its ability to up-regulate iNOS and other proinflammatory factors via NF-κB [244]. In contrast, elevated iNOS expression mediated by NPR-A (a.k.a., NPR1) caused vasodilation and vascular dysfunction, which are associated with a variety of complications in a model of endotoxic shock [245].
Similarly, contrasting effects of NOS isoforms occur during IR injury, where eNOS is protective, but iNOS is detrimental. In most IR models, eNOS has a positive effect, and iNOS is associated with toxicity. In part, this is because reperfusion injury focuses on the endothelium. In this context, low NO flux generated by eNOS prevents leukocyte recruitment and associated tissue damage through scavenging of ROS in the early phase [246]. However, when iNOS expression increases, NO levels rise and induce tissue injury, especially to the endothelial cells. NO potentiates the hepatic oxidative injury in warm IR [243]. In IR injury, preservation of the endothelium is critical for damage control. Thus, the beneficial effects of specific NOS isoforms depend on the type of primary damaging event. During sepsis or infection, the key initial events involve monocyte infiltration and concomitant elevated levels of NO. In contrast, IR injury occurs at the endothelium, where low flux NO protects against ROS-mediated endothelial damage. Elevated levels of NO generated from iNOS are deleterious when expressed in or near vascular beds. A comparison of these acute models of inflammation suggests that the location and temporal factors determine the outcome of eNOS and iNOS expression. When associated with immune cells, eNOS is detrimental, and iNOS is beneficial. However, when eNOS and iNOS are associated with vascular beds, the reverse is true, and eNOS mediates a positive outcome, and iNOS is detrimental. It should be noted that iNOS transfection does prevent hyperplasia of the vascular smooth muscle cell in restenosis [247, 248]. Thus, careful evaluation of the context dependence of NOS isoforms should be considered when assessing outcome.
Similar NO dichotomies occur in other inflammatory diseases. For example, in cancer, iNOS has positive and negative effects on prognosis [249]. As discussed in Introduction, it was shown that NO is involved in antitumorigenic processes, especially with respect to leukemias [250]. Mouse models show that iNOS and p53 knockout mice are prone to increased leukemias, primarily because of the role of NO during p53-mediated apoptosis. Studies suggest that iNOS expression in the liver can mediate apoptosis of circulating cancer cells, thus reducing metastatic burden. However, many cancers have p53 mutations. In this context, NO potentiates the oncogenic behavior of mutated p53. Many solid tumors have shown that iNOS within the tumor leads to poor patient prognosis [249]. In animal models, iNOS correlated with increased aggressiveness of colon cancers [251]. As iNOS produces different levels of NO, specific levels of NO generated by iNOS can activate HIF-1α, Akt, and ERK pathways, which then lead to increased proliferation, antiapoptosis, and metastatic mechanisms such as epithelial mesenchymal transition [249]. This would lead to increased proliferation, dissemination, and a higher-grade tumor. In addition, NO would lead to depression of lymphocytes with increased maturation of MDSCs and DCs as well as conversion of Treg to CD4+/CD25+ [251]. Taken together, these observations suggest that immunosuppression, consistent with the immune state that is present during wound-healing, serves to promote a more aggressive tumor phenotype.
CONCLUSIONS
The multifaceted role(s) of redox biology demonstrate complex and yet defined functions for reactive species in the immune response (Fig. 9). As immunotoxins, the varieties of RNS and ROS intermediates serve as arsenals that fight different pathogens. In addition, the development of resistance to these reactive intermediates suggests a continuous evolution of pathogen and host responses. The difference between murine models and humans with respect to regulation of these molecules indicates that adaptation to different biological stressors may have provided increased versatility in the overall scheme to fight pathogens and tumors. Regulation of immunity suggests that concentration, timing, and location of redox regulators determine outcome. The participation of NO and redox-related molecules during inflammation and tissue-restoration processes indicates the importance of redox-active molecules in coordinating these events. The identification of context-dependent outcome, with respect to redox intermediates, suggests that “the devil is in the details”, and within such details are new ways to look at disease and new therapeutic opportunities.
Figure 9. A summary of levels of NO required to elicit different mechanisms of the inflammation and tissue-restoration response.
Footnotes
- ADAM17
- a disintegrin and metalloproteinase domain 17
- ADMA
- asymmetrical dimethylarginine
- AG
- arginase
- Akt-/ERK-P
- Akt-/ERK-phosphorylation
- ARE
- antioxidant response element
- BH4
- tetrahydrobiopterin
- CBS
- cystathione β-synthase
- CO
- carbon monoxide
- CO2
- carbon dioxide
- DETANO
- diethylenetriamine-NO
- DUOX 1/2
- dual oxidase 1/2
- eNOS
- endothelial NOS
- FA
- fatty acids
- FKHD
- forkhead
- H2O2
- hydrogen peroxide
- H2S
- hydrogen sulfide
- HCys
- homocysteine
- HIF-1α
- hypoxia-inducible factor 1-α
- IR
- ischemia reperfusion
- LPG
- lipophosphoglycan
- MDSC
- myeloid-derived suppressor cells
- MMP-9
- matrix metalloprotease 9
- MSC
- mesenchymal stem cell
- N2O3
- nitrogen trioxide
- NO2
- nitrogen dioxide
- NO2–
- nitrite
- NOHA
- N-hydroxyarginine
- nNOS
- neuronal NOS
- NOX1/2/3/4/5
- NADPH oxidase 1/2/3/4/5
- NPR
- natriuretic peptide receptor
- NRF2
- nuclear factor (erythroid-derived 2)-like 2
- O2–
- superoxide anion
- ONOO–
- peroxynitrite
- PPARγ
- peroxisome proliferator-activated receptor γ
- PTP
- protein tyrosine phosphatase
- RNS
- reactive NOS
- SAG
- sodium antimony gluconate
- SAM
- s-adenosyl-methionine
- SHP-1
- Src homology 2-containing protein tyrosine phosphatase, nonreceptor type 6
- Treg
- T regulatory cell
REFERENCES
- 1. Colton C., Gilbert D. (1999) Reactive Oxygen Species in Biological Systems, New York, NY, USA, Springer [Google Scholar]
- 2. Halliwell B., Gutteridge J. M. C. (1999) Free Radicals in Biology and Medicine, Oxford, UK, Oxford University Press [Google Scholar]
- 3. Forman H. J., Fukuto J. M., Torres M. (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 287, C246–C256 [DOI] [PubMed] [Google Scholar]
- 4. Forman H. J., Torres M., Fukuto J. (2003) Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles, Dordrecjt, The Netherlands; Boston, MA, USA, Kluwer Academic Publishers [Google Scholar]
- 5. Forman H. J., Torres M., Fukuto J. (2002) Redox signaling. Mol. Cell. Biochem. 234-235, 49–62 [PubMed] [Google Scholar]
- 6. Schwentker A., Billiar T. R. (2003) Nitric oxide and wound repair. Surg. Clin. North Am. 83, 521–530 [DOI] [PubMed] [Google Scholar]
- 7. Schwentker A., Vodovotz Y., Weller R., Billiar T. R. (2002) Nitric oxide and wound repair: role of cytokines? Nitric Oxide 7, 1–10 [DOI] [PubMed] [Google Scholar]
