Abstract
Insect damage on fossil leaves from the Central Rocky Mountains, United States, documents the response of herbivores to changing regional climates and vegetation during the late Paleocene (humid, warm temperate to subtropical, predominantly deciduous), early Eocene (humid subtropical, mixed deciduous and evergreen), and middle Eocene (seasonally dry, subtropical, mixed deciduous and thick-leaved evergreen). During all three time periods, greater herbivory occurred on taxa considered to have short rather than long leaf life spans, consistent with studies in living forests that demonstrate the insect resistance of long-lived, thick leaves. Variance in herbivory frequency and diversity was highest during the middle Eocene, indicating the increased representation of two distinct herbivory syndromes: one for taxa with deciduous, palatable foliage, and the other for hosts with evergreen, thick-textured, small leaves characterized by elevated insect resistance. Leaf galling, which is negatively correlated with moisture today, apparently increased during the middle Eocene, whereas leaf mining decreased.
Climate change affects herbivorous insects directly and also indirectly, through the turnover of plant hosts (1, 2). In any climate, insect herbivores either adapt to the range of defense strategies that plants present (3), migrate, or suffer extinction. The fossil record offers a unique opportunity to examine the interplay of insect herbivory, plant defense, and climate. Nevertheless, investigations of the effects of past climate change and other disturbances on insects are few. Most studies have focused on the Pleistocene and Holocene, when climatic fluctuations had profound influences on the geographic distributions of extant plant and insect species (4–6). The potential now exists, in considerably more ancient deposits, to study herbivorous insects in particular, even in those time intervals or strata from which insect body fossils are virtually absent, by examination of feeding damage on fossil plants (7–9).
Studies of extant plant–insect associations provide predictions regarding how ancient herbivory may be related to past temperature and rainfall and to characteristics of host plants. Plant and insect diversity generally correlate positively with decreasing latitude (10, 11), with some important exceptions (12). Additionally, tropical plants endure more intense herbivory than their temperate counterparts, despite greater structural and chemical defenses (13, 14). Seasonal dry cycles are associated with a lower intensity of insect folivory (15) and with depressed populations of insect herbivores (16–19). However, seasonal herbivory peaks may be favored in dry forests when parasitoid numbers are reduced (20).
Moisture seems to have a strong effect on specialized herbivore guilds, with the best documentation based on galling insects, whose diversity and abundance are negatively correlated with moisture (21–23). Apparently, wet environments select against gallers by facilitating predator attacks and pathogen growth (20, 24). We emphasize that very few, if any, quantitative studies exist that directly compare herbivory among forests in relation to rainfall or other climate variables, rather than to latitude or altitude per se. Another observation is that plant species with small leaves tend to expand their leaves faster than do species with large leaves and therefore may suffer lower herbivory because the juvenile window of maximum vulnerability is shortened (25).
The availability to plants of critical resources such as nutrients and water limits growth rates, and the combination of resource availability and growth rate appears to select for varied but predictable defense strategies (26–28). Plant species in environments with abundant resources are more likely to have evolved rapid growth rates, short-lived leaves, inexpensive and mobile defenses, and high nutritional value, traits that result in high herbivory rates. The opposite trends are thought to hold for resource-limited environments (26). Supporting these observations are several field studies that show a general correlation of leaf lifetime and defense capability (27, 29–32). In other studies using leaves from a range of biomes (33, 34), leaf lifespan consistently exhibited significant positive correlations with the ratio of leaf mass to surface area (a general indicator of herbivory resistance) and negative correlations with the concentration of nitrogen, a critical nutrient for herbivores (35). These data imply the possible generality of greater herbivory on short-lived vs. long-lived leaves, which can be tested by using the fossil record if correct inferences can be made about leaf lifespan in the past.
In an earlier study (8), well preserved fossil leaves and associated insect damage were examined from the humid-temperate to subtropical latest Paleocene and the humid-subtropical early Eocene of southwestern Wyoming. Plant diversity and insect herbivory rose significantly with temperature, in accord with modern analog observations based on latitudinal trends. Here, we refine and extend the previous work to include the less humid, more seasonally dry, subtropical but cooler climates of the middle Eocene. Increased rainfall seasonality is well documented by thick sequences of evaporitic rocks from the late early Eocene of Wyoming (36) and is supported by climatic interpretations of the overlying, latest early and middle Eocene Green River flora (37–39). In contrast to the middle early Eocene, when evergreen taxa were present but not notably sclerophyllous, it has been observed that the leaves of many Green River species are small and possess a thick texture of dense aspect, evidenced by a prominent carbon residue in fossils (37). These changes probably reflect the drier climates of the middle Eocene. As precipitation decreases, leaf thickness in many habitats today exhibits a tendency to increase (40), whereas leaf size decreases (41). Additional, unknown factors such as soil quality could also have affected leaf size and texture. Leaf thickness and density correlate with leaf lifespan in modern vegetation (34, 40), and we infer that many species in the Green River flora were evergreen, with long-lived leaves. Thin-leaved, deciduous species also were present, such as sycamores and poplars (37).
We test the hypothesis that herbivory was consistently greater on short-lived vs. long-lived leaves throughout the study interval and that these differences were best expressed during the middle Eocene, because of the thick leaf texture, small leaf size, and possibly high insect resistance of some plant hosts. We also examine the overall response of herbivory to climate change.
Methods
Insect body fossils are virtually absent from the study area during the late Paleocene and through most of the early Eocene (P.W. and C.C.L., unpublished observation). Even when present, body fossils do not provide direct associational evidence with plants, and trophic information can not always be inferred (9). To bypass these problems, we used a method based on quantitative analysis of fossilized insect damage, which can provide direct associational evidence of diverse insect feeding and is often accessible when well preserved fossil plants are present (8, 9).
We recorded the presence or absence of 40 discrete morphologies of insect damage, hereafter termed “damage types,” within four functional feeding groups (external foliage feeding, mining, galling, and piercing-and-sucking), on all 2,435 identified and nonfragmentary dicot leaves found at six quarry sites (Fig. 1). These leaves represent 58 species. Leaflets of compound leaves were counted and analyzed as if they were simple leaves; the Webb size class of each leaf was also recorded (41). The damage types were the same as those used in the earlier study (8), with some minor modifications and additions. The six sites feature excellent preservation and floral diversity relative to contemporaneous strata in the region (39). Two sites were from the fluvial, late Paleocene Fort Union Formation (749 leaves; 10 dicot species; ≈56 Ma), and two were found in the fluvial, early Eocene Wasatch Formation (792 leaves; 22 species; ≈53 Ma), all four from southwestern Wyoming and discussed elsewhere (8). The two newly analyzed sites, collected in 1996, were from the uppermost portion of the upper member of the lacustrine Green River Formation in northeastern Utah (894 leaves; 28 species), which has a radiometric age of ≈43 Ma (43) (middle Eocene). Each pair of sites was combined for analysis to raise specimen counts and to balance site peculiarities, and we refer hereafter to the three combined pairs, each representing one of the three time periods, as “samples.” Voucher specimens are housed in the National Museum of Natural History, Smithsonian Institution (USNM; late Paleocene and early Eocene specimens), and the Denver Museum of Nature and Science (DMNH; middle Eocene specimens).
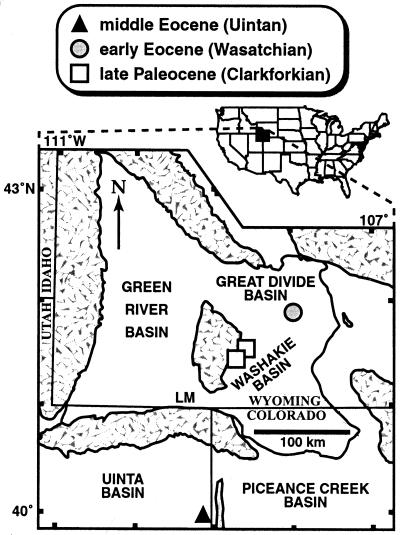
Figure 1.
Sampling areas. Symbols indicate census sites, which total six, with two each within the symbols for the late Paleocene and middle Eocene. Patterned areas are uplifts. Late Paleocene sites: USNM locs. (localities) 41270, 41.5658°N, 108.5765°W, and 41300, 41.4605°N, 108.6988°W, Fort Union Formation, Washakie Basin. Early Eocene sites: USNM locs. 41342, 41.9263°N, 107.9899°W, and 41352, 41.9091°N, 107.9951°W, Wasatch Formation, Great Divide Basin. Middle Eocene sites: DMNH locs. 323 and 1732, both 39.9411°N, 109.1347°W, Green River Formation, Uinta Basin. LM, Little Mountain flora (Bridgerian), mentioned in the text (39). “Clarkforkian,” “Wasatchian,” “Bridgerian,” and “Uintan” are successive North American land mammal “ages” (biozones) (42). See ref. 39 for additional locality information. Redrawn after ref. 39.