- 8. Leffler C. W., Parfenova H., Jaggar J. H., Wang R. (2006) Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J. Appl. Physiol. 100, 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakao A., Sugimoto R., Billiar T. R., McCurry K. R. (2009) Therapeutic antioxidant medical gas. J. Clin. Biochem. Nutr. 44, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ignarro L. J. (1996) Physiology and pathophysiology of nitric oxide. Kidney Int. Suppl. 55, S2–S5 [PubMed] [Google Scholar]
- 11. Moncada S., Palmer R. M. J., Higgs E. A. (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43, 109–142 [PubMed] [Google Scholar]
- 12. Murad F. (1994) The nitric oxide-cyclic GMP signal transduction system for intracellular and intercellular communication. Recent Prog. Horm. Res. 49, 239–248 [DOI] [PubMed] [Google Scholar]
- 13. Hibbs J. B., Taintor R. R., Vavrin Z., Rachlin E. M. (1988) Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157, 87–94 [DOI] [PubMed] [Google Scholar]
- 14. Hibbs J. B. J., Taintor R. R., Vavrin Z. (1987) Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235, 473–476 [DOI] [PubMed] [Google Scholar]
- 15. Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. (1981) Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 78, 7764–7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leaf C. D., Wishnok J. S., Tannenbaum S. R. (1989) Mechanisms of endogenous nitrosation. Cancer Surv. 8, 323–334 [PubMed] [Google Scholar]
- 17. Tannenbaum S. R. (1987) Endogenous formation of N-nitroso compounds: a current perspective. IARC Sci. Publ. 84, 292–296 [PubMed] [Google Scholar]
- 18. Hibbs J. B. J., Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P., et al. (1992) Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J. Clin. Invest. 89, 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yim C. Y., McGregor J. R., Kwon O. D., Bastian N. R., Rees M., Mori M., Hibbs J. B. J., Samlowski W. E. (1995) Nitric oxide synthesis contributes to IL-2-induced antitumor responses against intraperitoneal Meth A tumor. J. Immunol. 155, 4382–4390 [PubMed] [Google Scholar]
- 20. Stuehr D. J., Marletta M. A. (1985) Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA 82, 7738–7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marletta M. A. (1988) Mammalian synthesis of nitrite, nitrate, nitric oxide and N-nitrosating agents. Chem. Res. Toxicol. 1, 249–257 [DOI] [PubMed] [Google Scholar]
- 22. Kosaka H., Wishnok J. S., Miwa M., Leaf C. D., Tannenbaum S. R. (1989) Nitrosation by stimulated macrophages. Inhibitors, enhancers and substrates. Carcinogenesis 10, 563–566 [DOI] [PubMed] [Google Scholar]
- 23. Miwa M., Stuehr D. J., Marletta M. A., Wishnok J. S., Tannenbaum S. R. (1987) Nitrosation of amines by stimulated macrophages. Carcinogenesis 8, 955–958 [DOI] [PubMed] [Google Scholar]
- 24. Stuehr D. J., Nathan C. F. (1989) A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169, 1543–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lancaster J. R. J., Hibbs J. B. J. (1990) EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc. Natl. Acad. Sci. USA 87, 1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Griffith O. W., Stuehr D. J. (1995) Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol. 57, 707–736 [DOI] [PubMed] [Google Scholar]
- 27. Fulton D., Gratton J. P., Sessa W. C. (2001) Post-translational control of endothelial nitric oxide synthase: why isn′t calcium/calmodulin enough? J. Pharmacol. Exp. Ther. 299, 818–824 [PubMed] [Google Scholar]
- 28. Thomas D. D., Ridnour L. A., Isenberg J. S., Flores-Santana W., Switzer C. H., Donzelli S., Hussain P., Vecoli C., Paolocci N., Ambs S., Colton C. A., Harris C. C., Roberts D. D., Wink D. A. (2008) The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 45, 18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Espey M. G., Miranda K. M., Pluta R. M., Wink D. A. (2000) Nitrosative capacity of macrophages is dependent on nitric-oxide synthase induction signals. J. Biol. Chem. 275, 11341–11347 [DOI] [PubMed] [Google Scholar]
- 30. Boucher J. L., Custot J., Vadon S., Delaforge M., Lepoivre M., Tenu J. P., Yapo A., Mansuy D. (1994) N ω-hydroxyl-L-arginine, an intermediate in the L-arginine to nitric oxide pathway, is a strong inhibitor of liver and macrophage arginase. Biochem. Biophys. Res. Commun. 203, 1614–1621 [DOI] [PubMed] [Google Scholar]
- 31. Ushio-Fukai M. (2006) Localizing NADPH oxidase-derived ROS. Sci. STKE 2006, re8 [DOI] [PubMed] [Google Scholar]
- 32. Pervaiz S., Taneja R., Ghaffari S. (2009) Oxidative stress regulation of stem and progenitor cells. Antioxid. Redox Signal. 11, 2777–2789 [DOI] [PubMed] [Google Scholar]
- 33. Babior B. M., Peters W. A. (1981) The O2-producing enzyme of human neutrophils. Further properties. J. Biol. Chem. 256, 2321–2323 [PubMed] [Google Scholar]
- 34. Lambeth J. D. (2007) Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic. Biol. Med. 43, 332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Decoursey T. E., Ligeti E. (2005) Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 62, 2173–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leto T. L., Morand S., Hurt D., Ueyama T. (2009) Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox Signal. 11, 2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chamulitrat W., Stremmel W., Kawahara T., Rokutan K., Fujii H., Wingler K., Schmidt H. H., Schmidt R. (2004) A constitutive NADPH oxidase-like system containing gp91phox homologs in human keratinocytes. J. Invest. Dermatol. 122, 1000–1009 [DOI] [PubMed] [Google Scholar]
- 38. Li B., Bedard K., Sorce S., Hinz B., Dubois-Dauphin M., Krause K. H. (2009) NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL-6 expression. J. Innate Immun. 1, 570–581 [DOI] [PubMed] [Google Scholar]
- 39. Moreland J. G., Davis A. P., Bailey G., Nauseef W. M., Lamb F. S. (2006) Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J. Biol. Chem. 281, 12277–12288 [DOI] [PubMed] [Google Scholar]
- 40. Ducharme G., Newell E. W., Pinto C., Schlichter L. C. (2007) Small-conductance Cl– channels contribute to volume regulation and phagocytosis in microglia. Eur. J. Neurosci. 26, 2119–2130 [DOI] [PubMed] [Google Scholar]
- 41. Henderson L. M., Chappell J. B., Jones O. T. (1988) Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 255, 285–290 [PMC free article] [PubMed] [Google Scholar]
- 42. Henderson L. M., Chappell J. B., Jones O. T. (1988) Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 251, 563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao T., Benard V., Bohl B. P., Bokoch G. M. (2003) The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J. Clin. Invest. 112, 1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dana R., Leto T. L., Malech H. L., Levy R. (1998) Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J. Biol. Chem. 273, 441–445 [DOI] [PubMed] [Google Scholar]
- 45. Abid M. R., Kachra Z., Spokes K. C., Aird W. C. (2000) NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 486, 252–256 [DOI] [PubMed] [Google Scholar]
- 46. Abid M. R., Spokes K. C., Shih S. C., Aird W. C. (2007) NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J. Biol. Chem. 282, 35373–35385 [DOI] [PubMed] [Google Scholar]
- 47. Stuehr D. J. (1997) Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 37, 339–359 [DOI] [PubMed] [Google Scholar]
- 48. Wink D. A., Mitchell J. B. (1998) The chemical biology of nitric oxide: insights into regulatory, cytotoxic and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 25, 434–456 [DOI] [PubMed] [Google Scholar]