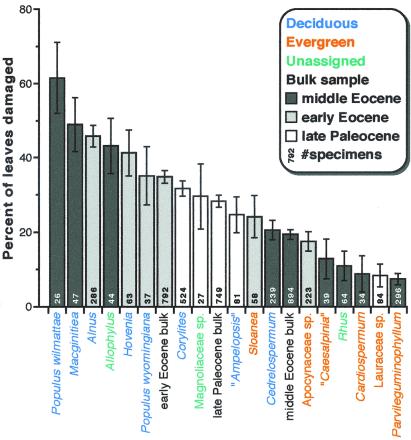
Proxy criteria were used to infer whether well sampled plant species (≥20 specimens) more likely bore short- or long-lived leaves. The categorizations were based on the combined, available evidence from reproductive strategy, leaf texture, leaf-margin state, and inference from living relatives. Three species could not be categorized in this manner. For convenience and recognizability, we will use the terms “deciduous” and “evergreen” for the two categories. Our usage of “deciduous” reflects an inference of relatively short leaf lifespan and high leaf turnover, and “evergreen” implies the opposite. The species assigned to these categories may or may not have been associated with synchronous or asynchronous abscission, as the respective terms “deciduous” and “evergreen” are conventionally applied. From this pool of species with more than 20 specimens, a cutoff of ≥25 specimens per species was found to be conservative and stable for comparisons of herbivory frequency and diversity among individual species. The 17 taxa remaining above the cutoff (Fig. 2) comprise 89.1% of total specimens.
Figure 2.
Herbivory frequency (percentage basis), coded by species, time period, and presumed category of leaf lifespan, for species with ≥25 specimens in individual samples and for all specimens in each of the three samples (bulk). Error bars indicate ±1σ of binomial error, based on the number of specimens (8). Only the generic or family name is given for plant hosts, except for congeneric species (see Tables 2 and 3, which are published as supplemental data, for nomenclature). Mean herbivory frequency is significantly higher for deciduous than for evergreen hosts (F[1,12] = 20.7, P < 0.001). The comparison is still significant at the 95% level at a cutoff of ≥20 specimens (F[1,16] = 4.36, P = 0.053). Even if Allophylus is coded as evergreen and Rhus as deciduous, significance remains (F[1,14] = 6.68, P = 0.022 at the 25-specimen cutoff). Deciduous means are higher than evergreens within each time period as well; these within-sample differences are significant in the two Eocene samples, but the number of species in the Paleocene is too low to support significance (Paleocene F[1,1] = 15.6, P = 0.158; early Eocene F[1,3] = 18.5, P = 0.023; middle Eocene F[1,4] = 9.37, P = 0.038). Average leaf area (mean natural log of mm2) from left to right: 7.40, 7.84, 7.40, 7.11, 7.27, 6.90, 7.35, 7.49, 8.17, 7.51, 7.54, 8.27, 5.35, 5.44, 6.93, 4.99, 6.20, 5.35, 7.42, 4.24.
Color photographs of selected examples of insect damage and annotated lists of the damage types and the plant hosts (with leaf lifespan categories, bases for categorization, and nomenclatural references), as well as both summary and complete data matrices, are shown in Fig. 5 and Tables 2–10, which are published as supplemental data on the PNAS web site, www.pnas.org.
The late Paleocene and early Eocene deposits were derived from low areas of floodplains, in climates that lacked any severe dry season or winter frost. Estimated mean annual paleotemperatures for these samples are ≈14°C and ≈21°C, respectively, with mean annual precipitation of ≈140 cm for both (39). Middle Eocene mean annual temperatures are interpreted as ≈15°C or probably higher with winter temperatures still above freezing, and annual rainfall is thought to have been less than 100 cm and seasonal (37, 38, 41, 44). The middle Eocene sample represents the vegetation of a mixture of habitats, including lakeshores, river valleys, and alluvial fans (37, 45). Middle Eocene sycamores (Macginitiea wyomingensis) and members of the poplar family (Populus wilmattae, Populus cinnamomoides, Salix cockerelli) were deciduous taxa that in all probability inhabited the mesic, low-lying habitats of the otherwise moisture-stressed landscape, given the habitat preferences of their extant and extinct relatives (37, 46, 47).
Several possible biases may have affected our data. First, the late Paleocene and early Eocene leaves were only minimally transported (39), whereas reconstructions and field observations suggest that the middle Eocene sample was possibly transported more than 10 km from shore (45). Despite the transport distance, the preservation of fine features of leaf venation and insect damage in the middle Eocene sample argues against any significant degradation of the herbivory signal among the preserved leaves. However, the increased transport is likely to decrease greatly the accuracy with which the relative abundance of the fossil assemblage represents that of the source forest (48), so that we cannot assess whether dry climates led to a greater abundance or diversity of deciduous host plants. A second issue is the geographic separation, by 173 km or more, of the middle Eocene sampling sites from the others (Fig. 1). However, all of the common species found in the middle Eocene sample are also present in a qualitative sample of latest early Eocene age (≈50 Ma) from the Green River Formation at Little Mountain, Wyoming (Fig. 1) that had a very similar paleoclimate (39), suggesting that there were no significant latitudinal or provincial effects on floral composition.
To analyze the diversities of damage types found both in the three bulk samples and on individual hosts, we constructed bootstrap curves with 5,000 replicates to adjust for the effects of uneven specimen counts among species (8). Single-classification analysis of variance was used for statistical comparisons of means (49), and all F-tests and related p-values reported were derived by using this technique unless otherwise stated. All percentage data, when used, were transformed before analysis as the arcsine, in degrees, of the square root of the proportion equal to the given percentage (49). Many statistical tests rendered significant results. However, an impractical scale of collecting, approaching an order of magnitude more specimens, would be needed to recover sufficient numbers of rare species and damage types for several tests or to make possible greater levels of significance.
Results
Damage Frequency.
Deciduous hosts bear significantly higher damage percentages than evergreens, both overall and within each time period (Fig. 2). The late Paleocene is the only time period for which this difference is not statistically significant, but there are only three species for comparison in that sample. Two deciduous taxa from the late Paleocene occupy the middle of the distribution shown in Fig. 2. However, the three deciduous hosts from the early Eocene exhibit greater damage frequencies than any of their Paleocene counterparts. Middle Eocene plant species occupy the extremes of the distribution. The maximum frequencies of herbivory are found on two deciduous species from the middle Eocene, M. wyomingensis and P. wilmattae. We also note high herbivory frequencies on the two middle Eocene hosts in the poplar family that were each represented by fewer than 25 specimens: P. cinnamomoides (23 specimens, 26% damaged) and S. cockerelli (24 specimens, 33% damaged).
The frequency of herbivory for bulk samples rises from the late Paleocene to the early Eocene (Fig. 2). This rise is attributed to warming temperatures (8). The bulk values for the middle Eocene are the lowest in the sequence, the result of minimal herbivory on abundant, small-leaved hosts (e.g., Cedrelospermum, Parvileguminophyllum; Fig. 2). Leaf size (mean natural log) correlates significantly with herbivory frequency for the eight middle Eocene hosts in Fig. 2 (r2 = 0.787, P = 0.003, n = 8), although there is no such correlation among the nine other hosts (r2 = 0.003). A preliminary analysis of the eight middle Eocene hosts from digital measurements shows that species leaf area (mean natural log) correlates significantly with the percentage of leaf area consumed (r2 = 0.597, P = 0.025, n = 8), suggesting a defensive role for small leaf size (25). With the exception of Cedrelospermum, all of the small-leaved hosts in the middle Eocene sample were also coded as evergreen (Fig. 2).
Damage Diversity.
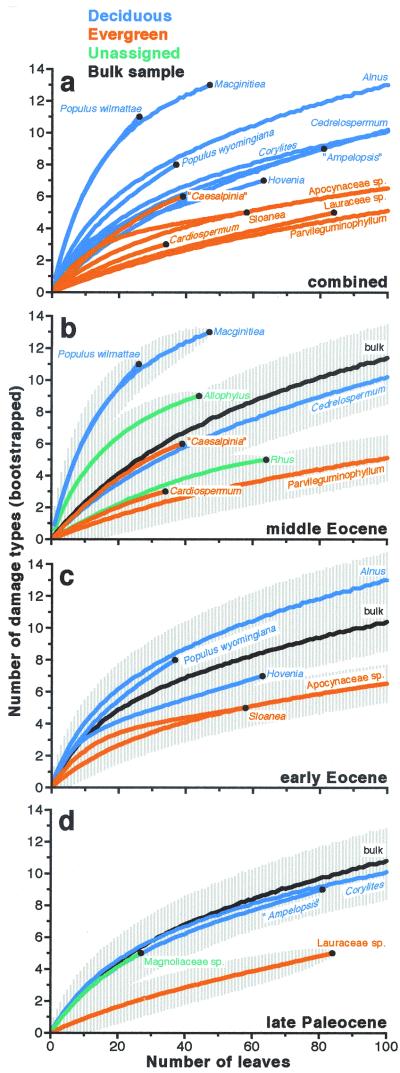
The bootstrap curves for the three bulk samples are nearly identical, a necessary result of there being approximately the same absolute number of damage types in each time period (Fig. 3, black curves). These bulk curves therefore provide a baseline for comparison of the bootstrap curves of individual species.
Figure 3.
Bootstrapped damage diversity for all host species with ≥25 specimens recovered in a time period, and for all specimens in the three bulk samples. The curves show the mean number of damage types, ±1σ, for each number of specimens, for 5,000 random subsamples without replacement during a subsampling run (ftp://geosci.uchicago.edu/pub/paleo/programs/source/wilfbootstrap.nb). The horizontal scale is reduced to 100 for greater detail. (a) Detail of all plant species assigned to evergreen and deciduous categories, error bars removed. Deciduous hosts bear significantly more damage types than evergreens (F[1,12] = 10.5, P = 0.007, computed at 25 specimens). (b–d) Individual plots for the three time periods, including bulk samples and ±1σ error bars. Deciduous hosts bear more damage types than evergreens within all three samples, although the significance levels are only 92–93%.