- 49. Wink D. A., Vodovotz Y., Grisham M. B., DeGraff W., Cook J. C., Pacelli R., Krishna M., Mitchell J. B. (1999) Antioxidant effects of nitric oxide. Methods Enzymol. 301, 413–424 [DOI] [PubMed] [Google Scholar]
- 50. Lymar S. V., Hurst K. (1995) Rapid reaction between peroxynitrite ion and cabon dioxide: implications for biological activity. J. Am. Chem. Soc. 117, 8867–8868 [Google Scholar]
- 51. Jourd′heuil D., Miranda K. M., Kim S. M., Espey M. G., Vodovotz Y., Laroux S., Mai C. T., Miles A. M., Grisham M. B., Wink D. A. (1999) The oxidative and nitrosative chemistry of the nitric oxide/superoxide reaction in the presence of bicarbonate. Arch. Biochem. Biophys. 365, 92–100 [DOI] [PubMed] [Google Scholar]
- 52. Abu-Soud H. M., Hazen S. L. (2000) Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 275, 37524–37532 [DOI] [PubMed] [Google Scholar]
- 53. Lintas C. L., Clark A., Fox J., Tannenbaum S. R., Newberne P. M. (1982) In vivo stability of nitrite and nitrosamine formation in the dog stomach: effect of nitrite and amine concentration and of ascorbic acid. Carcinogenesis 3, 161–165 [DOI] [PubMed] [Google Scholar]
- 54. Williams D. L. H. (1988) Nitrosation, Cambridge, UK, Cambridge University Press [Google Scholar]
- 55. Akaike T., Maeda H. (2000) Nitric oxide and virus infection. Immunology 101, 300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Delledonne M., Polverari A., Murgia I. (2003) The functions of nitric oxide-mediated signaling and changes in gene expression during the hypersensitive response. Antioxid. Redox Signal. 5, 33–41 [DOI] [PubMed] [Google Scholar]
- 57. Pacelli R., Wink D. A., Cook J. A., Krishna M. C., DeGraff W., Friedman N., Tsokos M., Samuni A., Mitchell J. B. (1995) Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J. Exp. Med. 182, 1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manchado M., Michan C., Pueyo C. (2000) Hydrogen peroxide activates the SoxRS regulon in vivo. J. Bacteriol. 182, 6842–6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wink D. A., Hanbauer I., Krishna M. C., DeGraff W., Gamson J., Mitchell J. B. (1993) Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc. Natl. Acad. Sci. USA 90, 9813–9817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaplan S. S., Lancaster J. R., Basford R. E., Simmons R. L. (1996) Effect of nitric oxide on staphylococcal killing and interactive effect with superoxide. Infect. Immun. 64, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ogawa R., Pacelli R., Espey M. G., Miranda K. M., Friedman N., Kim S. M., Cox G., Mitchell J. B., Wink D. A., Russo A. (2001) Comparison of control of Listeria by nitric oxide redox chemistry from murine macrophages and NO donors: insights into listeriocidal activity of oxidative and nitrosative stress. Free Radic. Biol. Med. 30, 268–276 [DOI] [PubMed] [Google Scholar]
- 62. Torres D., Barrier M., Bihl F., Quesniaux V. J., Maillet I., Akira S., Ryffel B., Erard F. (2004) Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect. Immun. 72, 2131–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Remer K. A., Jungi T. W., Fatzer R., Tauber M. G., Leib S. L. (2001) Nitric oxide is protective in listeric meningoencephalitis of rats. Infect. Immun. 69, 4086–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gregory S. H., Wing E. J., Hoffman R. A., Simmons R. L. (1993) Reactive nitrogen intermediates suppress the primary immunologic response to Listeria. J. Immunol. 150, 2901–2909 [PubMed] [Google Scholar]
- 65. MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N., et al. (1995) Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81, 641–650 [DOI] [PubMed] [Google Scholar]
- 66. Chan J., Flynn J. L. (1999) Nitric oxide in Mycobacterium tuberculosis infection. In Nitric oxide and Infection (Fang F., ed.), New York, NY, USA, Plenum Publishers, 281–310 [Google Scholar]
- 67. Yu K., Mitchell C., Xing Y., Magliozzo R. S., Bloom B. R., Chan J. (1999) Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79, 191–198 [DOI] [PubMed] [Google Scholar]
- 68. St John G., Brot N., Ruan J., Erdjument-Bromage H., Tempst P., Weissbach H., Nathan C. (2001) Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl. Acad. Sci. USA 98, 9901–9906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sciorati C., Rovere P., Ferrarini M., Paolucci C., Heltai S., Vaiani R., Clementi E., Manfredi A. A. (1999) Generation of nitric oxide by the inducible nitric oxide synthase protects γ δ T cells from Mycobacterium tuberculosis-induced apoptosis. J. Immunol. 163, 1570–1576 [PubMed] [Google Scholar]
- 70. Manfredi A. A., Heltai S., Rovere P., Sciorati C., Paolucci C., Galati G., Rugarli C., Vaiani R., Clementi E., Ferrarini M. (1998) Mycobacterium tuberculosis exploits the CD95/CD95 ligand system of γδ T cells to cause apoptosis. Eur. J. Immunol. 28, 1798–1806 [DOI] [PubMed] [Google Scholar]
- 71. Jung Y. J., LaCourse R., Ryan L., North R. J. (2002) Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J. Exp. Med. 196, 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vandal O. H., Roberts J. A., Odaira T., Schnappinger D., Nathan C. F., Ehrt S. (2009) Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 191, 625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Akaki T., Tomioka H., Shimizu T., Dekio S., Sato K. (2000) Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis. Clin. Exp. Immunol. 121, 302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bryk R., Griffin P., Nathan C. (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215 [DOI] [PubMed] [Google Scholar]
- 75. Anstey N. M., Weinberg J. B., Hassanali M. Y., Mwaikambo E. D., Manyenga D., Misukonis M. A., Arnelle D. R., Hollis D., McDonald M. I., Granger D. L. (1996) Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Perkins D. J., Kremsner P. G., Schmid D., Misukonis M. A., Kelly M. A., Weinberg J. B. (1999) Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy Gabonese children with prior mild or severe malaria. Infect. Immun. 67, 4977–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gyan B., Troye-Blomberg M., Perlmann P., Bjorkman A. (1994) Human monocytes cultured with and without interferon-γ inhibit Plasmodium falciparum parasite growth in vitro via secretion of reactive nitrogen intermediates. Parasite Immunol. 16, 371–375 [DOI] [PubMed] [Google Scholar]
- 78. Rockett K. A., Awburn M. M., Cowden W. B., Clark I. A. (1991) Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect. Immun. 59, 3280–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Serirom S., Raharjo W. H., Chotivanich K., Loareesuwan S., Kubes P., Ho M. (2003) Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am. J. Pathol. 162, 1651–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kun J. F., Mordmuller B., Perkins D. J., May J., Mercereau-Puijalon O., Alpers M., Weinberg J. B., Kremsner P. G. (2001) Nitric oxide synthase 2(Lambarene) (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 184, 330–336 [DOI] [PubMed] [Google Scholar]
- 81. Skorokhod O. A., Schwarzer E., Ceretto M., Arese P. (2007) Malarial pigment hemozoin, IFN-γ, TNF-α, IL-1β and LPS do not stimulate expression of inducible nitric oxide synthase and production of nitric oxide in immuno-purified human monocytes. Malar. J. 6, 73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Luckhart S., Vodovotz Y., Cui L., Rosenberg R. (1998) The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA 95, 5700–5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ali M., Al-Olayan E. M., Lewis S., Matthews H., Hurd H. (2010) Naturally occurring triggers that induce apoptosis-like programmed cell death in Plasmodium berghei ookinetes. PLoS ONE 5, e12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Luckhart S., Crampton A. L., Zamora R., Lieber M. J., Dos Santos P. C., Peterson T. M., Emmith N., Lim J., Wink D. A., Vodovotz Y. (2003) Mammalian transforming growth factor β1 activated after ingestion by Anopheles stephensi modulates mosquito immunity. Infect. Immun. 71, 3000–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Das P., Lahiri A., Lahiri A., Chakravortty D. (2010) Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 6, e1000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Buga G. M., Singh R., Pervin S., Rogers N. E., Schmitz D. A., Jenkinson C. P., Cederbaum S. D., Ignarro L. J. (1996) Arginase activity in endothelial cells: inhibition by NG-hydroxy-L-arginine during high-output NO production. Am. J. Physiol. 271, H1988–H1998 [DOI] [PubMed] [Google Scholar]
- 87. Wanasen N., Soong L. (2008) L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol. Res. 41, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kavoosi G., Ardestani S. K., Kariminia A., Abolhassani M., Turco S. J. (2006) Leishmania major: reactive oxygen species and interferon γ induction by soluble lipophosphoglycan of stationary phase promastigotes. Exp. Parasitol. 114, 323–328 [DOI] [PubMed] [Google Scholar]
- 89. Kavoosi G., Ardestani S. K., Kariminia A. (2009) The involvement of TLR2 in cytokine and reactive oxygen species (ROS) production by PBMCs in response to Leishmania major phosphoglycans (PGs). Parasitology 136, 1193–1199 [DOI] [PubMed] [Google Scholar]
- 90. Chakrabarti G., Basu A., Manna P. P., Mahato S. B., Mandal N. B., Bandyopadhyay S. (1999) Indolylquinoline derivatives are cytotoxic to Leishmania donovani promastigotes and amastigotes in vitro and are effective in treating murine visceral leishmaniasis. J. Antimicrob. Chemother. 43, 359–366 [DOI] [PubMed] [Google Scholar]
- 91. Mookerjee Basu J., Mookerjee A., Sen P., Bhaumik S., Sen P., Banerjee S., Naskar K., Choudhuri S. K., Saha B., Raha S., Roy S. (2006) Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 50, 1788–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Miranda K., Pace D. A., Cintron R., Rodrigues J. C., Fang J., Smith A., Rohloff P., Coelho E., de Haas F., de Souza W., Coppens I., Sibley L. D., Moreno S. N. (2010) Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol. Microbiol. 76, 1358–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Silva N. M., Vieira J. C., Carneiro C. M., Tafuri W. L. (2009) Toxoplasma gondii: the role of IFN-γ, TNFRp55 and iNOS in inflammatory changes during infection. Exp. Parasitol. 123, 65–72 [DOI] [PubMed] [Google Scholar]
- 94. Xu W., Zheng S., Dweik R. A., Erzurum S. C. (2006) Role of epithelial nitric oxide in airway viral infection. Free Radic. Biol. Med. 41, 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Robinson N. M., Zhang H. Y., Bevan A. L., De Belder A. J., Moncada S., Martin J. F., Archard L. C. (1999) Induction of myocardial nitric oxide synthase by Coxsackie B3 virus in mice. Eur. J. Clin. Invest. 29, 700–707 [DOI] [PubMed] [Google Scholar]
- 96. Noda S., Tanaka K., Sawamura S., Sasaki M., Matsumoto T., Mikami K., Aiba Y., Hasegawa H., Kawabe N., Koga Y. (2001) Role of nitric oxide synthase type 2 in acute infection with murine cytomegalovirus. J. Immunol. 166, 3533–3541 [DOI] [PubMed] [Google Scholar]
- 97. Karupiah G., Chen J. H., Mahalingam S., Nathan C. F., MacMicking J. D. (1998) Rapid interferon γ-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 188, 1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kline J. N., Hunninghake G. M., He B., Monick M. M., Hunninghake G. W. (1998) Synergistic activation of the human cytomegalovirus major immediate early promoter by prostaglandin E2 and cytokines. Exp. Lung Res. 24, 3–14 [DOI] [PubMed] [Google Scholar]
- 99. Hayes M. M., Lane B. R., King S. R., Markovitz D. M., Coffey M. J. (2002) Prostaglandin E(2) inhibits replication of HIV-1 in macrophages through activation of protein kinase A. Cell. Immunol. 215, 61–71 [DOI] [PubMed] [Google Scholar]
- 100. Neves-Souza P. C., Azeredo E. L., Zagne S. M., Valls-de-Souza R., Reis S. R., Cerqueira D. I., Nogueira R. M., Kubelka C. F. (2005) Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue fever in patients and during in vitro infection. BMC Infect. Dis. 5, 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Takhampunya R., Padmanabhan R., Ubol S. (2006) Antiviral action of nitric oxide on Dengue virus type 2 replication. J. Gen. Virol. 87, 3003–3011 [DOI] [PubMed] [Google Scholar]
- 102. Mendes-Ribeiro A. C., Moss M. B., Siqueira M. A., Moraes T. L., Ellory J. C., Mann G. E., Brunini T. M. (2008) Dengue fever activates the L-arginine-nitric oxide pathway: an explanation for reduced aggregation of human platelets. Clin. Exp. Pharmacol. Physiol. 35, 1143–1146 [DOI] [PubMed] [Google Scholar]
- 103. Chaturvedi U. C., Nagar R. (2009) Nitric oxide in Dengue and Dengue hemorrhagic fever: necessity or nuisance? FEMS Immunol. Med. Microbiol. 56, 9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pande V., Ramos M. J. (2003) Nuclear factor κ B: a potential target for anti-HIV chemotherapy. Curr. Med. Chem. 10, 1603–1615 [DOI] [PubMed] [Google Scholar]
- 105. Lall M. S., Jain R. P., Vederas J. C. (2004) Inhibitors of 3C cysteine proteinases from Picornaviridae. Curr. Top. Med. Chem. 4, 1239–1253 [DOI] [PubMed] [Google Scholar]
- 106. Liang P. H. (2006) Characterization and inhibition of SARS-coronavirus main protease. Curr. Top. Med. Chem. 6, 361–376 [DOI] [PubMed] [Google Scholar]
- 107. Best S. M. (2008) Viral subversion of apoptotic enzymes: escape from death row. Annu. Rev. Microbiol. 62, 171–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zell R., Markgraf R., Schmidtke M., Gorlach M., Stelzner A., Henke A., Sigusch H. H., Gluck B. (2004) Nitric oxide donors inhibit the coxsackievirus B3 proteinases 2A and 3C in vitro, virus production in cells, and signs of myocarditis in virus-infected mice. Med. Microbiol. Immunol. (Berl.) 193, 91–100 [DOI] [PubMed] [Google Scholar]
- 109. Ascenzi P., Salvati L., Bolognesi M., Colasanti M., Polticelli F., Venturini G. (2001) Inhibition of cysteine protease activity by NO-donors. Curr. Protein Pept. Sci. 2, 137–153 [DOI] [PubMed] [Google Scholar]
- 110. Basu A., Sehajpal P. K., Ogiste J. S., Lander H. M. (1999) Targeting cysteine residues of human immunodeficiency virus type 1 protease by reactive free radical species. Antioxid. Redox Signal. 1, 105–112 [DOI] [PubMed] [Google Scholar]
- 111. Sehajpal P. K., Basu A., Ogiste J. S., Lander H. M. (1999) Reversible S-nitrosation and inhibition of HIV-1 protease. Biochemistry 38, 13407–13413 [DOI] [PubMed] [Google Scholar]
- 112. Saavedra J. E., Srinivasan A., Bonifant C. L., Chu J., Shanklin A. P., Flippen-Anderson J. L., Rice W. G., Turpin J. A., Davies K. M., Keefer L. K. (2001) The secondary amine/nitric oxide complex ion R(2)N[N(O)NO](–) as nucleophile and leaving group in S9N)Ar reactions. J. Org. Chem. 66, 3090–3098 [DOI] [PubMed] [Google Scholar]