Like the frequency data (Fig. 2), the bootstrap curves of species (Fig. 3) indicate greater herbivory on deciduous forms, a significant trend that is particularly striking in a plot that combines all three samples (Fig. 3a). Maximum damage diversity climbs from the late Paleocene to the early Eocene, in parallel with the frequency data (Fig. 3 c and d; ref. 8). Error bars of one standard deviation indicate the presence of the same two statistical domains of damage diversity in both time periods: (a) bootstrap curves that group around the bulk plots (Corylites, Cedrelospermum), separated from (b) bootstrap curves of species bearing low damage diversity that group below the bulk plots with an intervening error-bar gap (Lauraceae sp., Sloanea).
Within the middle Eocene data (Fig. 3b), a third domain of high damage diversity appears, represented, as in the frequency data (Fig. 2), by M. wyomingensis and P. wilmattae. These two taxa were attacked by an augmented diversity of insects, whose traces include 14 of the 20 types of external feeding found in the middle Eocene sample, three of four galling types, and two of three mining types, all found on only 73 leaves.
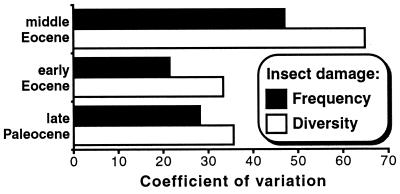
Variances for both frequency (Fig. 2) and diversity (Fig. 3) data are substantially higher for the middle Eocene (Fig. 4). When variance is standardized to the mean by using the coefficient of variation, damage diversity exhibits greater variation than does damage frequency in all three time periods (Fig. 4). Complementing the pattern seen in the frequency data, leaf size (average natural log) correlates significantly with damage diversity (at 25 specimens) for the eight middle Eocene hosts in Fig. 3 (r2 = 0.770, P = 0.004, n = 8), although there is no such correlation among the nine other hosts (r2 = 0.014). Damage frequency (Fig. 2) and diversity (Fig. 3) are significantly correlated (r2 = 0.826, P < 10−6, n = 17, damage diversity values at 25 bootstrapped specimens).
Figure 4.
The coefficients of variation (100σ/mean; ref. 49) for damage frequency and diversity (Figs. 2 and 3). The damage diversity values used were the bootstrap means for the 25th specimen of each species (Fig. 3).
Galling and Mining.
Our data suggest an increase in galling and a decrease in mining through the study interval (Table 1). Although sample sizes are low because of the rarity of these damage types, we make preliminary observations here because significantly larger samples would require an unprecedented collection effort. In the variety of measures shown in Table 1 that take host data into account, the trends are unidirectional, such as the percentages of host species galled or mined. We also constructed a proxy measure for galling and mining diversity, the damage ratio (Table 1), as follows. Host specificity characterizes most known lineages of gallers and miners to a high degree (22, 23, 50–52). Galls and mines with similar appearances as compression fossils may well represent different ancient species of insect feeders, especially if they occur on different hosts. As an approximation of a condition of high host specificity, the damage ratio was generated by counting each type of galling or mining damage once for each occurrence on a distinct host plant in a sample and dividing by the number of host plant species, both with and without singletons. The same unidirectional trends emerge (Table 1). Also corroborating this pattern are simple counts of the absolute numbers of gall and mine damage types in the three fossil samples (Table 1). An exception to the unidirectional trends is the maximum in galling frequency in the early Eocene sample, when counted without regard to host data: eleven of the 16 galled leaves in that sample were found on the most abundant host (Alnus sp.), and ten of these were the same type of galling damage, suggesting a single galling species. The absolute frequencies of galled and mined leaves were very low overall but compatible with site-specific data from living forests. Galling frequencies on extant oak leaves range from 0.10–5.9% (53, 54), and mining frequencies from 0.01–8.0% have been reported from various living plant taxa (55–57).
Table 1.
Galling and mining data
| Galls
|
Mines
|
|||||
|---|---|---|---|---|---|---|
| Late Pal. | Early Eoc. | Middle Eoc. | Late Pal. | Early Eoc. | Middle Eoc. | |
| Damage types | 0 | 3 | 4 | 5 | 3 | 3 |
| Leaves damaged | 0 | 16 | 12 | 9 | 7 | 3 |
| Leaves damaged, % | 0 | 2.02 | 1.34 | 1.20 | 0.884 | 0.336 |
| Host species damaged, % | 0 | 13.6 | 25.0 | 30.0 | 13.6 | 10.7 |
| Host species damaged, no singletons, % | 0 | 17.6 | 23.5 | 42.9 | 17.6 | 11.8 |
| Damage types, host basis | 0 | 5 | 8 | 5 | 4 | 3 |
| Damage ratio | 0 | 0.227 | 0.286 | 0.500 | 0.182 | 0.107 |
| Damage types, host basis, no singletons | 0 | 5 | 6 | 5 | 4 | 3 |
| Damage ratio, no. singletons | 0 | 0.294 | 0.353 | 0.714 | 0.235 | 0.176 |
The damage ratio is the sum of the numbers of gall or mine damage types found on each host species (number of damage types, host basis), divided by the number of host species. Although no galls occurred in the late Paleocene sample, we have recovered three galls from qualitatively sampled localities at the same stratigraphic level as USNM loc. 41270 (Fig. 1; 8). Number of host species in the three successive samples: 10, 22, and 28. Singletons are host species represented by only one specimen, which are unevenly distributed among the three samples. Number of nonsingleton hosts: 7, 17, and 17. Pal., Paleocene; Eoc., Eocene.