- 113. Maynard A. T., Huang M., Rice W. G., Covell D. G. (1998) Reactivity of the HIV-1 nucleocapsid protein p7 zinc finger domains from the perspective of density-functional theory. Proc. Natl. Acad. Sci. USA 95, 11578–11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Dukes C. S., Yu Y., Rivadeneira E. D., Sauls D. L., Liao H. X., Haynes B. F., Weinberg J. B. (1995) Cellular CD44S as a determinant of human immunodeficiency virus type 1 infection and cellular tropism. J. Virol. 69, 4000–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vásquez-Vivar J., Hogg N., Martasek P., Karoui H., Pritchard K. A. J., Kalyanaraman B. (1999) Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J. Biol. Chem. 274, 26736–26742 [DOI] [PubMed] [Google Scholar]
- 116. Vásquez-Vivar J., Kalyanaraman B., Martasek P., Hogg N., Masters B. S., Karoui H., Tordo P., Pritchard K. A. J. (1998) Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. USA 95, 9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Xia Y., Zweier J. L. (1997) Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. USA 94, 6954–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Morris S. M. J. (2009) Recent advances in arginine metabolism: roles and regulation of the arginases. Br. J. Pharmacol. 157, 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Morris S. M. J. (2004) Recent advances in arginine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 7, 45–51 [DOI] [PubMed] [Google Scholar]
- 120. Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. (2010) Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366 [DOI] [PubMed] [Google Scholar]
- 121. Wallace H. M. (2009) The polyamines: past, present and future. Essays Biochem. 46, 1–9 [DOI] [PubMed] [Google Scholar]
- 122. Mori M. (2007) Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J. Nutr. 137, 1616S–1620S [DOI] [PubMed] [Google Scholar]
- 123. Di Costanzo L., Ilies M., Thorn K. J., Christianson D. W. (2010) Inhibition of human arginase I by substrate and product analogues. Arch. Biochem. Biophys. 496, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Matthiesen S., Lindemann D., Warnken M., Juergens U. R., Racke K. (2008) Inhibition of NADPH oxidase by apocynin inhibits lipopolysaccharide (LPS) induced up-regulation of arginase in rat alveolar macrophages. Eur. J. Pharmacol. 579, 403–410 [DOI] [PubMed] [Google Scholar]
- 125. Von Knethen A., Brune B. (2002) Activation of peroxisome proliferator-activated receptor γ by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J. Immunol. 169, 2619–2626 [DOI] [PubMed] [Google Scholar]
- 126. Hardy S. J., Ferrante A., Poulos A., Robinson B. S., Johnson D. W., Murray A. W. (1994) Effect of exogenous fatty acids with greater than 22 carbon atoms (very long chain fatty acids) on superoxide production by human neutrophils. J. Immunol. 153, 1754–1761 [PubMed] [Google Scholar]
- 127. Kim C., Kim J. Y., Kim J. H. (2008) Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep. 41, 555–559 [DOI] [PubMed] [Google Scholar]
- 128. Brusko T. M., Wasserfall C. H., Agarwal A., Kapturczak M. H., Atkinson M. A. (2005) An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J. Immunol. 174, 5181–5186 [DOI] [PubMed] [Google Scholar]
- 129. Ryter S. W., Otterbein L. E. (2004) Carbon monoxide in biology and medicine. Bioessays 26, 270–280 [DOI] [PubMed] [Google Scholar]
- 130. Wang X. M., Kim H. P., Nakahira K., Ryter S. W., Choi A. M. (2009) The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. 182, 3809–3818 [DOI] [PubMed] [Google Scholar]
- 131. Wu S. C., Zhang Y. (2010) Active DNA demethylation: many roads lead to Rome. Nat. Rev. Mol. Cell Biol. 11, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Qu K., Lee S. W., Bian J. S., Low C. M., Wong P. T. (2008) Hydrogen sulfide: neurochemistry and neurobiology. Neurochem. Int. 52, 155–165 [DOI] [PubMed] [Google Scholar]
- 133. Pogribna M., Melnyk S., Pogribny I., Chango A., Yi P., James S. J. (2001) Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am. J. Hum. Genet. 69, 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang H., Bhatia M. (2008) Hydrogen sulfide: a novel mediator of leukocyte activation. Immunopharmacol. Immunotoxicol. 30, 631–645 [DOI] [PubMed] [Google Scholar]
- 135. Li L., Hsu A., Moore P. K. (2009) Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation—a tale of three gases! Pharmacol. Ther. 123, 386–400 [DOI] [PubMed] [Google Scholar]
- 136. Messmer U. K., Ankarcrona M., Nicotera P., Brune B. (1994) p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 355, 23–26 [DOI] [PubMed] [Google Scholar]
- 137. Sandau K. B., Fandrey J., Brune B. (2001) Accumulation of HIF-1α under the influence of nitric oxide. Blood 97, 1009–1015 [DOI] [PubMed] [Google Scholar]
- 138. Zhou J., Fandrey J., Schumann J., Tiegs G., Brune B. (2003) NO and TNF-α released from activated macrophages stabilize HIF-1α in resting tubular LLC-PK1 cells. Am. J. Physiol. Cell Physiol. 284, C439–C446 [DOI] [PubMed] [Google Scholar]
- 139. Ridnour L. A., Thomas D. D., Switzer C., Flores-Santana W., Isenberg J. S., Ambs S., Roberts D. D., Wink D. A. (2008) Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide 19, 73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ridnour L. A., Thomas D. D., Donzelli S., Espey M. G., Roberts D. D., Wink D. A., Isenberg J. S. (2006) The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 8, 1329–1337 [DOI] [PubMed] [Google Scholar]
- 141. Thomas D. D., Espey M. G., Ridnour L. A., Hofseth L. J., Mancardi D., Harris C. C., Wink D. A. (2004) Hypoxic inducible factor 1α, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc. Natl. Acad. Sci. USA 101, 8894–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ridnour L. A., Isenberg J. S., Espey M. G., Thomas D. D., Roberts D. D., Wink D. A. (2005) Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA 102, 13147–13152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lancaster J. R. (1994) Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc. Natl. Acad. Sci. USA 91, 8137–8141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Thomas D. D., Ridnour L. A., Espey M. G., Donzelli S., Ambs S., Hussain S. P., Harris C. C., DeGraff W., Roberts D. D., Mitchell J. B., Wink D. A. (2006) Superoxide fluxes limit nitric oxide-induced signaling. J. Biol. Chem. 281, 25984–25993 [DOI] [PubMed] [Google Scholar]
- 145. Brüne B. (2005) The intimate relation between nitric oxide and superoxide in apoptosis and cell survival. Antioxid. Redox Signal. 7, 497–507 [DOI] [PubMed] [Google Scholar]
- 146. Niedbala W., Cai B., Liew F. Y. (2006) Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 65 (Suppl. 3), iii37–iii40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Niedbala W., Cai B., Liu H., Pitman N., Chang L., Liew F. Y. (2007) Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc. Natl. Acad. Sci. USA 104, 15478–15483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Weinberg J. B. (1998) Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol. Med. 4, 557–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Park K. S., Guo Z., Shao L., Du Q., Geller D. A. (2009) A far-upstream Oct-1 motif regulates cytokine-induced transcription of the human inducible nitric oxide synthase gene. J. Mol. Biol. 390, 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Du Q., Park K. S., Guo Z., He P., Nagashima M., Shao L., Sahai R., Geller D. A., Hussain S. P. (2006) Regulation of human nitric oxide synthase 2 expression by Wnt β-catenin signaling. Cancer Res. 66, 7024–7031 [DOI] [PubMed] [Google Scholar]
- 151. Du Q., Zhang X., Cardinal J., Cao Z., Guo Z., Shao L., Geller D. A. (2009) Wnt/β-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-κB activation in cancer cells. Cancer Res. 69, 3764–3771 [DOI] [PubMed] [Google Scholar]
- 152. Ganster R. W., Taylor B. S., Shao L., Geller D. A. (2001) Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-κ B. Proc. Natl. Acad. Sci. USA 98, 8638–8643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tzeng E., Billiar T. R., Robbins P. D., Loftus M., Stuehr D. J. (1995) Expression of human inducible nitric oxide synthase in a tetrahydrobiopterin (H4B)-deficient cell line: H4B promotes assembly of enzyme subunits into an active dimer. Proc. Natl. Acad. Sci. USA 92, 11771–11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lähde M., Korhonen R., Moilanen E. (2000) Regulation of nitric oxide production in cultured human T84 intestinal epithelial cells by nuclear factor-κ B-dependent induction of inducible nitric oxide synthase after exposure to bacterial endotoxin. Aliment. Pharmacol. Ther. 14, 945–954 [DOI] [PubMed] [Google Scholar]
- 155. Watkins D. N., Peroni D. J., Basclain K. A., Garlepp M. J., Thompson P. J. (1997) Expression and activity of nitric oxide synthases in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 16, 629–639 [DOI] [PubMed] [Google Scholar]
- 156. Hori K., Burd P. R., Furuke K., Kutza J., Weih K. A., Clouse K. A. (1999) Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood 93, 1843–1850 [PubMed] [Google Scholar]
- 157. Kaku Y., Nanri H., Sakimura T., Ejima K., Kuroiwa A., Ikeda M. (1997) Differential induction of constitutive and inducible nitric oxide synthases by distinct inflammatory stimuli in bovine aortic endothelial cells. Biochim. Biophys. Acta 1356, 43–52 [DOI] [PubMed] [Google Scholar]
- 158. MacNaul K. L., Hutchinson N. I. (1993) Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem. Biophys. Res. Commun. 196, 1330–1334 [DOI] [PubMed] [Google Scholar]
- 159. Maier R., Bilbe G., Rediske J., Lotz M. (1994) Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim. Biophys. Acta 1208, 145–150 [DOI] [PubMed] [Google Scholar]
- 160. Hoebe K. H., Witkamp R. F., Fink-Gremmels J., Van Miert A. S., Monshouwer M. (2001) Direct cell-to-cell contact between Kupffer cells and hepatocytes augments endotoxin-induced hepatic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G720–G728 [DOI] [PubMed] [Google Scholar]
- 161. Ohshima H., Tsuda M., Adachi H., Ogura T., Sugimura T., Esumi H. (1991) L-arginine-dependent formation of N-nitrosamines by the cytosol of macrophages activated with lipopolysaccharide and interferon-γ. Carcinogenesis 12, 1217–1220 [DOI] [PubMed] [Google Scholar]
- 162. Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. (1990) Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann. Surg. 212, 462–469, discussion 470–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Billiar T. R., Curran R. D., Ferrari F. K., Williams D. L., Simmons R. L. (1990) Kupffer cell:hepatocyte cocultures release nitric oxide in response to bacterial endotoxin. J. Surg. Res. 48, 349–353 [DOI] [PubMed] [Google Scholar]
- 164. Freeswick P. D., Wan Y., Geller D. A., Nussler A. K., Billiar T. R. (1994) Remote tissue injury primes hepatocytes for nitric oxide synthesis. J. Surg. Res. 57, 205–209 [DOI] [PubMed] [Google Scholar]
- 165. Nussler A. K., Di Silvio M., Liu Z. Z., Geller D. A., Freeswick P., Dorko K., Bartoli F., Billiar T. R. (1995) Further characterization and comparison of inducible nitric oxide synthase in mouse, rat, and human hepatocytes. Hepatology 21, 1552–1560 [PubMed] [Google Scholar]
- 166. Fernández-Vizarra P., Fernandez A. P., Castro-Blanco S., Encinas J. M., Serrano J., Bentura M. L., Munoz P., Martinez-Murillo R., Rodrigo J. (2004) Expression of nitric oxide system in clinically evaluated cases of Alzheimer′s disease. Neurobiol. Dis. 15, 287–305 [DOI] [PubMed] [Google Scholar]
- 167. Johnson J. L., Moore E. E., Hiester A. A., Tamura D. Y., Zallen G., Silliman C. C. (1999) Disparities in the respiratory burst between human and rat neutrophils. J. Leukoc. Biol. 65, 211–216 [DOI] [PubMed] [Google Scholar]
- 168. Badger A. M. (1986) Enhanced superoxide production by rat alveolar macrophages stimulated in vitro with biological response modifiers. J. Leukoc. Biol. 40, 725–736 [DOI] [PubMed] [Google Scholar]
- 169. Colton C., Wilt S., Gilbert D., Chernyshev O., Snell J., Dubois-Dalcq M. (1996) Species differences in the generation of reactive oxygen species by microglia. Mol. Chem. Neuropathol. 28, 15–20 [DOI] [PubMed] [Google Scholar]
- 170. Turpaev K., Bouton C., Drapier J. C. (2004) Nitric oxide-derived nitrosating species and gene expression in human monocytic cells. Biochemistry 43, 10844–10850 [DOI] [PubMed] [Google Scholar]
- 171. Turpaev K., Bouton C., Diet A., Glatigny A., Drapier J. C. (2005) Analysis of differentially expressed genes in nitric oxide-exposed human monocytic cells. Free Radic. Biol. Med. 38, 1392–1400 [DOI] [PubMed] [Google Scholar]
- 172. Turpaev K., Glatigny A., Bignon J., Delacroix H., Drapier J. C. (2010) Variation in gene expression profiles of human monocytic U937 cells exposed to various fluxes of nitric oxide. Free Radic. Biol. Med. 48, 298–305 [DOI] [PubMed] [Google Scholar]
- 173. Zhang Z., Kolls J. K., Oliver P., Good D., Schwarzenberger P. O., Joshi M. S., Ponthier J. L., Lancaster J. R. J. (2000) Activation of tumor necrosis factor-α-converting enzyme-mediated ectodomain shedding by nitric oxide. J. Biol. Chem. 275, 15839–15844 [DOI] [PubMed] [Google Scholar]
- 174. Baeuerle P. A., Henkel T. (1994) Function and activation of NF-κ B in the immune system. Annu. Rev. Immunol. 12, 141–179 [DOI] [PubMed] [Google Scholar]
- 175. Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. (1990) Intracellular thiols regulate activation of nuclear factor κ B and transcription of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 87, 9943–9947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jacobs A. T., Ignarro L. J. (2003) Nuclear factor-κ B and mitogen-activated protein kinases mediate nitric oxide-enhanced transcriptional expression of interferon-β. J. Biol. Chem. 278, 8018–8027 [DOI] [PubMed] [Google Scholar]
- 177. Van Dervort A. L., Yan L., Madara P. J., Cobb J. P., Wesley R. A., Corriveau C. C., Tropea M. M., Danner R. L. (1994) Nitric oxide regulates endotoxin-induced TNF-α production by human neutrophils. J. Immunol. 152, 4102–4109 [PubMed] [Google Scholar]
- 178. Wu F., Cepinskas G., Wilson J. X., Tyml K. (2001) Nitric oxide attenuates but superoxide enhances iNOS expression in endotoxin- and IFNγ-stimulated skeletal muscle endothelial cells. Microcirculation 8, 415–425 [DOI] [PubMed] [Google Scholar]
- 179. Li Q., Engelhardt J. F. (2006) Interleukin-1β induction of NFκB is partially regulated by H2O2-mediated activation of NFκB-inducing kinase. J. Biol. Chem. 281, 1495–1505 [DOI] [PubMed] [Google Scholar]
- 180. Grumbach I. M., Chen W., Mertens S. A., Harrison D. G. (2005) A negative feedback mechanism involving nitric oxide and nuclear factor κ-B modulates endothelial nitric oxide synthase transcription. J. Mol. Cell. Cardiol. 39, 595–603 [DOI] [PubMed] [Google Scholar]