The maximum in galling by most measures during the middle Eocene (Table 1) is consistent with studies that indicate a higher diversity of gallers in dry habitats (22, 24). Regarding mining, observations on the physiological needs of leaf miners consistently point to the importance of desiccation avoidance (51, 58, 59), which suggests that a mining decrease may be tied to a relative lack of water within the leaf tissue of thick-textured evergreens (30) and the increasing representation of such host plants in the three successive samples.
Discussion
Our data suggest that climate change and plant defense had linked effects on insect herbivory over an extended period of time and within a single region. For the most part, our results can be correlated to observations made in living forests, implying that some recently documented characteristics of plant–insect associations may be ancient. However, we caution against an oversimplified comparison because of the vastly different time scales, techniques, and limits of observation, as well as the lack of direct modern analogs. Even within extant communities, insect feeding can be highly variable (60). Data from other regions and time periods are needed to assess the generality and broader evolutionary implications of our results.
Higher damage frequency and diversity on deciduous vs. evergreen taxa were found throughout the study interval (Figs. 2 and 3), in agreement with recent work showing that short-lived leaves are more susceptible to insect damage (26, 27, 29, 30, 32). The two end-member life strategies for plants were a short leaf lifespan with low investment in defense and a long leaf lifespan with high investment in defense. The first strategy is an opportunistic, rapid-growth lifestyle that capitalizes on favorable conditions while sacrificing defensive capabilities, whereas the second adaptive route emphasizes slow growth and high defense (26, 27, 61, 62). A range of defense strategies with these end-members stayed in place for more than 10 million years, even as individual host species turned over.
Climate change apparently had significant effects on herbivory. Foliage consumption increased from the late Paleocene to the early Eocene thermal maximum, suggesting a correlation between temperature and herbivory that is independent of latitude (8, 63). The seasonally dry climates of the middle Eocene were associated with an influx of thick- and small-leaved host plants and increased variance in the frequency and diversity of insect damage. Small leaf size, with the possibility of rapid leaf expansion (25), may have been an important defense strategy for many host plants, both evergreen and deciduous.
The most heavily consumed taxa from the middle Eocene were M. wyomingensis and P. wilmattae, deciduous trees with large leaves that occupied low-lying habitats with relatively high water availability. Exceptionally elevated herbivory was one of the costs for fast growth rates and access to mesic environments, as is the case for living sycamores and poplars from moist corridors of seasonally dry, warm temperate and subtropical areas of the southwestern United States and northwestern Mexico (64, 65). This intense damage is also consistent with herbivory data from deciduous plants in modern dry Neotropical forests (14, 29, 66). Although few studies exist, it has been hypothesized that the high rates of herbivory on deciduous species from dry forests may be due to greater densities of herbivores because of lower plant defenses and fewer insect predators and parasitoids (14, 20).
In contrast to the deciduous hosts, the evergreen strategy of long leaf lifespan and high defense was also employed during the middle Eocene, and the elevated variance in herbivory indicates that both deciduous and evergreen strategies were well represented. Evergreens, with their stronger defenses, became hosts to fewer species and numbers of insects, for which the higher biological costs of feeding were compensated by a relatively dependable food supply. Thus, a significant component of the insect population avoided evergreen taxa and instead consumed species with palatable, short-lived leaves.
Supplementary Material
Acknowledgments
For invaluable field, laboratory, and logistical assistance, we thank A. Ash, A. Daniels, F. Marsh, L. McMath, B. Miljour, R. Schrott, K. Werth, the Denver Leaf Whackers, and the staff of Western Wyoming Community College. We are also grateful to two anonymous colleagues for reviews; R. Burnham, R. Horwitt, D. Jablonski, and D. Smith for helpful critiques of drafts; A. Knoll and D. Schoolmaster for discussions; and the Bureau of Land Management office in Vernal, Utah, for land access. P.W. received support from the Michigan Society of Fellows, the Petroleum Research Fund of the American Chemical Society, a Smithsonian Institution Postdoctoral Fellowship, and the Evolution of Terrestrial Ecosystems Program of the Smithsonian Institution (ETE). C.C.L. acknowledges support from the Smithsonian Scholarly Studies Program, the Walcott Fund of the National Museum of Natural History, and the National Geographic Society. P.D.C. was funded through the National Science Foundation. This is ETE contribution no. 75.