- 181. Lee J. S., Kahlon S. S., Culbreth R., Cooper A. D. J. (1999) Modulation of monocyte chemokine production and nuclear factor κ B activity by oxidants. J. Interferon Cytokine Res. 19, 761–767 [DOI] [PubMed] [Google Scholar]
- 182. Schreck R., Albermann K., Baeuerle P. A. (1992) Nuclear factor κ B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic. Res. Commun. 17, 221–237 [DOI] [PubMed] [Google Scholar]
- 183. Marshall H. E., Hess D. T., Stamler J. S. (2004) S-Nitrosylation: physiological regulation of NF-κB. Proc. Natl. Acad. Sci. USA 101, 8841–8842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Pande V., Sousa S. F., Ramos M. J. (2009) Direct covalent modification as a strategy to inhibit nuclear factor-κ B. Curr. Med. Chem. 16, 4261–4273 [DOI] [PubMed] [Google Scholar]
- 185. Toledano M. B., Leonard W. J. (1991) Modulation of transcription factor NF-κ B binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 88, 4328–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kanayama A., Inoue J., Sugita-Konishi Y., Shimizu M., Miyamoto Y. (2002) Oxidation of Iκ Bα at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-κ B activation. J. Biol. Chem. 277, 24049–24056 [DOI] [PubMed] [Google Scholar]
- 187. Kabe Y., Ando K., Hirao S., Yoshida M., Handa H. (2005) Redox regulation of NF-κB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid. Redox Signal. 7, 395–403 [DOI] [PubMed] [Google Scholar]
- 188. Flohé L., Brigelius-Flohe R., Saliou C., Traber M. G., Packer L. (1997) Redox regulation of NF-κ B activation. Free Radic. Biol. Med. 22, 1115–1126 [DOI] [PubMed] [Google Scholar]
- 189. Connelly L., Palacios-Callender M., Ameixa C., Moncada S., Hobbs A. J. (2001) Biphasic regulation of NF-κ B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J. Immunol. 166, 3873–3881 [DOI] [PubMed] [Google Scholar]
- 190. Hamilton T. A., Ohmori Y., Tebo J. M., Kishore R. (1999) Regulation of macrophage gene expression by pro- and anti-inflammatory cytokines. Pathobiology 67, 241–244 [DOI] [PubMed] [Google Scholar]
- 191. Martinez F. O., Sica A., Mantovani A., Locati M. (2008) Macrophage activation and polarization. Front. Biosci. 13, 453–461 [DOI] [PubMed] [Google Scholar]
- 192. Colton C. A. (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Colton C. A., Wilcock D. M. (2010) Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets 9, 174–191 [DOI] [PubMed] [Google Scholar]
- 194. Swierkosz T. A., Mitchell J. A., Warner T. D., Botting R. M., Vane J. R. (1995) Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br. J. Pharmacol. 114, 1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Perkins D. J., Kniss D. A. (1999) Blockade of nitric oxide formation down-regulates cyclooxygenase-2 and decreases PGE2 biosynthesis in macrophages. J. Leukoc. Biol. 65, 792–799 [DOI] [PubMed] [Google Scholar]
- 196. Endres S., Fulle H. J., Sinha B., Stoll D., Dinarello C. A., Gerzer R., Weber P. C. (1991) Cyclic nucleotides differentially regulate the synthesis of tumor necrosis factor-α and interleukin-1 β by human mononuclear cells. Immunology 72, 56–60 [PMC free article] [PubMed] [Google Scholar]
- 197. Kreckler L. M., Gizewski E., Wan T. C., Auchampach J. A. (2009) Adenosine suppresses lipopolysaccharide-induced tumor necrosis factor-α production by murine macrophages through a protein kinase A- and exchange protein activated by cAMP-independent signaling pathway. J. Pharmacol. Exp. Ther. 331, 1051–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Von Bülow V., Rink L., Haase H. (2005) Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-α and IL-1 β production in monocytes by elevation of guanosine 3′,5′-cyclic monophosphate. J. Immunol. 175, 4697–4705 [DOI] [PubMed] [Google Scholar]
- 199. Sosroseno W., Musa M., Ravichandran M., Fikri Ibrahim M., Bird P. S., Seymour G. J. (2006) The role of cyclic-AMP on arginase activity by a murine macrophage cell line (RAW264.7) stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 21, 347–352 [DOI] [PubMed] [Google Scholar]
- 200. Edwards J. P., Emens L. A. (2010) The multikinase inhibitor Sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE(2) in murine macrophages. Int. Immunopharmacol. 10, 1220–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Chuang T. H., Ulevitch R. J. (2004) Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 5, 495–502 [DOI] [PubMed] [Google Scholar]
- 202. Baetz A., Frey M., Heeg K., Dalpke A. H. (2004) Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J. Biol. Chem. 279, 54708–54715 [DOI] [PubMed] [Google Scholar]
- 203. Yazdanpanah B., Wiegmann K., Tchikov V., Krut O., Pongratz C., Schramm M., Kleinridders A., Wunderlich T., Kashkar H., Utermohlen O., Bruning J. C., Schutze S., Kronke M. (2009) Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature 460, 1159–1163 [DOI] [PubMed] [Google Scholar]
- 204. Devey L., Mohr E., Bellamy C., Simpson K., Henderson N., Harrison E. M., Ross J. A., Wigmore S. J. (2009) c-Jun terminal kinase-2 gene deleted mice overexpress hemeoxygenase-1 and are protected from hepatic ischemia reperfusion injury. Transplantation 88, 308–316 [DOI] [PubMed] [Google Scholar]
- 205. Otterbein L. E., Soares M. P., Yamashita K., Bach F. H. (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 24, 449–455 [DOI] [PubMed] [Google Scholar]
- 206. Dulak J., Loboda A., Zagorska A., Jozkowicz A. (2004) Complex role of heme oxygenase-1 in angiogenesis. Antioxid. Redox Signal. 6, 858–866 [DOI] [PubMed] [Google Scholar]
- 207. Singh S., Vrishni S., Singh B. K., Rahman I., Kakkar P. (2010) Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic. Res. 44, 1267–1288 [DOI] [PubMed] [Google Scholar]
- 208. Kim Y. M., Bergonia H. A., Muller C., Pitt B. R., Watkins W. D., Lancaster J. R. (1995) Nitric oxide and intracellular heme. Adv. Pharmacol. 34, 277–291 [DOI] [PubMed] [Google Scholar]
- 209. Tsoyi K., Kim H. J., Shin J. S., Kim D. H., Cho H. J., Lee S. S., Ahn S. K., Yun-Choi H. S., Lee J. H., Seo H. G., Chang K. C. (2008) HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell. Signal. 20, 1839–1847 [DOI] [PubMed] [Google Scholar]
- 210. Turpaev K., Drapier J. C. (2009) Stimulatory effect of benzylidenemalononitrile tyrphostins on expression of NO-dependent genes in U-937 monocytic cells. Eur. J. Pharmacol. 606, 1–8 [DOI] [PubMed] [Google Scholar]
- 211. Lee T. S., Chau L. Y. (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 8, 240–246 [DOI] [PubMed] [Google Scholar]
- 212. Ryter S. W., Morse D., Choi A. M. (2004) Carbon monoxide: to boldly go where NO has gone before. Sci. STKE 2004, RE6 [DOI] [PubMed] [Google Scholar]
- 213. Ryter S. W., Choi A. M. (2007) Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found. Symp. 280, 165–175, discussion 175–181 [PubMed] [Google Scholar]
- 214. Shen H. M., Pervaiz S. (2006) TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 20, 1589–1598 [DOI] [PubMed] [Google Scholar]
- 215. Sharma P., Chakraborty R., Wang L., Min B., Tremblay M. L., Kawahara T., Lambeth J. D., Haque S. J. (2008) Redox regulation of interleukin-4 signaling. Immunity 29, 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Rawlings J. S., Rosler K. M., Harrison D. A. (2004) The JAK/STAT signaling pathway. J. Cell Sci. 117, 1281–1283 [DOI] [PubMed] [Google Scholar]