Abbreviation
- Ma
million years before present
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Coley P D. Clim Change. 1998;39:455–472. [Google Scholar]
- 2.Ayres M P, Lombardero M J. Sci Total Environ. 2000;262:263–286. doi: 10.1016/s0048-9697(00)00528-3. [DOI] [PubMed] [Google Scholar]
- 3.Feeny P P. In: Biochemical Interaction Between Plants and Insects. Wallace J, Mansell R L, editors. New York: Plenum; 1976. pp. 1–40. [Google Scholar]
- 4.Delcourt P A, Delcourt H A. Long-Term Forest Dynamics of the Temperate Zone: A Case Study of Late Quaternary Forests in Eastern North America. New York: Springer; 1987. [Google Scholar]
- 5.Elias S A. Quaternary Insects and Their Environments. Washington, DC: Smithsonian Inst. Press; 1992. [Google Scholar]
- 6.Coope G R, Wilkins A S. Philos Trans R Soc London B. 1994;344:19–26. [Google Scholar]
- 7.Crane P R, Jarzembowski E A. J Nat Hist. 1980;14:629–636. [Google Scholar]
- 8.Wilf P, Labandeira C C. Science. 1999;284:2153–2156. doi: 10.1126/science.284.5423.2153. [DOI] [PubMed] [Google Scholar]
- 9.Labandeira C C, Johnson K R, Lang P. In: The Hell Creek Formation and the Cretaceous-Tertiary boundary in the Northern Great Plains: An Integrated Record of the End of the Cretaceous. Hartman J H, Johnson K R, Nichols D J, editors. 2001. in press. [Google Scholar]
- 10.Erwin T L. Coleopterists' Bull. 1982;36:74–75. [Google Scholar]
- 11.Richards P W. The Tropical Rain Forest. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 12.Price P W. Insect Ecology. New York: Wiley; 1997. [Google Scholar]
- 13.Coley P D, Aide T M. In: Plant Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Price P W, Lewinsohn T M, Fernandes G W, Benson B B, editors. New York: Wiley; 1991. pp. 25–49. [Google Scholar]
- 14.Coley P D, Barone J A. Annu Rev Ecol Syst. 1996;27:305–335. [Google Scholar]
- 15.Windsor D M. In: The Ecology of Arboreal Folivores. Montgomery G G, editor. Washington, DC: Smithsonian Inst. Press; 1978. pp. 101–113. [Google Scholar]
- 16.Janzen D H. Ecology. 1973;54:687–708. [Google Scholar]
- 17.Frith C B, Frith D W. Aust J Ecol. 1985;10:237–248. [Google Scholar]
- 18.Marquis R J, Braker H E. In: La Selva: Ecology and Natural History of a Neotropical Rain Forest. McDade L A, Bawa K S, Hespenheide H A, Hartshorn G S, editors. Chicago: Univ. Chicago Press; 1994. pp. 261–281. [Google Scholar]
- 19.Leigh E G, Jr, Windsor D M. In: The Ecology of a Tropical Forest: Seasonal Rhythms and Long-Term Changes. Leigh E G Jr, Rand A S, Windsor D M, editors. Washington, DC: Smithsonian Inst. Press; 1996. pp. 111–122. [Google Scholar]
- 20.Godfray H C J. Parasitoids: Behavioral and Evolutionary Ecology. Princeton: Princeton Univ. Press; 1994. [Google Scholar]
- 21.Fernandes G W, Price P W. In: Plant Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Price P W, Lewinsohn T M, Fernandes G W, Benson B B, editors. New York: Wiley; 1991. pp. 91–115. [Google Scholar]
- 22.Price P W, Fernandes G W, Lara A F, Brawn J, Barrios H, Wright M G, Ribeiro S P, Rothcliff N. J Biogeogr. 1998;25:581–591. [Google Scholar]
- 23.Wright M G, Samways M J. Oecologia. 1998;115:427–433. doi: 10.1007/s004420050537. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes G W, Price P W. Oecologia. 1992;90:14–20. doi: 10.1007/BF00317803. [DOI] [PubMed] [Google Scholar]
- 25.Moles A T, Westoby M. Oikos. 2000;90:517–524. [Google Scholar]
- 26.Coley P D, Bryant J P, Chapin F S., III Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- 27.Coley P D. Oecologia. 1988;74:531–536. doi: 10.1007/BF00380050. [DOI] [PubMed] [Google Scholar]
- 28.Marques E S A, Price P W, Cobb N S. Environ Entomol. 2000;29:696–703. [Google Scholar]
- 29.Stanton N. Biotropica. 1975;7:8–11. [Google Scholar]
- 30.Coley P D. Ecol Monogr. 1983;53:209–233. [Google Scholar]
- 31.Lowman M D. J Ecol. 1992;80:433–447. [Google Scholar]
- 32.Basset Y. Acta Oecologica. 1994;15:181–191. [Google Scholar]
- 33.Reich P B, Walters M B, Ellsworth D S. Proc Natl Acad Sci USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ackerly D D, Reich P B. Am J Bot. 1999;86:1272–1281. [PubMed] [Google Scholar]
- 35.Mattson W J., Jr Annu Rev Ecol Syst. 1980;11:119–161. [Google Scholar]
- 36.Roehler H W. US Geol Surv Prof Paper. 1993;1506-F:1–74. [Google Scholar]
- 37.MacGinitie H D. Univ Calif Publ Geol Sci. 1969;83:1–140. [Google Scholar]
- 38.Wolfe J A. In: The Carbon Cycle and Atmospheric CO2: Natural Variations Archean to Present. Sundquist E T, Broeker W S, editors. Washington, DC: Am. Geophys. Union; 1985. pp. 357–375. [Google Scholar]
- 39.Wilf P. Geol Soc Am Bull. 2000;112:292–307. [Google Scholar]
- 40.Givnish T J. New Phytol. 1987;106:131–160. [Google Scholar]
- 41.Wilf P, Wing S L, Greenwood D R, Greenwood C L. Geology. 1998;26:203–206. [Google Scholar]
- 42.Woodburne M O. Cenozoic Mammals of North America: Geochronology and Biostratigraphy. Berkeley, CA: Univ. Calif. Press; 1987. [Google Scholar]
- 43.Remy R R. US Geol Surv Bull. 1992;1787-BB:1–79. [Google Scholar]
- 44.Wing S L, Greenwood D R. Philos Trans R Soc London B. 1993;341:243–252. [Google Scholar]
- 45.Franczyk K J, Fouch T D, Johnson R C, Molenaar C M, Cobban W A. US Geol Surv Bull. 1992;1787-Q:1–37. [Google Scholar]
- 46.Hickey L J, Doyle J A. Bot Rev. 1977;43:3–104. [Google Scholar]
- 47.Hickman J C. The Jepson Manual: Higher Plants of California. Berkeley, CA: Univ. Calif. Press; 1993. [Google Scholar]
- 48.Burnham R J, Wing S L, Parker G G. Paleobiology. 1992;18:30–49. [Google Scholar]
- 49.Sokal R R, Rohlf F J. Biometry. New York: Freeman; 1995. [Google Scholar]
- 50.Abrahamson W G, Melika G, Scrafford R, Csoka G. Am J Bot. 1998;85:1159–1165. [PubMed] [Google Scholar]
- 51.Connor E F, Taverner M P. Oikos. 1997;79:6–25. [Google Scholar]
- 52.Powell J A, Mitter C, Farrell B D. In: Lepidoptera, Moths and Butterflies. Kristensen N P, editor. Vol. 1. Berlin: Walter de Gruyter; 1999. pp. 403–422. [Google Scholar]
- 53.Boecklen W J, Larson K C. Gen Tech Rep NC (North Cent For Exp Stn) 1994;NC-174:110–120. [Google Scholar]
- 54.Boecklen W J, Spellenberg R. Oecologia. 1990;85:92–100. doi: 10.1007/BF00317348. [DOI] [PubMed] [Google Scholar]
- 55.Hinckley A D. Environ Entomol. 1972;1:358–361. [Google Scholar]
- 56.Faeth S H, Simberloff D. Ecology. 1981;62:625–635. [Google Scholar]
- 57.Boomsma J J, Timmermans H, Cörvers C P M, Kabout J. Holarctic Ecol. 1987;10:206–218. [Google Scholar]
- 58.Willmer P G. Adv Insect Physiol. 1982;16:1–57. [Google Scholar]
- 59.Givnish T J. J Ecol. 1999;87:193–210. [Google Scholar]
- 60.Lowman M D. Proc Linn Soc NSW. 1995;115:77–87. [Google Scholar]
- 61.Ernest K A. Biotropica. 1989;21:194–199. [Google Scholar]
- 62.Feeny P P. Recent Adv Phytochem. 1976;10:1–40. [Google Scholar]
- 63.Coley P D. Science. 1999;284:2098–2099. [Google Scholar]
- 64.Smith R H. Arborist's News. 1944;9:9–16. [Google Scholar]
- 65.Brown L R, Eads C O. Univ Calif Agr Exp Stn Bull. 1965;818:1–38. [Google Scholar]
- 66.Filip V, Dirzo R, Maass J M, Sarukhán J. Biotropica. 1995;27:78–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.