- 217. Rhee S. G., Bae Y. S., Lee S. R., Kwon J. (2000) Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 2000, pe1 [DOI] [PubMed] [Google Scholar]
- 218. Tonks N. K. (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7, 833–846 [DOI] [PubMed] [Google Scholar]
- 219. Barford D. (2004) The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 14, 679–686 [DOI] [PubMed] [Google Scholar]
- 220. Chen Y. Y., Chu H. M., Pan K. T., Teng C. H., Wang D. L., Wang A. H., Khoo K. H., Meng T. C. (2008) Cysteine S-nitrosylation protects protein-tyrosine phosphatase 1B against oxidation-induced permanent inactivation. J. Biol. Chem. 283, 35265–35272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Blanchette J., Abu-Dayyeh I., Hassani K., Whitcombe L., Olivier M. (2009) Regulation of macrophage nitric oxide production by the protein tyrosine phosphatase Src homology 2 domain phosphotyrosine phosphatase 1 (SHP-1). Immunology 127, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bonaparte K. L., Hudson C. A., Wu C., Massa P. T. (2006) Inverse regulation of inducible nitric oxide synthase (iNOS) and arginase I by the protein tyrosine phosphatase SHP-1 in CNS glia. Glia 53, 827–835 [DOI] [PubMed] [Google Scholar]
- 223. Serafini P., Meckel K., Kelso M., Noonan K., Califano J., Koch W., Dolcetti L., Bronte V., Borrello I. (2006) Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203, 2691–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Talmadge J. E. (2007) Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin. Cancer Res. 13, 5243–5248 [DOI] [PubMed] [Google Scholar]
- 225. Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A. I., Zhao R. C., Shi Y. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 [DOI] [PubMed] [Google Scholar]
- 226. Vodovotz Y., Chesler L., Chong H., Kim S. J., Simpson J. T., DeGraff W., Cox G. W., Roberts A. B., Wink D. A., Barcellos-Hoff M. H. (1999) Regulation of transforming growth factor β1 by nitric oxide. Cancer Res. 59, 2142–2149 [PubMed] [Google Scholar]
- 227. Ren G., Su J., Zhang L., Zhao X., Ling W., L′huillie A., Zhang J., Lu Y., Roberts A. I., Ji W., Zhang H., Rabson A. B., Shi Y. (2009) Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27, 1954–1962 [DOI] [PubMed] [Google Scholar]
- 228. Meisel R., Zibert A., Laryea M., Gobel U., Daubener W., Dilloo D. (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103, 4619–4621 [DOI] [PubMed] [Google Scholar]
- 229. Däubener W., MacKenzie C. R. (1999) IFN-γ activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv. Exp. Med. Biol. 467, 517–524 [DOI] [PubMed] [Google Scholar]
- 230. Maghzal G. J., Thomas S. R., Hunt N. H., Stocker R. (2008) Cytochrome b5, not superoxide anion radical, is a major reductant of indoleamine 2,3-dioxygenase in human cells. J. Biol. Chem. 283, 12014–12025 [DOI] [PubMed] [Google Scholar]
- 231. Pesce J. T., Ramalingam T. R., Mentink-Kane M. M., Wilson M. S., El Kasmi K. C., Smith A. M., Thompson R. W., Cheever A. W., Murray P. J., Wynn T. A. (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Reljic R., Stylianou E., Balu S., Ma J. K. (2010) Cytokine interactions that determine the outcome of Mycobacterial infection of macrophages. Cytokine 51, 42–46 [DOI] [PubMed] [Google Scholar]
- 233. Doyle A. G., Herbein G., Montaner L. J., Minty A. J., Caput D., Ferrara P., Gordon S. (1994) Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-γ. Eur. J. Immunol. 24, 1441–1445 [DOI] [PubMed] [Google Scholar]
- 234. Wilcock D. M., Colton C. A. (2009) Immunotherapy, vascular pathology, and microhemorrhages in transgenic mice. CNS Neurol. Disord. Drug Targets 8, 50–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hellwig-Bürgel T., Stiehl D. P., Wagner A. E., Metzen E., Jelkmann W. (2005) Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J. Interferon Cytokine Res. 25, 297–310 [DOI] [PubMed] [Google Scholar]
- 236. Maulik N., Das D. K. (2002) Redox signaling in vascular angiogenesis. Free Radic. Biol. Med. 33, 1047–1060 [DOI] [PubMed] [Google Scholar]
- 237. Gately S. (2000) The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 19, 19–27 [DOI] [PubMed] [Google Scholar]
- 238. Fulton D., Fontana J., Sowa G., Gratton J. P., Lin M., Li K. X., Michell B., Kemp B. E., Rodman D., Sessa W. C. (2002) Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J. Biol. Chem. 277, 4277–4284 [DOI] [PubMed] [Google Scholar]
- 239. Ridnour L. A., Windhausen A. N., Isenberg J. S., Yeung N., Thomas D. D., Vitek M. P., Roberts D. D., Wink D. A. (2007) Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 104, 16898–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. McCartney-Francis N. L., Song X., Mizel D. E., Wahl S. M. (2001) Selective inhibition of inducible nitric oxide synthase exacerbates erosive joint disease. J. Immunol. 166, 2734–2740 [DOI] [PubMed] [Google Scholar]
- 241. Wahl S. M., McCartney-Francis N., Chan J., Dionne R., Ta L., Orenstein J. M. (2003) Nitric oxide in experimental joint inflammation. Benefit or detriment? Cells Tissues Organs 174, 26–33 [DOI] [PubMed] [Google Scholar]
- 242. Amin A. R., Di Cesare P. E., Vyas P., Attur M., Tzeng E., Billiar T. R., Stuchin S. A., Abramson S. B. (1995) The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for up-regulated neuronal nitric oxide synthase. J. Exp. Med. 182, 2097–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Chen T., Zamora R., Zuckerbraun B., Billiar T. R. (2003) Role of nitric oxide in liver injury. Curr. Mol. Med. 3, 519–526 [DOI] [PubMed] [Google Scholar]
- 244. Connelly L., Jacobs A. T., Palacios-Callender M., Moncada S., Hobbs A. J. (2003) Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J. Biol. Chem. 278, 26480–26487 [DOI] [PubMed] [Google Scholar]
- 245. Panayiotou C. M., Baliga R., Stidwill R., Taylor V., Singer M., Hobbs A. J. (2010) Resistance to endotoxic shock in mice lacking natriuretic peptide receptor-A. Br. J. Pharmacol. 160, 2045–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kubes P., Suzuki M., Granger D. N. (1991) Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. USA 88, 4651–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Kibbe M. R., Tzeng E., Gleixner S. L., Watkins S. C., Kovesdi I., Lizonova A., Makaroun M. S., Billiar T. R., Rhee R. Y. (2001) Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J. Vasc. Surg. 34, 156–165 [DOI] [PubMed] [Google Scholar]
- 248. Kibbe M. R., Billiar T. R., Tzeng E. (2000) Gene therapy for restenosis. Circ. Res. 86, 829–833 [DOI] [PubMed] [Google Scholar]
- 249. Wink D. A., Ridnour L. A., Hussain S. P., Harris C. C. (2008) The reemergence of nitric oxide and cancer. Nitric Oxide 19, 65–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Hussain S. P., Trivers G. E., Hofseth L. J., He P., Shaikh I., Mechanic L. E., Doja S., Jiang W., Subleski J., Shorts L., Haines D., Laubach V. E., Wiltrout R. H., Djurickovic D., Harris C. C. (2004) Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 64, 6849–6853 [DOI] [PubMed] [Google Scholar]
- 251. Hussain S. P., He P., Subleski J., Hofseth L. J., Trivers G. E., Mechanic L., Hofseth A. B., Bernard M., Schwank J., Nguyen G., Mathe E., Djurickovic D., Haines D., Weiss J., Back T., Gruys E., Laubach V. E., Wiltrout R. H., Harris C. C. (2008) Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 68, 7130–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]